Abstract
Background
This is an updated version of the review which was first published in the Cochrane Database of Systematic Reviews in 2006. Long‐term central venous catheters (CVCs), including tunnelled CVCs (TCVCs) and totally implanted devices or ports (TIDs), are increasingly used when treating oncology patients. Despite international guidelines on sterile insertion and appropriate CVC maintenance and use, infection remains a common complication. These infections are mainly caused by Gram positive bacteria. Antimicrobial prevention strategies aimed at these micro‐organisms could potentially decrease the majority of CVC infections. The aim of this review was to evaluate the efficacy of antibiotics in the prevention of Gram positive infections in long‐term CVCs.
Objectives
To determine the efficacy of administering antibiotics prior to the insertion of long‐term CVCs, or flushing or locking long‐term CVCs with a combined antibiotic and heparin solution, or both, to prevent Gram positive catheter‐related infections in adults and children receiving treatment for cancer.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (to June 2013) and the MEDLINE and EMBASE databases (1966 to 2013).
Selection criteria
Randomised controlled trials (RCTs) comparing prophylactic antibiotics given prior to long‐term CVC insertion with no antibiotics, RCTs comparing a combined antibiotic and heparin solution with a heparin‐only solution to flush or lock newly inserted long‐term CVCs, and RCTs comparing a combination of these interventions in adults and children receiving treatment for cancer.
Data collection and analysis
Two authors independently selected studies, classified them and extracted data on to a pre‐designed data collection form. We pooled data using the RevMan software version 5.2 and used random‐effects (RE) model methods for meta‐analyses.
Main results
We included 11 trials with a total of 828 oncology patients (adults and children). We assessed most included studies to be at a low or unclear risk of bias. Five trials compared the use of antibiotics (vancomycin, teicoplanin or ceftazidime) given before the insertion of the long‐term CVC with no antibiotics, and six trials compared antibiotics (vancomycin, amikacin or taurolidine) and heparin with a heparin‐only solution for flushing or locking the long‐term CVC after use. Administering an antibiotic prior to insertion of the CVC did not significantly reduce Gram positive catheter‐related sepsis (CRS) (five trials, 360 adults; risk ratio (RR) 0.72, 95% confidence interval (CI) 0.33 to 1.58; I² = 5 2%; P = 0.41).
Flushing and locking long‐term CVCs with a combined antibiotic and heparin solution significantly reduced the risk of Gram positive catheter‐related sepsis compared with a heparin‐only solution (468 participants, mostly children; RR 0.47, 95% CI 0.28 to 0.80; I² = 0%; P = 0.005). For a baseline infection rate of 15%, this reduction translated into a number needed to treat (NNT) of 12 (95% CI 9 to 33) to prevent one catheter‐related infection. We considered this evidence to be of a moderate quality.
Authors' conclusions
There was no benefit to administering antibiotics before the insertion of long‐term CVCs to prevent Gram positive catheter‐related infections. Flushing or locking long‐term CVCs with a combined antibiotic and heparin solution appeared to reduce Gram positive catheter‐related sepsis experienced in people at risk of neutropenia through chemotherapy or disease. Due to insufficient data it was not clear whether this applied equally to TCVCs and totally implanted devices (TIDs), or equally to adults and children. The use of a combined antibiotic and heparin solution may increase microbial antibiotic resistance, therefore it should be reserved for high risk people or where baseline CVC infection rates are high (> 15%). Further research is needed to identify high risk groups most likely to benefit.
Keywords: Adult; Child; Humans; Antibiotic Prophylaxis; Anti‐Bacterial Agents; Anti‐Bacterial Agents/administration & dosage; Anticoagulants; Anticoagulants/administration & dosage; Catheter‐Related Infections; Catheter‐Related Infections/prevention & control; Catheterization, Central Venous; Catheterization, Central Venous/adverse effects; Gram‐Positive Bacterial Infections; Gram‐Positive Bacterial Infections/prevention & control; Heparin; Heparin/administration & dosage; Neoplasms; Neoplasms/therapy; Randomized Controlled Trials as Topic
Antibiotics for preventing early central venous catheter Gram positive infections in people with cancer
What is the problem?
People with cancer who undergo anti‐cancer treatment (chemotherapy) often have a tube inserted into a large vein (central venous catheter or CVC) through which their chemotherapy is given. As chemotherapy is usually administered at regular intervals over several months to years, long‐term, semi‐permanent, tunnelled CVCs (TCVCs) or totally implanted devices (TIDs) are frequently used. Despite sterile insertion and post‐insertion care, these long‐term CVCs may become infected. These infections are usually caused by Gram positive bacteria.
Flushing or locking means to instil a solution to dwell in the tube when it is not in use. Usually, after use, the tube is flushed or locked with a saline or heparin‐saline solution to prevent clot formation within the tube.
What was the aim of this review?
The aim of this review was to determine whether giving antibiotics before inserting the tube, or giving antibiotics with the solution used to flush and lock the tube, can prevent Gram positive bacterial infections.
What are the findings?
We searched the literature from 1966 to 2013 for relevant studies (randomised controlled trials only).
We included five studies (involving 360 children and adults) that compared antibiotics given before the insertion of the CVC with no antibiotics before insertion. We found that giving an antibiotic before inserting a tunnelled CVC did not prevent Gram positive catheter‐related infections.
We included six studies (involving 468 people, mainly children) that tested flushing or locking the newly inserted CVC with a combination of an antibiotic and heparin compared with heparin only. We found that flushing the catheter with a solution containing an antibiotic and heparin reduced the number of catheter‐related infections. This practice is most likely to be of value where the risk of such infections is high. We considered this evidence to be of a moderate quality.
Summary of findings
Summary of findings for the main comparison.
Summary of findings: prophylactic antibiotics before long‐term CVC insertion
| Antibiotics compared with no antibiotics prior to long‐term CVC insertion to prevent catheter‐related infections | ||||||
|
Patient or population: adults with a newly inserted long‐term CVC who were at risk of neutropenia due to chemotherapy or disease Settings: inpatient and outpatient Intervention: intravenous antibiotics (vancomycin, teicoplanin or ceftazidime) Comparison: placebo or no antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antibiotics | |||||
| Catheter‐related sepsis | 200 per 1000 | 144 per 1000 (66 to 316) | RR 0.72 (0.33 to 1.58) | 360 (5) | ⊕⊕⊕⊝ moderate | The difference between the comparison groups was not significant (P = 0.41). We downgraded this evidence to moderate due to the substantial heterogeneity (I² = 52%) between studies. |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 2.
Summary of findings: antibiotic and heparin versus heparin only flush or lock solution
| Antibiotic and heparin solution compared with a heparin only solution for flushing or locking long‐term CVCs to prevent Gram positive catheter‐related sepsis | ||||||
|
Patient or population: adults and children with a newly inserted long‐term CVC who were at risk of neutropenia due to chemotherapy or disease Settings: inpatient and outpatient Intervention: antibiotic (vancomycin, vancomycin and amikacon, or taurolidine) plus heparin solution Comparison: heparin only solution | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Heparin‐only | Antibiotic/heparin | |||||
| Catheter‐related sepsis | 200 per 1000 | 94 per 1000 (56 to 160) | RR 0.47 (0.28 to 0.80) | 468 (6) | ⊕⊕⊕⊝ moderate | Data consistent across included studies; I² = 0%; P = 0.005. For an assumed risk of 15%, the NNT = 12 (9 to 33). We downgraded this evidence to moderate as the sample was clinically heterogeneous. |
| *The basis for the assumed risk is the mean control group risk across included studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; NNT: number needed to treat | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
Description of the condition
This is an updated version of the review which was first published in the Cochrane Database of Systematic Reviews in 2006.
People undergoing treatment for cancer need adequate venous access because of the frequent administration of chemotherapy and requirements for intravenous fluids, blood products and other medications. To limit the discomfort of short‐term venous access, long‐term central venous catheters (CVCs), including tunnelled central venous catheters (TCVCs) and totally implanted devices or ports (TIDs), are used in more than two thirds of children and adults undergoing chemotherapy (Groeger 1993; Ingram 1991; Simon 2006). However, the use of long‐term CVCs is limited by the risk of blood clot formation and infection. The risk of infection ranges from 1.4 (Bagnall‐Reeb 2004; Press 1984; Schinabeck 2003) to 2.2 (Groeger 1993; Sarper 2006) infections per 1000 catheter days. The duration of antimicrobial therapy to treat these infections ranges from seven to 21 days. Success rates of 60% to 91% are reported, although often the device has to be removed (Bagnall‐Reeb 2004). Approximately one third of people experience an episode of infection while having a long‐term CVC in place. Seventy per cent of the organisms that are cultured are Gram positive organisms, mainly coagulase negative staphylococci (Staphylococcus aureus and enterococci). Other organisms include Gram negative organisms (15%) (mainly E coli), fungal organisms (8%) (mainly Candida species) and anaerobic organisms (7%) (O'Grady 2002).
The adherence to and colonization of CVCs with micro‐organisms is facilitated by the formation of a very thin biofilm inside the catheter lumen. This process is influenced by several factors such as the production of fibroglycocalyx (extracellular slime) by coagulase negative staphylococci. In addition, the host reaction to the CVC results in the formation of a thrombin sleeve rich in clotting factors such as fibronectin, fibrinogen and fibrin, which contributes to the formation of the biofilm (Bagnall‐Reeb 2004; Darouiche 1999). This means that adequate antibiotic treatment may lead to resolution of the CVC infection only in certain cases (that is when caused by coagulase negative staphylococci) whereas in other cases (that is when caused by Pseudomonas, Staphylococcus aureus or fungi) this will be much more difficult to clear and therefore removal of the catheter is necessary (Simon 2006).
The organisms responsible for catheter colonization and infection come from four sources. These are the skin, the catheter hub (the part through which the catheter is tunnelled under the skin), haematogenous seeding (infections originating outside the catheter can reach the CVC via the bloodstream) and contamination of the intravenous fluids given to the patient (for example intravenous total parenteral nutrition) (Hachem 2002).
Early catheter‐related infections (infections that develop within 45 days after placement of the catheter) are mostly due to organisms from the skin insertion site. This is the time period during which many manipulations of the CVC are necessary due to the intensity of the chemotherapy. After 45 days the catheter hub becomes a far more important source of infection (Abbas 2004; Shaul 1998). International guidelines have been developed to prevent catheter‐related infections (CPAC 1990; O'Grady 2002). These include guidelines for catheter insertion and care and handling, as well as restrictions on the number of catheter interruptions (the number of times per day one is allowed to open the catheter, to give medication or to draw blood). Most recently, a clinical care management bundle (including hand hygiene, barrier precautions for insertion, chlorhexidine skin antisepsis, optimal catheter site selection and assessment of CVC necessity) sets the standard for CVC care (Schiffer 2013).
Description of the intervention
Standard maintenance of long‐term CVCs includes flushing the lumen with a saline solution following access or closing the CVC with a locking solution which is instilled into the lumen of the CVC after chemotherapy and left to dwell in the CVC until the next use. There are conflicting data about the relative value of adding prophylactic heparin to saline flushes (Schiffer 2013); however, heparinised saline is commonly used. Adding an antibiotic to the flush solution may prevent biofilm formation and eliminate bacteria introduced into the CVC via the skin or during CVC access, from any source. Antibiotics which have activity against Gram positive organisms and which have been evaluated for this purpose include vancomycin, taurolidine, teicoplanin and minocycline. Systemic antibiotics may be given intravenously before the insertion of the CVC in an attempt to reduce early infections; however, in the original version of this review we found no evidence to support the use of antibiotics in this way.
How the intervention might work
Oncology patients are at increased risk of infection due to the immunosuppressive effects of chemotherapy or their disease, for example with haematological malignancy. Administering antibiotics prophylactically may reduce the likelihood that Gram positive bacteria, introduced at the time of CVC insertion or following access, will thrive and lead to a catheter‐related infection.
Why it is important to do this review
This is an updated version of the original review in which we found weak evidence to support adding an antibiotic with activity against Gram positive organisms to the standard flush or lock solution, and no evidence to support the use of systemic antibiotics prior to long‐term CVC insertion. There remains uncertainty as to whether antibiotic prophylaxis is of benefit to adults and children at high risk of catheter‐related infections. By updating this review and incorporating new evidence we hoped to clarify the role of prophylactic antibiotics to prevent Gram positive infections in long‐term CVCs.
Objectives
To determine the efficacy of administering antibiotics prior to the insertion of long‐term CVCs, or flushing or locking long‐term CVCs with a combined antibiotic and heparin solution, or both, to prevent Gram positive catheter‐related infections in adults and children receiving treatment for cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trial (RCTs) comparing antibiotics with placebo prior to insertion of the long‐term CVC to reduce Gram positive infections related to the CVC.
RCTs comparing an antibiotic flush or lock solution with a standard solution to reduce Gram positive infections related to the CVC.
RCTs combining the first two comparisons.
Types of participants
Adults and children with newly inserted long‐term CVCs (TCVCs or TIDs) to facilitate chemotherapy.
Types of interventions
Intravenous antibiotics for Gram positive organisms, e.g. vancomycin, taurolidine, teicoplanin and minocycline, administered before long‐term CVC insertion.
An antibiotic solution administered as a catheter flush or lock solution after catheter insertion and use.
Types of outcome measures
Catheter‐related sepsis (CRS) or proxy outcomes, to include the following.
Catheter‐related blood stream infections (CRBSI), defined as an isolation of the same organism from a percutaneous blood culture and from one of the following: an exudate at the catheter site, a semi‐quantitative catheter segment culture following catheter removal, or quantitative blood culture with recovery of at least a five‐fold higher colony count from blood obtained through the catheter than from a percutaneous blood culture (Mermel 2001; O'Grady 2002).
Exit‐site infections, defined as evidence of cellulitis around the exit site.
Tunnel infections, defined as spreading cellulitis overlying the tunnel tract of subcutaneously tunnelled catheters.
A catheter‐related infection diagnosed following a temporal succession of catheter flushing by the onset of chills and fever and a positive blood culture (bloodstream infection (BSI)).
If studies reported CRBSI and proxy outcomes, we preferentially used the CRBSI data in our meta‐analyses.
Search methods for identification of studies
Electronic searches
For the original review, the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (to July 2006), MEDLINE and EMBASE (from 1966 to September 2006) were searched. For this updated review, these databases were searched from September 2006 to June 2013. The search strategies are outline in Appendix 1; Appendix 2; Appendix 3.
Searching other resources
We handsearched the following conference proceedings: International Society for Paediatric Oncology (SIOP) (1995 to 2005), Multinational Association of Supportive Care in Cancer (MASCC) (1995 to 2005), American Society of Clinical Oncology (ASCO) (1995 to 2005), Interscience Conference of Antimicrobial agents and Chemotherapy (ICAAC) (1995 to 2005). No extra information was obtained from the conference proceedings. For this updated review, we did not handsearch conference proceedings however we handsearched reference lists of included studies and other related publications.
Data collection and analysis
Selection of studies
Two authors independently identified and classified the eligible studies. For the original review this was performed by Marianne van der Wetering (MvdW) and Job van Woensel (JvW) and for the updated review by Theresa Lawrie (TAL) and MvdW.
Data extraction and management
We extracted data on to a pre‐designed data extraction and collection form. In addition, we recorded the following information for each study, where possible:
study location, accrual dates;
participant inclusion and exclusion criteria;
type of long‐term CVCs used, site, technique and timing of insertion;
type of intervention(s), dose and timing of administration;
methods of randomisation and allocation concealment;
baseline characteristics of participants including age, type of cancer and previous chemotherapy;
types of outcomes.
Assessment of risk of bias in included studies
For the updated review, we retained the original methods for assessing risk of bias. We assessed the methodological quality (quality of randomisation, blinding and analysis) according to the van Tulder 1997 criteria and assessed allocation concealment according to the Cochrane Handbook for Systematic Reviews of Interventions (2006 version) as follows. (A) Adequate: some form of centralised or pharmacy controlled randomisation scheme, or the use of pre‐coded identical containers administered sequentially to participants or the use of sequentially numbered sealed opaque envelopes, alternatively using an on‐site computer with a locked file which could only be accessed after entering participant details, or a mixture of these approaches and including innovative schemes provided that the method appears impervious to allocation bias. (B) Uncertain: when only terms such as lists, tables, sealed envelopes or randomly assigned were mentioned in the text, or any trial where intervention or placebo assignments were mentioned without specifying the method of allocation. (C) Inadequate: when quasi‐randomisation methods were used, e.g. alternation, date of birth, case record, day of the week, enrolment order or when an open system of random numbers or unblinded assignment was used.
We contacted the authors for additional information, where necessary, and resolved disagreements between review authors by discussion.
Measures of treatment effect
All review outcomes required dichotomous data, for which we presented the results as summary risk ratios (RR) with 95% confidence intervals (CIs) (RevMan 2012).
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We considered heterogeneity to be substantial if the I² was equal to or greater than 50%.
Data synthesis
We grouped studies according to the interventions evaluated and analysed these groups separately, as follows:
studies of intravenous antibiotic prophylaxis prior to insertion of the long‐term CVC versus placebo or no antibiotics; and
studies of antibiotic flush or lock solutions versus standard (heparin only) flush or lock solutions following long‐term CVC insertion.
We pooled data in the meta‐analyses using RevMan 5.2 (RevMan 2012). Where the results for catheter‐related sepsis were separated into Gram positive and Gram negative infections, we included the Gram positive data only. We used the random‐effects model for all meta‐analyses due to substantial heterogeneity between studies with regard to design, interventions and populations.
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity we investigated it using subgroup analyses and sensitivity analyses, where possible. Potential reasons for heterogeneity included types of participants (adults versus children), types of antibiotics (vancomycin versus others) and types of CVCs.
Sensitivity analysis
We performed sensitivity analyses where there was a high risk of bias associated with the quality of one of the included studies (Ljungman 1997). Where six or more trials contributed to a meta‐analysis, we visually assessed the risk of publication bias using funnel plots.
Results
Description of studies
Results of the search
For the original review, we identified the abstracts of 40 potentially relevant studies and on screening excluded 20 of these. Of the remaining 20 studies, we classified 11 as excluded and nine as included. Following the 2013 search, we identified an additional 16 records for classification (see Figure 1). Of these, we included two studies (four citations) and excluded 11 studies (12 citations). Thus, for this updated review there were 11 included studies and 22 excluded studies in total.
Figure 1.
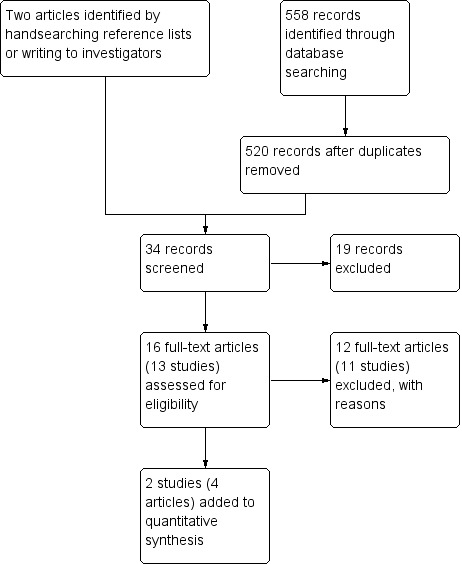
Study flow diagram of updated (June 2013) search.
Included studies
The 11 studies enrolled 828 participants. Five studies were conducted in adults (N = 360) (Di Carlo 2011; Lim 1993; Ljungman 1997; Ranson 1990; Vassilomaniakis 1995), four studies in children (N = 321) (Handrup 2013; Henrickson 2000; Rackoff 1995; Schwartz 1990) and two studies enrolled both (N = 147) (Barriga 1997; Daghistani 1996). Eight trials included participants with solid tumours or haematological malignancies, two trials included participants with haematological malignancies only (Lim 1993; Ljungman 1997) and one trial included participants with solid tumours only (Di Carlo 2011). We only included studies of newly inserted catheters except for one study (Henrickson 2000) which also enrolled an unspecified number of children with TCVCs already in situ. Most studies evaluated infections in TCVCs, however two studies (Di Carlo 2011; Handrup 2013) used totally implantable devices (TIDs). The latter study used both TCVCs and TIDs.
Five studies evaluated the administration of antibiotics prior to CVC insertion. The antibiotics used in these studies were as follows:
vancomycin (Ranson 1990; Vassilomaniakis 1995);
teicoplanin (Lim 1993; Ljungman 1997);
ceftazidime (Di Carlo 2011).
Six studies evaluated flushing or locking the TCVC with a combination of an antibiotic and heparin. Antibiotics used in these studies were as follows:
vancomycin (Barriga 1997; Henrickson 2000; Rackoff 1995; Schwartz 1990);
vancomycin and amikacin (Daghistani 1996);
taurolidine (antimicrobial) (Handrup 2013).
Most studies evaluated and reported catheter‐related infections over the lifespan of the CVC. Three studies (Di Carlo 2011; Ljungman 1997; Ranson 1990) reported early catheter‐related infections, occurring within 21 to 30 days of insertion. Most studies reported CRBSIs (Handrup 2013; Henrickson 2000; Lim 1993; Ljungman 1997; Schwartz 1990) or BSIs (Barriga 1997; Daghistani 1996; Rackoff 1995); one study reported surgical site infections (Di Carlo 2011) and two studies did not have clearly defined outcome measures (Ranson 1990; Vassilomaniakis 1995).
Excluded studies
We excluded 22 studies (11 for the original review and 11 for the updated review) for the following reasons:
participants were ill neonates and not people with cancer (two studies: Garland 2005; Ocete 1998);
non‐tunnelled CVCs were used (six studies: Carratala 1999; Chatzinikolaou 2003b; Hanna 2004; Jaeger 2005; Raad 1998; Schierholz 2010);
studies were not RCTs (six studies: Al Sibai 1987; Chatzinikolaou 2003a; Dawson 2000; Fourcade 2001; Rubie 1995; Scaife 2010; Simon 2008);
RCT did not evaluate newly inserted catheters (three studies: Akyuz 2012; Dumichen 2012; Ferreira Chacon 2011);
RCT did not evaluate prophylactic antibiotics (four studies: Chambers 2005; Hitz 2012; Abdelkefi 2005; Raad 2005).
Risk of bias in included studies
The methodology of the included studies was mostly of a reasonable quality, however sample sizes were relatively small and ranged from 27 (Vassilomaniakis 1995) to 108 participants (Di Carlo 2011). All studies described the eligibility criteria sufficiently and included adults or children, or both, who were at risk of neutropenia due to their disease or chemotherapy. Most studies excluded participants already receiving antibiotics except those used orally for selective gut decontamination (that is the use of oral antibiotics before a neutropenic episode is expected in which the potentially pathogenic aerobic organisms are eliminated without affecting the non‐pathogenic anaerobic organisms). All studies evaluated participants with newly inserted CVCs, however Henrickson 2000 also included an unspecified number of participants with CVCs already in situ.
Randomisation was adequately described in six studies with adequate concealment of treatment allocation (A) (Figure 2). Two studies used a quasi‐randomisation method (B) (Lim 1993; Vassilomaniakis 1995), two did not specify the method of randomisation (B) (Di Carlo 2011; Ljungman 1997) and one study did not specify how allocation concealment was achieved (B) (Handrup 2013). In all studies the experimental and control interventions were explicitly described. In five trials the participants were not blinded to the treatment (Di Carlo 2011; Handrup 2013; Lim 1993; Ljungman 1997; Vassilomaniakis 1995) and in five trials the outcome assessor was not blinded to the intervention (Di Carlo 2011; Lim 1993; Ljungman 1997; Ranson 1990; Vassilomaniakis 1995).
Figure 2.
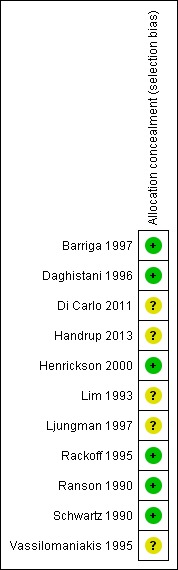
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
In Vassilomaniakis 1995, randomisation was initially performed but later all participants were included in the experimental group; therefore we only used the first part of the study in our analyses. In Ljungman 1997, open randomisation was performed and the study was stopped after an interim analysis. We considered the latter study to be at high risk of bias and performed sensitivity analyses with and without these data.
There was no evidence of publication bias (see Figure 3). Further details regarding the risk of bias and assessment of methodological quality can be found in Table 5, Table 6 and Table 7.
Table 1.
Criteria for the assessment of methodological quality of included studies
| Item ID | Description | Implementation |
| Patient selection | Note: all criteria were scored yes (+), no (‐) or don't know | |
| a | Were the eligibility criteria specified? | Patient inclusion/exclusion criteria must have been described appropriately according to the reviewer |
| b1 | Was a method of randomisation applied? | A random (unpredictable) allocation must have been applied |
| b2 | Was the treatment allocation concealed? | Allocation should have been performed by an independent person not responsible for determining eligibility for inclusion. |
| c | Were the groups similar at baseline with regard to the most important prognostic indicators? | Groups must be similar at baseline with regard to at least three of the four prognostic indicators of age sex duration of symptoms and value of main outcome measures |
| Intervention | ||
| d1 | Was the experimental intervention explicitly described? | Adequate description of the experimental intervention so that treatment can be replicated |
| d2 | Was the control intervention explicitly described? | Adequate description of the control intervention so that treatment can be replicated |
| e | Were co‐interventions avoided or similar for all groups? | Co‐interventions should either have been avoided in the trial design or be similar in the 2 groups |
| f | Was the patient blinded for the intervention? | Adequate information about blinding must have been provided |
| Outcome measurement | ||
| g | Was the outcome assessor blinded to the intervention? | Adequate information about blinding must have been provided |
| h | Were the outcome measures relevant? | At least one of the following outcome measures must be included: catheter‐related sepsis, exit infections, tunnel infections and time to first infection |
| i | Were complications described? | Any adverse events should be noted |
| j | Was the dropout loss to follow up described and acceptable? | Included people who did not complete the follow up period or were not included in the analysis should be described, if the percentage of dropouts is less than 20% then a '+' is scored |
| k | Was a follow‐up measurement performed? | Outcome assessment after randomization |
| l | Was the timing of the outcome similar for all groups? | Timing of outcome assessment should have started from the moment of treatment allocation and be identical for all intervention groups and all important outcome measures |
| S tatistics | ||
| m | Was the sample size described for each group? | Sample size should have been presented for each group at randomisation and for the most important outcome measures |
| n | Did the analysis include an intention‐to‐treat analysis? | For all randomised people the most important moments of effect measurement should have been reported |
| o | Were point estimates and measures of variability presented for the primary outcome measures? | For continuous data mean, median, standard deviation with 95 % confidence interval should be presented. For nominal and ordinal outcomes the number of people to whom the outcome measure applies and the total number of people must be presented |
Table 2.
Internal validity scores (b1, b2, c, e, f, g, j, l, n)
| reference | b1 | b2 | c | e | f | g | j | l | n |
| Vassilomaniakis 1995 | + | ‐ | + | + | ‐ | ‐ | + | + | + |
| Ranson 1990 | + | + | + | + | + | +? | + | + | +? |
| Lim 1993 | + | ‐ | + | + | ‐ | +? | + | + | + |
| Barriga 1997 | + | + | + | + | + | + | + | + | + |
| Rackoff 1995 | + | + | + | + | + | + | + | + | + |
| Schwartz 1990 | + | + | + | + | + | + | + | + | + |
| Henrickson 2000 | + | + | + | + | + | + | + | + | + |
| Daghistani 1996 | + | + | + | + | + | +? | + | + | + |
| Ljungman 1997 | + | ‐ | ‐ | ‐ | ‐ | ‐ | + | + | + |
| Di Carlo 2011 | + | + | + | + | ‐ | ‐ | + | + | ? |
| Handrup 2013 | + | ‐ | + | + | ‐ | ‐ | + | + | + |
Table 3.
External validity (a, d1, d2, h, i, k, m, o)
| Reference | a | d1 | d2 | h | i | k | m | o |
| Vassilomaniakis 1995 | + | + | + | +? | ‐ | + | + | + |
| Ranson 1990 | + | + | + | +? | ‐ | + | + | + |
| Lim 1993 | + | + | + | + | ‐ | + | + | ‐ |
| Barriga 1997 | + | + | + | + | ‐ | + | + | + |
| Rackoff 1995 | + | + | + | + | ‐ | + | + | + |
| Schwartz 1990 | + | + | + | + | ‐ | + | + | + |
| Henrickson 2000 | + | + | + | + | ‐ | + | + | + |
| Daghistani 1996 | + | + | + | + | ‐ | + | + | + |
| Ljungman 1997 | + | + | + | + | ‐ | + | + | + |
| Di Carlo 2011 | + | + | + | + | ‐ | + | + | + |
| Handrup 2013 | + | + | + | + | ‐ | + | + | + |
Figure 3.
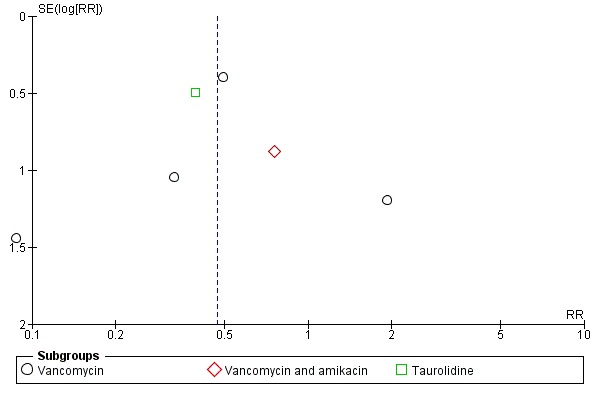
Funnel plot of comparison: 2 Antibiotic and heparin flush or lock solution versus heparin only, outcome: 2.1 Catheter‐related sepsis.
Effects of interventions
Antibiotics before long‐term central venous catheter (CVC) insertion
We included five studies in this meta‐analysis: two used vancomycin, two used teicoplanin, and one used ceftazadime prophylaxis versus control (placebo or no antibiotic). All five studies were conducted in adults. There was no significant difference in the risk of CRS between the prophylactic antibiotic and control groups (360 adults; RR 0.72, 95% CI 0.33 to 1.58; I² = 52%; P = 0.41) (Analysis 1.1). Differences were not significant for any of the antibiotic subgroups either. In the sensitivity analysis, we removed a study that was at high risk of bias (Ljungman 1997), which made little difference to the overall effect (295 adults; RR 0.54, 95% CI 0.23 to 1.27; I² = 61%).
Analysis 1.1.
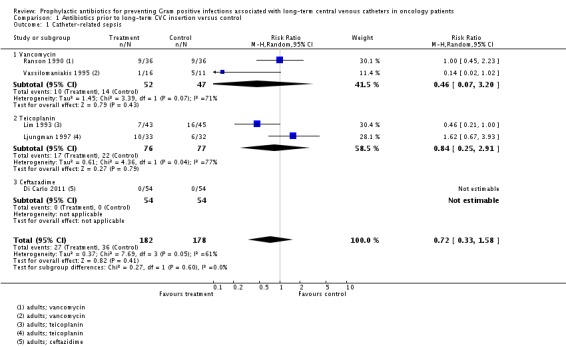
Comparison 1 Antibiotics prior to long‐term CVC insertion versus control, Outcome 1 Catheter‐related sepsis.
Antibiotic and heparin flush or lock solutions versus heparin only solutions
We included six studies that were conducted mainly in children in this meta‐analysis. Most used a vancomycin and heparin solution; one used a vancomycin, amikacin and heparin solution (Daghistani 1996) and one used a taurolidine and heparin solution (Handrup 2013). The combined antibiotic and heparin solution was associated with significantly less CRS than the heparin only solution (468 participants; RR 0.47, 95% CI 0.28 to 0.80; I² = 0%; P = 0.005) (Analysis 2.1). Using these data, the number needed to treat (NNT) to prevent CRS in one patient, for an assumed baseline rate of 15%, would be 12 participants (95% CI 9 to 33).
Analysis 2.1.
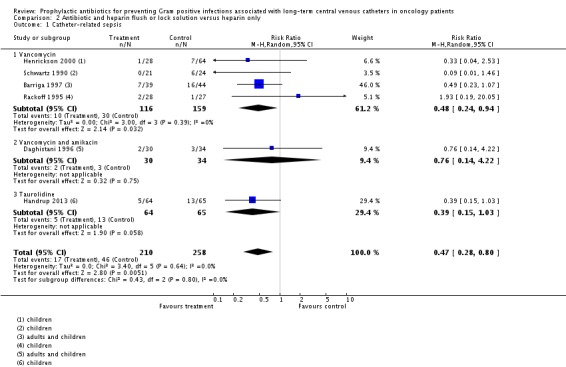
Comparison 2 Antibiotic and heparin flush or lock solution versus heparin only, Outcome 1 Catheter‐related sepsis.
When we excluded the studies which enrolled both adults and children (Barriga 1997; Daghistani 1996) and restricted our analyses to children only (N = 321), the RR was similar to the overall result (RR 0.41, 95% CI 0.18 to 0.89), in favour of antibiotics.
Henrickson 2000 included some participants with existing CVCs; we performed a sensitivity analysis by excluding this study from the analysis and the results remained true to the overall finding.
Discussion
Summary of main results
Administering antibiotics before the insertion of long‐term CVCs did not reduce the risk of subsequent catheter‐related infections (Table 1). Combining antibiotics with heparin in a solution to flush or lock long‐term CVCs approximately halved the risk of subsequent catheter‐related infections in oncology patients (Table 2).
Overall completeness and applicability of evidence
In this review, we included studies that enrolled adults, children, or both. However, as the first meta‐analysis included studies comprising adults only (Analysis 1.1) it is possible that the results of this meta‐analysis are not generalisable to children. Similarly, the second meta‐analysis (Analysis 2.1) included studies that were mainly conducted in children. Therefore, it is possible that the associated evidence, which indicates a beneficial effect of adding antibiotics to the standard flush or lock solution, may not be generalisable to adults. We consider the prevention, detection and treatment of infections in CVCs to be comparable in adults and children and therefore consider this evidence to be applicable to both.
We included studies evaluating the risk of CRS in TIDs and TCVCs. We were unable to distinguish between infection rates for TCVCs and TIDs due to insufficient data. Ports may be associated with a lower risk of CRS, however we pooled the data on ports and TCVCs as both are tunnelled central venous catheters and both are used to administer chemotherapy. One included study evaluated participants with TCVCs or TIDs (Handrup 2013) and one study evaluated participants with TIDs only (Di Carlo 2011). In the latter study no early infections occurred in the 108 participants that were included. In Handrup 2013, which comprised mainly TIDs, long‐term infection rates in the control group were comparable to those reported in the TCVC studies.
Although the risk of infection is considered to be greatest during the first 45 to 100 days after placement (Abbas 2004; Salzman 1995), few of the included studies defined or evaluated early CRS. Baseline infection rates differ between institutions and should always be assessed before the introduction of antibiotic prophylaxis.
Quality of the evidence
Overall, we consider the evidence synthesized in this review to be of a moderate quality. Analysis 1.1 suffered from substantial heterogeneity due to the small sample sizes and inconsistent findings of the included studies. We considered Analysis 2.1 to be high quality evidence with respect to children, however we downgraded the evidence to moderate quality due to the clinical heterogeneity of the studies (types of antibiotics, CVCs and participants).
Potential biases in the review process
We attempted to reduce bias in this review by excluding studies in which long‐term CVCs were already in situ, that is were not newly inserted. Catheters that are in situ and in use prior to enrolment were likely to be pre‐colonized with bacteria. Including such studies may have led to spurious findings or higher rates of infections observed and would have introduced another variable by which to adjust the results.
Some included studies reported Gram negative and Gram positive CRS (for example Barriga 1997; Handrup 2013; Henrickson 2000). In these instances we only used the Gram positive data. However, the antibiotic group in Handrup 2013 and Henrickson 2000 also experienced lower rates of Gram negative CRS. Had we included these data, the RR would have more strongly favoured the antibiotic group in Analysis 2.1.
Like Snaterse 2010, we did not differentiate between flush and lock solutions in our meta‐analyses as we considered them to have the same effect on the catheter lumen. Similarly, we combined the results of studies using various antibiotics with activity against Gram positive organisms into one meta‐analysis.
Agreements and disagreements with other studies or reviews
The original review found weak evidence in favour of antibiotic flush solutions and no evidence to support systemic antibiotics. There remains no demonstrable benefit from prophylactic intravenous antibiotics before long‐term CVC insertion. However, evidence from our updated meta‐analysis supports a beneficial effect of an antibiotic and heparin solution for flushing or locking long‐term CVCs. In a 2010 review, Snaterse 2010 points out that the lack of specificity in the outcomes measured in many of the included studies may lead to overestimation of the effect. We agree that more evidence is needed.
Authors' conclusions
Flushing or locking long‐term CVCs with an antibiotic and heparin solution appears to reduce Gram positive catheter‐related sepsis experienced in people at risk of neutropenia through chemotherapy or disease. Due to insufficient data it is not clear whether this applies equally to TCVCs and TIDs, or equally to adults and children. The use of an antibiotic and heparin solution may be of value in high risk people and where baseline CVC infection rates are high (> 15%). However, routine antibiotic administration, irrespective of risk, is likely to increase microbial resistance.
Although some of the included studies stratified risk groups (for example neutropenic and non‐neutropenic) none analysed these separately due to insufficient numbers. A large multicentre study to investigate the role of antibiotics for different risk groups is needed. Such a trial would also be valuable in identifying high risk groups that are most likely to benefit from antibiotic prophylaxis. It was not possible to draw any conclusions about the types of CVCs and antibiotics; further research would be valuable.
Antibiotic coatings for long‐term tunnelled CVCs are currently under investigation and studies comparing these new types of catheters with antibiotic and heparin lock solutions are required. Due to the risk of developing microbial resistance, research into non‐antibiotic solutions to reduce catheter‐related infections is warranted. Ethanol (70%) lock solutions to prevent catheter‐related infections are currently being investigated in both adults and children.
Acknowledgements
We wish to thank the Managing Editors, Gail Quinn and Clare Jess, at the Cochrane Gynaecological Cancer Group for their administrative support; Jane Hayes for conducting the updated search; and our funders for the research grant which made this systematic review possible.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Gynaecological Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search strategy for CENTRAL
#1 MeSH descriptor: [Neoplasms] explode all trees #2 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or oncolog* or leukemia* or leukaemia* or lymphoma* or metasta* or bone marrow transplant*) #3 #1 or #2 #4 MeSH descriptor: [Catheters] explode all trees #5 MeSH descriptor: [Catheterization] explode all trees #6 MeSH descriptor: [Catheter‐Related Infections] this term only #7 (catheter* or central venous line* or central venous device* or CVC* or TCVC*) #8 #4 or #5 or #6 or #7 #9 MeSH descriptor: [Antibiotic Prophylaxis] this term only #10 MeSH descriptor: [Anti‐Infective Agents] explode all trees #11 MeSH descriptor: [Gram‐Positive Bacterial Infections] explode all trees and with qualifiers: [Drug therapy ‐ DT] #12 antibiotic* #13 #9 or #10 or #11 or #12 #14 #3 and #8 and #13
Appendix 2. Search strategy for MEDLINE
MEDLINE Ovid
1 exp Neoplasms/ 2 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or oncolog* or leukemia* or leukaemia* or lymphoma* or metasta* or bone marrow transplant*).mp. 3 1 or 2 4 exp Catheters/ 5 Catheter‐Related Infections/ 6 exp Catheterization/ 7 (catheter* or central venous line* or central venous device* or CVC* or TCVC*).mp. 8 4 or 5 or 6 or 7 9 Antibiotic Prophylaxis/ 10 exp Anti‐Infective Agents/ 11 exp Gram‐Positive Bacterial Infections/dt [Drug Therapy] 12 antibiotic*.mp. 13 9 or 10 or 11 or 12 14 randomized controlled trial.pt. 15 controlled clinical trial.pt. 16 randomized.ab. 17 placebo.ab. 18 clinical trials as topic.sh. 19 randomly.ab. 20 trial.ti. 21 14 or 15 or 16 or 17 or 18 or 19 or 20 22 3 and 8 and 13 and 21 23 exp animals/ not humans.sh. 24 22 not 23
key: mp = title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier pt = publication type ab = abstract sh = subject heading ti = title
Appendix 3. Search strategy for EMBASE
EMBASE Ovid
1 exp neoplasm/ 2 (cancer* or tumor* or tumour* or neoplas* or carcinoma* or adenocarcinoma* or oncolog* or leukemia* or leukaemia* or lymphoma* or metasta* or bone marrow transplant*).mp. 3 1 or 2 4 exp catheter/ 5 catheter infection/ 6 catheterization/ 7 (catheter* or central venous line* or central venous device* or CVC* or TCVC*).mp. 8 4 or 5 or 6 or 7 9 antibiotic prophylaxis/ 10 exp antiinfective agent/ 11 Gram positive infection/dt [Drug Therapy] 12 antibiotic*.mp. 13 9 or 10 or 11 or 12 14 crossover procedure/ 15 double‐blind procedure/ 16 randomized controlled trial/ 17 single‐blind procedure/ 18 random*.mp. 19 factorial*.mp. 20 (crossover* or cross over* or cross‐over*).mp. 21 placebo*.mp. 22 (double* adj blind*).mp. 23 (singl* adj blind*).mp. 24 assign*.mp. 25 allocat*.mp. 26 volunteer*.mp. 27 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 28 3 and 8 and 13 and 27 29 (exp animal/ or nonhuman/ or exp animal experiment/) not human/ 30 28 not 29
key: mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
Data and analyses
Comparison 1.
Antibiotics prior to long‐term CVC insertion versus control
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related sepsis | 5 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.33, 1.58] |
| 1.1 Vancomycin | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.07, 3.20] |
| 1.2 Teicoplanin | 2 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.25, 2.91] |
| 1.3 Ceftazadime | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2.
Antibiotic and heparin flush or lock solution versus heparin only
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter‐related sepsis | 6 | 468 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.28, 0.80] |
| 1.1 Vancomycin | 4 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.24, 0.94] |
| 1.2 Vancomycin and amikacin | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.14, 4.22] |
| 1.3 Taurolidine | 1 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.15, 1.03] |
What's new
Last assessed as up‐to‐date: 28 June 2013.
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 3, 2003
| Date | Event | Description |
|---|---|---|
| 1 April 2015 | Amended | Contact details updated. |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
| 6 November 2013 | New citation required but conclusions have not changed | New evidence supports conclusions of previous review |
| 17 September 2013 | New search has been performed | Two additional studies added (Di Carlo 2011; Handrup 2013) |
| 28 June 2013 | New search has been performed | New search performed |
| 6 September 2011 | Amended | PLS amended |
| 9 November 2006 | New citation required and conclusions have changed | Substantive amendment |
| 9 November 2006 | Amended | Minor update: 09/11/06 New studies sought but none found: 01/09/06 New studies found and included or excluded: 08/09/06 Conclusions changed: 19/09/06 Updating the search from July 2001 to July 2006 revealed no new RCTs in tunnelled central venous catheters. However, the improved method of testing heterogeneity I, has resulted in a change to the conclusions. Vanco prophylaxis at insertion of the catheter is not beneficial. Flushing the catheter is beneficial in high risk patients. In the excluded studies some new RCTs have been included. These were all RCTs with non‐tunnelled central venous catheters. |
| 8 September 2006 | New citation required and conclusions have changed | Original search performed. |
Differences between protocol and review
Types of studies expanded to include lock solutions as well as flush solutions.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barriga 1997
| Methods | Double blind randomisation | |
| Participants | N = 83 adults and paediatric patients with various malignancies, mainly leukaemia; 143 febrile episodes recorded | |
| Interventions | Vancomycin/heparin versus heparin‐only flush (25 ug/ml vanco and 25 units/ml heparin) | |
| Outcomes | *Bacteraemia (BSI) *Vanco‐sensitive organism bacteraemia | |
| Notes | A difference was stated in neutropenia and non‐neutropenia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Daghistani 1996
| Methods | Double blind randomisation | |
| Participants | N = 61 adult and paediatric patients Various malignancies | |
| Interventions | Vancomycin/amikacin/heparin flush (25 ug/ml vanco, 25 ug/ml amikin and 100 units/ml hep) versus heparin only flush | |
| Outcomes | *Catheter‐related sepsis (BSI) *Cellulitis | |
| Notes | The only study in which amikacin was added | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Di Carlo 2011
| Methods | Consecutive people randomised; allocation by sealed envelopes | |
| Participants | N = 108 adult patients receiving a TID (Port‐a‐cath) to facilitate chemotherapy | |
| Interventions | Ceftazidime (1g IVI 10 min before skin incision) versus no antibiotic (control) | |
| Outcomes | *Surgical site infections (superficial and deep) *Infection considered if T°>37.5°C, WCC >10x10/L, and one or more of: pain, swelling, redness, or heat |
|
| Notes | Outcomes assessed for 30 days after insertion | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear; method of randomisation not described; allocation by sealed envelopes; not blinded |
Handrup 2013
| Methods | Open‐label RCT; computer generated randomisation code in blocks of 20 | |
| Participants | n = 112 children aged 0‐19 years receiving a newly placed TCVC to facilitate chemotherapy (49% haematological, 51% solid tumours) 129 TCVCs inserted, including 113 TIDs and 16 TCVCs with external lines (TEs) |
|
| Interventions | Taurolidine/heparin/sodium citrate lock solution versus heparin lock solution | |
| Outcomes | *CRBSI *Exit‐site and tunnel infections *Mechanical complications |
|
| Notes | Followed up (catheters in situ) from 12 to 1176 days Type of CVC was a risk factor for CRBSI (TIDs were less likely to get infected than TEs) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Henrickson 2000
| Methods | Double blind randomisation Stratified for risk groups | |
| Participants | N = 126 paediatric patients (44% ALL, 40% solid, 7 % BMT). There were 153 assessable TCVCs | |
| Interventions | Vancomycin/heparin versus heparin only flush (25 ug/ml vanco and 100 units/ml heparin) | |
| Outcomes | *Exit‐site infection *Bacteraemia (CRBSI) *Time to first infection | |
| Notes | The third group included vanco/heparin ciprofloxacin Quantitative cultures were done |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Lim 1993
| Methods | Method of randomisation not clear | |
| Participants | N = 88 adult oncology patients with haematological malignancies Baseline characteristics reported ‐ no significant difference |
|
| Interventions | Teicoplanin before insertion 400 mg before insertion catheter versus control | |
| Outcomes | *Soft tissue infection *Catheter‐related sepsis (CRS) | |
| Notes | All episodes of CRS occurred in people who were neutropenic | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Ljungman 1997
| Methods | Method of randomisation not clear | |
| Participants | N = 66 adult oncology patients, BMT and leukaemia patients | |
| Interventions | Teicoplanin prior to insertion and 24 hrs after insertion | |
| Outcomes | *Bacteraemia (BSI) *Exit‐site infection | |
| Notes | At interim analysis, the pre‐set efficacy could not be met, therefore they stopped study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Rackoff 1995
| Methods | Double blind randomisation | |
| Participants | N = 55 paediatric patients, one centre (total group was 63 patients, 8 were receiving TPN) Analysis was done on the oncology patients only | |
| Interventions | Vancomycin/heparin versus heparin only flush (25 ug/ml vanco and 100 units/ml heparin) | |
| Outcomes | *Bacteraemia with a vanco sensitive organism (CRBSI) *Time to first infection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Ranson 1990
| Methods | Double blind randomisation | |
| Participants | N = 98 adults A) N = 48 and 35 catheters, acute leukaemia and BMT B) N = 50 and 37 catheters (solid tumour) | |
| Interventions | Vancomycin versus control (2 doses one prior to insertion, one after positioning of the catheter) 500 mg vanco | |
| Outcomes | *Catheter‐related sepsis in first 30 days *Tunnel sepsis *Coagulase negative staphylococcal bacteraemia | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Schwartz 1990
| Methods | Double blind randomisation | |
| Participants | N = 45 paediatric patients | |
| Interventions | Vancomycin/heparin versus heparin only flush (25 ug/ml vanco and 100 units/ml heparin) | |
| Outcomes | *Bacteraemia (quantitative culture) *Time to first infection | |
| Notes | Statistics on the number of children not catheters Quantitative blood cultures |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Vassilomaniakis 1995
| Methods | Randomisation by cards in closed envelopes | |
| Participants | N = 40 adult patients | |
| Interventions | Vancomycin versus control (vanco in 3 doses of 500 mg 1 hr prior to insertion, 6 and 12 h afterwards) | |
| Outcomes | *Exit‐site infection *CRBSI *Gram positive infections | |
| Notes | Only initially randomised | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
CRS = catheter‐related sepsis; BMT = bone marrow transplant; TCVC = tunnelled central venous catheter; TPN = total parenteral nutrition; CVC = central venous catheter; TID = totally implantable device; CRBSI = catheter‐related blood stream infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelkefi 2005 | An RCT of low‐dose heparin prophylaxis not antibiotic prophylaxis to reduce non‐tunnelled CVC‐related infections in haemato‐oncological disease |
| Akyuz 2012 | RCT of taurolidine lock solution versus heparin lock solution in children undergoing treatment for cancer. This study did not specifically include patients with newly inserted TCVCs |
| Al Sibai 1987 | 146 patients with malignant disease received 160 Hickman catheters. 70 of these patients received prophylactic antibiotics during and after insertion of the catheter. The catheter infection rate dropped from 0.5‐0.25 per 100 days. Excluded because the antibiotic use and duration were at the discretion of the attending physician, and the results were retrospectively analysed |
| Carratala 1999 | Adult haematology patients with non‐tunnelled CVC's received 10 U heparin per ml (N = 57) or 10 U heparin + 25 µg vancomycin per ml (N = 60) allowed to dwell in catheter 1 hour every 2 days. Catheter‐related bacteraemia in 7% of people in control group and 0% in experimental group (P = 0.05). Mainly excluded because non‐tunnelled catheters |
| Chambers 2005 | RCT of sustained release chlorhexidine dressings (not antibiotics) versus standard dressings for TCVCs in neutropenic people |
| Chatzinikolaou 2003a | Prospective cohort study. M‐EDTA was used as lock solution in indwelling ports in 14 children. No catheter‐related infections were observed. In 48 control participants locked with heparin 10 port infections were observed. Not included because cohort study |
| Chatzinikolaou 2003b | Haemodialysis catheters in people with cancer. 66 people impregnated catheters with minocycline and rifampin and 64 non‐impregnated catheters. 0 catheter‐related infections in the impregnated group and 7 in the non‐impregnated group, duration catheter 8 days. Excluded because this concerns non‐tunnelled catheters and the duration of insertion was short |
| Dawson 2000 | 143 paediatric oncology patients, with 176 TCVC. Intervention cephalothin 100 mg/kg iv or vancomycin 20‐25 mg/kg iv prior to insertion of the catheter. Rate of infections <30 days dropped 40%. No randomisation performed and intervention period was compared to pre‐intervention period |
| Dumichen 2012 | RCT of taurolidine citrate versus heparin as a catheter lock solution in 71 paediatric oncology patients. The lock solution was not used immediately after TCVC insertion in most participants (given up to 2 months after insertion in some cases) |
| Ferreira Chacon 2011 | RCT of minocycline/EDTA versus heparin lock solution in children with TCVCs for chemotherapy, however TCVCs were not newly placed |
| Fourcade 2001 | Prospective cohort study using antibiotic lock technique to prevent bacteraemia in chronic haemodialysis catheters. The incidence of bacteraemia dropped from 4.6 per 1000 catheter days to 0.88 per 1000 catheter days. Not RCT, comparison with historical control, non‐tunnelled catheters |
| Garland 2005 | Prospective RCT in critically ill neonates. Vanco lock solution was used in 42 infants and heparin lock in 43 infants. Two people in the vanco/heparin lock group developed a catheter‐related infection, 13 people in the control group developed a catheter‐related infection, RR 0.13 (95% CI 0.01 to 0.57) highly significant, duration catheter 20 days. Excluded because it concerns non‐tunnelled catheters and done in neonates, which is not the appropriate group for our inclusion criteria |
| Hanna 2004 | Prospective RCT at MD Anderson in cancer patients, 356 catheters placed, 182 impregnated with minocyclin and rifampin, 174 non‐impregnated. Mean duration of the catheter 66 days. Three catheter‐related infections in the MR group and 14 in the non‐impregnated group, highly significant. Not included because these are non‐tunnelled catheters and baseline risk for these catheters is higher than the tunnelled catheters. Also, we included studies of newly inserted tunnelled central venous catheters only |
| Hitz 2012 | RCT of TCVCs coated with athrombogenic coating versus no coating in cancer patients, not an RCT of prophylactic antibiotics |
| Jaeger 2005 | RCT of chlorhexidine/sulfadiazine impregnated CVCs versus standard CVCs in leukaemia patients. This study did not use tunnelled catheters |
| Ocete 1998 | Single‐centre trial; 2 groups control group ‐ 61 newborns and experimental group 85 newborns, all receiving a central catheter (umbilical artery, umbilical vein and/or silastic). The study group received prophylactic vancomycin 25 ug/ml. All participants received parenteral nutrition. Results CNS 21/61 in the control group and 19/85 in the vancomycin group (P < 0.05). The patient group is not the group studied in this review. Methods of the study poor. Not specified how often the prophylactic vanco was given. Clinical criteria were used to determine if the neonate was infected, and then peripheral and central cultures were done. Not specified if quantitative or qualitative cultures were done. Trial not blinded, no tunnelled catheters used, inappropriate patient group |
| Raad 1998 | Crossover study: 26 people with melanoma on IL2 treatment enrolled. All people received a double lumen non‐tunnelled silicone catheter in subclavian vein. People randomised to receive prophylactic antibiotics novobiocin 500 mg + rifampin 300 mg orally. Significant results 41% in control group catheter‐related bacteraemia and 6% in experimental group, excluded because of non‐tunnelled catheters. Very specific group with high incidence of infection, not representative of the participant group for this Cochrane review |
| Raad 2005 | RCT evaluating dalbavancin versus vancomycin for the treatment of adults with CRBSIs |
| Rubie 1995 | 163 paediatric patients with cancer had 180 subcutaneous ports inserted. Over time a change of policy was made from only flushing with heparin to a V/H solution. The infection rate dropped from 31% to 4%. This study was not randomised and the results were retrospectively analysed |
| Scaife 2010 | A retrospective study of perioperative antibiotic prophylaxis for TCVCs implanted to facilitate chemotherapy in adults |
| Schierholz 2010 | RCT evaluating an antibiotic‐releasing CVC (rifampicin‐miconazole) versus a standard CVC in adults (38% with cancer). Non‐tunnelled CVCs were compared |
| Simon 2008 | Not a RCT. A prospective cohort study of heparin versus taurolidine lock solution in 188 adults receiving chemotherapy for cancer. The taurolidine lock solution significantly reduced the rate of CRBSIs |
CRS = catheter‐related sepsis; BMT = bone marrow transplant; TCVC = tunnelled central venous catheter; TPN = total parenteral nutrition; CVC = central venous catheter; TID = totally implantable device; CRBSI = catheter‐related blood stream infection
Contributions of authors
MD van de Wetering: reference search, article retrieval, assessment of studies for inclusion or exclusion, data extraction, analysis, manuscript preparation.
J van Woensel: reference search, assessment of studies for inclusion or exclusion, data extraction, reviewing of manuscript.
TA Lawrie: assessment of studies for inclusion or exclusion for the updated review, data extraction, analysis, and manuscript preparation.
Sources of support
Internal sources
-
SKK Stichting kindergeneeskundig kankeronderzoek (Childrens Oncology Research Fund) Amsterdam, Netherlands.
(original review)
-
Department of Health, UKNIHR Cochrane Programme Grant support from 'Optimising care, diagnosis and treatment pathways to ensure cost effectiveness and best practice in gynaecological cancer. Improving the evidence for the NHS.' CPG‐10/4001/12, UK.
(updated review)
External sources
No sources of support supplied
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
- Barriga FJ, Varas M, Potin M, Sapunar F, Rojo H, Martinez A, et al. Efficacy of a vancomycin solution to prevent bacteraemia associated with an indwelling central venous catheter in neutropenic and non‐neutropenic cancer patients. Medical and Pediatric Oncology 1997;28:196‐200. [DOI] [PubMed] [Google Scholar]
- Daghistani D, Horn M, Rodriguez Z, Schoenike S, Toledano S. Prevention of indwelling central venous catheter sepsis. Medical and Pediatric Oncology 1996;26:405‐8. [DOI] [PubMed] [Google Scholar]
- Carlo I, Toro A, Pulvirenti E, Palermo F, Scibilia G, Cordio S. Could antibiotic prophylaxis be not necessary to implant totally implantable venous access devices? Randomized prospective study. Surgical Oncology 2011;20:20‐5. [DOI] [PubMed] [Google Scholar]
- Handrup MM, Fuursted K, Funch P, Moller JK, Schroder H. Biofilm formation in long‐term central venous catheters in children with cancer: A randomized controlled open‐labelled trial of taurolidine versus heparin. APMIS 2012;120:794‐801. [DOI] [PubMed] [Google Scholar]; Handrup MM, Moller JK, Schroder H. Central venous catheters and catheter locks in children with cancer: A prospective randomized trial of taurolidine versus heparin. Pediatric Blood and Cancer. 2011; Vol. Conference: 43rd Congress of the International Society of Paediatric Oncology, SIOP 2011, Auckland, New Zealand, 28 to 30 October. [Google Scholar]; Handrup MM, Møller JK, Schrøder H. Central venous catheters and catheter locks in children with cancer: a prospective randomized trial of taurolidine versus heparin. Pediatric Blood Cancer 2013;60(8):1292‐8. [DOI] [PubMed] [Google Scholar]
- Henrickson KJ, Axtell RA, Hoover SM, Kuhn SM, Pritchett J, Kehl SC, et al. Prevention of central venous catheter related infections and thrombotic events in immuno compromised children by the use of vancomycin/ciprofloxacin/heparin flush solution: a randomized multicenter double blind trial. Journal of Clinical Oncology 2000;18(6):1269‐78. [DOI] [PubMed] [Google Scholar]
- Lim SH, Smith MP, Machin SJ, Goldstone AH. A prospective randomized study of prophylactic teicoplanin to prevent early Hickman catheter related sepsis in patients receiving intensive chemotherapy for haematological malignancies. European Journal of Haematology 1993;Suppl 54:10‐3. [DOI] [PubMed] [Google Scholar]
- Ljungman P, Hagglund H, Bjorkstrand B, Lonnqvist B, Ringden O. Peroperative teicoplanin for prevention of gram‐positive infections in neutropenic patients with indwelling central venous catheters: a randomized controlled study. Support Care Cancer 1997;5:485‐8. [DOI] [PubMed] [Google Scholar]
- Rackoff WR, Weiman M, Jakobowski D, Hirschl R, Stallings V, Bilodeau, et al. A randomized controlled trial of the efficacy of a heparin and vancomycin flush solution in preventing central venous catheter infections in children. Journal of Paediatrics 1995;127(1):147‐51. [DOI] [PubMed] [Google Scholar]
- Ranson MR, Oppenheim BA, Jackson A, Kamthan AG, Scarffe JH. Double blind placebo controlled study of vancomycin prophylaxis for central venous catheter insertion in cancer patients. The Journal of Hospital Infection 1990;15:95‐102. [DOI] [PubMed] [Google Scholar]
- Schwartz C, Henrickson K, Roghmann K, Powell K. Prevention of bacteraemia attributed to luminal colonization of tunnelled central venous catheters with vancomycin susceptible organisms. Journal of Clinical Oncology 1990;8(9):1591‐7. [DOI] [PubMed] [Google Scholar]
- Vassilomaniakis M, Platniotis G, Koumakis G. Central venous catheter related infections after bone marrow transplantation in patients with malignancies: a prospective study with short course vancomycin prophylaxis. Bone Marrow Transplantation 1995;15:77‐80. [PubMed] [Google Scholar]
References to studies excluded from this review
- Abdelkefi A, Torjman L, Ladeb S, Othman T B, Achour W, Lakhal A, et al. Randomized trial of prevention of catheter‐related bloodstream infection by continuous infusion of low‐dose unfractionated heparin in patients with hematologic and oncologic disease. Journal of Clinical Oncology 2005;23:7864‐70. [DOI] [PubMed] [Google Scholar]
- Akyuz C Kupeli S Yagci‐Kupeli B Buyukpamukcu M. Prophylactic taurolidine use in central venous catheters of pediatric cancer patients: A prospective randomized study from single center. Pediatric Blood and Cancer. 2010; Vol. 42nd Congress of the International Society of Pediatric Oncology, SIOP 2010, Boston, MA United States, 21 to 24 October. [Google Scholar]; Akyuz C, Kupeli S, Yagci‐Kupeli B, Buyukpamukcu M. Prophylactic taurolidine use in port catheters of pediatric cancer patients: a prospective randomised controlled study from single center. European Journal of Clinical and Medical Oncology submitted 2012 (authors' manuscript).
- Al Sibai MB, Harder EJ, Faskin RW, Johnson GW, Padmos MA. The value of prophylactic antibiotics during the insertion of long‐term indwelling silastic right atrial catheters in cancer patients. Cancer 1987;60:1891‐5. [DOI] [PubMed] [Google Scholar]
- Carratala J, Niubo J, Fernandez‐Sevilla A, Juve E, Castellsague X, Berlanga J, et al. Randomized, double‐blind trial of an antibiotic‐lock technique for prevention of Gram‐positive central venous catheter‐related infection in neutropenic patients with cancer. Antimicrobial Agents and Chemotherapy 1999;43(9):2200‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers ST, Sanders J, Patton WN, Ganly P, Birch M, Crump JA, et al. Reduction of exit‐site infections of tunnelled intravascular catheters among neutropenic patients by sustained‐release chlorhexidine dressings: results from a prospective randomized controlled trial. The Journal of Hospital Infection 2005;61:53‐61. [DOI] [PubMed] [Google Scholar]
- Chatzinikolaou I, Zipf T, Hanna H, Umphrey J, Roberts W, Sheretz R, et al. Minocycline‐ethylenediamine‐tetraacetate lock solution for the prevention of implantable port infections in children with cancer. Clinical Infectious Diseases 2003;36:116‐9. [DOI] [PubMed] [Google Scholar]
- Chatzinilolaou I, Finkel K, Hanna H, Boktour M, Foringer J, Ho T, et al. Antibiotic coated hemodialysis catheters for the prevention of vascular catheter‐related infections: a prospective randomized study. American Journal of Medicine 2003;115:352‐7. [DOI] [PubMed] [Google Scholar]
- Dawson S, Fitzgerald P, Langer JC, Walton M, Winthrop A, Lau G, et al. A preoperative protocol for the prevention of infection in children with tunnelled right atrial catheters. Oncology Repertorium 2000;7:1239‐42. [DOI] [PubMed] [Google Scholar]
- Dumichen MJ, Seeger K, Lode HN, Kuhl JS, Ebell W, Degenhardt P, et al. Randomized controlled trial of taurolidine citrate versus heparin as catheter lock solution in paediatric patients with haematological malignancies. The Journal of Hospital Infection 2012;80:304‐9. [DOI] [PubMed] [Google Scholar]
- Ferreira Chacon JM, Hato de Almeida E, Lourdes Simoes R, Lazzarin C Ozorio V, Alves BC, Mello de Andrea ML, et al. Randomized study of minocycline and edetic acid as a locking solution for central line (port‐a‐cath) in children with cancer. Chemotherapy 2011;57:285‐91. [DOI] [PubMed] [Google Scholar]
- Fourcade J, Mallaval FO, Raffenot D, Mercier D, Maret J, Morel B. Value of an antibiotic lock for the prevention of bacteraemia recurrence from central catheters in chronic hemodialysis. Nephrologie 2001;22(8):457‐8. [PubMed] [Google Scholar]
- Garland JS, Alex CP, Henrickson KJ, McAuliffe TL, Maki DG. A vancomycin‐heparin lock solution for the prevention of nosocomial bloodstream infection in critically ill neonates with peripherally inserted central venous catheters: a prospective randomized trial. Pediatrics 2005;116:198‐205. [DOI] [PubMed] [Google Scholar]
- Hanna H, Benjamin R, Chatzinikolaou I, Alakech B, Richardson D, Mansfield P, et al. Long‐term silicone central venous catheters impregnated with minocycline and rifampin decrease rates of catheter‐related bloodstream infection in cancer patients: a prospective randomized clinical trial. Journal of Clinical Oncology 2004;22(15):3163‐71. [DOI] [PubMed] [Google Scholar]
- Hitz F, Klingbiel D, Omlin A, Riniker S, Zerz A, Cerny T. Athrombogenic coating of long‐term venous catheter for cancer patients: A prospective, randomised, double‐blind trial. Annals of Hematology 2012;91:613‐20. [DOI] [PubMed] [Google Scholar]
- Jaeger K, Zenz S, Juttner B, Ruschulte H, Kuse E, Heine J, et al. Reduction of catheter‐related infections in neutropenic patients: A prospective controlled randomized trial using a chlorhexidine and silver sulfadiazine‐impregnated central venous catheter. Annals of Hematology 2005;84:258‐62. [DOI] [PubMed] [Google Scholar]
- Ocete E, Ruiz‐Extremera A, Goicoechea A, Lozano E, Robles C, Rey ML, et al. Low dosage prophylactic vancomycin in central venous catheters for neonates. Early Human Development 1998;Suppl 53:S181‐6. [DOI] [PubMed] [Google Scholar]
- Raad II, Hachem RY, Abi‐Said D, Rolston KV, Whimbey E, Buzaid AC, et al. A prospective crossover randomized trial of novobiocin and rifampin prophylaxis for the prevention of intravascular catheter infections in cancer patients treated with interleukin 2. Cancer 1998;82(2):403‐11. [DOI] [PubMed] [Google Scholar]
- Raad I, Darouiche R, Vazquez J, Lentnek A, Hachem R, Hanna H, et al. Efficacy and safety of weekly dalbavancin therapy for catheter‐related bloodstream infection caused by gram‐positive pathogens. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2005;40:374‐80. [DOI] [PubMed] [Google Scholar]
- Rubie H, Juricic M, Claeyssens S, Krimou A, Lemozy J, Izard P, et al. Morbidity using subcutaneous ports and efficacy of vancomycin flushing in cancer. Archives of Diseases in Childhood 1995;72:325‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife CL, Gross ME, Mone MC, Hansen HJ, Litz CL, Nelson ET, et al. Antibiotic prophylaxis in the placement of totally implanted central venous access ports. The American Journal of Surgery 2010;200:719‐23. [DOI] [PubMed] [Google Scholar]
- Schierholz JM, Nagelschmidt K, Nagelschmidt M, Lefering R, Yucel N, Beuth J. Antimicrobial central venous catheters in oncology: Efficacy of a rifampicin‐miconazole‐releasing catheter. Anticancer Research 2010;30:1353‐8. [PubMed] [Google Scholar]
- Simon A, Ammann RA, Wiszniewsky G, Bode U, Fleischhack G, Besuden MM. Taurolidine‐citrate lock solution (TauroLock) significantly reduces CVAD‐associated grampositive infections in pediatric cancer patients. BMC Infectious Diseases 2008;8:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
- Abbas AA, Fryer CJ, Paltiel C, Chedid F, Felimban SK, Yousef AA, et al. Factors influencing central line infections in children with acute lymphoblastic leukemia: results of a single institutional study. Pediatric Blood and Cancer 2004;42:325‐31. [DOI] [PubMed] [Google Scholar]
- Bagnall‐Reeb H. Evidence for the use of the antibiotic lock technique. Journal of Infusion Nursing 2004;27(2):118‐22. [DOI] [PubMed] [Google Scholar]
- Hospital Infection Control Practices Advisory Committee. Recommendations for the prevention of nosocomial intravascular device related infections. American Journal of Infection Control 1990;24(4):277‐93. [PubMed] [Google Scholar]
- Darouiche RO. Prevention of vascular catheter related infections. The Netherlands Journal of Medicine 1999;55:92‐9. [DOI] [PubMed] [Google Scholar]
- Groeger JS, Lucas AB, Coit D, LaQuaglia M, Brown AE, Turnbull A, et al. A prospective, randomized evaluation of the effect of silver impregnated subcutaneous cuffs for preventing tunnelled chronic venous access catheter infections in cancer patients. Annals of Surgery 1993;91:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachem R, Raad I. Prevention and management of longterm catheter related infections in cancer patients. Cancer Investigation 2002;20(7 and 8):1105‐13. [DOI] [PubMed] [Google Scholar]
- Ingram J, Weitzman S, Greenberg ML, Parkin P, Filler R. Complications of indwelling venous access lines in the pediatric hematology patient: a prospective comparison of external catheters and subcutaneous ports. American Journal of Pediatric Hematology and Oncology 1991;13:130‐6. [DOI] [PubMed] [Google Scholar]
- Mermel LA, Farr BM, Sherertz RJ, Raad II, O'Grady N, Harris JS, et al. Guidelines for the management of Intravascular catheter‐related infections. Clinical Infectious Diseases 2001;32:1249‐72. [DOI] [PubMed] [Google Scholar]
- O'Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter related infections. Pediatrics 2002;110:51‐75. [DOI] [PubMed] [Google Scholar]
- Press OW, Ramsey PG, Larson EB. Hickman catheter infections in patients with malignancies. Medicine 1984;63:189. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
- Salzman M, Rubin L. Intravenous catheter‐related infections. Advances in Pediatric Infectious Diseases 1995;10:337‐68. [PubMed] [Google Scholar]
- Sarper N, Zengin E, Corapcioglu F, Tugay M. Totally implantable central venous access devices in children with hemato oncologic malignancies: Evaluation of complications and comparison of incidence of febrile episodes with similar patients without central venous access devices. Pediatric Hematology and Oncology 2006;23:459‐70. [DOI] [PubMed] [Google Scholar]
- Schiffer CA, Mangu PB, Wade JC, Camp‐Sorrel D, Cope DG, El‐Rayes BF, et al. Central venous catheter care for the patient with cancer: American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology 2013;31(10):1357‐70. [DOI] [PubMed] [Google Scholar]
- Schinabeck MK, Ghannoum MA. Catheter related infections‐diagnosis treatment and prevention. Clinical Microbiology Newsletter 2003;25(15):113‐9. [Google Scholar]
- Shaul DB, Scheer B, Rokhsar S, Jones VA, Chan LS, Boody BA, et al. Risk factors for early infection of central venous catheters in pediatric patients. Journal of the American College of Surgeons 1998;186(6):654‐8. [DOI] [PubMed] [Google Scholar]
- Simon A, Bode U, Beutel K. Diagnosis and treatment of catheter‐related infections in paediatric oncology: an update. Clinical Microbiology and Infection 2006;12:606‐20. [DOI] [PubMed] [Google Scholar]
- Snaterse M, Ruger W, Scholte OP, Reimer WJM, Lucas C. Antibiotic‐based catheter lock solutions for prevention of catheter‐related bloodstream infection: a systematic review of randomised controlled trials. The Journal of Hospital Infection 2010;75:1‐11. [DOI] [PubMed] [Google Scholar]
- Tulder MW, Assendelft WJ, Koes BW, Bouter LM. Method guidelines for systematic reviews in the Cochrane Collaboration Back review group for spinal disorders. Spine 1997;22:2323‐30. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Wetering MD, Woensel JB, Kremer LC, Caron HN. Prophylactic antibiotics for preventing early Gram‐positive central venous catheter infections in oncology patients, a Cochrane systematic review. Cancer Treatment Review 2005;3:186‐96. [DOI] [PubMed] [Google Scholar]
- Wetering MD, Woensel JBM. Prophylactic antibiotics for preventing early central venous catheter Gram positive infections in oncology patients. Cochrane Database of Systematic Reviews 2007, Issue Issue 1. Art. No.: CD003295. DOI: 10.1002/14651858.CD003295.pub2. [DOI: 10.1002/14651858.CD003295.pub2] [DOI] [Google Scholar]


