Abstract
Background
Uterine carcinosarcomas are uncommon with about 35% not confined to the uterus at diagnosis. The survival of women with advanced uterine carcinosarcoma is poor with a pattern of failure indicating greater likelihood of upper abdominal and distant metastatic recurrence.
Objectives
To evaluate the effectiveness and safety of adjuvant radiotherapy and/or systemic chemotherapy in the management of uterine carcinosarcoma.
Search methods
We searched the Cochrane Gynaecological Cancer Group Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL), 2012, Issue 10, MEDLINE and EMBASE up to November 2012. We also searched registers of clinical trials, abstracts of scientific meetings, reference lists of included studies and contacted experts in the field.
Selection criteria
Randomised controlled trials (RCTs) comparing adjuvant radiotherapy and/or chemotherapy in women with uterine carcinosarcoma.
Data collection and analysis
Two review authors independently abstracted data and assessed risk of bias. Hazard ratios (HRs) for overall survival (OS) and progression‐free survival (PFS) and risk ratios (RRs) comparing adverse events in women who received radiotherapy and/or chemotherapy were pooled in random‐effects meta‐analyses.
Main results
Three trials met the inclusion criteria and these randomised 579 women, of whom all were assessed at the end of the trials. Two trials assessing 373 participants with stage III to IV persistent or recurrent disease, found that women who received combination therapy had a significantly lower risk of death and disease progression than women who received single agent ifosfamide, after adjustment for performance status (HR = 0.75, 95% confidence interval (CI): 0.60 to 0.94 and HR = 0.72, 95% CI: 0.58 to 0.90 for OS and PFS respectively). There was no statistically significant difference in all reported adverse events, with the exception of nausea and vomiting, where significantly more women experienced these ailments in the combination therapy group than the Ifosamide group (RR = 3.53, 95% CI: 1.33 to 9.37).
In one trial there was no statistically significant difference in the risk of death and disease progression in women who received whole body irradiation and chemotherapy, after adjustment for age and FIGO stage (HR = 0.71, 95% CI: 0.48 to 1.05 and HR = 0.79, 95% CI: 0.53 to 1.18 for OS and PFS respectively). There was no statistically significant difference in all reported adverse events, with the exception of haematological and neuropathy morbidities, where significantly less women experienced these morbidities in the whole body irradiation group than the chemotherapy group (RR= 0.02, 95% CI: 0.00 to 0.16) for haematological morbidity and all nine women in the trial experiencing neuropathy morbidity were in the chemotherapy group).
Authors' conclusions
In advanced stage metastatic uterine carcinosarcoma as well as recurrent disease adjuvant combination, chemotherapy with ifosfamide should be considered. Combination chemotherapy with ifosfamide and paclitaxel is associated with lower risk of death compared with ifosfamide alone. In addition, radiotherapy to the abdomen is not associated with improved survival.
Plain language summary
The addition of chemotherapy and/or radiation treatment after surgery in carcinosarcoma of the womb
Carcinosarcomas of the uterus (womb) are uncommon cancers accounting for 4.3% of all cancers of the womb. These rare cancers have poor prognosis; one of the reasons for the poor survival outcome is the fact that over a third of these cancers (carcinosarcomas) have already spread beyond the womb at the time of diagnosis.
The main treatment is surgery to remove the cancer, however, because of the high rates of both local and distant recurrence after surgery, effective adjuvant therapies are needed. This review has shown that women with high stage disease (stage III‐IV persistent or recurrent disease) who received combination chemotherapy including ifosfamide had a lower risk of death and disease progression than women who received ifosfamide alone, after adjustment for performance status.
In addition, radiotherapy to the abdomen was not associated with improved survival, as we found in one trial that there was no difference in the risk of death and disease progression in women who received whole abdominal irradiation and chemotherapy, after adjustment for age and stage of disease. Previous studies have shown that doxorubicin, despite being established in the treatment of uterine carcinoma, does not seem to be highly active.
Adverse events were comprehensively reported for the comparisons of combination therapy and ifosfamide and whole body irradiation and chemotherapy. More women experienced side effects when they received combination therapy than ifosamide alone and chemotherapy than whole body irradiation. The effect of therapy on quality of life was not reported in any of the trials.
Background
Description of the condition
Uterine carcinosarcomas are uncommon accounting for 4.3% of all cancers of the uterine corpus in Western Populations (El Nashar 2011; Young 1981). The worldwide annual incidence is between 0.5 and 3.3 cases per 100,000 women (Harlow 1986; Brooks 2004). In the UK the incidence of sarcoma is quoted to be 1 per 100,000 women and of these, 87% are carcinosarcomas (Olah 1992). Uterine carcinosarcomas, also called malignant mixed mesodermal tumours (MMT) or malignant mixed mullerian tumours (MMMT) are rare tumours with both malignant epithelial and malignant mesenchymal components. Surveillance Epidemiology and End Results (SEER) programme data also demonstrated that carcinosarcoma was the predominant uterine sarcoma (0.82/100,000) followed by leiomyosarcoma (0.64/100,000) and endometrial stromal sarcoma (0.19/100,000) (Harlow 1986).
Uterine carcinosarcomas tend to be aggressive with poor prognosis in comparison to uterine adenocarcinomas (Barwick 1979; Gadducci 2002; Toyoshima 2004). About 35% of carcinosarcomas are not confined to the uterus at diagnosis, and in most reports the median overall survival was about 21 months (Gadducci 2002). The most important prognostic factor is the extent of the tumour at the time of diagnosis; the prognosis being very poor when the tumour has extended beyond the uterus (Sartori 1997). There has been no significant improvement in survival suggested by some reports (Callister 2004; Chi 1997; Le 2001; Sutton 2000).
Carcinosarcomas can be subdivided histologically into homologous and heterologous types and it is important to differentiate the tumours that are monoclonal, that is those derived from a single stem cell, from true mixed cell tumours (Zelmanowicz 1998). This histological distinction (McCluggage 2002) is significant as the natural history of true mixed carcinosarcomas appear to be more aggressive. There is convincing recent evidence that most cases of uterine carcinosarcoma are monoclonal in origin (Szukala 1999; Toyoshima 2004). These data indicate that uterine carcinosarcoma may be metaplastic, with the implication that the sarcomatous components are derived from the carcinomatous elements (McCluggage 2002).
Histological diagnosis and clinical staging (based on findings at surgery) usually follows primary treatment which is surgical. In a prospective multi‐centre Gynecologic Oncology Group (GOG) study of carcinosarcomas 61 of the 301 patients (20%) with clinical Stage I and II disease were reassigned to pathological Stages III and IV on the basis of lymph node metastases. The study also revealed a recurrence rate of 53% for all carcinosarcomas, with 44% for homologous and 63% for heterologous tumours (Major 1993).
Description of the intervention
As with uterine adenocarcinomas, the mainstay of treatment is surgical removal of the tumour (Menczer 2005), however, the high rates of both local and distant relapse after surgery suggests a need for effective adjuvant therapies (Galaal 2009; Sutton 2000). The survival of patients with advanced uterine carcinosarcoma is poor with a pattern of failure indicating a higher likelihood of upper abdominal and distant metastatic recurrence (Galaal 2009; Spanos 1986). These patients are less likely to benefit from local adjuvant therapy and therefore consideration for systemic adjuvant chemotherapy as well as whole body irradiation has been considered in several studies (Chi 1997; Menczer 2005; Ramondetta 2003; Sutton 1989).
How the intervention might work
Several chemotherapeutic agents have been examined as single agent therapy in uterine carcinosarcoma with response rates as follows: 16% to19% with adriamycin (Omura 1983), 32% to 36% with ifosfamide (Sutton 1989; Sutton 2000), 19% with cisplatin (Thigpen 2004), and 18% with paclitaxel (Curtin 2001). Doxorubicin, despite being established in the treatment of uterine carcinoma, does not seem to be highly active in uterine carcinosarcoma (Omura 1983). Combination chemotherapeutic agents have been used in uterine carcinosarcoma with combination therapy appearing to be superior to single‐agent treatment in terms of improvement in progression‐free and overall survival. However, these combination therapies may be associated with increased toxicity (Homesley 2007; Sutton 2000; Van Rijswijk 1994). Whole abdominal irradiation has been investigated in a retrospective study on early staged uterine carcinosarcoma in the adjuvant setting. This study suggested that the addition of whole abdominal irradiation did not improve survival (Chi 1997).
Why it is important to do this review
Carcinosarcoma is a disease with a high recurrence rate (40% to 60%), and a tendency to distant metastasis, therefore, an effective systemic therapy may improve the outcomes of this disease. Several chemotherapeutic agents have been shown to produce objective response rates in patients with advanced carcinosarcoma. In addition, whole abdominal irradiation has been used in the adjuvant setting (Chi 1997; Ramondetta 2003). These treatment modalities may be associated with some costs in terms of toxicity and quality of life (QoL). Therefore, there is a need to assess the effectiveness and safety rigorously.
Objectives
To evaluate the role of adjuvant radiotherapy and/or systemic chemotherapy in the management of uterine carcinosarcoma.
Specifically, we wanted to address the following questions.
Is adjuvant systemic chemotherapy more effective than adjuvant radiotherapy?
Is adjuvant systemic combination chemotherapy more effective than single agent chemotherapy?
Is adjuvant radiotherapy and/or systemic chemotherapy well tolerated?
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs)
Types of participants
Women of any age with a histological diagnosis of uterine carcinosarcoma of any International Federation of Gynecology and Obstetrics (FIGO) stage (FIGO 2009).
Types of interventions
Intervention
Surgery followed by radiotherapy and/or systemic chemotherapy.
Comparison
Additionally, we considered any direct comparison between:
adjuvant radiotherapy or combination chemotherapy;
adjuvant single drug chemotherapy versus combination chemotherapy;
surgery alone or best supportive care.
Types of outcome measures
Primary outcomes
Overall survival (OS): Survival until death from all causes. Survival was assessed from the time when women were enrolled in the study.
Secondary outcomes
Progression‐free survival (PFS).
Quality of life (QoL), measured using a scale that has been validated through reporting of norms in a peer‐reviewed publication.
Grades 3 and 4 chemotherapeutic and radiotherapeutic toxicity, classified according to CTCAE 2006, was extracted and grouped as:
haematological (leucopenia, anaemia, thrombocytopenia, neutropenia, haemorrhage);
gastrointestinal (nausea, vomiting, anorexia, diarrhoea, liver, proctitis);
genitourinary;
skin (stomatitis, mucositis, alopecia, allergy);
neurological (peripheral and central);
pulmonary.
Search methods for identification of studies
Papers in all languages were sought and translations carried out when necessary.
Electronic searches
See: Cochrane Gynaecological Cancer Group methods used in reviews. The following electronic databases were searched.
The Cochrane Gynaecological Cancer Collaborative Review Group's Trial Register
Cochrane Central Register of Controlled Trials (CENTRAL), 2012, Issue 10
MEDLINE
EMBASE
The MEDLINE, EMBASE and CENTRAL search strategies based on terms related to the review topic are presented in Appendix 1, Appendix 2 and Appendix 3 respectively.
Databases were searched from January 1966 until November 2012.
All relevant articles found were identified on PubMed and using the 'related articles' feature; a further search was carried out for newly published articles.
Searching other resources
Unpublished and Grey literature
Metaregister, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov, www.cancer.gov/clinicaltrials, NHMRC Clinical Trials Register and the UKCCCR Register of Cancer Trials were searched for ongoing trials.
Handsearching
The citation list of relevant publications, abstracts of scientific meetings and list of included studies were checked through handsearching and experts in the field contacted to identify further reports trials. Reports of conferences were handsearched in the following sources.
Gynecologic Oncology (Annual Meeting of the American Society of Gynecologic Oncologist)
International Journal of Gynecological Cancer (Annual Meeting of the International Gynecologic Cancer Society )
British Journal of Cancer
British Cancer Research Meeting
Annual Meeting of the International Gynecologic Cancer Society
Annual Meeting of the American Society of Gynecologic Oncologist
British Gynaecological Cancer Society (BGCS)
Annual Meeting of European Society of Medical Oncology (ESMO)
Annual Meeting of the American Society of Clinical Oncology (ASCO)
European Society of Gynecological Cancer (ESGO)
Reference lists
The reference lists of all relevant trials obtained by this search were handsearched for further trials.
Correspondence
Authors of relevant trials were contacted to ask if they knew of further data which may or may not have been published.
Data collection and analysis
Selection of studies
All titles and abstracts retrieved by electronic searching were downloaded to the reference management database Endnote, duplicates were removed and the remaining references were independently examined by three review authors (KG, KG1, EH). These three review authors screened titles and abstracts of references identified from the search and eliminated articles that were obviously not relevant to the search question. When all authors determined that the trial was not eligible for inclusion no further action was taken. When any of the authors determined that the article may have been eligible for inclusion, the full text article was obtained. Each review author then independently determined if these trials were eligible for inclusion. Disagreements about inclusions were resolved by discussion. Further information was sought from the study authors when papers contained insufficient information to make a decision about eligibility. No attempt was made to blind review authors to article authors or journals.
Data extraction and management
For included trials, data were abstracted as recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Data were independently extracted by two review authors and included the following details.
Author, year of publication and journal citation (including language)
Country
Setting
Inclusion and exclusion criteria
Study design, methodology
Study population (participant characteristics, age, stage and postoperative residual disease)
Number of participants in each arm of the trial
Total number of intervention groups
Uterine carcinosarcoma details (FIGO stage, histology, tumour grade)
Type of intervention (chemotherapy agents and radiotherapy, dosage and timing of administration relative to surgery)
Length of follow‐up
Withdrawals from treatment protocol
Number of participants who experienced delays in treatment or received all, part, or none of the proposed treatment
Risk of bias in study (see below)
-
Outcomes: OS, PFS, QoL and adverse events
for each outcome: outcome definition
unit of measurement (if relevant)
for scales: upper and lower limits, and whether high or low score is good
results: Number of participants allocated to each intervention group
for each outcome of interest: Sample size; Missing participants
Data on outcomes were extracted as below.
For time‐to‐event (Overall and progression‐free survival) data, we extracted the log of the hazard ratio [log(HR)] and its standard error from trial reports. If these were not reported, we attempted to estimate them from other reported statistics using the methods of Parmer 1998.
-
For dichotomous outcomes (e.g. adverse events), we extracted the number of patients in each group who experienced the outcome of interest and the number of patients assessed at endpoint, in order to estimate a risk ratio (RR).
The scales, grades and sites of acute toxicity information were extracted from the trials.The toxicity scales used varied from trial to trial and reporting of toxicity was otherwise inconsistent. Also, the type and severity of side effects will depend on the drugs being used in the individual trials. However, there was some commonality in the types of toxicity documented, namely: nausea and vomiting; diarrhoea/other gastrointestinal, leucopenia, thrombocytopenia, anaemia, alopecia, fever/infection, neurological toxicity and renal/GU and in the scales of toxicity used. By analysing the common types, it is possible to get an indication of the relative levels of serious toxicity associated with chemotherapy regimens and radiation therapy.
Both unadjusted and adjusted statistics were extracted, if reported.
Where possible, all data extracted were those relevant to an intention‐to‐treat (ITT) analysis, in which participants were analysed in groups to which they were assigned.
The time points at which outcomes were collected and reported were noted.
Data were abstracted independently by two review authors (KG, KG1) onto a data abstraction form specially designed for the review. Differences between review authors were resolved by discussion or by appeal to a third review author (AB) when necessary. Where appropriate, authors were contacted for further information and data were updated.
Assessment of risk of bias in included studies
The risk of bias in included RCTs was assessed using The Cochrane Collaboration's tool and the criteria specified in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This included an assessment of:
sequence generation;
allocation concealment;
blinding (of participants, healthcare providers and outcome assessors);
-
incomplete outcome data: we coded a satisfactory level of loss to follow‐up for each outcome as:
'Yes', if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms;
'No', if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms;
'Unclear' if loss to follow‐up was not reported;
selective reporting of outcomes;
other possible sources of bias.
The 'Risk of bias' tool was applied independently by two review authors (KG1, KG) and differences resolved by discussion or by appeal to a third review author (AB). Results are presented in the 'Risk of bias' table and also in both a 'Risk of bias' graph and a 'Risk of bias' summary section. Results of meta‐analyses were interpreted in light of the findings with respect to risk of bias.
Measures of treatment effect
We used the following measures of the effect of treatment.
For time‐to‐event data, we used the hazard ratio (HR), when possible.
For dichotomous outcomes, we used the risk ratio (RR).
Dealing with missing data
We did not impute missing outcome data; if only imputed data were reported, we contacted trial authors to request data on the outcomes only among participants who were assessed.
Assessment of heterogeneity
Heterogeneity between trials was assessed by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials which cannot be ascribed to sampling variation (Higgins 2003; Higgins 2011), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). If there was evidence of substantial heterogeneity, the possible reasons for this were investigated and reported.
Data synthesis
If sufficient, clinically similar studies were available, their results were pooled in meta‐analyses. Adjusted summary statistics were used where available, otherwise, unadjusted results were used.
For time‐to‐event data, HRs were pooled using the generic inverse variance facility of RevMan 5.
For any dichotomous outcomes, the RR was calculated for each trial and these were then pooled.
Random‐effects models with inverse variance weighting were used for all meta‐analyses (DerSimonian 1986).
Results
Description of studies
see Characteristics of included studies; Characteristics of excluded studies
Results of the search
The original search strategy identified 445 references in MEDLINE, 745 in EMBASE and 10 in CENTRAL. When the search results were merged into Endnote and duplicates removed, 895 unique references remained. The title and abstract screening of these references identified 15 trials as potentially eligible for this review. The full text screening of these 15 references excluded 12 for the reasons described in the table Characteristics of excluded studies. The remaining three randomised controlled trials (RCTs) Homesley 2007; Sutton 2000; Wolfson 2007) met our inclusion criteria and are described in the table Characteristics of included studies.
Searches of the grey literature identified one additional relevant ongoing trial (EORTC‐55874).
The updated search from May 2010 to October 2012 identified another 77 references in MEDLINE, 348 in EMBASE and no additional references in CENTRAL, but did not identify any new eligible trials.
Included studies
The three included trials (Homesley 2007; Sutton 2000; Wolfson 2007) randomised 579 women, of whom all (100%) were assessed at the end of the trials. Two trials (Homesley 2007; Sutton 2000) reported the comparison of combination chemotherapy versus single agent chemotherapy in the adjuvant setting for advanced or recurrent uterine carcinosarcoma. Both these trials used ifosfamide as a single agent and in the comparison arm in combination with other chemotherapeutic agents that showed activity in uterine carcinosarcoma, paclitaxel, cisplatin.
The other trial (Wolfson 2007) randomised previously untreated patients with stages I, II, III, and IV primary carcinosarcomas of the uterus or cervix to whole abdominal irradiation (WAI) and combination chemotherapy with cisplatin and Ifosfamide with mesna (CIM).
Design
Phase III RCT.
Participants
One hundred and seventy‐nine women with histologically‐confirmed stage III or IV, persistent or recurrent uterine carcinosarcoma not amenable to curative intent by other means. Median age for both arms was 64 years.
Interventions
Ninety‐one women were randomised to arm one (single agent ifosfamide),18% had stage III, 31% stage IV and 52% had recurrent/persistent disease.
Eighty‐eight women were randomised to arm two (ifosfamide + paclitaxel),18% had stage III, 29% had stage IV disease and 52% had recurrent/persistent disease. Because of the reported toxicity when a five‐day schedule of ifosfamide was used (Sutton 2000), a three‐day schedule was used in this trial in both arms.
The trial reported 150 (84%) deaths and 162 (91%) disease recurrences.
Design
Phase III RCT.
Participants
One hundred and ninety‐four women with histologically‐confirmed advanced or recurrent carcinosarcoma no longer amenable to control by surgery and/or radiotherapy. All patients had to have measurable disease that could be defined in at least two dimensions by palpation or imaging. Median age for arm one was 67 years (range 32 to 84), 66 years for arm two (range 35 to 83). The two intervention arms were balanced for age, grade, and Gynecologic Oncology Group (GOG) performance status.
Intervention
One hundred and two women were in arm one (ifosfamide) and 92 women in arm two (ifosfamide + cisplatin). Each patient received eight cycles of therapy unless there was disease progression or toxicity. The dose of the combination regimen was reduced by 20% early in this trial (day one) because of toxicity.
The trial reported 175 (90%) deaths and 182 (94%) disease recurrences.
Design
Phase III RCT.
Participants
Two hundred and six women with previously untreated stages I, II, III, and IV primary carcinosarcomas of the uterus or cervix (without any demonstrable parenchymal hepatic involvement or extra‐abdominal distant disease). Forty‐three per cent of patients randomised to the whole abdominal irradiation (WAI) arm had stage III, and 46% in the CIM arm had stage III.
Median age for WAI was 68 years and 65 years for CIM arm.
Interventions
One hundred and five women were randomised to the WAI arm and 101 to the CIM arm. Forty‐three per cent of patients randomised to the WAI arm had stage III, and 46% in the CIM arm had stage III.
The trial reported 122 (59%) deaths and 132 (64%) disease recurrences.
Outcomes reported
All three trials reported overall and recurrence‐free survival and used appropriate statistical techniques (hazard ratios (HRs) to correctly allow for censoring). Prognostic factors were adjusted for in the analysis of survival outcomes in each trial.
The HR in the trial of Homesley 2007 was adjusted for performance status.
The HR in the trial of Sutton 2000 was adjusted for performance status.
The HR in the trial of Wolfson 2007 was adjusted for age and FIGO stage.
For the distribution of these factors at baseline for each trial by treatment arm see the table Characteristics of included studies.
Grades 3 and 4 severe adverse events (haematological, gastrointestinal, genitourinary, skin, neurological, pulmonary) were reported in all trials.
Excluded studies
We excluded 12 references after obtaining the full text (see Characteristics of excluded studies). Nine references (Currie 1996; Curtin 2001; Fowler 2002; Miller 2005; Powell 2010; Ramondetta 2003; Resnik 1995; Sutton 1989; Asbury 1998) reported the results of non‐comparative phase II trials; two references (Perez 1979; Toyoshima 2004) were retrospective studies and one reference (Sutton 2005) was a study that did not have a concurrent control group.
Risk of bias in included studies
Two trials (Homesley 2007; Wolfson 2007) were at low risk of bias: they satisfied four of the criteria that we used to assess risk of bias. The trial of (Sutton 2000) was at high risk of bias as it only satisfied two criteria ‐ see Figure 1, Figure 2.
1.
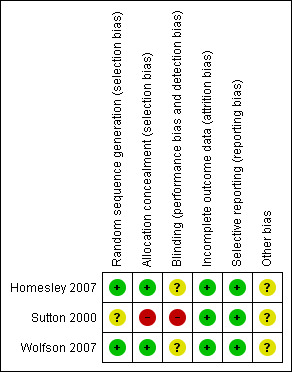
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
2.
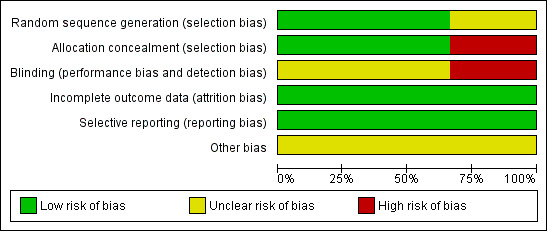
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Two trials (Homesley 2007; Wolfson 2007) reported the method of generation of the sequence of random numbers used to allocate women to treatment arms and concealment of this allocation sequence from patients and healthcare professionals involved in the trial. In the trial of (Sutton 2000) it was unclear whether the method of assigning women to treatment groups was carried out using an adequate method of sequence generation and there was no attempt to conceal the allocation. None of the trials reported whether the patients, healthcare professionals or outcome assessors were blinded. It was highly likely that all trials reported all the outcomes that they assessed, but it was not clear whether any other bias may have been present. At least 96% of women who were enrolled were assessed at endpoint in all three trials.
Effects of interventions
All meta‐analyses pooled data from two of the included trials (Homesley 2007; Sutton 2000), comparing ifosfamide and combination therapy. The trial of Wolfson 2007 compared whole body irradiation and chemotherapy in single trial analyses.
Meta‐analyses of survival are based on hazard ratios (HRs) that were adjusted for important prognostic variables.
For dichotomous outcomes, we were unable to estimate a risk ratio (RR) for comparison of whole body irradiation and chemotherapy if one or both treatment groups experienced no events, as in the trial of Wolfson 2007 for hepatic and neuropathy adverse event outcomes and in the Sutton 2000 trial for cardiovascular adverse events.
1. Combination therapy versus ifosfamide
Two trials (Homesley 2007; Sutton 2000)
Overall survival
(See Analysis 1.1)
1.1. Analysis.
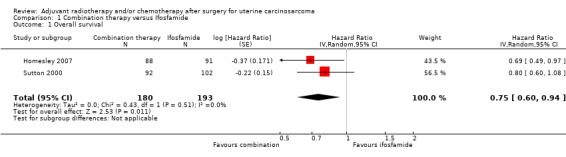
Comparison 1 Combination therapy versus Ifosfamide, Outcome 1 Overall survival.
Meta‐analysis of two trials, assessing 373 participants, found that women who received combination therapy had a significantly lower risk of death than women who received ifosfamide, after adjustment for performance status (HR = 0.75, 95% confidence interval (CI) 0.60 to 0.94). The percentage of the variability in effect estimates that is due to heterogeneity rather than sampling error (chance) is not important (I2 = 0%).
Progression‐free survival
(See Analysis 1.2)
1.2. Analysis.
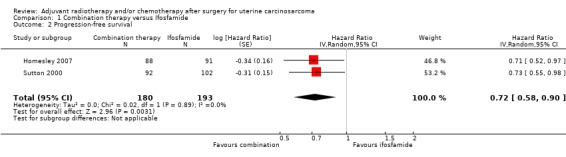
Comparison 1 Combination therapy versus Ifosfamide, Outcome 2 Progression‐free survival.
Meta‐analysis of two trials, assessing 373 participants, found that women who received combination therapy had a significantly lower risk of disease progression than women who received ifosfamide, after adjustment for performance status (HR= 0.72, 95% CI 0.58 to 0.90). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance is not important (I2 = 0%).
Adverse events
Severe nausea/vomiting
(See Analysis 1.3)
1.3. Analysis.
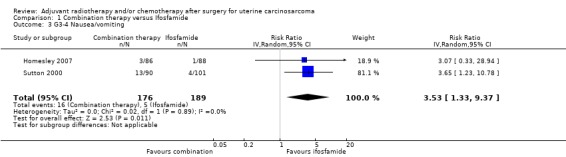
Comparison 1 Combination therapy versus Ifosfamide, Outcome 3 G3‐4 Nausea/vomiting.
Meta‐analysis of two trials, assessing 365 participants, found that women who received combination therapy had a significantly higher risk of severe nausea or vomiting than women who received ifosfamide (risk ratio) (RR) = 3.53, 95% CI 1.33 to 9.37). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance is not important (I2 = 0%).
Diarrhoea and other gastrointestinal morbidities
(See Analysis 1.4)
1.4. Analysis.
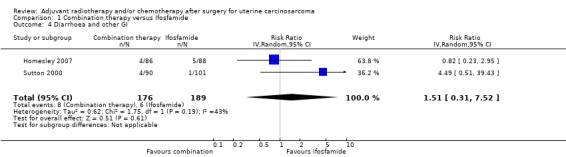
Comparison 1 Combination therapy versus Ifosfamide, Outcome 4 Diarrhoea and other GI.
Meta‐analysis of two trials, assessing 365 participants, showed no statistically significant difference in the risk of diarrhoea and other gastrointestinal morbidities in women who received combination therapy and those who received ifosfamide (RR 1.51, 95% CI 0.31 to 7.52). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance may represent moderate heterogeneity (I2 = 58%).
Haematological morbidities
(See Analysis 1.5)
1.5. Analysis.
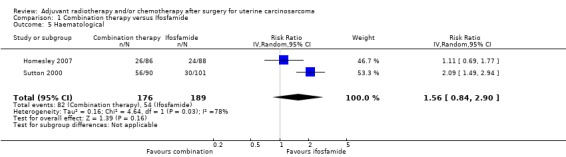
Comparison 1 Combination therapy versus Ifosfamide, Outcome 5 Haematological.
Meta‐analysis of two trials, assessing 365 participants, showed no statistically significant difference in the risk of haematological morbidity in women who received combination therapy and those who received ifosfamide (RR = 1.56, 95% CI 0.84 to 2.90). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance may represent substantial heterogeneity (I2 = 78%).
Genitourinary morbidities
(See Analysis 1.6)
1.6. Analysis.
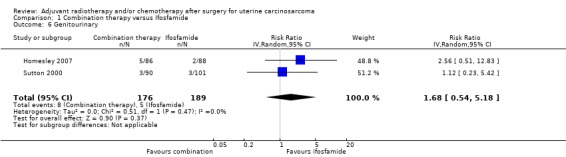
Comparison 1 Combination therapy versus Ifosfamide, Outcome 6 Genitourinary.
Meta‐analysis of two trials, assessing 365 participants, showed no statistically significant difference in the risk of genitourinary morbidity in women who received combination therapy and those who received ifosfamide (RR = 1.68, 95% CI 0.54 to 5.18). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance is not important (I2 = 0%).
Cardiovascular morbidities
(See Analysis 1.7)
1.7. Analysis.
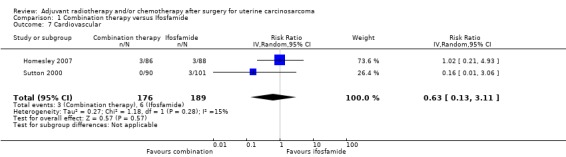
Comparison 1 Combination therapy versus Ifosfamide, Outcome 7 Cardiovascular.
Two trials (Homesley 2007; Sutton 2000), found no statistically significant difference in the risk of cardiovascular morbidity in women who received combination therapy and those who received ifosfamide (RR = 0.63, 95% CI: 0.13 to 3.11). In the trial of Homesley 2007 and the Sutton 2000 trial reported only three cases of cardiovascular morbidity, which were of woman in the Ifosamide group).
Hepatic morbidities
(See Analysis 1.8)
1.8. Analysis.
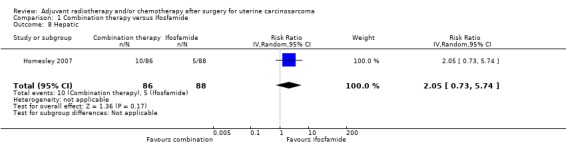
Comparison 1 Combination therapy versus Ifosfamide, Outcome 8 Hepatic.
One trial reported on hepatic toxicity (Homesley 2007); it reported that the single agent Ifosfamide was associated with less hepatic toxicity. However, there was no statistically significant difference in the risk of hepatic morbidity in women who received combination therapy and those who received ifosfamide (RR = 2.05, 95% CI 0.73 to 5.74).
Neuropathy
(See Analysis 1.9)
1.9. Analysis.
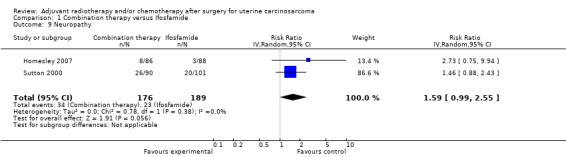
Comparison 1 Combination therapy versus Ifosfamide, Outcome 9 Neuropathy.
Meta‐analysis of two trials, assessing 365 participants, found that women who received combination therapy had a significantly higher risk of neuropathy than women who received ifosfamide (RR = 1.59, 95% CI 0.99 to 2.55). The percentage of the variability in effect estimates that is due to heterogeneity rather than by chance is not important (I2 = 0%).
2. Whole body irradiation versus combination chemotherapy
One trial (Wolfson 2007)
Overall survival
(see Analysis 2.1)
2.1. Analysis.
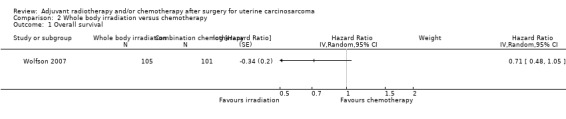
Comparison 2 Whole body irradiation versus chemotherapy, Outcome 1 Overall survival.
There was no statistically significant difference in the risk of death in women who received whole body irradiation and chemotherapy, after adjustment for age and FIGO stage (HR = 0.71, 95% CI: 0.48 to 1.05).
Progression‐free survival
(see Analysis 2.2)
2.2. Analysis.
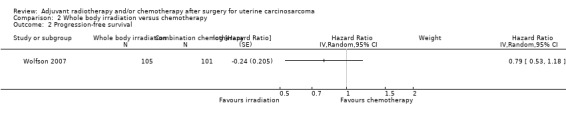
Comparison 2 Whole body irradiation versus chemotherapy, Outcome 2 Progression‐free survival.
There was no statistically significant difference in the risk of disease progression in women who received whole body irradiation and chemotherapy, after adjustment for age and FIGO stage (HR = 0.79, 95% CI: 0.53 to 1.18).
Adverse events
Gastrointestinal morbidities
(see Analysis 2.3)
2.3. Analysis.
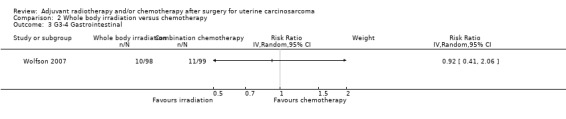
Comparison 2 Whole body irradiation versus chemotherapy, Outcome 3 G3‐4 Gastrointestinal.
There was no statistically significant difference in the risk of gastrointestinal morbidity in women who received whole body irradiation and chemotherapy (RR = 0.92, 95% CI: 0.41 to 2.06).
Haematological morbidities
(see Analysis 2.4)
2.4. Analysis.
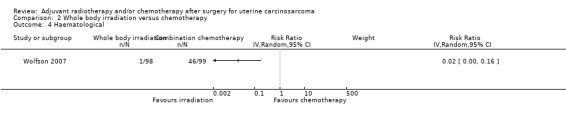
Comparison 2 Whole body irradiation versus chemotherapy, Outcome 4 Haematological.
Women who received whole body irradiation after surgery for treatment of uterine carcinosarcoma were associated with significantly less chance of haematological morbidity compared with those who received chemotherapy (RR = 0.02, 95% CI: 0.00 to 0.16).
Genitourinary morbidities
(see Analysis 2.5)
2.5. Analysis.
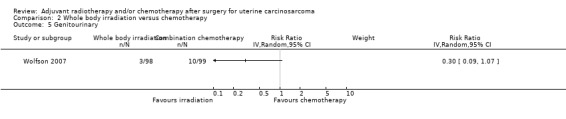
Comparison 2 Whole body irradiation versus chemotherapy, Outcome 5 Genitourinary.
There was no statistically significant difference in the risk of genitourinary morbidity in women who received whole body irradiation and chemotherapy (RR = 0.30, 95% CI: 0.09 to 1.07).
Cardiovascular morbidities
(see Analysis 2.6)
2.6. Analysis.
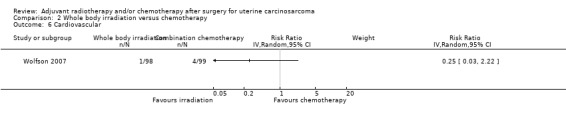
Comparison 2 Whole body irradiation versus chemotherapy, Outcome 6 Cardiovascular.
There was no statistically significant difference in the risk of cardiovascular morbidity in women who received whole body irradiation and chemotherapy (RR = 0.25, 95% CI: 0.03 to 2.22).
Hepatic morbidities
There was no statistically significant difference in the risk of hepatic morbidity in women who received whole body irradiation and chemotherapy. The trial reported only two cases of hepatic morbidity in women who were in the whole body irradiation group.
Neuropathy
Women who received whole body irradiation after surgery for treatment of uterine carcinosarcoma were associated with significantly less chance of neuropathy compared to those who received chemotherapy. The trial reported nine cases of neuropathy morbidity, all of women in the chemotherapy group.
Discussion
Summary of main results
We found three trials, enrolling 579 women, that met our inclusion criteria. Two of these trials (Homesley 2007; Sutton 2000) compared combination chemotherapy and ifosfamide alone, whereas the other trial (Wolfson 2007), compared whole body irradiation and combination chemotherapy in women with uterine carcinosarcoma.
When we combined the findings from the two similar trials, adjusted for important prognostic factors, we found that the risk of death and disease progression was lower among women who received combination therapy than among women who received ifosfamide alone (HR = 0.75, 95% CI 0.60 to 0.94 and HR = 0.72, 95% CI 0.58 to 0.90 for overall and progression‐free survival respectively). Risk of adverse events was not significantly different for most outcomes but the rate of nausea and vomiting was higher in women who received combination therapy (RR = 3.53, 95% CI 1.33, 9.37).
The other trial (Wolfson 2007) found no significant difference between whole abdominal irradiation and combination chemotherapy in terms of overall and progression‐free survival, but did seem to suggest that in general whole body irradiation was associated with less morbidity than combination chemotherapy, where haematological and neuropathic morbidities were significantly lower in women who received whole body irradiation compared to those who received combination chemotherapy (RR= 0.02, 95% CI 0.00 to 0.16 for haematological morbidity and all nine women in the trial experiencing neuropathy morbidity were in the chemotherapy group).
The trials had many strengths. They gave HRs that correctly allowed for censoring and they provided information about adverse events. Both trials recruited a satisfactory number of participants and a reasonably large number of events were observed in the two survival outcomes, but the number of women with adverse events was generally low so lacked statistical power to detect a difference.
Overall completeness and applicability of evidence
To date, two RCTs have compared the effect of ifosfamide with combination of ifosfamide and other chemotherapeutic agents. These trials suggested that combination chemotherapy may be better than single agent therapy in the treatment of advanced staged and recurrent uterine carcinosarcoma. The combination of ifosfamide and paclitaxel was associated with significant improvement in overall and progression‐free survival. The evidence from a single RCT suggested no benefit of whole abdominal irradiation over combination chemotherapy.
We were unable to report on Quality‐of‐life (QoL) as none of the included trials had QoL assessments as a component of the trials. Treatment‐related morbidity very often degrades the quality of the time that patients live, which is especially important after the completion of treatment for advanced cancer where patients have poor prognosis and will want to enjoy a comfortable standard of living during their final months.
Quality of the evidence
The amount of available evidence does allow robust conclusions for the comparison of combination therapy and ifosfamide, as there is consistency and commonality in the results, but the comparison of whole body irradiation is restricted to single trial analyses.
The reporting of the methodological quality of the trials showed that two trials (Homesley 2007; Wolfson 2007) were at low risk of bias while the trial of Sutton 2000 was at high risk of bias as it only satisfied two of the criteria used to assess risk of bias.
All three trials reported a hazard ratio (HR), which is the best statistic to summarise the difference in risk in two treatment groups over the duration of a trial, when there is "censoring" i.e. the time to death (or disease progression) is unknown for some women as they were still alive (or disease‐free) at the end of the trial.
The two similar trials (Homesley 2007; Sutton 2000) gave consistent evidence about all outcomes as individual trials were robust to the findings of the meta‐analyses, although in some instances point estimates were not necessarily similar. For survival outcomes, there is evidence that combination therapy delays death and disease progression compared with single agent ifosfamide, but we are not sure how safe combination therapy is as there were relatively low numbers of adverse events and it was associated with significantly more grades three and four nausea and vomiting. A substantial number of women experienced disease progression and death, which helps to ensure high quality evidence.
Potential biases in the review process
A comprehensive search was performed, including a thorough search of the grey literature and all studies were sifted and data independently extracted by three review authors. We restricted the included studies to RCTs as they provide the strongest level of evidence available. Hence we have attempted to reduce bias in the review process.
The greatest threat to the validity of the review is likely to be the possibility of publication bias i.e. studies that did not find the treatment to have been effective may not have been published. We were unable to assess this possibility as we found only three included trials.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, this is the first systematic review on the effects of adjuvant treatments for uterine carcinosarcoma. There are large number of trials other than RCTs studying adjuvant chemotherapeutic agents at present. These phase II trials, observational studies or non‐controlled trials were limited by small numbers and methodological limitations. There are several non systematic reviews on uterine sarcomas and/or uterine carcinosarcoma mainly addressing clinicopathological and prognostic factors (Arrastia 1997).
Authors' conclusions
Implications for practice.
The optimum chemotherapeutic regimen for advanced uterine carcinosarcoma is still to be defined, although our review has provided evidence that combination chemotherapy improves survival. The combination of ifosfamide and paclitaxel was associated with significant improvement in overall and progression‐free survival. While the addition of cisplatin to ifosfamide appears to offer a small improvement in progression‐free survival over ifosfamide alone; the added toxicity may not justify the use of this combination.
In advanced stage metastatic uterine carcinosarcoma as well as recurrent disease, adjuvant combination chemotherapy with ifosfamide should be considered.
Implications for research.
There is a need for a trial that randomly assigns women with advanced uterine carinosarcoma and no prior treatment to receive either ifosfamide and paclitaxel or platinum and paclitaxel.
In addition, we need a randomised controlled trial to determine the effectiveness of adjuvant chemotherapy in early stage uterine carcinosarcoma.
Future trials need to clarify which groups of patients would benefit from which treatment by stratifying patients at trial entry for prior therapies, performance status, site of disease and co‐morbidity.
quality of life (QoL) and symptom scores should be assessed as well as primary outcomes such as overall survival (OS) and progression‐free survival (PFS).
We need to determine the best dose of combination chemotherapy with the least adverse effects in advanced uterine carcinosarcoma.
Further studies (phase II trial) should be set up to investigate the use of newer chemotherapeutic agents in uterine carcinosarcoma.
What's new
| Date | Event | Description |
|---|---|---|
| 5 December 2018 | Review declared as stable | No new trials expected in this topic area. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 1, 2011
| Date | Event | Description |
|---|---|---|
| 1 April 2015 | Amended | Contact details updated. |
| 24 February 2015 | Amended | Contact details updated. |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
| 29 January 2013 | Amended | Contact details updated |
| 22 January 2013 | New search has been performed | New authors included. |
| 6 November 2012 | New citation required but conclusions have not changed | New search conducted November 2012. No new trials identified. |
Acknowledgements
We thank Chris Williams and Jo Morrison for clinical and editorial advice, Jane Hayes for designing the search strategy and Gail Quinn and Clare Jess for their contribution to the editorial process. Ram Athavale helped design the protocol. We also thank Katherine Dean for methodological expertise and advice at the protocol stage.
Appendices
Appendix 1. MEDLINE search strategy
Medline 1950 to November 2012
exp Uterine Neoplasms/
(uter* or endometri*).mp.
1 or 2
exp Carcinosarcoma/
carcinosarcoma*.mp.
Mixed Tumor, Mullerian/
Mixed Tumor, Mesodermal/
(mixed and tumo* and (mullerian or mesodermal)).mp.
4 or 5 or 6 or 7 or 8
3 and 9
exp Radiotherapy/
radiotherap*.mp.
radiation.mp.
radiotherapy.fs.
11 or 12 or 13 or 14
exp Antineoplastic Agents/
Antineoplastic Combined Chemotherapy Protocols/
Chemotherapy, Adjuvant/
chemotherap*.mp.
drug therapy.fs.
16 or 17 or 18 or 19 or 20
15 or 21
10 and 22
key: mp=title, original title, abstract, name of substance word, subject heading word, unique identifier, fs=floating subheading
Appendix 2. EMBASE search strategy
Embase Ovid 1980 to November 2012
exp uterus cancer/
(uter* or endometr*).mp.
1 or 2
exp mixed tumor/
carcinosarcoma.mp.
(mixed and tumo* and (mullerian or mesodermal)).mp.
4 or 5 or 6
3 and 7
cancer radiotherapy/
exp radiotherapy/
radiotherap*.mp.
radiation.mp.
rt.fs.
9 or 10 or 11 or 12 or 13
exp chemotherapy/
exp antineoplastic agent/
combination chemotherapy/
chemotherap*.mp.
dt.fs.
15 or 16 or 17 or 18 or 19
14 or 20
8 and 21
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name, fs=floating subheading
Appendix 3. CENTRAL search strategy
CENTRAL Issue 10, 2012
MeSH descriptor Uterine Neoplasms explode all trees
uter* or endometri*
(#1 OR #2)
MeSH descriptor Carcinosarcoma explode all trees
carcinosarcoma*
MeSH descriptor Mixed Tumor, Mullerian explode all trees
MeSH descriptor Mixed Tumor, Mesodermal explode all trees
mixed and tumo* and (mullerian or mesodermal)
(#4 OR #5 OR #6 OR #7 OR #8)
(#3 AND #9)
MeSH descriptor Radiotherapy explode all trees
radiotherap*
radiation
Any MeSH descriptor with qualifier: RT
(#11 OR #12 OR #13 OR #14)
MeSH descriptor Antineoplastic Agents explode all trees
MeSH descriptor Antineoplastic Combined Chemotherapy Protocols explode all trees
MeSH descriptor Chemotherapy, Adjuvant explode all trees
chemotherap*
Any MeSH descriptor with qualifier: DT
(#16 OR #17 OR #18 OR #19 OR #20)
(#15 OR #21)
(#10 AND #22)
Data and analyses
Comparison 1. Combination therapy versus Ifosfamide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 2 | 373 | Hazard Ratio (Random, 95% CI) | 0.75 [0.60, 0.94] |
| 2 Progression‐free survival | 2 | 373 | Hazard Ratio (Random, 95% CI) | 0.72 [0.58, 0.90] |
| 3 G3‐4 Nausea/vomiting | 2 | 365 | Risk Ratio (IV, Random, 95% CI) | 3.53 [1.33, 9.37] |
| 4 Diarrhoea and other GI | 2 | 365 | Risk Ratio (IV, Random, 95% CI) | 1.51 [0.31, 7.52] |
| 5 Haematological | 2 | 365 | Risk Ratio (IV, Random, 95% CI) | 1.56 [0.84, 2.90] |
| 6 Genitourinary | 2 | 365 | Risk Ratio (IV, Random, 95% CI) | 1.68 [0.54, 5.18] |
| 7 Cardiovascular | 2 | 365 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.13, 3.11] |
| 8 Hepatic | 1 | 174 | Risk Ratio (IV, Random, 95% CI) | 2.05 [0.73, 5.74] |
| 9 Neuropathy | 2 | 365 | Risk Ratio (IV, Random, 95% CI) | 1.59 [0.99, 2.55] |
Comparison 2. Whole body irradiation versus chemotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2 Progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3 G3‐4 Gastrointestinal | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4 Haematological | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5 Genitourinary | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6 Cardiovascular | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7 Hepatic | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8 Neuropathy | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
2.7. Analysis.
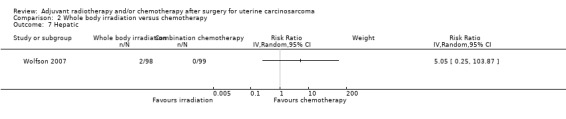
Comparison 2 Whole body irradiation versus chemotherapy, Outcome 7 Hepatic.
2.8. Analysis.
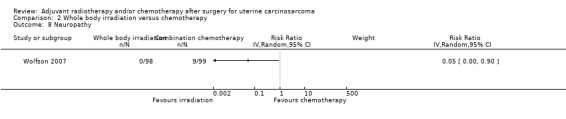
Comparison 2 Whole body irradiation versus chemotherapy, Outcome 8 Neuropathy.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Homesley 2007.
| Methods | Phase III Randomised Controlled Trial | |
| Participants | 179 women with histologically confirmed stage III or IV, persistent or recurrent uterine carcinosarcoma not amenable to treatment with curative intent by other means. 91 were randomised to arm 1 (ifosfamide alone). 88 were randomised to arm 2 (ifosfamide + paclitaxel). Median age for both arms was 64 years. In arm 1 (single agent ifosfamide) 18% had stage III, 31% stage IV and 52% had recurrent/persistent disease In arm 2 (ifosfamide + paclitaxel) 18% had stage III, 29% had stage IV disease and 52% had recurrent/persistent disease All women had measurable disease by palpation or radiologically (X‐ray, MRI, CT or USS) with minimum measurement of 1 cm. Patients had to have adequate bone marrow function with an absolute neutrophil count > 1,500 mL/uL, platelets >100,000 uL, creatinine clearance of > 50 mL/min, bilirubin < 1.5x normal, AST < 3x normal and serum albumin > 3 g/dL. GOG performance status 0, 1, or 2 At least 6 weeks must have passed since RT to the site of current measurable disease. Exclusion: patients who received prior chemotherapy for uterine carcinosarcoma. Patients with septicaemia, severe infection, acute hepatitis, GI bleed or performance status 3‐4. Patients having other invasive malignancies, prior or current evidence of cancer within the last 5 years, or any cancer therapy determined to be a contraindication for the study protocol. History of congestive cardiac failure unstable angina or myocardial infarction in the last 6 months. |
|
| Interventions | Arm 1: Ifosfamide 2 mg/m2 intravenously (IV) daily for three days every 21 days for eight cycles. The starting dose was 1.2 mg/m2 for patients who had received prior radiotherapy (RT). Mesna was administered either IV or orally. The IV administration consisted of 2 g during 12 hours beginning 1 minutes before the ifosfamide infusion; for oral administration, the total dose was 4 g in three divided doses of 1.33 g administered one hour before and eight hours after the ifosfamide infusion for three days Arm 2: Ifosfamide 1.6 mg/m2 daily for three days (or was reduced to 1.2 mg/m2 for patients who had received prior radiotherapy. Paclitaxel 135mg/m2 during three hours IV was administered on day 1. Mesna was to be administered as per Arm 1. The premedication for paclitaxel was dexamethasone 20 mg orally or IV 12 and 6 hours before paclitaxel, diphenhydramine 50 mg IV 30 minutes before paclitaxel. Patients on both arms were to receive a maximum of eight cycles of protocol therapy unless disease progression or adverse effects prohibited additional treatment. | |
| Outcomes |
Primary outcome measure:
a) Complete response was the disappearance of all gross evidence of disease lasting for at least 4 weeks b) Partial response was a reduction of 50% or greater in the bi‐dimensional measurement of each lesion sustained for at least 4 weeks c) Increasing disease was a 50% or greater increase in any lesion or the appearance of any new lesion (s) within 8 weeks of entry onto the study d) Stable disease was any condition not meeting any of the above criteria Secondary outcomes: Adverse events believed to be related protocol treatment, assessed according to the GOG Common Toxicity Criteria. |
|
| Notes | The median duration of follow up was 20 months. Overall the response rate (complete + partial response) was 45% in ifosfamide + paclitaxel arm and 29% in ifosfamide only arm. The odds of response stratified by performance status were 2.21 greater in arm 2 (P = 0.017). The median overall survival was 13.5 months in the combination arm and 8.4 months in the ifosfamide only arm. Median PFS and OS, respectively, for arm 1 compared with arm 2 were 3.6 versus 5.8 months and 8.4 versus 13.5 months, respectively. Intention‐to‐treat principle was applied to the treatment group comparisons of overall survival, progression‐free survival and clinical response. Alog rank test stratified by performance status was used to test the independence of treatment with PFS and OS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random assignment was carried out with equal probability of assignment to each treatment regimen using a balanced block randomisation to balance assigned treatment regimens within strata defined by institution and performance status (0 to 1 or 2)". |
| Allocation concealment (selection bias) | Low risk | "The treatment assignment was concealed from institution and the patient until telephone registration with verification of eligibility". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 179/179 (100%) for time‐to‐event outcomes and 174/179 (97%) for all adverse events outcomes. 214 women were randomised, but 35 were found to be ineligible. |
| Selective reporting (reporting bias) | Low risk | All important survival and adverse event outcomes have been reported. Survival outcomes have been analysed using appropriate statistical techniques to account for censoring. |
| Other bias | Unclear risk | Insufficient information to assess whether an additional risk of bias exists. |
Sutton 2000.
| Methods | Phase III Randomised Controlled Trial "Treatment was randomly allocated in an unblinded fashion without concealment" |
|
| Participants | 194 women with histologically confirmed advanced or recurrent carcinosarcoma no longer amenable to control by surgery and/or radiotherapy. 102 were in arm 1 (ifosfamide) and 92 in arm 2 (ifosfamide + cisplatin). Median age for arm 1 was 67 years (range 32‐84), 66 years for arm 2 (range 35‐83). Inclusion criteria: All patients had to have measurable disease which could be defined in at least two dimensions by palpation, X ray, computed tomography, or ultrasound. White blood count ≥ 3000/mcl, platelet count ≥ 100,000/mcl, blood urea nitrogen level ≥ 30 mg/dL, creatinine ≥ 1.5 mg/dL or creatinine clearance ≥ 50 mL/min, serum bilirubin ≤ 1.5 times normal, SGOT (ALT) ≤ 3 times normal, serum albumin ≥3 g/dL, and a GOG performance status (PS) of 0 to 2 (Karnofsky score ≥60%). Exclusion criteria: Patients must have received no prior chemotherapy. Patients with extensive hepatic metastases, acute hepatitis, septicaemia, severe infection, or gastrointestinal bleeding were ineligible. Patients with previous or concomitant malignancy other than non‐melanoma skin cancer were ineligible. Written informed consent was obtained from all patients prior to study entry, fulfilling all institutional, state, and federal regulations. Slides documenting the primary cancer were submitted for central pathologic review. |
|
| Interventions | Arm 1: ifosfamide alone (1.5 g/m2/day) for 5 days with mesna uroprotection , each course given intravenously every 3 weeks. Ifosfamide doses were reduced by 20% to 1.2 g/m2/day in patients who had previous pelvic radiotherapy. Mesna was administered as a continuous infusion in a dose identical to that of ifosfamide. Each patient received eight cycles of therapy unless there was disease progression or undue toxicity. Ifosfamide doses were reduced 20% for grade 4 white blood count or platelet toxicity, grade 3 or 4 hepatic toxicity, or grade 1 renal or neurologic toxicity. Arm 2: Ifosfamide plus cisplatin (20 mg/m2/day) times 5 days. Because of the unexpected toxicity observed early in the trial in patients receiving combination therapy, doses of both drugs were reduced 20% by giving a 4‐day instead of 5‐day course of therapy. | |
| Outcomes |
Primary outcome measure:
a) Complete response, b) Partial response, c) Increasing disease d) Stable disease, Adverse effects were graded using standard GOG criteria. Eligible patients receiving at least one course of treatment were included in the assessment of adverse effects. |
|
| Notes | All eligible patients were included in the analysis of overall and progression‐free survival. All causes of death were considered events in overall and progression‐free survival analysis This randomised Phase III study was designed to detect an increase in response from 34% to 54% or a 50% increase of 2.8 months in median progression‐free survival with 80% power and type I error set at 0.05 for a one‐tailed test. Also, detectable with 76% power and type I error set at 0.05 (one‐tailed) a 50% increase of 6.2 months in median survival was planned. Final analysis required the observation of 136 deaths for survival and 150 failures for PFS. Site of measurable disease appear to be imbalanced between the two treatment arms. 37% of patients on the ifosfamide arm compared to 59% of patients on the combination arm had measurable disease limited to the pelvis. The median number of treatment cycles was 4 with 0‐8 range. Treatment may have contributed to the deaths of 6 patients treated with full doses of ifosfamide and cisplatin for 5 days. The proportion of patients responding to ifosfamide alone versus ifosfamide– cisplatin therapy was (0.36 versus 0.54) overall, (0.47 versus 0.61) for pelvic, (0.21 versus 0.54) for lung, and (0.33 versus 0.40) for “other” metastatic sites of measurable disease. The median progression‐free survivals for the ifosfamide was 4 months and for the combination arm it was 6 months (relative risk, 0.73; 95% upper confidence limit, 0.94; P 5 0.02). There was no statistically significant difference between the two treatment groups with regard to survival The median duration overall survival for arms was 7.6 and 9.4 months, respectively (relative risk, 0.80, 95% upper confidence limit, 1.03; P 5 0.07). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported, "Treatment was randomly allocated". |
| Allocation concealment (selection bias) | High risk | "Treatment was randomly allocated ... without concealment". |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Treatment was randomly allocated in an unblinded fashion". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 194/194 (100%) for time‐to‐event outcomes and 191/194 (98%) for all adverse events outcomes. 224 women were randomised, but 30 were found to be ineligible. |
| Selective reporting (reporting bias) | Low risk | All important survival and adverse event outcomes have been reported. Survival outcomes have been analysed using appropriate statistical techniques to account for censoring. |
| Other bias | Unclear risk | Insufficient information to assess whether an additional risk of bias exists. |
Wolfson 2007.
| Methods | Phase III Randomised Controlled Trial | |
| Participants | 232 enrolled in the study, 25 were excluded based on review of histology and one patient was excluded due to inappropriate residual disease at determined by GOG Gyne/oncology committee review. All were previously untreated patients with stages I, II, III, and IV primary carcinosarcomas of the uterus or cervix (without any demonstrable parenchymal hepatic involvement or extra‐abdominal distant disease) 206 patients were in the study, 105 were randomised to whole abdominal irradiation (WAI) arm and 101 to Cispltain, Ifosfamide with mesna (CIM) arm. 43% of patients randomised to WAI arm had stage III, and 46% in the CIM arm had stage III Median age for WAI was 68 years and 65 years for CIM arm. Eligibility required a patient to have a TAH, BSO, and maximal resection of all gross intra‐abdominal/pelvic disease, including macroscopically involved pelvic and para‐aortic nodes, leaving no residual disease any larger than 1 cm. Peritoneal cytology and RPLND were optional if there were no intraoperative clinical manifestations of residual disease within the abdomen and pelvis. Adequate hematologic (WBC ≥ 3000/μL, platelets ≥1 00,000/μL, and granulocytes ≥ 1500/μL), renal (serum creatinine ≤ 1.5 mg% or creatinine clearance ≥ 50 mL/min), and hepatic (serum bilirubin ≤1.5 × the institutional value, serum AST≤3 × the institutional value, and serum albumin ≥ 3) functions were required. In addition, eligible patients were required to have a GOG Performance Status of 0, 1, or 2 and a normal chest X‐ray (no other imaging studies were required).patients who had received prior hormonal manipulation (not evaluated in this study) were also eligible for entry. Exclusion criteria: patients who had a prior history of receiving radiotherapy and/ or chemotherapy or who had a concomitant malignancy (other than non‐melanoma skin cancer) within 5 years of being diagnosed with uterine CS were ineligible. |
|
| Interventions |
Arm 1: Whole Abdominal Irradiation (WAI) was to be delivered by external beam radiation therapy (EBRT) to the abdomen and pelvis that involved a minimum beam energy of 4 MV photons and utilised an anterior/posterior (AP) and posterior/anterior (PA) summated technique. The field borders for WAI involved 1 cm margins on the diaphragm superiorly, the inguinal ligament inferiorly, and the lateral aspect of the peritoneal margin laterally. At the outset of this protocol, the whole abdomen was treated to a total dose of 30 Gy at 1 Gy per fraction, two fractions per day, and 5 days each week with a minimum of 6 h between morning and afternoon fractions (hyperfractionation). Due to slow patient accrual, in August of 1996, the dose fractionation schedule was changed to once‐daily fractions of 1.5 Gy for 5 days each week to the same total dose to the whole abdomen of 30 Gy. Whole abdominal irradiation (WAI).
The true pelvis was treated with a boost requiring a four‐field “box” set‐up that included not only AP/PA beam portals but also opposed lateral fields. The pelvic field borders included the S1/S2 interspace superiorly, the mid‐level portion of the obturator foramen inferiorly, and 1 cm beyond the widest aspect of the true pelvic laterally. At the initiation of this study, the true pelvis as a boost was treated to a total dose of 20 Gy at 1 Gy per fraction, two fractions per day for 5 days each week with the same 6 h time interval between fractions as was initially done for the WAI (cumulative true pelvic dose of 50 Gy). As stated above, the fractionation schedule was also changed in August of 1996 for this portion of radiotherapy to once‐daily fractions of 1.8 Gy for 5 days each week to a total dose of 19.8 Gy (cumulative true pelvic dose of 49.8 Gy). Arm 2: Cispltain, Ifosfamide with mesna (CIM) comprised intravenous (IV) cisplatin (20 mg/m2/day × 4 days) that was to be followed by a 1 h IV administration of ifosfamide (1.5g/m2/day IV × 4 days) with mesna (120 mg/m2 IV bolus over 15 min on day one, followed by 1.5 g/m2/day IV continuous infusion × 4 days beginning with day one) every 3 weeks for three cycles. It was recommended that hydration be maintained by IV administration of 1 L over several hours preferably with either normal or one‐half normal saline prior to initiation of chemotherapy in order to maintain urine output of at least 100 mL/h. IV fluid and electrolyte repletion was permitted as medically indicated during the 4‐day course of chemotherapy. Cisplatin administration was required prior to ifosfamide therapy and was to be reconstituted at a concentration of approximately 1 mg/mL and infused at a rate of 1 mg/min. Dose modifications for toxicities of cisplatin and ifosfamide were permitted but not for mesna administration. Protocol therapy was to be started within 8 weeks following initial surgery. |
|
| Outcomes |
The primary outcome measures for assessing treatment efficacy
Secondary measures: Adverse events of treatment were classified as being either acute or late toxicities. A toxicity that occurred during study therapy was identified as acute, while those that either persisted or developed after completion of treatment were separately identified as late or chronic toxicities. |
|
| Notes | After protocol treatment, patients were evaluated every 3 months for the first 2 years and then every 6 months thereafter by a treating physician with CBC, serum creatinine, serum bilirubin, serum AST, and CA‐125 level (required prior to study entry). A chest X‐ray was required every 6 months following completion of study treatment for the first 2 years and then yearly thereafter. The estimated crude probability of recurring within 5 years was 58% (WAI) and 52% (CIM). The estimated crude probability of surviving at least 5 years following diagnosis was approximately 35% for those randomised to WAI vs. 45% for those randomised to CIM. The median duration of follow‐up for patients alive at last contact was 5 years and 3 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The list of treatment assignments was created by concatenating randomly selected balanced blocks of permuted treatments". |
| Allocation concealment (selection bias) | Low risk | "The complete list of treatment assignments remained concealed and only the next unassigned treatment was revealed after each patient was successfully registered onto the study". |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 206/206 (100%) for time‐to‐event outcomes and 197/206 (96%) for all adverse events outcomes. 232 women were randomised, but 26 were found to be ineligible. |
| Selective reporting (reporting bias) | Low risk | All important survival and adverse event outcomes have been reported. Survival outcomes have been analysed using appropriate statistical techniques to account for censoring. |
| Other bias | Unclear risk | Insufficient information to assess whether an additional risk of bias exists. |
ALT: alanine transaminase AP: anterior/posterior AST: aspartate aminotransferase (SGOT) BSO: bilateral salpingo‐oophorectomy CIM: cisplatin and Ifosfamide with mesna CT: computed tomography EBRT: external beam radiation therapy GI: gastrointestinal GOG: Gynecologic Oncology Group MRI: magnetic resonance imaging OS: overall survival PA: posterior/anterior PFS: progression‐free survival RPLND: Retroperitoneal Lymph Node Dissection SGOT: glutamic oxaloacetic transaminase (AST) TAH: total abdominal hysterectomy USS: ultrasound scan WAI: whole abdominal irradiation WBC: white blood cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Asbury 1998 | A phase II trial of amonafide in patients with mixed mesodermal tumours of the uterus. Amonafide‐a drug that acts through intercalation of tumour DNA‐was used to treat 16 patients who had measurable, advanced mixed mesodermal tumours of the uterus. The starting dose was 300 mg/m2 intravenously over 1 hour for 5 consecutive days every 3 weeks. Outcome: This dose schedule was associated with poor response rate and substantial toxicity. No comparison group. |
| Currie 1996 | Phase II trial of hydroxyurea, dacarbazine (DTIC), and etoposide (VP‐16). Outcome: Hydroxyurea, dacarbazine (DTIC), and etoposide (VP‐16) shows moderate activity in patients with advanced or recurrent carcinosarcoma. No comparison group. |
| Curtin 2001 | Phase II study of paclitaxel in patients with advanced or recurrent uterine carcinosarcoma who failed to respond to local therapy. Outcome: Overall, paclitaxel shows 18.2% response rate in patients with uterine carcinosarcoma. No comparison group. |
| Fowler 2002 | Phase II group‐wide study of the Gynecologic Oncology Group to determine the toxicity and objective response rate of trimetrexate (TMTX) in patients with advanced, persistent, or recurrent mixed mesodermal tumours of the uterus. Outcome: Oral TMTX has insignificant activity in uterine carcinosarcoma. No comparison group. |
| Miller 2005 | Phase II trial of topotecan at a target dose of 1.5 mg/m2 was administered IV daily for 5 days, every 3 weeks, in persistent or recurrent carcinosarcoma of uterus. Outcome: Topotecan at this dose and schedule does not appear to have major activity in patients with advanced or recurrent uterine. carcinosarcoma previously treated with chemotherapy. No comparison group. |
| Perez 1979 | Retrospective study of 54 patients with uterine carcinosarcoma, patients with stage I‐II were treated with surgery alone or pre‐operative intracavity irradiation, or combination of pre‐operative intracavity irradiation and external irradiation. Patients with stage III‐IV were treated with surgery alone or combination of surgery and post operative irradiation or irradiation alone. Outcome: Patients with stage I‐II treated with pre‐operative irradiation showed reduced pelvic recurrence rate. None of patients with stage III‐IV survived. |
| Powell 2010 | Retrospective study of 46 eligible patients with advanced stage (III or IV), persistent or recurrent uterine carcinosarcoma (Malignant mixed Mullerian tumour) and no prior chemotherapy. Patients received paclitaxel at 175 mg/m2 intravenously (IV) over 3 hours plus carboplatin IV over 30 minutes every 3 weeks until disease progression or adverse effects occurred. No comparison group. |
| Ramondetta 2003 | A phase II trial of cisplatin, ifosfamide, and mesna in patients with advanced or recurrent uterine carcinosarcoma. Outcome: None of the patients had complete response, there was partial response in two and stable disease in one patient. Partial response duration was 6 and 9 months. No comparison group. |
| Resnik 1995 | Phase II study of etoposide, cisplatin, and doxorubicin chemotherapy in Mixed Mullerian Tumors (MMT) of the uterus, in 54 patients, 23 with early stage I‐II, and 19 with stage III‐IV. Outcome: median survival was 18 months. No comparison group. |
| Sutton 1989 | Phase II trial of ifosfamide and mesna in mixed mesodermal tumours of the uterus. Outcome: Response rate of 32% with 17.9% showing complete response. No comparison group. |
| Sutton 2005 | Study on adjuvant ifosfamide and cisplatin after primary surgery for stage I or II carcinosarcoma of the uterus. Ifosfamide was given at 1.5g/m2 I.V, 20mg/m2 cisplatin, followed by 120mg/m2 mesna for five days every 21 day for 3 cycles. Outcome: Two years progression‐free survival was 69% and overall survival was 82%. The five year overall survival was 62%. No comparison group. |
| Toyoshima 2004 | A retrospective review of six patients with uterine carcinosarcoma treated with paclitaxel and carboplatin. Outcome: Four patients had complete response and two had progressive disease. Median progression‐free survival was 18 months, median overall survival was 25 months. No comparison group. |
DTIC: hydroxyurea, dacarbazine TMTX: trimetrexate
Differences between protocol and review
Searches
In the protocol, we stated:
"The main investigators of any relevant ongoing trials will be contacted for further information, as will any major co‐operative trials groups active in this area."
However, we did not find any relevant ongoing trials or active trials groups, so we did not make these contacts.
Continuous outcome data
Continuous outcome data were not reported in any of the trials so the following sections in the protocol which discussed the handling of data for continuous outcomes were removed as they were unnecessary.
Data extraction and management
"For continuous outcomes (e.g. quality of life measures), we will extract the final value and standard deviation of the outcome of interest and the number of patients assessed at endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference between treatment arms and its standard error."
Measures of treatment effect
"For continuous outcomes, we will use the mean difference between treatment arms."
Data synthesis
"For continuous outcomes, the mean differences between the treatment arms at the end of follow‐up will be pooled if all trials measured the outcome on the same scale, otherwise standardised mean differences will be pooled".
There were also no multiple treatment groups in any of the three included trials and it was not possible to make indirect comparisons so the following methods were removed from the data synthesis section of the review:
If any trials have multiple treatment groups, the ‘shared’ comparison group will be divided into the number of treatment groups and comparisons between each treatment group and the split comparison group will be treated as independent comparisons.
If possible, indirect comparisons, using the methods of Bucher 1997 will be used to compare competing interventions that have not been compared directly with each other.
Assessment of reporting biases
Reporting biases were not assessed as there was an insufficient number of included trials in which to compute funnel plots to assess the potential for small study effects such as publication bias so the following was removed from the review.
Funnel plots corresponding to meta‐analysis of the primary outcome will be examined to assess the potential for small study effects such as publication bias. If these plots suggest that treatment effects may not be sampled from a symmetric distribution, as assumed by the random‐effects model, further meta‐analyses will be performed using fixed‐effect models.
Subgroup analysis and investigation of heterogeneity
It was not possible to perform subgroup analysis so the following was removed from the review.
"Sub‐group analyses will be performed, grouping the trials by:
Disease‐free interval"
Factors such as age, stage, length of follow‐up, adjusted/unadjusted analysis will be considered in interpretation of any heterogeneity.
Sensitivity analysis
Sensitivity analyses were not performed as there were an insufficient number of trials in the review. The following was removed from the sensitivity analysis section.
"Sensitivity analyses will be performed excluding trials which did not report (i) concealment of allocation and (ii) blinding of the outcome assessor."
Contributions of authors
Khadra Galaal is the main author of the review. The protocol was developed by Keith Godfrey. Khadra Galaal, and Keith Godfrey sifted references, sought and obtained unpublished data from investigators and prepared the tables. All authors had input in the write up of the review; Khadra Galaal drafted the clinical sections and Ali Kucukmetin, Raj Naik, Tito Lopes, Nagindra Das assisted with the clinical sections of the review & update. Andrew Bryant drafted the methodological and statistical sections. This updated version was written by Esther van der Heijden and Khadra Galaal.
The review authors met regularly to discuss the development of the review.
Sources of support
Internal sources
Northern Gynaecological Oncology Centre, UK.
External sources
-
Department of Health, UK.
NHS Cochrane Collaboration programme Grant Scheme CPG‐506
Declarations of interest
None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Homesley 2007 {published and unpublished data}
- Homesley HD, Filiaci V, Markman M, Bitterman P, Eaton L, Kilgore LC, et al. Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology Feb 10 2007;25(5):526‐31. [DOI] [PubMed] [Google Scholar]
Sutton 2000 {published data only}
- Sutton G, Brunetto VL, Kilgore L, Soper JT, McGehee R, Olt G, et al. A phase III trial of ifosfamide with or without cisplatin in carcinosarcoma of the uterus: A Gynecologic Oncology Group Study. Gynecologic Oncology 2000;79(2):147‐53. [DOI] [PubMed] [Google Scholar]
Wolfson 2007 {published and unpublished data}
- Wolfson AH, Brady MF, Rocereto T, Mannel RS, Lee YC, Futoran RJ, et al. A gynecologic oncology group randomized phase III trial of whole abdominal irradiation (WAI) vs. cisplatin‐ifosfamide and mesna (CIM) as post‐surgical therapy in stage I‐IV carcinosarcoma (CS) of the uterus. Gynecologic Oncology 2007;107(2):177‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Asbury 1998 {published data only}
- Asbury R, Blessing JA, Podczaski E, Ball H. A phase II trial of amonafide in patients with mixed mesodermal tumors of the uterus: a Gynecologic Oncology Group study. American Journal of Clinical Oncology 1998;21(3):306‐7. [DOI] [PubMed] [Google Scholar]
Currie 1996 {published data only}
- Currie JL, Blessing JA, McGehee R, Soper JT, Berman M. Phase II trial of hydroxyurea, dacarbazine (DTIC), and etoposide (VP‐16) in mixed mesodermal tumors of the uterus: a Gynecologic Oncology Group study. Gynecologic Oncology 1996;61(1):94‐6. [DOI] [PubMed] [Google Scholar]
Curtin 2001 {published data only}
- Curtin JP, Blessing JA, Soper JT, DeGeest K. Paclitaxel in the treatment of carcinosarcoma of the uterus: a Gynecologic Oncology Group study. Gynecologic Oncology 2001;83(2):268‐70. [DOI] [PubMed] [Google Scholar]
Fowler 2002 {published data only}
- Fowler JM, Blessing JA, Burger RA, Malfetano JH. Phase II evaluation of oral trimetrexate in mixed mesodermal tumors of the uterus: a Gynecologic Oncology Group study. Gynecologic Oncology 2002;85(2):311‐4. [DOI] [PubMed] [Google Scholar]
Miller 2005 {published data only}
- Miller DS, Blessing JA, Schilder J, Munkarah A, Lee YC. Phase II evaluation of topotecan in carcinosarcoma of the uterus: A Gynecologic Oncology Group study. Gynecologic Oncology 2005;98(2):217‐21. [DOI] [PubMed] [Google Scholar]
Perez 1979 {published data only}
- Perez CA, Askin F, Balgan RJ, Kao MS, Kraus FT, Perez BM, et al. Effects of irradiation on mixed mullian tumors of the uterus. Cancer 1979;43(4):1274‐84. [DOI] [PubMed] [Google Scholar]
Powell 2010 {published data only}
- Powell MA, Filiaci VL, Rose PG, Mannel RS, Hanjani P, DeGeest K, et al. A phase II evaluation of paclitaxel and carboplatin in the treatment of carcinosarcoma of the uterus: a GynecologicOncology Group Study. Journal of Clinical Oncolcogy 2010;28(16):2727‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramondetta 2003 {published data only}
- Ramondetta LM, Burke TW, Jhingran A, Schmandt R, Bevers MW, Wolf JK, et al. A phase II trial of cisplatin, ifosfamide, and mesna in patients with advanced or recurrent uterine malignant mixed mullerian tumors with evaluation of potential molecular targets.[see comment]. Gynecologic Oncology 2003 Sep;90(3):529‐36. [DOI] [PubMed] [Google Scholar]
Resnik 1995 {published data only}
- Resnik E, Chambers SK, Carcangiu ML, Kohorn EI, Schwartz PE, Chambers JT. A phase II study of etoposide, cisplatin, and doxorubicin chemotherapy in mixed müllerian tumors (MMT) of the uterus. Gynecologic Oncology 1995;56(3):370‐5. [DOI] [PubMed] [Google Scholar]
Sutton 1989 {published data only}
- Sutton GP, Blessing JA, Rosenshein N, Photopulos G, DiSaia PJ. Phase II trial of ifosfamide and mesna in mixed mesodermal tumors of the uterus (a Gynecologic Oncology Group study). American Journal of Obstetrics and Gynecology 1989;161(2):309‐12. [DOI] [PubMed] [Google Scholar]
Sutton 2005 {published data only}
- Sutton G, Kauderer J, Carsonc LF, Lentz SS, Whitney CW, Gallion H. Adjuvant ifosfamide and cisplatin in patients with completely resectedstage I or II carcinosarcomas (mixed mesodermal tumors) of the uterus:a Gynecologic Oncology Group study. Gynecologic Oncology 2005;96(3):630–4. [DOI] [PubMed] [Google Scholar]
Toyoshima 2004 {published data only}
- Toyoshima M, Akahira JI, Matsunaga G, Niikura H, Ito K, Yaegashi N, et al. Clinical experience with combination paclitaxel and carboplatin therapy for advanced or recurrent carcinosarcoma of the uterus. Gynecologic Oncology 2004;94(3):774‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Arrastia 1997
- Arrastia CD, Fruchter RG, Clark M, Maiman M, Remy JC, Macasaet M, et al. Uterine carcinosarcomas: Incidence and trends in management and survival. Gynecologic Oncology 1997;65(1):158‐63. [DOI] [PubMed] [Google Scholar]
Barwick 1979
- Barwick KW, LiVolsi VA. Malignant mixed mullerian tumors of the uterus. A clinicopathologic assessment of 34 cases. American Journal of Surgical Pathology 1979;3(2):125‐35. [DOI] [PubMed] [Google Scholar]
Brooks 2004
- Brooks SE, Zhan M, Cote T, Baquet CR. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma 1989‐1999. Gynecologic Oncology 2004;93(1):204‐8. [DOI] [PubMed] [Google Scholar]
Bucher 1997
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50(6):683‐91. [DOI] [PubMed] [Google Scholar]
Callister 2004
- Callister M, Ramondetta LM, Jhingran A, Burke TW, Eifel PJ. Malignant mixed mullerian tumors of the uterus: analysis of patterns of failure, prognostic factors, and treatment outcome. International Journal of Radiation Oncology Biology Physics 2004;58(3):786‐96. [DOI] [PubMed] [Google Scholar]
Chi 1997
- Chi DS, Mychalczak B, Saigo PE, Rescigno J, Brown CL. The role of whole‐pelvic irradiation in the treatment of early‐stage uterine carcinosarcoma. [Review] [21 refs]. Gynecologic Oncology 1997 Jun;65(3):493‐8. [DOI] [PubMed] [Google Scholar]
CTCAE 2006
- CTCAE. Common Terminology Criteria for Adverse Events. (http://ctep.cancer.gov/forms/CTCAEv3.pdf) 9th August 2006; Vol. v3.0 (CTCAE).
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ . Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
El Nashar 2011
- El‐Nashar SA, Mariani A. Uterine carcinosarcoma. Clinical Obstetetrics and Gynecology 2011 Jun;54(2):292‐304. [DOI] [PubMed] [Google Scholar]
FIGO 2009
- FIGO Committee on Gynecologic Oncology. Revised FIGO staging for carcinoma of vulva, cervix, and endometrium. International Journal of Gynecology and Obstetrics 2009;105(2):103‐4. [DOI] [PubMed] [Google Scholar]
Gadducci 2002
- Gadducci A, Sartori E, Landoni F, Zola P, Maggino T, Cosio S, et al. The prognostic relevance of histological type in uterine sarcomas: a Cooperation Task Force (CTF) multivariate analysis of 249 cases. European Journal of Gynaecological Oncology 2002;23(4):295‐9. [PubMed] [Google Scholar]
Galaal 2009
- Galaal K, Kew FM, Tam KF, Lopes AD, Meirovitz M, Naik R, et al. Evaluation of prognostic factors and treatment outcomes in uterine carcinosarcoma. European Journal of Obstetrics, Gynecology and Reproductive Bbiology 2009;143(2):88‐92. [DOI] [PubMed] [Google Scholar]
Harlow 1986
- Harlow BL, Weiss NS, Lofton S. The epidemiology of sarcomas of the uterus. Journal of the National Cancer Institute 1986;76(3):339‐402. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. The Cochrane Collaboration 2011. Available from www.cochrane‐handbook.org, [updated March 2011]. [Google Scholar]
Le 2001
- Le T. Adjuvant pelvic radiotherapy for uterine carcinosarcoma in a high risk population. European Journal of Surgical Oncology 2001;27(3):282‐5. [DOI] [PubMed] [Google Scholar]
Major 1993
- Major FJ, Blessing JA, Silverberg SG, Morrow CP, Creasman WT, Currie JL, et al. Prognostic factors in early‐stage uterine sarcoma. A Gynaecologic Oncology Group study. Cancer 1993;7(Suppl 4):1702‐9. [DOI] [PubMed] [Google Scholar]
McCluggage 2002
- McCluggage WG. Uterine carcinosarcomas (malignant mixed mullerian tumours) are metaplastic carcinomas. International Journal of Gynecological Cancer 2002;12(6):687‐90. [DOI] [PubMed] [Google Scholar]
Menczer 2005
- Menczer J, Levy T, Piura B, Chetrit A, Altaras M, Meirovitz M, et al. A comparison between different postoperative treatment modalities of uterine carcinosarcoma. Gynecologic Oncology 2005;97(1):166‐70. [DOI] [PubMed] [Google Scholar]
Olah 1992
- Olah KS, Dunn JA, Gee H. Leiomyosarcomas have a poorer prognosis than mixed mesodermal tumours when adjusting for known prognostic factors: results of a retrospective study of 423 cases of uterine sarcoma. British Journal of Obstetrics and Gynaecology 1992;99:590‐4. [DOI] [PubMed] [Google Scholar]
Omura 1983
- Omura GA, Blessing JA, Major F, Silverberg S. A. randomised trial of adriamycin versus no adjuvant chemotherapy in stage I and stage II uterine sarcomas. Proceedings of the American Society of Clinical Oncology. 1983; Vol. C‐580:149.
Parmer 1998
- ParmarMK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Sartori 1997
- Sartori E, Bazzurini L, Gadducci A, Landoni F, Lissoni A, Maggino T, et al. Carcinosarcoma of the uterus: a clinicopathological multicenter CTF study. Gynecologic Oncology 1997;67(1):70‐5. [DOI] [PubMed] [Google Scholar]
Spanos 1986
- Spanos WJ Jr, Peters LJ, Oswald MJ. Patterns of recurrence in malignant mixed mullerian tumor of the uterus. Cancer 1986;57(1):155‐9. [DOI] [PubMed] [Google Scholar]
Szukala 1999
- Szukala SA, Marks JR, Burchette JL, Elbendary AA, Krigman HR. Co‐expression of p53 by epithelial and stromal elements in carcinosarcomaof the female genital tract: an immunohistochemical study of19 cases. International Journal of Gynecological Cancer 1999;9(2):131‐6. [DOI] [PubMed] [Google Scholar]
Thigpen 2004
- Thigpen JT, Blessing JA, DeGeest K, Look KY, Homesley HD. Cisplatin as initial chemotherapy in ovarian carcinosarcomas: A Gynecologic Oncology Group study. Gynecologic Oncology 2004;93(2):336‐9. [DOI] [PubMed] [Google Scholar]
Van Rijswijk 1994
- Rijswijk REN, Tognon G, Burger CW, Baak JP, Kenemans P, Vermorken JB. The effect of chemotherapy on the different components of advanced carcinosarcomas (malignant mixed mesodermal tumors) of the female genital tract. International Journal of Gynecological Cancer 1994;4(1):52‐60. [DOI] [PubMed] [Google Scholar]
Young 1981
- Young JL, Percy CL, Asire AJ, Berg JW, Cusano MM, Gloeckler LA, et al. Cancer incidence and mortality in the United States, 1973‐77. National Cancer Institute Monograph 1981;57:1‐187. [PubMed] [Google Scholar]
Zelmanowicz 1998
- Zelmanowicz A, Hildesheim A, Sherman ME, Sturgeon SR, Kurman RJ, Barrett RJ, et al. Evidence for a common etiology for endometrial carcinomas and malignant mixed mullerian tumors. Gynecologic Oncology 1998;69( 3):253‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Galaal 2011
- Galaal K, Godfrey K, Naik R, Kucukmetin A, Bryant A. Adjuvant radiotherapy and/or chemotherapy after surgery for uterine carcinosarcoma. Cochrane Database of Systematic Reviews 2011, Issue 1. [DOI: 10.1002/14651858.CD006812.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


