Abstract
Purpose of review:
Over half of the older adults in U.S. have multimorbidity, defined broadly as the presence of 2 or more chronic diseases in an individual. Multimorbidity has significant overlap with disability and frailty. In this review, we broadly review the concepts of multimorbidity, disability, and frailty, as well as their interrelationships, and ability to predict future adverse health outcomes in older adults.
Recent findings:
Depending on the study, the prevalence of individuals with all three of multimorbidity, disability, and frailty ranges from 2–20%. Multimorbidity and patterns of multimorbidity are predictive of functional limitations, disability, health care usage, and mortality. The degree to which multimorbidity predicts these outcomes depends on many factors but partly upon the population examined and the presence of frailty and disability.
Summary:
Multimorbidity is an emerging public health concern that is observed with and predictive of disability and frailty.
Keywords: frailty, comorbidity, multimorbidity, disability, older adults, geriatric syndrome
Introduction
The U.S. older adult population (age 65+ years) is growing rapidly and is estimated to comprise approximately 400 million by the year 2050 [1]. Many older adults fall burden to multiple chronic diseases (comorbidity/multimorbidity), disability, and frailty. Although these conditions are distinct from one another, there is considerable overlap among them, and the terms, multimorbidity, disability, and frailty are sometimes used interchangeably. We begin this paper by distinguishing between comorbidity and multimorbidity (a broader term) and then examine multimorbidity in relation to disability and frailty before examining the relationships among all three conditions.
Distinction between Comorbidity and Multimorbidity
Multimorbidity is a term which has been increasingly used in recent years as opposed to the term comorbidity which was introduced over 40 years ago [2]. Multimorbidity has been broadly defined as the co-existence of two or more chronic conditions, where one is not necessarily more dominant than the other chronic conditions [3]. Comorbidity has been defined as the coexistence of medical conditions occurring in one individual in which an index disease occurs first [4, 5]. There are no consensus definitions of either comorbidity or multimorbidity; and, distinguishing between the two terms has been noted to be important in order to clarify concepts and further examine the implications of the presence of multiple chronic conditions [4].
Multimorbidity
The prevalence of multimorbidity differs depending on the definition; however, it is reported that the prevalence of multimorbidity is greater than 60% in Medicare beneficiaries and more than 80% in adults greater than 85 years old [5]. Across definitions, the number of conditions, length of time each condition has been present, and types and diseases used to define multimorbidity is also not consistent. A disease has been classified as a non-reversible pathological alteration [6]; and, more recently the term “non-communicable disease” has been used to capture the concept of chronic disease [7, 8]. Multimorbidity has been defined as any cooccurrence of diseases within the same person [9–13]. Measures of multimorbidity across the literature fall into two broad types: 1) those that utilize a simple count of health conditions, ascertained by self-report of doctor-diagnosis and/or a clinician’s assessment, or 2) those that utilize a scale or index to assess the burden of multiple diseases on outcomes such as mortality, morbidity, use of health care resources [14]. The first type of multimorbidity measure is the most commonly used; and the number of conditions has ranged from 2 or more diagnoses to as many as 5 chronic diseases [5, 12, 15]. In addition to the disease count, the concept of chronicity (length of time a chronic condition has been present) is also not uniformly defined [14]. Goodman et al. [16] illustrated that there are differences across studies in definitions of “chronic,” from “long duration or course” to a more general duration of greater than 3 months, or lasting one year at a minimum [12, 17, 18]. In addition to the typical age-related diagnoses such as arthritis, chronic respiratory conditions, diabetes, and heart disease, the U.S. Department of Health and Human Services Strategic Framework [12] includes other conditions such as developmental disabilities, cognitive impairment, dementia, and substance abuse to the list [12].
In addition to the number of diseases, a combination or cluster of specific conditions present in an individual has been found in some studies to have an impact overall health status, function, and health care utilization [19, 20]. Jackson et al. found three multimorbidity patterns among older women (76–81 years) to be differentially predictive of future decline as measured by activities of daily living (ADL) and instrumental activities of daily living (IADL): musculoskeletal/somatic, neurological/mental health, and cardiovascular [21]. Over time, women with a high score for the cardiovascular pattern had significantly worse declines in ADLs, while those with neurological and mental health patterns had the greatest functional declines in IADL. Goodman et al. examined multimorbidity patterns in national U.S. datasets and found that the most common patterns included physical and mental health conditions and a high prevalence of hypertension observed in combination with other diseases [19]. Of note, the most prevalent dyads, or combinations of two chronic diseases, were the combinations of chronic obstructive pulmonary disease (COPD) + chronic kidney disease (4.9% prevalence) and stroke + chronic kidney disease (2% prevalence), which have high per capita associated health care costs ($45,011 and $51,715, respectively). Garin and colleagues in an international, multi-continent study that used nationally representative data sets of predominantly older adults, identified similar and regional multimorbidity patterns, suggestive of underlying causal and developmental factors for multimorbidity [20]. Selected multimorbidity combinations were common across countries (a cardiorespiratory pattern of angina, asthma, and COPD) while others were only observed in specific countries (mental-articular pattern of arthritis and depression, which was found only in China, Ghana and India).
Another study by Whitson et al. examined multimorbidity classes using latent class analysis (LCA) in a nationally representative sample of Medicare beneficiaries during the years 1999–2007 [22]. They found that the LCA identified six classes, with the majority of individuals (33%) belonging to the minimal disease class, having fewer than two of the 13 conditions examined. Many individuals could not be assigned with confidence to any particular class, suggesting substantial heterogeneity in the multimorbidity patterns. An estimate of the misclassification error (0.36) approximates that 1 in 3 individuals with a multimorbidity pattern could not be assigned with confidence to one of the six classes due to a poor fit for any class or to a similar probability of belonging to multiple classes. This suggests a substantial heterogeneity in the multimorbidity patterns and limitation to the use of LCA multimorbidity classes. While health care usage was higher in classes with greater comorbidity, this study found that the number of conditions within an individual predicted hospitalization and emergency department usage at least as well as the LCA of class membership. Therefore, while awareness of and observing patterns in multimorbidity may be an initial step in understanding population health and policy planning, the authors concluded that a count of conditions might be preferable for predicting usage of health care services.
Multimorbidity and Disability
The Disablement Process Model and Definitions
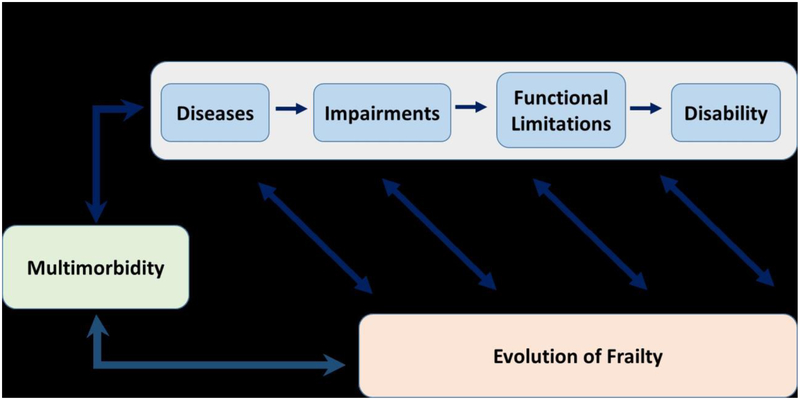
This section considers recent literature on the association between multimorbidity and disability within the context of the Disablement Process Model (DPM), a generalized framework for examining the pathway by which disease leads to subsequent disability (23–26). The DPM initially developed by Nagi [23, 24] comprises a disease-disability pathway that incorporates four sequential stages from 1) disease to 2) impairment to 3) functional limitation to 4) disability (see Figure 1). Verbrugge and Jette [25] further elaborated the DPM to include modifying factors outside the main disease-disability pathway that can alter the occurrence and/or rate of progression across stages toward disability. Disease is the presence of physiological abnormalities, often expressed as signs and symptoms, which are discovered and labeled as disease. Impairment is the presence of structural defects or dysfunction in specific organ systems, such as the musculoskeletal or neurosensory system. Functional limitation is present when an individual experiences difficulty performing basic actions – physical, cognitive, or emotional – that are used in a wide variety of situations in routine daily life. Examples of such actions include getting up from a chair, walking, remembering things that happened recently, and expressing positive emotion. Disability is present when an individual has difficulty performing his/her day-to-day social role activities as a result of a physical or health problem. These socially defined activities are carried out within specific sociocultural and physical settings. Examples of such activities include personal grooming, dressing, shopping, and managing finances.
Figure 1.
Overview of the associations among multimorbidity, disability, and the evolution of frailty. With the disablement process model as a framework, diseases lead to impairments, which lead to functional limitations, which ultimately lead to disability. At all stages of the disablement process, there is increased risk for the development of frailty; and, frailty may also influence the development of disability at each stage. Both the disability process and frailty are consequences of and contribute to multimorbidity.
Disease is the primary driver of progression to functional limitation, which, in turn is the primary driver of disability [26]. Personal, social, and environmental factors outside the main disease-disability pathway can alter progression along this pathway. Dependence occurs when an individual not only has difficulty performing his/her expected social role activities but is unable to do them without assistance.
Disability researchers, however, have not used the standardized DPM terminology consistently. Consequently, the literature often treats terms such as functional limitation and disability or dependence and disability as interchangeable even though their meanings are distinct. The term “physical function” also appears in the literature and refers variously to physical functional limitations, limitations in Activities of Daily Living (ADL), or some combination of the two. Use of the DPM to identify optimal targets for interventions to prevent or slow progression toward disability also appears infrequently in the literature. A recent exception is a paper in the physical therapy literature that uses the DPM to identify modifiable factors associated with walking speed in an older bi-ethnic cohort [27].
Types of Disability and Physical Functional Limitations Assessed
ADL Disability refers to difficulty performing Activities of Daily Living required to maintain one’s person, such as eating, bathing, and toileting.
IADL Disability refers to difficulty performing Instrumental Activities of Daily Living (IADL) required to maintain one’s household and connection to the community, such as doing housework, managing finances, shopping, and using transportation.
Mobility Disability refers to difficulty moving from one place to another, such as walking a quarter of a mile or walking up 10 steps without rest. Within the DPM, this type of disability is more appropriately termed functional limitation.
Functional Limitation, or Physical Function, refers to difficulty performing discrete tasks, such as stooping, crouching, or bending; getting up from a chair after sitting for a long period; reaching or extending arms above shoulder level; standing in place for long periods; and handling or fingering small objects. Some measures of physical function such as the Medical Outcomes Study Short Form-36 (MOS SF-36) include items that measure both functional limitations and ADLs/IADLs.
Multimorbidity and Disability
We identified seven studies conducted during the past several years that examined the association of multimorbidity with disability or functional limitations which, according to the disablement process model, drive disability. All of these studies used existing data from large, population-based studies -- four conducted in the U.S. [28–31] and one each in Australia [21], China [32], and Taiwan [33]. All of them treated multimorbidity as the presence of at least two or more health conditions, typically assessed by self-reported doctor-diagnosis or treatment. The number of health conditions assessed varied across studies, ranging from eight [30, 31] to 31 [21]. The majority of studies examined multimorbidity combinations, or patterns, to see if different combinations were differentially associated with disability or functional limitations; two focused on the magnitude of the association of increasing numbers of multimorbid conditions with disability [32, 33] or physical function [30].
Only one study assessed disability consistently with DPM terminology [31]. The other four studies assessed disability variously as: 1) either difficulty or needing help to perform ADLs and/or IADLs [28]; 2) having difficulty and requiring assistance from a person or assistive device [33]; 3) needing full or partial assistance [32]; or 4) scored difficulty performing ADL and IADL tasks (score of 1) and needing help (score of 2) and then summing the item responses to obtain a total ADL and IADL score which combined difficulty and dependence [21]. Both studies [29, 30] that examined physical function used assessments with items similar to those in the Nagi functional limitations scale [23], although one [29] did this using the MOS SF-36 Physical Function scale.
In spite of variation in their methodologies, key findings from these studies provide important insights into the association of multimorbidity with disability and functional limitations. An analysis of longitudinal data (1999–2011) in the Panel Study of Income Dynamics (PSID) [31] found that for white and African American women and white men, having multimorbidity was not associated with reduced life expectancy (LE), but substantially increased percent of remaining life with disability (DLE), particularly among those with combinations of cardiovascular disease related conditions. For example, the DLE for white women with diabetes + hypertension + stroke was 90.2% and for those with diabetes + heart disease + depression was 64.8%. For African American men, there was no evidence that multimorbidity affected either LE or DLE, although presence of any individual condition substantially increased DLE. This finding for African American men is consistent with the investigators’ hypothesis about racial differences in the risk of disability and death associated with multimorbid health conditions. Specifically, investigators reasoned that because African Americans compared with whites are more likely to have additional health conditions over and above those measured in the current study and, thus, have a greater background risk of death and disability. As a consequence, the health conditions measured may have a less substantial effect on increased risk of disability and death among African Americans compared with whites. This hypothesis, however, was not supported among African American women. Factor analysis applied to baseline data (2002) from the Australian Longitudinal Study of Women’s Health identified three multimorbidity patterns: 1) musculoskeletal/somatic (MSO), 2) neurological/mental health (NMH); and 3) cardiovascular (CVD) [21]. Factor scores were created for each participant, and those in the upper and middle tertile scores for each factor were compared to a reference group comprised by participants in the lowest tertile on all three factors. Surveys were conducted every 3 years thereafter, 2005 – 2011. In 2005, women in the highest factor tertile of all three multimorbidity patterns had significantly higher mean ADL and IADL scores relative to the reference group. Both ADL and IADL scores increased over time, indicating increasing disability/dependence. Compared with the reference group, women with high CVD and NMH factor scores, but not those with high MSO factor scores, had significantly greater increases over in mean ADL scores, with the greatest increase among women in the high CVD tertile. Women in the top two tertiles of the NMH pattern factor score had the greatest increases over time in mean IADL scores.
A study using data from the Longitudinal Health and Retirement Study (2010 and 2012) found 14 unique multimorbidity combinations in 2010 of sufficient size to support regression analyses comparing their association with a combined ADL-IADL disability index to that of reference groups with none or only one condition [28]. All 14 combinations included hypertension (HTN), arthritis (ARTH), or both. The most prevalent combinations were ARTH + HTN (17%) and ARTH + HTN + CVD (8%). High depressive symptoms (DSYM) were present in only one combination. (ARTH + HTN + DSYM), but this combination was associated with the highest ADL-IADL Index score (0.92) in 2012, 30–80% higher than that for any other combination. In regression analyses, the ADL-IADL index compared to those in the reference groups remained significantly higher for this combination, even after adjustment for demographic factors and body mass index. Further, relative to presence of ARTH + HTN only, including DSYM in this combination was associated with a 3.8 times greater ADL-IADL index score tin adjusted models.
A study using data from a representative community-based sample in Shanghai, China, and multiple logistic regression models adjusted for sociodemographic characteristics, found a consistent, graded association between number of multimorbid chronic conditions and ADL and IADL disability, even without taking into account severity or heterogeneity of diseases [32]. For ADL disability, odds ratios (ORs) ranged from 1.53 for one condition to 5.61 for ≥ 4 conditions. For IADL disability, ORs ranged from1.51 for 1 condition to 5.51 for ≥ 4 conditions.
Another study examined the separate impact of multimorbidity and geriatric conditions (i.e., “a collection of symptoms or a condition prevalent among frail older adults that are likely multifactorial in etiology”) on incident disability in young-old (65–79 years) and old-old (≥80 years) adults in the nationally representative Survey of Health and Living Status of the Elderly in Taiwan (2003 and 2007) [33]. Multimorbidity was assessed in 2003 based on 11 chronic conditions; five geriatric conditions were also assessed: cognitive impairment, DSYM, falls, urinary incontinence, and pain. Among the young-old, both multimorbidity and having multiple geriatric conditions were associated with incident disability. Among the old-old, multimorbidity was not significantly associated with incident disability; but with only one geriatric condition vs. none, the relative risk (RR) of incident disability was 2.38 and with ≥2 geriatric conditions present was 4.76.
A study carried out with long-term participants, 80+ years old in the Women’s Health Initiative studies examined the association of multimorbidity and coronary disease comorbidity (i.e., CHD plus one other disease) with physical function using the MOS SF-36 Physical Function scale (range: 0–100; higher scores indicate better function) [29]. After adjustment for covariates highly associated with multimorbidity, mean Physical Function score for women with 0–1 health conditions was 74. Score decrements with increasing number of multimorbid conditions ranged from −8 for two conditions to −19 for ≥4 conditions. Women with CHD paired with one other health condition vs. those with CHD alone had poorer physical function. The largest mean decrements for CHD comorbidity pairs occurred for three geriatric conditions: hip fracture (−22), cognitive impairment (−20), and frequent faller (−19). The influence of five modifiable risk factors on Physical Function score was also examined to identify potential targets for intervention to prevent or mitigate poor physical functioning. For each additional MET-hour/week of physical activity, the mean Physical Function score improved by 0.31 points. Decrements in mean score associated with overweight, obesity, current smoking, and high blood pressure and/or cholesterol ranged from −17 for obesity to −5 for high blood pressure and/or cholesterol.
Finally, a study using data from the Health and Retirement Study biennial surveys (1992–2010) found that age modified the association between number of chronic diseases and physical function [30]. Relative to younger participants (60–69 years), older participants had more functional limitations as the number of diseases increased. An average 70 year-old participant with no diseases had 0.89 physical functional limitations; but this increased to 1.72 with one disease and 3.82 with ≥3 diseases. Participants who remained disease-free during the follow-up period, experienced little change in physical functioning until after age 80. The average functional limitation score for any combination of two diseases was 2.90. In contrast, the highest functional limitations scores were observed for the combinations of memory-related + pulmonary diseases (5.94) and memory-related diseases + stroke (5.75), suggesting that different combinations of diseases may have differing impacts on physical functioning. The authors suggested that the observed age modification of the association between number of diseases and physical functioning could likely be attributed to increasing disease-induced impairments as well as deterioration of compensatory mechanisms with aging, an explanation consistent with the DPM framework.
These recent findings provide evidence that: 1) an increasing number of multimorbid conditions, regardless of their severity and type, increases the risk of disability and physical functional limitations; and 2) that various discrete combinations of multimorbid conditions differentially affect risk of disability and physical functional limitations. Even though none of the studies examined pathways through which multimorbid conditions or their unique combinations impact disability and functional limitations, findings can inform clinicians and public health providers about the critical need to prevent development of multimorbidity and alert them to multimorbidity combinations with particularly high impact on risk of functional limitations and disability in population groups similar to those for whom they provide care.
Multimorbidity & Frailty
Frailty is a geriatric syndrome of physical vulnerability and increased risk of physical decline with aging. Although there are several proposed definitions to describe this syndrome, perhaps the most often used are the frailty phenotype [34] and the frailty index [35]. Although a thorough discussion on these definitions is beyond the scope of this review, it is important to have a general understanding of the most commonly definitions in order to interpret papers reviewing the intersection between frailty, comorbidity, and disability. The frailty phenotype focuses on the characterization of a physical frailty syndrome and proposes that frailty is characterized by unintentional weight loss, exhaustion, low physical activity, muscle weakness, and slow walking speed [34]. The frailty index proposes that frailty can be seen as the accumulation of deficits in multiple systems, including medical diseases/conditions, age-related deficits (i.e., hearing loss), laboratory abnormalities, and disabilities. Although the two definitions take a different point of view at the measurement of frailty, both are strongly predictive of poor outcomes [36].
Frailty and multimorbidity are related but distinct clinical entities. A review study examined the overlap between frailty (defined as either the frailty phenotype [34] or the frailty index [35]) and multimorbidity (defined as the co-occurrence of several diseases in the same individual) in 41 studies [6]. Based on this review, a visual representation was created supported by the evidence demonstrating that both frailty and multimorbidity are predictors and outcomes of each other, as well as both predictors of disability and mortality. In this flowchart of the “system failure process,” non-communicable diseases lead to both multimorbidity and disability. Multimorbidity and disability both ultimately lead to frailty by way of “health deficits,” which are the deficits counted by the frailty index model of frailty [35]. However, this model does not incorporate impairments or functional impairments as does the DPM described above [23]. In Figure 1 we provide a model of the interrelationships between the disablement process, multimorbidity, and the evolution of frailty. In this depiction, we propose disability is a process as described by the DPM, and that at all stages there is increased risk for the development of frailty. Conversely frailty contributes to the development of disability at all stages along the DPM. Both the disability process and frailty are consequences of and contribute to multimorbidity.
In a French study, examining the relationship between multimorbidity and frailty [37], frailty was assessed by the Fried phenotype [34] and multimorbidity was defined as having at least two of major chronic diseases, including cardiac or cerebrovascular disease, diabetes, chronic respiratory disease, arthralgia, or depression. The prevalence of frailty was 11–12%, and the prevalence of multimorbidity was 15–17%, respectively, in two cohorts examined in France. Thirty to forty percent of those examined had both frailty and multimorbidity simultaneously. Similar factors (older age, functional decline, poor mental health, financial difficulties) were associated with both frailty and multimorbidity. Therefore, this international study concluded similarly to those conducted in the U.S. that frailty and multimorbidity are interrelated but distinct [38].
A systematic review [39] aimed to examine the effectiveness of comprehensive care programs as potential interventions for frailty and multimorbidity [40]. A comprehensive care program was defined as any program that follows the Chronic Care Model [41]. The objective was to describe the programs targeting these populations and estimate their effectiveness regarding improvement of patient and caregiver related outcomes, healthcare utilization and costs. Nineteen publications were included in the analysis, which revealed that comprehensive care may result in more patient satisfaction, less depressive symptoms, better health-related quality of life or functioning; however, the evidence is insufficient. Further, no evidence found a benefit of comprehensive care on caregiver-related outcomes. More good quality studies are needed to determine which specific target groups will benefit from comprehensive care [3].
Relationship Between Frailty, Comorbidity, and Disability
Perhaps the first description of the overlap of frailty, comorbidity and disability was by Fried and colleagues in the initial description of the frailty phenotype [34]. In the Cardiovascular Health Study (CHS), it was shown that a minority of older adults, only 20%, had all three conditions of comorbidity, disability, and frailty [38]. Interestingly, 27% of those characterized as frail had neither comorbidity nor disability. In a more recent analysis, Boeckxstaens et al. performed a cross sectional analysis of 567 adults aged 80+ years enrolled in the BELFRAIL cohort in Belgium in order to compare relationships of different measures of multimorbidity with disability and frailty [15]. In a Venn diagram of frailty (defined using Fried criteria), disability (based on ADLs), and multimorbidity (defined using disease count, cumulative illness rating scale [CIRS] [42], and Charlson comorbidity index [CCI] [43]), only 2.3% had all three conditions. ADL disability was associated with disease count (OR of 2.1, 95% CI: 1.4–3.4), CIRS (OR OF 4.0, 95% CI: 2.5–5.6), and CCI (OR of 1.8, 95% CI: 1.2–2.7). Frailty was also associated with the CCI (OR of 2.4, 95%CI: 1.2–4.6) and CIRS (OR OF 2.6, 95% CI: 1.3–5.3). The authors concluded that there was similar ability of disease count, CIRS, and CCI to identify patients with disability and frailty.
A large study conducted in 4,414 participants in Iceland examined frailty (defined by Fried criteria), multimorbidity (based on the presence of 13 comorbid conditions), and disability (ADLs) [9]. Differences among the following groups were examined: 1) non-frail (the reference group); 2) frail only; 3) frail with disability; 4) frail with multimorbidity; and 5) frail with disability and multimorbidity. Frailty prevalence was 11%, and individuals with frailty were at increased risk for mortality (OR = 1.40, 95% CI: 1.15–1.69) and nursing home admission (OR = 1.50, 95% CI: 1.16–1.93) overall; however, risk differed by the subgroups examined. Interestingly, while the frail only group did have poorer cognition and increased inflammation, this group did not have increased risk for mortality or nursing home admission. The other frail subgroups (those with disability and multimorbidity or both) had significantly poorer cognition, increased inflammation, and increased risk for mortality and nursing home admission. Therefore, the authors concluded that the increased risk associated with frailty may be driven primarily by increased disability and disease burden.
A smaller (n=121) study of centenarians conducted in China showed overlap between frailty, ADL disability, comorbidity, and self-rated health [44]. This study showed that 24% of this population were characterized as frail. Frailty co-occurred with poor self-rated health in 32.4% of participants, with disability in 31.4%, and with comorbidity in 26.0%. Nine percent of individuals examined had all four entities of frailty, poor self-rated health, disability, and comorbidity. In a French study, the relationship between multimorbidity and frailty was assessed [37]. Frailty was assessed by the Fried phenotype [34] and multimorbidity was defined as having at least two of major chronic diseases, including cardio or cerebrovascular disease, diabetes, chronic respiratory disease, arthralgia, or depression. The prevalence of frailty in two cohorts was 11–12%, and the prevalence of multimorbidity was 15–17%, respectively. Thirty to forty percent had both frailty and multimorbidity. Similar factors (older age, functional decline, poor mental health, financial difficulties) were associated with frailty and multimorbidity. Therefore, these international studies concluded similarly to those conducted in the U.S. that frailty, comorbidity, and disability are interrelated but distinct [38].
Predictive Value of Multimorbidity
Multimorbidity contributes to frailty, disability, increased health care costs, including polypharmacy, and potential for substandard care given competing health priorities due to clinical practice guidelines which are single disease focused [5, 12, 3, 45, 46, 30]. On a practical level, this contributes to difficulty in setting clinical priorities and targeted interventions for individuals with multimorbidity [45]. This becomes a major concern as the burden on multimorbidity increases as the population ages. Measuring multimorbidity is integral to public health surveillance efforts and research investigations [3].
However, we also know that frailty and disability also lend increased risk for these poor health outcomes. Some studies we reviewed aimed to determine the relative predictive ability of frailty, comorbidity or disability, in order to determine which clinical entity might best inform clinical decision making for prognostication. In a study by Ritt et al., it was found that frailty (defined using a frailty index [35], a clinical frailty scale using seven categories [47], and the FRAIL scale [48]) was a better predictor than disability to predict mortality [49]. Comorbidity was measured in this study using the cumulative illness rating scale for geriatrics (CIRS-G) [50] and the comorbidity domain of the frailty index [35]. Two disability instruments were used, including Katz activities of daily living [51] and the instrumental activities of daily living from domains of the frailty index [35]. The authors concluded that while all of the instruments were suitable tools to predict risk for mortality, the clinical frailty scale [47] performed slightly better than the others for mortality prediction, and, in individuals 83 years or older, the frailty index [35] was also predictive of mortality. Another study found that the clinical frailty scale predicts individuals at risk for disability and mortality after critical illness. Bagshaw et al. found that those who were frail were more likely to require help to live at home compared to non-frail and had higher in-hospital mortality compared to non-frail patients (OR = 1.81, 95% CI: 1.09–3.01) [52].
While these studies found that frailty was the better predictor of mortality, other studies have suggested that multimorbidity and chronological age are robust predictors of death. Kusumastuti et al. found that chronological age held most of the discrimination ability to predict mortality; and, any added value of comorbidity, frailty, and subjective health decreased with increasing age [53]. Nunes et al. performed a systematic review to examine the association between multimorbidity and mortality in older adults [54]. Multimorbidity was associated with mortality with hazard ratio of 1.44 (95%CI 1.34–1.55). Number of morbidities was also positively associated with risk of death (HR of 1.20, 95% CI: 1.1–1.3). Disability was found to mediate the effect of multimorbidity on mortality.
Conclusion
Multimorbidity and its impact on an aging population is an emerging public health concern. While these are considered distinct clinical entities, there is significant overlap among multimorbidity, disability, and frailty, and they are often predictive of each other. Although there is lack of consensus across definitions for all three conditions, there is a vast accumulation of evidence which has examined the associations among multimorbidity, disability, and frailty in diverse populations, both in the U.S. and Internationally. Clinicians and researchers should consider the particular population and outcomes are of interest when considering how to measure multimorbidity, disability, and frailty; and, researchers should generally consider all three clinical conditions when studying geriatric populations given their considerable interrelationships.
Footnotes
Compliance with Ethical Guidelines
Conflict of Interest
Sara Espnoza, Myla Quiben and Helen Hazuda declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Bureau USC. An aging nation: the older population in the United States. Population estimates and projections 2014. [Google Scholar]
- 2.Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. Journal of chronic diseases. 1970;23(7):455–68. [DOI] [PubMed] [Google Scholar]
- 3.Boyd CM, Fortin M. Future of multimorbidity research: how should understanding of multimorbidity inform health system design? Public Health Reviews. 2010;32(2):451. [Google Scholar]
- 4.van den Akker M, Buntinx F, Knottnerus JA. Comorbidity or multimorbidity: what’s in a name? A review of literature. The European Journal of General Practice. 1996;2(2):65–70. [Google Scholar]
- 5.Salive ME. Multimorbidity in older adults. Epidemiologic reviews. 2013;35(1):75–83. [DOI] [PubMed] [Google Scholar]
- • 6.Villacampa-Fernández P, Navarro-Pardo E, Tarín JJ, Cano A. Frailty and multimorbidity: Two related yet different concepts. Maturitas. 2017;95:31–5.This review article provides an overview of frailty and multimorbidity and provides a comprehensive discussion of their interrelationships. A flowchart of these interrelationships is proposed, which includes disability in addition to frailty and multimorbidity.
- 7.Hunter DJ, Reddy KS. Noncommunicable diseases. New England Journal of Medicine. 2013;369(14):1336–43. [DOI] [PubMed] [Google Scholar]
- 8.Organization WH. Global status report on noncommunicable diseases 2014. Geneva, Switzerland: 2014. [Google Scholar]
- 9.Aarts S, Patel K, Garcia M, Van Den Akker M, Verhey F, Metsemakers J et al. Co-presence of multimorbidity and disability with frailty: an examination of heterogeneity in the frail older population. The Journal of frailty & aging. 2015;4(3):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesari M, Pérez-Zepeda MU, Marzetti E. Frailty and Multimorbidity: Different Ways of Thinking About Geriatrics. Journal of the American Medical Directors Association. 2017;18(4):361–4. [DOI] [PubMed] [Google Scholar]
- 11.Radner H, Yoshida K, Smolen JS, Solomon DH. Multimorbidity and rheumatic conditions—enhancing the concept of comorbidity. Nature Reviews Rheumatology. 2014;10(4):252. [DOI] [PubMed] [Google Scholar]
- 12.Services USDoHaH. Multiple Chronic Conditions - A Strategic Framework: Optimum Health and Quality of Life for Individuals with Multiple Chronic Conditions. Washington, D.C. 2010. [Google Scholar]
- 13.Marengoni A, Angleman S, Melis R, Mangialasche F, Karp A, Garmen A et al. Aging with multimorbidity: a systematic review of the literature. Ageing research reviews. 2011;10(4):430–9. [DOI] [PubMed] [Google Scholar]
- 14.Huntley AL, Johnson R, Purdy S, Valderas JM, Salisbury C. Measures of multimorbidity and morbidity burden for use in primary care and community settings: a systematic review and guide. The Annals of Family Medicine. 2012;10(2):134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boeckxstaens P, Vaes B, Legrand D, Dalleur O, De Sutter A, Degryse J-M. The relationship of multimorbidity with disability and frailty in the oldest patients: a cross-sectional analysis of three measures of multimorbidity in the BELFRAIL cohort. The European journal of general practice. 2015;21(1):39–44. [DOI] [PubMed] [Google Scholar]
- 16.Goodman RA, Posner SF, Huang ES, Parekh AK, Koh HK. Defining and Measuring Chronic Conditions: Imperatives for Research, Policy, Program, and Practice. Preventing Chronic Disease. 2013;10:E66. doi: 10.5888/pcd10.120239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alwan A Global status report on noncommunicable diseases 2010. World Health Organization; 2011. [Google Scholar]
- 18.Statistics NCfH. Health, United States, 2010: With Special Feature on Death and Dying. Hyattsville, MD: 2011. [PubMed] [Google Scholar]
- 19.Goodman RA, Ling SM, Briss PA, Parrish RG, Salive ME, Finke BS. Multimorbidity patterns in the United States: implications for research and clinical practice. Oxford University Press; US; 2015. [DOI] [PubMed] [Google Scholar]
- 20.Garin N, Koyanagi A, Chatterji S, Tyrovolas S, Olaya B, Leonardi M et al. Global multimorbidity patterns: a cross-sectional, population-based, multi-country study. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2015;71(2):205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • 21.Jackson CA, Jones M, Tooth L, Mishra GD, Byles J, Dobson A. Multimorbidity patterns are differentially associated with functional ability and decline in a longitudinal cohort of older women. Age and ageing. 2015;44(5):810–6.Used factor analysis to identify three multimorbidity patterns (musculoskeletal/somatic, neurological/mental health, and cardiovascular) and showed that these multimorbidity patters were differentially associated with ADL and IADL disability/dependence over time.
- 22.Whitson HE, Johnson KS, Sloane R, Cigolle CT, Pieper CF, Landerman L et al. Identifying Patterns of multimorbidity in older americans: application of latent class analysis. Journal of the American Geriatrics Society. 2016;64(8):1668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagi SZ. Disability concepts revisited: Implications for prevention In: Pope AMT AR, editor. Disability in America: Toward a National Agenda for Prevention. Washington, D.C.: National Academy Press; 1991. p. 309–27. [Google Scholar]
- 24.Pope AM, Tarlov AR. Disability in America: Toward a national agenda for prevention. National Academies Press; 1991. [Google Scholar]
- 25.Verbrugge LM, Jette AM. The disablement process. Social science & medicine. 1994;38(1):1–14. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence RH, Jette AM. Disentangling the disablement process. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 1996;51(4):S173–S82. [DOI] [PubMed] [Google Scholar]
- • 27.Quiben MU, Hazuda HP. Factors contributing to 50-ft walking speed and observed ethnic differences in older community-dwelling Mexican Americans and European Americans. Physical therapy. 2015;95(6):871–83.Utilized a detailed DPM developed in the San Antonio Longitudinal Study of Aging to identify contextual/demographic, lifestyle/anthropometric characteristics, chronic diseases and impairments in the pathway leading to the functional limitation of slow walking speed.
- •• 28.Quiñones AR, Markwardt S, Botoseneanu A. Multimorbidity combinations and disability in older adults. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2016;71(6):823–30.Identified 14 unique multimorbidity combinations in the Longitudinal Health and Retirement Study in 2010 and reports their composition and prevalence as well as their longitudinal association in 2012 with a combined ADL-IADL disability index. Comparisons of various multimorbid combinations were performed and demonstrate the differential impact of various combinations on ADL-IADL disability.
- •• 29.Rillamas-Sun E, LaCroix AZ, Bell CL, Ryckman K, Ockene JK, Wallace RB. The impact of multimorbidity and coronary disease comorbidity on physical function in women aged 80 years and older: The Women’s Health Initiative. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2016; 71(Suppl_1):S54–S61.Reported decrements in MOS SF-36 Physical Function Score associated with increasing numbers of multimorbid conditions relative to 0 or 1 as well as decrements associated with potentially modifiable risk factors for poor physical functioning. Also examined CHD comorbidity pairs and reported that the largest decrements in physical functioning occurred for the three pairs involving geriatric conditions (hip fracture, cognitive impairment, and frequent faller), underscoring the importance of including geriatric conditions in measures of multimorbidity among older adults.
- • 30.Stenholm S, Westerlund H, Head J, Hyde M, Kawachi I, Pentti J et al. Comorbidity and functional trajectories from midlife to old age: the Health and Retirement Study. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2014;70(3):332–8.Found that age modifies the association between number of chronic diseases and functional limitations; older participants (≥ 70 years old) relative to younger participants (60–69 years) had more functional limitations as the number of diseases increased. The explanation for this age modification suggested by authors is consistent with the DPM.
- •• 31.Laditka JN, Laditka SB. Associations of multiple chronic health conditions with active life expectancy in the United States. Disability and rehabilitation. 2016;38(4):354–61.Examined the longitudinal association of multimorbidity with remaining LE and remaining DLE separately in White and African American men and women. Multimorbidity was not associated with LE, but significanlty increased DLE for all sex-race groups except African American men.
- 32.Su P, Ding H, Zhang W, Duan G, Yang Y, Chen R et al. The association of multimorbidity and disability in a community-based sample of elderly aged 80 or older in Shanghai, China. BMC geriatrics. 2016;16(1):178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •• 33.Lu FP, Chang WC, Wu SC. Geriatric conditions, rather than multimorbidity, as predictors of disability and mortality among octogenarians: A population‐based cohort study. Geriatrics & gerontology international. 2016;16(3):345–51.Examined the separate impact of multimorbid chronic health conditions and multiuple geriatric conditions on disability in young-old (65–79 years) and old-old (≥ 80 years). The study found that both multimorbid chronic health conditions and geriatric conditions were associated with incident disability in the young-old, but only geriatric conditions wre associated with incident disability in the old-old.
- 34.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J et al. Frailty in older adults evidence for a phenotype. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2001;56(3):M146–M57. [DOI] [PubMed] [Google Scholar]
- 35.Rockwood K, Mitnitski A. Frailty in relation to the accumulation of deficits. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62(7):722–7. [DOI] [PubMed] [Google Scholar]
- 36.Rockwood K, Andrew M, Mitnitski A. A comparison of two approaches to measuring frailty in elderly people. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2007;62(7):738–43. [DOI] [PubMed] [Google Scholar]
- 37.Le Cossec C, Perrine A-L, Beltzer N, Fuhrman C, Carcaillon-Bentata L. Pre-frailty, frailty, and multimorbidity: Prevalences and associated characteristics from two French national surveys. The journal of nutrition, health & aging. 2016;20(8):860–9. [DOI] [PubMed] [Google Scholar]
- 38.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2004;59(3):M255–M63. [DOI] [PubMed] [Google Scholar]
- 39.Hopman P, De Bruin SR, Forjaz MJ, Rodriguez-Blazquez C, Tonnara G, Lemmens LC et al. Effectiveness of comprehensive care programs for patients with multiple chronic conditions or frailty: A systematic literature review. Health policy. 2016;120(7):818–32. [DOI] [PubMed] [Google Scholar]
- 40.Wagner EH, Bennett SM, Austin BT, Greene SM, Schaefer JK, Vonkorff M. Finding common ground: patient-centeredness and evidence-based chronic illness care. Journal of Alternative & Complementary Medicine. 2005;11(supplement 1):s-7–s-15. [DOI] [PubMed] [Google Scholar]
- 41.Victoria J Barr SRBM-LLUADDR, Sandy S. The Expanded Chronic Care Model: An Integration of Concepts and Strategies from Population Health Promotion and the Chronic Care Model. Healthcare Quarterly. 2003;7(1):73–82. [DOI] [PubMed] [Google Scholar]
- 42.Hudon C, Fortin M, Vanasse A. Cumulative Illness Rating Scale was a reliable and valid index in a family practice context. Journal of clinical epidemiology. 2005;58(6):603–8. [DOI] [PubMed] [Google Scholar]
- 43.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–83. [DOI] [PubMed] [Google Scholar]
- 44.Lau BHP, Kwan J, Cheung KSL. Overlap of Frailty, Comorbidity, Disability, and Poor Self- Rated Health in Community-Dwelling Near-Centenarians and Centenarians. Journal of the American Geriatrics Society. 2016;64(4):900–1. [DOI] [PubMed] [Google Scholar]
- 45.Uhlig K, Leff B, Kent D, Dy S, Brunnhuber K, Burgers JS et al. A framework for crafting clinical practice guidelines that are relevant to the care and management of people with multimorbidity. Journal of general internal medicine. 2014;29(4):670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saum KU, Schöttker B, Meid AD, Holleczek B, Haefeli WE, Hauer K et al. Is polypharmacy associated with frailty in older People? Results from the ESTHER cohort study. Journal of the American Geriatrics Society. 2017;65(2). [DOI] [PubMed] [Google Scholar]
- 47.Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I et al. A global clinical measure of fitness and frailty in elderly people. Canadian Medical Association Journal. 2005;173(5):489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Kan GA, Rolland Y, Bergman H, Morley J, Kritchevsky S, Vellas B. The IANA Task Force on frailty assessment of older people in clinical practice. The Journal of Nutrition Health and Aging. 2008;12(1):29–37. [DOI] [PubMed] [Google Scholar]
- 49.Ritt M, Ritt JI, Sieber CC, Gassmann K-G. Comparing the predictive accuracy of frailty, comorbidity, and disability for mortality: a 1-year follow-up in patients hospitalized in geriatric wards. Clinical interventions in aging. 2017;12:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH et al. Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry research. 1992;41(3):237–48. [DOI] [PubMed] [Google Scholar]
- 51.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. STUDIES OF ILLNESS IN THE AGED. THE INDEX OF ADL: A STANDARDIZED MEASURE OF BIOLOGICAL AND PSYCHOSOCIAL FUNCTION. Jama. 1963;185:914–9. [DOI] [PubMed] [Google Scholar]
- 52.Bagshaw SM, Stelfox HT, McDermid RC, Rolfson DB, Tsuyuki RT, Baig N et al. Association between frailty and short-and long-term outcomes among critically ill patients: a multicentre prospective cohort study. Canadian Medical Association Journal. 2014;186(2):E95–E102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kusumastuti S, Gerds TA, Lund R, Mortensen EL, Westendorp RG. Discrimination ability of comorbidity, frailty, and subjective health to predict mortality in community-dwelling older people: Population based prospective cohort study. European Journal of Internal Medicine. 2017. [DOI] [PubMed] [Google Scholar]
- 54.Nunes BP, Flores TR, Mielke GI, Thumé E, Facchini LA. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Archives of gerontology and geriatrics. 2016;67:130–8. [DOI] [PubMed] [Google Scholar]