Abstract
Paired associative stimulation (PAS) has been shown to increase corticospinal excitability (CSE) providing a promising adjuvant therapeutic approach for stroke. Combining PAS with movement of the stimulated limb may further increase enhancement of CSE, however, individuals with moderate to severe stroke may not be able to engage in the necessary repetitive voluntary movements of the paretic limb. The objective of this study was to investigate the feasibility of contralaterally coordinated PAS (ccPAS) applied to the resting hand extensors during fast extension of the contralateral hand. A potential dependency of CSE modulation on the phase of the movement of the opposite hand was evaluated. Eleven participants each completed three session: PAS applied to the resting right hand during the preparation phase of the extension of the contralateral (left) hand; PAS applied during the execution phase of the left hand extension; and PAS applied with both hands at rest. Motor evoked potentials (MEPs) were evoked from the right extensor digitorum communis (EDC) and flexor digitorum superficialis (FDS) muscles prior and immediately after each session. PAS delivered during the muscle contraction of the left hand and PAS delivered at rest both increased the MEP amplitude in the right EDC. PAS delivered before the left hand movement onset led to a decrease in the MEP amplitude measured in the right EDC muscle. We conclude that PAS induced bidirectional changes in the amplitude of MEPs that were dependent on the phase of the movement of the opposite hand.
I. Introduction
To date, there has been little success developing rehabilitation treatments designed to ameliorate moderate to severe paralysis of the hand caused by stroke. It has been hypothesized that treatments designed to enhance the excitability of the motor cortex may prove to be effective adjuvants to existing therapies. Task-oriented functional electrical stimulation (FES) has shown promise in promoting functional recovery in stroke by combining FES and repetitive training of the affected limb [1]. However, combining FES with repetitive task practice of the affected limb may not be possible for those with severe impairments. Contralaterally controlled FES (ccFES), in which stroke patients use their unaffected hand to control the stimulation of the paretic limb, has been shown to improve hand function and does not require functional movements of the affected hand making it suitable for more impaired individuals. Though not explicitly tested, functional improvements due to ccFES are thought to be associated in part with the induction of increased cortical excitability via temporal correlation between peripheral and central neural activity [2]. In this case central activity refers to activation generated by voluntary contraction, however it also possible to generate central activation through non-invasive transcranial stimulation.
Paired associative stimulation (PAS) refers to the pairing of electrical peripheral nerve stimulation with stimulation of the motor cortex (M1) via transcranial magnetic stimulation (TMS) [3]. Repetitive pairs of stimuli delivered in a single session have been shown to result in changes in motor evoked potentials (MEPs) indicating modulation of corticospinal excitability (CSE). Directionality of modulation of CSE induced by PAS has been shown to depend on the inter-stimulus interval (ISI) between the peripheral stimulation and TMS pulse [4]. Importantly the induction of excitability via PAS has been shown to be at least partially preserved in the lesion hemisphere of patients post stroke [5]. To date the majority of PAS studies have investigated modulation of CSE while the targeted (for peripheral nerve stimulation) muscle is at rest [3, 5]. Studies combining PAS with voluntary activation of the targeted muscle indicate the addition of voluntary activation results in a greater increase in CSE and greater consistency of effects across subjects compared to PAS delivered at rest [6, 7].
The purpose of this study is to investigate the effects of PAS delivered during movement of the opposite hand on CSE of the hemisphere ipsilateral to the moving hand. Critical to the effective application of contralaterally coordinated (ccPAS) might be the timing of the stimuli relative to voluntary activation, as ipsilateral M1 excitability during a unilateral hand movement is known to vary with movement phase [8]. Therefore, we specifically investigated the impact of the timing of PAS delivery relative to the onset of contralateral hand opening on M1 excitability as measured by MEP amplitude in finger extensors of healthy individuals.
II. Methods
A. Electromyography (EMG) recording
Wireless surface electrodes (Trigno™ electrodes, Delsys Inc.) were placed over the left and right extensor digitorum communis (EDC) and flexor digitorum superficialis (FDS) muscles. EMG signals were amplified (x1000), band-pass filtered (10 – 300Hz), and digitized at a frequency of 1000 Hz. EMG signals were stored for further analysis to quantify the reaction time and MEP amplitude using custom-built MATLAB analysis software.
B. Stimulation
1). Peripheral electrical stimulation
The right EDC muscle was stimulated using a constant-current square-wave pulse of 1000μs duration (DS7A stimulator, Digitimer Ltd, Welwyn Garden City, UK) delivered through bipolar surface electrodes placed over the EDC muscle. The stimulation intensity was set at 300% of the perceptual threshold [3].
2). Neuronavigated TMS
To assure TMS precision, a canonical high-resolution anatomical MRI was co-registered with the subject’s head for frameless neuro-navigation. Throughout testing, the TMS coil (Magstim Rapid 2, Air Film) was held tangentially to the scalp with the handle posterior 45° off the sagittal plane. Following a rough mapping to determine the hotspot for the right EDC muscle in M1, the resting motor threshold (RMT) was defined as the minimum intensity required to elicit MEPs >50μV in the EDC muscle on 3 of 6 consecutive trials. The TMS intensity was then set throughout all sessions to be 120% of RMT intensity.
3). PAS
The PAS protocol that was implemented in all the study conditions comprised 240 pairs of peripheral electrical stimulation applied to the right EDC muscle followed by TMS pulse delivered to the contralateral (left) motor cortex M1 with the inter-stimulus interval of 25 ms [3]. The PAS stimulation rate was set to be 0.2 Hz based on previous evidence that this frequency is most effective for inducing potentiation of M1 [10].
C. Experimental protocol
Participants were seated in a comfortable reclining chair with their hands and forearms rested on the armrests. All subjects were instructed to remain relaxed and focus their attention on words on a screen placed in front of them. Following the determination of stimulation parameters, subjects performed a simple reaction time task (25 trials).Subjects were instructed to respond to a visual cue (“Move”) presented on the screen with immediate full extension of their left hand fingers (while minimizing activation of wrist muscles), hold the hand fully extended for about 1–2 seconds, then return to a relaxed posture at the appearance of a cue to “Relax”. Following baseline removal and rectifying, EMG envelopes were generated by a root mean square (RMS) filter. The reaction time (RT) was quantified as the EMG onset, calculated as the time point when EMG activity exceeded three standard deviations above baseline amplitude (taken as the mean of 1000 ms window prior to the “Move” cue). Additionally, all RTs were inspected visually to ensure an accurate detection.
For all conditions, PAS was administrated on the right resting hand (Fig. 1). In PAS at rest, subjects were instructed to relax their both hands at all time (no cues were shown). In conditions where PAS was combined with contralateral hand movement, subjects were engaged in an identical hand extension task to the one described for determination of the reaction time. Using the predetermined RT, PAS was either delivered in 1) the contralateral movement preparation phase (the TMS pulse in each trial preceded the subject’s mean RT by 100ms, PAS RT-100) or 2) the contralateral movement execution phase (the TMS pulse in each trial was delivered at 50ms following the subject’s mean RT, PAS RT+50). To assess changes in CSE, 20 MEPs were collected prior to (PRE) and directly following (POST) the PAS session.
Figure 1.
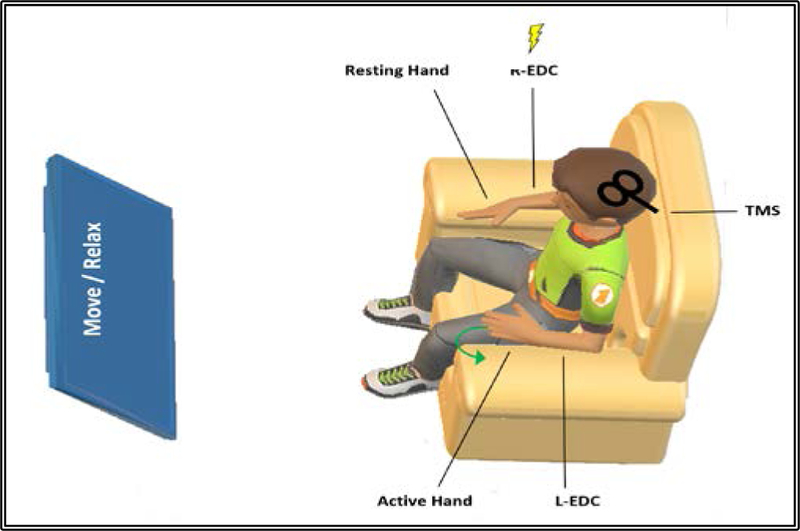
Experimental protocol: 240 pairs of peripheral electrical stimulation applied to the right resting EDC muscle (R-EDC) followed by TMS stimulation over the identified EDC hotspot at rate of 0.2 Hz. For PAS interventions that required left EDC (L-EDC) movements, participants were instructed to extend their left hand fingers upon the visual cue (Move), and then relax upon the visual cue (Relax).
D. Cortical excitability assessment
To evaluate changes in CSE in targeted EDC and untargeted FDS muscles, MEP amplitudes were calculated as the peak-to-peak amplitude of the EMG signal 20–50ms following the TMS pulse and averaged within collection blocks. Individual MEPs were excluded if the MEP amplitude exceeded three standard deviations of the block average [11].
E. Data analysis
To assess the induced PAS effect, we conducted a 3×2 repeated measure analysis of variance (ANOVA) using averaged MEP amplitudes as a dependent variable, separately for each of the two muscles. The ANOVA had within-subjects factors of Condition (PAS RT-100, PAS RT+50, Rest) and Time (PRE, POST), with α=0.05. If necessary, Greenhouse-Geisser method was used to correct for non-sphericity. Additionally, POST MEP values normalized to PRE, were analyzed with a one-way ANOVA with a within-subjects factor Condition to determine whether PAS-induced changes in MEP amplitude were significant. Post-hoc analysis was done using Holm-Bonferroni correction for multiple comparisons. All data are reported as the mean ± SEM.
III. Results
A. Participants
Following screening for TMS contraindications [9], eleven young, right-handed, healthy adults (6M, 5F; mean age 24.36 ± SD 2.29 years; range 22–30 years) were recruited and consented in accordance with the Institutional Review Boards of NJIT and Rutgers University. All participants completed all PAS sessions, which were assigned randomly and separated by one week to avoid any ordering or carry-over effects.
B. Session to session variability
To ensure stable and comparable cortical excitability baseline of subjects between sessions, one-way repeated measures ANOVA of the PRE MEPs with factor of Condition (PAS RT-100, PAS RT+50 and Rest) was conducted for each muscle individually. Results confirmed no effect of Condition in the EDC (F(2, 20) = 1.61, p = 0.23) or FDS muscle (F(2, 20) = 1.42, p = 0.26).
C. TMS pulse timing relative to the ipsilateral hand reaction time during PAS
The mean per-trial timing of the TMS pulse relative to the per-trial RT for the PAS sessions with ipsilateral hand movement was −84.3 ms (SD: 36.7) for ccPAS RT-100 condition and 48.4 ms (SD: 23.2) for the ccPAS RT+50 session. The mean RT during PAS sessions was 279.8 ms (SD: 21.8) for ccPAS RT-100 and 300.5 ms (SD: 31.6) for ccPAS RT+50.
D. Changes in MEPs amplitude following PAS
1). Changes due to PAS in the targeted right EDC muscle
The repeated measures ANOVA on the averaged MEP amplitudes revealed a significant Condition x Time interaction for the PAS targeted EDC muscle (F(2, 20) = 23.41, p = 0.001, η2 = 0.7) as well as a significant effect of Time (F(1, 10) = 19.42, p = 0.001, η2= 0.66) and no effect of Condition (F(2, 20) = 0.12, p = 0.89). Two-tailed paired t-tests were used to compare MEPs between PRE and POST (Fig. 3.A). There was a significant increase in the MEP amplitude for PAS RT+50 (46.57 ± 6.05%, t(10) = −4.369, p = 0.001). Conventional PAS at rest was also found to have a significant PRE to POST increase (29.26 ± 4.84%), t(10) = −5.97, p = 0.001). For PAS RT-100, the paired t-test showed a significant PRE to POST decrease in average MEP amplitude (−16.68 ± 5.91%, t(10) = 2.56, p = 0.03).
Figure 3.
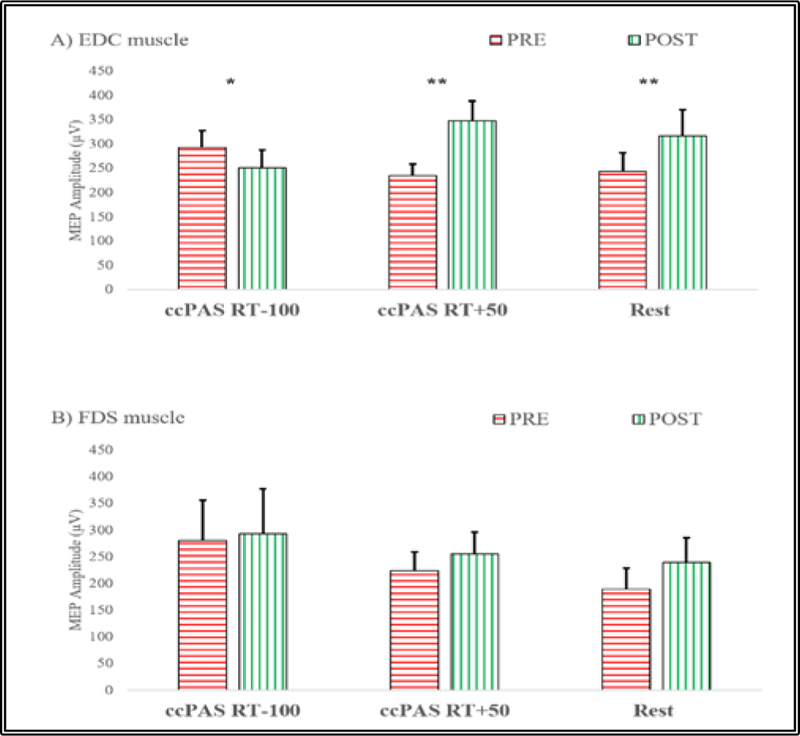
Effect of PAS on MEP amplitudes (mean±SEM) before (PRE) and after (POST) PAS sessions in: A) targeted right EDC muscle: MEP decrease was observed when the TMS was triggered 100 ms before the estimated movement onset in the left EDC while MEP increase was observed when TMS was delivered 50ms after the estimated movement onset in the left EDC as well as in the PAS_Rest condition. B) untargeted right FDS: no significant changes were observed. (Paired t-test, * denotes p<0.05, ** denotes p<0.01).
The repeated measures ANOVA of the POST MEPs normalized to baseline revealed a significant effect of Condition for the PAS-targeted EDC muscle (F(2, 20) = 44.36, p= 0.0005, η2 = 0.82). Paired t-test analyses of normalized MEP changes for PAS RT+50 and PAS RT-100 compared to PAS at Rest were conducted. PAS in the RT+50 condition induced a larger increase in CST excitability than PAS delivered at rest (t(10) = −2.42, p = 0.036), while CSE excitability decreased significantly after PAS in the RT-100 condition when compared to PAS at rest (t(10) = 5.99, p= 0.001).
2). Changes due to PAS in the untargeted right FDS muscle
A repeated measures ANOVA of the averaged MEP amplitudes in the right FDS muscle untargeted by PAS revealed a significant effect of Time (F(1, 10) =6.98, p=0.025, η2=0.41) (see Fig. 3.B), but no significant effect of Condition (F(2, 20) =0.94, p=0.41), or significant Condition x Time interaction (F(2, 20) =1.42, p=0.27). Paired t-tests to compare PRE to POST changes in each condition were not significant when corrected for multiple comparisons ccPAS RT-100 (t(10) = −1.06, p=0.31), ccPAS+50 (t(10) =−1.97, p=0.08) and PAS at rest (t(10) =−2.32, p=0.04).
IV. Discussion
Inducing cortical excitability changes in M1 using PAS can be a promising therapeutic intervention for stroke functional recovery [5]. The aim of this study was to determine the feasibility of inducing M1 excitability changes with ccPAS. This was investigated by examining the effect of delivering PAS stimuli during the preparation or execution phases of the contralateral hand movement on corticospinal excitability. Our results emphasize the key role of ipsilateral M1 activity during unilateral hand movements on PAS induced effects. Triggering PAS during the execution of a contralateral hand movement led to a robust increase of the cortical excitability in the stimulated M1 (ipsilateral to the moving hand). Compared to administrating PAS at rest, excitability changes in M1 were significantly higher. These PAS corticospinal excitability changes could resemble a long-term potentiation-like effect (LTP-like) [3]. Interestingly, although a well-known facilitatory PAS protocol (with a 25ms between peripheral and central stimulation) was implemented, when PAS was delivered during the preparation phase of a contralateral hand movement, a decrease in the M1 excitability was found. This may indicate a paradoxical induction of a long-term depression-like effects (LTD-like) usually associated with PAS protocols using 10ms ISI [4].
Possible neural mechanisms underlying these PAS-induced effects in the targeted M1 might be related to the temporal changes in the interhemispheric inhibition that is directed from right M1 towards left M1. Several TMS and fMRI studies have reported the significant role of the ipsilateral motor cortex during a contralateral hand movement. The co-activation of the ipsilateral motor cortex is believed to have a key role in processing and controlling the unilateral movement. It has been shown that the activation of the ipsilateral motor cortex during a unilateral hand movement is being affected by several factors, such as movement phase, rhythm, and contraction level and task complexity [12]. In a simple reaction-time task, the resting ipsilateral M1 activity assessed with TMS undergoes deep inhibition 80–120ms before the initiation of unilateral hand movement, while the contralateral M1 activity increased [8]. As the movement is initiated, MEPs of the ipsilateral hand were shown to be increased when the contralateral homologues muscle was voluntarily contracted [13]. Inhibition of the ipsilateral hemisphere during movement preparation followed by facilitation during execution may explain the effects seen in the current study. Further investigations are needed to assess the feasibility of ccPAS in chronic stroke patients as they demonstrate interhemispheric imbalance [14]. Resting and movement related power changes in cortical alpha and beta range oscillations have been previously linked to modulation of MEP amplitude [15]. Their role in ccPAS will be the subject of future investigations combining PAS and electroencephalography.
V. Conclusion
The findings of this feasibility study may have important implication for the use of PAS applied during movement of the contralateral limb as an adjuvant therapy for severely impaired stroke patients. Further investigation into the underlying mechanisms and optimal parameters for administration of ccPAS should be considered.
Figure 2.
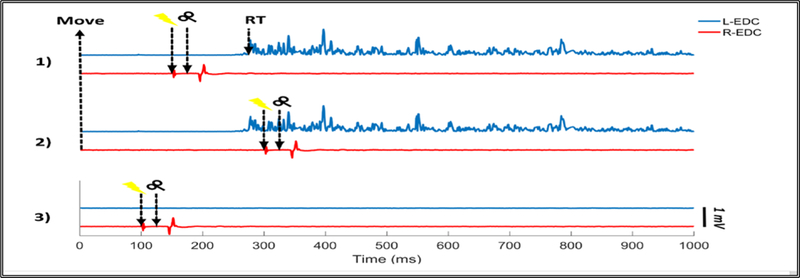
Stimulation timing relative to subject’s reaction time (RT) for one trial in: 1) ccPAS-100, in which TMS pulse was applied to the left ipsilateral M1 100 ms prior to the EMG onset of the left EDC muscle (L-EDC). 2) ccPAS+50, where TMS pulse was applied to the left M1 50 ms after the EMG onset of the L-EDC. 3) Conventional PAS, with both hands at rest. In each of the three conditions, peripheral electrical stimulation was applied 25 ms before the TMS pulse. Electrical stimulation artifact was reduced using template subtraction method.
Acknowledgments
*Research supported by King Saud University (AA), NIH grants F31NS092268 (MY), R01NS085122 (ET), R01HD58301 (SA) & by NIDILRR grant HHS90RE5021 (SA).
Contributor Information
Ahmad O. Alokaily, Department of Biomedical Engineering, New Jersey Institute of Technology, Newark, NJ, USA; Department of Biomedical Technology, King Saud University, Riyadh, Saudi Arabia (aoa45@njit.edu).
Mathew Yarossi, Department of Physical Therapy, Movement, and Rehabilitation Science and the Department of Electrical & Computer Engineering, Northeastern University, Boston, MA (m.yarossi@northeastern.edu)..
Gerard G. Fluet, School of Health Professions, Rutgers Biomedical Health Sciences, Newark, NJ, USA (fluetge@shp.rutgers.edu).
Eugene Tunik, Department of Physical Therapy, Movement, and Rehabilitation Science, and Department of Bioengineering, Northeastern University, Boston, MA (e.tunik@northeastern.edu)..
Sergei V. Adamovich, Department of Biomedical Engineering, New Jersey Institute of Technology, Newark, NJ, USA; School of Health Professions, Rutgers Biomedical Health Sciences, Newark, NJ, USA (sergei.adamovich@njit.edu)..
References
- [1].Dang B, Chen W, He W, & Chen G (2017). Rehabilitation Treatment and Progress of Traumatic Brain Injury Dysfunction. Neural plasticity, 2017. [DOI] [PMC free article] [PubMed]
- [2].Knutson JS, Harley MY, Hisel TZ, Hogan SD, Maloney MM, & Chae J (2012). Contralaterally controlled functional electrical stimulation for upper extremity hemiplegia: an early-phase randomized clinical trial in subacute stroke patients. Neurorehabilitation and neural repair, 26(3), 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stefan K, Kunesch E, Cohen LG, Benecke R, & Classen J (2000). Induction of plasticity in the human motor cortex by paired associative stimulation. Brain, 123(3), 572–584. [DOI] [PubMed] [Google Scholar]
- [4].Carson RG, & Kennedy NC (2013). Modulation of human corticospinal excitability by paired associative stimulation. Frontiers in human neuroscience, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Castel-Lacanal E, Marque P, Tardy J, de Boissezon X, Guiraud V, Chollet F & Simonetta-Moreau M (2009). Induction of cortical plastic changes in wrist muscles by paired associative stimulation in the recovery phase of stroke patients. Neurorehabilitation and neural repair, 23(4), 366–372. [DOI] [PubMed] [Google Scholar]
- [6].Mrachacz-Kersting N, Fong M, Murphy BA, & Sinkjaer T (2007). Changes in excitability of the cortical projections to the human tibialis anterior after paired associative stimulation. Journal of neurophysiology, 97(3), 1951–1958. [DOI] [PubMed] [Google Scholar]
- [7].Kujirai K, Kujirai T, Sinkjaer T, & Rothwell JC (2006). Associative plasticity in human motor cortex during voluntary muscle contraction. Journal of neurophysiology, 96(3), 1337–1346. [DOI] [PubMed] [Google Scholar]
- [8].Leocani L, Cohen LG, Wassermann EM, Ikoma K, & Hallett M (2000). Human corticospinal excitability evaluated with transcranial magnetic stimulation during different reaction time paradigms. Brain, 123(6), 1161–1173. [DOI] [PubMed] [Google Scholar]
- [9].Rossi S, Hallett M, Rossini PM, & Pascual-Leone A (2011). Screening questionnaire before TMS: an update. Clinical Neurophysiology, 122(8), 1686. [DOI] [PubMed] [Google Scholar]
- [10].Wischnewski M, & Schutter DJ (2016). Efficacy and time course of paired associative stimulation in cortical plasticity: Implications for neuropsychiatry. Clinical Neurophysiology, 127(1), 732–739. [DOI] [PubMed] [Google Scholar]
- [11].Yarossi M, Manuweera T, Adamovich SV, & Tunik E (2017). The Effects of Mirror Feedback during Target Directed Movements on Ipsilateral Corticospinal Excitability. Frontiers in human neuroscience, 11, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Beaulé V, Tremblay S, & Théoret H (2012). Interhemispheric control of unilateral movement. Neural plasticity, 2012. [DOI] [PMC free article] [PubMed]
- [13].Stedman A, Davey NJ, & Ellaway PH (1998). Facilitation of human first dorsal interosseous muscle responses to transcranial magnetic stimulation during voluntary contraction of the contralateral homonymous muscle. Muscle & nerve, 21(8), 1033–1039. [DOI] [PubMed] [Google Scholar]
- [14].Dodd KC, Nair VA, & Prabhakaran V (2017). Role of the Contralesional vs. Ipsilesional Hemisphere in Stroke Recovery. Frontiers in human neuroscience, 11, 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Karabanov A, Thielscher A, & Siebner HR (2016). Transcranial brain stimulation: closing the loop between brain and stimulation. Current opinion in neurology, 29(4), 397. [DOI] [PMC free article] [PubMed] [Google Scholar]


