Abstract
Study Objectives:
We previously presented results from a randomized controlled trial that examined the effects of antidepressant medication plus cognitive behavioral therapy for insomnia (CBT-I) among patients with major depressive disorder (MDD) and insomnia. The current secondary analysis aims to examine whether circadian preference moderated the reduction in depression and insomnia symptom severity during this trial.
Methods:
A total of 139 adult participants with MDD and insomnia disorder were treated with antidepressant medication and randomized to receive 7 sessions of CBT-I or a control therapy (CTRL). Circadian preference (eveningness) was measured using the Composite Scale of Morningness (CSM). Depression symptom severity was assessed using the Hamilton Depression Rating Scale (HDRS); insomnia symptom severity was assessed using the Insomnia Severity Inventory (ISI). The moderating role of circadian preference on changes in HRSD and ISI was assessed via latent growth models within the framework of structural equation modeling.
Results:
Greater evening preference was associated with smaller reduction in HDRS (P = .03) from baseline to week 6 across treatment groups. The interaction between CSM and treatment group was also significant (P = .02), indicating that participants with greater evening preference in the CTRL group had significantly smaller HDRS reduction than those with greater evening preference in the CBT-I group. Circadian preference did not share significant associations with ISI (all P > .30).
Conclusions:
Individuals with MDD and insomnia who have an evening preference are at increased risk for poor response to pharmacological depression treatment augmented with either CBT-I or CTRL behavioral insomnia treatment. However, evening types have better depression outcomes when treated with CBT-I than with CTRL for insomnia.
Citation:
Asarnow LD, Bei B, Krystal A, Buysse DJ, Thase ME, Edinger JD, Manber R. Circadian preference as a moderator of depression outcome following cognitive behavioral therapy for insomnia plus antidepressant medications: a report from the triad study. J Clin Sleep Med. 2019;15(4):573–580.
Keywords: CBT-I, circadian, depression, eveningness, insomnia
BRIEF SUMMARY
Current Knowledge/Study Rationale: Evening circadian preference is associated with increased vulnerability to both depression and insomnia and evidence that one of the benefits of cognitive behavioral therapy for insomnia is reduction in depressive symptom severity. Prior research indicates that eveningness may be associated with less reduction in depressive symptom severity following cognitive behavioral therapy for insomnia.
Study Impact: This is the first study to investigate the role of circadian preference in response to treatment for depression and insomnia in a randomized controlled treatment trial. The present study findings suggest that clinical care using antidepressant medications for individuals with comorbid depression and insomnia could be enhanced by (1) evaluating circadian preference and (2) augmenting antidepressant medications with cognitive behavioral therapy for insomnia for those expressing an evening circadian preference.
INTRODUCTION
Sleep and its regulatory systems, including the circadian and arousal systems, are closely related to mental illness and mental health treatments.1 During major depressive episodes, as many as 67% to 84% of adults report difficulties initiating or maintaining sleep.2–4 Insomnia symptoms are associated with greater functional impairment5 and depressive symptom severity.6 Poor sleep is a risk factor for a future depressive episode among nondepressed individuals7 and those who are in remission from a previous depressive episode.8
Circadian preference for rest and activity is also relevant to depression. An evening circadian preference, also referred to as eveningness, is characterized by a preference for later sleep onset and offset. Individuals with evening preference have higher suicide risk9,10 and greater depressive symptoms severity.11–13 These associations were found in community,14–16 clinically depressed,10,17 and admitted psychiatric inpatient18 samples. In an observational study of 253 individuals with major depressive disorder (MDD) recruited from a psychiatric outpatient clinic, eveningness was associated with higher risk of nonremission from depression at 5-year naturalistic follow-up, independent of insomnia severity.19 In a small study of 30 adults with depressive disorder who received fluoxetine, sleep duration and timing were experimentally manipulated in order to examine effects on circadian timing relative to sleep onset and on depressive symptoms.20 The study randomized participants to one of three sleep conditions: (1) 8 hours in bed at habitual bed time, (2) 6 hours in bed with a 2-hour advance of habitual rise time, or (3) 6 hours in bed with 2-hour delay of habitual rise time. Sleep restriction with delayed timing was associated with a poorer antidepressant treatment response across 8 weeks of the study relative to the habitual sleep duration and timing condition.20 These studies suggest that preference for delayed schedule are relevant to depression severity and response to antidepressant treatment.
Eveningness is also associated with worse self-reported sleep quality,10,21,22 shorter sleep duration, and more nonrefreshing sleep on working days.23,24 Among adults with insomnia, those with evening preference report more variability in their sleep schedules and greater distress than expected in association with the level of reported insomnia severity. Despite reporting more total sleep time, they also report more concern about the consequences of insomnia and their ability to control sleep.25 These studies suggest that individuals with evening preference might be more sensitive to the effects of sleep loss or more averse to perceived sleep disruption than individuals with higher scores on measures of circadian preferences. These observations further suggest that having a circadian preference toward eveningness may affect responses to cognitive behavioral therapy for insomnia (CBT-I), which typically includes changes in bedtime, wake time, and/or total time in bed.
Evidence indicates that evening preference is associated with increased vulnerability to both depression and insomnia, and that one of the benefits of CBT-I is reduction in depressive symptom severity.26–28 Thus, we previously examined the possibility that evening preference might dampen this benefit. In a large sample of outpatients with insomnia receiving group CBT-I in a sleep disorder center, we found that sleep improved among individuals of all circadian preferences, but greater preference toward eveningness was associated with significantly less reduction in depressive symptom severity.29 However, because there was no control therapy, it was not possible to ascertain whether those with eveningness benefit less in terms of depression severity than those not receiving CBT-I. Thus, although the aforementioned studies suggest that circa-dian preference may be an important factor to consider when treating depression, no study to date has investigated the role of circadian preference in response to treatment for depression in the context of a well-powered randomized controlled treatment trial.
The current study is a secondary analysis of data collected during the Treatment of Insomnia and Depression (TRIAD) study. The primary aim of the TRIAD study was to examine whether CBT-I enhances depression treatment outcomes among patients with comorbid MDD and insomnia. Although insomnia symptoms were significantly improved by the addition of CBT-I compared to a control insomnia therapy, there was no differential improvement in remission from depression.30 This negative finding underscores the need for identifying potential moderators of treatment response. The present study aims to examine whether circadian preference moderated reductions in both depressive and insomnia symptoms following CBT-I or control treatment within the TRIAD study sample.
METHODS
Details regarding the design and methods of the TRIAD study are published elsewhere.31 We include here methodological information that is most relevant to the current investigation. TRIAD was a three-site randomized controlled trial conducted at Duke University (Durham, North Carolina), Stanford University (Palo Alto, California), and the University of Pittsburgh (Pittsburgh, Pennsylvania).
Participants
Recruitment occurred between March 2009 and August 2013, using community advertisements. Eligible participants were (1) 18 to 75 years of age, (2) fluent in English, (3) met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) MDD criteria based on the Structured Clinical Interview for DSM-IV,30 (4) scored higher than 15 on the 17-item Hamilton Rating Scale for Depression,32 (5) met DSM-IV-TR criteria for insomnia (primary insomnia or insomnia due to another mental disorder) based on the Duke Structured Interview for Sleep Disorders,33 and (6) scored higher than 10 on the Insomnia Severity Index (ISI).34 Participants were excluded if they were involved in another active treatment for depression or insomnia, had failure or intolerance for past adequate trials of all study medications, had a history of treatment with CBT-I, and had conditions incompatible with study pharmacotherapy (eg, pregnancy). If participants had another sleep disorder, most relevant to this article, individuals with severe circadian rhythm sleep-wake disorders (CRSWD) such as advanced sleep-wake phase disorder or delayed sleep-wake phase disorder with extreme habitual bedtimes and rise times (outside of 8:00 pm to 3:00 am and 4:00 am to 11:00 am respectively) they were excluded. Participants with a fixed night shift work schedule between midnight and 5:00 am and those with rotating work schedules that require night shifts were also excluded. For other sleep disorder exclusion criteria see the main article for more details.31 Individuals were also excluded if they consumed more than 3 cups of caffeinated beverages per day, more than 14 alcoholic drinks per week, or reported using any illicit drugs; had conditions that would have necessitated medical care not included in the study or confounded the interpretation of study results or took medications with known impact on mood or sleep (see main article appendix for more details);31 or had current active suicidal potential, psychotic features, seasonal pattern of depression, or onset of current depressive episode within 2 months of childbirth. In the current study, nine participants who did not complete circadian preference assessment were excluded from analyses. Missing participants did not differ in Hamilton Rating Scale for Depression (HRSD) or ISI and were equally distributed by treatment arm.
Procedures
Study protocols were approved by each University's institutional review board and were identical for all three sites. After providing written consent, participants were screened for study eligibility. Eligible participants were randomized centrally (1:1 in random blocks of two and four, stratified by study site) to either 8 weeks of CBT-I or control therapy. Participants in both treatment conditions received antidepressant medication, managed by a study psychiatrist during medication management visits conducted every 2 weeks over 16 weeks (8 visits in total, including the baseline visit when medications were dispensed).
Following principles used in the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, pharmacotherapy allowed choice of the first medication to be tried and one switch to another medication.35 Medications prescribed were escitalopram, sertraline, and desvenlafaxine. Medications were equally distributed across treatment groups.31 Study psychiatrists were masked to treatment condition and did not address sleep issues.
Both the CBT-I and control therapy were conducted in individual, in-person format by licensed therapists who were trained by authors RM and JE to competency in delivery. CBT-I components included sleep education, sleep restriction, stimulus control, strategies for reducing somatic and sleep-related cognitive arousal, cognitive restructuring of sleep-related thoughts, and relapse prevention.36–38 The control therapy included the sleep education module from the CBT-I condition, and systematic pairing of sleep-related distressing situations with emotionally neutral images, a treatment previously used as a credible insomnia control therapy.27,39 Sleep therapists in both arms did not discuss issues unrelated to sleep or adherence with treatment recommendations. In particular, therapists did not discuss depressive cognitions.
Following the 16-week treatment phase, participants were transitioned to community care and were followed up for assessments every 4 months over 2 years.
Measurements
Depression Symptom Severity
The 17-item HRSD32 is an interviewer-administered semistructured interview and is one of the most widely used measures in depression treatment studies.40,41 Higher scores on the HRSD indicate greater depressive symptom severity. In this study, the HRSD was administered by blind raters, and the Cronbach α for the HRSD ranged from .72 to .84 across all time points. The HRSD was collected at baseline, biweekly during the acute treatment phase (nine repeated assessments in total). The HRSD scores used in this study excluded the sleep items.
Insomnia Symptom Severity
The seven-item ISI34 is an index of the global severity of insomnia, including perceived daytime consequences and distress from sleep difficulties; higher scores on the ISI indicates greater insomnia severity. In this study, Cronbach α for the ISI ranged from .87 to .90 across all time points.
Circadian Preference
The 13-item Composite Scale of Morningness42 (CSM) assessed preferred timing for various activities and ease of rising in the morning, with higher scores indicating greater morning preference. Each item was given a score from 1 to 4 when the response patterns were limited to four and from 1 to 5 for all the items implying five response patterns; the final score was cumulative and varied from a minimum of 13 to a maximum of 55, with lower scores representing a stronger evening preference. CSM was treated as a linear variable in all analyses. Circadian preference was assessed only at baseline. The Cronbach α in the study sample was .85.
Statistical Analyses
Changes in depression symptom severity (HRSD) and insomnia symptom severity (ISI) were assessed by latent growth models within the framework of structural equation modeling. These analyses did not include treatment arm. Changes in ISI and HRSD from baseline to the end of the intervention were nonlinear, with faster reductions in both symptom types early in treatment, and slower changes later in treatment. To address this nonlinear pattern, the latent growth factors included two linear “slopes” representing the rates of change over time during the earlier (slope 1; baseline to week 6) and later (slope 2; week 6 to the end of treatment) phases of the intervention respectively. The models also included an intercept representing pre-treatment symptom severity. The “bend” defining earlier and later phases was determined by comparing the model fit for inflections at weeks 4, 6, and 8. For both the ISI and HRSD, the model with a “bend” at week 6 was the best fit, and was therefore chosen for the final models. Time scores (ie, latent factor loadings) were specified such that unstandardized estimates of the slopes can be interpreted as changes in raw outcome scores per 2-week period. To examine whether CSM scores moderated the effects of CBT-I on HRSD, and separately on ISI trajectories, latent growth models with CSM, treatment condition (Arm), and their interaction were used to predict slope 1 and slope 2, with age and sex as covariates. Significant interactions, which indicate moderating effects, were further examined using simple slope analyses to test whether the individual simple slopes of the models differ.
RESULTS
Sample Characteristics
We included a total of 139 participants in the analyses (66.2% females, age mean 43.4 years, standard deviation [SD] 12.9). The parent study enrolled 150 participants. For this article, we excluded 9 participants who did not complete CSM and 2 participants whose postrandomization data revealed they were enrolled in error; psychotic symptoms developed in one participant before treatment began and bipolar symptoms developed in the other participant at week 4 (see primary outcome paper).31 Of the participants in the current study, 90.6% were Caucasian, 45.7% were employed, and 37.0% were married or living with a partner. The average baseline HRSD was 17.29 (SD = 3.36), and the average baseline ISI was 18.91 (SD = 4.15). Sleep diary data revealed the following mean baseline values: sleep efficiency was 66.8% (SD = 16.2), total sleep time was 5.7 hours (SD = 1.2), Bedtime was 12:10 am (SD = 1.5; in hours past 00:00) and rise time was 7:36 am (SD = 1.8; in hours past 00:00). Mean CSM score was 31.1 (SD = 7.7), classifying those with scores that were one standard deviation above the mean as having “morningness” and those with scores that were one standard deviation below the mean as having “eveningness” resulted in 13.7% classified as “Eveningness” (n = 19), 79.9% as “intermediate” type (n = 111), and 6.5% as “Morningness” type (n = 9). Circadian preference categories were used in order to illustrate the direction of findings for the continuous CSM variable. We chose to define preference categories based on one SD of CSM scores rather than based on traditional CSM cutoffs because in our sample, which excluded individuals with advanced sleep-wake phase disorder and delayed sleep-wake phase disorder in the context of extremes of sleep onset and offset times, the traditional cutoff yielded small cell sizes for the evening and morning types, particularly morning types, which would have rendered the results unstable. Traditional cutoffs for CSM define a score 22 or lower as eveningness chronotype, whereas in our classification the cutoff score was 23 or lower. Traditional cutoffs for CSM defined morningness type is a score of 44 or higher, whereas in our classification the cutoff was 38 or higher. Across the 16-week and 9 time points, data completion rate for the HRSD (the primary outcome) was 73.5%.
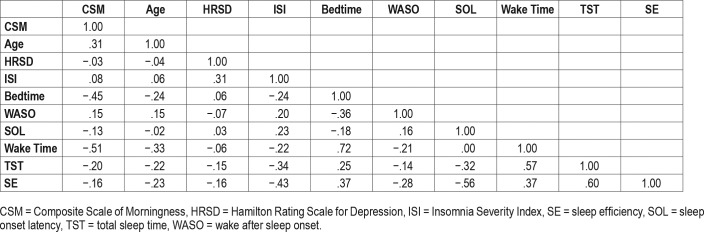
As shown in Table 1, at baseline, morning preference was associated with older age, earlier bedtime and wake time, and shorter total sleep time. CSM was not significantly associated with HRSD or ISI scores at baseline.
Table 1.
Correlations among baseline variables.
Insomnia and Depressive Symptom Change Trajectory
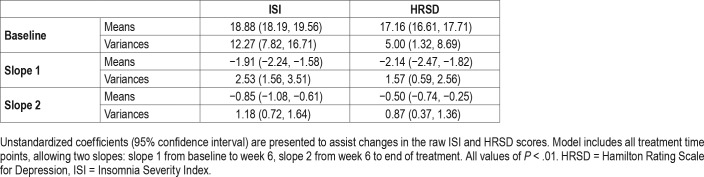
Piecewise latent growth models with two slopes bending at week 6 provided good fit for both HRSD and ISI data (HRSD: χ2 [36] = 44.90, P = .15, Confirmatory Fit Index = 0.98, Root Mean Square Error of Approximation = 0.042, Standard -ized Root Mean Square Residual = 0.079. ISI: χ2 [35] = 64.71, P = .02, Confirmatory Fit Index = 0.96, Root Mean Square Error of Approximation = 0.078, Standardized Root Mean Square Residual = 0.074). Model estimated trajectories are summarized in Table 2.
Table 2.
Overall growth model without covariates.
During the first 6 weeks, HRSD decreased at a rate of 2.14 points every 2 weeks, followed by a slower rate of 0.50 points every 2 weeks thereafter. Both slopes for HRSD were significantly less than 0 (P < .01), suggesting that for the overall sample, depressive symptom severity decreased significantly during both early and later treatment.
Similar to the HRSD trajectory, ISI decreased at a rate of 1.91 points every 2 weeks during the first 6 weeks, followed by a slower rate of 0.85 points every 2 weeks thereafter. Both slopes were significantly less than 0 (P < .01), suggesting that overall, insomnia symptom severity decreased significantly during both early and later treatment.
The Role of Circadian Preference in Depression Symptom Change Trajectory
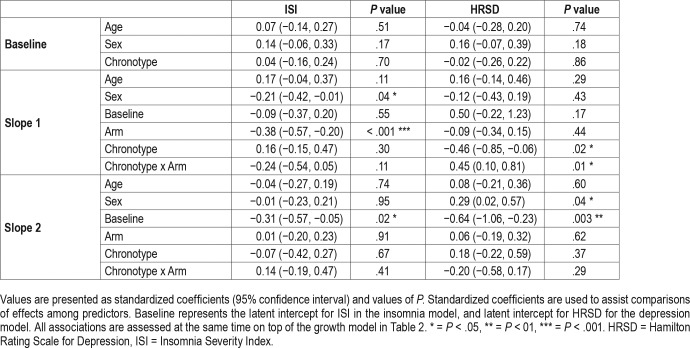
The moderation analysis revealed that greater evening preference (ie, lower score on CSM) was significantly associated with less reduction in HRSD (ie, smaller slope; P = .03) from baseline to week 6 across the whole sample (main effect). The interaction between CSM scores and Arm was also significant (P = .02), indicating that insomnia treatments had differential effects on depression symptom reduction during the first 6 weeks of treatment, depending on CSM scores. To illustrate this interaction term we categorized CSM, classifying those with scores that were 1 SD above the mean as “morningness” and those with scores that were 1 SD below the mean as “eveningness.” Figure 1 displays that, during the first 6 weeks of treatment, those in the “eveningness” category had better depression outcome if assigned to CBT-I compared with CTRL and that the CTRL was particularly ineffective in reducing depression symptoms those with “eveningness.”
Figure 1. Simple slopes for model-adjusted values with age held at its mean and effect of sex weighted based on sample proportion.
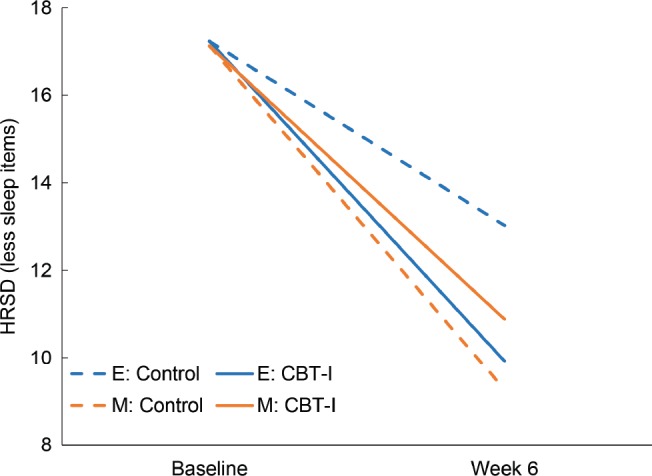
This figure is designed to illustrate the interaction term between CSM and “treatment arm” that were found in the main analyses, using CSM as a continuous variable. Categories of circadian preference were based on one standard deviation above and below the mean CSM scores. E = CSM scores ≤ 23; evening preference; M = CSM scores ≥ 38; morning preference. CBT-I = cognitive behavioral therapy for insomnia, CSM = Composite Scale of Morningness, HRSD = Hamilton Rating Scale for Depression
From week 6 onward, CSM did not have either a main effect, or a moderating effect on HRSD change trajectory (both P > .30). From week 6 to end of treatment sex did significantly moderate HRSD change trajectory (P = .04), such that women had less steep change in depression symptom trajectory.
The Role of Circadian Preference in Insomnia Symptom Change Trajectory
Controlling for age and sex, final models evaluated both the main and moderating effect of CSM in ISI reduction related to insomnia treatment. As shown in Table 3, CSM scores did not share significant associations with ISI score trajectory based on either baseline ISI or its two change slopes (all P > .30). The interaction between CSM scores and treatment arm was also not significant for either ISI change slopes (P = .11 and .41 respectively), suggesting that insomnia treatment effects on ISI did not depend on CSM scores.
Table 3.
Summary of final models for predicting insomnia and depression trajectories.
From baseline to week 6, sex significantly moderated ISI change trajectory (P = .04), such that women had a steeper change in insomnia symptom trajectory.
DISCUSSION
Using data from the TRIAD study, a randomized controlled trial that examined the effects of antidepressant medication plus CBT-I among patients with MDD and insomnia, we found that stronger evening preference was associated with higher depressive symptom severity at 6 weeks unless the patient was randomized to CBT-I; the CTRL treatment was particularly ineffective among those with stronger evening preference. This suggests that offering CBT-I to those with greater eveningness (eg, those with scores 23 or lower on the CSM) may mitigate their risk for poor response to antidepressant medications alone.
There are several possible explanations for this finding. One reason that treatment of insomnia may be particularly relevant to those with evening circadian preference is the possibility that among those with evening preference, CBT-I leads to greater reduction in distress about poor sleep than the control therapy. This assertion is based on past findings that those with evening circadian preference are more distressed by sleep difficulties, even when their sleep is not objectively worse than that the other circadian types.10,21,22,25 To test this potential explanation, we conducted exploratory moderation analyses of the effect of circadian preference on the daytime symptoms of insomnia (derived from relevant items on the ISI) but found no significant main or interaction effects. However, because this study was not optimally designed to test this potential mechanism we cannot rule out the possibility that differential effect on distress plays a role in explaining the findings. Another possible reason that treatment of insomnia may be particularly relevant to those with greater evening preference is that CBT-I aims to regularize sleep schedule, usually leading to an earlier rise time that could result in increased morning light exposure. Both more regular rise time and morning light exposure are tied to alignment between sleep/wake behaviors and circadian rhythms, which has been previously linked to depression symptom severity among those with a delayed sleep schedule.43 A shift to a more regular and advanced sleep schedule may strengthen the amplitude of the circadian rhythm and may result in circadian phase advance, both of which are circadian factors associated with improved depression symptoms and both of which may be particularly relevant for evening types.44–47 Through advancing rise time, CBT-I often leads to greater morning light exposure, which has demonstrated antidepressant effect and might have a particularly significant antidepressant effect among those with an evening circadian preference.48,49
The current study is the first to directly assess whether circa-dian preference moderated reduction in depressive symptoms following combined treatment with antidepressant and CBT-I. We found only one other study that examined eveningness tendency as a predictor of depression severity outcome following CBT-I. In this sample of 419 adult patients with insomnia disorder from a sleep disorders clinic, greater eveningness was a risk factor for nonremission of depression following group CBT-I, independent of baseline depression symptom severity.29 This previous study did not include individuals with comorbid MDD and insomnia (although comorbid psychiatric disorders were not excluded), did not provide antidepressant treatment, although some patients may have received it as part of their usual care, and did not include a control condition to assess the differential effect of CBT-I. Given the paucity of research in this area, further research is needed to (1) replicate the findings in the current secondary analysis of the TRIAD study and (2) identify and test possible mechanisms driving the moderation effect of circadian preference on depression treatment outcome.
We found that circadian preference did not moderate insomnia symptom severity trajectory. In other words, participants benefitted from insomnia treatment regardless of circadian preference. This finding is consistent with the sleep disorders clinic sample study, which also found that all circadian types benefited from CBT-I.29 It also indicates that insomnia symptom severity differences are likely not the mechanism by which circadian preference moderates antidepressant medication treatment response.
Interestingly, we found that baseline subjectively assessed circadian preference was not associated with more severe depression or insomnia symptoms. Previous studies found that individuals with eveningness have similar insomnia severity (or sleep symptom severity) but greater depression severity in both depressed patient samples and patient samples with insomnia.25,29 In the current study the correlation between depression severity and eveningness scores was weak (P = .08); however, there is a signal in the same direction. It is important to note that the sample in the current study had greater eveningness type than the sample from our sleep disorders clinic database29 (mean CSM in the clinic study was 35.70 (SD 9.13) and 31.14 (SD 7.71) in the current sample) and the standard deviation in CSM scores was lower. It is also possible, based on the substantial differences between the two samples, that a sample with co-morbid depression and insomnia is more likely to have greater eveningness; this is another important area for future research.
Although we did not have a priori hypotheses regarding sex as a moderator of depression or insomnia symptom trajectories, we found that women had greater insomnia symptom improvement than men during the first 6 weeks of treatment, and less depression symptom improvement than men in the latter half of treatment. Of note, there were more women (n = 108) than men (n = 40) in the sample and there were no baseline differences in depression or insomnia severity between men and women in the sample. These unexpected findings are difficult to interpret. There are some limited data that suggest women respond better to selective serotonin reuptake inhibitors than men.50,51 It is possible that women responded more quickly to the selective serotonin reuptake inhibitors than men, thus improving their insomnia symptoms and depression symptoms in the first 6 weeks. Although sex was not a significant moderator of depression symptom change from baseline to week 6 (P > .05) there was a negative slope; see Table 3 from baseline to week 6. While speculative, it is possible that this initial “bump” in treatment response was then followed by slower depression symptom improvement in the latter half of treatment. This finding requires more future research to be adequately contextualized.
Several limitations should be noted in the current study. The TRIAD study excluded some comorbid sleep and psychiatric disorders, including CRSWD, and most of the sample was Caucasian; particularly relevant to this article, we excluded two individuals because they met criteria for CRSWD and three additional people who did not meet criteria for CRSWD, who had extreme bedtimes/rise times. These exclusions limit the generalizability of the findings to those with severe CRSWD and/or more extreme sleep/wake schedules, other racial/ethnic groups and some psychiatric comorbid presentations. The current study was underpowered to detect differences between the treatment arms by circadian preferences and therefore results from simple slope analyses of differences between categorical morningness and eveningness in the CBT-I versus CTRL are not interpretable. Another potential limitation to generalizability is the demanding screening process, resulting in randomization of only 40% of consenting participants. Most individuals with depression report poor sleep, but may not meet criteria for insomnia disorder. Although evening preference is a risk for insomnia disorder, it is not clear if the results will generalize to individuals with depression and evening preference who do not meet criteria for insomnia. It is also not clear if the results will generalize to augmentation of antidepressant medications with pharmacotherapy for insomnia, or augmentation of psychotherapy for depression with insomnia therapy.
In conclusion, using a well-characterized clinical sample of individuals with depression and insomnia and standardized treatment protocols for both disorders, delivered simultaneously, the current study found that individuals with comorbid depression and insomnia and an evening preference are at increased risk for poor response to antidepressant medications and do better when their insomnia is treated with CBT-I. Given that the main outcome article from the TRIAD study did not find that CBT-I augmented depression treatment outcome, it is clinically relevant to better understand which patients could benefit from the addition of CBT-I treatment. Identifying individuals who are most likely to benefit from CBT-I is especially important given the additional cost and time commitment required for the patient and the health system. The findings from the current study suggest that circadian preference may be a salient dimension in treatment planning for individuals with MDD and insomnia. Moreover, the findings suggest that clinical care using antidepressant medications for individuals with comorbid depression and insomnia could be enhanced by (1) evaluating circadian preference and (2) augmenting antidepressant medications with CBT-I for those identified as having a more evening circadian preference.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This work was supported by MH078924, MH078961, MH079256 and MH019938. The Treatment of Insomnia and Depression (TRIAD) study was a multi-site randomized control trial conducted at Duke University (Durham, North Carolina), Stanford University (Palo Alto, California), and the University of Pittsburgh (Pittsburgh, Pennsylvania). TRIAD was a clinical trial (ClinicalTrials.gov identifier NCT00767624). The authors report no financial conflicts of interest.
ABBREVIATIONS
- CBT-I
cognitive behavioral therapy for insomnia
- CSM
Composite Scale of Morningness
- CTRL
control therapy
- HRSD
Hamilton Rating Scale for Depression
- ISI
Insomnia Severity Index
- MDD
major depressive disorder
- TRIAD
Treatment of Insomnia and Depression study
REFERENCES
- 1.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamilton M. Frequency of symptoms in melancholia (depressive illness) Br J Psychiatry. 1989;154:201–206. doi: 10.1192/bjp.154.2.201. [DOI] [PubMed] [Google Scholar]
- 3.Emslie GJ, Kennard BD, Mayes TL, et al. Insomnia moderates outcome of serotonin-selective reuptake inhibitor treatment in depressed youth. J Child Adolesc Psychopharmacol. 2012;22(1):21–28. doi: 10.1089/cap.2011.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 5.Soehner AM, Harvey AG. Prevalence and functional consequences of severe insomnia symptoms in mood and anxiety disorders: results from a nationally representative sample. Sleep. 2012;35(10):1367–1375. doi: 10.5665/sleep.2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor DJ, Lichstein KL, Durrence HH, Reidel BW, Bush AJ. Epidemiology of insomnia, depression, and anxiety. Sleep. 2005;28(11):1457–1464. doi: 10.1093/sleep/28.11.1457. [DOI] [PubMed] [Google Scholar]
- 7.Baglioni C, Battagliese G, Feige B, et al. Insomnia as a predictor of depression: a meta-analytic evaluation of longitudinal epidemiological studies. J Affect Disord. 2011;135(1–3):10–19. doi: 10.1016/j.jad.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Perlis ML, Giles DE, Buysse DJ, Tu X, Kupfer DJ. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord. 1997;42(2–3):209–212. doi: 10.1016/s0165-0327(96)01411-5. [DOI] [PubMed] [Google Scholar]
- 9.Gau SS, Shang CY, Merikangas KR, Chiu YN, Soong WT, Cheng AT. Association between morningness-eveningness and behavioral/emotional problems among adolescents. J Biol Rhythms. 2007;22(3):268–274. doi: 10.1177/0748730406298447. [DOI] [PubMed] [Google Scholar]
- 10.Selvi Y, Aydin A, Boysan M, Atli A, Agargun MY, Besiroglu L. Associations between chronotype, sleep quality, suicidality, and depressive symptoms in patients with major depression and healthy controls. Chronobiol Int. 2010;27(9–10):1813–1828. doi: 10.3109/07420528.2010.516380. [DOI] [PubMed] [Google Scholar]
- 11.Chelminski I, Ferraro FR, Petros TV, Plaud JJ. An analysis of the “eveningness-morningness” dimension in “depressive” college students. J Affect Disord. 1999;52(1–3):19–29. doi: 10.1016/s0165-0327(98)00051-2. [DOI] [PubMed] [Google Scholar]
- 12.Hidalgo MP, Caumo W, Posser M, Coccaro SB, Camozzato AL, Chaves ML. Relationship between depressive mood and chronotype in healthy subjects. Psychiatry Clin Neurosci. 2009;63(3):283–290. doi: 10.1111/j.1440-1819.2009.01965.x. [DOI] [PubMed] [Google Scholar]
- 13.Hirata FC, Lima MC, de Bruin VM, Nobrega PR, Wenceslau GP, de Bruin PF. Depression in medical school: the influence of morningness-eveningness. Chronobiol Int. 2007;24(5):939–946. doi: 10.1080/07420520701657730. [DOI] [PubMed] [Google Scholar]
- 14.Maglione JE, Ancoli-Israel S, Peters KW, et al. Depressive symptoms and circadian activity rhythm disturbances in community-dwelling older women. Am J Geriatr Psychiatry. 2014;22(4):349–361. doi: 10.1016/j.jagp.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merikanto I, Kronholm E, Peltonen M, Laatikainen T, Vartiainen E, Partonen T. Circadian preference links to depression in general adult population. J Affect Disord. 2015;188:143–148. doi: 10.1016/j.jad.2015.08.061. [DOI] [PubMed] [Google Scholar]
- 16.Antypa N, Vogelzangs N, Meesters Y, Schoevers R, Penninx BW. Chronotype Associations with Depression and Anxiety Disorders in a Large Cohort Study. Depress Anxiety. 2016;33(1):75–83. doi: 10.1002/da.22422. [DOI] [PubMed] [Google Scholar]
- 17.Gaspar-Barba E, Calati R, Cruz-Fuentes CS, et al. Depressive symptomatology is influenced by chronotypes. J Affect Disord. 2009;119(1–3):100–106. doi: 10.1016/j.jad.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Muller MJ, Kundermann B, Cabanel N. Eveningness and poor sleep quality independently contribute to self-reported depression severity in psychiatric inpatients with affective disorder. Nord J Psychiatry. 2016;70(5):329–334. doi: 10.3109/08039488.2015.1112832. [DOI] [PubMed] [Google Scholar]
- 19.Chan JW, Lam SP, Li SX, et al. Eveningness and insomnia: independent risk factors of nonremission in major depressive disorder. Sleep. 2014;37(5):911–917. doi: 10.5665/sleep.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swanson LM, Burgess HJ, Huntley ED, et al. Relationships between circadian measures, depression, and response to antidepressant treatment: a preliminary investigation. Psychiatry Res. 2017;252:262–269. doi: 10.1016/j.psychres.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung MH, Chang FM, Yang CC, Kuo TB, Hsu N. Sleep quality and morningness-eveningness of shift nurses. J Clin Nurs. 2009;18(2):279–284. doi: 10.1111/j.1365-2702.2007.02160.x. [DOI] [PubMed] [Google Scholar]
- 22.Barclay NL, Eley TC, Buysse DJ, Archer SN, Gregory AM. Diurnal preference and sleep quality: same genes? A study of young adult twins. Chronobiol Int. 2010;27(2):278–296. doi: 10.3109/07420521003663801. [DOI] [PubMed] [Google Scholar]
- 23.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 24.Roenneberg T, Kuehnle T, Juda M, et al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11(6):429–438. doi: 10.1016/j.smrv.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Ong JC, Huang JS, Kuo TF, Manber R. Characteristics of insomniacs with self-reported morning and evening chronotypes. J Clin Sleep Med. 2007;3(3):289–294. [PMC free article] [PubMed] [Google Scholar]
- 26.Manber R, Edinger JD, Gress JL, San Pedro-Salcedo MG, Kuo TF, Kalista T. Cognitive behavioral therapy for insomnia enhances depression outcome in patients with comorbid major depressive disorder and insomnia. Sleep. 2008;31(4):489–495. doi: 10.1093/sleep/31.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manber R, Bernert RA, Suh S, Nowakowski S, Siebern AT, Ong JC. CBT for insomnia in patients with high and low depressive symptom severity: adherence and clinical outcomes. J Clin Sleep Med. 2011;7(6):645–652. doi: 10.5664/jcsm.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clarke G, McGlinchey EL, Hein K, et al. Cognitive-behavioral treatment of insomnia and depression in adolescents: a pilot randomized trial. Behav Res Ther. 2015;69:111–118. doi: 10.1016/j.brat.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bei B, Ong JC, Rajaratnam SM, Manber R. Chronotype and improved sleep efficiency independently predict depressive symptom reduction after group cognitive behavioral therapy for insomnia. J Clin Sleep Med. 2015;11(9):1021–1027. doi: 10.5664/jcsm.5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spitzer M, Robert L, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 31.Manber R, Buysse DJ, Edinger J, et al. Efficacy of cognitive-behavioral therapy for insomnia combined with antidepressant pharmacotherapy in patients with comorbid depression and insomnia: a randomized controlled trial. J Clin Psychiatry. 2016;77(10):e1316–e1323. doi: 10.4088/JCP.15m10244. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6(4):278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 33.Edinger JD, Wyatt JK, Olsen MK, et al. Reliability and validity of the Duke Structured Interview for sleep disorders for insomnia screening. Sleep. 2009;32(Abstract Suppl):A265. [Google Scholar]
- 34.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR* D: implications for clinical practice. Am J Psychiatry. 2006;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 36.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10(1):45–56. [PubMed] [Google Scholar]
- 37.Manber R, Carney CE. Treatment Plans and Interventions for Insomnia: A Case Formulation Approach. New York, NY: Guilford Publications; 2015. [Google Scholar]
- 38.Bootzin RR, Epstein DR. Understanding and treating insomnia. Annu Rev Clin Psychol. 2011;7:435–458. doi: 10.1146/annurev.clinpsy.3.022806.091516. [DOI] [PubMed] [Google Scholar]
- 39.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285(14):1856–1864. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- 40.Miller IW, Bishop S, Norman WH, Maddever H. The Modified Hamilton Rating Scale for Depression: reliability and validity. Psychiatry Res. 1985;14(2):131–142. doi: 10.1016/0165-1781(85)90057-5. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith CS, Reilly C, Midkiff K. Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. J Appl Psychol. 1989;74(5):728–738. doi: 10.1037/0021-9010.74.5.728. [DOI] [PubMed] [Google Scholar]
- 43.Murray JM, Sletten TL, Magee M, et al. Prevalence of circadian misalignment and its association with depressive symptoms in delayed sleep phase disorder. Sleep. 2017;40(1) doi: 10.1093/sleep/zsw002. [DOI] [PubMed] [Google Scholar]
- 44.Linkowski P, Mendlewicz J, Kerkhofs M, et al. 24-hour profiles of adrenocorticotropin, cortisol, and growth hormone in major depressive illness: effect of antidepressant treatment. J Clin Endocr Metab. 1987;65(1):141–152. doi: 10.1210/jcem-65-1-141. [DOI] [PubMed] [Google Scholar]
- 45.Szuba MP, Guze BH, Baxter LR., Jr Electroconvulsive therapy increases circadian amplitude and lowers core body temperature in depressed subjects. Biol Psychiatry. 1997;42(12):1130–1137. doi: 10.1016/s0006-3223(97)00046-2. [DOI] [PubMed] [Google Scholar]
- 46.Bailey SL, Heitkemper MM. Circadian rhythmicity of cortisol and body temperature: morningness-eveningness effects. Chronobiol Int. 2001;18(2):249–261. doi: 10.1081/cbi-100103189. [DOI] [PubMed] [Google Scholar]
- 47.Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness–eveningness. J Sleep Res. 2000;9(2):117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- 48.Benedetti F, Colombo C, Pontiggia A, Bernasconi A, Florita M, Smeraldi E. Morning light treatment hastens the antidepressant effect of citalopram: a placebo-controlled trial. J Clin Psychiatry. 2003;64(6):648–653. doi: 10.4088/jcp.v64n0605. [DOI] [PubMed] [Google Scholar]
- 49.Lewy AJ, Sack RL, Miller LS, Hoban TM. Antidepressant and circadian phase-shifting effects of light. Science. 1987;235(4786):352–354. doi: 10.1126/science.3798117. [DOI] [PubMed] [Google Scholar]
- 50.Kornstein SG, Schatzberg AF, Thase ME, et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157(9):1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- 51.Young EA, Kornstein SG, Marcus SM, et al. Sex differences in response to citalopram: a STAR*D report. J Psychiatr Res. 2009;43(5):503–511. doi: 10.1016/j.jpsychires.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]