Abstract
Background
It is unclear whether people with type 2 diabetes mellitus on insulin monotherapy who do not achieve adequate glycaemic control should continue insulin as monotherapy or can benefit from adding oral glucose‐lowering agents to the insulin therapy.
Objectives
To assess the effects of insulin monotherapy compared with the addition of oral glucose‐lowering agents to insulin monotherapy for people with type 2 diabetes already on insulin therapy and inadequate glycaemic control.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, ClinicalTrials.gov, the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and reference lists of articles. The date of the last search was November 2015 for all databases.
Selection criteria
Randomised controlled clinical trials of at least two months' duration comparing insulin monotherapy with combinations of insulin with one or more oral glucose‐lowering agent in people with type 2 diabetes.
Data collection and analysis
Two review authors independently selected trials, assessed risk of bias, extracted data and evaluated overall quality of the evidence using GRADE. We summarised data statistically if they were available, sufficiently similar and of sufficient quality. We performed statistical analyses according to the statistical guidelines in the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
We included 37 trials with 40 treatment comparisons involving 3227 participants. The duration of the interventions ranged from 2 to 12 months for parallel trials and two to four months for cross‐over trials.
The majority of trials had an unclear risk of bias in several risk of bias domains. Fourteen trials showed a high risk of bias, mainly for performance and detection bias. Insulin monotherapy, including once‐daily long‐acting, once‐daily intermediate‐acting, twice‐daily premixed insulin, and basal‐bolus regimens (multiple injections), was compared to insulin in combination with sulphonylureas (17 comparisons: glibenclamide = 11, glipizide = 2, tolazamide = 2, gliclazide = 1, glimepiride = 1), metformin (11 comparisons), pioglitazone (four comparisons), alpha‐glucosidase inhibitors (four comparisons: acarbose = 3, miglitol = 1), dipeptidyl peptidase‐4 inhibitors (DPP‐4 inhibitors) (three comparisons: vildagliptin = 1, sitagliptin = 1, saxagliptin = 1) and the combination of metformin and glimepiride (one comparison). No trials assessed all‐cause mortality, diabetes‐related morbidity or health‐related quality of life. Only one trial assessed patients' treatment satisfaction and showed no substantial differences between the addition of either glimepiride or metformin and glimepiride to insulin compared with insulin monotherapy.
Insulin‐sulphonylurea combination therapy (CT) compared with insulin monotherapy (IM) showed a MD in glycosylated haemoglobin A1c (HbA1c) of ‐1% (95% confidence interval (CI) ‐1.6 to ‐0.5); P < 0.01; 316 participants; 9 trials; low‐quality evidence. Insulin‐metformin CT compared with IM showed a MD in HbA1c of ‐0.9% (95% CI ‐1.2 to ‐0.5); P < 0.01; 698 participants; 9 trials; low‐quality evidence. We could not pool the results of adding pioglitazone to insulin. Insulin combined with alpha‐glucosidase inhibitors compared with IM showed a MD in HbA1c of ‐0.4% (95% CI ‐0.5 to ‐0.2); P < 0.01; 448 participants; 3 trials; low‐quality evidence). Insulin combined with DPP‐4 inhibitors compared with IM showed a MD in HbA1c of ‐0.4% (95% CI ‐0.5 to ‐0.4); P < 0.01; 265 participants; 2 trials; low quality evidence. In most trials the participants with CT needed less insulin, whereas insulin requirements increased or remained stable in participants with IM.
We did not perform a meta‐analysis for hypoglycaemic events because the included studies used different definitions.. In most trials the insulin‐sulphonylurea combination resulted in a higher number of mild episodes of hypoglycaemia, compared to the IM group (range: 2.2 to 6.1 episodes per participant in CT versus 2.0 to 2.6 episodes per participant in IM; low‐quality evidence). Pioglitazone CT also resulted in more mild to moderate hypoglycaemic episodes compared with IM (range 15 to 90 episodes versus 9 to 75 episodes, respectively; low‐quality evidence. The trials that reported hypoglycaemic episodes in the other combinations found comparable numbers of mild to moderate hypoglycaemic events (low‐quality evidence).
The addition of sulphonylureas resulted in an additional weight gain of 0.4 kg to 1.9 kg versus ‐0.8 kg to 2.1 kg in the IM group (220 participants; 7 trials; low‐quality evidence). Pioglitazone CT caused more weight gain compared to IM: MD 3.8 kg (95% CI 3.0 to 4.6); P < 0.01; 288 participants; 2 trials; low‐quality evidence. Metformin CT was associated with weight loss: MD ‐2.1 kg (95% CI ‐3.2 to ‐1.1), P < 0.01; 615 participants; 7 trials; low‐quality evidence). DPP‐4 inhibitors CT showed weight gain of ‐0.7 to 1.3 kg versus 0.6 to 1.1 kg in the IM group (362 participants; 2 trials; low‐quality evidence). Alpha‐glucosidase CT compared to IM showed a MD of ‐0.5 kg (95% CI ‐1.2 to 0.3); P = 0.26; 241 participants; 2 trials; low‐quality evidence.
Users of metformin CT (range 7% to 67% versus 5% to 16%), and alpha‐glucosidase inhibitors CT (14% to 75% versus 4% to 35%) experienced more gastro‐intestinal adverse effects compared to participants on IM. Two trials reported a higher frequency of oedema with the use of pioglitazone CT (range: 16% to 18% versus 4% to 7% IM).
Authors' conclusions
The addition of all oral glucose‐lowering agents in people with type 2 diabetes and inadequate glycaemic control who are on insulin therapy has positive effects on glycaemic control and insulin requirements. The addition of sulphonylureas results in more hypoglycaemic events. Additional weight gain can only be avoided by adding metformin to insulin. Other well‐known adverse effects of oral glucose‐lowering agents have to be taken into account when prescribing oral glucose‐lowering agents in addition to insulin therapy.
Plain language summary
Combinations of insulin and oral glucose‐lowering drugs for people with type 2 diabetes on insulin treatment
Introduction
Many guidelines on type 2 diabetes recommend a glycosylated haemoglobin A1c (HbA1c) level below 7%. HbA1c levels in the blood express glucose or glycaemic control over a longer time period (two to three months). During the course of type 2 diabetes it will get more difficult to reach these levels with 'lifestyle' modification (diet, exercise or both) and oral glucose‐lowering agents alone. Finally, a substantial number of people will need insulin therapy for better glycaemic control. Insulin therapy can be initiated as insulin alone, called monotherapy (which means that oral glucose‐lowering medication will be stopped) or in combination with oral glucose‐lowering agents. In the former case, oral blood glucose‐lowering agents can be added at a later stage, if insulin monotherapy fails to achieve a good HbA1c level. Hypoglycaemia and weight gain are the most common and well known side effects of insulin therapy. Adding oral agents to insulin could reduce the required insulin dose and thus decrease these insulin‐related side effects. However, there could be other side effects specific to the various oral blood glucose‐lowering drugs.
Review question
To assess the effects of insulin monotherapy and the addition of an oral antidiabetic drug in people with type 2 diabetes already treated with insulin but not having good glycaemic control.
Background
It is unclear whether people with type 2 diabetes mellitus on insulin alone who do not achieve good glucose levels should continue with insulin alone or can benefit from adding an oral antidiabetic drug to their insulin therapy.
Study characteristics
All 37 included studies were randomised controlled trials (clinical studies where people are randomly put into one of two or more treatment groups). Their duration ranged from 2 to 12 months. The total number of participants was 3227. Several types of insulin monotherapy (once‐daily long‐ or intermediate‐acting insulin, twice‐daily premixed insulin, multiple injection therapy with short‐acting insulin) were compared with different types of additional antidiabetic tablets: sulphonylureas (such as glibenclamide/glyburide), metformin, alpha‐glucosidase inhibitors (such as acarbose), pioglitazone and DPP‐4 inhibitors (such as saxagliptin).
Key results
The addition of oral agents to insulin monotherapy reduced HbA1c by 0.4% to 1%. Most combinations of oral antidiabetic agents with insulin resulted in a reduction in the necessary insulin dose per day whereas the insulin dose per day had to be increased or remained stable in participants with insulin monotherapy. In studies reporting hypoglycaemic episodes severe events were rare and mild to moderate hypoglycaemia was observed in similar numbers when comparing insulin monotherapy to the addition of oral antidiabetic agents to insulin. However, most studies adding sulphonylureas to insulin reported more hypoglycaemic episodes. Moreover, the addition of sulphonylureas to insulin resulted in an additional weight gain of 0.4 kg to 1.9 kg compared with ‐0.8 kg to 2.1 kg in the insulin monotherapy groups. Pioglitazone insulin combination therapy caused on average an increase in weight of 3.8 kg compared with insulin monotherapy. The difference in average weight gain with metformin insulin combination therapy compared with insulin monotherapy was 2.1 kg less in favour of the combination therapy. Gastro‐intestinal side effects such as flatulence and diarrhoea were mostly reported with metformin and alpha‐glucosidase inhibitors. Addition of pioglitazone to insulin compared with insulin monotherapy resulted in more cases of oedema (fluid retention in the body) and heart failure. Only one study assessed participants' treatment satisfaction and showed no substantial differences between the addition of glimepiride or metformin and glimepiride to insulin compared with insulin monotherapy. No study assessed all‐cause mortality, diabetes‐related morbidity or health‐related quality of life.
This evidence is up to date as of November 2015.
Quality of the evidence
Almost a third of the studies had 30 or fewer participants. A lot of studies seemed to be underpowered and thus were probably not able to answer their own research question. This could mean that potentially important differences between intervention and control groups were not detected. Only five studies had a follow‐up of 12 months.
Summary of findings
Background
Description of the condition
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of these defects is chronic hyperglycaemia and disturbances of carbohydrate, fat and protein metabolism. Long‐term complications of diabetes mellitus include retinopathy, nephropathy and neuropathy. The risk of cardiovascular disease is increased.
The United Kingdom Prospective Diabetes Study (UKPDS) and its 10‐year follow‐up afterwards showed that tight glycaemic control can significantly reduce the development and progression of microvascular complications (Holman 2008; UKPDS 33; UKPDS 34). There is some inconsistency in the evidence of the effects of intensive treatment on macrovascular outcomes and mortality. Several large long‐term clinical trials comparing standard with intensive therapy did not show a significant reduction of cardiovascular outcomes and mortality (ACCORD 2008; ADVANCE 2008; Duckworth 2009; Kooy 2009). Intensive glycaemic control reduced the risk of microvascular complications but increased the risk of hypoglycaemia. Many guidelines on type 2 diabetes recommend a glycosylated haemoglobin A1c (HbA1c) below 7% for the majority of people with type 2 diabetes, however, a patient‐centred approach is more and more advocated, with the intent to encourage an appreciation of the variable and progressive nature of type 2 diabetes, the specific role of each drug, the patient and disease factors that drive clinical decision making and the constraints imposed by age and comorbidity (Inzucchi 2012). During the course of type 2 diabetes it will get more difficult to reach the HbA1c target levels with 'lifestyle' modification (diet, exercise or both) and oral glucose‐lowering agents alone. Finally, a substantial number of individuals will need insulin therapy for better glycaemic control (Turner 1999; Wright 2002).
Description of the intervention
Since the natural course of type 2 diabetes causes progressive decline of the pancreatic ß‐cell function, finally oral glucose‐lowering agents may not suffice and exogenous insulin will be required in a substantial number of people. At that stage insulin therapy can be initiated as insulin alone, that is monotherapy (which means that oral glucose‐lowering medication will be stopped) or in combination with oral glucose‐lowering agents. In the former category of people, oral blood glucose‐lowering agents can be added at a later stage, if monotherapy fails to achieve a sufficient HbA1c level. The latter intervention is the intervention under study in this review.
Adverse effects of the intervention
Hypoglycaemia, injection site reactions and weight gain are the most common and well known adverse effects of insulin therapy. Experimental and observational trials have shown that exogenous insulin may lead to increased atherosclerosis (Muis 2005; Ruige 1998; Stout 1990). Weight gain is another frequently reported adverse effect of insulin, with weight gain ranges from 0.3 kg to 3.8 kg. Several Dutch trials reported no effect or no negative effects on health‐related quality of life after starting insulin treatment (De Grauw 2001; De Sonnaville 1998; Goddijn 1999; Goudswaard 2004a). On the other hand, many people with type 2 diabetes (and healthcare providers) are reluctant to initiate insulin therapy. People with type 2 diabetes may be afraid of hypoglycaemia and weight gain, they may be uncomfortable with daily injections, they might experience restrictions in lifestyle and feelings of guilt and failure (Brunton 2005; Hunt 1997; Korytkowski 2002; Snoek 2002). In addition, primary care patients treated with insulin reported higher diabetes‐related distress compared with oral‐ or diet‐treated patients, which is stable over time and might be difficult to alter (Delahanty 2007; Karlsen 2014).
How the intervention might work
In the 1990s three reviews were executed comparing insulin monotherapy with insulin‐oral glucose‐lowering agents combination therapy (Johnson 1996; Peters 1991; Pugh 1992). The reviews did not distinguish between insulin‐treated and insulin‐naive participants. Besides, they only focused on sulphonylureas in combination with insulin therapy and excluded trials with other oral agents. Their conclusions differed. Peters 1991 concluded that combination therapy has no additional value for insulin‐treated people with type 2 diabetes, since it improved glycaemic control only slightly and it did not produce normal blood glucose concentrations. But Pugh 1992 and Johnson 1996 concluded that insulin combination therapy with sulphonylureas was more appropriate than insulin monotherapy because it was more efficacious and may be more cost‐effective. A more comprehensive review on the combination of insulin and oral glucose‐lowering agents in insulin‐naive and insulin‐treated patients did not meet Cochrane criteria (Yki‐Jarvinen 2001). It showed that in most trials glycaemic control was better and less insulin was required with the combination of insulin and oral glucose‐lowering agents compared with insulin alone. Notably, the difference in the required insulin dose between insulin monotherapy and the combination therapy was smaller in participants who were already being treated with insulin than in insulin‐naive participants.
Why it is important to do this review
In 2004 a Cochrane Review was published on the comparison of insulin monotherapy versus combinations of insulin and oral glucose‐lowering agents in insulin‐naive people with type 2 diabetes with poor glycaemic control despite maximal dosages of oral glucose‐lowering agents (Goudswaard 2004b). The authors concluded that bedtime Neutral Protamine Hagedorn (NPH) insulin combined with oral glucose‐lowering agents provides comparable glycaemic control to insulin monotherapy, but with less weight gain if metformin was used.
Up to now, no definitive answer has been available with regard to the comparison of insulin monotherapy versus combinations of insulin and oral glucose‐lowering agents in people with type 2 diabetes already on insulin therapy. In other words: is the adding of an oral glucose‐lowering agent to insulin beneficial with regard to outcomes such as glycaemic control, weight gain, hypoglycaemia, insulin dosage, health‐related quality of life and other outcome parameters? This systematic review will try to clarify the benefits of adding an oral blood glucose‐lowering agent to insulin monotherapy in people already on insulin therapy.
Objectives
To assess the effects of insulin monotherapy compared with the addition of oral glucose‐lowering agents to insulin monotherapy for people with type 2 diabetes already on insulin therapy and inadequate glycaemic control.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) with a minimal follow‐up period of two months.
Types of participants
Participants with type 2 diabetes mellitus (according to the appropriate diagnostic criteria at the time) already on insulin therapy and inadequate glycaemic control. To be consistent with changes in classification and diagnostic criteria of type 2 diabetes mellitus through the years, the diagnosis should have been established using the standard criteria valid at the time of the beginning of the trial (for example ADA 1997; ADA 1999; WHO 1980; WHO 1985; WHO 1998). Ideally, diagnostic criteria should have been described. If necessary, we used the study authors' definition of diabetes mellitus.
Types of interventions
Intervention
Combinations of insulin with one or more oral glucose‐lowering agent(s).
Control
Insulin monotherapy.
Types of outcome measures
Primary outcomes
All‐cause mortality
Diabetes‐related morbidity
Adverse events
Secondary outcomes
Health‐related quality of life
Patient satisfaction
Glycosylated haemoglobin A1c (HbA1c)
Fasting glucose
Lipids
Insulin dose
Method and timing of outcome measurement
Mortality: defined as all‐cause and diabetes‐related (cardiovascular mortality, mortality due to end‐stage renal disease or due to amputation) and measured at baseline and follow‐up with a minimum duration of two months.
Diabetes‐related morbidity: defined as myocardial infarction, angina, heart failure, stroke, renal failure, amputation (of at least one digit), vitreous haemorrhage, retinal photocoagulation, blindness in at least one eye, or cataract extraction and measured at baseline and follow‐up with a minimum duration of two months.
Adverse events such as hypoglycaemic episodes, weight gain, gastrointestinal symptoms, heart failure and measured at baseline and follow‐up with a minimum duration of two months.
Health‐related quality of life: evaluated by a validated instrument and measured at baseline and follow‐up with a minimum duration of two months.
Patient satisfaction: evaluated by a validated instrument and measured at baseline and follow‐up with a minimum duration of two months.
HbA1c: measured at baseline and follow‐up with a minimum duration of two months.
Fasting glucose: defined as after a period of eight hours of not eating or drinking with the exception of water and measured at baseline and follow‐up with a minimum duration of two months.
Lipids: defined as total cholesterol, high‐density lipoprotein (HDL)‐cholesterol, low‐density lipoprotein (LDL)‐cholesterol, triglycerides and measured at baseline and follow‐up with a minimum duration of two months.
Insulin dose: defined as once‐daily long‐acting, once‐daily intermediate‐acting, twice‐daily premixed insulin, and basal‐bolus regimen (multiple injections) and measured at baseline and follow‐up with a minimum duration of two months.
Summary of findings
We present a 'Summary of findings' table to report the following outcomes, listed according to priority.
All‐cause mortality.
Diabetes‐related mortality.
Diabetes‐related morbidity.
Health‐related quality of life.
Patient satisfaction.
Adverse events.
HbA1c.
Search methods for identification of studies
Electronic searches
We searched the following sources from inception of each database to the specified date and placed no restrictions on the language of publication.
Cochrane Central Register of Controlled Trials (CENTRAL issue 10, October 2015, 18.11.2015)
Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations and Ovid MEDLINE(R) 1946 to Present (18.11.2015)
Embase 1974 to 2015 November 17 (18.11.2015)
ClinicalTrials.gov (18.11.2015)
-
World Health Organization (WHO) ICTRP (International Clinical Trials Registry Platform ‐ http://apps.who.int/trialsearch/) (18.11.2015), including:
Australian New Zealand Clinical Trials Registry (2 November 2015)
Chinese Clinical Trial Registry (2 November 2015)
ClinicalTrials.gov (2 November 2015)
EU Clinical Trials Register (EU‐CTR) (2 November 2015)
ISRCTN (2 November 2015)
The Netherlands National Trial Register (3 November 2015)
Brazilian Clinical Trials Registry (ReBec) (13 October 2015)
Clinical Trials Registry ‐ India (13 October 2015)
Clinical Research Information Service ‐ Republic of Korea (13 October 2015)
Cuban Public Registry of Clinical Trials (13 October 2015)
German Clinical Trials Register (13 October 2015)
Iranian Registry of Clinical Trials (4 August 2015)
Japan Primary Registries Network (19 October 2015)
Pan African Clinical Trial Registry (13 October 2015)
Sri Lanka Clinical Trials Registry (13 October 2015)
Thai Clinical Trials Register (TCTR) (13 October 2015)
We also searched the excluded trials from the Cochrane Review with the same objective as ours except in insulin‐naive people with type 2 diabetes (Goudswaard 2004b).
If we had detected additional relevant key words during any of the electronic or other searches, we would have modified the electronic search strategies to incorporate these terms and document the changes.
Searching other resources
We tried to identify additional trials by searching the reference lists of included trials.
Data collection and analysis
Selection of studies
One review author (MA) first screened titles and abstracts to remove duplicates and obviously irrelevant records. Then two review authors (MA, AG or RV) independently scanned the abstract, title, or both, of the retriever records, to determine which trials should be assessed further. We investigated all potentially‐relevant articles as full text. Full articles were retrieved for further assessment if the information given suggested that the trial included participants with type 2 diabetes mellitus, compared insulin with a combination of insulin with oral glucose lowering agent(s), assessed one or more relevant clinical outcome measure(s), and used random allocation to the comparison groups. We resolved any discrepancies through consensus or recourse to a third review author (KG or GR). If resolution of a disagreement was not possible, we added the article to those 'awaiting assessment' and we contacted trial authors for clarification. We present an adapted Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram showing the process of trial selection (Figure 1) (Liberati 2009).
1.
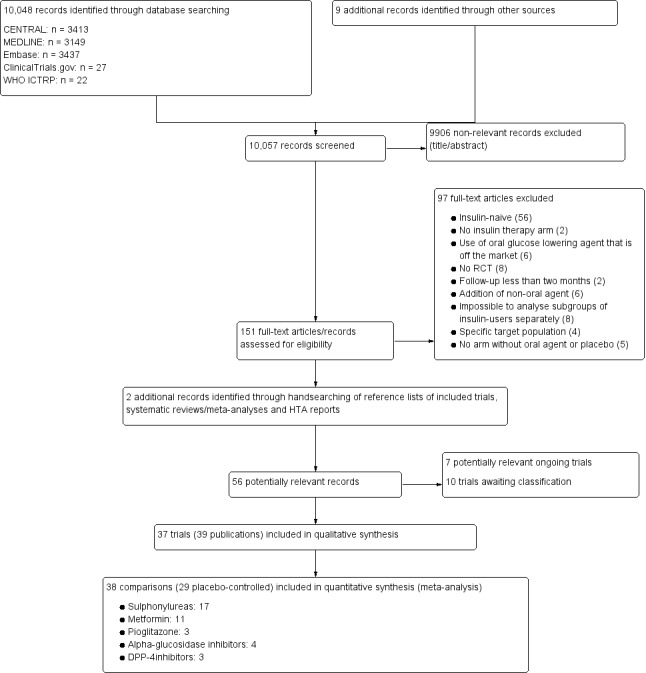
Study flow diagram.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary trial, we maximised yield of information by collating all available data and used the most complete data set aggregated across all known publications. In case of doubt, we prioritised the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Data extraction and management
For trials that fulfilled inclusion criteria, two review authors (MA and AK or MD or AG or RV) independently abstracted relevant population and intervention characteristics using standard data extraction templates (for details see Characteristics of included studies; Table 6; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8) with any disagreements to be resolved by discussion, or, if required, by a third party (KG).
1. Overview of study populations.
| Intervention(s) and comparator(s) | Sample sizea | Screened/eligible (N) | Randomised (N) | Safety (N) | ITT (N) | Finishing study (N) | Randomised finishing study (%) | Follow‐up timeb | |
| (1) Avilés 1999 | I: metformin | ‐ | 54 | 22 | 22 | ‐ | 21 | 95 | 24 weeks |
| C: placebo | 23 | 23 | ‐ | 22 | 96 | ||||
| total: | 45 | 45 | ‐ | 43 | |||||
| (2) Barnett 2013 | I:saxagliptin | ‐ | 141 | 95 | 95 | ‐ | 89 | 94 | 52 weeks |
| C:placebo | 46 | 46 | ‐ | 45 | 98 | ||||
| total: | 141 | 141 | ‐ | 134 | |||||
| (3) Casner 1988 | I: glibenclamide | 42 needed, 50 recruitment goal | 83 | 31 | 31 | 31 | 31 | 100 | 1 year |
| C: placebo | 33 | 33 | 33 | 33 | 100 | ||||
| total: | 64 | 64 | 64 | 64 | |||||
| (4) Chiasson 1994c | I: acarbose | 76 total, 38 per group | 91 | 41 | 41 | 40 | 35 | 85 | 1 year |
| C: placebo | 50 | 50 | 42 | 44 | 88 | ||||
| total: | 91 | 91 | 82 | 79 | |||||
| (5) Coniff 1995 | I: acarbose | ‐ | ‐ | 103 | 103 | ‐ | 103 | 100 | 24 weeks |
| C: placebo | 104 | 104 | ‐ | 104 | 100 | ||||
| total: | 207 | 207 | ‐ | 207 | |||||
| (6) Feinglos 1998d | I: glipizide | ‐ | 37 | ‐ | 7 months | ||||
| C: placebo | ‐ | ||||||||
| total: | 29 | 29 | ‐ | 29 | 100 | ||||
| (7) Fonseca 2007 | I: vildagliptin | ‐ | 461 | 144 | 144 | 114 | 79 | 24 weeks | |
| C: placebo | 152 | 152 | 124 | 82 | |||||
| total: | 296 | 296 | 290 | 238 | |||||
| (8) Fritsche 2000d | I: metformin | ‐ | ‐ | 24 weeks | |||||
| C: placebo | |||||||||
| total: | 13 | 13 | ‐ | 13 | 100 | ||||
| (9) Giugliano 1993 | I: metformin | ‐ | 50 | 27 | 27 | ‐ | 27 | 100 | 7 months |
| C: placebo | 23 | 23 | 23 | 100 | |||||
| total: | 50 | 50 | 50 | ||||||
| (10) Groop 1985d | I: glibenclamide | ‐ | 14 | 16 weeks (after 8 weeks run‐in) | |||||
| C: placebo | |||||||||
| total: | 14 | 14 | ‐ | 13 | 93 | ||||
| (11) Hermann 2001 | I: metformin | 30, 15 in each group | 37 | 16 | 16 | 16 | 12 | 75 | 12 months |
| C: placebo | 19 | 19 | 19 | 18 | 95 | ||||
| total: | 35 | 35 | 35 | 30 | |||||
| (12) Hong 2012 | I: sitagliptin | 140 | ‐ | 70 | 61 | 61 | 61 | 87 | 24 weeks |
| C: insulin increase | 70 | 63 | 63 | 63 | 90 | ||||
| total: | 140 | 124 | 124 | 124 | |||||
| (13) Hirsch 1999 | I: metformin | ‐ | ‐ | 25 | 25 | ‐ | 22 | 88 | 5 months |
| C: placebo | 25 | 25 | 25 | 100 | |||||
| total: | 50 | 50 | 47 | ||||||
| (14) Kitabchi 1987d | I: tolazamide | ‐ | 12 | 6 months | |||||
| C: insulin alone | |||||||||
| total: | 12 | 12 | ‐ | 12 | 100 | ||||
| (15) Krawczyk 2005 | I: metformin | ‐ | ‐ | 20 | 20 | 20 | 20 | 100 | 6 months |
| C: insulin alone | 20 | 20 | 20 | 20 | 100 | ||||
| total: | 40 | 40 | 40 | 40 | |||||
| (16) Kyllastinen 1985d | I: glibenclamide | ‐ | 11 | 4 months | |||||
| C: placebo | |||||||||
| total: | 9 | 9 | ‐ | 9 | 100 | ||||
| (17) Lewitt 1989d | I: glibenclamide | ‐ | 31 | 6 months | |||||
| C: placebo | |||||||||
| total: | 31 | 31 | ‐ | 31 | 100 | ||||
| (18) Lindstrom 1999d | I: glibenclamide | ‐ | ‐ | 6 months | |||||
| C: placebo | |||||||||
| total: | 15 | 15 | ‐ | 15 | 100 | ||||
| (19) Longnecker 1986d | I: tolazamide | ‐ | 12 | 20 weeks | |||||
| C: placebo | |||||||||
| total: | 12 | 12 | ‐ | 11 | 92 | ||||
| (20) Mattoo 2005 | I: pioglitazone | 250 (125 per treatment) | 385 | 142 | 142 | 142 | 128 | 90 | 6 months |
| C: placebo | 147 | 147 | 147 | 135 | 92 | ||||
| total: | 289 | 289 | 289 | 263 | |||||
| (21) Mauerhoff 1986 | I: glibenclamide | ‐ | 22 | 11 | 11 | ‐ | 11 | 100 | 19 weeks |
| C: placebo | 11 | 11 | 11 | 100 | |||||
| total: | 22 | 22 | 22 | ||||||
| (22) Mezitis 1992 | I: glibenclamide | ‐ | ‐ | 10 | 10 | ‐ | 10 | 100 | 20 weeks |
| C: placebo | 10 | 10 | 10 | 100 | |||||
| total: | 20 | 20 | ‐ | 20 | |||||
| (23) Mudaliar 2010 | I: pioglitazone | ‐ | ‐ | 12 | 12 | ‐ | 12 | 100 | 12‐16 weeks |
| C: placebo | 13 | 13 | 13 | 100 | |||||
| total: | 25 | 25 | 25 | ||||||
| (24) Nemoto 2011 | I: miglitol | ‐ | 276 | 107 | 107 | 107 | 100 | 93 | 16‐22 weeks |
| C: placebo | 100 | 100 | 100 | 97 | 97 | ||||
| total: | 207 | 207 | 207 | 197 | |||||
| (25) Osei 1984 | I: glibenclamide | ‐ | 22 | 10 | 10 | ‐ | 6 | 60 | 16 weeks |
| C: placebo | 12 | 12 | 11 | 92 | |||||
| total: | 22 | 22 | 17 | ||||||
| (26) Quartraro 1986 | I: gliclazide | ‐ | 40 | 15 | 15 | ‐ | 15 | 100 | 12 months |
| C: insulin alone | 15 | 15 | 15 | 100 | |||||
| total: | 30 | 30 | 30 | ||||||
| (27) Reich 1987e | I: glibenclamide | ‐ | 20 | 10 | 10 | ‐ | 9 | 90 | 4 months |
| C: placebo | 10 | 10 | 10 | 100 | |||||
| total: | 20 | 20 | 19 | ||||||
| (28) Relimpio 1998 | I: metformin | ‐ | 60 | 31 | 31 | 31 | 24 | 77 | 4 months |
| C: insulin dose increase | 29 | 29 | 23 | 23 | 79 | ||||
| total: | 60 | 60 | 60 | 47 | |||||
| (29) Robinson 1998d | I: metformin | ‐ | ‐ | 30 weeks | |||||
| C: placebo | |||||||||
| total: | 20 | 20 | ‐ | 19 | 95 | ||||
| (30) Rosenstock 2002 | I1: pioglitazone 15 mg | ‐ | ‐ | 191 | 191 | 191 | 161 | 89 | 16 weeks |
| I2: pioglitazone 30 mg | 188 | 188 | 188 | 172 | 92 | ||||
| C: placebo | 187 | 187 | 187 | 164 | 88 | ||||
| total: | 566 | 566 | 566 | 497 | |||||
| (31) Schade 1987d | I: glibenclamide | 16 | 16 | 32 weeks | |||||
| C: placebo | |||||||||
| total: | 16 | 16 | 16 | 15 | 94 | ||||
| (32) Schiel 2007 | I1: glimepiride | 60 (20 participants in each group) | 54 | 17 | 17 | 17 | 17 | 100 | 20 weeks |
| I2: glimepiride + metformin | 18 | 18 | 16 | 16 | 89 | ||||
| C: insulin alone | 17 | 17 | 17 | 17 | 100 | ||||
| total: | 52 | 52 | 50 | 50 | |||||
| (33) Simpson 1990 | I: glipizide | ‐ | 20 | 9 | ‐ | 4 months | |||
| C: placebo | 11 | ||||||||
| total: | 20 | 20 | 19 | 95 | |||||
| (34) Stenman 1988d | I: glibenclamide | ‐ | 16 | 5 months | |||||
| C: placebo | |||||||||
| total: | 16 | 16 | ‐ | 15 | 94 | ||||
| (35) Strowig 2002 | I1: metformin | ‐ | 92 | 30 | 30 | ‐ | 27 | 90 | 4 months |
| I2: troglitazone | 31 | 31 | 30 | 97 | |||||
| C: insulin alone | 31 | 31 | 31 | 100 | |||||
| total: | 92 | 92 | 88 | ||||||
| (36) Wulffelé 2002 | I: metformin | 390 | 745 | 196 | 195 | 196 | 171 | 87 | 16 weeks |
| C: placebo | 194 | 193 | 194 | 182 | 94 | ||||
| total: | 390 | 388 | 390 | 353 | |||||
| (37) Yilmaz 2007 | I1: acarbose | ‐ | ‐ | 15 | 15 | ‐ | 15 | 100 | 6 months |
| I2: metformin | 17 | 17 | 17 | 100 | |||||
| I3: rosiglitazone | 15 | 15 | 15 | 100 | |||||
| C: insulin alone | 19 | 19 | 19 | 100 | |||||
| total: | 66 | 66 | 66 | ||||||
| Grand total | All interventions | 1856f | 1677f | ||||||
| All comparators | 1558f | 1460f | |||||||
| All interventions and comparators | 3227 | 2951 | |||||||
aAccording to power calculation in study publication or report bDuration of intervention and/or follow‐up under randomised conditions until end of study cSubgroup of participants using insulin dCross‐over study eThree participants in the intervention group discontinued insulin fParticipants in cross‐over trials were counted both in the intervention and comparator groups
‐ denotes not reported
C: comparator; I: intervention; ITT: intention‐to‐treat; N/A: not applicable
We provide information including trial identifier about potentially relevant ongoing trials in the table 'Characteristics of ongoing studies'.
We sent an email request to authors of included trials to enquire whether they were willing to answer questions regarding their trials. Thereafter, we sought relevant missing information on the trial from the authors of the article, if required.
Assessment of risk of bias in included studies
Two review authors (MA and AK or MD or AG) assessed each trial independently. Possible disagreement was resolved by consensus, or with consultation with a third party in case of disagreement. We explored the influence of individual bias criteria in a sensitivity analysis (see Sensitivity analysis). In case of disagreement, we consulted the rest of the group and made a judgement based on consensus. We investigated risk of bias due to carry‐over effect in cross‐over trials during data‐extraction.
We used the Cochrane 'risk of bias' assessment tool (Higgins 2011b; Higgins 2011a) and investigated the following risk of bias criteria.
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other potential sources of bias.
We judged 'risk of bias' criteria as 'low risk', 'high risk' or 'unclear risk' and evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We present a 'Risk of bias' graph (Figure 2) and a 'Risk of bias summary' figure (Figure 3). We assessed the impact of individual risk of bias domains on trial results at the endpoint and trial levels. In case of high risk of selection bias, all endpoints investigated in the associated trial were marked as 'high risk'.
2.
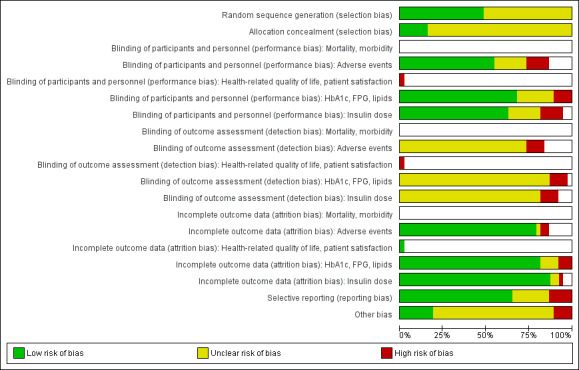
'Risk of bias' graph (blank cells indicate that the particular outcome was not investigated in some studies)
3.
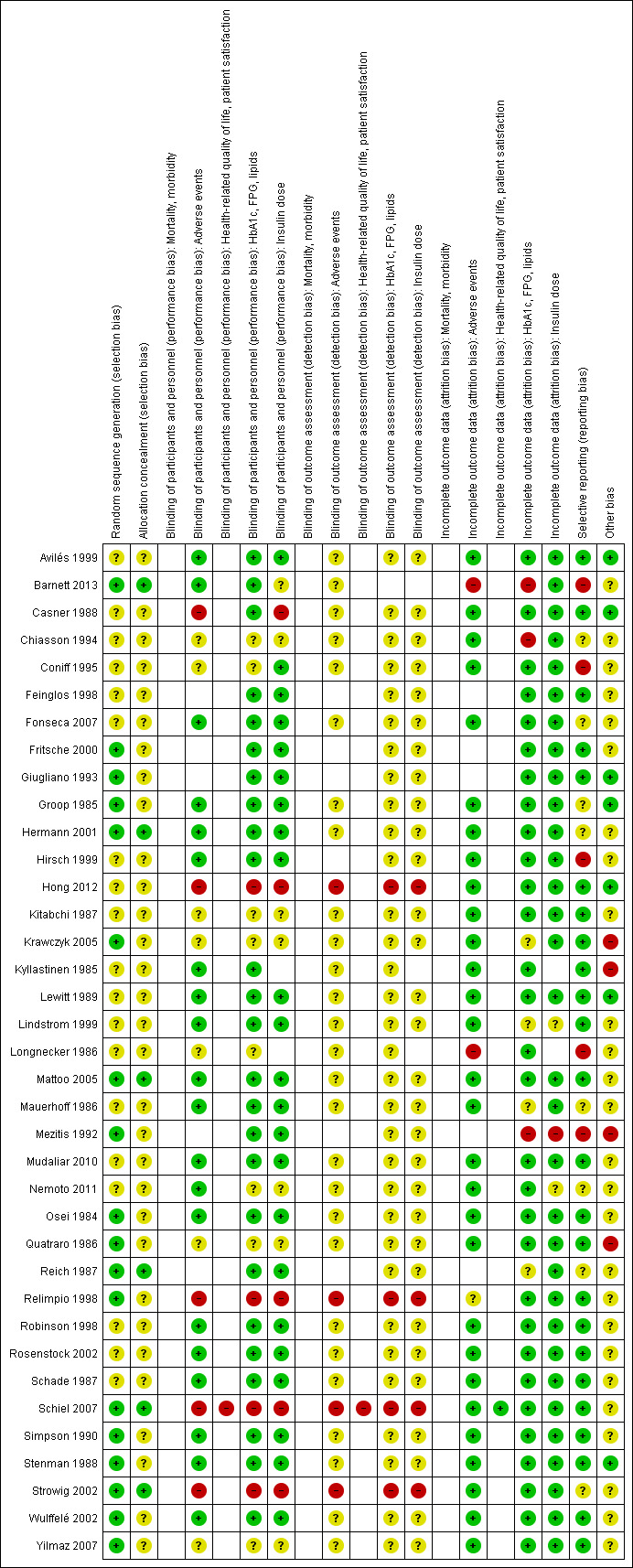
'Risk of bias' summary (blank cells indicate that the study did not report that particular outcome)
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessors) we evaluated the risk of bias separately for each outcome (Hróbjartsson 2013). We noted whether outcomes were measured subjectively or objectively, for example if body weight was measured by participants or trial personnel.
We considered the implications of missing outcome data from individual participants per outcome such as high dropout rates (e.g. above 15%) or disparate attrition rates (e.g. difference of 10% or more between trial arms).
We defined the following endpoints as subjective outcomes.
Health‐related quality of life.
Patient satisfaction.
We defined the following endpoints as objective outcomes.
All‐cause mortality.
Diabetes‐related morbidity.
Adverse events.
HbA1c.
Fasting glucose.
Lipids.
Insulin dose.
Measures of treatment effect
Continuous data
The results are expressed as mean differences (MD) with 95% confidence intervals (CIs).
For trials that did not provide HbA1c change‐from‐baseline values, we computed these data from baseline and post‐treatment values, if necessary extracted from graphs. When standard deviations of mean differences from the main outcome HbA1c were not provided in 11 publications (Barnett 2013; Casner 1988; Fonseca 2007; Giugliano 1993; Hirsch 1999; Mattoo 2005; Osei 1984; Quatraro 1986; Relimpio 1998; Strowig 2002; Yilmaz 2007), we computed these data assuming a general correlation coefficient that was derived from baseline and post‐treatment outcomes for HbA1c in trials that presented accompanying standard deviations. We computed matching standard deviations in SPSS 15.0 with a formula (formula 1), which included a general correlation coefficient of 0.5. This figure was 0.1 point lower than the correlation coefficient that was calculated from trials that provided information on change scores including standard deviations, and which appeared to be 0.6 in most trials (formula 2) (Armitage 2002). We used the same method and formula for assessing the standard deviation of the differences of fasting glucose for seven trials (Avilés 1999; Casner 1988; Mattoo 2005; Relimpio 1998; Schiel 2007; Strowig 2002; Yilmaz 2007), weight for six trials (Barnett 2013; Casner 1988; Krawczyk 2005; Mauerhoff 1986; Relimpio 1998; Strowig 2002), total cholesterol for five trials (Giugliano 1993; Osei 1984; Relimpio 1998; Strowig 2002; Yilmaz 2007), HDL‐cholesterol for six trials (Giugliano 1993; Mattoo 2005; Osei 1984; Relimpio 1998; Strowig 2002; Yilmaz 2007) and triglycerides for six trials (Fonseca 2007; Giugliano 1993; Osei 1984; Relimpio 1998; Strowig 2002; Yilmaz 2007). We included a correlation coefficient of 0.3 for fasting glucose, 0.9 for weight gain, 0.8 for HDL‐cholesterol and 0.6 for total cholesterol and triglycerides.
Formula 1: SPSS syntax for computing standard deviations of changes from baseline values of HbA1c: SD = sqrt (sd_tr_b2 + sd_tr_p2 ‐ (2 x corr x sd_tr_b x sd_tr_p)).
Abbreviations:
sd = standard deviation
sqrt = square root
sd_tr_b = standard deviation of mean baseline HbA1c
sd_tr_p = standard deviation of mean post‐treatment HbA1c
corr = correlation coefficient between baseline and post‐treatment values of HbA1c
Formula 2: SPSS syntax for computing correlation coefficient between baseline and post‐treatment values of HbA1c:
corr_tr = (hba1cbsd2 + hba1cptsd2 ‐ sddiff_tr2) / (2 x hba1cbsd x hba1cptsd).
Abbreviations:
corr_tr = correlation coefficient between baseline and post‐treatment values of HbA1c
hba1cbsd = standard deviation of mean baseline HbA1c
hba1cptsd = standard deviation of mean post‐treatment HbA1c
sddiff_tr = standard deviation of change from baseline HbA1c
Unit of analysis issues
We pooled the results of mean difference and standard error of the parallel group and the cross‐over trials using the generic inverse variance (GIV) method. In addition, we also used the non‐GIV method in order to give insight into the number of participants included in each trial and the range of mean values. For the cross‐over trials we calculated the correlation coefficient for within‐participants difference based on the trial results of Robinson 1998, and estimated the standard error as described in chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c). We then imputed this correlation coefficient in the other trials. When standard deviations of mean differences were not provided in the publications, we computed these data assuming a correlation coefficient that was derived from intervention and control outcomes in trials that presented accompanying standard deviations (HbA1c: Fritsche 2000; Kitabchi 1987; Kyllastinen 1985; Lewitt 1989; Schade 1987; Stenman 1988; fasting glucose: Fritsche 2000; Kyllastinen 1985; Lewitt 1989; Longnecker 1986; Robinson 1998; Stenman 1988; weight: Kitabchi 1987; Kyllastinen 1985; Lindstrom 1999; Robinson 1998; Schade 1987; Stenman 1988; total cholesterol: Groop 1985; Kitabchi 1987; Lindstrom 1999; Stenman 1988; HDL‐cholesterol: Groop 1985; Lindstrom 1999; Stenman 1988; triglycerides: Groop 1985; Kitabchi 1987; Lindstrom 1999; Stenman 1988).
Formula 3: SPSS syntax for computing correlation coefficient in cross‐over trials
corr_tr = (sd_tr_pa2 + sd_tr_pb2 ‐ sddiff_tr2) / (2 x sd_tr_pa x sd_tr_pb).
Abbreviations:
corr_tr = correlation coefficient between intervention and control values
sd_tr_pa = standard deviation of mean value after intervention
sd_tr_pb = standard deviation of mean value after control
sddiff_tr = standard deviation of within‐participant difference between intervention and control measurements
Dealing with missing data
We carefully evaluated important numerical data such as screened, eligible and randomised participants, as well as intention‐to‐treat (ITT) and per‐protocol (PP) population. We investigated attrition rates, for example dropouts, losses to follow‐up and withdrawals. We critically appraised issues of missing data, ITT and PP and compared them to specification of primary outcome parameters and power calculation.
Assessment of heterogeneity
We assessed clinical heterogeneity by comparing the trials with regard to different clinical parameters: patient characteristics, duration of disease, interventions and outcome. In the event of substantial clinical or methodological heterogeneity, we did not combine trial results by means of meta‐analysis. We identified statistical heterogeneity by visual inspection of the forest plots, by using a standard Chi² test and a significance level of α = 0.1, in view of the low power of such tests. Heterogeneity was specifically examined with the I² statistic (Higgins 2002; Higgins 2003), where I² values of 75% and more indicate a considerable level of heterogeneity (Deeks 2011). When heterogeneity was found, we attempted to determine potential reasons for it by examining individual trial characteristics and those of subgroups of the main body of evidence. We did not report the results of meta‐analysis with a considerable level of statistical heterogeneity (I² greater than 75%).
Assessment of reporting biases
If we included 10 trials or more investigating a particular outcome, we used funnel plots to assess small trial effects. Several explanations can be offered for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias. We therefore interpreted results carefully Sterne 2011).
Data synthesis
We summarised data statistically if they were available, sufficiently similar and of sufficient quality. We performed statistical analyses according to the statistical guidelines referenced in the latest version of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Unless there was good evidence for homogeneous effects across trials, we primarily summarised low risk of bias data using a random‐effects model (Wood 2008). We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual trial (Riley 2011). In addition, we performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011.
Quality of evidence
We present the overall quality of the evidence for each outcome as specified in types of outcome measures under 'Summary of findings' according to the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity such as directness of results. Two review authors (MA and AK or MD or AG) independently rated the quality for each outcome. We present a summary of the evidence in a 'Summary of findings' table, which provides key information about the best estimate of the magnitude of the effect, in relative terms and absolute differences for each relevant comparison of alternative management strategies, numbers of participants and trials addressing each important outcome, and the rating of the overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). We present results on the outcomes as described in the Types of outcome measures section. If meta‐analysis was not possible, we presented results in a narrative format in the 'Summary of findings' table.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses if one of the primary outcome parameters demonstrated statistically significant differences between intervention groups. In any other case, subgroup analyses would have been clearly marked as a hypothesis‐generating exercise.
We planned to carry out the following subgroup analyses.
Different oral glucose‐lowering agent(s) and different types of insulin.
Timing and frequency of insulin injections.
Sensitivity analysis
We planned to perform sensitivity analyses in order to explore the influence of very long trials (defined as equal to or greater than 24 weeks or six months) and the influence of trials with high risk of bias (defined as high risk of performance bias and detection bias because of not blinding researchers, or high risk of attrition bias because of incomplete outcome data, or both) on the effect size, to establish how much they dominated the results. Moreover, we compared the results of trials with a parallel design with the results of trials with a cross‐over design. We also planned to perform sensitivity analyses by restricting the analysis to published trials or restricting the analysis to trials using the following filters: diagnostic criteria; imputation; language of publication; source of funding (industry versus other); and country.
We also tested the robustness of the results by repeating the analysis using different measures of effect size (RR, odds ratio (OR), etc.) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies and Table 6.
Results of the search
The search strategy provided 10,048 citations. After exclusion of duplicates and trials not related to the objective of the review, two review authors (MA, AG or RV) independently assessed the remaining abstracts. One of the authors of this review (AG) has conducted a similar Cochrane Review, that also compares insulin monotherapy to insulin combined with oral glucose‐lowering agents, though in insulin‐naive type 2 diabetes patients (Goudswaard 2004b). One author (MA) scanned the title, abstract and text of the excluded trials of that review. Seven excluded trials were related to the objective of the current review and did not appear in the search. We found two additional records in the references of included articles. We obtained the full text of 151 potentially relevant trials, of which 37 (39 publications) fulfilled the inclusion criteria (for details see Figure 1).
Included studies
All 37 included trials were randomised controlled trials, of which 26 had a parallel design and 11 a cross‐over design (Feinglos 1998; Fritsche 2000; Groop 1985; Kitabchi 1987; Kyllastinen 1985; Lewitt 1989; Lindstrom 1999; Longnecker 1986; Robinson 1998; Schade 1987; Stenman 1988). Thirteen trials were conducted in the United States of America, three were conducted in Finland, two each in the United Kingdom, Sweden, Germany and Italy, and one each in Canada, Poland, Turkey, Australia, Belgium, Spain, Korea, Japan and the Netherlands. Another four trials were conducted in two or more countries. All trials were, if stated, conducted in secondary care. All were published in English, except one in Polish (Krawczyk 2005). More than 80% of the trials were sponsored by pharmaceutical companies.
The total number of participants was 3227 (range 9 to 566), with 0% to 100% men. Gender was not reported in four trials (Coniff 1995; Hirsch 1999; Mezitis 1992; Quatraro 1986). Participants ranged from 29 to 83 years of age and the duration of diabetes ranged from less than 1 to 31 years.
We evaluated 37 trials providing 40 comparisons between insulin monotherapy and insulin‐oral glucose‐lowering agents combination therapy. Insulin monotherapy was compared to insulin therapy in combination with:
sulphonylureas; n = 17 comparisons (glibenclamide = 11, glipizide = 2, tolazamide = 2, gliclazide = 1, glimepiride = 1);
metformin; n = 11 comparisons;
combination of metformin and sulphonylureas; n = 1 comparison;
pioglitazone; n = 4 comparisons;
alpha‐glucosidase inhibitors; n = 4 comparisons (acarbose n = 3, miglitol n = 1);
dipeptidyl peptidase‐4 inhibitors (DPP 4‐inhibitors); n = 3 comparisons (vildagliptin n = 1, sitagliptin n = 1, saxagliptin n = 1).
One trial on pioglitazone (Rosenstock 2002) compared the combination of insulin therapy with pioglitazone 15 mg as well as pioglitazone 30 mg to placebo. Insulin therapy was applied as a once‐daily, twice‐daily, and/or a multiple‐daily injection regimen. In almost all trials that reported the insulin regimens, participants received a different number of injections per day. Nine trials included participants who used a once‐daily insulin regimen (Barnett 2013; Longnecker 1986; Mattoo 2005; Mudaliar 2010; Osei 1984; Quatraro 1986; Reich 1987; Simpson 1990; Stenman 1988), in the other trials all participants received two or more injections per day. The total trial duration of all trials ranged from 2 to 12 months. The mean follow‐up of an intervention period of the cross‐over trials varied from two to four months.
All except one trial (Mauerhoff 1986) reported glycaemic control as mean values of glycosylated haemoglobin A1c (HbA1c). Ten trials provided change‐from‐baseline values for HbA1c with standard deviations or errors (Coniff 1995; Fonseca 2007; Fritsche 2000; Hong 2012; Nemoto 2011; Relimpio 1998; Robinson 1998; Rosenstock 2002; Schiel 2007; Wulffelé 2002;). Fasting blood glucose values were not reported in two trials (Coniff 1995; Mezitis 1992). Eleven trials provided change‐from‐baseline values for body weight with standard deviations or errors (Coniff 1995; Fonseca 2007; Fritsche 2000; Hong 2012; Mattoo 2005; Mudaliar 2010; Relimpio 1998; Robinson 1998; Strowig 2002; Wulffelé 2002; Yilmaz 2007). Change in insulin requirement was reported in all trials, except one (Chiasson 1994). No trial assessed patient‐reported outcomes like general well‐being or health‐related quality of life. Only one trial assessed patient treatment satisfaction (Schiel 2007). Eight trials reported data on total cholesterol, HDL‐cholesterol and/or triglycerides. All but 13 trials (Fritsche 2000; Giugliano 1993; Groop 1985; Kitabchi 1987; Krawczyk 2005; Kyllastinen 1985; Lewitt 1989; Lindstrom 1999; Longnecker 1986; Mezitis 1992; Mudaliar 2010; Osei 1984; Quatraro 1986) in some way provided information on hypoglycaemic events. Almost half of the trials reported information on other adverse effects.
Further details are listed in the Table Characteristics of included studies.
Excluded studies
Reasons for exclusion of trials are given in Characteristics of excluded studies. Main reasons for exclusion were that participants were insulin‐naive, trials used a non‐appropriate trial design, and non‐oral agents were added to insulin therapy.
Ongoing studies
We found seven ongoing trials, four with a subgroup for the combination insulin‐DPP IV inhibitor (sitagliptin = 1, vildagliptin = 1, saxagliptin = 2) versus insulin monotherapy, one with a thiazolidinedione (rosiglitazone) combined therapy and one with the combination insulin‐ipragliflozin (approved in Japan). More details of these trials are given in Characteristics of ongoing studies.
Risk of bias in included studies
All trials included in this review had some methodological weaknesses according to the criteria set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b), and thus showed unclear or high risk of bias in several risk of bias domains (Figure 2; Figure 3).
Allocation
Only six trials (Barnett 2013; Hermann 2001; Mattoo 2005; Reich 1987; Schiel 2007; Strowig 2002) fully reported the method of randomisation and allocation concealment. For the remaining trials it was not possible to judge whether the sequence generation was adequate and if the allocation to the intervention and control groups was concealed.
Blinding
The method of blinding was stated as open in four trials (Hong 2012; Relimpio 1998; Schiel 2007; Strowig 2002). The majority of the trials were double‐blinded. Mostly, it was unclear whether the researcher or the outcome assessor was blinded in addition to the participant. Risk of performance and detection bias was high for some outcomes in five trials (Casner 1988; Hong 2012; Relimpio 1998; Schiel 2007; Strowig 2002).
Incomplete outcome data
Eleven trials (Feinglos 1998; Fritsche 2000; Giugliano 1993; Kitabchi 1987; Krawczyk 2005; Lindstrom 1999; Mauerhoff 1986; Mezitis 1992; Mudaliar 2010; Quatraro 1986; Simpson 1990), with rather small trial populations ranging from 12 to 50 participants, did not mention whether there were dropouts or whether there was excessive loss to follow‐up. In thirteen trials (Avilés 1999; Barnett 2013; Casner 1988; Coniff 1995; Groop 1985; Hirsch 1999; Kyllastinen 1985; Lewitt 1989; Longnecker 1986; Osei 1984; Robinson 1998; Stenman 1988; Strowig 2002), dropouts were reported but no intention‐to‐treat analysis was executed or it was unclear whether it was done. In thirteen trials (Chiasson 1994; Fonseca 2007; Hermann 2001; Hong 2012; Mattoo 2005; Nemoto 2011; Reich 1987; Relimpio 1998; Rosenstock 2002; Schade 1987; Schiel 2007; Wulffelé 2002; Yilmaz 2007) dropouts were reported and intention‐to‐treat analysis was performed.
Selective reporting
We judged five trials (Barnett 2013; Coniff 1995; Hirsch 1999; Longnecker 1986; Mezitis 1992) to be at a high risk of bias for selective reporting, because some predefined outcomes were not reported. These outcomes (like level of liver enzymes or hormones) were often unimportant for the objective of this review.
Other potential sources of bias
The sample size of trials ranged from 9 to 566 participants. Thirteen of the 37 trials had 30 or fewer participants. Only eight trials (Casner 1988; Chiasson 1994; Hermann 2001; Hong 2012; Mattoo 2005; Schade 1987; Schiel 2007; Wulffelé 2002) discussed power calculations. This might mean that potential significant differences across groups were difficult to detect. Follow‐up periods differed between trials, ranging from 2 to 12 months. The outcome values of the trials with a short follow‐up might have been different if the trial had been continued for a longer period. In all cross‐over trials a cross‐over design was suitable and no risks of a carry‐over effect were found. Most trials were funded by pharmaceutical companies and often the overall outcome was in favour of the product of the sponsoring company.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5
Summary of findings for the main comparison. Summary of findings: sulphonylureas.
| Combinations of insulin and sulphonylureas compared with insulin monotherapy for diabetes mellitus | |||||
|
Patient: participants with type 2 diabetes mellitus Settings: mostly secondary care outpatients and secondary care inpatients Intervention: sulphonylureas plus insulin Comparison: insulin monotherapy | |||||
| Outcomes | Insulin monotherapy | Insulin plus sulphonylureas | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| All‐cause mortality | See comment | See comment | See comment | See comment | Not investigated |
| Diabetes‐related mortality | See comment | See comment | See comment | See comment | Not investigated |
| Diabetes‐related morbidity | See comment | See comment | See comment | See comment | Not investigated |
| Health‐related quality of life | See comment | See comment | See comment | See comment | Not investigated |
| Patient satisfaction | See comment | See comment | See comment | See comment | Not investigated |
|
Adverse events: a. mild hypoglycaemia (episodes per participant) Follow‐up: 12 weeks to 12 months b. weight gain (kg) Follow‐up: 8 weeks to 12 months |
a. range 2.0‐2.6 b. the mean weight gain across control groups ranged from ‐0.8 kg to 2.1 kg |
a. range 2.2‐6.1 b. the mean weight gain across intervention groups ranged from 0.4 kg to 1.9 kg |
a. 239 (8) b. 220 (7) |
a. ⊕⊕⊝⊝
lowa b. ⊕⊕⊝⊝ lowa |
a. Serious hypoglycaemic episodes were rare |
|
HbA1c, change from baseline (%) Follow‐up: 2 to 12 months |
The mean change in HbA1c ranged across control groups from ‐1.5% to 3% | The mean change in HbA1c in the intervention groups was 1% lower (1.6% lower to 0.5% lower) | 316 (9) | ⊕⊕⊝⊝ lowb | ‐ |
| CI: confidence interval; HbA1c: glycosylated haemoglobin A1c | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded by two levels because of risk of performance and detection bias and indirectness bDowngraded by two levels because of risk of performance bias and indirectness
Summary of findings 2. Summary of findings: metformin.
| Combinations of insulin and metformin compared with insulin monotherapy for diabetes mellitus | |||||
|
Patient: participants with type 2 diabetes mellitus Settings: mostly secondary care outpatients and secondary care inpatients Intervention: metformin plus insulin Comparison: insulin monotherapy | |||||
| Outcomes | Insulin monotherapy | Insulin plus metformin | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| All‐cause mortality | See comment | See comment | See comment | See comment | Not investigated |
| Diabetes‐related mortality | See comment | See comment | See comment | See comment | Not investigated |
| Diabetes‐related morbidity | See comment | See comment | See comment | See comment | Not investigated |
| Health‐related quality of life | See comment | See comment | See comment | See comment | Not investigated |
| Patient satisfaction | See comment | See comment | See comment | See comment | Not investigated |
|
Adverse events: a. mild hypoglycaemia (episodes per participant) Follow‐up: 12 weeks to 12 months b. weight gain (kg) Follow‐up: 12 weeks to 12 months |
a. see comment b. the mean weight gain across control groups ranged from 0 kg to 4.4 kg |
a. see comment b. the mean weight gain across intervention groups was 2.1 kg lower (3.2 kg lower to 1.1 kg lower) |
a. 590 (8) b. 615 (7) |
a. ⊕⊕⊝⊝
lowa b.⊕⊕⊝⊝ lowa |
a. comparable occurrences of hypoglycaemic events, severe hypoglycaemic episodes were rare |
|
HbA1c, change from baseline (%) Follow‐up: 3.5 to 6 months |
The mean change in HbA1c across control groups ranged from ‐1.6% to 0.5% | The mean change in HbA1c in the intervention groups was 0.9% lower (1.2% lower to 0.5% lower) | 698 (9) | ⊕⊕⊝⊝ lowb | ‐ |
| CI: confidence interval; HbA1c: glycosylated haemoglobin A1c | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded by two levels because of risk of performance and detection bias and indirectness bDowngraded by two levels because of risk of performance bias and indirectness
Summary of findings 3. Summary of findings: pioglitazone.
| Combinations of insulin and pioglitazone compared with insulin monotherapy for diabetes mellitus | |||||
|
Patient: participants with type 2 diabetes mellitus Settings: mostly secondary care outpatients and clinical research centre Intervention: pioglitazone plus insulin Comparison: insulin monotherapy | |||||
| Outcomes | Insulin monotherapy | Insulin plus pioglitazone | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| All‐cause mortality | See comment | See comment | See comment | See comment | Not investigated |
| Diabetes‐related mortality | See comment | See comment | See comment | See comment | Not investigated |
| Diabetes‐related morbidity | See comment | See comment | See comment | See comment | Not investigated |
| Health‐related quality of life | See comment | See comment | See comment | See comment | Not investigated |
| Patient satisfaction | See comment | See comment | See comment | See comment | Not investigated |
|
Adverse events: a. mild to moderate hypoglycaemia (episodes per participant) Follow‐up: 16 weeks to 6 months b. weight gain (kg) Follow‐up: 16 weeks to 6 months c. oedema (%) Follow‐up: 16 weeks to 6 months |
a. range 9‐75 b. the mean weight gain across control groups ranged from 0.2 kg to 1.7 kg c. range 4%‐7% |
a. range 15‐90 b. the mean weight gain in the intervention groups was3.8 kg higher (3.0 kg higher to 4.6 kg higher) c. range 16%‐18% |
a. 760 (2) b. 288 (2) c. 760 (2) |
a. ⊕⊕⊝⊝
lowa b. ⊕⊕⊝⊝ lowb c. ⊕⊕⊝⊝ lowb |
a. the proportion of all hypoglycaemic episodes was higher in the pioglitazone‐insulin combination group compared to insulin monotherapy; serious hypoglycaemic episodes were rare b. the minimum of 1.9 kg weight gain is clinically relevant, because it may have been partially caused by oedema c. pioglitazone was associated with a higher frequency of oedema which increased with dose. In addition, Rosenstock 2002 reported congestive heart failure for two participants receiving 15 mg pioglitazone and two participants receiving 30 mg pioglitazone. |
| HbA1c, change from baseline (%) Follow‐up: 12 weeks to 6 months | See comment | See comment | 785 (3) | ⊕⊕⊝⊝ lowc | The mean difference in HbA1c for insulin‐pioglitazone combination therapy ranged from ‐0.5% to ‐1.0% and for insulin monotherapy from ‐0.6% to 0% |
| CI: confidence interval; HbA1c: glycosylated haemoglobin A1c | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded by two levels because of risk of performance bias, indirectness and imprecision bDowngraded by two levels because of unclear risk of bias in several risk of bias domains, indirectness and imprecision cDowngraded by two levels because of unclear risk of bias in several risk of bias domains, indirectness and imprecision
Summary of findings 4. Summary of findings: alpha‐glucosidase inhibitors.
| Combinations of insulin and alpha‐glucosidase inhibitors compared with insulin monotherapy for diabetes mellitus | |||||
|
Patient: participants with type 2 diabetes mellitus Settings: mostly secondary care outpatients Intervention: alpha‐glucosidase inhibitors plus insulin Comparison: insulin monotherapy | |||||
| Outcomes | Insulin monotherapy | Insulin plus alpha‐glucosidase inhibitors | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| All‐cause mortality | See comment | See comment | See comment | See comment | Not investigated |
| Diabetes‐related mortality | See comment | See comment | See comment | See comment | Not investigated |
| Diabetes‐related morbidity | See comment | See comment | See comment | See comment | Not investigated |
| Health‐related quality of life | See comment | See comment | See comment | See comment | Not investigated |
| Patient satisfaction | See comment | See comment | See comment | See comment | Not investigated |
|
Adverse events: a. mild hypoglycaemia (% of participants) Follow‐up: 24 weeks to 12 months b. weight gain (kg) Follow‐up: 24 weeks to 12 months |
a. range 0%‐35% b. The mean weight gain across control groups ranged from +0.7 kg to +3.6 kg |
a. range 0%‐39% b. the mean weight gain in the intervention groups was0.5 kg lower (1.2 kg lower to 0.3 kg higher) |
a. 583 (4) b. 241 (2) |
a) ⊕⊝⊝⊝lowa b) ⊕⊝⊝⊝lowa |
a. serious hypoglycaemic episodes were rare |
|
HbA1c, change from baseline (%) Follow‐up: 3 to 6 months |
The mean change in HbA1c across control groups ranged from ‐1.1% to 0.04% | The mean change in HbA1c in the intervention groups was 0.4% lower (0.5% lower to 0.2% lower) | 448 (3) | ⊕⊝⊝⊝lowa | ‐ |
| CI: confidence interval; HbA1c: glycosylated haemoglobin A1c | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded by two levels because of unclear or high risk of bias in several risk of bias domains, indirectness and imprecision
Summary of findings 5. Summary of findings: DPP‐4 inhibitors.
| Combinations of insulin and dipeptidyl peptidase 4 inhibitors compared with insulin monotherapy for diabetes mellitus | |||||
|
Patient: participants with type 2 diabetes mellitus Settings: mostly secondary care outpatients Intervention: dipeptidyl peptidase 4 (DPP‐4) inhibitors plus insulin Comparison: insulin monotherapy | |||||
| Outcomes | Insulin monotherapy | Insulin + DPP4‐inhibitor | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| All‐cause mortality | See comment | See comment | See comment | See comment | Not investigated |
| Diabetes‐related mortality | See comment | See comment | See comment | See comment | Not investigated |
| Diabetes‐related morbidity | See comment | See comment | See comment | See comment | Not investigated |
| Health‐related quality of life | See comment | See comment | See comment | See comment | Not investigated |
| Patient satisfaction | See comment | See comment | See comment | See comment | Not investigated |
|
Adverse events: a. hypoglycaemia (% of participants) Follow‐up: 24 weeks to 52 weeks b. weight gain (kg) Follow‐up: 24 weeks to 52 weeks |
a. range 5%‐30% (0%‐5% severe) b. the mean weight gain across control groups ranged from 0.6 kg to 1.1 kg |
a. range 8%‐23% (0%‐2% severe) b. the mean weight gain in the intervention groups ranged from ‐0.7 kg to 1.3 kg compared to 0.6 kg to 1.1 kg in the insulin (+ placebo) monotherapy group |
a. 503 (3) b. 362 (2) |
a) ⊕⊕⊝⊝
lowa b) ⊕⊕⊝⊝ lowa |
|
|
HbA1c, change from baseline (%) Follow‐up: 24 weeks to 52 weeks |
The mean change in HbA1c across control groups ranged from ‐0.2% to ‐0.3% | The mean change in HbA1c in the intervention groups was 0.4% lower (0.5% lower to 0.4% lower) | 265 (2) | ⊕⊕⊝⊝ lowa | |
| CI: confidence interval; DPP‐4: dipeptidyl peptidase 4; HbA1c: glycosylated haemoglobin A1c | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded by two levels because of unclear or high risk of bias in several risk of bias domains, indirectness and imprecision
We categorised comparisons according to the oral glucose‐lowering agent that was added to insulin therapy. In the included trials we distinguished five groups of oral glucose‐lowering agents: sulphonylureas, metformin, pioglitazone, alpha‐glucosidase inhibitors and dipeptidyl‐peptidase (DPP) 4‐inhibitors. Categorisation regarding mode of insulin therapy (once‐daily, twice‐daily, or multiple daily injections) was not possible due to the often mixed use of number of insulin injections in participants in a trial or due to lack of reporting. We used a random‐effects model for the meta‐analyses.
None of the included trials assessed the primary outcomes of all‐cause mortality, diabetes‐related mortality or diabetes‐related morbidity.
None of the included trials assessed the secondary outcome, health‐related quality of life. Only one trial assessed patient satisfaction.
Insulin monotherapy versus insulin plus sulphonylurea
Primary outcomes
Adverse events: hypoglycaemia
Heterogeneity in the definitions used between trials, and the quality of reporting of hypoglycaemia precluded the pooling of data.
Eight trials reported hypoglycaemic events, quantitatively or qualitatively (Casner 1988; Feinglos 1998; Mauerhoff 1986; Reich 1987; Schade 1987; Schiel 2007; Simpson 1990; Stenman 1988). Feinglos 1998 only reported the number of hypoglycaemic events for the total group: 69 mild events, six moderate events (glucose ranging from 1.2 to 3.0 mmol/L) and one severe event requiring assistance from another individual. Simpson 1990 reported that four out of nine participants on combination therapy had to reduce their treatment drug because of hypoglycaemic symptoms. Stenman 1988 reported more mild hypoglycaemic events with combination therapy (6.1 ± 1.0 events per participant; n = 13) than with insulin monotherapy (2.6 ± 1.0 events per patient; n = 8; P < 0.01). No severe hypoglycaemic reactions requiring medical treatment occurred in this trial. Mauerhoff 1986 and Schade 1987 also counted more hypoglycaemic events with combination therapy than with insulin monotherapy (107 versus 25 and 6 versus 1, respectively). However, Reich 1987 counted more events in insulin monotherapy than with combination therapy (10 versus 5 (of which three were biochemically confirmed)). Schiel 2007 reported a similar number of mild hypoglycaemic episodes per participant (glimepiride 2.2 (37 episodes) versus insulin monotherapy 2.0 (34 episodes)). No episodes of severe hypoglycaemia (i.e. the need for intravenous glucose or glucagon injection) were reported in this trial. Casner 1988 qualitatively reported similar rates of mild hypoglycaemia for both regimens.
We rated this as low‐quality evidence, because of indirectness and risk of bias. In most trials randomisation and allocation concealment were unclear, in all trials blinding of the outcome assessor was unclear, and four of the eight trials were funded by a pharmaceutical company. In addition, heterogeneity in the definitions used between trials precluded pooling of data. Serious hypoglycaemic episodes were rare.
Other adverse events
One trial investigating the addition of sulphonylurea to insulin therapy reported one myocardial infarction during the insulin‐sulphonylurea combination period (Schade 1987).
Adverse events: weight gain
Seven trials (intervention period ranging from two months to one year) reported data on weight change. In six comparisons (Casner 1988; Kyllastinen 1985; Lindstrom 1999; Mauerhoff 1986; Schade 1987; Stenman 1988) the addition of glibenclamide was compared with insulin monotherapy and in one trial tolazamide was added (Kitabchi 1987). The addition of sulphonylureas to insulin resulted in an additional weight gain of 0.4 kg to 1.9 kg compared to ‐0.8 kg to 2.1 kg in the insulin monotherapy group (220 participants; 7 trials; low‐quality evidence; Analysis 1.1). The mean difference (MD) in weight change from baseline for the insulin‐sulphonylurea combination therapy compared to insulin monotherapy of the trials with a parallel design (1.1 kg (95% CI ‐3.1 to 5.3; P = 0.60; 86 participants; 2 trials) showed a weight gain, whereas the MD in weight change from baseline of the trials with a cross‐over design ranged between ‐1 kg to 0.4 kg (134 participants; 5 trials; Analysis 1.1; Analysis 1.2).
1.1. Analysis.
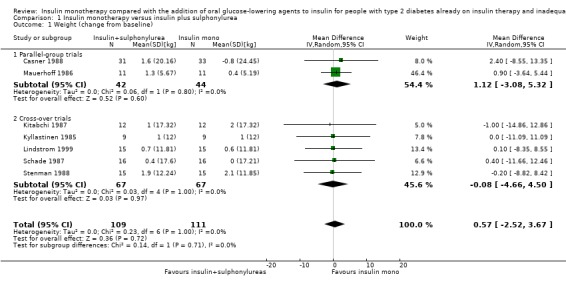
Comparison 1 Insulin monotherapy versus insulin plus sulphonylurea, Outcome 1 Weight (change from baseline).
1.2. Analysis.
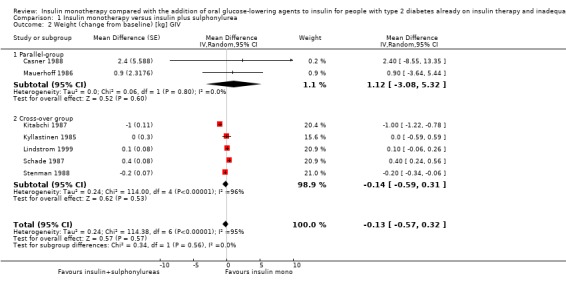
Comparison 1 Insulin monotherapy versus insulin plus sulphonylurea, Outcome 2 Weight (change from baseline) [kg] GIV.
The sensitivity analysis for the effect of trial duration indicated that, after excluding the only long‐term trial (Casner 1988), the effect on weight remained largely the same. Also, the sensitivity analysis excluding trials with high risk of bias (Casner 1988; Kitabchi 1987; Kyllastinen 1985; Stenman 1988) indicated that these trials had only very modest effects on the association between insulin‐sulphonylurea combination therapy and change in weight.
We rated this as low‐quality evidence, because of indirectness and risk of bias. In most trials randomisation and allocation concealment were unclear, in all trials blinding of the outcome assessor was unclear, and four of the six trials were funded by a pharmaceutical company.
Secondary outcomes
HbA1c and fasting glucose
In 12 comparisons the addition of glibenclamide to insulin therapy was compared to insulin monotherapy. In two comparisons (Feinglos 1998; Simpson 1990) glipizide was added, in two comparisons tolazamide was added (Kitabchi 1987; Longnecker 1986), in one comparison glimepiride was added (Schiel 2007) and in one comparison gliclazide was added (Quatraro 1986). We pooled data in a meta‐analysis on HbA1c from nine comparisons (glibenclamide n = 6, gliclazide n = 1, glimepiride n = 1, tolazamide n = 1), with the intervention period ranging from 2 to 12 months (Analysis 1.4). Insulin‐sulfphonylurea combination therapy compared with insulin monotherapy was associated with a pooled MD in lowering of HbA1c of ‐1.0% (95% CI ‐1.6 to ‐0.5; P = 0.0003; participants = 316 participants; 9 trials; Analysis 1.3 and Analysis 1.4). In one trial (Casner 1988) metabolic control (glycohaemoglobin) increased less in the intervention than in the control group after a follow‐up of one year. In addition, it was not clear whether glycohaemoglobin referred to HbA or HbA1c. After exclusion of this trial the MD did not change substantially.
1.4. Analysis.
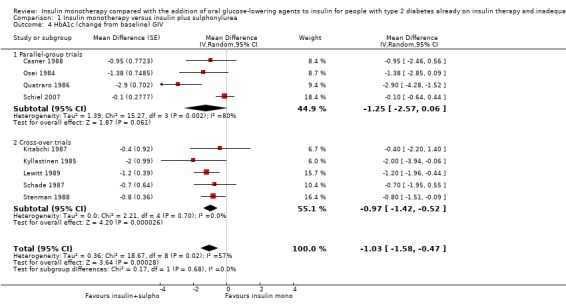
Comparison 1 Insulin monotherapy versus insulin plus sulphonylurea, Outcome 4 HbA1c (change from baseline) GIV.
1.3. Analysis.
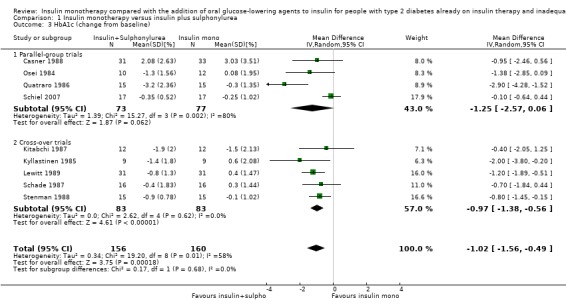
Comparison 1 Insulin monotherapy versus insulin plus sulphonylurea, Outcome 3 HbA1c (change from baseline).
Insulin‐sulphonylurea combination was also associated with a MD in lowering of fasting glucose of ‐2.29 mmol/L (95% CI ‐3.23 to ‐1.35; P < 0.00001; 205 participants; 6 trials; Analysis 1.5 and Analysis 1.6). This was calculated with pooled data from three different sulphonylurea compounds (glibenclamide, glimepiride, tolazamide).
1.5. Analysis.
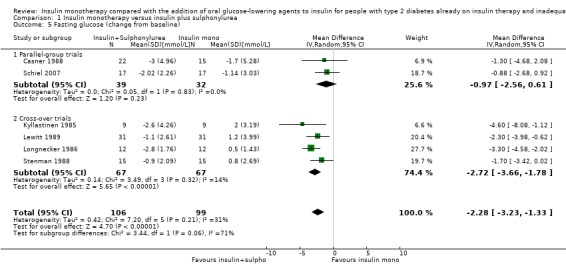
Comparison 1 Insulin monotherapy versus insulin plus sulphonylurea, Outcome 5 Fasting glucose (change from baseline).
1.6. Analysis.
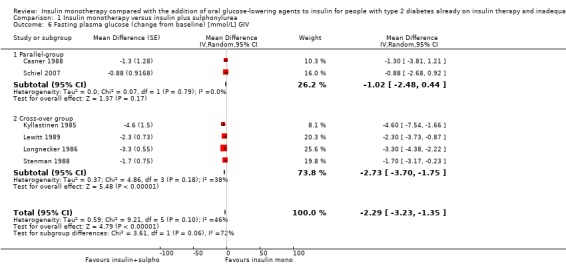
Comparison 1 Insulin monotherapy versus insulin plus sulphonylurea, Outcome 6 Fasting plasma glucose (change from baseline) [mmol/L] GIV.
The sensitivity analysis excluding long‐term trials (Casner 1988; Quatraro 1986) indicated that there was some impact of long‐term trials on the effect on HbA1c. Without these trials, insulin‐sulphonylurea combination therapy was associated with a MD in lowering of HbA1c of ‐0.8% (95% CI ‐1.2 to ‐0.3; P = 0.001) compared to insulin monotherapy. There was no impact of the long‐term trial (Casner 1988) on the effect on fasting glucose; the MD in lowering of fasting glucose was ‐2.41 mmol/L (95% CI ‐3.44 to ‐1.37; P < 0.00001).
All trials pooled in the meta‐analysis on HbA1c and fasting glucose, except Schade 1987, had a high risk of bias in some domain. The MD in HbA1c of the trials with a parallel design was higher compared to the MD in HbA1c of the trials with a cross‐over design (‐1.3% (95% CI ‐2.6 to 0.1; P = 0.06; 150 participants; 4 trials) versus ‐1% (95% CI ‐1.4 to ‐0.5; P < 0.00001; 166 participants; 5 trials) (Analysis 1.4). We had to impute SDs for all cross‐over trials.
In contrast, for fasting glucose, the pooled effect of trials with a parallel design was substantially lower ‐1.02 mmol/L (95% CI ‐2.48 to 0.44; P = 0.17; 71 participants; 2 trials) compared to the pooled effect of the trials with a cross‐over design ‐2.73 mmol/L (95% CI ‐3.70 to ‐1.75; P < 0.00001; 134 participants; 4 trials; Analysis 1.5 and Analysis 1.6).
We rated this as low‐quality evidence, because of indirectness and risk of bias. In most trials randomisation and allocation concealment were unclear, in all trials blinding of the outcome assessor was unclear, and four of the nine trials were funded by a pharmaceutical company.
Lipids
We pooled data from five trials in a meta‐analysis on total cholesterol (Groop 1985; Kitabchi 1987; Lindstrom 1999; Osei 1984; Stenman 1988). Insulin‐sulphonylurea combination therapy compared to insulin monotherapy was associated with a MD in change from baseline in total cholesterol of ‐0.04 mmol/L (95% CI ‐0.2 to 0.1; P = 0.52, 132 participants; 5 trials; Analysis 1.7 and Analysis 1.8). The same trials showed comparable results for HDL‐cholesterol and triglycerides: the MD was ‐0.1 mmol/L (95% CI ‐0.2 to 0.1; P = 0.31; 108 participants; Analysis 1.9 and Analysis 1.10) and 0.04 mmol/L (95% CI ‐0.2 to 0.3; P = 0.76; 132 participants; Analysis 1.11 and Analysis 1.12), respectively.
1.7. Analysis.
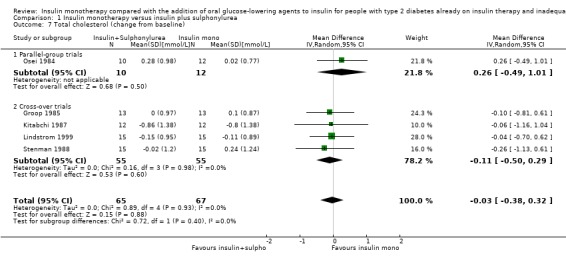
Comparison 1 Insulin monotherapy versus insulin plus sulphonylurea, Outcome 7 Total cholesterol (change from baseline).
1.8. Analysis.
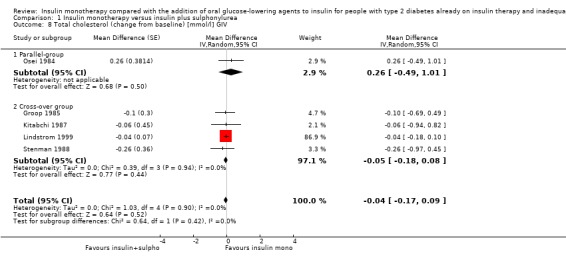
Comparison 1 Insulin monotherapy versus insulin plus sulphonylurea, Outcome 8 Total cholesterol (change from baseline) [mmol/l] GIV.
1.9. Analysis.
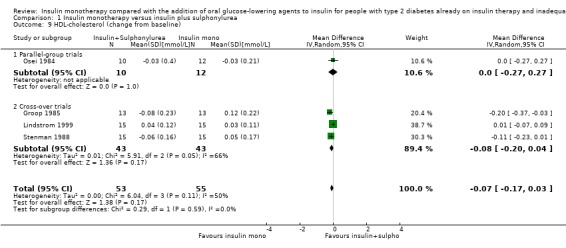
Comparison 1 Insulin monotherapy versus insulin plus sulphonylurea, Outcome 9 HDL‐cholesterol (change from baseline).
1.10. Analysis.
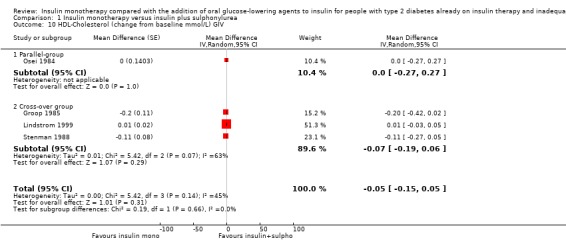
Comparison 1 Insulin monotherapy versus insulin plus sulphonylurea, Outcome 10 HDL‐Cholesterol (change from baseline mmol/L) GIV.
1.11. Analysis.
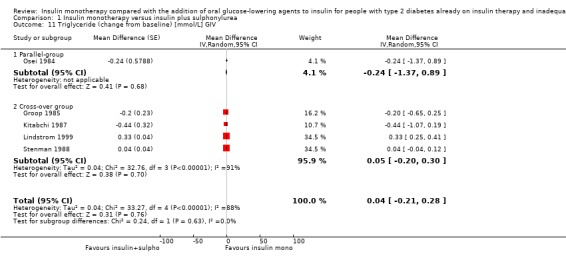
Comparison 1 Insulin monotherapy versus insulin plus sulphonylurea, Outcome 11 Triglyceride (change from baseline) [mmol/L] GIV.
1.12. Analysis.
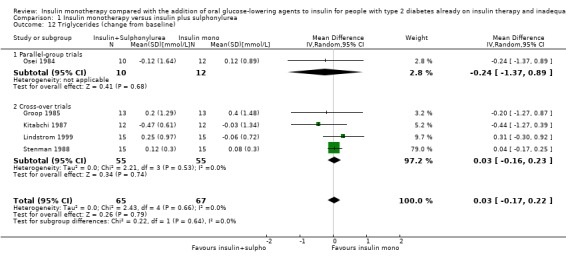
Comparison 1 Insulin monotherapy versus insulin plus sulphonylurea, Outcome 12 Triglycerides (change from baseline).
Insulin dose
Heterogeneity in type of insulin (short‐, intermediate‐ and long‐acting), units of quantification and the quality of reporting precluded the pooling of data.
Kyllastinen 1985; Longnecker 1986; Osei 1984 reported a fixed insulin dose during the trial period. Casner 1988 reported a decreased mean insulin dose over the trial period in the insulin‐sulphonylurea therapy group of ‐4 U and an increase in the insulin dose in the placebo group of 12 U. Almost no change in mean insulin amount was reported by Mauerhoff 1986: ‐0.04 difference in insulin dose in the insulin‐sulphonylurea‐treated participants versus 0.02 in the placebo‐treated participants. Kitabchi 1987 reported a mean (SD) insulin amount of 0.7 (0.01) U/kg body weight in the insulin‐sulphonylurea therapy group versus 0.9 (0.08) U/kg body weight in the placebo group at six months. In case of unexplained hypoglycaemia, insulin dose was changed in the trial by Lewitt 1989 and Stenman 1988. Lewitt 1989 reported a mean decrease in insulin amount of ‐4 U after the insulin‐sulphonylurea period versus ‐1 U after the placebo period. Stenman 1988 reported a 2 U to 4 U/visit increase by reported hypoglycaemia or if fasting glucose was less than 6.0 mmol/L, after the insulin‐sulphonylurea period a mean (SD) of 24 (3) U was found compared with 32 (4) U after the placebo period. Lindstrom 1999 reported a mean (SD) of 54 (7) U at the end of the run‐in period, which decreased to 45 (8) U after the insulin‐sulphonylurea therapy period versus an increase to 61 (6) after the placebo period. Quatraro 1986 reported a mean decrease of 33 U (mean (SD): 57 (4) U) after insulin‐SU therapy versus a mean decrease of 3 U (mean (SD): 85 (6) U) after placebo treatment. Schade 1987 reported comparable mean (SD) insulin dose after the insulin‐sulphonylurea period (54 (6) U) and the placebo period (55 (6) U). Schiel 2007 reported a mean (95%CI) decrease in insulin dose in the insulin‐sulphonylurea therapy group from 36 (10 to 62) to 26 (10 to 54) compared with no change in the placebo group from 31 (14 to112) U at both measurements.
Insulin monotherapy versus insulin plus metformin
Primary outcomes
Adverse events: hypoglycaemia
Heterogeneity in the definitions used between trials, and the quality of reporting of hypoglycaemia precluded the pooling of data.
All but three trials (Fritsche 2000; Giugliano 1993; Krawczyk 2005) reported hypoglycaemic events, quantitatively or qualitatively. Hirsch 1999 and Robinson 1998 only reported that no severe hypoglycaemia occurred. Relimpio 1998 reported qualitatively that some minor hypoglycaemic events took place in both groups. Avilés 1999 reported three hypoglycaemic events in the insulin‐metformin group (with blood glucose levels ranging from 3.1 to 3.9 mmol/L). Yilmaz 2007 reported a similar occurrence of hypoglycaemic events (n = 2 in both groups). None of the participants experienced severe hypoglycaemia in this trial. Hermann 2001 reported two versus zero hypoglycaemic events in the combination therapy group compared with the insulin monotherapy group. Also, Wulffelé 2002 reported comparable occurrences of hypoglycaemic events per person per month in both treatment groups (P = 0.477). Eight events in the metformin group and four in the control group required partner assistance, and none required medical assistance. Strowig 2002 reported less mild hypoglycaemia with insulin plus metformin compared to insulin alone (0.6 versus two episodes per participant per month; P < 0.01). In this trial, one participant taking insulin alone experienced six episodes of hypoglycaemia severe enough to require assistance, including emergency medical treatment.
We rated this as low‐quality evidence, because of indirectness and risk of bias. In most trials randomisation and allocation concealment were unclear, in all trials blinding of the outcome assessor was unclear, five of the eight included trials were funded by a pharmaceutical company, and in one trial funding was unclear. In addition, heterogeneity in the definitions used between trials precluded pooling; serious hypoglycaemic episodes were rare. In the largest trial (n = 353; Wulffelé 2002) no substantial difference in hypoglycaemic episodes between groups was found.
Other adverse events
Seven trials regarding the addition of metformin reported the number of gastro‐intestinal symptoms. The percentage of participants treated with insulin‐metformin combination therapy having gastro‐intestinal complaints ranged from 7% to 67%, and was mostly higher than in the insulin monotherapy group (Avilés 1999; Giugliano 1993; Hermann 2001; Hirsch 1999; Strowig 2002; Wulffelé 2002; Yilmaz 2007). Some trials mentioned that gastro‐intestinal complaints resolved spontaneously. Hermann 2001 reported one myocardial infarction in the metformin‐treated group.
Adverse events: weight gain
Data from seven trials (intervention period ranging from three to six months) were pooled in a meta‐analysis on weight (Avilés 1999; Krawczyk 2005; Relimpio 1998; Robinson 1998; Strowig 2002; Wulffelé 2002; Yilmaz 2007). Insulin‐metformin combination therapy compared to insulin monotherapy was associated with a MD of 2.1 kg less weight gain (95% CI ‐3.2 to ‐1.1 kg; P = 0.0001; 615 participants; 7 trials; Analysis 2.1 and Analysis 2.2).
2.1. Analysis.
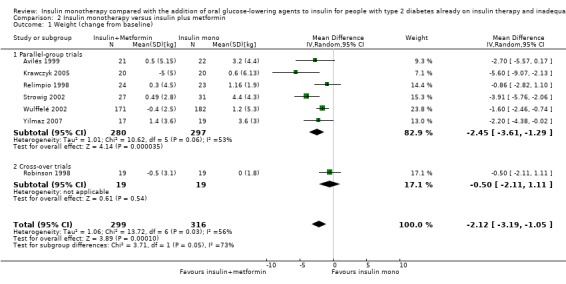
Comparison 2 Insulin monotherapy versus insulin plus metformin, Outcome 1 Weight (change from baseline).
2.2. Analysis.
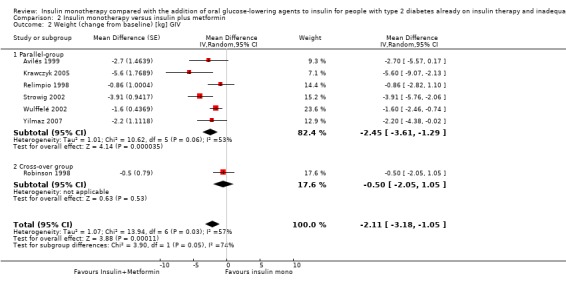
Comparison 2 Insulin monotherapy versus insulin plus metformin, Outcome 2 Weight (change from baseline) [kg] GIV.
A sensitivity analysis for the effect of trial duration indicated that after excluding long‐term trials (Avilés 1999; Krawczyk 2005; Yilmaz 2007) there was still less weight gain in the intervention group (‐1.7 kg (95% CI ‐2.9 to ‐0.4); P = 0.009; 496 participants; 4 trials). All trials pooled in the meta‐analysis on weight, except Wulffelé 2002, had some high or unclear risk of bias. The only cross‐over trial that was used for pooling in the meta‐analysis (Robinson 1998) had a similar result on weight gain compared to the trials with a parallel design.
We rated this as low‐quality evidence, because of indirectness and risk of bias. In most trials randomisation and allocation concealment were unclear, in all trials blinding of the outcome assessor was unclear, five of the seven included trials were funded by a pharmaceutical company, and in one trial funding was unclear.
Secondary outcomes
HbA1c and fasting glucose
Eleven trials compared the addition of metformin to insulin therapy with insulin monotherapy. We pooled data from nine comparisons in a meta‐analysis, with the intervention period ranging from two to six months (Avilés 1999; Fritsche 2000; Giugliano 1993; Hirsch 1999; Relimpio 1998; Robinson 1998; Strowig 2002; Wulffelé 2002; Yilmaz 2007). Insulin‐metformin combination therapy compared to insulin monotherapy was associated with a MD in lowering of HbA1c of ‐0.9% (95% CI ‐1.2 to ‐0.5); P < 0.00001; 698 participants; 9 trials; Analysis 2.3 and Analysis 2.4).
2.3. Analysis.
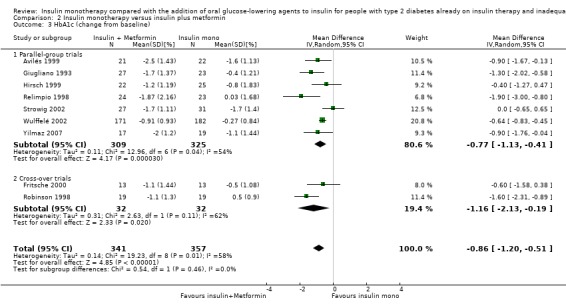
Comparison 2 Insulin monotherapy versus insulin plus metformin, Outcome 3 HbA1c (change from baseline).
2.4. Analysis.
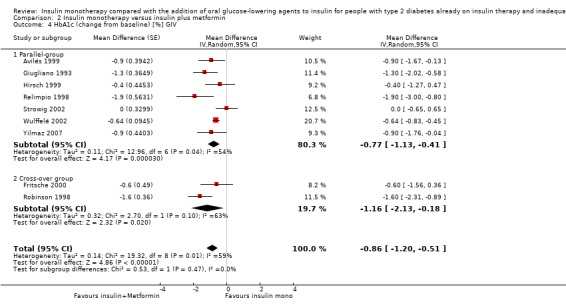
Comparison 2 Insulin monotherapy versus insulin plus metformin, Outcome 4 HbA1c (change from baseline) [%] GIV.
Because of considerable heterogeneity it was not possible to pool the results of insulin‐metformin combination therapy on fasting glucose. However, the MD in fasting glucose (insulin‐metformin combination therapy compared with insulin monotherapy; n = 6 trials) ranged from ‐5.7 to 1.1 mmol/L. The sensitivity analysis excluding long‐term trials (Avilés 1999; Giugliano 1993; Yilmaz 2007) indicated that these trials had hardly any impact on the association between insulin‐metformin combination therapy and lowering of HbA1c (MD ‐0.8% (95% CI ‐1.3 to ‐0.3); P = 0.001).The sensitivity analysis excluding trials with high risk of bias (Hirsch 1999; Relimpio 1998; Strowig 2002) indicated that these trials also had almost no impact on the effect of insulin‐metformin combination therapy on HbA1c (MD ‐1.0% (95% CI ‐1.3 to ‐0.6); P < 0.0001; 546 participants; 6 trials). The MD in HbA1c of the trials with a parallel design was smaller compared to the MD in HbA1c of the trials with a cross‐over design (‐0.8%, 95% CI ‐1.1 to ‐0.4; P < 0.0001; 634 participants; 7 trials; Analysis 2.4 versus ‐1.2%, 95% CI ‐2.1 to ‐0.2; P = 0.02; 64 participants; 2 trials; Analysis 2.3 and Analysis 2.4).
We rated this as low‐quality evidence, because of indirectness and risk of bias. In most trials randomisation and allocation concealment were unclear, in all trials blinding of the outcome assessor was unclear, five of the nine included trials were funded by a pharmaceutical company, and in one trial funding was unclear.
Lipids
We pooled data from eight trials in a meta‐analysis on total cholesterol (Avilés 1999; Fritsche 2000; Giugliano 1993; Relimpio 1998; Robinson 1998; Strowig 2002; Wulffelé 2002; Yilmaz 2007). Insulin‐metformin combination therapy compared to insulin monotherapy was associated with a MD in decrease of total cholesterol of ‐0.3 mmol/L (95% CI ‐0.5 to ‐0.1; P = 0.01; 651 participants; Analysis 2.5 and Analysis 2.6). The same pooled trials showed the following MDs for differences in HDL‐cholesterol and triglycerides: MD for HDL‐cholesterol 0.0 mmol/L (95% CI ‐0.1 to 0.0); P = 0.65; 651 participants; Analysis 2.7 and Analysis 2.8 and MD for triglycerides ‐0.2 mmol/L (95% CI ‐0.4 to 0.1); P = 0.26; 651 participants; Analysis 2.9 and Analysis 2.10.
2.5. Analysis.
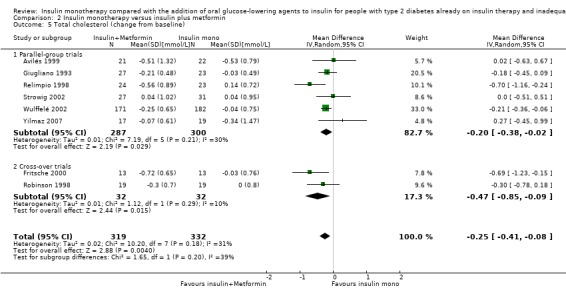
Comparison 2 Insulin monotherapy versus insulin plus metformin, Outcome 5 Total cholesterol (change from baseline).
2.6. Analysis.
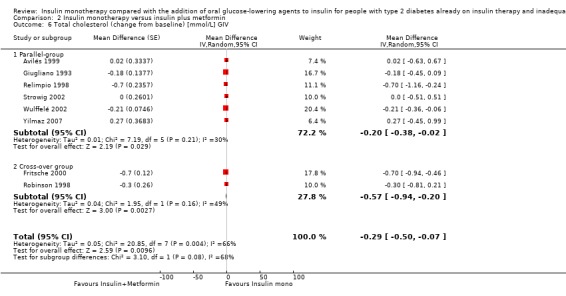
Comparison 2 Insulin monotherapy versus insulin plus metformin, Outcome 6 Total cholesterol (change from baseline) [mmol/L] GIV.
2.7. Analysis.
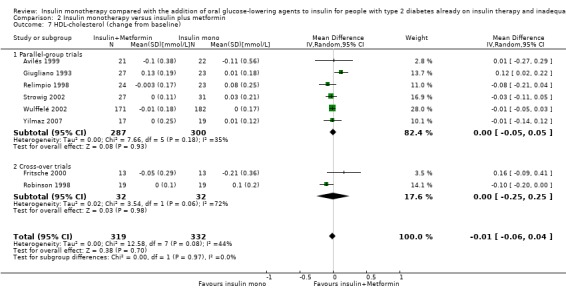
Comparison 2 Insulin monotherapy versus insulin plus metformin, Outcome 7 HDL‐cholesterol (change from baseline).
2.8. Analysis.
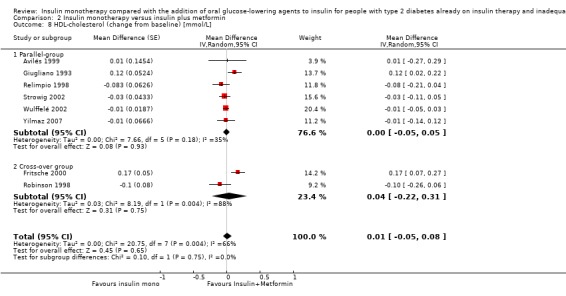
Comparison 2 Insulin monotherapy versus insulin plus metformin, Outcome 8 HDL‐cholesterol (change from baseline) [mmol/L].
2.9. Analysis.
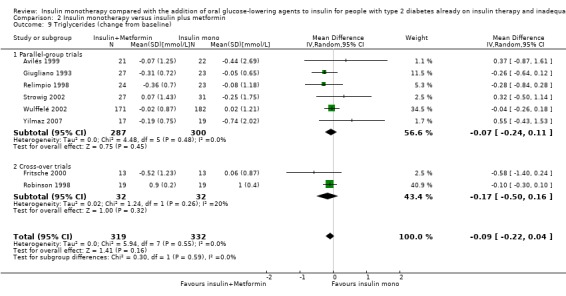
Comparison 2 Insulin monotherapy versus insulin plus metformin, Outcome 9 Triglycerides (change from baseline).
2.10. Analysis.
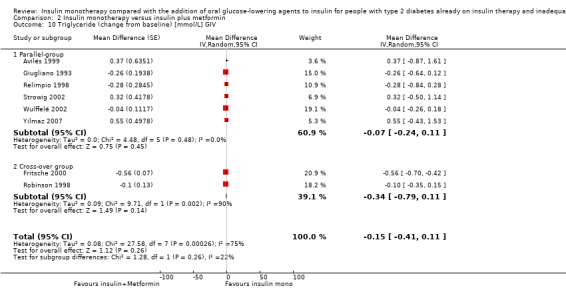
Comparison 2 Insulin monotherapy versus insulin plus metformin, Outcome 10 Triglyceride (change from baseline) [mmol/L] GIV.
Insulin dose
Heterogeneity in type of insulin (short‐, intermediate‐ and long‐acting), units of quantification and the quality of reporting precluded the pooling of data.
Ten of the 11 trials provided information about insulin requirements. One trial did not report the numbers of insulin doses, it only mentioned that there were no differences in insulin requirements (Hirsch 1999). Insulin doses were titrated to predetermined glycaemic targets based on fasting, postprandial or both glucose values in five trials (Avilés 1999; Fritsche 2000; Hermann 2001; Strowig 2002; Wulffelé 2002). Avilés 1999 reported a decrease in insulin dose in participants treated with metformin of 5 U/d (95% CI ‐17 to 8; P > 0.2) and an increase in insulin dose of 23 U/d (95% CI 11 to 35; P < 0.001) in participants treated with placebo. Also, Strowig 2002 and Wulffelé 2002 reported a decrease in mean total daily insulin dose in the insulin plus metformin group (from 83 U at baseline to 82 U at week 16 and a change of 7 U (95% CI ‐6 to ‐9), respectively) and an increase in total daily insulin dosage in the group treated with insulin alone (from 80 U to 135 U and a change of 1 U (95% CI 0.3 to 3), respectively). Fritsche 2000 (cross‐over trial) reported that total insulin requirements decreased by one‐third during the metformin treatment (from 53 U (SD 10) to 35 U (SD 7); P = 0.006), while insulin requirements were unchanged during the placebo phase (metformin versus placebo; P = 0.02). In contrast, Hermann 2001 reported no change in mean daily insulin dose after treatment, neither after metformin treatment (0.8 U/kg/day at baseline versus 0.8 U/kg/day after treatment) nor after treatment with placebo (0.7 U/kg/day at baseline versus 0.8 U/kg/day after treatment). In two trials insulin doses were titrated if hypoglycaemia occurred (Relimpio 1998; Yilmaz 2007). Relimpio 1998 reported a small reduction in insulin dose during the trial in the insulin plus metformin group (from 0.63 U/kg (SD 0.1) at baseline to 0.6 U/kg (SD 0.2) at the end of trial), while in participants assigned to insulin there was an increase in insulin dose from 0.7 U/kg (SD 0.1) at baseline to 0.8 U/kg (SD 0.14) at the end of trial. Also, Yilmaz 2007 reported a decrease in participants treated with insulin in combination with metformin (4.2/day; P < 0.001) and an increase in total daily insulin dose in participants treated with insulin alone (12.8 U/day; P < 0.001). Three trials did not report any targets to which insulin doses were titrated (Giugliano 1993; Krawczyk 2005; Robinson 1998). Giugliano 1993 reported a decrease in daily insulin dose in participants treated with insulin in combination with metformin (from 90 U (SD 9) at baseline to 68 U (SD 18) at six months' follow‐up) while the daily insulin dose remained constant in the participants treated with insulin and placebo (from 88 U (SD 9) at baseline to 86 U(SD 9) at six months' follow‐up). Krawczyk 2005 also reported a decrease in insulin requirement in participants treated with insulin and metformin (from 0.6 U/kg (SD 0.1) at baseline to 0.6 U/kg (SD 0.2) at follow‐up; P < 0.05). But in this trial, insulin requirements significantly increased from 0.6 U/kg (SD .0.1) at baseline to 0.6 U/kg (SD 0.2) at follow‐up (P < 0.05) in the control group. Robinson 1998 (cross‐over trial) reported a relatively constant daily insulin dosage in the insulin plus metformin phase as well as the insulin plus placebo phase (mean change during metformin ‐2 U; mean change during placebo +0.6 U).
Insulin monotherapy versus insulin plus glimepiride versus insulin plus metformin and glimepiride
Primary outcomes
Adverse events: hypoglycaemia
Schiel 2007 reported a comparable number of mild hypoglycaemic events with the addition of glimepiride and metformin to insulin (2.3 episodes per participant) and with insulin monotherapy (2.0 episodes per participant). No episodes of severe hypoglycaemia (that is, the need for intravenous glucose of glucagon injection) were reported.
Adverse events: weight gain
Schiel 2007 reported minor differences regarding weight gain between the addition of glimepiride and metformin to insulin and insulin monotherapy.
Secondary outcomes
Patient satisfaction
Schiel 2007 assessed patients' treatment satisfaction with the 'Diabetes Treatment Satisfaction Questionnaire'. They found no statistically significant differences between the addition of glimepiride to insulin, the addition of both metformin and glimepiride to insulin or insulin monotherapy.
HbA1c and fasting glucose
Insulin plus metformin and glimepiride (Schiel 2007) showed a greater reduction of HbA1c (‐0.7% (SD 0.9)) than the addition of glimepiride to insulin alone (‐0.4% (SD 0.5)) or compared with insulin monotherapy (‐0.3% (SD 1.0)). The same applied to fasting glucose levels.
Lipids
Data on lipids were not collected.
Insulin dose
Schiel 2007 reported a decrease in insulin requirements in the insulin plus glimepiride and metformin group (from 65 U/day (SD 32) to 54 U/day (SD 37); P = 0.009), whereas there was an increase in the insulin monotherapy group (65 U/day (SD 34) to 71 U/day (SD 35); P = 0.009).
Insulin monotherapy versus insulin plus pioglitazone
Primary outcomes
Adverse events: hypoglycaemia
Heterogeneity in the definitions used between trials, and the quality of reporting of hypoglycaemia precluded the pooling of data.
Two trials reported, quantitatively, hypoglycaemic events. Mattoo 2005 reported more subjective hypoglycaemia with insulin plus pioglitazone than with insulin monotherapy (90 events (63%) versus 75 (51%); P < 0.05). They reported that they found no differences in the number of clinical hypoglycaemic episodes (blood glucose < 2.8 mmol/L), but numbers were not reported. Rosenstock 2002 reported that 29 participants (15%) in the 30 mg pioglitazone group, 15 participants (8%) in the 15 mg pioglitazone group, and nine participants (5%) in the placebo group reported hypoglycaemia. All hypoglycaemic episodes were considered mild or moderate.
We rated this as low‐quality evidence, because of serious risk of bias. Only one trial reported adequate randomisation and allocation concealment, blinding of the outcome assessor was unclear in all of the trials, and all of the trials were funded by a pharmaceutical company.
Other adverse events
Two trials regarding the addition of pioglitazone reported a higher frequency of oedema with the use of pioglitazone, which increased with dose. Mattoo 2005 reported percentages of 16% versus 4% (pioglitazone 30 mg versus placebo) and Rosenstock 2002 reported 18% and 13% versus 7% (pioglitazone 30 mg and 15 mg versus placebo). In addition, Rosenstock 2002 reported congestive heart failure for two participants receiving 15 mg pioglitazone and two participants receiving 30 mg pioglitazone.
We rated this as low quality evidence, because of indirectness, imprecision and risk of bias; only one from the two included trials reported adequate randomisation and allocation concealment, in all trials blinding of the outcome assessor was unclear and all trials were funded by a pharmaceutical company.
Adverse events: weight gain
We pooled data from two trials (intervention period ranging from three to six months) in a meta‐analysis on weight (Mattoo 2005; Mudaliar 2010). Insulin‐pioglitazone combination compared to insulin monotherapy was associated with more weight gain (MD 3.8 kg (95% CI 3.0 to 4.6); P < 0.00001; 288 participants; 2 trials; Analysis 3.3). The multi‐intervention trial (Rosenstock 2002) showed a greater increase in weight (1.9 kg to 5.3 kg) with combination therapy than with insulin monotherapy (‐0.04 kg to 0.9 kg). Weight increased with increasing dosage of pioglitazone.
3.3. Analysis.
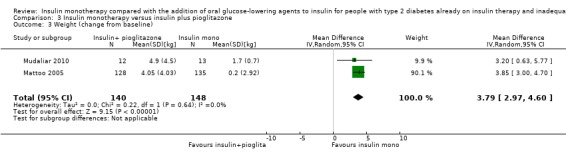
Comparison 3 Insulin monotherapy versus insulin plus pioglitazone, Outcome 3 Weight (change from baseline).
We rated this as low‐quality evidence, because of indirectness, imprecision and risk of bias. Only one of the three trials reported adequate randomisation and allocation concealment, in all trials blinding of the outcome assessor was unclear, and all trials were funded by a pharmaceutical company. Although the number of included participants in the meta analysis was low, the minimum of 1.9 kg weight gain is clinically relevant, because it may be partially caused by oedema.
Secondary outcomes
HbA1c and fasting glucose
Three trials (Mattoo 2005; Mudaliar 2010; Rosenstock 2002) compared the addition of pioglitazone to insulin therapy with insulin monotherapy. Because of missing SDs we could not perform a meta‐analysis. The mean difference in HbA1c for insulin‐pioglitazone combination therapy ranged from ‐0.5% to ‐1.0% and for insulin monotherapy from ‐0.6% to 0% (785 participants; Analysis 3.1).
3.1. Analysis.
Comparison 3 Insulin monotherapy versus insulin plus pioglitazone, Outcome 1 HbA1c (change from baseline).
We pooled data from three comparisons, with the intervention period ranging from three to six months, in a meta‐analysis to investigate the effect on fasting glucose (Mattoo 2005; Mudaliar 2010; Rosenstock 2002). We only included the results of the comparison pioglitazone 30 mg versus placebo from Rosenstock 2002. We did not use the results of the comparison between the addition of pioglitazone 15 mg versus placebo in the meta‐analysis because it comprised the same placebo group. Insulin‐pioglitazone combination therapy showed a greater variation in change in fasting glucose (‐1.5 mmol/L to 2.7 mmol/L) compared to insulin monotherapy (‐0.6 mmol/L to 0.7 mmol/L) (624 participants; 3 trials; Analysis 3.2).
3.2. Analysis.
Comparison 3 Insulin monotherapy versus insulin plus pioglitazone, Outcome 2 Fasting glucose (change from baseline).
The sensitivity analysis excluding the long‐term trial (Mattoo 2005) indicated that this trial had only a small impact on the association between insulin‐pioglitazone combination therapy and lowering of fasting glucose (‐2.1 mmol/L (95% CI ‐3.8 to ‐0.4); P = 0.02).
We rated this as low‐quality evidence, because of indirectness, imprecision and risk of bias. Only one of the three trials reported adequate randomisation and allocation concealment, in all trials blinding of the outcome assessor was unclear, and all trials were funded by a pharmaceutical company.
Lipids
Two trials reported data on HDL‐cholesterol and LDL‐cholesterol (Mattoo 2005; Rosenstock 2002), Rosenstock 2002 also reported data on total cholesterol and triglycerides.
Mattoo 2005 reported a small mean (SE) increase in HDL‐cholesterol in participants treated with insulin‐pioglitazone combination therapy (from 1.2 (0.03) to 1.4 (0.02) mmol/L) and a small mean (SE) decrease in the placebo group (from 1.2 (0.03) to 1.2 (0.02) mmol/L). For LDL‐cholesterol a small mean (SE) reduction in both groups was found (from 3.2 (0.1) to 3.2 (0.1) mmol/L in the insulin‐pioglitazone combination therapy group versus 3.2 (0.1) to 3.1 (0.1) mmol/L in the placebo groups.
Rosenstock 2002 investigated two different doses of pioglitazone (15 mg and 30 mg). In this trial a mean change (SD) in total cholesterol of 1.4 (1.1) mg/dL in the 15 mg pioglitazone group and of 0.4 (1.1) mg/dL in the 30 mg pioglitazone group versus ‐0.7 (1.1) mg/dL in the placebo group was found. For HDL‐cholesterol mean change (SD) of 7.1 (1.6) mg/dL in the 15 mg pioglitazone group and of 9.1 (1.6) mg/dL in the 30 mg pioglitazone group versus ‐0.2 (1.6) mg/dL in the placebo group was found. For LDL‐cholesterol a mean change (SD) of 5.1 (1.7) mg/dL in the 15 mg pioglitazone group and of 2.8 (1.8) mg/dL in the 30 mg pioglitazone group versus ‐1.4 (1.7) mg/dL in the placebo group was found. For triglycerides a mean (SD) change of 5.4 (6.6) mg/dL in the 15 mg pioglitazone group and of ‐10.4 (6.5) mg/dL in the 30 mg pioglitazone group versus 13.3 (6.6) mg/dL in the placebo group was found.
Insulin dose
Heterogeneity in type of insulin (short, intermediate and long acting), units of quantification and the quality of reporting precluded the pooling of data.
All trials provided information about insulin requirements. Insulin doses were titrated to predetermined glycaemic targets based on fasting, postprandial or both glucose values in two trials (Mattoo 2005; Mudaliar 2010). Mattoo 2005 reported a mean (SE) reduction in insulin dose in participants treated with insulin in combination with pioglitazone of ‐0.2 U/d/kg (0.02) ‐ P < 0.002; in participants treated with insulin in combination with placebo they found no change in insulin dose (from 0.9 U/d/kg (0.03) at baseline to 0.9 U/d/kg (0.02) at trial end point). Mudaliar 2010 also reported a decrease in insulin dose in the insulin plus pioglitazone group (from 105 U (SD 22) to 92 U (SD19)) but they found an increase in insulin dose in the insulin plus placebo group (from 114 U (SD 11) to 127 U (SD 16). Rosenstock 2002 investigated two different doses of pioglitazone (15 mg and 30 mg). In this trial insulin doses were only titrated if hypoglycaemia occurred. Total daily insulin dose remained stable in participants with insulin monotherapy (71 U (SD 34) at screening versus 70 U (SD 34) at the end of treatment), but decreased in participants with insulin‐pioglitazone combination therapy (pioglitazone 15 mg: 70 U (SD 34) at screening versus 67 U (SD 34) at the end of treatment; pioglitazone 30 mg: 72 U(SD 39) at screening versus 64 U (SD33) at the end of treatment.
Insulin monotherapy versus insulin plus alpha‐glucosidase inhibitor
Primary outcomes
Adverse events: hypoglycaemia
Heterogeneity in the definitions used between trials, and the quality of reporting of hypoglycaemia precluded the pooling of data.
All trials reported, quantitatively, hypoglycaemic events. Chiasson 1994 reported one severe hypoglycaemic event in the insulin‐acarbose group against three episodes in the insulin monotherapy group. Coniff 1995 and Yilmaz 2007 reported that hypoglycaemic episodes were not statistically significantly different between the treatment groups, both trials reported no severe hypoglycaemic events. Nemoto 2011 also reported no statistically significant difference in the incidence of hypoglycaemia between the insulin‐miglitol group and the insulin monotherapy group (39% versus 35%); all hypoglycaemic events in this trial were mild.
We rated this as low‐quality evidence, because of indirectness, imprecision and risk of bias. In most trials randomisation and allocation concealment were unclear, in all trials blinding of the outcome assessor was unclear, three of the four trials were funded by a pharmaceutical company, in one trial there was a high risk of bias because of selective reporting (Coniff 1995), and in another because of incomplete outcome data (Chiasson 1994).
Other adverse events
All trials regarding the addition of alpha‐glucosidase inhibitors reported higher frequencies of gastro‐intestinal complaints in the alpha‐glucosidase inhibitor group than under insulin monotherapy (Chiasson 1994; Coniff 1995; Nemoto 2011; Yilmaz 2007). Coniff 1995 and Nemoto 2011 reported percentages: flatulence 75% versus 35% and 21% versus 12%, diarrhoea 33% versus 13% and 14% versus 4%, respectively.
Adverse events: weight gain
Data from two trials were pooled in a meta‐analysis on weight (Coniff 1995; Yilmaz 2007). Insulin‐acarbose combination therapy compared to insulin monotherapy showed a MD of ‐0.5 kg weight change (95% CI ‐1.2 to 0.3); P = 0.26; 241 participants; 2 trials; Analysis 4.1).
4.1. Analysis.
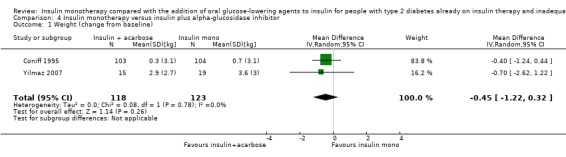
Comparison 4 Insulin monotherapy versus insulin plus alpha‐glucosidase inhibitor, Outcome 1 Weight (change from baseline).
We rated this as low‐quality evidence, because of indirectness, imprecision and risk of bias. In most trials randomisation and allocation concealment were unclear, in all trials blinding of the outcome assessor was unclear, both trials were funded by a pharmaceutical company, and in one trial there was bias because of selective reporting (Coniff 1995).
Secondary outcomes
HbA1c and fasting glucose
Three trials compared (Chiasson 1994; Coniff 1995; Yilmaz 2007) the addition of acarbose to insulin therapy and one trial compared the addition of miglitol (Nemoto 2011) to insulin therapy with insulin monotherapy. We pooled data from three comparisons, with an intervention period of three to six months, in a meta‐analysis (Coniff 1995; Nemoto 2011; Yilmaz 2007). Insulin‐alpha‐glucosidase inhibitor combination therapy was associated with a MD in lowering of HbA1c of ‐0.4% (95% CI ‐0.5 to ‐0.2); P < 0.00001; 448 participants; 3 trials; Analysis 4.2).
4.2. Analysis.
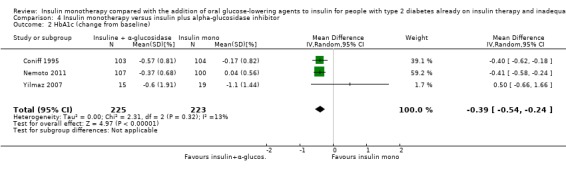
Comparison 4 Insulin monotherapy versus insulin plus alpha‐glucosidase inhibitor, Outcome 2 HbA1c (change from baseline).
Chiasson 1994 and Yilmaz 2007, when comparing combination‐therapy with insulin monotherapy, showed a MD of fasting glucose levels of 0.3 mmol/L (95% CI ‐0.7 to 1.4); P = 0.55; 113 participants; 2 trials; Analysis 4.3).
4.3. Analysis.
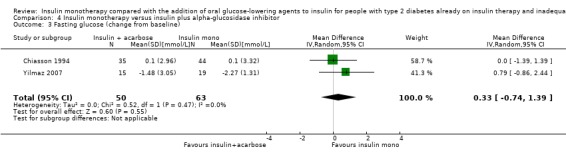
Comparison 4 Insulin monotherapy versus insulin plus alpha‐glucosidase inhibitor, Outcome 3 Fasting glucose (change from baseline).
We rated this as low‐quality evidence, because of indirectness, imprecision and risk of bias. In most trials randomisation and allocation concealment were unclear, in all trials blinding of the outcome assessor was unclear, all included trials were funded by a pharmaceutical company, and in one trial there was bias because of selective reporting (Coniff 1995).
Lipids
Yilmaz 2007 assessed total cholesterol, HDL‐cholesterol and triglycerides at baseline and at the trial end point (six months). In this trial the mean (SD) total cholesterol decreased more in the insulin‐only group (from 5.4 mmol/L (1.8) to 5.1 mmol/L (1.2)) compared to the insulin‐acarbose combination therapy group (from 5.1 mmol/L (1.5) to 5.0 mmol/L (1.1)).
HDL‐cholesterol did not change substantially in both groups after six months (in the insulin‐only group from 1.3 mmol/L (0.2) to 1.3 mmol/L (0.2) and in the insulin‐acarbose combination therapy group from 1.2 mmol/L (0.3) to 1.1 mmol/L (0.3).
Triglycerides were reduced in both groups at the trial end (in the insulin only group from 2.5 mmol/L (2.4) to 1.8 mmol/L (0.8) and in the insulin‐acarbose combination therapy group from 2.1 mmol/L (1.4) to 1.8 mmol/L (0.9).
Insulin dose
Heterogeneity in type of insulin (short‐, intermediate‐ and long‐acting), units of quantification and the quality of reporting precluded the pooling of data.
Three of the four trials provided information about insulin requirements (Coniff 1995; Nemoto 2011; Yilmaz 2007). Insulin doses were titrated if hypoglycaemia occurred. Coniff 1995 reported a decrease in total daily insulin dose of 7 U (SD 2) in the insulin plus acarbose group (at baseline 57 U (SD 3)), whereas total daily insulin remained constant in the insulin plus placebo group (baseline: 62 U (SD3); change: 1 U (SD 2). In Nemoto 2011 the mean reduction of insulin dosage to avoid hypoglycaemia was 5 U in participants treated with insulin and miglitol and 2 U in participants treated with insulin and placebo. Yilmaz 2007 reported a decrease in participants treated with insulin in combination with acarbose (3 U/day; P = 0.035) and an increase in total daily insulin dose in participants treated with insulin alone (13 U/day; P < 0.001).
Insulin monotherapy versus insulin plus dipeptidyl peptidase 4 (DPP‐4) inhibitor
Primary outcomes
Adverse events: hypoglycaemia
Heterogeneity in the definitions used between trials, and the quality of reporting of hypoglycaemia precluded the pooling of data.
Barnett 2013 reported lower rates of confirmed hypoglycaemia in the insulin‐saxagliptin group compared to the placebo group (8 versus 5) and no severe episodes. Hong 2012 reported a higher percentage of hypoglycaemia in the insulin increase group compared to the insulin‐sitagliptin combination group (18% versus 8%; severe 5% versus 2%). Fonseca 2007 reported highest rates of confirmed hypoglycaemia (2 versus 3 events per patient‐year; P < 0.001; 23% versus 30% of the participants) and severe hypoglycaemia (0 versus 0.1 events per patient‐year; P = 0.032; absolute number of events n = 6) with insulin‐vildagliptin therapy compared to insulin monotherapy.
We rated this as low‐quality evidence, because of indirectness, imprecision and risk of bias. Randomisation and allocation concealment were unclear in these trials, in two trials, blinding of the outcome assessor was unclear and one trial was without blinding, and two trials were funded by a pharmaceutical company.
Adverse events: weight gain
Two trials reported data on body weight change with conflicting results. Hong 2012 reported a weight loss for participants in the insulin‐sitagliptin group of 0.7 kg (0.1 SD) versus a weight gain of 1.1 kg (0.4 SD) in the insulin monotherapy group. In contrast, in the trial of Fonseca 2007 the body weight of participants in both groups increased during the intervention period (insulin‐vildagliptin: 1.3 kg (0.3 SD) versus insulin‐placebo: 0.6 kg (0.3 SD) (Analysis 5.1).
5.1. Analysis.
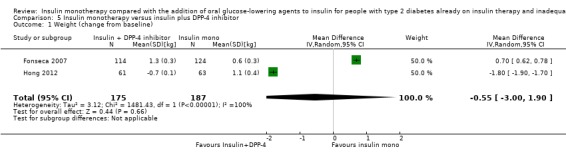
Comparison 5 Insulin monotherapy versus insulin plus DPP‐4 inhibitor, Outcome 1 Weight (change from baseline).
We rated this as low‐quality evidence, because of indirectness, imprecision and risk of bias. Randomisation and allocation concealment was unclear, in one trial, blinding of the outcome assessor was unclear, and another trial was without blinding and funded by a pharmaceutical company.
Secondary outcomes
HbA1c and fasting glucose
The pooled effect of insulin and DPP‐4 inhibitor combination therapy compared to insulin monotherapy on HbA1c showed a MD of ‐0.4% (95% CI ‐0.5 to ‐0.4; 265 participants; 2 trials; Analysis 5.2) (Barnett 2013; Hong 2012).
5.2. Analysis.
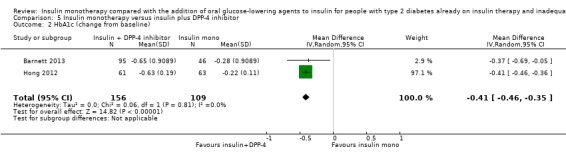
Comparison 5 Insulin monotherapy versus insulin plus DPP‐4 inhibitor, Outcome 2 HbA1c (change from baseline).
Fonseca 2007 was the only trial with data on fasting plasma glucose. In this trial with 238 participants the MD was ‐0.6 mmol/L (95% CI ‐1.6 to 0.4) for insulin‐vildagliptin therapy compared to insulin monotherapy.
We rated this as low‐quality evidence, because of indirectness, imprecision and risk of bias. Randomisation and allocation concealment was unclear, in one trial blinding of the outcome assessor was unclear and the other was without blinding, and both were funded by a pharmaceutical company.
Lipids
No trials assessed change in lipids.
Insulin dose
Heterogeneity in type of insulin (short‐, intermediate‐ and long‐acting), units of quantification and the quality of reporting precluded the pooling of data.
Barnett 2013 reported a similar increase in mean (95% CI) insulin dose for participants treated with saxagliptin (6 U (4 to 7)) and those treated with placebo (7 U (5 to 9)). Fonseca 2007 reported an adjusted mean change from baseline to endpoint of 1 U/day (SD 2) in vildagliptin treated participants and 4 U/day (SD 2) in participants receiving placebo (between‐group difference P = 0.315). Hong 2012 reported an overall mean decrease (95% CI) in insulin dose from baseline of 3 U (1 to 5) in the sitagliptin group versus an increase of 10 U (5 to 15) in the insulin increase group.
Sensitivity analyses
Repeating all meta‐analyses with a fixed‐effect model only yielded small differences compared to the results of the random‐effects model. The results of the sensitivity analyses investigating the impact of very long trials and trials with high risk of bias are reported in the relevant paragraphs. Also, the results of the comparisons between trials with a parallel design and trials with a cross‐over design are reported in the relevant paragraphs.
Subgroup analyses
We planned two subgroup analyses:
different oral glucose lowering agent(s) and different types of insulin; and
timing and frequency of insulin injections.
We divided our results into groups of the added oral glucose‐lowering agents. Subgroup analyses regarding the diverse types, timing and frequency of insulin were not feasible. The required information was often not reported or the participant groups used different insulin types and regimens in one trial.
Reporting bias
We did not create funnel plots, because we were not able to include 10 trials or more for a given outcome.
Discussion
Summary of main results
We included 37 RCTs with 3227 participants in this review. The addition of an oral glucose‐lowering agent to the treatment of people with type 2 diabetes, who were already on insulin therapy, had a beneficial effect on glycosylated haemoglobin A1c (HbA1c) levels . Sulphonylureas had a positive effect on fasting glucose levels. Combination therapy also led to a reduction of the insulin requirements in most trials on insulin‐sulphonylurea, insulin‐metformin, insulin‐pioglitazone, as well as insulin‐alpha‐glucosidase inhibitor combination therapy. Besides the benefits of an improved glycaemic control and lower required insulin doses, the addition of oral glucose‐lowering agents had some unwanted effects. Insulin therapy in combination with pioglitazone resulted in a higher frequency of hypoglycaemic events compared to insulin monotherapy. The combination with pioglitazone caused more weight gain compared to insulin monotherapy. On the other hand, weight gain appeared less when metformin was added. A substantial proportion of participants using metformin experienced gastrointestinal adverse effects. These adverse effects partly resolved spontaneously, however. Alpha‐glucosidase inhibitor‐users also experienced some gastro‐intestinal adverse effects. A substantial proportion of the pioglitazone‐users developed oedema, in a few cases combined with heart failure.
Overall completeness and applicability of evidence
The included trials shared an outcome measure of glycaemic control, often accompanied by some other diverse outcome measures. None of the included trials reported the effects on the primary outcomes, diabetes‐related morbidity and all‐cause mortality, as well as on health‐related quality of life and only one reported patient satisfaction. Although the trials were of overall low quality, they could answer questions regarding some outcome measures. One of our objectives was to distinguish the effects between the different oral glucose‐lowering agents and insulin regimens. The categorisation in insulin schemes was not possible, since most trials included participants with several kinds of insulin regimens without discriminating between them or without specification of the insulin regimens. In 11 of the 17 trials that investigated sulphonylureas, glibenclamide was added. Glibenclamide is associated with an increased risk of hypoglycaemic events compared to other sulphonylureas (Gangji 2007). So, the number of hypoglycaemic events in this review might be higher than expected with the use of other sulphonylureas.
There are some general barriers for the applicability of the evidence from this review. Abnormal liver and renal function limits the use of many oral agents. Therefore, in most trials participants with renal or liver failure were excluded. In the included trials, patient care reflected trial circumstances instead of routine daily care. This could mean that the participants received a more structured care which may have resulted in more educated and compliant participants. These features may have contributed to the reaching of better glycaemic control and other positive consequences, such as less weight gain and less hypoglycaemia.
The participants ranged in age from 29 to 83 years, and the duration of diabetes ranged from less than 1 to 31 years. These numbers are fairly comparable to the numbers of the type 2 diabetes patients with insufficient glycaemic control on insulin therapy that are currently treated for diabetes in daily practice.
The addition of alpha‐glucosidase inhibitors had a small effect on HbA1c (‐0.4%), but resulted in a reduction of insulin requirement ranging from 3 to 7 units per day. The baseline HbA1c was approximately 7.6% in the group of alpha‐glucosidase inhibitors, compared to 10.7% in the sulphonylurea, 9.0% in the metformin and DPP‐4, and 8.3% in the pioglitazone group. Apparently, the participants receiving alpha‐glucosidase inhibitors had a better glycaemic control at baseline than the other groups. It is not likely that a pronounced lowering of HbA1c would still be possible in these participants. This indicates the importance of perceiving features of a trial population when interpreting results.
Quality of the evidence
All included trials were RCTs, of which 26 had a parallel design and 11 had a cross‐over design. The total number of participants was 3227 (range 9 to 566), with 0% to 100% men. A third of the trials had 30 or fewer participants, partly due to the number of participants in the cross‐over trials (mean n = 18 (range 9 to 33)). A lot of trials seemed to have been underpowered and only eight trials discussed power calculations. This might mean that potential significant differences across groups were not detected.
Follow‐up periods differed between trials, ranging from 2 to 12 months. Only five trials had a follow‐up of 12 months. The outcome values of the trials with a short follow‐up might have been different if the trial had continued for longer. We performed sensitivity analyses in which we explored the influence of very long trials on the effect size, to establish how much they dominated the results. These analyses showed that a longer follow‐up somewhat strengthened the effects, but that it did not change the direction. Long‐term effects on diabetes‐related morbidity and all‐cause mortality particularly remained unclear.
Many trials had a serious risk of bias in some risk of bias domains, in addition randomisation, allocation concealment and blinding of the outcome assessor was often unclear. Most trials were funded by pharmaceutical companies and often the overall outcome was in favour of the product of the sponsoring company. Some argue that systematic bias favours products which are made by the company funding the research. Explanations include the selection of an inappropriate comparator to the product being investigated and publication bias (Lexchin 2003).
Potential biases in the review process
It is possible that trials concerning our objective were not published. Only one author did the first rough selection of possible appropriate trials in the references obtained by the searches. This approach might have caused some selection bias.
Agreements and disagreements with other studies or reviews
Yki‐Jarvinen 2001 draws some similar conclusions in a non‐systematic review with 25 comparisons in previously insulin‐treated type 2 diabetes patients. She showed better glycaemic control with the addition of metformin, sulphonylurea and thiazolidinediones compared to the treatment of insulin‐treated type 2 diabetes patients. Metformin was associated with less weight gain, whereas the addition of sulphonylureas showed no difference and the combination with thiazolidinediones caused more weight gain than insulin alone. The occurrence of hypoglycaemia was sparsely described in the trials included in Yki‐Jarvinen 2001. The trials reported more hypoglycaemic events with sulphonylurea (one trial) and thiazolidinediones (three trials) and fewer with metformin (one trial). There was no definite conclusion in favour of one treatment over the other with respect to the effects on lipids.
Goudswaard 2004b executed a Cochrane Review in insulin‐naive patients with the same objectives as ours. They did not include trials that observed combination therapy with thiazolidinediones. In contrast to our finding that combination therapy gives better glycaemic control, they found similar glycaemic control in combination therapy and insulin monotherapy. They demonstrated, as we did, a beneficial effect of metformin and no effect of sulphonylurea on weight gain. An explanation of the difference between Goudswaard 2004b and our review could be the difference in the history of insulin therapy of the included participants. The participants in our review experienced a period of failing oral agents after which insulin monotherapy was started and the majority was included at the moment they did not reach the aimed glycaemic control with insulin monotherapy. Unfortunately we did not have enough data about the duration of insulin therapy at the moment of inclusion. Goudswaard concluded that the start of insulin treatment with or without the continuation of oral agents had positive effects on glycaemic control. However, the additional effect of the combination of insulin with oral glucose‐lowering agents on glycaemic control in insulin‐naive patients was small. To conclude: the effectiveness of adding oral glucose‐lowering agents to insulin therapy is differential depending on whether they are administered in insulin‐naive patients or in patients who are already on insulin therapy.
Authors' conclusions
Implications for practice.
Adding oral glucose‐lowering agents to insulin therapy in people with type 2 diabetes with inadequate glycaemic control reduces glycosylated haemoglobin A1c (HbA1; range: ‐0.4% to ‐1.0%; low quality evidence). In most trials the participants with combination therapy needed less insulin, whereas insulin requirements increased or remained stable in participants with insulin monotherapy. Because adding an oral glucose‐lowering agent may also lead to weight gain, more hypoglycaemic events and other adverse effects like gastro‐intestinal complaints, oedema and heart failure (all low quality evidence), it is important that clinicians meticulously weight the advantages of combination therapy against possible negative effects in every individual patient. The evidence presented in this review is of ‘low quality’, therefore there is still uncertainty about the estimate of effect presented, and further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Implications for research.
As the majority of the included trials seemed to be underpowered and follow‐up was often short (< 12 months in all but five of the trials). Multi‐centre trials with a long follow‐up focusing on diabetes‐related morbidity and mortality and all‐cause mortality as outcome measures are needed.
None of the included trials assessed health‐related quality of life. Future trials investigating the effects on patient‐reported outcomes, like health‐related quality of life, health status, wellbeing and treatment satisfaction are also needed. We have found seven ongoing trials, four with a subgroup for the combination insulin‐DPP IV inhibitor versus insulin monotherapy, one with a thiazolidinedione combined therapy and one with the combination insulin‐ipragliflozin (approved in Japan).
What's new
| Date | Event | Description |
|---|---|---|
| 12 March 2020 | Amended | Clarification message added to the Declarations of interest statement about the review's compliance with the Cochrane Commercial Sponsorship Policy. |
Notes
Part of the background, the methods section, appendices, additional tables and figures 1 to 3 of this review are based on a standard template established by Cochrane Metabolic and Endocrine Disorders.
Acknowledgements
The authors gratefully acknowledge the contribution of Gudrun Paletta, Karla Bergerhoff and Bernd Richter of Cochrane Metabolic and Endocrine Disorders for their valuable assistance in the preparation of the protocol and this review. We also acknowledge the assistance of Rob Scholten of Cochrane Netherlands for his help with performing the generic inverse variance analysis.
Appendices
Appendix 1. Search strategies
| Cochrane Central Register of Controlled Trials (CENTRAL) |
| #1 [mh "Diabetes mellitus"] #2 diabet*:ti,ab,kw #3 (IDDM or NIDDM or MODY or T1DM or T2DM or T1D or T2D):ti,ab,kw #4 ((non and insulin* and depend*) or (noninsulin* and depend*) or (non and insulin?depend*) or noninsulin?depend*):ti,ab,kw #5 ((insulin* and depend*) or insulin?depend*):ti,ab,kw #6 {or #1‐#5} #7 [mh "Diabetes insipidus"] #8 (diabet* and insipidus):ti,ab,kw #9 #7 or #8 #10 #6 not #9 #11 [mh "Drug Therapy, Combination"] #12 combin*:ti,ab,kw #13 #11 or #12 #14 [mh "Sulfonylurea compounds"] #15 [mh Biguanides] #16 [mh Acarbose] #17 [mh Thiazolidinediones] #18 [mh Tolbutamide] #19 [mh Metformin] #20 [mh Chlorpropamide] #21 [mh "Dipeptidyl‐Peptidase IV inhibitors"] #22 [mh Glyburide] #23 [mh Tolazamide] #24 [mh Carbutamide] #25 [mh Acetohexamide] #26 [mh Buformin] #27 [mh Chlorhexidine] #28 [mh Chloroguanide] #29 [mh Phenformin] #30 (biguanid* or sulfonylurea* or sulphonylurea* or acarbos*):ti,ab,kw #31 (gliglacid* or glimepirid* or glibornurid* or gliguidon* or glisoxepid* or glipizid* or gliburid* or glyburid* or tolazamid*):ti,ab,kw #32 (tolbutamid* or carbutamid* or chlorpropamid* or acetohexamid* or glibenclamid*):ti,ab,kw #33 (metformin* or buformin* or chlorhexidin* or chlorguanid or phenformin*):ti,ab,kw #34 (miglitol* or nateglinid* or repaglinid* or meglitinid* or glucobay):ti,ab,kw #35 (troglitazone* or rosiglitazon* or pioglitazon* or thiazolidinedion* or glitazon*):ti,ab,kw #36 (antidiabet* near/3 drug*):ti,ab,kw #37 (antidiabet* near/3 herb*):ti,ab,kw #38 (antidiabet* near/3 agent*):ti,ab,kw #39 (antidiabet* near/3 compound*):ti,ab,kw #40 (anti and (diabet* near/3 drug*)):ti,ab,kw #41 (anti and (diabet* near/3 herb*)):ti,ab,kw #42 (anti and (diabet* near/3 agent*)):ti,ab,kw #43 (anti and (diabet* near/3 compound*)):ti,ab,kw #44 (oral near/6 hypoglyc?emic):ti,ab,kw #45 (oral near/6 antidiabet*):ti,ab,kw #46 (oral near/6 antihyperglyc?emic):ti,ab,kw #47 alpha‐glucosidase‐inhibitor*:ti,ab,kw #48 (hypoglyc?emic near/3 drug*):ti,ab,kw #49 (hypoglyc?emic near/3 herb*):ti,ab,kw #50 (hypoglyc?emic near/3 agent*):ti,ab,kw #51 (hypoglyc?emic near/3 compound*):ti,ab,kw #52 (sitagliptin* or vildagliptin*):ti,ab,kw #53 [mh "Glucagon‐Like Peptides"] #54 (glucagon‐like and peptid*):ti,ab,kw #55 (GLP1 or (GLP and 1)):ti,ab,kw #56 (DPP‐4 or DPP‐IV or DPP4 or DPPIV):ti,ab,kw #57 {or #14‐#30} #58 {or #13‐#50} #59 {or #51‐#56} #60 {or #57‐#59} #61 [mh Insulin] #62 insulin*:ti,ab,kw #63 #61 or #62 #64 #10 and #13 and #60 and #63 |
| MEDLINE (Ovid SP) |
| 1. exp Drug therapy combination/ 2. combin*.tw,ot. 3. 1 or 2 4. exp Diabetes Mellitus/ 5. diabet$.tw,ot. 6. (IDDM or NIDDM or MODY or T1DM or T2DM).tw,ot. 7. (non insulin$ depend$ or noninsulin$ depend$ or non insulin?depend$ or noninsulin?depend$).tw,ot. 8. (insulin$ depend$ or insulin?depend$).tw,ot. 9. or/4‐8 10. exp Diabetes Insipidus/ 11. diabet$ insipidus.tw,ot. 12. 10 or 11 13. 9 not 12 14. exp Sulfonylurea Compounds/ 15. exp Biguanides/ 16. exp Acarbose/ 17. exp Thiazolidinediones/ 18. exp Tolbutamide/ or exp Metformin/ or exp Chlorpropamide/ or exp Dipeptidyl‐Peptidase IV Inhibitors/ 19. exp Glyburide/ or exp Tolazamide/ or exp Carbutamide/ or exp Acetohexamide/ 20. exp Buformin/ or exp Chlorhexidine/ or exp Chloroguanide/ or exp Phenformin/ 21. (biguanid$ or sulfonylurea$ or sulphonylurea$ or acarbos$).tw,ot. 22. (gliglacide$ or glimepirid$ or glibornurid$ or gliguidon$ or glisoxepid$ or glipizid$ or gliburid$ or glyburid$ or tolazamid$).tw,ot. 23. (tolbutamid$ or carbutamid$ or chlorpropamid$ or acetohexamid$ or glibenclamid$ or glimepirid$).tw,ot. 24. (metformin$ or buformin$ or chlorhexidin$ or chlorguanid$ or phenformin$).tw,ot. 25. (miglitol$ or nateglinid$ or meglitinid$ or glucobay).tw,ot. 26. (troglitazon$ or rosiglitazon$ or pioglitazon$ or thiazolidinedion$ or glitazon$).tw,ot. 27. repaglinid$.tw,ot. 28. ( (antidiabet$ or anti diabet$) adj3 (drug$ or herb$ or agent$ or compound$)).tw,ot. 29. (oral adj6 (hypoglycemic or antidiabetic or antihyperglycemic)).tw,ot. 30. alpha‐glucosidase‐inhibitor$.tw,ot. 31. (hypoglyc?emic adj3 (drug$ or herb or agent$ or compound$)).tw,ot. 32. (sitagliptin$ or vildagliptin$).tw,ot. 33. exp Glucagon‐Like Peptide 1/ 34. glucagon‐like peptid$ 1.tw,ot. 35. (GLP1 or GLP 1).tw,ot. 36. (DPP‐4 or DPP‐IV or DPP4 or DPPIV).tw,ot. 37. or/14‐36 38. exp Insulin/ 39. insulin$.tw,ot. 40. 38 or 39 41. 3 and 13 and 37 and 40 42. Meta‐analysis.pt. 43. exp Review/ 44. exp Technology Assessment, Biomedical/ 45. exp Meta‐analysis/ 46. exp Meta‐analysis as topic/ 47. hta.tw,ot. 48. (health technology adj6 assessment$).tw,ot. 49. (meta analy$ or metaanaly$ or meta?analy$).tw,ot. 50. ( (review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systemat$)).tw,ot. 51. or/42‐50 52. randomized controlled trial.pt. 53. controlled clinical trial.pt. 54. randomi?ed.ab. 55. placebo.ab. 56. drug therapy.fs. 57. randomly.ab. 58. trial.ab. 59. groups.ab. 60. or/52‐59 61. 51 or 60 62. 41 and 61 63. (animals not (animals and humans)).sh. 64. 62 not 63 65. (comment or editorial or historical‐article).pt. 66. 64 not 65 |
| Embase (Ovid SP) |
| 1. exp Drug combination/ 2. combin*.tw,ot. 3. 1 or 2 4. exp Diabetes Mellitus/ 5. diabet$.tw,ot. 6. (non insulin* depend* or noninsulin* depend* or non insulin?depend* or noninsulin?depend*).tw,ot. 7. (insulin* depend* or insulin?depend*).tw,ot. 8. (IDDM or NIDDM or MODY or T1DM or T2DM or T1d or T2D).tw,ot. 9. or/4‐8 10. exp Diabetes Insipidus/ 11. diabet* insipidus.tw,ot. 12. 10 or 11 13. 9 not 12 14. exp sulfonylurea derivative/ 15. exp biguanide derivative/ 16. exp acarbose/ 17. exp 2,4 thiazolidinedione derivative/ 18. (biguanid* or sulfonylurea* or sulphonylurea* or acarbos*).tw,ot. 19. (gliglacide* or glimeprid* or glibornurid* or gliguidon* or glisoxepid* or glipizid* or gliburid* or glyburid* or tolazamid*).tw,ot. 20. (metformin* or buformin* or chlorhexidin* or chlorguanid* or phenformin*).tw,ot. 21. (troglitazon* or rosiglitazon* or pioglitazon* or thiazolidinedion* or glitazon*).tw,ot. 22. (miglitol* or nateglinid* or repaglinid* or meglitinid* or glucobay).tw,ot. 23. ( (antidiabet* or anti diabet*) adj3 (drug* or herb* or agent* or compound*)).tw,ot. 24. (oral adj6 (hypoglyc?emic or antidiabetic or antihyperglyc?emic)).tw,ot. 25. alpha‐glucosidase‐inhibitor*.tw,ot. 26. (hypoglyc?emic adj3 (drug* or herb* or agent* or compound*)).tw,ot. 27. (sitagliptin* or vildagliptin*).tw,ot. 28. exp glucagon like peptide 1/ 29. glucagon‐like peptid* 1.tw,ot. 30. (GLP 1 or GLP1).tw,ot. 31. (DPP‐4 or DPP‐IV or DPP4 or DPPIV).tw,ot. 32. (biguanid* or sulfonylurea* or sulphonylurea* or acarbos*).tw,ot. 33. exp glimepiride/ 34. exp glibornuride/ 35. exp glisoxepide/ 36. glibenclamide/ or metformin/ or glipizide/ or tolbutamide/ 37. exp tolazamide/ 38. exp carbutamide/ 39. exp chlorpropamide/ 40. exp acetohexamide/ 41. exp buformin/ 42. (tolbutamid* or carbutamid* or chlorpropamid* or acetohexamid* or glibenclamid* or glimepirid*).tw,ot. 43. exp chlorhexidine/ 44. proguanil/ 45. exp phenformin/ 46. exp miglitol/ 47. exp nateglinide/ 48. exp meglitinide/ 49. exp troglitazone/ 50. exp rosiglitazone/ 51. exp pioglitazone/ 52. exp 2,4 thiazolidinedione derivative/ 53. exp glitazone derivative/ 54. exp alpha glucosidase inhibitor/ 55. exp sitagliptin/ 56. exp vildagliptin/ 57. or/14‐56 58. exp insulin/ 59. insulin*.tw,ot. 60. 58 or 59 61. exp Randomized Controlled Trial/ 62. exp Controlled Clinical Trial/ 63. exp Clinical Trial/ 64. exp Comparative Study/ 65. exp Drug comparison/ 66. exp Randomization/ 67. exp Crossover procedure/ 68. exp Double blind procedure/ 69. exp Single blind procedure/ 70. exp Placebo/ 71. exp Prospective Study/ 72. ( (clinical or control$ or comparativ$ or placebo$ or prospectiv$ or randomi?ed) adj3 (trial$ or stud$)).ab,ti. 73. (random$ adj6 (allocat$ or assign$ or basis or order$)).ab,ti. 74. ( (singl$ or doubl$ or trebl$ or tripl$) adj6 (blind$ or mask$)).ab,ti. 75. (cross over or crossover).ab,ti. 76. or/61‐75 77. exp meta analysis/ 78. (metaanaly$ or meta analy$ or meta?analy$).ab,ti,ot. 79. ( (review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systematic$)).ab,ti,ot. 80. exp Literature/ 81. exp Biomedical Technology Assessment/ 82. hta.tw,ot. 83. (health technology adj6 assessment$).tw,ot. 84. or/77‐83 85. 76 or 84 86. 3 and 13 and 57 and 60 and 85 87. (comment or editorial or historical‐article).pt. 88. 86 not 87 89. limit 88 to human |
| WHO ICTRP (Standard search) |
| diabet* AND insulin monotherapy OR diabet* AND insulin alone |
| ClinicalTrials.gov (Advanced search) |
|
Search Terms: (((diabetes OR diabetic) AND ("type 2" OR "type II")) OR T2D OR T2DM) AND ("insulin monotherapy" OR "insulin alone") Study Type: Interventional Studies |
Appendix 2. Description of interventions
| Intervention(s) (route, frequency, total dose/day) | Comparator(s) (route, frequency, total dose/day) | |
| Avilés 1999 | Insulin as before treatment of at least 50 U insulin/day + metformin, oral, 2500 mg/day, 5 tablets of 500 mg daily | Insulin as before treatment of at least 50 U insulin/day + placebo, 5 tablets daily |
| Barnett 2013 | Insulin (premixed 67.4%, intermediate 16.8%, long‐acting 11.6%, intermediate + long‐acting 1.1%, intermediate + premixed 1.1, long‐acting + premixed 1.1) 53.6 U (range 30‐150) daily dose + saxagliptin, oral, 5 mg daily | Insulin (premixed 54.3%, intermediate 21.7%, long acting 13.0%, intermediate + long‐acting 6.5%, intermediate + premixed 4.3, long‐acting + premixed 0) 55.3 U (range 30‐149) daily dose + placebo daily |
| Casner 1988 | NPH insulin twice a day, total of at least 25 U insulin/day + glibenclamide, oral, 3.39 ± 0.22 mg/day | NPH insulin twice a day, total of at least 25 U insulin/day + placebo, oral, 3.28 ± 0.28 mg/day |
| Chiasson 1994 | Insulin (type and dose not mentioned) + acarbose, oral, 3 times a day, 50 mg to 200 mg/day | Insulin (type and dose not mentioned) + placebo, oral, 3 times a day |
| Coniff 1995 | Insulin (type and dose not mentioned) + acarbose, oral, 3 times a day 50 mg to 300 mg/day | Insulin (type and dose not mentioned) + placebo, oral, 3 times a day |
| Feinglos 1998 | Insulin (regular or NPH: 1 participant; daily, 26 participants; twice a day, 2 participants: 3 times a day, dose not mentioned) + glipizide, oral, 5‐40 mg/day, daily or twice a day | Insulin (regular or NPH: 1 participant; daily, 26 participants twice a day, 2 participants 3 times a day, dose not mentioned) + placebo, oral |
| Fonseca 2007 | Injectable insulin (type not mentioned) of at least 30 U/day + vildagliptin, oral, 100 mg/day, twice a day 50 mg | Injectable insulin (type not mentioned) of at least 30 U/day + placebo, oral, twice a day |
| Fritsche 2000 | NPH insulin twice a day (dose not mentioned) + metformin, oral, daily 850 mg ‐ 3 times a day | NPH insulin twice a day (dose not mentioned) + placebo, oral, daily ‐ 3 times a day |
| Giugliano 1993 | Regular and lente insulin, mean dose 90 (9 SD) U/day, twice a day + metformin, oral, 850 mg twice daily | Regular and lente insulin, mean dose 90 (9 SD) U/day, twice a day + placebo, oral, twice daily |
| Groop 1985 | Insulin (type and dose not mentioned) + glibenclamide, oral, 10 mg/day, twice a day 5 mg | Insulin (type and dose not mentioned) + placebo, oral, twice a day |
| Hermann 2001 | Insulin (rapid‐acting + NPH or premixed) 0.75 (0.28 SD) U/kg/day + metformin, oral, 2 weeks daily 850 mg, thereafter twice a day 850 mg | Insulin (rapid‐acting + NPH or premixed) 0.73 (0.23 SD) U/kg/day + placebo, oral, 2 weeks daily, thereafter twice a day |
| Hirsch 1999 | Insulin (type and dose not mentioned) + metformin, oral, 2.5 gram/day, frequency not stated | Insulin (type and dose not mentioned) + placebo, oral, frequency not stated |
| Hong 2012 | Insulin (glargine, glargine + rapid‐acting, NPH + regular) at least 10 U/day + sitagliptin, oral, 100 mg/day |
Increase insulin (glargine, glargine + rapid‐acting, NPH + regular) by ≥ 10% at random at the start of the intervention and again by ≥ 10% at 12 weeks if their HbA1c was not within target (≤ 7%) If glargine insulin 20 U daily was used, the dose increased to ≥ 22 U daily and to ≥ 24 U daily at 12 weeks. If 20‐10 U mixed insulin was used twice a day, the dose was increased to ≥ 22‐11 U twice a day and ≥ 24‐12 U twice a day at 12 weeks |
| Kitabchi 1987 | Insulin (NPH, dose not mentioned) + tolazamide, oral, 1000 mg/day, twice a day 500 mg | NPH insulin alone |
| Krawczyk 2005 | Insulin (type not mentioned) 57.5 (15.8 SD) U + metformin 1500 mg once daily | Insulin alone (type not mentioned) 59.5 (15.4 SD) U |
| Kyllastinen 1985 | Insulin (type not mentioned) mean dose 58 (3 SD) U daily + glibenclamide, oral, 10 mg/day, twice a day 5 mg | Insulin (type not mentioned) mean dose 58 (3 SD) U daily + placebo, oral, twice daily |
| Lewitt 1989 | Insulin (14 participants on daily, 17 on twice a day, 12 mixed, 19 premixed or intermediate‐acting insulin) mean dose 47 (21 SD) U + glibenclamide, oral, 15 mg/day, 10 mg in the morning and 5 mg in the evening | Insulin (14 participants on daily, 17 on twice a day, 12 mixed, 19 premixed or intermediate‐acting insulin) mean dose 47 (21 SD) U + placebo, oral, twice daily |
| Lindström 1999 | Soluble insulin (total dose 100 U/ml 3 times a day) + intermediate‐acting insulin (100 U/ml daily) + glibenclamide, oral, 10.5 mg/day, 3 times daily 3.5 mg | Soluble insulin (total dose 100 U/ml three times a day) + intermediate‐acting insulin (100 U/ml daily) +placebo, oral 3 times daily |
| Longnecker 1986 | Insulin (5 participants daily insulin injection: 1 participant on isophane insulin suspension alone (40 U daily), 4 participants on isophane insulin suspension (48 (8 SD) U) + regular insulin injections (23 (9 SD) U); 6 participants twice a day insulin injections: mean morning dose 38 (6 SD) U (regular, isophane suspension, or both) + mean afternoon dose 25 (7 SD) U (regular, isophane suspension, or both) + tolazamide, oral, 1000 mg, twice daily 250 mg | Insulin (5 participants daily insulin injection: 1 participant on isophane insulin suspension alone (40 U daily), 4 participants on isophane insulin suspension (48 (8 SD) U) + regular insulin injections (23 (9 SD) U); 6 participants DIB insulin injections: mean morning dose 38 (6 SD) U (regular, isophane suspension, or both) + mean afternoon dose 25 (7 SD) U (regular, isophane suspension, or both) + placebo, oral, twice daily |
| Mattoo 2005 | Insulin (type and dose not mentioned) + pioglitazone 30 mg | Insulin (type and dose not mentioned) + placebo, oral |
| Mauerhoff 1986 | Usual insulin (type and dose not mentioned) + Hb420 (galenic form of glibenclamide) 7 mg before breakfast and 3.5 mg before supper | Usual insulin (type and dose not mentioned) + placebo, oral |
| Mezitis 1992 | Biosynthetic human insulin (dose not mentioned) + glibenclamide, oral, twice daily 2 tablets (dose not mentioned)/day | Biosynthetic human insulin + placebo, oral |
| Mudaliar 2010 | Insulin (type and dose not mentioned) + pioglitazone, oral, 30 mg for 4 weeks, then titrated to 45 mg | Insulin (type and dose not mentioned) + placebo, oral |
| Nemoto 2011 | Insulin (type and dose not mentioned) + miglitol, oral, 50 mg 3 times a day | Insulin (type and dose not mentioned) + placebo, oral, 3 times a day |
| Osei 1984 | Insulin (intermediate‐acting insulin alone or in combination with short‐acting insulin twice a day (dose not mentioned)) + glibenclamide, oral, 5‐20 mg once daily | Insulin (intermediate‐acting insulin alone or in combination with short‐acting insulin twice a day (dose not mentioned)) + placebo, oral |
| Quartraro 1986 | Monocomponent porcine insulin (mean dose 95 (2 SD) U daily) + gliclazide 40‐240 mg once daily | Insulin alone (mono component porcine insulin (mean dose 95 (2 SD) U daily)) |
| Reich 1987 | Insulin (intermediate‐acting, dose not mentioned) + glibenclamide, oral, 5‐40 mg once daily | Insulin (intermediate‐acting, dose not mentioned) + placebo, oral |
| Relimpio 1998 | Insulin (premixed formulation of soluble and NPH or NPH only, twice a day in 23 participants and 3 times a day in 1 participant) + metformin 1275‐2550 mg once daily | Insulin dose increase (premixed formulation of soluble and NPH or NPH only, twice a day in 22 participants and 3 times a day in 1 participant), insulin increase by 10% |
| Robinson 1998 | Trial 1: insulin (type and dose not mentioned) + metformin 1000 mg/day increasing to 2000 mg /day | Insulin (type and dose not mentioned) + placebo |
| Rosenstock 2002 | I1: insulin (type and dose not mentioned) + pioglitazone 15 mg once daily | Insulin (type and dose not mentioned) + placebo once daily |
| I2: insulin (type and dose not mentioned) + pioglitazone 30 mg once daily | ||
| Schade 1987 | Insulin (intermediate‐acting or short‐acting) mean dose at least 28 U/day (daily or twice a day) + glibenclamide 20 mg once daily | Insulin (intermediate‐acting or short‐acting, mean dose at least 28 U/day, daily or twice a day) + placebo, oral |
| Schiel 2007 | I1: insulin glargine (before treatment dose) + glimepiride 3 mg/day | Insulin alone (75/25 or 70/30 premixed human insulin) |
| I2: insulin glargine (before treatment dose) + glimepiride 3 mg/day + metformin 1700 mg/day | ||
| Simpson 1990 | Usual insulin soluble + isophane twice a day or soluble + zinc‐complexed insulin daily or twice a day) + glipizide 10 mg twice a day | Usual insulin soluble + isophane twice a day or soluble + zinc‐complexed insulin daily or twice a day) + placebo, oral |
| Stenman 1988 | Insulin (intermediate‐acting daily or twice a day or intermediate‐ and short‐acting daily) + glibenclamide, oral, 15 mg once daily | Insulin (intermediate‐acting daily or twice a day or intermediate‐ and short‐acting daily) + placebo, oral |
| Strowig 2002 | I1: insulin (type and dose not mentioned) + metformin, oral, 2000 mg once daily | Insulin alone (type and dose not mentioned) |
| I2: insulin (type and dose not mentioned) + troglitazonea, oral, 600 mg once daily | ||
| Wulffelé 2002 | Insulin (Actrapid + Insulatard 4 times daily or mixed Actrapid (10%‐50%) + Insulatard (90%‐50%) twice a day) + metformin, oral, 3 x 850 mg once daily | Insulin (Actrapid + Insulatard 4 times daily or mixed Actrapid (10%‐50%) + Insulatard (90%‐50%) twice a day) + placebo, oral |
| Yilmaz 2007 | I1: insulin (mixed 30% insulin aspart + 70% NPH insulin twice a day) + acarbose, oral, 300 mg once daily | Insulin alone (mixed 30% insulin aspart + 70% NPH insulin twice a day) |
| I2: insulin (mixed 30% insulin aspart + 70% NPH insulin twice a day) + metformin, oral, 1700 mg once daily | ||
| I3: insulin (mixed 30% insulin aspart + 70% NPH insulin twice a day) + rosiglitazonea, oral, 8 mg once daily | ||
|
aoff the market I: intervention; NPH: Neutral Protamine Hagedorn; SD: standard deviation; U: units | ||
Appendix 3. Baseline characteristics (I)
| Intervention(s) and comparator(s) | Duration of intervention | Description of participants | Country | Setting | Ethnic groups (%) | Duration of diabetes (mean/range years (SD), or as reported) | |
| Avilés 1999 | I: metformin | 24 weeks | Participants with poorly controlled type 2 diabetes on insulin therapy | USA | Secondary care outpatients | White: 49 African‐American: 23 Hispanic :23 Other: 5 | 9.2 (6.4) |
| C: placebo | White: 68 African‐American: 18 Hispanic: 14 | 10.1 (4.7) | |||||
| Barnett 2013 | I: saxagliptin | 52 weeks | Inadequately controlled type 2 diabetes individuals with insulin alone (subgroup in the publication) | Canada, Hungary, India, Mexico, Poland, Russia, South Africa, UK | Multiple choices possible | White: 84.2 Black: 6.3 Asian: 7.4 Other: 2.1 |
12.1 (6.3) |
| C: placebo | White: 76.1 Black: 10.9 Asian: 6.5 Other: 6.5 |
10.3 (6.4) | |||||
| Casner 1988 | I: glibenclamide | 12 months | Participants with type 2 diabetes on insulin therapy | USA | Unclear | Hispanic: 84 Non‐Hispanic white: 10 Non‐Hispanic black: 6 | 14.3 (0.1) |
| C: placebo | Hispanic: 94 Non‐Hispanic white: 6 | 10.9 (0.1) | |||||
| Chiasson 1994 | I: acarbose | 12 months | Participants with type 2 diabetes (26% pretreated with insulin, only results of this subgroup is used) | Canada | Secondary care outpatients | ‐ | ‐ |
| C: placebo | ‐ | ‐ | |||||
| all: | White: 92 | 12.9 (0.8 SE) | |||||
| Coniff 1995 | I: acarbose | 24 weeks | Insulin pretreated participants with type 2 diabetes | Canda, USA | Not stated | ‐ | ‐ |
| C: placebo | |||||||
| Feinglos 1998 | I: glipizide | 3 months | Participants with type 2 diabetes taking insulin | USA | Secondary care inpatients and outpatients | ‐ | |
| C: placebo | |||||||
| all: | 15 (3 ‐ 30) | ||||||
| Fonseca 2007 | I: vildagliptin | 24 weeks | Participants with type 2 diabetes inadequately controlled by insulin | Finland, Germany, Spain, USA | Unclear | Black: 15 White: 70 Hispanic + Latino: 12 Other: 3 | 14.4 (8.6) |
| C: placebo | Black: 11 White: 72 Hispanic + Latino: 15 Other: 2 | 14.9 (8.4) | |||||
| Fritsche 2000 | I: metformin | 10 weeks | Severely obese participants with type 2 diabetes on intensified insulin therapy | Germany | Secondary care inpatients and outpatients | ‐ | |
| C: placebo | |||||||
| all: | ‐ | 10 (8) | |||||
| Giugliano 1993 | I: metformin | 6 months | Obese type 2 diabetes on insulin therapy | Italy | Secondary care outpatients | ‐ | 11.9 (1.2) |
| C: placebo | 11.5 (1.2) | ||||||
| Groop 1985 | I: glibenclamide | 8 weeks | Poorly controlled participants with type 2 diabetes on insulin | Finland | Secondary care outpatients | ‐ | |
| C: placebo | |||||||
| all: | 8 (1) | ||||||
| Hermann 2001 | I: metformin | 12 months | Overweight and obese type 2 diabetes participants on insulin therapy | Sweden | Secondary care outpatients | ‐ | 13 (3 ‐ 31) |
| C: placebo | 13 (4 ‐ 25) | ||||||
| Hirsch 1999 | I: metformin | 5 months | Participants with type 2 diabetes and sub optimal insulin therapy | USA | Secondary care outpatients | ‐ | ‐ |
| C: placebo | ‐ | ‐ | |||||
| Hong 2012 | I: Sitagliptin | 24 weeks | Participants with uncontrolled type 2 diabetes on insulin therapy | Korea | Secondary care outpatients | ‐ | 15.9 (10.5) |
| C: insulin increase | 15.8 (9.9) | ||||||
| Kitabchi 1987 | I: tolazamide | 3 months | Obese insulin‐requiring participants with type 2 diabetes | USA | Secondary care inpatients and outpatients | ‐ | |
| C: insulin alone | |||||||
| all: | 10 (1) | ||||||
| Krawczyk 2005 | I: metformin | 6 months | Obese insulin‐requiring participants with type 2 diabetes | Poland | Secondary care outpatients | ‐ | 12.2 (4.8) |
| C: insulin alone | 11.9 (5.9) | ||||||
| Kyllastinen 1985 | I: glibenclamide | 2 months | Elderly participants with type 2 diabetes on insulin therapy | Finland | Secondary care outpatients | ‐ | |
| C: placebo | |||||||
| all: | 11 (1) | ||||||
| Lewitt 1989 | I: glibenclamide | 3 months | Insulin‐treated type 2 diabetes participants | Australia | Secondary care outpatients | ‐ | ‐ |
| C: placebo | |||||||
| Lindström 1999 | I: glibenclamide | 3 months | Type 2 diabetes participants on insulin monotherapy | Sweden | Unclear | ‐ | |
| C: placebo | |||||||
| all: | 10.5 (1.2) | ||||||
| Longnecker 1986 | I: tolazamide | 8 weeks | Type 2 diabetes participants on insulin monotherapy | USA | Secondary care outpatients | ‐ | |
| C: placebo | |||||||
| all: | 12 (2) | ||||||
| Mattoo 2005 | I: pioglitazone | 6 months | Type 2 diabetes participants on insulin monotherapy | Australia, Belgium, Canada, New Zealand, Romania, Spain | Secondary care outpatients | White: 97 | 13.6 (6.8) |
| C: placebo | White: 97 | 13.4 (6.1) | |||||
| Mauerhoff 1986 | I: glibenclamide | 16 weeks | Type 2 diabetes participants on insulin monotherapy | Belgium | Secondary care outpatients | ‐ | 11 (2) |
| C: placebo | 10 (2) | ||||||
| Mezitis 1992 | I: glibenclamide | 20 weeks | Type 2 diabetes participants on insulin monotherapy | USA | Secondary care outpatients | ‐ | ‐ |
| C: placebo | |||||||
| Mudaliar 2010 | I: pioglitazone | 12 to 16 weeks | Type 2 diabetes participants on insulin monotherapy | USA | Clinical research centre | ‐ | ‐ |
| C: placebo | |||||||
| Nemoto 2011 | I: miglitol | 24 weeks | Type 2 diabetes participants on insulin monotherapy | Japan | Secondary care outpatients | ‐ | |
| C: placebo | |||||||
| all: | 15.1 (8.5) | ||||||
| Osei 1984 | I: glibenclamide | 16 weeks | Type 2 diabetes participants on insulin monotherapy | USA | Secondary care inpatients and outpatients | ‐ | 12.3 (1.2) |
| C: placebo | 12.9 (1.6) | ||||||
| Quartraro 1986 | I: gliclazide | 12 months | Type 2 diabetes participants on insulin monotherapy | Italy | Secondary care inpatients and outpatients | ‐ | 12.1 (1.4 SE) |
| C: insulin alone | 11.8 (1.3 SE) | ||||||
| Reich 1987 | I: glibenclamide | 4 months | Type 2 diabetes participants on insulin monotherapy | USA | Secondary care inpatients and outpatients | ‐ | 9.0 (3.1) |
| C: placebo | 9.7 (3.3) | ||||||
| Relimpio 1998 | I: metformin | 4 months | Type 2 diabetes participants on insulin monotherapy | Spain | Secondary care outpatients | ‐ | 15.4 (7.7) |
| C: insulin dose increase | 15.3 (6.0) | ||||||
| Robinson 1998 | I: metformin | 12 weeks | Type 2 diabetes participants on insulin monotherapy | UK | Secondary care outpatients | ‐ | Study 1: 15 (7) Study 2: 14 (6) |
| C: placebo | |||||||
| Rosenstock 2002 | I1: pioglitazone 15 mg | 16 weeks | Type 2 diabetes participants on insulin ± oral antidiabetic medication. Those participants on insulin + oral antidiabetic medication discontinued the oral drug at the beginning of the screening period. In addition the run‐in period for those participants was 6 weeks instead of 3 for the participants on insulin monotherapy. | USA | Unclear | White: 75 | ‐ |
| I2: pioglitazone 30 mg | White: 73 | ‐ | |||||
| C1: placebo | White: 71 | ‐ | |||||
| Schade 1987 | I: glibenclamide | 16 weeks | Type 2 diabetes participants on insulin monotherapy | USA | Secondary care outpatients | ‐ | ‐ |
| C: placebo | ‐ | ||||||
| all: | 10 (1) | ||||||
| Schiel 2007 | I1: glimepiride | 16 weeks | Type 2 diabetes participants on insulin monotherapy | Germany | Secondary care outpatients | ‐ | 15.3 (8.4) |
| I2: glimepiride + metformin | 14.2 (8.0) | ||||||
| C1: insulin alone | 16.3 (6.7) | ||||||
| Simpson 1990 | I: glipizide | 8 weeks | Type 2 diabetes participants on insulin monotherapy | UK | Secondary care outpatients | ‐ | 9 (2 ‐ 18) |
| C: placebo | 10 (1 ‐ 20) | ||||||
| Stenman 1988 | I: glibenclamide | 4 months | Type 2 diabetes participants on insulin monotherapy | Finland | Secondary care inpatients and outpatients | ‐ | ‐ |
| C: placebo | ‐ | ||||||
| all: | 9.8 (4.7) | ||||||
| Strowig 2002 | I1: metformin | 4 months | Type 2 diabetes participants on insulin monotherapy | USA | Unclear | White: 52 Afro‐American: 15 Hispanic: 30 Other: 4 |
7.6 (4.1) |
| I2: troglitazone | White: 57 Afro‐American: 17 Hispanic: 27 | 11.6 (6.8) | |||||
| C1: insulin alone | White: 55 Afro‐American: 29 Hispanic: 16 | 10.5 (7.3) | |||||
| Wulffelé 2002 | I: metformin | 16 weeks | Type 2 diabetes participants on insulin monotherapy | The Netherlands | Secondary care outpatients | ‐ | 14.0 (8.4) |
| C1: placebo | 12.0 (8.0) | ||||||
| Yilmaz 2007 | I1: acarbose | 6 months | Type 2 diabetes participants on insulin monotherapy | Turkey | Secondary care outpatients | ‐ | 13.9 (7.2) |
| I2: metformin | 12.1 (7.7) | ||||||
| I3: rosiglitazone | 12.1 (7.9) | ||||||
| C1: insulin alone | |||||||
| ‐ denotes not reported C: comparator; I: intervention; SD: standard deviation; SE: standard error | |||||||
Appendix 4. Baseline characteristics (II)
| Intervention(s) and comparator(s) | Sex (female %) | Age (mean years (SD); (range)) | HbA1c (mean % (SD); (range)) | Comedications/Cointerventions (%) | Comorbidities (%) | |
| Avilés 1999 | I: metformin | 71 | 53.1 (9.4); (35‐69) | 9 (1.4); ‐ | ‐ | ‐ |
| C: placebo | 55 | 54.6 (7.8); (36‐70) | 9.1 (1.5); ‐ | |||
| Barnett 2013 | I: saxagliptin | 60 | 58.7 (10.0); (29‐77) | 8.7 (0.9); (7‐11) | ‐ | ‐ |
| C: placebo | 59 | 57.8 (10.9); (30‐75) | 8.7 (0.8); (7‐11) | |||
| Casner 1988 | I: glibenclamide | 65 | 55.8 (0.1); ‐ | 10.9 (0.5 SE); ‐ | ‐ | ‐ |
| C: placebo | 79 | 60.0 (0.1); ‐ | 11.4 (0.4 SE); ‐ | |||
| Chiasson 1994 | I: acarbose | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | |||
| all: | 39 | 56.6 (0.9); ‐ | 7.7 (0.2 SE); ‐ | |||
| Coniff 1995 | I: acarbose | ‐ | ‐ | 6.4 (0.1 SE); ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | 6.6 (0.1 SE); ‐ | |||
| Feinglos 1998 | I: glipizide | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | |||
| all: | 59 | 56 (39 ‐ 68); (39‐68) | 12.1 (5.4‐21.2); | |||
| Fonseca 2007 | I: vildagliptin | 52 | 59.6 (10.3); ‐ | 8.4 (1.0); ‐ | ‐ | ‐ |
| C: placebo | 45 | 58.9 (10.8); ‐ | 8.4 (1.1); ‐ | |||
| Fritsche 2000 | I: metformin | 8.5 (0.4 SE); ‐ | ‐ | ‐ | ||
| C: placebo | 8.1 (0.4 SE); ‐ | |||||
| all: | 69 | 51 (9); ‐ | ||||
| Giugliano 1993 | I: metformin | 11.5 (1.2); ‐ | Antihypertensive drugs (ACE‐inhibitor or calcium antagonist): 5 | ‐ | ||
| C: placebo | 11.7 (1.3); ‐ | Antihypertensive drugs (ACE‐inhibitor or calcium antagonist): 4 | ||||
| all: | 62 | 60 (2); ‐ | ||||
| Groop 1985 | I: glibenclamide | ‐ | Antihypertensive drugs (n = 4) | Background retinopathy and signs of sensory neuropathy (n = 5) | ||
| C: placebo | ||||||
| all: | 46 | 56 (1); (49‐61) | 31 | 38 | ||
| Hermann 2001 | I: metformin | 56 | 56.9 (10.2); ‐ | 9.1 (1.3); ‐ | ‐ | Hypertension (n = 10) 27 Ischaemic heart disease (n = 7) 19 diabetic nephropathy (n = 1) 3 |
| C: placebo | 37 | 58.1 (9.7); ‐ | 8.7 (1.0); ‐ | |||
| Hirsch 1999 | I: metformin | ‐ | ‐ | 8.6 (0.2 SE); ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | 9.0 (0.4 SE); ‐ | |||
| Hong 2012 | I: sitagliptin | 53.7 | 58.8 (14.3); ‐ | 9.2 (1.0); ‐ | Sulphonylurea: 24.6 Glinides (short‐acting insulin secretagogues): 13.1 Metformin: 45.9 Thiazolidinedione: 6.6 Alpha‐glucosidase inhibitor: 31.1 Glargine only: 47.5 Glargine plus rapid acting insulin: 23 NPH plus regular insulin: 29.5 | Retinopathy: 16.4 Neuropathy: 21.3 |
| C: insulin increase | 50.9 | 59.6 (13.0); ‐ | 9.2 (1.1); ‐ | Sulphonylurea: 23.8 Glinides (short acting insulin secretagogues): 15.9 Metformin: 41.3 Thiazolidinedione: 3.2 Alpha‐glucosidase inhibitor: 42.9 Glargine only: 49.2 Glargine plus rapid‐acting insulin: 17.5 NPH plus regular insulin: 33.3 | Retinopathy: 14.3 Neuropathy: 23.8 | |
| Kitabchi 1987 | I: tolazamide | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: insulin alone | ‐ | ‐ | ‐ | |||
| all: | 100 | 51 (3); (34‐66) | 10.7 (0.7 SE); (8.7‐15.5) | |||
| Krawczyk 2005 | I: metformin | 60 | 55.8 (8.1); ‐ | 8.6 (1.9); ‐ | ‐ | ‐ |
| C: insulin alone | 40 | 58.4 (6.0); ‐ | 8.4 (1.6); ‐ | |||
| Kyllastinen 1985 | I: glibenclamide | 13.8 (0.6 SE); ‐ | Dose kept constant throughout trial | Heart failure: 89 Hypertension: 44 Hypothyroidism: 11 Psychiatric illness: 11 Chronic obstipation: 33 Retinopathy: 22 Elevated creatinine: 33 | ||
| C: placebo | 13.5 (0.8 SE); ‐ | ‐ | ‐ | |||
| all: | 78 | 73 (2); (66‐80) | ||||
| Lewitt 1989 | I: glibenclamide | ‐ | ‐ | |||
| C: placebo | ||||||
| all: | 15 | 67 (5); (59‐78) | 9.9 (1.3); (7.3‐13.3) | |||
| Lindström 1999 | I: glibenclamide | ‐ | ‐ | ‐ | ||
| C: placebo | ‐ | |||||
| all: | 33 | 59 (2); (48‐71) | ‐ | |||
| Longnecker 1986 | I: tolazamide | 92 | 62 (2.4); (48‐76) | ‐ | ‐ | |
| C: placebo | 100 | 54 (2.9); (43‐65) | ||||
| all: | 12.7 (0.8); (9‐16.3) | |||||
| Mattoo 2005 | I: pioglitazone | 56 | 58.8 (7.4); ‐ | 8.9 (0.1 SE); ‐ | ‐ | ‐ |
| C: placebo | 57 | 58.9 (6.9); ‐ | 8.8 (0.1 SE); ‐ | |||
| Mauerhoff 1986 | I: glibenclamide | 63 | 62 (2); ‐ | ‐; ‐ | ‐ | ‐ |
| C: placebo | 27 | 59 (4); ‐ | ‐; ‐ | |||
| Mezitis 1992 | I: glibenclamide | ‐ | ‐; 46‐68 | 8.7; (4.4‐8.2) | ‐ | ‐ |
| C: placebo | ‐ | ‐; 46‐68 | 8.6; (4.4‐8.2) | |||
| Mudaliar 2010 | I: pioglitazone | 7.6 (0.3); ‐ | ‐ | ‐ | ||
| C: placebo | 7.8 (0.3); ‐ | |||||
| all: | 19 | 58 (2); ‐ | ‐; 7.5‐10 | |||
| Nemoto 2011 | I: miglitol | ‐ | ‐ | |||
| C: placebo | ||||||
| all: | 42 | 59.9 (10.7); ‐ | 7.9 (1); ‐ | |||
| Osei 1984 | I: glibenclamide | 60 | 58.6 (2.7); ‐ | 10.9 (0.6 SE); ‐ | ‐ | ‐ |
| C: placebo | 83 | 56.3 (1.2); ‐ | 10.4 (0.4 SE); ‐ | |||
| Quartraro 1986 | I: gliclazide | ‐ | 56 (1.9 SE); (39‐70) | 12.0 (0.6) | ‐ | ‐ |
| C: insulin alone | 57 (1.9 SE); (39‐70) | 11.8 (0.4) | ||||
| Reich 1987 | I: glibenclamide | 0 | 58.7 (2.8); (39‐69) | 8.9 (0.7 SE); (7.2‐13.4) | ‐ | ‐ |
| C: placebo | 0 | 56.8 (3.7); (29‐69) | 10.0 (1.0 SE); (6.7‐12.8) | |||
| Relimpio 1998 | I: metformin | 79 | 65.4 (7.9); ‐ | 9.6 (1.4); ‐ | ACE inhibitors: 42 Thiazides: 8 Fibric acid: 8 HMG‐CoA inhibitors: 4 | ‐ |
| C: insulin dose increase | 65 | 66.7 (6.2); ‐ | 9.6 (1.2); ‐ | ACE inhibitors: 30 Thiazides: 17 Fibric acid: 17 HMG‐CoA inhibitors: 22 | ‐ | |
| Robinson 1998 | I: metformin | Study 1: 63 | Study 1: 61.3 (7.1); ‐ | Study 1: 8.9 (1.0 SE); ‐ | ‐ | Trial 1: Retinopathy (n = 9) 47 Neuropathy (n = 6) 32 Proteinuria (n = 1) |
| C: placebo | ‐ | |||||
| Rosenstock 2002 | I1: pioglitazone 15 mg | 54 | 56.9 (10.4); (29‐75) | 9.8 (0.1 SE); ‐ | Oral anti‐diabetic medication before study: total 12
evenly distributed among the treatment arms (metformin: 8, glibenclamide: 2, glipizide: 2) Lipid‐lowering drugs: 28 |
‐ |
| I2: pioglitazone 30 mg | 50 | 57.5 (9.9); (29‐75) | 9.8 (0.1 SE); ‐ | Lipid‐lowering drugs: 22 | ‐ | |
| C1: placebo | 55 | 56.8 (3.7); (29‐75) | 9.8 (0.1 SE); ‐ | Lipid‐lowering drugs: 21 | ‐ | |
| Schade 1987 | I: glibenclamide | ‐ | ‐ | |||
| C: placebo | ||||||
| all: | 56 | 51 (3); (35‐66) | 10.6 (0.4); ‐ | |||
| Schiel 2007 | I1: glimepiride | 53 | 61.7 (10.7); ‐ | 8.2 (0.7); ‐ | ‐ | ‐ |
| I2: glimepiride + metformin | 44 | 65.4 (8.5); ‐ | 8.1 (0.9); ‐ | |||
| C1: insulin alone | 47 | 69.8 (6.4); ‐ | 8.1 (0.8); ‐ | |||
| Simpson 1990 | I: glipizide | 65 (‐); (53 ‐ 75) | 10.6 (‐); (10‐16.4) | ‐ | ‐ | |
| C: placebo | 62 (‐); (50 ‐ 78) | 12.0 (‐); (7.6‐4.9) | ||||
| all: | 32 | |||||
| Stenman 1988 | I: glibenclamide | ‐ | ‐ | |||
| C: placebo | ‐ | |||||
| all: | 13 | 58 (6.6); (45‐68) | 9.2 (0.2); (9.9‐15.8) | Coronary heart disease (n = 1) 7 Hypertension (n = 3) 20 | ||
| Strowig 2002 | I1: metformin | 44 | 51.8 (10.5); ‐ | 8.8 (1.2); ‐ | Lipid‐lowering drugs: 30 | ‐ |
| I2: troglitazone | 57 | 51.7 (8.0); ‐ | 8.5 (1.2); ‐ | Lipid‐lowering drugs: 43 | ||
| C1: insulin alone | 52 | 54.4 (9.1); ‐ | 8.7 (1.6); ‐ | Lipid‐lowering drugs: 26 | ||
| Wulffelé 2002 | I: metformin | 56 | 63.2 (9.8); ‐ | 7.9 (1.2); ‐ | ‐ | ‐ |
| C: placebo | 50 | 58.9 (11.1); ‐ | 7.9 (1.2); ‐ | |||
| Yilmaz 2007 | I1: acarbose | 53 | 62.6 (6.6); (34‐80) | ‐ | ‐ | ‐ |
| I2: metformin | 66 | 57.7 (8.5); (34‐80) | ‐ | |||
| I3: rosiglitazone | 88 | 57.6 (8.8); (34‐80) | ‐ | |||
| C1: insulin alone | 63 | 61.5 (12.0); (34‐80) | ‐ | |||
| ‐ denotes not reported C: comparator; HbA1c: glycosylated haemoglobin A1c; I: intervention; NPH: Neutral Protamine Hagedorn; SD: standard deviation; SE: standard error | ||||||
Appendix 5. Matrix of study endpoints
|
Characteristic Study ID |
Primarya endpoint (s) | Secondaryb endpoint (s) | Otherc endpoint (s) |
| Avilés 1999 | ‐ | ‐ | Glycaemic control, insulin dose requirements, study drug tolerance, body weight, blood pressure, lipid and lipoprotein profiles, C‐peptide |
| Barnett 2013 | Change in HbA1c from baseline to 52 weeks follow‐up | Mean change from baseline in total daily insulin dose at 52 weeks | Safety end points including adverse events, hypoglycaemia and weight gain |
| Casner 1988 | ‐ | ‐ | Fasting blood glucose, HbA1c, C‐peptide, glucose tolerance test, side effects, compliance |
| Chiasson 1994 | ‐ | ‐ | HbA1c, glucose, C‐peptide, lipids, glucose tolerance test, safety, blood count, biochemistry, vitamins/minerals, hypoglycaemia |
| Coniff 1995 | HbA1c, insulin requirements | Glucose tolerance test, postprandial glucose area under the curve | Lipids, hypoglycaemia |
| Feinglos 19987 | ‐ | ‐ | Glucose, HbA1c, C‐peptide, plasma insulin, lipids |
| Fonseca 2007 | HbA1c | Fasting glucose, insulin dose, insulin injections, lipids, body weight | ‐ |
| Fritsche 2000 | ‐ | ‐ | Glucose tolerance test, HbA1c, glucose, C‐peptide, insulin dose, lipids |
| Giugliano 1993 | ‐ | ‐ | Body weight, blood pressure, HbA1c, lipids, glucose profile, safety |
| Groop 1985 | ‐ | ‐ | Body weight, glucose, HbA1c, lipids, glucose tolerance test, serum free insulin, plasma glucagon, insulin tolerance test, glucose profiles |
| Hermann 2001 | HbA1c | ‐ | Fasting glucose, body weight, BMI, waist‐hip ratio, blood pressure, insulin dose, C‐peptide, lipids, biochemistry, adverse events, compliance |
| Hong 2012 | Change in HbA1c (from baseline to 24 weeks) | Proportion of participants achieving HbA1c < 7%, body weight, waist circumference, change in insulin dose, change in C‐peptide, safety (AE, SAE, hypoglycaemia, liver/renal function) | ‐ |
| Hirsch 1999 | ‐ | ‐ | HbA1c, glucose, body weight, blood pressure, insulin dose, insulin levels, C‐peptide, hypoglycaemia |
| Kitabchi 1987 | ‐ | ‐ | HbA1c, glucose, body weight, C‐peptide, insulin dose, insulin antibodies, chemistry, triglycerides |
| Krawczyk 2005 | ‐ | ‐ | HbA1c, glucose, insulin dose, waist‐hip ratio, body weight |
| Kyllastinen 1985 | ‐ | ‐ | Glucose, HbA1c, insulin dose, C‐peptide, body weight, biochemistry, lipids |
| Lewitt 1989 | ‐ | ‐ | HbA1c, glucose, BMI, C‐peptide, insulin dose |
| Lindström 1999 | ‐ | ‐ | Insulin dose, glucose, HbA1c, plasma insulin, C‐peptide, lipoproteins, IGF‐1, testosterone, SHBG |
| Longnecker 1986 | ‐ | ‐ | HbA1c, glucose, C‐peptide, body weight, compliance, side effects |
| Mattoo 2005 | ‐ | ‐ | HbA1c, glucose, lipids, CRP, hypoglycaemia, body weight, cardiovascular risk markers |
| Mauerhoff 1986 | ‐ | ‐ | Glucose, C‐peptide, insulin dose, hypoglycaemia |
| Mezitis 1992 | ‐ | ‐ | Insulin dose, C‐peptide, HbA1c, lipids, glucose profiles |
| Mudaliar 2010 | ‐ | ‐ | HbA1c, glucose, insulin dose, weight, total body water, extracellular fluid, renal and hormonal measures |
| Nemoto 2011 | ‐ | ‐ | Meal tolerance test, HbA1c, 1,5 AG, glycoalbumin, hypoglycaemia, safety |
| Osei 1984 | ‐ | ‐ | Compliance, dietary intake, weight, glucose, HbA1c, tolerance tests |
| Quartraro 1986 | ‐ | ‐ | Glucose profile, HbA1c, insulin dose, weight, C‐peptide |
| Reich 1987 | ‐ | ‐ | HbA1c, glucose, chemistry, urinalyses |
| Relimpio 1998 | ‐ | ‐ | Weight, BMI, blood pressure, HbA1c, lipids, insulin dose, biochemistry, compliance |
| Robinson 1998 | ‐ | ‐ | Glucose, HbA1c, creatinine, lipids, blood pressure, insulin dose, hypoglycaemia |
| Rosenstock 2002 | ‐ | ‐ | HbA1c, glucose, C‐peptide, lipids, ECG, chemistry, haematology, vital signs, adverse events |
| Schade 1987 | ‐ | ‐ | HbA1c, glucose, insulin dose, C‐peptide, free insulin, erythrocyte‐glucose binding, compliance |
| Schiel 2007 | HbA1c | Glucose, treatment satisfaction, hypoglycaemia, adverse events | Blood pressure, creatinine, liver enzymes |
| Simpson 1990 | ‐ | ‐ | HbA1c, C‐peptide, serum insulin, lipids, glucose, body weight, BMI |
| Stenman 1988 | ‐ | ‐ | HbA1c, glucose, body weight, lipids, C‐peptide, free insulin |
| Strowig 2002 | ‐ | ‐ | HbA1c, glucose, liver enzymes, body weight, chemistry, C‐peptide, lipids, waist‐hip ratio |
| Wulffelé 2002 | HbA1c, insulin dose | BMI, body weight, lipids, blood pressure | Hypoglycaemia |
| Yilmaz 2007 | HbA1c | Insulin dose, body weight, waist‐hip ratio, lipids | Hypoglycaemia, side effects |
|
a,bverbatim statement in the publication
cnot explicitly stated as primary or secondary endpoint (s) in the publication ‐ denotes not reported BMI: body mass index; HbA1c: glycosylated haemoglobin A1c | |||
Appendix 6. Adverse events (I)
| Intervention(s) and comparator(s) | Participants included in analysis (N) | Deaths (N) | Deaths (%) | Participants with adverse events (N) | Participants with adverse events (%) | Participants with severe/serious adverse events (N) | Participants with severe/serious adverse events (%) | |
| Avilés 1999 | I: metformin | 22 | ‐ | ‐ | 3 | 14 | ‐ | ‐ |
| C: placebo | 23 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Barnett 2013 | I: saxagliptin | 95 | 1 | 1.1 | 61 | 64.2 | 9 | 9.5 |
| C: placebo | 46 | 0 | 0 | 32 | 69.6 | 3 | 6.5 | |
| Casner 1988 | I: glibenclamide | 31 | ‐ | ‐ | ‐ | ‐ | 0 | |
| C: placebo | 33 | ‐ | ‐ | ‐ | ‐ | 0 | ||
| Chiasson 1994 | I: acarbose | 35 | ‐ | ‐ | 1 | 2.4 | 0 | 0 |
| C: placebo | 44 | ‐ | ‐ | 3 | 2.9 | 0 | 0 | |
| Coniff 1995 | I: acarbose | 103 | ‐ | ‐ | 78 | 76 | 0 | 0 |
| C: placebo | 104 | ‐ | ‐ | 36 | 35 | 0 | 0 | |
| Feinglos 1998 | I: glipizide | ‐ | ‐ | ‐ | ||||
| C: placebo | ‐ | |||||||
| all: | 29 | ‐ | ‐ | 69 episodes of hypoglycaemia | ‐ | 1 | ‐ | |
| Fonseca 2007 | I: vildagliptin | 144 | 1 | 0.7 | ‐ | 81.3 | ‐ | 8.3 |
| C: placebo | 152 | 1 | 0.7 | ‐ | 82.9 | ‐ | 9.2 | |
| Fritsche 2000 | I: metformin | ‐ | ‐ | ‐ | 0 | 0 | 0 | 0 |
| C: placebo | ‐ | ‐ | ‐ | 0 | 0 | 0 | 0 | |
| all: | 13 | ‐ | ‐ | 0 | 0 | 0 | 0 | |
| Giugliano 1993 | I: metformin | 27 | ‐ | ‐ | 2 | 7.4 | 0 | 0 |
| C: placebo | 23 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Groop 1985 | I: glibenclamide | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| all: | 13 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Hermann 2001 | I: metformin | 16 | ‐ | ‐ | 9 | ‐ | ‐ | ‐ |
| C: placebo | 19 | ‐ | ‐ | 6 | ‐ | ‐ | ‐ | |
| Hong 2012 | I: sitagliptin | 61 | ‐ | ‐ | ‐ | 34.4 | 1 | ‐ |
| C: insulin increase | 63 | ‐ | ‐ | ‐ | 36.5 | 4 | ‐ | |
| Hirsch 1999 | I: metformin | 25 | ‐ | ‐ | 3 | 12 | 0 | 0 |
| C: placebo | 25 | ‐ | ‐ | 0 | ‐ | 0 | 0 | |
| Kitabchi 1987 | I: tolazamide | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C: NPH alone | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| all: | 12 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Krawczyk 2005 | I: metformin | 20 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: insulin alone | 20 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Kyllastinen 1985 | I: glibenclamide | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| all: | 9 | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Lewitt 1989 | I: glibenclamide | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| all: | 31 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Lindström 1999 | I: glibenclamide | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| all: | 15 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Longnecker 1986 | I: tolazamide | ‐ | ‐ | ‐ | 0 | 0 | 0 | 0 |
| C: placebo | ‐ | ‐ | ‐ | 0 | 0 | 0 | 0 | |
| all: | 11 | ‐ | ‐ | 0 | 0 | 0 | 0 | |
| Mattoo 2005 | I: pioglitazone | 128 | 0 | 0 | 109 | 76.8 | ‐ | ‐ |
| C: placebo | 135 | 1 | 0.7 | 98 | 66.7 | ‐ | ‐ | |
| Mauerhoff 1986 | I: glibenclamide | 11 | 0 | 0 | 107 episodes | ‐ | ‐ | ‐ |
| C: placebo | 11 | 0 | 0 | 25 episodes | ‐ | ‐ | ‐ | |
| Mezitis 1992 | I: glibenclamide | 10 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 10 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| all: | 20 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Mudaliar 2010 | I: pioglitazone | 12 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 13 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Nemoto 2011 | I: miglitol | 107 | ‐ | ‐ | 122 episodes | 78.5 | ‐ | ‐ |
| C: placebo | 100 | ‐ | ‐ | 91 episodes | 76 | ‐ | ‐ | |
| Osei 1984 | I: glibenclamide | 6 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 11 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Quartraro 1986 | I: gliclazide | 15 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: insulin alone | 15 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Reich 1987 | I: glibenclamide | 10 | 1 | 10 | 3 (5 episodes) | 30 | ‐ | ‐ |
| C: placebo | 10 | 0 | 0 | 10 episodes | ‐ | ‐ | ‐ | |
| Relimpio 1998 | I: metformin | 24 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: insulin dose increase | 23 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Robinson 1998 | I: metformin | ‐ | ‐ | ‐ | ‐ | ‐ | 0 | 0 |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | 0 | 0 | |
| all: | 19 | ‐ | ‐ | ‐ | ‐ | 0 | 0 | |
| Rosenstock 2002 | I1: pioglitazone 15 mg | 191 | ‐ | ‐ | ‐ | 78.4 | ‐ | ‐ |
| I2: pioglitazone 30 mg | 188 | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| C: placebo | 187 | ‐ | ‐ | ‐ | 74.3 | ‐ | ||
| Schade 1987 | I: glibenclamide | 16 | 6 | 37.5 | 0 | 0 | ||
| C: placebo | 16 | 1 | 6.3 | 0 | 0 | |||
| Schiel 2007 | I1: glimepiride | 17 | ‐ | ‐ | ‐ | 59 | 0 | 0 |
| I2: glimepiride + metformin | 18 | ‐ | ‐ | ‐ | 72 | 0 | 0 | |
| C: insulin | 17 | ‐ | ‐ | ‐ | 77 | 0 | 0 | |
| Simpson 1990 | I: glipizide | 9 | ‐ | ‐ | 4 | 44.4 | ‐ | ‐ |
| C: placebo | 10 | ‐ | ‐ | 0 | 0 | ‐ | ‐ | |
| Stenman 1988 | I: glibenclamide | 15 | 0 | 0 | 13 | 86.7 | 0 | 0 |
| C: placebo | 15 | 0 | 0 | 8 | 53.3 | 0 | 0 | |
| Strowig 2002 | I1: metformin | 27 | 0 | 0 | ‐ | ‐ | 2 | 6.7 |
| I2: troglitazone | 30 | 0 | 0 | ‐ | ‐ | 0 | 0 | |
| C: insulin | 31 | 0 | 0 | ‐ | ‐ | 1 | 3.2 | |
| Wulffelé 2002 | I: metformin | 171 | 1 | 0.6 | 1 episode per participant per month: 31 2 episodes per participant per month: 12 3 episodes per participant per month: 9 ≥ 4 episodes per participant per month: 11 |
1 episode per participant per month: 18 2 episodes per participant per month: 7 3 episodes per participant per month: 5 ≥ 4 episodes per participant per month: 6 |
8 episodes | ‐ |
| C: placebo | 182 | 0 | 0 | 1 episode per participant per month: 30 2 episodes per participant per month: 11 3 episodes per participant per month: 11 ≥ 4 episodes per participant per month: 7 |
1 episode per participant per month: 16 2 episodes per participant per month: 6 3 episodes per participant per month: 6 ≥ 4 episodes per participant per month: 4 |
4 episodes | ||
| Yilmaz 2007 | I1: acarbose | 15 | 0 | 0 | 3 | 20 | 0 | 0 |
| I2: metformin | 17 | 0 | 0 | 5 | 29.4 | 0 | 0 | |
| I3: rosiglitazone | 15 | 0 | 0 | 3 | 20 | 0 | 0 | |
| C: insulin alone | 19 | 0 | 0 | 3 | 15.8 | 0 | 0 | |
| ‐ denotes not reported C: comparator; I: intervention; NPH: Neutral Protamine Hagedorn | ||||||||
Appendix 7. Adverse events (II)
| Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants discontinuing study due to adverse events (N) | Participants discontinuing study due to adverse event (%) | Participants hospitalised (N) | Participants hospitalised (%) | Participants with outpatient treatment (N) | Participants with outpatient treatment (%) | |
| Avilés 1999 | I: metformin | 21 | 0 | 0 | 0 | 0 | ‐ | ‐ |
| C: placebo | 22 | 0 | 0 | 0 | 0 | ‐ | ‐ | |
| Barnett 2013 | I: saxagliptin | 95 | 3 | 3.2 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 46 | 1 | 2.2 | ‐ | ‐ | ‐ | ‐ | |
| Casner 1988 | I: glibenclamide | 31 | 0 | 0 | 0 | ‐ | ‐ | ‐ |
| C: placebo | 33 | 0 | 0 | 0 | ‐ | ‐ | ‐ | |
| Chiasson 1994 | I: acarbose | 35 | 11 | 27 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 44 | 12 | 23 | ‐ | ‐ | ‐ | ‐ | |
| Coniff 1995 | I: acarbose | 103 | 9 | 9 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 104 | 4 | 4 | ‐ | ‐ | ‐ | ‐ | |
| Feinglos 1998 | I: glipizide | ‐ | 0 | 0 | ‐ | ‐ | ‐ | |
| C: placebo | ‐ | 0 | 0 | ‐ | ||||
| all: | 29 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Fonseca 2007 | I: vildagliptin | 114 | ‐ | 6.3 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 124 | ‐ | 0.7 | ‐ | ‐ | ‐ | ‐ | |
| Fritsche 2000 | I: metformin | ‐ | ‐ | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | 0 | ‐ | ‐ | ‐ | ‐ | |
| all: | 13 | ‐ | 0 | ‐ | ‐ | ‐ | ‐ | |
| Giugliano 1993 | I: metformin | 27 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 23 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Groop 1985 | I: glibenclamide | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Hermann 2001 | I: metformin | 16 | 4 | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 19 | 1 | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Hirsch 1999 | I: metformin | 25 | 3 | 12 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 25 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Hong 2012 | I: sitagliptin | 61 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: insulin increase | 63 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Kitabchi 1987 | I: tolazamide | ‐ | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: NPH alone | ‐ | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| all: | 12 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Krawczyk 2005 | I: metformin | 20 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: insulin alone | 20 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Kyllastinen 1985 | I: glibenclamide | ‐ | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| all: | 9 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Lewitt 1989 | I: glibenclamide | ‐ | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| all: | 31 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Lindström 1999 | I: glibenclamide | ‐ | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| all: | 15 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Longnecker 1986 | I: tolazamide | ‐ | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| all: | 11 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Mattoo 2005 | I: pioglitazone | 128 | 7 | 4.9 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 135 | 3 | 2 | ‐ | ‐ | ‐ | ‐ | |
| Mauerhoff 1986 | I: glibenclamide | 11 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 11 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Mezitis 1992 | I: glibenclamide | 10 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 10 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Mudaliar 2010 | I: pioglitazone | 12 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 13 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Nemoto 2011 | I: miglitol | 107 | 7 | 6.5 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 100 | 3 | 3 | ‐ | ‐ | ‐ | ‐ | |
| Osei 1984 | I: glibenclamide | 6 | 4 | 40 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 11 | 1 | 8.3 | ‐ | ‐ | ‐ | ‐ | |
| Quartraro 1986 | I: gliclazide | 15 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: insulin alone | 15 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Reich 1987 | I: glibenclamide | 10 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 10 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Relimpio 1998 | I: metformin | 24 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: insulin dose increase | 23 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Robinson 1998 | I: metformin | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| all: | 19 | 1 | 5 | ‐ | ‐ | ‐ | ‐ | |
| Rosenstock 2002 | I1: pioglitazone 15 mg | 191 | ‐ | 2.6 | ‐ | ‐ | ‐ | ‐ |
| I2: pioglitazone 30 mg | 188 | ‐ | 3.2 | ‐ | ‐ | ‐ | ‐ | |
| C: placebo | 187 | ‐ | 1.6 | ‐ | ‐ | ‐ | ‐ | |
| Schade 1987 | I: glibenclamide | 16 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 16 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Schiel 2007 | I1: glimepiride | 17 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| I2: glimepiride + metformin | 18 | 2 | 11.1 | ‐ | ‐ | ‐ | ‐ | |
| C: insulin | 17 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Simpson 1990 | I: glipizide | 9 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 10 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Stenman 1988 | I: glibenclamide | 15 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 15 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Strowig 2002 | I1: metformin | 27 | 2 | 6.7 | ‐ | ‐ | ‐ | ‐ |
| I2: troglitazone | 30 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| C: insulin | 31 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| Wulffelé 2002 | I: metformin | 171 | 20 | 10.2 | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 182 | 6 | 3.1 | ‐ | ‐ | ‐ | ||
| Yilmaz 2007 | I1: acarbose | 15 | 0 | 0 | ‐ | ‐ | ‐ | ‐ |
| I2: metformin | 17 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| I3: rosiglitazone | 15 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| C: insulin alone | 19 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | |
| ‐ denotes not reported C: comparator; I: intervention; NPH: Neutral Protamine Hagedorn | ||||||||
Appendix 8. Adverse events (III)
| Intervention(s) and comparator(s) | Participants included in analysis (N) | Participants with hypoglycaemic episodes (N) | Participants with hypoglycaemic episodes (%) | Participants with nocturnal hypoglycaemic episodes (N/%) | Participants with severe/serious hypoglycaemic episodes (N/%) | Definition of severe/serious hypoglycaemia | Participants with specific adverse events (N) | Participants with specific adverse events (%) | |
| Avilés 1999 | I: metformin | 21 | 3 | 13.6 | ‐ | 0 | ‐ | ‐ | ‐ |
| C: placebo | 22 | 0 | 10 | ‐ | 0 | ‐ | ‐ | ||
| Barnett 2013 | I: saxagliptin | 95 | Reported: 22 Confirmed: 8 |
23.2 8.4 |
‐ | ‐ | ‐ | Urine tract infection: 7 Nasopharyngitis: 6 Upper resp. tract infection: 6 Headache: 5 Bronchitis: 4 Pharyngitis: 3 Influenza: 3 Hypertension: 4 Pain in extremity: 3 | Urine tract infection: 7.4 Nasopharyngitis: 6.3 Upper resp. tract infection: 6.3 Headache: 5.3 Bronchitis: 4.2 Pharyngitis: 3.2 Influenza: 3.2 Hypertension: 4.2 Pain in extremity: 3.2 |
| C: placebo | 46 | Reported: 15 Confirmed: 5 |
32.6 10.9 |
‐ | ‐ | Urine tract infection: 1 Nasopharyngitis: 1 Upper resp. tract infection: 5 Headache: 1 Bronchitis: 0 Pharyngitis: 3 Influenza: 2 Hypertension: 4 Pain in extremity: 2 | Urine tract infection: 2.2 Nasopharyngitis: 2.2 Upper resp. tract infection: 10.9 Headache: 2.2 Bronchitis: 0 Pharyngitis: 6.5 Influenza: 4.3 Hypertension: 8.7 Pain in extremity: 4.3 |
||
| Casner 1988 | I: glibenclamide | 31 | "complains compatible with mild hypoglycaemia were not different between the two groups." | ‐ | ‐ | 0 | ‐ | ‐ | ‐ |
| C: placebo | 33 | ‐ | ‐ | ‐ | 0 | ‐ | ‐ | ||
| Chiasson 1994 | I: acarbose | 35 | ‐ | 2.4 | ‐ | /2.4 | Required correction of hypoglycaemia | ‐ | Flatulence: 73.2 Diarrhoea: 43.6 Abdominal discomfort: 25.0 |
| C: placebo | 44 | ‐ | 6.0 | ‐ | /6.0 | ‐ | Flatulence: 39.0 Diarrhoea: 20.3 Abdominal discomfort: 8.8 | ||
| Coniff 1995 | I: acarbose | 103 | 0 | 0 | ‐ | ‐ | ‐ | Digestive system: 78 Flatulence: 34 | Digestive system: 76 Flatulence: 33 |
| C: placebo | 104 | 0 | 0 | ‐ | ‐ | Digestive system: 36 Flatulence: 14 | Digestive system: 35 Flatulence: 13 | ||
| Feinglos 1998 | I: glipizide | ‐ | ‐ | ‐ | ‐ | ‐ | Requiring assistance from another person | ‐ | |
| C: placebo | ‐ | ‐ | ‐ | ‐ | |||||
| all: | 29 | 69 total episodes, number of participants not mentioned | ‐ | ‐ | 1/ | ‐ | ‐ | ||
| Fonseca 2007 | I: vildagliptin | 114 | ‐ | 22.9 | ‐ | 0 | Requiring assistance of another party | ‐ | ‐ |
| C: placebo | 124 | ‐ | 29.6 | ‐ | ‐ | ‐ | ‐ | ||
| Fritsche 2000 | I: metformin | ‐ | 0 | 0 | 0 | 0 | ‐ | ‐ | ‐ |
| C: placebo | ‐ | 0 | 0 | 0 | 0 | ‐ | ‐ | ||
| all: | 13 | 0 | 0 | 0 | 0 | ‐ | ‐ | ||
| Giugliano 1993 | I: metformin | 27 | 0 | 0 | ‐ | ‐ | ‐ | Diarrhoea: 2 | Diarrhoea: 7.4 |
| C: placebo | 23 | 0 | 0 | ‐ | ‐ | Diarrhoea: ‐ | Diarrhoea: ‐ | ||
| Groop 1985 | I: glibenclamide | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| all: | 13 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Hermann 2001 | I: metformin | 16 | 2 | ‐ | ‐ | 0 | ‐ | Diarrhoea: 6 Flatulence: 1 Epigastic pain: 0 Anorexia: 2 Constipation: 1 Sweating: 0 GI‐event: 8 | ‐ |
| C: placebo | 19 | 0 | ‐ | ‐ | 0 | Diarrhoea: 1 Flatulence: 3 Epigastric pain: 1 Anorexia: 1 Constipation: 1 Sweating: 1 GI‐event: 5 | ‐ | ||
| Hirsch 1999 | I: metformin | 25 | ‐ | ‐ | ‐ | 0 | ‐ | Gastro‐intestinal side effects: 3 | Gastro‐intestinal side effects: 12.0 |
| C: placebo | 25 | ‐ | ‐ | ‐ | 0 | Gastro‐intestinal side effects: 0 | Gastro‐intestinal side effects: 0 | ||
| Hong 2012 | I: sitagliptin | 61 | ‐ | 8.2 | ‐ | 1.6 | Any episode requiring assistance from another party with plasma glucose value < 3.0 mmol/L (54 mg/dL) | ‐ | ‐ |
| C: insulin increase | 63 | ‐ | 17.5 | ‐ | 4.8 | ‐ | ‐ | ||
| Kitabchi 1987 | I: tolazamide | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: NPH alone | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Krawczyk 2005 | I: metformin | 20 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: insulin alone | 20 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Kyllastinen 1985 | I: glibenclamide | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Lewitt 1989 | I: glibenclamide | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Lindström 1999 | I: glibenclamide | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| all: | 15 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Longnecker 1986 | I: tolazamide | ‐ | 0 | 0 | 0 | ‐ | ‐ | ‐ | |
| C: placebo | ‐ | 0 | 0 | 0 | ‐ | ‐ | |||
| all: | 11 | 0 | 0 | 0 | ‐ | ‐ | |||
| Mattoo 2005 | I: pioglitazone | 128 | 109 | 63.4 | ‐ | ‐ | Requiring assistance | Oedema: 20 | Oedema: 14.1 |
| C: placebo | 135 | 98 | 51.0 | ‐ | ‐ | Oedema: 5 | Oedema: 3.4 | ||
| Mauerhoff 1986 | I: glibenclamide | 11 | 107 episodes | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 11 | 25 episodes | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Mezitis 1992 | I: glibenclamide | 10 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 10 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Mudaliar 2010 | I: pioglitazone | 12 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 13 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Nemoto 2011 | I: miglitol | 107 | 122 episodes | 39.3 | 13 episodes | ‐ | ‐ | ‐ | Flatulence: 20.6 Diarrhoea: 14.0 Abdominal distension: 15.0 |
| C: placebo | 100 | 91 episodes | 35.0 | 29 episodes | ‐ | ‐ | Flatulence: 12.0 Diarrhoea: 4.0 Abdominal distension: 4.0 | ||
| Osei 1984 | I: glibenclamide | 6 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 11 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Quartraro 1986 | I: gliclazide | 15 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: insulin alone | 15 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Reich 1987 | I: glibenclamide | 10 | 3 (5 episodes) | 30.0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 10 | 10 episodes | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| Relimpio 1998 | I: metformin | 24 | ‐ | ‐ | ‐ | 0 | ‐ | ‐ | ‐ |
| C: insulin dose increase | 23 | ‐ | ‐ | ‐ | 0 | ‐ | ‐ | ||
| Robinson 1998 | I: metformin | ‐ | ‐ | ‐ | ‐ | 0 | ‐ | ‐ | Diarrhoea: 5.0 Mild abdominal bloating: 5.0 |
| C: placebo | ‐ | ‐ | ‐ | ‐ | 0 | ‐ | Diarrhoea: 0 Mild abdominal bloating: 0 | ||
| all: | 19 | ‐ | ‐ | ‐ | 0 | ‐ | ‐ | ||
| Rosenstock 2002 | I1: pioglitazone 15 mg | 191 | ‐ | 7.9 | ‐ | 0 | ‐ | ‐ | Oedema: 12.6 |
| I2: pioglitazone 30 mg | 188 | ‐ | 15.4 | ‐ | 0 | ‐ | Oedema: 17.6 | ||
| C: placebo | 187 | ‐ | 4.8 | ‐ | 0 | ‐ | Oedema: 7.0 | ||
| Schade 1987 | I: glibenclamide | 16 | 6 | 37.5 | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 16 | 1 | 6.3 | ‐ | ‐ | ‐ | ‐ | ||
| Schiel 2007 | I1: glimepiride | 17 | ‐ | 59 | ‐ | 0 | Need for intravenous glucose or glucagon | 0 | 0 |
| I2: glimepiride + metformin | 18 | ‐ | 72 | ‐ | 0 | Gastrointestinal discomfort: 2 | Gastrointestinal discomfort: 11.1 | ||
| C: insulin | 17 | ‐ | 77 | ‐ | 0 | 0 | 0 | ||
| Simpson 1990 | I: glipizide | 9 | 4 | 44.4 | ‐ | ‐ | ‐ | ‐ | ‐ |
| C: placebo | 10 | 0 | 0 | ‐ | ‐ | ‐ | ‐ | ||
| Stenman 1988 | I: glibenclamide | 15 | 13 | 86.7 | ‐ | 0 | ‐ | ‐ | ‐ |
| C: placebo | 15 | 8 | 53.3 | ‐ | 0 | ‐ | ‐ | ||
| Strowig 2002 | I1: metformin | 27 | 0.6 episodes per participant per month | ‐ | ‐ | 0 | Third party assistance | ‐ | Gastrointestinal side effects: 67 |
| I2: troglitazone | 30 | 1.7 episodes per participant per month | ‐ | ‐ | 0 | ‐ | Gastrointestinal side effects:36.7 | ||
| C: insulin | 31 | 2 episodes per participant per month | ‐ | ‐ | 1/3.2 | ‐ | Gastrointestinal side effects: 13 | ||
| Wulffelé 2002 | I: metformin | 171 | ‐ | 36.8 | ‐ | ‐ | Third party assistance | Diarrhoea: 9 Flatulence: 4 Pruritus: 1 Headaches: 1 Pyrosis: 0 Nausea: 1 | ‐ |
| C: placebo | 182 | ‐ | 32.4 | ‐ | ‐ | Diarrhoea: 2 Flatulence: 1 Pruritus: 0 Headaches: 0 Pyrosis: 1 Nausea: 0 | ‐ | ||
| Yilmaz 2007 | I1: acarbose | 15 | 1 | 6.7 | ‐ | 0 | Unable to treat themselves | Flatulence and bloating: 2 | Flatulence and bloating: 13.3 |
| I2: metformin | 17 | 2 | 11.8 | ‐ | 0 | Gastrointestinal side effects: 3 | Gastrointestinal side effects: 17.7 | ||
| I3: rosiglitazone | 15 | 2 | 13.3 | ‐ | 0 | Pretibial oedema: 1 | Pretibial oedema: 6.7 | ||
| C: insulin alone | 19 | 2 | 10.5 | ‐ | 0 | Pretibial oedema: 1 | Pretibial oedema: 5.3 | ||
| ‐ denotes not reported C: comparator; I: intervention; NPH: Neutral Protamine Hagedorn | |||||||||
Data and analyses
Comparison 1. Insulin monotherapy versus insulin plus sulphonylurea.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (change from baseline) | 7 | 220 | Mean Difference (IV, Random, 95% CI) | 0.57 [‐2.52, 3.67] |
| 1.1 Parallel‐group trials | 2 | 86 | Mean Difference (IV, Random, 95% CI) | 1.12 [‐3.08, 5.32] |
| 1.2 Cross‐over trials | 5 | 134 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐4.66, 4.50] |
| 2 Weight (change from baseline) [kg] GIV | 7 | Mean Difference (Random, 95% CI) | ‐0.13 [‐0.57, 0.32] | |
| 2.1 Parallel‐group | 2 | Mean Difference (Random, 95% CI) | 1.12 [‐3.08, 5.32] | |
| 2.2 Cross‐over group | 5 | Mean Difference (Random, 95% CI) | ‐0.14 [‐0.59, 0.31] | |
| 3 HbA1c (change from baseline) | 9 | 316 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐1.56, ‐0.49] |
| 3.1 Parallel‐group trials | 4 | 150 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐2.57, 0.06] |
| 3.2 Cross‐over trials | 5 | 166 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.38, ‐0.56] |
| 4 HbA1c (change from baseline) GIV | 9 | Mean Difference (Random, 95% CI) | ‐1.03 [‐1.58, ‐0.47] | |
| 4.1 Parallel‐group trials | 4 | Mean Difference (Random, 95% CI) | ‐1.25 [‐2.57, 0.06] | |
| 4.2 Cross‐over trials | 5 | Mean Difference (Random, 95% CI) | ‐0.97 [‐1.42, ‐0.52] | |
| 5 Fasting glucose (change from baseline) | 6 | 205 | Mean Difference (IV, Random, 95% CI) | ‐2.28 [‐3.23, ‐1.33] |
| 5.1 Parallel‐group trials | 2 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐2.56, 0.61] |
| 5.2 Cross‐over trials | 4 | 134 | Mean Difference (IV, Random, 95% CI) | ‐2.72 [‐3.66, ‐1.78] |
| 6 Fasting plasma glucose (change from baseline) [mmol/L] GIV | 6 | Mean Difference (Random, 95% CI) | ‐2.29 [‐3.23, ‐1.35] | |
| 6.1 Parallel‐group | 2 | Mean Difference (Random, 95% CI) | ‐1.02 [‐2.48, 0.44] | |
| 6.2 Cross‐over group | 4 | Mean Difference (Random, 95% CI) | ‐2.73 [‐3.70, ‐1.75] | |
| 7 Total cholesterol (change from baseline) | 5 | 132 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.38, 0.32] |
| 7.1 Parallel‐group trials | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.49, 1.01] |
| 7.2 Cross‐over trials | 4 | 110 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.50, 0.29] |
| 8 Total cholesterol (change from baseline) [mmol/l] GIV | 5 | Mean Difference (Random, 95% CI) | ‐0.04 [‐0.17, 0.09] | |
| 8.1 Parallel‐group | 1 | Mean Difference (Random, 95% CI) | 0.26 [‐0.49, 1.01] | |
| 8.2 Cross‐over group | 4 | Mean Difference (Random, 95% CI) | ‐0.05 [‐0.18, 0.08] | |
| 9 HDL‐cholesterol (change from baseline) | 4 | 108 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.17, 0.03] |
| 9.1 Parallel‐group trials | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.27, 0.27] |
| 9.2 Cross‐over trials | 3 | 86 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.20, 0.04] |
| 10 HDL‐Cholesterol (change from baseline mmol/L) GIV | 4 | Mean Difference (Random, 95% CI) | ‐0.05 [‐0.15, 0.05] | |
| 10.1 Parallel‐group | 1 | Mean Difference (Random, 95% CI) | 0.0 [‐0.27, 0.27] | |
| 10.2 Cross‐over group | 3 | Mean Difference (Random, 95% CI) | ‐0.07 [‐0.19, 0.06] | |
| 11 Triglyceride (change from baseline) [mmol/L] GIV | 5 | Mean Difference (Random, 95% CI) | 0.04 [‐0.21, 0.28] | |
| 11.1 Parallel‐group | 1 | Mean Difference (Random, 95% CI) | ‐0.24 [‐1.37, 0.89] | |
| 11.2 Cross‐over group | 4 | Mean Difference (Random, 95% CI) | 0.05 [‐0.20, 0.30] | |
| 12 Triglycerides (change from baseline) | 5 | 132 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.17, 0.22] |
| 12.1 Parallel‐group trials | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐1.37, 0.89] |
| 12.2 Cross‐over trials | 4 | 110 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.16, 0.23] |
Comparison 2. Insulin monotherapy versus insulin plus metformin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (change from baseline) | 7 | 615 | Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐3.19, ‐1.05] |
| 1.1 Parallel‐group trials | 6 | 577 | Mean Difference (IV, Random, 95% CI) | ‐2.45 [‐3.61, ‐1.29] |
| 1.2 Cross‐over trials | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐2.11, 1.11] |
| 2 Weight (change from baseline) [kg] GIV | 7 | Mean Difference (Random, 95% CI) | ‐2.11 [‐3.18, ‐1.05] | |
| 2.1 Parallel‐group | 6 | Mean Difference (Random, 95% CI) | ‐2.45 [‐3.61, ‐1.29] | |
| 2.2 Cross‐over group | 1 | Mean Difference (Random, 95% CI) | ‐0.5 [‐2.05, 1.05] | |
| 3 HbA1c (change from baseline) | 9 | 698 | Mean Difference (IV, Random, 95% CI) | ‐0.86 [‐1.20, ‐0.51] |
| 3.1 Parallel‐group trials | 7 | 634 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.13, ‐0.41] |
| 3.2 Cross‐over trials | 2 | 64 | Mean Difference (IV, Random, 95% CI) | ‐1.16 [‐2.13, ‐0.19] |
| 4 HbA1c (change from baseline) [%] GIV | 9 | Mean Difference (Random, 95% CI) | ‐0.86 [‐1.20, ‐0.51] | |
| 4.1 Parallel‐group | 7 | Mean Difference (Random, 95% CI) | ‐0.77 [‐1.13, ‐0.41] | |
| 4.2 Cross‐over group | 2 | Mean Difference (Random, 95% CI) | ‐1.16 [‐2.13, ‐0.18] | |
| 5 Total cholesterol (change from baseline) | 8 | 651 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.41, ‐0.08] |
| 5.1 Parallel‐group trials | 6 | 587 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.38, ‐0.02] |
| 5.2 Cross‐over trials | 2 | 64 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.85, ‐0.09] |
| 6 Total cholesterol (change from baseline) [mmol/L] GIV | 8 | Mean Difference (Random, 95% CI) | ‐0.29 [‐0.50, ‐0.07] | |
| 6.1 Parallel‐group | 6 | Mean Difference (Random, 95% CI) | ‐0.20 [‐0.38, ‐0.02] | |
| 6.2 Cross‐over group | 2 | Mean Difference (Random, 95% CI) | ‐0.57 [‐0.94, ‐0.20] | |
| 7 HDL‐cholesterol (change from baseline) | 8 | 651 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 7.1 Parallel‐group trials | 6 | 587 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.05, 0.05] |
| 7.2 Cross‐over trials | 2 | 64 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.25, 0.25] |
| 8 HDL‐cholesterol (change from baseline) [mmol/L] | 8 | Mean Difference (Random, 95% CI) | 0.01 [‐0.05, 0.08] | |
| 8.1 Parallel‐group | 6 | Mean Difference (Random, 95% CI) | ‐0.00 [‐0.05, 0.05] | |
| 8.2 Cross‐over group | 2 | Mean Difference (Random, 95% CI) | 0.04 [‐0.22, 0.31] | |
| 9 Triglycerides (change from baseline) | 8 | 651 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.22, 0.04] |
| 9.1 Parallel‐group trials | 6 | 587 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.24, 0.11] |
| 9.2 Cross‐over trials | 2 | 64 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.50, 0.16] |
| 10 Triglyceride (change from baseline) [mmol/L] GIV | 8 | Mean Difference (Random, 95% CI) | ‐0.15 [‐0.41, 0.11] | |
| 10.1 Parallel‐group | 6 | Mean Difference (Random, 95% CI) | ‐0.07 [‐0.24, 0.11] | |
| 10.2 Cross‐over group | 2 | Mean Difference (Random, 95% CI) | ‐0.34 [‐0.79, 0.11] |
Comparison 3. Insulin monotherapy versus insulin plus pioglitazone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HbA1c (change from baseline) | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Fasting glucose (change from baseline) | 3 | 624 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐3.61, 3.46] |
| 3 Weight (change from baseline) | 2 | 288 | Mean Difference (IV, Random, 95% CI) | 3.79 [2.97, 4.60] |
Comparison 4. Insulin monotherapy versus insulin plus alpha‐glucosidase inhibitor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (change from baseline) | 2 | 241 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.22, 0.32] |
| 2 HbA1c (change from baseline) | 3 | 448 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.54, ‐0.24] |
| 3 Fasting glucose (change from baseline) | 2 | 113 | Mean Difference (IV, Random, 95% CI) | 0.33 [‐0.74, 1.39] |
Comparison 5. Insulin monotherapy versus insulin plus DPP‐4 inhibitor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight (change from baseline) | 2 | 362 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐1.00, 1.90] |
| 2 HbA1c (change from baseline) | 2 | 265 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.46, ‐0.35] |
| 3 Fasting glucose (change from baseline) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
5.3. Analysis.
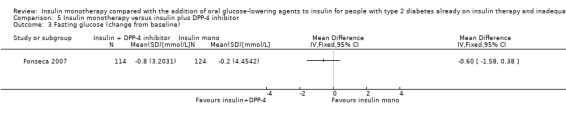
Comparison 5 Insulin monotherapy versus insulin plus DPP‐4 inhibitor, Outcome 3 Fasting glucose (change from baseline).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Avilés 1999.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: type 2 diabetes diagnosed after 30 years of age and treated for at least 2 years with at least 50 units of insulin per day, age at enrolment younger than 70 years and HbA1c level ≥ 8%. Exclusion criteria: pregnant women; women trying to become pregnant; patients with a serum creatinine concentration greater than 132.6 mmol/L (1.5 mg/dL) or hepatic enzyme levels greater than twice the upper limit of normal; and patients with medical conditions that could promote lactic acidoses such as renal or hepatic disease, congestive heart failure or chronic obstructive pulmonary disease. Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: insulin: intervention 96.2 ± 44.9 U/day, control 96.9 ± 43.3 U/day Titration period: 8 weeks |
|
| Outcomes |
Outcomes reported in abstract of publication: Primary outcome(s) (as stated in the publication): glycaemic control (HbA1c, fasting plasma glucose), C‐peptide, body weight, lipids, insulin dose, adverse events (hypoglycaemia) |
|
| Study details |
Run‐in period: 8 weeks to titrate metformin in maximal dosage Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Bristol‐Myers‐Squibb Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To evaluate the efficacy of metformin in combination with insulin in patients with type 2 diabetes mellitus poorly controlled with insulin therapy alone". | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk | Quote from publication: "Patients who met the inclusion criteria were randomly assigned in a double‐blind fashion to receive metformin or placebo in addition to their current insulin therapy" |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk | Quote from publication: "Patients who met the inclusion criteria were randomly assigned in a double‐blind fashion to receive metformin or placebo in addition to their current insulin therapy" |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk | Quote from publication: "Patients who met the inclusion criteria were randomly assigned in a double‐blind fashion to receive metformin or placebo in addition to their current insulin therapy" |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: it is unclear if the outcome assessor were blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: it is unclear if the outcome assessor were blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: it is unclear if the outcome assessor were blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: in table 3 of the article the incidence of adverse events is listed for both groups |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: data were collected, analysed and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: was reported |
| Selective reporting (reporting bias) | Low risk | Comment: data on blood pressure, medical history and the physical examination is not reported. However this is not likely to bias the results of the other outcomes |
| Other bias | Low risk | Comment: no incomplete outcome data (attrition bias) Diabetes‐related mortality |
Barnett 2013.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 2:1 Equivalence design Controlled clinical trial (CCT): a Phase IIIb, extension of RCT |
|
| Participants |
Inclusion criteria: Men and women aged 18–78 years with T2DM, fasting
C‐peptide C 0.8 ng/mL, body mass index (BMI) B45 kg/m2, and inadequate glycaemic control (HbA1c 7.5%–11.0%) on a stable regimen of insulin (30–150 U/day, with B 20% variation in total daily dose for C 8 weeks before screening). Exclusion criteria: poorly controlled diabetes (e.g. marked polyuria and polydipsia with 10% weight loss during the 3 months before screening); history of diabetic ketoacidosis or hyperosmolar nonketotic coma; history of significant cardiovascular disease or haemoglobinopathy; contraindications to DPP‐4 inhibitors, metformin, or insulin; or pregnancy, breast feeding, or not using an acceptable method of birth control Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: unclear Treatment before study: intermediate‐acting insulin, long‐acting (basal) insulin, or a premixed formulation in which rapid‐ or short‐acting insulin constituted one component was permitted. Participants could also be taking metformin if the daily dose was stable for C 8 weeks before screening. Titration period: 4 weeks |
|
| Outcomes | Primary outcome(s) (as stated in the publication): mean change from baseline to 52 weeks in HbA1c | |
| Study details |
Total study duration: 56 weeks Run‐in period: 4 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Bristol‐Myers‐Squibb and AstraZeneca Publication status: peer‐reviewed journal |
|
| Stated aim of study | To evaluate the safety and efficacy of the DPP4 inhibitor saxagliptin vs placebo as add‐on therapy in type 2 diabetes inadequately controlled with insulin with or without metformin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Patients were stratified based on metformin use at enrolment and randomised 2:1 via an interactive voice response system using a blocked randomisation schedule to receive saxagliptin 5 mg (Onglyza, Bristol‐Myers Squibb Company, Princeton, NJ, USA, and AstraZeneca Pharmaceuticals LP, Wilmington, DE, USA) or placebo once daily as add‐on to baseline therapy with insulin or insulin plus metformin." |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "To maintain blinding to patients and physicians, saxagliptin and placebo tablets were identical in appearance, and bottles were printed with a blinded label." |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk | Quote from publication: "To maintain blinding to patients and physicians, saxagliptin and placebo tablets were identical in appearance, and bottles were printed with a blinded label." |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk | Comment: FGP and lipids not assessed |
| Blinding of participants and personnel (performance bias) Insulin dose | Unclear risk | Quote from publication: "To maintain blinding to patients and physicians, saxagliptin and placebo tablets were identical in appearance, and bottles were printed with a blinded label." |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | High risk | Comment: no exact SDs are reported as text only in the figures |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | High risk |
Comment: no exact SDs are reported as text only in the figures Comment: incomplete for FPG and lipids |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: available |
| Selective reporting (reporting bias) | High risk | Comment: no exact SDs are reported as text only in the figures |
| Other bias | Unclear risk | Comment: sponsoring by a pharmaceutical company |
Casner 1988.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with non‐insulin‐dependent diabetes previously treated with an oral hypoglycaemic agent, currently receiving at least 25 U insulin/day with a fasting blood glucose value of 140 mg/dL or greater. Exclusion criteria: history of allergic reactions to sulphonylurea therapy or a serious debilitating disease that would limit ability to participate in the study Diagnostic criteria: NDDG 1979 |
|
| Interventions |
Number of study centres: 1 Treatment before study: insulin: intervention 65.9 U/day and control 66.9 U/day Titration period: variable |
|
| Outcomes | Primary outcome(s) (as stated in the publication): glycaemic control (HbA1c, oral glucose tolerance test, fasting blood glucose), side effects, C‐peptide, insulin dose, weight | |
| Study details |
Total study duration: 1 year Run‐in period: variable Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Upjohn Company and an intramural grant from Texas Tech Health Sciences Center Publication status: peer‐reviewed journal |
|
| Stated aim of study | Can the combination of a sulphonylurea and insulin improve glycaemic control? If this is true, can it be done with lower doses of exogenous insulin? Can the effect be maintained over a long period of time? | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | High risk |
Quote from publication: "Patients and physicians were blinded regarding laboratory results and study medication but not on insulin" Comment: so patients were not blinded for their weight gain (this is the only adverse event mentioned) |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "Patients and physicians were blinded regarding laboratory results and study medication but not on insulin" Comment: |
| Blinding of participants and personnel (performance bias) Insulin dose | High risk | Quote from publication: "Patients and physicians were blinded regarding laboratory results and study medication but not on insulin" |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: it is unclear if the outcome assessment was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: it is unclear if the outcome assessment was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: it is unclear if the outcome assessment was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on weight gain was reported for both groups |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: data were reported for both groups |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data were reported for both groups |
| Selective reporting (reporting bias) | Low risk | Comment: data which was collected was also reported |
| Other bias | Low risk | Comment: no other concerns |
Chiasson 1994.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with non‐insulin‐dependent diabetes of at least 6 months, HbA1c > 7%, normal renal and hepatic function Exclusion criteria: poor controlled hypertension, documented gastrointestinal disease, taking medication likely to alter gut motility or absorption, taking medications to lower lipid levels Diagnostic criteria: WHO 1985 |
|
| Interventions |
Number of study centres: 7 Treatment before study: insulin, dose is not stated in the publication Titration period: 6 weeks |
|
| Outcomes | Primary outcome(s) (as stated in the publication): glycaemic control (HbA1c, oral glucose tolerance test, fasting blood glucose), side effects, hypoglycaemia, C‐peptide, lipids, blood count, biochemistry, vitamins, minerals | |
| Study details |
Total study duration: 12 months Run‐in period: 6 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Miles Canada Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "to evaluate the long‐term efficacy of acarbose, an alpha‐glucosidase inhibitor, in improving glycaemic control in patients with non‐insulin‐dependent diabetes mellitus" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Unclear risk | Comment: it is not clear whether the physician or the outcome assessor is besides the participants blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Unclear risk |
Quote from publication: (from the abstract) " Design: a 1‐year, multicenter, randomised, double‐blind, placebo‐controlled study" (from the main text) "Acarbose or placebo was taken with the first sip of the liquid meal" Comment: it is not clear whether the physician or the outcome assessor is besides the participants blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Unclear risk |
Quote from publication: (from the abstract) " Design: a 1‐year, multicenter, randomised, double‐blind, placebo‐controlled study" (from the main text) "Acarbose or placebo was taken with the first sip of the liquid meal" Comment: it is not clear whether the physician or the outcome assessor is besides the participants blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: it is not clear whether the physician or the outcome assessor is besides the participants blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: it is not clear whether the physician or the outcome assessor is besides the participants blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: it is not clear whether the physician or the outcome assessor is besides the participants blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: none |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | High risk | Comment: data on lipids is only mentioned, no analyses is performed or shown |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data were presented for both groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: more figures than tables, that causes unclear reporting |
| Other bias | Unclear risk | Comment: funded in part by a pharmaceutical company |
Coniff 1995.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes of at least 6 months, stable body weight, not receiving sulphonylurea for at least 2 months Exclusion criteria: not stated Diagnostic criteria: WHO 1985 |
|
| Interventions |
Number of study centres: multicentre, number of centres not mentioned, 4 centres are mentioned in the acknowledgements Treatment before study: insulin: 56.8 (SE3.4) IU/day (intervention), 62.2 (SE3.3) IU/day Titration period: 6 weeks |
|
| Outcomes |
Primary outcome(s) (as stated in the publication): glycaemic control (HbA1c, glucose tolerance tests, fasting blood glucose), insulin requirements Secondary outcomes (as stated in the publication): lipids, hypoglycaemic episodes |
|
| Study details |
Total study duration: 6 weeks pretreatment, 24 weeks double‐blind, 6 weeks follow‐up (discontinuation acarbose) Run‐in period: 6 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Miles pharmaceutical division Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To determine whether a forced titration of acarbose (from 50 to 300 mg three times daily) administered over a 24‐week period, in conjunction with diet and insulin therapy, improves glycaemic control and reduce daily insulin requirements in insulin‐requiring type II diabetes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Unclear risk |
Quote from publication: (abstract and main text): "This double‐blind, randomised, multicenter, placebo‐controlled study..." (main text) "The double‐blind endpoint was defined as last visit observation for each patients" Comment: at all measurement occasions the efficacy and safety tests were assessed. However it is not clear whether the physician or the outcome assessor was blinded in addition to the participants |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Unclear risk |
Quote from publication: (abstract and main text): "This double‐blind, randomised, multicenter, placebo‐controlled study..." (main text)"The double‐blind endpoint was defined as last visit observation for each patients" Comment: at all measurement occasions the efficacy and safety tests were assessed. However it is not clear whether the physician or the outcome assessor was blinded in addition to the participants |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk | Comment: data are reported |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk |
Quote from publication: (abstract and main text): "This double‐blind, randomised, multicenter, placebo‐controlled study..." (main text) "The double‐blind endpoint was defined as last visit observation for each patient" Comment: at all measurement occasions the efficacy and safety tests were assessed. However it is not clear whether the physician or the outcome assessor was blinded in addition to the participants |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk |
Quote from publication: (abstract and main text): "This double‐blind, randomised, multicenter, placebo‐controlled study..." (main text) "The double‐blind endpoint was defined as last visit observation for each patient" Comment: at all measurement occasions the efficacy and safety tests were assessed. However it is not clear whether the physician or the outcome assessor was blinded in addition to the participants |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk |
Quote from publication: (abstract and main text): "This double‐blind, randomised, multicenter, placebo‐controlled study..." (main text)"The double‐blind endpoint was defined as last visit observation for each patients" Comment: at all measurement occasions the efficacy and safety tests were assessed. However it is not clear whether the physician or the outcome assessor was blinded in addition to the participants |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote from publication: "Most adverse events involved.....There were no significant differences between treatment groups in the incidence of adverse events related to other body systems " |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data were reported |
| Selective reporting (reporting bias) | High risk | Comment: serum lipids and SGOT and SGPT values were not reported |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Feinglos 1998.
| Methods |
Cross‐over randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: insulin‐requiring type 2 diabetes, total daily insulin dose ≥ 40 units, insulin monotherapy ≥ 1 year prior to the study Exclusion criteria: not stated Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: insulin NPH and regular 80.8 (range 40‐210) U/day Titration period: 1 week |
|
| Outcomes | Primary outcome(s) (as stated in the publication): glycaemic control (plasma glucose, HbA1c), C‐peptide, plasma free insulin levels, lipoprotein (TC, TG, HDL, LDL, VLDL), insulin dose, BMI | |
| Study details |
Total study duration: 8 months Run‐in period: 1 week Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Pfizer Pharmaceuticals, Lifescan, National Center for Research Resources Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To determine the effect(s) on glucose control, insulin dose and circulating insulin levels of the addition of a sulphonylurea (glipizide) to the treatment regimen of patients with insulin‐requiring type 2 diabetes mellitus." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "This study was a double‐blind crossover comparison of insulin and placebo vs. insulin and glipizide." Comment: probably the participant and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "This study was a double‐blind crossover comparison of insulin and placebo vs. insulin and glipizide." Comment: probably the participant and the personnel were blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: it is unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: it is unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: all outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: all outcome data were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest reported |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Fonseca 2007.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: participants had to have received only injectable insulin for at least 3 months, at a dose of at least 30 U/day for a minimum of 4 weeks prior to enrolment. Age 18‐80 years, HbA1c 7.5‐11.0%, fasting plasma glucose < 15 mmol/L and BMI 22‐45 kg/m² Exclusion criteria: people with type 1 diabetes, diabetes resulting from pancreatic injury or secondary forms of diabetes. People with acute metabolic diabetic complications within the past 6 months, serious cardiac conditions or clinically significant liver disease. Any of the following laboratory abnormalities: alanine transaminase > 3 x the upper limit of normal; direct bilirubin > 1.3 x the upper limit of normal; serum creatinine > 220 μmol/L; fasting triacylglycerol > 7.9 mmol/L Diagnostic criteria: based on the investigator's diagnosis and on the patient's medical record |
|
| Interventions |
Number of study centres: 68 Treatment before study: insulin: intervention 81.2 ± 44.8 U/day and control 81.9 ± 49.4 U/day Titration period: 4 weeks |
|
| Outcomes | Primary outcome(s) (as stated in the publication): glycaemic control (HbA1c, fasting plasma glucose), insulin dose and number of injections, fasting lipids, bodyweight | |
| Study details |
Total study duration: 24 weeks Run‐in period: 4 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Novartis Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To assess the efficacy and tolerability of vildagliptin added to added to insulin therapy in patients with type 2 diabetes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "This was a 24‐week, randomised, double‐blind, placebo‐controlled, parallel‐group study...." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "All assessments were made by central laboratories." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "This was a 24‐week, randomised, double‐blind, placebo‐controlled, parallel‐group study...." Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on weight gain and hypoglycaemia were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: data on all outcome measures were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose was collected and reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: post‐treatment BMI not reported |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Fritsche 2000.
| Methods |
Cross‐over randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: not clearly defined: severe obesity, moderate glycaemic control, intensive insulin therapy with regular specialist consultations for regimen adaptation for at least 6 months prior to inclusion Exclusion criteria: not stated Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: intervention: insulin: NPH 26 ± 6 U/day and regular 27 ± 5 U/day control: insulin: NPH 20 ± 4 U/day and regular 26 ± 4 U/day Titration period: 6 weeks |
|
| Outcomes | Primary outcome(s) (as stated in the publication): glycaemic control (HbA1c, blood glucose levels, OGTT), insulin dose, lipids, C‐peptide, lactate | |
| Study details |
Total study duration: 24 weeks Run‐in period: 6 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Lipha Pharmaceuticals (medication) Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To examine the effect of adjunct metformin in 13 severely obese type 2 diabetes patients in sub optimal glucaemic control pretreated with intensified insulin therapy" on glycaemic control, insulin dosage, lipid profile and bodyweight | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Patients were randomly assigned to either metformin or placebo treatment (double‐blind)...." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "patients were randomised in a double‐blind fashion..." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "patients were randomised in a double‐blind fashion..." Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: all outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: all outcome data were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | Unclear risk | Comment: funding by a pharmaceutical company |
Giugliano 1993.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: age at diagnosis > 40 years, duration of disease > 3 years, duration of previous response to oral drugs > 1 year, inadequate metabolic control even when on maximal doses of sulphonylurea Exclusion criteria: age > 70 years, creatinine > 1.2 mg/dL, ischaemic of wasting disease, acute severe diseases Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1? Treatment before study: intervention: insulin: lente + regular 2 dd 90 ± 9 U/day; control: insulin lente + regular 2 dd 88 ± 9.4 U/day Titration period: 4 weeks |
|
| Outcomes | Primary outcome(s) (as stated in the publication): glycaemic control (HbA1c, daily glucose levels), lipids, blood pressure, body weight, insulin dose, beta‐cell function (C‐peptide, insulin) | |
| Study details |
Total study duration: 7 months Run‐in period: 4 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: not reported Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To evaluate the effectiveness and safety of metformin in obese type 2 diabetic patients poorly controlled by conventional insulin therapy." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Phase II (double‐blind) patients were randomly assigned to continue to receive placebo or to treatment with metformin for six months" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "Phase II (double‐blind) patients were randomly assigned to continue to receive placebo or to treatment with metformin for six months Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "Phase II (double‐blind) patients were randomly assigned to continue to receive placebo or to treatment with metformin fro six months" Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: all outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: no |
| Other bias | Low risk | Comment: no |
Groop 1985.
| Methods |
Cross‐over randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: onset of non‐ketotic diabetes after the age of 35 years, treated with oral antidiabetic drugs for at least 1 year before insulin therapy was started due to secondary drug failure. Exclusion criteria: not stated Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: insulin 0.75 ± 0.11 IU/kg per day Titration period: none |
|
| Outcomes | Primary outcome(s) (as stated in the publication): insulin dose and bodyweight, glycaemic control (HbA1c, FBG, blood glucose profile), serum insulin levels, C‐peptide, lipids | |
| Study details |
Total study duration: 24 weeks (2 x 8 = 16 weeks intervention) Run in period: 8 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: none Publication status: peer review journal |
|
| Stated aim of study | Quote from publication: "To investigate the metabolic effects of the combination of insulin and sulphonylurea (glibenclamide) during controlled long‐term therapy in NIDDM patients whose hyperglycaemia could not be controlled by insulin alone." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "...the patients were randomly allocated to 8 weeks of treatment with ....." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "In a double‐blind cross‐over study we compared the effect of...." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "In a double‐blind cross‐over study we compared the effect of...." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "In a double‐blind cross‐over study we compared the effect of...." Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data of adverse events (myocardial infarct, weight gain) were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: all outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: all data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: unclear graphical reporting of data |
| Other bias | Low risk | Comment: none |
Hermann 2001.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes with insulin therapy > 1 year. BMI > 27 kg/m2 for men and > 25 kg/m2 for women. HbA1c value higher than the upper reference limit + 2%. Insulin dose 0.4‐1.0 U/kg/day. C‐peptide after 1 mg glucagon intravenously > 0.6 nmol/L Exclusion criteria: treatment with oral antidiabetic agents within the last 6 months. Presence of any of the usual contraindications for metformin. Abnormal serum creatinine concentration. Transaminases > 2 x the upper reference limit. Overconsumption of alcohol Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 3 Treatment before study: insulin: intervention 0.75 ± 0.28 U/kg/day and control 0.73 ± 0.23 U/kg/day Titration period: 2 weeks |
|
| Outcomes |
Primary outcome(s) (as stated in the publication): glycaemic control (HbA1c, FBG), bodyweight and BMI, insulin dose, lipids Secondary outcomes (as stated in the publication): waist‐hip ratio, blood pressure, fibrinogen, C‐peptide, serum B12, compliance |
|
| Study details |
Total study duration: 15 months Run‐in period: 12 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: none Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To assess the adjunct effect of metformin to insulin in type 2 diabetes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "...the patients entered a 12‐month double‐blind treatment phase randomly allocated to metformin or placebo in parallel groups and as adjunct to their current insulin therapy" |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Randomization was performed by center in block of four." |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "The study was a 12‐month double‐blind placebo‐controlled trial..." Comment: probably the participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "The study was a 12‐month double‐blind placebo‐controlled trial..." Comment: probably the participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "The study was a 12‐month double‐blind placebo‐controlled trial..." Comment: probably the participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on adverse events were reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: data were reported graphically, unclear |
| Other bias | Unclear risk | Comment: medication was funded by a pharmaceutical company |
Hirsch 1999.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes with less than optimal control on insulin therapy alone. Exclusion criteria: not stated Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: insulin: dose not stated Titration period: not stated |
|
| Outcomes | Primary outcome(s) (as stated in the publication): glycaemic control (HbA1c, FPG), bodyweight, blood pressure, insulin dose, fasting insulin levels, fasting C‐peptide, hypoglycaemia | |
| Study details |
Total study duration: 5 months Run‐in period: no Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Bristol‐Myers‐Squibb Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To prospectively evaluate the efficacy of metformin added to insulin for patients with type 2 diabetes with less than optimal glycaemic control on insulin alone." | |
| Notes | Publication is a letter | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "... a 5‐month single‐center prospective double‐blind placebo‐controlled study" Comment: probably the participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "... a 5‐month single‐center prospective double‐blind placebo‐controlled study" Comment: probably the participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "... a 5‐month single‐center prospective double‐blind placebo‐controlled study" Comment: probably the participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on adverse events were reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: the outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | High risk | Comment: short report of the trial |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Hong 2012.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: type 2 diabetes mellitus; age 30–70 years; HbA1c 7.5%–11.0%; fasting plasma glucose (FPG) < 15 mmol/L (270 mg/dL) and body mass index (BMI) 18–35 kg/m2. Female participants had to be non‐fertile or of childbearing potential using a medically approved birth control method. Exclusion criteria: type 1 diabetes, gestational diabetes or diabetes with identifiable secondary causes, significant renal impairment (estimated creatinine clearance < 50 ml/min) or elevated (> 100) alanine or aspartate aminotransferase (ALT or AST). Participants who were taking medications, aside from antidiabetic medications, known to affect glycaemic control, such as glucocorticoids were also excluded. Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 2 Treatment before study: insulin injections for at least 3 months; at a dose of at least 10 U/day and for a minimum of 4 weeks prior to enrolment. Titration period: none |
|
| Outcomes |
Primary outcome(s) (as stated in the publication): change in HbA1c (from baseline to 24 weeks) Secondary outcomes (as stated in the publication): proportion of participants achieving HbA1c < 7%, body weight, waist circumference, change in insulin dose, change in C‐peptide, safety (AE, SAE, hypoglycaemia, liver/renal function) |
|
| Study details |
Total study duration: 24 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: non commercial; National Research Foundation Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "This study compared the efficacy and tolerability of adding sitagliptin, an oral dipeptidyl peptidase‐4 inhibitor, and an up to 20% increase in insulin dose in patients with uncontrolled type 2 diabetes on insulin therapy." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not mentioned in the publication |
| Allocation concealment (selection bias) | Unclear risk | Comment: not mentioned in the publication |
| Blinding of participants and personnel (performance bias) Adverse events | High risk | Comment: participants and personnel were not blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | High risk | Comment: participants and personnel were not blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | High risk | Comment: participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) Adverse events | High risk | Comment: the outcome assessor was not blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | High risk | Comment: the outcome assessor was not blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | High risk | Comment: the outcome assessor was not blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: all end points shown |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: all end points shown |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: all end points shown |
| Selective reporting (reporting bias) | Low risk | Comment: none |
| Other bias | Low risk | Comment: none |
Kitabchi 1987.
| Methods |
Cross‐over randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: obese (130%‐200% IBW) women with type 2 diabetes mellitus on prior insulin therapy who were poorly controlled but without severe diabetic complications. Exclusion criteria: history of diabetic ketoacidosis Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: insulin 0.89 ± 0.07 U/kg/BW Titration period: none |
|
| Outcomes | Primary outcome(s) (as stated in the publication): glycaemic control (HbA1c, FBG, 2‐hour post prandial glucose), bodyweight, insulin requirement, total cholesterol, triglyceride, C‐peptide | |
| Study details |
Total study duration: 6 months Run‐in period: no Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: NIH, Abe Goodman Fund for Diabetes Research, Eli Lilly, Upjohn Company Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To assess the efficacy of combined NPH and tolazamide in enhancing insulin secretion and tissue sensitivity in obese patients with type 2 diabetes mellitus who were maintained at the same weight and glycaemic index during both phases of the study." | |
| Notes | Several young participants with long‐term insulin use and without severe obesity | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Unclear risk |
Quote from publication: "In a randomised cross‐over trial, ..." Comment: unclear if it was a blinded trial |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Unclear risk |
Quote from publication: "In a randomised cross‐over trial, ..." Comment: unclear if it was a blinded trial |
| Blinding of participants and personnel (performance bias) Insulin dose | Unclear risk |
Quote from publication: "In a randomised cross‐over trial, ..." Comment:unclear if it was a blinded trial |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk |
Quote from publication: "In a randomised cross‐over trial, ..." Comment: unclear if it was a blinded trial |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk |
Quote from publication: "In a randomised cross‐over trial, ..." Comment: unclear if it was a blinded trial |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk |
Quote from publication: "In a randomised cross‐over trial, ..." Comment: unclear if it was a blinded trial |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on weight gain were reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were reported |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes of interest were reported |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Krawczyk 2005.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: type 2 diabetes patients, diabetes duration of at least 5 years, BMI > 30, Insulin > 40 IU/day Exclusion criteria: contraindication for metformin, body weight change > 5 kg last year Diagnostic criteria: age > 35 years at diagnosis and at least 1 year of effective treatment with oral glucose‐lowering agents |
|
| Interventions |
Number of study centres: 1 Treatment before study: insulin 2dd, 59.5 (15.4) IU/day intervention, 55.6 (16.3) IU/day control Titration period: none |
|
| Outcomes | Primary outcome(s) (as stated in the publication): glycaemic control (HbA1c, glucose), bodyweight, insulin dose, WHR | |
| Study details |
Total study duration: 6 months Run‐in period: none Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: Polish Funding source: not reported Publication status: unknown |
|
| Stated aim of study | Quote from publication: "To assess the influence of adding metformin to insulin monotherapy on metabolic control in type 2 diabetes patients." | |
| Notes | Statistical methods not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: randomisations in two groups of 20 participants |
| Allocation concealment (selection bias) | Unclear risk | Comment: randomisations in blocks of 4 (20 participants per group) |
| Blinding of participants and personnel (performance bias) Adverse events | Unclear risk | Comment: it is unclear if the study was blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Unclear risk | Comment: it is unclear if the study was blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Unclear risk | Comment: it is unclear if the study was blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: it is unclear if the study was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: it is unclear if the study was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: it is unclear if the study was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: weight gain was reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Unclear risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | High risk | Comment: statistical methods were not described |
Kyllastinen 1985.
| Methods |
Cross‐over randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: elderly patients, type 2 diabetes inadequately controlled by insulin Exclusion criteria: surgical operation; lack of co‐operation Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: insulin 58 ± 3 IU/day (n = 1 once daily, n = 8 twice daily) Titration period: none |
|
| Outcomes | Primary outcome(s) (as stated in the publication): glycaemic control (HbA1c, FPG), daily insulin dose, C‐peptide, bodyweight, triglyceride, total cholesterol, HDL‐cholesterol, Na, K, creatinine, chloride | |
| Study details |
Total study duration: 4 months Run‐in period: none Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: none Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To determine whether glibenclamide could improve glycaemic control in patients not adequately controlled by insulin." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "A double blind, cross over trial was assigned ...after randomisation patients were given either glibenclamide or placebo....5 patients started with glibenclamide and 4 with placebo" Comment: probably the participants and personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "A double blind, cross over trial was assigned...after randomisation patients were given either glibenclamide or placebo....5 patients started with glibenclamide and 4 with placebo" Comment: probably the participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on weight gain were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | High risk | Comment: only 9 participants included |
Lewitt 1989.
| Methods |
Cross‐over randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: insulin treated participants who were not ketosis prone and had previous primary or secondary oral hypoglycaemic failure. Exclusion criteria: combined insulin‐sulphonylurea therapy Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: insulin 47.3 ± 21.3 U/day. Insulin regimen: once or twice daily Titration period: none |
|
| Outcomes | Primary outcome(s) (as stated in the publication): glycaemic control (HbA1c, self‐monitored fasting and postprandial glucose), BMI, C‐peptide, insulin dosage | |
| Study details |
Total study duration: 6 months Run‐in period: none Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: none Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To determine which patient characteristics best predict a beneficial response to combined Insulin‐gliburide therapy." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "Glyburide was compared with placebo in a double‐blind crossover design" Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "Glyburide was compared with placebo in a double‐blind crossover design" Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "Glyburide was compared with placebo in a double‐blind crossover design" Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on weight gain were reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | Low risk | Comment: none |
Lindstrom 1999.
| Methods |
Cross‐over randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: participants with type 2 diabetes with insulin monotherapy for 6‐36 months. Exclusion criteria: not stated Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: unclear Treatment before study: insulin 54.5 ± 6.9 U/day (at the end of the run‐in period) Insulin regimen: four times daily, regular plus intermediate insulin Titration period: 4‐8 weeks |
|
| Outcomes | Primary outcome(s) (as stated in the publication): glycaemic control (blood glucose, HbA1c), insulin dose, C‐peptide, lipoproteins, IGF‐1, SHBG, serum testosterone | |
| Study details |
Total study duration: 7‐8 months Run‐in period: 4‐8 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Swedish Medical Research Council, Swedish Diabetes Association, County Council of Östergötland, Novo Nordisk Insulin Fund Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To study whether changes in endogenous insulin secretion at the same glycaemic control affect the plasma concentration of lipoproteins in patients with type 2 diabetes mellitus." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "...in this randomised double‐blind crossover study." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "...in this randomised double‐blind crossover study." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "...in this randomised double‐blind crossover study." Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on weight gain were reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Unclear risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Unclear risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | Unclear risk | Comment: also funded by pharmaceutical company |
Longnecker 1986.
| Methods |
Cross‐over randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: severely hyperglycaemic patients with type 2 diabetes with insulin monotherapy failed to sulphonylurea therapy Controls were nondiabetic women comparable with patients in age and weight. Exclusion criteria: not stated Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: insulin 64 ± 5.6 U/day (insulin regimen: once or twice daily, regular and/or isophane insulin) Titration period: 1 week |
|
| Outcomes | Primary outcome(s) (as stated in the publication): glycaemic control (HbA1c, fasting blood glucose), plasma glucose and C‐peptide before and after standardised meal, weight, C‐peptide, drug compliance, side effects | |
| Study details |
Total study duration: 20 weeks Run‐in period: 1 week Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Public Health Service Grant, ADA, Upjohn Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To evaluate the efficacy of adding tolazamide, an oral agent, to insulin in a group of severely hyperglycaemic patients with NIDDM, all of whom had previously failed to respond to therapy with oral sulfonylurea agent alone." | |
| Notes | Carry‐over effect not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Unclear risk |
Quote from publication: (from the abstract) "Using a double‐blind crossover design, ..." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Unclear risk |
Quote from publication: (from the abstract) "Using a double‐blind crossover design, ..." Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | High risk | Comment: the effects on weight and side effects were not reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome date were collected and reported |
| Selective reporting (reporting bias) | High risk | Comment: the effects on weight and side effects were not reported |
| Other bias | Unclear risk | Comment: only 12 participants were included |
Mattoo 2005.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes with insulin therapy with or without oral antihyperglycaemic agents for ≥ 3 months, HbA1c ≥ 7,5% and ≥ 30 years at the time of diagnosis Exclusion criteria: type 1 DM, signs or symptoms of any chronic condition or drug or alcohol abuse, previous TZD, glucocorticoid, nicotinic acid or therapy for a malignancy (except basal cell or squamous cell cancer), breastfeeding, pregnancy, women of childbearing potential Diagnostic criteria: WHO |
|
| Interventions |
Number of study centres: 39 Treatment before study: intervention: insulin 0.96 (0.03) U/day/kg, control insulin 0.92 (0.03) U/day/kg. Insulin regimen: once, twice, thrice or four times a day. Titration period: 2 weeks lead‐in (at the end oral agents were stopped) and 3 months insulin intensification period |
|
| Outcomes | Primary outcome(s) (as stated in the publication): glycaemic control (HbA1c, fasting blood glucose), lipids, hs‐CRP, PAI‐1, hypoglycaemia, bodyweight, insulin dose | |
| Study details |
Total study duration: 6 months Run‐in period: 2 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Eli Lilly, Takeda Europe Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To determine the effect of pioglitazone 30 mg plus insulin versus placebo plus insulin on glycaemic control, the serum lipid profile, and selected cardiovascular risk factors in patients with type 2 diabetes mellitus whose disease was inadequately controlled with insulin therapy alone despite efforts to intensify such treatment." | |
| Notes | Some participants also used oral antihyperglycaemic agents before study. Users and non‐users were separated in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "randomised" |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "randomised with equal probability.... according to a central randomisation table generated by the study sponsor and administered by an automated interactive voice response system at all sites" |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "this 6‐month, randomised, double blind, prospective, multicenter, placebo‐controlled, parallel‐group study was ...." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "this 6‐month, randomised, double blind, prospective, multicenter, placebo‐controlled, parallel‐group study was ...." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "this 6‐month, randomised, double blind, prospective, multicenter, placebo‐controlled, parallel‐group study was ...." Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on adverse events and weight gain were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | Unclear risk | Comment: funded by pharmaceutical companies |
Mauerhoff 1986.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes with insulin therapy for ≥ 1 year. Exclusion criteria: abnormal renal and hepatic functions, C‐peptide > 0.2 pmol/mL Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: intervention: insulin 0.50 (0.07) U/day/kg, control insulin 0.44 (0.05) U/day/kg Titration period: 3 weeks lead‐in |
|
| Outcomes | Primary outcome(s) (as stated in the publication): plasma glucose and C‐peptide after standardised breakfast, HbA1c (no assessment for technical reasons), fasting cholesterol and triglyceride, hypoglycaemia, insulin requirements | |
| Study details |
Total study duration: 16 weeks Run‐in period: 3 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Hoechst Belgium Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "We have studied the effect of the combination of a sulphonylurea (Hb 420 or glibenclamide) with insulin in 22 type 2 diabetic patients, treated with insulin and with residual insulin secretion." | |
| Notes | Use of intervention medication Hb420 (galenic form of glibenclamide) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "The study was carried out double‐blind......" Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "The study was carried out double‐blind......" Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "The study was carried out double‐blind......" Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on number of hypoglycaemia were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Unclear risk | Comment: HbA1c analyses not performed for technical reasons |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: HbA1c analyses not performed for technical reasons |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Mezitis 1992.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
HbA1c before randomisation (% (SD)): intervention 8.7, control 8.6 Inclusion criteria: people with type 2 diabetes with insulin therapy for ≥ 1 year. Exclusion criteria: endocrinologic disease other than diabetes mellitus, history of allergies to sulphonamides and/or insulin, history of impaired gastric emptying, active hepatic disease, renal disease significantly impairing creatinine clearance, current treatment with steroids, oestrogens, progestogens, beta‐blockers, Ca‐channel antagonists, diuretics, monoamine oxidase inhibitors, clonidine, probenecid, anticoagulants, NSAID Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: insulin monotherapy, dosages not stated Titration period: yes, duration not stated |
|
| Outcomes | Primary outcome(s) (as stated in the publication): urinary C‐peptide, HbA1c, lipids, insulin requirement, glycaemic profiles in response to test meals | |
| Study details |
Total study duration: 20 weeks Run‐in period: unclear Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: UpJohn, Boehringer Mannheim, Becton Dickinson Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To investigate the effects of addition of glibenclamide to the regimen of insulin‐treated non‐insulin‐dependent diabetes mellitus (NIDDM) patients with regard to their overall insulin requirements and dosage schedule and to assess persistence of these effects." | |
| Notes | A lot of data were missing: dose of glibenclamide, incomplete study design and baseline data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "random assignment to equal‐sized parallel‐groups" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "The study was double‐blinded with random assignment to equal‐sized parallel‐groups..." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "The study was double‐blinded with random assignment to equal‐sized parallel‐groups..." Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | High risk | Comment: outcomes of C‐peptide, lipids and HbA1c assays were not reported |
| Incomplete outcome data (attrition bias) Insulin dose | High risk | Comment: missing data on dose of glibenclamide |
| Selective reporting (reporting bias) | High risk | Comment: outcomes of C‐peptide, lipids and HbA1c assays were not reported |
| Other bias | High risk | Comment: no data on adverse events and population size; funded by a pharmaceutical company |
Mudaliar 2010.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: obese people with type 2 diabetes on insulin therapy alone and with HbA1c between 7.5% and 10%. Exclusion criteria: history of peripheral oedema, cardiac, hepatic or renal problems, or had been treated with NSAIDs or diuretics within 21 days of screening Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: insulin monotherapy (IU/day (SD)): intervention: 105 (22), control 114 (11) Titration period: 4 weeks: weight maintenance, carbohydrate diet |
|
| Outcomes | Primary outcome(s) (as stated in the publication): HbA1c, fasting plasma glucose, insulin dose, weight, total body water, extracellular fluid, renal measures, hormonal measures | |
| Study details |
Total study duration: 16‐20 weeks Run‐in period: 4 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Takeda pharmaceuticals, Veterans Medical Research Foundation, Department of Veterans Affairs and the VA San Diego Healthcare System, University of California San Diego Clin Res. Centre NIH Grant Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To evaluate the effects of intensive insulin therapy alone or with added pioglitazone on renal salt/water balance and body fluid compartment shifts in type 2 diabetes." | |
| Notes | Funded by pharmaceutical company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "After the completion of the baseline studies, subjects were randomised (in a double‐blind manner) to..." Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "After the completion of the baseline studies, subjects were randomised (in a double‐blind manner) to..." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "After the completion of the baseline studies, subjects were randomised (in a double‐blind manner) to..." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "After the completion of the baseline studies, subjects were randomised (in a double‐blind manner) to..." Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on weight gain were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | Unclear risk | Comment: funding by pharmaceutical company; performed in clinical research centre |
Nemoto 2011.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes on insulin therapy alone, HbA1c ≥ 6.5%, outpatients, age at least 20 years Exclusion criteria: not stated Diagnostic criteria: plasma glucose level at either 1 or 2 h after meal was 180 mg/dL or higher; HbA1c ≥ 6.5% |
|
| Interventions |
Number of study centres: not stated Treatment before study: insulin monotherapy (U/day (SD)): 31.7 (17.6) Titration period: 4‐10 weeks observation period |
|
| Outcomes |
Primary outcome(s) (as stated in the publication): meal tolerance test, plasma glucose AUC Secondary outcomes (as stated in the publication): HbA1c, 1,5 AG, glycoalbumin, hypoglycaemic symptoms ADDITIONAL OUTCOMES: safety |
|
| Study details |
Total study duration: 16‐22 weeks (4‐10 weeks observation + 12 weeks treatment) Run‐in period: 4‐10 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Sanwa Kagaku Kenkyusyo Co Ltd Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To investigate the efficacy of combination therapy with miglitol and insulin" in people with type 2 diabetes receiving insulin therapy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "The enrolled patients were randomised to groups treated with miglitol or with placebo" Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "We conducted a placebo‐controlled double‐blind comparative study..." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Unclear risk |
Quote from publication: "We conducted a placebo‐controlled double‐blind comparative study..." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Unclear risk |
Quote from publication: "We conducted a placebo‐controlled double‐blind comparative study..." Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: adverse events were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: data on HbA1c were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Unclear risk | Comment: part of the results only described in figures, not in numbers |
| Selective reporting (reporting bias) | Unclear risk | Comment: part of the results only described in figures, not in numbers |
| Other bias | Unclear risk | Comment: funding unclear, possibly commercial |
Osei 1984.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes with serum glucose levels > 200 mg/dL and daily insulin requirement > 30 IU Exclusion criteria: renal and hepatic disease, allergies to sulphonylurea Diagnostic criteria: NDDG |
|
| Interventions |
Number of study centres: 1 Control (route, total dose/day, frequency): placebo, oral Treatment before study: intervention: insulin 60.3 (7.1) U/day, control insulin 50.27 (5.0) U/day (insulin regimen: once or twice daily, short‐acting and/or intermediate insulin) Titration period: 4 weeks |
|
| Outcomes | Primary outcome(s) (as stated in the publication): fasting glucose, HbA1c and C‐peptide, after OGTT serum glucose and C‐peptide, lipids, lipoproteins, weight, dietary intake, compliance | |
| Study details |
Total study duration: 16 weeks Run‐in period: 4 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: UpJohn, Core Laboratory, Central Ohio Diabetes Association Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To evaluate, in a double‐blind, placebo‐controlled manner, glucose and lipoprotein responses after long‐term use of combination therapy in the management of insulin‐treated patients with poorly controlled type 2 diabetes mellitus." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Patients were randomly assigned in a double‐blind manner to receive either glyburide or placebo..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "Patients were randomly assigned in a double‐blind manner..." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "Patients were randomly assigned in a double‐blind manner..." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "Patients were randomly assigned in a double‐blind manner..." Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on weight gain were reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Quatraro 1986.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes on insulin therapy Exclusion criteria: not stated Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: intervention: insulin 90 (6) U/day, control insulin 88 (5) U/day Insulin regimen: twice or thrice daily, porcine lente and/or rapid‐acting insulin Titration period: 2 months + 1‐2 weeks inpatient period |
|
| Outcomes | Primary outcome(s) (as stated in the publication): diurnal glucose profile, HbA1c, C‐peptide, glucagon stimulated C‐peptide, insulin dose, weight | |
| Study details |
Total study duration: 14 months Run‐in period: 2 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: none Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "We studied the influence of chronic sulphonylurea treatment on glucose metabolism and beta‐cell secretory activity in diabetic patients requiring insulin after secondary failure to oral drugs." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "They were allocated at random into two groups, each consisting of 15 subjects..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Unclear risk | Comment: unclear if the study was blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the study was blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Unclear risk | Comment: unclear if the study was blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the study was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the study was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the study was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote from publication: "weight remained stable throughout the study period." |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | High risk | Comment: different participant groups reported in figure and table |
Reich 1987.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes on insulin therapy Exclusion criteria: significant surgery within 3 months before entry into the study, major organ or system disease, medication use affecting glucose metabolism or sulphonylurea activity Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 2 Treatment before study: intervention: 36.5 (6.3) insulin, placebo: insulin 48.2 (4.0) U/day. Insulin regimen: only intermediate insulin Titration period: 12 days hospitalisation (after 6 weeks optimisation glycaemic control with insulin) |
|
| Outcomes | Primary outcome(s) (as stated in the publication): serum and urinary glucoses, HbA1c, urinary C‐peptide, insulin dose, number of tablets prescribed, compliance | |
| Study details |
Total study duration: 5.5 months Run‐in period: 12 days Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Upjohn Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To test the effect of combined glibenclamide‐insulin therapy over 4 months in 20 patients after achieving good control of fasting glucose with diet and intermediate insulin alone." | |
| Notes | Participants had adequate glycaemic control | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "randomised" |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "randomised by the hospital pharmacy" |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: (from abstract) "A placebo‐controlled, double‐blinded design..." Comment: Probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: (from abstract) "A placebo‐controlled, double‐blinded design..." Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Unclear risk | Comment: not all outcome values were reported, only graphical |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: insulin dose was collected and reported |
| Selective reporting (reporting bias) | Unclear risk |
Comment: not all outcome values were reported, only graphical Comment: dropouts were described; intention‐to‐treat |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Relimpio 1998.
| Methods |
Parallel open‐label randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: poorly controlled (HbA1c > 8%) people with type 2 diabetes on insulin therapy (≥ 0.5 IU/kg body weight in 2 or more daily doses)) Exclusion criteria: life‐threatening condition, contraindication for biguanides, serum creatinine < 132.6 μmol/L Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: intervention: 47.9 (10) insulin, control: insulin 51.8 (9.6) U/day. Insulin regimen: twice or more daily, premixed soluble and NPH insulin or NPH insulin alone Titration period: 4 weeks increase metformin dose (1,275 g ‐> 2,550 g) |
|
| Outcomes | Primary outcome(s) (as stated in the publication): HbA1c, glucose, lipids, blood pressure, height, weight, BMI, insulin dose, serum creatinine, albumin excretion rate, uric acid, compliance | |
| Study details |
Total study duration: 4 months Run‐in period: 4 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Novo Nordisk Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To compare the effect of adding metformin to insulin therapy with a moderate increase in insulin dose alone in insulin‐treated, poorly controlled type 2 diabetes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "... and were subsequently randomised (31 to insulin + metformin and 29 to insulin dose increase)" |
| Allocation concealment (selection bias) | Unclear risk | "patients were randomised into two study groups following an experimental design of open‐label randomisation." |
| Blinding of participants and personnel (performance bias) Adverse events | High risk | Quote from publication: "open‐label randomisation" |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | High risk | Quote from publication: "open‐label randomisation" |
| Blinding of participants and personnel (performance bias) Insulin dose | High risk | Quote from publication: "open‐label randomisation" |
| Blinding of outcome assessment (detection bias) Adverse events | High risk | Quote from publication: "open‐label randomisation" |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | High risk | Quote from publication: "open‐label randomisation" |
| Blinding of outcome assessment (detection bias) Insulin dose | High risk | Quote from publication: "open‐label randomisation" |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | Comment: data on weight gain were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Robinson 1998.
| Methods |
Two cross‐over randomised controlled clinical trials Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: study I: DM2 patients with insulin monotherapy for at least 1 year after secondary failure of maximum dose oral antihyperglycaemic agents; study II: see study I plus taking metformin (100‐2550 mg/day as the sole oral antihyperglycaemic agent for at least 1 year Exclusion criteria: women of childbearing age, unable to give informed consent Diagnostic criteria: fasting plasma glucose > 7.8 mmol/L on 2 occasions |
|
| Interventions |
Number of study centres: 2 Treatment before study: study I: insulin 71 (47) U/day, study II : insulin 41 (16) U/day + metformin 1000‐2550 Titration period: 6 weeks |
|
| Outcomes | Primary outcome(s) (as stated in the publication): HbA1c, glucose, lipids, blood pressure, insulin dose, hypoglycaemia | |
| Study details |
Total study duration: 12 weeks (intervention period study I) Run‐in period: 6 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Lipha Pharmaceuticals Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To test the hypothesis that metformin therapy, given as an adjunct to insulin therapy, improves metabolic control in insulin‐treated type 2 diabetes mellitus patients with sub optimal glycaemic control." | |
| Notes | 2 studies, only study I fulfilled our inclusion criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "patients were randomised..." Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: (from abstract) "Two randomised double‐blind placebo‐controlled crossover studies were run." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: (from abstract) "Two randomised double‐blind placebo‐controlled crossover studies were run." Comment:probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: (from abstract) "Two randomised double‐blind placebo‐controlled crossover studies were run." Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on weight gain were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were described |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Rosenstock 2002.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes on insulin therapy (≥ 30 IU/day) for 4 months, 30‐75 years, HbA1c ≥ 8.0%, fasting C‐peptide > 0.7μg/l Exclusion criteria: history of ketoacidosis; unstable retinopathy, nephropathy or neuropathy; impaired hepatic and renal function; anaemia; unstable cardiovascular or cerebrovascular condition; concomitant lipid lowering medication must be taken for a stable dose > 60 days Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 79 Treatment before study: pioglitazone 15: 70.2 (34.0) insulin, pioglitazone 30: insulin 72.3 (38.5), placebo: insulin 70.7 (33.5) U/day (insulin regimen: 88% monotherapy) Titration period: insulin monotherapy: 2 weeks screening + 1 week single‐blind placebo insulin with oral anti‐diabetic (OAD) medication: 2 weeks screening + 4 weeks single‐blind placebo (= washout) |
|
| Outcomes | Primary outcome(s) (as stated in the publication): HbA1c, glucose, C‐peptide, lipids, insulin dose, safety profile: chemistry, haematology, urine analysis, vital signs, ECG, adverse events | |
| Study details |
Total study duration: 16 weeks Run‐in period: 3 weeks for insulin users and 6 weeks for insulin + OAD users (last group discontinued oral agent at the beginning of the screening period Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Takeda Pharmaceuticals Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To evaluate the ability of two doses of pioglitazone in combination with a stable insulin regimen to improve glycaemic control in patient whose type 2 diabetes remained poorly controlled despite current insulin therapy." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: not possible to judge whether the sequence generation was adequate. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "... the 16‐weeks double‐blind treatment period" Comment: Probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "... the16‐weeks double‐blind treatment period" Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "During the single‐blinded period, patients received their stable insulin regimens" Quote from publication: "... the16‐weeks double‐blind treatment period" Comment: the single‐blind period refers to the run‐in period and the 16‐weeks to the treatment period. Probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on weight gain and drug‐related adverse events were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Schade 1987.
| Methods |
Cross‐over randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes on insulin therapy (≥ 28 IU/day ≥ 3 months), C‐peptide‐positive on stimulation of endogenous insulin secretion (OGTT) Exclusion criteria: hepatic or renal disease or other major diseases that might impair participation, use of sulphonylurea or other drugs that alter glucose control within 1 month of the study Diagnostic criteria: NDDG |
|
| Interventions |
Number of study centres: > 1? Treatment before study: 55.4 (6.0) IU/day (insulin regimen: 10/16 twice daily) Titration period: 4 weeks (washout) |
|
| Outcomes | Primary outcome(s) (as stated in the publication): OGTT, insulin‐ C‐peptide, fasting blood glucose, HbA1c, ery‐glu binding capacity, compliance, insulin dose, weight | |
| Study details |
Total study duration: 32 weeks Run‐in period: 4 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Upjohn, NIH Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To examine the potential beneficial effect of the addition of a second‐generation sulphonylurea to insulin therapy for poorly controlled type 2 diabetes." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "The order of the two treatment regimens was randomised..." Comment: not possible to judge whether the sequence generation was adequate |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk | Quote from publication: "...neither the patient nor the investigators knew until the completion of the study which treatment regimen the patients were receiving during each 16‐weeks period." |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk | Quote from publication: "...neither the patient nor the investigators knew until the completion of the study which treatment regimen the patients were receiving during each 16‐weeks period." |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk | Quote from publication: "...neither the patient nor the investigators knew until the completion of the study which treatment regimen the patients were receiving during each 16‐weeks period." |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk |
Quote from publication: "...neither the patient nor the investigators knew until the completion of the study which treatment regimen the patients were receiving during each 16‐weeks period." Comment: unclear if "end of the study" meant the end of the treatment period or after outcome analysis |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk |
Quote from publication: "...neither the patient nor the investigators knew until the completion of the study which treatment regimen the patients were receiving during each 16‐weeks period." Comment: unclear if "end of the study" meant the end of the treatment period or after outcome analysis |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk |
Quote from publication: "...neither the patient nor the investigators knew until the completion of the study which treatment regimen the patients were receiving during each 16‐weeks period." Comment: unclear if "end of the study" meant the end of the treatment period or after outcome analysis |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on weight gain were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Schiel 2007.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes with inadequate glycaemic control (HbA1c ≥ 8.0% or FBG ≥ 6.6 mmol/L) on premix insulin therapy (≤ 5 years), diabetes duration > 5 years, BMI 27‐35 Exclusion criteria: impaired hepatic or renal function, pregnancy, unable to understand the study, to cope or attend follow‐up visits Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: mix insulin 75/25 or 70/30 A: 77.6 (32.1) , B: 64.9 (32.1), C: 65.2 (34.2) IU/day Titration period: 4 weeks |
|
| Outcomes | Primary outcome(s) (as stated in the publication): fasting blood glucose, HbA1c, treatment satisfaction, hypoglycaemia, adverse events, blood pressure, creatinine, liver enzymes | |
| Study details |
Total study duration: 20 weeks (4 + 16 wks) Run‐in period: 4 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Sanofi‐Aventis Publication status: peer review journal |
|
| Stated aim of study | Quote from publication: "To establish whether insulin plus OADs is effective in type 2 diabetes patients previously poorly controlled on premixed insulin therapy." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Randomization was performed..." |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Randomization was performed by a central computer and patients were assigned (1:1:1) to one of the three treatment groups." |
| Blinding of participants and personnel (performance bias) Adverse events | High risk | Quote from publication: "In a open, controlled, randomised, parallel‐group, single‐centre, 16‐weeks pilot study..." |
| Blinding of participants and personnel (performance bias) Health‐related quality of life, patient satisfaction | High risk | Quote from publication: "In a open, controlled, randomised, parallel‐group, single‐centre, 16‐weeks pilot study..." |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | High risk | Quote from publication: "In a open, controlled, randomised, parallel‐group, single‐centre, 16‐weeks pilot study..." |
| Blinding of participants and personnel (performance bias) Insulin dose | High risk | Quote from publication: "In a open, controlled, randomised, parallel‐group, single‐centre, 16‐weeks pilot study..." |
| Blinding of outcome assessment (detection bias) Adverse events | High risk | Quote from publication: "In a open, controlled, randomised, parallel‐group, single‐centre, 16‐weeks pilot study..." |
| Blinding of outcome assessment (detection bias) Health‐related quality of life, patient satisfaction | High risk | Quote from publication: "In a open, controlled, randomised, parallel‐group, single‐centre, 16‐weeks pilot study..." |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | High risk | Quote from publication: "In a open, controlled, randomised, parallel‐group, single‐centre, 16‐weeks pilot study..." |
| Blinding of outcome assessment (detection bias) Insulin dose | High risk | Quote from publication: "In a open, controlled, randomised, parallel‐group, single‐centre, 16‐weeks pilot study..." |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on weight gain and hypoglycaemia were collected and reported |
| Incomplete outcome data (attrition bias) Health‐related quality of life, patient satisfaction | Low risk | Comment: data on patient satisfaction were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Simpson 1990.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes on insulin therapy, inadequate controlled (fasting glucose > 8.0 mmol/L Exclusion criteria: not stated Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: intervention: 36 (10‐62) insulin , placebo: insulin 31 (14‐112) U/day. Insulin regimen: once (n = 5) or twice daily (n = 15) Titration period: 2 months minimum run‐in period in which oral medication was stopped |
|
| Outcomes | Primary outcome(s) (as stated in the publication): serum and urinary glucoses, HbA1c, urinary C‐peptide, insulin dose, number of tablets prescribed, compliance | |
| Study details |
Total study duration: 2 months run‐in and 8 weeks intervention Run‐in period: 2 months Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Farmaitalia Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To assess the relative contribution of endogenous insulin secretion and peripheral insulin sensitivity when SU was given in combination with insulin to 'secondary failure' type 2 diabetes patients." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "patients were randomised to receive either glipizide 10 mg twice daily or placebo, in addition to usual insulin" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk | Quote from publication: "The treatment was blind to both the patient and investigators" |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk | Quote from publication: "The treatment was blind to both the patient and investigators" |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk | Quote from publication: "The treatment was blind to both the patient and investigators" |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on weight gain were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Stenman 1988.
| Methods |
Cross‐over randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes failing on oral antidiabetic agents Exclusion criteria: hepatic, renal or pulmonary dysfunction; secondary diabetes Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: intervention: 34 (4) insulin, placebo: insulin 26 (3) U/day. Insulin regimen: once or twice daily intermediate or intermediate mixed with short‐acting insulin Titration period: 4 months (2 weeks in hospital) initiation insulin therapy |
|
| Outcomes | Primary outcome(s) (as stated in the publication): HbA1c, fasting glucose, urinary glucose, C‐peptide after glucagon, insulin dose, lipids, body weight, free insulin | |
| Study details |
Total study duration: 8 months: 4 months run‐in and 4 months intervention Run‐in period: 2 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Sigrid Juselius Foundation, Finnish Medical Association, Signe and Ane Gyllenberg foundation Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To examine the effect of the addition of glibenclamide to insulin therapy on glycaemic control and beta cell function." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "...the patients were randomised to a double‐blind treatment with insulin in combination with glibenclamide or insulin and placebo for four months" |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "....the patients were randomised to a four‐months double‐blind cross‐over treatment...." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "....the patients were randomised to a four‐months double‐blind cross‐over treatment...." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "....the patients were randomised to a four‐months double‐blind cross‐over treatment...." Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on weight gain and hypoglycaemia were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: some data were only reported graphically |
| Other bias | Low risk | Comment: none |
Strowig 2002.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes on insulin therapy (≥ 30 IU/day), 24‐70 years, HbA1c ≥ 7.0%, normal renal and hepatic function Exclusion criteria: not stated Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: metformin insulin 82.9 U/day, troglitazone insulin 96.5 U/day, control insulin 80.3 U/day. Insulin regimen: 2 or more daily, 70/30 mix insulin or intermediate and short‐acting insulin Titration period: 4 weeks |
|
| Outcomes | Primary outcome(s) (as stated in the publication): insulin dose, lipids, HbA1c, glucose, C‐peptide, body weight, Alat, Asat, medical history, physical exam, waist‐hip measurement, 3‐day food record, serum chemistry | |
| Study details |
Total study duration: 4 weeks run‐in and 12 weeks intervention Run‐in period: 4 weeks Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: Bristol Myers Squibb Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To evaluate the safety and efficacy of treatment with insulin alone, insulin plus metformin, or insulin plus troglitazone for 4 months in type 2 DM." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "Subjects ... were randomly assigned ..." |
| Allocation concealment (selection bias) | Low risk | Quote from publication: "Random assignment was determined by the sponsor who provided sealed sequentially numbered envelopes" |
| Blinding of participants and personnel (performance bias) Adverse events | High risk | Quote from publication: "...were randomly assigned in an unmasked fashion..." |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | High risk | Quote from publication: "...were randomly assigned in an unmasked fashion..." |
| Blinding of participants and personnel (performance bias) Insulin dose | High risk | Quote from publication: "...were randomly assigned in an unmasked fashion..." |
| Blinding of outcome assessment (detection bias) Adverse events | High risk | Quote from publication: "...were randomly assigned in an unmasked fashion..." |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | High risk | Quote from publication: "...were randomly assigned in an unmasked fashion..." |
| Blinding of outcome assessment (detection bias) Insulin dose | High risk | Quote from publication: "...were randomly assigned in an unmasked fashion..." |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on adverse events were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: medical history and physical exam were not reported |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Wulffelé 2002.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with type 2 diabetes on insulin therapy Exclusion criteria: significant surgery within 3 months before entry into the study, major organ or system disease, medication use affecting glucose metabolism or sulphonylurea activity Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 3 Treatment before study: intervention: 71 (33) insulin, placebo: insulin 70 (33) U/day. Insulin regimen: 4 times daily intermediate plus short‐acting insulin or twice daily mix‐insulin Titration period: 12 weeks (optimisation of insulin therapy, cessation of metformin, cessation of antihypertensive and lipid‐lowering medication) |
|
| Outcomes | Primary outcome(s) (as stated in the publication): glucose, HbA1c, lipids, insulin dose, blood pressure, weight, BMI, waist‐hip‐ratio | |
| Study details |
Total study duration: 16 weeks Run‐in period: 12 weeks Study terminated early (for benefit/because of adverse events): no (results of the interim analysis) |
|
| Publication details |
Language of publication: English Funding source: Byk, Lifescan, Merck Lipha, MSD, Novo Nordsik Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To investigate the metabolic effects of metformin, as compared with placebo, in type 2 diabetes patients intensively treated with insulin." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "...all subject were randomly assigned..." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "...all subjects were randomly assigned in a double‐blind fashion..." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Low risk |
Quote from publication: "...all subjects were randomly assigned in a double‐blind fashion..." Comment: probably the participants and the personnel were blinded |
| Blinding of participants and personnel (performance bias) Insulin dose | Low risk |
Quote from publication: "...all subjects were randomly assigned in a double‐blind fashion..." Comment: probably the participants and the personnel were blinded |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear if the outcome assessor was blinded |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on adverse events were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were collected and reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | Unclear risk | Comment: funded by a pharmaceutical company |
Yilmaz 2007.
| Methods |
Parallel randomised controlled clinical trial Randomisation ratio: 1:1:1 Superiority design |
|
| Participants |
Inclusion criteria: people with poorly controlled type 2 diabetes on insulin monotherapy Exclusion criteria: severe hypertension, repeated hypoglycaemic episodes, severe cardiovascular and cerebrovascular disease, renal or hepatic failure, acute diabetic complications, incipient heart failure, pregnancy, breastfeeding Diagnostic criteria: not stated |
|
| Interventions |
Number of study centres: 1 Treatment before study: insulin MIX 30/70 aspart/NPH Titration period: not stated |
|
| Outcomes |
Primary outcome(s) (as stated in the publication): change in HbA1c Secondary outcomes (as stated in the publication): changes in insulin dosage, body weight, waist‐to‐hip ratio, lipids ADDITIONAL OUTCOMES: incidence of hypoglycaemia and side effects |
|
| Study details |
Total study duration: 6 months Run‐in period: unclear Study terminated early (for benefit/because of adverse events): no |
|
| Publication details |
Language of publication: English Funding source: not stated Publication status: peer‐reviewed journal |
|
| Stated aim of study | Quote from publication: "To investigate the glucose lowering effects of insulin alone, insulin plus metformin, insulin plus rosiglitazone, or insulin plus acarbose in subjects with type 2 diabetes mellitus and determine the type of treatment that would influence the serum level of total cholesterol, LDL‐C, HDL‐C, CRP and fibrinogen in these patients." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from publication: "After the initial assessment, subjects were randomly assigned to continue insulin therapy alone, or add acarbose (300 mg/day), or metformin (1,700 mg/day), or rosiglitazone (8 mg/day) to insulin therapy." |
| Allocation concealment (selection bias) | Unclear risk | Comment: not possible to judge whether the allocation to the intervention and control group was concealed |
| Blinding of participants and personnel (performance bias) Adverse events | Unclear risk | Comment: unclear, not described |
| Blinding of participants and personnel (performance bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear, not described |
| Blinding of participants and personnel (performance bias) Insulin dose | Unclear risk | Comment: unclear, not described |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: unclear, not described |
| Blinding of outcome assessment (detection bias) HbA1c, FPG, lipids | Unclear risk | Comment: unclear, not described |
| Blinding of outcome assessment (detection bias) Insulin dose | Unclear risk | Comment: unclear, not described |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: data on weight gain were collected and reported |
| Incomplete outcome data (attrition bias) HbA1c, FPG, lipids | Low risk | Comment: outcome data were collected and reported |
| Incomplete outcome data (attrition bias) Insulin dose | Low risk | Comment: data on insulin dose were reported |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes of interest were reported |
| Other bias | Unclear risk | Comment: funding not disclosed; differences in baseline data between groups; people with heart failure excluded |
Note: where the judgement is 'Unclear' and the description is blank, the study did not report that particular outcome.
NPH: Neutral Protamine Hagedorn
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abraira 1998 | Mixed population: insulin‐naive and previously insulin‐treated |
| Al‐Shaikh 2006 | Insulin‐naive participants |
| Altuntas 2003 | Insulin‐naive participants |
| Bachman 1988 | Insulin‐naive participants |
| Barranco 2006 | No insulin therapy arm |
| Berhanu 2007 | Impossible to analyse subgroup of insulin‐users separately |
| Bianchi 1996 | Trial on combination of benfluorex with insulin |
| Buse 1998 | Use of troglitazone (off the market) |
| Carta 1984 | Insulin‐naive participants |
| Castillo 1987 | Insulin‐naive participants |
| Charbonnel 2010 | Impossible to analyse subgroup of insulin‐users separately |
| Chazan 2001 | Insulin‐naive participants |
| Chow1995 | Insulin‐naive participants |
| Clauson 1995 | Insulin‐naive participants |
| Cortes 1993 | Well‐controlled population |
| Dashora 2007 | Impossible to analyse subgroup of insulin‐users separately |
| Davidson 2006 | No arm without oral agent or placebo |
| De Luis 2001 | No RCT |
| Douek 2005 | Insulin‐naive participants |
| Fonseca 2006 | Insulin‐naive participants |
| Fonseca 2008 | Observational phase of RCT |
| Garg 2007 | No RCT |
| Gerstein 2006 | Insulin‐naive participants |
| Goudswaard 2004a | Insulin‐naive participants |
| Greco 1998 | No RCT |
| Groop 1984 | Insulin‐naive participants |
| Groop 1991 | Insulin‐naive participants |
| Gutniak 1987 | Insulin‐naive participants |
| Hamelbeck 1982 | Insulin‐naive participants |
| Hermanns 2004 | Follow‐up less than 2 months |
| Hollander 2003 | Adding of non‐oral agent pramlintide |
| Hollander 2003a | Adding of non‐oral agent pramlintide |
| Hollander 2004 | Adding of non‐oral agent pramlintide |
| Hollander 2007 | Adding of rosiglitazone (off the market) |
| Holman 1987 | Insulin‐naive participants |
| Home 2007 | Insulin‐naive participants |
| Inoue1998 | Insulin‐naive participants |
| Jacob 2007 | Impossible to analyse subgroup of insulin‐users separately |
| Jacober 2006 | Insulin‐naive participants |
| Janka 2005 | Insulin‐naive participants |
| Janka 2007 | Insulin‐naive participants |
| Juurinen 2008 | No RCT |
| Juurinen 2009 | Impossible to analyse subgroup of insulin‐users separately |
| Kabadi 2003 | Insulin‐naive participants |
| Kanda 1996 | Insulin‐naive participants |
| Kandalintseva 2008 | Rosiglitazone combination therapy (off the market) |
| Karlander 1991 | Insulin‐naive participants |
| Kilo 2003 | Insulin‐naive participants |
| Kokic 2003 | Insulin‐naive participants |
| Kothny 2013 | Impossible to analyse subgroup of insulin‐users separately |
| Kvapil 2006 | Insulin‐naive participants |
| LAPToP 2004 | Insulin‐naive participants |
| Lins 1988 | Insulin‐naive participants |
| Liu 2003 | Rosiglitazone combination therapy (off the market) |
| Lotz 1988 | Insulin‐naive participants |
| Lund 2009 | Insulin‐naive participants |
| Lundershausen 1987 | Insulin‐naive participants |
| Makimattila 1999 | Insulin‐naive participants |
| Mohan 1990 | No RCT |
| Morrow 2011 | Combination with ligarglutide (non‐oral agent) |
| Olsson 2002 | Insulin‐naive participants |
| Ose 2005 | Not RCT |
| Ozbek 2006 | Well‐controlled population |
| Pan 2007 | Insulin‐naive participants |
| Peacock 1984 | Insulin‐naive participants |
| Peyrot 2008 | Addition of a non‐oral agent pramlintide |
| Ponssen 2000 | Mixed group: insulin‐naive and previously insulin‐treated participants |
| Pontiroli 1990 | Insulin‐naive participants |
| Poulsen 2003 | Control group mixed: insulin‐ and insulin + metformin‐treated |
| Raskin 2001 | Addition of rosiglitazone (off the market) |
| Ravnik 1989 | Insulin‐naive participants |
| Ravnik 1995 | Insulin‐naive participants |
| Raz 2005 | Insulin‐naive participants |
| Riddle 1989 | Mixed group: insulin‐naive and previously insulin‐treated participants |
| Riddle 1992 | Insulin‐naive participants |
| Riddle 1998 | Insulin‐naive participants |
| Riddle 2007 | Adding of a non‐oral agent pramlintide |
| Rosak 1985 | Follow‐up less than 2 months |
| Sachse 1984 | Insulin‐naive participants |
| Sangiorgio 2000 | Not RCT |
| Schmidt 1986 | Insulin‐naive participants |
| Schnell 2006 | Insulin‐naive participants |
| Schwartz 1998 | Use of troglitazone (off the market) |
| Sun 1995 | Insulin‐naive participants |
| Tamemoto 2007 | Insulin‐naive participants |
| Vilsboll 2010 | Impossible to analyse subgroup of insulin‐users separately |
| Willey 1994 | Mixed group: insulin‐ and insulin + metformin‐treated participants; trial on combination of insulin and dexfenfluramine |
| Wolffenbuttel 1991 | Insulin‐naive participants |
| Wolffenbuttel 1999 | Insulin‐naive participants |
| Wong 2005 | Special population: type 2 diabetes participants with peritoneal dialysis |
| Wright 2002 | Newly diagnosed type 2 diabetes participants |
| Yamada 2007 | No arm without oral agent or placebo |
| Yki‐Jarvinen 1992 | Insulin‐naive participants |
| Yki‐Jarvinen 1999 | Insulin‐naive participants |
| Yki‐Jarvinen 2013 | Impossible to analyse subgroup of insulin‐users separately |
| Yokoyama 2011 | Two arms: continuation and discontinuation of glimepiride |
| Zargar 2002 | Insulin‐naive participants |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Fargren 2014.
| Methods | A single‐centre, double‐blind, randomised, placebo‐controlled cross‐over study |
| Participants | Participants with type 2 diabetes, mean age 59 + 6 (SD) years and mean HbA1c 7.7% + 0.8%, treated with exogenous insulin with or without oral antihyperglycaemic agents |
| Interventions | Participants received vildagliptin (50 mg twice a day) or placebo as add‐on to insulin for 4 weeks in random order with a 4‐week washout in between |
| Outcomes | Glucose, glucagon |
| Notes | ‐ |
Kaku 2014.
| Methods | Randomised, double‐blind, 12‐week comparative trial, followed by a 40‐week, open‐label phase |
| Participants | 179 participants with type 2 diabetes |
| Interventions | Alogliptin and insulin versus placebo and insulin |
| Outcomes | HbA1c, body weight, hypoglycaemia |
| Notes | ‐ |
Mathieu 2015.
| Methods | Multicenter, randomised, double‐blind, placebo‐controlled, 24‐week clinical trial |
| Participants | 660 participants with type 2 diabetes and inadequate glycaemic control on insulin, with or without metformin (> 1500 mg/day) or sulfonylurea, for > 10 weeks. Participants could remain on metformin but not sulphonylurea after randomisation |
| Interventions | Sitagliptin 100 mg/day or placebo was administered concurrently with insulin glargine titration, targeting a fasting glucose of 4.0‐5.6 mmol/L (72‐100 mg/dL) |
| Outcomes | Insulin dose, HbA1c, hypoglycaemia, adverse effects |
| Notes | Subgroup analysis with insulin monotherapy needs to be checked |
McGill 2015.
| Methods | Data for participants in two phase 3 trials with linagliptin who were receiving insulin were analysed separately (n = 811) |
| Participants | Type 2 diabetes mellitus participants with chronic kidney disease |
| Interventions | Linagliptin + insulin vs placebo + insulin |
| Outcomes | HbA1c, (severe) hypoglycaemia |
| Notes | Inadequate control needs to be checked |
Ning 2014.
| Methods | Multicentre, double‐blind, placebo‐controlled trial |
| Participants | Participants with type 2 diabetes inadequately controlled (HbA1c 7.5%‐11.0%) on stable therapy with long‐acting, intermediate‐acting or premixed insulin, with or without concomitant metformin |
| Interventions | Vildagliptin 50 mg twice a day (n = 146) or placebo (n = 147) |
| Outcomes | HbA1c, fasting blood glucose, BMI |
| Notes | Need to determine if subgroup analysis with insulin monotherapy is possible |
Rosenstock 2009.
| Methods | 26‐week, double‐blind, placebo‐controlled study |
| Participants | People with type 2 diabetes inadequately controlled with insulin alone or combined with metformin |
| Interventions | Participants received alogliptin 12.5 mg (n = 131), alogliptin 25 mg (n = 129) or placebo (n = 130) once daily, as add‐on to stable insulin therapy with or without metformin |
| Outcomes | HbA1c, body weight, hypoglycaemia, incidences of overall adverse events, and of gastrointestinal, dermatological and infection‐related events |
| Notes | Need to determine whether subgroup analysis for insulin monotherapy group is possible |
Sato 2015.
| Methods | 24‐week, prospective, randomised, open‐labelled, controlled trial |
| Participants | Participants with type 2 diabetes who were suboptimally controlled despite receiving at least twice‐daily injections of insulin were enrolled in the trial |
| Interventions | The participants were randomised to continuation of insulin treatment (insulin group) or addition of sitagliptin 50 mg‐100 mg daily to insulin treatment |
| Outcomes | HbA1c, body weight, hypoglycaemia, treatment satisfaction |
| Notes | ‐ |
Sheu 2015.
| Methods | Randomised double‐blind trial |
| Participants | Asian participants with type 2 diabetes mellitus inadequately controlled by basal insulin with/without oral agents |
| Interventions | Treatment with linagliptin 5 mg once daily or placebo. Basal insulin dose remained stable for 24 weeks, after which adjustments could be made according to the investigator's discretion to improve glycaemic control |
| Outcomes | HbA1c, adverse events |
| Notes | Need to determine if subgroup analysis with insulin monotherapy is possible |
Srivanichakorn 2015.
| Methods | 8‐week randomised controlled trial |
| Participants | Participants with type 2 diabetes who had been treated with insulin for at least 3 years plus moderate to high doses of sulphonylureas |
| Interventions | Withdrawal of sulphonylureas (insulin monotherapy) (n = 16) or continuation (n = 16) of sulphonylureas |
| Outcomes | HbA1c, insulin secretion |
| Notes | Need to determine whether participants were adequately controlled |
Takahashi 2015.
| Methods | Randomised controlled trial |
| Participants | Type 2 diabetes participants receiving twice‐daily injections of premix analog insulin |
| Interventions | Group A, in which participants were switched to long‐acting insulin glargine at 80% of the insulin dose in the previous treatment regimen (23 participants), or group B (21 participants), given 50% of the previous dose, concurrently with the oral dipeptidyl‐peptidase 4 inhibitor sitagliptin |
| Outcomes | HbA1c, lipids, hyperglycaemia and nocturnal hypoglycaemia |
| Notes | Need to determine if participants were adequately controlled before randomisation |
BMI: body mass index; HbA1c: glycosylated haemoglobin A1c
Characteristics of ongoing studies [ordered by study ID]
EUCTR2011‐004622‐96‐ES.
| Trial name or title | Study to test the safety, tolerability, and effectiveness of sitagliptin when compared to placebo in reducing the amount of insulin (with or without metformin) needed per day, to control blood sugar, over a 24‐week period |
| Methods | A phase III, multicenter, randomised, double‐blind, placebo‐controlled clinical trial to study the safety and insulin‐sparing efficacy of the addition of sitagliptin in ‐ Study to test the safety, tolerability, and effectiveness of sitagliptin when compared to placebo |
| Participants | Participants with type 2 diabetes mellitus who have inadequate glycaemic control on insulin alone or in combination with metformin |
| Interventions | Sitagliptin compared with placebo on the change in insulin dose in IU per day in people with type 2 diabetes mellitus (T2DM) with inadequate glycaemic control on insulin with or without metformin, who titrate insulin glargine (treat‐to‐target) |
| Outcomes | Daily insulin dose, HbA1c, fasting plasma glucose, body weight |
| Starting date | 23 December 2011 |
| Contact information | |
| Notes | Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc |
IRCT201204229536N1.
| Trial name or title | The comparison glycaemic control in two group patient treated with insulin alone and insulin plus sulfonylurea [title as provided by study investigators] |
| Methods | |
| Participants | |
| Interventions | Insulin 0.2 U/kg /day and sulphonylurea with previous dose vs insulin 0.3 U/kg /day |
| Outcomes | Blood glucose, body weight, hypoglycaemic events |
| Starting date | |
| Contact information | |
| Notes |
JPRN‐UMIN000011851.
| Trial name or title | The efficacy of vildagliptin on type 2 diabetic patients with basal‐bolus insulin therapy in the short‐term hospitalisation (single‐center, randomised, open‐label, controlled study) |
| Methods | Single‐center, randomised, open‐label, controlled study |
| Participants | Type 2 diabetes participants |
| Interventions | Insulin + vildagliptin vs insulin alone |
| Outcomes | Blood glucose, lipids |
| Starting date | |
| Contact information | |
| Notes |
JPRN‐UMIN000018784.
| Trial name or title | Comparison of the effects of insulin monotherapy and combination therapy with ipragliflozin and insulin on glucose toxicity in type 2 diabetes mellitus : a randomised controlled trial |
| Methods | Not specified |
| Participants | |
| Interventions | Combination therapy with ipragliflozin and insulin group. Oral administration of 50 mg ipragliflozin once a day, after breakfast. Multiple injection of rapid‐acting insulin and long‐acting insulin. Insulin monotherapy group: multiple injection of rapid‐acting insulin and long‐acting insulin |
| Outcomes | Glucose, insulin dose, lipids, body weight |
| Starting date | |
| Contact information | |
| Notes |
NCT00329225.
| Trial name or title | Rosiglitazone in subjects with type 2 diabetes mellitus who are inadequately controlled on insulin |
| Methods | A multicenter, randomised, double‐blind, parallel‐group, placebo‐controlled clinical evaluation of insulin plus rosiglitazone (2 mg and 4 mg) compared to insulin plus placebo for 24 weeks |
| Participants | Participants with type 2 diabetes mellitus who are inadequately controlled on insulin |
| Interventions | Rosiglitazone + insulin vs insulin + placebo |
| Outcomes | HbA1c, fasting plasma glucose, lipids |
| Starting date | 22 May 2006 |
| Contact information | |
| Notes | GlaxoSmithKline |
NCT00757588.
| Trial name or title | Safety and efficacy of saxagliptin plus insulin with or without metformin |
| Methods | Multicenter double‐blind parallel‐group randomised controlled trial, phase 3 trial |
| Participants | Type 2 diabetes with inadequate glycaemic control |
| Interventions | Saxagliptin (5 mg) + insulin vs placebo for saxagliptin + insulin |
| Outcomes | HbA1c, fasting glucose, insulin dose, blood pressure, (serious) adverse events (hypoglycaemia) |
| Starting date | 22 September 2008 |
| Contact information | |
| Notes | AstraZeneca |
NCT02104804.
| Trial name or title | Evaluate the efficacy and aafety of aaxagliptin added to insulin monotherapy or to insulin combined with metformin in Chinese subjects with type 2 diabetes who have inadequate glycaemic control |
| Methods | Double‐blind parallel‐group randomised controlled trial, phase 3 trial |
| Participants | Type 2 diabetes participants who have inadequate glycaemic control on insulin alone or on insulin in combination with metformin |
| Interventions | Saxagliptin (5 mg) + insulin vs placebo for saxagliptin + insulin |
| Outcomes | HbA1c, fasting glucose, insulin dose, (serious) adverse events (hypoglycaemia) |
| Starting date | 2 April 2014 |
| Contact information | |
| Notes | AstraZeneca |
HbA1c: glycosylated haemoglobin A1c
Differences between protocol and review
Terminology was changed from 'oral hypoglycaemic agents' to 'oral glucose‐lowering agents'.
Type of outcome measures
We deleted from secondary outcome measures 'the percentage of participants achieving good glycaemic control (HbA1c less than 7%) without hypoglycaemic events', blood pressure and body‐mass index, because reporting about these outcomes was rare.
Data collection and analysis
We changed second reviewer from AG to AK for data extraction, assessment of risk of bias and data entry. Dichotomous data were not identified, therefore this part was omitted. RV and MA, contributed equally to the data analyses and the review development.
Analyses of cross‐over trials were specified.
We changed the sensitivity analyses. We did not repeat the analysis excluding unpublished trials, because only published trials were included. We did not repeat the analysis excluding trials using different filters i.e. diagnostic criteria, language of publication, source of funding (industry versus other) and country. Not all trials reported diagnostic criteria and only one trial was not published in English. Almost all trials were funded by a pharmaceutical company. The trials were mainly performed in Western‐European countries and the United States. We did not expect large differences in the results between these countries.
Declarations of interest
Changed from 'None known' to 'GR has received research grants from Sanofi‐Aventis and honoraria form Novo Nordisk for lecturing and attending (inter‐)national board meetings. In 1999, AG has received an unrestricted research grant from Novo Nordisk. MA received a reimbursement from Sanofi‐Aventis for attending a congress and printing her thesis' and royalties from publisher Bohn Stafleu van Loghum for authorship of a book: "Leven met diabetes mellitus type 2".
Contributions of authors
Rimke C Vos (RV): methodology, statistical analysis and review development. Marielle JP van Avendonk (MA): protocol development, searching for trials, trial selection, quality assessment of trials, data extraction, methodology, statistics and review development. Hanneke Jansen (HJ): quality assessment of trials, data extraction and review development. Alex N Goudswaard (AG): protocol development, trial selection, quality assessment, data extraction and review development. Maureen Van den Donk (MD): methodology, statistics, data extraction and review development. Kees Gorter (KG): data extraction and review development. Anneloes Kerssen (AK): data extraction and review development. Guy EHM Rutten (GR): review development.
Sources of support
Internal sources
University Medical Center Utrecht, Netherlands.
External sources
No sources of support supplied
Declarations of interest
RV: an unrestricted grant for a study in type 2 diabetes patients on insulin therapy (support of self‐managment by triggers) is received by Sanofi. RV did not control the grant or receive direct payment from Sanofi. The case was referred to the Funding Arbiters who determined Sanofi’s grant funding of RV’s institution was not a relevant conflict of interest. An unrestricted grant is received for a study on the long term effects of a self management education course for patients with type 2 diabetes by the European Foundation for the Study of Diabetes. MA: received a reimbursement from Sanofi‐Aventis for attending a congress and printing her thesis (2010) and she received royalties from publisher Bohn Stafleu van Loghum for authorship of a book: "Leven met diabetes mellitus type 2". HJ: has no conflict of interest. AG: received an unrestricted research grant from Novo Nordisk in 1999. MD: has no conflict of interest. KG: has no conflict of interest. AK: has no conflict of interest. GR: received a grant for a study project from Sanofi‐aventis. Besides he received honoraria from Novo Nordisk for lecturing and attending (inter‐) national board meetings.
Edited (no change to conclusions)
References
References to studies included in this review
Avilés 1999 {published data only}
- Avilés‐Santa L, Sinding J, Raskin P. Effects of metformin in patients with poorly controlled, insulin‐treated type 2 diabetes mellitus. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1999;131:182‐8. [DOI] [PubMed] [Google Scholar]
Barnett 2013 {published data only}
- Barnett AH, Charbonnel B, Li J, Donovan M, Fleming D, Iqbal N. Saxagliptin add‐on therapy to insulin with or without metformin for type 2 diabetes mellitus: 52‐week safety and efficacy. Clinical drug investigation 2013;33(10):707‐17. [PUBMED: 23949898] [DOI] [PubMed] [Google Scholar]
Casner 1988 {published data only}
- Casner PR. Insulin‐glyburide combination therapy for non‐insulin‐dependent diabetes mellitus: a long‐term double‐blind, placebo‐controlled trial. Clinical Pharmacology and Therapeutics 1988;44:594‐603. [DOI] [PubMed] [Google Scholar]
Chiasson 1994 {published data only}
- Chiasson J‐L, Josse R, Hunt J, Palmason C, Rodger W, Ross S, et al. The efficacy of acarbose in the treatment of patients with non‐insulin‐dependent diabetes mellitus. Annals of Internal Medicine 1994;121:928‐35. [DOI] [PubMed] [Google Scholar]
Coniff 1995 {published data only}
- Coniff RF, Shapiro JA, Seaton TB, Hoogwerf BJ, Hunt JA. A double‐blind placebo‐controlled trial evaluating the safety and efficacy of acarbose for the treatment of patients with insulin‐requiring type 2 diabetes. Diabetes Care 1995;18(7):928‐32. [DOI] [PubMed] [Google Scholar]
Feinglos 1998 {published data only}
- Feinglos MN, Thacker CR, Lobaugh B, DeAtkine DD, McNeill DB, English JS, et al. Combination insulin and sulfonylurea therapy in insulin‐requiring type 2 diabetes mellitus. Diabetes Research and Clinical Practice 1998;39:193‐9. [DOI] [PubMed] [Google Scholar]
Fonseca 2007 {published data only}
- Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia 2007;50:1148‐55. [DOI] [PubMed] [Google Scholar]
Fritsche 2000 {published data only}
- Fritsche A, Schmulling RM, Haring HU, Stumvoll M. Intensive insulin therapy combined with metformin in obese type 2 diabetic patients. Acta Diabetologica 2000;37:13‐8. [DOI] [PubMed] [Google Scholar]
Giugliano 1993 {published data only}
- Giugliano D, Quatraro A, Consoli G, Minei A, Ceriello A, Rosa N, et al. Metformin for obese, insulin‐treated diabetic patients: improvement in glycaemic control and reduction of metabolic risk factors. European Journal of Clinical Pharmacology 1993;44:107‐12. [DOI] [PubMed] [Google Scholar]
Groop 1985 {published data only}
- Groop L, Harno K, Nikkila EA, Pelkonen R, Tolppannen EM. Transient effect of the combination of insulin and sulfonylurea (glibenclamide) on glycemic control in non‐insulin dependent diabetics poorly controlled on insulin alone. Acta Medica Scandinavica 1985;217:33‐9. [DOI] [PubMed] [Google Scholar]
Hermann 2001 {published data only}
- Hermann LS, Kalén J, Katzman P, Lager I, Nilsson A, Norrhamn O, et al. Long‐term glycaemic improvement after addition of metformin to insulin in insulin‐treated obese type 2 diabetes patients. Diabetes, Obesity and Metabolism 2001;3:428‐34. [DOI] [PubMed] [Google Scholar]
Hirsch 1999 {published data only}
- Hirsch IB. Metformin added to insulin therapy in poorly controlled type 2 diabetes. Diabetes Care 1999;22(5):854. [DOI] [PubMed] [Google Scholar]
Hong 2012 {published data only}
- Hong ES, Khang AR, Yoon JW, Kang SM, Choi SH, Park KS, et al. Comparison between sitagliptin as add‐on therapy to insulin and insulin dose‐increase therapy in uncontrolled Korean type 2 diabetes: CSI study. Diabetes, Obesity & Metabolism 2012;14(9):795‐802. [PUBMED: 22443183] [DOI] [PubMed] [Google Scholar]
Kitabchi 1987 {published data only}
- Kitabchi AE, Soria AG, Radparvar A, Lawson‐Grant V. Combined therapy of insulin and tolazamide decreases insulin requirement and serum triglycerides in obese patients with non‐insulin‐dependent diabetes mellitus. American Journal Medical Science 1987;294(1):10‐4. [DOI] [PubMed] [Google Scholar]
Krawczyk 2005 {published data only}
- Krawczyk A, Gajer G, Greszczak W, Strojek K. The impact of metformin on metabolic control in obese type 2 diabetes patients treated with insulin. Preliminary observation [Wplyw metforminy na wyrównanie metaboliczne u otylych chorych na cukrzyce typu 2 leczonych insulina. Obserwacja wstepna]. Diabetologia Doswiadczalna i Kliniczna 2005;5(1):47‐51. [Google Scholar]
Kyllastinen 1985 {published data only}
- Kyllastinen M, Groop L. Combination of insulin and glibenclamide in the treatment of elderly non‐insulin dependent (type 2) diabetic patients. Annals of Clinical Research 1985;17:100‐4. [PubMed] [Google Scholar]
Lewitt 1989 {published data only}
- Lewitt MS, Yu VKF, Rennie GC, Carter JN, Marel GM, Yue DKS, et al. Effects of combined insulin‐sulfonylurea therapy in type II patients. Diabetes Care 1989;12(6):379‐83. [DOI] [PubMed] [Google Scholar]
Lindstrom 1999 {published data only}
- Lindstrom T, Nystrom FH, Olsson AG, Ottoson AM, Arnqvist HJ. The lipoprotein profile differs during insulin treatment alone and combination therapy with insulin and sulphonylurea in patients with type 2 diabetes mellitus. Diabetic Medicine 1999;16:820‐6. [DOI] [PubMed] [Google Scholar]
Longnecker 1986 {published data only}
- Longnecker MP, Elsenhans VD, Leiman SM, Owen OE, Boden G. Insulin and a sulfonylurea agent in non‐insulin‐dependent diabetes mellitus. Archives of Internal Medicine 1986;146:673‐6. [PubMed] [Google Scholar]
Mattoo 2005 {published data only}
- Mattoo V, Eckland D, Widel M, Duran S, Fajardo C, Strand J, et al. Metabolic effects of pioglitazone in combination with insulin in patients with type 2 diabetes mellitus whose disease is not adequately controlled with insulin therapy: results of a six‐month, randomized, double‐blind, prospective, multicenter, parallel‐group study. Clinical Therapeutics 2005;27(5):554‐67. [DOI] [PubMed] [Google Scholar]
Mauerhoff 1986 {published data only}
- Mauerhoff T, Ketelslegers JM, Lambert AE. Effect of glibenclamide in insulin‐treated diabetic patients with a residual insulin secretion. Diabete & Metabolisme 1986;12:34‐8. [PubMed] [Google Scholar]
Mezitis 1992 {published data only}
- Mezitis NHE, Heshka S, Saitas V, Bailey T, Costa R, Pi‐Sunyer FX. Combination therapy for NIDDM with biosynthetic human insulin and glyburide. Diabetes Care 1992;15(2):265‐9. [DOI] [PubMed] [Google Scholar]
Mudaliar 2010 {published data only}
- Mudaliar S, Chang AR, Aroda VR, Chao E, Burke P, Baxi, et al. Effects of intensive insulin therapy alone and with added pioglitazone on renal salt/water balance and fluid compartment shifts in type 2 diabetes. Diabetes, Obesity and Metabolism 2010;12:133‐8. [DOI] [PubMed] [Google Scholar]
- Shah PK, Mudaliar S, Chang AR, Aroda V, Andre M, Burke P, et al. Effects of intensive insulin therapy alone and in combination with pioglitazone on body weight, composition, distribution and liver fat content in patients with type 2 diabetes. Diabetes, Obesity and Metabolism 2011;13:505‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nemoto 2011 {published data only}
- Nemoto M, Tajima N, Kawamori R. Efficacy of combined use of miglitol in type 2 diabetes patients receiving insulin therapy‐placebo‐controlled double‐blind comparative study. Acta Diabetologica 2011;48:15‐20. [DOI] [PubMed] [Google Scholar]
Osei 1984 {published data only}
- Falko JM, Osei K. Combination insulin/glyburide therapy in type II diabetes mellitus. Effects on lipoprotein metabolism and glucoregulation. American Journal of Medicine 1985;79(Suppl 3B):92‐101. [DOI] [PubMed] [Google Scholar]
- Osei K, O'Dorisio TM, Falko JM. Concomitant insulin and sulfonylurea therapy in patients with type II diabetes. American Journal of Medicine 1984;77:1002‐9. [DOI] [PubMed] [Google Scholar]
Quatraro 1986 {published data only}
- Quatraro A, Consoli G, Ceriello A, Giugliano D. Combined insulin and sulfonylurea therapy in non‐insulin‐dependent diabetics with secondary failure to oral drugs: a one year follow‐up. Diabete & Metabolisme 1986;12:315‐8. [PubMed] [Google Scholar]
Reich 1987 {published data only}
- Reich A, Abraira C, Lawrence AM. Combined glyburide and insulin therapy in type II diabetes. Diabetes Research 1987;6:99‐104. [PubMed] [Google Scholar]
Relimpio 1998 {published data only}
- Relimpio F, Pumar A, Losada F, Mangas MA, Acosta D, Astorga R. Adding metformin versus insulin dose increase in insulin‐treated but poorly controlled type 2 diabetes mellitus: an open‐label randomized trial. Diabetic Medicine 1998;15:997‐1002. [DOI] [PubMed] [Google Scholar]
Robinson 1998 {published data only}
- Robinson AC, Burke J, Robinson S, Johnston DG, Elkeles RS. The effects of metformin on glycemic control and serum lipids in insulin‐treated NIDDM patients with suboptimal metabolic control. Diabetes Care 1998;21(5):701‐5. [DOI] [PubMed] [Google Scholar]
Rosenstock 2002 {published data only}
- Rosenstock J, Einhorn D, Hershon K, Glazer NB, Yu S, Pioglitazone 014 Study Group. Efficacy and safety of pioglitazone in type 2 diabetes: a randomised, placebo‐controlled study in patients receiving stable insulin therapy. International Journal of Clinical Practice 2002;56(4):251‐57. [PubMed] [Google Scholar]
Schade 1987 {published data only}
- Schade DS, Mitchell WJ, Griego G. Addition of sulfonylurea to insulin treatment in poorly controlled type II diabetes. A double‐blind, randomized clinical trial. Journal of the American Medical Association 1987;257(18):2441‐5. [PubMed] [Google Scholar]
Schiel 2007 {published data only}
- Schiel R, Muller UA. Efficacy and treatment satisfaction of once‐daily insulin glargine plus one or two oral antidiabetic agents versus continuing premixed human insulin in patients with type 2 diabetes previously on long‐term conventional insulin therapy: The Switch pilot study. Experimental and Clinical Endocrinology & Diabetes 2007;115:627‐33. [DOI] [PubMed] [Google Scholar]
Simpson 1990 {published data only}
- Simpson HCR, Sturley R, Stirling CA, Reckless JPD. Combination of insulin with glipizide increases peripheral glucose disposal in secondary failure type 2 diabetic patients. Diabetic Medicine 1990;7:143‐7. [DOI] [PubMed] [Google Scholar]
Stenman 1988 {published data only}
- Stenman S, Gropp PH, Saloranta C, Totterman KJ, Fyhrqvist F, Groop L. Effects of the combination of insulin and glibenclamide in type 2 (non‐insulin‐dependent) diabetic patients with secondary failure to oral glucose lowering agents. Diabetologia 1988;31:206‐13. [DOI] [PubMed] [Google Scholar]
Strowig 2002 {published data only}
- Strowig S, Avilés‐Santa ML, Raskin P. Comparison of insulin monotherapy and combination therapy with insulin and metformin or insulin and troglitazone in type 2 diabetes. Diabetes Care 2002;25(10):1691‐8. [DOI] [PubMed] [Google Scholar]
Wulffelé 2002 {published data only}
- Wulffelé MG, Kooy A, Lehert P, Bets D, Ogterop JC, Borger van der Burg B, et al. Combination of insulin and metformin in the treatment of type 2 diabetes. Diabetes Care 2002;25:2133‐40. [DOI] [PubMed] [Google Scholar]
Yilmaz 2007 {published data only}
- Yilmaz H, Gursoy A, Sahin M, Guvener Demirag N. Comparison of insulin monotherapy and combination therapy with insulin and metformin or insulin and rosiglitazone or insulin and acarbose in type 2 diabetes. Acta Diabetologica 2007;44:187‐92. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abraira 1998 {published data only}
- Abraira C, Henderson WG, Colwell JA, Nuttall FQ, Comstock JP, Emanuele NV, et al. Response to intensive therapy steps and to glipizide dose in combination with insulin in type 2 diabetes. VA feasibility study on glycemic control and complications. Diabetes Care 1998;21(4):574‐9. [DOI] [PubMed] [Google Scholar]
Al‐Shaikh 2006 {published data only}
- Al‐Shaikh AR. Comparison of basal insulin added to oral agents versus twice‐daily premixed insulin as initial insulin therapy for type 2 diabetes. Pakistan Journal of Medical Science 2006;22(1):14‐7. [Google Scholar]
Altuntas 2003 {published data only}
- Altuntas Y, Ozen B, Ozturk B, Sengul A, Ucak S, Ersoy O, et al. Comparison of additional metformin or NPH insulin to mealtime insulin lispro with mealtime human insulin therapy in secondary OAD failure. Diabetes, Obesity and Metabolism 2003;5:371‐8. [DOI] [PubMed] [Google Scholar]
Bachman 1988 {published data only}
- Bachmann W, Lotz N, Mehnert H, Rosak C, Schoffling K. Effectiviness of combined glibenclamide and insulin treatment of patients with secondary sulphonylurea failure: a controlled multi‐center double‐blind trial [Wirksamheit der kombinationsbehandlung mit glibenclamid und insulin bei sulfonylharnstoff‐sekundarversagen]. Deutsche Medizinische Wochenschrift 1988;113:631‐6. [DOI] [PubMed] [Google Scholar]
Barranco 2006 {published data only}
- Barranco C. Early use of combination therapy improves outcome of patients with type 2 diabetes. Endocrinology & Metabolism 2006;2(4):186‐7. [Google Scholar]
Berhanu 2007 {published data only}
- Berhanu P, Perez A, Yu S. Effect of pioglitazone in combination with insulin therapy on glycaemic control, insulin dose requirement and lipid profile in patients with type 2 diabetes previously poorly controlled with combination therapy. Diabetes, Obesity & Metabolism 2007;9:512‐20. [DOI] [PubMed] [Google Scholar]
Bianchi 1996 {published data only}
- Bianchi R, Vries DE, Bravenboer B, Erkelens DW. Effect of benfluorex in addition to insulin therapy in obese type II diabetic patients with secondary failure to conventional oral treatment. Diabetes Nutrition Metabolism. 1996;9:81‐8. [Google Scholar]
Buse 1998 {published data only}
- Buse JB, Gumbiner BG, Mathias NP, Nelson DM, Faja BW, Whitcomb RW, Troglitazone Insulin study group. Troglitazone use in insulin‐treated type 2 diabetic patients. Diabetes Care 1998;21:1455‐61. [DOI] [PubMed] [Google Scholar]
Carta 1984 {published data only}
- Carta Q, Trovati M, Dani F, Caselle MT, Vitali S, Cavalot F, et al. Insulin or insulin + oral agents in the treatment of type II diabetics after oral agents failure. [Insulina o insulina + farmaci ipoglicemizzanti orali nella terapia del diabete di tipo II di difficile compenso metabolico]. Minerva Endocrinologia 1984;9:241‐5. [PubMed] [Google Scholar]
Castillo 1987 {published data only}
- Castillo M, Scheen AJ, Paolisso G, Lefebvre PJ. The addition of glipizide to insulin therapy in type‐II diabetic patients with secondary failure to sulfonylureas is useful only in the presence of a significant residual insulin secretion. Acta Endocrinologica 1987;116:364‐72. [DOI] [PubMed] [Google Scholar]
Charbonnel 2010 {published data only}
- Charbonnel B, DeFronzo R, Davidson J, Schmitz O, Birkeland K, Pirags V, et al. Pioglitazone use in combination with insulin in the prospective Pioglitazone Clinical Trial in Macrovascular Events Study (PROactive19). Journal of Clinical Endocrinology & Metabolism 2010;95:2163‐71. [DOI] [PubMed] [Google Scholar]
Chazan 2001 {published data only}
- Chazan ACS, Gomes MB. Glicazide and bedtime insulin are more efficient than insulin alone for type 2 diabetic patients with sulfonylurea secondary failure. Brazilian Journal of Medical and Biological Research 2001;34:49‐56. [DOI] [PubMed] [Google Scholar]
Chow1995 {published data only}
- Chow C, Tsang LWW, Sorensen JP, Cockram CS. Comparison of insulin with or without continuation of oral hypoglycemic agents in the treatment of secondary failure in NIDDM patients. Diabetes Care 1995;18(3):307‐14. [DOI] [PubMed] [Google Scholar]
Clauson 1995 {published data only}
- Clauson P, Karlander S, Steen L, Efendic S. Daytime glibenclamide and bedtime NPH insulin compared to intensive insulin treatment in secondary sulphonylurea failure: a 1‐year follow‐up. Diabetic Medicine 1996;13:471‐7. [DOI] [PubMed] [Google Scholar]
Cortes 1993 {published data only}
- Cortes Aguirre H, Espinosa Lopez FR, Angluo Ververa JA, Diaz Torres J. A comparative study of insulin and glyburide versus glyburide or insulin in the chronic control of patients with type‐2 diabetes [Estudio comparativo de insulina y glyburida contra gliburia o insulina, en el control crónico de pacientes con diabetes tipo II]. Gaceta Medica de Mexico 1993;129(6):383‐6. [PubMed] [Google Scholar]
Dashora 2007 {published data only}
- Dashora UK, Sibal L, Ashwell SG, Home PD. Insulin glargine in combination with nateglinide in people with type 2 diabetes: a randomized placebo‐controlled trial. Diabetic Medicine 2007;24:344‐9. [DOI] [PubMed] [Google Scholar]
Davidson 2006 {published data only}
- Davidson J, Perez A, Zhang J and The Pioglitazone 343 Study group. Addition of pioglitazone to stable insulin therapy in patients with poorly controlled type 2 diabetes: results of a double‐blind, multi‐centre, randomized study. Diabetes, Obesity and Metabolism 2006;8:164‐74. [DOI] [PubMed] [Google Scholar]
De Luis 2001 {published data only}
- Luis DA, Aller R, Cuellar L, Terroba C, Ovalle H, Izaola O, et al. Effect of repaglinide addition to NPH insulin monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2001;24:1844‐5. [DOI] [PubMed] [Google Scholar]
Douek 2005 {published data only}
- Douek IF, Allen SE, Ewings P, Gale EAM, Bingley PJ for the Metformin Trial Group. Continuing metformin when starting insulin in patients with type 2 diabetes: a double‐blind randomized placebo‐controlled trial. Diabetic Medicine 2005;22:634‐40. [DOI] [PubMed] [Google Scholar]
Fonseca 2006 {published data only}
- Fonseca VA, Theuma P, Mundaliar S, Leissinger CA, Clejan S, Henry RR. Diabetes treatments have differential effects on nontraditional cardiovascular risk factors. Journal of Diabetes and Its Complications 2006;20:14‐20. [DOI] [PubMed] [Google Scholar]
Fonseca 2008 {published data only}
- Fonseca V, Baron M, Shao Q, Dejager S. Sustained efficacy and reduced hypoglycaemia during one year of treatment with vildagliptine added to insulin in patients with type 2 diabetes mellitus. Hormone and Metabolic Research 2008;40:427‐30. [DOI] [PubMed] [Google Scholar]
Garg 2007 {published data only}
- Garg R, Gopal J, Jones GR. Rosiglitazone: safety and efficacy in combination with insulin in poorly controlled type2 diabetes mellitus patients treated with insulin alone. Journal of Diabetes and its Complications 2007;21:1‐6. [DOI] [PubMed] [Google Scholar]
Gerstein 2006 {published data only}
- Gerstein HC, Yale JF, Harris SB, Issa M, Stewart JA, Dempsey E. A randomized trial of adding insulin glargine vs. avoidance of insulin in people with type 2 diabetes in either no oral glucose‐lowering agents or submaximal doses of metformin and/or sulfonylureas. The Canadian INSIGHT (Implementing new Strategies with Insulin Glargine for Hyperglycaemia Treatment) Study. Diabetic Medicine 2006;23:736‐42. [DOI] [PubMed] [Google Scholar]
Goudswaard 2004a {published data only}
- Goudswaard AN, Stolk RP, Zuithoff P, Valk HW, Rutten GE. Starting insulin in type 2 diabetes: continue oral glucose lowering agents? A randomized trial in primary care. Journal of Family Practice 2004;53(5):393‐9. [PubMed] [Google Scholar]
Greco 1998 {published data only}
- Greco D, Taravella V, Sinagra D, Galluzzo A. Considerations on combined use of insulin and oral antidiabetic agents in the treatment of type 2 diabetes mellitus [Considerazioni sull'uso della "terapia combinata" insulina + antidiabetici orali nel trattamento del diabete mellito di tipo 2]. L'Internista 1998;6:95‐100. [Google Scholar]
Groop 1984 {published data only}
- Groop L, Harno K, Tolppanen E. The combination of insulin and sulphonylurea in the treatment of secondary drug failure in patients with type II diabetes. Acta Endocrinologica 1984;106:97‐101. [DOI] [PubMed] [Google Scholar]
Groop 1991 {published data only}
- Groop L, Widén E. Treatment strategies for secondary sulfonylurea failure. Should we start insulin or add metformin? Is there place for intermittent insulin therapy?. Diabete & Metabolisme 1991;17:218‐23. [PubMed] [Google Scholar]
Gutniak 1987 {published data only}
- Gutniak M, Karlander S, Efendic S. Glyburide decreases insulin requirement, increases beta‐cell response to mixed meal, and does not affect insulin sensitivity: effects of short‐ and long‐term combined treatment in secondary failure to sulfonylurea. Diabetes Care 1987;10(5):545‐54. [DOI] [PubMed] [Google Scholar]
Hamelbeck 1982 {published data only}
- Hamelbeck H, Klein W, Zoltobrocki M, Schoffling K. Glibenclamide‐insulin combination in the management of secondary failure of sulfonylurea medication [Glibenclamid‐Insulin‐Kombinationsbehandlung beim Sekundarversagen der Sulfonylharnstoff‐Therapie]. Deutsche Medizinische Wochenschrift 1982;107:1581‐3. [DOI] [PubMed] [Google Scholar]
Hermanns 2004 {published data only}
- Hermanns N, Burkert A, Haak T. The addition of acarbose to insulin lispro reduces acute glycaemic responses in patients with type 2 diabetes. Experimental and Clinical Endocrinology and Diabetes 2004;112:310‐4. [DOI] [PubMed] [Google Scholar]
Hollander 2003 {published data only}
- Hollander P, Ratner R, Fineman M, Strobel S, Shen L, Maggs D, et al. Addition of pramlintide to insulin therapy lowers hbA1c in conjunction with weight loss in patients with type 2 diabetes approaching glycaemic targets. Diabetes, Obesity and Metabolism 2003;5:408‐14. [DOI] [PubMed] [Google Scholar]
Hollander 2003a {published data only}
- Hollander PA, Levy P, Fineman MS, Maggs DG, Shen LZ, Strobel SA, et al. Pramlintide as an adjunct to insulin therapy improves long‐term glycemic and weight control in patients with type 2 diabetes. Diabetes Care 2003;26(3):784‐90. [DOI] [PubMed] [Google Scholar]
Hollander 2004 {published data only}
- Hollander P, Maggs DG, Ruggles JA, Fineman M, Shen L, Kolterman OG, et al. Effect of pramlintide on weight in overweight and obese insulin‐treated type 2 diabetes patients. Obesity Research 2004;12(4):661‐8. [DOI] [PubMed] [Google Scholar]
Hollander 2007 {published data only}
- Hollander P, Dahong Y, Chou HS. Low‐dose rosiglitazone in patients with insulin‐requiring type 2 diabetes. Archives of Internal Medicine 2007;167:1284‐90. [DOI] [PubMed] [Google Scholar]
Holman 1987 {published data only}
- Holman RR, Steemson J, Turner RC. Sulfonylurea failure in type 2 diabetes: treatment with a basal insulin supplement. Diabetic Medicine 1987;4:457‐62. [DOI] [PubMed] [Google Scholar]
Home 2007 {published data only}
- Home PD, Bailey CJ, Donaldson J, Chen H, Stewart MW. A double‐blind randomized study comparing the effects of continuing or not continuing rosiglitazone + metformin therapy when starting insulin therapy in people with Type 2 diabetes. Diabetic Medicine 2007;24:618‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Inoue1998 {published data only}
- Inoue M, Satou T, Shimizu T, Tumagari K, Ishihara H, Chino T, et al. Efficacy of sulfonylureas plus α‐glucosidase inhibitor plus bedtime NPH insulin combination therapy in type II diabetes patients with secondary failure using sulfonylureas. Journal of the Japan Diabetes Society 1998;41(8):683‐90. [Google Scholar]
Jacob 2007 {published data only}
- Jacob AN, Salinas K, Adams‐Huet B, Raskin P. Weight gain in type 2 diabetes mellitus. Diabetes, Obesity and Metabolism 2007;9:386‐93. [DOI] [PubMed] [Google Scholar]
Jacober 2006 {published data only}
- Jacober SJ, Scism‐Bacon JL, Zagar AJ. A comparison of intensive mixture therapy with basal insulin therapy in insulin‐naive patients with type 2 diabetes receiving oral antidiabetes agents. Diabetes, Obesity and Metabolism 2006;8:448‐55. [DOI] [PubMed] [Google Scholar]
Janka 2005 {published data only}
- Janka HU, Plewe G, Riddle MC, Kliebe‐Frisch C, Schweitzer MA, Yki‐Jarvinen H. Comparison of basal insulin added to oral agents versus twice‐daily premixed insulin as initial insulin therapy or type 2 diabetes. Diabetes Care 2005;28:254‐9. [DOI] [PubMed] [Google Scholar]
Janka 2007 {published data only}
- Janka HU, Plewe G, Busch K. Combination of oral antidiabetic agents with basal insulin versus premixed insulin alone in randomized elderly patients with type 2 diabetes mellitus. Journal of American Geriatrics Society 2007;55:182‐8. [DOI] [PubMed] [Google Scholar]
Juurinen 2008 {published data only}
- Juurinen L, Kotronen A, Granér M, Yki‐Jarvinen H. Rosiglitazone reduces liver fat and insulin requirements and improves hepatic insulin sensitivity and glycemic control in patients with type 2 diabetes requiring high insulin doses. Journal of Clinical Endocrinology and Metabolism 2008;93(1):118‐24. [DOI] [PubMed] [Google Scholar]
Juurinen 2009 {published data only}
- Juurinen L, Tiikkainen M, Saltevot J, Nikkila K, Lanki H, Leppavuori E. Nateglinide combination therapy with basal insulin and metformin in patients with type 2 diabetes. Diabetic Medicine 2009;26:409‐15. [DOI] [PubMed] [Google Scholar]
Kabadi 2003 {published data only}
- Kabadi MU, Kabadi UM. Efficacy of sulfonylureas with insulin in type 2 diabetes mellitus. The Annals of Pharmacotherapy 2003;37:1572‐6. [DOI] [PubMed] [Google Scholar]
Kanda 1996 {published data only}
- Kanda T, Imano E, Matsushima H, Wada M, Kubota M, Yamasaki Y, et al. The usefulness of combination therapy with preprandial rapid‐acting insulin injections and α‐glucosidase inhibitor in non‐obese NIDDM patients with secondary failure on oral hypoglycemic agents. Journal of Japanese Diabetes Association 1996;39(3):183‐90. [Google Scholar]
Kandalintseva 2008 {published data only}
- Kandalintseva OA, Ametov AS. Effects of combined treatment with rosiglitazone and NPH insulin on carbohydrate metabolism in patients with type 2 diabetes mellitus. Terapevticeskij Arhiv 2008;80(12):40‐4. [PubMed] [Google Scholar]
Karlander 1991 {published data only}
- Karlander SG, Gutniak MKM, Efendic S. Effects of combination therapy with glyburide and insulin on serum lipid levels in NIDDM patients with secondary sulfonylurea failure. Diabetes Care 1991;14:963‐7. [DOI] [PubMed] [Google Scholar]
Kilo 2003 {published data only}
- Kilo C, Mezitis N, Jain R, Mersey J, McGill J, Raskin P. Starting patients with type 2 diabetes on insulin therapy using once‐daily injections of biphasic insulin aspart 70/30, biphasic human insulin 70/30, or NPH insulin in combination with metformin. Journal of Diabetes and Its Complications 2003;17:307‐13. [DOI] [PubMed] [Google Scholar]
Kokic 2003 {published data only}
- Kokic S, Bukovic D, Radman M, Capkun V, Gabric N, Lesko V, et al. Lispro insulin and metformin versus other combination in the diabetes mellitus type 2 management after secondary oral antidiabetic drug failure. Collegium Antropologicum 2003;27:181‐7. [PubMed] [Google Scholar]
Kothny 2013 {published data only}
- Kothny W, Foley J, Kozlovski P, Shao Q, Gallwitz B, Lukashevich V. Improved glycaemic control with vildagliptin added to insulin, with or without metformin, in patients with type 2 diabetes mellitus. Diabetes, Obesity & Metabolism 2013;15(3):252‐7. [PUBMED: 23039321] [DOI] [PubMed] [Google Scholar]
Kvapil 2006 {published data only}
- Kvapil M, Swatko A, Hilberg C, Shestakova M. Biphasic insulin aspart 30 plus metformin: an effective combination in type 2 diabetes. Diabetes, Obesity and Metabolism 2006;8:39‐48. [DOI] [PubMed] [Google Scholar]
LAPToP 2004 {published data only}
- LAPToP Studie. [Lantus in der BOT ist dem Standard‐Mischinsulin uberlegen]. Krankenpflege 2004; Vol. 42:172. [PubMed]
Lins 1988 {published data only}
- Lins P, Lundland S, Persson‐Trotzig E, Adamson U. Glibenclamide improves the response to insulin treatment in non‐insulin‐dependent diabetics with second failure to sulfonylurea therapy. Acta Medica Scandinavica 1988;223:171‐9. [DOI] [PubMed] [Google Scholar]
Liu 2003 {published data only}
- Liu HX, Qin AP, He BX. Clinical observation of efficacy of rosiglitazone combined with insulin in treatment of elderly type 2 diabetes mellitus. Journal of Chinese Physician 2003;5(11):1502‐4. [Google Scholar]
Lotz 1988 {published data only}
- Lotz N, Bachmann W, Ladik T, Mehnert H. The combination of insulin and sulfonylureas in the treatment of type 2 diabetes with 'secondary failure of sulfonylurea therapy' ‐ long term results [Die Kombinationstherapie Insulin/Sulfonylharnstoff in der Langzeittherapie des type‐II‐Diabetes nach 'Sekundarversagen']. Klinische Wochenschrift 1988;66:1079‐84. [DOI] [PubMed] [Google Scholar]
Lund 2009 {published data only}
- Lund SS, Tarnow L, Frandsen M, Nielsen BB, Hansen BV, Pedersen O, et al. Combining insulin with metformin or an insulin secretagogue in non‐obese patients with type 2 diabetes: 12 month, randomised, double blinded trial. BMJ 2009;339(b4324):DOI:10.1136/bmj.b4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundershausen 1987 {published data only}
- Lundershausen R, Orban S, Pissarek D, Panzram G. Long‐term effect of glibenclamide‐insulin combination therapy in the management of secondary drug failure ‐ results of a double‐blind one‐year follow‐up study [Langzeiteffekt der glibenclamid‐insulin‐kombinationsbehandlung beim sekundarversagen der sulfonylharnstofftherapie ‐ ergebnisse einer einjahrigen doppelblindstudie]. Wiener Klinische Wochenschrift 1987;99:603‐8. [PubMed] [Google Scholar]
Makimattila 1999 {published data only}
- Makimatilla S, Nikkila K, Yki‐Jarvinen H. Causes of weight gain during insulin therapy with and without metformin in patients with type II diabetes mellitus. Diabetologia 1999;42:406‐12. [DOI] [PubMed] [Google Scholar]
Mohan 1990 {published data only}
- Mohan V, Snehalatha C, Ramachandran A, Viswanathan M. Combination therapy of glibenclamide and insulin in NIDDM patients with secondary failure to oral drugs. Journal of the Association of Physicians of India 1990;38:537‐40. [PubMed] [Google Scholar]
Morrow 2011 {published data only}
- Morrow L, Hompesch M, Guthrie H, Chang D, Chatterjee DJ. Co‐administration of liraglutide with insulin detetmir demonstrates additive pharmacodynamic effects with no pharmacokinetic interaction. Diabetes, Obesity and Metabolism 2011;13:75. [DOI] [PubMed] [Google Scholar]
Olsson 2002 {published data only}
- Olsson PO, Lindstrom T. Combination‐therapy with bedtime NPH insulin and sulphonylurea gives similar glycaemic control but lower weight gain than insulin twice daily in patients with type 2 diabetes. Diabetes and Metabolism 2002;28:272‐7. [PubMed] [Google Scholar]
Ose 2005 {published data only}
- Ose H, Fukui M, Kitagawa Y, Hirata C, Ichio N, Kadono M, et al. Efficacy of glimepiride in patients with poorly controlled insulin‐treated type 2 diabetes mellitus. Endocrine Journal 2005;52(5):563‐9. [DOI] [PubMed] [Google Scholar]
Ozbek 2006 {published data only}
- Ozbek M, Erdogan M, Karadeniz M, Cetinkalp S, Ozgen AG, Saygili F, et al. Preprandial repaglinide decreases exogenous insulin requirements and HbA1c levels in type 2 diabetic patients taking intensive insulin treatment. Acta Diabetologica 2006;43:148‐51. [DOI] [PubMed] [Google Scholar]
Pan 2007 {published data only}
- Pan C, Sinnassamy P, Chung K, Kim K, on behalf of the LEAD Study Investigators Group. Insulin glargine versus NPH insulin therapy ins Asian type 2 diabetes patients. Diabetes Research and Clinical Practice 2007;76:111‐8. [DOI] [PubMed] [Google Scholar]
Peacock 1984 {published data only}
- Peacock I, Tattersall RB. The difficult choice of treatment for poorly controlled maturity onset diabetes: tablets or insulin?. British Medical Journal 1984;288:1956‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peyrot 2008 {published data only}
- Peyrot M, Rubin RR, Polonsky WH. Diabetes distress and its association with clinical outcomes in patients with type 2 diabetes treated with pramlintide as an adjunct to insulin therapy. Diabetes Technology & Therapeutics 2008;10(6):461‐6. [DOI] [PubMed] [Google Scholar]
Ponssen 2000 {published data only}
- Ponssen HH, Elte JWF, Lehert P, Schouten JP, Bets D. Combined metformin and insulin therapy for patients with type 2 diabetes mellitus. Clinical Therapeutics 2000;22(6):709‐18. [DOI] [PubMed] [Google Scholar]
Pontiroli 1990 {published data only}
- Pontiroli AE, Dino G, Capra F, Pozza G. Combined therapy with glibenclamide and ultralente insulin in lean patients with NIDDM with secondary failure of sulfonylureas. Follow up at two years. Diabete & Metabolisme 1990;16:323‐7. [PubMed] [Google Scholar]
Poulsen 2003 {published data only}
- Poulsen MK, Henriksen JE, Hother‐Nielsen O, Beck‐Nielsen H. The combined effect of triple therapy with rosiglitazone, metformin, and insulin aspart in type 2 diabetic patients. Diabetes Care 2003;26(12):3273‐9. [DOI] [PubMed] [Google Scholar]
Raskin 2001 {published data only}
- Raskin P, Rendell M, Riddle MC, Dole JF, Freed MI, Rosenstock J, for the Rosiglitazone Clinical Trials Study Group. A randomized trial of rosiglitazone therapy inpatients with inadequately controlled insulin‐treated type 2 diabetes. Diabetes Care 2001;24:1226‐32. [DOI] [PubMed] [Google Scholar]
Ravnik 1989 {published data only}
- Ravnik‐Oblak M. The rationality and effectiveness of insulin therapy in elderly patients with type II diabetes. Diabetologia Croatica 1989;18:163‐9. [Google Scholar]
Ravnik 1995 {published data only}
- Ravnik‐Oblak M, Mrevlje F. insulin versus a combination of insulin and sulfonylurea in the treatment of NIDDM patients with secondary oral failure. Diabetes Research and Clinical Practice 1995;30:27‐35. [DOI] [PubMed] [Google Scholar]
Raz 2005 {published data only}
- Raz I, Stranks S, Filipczak R, Joshi P, Lertoft B, Rastam J, et al. Efficacy and safety of biphasic insulin aspart 30 combined with pioglitazone in type 2 diabetes poorly controlled on glibenclamide (glyburide) monotherapy or combination therapy: an 18‐week randomized, open‐label study. Clinical Therapeutics 2005;27(9):1432‐43. [DOI] [PubMed] [Google Scholar]
Riddle 1989 {published data only}
- Riddle MC, Hart JS, Bouma DJ, Phillipson BE, Youker G. Efficacy of bedtime NPH insulin with daytime sulfonylurea for subpopulation of type II diabetic subjects. Diabetes Care 1989;12(9):623‐9. [DOI] [PubMed] [Google Scholar]
Riddle 1992 {published data only}
- Riddle M, Hart J, Bingham P, Garrison C, McDaniel P. Combined therapy for obese type 2 diabetes: suppertime mixed insulin with daytime sulfonylurea. American Journal of Medical Sciences 1992;303(3):151‐6. [DOI] [PubMed] [Google Scholar]
Riddle 1998 {published data only}
- Riddle MC, Schneider J, The Glimepiride Combination Group. Beginning insulin treatment of obese patients with evening 70/30 insulin plus glimepiride versus insulin alone. Diabetes Care 1998;21(7):1052‐7. [DOI] [PubMed] [Google Scholar]
Riddle 2007 {published data only}
- Riddle M, Frias J, Zhang B, Maier H, Brown C, Lutz K, et al. Pramlintide improved glycemic control and reduced weight in patients with type 2 diabetes using basal insulin. Diabetes care 2007;30(11):2794‐9. [DOI] [PubMed] [Google Scholar]
Rosak 1985 {published data only}
- Rosak C, Schwarz O, Althoff PH, Schoffling K, Schmidt FH. Combination treatment of type II diabetics with insulin and glibenclamide following secondary failure [Kombinierte behandlung von typ‐II‐diabetikern mit insulin und glibenclamid nach tablettensekundarversagen]. Deutsche Medizinische Wochenschrift 1985;110:1975‐80. [DOI] [PubMed] [Google Scholar]
Sachse 1984 {published data only}
- Sachse G, Maser E, Federlin K. Combination of insulin and glibenclamide in secondary failure of sulphonylurea treatment? [Kombinationstherapie mit insulin und sulfonylharnstoffen bei sekundarversagen der sulfonylharnstofftherapie?]. Deutsche Medizinische Wochenschrift 1984;109:419‐21. [DOI] [PubMed] [Google Scholar]
Sangiorgio 2000 {published data only}
- Sangiorgio L, Attardo T, Condorelli L, Lunetta M. Effects of the treatment with acarbose in elderly overweight type 2 diabetic patients in poor glycemic control with oral hypoglycemic agents or insulin. Archives Gerontology and Geriatrics 2000;31:27‐34. [DOI] [PubMed] [Google Scholar]
Schmidt 1986 {published data only}
- Schmidt FH, Klujko J, Kuhnle HF, Reiter J. The mode of action of the combination glibenclamide/insulin in type‐II‐diabetics [Untersuchungen zum wirkungsmechanismus de kombinierten gabe von glibenclamid und insulin bei type‐II‐diabetikern mit sekundarversagen auf die orale behandlung]. Wiener Klinische Wochenschrift 1986;64:1021‐28. [DOI] [PubMed] [Google Scholar]
Schnell 2006 {published data only}
- Schnell O, Mertes G, Standl E, on behalf of the Acarbose‐Insulin Combination Study Group. Acarbose and metabolic control in patients with type 2 diabetes with newly initiated insulin therapy. Diabetes, Obesity and Metabolism 2007;9:853‐8. [DOI] [PubMed] [Google Scholar]
Schwartz 1998 {published data only}
- Schwartz S, Raskin P, Fonseca V, Graveline JF, Troglitazone and Exogenous Insulin Study Group. Effect of troglitazone in insulin‐treated patients with type II diabetes mellitus. New England Journal of Medicine 1998;338:861‐6. [DOI] [PubMed] [Google Scholar]
Sun 1995 {published data only}
- Sun Y, Xiong Y, Yang J, et al. The effectiveness of combined insulin and sulfonylurea in treating non‐insulin dependent diabetic patients. Zhonghua Nei Ke Za Zhi 1995;34(4):246‐9. [PubMed] [Google Scholar]
Tamemoto 2007 {published data only}
- Tamemoto H, Ikoma A, Saitoh T, Ishikawa A, Kawakami M. Comparison of once‐daily glargine plus sulfonylurea with twice‐daily 70/30 aspart premix in insulin‐naive Japanese patients with diabetes. Diabetes Technology & Therapeutics 2007;9(3):246‐53. [DOI] [PubMed] [Google Scholar]
Vilsboll 2010 {published data only}
- Vilsboll T, Rosenstock J, Yki‐Jarvinen H, Cefalu WT, Chen Y, Luo E, et al. Efficacy and safety of sitagliptin when added to insulin therapy in patients with type 2 diabetes. Diabetes, Obesity and Metabolism 2010;12:167‐77. [DOI] [PubMed] [Google Scholar]
Willey 1994 {published data only}
- Willey KA, Molyneaux LM, Yue DK. Obese patients with type 2 diabetes poorly controlled by insulin and metformin: effects of adjunctive dexflenfluramine therapy on glycaemic control. Diabetic Medicine 1994;11:701‐4. [DOI] [PubMed] [Google Scholar]
Wolffenbuttel 1991 {published data only}
- Wolffenbuttel BHR, Rondas‐Colbers GJWM, Menheere PPCA, Sels JPJE, Nieuwenhuijzen Kruseman AC. The effects of insulin in combination with glibenclamide on glucose and lipid metabolism in patients with type II diabetes mellitus [De effecten van insuline in combinatie met glibenclamide op glucose‐ en vetstofwisseling bij patiënten met diabetes mellitus type II]. Nederlands Tijdschrift Voor Geneeskunde 1991;135(24):1080‐4. [PubMed] [Google Scholar]
Wolffenbuttel 1999 {published data only}
- Wolffenbuttel BHR, Sels JPJE, Rondas‐Colbers JWM, Menheere PPCA. Prognostic factors for successful insulin therapy in subjects with type 2 diabetes. Netherlands Journal of Medicine 1999;54:63‐9. [DOI] [PubMed] [Google Scholar]
Wong 2005 {published data only}
- Wong TY, Szeto C, Chow K, Leung C, Lam CW, Li PK. Rosiglitazone reduces insulin requirement and C‐reactive protein levels in type 2 diabetic patients receiving peritoneal dialysis. American Journal of Kidney Diseases 2005;46(4):713‐9. [DOI] [PubMed] [Google Scholar]
Wright 2002 {published data only}
- Wright A, Burden ACF, Paisey RB, Cull CA, Holman RR for the UK Prospective Diabetes Study group. Sulfonylurea inadequacy. Efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the U.K. Prospective Diabetes Study (UKPDS 57). Diabetes Care 2002;25:330‐6. [DOI] [PubMed] [Google Scholar]
Yamada 2007 {published data only}
- Yamada S, Watanabe M, Funae O, Atsumi Y, Suzuki R, Yajima K, et al. Effect of combination therapy of a rapid‐acting insulin secretagogue (glinide) with premixed insulin in type 2 diabetes mellitus. Internal Medicine 2007;46.0415:1893‐7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Yki‐Jarvinen 1992 {published data only}
- Yki‐Jarvinen H, Kauppila M, Kujansuu E, Lahti J, Marjanen T, Niskanen L, et al. Comparison of insulin regimens in patients with non‐insulin‐dependent diabetes mellitus. New England Journal of Medicine 1992;327:1426‐33. [DOI] [PubMed] [Google Scholar]
Yki‐Jarvinen 1999 {published data only}
- Yki‐Jarvinen H, Ryysy L, Nikkila K, Tulokas T, Vanamo R, Heikkila M. Comparison of bedtime insulin regimens in patients with type 2 diabetes mellitus. Annals of Internal Medicine 1999;130(5):389‐96. [DOI] [PubMed] [Google Scholar]
Yki‐Jarvinen 2013 {published data only}
- Yki‐Jarvinen H, Duran‐Garcia S, Pinnetti S, Bhattacharya S, Thiemann S, Patel S, et al. Efficacy and safety of linagliptin as add‐on therapy to basal insulin in patients with type 2 diabetes. Canadian Journal of Diabetes 2012;1:S39. [Google Scholar]
- Yki‐Jarvinen H, Rosenstock J, Duran‐Garcia S, Pinnetti S, Bhattacharya S, Thiemann S, et al. Effects of adding linagliptin to basal insulin regimen for inadequately controlled type 2 diabetes: a >/=52‐week randomized, double‐blind study. Diabetes care 2013;36(12):3875‐81. [PUBMED: 24062327] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yokoyama 2011 {published data only}
- Yokoyama H, Sone H, Yamada D, Honjo J, Haneda M. Contribution of glimepiride to basal‐prandial insulin therapy in patients with type 2 diabetes. Diabetes Research and Clinical Practice 2011;91:148‐53. [DOI] [PubMed] [Google Scholar]
Zargar 2002 {published data only}
- Zargar AH, Masoodi SR, Laway BA, Wani AI, Bashir MI. Response to regimens of insulin therapy in type 2 diabetes mellitus subjects with secondary failure. Journal of the Association of Physicians of India 2002;50:641‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
Fargren 2014 {published data only}
- Farngren J, Persson M, Schweizer A, Foley J, Ahren B. Glucagon dynamics during hypoglycaemia and food‐re‐challenge following treatment with vildagliptin in insulin‐treated patients with type 2 diabetes. Diabetes, Obesity & Metabolism 2014;16:812‐8. [DOI] [PubMed] [Google Scholar]
Kaku 2014 {published data only}
- Kaku K, Mori M, Kanoo T, Katou M, Seino Y. Efficacy and safety of alogliptin added to insulin in Japanese patients with type 2 diabetes: a randomized, double‐blind, 12‐week, placebo‐controlled trial followed by an open‐label, long‐term extension phase. Expert Opinion on Pharmacotherapy 2014;15:2121‐30. [DOI] [PubMed] [Google Scholar]
Mathieu 2015 {published data only}
- Mathieu C, Shankar R, Lorber D, Umpierrez G, Wu F, Xu L, et al. A randomized clinical trial to evaluate the efficacy and safety of co‐administration of sitagliptin with intensively titrated insulin glargine. Diabetes Therapy 2015;6:127‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
McGill 2015 {published data only}
- McGill J, Yki‐Jarvinen H, Crowe S, Woerle HJ, Eynatten M. Combination of the dipeptidyl peptidase‐4 inhibitor linagliptin with insulin‐based regimens in type 2 diabetes and chronic kidney disease. Diabetes & Vascular Disease Research 2015;12:249‐57. [DOI] [PubMed] [Google Scholar]
- McGill JB, Yki‐Jarvinen H, Duran‐Garcia S, Thiemann S, Hehnke U, Pinnetti S, et al. Efficacy and safety of linagliptin as add‐on to basal insulin in patients with type 2 diabetes and renal impairment. Diabetes 2014;63:A264. [Google Scholar]
Ning 2014 {published data only}
- Ning G, Li L, Ma J, Lv X, Yang M, Wang W, et al. Vildagliptin as add‐on to insulin improves glycaemic control without increased risk of hypoglycaemia in Asian (predominantly Chinese) patients with type 2 diabetes mellitus. Diabetologia 2014;57:S330. [DOI] [PubMed] [Google Scholar]
- Ning G, Wang W, Li L, Ma J, Lv X, Yang M, et al. Vildagliptin as an add‐on therapy to insulin improves glycemic control without an increased risk of hypoglycemia: a dedicated Asian study, in a predominantly Chinese population. Diabetes 2014;63:A274. [DOI] [PubMed] [Google Scholar]
Rosenstock 2009 {published data only}
- Rosenstock J, Rendell MS, Gross JL, Fleck PR, Wilson CA, Mekki Q. Alogliptin added to insulin therapy in patients with type 2 diabetes reduces HbA1c without causing weight gain or increased hypoglycaemia. Diabetes, Obesity and Metabolism 2009;11:1145‐52. [DOI] [PubMed] [Google Scholar]
Sato 2015 {published data only}
- Sato S, Saisho Y, Kou K, Meguro S, Tanaka M, Irie J, et al. Efficacy and safety of sitagliptin added to insulin in Japanese patients with type 2 diabetes: the EDIT randomized trial. PLoS ONE 2015;10:e0121988. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheu 2015 {published data only}
- Sheu WH, Park S, Gong Y, Pinnetti S, Bhattacharya S, Patel S, et al. Linagliptin improves glycaemic control after 1 year as add‐on therapy to basal insulin in Asian patients with type 2 diabetes mellitus. Current Medical Research and Opinion 2015;31:503‐12. [DOI] [PubMed] [Google Scholar]
Srivanichakorn 2015 {published data only}
- Srivanichakorn W, Sriwijitkamol A, Kongchoo A, Sriussadaporn S, Plengvidhya N, Lertwattanarak R, et al. Withdrawal of sulfonylureas from patients with type 2 diabetes receiving long‐term sulfonylurea and insulin combination therapy results in deterioration of glycemic control: a randomized controlled trial. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 2015;8:137‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Takahashi 2015 {published data only}
- Takahashi H, Sakai K, Kawanishi K, Suzuki J, Igawa K, Sankoda K, et al. Efficacy of switching from premix analog insulin twice daily injection to insulin glargine once daily injection with sitagliptin. Diabetology International 2015;6:33‐8. [Google Scholar]
References to ongoing studies
EUCTR2011‐004622‐96‐ES {published data only}
- EUCTR2011‐004622‐96‐ES. Study to test the safety, tolerability, and effectiveness of sitagliptin when compared to placebo in reducing the amount of insulin (with or without metformin) needed per day, to control blood sugar, over a 24‐week period. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2011‐004622‐96‐ES (accessed 28 April 2016).
IRCT201204229536N1 {published data only}
- IRCT201204229536N1. The comparison glycemic control in two group patient treated with insulin alone and insulin plus sulfunyl urea. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201204229536N1 (accessed 28 April 2016).
JPRN‐UMIN000011851 {published data only}
- JPRN‐UMIN000011851. The efficacy of vildagliptin on type 2 diabetic patients with basal‐bolus insulin therapy in the short‐term hospitalization (single‐center, randomized, open‐label, controlled study). http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000011851 (accessed 28 April 2016).
JPRN‐UMIN000018784 {published data only}
- JPRN‐UMIN000018784. Comparison of the effects of insulin monotherapy and combination therapy with ipragliflozin and insulin on glucose toxicity in type 2 diabetes mellitus : a randomized controlled trial. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000018784 (accessed 28 April 2016).
NCT00329225 {published data only}
- NCT00329225. Rosiglitazone in subjects with type 2 diabetes mellitus who are inadequately controlled on insulin. https://clinicaltrials.gov/ct2/show/NCT00329225 (accessed 28 April 2016).
NCT00757588 {published data only}
- NCT00757588. Safety and efficacy of saxagliptin plus insulin with or without metformin. https://clinicaltrials.gov/ct2/show/NCT00757588 (accessed 28 April 2016).
NCT02104804 {published data only}
- NCT02104804. Evaluate the efficacy and safety of saxagliptin added to insulin monotherapy or to insulin combined with metformin in Chinese subjects with type 2 diabetes who have inadequate glycaemic control. https://clinicaltrials.gov/ct2/show/NCT02104804 (accessed 28 April 2016).
Additional references
ACCORD 2008
- The Action to Control Cardiovascular Risk in Diabetes Study Group. Effects of intensive glucose lowering in type 2 diabetes. New England Journal of Medicine 2008;358(24):2545‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
ADA 1997
- American Diabetes Association. Report on the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1997;20 Suppl 1:S5‐20. [DOI] [PubMed] [Google Scholar]
ADA 1999
- The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1999;22 Suppl 1:S5‐19. [DOI] [PubMed] [Google Scholar]
ADVANCE 2008
- The ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. New England Journal of Medicine 2008;358(24):2560‐72. [DOI] [PubMed] [Google Scholar]
Armitage 2002
- Armitage P, Berry G, Matthews JNS. Paragraph 5.3. Statistical Methods in Medical Research. 4th Edition. Blackwell Science, 2002. [Google Scholar]
Brunton 2005
- Brunton S, Carmichael B, Funnell M, Lorber D, Rakel R, Rubin R. Type 2 diabetes: the role of insulin. The Journal of Family Practice 2005;54(5):445‐52. [PubMed] [Google Scholar]
De Grauw 2001
- Grauw WJ, Lisdonk EH, Gerwen WH, Hoogen HJ, Weel C. Insulin therapy in poorly controlled type 2 diabetic patients: does it affect quality of life?. British Journal of General Practice 2001;51(468):527‐32. [PMC free article] [PubMed] [Google Scholar]
De Sonnaville 1998
- Sonnaville JJ, Snoek FJ, Colly LP, Deville W, Wijkel D, Heine RJ. Well‐being and symptoms in relation to insulin therapy in type 2 diabetes. Diabetes Care 1998;21(6):919‐24. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Delahanty 2007
- Delahanty LM, Grant RW, Wittenberg E, Bosch JL, Wexler DJ, Cagliero E, et al. Association of diabetes‐related emotional distress with diabetes treatment in primary care patients with type 2 diabetes. Diabetic Medicine 2007;24(1):48–54. [DOI] [PubMed] [Google Scholar]
Duckworth 2009
- Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. New England Journal of Medicine 2009;360(2):129‐39. [DOI] [PubMed] [Google Scholar]
Gangji 2007
- Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM. A systematic review and meta‐analysis of hypoglycaemia and cardiovascular events. Diabetes Care 2007;30(2):389‐94. [DOI] [PubMed] [Google Scholar]
Goddijn 1999
- Goddijn PP, Bilo HJ, Feskens EJ, Groeniert KH, Zee KI, Meyboom‐de Jong B. Longitudinal study on glycaemic control and quality of life in patients with type 2 diabetes mellitus referred for intensified control. Diabetes Medicine 1999;16(1):23‐30. [DOI] [PubMed] [Google Scholar]
Goudswaard 2004b
- Goudswaard AN, Furlong NJ, Rutten GE, Stolk RP, Valk GD. Insulin monotherapy versus combinations of insulin with oral glucose lowering agents in patients with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD003418.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society. Series A, (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org..
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Holman 2008
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10‐year follow‐up of intensive glucose control in type 2 diabetes. New England Journal of Medicine 2008;359(15):1577‐89. [DOI] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hunt 1997
- Hunt LM, Valenzuela MA, Pugh JA. NIDDM patients' fears and hopes about insulin therapy. The basis of patient reluctance. Diabetes Care 1997;20(3):292‐8. [DOI] [PubMed] [Google Scholar]
Inzucchi 2012
- Inzucchi SE, Bergenstal RM, Buse JB. Management of hyperglycemia in type 2 diabetes: a patient‐centered approach: position statement of the American Diabetes Association (ADA)and the European Association for the study of Diabetes (EASD). Diabetes Care 2012;35:1364‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 1996
- Johnson JL, Wolf SL, Kabadi UM. Efficacy of insulin and sulfonylurea combination therapy in type II diabetes. A meta‐analysis of the randomized placebo‐controlled trials. Archives of Internal Medicine 1996;156(3):259‐64. [PubMed] [Google Scholar]
Karlsen 2014
- Karlsen B, Bru E. The relationship between diabetes‐related distress and clinical variables and perceived support among adults with type 2 diabetes: a prospective study. International Journal of Nursing Studies 2014;51:438‐47. [DOI] [PubMed] [Google Scholar]
Kooy 2009
- Kooy A, Jager J, Lehert P, Bets D, Wulffele MG, Donker AJM, et al. Long‐term effects of metformin on metabolism and microvascular and macrovascular disease in patients With type 2 diabetes mellitus. Archives of Internal Medicine 2009;169(9):616‐25. [DOI] [PubMed] [Google Scholar]
Korytkowski 2002
- Korytkowski M. When oral agents fail: practical barriers to starting insulin. International Journal of Obesity Related Metabolic Disorders 2002;26 Suppl 3:S18‐24. [DOI] [PubMed] [Google Scholar]
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326:1167‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muis 2005
- Muis MJ, Bots ML, Bilo HJ, Hoogma RP, Hoekstra JB, Grobbee DE. High cumulative insulin exposure: a risk factor of atherosclerosis in type 1 diabetes?. Atherosclerosis 2005;181(1):185‐92. [DOI] [PubMed] [Google Scholar]
Peters 1991
- Peters AL, Davidson MB. Insulin plus a sulfonylurea agent for treating type 2 diabetes. Annals of Internal Medicine 1991;115(1):45‐53. [DOI] [PubMed] [Google Scholar]
Pugh 1992
- Pugh JA, Wagner ML, Sawyer J, Ramirez G, Tuley M, Friedberg SJ. Is combination sulfonylurea and insulin therapy useful in NIDDM patients? A meta analysis. Diabetes Care 1992;15(8):953‐9. [DOI] [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Ruige 1998
- Ruige JB, Assendelft WJ, Dekker JM, Kostense PJ, Heine RJ, Bouter LM. Insulin and risk of cardiovascular disease: a meta‐analysis. Circulation 1998;97(10):996‐1001. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Snoek 2002
- Snoek FJ. Breaking the barriers to optimal glycaemic control‐‐what physicians need to know from patients' perspectives. International Journal of Clinical Practice 2002;129(Supplement):80‐4. [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Stout 1990
- Stout RW. Insulin and atheroma. 20‐yr perspective. Diabetes Care 1990;13(6):631‐54. [DOI] [PubMed] [Google Scholar]
Turner 1999
- Turner RC, Cull CA, Frighi V, Holman RR. Glycaemic control with diet, sulfonylurea, metformin or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). UK Prospective Diabetes Study (UKPDS) Group. Journal of American Medical Association 1999;281(21):2005‐12. [DOI] [PubMed] [Google Scholar]
UKPDS 33
- UK Prospective Diabetes Study. Intensive blood‐glucose control with sulfonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837‐53. [PubMed] [Google Scholar]
UKPDS 34
- UK Prospective Diabetes Study (UKPDS). Effect of intensive blood‐glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet 1998;352(9131):854‐65. [PubMed] [Google Scholar]
WHO 1980
- World Health Organization (WHO) Expert Committee on Diabetes Mellitus. Second report. Technical Report Series 646. World Health Organization, Geneva 1980. [PubMed]
WHO 1985
- World Health Organization (WHO) Expert Committee on Diabetes Mellitus. Technical Report Series 727. World Health Organization, Geneva 1985. [PubMed]
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman D, G, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yki‐Jarvinen 2001
- Yki‐Jarvinen H. Combination therapies with insulin in type 2 diabetes. Diabetes Care 2001;24(4):758‐67. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Van Avendonk 2008
- Avendonk MJ, Gorter K, Goudswaard AN, Rutten GEHM, Donk M. Combinations of insulin and oral hypoglycaemic agents for people with type 2 diabetes mellitus on insulin treatment. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006992] [DOI] [Google Scholar]


