Abstract
Background
Cervical cancer is the second most common cancer among women up to 65 years of age and is the most frequent cause of death from gynaecological cancers worldwide. A woman's risk of developing cervical cancer by 65 years of age ranges from 0.69% in developed countries to 1.38% in developing countries. Although screening by Pap smear should mean early detection at a curable stage for most women, many still present with advanced or metastatic disease with a worse prognosis. The addition of platinum‐based chemotherapy to radiotherapy has improved outcome compared to radiotherapy alone; however, 30% to 50% fail to respond to treatment or develop recurrent disease. There are no standard treatment options for these patients, although platinum‐based chemotherapy is frequently used and trials are on‐going.
Objectives
To compare different types and combinations of cytotoxic chemotherapy for the treatment of metastatic/recurrent cervical cancer.
Search methods
We searched the Cochrane Gynaecological Cancer Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 1, 2012), MEDLINE (1950 to January 2012) and EMBASE (1980 to January 2012). The reference lists from these and those of review articles were also checked.
Selection criteria
All randomised controlled trials (RCTs) involving chemotherapy for metastatic/recurrent cervical cancer. Trials involving radiotherapy, chemoradiotherapy, intra‐arterial chemotherapy, biological agents or immunomodulators were excluded.
Data collection and analysis
Three review authors independently reviewed trials for inclusion and data extraction and assessed risk of bias.
Main results
There were no data comparing best supportive care with chemotherapy. Cisplatin‐based regimens are the most widely used and therefore we have concentrated on these trials. In terms of response rates some non‐platinum regimens are equivalent but toxicity is higher. The most common cisplatin regimen was 50 mg/m2 day 1 q21days. Higher doses had similar survivals. There was no direct comparison between single‐agent cisplatin and carboplatin. Overall survival (OS) and progression‐free survival (PFS) were not adequately reported and quality of life (QoL) outcomes were incompletely documented. Combination regimens were more toxic than single agents, but in the limited reported data this did not appear to adversely affect QoL.
No significant difference in response rate by site of recurrence was found, although there was a trend towards improved response when the main site of disease was beyond the previously irradiated pelvis.
Authors' conclusions
Combination cisplatin‐based chemotherapy could be a viable option for patients of good performance status with recurrent/metastatic cervical cancer, but further trials that report adequate survival and QoL data are sought. Response rates and improvements in survival are low. Cisplatin‐based combinations have significant toxicity. Outcomes are poor and novel cytotoxic/biological agents and optimal scheduling need further investigation. Future trials need to stratify for and perform planned subgroup analysis with respect to previous treatment and site of recurrence.
Plain language summary
Chemotherapy primarily aimed at improving length of life while maintaining quality of life for incurable cervical cancer
Cervical cancer often affects young women. Cancer that has come back after initial treatment (recurrent) or has already spread around the body at diagnosis (metastatic) is incurable so chemotherapy is aimed at improving length of life, while maintaining good quality of life. A literature search was conducted identifying 30 potential trials; four trials were excluded. The 26 clinical trials included in this review encompass a large range of different drugs, doses and combinations in a mixed group of patients over a long time period (1976 to 2011), making it difficult to compare treatment options. Although there are no trials directly comparing chemotherapy with symptomatic management alone, chemotherapy is widely used in this setting and assumed to be of benefit. Cisplatin and carboplatin chemotherapy were shown to shrink the cancer in 10% to 30% of patients and are widely used in current practice. Cisplatin chemotherapy when combined with other drugs has been shown to prolong survival by a few months compared with cisplatin alone, but with the cost of increased side effects. Other chemotherapy has been used, but has been found to be less effective or more toxic. Quality of life for patients on chemotherapy appears to be similar for cisplatin and cisplatin‐based combinations. Nearly all patients in these studies were relatively fit and well prior to starting treatment, despite their cancer; these results may not be the same in patients who are not fit and well.
Background
Description of the condition
Cervical carcinoma arises in the uterine cervix and 60% to 70% of cases are squamous cell carcinomas, approximately 25% to 30% are adenocarcinomas or adenosquamous carcinomas, with a very small number of rarer cancers, such as small cell tumours and neuroendocrine tumours (Jemal 2008; Meta‐analysis Collaboration 2008).
Cervical cancer is the second most common cancer among women aged up to 65 years and is the most frequent cause of death from gynaecological cancers worldwide. A woman's risk of developing cervical cancer by 65 years of age ranges from 0.69% in developed countries to 1.38% in developing countries (GLOBOCAN 2008). In Europe, about 60% of women with cervical cancer are alive five years after diagnosis (EUROCARE‐3). The stage of disease at diagnosis determines survival rates; lesions less than 4 cm in diameter and confined to the cervix (FIGO (International Federation of Gynecology and Obstetrics) Stage Ib1) at presentation have a five‐year survival rate of 92%, while women with cancer spread beyond the true pelvis to adjacent organs (FIGO Stage IVa) have a five‐year survival rate of only 17% (Jemal 2008; Meta‐analysis Collaboration 2008).
The overwhelming risk factor for development of cervical cancer is the presence of human papilloma virus (HPV), particularly subtypes 16 and 18. Vaccines against HPV have become available and introduced in some countries. In the future it is hoped this will significantly reduce the incidence of cervical cancer. HPV causes the cervical epithelium to become increasingly abnormal (graded as cervical intra‐epithelial neoplasia (CIN) grades I to III), this then becomes invasive in 30% to 70% of women over 10 to 12 years, although this process can be much faster in a small minority (< one year). Invasive cancer then spreads directly by invading adjacent structures and metastasising via regional lymph nodes and, less commonly, via the bloodstream to distant sites such as the lungs.
Description of the intervention
Early‐stage cancer confined to the cervix, or with extension into upper vagina (Stage I to IIa), can be successfully treated by radical surgery (with or without neoadjuvant chemotherapy) or concomitant chemoradiation, giving five‐year survival rates of 80% to 90% (Eifel 2001). A European Organisation for Research and Treatment of Cancer (EORTC) trial (EORTC 55994) of chemoradiotherapy versus neoadjuvant chemotherapy plus surgery is on‐going. For more advanced cancer, Stages IIb to IVa the treatment of choice is chemoradiotherapy; as a Cochrane meta‐analysis has shown that the addition of platinum‐based chemotherapy significantly improves survival compared to radiotherapy alone (Green 2005). Combined chemoradiotherapy will cure 50% to 70% of patients with locally advanced carcinoma of the cervix (Meta‐analysis Collaboration 2008; Willmott 2009) and can be used for local control of metastatic disease.
Palliative chemotherapy is used for the management of Stage IVb patients whose disease has spread to distant sites, or for patients with inoperable recurrent or persistent disease (de la Motte Rouge 2006; Pectasides 2008). A number of chemotherapeutic options have been used either as single agents or in combination including cisplatin, adriamycin, ifosfamide, paclitaxel, irinotecan, topotecan, vinorelbine and gemcitabine with responses in the range of 15% to 46% (Moore 2006; Tewari 2005).
How the intervention might work
The aim of chemotherapy is to slow cancer growth, improve survival with minimal toxicity so there is an improvement in quality of life (QoL).
Why it is important to do this review
There are no standard treatments for patients with persistent, recurrent or metastatic cervical cancer. In this group of patients, who are often young and otherwise fit and well, it is important to establish chemotherapy regimens that have the greatest chance of response with tolerable side effects and an improvement in QoL. In the era of chemoradiation as primary management for locally advanced cervical cancer the role of chemotherapy in this setting becomes even more important to establish.
Objectives
To investigate the effectiveness of single‐agent and combination chemotherapy in the treatment of patients with metastatic or recurrent cervical cancer with regards to progression‐free survival (PFS), overall survival (OS), adverse effects of treatment and QoL.
Methods
Criteria for considering studies for this review
Types of studies
Randomised clinical trials (RCTs).
Types of participants
Adult women (aged 18 years or over) with metastatic (FIGO Stage IVb) or recurrent cervical carcinoma who are suitable for chemotherapeutic treatment.
Types of interventions
Single‐agent cytotoxic chemotherapy.
Multi‐agent cytotoxic chemotherapy.
Best supportive care.
For the purposes of this review chemotherapy refers to any cytotoxic drug given intravenously or orally with the intent of producing tumour regression as defined by World Health Organization (WHO) criteria for assessing response (Miller 1981); thus biological and immunomodulators were excluded. Trials involving concomitant radiotherapy were excluded because this could complicate assessment of response to chemotherapy, which is the primary objective of the review, particularly as most trials involving radiotherapy compare radiotherapy with radiotherapy plus chemotherapy. Intra‐arterial chemotherapy regimens were excluded as this was felt to be a local rather than systemic therapy and so not comparable. We included trials that used both platinum and non‐platinum chemotherapy and planned to subgroup based on whether the chemotherapy was platinum or non‐platinum based (see Subgroup analysis and investigation of heterogeneity). Best supportive care is not a clearly defined term, but is generally considered to include any interventions that palliate symptoms and optimise QoL, without treating the underlying cancer, such as pain relief or psychological support (www.WHO.int). Where there is no standard treatment option for a particular disease new treatments are often compared with best supportive care (i.e. symptom control) alone.
Types of outcome measures
Primary outcomes
Response rate (percentage of patients with evidence of reduction in tumour size following treatment, usually assessed by computerised tomography (CT) scan within one to three months).
OS (length of time from completing treatment to death from whatever cause).
Secondary outcomes
Time to progression (length of time from start of chemotherapy to evidence of cancer progression).
QoL, measured using a scale that has been validated through reporting of norms in a peer‐reviewed publication.
-
Toxicity, classified according to CTCAE 2006:
haematological (leukopenia, anaemia, thrombocytopenia, neutropenia, haemorrhage);
gastrointestinal (nausea, vomiting, anorexia, diarrhoea, liver, proctitis);
genitourinary;
skin (stomatitis, mucositis, alopecia, allergy);
neurological (peripheral and central);
pulmonary;
other.
Search methods for identification of studies
Papers in all languages were sought and translations carried out when necessary.
Electronic searches
See Cochrane Gynaecological Cancer Group methods used in reviews. The following electronic databases were searched:
the Cochrane Gynaecological Cancer Review Group's Trial Register
the Cochrane Central Register of Controlled Trials (CENTRAL), Issue 1, 2012
MEDLINE to January 2012
EMBASE to January 2012
The MEDLINE, EMBASE and CENTRAL search strategies based on terms related to the review topic are presented in Appendix 1, Appendix 2 and Appendix 3, respectively.
All relevant articles found were identified on PubMed and using the 'related articles' feature, a further search was carried out for newly published articles.
Searching other resources
Unpublished and grey literature
Meta‐Register, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov and www.cancer.gov/clinicaltrials were searched for ongoing trials.
Reference lists and Correspondence
The citation lists of included studies were checked and experts in the field contacted to identify further reports of trials.
Data collection and analysis
Selection of studies
All titles and abstracts retrieved by electronic searching were downloaded to the reference management database Endnote, duplicates were removed and the remaining references were examined by three review authors (KS, JF and MF) independently. Those studies which clearly did not meet the inclusion criteria were excluded and copies of the full text of potentially relevant references were obtained. The eligibility of retrieved papers were assessed independently by three review authors (KS, JF and MF). Disagreements were resolved by discussion between the three review authors. Reasons for exclusion are documented. No blinding of review authors to article author or journal title occurred.
Data extraction and management
For included studies, data were abstracted as recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This included data on the following:
author, year of publication and journal citation (including language);
country;
setting;
inclusion and exclusion criteria;
study design, methodology;
patient characteristics (age, histology, grade, extent of disease, previous therapy, performance status, whether disease lies within an area previously treated with radiotherapy);
-
cervical cancer details at diagnosis:
FIGO Stage;
histological cell type;
tumour grade;
number of participants in each arm of the trial;
number excluded from analysis;
type of intervention (drug, dose, regimen, frequency, number of cycles);
proportion of participants who received all/ part/none of the intended treatment;
delays in treatment;
risk of bias in study (see below);
length of follow‐up;
-
outcomes: response rate, OS, time to progression, QoL and toxicity:
for each outcome: outcome definition (with diagnostic criteria if relevant);
unit of measurement (if relevant);
for scales: upper and lower limits, and whether high or low score is good;
results: number of participants allocated to each intervention group;
for each outcome of interest: sample size; missing participants;
the time points at which outcomes were collected and reported will be noted.
Data on outcomes were extracted as below:
for time to event (OS and time to progression) data, we planned to extract the log of the hazard ratio [log(HR)] and its standard error from trial reports; if these were not reported, we attempted to estimate them from other reported statistics using the methods of Parmar 1998;
for dichotomous outcomes (e.g. response rate, toxicity), we extracted the number of patients in each treatment arm who experienced the outcome of interest and the number of patients assessed at end point, in order to estimate a risk ratio (RR);
for continuous outcomes (e.g. QoL), we planned to extract the final value and standard deviation of the outcome of interest and the number of patients assessed at end point in each treatment arm at the end of follow‐up, in order to estimate the mean difference (if trials measured outcomes on the same scale) or standardised mean difference (if trials measured outcomes on different scales) between treatment arms and its standard error.
Where possible, all data extracted were those relevant to an intention‐to‐treat analysis, in which participants were analysed in groups to which they were assigned.
Data were abstracted independently by three review authors (KS, JF and MF) onto a data abstraction form specially designed for the review. Differences between review authors were resolved by discussion or arbitration of a third party (CW or PC).
Assessment of risk of bias in included studies
The risk of bias in included RCTs was assessed using The Cochrane Collaboration's tool and the criteria specified in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This included assessment of:
-
selection bias:
random sequence generation;
allocation concealment;
-
performance bias:
blinding of participants and personnel (patients and treatment providers);
-
detection bias:
blinding of outcome assessment;
-
attrition bias:
-
incomplete outcome data: we recorded the proportion of participants whose outcomes were not reported at the end of the study; we coded a satisfactory level of loss to follow‐up for each outcome as:
low risk of bias, if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms;
high risk of bias, if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms;
unclear risk of bias if loss to follow‐up was not reported;
-
-
reporting bias:
selective reporting of outcomes;
other possible sources of bias.
The 'Risk of bias' tool was applied independently by three review authors (KS, JF and MF) and differences resolved by discussion or by appeal to a third review author (CW or PC). Results are presented in a 'Risk of bias' summary graph. Results of meta‐analyses were interpreted with consideration of the findings with respect to risk of bias.
Measures of treatment effect
We used the following measures of the effect of treatment:
for time to event data, we planned to use the HR;
for dichotomous outcomes, we used the RR;
for continuous outcomes, we planned to use the mean difference between treatment arms.
Dealing with missing data
We did not impute missing outcome data for the primary outcome.
Assessment of heterogeneity
Heterogeneity between trials was assessed by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials that cannot be ascribed to sampling variation (Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there was evidence of substantial heterogeneity, the possible reasons for this were investigated and reported.
Assessment of reporting biases
There was an insufficient number of included trials to assess the potential for small study effects such as publication bias adequately.
Data synthesis
If sufficient, clinically similar studies were available, their results were pooled in meta‐analyses.
For time‐to‐event data, HRs would have been pooled using the generic inverse variance facility of RevMan 5 (RevMan 2011).
For any dichotomous outcomes, the RR was calculated for each trial and these were then pooled.
For continuous outcomes, the mean differences between the treatment arms at the end of follow‐up would have been pooled if all trials measured the outcome on the same scale, otherwise standardised mean differences would have been pooled.
Random‐effects models with inverse variance weighting was used for all meta‐analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
We included trials that used both platinum and non‐platinum chemotherapy and planned subgroup analyses, grouping the trials based on whether the chemotherapy was platinum or non‐platinum based.
Sensitivity analysis
We planned to perform sensitivity analyses excluding trials at high risk of bias
Results
Description of studies
Results of the search
Five hundred and sixty eight references were identified by searching the electronic databases (excluding duplicates). Reviewing the abstracts for these references identified 30 potentially eligible studies. Three trials were excluded (Baker 1978; Greggi 2000; Kumar 1998) owing to non‐randomisation of participants. One trial was excluded as it was a trial of differing Bryostatin‐1 schedules showing no activity (Armstrong 2003). Thus 26 eligible trials were included in this review (see Characteristics of included studies), comprising of 3766 randomised participants of which 631 were excluded from analysis in the published papers or abstracts. Thirteen trials were sufficiently similar and were included in meta‐analyses. No unpublished data from completed trials were identified.
Included studies
The RCTs included in this review dated from the 1970s to the present day with the number of patients ranging from five to 581. The trials included patients with locally recurrent, persistent or metastatic disease. Most of the patients had previously received radiotherapy to the primary site; however, since 1999 chemoradiotherapy has been the gold standard treatment for locally advanced disease and so patients included in more recent trials were likely to have previously received concurrent chemotherapy (generally platinum‐based) with radiotherapy.
The trials used a wide variety of designs and chemotherapy schedules. Five trials compared single‐agent platinum with a platinum‐containing combination (Alberts 1987; Cadron 2005; Long 2005; Moore 2004; Omura 1997) generally cisplatin, four trials compared single‐agent platinum with different formulations or doses (Bonomi 1985; Lira‐Puerto 1991; McGuire 1989; Thigpen 1989), two trials compared single‐agent platinum with a non‐platinum (Long 2006; Pfeiffer 1998), 10 trials compared a variety of non‐platinum‐containing regimens (Bond 1976; Freedman 1980; Greenberg 1977; Malkasian 1981; Moseley 1976; Omura 1978; Omura 1981; Palo 1976; Wallace 1978; Weiss 1992), four trials compared differing platinum combinations (Bloss 2002; Edmonson 1988; Monk 2008; Mountzios 2009) and the remaining trial compared non‐platinum single‐agent with platinum‐containing combination (Bezwoda 1986).
Outcomes were incompletely reported, with only response rate and some adverse event components being reported to an adequate level to include in meta‐analyses. Time to event data were poorly reported with most trials presenting median and quartile statistics. Median survival in all trials ranged from four months to 17 months. The intention was to compare survival data and time to progression data by comparing HRs, but these were only reported in two studies. Where data were not reported, we had hoped to be able to calculate them using the method described by Parmar 1998, by extracting the log rank P value, number of events and the number of subjects in the treatment and control groups; however, insufficient trial data (mean survival, probabilities or individual patient data) had been reported for this. Michiels 2005 showed that median survival times are not reasonable surrogate measures for meta‐analysis of survival. Eighteen of the 26 trials did not perform intention‐to‐treat analyses.
It had been anticipated that the majority of trials would be reporting toxicity with reference to Common Toxicity Criteria version 3.0 (CTCv3.0); however, this only became available in 2003 (replacing version 2.0, which ran from 1998 to 2003) and the majority of included trials (18/26) were published prior to 2003. Of these pre‐1998 studies eight made no attempt to grade toxicity (Bezwoda 1986; Bond 1976; Edmonson 1988; Freedman 1980; Greenberg 1977; Malkasian 1981; Moseley 1976; Omura 1978), two used an unspecified grading system (Palo 1976; Wallace 1978), and remainder used Eastern Cooperative Oncology Group (ECOG) (Lira‐Puerto 1991), Southwest Oncology Group (SWOG) (Alberts 1987; Weiss 1992) and Gynecologic Oncology Group (GOG) (Bonomi 1985; McGuire 1989; Omura 1981; Omura 1997; Thigpen 1989). None of the included trials published after 2003 (8/23) used CTCv3.0 and only two (Long 2005; Monk 2008) used CTCv2.0. The remainder using WHO (Mountzios 2009; Pfeiffer 1998), SWOG (Weiss 1992) or GOG (Bloss 2002; Moore 2004) scales. This wide variety made it very difficult to compare toxicities between regimens despite an attempt to convert all the differing grading scales into the CTCv2.0 equivalent.
Excluded studies
Four references were excluded, after obtaining the full text, for the following reasons:
Baker 1978, Greggi 2000 and Kumar 1998 were excluded as they were not randomised trials;
Armstrong 2003 was excluded as trial drugs were not cytotoxic agents.
For further details of all the excluded trials see the Characteristics of excluded studies.
Risk of bias in included studies
For risk of bias summary see Figure 1 and Figure 2.
1.
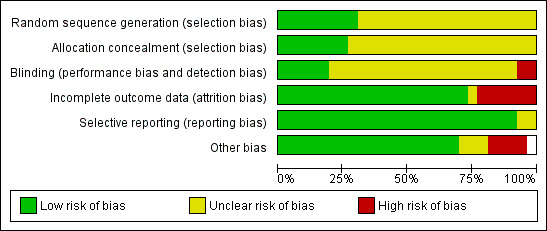
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
2.
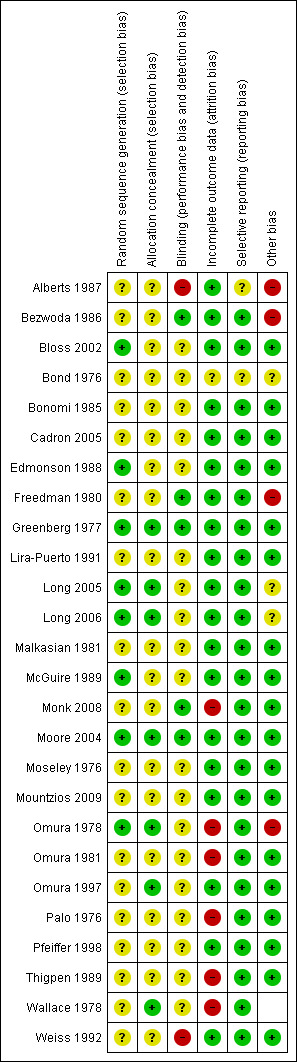
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
All included trials were described as randomised or involved the randomised assignment of treatment; however, there was only sufficient detail given in 10 (Bloss 2002; Greenberg 1977; Long 2005; Long 2006; McGuire 1989; Moore 2004; Omura 1978; Omura 1997; Thigpen 1989; Wallace 1978) out of the 26 trials to confirm that the randomisation procedure was adequate. Of these trials only five also gave details of allocation concealment, which was by central randomisation (Greenberg 1977; Long 2005; Omura 1997) or closed envelope technique (Omura 1978; Wallace 1978).
Blinding
Adequacy of blinding could not be assessed in any of the trials. Many trials reported using physical examination as part of the assessment of response introducing potential for significant bias. Whether or not the outcome assessor was blinded was not reported in any of the trials.
Incomplete outcome data
Twelve trials excluded 5% or less of their participants (Alberts 1987; Bloss 2002; Edmonson 1988; Freedman 1980; Greenberg 1977; Lira‐Puerto 1991; Long 2005; Long 2006; Malkasian 1981; Mountzios 2009; Omura 1997; Weiss 1992), eight trials excluded 6% to 19% (Bezwoda 1986; Cadron 2005; McGuire 1989; Monk 2008; Moore 2004; Omura 1981; Pfeiffer 1998; Thigpen 1989) and five trials excluded 20% to 49% (Bonomi 1985; Moseley 1976; Omura 1978; Palo 1976; Wallace 1978). In one trial (Bond 1976) the total number of participants was not given neither was it stated how many patients were excluded from the final analysis. Frequent reasons for exclusion were given as ineligibility, loss to follow‐up, inadequate data, lack of easily assessable disease and missing data.
In the older trials particularly there was a large drop‐out rate, such that in three trials nearly half the patients randomised were not evaluated (Bond 1976; Omura 1978; Wallace 1978). Patients were sometimes excluded because of a lack of measurable disease.
Selective reporting
All trials reported response rates but survival data reporting was inconsistent. Twenty‐one of 26 trials reported OS with two further trials reporting median survival (Alberts 1987; Bond 1976), but were not reported in sufficient detail to allow inclusion in meta‐analyses. Survival data were not reported in four trials (Moseley 1976; Omura 1981; Omura 1997; Weiss 1992). PFS was only reported in 11 trials (Bloss 2002; Bonomi 1985; Monk 2008; Moore 2004; Mountzios 2009; Omura 1981; Omura 1997; Palo 1976; Pfeiffer 1998; Thigpen 1989; Wallace 1978).
Where full survival data were not reported there was no justification for the choice of end points. Only two trials (Long 2005; Monk 2008) reported HRs and there was insufficient detail in the other trials to calculate using Parmars methods (Michiels 2005; Parmar 1998). This is discussed in more detail in the Results section.
Other potential sources of bias
A variety of first‐line treatment was given prior to these trials with a historical bias. In the older trials (patients enrolled pre‐1999) the majority of first‐line treatment was radiotherapy whereas post‐1999 most patients received primary chemoradiotherapy. In some trials (Bond 1976; Edmonson 1988; Palo 1976) cross‐over was allowed between arms.
Effects of interventions
In the meta‐analysis comparing various forms of chemotherapy, two trials (Bezwoda 1986; Greenberg 1977) assessing response rate and three trials (Alberts 1987; Omura 1997; Moore 2004) assessing toxicity had no responses in one of the arms, so we added 0.5 to these cells to allow calculation of an RR. This is the default zero‐cell correction within RevMan (RevMan 2011), and biases the result of the meta‐analysis towards no difference between the two types of chemotherapy being compared.
The methods of assessing response rates were variable, many of the older trials used clinical evaluation to a large extent with some radiological imaging (e.g. pelvic x‐ray) while more modern trials focused on CT or magnetic resonance imaging (MRI). Criteria for determining response also vary; however, three (Long 2005; Moore 2004; Omura 1997) of the five trials included in the meta‐analysis used the GOG definition based on the best response achieved at any point during treatment that had to be maintained for at least four weeks (complete response: disappearance of all lesions for at least four weeks; partial response: a greater than 50% reduction in the product of bi‐dimensional measurement of each lesion for at least four weeks; progressive disease: a greater than 50% increase in the product of the bi‐dimensional measurement of any lesion). One study (Alberts 1987) used SWOG criteria where the difference in size of lesions had to be 25% or greater to be progressive or partially responsive disease. Cadron 2005 used WHO criteria for determining response but gave no further details as to what this entailed. Response rates are early, easily measurable indicators often used as a surrogate for benefit but do not necessarily correlate with a survival advantage.
In Analysis 1.3, Analysis 1.4, Analysis 1.5, Analysis 1.6 and Analysis 1.7 complete and partial response were grouped together and were deemed 'response'.
1.3. Analysis.
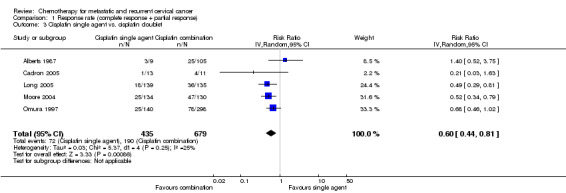
Comparison 1 Response rate (complete response + partial response), Outcome 3 Cisplatin single agent vs. cisplatin doublet.
1.4. Analysis.
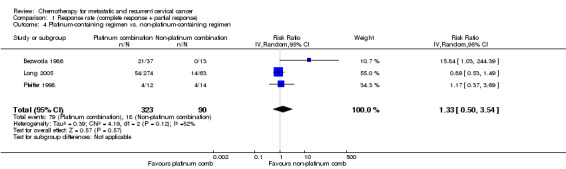
Comparison 1 Response rate (complete response + partial response), Outcome 4 Platinum‐containing regimen vs. non‐platinum‐containing regimen.
1.5. Analysis.
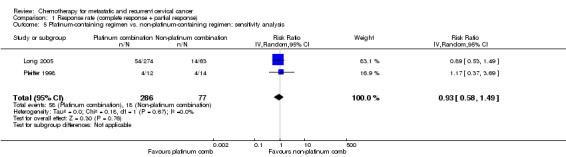
Comparison 1 Response rate (complete response + partial response), Outcome 5 Platinum‐containing regimen vs. non‐platinum‐containing regimen: sensitivity analysis.
1.6. Analysis.
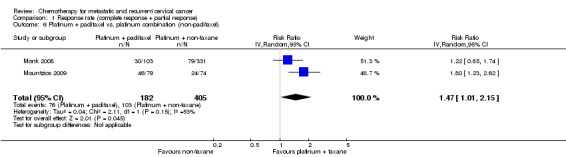
Comparison 1 Response rate (complete response + partial response), Outcome 6 Platinum + paclitaxel vs. platinum combination (non‐paclitaxel).
1.7. Analysis.
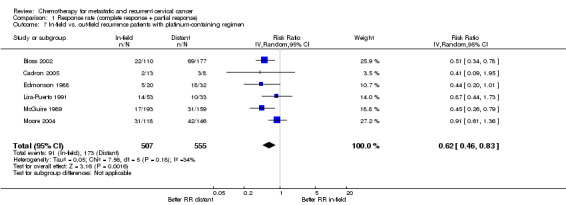
Comparison 1 Response rate (complete response + partial response), Outcome 7 In‐field vs. out‐field recurrence patients with platinum‐containing regimen.
Single‐agent versus combination chemotherapy
Meta‐analysis of 10 RCTs (Alberts 1987; Bezwoda 1986; Cadron 2005; Greenberg 1977; Long 2006; Malkasian 1981; Moore 2004; Omura 1981; Omura 1997; Wallace 1978), assessing 1438 participants, did not find any statistically significant difference in response rate between women who received single‐agent chemotherapy and those who received combination therapy (RR 0.94; 95% CI 0.57 to 1.55). The percentage of the variability in effect estimates that was because of heterogeneity between studies rather than chance may represent substantial heterogeneity (I2 = 67%). A wide variety of chemotherapy agents and doses were used across trials (see Table 1). Many of the trials were from an era when activity of agents was not known and so included inactive agents. The trials of Bezwoda 1986 and Greenberg 1977 were removed because of this in a sensitivity analysis, but the results were largely unchanged (RR 0.94; 95% CI 0.59 to 1.50; I2 = 66%). In addition many of these older trials were of poor quality, where more stringent reporting guidelines were not introduced until after these trials were completed.
1. Comparison of median survival: any single agent vs. any combination.
| Study | Single agent | Combination | ||||
| n | Median OS (months) |
Median PFS (months) |
n | Median OS (months) |
Median PFS (months) |
|
|
Alberts 1987 C vs. MC vs. MVBC |
9 | 17 (C) | nr | 54 (MVBC) 51 (MC) |
6.9 (MVBC) 7 (MC) |
5.4 (MVBC) 7.2 (MC) |
|
Bezwoda 1986 H vs. CMeth |
13 | 4 (H) | nr | 37 | 9 (CMeth) | nr |
|
Cadron 2005 C vs. CIF |
11 | 13 (C) | nr | 10 | 12.3 (CIF) | nr |
|
Greenberg 1977 A vs. AB |
9 | 4 (A) | nr | 11 | 4.3 (AB) | nr |
|
Long 2005 C vs. CTop vs. MVAC |
146 | 6.5 (C) | 2.9 (C) | 63 (MVAC) 147 (CTop) |
9.4 (MVAC) 9.4 (CTop) |
4.4 (MVAC) 4.6 (CTop) |
|
Malkasian 1981 A vs. VF |
20 | 5.9 (A) | 2.8 (A) | 21 | 9 (VF) | 2.9 (VF) |
|
Moore 2004 C vs. CP |
134 | 8.9 (C) | 3 | 130 | 9.9 (CP) | 4.9 |
|
Omura 1981 cyclo vs. AFV |
30 | 6.5 (cyclo) | nr | 31 | 7.6 (AFV) | nr |
|
Omura 1997 C vs. CMito vs. CI |
140 | 8 (C) | 3.2 | 147 (CMito) 151 (CI) |
7.3 8.3 |
nr (CMito) 4.6 (CI) |
|
Wallace 1978 A vs. AV vs. Acyclo |
63 | 5.9 (A) | 3.3 | 63 (AV) 52 (Acyclo) |
5.5 (AV) 7.3 (Acyclo) |
3.4 (AV) 3.9 (Acyclo) |
A: adriamycin; AB: adriamycin/bleomycin; Acyclo: adriamycin/cyclophosphamide; AFV: adriamycin/5‐fluorouracil/vincristine; AV: adriamycin/vincristine; C: cisplatin; CI: cisplatin/ifosfamide; CIF: cisplatin/ifosfamide/5‐fluorouracil; CMeth: cisplatin/methotrexate; CMito: cisplatin/mitolactol; CP: cisplatin/paclitaxel; CTop: cisplatin/topotecan; cyclo: cyclophosphamide; H: hydroxyurea; M: mitomycin; MC: mitomycin/cisplatin; MVAC: methotrexate/vinblastine/doxorubicin/cisplatin; MVBC: mitomycin/vincristine/bleomycin/cisplatin; nr: not reported; OS: overall survival; PFS: progression‐free survival; VF: vincristine/5‐fluorouracil.
There were no data comparing best supportive care with chemotherapy. Bezwoda 1986 compared hydroxyurea with cisplatin methotrexate and found no response to hydroxyurea and a difference in OS of nine versus four months. Moseley 1976 compared different CCNU‐based combinations again with no response (see Analysis 1.1; Analysis 1.2).
1.1. Analysis.
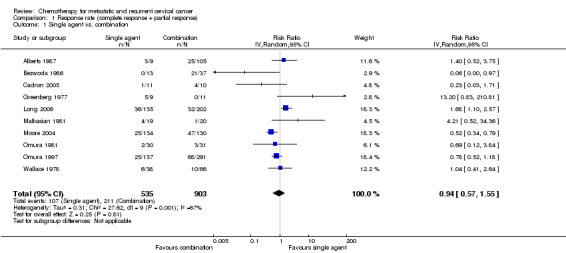
Comparison 1 Response rate (complete response + partial response), Outcome 1 Single agent vs. combination.
1.2. Analysis.
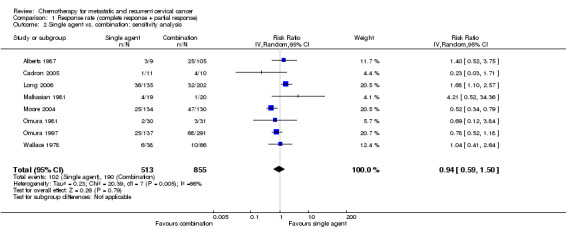
Comparison 1 Response rate (complete response + partial response), Outcome 2 Single agent vs. combination: sensitivity analysis.
Cisplatin single‐agent versus cisplatin combination chemotherapy
Meta‐analysis of five RCTs (Alberts 1987; Cadron 2005; Long 2005; Moore 2004; Omura 1997), assessing 1114 participants, found that the proportion of women who responded to treatment was significantly lower in the group who received chemotherapy as a single agent than in the group who received combination chemotherapy (RR 0.60; 95% CI 0.44 to 0.81) (Table 2). The percentage of the variability in effect estimates that was because of heterogeneity between studies rather than sampling error (chance) was of little importance (I2 = 25%) (Analysis 1.3).
2. Comparison of median survival: cisplatin single agent vs. cisplatin combination.
| Study | Single‐agent cisplatin | Cisplatin combination | ||||
| n | Median OS (months) |
Median PFS (months) |
n | Median OS (months) |
Median PFS (months) |
|
|
Alberts 1987 C vs. MC vs. MVBC |
9 | 17 | nr | 54 (MVBC) 51 (MC) |
6.9 (MVBC) 7 (MC) |
5.4 (MVBC) 7.2 (MC) |
|
Cadron 2005 C vs. CIF |
11 | 13 | nr | 10 | 12.3 | nr |
|
Long 2005 C vs. CT |
146 | 6.5 | 2.9 | 147 | 9.4 | 4.6 |
|
Moore 2004 C vs. CP |
134 | 8.9 | 3 | 130 | 9.9 | 4.9 |
|
Omura 1997 C vs. CMito vs. CI |
140 | 8 | 3.2 | 147 (CMito) 151 (CI) |
7.3 8.3 |
nr (CMito) 4.6 (CI) |
C: cisplatin; CI: cisplatin/ifosfamide; CIF: cisplatin/ifosfamide/5‐fluorouracil; CMito: cisplatin/mitolactol; CP: cisplatin/paclitaxel; CT: cisplatin/topotecan; MC: mitomycin/cisplatin; MVBC: mitomycin/vincristine/bleomycin/cisplatin; nr: not reported; OS: overall survival; PFS: progression‐free survival.
Platinum versus non‐platinum chemotherapy
Meta‐analysis of three RCTs (Bezwoda 1986; Long 2005; Pfeiffer 1998), assessing 413 participants, did not find any statistically significant difference in response rate between women who received platinum‐based chemotherapy and those who received non‐platinum chemotherapy (RR 1.33; 95% CI 0.50 to 3.54). The percentage of the variability in effect estimates that was because of heterogeneity between studies rather than chance may represent moderate heterogeneity (I2 = 52%) (Table 3). Exclusion of the Bezwoda 1986 trial data (which was generally of poor quality and showed no response at all to hydroxyurea) following a sensitivity analysis removed all of the heterogeneity (I2 = 0%). It should be noted that the MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) arm of the Long 2005 trial was stopped early because of toxicity and patient numbers are small (see Analysis 1.4; Analysis 1.5).
3. Comparison of median survival: platinum vs. non‐platinum‐containing regimens.
| Study | Platinum containing | Non‐platinum containing | ||||
| n | Median OS (months) |
Median PFS (months) |
n | Median OS (months) |
Median PFS (months) |
|
|
Bezwoda 1986 H vs. CMeth |
37 | 11 (CMeth) | nr | 13 | 4 (H) | nr |
|
Long 2005/Long 2006 C vs. CT vs. MVAC |
146 147 |
6.5 (C) 9.4 (CT) |
2.9 (C) 4.6 (CT) |
63 | 9.4 (MVAC) | 4.4 |
|
Pfeiffer 1998 Carbo vs. Ten |
12 | 9.3 (Carbo) | 4.6 | 14 | 9.6 (Ten) | 4.0 |
C: cisplatin; Carbo: carboplatin; CMeth: cisplatin/methotrexate; CT: cisplatin/topotecan; H: hydroxyurea; MVAC: methotrexate/vinblastine/doxorubicin/cisplatin; nr: not reported; OS: overall survival; PFS: progression‐free survival; Ten: teniposide.
Cisplatin combination with or without paclitaxel
Meta‐analysis of two RCTs (Monk 2008; Mountzios 2009), assessing 587 participants, found that the proportion of women who responded to treatment was statistically significantly lower in the group who received platinum plus paclitaxel than in the group who received combination platinum‐containing regimens that did not include paclitaxel (RR 1.47; 95% CI 1.01 to 2.15). The percentage of the variability in effect estimates that was because of heterogeneity between studies rather than chance may represent moderate heterogeneity (I2 = 52%) (Table 4). However, in one trial a paclitaxel triplet was compared with a no‐paclitaxel doublet and so this explains some of the heterogeneity. Thus a combined plot should be interpreted with caution (see Analysis 1.6).
4. Comparison of median survival: cisplatin combination including or excluding a taxane.
| Study | Cisplatin + other | Cisplatin + taxane | P value | ||||
| n | Median OS (months) |
Median PFS (months) |
n | Median OS (months) |
Median PFS (months) |
||
|
Monk 2008 CV vs. CT vs. CG vs. CP |
117 (CV) 118 (CT) 119 (CG) |
10‐10.3 | 4.9 (CV) 4.6 (CT) 4.7 (CG) |
118 (CP) | 12.9 | 5.8 | ns |
|
Mountzios 2009 CI vs. CIP |
74 (CI) | 13.2 | 6.3 | 79 (CIP) | 15.4 | 7.9 | 0.048 (OS) 0.023 (PFS) |
CG: cisplatin/gemcitabine; CI: cisplatin/ifosfamide; CIP: cisplatin/ifosfamide/paclitaxel; CP: cisplatin/paclitaxel; CT: cisplatin/topotecan; CV: cisplatin/vinorelbine; OS: overall survival; PFS: progression‐free survival; ns: not significant
In‐field versus out‐of‐field responses with platinum‐containing chemotherapy
Some authors hypothesised that response rates to chemotherapy will be less in previously irradiated areas therefore some trials performed a subgroup analysis to compare in‐radiotherapy‐field versus out‐of‐field response rates. In order to attempt to explore this it was decided to only look at platinum‐containing regimens, which were selected because they are the most widely used clinically and have demonstrable activity. Meta‐analysis of six RCTs (Bloss 2002; Cadron 2005; Edmonson 1988; Lira‐Puerto 1991; McGuire 1989; Moore 2004), assessing 1062 participants, found that the proportion of women who responded to treatment was significantly lower for recurrences within the pelvic field compared with disease outside of the pelvic radiotherapy field (RR 0.62; 95% CI 0.46 to 0.83). The percentage of the variability in effect estimates that was because of heterogeneity between studies rather than chance may represent moderate heterogeneity (I2 = 34%). Two trials were excluded from the meta‐analysis because of incomplete data (Bezwoda 1986; Pfeiffer 1998). Of note the majority of patients had not received prior chemotherapy (77.2%) with their radiotherapy. Where patients had had previous chemotherapy this was generally cisplatin as part of chemoradiotherapy, rather than chemotherapy alone with palliative intent (see Analysis 1.7 and Table 5).
5. Comparison of different single‐agent cisplatin regimens.
| Study | Platinum / dose (mg/m2) | n | RR (%) | OS (months) |
PFS (months) |
TP (months) |
| Alberts 1987 | C 50 d1 q21 | 9 | 33 | 17 | nr | nr |
| Bonomi 1985 | C 50 d1 q21 C 100 d1 q21 C 20 d1‐5 q21 |
150 166 128 |
20 31.4 25 |
7.1 7.0 6.1 |
nr | 3.7 4.6 3.9 |
| Cadron 2005 | C 37.5 d1, 2 q28 | 11 | 9 | 13 | nr | nr |
| Lira‐Puerto 1991 | Carbo 400 d1 q28 I 300 d1 q 28 |
48 41 |
26 30 |
7.5 7.6 |
nr | 5.5 6 |
| Long 2005 | C 50 d1 q21 | 146 | 13 | 6.5 | 2.9 | nr |
| McGuire 1989 | Carbo 400/340 d1 q28 I 270/230 d1 q28 |
175 177 |
15.4 10.8 |
6.5 5.6 |
nr | 2.7 3.0 |
| Moore 2004 | C 50 d1 q21 | 134 | 19 | 8.9 | 3.0 | nr |
| Omura 1997 | C 50 d1 q21 | 140 | 17.8 | 8 | nr | nr |
| Thigpen 1989 | C 50 d1 (24 h) q21 C 50 d1 (1 mg/minute) q21 |
163 168 |
18 17 |
6.4 6.2 |
nr | 3.5 2.9 |
| Pfeiffer 1998 | Carbo 400 d1 q28 | 12 | 33 | 9.3 | 4.6 | 4 |
C: cisplatin; Carbo: carboplatin; d: day; h: hour; I: iproplatin; nr: not reported; OS: overall survival; PFS: progression‐free survival; RR: risk ratio; TP: time to progression.
Overall survival, progression‐free survival and time to progression
OS and PFS was incompletely reported and there was insufficient information across trials to allow pooling of results.
OS with cisplatin alone was 6.5 to 9 months (> 11 trials) with PFS of approximately three months (additional tables). With cisplatin combination OS was 7 to 10 months with PFS of 4.6 to 4.9 months. The Alberts 1987 and Cadron 2005 trials were excluded as the numbers were small and appeared to be outliers to other studies with greater numbers.
When comparing cisplatin‐containing regimens with non‐platinum‐containing regimens there was marked heterogeneity owing to different activities. Again ranges of OS were 4 to 9.6 months and PFS 2.9 to 4.6 months.
The addition of a taxane led to OS of 12.9 to 15.4 months with PFS of 5.8 to 7.9 months. However, these were based on more recent trials (Monk 2008; Mountzios 2009) rather than older trials.
Some trials compared different cisplatin or carboplatin doses. The most commonly used was cisplatin 50 mg/m2 day one every 21 days with median OS of eight months and median response rates of 19%. This is a relatively low dose compared with cisplatin doses in other tumour sites. Higher doses of cisplatin were explored by Bonomi 1985 yielding a higher response rate of 31% but no difference in OS (seven months). However, this was one study with limited power to detect differences owing to low patients numbers and high drop‐out rates and so the question remains unanswered. Carboplatin gave similar median response rates of 26% with median OS of 7.5 months.
HRs were only reported in two trials (Monk 2008; Long 2005). Long 2005 found a survival advantage of cisplatin/topotecan over cisplatin alone in patients with recurrent or metastatic cervical cancer; with a PFS of 2.9 versus 4.6 months (HR 0.76) and OS of 6.5 versus 9.4 months (HR 0.76). Monk 2008 compared three chemotherapy doublets (cisplatin/vinorelbine, cisplatin/gemcitabine and cisplatin/topotecan) with the standard of cisplatin/paclitaxel. They found that no one doublet was statistically significantly superior but there was a trend in PFS, OS and response rate favouring the control arm of cisplatin/paclitaxel (Table 6).
6. Comparison of four cisplatin‐containing doublets.
| Regimen | Hazard ratio PFS | Hazard ratio OS |
| CV vs. CP | 1.357 | 1.147 |
| CG vs. CP | 1.394 | 1.322 |
| CT vs. CP | 1.268 | 1.255 |
*From Monk 2008. CG: cisplatin/gemcitabine; CP: cisplatin/paclitaxel; CT: cisplatin/topotecan; CV: cisplatin/vinorelbine; OS: overall survival; PFS: progression‐free survival.
Toxicity
Among all the studies there were 23 deaths from non‐cancer‐related causes. Fifteen of these were reported as treatment‐related with seven deaths from neutropenic sepsis (often with secondary acute renal failure), two from haemorrhage owing to severe thrombocytopenia, two from pulmonary toxicity owing to bleomycin, one from acute renal failure following cisplatin and one death owing to ifosfamide‐induced encephalopathy. Four deaths owing to severe haematological toxicity were reported in patients receiving MVAC in the GOG179 trial (Long 2006) causing this arm in the trial to be closed early after only sixty‐three patients had been treated with the regimen. Eight other non‐cancer deaths were reported although not linked directly to treatment by the authors, including two fatal pulmonary emboli (Long 2005) and six deaths in one trial (Edmonson 1988) owing to cardiac arrests and cerebrovascular accidents in patients who had been receiving cisplatin/bleomycin (two deaths) or cisplatin/bleomycin/doxorubicin/cyclophosphamide (four deaths)).
Because of the heterogeneity in types and combinations of cytotoxic agents used, with many trials only containing small patient numbers and with a wide variety of toxicity scoring systems used it was difficult to analyse and compare the toxicity data. With these provisos an attempt was made to compare toxicities between trials were reported in sufficient detail. This was only possible for the trials involving single‐agent cisplatin versus cisplatin‐containing combinations.
Cisplatin single‐agent versus cisplatin‐combination chemotherapy
Unsurprisingly cisplatin combinations generally tended to produce higher rates of toxicity than cisplatin alone.
Grade 3/4 neutropenia
Meta‐analysis of four RCTs (Alberts 1987; Long 2005; Moore 2004; Omura 1997), assessing 1073 participants, found that there was significantly less risk of severe neutropenia in women who received chemotherapy as a single agent than those who received combination chemotherapy (RR 0.04; 95% CI 0.02 to 0.12). The percentage of the variability in effect estimates that was because of heterogeneity between studies rather than chance may represent moderate heterogeneity (I2 = 32%) (Analysis 2.1).
2.1. Analysis.
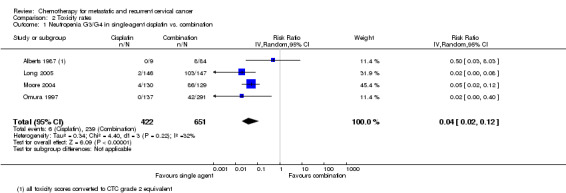
Comparison 2 Toxicity rates, Outcome 1 Neutropenia G3/G4 in single‐agent cisplatin vs. combination.
Grade 3/4 thrombocytopenia
Meta‐analysis of four RCTs (Alberts 1987; Long 2005; Moore 2004; Omura 1997), assessing 1104 participants, found that there was significantly less risk of severe thrombocytopenia in women who received chemotherapy as a single agent than those who received combination chemotherapy (RR 0.16; 95% CI 0.05 to 0.48). The percentage of the variability in effect estimates that was because of heterogeneity between studies rather than chance may represent moderate heterogeneity (I2 = 49%) (Analysis 2.2).
2.2. Analysis.
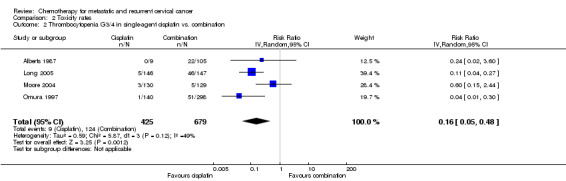
Comparison 2 Toxicity rates, Outcome 2 Thrombocytopenia G3/4 in single‐agent cisplatin vs. combination.
Grade 3/4 infection
Meta‐analysis of two RCTs (Long 2005; Moore 2004), assessing 552 participants, found that there was significantly less risk of severe infection in women who received chemotherapy as a single agent than those who received combination chemotherapy (RR 0.42; 95% CI 0.22 to 0.81). The percentage of the variability in effect estimates that was because of heterogeneity between studies rather than chance was not important (I2 = 0%) (see Analysis 2.3).
2.3. Analysis.
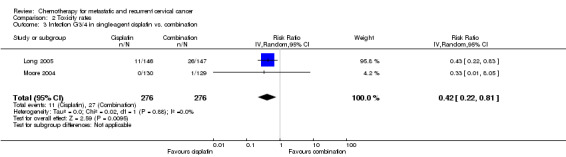
Comparison 2 Toxicity rates, Outcome 3 Infection G3/4 in single‐agent cisplatin vs. combination.
Grade 3/4 renal dysfunction
Meta‐analysis of three RCTs (Long 2005; Moore 2004; Omura 1997), assessing 980 participants, did not find any statistically significant difference in the risk of severe renal dysfunction between women who received single‐agent chemotherapy and those who received combination chemotherapy (RR 0.81; 95% CI 0.46 to 1.41). The percentage of the variability in effect estimates that was because of heterogeneity between studies rather than chance was not important (I2 = 0%) (see Analysis 2.4).
2.4. Analysis.
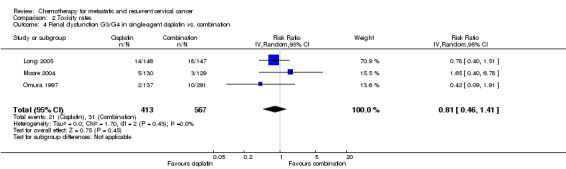
Comparison 2 Toxicity rates, Outcome 4 Renal dysfunction G3/G4 in single‐agent cisplatin vs. combination.
Grade 3/4 neuropathy
Meta‐analysis of two RCTs (Long 2005; Moore 2004), assessing 552 participants, did not find any statistically significant difference in the risk of severe neuropathy between women who received single‐agent chemotherapy and those who received combination chemotherapy (RR 1.39; 95% CI 0.45 to 4.33). The percentage of the variability in effect estimates that was because of heterogeneity between studies rather than chance was not important (I2 = 0%) (Analysis 2.5).
2.5. Analysis.
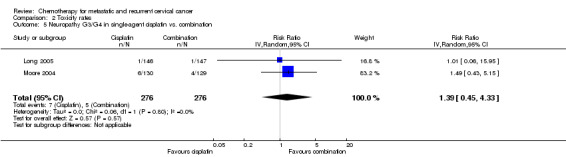
Comparison 2 Toxicity rates, Outcome 5 Neuropathy G3/G4 in single‐agent cisplatin vs. combination.
In those trials using carboplatin at 400 mg/m2 there was a tendency for severe toxicity in those patients with pre‐existing renal impairment highlighting the importance of calculating carboplatin dose based on creatinine clearance using the Calvert formula rather than a milligram per square metre of body surface area (mg/m2) dose as with other cytotoxic agents (see Analysis 2.5).
Quality of life
The effect of treatment of QoL is of key importance when deciding on treatment of patients where cure is not a realistic goal. Only three trials (Long 2005; Monk 2008; Moore 2004) out of 26 reported any QoL information on trial patients. Similar tools were used by these three trials for monitoring (Functional Assessment of Cancer Therapy ‐ General (FACT‐G), Functional Assessment of Cancer Therapy ‐ Cervix (FACT‐Cx), NEUROTOX, Brief Pain Inventory (BPI)).
Long 2005 and Moore 2004 found that patients with metastatic or recurrent disease had reduced QoL scores compared to the general population prior to starting treatment (particularly with respect to loss of function and physical wellbeing). In the trial of cisplatin with or without topotecan (Long 2005) there was an association between higher QoL scores at baseline and OS with those in the highest scoring quartile having a 47% lower hazard of death when compared with the lowest scoring quartile (HR 0.53; 95% CI 0.36 to 0.78; P = 0.001). Among patients who complete protocol therapy the serial measurements of QoL scores tend to remain stable no matter which treatment arm they had been allocated to. There was also no difference in QoL scores when comparing single‐agent cisplatin with different cisplatin combinations at any time point. The authors hypothesised that improved response rates compensate for the increased toxicity of combination regimens.
Discussion
Summary of main results
There are no data comparing best supportive care with chemotherapy.
Survival data were incompletely reported so there is an absence of evidence for important outcomes such as OS and PFS.
Numerous older trials had small patient numbers and significant potential for bias.
Large numbers of different chemotherapy agents and combinations have been explored making for heterogeneous data. It is thus difficult to interpret pooled results satisfactorily. Cisplatin‐based regimens are the most widely used currently and historically and therefore this review has concentrated on these trials.
In terms of response rates some non‐platinum regimens were equivalent but toxicity was significantly higher, for example the MVAC arm was closed early owing to four treatment‐related deaths (of 63 patients) (Long 2006).
The most widely used cisplatin regimen was 50 mg/m2 day 1 q21days. Some trials hinted that higher doses had similar survivals, but this could not be confirmed or refuted owing to poor reporting of time to event data. There was no direct comparison between cisplatin and carboplatin but response rate with single‐agent carboplatin was similar to single‐agent cisplatin.
Evidence to suggest that cisplatin combination improves response rates when compared with single‐agent cisplatin, but further trials are needed to ascertain whether this is the case for OS and PFS. However no one combination is significantly different from others (Monk 2008), although some outcomes may be improved with cisplatin/paclitaxel. Thus toxicity profile, scheduling and co‐morbidity are important when individualising therapy.
Combination regimens are more toxic than single‐agent regimens but in the limited reported data this does not appear to impact on QoL, although further trials reporting QoL outcomes are required.
Nine of the 23 included trials performed subgroup analyses comparing response rates between previously irradiated and non‐irradiated sites. Eight of these found no significant difference in response rate by site of recurrence although there was a trend towards improved response when the main site of disease was beyond the previously irradiated pelvis.
Overall completeness and applicability of evidence
This review included patients enrolled into trials with local relapse, distant relapse and primary metastatic disease. The relapsed patients may have had primary treatment with surgery, radiotherapy or chemoradiotherapy. There are no data comparing responses in these different groups. Since 1999, cisplatin‐based chemoradiotherapy has been the preferred treatment in patients presenting with Stage 1b2 or above. In this analysis less than 30% of patients had received prior chemoradiotherapy and thus the generalisability of these results remains uncertain. It is likely that response will be less especially in patients with short disease‐free intervals or in previously irradiated sites. Conversely there are no data to suggest that there is a lack of response in all patients who have received prior chemoradiotherapy.
There is no direct evidence documenting a comparison of chemotherapy and best supportive care. Cisplatin‐based combinations appear to have the highest response rates but median OS remains poor at nine to 12 months with PFS of four to five months. Thus metastatic cervical cancer remains relatively chemo‐insensitive.
There is a lack of data on QoL and also survival outcomes. Optimising QoL is essential in this group of palliative patients. Only three trials reported QoL data and new trials need to include this as an outcome. The limited data reported have indicated that cisplatin combination chemotherapy is not detrimental to patient experience, but in the absence of adequate QoL and survival data results need to be interpreted with caution.
Quality of the evidence
The quality of the evidence is low as important outcomes such as OS were incompletely reported and the chemotherapeutic agents used in the different trials varied markedly making comparisons difficult and the presence of heterogeneity across trials a problem. More recent trials, which are most relevant to our results and reflect current practice, are of a more acceptable quality, but most still did not report outcomes satisfactorily.
For example, the most widely used cisplatin regimen was 50 mg/m2 day 1 q21days. It is not possible to comment on optimal cisplatin owing to inadequate survival data. There were no direct comparisons between cisplatin and carboplatin. Although response rates with single‐agent carboplatin were similar to single‐agent cisplatin there is insufficient evidence to conclude their equivalence. Further trials on the optimal platinum compound and dose would be clinically useful.
Only in very recent studies, comparing cisplatin combinations, are survival and QoL outcomes reported adequately. Toxicity was reported using different scales and the data were very heterogeneous
Potential biases in the review process
An extensive literature search was performed in EMBASE, MEDLINE, LILACS, CENTRAL, Physician Data Query and Meta‐Register. No un‐published data were identified. In order to minimise bias a thorough search of grey literature was performed. Strict inclusion and exclusion criteria were also used to make sure that all appropriate trials were included. We restricted the included studies to RCTs as they provided the strongest level of evidence available. Hence we have attempted to reduce bias in the review process.
The greatest threat to the validity of the review is likely to be the possibility of publication bias, that is studies that did not find the treatment to have been effective may not have been published. We were unable to assess this possibility as the number of trials in each comparison was limited.
Agreements and disagreements with other studies or reviews
Other reviews and meta‐analyses of chemotherapy in metastatic or recurrent cervical cancer have been undertaken, all of which concur with the importance of basing treatment on a platinum compound and highlighting the improved PFS when cisplatin used in combination rather than as a single agent (Tewari 2005).
Pectasides 2008 reviewed chemotherapy for recurrent cervical cancer, including Phase II non‐randomised data that has not been included in this review. His conclusions of single‐agent and combination therapy were similar to those of this review. That is, cisplatin appears to have the highest response rates and analogues of cisplatin, such as carboplatin or iproplatin, were active. Doxorubicin, paclitaxel and topotecan showed significant activity as single agents with 15% to 20% response rates. For combination chemotherapy cisplatin has been combined with 5‐fluorouracil (5‐FU) Weiss 1990, bleomycin, ifosfamide, gemcitabine, vinorelbine, paclitaxel and topotecan in Phase II and III trials. Many of these trials showed an advantage in terms of response rate for combination when compared with single‐agent cisplatin and in some there was also a PFS advantage (cisplatin plus ifosfamide (Omura 1997), cisplatin plus paclitaxel (Moore 2004) and cisplatin plus topotecan (Long 2006)). Only cisplatin plus topotecan showed a survival advantage over single‐agent cisplatin.
Many centres are using carboplatin‐based combinations in this group of patients despite there being no randomised evidence published to support this. Thus as part of this review of RCTs the Phase II data is not represented. Non‐randomised Phase II data suggest that carboplatin in combination with paclitaxel is active and well tolerated (Pectasides 2008; Tinker 2005). Moore 2007 published a retrospective study comparing cisplatin with carboplatin in combination with paclitaxel suggesting equivalent efficacy. One Phase III randomised trial (Saito 2010) is currently recruiting comparing carboplatin/paclitaxel with cisplatin/paclitaxel, which will provide further information on whether carboplatin can be substituted for cisplatin, improving toxicity profile without compromising outcome. This is a particularly important question because of the high frequency of renal impairment in patients with locally recurrent disease limiting their suitability for cisplatin‐containing regimens.
Mutch 2003 reviewed the role of gemcitabine in cervical cancer; the majority of the data being non‐randomised Phase II trials that are not represented in this paper. They conclude that gemcitabine has demonstrated little activity as a single agent but that it appears to act synergistically with cisplatin. Monk 2008 found similar results in terms of response rate, PFS and OS between cisplatin plus gemcitabine, cisplatin plus paclitaxel, cisplatin plus vinorelbine and cisplatin plus topotecan.
Cisplatin‐based combinations are currently the most widely used internationally, making the results of this review applicable.
Authors' conclusions
Implications for practice.
When treating patients with metastatic or recurrent cervical cancer combination cisplatin‐based chemotherapy could be a viable treatment option, but further trials that report adequate survival and QoL data are sought. The role of carboplatin in this context is not established. There is a trend to improved outcome with cisplatin plus paclitaxel combinations but the different toxicity profiles of the various combinations should be discussed individually.
Response rates and improvements in survival were low, therefore chemotherapy may not be appropriate for all patients, especially those with poorer performance status. The majority of patients in these trials were performance status 0 to 1, only small numbers of performance status 2 to 3 patients were included. Cisplatin‐based combination chemotherapy had significant toxicity.
Implications for research.
There is one ongoing randomised Phase III trial that should address the question of substitution of cisplatin by carboplatin in patients with metastatic and recurrent cervical cancer (Saito 2010). QoL, toxicity and survival will be critical end points for this trial and others.
In other tumour sites weekly regimens have shown better responses and toxicity profiles. This should be explored for cervical cancer.
Overall response rates and survival are poor and novel cytotoxic and biological agents need investigation. The evaluation of existing biological agents was outside the scope of this review; however, agents targeting angiogenesis, epidermal growth factor receptors, histone deacetylases, COX‐2 and m‐TOR are currently in clinical development. Far fewer molecularly targeted agents have been trialed in cervical cancer compared with other tumour sites, especially at the Phase III level and therefore none of these agents are currently approved for use in clinical practice.
Future trials need to stratify for and perform planned subgroup analysis with respect to previous treatment and site of recurrence.
Future trials should report HRs for survival data to facilitate meta‐analysis of pooled data.
What's new
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 2007 Review first published: Issue 10, 2012
| Date | Event | Description |
|---|---|---|
| 27 March 2014 | Amended | Contact details updated. |
Acknowledgements
We thank Jo Morrison for clinical and editorial advice, Jane Hayes for designing the search strategy and Gail Quinn and Clare Jess for their contribution to the editorial process. We are also grateful to Marielena Trivella and Phil Wiffen from the UK Cochrane Centre.
Appendices
Appendix 1. MEDLINE search strategy
1950 to January 2012
1 exp Uterine Cervical Neoplasms/ 2 (cervi* adj5 (cancer* or tumor* or tumour* or carcinoma* or neoplasm* or malignan*)).mp. 3 1 or 2 4 metasta*.mp. 5 recur*.mp. 6 (FIGO and IVB).mp. 7 4 or 5 or 6 8 3 and 7 9 exp Antineoplastic Agents/ 10 Antineoplastic Combined Chemotherapy Protocols/ 11 chemotherap*.mp. 12 drug therapy.fs. 13 cisplatin.mp. 14 carboplatin.mp. 15 gemcitabine.mp. 16 ifosfamide.mp. 17 paclitaxel.mp. 18 docetaxel.mp. 19 irinotecan.mp. 20 capecitabine.mp. 21 (5‐FU or 5‐Fluorouracil).mp. 22 topotecan.mp. 23 methotrexate.mp. 24 vinorelbine.mp. 25 doxorubicin.mp. 26 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 27 8 and 26 28 randomized controlled trial.pt. 29 controlled clinical trial.pt. 30 randomized.ab. 31 placebo.ab. 32 clinical trials as topic.sh. 33 randomly.ab. 34 trial.ti. 35 28 or 29 or 30 or 31 or 32 or 33 or 34 36 27 and 35
key:
mp = title, original title, abstract, name of substance word, subject heading word, unique identifier, fs = floating subheading, pt = publication type, ab = abstract, sh = subject heading, ti = title
Appendix 2. EMBASE search strategy
EMBASE Ovid 1980 to January 2012
1 exp uterine cervix tumor/ 2 (cervi* adj5 (cancer* or tumor* or tumour* or carcinoma* or neoplasm* or malignan*)).mp. 3 1 or 2 4 metasta*.mp. 5 recur*.mp. 6 (FIGO and IVB).mp. 7 4 or 5 or 6 8 exp cancer chemotherapy/ 9 exp antineoplastic agent/ 10 chemotherap*.mp. 11 dt.fs. 12 cisplatin.mp. 13 carboplatin.mp. 14 gemcitabine.mp. 15 ifosfamide.mp. 16 paclitaxel.mp. 17 docetaxel.mp. 18 irinotecan.mp. 19 capecitabine.mp. 20 (5‐FU or 5‐Fluorouracil).mp. 21 topotecan.mp. 22 methotrexate.mp. 23 vinorelbine.mp. 24 doxorubicin.mp. 25 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 26 3 and 7 and 25 27 random*.mp. 28 factorial*.mp. 29 crossover*.mp. 30 cross over*.mp. 31 cross‐over*.mp. 32 placebo*.mp. 33 (doubl* adj blind*).mp. 34 (singl* adj blind*).mp. 35 assign*.mp. 36 volunteer*.mp. 37 crossover procedure/ 38 double blind procedure/ 39 randomized controlled trial/ 40 single blind procedure/ 41 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 42 26 and 41
key ‐ mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer
Appendix 3. CENTRAL search strategy
CENTRAL Issue 1, 2012
#1 MeSH descriptor Uterine Cervical Neoplasms explode all trees #2 cervi* near/5 (cancer* or tumor* or tumour* or carcinoma* or neoplasm* or malignan*) #3 (#1 OR #2) #4 metasta* #5 recur* #6 FIGO and 1VB #7 (#4 OR #5 OR #6) #8 (#3 AND #7) #9 MeSH descriptor Antineoplastic Agents explode all trees #10 MeSH descriptor Antineoplastic Combined Chemotherapy Protocols explode all trees #11 chemotherap* #12 Any MeSH descriptor with qualifier: DT #13 cisplatin #14 carboplatin #15 gemcitabine #16 ifosfamide #17 paclitaxel #18 docetaxel #19 irinotecan #20 capecitabine #21 5‐FU or 5‐Fluorouracil #22 topotecan #23 methotrexate #24 vinorelbine #25 doxorubicin #26 (#9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25) #27 (#8 AND #26)
Data and analyses
Comparison 1. Response rate (complete response + partial response).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Single agent vs. combination | 10 | 1438 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.57, 1.55] |
| 2 Single agent vs. combination: sensitivity analysis | 8 | 1368 | Risk Ratio (IV, Random, 95% CI) | 0.94 [0.59, 1.50] |
| 3 Cisplatin single agent vs. cisplatin doublet | 5 | 1114 | Risk Ratio (IV, Random, 95% CI) | 0.60 [0.44, 0.81] |
| 4 Platinum‐containing regimen vs. non‐platinum‐containing regimen | 3 | 413 | Risk Ratio (IV, Random, 95% CI) | 1.33 [0.50, 3.54] |
| 5 Platinum‐containing regimen vs. non‐platinum‐containing regimen: sensitivity analysis | 2 | 363 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.58, 1.49] |
| 6 Platinum + paclitaxel vs. platinum combination (non‐paclitaxel) | 2 | 587 | Risk Ratio (IV, Random, 95% CI) | 1.47 [1.01, 2.15] |
| 7 In‐field vs. out‐field recurrence patients with platinum‐containing regimen | 6 | 1062 | Risk Ratio (IV, Random, 95% CI) | 0.62 [0.46, 0.83] |
Comparison 2. Toxicity rates.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Neutropenia G3/G4 in single‐agent cisplatin vs. combination | 4 | 1073 | Risk Ratio (IV, Random, 95% CI) | 0.04 [0.02, 0.12] |
| 2 Thrombocytopenia G3/4 in single‐agent cisplatin vs. combination | 4 | 1104 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.05, 0.48] |
| 3 Infection G3/4 in single‐agent cisplatin vs. combination | 2 | 552 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.22, 0.81] |
| 4 Renal dysfunction G3/G4 in single‐agent cisplatin vs. combination | 3 | 980 | Risk Ratio (IV, Random, 95% CI) | 0.81 [0.46, 1.41] |
| 5 Neuropathy G3/G4 in single‐agent cisplatin vs. combination | 2 | 552 | Risk Ratio (IV, Random, 95% CI) | 1.39 [0.45, 4.33] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alberts 1987.
| Methods | Randomised Phase II trial | |
| Participants | 119 women with incurable squamous cell carcinoma unsuitable for surgery or radiotherapy. No previous chemotherapy exposure | |
| Interventions | Arm 1: mitomycin C 10 mg/m2 iv d2, 44, vincristine 0.5 mg/m2 iv d2, 4, 44, 46, bleomycin 30 mg continuous iv infusion over 24 h for 4 days d1‐4, 43‐46, cisplatin 50 mg/m2 iv d1, 22, 43, 64 Arm 2: mitomycin C 12 mg/m2 iv d1, 43, cisplatin 50 mg/m2 iv d1, 22, 43, 64 Arm 3: cisplatin 50 mg/m2 iv d1, 22, 43, 64 |
|
| Outcomes | Response rate Response duration Median survival Toxicity (SWOG) |
|
| Notes | 5 ineligible patients (reasons not stated). Arm 3 stopped early owing to slow accrual. Not clear whether ITT analysis performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not documented |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not documented |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 ineligible patients (reasons not stated). Arm 3 stopped early owing to slow accrual. Not clear whether ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information |
| Other bias | High risk | Cisplatin arm stopped early despite appearing to have better results so only 9 patients evaluable |
Bezwoda 1986.
| Methods | Randomised Phase II trial 1982 to 1984 |
|
| Participants | 50 women with recurrent or metastatic cervical cancer | |
| Interventions | Arm 1: hydroxyurea 1.5 g/m2 po d1‐10, 14‐d rest period then maintenance with 1 g/m2 po d1‐14 q28 Arm 2: cisplatin 20 mg/m2 iv daily d1‐3 and methotrexate 100 mg/m2 iv d3 with leucovorin rescue |
|
| Outcomes | OS Response rates Toxicity |
|
| Notes | 3 patients were excluded (refused chemotherapy after randomisation). No ITT analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not documented |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 patients were excluded |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | High risk | Hydroxyurea (arm 1) arm stopped early owing to lack of response, only 12 patients randomised to cisplatin + methotrexate arm (arm 2), the remainder entered that arm as only treatment option once the hydroxyurea arm closed. Thus many randomised and trial results not used in meta‐analyses |
Bloss 2002.
| Methods | Randomised Phase II trial | |
| Participants | 303 women with advanced, recurrent or persistent squamous cell cancer of the cervix not suitable for curative treatment with radiotherapy or surgery | |
| Interventions | Arm 1: cisplatin 50 mg/m2 d1 and ifosfamide 5 g/m2 over 24 h with mesna 6 g/m2 q21 max 6 cycles Arm 2: cisplatin 50 mg/m2 d1 and ifosfamide 5 g/m2 over 24 h with mesna 6 g/m2 and bleomycin 30 U over 24 h q21 maximum 6 cycles |
|
| Outcomes | Response rate OS PFS Toxicity |
|
| Notes | 16 patients ineligible (11 wrong histology, 1 wrong primary tumour site, 2 inadequate pathology material, 1 inadequate renal function, 1 inadequate performance status). All patients were included in ITT analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised with equal probability |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 patients ineligible (11 ‐ wrong histology, 1 ‐ wrong primary tumour site, 2 ‐ inadequate pathology material, 1 ‐ inadequate renal function, 1 ‐ inadequate performance status). All patients were included in ITT analysis |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
Bond 1976.
| Methods | Randomised Phase II trial | |
| Participants | Number of participants not stated in the paper. Number included in the analysis was 41. Women with histologically confirmed squamous cell carcinoma of the cervix. Most had previously received radical radiotherapy followed by recurrence of local disease, distant metastases or both | |
| Interventions | Arm 1: doxorubicin 50 mg iv, vincristine 2 mg iv, methotrexate 50 mg iv all d1 q28 Arm 2: doxorubicin 50 mg iv, vincristine 2 mg iv, methotrexate 50 mg iv, bleomycin 15 mg iv all d1 q28 |
|
| Outcomes | Toxicity Response rates Median survival |
|
| Notes | Standard doses of drugs given regardless of height, weight or surface area in order to avoid risk of incorrect dose calculation when treatment administered by junior staff. No ITT analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not documented |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of participants not stated in the paper. Number included in the analysis was 41 |
| Selective reporting (reporting bias) | Unclear risk | Total number of trial participants not stated only those evaluable |
| Other bias | Unclear risk | No comparison of patient characteristics between the 2 groups given |
Bonomi 1985.
| Methods | Randomised Phase II trial | |
| Participants | 581 women with squamous cell carcinoma of the cervix considered incurable with surgery or radiation | |
| Interventions | Arm 1: cisplatin 50 mg/m2 iv q21 Arm 2: cisplatin 100 mg/m2 q21 Arm 3: cisplatin 20 mg/m2 iv daily d1‐5 q21 All arms to a maximum dose of 400 mg/m2 |
|
| Outcomes | Response rates Toxicity OS PFS |
|
| Notes | 54 excluded (5 no histological confirmation of tumour, 32 wrong cell type, 3 inadequate renal function, 8 second or wrong primary tumour, 2 inadequate performance status, 4 improper pre‐protocol treatment). There were a further 30 women who could not be evaluated (8 inadequate pathological material, 3 improper randomisation, 2 clerical error, 11 never received cisplatin, 1 removed by investigator, 5 inadequate data). ITT analysis not performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not documented |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 54 excluded. There were a further 30 women who could not be evaluated. ITT analysis not performed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No hint at any other possible biases |
Cadron 2005.
| Methods | Randomised Phase III trial | |
| Participants | 24 women with histologically confirmed cervical cancer with distant metastases after surgery or recurrence after radiotherapy | |
| Interventions | Arm 1: cisplatin 37.5 mg/m2 iv d1‐2 q28 Arm 2: cisplatin 37.5 mg/m2 iv d1‐2 and Ifosfamide 2 g/m2 iv d1‐2, mesna 0.5 g/m2, 5‐fluorouracil 500 mg/m2 d1‐2, folinic acid 30 mg/m2 d1‐2 q28 |
|
| Outcomes | Response rates Toxicity (WHO) OS |
|
| Notes | 3 patients ineligible (did not receive chemotherapy owing to rapid progression). ITT analysis performed. Trial stopped early owing to poor accrual | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not documented |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
Edmonson 1988.
| Methods | Randomised Phase II trial 1980 to 1985 |
|
| Participants | 91 women with advanced cervical carcinoma | |
| Interventions | Arm 1: bleomycin 20 U/m2 iv 3‐d infusion, cisplatin 60 mg/m2 d1 iv, doxorubicin 40 mg/m2 iv D1, cyclophosphamide 400 mg/m2 iv d1 q28 Arm 2: bleomycin 20 U/m2 iv 3‐d infusion, cisplatin 60 mg/m2 d1 iv q21 Bleomycin omitted after 4 cycles in both arms |
|
| Outcomes | Response rates OS |
|
| Notes | 1 patient excluded as ineligible owing to second primary cancer. No ITT analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dynamic allocation system |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
Freedman 1980.
| Methods | Randomised Phase II trial 1975 |
|
| Participants | 59 women with recurrent squamous cell cervical cancer | |
| Interventions | Arm 1: sequential cyclophosphamide 8 mg/kg iv d1‐5 q28 then adriamycin 80 mg/m2 iv d1 q28 then hexamethylmelamine 8 mg/kg po daily continuously Arm 2: sequential adriamycin 80 mg/m2 iv d1 q28 then cyclophosphamide 8 mg/kg iv d1‐5 q28 then hexamethylmelamine 8 mg/kg po daily continuously Arm 3: sequential hexamethylmelamine 8 mg/kg po daily continuously then cyclophosphamide 8 mg/kg iv d1‐5 q28 then adriamycin 80 mg/m2 iv d1 q28 Not stated prospectively what criteria would be for switching treatment (i.e. number of cycles versus progressive disease) |
|
| Outcomes | Response rates Survival time after chemotherapy start Survival from time of treatment change |
|
| Notes | 1 patient excluded (died prior to starting chemotherapy). No ITT analysis performed. Cross‐over between arms allowed. Reasons for switching chemotherapy agents varied and included disease progression, toxicity and cumulative doxorubicin dose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified by ease of disease evaluation then randomised |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not documented, but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | High risk | Patients allowed to switch between arms for variety of reasons including toxicity, cumulative doxorubicin dose and disease progression |
Greenberg 1977.
| Methods | Randomised Phase II trial | |
| Participants | 20 women with recurrent or metastatic epidermoid cervical cancer | |
| Interventions | Arm 1: adriamycin 30 mg/m2 iv cycle 1 then 45 mg/m2 iv cycle 2; 3rd and subsequent cycles 60 mg/m2 iv q21 Arm 2: bleomycin 10 U/m2 weekly plus adriamycin 30 mg/m2 iv cycle 1 then 45 mg/m2 iv cycle 2; 3rd and subsequent cycles 60 mg/m2 iv q21 |
|
| Outcomes | Response rates Toxicity OS |
|
| Notes | No patients excluded. ITT analysis performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not documented |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by Statistical Office of Western Cancer Study Group |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
Lira‐Puerto 1991.
| Methods | Randomised Phase II trial 1984 to 1987 |
|
| Participants | 89 women with recurrent measurable squamous cell cervical cancer | |
| Interventions | Arm 1: carboplatin 400 mg/m2 iv d1, 1 dose escalation if haematological toxicity ≤ grade 1 Arm 2: iproplatin 300 mg/m2 iv d1, 1 dose escalation if haematological toxicity ≤ grade 1 Cycle length not stated |
|
| Outcomes | Response rates Toxicity OS |
|
| Notes | 3 patients excluded (1 patient refused treatment after randomisation, 2 patients excluded as recurrence subsequently deemed to be benign fibrosis). ITT analysis not performed but excluded patients eligible for toxicity evaluation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not documented |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
Long 2005.
| Methods | Randomised Phase III trial 1999 to 2002 |
|
| Participants | 294 women with histologically confirmed advanced (Stage IVb), recurrent or persistent squamous cell, adenocarcinoma or adenosquamous cervical cancer unsuitable for curative treatment with surgery or radiotherapy, or both | |
| Interventions | Arm 1: cisplatin 50 mg/m2 iv d1 q21 Arm 2: cisplatin 50 mg/m2 iv d1 and topotecan 0.75 mg/m2 iv d1‐3 q21 |
|
| Outcomes | Response rates Toxicity OS |
|
| Notes | 8 patients excluded (1 institutional review board approval error, 2 second active malignancy present, 2 wrong histological type, 1 primary tumour other than cervical cancer, 2 inadequate tissue to confirm metastases). ITT analysis performed Trial originally had 3 arms but third arm (MVAC) was closed early owing to toxicity and is reported in detail in separate papers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients assigned with equal probability using a fixed‐block design |
| Allocation concealment (selection bias) | Low risk | GOG Statistical and Data Centre performed randomisation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8 patients excluded but balanced across groups. ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Unclear risk | MVAC arm closed early owing to toxic deaths |
Long 2006.
| Methods | Randomised Phase III trial 1999 to 2001 |
|
| Participants | 186 women with histologically confirmed advanced (Stage IVb), recurrent or persistent squamous cell, adenocarcinoma or adenosquamous cervical cancer unsuitable for curative treatment with surgery or radiotherapy, or both | |
| Interventions | Arm 1: cisplatin 50 mg/m2 iv d1 q21 Arm 2: cisplatin 50 mg/m2 iv d1 and topotecan 0.75 mg/m2 iv d1‐3 q21 Arm 3: methotrexate 30 mg/m2 iv d1, 15, 22, vinblastine 3 mg/m2 iv d2, 15, 22, doxorubicin 30 mg/m2 iv d2, cisplatin 70 mg/m2 iv d2 q28 Maximum 6 cycles given unless maximum cumulative dose of doxorubicin achieved or progressive disease or unacceptable toxicity |
|
| Outcomes | Response rates Toxicity OS |
|
| Notes | 6 patients ineligible (1 clerical error, 2 wrong cell type, 1 wrong primary, 1 inadequate pathology tissue, 1 second primary tumour. ITT analysis performed This paper reports the third arm of trial reported by Long 2006 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients assigned with equal probability using a fixed‐block design |
| Allocation concealment (selection bias) | Low risk | GOG Statistical and Data Centre randomly assigned patients |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 6 patients excluded balanced across arms. ITT analysis |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Unclear risk | MVAC arm closed early owing to toxic deaths |
Malkasian 1981.
| Methods | Randomised Phase II trial | |
| Participants | 41 women with recurrent and metastatic cervical cancer who had had as much surgery as was possible and radiotherapy where appropriate | |
| Interventions | Arm 1: 5‐fluorouracil 450 mg/m2 iv d1‐5 and vincristine 1.5 mg/m2 d1 and d5 q35 Arm 2: doxorubicin 60 mg/m2 iv d1 q21 |
|
| Outcomes | Response rates Toxicity OS Time to treatment failure |
|
| Notes | 2 patients excluded (1 refused treatment after randomisation, 1 immediately lost to follow‐up). ITT analysis was not performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not documented |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 patients excluded. No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
McGuire 1989.
| Methods | Randomised Phase III trial 1984 to 1987 |
|
| Participants | 394 patients with histologically confirmed squamous carcinoma of the cervix, recurrent disease following primary therapy or metastases. No previous chemotherapy allowed | |
| Interventions | Arm 1: carboplatin 400 mg/m2 d1 q28 Arm 2: iproplatin 270 mg/m2 d1 q28 |
|
| Outcomes | Response rates Response duration Toxicity (GOG) OS |
|
| Notes | 23 ineligible (7 non‐squamous histology, 4 other primaries, 8 previous other malignancy, 3 no pathological documentation of primary tumour, 1 previous chemotherapy). 10 additional patients not evaluable for toxicity or response. 7 did not receive treatment. 3 lost to follow‐up. ITT analysis not performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block design with balanced assignments |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 23 ineligible and 10 additional patients not evaluable for toxicity or response balanced across groups. ITT analysis not performed |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
Monk 2008.
| Methods | Randomised Phase III trial 2003 to 2007 |
|
| Participants | 513 women with advanced, recurrent or persistent squamous cell cervical cancer not suitable for surgery or radiotherapy | |
| Interventions | Arm 1: cisplatin 50 mg/m2 d2 plus paclitaxel 135 mg/m2 over 24 h d1 q21 Arm 2: cisplatin 50 mg/m2 d1 plus vinorelbine 30 mg/m2 d1, 8 q21 Arm 3: cisplatin 50 mg/m2 plus gemcitabine 1000 mg/m2 d1, 8 q21 Arm 4: cisplatin 50 mg/m2 plus topotecan 0.75 mg/m2 d1‐3 q21 |
|
| Outcomes | OS PFS Response rates Toxicity (WHO) QoL |
|
| Notes | First 41 patients enrolled were excluded from primary analysis. another 38 patients later found to be ineligible. 434 evaluable for efficacy. 9 patients never treated so excluded from toxicity assessment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not documented |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | First 41 patients enrolled were excluded from primary analysis. Another 38 patients later found to be ineligible |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
Moore 2004.
| Methods | Randomised Phase III trial | |
| Participants | 280 women with squamous cell Stage IVb, recurrent or persistent cervical cancer. No previous chemotherapy allowed | |
| Interventions | Arm 1: cisplatin 50 mg/m2 iv d1 q21 Arm 2: cisplatin 50 mg/m2 iv d1 and paclitaxel 135 mg/m2 iv d1 q21 |
|
| Outcomes | Response rates OS PFS Toxicity (WHO) |
|
| Notes | 16 ineligible patients (7 non‐squamous histology, 1 incorrect stage, 8 inadequate pathology). A further 5 patients did not receive chemotherapy, but were included in the ITT analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | With equal probability block design balancing sequences of assigned arms within institutions |
| Allocation concealment (selection bias) | Low risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 ineligible patients A further 5 patients did not receive chemotherapy, but were included in the ITT analysis |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
Moseley 1976.
| Methods | Randomised Phase II trial | |
| Participants | 5 women with squamous cell cervical cancer considered to be unsuitable for surgery. These women were part of a larger trial of 35 patients with squamous cell cancer in a variety of primary sites | |
| Interventions | Arm 1: CCNU (lomustine) 100‐130 mg/m2 po depending on bone marrow reserves d1 plus bleomycin 0.5 mg/kg im or iv d8, 11, 15, 18, 22, 25, 29, 32, 36, 39 q42 days Arm 2: CCNU 100‐130 mg/m2 po depending on bone marrow reserves d1 plus vinblastine 0.1 mg/kg iv d8, 15 plus methotrexate 0.5 mg/kg iv (maximum 25 mg) d8, 15 plus bleomycin 0.5 mg/kg im or iv d8, 15, 18, 22, 25, 29, 32 q56 days Maximum cumulative dose of bleomycin is 300 mg |
|
| Outcomes | Response rates Toxicity |
|
| Notes | 1 patient dropped out after cycle 1 day 1 treatment (single‐dose CCNU) to have treatment elsewhere and was considered unevaluable. No ITT analysis performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not documented |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
Mountzios 2009.
| Methods | Randomised Phase II clinical trial | |
| Participants | 153 women with metastatic, persistent or recurrent cervical cancer. performance status 0 to 2 | |
| Interventions | Arm 1: ifosfamide 1.5 g/m2 d1‐3 plus cisplatin 70 mg/m2 d2 q28 Arm 2: ifosfamide 1.5 g/m2 d1‐3 plus cisplatin 70 mg/m2 d2 plus paclitaxel 175 mg/m2 d1 q28 |
|
| Outcomes | Response rates PFS OS Toxicity |
|
| Notes | 4 patients excluded (3 had had previous chemotherapy, 1 previous primary cancer) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not documented |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
Omura 1978.
| Methods | Randomised Phase II trial | |
| Participants | 284 women with biopsy‐confirmed cancer of the genital tract not amenable to surgery or radiotherapy. 231 of these had cervical cancer. Previous chemotherapy allowed unless contained a nitrosourea | |
| Interventions | Arm 1: CCNU (lomustine) 100 mg/m2 q6 weeks Arm 2: methyl‐CCNU 150 mg/m2 q6 weeks |
|
| Outcomes | Response rates Toxicity |
|
| Notes | 82 patients with cervical cancer were ineligible or unevaluable. In the whole trial 102 patients were excluded owing to missing data in 77, chemotherapy violation in 22, clerical error in 2, and inadequate pathology material in 3. No ITT analysis was performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | closed envelope technique |
| Allocation concealment (selection bias) | Low risk | closed envelope technique |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 82 patients with cervical cancer were ineligible or women not evaluable. Further 102 patients were excluded as not evaluable |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | High risk | 102 patients of 284 not evaluable |
Omura 1981.
| Methods | Randomised Phase II trial | |
| Participants | 75 women with metastatic or recurrent squamous cell cervical cancer not amenable to surgery or radiotherapy | |
| Interventions | Arm 1: doxorubicin 50 mg/m2 iv d1, vincristine 1.5 mg/m2 iv d1, 8, 5‐fluorouracil 500 mg/m2 iv d1, 8 q21 Arm 2: cyclophosphamide 1100 mg/m2 iv d1 q21 |
|
| Outcomes | Response rates OS PFS Toxicity |
|
| Notes | 14 patients excluded (1 incomplete data, 2 ineligible for trial, 3 major protocol violation, 5 lost to follow‐up, 1 patient refused treatment after randomisation, 1 inter‐current illness preventing chemotherapy administration, 1 only evaluable for toxicity) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not documented |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14 patients excluded balanced across groups |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
Omura 1997.
| Methods | Randomised Phase II trial | |
| Participants | 454 women with Stage IVb, persistent or recurrent squamous cell cervical cancer not amenable to curative surgery or radiotherapy | |
| Interventions | Arm 1: cisplatin 50 mg/m2 iv q21 Arm 2: cisplatin 50 mg/m2 iv, mitolactol 180 mg/m2 po od d2‐6 q21 Arm 3: cisplatin 50 mg/m2 iv, ifosfamide 5 g/m2 iv over 24 h with mesna 6 g/m2 q21 Maximum 6 cycles |
|
| Outcomes | Response rates OS PFS Toxicity |
|
| Notes | 16 patients excluded (ineligible on grounds of: 2 wrong stage, 9 wrong cell type, 2 wrong primary site, 2 previous chemotherapy, 1 second primary cancer). 10 further patients never received chemotherapy but were included in an ITT analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Central randomisation with equal probity |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 16 patients excluded and 10 further patients never received chemotherapy but were included in an ITT analysis balanced across groups |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
Palo 1976.
| Methods | Randomised Phase II trial | |
| Participants | 48 women with cervical cancer | |
| Interventions | Arm 1: adriamycin 75 mg/m2 d1 + bleomycin 15 mg/m2 d1, 8 q21 Arm 2: cyclophosphamide 1.2 g/m2 d1 + vincristine 1.4 mg/m2 d1, 8 q21 |
|
| Outcomes | Response rates OS PFS Toxicity |
|
| Notes | 14 patients excluded from analysis (3 lack of measurable disease, 11 lost to follow‐up). 18 patients also allowed to cross‐over between arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not documented |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 14 patients excluded from analysis balanced across group |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
Pfeiffer 1998.
| Methods | Randomised Phase II trial March 1990 to May 1994 |
|
| Participants | 28 women with recurrent or advanced cervical cancer | |
| Interventions | Arm 1: carboplatin 400 mg/m2 iv d1 q28 Arm 2: teniposide 125 mg/m2 iv d1‐3 q28 |
|
| Outcomes | Response rates OS PFS Toxicity (WHO) |
|
| Notes | 2 patients ineligible (1 severe renal impairment, 1 poor performance status). No ITT analysis performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not documented |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
Thigpen 1989.
| Methods | Randomised Phase II trial April 1982 to July 1985 |
|
| Participants | 331 women with advanced or recurrent squamous cell cervical cancer no longer amenable to control by surgery or radiotherapy | |
| Interventions | Arm 1: cisplatin 50 mg/m2 iv rate 1 mg/minute q21 days Arm 2: cisplatin 50 mg/m2 iv rate over 24 h q21 days |
|
| Outcomes | Response rates OS PFS Toxicity |
|
| Notes | 63 patients deemed ineligible (7 other primary site, 4 second primary, 26 wrong histological subtype, 26 no documentation of cervical primary, 1 elevated blood nitrogen urea at entry, 1 GOG performance status 4 at entry). 6 further patients with unevaluable (1 clerical error at entry, 1 never treated, 1 inadequate pathology, 2 inadequate data submitted, 1 not documented reason for unevaluability) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Latin square arrangement balancing the sequence of assigned regimens within and across institutions |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 63 patients excluded as ineligible and further 6 patients were unevaluable balanced across groups |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
Wallace 1978.
| Methods | Randomised Phase II trial September 1974 to June 1976 |
|
| Participants | 326 women with advanced, recurrent or persistent squamous cell cervical cancer not suitable for surgery or radiotherapy | |
| Interventions | Arm 1: adriamycin 60 mg/m2 iv q21 Arm 2: adriamycin 60 mg/m2 iv, vincristine 1.5 mg/m2 iv q21 Arm 3: adriamycin 60 mg/m2 iv, cyclophosphamide 500 mg/m2 iv q21 |
|
| Outcomes | OS PFS Response rates Toxicity (WHO) |
|
| Notes | 65 patients ineligible (more than half for wrong histological subtype, remainder because of wrong primary, 2nd primary, elevated urea or creatinine, poor risk at entry or no evidence of progression after previous treatment; breakdown of numbers not given). Further 69 patients deemed to be not evaluable (inadequate data collection, therapy not initiated, insufficient follow‐up after first course, improper randomisation, clerical errors and others; no specific breakdown in numbers) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not documented |
| Allocation concealment (selection bias) | Low risk | Closed envelope technique |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not documented but OS unlikely to be affected by blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 65 patients ineligible and further 69 patients deemed to be not evaluable balanced across groups |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
Weiss 1992.
| Methods | Randomised Phase II | |
| Participants | 44 women with persistent, recurrent or metastatic Squamous cell cancer cervix 1987 to 1990 |
|
| Interventions | Arm 1: didemnin B 2.6‐3.5 mg/m2 iv over 30 minutes d1 q28 (subsequent dose escalations if no toxicity) Arm 2: trimetrexate 8 mg/m2 if previous radiotherapy or 12 mg/m2 if no radiotherapy iv over 10 minutes d1‐5 q21 (dose escalations to 15 mg/m2 and 18 mg/m2 respectively permitted on subsequent cycles if no toxicity) |
|
| Outcomes | Overall response rate | |
| Notes | 0% response rate in either arm at any dose 1 patient ineligible as baseline imaging not done within 14 days of starting treatment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not documented |
| Allocation concealment (selection bias) | Unclear risk | Not documented |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not documented |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | Published report included all pre‐specified outcomes |
| Other bias | Low risk | No hint at any other possible biases |
d: day; h: hour; GOG: Gynecologic Oncology Group; im: intramuscular; ITT: intention to treat; iv: intravenous; OS: overall survival; po: orally; QoL: quality of life; SWOG: Southwest Oncology Group; MVAC: methotrexate/vinblastine/doxorubicin/cisplatin; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Armstrong 2003 | Comparison of 2 Bryostatin‐1 schedules, neither active |
| Baker 1978 | Not an RCT |
| Greggi 2000 | Not an RCT |
| Kumar 1998 | No evidence that patients randomised between treatment arms |
RCT: randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Saito 2010.
| Trial name or title | JCOG0505 |
| Methods | Phase III RCT |
| Participants | Women with IVB persistent or recurrent cervical cancer not amenable to curative treatment with local therapy |
| Interventions | Arm 1: paclitaxel 135 mg/m2 over 24 h d1 plus carboplatin AUC 5 d1 q21 Arm 2: paclitaxel 135 mg/m2 over 24 h d1 plus cisplatin 50 mg/m2 2 h d2 q21 |
| Outcomes | OS PFS Toxicity Unplanned hospital admissions (surrogate for QoL) |
| Starting date | 21 February 2006 |
| Contact information | kitagawa.ryo@east.ntt.co.jp |
| Notes | Planned sample size 250 |
AUC: area under curve; d: day; h: hour; OS: overall survival; PFS: progression‐free survival; QoL: quality of life; RCT: randomised controlled trial.
Differences between protocol and review
There were no data comparing best supportive care with chemotherapy.
It was not possible to undertake a meta‐analysis of survival data.
Only three trials reported any QoL data and this was limited.
Toxicity data was reported using a variety of scales making comparisons difficult to perform.
A formal meta‐analysis could only be performed for response rate and some toxicity outcomes.
Contributions of authors
KS, JF and MF prepared the protocol, assisted the literature search, analysed the data and wrote the review.
Sources of support
Internal sources
-
Gynaecological Cochrane Study Group, UK.
Helping with running of searches and using Archie.
External sources
-
Bristol Royal Infirmary Library, UK.
Obtaining papers.
Declarations of interest
No declarations of interest to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Alberts 1987 {published data only}
- Alberts DS, Kronmal R, Baker LH, Stock‐Novack DL, Surwit EA, Boutselis JG, et al. Phase II randomized trial of cisplatin chemotherapy regimens in the treatment of recurrent or metastatic squamous cell cancer of the cervix: a Southwest Oncology Group Study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 1987;5(11):1791‐5. [PUBMED: 2445932] [DOI] [PubMed] [Google Scholar]
Bezwoda 1986 {published data only}
- Bezwoda WR, Nissenbaum M, Derman DP. Treatment of metastatic and recurrent cervix cancer with chemotherapy: a randomised trial comparing hydroxyurea with cisdiamminedichloro‐platinum plus methotrexate. Medical and Pediatric Oncology 1986;14(1):17‐9. [PUBMED: 3512970] [DOI] [PubMed] [Google Scholar]
Bloss 2002 {published data only}
- Bloss JD, Blessing JA, Behrens BC, Mannel RS, Rader JS, Sood AK, et al. Randomized trial of cisplatin and ifosfamide with or without bleomycin in squamous carcinoma of the cervix: a Gynecologic Oncology Group study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 2002;20(7):1832‐7. [PUBMED: 11919241] [DOI] [PubMed] [Google Scholar]
Bond 1976 {published data only}
- Bond WH, Arthur K, Banks AJ, Freeman WE, Holme GM, Newsholme GA, et al. Combination chemotherapy in the treatment of advanced squamous cell carcinoma of the cervix. Clinical Oncology 1976;2(2):173‐8. [PUBMED: 60188] [PubMed] [Google Scholar]
Bonomi 1985 {published data only}
- Bonomi P, Blessing JA, Stehman FB, DiSaia PJ, Walton L, Major FJ. Randomized trial of three cisplatin dose schedules in squamous‐cell carcinoma of the cervix: a Gynecologic Oncology Group study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 1985;3(8):1079‐85. [PUBMED: 3894589] [DOI] [PubMed] [Google Scholar]
Cadron 2005 {published data only}
- Cadron I, Jakobsen A, Vergote I. Report of an early stopped randomized trial comparing cisplatin vs. cisplatin/ifosfamide/ 5‐fluorouracil in recurrent cervical cancer. Gynecologic and Obstetric Investigation 2005;59(3):126‐9. [PUBMED: 15604591] [DOI] [PubMed] [Google Scholar]
Edmonson 1988 {published data only}
- Edmonson JH, Johnson PS, Wieand HS, Malkasian GD, Cullinan SA, Brown LD, et al. Phase II studies of bleomycin, cyclophosphamide, doxorubicin and cisplatin, and bleomycin and cisplatin in advanced cervical carcinoma. American Journal of Clinical Oncology 1988;11(2):149‐51. [PUBMED: 2451883] [DOI] [PubMed] [Google Scholar]
Freedman 1980 {published data only}
- Freedman RS, Herson J, Wharton JT, Rutledge FN. Single‐agent chemotherapy for recurrent carcinoma of the cervix. Cancer Clinical Trials 1980;3(4):345‐50. [PUBMED: 6775827] [PubMed] [Google Scholar]
Greenberg 1977 {published data only}
- Greenberg BR, Kardinal CG, Pajak TF, Bateman JR. Adriamycin versus adriamycin and bleomycin in advanced epidermoid carcinoma of the cervix. Cancer Treatment Reports 1977;61(7):1383‐4. [PUBMED: 73417] [PubMed] [Google Scholar]
Lira‐Puerto 1991 {published data only}
- Lira‐Puerto V, Silva A, Morris M, Martinez R, Groshen S, Morales‐Canfield F, et al. Phase II trial of carboplatin or iproplatin in cervical cancer. Cancer Chemotherapy and Pharmacology 1991;28(5):391‐6. [PUBMED: 1914084] [DOI] [PubMed] [Google Scholar]
Long 2005 {published data only}
- Brave M, Dagher R, Farrel A, Ramchandani R, Gobburu J, Booth R, et al. Topotecan in combination with cisplatin for the treatment of stage IVB, recurrent or persistent cervical cancer. Oncology 2006;20:1401‐16. [PubMed] [Google Scholar]
- Long HJ 3rd, Bundy BN, Grendys EC Jr, Benda JA, McMeekin DS, Sorosky J, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 2005;23(21):4626‐33. [PUBMED: 15911865] [DOI] [PubMed] [Google Scholar]
- Monk BJ, Huang HQ, Cella D, Long HJ 3rd. Quality of life outcomes from a randomised phase III trial of cisplatin with or without topotecan in advanced carcinoma of the cervix: a Gynecologic Oncology Group study. Journal of Clinical Oncology 2005;23(21):4617‐25. [DOI] [PubMed] [Google Scholar]
Long 2006 {published data only}
- Long HJ 3rd, Monk BJ, Huang HQ, Grendys EC Jr, McMeekin DS, Sorosky J, et al. Clinical results and quality of life analysis for the MVAC combination (methotrexate, vinblastine, doxorubicin, and cisplatin) in carcinoma of the uterine cervix: a Gynecologic Oncology Group study. Gynecologic Oncology 2006;100(3):537‐43. [PUBMED: 16216315] [DOI] [PubMed] [Google Scholar]
Malkasian 1981 {published data only}
- Malkasian GD Jr, Decker DG, Green SJ, Edmonson JH, Jefferies JA, Webb MJ. Treatment of recurrent and metastatic carcinoma of the cervix: comparison of doxorubicin with a combination of vincristine and 5‐fluorouracil. Gynecologic Oncology 1981;11(2):235‐9. [PUBMED: 7011913] [DOI] [PubMed] [Google Scholar]
McGuire 1989 {published data only}
- McGuire WP 3rd, Arseneau J, Blessing JA, DiSaia PJ, Hatch KD, Given FT Jr, et al. A randomized comparative trial of carboplatin and iproplatin in advanced squamous carcinoma of the uterine cervix: a Gynecologic Oncology Group study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 1989;7(10):1462‐8. [PUBMED: 2674333] [DOI] [PubMed] [Google Scholar]
Monk 2008 {published data only}
- Monk BJ. A randomised phase III trial of four cisplatin containing doublet combinations in stage IVB, recurrent or persistent cervical carcinoma: a GOG study. 01/06/2008. [DOI] [PMC free article] [PubMed]
Moore 2004 {published data only}
- McQuellon RP, Thaler HT, Cella D, Moore D. Quality of life outcomes from a randomised trial of cisplatin versus cisplatin plus paclitaxel in advanced cervical cancer: a Gynecologic Oncology Group study. Gynecologic Oncology 2006;101(2):296‐304. [DOI] [PubMed] [Google Scholar]
- Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 2004;22(15):3113‐9. [PUBMED: 15284262] [DOI] [PubMed] [Google Scholar]
Moseley 1976 {published data only}
- Moseley HS, Sasaki T, McConnell DB, Merhoff GC, Wilson WL, Grage TB, et al. A randomized pilot study comparing two regimens in the treatment of squamous cell carcinoma. Journal of Surgical Oncology 1976;8(1):35‐42. [PUBMED: 55523] [DOI] [PubMed] [Google Scholar]
Mountzios 2009 {published data only}
- Mountzios G, Dimpoulos MA, Bamias A, Vourli G, Kalofonos H, Aravantinos G, et al. Randomise multicentre phase II trial of cisplatin and ifosfamide with or without paclitaxel in recurrent or metastatic carcinoma of the uterine cervix: a Hellenic Co‐operative Oncology Group (HeCOG) study. Annals of Oncology 2009;20:1362‐8. [DOI] [PubMed] [Google Scholar]
Omura 1978 {published data only}
- Omura GA, Shingleton HM, Creasman WT, Blessing JA, Boronow RC. Chemotherapy of gynecologic cancer with nitrosoureas: a randomized trial of CCNU and methyl‐CCNU in cancers of the cervix, corpus, vagina, and vulva. Cancer Treatment Reports 1978;62(5):833‐5. [PUBMED: 350400] [PubMed] [Google Scholar]
Omura 1981 {published data only}
- Omura GA, Velez‐Garcia E, Birch R. Phase II randomized study of doxorubicin, vincristine, and 5‐FU versus cyclophosphamide in advanced squamous cell carcinoma of the cervix. Cancer Treatment Reports 1981;65(9‐10):901‐3. [PUBMED: 7196801] [PubMed] [Google Scholar]
Omura 1997 {published data only}
- Omura GA, Blessing JA, Vaccarello L, Berman ML, Clarke‐Pearson DL, Mutch DG, et al. Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Group study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 1997;15(1):165‐71. [PUBMED: 8996138] [DOI] [PubMed] [Google Scholar]
Palo 1976 {published data only}
- Palo GM, Bajetta E, Beretta G, Bonadonna G. Adriamycin plus bleomycin versus cyclophosphamide plus vincristine in advanced carcinoma of the uterine cervix. Tumori 1976;62(1):113‐22. [PUBMED: 65039] [DOI] [PubMed] [Google Scholar]
Pfeiffer 1998 {published data only}
- Pfeiffer P, Thomsen K, Bertelsen K. Teniposide or carboplatin in patients with recurrent or advanced cervical carcinoma. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society 1997;7(2):1. [Google Scholar]
- Thomsen TK, Pfeiffer P, Bertelsen K. Teniposide or carboplatin in patients with recurrent or advanced cervical carcinoma: a randomized phase II trial. International Journal of Gynecological Cancer 1998;8(4):310‐14. [Google Scholar]
Thigpen 1989 {published data only}
- Thigpen JT, Blessing JA, DiSaia PJ, Fowler WC Jr, Hatch KD. A randomized comparison of a rapid versus prolonged (24 hr) infusion of cisplatin in therapy of squamous cell carcinoma of the uterine cervix: a Gynecologic Oncology Group study. Gynecologic Oncology 1989;32(2):198‐202. [PUBMED: 2910782] [DOI] [PubMed] [Google Scholar]
Wallace 1978 {published data only}
- Wallace HJ Jr, Hreshchyshyn MM, Wilbanks GD, Boronow RC, Fowler WC Jr, Blessing JA. Comparison of the therapeutic effects of adriamycin alone versus adriamycin plus vincristine versus adriamycin plus cyclophosphamide in the treatment of advanced carcinoma of the cervix. Cancer Treatment Reports 1978;62(10):1435‐41. [PUBMED: 709549] [PubMed] [Google Scholar]
Weiss 1992 {published data only}
- Weiss G, Liu P, O'Sullivan J, Alberts D, Brown T, Neefe J, Hutchins L. A randomised phase II trial of trimetrexate or didemnin for the treatment of metastatic or recurrent squamous carcinoma of the uterine cervix: a SWOG trial. Gynecologic Oncology 1992;45:303‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Armstrong 2003 {published data only}
- Armstrong D, Blessing J, Rader J, Sorosky J. A randomised Phase II evaluation of bryostatin‐1 in persistent or recurrent squamous cell carcinoma of the cervix: a Gynaecological Oncology Group study. Investigational New Drugs 2003;21(4):453‐7. [DOI] [PubMed] [Google Scholar]
Baker 1978 {published data only}
- Baker LH, Opipari MI, Wilson H, Bottomley R, Coltman CA Jr. Mitomycin C, vincristine, and bleomycin therapy for advanced cervical cancer. Obstetrics and Gynecology 1978;52(2):146‐50. [PUBMED: 79991] [PubMed] [Google Scholar]
Greggi 2000 {published data only}
Kumar 1998 {published data only}
- Kumar L, Pokharel YH, Kumar S, Singh R, Rath GK, Kochupillai V. Single agent versus combination chemotherapy in recurrent cervical cancer. The Journal of Obstetrics and Gynaecology Research 1998;24(6):401‐9. [PUBMED: 10063235] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Saito 2010 {published data only}
- Saito I, Kitagawa R, Fukuda H, Shibata T, Katsumata N, Konishi I, et al. A phase III trial of paclitaxel plus carboplatin versus paclitaxel plus cisplatin in stage IVB persistent or recurrent cervical cancer: a Gynecologic Cancer Study group/Japan Clinical Oncology Group study (JCOG0505). Japanese Journal of Clinical Oncology 2010;40(1):90‐3. [DOI] [PubMed] [Google Scholar]
Additional references
CTCAE 2006
- CTCAE. Common Terminology Criteria for Adverse Events V3.0, August 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf (accessed 5 August 2012).
de la Motte Rouge 2006
- Motte Rouge T, Pautier P, Hamy AS, Duvillard P, Bruna A, Castaigne D, et al. Medical treatment of metastatic or recurrent cancer of the cervix. Bull Cancer 2006;93(3):263‐70. [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Eifel 2001
- Eifel PJ, Berek JS, Thigpen JT. Gynaecological Cancers. Section 2. Cancer of the cervix, vagina, vulva. In: DeVita VT, Hellman S, Rosenberg SA editor(s). Cancer: Principles and Practice of Oncology. 6. Vol. 2, Philadelphia: Lippincott‐Raven, 2001:1526‐1556. [Google Scholar]
EUROCARE‐3
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, et al. EUROCARE‐3: survival of cancer patients diagnosed 1990‐94‐ results and commentary. Annals of Oncology 2003;14(Suppl 5):V61‐118. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2008
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C. Estimates of worldwide burden of cancer in 2008; GLOBOCAN. International Journal of Cancer 2010;127(12):2893‐917. [DOI] [PubMed] [Google Scholar]
Green 2005
- Green J, Kirwan J, Tierney J, Vale C, Symonds P, Fresco L, et al. Concomitant chemotherapy and radiotherapy for cancer of the uterine cervix. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD002225.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jemal 2008
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics 2008. CA: A Cancer Journal for Clinicians 2008;58(2):71‐96. [DOI] [PubMed] [Google Scholar]
Meta‐analysis Collaboration 2008
- Chemoradiotherapy for Cervical Cancer Meta‐analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta‐analysis of individual patient data from 18 randomized trials. Journal of Clinical Oncology 2008;26(35):5802‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michiels 2005
- Michiels S, Piedbois P, Burdett S, Syz N, Stewart L, Pignon JP. Meta‐analysis when only the median survival times are known: a comparison with individual patient data results. International Journal of Technology Assessment in Healthcare 2005;21(1):119‐25. [DOI] [PubMed] [Google Scholar]
Miller 1981
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207‐14. [DOI] [PubMed] [Google Scholar]
Moore 2006
- Moore DH. Chemotherapy for recurrent cervical carcinoma. Current Opinions in Oncology 2006;18(5):516‐9. [DOI] [PubMed] [Google Scholar]
Moore 2007
- Moore K. A comparison of cisplatin/paclitaxel and carboplatin/paclitaxel in stage IV recurrent or persistent cervix cancer. Gynaecological Oncology 2007;105:299‐303. [Google Scholar]
Mutch 2003
- Mutch D, Bloss J. Gemcitabine in cervical cancer. Gynaecological Oncology 2003;90(2 part 2):S8‐25. [DOI] [PubMed] [Google Scholar]
National Cancer Institute 2012
- National Cancer Institute. Cervical cancer, 2012. http://www.cancer.gov/cancertopics/types/cervical (accessed 5 August 2012).
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Pectasides 2008
- Pectasides D, Kamposioras K, Papaxoinis D, Pectasides E. Chemotherapy for recurrent cervical cancer. Cancer Treatment Reviews 2008;34(7):603‐13. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Tewari 2005
- Tewari KS, Monk BJ. Gynaecological group trials of chemotherapy for metastatic and recurrent cervical cancer. Current Oncology Reports 2005;7(6):419‐34. [DOI] [PubMed] [Google Scholar]
Tinker 2005
- Tinker A, Bhagat K, Swenerton K, Hoskins P. Carboplatin and paclitaxel for advanced and recurrent cervical carcinoma; the BCCA experience. Gynaecological Oncology 2005;98(1):54‐8. [DOI] [PubMed] [Google Scholar]
Weiss 1990
- Weiss GR, Green S, Hannigan EV, Boutselis JG, Surwit EA, Wallace DL, et al. A Phase II trial of carboplatin for recurrent or metastatic squamous carcinoma of the uterine cervix. A SWOG study. Gynaecological Oncology 1990;39(3):332‐6. [DOI] [PubMed] [Google Scholar]
Willmott 2009
- Willmott LJ, Monk B. Cervical cancer therapy. Expert Review of Anticancer Therapy 2009;9(7):895‐903. [DOI] [PubMed] [Google Scholar]


