Abstract
Study Objectives:
Obstructive sleep apnea (OSA) is a global health issue and is associated with obesity and oropharyngeal crowding. Global data are limited on the effect of ethnicity and sex on these relationships. We compare associations between the apnea-hypopnea index (AHI) and these risk factors across ethnicities and sexes within sleep clinics.
Methods:
This is a cross-sectional, multicenter study of patients with OSA from eight sleep centers representing the Sleep Apnea Global Interdisciplinary Consortium (SAGIC). Four distinct ethnic groups were analyzed, using a structured questionnaire: Caucasians (Australia, Iceland, Germany, United States), African Americans (United States), Asians (Taiwan), and South Americans (Brazil). Regression analyses and interaction tests were used to assess ethnic and sex differences in relationships between AHI and anthropometric measures (body mass index [BMI], neck circumference, waist circumference) or Mallampati score.
Results:
Analyses included 1,585 individuals from four ethnic groups: Caucasian (60.6%), African American (17.5%), Asian (13.1%), and South American (8.9%). BMI was most strongly associated with AHI in South Americans (7.8% increase in AHI per 1 kg/m2 increase in BMI; P < .0001) and most weakly in African Americans (1.9% increase in AHI per 1 kg/m2 increase in BMI; P = .002). In Caucasians and South Americans, associations were stronger in males than females. Mallampati score differed between ethnicities but did not influence AHI differently across groups.
Conclusions:
We demonstrate ethnic and sex variations in associations between obesity and OSA. For similar BMI increases, South American patients show greatest AHI increases compared to African Americans. Findings highlight the importance of considering ethnicity and sex in clinical assessments of OSA risk.
Citation:
Sutherland K, Keenan BT, Bittencourt L, Chen NH, Gislason T, Leinwand S, Magalang UJ, Maislin G, Mazzotti DR, McArdle N, Mindel J, Pack AI, Penzel T, Singh B, Tufik S, Schwab RJ, Cistulli PA; for the SAGIC Investigators. A global comparison of anatomic risk factors and their relationship to obstructive sleep apnea severity in clinical samples. J Clin Sleep Med. 2019;15(4):629–639.
Keywords: anthropometry, ethnicity, Mallampati score, obesity, obstructive sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea (OSA) is a global problem, but little is known about the influence of ethnicity and sex on OSA in clinical populations. This study uses global sleep clinical populations to examine differences in associations between OSA severity and clinical anatomic risk factors.
Study Impact: In clinical populations, we found South Americans and Asians are most susceptible to effects of obesity on OSA severity, whereas African Americans are least affected. Men are more susceptible than women to obesity effects in Caucasian and South American clinical populations. Relationships between Mallampati score and OSA severity or obesity were similar among ethnic groups. Our study highlights the importance of ethnicity and sex in OSA clinical presentations, and the need for personalized strategies for OSA management.
INTRODUCTION
Obstructive sleep apnea (OSA) is a chronic sleep disorder in which repetitive pharyngeal collapse leads to intermittent hypoxemia, sleep fragmentation, sympathetic activation, and intrathoracic pressure swings. OSA is associated with significant comorbidity, including excessive daytime sleepiness, impaired quality of life,1 increased motor vehicle accident risk,2 hyper-tension,3,4 cardiovascular disease, stroke,5 type 2 diabetes,6 and mortality.7 The substantial global health burden associated with OSA highlights the importance of accurate diagnosis and treatment. General population studies suggest high OSA prevalence across the globe, with estimates of 9% to 38% in adults.8 OSA prevalence is similar across different global regions, including Asia, Latin America, North America, Oceania, and Europe.9–12
Obesity is a well-recognized clinical risk factor for OSA and is a strong predictor of its presence across different populations.11–13 The Mallampati score, a four-point grading system of the visibility of oropharyngeal structures behind the tongue, is also proposed as a simple clinical assessment of OSA risk.14 Although clinical anatomic risk factors are common to different OSA populations, their relative effect on OSA severity may vary with ethnicity due to differences in disease pathogenesis. Craniofacial skeletal dimensions interact with upper airway soft-tissue sizes to contribute to OSA.15 Compared to Caucasians with OSA, Chinese patients with OSA have more craniofacial restriction and less obesity.16 This craniofacial restriction may make Chinese patients more susceptible to the effects of obesity. Conversely, when compared to African Americans, Caucasian patients with apnea show evidence of craniofacial skeletal restriction, whereas African-American patients with apnea do not. This could result in African Americans being less vulnerable to effects of oropharyngeal obesity.17,18 Asians have a higher Mallampati score compared to Caucasians in clinical populations.19 High Mallampati score may be related to central adiposity,20 but could also reflect oropharyngeal crowding as a result of craniofacial restriction. This relationship may vary between ethnicities; hence, Mallampati score could relate to different aspects of OSA pathogenesis in different populations.
Clinical anatomic risk factors may also differentially reflect OSA severity between sexes. OSA tends to be more severe in men than women for the same body mass index (BMI)21,22 and differences in relationships between regional body fat distributions and OSA have been reported.23,24 In United States samples, the relationship between anthropo-metric measures of obesity and OSA severity appear stronger in men compared to women.25,26 Mallampati score has also been associated with OSA severity in men, but not women.20 Thus, both ethnicity and sex likely influence the relationship between clinical anatomic risk factors and severity of OSA. However, there are limited direct multiethnic studies with consideration of sex differences. Understanding the relationship between OSA and anatomic risk factors in different ethnicities and within sexes may improve the clinical assessment and management of individual patients who present to the sleep clinic.
We aimed to compare associations of clinical anatomic risk factors (anthropometry for overall and regional obesity and Mallampati score) with OSA severity among different clinical sleep populations across the globe, as well as between sexes. We hypothesized that the strength of association between OSA severity and clinical anatomic risk factors will vary between the different ethnic groups, and with sex. Specifically, we hypothesized that Mallampati score and obesity measures would have a greater influence on OSA severity in Asian patients with OSA, given the known role of craniofacial restriction in OSA in this population. Finally, we hypothesized that the relationship between clinical anatomic risk factors and OSA severity will be stronger in men compared to women.
MATERIALS
Study Sample
This is a cross-sectional, clinical, multicenter study of the Sleep Apnea Global Interdisciplinary Consortium (SAGIC). SAGIC is a collaborative effort of international sleep centers actively recruiting to establish a well-phenotyped multinational cohort for investigation of ethnic differences in OSA. Further information on SAGIC can be found in the supplemental material and at the SAGIC webpage (www.med.upenn.edu/sleepctr/sagic.html). The current study included individuals with OSA (apnea-hypopnea index [AHI] > 5 events/h) recruited from eight clinical sleep centers participating in SAGIC. Anthropometry was conducted using standardized protocols for height, weight, and neck, waist, and hip circumferences (details of methodology are available in the supplemental material). Modified Mallampati score was collected and graded using a photographic method.27 AHI was obtained from overnight sleep studies (the definition of events and information about how sleep studies were conducted are available in the supplemental material). Scoring concordance studies between eight SAGIC centers has previously confirmed strong agreement in scoring of respiratory events on both in-laboratory28 and home29 sleep studies. Data collection was approved by the local Institutional Review Boards at each participating institution.
SAGIC allows comparison of sleep clinic patients from different countries, thereby providing differences in genetic, cultural, and environmental aspects of ethnicity. Ethnicity encompasses a broad range of factors, including genetic and racial background, physical appearance, culture or nationality, and environment, and therefore can be difficult to define.30,31 In the current study, ethnicity was recorded by a self-report questionnaire (supplemental material), which asked for participants to select their ethnicity based on a mixture of culture, religion, skin color, and language. To limit interference of regional differences in analyses of ethnic differences, we restricted inclusion of individuals in four ethnic groups to those who were recruited from specific SAGIC sites: Caucasians recruited from Australia, Germany, Iceland, United States (97.3% of all participants reporting “Caucasian”), Asians recruited from Taiwan (84.5% of all participants reporting “Asian”), African Americans recruited from United States (98.6% of all participants reporting African ancestry), and South Americans recruited from Brazil (95.3% of all individuals reporting Central/South American ancestry). We further examined differences in the Caucasian sample by country to assess for cultural or geographical effects.
Statistical Analysis
Continuous variables were summarized using mean and standard deviation and compared among groups using analysis of variance or using median and interquartile range and compared using nonparametric Kruskal-Wallis tests. Categorical variables were summarized using frequencies and percentages and compared among groups using chi-square tests. Subsequent pairwise comparisons were performed to define between group differences. We examined the association between clinical anatomic risk factors and natural log-transformed AHI both within and across ethnic groups using linear regression. Results are presented with respect to the original AHI scale as exponentiated β-coefficients (eβ), equal to the expected proportional change in AHI per 1 unit increase in a given anthropo-metric measure (or scaled per 0.1 units for ratios). To test for differential associations across ethnic groups, we performed interaction tests within the full sample. Interaction tests examined the significance of the product term (ethnicity × clinical anatomic risk factor) in a regression model that also included the corresponding main effects (ethnicity, clinical anatomic risk factor). A significant interaction suggests the association between the given clinical anatomic risk factor and AHI differs by ethnicity. Adjustment for age and sex were made in all analyses, as appropriate. In addition to analyses with AHI as an outcome, we assess the association between anthropometric measures and Mallampati score using ordinal logistic regression models, using similar methods.
To understand the effect of sex on associations between AHI and clinical risk factors, we performed additional interaction tests using models similar to those previously described. Specifically, within each ethnic group, we examined the pairwise interaction between sex and clinical risk factors by evaluating the product term (sex × clinical risk factor) in a model including main effects. In addition, to evaluate whether these sex-specific effects differed across ethnic groups, we assessed the three-way interaction between ethnicity, sex, and the clinical risk factors in a model including all main effects and pairwise interactions. For context, associations between clinical risk factors and AHI are presented stratified by sex within each ethnic group.
Statistical significance was based on a Bonferroni-corrected P < .006, adjusted for eight variables; Mallampati score and anthropometric obesity measures (BMI, weight, waist circumference, neck circumference, hip circumference, neck-to-waist ratio and waist-to-hip ratio). Statistical analyses were performed using Stata/SE Version 14.1 (StataCorp LP, College Station, Texas, United States) and SAS Version 9.4 (SAS Institute Inc., Cary, North Carolina, United States).
RESULTS
Sample Characteristics of the Four Ethnic Groups
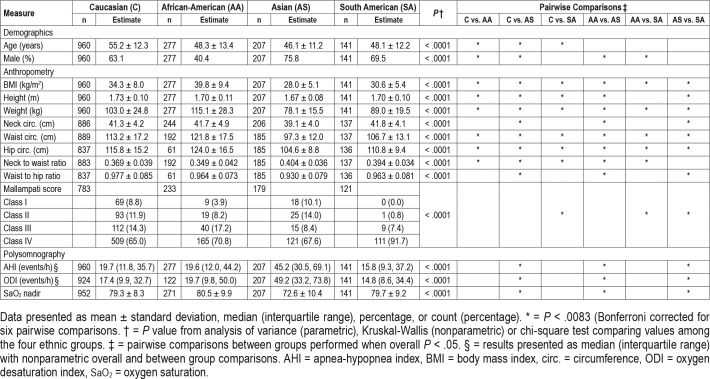
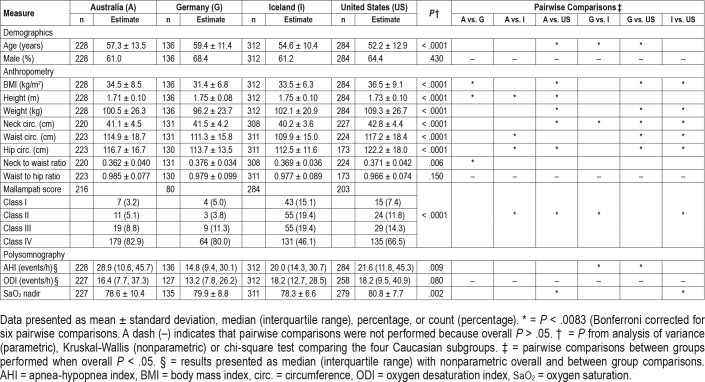
A total of 1,585 patients with OSA belonging to one of four ethnicity groups (Caucasian [60.6%], African American [17.5%], Asian [13.1%], and South American [8.9%]) with available age, sex, and BMI data were included. Descriptive characteristics of these four groups are shown in Table 1. The Asian group was youngest, predominantly male, with the lowest obesity measures, but presented with highest AHI, oxygen desaturation index, and lowest nadir oxygen saturation. Caucasians were generally older than other ethnic groups and African Americans had the highest BMI and greatest proportion of females. There was a significant difference in the distribution of the Mallampati score between ethnic groups, with South Americans demonstrating predominantly class III or IV.
Table 1.
Descriptive characteristics of the four ethnic groups recruited through the Sleep Apnea Global Interdisciplinary Consortium.
Relationship Between Anthropometry and AHI Among Ethnic Groups
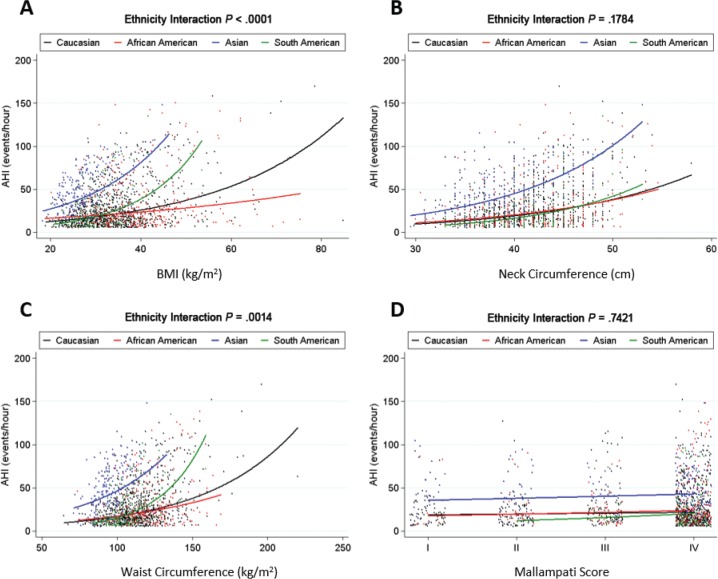
The associations between anthropometry measures of BMI, neck circumference, waist circumference, and AHI across ethnic groups are shown in Figure 1. Detailed information and additional anthropometric variables are presented in Table S1 in the supplemental material. A description of how to interpret the information in the figures is provided in the legend for Figure 1, which is applicable to all other figures. There were positive relationships between BMI, neck and waist circumference, and AHI within each ethnicity (P < .002). In addition, based on interaction tests, there was evidence for ethnic differences in the relationship between AHI and both BMI (P < .0001) and waist circumference (P = .002). The relationship between AHI and neck circumference did not differ by ethnicity, although there was a differential effect of neck-to-waist ratio (P = .001; Table S1). For BMI, the strongest relationship with AHI was in the South American and Asian groups (Figure 1), with proportional increases in AHI of 7.8% and 5.7%, respectively, for each 1 kg/m2 BMI increase, compared to an expected increase of 3.7% for Europeans and an even smaller effect of only 1.9% in African Americans. Similar associations were observed for neck and waist circumference, with the largest effects within South Americans.
Figure 1. Differential effects of clinical anatomic risk factors on AHI severity across ethnicities.
Individual data points in the analysis (small dots) and the regression line (solid lines) for the relationship between BMI and AHI within each of the four ethnic groups. A significant interaction value of P indicates that the slope of this line differs between ethnic groups. Each ethnic group is represented by a color: Caucasian (black), African American (red), Asian (blue), and South American (green). A steeper regression line (a sharper upward curve of the line) indicates a stronger influence of the anthropometric variable on AHI, such that an increase in that anthropometric variable will result in a greater increase in AHI. Associations between AHI and (A) BMI, (B) neck circumference, (C) waist circumference, and (D) Mallampati score are shown. A significant interaction was observed between BMI and ethnicity (P < .0001), with BMI showing the strongest positive relationship with AHI among South American and Asian groups, as indicated by the sharper curve of the line. A similar pattern was observed for waist circumference (P = .0014). This indicates more severe sleep apnea in South Americans and at lower levels of BMI and waist circumference. There was no evidence of a differential relationship between AHI and neck circumference or Mallampati score between ethnic groups. Models were fit with natural log-transformed AHI as an outcome and results transformed back to the original AHI scale for graphical presentation. Analyses were adjusted for confounding effects of age and sex. AHI = apnea-hypopnea index, BMI = body mass index.
Relationship Between Mallampati Score and AHI Among Ethnic Groups
Associations between modified Mallampati score and AHI across ethnic groups are shown in Table S1 and Figure 1. There were trending, but nonsignificant associations between increased Mallampati score and AHI, with proportional increases ranging from 5% to 31% for an increase in Mallampati class. There was no evidence of significant interaction of ethnicity on the relationship between Mallampati score and AHI (P = .742).
Relationship Between Mallampati Score and Obesity Among Ethnic Groups
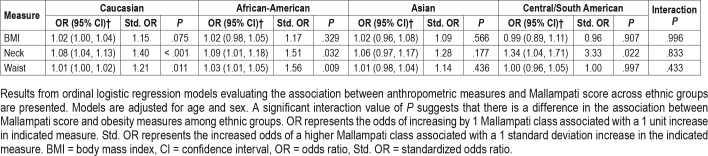
To assess whether Mallampati score may be differentially affected by increases in obesity, we assessed the effect of anthropometric measures across ethnic groups (Table 2). Within some ethnicities there was evidence of a positive relationship between Mallampati score and anthropometric measures. In particular, increased neck circumference was associated with greater odds of having a higher Mallampati class in Caucasians (P < .001), African Americans (P = .032), and South Americans (P = .022), but not Asians (P = .177). However, as indicated by the nonsignificant interaction terms, there were no differences between ethnic groups in the relationship between anthropo-metric measures and Mallampati score.
Table 2.
Association between obesity measures and Mallampati score among ethnic groups.
Sex Comparison of Clinical Anatomic Risk Factors and AHI Relationships Within Ethnicities
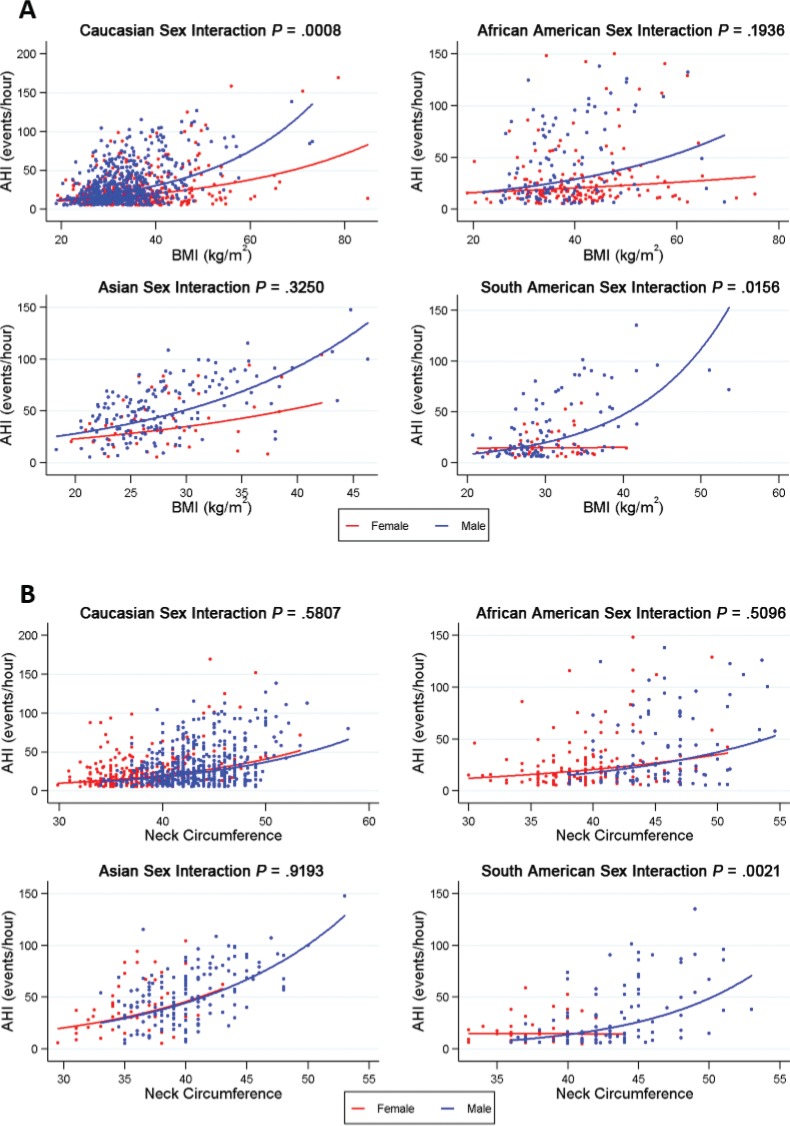
Associations of BMI and neck circumference with AHI in males and females within each ethnicity are shown in Figure 2 (detailed presentation and additional clinical anatomic risk factors are available in Table S2 in the supplemental material). There were no significant sex-related differences in the relationship between clinical anatomic risk factors and AHI in the Asian or African-American groups (Table S2). There was evidence for differential relationships between males and females in the association between AHI and BMI in the Caucasian (P = .001) and South American (P = .015) groups, with a stronger positive association with AHI in males (Figure 2A). There was also a significant interaction between sex and neck circumference (P = .002) in South Americans, with stronger effects in men compared to women (Figure 2B). There was no effect of sex on the associations between Mallampati score and AHI across the ethnic groups assessed (Table S2). Assessments of three-way interaction between ethnicity, sex, and clinical anatomic risk factors suggested that sex-specific effects on the relationship of neck circumference (P = .028) and waist-to-hip ratio (P = .016) to AHI differed across ethnic groups.
Figure 2. Effect of sex on the relationship between BMI and neck circumference and AHI within each ethnicity group.
The associations between (A) BMI and (B) neck circumference with AHI in males (blue) and females (red) are illustrated within each ethnicity group. For BMI, there was a significant interaction with sex in the Caucasian group (P = .001) and a nominal interaction in the South American group (P = .016). In both cases, BMI was more strongly associated with AHI among males compared to females. For neck circumference, there was an interaction with sex in the South American group only (P = .002), with an association between neck circumference and AHI in males only. Models were adjusted for age and fit with natural log transformed AHI as an outcome; results are transformed back to the original AHI scale for graphical presentation. AHI = apnea-hypopnea index, BMI = body mass index.
Relationship Between Clinical Anatomic Risk Factors and AHI in Caucasians From Four Regions
To assess for cultural or geographical differences in the Caucasian group, the sample of 960 participants was divided into their four primary countries of residence: Australia (n = 228), Germany (n = 136), Iceland (n = 312), and United States (n = 284). The descriptive characteristics of these four groups are shown in Table 3. Participants from these four regions differed in age, AHI, most anthropometric measurements, and Mallampati scores. The United States group was the most obese, with lowest BMI in participants from the two European countries. AHI values were highest for the United States and Australian groups.
Table 3.
Descriptive characteristics of four Caucasian subgroups from different regions.
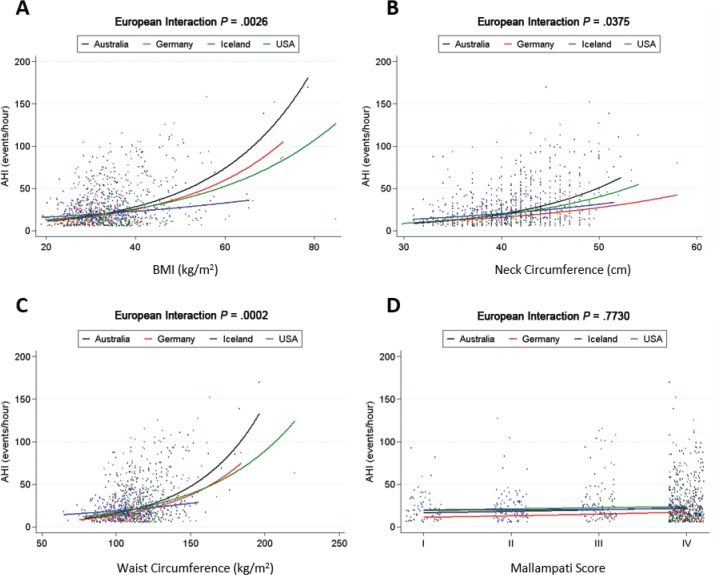
Associations between four clinical anatomic risk factors and AHI are shown in Figure 3 (detailed presentation and additional anthropometric variables are available in Table S3 in the supplemental material). Interaction P values suggested significant or nominal differences in the strength of these relationships between countries for BMI (P = .003), neck circumference (P = .038), and waist circumference (P < .001). For BMI, the strongest relationship to AHI was in the Australian group, with a proportional increase in AHI of 4.9% for a 1 kg/m2 increase in BMI, followed by 4.4% and 3.6% increases in the German and United States subgroups, respectively, for the same 1 kg/m2 increase. Icelanders had the weakest association between BMI and AHI. Similar patterns were observe for neck and waist circumference (Figure 3). An examination of sex differences in anthropometry and AHI relationships are available in the supplemental material (Table S4, Figure S2). Sex differences were generally not evident in the relationship between AHI and anthropometry within Caucasian geographical groups.
Figure 3. Differential effect of clinical anatomic risk factors on AHI severity across Caucasians from different countries.
Associations between AHI and (A) BMI, (B) neck circumference, (C) waist circumference, and (D) Mallampati score are shown. The lines represent the four Caucasian subgroups based on country of residence: Australia (black), Germany (red), Iceland (blue) and the United States (green). We observed a significant interaction between BMI (P < .003) and waist circumference (P < .001) and country subgroups with the weakest association with AHI in Iceland, compared to intermediate effects in Germany and the United States, and stronger effects in Australia. There was a nominal interaction with neck circumference and country subgroup (P = .038). Models were fit with natural log-transformed AHI as an outcome and results transformed back to the original AHI scale for graphical presentations. Analyses were adjusted for confounding effects of age and sex. AHI = apnea-hypopnea index, BMI = body mass index.
DISCUSSION
Although OSA is clearly a global problem, there are remarkably limited data comparing different clinical populations in terms of key clinical risk factors. To our knowledge, this is the first study to examine multiethnic and sex-specific variations in the relationships between clinical anatomic risk factors (anthropometric obesity measurements and Mallampati score) and OSA severity in sleep clinic patients from across the globe. Previous interethnic studies have been restricted to ethnic groups within a single country. We additionally examined potential cultural or geographic differences among Caucasians. Understanding variations in clinical anatomic risk factors across populations will improve understanding of OSA presentations in sleep clinics across the world. We have shown that overall obesity levels (BMI) have the greatest influence on AHI in the South American group, followed by the Asian group, suggesting that weight gain is an important contributor to AHI severity in these specific populations. Conversely, increased obesity in the African-American group showed the least effect on AHI severity. In terms of oropharyngeal crowding (Mallampati score), although the distribution of Mallampati classes differed between ethnic groups, we did not find any evidence of a differential relationship with OSA severity between groups.
Although it has been recognized that there are likely ethnic differences in OSA etiology and consequences, data are limited and previous interethnic comparisons have predominantly involved comparing Caucasians to one other group,32 and have predominantly been assessed in population samples, which are likely to be different to clinical samples due to referral and other biases. In contrast, we compared OSA anatomic risk factors across four ethnic groups and between sexes presenting to the sleep clinic. We additionally examined the relationship between oropharyngeal crowding (Mallampati score) and anthropometric measures between ethnicities to determine whether Mallampati score may differentially relate to OSA severity. Furthermore, we compared the Caucasian populations by region of residence. In the Caucasians, the strongest relationship between AHI and BMI was in the Australian group, with the weakest relationship in Icelanders.
A key strength of the SAGIC is recruitment from international sleep clinics using standardized methodologies, enabling a reliable phenotypic description of patients with OSA from different populations. We have examined more than 1,500 patients with OSA, divided into four ethnic groups, as well as four regional groups of Caucasians. We selected these particular ethnic groups for initial comparison, as we anticipate they likely represent a spectrum in terms of OSA risk factor phenotypes—from predominantly soft tissue to predominantly skeletal. Compared to Caucasian patients with OSA, Asian patients display more craniofacial restriction.16 However, Caucasian participants display more craniofacial structure-related OSA risk compared to African-Americans patients.17,18 We additionally included a South American group recruited from São Paulo, Brazil. The Brazilian population is a highly heterogeneous population as a result of centuries of intermixing between different ancestral populations (including Indigenous, European, Japanese, and African), with diverse patterns of admixture within individuals.33,34 Therefore, we have included this group as one with “heterogeneous ancestry.” Future studies need to address this heterogeneity using ancestral genetic markers and admixture analysis to specifically determine ethnic origins and assess ancestry-specific effects.
In this study, we have targeted clinical anatomic risk factors (anthropometry and Mallampati score) to examine the differential relationships between obesity and oropharyngeal crowding between different clinical populations. Our finding of the strongest influence of obesity (BMI) on OSA severity in the admixed South American clinical population (Brazil) is interesting. This population has not previously been directly compared to the other ethnic groups. The second strongest association with BMI in the Asian group was one we expected to see and confirms effects shown in other recent investigations.35 Analysis of data from the Multi-Ethnic Study of Atherosclerosis, or MESA, a general population sample from the United States examined relationships between AHI and BMI and waist circumference in white, black, Hispanic, and Chinese Americans.35 This analysis identified Chinese Americans as having the largest AHI increase from a greater BMI, with whites and blacks having a similar relationship and the weakest association in the Hispanic group. There are differences between this study and that reported here in the populations examined, although it is interesting to note that the stronger relationship between AHI and BMI in Chinese Americans is reflected in our Taiwanese clinical sample. As our study recruitment was from sleep clinic samples, it contained a greater spectrum and severity of OSA compared to the MESA study, which examined population cohorts. Our study is a global clinical study and therefore likely captures more aspects of ethnicity when compared to different races from a single country. Although there are differences between these studies, they show a consistent finding of Asian populations being at greater risk of increasing OSA severity with increasing obesity.
A novel aspect of our investigation is concurrently studying an additional anatomic risk factor, Mallampati score, as a clinical assessment of oropharyngeal crowding. Mallampati score is often a component of clinical prediction models.19,36 The distribution of Mallampati scores also differed between ethnic groups, with the South Americans having the largest proportion of Mallampati class IV. To our knowledge, a multiethnic comparison of Mallampati score and relationship to either OSA severity or obesity has not been made. We did not find evidence of a differential relationship between Mallampati score and OSA severity across ethnic groups. In addition to effects of Mallampati score on AHI, another novel aspect of our investigation was exploring ethnic-specific relationships between obesity measures and Mallampati score. Mallampati score relates to tongue volume and measures of central obesity,20,37 and oropharyngeal crowding may also be related to aspects of craniofacial restriction. It is interesting that the South American group with the greatest susceptibility to obesity also had the highest prevalence of Mallampati class IV. Whether this is a reflection of particular aspects of craniofacial restriction in this group requires further investigation.
Our study also describes differences in clinical features in clinical populations across the globe. Our Asian group (from Taiwan) were more likely to be male and had the highest AHI and lowest oxygen desaturation, despite being younger and less obese. These findings are consistent with previous studies that showed higher AHI and lower oxygen levels in Asian patients with OSA compared to Caucasian patients.16,38,39 Given the early age of onset in Asians, a greater genetic contribution could be speculated; however, no family aggregation studies have been conducted in Asian populations. This is an important future direction. Our African-American group was the only ethnicity with a higher percentage of females than males, consistent with a previous clinical observation.40 We also found African Americans to be younger and have higher AHI than Caucasians.18,40,41 Compared to Caucasians, South Americans were equivalent in OSA severity, but were overall less obese.
The stronger relationship between AHI and obesity in South Americans (Brazil) and Asians (Taiwan) has important public health and clinical implications for these countries. The prevalence of being overweight and obese is increasing across the globe, including in Asian countries and Latin America.42 Obesity clearly contributes to OSA and our data suggest these populations are more susceptible to the effect of weight gain on OSA severity. Accordingly, sleep clinics in Asian countries and Brazil may need to particularly monitor for weight changes in their patients. This could also suggest that weight loss therapy may be more effective as an OSA treatment in these populations. Conversely, although both sleep-disordered breathing and obesity may be more prevalent in African Americans compared to Caucasians,41,43 overall obesity may not necessarily have the same effect on OSA and other mechanisms may play a role in OSA risk for these populations.
Sex differences were also most evident in the South Americans, who had the strongest sex interactions with AHI across obesity measures. In particular, females did not show a relationship between body circumferences and AHI, whereas males had a strong relationship with increasing waist circumference. Caucasians showed a sex difference in the BMI and AHI relationship only; no sex differences were apparent in the Asian or African-American groups. All sex differences indicated a stronger relationship between obesity and AHI in men. It has been reported that men tend to have more severe OSA for the same level of BMI.22,25,44 Our findings suggest that this sex effect is not equally present across ethnic groups, as it was not seen in African Americans or Asians. Although women have less neck fat than men,45 this did not differentially affect the relationship between the neck circumference and OSA severity in most groups. Previous studies suggest sex differences in anthropometric predictors of OSA23,24; however, this is the first study to compare sex interactions in relation to OSA severity within different ethnic groups. These sex differences may also have important clinical implications in the treatment of OSA.
The SAGIC cohort contains a large number of Caucasian participants from different countries, which likely vary in cultural and other characteristics that could influence OSA and obesity relationships. We observed differences in anthropometric characteristics between the four Caucasian groups, in line with recognized differences in obesity rates between regions.46 OSA and obesity were clearly related in all Caucasian subgroups, but groups differed in the strength of this relationship. The Australian group had the strongest relationship between obesity measures and OSA. Further investigation is needed in conjunction with other phenotypes to better understand the reason for these regional differences, which may also include regional variations in sleep clinic referral patterns. Regardless, the differences between Caucasian groups from different countries could suggest that it may not be valid to combine multicountry Caucasians together in subsequent investigations.
Although we present a novel multiethnic and sex comparison of OSA, there are limitations to our study. Ethnicity was classified by self-report, which may not fully or accurately represent genetic ancestry as ethnicity and race are not completely overlapping concepts.47–49 In this study, we have focused on specific ethnic groups (encompassing cultural, social, and geopolitical factors) in limiting to those from a single country. These findings may not be applicable to people from the racial/ethnic groups studied in our investigation if they come from different countries. However, future SAGIC studies have the potential to compare ancestral mixture after genotyping of collected samples is completed. This will help understand the relative effect of ethnicity versus genetic ancestry or race. We have adjusted our statistical models for confounders of age and sex; however, other examinations of the relationship between obesity and OSA severity have also controlled for comorbidities (ie, hypertension, diabetes, cardiovascular disease).35 Our results remained similar when including these covariates (available in a large subset of the population) as possible confounders (data not shown). Ultimately, we believe that comorbidities are consequences of obesity and OSA and thus do not meet the traditional definition of confounding and adjusting for consequences could lead to biased estimates of effect sizes. Reflecting the general risk factors for OSA, some of our groups consist of a relatively small sample sizes (particularly females). Although in-laboratory sleep testing was performed in most participants, the Icelandic sleep data were obtained via an ambulatory type 3 device. Ambulatory testing may underestimate the AHI when compared to polysomnography, and could potentially explain weaker relationships in this group. The analysis sample is a clinical cohort recruited from international sleep centers; therefore, there may be some bias related to region-specific referral patterns and medical awareness of OSA. Furthermore, there may also be some biases related to recruitment of patients from clinical sleep centers, which likely differ from cases in the general population.50 Therefore, our study results relate to presentations in clinical sleep populations. Our study relied on anthropometric measures of obesity. The relationship between body fat percentage and visceral fat with BMI and waist circumference varies with ethnicity.51,52 Therefore, the relative effect of anthropometric measures on underlying fat distribution may not be equivalent in the different populations.
CONCLUSIONS
OSA has a complex etiology, which is likely influenced by ethnicity. Common phenotyping procedures through the SAGIC allowed us to study the influence of ethnicity on OSA and the effect of clinical anatomic risk factors in different clinical populations across the globe. South Americans showed stronger relationships between obesity and AHI, followed by Asians, whereas African Americans had the weakest. Caucasians showed intermediate effects of obesity on OSA. Although Mallampati class distribution differed between ethnic groups, oropharyngeal crowding was not differentially related to OSA severity. This differential influence of obesity may be due to the relative effect of other intermediate phenotypes, such as craniofacial structure. We also show that sex has differential effects on the relationship between obesity and OSA severity among certain ethnic groups. Our data provide a unique comparison of different clinical OSA populations and highlights the importance of understanding variations in OSA phenotypes across different ethnic populations and sexes, as a precursor to the development of personalized approaches to diagnosis and management.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. Sarah Leinwand is deceased; she made a valuable contribution to the data collection for this study. Support for the REDCap database at Ohio State University was provided by Grant UL1TR001070 from the National Center for Advancing Translational Sciences. Dr. Lia Bittencourt has a grant number 401569/2016-0 from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq). Research at the University of Pennsylvania was supported by a Program Project Grant from the National Institutes of Health (P01 HL094307). Dr. Cistulli has an appointment to an endowed academic Chair at the University of Sydney that was established from ResMed funding. He has received research support from ResMed, SomnoMed, and Zephyr Sleep Technologies. He has been a consultant / adviser to Zephyr Sleep Technologies, NovoNordisk, and Bayer. Dr. Pack is The John L. Miclot Professor of Medicine at the University of Pennsylvania; funds for this endowment were provided by the Philips Respironics Foundation. Dr. Penzel reports grant support from Cidelec, Itamar, Resmed, Philips, Heinen and Loewenstein, and Weinmann, as well as speaker support from Itamar, SomnoMed, and Weinmann. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the participants, study recruiters, technologists and research team involved in the SAGIC project at each site.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- OSA
obstructive sleep apnea
- SAGIC
Sleep Apnea Global Interdisciplinary Consortium
REFERENCES
- 1.Baldwin CM, Griffith KA, Nieto FJ, O'Connor GT, Walsleben JA, Redline S. The association of sleep-disordered breathing and sleep symptoms with quality of life in the Sleep Heart Health Study. Sleep. 2001;24(1):96–105. doi: 10.1093/sleep/24.1.96. [DOI] [PubMed] [Google Scholar]
- 2.Rakel RE. Clinical and societal consequences of obstructive sleep apnea and excessive daytime sleepiness. Postgrad Med. 2009;121(1):86–95. doi: 10.3810/pgm.2009.01.1957. [DOI] [PubMed] [Google Scholar]
- 3.Lavie P, Herer P, Hoffstein V. Obstructive sleep apnoea syndrome as a risk factor for hypertension: population study. BMJ. 2000;320(7233):479–482. doi: 10.1136/bmj.320.7233.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nieto FJ, Young TB, Lind BK, et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 5.Arzt M, Young T, Finn L, Skatrud JB, Bradley TD. Association of sleep-disordered breathing and the occurrence of stroke. Am J Respir Crit Care Med. 2005;172(11):1447–1451. doi: 10.1164/rccm.200505-702OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reichmuth KJ, Austin D, Skatrud JB, Young T. Association of sleep apnea and type II diabetes: a population-based study. Am J Respir Crit Care Med. 2005;172(12):1590–1595. doi: 10.1164/rccm.200504-637OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10(4):355–362. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senaratna CV, Perret JL, Lodge CJ, et al. Prevalence of obstructive sleep apnea in the general population: a systematic review. Sleep Med Rev. 2017;34:70–81. doi: 10.1016/j.smrv.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Tufik S, Santos-Silva R, Taddei JA, Bittencourt LR. Obstructive sleep apnea syndrome in the Sao Paulo Epidemiologic Sleep Study. Sleep Med. 2010;11(5):441–446. doi: 10.1016/j.sleep.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 10.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170(10):1108–1113. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 12.Ip MS, Lam B, Lauder IJ, et al. A community study of sleep-disordered breathing in middle-aged Chinese men in Hong Kong. Chest. 2001;119(1):62–69. doi: 10.1378/chest.119.1.62. [DOI] [PubMed] [Google Scholar]
- 13.Young T, Shahar E, Nieto FJ, et al. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002;162(8):893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 14.Nuckton TJ, Glidden DV, Browner WS, Claman DM. Physical examination: Mallampati score as an independent predictor of obstructive sleep apnea. Sleep. 2006;29(7):903–908. doi: 10.1093/sleep/29.7.903. [DOI] [PubMed] [Google Scholar]
- 15.Tsuiki S, Isono S, Ishikawa T, Yamashiro Y, Tatsumi K, Nishino T. Anatomical balance of the upper airway and obstructive sleep apnea. Anesthesiology. 2008;108(6):1009–1015. doi: 10.1097/ALN.0b013e318173f103. [DOI] [PubMed] [Google Scholar]
- 16.Lee RW, Vasudavan S, Hui DS, et al. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep. 2010;33(8):1075–1080. doi: 10.1093/sleep/33.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cakirer B, Hans MG, Graham G, Aylor J, Tishler PV, Redline S. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African-Americans. Am J Respir Crit Care Med. 2001;163(4):947–950. doi: 10.1164/ajrccm.163.4.2005136. [DOI] [PubMed] [Google Scholar]
- 18.Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med. 1997;155(1):186–192. doi: 10.1164/ajrccm.155.1.9001310. [DOI] [PubMed] [Google Scholar]
- 19.Lam B, Ip MS, Tench E, Ryan CF. Craniofacial profile in Asian and white subjects with obstructive sleep apnoea. Thorax. 2005;60(6):504–510. doi: 10.1136/thx.2004.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson TM, Patel MR. Waist circumference and sleep disordered breathing. Laryngoscope. 2008;118(2):339–347. doi: 10.1097/MLG.0b013e3181587d7c. [DOI] [PubMed] [Google Scholar]
- 21.Mohsenin V. Gender differences in the expression of sleep-disordered breathing: role of upper airway dimensions. Chest. 2001;120(5):1442–1447. doi: 10.1378/chest.120.5.1442. [DOI] [PubMed] [Google Scholar]
- 22.Hader C, Schroeder A, Hinz M, Micklefield GH, Rasche K. Sleep disordered breathing in the elderly: comparison of women and men. J Physiol Pharmacol. 2005;56(Suppl4):85–91. [PubMed] [Google Scholar]
- 23.Lim YH, Choi J, Kim KR, et al. Sex-specific characteristics of anthropometry in patients with obstructive sleep apnea: neck circumference and waist-hip ratio. Ann Otol Rhinol Laryngol. 2014;123(7):517–523. doi: 10.1177/0003489414526134. [DOI] [PubMed] [Google Scholar]
- 24.Simpson L, Mukherjee S, Cooper MN, et al. Sex differences in the association of regional fat distribution with the severity of obstructive sleep apnea. Sleep. 2010;33(4):467–474. doi: 10.1093/sleep/33.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cairns A, Poulos G, Bogan R. Sex differences in sleep apnea predictors and outcomes from home sleep apnea testing. Nat Sci Sleep. 2016;8:197–205. doi: 10.2147/NSS.S101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165(20):2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 27.Schwab RJ, Leinwand SE, Bearn CB, et al. Digital morphometrics: a new upper airway phenotyping paradigm in OSA. Chest. 2017;152(2):330–342. doi: 10.1016/j.chest.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magalang UJ, Chen NH, Cistulli PA, et al. Agreement in the scoring of respiratory events and sleep among international sleep centers. Sleep. 2013;36(4):591–596. doi: 10.5665/sleep.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magalang UJ, Arnardottir ES, Chen NH, et al. Agreement in the scoring of respiratory events among international sleep centers for home sleep testing. J Clin Sleep Med. 2016;12(1):71–77. doi: 10.5664/jcsm.5398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mateos P. A review of name-based ethnicity classification methods and their potential in population studies. Popul Space Place. 2007;13(4):243–263. [Google Scholar]
- 31.Villaneuva AT, Buchanan PR, Yee BJ, Grunstein RR. Ethnicity and obstructive sleep apnoea. Sleep Med Rev. 2005;9(6):419–436. doi: 10.1016/j.smrv.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Sutherland K, Lee RW, Cistulli PA. Obesity and craniofacial structure as risk factors for obstructive sleep apnoea: impact of ethnicity. Respirology. 2012;17(2):213–222. doi: 10.1111/j.1440-1843.2011.02082.x. [DOI] [PubMed] [Google Scholar]
- 33.Guindalini C, Colugnati FA, Pellegrino R, Santos-Silva R, Bittencourt LR, Tufik S. Influence of genetic ancestry on the risk of obstructive sleep apnoea syndrome. Eur Respir J. 2010;36(4):834–841. doi: 10.1183/09031936.00146809. [DOI] [PubMed] [Google Scholar]
- 34.Parra FC, Amado RC, Lambertucci JR, Rocha J, Antunes CM, Pena SD. Color and genomic ancestry in Brazilians. Proc Natl Acad Sci U S A. 2003;100(1):177–182. doi: 10.1073/pnas.0126614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen X, Wang R, Lutsey PL, et al. Racial/ethnic differences in the associations between obesity measures and severity of sleep-disordered breathing: the Multi-Ethnic Study of Atherosclerosis. Sleep Med. 2016;26:46–53. doi: 10.1016/j.sleep.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deflandre E, Degey S, Brichant JF, Poirrier R, Bonhomme V. Development and validation of a morphologic obstructive sleep apnea prediction score: the DES-OSA score. Anesth Analg. 2016;122(2):363–372. doi: 10.1213/ANE.0000000000001089. [DOI] [PubMed] [Google Scholar]
- 37.Ahn SH, Kim J, Min HJ, et al. Tongue volume influences lowest oxygen saturation but not apnea-hypopnea index in obstructive sleep apnea. PLoS One. 2015;10(8):e0135796. doi: 10.1371/journal.pone.0135796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li KK, Kushida C, Powell NB, Riley RW, Guilleminault C. Obstructive sleep apnea syndrome: a comparison between Far-East Asian and white men. Laryngoscope. 2000;110(10Pt1):1689–1693. doi: 10.1097/00005537-200010000-00022. [DOI] [PubMed] [Google Scholar]
- 39.Ong KC, Clerk AA. Comparison of the severity of sleep-disordered breathing in Asian and Caucasian patients seen at a sleep disorders center. Respir Med. 1998;92(6):843–848. doi: 10.1016/s0954-6111(98)90386-9. [DOI] [PubMed] [Google Scholar]
- 40.Pranathiageswaran S, Badr MS, Severson R, Rowley JA. The influence of race on the severity of sleep disordered breathing. J Clin Sleep Med. 2013;9(4):303–309. doi: 10.5664/jcsm.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med. 1995;152(6Pt1):1946–1949. doi: 10.1164/ajrccm.152.6.8520760. [DOI] [PubMed] [Google Scholar]
- 42.Stevens GA, Singh GM, Lu Y, et al. National, regional, and global trends in adult overweight and obesity prevalences. Popul Health Metr. 2012;10(1):22. doi: 10.1186/1478-7954-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 44.Vagiakis E, Kapsimalis F, Lagogianni I, et al. Gender differences on polysomnographic findings in Greek subjects with obstructive sleep apnea syndrome. Sleep Med. 2006;7(5):424–430. doi: 10.1016/j.sleep.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Whittle AT, Marshall I, Mortimore IL, Wraith PK, Sellar RJ, Douglas NJ. Neck soft tissue and fat distribution: comparison between normal men and women by magnetic resonance imaging. Thorax. 1999;54(4):323–328. doi: 10.1136/thx.54.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finucane MM, Stevens GA, Cowan MJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377(9765):557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mersha TB, Abebe T. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum Genomics. 2015;9:1. doi: 10.1186/s40246-014-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee YL, Teitelbaum S, Wolff MS, Wetmur JG, Chen J. Comparing genetic ancestry and self-reported race/ethnicity in a multiethnic population in New York City. J Genet. 2010;89(4):417–423. doi: 10.1007/s12041-010-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hall JB, Dumitrescu L, Dilks HH, Crawford DC, Bush WS. Accuracy of administratively-assigned ancestry for diverse populations in an electronic medical record-linked biobank. PLoS One. 2014;9(6):e99161. doi: 10.1371/journal.pone.0099161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arnardottir ES, Bjornsdottir E, Olafsdottir KA, Benediktsdottir B, Gislason T. Obstructive sleep apnoea in the general population: highly prevalent but minimal symptoms. Eur Respir J. 2016;47(1):194–202. doi: 10.1183/13993003.01148-2015. [DOI] [PubMed] [Google Scholar]
- 51.Rush EC, Goedecke JH, Jennings C, et al. BMI, fat and muscle differences in urban women of five ethnicities from two countries. Int J Obes (Lond) 2007;31(8):1232–1239. doi: 10.1038/sj.ijo.0803576. [DOI] [PubMed] [Google Scholar]
- 52.Carroll JF, Chiapa AL, Rodriquez M, et al. Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity (Silver Spring) 2008;16(3):600–607. doi: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.