Abstract
Study Objectives:
To determine the optimal Actiwatch 2 setting configuration for the estimation of total sleep time (TST) in persons with suspected idiopathic hypersomnia.
Methods:
Thirty-three patients with a diagnosis of idiopathic hypersomnia (28 female; mean age = 33.7 ± 10.5) underwent ad libitum polysomnography with concurrent use of the Actiwatch 2. Actiwatch 2 sleep-wake activity threshold (SWAT; Low, Medium, and High) and sleep immobility onset and offset (SIOO; 5, 10, 15, 20, 25, and 30 epoch) duration were modified during data processing. The resultant 18 unique setting combinations were subsequently evaluated using Bland-Altman and epoch comparison analyses to determine optimal settings relative to polysomnography.
Results:
Low SWAT + 25 Epoch SIOO displayed the least divergence from polysomnography (mean difference 3.4 minutes). Higher SWAT and lower SIOO increased sensitivity and accuracy, but at the expense of reducing specificity and the ability to accurately estimate TST.
Conclusions:
These results demonstrate that actigraphic settings should be carefully considered when estimating sleep duration. The Low + 25 Epoch configuration is indicated as most optimal for estimating TST in persons with suspected idiopathic hypersomnia.
Commentary:
A commentary on this article appears in this issue on page 539.
Citation:
Cook JD, Eftekari SC, Leavitt LA, Prairie ML, Plante DT. Optimizing actigraphic estimation of sleep duration in suspected idiopathic hypersomnia. J Clin Sleep Med. 2019;15(4):597–602.
Keywords: actigraphy, Actiwatch, hypersomnolence, idiopathic hypersomnia
BRIEF SUMMARY
Current Knowledge/Study Rationale: Current sleep medicine nosology considers actigraphy to be an alternative method to quantify excessive sleep duration in idiopathic hypersomnia, yet standardized setting parameters have not been established. Considering actigraphic estimates of total sleep time (TST) are highly dependent upon device settings, TST assessment in persons with suspected idiopathic hypersomnia can be enhanced by establishing an optimal parameter configuration.
Study Impact: These results demonstrate the necessity of standardized actigraphic settings for estimations of TST. The Low + 25 Epoch parameter configuration is indicated as most optimal for estimating TST for persons with suspected idiopathic hypersomnia.
INTRODUCTION
Idiopathic hypersomnia (IH) is a chronic, debilitating disorder that is characterized by the cardinal symptoms of excessive daytime sleepiness with normal or prolonged sleep duration.1,2 Standard clinical assessment utilizes nocturnal polysomnography (PSG) and daytime Multiple Sleep Latency Test (MSLT) to objectively assess excessive daytime sleepiness and sleep duration.3,4 Currently, the International Classification of Sleep Disorders, Third Edition (ICSD-3) recognizes a MSLT mean sleep onset latency (mSOL) < 8 minutes and/or prolonged sleep duration through total sleep time (TST) ≥ 660 minutes across a 24-hour period as objective indications of IH.4–7
Notably, the ICSD-3 now also recognizes actigraphy, a movement-based measurement used to estimate habitual sleep-wake patterns,8,9 as a viable alternative to electroencephalography (EEG)-based methods for quantifying TST within the diagnostic framework of IH.4–7 Although actigraphy has demonstrated utility as a method to estimate TST across a variety of disorders including those with IH,8–15 ability of the device to estimate sleep duration is highly dependent on its parameter configurations.15–17 Currently, there are no standardized actigraphic parameters for the evaluation of TST in IH. In this context, establishing optimal actigraphic configurations to estimate TST in IH is an important step for research and clinical care in the disorder. Thus, this investigation aimed to determine optimal actigraphic parameter configurations to estimate TST in persons with IH relative to gold-standard PSG.
METHODS
Participants and Study Design
Thirty-three patients with a diagnosis of IH were drawn from a larger study examining multidimensional hypersomnolence assessments in patients referred for PSG/MSLT. All participants were clinical patients at Wisconsin Sleep, the sleep clinic and laboratory affiliated with the University of Wisconsin-Madison. For this study, participants concurrently wore the Actiwatch 2 (AW2; Phillips Respironics, Murrysville, Pennsylvania, United States) on their nondominant wrist during overnight, ad libitum PSG, conducted without a prescribed wake time. The clinical diagnosis of IH was made by the patient's treating physician and confirmed by post hoc chart review. Participants also completed the Epworth Sleepiness Scale (ESS),18 Pittsburgh Sleep Quality Index (PSQI),19 Hypersomnia Severity Index,20 and Inventory of Depression Symptomatology – Self Report (IDS-SR)21,22 during their PSG/MSLT. All participants provided informed consent, with the first consent occurring on November 17, 2016 and the last on April 12, 2018, and this study was approved by the Health Sciences Institutional Review Board of the University of Wisconsin-Madison.
PSG Collection and Scoring
PSG data were collected following American Academy of Sleep Medicine (AASM) guidelines using a standard six-channel EEG montage paired with other recording sensors including electrooculogram, submental electromyogram, electrocardiogram, bilateral tibial electromyogram, respiratory inductance plethysmography, pulse oximetry, and a position sensor (Alice Sleepware; Phillips Respironics). A registered sleep technologist, blind to the AW2 staging output, staged all PSG recordings using 30-second epochs according to AASM criteria.23 All participants were scheduled for routine clinical testing and arrived at the sleep laboratory at approximately 7:00 pm. After PSG setup, participants underwent an ad libitum protocol, whereby participants freely determined the onset (lights-off) and termination (lights-on) of their nocturnal assessment. During their sleep period, participants were minimally disturbed with technicians entering the room only if technical issues arose that would inhibit sleep staging.
AW2 Collection, Processing, Setting Modifications, and Statistical Analysis
Participants concurrently wore the AW2 on their nondominant wrist for the duration of the ad libitum PSG. Polysomnographic and accelerometer data were collected within a local network of computers that were time synchronized to an external atomic clock through frequent restart.24 Although the AW2 software allows the user to choose between five durations of epoch resolution, this investigation chose to collect and analyze the AW2 data using a 30-second epoch resolution to remain congruent with PSG. PSG lights-off and lights-on time stamps were used to set the rest interval within the AW2 data.
Actigraphic data were extracted using Actiware (Phillips Respironics). Within the Actiware software, two modifiable parameters exist that can affect data output: the sleep-wake activity threshold (SWAT) and sleep immobility onset and offset (SIOO). SWAT determines the classification of sleep versus wake based on a calculated amount of movement from a weighted summation algorithm incorporating the four epochs before and after the epoch under evaluation25:
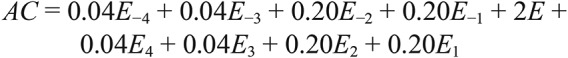 |
where AC is activity count and E is the epoch under evaluation.
This calculated AC value is then compared against the set SWAT with values exceeding the threshold being classified as wake and those below the threshold as sleep. The AW2 software includes Low (20 AC), Medium (40 AC), and High (80 AC) SWAT options, which were used in this investigation.
SIOO determines the sleep period within the overall rest interval by identifying when sleep begins and ends based on a set duration of epochs that are subthreshold (immobile) to the SWAT setting. Within this specific setting parameter, users have the option to choose between immobile minutes or number of 30-second epochs, with this investigation utilizing the number of 30-second epochs to remain congruent with PSG staging. SIOO durations were extracted using six different variations: 5 (5E), 10 (10E), 15 (15E), 20 (20E), 25 (25E), and 30 (30E) 30-second epochs.
Overall, the combination of SWAT and SIOO variations resulted in 18 unique setting combinations that were evaluated against PSG. Data for each setting combination were exported from Actiware and sleep variable tabulations were performed utilizing inhouse custom MATLAB (Mathworks; Natick, Massachusetts, United States) scripts.24
Bland-Altman mean difference analysis26 was used to compare sleep variables for each of the AW2 setting combinations relative to PSG. Further analyses explored the overall congruency between individually staged epochs. Sensitivity (ability to correctly detect PSG-scored sleep epochs), specificity (ability to correctly detect PSG-scored wake epochs), and accuracy (ability to correctly detect PSG-scored sleep and wake epochs) were computed for each of the AW2 setting combinations.
Epoch-by-epoch comparisons were executed using MATLAB with all other statistical analyses performed using JMP Pro 14 (SAS; Cary, North Carolina, United States). Alpha equaled .05 for statistical significance for all comparisons. Results are presented as mean ± standard deviation unless otherwise noted.
RESULTS
Sample Demographics, Characteristics, and PSG/MSLT Sleep Variables
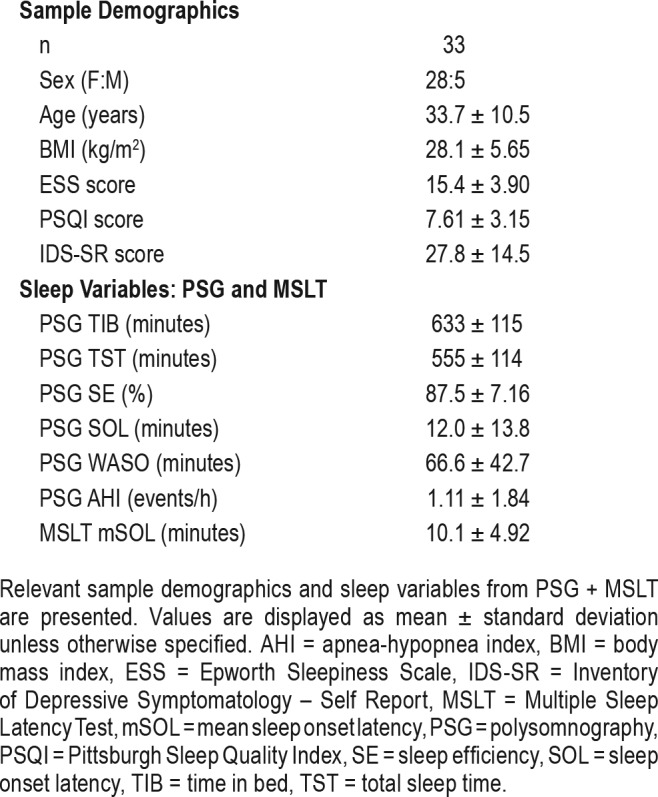
Sample characteristics and PSG/MSLT values are presented in Table 1. The sample was predominantly female (28F:5M) and consisted of young to middle aged individuals (age = 33.7 ± 10.5). Approximately half of patients (n = 17) were on psychotropic medications at the time of PSG/MSLT evaluation. Regarding antidepressant use, 12 patients were taking selective serotonin reuptake inhibitors, one was taking a serotonin-norepinephrine reuptake inhibitor, and one was taking a tricyclic antidepressant at the time of the study. Participants endorsed clinically significant levels of self-reported daytime sleepiness (ESS > 10; mean ESS = 15.4 ± 3.9),18 a global assessment of sleep quality representative of significant disturbance (PSQI > 5; mean PSQI = 7.61 ± 3.15),19 and depression symptomatology of moderate severity (IDS-SR > 25; mean IDS-SR = 27.8 ± 14.5).27 PSG ruled out other causes of somnolence including sleep-disordered breathing and periodic limb movement disorder (Table 1). Mean sleep duration was 9.25 hours on overnight PSG.
Table 1.
Sample demographics and sleep variables.
All participants received a clinical diagnosis of IH by their treating physician. Eighteen participants met diagnostic criteria for IH with MSLT mSOL below 8 minutes and/or EEG-defined sleep duration > 660 minutes during in-laboratory PSG/MSLT assessment. An additional 5 participants met objective criteria for IH due to habitual sleep duration > 660 minutes per day quantified by actigraphy preceding in-laboratory assessment (with TST defined using default Actiware settings). The remainder of the participants received an IH diagnosis from their treating providers following ICSD-3–allowed clinical judgment,5 with patients in this category often having MSLT mSOL or TST values that have historically been considered “gray” areas (eg, MSLT mSOL 8 to 10 minutes and/or TST 10 to 11 hours).
AW2 Versus PSG: Bland-Altman Mean Difference Analysis
A complete summary of the Bland-Altman mean difference analyses is presented in Figure 1. Results demonstrated wide variation in AW2 congruency with PSG when measuring TST. Overall, the Low SWAT setting coupled with longer SIOO durations demonstrated the least divergence from PSG, with higher SWAT and shorter SIOO durations resulting in greater divergence. The most optimal setting combinations indicated by this approach were the Low + 25E and Low + 30E, with the Low + 25E slightly overestimating TST (mean difference = 3.4 ± 8.72 minutes; P = .70) and Low + 30E slightly underestimating TST (mean difference = −4.4 ± 11.1 minutes; P = .69). Of settings tested, the High + 5E displayed the largest divergence from PSG, with results illustrating a significant overestimation of TST (mean difference = 53.6 ± 6.37 minutes; P < .0001). Post hoc analysis demonstrated similar results for patients taking or not taking psychotropic medications at the time of testing, though Low + 20E most closely approximated TST for patients not on psychotropic medications (mean difference −5.2 ± 13.7 minutes), whereas Low +30E most closely approximated TST for those taking medications (mean difference 7.3 ± 14.4 minutes). Results were also very similar in the subset of patients who met criteria for IH based only on MSLT and/ or in-laboratory EEG defined TST, with the Low +25E setting underestimating sleep duration by only 2.9 minutes.
Figure 1. Bland-Altman mean difference summary: TST.
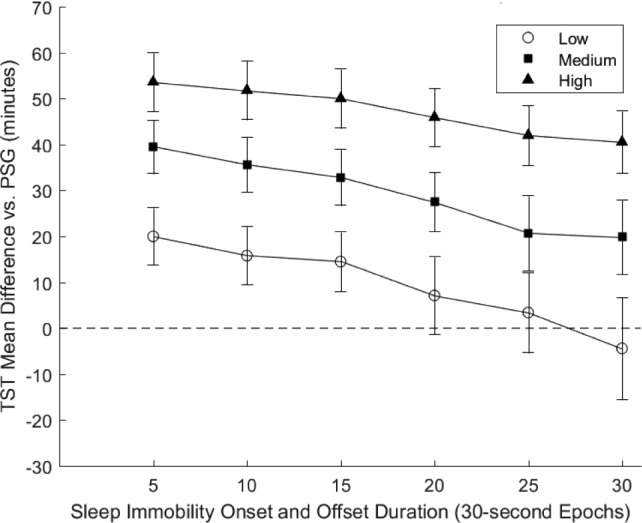
TST mean difference values are presented for the 18 setting combinations. The x-axis is the sleep immobility onset and offset duration. The y-axis is the mean difference value for the setting combination relative to PSG, with positive values corresponding to overestimations. Each line on the graph references a different sleep-wake activity threshold configuration. Unfilled circles = Low (20 AC). Filled squares = Medium (40 AC). Filled triangles = High (80 AC). The dashed horizontal line represents no deviation from PSG TST. AC = activity count, PSG = polysomnography, TST = total sleep time.
AW2 Versus PSG: Epoch Comparisons
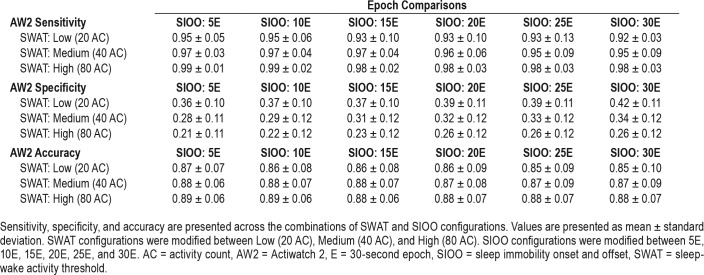
A complete presentation of the sensitivity, specificity, and accuracy calculations are displayed in Table 2.
Table 2.
Epoch comparisons: sensitivity, specificity, and accuracy.
All setting combinations demonstrated relatively good ability to identify true sleep epochs (sensitivity), with each variation permutation demonstrating sensitivity greater than 0.9. Overall, the High SWAT setting coupled with shorter SIOO durations resulted in the greatest sensitivities, with High + 5E displaying the highest (0.99 ± 0.01) and Low + 30E exhibiting the lowest (0.92 ± 0.13) sensitivity.
All setting combinations demonstrated relatively poor ability to identify true wake epochs (specificity), with each variation demonstrating specificity below 0.5. Overall, the Low SWAT setting coupled with longer SIOO resulted in the greatest specificities, with Low + 30E displaying the highest (0.42 ± 0.11) and High + 5E exhibiting the lowest (0.21 ± 0.11) specificity.
The accuracy component of the epoch comparisons paralleled the sensitivity results, where each variation demonstrated good accuracy relative to PSG. Overall, the setting combinations displayed relatively similar accuracy values, with a general trend of higher SWAT and shorter SIOO durations resulting in higher accuracy.
DISCUSSION
This investigation aimed to define optimal setting parameters for a widely used actigraph to estimate TST in persons with IH. The results from this investigation are important for several reasons. First, these results corroborate the existing literature that demonstrates alterations in configuration parameters result in wide variability in actigraphic estimation of TST. Second, these results highlight the importance of device specificity (ability to correctly detect polysomnography-scored wake epochs) in actigraphic estimation of sleep duration in IH. Last, these results provide specific parameters for a common, commercially available actigraph that most closely approximate TST in IH, which can be used in future validation studies.
Daily excessive sleep duration (ESD) is a fundamental component of IH presentation and classification,5 yet objectively measuring total sleep duration across a 24-hour period can be problematic. Prolonged and/or continuous EEG recordings can be used to measure ESD, but few laboratories employ this technique because of its prohibitive cost and logistical complexities.1 Moreover, longitudinal assessment of daily ESD can be quite useful when distinguishing IH from other behavioral and/or psychological causes of hypersomnolence, such as insufficient sleep time and clinophilia.28 Thus, wrist actigraphy provides a longitudinal alternative to extended-duration EEG recordings in estimating sleep duration in IH at a fraction of the cost.
Although the ICSD-3 allows the use of actigraphy averaged over at least 7 days of unrestricted sleep to identify increased sleep duration in the diagnosis of IH, this criterion exists in the absence of diagnostic validation. The ICSD-3 notes that the use of actigraphy to diagnose IH has not been validated against healthy controls participants,5 but more fundamentally, the optimal actigraphic settings to estimate sleep duration in IH have not been established. As noted by a recent AASM-commissioned review of actigraphy, device-setting parameters are an important component of estimating sleep variables using this technology.29 Thus, this study is an important step toward validating the use of actigraphy to estimate sleep duration in IH; however, further work is clearly indicated to validate this approach over extended periods of time and against healthy individuals.
Two complementary approaches were used in this investigation to determine optimal actigraphic parameters relative to PSG. Bland-Altman (mean difference) results indicated better congruency with PSG for lower SWAT coupled with longer SIOO configurations. Congruent with the prior literature, altering SWAT and SIOO resulted in a wide range of predominantly overestimates of sleep duration relative to PSG. In this context, it is noteworthy that these optimal settings were associated with higher specificity (ability to correctly detect PSG-scored sleep and wake epochs), rather than sensitivity (ability to correctly detect PSG-scored sleep epochs) or accuracy (ability to correctly detect PSG-scored sleep and wake epochs). On average, persons with IH have sleep efficiencies similar to those of healthy control participants,30 and thus most epochs within a given night are far more likely to be sleep epochs than wake epochs. Within such a population, our results corroborate the notion that the ability to correctly identify epochs of wake within a period consisting predominantly of sleep, is ultimately a more critical factor in accurately estimating TST. Given this and the general shortcomings of actigraphy in its ability to identify true wake, it is critical for future actigraphic validations to consider the sleep characteristics of the group under investigation when determining optimal actigraphic settings relative to PSG.
There are limitations of this study that merit discussion. First, our convenience sample was predominantly female, which may limit the generalizability of results. Second, our sample was composed entirely of adults and, as such, the results may not apply to children presenting with symptoms of hypersomnolence. Third, only a single night of assessment was used in this design and, as such, the results may not extend to longitudinal actigraphic assessment in IH. Further research is warranted to evaluate the utility of these findings across an extended evaluation period in the disorder. Fourth, this investigation only evaluated one widely used actigraph and the results may not generalize to other actigraphs that use different scoring algorithms and/or setting parameters. Further research would be required to establish optimal parameter configurations for alternative actigraphs in the estimation of TST in IH.
In conclusion, the results from this investigation suggest that combining the Low SWAT with the 25-epoch SIOO parameters, while using a 30-second epoch resolution, is most appropriate for AW2 estimation of TST in persons with suspected IH. These findings will inform future clinical research in the use of actigraphy to accurately quantify excessive sleep duration in the disorder.
DISCLOSURE STATEMENT
Dr. Plante has received research support from NIH, the Brain and Behavior Research Foundation, and the American Sleep Medicine Foundation; and he has been a consultant for Teva Pharmaceuticals Australia and Jazz Pharmaceuticals. The other authors report no conflicts of interest. This research was supported by a grant from the American Sleep Medicine Foundation (138-SR-16) to DTP.
ACKNOWLEDGMENTS
The authors thank Erika Dallman, Megan Sippy, Sara Khan, and Sam Boroumand for their assistance with data collection. We additionally thank the research participants for their time and effort, as well as the clinical and laboratory staff at Wisconsin Sleep for their assistance.
ABBREVIATIONS
- AASM
American Academy of Sleep Medicine
- AC
activity count
- AHI
apnea-hypopnea index
- AW2
Actiwatch 2
- BMI
body mass index
- E
epochs
- EEG
electroencephalography
- ESD
excessive sleep duration
- ESS
Epworth Sleepiness Scale
- ICSD
International Classification of Sleep Disorders
- IDS-SR
Inventory of Depression Symptomatology – Self Report
- IH
idiopathic hypersomnia
- mSOL
mean sleep onset latency
- MSLT
Multiple Sleep Latency Test
- PSQI
Pittsburgh Sleep Quality Index
- PSG
polysomnography
- SIOO
sleep immobility onset and offset
- SWAT
sleep-wake activity threshold
- TST
total sleep time
- WASO
wake after sleep onset
REFERENCES
- 1.Billiard M, Sonka K. Idiopathic hypersomnia. Sleep Med Rev. 2016;29:23–33. doi: 10.1016/j.smrv.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Trotti LM. Idiopathic hypersomnia. Sleep Med Clin. 2017;12(3):331–344. doi: 10.1016/j.jsmc.2017.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boon P, Pevernagie D, Schrans D. Hypersomnolence and narcolepsy; a pragmatic diagnostic neurophysiological approach. Acta Neurol Belg. 2002;102(1):11–18. [PubMed] [Google Scholar]
- 4.Khan Z, Trotti LM. Central disorders of hypersomnolence: focus on the narcolepsies and idiopathic hypersomnia. Chest. 2015;148(1):262–273. doi: 10.1378/chest.14-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 6.Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–1394. doi: 10.1378/chest.14-0970. [DOI] [PubMed] [Google Scholar]
- 7.Sowa NA. Idiopathic hypersomnia and hypersomnolence disorder: a systematic review of the literature. Psychosomatics. 2016;57(2):152–164. doi: 10.1016/j.psym.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Martin JL, Blackwell T, et al. The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med. 2015;13(Suppl 1):S4–S38. doi: 10.1080/15402002.2015.1046356. [DOI] [PubMed] [Google Scholar]
- 9.de Souza L, Benedito-Silva AA, Pires ML, Poyares D, Tufik S, Calil HM. Further validation of actigraphy for sleep studies. Sleep. 2003;26(1):81–85. doi: 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- 10.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 11.Filardi M, Pizza F, Martoni M, Vandi S, Plazzi G, Natale V. Actigraphic assessment of sleep/wake behavior in central disorders of hypersomnolence. Sleep Med. 2015;16(1):126–130. doi: 10.1016/j.sleep.2014.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Kushida CA, Chang A, Gadkary C, Guilleminault C, Carrillo O, Dement WC. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2(5):389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 13.Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514–1527. doi: 10.1378/chest.10-1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meltzer LJ, Montgomery-Downs HE, Insana SP, Walsh CM. Use of actigraphy for assessment in pediatric sleep research. Sleep Med Rev. 2012;16(5):463–475. doi: 10.1016/j.smrv.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15(4):259–267. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Chae KY, Kripke DF, Poceta JS, et al. Evaluation of immobility time for sleep latency in actigraphy. Sleep Med. 2009;10(6):621–625. doi: 10.1016/j.sleep.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Meltzer LJ, Hiruma LS, Avis K, Montgomery-Downs H, Valentin J. Comparison of a commercial accelerometer with polysomnography and actigraphy in children and adolescents. Sleep. 2015;38(8):1323–1330. doi: 10.5665/sleep.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 19.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan KA, Plante DT, Cook JD, Soehner AM, Harvey AG. Validation of the Hypersomnia Severity Index (HSI) [abstract] Sleep. 2015;38(Abstract Suppl):A432. [Google Scholar]
- 21.Corruble E, Legrand JM, Duret C, Charles G, Guelfi JD. IDS-C and IDS-SR: psychometric properties in depressed in-patients. J Affect Disord. 1999;56(2–3):95–101. doi: 10.1016/s0165-0327(99)00055-5. [DOI] [PubMed] [Google Scholar]
- 22.Trivedi MH, Rush AJ, Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol Med. 2004;34(1):73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- 23.Berry RB, Brooks R, Gamaldo CE, et al. for the American Academy of Sleep Medicine. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology, and Technical Specifications. Darien, IL: American Academy of Sleep Medicine; 2014. Version 2.1. [Google Scholar]
- 24.Cook JD, Prairie ML, Plante DT. Ability of the multisensory Jawbone UP3 to quantify and classify sleep in patients with suspected central disorders of hypersomnolence: a comparison against polysomnography and actigraphy. J Clin Sleep Med. 2018;14(5):841–848. doi: 10.5664/jcsm.7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips Respironics. Actiware: Instruction Manual. Murrysville, PA: Phillips Respironics; 2015. [Google Scholar]
- 26.Altman DG, Bland JM. Measurement in medicine: the analysis of method comparison studies. J R Stat Soc Ser D Statistician. 1983;32(3):307–317. [Google Scholar]
- 27.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54(5):573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 28.Dauvilliers Y, Lopez R, Ohayon M, Bayard S. Hypersomnia and depressive symptoms: methodological and clinical aspects. BMC Med. 2013;11:78. doi: 10.1186/1741-7015-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith MT, McCrae CS, Cheung J, et al. Use of actigraphy for the evaluation of sleep disorders and circadian rhythm sleep-wake disorders: an American Academy of Sleep Medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2018;14(7):1209–1230. doi: 10.5664/jcsm.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Plante DT. Nocturnal sleep architecture in idiopathic hypersomnia: a systematic review and meta-analysis. Sleep Med. 2018;45(5):17–24. doi: 10.1016/j.sleep.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]