Abstract
Study Objectives:
The objective of this study was to measure fatigue and hypersomnolence in patients with obstructive sleep apnea (OSA) treated with a mandibular advancement device (MAD), using Epworth Sleepiness Scale (ESS) for hypersomnolence and Checklist Individual Strength questionnaire (CIS20R) for fatigue.
Methods:
This was a single-center, prospective cohort study. A total of 58 patients with OSA filled out ESS and CIS20R questionnaires at baseline and after 3 months of MAD treatment. A total of 39 full datasets were collected. Statistical analysis for reliability of the questionnaires, comparison between baseline and 3-month follow-up, correlation between the changes in the values of the two questionnaires, and changes in apnea-hypopnea index (AHI) were performed.
Results:
CIS20R showed excellent reliability in this patient group at baseline and 3-month follow-up (Cronbach α = .97), ESS showed a marginally good reliability (Cronbach α = .82). The CIS20R (82/140) expressed high levels of fatigue at baseline, and ESS showed a normal level of daytime sleepiness. AHI, ESS, and CIS20R were significantly reduced under MAD treatment. A significant correlation between ESS and CIS20R was observed. No significant correlation between any of the questionnaires and the change in AHI was found.
Conclusions:
The CIS20R questionnaire results showed a high level of fatigue in the patients with OSA, and the questionnaire can be used to evaluate changes in fatigue due to MAD treatment after 3 months. The ESS failed to show similar characteristics. Therefore, a combination of ESS for hypersomnolence with CIS20R for fatigue is proposed for the follow-up of patients with OSA treated with MAD.
Citation:
Kazemeini E, Braem MJ, Moorkens G, Balina S, Kastoer C, Op de Beeck S, Vanderveken OM, Dieltjens M. Scoring of hypersomnolence and fatigue in patients with obstructive sleep apnea treated with a titratable custom-made mandibular advancement device. J Clin Sleep Med. 2019;15(4):623–628.
Keywords: checklist individual strength, Epworth Sleepiness Scale, fatigue, hypersomnolence, sleep-disordered breathing
BRIEF SUMMARY
Current Knowledge/Study Rationale: Currently, hypersomnolence and fatigue are often assessed using the Epworth Sleepiness Scale (ESS) and no clear distinction between these two symptoms can be made. The current study is the first to apply the Checklist Individual Strength (CIS20R) as a follow-up tool for measuring fatigue during the treatment of patients with obstructive sleep apnea and to compare the results with the ESS score.
Study Impact: The results showed an excellent reliability of CIS20R and demonstrated the severity of fatigue present in the study population with obstructive sleep apnea and also the effects of 3 months of MAD treatment on fatigue. The ESS score was clearly limited to the representation of hypersomnolence.
INTRODUCTION
Within the range of sleep-disordered breathing, obstructive sleep apnea (OSA) is highly prevalent.1 OSA is caused by recurring collapse of the upper airway during sleep, resulting in complete (apnea) or partial (hypopnea) cessation of airflow.2 This gives rise to sleep disturbances and sleep fragmentation.3 OSA severity is expressed by the apnea-hypopnea index (AHI), defined as the number of apneas and hypopneas per hour of total sleep time.4
Most patients in whom OSA has been diagnosed complain about a combination of hypersomnolence and fatigue,5 as well as other symptoms such as socially disturbing snoring, gasping, and choking. Hypersomnolence and fatigue are often mistaken for each other although they are two distinct symptoms; thus, a clear discrimination between both symptoms ought to be crucial in OSA treatment decision making and the evaluation of the effect of OSA therapies.
The Epworth Sleepiness Scale (ESS) is a widely used questionnaire to quantify the degree of hypersomnolence in patients with OSA.6 With this questionnaire, the patients are asked to rate their probability of falling asleep or dozing off on a scale of 0 to 3 for eight day-life situations. The ESS score is the sum of the eight item scores and can range from 0 to 24. However, recent results showed that the ESS had a poor test-retest reliability and should not be relied upon for decision making and providing treatment services in sleep disorders.7
Fatigue is defined as the self-reported feeling of tiredness or exhaustion and is a commonly reported symptom among patients with chronic conditions8 such as OSA.9 It is an important parameter of health care outcome evaluations, with a considerable effect on multiple levels of public and occupational health care. Poor personal performance,10 reduced productivity during work,10 an increase in sick leave as well as work disability,10 and a rise in the incidence of motor vehicle11 and occupational accidents12 all are translated into a substantial economic burden related to fatigue. Fatigue can also lead to decreased quality of life and other related health problems.13 The Checklist Individual Strength (CIS20R) questionnaire can be used to estimate the fatigue present in a population.14 It is a 20-item questionnaire with each question to be scored on a 7-point Likert scale ranging between “yes, this is true” to “no, this is not true.” The total score is calculated as the sum of the responses to the different statements, with a maximum CIS20R score of 140.
Because of the socioeconomic burden of OSA, adequate treatment is of utmost importance. The standard noninvasive treatment for OSA is continuous positive airway pressure, stabilizing the upper airway through a pneumatic splint.15 Mandibular advancement devices (MAD) stabilize the upper airway by moving the lower jaw forward. These appliances have shown promising results not only in decreasing AHI, but also in improvement in sleepiness as measured by the ESS.16
The objective of this study was to investigate the application of the CIS20R questionnaire in patients with OSA treated with MAD and to compare it with the ESS questionnaire. The first part of this paper focuses on the reliability of the CIS20R and ESS questionnaires in this specific patient population. Next, the efficacy of MAD treatment was analyzed after 3 months of follow-up in terms of reduction in AHI, as well as reduction in hypersomnolence and fatigue. Finally, the correlation between the changes in AHI and the changes in ESS or CIS20R and its subscales were tested.
METHODS
This study was designed as a single-center, prospective cohort study. Patients had a diagnosis of OSA if the AHI was higher than 5 events/h of sleep on a type 1 polysomnography (PSG). The patients were referred to the Special Care Dentistry unit of the Antwerp University Hospital for routine MAD treatment and were consecutively included after further confirmation of eligibility for MAD treatment. All participants provided written informed consent and the study was approved by the Ethical Committee of the Antwerp University Hospital.
At baseline the patients were asked to fill out both ESS6 and CIS20R14 questionnaires. A custom-made titratable commercially available duo-bloc MAD allowing adjustments in mandibular protrusion (SomnoMed Flex, SomnoMed Ltd, Australia), was fitted in maximal comfortable protrusion. Thereafter, the patients were instructed to further adjust mandibular protrusion until resolution of the self-reported symptoms such as snoring and hypersomnolence, or until physiological limits were reached. Three months after the fitting of the MAD, the patients were asked to fill out both questionnaires again. According to the Belgian reimbursement modalities, a follow-up home sleep-test (polygraphy or PG) with the MAD in situ was carried out to assess the residual AHI under MAD treatment. No corrections were made for the differences in total sleep time (PSG) versus registration time (PG).
ESS Questionnaire
Hypersomnolence was assessed using the ESS, an eight-item questionnaire that takes about 3 minutes to fill out. It reflects the chance of each person's general level of sleepiness during eight situations of daily life. The participant selects the most appropriate integer score ranging from 0 (would never doze) to 3 (high chance of dozing) for each of the 8 questions. The results are then summed up, with 0 being the minimal and 24 the maximum total ESS score. The higher the ESS score, the higher that person's average sleep propensity in daily life. In general, according to the recent update by Johns,6 ESS scores need to be interpreted as follows: 0–5 = lower normal daytime sleepiness, 6–10 = higher normal daytime sleepiness, 11–12 = mild excessive daytime sleepiness, 13–15 = moderate excessive daytime sleepiness, 16–24 = severe excessive daytime sleepiness.
Checklist Individual Strength Questionnaire
Fatigue severity was assessed using the CIS20R, a 20-item questionnaire that takes approximately 5 to 10 minutes to complete. Each question has a statement to be scored on a seven-point Likert scale ranging between “yes, this is true” to “no, this is not true.” The total score is calculated as the sum of the responses to the different statements. The maximum CIS20R score is 140, with a score of 76/140 or higher indicating that the patient is at risk for prolonged absence at work.17 The scale is subdivided in four dimensions of fatigue: fatigue severity (CISFatigue; 8 items, maximum score 56), concentration problems (CISConcentration; 5 items, maximum score 35), reduced motivation (CISMotivation; 4 items, maximum score 28), and activity (CISActivity; 3 items, maximum score 21).
For the purpose of this study, the main focus will be on CISFatigue, with a score ≤ 26 = normal, 27–34 = mild fatigue, and ≥ 35 = severe fatigue.14
Statistical Analysis
Statistical analysis was conducted using R software (R Foundation for Statistical Computing, Vienna, Austria). Data are represented as mean ± standard deviation (SD) unless reported otherwise. The parameters used in the main analysis were AHI, ESS score, CIS20R, CISFatigue, CISConcentration, CISMotivation, and CISActivity. Normality was tested using the Shapiro-Wilk test. To evaluate the internal consistency of the ESS and the CIS20R questionnaire in patients with OSA, we used a Cronbach α test for the ESS, CIS20R, and CIS20R sub-scales. In general, a Cronbach α reliability coefficient of .70 or higher is considered acceptable and a value of .90 and above is considered excellent. A complementary test to Cronbach α is Gutman split-half, which randomly divides the test into two halves, applies each half to the same group of people and then gives the correlation between the two associated results.
The change from baseline to 3 months follow-up was tested using a paired t test for normally distributed parameters and a Wilcoxon signed rank test for non-normally distributed parameters. A Spearman correlation test was used to assess the correlation between ESS and CIS20R at baseline, 3 months after treatment and the absolute difference between the two time-lines (Δ). The same test was used to determine the correlation among ΔAHI and all other parameters.
RESULTS
Study Population
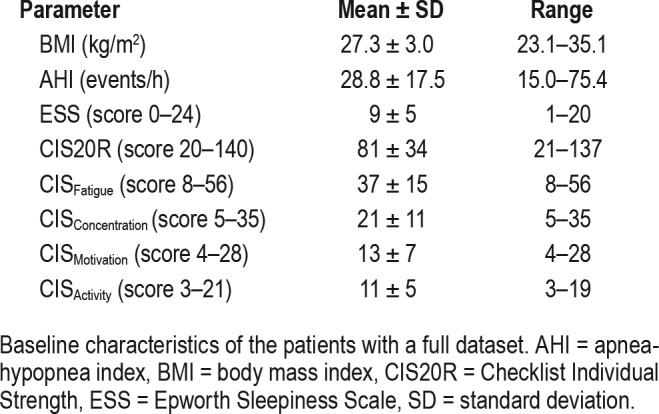
A total of 58 patients in whom OSA was diagnosed were asked to fill out ESS and CIS20R questionnaires at baseline and after 3 months of MAD treatment. Seventeen patients did not complete the 3-month follow-up whereas two other questionnaires were incomplete. In 6 patients the CIS20R questionnaire was incomplete, whereas in 13 patients the data on OSA severity under MAD therapy were not available. In total, 39 complete datasets were obtained. The baseline characteristics of the patients are summarized in Table 1. The study population included 29 men (74%) and 10 women (26%) with a mean body mass index (BMI) at baseline of 27.3 ± 3.0 kg/m2. On average, the baseline AHI was 28.8 ± 17.5 events/h indicating moderate to severe OSA. The ESS showed a higher normal hypersomnolence (ESS = 9/24), whereas the CIS20R indicated that patients were at risk of prolonged absence at work (CIS20R = 82/140) and the CIS Fatigue indicated the presence of severe fatigue (CISFatigue = 37/56) at baseline. The baseline characteristics of the 19 patients with incomplete dataset did not differ significantly from those of the 39 patients included in the analysis, except for the AHI (P = .002) which can be explained by the relatively large range for this group (3.2–75.6 events/h).
Table 1.
Baseline characteristics (n = 39).
Internal Consistency and Reliability
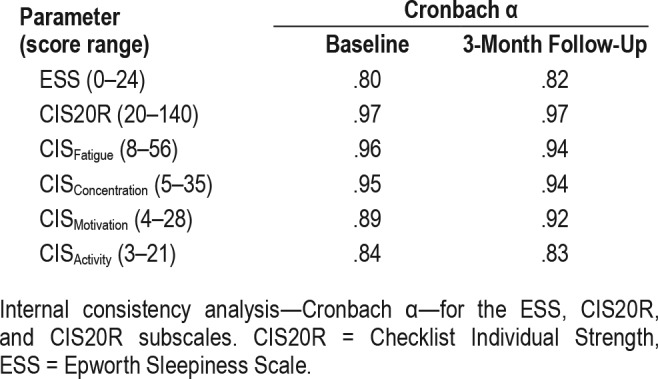
Table 2 provides a summary of the Cronbach α. The CIS20R questionnaire and its subscales on fatigue and concentration showed excellent internal consistency (Cronbach α ≥ .90), both at baseline and at 3-month follow-up. The results for ESS showed a lower, but still acceptable, internal consistency (Cronbach α = .80). Gutman split-half correlation in this patient group also showed an excellent reliability for CIS20R both at baseline (r = .97) and at 3-month follow-up (r = .97). For ESS a marginally good reliability at baseline (r = .82) and with MAD treatment (r = .83) was observed.
Table 2.
Internal consistency analysis.
Efficacy of MAD Treatment
Figure 1 summarizes the evolution of the AHI, ESS, and CIS20R and its subscales during MAD treatment. There was a statically significant decrease in AHI at the follow-up PG with the MAD in situ as compared to baseline: AHI decreased from 28.9 ± 17.6 events/h at baseline to 10.0 ± 11.8 events/h with MAD (P = .00002).
Figure 1. Summary of the efficacy of MAD treatment.
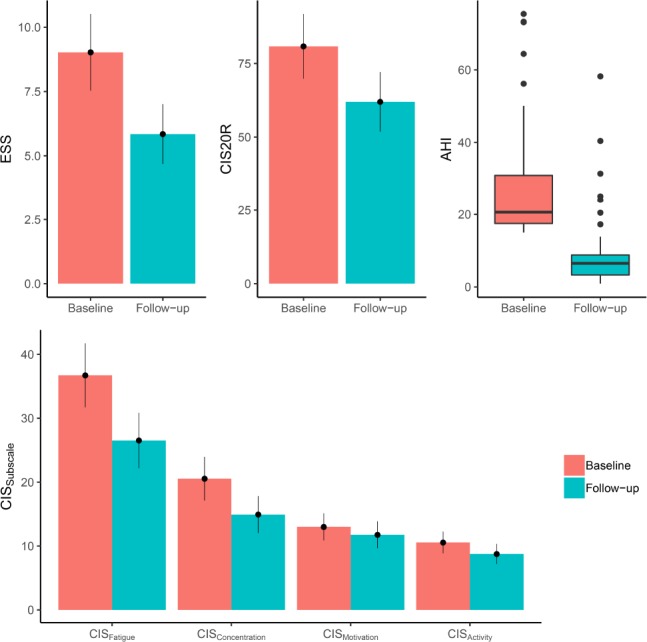
Summary of the efficacy of MAD treatment on AHI, CIS20R, CIS20R subscales, and the ESS. AHI = apnea-hypopnea index, CIS20R = Checklist Individual Strength, ESS = Epworth Sleepiness Scale, MAD = mandibular advancement device.
The ESS score significantly decreased from 9 ± 5 at baseline to 6 ± 4 at 3-month follow-up (P = .003) (Figure 1). Furthermore, the CIS20R reduced from 82 ± 34 at baseline to 63 ± 31 with MAD (P = .024). The severe fatigue (CISFatigue = 37 ± 15) at baseline was significantly reduced to mild fatigue (CISFatigue = 27 ± 13) upon MAD treatment (P = .007). CISConcentration and CISActivity subscales significantly reduced under MAD treatment as well, but the CISMotivation did not. Patient weight and BMI did not significantly change during MAD treatment.
Correlation Between Changes in AHI and Changes in ESS or CIS20R and its Subscales
Correlation analyses between the changes in the different parameters (ΔAHI, ΔESS, ΔCIS20R, and the changes in its sub-scales) are depicted in Figure 2. These correlation analyses revealed a significant correlation between the ΔESS and the ΔCIS20R (P = .001, rs = .5), the ΔESS and ΔCISFatigue (P = .006, rs = .43), and ΔESS with ΔCIS Concentration (P = .005, r s = .43), Figure 2. The analysis failed to demonstrate a correlation between ΔESS and ΔCISMotivation (P = .035, rs = .33) and ΔESS and ΔCISActivity (P = .086, rs = .27).
Figure 2. Correlation plot.
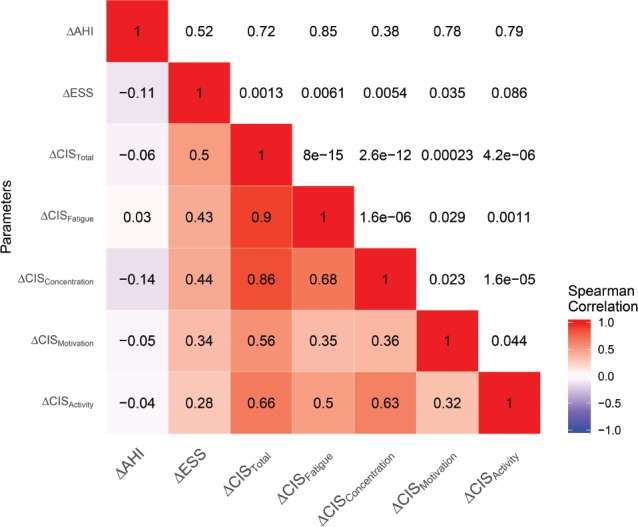
The correlations coefficients are shown on the lower left side, and range from −1 to 1. Negative correlations are shown in blue and positive correlations in red. The P values are shown on the upper right side of the correlation plot. Δ = absolute difference between baseline and 3-month follow-up, AHI = apnea-hypopnea index, CIS = Checklist Individual Strength, ESS = Epworth Sleepiness Scale.
Despite observing a significant reduction in AHI, ESS, and CIS20R after 3 months of MAD therapy compared to their baseline values according to the comparison analysis, no significant correlation was found between the ΔAHI following MAD treatment and ΔESS (P = .52, rs = −.1), ΔCIS20R (P = .72, rs = −.59) or its subscales.
DISCUSSION
OSA is a chronic disorder with important socioeconomic consequences due to the related daytime symptoms and comorbid disorders. Hypersomnolence and fatigue are two of the main self-reported complaints of patients with an OSA diagnosis and the current study offers a way to differentiate between these two distinct but not necessarily mutually exclusive symptoms. The CIS20R questionnaire is already validated and used in the follow-up of various chronic diseases where it proved to be a valid and reliable tool for the assessment of fatigue in those patient groups. Now, the CIS20R is introduced into the follow-up of OSA treatment with MAD. This paper focuses on the internal consistency and reliability of the CIS20R and the ESS in patients treated with MAD (before and after the treatment implementation), on the efficacy of the therapy using both questionnaires and on the OSA severity as well as on the correlation between the decrease in AHI, the decrease in ESS, and CIS20R and its subscales.
Internal Consistency and Reliability
A good questionnaire should show reliability and internal consistency in any patient group. Cronbach α offers an easy way to measure whether or not a questionnaire is reliable. It is used under the assumption that a questionnaire has multiple questions, all asking different aspects, for example, about fatigue, but when combined, it can measure the overall fatigue. In a study on patients with rheumatoid arthritis,18 the CIS20R was compared with 12 questionnaires on fatigue. The results of that study confirmed CIS20R to be a reliable instrument for identification of cognitive and physical fatigue with an excellent internal consistency and reliability.18 The internal consistency scores in our study population were very high, with Cronbach α = .97 for CIS20R and Cronbach α ranging from .83 to .96 for the subscales, both at baseline and after 3 months of MAD treatment.
Efficacy of MAD Treatment
The current study showed that MAD treatment yields to a significant improvement in AHI, ESS, and CIS20R scores and all its subscales, except for CISMotivation. However, the Cronbach α did show a high internal consistency for the motivation sub-scale, indicating that motivational complaints are not primor-dial in patients with OSA.
The total CIS20R score at baseline showed that the patients were at risk for prolonged absence at work (CIS20R = 82/140). This score was significantly reduced under MAD treatment, alleviating this risk after 3 months. The fatigue subscale could be reduced from severe fatigue (CISFatigue = 37 ± 15) to mild fatigue (CISFatigue = 27 ± 13) upon MAD treatment.
Correlation Between Changes in AHI and Changes in ESS or CIS20R and its Subscales
A significant correlation was found between the changes in ESS and changes in CIS20R scores following MAD treatment. However, no correlation between the improvement in AHI and the changes in any of the two questionnaires, CIS20R and ESS, or the CIS20R subscales could be demonstrated. This suggests that the objective improvement in AHI is not always reflected in a comparable reduction in self-reported complaints or vice versa. Further research and analysis are needed to reveal possible causes and/or explanations for this phenomenon, because the current study reports on a limited group of patients for a relatively short period of time. Long-term follow- up on larger study populations will be required to further delineate the application of CIS20R in OSA treatment.
Clinical Implications
Because fatigue is one of the main complaints of OSA, this paper focused on the CISFatigue subscale. This fatigue subscale showed a high consistency both at baseline (Cronbach α = .96) and at 3-month follow-up (Cronbach α = .94) and as such indicates that the CISFatigue subscale is an accurate tool in the follow-up of the symptom fatigue in patients with OSA treated with MAD.
A prominent finding of the current study is that the ESS questionnaire failed to express the severe fatigue as indicated by the CIS20R and CISFatigue scores in the OSA study population at baseline. This conclusion stresses that fatigue and hypersomnolence are two distinct symptoms of OSA. Fatigue is defined as the self-reported feeling of tiredness or exhaustion whereas hypersomnolence describes the tendency to fall asleep during normal daytime activities. In order to differentiate between fatigue and hypersomnolence in patients with OSA, the Fatigue Severity Scale and the Multiple Sleep Latency Test were investigated in another study. The results showed that perceived severe fatigue was a common feature among many sleep disorders including OSA, but there was no correlation with the severity of the sleep disorder or with hypersomnolence.9 This all points out the necessity to routinely combine the ESS questionnaire with a more specific tool for exploring fatigue, such as the CIS20R questionnaire, in order to be able to more accurately assess this complaint, both at baseline as well as during treatment.
Another important finding is the high score on fatigue in patients with OSA at baseline, with a CIS20R = 82/140, indicating that these patients were at risk for prolonged absence at work.10 This score is higher than the one reported in patients with multiple sclerosis (CIS20R = 79/140)19 as well as above the cutoff point of 76/140 for severe problematic fatigue.17 This alarming finding is also reflected in the CISFatigue score of 37 ± 15 at baseline, which is 2 points above the cutoff point of 35 reflecting severe fatigue.17 The obtained score is in the same order of magnitude as scores obtained regarding the physical and psychosocial aspects of fatigue in rheumatoid arthritis20 and in patients with multiple sclerosis,19 among others. The presence of such high levels of fatigue has been proven to cause serious hazardous effects on economy, occupation, and health and should not be neglected. In a large-scale study 12,000 employees from various organizations were evaluated with a follow-up period of 3 years, studying physical, psychological, behavioral and work-related aspects of fatigue, burnout, sick leave, and recovery requirement. In this study it was shown that employees with, for example, a somatic reason for fatigue scored higher on CIS20R with 89.7/140 and all its subscales (CISFatigue = 39.6/58) while also showing a higher percentage of sick leave and lower working hours compared to other groups of employees.10 It must be noted, however, that there is one report that warns for the risk of false-positive results in CIS20R: 17% of a group that was considered not fatigued as based on other clinical measures, was categorized in the severely fatigued group using CIS20R.
Overall, the current findings indicate that both the overall CIS20R score as well as the subscale CISFatigue reveal an excellent consistency and reliability. Three months after start of the MAD treatment, the AHI, the ESS, and the CIS20R were significantly reduced. Although both ESS and CIS20R significantly improved following MAD treatment, no correlation could be demonstrated between the changes in AHI and the changes in either questionnaire scores.
The current results indicate that CIS20R is a reliable tool to demonstrate treatment efficacy and improvement in health outcome characteristics upon MAD treatment. It could be of interest to use CIS20R in combination with the routinely used ESS in future studies evaluating treatment outcome among various available therapies for OSA.
DISCLOSURE STATEMENT
All authors have read and agreed on the final manuscript of this paper as submitted. Authors were partially supported by the following: OV—Senior Clinical Fellowship Grant (Fundamenteel Klinisch Mandaat) from Research Foundation - Flanders -Vlaanderen (FWO); MD—Postdoctoral Research Grant from Research Foundation - Flanders - Vlaanderen (FWO). OV reports receiving research support at the University Hospital of Antwerp from Philips, Somnomed, Inspire Medical Systems and Nightbalance and lecture fees from Somnomed and Inspire Medical Systems. He has consulted for Galvani and Liva Nova and is on the advisory board for Zephyr and Somnomed. MB reports receiving research support at the University Hospital of Antwerp from Somnomed. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge the secretarial support by Ms. Nadine De Kerpel for the distribution and collection of the questionnaires.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CIS20R
Checklist Individual Strength
- ESS
Epworth Sleepiness Scale
- MAD
mandibular advancement device
- OSA
obstructive sleep apnea
- PSG
polysomnography
REFERENCES
- 1.Jennum P, Riha RL. Epidemiology of sleep apnoea/hypopnoea syndrome and sleep-disordered breathing. Eur Respir J. 2009;33(4):907–914. doi: 10.1183/09031936.00180108. [DOI] [PubMed] [Google Scholar]
- 2.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):144–513. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colt HG, Haas H, Rich GB. Hypoxemia vs sleep fragmentation as cause of excessive daytime sleepiness in obstructive sleep apnea. Chest. 1991;100(6):1542–1548. doi: 10.1378/chest.100.6.1542. [DOI] [PubMed] [Google Scholar]
- 4.Epstein LJ, Kristo D, Strollo PJ, Jr, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 5.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 6.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 7.Taylor E, Zeng I, O'Dochartaigh C. The reliability of the Epworth Sleepiness Score in a sleep clinic population. J Sleep Res. 2018;28(2):e12687. doi: 10.1111/jsr.12687. [DOI] [PubMed] [Google Scholar]
- 8.Nordin Å, Taft C, Lundgren-Nilsson Å, Dencker A. Minimal important differences for fatigue patient reported outcome measures—a systematic review. BMC Med Res Methodol. 2016;16:62. doi: 10.1186/s12874-016-0167-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichstein KL, Means MK, Noe SL, Aguillard RN. Fatigue and sleep disorders. Behav Res Ther. 1997;35(8):733–740. doi: 10.1016/s0005-7967(97)00029-6. [DOI] [PubMed] [Google Scholar]
- 10.Beurskens AJ, Bultmann U, Kant I, Vercoulen JH, Bleijenberg G, Swaen GM. Fatigue among working people: validity of a questionnaire measure. Occup Environ Med. 2000;57(5):353–357. doi: 10.1136/oem.57.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martiniuk AL, Senserrick T, Lo S, et al. Sleep-deprived young drivers and the risk for crash: the DRIVE prospective cohort study. JAMA Pediatr. 2013;16(7):7, 647–655. doi: 10.1001/jamapediatrics.2013.1429. [DOI] [PubMed] [Google Scholar]
- 12.Swaen GMH, van Amelsvoort LG, Bültmann U, Kant I. Fatigue as a risk factor for being injured in an occupational accident: results from the Maastricht Cohort Study. Occup Environ Med. 2003;60(Suppl 1):i88–i92. doi: 10.1136/oem.60.suppl_1.i88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flechtner H, Bottomley A. Fatigue and quality of life: lessons from the real world. Oncologist. 2003;8(Suppl 1):5–9. doi: 10.1634/theoncologist.8-suppl_1-5. [DOI] [PubMed] [Google Scholar]
- 14.Worm-Smeitink M, Gielissen M, Bloot L, et al. The assessment of fatigue: Psychometric qualities and norms for the Checklist individual strength. J Psychom Res. 2017;98:40–46. doi: 10.1016/j.jpsychores.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Weaver TE, Grunstein RR. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187(8):879–887. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 17.Bultmann U, de Vries M, Beurskens AJ, Bleijenberg G, Vercoulen JH, Kant I. Measurement of prolonged fatigue in the working population: determination of a cutoff point for the checklist individual strength. J Occup Health Psychol. 2000;5(4):411–416. doi: 10.1037//1076-8998.5.4.411. [DOI] [PubMed] [Google Scholar]
- 18.Hewlett S, Dures E, Almeida C. Measures of fatigue: Bristol Rheumatoid Arthritis Fatigue Multi-Dimensional Questionnaire (BRAF MDQ), Bristol Rheumatoid Arthritis Fatigue Numerical Rating Scales (BRAF NRS) for severity, effect, and coping, Chalder Fatigue Questionnaire (CFQ), Checklist Individual Strength (CIS20R and CIS8R), Fatigue Severity Scale (FSS), Functional Assessment Chronic Illness Therapy (Fatigue) (FACIT-F), Multi-Dimensional Assessment of Fatigue (MAF), Multi-Dimensional Fatigue Inventory (MFI), Pediatric Quality Of Life (PedsQL) Multi-Dimensional Fatigue Scale, Profile of Fatigue (ProF), Short Form 36 Vitality Subscale (SF-36 VT), and Visual Analog Scales (VAS) Arthritis Care Res. 2011;63(Suppl 11):S263–S286. doi: 10.1002/acr.20579. [DOI] [PubMed] [Google Scholar]
- 19.Rietberg MB, Van Wegen EEH, Kwakkel G. Measuring fatigue in patients with multiple sclerosis: reproducibility, responsiveness and concurrent validity of three Dutch self-report questionnaires. Disabil Rehabil. 2010;32(22):1870–1876. doi: 10.3109/09638281003734458. [DOI] [PubMed] [Google Scholar]
- 20.van Hoogmoed D, Fransen J, Bleijenberg G, van Riel P. Physical and psychosocial correlates of severe fatigue in rheumatoid arthritis. Rheumatology (Oxford) 2010;49(7):1294–1302. doi: 10.1093/rheumatology/keq043. [DOI] [PubMed] [Google Scholar]


