Abstract
Background
The use of anthracyclines is limited by the occurrence of cardiotoxicity. In an effort to prevent this cardiotoxicity, different anthracycline derivates have been studied.
Objectives
To determine the occurrence of cardiotoxicity with the use of different anthracycline derivates in cancer patients.
Search methods
We searched The Cochrane Central Register of Controlled Trials (CENTRAL), (The Cochrane Library, Issue 2, 2009), MEDLINE (1966 to 29 May 2009) and EMBASE (1980 to 2 June 2009). In addition, we searched reference lists of relevant articles, conference proceedings and ongoing‐trials‐databases.
Selection criteria
Randomised controlled trials (RCTs) in which different anthracycline derivates were compared in cancer patients (children and adults).
Data collection and analysis
Two authors independently performed study selection, assessment of risk of bias and data‐extraction including adverse effects.
Main results
We identified five RCTs of varying quality addressing epirubicin versus doxorubicin (1036 patients) with the same dose. The meta‐analysis showed no evidence for a significant difference in the occurrence of clinical heart failure between the treatment groups (RR = 0.36, 95% CI 0.12 to 1.11). However, there is some suggestion of a lower rate of clinical heart failure in patients treated with epirubicin.
We identified two RCTs with varying quality addressing liposomal‐encapsulated doxorubicin versus conventional doxorubicin (521 patients). The meta‐analysis showed a significantly lower rate of both clinical heart failure and clinical and subclinical heart failure combined in patients treated with liposomal‐encapsulated doxorubicin (RR = 0.20, 95% CI 0.05 to 0.75 and RR = 0.38, 95% CI 0.24 to 0.59 respectively). It should be noted that in one of the studies patients in the liposomal‐encapsulated doxorubicin group received a higher cumulative anthracycline dose than patients in the doxorubicin group.
For the other possible combinations of different anthracycline derivates only one RCT (epirubicin versus liposomal‐encapsulated doxorubicin) or no RCT was identified.
Authors' conclusions
We are not able to favour either epirubicin or doxorubicin when given with the same dose. Based on the currently available evidence on heart failure, we conclude that in adults with a solid tumour liposomal‐encapsulated doxorubicin should be favoured over doxorubicin. For both epirubicin versus doxorubicin and liposomal‐encapsulated doxorubicin versus conventional doxorubicin no conclusions can be made about the effects of treatment in children treated with anthracyclines and also not in patients diagnosed with leukaemia. More research is needed. For other combinations of anthracycline derivates not enough evidence was available to make definitive conclusions about the occurrence of cardiotoxicity in patients treated with anthracyclines.
Keywords: Adult; Child; Humans; Antibiotics, Antineoplastic; Antibiotics, Antineoplastic/administration & dosage; Antibiotics, Antineoplastic/adverse effects; Cardiac Output, Low; Cardiac Output, Low/chemically induced; Doxorubicin; Doxorubicin/administration & dosage; Doxorubicin/adverse effects; Epirubicin; Epirubicin/administration & dosage; Epirubicin/adverse effects; Heart; Heart/drug effects; Liposomes; Neoplasms; Neoplasms/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Different anthracycline derivates for reducing cardiotoxicity in cancer patients
Anthracyclines are among the most effective chemotherapy treatments available for various types of cancer. However, there is a risk of damage to the heart depending on the cumulative dose. In an effort to prevent heart damage different anthracycline derivates (like doxorubicin, daunorubicin, and epirubicin) are being used.
The authors found that for the use of many different combinations of anthracycline derivates there was no high quality evidence available and it was impossible to draw conclusions.
For the use of epirubicin versus doxorubicin, there was some suggestion of a lower rate of clinical heart failure in patients treated with epirubicin. There is no evidence which suggests a difference in anti‐tumour response rate and survival between epirubicin and doxorubicin. No conclusions can be made regarding adverse effects. There are no data for children and patients with leukaemia. Further research is needed. For the use of doxorubicin versus liposomal‐encapsulated doxorubicin, the authors found a significantly lower rate of both clinical heart failure and subclinical heart failure (i.e. various cardiac abnormalities, diagnosed with different diagnostic methods like echocardiography in asymptomatic patients) in patients treated with liposomal‐encapsulated doxorubicin. There is no evidence which suggests a difference in anti‐tumour response rate and survival between doxorubicin and liposomal‐encapsulated doxorubicin. A lower rate of adverse effects was identified in patients treated with liposomal‐encapsulated doxorubicin. There are no data for children and patients with leukaemia. Further research is needed.
Background
Anthracyclines are among the most effective chemotherapeutic agents and have gained widespread use in the treatment of numerous solid tumours and hematologic malignancies in both adult and paediatric patients. However, their use is limited by a dose‐dependent cardiotoxicity (Bonadonna 1969; Lefrak 1973).
According to the time of presentation, the heart damage after anthracycline therapy can be divided into early and late cardiotoxicity: early cardiotoxicity refers to heart damage that develops during anthracycline therapy or in the first year after its completion, and late cardiotoxicity manifests itself at least one year after the completion of anthracycline therapy (Shan 1996). The risk of developing heart failure remains a lifelong threat, especially for children and young adults who have a long life‐expectancy after successful antineoplastic treatment. The risk of developing clinical heart failure 20 years after anthracycline therapy for childhood cancer is estimated to be approximately 5.5 per cent (Van Dalen 2006a).
Heart damage can become manifest in patients as either subclinical cardiotoxicity or clinical cardiotoxicity. The term subclinical cardiotoxicity is used to describe various cardiac abnormalities, diagnosed with different diagnostic methods in asymptomatic patients. Examples are histological abnormalities according to the Billingham score (Billingham 1978) or abnormalities in cardiac function measured by echocardiography or radionuclide ventriculography. Clinical cardiotoxicity is defined on the basis of symptoms of clinical heart failure, confirmed by an abnormal diagnostic test. In the end stage of clinical heart failure, heart transplantation is the only remaining option to avoid cardiac death.
In the literature, there is a wide variation in the reported frequency of both clinical and subclinical cardiotoxicity; in children, the prevalence of subclinical cardiac dysfunction has been reported to be more than 57% at a median of 6.4 years after treatment (Kremer 2002a) and the incidence of clinical heart failure as high as 16% 0.9 to 4.8 years after treatment (Kremer 2002b). In adults the prevalence of subclinical cardiac dysfunction has been reported to be 36% during anthracycline therapy (Nousiainen 2002) and the incidence of clinical heart failure 30% at a median of 37 months after treatment (Meinardi 2002). However, we did not perform systematic reviews on the frequency of anthracycline‐induced cardiotoxicity in adults. Possible risk factors (Kremer 2002b; Ng 2006; Simbre 2005) are the type of anthracycline used, the cumulative anthracycline dose, and the presence of additional risk factors for developing heart damage such as radiation therapy involving the heart region, type of tumour, exposure to cyclophosphamide, iphosphamide, amsacrine, trastuzumab or taxanes or the presence of pre‐existing heart damage. There also seems to be a higher risk for females, children and elderly people.
Clinicians confront a clinical dilemma as they balance the efficacy of higher anthracycline doses against the cardiotoxicity associated with these higher doses. In an effort to prevent or reduce this toxicity, extensive research has been devoted to the identification of anthracycline derivates with less cardiotoxic effects than doxorubicin, such as daunorubicin, epirubicin and idarubicin (Muggia 1991) and liposomal anthracyclines (Batist 2001).
An important question regarding any anthracycline derivate is whether it has a lower cardiotoxic effect without reducing the anti‐tumour efficacy and without negative effects on toxicities other than cardiac damage, such as alopecia, nausea, vomiting, stomatitis, diarrhoea, fatigue, anaemia, leukopenia and thrombocytopenia.
This is an update of the first systematic review on the cardiotoxicity of different anthracycline derivates.
Objectives
Primary objective
To determine the cardiotoxicity of any type of anthracycline derivate in patients with cancer when compared to another type of anthracycline derivate.
Secondary objectives:
To determine possible effects of these anthracycline derivates on tumour response and patient survival (i.e. antitumour efficacy).
To determine possible effects of these anthracycline derivates on toxicities other than cardiac damage as well as quality of life (QOL).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing the occurrence of heart damage with the use of any type of anthracycline derivate with another type of anthracycline derivate.
Types of participants
Patients with cancer (both adults and children) who received anthracycline chemotherapy.
Types of interventions
Different types of anthracycline derivates with the same infusion duration and peak dose (i.e. the maximal dose received in one week). Chemotherapy other than anthracyclines and radiotherapy involving the heart region should be the same in both treatment groups. The cumulative anthracycline dose received in both treatment groups should have been mentioned, since otherwise it was impossible to correctly interpret the results of the study.
Types of outcome measures
Primary outcomes
Anthracycline‐induced heart failure (i.e. clinical heart failure (as defined by the authors) and subclinical cardiac dysfunction (defined as either histological abnormalities according to the Billingham‐score on myocardial biopsy (Billingham 1978) or abnormalities in cardiac function measured by echocardiography or radionuclide ventriculography)). If possible, both early and late cardiotoxicity were assessed (early cardiotoxicity refers to heart damage that develops during anthracycline therapy or in the first year after its completion, and late cardiotoxicity manifests itself at least one year after the completion of anthracycline therapy).
Secondary outcomes
Secondary outcomes included potential adverse effects of the different types of anthracycline derivates on:
Tumour response (defined as the number of complete and partial remissions)
Patient survival (progression‐free survival (PFS) and overall survival (OS))
Toxicities other than cardiac damage
QOL
Search methods for identification of studies
Electronic searches
See: Review Group search strategy.
The electronic databases of CENTRAL (Cochrane Library, Issue 2, 2009), MEDLINE/PubMed (from 1966 to 29 May 2009), and EMBASE/Ovid (from 1980 to 2 June 2009) were searched. The search strategies for the different databases (using a combination of subject headings and text word terms) are stated in Appendix 1, Appendix 2 and Appendix 3. The search strategies were designed and executed by the author team. For the update of this review we adapted the search strategies used in the original version of the review (until April 2005). The exact changes are stated in the appendices.
Searching other resources
Information about trials not registered in CENTRAL, MEDLINE, or EMBASE, either published or unpublished, was located by searching the reference lists of relevant articles and review articles. In addition, the conference proceedings of the International Society for Paediatric Oncology (SIOP) and the American Society of Clinical Oncology (ASCO) were handsearched from 2000 to 2008. We searched for ongoing trials by scanning the ISRCTN register and the National Institute of Health Register (both screened June 2009 on www.controlled‐trials.com). If possible, we contacted the investigators of possible eligible trials of which the available data did not provide all data needed to assess whether the study was truly eligible for inclusion in the review. Language restriction was not imposed.
Data collection and analysis
Selection of studies
After employing the search strategy described previously, identification of studies meeting the inclusion criteria was undertaken by two authors independently. Discrepancies between authors were resolved by consensus. No third party arbitration was needed. Any study seemingly meeting the inclusion criteria on grounds of the title, or abstract, or both, was obtained in full for closer inspection.
Data extraction and management
Data extraction was performed independently by two authors using standardised forms. Data of the characteristics of participants (such as age, sex, tumour type), of interventions (such as individual peak dose, cumulative dose), of outcome measures and of length of follow‐up were extracted. Discrepancies were resolved by consensus. No third party arbitration was needed.
Assessment of risk of bias in included studies
The risk of bias in the included trials was assessed by two authors independently according to the following criteria: method of randomization, concealment of treatment allocation, blinding of the care provider, blinding of the patients, blinding of the outcome assessor (for each outcome separately), and completeness of follow‐up (for each outcome separately). The adequacy of allocation concealment was assessed using the criteria proposed by Schulz and colleagues (Schulz 1995). See additional Table 1 for the complete criteria list for the assessment of risk of bias. Discrepancies were resolved by consensus. No third party arbitration was needed.
1. Criteria list for the assessment of risk of bias in included studies.
| Item ID | Description | Implementation |
| Patient selection | Note: all criteria were scored yes (+), no (‐) or unclear (?) | |
| a | Was the allocation of participants to treatment groups randomised? | A random (unpredictable) assignment sequence must have been applied. |
| b | Was the treatment allocation concealed? | Allocation must have been performed by a person not responsible for determining eligibility of patients for inclusion. |
| Interventions | ||
| c | Was the care provider blinded to the intervention? | Adequate information about blinding must have been provided. |
| d | Was the patient blinded to the intervention? | Adequate information about blinding must have been provided. |
| Outcome assessments (for each outcome separately) | ||
| e | Was the outcome assessor blinded to the intervention? | Adequate information about blinding must have been provided. |
| f | Were patients lost to follow‐up described and acceptable? | For each outcome measure the number of evaluated patients must be mentioned. If the percentage of non‐evaluable patients does not exceed 20% a "yes" (+) is scored. |
Data synthesis
Data were entered into RevMan and analysed according to the guidelines of the Cochrane Handbook (Higgins 2005). Dichotomous variables were related to risk using the relative risk / risk ratio (RR). If possible, data were extracted by allocation intervention, irrespective of compliance with the allocated intervention, in order to allow an 'intention‐to‐treat' analysis. If this was not possible, this was stated. Heterogeneity was assessed both by visual inspection of the forest plots and by a formal statistical test for heterogeneity, i.e. the I2‐test (I2 > 50% was considered substantial heterogeneity). If there was evidence of substantial heterogeneity, this was reported. We used a random effects model throughout the review. All results are presented with the corresponding 95% confidence interval (95% CI). If pooling was not possible we provided descriptive results for these studies. We used the generic inverse variance function of RevMan to combine logs of the hazard ratios (HR) for progression‐free survival and overall survival. Where necessary, Parmar's method was used to extract the log of the HR and its standard error (SE) (Parmar 1998). Otherwise, survival was summarised descriptively. An outcome measure was only included in this systematic review if it was the intention of the study to perform the necessary assessments in all randomised patients (i.e. not only optional or only performed in some centers). When less than 50% of the patients of a study had an acceptable follow‐up for a particular outcome measure, due to associated the high risk of attrition bias, we did not report the results of this outcome measure. The risk of bias in studies included in the analyses was taken into account in the interpretation of the review's results. For all outcomes for which pooling was possible we performed sensitivity analyses for all risk of bias criteria separately. We excluded studies with a high risk of bias and studies for which the presence of bias was unclear and compared the results of studies with a low risk of bias with the results of all available studies. It was our intention to perform subgroup analyses for children and adults and for leukaemias and solid tumours, but unfortunately this was not possible (see Results for reasons).
Results
Description of studies
After performing the searches of the electronic databases of CENTRAL, MEDLINE/PubMed and EMBASE/Ovid (2925 references: 684 identified in the update) we included a total of seven articles which fulfilled all the criteria for considering studies for this review (no new studies were identified in the update). From the currently available data of one study it is unclear if this study is eligible for inclusion in this review (identified in the update; see Studies awaiting classification). Fifty‐two articles were excluded after assessing the full text article for reasons described in the Characteristics of excluded studies. The remaining 2865 articles were excluded based on the title and / or abstract since they were not a RCT, were laboratory studies, were animal studies, did not include patients with cancer, did not describe anthracycline therapy with different derivates, were duplicate publications, there was a difference in anthracycline peak dose and / or infusion duration between the treatment groups, there was a difference in chemotherapy other than anthracyclines and / or radiotherapy involving the heart region between the treatment groups, and / or did not have heart failure as an outcome measure.
From scanning the reference lists of relevant articles and reviews one additional article was included in this review. From the currently available data of one study it is unclear if this study is eligible for inclusion in this review (identified in the update; see Characteristics of studies awaiting classification) Ten other articles were added to the Characteristics of excluded studies.
No extra information was obtained from scanning the conference proceedings of SIOP and ASCO, although two additional studies were excluded and thus added to the Characteristics of excluded studies (one identified in the update). Also, from the currently available data of one study it is unclear if this study is eligible for inclusion in this review (identified in the update; see Studies awaiting classification).
Searching the ongoing trial databases identified nine studies, of which one was eligible for this review (identified in the update; see Characteristics of ongoing studies). From five other trials (three identified in the update) we were not able to obtain all the information necessary to assess the eligibility of these trials (see Studies awaiting classification). After obtaining additional information it became clear that the other three studies were not eligible for inclusion in the review (all identified in the update; see Excluded studies).
Therefore, the total number of identified RCTs was eight. Five studies addressed doxorubicin versus epirubicin, two studies addressed conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet) and one study addressed epirubicin versus liposomal‐encapsulated doxorubicin (myocet). For the other combinations of different anthracycline derivates no adequate RCTs were identified.
Description of studies addressing doxorubicin versus epirubicin
Our analysis of the cardiotoxicity of doxorubicin when compared with that of epirubicin included five trials (Brambilla 1986; FESG 1988; Gasparini 1991; IMBSWE 1988; Mouridsen 1984) with a total of 1036 patients. Five‐hundred‐and‐fifteen patients were randomised to treatment with doxorubicin, whereas 521 patients were randomised to treatment with epirubicin. There were no important differences in cumulative anthracycline doses received in both treatment arms of the different RCTs. All studies included adult patients with a solid tumour. In four studies patients were diagnosed with breast cancer (Brambilla 1986; FESG 1988; Gasparini 1991; IMBSWE 1988), and in the other study with soft tissue sarcoma (Mouridsen 1984). In 2 studies the follow‐up of the included patients was more than one year (Brambilla 1986; FESG 1988) and in one study this was possible for at least part of the included patients (IMBSWE 1988). Therefore it is possible that these studies included cases of both early and late cardiotoxicity. In the other studies the length of follow‐up was not mentioned and as a result we don't know if the cases of cardiotoxicity in these studies are early or late. However, based on the fact that all patients included in these trials had advanced or metastatic disease and the associated effect on survival duration, we presume that cases of heart failure in these trials were early cardiotoxicity.
Description of studies addressing conventional doxorubicin versus liposomal‐encapsulated doxorubicin
Our analysis of the cardiotoxicity of conventional doxorubicin when compared with that of liposomal‐encapsulated doxorubicin included two trials (Batist 2001; Harris 2002) with a total of 521 patients. Two‐hundred‐and‐seventy‐one patients were randomised to treatment with doxorubicin, whereas 250 patients were randomised to treatment with liposomal‐encapsulated doxorubicin. Both studies mentioned the cumulative anthracycline dose patients in the treatment groups received: in the study of Batist 2001 patients in both treatment groups received a median cumulative dose of 360 mg/m2, whereas in the study of Harris 2002 patients in the doxorubicin group received a median cumulative dose of 570 mg/m2 and patients in the liposomal‐encapsulated doxorubicin group received a cumulative dose of 785 mg/m2. Both studies included adult patients with breast cancer. In one study the follow‐up of the included patients was more than one year (Batist 2001) and therefore it is possible that this study included cases of both early and late cardiotoxicity. In the other study the length of follow‐up was not mentioned and as a result we don't know if the cases of cardiotoxicity in this study are early or late. However, based on the fact that all patients included in this trial had metastatic disease and the associated effect on survival duration, we presume that cases of heart failure in this trial were early cardiotoxicity.
Description of the study addressing epirubicin versus liposomal‐encapsulated doxorubicin
Our analysis of the cardiotoxicity of epirubicin when compared with that of liposomal‐encapsulated doxorubicin included one trial (Chan 2004) with a total of 160 patients. Eighty patients were randomised to treatment with epirubicin, whereas 80 patients were randomised to treatment with liposomal‐encapsulated doxorubicin. The cumulative anthracycline dose patients in both treatment groups received was comparable. All patients included in this study were adults with breast cancer. The follow‐up of at least part of the included patients was more than one year and therefore it is possible that this study included cases of both early and late cardiotoxicity.
Risk of bias in included studies
See additional Table 1 for the criteria list for the assessment of risk of bias.
Risk of bias in studies addressing doxorubicin versus epirubicin
In all five studies the allocation of patients to the treatment groups was randomised, but none of the studies did specify the presence of a concealed treatment allocation (Brambilla 1986; FESG 1988; Gasparini 1991; IMBSWE 1988; Mouridsen 1984).
It was unclear if the care provider and patients were blinded to treatment in all five studies.
For blinding of the outcome assessor we scored each different outcome, with the exception of overall survival, since for that outcome blinding was not relevant. For all evaluated outcomes (i.e. clinical heart failure, subclinical heart failure, response rate, progression‐free survival and adverse effects) it was unclear if the outcome assessor was blinded to treatment in all studies evaluating the outcome.
Patients lost to follow‐up were also scored for each different outcome. For clinical heart failure the number of patients lost to follow‐up was described and acceptable (i.e. less than 20%) in three studies (FESG 1988; Gasparini 1991; IMBSWE 1988), in one study it was unclear (Brambilla 1986) and in one study it was unacceptable (Mouridsen 1984). For subclinical heart failure (both as a dichotomous and continuous outcome) the number of patients lost to follow‐up was unacceptable in the one study describing this outcome (Brambilla 1986). For response rate the number of patients lost to follow‐up was described and acceptable in four studies (Brambilla 1986; FESG 1988; Gasparini 1991; IMBSWE 1988), whereas in one study it was not (Mouridsen 1984). For progression‐free survival the number of patients lost‐to‐follow‐up was described and acceptable in three studies (FESG 1988; Gasparini 1991; IMBSWE 1988), whereas in two studies it was not (Brambilla 1986; Mouridsen 1984). Overall survival was evaluated in five studies, in four of them the number of patients lost to follow‐up was described and acceptable (Brambilla 1986; FESG 1988; Gasparini 1991; IMBSWE 1988), whereas in the other study it was not (Mouridsen 1984). Finally, for the assessment of adverse effects the number of patients lost‐to‐follow‐up was described and acceptable in two studies (Gasparini 1991; IMBSWE 1988), whereas in the other study it was not (FESG 1988). Please note that in the study of IMBSWE 1988 the number of patients lost‐to‐follow‐up was described and acceptable for all adverse effects, with the exception of alopecia.
See additional Table 2 for the exact scores per included study.
2. Risk of bias assessment in included studies.
| Study | a | b | c | d | e | f | Intervention |
| Batist 2001 | + | ? | ? | ? | Clinical heart failure, subclinical heart failure, tumour response, PFS: +; adverse effects: ? | Clinical heart failure, subclinical heart failure, tumour response, PFS, OS, adverse effects: + | Doxorubicin versus liposomal‐encapsulated doxorubicin (myocet). |
| Brambilla 1986 | + | ? | ? | ? | Clinical heart failure, subclinical heart failure, tumour response, PFS: ? | Clinical heart failure: ?; subclinical heart failure, PFS: ‐; tumour response, OS: + | Doxorubicin versus epirubicin. |
| Chan 2004 | + | ? | ? | ? | Clinical heart failure, subclinical heart failure, tumour response, PFS, adverse effects: ? | Clinical heart failure, subclinical heart failure, tumour response, PFS, OS, adverse effects: + | Epirubicin versus liposomal‐encapsulated doxorubicin (myocet). |
| FESG 1998 | + | ? | ? | ? | Clinical heart failure, tumour response, PFS, adverse effects: ? | Clinical heart failure, tumour response, PFS, OS: +; adverse effects: ‐ | Doxorubicin versus epirubicin. |
| Gasparini 1991 | + | ? | ? | ? | Clinical heart failure, tumour response, PFS, adverse effects: ? | Clinical heart failure, tumour response, PFS, OS, adverse effects: + | Doxorubicin versus epirubicin. |
| Harris 2002 | + | ? | ? | ? | Clinical heart failure, adverse effects: ?; subclinical heart failure, tumour response, PFS: + | Clinical heart failure, subclinical heart failure, tumour response, PFS, OS, adverse effects: + | Doxorubicin versus liposomal‐encapsulated doxorubicin (myocet). |
| IMBSWE 1988 | + | ? | ? | ? | Clinical heart failure, tumour response, adverse effects: ? | Clinical heart failure, tumour response, PFS, OS, adverse effects (with the exception of alopecia: ‐): + | Doxorubicin versus epirubicin. |
| Mouridsen 1984 | + | ? | ? | ? | Clinical heart failure, tumour response, PFS: ? | Clinical heart failure, tumour response, PFS, OS: ‐ | Doxorubicin versus epirubicin. |
PFS = progression‐free survival; OS = overall survival
In conclusion, the risk of bias in the included studies varied and bias could not be ruled out in the following percentages of included studies: selection bias (based on method of randomisation and concealment of allocation) 100%, performance bias (based on blinding of the care provider and patient) 100%, detection bias (based on blinding of the outcome assessor) 100% for all evaluated outcomes, and finally attrition bias (based on the completeness of follow‐up) 40% for clinical heart failure, 100% for subclinical heart failure (both as a dichotomous and continuous outcome), 20% for response rate, 40% for progression‐free survival, 20% for overall survival, and 33% for adverse effects.
Risk of bias in studies addressing conventional doxorubicin versus liposomal‐encapsulated doxorubicin
In both studies the allocation of patients to the treatment groups was randomised, but it was unclear if the treatment allocation was concealed (Batist 2001; Harris 2002). It was unclear if the care provider and patients were blinded to treatment in both studies.
For blinding of the outcome assessor we scored each different outcome, with the exception of overall survival, since for that outcome blinding was not relevant. In one of the two studies evaluating clinical heart failure it was unclear if the outcome assessor was blinded to treatment (Harris 2002). The outcome assessor was blinded to treatment in both studies evaluating subclinical heart failure, response rate and progression‐free survival. It was unclear if the outcome assessor was blinded to treatment in both studies evaluating adverse effects.
Patients lost to follow‐up were also scored for each different outcome. For clinical heart failure, subclinical heart failure, tumour response, progression‐free survival, overall survival and adverse effects the number of patients lost to follow‐up was described and acceptable (i.e. less than 20%) in both studies.
See additional Table 2 for the exact scores per included study.
In conclusion, bias could not be ruled out in the following percentages of included studies: selection bias (based on method of randomisation and concealment of allocation) 100%, performance bias (based on blinding of the care provider and patient) 100%, detection bias (based on blinding of the outcome assessor) 50% for clinical heart failure, 0% for subclinical heart failure, response rate and progression‐free survival and 100% for adverse effects, and finally attrition bias (based on the completeness of follow‐up) 0% for all evaluated outcomes.
Risk of bias in the study addressing epirubicin versus liposomal‐encapsulated doxorubicin
The allocation of patients to the treatment groups was randomised, but it was unclear if the treatment allocation was concealed (Chan 2004). It was unclear if the care provider and patients were blinded to treatment.
For blinding of the outcome assessor we scored each different outcome, with the exception of overall survival, since for that outcome blinding was not relevant. For clinical heart failure, subclinical heart failure, tumour response, progression‐free survival, and adverse effects it was unclear if the outcome assessor was blinded to treatment.
Patients lost to follow‐up were also scored for each different outcome. For all outcomes evaluated in this study (i.e. the above mentioned and overall survival) the number of patients lost to follow‐up was described and acceptable (i.e. less than 20%). See additional Table 2 for the exact scores per included study.
In conclusion, in this study selection bias, performance bias, and detection bias (for all evaluated outcomes) could not be ruled out.
Effects of interventions
Not all articles allowed data extraction for all outcomes (see Characteristics of included studies for a more detailed description of the extractable outcomes of each study).
Studies addressing doxorubicin versus epirubicin
Clinical heart failure
We could collect data on clinical heart failure from five trials with a total of 1036 adult patients with a solid tumour (Brambilla 1986; FESG 1988; Gasparini 1991; IMBSWE 1988; Mouridsen 1984). There were three cases of clinical heart failure among 521 patients randomised to epirubicin and 12 cases among 515 patients randomised to doxorubicin. The meta‐analysis showed no significant difference in the occurrence of clinical heart failure in the treatment groups (RR = 0.36, 95% CI 0.12 to 1.11, P = 0.07). However, there is some suggestion of a lower rate of clinical heart failure in patients treated with epirubicin. No heterogeneity was detected (I2 = 0%).
In two studies the follow‐up of the included patients was more than one year (Brambilla 1986; FESG 1988) and in one study it was possible that part of the included patients had a follow‐up of more than one year (IMBSWE 1988), therefore it is possible that these studies included cases of both early and late cardiotoxicity. In the other studies the length of follow‐up was not mentioned (Gasparini 1991; Mouridsen 1984) and as a result we don't know if the cases of cardiotoxicity in these studies are early or late. However, based on the fact that all patients included in these trials had metastatic or advanced disease and the associated effect on survival duration, we presume that cases of heart failure in these trials were early cardiotoxicity.
Clinical and subclinical heart failure combined
Data on clinical and subclinical heart failure combined could be extracted from one trial including adult patients with a solid tumour (Brambilla 1986). However, due to the high risk of attrition bias (less than 50% of the patients had an acceptable follow‐up), results of this study are not reported.
Subclinical heart failure as a continuous outcome
We could collect data on subclinical heart failure described as a continuous outcome from one trial including adult patients with a solid tumour (Brambilla 1986). However, due to the high risk of attrition bias (less than 50% of the patients had an acceptable follow‐up), results of this study are not reported.
Tumour response
Data on response rate could be extracted from five trials with a total of 1036 adult patients with a solid tumour (Brambilla 1986; FESG 1988; Gasparini 1991; IMBSWE 1988; Mouridsen 1984). These trials used comparable criteria to assess tumour response (see Characteristics of included studies). There were 210 complete or partial responses among 521 patients randomised to epirubicin and 221 among 515 patients randomised to doxorubicin. The meta‐analysis showed no significant difference in the response rate between the treatment groups (RR = 0.94, 95% CI 0.82 to 1.08, P = 0.40). No heterogeneity was detected (I2 = 0%). Only one study mentioned that the response rate was determined by at least two observers (Mouridsen 1984).
Please note that due to the nature of this measurement (i.e. the percentage of patients with a remission) a high event rate is favourable. Therefore, in the figure of this analysis, "favours doxorubicin" is on the left and "favours epirubicin" is on the right, as opposed to the figures of the other analyses.
Survival
Data on progression‐free survival were presented in 5 trials, but only 2 trials with a total of 446 adults with a solid tumour (FESG 1988; Mouridsen 1984) could be included in the meta‐analysis. The meta‐analysis showed no significant difference between the treatment groups (HR=1.05; 95% CI 0.76 to 1.44; P = 0.78). However, unexplained heterogeneity was detected (I2 = 59%).
We excluded the study of Brambilla 1986 from this analysis due to the high risk of attrition bias (less than 50% of the patients had an acceptable follow‐up). We excluded the studies of Gasparini 1991 and IMBSWE 1988 from this analysis because we were not able to reliably extract data needed to use Parmar's method for the assessment of survival for this study. However, for descriptive results see additional Table 3. In all individual studies no significant differences between the treatment arms were identified.
3. Descriptive results of survival: epirubicin versus doxorubicin.
| Study | Progression‐free survival | Overall survival |
| Brambilla 1986 | Not evaluated in review | Median: in epirubicin group > 16 months (7‐25+), in doxorubicin group > 18 months (6‐28+) (no significant difference) |
| FESG 1988 | Median: in epirubicin group 220 days (30‐1230), in doxorubicin group 270 days (30‐1380) (no significant difference) | Median: in epirubicin group 450 days (20‐1582), in doxorubicin group 530 days (36‐1681) (no significant difference) |
| Gasparini 1991 | In epirubicin group range 2 to 11 months, in doxorubicin group 3 to 14 months (P=0.91) | Median: in epirubicin group 12 months, in doxorubicin group 11 months (no significant difference) |
| IMBSWE 1988 | Median: in epirubicin group 273 days, in doxorubicin group 314 days (P=0.59) | Median: in epirubicin group 591 days, in doxorubicin group 613 days (P=0.75) |
| Mouridsen 1987 | No significant differences between both treatment groups (P=0.41) | No significant differences between both treatment groups (P=0.90) |
Data on overall survival were presented in 5 trials, but only 2 trials with a total of 245 adults with a solid tumour (Gasparini 1991; Mouridsen 1984) could be included in the meta‐analysis. The meta‐analysis showed no significant difference between the treatment groups (HR=0.95; 95% CI 0.65 to 1.39; P=0.79). No heterogeneity was detected (I2=0%).
We excluded the studies of Brambilla 1986, FESG 1988 and IMBSWE 1988 from this analysis because we were not able to reliably extract data needed to use Parmar's method for the assessment of survival for this study. However, for descriptive results see additional Table 3. In all individual studies no significant differences between the treatment arms were identified.
Adverse effects
Since all patients receiving chemotherapy will suffer from side effects, we decided to analyse only the severe and life threatening effects. We defined this as grade 3 or 4 toxicity. All studies used the WHO criteria (Miller 1981; WHO Handbook 1979). Therefore, it was possible to perform meta‐analyses for adverse effects for which more than 1 RCT was available. For adverse effects for which 1 RCT was available, we provide descriptive results (all the mentioned RR, 95%CI and P‐values are calculated in RevMan with the random effects model).
Anaemia
Data on anaemia could be extracted from two trials with a total of 546 adult patients with a solid tumour (Gasparini 1991; IMBSWE 1988). However, in one study there were no cases of anaemia in both treatment groups and therefore, the not significantly different results of this study were not estimable for analysis of the RR (Gasparini 1991). As a result, pooling of results was not possible. In the other study (IMBSWE 1988), there was one case of anaemia grade 3 or 4 among 250 patients randomised to epirubicin and nine among 247 patients randomised to doxorubicin. This was a significant difference in favour of epirubicin (RR = 0.11, 95% CI 0.01 to 0.86, P = 0.04).
Leukopenia
Data on leukopenia could be extracted from two trials with a total of 546 adult patients with a solid tumour (Gasparini 1991; IMBSWE 1988). There were 23 cases of leukopenia grade 3 or 4 among 275 patients randomised to epirubicin and 46 among 271 patients randomised to doxorubicin. The meta‐analysis showed a significantly lower rate of leukopenia grade 3 or four in patients treated with epirubicin as compared to patients treated with doxorubicin (RR = 0.50, 95% CI 0.31 to 0.80, P = 0.004). No heterogeneity was detected (I2 = 0%).
Nausea and vomiting
Data on nausea / vomiting could be extracted from two trials with a total of 546 adult patients with a solid tumour (Gasparini 1991; IMBSWE 1988). There were 79 cases of nausea / vomiting grade 3 or 4 among 275 patients randomised to epirubicin and 102 among 271 patients randomised to doxorubicin. The meta‐analysis showed a significantly lower rate of nausea / vomiting grade 3 or 4 in patients treated with epirubicin as compared to patients treated with doxorubicin (RR = 0.77, 95% CI 0.61 to 0.97, P = 0.03). No heterogeneity was detected (I2 = 0%).
Alopecia
Data on alopecia could be extracted from three trials with a total of 796 adult patients with a solid tumour (Gasparini 1991; FESG 1988; IMBSWE 1988). There were 128 cases of alopecia grade 3 or 4 among 402 patients randomised to epirubicin and 139 among 394 patients randomised to doxorubicin. The meta‐analysis showed no significant difference in the occurrence of alopecia grade 3 or 4 between the treatment groups (RR=0.85, 95% CI 0.52 to 1.38, P=0.51). However, unexplained heterogeneity was detected (I2 = 71%).
Thrombocytopenia
Two trials with a total of 546 adult patients with a solid tumour evaluated thrombocytopenia grade 3 or 4. However, in one study (Gasparini 1991) there were no cases of thrombocytopenia grade 3 or 4 in both treatment groups and therefore, the results of this study are not estimable for analysis of the RR. As a result, pooling of results was not possible. In the other study (IMBSWE 1988) there were four cases of thrombocytopenia grade 3 or 4 in both treatment groups (RR = 0.99, 95% CI 0.25 to 3.91, P=0.99), so in both studies no significant difference in the occurrence of thrombocytopenia grade 3 or 4 between the treatment groups was detected.
Infection
One trial with a total of 49 adult patients with a solid tumour evaluated infection grade 3 or 4 (Gasparini 1991). However, there were no cases of infection grade 3 or 4 in both treatment groups and therefore, the results of this study are not estimable for analysis of the RR, but no significant difference in the occurrence of infection grade 3 or 4 between the treatment groups was detected.
Stomatitis and mucositis
Two trials with a total of 546 adult patients with a solid tumour evaluated stomatitis / mucositis grade 3 or 4. However, in one study (Gasparini 1991) there were no cases of stomatitis / mucositis grade 3 or 4 in both treatment groups and therefore, the results of this study are not estimable for analysis of the RR. As a result, pooling of results was not possible. In the other study (IMBSWE 1988) there were six cases of stomatitis / mucositis grade 3 or 4 among 250 patients randomised to epirubicin and 8 among 247 patients randomised to doxorubicin. This was not a significant difference (RR = 0.74, 95% CI 0.26 to 2.10, P = 0.57), so in both studies no significant difference in the occurrence of stomatitis / mucositis grade 3 or 4 between the treatment groups was detected.
Thrombophlebitis
One trial with a total of 49 adult patients with a solid tumour evaluated thrombophlebitis grade 3 or 4 (Gasparini 1991). However, there were no cases of thrombophlebitis grade 3 or 4 in both treatment groups and therefore, the results of this study are not estimable for analysis of the RR, but no significant difference in the occurrence of thrombophlebitis grade 3 or 4 between the treatment groups was detected.
Other adverse effects
For hepatic dysfunction related to drug administration (Gasparini 1991), and renal dysfunction related to drug administration (Gasparini 1991), there were no cases in both treatment groups of the one study evaluating the adverse effect, and thus no significant differences in the occurrence of the evaluated outcome.
Quality of life
None of the studies evaluated QOL.
Subgroup analyses
Since all patients were adults with a solid tumour, subgroup analyses for children versus adults and leukaemias versus solid tumours were not performed.
Sensitivity analyses
The results of the sensitivity analyses for the risk of bias criteria were consistent among the trials and did not differ from the overall analyses.
Studies addressing conventional doxorubicin versus liposomal‐encapsulated doxorubicin
Clinical heart failure
We collected data on clinical heart failure from two trials with a total of 521 adult patients with breast cancer (Batist 2001; Harris 2002). There were 14 cases of clinical heart failure among 271 patients randomised to conventional doxorubicin and two cases among 250 patients randomised to liposomal‐encapsulated doxorubicin. The meta‐analysis showed a statistically significant lower rate of clinical heart failure in patients treated with liposomal‐encapsulated doxorubicin as compared to treatment with conventional doxorubicin (RR = 0.20, 95% CI 0.05 to 0.75, P = 0.02). No heterogeneity was detected (I2 = 0%).
In one study the follow‐up of the included patients was more than one year (Batist 2001) and therefore it is possible that this study included cases of both early and late cardiotoxicity. In the other study the length of follow‐up was not mentioned and as a result we do not know if the cases of cardiotoxicity in these studies are early or late. However, based on the fact that all patients included in this trial had metastatic disease and the associated effect on survival duration, we presume that cases of heart failure in these trials were early cardiotoxicity.
Clinical and subclinical heart failure combined
Data on clinical and subclinical heart failure combined could be extracted from 2 trials with a total of 521 adult patients with breast cancer (Batist 2001; Harris 2002). There were 67 cases of clinical and subclinical heart failure combined among 271 patients randomised to conventional doxorubicin and 23 cases among 250 patients randomised to liposomal‐encapsulated doxorubicin. The meta‐analysis showed a statistically significant lower rate of clinical and subclinical heart failure combined in patients treated with liposomal‐encapsulated doxorubicin as compared to treatment with conventional doxorubicin (RR = 0.38, 95% CI 0.24 to 0.59, P < 0.0001). No heterogeneity was detected (I2 = 0%).
In one study the follow‐up of the included patients was more than one year (Batist 2001) and therefore it is possible that this study included cases of both early and late cardiotoxicity. In the other study the length of follow‐up was not mentioned and as a result we don't know if the cases of cardiotoxicity in these studies are early or late. However, based on the fact that all patients included in this trial had metastatic disease and the associated effect on survival duration, we presume that cases of heart failure in these trials were early cardiotoxicity.
For both studies it should be noted that patients who suffered from clinical heart failure are also included in the meta‐analysis of clinical heart failure as mentioned above.
Tumour response
Data on response rate could be extracted from two trials with a total of 521 adult patients with breast cancer (Batist 2001; Harris 2002). These trials used comparable criteria to assess tumour response (see Characteristics of included studies). There were 96 complete or partial responses among 271 patients randomised to conventional doxorubicin and 89 among 250 patients randomised to liposomal‐encapsulated doxorubicin. The meta‐analysis showed no significant difference in the response rate between the treatment groups (RR = 1.01, 95% CI 0.80 to 1.26, P = 0.95). No heterogeneity was detected (I2 = 0%). None of the studies mentioned that the response rate was determined by at least two observers.
Please note that due to the nature of this measurement (i.e. the percentage of patients with a remission) a high event rate is favourable. Therefore, in the figure of this analysis, "favours doxorubicin" is on the left and "favours liposomal‐encapsulated doxorubicin" is on the right, as opposed to the figures of the other analyses.
Survival
Data on survival could be extracted from two trials with a total of 521 adult patients with breast cancer (Batist 2001; Harris 2002). Both studies presented HRs with 95% CIs, making it possible to use Parmar's method for the assessment of survival (Parmar 1998).
For the progression‐free survival the meta‐analysis showed no significant difference between patients treated with liposomal‐encapsulated doxorubicin and patients treated with conventional doxorubicin (HR = 1.01, 95% CI 0.83 to 1.24, P=0.89). No heterogeneity was detected (I² =0%).
For the overall survival the meta‐analysis also showed no significant difference between patients treated with liposomal‐encapsulated doxorubicin and patients treated with conventional doxorubicin (HR = 1.12, 95% CI 0.83 to 1.53, P = 0.46). No heterogeneity was detected (I² = 50%).
Adverse effects
Data on adverse effects could be extracted from two trials with a total of 521 adult patients with breast cancer (Batist 2001; Harris 2002). Since all patients receiving chemotherapy will suffer from side effects, we decided to analyse only the severe and life threatening effects. We defined this as grade 3 or 4 toxicity. Both studies used the CTC (common toxicity criteria) of the National Cancer Institute. Therefore it was possible to perform meta‐analyses.
Anaemia
There were 73 cases of anaemia grade > =3 among 271 patients randomised to conventional doxorubicin and 56 among 250 patients randomised to liposomal‐encapsulated doxorubicin. The meta‐analysis showed no significant difference in the occurrence of anaemia grade > =3 between the treatment groups (RR = 0.83, 95% CI 0.61 to 1.13, P = 0.23). No heterogeneity was detected (I2 =0 %).
Thrombocytopenia
There were 20 cases of thrombocytopenia grade >=3 among 271 patients randomised to conventional doxorubicin and also 20 among 250 patients randomised to liposomal‐encapsulated doxorubicin. The meta‐analysis showed no significant difference in the occurrence of thrombocytopenia grade > =3 between the treatment groups (RR = 1.09, 95% CI 0.60 to 1.97, P = 0.78). No heterogeneity was detected (I2 = 0%). Please note that thrombocytopenia grade >=3 in this meta‐analysis was defined as platelets < 20*109/L, whereas according to the CTC‐criteria grade 3 starts with platelets <50*109. The study of Batist 2001 also reported patients with platelets <50*109 and again no significant difference between the treatment groups was identified (P = 0.78 as reported by the authors).
Neutropenia
There were 184 cases of neutropenia grade 4 among 271 patients randomised to conventional doxorubicin and 140 among 250 patients randomised to liposomal‐encapsulated doxorubicin. The meta‐analysis showed a significantly lower rate of neutropenia grade 4 in patients treated with liposomal‐encapsulated doxorubicin as compared to patients treated with conventional doxorubicin (RR = 0.82, 95% CI 0.72 to 0.94, P = 0.005). No heterogeneity was detected (I2 =0%). The study of Batist 2001 also reported patients with prolonged neutropenia grade 4 (defined as seven days or longer) and again no significant difference between the treatment groups was identified (P = 0.18 as reported by the authors).
Neutropenic fever
There were 31 cases of neutropenic fever (i.e fever > = 38ºC, neutropenia grade 4 and IV antibiotics and/or hospitalisation) among 271 patients randomised to conventional doxorubicin and 25 among 250 patients randomised to liposomal‐encapsulated doxorubicin. The meta‐analysis showed no significant difference in the occurrence of neutropenic fever between the treatment groups (RR = 0.88, 95% CI 0.53 to 1.45, P = 0.61). No heterogeneity was detected (I2 = 0%).
Nausea and vomiting
There were 53 cases of nausea / vomiting grade > =3 among 271 patients randomised to conventional doxorubicin and 32 among 250 patients randomised to liposomal‐encapsulated doxorubicin. The meta‐analysis showed a significantly lower rate of nausea / vomiting grade >=3 in patients treated with liposomal‐encapsulated doxorubicin as compared to patients treated with conventional doxorubicin (RR = 0.65, 95% CI 0.44 to 0.98, P = 0.04). No heterogeneity was detected (I2 = 0%).
Stomatitis and mucositis
There were 28 cases of stomatitis / mucositis grade > =3 among 271 patients randomised to conventional doxorubicin and 15 among 250 patients randomised to liposomal‐encapsulated doxorubicin. The meta‐analysis showed no significant difference in the occurrence of stomatitis / mucositis grade > =3 between the treatment groups (RR = 0.58, 95% CI 0.32 to 1.05, P = 0.07). No heterogeneity was detected (I2 = 0%).
Diarrhoea
There were 17 cases of diarrhoea grade >=3 among 271 patients randomised to conventional doxorubicin and 5 among 250 patients randomised to liposomal‐encapsulated doxorubicin. The meta‐analysis showed a significantly lower rate of diarrhoea grade >=3 in patients treated with liposomal‐encapsulated doxorubicin as compared to patients treated with conventional doxorubicin (RR = 0.33, 95% CI 0.12 to 0.87, P = 0.03). No heterogeneity was detected (I2 = 0%).
Asthenia and fatigue
There were 30 cases of asthenia / fatigue grade 3 among 271 patients randomised to conventional doxorubicin and 24 among 250 patients randomised to liposomal‐encapsulated doxorubicin. The meta‐analysis showed no significant difference in the occurrence of asthenia / fatigue grade 3 between the treatment groups (RR = 0.85, 95% CI 0.52 to 1.41, P = 0.54). No heterogeneity was detected (I2 = 0%).
Cutaneous
There were two cases of cutaneous toxicity grade 3 among 271 patients randomised to conventional doxorubicin and one among 250 patients randomised to liposomal‐encapsulated doxorubicin. The meta‐analysis showed no significant difference in the occurrence of cutaneous toxicity grade 3 between the treatment groups (RR = 0.68, 95% CI 0.08 to 5.45, P = 0.71). No heterogeneity was detected (I2 =0%).
Infection
There were 26 cases of infection grade > =3 among 271 patients randomised to conventional doxorubicin and 21 among 250 patients randomised to liposomal‐encapsulated doxorubicin. The meta‐analysis showed no significant difference in the occurrence of infection grade >=3 between the treatment groups (RR=0.78, 95% CI 0.21 to 2.89, P=0.71). However, unexplained heterogeneity was detected (I2 = 78%).
Quality of life
None of the studies evaluated QOL.
Subgroup analyses
Since all patients were adults with a solid tumour, subgroup analyses for children versus adults and leukaemias versus solid tumours were not performed.
Study addressing epirubicin versus liposomal‐encapsulated doxorubicin
Due to the absence of more than one RCT, for epirubicin versus liposomal‐encapsulated doxorubicin pooling of results was not possible. We therefore provide descriptive results of this study. All the mentioned RR, 95% CI and P‐values are calculated in RevMan with the random effects model.
Clinical heart failure
In the study of Chan 2004 there were no cases of clinical heart failure in both treatment groups and therefore, the results of this study are not estimable for analysis of the RR. However, no significant difference in the occurrence of clinical heart failure between the treatment groups was identified. All patients included in this study were adults with breast cancer.
Clinical and subclinical heart failure combined
In the study of Chan 2004 there were eight cases of clinical and subclinical heart failure combined among 80 patients randomised to epirubicin and nine cases among 80 patients randomised to liposomal‐encapsulated doxorubicin. The analysis showed no significant difference in the occurrence of clinical and subclinical heart failure between the treatment groups (RR = 1.13, 95% CI 0.46 to 2.77, P = 0.80). All patients included in this study were adults with breast cancer. The follow‐up of at least part of the included patients was more than one year and therefore it is possible that this study included cases of both early and late cardiotoxicity.
Tumour response
In the study of Chan 2004 there were 31 complete or partial responses among 80 patients randomised to epirubicin and 37 among 80 patients randomised to liposomal‐encapsulated doxorubicin. The analysis showed no significant difference in the response rate between the treatment groups (RR = 1.19, 95% CI 0.83 to 1.72, P = 0.34). It was not mentioned that the response rate was determined by at least two observers. All patients included in this study were adults with breast cancer. Please note that due to the nature of this measurement (i.e. the percentage of patients with a remission) a high event rate is favourable.
Survival
In the study of Chan 2004 a significant difference in progression‐free survival in favour of liposomal‐encapsulated doxorubicin was identified. Patients randomised to epirubicin had a median progression‐free survival of 5.6 months and patients randomised to liposomal‐encapsulated doxorubicin 7.7 months (HR = 1.52, 95% CI 1.06 to 2.20 as reported by the authors). However, no significant differences in overall survival were found between the treatment arms. Patients randomised to epirubicin had a median overall survival of 16 months and patients randomised to liposomal‐encapsulated doxorubicin 18.3 months (HR = 1.15, 95% CI 0.77 to 1.72 as reported by the authors).
Adverse effects
Since all patients receiving chemotherapy will suffer from side effects, we decided to analyse only the severe and life threatening effects. We defined this as grade 3 or 4 toxicity. Chan 2004 used the common toxicity criteria (CTC) of the National Cancer Institute. Results are shown in additional Table 4. Neutropenia grade 4 occurred significantly more often in the patients randomised to treatment with liposomal‐encapsulated doxorubicin. For all the other evaluated adverse effects no significant differences between the treatment groups were identified.
4. Adverse effects: epirubicin versus liposomal‐encapsulated doxorubicin (myocet).
| Adverse effect | n/N myocet patients | n/N epirubicin patients | RR (95% CI) | P‐value |
| Anaemia grade >=3 | 19/80 | 11/80 | 1.73 (0.88 ‐ 3.39) | 0.11 |
| Thrombocytopenia grade >=3 | 3/80 | 2/80 | 1.50 (0.26 ‐ 8.74) | 0.65 |
| Neutropenia grade 4 | 66/80 | 52/80 | 1.17 (1.05 ‐ 1.53) | 0.01 |
| Prolonged neutropenia grade 4 (>=7 days) | 20/80 | 24/80 | 0.83 (0.50 ‐ 1.38) | 0.48 |
| Febrile neutropenia (fever >=38 C, neutropenia grade 4, IV antibiotics and/or hospitalisation) | 4/80 | 1/80 | 4.00 (0.46 to 35.01) | 0.21 |
| Infection grade >=3 | 5/80 | 1/80 | 5.00 (0.60 ‐ 41.85) | 0.14 |
| Nausea / vomiting grade >=3 | 16/80 | 15/80 | 1.07 (0.57 ‐ 2.01) | 0.84 |
| Stomatitis / mucositis grade 3 | 5/80 | 0/80 | 11.00 (0.62 ‐ 195.69) | 0.10 |
| Diarrhoea grade 3 | 1/80 | 1/80 | 1.00 (0.06 ‐ 15.71) | 1.00 |
| Asthenia / fatigue grade 3 | 0/80 | 1/80 | 0.33 (0.01 ‐ 8.06) | 0.50 |
| Cutaneous grade 3 | 0/80 | 1/80 | 0.33 (0.01 ‐ 8.06) | 0.50 |
| Injection site toxicity grade 3 | 0/80 | 1/80 | 0.33 (0.01 ‐ 8.06) | 0.50 |
IV = intravenous; n = number of patients with adverse effect; N = total number of patients in group; RR = risk ratio/relative risk; CI = confidence interval
Quality of life
QOL was not evaluated in this study.
Subgroup analyses
Since all patients were adults with a solid tumour, subgroup analyses for children versus adults and leukaemias versus solid tumours were not performed.
Discussion
Heart damage due to anthracycline chemotherapy is a considerable and serious problem. It reduces QOL and can even cause premature death. Also, when heart damage occurs during therapy the maximum cumulative dose of anthracyclines needs to be limited and as a result the efficacy of anthracycline chemotherapy will be reduced. This is an update of the first systematic review evaluating the existing evidence on different anthracycline derivates for reducing cardiotoxicity. Only RCTs were included since it is widely recognized that a RCT is the only study design which can be used to obtain unbiased evidence on the use of anthracycline derivates, provided that the design and execution are adequate.
We could identify RCTs for three combinations of different anthracycline derivates, i.e. epirubicin versus doxorubicin, liposomal‐encapsulated doxorubicin (myocet) versus doxorubicin and liposomal‐encapsulated doxorubicin (myocet) versus epirubicin. For the other 25 combinations of different anthracycline derivates (see search strategy) no adequate RCTs could be identified.
For epirubicin versus doxorubicin five trials were identified.
Our meta‐analysis of five trials showed no evidence for a significant difference in the occurrence of clinical heart failure between the treatment groups (RR=0.36, 95% CI 0.12 to 1.11, P = 0.07). However, based on the low value of the RR and the wide 95% CI there is some suggestion of a lower rate of clinical heart failure in patients treated with epirubicin as compared to patients treated with doxorubicin. The reason that this difference is not statistically significant could be a result of a low power of the included studies. No results are available on the occurrence of clinical and subclinical heart failure combined in patients treated with either epirubicin or doxorubicin, since none of the included studies adequately evaluated subclinical heart failure.
Our meta‐analysis of tumour response showed no significant difference in response rate between the treatment groups (RR = 0.94, 95% CI 0.82 to 1.08, P=0.40). The same was true for our meta‐analyses of both progression‐free and overall survival (HR = 1.05; 95% CI 0.76 to 1.44; P = 0.78 and HR = 0.95; 95% CI 0.65 to 1.39; P = 0.79 respectively). However, please note that in the meta‐analysis of progression‐free survival substantial heterogeneity was detected. Individual studies not included in the meta‐analysis also showed no significant differences in survival between the treatment groups.
For three evaluated adverse effects it was possible to perform a meta‐analysis. A significantly lower rate of leukopenia and nausea/vomiting was identified in patients treated with epirubicin as compared to patients treated with doxorubicin. No significant difference in the occurrence of alopecia between the treatment groups was identified; please note that for this outcome substantial heterogeneity was present. For the other evaluated adverse effects pooling of results was not possible. As a result, this review does not allow for any definitive conclusions regarding those adverse effects in patients treated with either epirubicin or doxorubicin. However, for thrombocytopenia, infection, stomatitis/mucositis, thrombophlebitis, and hepatic or renal dysfunction related to drug administration, results were consistent among the individual studies evaluating the outcome (either one or two studies). None of the studies identified a significant difference in the occurrence of the evaluated adverse effects between the treatment groups. For anaemia the results were not consistent among the individual studies evaluating the outcome. The reason that some studies did not identify a significant difference between the treatment groups could be due to the fact that the number of patients included in these studies were too small to detect a difference between the treatment groups (i.e. low power).
The cumulative anthracycline dose received in both treatment groups was comparable. Therefore, the results of the different outcomes are direct comparisons of equimolar doses of epirubicin and doxorubicin. No conclusions can be made regarding treatment with epirubicin and doxorubicin with different cumulative doses.
The risk of bias in the included studies varied; in many studies bias could not be ruled out due to a lack of reporting. However, at the moment this is the best available evidence of RCTs evaluating epirubicin and doxorubicin.
For liposomal‐encapsulated doxorubicin versus conventional doxorubicin two trials were identified.
Our meta‐analysis of the two trials showed a significantly lower rate of both clinical heart failure and clinical and subclinical heart failure combined in patients treated with liposomal‐encapsulated doxorubicin as compared to patients treated with doxorubicin (RR = 0.20, 95% CI 0.05 to 0.75, P = 0.02 and RR = 0.38, 95% CI 0.24 to 0.59, P < 0.0001 respectively).
However, an important question regarding any cardioprotective intervention during anthracycline therapy is whether the intervention could selectively decrease the heart damage without reducing the anti‐tumour efficacy (i.e. tumour response and patient survival) and without negative effects on toxicities other than cardiac damage. Our meta‐analysis of two trials for response rate showed no significant difference between the treatment groups (RR = 1.01, 95% CI 0.80 to 1.26, P = 0.95). The same was true for our meta‐analyses of both progression‐free and overall survival (HR=1.01, 95% CI 0.83 to 1.24, P=0.89 and HR = 1.12, 95% CI 0.83 to 1.53, P = 0.46 respectively). However, it should be noted that in the study of Harris 2002 there was a non‐significant trend toward a shorter overall survival in patients treated with liposomal‐encapsulated doxorubicin (P = 0.09). The authors state that although this finding cannot be ignored, it seems unlikely, given the other efficacy parameters, that this is a consequence of reduced efficacy of liposomal‐encapsulated doxorubicin compared with conventional doxorubicin. It is possible that the excess of progesterone receptor positivity in the conventional doxorubicin arm may denote a better prognostic group. Other unmeasured prognostic factors (e.g. HER‐2 expression) also may have played a role in the natural history of the disease. In the study of Batist 2001 there were no significant differences in progesterone receptor positivity between the treatment groups.
For all adverse effects it was possible to perform a meta‐analysis. For neutropenia, nausea/vomiting and diarrhoea a significantly lower rate of the adverse effect was observed in patients treated with liposomal‐encapsulated doxorubicin as compared to patients treated with doxorubicin. For anaemia, thrombocytopenia, neutropenic fever, stomatitis/mucositis, asthenia/fatigue, cutaneous toxicity and infection no significant differences between the treatment groups were identified. Please note, that there was heterogeneity present in the analysis of infection.
It should be emphasised that the cumulative anthracycline dose received in both treatment groups was the same in the study of Batist 2001, whereas in the study of Harris 2002 patients in the liposomal‐encapsulated doxorubicin group received a higher cumulative anthracycline dose than patients in the doxorubicin group. So despite a higher cumulative anthracycline dose received in the liposomal‐encapsulated doxorubicin group, there was still a lower rate of both heart failure and adverse effects in the liposomal‐encapsulated doxorubicin group as compared to the doxorubicin group. However, no significant differences in both tumour response and survival were identified between both treatment groups, whereas it might be expected that those outcomes would improve with a higher cumulative anthracycline dose. As a result, we are not able to provide definitive conclusions on tumour response and survival.
The risk of bias in the included studies varied; in many studies bias could not be ruled out due to a lack of reporting. However, at the moment this is the best available evidence of RCTs evaluating liposomal‐encapsulated doxorubicin and doxorubicin.
For liposomal‐encapsulated doxorubicin versus epirubicin one trial was identified. Pooling of results was therefore not possible, and as a result this review does not allow for any definitive conclusions regarding the effects of treatment with liposomal‐encapsulated doxorubicin or epirubicin. No significant difference in the occurrence of both clinical heart failure and clinical and subclinical heart failure combined was identified. The same was true for response rate and overall survival, whereas progression‐free survival was significantly better in patients treated with liposomal‐encapsulated doxorubicin as compared to patients treated with doxorubicin. We cannot explain this difference.
This review does not allow for any definitive conclusions regarding adverse effects in patients treated with either epirubicin or liposomal‐encapsulated doxorubicin. However, only for neutropenia a significant difference in favour of epirubicin was identified. For all other evaluated adverse effects no significant difference between the treatment groups was found.
The reason that in this study for most evaluated outcomes no significant difference between the treatment groups was identified could be due to the fact that the number of patients included in this study was too small to detect a difference between the treatment groups (i.e. low power).
The cumulative anthracycline dose received in both treatment groups was comparable. Therefore, the results of the different outcomes are direct comparisons of equimolar doses of liposomal‐encapsulated doxorubicin and epirubicin. No conclusions can be made regarding treatment with epirubicin and liposomal‐encapsulated doxorubicin with different cumulative doses.
The risk of bias in the included trial was unclear due to a lack of reporting, only the presence of attrition bias was ruled out. However, at the moment this is the best available evidence of RCTs evaluating epirubicin and liposomal‐encapsulated doxorubicin.
Regarding early and late cardiotoxicity, we must conclude the following for all 3 comparisons. In some studies the follow‐up of (at least part of) the included patients was more than 1 year, therefore it is possible that these studies included cases of both early and late cardiotoxicity. In the other studies the length of follow‐up was not mentioned and as a result we don't know if the cases of cardiotoxicity in these studies are early or late. However, based on the fact that all patients included in these trials had metastatic or advanced disease and the associated effect on survival duration, we presume that cases of heart failure in these trials were early cardiotoxicity.
For all three comparisons of different anthracycline derivates it should be emphasised that all included patients were adults with a solid tumour, mainly breast cancer. As a result no conclusions can be made about the effects of treatment with epirubicin and doxorubicin in children treated with anthracyclines and also not in patients diagnosed with leukaemia.
It should be kept in mind that the inclusion of studies for this systematic review was limited to RCTs describing cardiotoxicity, and as a result, the analyses of response rate, survival, adverse effects and QOL were possibly based on only a subgroup of trials comparing different anthracycline derivates.
We are awaiting the results of the currently ongoing study evaluating liposomal‐encapsulated doxorubicin versus conventional doxorubicin in adults with B‐cell lymphoma (see Ongoing studies table). We are also awaiting (additional) results of the eight trials currently awaiting assessment (see Studies awaiting classification table): epirubicin versus doxocrubicin (n=3; all in patients with breast cancer), liposomal‐encapsulated doxorubicin versus conventional doxorubicin (n = 2; one study in adults with AIDS‐related Kaposi's sarcoma and one in adults with lymphoma), liposomal‐encapsulated doxocrubicin versus epirubicin (n = 2; all in patients with breast cancer) and liposomal‐encapsulated doxocrubicin versus liposomal daunorubicin (n=1; patients with AIDS‐related Kaposi's sarcoma). From the currently available data it is unclear if these studies are eligible for inclusion in this systematic review.
Authors' conclusions
Implications for practice.
Combinations of different anthracycline derivates for which no adequate RCTs were identified
For all combinations of different anthracycline derivates for which no adequate RCTs were identified, no conclusions can be made about possible differences in preventing anthracycline‐induced heart damage. Based on the current available evidence, we are not able to give recommendations for clinical practice.
Epirubicin versus doxorubicin
Based on the current available evidence, we are not able to favour either epirubicin or doxorubicin when given in equimolar doses. No conclusions can be made regarding treatment with epirubicin and doxorubicin with different cumulative doses.
It should be emphasised that all patients included in these studies were adults with advanced solid tumours. As a result no conclusions can be made about the effects of treatment with epirubicin and doxorubicin in children treated with anthracyclines and also not in patients diagnosed with leukaemia.
Liposomal‐encapsulated doxorubicin versus conventional doxorubicin
Based on our meta‐analysis which clearly shows that treatment with liposomal‐encapsulated doxorubicin reduces the risk of both clinical and subclinical heart failure as compared to treatment with doxorubicin despite the fact that patients treated with liposomal‐encapsulated doxorubicin received a higher cumulative anthracycline dose than patients treated with doxorubicin, we conclude that in adults with a solid tumour liposomal‐encapsulated doxorubicin should be favoured over doxorubicin. However, until more evidence becomes available on tumour response and survival in patients treated with liposomal‐encapsulated doxorubicin or doxorubicin in equimolar doses, we recommend the use of a higher cumulative liposomal‐encapsulated doxorubicin dose as compared to the standard cumulative doxorubicin dose. Despite the higher cumulative anthracycline dose received in the liposomal‐encapsulated doxorubicin group, patients treated with liposomal‐encapsulated doxorubicin suffered from less side effects than patients treated with doxorubicin.
It should be emphasised that all patients included in these studies were adults with advanced breast cancer. As a result no conclusions can be made about the effects of treatment with liposomal‐encapsulated doxorubicin and doxorubicin in children treated with anthracyclines and also not in patients diagnosed with leukaemia.
Liposomal‐encapsulated doxorubicin versus epirubicin
Since pooling of results was not possible for the comparison of epirubicin versus liposomal‐encapsulated doxorubicin, no definitive conclusions can be made about the occurrence of anthracycline‐induced heart damage with the use of these anthracycline derivates. Based on the currently available evidence, we are not able to give recommendations for clinical practice.
Implications for research.
Combinations of different anthracycline derivates for which no adequate RCTs were identified
Before any conclusions can be made about the occurrence of anthracycline‐induced heart damage with the use of the anthracycline derivates for which no adequate RCTs were identified, high quality RCTs need to be undertaken. These RCTs should be performed in homogeneous study populations treated for either a haematological malignancy or a solid tumour. Also, since data obtained in adults cannot be extrapolated to children, they should be evaluated in children. The number of included patients should be sufficient to obtain the power needed for the results to be reliable and also, there should be adequate reporting of the occurrence of cardiotoxicity in relation to follow‐up time. We are awaiting results of the trial currently awaiting assessment which compares liposomal‐encapsulated doxocrubicin with liposomal daunorubicin.
Epirubicin versus doxorubicin
Future trials in adults on epirubicin versus doxorubicin in equimolar doses should be performed in homogeneous study populations treated for either a haematological malignancy or a solid tumour. Also, since data obtained in adults cannot be extrapolated to children, epirubicin and doxorubicin in equimolar doses should be evaluated in children. Epirubicin and doxorubicin with different cumulative doses could also be evaluated in high quality RCTs. The number of included patients in all RCTs should be sufficient to obtain the power needed for the results to be reliable and also, there should be adequate reporting of the occurrence of cardiotoxicity in relation to follow‐up time. We are awaiting results of the three trials currently awaiting assessment.
Liposomal‐encapsulated doxorubicin versus conventional doxorubicin
Future trials in adults on doxorubicin versus liposomal‐encapsulated doxorubicin should be performed in homogeneous study populations treated for either a haematological malignancy or a solid tumour. Also, since data obtained in adults cannot be extrapolated to children, doxorubicin and liposomal‐encapsulated doxorubicin should be evaluated in children. The number of included patients in all RCTs should be sufficient to obtain the power needed for the results to be reliable and also, there should be adequate reporting of the occurrence of cardiotoxicity in relation to follow‐up time. We are awaiting the results of the currently ongoing study and also results of the two trials currently awaiting assessment.
Liposomal‐encapsulated doxorubicin versus epirubicin
Before any definitive conclusions can be made about the possible difference between epirubicin and liposomal‐encapsulated doxorubicin in preventing anthracycline‐induced heart damage, more high quality RCTs need to be undertaken. These RCTs should be performed in homogeneous study populations treated for either a haematological malignancy or a solid tumour. Also, since data obtained in adults cannot be extrapolated to children, they should be evaluated in children. The number of included patients should be sufficient to obtain the power needed for the results to be reliable and also, there should be adequate reporting of the occurrence of cardiotoxicity in relation to follow‐up time. We are awaiting results of the two trials currently awaiting assessment.
What's new
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 24 February 2015 | Amended | Contact details updated. |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
| 27 November 2009 | New citation required but conclusions have not changed | Unfortunately, no new studies could be included in the review. However, eight studies are awaiting classification as the current available data is unclear regarding inclusion (six studies are identified in the update): epirubicin versus doxocrubicin (n = 3), liposomal‐encapsulated doxorubicin versus conventional doxorubicin (n = 2), liposomal‐encapsulated doxocrubicin versus epirubicin (n = 2) and liposomal‐encapsulated doxocrubicin versus liposomal daunorubicin (n = 1). Also, we identified an ongoing trial evaluating liposomal‐encapsulated doxorubicin versus conventional doxorubicin (new in the update). |
| 27 November 2009 | New search has been performed | The search for eligible studies was updated to May/June 2009 using an updated search strategy. |
| 14 October 2008 | Amended | Converted to new review format. |
| 17 July 2006 | New search has been performed | Minor update |
| 29 June 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Heleen van der Pal for her help in preparing the protocol of this review. Also, we would like to thank Leon Bax for translating Japanese articles and all researchers who provided additional information on their studies. Finally, we would like to thank our funders for the financial support which made it possible to perform this systematic review.
For the comparison of epirubicin versus doxorubicin, the hazard ratio and associated statistics were calculated using an Excel spreadsheet developed by the Matthew Sydes (Cancer Division) in collaboration with the Meta‐analysis Group of the MRC Clinical Trials Unit, London. For the comparison of liposomal‐encapsulated doxorubicin and doxorubicin, the hazard ratio and associated statistics were calculated using an Excel spreadsheet provided by Heather Dickinson.
Appendices
Appendix 1. Search strategy for MEDLINE/PubMed
For idarubicin the following subject headings and text words were used (in both the original version of the review and the update): 4‐demethoxydaunorubicin OR 4 demethoxydaunorubicin OR 4‐desmethoxydaunorubicin OR 4 desmethoxydaunorubicin OR IMI 30 OR IMI30 OR IMI‐30 OR idarubicin hydrochloride OR hydrochloride, idarubicin OR NSC 256439 OR NSC‐256439 OR NSC256439 OR idarubicin OR idarubic*.
For liposomal idarubicin the following subject headings and text words were used (in both the original version of the review and the update): (4‐demethoxydaunorubicin OR 4 demethoxydaunorubicin OR 4‐desmethoxydaunorubicin OR 4 desmethoxydaunorubicin OR IMI 30 OR IMI30 OR IMI‐30 OR idarubicin hydrochloride OR hydrochloride, idarubicin OR NSC 256439 OR NSC‐256439 OR NSC256439 OR idarubicin OR idarubic*) AND (pegylated OR pegyl* OR encapsulated OR encapsul* OR liposomal OR liposom*).
For epirubicin the following subject headings and text words were used (in both the original version of the review and the update): 4'‐epiadriamycin OR 4' epiadriamycin OR 4'‐epidoxorubicin OR 4' epidoxorubicin OR 4'‐epi‐doxorubicin OR 4' epi doxorubicin OR 4'‐epi‐adriamycin OR 4' epi adriamycin OR 4'‐epi‐DXR OR 4' epi DXR OR epirubicin hydrochloride OR hydrochloride, epirubicin OR farmorubicin OR IMI‐28 OR IMI 28 OR IMI28 OR NSC 256942 OR NSC‐256942 OR NSC256942 OR epirubicin OR epirubic*.
For liposomal epirubicin the following subject headings and text words were used (in both the original version of the review and the update): (4'‐epiadriamycin OR 4' epiadriamycin OR 4'‐epidoxorubicin OR 4' epidoxorubicin OR 4'‐epi‐doxorubicin OR 4' epi doxorubicin OR 4'‐epi‐adriamycin OR 4' epi adriamycin OR 4'‐epi‐DXR OR 4' epi DXR OR epirubicin hydrochloride OR hydrochloride, epirubicin OR farmorubicin OR IMI‐28 OR IMI 28 OR IMI28 OR NSC 256942 OR NSC‐256942 OR NSC256942 OR epirubicin OR epirubic*) AND (pegylated OR pegyl* OR encapsulated OR encapsul* OR liposomal OR liposom*).
For doxorubicin the following subject headings and text words were used (in both the original version of the review and the update): adriablastine OR adriblastin OR adriablastin OR adriamycin OR DOX‐SL OR DOX SL OR DOXSL OR doxorubicin hydrochloride OR hydrochloride doxorubicin OR doxorubic* OR adriamyc*.
For liposomal doxorubicin the following subject headings and text words were used (in the original version of the review): ((adriablastine OR adriblastin OR adriablastin OR adriamycin OR DOX‐SL OR DOX SL OR DOXSL OR doxorubicin hydrochloride OR hydrochloride doxorubicin OR doxorubic* OR adriamyc*) AND (pegylated OR pegyl* OR encapsulated OR encapsul* OR liposomal OR liposom*)) OR (doxil OR caelyx OR liposomal doxorubicin OR doxorubicin, liposomal). For the update we added the following to the search: OR myocet.
For daunorubicin the following subject headings and text words were used (in both the original version of the review and the update): dauno‐rubidomycine OR dauno rubidomycin OR rubidomycin OR rubomycin OR daunomycin OR cerubidine OR daunoblastin OR daunoblastine OR daunorubicin hydrochloride OR hydrochloride, daunorubicin OR daunorubic* OR rubidomyc* OR NSC‐82151 OR NSC 82151 OR NSC82151.
For liposomal daunorubicin the following subject headings and text words were used (in both the original version of the review and the update): ((dauno‐rubidomycine OR dauno rubidomycin OR rubidomycin OR rubomycin OR daunomycin OR cerubidine OR daunoblastin OR daunoblastine OR daunorubicin hydrochloride OR hydrochloride, daunorubicin OR daunorubic* OR rubidomyc* OR NSC‐82151 OR NSC 82151 OR NSC82151) AND (pegylated OR pegyl* OR encapsulated OR encapsul* OR liposomal OR liposom*)) OR (daunoxome OR daunosom*).
Subject headings and text words of each type of anthracycline derivate were combined with an other type of anthracycline derivate, i.e. idarubicin versus liposomal idarubicin, idarubicin versus epirubicin, idarubicin versus liposomal epirubicin, idarubicin versus doxorubicin, idarubicin versus liposomal doxorubicin, idarubicin versus daunorubicin, idarubicin versus liposomal daunorubicin, liposomal idarubicin versus epirubicin, liposomal idarubicin versus liposomal epirubicin, liposomal idarubicin versus doxorubicin, liposomal idarubicin versus liposomal doxorubicin, liposomal idarubicin versus daunorubicin, liposomal idarubicin versus liposomal daunorubicin, epirubicin versus doxorubicin, epirubicin versus liposomal doxorubicin, epirubicin versus daunorubicin, epirubicin versus liposomal daunorubicin, epirubicin versus liposomal epirubicin, liposomal epirubicin versus doxorubicin, liposomal epirubicin versus liposomal doxorubicin, liposomal epirubicin versus daunorubicin, liposomal epirubicin versus liposomal daunorubicin, doxorubicin versus liposomal doxorubicin, doxorubicin versus daunorubicin, doxorubicin versus liposomal daunorubicin, liposomal doxorubicin versus daunorubicin, liposomal doxorubicin versus liposomal daunorubicin, and daunorubicin versus liposomal daunorubicin.
All the above mentioned combinations were combined with the following subject headings and text words for heart damage(in the original version of the review): heart OR heart diseases OR heart disease OR disease, heart OR diseases, heart OR cardiac diseases OR cardiac disease OR diseases, cardiac OR disease, cardiac OR cardiotoxicity OR cardiomyopathy OR heart failure, congestive OR heart failure OR cardiomyopathy, congestive OR ventricular dysfunction OR ventricular dysfunction, left OR ventricular dysfunction, right. For the update we added the following to the search: OR shortening fraction OR ejection fraction OR echocardiography OR radionuclide angiography OR radionuclide ventriculography OR ventriculography, radionuclide OR gated blood‐pool imaging OR blood pool scintigraphy OR gated radionuclide ventriculography OR ventriculography, first pass OR cardiotox* OR cardiomyop* OR echocardiogr* OR ventriculogr* OR scintigr* OR MUGA OR LVEF OR LVSF OR endomyocardial biopsy OR angiocardiography OR cardiomyopathies.
Finally, the results of this search were combined with the highly sensitive search strategy as described in the Cochrane Handbook (for the original review:Higgins 2005 (all phases); for the update: Higgins 2009 (sensitivity‐maximaizing version)).
Appendix 2. Search strategy for EMBASE/Ovid
For idarubicin the following subject headings and text words were used (in both the original version of the review and the update): exp IDARUBICIN DERIVATIVE/ or exp IDARUBICIN/ or idarubicin.mp or (idarubicin derivative or IMI 30 or NSC 256439 or idarubicin hydrochloride or 4 demethoxydaunorubicin derivative).mp or idarubic$.mp.
For liposomal idarubicin the following subject headings and text words were used (in both the original version of the review and the update): (exp IDARUBICIN DERIVATIVE/ or exp IDARUBICIN/ or idarubicin.mp or (idarubicin derivative or IMI 30 or NSC 256439 or idarubicin hydrochloride or 4 demethoxydaunorubicin derivative).mp or idarubic$.mp) and (liposomal or encapsulated or pegylated).mp.
For epirubicin the following subject headings and text words were used (in both the original version of the review and the update): epirubicin.mp or exp EPIRUBICIN/ or epirubic$.mp or (4' epiadriamycin or 4' epidoxorubicin).mp or (farmorubicin or 4' epi DXR or epirubicin hydrochloride).mp OR (IMI 28 or NSC 256942).mp.
For liposomal epirubicin the following subject headings and text words were used (in both the original version of the review and the update): (epirubicin.mp or exp EPIRUBICIN/ or epirubic$.mp or (4' epiadriamycin or 4' epidoxorubicin).mp or (farmorubicin or 4' epi DXR or epirubicin hydrochloride).mp OR (IMI 28 or NSC 256942).mp) and (liposomal or encapsulated or pegylated).mp.
For doxorubicin the following subject headings and text words were used (in both the original version of the review and the update): (adriamycin or doxorubicin).mp or exp Doxorubicin/ or (doxorubic$ or adriamyc$).mp or adriablastine.mp or (adriblastin or adriablastin or doxorubicin hydrochloride or DOX SL).mp.
For liposomal doxorubicin the following subject headings and text words were used (in the original version of the review): (((adriamycin or doxorubicin).mp or exp Doxorubicin/ or (doxorubic$ or adriamyc$).mp or adriablastine.mp or (adriblastin or adriablastin or doxorubicin hydrochloride or DOX SL).mp) and (liposomal or encapsulated or pegylated).mp) or (caelyx or doxil or liposomal doxorubicin).mp. For the update we added the following to the search: OR myocet.
For daunorubicin the following subject headings and text words were used (in both the original version of the review and the update): exp DAUNORUBICIN DERIVATIVE/ or daunorubicin.mp or exp DAUNORUBICIN/ or (daunorubidomycine or rubidomycin or rubomycin or daunomycin or daynorubicin hydrochloride or daunoblastin or daunoblastine or cerubidine or NSC 82151).mp or (rubidomyc$ or daunorubic$).mp.
For liposomal daunorubicin the following subject headings and text words were used (in both the original version of the review and the update): ((exp DAUNORUBICIN DERIVATIVE/ or daunorubicin.mp or exp DAUNORUBICIN/ or (daunorubidomycine or rubidomycin or rubomycin or daunomycin or daynorubicin hydrochloride or daunoblastin or daunoblastine or cerubidine or NSC 82151).mp or (rubidomyc$ or daunorubic$).mp) and (liposomal or encapsulated or pegylated).mp) or daunoxome.mp or daunosom$.mp.
Subject headings and text words of each type of anthracycline derivate were combined with an other type of anthracycline derivate, i.e. idarubicin versus liposomal idarubicin, idarubicin versus epirubicin, idarubicin versus liposomal epirubicin, idarubicin versus doxorubicin, idarubicin versus liposomal doxorubicin, idarubicin versus daunorubicin, idarubicin versus liposomal daunorubicin, liposomal idarubicin versus epirubicin, liposomal idarubicin versus liposomal epirubicin, liposomal idarubicin versus doxorubicin, liposomal idarubicin versus liposomal doxorubicin, liposomal idarubicin versus daunorubicin, liposomal idarubicin versus liposomal daunorubicin, epirubicin versus doxorubicin, epirubicin versus liposomal doxorubicin, epirubicin versus daunorubicin, epirubicin versus liposomal daunorubicin, epirubicin versus liposomal epirubicin, liposomal epirubicin versus doxorubicin, liposomal epirubicin versus liposomal doxorubicin, liposomal epirubicin versus daunorubicin, liposomal epirubicin versus liposomal daunorubicin, doxorubicin versus liposomal doxorubicin, doxorubicin versus daunorubicin, doxorubicin versus liposomal daunorubicin, liposomal doxorubicin versus daunorubicin, liposomal doxorubicin versus liposomal daunorubicin, and daunorubicin versus liposomal daunorubicin.
All the above mentioned combinations were combined with the following subject headings and text words for heart damage(in the original version of the review): heart failure.mp or exp Heart Failure/ or exp Heart Ventricle Failure/ or exp Heart Ventricle Function/ or exp Congestive Heart Failure/ or cardiotoxicity.mp or exp CARDIOTOXICITY/ or exp Congestive Cardiomyopathy/ or exp CARDIOMYOPATHY/ or exp Heart Disease/ or exp Heart Failure/ or exp HEART LEFT VENTRICLE FUNCTION/ or exp HEART RIGHT VENTRICLE FUNCTION/ or exp HEART RIGHT VENTRICLE FAILURE/ or exp HEART/ or exp FORWARD HEART FAILURE/. For the update we added the following to the search: heart.mp or exp HEART EJECTION FRACTION/ or exp HEART RIGHT VENTRICLE EJECTION FRACTION/ or exp HEART FUNCTION/ or exp HEART FUNCTION TEST/ or exp HEART LEFT VENTRICLE FAILURE/ or exp HEART VENTRICULOGRAPHY/ or exp HEART LEFT VENTRICLE EJECTION FRACTION/ or congestive heart failure.mp or cardiomyopathy.mp or heart disease.mp or cardiac disease.mp or ventricular dysfunction.mp or shortening fraction.mp or ejection fraction.mp or (MUGA or LVEF or LVSF).mp or echocardiography.mp or exp ECHOCARDIOGRAPHY/ or radionuclide angiography.mp or radionuclide ventriculography.mp or exp Radioisotope Ventriculography/ or gated blood‐pool imaging.mp or endomyocardial biopsy.mp or exp Heart Muscle Biopsy/ or angiocardiography.mp or exp ANGIOCARDIOGRAPHY/ or blood pool scintigraphy.mp or (cardiotox$ or cardiomyop$ or echocardiogr$ or ventriculogr$ or scintigr$).mp.
Finally, the results of this search were combined with the search for randomised controlled trials. The following subject headings and text words were used (in the original version of the review; based on the highly sensitive search strategy for identifying reports of randomised controlled trials as described in the Cochrane Handbook (Higgins 2005)): Randomized Controlled Trial/ or exp RANDOMIZATION/ or Controlled Study/ or Clinical Trial/ or Single Blind Procedure/ or Double Blind Procedure/ or exp PLACEBO/ or exp Comparative Study/ or exp Prospective Study/. For the update we used the following strategy (based on the highly sensitive search strategy for identifying reports of randomised controlled trials as described in the Cochrane Handbook (Higgins 2009)): (Randomized Controlled Trial/ or Controlled Clinical Trial/ or randomized.ti,ab. or placebo.ti,ab. or randomly.ti,ab. or trial.ti,ab. or groups.ti,ab. or drug therapy.sh.) not (animal/ not human/).
[mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]; [ti,ab=title, abstract]; [sh=subject heading]
Appendix 3. Search strategy for The Cochrane Register of Controlled Trials (CENTRAL)
For idarubicin the following subject headings and text words were used (in the original version of the review): idarubicin OR 4‐demethoxydaunorubicin OR 4 demethoxydaunorubicin OR 4‐desmethoxydaunorubicin OR 4 desmethoxydaunorubicin OR idarubicin hydrochloride OR idarubic*. For the update we added the following to the search: OR IMI 30 OR IMI30 OR IMI‐30 OR NSC 256439 OR NSC‐256439 OR NSC256439.
For liposomal idarubicin the following subject headings and text words were used (in the original version of the review): (pegylated OR pegyl* OR encapsulated OR encapsul* OR liposomal OR liposom*) and (idarubicin OR 4‐demethoxydaunorubicin OR 4 demethoxydaunorubicin OR 4‐desmethoxydaunorubicin OR 4 desmethoxydaunorubicin OR idarubicin hydrochloride OR idarubic*). For the update we added the following to the search: OR IMI 30 OR IMI30 OR IMI‐30 OR NSC 256439 OR NSC‐256439 OR NSC256439
For epirubicin the following subject headings and text words were used (in the original version of the review): 4'‐epiadriamycin OR 4' epiadriamycin OR 4'‐epidoxorubicin OR 4' epidoxorubicin OR 4'‐epi‐doxorubicin OR 4' epi doxorubicin OR 4'‐epi‐adriamycin OR 4' epi adriamycin OR 4'‐epi‐DXR OR 4' epi DXR OR epirubicin hydrochloride OR farmorubicin OR epirubicin OR epirubic*. For the update we added the following to the search: OR IMI‐28 OR IMI 28 OR IMI28 OR NSC 256942 OR NSC‐256942 OR NSC256942.
For liposomal epirubicin the following subject headings and text words were used (in the original version of the review): (pegylated OR pegyl* OR encapsulated OR encapsul* OR liposomal OR liposom*) and (4'‐epiadriamycin OR 4' epiadriamycin OR 4'‐epidoxorubicin OR 4' epidoxorubicin OR 4'‐epi‐doxorubicin OR 4' epi doxorubicin OR 4'‐epi‐adriamycin OR 4' epi adriamycin OR 4'‐epi‐DXR OR 4' epi DXR OR epirubicin hydrochloride OR farmorubicin OR epirubicin OR epirubic*). For the update we added the following to the search: OR IMI‐28 OR IMI 28 OR IMI28 OR NSC 256942 OR NSC‐256942 OR NSC256942.
For doxorubicin the following subject headings and text words were used (in the original version of the review): adriablastine OR adriblastin OR adriablastin OR adriamycin OR doxorubicin hydrochloride OR doxorubicin OR doxorubic* OR adriamycin*. For the update we added the following to the search: OR DOX‐SL OR DOX SL OR DOXSL OR hydrochloride doxorubicin; for the update we changed adriamycin* into adriamyc*.
For liposomal doxorubicin the following subject headings and text words were used (in the original version of the review): (pegylated OR pegyl* OR encapsulated OR encapsul* OR liposomal OR liposom*) AND (adriablastine OR adriblastin OR adriablastin OR adriamycin OR doxorubicin hydrochloride OR doxorubicin OR doxorubic* OR adriamycin*). For the update we added the following to the search: OR DOX‐SL OR DOX SL OR DOXSL OR hydrochloride doxorubicin OR doxil OR caelyx OR liposomal doxorubicin OR myocet; for the update we changed adriamycin* into adriamyc*.
For daunorubicin the following subject headings and text words were used (in the original version of the review): daunorubidomycine OR rubidomycin OR rubomycin OR daunomycin OR cerubidine OR daunoblastin OR daunoblastine OR daunorubicin hydrochloride OR daunorubicin OR daunorubic* OR rubidomyc*. For the update we added the following to the search: OR NSC‐82151 OR NSC 82151 OR NSC82151; for the update we changed daunorubidomycine in dauno‐rubidomycine OR dauno rubidomycin.
For liposomal daunorubicin the following subject headings and text words were used (in the original version of the review): daunoxome OR daunosom* OR ((pegylated OR pegyl* OR encapsulated OR encapsul* OR liposomal OR liposom*) AND (daunorubidomycine OR rubidomycin OR rubomycin OR daunomycin OR cerubidine OR daunoblastin OR daunoblastine OR daunorubicin hydrochloride OR daunorubicin OR daunorubic* OR rubidomyc*). For the update we added the following to the search: OR NSC‐82151 OR NSC 82151 OR NSC82151; for the update we changed daunorubidomycine in dauno‐rubidomycine OR dauno rubidomycin.
Subject headings and text words of each type of anthracycline derivate were combined with an other type of anthracycline derivate, i.e. idarubicin versus liposomal idarubicin, idarubicin versus epirubicin, idarubicin versus liposomal epirubicin, idarubicin versus doxorubicin, idarubicin versus liposomal doxorubicin, idarubicin versus daunorubicin, idarubicin versus liposomal daunorubicin, liposomal idarubicin versus epirubicin, liposomal idarubicin versus liposomal epirubicin, liposomal idarubicin versus doxorubicin, liposomal idarubicin versus liposomal doxorubicin, liposomal idarubicin versus daunorubicin, liposomal idarubicin versus liposomal daunorubicin, epirubicin versus doxorubicin, epirubicin versus liposomal doxorubicin, epirubicin versus daunorubicin, epirubicin versus liposomal daunorubicin, epirubicin versus liposomal epirubicin, liposomal epirubicin versus doxorubicin, liposomal epirubicin versus liposomal doxorubicin, liposomal epirubicin versus daunorubicin, liposomal epirubicin versus liposomal daunorubicin, doxorubicin versus liposomal doxorubicin, doxorubicin versus daunorubicin, doxorubicin versus liposomal daunorubicin, liposomal doxorubicin versus daunorubicin, liposomal doxorubicin versus liposomal daunorubicin, and daunorubicin versus liposomal daunorubicin.
All the above mentioned combinations were combined with the following subject headings and text words for heart damage(in the original version of the review): heart OR heart diseases OR heart disease OR cardiac diseases OR cardiac disease OR cardiotoxicity OR cardiomyopathy OR heart failure OR congestive heart failure OR ventricular dysfunction. For the update we added the following to the search: congestive cardiomyopathy OR shortening fraction OR ejection fraction OR echocardiography OR radionuclide angiography OR radionuclide ventriculography OR gated blood‐pool imaging OR blood pool scintigraphy OR gated radionuclide ventriculography OR cardiotox* OR cardiomyop* OR echocardiogr* OR ventriculogr* OR scintigr* OR MUGA OR LVEF OR LVSF OR endomyocardial biopsy OR angiocardiography OR cardiomyopathies.
Data and analyses
Comparison 1. Doxorubicin versus epirubicin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical heart failure | 5 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.12, 1.11] |
| 2 Tumour response | 5 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.82, 1.08] |
| 3 Progression‐free survival | 2 | Hazard ratio (Random, 95% CI) | 1.05 [0.76, 1.44] | |
| 4 Overall survival | 2 | Hazard ratio (Random, 95% CI) | 0.95 [0.65, 1.39] | |
| 5 Adverse effects: leukopenia grade 3 or 4 | 2 | 546 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.31, 0.80] |
| 6 Adverse effects: nausea / vomiting grade 3 or 4 | 2 | 546 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.61, 0.97] |
| 7 Adverse effects: alopecia grade 3 or 4 | 3 | 796 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.38] |
1.1. Analysis.
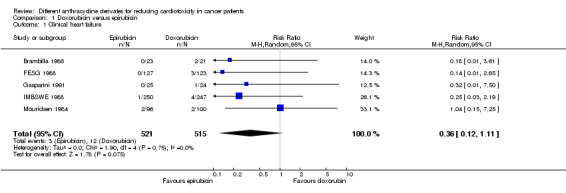
Comparison 1 Doxorubicin versus epirubicin, Outcome 1 Clinical heart failure.
1.2. Analysis.
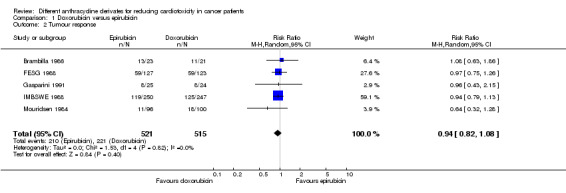
Comparison 1 Doxorubicin versus epirubicin, Outcome 2 Tumour response.
1.3. Analysis.
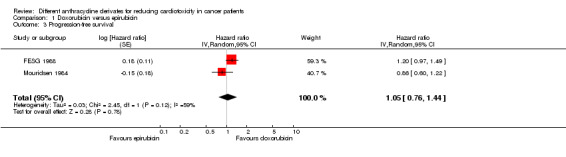
Comparison 1 Doxorubicin versus epirubicin, Outcome 3 Progression‐free survival.
1.4. Analysis.
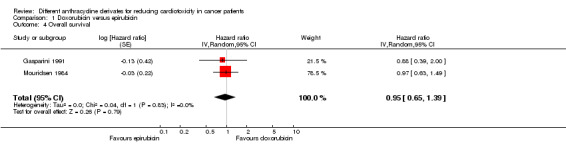
Comparison 1 Doxorubicin versus epirubicin, Outcome 4 Overall survival.
1.5. Analysis.
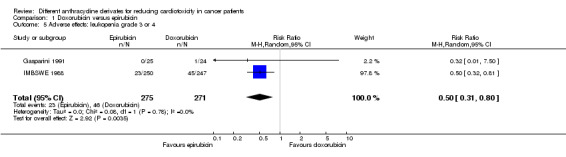
Comparison 1 Doxorubicin versus epirubicin, Outcome 5 Adverse effects: leukopenia grade 3 or 4.
1.6. Analysis.
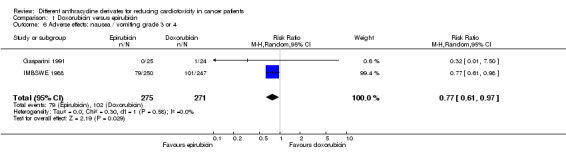
Comparison 1 Doxorubicin versus epirubicin, Outcome 6 Adverse effects: nausea / vomiting grade 3 or 4.
1.7. Analysis.
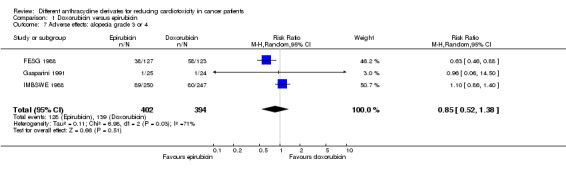
Comparison 1 Doxorubicin versus epirubicin, Outcome 7 Adverse effects: alopecia grade 3 or 4.
Comparison 2. Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical heart failure | 2 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.05, 0.75] |
| 2 Heart failure combined | 2 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.24, 0.59] |
| 3 Tumour response | 2 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.80, 1.26] |
| 4 Progression‐free survival | 2 | Hazard ratio (Random, 95% CI) | 1.01 [0.83, 1.24] | |
| 5 Overall survival | 2 | Hazard ratio (Random, 95% CI) | 1.12 [0.83, 1.53] | |
| 6 Adverse effects: anaemia grade >=3 | 2 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.61, 1.13] |
| 7 Adverse effects: thrombocytopenia grade >=3 | 2 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.60, 1.97] |
| 8 Adverse effects: neutropenia grade 4 | 2 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.72, 0.94] |
| 9 Adverse effects: neutropenic fever (fever >=38, neutropenia grade 4, IV antibiotics and/or hospitalisation) | 2 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.53, 1.45] |
| 10 Adverse effects: nausea/vomiting grade >=3 | 2 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.98] |
| 11 Adverse effects: stomatitis/mucositis grade >=3 | 2 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.32, 1.05] |
| 12 Adverse effects: diarrhoea grade >=3 | 2 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.12, 0.87] |
| 13 Adverse effects: asthenia/fatigue grade 3 | 2 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.41] |
| 14 Adverse effects: cutaneous grade 3 | 2 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.08, 5.45] |
| 15 Adverse effects: infection grade >=3 | 2 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.21, 2.89] |
2.1. Analysis.
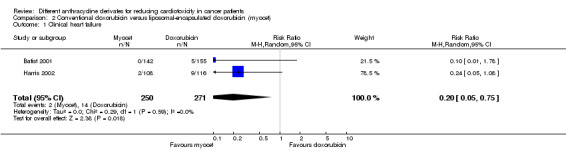
Comparison 2 Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet), Outcome 1 Clinical heart failure.
2.2. Analysis.
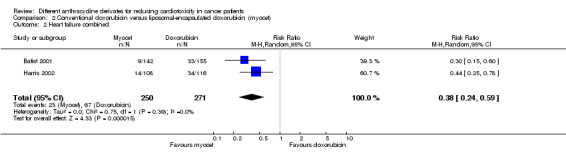
Comparison 2 Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet), Outcome 2 Heart failure combined.
2.3. Analysis.
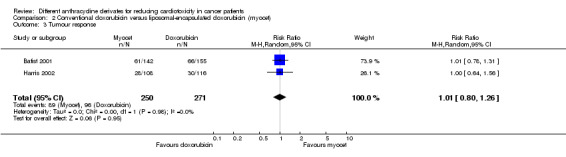
Comparison 2 Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet), Outcome 3 Tumour response.
2.4. Analysis.
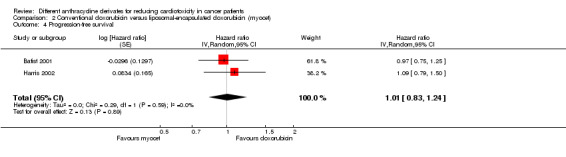
Comparison 2 Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet), Outcome 4 Progression‐free survival.
2.5. Analysis.
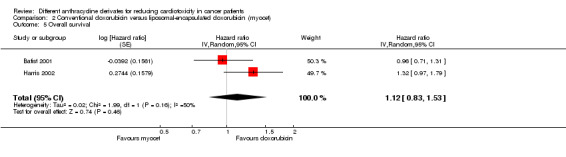
Comparison 2 Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet), Outcome 5 Overall survival.
2.6. Analysis.
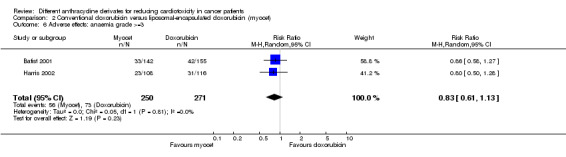
Comparison 2 Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet), Outcome 6 Adverse effects: anaemia grade >=3.
2.7. Analysis.
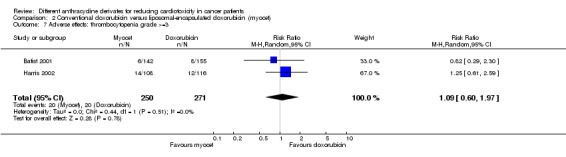
Comparison 2 Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet), Outcome 7 Adverse effects: thrombocytopenia grade >=3.
2.8. Analysis.
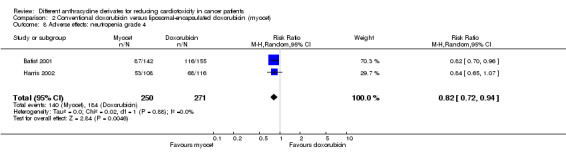
Comparison 2 Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet), Outcome 8 Adverse effects: neutropenia grade 4.
2.9. Analysis.
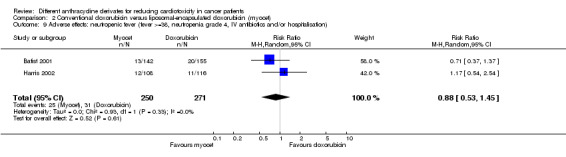
Comparison 2 Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet), Outcome 9 Adverse effects: neutropenic fever (fever >=38, neutropenia grade 4, IV antibiotics and/or hospitalisation).
2.10. Analysis.
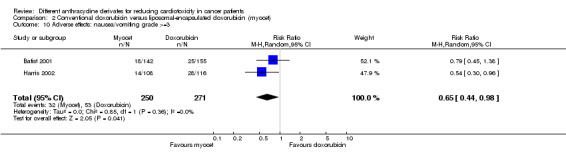
Comparison 2 Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet), Outcome 10 Adverse effects: nausea/vomiting grade >=3.
2.11. Analysis.
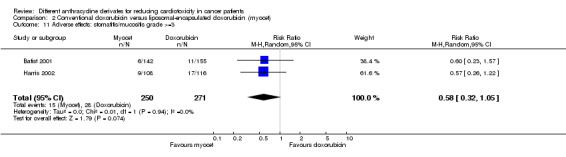
Comparison 2 Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet), Outcome 11 Adverse effects: stomatitis/mucositis grade >=3.
2.12. Analysis.
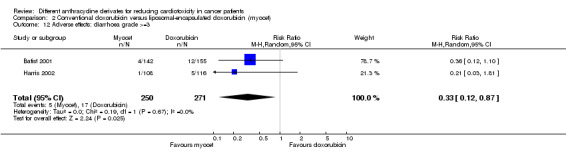
Comparison 2 Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet), Outcome 12 Adverse effects: diarrhoea grade >=3.
2.13. Analysis.
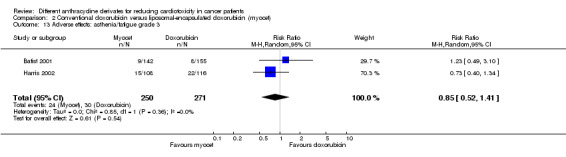
Comparison 2 Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet), Outcome 13 Adverse effects: asthenia/fatigue grade 3.
2.14. Analysis.
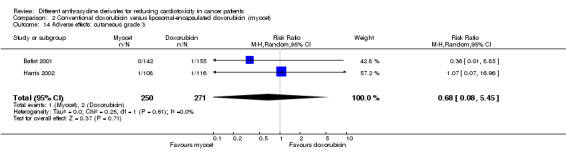
Comparison 2 Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet), Outcome 14 Adverse effects: cutaneous grade 3.
2.15. Analysis.
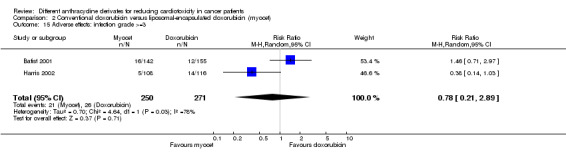
Comparison 2 Conventional doxorubicin versus liposomal‐encapsulated doxorubicin (myocet), Outcome 15 Adverse effects: infection grade >=3.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Batist 2001.
| Methods | Randomisations were performed on 1:1 basis with a balanced block design (stratified according to prior exposure to doxorubicin). | |
| Participants | 297 patients (aged 22 to 88 years; sex nm) with metastatic breast cancer treated with cyclophosphamide and either liposomal‐encapsulated doxorubicin (myocet) or doxorubicin. Prior anthracycline therapy in 14 patients in the liposomal‐encapsulated doxorubicin group (median cumulative doxorubicin dose of 240 mg/m2; range 50 to 294 mg/m2) and 15 patients in the doxorubicin group (median cumulative doxorubicin dose of 240 mg/m2; range 63 to 270 mg/m2). Prior cardiac radiotherapy possible for 15 patients in the liposomal‐encapsulated doxorubicin group and 19 in the doxorubicin group (all less than 35 Gy on the mediastinum). Prior cardiac dysfunction in 5 patients in the liposomal‐encapsulated doxorubicin group and 3 patients in the doxorubicin group. | |
| Interventions | Liposomal‐encapsulated doxorubicin (n=142; median cumulative dose 360 mg/m2; range 60 to 2220 mg/m2) or doxorubicin (n=155; median cumulative dose 360 mg/m2; range 60 to 660 mg/m2) every 3 weeks (both peak dose 60 mg/m2 and infusion duration of 1 hour). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as clinical evidence of congestive heart failure; subclinical heart failure defined as a decrease in resting LVEF of 20 EF units or more from baseline to a final value of 50% or more or a decrease of 10 EF units or more from baseline to a final value of less than 50% as measured by MUGA scan). Tumour response (i.e. CR defined as the complete disappearance of all evidence of disease, including disease‐related signs and symptoms, for at least 6 weeks; PR was defined as a 50% or greater decrease in the sum of the products of the 2 longest perpendicular diameters of all measured lesions for at least 6 weeks with no evidence of progressive disease). Survival. Adverse effects (according to NCI‐CTC criteria). | |
| Notes | One patient randomised to doxorubicin was withdrawn from the study before the first dose of chemotherapy; however, we performed an intention‐to‐treat analysis. Length of follow‐up minimal 1 year (median 20 months). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Not mentioned in article. |
Brambilla 1986.
| Methods | Randomisations were performed with random permuted blocks of length 4 (stratified according to site of dominant lesion). | |
| Participants | 44 patients (aged 28 to 69 years; all females) with advanced breast cancer treated with either doxorubicin or epirubicin and eventual radiotherapy to side of initial disease (3 in each group). No prior anthracycline therapy. Prior cardiac radiotherapy in 1 patient in the doxorubicin group and 4 patients in the epirubicin group (all left chest wall irradiation). No prior cardiac dysfunction. | |
| Interventions | Doxorubicin (n=21; median cumulative dose 540 mg/m2; range 225 to 650 mg/m2) or epirubicin (n=23; median cumulative dose 565 mg/m2; range 150 to 600 mg/m2) every 3 weeks (both peak dose 75 mg/m2 and bolus infusion). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as symptoms and signs of left ventricular failure; subclinical heart failure defined as a fall in MAS as measured by echocardiography or a fall in LVEF as measured by radionuclide angiography). Tumour response (according to WHO criteria). Survival. | |
| Notes | Median length of follow‐up 22 months (range 14 to 30 months). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Not mentioned in article. |
Chan 2004.
| Methods | Method of randomisation not clear (stratified according to country of treatment centre). | |
| Participants | 160 patients (aged 19 to 82 years; all females) with metastatic breast cancer treated with cyclophosphamide and either liposome‐encapsulated doxorubicin (myocet) or doxorubicin. No prior anthracycline therapy. Prior cardiac radiotherapy possible for 47 patients in the liposomal‐encapsulated doxorubicin group and 53 in the epirubicin group (all less than 35 Gy on the mediastinum). No prior cardiac dysfunction. | |
| Interventions | Liposomal‐encapsulated doxorubicin (n=80) or epirubicin (n=80) every 3 weeks (both peak dose 75 mg/m2 and infusion duration of 1 hour; median cumulative dose nm; cumulative anthracycline dose received in both treatment groups is comparable). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as clinical evidence of congestive heart failure; subclinical heart failure defined as a decrease in resting LVEF of 20 units or more from baseline to a final value of 50% or more or a decrease of 10 units or more from baseline to a final value of less than 50% as measured by echocardiography). Tumour response (i.e. CR defined as the disappearance of all evidence of disease for 6 weeks or longer; PR defined as a 50% or more decrease in the sum of the products of the 2 longest perpendicular diameters of all measured lesions for 6 weeks or longer , with no evidence of progressive disease). Survival. Adverse effects (according to NCI‐CTC criteria). | |
| Notes | Six patients (4 in the liposomal‐encapsulated doxorubicin group and 2 in the epirubicin group) never received treatment; however, we performed an intention‐to‐treat analysis. The median length of follow‐up was 21 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Not mentioned in article. |
FESG 1988.
| Methods | Method of randomisation not clear. | |
| Participants | 250 patients (aged 26 to 70 years; sex nm) with advanced breast cancer treated with fluorouracil, cyclophosphamide and either epirubicin or doxorubicin. No prior anthracycline therapy. Prior cardiac radiotherapy in 65 patients in the epirubicin group and 59 patients in the doxorubicin group. No prior cardiac dysfunction. | |
| Interventions | Doxorubicin (n=123) or epirubicin (n=127) every 3 weeks (both peak dose of 50 mg/m2 and infusion duration nm; cumulative anthracycline dose nm; cumulative anthracycline dose received in both treatment groups is comparable). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as congestive heart failure). Tumour response (according to WHO criteria). Survival. Adverse effects (according to WHO criteria). | |
| Notes | The data presented in this table are for 230 patients which were evaluable for efficacy (113 in the doxorubicin group and 117 in the epirubicin group). However, we performed an intention‐to‐treat analysis. Median follow‐up 41 months (range 27 to 52 months). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Not mentioned in article. |
Gasparini 1991.
| Methods | Randomisations were performed using a permuted blocks design (stratified according to number of organ sites involved, dominant site of disease and performance status). | |
| Participants | 49 patients (aged 30 to 77 years; sex nm) with advanced or metastatic breast cancer treated with either epirubicin or doxorubicin. No prior anthracycline therapy. Prior cardiac radiotherapy possible for 14 patients in the epirubicin group and 12 patients in the doxorubicin group. No prior cardiac dysfunction. | |
| Interventions | Doxorubicin (n=24; median cumulative dose 240 mg/m2; range 160 to 860 mg/m2) or epirubicin (n=25; median cumulative dose 220 mg/m2; range 160‐860 mg/m2) weekly (both peak dose 20 mg/m2 and bolus infusion). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as congestive heart failure). Tumour response (according to WHO criteria). Survival. Adverse effects (according to WHO criteria). | |
| Notes | Length of follow‐up nm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Not mentioned in article. |
Harris 2002.
| Methods | Randomisations were performed using a balanced block design (stratified according to prior doxorubicin and institution). | |
| Participants | 224 patients (aged 26 to 85 years; sex nm) with metastatic breast cancer treated with either liposomal‐encapsulated doxorubicin (TLC‐D99; myocet) or doxorubicin. Prior anthracycline therapy in 18 patients in the liposomal‐encapsulated doxorubicin group (median cumulative doxorubicin dose 240 mg/m2; range 167 to 300 mg/m2) and 21 patients in the doxorubicin group (median cumulative doxorubicin dose 240 mg/m2; range 70 to 360 mg/m2). Prior cardiac radiotherapy possible for 47 patients in the liposomal‐encapsulated doxorubicin group and 44 patients in the doxorubicin group (all less than 35 Gy on the mediastinum). No prior cardiac dysfunction. | |
| Interventions | Liposomal‐encapsulated doxorubicin (n=108, median cumulative dose 785 mg/m2) or doxorubicin (n=116; cumulative dose 570 mg/m2) every 3 weeks (both peak dose 75 mg/m2 and 1 hour infusion duration). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as clinical evidence of congestive heart failure; subclinical heart failure defined as a decrease in resting LVEF of 20 points or more from baseline to a final value of 50% or more or a decrease of 10 points or more from baseline to a final value of less than 50% as measured by MUGA‐scan). Tumour response (i.e. CR defined as the complete disappearance of all evidence of disease, including disease‐related signs and symptoms, for at least 6 weeks; PR was defined as a 50% or greater decrease in the sum of the products of the 2 longest perpendicular diameters of all measured lesions for at least 6 weeks with no evidence of progressive disease). Survival. Adverse effects (according to NCI‐CTC criteria). | |
| Notes | Two patients randomised to the liposomal‐encapsulated doxorubicin group were accidently treated with conventional doxorubicin and one patient in this group never received study treatment. However, we performed an intention‐to‐treat analysis. Length of follow‐up nm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Not mentioned in article. |
IMBSWE 1988.
| Methods | Method of randomisation not clear. | |
| Participants | 497 patients (aged 28 to 75 years; sex nm) with advanced breast cancer treated with fluorouracil, cyclophosphamide and either epirubicin or doxorubicin. No prior anthracyclines. Prior cardiac radiotherapy possible for 90 patients in the epirubicin group and 83 patients in the doxorubcin group. No prior cardiac dysfunction. | |
| Interventions | Doxorubicin (n=247; median cumulative dose 311 mg/m2; range 50 to 600 mg/m2) or epirubicin (n=250; median cumulative dose 330 mg/m2; range 25 to 700 mg/m2) every 3 weeks (both peak dose 50 mg/m2 and infusion duration nm). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as congestive heart failure). Tumour response (according to WHO criteria). Survival. Adverse effects (according to WHO criteria). | |
| Notes | The data presented in this table are for the 443 evaluable patients (221 in the doxorubicin group and 222 in the epirubicin group). However, we performed an intention‐to‐treat analysis. Length of follow‐up 28 to 1400 days. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Not mentioned in article. |
Mouridsen 1984.
| Methods | Method of randomisation not clear (stratified according to institution). | |
| Participants | 196 patients (aged 16 to 80 years; 70 women and 80 men) with locally advanced or metastatic soft tissue sarcoma treated with either doxorubicin or epirubicin. No prior anthracycline therapy. Prior cardiac radiotherapy possible for 18 patients in the doxorubicin group and 27 in the epirubicin group. No prior cardiac dysfunction. | |
| Interventions | Doxorubicin (n=100; median cumulative dose 342 mg/m2; range 141 to 916 mg/m2) or epirubicin (n=96; median cumulative dose 355 mg/m2; range 144 to 1683 mg/m2) every 3 weeks (both peak dose 75 mg/m2 and bolus infusion). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as cardiac dysfunction). Tumour response (according to WHO criteria). Survival. | |
| Notes | The data presented in this table are for 150 of the 196 patients who were evaluable, i.e. 75 patients in each group. However, we performed an intention‐to‐treat analysis. Length of follow‐up nm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | Not mentioned in article. |
nm = not mentioned; LVEF = left ventricular ejection fraction; EF = ejection fraction; MUGA = multiple gated acquisition scan; CR = complete remission; PR = partial remission; NCI‐CTC = national cancer institute‐common toxicity criteria; MAS = minor axis shortening; WHO = world health organisation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Al‐Ismael 1987 | Difference in anthracycline peak dose between intervention and control group. |
| Armand 1984 | Duplicate publication of FESG 1988. |
| Aviles 1993 | No randomized controlled trial. |
| Aviles 1995 | Difference in anthracycline peak dose between intervention and control group. |
| Aviles 2005 | Difference in anthracycline peak dose between intervention and control group. |
| Baldini 2004 | Results of two randomized controlled trials presented, both evaluating only one anthracycline derivate. |
| Benjamin 1978 | No randomized controlled trial. |
| Bertini 1997 | Difference in anthracycline peak dose between intervention and control group. |
| Bezwoda 1986 | Difference in anthracycline peak dose between intervention and control group. |
| Bhutani 2002 | Difference in anthracycline peak dose between intervention and control group. |
| Bonadonna 1984 | Duplicate publication of Brambilla 1986. |
| Bonfante 1986 | Duplicate publication of Brambilla 1986. |
| Bontenbal 1998 | Difference in anthracycline peak dose between intervention and control group. |
| Brugiatelli 1993 | Difference in anthracycline peak dose between intervention and control group; duplicate publication of Federico 1998. |
| Burton 2005 | Difference in anthracycline peak dose between intervention and control group. |
| Casper 1987A | Difference in anthracycline peak dose between intervention and control group; duplicate publication of Casper 1987B. |
| Casper 1987B | Difference in anthracycline peak dose between intervention and control group. |
| Cottin 1998 | Difference in anthracycline peak dose between intervention and control group; probably no randomized controlled trial. |
| Creutzig 1988 | Duplicate publication of Creutzig 2001C. |
| Creutzig 2001A | Duplicate publication of Creutzig 2001C. |
| Creutzig 2001B | Duplicate publication of Creutzig 2001C. |
| Creutzig 2001C | Difference in anthracycline peak dose between intervention and control group. |
| De Lena 1987 | Not all patients in the intervention group (i.e. epirubicin) received the same anthracycline peak dose as patients in the control group (i.e. doxorubicin). |
| De Lena 1989 | Duplicate publication of De Lena 1987. |
| Federico 1998 | Difference in anthracycline peak dose between intervention and control group. |
| Gebbia 2003 | Difference in anthracycline peak dose between intervention and control group. |
| Gregory 2000 | Difference in anthracycline peak dose between intervention and control group. |
| Heidemann 1990 | Duplicate publication Heidemann 1993. |
| Heidemann 1993 | Cumulative anthracycline dose patients received in both treatment groups was not mentioned. |
| Hernadi 1988 | Cumulative anthracycline dose patients received in both treatment groups was not mentioned. |
| Homesley 1992 | Difference in anthracycline peak dose between intervention and control group. |
| Hortobagyi 1989 | Difference in anthracycline peak dose between intervention and control group. |
| Jain 1985 | Difference in anthracycline peak dose between intervention and control group; duplicate publication of Casper 1987B. |
| Keiling 1986 | Duplicate publication of FESG 1988. |
| Klener 1973 | No randomized controlled trial; difference in anthracycline peak dose between intervention and control group. |
| Lahtinen 1991 | Cumulative anthracycline dose patients received in both treatment groups was not mentioned. |
| Lawton 1993 | Cumulative anthracycline dose patients received in both treatment groups was not mentioned. |
| Lopez 1989 | Duplicate publication IMBSWE 1988. |
| Lotrionte 2009 | Difference in anthracycline peak dose between intervention and control group; difference in treatment other than anthracyclines between intervention and control group; protocol of planned study. |
| Mandelli 1991 | Difference in anthracycline peak dose between intervention and control group. |
| Maung 2002 | Difference in anthracycline peak dose between intervention and control group. |
| Moser 2005 | Results of 4 different trials presented; in none of these trials a randomisation of different anthracycline derivates was performed. |
| Mouridsen 1987 | Cardiotoxicity data not presented in this publication. |
| Nair 1998 | Difference in anthracycline peak dose between intervention and control group. |
| Namer 2001 | Results of patients treated with doxorubicin and epirubicin not presented seperately. |
| NCT00531973 | Registration in an ongoing trials database of the Lotrionte 2009 study protocol. |
| NCT00589082Latagliata2008 | Difference in anthracycline peak dose between intervention and control group. |
| NCT00854568 | Difference in anthracycline peak dose between intervention and control group. |
| Neri 1989 | No randomized controlled trial. |
| Nielsen 1998 | Difference in anthracycline peak dose between intervention and control group. |
| Nielsen 2000 | Update of studies (Nielsen 1988 and Mouridsen 1987); as mentioned elsewhere, both studies were excluded from this review. |
| Nikkanen 1988 | Cardiotoxicity not mentioned. |
| O'Brian 2004 | Difference in anthracycline peak dose between intervention and control group. |
| Perez 1991 | Difference in anthracycline peak dose between intervention and control group. |
| Pinedo 1987 | Cardiotoxicity not mentioned. |
| Rifkin 2006 | Difference in anthracycline peak dose between intervention and control group. |
| Sculier 1995 | Difference in anthracycline peak dose between intervention and control group. |
| Smith 1987 | Cumulative anthracycline dose patients received in both treatment groups was not mentioned. |
| Stohr 2006 | Difference in anthracycline peak dose and infusion duration between intervention and control group; difference in treatment other than anthracyclines between intervention and control group. |
| Suzuki 1984 | Animal study. |
| Swenson 2003 | Describes part of the patients of Batist 2001; no cardiac data presented. |
| Tanaka 1983 | No randomized controlled trial. |
| Toda 1989 | No randomized controlled trial. |
| Tsushima 1991 | Difference in anthracycline peak dose between intervention and control group. |
| Ventura 2005 | No randomized controlled trial. |
| Yates 1982 | No definition of cardiotoxicity provided. |
| Zinzani 1995 | Difference in anthracycline peak dose between intervention and control group. |
Characteristics of studies awaiting assessment [ordered by study ID]
Burnell 2007.
| Methods | Method of randomisation not clear. |
| Participants | Female patients with node‐positive or high‐risk node‐negative breast cancer (maximal age 60 years). |
| Interventions | Doxorubicin versus epirubicin. |
| Outcomes | Survival and toxicity (including cardiotoxicity) (definitions not provided). |
| Notes | We were not able to contact the investigators of this trial, therefore we are not certain if this trial fulfilled all criteria for considering studies for this review. |
Knobloch 2008.
| Methods | Unclear if this is a randomized controlled trial. |
| Participants | Female patients with breast cancer, ovarial cancer, corpus carcinoma or collum carcinoma (age not specified). |
| Interventions | Epirubicin versus liposomal doxorubicin (caelyx). |
| Outcomes | Cardiotoxicity (definitions not provided). |
| Notes | We were not able to contact the investigators of this trial, therefore we are not certain if this trial fulfilled all criteria for considering studies for this review. |
NCT00002985.
| Methods | Method of randomisation not clear. |
| Participants | Patients with AIDS‐related Kaposi's sarcoma (age not specified). |
| Interventions | Liposomal daunorubicin (daunoxome) versus liposomal doxorubicin (doxil). |
| Outcomes | Clinical benefit, tumour response and safety (definitions not provided). |
| Notes | We were not able to contact the investigators of this trial, therefore we are not certain if this trial fulfilled all criteria for considering studies for this review. |
NCT00022516.
| Methods | Method of randomisation not clear. |
| Participants | Patients with breast cancer (stage I, II or III) (age not specified). |
| Interventions | Doxorubicin versus epirubicin. |
| Outcomes | Toxic effects, survival and quality of life (definitions not provided). |
| Notes | We were not able to contact the investigators of this trial, therefore we are not certain if this trial fulfilled all criteria for considering studies for this review. |
NCT00431795.
| Methods | Method of randomisation not clear. |
| Participants | Female patients with advanced breast cancer (aged between 18 and 75 years). |
| Interventions | Epirubicin versus liposomal doxorubicin (caelyx). |
| Outcomes | Tumour response and toxicities (definitions not provided). |
| Notes | We were not able to contact the investigators of this trial, therefore we are not certain if this trial fulfilled all criteria for considering studies for this review. |
NCT00516425.
| Methods | Method of randomisation not clear. |
| Participants | Female patients with invasive breast cancer (minimal age 70 years). |
| Interventions | Doxorubicin versus epirubicin. |
| Outcomes | Survival and safety (definitions not provided). |
| Notes | We were not able to contact the investigators of this trial, therefore we are not certain if this trial fulfilled all criteria for considering studies for this review. |
NCT00536393.
| Methods | Method of randomisation not clear. |
| Participants | Patients with disseminated high grade lymphoma (aged between 60 and 75 years). |
| Interventions | Doxorubicin versus liposomal doxorubicin. |
| Outcomes | Tumour response, survival, toxicities (including cardiotoxicity) (definitions not provided). |
| Notes | We were not able to contact the investigators of this trial, therefore we are not certain if this trial fulfilled all criteria for considering studies for this review. |
Northfelt 1996.
| Methods | Method of randomisation not clear. |
| Participants | Patients with AIDS‐related Kaposi's sarcoma (aged between 29 and 46 years). |
| Interventions | Doxorubicin versus liposomal doxorubicin. |
| Outcomes | Toxicity (according to WHO criteria). |
| Notes | We were not able to contact the investigators of this trial, therefore we are not certain if this trial fulfilled all criteria for considering studies for this review. |
WHO = world health organisation
Characteristics of ongoing studies [ordered by study ID]
NCT00575406.
| Trial name or title | Multicentre study to determine the cardiotoxicity of R‐CHOP (rituximab, cyclophosphamide, doxorubicin, vincristin and prednisolone) compared to R‐COMP (rituximab, cyclophosphamide, liposomal doxorubicin, vincristin and prednisolone) in patients with diffuse large B‐cell lymphoma (NHL‐14). |
| Methods | Method of randomisation not clear. |
| Participants | Patients with diffuse large B‐cell lymphoma (minimal age 18 years). |
| Interventions | Doxorubicin versus liposomal doxorubicin (both given in a short infusion with a peak dose of 50 mg/m2). |
| Outcomes | Cardiotoxicity (definition not provided). |
| Starting date | December 2007 |
| Contact information | Prof. dr. MA Fridrik (michael.fridrik@akh.linz.at) |
| Notes |
Contributions of authors
Elvira van Dalen designed the study. She developed the search strategy and undertook the searches in the different electronic databases. She searched for unpublished and ongoing studies and identified the studies meeting the inclusion criteria. She performed the data‐extraction and risk of bias assessment of the included studies. She analyzed the data and interpreted the results. She wrote and revised the manuscript.
Erna Michiels identified the studies meeting the inclusion criteria. She performed the data‐extraction and risk of bias assessment of the included studies. She contributed to the data‐analysis and the interpretation of the results.She critically reviewed the manuscript.
Huib Caron contributed to the data‐analysis and the interpretation of the results. He critically reviewed the manuscript.
Leontien Kremer designed the study. She performed the data‐extraction and risk of bias assessment of the included studies. She contributed to the data‐analysis and the interpretation of the results. She critically reviewed the manuscript.
All authors approved the final version.
Sources of support
Internal sources
Dutch Cochrane Centre, Netherlands.
External sources
Foundation of Pediatric Cancer Research (SKK) Amsterdam, Netherlands.
Stichting Steun Emma Kinderziekenhuis AMC, Netherlands.
Stichting Kinderen Kankervrij (KIKA), Netherlands.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Batist 2001 {published data only}
- Batist G, Ramakriskan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome‐encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized multicenter trial of metastatic breast cancer. Journal of Clinical Oncology 2001;19:1444‐54. [DOI] [PubMed] [Google Scholar]
Brambilla 1986 {published data only}
- Brambilla C, Rossi A, Bonfante V, Ferrari L, Villani F, Crippa F, et al. Phase II study of doxorubicin versus epirubicin in advanced breast cancer. Cancer Treatment Reports 1986;70:261‐6. [PubMed] [Google Scholar]
Chan 2004 {published data only}
- Chan S, Davidson N, Juozaityte E, Erdkamp F, Pluzanska A, Azarnia N, et al. Phase III trial of liposomal doxorubicin and cyclophosphamide compared with epirubicin and cyclophosphamide as first‐line therapy for metastatic breast cancer. Annals of Oncology 2004;15:1527‐34. [DOI] [PubMed] [Google Scholar]
FESG 1988 {published data only}
- French Epirubicin Study Group. A prospective randomized phase III trial comparing combination chemotherapy with cyclophosphamide, fluorouracil and either doxorubicin or epirubicin. Journal of Clinical Oncology 1988;6:679‐88. [DOI] [PubMed] [Google Scholar]
Gasparini 1991 {published data only}
- Gasparini G, Dal Fior S, Panizzoni GA, Favretto S, Pozza F. Weekly epirubicin versus doxorubicin as second line therapy in advanced breast cancer, a randomized clinical trial. American Journal of Clinical Oncology 1991;14:38‐44. [DOI] [PubMed] [Google Scholar]
Harris 2002 {published data only}
- Harris L, Batist G, Belt R, Rovira D, Navari R, Azarnia N, et al. Liposome‐encapsulated doxorubicin compared with conventional doxorubicin in a randomized multicenter trial as first‐line therapy of metastatic breast carcinoma. Cancer 2002;94:25‐36. [DOI] [PubMed] [Google Scholar]
IMBSWE 1988 {published data only}
- Italian Multicentre Breast Study with Epirubicin. Phase III randomized study of fluorouracil, epirubicin and cyclophosphamide v fluorouracil, doxorubicin and cyclophosphamide in advanced breast cancer: an Italian multicentre trial. Journal of Clinical Oncology 1988;6:976‐82. [DOI] [PubMed] [Google Scholar]
Mouridsen 1984 {published data only}
- Mouridsen HT, Somers R, Santoro A, Mulder JH, Bramwell V, Oosterom AT, et al. Doxorubicin vs epirubicin in advanced soft tissue sarcomas, an EORTC randomized phase II study. Bonadonna G (ed) Advances in anthracycline chemotherapy: epirubicin, Masson, Milan. 1984:95‐103. [Google Scholar]
References to studies excluded from this review
Al‐Ismael 1987 {published data only}
- Al‐Ismael DA, Whittaker JA, Gouch J. Combination chemotherapy including epirubicin for the management of non‐hodgkin's lymphoma. European Journal of Cancer and Clinical Oncology 1987;23:1379‐84. [DOI] [PubMed] [Google Scholar]
Armand 1984 {published data only}
- Armand JP. Bonadonna G (ed) Phase II and phase III studies with epirubicin in breast cancer in France. Advances in anthracycline chemotherapy: epirubicin. Masson, Milan: 75‐82, 1984. [Google Scholar]
Aviles 1993 {published data only}
- Aviles A, Arevila N, Diaz Maqueo JC, Gomez T, Garcia R, Jesus Nambo MA. Late cardiac toxicity of doxorubicin, epirubicin, and mitoxantrone therapy for Hodgkin's disease in adults. Leukemia and Lymphoma 1993;11:275‐9. [DOI] [PubMed] [Google Scholar]
Aviles 1995 {published data only}
- Aviles A, Soto B, Guzman R, Garcia EL, Jesus Nambo M, Diaz‐Maqueo JC. Results of a randomized study of early stage hodgkin's disease using ABVD, EBVD, or MBVD. Medical and Pediatric Oncology 1995;24:171‐5. [DOI] [PubMed] [Google Scholar]
Aviles 2005 {published data only}
- Aviles A, Neri N, Nambo JM, Huerta‐Guzman J, Talavera A, Cleto S. Late cardiac toxicity secondary to treatment in Hodgkin's disease. A study comparing doxorubicin, epirubicin and mitoxantrone in combined therapy. Leukemia and Lymphoma 2005;46:1023‐28. [DOI] [PubMed] [Google Scholar]
Baldini 2004 {published data only}
- Baldini E, Gardin G, Evangelista G, Prochilo T, Collecchi P, Lionetti R. Long‐term results of combined modality therapy for inflammatory breast carcinoma. Clinical Breast Cancer 2004;5:358‐63. [DOI] [PubMed] [Google Scholar]
Benjamin 1978 {published data only}
- Benjamin RS, Mason J, Billingham ME. Cardiac toxicity of adriamycin‐DNA complex and rubidazone: evaluation by electrocardiogram and endomyocardial biopsy. Cancer Treatment Reports 1978;62:935‐9. [PubMed] [Google Scholar]
Bertini 1997 {published data only}
- Bertini M, Freilone R, Botto B, Calvi R, Gallamini A, Gatti AM, et al. Idarubicin in patients with diffuse large cell lymphomas: a randomized trial comparing VACOP‐B (A=doxorubicin) vs VICOP‐B (I=idarubicin). Haematologica 1997;82:309‐13. [PubMed] [Google Scholar]
Bezwoda 1986 {published data only}
- Bezwoda WR. Treatment of advanced ovarian cancer: a randomised trial comparing adriamycin or 4'epi‐adriamycin in combination with cisplatin and cyclophosphamide. Medical and Pediatric Oncology 1986;14:26‐9. [DOI] [PubMed] [Google Scholar]
Bhutani 2002 {published data only}
- Bhutani M, Kumar L, Vora A, Bhardwaj N, Kumar Pathak A, Singh R, et al. Randomized study comparing 4'‐epi‐doxorubicin (epirubicin) versus doxorubicin as a part of induction treatment in adult acute lymphoblastic leukemia. American Journal of Hematology 2002;71:241‐7. [DOI] [PubMed] [Google Scholar]
Bonadonna 1984 {published data only}
- Bonadonna G, Brambilla C, Rossi A, Bonfante V, Ferrari L, Crippa F, et al. Epirubicin in advanced breast cancer, the experience of the Milan Cancer Institute. Bonadonna G (ed) Advances in anthracycline chemotherapy: epirubicin, Masson, Milan. 1984:63‐70. [Google Scholar]
Bonfante 1986 {published data only}
- Bonfante V, Ferrari L, Brambilla C, Rossi A, Villani F, Crippa F, et al. New anthracycline analogs in advanced breast cancer. European Journal of Cancer and Clinical Oncology 1986;22:1379‐85. [DOI] [PubMed] [Google Scholar]
Bontenbal 1998 {published data only}
- Bontenbal M, Andersson M, Wildiers J, Cocconi G, Jassem J, Paridaens R, et al. Doxorubicin vs epirubicin, report of a second‐line randomized phase II/III study in advanced breast cancer. British Journal of Cancer 1998;77:2257‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brugiatelli 1993 {published data only}
- Brugiatelli M, Federico M, Gobbi PG, Avanzini P, Callea V, Cavanna L, et al. Epidoxorubixin vs idarubicin containing regimens in intermediate and high grade non‐hodgkin's lymphoma: preliminary results of a multicentric randomized trial. Haematologica 1993;78:306‐12. [PubMed] [Google Scholar]
Burton 2005 {published data only}
- Burton C, Smith P, Vaughan‐Hudson G, Qian W, Hoskin P, Cunningham D, et al. Comparison of CHOP versus CIOP in good prognosis younger patients with histologically aggressive non‐Hodgkin lymphoma. British Journal of Hematology 2005;130:536‐41. [DOI] [PubMed] [Google Scholar]
Casper 1987A {published data only}
- Casper ES. The cardiotoxic effect of epirubicin and doxorubicin (adriamycin), a clinical and radionuclide cine‐angiographic comparison. Clinical Trials Journal 1987;24:57‐67. [Google Scholar]
Casper 1987B {published data only}
- Casper ES. Epirubicin and doxorubicin in advanced breast cancer, a comparative evaluation of epirubicin and doxorubicin. Clinical Trials Journal 1987;24:139‐41. [Google Scholar]
Cottin 1998 {published data only}
- Cottin Y, Touzery C, Dalloz F, Coudert B, Toubeau M, Riedinger A, et al. Comparison of epirubicin and doxorubicin cardiotoxicity induced by low doses: evaluation of the diastolic and systolic parameters studied by radionuclide angiography. Clinical Cardiology 1998;21:665‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Creutzig 1988 {published data only}
- Creutzig U, Hofmann J, Ritter J, Schellong G. Therapy realization and complications in the BFM‐83 therapy study of acute myelogenous leukemia [Therapierealisierung und komplikationen in der therapiestudie BFM‐83 fur die akute myeloische leukamie]. Klinische Padiatrie 1988;200:190‐9. [DOI] [PubMed] [Google Scholar]
Creutzig 2001A {published data only}
- Creutzig U, Ritter J, Zimmermann M, Hermann J, Gadner H, Blutters Sawatzki D, et al. Idarubicin improves blast cell clearance during induction therapy in children with AML: results of study AML‐BFM 93. Leukemia 2001;15:348‐54. [DOI] [PubMed] [Google Scholar]
Creutzig 2001B {published data only}
- Creutzig U, Berthold F, Boos J, Fleischhack G, Gadner H, Gnekow A, et al. Improved treatment results in children with AML: Results of study AML‐BFM 93 [Verbesserung der prognose bei kindern mit AML: ergebnisse der studie AML‐BFM 93]. Klinische Padiatrie 2001;213:175‐85. [DOI] [PubMed] [Google Scholar]
Creutzig 2001C {published data only}
- Creutzig U, Ritter J, Zimmermann M, Reinhardt D, Hermann J, Berthold F, et al. Improved treatment results in high‐risk pediatric acute myeloid leukemia patients after intensification with high‐dose cytarabine and mitoxantrone: results of study acute myeloid leukemia‐berlin‐frankfurt‐munster 93. Journal of Clinical Oncology 2001;19:2705‐13. [DOI] [PubMed] [Google Scholar]
De Lena 1987 {published data only}
- Lena M, Colucci G, Marzullo F, Lorusso V, Brandi M. Randomized trial with two CHOP‐B regimens (with doxorubicin or epirubicin) in poor prognosis non‐hodgkin's lymphomas. Clinical Trials Journal 1987;24:223‐9. [Google Scholar]
De Lena 1989 {published data only}
- Lena M, Maiello E, Lorusso V, Brandi M, Calabrese P, Romito S, et al. Comparison of CHOP‐B vs CEOP‐B in 'poor prognosis' non‐hodgkin's lymphomas, a randomized trial. Medical Oncology & Tumor Pharmacotherapy 1989;6:163‐9. [DOI] [PubMed] [Google Scholar]
Federico 1998 {published data only}
- Federico M, Clo V, Brugiatelli M, Carotenuto M, Gobbi PG, Vallisa D, et al. Efficacy of two different ProMACE‐CytaBOM derived regimens in advanced agressive non‐hodgkin's lymphoma. Final report of a multicenter trial conducted by GISL. Haematologica 1998;83:800‐11. [PubMed] [Google Scholar]
Gebbia 2003 {published data only}
- Gebbia V, Blasi L, Borsellino N, Caruso M, Leonardi V, Agostara B, et al. Paclitaxel and epidoxorubicin or doxorubicin versus cyclophosphamide and epidoxorubicin as first‐line chemotherapy for metastatic breast carcinoma: a randomized phase II study. Anticancer Research 2003;23:765‐72. [PubMed] [Google Scholar]
Gregory 2000 {published data only}
- Gregory RK, Hill ME, Moore J, A'Hern RP, Johnston SRD, Blake P, et al. Combining platinum, paclitaxel and anthracycline in patients with advanced gynaecological malignancy. European Journal of Cancer 2000;36:503‐7. [DOI] [PubMed] [Google Scholar]
Heidemann 1990 {published data only}
- Heidemann E, Steinke B, Hartlapp J, Schumacher K, Possinger K, Kunz S, et al. Randomized clinical trial comparing mitoxantrone with epirubicin and with doxorubicin, each combined with cyclophosphamide in the first‐line treatment of patients with metastatic breast cancer. Onkologie 1990;13:24‐7. [DOI] [PubMed] [Google Scholar]
Heidemann 1993 {published data only}
- Heidemann E, Steinke B, Hartlapp J, Schumacher K, Possinger K, Kunz S, et al. Prognostic subgroups: the key factor for treatment outcome in metastatic breast cancer, results of a three‐arm randomized multicenter trial comparing doxorubicin, epirubicin and mitoxantrone each in combination with cyclophosphamide. Onkologie 1993;16:344‐53. [Google Scholar]
Hernadi 1988 {published data only}
- Hernadi Z, Juhasz B, Poka R, Lampe LG. Randomised trial comparing combinations of cyclophosphamide and cisplatin without or with doxorubicin or 4‐epi‐doxorubicin in the treatment of advanced ovarian cancer. International Journal of Gynecology and Obstetrics 1988;27:199‐204. [DOI] [PubMed] [Google Scholar]
Homesley 1992 {published data only}
- Homesley HD, Harry DS, O'Toole RV, Hoogstraten B, Franklin EW, Cavanagh D, et al. Randomized comparison of cisplatin plus epirubicin or doxorubicin for advanced epithelial ovarian carcinoma. American Journal of Clinical Oncology 1992;15:129‐34. [DOI] [PubMed] [Google Scholar]
Hortobagyi 1989 {published data only}
- Hortobagyi GN, Yap HY, Kau SW, Fraschini G, Ewer MS, Chawla SP. A comparative study of doxorubicin and epirubicin in patients with metastatic breast cancer. American Journal of Clinical Oncology 1989;12:57‐62. [DOI] [PubMed] [Google Scholar]
Jain 1985 {published data only}
- Jain KK, Casper ES, Geller NL, Hakes TB, Kaufman RJ, Currie V, et al. A prospective randomized comparison of epirubicin and doxorubicin in patients with advanced breast cancer. Journal of Clinical Oncology 1985;3:818‐26. [DOI] [PubMed] [Google Scholar]
Keiling 1986 {published data only}
- Keiling R, Armand PP, Hurteloup P, Cappelaere P. French FAC vs FEC study in advanced breast cancer [Die franzosische FAC‐vs‐FEC‐studie bei fortgeschrittenen mammakarzinomen]. Onkologie 1986;9:8‐10. [DOI] [PubMed] [Google Scholar]
Klener 1973 {published data only}
- Klener P, Donner L, Kozena J. Daunorubicin and adriamycin in the treatment of leukemia. Neoplasma 1973;20:87‐96. [PubMed] [Google Scholar]
Lahtinen 1991 {published data only}
- Lahtinen R, Kuikka J, Nousiainen T, Uusitupa M, Lansimies E. Cardiotoxicity of epirubicin and doxorubicin: a double‐blind randomized study. European Journal of Haematology 1991;46:301‐5. [DOI] [PubMed] [Google Scholar]
Lawton 1993 {published data only}
- Lawton PA, Spittle MF, Ostrowski MJ, Young T, Madden F, Folkes A, et al. A comparison of doxorubicin, epirubicin and mitozantrone as single agents in advanced breast carcinoma. Clinical Oncology 1993;5:80‐4. [DOI] [PubMed] [Google Scholar]
Lopez 1989 {published data only}
- Lopez M, Papaldo P, Lauro L, Vici P, Carpano S, Conti EMS. 5‐Fluorouracil, adriamycin, cyclophosphamide (FAC) vs 5‐fluorouracil, epirubicin, cyclophosphamide (FEC) in metastatic breast cancer. Oncology 1989;46:1‐5. [DOI] [PubMed] [Google Scholar]
Lotrionte 2009 {published data only}
- Lotrionte M, Palazzoni G, Natali R, Comerci G, Abbate A, Persio S, et al. Appraising cardiotoxicity associated with liposomal doxorubicin by means of tissue Doppler echocardiography end‐points: rationale and design of the LITE (Liposomal doxorubicin‐Investigational chemotherapy‐Tissue Doppler imaging Evaluation) randomized pilot study. International Journal of Cardiology 2009;135:72‐7. [DOI] [PubMed] [Google Scholar]
Mandelli 1991 {published data only}
- Mandelli F, Petti MC, Ardia A, Pietro N, Raimondo F, Ganzina F, et al. A randomised clinical trial comparing idarubicin and cytarabine to daunorubicin and cytarabine in the treatment of acute non‐lymphoid leukaemia, a multicentric study from the italian co‐operative group GIMEMA. European Journal of Cancer 1991;27:750‐5. [DOI] [PubMed] [Google Scholar]
Maung 2002 {published data only}
- Maung K. Pegylated liposomal doxorubicin (doxil) versus doxorubicin for first‐line treatment of metastatic breast cancer. Clinical Breast Cancer 2002;3:183‐4. [Google Scholar]
Moser 2005 {published data only}
- Moser EC, Noordijk EM, Carde P, Tirelli U, Baars JW, Thomas J, et al. Late non‐neoplastic events in patients with aggressive non‐Hodgkin's lymphoma in four randomized European Organisation for Research and Treatment of Cancer trials. Clinical Lymphoma and Myeloma 2005;6:122‐30. [DOI] [PubMed] [Google Scholar]
Mouridsen 1987 {published data only}
- Mouridsen HT, Bastholt L, Somers R, Santoro A, Bramwell V, Mulder JH, et al. Adriamycin versus epirubicin in advanced soft tissue sarcomas, a randomized phase II / phase III study of the EORTC soft tissue and bone sarcoma group. European Journal of Cancer & Clinical Oncology 1987;23:1477‐83. [DOI] [PubMed] [Google Scholar]
Nair 1998 {published data only}
- Nair R, Ramakrishnan G, Nair NN, Saikia TK, Parikh PM, Joshi SR, et al. A randomized comparison of the efficacy and toxicity of epirubicin and doxorubicin in the treatment of patients with non‐hodgkin's lymphoma. Cancer 1998;82:2282‐88. [DOI] [PubMed] [Google Scholar]
Namer 2001 {published data only}
- Namer M, Soler‐Michel P, Turpin F, Chinet‐Charrot P, Gislain C, Pouillart P, et al. Results of a phase III prospective, randomised trial, comparing mitoxantrone and vinorelbine (MV) in combination with standard FAC/FEC in front‐line therapy of metastatic breast cancer. European Journal of Cancer 2001;37:1132‐40. [DOI] [PubMed] [Google Scholar]
NCT00531973 {published data only}
- Liposomal doxorubicin‐investigational chemotherapy‐tissue doppler imaging evaluation (LITE) randomized pilot study. NCT00531973 on www.controlled‐trials.com.
NCT00589082Latagliata2008 {published data only}
- DaunoXome (liposomal daunorubicin) plus ara‐C versus daunorubicin plus ara‐C in elderly AML patients; a randomized phase III study. NCT00589082 on www.controlled‐trials.com.
- Latagliata R, Breccia M, Fazi P, Iacobelli S, Martinelli G, Raimondo F, et al. Liposomal daunorubicin versus standard daunorubicin: long term follow‐up of the GIMEMA GSI 103 AMLE randomized trial in patients older than 60 years with acute myelogenous leukaemia. British Journal of Haematology 2008;143:681‐9. [DOI] [PubMed] [Google Scholar]
NCT00854568 {published and unpublished data}
- A randomized phase III study comparing CHOP verus CEOP‐induced cardiotoxicity in patients with aggressive B‐cell lymphoma. NCT00854568 on www.controlled‐trials.com.
Neri 1989 {published data only}
- Neri B, Cini‐Neri G, Bandinelli M, Pacini P, Bartalucci S, Ciapini A. Doxorubicin and epirubicin cardiotoxicity: experimental and clinical aspects. International Journal of Clinical Pharmacology, Therapy and Toxicology 1989;27:217‐21. [PubMed] [Google Scholar]
Nielsen 1998 {published data only}
- Nielsen OS, Dombernowsky P, Mouridsen H, Crowther D, Verweij J, Buesa J, et al. High‐dose epirubicin is not an alternative to standard‐dose doxorubicin in the treatment of advanced soft tissue sarcomas, a study of the EORTC soft tissue and bone sarcoma group. British Journal of Cancer 1998;78:1634‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielsen 2000 {published data only}
- Nielsen OS, Dombernowsky P, Mouridsen H, Daugaard S, Glabbeke M, Kirkpatrick A, et al. Epirubicine is not superior to doxorubicin in the treatment of advanced soft tissue sarcomas, the experience of the EORTC soft tissue and bone sarcoma group. Sarcoma 2000;4:31‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nikkanen 1988 {published data only}
- Nikkanen TAV, Jakobsson M, Jarvinen M, Liipo K, Ojala A, Paloheimo S, et al. Doxorubicin and 4'epidoxorubicin in combination chemotherapy of extensive small cell lung cancer. Acta Oncologica 1988;27:75‐6. [DOI] [PubMed] [Google Scholar]
O'Brian 2004 {published data only}
- O'Brian ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trials of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for the first‐line treatment of metastatic breast cancer. Annals of Oncology 2004;15:440‐9. [DOI] [PubMed] [Google Scholar]
Perez 1991 {published data only}
- Perez DJ, Harvey VJ, Robinson BA, Atkinson CH, Dady PJ, Kirk AR, et al. A randomized comparison of single‐agent doxorubicin and epirubicin as first‐line cytotoxic therapy in advanced breast cancer. Journal of Clinical Oncology 1991;9:2148‐52. [DOI] [PubMed] [Google Scholar]
Pinedo 1987 {published data only}
- Pinedo HM, Mouridsen HT, Somers R, Santor A, Mulder YH, Blanwell V, et al. A randomized trial comparing the effects of epirubicin and doxorubicin in soft tissue sarcoma. Clinical Trials Journal 1987;24:231‐41. [Google Scholar]
Rifkin 2006 {published data only}
- Rifkin RM, Gregory SA, Mohrbacher A, Hussein MA. Pegylated liposomal doxorubicin, vincristine, and dexamethasone provide significant reduction in toxicity compared with doxorubicin, vincristine, and dexamethasone in patients with newly diagnosed multiple myeloma: A phase III multicenter randomized trial. Cancer 2006;106:848‐58. [DOI] [PubMed] [Google Scholar]
- Rifkin RM, Hussein M, Iskandar R, O'Sullivan A, Thompson D, Forlenza J, et al. Economic evaluation of DVd versus VAd in the treatment of multiple myeloma: Results from a phase III multicenter randomized clinical trial. 2006 ASCO Annual Meeting Proceedings Part I. Vol 24. No. 18S (June 20 Supplement): 6050. 2006.
Sculier 1995 {published data only}
- Sculier JP, Bureau G, Giner V, Thiriaux J, MIchel J, Berchier MC, et al. Induction chemotherapy with ifosfamide, etoposide, and anthracycline for small cell lung cancer: experience of the european lung cancer working party. Seminars in Oncology 1995;22:18‐22. [PubMed] [Google Scholar]
Smith 1987 {published data only}
- Smith AP, Anderson EG, Chappell AG. Epirubicin and small cell lung cancer, a pilot study of the efficacy and toxicity of epirubicin and doxorubicin (adriamycin). Clinical Trials Journal 1987;24:195‐200. [Google Scholar]
Stohr 2006 {published data only}
- Stohr W, Paulides M, Brecht I, Kremers A, Treuner J, Langer T, et al. Comparison of epirubicin and doxorubicin cardiotoxicity in children and adolescents treated within the German Cooperative Soft Tissue Sarcoma Study (CWS). Journal of Cancer Research and Clinical Oncology 2006;132:35‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Suzuki 1984 {published data only}
- Suzuki T, Yamamoto H, Iwasaki T, Okamoto S, Iizuka T, Kanda H, et al. A comparative study of 4'epi‐doxorubicin and doxorubicin cardiotoxicities. Japanese Journal of Cancer Chemotherapy 1984;11:2170‐76. [PubMed] [Google Scholar]
Swenson 2003 {published data only}
- Swenson E, Bolcsak LE, Batist G, Guthrie Jr TH, Tkaczuk KH, Boxenbaum H, et al. Pharmacokinetics of doxorubicin administered i.v. as Myocet (TLC D‐99; liposome‐encapsulated doxorubicin citrate) compared with conventional doxorubicin when given in combination with cyclophosphamide in patients with metastatic breast cancer. Anti‐Cancer Drugs 2003;14:239‐46. [DOI] [PubMed] [Google Scholar]
Tanaka 1983 {published data only}
- Tanaka T, Suzuki K, Ota M, Hiyoshi Y, Yoshioka F, Kato H. Effects of adriamycin and daunomycin on cardiac function. Japanese Journal of Cancer Chemotherapy 1983;10:2393‐98. [PubMed] [Google Scholar]
Toda 1989 {published data only}
- Toda K, Asaishi K, Okazaki M, Okazaki Y, Okazaki A, Sato H, et al. Intra‐arterial infusion chemotherapy for advanced breast cancer‐effects and side effects of adriamycin, 4'‐epi‐adriamycin and THP‐adriamycin. Japanese Journal of Cancer & Chemotherapy 1989;16:3011‐14. [PubMed] [Google Scholar]
Tsushima 1991 {published data only}
- Tsushima K, Sakata Y, Suzuki H, Saitoh S, Sugimoto N, Itoh T, et al. A randomized controlled study of 5‐fluorouracil / doxorubicin / mitomycin C / OK‐432 (FAM‐OK) therapy and 5‐flourouracil / epirubicin / mitomycin C / OK‐432 (FEM‐OK) therapy in advanced gastric cancer. Journal of the Japanese Society for Cancer Therapy 1991;26:1317‐24. [Google Scholar]
Ventura 2005 {published data only}
- Ventura GJ. Cardiotoxicity of epirubicin versus doxorubicin: cost and clinical results. Journal of Clinical Oncology 2005;23:2873‐4. [DOI] [PubMed] [Google Scholar]
Yates 1982 {published data only}
- Yates J, Glidewell O, Wiernik P, Cooper M R, Steinberg D, Dosik H, et al. Cytosine arabinoside with daunorubicin or adriamycin for therapy of acute myelocytic leukemia: a CALGB study. Blood 1982;60:454‐62. [PubMed] [Google Scholar]
Zinzani 1995 {published data only}
- Zinzani PL, Martelli M, Storti S, Musso M, Cantonetti M, Leone G, et al. Phase III comparative trial using CHOP vs CIOP in the treatment of advanced intermediate‐grade non‐hodgkin's lymphoma. Leukemia and Lymphoma 1995;19:329‐35. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Burnell 2007 {published data only}
- Burnell MJ, Levine MN, Chapman JA, Bramwell V, Vandenberg T, Chalchal HI, et al. A phase III adjuvant trial of sequenced EC + filgrastim + epoetin‐alpha followed by paclitaxel compared to sequenced AC followed by paclitaxel compared to CEF in women with node‐positive or high‐risk node‐negative breast cancer (NCIC CTG MA.21). 2007 ASCO Annual Meeting Proceedings Part I. Vol 25, No. 18S (June 20 Supplement): 550. 2007.
Knobloch 2008 {published data only (unpublished sought but not used)}
- Knobloch K, Tepe J, Rossner D, Lichtinghagen R, Luck HJ, Busch KH, et al. Combined NT‐pro‐BNP and CW‐Doppler ultrasound cardiac output monitoring (USCOM) in epirubicin and liposomal doxorubicin therapy. International Journal of Cardiology 2008;128:316‐25. [DOI] [PubMed] [Google Scholar]
NCT00002985 {published data only}
- Double‐Blind Randomized Evaluation of Clinical Benefits of DOXIL in Patients With AIDS‐Related Kaposi's Sarcoma Treated With DOXIL or DaunoXome. NCT00002985 on www.controlled‐trials.com.
NCT00022516 {published data only}
- Maintenance Chemotherapy In Hormone Non‐Responsive Breast Cancer, Assessment of Vascular Endothelial Growth Factor (VEGF), Soluble Her2 Protein (NRP, HER2‐ECD) and Vascular Cellular Adhesion Molecule‐1 (VCAM‐1) in Serum Samples. NCT0022516 on www.controlled‐trials.com.
NCT00431795 {published data only (unpublished sought but not used)}
- A multicentre randomized phase II study of second line chemotherapy with epirubicin (farmorubicin) versus the pegylated liposomal doxorubicin in advanced breast cancer patients. NCT00431795 on www.controlled‐trials.com.
NCT00516425 {published data only (unpublished sought but not used)}
- Adjuvant cytotoxic chemotherapy in older women. NCT00516425 on www.controlled‐trials.com.
NCT00536393 {published data only (unpublished sought but not used)}
- Phase III study of treatment of disseminated and aggresive lymphoma R CHOP versus R CLOP (with liposomal doxorubicin). NCT00536393 on www.controlled‐trials.com.
Northfelt 1996 {published data only (unpublished sought but not used)}
- Northfelt DW, Martin FJ, Working P, Volberding PA, Russell J, Newman M, et al. Doxorubicin encapsulated in liposomes containing surface‐bound polyethylene glycol: pharmacokinetics, tumor localization, and safety in patients with AIDS‐related Kaposi's sarcoma. Journal of Clinical Pharmacology 1996;36:55‐63. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00575406 {published and unpublished data}
- Multicentre study to determine the cardiotoxicity of R‐CHOP (rituximab, cyclophosphamide, doxorubicin, vincristin and prednisolone) compared to R‐COMP (rituximab, cyclophosphamide, liposomal doxorubicin, vincristin and prednisolone) in patients with diffuse large B‐cell lymphoma (NHL‐14). NCT00575406 on www.controlled‐trials.com.
Additional references
Billingham 1978
- Billingham ME, Mason JW, Bristow MR, Daniels JR. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treatment Reports 1978;62:865‐72. [PubMed] [Google Scholar]
Bonadonna 1969
- Bonadonna G, Monfardini S. Cardiac toxicity of daunorubicin. Lancet 1969;1:837. [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. In: The Cochrane Library, Issue 3, 2005. Chichester (UK): John Wiley & Sons Ltd, 2005.
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2009]. The Cochrane Collaboration 2009. Available from www.cochrane‐handbook.org.
Kremer 2002a
- Kremer LC, Pal HJ, Offringa M, Dalen EC, Voute PA. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Annals of Oncology 2002;13:819‐29. [DOI] [PubMed] [Google Scholar]
Kremer 2002b
- Kremer LC, Dalen EC, Offringa M, Voute PA. Frequency and risk factors of anthracycline‐induced clinical heart failure in children: a systematic review. Annals of Oncology 2002;13:503‐12. [DOI] [PubMed] [Google Scholar]
Lefrak 1973
- Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973;32:302‐14. [DOI] [PubMed] [Google Scholar]
Meinardi 2002
- Meinardi MT, Graaf WTA, Gietema JA, Berg MP, Sleijfer DT, Vries EGE, et al. Evaluation of long term cardiotoxicity after epirubicin containing adjuvant chemotherapy and locoregional radiotherapy for breast cancer using various detection techniques. Heart 2002;88:81‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 1981
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207‐14. [DOI] [PubMed] [Google Scholar]
Muggia 1991
- Muggia FM, Green MD. New anthracycline antitumor antibiotics. Critical Reviews in Oncology/Hematology 1991;11:43‐64. [DOI] [PubMed] [Google Scholar]
Ng 2006
- Ng R, Better N, Green MD. Anticancer agents and cardiotoxicity. Seminars in Oncology 2006;33:2‐14. [DOI] [PubMed] [Google Scholar]
Nousiainen 2002
- Nousiainen T, Jantunen E, Vanninen E, Hartikainen J. Early decline in left ventricular ejection fraction predicts doxorubicin cardiotoxicity in lymphoma patients. British Journal of Cancer 2002;86:1697‐1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Shan 1996
- Shan K, Lincoff AM, Young JB. Anthracycline‐induced cardiotoxicity. Annals of Internal Medicine 1996;125:47‐58. [DOI] [PubMed] [Google Scholar]
Simbre 2005
- Simbre II VC, Duffy SA, Dadlani GH, Miller TL, Lipshultz SE. Cardiotoxicity of cancer chemotherapy, implications for children. Pediatric Drugs 2005;7:187‐202. [DOI] [PubMed] [Google Scholar]
Van Dalen 2006a
- Dalen EC, Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: a long‐term follow‐up study. European Journal of Cancer 2006;42(18):3191‐8. [DOI] [PubMed] [Google Scholar]
WHO Handbook 1979
- World Health Organization. WHO Handbook for reporting results of cancer treatment. Geneva: World Health Organization, 1979.
References to other published versions of this review
Van Dalen 2006b
- Dalen EC, Michiels EMC, Caron HN, Kremer LCM. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI] [PubMed] [Google Scholar]


