Abstract
Background
Induction test of atrial fibrillation (AF) is one of endpoint measures in catheter ablation (CA). However, its predictive value in long‐term outcome remains controversial.
Methods
Ninety‐eight patients (61 years, 77 males) with persistent AF who underwent pulmonary vein antrum isolation‐based CA were retrospectively analyzed. We determined whether inducibility of AF/atrial tachyarrhythmias (AT) by atrial burst pacing at the end of CA and other characteristics were associated with the recurrence of AF/AT. Atrial burst pacing was performed with 30‐beat from the coronary sinus; increasing from 240 to 320 ppm. Inducibility was defined as AF/AT lasting ≥5 minutes following atrial burst pacing.
Results
AF/AT was induced in 50 patients (51%). During 1 year of follow‐up, 71 patients (72.4%) had no recurrence of AF/AT. A logistic regression analysis showed that female gender (OR 3.8; P = 0.02), multiple sessions (OR 3.5; P = 0.02), and early recurrence of AF/AT (OR 5.3; P = 0.004) were associated with clinical recurrence. AF/AT Inducibility was not associated with clinical recurrence (P = 0.65). A subanalysis in patients with enlarged LA (LA diameter ≥45 mm, n = 40) showed that AF/AT inducibility was associated with recurrence (OR 8.1; P = 0.04). The positive and negative predictive values of AF/AT inducibility for AF/AT recurrence were 41 and 89%, respectively. Negative predictive value was increased to 92.3% when the inducibility was defined as AF/AT of ≥30 seconds following atrial burst pacing.
Conclusions
AF/AT inducibility cannot predict long‐term clinical recurrence in patients with persistent AF. However, it may have a prognostic value especially in patients with enlarged LA.
Keywords: atrial burst pacing, atrial fibrillation, catheter ablation, predictive value, pulmonary vein isolation
1. INTRODUCTION
Although technologes in catheter ablation (CA) of atrial fibrillation (AF) have been dramatically developed, recurrent cases are still important issues. Pulmonary vein antrum isolation (PVAI) is well accepted as a basic ablation strategy for AF,1, 2 but number of report indicated that PVAI alone could not achieve sufficient therapeutic outcomes in persistent/long‐standing persistent AF cases.3, 4
Unlike CA of other tachyarrhythmias, there are no clear electrophysiological endpoint measures in AF catheter ablation beyond the completion of PVAI. Hence, induction test of AF by atrial burst pacing is used as one of possible endpoint measures at the end of CA. Previous studies demonstrated that the noninducibility of AF by atrial burst pacing identified patients who were less likely to have recurrent AF during follow‐up.5, 6, 7, 8 However, most of these studies limited to patients with paroxysmal AF. Therefore, it remains undetermined whether the response to the atrial burst pacing can predict long‐term outcomes in persistent AF.6, 7, 9, 10 In addition, the differences in stimulation protocol and definition of inducibility among studies might lead to the discordant conclusions regarding the predictive value of the atrial burst pacing in long‐term outcomes.5, 6, 7, 8, 9, 10, 11, 12, 13
The purpose of this study was to evaluate the prognostic value of AF induction by atrial burst pacing in patients with persistent/long‐standing persistent AF.
2. METHODS
2.1. Study population
This study enrolled 98 consecutive patients with drug‐refractory persistent/long‐standing persistent AF referred to our institution for AF ablation between January 2013 and December 2016. We enrolled both 1st session cases and multiple session cases. In multiple session cases, results of the last session during the studied period were analyzed. The definition of persistent AF and long‐standing persistent AF was followed by the American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines.14 In short, persistent AF was defined as episodes lasting more than 7 days and long‐standing persistent AF was defined as episodes lasting more than 12 months.
Antiarrhythmic drugs were discontinued for 2.1 ± 2.5 days before the procedure. Inducibility of AF/atrial tachycardia (AT) by atrial burst pacing at the end of CA procedure and other covariates were retrospectively validated regarding clinical AF/AT recurrence at 1 year follow‐up. In order to validate the diagnostic accuracy of atrial burst pacing in different clinical backgrounds, we also performed subanalyses of the following four categories; enlarged left atrium (LA), multiple sessions, extra‐PVAI procedure, and long‐standing persistent AF. This study was approved by the institutional review board for ethics at our institution (approval no. 29‐44).
2.2. Electrophysiological study
The presence of LA thrombi was excluded by contrast‐enhanced computed tomography. When a contrast defect in the left atrial appendage was suspected, we excluded the presence of LA thrombi by transesophageal echocardiography. All patients underwent an electrophysiological study in the fasting state under conscious sedation. At the beginning of the procedure, a bolus injection of pentazocine 7.5‐15 mg and hydroxyzine pamoate 12.5‐25 mg was performed. During the procedure, continuous infusion of dexmedetomidine hydrochloride 0.3‐0.6 μg/kg/h was performed. During the ablation attempt, a bolus injection of thiamylal sodium 25 mg was added as needed. A 20 pole catheter was inserted through the right jugular vein (BeeAT® Japan Lifeline, Tokyo). The proximal portion of the catheter was positioned along the superior vena cava (SVC) and crista terminalis (CT), and the distal portion was positioned in the coronary sinus (CS) for pacing and internal cardioversion.
Following a transseptal puncture under guidance with an intracardiac echocardiography catheter (5.5‐10 MHz, 8Fr, AcuNav; Biosense Webster, Diamond Bar, CA, USA), two or three long sheaths (SL1®; AF Division, St. Jude Medical, Minneapolis, MN, USA) were introduced into the LA via the same transseptal puncture site. After left atriography was performed, mapping catheters (20 pole circular catheter or PentaRay NAV® catheter; Biosense Webster) and an ablation catheter were positioned in the pulmonary veins. The electrophysiological studies were performed under the support of an electroanatomical mapping system with the CARTO system (Biosense Webster) or Ensite Velocity system (St. Jude Medical).
2.3. Catheter ablation
We performed PVAI‐based ablation in all patients. A 3.5 mm open‐irrigated‐tip ablation catheter was used, and the ablation catheter was irrigated with heparinized saline. Radiofrequency energy was delivered with a maximum temperature setting of 43°C and a power of 20‐35 W. We defined successful PVAI as the loss of PV potentials during sinus rhythm or CS pacing (entrance block) and local PV capture without left atrial capture by pacing from a circular mapping catheter or an ablation catheter placed at the PVs just distal to the radiofrequency ablation lesions (exit block).
In cases with documented non‐PV triggers after the completion of PVAI, we added ablation targeting the trigger sites and aimed at complete elimination of the AF inducibility from the targeted sites. Linear ablation or defragmentation or both were also added after PVAI based on the electrophysiological findings in each case.
2.4. Postcatheter ablation pacing protocol
Following completion of ablation procedure, patients underwent a uniform atrial burst pacing protocol from the CS ostium. The atrial burst pacing was performed with 30 beats at an amplitude of 10 V and pulse width of 1 ms; increasing from 240 to 320 ppm in steps of 20 ppm or failure to 1:1 atrial capture. Inducibility of AF/AT was defined as AF/AT lasting ≥5 minutes or as AF/AT of 30 seconds in separate analyses. Induced arrhythmias were pace‐terminated or electrically cardioverted to sinus rhythm.
2.5. Follow‐up
It was at the discretion of the treating physicians whether previously tolerated antiarrhythmic drugs were restarted/continued or not after the CA.
Patients were seen in our outpatient clinic at least at 1 month, 3 months, 6 months, and 12 months after the procedure. A clinical evaluation and a 12 lead surface ECG recording were performed at each visit. In addition, whenever patients experienced symptoms suggestive of an arrhythmia, they were instructed to visit our outpatient clinic or the referring physician in order to record a 12 lead surface ECG. In cases of patients with permanent pace maker/intracardiac defibrillator, routine device interrogation was also performed to detect asymptomatic arrhythmias. Holter recording (24 hour) was performed at least once during the period of 6‐12 months after the procedure by the referring physician. The first 3 months after the procedure were regarded as a blanking period for episodes. Early recurrence of AF (ERAF) was defined as the finding of any atrial tachyarrhythmia lasting >30 seconds during the 3 month blanking period. Arrhythmia recurrence was defined as the detection of AF/AT lasting >30 seconds after a blanking period.
2.6. Statistical analysis
The data are expressed as mean ± standard deviation (SD) for continuous variables and counts and percentages for categorical variables. A comparison of categorical variables between pairs of groups was carried out using the chi‐square test or Fisher('s exact test. A comparison of continuous variables between pairs of groups was carried out using Student')s t test. We carried out a univariate analysis and a multivariate analysis of continuous variables between pairs of groups using a logistic regression analysis. Variables selected to be tested in the multivariate analysis were those with P < 0.1 in the univariate analysis. All tests were two‐sided, and P < 0.05 was considered significant. Analyses were conducted using software program JMP (SAS, Cary, NC, USA).
3. RESULTS
3.1. Patient characteristics
A total of 98 patients were enrolled in this study, 77 men (78.6%) and 21 women (21.4%), and age 61 ± 10 years. There were 63 persistent AF patients (64.3%) and 35 long‐standing persistent AF patients (35.7%). At 1 year follow‐up of CA, 71 patients did not have AF/AT recurrence. We divided the patients into two groups; recurrence group (n = 27, 27.6%) and no‐recurrence group (n = 71, 72.4%).
Table 1 summarizes the baseline characteristics of these two groups. There were no significant differences in age or comorbidity such as diabetes mellitus, hypertension, and history of congestive heart failure between the two groups. AF type (persistent AF or long‐standing persistent AF), left atrial diameter (LAD), left ventricular ejection fraction (LVEF), and biomarkers such as brain natriuretic peptide (BNP), C‐reactive protein (CRP) were also comparable between the two groups. The use of antiarrhythmic drugs, beta blockers, and extra‐PVAI procedure did not differ between groups. The recurrence group had more female (P = 0.006), structural heart disease (P = 0.07), multiple sessions (P = 0.008), and ERAF (P = 0.003) compared with the no‐recurrence group. ERAF was associated with AF/AT recurrence in the 74 patients with 1st session case (P = 0.005), but not in the 24 patients with multiple sessions (P = 0.67). Of note, burst pacing positivity and maximum burst rate that did not induce sustained AF were not correlated with clinical recurrence.
Table 1.
Patient characteristics
| Characteristics | Recurrence (n = 27) | No‐recurrence (n = 71) | P |
|---|---|---|---|
| Age (y) | 62.3 ± 11.1 | 60.3 ± 9.6 | 0.38 |
| Female | 11 (40.7) | 10 (14.1) | 0.006 |
| Long‐standing persistent AF | 8 (29.6) | 27 (38.0) | 0.49 |
| Diabetes mellitus | 2 (7.4) | 14 (19.7) | 0.22 |
| Hypertension | 17 (63.0) | 40 (56.3) | 0.65 |
| Structural heart disease | 6 (22.2) | 5 (7.0) | 0.07 |
| History of congestive heart failure | 7 (25.9) | 15 (21.1) | 0.60 |
| Plasma BNP (pg/mL) | 212 ± 465 | 127 ± 153 | 0.36 |
| CRP (mg/dL) | 0.27 ± 0.42 | 0.19 ± 0.23 | 0.35 |
| LAD (mm) | 43.2 ± 8.9 | 43.5 ± 7.1 | 0.88 |
| LVEF (%) | 64.9 ± 10.5 | 63.4 ± 9.9 | 0.52 |
| AAD | 21 (77.8) | 63 (88.7) | 0.2 |
| Beta blockers | 20 (74.1) | 44 (61.9) | 0.34 |
| Multiple sessions | 12 (44.4) | 12 (16.9) | 0.008 |
| Extra‐PVAI procedure | 19 (70.4) | 37 (52.1) | 0.12 |
| Burst pacing positive | 15 (55.6) | 35 (49.3) | 0.65 |
| Max Burst Rate (ppm) (AF noninducible) | 290 ± 43 | 291 ± 39 | 0.89 |
| ERAF | 12 (44.4) | 10 (14.1) | 0.003 |
BNP, brain natriuretic peptide; CRP, C‐reactive protein; LAD, left atrial diameter; LVEF, left ventricular ejection fraction; AAD, antiarrhythmic drugs; PVAI, pulmonary vein antrum isolation; ERAF, early recurrence of atrial fibrillation.
A multivariate analysis revealed that female gender, multiple sessions, and ERAF were significantly associated with long‐term AF/AT recurrence during 1 year of follow‐up (OR 3.8, 3.5 and 5.3, P = 0.02, P = 0.02 and P = 0.004, respectively) although the sample size of analysis was small for statistical reliance.
Among 98 studied patients, 50 patients (51%) had inducibility of AF/AT by burst pacing at the end of procedure, which was not associated with clinical recurrence. The sensitivity and specificity of burst positivity for clinical recurrence were 55.6% and 50.7%, respectively. The positive predictive value of burst pacing was as low as 30%, whereas the negative predictive value was relatively high as 75% (Figure 1A). Among 50 patients with burst pacing positivity, AT and AF were induced in 13 and 37 patients, respectively. Of 13 patients with induced AT, six patients (46%) had AF/AT recurrence (three AF and three AT). The consistency of the type of arrhythmia was observed in three patients (50%). On the other hand, of 37 patients with induced AF, nine patients (24%) had AF/AT recurrence (eight AF and one AT). The consistency of the type of arrhythmia was highly observed in eight patients (89%). Thus, inducible AT at the end of procedure was associated with an increased risk of recurrent AF/AT whereas the form of recurrent tachyarrhythmia was not necessarily an AT but an AF.
Figure 1.
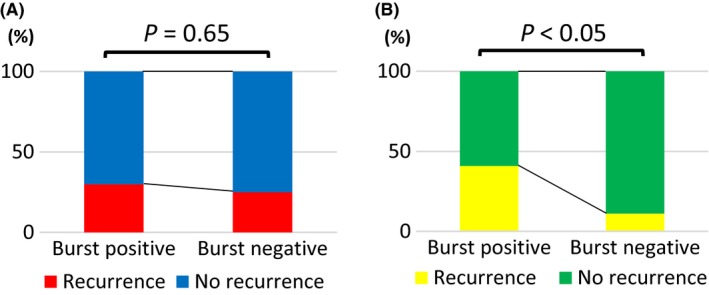
Predictive value analysis. A, All patients. B, Patients with enlarged left atrium
3.2. Subgroup analysis
We performed subanalyses of the following four categories; enlarged LA, multiple sessions, extra‐PVAI procedure, and long‐standing persistent AF. In each subanalysis group, we compared the burst pacing positive rate in recurrence group with that in no‐recurrence group and found that the inducibility of AT/AF by burst pacing was associated with clinical recurrence only in the “enlarged LA” subgroup. Calculated odds ratio for burst positivity in recurrent patients in each subgroup are shown in a comparative forest plot (Figure 2). We also performed subgroup analyses with different cut‐off values of LA dilatation (LAD ≥40 mm, LAD ≥43 mm, LAD ≥45 mm, and LAD ≥50 mm) and compared the predictive value of the induction test with atrial burst pacing. The cut‐off value of LAD ≥45 mm and LAD ≥50 mm showed statistical significance, but the sample size was too small with the cut‐off value of LAD ≥50 mm (N = 18). Therefore, we focused on a subgroup analysis in 40 patients with enlarged LA (LAD ≥45 mm, mean LAD 50.4 ± 4.9 mm). During 1 year of follow‐up, 29 patients did not have AF/AT recurrence. We divided the patients into two groups; recurrence group (n = 11, 27.5%) and no‐recurrence group (n = 29, 72.5%). Table 2 summarizes the baseline characteristics of the two groups. There were no significant differences in age, gender or comorbidity such as diabetes mellitus, hypertension, structural heart disease, and history of congestive heart failure between the two groups. AF type (persistent AF or long‐standing persistent AF), LAD, LVEF, and biomarkers such as BNP, CRP were also comparable between the two groups. The use of antiarrhythmic drugs, beta blockers, ERAF, extra‐PVAI procedure, and maximum burst rate which did not induce sustained AF did not differ between groups. The recurrence group had more multiple sessions (P = 0.008) and burst pacing positivity (P = 0.07) compared with the no‐recurrence group.
Figure 2.
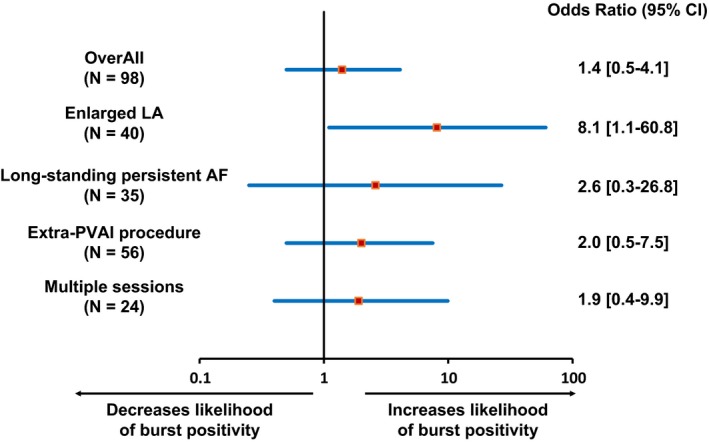
Odds ratio of atrial burst pacing positivity with regard to long‐term recurrence in various subgroups
Table 2.
Characteristics in patients with enlarged left atrium
| Characteristics | Recurrence (n = 11) | No‐recurrence (n = 29) | P |
|---|---|---|---|
| Age (y) | 65.8 ± 9.0 | 61.2 ± 8.2 | 0.13 |
| Female | 3 (27.3) | 5 (17.2) | 0.66 |
| Long‐standing persistent AF | 7 (63.6) | 15 (51.7) | 0.72 |
| Diabetes mellitus | 2 (18.2) | 7 (24.1) | 1.0 |
| Hypertension | 6 (54.6) | 21 (72.4) | 0.45 |
| Structural heart disease | 3 (27.3) | 4 (13.8) | 0.37 |
| History of congestive heart failure | 3 (27.3) | 5 (17.2) | 0.66 |
| Plasma BNP (pg/mL) | 328 ± 710 | 161 ± 175 | 0.46 |
| CRP (mg/dL) | 0.28 ± 0.5 | 0.23 ± 0.23 | 0.76 |
| LAD (mm) | 50.6 ± 5.9 | 50.2 ± 4.6 | 0.82 |
| LVEF (%) | 62.3 ± 14.4 | 60.7 ± 10.8 | 0.71 |
| AAD | 8 (72.7) | 26 (89.7) | 0.32 |
| Beta blockers | 8 (72.7) | 21 (72.4) | 1.0 |
| Multiple sessions | 7 (63.6) | 5 (17.2) | 0.008 |
| Extra‐PVAI procedure | 10 (90.9) | 20 (69.0) | 0.23 |
| Burst pacing positive | 9 (81.8) | 13 (44.8) | 0.07 |
| Max Burst Rate (ppm) (AF noninducible) | 274 ± 49 | 294 ± 42 | 0.19 |
| ERAF | 4 (36.4) | 7 (24.1) | 0.46 |
The multivariate analysis revealed that multiple sessions and burst pacing positivity were significantly associated with AF/AT recurrence (OR 11.7 and 8.1, P = 0.008 and P = 0.04, respectively) although the sample size of analysis was small for statistical reliance.
We also analyzed predictive value in the subgroup population with enlarged LA. At the end of procedure, 22 patients (55%) had inducibility of AF/AT by burst pacing. The sensitivity and specificity of burst positivity for clinical recurrence were 81.8% and 55.2%, respectively. The negative predictive value of burst pacing for clinical recurrence was as high as 88.9% although the positive predictive value was 40.9% (Figure 1B).
We evaluated the AF/AT inducibility defined as AF/AT of 30 seconds among these 40 patients with enlarged LA. The shorter definition of inducibility increased the negative predictive value (92.3%) but decreased the positive predictive value (37.0%). Comparison of predictive values in each studied population and definition of positivity are summarized in Table 3.
Table 3.
Comparison of predictive values of atrial burst pacing
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| All patients | 55.6% | 50.7% | 30% | 75% |
| Patients with enlarged LA | 81.8% | 55.2% | 40.9% | 88.9% |
| Shorter definition of AF/AT in patients with enlarged LA | 90.9% | 41.4% | 37% | 92.3% |
LA, left atrium; PPV, positive predictive value; NPV, negative predictive value.
4. DISCUSSION
4.1. Main findings
The main findings of this study are as follows: (a) Approximately, half of the patients with persistent AF had inducibility of AF/AT by atrial burst pacing at the end of PVAI‐based procedure. (b) The AF/AT inducibility was not associated with clinical recurrence and had high false positive rate whereas other clinical variables such as gender or ERAF were more associated. (c) A subanalysis in patients with enlarged LA showed that the AF/AT inducibility was associated with clinical recurrence and had a high negative predictive value. (d) The shorter definition of inducibility increased the negative predictive value but decreased the positive predictive value.
To date, several investigators validated whether the induction test with atrial burst pacing had predictive value in long‐term recurrence.5, 6, 7, 8, 9, 10, 11, 12, 13 However, its predictive value remains controversial due to limited predictive accuracy with high false positiveness in addition to inconsistent studied AF type or population, different induction protocol, and different definition of inducibility among the studies. It is noteworthy that most of the studies consistently showed relatively high negative predictive value despite low positive predictive value (Table 4). Therefore, additional substrate modification after PVAI should not be performed based on AF inducibility by atrial burst pacing considering its low positive predictive value for clinical recurrence.
Table 4.
Summary of previous studies with regard to burst pacing protocol & predictive values
| Author & Year | AF type & sample size | Induction Protocol | Definition of inducibility | PPV | NPV |
|---|---|---|---|---|---|
| Oral et al5 2004 | pAF (100) | Burst (≥15 s) | AF >1 min | 33% | 85% |
| Essebag et al6 2005 | pAF (60), non‐pAF (42) | Burst (5 s at 200 ms) | AF/AT > 10 s | 47% | 72% |
| Richter et al7 2006 | pAF (166), non‐pAF (68) | Decremental burst | AF >1 min | 54% | 69% |
| Jaïs et al11 2006 | pAF (74) | Decremental burst (10 s) | AF ≥10 min | NR | NR |
| Chang et al8 2007 | pAF (88) | Decremental burst (5‐10 s) | AF/AT >1 min | 55% | 81% |
| Satomi et al12 2008 | pAF (60) | Decremental burst (10 s) | AF >10 min | 41% | 58% |
| Crawford et al13 2010 | pAF (112) | Decremental burst (10s) | AF ≥1 min | 40% | 76% |
| Leong‐Sit et al9 2013 | pAF (78), non‐pAF (66) | Decremental burst (15 beats) | AF/AT ≥2 min | 49% | 51% |
| Santangeli et al10 2018 | pAF (201), non‐pAF (104) | Decremental burst (15 beats) | AF/AT ≥2 min | 31% | 76% |
| The Present study | Non‐pAF (98) | Decremental burst (30 beats) | AF/AT ≥5 min | 30% | 75% |
| Non‐pAF with enlarged LA (40) | Decremental burst (30 beats) | AF/AT ≥5 min | 41% | 89% |
The main contributing factors in recurrence of AF are as following: (a) reconnection of PVAI lines/linear ablation lines, (b) existence of non‐PV triggers, and (c) existence of atrial arrhythmic substrates.
Previous studies reported an issue of reconnections of the PVAI lines in the remote phase,15, 16 which is not predictable by the response to atrial burst pacing in the acute phase. Reconnection of linear ablation lines in the atria is also not predictable, and these factors constitute critical portion of AF recurrence. In the present study, ERAF was strongly associated with AF/AT recurrence in the overall studied population. ERAF may partly be associated with reconnections of the ablated lines.17 Interestingly, ERAF was not associated with AF/AT recurrence in the 24 patients with multiple sessions (P = 0.67), which may be attributable to a transient inflammatory effect rather than reconnections of PVAI line in this population.
The existence of non‐PV triggers is another important issue, which is also not predictable by the response to atrial burst pacing. In the present study, multiple sessions were associated with AF/AT recurrence. The previous studies indicated the importance of non‐PV triggers in multiple session cases.18 In addition, female gender was also correlated with AF/AT recurrence. The previous studies demonstrated that female gender was significantly correlated with non‐PV triggers of AF.19
The response to atrial burst pacing may reflect the residual substrates in the atria that maintain AF. Therefore, atrial burst pacing can evaluate only a small part of the pathology regarding AF recurrence. Our results demonstrated that the response to atrial burst pacing was correlated with clinical recurrence in cases with enlarged LA in which atrial substrate is highly possible.
Predictive value of atrial burst pacing may also be limited due to methodological reasons. One is the issue of induction reproducibility. As shown in the previous study,9 there is concern that the AF/AT inducibility may be a nonspecific response. Indeed, poor reproducibility could be seen in our preliminary data. Furthermore, susceptible atrial pacing site for AF induction may be different among patients whereas CS ostium is the pacing site in this study.
With regard to predictors of long‐term outcome, clinical characteristics and clinical course such as gender, ERAF, and re‐session cases had significant correlation with clinical recurrence in a multivariate analysis. Thus, prediction of long‐term outcome after AF‐CA needs a comprehensive judgment of multiple factors, where response to atrial burst pacing can be an index in a selected population.
4.2. Limitations
There were several limitations in our study. First, the study population was relatively small due to the single‐center, observational study. Although the sample size confined to persistent AF cases was larger than in previous studies (Table 4),6, 7, 9, 10 the present findings need to be confirmed in multi‐center studies. Second, induction protocol and definition of inducibility are different from the previous studies. In the present study, we found that the shorter definition of inducibility increased the negative predictive value while it also increased the false positive rate (Table 3). It is possible that the results depend on the studied population or conditions. However, a previous study reported almost the same negative predictive value and false positive rate as our results despite different induction protocol and definition of inducibility in which sample size confined to persistent AF was similar to our study.10 Third, the residual substrates in the atrium were not evaluated quantitatively in the present study. Chang et al reported that the induction positive cases had a lower biatrial voltage compared with the induction negative cases in paroxysmal AF patients.8 Further studies are needed in order to validate the relationship between low voltage area and induction positivity in persistent AF patients.
5. CONCLUSIONS
AF/AT inducibility at the end of ablation session does not predict long‐term clinical recurrence in patients with persistent AF. However, it may have a prognostic value especially as a negative predictor in patients with enlarged LA, probably reflecting a successful substrate modification.
CONFLICT OF INTEREST
Authors declare no conflict of interests for this article.
Supporting information
ACKNOWLEDGEMENTS
This study was partly supported by a research grant provided from Bristol‐Myers Squibb, Inc (Kyushu University Registration Code #GAKF850098).
Kawai S, Mukai Y, Inoue S, et al. Predictive value of the induction test with atrial burst pacing with regard to long‐term recurrence after ablation in persistent atrial fibrillation. J Arrhythmia. 2019;35:223–229. 10.1002/joa3.12150
REFERENCES
- 1. Häissaguerre M, Jaïs P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- 2. Chen SA, Hsieh MH, Tai CT, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999;100:1879–86. [DOI] [PubMed] [Google Scholar]
- 3. Scherr D, Khairy P, Miyazaki S, et al. Five‐year outcome of catheter ablation of persistent atrial fibrillation using termination of atrial fibrillation as a procedural endpoint. Circ Arrhythm Electrophysiol. 2015;8:18–24. [DOI] [PubMed] [Google Scholar]
- 4. Elayi CS, Verma A, Di Biase L, et al. Ablation for longstanding permanent atrial fibrillation: results from a randomized study comparing three different strategies. Heart Rhythm. 2008;5:1658–64. [DOI] [PubMed] [Google Scholar]
- 5. Oral H, Chugh A, Lemola K, et al. Noninducibility of atrial fibrillation as an end point of left atrial circumferential ablation for paroxysmal atrial fibrillation. Circulation. 2004;110:2797–801. [DOI] [PubMed] [Google Scholar]
- 6. Essebag V, Baldessin F, Reynolds MR, et al. Non‐inducibility post‐pulmonary vein isolation achieving exit block predicts freedom from atrial fibrillation. Eur Heart J. 2005;26:2550–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Richter B, Gwechenberger M, Filzmoser P, Marx M, Lercher P, Gössinger HD. Is inducibility of atrial fibrillation after radio frequency ablation really a relevant prognostic factor? Eur Heart J. 2006;27:2553–9. [DOI] [PubMed] [Google Scholar]
- 8. Chang SL, Tai CT, Lin YJ, et al. The efficacy of inducibility and circumferential ablation with pulmonary vein isolation in patients with paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2007;18:607–11. [DOI] [PubMed] [Google Scholar]
- 9. Leong‐Sit P, Robinson M, Zado ES, et al. Inducibility of atrial fibrillation and flutter following pulmonary vein ablation. J Cardiovasc Electrophysiol. 2013;24:617–23. [DOI] [PubMed] [Google Scholar]
- 10. Santangeli P, Zado ES, Garcia FC, et al. Lack of prognostic value of atrial arrhythmia inducibility and change in inducibility status after catheter ablation of atrial fibrillation. Heart Rhythm. 2018;15(5):660–5. [DOI] [PubMed] [Google Scholar]
- 11. Jaïs P, Hocini M, Sanders P, et al. Long‐term evaluation of atrial fibrillation ablation guided by noninducibility. Heart Rhythm. 2006;3:140–5. [DOI] [PubMed] [Google Scholar]
- 12. Satomi K, Tilz R, Takatsuki S, et al. Inducibility of atrial tachyarrhythmias after circumferential pulmonary vein isolation in patients with paroxysmal atrial fibrillation: clinical predictor and outcome during follow‐up. Europace. 2008;10:949–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crawford T, Chugh A, Good E, et al. Clinical value of noninducibility by high‐dose isoproterenol versus rapid atrial pacing after catheter ablation of paroxysmal atrial fibrillation. J Cardiovasc Electrophysiol. 2010;21:13–20. [DOI] [PubMed] [Google Scholar]
- 14. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society: developed in collaboration with the Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;64(21):2246–80. [DOI] [PubMed] [Google Scholar]
- 15. Cappato R, Negroni S, Pecora D, et al. Prospective assessment of late conduction recurrence across radiofrequency lesions producing electrical disconnection at the pulmonary vein ostium in patients with atrial fibrillation. Circulation. 2003;108:1599–604. [DOI] [PubMed] [Google Scholar]
- 16. Callans DJ, Gertenfeld EP, Dixit S, et al. Efficacy of repeat pulmonary vein isolation procedures in patients with recurrent atrial fibrillation. J Cardiovasc Electrophysiol. 2004;15:1050–5. [DOI] [PubMed] [Google Scholar]
- 17. Das M, Wynn GJ, Morgan M, et al. Recurrence of atrial tachyarrhythmia during the second month of the blanking period is associated with more extensive pulmonary vein reconnection at repeat electrophysiology study. Circ Arrhythm Electrophysiol. 2015;8:846–52. [DOI] [PubMed] [Google Scholar]
- 18. Lin D, Santangeli P, Zado ES, et al. Electrophysiologic findings and long‐term outcomes in patients undergoing third or more catheter ablation procedures for atrial fibrillation. J Cardiovasc Electrophysiol. 2015;26(4):371–229. [DOI] [PubMed] [Google Scholar]
- 19. Lee SH, Tai CT, Hsieh MH, et al. Predictors of non‐pulmonary vein ectopic beats initiating paroxysmal atrial fibrillation. J Am Coll Cardiol. 2005;46:1054–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


