Graphical Abstract
Biotinylated bleomycin A5 was attached to streptavidin-derivatized microbubbles and a solution containing the conjugate was passed over a monolayer of cultured MCF-7 cells. The bleomycin-derivatized microbubbles adhered to the MCF-7 cells, and the association could be monitored by the use of a microscope. Three other cancer cell lines gave similar results. The bleomycin-microbubble conjugate did not bind to a normal breast cell line (MCF-10A), nor to the matched non-cancer cell lines corresponding to the other cancer cell lines targeted by bleomycin. No binding to any tested cell line was observed when the microbubbles lacked conjugated bleomycin A5, or when the microbubble contained a bleomycin A5 analogue lacking the carbohydrate moiety.
The bleomycins (BLM A5, 1) are clinically used antitumor agents efficacious in the treatment of squamous cell carcinomas and malignant lymphomas.1 Their anti-tumor activity is believed to result from their ability to mediate the selective oxidative cleavage of DNA,2 and possibly also RNA.3
One property of bleomycin that has been recognized for decades is its ability to target tumors; this has been documented in numerous tumor imaging studies that employed BLMs bound to radionuclides.4,5 The importance of tumor targeting to the therapeutic efficacy of BLM is underscored by the therapeutic dose of BLM (~5 μmoles), which is quite low relative to the doses of many clinically used agents. Understanding the molecular nature of tumor targeting by BLM would facilitate the synthesis of analogues with improved properties, and might also enable the selective delivery of other probes and drugs to tumor cells.
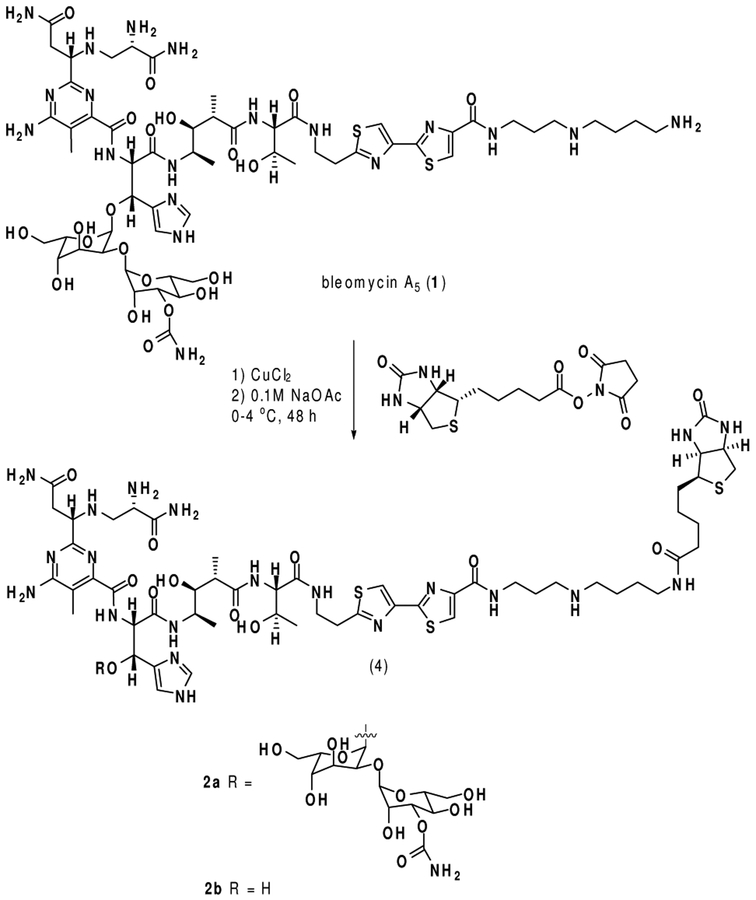
Microbubbles, consisting of a shell enclosing a gas core, are used in ultrasonography as contrast agents and intravascular blood flow tracers. The shell is usually composed of albumin, carbohydrates or lipids. The gas core is usually air, nitrogen or a perfluorocarbon.6 While individual microbubbles may exist in varying sizes, they are usually smaller than red blood cells and their mean diameter is typically within the range 1–4 μm. This small size permits free flow of the microbubbles through the (micro)circulation. In recent years, microbubbles have been modified with ligands that bind specific receptors expressed by cell types of interest, including inflamed and cancer cells.7 The majority of ligands to date have been monoclonal antibodies. Presently we describe the conjugation of BLM A5 to microbubbles, demonstrate that the conjugate adheres selectively to cancer cells, and report that the carbohydrate moiety of BLM A5 is required for tumor cell targeting.
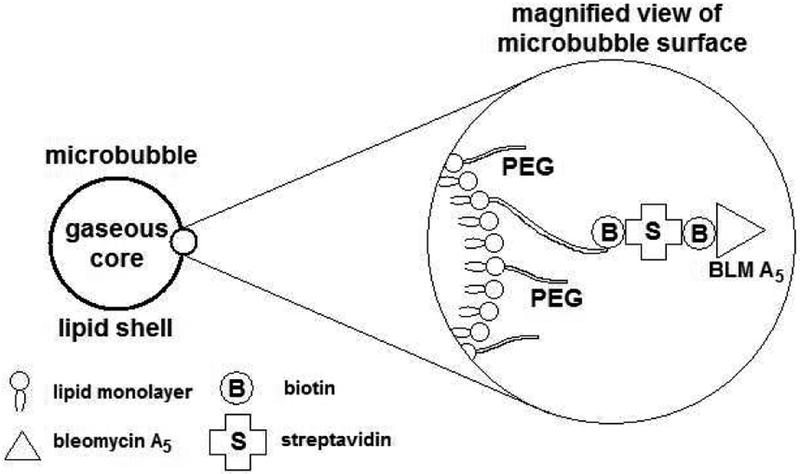
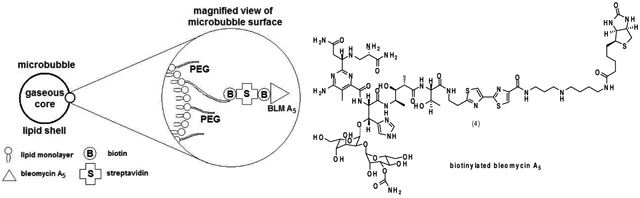
The C-terminus of BLM A5 (1) was acylated with biotin, as shown in Figure 1, to afford BLM A5-biotin conjugate 2a.8 The preparation of BLM A5-conjugated microbubbles was then accomplished by admixture of BLM-biotin 2a to commercially available microbubbles derivatized with streptavidin.9 A schematic representation of the resulting targeted microbubble is shown in Figure 2.
Figure 1.
Synthesis of biotinylated derivatives of bleomycin A5 and deglycobleomycin A5.
Figure 2.
Constitution of the bleomycin A5-microbubble conjugate.
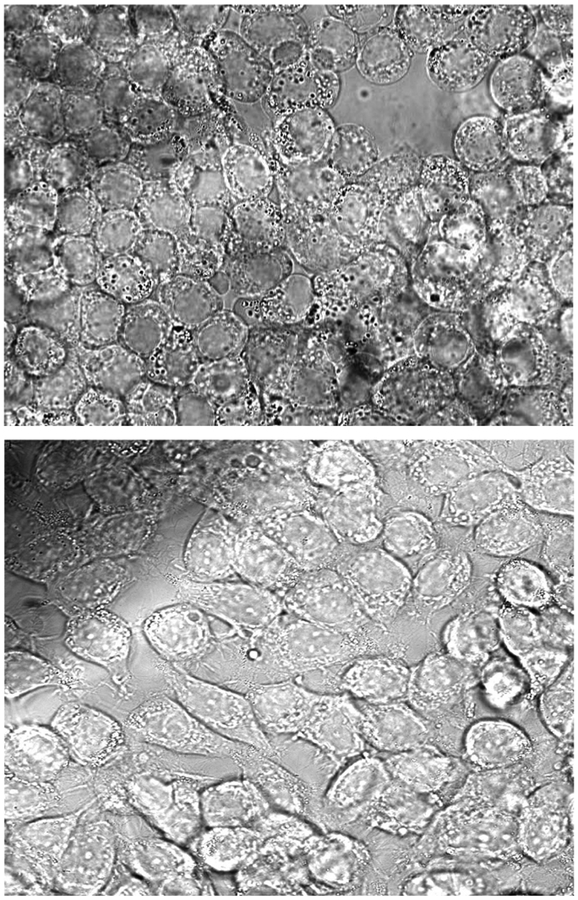
MCF-7 human breast carcinoma cells were grown on sterile glass cover slips (40 mm) and incubated at 37 °C until they reached 40–60% confluency in a 5% fetal bovine serum/RPMI 1640 medium. They were then assembled into a parallel plate flow chamber with a constant temperature maintained at 37 °C. The suspension containing the BLM-microbubble conjugate was introduced into the flow chamber at a controlled rate of 0.01 mL/min, and possible attachment of the microbubbles to the cultured MCF-7 cells was imaged using an inverted microscope fitted with a camera. As shown in Figure 3, the BLM microbubble conjugate adhered to the cultured MCF-7 cells, but microbubbles lacking attached BLM 2a failed to adhere to the cultured MCF-7 cells.
Figure 3.
Monolayers of cultured MCF-7 breast cancer cells treated with the BLM A5-microbubble conjugate (top) or with underivatized microbubbles (bottom).
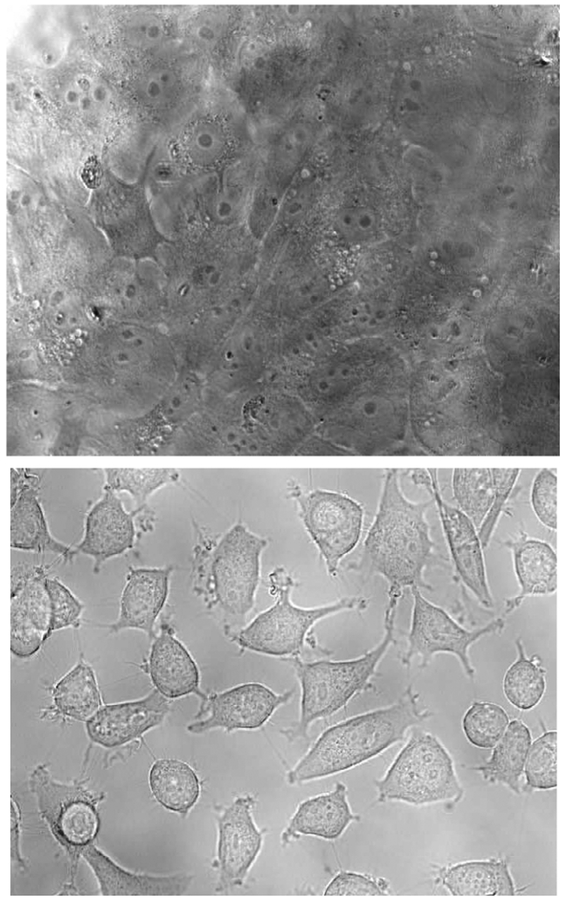
To judge the ability of the BLM A5-microbubble conjugate to bind selectively to tumor cells, the experiment was repeated using the “normal” breast cell line MCF-10A. As shown in Figure 4, there was no adhesion of the BLM A5 microbubble-conjugate to the cultured MCF-10A cells. Thus the BLM A5-microbubble conjugate adhered selectively to the cancer cell line.10
Figure 4.
Monolayers of cultured MCF-10A breast cells treated with the BLM A5 microbubble conjugate (top) and of cultured MCF-7 cells treated with the deglyco BLM A5 microbubble conjugate (bottom).
The realization of a system for monitoring the selective interaction of BLM A5 with tumor cells provides a vehicle for evaluating those structural elements of BLM that contribute to tumor cell targeting. Accordingly, biotinylated deglyco BLM A5 (2b) was prepared to permit a direct assessment of the role of the carbohydrate moiety of BLM in tumor cell targeting. BLM 2b was conjugated to the same microbubbles and employed in experiments designed to test adhesion to cultured MCF-7 and MCF-10A cells. As shown in Figure 4 for the cultured MCF-7 cells, no adhesion of the deglyco BLM A5 microbubble conjugate to either cell line was observed.
The foregoing experiments enable direct, visual observation of the binding of a BLM conjugate to cultured tumor cells and demonstrate that BLM adheres selectively to tumor, as compared with the same “normal” cells. Also established is the requirement for the carbohydrate moiety of BLM to support tumor cell targeting. Not yet resolved by these experiments is the related question of the sufficiency of the BLM carbohydrate moiety to support tumor cell targeting, a finding that could enable novel strategies for selective drug delivery and antitumor therapy.
Supplementary Material
Footnotes
Supporting Information Aavailable: Experimental details. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).(a) Umezawa H Antibiot. Chemother 1978, 23, 76. [DOI] [PubMed] [Google Scholar]; (b) Bleomycin Chemotherapy, Sikic BI, Rozencweig M Carter SK, Eds.; Academic Press: Orlando, FL, 1985. [Google Scholar]; (c) Levi JA; Raghavan D; Harvey V; Thompson D; Sandeman T; Gill G; Stuart-Harris R; Snyder R; Byrne M; Kerestes ZJ Clin. Oncol 1993, 11, 1300. [DOI] [PubMed] [Google Scholar]
- (2).(a) Stubbe J; Kozarich JW Chem. Rev 1987, 87, 1107. [Google Scholar]; (b) Kane SA; Hecht SM Prog. Nucleic Acid Res. Mol. Biol 1994, 49, 313. [DOI] [PubMed] [Google Scholar]; (c) Claussen CA; Long EC Chem.. Rev. 1999, 99, 2797. [DOI] [PubMed] [Google Scholar]; (d) Hecht SM J. Nat. Prod 2000, 63, 158. [DOI] [PubMed] [Google Scholar]; (e) Chen J; Stubbe J Nature Rev. 2005, 5, 102. [Google Scholar]
- (3).(a) Carter BJ; de Vroom E; Long EC; van der Marel GA; van Boom JH; Hecht SM Proc. Natl. Acad. Sci. U. S. A 1990, 87, 9373. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Holmes CE; Carter BJ; Hecht SM Biochemistry 1993, 32, 4293. [DOI] [PubMed] [Google Scholar]; (c) Hecht SM Bioconjugate Chem. 1994, 5, 513. [DOI] [PubMed] [Google Scholar]; (d) Abraham AT; Lin J-J; Newton DL; Rybak S; Hecht SM Chem. Biol 2003, 10, 45. [DOI] [PubMed] [Google Scholar]; (e) Tao Z-F; Konishi K; Keith G; Hecht SM J. Am. Chem. Soc 2006, 128, 14806. [DOI] [PubMed] [Google Scholar]
- (4).Jones SE; Lilien DL; O’Mara RE; Durie BG; Salmon SE Med. Ped. Oncol 1975, 1, 11. [DOI] [PubMed] [Google Scholar]; (b) Silverstein MJ; Verma RC; Greenfield L; Morton DL Cancer 1976, 37, 36. [DOI] [PubMed] [Google Scholar]; (c) van de Poll MA; Versluis A; Rasker JJ; Jurjens H; Woldring MG Nuclear-Medizin 1976, 15, 86. [PubMed] [Google Scholar]; (d) Rasker JJ; Beekhuis H; van de Wal AM; van der Heide JNH; Woldring MG Thorax 1976, 31, 641. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Oyama K; Machida K; Watari T; Hayashi S; Akaike A Radioisotopes 1976, 25, 567. [DOI] [PubMed] [Google Scholar]; (f) Burton IE; Todd JH; Turner RL Brit. J. Radiol 1977, 50, 508. [DOI] [PubMed] [Google Scholar]; (g) Tonami N; Kobayashi S; Maeda T; Hisada K Jap. J. Nucl. Med 1977, 14, 217. [PubMed] [Google Scholar]; (h) Firusian N; Makoski HB Strahlentherapie 1977, 153, 331. [PubMed] [Google Scholar]; (i) Bekerman C; Moran EM; Hofer PB; Hendrix RW; Gottschalk A Radiology 1977, 123, 687. [DOI] [PubMed] [Google Scholar]; (j) Stern PH; Helpern SE, Hagan PL; Howell SB; Dabbs J; Gordon RM J. Nat. Cancer Inst. 1981, 66, 807. [PubMed] [Google Scholar]
- (5).(a) DeRiemer LH; Meares CF; Goodwin DA; Diamanti CI J. Med. Chem 1979, 22, 1019. [DOI] [PubMed] [Google Scholar]; (b) Goodwin DA; Meares CF; DeRiemer LH; Diamanti CI; Goode RL; Baumert JE Jr.; Sartorius DJ; Lantieri RL; Fawcett HD J. Nucl. Med 1981, 22. [PubMed] [Google Scholar]
- (6).Linder J Nat. Rev. Drug Discov. 2004, 3, 527. [DOI] [PubMed] [Google Scholar]
- (7).(a) Klibanov A Invest. Radiol 2006, 41, 354. [DOI] [PubMed] [Google Scholar]; (b) Willmann J; Paulmurugan R; Chen K; Gheysens O; Rodriguez-Porcel M; Lutz A; Chen I; Chen X; Gambhir S Radiology 2008, 246, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Cu(II)•BLM A5 was treated with the N-hydroxysuccinmide ester of biotin, as outlined in Figure 1, in analogy with attachment of BLM A5 to a solid support (Abraham, A. T.; Zhou, X.; Hecht, S. M. J. Am. Chem. Soc. 2001, 123, 5167). Following the removal of Cu2+ by treatment with EDTA, the BLM A5-biotin conjugate (2a) was purified by C18 reversed phase HPLC.
- (9).TargestarB Ultrasound Contrast Agent, containing streptavidin attached through a PEG spacer, was used for attachment of BLM 2a and deglyco BLM 2b. These microbubbles have a consistent mean diameter of ~ 2.5 μm with ~ 1.5 × 106 streptavidin molecules/microbubbles. A 1-mL aqueous suspension of 1.5 × 108 streptavidin-derivatized microbubbles was treated with 50 μL of aq. 500 μM BLM 2a or 2b. The combined solution was agitated gently for 20 min, then free BLM was removed by repeated centrifugation (400 × g, 3 min, 10 °C) and washing with buffer.
- (10).Analogous experiments were carried out using other sets of cultured cancer and normal cells. Selective adhesion of the BLM A5 microbubble conjugate was observed using SW480 colon carcinoma cells (but not CRL-1541 colon cells), DU145 prostate carcinoma cells (but not CRL-2221 prostate cells) and A549 lung carcinoma cells (but not CCL-75 lung cells).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.