Abstract
Background
Aortic valve stenosis is the most common type of valvular heart disease in the USA and Europe. Aortic valve stenosis is considered similar to atherosclerotic disease. Some studies have evaluated statins for aortic valve stenosis.
Objectives
To evaluate the effectiveness and safety of statins in aortic valve stenosis.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, LILACS ‐ IBECS, Web of Science and CINAHL Plus. These databases were searched from their inception to 24 November 2015. We also searched trials in registers for ongoing trials. We used no language restrictions.
Selection criteria
Randomised controlled clinical trials (RCTs) comparing statins alone or in association with other systemic drugs to reduce cholesterol levels versus placebo or usual care.
Data collection and analysis
Primary outcomes were severity of aortic valve stenosis (evaluated by echocardiographic criteria: mean pressure gradient, valve area and aortic jet velocity), freedom from valve replacement and death from cardiovascular cause. Secondary outcomes were hospitalisation for any reason, overall mortality, adverse events and patient quality of life.
Two review authors independently selected trials for inclusion, extracted data and assessed the risk of bias. The GRADE methodology was employed to assess the quality of result findings and the GRADE profiler (GRADEPRO) was used to import data from Review Manager 5.3 to create a 'Summary of findings' table.
Main results
We included four RCTs with 2360 participants comparing statins (1185 participants) with placebo (1175 participants). We found low‐quality evidence for our primary outcome of severity of aortic valve stenosis, evaluated by mean pressure gradient (mean difference (MD) ‐0.54, 95% confidence interval (CI) ‐1.88 to 0.80; participants = 1935; studies = 2), valve area (MD ‐0.07, 95% CI ‐0.28 to 0.14; participants = 127; studies = 2), and aortic jet velocity (MD ‐0.06, 95% CI ‐0.26 to 0.14; participants = 155; study = 1). Moderate‐quality evidence showed no effect on freedom from valve replacement with statins (risk ratio (RR) 0.93, 95% CI 0.81 to 1.06; participants = 2360; studies = 4), and no effect on muscle pain as an adverse event (RR 0.91, 95% CI 0.75 to 1.09; participants = 2204; studies = 3; moderate‐quality evidence). Low‐ and very low‐quality evidence showed uncertainty around the effect of statins on death from cardiovascular cause (RR 0.80, 95% CI 0.56 to 1.15; participants = 2297; studies = 3; low‐quality evidence) and hospitalisation for any reason (RR 0.84, 95% CI 0.39 to 1.84; participants = 155; study = 1; very low‐quality evidence). None of the four included studies reported on overall mortality and patient quality of life.
Authors' conclusions
Result findings showed uncertainty surrounding the effect of statins for aortic valve stenosis.The quality of evidence from the reported outcomes ranged from moderate to very low. These results give support to European and USA guidelines (2012 and 2014, respectively) that so far there is no clinical treatment option for aortic valve stenosis.
Plain language summary
Statins for aortic valve stenosis
Review question
What is the evidence regarding the effect of statins in people suffering from aortic valve stenosis?
Background
The heart is responsible for pumping blood throughout the body. It has four valves that control the blood flow within it. One of the valves is the aortic valve that controls the flow of blood from the left ventricle chamber to the body. Aortic valve stenosis is a disease characterised by the narrowing of this valve. This is the most common type of valvular heart disease in the USA and Europe. Its incidence rises with age and 2% to 7% of adults over 65 years old have aortic valve stenosis. Aortic valve stenosis is considered similar to atherosclerotic disease and it is known to have a long asymptomatic period for several decades. When it manifests clinically, symptoms such as syncope (brief lapse of consciousness), angina and dyspnoea (shortness of breath) may lead to death. Some prospective and retrospective trials have shown that statins can delay the progression of aortic valve stenosis. Statins are considered very useful drugs to lower high cholesterol.
Study characteristics
The evidence is current up to 24 November 2015. We searched electronic databases for reports of randomised controlled trials comparing statins alone or in combination with other types of lipid‐lowering drugs in the treatment of aortic valve stenosis.
Key results
We evaluated the severity of aortic valve stenosis according to the following echocardiographic criteria: mean pressure gradient, valve area and aortic jet velocity. We also evaluated freedom from valve replacement and death from cardiovascular cause. There were no differences in effect for mean pressure gradient, valve area, freedom from valve replacement and death from cardiovascular cause in the statin group when compared with the placebo group. We were not able to conduct a meta‐analysis to assess the aortic jet velocity as only one study analysed this outcome. We also checked the safety of statins by analysing other adverse events among them muscle pain. Muscle pain is the most prevalent adverse event that can limit the use of statin. Muscle pain did not differ in the statin group when compared with the placebo group. Results of four randomised controlled trials with 2360 participants showed that statins did not delay the progression of aortic valve stenosis.
Quality of evidence
The quality of the evidence quality ranged from moderate to very low across the different outcomes due to the limitations of the original studies. Included studies had at least one methodological limitation.
Conclusions
Based on the evidence in this review, there is uncertainty surrounding the effect of statins for aortic valve stenosis. These results give support to European and USA guidelines (2012 and 2014, respectively) that so far there is no clinical treatment option for aortic valve stenosis. An alternative might be to broaden the knowledge of the pathophysiology of this disease and include risk factors such as calcium, heredity, vitamin D, inflammation, oxidative stress, diabetes, hypertension among others that could contribute to aortic valve stenosis. High‐quality randomised trials to include risk factors for aortic valve stenosis are needed.
Summary of findings
Summary of findings for the main comparison. Statin versus Placebo for aortic valve stenosis.
| Statin versus Placebo for aortic valve stenosis | |||||
| Patient or population: patients with aortic valve stenosis Settings: Outpatients and hospitalisation Intervention: Statin versus Placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Statin | ||||
|
Mean pressure gradient (mmHg) Better indicated by lower scores. Follow‐up: median 2.4 to 4.5 years |
The mean mean pressure gradient in the control groups was 34 to 35 mmHg | The mean mean pressure gradient in the intervention groups was 0.54 lower (1.88 lower to 0.8 higher) | MD ‐0.54 (‐1.88 to 0.80) | 1935 (2 studies) | ⊕⊕⊝⊝ low1,2 |
|
Valve area (cm2)
Better indicated by higher scores. Follow‐up: median 2.4‐ to 3.5 years |
The mean valve area in the control groups was 1 to 1.5 cm2 | The mean valve area in the intervention groups was 0.07 lower (0.28 lower to 0.14 higher) | MD ‐0.07 (‐0.28 to 0.14) | 127 (2 studies) | ⊕⊕⊝⊝ low2,3 |
| Aortic jet velocity (m/s) Follow‐up: median 2.1 years | The mean aortic jet velocity in the control groups was 3.45 m per second | The mean aortic jet velocity in the intervention groups was 0.06 lower (0.26 lower to 0.14 higher) | MD ‐0.06 (‐0.26 to 0.14) | 155 (1 study) | ⊕⊕⊝⊝ low2,3 |
| Freedom from valve replacement Follow‐up: median 2.1 to 4.5 years | Study population | RR 0.93 (0.81 to 1.06) | 2360 (4 studies) | ⊕⊕⊕⊝ moderate2 | |
| 281 per 1000 | 261 per 1000 (227 to 298) | ||||
| Moderate population | |||||
| 222 per 1000 | 206 per 1000 (180 to 235) | ||||
| Death from cardiovascular cause Follow‐up: median 2.1 to 4.5 years | Study population | RR 0.80 (0.56 to 1.15) | 2297 (3 studies) | ⊕⊕⊝⊝ low2,4 | |
| 56 per 1000 | 45 per 1000 (31 to 64) | ||||
| Moderate population | |||||
| 39 per 1000 | 31 per 1000 (22 to 45) | ||||
| Hospitalisation for any reason Follow‐up: median 2.1 years | 154 per 1000 | 129 per 1000 (60 to 283) | RR 0.84 (0.39 to 1.84) | 155 (1 study) | ⊕⊝⊝⊝ very low2,3,4 |
| Adverse events ‐ Muscle pain Follow‐up: median 2.4 to 4.5 years | Study population | RR 0.91 (0.75 to 1.09) | 2204 (3 studies) | ⊕⊕⊕⊝ moderate2 | |
| 168 per 1000 | 153 per 1000 (126 to 183) | ||||
| Moderate population | |||||
| 30 per 1000 | 27 per 1000 (22 to 33) | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 Downgraded by one due to randomisation and allocation being unclear for Rossebø 2008. 2 Downgraded by one due to possible risk of publication bias as only a small number of studies were identified. 3 Downgraded by one due to imprecision: small sample size with effect size crossing the line of no effect. 4 Downgraded by one due to imprecision: very few events < 300.
Background
Aortic valve stenosis is the main type of heart valve disease observed in high‐income countries.Calcified aortic stenosis is the main etiology of this disease. There are two more causes of aortic valve stenosis, namely congenital and rheumatic diseases (Vahanian 2012). Currently, aortic valve stenosis is the most common indication for surgical valve replacement in North America and Europe (Vahanian 2010). The number of cases of aortic valve replacements has doubled in the past decade in the USA, due to the ageing population (Dweck 2012). When aortic valve replacement is combined with bypass surgery, operative mortality ranges from 5% to 7% (Vahanian 2010). In the last few years, transcatheter aortic valve implantation (TAVI) has been used as an alternative for patients with contraindications to surgery or for high‐risk patients (Vahanian 2012).
Description of the condition
Aortic valve stenosis is the most prevalent valve illness in adults and the third cause of cardiovascular disorders (after arterial hypertension and coronary artery disease) (Parolari 2011). Aortic valve stenosis is characterised by a reduction of the aortic valve area, and it is mainly caused by the calcification of its three leaflets (Vahanian 2012). These leaflets are the part of the aortic valve designed to open in the direction of the blood flow. The incidence of aortic valve stenosis ranges from 2% to 7% in adults over 65 years old (Vahanian 2012). This calcification process is known to be constant and is evidenced by lipid deposition, inflammation and the accumulation of calcium, and is similar to coronary atherosclerosis in many aspects (Dweck 2012). Other causes of aortic valve stenosis, with varying prevalence rates in different populations, include bicuspid aortic valve and rheumatic disease. Patients with bicuspid aortic valve have shown a higher tendency to have calcification than patients with tricuspid aortic valve. Bicuspid aortic valve is more common in younger adults and its incidence ranges from 0.5% to 2.0% in the population (Siu 2010). Rheumatic disease remains an important public health problem in low‐ and middle‐income countries with a prevalence of around 1% among school children in Africa, Asia and Latin America (Canterin 2010). The prevalence of aortic disease among people with rheumatic disease in these countries ranges from 5% to 24% in the population and its calcification process is less common (Canterin 2009). Aortic valve stenosis is a progressive disease which may last for decades, and which causes obstruction of the left ventricular outflow tract leading to ventricular hypertrophy and subsequently heart failure (Dweck 2012). The onset of typical symptoms such as dyspnoea (shortness of breath), angina and syncope (brief lapse of consciousness) is closely related to the severity of the illness (Vahanian 2010). The degree of valve obstruction can be assessed by Doppler echocardiography, the gold standard for the disease, which is used to measure the valve area, mean pressure gradient and aortic jet velocity (Vahanian 2012; Nishimura 2014). The echocardiographic criteria for aortic valve stenosis (Vahanian 2012, Nishimura 2014) are shown in Table 2.
1. Echocardiografic criteria for aortic valve stenosis (Vahanian 2012; Nishimura 2014).
|
Mild Aortic stenosis |
Moderate Aortic stenosis |
Severe Aortic stenosis |
|
| Valve area | 1.5 cm² | 1.0 to 1.5 cm² | < 1.0 cm² |
|
Mean pressure gradient |
< 20 mmHg | 20 to 39 mmHg | > 40 mmHg |
| Aortic jet velocity | < 2.0 to 2.9 m per second |
3.0 to 3.9 m per second |
> 4.0 m per second |
Description of the intervention
Statins are lipid‐lowering drugs recommended for the treatment of some disease such as diabetes, acute infarction myocardial and dyslipidaemia (abnormal blood levels of cholesterol) (Stone 2014). Randomised controlled trials (RCTs) have demonstrated the importance of statins in decreasing cardiovascular events (Ridker 2008). Statins act inside the liver by blocking hydroxymethylglutaryl coenzyme A (HMG CoA) reductase enzyme which plays a key role in cholesterol synthesis (Vaughan 2004; Kapur 2008). Besides inhibiting the enzyme responsible for the formation of cholesterol, statins have several pleiotropic effects such as nitric oxide increase, endothelial function improvement, antioxidant, anti‐thrombotic and anti‐inflammatory actions, atherosclerotic plaque stabilisation and anticoagulant action (Kapur 2008). Most people have shown good tolerance to statins. However, adverse effects such as liver dysfunction and myopathy may occur. Muscle pain is the main symptom of myopathy that can limit the use of statins. It occurs in 1.5 % to 5% of patients treated with statins (Mancini 2011). A recent systematic review (Macedo 2014) regarding the safety of statins in 86 included studies demonstrated the presence of the following adverse effects: myopathy, liver enzymes and diabetes (respectively: odds ratio (OR) 2.63 [95% confidence interval (CI) 1.50 to 4.61]; OR 1.54 [95% CI 1.47 to 1.62]; OR 1.31 [95% CI 0.99 to 1.73]). However, the review considered them minor adverse effects when compared with the beneficial effects of statins in major cardiovascular events (Macedo 2014).
How the intervention might work
Calcified aortic valve stenosis has recently come to be considered an active and progressive inflammatory process, and similar to coronary atherosclerosis in many aspects (Dweck 2012). What occurs is a build‐up of fat on the valve leaflets promoting oxidation of low‐density lipoprotein (LDL) by macrophages, activation of myofibroblasts, infiltration of T lymphocytes and cell proliferation from pro‐inflammatory cytokines IL 1(beta), as well as tumour necrosis factor (TNF alpha) (Liebe 2006). Considering the possible relationship between aortic valve stenosis and atherosclerotic disease, statins may halt the progression, or even induce regression of aortic valve stenosis by inhibiting HMG‐CoA reductase, which decreases fat deposition on the valve leaflets (Pedersen 2008). Statins also have anti‐inflammatory actions such as reducing cytokines, blocking smooth muscle cell and T cell depletion, as well as blocking macrophage‐oxidised LDL which hinders foam cells formation (Liebe 2006; Dweck 2012). Studies to verify the possible benefit of statins for the treatment of aortic valve stenosis have already been conducted. A retrospective non‐randomised observational study with the lowest level of evidence showed that statin therapy was successful in delaying the progression of aortic valve stenosis based on a slow reduction in the valve area (P = 0.02) and a small increase in peak pressure gradient (P = 0.01) (Novaro 2001; Pedersen 2008). Other randomised control trials (SALTIRE, SEAS, ASTRONOMER) have also evaluated the role of various statins in halting the progression of aortic valve stenosis over the past 10 years (Cowell 2005; Rossebø 2008; Chan 2010). The SALTIRE study (A randomised trial of intensive lipid‐lowering therapy in calcific aortic stenosis, Cowell 2005) evaluated the effect of 80 mg of atorvastatin in the progression of aortic valve stenosis. A total of 155 patients (mostly men) with a mean age of 68 years were included. The trial did not show any benefit of atorvastatin to slow the progression and calcification of aortic valve stenosis (P = 0.93) (Cowell 2005). The SEAS trial (Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis, Rossebø 2008) evaluated the effect of the association of 40 mg of simvastatin plus 10 mg of ezetimibe in 1873 patients (mostly men) with mild to moderate aortic valve stenosis and a mean age of 67 years (Rossebø 2008). The conclusion of this trial was that simvastatin plus ezetimibe did not reduce the progression of aortic valve stenosis (P = 0.77). The ASTRONOMER trial (Effect of lipid lowering with rosuvastatin on progression of aortic stenosis, Chan 2010) evaluated the effect of 40 mg of rosuvastatin in 269 patients (mostly men) with mild to moderate aortic stenosis and a mean age 58 years. There were no significant differences between the two groups (rosuvastatin versus placebo) in the progression of the disease (mean pressure gradient and aortic valve area) (P = 0.32), cardiovascular death and the need for aortic valve replacement (P = 0.446), as well as adverse events (P = 0.64) (Chan 2010). Currently, there is no recommended dosage of statins to treat aortic valve stenosis (Vahanian 2012; Nishimura 2014).
Why it is important to do this review
The current evidence shows there is still no effective clinical treatment to halt the progression of aortic valve stenosis (Cowell 2005, Rossebø 2008, Chan 2010, Van der Linde 2011). Many patients with aortic valve stenosis who are eventually submitted to invasive procedures such as valve replacement are often diagnosed in the advanced stages of the disease (Katz 2010). Many of these patients also suffer from common chronic diseases such as diabetes, hypertension, dyslipidaemia, as well as the risks associated with smoking (Rajamannan 2008), which hinder post‐operative recovery. They are also subjected to long periods of hospitalisation which represents high costs for health services. Therefore, in order to promote a new rational decision‐making process regarding clinical interventions such as statins for the treatment of aortic valve stenosis, we proposed a systematic review of randomised controlled trials (Mulrow 1994) using Cochrane criteria (Higgins 2011). This review is important as it uses the best available evidence to assess the effects of statins on the progression of aortic valve stenosis, thus limiting the risk of systematic and random errors in the analyses and providing credible results (Antman 1992; Oxman1993; Higgins 2011).
Objectives
General objective
To evaluate the effectiveness and safety of statins in aortic valve stenosis.
Specific objectives
To evaluate the effectiveness of statins in the following subgroups: mild aortic valve stenosis, moderate aortic valve stenosis and severe aortic valve stenosis.
To investigate the safety of statins in aortic valve stenosis by analysing the adverse effects observed in the included studies.
To assess the quality of life of patients suffering from aortic valve stenosis and undergoing treatment with statins.
Methods
Criteria for considering studies for this review
Types of studies
Published or ongoing randomised controlled clinical trials (RCTs) in which statins alone or in association with other systemic drugs to reduce cholesterol levels compared with placebo or control groups were eligible for inclusion. We considered studies with at least one‐year follow‐up.
Types of participants
Adults (age ranged 18 to 85 years) of both sexes suffering from aortic valve stenosis and diagnosed by means of clinical and echocardiographic criteria. For more details, see Table 2. We considered only patients with tricuspid and bicuspid aortic valve disease. We excluded rare causes of valve disease and rheumatic disease.
Types of interventions
Statins alone or in association with other systemic drugs to reduce cholesterol levels versus placebo or control groups.
Types of outcome measures
Primary outcomes
Severity of aortic valve stenosis (evaluated by echocardiographic criteria such as mean pressure gradient, valve area and aortic jet velocity)
Freedom from valve replacement
Death from cardiovascular cause
Secondary outcomes
Hospitalisation for any reason
Overall mortality
Adverse events
Patient quality of life
Search methods for identification of studies
We searched for every published or ongoing randomised controlled clinical trial in which statins, alone or in association with other systemic drugs, to reduce cholesterol levels compared with placebo or control groups (date of the search: 24 November, 2015). Searches were conducted by Information Scientists of the Cochrane Heart Group and the Brazilian Cochrane Center .
Electronic searches
We searched the following electronic databases.
Cochrane Central Register of Controlled Trials (CENTRAL, 2015, Issue 10 2015) in the Cochrane Library.
MEDLINE (Ovid, 1946 to 24 November, 2015).
Embase (Embase CLASSIC and Embase Ovid, 1947 to 23 November, 2015).
Web of Science (Thomson Reuter, 1970 to 23 November, 2015).
CINAHL Plus (EBSCO ‐1937 to 24 November, 2015).
LILACS‐IBECS (1982 to 24 November, 2015).
Details of the search strategies used can be found in Appendix 1. We used medical subject headings (MeSH) for MEDLINE. The terms used to search MEDLINE were modified when necessary to search the other databases listed. No language or date restrictions were applied to the searches. The following RCT filter was applied to MEDLINE (OVID) and adaptations of it were used for Embase (OVID) and Web of Science: Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision) (Higgins 2011).
Searching other resources
We checked the reference lists of the included studies to obtain other articles that were not found by the search strategy. We contacted pharmaceutical companies and authors of published articles to get relevant information. We also searched the WHO's International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/), ClinicalTrials.gov (www.clinicaltrials.gov) and Current Controlled Trials Register (www.controlled‐trials.com/) to identify ongoing trials using the terms "aortic valve stenosis" as condition and "Hydroxymethylglutaryl CoA Reductases" as intervention. We searched Grey literature in OpenGrey (System for Information on Grey Literature in Europe) (http://www.opengrey.eu/) and materials from international congresses of the European Society of Cardiology (www.escardio.org), American Heart Association (AHA) (www.heart.org) and American College of Cardiology (ACC) (www.acc.org) with analogous terms. We imposed no language restrictions.
Data collection and analysis
Two review authors (LT, AFTG) independently collected data using a standard form and two review authors (LT, CRM) independently input the data into the Review Manager (RevMan 5.3) software (RevMan 2014).
Selection of studies
Two review authors (LT, AFTG) independently selected the studies based on a search strategy conducted in the database. Two authors (LT, AFTG) independently identified potentially eligible studies by reading all the titles and abstracts of the articles found. We obtained the full text of relevant studies and selected studies that satisfied the inclusion criteria. The CONSORT (Consolidated Standards of Reporting Trials) statement was used to identify the RCTs when selecting the studies (Schulz 2010) and the PRISMA (Preferred Reporting Items for Systematic reviews and Meta‐Analyses) statement was applied to check and report each step of the review process (Moher 2009). We resolved disagreements by consensus and asked a third review author (ANA) to make a decision when necessary. There was a high degree of agreement between the authors. The excluded studies were presented and justified in a standard form. For more details, see below Characteristics of excluded studies section.
Data extraction and management
Two review authors (LT, AFTG) independently extracted the data from the included studies. We used a standard form to extract the following data: study objective, study design, study place, date, country, follow‐up, participants (total number, age, gender, comorbidities, inclusion and exclusion criteria, losses and withdrawals), severity of the disease, aortic valve morphology, statistical power of the study, sample size calculation, intervention (statins, types of statins, dosage, association with other drugs to reduce cholesterol levels, side effects, route of administration and placebo), results (outcome measures, adverse events and risk of bias), and funding sources. We solved any disagreement by consensus involving a third author when necessary (ANA). We contacted the authors of the studies when the extracted data were insufficient or unclear (Higgins 2011). We summarised the extracted data in a meta‐analysis using the Review Manager (RevMan 5.3) software (RevMan 2014).
Assessment of risk of bias in included studies
Two review authors (LT, AFTG) independently assessed the risk of bias by applying the Cochrane Collaboration 'Risk of bias' tool (Higgins 2011). The differences were settled through discussion or by resorting to a third review author (CRM or ANA). We analysed the following items associated with the risk of bias in the included studies: random sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data and selective outcome reporting among other sources of bias. We classified each of these criteria as high, low or unclear bias categories according to the Cochrane Collaboration tool and compiled them in a 'Risk of bias' graph and 'Risk of bias' summary.
Measures of treatment effect
We calculated the mean difference (MD) between the treatment groups for continuous outcomes (e.g. valve area). We calculated risk ratio (RR) in both groups for dichotomous outcomes (e.g. death from cardiovascular cause). (Higgins 2011). All outcomes were reported with 95% confidence intervals (CIs).
Unit of analysis issues
Cross‐over trials and cluster‐randomised trials were not eligible for inclusion because the range of this review was a progressive disease (aortic valve stenosis), and the unit of randomisation was the individual. Four RCTs were included (Cowell 2005; Rossebø 2008; Chan 2010; Van der Linde 2011) and the participants were individually randomised into treatment groups. The result of the intervention was evaluated and analysed for each outcome in this review.
Dealing with missing data
We contacted the study authors to clarify and recover missing data. Only assessable data were considered in this review and the impact of missing data are addressed in the Discussion section of this review.
Assessment of heterogeneity
The presence of statistical heterogeneity was analysed using the I2 statistical test and the Chi2 test for each outcome. When no heterogeneity was found, we performed a fixed‐effect model. When substantial heterogeneity (I² statistic above 50%) was found, we used the random‐effects model in order to quantify the effect of the findings and verify if they were statistically significant (Higgins 2011). The reasons for the presence of these large differences are explored in the Discussion.
Assessment of reporting biases
We strived to include both published and unpublished studies during the selection process; however the funnel plots and the test of asymmetry to assess possible publication bias were not done due to the insufficient number of trials found (less than10) (Higgins 2011).
Data synthesis
We carried out a meta‐analysis of the data using the Review Manager software, version 5.3 (RevMan 2014). Had there been studies with substantial heterogeneity and for which a meta‐analysis could not be made, we would have prepared a narrative synthesis of the data. We used fixed‐effect and random‐effects models to measure the effect size of the results. We used the fixed‐effect model when the studies did not show heterogeneity. We planned to use the random‐effects model if the studies showed substantial heterogeneity (an I² statistic above 50%) (Higgins 2011). We presented the effect size of the included studies in the forest plots with their respective confidence intervals (CIs).
Subgroup analysis and investigation of heterogeneity
The number of included studies was insufficient to carry out subgroup analysis (less than 10). Therefore, we were unable to evaluate the effectiveness of statins in the following subgroups: mild aortic valve stenosis, moderate aortic valve stenosis and severe aortic valve stenosis as we mentioned in the objective section of this review. In future updates, we plan to conduct subgroup analyses according to age, gender, type of statin and severity of illness, if we have a sufficient number of eligible studies (more than 10).
Sensitivity analysis
The number of included studies was not enough to carry out sensitivity analysis (less than 10). We would have used sensitivity analysis to test the robustness of any results that appeared to be based on heterogeneous combinations of the studies. Had we included more than 10 studies, we would have applied the sensitivity analysis including and excluding lower quality studies to assess the robustness of the observed effects. In this analysis, we would have considered death from cardiovascular cause as the most important outcome. We hope to be able to plan sensitivity analysis in future updates if we have a sufficient number of eligible studies (more than 10).
Overall quality of the body of evidence: 'Summary of findings' table
We generated a 'Summary of findings' table using GRADEPRO software (GRADE PRO 2011). This table evaluated the overall quality of the body of evidence for the review outcomes using GRADE criteria (study limitations [i.e. risk of bias] consistency of effect, imprecision, indirectness and publication bias). The outcomes analysed in the 'Summary of findings' table were mean pressure gradient, freedom from valve replacement, death from cardiovascular cause, hospitalisation for any reason, aortic jet velocity, valve area and severe adverse events (muscle pain). The GRADE assessments were incorporated into the reported result findings for each outcome.
Results
Description of studies
Results of the search
The database searches generated 946 citations and 645 after de‐duplication. The de‐duplication is a tool to identify indexed articles in more than one of the databases. We found 450 citations in the LILACS‐IBECS database . We found two citations by searching other resources. We identified nine eligible papers for inclusion or exclusion screening the titles and abstracts of the 1097 records. Of these nine papers, four were included (Cowell 2005; Rossebø 2008; Chan 2010; Chan 2011). We excluded three papers (Ditchl 2008; Chan 2011; Panahi 2013). We identified only two ongoing trials (Schuler 2005; Kindo 2008).The flow of studies throughout the review is presented in the PRISMA diagram in Figure 1.
1.
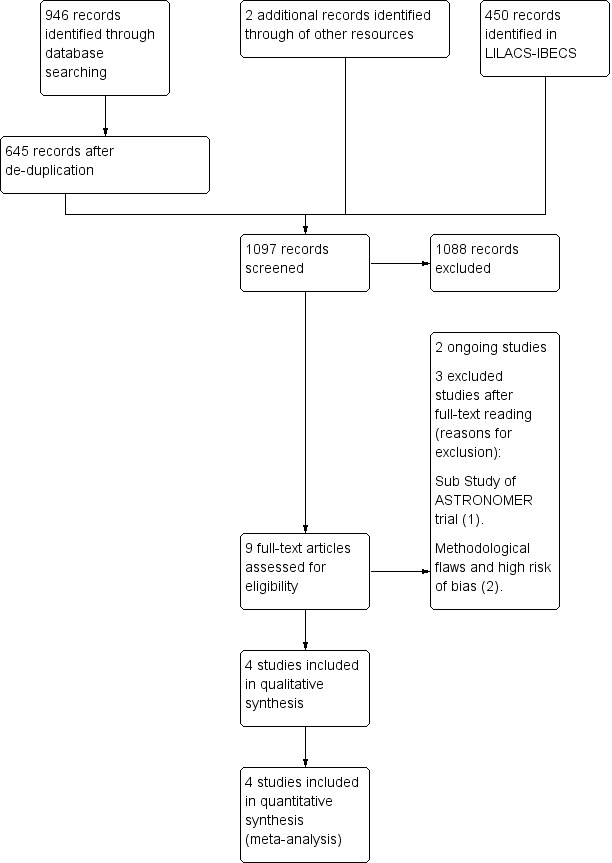
Study flow diagram.
Included studies
Four randomised controlled trials (RCTs) were included in this review (Cowell 2005; Rossebø 2008; Chan 2010; Van der Linde 2011). A total of 2360 adults with aortic valve stenosis were randomised. All participants, mostly men (66%) and white, were followed up for at least two years. Three of the studies analysed tricuspid aortic valve (Cowell 2005; Rossebø 2008; Chan 2010) and the other study analysed mostly younger patients with bicuspid aortic valve (Van der Linde 2011). Rosuvastatin was the intervention drug of two studies (Chan 2010; Van der Linde 2011). Another study used atorvastatin as intervention drug (Cowell 2005). Only one study used a statin in association with other systemic drugs to reduce cholesterol levels (simvastatin plus ezetimibe) as the intervention drugs (Rossebø 2008). Two studies had participants with other comorbidities such as hypertension, coronary heart disease, β‐blocker and aspirin use, high levels of low‐density lipoprotein (LDL) and who were current smokers (Cowell 2005; Rossebø 2008). For further information about study design, sites, follow‐up, participants, comorbidities, inclusion and exclusion criteria, aortic valve morphology, severity of aortic valve stenosis, intervention and outcomes for each of the included studies in this review see Characteristics of included studies.
We also identified two ongoing trials (Schuler 2005; Kindo 2008). One trial is analysing the effect of 40 mg of fluvastatin per day in asymptomatic aortic valve stenosis. The primary outcomes of this study are the progression of calcified aortic valve stenosis measured by transthoracic echocardiography (mean pressure gradient, valve area and aortic jet velocity) and catheterisation (peak‐to‐peak gradient, left ventricular function and compliance) (Schuler 2005).The recruitment status of this study is unknown as the authors have not updated it. Another trial is analysing the effect 80 mg of atorvastatin per day in surgical aortic valve stenosis and is currently recruiting participants. The primary outcomes of this study are changes on inflammatory markers after aortic valve replacement and changes in left ventricular mass (Kindo 2008). For more details, see Characteristics of ongoing studies.
No studies are awaiting classification.
Excluded studies
Three of the nine eligible studies were excluded after full‐text reading (Ditchl 2008; Chan 2011; Panahi 2013). For more details, see Characteristics of excluded studies. The main reason to exclude these studies was the type of design (a sub‐study of ASTRONOMER trial) (Chan 2011), methodological flaws and high risk of bias (Ditchl 2008; Panahi 2013).
Risk of bias in included studies
We assessed the risk of bias in the included studies as 'low', 'high' or 'unclear'. For more details, see below Figure 2 , Figure 3 and Characteristics of included studies section.
2.
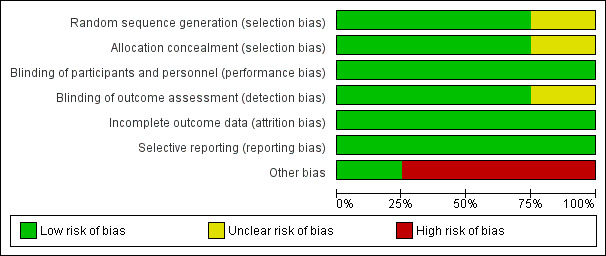
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
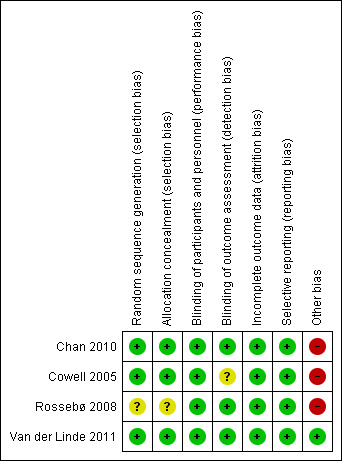
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Three studies mentioned the method of sequence generation and they were considered as low risk of bias (Cowell 2005; Chan 2010; Van der Linde 2011). Two of these studies used a computer program which did not have access to the rest of the data (Chan 2010; Van der Linde 2011). One of these studies used a minimisation technique with a computer program (Cowell 2005). One study did not mention how the random sequence generation was made and it was considered as having an unclear risk of bias (Rossebø 2008).
Allocation concealment
Three studies were considered as low risk of bias for allocation concealment (Cowell 2005; Chan 2010; Van der Linde 2011). One of these studies used a randomisation number from the study database via a secure Internet line (Chan 2010). Another study used numbered containers (Cowell 2005) and Van der Linde 2011 used a randomisation number that was sent to the site co‐ordinator by the pharmacology department and the study medication was sent to the participants. Rossebø 2008 did not mention how the allocation concealment was made and it was considered as unclear risk of bias (Rossebø 2008).
Blinding
Blinding of participants and personnel
We considered all four studies as low risk of bias for blinding of participants and personnel (Cowell 2005; Rossebø 2008; Chan 2010; Van der Linde 2011). All four studies were reported to be double‐blind, placebo‐controlled trials.
Blinding of outcome assessment
Three studies were classified as low risk of bias for blinding of outcome assessment (Rossebø 2008; Chan 2010; Van der Linde 2011). All members of the committees of these studies who evaluated the outcomes were blinded. One of the studies was classified as unclear risk of bias because it did not mention if the investigators were blinded to evaluate the data. This paper just mentioned what each author did in the study (Cowell 2005).
Incomplete outcome data
We considered all four studies as low risk of bias for incomplete outcome data (Cowell 2005; Rossebø 2008; Chan 2010; Van der Linde 2011). Withdrawn participants were well‐described in each study.
Selective reporting
We classified all four studies as low risk of bias for selective reporting (Cowell 2005; Rossebø 2008; Chan 2010; Van der Linde 2011). These four studies evaluated their primary and secondary outcomes and reported the results. Three of these four studies reported the study protocol (Cowell 2005; Rossebø 2008; Chan 2010).
Other potential sources of bias
We considered only one study as low risk of bias for other potential sources of bias (Van der Linde 2011). Three industry funded studies were considered high risk of bias (Cowell 2005; Rossebø 2008; Chan 2010). One of the studies received funding from the Canadian Institute of Health Research and Astra Zeneca Canada, but Astra Zeneca had no input into the study design and analysis (Chan 2010). In another study, the drug was provided by Pfizer which had no access to the rest of the data (Cowell 2005). The PROCAS trial did not received funding from any organisation (Van der Linde 2011). The SEAS trial received funding from Merck, but the scientific responsibility of the study remained with the independent steering committee (Rossebø 2008).
Effects of interventions
See: Table 1
Primary outcomes
1. Severity of aortic valve stenosis
1.1 Mean pressure gradient
Two studies included mean pressure gradient (Rossebø 2008; Van der Linde 2011). The mean pressure gradient did not differ in the participants using statin compared with the participants using placebo (mean difference (MD) ‐0.54, 95% confidence interval (CI) ‐1.88 to 0.80; studies = 2; participants = 1935; low‐quality evidence) Analysis 1.1. There was low heterogeneity in this comparison (I² = 37%).
1.1. Analysis.
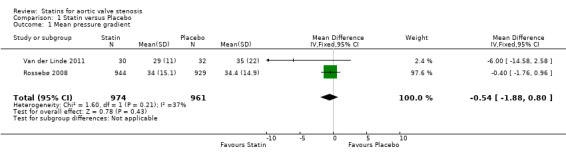
Comparison 1 Statin versus Placebo, Outcome 1 Mean pressure gradient.
1.2 Valve area
Two studies included valve area (Chan 2010; Van der Linde 2011). The valve area did not differ in the participants using statin compared with the participants using placebo (MD ‐0.07, 95% CI ‐0.28 to 0.14; studies = 2; participants = 127; low‐quality evidence ) Analysis 1.2 . There was low heterogeneity in this comparison (I² = 10%).
1.2. Analysis.
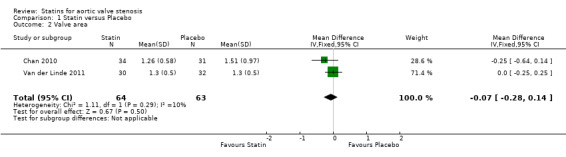
Comparison 1 Statin versus Placebo, Outcome 2 Valve area.
1.3 Aortic jet velocity
Only one study included aortic jet velocity as outcome (MD ‐0.06, 95% CI ‐0.26 to 0.14; participants = 155; study = 1; low‐quality evidence) (Cowell 2005). There were no more studies for comparison Analysis 1.3. Heterogeneity was not applicable.
1.3. Analysis.
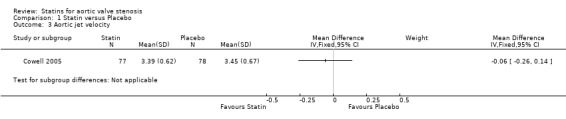
Comparison 1 Statin versus Placebo, Outcome 3 Aortic jet velocity.
2. Freedom from valve replacement
Four studies included freedom from valve replacement (Cowell 2005; Rossebø 2008; Chan 2010; Van der Linde 2011). Freedom from valve replacement did not differ in the participants using statin (309/1185, 26%) compared with the participants using placebo (330/1175, 28%) (risk ratio (RR) 0.93, 95% CI 0.81 to 1.06; studies = 4; participants = 2360; moderate‐quality evidence) Analysis 1.4. There was no substantial heterogeneity in this comparison (I² = 0%).
1.4. Analysis.
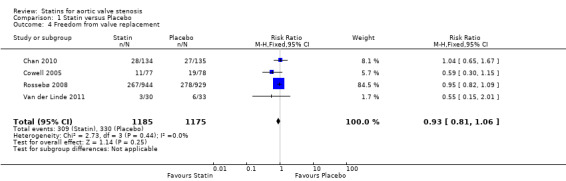
Comparison 1 Statin versus Placebo, Outcome 4 Freedom from valve replacement.
3. Death from cardiovascular cause
Three studies included death from cardiovascular cause (Cowell 2005; Rossebø 2008; Chan 2010). Death from cardiovascular cause did not differ in the participants using statin (52/1155, 4.5 %) compared with the participants using placebo (64/ 1142, 5.6%) (RR 0.80, 95% CI 0.56 to 1.15; studies = 3; participants = 2297; low‐quality evidence) Analysis 1.5.There was no substantial heterogeneity in this comparison (I² = 0%).
1.5. Analysis.
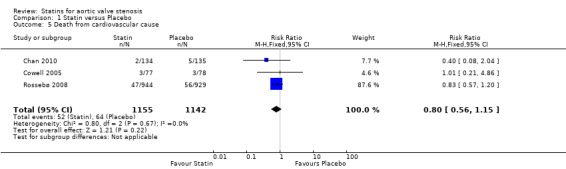
Comparison 1 Statin versus Placebo, Outcome 5 Death from cardiovascular cause.
Secondary Outcomes
1. Hospitalisation for any reason
Only one study included hospitalisation for any reason as outcome (RR 0.84, 95% CI 0.39 to 1.84; participants = 155; study = 1; very low‐quality evidence) (Cowell 2005). This outcome occurred in the participants using statin (10/77, 12.9%) compared with the participants using placebo (12/ 78, 15.3%) Analysis 1.6. There were no more studies for comparison. Heterogeneity was not applicable.
1.6. Analysis.
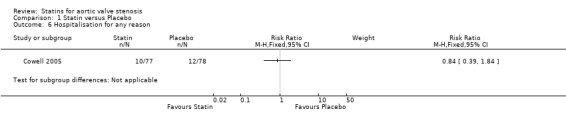
Comparison 1 Statin versus Placebo, Outcome 6 Hospitalisation for any reason.
2. Overall mortality
Studies did not report data on overall mortality as an outcome.
3. Adverse events
We evaluated a total of five adverse events that were present in at least two studies (muscle pain, hepatic enzymes, hepatitis, gastrointestinal condition and creatine kinase). We considered muscle pain as the main adverse event because it is the most prevalent adverse event that can limit the use of statin.
3.1 Muscle pain
Three studies included muscle pain (Rossebø 2008; Chan 2010; Van der Linde 2011). Muscle pain did not differ in the participants using statin (169/1107, 15.26%) compared with the participants using placebo (184/1097, 16.77%) (RR 0.91, 95% CI 0.75 to 1.09; studies = 3; participants = 2204; moderate‐quality evidence) Analysis 1.7. There was no substantial heterogeneity in this comparison (I² = 0%).
1.7. Analysis.
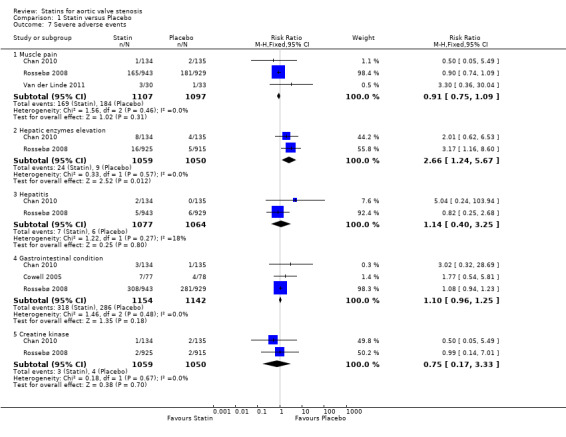
Comparison 1 Statin versus Placebo, Outcome 7 Severe adverse events.
3.2 Hepatic enzymes elevation
Two studies included hepatic enzymes elevation (Rossebø 2008; Chan 2010). Hepatic enzymes elevation differed in the participants using statin (24/1059, 2.26%) compared with the participants using placebo (9/1050, 0.85%) (RR 2.66, 95% CI 1.24 to 5.67; studies = 2; participants = 2109) Analysis 1.7. There was no substantial heterogeneity in this comparison (I² = 0%).
3.3 Hepatitis
Two studies included hepatitis (Rossebø 2008; Chan 2010). Hepatitis did not differ in the participants using statin (7/1077, 0.64 %) compared with the participants using placebo (6/1064, 0.56%) (RR 1.14, 95% CI 0.40 to 3.25; studies = 2; participants = 2141) Analysis 1.7. There was low heterogeneity in this comparison (I² = 18%).
3.4 Gastrointestinal condition, gastrointestinal serious event or gastrointestinal symptoms
Three studies included gastrointestinal condition, gastrointestinal serious event or gastrointestinal symptoms respectively (Cowell 2005; Rossebø 2008; Chan 2010). Gastrointestinal condition, gastrointestinal serious event or gastrointestinal symptoms did not differ in the participants using statin (318/1154, 27%) compared with the participants using placebo (286/1142, 25%) (RR 1.10, 95% CI 0.96 to 1.25; studies = 3; participants = 2296) Analysis 1.7. There was no substantial heterogeneity in this comparison (I² = 0%).
3.5 Creatine kinase elevation
Two studies included creatine kinase elevation (Rossebø 2008; Chan 2010). Creatine kinase elevation did not differ in the participants using statin (3/1059, 0.28%) compared with the participants using placebo (4/1050, 0.38%) (RR 0.75, 95% CI 0.17 to 3.33; studies = 2; participants = 2109) Analysis 1.7. There was no substantial heterogeneity in this comparison (I² = 0%).
4. Patient quality of life
Studies did not report data on patient quality of life as an outcome.
Overall quality of the body of evidence: 'Summary of findings' table
The quality of evidence of our the obtained results was very low to moderate. For more details, see Table 1.
Discussion
The general objective of this review was to evaluate the effectiveness and safety of statins in aortic valve stenosis. We evaluated the effectiveness of statins in aortic valve stenosis through the proposed outcomes found in the included studies. We checked the safety of statins as an important factor for the success of the treatment by analysing the adverse events found in the included studies. Though we had intended to conduct a subgroup analysis (mild, moderate and severe aortic valve stenosis groups) to check the effect of statins on the different subgroups, we were unable to do so due to insufficient number of trials (less than 10). None of the included studies reported on quality of life and overall mortality in their outcomes.
Summary of main results
Only four randomised controlled trials from the electronic searches were included in this review (Cowell 2005; Rossebø 2008; Chan 2010; Van der Linde 2011). A total of 2360 adults were randomised (age ranged 18 to 85 years). We analysed primary and secondary outcomes considered for this review in each included study. We did not identify differences between the group using statins alone or statins in association with other drugs to reduce cholesterol levels when compared with the placebo group for the following primary outcomes: mean pressure gradient (low‐quality evidence) Analysis 1.1, valve area (low‐quality evidence) Analysis 1.2, freedom from valve replacement (moderate‐quality evidence) Analysis 1.4 and death from cardiovascular cause (low‐quality evidence) Analysis 1.5. We did not carry out meta‐analysis to assess aortic jet velocity as only one study included this outcome (low‐quality evidence) Analysis 1.3. For secondary outcomes, we identified differences between the group using statins alone or statins in association with other drugs to reduce cholesterol levels when compared with the placebo group only for the adverse events. We analysed five adverse events, among which was muscle pain. We considered muscle pain as the main adverse event because it is the most prevalent adverse event that can limit the use of statin (moderate‐quality evidence) Analysis 1.7. The quality of evidence of the obtained results for the analysed outcomes was very low to moderate. For more details, see Table 1. We did not carry out meta‐analysis to assess other secondary outcomes as hospitalisation for any reason (very low‐quality evidence), as only one study reported this outcome Analysis 1.6. None of the four included studies evaluated the secondary outcomes of overall mortality nor patient quality of life. We could not find sufficient number of trials to conduct subgroup analysis and sensitivity analysis (more than 10). The findings of this systematic review did not show that statins may be able to delay the progression of aortic valve stenosis.
Overall completeness and applicability of evidence
The general objective of this review was to evaluate the effectiveness and safety of statins in aortic valve stenosis. We summarised the studies with the best evidence available about this subject. Considering the analysed outcomes, our results are applicable for people suffering from aortic valve stenosis. However, we were unable to find enough studies in the electronic database search that allowed us to assess all objectives of the review. All types of participants and interventions cited in the method section were investigated in the included studies, but we could not carry out a meta‐analysis of the outcomes aortic jet velocity and hospitalisation for any reason as both outcomes were only reported in one study (Cowell 2005). We did not find the outcomes overall mortality and patient quality of life reported in the included studies. Two studies included participants who were current smokers and had other comorbidities such as hypertension, coronary heart disease, β‐blocker and aspirin use, high levels of LDL (Cowell 2005; Rossebø 2008). These comorbidities could have adversely affected the analysis of the results and they require analyses of their effect on each of these subgroups. These difficulties encountered during the review process did not allow us to find a high‐quality evidence in our findings, but our obtained results confirm the current international guidelines which do not recommend statins to slow down the progression of aortic valve stenosis.
Quality of the evidence
The GRADE approach was employed to assess the quality of result findings and the GRADE profiler (GRADEPRO) allowed us to import data from Review Manager 5.3 to create 'Summary of findings' tables. The quality of evidence ranged from moderate to very low across the different outcomes, mainly due to risk of bias and imprecise results. One of the four included studies was low risk of bias for the following criteria: random sequence generation; allocation concealment; blinding of participants; personnel and outcome assessors; incomplete outcome data and selective reporting among other sources of bias (Van der Linde 2011). One study had unclear risk of bias for random sequence generation and allocation concealment (selection bias) (Rossebø 2008). Three studies were high risk of bias for other bias because they were industry funded trials (Cowell 2005; Rossebø 2008; Chan 2010). We could not elucidate the risk of bias for these studies even having contacted their authors. Overall, the studies were small and had short follow‐ups (Cowell 2005; Chan 2010; Van der Linde 2011) which may have meant a lack of rigor in their methods and may have produced inconsistent results. Regarding the findings of the studies, particularly the continuous outcomes of the severity of aortic valve stenosis were not affected by dropouts. In the largest trial (Rossebo et al, 1873 of the 2360 patients considered in the meta‐analysis), 1857 patients (99%) were followed until the end of the study (median 52.2 months) and 1693 (90%) were included in an echocardiographic substudy (Rossebø 2008). In the Van der Linde study, 55/63 (87%) of patients completed follow‐up (Van der Linde 2011). In Cowell 2005, 11/155 patients (7%) discontinued treatment because of adverse effects and 134/155 (86.5%) completed the echocardiographic follow‐up. In Chan 2010, the discontinuation rate was high (123/269, 45%). There were 51 cases due to adverse events, but only three patients were lost during the follow‐up. Per‐protocol analysis yielded similar results to intention‐to‐treat analysis. We were unable to assess the publication bias in funnel plots due to limited number of the included studies (less than 10). All of these considerations were taken into account when interpreting the results of this systematic review.
Potential biases in the review process
We attempted to include all relevant studies in this review from an electronic sensitised search. Two review authors independently selected, collected and analysed the data in order to minimise bias. One limitation was that we could not find sufficient studies to perform sensitivity analysis, subgroup analysis, or to assess publication bias. Another limitation was that we could not carry out meta‐analysis to assess the secondary outcome of hospitalisation for any reason as only one trial reported this outcome, and the secondary outcomes of overall mortality and patient quality of life were not reported in any of the trials.
Agreements and disagreements with other studies or reviews
A meta‐analysis published by Teo KK et al in 2011 (2344 participants) (Teo 2011) did not find differences of effect between statin or placebo groups for similar outcomes such as aortic valve stenosis severity, freedom from valve replacement and cardiovascular death either. However, we analysed the safety of statins through their adverse events in this paper. We found differences in one adverse event between statin and placebo group: hepatic enzymes (RR 2.66, 95% CI 1.24 to 5.67; studies = 2; participants = 2109). Nevertheless, a recent review demonstrated that drug‐induced liver injury statin is rare (22 cases from 1188 drug‐induced liver injury cases, eight years of follow‐up) (Russo 2014).
Authors' conclusions
Implications for practice.
We found only four randomised controlled trials in the electronic search which analysed the role of statins on the progression of aortic valve stenosis. Some of these studies had limitations such as follow‐up, sample size and the randomisation process. Some objectives of the review could not be accomplished due to the insufficient number of the included studies. This also affected the quality of our obtained results which did not show statins can delay the progression of aortic valve stenosis. The quality of evidence of the obtained results was very low to moderate. Although the first experimental and retrospective studies found some benefit of statins in aortic valve stenosis in the past decade, European and USA guidelines do not recommend this intervention (Vahanian 2012; Nishimura 2014). Our findings are consistent with these guidelines. However, we believe this issue is not over, mainly because the available evidence is based on studies which have limitations such as follow‐up, randomisation process, sample size and very elderly participants with many comorbidities.
Implications for research.
We believe the research for a clinical treatment for aortic valve stenosis should continue to be encouraged. The current option for the treatment of aortic valve stenosis is still aortic replacement surgery or transcatheter aortic‐valve implantation (TAVI). However, because this medical condition has a long progression, when patients are submitted to these procedures they are symptomatic, have severe aortic valve stenosis, and high mortality. For future clinical treatment research on aortic valve stenosis, an alternative might be to broaden the pathophysiology knowledge of this disease and search for other risk factors such as accumulation of calcium, heredity, diabetes, vitamin D, inflammation, oxidative stress, hypertension, among others that could contribute to its development. A suggestion regarding these risk factors, for example, could be to compare individuals suffering from hypertension and diabetes with individuals suffering only from diabetes. In this comparison, it could be assessed if the drug for hypertension would prevent the development of aortic valve stenosis. Searching for such risk factors for aortic valve stenosis might promote future clinical trials.
Acknowledgements
We thank the staff of the Cochrane Heart Group for their help and assistance with the systematic review. We also thank Dr Álvaro Nagib Atallah (Director) and the staff of the Brazilian Cochrane Centre for their orientation and assistance with the review. We thank Patricia Moerbeck Casadei for her assistance with the English version of this study. We are grateful to Nicole Martin and Jo Abbot for conducting the searches for this review.
Appendices
Appendix 1. Search strategies
CENTRAL
#1MeSH descriptor Aortic Valve Stenosis explode all trees #2MeSH descriptor Heart Valve Diseases, this term only #3(aortic near/2 stenos?s) #4((heart or aortic) near/2 valv* near/2 disease*) #5(bicuspid near/2 calcification) #6(tricuspid near/2 calcification) #7((aortic or valve) near/2 calcification) #8(bicuspid near/2 valve*) #9 (tricuspid near/2 valve*) #10(calcinos?s) #11(calci* near/3 stenos?s) #12(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11) #13MeSH descriptor Anticholesteremic Agents explode all trees #14MeSH descriptor Hydroxymethylglutaryl CoA Reductases explode all trees #15(hydroxymethylglutaryl* near/5 inhibitor*) #16 (hmg‐coa* near/5 statin*) #17(hmg‐coa* near/5 inhibit*) #18(3‐hydroxy‐3‐methylpentanedioic acid) #19(beta‐hydroxy‐beta‐methylglutarate) #20(3‐hydroxy‐3‐methylglutaric acid) #21(statin*) #22(altoc?r or altoprev or artein or atorvastatin) #23(baycol or bristacol or "bay w 6228" or "bay w6228") #24(canef or cerivastatin or certa or compactin or cranoc or crestor or ci‐981 or ci981 or cs‐500 or cs500 or cs‐514 or cs514) #25(dalvastatin or denan) #26(elisor or epatostantin or eptastatin* or epistatin or fluindostatin or fluvastatin or gerosim or itavastatin) #27(lescol or leucol or lipemol or lipitor or lipibec or liplat or lipex or lipobay or lipovas or lipostat or livalo or loc?ol or lodales or lovacol or lovastatin or l‐654969 or l‐644128 or l644128) #28 (mevastatin or mevastin or mevinolin or mona?olin or methylcompactin or mk‐803 or mk803 or mk‐0803 or mk0803 or msd‐803 or mevacor or mk‐733 or mk733 or meglutol or mevalotin or mevinacor or medostatin or ml‐236b or ml236b or medipo) #29 (nk‐104 or nk104 or nks‐104 or nks104 or nisvastatin or neolipid) #30(pravastatin or prareduct or pravachol or pravacol or pravasin* or pitavastatin or pitava or pravachol) #31(rms‐431 or rms431 or ribar or rivastatin or rosuvastatin or RG‐12561) #32 (sanaprav or selektine or simvastatin or sinvacor or s?nvinolin or sortis or sq‐31000 or sq31000 sq‐31,000 or sq31,000 or sri‐62320 or sri62320 or s‐4522 or s4522) #33(tahor or torvast) #34(vast?n or xu‐62320 or xu62320 or ym‐548 or ym548 or zarator or zenas or zocor? or zd‐4522 or zd4522) #35(#13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34) #36(#12 AND #35)
MEDLINE
1. exp Aortic Valve Stenosis/ 2. Heart Valve Diseases/ 3. (aortic adj2 stenos?s).tw. 4. ((heart or aortic) adj2 valv* adj2 disease*).tw. 5. (bicuspid adj2 calcification).tw. 6. (tricuspid adj2 calcification).tw. 7. ((aortic or valve) adj2 calcification).tw. 8. (bicuspid adj2 valve*).tw. 9. (tricuspid adj2 valve*).tw. 10. calcinos?s.tw. 11. (calci* adj3 stenos?s).tw. 12. or/1‐11 13. exp Anticholesteremic Agents/ 14. exp Hydroxymethylglutaryl CoA Reductases/ 15. (hydroxymethylglutaryl* adj5 inhibitor*).tw. 16. (hmg‐coa* adj5 statin*).tw. 17. (hmg‐coa* adj5 inhibit*).tw. 18. 3‐hydroxy‐3‐methylpentanedioic acid.tw. 19. beta‐hydroxy‐beta‐methylglutarate.tw. 20. 3‐hydroxy‐3‐methylglutaric acid.tw. 21. statin*.tw. 22. (altoc?r or altoprev or artein or atorvastatin).tw. 23. (baycol or bristacol or "bay w 6228" or "bay w6228").tw. 24. (canef or cerivastatin or certa or compactin or cranoc or crestor or ci‐981 or ci981 or cs‐500 or cs500 or cs‐514 or cs514).tw. 25. (dalvastatin or denan).tw. 26. (elisor or epatostantin or eptastatin* or epistatin or fluindostatin or fluvastatin or gerosim or itavastatin).tw. 27. (lescol or leucol or lipemol or lipitor or lipibec or liplat or lipex or lipobay or lipovas or lipostat or livalo or loc?ol or lodales or lovacol or lovastatin or l‐654969 or l‐644128 or l644128).tw. 28. (mevastatin or mevastin or mevinolin or mona?olin or methylcompactin or mk‐803 or mk803 or mk‐0803 or mk0803 or msd‐803 or mevacor or mk‐733 or mk733 or meglutol or mevalotin or mevinacor or medostatin or ml‐236b or ml236b or medipo).tw. 29. (nk‐104 or nk104 or nks‐104 or nks104 or nisvastatin or neolipid).tw. 30. (pravastatin or prareduct or pravachol or pravacol or pravasin* or pitavastatin or pitava or pravachol).tw. 31. (rms‐431 or rms431 or ribar or rivastatin or rosuvastatin or RG‐12561).tw. 32. (sanaprav or selektine or simvastatin or sinvacor or s?nvinolin or sortis or sq‐31000 or sq31000 sq‐31,000 or sq31,000 or sri‐62320 or sri62320 or s‐4522 or s4522).tw. 33. (tahor or torvast).tw. 34. (vast?n or xu‐62320 or xu62320 or ym‐548 or ym548 or zarator or zenas or zocor? or zd‐4522 or zd4522).tw. 35. or/13‐34 36. 12 and 35 37. randomized controlled trial.pt. 38. controlled clinical trial.pt. 39. randomized.ab. 40. placebo.ab. 41. drug therapy.fs. 42. randomly.ab. 43. trial.ab. 44. groups.ab. 45. 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 46. exp animals/ not humans.sh. 47. 45 not 46 48. 36 and 47
Embase
1. exp Aortic Valve Stenosis/ 2. Heart Valve Diseases/ 3. (aortic adj2 stenos?s).tw. 4. ((heart or aortic) adj2 valv* adj2 disease*).tw. 5. (bicuspid adj2 calcification).tw. 6. (tricuspid adj2 calcification).tw. 7. ((aortic or valve) adj2 calcification).tw. 8. (bicuspid adj2 valve*).tw. 9. (tricuspid adj2 valve*).tw. 10. calcinos?s.tw. 11. (calci* adj3 stenos?s).tw. 12. or/1‐11 13. exp Anticholesteremic Agents/ 14. exp Hydroxymethylglutaryl CoA Reductases/ 15. (hydroxymethylglutaryl* adj5 inhibitor*).tw. 16. (hmg‐coa* adj5 statin*).tw. 17. (hmg‐coa* adj5 inhibit*).tw. 18. 3‐hydroxy‐3‐methylpentanedioic acid.tw. 19. beta‐hydroxy‐beta‐methylglutarate.tw. 20. 3‐hydroxy‐3‐methylglutaric acid.tw. 21. statin*.tw. 22. (altoc?r or altoprev or artein or atorvastatin).tw. 23. (baycol or bristacol or "bay w 6228" or "bay w6228").tw. 24. (canef or cerivastatin or certa or compactin or cranoc or crestor or ci‐981 or ci981 or cs‐500 or cs500 or cs‐514 or cs514).tw. 25. (dalvastatin or denan).tw. 26. (elisor or epatostantin or eptastatin* or epistatin or fluindostatin or fluvastatin or gerosim or itavastatin).tw. 27. (lescol or leucol or lipemol or lipitor or lipibec or liplat or lipex or lipobay or lipovas or lipostat or livalo or loc?ol or lodales or lovacol or lovastatin or l‐654969 or l‐644128 or l644128).tw. 28. (mevastatin or mevastin or mevinolin or mona?olin or methylcompactin or mk‐803 or mk803 or mk‐0803 or mk0803 or msd‐803 or mevacor or mk‐733 or mk733 or meglutol or mevalotin or mevinacor or medostatin or ml‐236b or ml236b or medipo).tw. 29. (nk‐104 or nk104 or nks‐104 or nks104 or nisvastatin or neolipid).tw. 30. (pravastatin or prareduct or pravachol or pravacol or pravasin* or pitavastatin or pitava or pravachol).tw. 31. (rms‐431 or rms431 or ribar or rivastatin or rosuvastatin or RG‐12561).tw. 32. (sanaprav or selektine or simvastatin or sinvacor or s?nvinolin or sortis or sq‐31000 or sq31000 sq‐31,000 or sq31,000 or sri‐62320 or sri62320 or s‐4522 or s4522).tw. 33. (tahor or torvast).tw. 34. (vast?n or xu‐62320 or xu62320 or ym‐548 or ym548 or zarator or zenas or zocor? or zd‐4522 or zd4522).tw. 35. or/13‐34 36. 12 and 35 37. random$.tw. 38. factorial$.tw. 39. crossover$.tw. 40. cross over$.tw. 41. cross‐over$.tw. 42. placebo$.tw. 43. (doubl$ adj blind$).tw. 44. (singl$ adj blind$).tw. 45. assign$.tw. 46. allocat$.tw. 47. volunteer$.tw. 48. crossover procedure/ 49. double blind procedure/ 50. randomized controlled trial/ 51. single blind procedure/ 52. 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 53. (animal/ or nonhuman/) not human/ 54. 52 not 53 55. 36 and 54
Web of Science
# 34 #33 AND #32 # 33 TS =((random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)) # 32 #31 AND #10 # 31 #30 OR #29 OR #28 OR #27 OR #26 OR #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 OR #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 # 30 TS =((vast?n or xu‐62320 or xu62320 or ym‐548 or ym548 or zarator or zenas or zocor? or zd‐4522 or zd4522)) # 29 TS =((tahor or torvast)) # 28 TS =((sanaprav or selektine or simvastatin or sinvacor or s?nvinolin or sortis or sq‐31000 or sq31000 sq‐31,000 or sq31,000 or sri‐62320 or sri62320 or s‐4522 or s4522)) # 27 TS =((rms‐431 or rms431 or ribar or rivastatin or rosuvastatin or RG‐12561)) # 26 TS =((pravastatin or prareduct or pravachol or pravacol or pravasin* or pitavastatin or pitava or pravachol)) # 25 TS =((nk‐104 or nk104 or nks‐104 or nks104 or nisvastatin or neolipid)) # 24 TS =((mevastatin or mevastin or mevinolin or mona?olin or methylcompactin or mk‐803 or mk803 or mk‐0803 or mk0803 or msd‐803 or mevacor or mk‐733 or mk733 or meglutol or mevalotin or mevinacor or medostatin or ml‐236b or ml236b or medipo)) # 23 TS =((lescol or leucol or lipemol or lipitor or lipibec or liplat or lipex or lipobay or lipovas or lipostat or livalo or loc?ol or lodales or lovacol or lovastatin or l‐654969 or l‐644128 or l644128)) # 22 TS =((elisor or epatostantin or eptastatin* or epistatin or fluindostatin or fluvastatin or gerosim or itavastatin)) # 21 TS =((dalvastatin or denan)) # 20 TS =((canef or cerivastatin or certa or compactin or cranoc or crestor or ci‐981 or ci981 or cs‐500 or cs500 or cs‐514 or cs514)) # 19 TS =((baycol or bristacol or "bay w 6228" or "bay w6228")) # 18 TS =((altoc?r or altoprev or artein or atorvastatin)) # 17 TS =((statin*)) # 16 TS =((3‐hydroxy‐3‐methylglutaric acid)) # 15 TS =((beta‐hydroxy‐beta‐methylglutarate)) # 14 TS =((3‐hydroxy‐3‐methylpentanedioic acid)) # 13 TS =((hmg‐coa* near/5 inhibit*)) # 12 TS =((hmg‐coa* near/5 statin*)) # 11 TS =((hydroxymethylglutaryl* near/5 inhibitor*)) # 10 #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 # 9 TS =((calci* near/3 stenos?s)) # 8 TS =((calcinos?s)) # 7 TS =((tricuspid near/2 valve*)) # 6 TS =((bicuspid near/2 valve*)) # 5 TS =(((aortic or valve) near/2 calcification)) # 4 TS =((tricuspid near/2 calcification)) # 3 TS =((bicuspid near/2 calcification)) # 2 TS =(((heart or aortic) near/2 valv* near/2 disease*)) # 1 TS=((aortic near/2 stenos?s))
CINAHL
S34 S12 and S33 S33 S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 S32 (vast?n or xu‐62320 or xu62320 or ym‐548 or ym548 or zarator or zenas or zocor? or zd‐4522 or zd4522) S31 (tahor or torvast) S30 (sanaprav or selektine or simvastatin or sinvacor or s?nvinolin or sortis or sq‐31000 or sq31000 sq‐31,000 or sq31,000 or sri‐62320 or sri62320 or s‐4522 or s4522) S29 (rms‐431 or rms431 or ribar or rivastatin or rosuvastatin or RG‐12561) S28 (pravastatin or prareduct or pravachol or pravacol or pravasin* or pitavastatin or pitava or pravachol) S27 (nk‐104 or nk104 or nks‐104 or nks104 or nisvastatin or neolipid) S26 (mevastatin or mevastin or mevinolin or mona?olin or methylcompactin or mk‐803 or mk803 or mk‐0803 or mk0803 or msd‐803 or mevacor or mk‐733 or mk733 or meglutol or mevalotin or mevinacor or medostatin or ml‐236b or ml236b or medipo) S25 (lescol or leucol or lipemol or lipitor or lipibec or liplat or lipex or lipobay or lipovas or lipostat or livalo or loc?ol or lodales or lovacol or lovastatin or l‐654969 or l‐644128 or l644128) S24 (elisor or epatostantin or eptastatin* or epistatin or fluindostatin or fluvastatin or gerosim or itavastatin) S23 (dalvastatin or denan) S22 (canef or cerivastatin or certa or compactin or cranoc or crestor or ci‐981 or ci981 or cs‐500 or cs500 or cs‐514 or cs514) S21 (baycol or bristacol or "bay w 6228" or "bay w6228") S20 (altoc?r or altoprev or artein or atorvastatin) S19 (statin*) S18 (3‐hydroxy‐3‐methylglutaric acid) S17 (beta‐hydroxy‐beta‐methylglutarate) S16 (3‐hydroxy‐3‐methylpentanedioic acid) S15 (hmg‐coa* N5 inhibit*) S14 (hmg‐coa* N5 statin*) S13 (hydroxymethylglutaryl* N5 inhibitor*) S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 S11 (calci* N3 stenos?s) S10 (calcinos?s) S9 (tricuspid N2 valve*) S8 (bicuspid N2 valve*) S7 ((aortic or valve) N2 calcification) S6 (tricuspid N2 calcification) S5 (bicuspid N2 calcification) S4 ((heart or aortic) N2 valv* N2 disease*) S3 (aortic N2 stenos?s) S2 (MH "Heart Valve Diseases") S1 (MH "Aortic Valve Stenosis+")
LILACS
#1 (mh:"Estenose da Valva Aórtica" OR "Aortic Stenosis" OR "Estenose da Valva Aórtica" OR "Estenosis de la Válvula Aórtica" OR "Aortic Valve Stenosis" OR MH:C14.280.484.150$ OR MH:C14.280.955.249$ OR "Estenose Aórtica") OR (MH:"Heart Valve Diseases" OR "Enfermedades de las Válvulas Cardíacas" OR "Doenças das Valvas Cardíacas" OR "Valvular Heart Diseases" OR "Doenças Valvares do Coração" OR "Doenças Valvares Cardíacas" OR MH:C14.280.484$) OR (TW:aortic stenos$ (436) OR TW:(heart or aortic) valv$ disease$ OR TW:(bicuspid calcification) OR TW:(tricuspid calcification) OR TW:((aortic or valve) calcification) OR TW:(bicuspid valve$) OR tw:(tricuspid valve$) OR TW:calcinos$ OR TW:(calci$ stenos$) )
#2 (MH:Anticolesterêmicos OR MH:"Anticholesteremic Agents" OR "Agentes Anticolesterémicos" OR "Anticholesteremic Agents" OR "Cholesterol Inhibitors" OR "Hypocholesteremic Agents" OR MH:D27.505.519.186.071.202$ OR MH:D27.505.954.557.500.202$ OR "Inibidores do Colesterol" OR "Agentes Hipocolesterêmicos") OR (MH:"Hidroximetilglutaril CoA Redutases" OR "Hydroxymethylglutaryl CoA Reductases" OR "Hidroximetilglutaril CoA Reductasas" OR "Hidroximetilglutaril CoA Redutases" OR "HMG CoA Reductases" OR MH:D08.811.682.047.385.415$) OR (TW:(hydroxymethylglutaryl$ Inhibitor$) OR TW:(hmg‐coa$ statin$) OR TW:(hmg‐coa$ inhibit$) OR TW:3‐hydroxy‐3‐methylpentanedioic acid OR TW:beta‐hydroxy‐beta‐methylglutarate) OR (TW:3‐hydroxy‐3‐methylglutaric acid OR TW:statin$ OR tw:(altoc$ or altoprev or artein or atorvastatin) OR TW:(baycol or bristacol or "bay w 6228" or "bay w6228") OR TW:(canef or cerivastatin or certa or compactin or cranoc or crestor or ci‐981 or ci981 or cs‐500 or cs500 or cs‐514 or cs514) OR TW:(dalvastatin or denan) OR TW:(elisor or epatostantin or eptastatin$ or epistatin or fluindostatin or fluvastatin or gerosim or itavastatin) OR TW:(lescol or leucol or lipemol or lipitor or lipibec or liplat or lipex or lipobay or lipovas or lipostat or livalo or loc$ or lodales or lovacol or lovastatin or l‐654969 or l‐644128 or l644128) OR TW:(mevastatin or mevastin or mevinolin or mona$olin or methylcompactin or mk‐803 or mk803 or mk‐0803 or mk0803 or msd‐803 or mevacor or mk‐733 or mk733 or meglutol or mevalotin or mevinacor or medostatin or ml‐236b or ml236b or medipo) OR tw:(nk‐104 or nk104 or nks‐104 or nks104 or nisvastatin or neolipid) OR tw:(pravastatin or prareduct or pravachol or pravacol or pravasin* or pitavastatin or pitava or pravachol) OR tw:(rms‐431 or rms431 or ribar or rivastatin or rosuvastatin or RG‐12561) OR TW:(sanaprav or selektine or simvastatin or sinvacor or sInvinolin or sortis or sq‐31000 or sq31000 sq‐31,000 or sq31,000 or sri‐62320 or sri62320 or s‐4522 or s4522) OR TW:(tahor or torvast) OR TW:(vast$ or xu‐62320 or xu62320 or ym‐548 or ym548 or zarator or zenas or zocor$ or zd‐4522 or zd4522))
#1 AND #2
(mh:"Estenose da Valva Aórtica" OR "Aortic Stenosis" OR "Estenose da Valva Aórtica" OR "Estenosis de la Válvula Aórtica" OR "Aortic Valve Stenosis" OR MH:C14.280.484.150$ OR MH:C14.280.955.249$ OR "Estenose Aórtica") OR (MH:"Heart Valve Diseases" OR "Enfermedades de las Válvulas Cardíacas" OR "Doenças das Valvas Cardíacas" OR "Valvular Heart Diseases" OR "Doenças Valvares do Coração" OR "Doenças Valvares Cardíacas" OR MH:C14.280.484$) OR (TW:aortic stenos$ (436) OR TW:(heart or aortic) valv$ disease$ OR TW:(bicuspid calcification) OR TW:(tricuspid calcification) OR TW:((aortic or valve) calcification) OR TW:(bicuspid valve$) OR tw:(tricuspid valve$) OR TW:calcinos$ OR TW:(calci$ stenos$) ) AND (MH:Anticolesterêmicos OR MH:"Anticholesteremic Agents" OR "Agentes Anticolesterémicos" OR "Anticholesteremic Agents" OR "Cholesterol Inhibitors" OR "Hypocholesteremic Agents" OR MH:D27.505.519.186.071.202$ OR MH:D27.505.954.557.500.202$ OR "Inibidores do Colesterol" OR "Agentes Hipocolesterêmicos") OR (MH:"Hidroximetilglutaril CoA Redutases" OR "Hydroxymethylglutaryl CoA Reductases" OR "Hidroximetilglutaril CoA Reductasas" OR "Hidroximetilglutaril CoA Redutases" OR "HMG CoA Reductases" OR MH:D08.811.682.047.385.415$) OR (TW:(hydroxymethylglutaryl$ Inhibitor$) OR TW:(hmg‐coa$ statin$) OR TW:(hmg‐coa$ inhibit$) OR TW:3‐hydroxy‐3‐methylpentanedioic acid OR TW:beta‐hydroxy‐beta‐methylglutarate) OR (TW:3‐hydroxy‐3‐methylglutaric acid OR TW:statin$ OR tw:(altoc$ or altoprev or artein or atorvastatin) OR TW:(baycol or bristacol or "bay w 6228" or "bay w6228") OR TW:(canef or cerivastatin or certa or compactin or cranoc or crestor or ci‐981 or ci981 or cs‐500 or cs500 or cs‐514 or cs514) OR TW:(dalvastatin or denan) OR TW:(elisor or epatostantin or eptastatin$ or epistatin or fluindostatin or fluvastatin or gerosim or itavastatin) OR TW:(lescol or leucol or lipemol or lipitor or lipibec or liplat or lipex or lipobay or lipovas or lipostat or livalo or loc$ or lodales or lovacol or lovastatin or l‐654969 or l‐644128 or l644128) OR TW:(mevastatin or mevastin or mevinolin or mona$olin or methylcompactin or mk‐803 or mk803 or mk‐0803 or mk0803 or msd‐803 or mevacor or mk‐733 or mk733 or meglutol or mevalotin or mevinacor or medostatin or ml‐236b or ml236b or medipo) OR tw:(nk‐104 or nk104 or nks‐104 or nks104 or nisvastatin or neolipid) OR tw:(pravastatin or prareduct or pravachol or pravacol or pravasin* or pitavastatin or pitava or pravachol) OR tw:(rms‐431 or rms431 or ribar or rivastatin or rosuvastatin or RG‐12561) OR TW:(sanaprav or selektine or simvastatin or sinvacor or sInvinolin or sortis or sq‐31000 or sq31000 sq‐31,000 or sq31,000 or sri‐62320 or sri62320 or s‐4522 or s4522) OR TW:(tahor or torvast) OR TW:(vast$ or xu‐62320 or xu62320 or ym‐548 or ym548 or zarator or zenas or zocor$ or zd‐4522 or zd4522))
Data and analyses
Comparison 1. Statin versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean pressure gradient | 2 | 1935 | Mean Difference (IV, Fixed, 95% CI) | ‐0.54 [‐1.88, 0.80] |
| 2 Valve area | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.28, 0.14] |
| 3 Aortic jet velocity | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Freedom from valve replacement | 4 | 2360 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.81, 1.06] |
| 5 Death from cardiovascular cause | 3 | 2297 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.56, 1.15] |
| 6 Hospitalisation for any reason | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 Severe adverse events | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Muscle pain | 3 | 2204 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.75, 1.09] |
| 7.2 Hepatic enzymes elevation | 2 | 2109 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.24, 5.67] |
| 7.3 Hepatitis | 2 | 2141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.40, 3.25] |
| 7.4 Gastrointestinal condition | 3 | 2296 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.96, 1.25] |
| 7.5 Creatine kinase | 2 | 2109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.33] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chan 2010.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | Men and women between 18 and 82 years of age (mean of age = 58 years). Total participants = 269 (from 23 Canadian Centres) with asymptomatic mild aortic valve stenosis defined by maximum aortic valve velocity between 2.5 and 4.0 m/s. Participants were followed for a minimum of 3 years to a maximum of 5 years (median follow‐up = 3.5 years). Baseline Characteristics of Participants: Men = 60.5% (rosuvastatin group) and 63% (placebo group). Tricuspid Aortic Valve = 29.1% (rosuvastatin group) and 34.1% (placebo group). Bicuspid Aortic Valve = 53.7% (rosuvastatin group) and 45.2% (placebo group). Uncertain Aortic Valve Morphology = 17.2% (rosuvastatin group) and 20.7% (placebo group). Mean Pressure Gradient, mean (± SD) mmHg = 22.5 (± 7.6) (rosuvastatin group) and 23.1(± 7.6) (placebo group). Peak Pressure Gradient, mean (± SD) mmHg = 40.8 (± 11.1) (rosuvastatin group) and 41.6 (± 10.9) (placebo group). Aortic Valve Area, mean (± SD) cm² = 1.49 (± 0.71) (rosuvastatin group) and 1.56 (± 0.70) (placebo group). Peak AS Velocity, mean (± SD) m/s = 3.16 (± 0.42) (rosuvastatin group) and 3.19 (± 0.42) (placebo group). LDL value (± SD) = 3.18 mmol/L (± 0.63 SD) (rosuvastatin group) and 3.12 mmol/L (± 0.74 SD) (placebo group). Systolic blood pressure, mean (± SD) mmHg = 128.8 (± 15.67) (rosuvastatin group) and 128.4 (± 15.94) (placebo group). Diastolic blood pressure, mean (± SD) = 76.5 (± 10.04) (rosuvastatin group) and 75.9 (± 10.92) (placebo group). Most of the participants were white. |
|
| Interventions | Rosuvastatin 40 mg daily (n = 134) or matching placebo (n = 135). Participants were randomised in 1:1 fashion in blocks of 4. | |
| Outcomes | Primary Outcome: Transvalvular aortic stenosis gradient and aortic valve area measured by Doppler echocardiography. Secondary Outcome: Aortic valve replacement and cardiovascular death. |
|
| Notes | The trial was supported by the Canadian Institute of Health Research with additional support from Astra Zeneca Canada Inc, but Astra Zeneca Canada had no input into the study design and data analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Rosuvastatin and placebo groups were randomised using a computer program at Astra Zeneca Canada Inc, which has no access to the rest of the data. |
| Allocation concealment (selection bias) | Low risk | A randomisation number was from the study database via a secure Internet line. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, site co‐ordinators, investigators and statisticians were all blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The members of Committee who evaluated all outcomes and serious adverse events were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants who withdrew from the study were well‐described and all the randomised patients were evaluated for outcomes. |
| Selective reporting (reporting bias) | Low risk | Study protocol is available and is well‐described. |
| Other bias | High risk | The study received funding from the Canadian Institute of Health Research and Astra Zeneca Canada, but Astra Zeneca Canada had no input into the study design and a data analysis. |
Cowell 2005.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | 155 participants were randomised (older than 18 years of age, mean of age = 68 years) with calcific aortic stenosis, an aortic jet velocity of at least 2.5 m per second and aortic valve calcification measured by Doppler Echocardiography. Participants were followed between 7 and 36 months (median of 25 months). Baseline Characteristics of Participants: Men = 68% (atorvastatin group) and 72% (placebo group). Tricuspid Aortic Valve = 96% (atorvastatin group) and 97% (placebo group). Bicuspid Aortic Valve = 4% (atorvastatin group) and 3% (placebo group). Peak Pressure Gradient, mean (± SD) mmHg = 47.8 (± 17.4) (atorvastatin group) and 49.5 (± 19.5) (placebo group). Aortic Valve Area, mean (± SD) cm² = 1.03 (± 0.40) (atorvastatin group) and 1.02 (± 0.41) (placebo group). Aortic Jet Velocity, mean (± SD) m/s = 3.39 (± 0.62) (atorvastatin group) and 3.45 (± 0.67) (placebo group). Most of the participants had hypertension and used aspirin. Current Smoker = 27% (atorvastatin group) and 28% (placebo group). Coronary heart disease = 23% (atorvastatin group) and 26% (placebo group). β‐blocker use = 27% (atorvastatin group) and 34% (placebo group). LDL value (± SD) = 137 mg/dL (± 34) (atorvastatin group) and 133 mg/dL (± 30) (placebo group). |
|
| Interventions | Atorvastatin 80 mg daily (n = 77) or matching placebo (n = 78). | |
| Outcomes | Primary Outcome: Progression of aortic valve stenosis by changes in aortic jet velocity on doppler echocardiography and progression of valvular calcification by computer tomography. Secondary Outcome: Aortic valve replacement, death from any cause, death from cardiovascular causes, hospitalisation for severe aortic valve, hospitalisation for any cause and hospitalisation for cardiovascular causes. |
|
| Notes | The study drug was provided by Pfizer, which has no access to the rest of the data.The authors received support from Pfizer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Atorvastatin and placebo groups were randomised using a minimisation technique with a dedicated, locked computer program (Edinburgh University). |
| Allocation concealment (selection bias) | Low risk | Numbered containers were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | There was no information to say that the investigators were blinded to evaluate the data.The paper just mentioned what each author did in the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of missing values was well‐balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Study protocol is available and well‐described. |
| Other bias | High risk | The study drug was provided by Pfizer, which had no access to the rest of the data.The authors received support from Pfizer. |
Rossebø 2008.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | Men and women between the ages of 45 and 85 years (mean of age = 67.9) who had asymptomatic, mild to moderate aortic valve stenosis, as assessed on echocardiography, with peak aortic jet velocity of 2.5 to 4 m/s. 1873 participants were randomised. The minimum follow‐up was 4 years (median = 52.2 months). Baseline Characteristics of Participants: Men = 61.5% (simvastatin plus ezetimibe group) and 61.2 % (placebo group). Bicuspid Aortic Valve = 5% (simvastatin plus ezetimibe group) and 6.3% (placebo group). Peak Pressure Gradient, mean (± SD) mmHg = 39.3 (± 13.9) (simvastatin plus ezetimibe group) and 39.6 (± 8.7) (simvastatin plus ezetimibe group). Mean Pressure Gradient, mean (± SD) mmHg = 22.7 (± 8.8) (simvastatin plus ezetimibe group) and 23 (± 13.8) (simvastatin plus ezetimibe group). Aortic Valve Area, mean (± SD) cm² = 1.29 (± 0.48) (simvastatin plus ezetimibe group) and 1.27 (± 0.46) (placebo group). Peak Aortic Jet Velocity, mean (± SD) m/s = 3.09 (± 0.55) (simvastatin plus ezetimibe arm) and 3.10 (± 0.54) (placebo arm). Most of the participants were white and had hypertension. Former smoking = 37% (simvastatin plus ezetimibe group) and 35 %(placebo group). Neoplasm = 11% (simvastatin plus ezetimibe group ) and 8.4% (placebo group). β‐blocker and Aspirin use: 28% (simvastatin plus ezetimibe group) and 25% (placebo group). Calcium antagonist use = 17% (simvastatin plus ezetimibe group) and 16% (placebo group). Diuretic (including spironolactone) = 22% (simvastatin plus ezetimibe group) and 28% (placebo group). The mean of left ventricular ejection fraction was 66% for both groups. LDL value, (± SD) = 139 mg/dL (± 35) (simvastatin plus ezetimibe group) and 140 mg/dL (±36) (placebo group). |
|
| Interventions | Simvastatin (40 mg) plus ezetimibe (10 mg) (n = 944) or matching placebo (n = 929). | |
| Outcomes | Primary outcome: death from cardiovascular causes, aortic valve replacement, congestive heart failure, non‐fatal myocardial infarction, hospitalisation for unstable angina, coronary artery bypass grafting, percutaneous coronary intervention, or non‐haemorrhagic stroke. Secondary outcome: All primary outcomes above and progression of aortic stenosis as seen on echocardiography and safety of the study drugs. |
|
| Notes | 173 study sites in seven European countries. The authors received support from Merck. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Does not mention how the random sequence generation was carried out. |
| Allocation concealment (selection bias) | Unclear risk | Does not mention how the allocation concealment was carried out. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The Committee members who evaluated all outcomes were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The number of withdrawals were reported. |
| Selective reporting (reporting bias) | Low risk | Study protocol is available and well‐described. |
| Other bias | High risk | The study was provided by Merck, but the scientific responsibility remained with the independent steering committee. |
Van der Linde 2011.
| Methods | Randomised, double‐blind, placebo‐controlled trial. | |
| Participants | Women and men between 18 and 45 years of age (mean of age 33 years). Total participants = 242; 63 randomised with congenital aortic valve stenosis and peak aortic valve velocity of 2.5 m/s. The median follow‐up was 2.4 years. Baseline Characteristics of Participants: Men: 70% (rosuvastatin group) and 73% (placebo group). Bicuspid Valve: 93% (rosuvastatin group) and 88% (placebo group). Tricuspid Valve: 7 % (rosuvastatin group) and 12% (placebo group). Mean Pressure Gradient, mean (± SD) mmHg = 27 (± 10) (rosuvastatin group) and 32 (± 17) (placebo group). Peak Pressure Gradient, mean (± SD) mmHg = 48 (± 18) (rosuvastatin group) and 56 (± 28) (placebo group). Aortic Valve Area, mean (± SD) cm² = 1.3 (± 0.4) (rosuvastatin group) and 1.3 (± 0.5) (placebo group). Peak AS Velocity, mean (± SD) m/s = 3.4 (± 0.7) (rosuvastatin group) and 3.6 (± 0.9) (placebo group). Most of the participants had a previous intervention (surgical valvulotomy or balloon valvuloplasty) and none or a grade 1 of aortic regurgitation. Systolic blood pressure, mean (± SD) mmHg = 129 (± 16) (rosuvastatin group) and 131 (±16) (placebo group). Diastolic blood pressure, mean (± SD) mmHg = 76 (± 10) (rosuvastatin group) and 78 (± 10) (placebo group). Aortic valve calcium = 40% (rosuvastatin group) and 36% (placebo group). Left ventricular hypertrophy = 20% (rosuvastatin group) and 33%(placebo group). LDL value, (± SD) = 106 mg/dL (± 31) (rosuvastatin group) and 104 mg/dL (± 35) (placebo group). |
|
| Interventions | Rosuvastatin 10 mg (n = 30) or matching placebo (n = 33). | |
| Outcomes | Primary outcome: progression of peak aortic valve velocity. Secondary outcome: temporal changes in the left ventricular mass, ascending aortic diameter and N‐terminal pro‐brain natriuretic peptide. |
|
| Notes | Study Site: 6 tertiary referral centres for congenital heart disease in The Netherlands and Belgium. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Rosuvastatin and placebo groups were randomised using a computer program at the Erasmus Medical University Center pharmacology department, which had no access to the rest of data. |
| Allocation concealment (selection bias) | Low risk | A randomisation number was sent by pharmacology department to the site co‐ordinator and the study medication to the participants. The participants were unaware of the treatment assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients, physicians and investigators were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The investigators that evaluated all outcomes were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Patients who withdrew from the study were well‐described all the randomised patients were evaluated. |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but all data were properly reported. |
| Other bias | Low risk | There was no other bias. The study did not received support from any organisations. |
LDL: low‐density lipoprotein SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chan 2011 | Sub study of ASTRONOMER trial. |
| Ditchl 2008 | Study with methodological flaws and high risk of bias. |
| Panahi 2013 | Study with methodological flaws and high risk of bias. |
Characteristics of ongoing studies [ordered by study ID]
Kindo 2008.
| Trial name or title | Interest of statin in surgical aortic stenosis: from myocardial preconditioning to ventricular reverse remodeling |
| Methods | Randomised controlled trial. Allocation: Randomised. Endpoint Classification: Efficacy study. Intervention Model: Parallel assignment. Masking: Single‐blind (participant). Primary Purpose: Treatment. Phase: 3. |
| Participants | Inclusion Criteria: 1. Age > or = 70 years and < 80 years. 2. Severe aortic valve stenosis. 3. Indication for aortic valve replacement by bioprosthesis. 4. Ejection fraction > or = 50%. 5. Without treatment with statin and no renal failure. 6. Informed consent signed. Exclusion Criteria: 1. Ischemic heart disease. 2. Concomitant surgery to aortic valve replacement. 3. Emergency surgery and known intolerance for statin. 4. Pregnant woman. |
| Interventions | Atorvastatin 80 mg per day. |
| Outcomes | Primary outcomes: Phase I: To study changes on inflammatory markers after aortic valve replacement. Phase II: To study changes in left ventricular mass at the end of the study (12 months). [ Time Frame: 1 year ] [ Designated as safety issue: No ]. Secondary outcomes: Phase I: To study changes on mitochondrial function, reactive oxygen species, and perioperative systolic and diastolic functions. Phase II: To study changes on clinical status, systolic and diastolic functions during the one year follow‐up. [ Time Frame: 1 year ] [Designated as safety issue: No ]. |
| Starting date | December 2008. |
| Contact information | Michel KINDO, MD. University Hospital, Strasbourg. France. E‐mail: michel.kindo@chru‐strasbourg.fr. |
| Notes | ClinicalTrials.gov Identifier: NCT00811330. This study is currently recruiting participants. First received: December 17, 2008. Last updated: June 18, 2015. Last verified: June 2015. |
Schuler 2005.
| Trial name or title | Statin therapy in asymptomatic aortic stenosis |
| Methods | Randomised controlled trial. Allocation: Randomised. Endpoint Classification: Efficacy study. Intervention Model: Single‐group assignment. Masking: Double‐blind (participant, investigator, outcomes assessor). Primary Purpose: Treatment. Phase: 2. |
| Participants | Inclusion Criteria: 1. Age 21 years to 80 years. 2. Gender: both. 3. Mild to moderate aortic stenosis. 4. No symptoms caused by aortic stenosis. 5. Written informed consent to participate in the study. 6. Aortic valve leaflet thickening with reduced systolic opening. 7. Reduced aortic valve area > 0.8 cm2 and < 1.5 cm2. 8. Maximum aortic jet velocity at rest > 2.5 m/s. Exclusion Criteria: 1. Symptoms caused by aortic stenosis. 2. Aortic valve area < 0.7 cm2. 3. Severe aortic regurgitation. 4. Reduced left ventricular ejection fraction (< 50%). 5. Any valve disease with indication for surgery. 6. Coronary artery disease. 7. Therapy refractory arterial hypertension. 8. Comorbid noncardiac diseases or other reasons which make a regular follow‐up impossible. 9. Other indication for treatment with statins. 10. Women of childbearing potential not using the contraception method(s) specified in this study (specify), as well as women who are breastfeeding. 11. Known sensitivity to study drug(s) or class of study drug(s). 12. Patients with severe medical condition(s) that in the view of the investigator prohibits participation in the study (specify as required). 13. Use of any other investigational agent in the last 30 days. |
| Interventions | 40 mg fluvastatin daily. |
| Outcomes | Primary outcomes: 1. Progression of calcified aortic stenosis measured by: [ Time Frame: 24 months ] [ Designated as safety issue: No ]. 2. Transthoracic echocardiography (peak max/ mean, velocity max and aortic valve area) [ Time Frame: 24 months ] [ Designated as safety issue: No ]. 3. Catheterisation (peak to peak gradient, left ventricular function and compliance) [ Time Frame: 24 months ] [ Designated as safety issue: No ]. Secondary outcomes: Number of cardiovascular events [ Time Frame: 24 months ] [ Designated as safety issue: No ]. |
| Starting date | January 2003. |
| Contact information | Claudia Walther, MD. University of Leipzig. Germany. Telephone: xx49‐341‐8651428. E‐mail: waltherc@medizin.uni‐leipzig.de. |
| Notes | ClinicalTrials.gov Identifier: NCT00176410. The recruitment status of this study is unknown because the information has not been verified recently. First received: September 13, 2005. Last updated: January 13, 2010. Last verified: September 2006. |
Differences between protocol and review
We included GRADE to assess the quality evidence of the review (GRADE PRO 2011).
Two new co‐authors were added (Cristiane Rufino Macedo and Jonathan Nyong).
We excluded two studies on the basis of high risk of bias and methodological flaws as instructed by the editorial staff. The low number of patients in these trials suggests that no significant change could occur in meta‐analysis results.
In the protocol of this review, we planned to assess if the effect of the treatment varies according to different populations (age, gender and severity of illness) or to characteristics of the intervention (type of statin). However, the numbers of the included studies identified by the electronic search were not enough to carry out subgroup analysis. We also did not conduct the planned sensitivity analysis to assess the robustness of the observed effects due to insufficient number of trials. Although we did not specify the number in the protocol, we consider that at least 10 included studies are required to carry out subgroup and sensitivity analyses. In future updates of this review, we intend to carry out subgroup analysis by age, gender, type of statin and severity of Illness (mild, moderate and severe aortic valve stenosis) and sensitivity analysis, provided we have more than 10 eligible studies.
We planned to calculate NNT (number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH)) (Higgins 2011) in the protocol, but it was not possible because we did not find any significant difference between the participants who received statin compared with the participants who received placebo for the following outcomes: mean pressure gradient, freedom from valve replacement, death from cardiovascular cause and valve area.
Contributions of authors
Luciana Thiago: is the primary author of the review, drafted the protocol, searched the references, entered data into RevMan, selected the included papers, extracted the data, organised and analysed data and drafted the manuscript.
Selma Rumiko Tsuji: Drafted the protocol.
Jonathan Nyong: Quality assured the report and data entry, organised the data into GRADE software and produced 'Summary of findings' table
Maria Eduarda dos Santos Puga: Developed the search strategy.
Aécio Flávio Teixeira de Góis: Extracted and analysed the data.
Cristiane Rufino Macedo: Entered the data into Revman and organised the data into GRADE software.
Orsine Valente: Drafted the protocol.
Álvaro Nagib Átallah: Organised and analysed the data.
Sources of support
Internal sources
Brazilian Cochrane Centre, Federal University of Sao Paulo and Marilia Medical, Brazil.
External sources
No sources of support supplied
Declarations of interest
There is no conflict of interest in this review.
New
References
References to studies included in this review
Chan 2010 {published data only}
- Chan KL, Teo K, Dumesnil JG, Ni A, Tam J, ASTRONOMER Investigators. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation 2010;121(2):306‐14. [DOI] [PubMed] [Google Scholar]
Cowell 2005 {published data only}
- Cowell SJ, Newby DE, Prescott RJ, Bloomfield P, Reid J, Northridge DB, et al. Scottish Aortic Stenosis and Lipid Lowering Trial, Impact on Regression (SALTIRE) Investigators. A randomized trial of intensive lipid‐lowering therapy in calcific aortic stenosis. New England Journal of Medicine 2005;352:2389‐97. [DOI] [PubMed] [Google Scholar]
Rossebø 2008 {published data only}
- Rossebø AB, Pedersen TR, Boman K, Brudi P, Chambers JB, Egstrup K, et al. SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. New England Journal of Medicine 2008;359(13):1343‐56. [DOI] [PubMed] [Google Scholar]
Van der Linde 2011 {published data only}
- Linde D, Yap SC, Dijk AP, Budts W, Pieper PG, Burgh PH, et al. Effects of rosuvastatin on progression of stenosis in adult patients with congenital aortic stenosis (PROCAS Trial). American Journal of Cardiology 2011;108(2):265‐71. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chan 2011 {published data only}
- Chan KL, Dumesnil JG, Tam J, Ni A, Teo K. Effect of rosuvastatin on C‐reactive protein and progression of aortic stenosis. American Heart Journal 2011;161(6):1133‐9. [DOI] [PubMed] [Google Scholar]
Ditchl 2008 {published data only}
- Ditchl W, Alber HF, Feuchtner GM, Hintringer F, Reinthaler M, Bartel T, et al. Prognosis and risk factors in patients with asymptomatic aortic stenosis and their modulation by atorvastatin (20 mg). American Journal of Cardiology 2008;102(6):743‐8. [DOI] [PubMed] [Google Scholar]
Panahi 2013 {published data only}
- Panahi Y, Sahebkar A, Taghipour HR, Dadjou Y, Pishgoo B, Rakhshankhah AS. Atorvastatin therapy is not associated with slowing the progression of aortic stenosis: findings of a randomized controlled trial. Clinical Laboratory 2013;59(3‐4):299‐305. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Kindo 2008 {published data only}
- Kindo M. Interest of Statin in Surgical Aortic Stenosis: From Myocardial Preconditioning to Ventricular Reverse Remodeling. ClinicalTrials.gov: NCT00811330.
Schuler 2005 {published data only}
- Schuler G. Statin Therapy in Asymptomatic Aortic Stenosis. ClinicalTrials.gov: NCT00176410.
Additional references
Antman 1992
- Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC. A comparison of results of meta‐analyses of randomized control trials and recommendations of clinical experts. Treatments for myocardial infarction. JAMA 1992;268:240‐8. [PubMed] [Google Scholar]
Canterin 2009
- Canterin FA, Leiballi E, Enache R, Popescu BA, Roçsca M, Cervesato E, et al. Hydroxymethylglutaryl coenzime‐A reductase inhibitors delay the progression of rheumatic aortic valve stenosis: a long‐term echocardiographic study. Journal of the American College of Cardiology 2009;53:1874‐9. [DOI] [PubMed] [Google Scholar]
Canterin 2010
- Canterin FA, Moura LM, Enache R, Leiballi E, Pavan D, Piazza R, et al. Effect of hydroxymethylglutaryl coenzyme‐A reductase inhibitors on the long‐term progression of rheumatic mitral valve disease. Circulation 2010;121(19):2130‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dweck 2012
- Dweck MR, Boon NA, Newby DE. Calcific aortic stenosis. Journal of the American College of Cardiology 2012;60(19):1854–63. [DOI] [PubMed] [Google Scholar]
GRADE PRO 2011 [Computer program]
- GRADE Working Group. GRADE Profiler. Version Version 3.2 for Windows. Brozek J, Oxman A, Schünemann H, 2011.
Higgins 2011
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kapur 2008
- Kapur NK. Clinical efficacy and safety of statins in managing cardiovascular risk. Vascular Health and Risk Management 2008;4(2):341‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Katz 2010
- Katz M, Tarasoutchi F, Grinberg M. Severe aortic stenosis in asymptomatic patients: the dilemma of clinical versus surgical treatment. Arquivos Brasileiros de Cardiologia 2010;95(4):542‐6. [DOI] [PubMed] [Google Scholar]
Liebe 2006
- Liebe V, Brueckmann M, Borggrefe M, Kaden JJ. Statin therapy of calcific aortic stenosis: hype or hope?. European Heart Journal 2006;27:773‐8. [DOI] [PubMed] [Google Scholar]
Macedo 2014
- Macedo AF, Taylor FC, Casas JP, Adler A, Merino DP, Ebrahim S. Unintended effects of statins from observational studies in the general population: systematic review and meta‐analysis. BMC Medicine 2014;12(51):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mancini 2011
- Mancini GBJ, Baker S, Bergeron J, Fitchett D, Frohlich J, Genest J, et al. Diagnosis, prevention, and management of statin adverse effects and intolerance: proceedings of a Canadian Working Group Consensus Conference. Canadian Journal of Cardiology 2011;27(5):635‐62. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. Annals of Internal Medicine 2009;151(4):264‐9. [DOI] [PubMed] [Google Scholar]
Mulrow 1994
- Mulrow CD. Rationale for systematic reviews. BMJ 1994;309:597‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nishimura 2014
- Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, et al. 2014 AHA/ACC Guideline for the Managementof Patients With Valvular Heart Disease. Journal of the American College of Cardiology 2014;63(22):e57–185. [DOI] [PubMed] [Google Scholar]
Novaro 2001
- Novaro GM, Tiong IY, Pearce GL, Lauer MS, Sprecher DL, Griffin BP. Effect of hydroxymethylglutaryl coenzyme A reductase inhibitors on the progression of calcific aortic stenosis. Circulation 2001;104:2205‐9. [DOI] [PubMed] [Google Scholar]
Oxman1993
- Oxman AD, Guyatt GH. The science of reviewing research. Annals of the New York Academy of Sciences 1993;703:125‐33. [DOI] [PubMed] [Google Scholar]
Parolari 2011
- Parolari A, Tremoli E, Cavallotti L, Trezzi M, Kassem S, Loardi C, Veglia F, et al. Do statins improve outcomes and delay the progression of non rheumatic calcific aortic stenosis?. Heart 2011;97(7):523‐9. [DOI] [PubMed] [Google Scholar]
Pedersen 2008
- Pedersen TR. Overview of clinical trials on calcific stenosis. European Heart Journal 2008;10 (Supplement E):E32‐40. [Google Scholar]
Rajamannan 2008
- Rajamannan NM. Update on the pathophysiology of aortic stenosis. European Heart Journal 2008;10(E Supl):4‐10. [Google Scholar]
RevMan 2014 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.. The Cochrane Collaboration, 2014.
Ridker 2008
- Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AMJ, Kastelein JJP, et al. Rosuvastatin to prevent vascular events in men and women with elevated C‐reactive protein. New England Journal of Medicine 2008;359(21):2195‐207. [DOI] [PubMed] [Google Scholar]
Russo 2014
- Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE, et al. The spectrum of statin hepatotoxicity: experience of the drug induced liver injury network. Hepatology 2014;60(2):379‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine 2010;152(11):726‐32. [DOI] [PubMed] [Google Scholar]
Siu 2010
- Siu SC, Silversides CK. Bicuspid valve aortic disease. Journal of the American College of Cardiology 2010;55:2789‐800. [DOI] [PubMed] [Google Scholar]
Stone 2014
- Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHAguideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129(Suppl 2):S1–45. [DOI] [PubMed] [Google Scholar]
Teo 2011
- Teo KK, Corsi DJ, Tam JW, Dumesnil JG, Chan KL. Lipid lowering on progression of mild to moderate aortic stenosis: meta‐analysis of the randomized placebo controlled clinical trials on 2344 patients. Canadian Journal of Cardiology 2011;27(6):800‐8. [DOI] [PubMed] [Google Scholar]
Vahanian 2010
- Vahanian A, Otto CM. Risk stratification of patients with aortic stenosis. European Heart Journal 2010;31:416‐23. [DOI] [PubMed] [Google Scholar]
Vahanian 2012
- Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Esquivias GB, Baumgartner H, et al. Guidelines on the management of valvular heart disease (version 2012). European Heart Journal 2012;33(19):2451‐96. [DOI] [PubMed] [Google Scholar]
Vaughan 2004
- Vaughan CJ, Gotto AM J. Update on Statins:2003. Circulation 2004;110:886‐92. [DOI] [PubMed] [Google Scholar]


