Abstract
Background
Globally, over 400,000 neonatal deaths in 2015 were attributed to sepsis, however, the incidence and etiologies of these infections are largely unknown in low-middle income countries. We aimed to determine incidence and etiology of community-acquired early-onset (<72 hours age) sepsis (EOS) using culture and molecular diagnostics.
Methods
This was a prospective observational study, in which we conducted a surveillance for pathogens using a combination of blood culture and a polymerase chain reaction (PCR)-based test. Blood culture was performed on all neonates with suspected EOS. Among the subset fulfilling criteria for protocol-defined EOS, blood and nasopharyngeal (NP) respiratory swabs were tested by quantitative real-time reverse-transcriptase PCR using a Taqman Array Card (TAC) with 15 bacterial and 12 viral targets. Blood and NP samples from 312 healthy newborns were also tested by TAC to estimate background positivity rates. We used variant latent-class methods to attribute etiologies and calculate pathogen-specific proportions and incidence rates.
Results
We enrolled 2,624 neonates with suspected EOS and from these 1,231 newborns met criteria for protocol-defined EOS (incidence- 39.3/1,000 live-births). Using the partially latent-class modelling, only 26.7% cases with protocol-defined EOS had attributable etiology, and the largest pathogen proportion were Ureaplasma spp. (5.4%; 95%CI: 3.6–8.0) and group B Streptococcus (GBS) (4.8%; 95%CI: 4.1–5.8), and no etiology was attributable for 73.3% of cases. Blood cultures were positive in 99/1,231 (8.0%) with protocol-defined EOS (incidence- 3.2/1,000 live-births). Leading pathogens on blood culture included GBS (35%) and viridans streptococci (24%). Ureaplasma spp. was the most common organism identified on TAC among cases with protocol-defined EOS.
Conclusion
Using a combination of blood culture and a PCR-based test the common pathogens isolated in neonates with sepsis were Ureaplasma spp. and GBS. Despite documenting higher rates of protocol-defined EOS and using a combination of tests, the etiology for EOS remains elusive.
Introduction
Sepsis was attributed to cause approximately 400,000 neonatal deaths in 2015 globally, half of which occurred in sub-Saharan Africa where 34.6% to 66.0% of neonatal deaths reportedly occur within first 24 hours of life.[1–3] There are few studies reporting on etiology of sepsis in the first three days of life from sub-Saharan African countries.[4–10] Ascertaining the etiology of neonatal sepsis in resource-limited settings is challenging, including due to births taking place outside healthcare facilities and inadequate laboratory facilities.
Furthermore, the yield from blood culture, the gold standard for determining sepsis etiology might be compromised due to inadequate blood volume sampling in newborns, challenges in timely processing of specimens and antecedent antibiotic therapy prior to sampling. In addition, blood culture excludes identification of pathogens not readily culturable.
In order to address this gap, we established a prospective surveillance for serious probable infections in neonates from a large academic hospital in Soweto, South Africa with the aim to evaluate the etiology and incidence of early-onset (first-three days of life) sepsis (EOS). Investigations included blood culture and molecular diagnostic assay on blood and nasopharyngeal respiratory samples in newborns with protocol-defined community-acquired EOS.
Materials and methods
Population and setting
We conducted the Sepsis Aetiology in Neonates in South Africa (SANISA) Study between August 2013 and September 2014 at Chris Hani Baragwanath Academic Hospital (CHBAH), a public hospital in Soweto, South Africa. CHBAH delivers two-thirds of annual births from the surrounding urban, low-to-middle income population of Soweto. The majority of Soweto births outside CHBAH occur in public clinics, and if any of these newborns require further medical management, they are referred to CHBAH. During the study period, CHBAH was the only public hospital admitting sick neonates in Soweto. The protocol in the neonatal unit is that all neonates born or referred with high obstetric risk factors e.g. preterm births, those requiring resuscitation at birth, and those with symptoms suggestive of sepsis have blood cultures done. Approximately 90% of all newborns admitted in the first 3 days of life have blood taken for culture. As part of hospital protocol, admissions to neonatal intensive care unit (NICU) are restricted to those requiring intubation for mechanical ventilation and/ or inotropes and are weighing ≥900 grams. Neonates weighing <900 grams are only offered nasal continuous positive airway pressure if they weigh ≥750 grams.
Study design and procedures
This was a prospective surveillance, to identify cases of physician-suspected infection in neonates <28 days of age, with the present analysis restricted to those <3 days of age. The primary objective was to characterize the etiology of EOS and determine its incidence rates. Neonates were eligible for inclusion if blood cultures were collected as per standard care for physician-suspected sepsis. Neonates with major congenital abnormalities, care redirected for anticipated demise, or born to mothers <18 years of age were excluded. Due to an inability to enroll patients at all times, we excluded neonates born from 3:00pm Friday to midnight Saturday from numerators and denominators. Clinical data were collected by medical record abstraction performed by trained study staff using a standardized form, which included maternal and neonatal demographics, sepsis risk factors, clinical presentation, care received, laboratory findings, and clinical outcome.
On admission, as per hospital protocol, physicians collected blood for bacterial culture from all neonates with suspected EOS. The standard of care was for lumbar puncture only to be performed in suspected EOS if there were signs suggestive of meningitis. For blood culture, up to 1ml whole blood was placed in pediatric blood culture bottles (Biomerieux, South Africa), and any remaining volume (minimum: 0.5ml) placed in an ethylenediaminetetraacetic acid (EDTA) tube for study-specific molecular testing. After maternal consent was obtained, study staff collected respiratory specimens (nasopharyngeal and oropharyngeal swabs, NP/OP) from neonates with suspected sepsis. The NP/OP swabs were pooled in universal transport media. Blood in EDTA tube and respiratory samples for molecular testing were stored at -20°C until transfer, which was within 24-hours of collection, for storage at -70°C.
Study definitions
All physician-suspected EOS cases enrolled were further evaluated to establish if they fulfilled criteria of protocol-defined EOS based on a predefined clinical and laboratory algorithm previously used in this population (S1 Table).[11] A case was defined as culture-confirmed EOS if a significant pathogen was grown from either blood or CSF. Cases were defined as severe if admitted to NICU, had lethargy (defined as absent spontaneous movement and/or decreased level of activity despite stimulation), or died.[12, 13] Prematurity was defined as gestational age <37 weeks, and low birthweight as <2,500 grams.
Laboratory analysis
Blood culture and CSF specimens were processed per National Health Laboratory Services protocol using the BacT/Alert automated culture system (BioMerieux, Marcy l’Etoile, France). If bacterial growth was detected, a Gram-stain was performed and the sample sub-cultured onto appropriate media. Classification of culture isolates as contaminants was similar to that used in the Aetiology of Neonatal Infection in South Asia (ANISA) study.[14] The organisms that were considered contaminants were coagulase-negative Staphylococcus spp, Bacillus spp, Micrococcus spp, Corynebacterium spp, Propionibacterium spp, and Diphtheroids. Study-specific blood and respiratory specimens underwent molecular testing using custom TaqMan Array Cards (TAC) (Thermo Fisher, Waltham, USA) which allowed simultaneous testing of 21 organisms (9 bacteria and 12 viruses) for respiratory specimens and 12 agents (11 bacteria and 1 virus) for blood samples (S2 Table). TAC designs and assays were adopted from the ANISA study and have been validated in previous studies.[15–17] A negative template control and a positive control consisting of combined RNA transcripts were included on each TAC.[18]
Study quality control identified an unexpected increase in blood TAC detection of Pseudomonas aeruginosa in samples collected between July and September, 2014 (30%; 70/234) positive); before July: 1% (7/698) positive). Investigation of these results suggested specimen contamination before processing. Therefore, we excluded all blood TAC results for P. aeruginosa during this 3-month period.
Assessment of background rates of pathogen-specific TAC positivity
Molecular tests such as TAC often have a background positivity rate in a healthy uninfected population; which may stem from organism carriage, presence of non-viable genetic material, or very low copy numbers of a pathogen. Such background rates typically vary by age, location and season. Because prior information on background positivity rates for the pathogens of interest was not available for Soweto newborns, in parallel to the active surveillance we enrolled 312 newborns at CHBAH without sepsis (a target of 25 per month), obtained blood and respiratory samples for TAC testing.
Statistical analysis
Incidence was calculated as cases per 1,000 live births. Administrative live birth data from CHBAH and Soweto community clinics were used as population denominators corrected for the excluded 32-hour period each week. Established patient referral protocols limited the chance that neonates living outside the hospital catchment area were admitted to CHBAH, especially within 72-hours of birth. Confidence intervals for observed incidence (i.e., culture-based incidence) were calculated based on a Poisson distribution.
The primary analytic objective was to estimate the proportion of sepsis episodes attributed to each pathogen evaluated in the study, referred to throughout as “pathogen proportions.” Because the study captured two specimen samples (blood and respiratory), conducted up to three tests per pathogen (blood culture, blood TAC, respiratory TAC), and because each of these tests had its own true positive rate (TPR or sensitivity) and false positive rate (FPR or 1-specificity) for detection of the true cause of the sepsis episode, we adapted the partial latent class model (pLCM) methods developed by Wu et al[19, 20] for application to our surveillance as detailed in the text in S1 Text. All protocol defined EOS cases with at least one laboratory test result available were included in the model input dataset, along with blood culture final determination (for neonates with sepsis) and observed binary (positive or negative) results of the TAC tests. When a culture contained multiple pathogens, a single pathogen was assigned based on physician case review. Healthy-neonate TAC results were used to establish initial prior values for pathogen-specific false positive rates. The 312 enrolled healthy-neonates were sufficient to provide initial estimation of false positive rates with a maximum standard error of 3%, and with an even smaller estimation error after model convergence. The pathogen proportions estimated, included the 27 pathogen targets tested by TAC, plus two additional classes: ‘other blood culture’, which included all significant pathogens cultured from blood culture but for which targets were not included on the TAC test (blood or respiratory), and “other/none” for episodes that could not be attributed to one of the evaluated pathogens. The primary output of the model was the population-level pathogen proportions (presented as means with 95% credible intervals); from these individual-level pathogen proportions could also be derived. Covariates included date of enrollment as a continuous variable, maternal HIV-infection status, and severity of infection. Performance of the pLCM model under a range of input dataset conditions was assessed by a series of simulations. Model convergence was assessed through trace and other diagnosis plots. Model assumptions were examined using interim model outputs by stopping the Gibbs sampler at random cycles. Model fit to input data was evaluated by comparing the fitted and observed number of positives for each TAC test and blood culture. Programming and computation were conducted using the R (version 3.2.5, R foundation for statistical computing, Vienna, Austria)), SAS (version 9.3, Cary, NC, USA), and STATA (version 13, College station, Texas, StataCorp LP) platform.
Ethical considerations
This study was approved by the Human Research Ethics Committee (M120552) of the University of the Witwatersrand, Johannesburg, South Africa and the U.S. CDC through a formal joint review arrangement. The local Ethics review limited enrolment to 320 healthy-newborns, due to considerations of invasive procedure in healthy-newborns without any direct potential for benefit. Written informed maternal consent was obtained for all enrollees.
Results
Patient recruitment and descriptive data
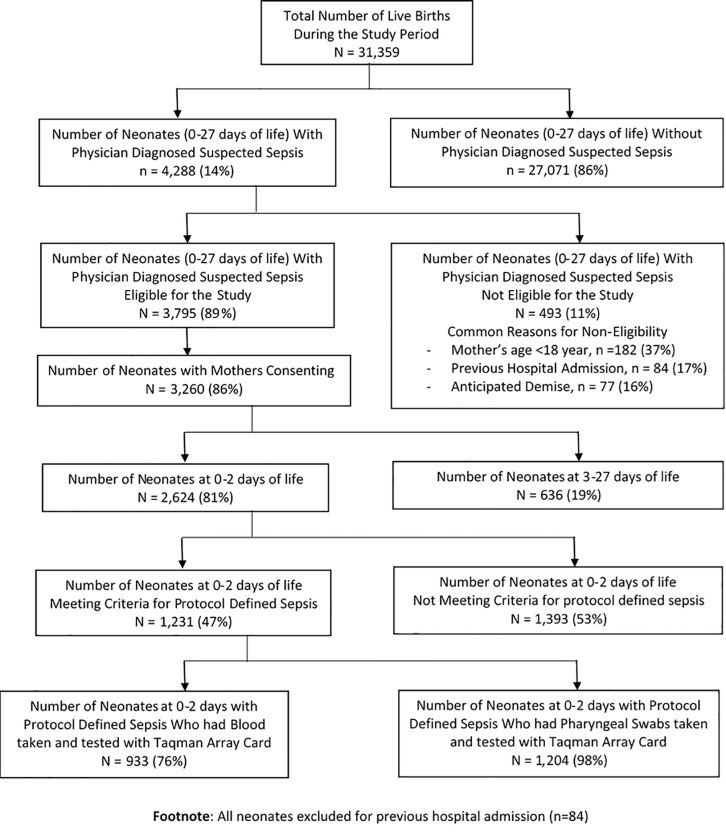
A total of 31,359 live births were documented in public health facilities in Soweto during the study period; of which 4,288 (14%) neonates (0–27 days) were hospitalized for physician diagnosed suspected sepsis, and of whom 3,795 (89%) were eligible for participation. The most frequent reason for exclusion (11% excluded; 493/4,288), was mother’s age <18 years (37%; 182/493), previous hospital admission (17%; 84/493) and anticipated imminent demise (16%; 77/493). Maternal consent was obtained for 86% of eligible cases; including 2,624 (81%) <3 days age, of whom 1,231 (47%) met our protocol-defined EOS definition (i.e. cases) (Fig 1).
Fig 1. Flow chart of enrolled neonates with physician diagnosed suspected and protocol defined sepsis.
Overall, among protocol-defined EOS cases, 55% were male, 56% had low birth weight, 47% born prematurely and 32% born to HIV-infected women. The most common signs of sepsis included tachypnea (32%), metabolic acidosis (36%), lethargy (15%), and hypothermia (15%); whilst fever was uncommon (0.6%). A total of 332 (27%) cases were categorized as severe. The majority of cases were enrolled on day 0 (84%) (Table 1).
Table 1. Characteristics of infants meeting case definition* for protocol-defined early-onset sepsis (n = 1,231)—SANISA Study: Soweto, South Africa.
| Neonates with Protocol Defined Sepsis |
||
|---|---|---|
| Characteristic | Numbers | Percent |
| Maternal characteristics | ||
| Mother: At least one prenatal visit | 1136 | 92.3 |
| Mother: HIV positive | 389 | 31.6 |
| HIV status available** | 1175 | 95.5 |
| Intrapartum characteristics | ||
| Delivery: cesarean section | 664 | 53.9 |
| Antibiotics received during labor / delivery | 103 | 8.4 |
| Prolonged rupture of membranes | 60 | 4.9 |
| Meconium stained liquor | 277 | 22.5 |
| Infant characteristics | ||
| Male sex | 672 | 54.6 |
| Birth weight categories (n = 1230) | ||
| <1000 grams | 114 | 9.3 |
| 1000–1499 grams | 222 | 18.0 |
| 1500–1999 grams | 195 | 15.8 |
| 2000–2499 grams | 155 | 12.6 |
| ≥2500 grams | 544 | 44.2 |
| Gestational age (n = 1218) | ||
| <28 weeks | 83 | 6.7 |
| 28–32 weeks | 305 | 24.8 |
| 33–36 weeks | 181 | 14.7 |
| ≥37 weeks | 649 | 52.7 |
| Day of life enrolled | ||
| Day 0 (date of birth) | 1035 | 84.1 |
| Day 1 | 192 | 15.6 |
| Day 2 | 4 | <1 |
| Sepsis signs | ||
| Poor feeding | 4 | <1 |
| Lethargy | 183 | 14.9 |
| Hypothermia (T<36° C) | 182 | 14.8 |
| Hyperthermia (>38° C) | 7 | <1 |
| Hypoglycemia | 163 | 13.2 |
| Hyperglycemia | 115 | 9.3 |
| Metabolic acidosis | 441 | 35.8 |
| Tachypnea (respiratory rate > 60/minute) | 398 | 32.3 |
| NICU admission | 88 | 7.2 |
| Length of stay†: median days (range) | 7 (0–182) | - |
| Death†† | 149 | 12.1 |
| Infection categorized as severe‡ | 332 | 27.0 |
* Case definition includes clinical and laboratory criteria based on Cutland, et al.[11]
** Infants born to mothers with unknown HIV status were included as no known HIV exposure
† Restricted to infants with known discharge status (alive or died); n = 1173
†† Excludes transferred patients; n = 1193
‡ Severe: infant with noted lethargy, NICU admission, or infection resulted in death
Culture results
All cases had a blood culture, the mean volume of which was 1.0ml (range: 0.10–1.6ml) per culture (available for 858 cases). Eleven cases received antibiotics before blood collection and a lumbar puncture was performed in 62 cases (5%). Overall, 4.2% (52/1,231) of blood cultures yielded contaminants, 8.0% (99/1,231) yielded any pathogen, of which 7% (7/99) were polymicrobial. The majority of pathogens cultured were Gram-positive (71%), including Group B streptococcus (GBS) (35%) followed by Streptococcus viridans (24%), and Enterococcus spp. (8%); Table 2. The most common Gram-negative pathogens were Escherichia coli (8%) and Acinetobacter spp. (4%). Three (5%) CSF cultures had a pathogen isolated, with Acinetobacter spp., GBS (also positive on blood), and one mixed sample of Enterococcus spp. and S. aureus identified.
Table 2. Blood culture isolates by pathogen among neonates with protocol defined early-onset sepsis* (n = 1,231)—SANISA Study, Soweto, South Africa.
| Pathogen** | Number of Isolates |
Percent |
|---|---|---|
| Gram Positives | 70 | 70.7 |
| Group B Streptococcus | 35 | 35.4 |
| Viridans streptococci | 24 | 24.2 |
| Enterococcus spp. | 8 | 8.1 |
| Staphylococcus aureus | 3 | 3.0 |
| Gram Negatives | 25 | 25.2 |
| Escherichia coli | 8 | 8.1 |
| Acinetobacter baumannii | 4 | 4.0 |
| Haemophilus influenza | 3 | 3.0 |
| Neisseria meningitidis | 2 | 2.0 |
| Sphingomonas paucimobilis | 2 | 2.0 |
| Enterobacter cloacae | 1 | 1.0 |
| Klebsiella pneumoniae | 1 | 1.0 |
| Morganella morganii | 1 | 1.0 |
| Proteus mirabilis | 1 | 1.0 |
| Pseudomonas aeruginosa | 1 | 1.0 |
| Salmonella spp. | 1 | 1.0 |
| Other Pathogens | 4 | 4.0 |
| Candida spp. (fungal) | 3 | 3.0 |
| Mucor spp. (fungal) | 1 | 1.0 |
| TOTAL | 99 | 100 |
The following were considered: Coagulase-negative Staphylococcus (CONS), Diphtheroids, and Bacillus spp. Total blood culture pathogens isolated: 99/1231 (8.0%); Total blood culture contaminants isolated: 52/1231 (4.2%)
*Case definition includes clinical and laboratory criteria based on Cutland, et al.[11]
** Polymicrobial: Cultures with ≥2 pathogens isolated had a single pathogen assigned based on physician case review [7/99 (7%)neonates with positive culture had polymicrobial cultures]
Molecular diagnostic results
Among cases, 37.3% of blood samples (348/933) tested positive for at least one target and 11.0% with multiple targets. The most common organism detected was Streptococcus pneumoniae (14.1%) followed by Ureaplasma spp. (9.2%). Among cases respiratory samples, 44.4% (535/1204) tested positive for at least one target and 14.8% for multiple targets. The most common organism detected was Ureaplasma spp. (19.9%), followed by Klebsiella pneumoniae (11.9%); (Table 3; stratified by case severity and maternal HIV-status in S3 Table and S4 Table respectively). There was poor concordance between blood TAC and cultures for all organisms, with level of concordance varying from 0% for most pathogens to 27.5% for GBS (Table 4).
Table 3. Pathogens detected using Taqman Array Card among neonates with protocol defined sepsis* who had blood (N = 933) and respiratory specimens (N = 1204) collected.
| Number of neonates tested positive for different organisms | ||
|---|---|---|
| Organism tested in Blood Specimens | n | % |
| Ureaplasma spp. | 86 | 9.2 |
| Group B Streptococcus | 65 | 7.0 |
| Staphylococcus aureus | 12 | 1.3 |
| E. coli/Shigella | 24 | 2.6 |
| Streptococcus pneumoniae | 132 | 14.1 |
| Klebsiella pneumoniae | 35 | 3.8 |
| Salmonella spp. | 29 | 3.1 |
| Neisseria meningitidis | 10 | 1.1 |
| Enterovirus | 2 | 0.2 |
| Pseudomonas aeruginosa† | 7 | 1.0 |
| Group A Streptococcus | 2 | 0.2 |
| Haemophilus influenza | 6 | 0.6 |
| All | 346 | 37.1 |
| Organisms tested in Respiratory Specimens | n | % |
| Ureaplasma spp. | 240 | 19.9 |
| Group B Streptococcus | 102 | 8.5 |
| E. coli/Shigella spp. | 105 | 8.7 |
| Enterovirus | 17 | 1.4 |
| Human metapneumovirus | 5 | 0.4 |
| Klebsiella pneumoniae | 143 | 11.9 |
| Human parechovirus | 4 | 0.3 |
| Cytomegalovirus | 69 | 5.7 |
| Bordetella pertussis | 6 | 0.5 |
| Rhinovirus | 8 | 0.7 |
| Streptococcus pneumoniae | 27 | 2.2 |
| Respiratory syncytial virus | 3 | 0.2 |
| Adenovirus | 0 | 0.0 |
| Chlamydia pneumoniae | 2 | 0.2 |
| Chlamydia trachomatis | 6 | 0.5 |
| Influenza A | 0 | 0.0 |
| Influenza B | 0 | 0.0 |
| Mycoplasma pneumoniae | 0 | 0.0 |
| Rubella virus | 11 | 0.9 |
| Parainfluenza virus 1 | 4 | 0.3 |
| Parainfluenza virus 2 | 0 | 0.0 |
| Parainfluenza virus 3 | 0 | 0.0 |
| All | 531 | 44.1 |
† Total case samples tested for Pseudomonas aeruginosa = 701
* Case definition includes clinical and laboratory criteria based on Cutland, et al.[11]
Table 4. Concordance between blood culture and Taqman Array Card (TAC) for detecting bacteria in neonates with early-onset sepsis†.
| Number with TAC or Culture Positive | TAC and Culture Positive n (%) |
Only TAC Positive n (%) |
Only Culture Positive n (%) |
|
|---|---|---|---|---|
| Escherichia coli | 21 | 2 (9.5) | 19 (90.5) | 0 |
| Group A streptococcus | 1 | 0 | 1 (100) | 0 |
| Group B streptococcus | 69 | 19 (27.5) | 46 (66.7) | 4 (5.8) |
| Haemophilus influenza | 7 | 1 (14.3) | 5 (71.4) | 1 (14.3) |
| Klebsiella pneumoniae | 36 | 0 | 35 (97.2) | 1 (2.8) |
| Neisseria meningitidis | 5 | 0 | 5 (100) | 0 |
| Pseudomonas aeruginosa | 8 | 0 | 7 (87.5) | 1 (12.5) |
| Salmonella species | 29 | 0 | 29 (100) | 0 |
| Staphylococcus aureus | 12 | 1 (8.3) | 11 (91.7) | 0 |
| Streptococcus pneumoniae | 132 | 0 | 132 (0) | 0 |
† Analysis limited to cases in which blood TAC and culture were done (N = 933)
Background positivity rates among newborns without sepsis
A total of 382 newborns 0–2 days of age without sepsis were screened for participation, 312 (82%) of whom consented. Among these, 20.1% of blood samples (61/304) tested positive for at least one target and 2.6% for more than one target. The most common organism detected was Streptococcus pneumoniae (9.9%) followed by Klebsiella pneumoniae (2.6%). Among respiratory samples, 54.8% (166/303) tested positive for at least one target and 19.5% for more than one target. The most common pathogen detected was E.coli/Shigella (19.5%) followed by Ureaplasma spp. (14.5%) (Table 5).
Table 5. Pathogen detection using Taqman Array Card among healthy infants who had blood (n = 304) and respiratory (n = 303) specimens collected.
| Number of healthy infants with positive test for different organisms | ||
|---|---|---|
| Organisms tested in Blood Specimens | N | % |
| Ureaplasma spp. | 6 | 2.0 |
| Group B Streptococcus | 5 | 1.6 |
| Staphylococcus aureus | 1 | 0.3 |
| E. coli/Shigella | 4 | 1.3 |
| Streptococcus pneumoniae | 30 | 9.9 |
| Klebsiella pneumoniae | 8 | 2.6 |
| Salmonella spp. | 7 | 2.3 |
| Neisseria meningitidis | 3 | 1.0 |
| Enterovirus | 1 | 0.3 |
| Pseudomonas aeruginosa | 6 | 2.0 |
| Group A Streptococcus | 0 | 0.0 |
| Haemophilus influenza | 0 | 0.0 |
| All | 61 | 19.5 |
| Organisms tested in Respiratory Specimens | n | % |
| Ureaplasma spp. | 44 | 14.5 |
| Group B Streptococcus | 40 | 13.2 |
| E. coli/Shigella spp. | 59 | 19.5 |
| Enterovirus | 26 | 8.6 |
| Human metapneumovirus | 1 | 0.3 |
| Klebsiella pneumoniae | 31 | 10.2 |
| Human parechovirus | 1 | 0.3 |
| Cytomegalovirus | 18 | 5.9 |
| Bordetella pertussis | 2 | 0.7 |
| Rhinovirus | 3 | 1.0 |
| Streptococcus pneumoniae | 15 | 5.0 |
| Respiratory syncytial virus | 3 | 1.0 |
| Adenovirus | 0 | 0.0 |
| Chlamydia pneumoniae | 0 | 0.0 |
| Chlamydia trachomatis | 0 | 0.0 |
| Influenza A | 0 | 0.0 |
| Influenza B | 0 | 0.0 |
| Mycoplasma pneumoniae | 0 | 0.0 |
| Rubella virus | 0 | 0.0 |
| Parainfluenza virus 1 | 0 | 0.0 |
| Parainfluenza virus 2 | 0 | 0.0 |
| Parainfluenza virus 3 | 0 | 0.0 |
| All | 161 | 53.1 |
Etiologic attribution
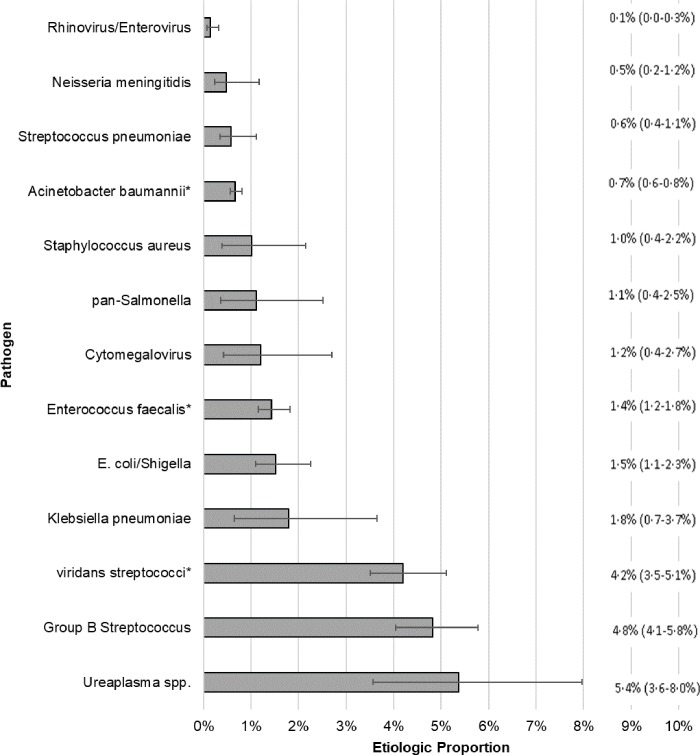
Of the 27 organisms considered, 10 were detected at a rate sufficient for etiologic modeling. Estimated TPR by organism and testing method and average FPR for TAC tests are detailed in Table 6. Overall, we were able to attribute etiology for 26.7% (95%CI: 22.8–31.8%) of cases. The three most commonly attributed pathogens being Ureaplasma (5.4%), GBS (4.8%), and Klebsiella pneumoniae (1.8%), which accounted for 70.0% of the attributable proportion; Fig 2. Individual case-level probabilities for Ureaplasma (median probability = 8.2%) were low compared to GBS (median probability = 24.5%). Pathogens in the “other culture” class accounted for an additional 8.7% of the attributable proportion with Streptococcus viridans (4.2%), Enterococcus faecalis (1.4%), and Acinetobacter baumannii (0.7%) being the most common; Fig 2.
Table 6. Estimated true positive rate (TPR) and average false positive rate (FPR) in percentages by pathogen and testing method.
| True Positive Rate | Average* False Positive Rate | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Blood Culture** | Blood Taqman Array Card | Respiratory Taqman Array Card | Blood Taqman Array Card | Respiratory Taqman Array Card | |||||||||||
| Pathogen | Mean | 2.5Q | 97.5Q | Mean | 2.5Q | 97.5Q | Mean | 2.5Q | 97.5Q | Mean | 2.5Q | 97.5Q | Mean | 2.5Q | 97.5Q |
| Cytomegalovirus | NA | NA | NA | NA | NA | NA | 75.40 | 41.86 | 99.93 | NA | NA | NA | 5.25 | 4.42 | 5.84 |
| E. coli/Shigella | 46.30 | 20.47 | 78.06 | 57.14 | 25.29 | 89.19 | 52.98 | 40.39 | 75.12 | 1.00 | 0.72 | 1.24 | 10.31 | 9.86 | 10.72 |
| Rhinovirus/Enterovirus | NA | NA | NA | 63.05 | 21.54 | 99.91 | 73.19 | 41.41 | 99.89 | 0.32 | 0.17 | 0.42 | 4.23 | 3.97 | 4.44 |
| Group B Streptococcus | 59.57 | 43.88 | 74.99 | 85.80 | 71.93 | 95.86 | 82.48 | 69.14 | 93.02 | 2.26 | 1.69 | 2.77 | 7.38 | 6.89 | 7.84 |
| Klebsiella pneumoniae | 8.54 | 0.52 | 30.92 | 43.81 | 20.82 | 83.12 | 56.44 | 40.42 | 95.53 | 2.29 | 1.79 | 2.73 | 10.69 | 9.77 | 11.29 |
| Neisseria meningitidis | 49.54 | 6.57 | 99.80 | 39.17 | 20.70 | 77.29 | NA | NA | NA | 0.49 | 0.28 | 0.62 | NA | NA | NA |
| Staphylococcus aureus | 32.51 | 5.94 | 94.38 | 60.61 | 23.87 | 95.42 | NA | NA | NA | 0.41 | 0.16 | 0.84 | NA | NA | NA |
| pan-Salmonella | 14.64 | 0.94 | 55.13 | 72.56 | 25.93 | 99.96 | NA | NA | NA | 2.35 | 1.63 | 2.82 | NA | NA | NA |
| Streptococcus pneumoniae | 14.87 | 0.02 | 85.09 | 72.58 | 36.28 | 99.93 | 62.79 | 40.53 | 99.62 | 12.37 | 11.83 | 12.85 | 3.46 | 3.11 | 3.74 |
| Ureaplasma spp. | NA | NA | NA | 87.87 | 58.86 | 99.98 | 79.00 | 59.86 | 99.24 | 2.38 | 1.45 | 3.28 | 13.72 | 12.48 | 14.54 |
True positive rates (TPR): The probability of the test having a positive test result if the case is caused by the pathogen
False positive rates (FPR): The probability of the test having a positive result either for a case, if the pathogen is not the cause, or for a control infant
2.5Q: 2.5% quantile; 97.5Q: 97.5% quantile
* Averaged across modeled covariates (case severity and maternal HIV status)
**TPR for blood culture is equivalent to a pathogen specific estimate of blood culture sensitivity
Fig 2. Estimated proportion of total cases caused by each pathogen with 95% confidence interval; Legend: *Pathogen proportion estimated indirectly from the “other blood culture” class.
Footnote: 73.3% (68.2–77.2%) of case episodes could not be attributed to an evaluated pathogen class.
The observed incidence (per 1,000 live births) of protocol-defined EOS was 39.3 with 12.1% case fatality risk; and the incidence of culture-confirmed EOS was 3.2 with 17.2% (17/99) case fatality risk. Using a combination of tests, the incidence of sepsis attributed to bacteria was 9.7 (95%CI: 8.2–11.5) with Gram-positive incidence (4.7; 95%CI: 4.0–5.7) approximately twice that of Gram-negative (2.8; 95%CI: 2.1–3.8). Overall and stratified by HIV-exposure, Ureaplasma, GBS, and Streptococci viridans all had an estimated incidence >1 per 1,000 live births (Table 7).
Table 7. Observed and estimated pathogen specific incidences per 1,000 live births (with 95% confidence limits) of sepsis* overall and by HIV exposure.
| Pathogen | Observed Incidence | Estimated Overall Incidence N = 1,231 |
Estimated Incidence (HIV Exposed) N = 389 |
Estimated Incidence (HIV unexposed) N = 842 |
|---|---|---|---|---|
| Ureaplasma spp. | NA | 2.11 (1.4–3.1) | 2.38 (1.3–4.1) | 2.01 (1.4–3.0) |
| Group B Streptococcus | 1.12 | 1.90 (1.6–2.3) | 1.26 (0.9–1.8) | 2.13 (1.8–2.5) |
| Viridans streptococci | 0.70 | 1.65 (1.4–2.0) | 1.93 (1.5–2.6) | 1.55 (1.2–2.0) |
| K. pneumoniae | 0.03 | 0.70 (0.3–1.4) | 0.95 (0.2–2.1) | 0.61 (0.2–1.5) |
| E. coli/Shigella spp. | 0.26 | 0.60 (0.4–0.9) | 0.93 (0.7–1.4) | 0.47 (0.3–0.8) |
| E. faecalis | 0.26 | 0.57 (0.5–0.7) | 0.45 (0.3–0.6) | 0.61 (0.5–0.8) |
| Cytomegalovirus | NA | 0.47 (0.2–1.1) | 0.58 (0.1–1.9) | 0.43 (0.1–1.0) |
| Salmonella spp. | 0.03 | 0.44 (0.2–1.0) | 0.95 (0.3–2.0) | 0.25 (0.1–0.8) |
| A. baumannii | 0.13 | 0.26 (0.2–0.3) | 0.28 (0.2–0.4) | 0.26 (0.2–0.3) |
| S. pneumoniae | — | 0.23 (0.1–0.4) | 0.38 (0.2–0.8) | 0.17 (0.1–0.4) |
| N. meningitidis | 0.06 | 0.19 (0.1–0.5) | — | 0.26 (0.1–0.6) |
| S. aureus | 0.10 | 0.07 (0.03–0.2) | — | 0.55 (0.2–1.2) |
| Rhinovirus/Enterovirus | NA | 0.06 (0.03–0.1) | — | 0.08 (0.0–0.2) |
The proportion of cases attributed to an etiology did not differ by HIV-exposure (HIV-exposed: 26.9%; 95%CI: 21.4–34.5%; no HIV-exposure: 26.7%; 95%CI: 22.1–31.9%). Estimated pathogen proportions were also similar between HIV-exposure groups with the exception of GBS, which was more common in those with no HIV-exposure (5.8%; 95%CI: 4.8–6.9) than HIV-exposed cases (2.8%; 95%CI 2.1–4.0%) (S5 Table).
The proportion of cases attributed to an etiology did not differ significantly between severe and non-severe cases (severe: 31.3%; 95%CI: 26.1–37.4%; non-severe: 26.8%; 95%CI: 22.8–31.8%). However, the pathogen proportion attributed to GBS and Acinetobacter baumannii were significantly higher among severe compared to non-severe cases (S1 Fig). Due to previously observed associations between Ureaplasma colonization and prematurity,[21] we modeled etiology limited to infants born at 30+ weeks gestation with no substantive change in results.
Discussion
The high incidence of protocol-defined EOS (39.3 per 1,000 live births) in our study is lower than reported in Asia (55.8 per 1,000 live births).[22] We could not compare these findings with studies from Africa, as we could not find studies that used similar definition of sepsis, and this highlights the need for establishing a harmonized case definition for future epidemiological studies on neonatal sepsis.[23] The incidence of culture proven sepsis in this study was 3.2/ 1000 live births, and the most common organisms isolated were Gram positives, similar to that reported from other sub-Saharan African countries though the common organism was GBS instead of being Staphylococcus aureus.[5, 8, 9] This difference could be due to this study mainly enrolling babies born in healthcare-facilities and 84% of cases being investigated within 24 hours of birth. In contrast, up to 50% of births in other African countries are born outside healthcare-facilities.[6, 9] Gram negatives accounted for only 25% of EOS overall, with Acinetobacter isolated only in 4%, contrary to that reported in India where Acinetobacter (27%), Klebsiella (16%) and Escherichia coli (14%) accounted for majority of cases.[24]
To address the possibly low sensitivity of blood culture for diagnosing neonatal sepsis, we also investigated suspected neonatal cases using molecular-based assays. The molecular based results, together with blood-culture attributed a putative pathogen to 26.7% of the protocol defined EOS sepsis cases compared to 8% based only on blood culture. Nevertheless, no pathogen was attributed to a high percentage (73.3%) of EOS cases. This could be due to the causative agent not being identifiable on blood culture or not included for detection on the PCR assay. Also, it is possible that the algorithm used was not specific for diagnosing EOS, highlighting the need for better tools by which to diagnose neonatal sepsis. A challenge of using the molecular assay for identifying pathogens, is the background positivity in healthy neonates for some of organisms included in the assay. The partial latent class etiology attribution model used in our study represents a new analytic approach, to interalia address this challenge. This analytical approach has also been applied in large studies of disease etiology[22, 25], and is seemingly pertinent when using molecular diagnostic tools which might not necessarily infer causality, and when using specimens from multiple sampling sites.[22, 26] Although our case definition might still have included some cases that were not truly neonatal sepsis, contributing to the modest attributable etiology, this is inherent to all studies on neonatal sepsis in the absence of a specific and sensitive gold standard clinical definition. Nevertheless, the molecular and analytic methods, including adjusting for “false positive” by inclusion of healthy-newborns, represent a huge advance with an increase in proportion of cases in whom an etiology could be attributed (26.7%) compared to by blood culture alone (8%).
The discordance for Ureaplasma spp. between TAC and culture results is largely due to current routine microbiological methods not able to grow Ureaplasma. The dominant association of Ureaplasma spp., which commonly colonizes the female urogenital tract,[27] and EOS was an unexpected finding in our study. These findings are similar to those reported in ANISA where Ureaplasma spp. was attributed as the common cause of EOS.[22] Contrary to our findings where we did not find differences in prevalence of Ureaplasma between survivors and non-survivors, ANISA study reported a high prevalence of Ureaplasma among non-survivors suggesting pathogenicity and virulence of this organism. Ureaplasma spp. has been previously described as a cause of neonatal pneumonia, bacteremia, and meningitis particularly in preterm births.[28] Further studies on the role of Ureaplasma in pathogenesis of EOS are needed to determine whether changes to current WHO empiric antibiotic treatment guidelines are warranted.
The use of molecular diagnostics in our study, resulted in a 70% increase in estimated incidence of early-onset invasive GBS disease (1.91/1,000 births) compared to culture-only based incidence (1.12/1,000 births) which was consistent with previous estimates from this site (1.28–1.55/1,000 births).[29] This finding highlights that the current global burden of early-onset disease estimates are likely an under-estimation of the role of GBS in neonatal sepsis when estimated using only blood culture as a diagnostic tool.[30] The discordance noted overall between the traditional blood culture results (8% of cases blood culture positive) and blood PCR-based test, TAC (37% of cases blood TAC positive) is most likely due to TAC detecting culturable and non-culturable organisms (including viruses and Ureaplasma). Also, the sensitivity of blood culture could be affected by multiple factors, including volume of blood inoculated, and antibiotic inhibition. TAC also having an advantage of being able to detect both viable and non-viable organisms, thus most likely why some of the healthy neonates had positive TAC results. Compared to NP/OP molecular results, blood TAC tests had higher mean cycle threshold (CT) values, consistent with low levels of pathogen DNA present in the blood. Moreover vastly different sample volumes were evaluated by each test (blood culture: an attempted 1 ml minimum; blood TAC: 300 μL), so the results would not be expected to have strong concordance unless very high genome quantities were present in the blood and all organisms were viable. Furthermore, the background positivity of antigen detection by TAQ in the healthy population, would inherently undermine the expected concordance between blood culture and the TAC readout.
Despite this study increasing our understanding on the causes of neonatal sepsis, the study has several limitations. First, there is no gold-standard case definition for neonatal sepsis potentially leading to case misclassification and limited comparability of study results. This highlights the need to develop more specific clinical criteria for diagnosing neonatal sepsis. To address this we used a case definition that was previously applied in this population, and stratified results by disease severity to increase specificity. Sample collection and blood-culture processing relied upon standard hospital practice, which may not reflect optimized methods of sample collection and laboratory conditions. This is highlighted by finding of an increase in Pseudomonas aeruginosa detection in TAC limited to a short period of three months, which we assessed as contamination of the sample after blood was inoculated into the blood culture bottles. Our assessment that this was related to contamination was supported by assessment of the patients showing no difference in severity based on detection of Pseudomonas aeruginosa. A limitation of the partial latent class etiologic attribution model [22, 26] is that it does not cater for cases with concurrent multiple pathogens. In this study, it is unlikely that this limitation would have affected the findings, as there were only 7 patients with multiple pathogens out of 99 patients with positive cultures.
Also, an exclusion criteria of our study was perceived imminent death of the newborn, which might have been due to severe infection and consequently we might have under-estimated the burden of invasive disease by excluding them. This was, however, done based on sensitivity issues about approaching the parents for study participation immediately after they were informed of the likely poor prognosis of their newborn. Another limitation of the study is that a number of patients who might have had sepsis were not included, these included babies who were not enrolled because they were born over weekends, or born to mothers with ages <18 years, or were enrolled as they had physician defined sepsis but did not have TAC performed because they did not meet study defined sepsis criteria. Ultimately, the inability to determine an etiology in over three-quarters of cases despite using a combination of tests suggests that significant diagnostic limitations remain or that mucosal infections without bacteremia/viremia or non-infectious processes are responsible for the sepsis-like presentation of a majority of newborns in the first days of life.
In conclusion, results from our study highlight the dominant role of GBS and possible role of Ureaplasma spp. in the pathogenesis of EOS. Furthermore, although molecular diagnostic provides an opportunity in improving our understanding on the etiology of EOS, the interpretation of such tests warrant careful analytical approaches. Further studies, including the use of minimal invasive tissue sampling on neonates dying of suspected sepsis is warranted to clarify the causes of neonatal death generally and that attributed to infection.[31]
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Legend: *Pathogen proportion estimated indirectly from the “other blood culture” class. Footnote: 68.8% (62.6–73.9%) of severe case episodes and 74.9% (69.3–79.4%) of non-severe episodes could not be attributed to an evaluated pathogen class.
(TIF)
Data Availability
The data underlying the study cannot be made publicly available due to ethical restrictions imposed by the University of Witwatersrand ethics committee. The minimal data set underlying is available upon request to the Respiratory and Meningeal Pathogens Research Unit Website (contact via www.RMPRU.com), or by request to the ethics committee at HREC-Medical@ResearchOffice.wits.ac.za, for researchers who meet the criteria for access to confidential data.
Funding Statement
Sithembiso C. Velaphi (SCV) was funded for this study by Centre for Disease Control and Prevention, Atlanta, USA through the South African Global Disease Detection Centre at National Institute of Communicable Diseases. The funder of the study contributed to the study design, data analysis, data interpretation and reviewing the report through its employees, authors MW, TP, MD, JW, NS, AD and SJS.
References
- 1.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: An updated systematic analysis with implications for the sustainable development goals. Lancet. 2016;388(10063):3027–3035. 10.1016/S0140-6736(16)31593-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klingenberg C, Olomi R, Oneko M, Sam N, Langeland N. Neonatal morbidity and mortality in a tanzanian tertiary care referral hospital. Ann Trop Paediatr. 2003;23(4):293–299. 10.1179/027249303225007806 [DOI] [PubMed] [Google Scholar]
- 3.Udo JJ, Anah MU, Ochigbo SO, Etuk IS, Ekanem AD. Neonatal morbidity and mortality in calabar, nigeria: A hospital-based study. Niger J Clin Pract. 2008;11(3):285–289. [PubMed] [Google Scholar]
- 4.Nathoo KJ, Mason PR, Chimbira TH. Neonatal septicaemia in harare hospital: Aetiology and risk factors. The puerperal sepsis study group. Cent Afr J Med. 1990;36(6):150–156. [PubMed] [Google Scholar]
- 5.Mugalu J, Nakakeeto MK, Kiguli S, Kaddu-Mulindwa DH. Aetiology, risk factors and immediate outcome of bacteriologically confirmed neonatal septicaemia in mulago hospital, uganda. Afr Health Sci. 2006;6(2):120–126. 10.5555/afhs.2006.6.2.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kayange N, Kamugisha E, Mwizamholya DL, Jeremiah S, Mshana SE. Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, mwanza-tanzania. BMC Pediatr. 2010;10:39 10.1186/1471-2431-10-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tann CJ, Nkurunziza P, Nakakeeto M, Oweka J, Kurinczuk JJ, Were J, et al. Prevalence of bloodstream pathogens is higher in neonatal encephalopathy cases vs. Controls using a novel panel of real-time pcr assays. PLoS One. 2014;9(5):e97259 10.1371/journal.pone.0097259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labi AK, Obeng-Nkrumah N, Bjerrum S, Enweronu-Laryea C, Newman MJ. Neonatal bloodstream infections in a ghanaian tertiary hospital: Are the current antibiotic recommendations adequate? BMC Infect Dis. 2016;16(1):598 10.1186/s12879-016-1913-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akindolire AE, Tongo O, Dada-Adegbola H, Akinyinka O. Etiology of early onset septicemia among neonates at the university college hospital, ibadan, nigeria. J Infect Dev Ctries. 2016;10(12):1338–1344. 10.3855/jidc.7830 [DOI] [PubMed] [Google Scholar]
- 10.Kabwe M, Tembo J, Chilukutu L, Chilufya M, Ngulube F, Lukwesa C, et al. Etiology, antibiotic resistance and risk factors for neonatal sepsis in a large referral center in zambia. Pediatr Infect Dis J. 2016;35(7):e191–198. 10.1097/INF.0000000000001154 [DOI] [PubMed] [Google Scholar]
- 11.Cutland CL, Madhi SA, Zell ER, Kuwanda L, Laque M, Groome M, et al. Chlorhexidine maternal-vaginal and neonate body wipes in sepsis and vertical transmission of pathogenic bacteria in south africa: A randomised, controlled trial. Lancet. 2009;374(9705):1909–1916. 10.1016/S0140-6736(09)61339-8 [DOI] [PubMed] [Google Scholar]
- 12.Young Infants Clinical Signs Study Group. Clinical signs that predict severe illness in children under age 2 months: A multicentre study. Lancet. 2008;371(9607):135–142. 10.1016/S0140-6736(08)60106-3 [DOI] [PubMed] [Google Scholar]
- 13.Zakariya BP, Bhat BV, Harish BN, Arun Babu T, Joseph NM. Risk factors and predictors of mortality in culture proven neonatal sepsis. Indian J Pediatr. 2012;79(3):358–361. 10.1007/s12098-011-0584-9 [DOI] [PubMed] [Google Scholar]
- 14.Hossain B, Weber MW, Hamer DH, Hibberd PL, Ahmed AS, Marzan M, et al. Classification of blood culture isolates into contaminants and pathogens on the basis of clinical and laboratory data. Pediatr Infect Dis J. 2016;35(5 Suppl 1):S52–54. [DOI] [PubMed] [Google Scholar]
- 15.Saha SK, Islam MS, Qureshi SM, Hossain B, Islam M, Zaidi AK, et al. Laboratory methods for determining etiology of neonatal infection at population-based sites in south asia: The anisa study. Pediatr Infect Dis J. 2016;35(5 Suppl 1):S16–22. 10.1097/INF.0000000000001101 [DOI] [PubMed] [Google Scholar]
- 16.Kodani M, Yang G, Conklin LM, Travis TC, Whitney CG, Anderson LJ, et al. Application of taqman low-density arrays for simultaneous detection of multiple respiratory pathogens. J Clin Microbiol. 2011;49(6):2175–2182. 10.1128/JCM.02270-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Diaz MH, Waller JL, Napoliello RA, Islam MS, Wolff BJ, Burken DJ, et al. Optimization of multiple pathogen detection using the taqman array card: Application for a population-based study of neonatal infection. PLoS One. 2013;8(6):e66183 10.1371/journal.pone.0066183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kodani M, Winchell JM. Engineered combined-positive-control template for real-time reverse transcription-pcr in multiple-pathogen-detection assays. J Clin Microbiol. 2012;50(3):1057–1060. 10.1128/JCM.05987-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Z, Deloria-Knoll M, Hammitt LL, Zeger SL, the Pneumonia Etiology Research for Child Health Core T. Partially latent class models for case–control studies of childhood pneumonia aetiology. Journal of the Royal Statistical Society: Series C (Applied Statistics). 2016;65(1):97–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deloria Knoll M, Fu W, Shi Q, Prosperi C, Wu Z, Hammitt LL, et al. Bayesian estimation of pneumonia etiology: Epidemiologic considerations and applications to the pneumonia etiology research for child health study. Clin Infect Dis. 2017;64(suppl_3):S213–S227. 10.1093/cid/cix144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005;18(4):757–789. 10.1128/CMR.18.4.757-789.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saha SK, Schrag SJ, El Arifeen S, Mullany LC, Shahidul Islam M, Shang N, et al. Causes and incidence of community-acquired serious infections among young children in south asia (anisa): An observational cohort study. Lancet. 2018;392(10142):145–159. 10.1016/S0140-6736(18)31127-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitchett EJA, Seale AC, Vergnano S, Sharland M, Heath PT, Saha SK, et al. Strengthening the reporting of observational studies in epidemiology for newborn infection (strobe-ni): An extension of the strobe statement for neonatal infection research. Lancet Infect Dis. 2016;16(10):e202–e213. 10.1016/S1473-3099(16)30082-2 [DOI] [PubMed] [Google Scholar]
- 24.Investigators of the delhi neonatal infection study (denis) collaboration. Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in delhi, india: A cohort study. Lancet Glob Health. 2016;4(10):e752–760. 10.1016/S2214-109X(16)30148-6 [DOI] [PubMed] [Google Scholar]
- 25.O'Brien KL, Baggett HC, Brooks WA, Feikin DR, Hammitt LL, Howie SRC, et al. Introduction to the epidemiologic considerations, analytic methods, and foundational results from the pneumonia etiology research for child health study. Clin Infect Dis. 2017;64(suppl_3):S179–S184. 10.1093/cid/cix142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine OS, O'Brien KL, Deloria-Knoll M, Murdoch DR, Feikin DR, DeLuca AN, et al. The pneumonia etiology research for child health project: A 21st century childhood pneumonia etiology study. Clin Infect Dis. 2012;54 Suppl 2:S93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez PJ. Perinatal transmission of ureaplasma urealyticum: Current concepts based on review of the literature. Clin Infect Dis. 1993;17(Suppl 1):S107–111. [DOI] [PubMed] [Google Scholar]
- 28.Sung TJ. Ureaplasma infections in pre-term infants: Recent information regarding the role of ureaplasma species as neonatal pathogens. Korean J Pediatr. 2010;53(12):989–993. 10.3345/kjp.2010.53.12.989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dangor Z, Cutland CL, Izu A, Kwatra G, Trenor S, Lala SG, et al. Temporal changes in invasive group b streptococcus serotypes: Implications for vaccine development. PLoS One. 2016;11(12):e0169101 10.1371/journal.pone.0169101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madrid L, Seale AC, Kohli-Lynch M, Edmond KM, Lawn JE, Heath PT, et al. Infant group b streptococcal disease incidence and serotypes worldwide: Systematic review and meta-analyses. Clin Infect Dis. 2017;65(suppl_2):S160–s172. 10.1093/cid/cix656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassat Q, Castillo P, Martinez MJ, Jordao D, Lovane L, Hurtado JC, et al. Validity of a minimally invasive autopsy tool for cause of death determination in pediatric deaths in mozambique: An observational study. PLoS Med. 2017;14(6):e1002317 10.1371/journal.pmed.1002317 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Legend: *Pathogen proportion estimated indirectly from the “other blood culture” class. Footnote: 68.8% (62.6–73.9%) of severe case episodes and 74.9% (69.3–79.4%) of non-severe episodes could not be attributed to an evaluated pathogen class.
(TIF)
Data Availability Statement
The data underlying the study cannot be made publicly available due to ethical restrictions imposed by the University of Witwatersrand ethics committee. The minimal data set underlying is available upon request to the Respiratory and Meningeal Pathogens Research Unit Website (contact via www.RMPRU.com), or by request to the ethics committee at HREC-Medical@ResearchOffice.wits.ac.za, for researchers who meet the criteria for access to confidential data.