Abstract
Background
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) account for one‐quarter of cases of acute respiratory failure in intensive care units (ICUs). A third to half of patients will die in the ICU, in hospital or during follow‐up. Mechanical ventilation of people with ALI/ARDS allows time for the lungs to heal, but ventilation is invasive and can result in lung injury. It is uncertain whether ventilator‐related injury would be reduced if pressure delivered by the ventilator with each breath is controlled, or whether the volume of air delivered by each breath is limited.
Objectives
To compare pressure‐controlled ventilation (PCV) versus volume‐controlled ventilation (VCV) in adults with ALI/ARDS to determine whether PCV reduces in‐hospital mortality and morbidity in intubated and ventilated adults.
Search methods
In October 2014, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Isssue 9), MEDLINE (1950 to 1 October 2014), EMBASE (1980 to 1 October 2014), the Latin American Caribbean Health Sciences Literature (LILACS) (1994 to 1 October 2014) and Science Citation Index‐Expanded (SCI‐EXPANDED) at the Institute for Scientific Information (ISI) Web of Science (1990 to 1 October 2014), as well as regional databases, clinical trials registries, conference proceedings and reference lists.
Selection criteria
Randomized controlled trials (RCTs) and quasi‐RCTs (irrespective of language or publication status) of adults with a diagnosis of acute respiratory failure or acute on chronic respiratory failure and fulfilling the criteria for ALI/ARDS as defined by the American‐European Consensus Conference who were admitted to an ICU for invasive mechanical ventilation, comparing pressure‐controlled or pressure‐controlled inverse‐ratio ventilation, or an equivalent pressure‐controlled mode (PCV), versus volume‐controlled ventilation, or an equivalent volume‐controlled mode (VCV).
Data collection and analysis
Two review authors independently screened and selected trials, assessed risk of bias and extracted data. We sought clarification from trial authors when needed. We pooled risk ratios (RRs) for dichotomous data and mean differences (MDs) for continuous data with their 95% confidence intervals (CIs) using a random‐effects model. We assessed overall evidence quality using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach.
Main results
We included three RCTs that randomly assigned a total of 1089 participants recruited from 43 ICUs in Australia, Canada, Saudi Arabia, Spain and the USA. Risk of bias of the included studies was low. Only data for mortality and barotrauma could be combined in the meta‐analysis. We downgraded the quality of evidence for the three mortality outcomes on the basis of serious imprecision around the effect estimates. For mortality in hospital, the RR with PCV compared with VCV was 0.83 (95% CI 0.67 to 1.02; three trials, 1089 participants; moderate‐quality evidence), and for mortality in the ICU, the RR with PCV compared with VCV was 0.84 (95% CI 0.71 to 0.99; two trials, 1062 participants; moderate‐quality evidence). One study provided no evidence of clear benefit with the ventilatory mode for mortality at 28 days (RR 0.88, 95% CI 0.73 to 1.06; 983 participants; moderate‐quality evidence). The difference in effect on barotrauma between PCV and VCV was uncertain as the result of imprecision and different co‐interventions used in the studies (RR 1.24, 95% CI 0.87 to 1.77; two trials, 1062 participants; low‐quality evidence). Data from one trial with 983 participants for the mean duration of ventilation, and from another trial with 78 participants for the mean number of extrapulmonary organ failures that developed with PCV or VCV, were skewed. None of the trials reported on infection during ventilation or quality of life after discharge.
Authors' conclusions
Currently available data from RCTs are insufficient to confirm or refute whether pressure‐controlled or volume‐controlled ventilation offers any advantage for people with acute respiratory failure due to acute lung injury or acute respiratory distress syndrome. More studies including a larger number of people given PCV and VCV may provide reliable evidence on which more firm conclusions can be based.
Plain language summary
Different methods of ventilation (controlling pressure vs volume) for people with acute respiratory failure due to lung injury
Review question
We reviewed available evidence for the safety and efficacy of controlling pressure versus controlling volume of air delivered during mechanical ventilation in critically ill adults with acute respiratory failure due to lung injury. We found three relevant studies.
Background
Acute respiratory failure due to acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) is a common reason for admission to intensive care units (ICUs) worldwide. A third to half of people with ALI/ARDS die in the ICU, in hospital or during follow‐up. People with ALI/ARDS are put on ventilator machines to give the lungs time to recover. However, lung damage can worsen if the volume of air delivered by these machines is too large, or if the pressure reached in the lungs during ventilation is too high.
We wanted to know whether controlling pressure in the lung during ventilation by varying the volume of air delivered (pressure‐controlled ventilation, or PCV) was better than allowing varying lung pressures when a fixed volume of air is delivered (volume‐controlled ventilation, or VCV).
Study characteristics
The three randomized trials compared PCV versus VCV in a total of 1089 adults with ALI/ARDS from 43 ICUs in five high‐income countries. None of the trials were industry‐funded. The evidence is current to October 2014.
Key results
We could not be sure whether the proportions of patients who died in hospital were very different between those treated with PCV and with VCV. For every 1000 persons treated with VCV, 636 deaths were reported. On the basis of our results, we could expect to see between 210 fewer deaths and 13 more deaths with PCV. We found that effects on mortality in the ICU and on mortality at 28 days were similarly uncertain. Our results include the possibility that VCV or PCV could be better for reducing the duration of ventilation or the development of traumatic lung damage caused by ventilation (barotrauma). None of the studies provided reliable information regarding to what extent failure of other organs would be impacted by the type of ventilation, nor did they provide information on differences in infection risk or quality of life following discharge from intensive care.
Quality of the evidence
The overall evidence for mortality was of moderate quality. For outcomes such as duration of ventilation, barotrauma and organ failure, evidence was limited by the small numbers studied, the different methods used in the studies or differences in reporting of results, which made interpretation difficult.
Conclusions
Available evidence is insufficient to confirm whether PCV offers any advantage over VCV in improving outcomes for people with acute lung injury on ventilator machines. More studies including a larger number of people given PCV and VCV may provide reliable evidence on which more firm conclusions can be based.
Summary of findings
Summary of findings for the main comparison. Pressure‐controlled ventilation vs volume‐controlled ventilation for acute respiratory failure due to acute lung injury (ALI) or acute respiratory distress syndrome (ARDS).
| What are the effects of pressure‐controlled ventilation vs volume‐controlled ventilation for acute respiratory failure due to acute lung injury (ALI) or acute respiratory distress syndrome (ARDS)? | ||||||
| Patient or population: patients with acute respiratory failure due to acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) Settings: intensive care units in high‐income countriesa Intervention: pressure‐controlled ventilation vs volume‐controlled ventilation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Volume‐controlled ventilation | Pressure‐controlled ventilation | |||||
| Mortality in hospital | 636 per 1000 | 528 per 1000 (426 to 649) | RR 0.83 (0.67 to 1.02) | 1089 (3 studies)a | ⊕⊕⊕⊝ Moderateb,c | |
| Mortality in ICU | 376 per 1000 | 316 per 1000 (267 to 373) | RR 0.84 (0.71 to 0.99) | 1062 (2 studies)d | ⊕⊕⊕⊝ Moderateb,e | |
| Mortality on follow‐up Follow‐up: 28 days | 323 per 1000 | 284 per 1000 (236 to 342) | RR 0.88 (0.73 to 1.06) | 983 (1 study)f | ⊕⊕⊕⊝ Moderateb,c | |
| Duration of mechanical ventilation | See comment | See comment | Not estimable | 983 (1 study)f | See comment | Skewed data presented as median (10 days) and interquartile ranges (6 days to 16 and 17 days) did not differ |
| Barotrauma | 94 per 1000 | 117 per 1000 (82 to 166) | RR 1.24 (0.87 to 1.77) | 1062 (2 studies)d | ⊕⊕⊝⊝ Lowg,h | |
| Organ failure | Not estimable | Not estimable | Not estimable | 79 (1 study)i | See comment | Skewed data for mean number of organ failures in survivors favoured PCV (P value 0.003) |
| Quality of life | See comment | See comment | Not estimable | 0 (0) | See comment | No trials measured this outcome |
| *The basis for the assumed risk is the median control group risk across studies, or the average risk, for pooled data; and the control group risk for single studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aEsteban 2000 (Spain); Meade 2008 (Australia, Canada, Saudi Arabia), Rappaport 1994 (USA). bNo serious study limitations: Esteban 2000 was judged at high risk of selection bias because of imbalances in proportions with renal failure at baseline; renal failure was independently associated with increased mortality. However removal of this trial did not alter the results significantly. Not downgraded. cSerious imprecision: 95% CIs of pooled estimates indicated appreciable benefit with PCV and non‐appreciable benefit with VCV, with no significant differences between ventilatory modes. Downgraded by 1.
dEsteban 2000; Meade 2008. eSerious imprecision: 95% CIs of risk ratios indicated appreciable and non‐appreciable benefit for PCV. Downgraded by 1.
fMeade 2008. gSerious inconsistency: Although no statistical heterogeneity was noted (I2 = 0%), the 2 trials differed in co‐interventions offered, with Meade 2008 permitting rescue interventions in the PCV arm that could have contributed to increased barotrauma. Downgraded by 1. hSerious imprecision: 95% CIs of effect estimates indicated appreciable benefit for VCV and non‐appreciable benefit for PCV with no significant difference between ventilatory modes. Downgraded by 1.
Background
Acute respiratory failure is the most common form of organ failure in critically ill patients. Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) account for one‐quarter of cases of acute respiratory failure in intensive care units (ICUs) (Rubenfield 2005). Earlier mortality rates among adults with ARDS were reported to range from 40% to 70% (Brun‐Bruisson 2004; Krafft 1996). More recent publications pertaining to ARDS have reported lower mortality rates of 17.7% and 23% (Caser 2014; Gates 2013; Matthay 2011). In the paediatric population, mortality due to ALI was even lower (18%) (Zimmerman 2009). Given the projected doubling of the annual incidence of ARDS over the next two decades (Rubenfield 2005), determining cost‐effective modalities that can decrease the mortality associated with ARDS is important.
The main principles behind mechanical ventilatory support in people with acute respiratory failure consist of preventing or reducing ongoing lung injury and supporting organ function until recovery. Despite several trials on different aspects of this disease, management of this complex clinical condition appears to be largely supportive and directed toward limiting or reversing the triggering insult. It is also increasingly evident that ventilatory therapy can aggravate the underlying lung injury—a condition described as ventilator‐induced or ventilator‐associated lung injury (VILI/VALI) (Ranieri 1999; Slutsky 2013).
Recommendations posit that, under conditions in which lung overdistension is likely to occur, tidal volume (the volume of air moved into or out of the lungs during quiet breathing) and airway pressure should be limited and the attendant increase in arterial carbon dioxide levels considered as an acceptable trade‐off to prevent lung damage (Slutsky 1993; Slutsky 2013). Whilst evidence is sufficient to suggest that lung protective ventilation, which includes low tidal volume (approximately 6 mL/kg) and a plateau airway pressure restricted to approximately 28 to 30 cm H2O (ARDSNet 2000), translates to better outcomes when compared with traditional ventilatory strategies for ALI/ARDS (Gattinoni 2008), it is unclear whether the mode of ventilation can influence the outcome. Although pressure‐targeted modes deliver tidal volumes that are determined by preset airway pressures, volume‐controlled modes deliver a preset volume with a provision to limit high airway pressures by using pressure‐limited alarm settings. We assessed whether pressure‐targeted ventilatory modes provide an advantage over volume‐targeted modes in patients with acute respiratory failure due to ALI/ARDS.
Description of the condition
The report of the American‐European Consensus Conference (AECC) (Bernard 1994) recommended that ALI /ARDS should be defined as a syndrome of inflammation and increased permeability associated with a constellation of clinical, radiological and physiological abnormalities that could not be explained by left atrial or pulmonary capillary hypertension. Acute respiratory distress syndrome, in very precise terms, is a subset of ALI representing a more severe form of ALI. However in practice, ARDS and ALI are considered as part of a continuum: ARDS represents severe lung injury with a ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) less than 200, regardless of the level of positive end‐expiratory pressure (PEEP), while ALI represents any lung injury that results in a PaO2/FiO2 between 201 and 300. Partial pressure of arterial oxygen (PaO2) is measured in mmHg, and FiO2 is expressed as a decimal between 0.21 and 1.00. Both of these conditions have bilateral infiltrates on frontal chest radiographs with pulmonary artery wedge pressure less than 18 mmHg when measured, or with no clinical evidence of left atrial hypertension.
The AECC definition has been criticized for several reasons—lack of explicit criteria to define acuity of this condition, high interobserver variability in interpreting chest radiographs and difficulty in ruling out cardiogenic pulmonary oedema given the declining use of pulmonary artery catheters. However this remained the standard for defining ARDS until the more recent Berlin definition was published (Ranieri 2012). This latest definition has addressed the limitations mentioned above and classifies ARDS as mild, moderate or severe on the basis of PaO2/FiO2. Conflicting views, based on prospective observational data, surround the validity of the Berlin definition and its ability to predict 28‐day mortality compared with the AECC definition (Caser 2014; Hernu 2013). We used the AECC definition for this review, as we anticipated that the included trials would have been completed before the time of publication of the Berlin definition.
Description of the intervention
Ventilation in patients with ALI/ARDS allows time for the lungs to heal. However the process of ventilation is not without complications (ARDSNet 2000). One major complication is ventilator‐associated/ventilator‐induced lung injury (VALI/VILI), which is clinically indistinguishable from ALI/ARDS. This may occur as a result of excessive pressure (barotrauma), alveolar overdistension (volutrauma), alveolar collapse and shearing due to repeated opening and closing of alveoli (atelectrauma) and alveolar inflammation or nosocomial infection (biotrauma) (Halter 2007), which may substantially contribute to mortality and morbidity in this group of patients.
In pressure‐controlled ventilation (PCV), the limiting pressure is set as the target pressure, and the delivered volume is determined by lung compliance and airway resistance. In this time‐cycled mode, the target pressure is set within safe pressure limits. The distinguishing characteristic of this mode is that constant pressure is delivered, resulting in a decelerating flow and a more even distribution of ventilation in patients with poor lung compliance (Al‐Sady 1985). In addition to the classic pressure‐controlled mode, different types of PCV are available, for example, pressure‐controlled inverse ratio ventilation, which has a higher inspiratory time when compared with expiratory time, resulting in inverse ratio ventilation; this mode has been proposed to improve arterial oxygenation through an increase in mean alveolar pressure and lower peak airway pressure seen as a low end‐inspiratory flow rate (Tharrat 1998).
In contrast, in the volume‐targeted mode (volume‐controlled ventilation, or VCV), the primary goal is to deliver a set volume. When pressure exceeds a preset pressure alarm limit during VCV, either the inward gas flow is stopped in disease processes with increased airway resistance, or the non‐delivered volume when the preset pressure is reached is dumped (e.g. if the set volume is 400 mL and the peak pressure alarm limit is reached when the administered volume reaches 300 mL, the remaining volume is dumped).
How the intervention might work
The importance of limiting tidal volume to decrease alveolar overdistension and VILI is well established (ARDSNet 2000). No other ventilatory strategy has clearly been shown to affect outcome. It is unclear at this stage whether VALI/VILI is caused/perpetuated by higher volumes (which may be achieved with pressure‐targeted modes at the same set pressures when lung compliance improves) or higher pressures (which may be reached when an attempt is made to deliver the set tidal volume in volume‐targeted modes). However, conflicting reports from studies have looked at respiratory mechanics and gas exchange parameters when comparing the two modes of ventilation mentioned above. Several studies (Cadi 2008; Davis 1996; Rappaport 1994) have shown improvement in lung compliance and oxygenation among patients placed on pressure‐controlled modes, whereas others (Lessard 1994) have shown no differences in these outcomes. Whilst these surrogate outcomes are important, it is crucial to determine whether clinically meaningful outcomes (e.g. mortality) are affected.
Why it is important to do this review
No consensus (MacIntyre 2011; Marini 2011) has yet been reached regarding whether PCV provides an advantage over VCV in the management of acute respiratory distress syndrome. Even within ICUs in a single institution, both modes of ventilation are sometimes used. This review systematically examined available evidence to assess whether the mode of ventilation impacts clinically important outcomes (or mortality as the primary outcome) in critically ill adults with ALI and ARDS.
See Appendix 1 for the list of acronyms used.
Objectives
To compare pressure‐controlled ventilation (PCV) versus volume‐controlled ventilation (VCV) in adults with ALI/ARDS to determine whether PCV reduces in‐hospital mortality and morbidity in intubated and ventilated adults.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐group randomized controlled trials (RCTs) and quasi‐RCTs irrespective of their language or publication status (published, unpublished or available only in abstract form). We excluded cross‐over trials.
Types of participants
We looked at adult patients admitted to an ICU for invasive mechanical ventilation with a diagnosis of acute respiratory failure or acute on chronic respiratory failure and fulfilling the criteria for ALI/ARDS as defined by the American‐European Consensus Conference (Bernard 1994). We also evaluated studies published before 1994 to establish consistency with this definition.
We excluded trials with the following participants.
Paediatric population as defined by study authors (unless children constituted less than 10% of the sample in each arm).
Ventilation for participants with chronic respiratory failure (obstructive sleep apnoea, chronic neuromuscular disorders, etc.).
Participants treated only with non‐invasive respiratory support (use of non‐invasive ventilatory support before invasive ventilatory support did not preclude inclusion).
Participants with acute respiratory failure comprising a mixed population of participants with and without ALI/ARDS (unless those without ALI/ARDS constituted less than 10% of the population).
Types of interventions
Pressure‐controlled, or pressure‐controlled inverse ratio, ventilation or an equivalent pressure‐controlled model compared with volume‐controlled ventilation or an equivalent volume‐controlled mode.
Types of outcome measures
Primary outcomes
In‐hospital mortality, including ICU mortality.
Mortality at 28 days.
Secondary outcomes
Total duration of ventilation (including duration of ventilation prerandomization, as studies may not report both total duration of ventilation and duration of ventilation following randomization).
Barotrauma (as evidenced by new‐onset pneumothorax, pneumomediastinum, pneumoperitoneum, pneumopericardium or subcutaneous emphysema following institution of mechanical ventilation, or as otherwise defined by study authors).
Development of other organ failure/dysfunction during ICU stay.
Length of stay in ICU and in hospital.
Number of participants with infective complications (ventilator‐associated pneumonia, sepsis) as defined by study authors.
Quality of life after discharge from hospital.
Search methods for identification of studies
Electronic searches
Electronic databases
On 1 October 2014, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Isssue 9), MEDLINE (1950 to 1 October 2014), EMBASE (1980 to 1 October 2014) and Science Citation Index‐Expanded (SCI‐EXPANDED) at the Institute for Scientific Information (ISI) Web of Science (1990 to 1 October 2014). The search strategy developed specifically for MEDLINE is detailed in Appendix 2; for EMBASE in Appendix 3; for CENTRAL in Appendix 4; and for ISI Web of Science in Appendix 5.
We searched additional databases such as the Latin American Caribbean Health Sciences Literature (LILACS) (1994 to 1 October 2014); the Indian Medlars Centre (indmed.nic.in/imcwebij.html) (1994 to 1 October 2014); and the South Asian Database of Controlled Clinical Trials (www.cochrane‐sadcct.org) (to 1 October 2014).
Clinical trials registries
We searched the following clinical trials registries to look for ongoing and completed trials.
In addition, we searched the World Health Organization International Clinical Trials Registry Platform Search Portal (www.who.int/trialsearch/) for prospectively registered trials across trials registers of the WHO ICTRP stable of contributory registers. The last search of these databases was conducted on 1 October 2014.
Searching other resources
Reference lists
One review author (BC) searched reference lists of all articles retrieved by the search, as well as the systematic reviews and meta‐analyses listed in the background, to identify relevant RCTs. We included all RCTs irrespective of their language or publication status (published article or abstract). In addition we searched conference proceedings for relevant abstracts; contacted individual researchers working in this field as well as organizations and pharmaceutical companies to identify unpublished and ongoing trials; and provided this information and the relevant dates in a table.
Conference proceedings
We searched electronically the following journals for abstracts of conference proceedings.
American Thoracic Society Conference proceedings (published in American Journal of Respiratory and Critical Care Medicine) from year 2009 to 2013.
Australian and New Zealand Intensive Care Society Annual Scientific Meeting proceedings (published in Anaesthesia and Intensive Care) from 2009 to 2012.
Annual Congress of the European Society of Intensive Care Medicine proceedings (published in Intensive Care Medicine) from 2007 to 2013.
Data collection and analysis
Selection of studies
Two review authors (BC, JVP) independently scanned all titles and abstracts identified by the search. We evaluated the full text of potentially relevant articles to determine whether they met the eligibility criteria for inclusion in the review. We considered trials as meeting the criteria for inclusion (randomized trials comparing pressure‐ vs volume‐controlled ventilation) provided one of the stated outcomes described in the protocol was presented. In cases of ambiguity or insufficient data, we contacted study authors to request clarification and additional information. We resolved disagreements by consensus or by consultation with the senior review author (GJ). We scrutinized each trial report to ensure that multiple publications from the same trial were included only once, and we linked all such reports to the original trial or study report in the reference list of included studies. We excluded studies that did not meet eligibility criteria and documented the reasons for exclusion.
Data extraction and management
BC and JVP independently extracted data using pretested data extraction forms (see Appendix 6); PT independently validated the extracted data. We extracted the following information from each included study.
General information: title, authors, source, contact address, country, published or unpublished and language and year of publication.
Trial characteristics: design and quality assessment criteria as detailed below.
Participants: inclusion and exclusion criteria, sample size, baseline characteristics and number allocated to each group. We also recorded Acute Physiology and Chronic Health Evaluation (APACHE II) scores, Simplified Acute Physiology Scores (SAPS II) and details of lung compliance at ICU admission.
Interventions: whether pressure‐ or volume‐controlled ventilation was provided; baseline plateau pressure and tidal volume set at admission; inspiratory time (Ti/T total) during which tidal volume was delivered.
Co‐interventions: concurrent interventions that might have influenced outcomes in this subset of participants (e.g. steroids, inhaled nitric oxide) if the data were provided. If heterogeneity was significant in the primary analysis pertaining to the intervention, sensitivity analysis was considered to assess the effects of co‐interventions.
Outcomes: in‐ICU mortality, in‐hospital mortality and mortality at longest follow‐up; extrapulmonary organ failure; ICU length of stay (mean ± standard deviation (SD)); hospital length of stay (mean ± SD); duration of ventilation; quality of life post discharge; infective complications; barotrauma; numbers experiencing each outcome; time to onset of each outcome; and numbers of dropouts and withdrawals with reasons.
For every outcome, we extracted the number analysed and the number randomly assigned to each treatment group to allow assessment of losses to follow‐up. We resolved disagreements about data extraction by careful review of the trial report and by discussion. A third review author (PT) adjudicated when disagreements were to be resolved through discussion. When data were insufficient or missing, we attempted to contact the trial authors.
For continuous outcomes, we extracted arithmetic mean values, standard deviations and the number of participants in each trial arm for whom the outcome was assessed. We noted whether numbers assessed in the trial referred to the numbers of participants who completed the trial or the numbers randomly assigned. When median values had been reported, we attempted to extract ranges, or interquartile ranges. Details on how this was done are provided in the Data synthesis section of the review.
Assessment of risk of bias in included studies
Two review authors (BC, JVP) assessed the methodological quality of each trial on the basis of the following six components: sequence generation, allocation concealment, blinding or masking, incomplete outcome data, selective outcome reporting and other biases. For each of these components, we assigned a judgement regarding risk of bias as 'low,' 'unclear' or 'high' (Higgins 2011a). We attempted to contact trial authors for clarification when methodological details were unclear. Blinding of clinicians or research personnel would not be feasible in this study. If statisticians were blinded to the results, this was recorded. We recorded follow‐up as adequate when more than 90% of randomly assigned participants were included in the final analysis, inadequate when ≤ 90% were included, and unclear when this information was not provided by the report or by trial authors. We recorded these assessments in 'Risk of bias' tables in RevMan 5.3, and summarized them in 'Risk of bias' graphs and summary figures. We used these assessments to perform a sensitivity analysis based on methodological quality when appropriate. We resolved conflicts in assessment through discussion; PT independently validated these assessments.
Measures of treatment effect
When outcomes were dichotomous, we used risk ratios; when outcomes were continuous, we used mean differences; each was provided with 95% confidence intervals (95% CIs).
Unit of analysis issues
No unit of analysis issues were encountered in the included trials. The methods that we intended to use to deal with cluster‐randomized trials, change data, count data and time‐to‐event data are described under Differences between protocol and review.
Dealing with missing data
We attempted to obtain missing data from trial authors. When possible, we extracted data to allow an intention‐to‐treat analysis in which all randomly assigned participants were analysed in the groups to which they were originally assigned. If a discrepancy was noted between the number randomly assigned and the number analysed for each treatment group, we calculated the percentage of loss to follow‐up in each group and planned to report this information. If dropouts exceeded 10% for any trial, we would have assigned the worse outcome to those lost to follow‐up for dichotomous outcomes and would have assessed the impact of this in sensitivity analyses including the results of completers.
If continuous data were reported with missing standard deviations, we would have calculated these, if possible, from other available data such as standard errors, or we would have imputed them using methods suggested in Higgins 2011b. We made no assumptions about loss to follow‐up for continuous data and analysed results for those who had completed the trials.
Assessment of heterogeneity
We assessed heterogeneity between trials by visually examining the forest plot to check for overlapping confidence intervals, by using the Chi2 test to evaluate homogeneity with a 10% level of significance and by determining the I2 statistic to assess inconsistency (percentage of variability in effect estimates that is due to heterogeneity rather than to sampling error). We acknowledge that thresholds for interpreting I2 can be misleading, as interpretation depends on several factors such as the magnitude and direction of effects and the strength of evidence for heterogeneity (e.g. P value for the Chi2 test, confidence interval for I2). When interpreting I2, we used the following recommendations as stated in Deeks 2011.
0% to 40%: might not be important.
30% to 60%: may represent moderate heterogeneity.
50% to 90%: may represent substantial heterogeneity.
75% to 100%: represents considerable heterogeneity.
As this statistical assessment of heterogeneity using the above guidelines posed problems in terms of overlapping ranges of I2 (e.g. 35% and 55%), in general we interpreted an I2 value ≥ 50% to denote substantial heterogeneity. We have detailed below measures used to deal with substantial heterogeneity.
In addition to assessing for statistical heterogeneity, we carefully scrutinized the included studies to look for significant variations in study methodology or in clinical factors that could contribute to methodological or clinical heterogeneity.
Assessment of reporting biases
We assessed the adequacy of all included studies in reporting data for prestated outcomes and in selectively reporting outcomes. We noted in the Characteristics of included studies section judgements based on risk of selective reporting as provided in the 'Risk of bias' tables that followed each study. We also reported risk of selective outcome reporting in the results under Assessment of risk of bias in included studies.
We attempted to identify published, unpublished and ongoing trials applying our search methods. We planned to assess the likelihood of potential publication bias by using funnel plots, provided that at least 10 trials were identified. We would have used a funnel plot to visually assess whether treatment estimates for the primary outcome were associated with study size. We intended to use a test based on arcsine transformation of observed risks, with explicit modelling of between‐study heterogeneity (Rücker 2008), and would have sought statistical advice on using the version of the arcsine test that includes random‐effects modelling (AS + RE) when Tau2 is greater than 1.0.
Data synthesis
We synthesized dichotomous data by using pooled and weighted risk ratios. We combined continuous data summarized by arithmetic means and standard deviations using mean differences. If continuous data were summarized using geometric means, we would have combined them on the log scale using the generic inverse variance method and reported them on the natural scale.
We planned to compare count data by using rate ratios when the total number of events in each group and the total amount of person‐time at risk in each group were provided, or by using risk ratios or mean differences when data were presented in a dichotomous or continuous form, respectively. We would have combined hazard ratios from survival data on the log scale using the inverse variance method and presented them on the natural scale.
One review author (BC) entered the data using RevMan 5.3. JVP and PT independently checked the accuracy of the data entered. If data could be meaningfully combined, we pooled risk ratios of the comparisons for dichotomous outcomes in Mantel‐Haenszel random‐effects meta‐analyses. We pooled mean differences between comparisons for continuous outcomes by using the inverse variance method; we have presented pooled estimates for both along with their 95% CIs. We would have estimated pooled effects for rare events (frequency of complications or adverse events) using the Peto odds ratio (Higgins 2011b).
Continuous data for outcomes such as duration of ventilation may be skewed when the mean is not at the centre of the distribution. If data from trials were skewed and this was not considerable, we used these data in the meta‐analysis. If the skew was considerable (ratio of observed mean minus lowest possible value divided by SD is less than one), we attempted to obtain appropriate data summaries or log‐transformed the data from all included studies to use the generic inverse variance method to pool data. If this was not possible, we presented these data in additional tables.
Subgroup analysis and investigation of heterogeneity
We uniformly used the random‐effects model (DerSimionian 1986) for assessment of treatment effects because this approach provides a more conservative estimate of treatment effects than is obtained by the fixed‐effect model (DeMets 1987).
If significant heterogeneity was detected and if data were sufficient, we would have undertaken the following subgroup analyses for each comparison.
ARDS versus ALI at entry to study.
Mean tidal volume delivered by pressure‐limited and volume‐limited modes (we would have adopted a threshold of 6 mL/kg as the desired target tidal volume and would have compared it with data from studies in which this target was not met).
Events of barotrauma between ventilatory supports with and without pressure limitation.
Mortality in hospital versus mortality in ICU and at longest follow‐up.
Trials using American European Consensus Conference criteria to define ARDS/ALI versus trials using other definitions.
We planned to formally compare effects between subgroups using the methods described in Deeks 2001.
Sensitivity analysis
We performed a sensitivity analysis to investigate the effects of interventions reported in trials at low risk of bias.
We planned to undertake sensitivity analyses if trials reported dropout rates of 10% or greater, to ascertain differences in outcomes of intention‐to‐treat (ITT) analyses (all dropouts would be assigned to the worst outcome for dichotomous outcomes) and analyses of completers. If results of these analyses differed significantly in terms of direction of effect, we planned to perform two additional analyses: a best‐case scenario favouring pressure‐controlled ventilation (i.e. no dropouts in this group had the unfavourable outcome, but all dropouts from the volume‐controlled group had the worst outcome) and a worst‐case scenario favouring control (i.e. all dropouts from the pressure‐controlled group had the unfavourable outcome, but no dropouts from the volume‐controlled group had this poor outcome).
Summarizing and interpreting results
We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach to interpret findings (Schünemann 2008) and used GRADE profiler (GRADE 2004) to import data from RevMan 5.3 to create 'Summary of findings' tables using information on quality of evidence, magnitude of effects of the interventions examined and sums of available data on all important outcomes from each study included in the comparison. The GRADE approach (Schunemann 2008) considers ‘quality’ to be a judgement of the extent to which we can be confident that the estimates of effect are correct. Evidence from randomized controlled studies initially was graded as high and was downgraded by one or two levels on each of five domains after full consideration of any limitations in the design of studies, directness (or applicability) of the evidence, consistency and precision of results and the possibility of publication bias. A GRADE quality level of 'high' reflects confidence that the true effect lies close to the estimate of the effect for a given outcome. A judgement of 'moderate' quality indicates that the true effect is likely to be close to the estimate of the effect, but acknowledges that it could be substantially different. Evidence of 'low' and 'very low' quality limits our confidence in the effect estimate (Balshem 2011).
Outcomes selected for the 'Summary of findings' tables include the following.
In‐hospital mortality.
Mortality in ICU.
Mortality on follow‐up (at 28 days).
Duration of mechanical ventilation.
Barotrauma.
Organ failure/dysfunction during ICU stay.
Quality of life after discharge from hospital.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Characteristics of studies awaiting classification.
Results of the search
Electronic searches retrieved a total of 8524 records, of which 8475 were excluded during abstract review for several reasons, which included alternate population, retrospective or cohort design, letter or case report format, use of alternate interventions, animal studies, physiological studies and reviews.
We identified 49 potentially relevant articles. Of these, 45 did not meet our inclusion criteria for reasons detailed in Characteristics of excluded studies. We included three trials in this review. Another trial (Keddissi 2000), which was reported only as a conference abstract, awaits assessment and is described in Characteristics of studies awaiting classification. We were unsuccessful in making contact with this study author to obtain more information regarding study design, quality and results.
Figure 1 details the selection process.
1.
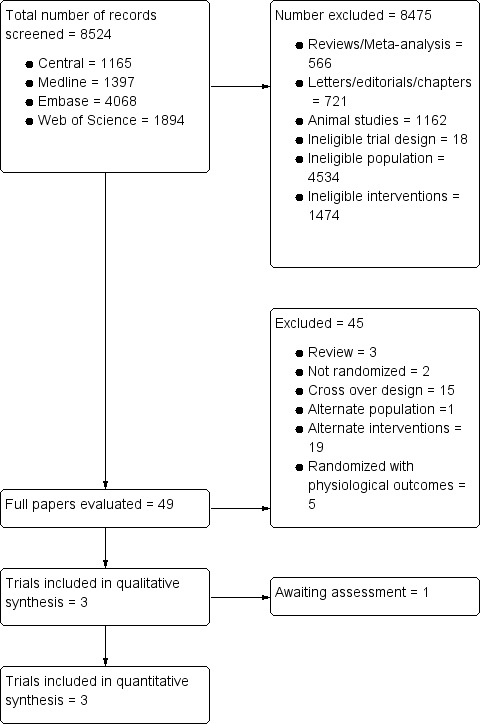
Flow diagram of study selection.
Included studies
The three included RCTs used a parallel‐group design. Two were multi‐centre trials (Esteban 2000; Meade 2008), and the latter was a multi‐country trial. These trials included a total of 1089 participants recruited from 43 ICUs in Australia, Canada, Saudi Arabia (Meade 2008), Spain (Esteban 2000) and USA (Esteban 2000). Sample sizes in the individual trials were 27 (Rappaport 1994), 62 (Esteban 2000) and 983 (Meade 2008).
The more recent trials (Esteban 2000; Meade 2008) used the AECC ARDS/ALI definition for inclusion, and Rappaport 1994 was conducted before this definition was published. However, criteria for inclusion in this study were consistent with the AECC ARDS/ALI definition of PaO2/FiO2 ratio < 150 with no clinical evidence of fluid overload.
Participants
The three included trials recruited adults of both genders with a mean age of 54 to 59 years in Esteban 2000 and Meade 2008; participants were younger in Rappaport 1994 (mean age 43 to 51 years). Esteban 2000 excluded pregnant women and people with head injury and burns and barotrauma. Meade 2008 also excluded pregnant women and those with underlying conditions that would prolong life support. Rappaport 1994 reported very few exclusion criteria. Baseline characteristics, including causes of lung injury (most often sepsis or pneumonia), APACHE II scores and other ventilatory and respiratory parameters, were balanced across PPV and VCV arms in Meade 2008 and in Rappaport 1994. In Esteban 2000, although randomization and allocation concealment were adequate, baseline imbalances were noted for proportions with renal failure (VCV 28% vs PCV 13%; P value 0.06); renal failure was an independent risk factor on logistic regression for increased mortality.
Interventions and co‐interventions
The three trials compared VCV with PCV. In Meade 2008, the PCV mode was referred to as the lung open ventilation group. Unique to this trial was the use of additional recruitment manoeuvres in the PCV arm. Meade 2008 allowed for plateau pressures up to 40 cm H2O in the PCV arm, in contrast to Esteban 2000, which targeted lower plateau pressures of < 36 cm H2O. Further in Meade 2008, plateau pressure in the control arm (VCV arm) was restricted to 30 cm H2O. Rappaport 1994 did not report ventilatory targets. In Esteban 2000, plateau inspiratory pressure was limited to ≤ 35 cm H2O. For both groups, adjustments to the inspiratory‐to‐expiratory time (I/E) ratio were made at the discretion of the attending physician, and 3:1 was the maximal I/E ratio allowed. Also, for both groups, IV sodium bicarbonate infusions were permitted if arterial pH was < 7.20. If pH remained < 7.20, despite this infusion, the tidal volume in the VCV group or the inspiratory pressure in the PCV group was increased until pH reached 7.20 or higher.
Outcomes
All trials reported in‐hospital mortality. In Rappaport 1994 this was the only outcome reported that was relevant to this review. Esteban 2000 and Meade 2008 also reported data for mortality in the ICU, and Meade 2008 additionally provided 28‐day mortality rates. Duration of ventilation, mean number of extrapulmonary organ failures after hospitalization and length of stay in hospital and in the ICU were reported in Meade 2008 as medians and interquartile ranges, hence these values could not be pooled. However, dichotomous data from these two trials (Esteban 2000; Meade 2008) for proportions developing barotrauma could be pooled in the meta‐analysis.
None of the included trials reported the numbers of participants with infective complications (ventilator‐associated pneumonia, sepsis) or measures of quality of life after hospital discharge.
Excluded studies
Of the 45 excluded studies (see Characteristics of excluded studies), 15 were cross‐over trials intended to establish physiological effects; two were non‐randomized trials; five were RCTs that assessed only physiological outcomes without addressing this review's objectives; 19 evaluated interventions not relevant to this review; and three (Koh 2007; McCallion 2005; Vines 2001) were reviews. Maxwell 2010 included trauma patients intubated for respiratory failure; only about 50% of study participants in the two arms had ARDS, and data for these participants were not reported separately.
Risk of bias in included studies
Full details of judgements regarding risk of bias and explanations for these for each study can be found in the 'Risk of bias' tables following each study in Characteristics of included studies. Although none of these trials was judged as entirely free of the risk of bias, the overall risk of bias in the included trials was low (see Figure 2 and Figure 3).
2.
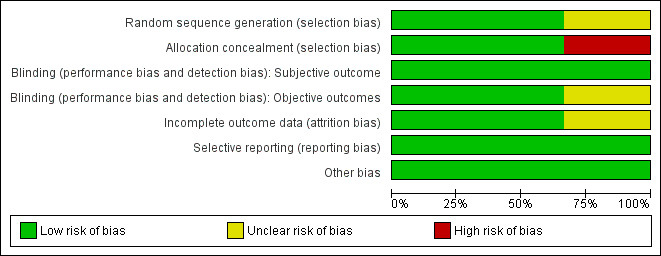
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
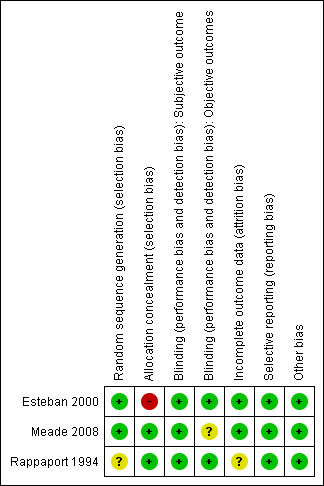
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Esteban 2000 and Meade 2008 were judged to be at low risk of bias for random sequence generation; Rappaport 1994 was assessed as having unclear risk, as the method of generating the random sequence was not reported.
Esteban 2000 and Rappaport 1994 used opaque sealed envelopes to conceal allocation. Rappaport 1994 was judged to be at low risk of bias for allocation concealment. However in Esteban 2000, as randomization was not stratified by risk factors for increased mortality, baseline imbalances for proportions with renal failure fell short of statistical significance; renal failure was independently associated with increased mortality and this could have biased mortality outcomes against those allocated to VCV, among whom more participants had renal failure at recruitment than among those allocated to PCV. This trial was judged as having high risk of selection bias.
Meade 2008 concealed randomization using a central computerized telephone system and stratified enrolment by site using variable permuted blocks. However at the end of the trial, a programming error that had disrupted the randomization blocks was picked up. Study authors reported that sensitivity analysis did not suggest that randomization was undermined. This trial was therefore judged as having low risk of selection bias.
Blinding
None of the trials reported subjective outcomes (e.g. quality of life after discharge from hospital). Rappaport 1994 and Esteban 2000 were judged as having low risk of performance and detection bias for the objective outcomes reported. The data analyst in the trial reported by Meade 2008 was blinded to analyses, hence we judged this trial to be at low risk of detection bias; however Meade 2008 used additional recruitment manoeuvres in the PCV arm that could have increased the risk of barotrauma in this arm. Hence, this trial was judged as having unclear risk of performance bias.
Incomplete outcome data
Meade 2008 and Esteban 2000 were judged as having low risk of attrition bias because no withdrawals or dropouts were reported in Esteban 2000, and because primary outcome data were available for all participants in Meade 2008, with only seven withdrawn and with intention‐to‐treat analyses performed. We judged Rappaport 1994 as having unclear risk of attrition bias, as participants recruited were few; three of 14 randomly assigned to VCV were withdrawn from the trial (found ineligible), and no outcome data were reported for them.
Selective reporting
Meade 2008 was prospectively registered, and no reporting biases were detected. Although the trial protocols for Esteban 2000 and Rappaport 1994 were not available, all prestated outcomes were reported.
Other potential sources of bias
The source of funding was unclear for Esteban 2000 and for Rappaport 1994. The funders of Meade 2008 had no other role in the trial. No other sources of bias were detected in the three trials.
Effects of interventions
See: Table 1
Primary outcomes
Mortality in hospital and in ICU
The three included trials provided data for all‐cause mortality in hospital, and although the pooled estimate favoured PCV over VCV (53% vs 64%), the 95% CI for this estimate did not rule out random error (RR 0.83, 95% CI 0.67 to 1.02; 1089 participants; Analysis 1.1; Figure 4).
1.1. Analysis.
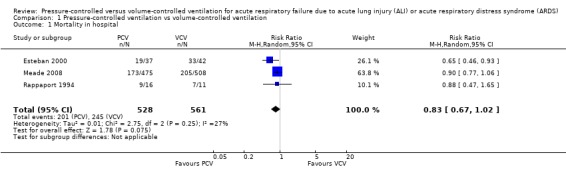
Comparison 1 Pressure‐controlled ventilation vs volume‐controlled ventilation, Outcome 1 Mortality in hospital.
4.
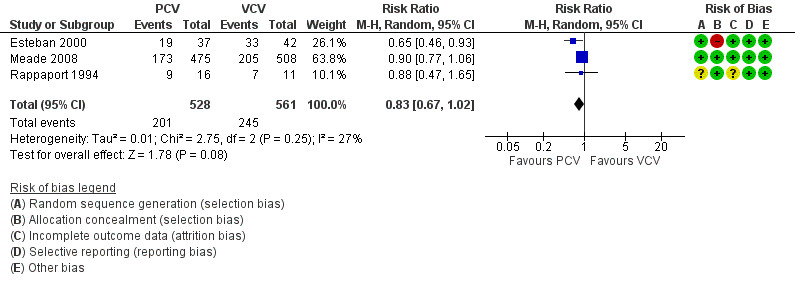
Forest plot of comparison: 1 Pressure‐controlled ventilation vs volume‐controlled ventilation, outcome: 1.1 Mortality in hospital.
Two trials (Esteban 2000; Meade 2008) also provided data for mortality in the ICU. PCV reduced risk of death in the ICU compared with VCV (32% vs 38%), but the upper limit of the 95% CI for the effect estimate indicated that this benefit may not always be clinically appreciable (RR 0.84, 95% CI 0.71 to 0.99; for the following reasons: the analysis involved only two trials (1062 participants). Further, in practice, hospital mortality has greater relevance than ICU mortality. (Analysis 1.2; Figure 5).
1.2. Analysis.
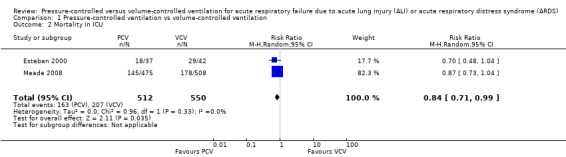
Comparison 1 Pressure‐controlled ventilation vs volume‐controlled ventilation, Outcome 2 Mortality in ICU.
5.
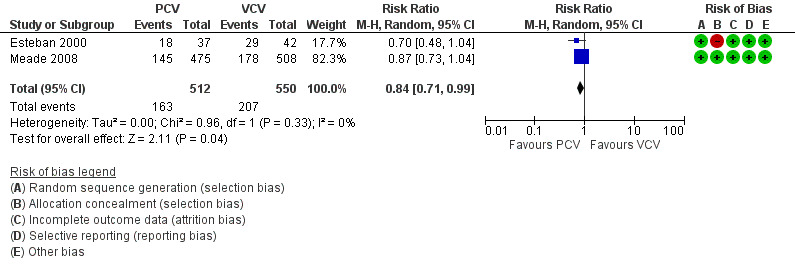
Forest plot of comparison: 1 Pressure‐controlled ventilation vs volume‐controlled ventilation, outcome: 1.2 Mortality in ICU.
Mortality at 28 days
Meade 2008 was the sole trial that reported this outcome. Although 28‐day mortality was lower in the PCV arm (28%) than in the VCV arm (32%), this difference was not significant (RR 0.88, 95% CI 0.73 to 1.06; 983 participants; Table 2).
1. Mortality at 28 days.
|
Comparison: pressure‐controlled ventilation vs volume‐controlled ventilation Outcome: mortality at end of follow‐up (28 days) | |||||
| Study ID | PCV | VCV | Risk ratio (95% CI) | ||
| Events | Total | Events | Total | ||
| Meade 2008 | 135 | 475 | 164 | 508 | 0.88 (0.73 to 1.06) |
| Total events = 299; total participants = 983. Test for overall effect Z = 1.31 (P value 0.19). | |||||
CI = Confidence interval.
PCV = Pressure‐controlled ventilation.
VCV = Volume‐controlled ventilation.
Secondary outcomes
Total duration of ventilation
Meade 2008 reported the duration of mechanical ventilation as median values with interquartile ranges among survivors of mechanical ventilation, and reported no significant differences between the two arms (Table 3).
2. Duration of ventilation.
|
Comparison: pressure‐controlled ventilation vs volume‐controlled ventilation Outcome: duration of ventilation (days) | |||||
| Study ID | Effect measure | PCV | LCV | P value | Comment |
| Meade 2008 | Median (interquartile range) | 10 (6‐17) | 10 (6‐16) | 0.92 | Among survivors of mechanical ventilation |
PCV = Pressure‐controlled ventilation.
VCV = Volume‐controlled ventilation.
Barotrauma
Two studies (Esteban 2000; Meade 2008) reported on barotrauma. The pooled estimate of the risk of barotrauma favoured VCV, but this difference between PCV and VCV was not statistically significant (12% vs 9%; RR 1.24, 95% CI 0.87 to 1.77; 1062 participants; Analysis 1.3). This estimate was largely influenced by Meade 2008, which contributed 91% weight to the estimate. It is possible that use of additional recruitment manoeuvres in the PCV arm of this study contributed to increased barotrauma in this arm.
1.3. Analysis.
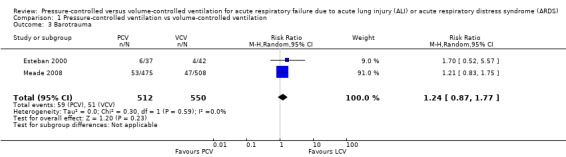
Comparison 1 Pressure‐controlled ventilation vs volume‐controlled ventilation, Outcome 3 Barotrauma.
Development of other organ failure/dysfunction during ICU stay
Esteban 2000 reported that the difference in the mean number of extrapulmonary organ failures significantly favoured PCV versus VCV, but data were skewed and the 95% CI for the mean difference in the number of extrapulmonary organ failures (MD 1.10, 95% CI ‐1.83 to 0.37; 79 participants; Table 4) does not support this interpretation.
3. Extrapulmonary organ failures.
|
Comparison: pressure‐controlled ventilation vs volume‐controlled ventilation Outcome: number of extrapulmonary organ failures | ||||
| Study ID |
PCV (N = 37) |
VCV (N = 42) |
Mean difference (95% CI) | P value |
| Mean (SD) | Mean (SD) | |||
| Esteban 2000 | 2.6 (1.5) | 3.7 (1.8) | 1.10 (‐1.83 to 0.37) | 0.005 |
CI = Confidence interval.
PCV = Pressure‐controlled ventilation.
SD = Standard deviation.
VCV = Volume‐controlled ventilation.
Length of stay in ICU and in hospital
Esteban 2000 and Meade 2008 reported length of ICU and hospital stays. Data from Esteban 2000 reported as means with standard deviations were skewed. Meade 2008 reported this outcome as medians with interquartile ranges. The results could not be pooled but are presented in Table 5 and in Table 6. The primary analyses in both trials did not reveal statistically or clinically important differences in the duration of in‐hospital or ICU stay.
4. Length of stay in ICU.
|
Comparison: pressure‐controlled ventilation vs volume‐controlled ventilation Outcome: length of stay in ICU (days) | |||||
| Study ID | Effect measure | PCV | VCV | P value | Comment |
| Esteban 2000 | Mean (SD) Number in each arm |
21 (15) 37 |
25 (19) 42 |
0.46 | Skewed data (unclear if only for survivors) |
| Meade 2008 | Median (interquartile range) | 13 (8‐23) | 13 (9‐23) | 0.98 | Skewed data among survivors |
PCV = Pressure‐controlled ventilation.
SD = Standard deviation.
VCV = Volume‐controlled ventilation.
5. Length of stay in hospital.
|
Comparison: pressure‐controlled ventilation vs volume‐controlled ventilation Outcome: length of stay in hospital (days) | |||||
| Study ID | Effect measure | PCV | VCV | P value | Comment |
| Esteban 2000 | Mean (SD) Number in each arm |
27 (20) 37 |
30 (24) 42 |
0.84 | Skewed data; unclear if only for survivors |
| Meade 2008 | Median (interquartile range) | 29 (17‐48) | 29 (16‐51) | 0.96 | Skewed data; among survivors |
PCV = Pressure‐controlled ventilation.
SD = Standard deviation.
VCV = Volume‐controlled ventilation.
Other secondary outcomes
None of the included studies reported on infective complications, and none described quality of life after hospital discharge.
Subgroup and sensitivity analyses
Data were available for meta‐analyses only for mortality and for barotrauma. As heterogeneity was not significant in the pooled estimates for these outcomes, subgroup analyses were not indicated.
Excluding data from Esteban 2000, which was at risk of bias because of confounding in sensitivity analyses for mortality outcomes, did not significantly alter pooled estimates. The pooled estimate for barotrauma from two trials (Esteban 2000; Meade 2008) was largely influenced by Meade 2008, which contributed 91% weight to the estimate. It was thought possible that use of additional recruitment manoeuvres in the PCV arm of this study contributed to increased barotrauma. However, removal of Meade 2008 from sensitivity analyses did not alter the results significantly. No trials were at high risk of attrition bias, and sensitivity analyses excluding data from these trials were not indicated.
Discussion
Summary of main results
See Table 1.
This review included three trials that compared pressure‐controlled versus volume‐controlled ventilation in 1089 people admitted to 43 intensive care units (ICUs) in high‐income countries with acute respiratory failure due to acute lung injury/acute respiratory distress syndrome (ALI/ARDS). Data for the outcomes sought in this review were available from the three trials only for mortality in hospital, and from two trials for mortality in the ICU and for barotrauma. All other outcomes could not be pooled because they were reported only in a single study, or because they were presented in a manner that precluded data synthesis.
Pressure‐controlled ventilation (PCV) probably reduces ICU mortality (32%, 95% CI 27% to 37%) compared with volume‐controlled ventilation (VCV) (38%; moderate‐quality evidence), and probably does not differ from VCV in terms of mortality in hospital or at 28 days (moderate‐quality evidence); however, the numbers evaluated were small, and further research may change these estimates.
It is uncertain whether the duration of mechanical ventilation differs with these ventilatory modes, given that this outcome was reported in only one trial with skewed data.
Risk of barotrauma may not differ between PCV and VCV (low‐quality evidence), but our confidence in this result was limited by differences in co‐administered interventions in the two trials that provided data for this outcome, and the small numbers were evaluated.
We are unsure whether development of organ failure is influenced by mode of ventilation. Limited data from one trial were skewed.
Pressure‐controlled ventilation (PCV) and VCV may not differ in terms of length of stay in hospital and in ICU, but we are unsure of this, as data from the two trials were skewed and could not be pooled.
We found no trials that evaluated infective complications or quality of life after discharge among those treated with PCV or VCV.
Overall completeness and applicability of evidence
Completeness
This review found only three trials that addressed the efficacy and safety of PCV versus VCV, and 28‐day mortality data were available from only one trial. Two of the three trials recruited small numbers (Rappaport 1994 27 participants; Esteban 2000 79 participants) and were clearly underpowered. Given the recently reported mortality of ARDS in the range of 15% to 25% in the control groups in ARDSNet trials (Caser 2014; Gates 2013; Matthay 2011), a very large number of participants would be required to show reduction in mortality by any intervention. Lack of available data from Keddissi 2000, which currently awaits assessment, also could have impacted our results. Some outcomes were reported only in single trials, and we were unable to provide pooled estimates for length of stay in hospital and in the ICU from two trials (Esteban 2000; Meade 2008). No data were available for infective complications such as ventilator‐associated pneumonia and sepsis, nor for quality of life after discharge, among those ventilated.
Because of these limitations, the evidence base is currently incomplete and is insufficient to provide robust evidence of the comparative effects of pressure‐controlled over volume‐controlled ventilation in ALI/ARDS.
Applicability
Despite the absence of statistical heterogeneity, some degree of clinical heterogeneity was present across the included studies. Meade 2008 employed additional recruitment manoeuvres in one treatment arm, which could have confounded the results. In Rappaport 1994, non‐protective ventilation was used and no data on target volumes were reported. Although lung‐protective ventilation strategies were not well understood at that time, it is possible that higher volumes in the VCV group may have contributed to greater mortality. Esteban 2000 used target volumes and pressures that were intermediate between protective and non‐protective. Additionally, these trials were conducted in high‐income settings, and the aetiology, disease progression and prognosis of ARDS may differ in low‐ and middle‐income settings (Agarwal 2006; George 2014), thereby affecting applicability of the results of these trials.
Quality of the evidence
The overall quality of the evidence for our primary outcomes of mortality in ICU, in hospital and at 28 days was moderate, and for barotrauma was low. For each of these outcomes, we downgraded the overall quality by one level for imprecision because the results of our analysis do not rule out either treatment as superior in reducing mortality. For the outcome of barotrauma, we also considered clinical heterogeneity to be a major issue despite the lack of statistical heterogeneity. The two studies contributing data to this outcome applied different co‐interventions; therefore we downgraded the quality of evidence further by one level to low quality because of clinical rather than statistical heterogeneity. We could not rate the quality of evidence for other outcomes of interest because relevant outcome data were not provided by the included studies.
Potential biases in the review process
We used standard methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b) and ensured compliance with Cochrane standards for the conduct of new reviews of interventions (MECIR 2011).
Agreements and disagreements with other studies or reviews
A Cochrane systematic review (Wheeler 2010, which is an updated version of McCallion 2005) comparing volume‐targeted ventilation versus pressure‐limited ventilation in newborn infants concluded that volume‐targeted ventilation reduced death and chronic lung disease in neonates. We are not aware of any published systematic review comparing these ventilatory modes in adults with ALI/ARDS.
Authors' conclusions
Implications for practice.
The quality of evidence in this Cochrane review is insufficient to permit robust conclusions regarding the effects of volume‐ or pressure‐controlled ventilation for ARDS.
Implications for research.
In the first instance, published studies on respiratory mechanics and gas exchange may be systematically reviewed before new outcome‐based studies are undertaken, to ascertain whether either of these strategies confers a physiological advantage over the other. If a potential advantage is identified, randomized controlled trials comparing PCV versus VCV, including participants from low‐ and middle‐income countries, would be needed to provide evidence for more definitive guidance. Given the decreasing mortality of ARDS, care should be taken to adequately power these trials to answer this question definitively. These trials should conform to the CONSORT statement and should stratify randomization by risk factors for mortality (and by severity of ARDS). Such trials should also restrict co‐interventions such as those described in the trials published so far. If randomization is stratified for baseline severity of ARDS and for prognostic risk factors, then mortality in ICU, in hospital or at 60 days is likely to provide meaningful data on the comparative efficacy of the two ventilatory modes. Reliable data on risk of barotrauma, infection and quality of life after discharge are required before it can be fully evaluated whether these ventilatory modes offer any advantage for adults with ALI/ARDS placed on mechanical ventilation.
Feedback
Error noted in mortality in the ICU
Summary
When you compared the mortality in the ICU, you stated that this benefit may not always be clinically appreciable because of the lower limit of the 95% confidence interval for the effect estimate. But I think the two groups have a difference because the test for overall effect was P = 0.04 < 0.05 (see Analysis 1.2). Can you please clarify this?
Reply
Thank you for alerting us to an error which appeared in the following statement in the Effects of interventions/ Mortality in hospital and in ICU section: “Two trials (Esteban 2000; Meade 2008) also provided data for mortality in the ICU. PCV reduced risk of death in the ICU compared with VCV (32% vs 38%), but the lower limit of the 95% CI for the effect estimate indicated that this benefit may not always be clinically appreciable (RR 0.84, 95% CI 0.71 to 0.99)”.
We have made the following changes to this statement:
a. We have changed 'lower limit' in the statement to 'upper limit' (see below)
b. To further clarify that the benefit may not always be clinically appreciable, we have added the following: "The analysis involved only two trials (1062 participants). Further, in practice, hospital mortality has greater relevance than ICU mortality."
The statement now reads in full: "Two trials (Esteban 2000; Meade 2008) also provided data for mortality in the ICU. PCV reduced risk of death in the ICU compared with VCV (32% vs 38%), but the upper limit of the 95% CI for the effect estimate indicated that this benefit may not always be clinically appreciable (RR 0.84, 95% CI 0.71 to 0.99; for the following reasons: the analysis involved only two trials (1062 participants). Further, in practice, hospital mortality has greater relevance than ICU mortality. (Analysis 1.2; Figure 5)."
Contributors
Summary contributor: Huang Haijun
Zhejiang Provincial Hospital of Traditional Chinese Medicine, Zhejiang, China
I do not have any affiliation with or involvement in any organization with a financial interest in the subject matter of my comment
Reply contributor: Binila Chacko
Medical Intensive Care Unit, Christian Medical College & Hospital, Vellore, India
Lead author of Chacko 2015
What's new
| Date | Event | Description |
|---|---|---|
| 17 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
| 13 December 2016 | Amended | Feedback received, responded to and incorporated in the review; error corrected in Effects of interventions/ Mortality in hospital and in ICU |
History
Protocol first published: Issue 11, 2010 Review first published: Issue 1, 2015
| Date | Event | Description |
|---|---|---|
| 15 September 2016 | Amended | Analysis 1.2: label corrected to show risk of death as lower for PCV (previously reversed); Figure 5 (analysis 1.2) also corrected |
Notes
December 2016
Feedback received, responded to and incorporated in the review; error corrected in Effects of interventions/ Mortality in hospital and in ICU
September 2016
Analysis 1.2: label corrected to show risk of death as lower for PCV (previously reversed); Figure 5 (analysis 1.2) also corrected.
Acknowledgements
This review is an output of a protocol development workshop conducted by the South Asian Cochrane Network & Centre and hosted at the Prof. BV Moses & ICMR Centre for Advanced Research & Training, CMC Vellore, Tamil Nadu, India.
We are grateful to Nicola Petrucci (content editor), Nathan Pace (statistical editor) and Karen Burns and Arash Afshari (peer reviewers) for help and editorial advice provided during preparation of the protocol for this systematic review. We also thank Peter JD Andrews, Ross Freebairn, Karen Burns and Yuh‐Chin T Huang (peer reviewers) and Robert Wylie (consumer referee) for help and editorial advice provided during preparation of this systematic review. We are grateful to Toby Lasserson (editor, Cochrane Central Editorial Unit) for helpful suggestions that were incorporated into the final version of this review. We thank Jane Cracknell, Managing Editor of the Cochrane Anaesthesia Group, for the patience, encouragement and support that she provided.
Appendices
Appendix 1. Acronyms
| Abbreviation | Full form | Definitiona |
| PaO2/FiO2 | Ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2). PaO2 is measured in mmHg and FiO2 is expressed as a decimal between 0.21 and 1.00 | |
| ALI | Acute lung injury |
Pulmonary artery wedge pressure < 18 mmHg when measured or no clinical evidence of left atrial hypertension |
| ARDS | Acute respiratory distress syndrome | 1. Ratio of partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FiO2) < 200, regardless of level of positive end‐expiratory pressure (PEEP) 2. Bilateral infiltrates on frontal chest radiographs 3. Pulmonary artery wedge pressure < 18 mmHg when measured or no clinical evidence of left atrial hypertension |
| VILI | Ventilator‐induced lung injury | Refers to aggravation of underlying lung injury by ventilatory therapy alone. This may occur as a result of excessive pressure (barotrauma), alveolar overdistension (volutrauma), trauma with repeated opening and closing of alveoli (atelectrauma) and alveolar inflammation or nosocomial infection (biotrauma) |
| VALI | Ventilator‐associated lung injury | Lung injury associated with ventilation, which is believed to occur as the result of repeated alveolar collapse and expansion (RACE) |
| aAs per the American‐European Consensus Conference. | ||
Appendix 2. MEDLINE (Ovid SP) search strategy
1. Pulmonary Ventilation/ or Ventilation/ or Positive‐Pressure Respiration/ or Respiration, Artificial/ or Ventilators, Mechanical/ or High‐Frequency Ventilation/ or High‐Frequency Jet Ventilation/
2. ((Pressure‐controlled or volume‐controlled) and ventilat*).mp. or ventilat*.ti.
3. 1 or 2
4. Respiratory Distress Syndrome, Adult/ or Respiratory Insufficiency/ or exp Severe Acute Respiratory Syndrome/ or Lung Injury/
5. (acute respiratory failure or acute lung injury or (acute adj3 distress syndrome)).mp. or (ALI or ARDS).ti,ab.
6. 5 or 4
7. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh.
8. 7 and 6 and 3
Appendix 3. EMBASE (Ovid SP)
1. lung ventilation/ or positive end expiratory pressure/ or artificial ventilation/ or ventilator/ or high frequency ventilation/ or high frequency ventilation/
2. ((Pressure‐controlled or volume‐controlled) and ventilat*).ti,ab. or ventilat*.ti.
3. 1 or 2
4. adult respiratory distress syndrome/ or respiratory failure/ or severe acute respiratory syndrome/ or lung injury/
5. (acute respiratory failure or acute lung injury or (acute adj3 distress syndrome) or (ALI or ARDS)).ti,ab.
6. 4 or 5
7. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) not (animals not (humans and animals)).sh.
8. 3 and 6 and 7
Appendix 4. CENTRAL
#1 MeSH descriptor Pulmonary Ventilation, this term only
#2 MeSH descriptor Positive‐Pressure Respiration explode all trees
#3 MeSH descriptor Respiration, Artificial explode all trees
#4 MeSH descriptor Ventilators, Mechanical explode all trees
#5 MeSH descriptor High‐Frequency Ventilation explode all trees
#6 MeSH descriptor High‐Frequency Jet Ventilation explode all trees
#7 ((Pressure‐controlled or volume‐controlled) and ventilat*):ti,ab
#8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
#9 MeSH descriptor Respiratory Distress Syndrome, Adult explode all trees
#10 MeSH descriptor Respiratory Insufficiency explode all trees
#11 MeSH descriptor Severe Acute Respiratory Syndrome explode all trees
#12 MeSH descriptor Lung Injury explode all trees
#13 (acute respiratory failure or acute lung injury or (acute near distress syndrome)):ti,ab or (ALI or ARDS):ti,ab
#14 (#9 OR #10 OR #11 OR #12 OR #13)
#15 (#8 AND #14)
Appendix 5. ISI Web of Science
# 1 TS=((Pressure‐controlled or volume‐controlled) SAME ventilat*) or TS=(respirat* SAME (pressure or artificial)) # 2 TI=(distress syndrome) or TS=(acute respiratory SAME (distress or Insufficiency or failure)) or TS=(acute lung injury) or TS=(ALI or ARDS) # TS=(random* or trial* or placebo* or multicenter* or prospective*) or TS=((single or double or triple or treble) SAME (mask* or blind)) # 4 #3 AND #2 AND #1
Appendix 6. Data abstraction form
Study selection, quality assessment and data extraction form
| First author | Journal/Conference proceedings, etc. | Year |
Study eligibility
| RCT/Quasi/CCT (delete as appropriate) | Relevant participants | Relevant interventions | Relevant outcomes |
| Yes/No/Unclear |
Yes/No/Unclear |
Yes/No/Unclear |
Yes/Noa/Unclear |
aIssue relates to selective reporting when study authors may have taken measurements for particular outcomes but not reported these within the paper(s). Review authors should contact trialists for information on possible non‐reported outcomes and reasons for exclusion from publication. Study should be listed in Studies awaiting classification until clarified. If no clarification is received after 3 attempts, study should be excluded.
CCT = Controlled clinical trial.
RCT = Randomized controlled trial.
| Do not proceed if any of the above answers is ‘No.’ If study is to be included in Excluded studies section of the review, record below the information to be inserted into Characteristics of excluded studies table. |
| Freehand space for comments on study design and treatment: |
References to trial
Check other references identified in searches. If further references to this trial are found, link the papers now and list below. All references to a trial should be linked under one Study ID in RevMan.
| Code each paper | Author(s) | Journal/Conference proceedings, etc. | Year |
| A | The paper listed above | ||
| B | Further papers | ||
Participants and trial characteristics
| Participant characteristics | ||
| Volume controlled | Pressure controlled | |
| Numbers of participants | ||
| Age (mean, median, range, etc.), years | ||
| Sex of participants (numbers/%, etc.) | ||
| Duration of symptoms before recruitment | ||
| APACHE II/III score | ||
| SAPS score | ||
| Lung injury score | ||
| P/F ratio at admission | ||
| pH at admission | ||
| Static compliance at admission | ||
| No extrapulmonary organ failure | ||
| Extrapulmonary organ failure | ||
| Primary ARDS | ||
| Extrapulmonary ARDS | ||
| Baseline plateau pressure | ||
| Baseline tidal volume set/achieved | ||
| Co‐morbidities IHD COPD DM Smoking Others |
||
| Cause of ARDS/ALI Sepsis Trauma Pulmonary infection Gastric aspiration Transfusion Reperfusion injury Pancreatitis Others |
||
ALI: Acute lung injury.
APACHE: Acute Physiology and Chronic Health Evaluation.
ARDS: Acute respiratory distress syndrome.
COPD: Chronic obstructive pulmonary disease.
DM: Diabetes mellitus.
IHD: Ischaemic heart disease.
P/F: PaO2/FiO2 ratio.
SAPS: Simplified Acute Physiology Score.
Trial characteristics (usually completed by just one review author)
| Trial characteristics | |
| Further details | |
| Single centre/Multi‐centre | |
| Country/Countries | |
| How was participant eligibility defined? | |
| How many people were randomly assigned? | |
| Number of participants in each intervention group | |
| Number of participants who received intended treatment | |
| Number of participants who were analysed | |
| Intervention used | |
| Duration of treatment (state weeks/months, etc.; if cross‐over trial, give length of time in each arm) | |
| Median (range) length of follow‐up reported in this paper (state weeks, months or years or if not stated) | |
| Time points when measurements were taken during the study | |
| Time points reported in the study | |
| Time points you are using in RevMan | |
| Trial design (e.g. parallel/cross‐over*) | |
| Other | |
Methodological quality
| Allocation of intervention | |
| State here method used to generate allocation and reasons for grading | Grade (circle) |
| |
Adequate (random) |
| Inadequate (e.g. alternate) | |
| Unclear | |
|
Concealment of allocation Process used to prevent foreknowledge of group assignment in an RCT, which should be seen as distinct from blinding | |
| State here method used to conceal allocation and reasons for grading | Grade (circle) |
| Adequate | |
| Inadequate | |
| Unclear | |
| Blinding | |
| Person responsible for participants' care | Yes/No |
| Participant | Yes/No |
| Outcome assessor | Yes/No |
| Other (please specify) | Yes/No |
|
Intention‐to‐treat An intention‐to‐treat analysis is one in which all participants in a trial are analysed according to the intervention to which they were allocated, whether or not they received it | |
| All participants entering trial | |
| ≤ 15% excluded | |
| > 15% excluded | |
| Not analysed as ‘intention‐to‐treat’ | |
| Unclear | |
Were withdrawals described? Yes ? No ? Not clear ?
Discuss if appropriate.
Data extraction
|
Outcomes relevant to your review Copy and paste from Types of outcome measures | |
| Reported in paper (circle) | |
| Mortality (in ICU) | Yes/No |
| Mortality (in hospital) | Yes/No |
| Mortality (90 day) | Yes/No |
| Physiological variables | Yes/No |
| Duration of mechanical ventilation | Yes/No |
| Ventilator‐free days | Yes/No |
| Number of participants with infective complications | Yes/No |
| Duration of ICU stay | Yes/No |
| Duration of hospitalization | Yes/No |
| Events of barotrauma | Yes/No |
| Other organ failure/dysfunction during ICU stay | Yes/No |
| Quality of life measures post discharge from hospital | Yes/No |
| For continuous data | |||||||
| Code of paper |
Outcomes (rename) |
Unit of measurement |
Intervention group | Control group | Details if only outcome described in text | ||
| N | Mean (SD) | N | Mean (SD) | ||||
| Quality of life measures post discharge from hospital | Score | ||||||
| Duration of mechanical ventilation | Days | ||||||
| Ventilator‐free days | Days | ||||||
| Duration of ICU stay | Days | ||||||
| Duration of hospitalization | Days | ||||||
| PaO2/FiO2 (P/F) ratio at 24 hours P/F ratio on day 4 P/F ratio on day 7 |
|||||||
| Compliance on day 1 Compliance on day 4 Compliance on day 7 |
mL/cm H2O | ||||||
| Arterial pH on day 1 Arterial pH on day 4 Arterial pH on day 7 |
|||||||
| For dichotomous data | |||
| Code of paper | Outcomes (rename) | Intervention group (N) N = number of participants, not number of events |
Control group (N) N = number of participants, not number of events |
| Mortality (in ICU) | |||
| In‐hospital mortality | |||
| Mortality at longest follow‐up (90 days) | |||
| Events of barotrauma | |||
| Other organ failure/dysfunction during ICU stay | |||
| Number of participants with infective complications | |||
| VAP | |||
| CRBSI | |||
CRBSI: Catheter‐related bloodstream infection.
ICU: Intensive care unit.
VAP: Ventilator‐associated pneumonia.
|
Other information that you feel is relevant to the results Indicate if any data were obtained from the primary author; if results were estimated from graphs, etc, or were calculated by you using a formula (this should be stated and the formula given). In general if results not reported in paper(s) are obtained, this should be made clear here to be cited in the review. |
| |
| Freehand space for writing actions such as contact with study authors and changes |
References to other trials
| Did this report include any references to published reports of potentially eligible trials not already identified for this review? | ||
| First author | Journal/Conference | Year of publication |
| Did this report include any references to unpublished data from potentially eligible trials not already identified for this review? If yes, give list contact name and details | ||
| | ||
Data and analyses
Comparison 1. Pressure‐controlled ventilation vs volume‐controlled ventilation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality in hospital | 3 | 1089 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.67, 1.02] |
| 2 Mortality in ICU | 2 | 1062 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.71, 0.99] |
| 3 Barotrauma | 2 | 1062 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.87, 1.77] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Esteban 2000.
| Methods | Multi‐centre, parallel‐group, randomized trial | |
| Participants |
Number of participants: 79 Age: older than 18 years; mean age: PCV 56 (SD 17) years; VCV 59 (SD 16) years Gender: both; females: PCV 35%; VCV 29% Inclusion criteria
Exclusion criteria
|
|
| Interventions |
Intervention Pressure‐controlled ventilation (PCV) with respiratory rate, ratio of inspiration to expiration or inspiratory pressure adjusted in an attempt to maintain PaCO2 at 35 to 45 mmHg, but hypercapnia accepted if this target could not be achieved with plateau pressure < 36 cm H2O (N = 37). Control Volume‐controlled ventilation (VCV) delivered with a square‐wave inspiratory flow. Respiratory rate, ratio of inspiration to expiration or tidal volume adjusted in an attempt to maintain PaCO2 at 35 to 45 mmHg, but hypercapnia accepted if this target could not be achieved with plateau pressure < 36 cm H2O (N = 42). For both groups, plateau inspiratory pressure was limited to ≤ 35 cm H2O. PEEP and FiO2 were titrated to maintain oxygen saturation of 89% to 92%, with the least FiO2 and PEEP level never < 5 cm H2O. |
|
| Outcomes |
Outcomes reported and used
Outcomes sought but not reported
|
|
| Notes |
Period of the study: February 1995 to January 1996 Country: Spain: 12 ICUs in 12 tertiary care hospitals Co‐interventions: For both groups, intravenous sodium bicarbonate was infused if arterial pH was < 7.20. If pH remained < 7.20 despite bicarbonate sodium infusion, tidal volume in the VCV group or inspiratory pressure in the PCV group was increased until pH reached ≥ 7.20 Source of funding: not reported Notes
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quotes from report: "Randomization was performed by permuted blocks according to the study centre" and "Patients were randomly assigned with the use of a random number table to receive either VCV or PCV" |
| Allocation concealment (selection bias) | High risk | Quote from report: "Patients were allocated to the two groups in a blinded fashion with the use of opaque, sealed, numbered envelopes, which were opened only when the patient fulfilled the inclusion criteria" Comment: Randomization was not stratified by risk factors for increased mortality. Baseline imbalances were noted for proportions with renal failure that fell short of statistical significance but could have biased mortality outcomes against those allocated to VCV when more participants had renal failure at recruitment than those allocated to PCV. Renal failure was an independent risk factor for increased mortality |
| Blinding (performance bias and detection bias) Subjective outcome | Low risk | No subjective outcomes were reported |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | The study was open‐label, but outcomes reported in this trial such as mortality, barotrauma and organ failure were objective and are at low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals or dropouts were reported |
| Selective reporting (reporting bias) | Low risk | Although the trial protocol was not available, all prestated outcomes of interest were reported |
| Other bias | Low risk | No other sources of bias were detected |
Meade 2008.
| Methods | Multi‐centre, parallel‐group, randomized controlled trial | |
| Participants |
Number of participants: 983 Age: mean (SD): PCV 54.5 (16.5) years; VCV 56.9 (16.5) years Gender: both; female: PCV 41%; VCV 40% Inclusion criteria Patients with both acute lung injury and ARDS, defined by onset of new respiratory symptoms within 28 days and bilateral opacifications on chest radiograph, and requiring a ratio of arterial oxygen tension to inspired oxygen fraction (PaO2/FiO2) ≤ 250 during invasive mechanical ventilation Exclusion criteria
|
|
| Interventions |
Intervention Pressure‐controlled ventilation (lung open ventilation strategy): included target tidal volumes of 6 mL/kg of predicted body weight, plateau pressures not exceeding 40 cm H2O, recruitment manoeuvres and higher positive end‐expiratory pressures (N = 475). Control Volume‐controlled ventilation: included target tidal volumes of 6 mL/kg of predicted body weight, plateau airway pressures not exceeding 30 cm H2O and conventional levels of positive end‐expiratory pressure (N = 508). |
|
| Outcomes |
Outcomes reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes |
Period of the study: August 2000 to March 2006 Country: 30 hospitals in Canada, Australia and Saudi Arabia Co‐interventions
Source of funding: grants from the Canadian Institutes for Health Research and Hamilton Health Sciences Foundation Dr Meade was a Peter Lougheed Scholar of the Medical Research Council of Canada during the period of this study. Funders had no role in designing or reporting this study. Notes
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from report: "stratified enrolment by site using variable permuted blocks" Comment: Method reported suggests that randomization list was computer generated |
| Allocation concealment (selection bias) | Low risk | Quotes from report: "We concealed randomization using a central computerized telephone system and stratified enrolment by site using variable permuted blocks"; "At the end of the trial, we noted an unexpected difference in the number of patients allocated to each group and found that in high‐volume hospitals with rapid enrolment and in newly participating centers, a programming error occurring late in the study had disrupted the specified randomization blocks. Sensitivity analyses indicated that this error did not undermine randomization" Comment: Sensitivity analyses suggest that risk of selection bias was low |
| Blinding (performance bias and detection bias) Subjective outcome | Low risk | No subjective outcomes were reported |
| Blinding (performance bias and detection bias) Objective outcomes | Unclear risk | This trial was at low risk of detection bias, as all outcomes were objective outcomes and the data analyst was blinded to treatment allocation. However, this unblinded trial used additional recruitment manoeuvres in the PCV arm that could have contributed to increased barotrauma in this arm |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were available for all participants; only 7 were withdrawn, and intention‐to‐treat analyses were used |
| Selective reporting (reporting bias) | Low risk | The trial was prospectively registered and all predefined outcomes were reported |
| Other bias | Low risk | No other sources of bias were detected |
Rappaport 1994.
| Methods | Randomized, parallel‐group, controlled trial | |
| Participants |
Number of participants: 27 Age: mean (SD): PCV: 43.1 (4.3); VCV: 51.6 (6.3) Gender: both; female: PCV 44%; VCV 36% Eligible were all patients receiving care in a medical ICU for acute, severe hypoxic respiratory failure (PaO2/FiO2 ratio < 150) during a 6‐month period Inclusion criteria
|
|
| Interventions |
Intervention Pressure‐controlled ventilation (N = 16) Control Volume‐controlled ventilation (N = 11) Ventilation was initiated within 24 hours of endotracheal intubation in both arms |
|
| Outcomes |
Outcome reported and used
Outcomes sought but not reported
Outcomes reported but not used
|
|
| Notes |
Period of study: dates not reported; conducted over 6 months Country: UCLA School of Medicine, Los Angeles, California, USA Co‐intervention: paralytic agents and sedatives (no difference between intervention arms) Source of funding: unclear Notes
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of generating random numbers was not mentioned |
| Allocation concealment (selection bias) | Low risk | "Patients were randomized via a blind, "envelope pull" method, to receive either pressure limited or volume controlled ventilation" |
| Blinding (performance bias and detection bias) Subjective outcome | Low risk | No subjective outcomes were reported |
| Blinding (performance bias and detection bias) Objective outcomes | Low risk | Risk of performance bias was low, as intervention arms did not differ in proportions given co‐interventions. Mortality was the sole outcome reported that was used in this review, and lack of blinding is unlikely to have introduced detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sample was small; 3/14 randomly assigned to VCV were withdrawn from the trial (found ineligible), and no outcome data were reported |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available but prestated outcomes were reported |
| Other bias | Low risk | We detected no other biases |
ARDS: Acute respiratory distress syndrome.
FiO2: Fraction of inspired oxygen.
ICU: Intensive care unit.
PaO2: Partial pressure of arterial oxygen.
PCV: Pressure‐controlled ventilation.
PEEP: Positive end‐expiratory pressure.
SD: Standard deviation.
VCV: Volume‐controlled ventilation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alvarez 1998 | Cross‐over study design; participants were stabilized on volume‐controlled ventilation (VCV) for 30 minutes before, between and at the end of PCV and PRVC to reach baseline conditions |
| Amato 1995 | Alternate interventions; intervention arm employed open lung ventilation, and ventilator modes included both pressure‐controlled modes and dual modes, that is, pressure‐ and volume‐controlled ventilation, whilst control arm used volume‐controlled ventilation |
| Betensley 2008 | Cross‐over design; alternate intervention (non‐invasive ventilation) |
| Brower 2004 | Alternate intervention; participants with acute lung injury and ARDS were randomly assigned to receive mechanical ventilation with lower or higher PEEP levels, which were set according to different tables of predetermined combinations of PEEP and fraction of inspired oxygen |
| Choi 2006 | Alternate intervention with physiological outcomes; participants were randomly assigned to mechanical ventilation with higher tidal volumes of 12 mL/kg ideal body weight and no positive end‐expiratory pressure (PEEP), or lower tidal volumes of 6 mL/kg and 10 cm H2O PEEP |
| Davis 1996 | Cross‐over study design; participants were ventilated at a tidal volume of 10 mL/kg; then in random sequence, ventilator mode was changed from volume control with a square flow waveform, pressure‐control ventilation with a decelerating flow waveform or volume‐controlled ventilation with a decelerating flow waveform |
| de Durante 2002 | Alternate intervention looking at effects of respiratory rate on PEEP in participants ventilated with limited ventilation strategy |
| Delgado 2009 | Alternate intervention comparing the effects of 2 strategies of setting PEEP on mortality |
| Edibam 2001 | Assessed only physiological outcomes in the short term |
| Edibam 2003 | Assessed only physiological outcomes in the short term |
| Eisner 2001 | Alternate intervention; looked at efficacy of low tidal volume strategy |
| Fang 2002 | Cross‐over study design |
| Ferguson 2002 | Post hoc analysis of pulmonary artery wedge pressure in participants with or at high risk for ARDS, enrolled in a randomized controlled trial of pressure‐ and volume‐limited ventilation |
| Forel 2006 | Alternate intervention; participants were randomly assigned to receive conventional therapy plus placebo or conventional therapy plus neuromuscular blocking agents for 48 hours. Both groups were ventilated with a lung‐protective strategy (tidal volume between 4 and 8 mL/kg ideal body weight, plateau pressure ≤ 30 cm H2O) |
| Gainnier 2003 | Alternate intervention; effects of PEEP and prone position in participants with ARDS |
| Gainnier 2004 | Alternate intervention; after randomization, participants received conventional therapy without NMBA (control group) or conventional therapy plus NMBA for the next 48 hours. Initial ventilator mode was volume‐assist/control |
| Ge 2004 | Assessed only physiological outcomes in the short term |
| Grasso 2007 | Cross‐over study design with physiological outcomes |
| Huh 2009 | Alternate intervention; decremental PEEP titration following alveolar recruitment manoeuvre or a table‐based PEEP (control) group |
| Kallet 2000 | Cross‐over study design |
| Kallet 2005 | Cross‐over repeated‐measures design |
| Kiehl 1996 | Not randomized; volume‐controlled vs biphasic positive airway pressure ventilation in leukopenic participants with severe respiratory failure |
| Knebel 1994 | Study looked at different interventions for weaning |
| Koh 2007 | Review |
| Lessard 1994 | Cross‐over study design; participants with moderate to severe ARDS had lungs ventilated with VC, PCV with a conventional ratio (I:E 1:2; PC 1/2) and PCIRV (I:E 2:1 and 3:1; PC 2/1 and PC 3/1, respectively) |
| Mancebo 1994 | Physiological outcomes |
| Maxwell 2010 | Population of participants with trauma, only 50% with ALI/ARDS |
| McCallion 2005 | Review; volume‐targeted vs pressure‐limited ventilation in the neonate |
| Mercat 1993 | Cross‐over study design; pressure‐controlled ventilation was compared with an inspiratory‐to‐expiratory time ratio (I/E) of 1/2 (PCV) and of 2/1 (PCIRV) versus volume‐controlled ventilation (VCV) with an I/E of 1/2 in participants with acute respiratory distress syndrome. Each ventilatory mode was applied for 1 hour in a randomly assigned order |
| Mercat 2008 | Alternate intervention; tidal volume was set at 6 mL/kg of predicted body weight in both strategies and participants were randomly assigned to a moderate PEEP strategy (5 to 9 cm H2O) (minimal distension strategy) or to a level of PEEP set to reach a plateau pressure of 28 to 30 cm H2O (increased recruitment strategy) |
| Parsons 2005 | Alternate intervention; participants were randomly assigned to 6 mL/kg or 12 mL/kg tidal volume strategy |
| Prella 2002 | Cross‐over study design; sequential ventilation in PCV and VCV with constant inspiratory/expiratory ratio, tidal volume, respiratory rate and total positive end‐expiratory pressure |
| Putensen 2001 | Alternate intervention; airway pressure release ventilation vs PCV |
| Rasenan 1991 | Cross‐over study design, focused mainly on physiological outcomes |
| Riverso 1998 | Cross‐over study design with physiological outcomes; participants were first ventilated in volume‐controlled mode and were then ventilated with pressure‐regulated volume control |
| Sarmiento 1998 | Cross‐over study design; participants were initially ventilated with VCV + PEEP. All sequentially underwent 3 ventilatory modes |
| Stewart 1998 | Alternate intervention; limited ventilation group vs conventional ventilation group |
| Talmor 2008 | Alternate intervention; PEEP assigned according to measurements of oesophageal pressure |
| Tuğrul 1997 | Cross‐over study design |
| Varpula 2003 | Outcomes physiological |
| Villar 2006 | Alternate intervention |
| Vines 2001 | Review |
| Wang 2002 | Cross‐over study design |
| Yang 2007 | Cross‐over physiological study |
| Zupancich 2005 | Alternate intervention with physiological outcomes; participants undergoing elective coronary artery bypass were randomly assigned to be ventilated after cardiopulmonary bypass disconnection with high tidal volume/low positive end‐expiratory pressure (10 to 12 mL/kg and 2 to 3 cm H2O, respectively) or low tidal volume/high positive end‐expiratory pressure (8 mL/kg and 10 cm H2O, respectively) |
ALI: Acute lung injury.
ARDS: Acute respiratory distress syndrome.
I:E: Inspiratory:Expiratory ratio.
NMBA: Neuromuscular blocking agent.
PCIRV: Pressure‐controlled inverse ratio ventilation.
PCV: Pressure‐controlled ventilation.
PEEP: Positive end‐expiratory pressure.
PRVC: Pressure regulated volume control.
VCV: Volume‐controlled ventilation.
Characteristics of studies awaiting assessment [ordered by study ID]
Keddissi 2000.
| Methods | Randomized controlled trial |
| Participants | Unclear |
| Interventions | Unclear |
| Outcomes | Unclear |
| Notes | Full‐text article/contact with study authors was not possible. We shall attempt to obtain full text to assess this trial for inclusion in an update of this review |
Differences between protocol and review
We made the following changes to the protocol (Chacko 2010).
Background
We added the Berlin definition (Ranieri 2012) to the section on Description of the condition.
Methods
Criteria for selecting studies
In Types of studies, we clarified that only parallel‐group RCTs and quasi‐RCTs were included, and that cross‐over trials were excluded. We had omitted to clearly state this intent in our protocol.
We added an exclusion criterion to the section Types of participants: "Trials of patients with acute respiratory failure comprising of a mixed population of patients with and without ALI/ARDS (unless those without ALI/ARDS constitute less than 10% of the population)." Again, we had omitted to clearly state this in our protocol.
In the section Types of outcome measures, we included data on 'Mortality in ICU' along with in‐hospital mortality as a primary outcome, as these data were presumed to also include mortality In hospital. An additional endpoint for assessing mortality on follow‐up at 28 days was included as a standard method to assess mortality across trials. We also added one relevant secondary outcome, 'Length of stay in ICU and in hospital.'
Data synthesis
In our protocol we had stated that data would be synthesized using random‐effects, inverse variance meta‐analysis. For the review we pooled data using the Mantel‐Haenszel method. This was based on guidance provided in Deeks 2011 indicating that estimates of standard errors of effect estimates using inverse variance methods may be poor when event rates are low or when study size is small; Mantel‐Haenszel methods have been shown to have better statistical properties in these situations.
Unit of analysis issues
If we had included trials randomized by clusters, and if results were adjusted for clustering, we would have combined adjusted measures of effects of these cluster‐randomized trials. If results had not been adjusted for clustering, we would have attempted to make these adjustments by multiplying the standard errors of estimates by the square root of the design effect when the design effect was calculated as DEff = 1 + (M ‐ 1) ICC, where M was the average cluster size and ICC was the intracluster coefficient. If this was not possible, we would not have combined data in a meta‐analysis but would have presented the results in an additional table.
If outcomes had been reported both at baseline and at follow‐up or at trial endpoints, we would have extracted both the mean change from baseline and the standard deviation of this mean for each treatment group, as well as the same for endpoint data. We would have used endpoint data preferentially but would have combined endpoint and change scores for outcomes from trials for each comparison if endpoint data were not available.
Had count data been reported in trials, we would have extracted the total number of events in each group and the total amount of person‐time at risk in each group. We also would have recorded the total number of participants in each group. If this information was not available, we would have attempted to extract alternative summary statistics such as risk ratios and confidence intervals, if available. Had count data been presented as dichotomous outcomes, we would have extracted the number of participants in each intervention group and the number of participants in each intervention group who experienced at least one event. Had count data been presented as continuous outcomes or as time‐to‐event outcomes, we would have attempted to extract the same information as outlined for continuous and time‐to‐event outcomes.
Had time‐to‐event outcomes been reported, we would have extracted estimates of the log hazard ratio and its standard error. If standard errors were not available, we would have extracted alternative statistics such as confidence intervals or P values.
Summarizing and interpreting results
Outcomes selected for the 'Summary of findings' tables were expanded to include mortality in ICU and on follow‐up (at 28 days).
Contributions of authors
Binila Chacko (BC) and John Victor Peter (JVP) conceived of the review, wrote the protocol, screened and selected studies, extracted data, assessed risk of bias, contacted study authors for additional data and helped write the review. In addition, BC co‐ordinated the review, entered and analysed data and drafted the 'Summary of findings' table and the final review. JVP also assisted in designing the approach to the review and in interpreting data.
Prathap Tharyan (PT) helped write the protocol, checked the appropriateness of included and excluded studies, checked extracted data and performed additional extraction, checked risk of bias assessments and data entry, finalized the 'Summary of findings' table and helped write the review.
Lakshmanan Jeyaseelan (LJ) helped write the protocol and checked data analysis and interpretation of results.
George John (GJ) provided perspectives on background and methods and helped interpret and write the review.
All review authors approved the final version of this review. PT served as the guarantor of this review.
Sources of support
Internal sources
-
Christian Medical College, Vellore, India.
All authors are salaried employees of CMC Vellore
External sources
-
Indian Council of Medical Research, India.
Funding for the Prof. BV Moses Centre at CMC Vellore during protocol development and for the initial period of review completion
-
Effective Health Care Research Consortium, UK.
Funding for the South Asian Cochrane Centre via a programme grant to the Liverpool School of Tropical Medicine (Paul Garner) from UKaid (Depertment for International Develpment) in aid of developing countries. PT Is a programme partner
Declarations of interest
Binila Chacko: none known.
John V Peter: none known.
Prathap Tharyan is a programme partner with the Effective Health Care Research Consortium (funded by DFID) (www.evidence4health.org/) and is a recipient of a grant under this project. This is one of the portfolios of reviews that were mentored under the terms of this grant. PT is contracted by The Cochrane Collaboration to help build capacity among authors from India and the South‐Asian region to undertake systematic reviews.
George John: none known.
Lakshmanan Jeyaseelan: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Esteban 2000 {published data only}
- Esteban A, Alía I, Gordo F, Pablo R, Suarez J, González G, et al. Prospective randomized trial comparing pressure‐controlled ventilation and volume‐controlled ventilation in ARDS. For the Spanish Lung Failure Collaborative Group. Chest 2000;117(6):1690‐6. [PUBMED: 10858404 ] [DOI] [PubMed] [Google Scholar]
Meade 2008 {published data only}
- Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Lung Open Ventilation Study Investigators. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end‐expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299(6):637‐45. [PUBMED: 18270352] [DOI] [PubMed] [Google Scholar]
Rappaport 1994 {published data only}
- Rappaport SH, Shpiner R, Yoshihara G, Wright J, Chang P, Abraham E. Randomized, prospective trial of pressure‐limited versus volume‐controlled ventilation in severe respiratory failure. Critical Care Medicine 1994;22(1):22‐32. [PUBMED: 8124968] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alvarez 1998 {published data only}
- Alvarez A, Subirana M, Benito S. Decelerating flow ventilation effects in acute respiratory failure. Journal of Critical Care 1998;13(1):21‐5. [DOI] [PubMed] [Google Scholar]
Amato 1995 {published data only}
- Amato MB, Barbas CS, Medeiros DM, Schettino Gde P, Lorenzi FG, Kairalla RA, et al. Beneficial effects of the "open lung approach" with low distending pressures in acute respiratory distress syndrome. A prospective randomized study on mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 1995;152(6 Pt 1):1835‐46. [DOI] [PubMed] [Google Scholar]
Betensley 2008 {published data only}
- Betensley AD, Khalid I, Crawford J, Pensler RA, DiGiovine B. Patient comfort during pressure support and volume controlled‐continuous mandatory ventilation. Respiratory Care 2008;53(7):897‐902. [PubMed] [Google Scholar]
Brower 2004 {published data only}
- Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. Higher versus lower positive end‐expiratory pressures in patients with the acute respiratory distress syndrome. The New England Journal of Medicine 2004;351(4):327‐36. [DOI] [PubMed] [Google Scholar]
Choi 2006 {published data only}
- Choi G, Wolthuis EK, Bresser P, Levi M, Poll T, Dzoljic M, et al. Mechanical ventilation with lower tidal volumes and positive end‐expiratory pressure prevents alveolar coagulation in patients without lung injury. Anesthesiology 2006;105(4):689‐95. [DOI] [PubMed] [Google Scholar]
Davis 1996 {published data only}
- Davis K, Branson RD, Campbell RS, Porembka DT. Comparison of volume control and pressure control ventilation: is flow waveform the difference?. The Journal of Trauma 1996;41(5):808‐14. [DOI] [PubMed] [Google Scholar]
de Durante 2002 {published data only}
- Durante G, Turco M, Rustichini L, Cosimini P, Giunta F, Hudson LD, et al. ARDSNet lower tidal volume ventilatory strategy may generate intrinsic positive end‐expiratory pressure in patients with acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2002;165(9):1271‐4. [DOI] [PubMed] [Google Scholar]
Delgado 2009 {published data only}
- Delgado M, Sanchez RD, Arribas I, Pena MT, Esther LR, AlvarezMM, Cambronero JA. Mortality at 28 day in patients with acute respiratory distress syndrome in two strategies of setting positive end‐expiratory pressure. Intensive Care Medicine 2009;35(0722):Suppl 1. [Google Scholar]
Edibam 2001 {published data only}
- Edibam C, RuttenA, Bersten A. Regional lung inflation increases with pressure controlled inverse ratio (PCIRV) compared to PC and volume controlled (VC) ventilation in acute lung injury (ALI) [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A767. [Google Scholar]
Edibam 2003 {published data only}
- Edibam C, Rutten AJ, Collins DV, Bersten AD. Effect of inspiratory flow pattern and inspiratory to expiratory ratio on nonlinear elastic behavior in patients with acute lung injury. American Journal of Respiratory and Critical Care Medicine 2003;167(5):702‐7. [DOI] [PubMed] [Google Scholar]
Eisner 2001 {published data only}
- Eisner MD, Thompson T, Hudson LD, Luce JM, Hayden D, Schoenfeld D, et al. Efficacy of low tidal volume ventilation in patients with different clinical risk factors for acute lung injury and the acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine 2001;164(2):231‐6. [DOI] [PubMed] [Google Scholar]
Fang 2002 {published data only}
- Fang Z, Niu S, Zhu L, Bai C. Distribution of ventilation and hemodynamic effects of different ventilatory patterns. Chinese Medical Journal 2002;115(2):188‐91. [PubMed] [Google Scholar]
Ferguson 2002 {published data only}
- Ferguson ND, Meade MO, Hallett DC, Stewart TE. High values of the pulmonary artery wedge pressure in patients with acute lung injury and acute respiratory distress syndrome. Intensive Care Medicine 2002;28(8):1073‐7. [DOI] [PubMed] [Google Scholar]
Forel 2006 {published data only}
- Forel JM, Roch A, Marin V, Michelet P, Demory D, Blache JL, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Critical Care Medicine 2006;34(11):2749‐57. [DOI] [PubMed] [Google Scholar]
Gainnier 2003 {published data only}
- Gainnier M, Michelet P, Thirion X, Arnal JM, Sainty JM, Papazian L. Prone position and positive end‐expiratory pressure in acute respiratory distress syndrome. Critical Care Medicine 2003;31(12):2719‐26. [DOI] [PubMed] [Google Scholar]
Gainnier 2004 {published data only}
- Gainnier M, Roch A, Forel JM, Thirion X, Arnal JM, Donati S, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Critical Care Medicine 2004;32(1):113‐9. [DOI] [PubMed] [Google Scholar]
Ge 2004 {published data only}
- Ge Y, Wan Y, Wang D, Su X, Li J, Chen J. [Treatment of acute respiratory distress syndrome using pressure and volume controlled ventilation with lung protective strategy]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue = Chinese Critical Care Medicine = Zhongguo Weizhongbing Jijiuyixue 2004;16(7):424‐7. [PubMed] [Google Scholar]
Grasso 2007 {published data only}
- Grasso S, Stripoli T, Michele M, Bruno F, Moschetta M, Angelelli G, et al. ARDSnet ventilatory protocol and alveolar hyperinflation: role of positive end‐expiratory pressure. American Journal of Respiratory and Critical Care Medicine 2007;176(8):761‐7. [DOI] [PubMed] [Google Scholar]
Huh 2009 {published data only}
- Huh JW, Jung H, Choi HS, Hong SB, Lim CM, Koh Y. Efficacy of positive end‐expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Critical Care (London, England) 2009;13(1):R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kallet 2000 {published data only}
- Kallet RH, Campbell AR, Alonso JA, Morabito DJ, Mackersie RC. The effects of pressure control versus volume control assisted ventilation on patient work of breathing in acute lung injury and acute respiratory distress syndrome. Respiratory Care 2000;45(9):1085‐96. [PubMed] [Google Scholar]
Kallet 2005 {published data only}
- Kallet RH, Campbell AR, Dicker RA, Katz JA, Mackersie RC. Work of breathing during lung‐protective ventilation in patients with acute lung injury and acute respiratory distress syndrome: a comparison between volume and pressure‐regulated breathing modes. Respiratory Care 2005;50(12):1623‐31. [PubMed] [Google Scholar]
Kiehl 1996 {published data only}
- Kiehl M, Schiele C, Stenzinger W, Kienast J. Volume‐controlled versus biphasic positive airway pressure ventilation in leukopenic patients with severe respiratory failure. Critical Care Medicine 1996;24(5):780‐4. [PUBMED: 8706453] [DOI] [PubMed] [Google Scholar]
Knebel 1994 {published data only}
- Knebel AR, Janson BSL, Malley JD, Wilson AG, Marini JJ. Comparison of breathing comfort during weaning with two ventilatory modes. American Journal of Respiratory and Critical Care Medicine 1994;149(1):14‐8. [DOI] [PubMed] [Google Scholar]
Koh 2007 {published data only}
- Koh SOK. Mode of mechanical ventilation: volume controlled mode. Critical Care Clinics 2007;23(2):161‐7. [DOI] [PubMed] [Google Scholar]
Lessard 1994 {published data only}
- Lessard MR, Guérot E, Lorino H, Lemaire F, Brochard L. Effects of pressure‐controlled with different I:E ratios versus volume‐controlled ventilation on respiratory mechanics, gas exchange, and hemodynamics in patients with adult respiratory distress syndrome. Anesthesiology 1994;80(5):983‐91. [PubMed] [Google Scholar]
Mancebo 1994 {published data only}
- Mancebo J, Vallverdú I, Bak E, Domínguez G, Subirana M, Benito S, et al. Volume‐controlled ventilation and pressure‐controlled inverse ratio ventilation: a comparison of their effects in ARDS patients. Monaldi Archives for Chest Disease = Archivio Monaldi Per Le Malattie Del Torace / Fondazione Clinica Del Lavoro, IRCCS [and] Istituto Di Clinica Tisiologica E Malattie Apparato Respiratorio, Università Di Napoli, Secondo Ateneo 1994;49(3):201‐7. [PubMed] [Google Scholar]
Maxwell 2010 {published data only}
- Maxwell RA, Green JM, Waldrop J, Dart BW, Smith PW, Brooks D, et al. A randomized prospective trial of airway pressure release ventilation and low tidal volume ventilation in adult trauma patients with acute respiratory failure. The Journal of Trauma 2010;69(3):501‐10. [DOI] [PubMed] [Google Scholar]
McCallion 2005 {published data only}
- McCallion N, Davis PG, Morley CJ. Volume‐targeted versus pressure‐limited ventilation in the neonate. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003666.pub2] [DOI] [PubMed] [Google Scholar]
Mercat 1993 {published data only}
- Mercat A, Graïni L, Teboul J L, Lenique F, Richard C. Cardiorespiratory effects of pressure‐controlled ventilation with and without inverse ratio in the adult respiratory distress syndrome. Chest 1993;104(3):871‐5. [DOI] [PubMed] [Google Scholar]
Mercat 2008 {published data only}
- Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. Expiratory Pressure (Express) Study Group. Positive end‐expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 2008;299(6):646‐55. [DOI] [PubMed] [Google Scholar]
Parsons 2005 {published data only}
- Parsons PE, Eisner MD, Thompson BT, Matthay MA, Ancukiewicz M, Bernard GR, et al. Lower tidal volume ventilation and plasma cytokine markers of inflammation in patients with acute lung injury. Critical Care Medicine 2005;33(1):1‐6; discussion 230‐2‐1‐6; discussion 230‐2. [DOI] [PubMed] [Google Scholar]
Prella 2002 {published data only}
- Prella M, Feihl F, Domenighetti G. Effects of short‐term pressure‐controlled ventilation on gas exchange, airway pressures, and gas distribution in patients with acute lung injury/ARDS: comparison with volume‐controlled ventilation. Chest 2002;122(4):1382‐8. [DOI] [PubMed] [Google Scholar]
Putensen 2001 {published data only}
- Putensen C, Zech S, Wrigge H, Zinserling J, Stüber F, Spiegel T, et al. Long‐term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. American Journal of Respiratory and Critical Care Medicine 2001;164(1):43‐9. [DOI] [PubMed] [Google Scholar]
Rasenan 1991 {published data only}
- Rasanen J, Cane RD, Downs JB, Hurst JM, Jousela IT, Kirby RR, et al. Airway pressure release ventilation during acute lung injury—a prospective multicenter trial. Critical Care Medicine 1991;19:1234‐41. [DOI] [PubMed] [Google Scholar]
Riverso 1998 {published data only}
- Riverso P, Bernardi PL, Corsa D, Morra MG, Paganini G, Parigi F. [A comparison of ventilation techniques in ARDS. Volume controlled vs pressure regulated volume control]. Minerva Anestesiologica 1998;64(7‐8):339‐43. [PubMed] [Google Scholar]
Sarmiento 1998 {published data only}
- Sarmiento X, Soler M, Velasco P, Xirgu J, Esquirol X, Tomasa MT, et al. Comparative study between volume controlled ventilation and pressure controlled ventilation in the acute respiratory distress syndrome. Medicina Intensiva 1998;22(9):404‐11. [Google Scholar]
Stewart 1998 {published data only}
- Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky SE, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome. Pressure‐ and Volume‐Limited Ventilation Strategy Group. The New England Journal of Medicine 1998;338(6):355‐61. [DOI] [PubMed] [Google Scholar]
Talmor 2008 {published data only}
- Talmor D, Sarge T, Malhotra A, O'Donnell CR, Ritz R, Lisbon A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. The New England Journal of Medicine 2008;359(20):2095‐104. [PUBMED: 19001507] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tuğrul 1997 {published data only}
- Tuğrul M, Camci E, Karadeniz H, Sentürk M, Pembeci K, Akpir K. Comparison of volume controlled with pressure controlled ventilation during one‐lung anaesthesia. British Journal of Anaesthesia 1997;79(3):306‐10. [DOI] [PubMed] [Google Scholar]
Varpula 2003 {published data only}
- Varpula T, Jousela I, Niemi R, Takkunen O, Pettilä V. Combined effects of prone positioning and airway pressure release ventilation on gas exchange in patients with acute lung injury. Acta Anaesthesiologica Scandinavica 2003;47(5):516‐24. [DOI] [PubMed] [Google Scholar]
Villar 2006 {published data only}
- Villar J, Kacmarek RM, Pérez‐Méndez L, Aguirre‐Jaime A. A high positive end‐expiratory pressure, low tidal volume ventilatory strategy improves outcome in persistent acute respiratory distress syndrome: a randomized, controlled trial. Critical Care Medicine 2006;34(5):1311‐8. [DOI] [PubMed] [Google Scholar]
Vines 2001 {published data only}
- Vines DL, Peters JI. Pressure control ventilation in acute lung injury. Clinical Pulmonary Medicine 2001;8(4):231‐7. [Google Scholar]
Wang 2002 {published data only}
- Wang SH, Wei TS. The outcome of early pressure‐controlled inverse ratio ventilation on patients with severe acute respiratory distress syndrome in surgical intensive care unit. American Journal of Surgery 2002;183(2):151‐5. [DOI] [PubMed] [Google Scholar]
Yang 2007 {published data only}
- Yang LY, Huang YCT, Macintyre NR. Patient‐ventilator synchrony during pressure‐targeted versus flow‐targeted small tidal volume assisted ventilation. Journal of Critical Care 2007;22(3):252‐7. [DOI] [PubMed] [Google Scholar]
Zupancich 2005 {published data only}
- Zupancich E, Paparella D, Turani F, Munch C, Rossi A, Massaccesi S, et al. Mechanical ventilation affects inflammatory mediators in patients undergoing cardiopulmonary bypass for cardiac surgery: a randomized clinical trial. The Journal of Thoracic and Cardiovascular Surgery 2005;130(2):378‐83. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Keddissi 2000 {published data only}
- Keddissi J, Eid A, Kinasewitz G. Effect of pressure control vs volume control ventilation with constant I:E ratio on gas exchange in ARDS [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A386. [Google Scholar]
Additional references
Agarwal 2006
- Agarwal R, Aggarwal AN, Gupta D, Behera D, Jindal SK. Etiology and outcomes of pulmonary and extrapulmonary acute lung injury/ARDS in a respiratory ICU in North India. Chest 2006;130(3):724‐9. [DOI] [PubMed] [Google Scholar]
Al‐Sady 1985
- Al‐Sady N, Bennett ED. Decelerating inspiratory flow waveform improves lung mechanics and gas exchange in patients on intermittent positive‐pressure ventilation. Intensive Care Medicine 1985;11:68‐75. [DOI] [PubMed] [Google Scholar]
ARDSNet 2000
- Acute Respiratory Distress Syndrome Network (ARDSNet). Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine 2000;342:1301‐8. [PUBMED: 10793162] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Bernard 1994
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, at al. Report of the American‐European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. Intensive Care Medicine 1994;20(3):225‐32. [8014293] [DOI] [PubMed] [Google Scholar]
Brun‐Bruisson 2004
- Brun‐Buisson C, Minelli C, Bertolini G, Brazzi L, Pimentel J, Lewandowski K, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Medicine 2004;30:51‐61. [PUBMED: 14569423] [DOI] [PubMed] [Google Scholar]
Cadi 2008
- Cadi P, Guenoun T, Journois D, ChevallierJ.‐M, Diehl J‐L, Safran D. Pressure‐controlled ventilation improves oxygenation during laparoscopic obesity surgery compared with volume‐controlled ventilation. British Journal of Anaesthesia 2008;100(5):709‐16. [DOI] [PubMed] [Google Scholar]
Caser 2014
- Caser EB, Zandonade E, Pereira E, Gama AM, Barbas CS. Impact of distinct definitions of acute lung injury on its incidence and outcomes in Brazilian ICUs: prospective evaluation of 7,133 patients. Critical Care Medicine 2014;42(3):574‐82. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care:Meta‐analysis in Context. London (UK): BMJ Publication Group, 2001. [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org. [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [PUBMED: 3616287] [DOI] [PubMed] [Google Scholar]
DerSimionian 1986
- DerSimionian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [PUBMED: 3802833] [DOI] [PubMed] [Google Scholar]
Gates 2013
- Gates S, Perkins GD, Lamb SE, Kelly C, Thickett DR, Young JD, et al. Beta‐Agonist Lung injury TrIal‐2 (BALTI‐2): a multicentre, randomised, double‐blind, placebo‐controlled trial and economic evaluation of intravenous infusion of salbutamol versus placebo in patients with acute respiratory distress syndrome. Health Technology Assessment 2013;17(38):v‐vi, 1‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gattinoni 2008
- Gattinoni L, Caironi P. Refining ventilatory treatment for acute lung injury and acute respiratory distress syndrome. JAMA 2008;299:691‐3. [PUBMED: 18270359] [DOI] [PubMed] [Google Scholar]
George 2014
- George T, Viswanathan S, Karnam AH, Abraham G. Etiology and outcomes of ARDS in a rural‐urban fringe hospital of South India. Critical Care Research and Practice 2014;2014:181593. [PUBMED: 24660060] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2004 [Computer program]
- GRADE Working Group. GRADE Profiler; version 3.2. GRADE Working Group, 2004.
Halter 2007
- Halter JM, Steinberg JM, Gatto LA, DiRocco JD, Pavone LA, Schiller HJ, et al. Effect of positive end‐expiratory pressure and tidal volume on lung injury induced by alveolar instability. Critical Care Medicine 2007;11:R20. [PUBMED: 17302983] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hernu 2013
- Hernu R, Wallet F, Thiollière F, Martin O, Richard JC, Schmitt Z, et al. An attempt to validate the modification of the American‐European consensus definition of acute lung injury/acute respiratory distress syndrome by the Berlin definition in a university hospital. Intensive Care Medicine 2013;39(12):2161‐70. [DOI: 10.1007/s00134-013-3122-6] [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org. [Google Scholar]
Krafft 1996
- Krafft P, Fridrich P, Pernerstorfer T, Fitzgerald RD, Koc D, Schneider B, et al. The acute respiratory distress syndrome: definitions, severity and clinical investigations. Intensive Care Medicine 1996;22:519‐29. [PUBMED: 8814466] [DOI] [PubMed] [Google Scholar]
MacIntyre 2011
- MacIntyre N. Counterpoint: is pressure assist‐control preferred over volume assist‐control mode for lung protective ventilation in patients with ARDS? No. Chest 2011;140(2):290‐2. [DOI] [PubMed] [Google Scholar]
Marini 2011
- Marini JJ. Point: is pressure assist‐control preferred over volume assist‐control mode for lung protective ventilation in patients with ARDS? Yes. Chest 2011;140(2):286‐90. [DOI] [PubMed] [Google Scholar]
Matthay 2011
- Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, et al. Randomized, placebo‐controlled clinical trial of an aerosolized β₂‐agonist for treatment of acute lung injury. American Journal of Respiratory and Critical Care Medicine 2011;184(5):561‐8. [PUBMED: 21562125] [DOI] [PMC free article] [PubMed] [Google Scholar]
MECIR 2011
- Chandler J, Churchill R, Higgins J, Lasserson T, Tovey D. Methodological Expectations of Cochrane Intervention Reviews (MECIR). Methodological standards for the conduct of new Cochrane Intervention Reviews. Version 2.1, 8 December 2011. http://www.editorial‐unit.cochrane.org/sites/editorial‐unit.cochrane.org/files/uploads/MECIR_conduct_standards%202.1.pdf. (accessed 4 November 2012).
Ranieri 1999
- Ranieri VM, Suter PM, Tortorella C, Tullio R, Dayer JM, Brienza A, et al. Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 1999;282(1):54‐61. [DOI] [PubMed] [Google Scholar]
Ranieri 2012
- Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307(23):2526‐33. [PUBMED: 22797452] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rubenfield 2005
- Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. New England Journal of Medicine 2005;353:1685‐93. [PUBMED: 16236739] [DOI] [PubMed] [Google Scholar]
Rücker 2008
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta‐analyses with binary outcomes. Statistics in Medicine 2008;27:746‐63. [PUBMED: 17592831] [DOI] [PubMed] [Google Scholar]
Schunemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, Guyatt GH. Interpreting results drawing conclusions. Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. www.cochrane‐handbook.org. The Cochrane Collaboration, 2008. [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. www.cochrane‐handbook.org. The Cochrane Collaboration, 2008. [Google Scholar]
Slutsky 1993
- Slutsky AS. Mechanical ventilation: American College of Chest Physicians’ Consensus Conference. Chest 1993;104:1833‐59. [8252973 ] [DOI] [PubMed] [Google Scholar]
Slutsky 2013
- Slutsky AS, Ranieri VM. Ventilator‐induced lung injury. New England Journal of Medicine 2013;369(22):2126‐36. [DOI] [PubMed] [Google Scholar]
Tharrat 1998
- Tharrat RS, Allen RP, AlbertsonTE. Pressure controlled inverse ratio ventilation in severe adult respiratory failure. Chest 1998;94:755‐62. [PUBMED: 3168572] [DOI] [PubMed] [Google Scholar]
Wheeler 2010
- Wheeler K, Klingenberg C, McCallion N, Morley CJ, Davis PG. Volume‐targeted versus pressure‐limited ventilation in the neonate. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD003666.pub3] [DOI] [PubMed] [Google Scholar]
Zimmerman 2009
- Zimmerman JJ, Akhtar SR, Caldwell E, Rubenfeld GD. Incidence and outcomes of pediatric acute lung injury. Pediatrics 2009;124(1):87‐95. [PUBMED: 19564287] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chacko 2010
- Chacko B, Peter JV, Tharyan P, John G, Jeyaseelan L. Pressure‐controlled versus volume‐controlled ventilation for acute respiratory failure due to acute lung injury (ALI) or acute respiratory distress syndrome (ARDS). Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD008807] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chacko 2015
- Chacko B, Peter JV, Tharyan P, John G, Jeyaseelan L. Pressure‐controlled versus volume‐controlled ventilation for acute respiratory failure due to acute lung injury (ALI) or acute respiratory distress syndrome (ARDS). Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD008807.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


