HEARING LOSS IN ADULTS IS ENCOUNTERED IN ALL MEDICAL SETTINGS and frequently influences medical encounters. This disorder constitutes a substantial burden on the adult population in the United States, yet screening for hearing loss is not routine,1 and treatments are often inaccessible because of the high cost or perceived ineffectiveness.
BURDEN OF HEARING LOSS
The Global Burden of Disease Study2 measured years lived with disability and found that hearing loss is the fourth leading cause of disability globally. In the United States, the prevalence of hearing loss doubles with every 10-year increase in age. Approximately half of persons in their seventh decade (60 to 69 years of age)3 and 80% who are 85 years of age or older4 have hearing loss that is severe enough to affect daily communication. Because of the aging population in this and other developed countries, hearing loss is likely to become an increasingly prevalent disability.
The primary effect of adult hearing loss is impaired communication, which can adversely affect relationships with family and friends and create difficulties in the workplace. Untreated hearing loss in adults also has indirect health, psychosocial, and economic effects and leads to social isolation and a reduced quality of life.5–7 As compared with age-matched adults with unimpaired hearing, older persons with hearing loss have higher rates of hospitalization,8 death,9,10 and falls and frailty,11,12 as well as higher rates of dementia13–15 and depression,16,17 even when known risks for these disorders are taken into account.13 On a societal level, because of their hearing loss, persons with hearing loss achieve significantly lower levels of education than do those with normal hearing; they also have higher levels of unemployment or underemployment and lower levels of income than those with normal hearing.18,19 Annual health care costs for middle-age U.S. adults with hearing loss are significantly higher than the costs of care for those without hearing loss.19
TYPES OF HEARING LOSS
Peripheral hearing loss is typically categorized as conductive (caused by impairment of the outer or middle ear) or sensorineural (caused by dysfunction in the cochlea or spiral ganglion). Hearing loss that has both conductive and sensorineural components is categorized as mixed. Conductive hearing loss results from obstruction or disease of the outer or middle ear that prevents transmission of sound energy to the inner ear. The causes of conductive hearing loss range from cerumen impaction and otitis media to fixation of one or more of the middle-ear bones, mainly fixation of the stapes due to otosclerosis. Medical or surgical treatment of most types of conductive hearing loss often results in full restoration of hearing.
ASSESSMENT OF HEARING
Audiologic testing is performed to assess hearing thresholds across the range of frequencies that are important for human communication. Auditory thresholds are typically measured for air- and bone-conducted pure-tone stimuli in order to differentiate conductive from sensorineural hearing loss and to characterize the pattern of hearing loss at various frequencies. The term “threshold shift” refers to a change in hearing thresholds between sequential audiologic tests; it may reflect improvement or worsening of hearing. Testing the perception of speech signals of low redundancy (monosyllabic words) presented at a comfortable listening level in the absence of background noise is another method of assessing hearing in adults.
The broad term “sensorineural hearing loss” has been used by clinicians because, until recently, diagnostic tests could not determine whether a lesion was in the sensory or the neural portion of the peripheral auditory system. This distinction is now made by measuring optoacoustic emissions, performed by simultaneously presenting tones of different frequencies and sound pressures to the external canal and detecting sound emissions from the cochlea itself; the results reflect the functioning of outer hair cells of the cochlea. Auditory brain-stem responses also test the neural component of audition by recording synchronous neural activity of the auditory nerve and auditory brain-stem nuclei.
STRUCTURES OF THE EAR INVOLVED IN HEARING LOSS
The peripheral auditory system consists of the outer ear, the middle ear, and the inner ear (cochlea); the inner ear contains the mechanosensory hair cells that convert sound energy into neural signals (Fig. 1). Cochlear hair cells are innervated by neurons of the spiral ganglion, which project centrally to the auditory nuclei of the brain stem through the auditory nerve. Sensory hair cells are susceptible to damage from a variety of stresses, and since hair cells in the mammalian cochlea are not regenerated after they are lost, the resulting hearing loss is permanent. In many cases, the death of hair cells results in degeneration of spiral ganglion neurons, which complicates the treatment of hearing loss with cochlear implants because these devices directly stimulate the spiral ganglion neurons.
Figure 1. Diseases Affecting the Auditory System.
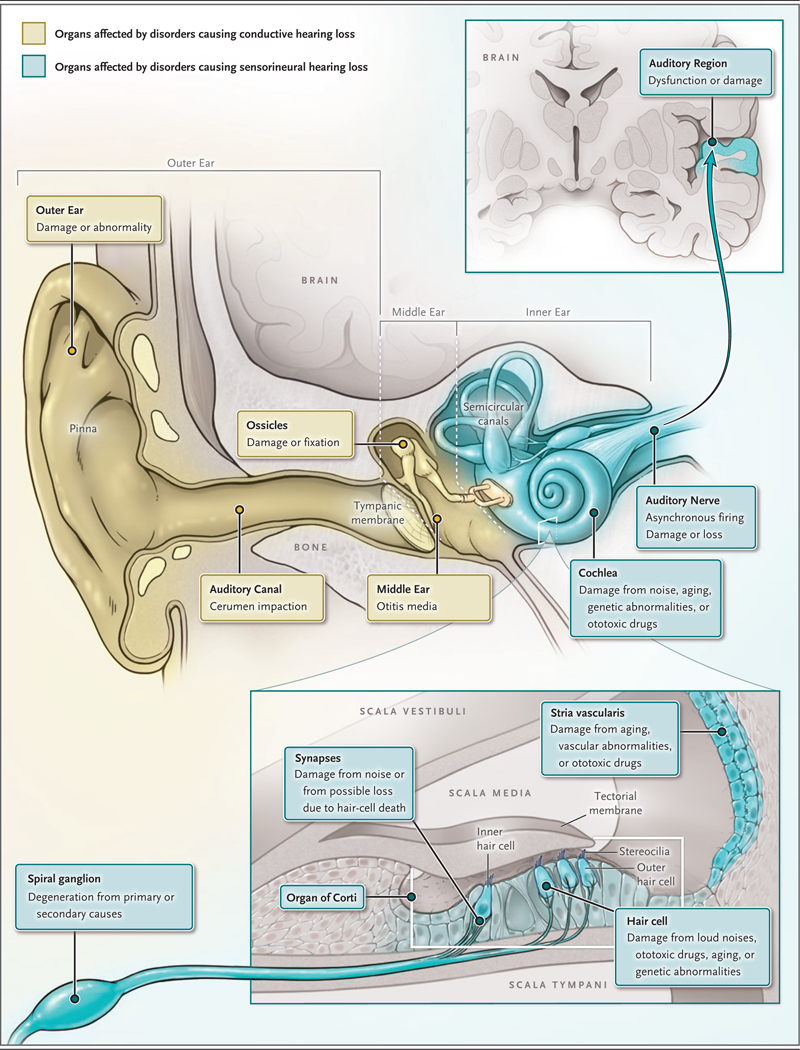
Shown are the auditory system and diseases affecting each component of the system, including the ossicular chain of the middle ear, the cochlea, the organ of Corti and hair cells, and the auditory nerve.
Sensory hearing loss is the result of damage to the organ of Corti (the sensory organ within the cochlea that houses the hair cells) or the stria vascularis, a portion of the inner ear that provides metabolic support for the organ of Corti and generates the electrochemical (endocochlear) potential that is the driving force for transduction of sound by sensory hair cells.20 In contrast, neural hearing loss is the result of loss or dysfunction of spiral ganglion neurons or of more proximal auditory structures. Auditory neuropathy is characterized by normal (or nearnormal) sensory hair-cell function coupled with abnormal neural responses and, usually, poorer word recognition than is typical in sensory hearing loss18,21; this type of hearing loss is caused by damage to the synapses that connect hair cells to spiral ganglion neurons or to asynchronous neural firing within the auditory nerve. For this reason, patients with auditory neuropathy may benefit less from hearing aids than those with sensory hearing loss.
CAUSES OF HEARING LOSS
Hearing loss can be caused by damage to any portion of the peripheral and central auditory systems. The main causes of sensorineural hearing loss are degenerative processes associated with aging, genetic mutations, noise exposure, exposure to therapeutic drugs that have ototoxic side effects, and chronic conditions.
AGE-RELATED DEGENERATIVE PROCESSES
The leading cause of adult onset hearing loss is he effects of aging on the auditory system. Hearing loss in older persons is caused not only by the degenerative effects of aging on the cochlea but also by the accumulated effects of exposure to noise and ototoxic drugs. Age-related hearing loss (presbycusis) is usually bilateral and symmetric and is most pronounced at higher frequencies (≥2000 Hz).19 A prominent feature of this type of hearing loss is reduction in the ability to understand speech,22,23 even if the sounds are loud enough. Sensory presbycusis refers to death or damage of the cochlear sensory hair cells with aging, and metabolic presbycusis refers to decreased functioning of the stria vascularis through age-related mechanisms that have not been fully determined.24,25
GENETIC MUTATIONS
More than 100 genes have known mutations that result in hearing loss that is not associated with disorders of other organs or with dysmorphic features (nonsyndromic hearing loss). Mutations in approximately 30 of these genes are associated with adult-onset or progressive hearing loss that is inherited as an autosomal dominant trait.26 In addition, more than 500 syndromes that include hearing loss have been described. Hereditary hearing loss is relatively common among newborns, affecting approximately 1 in 1000 live births,27 but it is difficult to estimate the heritability of adult-onset hearing loss, since genetic and environmental factors are not easily separable. Estimates of the heritability of adult-onset hearing loss range from 25 to 55%.28 The majority of monogenetic causes of hearing loss involve mutations in genes that are required for normal functioning of the cochlea, and several of these genes specifically affect the functioning of sensory hair cells.29 In addition, the genetics of susceptibility to age-related and noise-induced hearing loss are beginning to be understood.30 The identification of genetic modifiers that enhance or reduce susceptibility to acquired hearing loss will be important for the development of therapies to preserve hearing.
NOISE EXPOSURE
Approximately 104 million people in the United States are exposed to levels of noise that can cause hearing loss,31 and 1 in 4 adults in the United States has measurable hearing loss caused by exposure to harmful noise.32 Even among people who report that their hearing is “excellent” or “good,” nearly 20% have audiometric evidence of noise-induced hearing loss.32 Workplaces such as factories and certain jobs in the military are associated with exposure to high noise levels; however, even people who do not work in these environments have a risk of noise exposure in daily life that they often underestimate. Loud sounds are pervasive in modern life, and noise exposure can occur in a variety of seemingly innocuous settings, such as concerts, movie theaters, and fitness classes with loud music, and through engagement in a range of activities, such as listening to music at home, participating in power sports (e.g., those involving motorcycles, all-terrain vehicles, speed boats, or snowmobiles), shooting, and using power tools.
Noise damages the sensory hair cells of the inner ear through the direct mechanical stress of intense sound pressure and by activation of stress-induced molecular pathways, including generation of reactive oxygen species and calcium overload.33 Noise-induced hearing loss can be temporary or permanent, depending on the intensity and duration of exposure. The term “temporary threshold shift” is used to describe objective changes in hearing acuity that can be measured on audiometry immediately after an episode of exposure to loud sounds (e.g., attendance at a concert) and that revert to preexposure levels after a few days to 2 weeks. Temporary threshold shifts are characterized subjectively by decreased hearing sensitivity, a feeling of fullness in the ears, tinnitus (ringing), and a perception that sounds are muffled. Prolonged or repeated exposure to noise can cause the death of sensory hair cells and permanent hearing loss, referred to as a “permanent threshold shift.” Death of hair cells can be followed by a slower loss of spiral ganglion neurons over a period of months or years.34
In the past, temporary threshold shifts were not thought to be associated with permanent damage to the auditory system; however, recent data indicate that noise exposures that result in temporary threshold shifts may cause permanent damage to the cochlea. In animal models, noise exposures that result in temporary threshold shifts lead to permanent loss of hair-cell ribbon synapses,34,35 specialized synaptic structures that release neurotransmitters from hair cells in response to sound. Although our understanding of this type of synaptic loss is incomplete, it has implications for hearing function and for recommendations regarding safe noise levels.36 It is not known whether synaptic loss is necessarily permanent, since in some animal models, there is partial recovery of the functioning of these structures.37,38 A reliable, noninvasive clinical measure of cochlear synaptic damage has not been developed, although this is an area of active research.39–41
EXPOSURE TO THERAPEUTIC DRUGS
Various chemicals and drugs adversely affect the auditory system; the main ones in clinical use are aminoglycoside antibiotics and cisplatin, both of which are toxic to sensory hair cells. Hearing loss develops in approximately 20% of patients receiving aminoglycosides,42,43 and the prevalence is as high as 56% among patients with cystic fibrosis,44,45 a population exposed to repeated courses of aminoglycoside therapy. Among adults who have received cisplatin, clinically significant hearing loss develops in approximately 60% of patients with testicular cancer46 and 65% of patients with head and neck cancer.47 Susceptibility to cisplatin-induced hearing loss depends on the cumulative dose of the drug, the age of the patient (children are more susceptible than adults), and status with respect to concurrent cranial irradiation.48 Patients who have severe hearing loss caused by ototoxic drugs are likely to be identified and referred for follow-up auditory testing, but many more patients with mild-to- moderate drug-induced hearing loss are not identified and hence do not receive treatment for their hearing loss.46
SMOKING, ADIPOSITY, AND CHRONIC DISEASES
Strong associations between hearing loss and cigarette smoking, adiposity, diabetes mellitus, and other risk factors for cardiovascular disease are supported by epidemiologic studies, but causality remains uncertain. For example, in the Beaver Dam Eye Study, involving persons between the ages of 43 and 84 years, smoking, central adiposity, and poorly controlled diabetes mellitus were associated with hearing loss in later life, suggesting that vascular changes may contribute to age-related hearing loss.49 On the basis of data from the National Health and Nutrition Examination Survey for the years 1999 through 2004, a study of patients with diabetes who were 20 to 69 years of age showed that low-density and high-density lipoprotein levels, as well as status with respect to coronary disease, peripheral neuropathy, and general health, were correlated with hearing impairment, whereas glycemic control, number of years since diagnosis, and type of hypoglycemic medication were not.50 These putative associations with chronic systemic diseases suggest that some contributors to hearing loss may be modifiable.
Autoimmune forms of ear disease are characterized by progressive, fluctuating hearing loss with a variable time course. Although typically bilateral, symptoms may be more prominent in one ear, and one possible cause of sudden, unilateral sensorineural hearing loss is an autoimmune disorder. Ear disease may be associated with rheumatoid arthritis, systemic lupus erythematosus, Cogan’s syndrome, sarcoidosis, or other autoimmune disorders.51 Glucocorticoids are the main treatment and are often used for long periods. The complications of this prolonged treatment led to a trial in which prednisone and methotrexate were compared with prednisone and placebo; the trial failed to show a benefit from the addition of methotrexate.52
SUDDEN HEARING LOSS
The prevalence of sudden, idiopathic hearing loss is 5 to 20 cases per 100,000 population, with approximately 4000 new cases per year in the United States. Sudden sensorineural hearing loss, defined as the onset of hearing loss over a period of 72 hours or less in one or both ears, is considered an otologic emergency because of evidence, albeit uncertain, that there is a benefit of early treatment with glucocorticoids. The audiometric criterion for diagnosis is a decrease in hearing of at least 30 dB, affecting at least three sound frequencies. The cause of sudden hearing loss is not known but is presumed to be viral, vascular, or autoimmune. The diagnostic workup involves testing to exclude identifiable causes of sudden hearing loss, such as acoustic neuroma and rare tumors of the internal auditory canal.
A clinical practice guideline from the American Academy of Otolaryngology-Head and Neck Surgery53 recommends that physicians evaluate patients presenting with a sudden onset of hearing loss in order to distinguish between sensorineural and conductive impairment, initially with a physical examination and tuning-fork tests, followed by audiometry when possible. Although high doses of oral glucocorticoids (typical dose, 1 mg per kilogram of body weight) and of intra-tympanic glucocorticoids have been used in the treatment of sudden, idiopathic hearing loss, spontaneous recovery in many patients and the lack of data from placebo-controlled trials make these treatment recommendations tentative. Nevertheless, most otolaryngologists recommend some form of glucocorticoid treatment for sudden hearing loss.53
PREVENTION OF HEARING LOSS
An epidemiologic study54 has shown that the age-adjusted prevalence of hearing loss is declining in the United States. Possible explanations for this decrease include a reduction in exposure to occupational noise as a result of fewer manufacturing jobs and more widespread use of hearing protection, cessation of smoking, and better management of cardiovascular risk factors. Although the prevalence of hearing loss increases with age, it is not inevitable. Adults should be made aware of the potentially damaging and slowly cumulative effects of exposure to loud sounds such as the common sources of exposure noted above. Hearing protection includes using ear protectors (earmuffs or earplugs), avoiding or limiting time spent in loud venues, and using personal music systems at moderate volumes, and wearing noise-canceling headphones or earphones.
Despite the high prevalence of hearing loss among older adults, routine auditory screening is not currently recommended for asymptomatic persons. A review of data on primary care screening for hearing loss, performed for the U.S. Preventive Services Task Force,1 concluded that additional research is needed to understand the potential effects of such screening on health outcomes but acknowledged that common screening tests, such as validated hearing-Ioss questionnaires and the whisper, finger-rub, or watch-tick test, or screening audiometry55 can identify patients at risk for hearing loss. Primary care physicians are often the first to suspect and identify hearing loss, and they can play a role in referring patients for hearing evaluation and management.56
TREATMENT OF SENSORINEURAL HEARING LOSS
Despite increasing knowledge of the biology of the inner ear, hearing restoration is not currently available for most cases of hearing loss.57 A review of novel therapies included 22 active clinical drug trials registered on the National Institutes of Health ClinicalTrials.gov website.58 Most of these proposed treatments address cell-death pathways and mitigate the effects of oxidative stressors on inner-ear hair cells. Commercially sponsored trials of antibiotic and chemotherapeutic medications for the treatment or prevention of ototoxicity are planned or are under way (e.g., ClinicalTrials.gov number, NCT02819856). One study uses a viral vector to deliver gene therapy to the inner ear.59,60 Pre-clinical studies in animal models suggest that viral-vector gene therapy may be valuable in treating monogenetic hereditary hearing loss. Restoration or partial restoration of hearing and balance functions have been achieved with the use of gene therapy in mouse models of human deafness, including models of some forms of the Usher syndrome.61–66
HEARING AIDS AND OTHER DEVICES
A hearing aid is defined by the Food and Drug Administration (FDA) as a “wearable sound- amplifying device that is intended to compensate for impaired hearing.” The goal of treatment with well-fitted hearing aids is to improve the audibility of even soft speech or music and other sounds while ensuring that sounds do not become uncomfortably loud. Hearing aids can be sophisticated instruments with a variety of customizable features that contribute to their high costs; whether performance improves with higher-cost devices is uncertain.67 A variety of noncustomized devices, termed “hearing assistive technologies,” are also available; they include amplified telephones, visual technologies such as captioning, video conferencing, and visual or vibrotactile alerts.68 Hearing aids are regulated by the FDA, and state laws may restrict access to them. The devices are sold through audiologists and hearing-aid dispensers. The costs of services related to hearing-aid fitting are often bundled with the cost of the device, so the specific costs of the services and the technology are not transparent.68
The frequency of use of hearing aids by adults with hearing loss is low.68 In a survey published in 2012, only 14.2% of adults with hearing loss reported wearing hearing aids.69 Although the cost of the devices, typically $1,400 to $2,200, is probably a factor, other deterrents to the adoption of hearing aids include stigma, perceived ineffectiveness, ongoing costs (for batteries and maintenance), lack of comfort, and cosmetic appearance.68 The United States is one of the few developed countries that does not offer government assistance for the purchase of hearing devices.68 However, even in countries where assistance is provided, hearing-aid use is not universal among candidates; for example, the rate of use is less than 15% in Finland and is 50% in Denmark.68 Efforts to increase use will therefore have to address multiple impediments.
COCHLEAR IMPLANTS
Persons with severe or total sensorineural hearing loss do not typically benefit from hearing aids, since inner-ear hair cells are not able to stimulate the auditory nerve in response to sound. In such cases, cochlear implants, which are surgically implanted devices that bypass the cochlear hair cells to electrically stimulate the auditory nerve, permit partial restoration of hearing and have been shown to improve speech perception and vocational, social, and psychological functioning,70–72 as well as the quality of life for adults, including older adults.73 Details of the function and use of cochlear implants can be found at https://www.nidcd.nih.gov/health/cochlear-implants.
The cost-effectiveness of cochlear implants has been established in developed countries71 and in some developing countries.71,74–77 These analyses have evaluated the lifetime costs of cochlear implants, including the costs of the device and of surgery and rehabilitation, versus the benefits, as determined on the basis of health-preference measures. Measures of cost-effectiveness have also been used to compare cochlear implants with other treatments, such as educational programs for deaf persons, and these assessments have shown that cochlear implants compare favorably with other treatments.
INCREASING ACCESS TO HEARING AIDS
Hearing loss is increasingly being viewed as a public health problem.68 In October 2015, the President’s Council of Advisors on Science and Technology recommended that the FDA create a new regulatory class for hearing aids that can be sold over the counter for persons with mild or moderate hearing loss.78 This recommendation was endorsed by the National Academies of Sciences, Engineering, and Medicine in their report titled “Hearing Health Care for Adults: Priorities for Improving Access and Affordability,” released in June 2016.68 They recommended that the FDA create a category of over-the-counter, wearable hearing devices that would be regulated to meet specific safety and quality standards and labeling specifications; the new FDA classification would preempt current state laws and regulations in order not to limit access to affordable hearing aids. Legislation has recently been signed into law that requires the FDA to create and regulate a category of over-the-counter hearing aids for adults who have mild to moderate hearing loss.79,80 Opening the market to these devices should increase the options available to patients, decrease costs, and increase access.81 Bulk purchasing by government agencies provides another opportunity to decrease costs. The Department of Veterans Affairs, for example, purchased approximately 20% of hearing aids on the U.S. market in 2013,82 at an average cost of $369 per hearing aid as compared with $1,400 to $2,200 on the open market.68
CONCLUSIONS
Hearing loss is a major source of disability in adults, associated with serious communication and psychosocial problems and high health care costs, with economic implications at the societal and individual levels. Technologies exist to ameliorate hearing loss, but cost, health policies, and regulations limit access to these therapies. Efforts are under way to improve access to auditory health care for adults. Recent advances in our understanding of the underlying causes of hearing loss have led to efforts to develop drugs and therapies that can prevent or reverse hearing loss.
Acknowledgments
Dr. Tucci reports receiving consulting fees and travel support from Roche Pharmaceuticals, consulting fees from Otic Pharma, and consulting fees from Otonomy and serving as chair of a data and safety monitoring board for Otonomy.
Footnotes
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Contributor Information
Lisa L. Cunningham, Section on Sensory Cell Biology, National Institute on Deafness and Other Communication Disorders, Bethesda, MD
Debara L. Tucci, Division of Head and Neck Surgery and Communication Sciences, Duke University Medical Center, Durham, NC
REFERENCES
- 1.Chou R, Dana T, Bougatsos C, Fleming C, Beil T. Screening for hearing loss in adults ages 50 years and older: a review of the evidence for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Health Policy and Research, 2011. [PubMed] [Google Scholar]
- 2.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388:1545–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and differences by demographic characteristics among US adults: data from the National Health and Nutrition Examination Survey, 1999–2004. Arch Intern Med 2008;168:1522–30. [DOI] [PubMed] [Google Scholar]
- 4.Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci 2011;66:582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bainbridge KE, Wallhagen MI. Hearing loss in an aging American population: extent, impact, and management. Annu Rev Public Health 2014;35:139–52. [DOI] [PubMed] [Google Scholar]
- 6.Kamil RJ, Lin FR. The effects of hearing impairment in older adults on communication partners: a systematic review. J Am Acad Audiol 2015;26:155–82. [DOI] [PubMed] [Google Scholar]
- 7.Mick P, Kawachi I, Lin FR. The association between hearing loss and social isolation in older adults. Otolaryngol Head Neck Surg 2014;150:378–84. [DOI] [PubMed] [Google Scholar]
- 8.Genther DJ, Frick KD, Chen D, Betz J, Lin FR. Association of hearing loss with hospitalization and burden of disease in older adults. JAMA 2013;309:2322–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Contrera KJ, Betz J, Genther DJ, Lin FR. Association of hearing impairment and mortality in the National Health and Nutrition Examination Survey. JAMA Otolaryngol Head Neck Surg 2015;141:944–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fisher D, Li CM, Chiu MS, et al. Impairments in hearing and vision impact on mortality in older people: the AGES- Reykjavik Study. Age Ageing 2014;43:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin FR, Ferrucci L. Hearing loss and falls among older adults in the United States. Arch Intern Med 2012;172:369–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Viljanen A, Kaprio J, Pyykko I, et al. Hearing as a predictor of falls and postural balance in older female twins. J Gerontol A Biol Sci Med Sci 2009;64:312–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin FR, Metter EJ, O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol 2011;68:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin FR, Yaffe K, Xia J, et al. Hearing loss and cognitive decline in older adults. JAMA Intern Med 2013;173:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallacher J, Ilubaera V, Ben-Shlomo Y, et al. Auditory threshold, phonologic demand, and incident dementia. Neurology 2012;79:1583–90. [DOI] [PubMed] [Google Scholar]
- 16.Li CM, Zhang X, Hoffman HJ, Cotch MF, Themann CL, Wilson MR. Hearing impairment associated with depression in US adults, National Health and Nutrition Examination Survey 2005–2010. JAMA Otolaryngol Head Neck Surg 2014;140: 293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mener DJ, Betz J, Genther DJ, Chen D, Lin FR. Hearing loss and depression in older adults. J Am Geriatr Soc 2013;61: 1627–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starr A, Rance G. Auditory neuropathy. Handb Clin Neurol 2015;129:495–508. [DOI] [PubMed] [Google Scholar]
- 19.Allen PD, Eddins DA. Presbycusis phenotypes form a heterogeneous continuum when ordered by degree and configuration of hearing loss. Hear Res 2010; 264:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wangemann P Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol 2006;576:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hood LJ. Auditory neuropathy/dyssynchrony disorder: diagnosis and management. Otolaryngol Clin North Am 2015;48:1027–40. [DOI] [PubMed] [Google Scholar]
- 22.van Rooij JC, Plomp R. Auditive and cognitive factors in speech perception by elderly listeners. III. Additional data and final discussion. J Acoust Soc Am 1992; 91:1028–33. [DOI] [PubMed] [Google Scholar]
- 23.Dubno JR, Lee FS, Matthews LJ, Ahlstrom JB, Horwitz AR, Mills JH. Longitudinal changes in speech recognition in older persons. J Acoust Soc Am 2008;123: 462–75. [DOI] [PubMed] [Google Scholar]
- 24.Dubno JR, Eckert MA, Lee FS, Matthews LJ, Schmiedt RA. Classifying human audiometric phenotypes of age-related hearing loss from animal models. J Assoc Res Otolaryngol 2013;14:687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuknecht HF, Gacek MR. Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol 1993;102:1–16. [DOI] [PubMed] [Google Scholar]
- 26.Griffith AJ, Friedman TB. Hereditary hearing loss In: Wackym PA, Snow JB Jr, eds. Ballenger’s otorhinolaryngology head and neck surgery. 18th ed. Shelton, CT: People’s Medical Publishing House, 2017: 329–45. [Google Scholar]
- 27.Morton NE. Genetic epidemiology of hearing impairment. Ann N Y Acad Sci 1991;630:16–31. [DOI] [PubMed] [Google Scholar]
- 28.Christensen K, Frederiksen H, Hoffman HJ. Genetic and environmental influences on self-reported reduced hearing in the old and oldest old. J Am Geriatr Soc 2001;49:1512–7. [DOI] [PubMed] [Google Scholar]
- 29.Richardson GP, de Monvel JB, Petit C. How the genetics of deafness illuminates auditory physiology. Annu Rev Physiol 2011;73:311–34. [DOI] [PubMed] [Google Scholar]
- 30.Friedman RA, Van Laer L, Huentel-man MJ, et al. GRM7 variants confer susceptibility to age-related hearing impairment. Hum Mol Genet 2009;18:785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hammer MS, Swinburn TK, Neitzel RL. Environmental noise pollution in the United States: developing an effective public health response. Environ Health Perspect 2014;122:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carroll YI, Eichwald J, Scinicariello F, et al. Vital signs: noise-induced hearing loss among adults — United States 2011–2012. MMWR Morb Mortal Wkly Rep 2017;66:139–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sha SH, Schacht J. Emerging therapeutic interventions against noise-induced hearing loss. Expert Opin Investig Drugs 2017;26:85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci 2006;26:2115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kujawa SG, Liberman MC. Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 2009;29:14077–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liberman MC, Kujawa SG. Cochlear synaptopathy in acquired sensorineural hearing loss: manifestations and mechanisms. Hear Res 2017;349:138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu L, Wang H, Shi L, et al. Silent damage of noise on cochlear afferent innervation in guinea pigs and the impact on temporal processing. PLoS One 2012; 7(11):e49550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi LJ, Chang Y, Li XW, Aiken SJ, Liu LJ, Wang J. Coding deficits in noise-induced hidden hearing loss may stem from incomplete repair of ribbon synapses in the cochlea. Front Neurosci 2016;10: 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liberman MC, Epstein MJ, Cleveland SS,Wang H, Maison SF. Toward a differential diagnosis of hidden hearing loss in humans. PLoS One 2016;11(9):e0162726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehraei G, Hickox AE, Bharadwaj HM, et al. Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. J Neurosci 2016;36:3755–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hickox AE, Larsen E, Heinz MG, Shinobu L, Whitton JP. Translational issues in cochlear synaptopathy. Hear Res 2017; 349:164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol 2000;5:3–22. [DOI] [PubMed] [Google Scholar]
- 43.Duggal P, Sarkar M. Audiologic monitoring of multi-drug resistant tuberculosis patients on aminoglycoside treatment with long term follow-up. BMC Ear Nose Throat Disord 2007;7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garinis AC, Cross CP, Srikanth P, et al. The cumulative effects of intravenous antibiotic treatments on hearing in patients with cystic fibrosis. J Cyst Fibros 2017;16: 401–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Al-Malky G, Dawson SJ, Sirimanna T, Bagkeris E, Suri R. High-frequency audiometry reveals high prevalence of aminoglycoside ototoxicity in children with cystic fibrosis. J Cyst Fibros 2015;14:248–54. [DOI] [PubMed] [Google Scholar]
- 46.Frisina RD, Wheeler HE, Fossa SD, et al. Comprehensive audiometric analysis of hearing impairment and tinnitus after cisplatin-based chemotherapy in survivors of adult-onset cancer. J Clin Oncol 2016;34:2712–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Theunissen EA, Zuur CL, Bosma SC, et al. Long-term hearing loss after chemo-radiation in patients with head and neck cancer. Laryngoscope 2014;124:2720–5. [DOI] [PubMed] [Google Scholar]
- 48.Lambert EM, Gunn GB, Gidley PW. Effects of radiation on the temporal bone in patients with head and neck cancer. Head Neck 2016;38:1428–35. [DOI] [PubMed] [Google Scholar]
- 49.Cruickshanks KJ, Nondahl DM, Dalton DS, et al. Smoking, central adiposity, and poor glycemic control increase risk of hearing impairment. J Am Geriatr Soc 2015;63:918–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bainbridge KE, Hoffman HJ, Cowie CC. Risk factors for hearing impairment among U.S. adults with diabetes: National Health and Nutrition Examination Survey 1999–2004. Diabetes Care 2011;34:1540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mijovic T, Zeitouni A, Colmegna I. Autoimmune sensorineural hearing loss: the otology-rheumatology interface. Rheumatology (Oxford) 2013;52:780–9. [DOI] [PubMed] [Google Scholar]
- 52.Harris JP, Weisman MH, Derebery JM, et al. Treatment of corticosteroid-responsive autoimmune inner ear disease with methotrexate: a randomized controlled trial. JAMA 2003;290:1875–83. [DOI] [PubMed] [Google Scholar]
- 53.Stachler RJ, Chandrasekhar SS, Archer SM, et al. Clinical practice guideline: sudden hearing loss. Otolaryngol Head Neck Surg 2012;1463 Suppl:S1–S35. [DOI] [PubMed] [Google Scholar]
- 54.Hoffman HJ, Dobie RA, Losonczy KG, Themann CL, Flamme GA. Declining prevalence of hearing loss in US adults aged 20 to 69 years. JAMA Otolaryngol Head Neck Surg 2017;143:274–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pacala JT, Yueh B. Hearing deficits in the older patient: “I didn’t notice anything.” JAMA 2012;307:1185–94. [DOI] [PubMed] [Google Scholar]
- 56.Bogardus ST Jr, Yueh B, Shekelle PG. Screening and management of adult hearing loss in primary care: clinical applications. JAMA 2003;289:1986–90. [DOI] [PubMed] [Google Scholar]
- 57.Rubel EW, Furrer SA, Stone JS. A brief history of hair cell regeneration research and speculations on the future. Hear Res 2013;297:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crowson MG, Hertzano R, Tucci DL. Emerging therapies for sensorineural hearing loss. Otol Neurotol 2017;38:792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fukui H, Raphael Y. Gene therapy for the inner ear. Hear Res 2013;297:99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Staecker H, Rodgers B. Developments in delivery of medications for inner ear disease. Expert Opin Drug Deliv 2013;10: 639–50. [DOI] [PubMed] [Google Scholar]
- 61.Askew C, Rochat C, Pan B, et al. Tmc gene therapy restores auditory function in deaf mice. Sci Transl Med 2015;7:295ra108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akil O, Seal RP, Burke K, et al. Restoration of hearing in the VGLUT3 knockout mouse using virally mediated gene therapy. Neuron 2012;75:283–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pan B, Askew C, Galvin A, et al. Gene therapy restores auditory and vestibular function in a mouse model of Usher syndrome type 1c. Nat Biotechnol 2017;35: 264–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Isgrig K, Shteamer JW, Belyantseva IA, et al. Gene therapy restores balance and auditory functions in a mouse model of Usher syndrome. Mol Ther 2017;25:780–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lentz JJ, Jodelka FM, Hinrich AJ, et al. Rescue of hearing and vestibular function by antisense oligonucleotides in a mouse model of human deafness. Nat Med 2013; 19:345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shibata SB, Ranum PT, Moteki H, et al. RNA interference prevents autosomaldominant hearing loss. Am J Hum Genet 2016;98:1101–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cox RM, Johnson JA, Xu J. Impact of advanced hearing aid technology on speech understanding for older listeners with mild to moderate, adult-onset, sensorineural hearing loss. Gerontology 2014;60:557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Blazer DG, Domnitz S, Liverman CT, eds. Hearing health care for adults: priorities for improving access and affordability. Washington, DC: National Academies Press, 2016. [PubMed] [Google Scholar]
- 69.Chien W, Lin FR. Prevalence of hearing aid use among older adults in the United States. Arch Intern Med 2012;172: 292–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wyatt JR, Niparko JK, Rothman ML, deLissovoy G. Cost effectiveness of the multichannel cochlear implant. Am J Otol 1995;16:52–62. [PubMed] [Google Scholar]
- 71.Saunders JE, Francis HW, Skarzynski PH. Measuring success: cost-effectiveness and expanding access to cochlear implantation. Otol Neurotol 2016;37(2):e135–40. [DOI] [PubMed] [Google Scholar]
- 72.Wilson BS, Dorman MF. Cochlear implants: a remarkable past and a brilliant future. Hear Res 2008;242:3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang L, Thompson CB, Clark JH, Ceh KM, Yeagle JD, Francis HW. Rehabilitation and psychosocial determinants of cochlear implant outcomes in older adults. Ear Hear 2017;38:663–71. [DOI] [PubMed] [Google Scholar]
- 74.McKinnon BJ. Cost effectiveness of cochlear implants. Curr Opin Otolaryngol Head Neck Surg 2014;22:344–8. [DOI] [PubMed] [Google Scholar]
- 75.Emmett SD, Francis HW. The socioeconomic impact of hearing loss in U.S. adults. Otol Neurotol 2015;36:545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Saunders JE, Barrs DM, Gong W, Wilson BS, Mojica K, Tucci DL. Cost effectiveness of childhood cochlear implantation and deaf education in Nicaragua: a disability adjusted life year model. Otol Neurotol 2015;36:1349–56. [DOI] [PubMed] [Google Scholar]
- 77.Emmett SD, Tucci DL, Bento RF, et al. Moving beyond GDP: cost effectiveness of cochlear implantation and deaf education in Latin America. Otol Neurotol 2016;37: 1040–8. [DOI] [PubMed] [Google Scholar]
- 78.PCAST recommends changes to promote innovation in hearing technologies. Washington, DC: The White House, October 26, 2015. (https://obamawhitehouse.archives.gov/blog/2015/10/26/%E2%80%8Bpcast-recommends-changes-promote-innovation-hearing-technologies). [Google Scholar]
- 79.Warren E, Grassley C. Over-the-counter hearing aids: the path forward. JAMA Intern Med 2017;177:609–10. [DOI] [PubMed] [Google Scholar]
- 80.President Trump signs OTC hearing aid legislation into law. Hearing Review. August 19, 2017. (http://www.hearingreview.com/2017/08/president-trump-signs-otc-hearing-aid-legislation-law/). [Google Scholar]
- 81.Strom KE. HR 2013 hearing aid dispenser survey: dispensing in the age of internet and big box retailers. Hearing Review. April 8, 2014. (http://www.hearingreview.com/2014/04/hr-2013-hearing-aid-dispenser-survey-dispensing-age-internet-big-box-retailers-comparison-present-past-key-business-indicators-dispensing-offices). [Google Scholar]
- 82.Strom KE. Hearing aid sales rise 5% in 2013; industry closes in on 3M unit mark. Hearing Review. February 26, 2014. (http://www.hearingreview.com/2014/02/staff-standpoint-hearing-aid-sales-rise-5-2013-industry-closes-3m-unit-mark). [Google Scholar]


