Abstract
Background
Preterm infants (< 37 weeks' postmenstrual age) are often delayed in attaining oral feeding. Normal oral feeding is suggested as an important outcome for the timing of discharge from the hospital and can be an early indicator of neuromotor integrity and developmental outcomes. A range of oral stimulation interventions may help infants to develop sucking and oromotor co‐ordination, promoting earlier oral feeding and earlier hospital discharge.
Objectives
To determine the effectiveness of oral stimulation interventions for attainment of oral feeding in preterm infants born before 37 weeks' postmenstrual age (PMA).
To conduct subgroup analyses for the following prespecified subgroups.
• Extremely preterm infants born at < 28 weeks' PMA.
• Very preterm infants born from 28 to < 32 weeks' PMA.
• Infants breast‐fed exclusively.
• Infants bottle‐fed exclusively.
• Infants who were both breast‐fed and bottle‐fed.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE via PubMed (1966 to 25 February 2016), Embase (1980 to 25 February 2016) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to 25 February 2016). We searched clinical trials databases, conference proceedings and the reference lists of retrieved articles.
Selection criteria
Randomised and quasi‐randomised controlled trials comparing a defined oral stimulation intervention with no intervention, standard care, sham treatment or non‐oral intervention in preterm infants and reporting at least one of the specified outcomes.
Data collection and analysis
One review author searched the databases and identified studies for screening. Two review authors screened the abstracts of these studies and full‐text copies when needed to identify trials for inclusion in the review. All review authors independently extracted the data and analysed each study for risk of bias across the five domains of bias. All review authors discussed and analysed the data and used the GRADE system to rate the quality of the evidence. Review authors divided studies into two groups for comparison: intervention versus standard care and intervention versus other non‐oral or sham intervention. We performed meta‐analysis using a fixed‐effect model.
Main results
This review included 19 randomised trials with a total of 823 participants. Almost all included trials had several methodological weaknesses. Meta‐analysis showed that oral stimulation reduced the time to transition to oral feeding compared with standard care (mean difference (MD) ‐4.81, 95% confidence interval (CI) ‐5.56 to ‐4.06 days) and compared with another non‐oral intervention (MD ‐9.01, 95% CI ‐10.30 to ‐7.71 days), as well as the duration of initial hospitalisation compared with standard care (MD ‐5.26, 95% CI ‐7.34 to ‐3.19 days) and compared with another non‐oral intervention (MD ‐9.01, 95% CI ‐10.30 to ‐7.71 days).
Investigators reported shorter duration of parenteral nutrition for infants compared with standard care (MD ‐5.30, 95% CI ‐9.73 to ‐0.87 days) and compared with another non‐oral intervention (MD ‐8.70, 95% CI ‐15.46 to ‐1.94 days). They could identify no effect on breast‐feeding outcomes nor on weight gain.
Authors' conclusions
Although the included studies suggest that oral stimulation shortens hospital stay, days to exclusive oral feeding and duration of parenteral nutrition, one must interpret results of these studies with caution, as risk of bias and poor methodological quality are high overall. Well‐designed trials of oral stimulation interventions for preterm infants are warranted. Such trials should use reliable methods of randomisation while concealing treatment allocation, blinding caregivers to treatment when possible and paying particular attention to blinding of outcome assessors.
Plain language summary
Effects of oral stimulation for oral feeding in preterm infants
Review questions
Do oral stimulation interventions that involve finger stimulation protocols in preterm infants born before 37 weeks' gestation:
• reduce time taken to achieve exclusive oral feeding and time spent in hospital?
• result in exclusive oral feeding, exclusive breast feeding or any direct breast feeding?
• increase sucking strength?
• increase rate of growth and improve development?
Background
Many preterm infants have delayed establishment of oral (suck) feeding and are fed at first with feeding tubes or with intravenous (parenteral) nutrition. Development of oral feeding skills needs careful co‐ordination of sucking, swallowing and breathing. In preterm infants, the development of oral feeding can be challenging because of long hospitalisations, breathing difficulties and other medical conditions associated with preterm birth. Unpleasant procedures such as ventilation or frequent suctioning of secretions from the mouth or nose can negatively impact feeding skills. International guidelines for the transition from tube feeding to oral feeding vary widely. Healthcare providers use a range of interventions to improve sucking and feeding skills in preterm infants, and studies report faster transition time from tube feeds to oral feeds, reduced length of stay in hospital and improvement in infants' sucking skills. No Cochrane review has assessed the intervention involving finger stimulation of the mouth before and during feeds.
Study characteristics
This review included randomised controlled trials (RCTs) that explored oral stimulation by finger stimulation only in preterm infants. Review authors identified studies to be included by searching electronic databases, clinical trials registers, peer‐reviewed journals and published conference proceedings.
Key results
We included 19 studies of poor quality with small numbers of participants. Study findings suggest that oral stimulation interventions can shorten the transition to oral feeding, reduce length of hospital stay and decrease time spent on parenteral nutrition. No studies looked at longer‐term outcomes of the interventions (i.e. beyond six months). Studies have reported no effect on breast feeding outcomes nor on weight gain.
Quality of evidence
These studies were small and most were of low or very low methodological quality. Review authors identified no high‐quality studies that could support the efficacy, effectiveness and safety of oral stimulation interventions. Larger, well‐designed RCTs are needed to help inform parents and caregivers about the possible benefits and harms of this intervention.
Summary of findings
Background
Preterm infants, particularly very preterm (< 32 weeks') infants, often have substantial delays in attaining independent oral feeding (American Academy of Pediatrics 2008; Eichenwald 2001; Engle 2007; Jadcherla 2010). Acquiring the skills needed for safe oral feeding is a complex process, and very preterm infants frequently have lengthy initial hospital stays until they can demonstrate the ability to show feeding and satiation cues; sustain suck, swallow and breathing throughout oral feeding; and maintain nutritional intake to support growth and development (Lau 2000a; Lau 2011; MacMullen 2000; Premji 2004). Several factors help to promote maturation, including practice, co‐ordination, increased strength and decreased fatigue (Amaizu 2008; Cunha 2009; Joung 2006; Lau 2000a). Although maturation of oral feeding functions will enhance their performance, it is co‐ordination of these activities in conjunction with swallowing and respiratory control that will ultimately lead to ‘readiness to oral feed’ in a safe and efficient manner (Lau 2011).
Development can be significantly disrupted by comorbidities present in preterm infants, such as respiratory disease (Lau 2015; Mandich 1996; Miller 2007), brain injury (Medoff‐Cooper 1996) and necrotising enterocolitis (NIH 2008), which limit opportunities for sucking and deprive the infant of essential sensory and motor experiences during a critical period of brain development when the central patterning of suck and feeding skill is refined (da Costa 2010b; da Costa 2010c; Howe 2007; Mizuno 2007; Stumm 2008; Thoyre 2003a; Thoyre 2003b). Medical interventions used with preterm infants, such as prolonged endotracheal intubation (Bier 1993), continuous positive airway pressure (CPAP), nasal cannulation and regular oropharyngeal, nasal or tracheal suction (White‐Traut 2005) may result in negative responses to oral feeding (Bingham 2009; Jadcherla 2010; Rocha 2007) and long‐term oral sensitivity (Dodrill 2004). Other factors, such as prefeeding behaviour state, feeding readiness and feeding experience, also influence feeding performance in preterm infants (Burklow 2002; Dodrill 2008a; Howe 2007; Joung 2006; Kinneer 1994; Pickler 2006).
Few preterm infants are adequate oral feeders from birth, and many receive enteral feeds by tube, necessitating longer hospital stays as they transition from tube (gavage) feeds to oral feeds. Occasionally, preterm infants do not have adequate oral intake at term corrected age, and they remain partially or exclusively tube fed for months or years. Pathways for facilitating the transition from tube to oral feeding in this population can vary between centres and are dependent on a variety of factors, such as age, weight, oral motor skills, feeding techniques and feeding experience (Cowen 2006; Dougherty 2008; Howe 2007). Initiation of oral feeding is often based on infant weight and postmenstrual age (PMA), but empirically derived guidelines for starting or progressing oral feeds are not available (Crowe 2006; Dodrill 2008c; Pickler 2006).
Should feeding commence earlier in this population, estimated economic data identify potential cost savings in the USA ranging from $3500 (Field 1982) to $280 million in hospitalisation charges per infant for board alone (Daley 2000). More recent figures estimate that a three‐day decrease in hospital stay for this population could result in savings of more than two billion dollars annually (Lessen 2011).
Description of the condition
Oral feeding is a complex skill that requires the integration of breathing, sucking and swallowing in the context of overall motor stability and incoming sensory stimuli (Arvedson 2010; da Costa 2010a; Fadavi 1997; Kelly 2007; Lau 2000a; Lefton‐Greif 2007; Ross 2002). This skill depends upon brainstem central pattern generators, whose activity is influenced by chemosensory and oral tactile input (Amaizu 2008; Bingham 2009; Lau 2011; Lau 2015; Wolf 1992). The ability to progress to successful feeding depends on the infant's ability to co‐ordinate the muscles of the jaw, lips, tongue, palate and pharynx, upper trunk and respiratory systems to support a safe swallow. It is also dependent on normal sensory functioning seen in primitive reflexes such as rooting, gag and an intact swallow reflex and intraoral and pharyngeal sensation.
Researchers have described the developmental stages of sucking in preterm infants during oral feeding (Amaizu 2008; Cunha 2009; Dodrill 2008b; Lau 2000a; Medoff‐Cooper 1993; Neiva 2007 (an additional reporting of Neiva 2006)). Varying components of sucking physiology, such as sucking amplitude, rate and pressure intensity; timing of sucking cycles; and proficiency and efficiency (Bingham 2009; Lau 2011; Medoff‐Cooper 2000; Neiva 2007 (an additional reporting of Neiva 2006); Poore 2008a; Stumm 2008), appear to mature over time at varying rates, depending on the factors outlined above (Amaizu 2008; Lau 1997; Pickler 2006). Experience with oral feeding appears to have a positive effect on the characteristics of sucking (Cunha 2009; Pickler 2006; Simpson 2002). One analysis of nutritive sucking function in very low and extremely low birth weight infants outlines how weakness of oral muscular function and minimal sucking skill can bring about weakness of intensity of sucking pressure, decreased time of the sucking stage in a sucking cycle and unstable intensity of sucking pressure and time, causing low efficiency of milk intake and smaller amounts of milk swallowed during each sucking period (Matsubara 2005). These problems lasted longer in an extremely low birth weight group than in a small group of full‐term infants. The presence of a persistently disorganised sucking pattern after 37 weeks can be predictive of neurodevelopmental outcomes at six months and 12 months (Tsai 2010). Although enteral milk feeding is critical for their optimal growth and development, few preterm infants feed adequately orally from birth. Consequently, these infants remain tube fed in hospital for protracted periods as they learn to feed orally, contributing to increased healthcare costs and heightened family stress (Swift 2010).
Description of the intervention
The intervention programmes referred to earlier in this review are designed to facilitate the development of oral motor and sensory skills required for sucking and swallowing. Such direct programmes often involve stroking perioral and intraoral structures in a specific way with a gloved finger for a specified time before feeding (Fucile 2002; Fucile 2002a; Fucile 2011; Lessen 2009; Pimenta 2008). Techniques such as stroking the cheeks are reported to enhance the sucking rate, and providing cheek and chin/jaw support may facilitate sucking efficiency during feeding (Boiron 2007). Positive effects on the rhythm of pharyngeal swallowing in response to oral sensorimotor programmes have been described (Boiron 2009, an additional reporting of Boiron 2007). Many of the interventions described involve some level of training and require skilled delivery by a nurse, an occupational therapist, a speech and language therapist, a parent or other developmental specialists.
How the intervention might work
These interventions are designed to reduce oral hypersensitivity, improve range of motion and strength of muscles for sucking (Fucile 2002), increase oral motor organisation (Case‐Smith 1989) and activate reflex behaviours that facilitate nutritive sucking (Leonard 1980; Neiva 2007 (an additional reporting of Neiva 2006)). In general, these techniques aim to normalise sensation by restoring reflexes and in turn elicit normal oral movements of lips, tongue, jaw and pharynx for development of sucking and swallowing. As well as facilitating the development of oral skills for eventual feeding, these interventions provide such beneficial effects as accelerated transition from tube feeding to independent oral feeding (McCain 2001; Pinelli 2005), enhanced sucking maturation (Boiron 2007; Harding 2006; Leonard 1980; Poore 2008b), earlier achievement of oral feeding (Boiron 2007; Harding 2006), reduction in bottle feeding stress (Pickler 1992), increased volume intake (Boiron 2007; Einarsson‐Brackes 1994), greater weight gain (Bernbaum 1983; Gaebler 1996) and fewer days of hospitalisation (Gaebler 1996; Harding 2006; Johnston 1999; Pinelli 2005).
Although no adverse effects of these interventions have been reported to date, effects that may be observed as indicators of feeding stress in this group include heart rate variability (McCain 1995; McCain 2010) and apnoeic episodes associated with feeding‐induced apnoea (Eichenwald 2001; Howe 2007; Thoyre 2003a; Thoyre 2003b). Other possible adverse effects include oral trauma to the mouth, oral infection or both. Silent aspiration of oral feeds is an ongoing concern that needs careful monitoring in this group (Miller 2007). The introduction of any implement or device into the oral cavity can cause an increase in salivary flow rate. For preterm infants who display weakness and inco‐ordination in the oropharyngeal system, and are unable to consistently control and swallow their own saliva, the sudden increase in saliva associated with the introduction of a soother or a gloved finger, for example, may be overwhelming and may increase stress and risk of aspiration of oral secretions (Wolf 1992).
Why it is important to do this review
Several other systematic reviews and meta‐analyses (Arvedson 2010; Crowe 2006Daley 2000; Pinelli 2005) have studied general approaches to feeding, including use of pacifiers, but none has yet evaluated the evidence for specific oral stimulation interventions based on finger stimulation protocols. Previous reviews have had a broad scope, resulting in wide heterogeneity and variability among participants, interventions and outcome measures (Arvedson 2010; Daley 2000), or review authors have looked only at non‐nutritive sucking activities (Cowen 2006; Pinelli 2005). Therefore, this review will compare only finger stimulation protocols as oral stimulation interventions and will determine their effects on outcomes such as neonatal intensive care unit (NICU)/hospital discharge, time to attainment of oral feeding, duration of parenteral feeding, suck/swallow maturation and anthropometrical measures such as weight gain, length and head circumference.
For the purposes of this review, we have revised the definition of oral stimulation from that proposed in the protocol (Greene 2012) (see Differences between protocol and review). Oral stimulation is currently defined as direct delivery of sensory stimulation by a finger stroking protocol to the perioral and/or oral area, designed to elicit movement responses in the lips, jaw, soft palate, pharynx, larynx and respiratory muscles to influence oropharyngeal and respiratory sensorimotor mechanisms, to improve function for sucking and feeding in preterm infants. Oral stimulation should occur before or during nutritive sucking (NS) and non‐nutritive sucking (NNS) events with tube feeds.
It is unclear whether oral stimulation interventions, specifically those using finger stimulation protocols, result in earlier exclusive oral feeding in preterm infants. It is important to determine whether exclusive oral feeding as a result of this intervention contributes to earlier NICU discharge and subsequent hospital discharge. This review is important because it will (1) assist healthcare providers in clarifying policy related to implementing treatment for preterm infants in appropriate clinical settings and (2) assist in promoting evidence‐based practice internationally in the treatment of preterm infants.
If these interventions are found to be effective, they could become a routine and standard part of delivery of care to preterm infants in NICU settings, facilitating earlier discharge and reducing costs of care associated with long hospital stay.
Objectives
Primary objectives
To examine the effectiveness of oral stimulation interventions for attainment of oral feeding in preterm infants born before 37 weeks' postmenstrual age (PMA).
To conduct subgroup analyses for the following prespecified subgroups.
Extremely preterm infants born at < 28 weeks' PMA.
Very preterm infants born from 28 to < 32 weeks' PMA.
Infants breast fed exclusively.
Infants bottle fed exclusively.
Infants who were both breast fed and bottle fed.
Methods
Criteria for considering studies for this review
Types of studies
We included all published and unpublished randomised controlled trials (RCTs) and quasi‐randomised controlled trials reported in any language. We classified as RCTs all trials that involved at least one test treatment aimed at improving oral motor function and one control treatment, with concurrent enrolment and follow‐up of both test‐treated and control‐treated groups. We classified as quasi‐RCTs all trials that involved at least one test treatment aimed at improving oral motor function and one control treatment, with concurrent enrolment and follow‐up of test‐treated and control‐treated groups, when the method of allocation was known but was not considered strictly random, for example, alternate allocation by day or date of birth or medical record number. We excluded cross‐over trials.
Types of participants
We included all trials of preterm infants of mixed ages in which the data allowed for extraction of participants up to 37 weeks' PMA. The intervention could occur at any time from date of birth. We did not exclude trials that included infants with comorbid impairments, such as neurological or structural impairments. Participants had to be deemed medically stable for the intervention. We excluded participants who presented with defined respiratory disease, as this particular subgroup is at increased risk of feeding disorders, and comparison between these infants and healthy preterm infants is difficult. We excluded trials of infants presenting with significant comorbid conditions that preclude the introduction of oral feeding.
Types of interventions
We included all trials involving oral stimulation interventions that occurred in any clinical setting with delivery by a trained person or team, including nurse, occupational therapist, speech and language therapist, other developmental specialist or parent. We considered any dosage, intensity, frequency, duration and timing of delivery of interventions. We made the following comparisons.
Oral stimulation intervention versus no intervention or standard care or sham treatment.
Oral stimulation intervention versus non‐oral intervention.
Oral stimulation intervention versus other oral stimulation delivered by a different method (e.g. dosage/intensity, frequency, duration and/or timing of delivery, mode of delivery, personnel delivering the intervention).
Types of outcome measures
We considered the following outcome measures as potential measures of success: outcome measures that signified improvement in feeding ability and oromotor function of the preterm infant and that reduced NICU and/or overall hospital stay.
Primary outcomes
Time (days) taken to achieve exclusive oral feeding, defined as ingestion of all nutrient volumes in a 24‐hour period without gavage (McCain 2001)
Time (days) spent in NICU
Total hospital stay (days)
Duration (days) of parenteral nutrition
Secondary outcomes
Exclusive oral feeding at 40 weeks' PMA
Exclusive direct breast feeding at 40 weeks' PMA
Any direct breast feeding at 40 weeks' PMA
Weight gain (g/kg/d)
Length (cm/d)
Head circumference (cm/d)
Maturation in sucking strength (measured by rate of milk intake (mL/min); suction amplitude (mmHg)/sucks/min)
Developmental outcomes ascertained by a validated instrument at 12 to 18 months
Adverse outcomes, such as sepsis, oral infection, oral trauma, apnoea or bradycardia episodes requiring intervention from the caregiver (stimulation, oronasal suction, increased delivery of oxygen, assisted ventilation), increased salivary flow (as measured by the presence of saliva beyond the level of the lips), oxygen dependence at 36 weeks' PMA, death during initial hospital stay
Necrotising enterocolitis (≥ Bell's stage 2)
Retinopathy of prematurity (any stage and ≥ stage 3)
Family satisfaction with intervention
Non‐compliance with intervention
We considered three time frames for follow‐up.
Immediate change.
Medium‐term change (three to six months).
Long‐term change (beyond six months).
Search methods for identification of studies
We included in the review published and unpublished studies of trials on humans reported in any language.
Electronic searches
We used the criteria and standard methods of The Cochrane Collaboration and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
We conducted a comprehensive search that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 1), in The Cochrane Library; MEDLINE via PubMed (1966 to current); Embase (1980 to current); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to current), using the following search terms: ((non‐nutritive suck*) OR pacifier OR dummy OR (myofunctional therapy) OR oromotor OR (oral motor) OR sensorimotor OR ((suck OR oral OR orocutaneous OR physical OR mechanical OR sensory OR somatosensory OR pre‐feeding) AND (stimulation OR training OR support)) AND (feed* OR growth)), (Note: Growth was included as a term only in The Cochrane Library), plus database‐specific limiters for RCTs and neonates (see Appendix 2 for the full search strategy for each database). We applied no language restrictions.
We searched clinical trials registries for ongoing and recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform www.whoint/ictrp/search/en/ and the ISRCTN Registry).
Searching other resources
We checked published abstracts from the following organisations.
American Speech‐Language‐Hearing Association: Perspectives Special Interest Group 13 (2001 to 2016).
Royal College of Speech and Language Therapists (1999 to 2016).
Neonatal Society via www.neonatalsociety.ac.uk (2001 to 2016).
British Association of Perinatal Medicine (guidelines/reports/newsletters only) (2003 to 2016).
Conference on Feeding and Eating in Infancy and Early Childhood, Institute of Child Health Great Ormond Street (2010 to 2016).
Abstracts for the following organisations were available via standard databases through our online searches.
American Dysphagia Research Society (DRS) and European Society for Swallowing Disorders (ESSD): Dysphagia Journal, from 1992 to 2016.
American Academy of Pediatrics: Paediatrics, Hospital Paediatrics.
American Society for Parenteral and Enteral Nutrition (ASPEN): Journal of Parenteral and Enteral Nutrition, Nutrition in Clinical Practice.
European Society for Swallowing Disorders (ESSD): Dysphagia Journal, DRS above.
Canadian Pediatric Society.
European Academy of Paediatrics: European Journal of Paediatrics, online archive from 2011 to 2016.
European Society for Paediatric Research: Pediatric Research, online archive from 1967 to 2016.
Personal communication with other relevant groups was not considered necessary.
Data collection and analysis
Selection of studies
We merged search results using reference management software (RefWorks) and removed duplicate records. Two review authors (ZG, MW) used a screening form to individually examine the titles and abstracts of identified studies. We classified studies for this review as 'include', 'unsure' or 'exclude'. We excluded reports that clearly did not meet the inclusion criteria and were not relevant. We resolved disagreements on inclusion of studies through discussion.
All review authors independently reviewed full texts of reports identified as 'include' or 'unsure'. We resolved disagreements on compliance with eligibility criteria through discussion. We determined that it was not necessary to contact any study authors.
Data extraction and management
We used a specifically devised data extraction form to extract data from study reports (Greene 2012). All review authors independently extracted data from each report to minimise errors and reduce potential risk of bias. We resolved disagreements through discussion.
Assessment of risk of bias in included studies
We analysed each study individually for bias across the five domains of bias and added this information to the Characteristics of included studies table. We evaluated the following issues and entered this information into the risk of bias table (Higgins 2008).
Sequence generation (checking for possible selection bias)
Was the allocation sequence adequately generated?
For each included study, we considered the method used to generate the allocation sequence as:
adequate (any truly random process, e.g. random number table, computer random number generator);
inadequate (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number); or
unclear.
Allocation concealment (checking for possible selection bias)
Was allocation adequately concealed?
For each included study, we considered the method used to conceal the allocation sequence as:
adequate (e.g. telephone or central randomisation, consecutively numbered sealed opaque envelopes);
inadequate (e.g. open random allocation, unsealed or non‐opaque envelopes, alternation, date of birth); or
unclear.
Blinding (checking for possible performance bias)
Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
For each included study, we considered the methods used to blind study participants and personnel from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or different classes of outcomes. We categorised methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel; or
adequate, inadequate or unclear for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
Were incomplete outcome data adequately addressed?
For each included study and for each outcome, we described completeness of data, including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion when reported and whether missing data were balanced across groups or were related to outcomes.
When sufficient information was reported or supplied by the trial authors, we included this missing data in the analyses and categorised the method as:
adequate (< 20% missing data);
inadequate (≥ 20% missing data); or
unclear.
Selective reporting bias
Are reports of the study free of the suggestion of selective outcome reporting?
For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed methods as:
adequate (when it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (when not all the study’s prespecified outcomes have been reported; when one or more reported primary outcomes that were not prespecified outcomes of interest are reported incompletely and so cannot be used; when the study fails to include results of a key outcome that would have been expected to have been reported); or
unclear.
We also considered other issues that may affect reporting bias, such as publication, time lag, language, duplicate publication and citation.
Other sources of bias
Was the study apparently free of other problems that could put it at high risk of bias?
For each included study, we described important concerns that we had about other possible sources of bias (e.g. whether we noted a potential source of bias related to the specific study design, whether the trial was stopped early because of some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
yes;
no; or
unclear.
If needed, we planned to explore the impact of the level of bias by undertaking sensitivity analyses.
We created a 'Risk of bias' table for each study in Review Manager 5.3 (RevMan 2015) (Figure 1).
1.
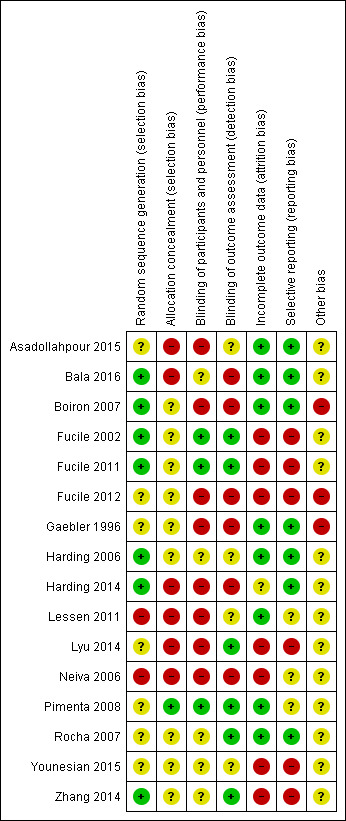
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
We calculated risk ratio (RR) and risk difference (RD) for dichotomous data, and mean difference (MD) for continuous data, with respective 95% confidence intervals (CIs), in Review Manager 5.3 (RevMan 2015).
Unit of analysis issues
The unit of analysis was the individual preterm infant.
Dealing with missing data
We planned to contact study authors to seek missing data if we judged that these data would be useful for the review.
Assessment of heterogeneity
If more than one trial was included in a meta‐analysis, we examined the treatment effects of individual trials and heterogeneity between trial results by inspecting the forest plots. We calculated the I² statistic for each analysis to quantify inconsistency across studies and to describe the percentage of variability in effect estimates that may be due to heterogeneity rather than to sampling error.
Data synthesis
We performed meta‐analyses when data were presented with sufficient information. We used mean difference (MD) for continuous outcomes when analysing interventions and outcomes of sufficient homogeneity. For dichotomous outcomes, we used risk ratio (RR) and risk difference (RD) and the fixed‐effect model for meta‐analysis.
Quality of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: days to full oral feeding, weight gain, days of parenteral nutrition, total hospital stay (days), exclusive direct breast feeding at discharge and any direct breast feeding at discharge.
Two authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomized controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
If sufficient data were available, we planned to undertake subgroup analyses of:
infants born at < 28 weeks' PMA;
infants born from 28 to < 32 weeks' PMA;
infants breast fed exclusively;
infants bottle fed exclusively; and
infants who were both breast fed and bottle fed.
Sensitivity analysis
We planned to perform sensitivity analyses based on methodological quality.
Results
Description of studies
Results of the search
The search yielded 2252 studies after duplicates were excluded (Figure 2). Screening of titles resulted in 94 trials for further scrutiny. Review authors determined that 17 studies were potentially eligible for inclusion in the review. On further inspection at data extraction, we had to exclude the stage 1 study, as data could not be extracted in relation to infants under 37 weeks' PMA (Howard 2003). Therefore, a total of 16 studies were eligible for full data extraction. All studies were published in English. Searching of conference proceedings revealed no abstracts apart from the ASHA (American Speech‐Language‐Hearing Association) Perspectives Special Interest Group 13 (2001 to 2016), for which a separate online search revealed 73 abstracts; we identified six as relevant to this topic, but all were reviews or summaries of the literature, and we excluded them on this basis (Gosa 2006; Faherty 2006; Lau 2014; Ross 2008b; Shaker 2010; Sheppard 2005).
2.
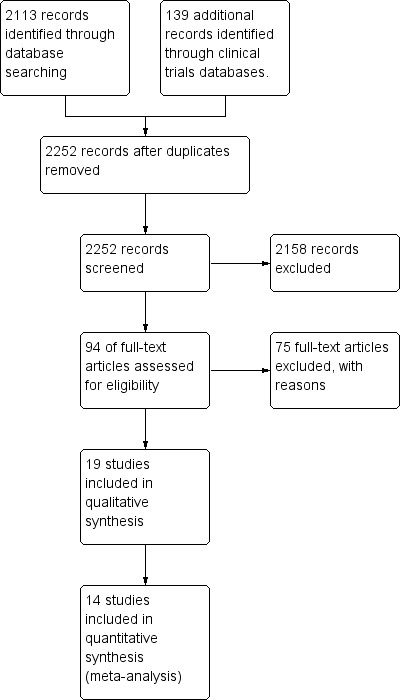
Study flow diagram.
Included studies
See Characteristics of included studies.
We included 16 RCTs (no quasi‐RCTs) that enrolled between 14 and 108 participants, for a total of 825 participants.
All trials reported finger stimulation protocols before feeds (gavage or oral) with or without other supports. Broadly, these fell into two comparison types (Table 3).
1. Comparison groups: RCT allocation.
| RCTs in Comparison group 1 | RCTs in Comparison group 2 |
|
Bala 2016 Boiron 2007 Gaebler 1996 Harding 2006 Harding 2014 Lyu 2014 Neiva 2006 Younesian 2015 Zhang 2014 |
Asadollahpour 2015 Fucile 2002 Fucile 2011 Fucile 2012 Lessen 2011 Pimenta 2008 Rocha 2007 |
Oral stimulation versus no intervention or standard care (Bala 2016; Boiron 2007; Gaebler 1996; Harding 2006; Harding 2014; Lyu 2014; Neiva 2006; Younesian 2015; Zhang 2014).
Oral stimulation versus another non‐oral stimulation intervention (Asadollahpour 2015; Fucile 2002; Fucile 2011; Fucile 2012; Lessen 2011; Pimenta 2008; Rocha 2007).
No studies assessed an oral stimulation intervention versus another oral stimulation intervention that differed in method (e.g. dosage/intensity, frequency, duration and/or timing of delivery, mode of delivery, personnel delivering the intervention).
Interventions
'Fucile protocol'
Nine trials replicated the 15‐minute finger stimulation protocol described by Fucile 2002 as their primary oral stimulation intervention (Asadollahpour 2015; Fucile 2011; Fucile 2012; Harding 2014; Lyu 2014; Pimenta 2008; Rocha 2007; Younesian 2015; Zhang 2014). This 'Fucile protocol' is a clearly described prefeeding finger stimulation protocol that involves 12 minutes of structured finger stroking and three minutes of pacifier sucking (i.e. 15 minutes once a day for one consecutive day one to 30 minutes before a tube feeding). Researchers clearly describe a sham stimulation for the control group.
Interventions in other trials
The other studies reported a range of interventions that differed in dose, frequency and method of delivery. Bala 2016 used an intervention described by Hwang 2010 ‐ a five‐minute prefeeding oral stimulation programme delivered before feeds five times a day, involving three minutes of manual perioral and intraoral stimulation, followed by two minutes on a pacifier. Gaebler 1996 described a different five‐minute oral stroking protocol that was completed three times daily, five days a week, before feeds. Harding 2006 described another finger stimulation protocol delivered by parents by which a finger or a pacifier could be used to elicit non‐nutritive sucking, then the finger or pacifier remained in the infant's mouth for the first 10 minutes of tube feeding from when feeding readiness was demonstrated until all feeds were given orally. This was done three times daily. The stimulation protocols reported by Neiva 2006 were vague and were not replicable; investigators simply stated that groups received no stimulation, received non‐nutritive sucking with a pacifier or received stimulation of non‐nutritive sucking with a gloved finger. Stimulation was done daily for 10 minutes, except on weekends. The finger stimulation protocol described by Boiron 2007 differs again from those described above, involving a 12‐minute finger stimulation programme with or without oral support during feeding. The protocol was delivered once a day 30 minutes before gavage feeds for the last 14 consecutive days of gavage feeds. Lessen 2011 described a five‐minute oral motor programme delivered from 29 weeks' PMA once a day for seven consecutive days.
Outcomes
Most trials reported outcome observations only for the short term (i.e. on discharge from NICU), with the exception of Pimenta 2008, which followed groups up to six months of age. Several primary and secondary outcome measures were not reported by any studies (i.e. time (days) spent in NICU (one of our primary outcomes)), and secondary outcomes included direct breast feeding at term corrected age, developmental outcomes at 12 to 18 months of age, retinopathy of prematurity, family satisfaction and non‐compliance with the intervention. Harding 2014 reported follow‐up at six months. Researchers noted numerous hospital readmissions, problems with oral feeding within that time frame and receptive and expressive language ratings on the Preschool Language Scales ‐ a standardised and validated assessment tool ‐ but these did not fall within the remit of our outcome measures.
Excluded studies
Risk of bias in included studies
Review authors noted variable risk of bias across all studies across all domains, with generally poorly described randomisation methods and poor allocation concealment and blinding of participants and outcome assessors (Figure 1). Only three studies performed reasonably well across the seven domains in terms of adequate sequence generation; adequate blinding of participants, personnel and outcome assessors; reports of complete data; and apparent low risk of selective reporting (Harding 2006; Pimenta 2008; Rocha 2007). The risk of bias graph (Figure 1) shows high risk of bias across the 16 studies for allocation concealment, blinding of participants and personnel, blinding of outcome assessment and incomplete outcome data.
Allocation
Seven studies adequately described their random sequence generation methods (Bala 2016; Boiron 2007; Fucile 2002; Fucile 2011; Harding 2006; Harding 2014; Zhang 2014). We could not determine the method used in the other 12 studies. Only one study described adequate allocation concealment (Pimenta 2008). We could not determine allocation concealment in 15 studies. Eight studies had unclear methods of allocation (Fucile 2002; Fucile 2011; Fucile 2012; Gaebler 1996; Harding 2006; Rocha 2007; Younesian 2015; Zhang 2014), and seven provided a poor or no description of allocation (Asadollahpour 2015; Bala 2016; Boiron 2007; Harding 2014; Lessen 2011; Lyu 2014; Neiva 2006).
Blinding
Only five studies described blinding of participants and personnel (Fucile 2002; Fucile 2011; Pimenta 2008; Rocha 2007; Zhang 2014). Only six studies described blinding of outcome assessors (Fucile 2002; Fucile 2011; Lyu 2014; Pimenta 2008; Rocha 2007; Zhang 2014).
Incomplete outcome data
Review authors noted missing data in several studies, particularly in relation to behavioural data taken at every intervention and adverse effects.Eight trials provided complete data (Asadollahpour 2015; Bala 2016; Boiron 2007; Gaebler 1996; Harding 2006; Lessen 2011; Pimenta 2008; Rocha 2007).
Selective reporting
Seven trials had low risk of reporting bias (Asadollahpour 2015; Bala 2016; Boiron 2007; Gaebler 1996; Harding 2006; Harding 2014; Rocha 2007); remaining studies had unclear or high risk of reporting bias.
Other potential sources of bias
Four trials had other biases (Boiron 2007; Fucile 2012; Gaebler 1996; Neiva 2006). It was unclear whether other sources of bias were present in the remainder.
Effects of interventions
Summary of findings for the main comparison. Summary of findings table 1. Oral stimulation intervention versus standard care.
| Comparison group 1 | ||||||
| Patient or population: preterm infants Setting: NICU Intervention: oral stimulation Comparison: standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with standard care | Risk with oral stimulation | |||||
| Days to full oral feeding | Mean days to full oral feeding: 0 | Mean days to full oral feeding in the intervention group: 5.22, undefined lower (6.86 lower to 3.59 lower) | ‐ | 376 (8 RCTs) | ⊕⊕⊝⊝ Low a,b,c,d,e,f,g,h | Heterogeneity (I2= 68%) between these studies was substantial, with high risk of bias overall between them. |
| Weight gain | Mean weight gain: 0 | Mean weight gain in the intervention group: 0.05, undefined lower (1.19 lower to 1.09 higher) | ‐ | 81 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b,e,f,g | |
| Total hospital stay (days) | Mean total hospital stay (days): 0 | Mean total hospital stay (days) in the intervention group: 5.26, undefined lower (7.34 lower to 3.19 lower) | ‐ | 301 (7 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,d,e,f | |
| Duration (days) of parenteral nutrition | Mean duration (days) of parenteral nutrition: 0 | Mean duration (days) of parenteral nutrition in the intervention group: 5.3, undefined lower (9.73 lower to 0.87 lower) | ‐ | 19 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c,f | |
| Exclusive direct breast feeding at discharge | 350 per 1000 | 641 per 1000 (366 to 847) | RR 1.83 (0.96 to 3.48) | 59 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b,c,e | |
| Any direct breast feeding at discharge | 348 per 1000 | 431 per 1000 (202 to 925) | RR 1.24 (0.58 to 2.66) | 110 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c,d | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aHigh risk of selection bias.
bHigh risk of performance bias.
cHigh risk of detection bias.
dSubstantial heterogeneity (50% to 90%).
eHigh risk of attrition bias.
fHigh risk of reporting bias.
gModerate heterogeneity (30% to 60%).
hConsiderable heterogeneity (75% to 100%).
Summary of findings 2. Summary of findings table 2. Oral stimulation intervention versus other non‐oral intervention.
| Comparison group 2 | ||||||
| Patient or population: preterm infants Setting: NICU Intervention: oral stimulation Comparison: non‐oral intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with non‐oral intervention | Risk with oral stimulation | |||||
| Time (days) to achieve exclusive oral feeding | Mean time (days) to achieve exclusive oral feeding: 0 | Mean time (days) to achieve exclusive oral feeding in the intervention group: 9.01, undefined lower (10.3 lower to 7.71 lower) | ‐ | 256 (5 RCTs) | ⊕⊕⊝⊝ Low1,4,5,6,7,8 | Heterogeneity (I2 = 25%) between studies was low, and issues with selection, performance and attrition bias were noted. |
| Total hospital stay (days) | Mean total hospital stay (days): 0 | Mean total hospital stay (days) in the intervention group: 2.94, undefined lower (4.36 lower to 1.51 lower) | ‐ | 352 (6 RCTs) | ⊕⊕⊝⊝ Low1,2,5,6 | |
| Duration (days) of parenteral nutrition | Mean duration (days) of parenteral nutrition: 0 | Mean duration (days) of parenteral nutrition in the intervention group: 8.7, undefined lower (15.46 lower to 1.94 lower) | ‐ | 98 (1 RCT) | ⊕⊕⊕⊝ Low | Only 1 study included Wide confidence interval Not fully blinded |
| Exclusive direct breast feeding at discharge | 500 per 1000 | 479 per 1000 (346 to 617) | RR 0.96 (0.72 to 1.28) | 196 (1 RCT) | ⊕⊕⊕⊝ Moderate | Only 1 study included |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1High risk of reporting bias.
2Moderate heterogeneity (30% to 60%).
3Substantial heterogeneity (50% to 90%).
4Considerable heterogeneity (75% to 100%).
5High risk of selection bias.
6High risk of performance bias.
7High risk of attrition bias.
8Low heterogeneity (0 to 40%).
Comparison group 1
Days to full oral feeding
1.1. Analysis.
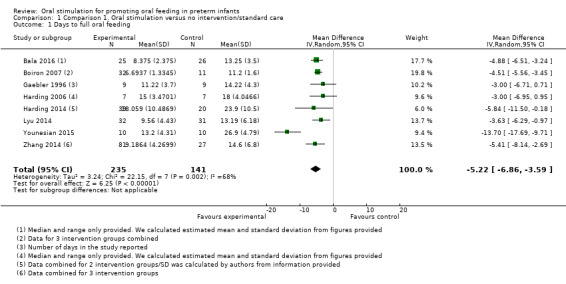
Comparison 1 Comparison 1. Oral stimulation versus no intervention/standard care, Outcome 1 Days to full oral feeding.
3.
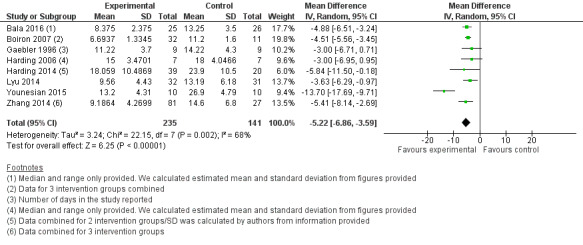
Comparison group 1. Analysis 1.1. Days to full oral feeding.
Meta‐analysis showed statistically significantly fewer days taken to attain full oral feeding in the intervention groups (MD ‐4.81, 95% CI ‐5.56 to ‐4.06, I2 = 68%, eight trials, 376 infants). Four of these studies (Harding 2014; Lyu 2014; Younesian 2015; Zhang 2014) followed the 'Fucile protocol', and the remaining studies (Bala 2016; Boiron 2007; Gaebler 1996; Harding 2006) used a range of different interventions. The GRADE rating for methodological quality was low (Table 1).
Weight gain
1.2. Analysis.
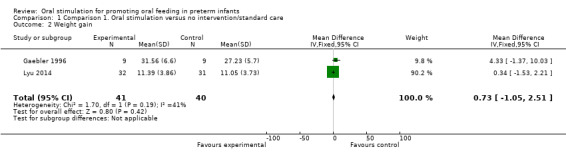
Comparison 1 Comparison 1. Oral stimulation versus no intervention/standard care, Outcome 2 Weight gain.
Only two studies used ‘weight gain’ as an outcome measure (Gaebler 1996; Lyu 2014). Other studies used varying terminology to describe weight: Younesian 2015 described weight change from four oral feeds a day/four to eight oral feeds a day/eight oral feeds a day until discharge (grams), and Zhang 2014 described % weight gain. Therefore, we included only two studies in the meta‐analysis, which showed no significant effect of the intervention on weight gain (MD 0.73 grams, 95% CI ‐1.05 to 2.51 grams, I2 = 41%, two trials, 81 infants).
The GRADE rating for methodological quality was low (Table 1).
Days in hospital
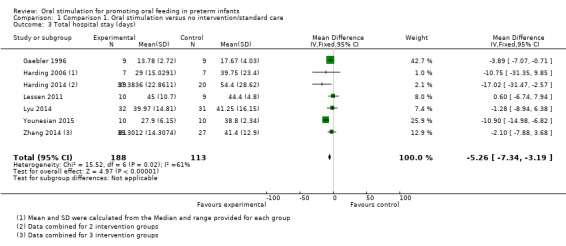
1.3. Analysis.
Comparison 1 Comparison 1. Oral stimulation versus no intervention/standard care, Outcome 3 Total hospital stay (days).
4.
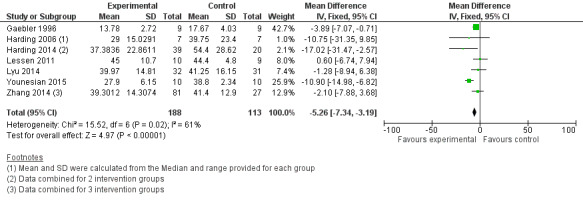
Comparison group 1. Analysis 1.3. Total hospital stay (days).
Meta‐analysis showed that the intervention group had a statistically significantly shorter hospital stay (MD ‐5.26 days, 95% CI ‐7.34 to ‐3.19 days, I2 = 61%, seven trials, 301 infants). The GRADE rating for methodological quality was very low (Table 1).
Duration of parenteral nutrition (days)
1.4. Analysis.
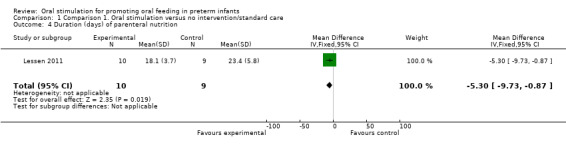
Comparison 1 Comparison 1. Oral stimulation versus no intervention/standard care, Outcome 4 Duration (days) of parenteral nutrition.
5.
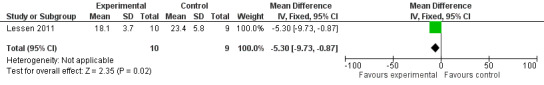
Comparison group 1. Analysis 1.4. Duration (days) of parenteral nutrition.
Lessen 2011 reported a statistically significant reduction in the number of days of parenteral nutrition in the intervention group (MD ‐5.30, 95% CI ‐9.73 to ‐0.87). The GRADE rating for methodological quality was very low (Table 1).
Exclusive direct breast feeding on discharge
1.5. Analysis.
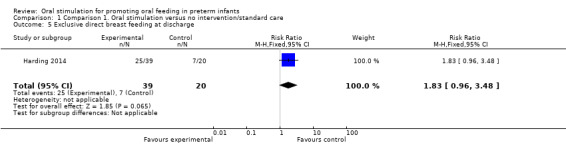
Comparison 1 Comparison 1. Oral stimulation versus no intervention/standard care, Outcome 5 Exclusive direct breast feeding at discharge.
Harding 2014 did not show a statistically significant difference in exclusive direct breast feeding on discharge with the intervention (RR 1.83, 95% CI 0.96 to 3.48). The GRADE rating for methodological quality was very low (Table 1).
Any/Partial direct breast feeding on discharge
1.6. Analysis.
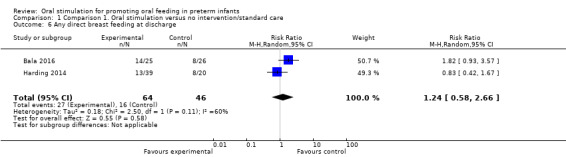
Comparison 1 Comparison 1. Oral stimulation versus no intervention/standard care, Outcome 6 Any direct breast feeding at discharge.
Meta‐analysis did not show a statistically significant effect on any or partial direct breast feeding on discharge with the intervention (RR 1.24, 95% CI 0.58 to 2.66, I2 = 60%, two trials, 100 infants). The GRADE rating for methodological quality was very low (Table 1).
Comparison group 2
Time (days) to achieve exclusive oral feeding
2.1. Analysis.
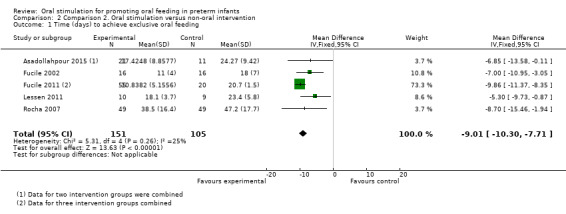
Comparison 2 Comparison 2. Oral stimulation versus non‐oral intervention, Outcome 1 Time (days) to achieve exclusive oral feeding.
6.
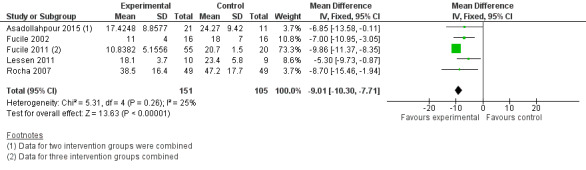
Comparison group 2. Analysis 2.1. Time (days) to achieve exclusive oral feeding.
Meta‐analysis showed a statistically significant reduction in days to achieve exclusive oral feeding with the intervention (MD ‐9.01 days, 95% CI ‐10.30 to ‐7.71, I2 = 25%, five trials, 256 infants). The GRADE rating for methodological quality was low (Table 2).
Days in hospital
2.2. Analysis.
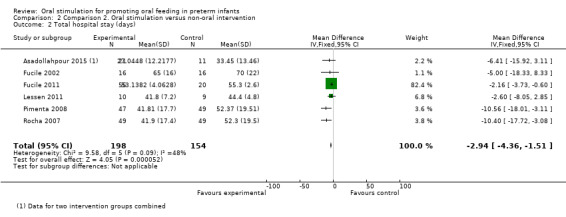
Comparison 2 Comparison 2. Oral stimulation versus non‐oral intervention, Outcome 2 Total hospital stay (days).
7.
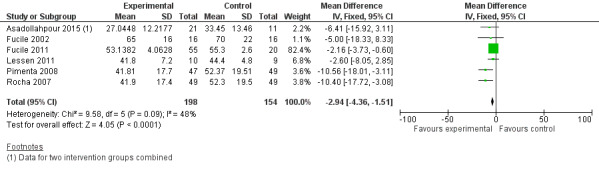
Comparison group 2. Analysis 2.2. Total hospital stay (days).
Meta‐analysis showed a statistically significant reduction in total hospital stay (days) for the intervention group (MD ‐2.94, 95% CI ‐4.36 to ‐1.51, I2 = 48%, six trials, 352 infants). The GRADE rating for methodological quality was low (Table 2).
Duration (days) parenteral nutrition
2.3. Analysis.
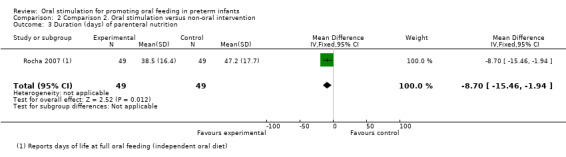
Comparison 2 Comparison 2. Oral stimulation versus non‐oral intervention, Outcome 3 Duration (days) of parenteral nutrition.
8.
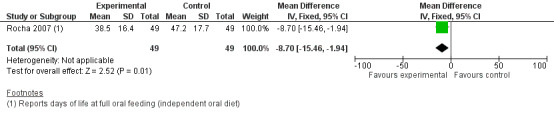
Comparison group 2. Analysis 2.3. Duration (days) of parenteral nutrition.
Rocha 2007 showed a statistically significantly shorter duration of parenteral nutrition in the intervention group (MD ‐8.70, 95% CI ‐15.46 to ‐1.94). The GRADE rating was low.
Weight gain
No studies described 'weight gain' as an outcome measure. Asadollahpour 2015 reported 'weight changes', Fucile 2011 reported 'weight at end of intervention' and Rocha 2007 described 'weight at discharge (g)'; however, these researchers did provide data for weight gain in the first and second weeks of the study for each group (g/kg/d). Therefore, meta‐analysis was not possible.
Length gain
This outcome was not reported by any trials.
Head circumference growth
This outcome was not reported by any trials.
Exclusive direct breast feeding at discharge
2.4. Analysis.
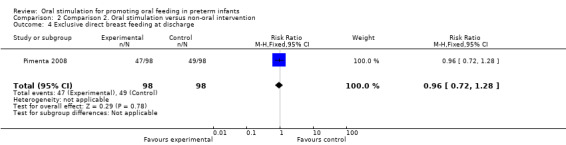
Comparison 2 Comparison 2. Oral stimulation versus non‐oral intervention, Outcome 4 Exclusive direct breast feeding at discharge.
Pimenta 2008 showed no statistically significant difference in exclusive direct breast feeding at discharge with the intervention (RR 0.96, 95% CI 0.72, 1.28). The GRADE rating was moderate.
Maturation in sucking strength
Twelve trials reported a wide variety of different and thereby incomparable suck, swallow and feeding measures, including sucking pressure (mmHg), number of bolus feeds per day, percentage milk ingested daily, number of swallows per minute, number of swallow bursts per minute, number of isolated swallows per minute, rate of milk transfer (mL/min), sucking pattern maturation, sucking frequency and amplitude, proficiency (% milk in first five minutes of feed), volume transfer (% volume consumed/total), volume loss, stage of sucking at different time frames, suction and expression amplitude; suck, swallow and respiratory co‐ordination; % nipple feeds engaged in, Revised Neonatal Oral Motor Assessment Scale (R‐NOMAS) scores at days 1, 3 and 5; proficiency (Gaebler 1996); NOMAS scores (Harding 2006; Harding 2014); oral feeding progression, oral feeding performance and efficiency (Lyu 2014); easy beginning of sucking, labial sealing, sucking rhythm, labial/tongue/jaw co‐ordination (Neiva 2006); numbers of bursts and pauses per minute, mean duration of bursts and pauses, number of sucks per second (Neiva 2007, an additional reporting of Neiva 2006); and rate of milk transfer (mL/min), proficiency and volume transfer at days 1 and 4, and at end of trial (Zhang 2014).
Length (cm/d)
No data are available for this outcome.
Head circumference (cm/d)
No data are available for this outcome.
Developmental outcomes ascertained by a validated instrument at 12 to 18 months
No data are available for this outcome.
Necrotising enterocolitis (≥ Bell's stage 2)
No data are available for this outcome.
Retinopathy of prematurity (any stage and ≥ stage 3)
No data are available for this outcome.
Family satisfaction with intervention
No data are available for this outcome.
Non‐compliance with intervention
No data are available for this outcome.
Adverse effects
No adverse effects such as sepsis, oral infection, oral trauma, apnoea or bradycardia episodes that require intervention from the caregiver (stimulation, oronasal suction, increase in delivery of oxygen, assisted ventilation), increase in salivary flow (as measured by the presence of saliva beyond the level of the lips), oxygen dependence at 36 weeks PMA or death during initial hospital stay were reported. Many studies did report adverse effects of apnoea and bradycardia that were self‐resolving and did not require intervention other than cessation of the oral stimulation intervention.
Discussion
Preterm infants who received oral stimulation rather than usual care took fewer days to attain full oral feeding (mean difference (MD) ‐4.81, 95% confidence interval (CI) ‐5.56 to ‐4.06), had a statistically significantly shorter hospital stay (MD ‐5.62, 95% CI ‐7.34 to ‐3.19) and had a statistically significant reduction in the number of days of parenteral nutrition (MD ‐5.30, 95% CI ‐9.73 to ‐0.87). Oral stimulation intervention in this group appeared to have no influence on breast feeding outcomes nor on weight gain compared with usual care.
Infants who received oral stimulation had a statistically significant reduction in the number of days it took to achieve exclusive oral feeding (MD ‐8.81, 95% CI ‐10.05 to ‐7.58), a statistically significant reduction in total hospital stay (days) (MD ‐2.94, 95% CI ‐4.36 to ‐1.51) and a statistically significantly shorter duration of parenteral nutrition (MD ‐8.70, 95% CI ‐15.46 to ‐1.94) compared with these outcomes following usual care. Oral stimulation intervention in this group did not appear to have an impact on breast feeding outcomes.
Summary of main results
We identified 19 randomised controlled trials (RCTs) that were eligible for inclusion in this review. All were of low methodological quality overall.
Investigators reported a range of oral stimulation interventions that appear beneficial for preterm infants in terms of reduced length of hospital stay and earlier transition to oral feeding, with reduced length of time on parenteral nutrition.
Overall completeness and applicability of evidence
The included studies reported positive outcomes involving length of hospital stay, transition times from tube (gavage) to oral feeding and duration of parenteral nutrition. These studies ranged in size but most were small, and they were often poorly designed. Study results should be interpreted with caution and methodological limitations should be assessed when potential use of an intervention is considered.
Quality of the evidence
Trends in the data appear to indicate that providing an oral stimulation intervention by a finger stimulation protocol reduces length of hospital stay, time taken to achieve oral feeding and time spent on parenteral nutrition, but all of the analyses are based on studies of limited methodological quality. Results of the data analysis are encouraging but must be interpreted with caution, given the high risk of bias encountered across virtually all of the included studies.
For comparison group 1, the quality of the evidence ranged from low (days to oral feeding, weight gain) to very low (total hospital stay, parenteral nutrition, breast feeding).
For comparison group 2, the quality of the evidence ranged from moderate (duration of parenteral nutrition, exclusive direct breast feeding at discharge) to low (time to exclusive oral feeding, total hospital stay, days of parenteral nutrition).
Potential biases in the review process
We strove to decrease biases in the review process. Two review authors (ZG, MW) individually examined the titles and abstracts of identified studies while using a screening form. All review authors were involved in the data extraction process. The Cochrane Neonatal Review Group was actively supportive at all stages from designing the database search strategy and conducting the database search to providing advice on methods and making revisions to same.
Our deviations from the protocol consisted of redefinition of oral stimulation interventions, re‐scoping of the review focus and application of the GRADE method in assessing the quality of evidence. Our deviations from the protocol were unlikely to introduce bias into the review process.
Agreements and disagreements with other studies or reviews
Not applicable.
Authors' conclusions
Implications for practice.
Small studies with variable risk of bias and poor methodological quality suggest that oral stimulation interventions shorten the time taken for preterm infants to achieve exclusive oral feeding, reduce length of hospital stay and reduce days on parenteral nutrition. The quality of these studies varied from moderate to very low; therefore, findings should be interpreted with caution. It is apparent however that using an oral stimulation intervention does have a statistically significant positive influence on the outcomes reported, despite varying levels of evidence, and should be considered for all infants in the neonatal intensive care unit (NICU).
Implications for research.
Well‐designed studies of oral stimulation interventions for preterm infants are warranted. Such studies should:
clearly define the intervention;
measure clinically important outcomes that are not limited to those determined before hospital discharge;
enrol adequate numbers of infants to reliably determine a difference in the primary outcome between groups;
use a reliable of method of randomisation;
conceal the treatment allocation;
blind caregivers to treatment when possible;
pay particular attention to blinding of outcome assessors; and
report all outcomes.
Methods used to assess sucking and feeding have not been standardised. This has led to lack of standardised reporting of clinically relevant outcomes for suck and swallow maturation. The terminology used to describe sucking and feeding skills should be made more uniform, so studies can be more comparable and outcomes more clinically relevant.
What's new
| Date | Event | Description |
|---|---|---|
| 6 February 2017 | Amended | Added external source of support |
Acknowledgements
Zelda Greene was supported by the Health Research Board (Ireland), Cochrane Training Fellowship 2010.
Yolanda Brosseau, Trials Search Co‐ordinator for the Neonatal Group, provided advice and assistance in devising the search strategy.
Dr William Maguire, Hull York Medical School & Centre for Reviews and Dissemination, University of York, UK, provided advice on methods.
Appendices
Appendix 1. Database search strategy
We used the search strategy recommended by the Cochrane Neonatal Review Group to find relevant studies for the review (http://www.neonatal.cochrane.org/en/index.html). We will use search terms and synonyms for 'oral stimulation', 'preterm infants' and filters to include clinical trials. We searched each database from inception to June 2013. We will search the following databases with specific search terms as outlined below:
ERIC, PsycINFO, PsycARTICLES, ASSIA, Linguistic and Language Behaviour Abstracts via CSA:
DE=( oral or stimulation or sucking) or DE=(feeding) or (pacifier) or (oral motor) or KW=(oromotor) or (enteral nutrition) or (parenteral nutrition) or DE=(motor manipulation) or (programme) or (myofunctional therapy) and KW=(premature or infant or neonate) or KW=(NICU) or (Intensive Care) or (low birthweight) DE = descriptors KW = Keywords
Academic Search Complete, CINAHL Plus, AMED, UK/EIRE Reference Centre via EBSCO:
AB Oral motor or SU feeding or SU sucking or SU pacifier or SU stimulation or SU mouth or SU rehabilitation or SU treatment or SU programme or SU oromotor AND SU neonatal or Su preterm infants or SU intensive care unit and SU Clinical trials
Science Direct and SCOPUS:
Oral stimulation OR feeding OR sucking OR oral motor exercises OR pacifiers OR stimulation OR treatment OR manipulation OR enteral feeding OR parenteral feeding AND premature infants OR neonate OR neonatal intensive care units OR health care costs
Social Science Citation Index via ISI Web of Science:
Topic=(oral) OR Topic=(stimulation) OR Topic=(feeding) OR Topic=(sucking) OR Topic=(pacifiers) OR Topic=(programme) OR Topic=(oral motor) OR Topic=(oromotor) OR Topic=(orofacial myology) OR Topic=(treatment) AND Topic=(preterm infant) OR Topic=(newborn infant) OR Topic=(neonate) OR Topic=(very low birth weight) OR Topic=(neonatal intensive care unit) Refined by: Subject Areas=(NEUROSCIENCES & NEUROLOGY) AND General Categories=(SOCIAL SCIENCES)
Highwire (Stanford University, http://highwire.stanford.edu/)
using the following key words; sucking stimulation, pacifiers, preterm, neonates, oral motor stimulation, feeding, neonatal intensive care,
REHABDATA (http://www.naric.com/research/rehab/)
using the following free text terms; oral stimulation, oral motor, feeding, sucking, infants, training programs, programs, rehabilitation, intervention, intensive care unit.
Searching other resources
We checked published conference proceedings of the following organisations:
American Academy of Pediatrics.
American Society for Parenteral and Enteral Nutrition.
American Speech‐Hearing‐Language Association.
British Association of Perinatal Medicine.
Canadian Pediatric Society.
Dysphagia Research Society (1992 to 2016).
European Academy of Paediatrics.
European Society for Paediatric Research.
Neonatal Nurses Association.
Royal College of Speech and Language Therapists (1999 to 2016).
The Neonatal Society.
Conference on Feeding and Eating in Infancy and Early Childhood (2010 to 2012).
Personal communication with other relevant groups will be considered if appropriate.
Appendix 2. Standard search methods
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti] OR comparative study) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
The Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Data and analyses
Comparison 1. Comparison 1. Oral stimulation versus no intervention/standard care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Days to full oral feeding | 8 | 376 | Mean Difference (IV, Random, 95% CI) | ‐5.22 [‐6.86, ‐3.59] |
| 2 Weight gain | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.73 [‐1.05, 2.51] |
| 3 Total hospital stay (days) | 7 | 301 | Mean Difference (IV, Fixed, 95% CI) | ‐5.26 [‐7.34, ‐3.19] |
| 4 Duration (days) of parenteral nutrition | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐5.30 [‐9.73, ‐0.87] |
| 5 Exclusive direct breast feeding at discharge | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.96, 3.48] |
| 6 Any direct breast feeding at discharge | 2 | 110 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.58, 2.66] |
Comparison 2. Comparison 2. Oral stimulation versus non‐oral intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time (days) to achieve exclusive oral feeding | 5 | 256 | Mean Difference (IV, Fixed, 95% CI) | ‐9.01 [‐10.30, ‐7.71] |
| 2 Total hospital stay (days) | 6 | 352 | Mean Difference (IV, Fixed, 95% CI) | ‐2.94 [‐4.36, ‐1.51] |
| 3 Duration (days) of parenteral nutrition | 1 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐8.70 [‐15.46, ‐1.94] |
| 4 Exclusive direct breast feeding at discharge | 1 | 196 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.72, 1.28] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Asadollahpour 2015.
| Methods | Country: Iran RCT Three study groups. Randomisation method not fully described No evidence of allocation concealment Blinding of personnel delivering the intervention unclear Blinding of outcome assessor unclear |
|
| Participants | Preterm infants from 26 to 32 weeks of gestational age fed through a tube with birth weight 1000 to 2000 grams NNS intervention group: N = 11 (6 male/5 female), GA 30.18 ± 1.77 weeks, birth weight 1406.36 grams Prefeeding oral stimulation group: N = 10 (5 male/5 female), GA 30.01 ± 1.76, birth weight 1343.01 grams Control group: N = 11 (5 male/6 female), GA 30.29±1.95, birth weight 1393.63 grams |
|
| Interventions |
|
|
| Outcomes |
Primary outcome: time to attain independent oral feeding Secondary outcomes:
|
|
| Notes | For birth weight, median values provided. Mean or standard deviation had to be calculated. Adverse events not recorded or reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random assignment was performed by 'a simple randomisation method', whereby infants were randomly assigned to NNS (n = 11), prefeeding oral stimulation (n = 10) and control (n = 11) groups. This was not clearly described. |
| Allocation concealment (selection bias) | High risk | Despite study authors reporting, "This intervention delivered by one speech therapist who was blinding to research", this SLT delivered all interventions and therefore was aware of allocation in all groups. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Same SLT delivered all interventions and sham interventions and was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Weight was measured by 'a nurse'. It is unclear whether the same nurse measured all infants, or whether the nurse on duty at the time of weigh in performed the measurements. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes are reported. No data were missing. |
| Selective reporting (reporting bias) | Low risk | All of the study’s prespecified outcomes and all expected outcomes of interest to the review were reported. |
| Other bias | Unclear risk | Information was insufficient to permit judgement. Adverse events were not recorded or reported. |
Bala 2016.
| Methods | Country: India RCT Two study groups. Randomisation method described No evidence of allocation concealment Blinding of personnel delivering the intervention unclear Blinding of outcome assessor unclear |
|
| Participants | 51 healthy stable neonates who had reached full gavage feeding, were in transition from gavage to spoon feeding and were receiving NNS and kangaroo mother care (KMC) as routine care Treatment group: 25 infants (10 male/15 female), gestational age 30.9 (1.7) weeks, birth weight 1285 (283) grams Control group: 26 infants (16 male/10 female), gestational age 30.3 (1.5) weeks, birth weight 1212 (323) grams |
|
| Interventions | Intervention is not directly described, but Hwang 2010 is cited as a reference for the protocol. Hwang 2010 describes a 5‐minute programme modified from existing literature, which involves 3 minutes of manual perioral and intraoral stimulation, followed by 2 minutes on a pacifier. Mothers were trained in oromotor stimulation (OMS) by principal investigator. Intervention group: OMS finger stimulation protocol plus standard care (NNS & KMC) delivered by mothers trained on approach by PI Control group: standard care described only as NNS and KMC |
|
| Outcomes |
Primary outcome: comparison of transition time from full gavage feed to partial and full spoon feed
Secondary outcome: assessment of total volume of milk by spoon at each feed and time required to complete full spoon feed and partial direct breast feed at discharge
|
|
| Notes | Study authors report, "No harms or unintended effects like desaturation, aspiration, apnoea, hypothermia, bradycardia, or infection were observed". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment was performed with computer‐generated random numbers. Sequentially numbered sealed opaque envelopes were opened by the principal investigator to assign infants to intervention groups. |
| Allocation concealment (selection bias) | High risk | Principal investigator was not blinded to allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | OMS was performed by mothers in the intervention group. It is unclear whether they were blinded to group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study authors state, "intervention and assessment could not be blinded due to its nature". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes are reported for all infants who achieved partial spoon feed, full spoon feed and partial breast feed at discharge. |
| Selective reporting (reporting bias) | Low risk | All of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported. |
| Other bias | Unclear risk | Information was insufficient to permit judgement. |
Boiron 2007.
| Methods | Country: France RCT Four study groups. Randomisation method described Allocation concealment unclear Blinding of personnel delivering the intervention unclear Blinding of outcome assessor unclear |
|
| Participants | 43 participants were recruited and participated in the study (23 males/20 females); all were born between 29 and less than 34 weeks and entered the protocol at between 32 and less than 34 weeks GA; no older than 4 days of age Treatment group 1 (stimulation and support): 9 participants (5 males/4 females), age range 32 to 34 weeks, mean GA 31.3 weeks, mean birth weight 1718 grams Treatment group 2 (stimulation): 11 participants (4 males/7 females), age range 32 to 24 weeks, mean GA 31.1 weeks, mean birth weight 1446 grams Treatment group 3 (support): 11 participants (7 male/4 female), age range 32 to 34 weeks, mean GA 31.6 weeks, mean birth weight 1714 grams Control group: 11 participants (7 male/4 female), age range 32 to 34 weeks, mean GA 31.1 weeks, mean birth weight 1442 grams |
|
| Interventions |
Treatment group 1: received oral stimulation and support Treatment group 2: received oral stimulation only Treatment group 3: received support only Control group: no intervention described; assumed standard care Infants in treatment group 1 received 12 minutes of a clearly described oral stimulation protocol 30 minutes before gavage feed for last 14 consecutive days of period of gavage, and oral support for 2 oral feeds a day for a maximum of 10 minutes per bottle during the transition period. Treatment groups 2 and 3 each received only 1 component of this programme. |
|
| Outcomes | All participants had a baseline sucking assessment with a pacifier and a transducer recording system. Five‐minute recordings were taken at 3, 7 and 14 days. Outcome measures:
|
|
| Notes | Adverse events were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A blocked randomisation process is described: "randomisation lists were computer generated with blocks of varying size". |
| Allocation concealment (selection bias) | Unclear risk | This is not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Investigators were not blinded to intervention groups. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Measures of sucking were made by investigators; it is unclear who decided to increase volume of oral feeding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes are reported. No data were missing. |
| Selective reporting (reporting bias) | Low risk | All of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported. |
| Other bias | High risk | Adverse events were not reported. It is unclear who decided to increase volume of oral feeding as intervention progressed. |
Fucile 2002.
| Methods | Country: USA RCT Random sequence generation: stratified random sampling technique used to ensure that groups were similar in mean gestational age and birth weight Allocation concealment unclear No blinding of personnel delivering the intervention. Researchers carried out both treatment and sham treatments. Caregivers and family blinded to intervention Blinding of outcome assessors unclear Treatment duration: 10 consecutive days |
|
| Participants | 32 participants in total: 19 females, 13 males 16 participants in each group Treatment group: N = 16, age range 28 ± 1.3 weeks, GA 26.4 to 29.9 weeks, birth weight 1044 ± 260 (740 to 1500) grams Control group: N = 16, age range 28.1 ± 1.1 weeks, GA 26.0 to 29.7 weeks, birth weight 959 ± 244 (560 to 1300) grams |
|
| Interventions | Nursing and medical staff were reported to be blinded to the intervention, as a screen was placed around the isolette during any intervention. Both groups were monitored from time of entry into the study until discharge from the hospital. Initiation and advancement of oral feeding were left to the discretion of the attending physician and nurses who were responsible for standard feeding care. Measures were taken at the introduction of oral feeds, at 1 oral feed per day, at 4 oral feeds per day and at 8 oral feeds per day. Interventions were started 48 hours after discontinuation of nasal CPAP. Intervention was not administered if infants were disturbed 30 minutes before the intervention, and it was stopped if infants were medically unstable and/or had any episodes of oxygen desaturation and/or apnoea/bradycardia during the intervention. Treatment group received a prefeeding oral stimulation programme consisting of a 12‐minute finger stroking protocol, followed by 3 minutes of sucking on a pacifier. Intervention lasted 15 minutes and was performed once a day for 10 consecutive days, 15 to 30 minutes before a tube feeding. Control group received sham stimulation identical to the prefeeding stimulation programme, except that they did not receive the 15‐minute finger stroking and pacifier portion of the protocol. |
|
| Outcomes |
|
|
| Notes | Both gestational age (GA) and postmenstrual age (PMA) are used in the report. GA is used to describe age at birth and age range of groups, PMA to describe age at feeding. Some adverse effects were reported in 1 case, in which bradycardia was observed but resolved spontaneously. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Infants were randomised into control or experimental groups in blocks of 4, stratified by gestational age (26 to 27 vs 28 to 29 weeks). |
| Allocation concealment (selection bias) | Unclear risk | This was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Researchers carried out both treatments and sham treatments. Caregivers and family were blinded to intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Advancement of oral feeding was done at the discretion of physicians, who were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | It is reported that groups were similar in baseline characteristics, such as number of infants who received breast feedings throughout the study, gastric residuals, oxygen requirement, episodes of oxygen desaturation and/or apnoea/bradycardia at the 3 monitored feeding sessions and behavioural state, although data are not provided to confirm. |
| Selective reporting (reporting bias) | High risk | Not all outcomes are fully reported as above. |
| Other bias | Unclear risk | Information provided was insufficient to permit judgement. |
Fucile 2011.
| Methods | Country: USA RCT Random sequence generation: infants randomised through stratified blocked randomisation Follow‐up: participants monitored from study start to hospital discharge Treatment duration: 14 days |
|
| Participants | 75 infants were enrolled: Group 1: N = 19 (12 male/7 female), 10 age 26 to 29 weeks GA, 9 age 30 to 32 weeks GA, birth weight not provided but weight at introduction of oral feeding was 2001.3 (63.3) grams Group 2: N = 18 (11 male/7 female), 8 were 26 to 29 weeks GA, 10 were 30 to 32 weeks GA, birth weight not provided but weight at introduction of oral feeding was 2065.6 (108.7) grams Group 3: N = 18 (10 male/8 female), 11 were 26 to 29 weeks GA, 7 were 30 to 32 weeks GA, birth weight not provided but weight at introduction of oral feeding was 1952.1 (48.7) grams Control group: N = 20 (16 male/4 female), 9 were 26 to 29 weeks GA, 11 were 30 to 32 weeks GA, birth weight not given but weight at introduction of oral feeding was 1885.2 (61.5) grams |
|
| Interventions |
Group 1, oral (O): twice‐daily finger stroking protocol of the cheeks, lips, gums and tongue for 12 minutes and NNS for 3 minutes as per previously described protocol Group 2, T/K: twice‐daily stroking of the head, neck, back, arms and legs for 10 minutes and passive range of motion to the limbs for 5 minutes Group 3, O + T/K: 15 minutes of O or T/K, each once a day, in random order Control intervention: Researcher placed her hands in the incubator but did not touch the infant for 15 minutes twice daily. Assigned interventions were started 48 hours after discontinuation of nasal CPAP and were administered in two 15‐minute sessions/d for 10 days over a 14‐day period. Sessions were provided 30 minutes before tube feedings, with a minimum 3‐hour interval between sessions, to clinically stable infants. Interventions were stopped if adverse effects were observed. All interventions were administered by the same researcher. A screen was placed around the incubator for all interventions. Data on the primary outcome were gathered from the charts of 10 additional infants to assess for any potential Hawthorne effect (Hawthorne group). |
|
| Outcomes |
|
|
| Notes | Both GA and PMA were used to describe participant age. Adverse events: Of 1100 administered interventions, 13 (1.1%) were stopped because of apnoea, bradycardia or oxygen desaturation episodes, all of which resolved spontaneously. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised in blocks (size not stated) stratified by gestational age (26 to 29 vs 30 to 32 weeks) and time ("every 3 months"). |
| Allocation concealment (selection bias) | Unclear risk | This was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of caregivers was attempted by sham procedure (therapist placed hands in incubator for 15 minutes) with screen placed around the incubator. Investigator was not blinded to the intervention but was not involved in outcome measurement. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Primary outcome was time to independent oral feeding; feeding advancement was done at the discretion of blinded physicians. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Information was provided regarding number of infants receiving co‐interventions (occupational, physical and/or speech therapy). Number of parental visits was not reported. |
| Selective reporting (reporting bias) | High risk | As above. Information was provided regarding number of infants receiving co‐interventions (occupational, physical and/or speech therapy). Number of parental visits was not reported. |
| Other bias | Unclear risk | Parental visit and therapy intervention information is required to inform interpretation of outcomes. |
Fucile 2012.
| Methods | Country: USA RCT Random sequence generation: infants randomised by stratified blocked randomisation Allocation concealment: no Blinding of personnel delivering the intervention: unclear Blinding of outcome assessors: unclear Treatment duration: 14 days |
|
| Participants | 75 infants were enrolled: Group 1, O: N = 19 (12 male/7 female), age range 29.6 weeks GA (SEM 0.4), 10 were 26 to 29 weeks GA, 9 were 30 to 32 weeks GA, birth weight 1359.7 (78.2) grams Group 2, T/K: N = 18 (11 male/7 female), 8 were 26 to 29 weeks GA, 10 were 30 to 32 weeks GA, birth weight 1325.4 (53.3) grams Group 3, O + T/K: N = 18 (10 male/8 female), 11 were 26 to 29 weeks GA, 7 were 30 to 32 weeks GA, birth weight 1329.6 (39.1) grams Control group: N = 20 (16 male/4 female), 9 were 26 to 29 weeks GA, 11 were 30 to 32 weeks GA, birth weight 1346.6 (39.3) grams |
|
| Interventions |
Group 1, oral (O): twice‐daily finger stroking protocol of the cheeks, lips, gums and tongue for 12 minutes and NNS for 3 minutes as per previously described protocol Group 2, T/K: twice‐daily stroking of the head, neck, back, arms and legs for 10 minutes and passive range of motion to the limbs for 5 minutes Group 3, O + T/K: 15 minutes of O or T/K, each once a day, in random order Control intervention: Researcher placed her hands in the incubator but did not touch the infant for 15 minutes twice daily. Assigned interventions were started 48 hours after discontinuation of nasal CPAP and were administered in two 15‐minute sessions/d for 10 days over a 14‐day period. Sessions were provided 30 minutes before tube feedings, with a minimum 3‐hour interval between sessions, to clinically stable infants. Interventions were stopped if adverse effects were observed. Participants were monitored from study start to hospital discharge. Nutritive sucking skills were assessed on a 5‐point stage of sucking scale, suck/swallow co‐ordination was assessed by a suck‐to‐swallow ratio and respiratory patterns were assessed with nipple‐bottle apparatus that simultaneously recorded suck, swallow and respiration. These measurements were monitored once during 3 oral feeding sessions, when infants were taking 1 to 2, 3 to 5 and 6 to 8 oral feedings per day. Management of feeding was left to the discretion of attending neonatologists. Nurses were responsible for standard feeding care. |
|
| Outcomes |
Also recorded were severity of illness, number of infants receiving all or partial breast feeding, number of co‐interventions (occupational/physical and/or speech therapy), number of parental visits, PMA, days of life, behavioural state during feeding measured on a 3‐point scale and episodes of apnoea, bradycardia and/or oxygen desaturation at the 3 monitored oral feeding sessions. |
|
| Notes | Adverse events were not reported although they were recorded as part of the protocol. Although not stated in the study, the profile of these study participants is the same as in Fucile 2011. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although it was not explicitly stated, this paper appears to report secondary outcomes for infants described in Fucile 2011, as identical numbers of infants are reported in each of the 4 groups. If so, infants in Fucile 2011 were randomised in blocks (size not stated) stratified by gestational age (26 to 29 and 30 to 32 weeks GA) and time (3‐month intervals). |
| Allocation concealment (selection bias) | Unclear risk | This was not described, although a screen was placed around the incubator for all interventions. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Caregivers were blinded. All interventions were administered by the same researcher, who therefore must have been aware of allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unblinded researcher assessed outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Some outcomes were reported for all 75 infants; some data were missing. |
| Selective reporting (reporting bias) | High risk | All of the study’s prespecified outcomes and all expected outcomes of interest to the review have not been reported. The protocol specifies that the following co‐variates were considered and recorded: severity of illness, number of infants receiving all or partial breast feeding, number of co‐interventions (occupational/physical and/or speech therapy), number of parental visits, PMA, days of life, behavioural state during feeding measured on a 3‐point scale and episodes of apnoea, bradycardia and/or oxygen desaturation at the 3 monitored oral feeding sessions. No outcomes were reported for these co‐variates. |
| Other bias | High risk | Although it was not stated in the study, the profile of these study participants is the same as in Fucile 2011. |
Gaebler 1996.
| Methods | Country: USA RCT Random sequence generation: no information Allocation concealment: no information Blinding of personnel delivering the intervention: no Blinding of outcome assessors: no |
|
| Participants | 18 participants Experimental group: N = 9 (6 male/3 female/9 Caucasian), mean birth age (range) 32.3 weeks GA (30 to 34), age (range) at start of study 34.3 weeks PCA (32 to 36), mean (range) birth weight 1836 (1605 to 2282) grams Control group: N = 9 (6 male/3 female/8 Caucasian/1 Black‐Caucasion), mean birth age (range) 32.4 weeks GA (31‐34), age (range) at start of study 34.1 weeks PCA (33 to 36), mean (range) birth weight 1729 (1410 to 1975) grams |
|
| Interventions | NPIA was administered within 24 hours of entry to study, between 30 and 90 minutes before a scheduled feeding by 1 of 4 occupational or physical therapists. Recommendations were made to nursing staff. Then all parents and nurses were provided with information regarding a 5‐minute stroking protocol. Parents of the experimental group were given further separate instruction about a 2‐minute oral motor protocol. They were instructed to carry it out 3 times a day, 5 days a week, before feedings, only until infants were nipple feeding all of their feedings for 24 hours. Parents were instructed to feed infants after they had administered the prefeeding protocol (stroking protocol or stroking, perioral and intraoral protocol). If parents were not able to administer the protocol, nursing staff did so. R‐NOMAS was administered within 48 hours of first nipple feed, then again on the following third and fifth days. They were discharged from the study once the infant managed all feeds orally for 24 hours. All protocols were to be carried out 5 minutes before feeding, 3 times a day for 5 days. Control group carried out a stroking protocol only, involving stroking baby in the isolette on back of head, across neck and shoulders, down head, down legs and down arms, 5 minutes before scheduled feeding. Experimental group was instructed to do the stroking protocol, then a 2‐minute oral motor stimulation protocol. Oral stimulation protocol was to take place outside the isolette if the infant was to be held for the feeding, otherwise inside the isolette. |
|
| Outcomes |
|
|
| Notes | Adverse effects and unwanted symptoms were not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details were provided about how infants were assigned to either group. |
| Allocation concealment (selection bias) | Unclear risk | No information was provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Protocols were posted on the isolettes, so therapists and nursing staff were aware of group assignments. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessors were 1 of the 4 researchers; all were aware of group assignments, as above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data were provided for all infants. |
| Selective reporting (reporting bias) | Low risk | All of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported. No data were missing. |
| Other bias | High risk | Parents were instructed to feed infants after they had administered the prefeeding protocol and to hold infants in a supported, flexed position for all feedings ‐ nipple or gavage ‐ to facilitate active sucking. This could have had an influence on ability to suck and feed, thereby introducing bias. Additionally, both parents and nursing staff/researchers carried out the interventions, which may have added variability in delivery of interventions. |
Harding 2006.
| Methods | Country: UK RCT Random sequence generation using stratified random sampling technique Allocation concealment reported Blinding of personnel delivering the intervention unclear Blinding of outcome assessors: unclear |
|
| Participants | 14 participants (3 male/11 female) ‐ paired groups Pair 1: GA 27 weeks, birth weight 1325 grams (intervention infant)/1085 grams (control infant) Pair 2: GA 29 weeks, birth weight 1325 grams (intervention infant)/1420 grams (control infant) Pair 3: GA 30 weeks, birth weight 1500 grams (intervention infant)/1650 grams (control infant) Pair 4: GA 32 weeks, birth weight 1920 grams (intervention infant)/1925 grams (control infant) Pair 5: GA 34 weeks, birth weight 1900 grams (intervention infant)/1925 grams (control infant) Pair 6: GA 34 weeks, birth weight 1875 grams (intervention infant)/1930 grams (control infant) Pair 7: GA 35 weeks, birth weight 2050 grams (intervention infant)/2205 grams (control infant) |
|
| Interventions | Intervention was delivered by parents. Experimental group: Parents provided 10 minutes of oral stimulation by gently stroking the bottom lip with a finger or a pacifier, then moving intraorally to stimulate the tongue with a gentle front‐to‐back movement until the finger/pacifier was prompting an NNS pattern. This was carried out during the first 10 minutes of a tube feed from the time infants demonstrated readiness to attempt oral feeding with no supplements until they received all feeds orally. Control group: No oral stimulation protocol was followed, but infants received usual developmental care approach from the unit, with an SLT providing verbal support and discussion of oral feeding. |
|
| Outcomes |
|
|
| Notes | No adverse effects were recorded or reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A 'matched pairs design' was used. Infants were matched for gestational age and as closely as possible for birth weight. A member of each pair was randomly allocated to treatment or control through a stratified random sampling technique. Allocation was completed by computer‐generated random number system. |
| Allocation concealment (selection bias) | Unclear risk | This was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Parents performed the intervention. It is unclear whether parents or medical/nursing staff were aware of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The assessment was conducted by the researcher and a speech & language therapist... who was unaware of the group allocation'. It is unclear whether the researcher and the speech and language therapist were aware of group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were reported for all enrolled infants. |
| Selective reporting (reporting bias) | Low risk | All of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported. |
| Other bias | Unclear risk | No adverse effects were recorded or reported. |
Harding 2014.
| Methods | Country: UK RCT Random allocation by computer‐generated distribution |
|
| Participants | 59 premature infants born between 26 and 35 weeks | |
| Interventions | Parents, nursing and therapy staff completed the interventions. Parents were encouraged to implement the programme a minimum of 3 times a day. Nursing/therapy staff completed interventions if parents were unable to be present. Group 1: NNS (i.e. perioral stimulation programme, as per Fuclie et al, 2002) before start of tube feeding Group 2: NNS at start of tube feeding Group 3: standard care |
|
| Outcomes |
|
|
| Notes | Adverse events were not reported. Investigators did not record how many intervention sessions were completed by parents/nurse/therapist per participant. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to 1 of 3 groups by computer‐generated distribution. |
| Allocation concealment (selection bias) | High risk | This was a non‐blinded study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was a non‐blinded study. Parents and therapy staff and nursing staff could all complete the interventions when necessary. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | This was a non‐blinded study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of 60 enrolled infants, 4 did not complete the study: 2 deteriorated with changes in health and did not progress with the intervention, 2 moved away from the area. No intention‐to‐treat analysis was completed. Acceptable reasons were provided for missing data, and all groups were equally balanced. |
| Selective reporting (reporting bias) | Low risk | All of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported. |
| Other bias | Unclear risk | Information was insufficient to permit judgement. |
Lessen 2011.
| Methods | Country: USA RCT Random sequence generation: yes Allocation concealment: unclear Blinding of personnel delivering the intervention: unclear Blinding of outcome assessors: unclear |
|
| Participants | A total of 19 participants were included: PIOMI (intervention) group: N = 10 (4 male/6 female), age range 28.1 ± 0.6 weeks PMA, birth weight 1017.3 ± 127.1 grams, weight at entry to study 1.0 ± 124.6 kg Control group: N = 9 (3 male/6 female), age range 28.0 ± 0.9 weeks PMA, birth weight 913 ± 87.8 grams, weight at entry to study 915 ± 145.2 grams Infants were enrolled if they were born between 26 and 29 weeks PMA and were appropriate for gestational age, were clinically stable but could be receiving oxygen via high‐flow nasal cannula and had no comorbidities. |
|
| Interventions |
Experimental group: received PIOMI (premature infant oral motor intervention), which is a 5‐minute oral motor programme that provides assisted movement to activate muscle contraction and provides movement against resistance to build strength. Each intervention was separated by a minimum of 9 hours and a maximum of 36 hours, with 24 hours being ideal. Control group: did not receive the 5‐minute oral stimulation intervention. PI or RA stood at the bedside during that time with both hands inside the isolette for 5 minutes, not touching the infant. Intervention took place over 7 consecutive days and outcomes were measured until discharge. Data collection began on the day the infant reached 29 weeks PMA (before oral feed commencement) and continued once a day for 7 consecutive days, ending at 30 weeks PMA. Oral feeding trial could then commence. Intervention was carried out before a feeding once a day for 7 consecutive days. A card on each participant's bed identified him/her as a participant in the study, but group assignments were blinded to nursing and medical staff and to parents by a curtain pulled around the infant's bed for both control (sham) and intervention groups. Feeding progression was tracked through a 6‐phase feeding progression protocol. The intervention was provided by the PI or by 1 of 3 research assistants (RAs). |
|
| Outcomes |
|
|
| Notes | Nine of 16 infants who received the PIOMI intervention experienced 1 to 3 mild apnoea/bradycardia episodes across the 7 days that were self‐corrected after pausing the intervention, and the intervention was continued with no further signs of intolerance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "All infants were randomly assigned in blocks of 2". Possible selection bias cannot be ruled out. |
| Allocation concealment (selection bias) | High risk | Infants allocated in "blocks of 2"; therefore next allocation of next infant was known. Also, "if subjects in either group were dropped, they were replaced by assigning the next enrolled subject to that group to maintain equal numbers in groups". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Allocation was concealed from medical and nursing staff and parents by screening researcher who performed intervention or sham. Blinding of some key study personnel was attempted, but researcher was not blinded to the groups; this is likely to introduce performance bias. It is unclear whether outcome assessor was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Progression of oral feeding was determined from bedside charts, but it is unclear who decided on progression of oral feeds. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eleven (of 30) enrolled infants were excluded post randomisation, and reasons were provided. |
| Selective reporting (reporting bias) | Unclear risk | All of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported. |
| Other bias | Unclear risk | Information was insufficient to permit judgement. |
Lyu 2014.
| Methods | Country: China RCT |
|
| Participants | Healthy preterm infants born between 29 and 34 weeks GA Intervention group: N = 32 (male 16/female16), GA 30.87 ± 1.47 weeks, weight 1597.38 ± 264.263 grams Control group: N = 31 (16 male/15 female), GA 30.92 ± 1.48 weeks, weight 1652.50 ± 327.468 grams |
|
| Interventions | Oral stimulation programme was developed by Fucile (2002) and consisted of 12 minutes of oral stimulation and 3 minutes of non‐nutritive sucking. Control group received routine feeding care. |
|
| Outcomes |
|
|
| Notes | Study authors also provided data on duration of parenteral feeding, although this is not listed as an outcome measure. Ten incidents in experimental groups due to delay or stopping halfway were recorded during the intervention process. Eight incidents were caused by delay because infants were disturbed by a medical or nursing intervention, and 2 sessions were halted after infants suffered an episode of bradycardia, which resolved spontaneously. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Infants were randomly assigned to experimental and control groups by computer‐generated random number assignment. Sample size ranged from 1 to 72 as a result of the random number generator feature in Microsoft Excel. Infants receiving numbers 1 to 36 were assigned to the experimental group, and those receiving numbers 37 to 72 were assigned to the control group. Selection bias may be present. |
| Allocation concealment (selection bias) | High risk | The order of the allocation sequence was saved and sealed in an envelope; researchers opened the envelope and recorded groups when infants met the inclusion criteria and after parental informed consent was obtained. Researchers were most likely aware of group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was not reported. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The nurse on duty, who was blind to group assignments, recorded the duration and volume of feeds in every observed oral feeding session. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Behavioural state feeding data, which were recorded at the start of the feeding session, were not reported. |
| Selective reporting (reporting bias) | High risk | Behavioural outcome data were omitted. |
| Other bias | Unclear risk | Information was insufficient to permit judgement. |
Neiva 2006.
| Methods | Country: Brazil RCT |
|
| Participants | 95 preterm infants Participants were divided into 3 groups. Weekly sucking evaluations (NNS and NS) were filmed in a standardised manner (not described) as performed by the researcher. First evaluation co‐incided with first oral feeding. Control group: N = 35 (15 male/20 female), birth age 30.2 (SD 1.82) weeks GA, age at start of study 31.4 (SD 1.5) weeks GA, birth weight 1389.1 (404.7) grams, weight at study entry 1283 (SD 372.2) grams Group 2 (NNS with pacifier): N = 30 (17 male/13 female), birth age 30.6 (SD 1.45) weeks GA, age at start of study 31.7 (SD 1.2) weeks GA, birth weight 1357 (SD 324.2) grams, weight at study entry 1294 (SD 338.5) grams Group 3 (NNS with gloved finger): N = 30 (15 male/15 female), birth age 30.6 (SD 1.4) weeks GA, age at start of study 31.7 (SD 1.3) weeks GA, birth weight 1425 (SD 298.4) grams, weight at study entry 1330 (SD 305.4) grams |
|
| Interventions |
Control: assumed to be standard care Stimulation with pacifier: orthodontic NUK pacifier used for premature infants daily, except on weekends, for 10 minutes at the same time as gavage feeds Stimulation with gloved finger: not described but delivered daily, except on weekends, for 10 minutes at the same time as orogastric feeds |
|
| Outcomes |
|
|
| Notes | It was difficult to interpret the data and present meaningful results. Language is a problem; study was published in English, but most likely this is not the first language of study authors. Data were difficult to interpret. Abbreviations were unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Infants were "distributed in a random manner", to ensure a balanced distribution of GA at birth and corrected GA in the 3 study groups. No other details were available. |
| Allocation concealment (selection bias) | High risk | All interventions and assessments were carried out by the researcher. No apparent attempts were made to conceal group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No reference is made to blinding of medical and nursing staff, family or primary caregivers. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcome assessor (i.e. researcher) was aware of group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Summary statistics were provided, but it is unclear how many infants they describe. |
| Selective reporting (reporting bias) | Unclear risk | All of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported, but individual data are not available. Results were difficult to interpret because several abbreviations used in the tables were not explained in the text nor in the Results section. |
| Other bias | Unclear risk | Report was difficult to interpret. |
Pimenta 2008.
| Methods | Country: Brazil RCT |
|
| Participants | 2 groups of healthy, stable, low birth weight, preterm infants were enrolled. 98 were enrolled; 96 remained in the study until they reached corrected age of 6 months. Group 1 (Experimental): N = 47, GA at birth 30.5 ± 1.2 weeks, GA (range) on reaching clinical stability 32 (28.6 to 35.5) weeks GA, birth weight 1204 ± 222 grams Group 2 (Control): N = 49, GA at birth 30.2 ± 1.8 weeks, GA (range) on reaching clinical stability 32.4 (27.5 to 34.4) weeks, birth weight 1125 ± 221 grams |
|
| Interventions | Experimental group received a standardised sensory‐motor‐oral stimulation programme and non‐nutritive sucking, delivered by 3 trained SLTs. Groups were followed until 6 months corrected age. Group 1 (Experimental): finger stimulation programme and NNS with a pacifier, as per Fucile 2002, performed once a day for 15 minutes during gavage feed for 10 days until oral diet commenced Group 2 (Control): sham stimulation during which the researcher stood around the incubator for the same length of time as group 1, while infants were positioned and gavage fed. No stimulation or pacifier was offered. |
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Infants were randomly assigned"; sequence generation was not described. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed, opaque, non‐translucent envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinding of medical staff at the neonatal intensive care unit and at the outpatient ward, of nursing staff who provided care to the infants, of the speech therapist who assessed infant capacity to begin sucking and of mothers was reported. Three speech therapists who delivered intervention or sham procedure to enrolled infants were not blinded to group allocation. Therefore, some key study personnel were not blinded, but as outcome assessors and all other personnel were blinded, non‐blinding of researchers is unlikely to introduce bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | A single external SLT who was double‐blinded performed clinical assessment of ability to initiate oral feeding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two infants in experimental group were lost to follow‐up at 6 months. Intention‐to‐treat analysis was reported. Reasons for loss to follow‐up were not given. |
| Selective reporting (reporting bias) | Unclear risk | All of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported. |
| Other bias | Unclear risk | It is unclear whether other biases were present. |
Rocha 2007.
| Methods | Country: Brazil RCT |
|
| Participants | Very low birth weight, healthy, stable preterm infants Experimental group: N = 49, GA 30.5 ± 1.7 weeks, birth weight 1195 ± 221 grams Control group: N = 49, GA 30.2 ± 1.8 weeks, birth weight 1125 ± 221 grams |
|
| Interventions |
Experimental group: received a stimulation protocol, as per Fucile 2002, plus non‐nutritive sucking that appears to last 15 minutes. Not clear when it took place and under what conditions. It appears that this was continued until the newborn began an exclusively oral diet ‐ for at least 10 days. Control group: received gavage tube diet with a sham procedure for 15 minutes, but this is not described |
|
| Outcomes |
Other outcomes reported but not addressed by study authors are days of life at introduction to oral feeds, days of life at full oral feeds, days of life at discharge, GA at introduction to initial oral feeds, GA at introduction of full oral feeds and GA at discharge. |
|
| Notes | No adverse events were recorded or reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A double‐blind randomised clinical trial was performed. Randomisation was stratified on the basis of gestation age ranges (26 to 28, 28.1 to 30, 30.1 to 32). Newborns were randomised when they reached a full enteral diet (i.e. 100 kcal/kg/d). |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to permit judgement of 'yes' or 'no'. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Procedures were performed by 3 SLTs, who used a previously standardised method; this is not directly described, but the Fucile 2002 protocol is cited. Therefore, staff could not have been blinded to group allocation. Staff members who measured the weight of newborns were unaware of newborn group status. Researchers had no influence on newborn hospital discharge date. Therefore, some key study personnel were not blinded, but as outcome assessors and some other personnel were blinded, non‐blinding of researchers is unlikely to introduce bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The newborn's capacity to begin an oral diet was clinically evaluated 3 times a day by an external experienced SLT blinded to which group the child belonged. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data are reported for all participants. |
| Selective reporting (reporting bias) | Low risk | All of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported. |
| Other bias | Unclear risk | This is unclear. |
Younesian 2015.
| Methods | RCT Country: Iran |
|
| Participants | 20 healthy preterm neonates Intervention group N = 10 (5 boys and 5 girls) GA 31.20 ± 0.78 weeks Control group N = 10 (5 boys and 5 girls) GA 30.90 ± 0.73 weeks All fed by tube |
|
| Interventions | Oral sensory motor stimulation programme (15‐minute stimulation programme, whose first 12 minutes included stroking the cheeks, lips, gums and tongue, and whose last 3 minutes included the newborn sucking on an index finger of the speech therapist, who was trained by the researchers) was given to the experimental group. This stimulation programme replicated that described in Fucile 2002. Interventions were started before the start of oral feeding and were applied once per day for 10 sequential days, 20 to 40 minutes before initiation of tube feeding. Control group received no stimulation except routine nursery care. | |
| Outcomes |
|
|
| Notes | Other co‐variates were taken into account, including infant's behavioural state at the beginning and at the end of feeding time via the preterm infant's behavioural scale, as well as bradycardia, apnoea and oxygen desaturation throughout oral feeding. Two sessions were implemented owing to medical instability. Two sessions were cancelled because infants had an episode of bradycardia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Convenience sampling was performed; participants were randomly assigned by a simple randomisation method. Method was not described; therefore, information was insufficient to permit a judgement of 'adequate' or 'inadequate'. |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Both nurses and physicians involved in infant management were blinded to group assignment, but breaking of blinding was possible if SLT was noted to be delivering intervention to other infants in the unit not involved in the study. The intervention SLT was aware of intervention group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Infants were weighed by the same nurse every day at 7 a.m. without clothes and diapers and before feeding. Practitioners who measured the weight of newborns were blinded to assigned group and hospital discharge time. It was not stated who recorded time to oral feeding and length of stay, and it is unclear whether they were blinded to group allocation. Commencement and advancement of oral feeding were assigned to the attending physician, who was reported to be blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Information on infants' behavioural state at the beginning and at the end of feeding time obtained via the Preterm Infants Scale is not reported. |
| Selective reporting (reporting bias) | High risk | Not all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported. |
| Other bias | Unclear risk | Adverse events were not reported. |
Zhang 2014.
| Methods | RCT Country: China Randomised groups |
|
| Participants | 108 preterm infants | |
| Interventions |
Group 1, NNS: sucked on pacifier for 5 minutes 7 to 8 times a day Group 2, oral stimulation (OS): 12‐minute peristimulation programme, as per Fucile 2002 Group 3: combined both of the above interventions |
|
| Outcomes |
|
|
| Notes | Behavioural state was measured at the start of the feeding session by the Anderson Behavioural State Scale. Episodes of apnoea, bradycardia and/or oxygen desaturation during the feeding session were also measured. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised by stratified blocked randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Information was insufficient to permit judgement. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Two experienced researchers were responsible for administration of all interventions. Initiation and advancement of oral feeding were left to the discretion of the physician; it is unclear whether the physician was blinded. It is unclear whether other personnel (nurses, parents) were blinded to allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Initiation and advancement of oral feeding were left to the discretion of the physician. Feeding variables (rate of transfer/proficiency/volume transfer at days 1, 4 and 7) and DA (day autonomous feeding achieved) were monitored by a second researcher, who was blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Study authors report, 'there was no difference in terms of behavioural state and numbers of episodes of apnoea, bradycardia or oxygen desaturations'; however, apart from severity of illness scores (Neonatal Medicine Index) provided in the table of baseline characteristics, no other data are provided to confirm this. Behavioural state data before and after feeds also are not reported. |
| Selective reporting (reporting bias) | High risk | This is the same as above. |
| Other bias | Unclear risk | Information was insufficient to permit judgement. |
CPAP: continuous positive airway pressure. DA: day autonomous feeding achieved. GA: gestational age. KMC: kangaroo mother care. NICU: neonatal intensive care unit. NNS: non‐nutritive sucking. NPIA: Neurobehavioral Preterm Infant Assessment. NS: nutritive sucking. OMS: oromotor stimulation. PCA: postconceptional age. PI: principal investigator. PIOMI: premature infant oral motor intervention. PLS: Preschool Language Scales. PMA: postmenstrual age. RA: research assistant. RCT: randomised controlled trial. R‐NOMAS: Revised Neonatal Oral Motor Assessment Scale. SEM: standard error of the mean. SLT: speech and language therapist.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 1986 | This is a descriptive literature review of interventions only. |
| Bache 2014 | Populations described were preterm infants with respiratory distress syndrome and chronic lung disease, making comparison with healthy preterm infants difficult. |
| Barlow 2008 | Researchers described an adapted pulsating pacifier for patterned orocutaneous stimulation, not a finger stimulation protocol. |
| Barlow 2014a | Outcomes were specific to non‐nutritive sucking parameters only, not to feeding. The population under investigation consists of preterm infants with respiratory distress syndrome and preterm infants of diabetic mothers. It is difficult to compare these infants with healthy preterm infants. |
| Barlow 2014b | Populations described are preterm infants with respiratory distress syndrome and chronic lung disease, making comparison with healthy preterm infants difficult. |
| Bingham 2010 | This was not a randomised controlled trial. It was a prospective observational study of 51 infants in various NICUs. |
| Bragelien 2007 | Method of sucking stimulation described as the intervention was based on 'Vojta's' technique (i.e. initiating reflex activity of striate and smooth muscle by stroking the chest and underneath the jaw). This was not a finger stimulation protocol by our definition. |
| Breton 2008 | This is a review of literature and current research. |
| Brown 2013 | This case study design did not involve oral stimulation. |
| Case‐Smith 1988 | This trial used a single‐study design. |
| Chang 2007 | Intervention described was not an oral stimulation intervention. |
| Chorna 2014 | A percentage of both intervention and control groups had 'white matter injury, all types' and 'white matter injury, severe', as reported, and these participants cannot be extracted from the rest of the group. Therefore, not all infants in both groups were 'healthy preterm infants', and this study cannot be compared with the other included studies, from which such infants were excluded. |
| Christensen 1976 | This study did not include premature infants and was not a randomised controlled trial. |
| Coker‐Bolt 2013 | This was not a randomised controlled trial. Two groups were compared, including 1 treatment group and 1 historical group, which did not receive treatment. |
| Collins 2004 | Intervention described is not an oral stimulation intervention. |
| Dawson 2013 | No oral stimulation intervention was reported. |
| De Curtis 1986 | This study used an inadequate design and inappropriate outcome measures. |
| Dieter 1997 | This study is a literature review. |
| Einarsson‐Brackes 1994 | This is not a randomised controlled trial. |
| Engebretson 1997 | Appropriate outcome measures were not included. |
| Ernst 1989 | Intervention involves use of pacifiers during tube feeds and did not involve a finger stimulation protocol. |
| Faherty 2006 | This is a review and discussion of the literature. |
| Fan 2013 | This trial did not report on any of our primary or secondary outcomes. |
| Fewtrell 2012 | This trial assessed bottle design ‐ not oral stimulation. |
| Field 1982 | Infants were given pacifiers during all tube feeds. Study did not involve a finger stimulation programme. |
| Finan 1996 | This study described development of a piece of equipment for assessing the sucking ability of preterm infants. |
| Fucile 2009 | A controlled flow vacuum free bottle system is not an oral stimulation intervention. |
| Gill 1988 | No relevant outcomes were measured. Behavioural state observations were reported. |
| Gill 1992 | No relevant outcomes were measured. Behavioural state observations were reported. |
| Glass 1994 | This is a review article. |
| Gosa 2006 | This is a review and discussion of current literature. |
| Hill 2000 | This was a cross‐over trial. |
| Howard 2003 | This study did not include preterm infants < 37 weeks. |
| Hwang 2010 | This was not a randomised controlled trial. |
| Kao 2010 | This study used a cross‐over design. |
| Kumar 2010 | Spoon feeding is not an oral stimulation intervention. |
| Lau 2000b | This was not a randomised controlled trial. |
| Lau 2012 | Intervention options involve pacifier sucking and swallowing or placing a milk bolus on the tongue, where the bolus rests before entering the pharynx. Neither is a finger stimulation protocol by our definition. |
| Lau 2014 | This is a review and discussion of current literature. |
| Loewy 2013 | Music therapy described involves presentation of audio only to premature infants and does not involve an oral stimulation intervention. |
| Luo 2012 | The study population consisted of mechanically ventilated preterm infants, making comparison with healthy preterm infants difficult. Additionally, outcomes did not include oral feeding, but rather time to reach full enteral feeding, birth weight recovery time, body weight growth rate, hospitalisation time, feeding tolerance and mechanical ventilation‐related complications. |
| Malhotra 1999 | This was not a randomised controlled trial. |
| Mattes 1996 | Intervention involved sweet tastes presented on a modified pacifier ‐ not a finger stimulation protocol. |
| McCain 1995 | This was not a randomised controlled trial. Infants served as their own controls. |
| McCain 2001 | Intervention involved semi‐demand gavage feeding with pacifier for NNS; this was already explored in a previous Cochrane review (Watson 2015). |
| McCain 2002 | Intervention involved semi‐demand gavage feeding with pacifier for NNS; this was already explored in a previous Cochrane review (Watson 2015). |
| McCain 2012 | Study looked at transition from gavage to nipple feeding for preterm infants with bronchopulmonary dysplasia, making comparison with healthy preterm infants difficult. Additionally, use of a pacifier was not consistent for all infants and appears to have been done only to bring the baby to an alert state for feeding trials, only if necessary; this was not an integral component of the intervention. |
| Moyses 2013 | This is a systematic review ‐ not an RCT. |
| Philbin 2011 | This study describes an assessment technique/process only. |
| Pickler 1996 | This was not a randomised controlled trial. |
| Pickler 2004 | This study used an inadequate randomised cross‐over design, by which participants were their own controls over 2 bottle feeds. |
| Poore 2008b | Intervention was the NTrainer device, which delivers digitally patterned orocutaneous stimulation via an adapted pacifier ‐ not a finger stimulation protocol. |
| Poore 2009 | This was not a randomised controlled trial. It was a descriptive review. |
| Puckett 2008 | This study did not test an oral stimulation intervention. |
| Rocha 2002 | This was not a randomised controlled trial. |
| Ross 2008a | This is a review and discussion of current literature. |
| Ross 2008b | This is a descriptive review article. |
| Ross 2011 | This study describes the development of an assessment protocol. |
| Ross 2013 | This is a systematic review. |
| Scheel 2005 | This was not a randomised controlled trial. |
| Shaker 2010 | This is a review and discussion of current literature. |
| Shaker 2013 | This is a descriptive review of current literature and practice. |
| Sheppard 2005 | This is a review of current literature. |
| Sheppard 2007 | This is a descriptive review of the literature. |
| Simpson 2002 | Intervention described is not an oral stimulation intervention. |
| Stade 2002 | Intervention described is not an oral stimulation intervention. |
| Standley 2000 | This was not a randomised controlled trial. |
| Standley 2003 | This was not a randomised controlled trial. |
| Standley 2010 | Investigators used the Pacifier Activated Lullaby intervention (music activated in cot on commencement of pacifier sucking), which does not involve a finger stimulation protocol. |
| Standley 2012 | This discussion paper reviews previously published data (Standley 2010a). |
| Thoyre 2012 | This study did not include oral stimulation and was not a randomised controlled trial. |
| Thukral 2012 | Researchers used skin‐to‐skin contact ‐ not oral stimulation. |
| Vianna 2011 | Investigators did not provide an oral stimulation intervention. |
| White 2013 | This is a review of current practice and does not involve oral stimulation. |
| White‐Traut 2002a | Intervention described (ATVV) was not an oral stimulation intervention. |
| White‐Traut 2002b | Intervention described (ATVV) was not an oral stimulation intervention. |
| White‐Traut 2013a | This was a case study. |
| White‐Traut 2013b | This was not an RCT; it is descriptive only. |
| Yildiz 2011b | Intervention groups included infants who were provided with pacifiers during gavage feeds, lullabies during gavage feeds or standard gavage feed care. No finger stimulation protocols were used. |
| Yildiz 2011a | This study looks at olfaction ‐ not an oral stimulation intervention. |
| Zimmerman 2009 | This is a general review of the literature. |
ATVV: auditory, tactile, visual and vestibular intervention. NICU: neonatal intensive care unit. NNS: non‐nutritive sucking. RCT: randomised controlled trial.
Differences between protocol and review
The protocol and the review differ in the following ways.
The title of the review has changed from 'Effects of oral stimulation for oral feeding in preterm infants' to 'Oral stimulation for promoting oral feeding in preterm infants'.
Review authors have redefined 'oral stimulation intervention' to provide a specific focus and to narrow the remit of the review for clearer reporting. Initial searching under the original definition (Greene 2012) resulted in an extremely heterogeneous group of studies describing a wide spectrum of incomparable interventions including semi‐demand gavage feeding, use of a pacifier with gavage feeds/direct active stimulation with pacifier, finger stimulation protocols before feeds (gavage or oral) with or without other supports, a device delivering timed electronic pulses via a nipple before feeds, body stroking protocols with or without oral stimulation, listening to music and sucking on a pacifier before feeds and sweet tastes on a pacifier with gavage feeds. Consultation among the Cochrane Neonatal Review Group Editors and the review authors resulted in agreement on narrowing the focus of this review to include only studies that described a 'finger stimulation' intervention. Additionally, several subsequent Cochrane reviews have addressed some of these interventions, for example, non‐nutritive sucking (Pinelli 2005) and semi‐demand feeding (Watson 2015), so these data have been examined elsewhere. Therefore, this current review has a narrower focus, and the definition of oral stimulation has been refined to reflect this change.
We have excluded preterm populations with defined respiratory disease. We identified in our search several studies involving preterm populations with defined respiratory disease. We agreed to exclude these participants, as this group is at increased risk of feeding and swallowing problems. We had not directly specified in the original protocol that we would exclude them. We believe that making comparisons between this group and healthy preterm infants would be difficult.
We added methods and a plan for Summary of findings tables and GRADE recommendations; these were not included in the original protocol.
Contributions of authors
All review authors contributed to the development of this review.
Sources of support
Internal sources
No sources of support supplied
External sources
Health Research Board Cochrane Training Fellowship, Ireland.
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been supported with federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
-
National Institute for Health Research, UK.
Editorial support for Cochrane Neonatal has been funded with funds from a UK National Institute of Health Research Grant (NIHR) Cochrane Programme Grant (13/89/12). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR, or the UK Department of Health.
Declarations of interest
Review authors have declared no conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Asadollahpour 2015 {published data only}
- Asadollahpour F, Yadegari F, Soleimani F, Khalesi N. The effects of non‐nutritive sucking and pre‐feeding oral stimulation on time to achieve independent oral feeding for preterm infants. Iranian Journal of Pediatrics 2015;25(3):e809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bala 2016 {published data only}
- Bala P, Rupinder K, Muckhopadhyay K, Kaur S. Oromotor stimulation for transition from gavage to full oral feeding in preterm neonates: a randomized controlled trial. Indian Pediatrics 2016;53(1):36‐8. [DOI] [PubMed] [Google Scholar]
Boiron 2007 {published data only}
- Boiron M, Nobrega L, Roux S, Henrot A, Saliba E. Effects of oral stimulation and oral support on non‐nutritive sucking and feeding performance in preterm infants. Developmental Medicine and Child Neurology 2007;49(6):439‐44. [DOI] [PubMed] [Google Scholar]
- Boiron M, Nobrega L, Roux S, Saliba E. Pharyngeal swallowing rhythm in response to oral sensorimotor programs in preterm infants. Journal of Neonatal Nursing 2009;15(4):123‐8. [Google Scholar]
Fucile 2002 {published data only}
- Fucile S, Gisel E, Lau C. Oral stimulation accelerates the transition from tube to oral feeding in preterm infants. Journal of Pediatrics 2002;141(2):230‐6. [DOI] [PubMed] [Google Scholar]
- Fucile S, Gisel EG, Lau C. Effect of an oral stimulation program on sucking skill maturation of preterm infants. Developmental Medicine and Child Neurology 2005;47(3):158‐62. [DOI] [PubMed] [Google Scholar]
Fucile 2011 {published data only}
- Fucile S, Gisel EG. Sensorimotor interventions improve growth and motor function in preterm infants. Neonatal Network 2010;29(6):359‐66. [DOI] [PubMed] [Google Scholar]
Fucile 2012 {published data only}
- Fucile S, McFarland DH, Gisel EG, Lau C. Oral and nonoral sensorimotor interventions facilitate suck‐swallow‐respiration functions and their coordination in preterm infants. Early Human Development 2012;88(6):345‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaebler 1996 {published data only}
- Gaebler CP, Hanzlik JR. The effects of a prefeeding stimulation program on preterm infants. American Journal of Occupational Therapy 1996;50(3):184‐92. [DOI] [PubMed] [Google Scholar]
Harding 2006 {published data only}
- Harding CM, Law J, Pring T. The use of non‐nutritive sucking to promote functional sucking skills in premature infants: an exploratory trial. Infant 2006;2(6):238‐43. [Google Scholar]
Harding 2014 {published data only}
- Harding C, Frank L, Someren V, Hilari K, Botting N. How does non‐nutritive sucking support infant feeding?. Infant Behaviour and Development 2014;37:457‐64. [DOI] [PubMed] [Google Scholar]
Lessen 2011 {published data only}
- Lessen BS. Effect of the premature infant oral motor intervention on feeding progression and length of stay in preterm infants. Advances in Neonatal Care 2011;11(2):129‐39. [DOI] [PubMed] [Google Scholar]
Lyu 2014 {published data only}
- Lyu T, Zhang Y, Hu X, Cao Y, Ren P. The effect of an early oral stimulation program on oral feeding of preterm infants. International Journal of Nursing Sciences 2014;1(1):42‐7. [Google Scholar]
Neiva 2006 {published data only}
- Neiva FC, Leone CR. Development of sucking rhythm and the influence of stimulation in premature infants. Pró‐Fono Revista de Atualizacao Cientifica 2007;19(3):241‐8. [DOI] [PubMed] [Google Scholar]
- Neiva FC, Leone CR. Sucking in preterm newborns and the sucking stimulation [Succao em recem‐nascidos pré‐termo e estimulacao da succao]. Pró‐Fono Revista de Atualizacao Cientifica 2006;18(2):141‐50. [DOI] [PubMed] [Google Scholar]
Pimenta 2008 {published data only}
- Pimenta HP, Moreira ME, Rocha AD, Gomes SC Jr, Pinto LW, Lucena SL. Effects of non‐nutritive sucking and oral stimulation on breastfeeding rates for preterm, low birth weight infants: a randomized clinical trial. Jornal de Pediatria 2008;84(5):423‐7. [DOI] [PubMed] [Google Scholar]
Rocha 2007 {published data only}
- Rocha AD, Moreira ME, Pimenta HP, Ramos JR, Lucena SL. A randomized study of the efficacy of sensory‐motor‐oral stimulation and non‐nutritive sucking in very low birthweight infant. Early Human Development 2007;83(6):385‐8. [DOI] [PubMed] [Google Scholar]
Younesian 2015 {published data only}
- Younesian S, Yadegari F, Soleimani F. Impact of oral sensory motor stimulation on feeding performance, length of hospital stay, and weight gain of preterm infants in NICU. Iran Red Crescent Medical Journal 2015;17(7):1‐6. [10.5812/ircmj.17(5)2015.13515.eCollection 2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2014 {published data only}
- Zhang Y, Lyu T, Hu X, Shi P, Cao Y, Latour JM. Effect of nonnutritive sucking and oral stimulation on feeding performance in preterm infants: a randomized controlled trial. Paediatric Critical Care Medicine 2014;15(7):608‐14. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anderson 1986 {published data only}
- Anderson J. Sensory intervention with the preterm infant in the neonatal intensive care unit. The American Journal of Occupational Therapy 1986;40(1):19‐26. [DOI] [PubMed] [Google Scholar]
Bache 2014 {published data only}
- Bache M, Pizon E, Jacobs J, Vaillant M, Lecomte A. Effects of pre‐feeding oral stimulation on oral feeding in preterm infants: a randomized clinical trial. Early Human Development 2014;90(3):125‐9. [PUBMED: 24461572] [DOI] [PubMed] [Google Scholar]
Barlow 2008 {published data only}
- Barlow SM, Finan DS, Lee J, Chu S. Synthetic orocutaneous stimulation entrains preterm infants with feeding difficulties to suck. Journal of Perinatology 2008;28(8):541‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barlow 2014a {published data only}
- Barlow SM, Lee J, Wang J, Oder A, Oh H, Hall S, et al. Effects of oral stimulus frequency spectra on the development of nonnutritive suck in preterm infants with respiratory distress syndrome or chronic lung disease, and preterm infants of diabetic mothers. Journal of Neonatal Nursing 2014;20(4):178‐88. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barlow 2014b {published data only}
- Barlow SM, Lee J, Wang J, Oder A, Hall S, Knox K, et al. Frequency‐modulated orocutaneous stimulation promotes non‐nutritive suck development in preterm infants with respiratory distress syndrome or chronic lung disease. Journal of Perinatology 2014;34(2):136‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bingham 2010 {published data only}
- Bingham PM, Ashikaga T, Abbasi S. Prospective study of non‐nutritive sucking and feeding skills in premature infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2010;95(3):F194‐200. [DOI] [PubMed] [Google Scholar]
Bragelien 2007 {published data only}
- Bragelien R, Rokke W, Markestad T. Stimulation of sucking and swallowing to promote oral feeding in premature infants. Acta Paediatrica 2007;96(10):1430‐2. [DOI] [PubMed] [Google Scholar]
Breton 2008 {published data only}
- Breton S, Steinwender S. Timing introduction and transition to oral feeding in preterm infants: current trends and practice. Newborn & Infant Nursing Reviews 2008;8(3):153‐9. [Google Scholar]
Brown 2013 {published data only}
- Brown LF, Pickler R. A guided feeding intervention for mothers of preterm infants: two case studies. Journal of Specialists in Pediatric Nursing 2013;18(2):98‐108. [DOI] [PubMed] [Google Scholar]
Case‐Smith 1988 {published data only}
- Case‐Smith J. An efficacy study of occupational therapy with high‐risk neonates. The American Journal of Occupational Therapy 1988;42(8):499‐506. [DOI] [PubMed] [Google Scholar]
Chang 2007 {published data only}
- Chang YL, Lin CP, Lin YJ, Lin CH. Effects of single hole and cross cut nipple units on feeding efficiency and physiologic parameters in preterm infants. Journal of Nursing Research 2007;15(3):215‐23. [DOI] [PubMed] [Google Scholar]
Chorna 2014 {published data only}
- Chorna OD, Slaughter JC, Wang L, Stark AR, Nathalie L. A pacifier activated music player with mother's voice improves oral feeding in preterm infants. Pediatrics 2014;133(3):462‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christensen 1976 {published data only}
- Christensen S, Dubignon J, Campbell D. Variations in intra‐oral stimulation and nutritive sucking. Child Development 1976;47(2):539‐42. [PubMed] [Google Scholar]
Coker‐Bolt 2013 {published data only}
- Coker‐Bolt P, Jarrard C, Woodard F, Merril P. The effects of oral motor stimulation on feeding behaviours of infants born with univentricle anatomy. Journal of Pediatric Nursing 2013;28(1):64‐71. [DOI] [PubMed] [Google Scholar]
Collins 2004 {published data only}
- Collins CT, Ryan P, Crowther CA, McPhee AJ, Paterson S, Hiller JE. Effects of bottles, cups and dummies on breast feeding in preterm infants. British Medical Journal 2004;329(7459):193‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dawson 2013 {published data only}
- Dawson JA, Myers LR, Moorhead A, Jacobs SE, Ong K, Salo F, et al. A randomised trial of two techniques for bottle feeding preterm infants. Journal of Paediatrics and Child Health 2013;49(6):462‐6. [DOI] [PubMed] [Google Scholar]
De Curtis 1986 {published data only}
- Curtis M, McIntosh N, Ventura V, Brooke O. Effect of nonnutritive sucking on nutrient retention in preterm infants. Journal of Pediatrics 1986;109(5):888‐90. [DOI] [PubMed] [Google Scholar]
Dieter 1997 {published data only}
- Dieter JN, Emory EK. Supplemental stimulation of premature infants: a treatment model. Journal of Pediatric Psychology 1997;22(3):281‐95. [DOI] [PubMed] [Google Scholar]
Einarsson‐Brackes 1994 {published data only}
- Einarsson‐Brackes LM, Deitz J, Price R, Glass R, Hays R. The effect of oral support on sucking efficiency in preterm infants. The American Journal of Occupational Therapy 1994;48(6):490‐8. [DOI] [PubMed] [Google Scholar]
Engebretson 1997 {published data only}
- Engebretson JC, Wardell DW. Development of a pacifier for low‐birth‐weight infants' nonnutritive sucking. Journal of Obstetric, Gynecologic and Neonatal Nursing 1997;26(6):660‐4. [DOI] [PubMed] [Google Scholar]
Ernst 1989 {published data only}
- Ernst JA, Rickard KA, Neal PR, Yu PL, Oei TO, Lemons JA. Lack of improved growth outcome related to nonnutritive sucking in very low birth weight premature infants fed a controlled nutrient intake: a randomized controlled trial. Pediatrics 1989;83(5):706‐16. [PubMed] [Google Scholar]
Faherty 2006 {published data only}
- Faherty AS. Assessment and management considerations for oral feeding of the premature infant on the neonatal intensive care unit. SIG 13 Perspectives on Swallowing and Swallowing Disorders (Dysphagia) 2006;15:3‐9. [Google Scholar]
Fan 2013 {published data only}
- Fan YC, Chung SC, Yang PH, Hung CC, Li HJ. The effect of oral training on vital signs of premature infants. Journal of Clinical Nursing 2013;22(11‐12):1771‐8. [PUBMED: 23279713] [DOI] [PubMed] [Google Scholar]
Fewtrell 2012 {published data only}
- Fewtrell MS, Kennedy K, Nicholl R, Khakoo A, Lucas A. Infant feeding bottle design, growth and behaviour: results from a randomised trial. BMC Research Notes 2012;16(5):150. [DOI: 10.1186/1756-0500-5-150] [DOI] [PMC free article] [PubMed] [Google Scholar]
Field 1982 {published data only}
- Field T, Ignatoff E, Stringer S, Brennan J, Greenberg R, Widmayer S, et al. Nonnutritive sucking during tube feedings: effects on preterm neonates in an intensive care unit. Pediatrics 1982;70(3):381‐4. [PubMed] [Google Scholar]
Finan 1996 {published data only}
- Finan DS, Barlow SM. The Actifier: a device for neurophysiological studies of orofacial control in human infants. Journal of Speech and Hearing Research 1996;39(4):833‐8. [PubMed] [Google Scholar]
Fucile 2009 {published data only}
- Fucile S, Gisel E, Schanler RJ, Lau C. A controlled‐flow vacuum‐free bottle system enhances preterm infants' nutritive sucking skills. Dysphagia 2009;24(2):145‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gill 1988 {published data only}
- Gill NE, Behnke M, Conlon M, McNeely JB, Anderson GC. Effect of nonnutritive sucking on behavioral state in preterm infants before feeding. Nursing Research 1988;37(6):347‐50. [PubMed] [Google Scholar]
Gill 1992 {published data only}
- Gill NE, Behnke M, Conlon M, Anderson GC. Nonnutritive sucking modulates behavioral state for preterm infants before feeding. Scandinavian Journal of Caring Sciences 1992;6(1):3‐7. [DOI] [PubMed] [Google Scholar]
Glass 1994 {published data only}
- Glass RP, Wolf LS. A global perspective on feeding assessment in the neonatal intensive care unit. The American Journal of Occupational Therapy 1994;48(6):514‐26. [DOI] [PubMed] [Google Scholar]
Gosa 2006 {published data only}
- Gosa M. Therapeutic considerations for children and infants with feeding tubes. SIG 13 Perspectives on Swallowing and Swallowing Disorders (Dysphagia) 2006;15:15‐20. [DOI: 10.1044/sasd15.3.15] [DOI] [Google Scholar]
Hill 2000 {published data only}
- Hill AS, Kurlowski TB, Garcia J. Oral support measures used in feeding the preterm infant. Nursing Research 2000;49(1):2‐10. [DOI] [PubMed] [Google Scholar]
Howard 2003 {published data only}
- Howard CR, Howard FM, Lanphear B, Eberly S, deBlieck EA, Oakes D, et al. Randomized clinical trial of pacifier use and bottle‐feeding or cupfeeding and their effect on breastfeeding. Pediatrics 2003;111(3):511‐8. [DOI] [PubMed] [Google Scholar]
Hwang 2010 {published data only}
- Hwang YS, Lin CH, Coster WJ, Bigsby R, Vergara E. Effectiveness of cheek and jaw support to improve feeding performance of preterm infants. The American Journal of Occupational Therapy 2010;64(6):886‐94. [DOI] [PubMed] [Google Scholar]
Kao 2010 {published data only}
- Kao HM, Lin CH, Chang YJ. Feeding with cross‐cut teats has better sucking effects and oxygenation in preterm infants with chronic lung disease. Journal of Clinical Nursing 2010;19(21‐22):3016‐22. [DOI] [PubMed] [Google Scholar]
Kumar 2010 {published data only}
- Kumar A, Dabas P, Singh B. Spoon feeding results in early hospital discharge of low birth weight babies. Journal of Perinatology 2010;30(3):209‐17. [DOI] [PubMed] [Google Scholar]
Lau 2000b {published data only}
- Lau C, Schanler RJ. Oral feeding in premature infants: advantage of a self‐paced milk flow. Acta Paediatrica 2000;89(4):453‐9. [DOI] [PubMed] [Google Scholar]
Lau 2012 {published data only}
- Lau C, Smith EO. Interventions to improve the oral feeding performance of preterm infants. Acta Paediatrica 2012;101(7):e269‐74. [DOI] [PubMed] [Google Scholar]
Lau 2014 {published data only}
- Lau C. Interventions to improve oral feeding performance of preterm infants. SIG 13 Perspectives on Swallowing and Swallowing Disorders (Dysphagia) 2014;23:23‐45. [DOI: ] [Google Scholar]
Loewy 2013 {published data only}
- Loewy J, Stewart K, Dassler A, Telsey A, Homel P. The effects of music therapy on vital signs, feeding, and sleep in premature infants. Pediatrics 2013;131(5):902‐18. [DOI] [PubMed] [Google Scholar]
Luo 2012 {published data only}
- Luo CC, Li RL, Zhang SY, Lin HQ. Application of non‐nutritive sucking in preterm infants requiring mechanical ventilation. Zhongguo Dang Dai Er Ke Za Zhi 2012;14(3):169‐71. [PUBMED: 22433400] [PubMed] [Google Scholar]
Malhotra 1999 {published data only}
- Malhotra N, Vishwambaran L, Sundaram KR, Narayanan I. A controlled trial of alternative methods of oral feeding in neonates. Early Human Development 1999;54(1):29‐38. [DOI] [PubMed] [Google Scholar]
Mattes 1996 {published data only}
- Mattes RD, Maone T, Wager‐Page S, Beauchamp G, Bernbaum J, Stallings V, et al. Effects of sweet taste stimulation on growth and sucking in preterm infants. Journal of Obstetric, Gynecologic and Neonatal Nursing 1996;25(5):407‐14. [DOI] [PubMed] [Google Scholar]
McCain 1995 {published data only}
- McCain GC. Promotion of preterm infant nipple feeding with nonnutritive sucking. Journal of Pediatric Nursing 1995;10(1):3‐8. [DOI] [PubMed] [Google Scholar]
McCain 2001 {published data only}
- McCain GC, Gartside PS, Greenberg JM, Lott JW. A feeding protocol for healthy preterm infants that shortens time to oral feeding. Journal of Pediatrics 2001;139(3):374‐9. [DOI] [PubMed] [Google Scholar]
McCain 2002 {published data only}
- McCain GC, Gartside PS. Behavioral responses of preterm infants to a standard‐care and semi‐demand feeding protocol. Newborn and Infant Nursing Reviews 2002;2(3):187‐93. [Google Scholar]
McCain 2012 {published data only}
- McCain GC, Moral T, Duncan RC, Fontaine JL, Pino LD. Transition from gavage to nipple feeding for preterm infants with bronchopulmonary dysplasia. Nursing Research 2013;61(6):380‐7. [DOI: 10.1097/NNR.0b013e318268cefb.] [DOI] [PubMed] [Google Scholar]
Moyses 2013 {published data only}
- Moyses HE, Johnson MJ, Leaf AA, Cornelius VR. Early parenteral nutrition and growth outcomes in preterm infants: a systematic review and meta‐analysis. American Journal of Clinical Nutrition 2013;97(4):816‐26. [DOI: 10.3945/ajcn.112.042028] [DOI] [PubMed] [Google Scholar]
Philbin 2011 {published data only}
- Philbin MK, Ross ES. The SOFFI reference guide: text, algorithms, and appendices: a manualized method for quality bottle‐feedings. Journal of Perinatal & Neonatal Nursing 2011;25(4):360‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pickler 1996 {published data only}
- Pickler RH, Frankel HB, Walsh KM, Thompson NM. Effects of nonnutritive sucking on behavioral organization and feeding performance in preterm infants. Nursing Research 1996;45(3):132‐5. [DOI] [PubMed] [Google Scholar]
Pickler 2004 {published data only}
- Pickler RH, Reyna BA. Effects of non‐nutritive sucking on nutritive sucking, breathing, and behavior during bottle feedings of preterm infants. Advances in Neonatal Care 2004;4(4):226‐34. [DOI] [PubMed] [Google Scholar]
Poore 2008b {published data only}
- Poore M, Zimmerman E, Barlow SM, Wang J, Gu F. Patterned orocutaneous therapy improves sucking and oral feeding in preterm infants. Acta Paediatrica 2008;97(7):920‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Poore 2009 {published data only}
- Poore MA, Barlow SM. Suck predicts neuromotor integrity and developmental outcomes. Perspectives on Speech Science and Orofacial Disorders 2009;19(1):44‐51. [Google Scholar]
Puckett 2008 {published data only}
- Puckett B, Grover VK, Holt T, Sankaran K. Cue‐based feeding for preterm infants: a prospective trial. American Journal of Perinatology 2008;25(10):623‐8. [DOI] [PubMed] [Google Scholar]
Rocha 2002 {published data only}
- Rocha NM, Martinez FE, Jorge SM. Cup or bottle for preterm infants: effects on oxygen saturation, weight gain, and breastfeeding. Journal of Human Lactation 2002;18(2):132‐8. [DOI] [PubMed] [Google Scholar]
Ross 2008a {published data only}
- Ross ES. Feeding in the NICU and issues that influence success. SIG 13 Perspectives on Swallowing and Swallowing Disorders (Dysphagia) 2008;17(3):94‐100. [DOI: 10.1044/sasd17.3.94] [DOI] [Google Scholar]
Ross 2008b {published data only}
- Ross ES. Feeding in the NICU and issues that influence success. Perspectives on Swallowing and Swallowing Disorders (Dysphagia) 2008;17(3):94‐100. [Google Scholar]
Ross 2011 {published data only}
- Ross ES, Philbin MK. Supporting oral feeding in fragile infants: an evidence‐based method for quality bottle‐feedings of preterm, ill, and fragile infants. Journal of Perinatal & Neonatal Nursing 2011;25(4):349‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ross 2013 {published data only}
- Ross ES, Browne JV. Feeding outcomes in preterm infants after discharge from the neonatal intensive care unit (NICU): a systematic review. Newborn and Infant Nursing Reviews 2013;12(2):87‐93. [Google Scholar]
Scheel 2005 {published data only}
- Scheel CE, Schanler RJ, Lau C. Does the choice of bottle nipple affect the oral feeding performance of very‐low‐birthweight (VLBW) infants?. Acta Paediatrica 2005;94(9):1266‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaker 2010 {published data only}
- Shaker CS. Improving feeding outcomes in the NICU: moving from volume‐driven to infant‐driven feeding. SIG 13 Perspectives on Swallowing and Swallowing Disorders (Dysphagia) 2010;19:68‐74. [DOI: ] [Google Scholar]
Shaker 2013 {published data only}
- Shaker S. Cue‐based coregulated feeding in the neonatal intensive care unit: supporting parents in learning to feed their preterm infant. Newborn and Infant Nursing Reviews 2013;13(1):51‐5. [Google Scholar]
Sheppard 2005 {published data only}
- Sheppard JJ. The role of oral sensorimotor therapy in the treatment of pediatric dysphagia. SIG 13 Perspectives on Swallowing and Swallowing Disorders (Dysphagia) 2005;14:6‐10. [DOI: 10.1044/sasd14.2.6] [DOI] [Google Scholar]
Sheppard 2007 {published data only}
- Sheppard JJ, Fletcher KR. Evidence‐based interventions for breast and bottle feeding in the neonatal intensive care unit. Seminars in Speech & Language 2007;28(3):204‐12. [DOI] [PubMed] [Google Scholar]
Simpson 2002 {published data only}
- Simpson C, Schanler RJ, Lau C. Early introduction of oral feeding in preterm infants. Pediatrics 2002;110(3):517‐22. [DOI] [PubMed] [Google Scholar]
Stade 2002 {published data only}
- Stade B, Bishop C. A semidemand feeding protocol reduced time to full oral feeding in healthy preterm infants. Evidence Based Nursing 2002;5(3):74. [DOI] [PubMed] [Google Scholar]
Standley 2000 {published data only}
- Standley JM. The effect of contingent music to increase non‐nutritive sucking of premature infants. Pediatric Nursing 2000;26(5):493‐5, 498‐9. [PubMed] [Google Scholar]
Standley 2003 {published data only}
- Standley JM. The effect of music reinforced‐nonnutritive sucking on feeding rate of premature infants. Journal of Pediatric Nursing 2003;18(3):169‐73. [DOI] [PubMed] [Google Scholar]
Standley 2010 {published data only}
- Standley JM, Cassidy J, Grant R, Cevasco A, Szuch C, Nguyen J, et al. The effect of music reinforcement for non‐nutritive sucking on nipple feeding of premature infants. Pediatric Nursing 2010;36(3):138‐45. [PubMed] [Google Scholar]
Standley 2012 {published data only}
- Standley JM. A discussion of evidence‐based music therapy to facilitate feeding skills of premature infants: the power of contingent music. The Arts in Psychotherapy 2012;39(5):379‐82. [Google Scholar]
Thoyre 2012 {published data only}
- Thoyre SM, Holditch‐Davis D, Schwartz TA, Melendez Roman CR, Nix W. Coregulated approach to feeding preterm infants with lung disease: effects during feeding. Nursing Research 2012;61(4):242‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thukral 2012 {published data only}
- Thukral A, Sankar MJ, Agarwal R, Gupta N, Deorari AK, Paul VK. Early skin‐to‐skin contact and breast‐feeding behavior in term neonates: a randomized controlled trial. Neonatology 2012;102(2):114‐9. [DOI] [PubMed] [Google Scholar]
Vianna 2011 {published data only}
- Vianna MN, Barbosa AP, Carvalhaes AS, Cunha AJ. Music therapy may increase breastfeeding rates among mothers of premature newborns: a randomized controlled trial. Jornal de Pediatria (Rio J) 2011;87(3):206‐12. [DOI] [PubMed] [Google Scholar]
White 2013 {published data only}
- White A, Parnell K. The transition from tube to full oral feeding (breast or bottle) ‐ a cue based developmental approach. Journal of Neonatal Nursing 2013;19(4):189‐97. [Google Scholar]
White‐Traut 2002a {published data only}
- White‐Traut RC, Nelson MN, Silvestri JM, Vasan U, Littau S, Meleedy‐Rey P, et al. Effect of auditory, tactile, visual and vestibular intervention on length of stay, alertness and feeding progression in preterm infants. Developmental Medicine and Child Neurology 2002;44(2):91‐7. [DOI] [PubMed] [Google Scholar]
White‐Traut 2002b {published data only}
- White‐Traut RC, Nelson MN, Silvestri JM, Vasan U, Patel M, Cardenas L. Feeding readiness behaviours and feeding efficiency in response to ATVV intervention. Newborn and Infant Nursing Reviews 2002;2(3):166‐73. [Google Scholar]
White‐Traut 2013a {published data only}
- White‐Traut R, Shapiro N, Healy‐Baker E, Menchavez L, Rankin K, Medoff‐Cooper B. Lack of feeding progression in a preterm infant: a case study. Advances in Neonatal Care 2013;13(2):175‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
White‐Traut 2013b {published data only}
- White‐Traut R, Pham T, Rankin K, Norr K, Shapiro N, Yoder J. Exploring factors related to oral feeding progression in premature infants. Advances in Neonatal Care 2013;13(4):288‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yildiz 2011a {published data only}
- Yildiz A, Arikan D, Gozum S, Tastekin A, Budancamanak I. The effect of the odor of breast milk on the time needed for transition from gavage to total oral feeding in preterm infants. Journal of Nursing Scholarship 2011;43(3):265‐73. [DOI] [PubMed] [Google Scholar]
Yildiz 2011b {published data only}
- Yildiz A, Arikan D. The effects of giving pacifiers to premature infants and making them listen to lullabies on their transition period for total oral feeding and sucking success. Journal of Clinical Nursing 2011;21(5‐6):644‐56. [DOI] [PubMed] [Google Scholar]
Zimmerman 2009 {published data only}
- Zimmerman EA, Barlow SM. The complexity of transitioning to oral feeds in preterm infants. Perspectives on Speech Science and Orofacial Disorders 2009;19(1):52‐7. [Google Scholar]
Additional references
Amaizu 2008
- Amaizu N, Shulman R, Schanler R, Lau C. Maturation of oral feeding skills in preterm infants. Acta Paediatrica 2008;97(1):61‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
American Academy of Pediatrics 2008
- American Adacemy of Pediatrics Committee on Fetus and Newborn. Hospital discharge of the high‐risk neonate. Pediatrics 2008;122(5):1119‐26. [DOI] [PubMed] [Google Scholar]
Arvedson 2010
- Arvedson J, Clark H, Lazarus C, Schooling T, Frymark T. Evidence‐based systematic review: effects of oral motor interventions on feeding and swallowing in preterm infants. American Journal of Speech‐Language Pathology 2010;19(4):321‐40. [DOI] [PubMed] [Google Scholar]
Bernbaum 1983
- Bernbaum JC, Pereira GR, Watkins JB, Peckham GJ. Nonnutritive sucking during gavage feeding enhances growth and maturation in premature infants. Pediatrics 1983;71(1):41‐5. [PubMed] [Google Scholar]
Bier 1993
- Bier JA, Ferguson A, Cho C, Oh W, Vohr BR. The oral motor development of low‐birth‐weight infants who underwent orotracheal intubation during the neonatal period. American Journal of Diseases of Children 1993;147(8):858‐62. [DOI] [PubMed] [Google Scholar]
Bingham 2009
- Bingham PM. Deprivation and dysphagia in premature infants. Journal of Child Neurology 2009;24(6):743‐9. [DOI] [PubMed] [Google Scholar]
Blencowe 2013
- Blencowe H, Cousens S, Chou D, Oestergaard M, Say L, Moller A, et al. Born too soon: the global epidemiology of 15 million preterm births. Reproductive Health 2013;10(Suppl 1):S2. [S2http://www.reproductive‐health‐journal.com/content/10/S1/S2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Burklow 2002
- Burklow KA, McGrath AM, Valerius KS, Rudolph C. Relationship between feeding difficulties, medical complexity, and gestational age. Nutrition in Clinical Practice 2002;17(6):373‐8. [DOI] [PubMed] [Google Scholar]
Case‐Smith 1989
- Case‐Smith J, Cooper P, Scala V. Feeding efficiency of premature neonates. American Journal of Occupational Therapy 1989;43(4):245‐50. [DOI] [PubMed] [Google Scholar]
Cowen 2006
- Cowen LJ. Feeding practices in the neonatal intensive care unit: the transition to full oral feeding. Dissertation Thesis Claremount Graduate University. Claremont: Claremont Graduate University, 2006:183.
Crowe 2006
- Crowe L, Chang A, Wallace K. Instruments for assessing readiness to commence suck feeds in preterm infants: effects on time to establish full oral feeding and duration of hospitalisation. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD005586] [DOI] [Google Scholar]
Cunha 2009
- Cunha M, Barreiros J, Goncalves I, Figueiredo H. Nutritive sucking pattern ‐ from very low birth weight preterm to term newborn. Early Human Development 2009;85(2):125‐30. [DOI] [PubMed] [Google Scholar]
da Costa 2010a
- Costa SP, Schans CP, Boelema SR, Meij E, Boerman MA, Bos AF. Sucking patterns in full term infants between birth and 10 weeks of age. Infant Behavior & Development 2010;33(1):61‐7. [DOI] [PubMed] [Google Scholar]
da Costa 2010b
- Costa SP, Schans CP, Zweens MJ, Boelema SR, Meij E, Boerman MA, et al. Development of sucking patterns in pre‐term infants with bronchopulmonary dysplasia. Neonatology 2010;98(3):268‐77. [DOI] [PubMed] [Google Scholar]
da Costa 2010c
- Costa SP, Schans CP, Zweens MJ, Boelema SR, Meij E, Boerman MA, et al. The development of sucking patterns in preterm, small‐for‐gestational age infants. The Journal of Pediatrics 2010;157(4):603‐9, e1‐3. [DOI] [PubMed] [Google Scholar]
Daley 2000
- Daley HK, Kennedy CM. Meta analysis: effects of interventions on premature infants feeding. Journal of Perinatal & Neonatal Nursing 2000;14(3):62‐77. [DOI] [PubMed] [Google Scholar]
Dodrill 2004
- Dodrill P, McMahon S, Ward E, Weir K, Donovan T, Riddle B. Long‐term oral sensitivity and feeding skills of low‐risk pre‐term infants. Early Human Development 2004;76(1):23‐37. [DOI] [PubMed] [Google Scholar]
Dodrill 2008a
- Dodrill P, Cleghorn G, Donovan T, Davies P. Growth patterns in preterm infants born appropriate for gestational age. Journal of Paediatrics and Child Health 2008;44(6):332‐7. [DOI] [PubMed] [Google Scholar]
Dodrill 2008b
- Dodrill P, Donovan T, Cleghorn G, McMahon S, Davies PS. Attainment of early feeding milestones in preterm neonates. Journal of Perinatology 2008;28(8):549‐55. [DOI] [PubMed] [Google Scholar]
Dodrill 2008c
- Dodrill P, McMahon S, Donovan T, Cleghorn G. Current management of transitional feeding issues in preterm neonates born in Queensland, Australia. Early Human Development 2008;84(10):637‐43. [DOI] [PubMed] [Google Scholar]
Dougherty 2008
- Dougherty D, Luther M. Birth to breast ‐ a feeding care map for the NICU: helping the extremely low birth weight infant navigate the course. Neonatal Network 2008;27(6):371‐7. [DOI] [PubMed] [Google Scholar]
Eichenwald 2001
- Eichenwald EC, Blackwell M, Lloyd JS, Tran T, Wilker RE, Richardson DK. Inter‐neonatal intensive care unit variation in discharge timing: influence of apnea and feeding management. Pediatrics 2001;108(4):928‐33. [DOI] [PubMed] [Google Scholar]
Engle 2007
- Engle WA, Tomashek KM, Wallman C. "Late‐preterm" infants: a population at risk. Pediatrics 2007;120(6):1390‐401. [DOI] [PubMed] [Google Scholar]
Fadavi 1997
- Fadavi S, Punwani IC, Jain L, Vidyasagar D. Mechanics and energetics of nutritive sucking: a functional comparison of commercially available nipples. The Journal of Pediatrics 1997;130(5):740‐5. [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- McMaster University. GRADEpro [www.gradepro.org]. McMaster University, 2014.
Greene 2012
- Greene Z, Walshe M, O'Donnell C. Effects of oral stimulation for oral feeding in preterm infants. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD009720] [DOI] [Google Scholar]
Harding 2009
- Harding C. An evaluation of the benefits of non‐nutritive sucking for premature infants as described in the literature. Archives of Disease in Childhood 2009;94(8):64‐70. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Greene S editor(s). Cochrane Handbook for Systematic Interventions. Chichester (UK): John Wiley & Sons, 2008:187‐241. [Google Scholar]
Howe 2007
- Howe TH, Sheu CF, Hinojosa J, Lin J, Holzman IR. Multiple factors related to bottle‐feeding performance in preterm infants. Nursing Research 2007;56(5):307‐11. [DOI] [PubMed] [Google Scholar]
Jadcherla 2010
- Jadcherla SR, Wang M, Vijayapal AS, Leuthner SR. Impact of prematurity and co‐morbidities on feeding milestones in neonates: a retrospective study. Journal of Perinatology 2010;30(3):201‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnston 1999
- Johnston L. Review: non‐nutritive sucking decreases length of hospital stay in premature infants. Evidence‐Based Nursing 1999;2(3):74. [Google Scholar]
Joung 2006
- Joung KH, Yoo IY, Kim HS, Kim S, Lee JH. Effects of non‐nutritive sucking on the physiological and behavioral states of pre‐term infants during tube feeding. Taehan Kanho Hakhoe Chi 2006;36(5):732‐41. [DOI] [PubMed] [Google Scholar]
Kelly 2007
- Kelly BN, Huckabee ML, Jones RD, Frampton CM. The first year of human life: coordinating respiration and nutritive swallowing. Dysphagia 2007;22(1):37‐43. [DOI] [PubMed] [Google Scholar]
Kinneer 1994
- Kinneer MD, Beachy P. Nipple feeding premature infants in the neonatal intensive‐care unit: factors and decisions. Journal of Obstetric, Gynecologic and Neonatal Nursing 1994;23(2):105‐12. [DOI] [PubMed] [Google Scholar]
Lau 1997
- Lau C, Sheena HR, Shulman RJ, Schanler RJ. Oral feeding in low birth weight infants. The Journal of Pediatrics 1997;130(4):561‐9. [DOI] [PubMed] [Google Scholar]
Lau 2000a
- Lau C, Alagugurusamy R, Schanler RJ, Smith EO, Shulman RJ. Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta Paediatrica 2000;89(7):846–52. [PubMed] [Google Scholar]
Lau 2011
- Lau C, Smith EO. A novel approach to assess oral feeding skills of preterm infants. Neonatology 2011;100(1):64‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau 2015
- Lau C. Development of suck and swallow mechanisms in infants. Annals of Nutrition and Metabolism 2015;66(Suppl 5):7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefton‐Greif 2007
- Lefton‐Greif MA, McGrath‐Morrow SA. Deglutition and respiration: development, coordination, and practical implications. Seminars in Speech and Language 2007;28(3):166‐79. [DOI] [PubMed] [Google Scholar]
Leonard 1980
- Leonard EL, Trykowski LE, Kirkpatrick BV. Nutritive sucking in high‐risk neonates after perioral stimulation. Physical Therapy 1980;60(3):299‐302. [DOI] [PubMed] [Google Scholar]
Lessen 2009
- Lessen BS. Effect of oral stimulation on feeding progression in preterm infants. Advances in Neonatal Care 2009;9(4):187. [DOI] [PubMed] [Google Scholar]
MacMullen 2000
- MacMullen NJ, Dulski LA. Factors related to sucking ability in healthy newborns. Journal of Obstetric, Gynecologic and Neonatal Nursing 2000;29(4):390‐6. [DOI] [PubMed] [Google Scholar]
Mandich 1996
- Mandich MB, Ritchie SK, Mullett M. Transition times to oral feeding in premature infants with and without apnea. Journal of Obstetric, Gynecologic and Neonatal Nursing 1996;25(9):771‐6. [DOI] [PubMed] [Google Scholar]
Matsubara 2005
- Matsubara M, Tamura Y, Ruchala P. Analysis of nutritive sucking function in very low and extremely low birthweight infants in Japan: a pilot study. Japan Journal of Nursing Science 2005;2(1):3‐7. [Google Scholar]
McCain 2010
- McCain GC, Knupp AM, Fontaine JL, Pino LD, Vasquez EP. Heart rate variability responses to nipple feeding for preterm infants with bronchopulmonary dysplasia: three case studies. Journal of Pediatric Nursing 2010;25(3):215‐20. [DOI] [PubMed] [Google Scholar]
Medoff‐Cooper 1993
- Medoff‐Cooper B, Verklan T, Carlson S. The development of sucking patterns and physiologic correlates in very‐low‐birth‐weight infants. Nursing Research 1993;42(2):100‐5. [PubMed] [Google Scholar]
Medoff‐Cooper 1996
- Medoff‐Cooper B, Gennaro S. The correlation of sucking behaviors and Bayley Scales of Infant Development at six months of age in VLBW infants. Nursing Research 1996;45(5):291‐6. [DOI] [PubMed] [Google Scholar]
Medoff‐Cooper 2000
- Medoff‐Cooper B, McGrath JM, Bilker W. Nutritive sucking and neurobehavioral development in preterm infants from 34 weeks PCA to term. The American Journal of Maternal Child Nursing 2000;25(2):64‐70. [DOI] [PubMed] [Google Scholar]
Miller 2007
- Miller CK, Willging JP. The implications of upper‐airway obstruction on successful infant feeding. Seminars in Speech and Language 2007;28(3):190‐203. [DOI] [PubMed] [Google Scholar]
Mizuno 2007
- Mizuno K, Nishida Y, Taki M, Hibino S, Murase M, Sakurai M, et al. Infants with bronchopulmonary dysplasia suckle with weak pressures to maintain breathing during feeding. Pediatrics 2007;120(4):e1035‐42. [DOI] [PubMed] [Google Scholar]
NIH 2008
- NIH, DHHS. Prematurity Research at the NIH. Washington, DC: Eunice Kennedy Shriver National Institute of Child Health and Human Development, 2008.
Pickler 1992
- Pickler RH, Higgins KE, Crummette BD. The effect of nonnutritive sucking on bottle‐feeding stress in preterm infants. Journal of Obstetric, Gynecologic and Neonatal Nursing 1993;22(3):230‐4. [DOI] [PubMed] [Google Scholar]
Pickler 2006
- Pickler RH, Best AM, Reyna BA, Gutcher G, Wetzel PA. Predictors of nutritive sucking in preterm infants. Journal of Perinatology 2006;26(11):693‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinelli 2005
- Pinelli J, Symington A. Non‐nutritive sucking for promoting physiologic stability and nutrition in preterm infants. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD001071.pub2] [DOI] [PubMed] [Google Scholar]
Poore 2008a
- Poore M, Barlow SM. Suck predicts neuromotor integrity and developmental outcomes. Perspectives on Speech Science and Orofacial Disorders 2009;19(1):44‐51. [Google Scholar]
Premji 2004
- Premji SS, McNeil DA, Scotland J. Regional neonatal oral feeding protocol: changing the ethos of feeding preterm infants. Journal of Perinatal and Neonatal Nursing 2004;18(4):371‐84. [DOI] [PubMed] [Google Scholar]
RevMan 2015 [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2015.
Ross 2002
- Ross ES, Browne JV. Developmental progression of feeding skills: an approach to supporting feeding in preterm infants. Seminars in Neonatology 2002;7(6):469‐75. [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GWG. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. www.guidelinedevelopment.org/handbook. Updated October 2013.
Stumm 2008
- Stumm SL, Barlow SM, Estep M, Lee J, Cannon S, Carlson J, et al. Respiratory distress syndrome degrades the fine structure of the non‐nutritive suck in preterm infants. Journal of Neonatal Nursing 2008;14(1):9‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Swift 2010
- Swift MC, Scholten I. Not feeding, not coming home: parental experiences of infant feeding difficulties and family relationships in a neonatal unit. Journal of Clinical Nursing 2010;19(1‐2):249‐58. [DOI] [PubMed] [Google Scholar]
Thoyre 2003a
- Thoyre SM, Carlson J. Occurrence of oxygen desaturation events during preterm infant bottle feeding near discharge. Early Human Development 2003;72(1):25‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thoyre 2003b
- Thoyre SM, Carlson JR. Preterm infants' behavioural indicators of oxygen decline during bottle feeding. Journal of Advanced Nursing 2003;43(6):631‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsai 2010
- Tsai SW, Chen CH, Lin MC. Prediction for developmental delay on Neonatal Oral Motor Assessment Scale in preterm infants without brain lesion. Pediatrics International 2010;52(1):65‐8. [DOI] [PubMed] [Google Scholar]
Watson 2015
- Watson J, McGuire W. Responsive versus scheduled feeding for preterm infants. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD005255.pub4] [DOI] [PubMed] [Google Scholar]
White‐Traut 2005
- White‐Traut RC, Berbaum ML, Lessen B, McFarlin B, Cardenas L. Feeding readiness in preterm infants: the relationship between preterm behavioral state and feeding readiness behaviors and efficiency during transition from gavage to oral feeding. MCN: The American Journal of Maternal Child Nursing 2005;30(1):52‐9. [PubMed] [Google Scholar]
WHO 2012
- World Health Organization Editors: Howson CP, Kinney MV, Lawn JE. Born too soon: the global action report on preterm birth. Geneva, Switzerland: World Health Organization, 2012.
Wilson‐Costello 2005
- Wilson‐Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics 2005;115(4):997‐1003. [DOI] [PubMed] [Google Scholar]
Wolf 1992
- Wolf LS, Glass RP. Feeding and Swallowing Disorders in Infancy: Assessment and Management. San Antonio: Texas: Therapy Skill Builders, 1992. [Google Scholar]


