Abstract
Background
Peripheral arterial disease (PAD) of the lower limb is common, with prevalence of both symptomatic and asymptomatic disease estimated at 13% in the over 50 age group. Symptomatic PAD affects about 5% of individuals in Western populations between the ages of 55 and 74 years. The most common initial symptom of PAD is muscle pain on exercise that is relieved by rest and is attributed to reduced lower limb blood flow due to atherosclerotic disease (intermittent claudication). The ankle brachial index (ABI) is widely used by a variety of healthcare professionals, including specialist nurses, physicians, surgeons and podiatrists working in primary and secondary care settings, to assess signs and symptoms of PAD. As the ABI test is non‐invasive and inexpensive and is in widespread clinical use, a systematic review of its diagnostic accuracy in people presenting with leg pain suggestive of PAD is highly relevant to routine clinical practice.
Objectives
To estimate the diagnostic accuracy of the ankle brachial index (ABI) ‐ also known as the ankle brachial pressure index (ABPI) ‐ for the diagnosis of peripheral arterial disease in people who experience leg pain on walking that is alleviated by rest.
Search methods
We carried out searches of the following databases in August 2013: MEDLINE (Ovid SP),Embase (Ovid SP), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO), Latin American and Caribbean Health Sciences (LILACS) (Bireme), Database of Abstracts of Reviews of Effects and the Health Technology Assessment Database in The Cochrane Library, the Institute for Scientific Information (ISI) Conference Proceedings Citation Index ‐ Science, the British Library Zetoc Conference search and Medion.
Selection criteria
We included cross‐sectional studies of ABI in which duplex ultrasonography or angiography was used as the reference standard. We also included cross‐sectional or diagnostic test accuracy (DTA) cohort studies consisting of both prospective and retrospective studies.
Participants were adults presenting with leg pain on walking that was relieved by rest, who were tested in primary care settings or secondary care settings (hospital outpatients only) and who did not have signs or symptoms of critical limb ischaemia (rest pain, ischaemic ulcers or gangrene).
The index test was ABI, also called the ankle brachial pressure index (ABPI) or the Ankle Arm Index (AAI), which was performed with a hand‐held doppler or oscillometry device to detect ankle vessels. We included data collected via sphygmomanometers (both manual and aneroid) and digital equipment.
Data collection and analysis
Two review authors independently replicated data extraction by using a standard form, which included an assessment of study quality, and resolved disagreements by discussion. Two review authors extracted participant‐level data when available to populate 2×2 contingency tables (true positives, true negatives, false positives and false negatives).
After a pilot phase involving two review authors working independently, we used the methodological quality assessment tool the Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2), which incorporated our review question ‐ along with a flow diagram to aid reviewers' understanding of the conduct of the study when necessary and an assessment of risk of bias and applicability judgements.
Main results
We screened 17,055 records identified through searches of databases. We obtained 746 full‐text articles and assessed them for relevance. We scrutinised 49 studies to establish their eligibility for inclusion in the review and excluded 48, primarily because participants were not patients presenting solely with exertional leg pain, investigators used no reference standard or investigators used neither angiography nor duplex ultrasonography as the reference standard. We excluded most studies for more than one reason.
Only one study met the eligibility criteria and provided limb‐level accuracy data from just 85 participants (158 legs). This prospective study compared the manual doppler method of obtaining an ABI (performed by untrained personnel) with the automated oscillometric method. Limb‐level data, as reported by the study, indicated that the accuracy of the ABI in detecting significant arterial disease on angiography is superior when stenosis is present in the femoropopliteal vessels, with sensitivity of 97% (95% confidence interval (CI) 93% to 99%) and specificity of 89% (95% CI 67% to 95%) for oscillometric ABI, and sensitivity of 95% (95% CI 89% to 97%) and specificity of 56% (95% CI 33% to 70%) for doppler ABI. The ABI threshold was not reported. Investigators attributed the lower specificity for doppler to the fact that a tibial or dorsalis pedis pulse could not be detected by doppler in 12 of 27 legs with normal vessels or non‐significant lesions. The superiority of the oscillometric (automated) method for obtaining an ABI reading over the manual method with a doppler probe used by inexperienced operators may be a clinically important finding.
Authors' conclusions
Evidence about the accuracy of the ankle brachial index for the diagnosis of PAD in people with leg pain on exercise that is alleviated by rest is sparse. The single study included in our review provided only limb‐level data from a few participants. Well‐designed cross‐sectional studies are required to evaluate the accuracy of ABI in patients presenting with early symptoms of peripheral arterial disease in all healthcare settings. Another systematic review of existing studies assessing the use of ABI in alternative patient groups, including asymptomatic, high‐risk patients, is required.
Plain language summary
Ankle brachial index for the diagnosis of lower limb peripheral arterial disease
Peripheral arterial disease (PAD) of the legs affects 13% of people over 50 years of age. Sometimes PAD is "silent" and people are unaware they have it, but PAD can cause pain in the legs, especially with walking, and this type of symptomatic PAD affects about 5% of people in the Western world between the ages of 55 and 74 years. In PAD, fatty deposits (atherosclerosis) and blood clots cause the arteries to narrow and block. This leads to poor blood flow to the muscles during exercise, causing the classical symptom of muscle pain during walking that goes away after rest (intermittent claudication). In severe cases of PAD, symptoms of rest pain, ulceration and gangrene may develop and, if untreated, can lead to lower limb amputation. People with PAD are also at higher risk for cardiovascular disease and stroke.
The ankle brachial index (ABI) is a test that is used to facilitate diagnosis of PAD. This test uses a device for measuring blood pressure with an inflatable cuff, and blood pressure measurements are taken at the upper arm and the ankle. The equipment can be manual or digital with automatic electronic calculation of blood pressure. The ABI is widely used for assessment of PAD by specialist nurses, physicians, surgeons and podiatrists working in hospitals. Dividing blood pressure recorded at the ankle by that recorded at the arm produces a ratio. Ratios of 0.90 to 1.30 are considered normal for adults, and ratios less than 0.8 indicate that PAD is present. Lower readings (< 0.7) suggest that the disease is severe and people might develop ulcers and gangrene. People with mild to moderate PAD can arrive at a diagnosis by several routes when using the ABI: during routine diabetic foot checks in general practice, in community health clinic or hospital settings, as a screening test for PAD in people who have no symptoms and during assessment of people presenting with exertional leg pain suggestive of PAD. Once a diagnosis of PAD is established, treatment will include prescribed secondary prevention therapy and lifestyle advice (exercise, smoking cessation, diet, weight), and for those with impaired quality of life, treatment may include supervised exercise therapy, or revascularisation, which commonly involves endovascular treatment rather than surgery.
In hospitals, other tests may be used to diagnose PAD. Duplex ultrasound (DUS) shows blood flow in the arteries and is non‐invasive, but only an experienced radiologist can achieve useful images. Hospital staff can use other tests to image the blood vessels, namely, computerised tomography angiography (CTA), magnetic resonance angiography (MRA) and catheter angiography.
The ABI test is non‐invasive and inexpensive and is widely used clinically; therefore, we have reviewed all available reports obtained from a wide search of databases of medical literature to estimate its accuracy in identifying PAD in people who experience pain on walking that goes away after rest. Two review authors independently assessed studies that met inclusion criteria of the review, including use of a cross‐sectional study design; enrolment of participants with pain on walking that got better with rest; and use of duplex ultrasonography or angiography to check that results of the ABI test were accurate. One study met our criteria and provided data from 85 participants (158 limbs). Investigators compared the manual doppler method of measuring ABI with the automated method. Researchers provided only data for legs as opposed to data for patients; we were therefore unable to recalculate the analysis at the whole‐participant level.
In conclusion, we found little evidence about the accuracy of the ankle brachial index for diagnosing PAD in people presenting with exertional leg pain. The study included in our review had some flaws, and well‐designed cross‐sectional studies are needed to measure the accuracy of the ABI for diagnosing PAD in patients with early symptoms.
Summary of findings
Summary of findings'. 'Summary of findings table.
| Accuracy of the ankle brachial index (ABI) in diagnosing symptomatic peripheral arterial disease (PAD) | ||||
| Population: | People with intermittent claudication | |||
| Setting | Primary and secondary care settings (hospital outpatients) | |||
| Index test | Ankle brachial index | |||
| Importance | The success of management strategies for PAD depends upon the quality of the diagnostic process, which involves careful assessment of the underlying pathology with diagnostic tests that possess a high level of accuracy, to allow detection and measurement of an arterial stenosis and its distribution in the blood vessels. | |||
| Reference standard | Duplex ultrasonography or angiography | |||
| Studies | Cross‐sectional or diagnostic cohort study | |||
| Test/subgroup | Sensitivity | Specificity | No. of participants (studies) | Quality (QUADAS‐2)a and comments |
| Cut‐off ABI ratio positivity Mild PAD: 0.7 to 0.9 Moderate PAD: 0.41 to 0.69 |
||||
|
Automated ABI: Manual ABI: |
97% (95% CI 93% to 99%) 95% (95% CI 89% to 97%) |
89% (95% CI 67% to 95%) 56% (95% CI 33% to 70%) |
85 (n = 158 legs) (1 study) | Unclear risk of bias: Vega 2011 may have included patients with severe PAD (stenosis > 50%); the threshold was not reported; time between conduct of the index test and use of the reference standard is not reported. One study, no pooled analysis, sensitivity and specificity data for limb level ‐ not for participant level, as reported by study authors |
aQUADAS‐2 is a tool used for assessment of the quality of diagnostic accuracy studies. This tool comprises four domains: patient selection, index test, reference standard and flow and timing. Each domain is assessed in terms of risk of bias; the first three domains are also assessed in terms of concerns regarding applicability.
ABI: ankle brachial index. PAD: peripheral arterial disease.
Background
Peripheral arterial disease (PAD) of the lower limbs is common, with prevalence of both symptomatic and asymptomatic disease estimated at 13% in the over 50 age group (Hirsch 2001). Symptomatic PAD affects about 5% of individuals in Western populations between the ages of 55 and 74 years (Khan 2007). The most common initial symptom of PAD is muscle pain on exercise that is relieved by rest and is attributed to reduced lower limb blood flow due to atherosclerotic disease (intermittent claudication; IC). Patients with more severe PAD may develop rest pain, ulceration and gangrene (critical limb ischaemia; CLI), which, if untreated, can lead to lower limb amputation (Hooi 2007; Twine 2009). The presence of PAD has been shown to be a marker of underlying cardiovascular disease.
A simple, non‐invasive test known as the ankle brachial index (ABI) ‐ or the ankle brachial pressure index (ABPI) ‐ can detect PAD. Healthcare providers can use the ABI to screen asymptomatic patients at increased risk of developing PAD, for example, people with diabetes, and to assess people presenting with leg pain suggestive of PAD. Clinicians can use a low ABI, even in the absence of symptoms, to identify people who are at increased risk of cardiac and cerebrovascular disease (Ankle Brachial Index Collaboration 2008; SIGN 2007).
Dividing the highest ankle pressure (obtained in the posterior tibial, dorsalis pedis and, when required, peroneal arteries) by the highest systolic arm pressure yields the ABI ratio. Classically, healthcare providers have used a doppler probe to detect signals within the arteries, but recently designed oscillometric and photophlethysmographic devices are now available. Current guidelines do not endorse the use of these newer devices but recommend the hand‐held doppler technique (NICE 2012). Ratios of 0.90 to 1.30 are normal for adults, ratios less than 0.9 are indicative of arterial stenosis and ratios less than 0.5 are associated with CLI (Bhasin 2007; MacLeod‐Roberts 1995; NICE 2012). Individuals with aorto‐iliac disease may have normal ABI at rest and low values after exercise. An 'exercise‐ABI' test can detect this and can be performed during secondary care.
A wide variety of healthcare professionals, including specialist nurses, physicians, surgeons and podiatrists working in primary and secondary care settings, frequently use the ABI to assess PAD. These providers normally check foot and leg pulses of people presenting with leg pain suggestive of PAD before performing an ABI to determine their presence or absence. Once PAD is diagnosed, first‐line management of the condition consists of cardiac risk factor management, which includes lifestyle advice, smoking cessation, statin and antiplatelet therapy, blood pressure control and screening for and treatment of diabetes (Bhasin 2007; Heald 2006). Supervised exercise programmes can lead to symptomatic improvement, and healthcare providers can perform arterial revascularisation, in the form of angioplasty or less commonly surgery, to treat those with incapacitating disease and significantly impaired quality of life (Cassar 2003; Chang 2011; de Backer 2012; Fokkenrood 2013; Lane 2014; NICE 2012; Rutherford 1997). Physicians may prescribe naftidrofuryl oxalate for patients in whom supervised exercise therapy has not been found to be effective and who do not wish to be referred for revascularisation (NICE 2012).
In secondary care, hospital staff may use a variety of non‐invasive imaging tests for patients with suspected PAD in whom revascularisation may be considered, including non‐invasive duplex ultrasonography, computerised tomography angiography (CTA) or magnetic resonance angiography (MRA). The National Institute for Health and Care Excellence suggests duplex ultrasonography as the first‐line approach for imaging PAD, and CTA or contrast‐enhanced MRA for those who need further imaging (NICE 2012).
The ABI test is non‐invasive and inexpensive and is in widespread clinical use; a systematic review of its diagnostic accuracy in people presenting with leg pain suggestive of PAD is highly relevant to routine clinical practice.
Target condition being diagnosed
Presence or absence of peripheral arterial disease of the lower limb.
Index test(s)
Healthcare providers use the ankle brachial index (ABI) to diagnose peripheral arterial disease (PAD), by dividing highest systolic pressure measured in the arteries at the ankle (dorsalis pedis and posterior tibial arteries, or peroneal if the others are non‐detectable) by highest systolic blood pressure at the arm (brachial artery).
Physicians can calculate an ankle brachial ratio in several ways. UK clinical guidelines recommend that the patient is rested in a supine position and that blood pressure is taken by using a sphygmomanometer with an appropriately sized cuff at the brachial artery and the posterior tibial, dorsalis pedis and, when possible, peroneal arteries. A doppler probe detects audible systolic pressure (Aboyans 2012; McDermott 2000; NICE 2012).
For each leg, the healthcare professional calculates the ABI by dividing the highest ankle pressure by the highest pressure reading taken from the arm (McDermott 2000). MacLeod‐Roberts 1995 presents a classification of ABI values.
In this review, we use the threshold of less than 0.90 to distinguish between positive (< 0.90) and negative (≥ 0.90) test results. Clinicians commonly use this threshold in clinical practice, and is cited in current guidelines (NICE 2012).
The position of the patient at the time blood pressure is taken is important: For each inch that the ankle is positioned below the heart, care providers have noted a 1 mmHg increase in systolic ankle blood pressure (MacLeod‐Roberts 1995).
False negatives commonly occur in people who have calcification of the ankle artery wall, which creates incompressibility and an artificially high reading. This may occur in some patients with diabetes (Bhasin 2007; MacLeod‐Roberts 1995).
Several automated blood pressure machines are available, and all are eligible for inclusion in the review.
Clinical pathway
Healthcare providers may follow several clinical pathways to diagnose mild to moderate PAD by using the ABI: They may measure the ABI in primary care to diagnose PAD in members of the general population who report symptoms of exertional leg pain. They sometimes use ABI in addition to routine diabetic foot checks in primary care, community health settings or hospital settings as a screening test for PAD in people who have no symptoms but are at high risk. Once a diagnosis of PAD is established, healthcare staff will prescribe secondary prevention therapy and will give lifestyle advice (exercise, smoking cessation, diet, weight); for those who have impaired quality of life, they may offer supervised exercise therapy, or revascularisation, which commonly involves endovascular treatment rather than surgery.
Role of index test(s)
Practitioners use the ABI test in healthcare settings to identify PAD in people who have suggestive symptoms, and can use this test to screen those at increased risk for PAD. An ABI < 0.90 is predictive of increased risk of cardiovascular disease (Ankle Brachial Index Collaboration 2008). This review aimed to include studies evaluating the diagnostic test accuracy of the ABI used in primary and secondary (outpatient only) care settings by a range of healthcare professionals, to evaluate people presenting with leg pain on exercise that is relieved by rest, which is suggestive of underlying PAD.
Alternative test(s)
Uses of the ABI in clinical practice are diverse, and care providers do not need to consider standard alternative tests.
Rationale
The success of management strategies for PAD depends upon the quality of the diagnostic process, which involves careful assessment of underlying pathology through diagnostic tests that possess a high level of accuracy.
Objectives
To estimate the diagnostic accuracy of the ankle brachial index (ABI) ‐ also known as the ankle brachial pressure index (ABPI) ‐ for the diagnosis of peripheral arterial disease in people who experience leg pain on walking that is alleviated by rest.
Secondary objectives
We also intended to investigate the effect of sources of heterogeneity on diagnostic accuracy, specifically, study setting, previous tests, types of equipment used, types of reference standards applied, different groups of patients examined (people with type 1 or type 2 diabetes and suspected aorto‐iliac disease) and duration of symptoms, by including them as co‐variates in the meta‐analysis, if sufficient studies provided relevant data. It was our intention that we would examine graphically other potential sources of heterogeneity for signs that they were a cause of heterogeneity.
Methods
Criteria for considering studies for this review
Types of studies
We included studies of ABI that used duplex ultrasonography or angiography as the reference standard. We included cross‐sectional or diagnostic test accuracy (DTA) cohort studies examining both prospective and retrospective studies. These studies had to report that all participants received a reference standard; investigators had to present cross‐tabulated results of the index test and the reference standard (2×2 table), or had to report sufficient information to allow the 2×2 table data to be back‐calculated.
Participants
Adults with leg pain on walking relieved by rest, who are tested in primary care settings or secondary care settings (hospital outpatients only) and do not have signs or symptoms of critical limb ischaemia (rest pain, ischaemic ulcers or gangrene). We excluded from the review patients who were free of exertional leg pain, as well as those with CLI.
Index tests
Ankle brachial index (ABI), also called ankle brachial pressure index (ABPI). We included data collected by sphygmomanometers (both manual and aneroid) as well as by digital equipment that used manual or automatic inflation. We included studies that used hand‐held doppler or oscillometry to detect ankle vessels.
Target conditions
Peripheral arterial disease of the lower limbs.
Reference standards
We included studies that used duplex ultrasonography or angiography as the reference standard test, and we noted instances in which different reference standards were used to verify the presence or absence of disease in the same study population.
Search methods for identification of studies
We applied no restrictions in terms of date, language of publication or publication status of studies. We used no diagnostic method search filters.
Electronic searches
We applied no restrictions in terms of language of publication or publication status.
We searched the following databases.
MEDLINE Ovid (1946 to July week 5 2013).
Embase (Ovid SP) (1980 to 2013 week 32).
Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCO (12 August 2013).
Latin American and Caribbean Health Sciences (LILACS) (Bireme) (13 August 2013).
Database of Abstracts of Reviews of Effects (DARE) and the Health Technology Assessment Database (HTA), in The Cochrane Library (2013, Issue 7).
Institute for Scientific Information (ISI) Conference Proceedings Citation Index ‐ Science (14 August 2013).
British Library Zetoc Conference search (29 August 2013).
We used the search strategies shown in Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6 and Appendix 7.
We also searched MEDION (www.mediondatabase.nl/) using the 'Systematic Reviews of Diagnostic Studies' search filter (29 August 2013) (Appendix 8).
Searching other resources
We reviewed the bibliographies of review articles identified by searches for potentially relevant studies.
Data collection and analysis
Selection of studies
One review author (FC) screened titles and abstracts retrieved by the electronic searches, and a second review author (AA) checked a random sample of 10% of the studies. We obtained full papers for potentially eligible studies, including those identified by non‐electronic means. Two review authors (FC, AA) independently applied exclusion criteria to the full papers and resolved disagreements by discussion. We used a flow diagram to show results of the decision‐making process.
Data extraction and management
Two review authors (FC, AA) independently used a standard form to replicate data extraction; this form included an assessment of study quality. Review authors corroborated their data extraction and quality assessment decisions and resolved disagreements by discussion. Review authors intended to extract participant‐level data to populate 2×2 contingency tables ‐ true positives (TPs), true negatives (TNs), false positives (FPs) and false negatives (FNs)) ‐ as reported. We also extracted details of test threshold(s) used for interpretation of results.
We collected data on mortality, adverse events, the nature of the equipment used (manual or automated) and the number of technical failures.
Assessment of methodological quality
After a pilot phase involving two review authors working independently, we used the Quality Assessment of Diagnostic Accuracy Studies‐2 (QUADAS‐2) (Whiting 2011), which incorporated our review question, a flow diagram to aid reviewers' understanding of the conduct of the study when necessary and an assessment of risk of bias and applicability judgements. We presented review‐specific signalling questions and appropriate items concerning the applicability of primary studies relative to the review, together with guidance about ratings, in Appendix 9. We resolved disagreements by discussion.
Statistical analysis and data synthesis
We intended to use 2×2 contingency tables populated with participant‐level data, rather than data on limbs, to estimate sensitivity and specificity for each study. When data were adequate, we intended to perform a bivariate random‐effects meta‐analysis of sensitivity and specificity. We anticipated that we would use these estimates to create receiver operating characteristic (ROC) and forest plots. As an ABI less than 0.90 is the accepted threshold used in clinical practice, we had planned to restrict the meta‐analysis to studies that used this threshold, so that our estimates of sensitivity and specificity would be derived directly from that threshold. We intended to add items investigated for heterogeneity as co‐variates to the bivariate model.
However, available data are based on limbs as the unit of analysis. If two datapoints were obtained from the same participant (one from each leg), these datapoints will tend to be more similar to each other than datapoints from different patients, thus changing the variance in data. However, estimating within‐study variance is a key part of meta‐analysis, and current methods do not allow for studies in which a participant may contribute data from more than one potential disease site. If studies do not provide participant‐level data, we have no correct way to estimate within‐study variance, and so meta‐analysis, whether bivariate or based on hierarchical summary receiver operating characteristic (HSROC) models (Harbord 2007), or univariate meta‐analysis for sensitivity and specificity, is not an option.
We intended to perform all analyses in R 7.1 (cran.r‐project.org) and SAS 9.3 (www.sas.com).
Investigations of heterogeneity
Our planned investigations into the effect of sources of heterogeneity on diagnostic accuracy focussed on patient groups (e.g. type 1 and type 2 diabetes, suspected aorto‐iliac disease), duration of symptoms, previous tests and types of equipment (automatic or manual) by including them as co‐variates in the meta‐analyses. We intended to examine other potential sources of heterogeneity graphically for signs that they were the source of heterogeneity. We planned to group estimates in plots according to items considered potential sources of heterogeneity, as detailed above, and to present these as forest and ROC plots for visual assessment of heterogeneity.
If we found sufficient studies, we planned to investigate heterogeneity by adding items as co‐variates to the meta‐analysis model, from a bivariate or univariate analysis, depending on results of the main analysis. However, we recognised the likelihood of having too few studies to perform meta‐regression with all items listed as potential sources of heterogeneity, and under these circumstances, we planned to limit ourselves to visual inspection of ROC and forest plots. We understand that some items are investigated better with individual participant data, as they are patient‐specific, rather than study‐specific, for example, duration of symptoms, and we planned to interpret any aggregate results cautiously.
Sensitivity analyses
We intended to conduct several sensitivity analyses to compare the diagnostic accuracy of ABI in those with and without diabetes, in those with and without coronary heart disease, in smokers versus non smokers and when manual versus automated methods are used to measure the ABI.
Assessment of reporting bias
Methods for dealing with publication bias in reviews of diagnostic accuracy studies are relatively underdeveloped. Consequently, we interpreted our results cautiously, and with awareness of the likelihood of publication bias, rather than by using funnel plots, which can be challenging to interpret in this context. We planned to consider using a funnel plot of the log of the diagnostic odds ratio (lnDOR), provided we found low heterogeneity in the lnDOR (Deeks 2005).
Results
Results of the search
See Figure 1.
1.
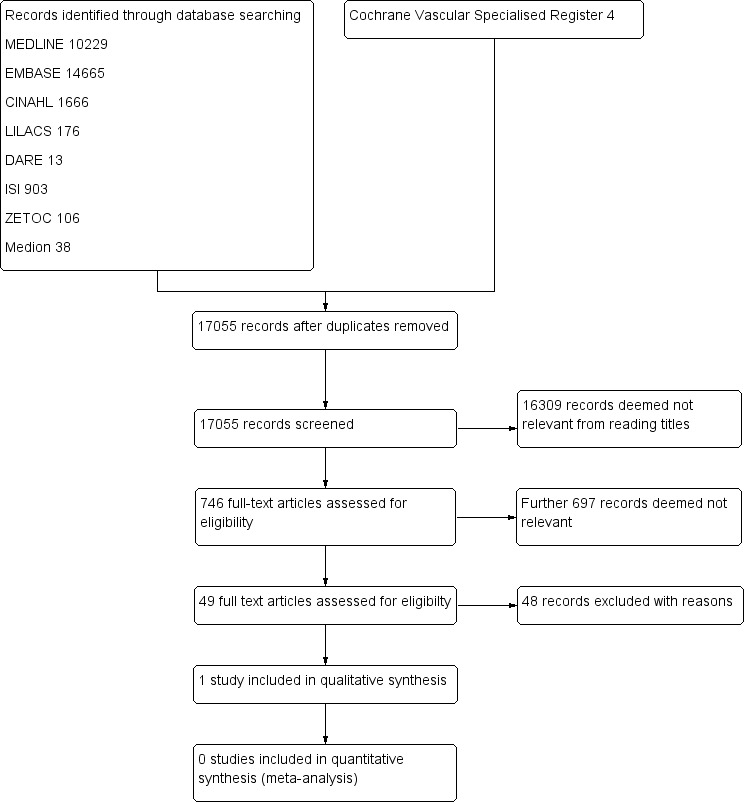
Study flow diagram.
We screened 17,055 records identified through searches of databases. We obtained and assessed for relevance 746 full‐text articles. A second review author (AA) checked a 10% random sample of these articles and reached 100% agreement with the first review author (FC). We scrutinised 49 studies to establish their eligibility for inclusion in the review.
We included only one study (Vega 2011) with a total of 85 participants and have described it in the Characteristics of included studies table.
We have listed the 48 studies excluded from the review, along with reasons for exclusion, in the Characteristics of excluded studies table. We excluded studies primarily because participants were not patients presenting solely with exertional leg pain, investigators used no reference standard or the reference standard used was neither angiography nor duplex ultrasonography. We have provided more than one reason for exclusion of most studies.
Methodological quality of included studies
2.
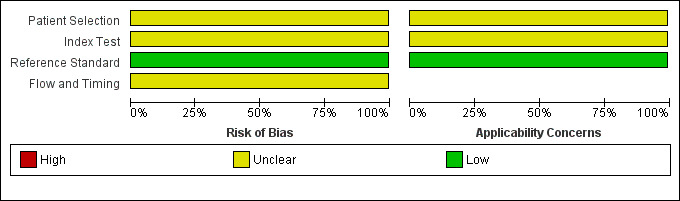
Risk of bias and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies.
3.
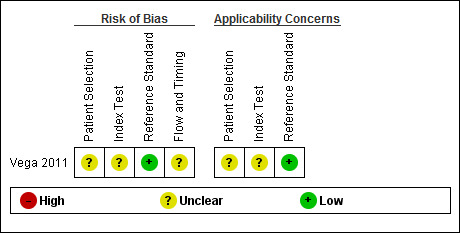
Risk of bias and applicability concerns summary: review authors' judgements about each domain for each included study.
The Characteristics of included studies table incorporates the methodological quality assessment. In Vega 2011, the risk of bias was generally 'low', but some items had 'unclear' risk of bias; we explain these below. QUADAS‐2 quality assessment items are grouped into four domains: patient selection, index test, reference standard and flow and timing.
The risk of bias arising from patient selection was unclear (Vega 2011). Although a consecutive sample of patients was reported, inappropriate exclusions may not have been avoided. Investigators included patients who had 'suspected advanced PAD'; consequently, the patient population may have been affected by disease spectrum bias.
Investigators described the execution of ABI tests well but did not state the ABI threshold used (Vega 2011); this led to a classification of "unclear risk of bias".
Vega 2011 did not report the time between ABI and angiography assessments, which might have led to misclassification due to progression of the disease to a more advanced state (disease progression bias).
We have presented in the Characteristics of included studies table details of the execution of index and reference standard tests used by Vega 2011.
Findings
The one included study (Vega 2011) did not report accuracy data at the participant level; therefore, we were unable to calculate estimates of sensitivity or specificity for individual participants. The accuracy estimates reported in the narrative synthesis below are those calculated and reported by Vega 2011. See also Table 1.
This prospective study compared the manual doppler method of obtaining an ABI with the automated oscillometric method (Vega 2011). In total, researchers recruited into the study 85 patients (76 men and nine women) with a mean (standard deviation) age of 68 (11) years, who were referred for angiography with 'symptoms of intermittent claudication'. Study participants had several co‐morbidities, including diabetes (52%), hypertension (76%), hypercholesterolaemia (43.5%), ischaemic heart disease (30%), percutaneous coronary intervention (12%), coronary surgery (6%), previous stroke (22%), carotid revascularisation (2.4%) and aortic aneurysm (6%), and included current smokers (32%) and previous smokers (46%).
Doctors with no specialist training performed ABI measurements, and researchers conducted the study in a hospital catheterisation laboratory in Spain. Investigators performed manual doppler ABI by using an 8 MHz doppler probe (Dopplex II MD2/SD, ArjoHuntleigh Inc., Addison, Illinois) model and a sphygmomanometer with cuffs of appropriate size. They obtained automated ABI measurements by using automated oscillometric equipment: Omron M4‐1 (Omron Healthcare Europe BV, Hoofddorp, The Netherlands). The threshold for a positive test result was a significant lesion of > 50% occlusion detected by catheter angiography (the reference standard). Researchers defined non‐significant PAD as < 50% obstruction.
According to Vega 2011, the reported accuracy of automated oscillometric ABI was not statistically significantly different from that of the manual doppler method, with reported sensitivity of 97% (95% confidence interval (CI) 93% to 99%) and specificity of 89% (95% CI 67% to 95%) for oscillometric ABI, compared with sensitivity of 95% (95% CI 89% to 97%) and specificity of 56% (95% CI 33% to 70%) for the manual doppler ABI. The reason for the lower specificity for the doppler was that the doppler could not detect a tibial or dorsalis pedis pulse in 12 legs with normal vessels or non‐significant lesions, among a total of 27 legs. However, with the automated method, investigators could not measure blood pressure in 70 legs, 69 of which were found to have severe angiographic lesions. The superiority of the automated oscillometric method for obtaining an ABI reading over the manual method in which inexperienced operators used a doppler probe may be a clinically important finding.
Researchers reported accuracy estimates with 'limbs' as the unit of analysis, and as participant‐level data are not available, we were unable to reproduce accuracy estimates. The number of significant lesions (occlusions > 50%) detected by angiography in this study population was 131 (83%).
Discussion
The ankle brachial index (ABI) test is cheap and non‐invasive, which makes it potentially valuable in health care. Unfortunately, evidence for the accuracy of the ABI test for the detection of peripheral arterial disease (PAD) in people presenting with leg pain on exercise that is alleviated by rest is sparse. We included in this review only one study, which evaluated automated versus manual ABI equipment, and provided limb‐level data from a total of 85 participants.
The main findings of the review are the following: (1) Although both ABI tests demonstrated high levels of sensitivity, the results came from a single small study in which participants with critical limb ischaemia (CLI) may have been included, and researchers reported no threshold for the ABI; (2) investigators reported no statistically significant differences in accuracy between automated and manual ABI equipment in this small group of participants, although automated ABI was associated with a greater number of technical failures than the hand‐held doppler, and these technical failures occurred in participants with severe angiographic lesions; and (3) in light of recruitment of patients from those already referred for angiography, it seems likely that participants included in the review may have had worse PAD than those recruited directly from a primary healthcare setting, and we would not extrapolate these findings to all patients presenting in primary care.
More than half of the participants in this study had received a diagnosis of diabetes mellitus, but we were unable to produce evidence to support the advice given in clinical guidelines (NICE 2012) that the use of ABI in assessment of PAD among people with diabetes is less reliable than among those who do not have diabetes mellitus, because data were not available for such an analysis. The single included study (Vega 2011) excluded patients with an ABI > 1.4, but investigators excluded only two patients for this reason and did not reveal whether these patients had diabetes.
The review excluded 48 studies, most of which were cross‐sectional studies evaluating the accuracy of ABI or comparing different ABI techniques in the diagnosis of PAD. Unfortunately, these studies usually included patients other than those presenting with exertional leg pain, and many did not use the reference standard of duplex ultrasonography or angiography. These shortcomings are important findings of this review, and in the recommendations for research section below, we make specific suggestions to inform the design of future DTA studies of ABI for the diagnosis of PAD in people with exertional leg pain.
Summary of main results
This review found a very small amount of evidence indicating that the ABI test is accurate in the diagnosis of symptomatic PAD among people with intermittent claudication (IC). The one included study suggests that automated equipment may be more accurate than manual methods when used by individuals with no specialist training. The accuracy of manual doppler varies with operator skill, and so trained individuals may obtain more accurate results with this method. The small number of participants who took part in the study led to our cautious interpretation of the data.
Strengths and weaknesses of the review
We identified only one study for inclusion. We restricted the inclusion criteria for this review to patients with leg pain on walking relieved by rest and use of duplex ultrasonography or angiography as the reference standard; this contributed to the exclusion of a large number of studies.
Vega 2011 included some patients with 'suspected advanced PAD' and did not present data for these participants separately. This may have led to higher estimates of accuracy than would be observed in a population that was strictly recruited on the basis of leg pain alone. In addition, researchers did not report the ABI threshold.
Vega 2011 reported accuracy data at limb level only; therefore we were unable to calculate estimates of sensitivity and specificity for individual participants. We attempted to contact the study authors to obtain participant‐level data, but we received no response.
Applicability of findings to the review question
The patient population recruited to the included study suggests that the findings may not answer the review question. Investigators recruited the study population from patients referred for angiography for peripheral arterial intermittent claudication or suspected advanced PAD (Vega 2011). The percentage of people with symptoms of IC and the percentage with more advanced PAD remain unclear, but it is likely that researchers included in this population people with critical limb ischaemia. Use of angiography as the reference standard by which to verify results of the ABI (index) test may mean that the spectrum of disease is worse than in the general population seeking a diagnosis for PAD, as angiography is an invasive test conducted in hospital vascular departments. Authors of the included study themselves cautioned that their findings should not be extrapolated to the general population because of the high prevalence of PAD reported among study participants (Vega 2011).
Authors of another systematic review evaluating the accuracy of ABI for PAD suggest that accuracy is dependent on the purpose of the examination; they found ABI to be highly accurate when used to detect serious stenosis (> 50%) (Dachun 2013). The American Heart Association (AHA) Scientific Statement on measurement and interpretation of the ABI reports that areas under the receiver operating characteristic (ROC) curve are higher for ABI measured by doppler than for ABI measured by oscillometric methods (Aboyans 2012). This narrative review provides evidence on the overall diagnostic ability of the ABI in a variety of populations and settings based on four studies that did not meet the eligibility criteria for the current review (Aboyans 2012).
Authors' conclusions
Implications for practice.
This review found little evidence on the value of the ankle brachial index (ABI) for detection of lower limb peripheral arterial disease (PAD) in patients with exertional leg pain. The paucity of studies assessing the accuracy of ABI in patients with leg pain and the small number of participants enrolled in only one included study mean that robust conclusions cannot be reached.
It is often written that the ABI is not a useful test for detecting PAD in those with diabetes (Bhasin 2007; MacLeod‐Roberts 1995) because incompressibility of calcified vessels produces false results. In the included study, the co‐morbidities of participants who were withdrawn, or in whom ABI measurement was not possible, are not reported, and uncertainty exists about the influence that any underlying disease may have on the accuracy of ABI in the diagnosis of PAD.
Implications for research.
Well‐designed primary studies are needed to evaluate the diagnostic accuracy of ABI in patients presenting specifically with exertional leg pain in both primary and secondary (outpatient) healthcare settings. Further systematic review of existing studies assessing the use of ABI in alternative patient groups, including high‐risk patients without leg pain and patients with atypical leg pain, is required. Additional primary studies will likely be required in these populations, including patients not previously diagnosed with PAD and asymptomatic patients with co‐morbidities such as diabetes mellitus.
We suggest that duplex ultrasonography is a more appropriate reference standard for a population of patients who present for assessment for the first time, as it is non‐invasive in nature and may be available outside the hospital setting. We recommend that this single reference standard should be used to validate the presence or absence of disease in the ABI test result for each individual patient.
A comparison of the accuracy between the manual doppler probe ABI and automated oscillometric ABI measurements has cost implications, deserves further consideration, and should include patients with co‐morbidities such as diabetes mellitus and should be performed by trained healthcare professionals.
Moreover, study authors must be careful about how they analyse their data, in particular, they need to account for participants contributing data from both legs and must provide participant‐level data to facilitate meta‐analyses in updates of this, and other, systematic reviews.
Acknowledgements
This review forms part of a National Institute of Health Research (NIHR) Cochrane programme grant (10/4001/14). Thanks to Ms Carole Marshall, programme secretary, for her help in obtaining the 746 reports of studies that we considered for inclusion in this review.
The review authors thank Professor Julie Brittenden, Professor of Vascular Surgery at the University of Glasgow, who provided content expertise during all stages of preparation of the review.
Appendices
Appendix 1. MEDLINE search strategy
Database: Ovid MEDLINE(R) <1946 to July Week 5 2013>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 Ankle Brachial Index/ (1231)
2 Oscillometry/ [Methods] (1841)
3 Blood Pressure Determination/ (16515)
4 Laser‐Doppler Flowmetry/ (7317)
5 oscillometr$.ti,ab. (1745)
6 (doppler adj2 (ultrasound or flow$ or method or device)).ti,ab. (24957)
7 ABI.ti,ab. (4022)
8 ABPI.ti,ab. (309)
9 AAI.ti,ab. (1016)
10 (ankle adj4 index).ti,ab. (3997)
11 (arm adj4 index).ti,ab. (877)
12 (brachial adj4 (index or pressure)).ti,ab. (4836)
13 (systolic adj5 ratio).ti,ab. (2833)
14 (pressure adj5 ratio).ti,ab. (5088)
15 (BP adj5 ratio).ti,ab. (618)
16 (four and limbs and pressure).ti,ab. (309)
17 or/1‐16 (63464)
18 (anterior tibial or dorsalis pedis or posterior tibial).ti,ab. (6189)
19 (ankle or arm or elbow or calf).ti,ab. (172149)
20 (lower and upper and (extremit$ or limb)).ti,ab. (9791)
21 or/18‐20 (185143)
22 (systolic or pressure).ti,ab. (588700)
23 21 and 22 (11891)
24 17 or 23 (71623)
25 exp Peripheral Vascular Diseases/di [Diagnosis] (8148)
26 Arterial Occlusive Diseases/di [Diagnosis] (4178)
27 exp Arteriosclerosis/di [Diagnosis] (13998)
28 exp Atherosclerosis/di [Diagnosis] (2178)
29 exp Peripheral Arterial Disease/di [Diagnosis] (498)
30 Intermittent Claudication/di [Diagnosis] (994)
31 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. (130870)
32 (arter$ adj4 ($occlus$ or steno$ or obstruct$ or lesio$ or block$ or obliter$)).ti,ab. (76067)
33 (vascular adj4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. (20139)
34 (vein* adj4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. (8880)
35 (veno* adj4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. (9793)
36 (peripher* adj4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. (12782)
37 (peripheral adj3 dis*).ti,ab. (28626)
38 arteriopathic.ti,ab. (152)
39 (claudic* or hinken* or IC).ti,ab. (53144)
40 CLI.ti,ab. (1175)
41 dysvascular*.ti,ab. (144)
42 (leg adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. (484)
43 (limb adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. (1330)
44 (lower adj3 extrem* adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. (1345)
45 or/25‐44 (320767)
46 24 and 45 (10229)
Appendix 2. Embase search strategy
Database: Embase <1980 to 2013 Week 32>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 ankle brachial index/ (4434)
2 oscillometry/ (6310)
3 blood pressure measurement/ (34214)
4 laser Doppler flowmetry/ (8539)
5 oscillometr$.ti,ab. (2398)
6 (doppler adj2 (ultrasound or flow$ or method or device)).ti,ab. (31994)
7 ABI.ti,ab. (6940)
8 ABPI.ti,ab. (455)
9 AAI.ti,ab. (1452)
10 (ankle adj4 index).ti,ab. (5541)
11 (arm adj4 index).ti,ab. (1137)
12 (brachial adj4 (index or pressure)).ti,ab. (6690)
13 (systolic adj5 ratio).ti,ab. (3654)
14 (pressure adj5 ratio).ti,ab. (6524)
15 (BP adj5 ratio).ti,ab. (895)
16 (four and limbs and pressure).ti,ab. (389)
17 or/1‐16 (98837)
18 (anterior tibial or dorsalis pedis or posterior tibial).ti,ab. (7231)
19 (ankle or arm or elbow or calf).ti,ab. (210874)
20 (lower and upper and (extremit$ or limb)).ti,ab. (14215)
21 or/18‐20 (228282)
22 (systolic or pressure).ti,ab. (736050)
23 21 and 22 (15587)
24 17 or 23 (109336)
25 peripheral vascular disease/di [Diagnosis] (2054)
26 artery disease/di [Diagnosis] (1691)
27 arteriolosclerosis/di [Diagnosis] (32)
28 arteriosclerosis/di [Diagnosis] (2186)
29 atherosclerosis/di [Diagnosis] (5485)
30 atherosclerotic plaque/di [Diagnosis] (2755)
31 peripheral occlusive artery disease/di [Diagnosis] (3610)
32 artery occlusion/di [Diagnosis] (2700)
33 intermittent claudication/di [Diagnosis] (870)
34 (atherosclero* or arteriosclero* or PVD or PAOD or PAD).ti,ab. (168115)
35 (arter$ adj4 ($occlus$ or steno$ or obstruct$ or lesio$ or block$ or obliter$)).ti,ab. (96829)
36 (vascular adj4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. (25100)
37 (vein* adj4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. (11516)
38 (veno* adj4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. (11947)
39 (peripher* adj4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)).ti,ab. (16599)
40 (peripheral adj3 dis*).ti,ab. (37189)
41 arteriopathic.ti,ab. (183)
42 (claudic* or hinken* or IC).ti,ab. (43148)
43 CLI.ti,ab. (1795)
44 dysvascular*.ti,ab. (165)
45 (leg adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. (585)
46 (limb adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. (1808)
47 (lower adj3 extrem* adj4 (obstruct* or occlus* or steno* or block* or obliter*)).ti,ab. (1656)
48 or/25‐47 (372990)
49 24 and 48 (14665)
Appendix 3. CINAHL search strategy
| S40 | S22 AND S39 | 1,666 |
| S39 | S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 OR S39 | 44,201 |
| S38 | TI ( (limb N4 (obstruct* or occlus* or steno* or block* or obliter*)) ) OR AB ( (limb N4 (obstruct* or occlus* or steno* or block* or obliter*)) ) | 159 |
| S37 | TI ( (leg N4 (obstruct* or occlus* or steno* or block* or obliter*)) ) OR AB ( (leg N4 (obstruct* or occlus* or steno* or block* or obliter*)) ) | 70 |
| S36 | TI dysvascular* OR AB dysvascular | 75 |
| S35 | TI CLI OR AB CLI | 123 |
| S34 | TI ( claudic* or hinken* or IC ) OR AB ( claudic* or hinken* or IC ) | 3,907 |
| S33 | TI arteriopathic OR AB arteriopathic | 9 |
| S32 | TI peripheral N3 dis* OR AB peripheral N3 dis* | 4,086 |
| S31 | TI ( (peripher* N4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)) ) OR AB ( (peripher* N4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)) ) | 1,159 |
| S30 | TI ( (veno* N4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)) ) OR AB ( (veno* N4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)) ) | 656 |
| S29 | TI ( (vein* N4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)) ) OR AB ( (vein* N4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)) ) | 696 |
| S28 | TI ( (vascular N4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)) ) OR AB ( (vascular N4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)) ) | 1,251 |
| S27 | TI ( (arter* N4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)) ) OR AB ( (arter* N4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)) ) | 6,063 |
| S26 | TI ( atherosclero* or arteriosclero* or PVD or PAOD or PAD ) OR AB ( atherosclero* or arteriosclero* or PVD or PAOD or PAD ) | 11,796 |
| S25 | (MH "Atherosclerosis") OR (MH "Intermittent Claudication/DI") | 3,829 |
| S24 | (MH "Arterial Occlusive Diseases+") OR (MH "Arteriosclerosis+/DI") | 24,816 |
| S23 | (MH "Peripheral Vascular Diseases+/DI") | 1,760 |
| S22 | S15 OR S21 | 12,571 |
| S21 | S19 AND S20 | 2,069 |
| S20 | TI ( systolic or pressure ) OR AB ( systolic or pressure ) | 69,646 |
| S19 | S16 OR S17 OR S18 | 31,950 |
| S18 | TI ( (lower and upper and (extremit* or limb)) ) OR AB ( (lower and upper and (extremit* or limb)) ) | 2,062 |
| S17 | TI ( ankle or arm or elbow or calf ) OR AB ( ankle or arm or elbow or calf ) | 28,839 |
| S16 | TI ( anterior tibial or dorsalis pedis or posterior tibial ) OR AB ( anterior tibial or dorsalis pedis or posterior tibial ) | 1,839 |
| S15 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 | 11,160 |
| S14 | TI ( four and limbs and pressure ) OR AB ( four and limbs and pressure ) | 23 |
| S13 | TI BP N5 ratio OR AB BP N5 ratio | 105 |
| S12 | TI pressure N5 ratio OR AB pressure N5 ratio | 653 |
| S11 | TI systolic N5 ratio OR AB systolic N5 ratio | 340 |
| S10 | TI ( (brachial N4 (index or pressure)) ) OR AB ( (brachial N4 (index or pressure)) ) | 953 |
| S9 | TI arm N4 index OR AB arm N4 index | 210 |
| S8 | TI ankle N4 index OR AB ankle N4 index | 871 |
| S7 | TI AAI OR AB AAI | 236 |
| S6 | TI ABPI OR AB ABPI | 83 |
| S5 | TI ABI OR AB ABI | 743 |
| S4 | TI ( (doppler N2 (ultrasound or flow* or method or device)) ) OR AB ( (doppler N2 (ultrasound or flow* or method or device)) ) | 2,041 |
| S3 | TI oscillometr* OR AB oscillometr* | 337 |
| S2 | MH Blood Pressure Determination | 5,576 |
| S1 | MH Ankle Brachial Index | 1,194 |
Appendix 4. LILACS search strategy
| Database : |
LILACS 13 August 2013 http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/ |
| Search on : | ((ankle OR arm or brachial) AND index) OR ((ultrasound OR flow$ OR method OR device) AND doppler) OR abi OR abpi OR aai [Words] and atherosclero$ OR arteriosclero$ OR pvd OR paod OR pad OR cli OR ((occlus$ OR steno$ OR obstruct$ OR lesion$ OR block$ OR obliter$) and leg or limb) OR claudic$ [Words] or (ankle brachial index or Oscillometry or Blood Pressure Determination or Laser‐Doppler Flowmetry) AND (Atherosclerosis OR (Peripheral Arterial Disease)) [Subject descriptor] |
| Total of references : | 176 |
Appendix 5. DARE (Database of Abstracts of Reviews of Effects) and the Health Technology Assessment Database (HTA) in The Cochrane Library
| #1 | MeSH descriptor: [Arteriosclerosis] explode all trees and with qualifiers: [Diagnosis ‐ DI] | 408 |
| #2 | MeSH descriptor: [Arteriolosclerosis] explode all trees and with qualifiers: [Diagnosis ‐ DI] | 0 |
| #3 | MeSH descriptor: [Arteriosclerosis Obliterans] explode all trees and with qualifiers: [Diagnosis ‐ DI] | 3 |
| #4 | MeSH descriptor: [Atherosclerosis] explode all trees and with qualifiers: [Diagnosis ‐ DI] | 45 |
| #5 | MeSH descriptor: [Arterial Occlusive Diseases] explode all trees and with qualifiers: [Diagnosis ‐ DI] | 563 |
| #6 | MeSH descriptor: [Intermittent Claudication] explode all trees and with qualifiers: [Diagnosis ‐ DI] | 44 |
| #7 | MeSH descriptor: [Ischemia] explode all trees and with qualifiers: [Diagnosis ‐ DI] | 48 |
| #8 | MeSH descriptor: [Peripheral Vascular Diseases] explode all trees and with qualifiers: [Diagnosis ‐ DI] | 254 |
| #9 | MeSH descriptor: [Vascular Diseases] explode all trees and with qualifiers: [Diagnosis ‐ DI] | 4377 |
| #10 | MeSH descriptor: [Leg] explode all trees and with qualifiers: [Blood supply ‐ BS] | 1090 |
| #11 | MeSH descriptor: [Femoral Artery] explode all trees | 736 |
| #12 | MeSH descriptor: [Popliteal Artery] explode all trees | 259 |
| #13 | MeSH descriptor: [Iliac Artery] explode all trees | 152 |
| #14 | MeSH descriptor: [Tibial Arteries] explode all trees | 29 |
| #15 | (atherosclero* or arteriosclero* or PVD or PAOD or PAD) | 17613 |
| #16 | (arter*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) | 4961 |
| #17 | (vascular) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) | 1416 |
| #18 | (vein*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) | 747 |
| #19 | (veno*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) | 1004 |
| #20 | (peripher*) near (*occlus* or steno* or obstruct* or lesio* or block* or obliter*) | 1380 |
| #21 | peripheral near/3 dis* | 3353 |
| #22 | arteriopathic | 17 |
| #23 | (claudic* or hinken*) | 1469 |
| #24 | (isch* or CLI) | 17265 |
| #25 | dysvascular* | 26 |
| #26 | leg near/4 (obstruct* or occlus* or steno* or block* or obliter*) | 187 |
| #27 | limb near/4 (obstruct* or occlus* or steno* or block* or obliter*) | 241 |
| #28 | (lower near/3 extrem*) near/4 (obstruct* or occlus* or steno* or block* or obliter*) | 146 |
| #29 | #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 | 42501 |
| #30 | MeSH descriptor: [Ankle Brachial Index] explode all trees | 68 |
| #31 | MeSH descriptor: [Oscillometry] explode all trees and with qualifiers: [Methods ‐ MT] | 31 |
| #32 | MeSH descriptor: [Blood Pressure Determination] this term only and with qualifiers: [Methods ‐ MT] | 301 |
| #33 | MeSH descriptor: [Laser‐Doppler Flowmetry] this term only and with qualifiers: [Methods ‐ MT] | 63 |
| #34 | oscillometr*:ti,ab,kw (Word variations have been searched) | 304 |
| #35 | (doppler near/2 (ultrasound or flow* or method or device)):ti,ab,kw (Word variations have been searched) | 2211 |
| #36 | ABI or ABPI or AAI:ti,ab,kw (Word variations have been searched) | 321 |
| #37 | ankle near/4 index:ti,ab,kw (Word variations have been searched) | 458 |
| #38 | arm near/4 index:ti,ab,kw (Word variations have been searched) | 114 |
| #39 | (brachial near/4 (index or pressure)):ti,ab,kw (Word variations have been searched) | 847 |
| #40 | systolic near/5 ratio:ti,ab,kw (Word variations have been searched) | 247 |
| #41 | pressure near/5 ratio:ti,ab,kw (Word variations have been searched) | 505 |
| #42 | pressure near/5 ratio:ti,ab,kw (Word variations have been searched) | 505 |
| #43 | BP near/5 ratio:ti,ab,kw (Word variations have been searched) | 71 |
| #44 | BP near/5 ratio:ti,ab,kw (Word variations have been searched) | 71 |
| #45 | four and limbs and pressure:ti,ab,kw (Word variations have been searched) | 135 |
| #46 | #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 | 4577 |
| #47 | anterior tibial or "dorsalis pedis" or "posterior tibial":ti,ab,kw (Word variations have been searched) | 226 |
| #48 | ankle or arm or elbow or calf:ti,ab,kw (Word variations have been searched) | 25464 |
| #49 | (lower and upper and (extremit* or limb)):ti,ab,kw (Word variations have been searched) | 792 |
| #50 | #47 or #48 or #49 | 26142 |
| #51 | systolic or pressure:ti,ab,kw (Word variations have been searched) | 67765 |
| #52 | #50 and #51 | 2741 |
| #53 | #46 or #52 | 6726 |
| #54 | #29 and #53 in Other Reviews and Technology Assessments | 13 |
Appendix 6. ISI Conference Proceedings Citation Index
| Topic=(ABI or ABPI or AAI or (ankle NEAR/4 index) or (arm NEAR/4 index) or (brachial NEAR/4 (index or pressure)) or (systolic NEAR/4 ratio) or (pressure NEAR/3 ratio) or (BP NEAR/3 ratio) or Oscillometr* or Blood Pressure Determination or Laser‐Doppler Flowmetry or (doppler NEAR/2 (ultrasound or flow* or method or device))) AND Topic=(Claudicat* or IC or CLI or atherosclero* or arteriosclero* or PVD or PAOD or PAD or (arter* NEAR/4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)) or (vascular NEAR/4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)) or (vein* NEAR/4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)) or (veno* NEAR/4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)) or (peripher* NEAR/4 (occlus* or steno* or obstruct* or lesio* or block* or obliter*)) or (peripheral NEAR/3 dis*) or dysvascular* or (leg NEAR/4 (obstruct* or occlus* or steno* or block* or obliter*)) or (limb NEAR/4 (obstruct* or occlus* or steno* or block* or obliter*)) or (lower NEAR/4 (obstruct* or occlus* or steno* or block* or obliter*))) Refined by: Document Types=( PROCEEDINGS PAPER OR MEETING ABSTRACT ) Timespan=All years. Databases=CPCI‐S, CCR‐EXPANDED, IC. Results: 903 |
Appendix 7. ZETOC Conference search
ABI in title 87
ABPI in title 5
“brachial index” in title 14
Appendix 8. Medion search
ABI in title 36
ABPI in title 0
Brachial in title 2
Appendix 9. Quality Assessment Checklist (QUADAS‐2)
| Domains, signalling questions (SQ) and applicability | Rating criteria |
| Domain 1: Patient selection | |
| A. Risk of bias | Describe the methods of patient selection given in the report: |
| SQ 1: Was a consecutive or random sample of patients enrolled? |
Yes: it is reported that a consecutive or a random sample was included Unclear: the precise method of sampling is not reported |
| SQ 2: Did the study avoid inappropriate exclusions? |
Yes: the study included all symptomatic outpatients (in primary or secondary care) without previous ABI test results No: the study included patients who had received an ABI test before, or were asymptomatic Unclear: the ABI test history of the patients in the study was not reported |
| B. Concerns regarding applicability | Give the paper's description of the inclusion/exclusion criteria, including setting, prior tests and symptoms |
| Domain 2: Index test | |
| A. Risk of bias | Give the paper's description of the index test and how it was conducted and interpreted, including the background of the person who carried out the test |
| SQ 1: Were index test results interpreted without knowledge of results of the reference standard? |
Yes: it is stated that the index tests were interpreted in a blind manner (i.e. without knowledge of results of the reference standard), or the index test was always performed and interpreted before the reference standard No: the results of the reference standard were known to the reader of the index test Unclear: it is not reported whether the index test was conducted without knowledge of results of the index test, or whether the index test was completed before the reference standard |
| SQ 2: If a threshold was used, was it prespecified? |
Yes: value for an abnormal test result is < 0.90, and this is clearly stated in the Methods section or elsewhere in the report No: values for a normal or abnormal test results are not reported (prespecified) Unclear: it is not clear at what point in time values for normal and abnormal test results were decided |
| SQ 3: Was the person conducting the test (measuring the ABI) trained to do so? |
Yes: it is stated that the person conducting the test was trained in ABI measurement No: it is clearly reported that the person conducting the test was not trained in ABI measurement Unclear: the expertise and background of the individuals conducting the index test are unclear |
| B. Concerns regarding applicability? |
High: the index test was conducted using hand‐held Dopplers as opposed to a stethoscope or other equipment not widely available Low: the index test was not conducted using hand‐held Dopplers, and the equipment was standard (as outlined in the protocol) Unclear: information about the equipment used to conduct the test is not presented |
| Domain 3: Reference standard | |
| A. Risk of bias | Give the reported definition of the reference standard and how it was conducted and interpreted |
| SQ 1: Is the reference standard likely to correctly classify the target condition? |
Yes: it is reported that duplex ultrasonography or angiography test results were interpreted by trained operatives No: it is reported that the duplex ultrasonography or angiography test results were not interpreted by trained operatives Unclear: it is not clear whether those individuals who interpreted the duplex ultrasonography or angiography test results were trained |
| SQ 2: Were the reference standard test results interpreted without knowledge of the index test results? |
Yes: the person classifying the reference standard test results was unaware of the ABI test results No: the person classifying the reference standard test results was aware of the ABI test results Unclear: not reported |
| SQ 3: Was the person conducting the reference standard test (duplex ultrasonography) trained? |
Yes: it is stated that the person conducting the reference standard test was trained in the interpretation of duplex ultrasonography
No: it is clear that the person conducting the reference standard test was not trained in the interpretation of duplex ultrasonography Unclear: the expertise and background of the reference standard test readers are unclear |
| Domain 4: Flow and timing | |
| A. Risk of bias | Describe the reasons why any patients recruited into the study did not contribute to the 2 × 2 table (i.e. patients who did not undergo the reference standard and/or the index test) referring to the flow diagram Give the time interval between the ABI and the reference standard tests |
| SQ 1: Was there an appropriate interval between the index test and the reference standard? |
Yes: the index and reference standard tests were all conducted within 2 weeks of each other No: some of the reference standard test results were not conducted within 2 weeks of each other Unclear: no information about the relative timing of the tests is provided |
| SQ 2: Did all the patients receive the same reference standard? |
Yes: a complete set of reference standard test results is available for all study patients No: the same reference standard test results are not available for all patients Unclear: insufficient information is available to make a judgement about the availability of reference standard |
| SQ 3: Were all patients included in the final analysis? |
Yes: all patients enrolled contributed to the 2 × 2 table No: not all patients enrolled contributed to the 2 × 2 table Unclear: it is not clear whether patients were recruited but not included in the study report of the 2 × 2 table |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Vega 2011.
| Study characteristics | |||
| Patient sampling | The sample of patients was reported to be consecutive. | ||
| Patient characteristics and setting | 85 patients (158 legs) referred to the catheterisation laboratory for angiography for peripheral arterial intermittent claudication or suspected advanced PAD and over 30 years of age were included. | ||
| Index tests | Doppler ABI measurement was performed with an 8 MHz doppler probe (Dopplex II MD2/SD, Huntleigh model) (ArjoHuntleigh Inc., Addison, Illinois), and a sphygmomanometry with cuffs of appropriate size. The oscillometer used was an Omron M4‐1 (Omron Healthcare Europe BV, Hoofddorp, The Netherlands). The ABI was calculated as the ratio of peak systolic pressure at the ankle and arms. Patients with non‐compressible arteries with ABI > 1.4 were excluded. If the oscillometric method still gave an error reading after 3 serial attempts with rehabilitation of cuff pressures, pressure was assumed to be < 60 mmHg and a '0 index' was assigned if it was not possible to detect flow with the doppler method (DM). The ABI threshold was not prospectively stated. |
||
| Target condition and reference standard(s) | Haemodynamically significant or severe PAD was defined as stenosis with > 50% obstruction, and non‐significant PAD as < 50% obstruction. Severity was determined simultaneously by visual comparison between healthy and diseased segments and by use of the Quantitiative Coronary Angiography (QCA) programme. Reference standard was the angiography digital subtraction technique with sequential images. | ||
| Flow and timing | We excluded 12 legs for a variety of reasons: 6 cases because of amputation, 4 because of painful ulcers, which ruled out examination, and 2 because ABI was > 1.4. The time between the conduct of the index test and use of the reference standard is not reported. |
||
| Comparative | |||
| Notes | Only 131 (83%) limbs had verification from angiography. Contacted study author to request participant‐level data and received no response | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test ABI | |||
| If a threshold was used, was it pre‐specified? | No | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Unclear | |||
ABI: ankle brachial index. DM: doppler method. PAD: peripheral arterial disease. QCA: quantitative coronary angiography.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akhtar 2009 | Study did not use duplex ultrasonography nor angiography as reference standard. Case‐control study comparing different techniques for measurement of the ABI (hand‐held doppler ABI vs palpatory ABI) in healthy, high‐risk asymptomatic and known PAD patients |
| Alnaeb 2007 | Study population not defined as presenting with exertional leg pain. Patients with diabetes, recruited from hospital vascular clinic, and control patients from an orthopaedic outpatient clinic used to compare ABI and photoplethysmography with duplex ultrasonography |
| Alnaeb 2008 | Study population not defined as presenting with exertional leg pain. PAD and non‐PAD patients recruited from vascular outpatient clinic for comparison of ABI and photoplethysmography with duplex ultrasonography |
| Armstrong 2010 | Study did not use duplex ultrasonography nor angiography as reference standard. Study comparing physical examination with ABI in people suspected of having or at high risk for PAD |
| Baxter 1993 | Study population not selected owing to exertional leg pain. Patients with lower limb claudication, rest pain or cellulitis assessed with ABI, colour doppler ultrasonography and arteriography. Separate data for claudication population sought from study author, who replied that data were not available |
| Beckman 2005 | Study did not use duplex ultrasonography nor angiography as reference standard. Comparison of 2 different types of ABI (oscillometric and continuous wave doppler ultrasonography) in participants referred to lab for evaluation of PAD |
| Benchimol 2009 | Study did not use duplex ultrasonography nor angiography as reference standard. Comparison of ABI measuring methods (automated vs doppler ultrasonography) in participants recruited by physicians in preventive medicine attending annual check‐up |
| Beutner 2012 | Study did not use duplex ultrasonography nor angiography as reference standard. Study compared 2 different ABI measurement methods in healthy participants and in participants with confirmed lower limb PAD before revascularisation |
| Bogomolov 2012 | Study did not use duplex ultrasonography nor angiography as reference standard. Unclear whether study population was selected for exertional leg pain. Study performed ABI and doppler examination in people with and without diabetes |
| Cacoub 2005 | Comparison of 2 types of ABI measurements (stethoflux and continuous wave doppler) in patients recruited consecutively from vascular laboratory |
| Carmo 2009 | Study did not use duplex ultrasonography nor angiography as reference standard. Comparison of ABI measured by stethoscope and doppler in people referred to haemodynamic laboratory of Fekicio Rocho Hospital for peripheral, renal, coronary and cerebral vascular territory diagnostic and interventional angiographic examinations |
| Clairotte 2009 | Unclear whether study population selected because of exertional leg pain. Study assessed oscillometric ABI vs doppler ABI in patients referred to physiology department for doppler ultrasound examination of PAD. Two‐dimensional ultrasound measurement reported but not used as reference standard |
| Cortez‐Cooper 2003 | Study did not use duplex ultrasonography nor angiography as reference standard. Study compared automated device with manual method for assessment of ABI in normotensive and hypertensive participants. |
| Eason 2005 | Study did not use duplex ultrasonography nor angiography as reference standard. Study in asymptomatic PAD population (patients with symptomatic PAD were excluded). PAD assessed via ABI and the San Diego claudication questionnaire |
| Ena 2011 | Study did not use duplex ultrasonography nor angiography as reference standard. Study compared ABI measured with upper arm automated blood pressure device vs ABI measured by hand‐held doppler in diabetic patients attending screening for PAD. |
| Espeland 2008 | Study did not use duplex ultrasonography nor angiography as reference standard. Population not presenting with exertional leg pain. Study compared different systolic blood pressure protocols for measuring ABI in a trial population of overweight/obese volunteers with type 2 diabetes. |
| Feigelson 1994 | Study population not identified as presenting with exertional leg pain. Participants were a population‐based cohort of adults screened for large‐vessel PAD. |
| Fronek 1999 | Index test was upper thigh/brachial index measurement rather than ankle/brachial index measurement. Study performed in patients referred to the Veterans' Administration Vascular Laboratory with suspected arterial occlusive disease of lower extremities. |
| Guo 2008 | Population not identified as presenting with exertional leg pain to primary care or outpatient clinic. Participants recruited from cardiology hospital ward |
| Heidrich 1998 | Index test involved measurement of BP in upper limb digital arteries. Study population consisted of people with primary or secondary Raynaud's disease or digital occlusions. |
| Hoyer 2012 | Index test was new portable photoplethysmography device for diagnosing ABI; reference standard was strain‐gauge plethysmography. |
| Hriljac 2004 | Study did not report the use of duplex ultrasonography nor angiography as reference standard. Unclear whether study population referred for exertional leg pain, Study assessed the added diagnostic utility of measuring ABI and pulse volume waveforms at rest and after exercise in patients referred to vascular laboratory by 'non‐vascular' specialists. |
| Johansson 2002 | Study did not use duplex ultrasonography nor angiography as reference standard. Population not identified as presenting with exertional leg pain. Study compared pulse oximetric method for assessment of toe pressure ABI with traditional ankle doppler pressure ABI in people with diabetes drawn from the case records of primary healthcare clinics. |
| Klein 2003 | Study population not identified as presenting with exertional leg pain. Participants were patients considered for fibula free flap transplantation or those who had undergone this procedure. Patients with a history of intermittent claudication were excluded. |
| Kollias 2011 | Study did not use duplex ultrasonography nor angiography as reference standard. Study investigated automated vs manual doppler method in patients with CVD risk factors attending hypertension or diabetes clinic. |
| Korno 2009 | Study did not use duplex ultrasonography nor angiography as reference standard. Study compared automated oscillometric ABI vs hand‐held doppler ABI in patients admitted for surgical treatment or for evaluation of venous disease to the department of vascular surgery, including participants with intermittent claudication or critical ischaemia. |
| Kurtoglu 2009 | Study population not identified as presenting with exertional leg pain. Participants were patients with extremity injury or with suspicion of peripheral artery injury admitted to trauma and emergency medicine department. |
| Lijmer 1996 | Patients referred by general practitioner for claudication or critical ischaemia ‐ separate ABI data for patients with IC requested from study authors but no reply received. |
| MacDougall 2008 | Study did not use duplex ultrasonography nor angiography as reference standard. Study investigated oscillometric ABI vs doppler ABI in 3 groups of patients: normal volunteers, patients with significant CV risk profiles and patients suspected of having PAD who were referred to a vascular lab. |
| Manzano 2006 | Study did not use duplex ultrasonography nor angiography as reference standard. Study population was not defined as people presenting with exertional leg pain. Study compared ABI vs Edinburgh Claudication Questionnaire in patients without typical intermittent claudication or known atherosclerotic disease. |
| McLafferty 1997 | Study population consisted of people who had undergone previous revascularisation procedures. |
| Mehlsen 2008 | Paper reports 2 studies, neither of which used duplex ultrasonography nor angiography as reference standard.
|
| Nam 2010 | Index test was ABI measured using photoplethysmography (currently not an endorsed method for the assessment of ABI). |
| Nexoe 2012 | Study did not use duplex ultrasonography nor angiography as reference standard. Study compared ABI measured in GP surgery vs ABI measured by experienced staff of Department of Nuclear Medicine in people presenting to GP surgery. |
| Niazi 2006 | Uncertain whether study population was recruited with exertional leg pain. Population recruited from a retrospective sample of people who had undergone an angiogram and ABI measurement; no indication for angiography given. Study authors contacted for further information but did not reply |
| Oksala 2010 | Study did not use duplex ultrasonography nor angiography as reference standard. Study compared different methods of calculating ABI from BP measurements in people 50 to 69 years of age with ≥ 1 cardiovascular risk factor; > 70 years of age; with claudication defined as pain in the calf during exercise. |
| Paez 2010 | Study population was not defined as presenting with exertional leg pain. Patients with ≥ 1 cardiovascular risk factor were recruited from the Heart Institute of Bucaramanga (Colombia). |
| Parmeswarin 2005 | Study did not use duplex ultrasonography nor angiography as reference standard. Study compared ABI with pulse oximetry test in patients with type 2 diabetes without known lower extremity arterial disease or symptoms of LEAD (typical IC or rest pain). |
| Potier 2008 | Study did not use duplex ultrasonography nor angiography as reference standard. Study compared doppler ABI and oscillometric measured ABI in diabetic and non‐diabetic patients. |
| Premalatha 2002 | Study population was not defined as presenting with exertional leg pain. Participants were diabetic hospital inpatients with severe foot infection. |
| Premanath 2010 | Study did not use duplex ultrasonography nor angiography as reference standard. Study population was not defined as presenting with exertional leg pain. Study compared different methods of measuring ABI in patients with diabetes attending a clinic, irrespective of their symptoms. |
| Ramos 2011 | Study did not use duplex ultrasonography nor angiography as reference standard. Study population was not defined as presenting with exertional leg pain. Study used ABI < 0.9 as an outcome to develop a predictive risk score for low ABI in people recruited from a primary healthcare setting, irrespective of symptoms. |
| Schroder 2006 | Study population was not defined as presenting with exertional leg pain. Participants were patients presenting at outpatient clinic with suspected vascular disease; < 40% had intermittent claudication (data not presented separately in paper, and study authors unable to provide data when contacted). |
| Stoffers 1996 | Unclear whether duplex ultrasonography or angiography was used as reference standard. Participants selected from Limburg POAD study with ABI < 0.95, typical IC complaints or leg complaints less typical of IC but with ≥ 1 foot pulse missing, Study compared ABI measurements with diagnostic conclusions of vascular laboratory from "sophisticated ultrasound measurements". Pressure measurements at rest and after treadmill walking test; technician interpreted acoustic and audiospectrum pulsewave signals from posterior tibial and dorsalis pedis arteries. No separate 2 × 2 data for the 22 patients in the total population who had IC. Contacted study author, who replied that data were not available |
| Vinyoles 2007 | Study did not use duplex ultrasonography nor angiography as reference standard. Study population was not defined as presenting with exertional leg pain. Study compared oscillometry ABI vs ABI measured with doppler ultrasound probe. |
| Williams 2005 | Study population was not defined as presenting with exertional leg pain. Study evaluated ABI and other methods of screening for lower limb PAD in diabetic and non‐diabetic participants with and without arterial disease and neuropathy. |
| Wohlfahrt 2011 | Study did not use duplex ultrasonography nor angiography as reference standard. Study population was not defined as presenting with exertional leg pain. Study compared doppler ABI vs oscillometric ABI in randomly selected general population sample. |
| Zhang 2010 | Study population was not defined as presenting with exertional leg pain. Study assessed lower limb PAD using ABI and duplex ultrasonography in diabetic individuals recruited from a secondary care setting, irrespective of symptoms. |
ABI: ankle brachial index. CV: cardiovascular. DTA: diagnostic test accuracy. IC: intermittent claudication. PAD: peripheral arterial disease.
Differences between protocol and review
The reference standard in our protocol was duplex ultrasonography, and patients were required to have their peripheral arterial disease (PAD) categorised as 1, 2 or 3 on the basis of the Rutherford index (Rutherford 1997), or as stage II on the basis of the Fontaine classification system (Fontaine 1954). None of the studies that we considered for inclusion in the review met these criteria. After discussion between the review authors and Cochrane Vascular editorial base, it was agreed that we should deviate from the protocol to include studies that had used angiography as the reference standard and to include studies in which participants presented with exertional leg pain, with or without subsequent categorisation by Fontaine 1954 or Rutherford 1997.
We were not able to search the Cochrane Register of Diagnostic Test Accuracy Studies.
Contributions of authors
The whole review team has contributed to the development of the protocol. KW developed the search strategy. FC and AA applied the eligibility criteria and extracted data from studies. FC completed RevMan. FMC assumed responsibility for plans for the analysis. All review authors contributed to the final report of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Programme Grant funding to Cochrane Vascular (10/4001/14). The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office.
Declarations of interest
FC: none known. KW: none known. AA: none known. FMC: none known.
This review forms part of a National Institute of Health Research (NIHR) Cochrane programme grant (10/4001/14) and is being conducted independently of our funders, the NIHR. The NIHR has had no input on the conduct or results of the review.
New
References
References to studies included in this review
Vega 2011 {published data only}
- Vega J, Romani S, Garciperez J, Vicente L, Pacheco N, Zamorano J, et al. Peripheral arterial disease: efficacy of the oscillometric method. Revista Espanola De Cardiologia 2011;64(7):619‐21. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akhtar 2009 {published data only}
- Akhtar B, Siddique S, Khan RA, Zulfiqar S. Detection of atherosclerosis by ankle brachial index: evaluation of palpatory method versus ultrasound Doppler technique. Journal of Ayub Medical College, Abbottabad 2009;21(1):11‐6. [PubMed] [Google Scholar]
Alnaeb 2007 {published data only}
- Alnaeb ME, Crabtree VP, Boutin A, Mikhailidis DP, Seifalian AM, Hamilton G. Prospective assessment of lower‐extremity peripheral arterial disease in diabetic patients using a novel automated optical device. Angiology 2007;58(5):579. [DOI] [PubMed] [Google Scholar]
Alnaeb 2008 {published data only}
- Alnaeb ME, Boutin A, Crabtree VP, Mikhailidis DP, Seifalian AM, Hamilton G. Assessment of lower extremity peripheral arterial disease using a novel automated optical device. Vascular and Endovascular Surgery 2008;41(6):522‐7. [DOI] [PubMed] [Google Scholar]
Armstrong 2010 {published data only}
- Armstrong DWJ, Tobin C, Matangi MF. The accuracy of the physical examination for the detection of lower extremity peripheral arterial disease. Canadian Journal of Cardiology 2010;26(10):e346‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baxter 1993 {published data only}
- Baxter GM, Polak JF. Lower limb colour flow imaging: a comparison with ankle:brachial measurements and angiography. Clinical Radiology 1993;47:91‐5. [DOI] [PubMed] [Google Scholar]
Beckman 2005 {published data only}
- Beckman JA, Higgins CO, Gerhard‐Herman M. Automated oscillometric determination of the ankle‐brachial index provides accuracy necessary for office practice. Hypertension 2005;47:35‐8. [DOI] [PubMed] [Google Scholar]
Benchimol 2009 {published data only}
- Benchimol D, Pillois X, Benchimol A, Houitte A, Sagardiluz P, Tortelier L, et al. Accuracy of ankle‐brachial index using an automatic blood pressure device to detect peripheral artery disease in preventive medicine. Archives of Cardiovascular Diseases 2009;102:519‐24. [DOI] [PubMed] [Google Scholar]
Beutner 2012 {published data only}
- Beutner F, Teren A, Gielen S, Schuler G, Wirkner K, Tiller D, et al. Automated photoplethysmography‐based determination of ankle‐brachial: a validation study against doppler sonography. Clinical Research in Cardiology 2012;101:875‐83. [DOI] [PubMed] [Google Scholar]
Bogomolov 2012 {published data only}
- Bogomolov MS, Sedov VM, Slobodianiuk VV. Reliability of doppler examination and measurement of ABI for diagnostic peripheral arterial disease. Khirurglia 2012;9:78‐81. [PubMed] [Google Scholar]
Cacoub 2005 {published data only}
- Cacoub P, Luizy F, Saadoun D, Michon‐Pasturel M, Hello C, Frances Y, et al. Screening for peripheral arterial disease by the ankle‐brachial pressure index (ABPI) using Stethoflux™ in comparison to continuous wave doppler. Circulation. 2005; Vol. 112, issue 17:U863‐U864.
Carmo 2009 {published data only}
- Carmo GAL, Mandil A, Nascimento BR, Arantes BD, Brittencourt JC, Falqueto EB, et al. Can we measure the ankle‐brachial index using only a stethoscope? A pilot study. Family Practice 2009;26:22‐6. [DOI] [PubMed] [Google Scholar]
Clairotte 2009 {published data only}
- Clairotte C, Retout S, Potier L, Roussel R, Escoubet B. Automated ankle‐brachial pressure index measurement by clinical staff for peripheral arterial disease diagnosis in nondiabetic and diabetic patients. Diabetes Care 2009;32(7):1231‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cortez‐Cooper 2003 {published data only}
- Cortez‐Cooper MY, Supak JA, Tanaka H. A new screening device for automatic measurements of arterial stiffness and ankle brachial index. American Journal of Hypertension 2003;16(81):138A. [DOI] [PubMed] [Google Scholar]
Eason 2005 {published data only}
- Eason SL, Petersen NJ, Suarez‐Almazor M, Davis B, Collins TC. Diabetes mellitus, smoking, and the risk for asymptomatic peripheral arterial disease: whom should we screen?. Journal of the American Board of Family Practice / American Board of Family Practice 2005;18:355‐61. [DOI] [PubMed] [Google Scholar]
Ena 2011 {published data only}
- Ena J, Lozano T, Verdú G, Argente CR, González VL. Accuracy of ankle‐brachial index obtained by automated blood pressure measuring devices in patients with diabetes mellitus. Diabetes Research and Clinical Practice 2011;92:329‐36. [DOI] [PubMed] [Google Scholar]
Espeland 2008 {published data only}
- Espeland MA, Regensteiner JG, Jaramillo SA, Gregg E, Knowler WC, Wagenknecht LE, et al. Measurement characteristics of the ankle‐brachial Index: results from action for health in diabetes study. Vascular Medicine 2008;13:225‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feigelson 1994 {published data only}
- Feigelson HS, Criqui MH, Fronek A, Langer RD, Molgaard CA. Screening for peripheral arterial disease: the sensitivity, specificity, and predictive value of noninvasive tests in a defined population. American Journal of Epidemiology 1994;140(6):526‐34. [DOI] [PubMed] [Google Scholar]
Fronek 1999 {published data only}
- Fronek A, Oglevie S, Curran B, Fronek K. Combination of pressure and velocity parameters in the non‐invasive diagnosis of aorto‐iliac disease. Vascular Medicine (London, England) 1999;4:77‐81. [DOI] [PubMed] [Google Scholar]
Guo 2008 {published data only}
- Guo X, Pang W, Zhao M, Luo Y, Sun Y, Hu D. Sensitivity and specificity of ankle‐brachial index for detecting angiographic stenosis of peripheral arteries. Ciculation Journal 2008;72:605‐10. [DOI] [PubMed] [Google Scholar]
Heidrich 1998 {published data only}
- Heidrich H, Blank B. Does the measurement of the digital arterial pressure using the doppler ultrasonic technique without testing for vasospasm to detect digital arterial occlusions make good sense?. Vasa‐Journal of Vascular Diseases 1998;27(3):158‐62. [PubMed] [Google Scholar]
Hoyer 2012 {published data only}
- Hoyer C, Sanderman J, Petersen LJ. Randomised diagnostic accuracy study of a fully automated portable device for diagnosing peripheral arterial disease by measuring the toe‐brachial index. European Journal of Vascular and Endovascular Surgery 2013;45(1):57‐64. [DOI] [PubMed] [Google Scholar]
Hriljac 2004 {published data only}
- Hriljac I, Olin JW, Teodorescu V, Gustavson SM, Halpern JL. Measurement of resting ankle brachial index may not accurately identify disease in patients with signs or symptoms of peripheral arterial disease. Journal of the American College of Cardiology 2004;43(5s2):A459 (Abstract No. 1046‐165). [Google Scholar]
Johansson 2002 {published data only}
- Johansson KE, Marklund BR, Fowelin JH. Evaluation of a new screening method for detecting peripheral arterial disease in a primary health care population of patients with diabetes mellitus. Diabetic Medicine 2002;19:307‐10. [DOI] [PubMed] [Google Scholar]
Klein 2003 {published data only}
- Klein S, Hage JJ, Horst CMAM, Lagerweij M. Ankle‐arm index versus angiography for the preassessment of the fibula free flap. Plastic Reconstruction Surgery 2003;111(2):735‐43. [DOI] [PubMed] [Google Scholar]
Kollias 2011 {published data only}
- Kollias A, Xilomenos A, Protogerou A, Dimakakos E, Stergiou GS. Automated determination of the ankle‐brachial index using an oscillometeric blood pressure monitor: validation vs. doppler measurement and cardiovascular risk factor profile. Hypertension Research 2011;34:825‐30. [DOI] [PubMed] [Google Scholar]
Korno 2009 {published data only}
- Korno M, Eldrup N, Sillesen H. Comparison of ankle‐brachial index measured by an automated oscillometric apparatus with that by standard doppler technique in vascular patients. European Journal of Vascular Surgery 2009;38:610‐5. [DOI] [PubMed] [Google Scholar]
Kurtoglu 2009 {published data only}
- Kurtoglu M, Dolay K, Karamustafaoglu B, Yanar H, Kuzkaya M. The role of the ankle brachial pressure index in the diagnosis of peripheral arterial injury. Turkish Journal of Trauma and Emergency Surgery 2009;15(5):448‐52. [PubMed] [Google Scholar]
Lijmer 1996 {published data only}
- Lijmer JG, Hunink MGM, Dungen JJAM, Loonstra J, Smit AJ. ROC analysis of non invasive tests for peripheral Arterial Disease. Ultrasound in Medicine and Biology 1996;22(4):391‐8. [DOI] [PubMed] [Google Scholar]
MacDougall 2008 {published data only}
- MacDougall AM, Tandon V, Wilson MP, Wilson TW. Oscillometric measurement of the ankle‐brachial Index. Canadian Journal of Cardiology 2008;24(1):49‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manzano 2006 {published data only}
- Manzano L, Vargus JA, Cepeda‐Rodrigo JM, Bianchi‐Llaves JL, Cabreu‐Roue M, Grilo‐Reina A, et al. Diagnostic value of atypical claudication for peripheral arterial disease diagnosed by a low ankle‐brachial index: MERITO study. European Heart Journal 2006;27(Suppl 1):671. [Google Scholar]
McLafferty 1997 {published data only}
- McLafferty RB, Moneta GL, Taylor LM Jr, Porter JM. Ability of ankle‐brachial index to detect lower‐extremity atherosclerotic disease progression. Archives of Surgery 1997;132(8):836‐40. [DOI] [PubMed] [Google Scholar]
Mehlsen 2008 {published data only}
- Mehlsen J, Wiinberg N, Bruce C. Oscillometric blood pressure measurement: a simple method in screening for peripheral arterial disease. Clinical Physiology and Functional Imaging 2008;28:426‐9. [DOI] [PubMed] [Google Scholar]
Nam 2010 {published data only}
- Nam SC, Seung HH, Sang HL, You SH, Je HW, Jae IB, et al. Factors affecting the validity of ankle‐brachial index in the diagnosis of peripheral arteria: obstructive disease. Angiology 2010;61(4):392‐6. [DOI] [PubMed] [Google Scholar]
Nexoe 2012 {published data only}
- Nexoe J, Damsbo B, Lund JO, Munck A. Measurement of blood pressure, ankle blood pressure and calculation of ankle brachial index in general practice. Family Practice 2012;29:345. [DOI] [PubMed] [Google Scholar]
Niazi 2006 {published data only}
- Niazi K, Khan TH, Easley KA. Diagnostic utility of the two methods of ankle brachial index in the detection of peripheral arterial disease of lower extremities. Catheterization and Cardiovascular Interventions 2006;68:788‐92. [DOI] [PubMed] [Google Scholar]
Oksala 2010 {published data only}
- Oksala NKJ, Viljamaa J, Saimanen E, Venermo M, on behalf of the ATTAC Study Group. Modified ankle‐brachial index detects more patients at risk in a Finnish primary health care. European Journal of Vascular Surgery 2010;39:227‐33. [DOI] [PubMed] [Google Scholar]
Paez 2010 {published data only}
- Páez EAN, Oróstegui AM, Hernández GHJ, Valencia ALI, Reyes SCI, Tapias VLF, et al. Validation of oscillometric measurement of ankle‐brachial index compared with arterial lower echo‐doppler for arterial disease [Validacion del indice tobillo brazo oscilometrico comparado con eco‐doppler arterial de miembros inferiores para enfermadad]. Revista Columbiana de Cardiologia 2010;17(4):157‐66. [Google Scholar]
Parmeswarin 2005 {published data only}
- Parameswaran GL, Brand K, Dolan J. Pulse oximetry as a potential screening tool for lower extremity arterial disease in asymptomatic patients with diabetes mellitus. Archives of Internal Medicine 2005;165:442‐6. [DOI] [PubMed] [Google Scholar]
Potier 2008 {published data only}
- Potier L, Clairotte C, Roussel R, Escoubet B. Automated oscillometric ankle‐brachial index measurement by nurses as a sensitive screening test for peripheral arterial disease (PAD): a doppler ultrasound validation. Diabetes 68th Scientific Sessions. 2008:Abstract number 2210‐PO.
Premalatha 2002 {published data only}
- Premalatha G, Ravkumar R, Sanjay R, Deepa R, Mohan V. Comparison of colour duplex ultrasound and ankle brachial pressure index measurements in peripheral vascular disease in type 2 diabetic patients with foot infections. Journal of the Association of Physicians in India 2002;50:1240‐4. [PubMed] [Google Scholar]
Premanath 2010 {published data only}
- Premanath M, Raghunath M. Ankle‐brachial index by oscillometery: a very useful method to assess peripheral arterial disease in diabetes. International Journal of Diabetes in Developing Countries 2010;30(2):97‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramos 2011 {published data only}
- Ramos R, Baena‐Diez JM, Quesada M, Solanas P, Subirana I, Sala J, et al. Derivation and validation of REASON: a risk score identifying candidates to screen for peripheral arterial disease using ankle brachial Index. Atherosclerosis 2010;214:474‐9. [DOI] [PubMed] [Google Scholar]
Schroder 2006 {published data only}
- Schroder F, Diehm N, Kareem S, Ames M, Pira A, Zwettler U, et al. A modified calculation of ankle‐brachial pressure index is far more sensitive in the detection of peripheral arterial disease. Journal of Vascular Surgery 2006;44:531‐6. [DOI] [PubMed] [Google Scholar]
Stoffers 1996 {published data only}
- Stoffers HEJH, Kester ADM, Kaiser V, Rinkens PELM, Kitslaar EHM, Knottnerus JA. The diagnostic value of the measurement of the ankle brachial systolic pressure index in primary health care. Journal of Clinical Epidemiology 1996;49(12):1401‐5. [DOI] [PubMed] [Google Scholar]
Vinyoles 2007 {published data only}
- Vinyoles E, Pujol E, Casermeiro J, Prado C, Jabalera S, Salido V. Ankle‐brachial index to detect peripheral arterial disease: concordance and validation study between Doppler and an oscillometric device [Indice tobillo‐brazo en la deteccion de arteriopatia periferica: estudio de validez y concordiancia entre Doppler t metodo oscilometrico]. Medicina Clinica 2007;128(3):92‐4. [DOI] [PubMed] [Google Scholar]
Williams 2005 {published data only}
- Williams DT, Harding KG, Price P. An evaluation of the efficacy of methods used in screening for lower limb arterial disease in diabetes. Diabetes Care 2005;28:2206‐10. [DOI] [PubMed] [Google Scholar]
Wohlfahrt 2011 {published data only}
- Wohlfahrt P, Ingrischova M, Karjcoviechova A, Palous D, Dolejsova M, Seidlerova J, et al. A novel oscillometric device for peripheral arterial disease screening in everyday practice. International Angiography 2010;30:256‐61. [PubMed] [Google Scholar]
Zhang 2010 {published data only}
- Zhang H, Li XY, Si XY, Lu XS, Liu ZY. Manifestations of lower extremity atherosclerosis in diabetic patients with high ankle‐brachial index. Chinese Medical Journal 2010;123(7):890‐4. [PubMed] [Google Scholar]
Additional references
Aboyans 2012
- Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Dieham C, et al. Measurement and interpretation of the ankle‐brachial index: a scientific statement from the American Heart Association. Circulation 2012;126(24):2890‐909. [DOI] [PubMed] [Google Scholar]
Ankle Brachial Index Collaboration 2008
- The Ankle Brachial Index Collaboration. Ankle Brachial Index combined with Framingham risk score to predict cardiovascular events and mortality. A meta‐analysis. JAMA 2008;300(2):197‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bhasin 2007
- Bhasin N, Scott DJA. Ankle Brachial Index: identifying cardiovascular risk and improving diagnostic accuracy. Journal of the Royal Society of Medicine 2007;100(1):4‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cassar 2003
- Cassar K, Bachoo P, Brittenden J. The effect of peripheral percutaneous transluminal angioplasty on quality of life in patients with intermittent claudication. European Journal of Vascular and Endovascular Surgery 2003;26(2):130‐6. [DOI] [PubMed] [Google Scholar]
Chang 2011
- Chang ZN, Lui ZY. Subintimal angioplasty for chronic lower limb arterial occlusion. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD009418] [DOI] [PubMed] [Google Scholar]
Dachun 2013
- Dachun X, Zou LZ, Xing Y, Hou L, Wei Y, Zhang J, et al. Diagnostic value of ankle‐brachial index in peripheral arterial disease: a meta analysis. Canadian Journal of Cardiology 2013;29:492‐8. [DOI] [PubMed] [Google Scholar]
de Backer 2012
- Backer TLM, Vander Stichele R, Lehert P, Bortel L. Naftidrofuryl for intermittent claudication. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD001368.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58(9):882‐93. [DOI] [PubMed] [Google Scholar]
Fokkenrood 2013
- Fokkenrood HJP, Bendermacher BLW, Lauret GJ, Willigendael EM, Prins MH, Teijink JAW. Supervised exercise therapy versus non‐supervised exercise therapy for intermittent claudication. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD005263.pub3] [DOI] [PubMed] [Google Scholar]
Fontaine 1954
- Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders [Die Chirurgische Behandlung Der Peripheren Durch‐blutungsstörungen]. Helvetica Chirurgica Acta 1954;21(5‐6):499‐533. [PubMed] [Google Scholar]
Harbord 2007
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta‐analysis of diagnostic accuracy studies. Biostatistics 2007;8(2):239‐51. [DOI] [PubMed] [Google Scholar]
Heald 2006
- Heald CL, Fowkes FG, Murray GD, Price JF, on behalf of the Ankle Brachial Index Collaboration. Risk of mortality and cardiovascular disease associated with the ankle‐brachial index: systematic review. Atherosclerosis 2006;189(1):61‐9. [DOI] [PubMed] [Google Scholar]
Hirsch 2001
- Hirsch AT, Criqui MH, Treat‐Jacobson D, Regensteiner JG, Creager MA, Olin JW, et al. Peripheral arterial disease detection awareness and treatment in primary care. JAMA 2001;286(11):1317‐24. [DOI] [PubMed] [Google Scholar]
Hooi 2007
- Hooi JD, Kester ADM, Stoffers HEJH, Rinkens PELM, Knottnerus JA, Ree JW. Asymptomatic peripheral arterial occlusive disease predicted cardiovascular morbidity and mortality in a 7 year follow‐up study. Journal of Clinical Epidemiology 2004;57(3):294‐300. [DOI] [PubMed] [Google Scholar]
Khan 2007
- Khan S, Flather M, Mister R, Delahunty N, Fowkes G, Bradbury A, et al. Characteristics and treatments of patients with peripheral arterial disease referred to UK vascular clinics: results of a prospective registry. European Journal of Vascular and Endovascular Surgery 2007;33(4):442‐50. [DOI] [PubMed] [Google Scholar]
Lane 2014
- Lane R, Ellis B, Watson L, Leng GC. Exercise for intermittent claudication. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD000990.pub3] [DOI] [PubMed] [Google Scholar]
MacLeod‐Roberts 1995
- MacLeod‐Roberts J. Vascular assessment. In: Merriman LM, Tollafield DR editor(s). Assessment of the Lower Limb. 1st Edition. Edinburgh: Churchill Livingstone, 1995:79‐112. [Google Scholar]
McDermott 2000
- McDermott MM, Criqui MH, Liu K, Guralnik JM, Greenland P, Martin GJ, et al. Lower ankle/brachial index, as calculated by averaging the dorsalis pedis and posterior tibial arterial pressures, and association with leg functioning in peripheral arterial disease. Journal of Vascular Surgery 2000;32(6):1164‐71. [DOI] [PubMed] [Google Scholar]
NICE 2012
- NHS National Institute for Health and Clinical Excellence. Lower limb peripheral arterial disease: diagnosis and management, August 2012. guidance.nice.org.uk/cg147 (accessed 7 September 2012).
Rutherford 1997
- Rutherford RB, Baker JD, Ernst C, Johnston KW, Porter JM, Ahn S, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. Journal of Vascular Surgery 1997;26(3):517‐38. [DOI] [PubMed] [Google Scholar]
SIGN 2007
- Scottish InterCollegiate Guidelines Network (SIGN). Diagnosis and management of peripheral arterial disease. A national clinical guideline, 2007. www.sign.ac.uk (accessed 7 September 2012).
Twine 2009
- Twine CP, Coulston J, Shandall A, McLain AD. Angioplasty versus stenting for superficial femoral artery lesions. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006767.pub2] [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2 Group. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [DOI] [PubMed] [Google Scholar]


