Abstract
Over the last thirty years, it has become increasingly clear the amount of bone (e.g. ‘bone quantity’) and the quality of the bone matrix (e.g. ‘bone quality’) both critically contribute to bone’s tissue-level mechanical behavior and the subsequent ability of bone to resist fracture. Although determining the tissue-level mechanical behavior of bone through mechanical testing is relatively straightforward in the laboratory, the destructive nature of such testing is unfeasible in humans and in animal models requiring longitudinal observation. Therefore, surrogate measurements are necessary for quantifying tissue-level mechanical behavior for the pre-clinical and clinical evaluation of bone strength and fracture risk in vivo.
A specific implementation of indentation known as reference point indentation (RPI) enables the mechanical testing of bone tissue without the need to excise and prepare the bone surface. However, this compromises the ability to carefully control the specimen geometry that is required to define the bone tissue material properties. Yet the versatility of such measurements in clinical populations is provocative, and to date there are a number of promising studies that have utilized this tool to discern bone pathologies and to monitor the effects of therapeutics on bone quality. Concurrently, on-going efforts continue to investigate the aspects of bone material behavior measured by RPI, and the compositional factors that contribute to these measurements. There are currently two variants, cyclic- and impact- RPI, that have been utilized in pre-clinical and clinical studies. This review surveys clinical studies that utilize RPI, with particular emphasis on the clinical instrument, as well as the endeavors to understand the fundamental mechanisms of such measurements. Ultimately, an improved awareness in the tradeoffs and limitations of in vivo RPI is critical towards the effective and successful utilization of this tool for the overall improvement of fragility determination in the clinic.
Keywords: Bone strength, bone quality, reference point indentation, Osteoprobe, Biodent, fracture risk
1. Introduction
The ability to resist fracture of whole bone is derived from the bone’s geometry and tissue-level mechanical behavior 1,2. In turn, the tissue-level mechanical behavior, such as crack-growth toughness, fracture toughness, material strength, and fatigue characteristics, are controlled by both bone mass and quality of the bone tissue 2. Bone mass is defined here as the quantity of bone that can be clinically measured by dual x-ray absorptiometry (DXA) 3, and bone quality is defined as the microstructural and compositional factors beyond those measurable by DXA 4,5 including but not limited to collagen crosslinking 6, trabecular architecture 7, degree of mineralization 8, hydration 9, and noncollagenous proteins 10.
Although the characterization of bone tissue mechanical behavior is relatively straightforward in the laboratory 11,12, mechanical testing requires the careful preparation of the test sample, and the testing process itself inevitably damages the sample. Although it also requires specimen preparation, indentation damages only the tissue surrounding the site of indentation, rather than the entire sample. Indentation measurements quantify the resistance of the material to plastic deformation, and this is commonly used to infer relative changes in the bone’s plasticity relating to microcracking13. Even though classical indentation approaches (i.e. hardness testing and depth-sensing indentation) were only done on ex vivo and prepared samples, the recent development of reference point indentation (RPI) provides the promise of measuring bone tissue mechanical behavior in humans. Two related RPI devices, BioDent and OsteoProbe (Active Life Scientific, Santa Barbara, CA), have been developed for research and clinical research use (Figure 1). The OsteoProbe in particular has seen accelerated adoption in clinical studies. However, significant questions remain as to how the two devices, especially OsteoProbe, relate to whole-bone fracture behavior and tissue material properties measured by traditional quasi-static mechanical testing. Here, we provide a technical overview of indentation methods in bone, review clinical and preclinical studies (cadaveric) utilizing reference point indentation analysis, and discuss the mechanisms and impact of these measurements as they apply to clinical studies.
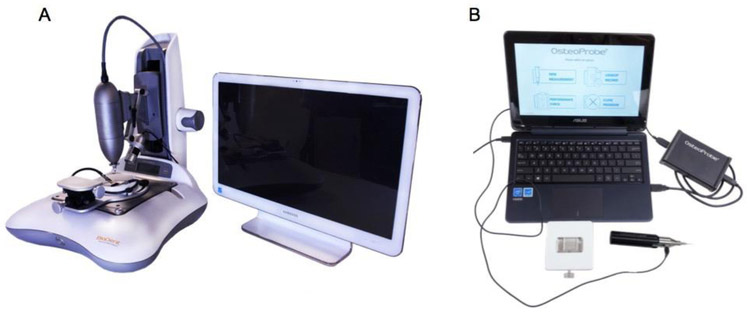
Figure 1:
(A) The cyclic reference point indentation system, BioDent, is shown here with the typical mounted setup. (B) The impact reference point indentation system, OsteoProbe, is typically operated as a hand-held device. Images obtained from http://www.activelifescientific.com.
2.1. Traditional Indentation Methods
Microscopy-based microhardness testing, the most traditional form of hardness testing used for bone, measures material resistance to plastic deformation under constant compressive load between 0.6 mN and 2 N 14. In general, indentation hardness testing applies a force P onto a prepared surface using a probe of defined geometry. The probe creates an impression of contact area Ac as determined by microscopy, and the hardness is calculated H = P/Ac. The most common probe geometries for microhardness testing are square-pyramidal (Vickers test) and rhombic-pyramidal (Knoop test). Traditional microhardness testing offers the advantage of being relatively straightforward; the major control parameters are indenter (probe) geometry, load, and dwell (hold) time. Microhardness testing in bone is also well-documented, though the appropriate force must be carefully chosen for the site of interest 15. Yet microscopy-based microhardness testing is unable to characterize purely elastic parameters. Though indentation hardness is generally well correlated with both elastic modulus and yield stress in bone 16, there is no convenient analytical method to derive either value from a hardness value alone. Additionally, this form of testing is impractical clinically, as hardness testing is subject to extremely stringent boundary conditions. In order for the area to be appropriately determined, the indenter must touch down perpendicularly to a carefully prepared specimen surface with very little tolerance for angle deviation. In bones, sample preparation is required to remove surface roughness at the indenter-bone contact surface 17. The proper preparation requires significant technical expertise and would not be sufficiently high-throughput for routine biopsies in a clinical pathology laboratory.
With the advancement of controller technology, depth-sensing indentation was developed to avoid the need for direct optical microscopic measurements. By utilizing traditional indenter geometries in conjunction with depth-sensing, it is then possible to generate a load-displacement curve and derive the indentation modulus, energy dissipation, and anisotropy in addition to hardness 14,18. The development of high-accuracy controllers also allowed these measurements to be obtained at the nano-scale 19, enabling the mapping of mechanical features down to the size of individual osteons 20. When applied to biopsies from clinical populations, nanoindentation analyses have revealed important insights in fragility in disparate populations such as those who suffered from atypical femoral fractures 21. However, depth-sensing methods still have many of the same limitations as traditional microhardness testing including the need for surface polishing embedding for relatively small samples.
In recent years, microhardness testing and depth-sensing indentation have been used to characterize a wide range of bones ex vivo. Boivin and colleagues performed hardness testing on biopsied iliac crest and calcanei 16, and found significantly decreased microhardness in osteoporotic patients. Ossicles obtained from patients with normal and inflamed middle ears revealed a relationship between mineralization and hardness 22. Similarly in cancellous bone, the Vickers microhardness of trabeculae in proximal femoral epiphyses was found to decrease with increasing severity of hip osteoarthritis 15.
2.2. Reference Point Indentation
The introduction of reference point indentation (RPI) has increased the feasibility of in vivo and in situ indentation testing of bone 23-26. Traditional hardness and depth-sensing indentation generate results that depend on precise measurement of indentation area, and they are thus highly sensitive to variations in indentation angle. This is nearly impossible to assure in vivo. RPI simplifies indentation by measuring indentation distance-based parameters of bone mechanical behavior and avoiding indentation area estimation altogether. Consequently, it is less susceptible to variations in contact angle 24,27 Furthermore, the reference probe design allows the test probe to reach the testing surface without having to make incisions on the superficial soft tissues 24,25,27,28. As a compromise, however, RPI generally does not allow the measurement of traditionally defined mechanical property parameters, and it utilizes a plastic (PMMA -polymethylmethacrylate) standard to ensure variance in tip geometry is not driving penetration depth. Emerging research and subsequent debate has gone into correlating RPI with compositional and whole-bone parameters. Two RPI devices have been developed, both by the same manufacturer (Active Life Scientific): BioDent 1000 and OsteoProbe RUO (Figure 1). The devices appear to be minimally invasive, and there are no documented adverse complications related to the RPI measurements of more than 2000 human subjects 29.
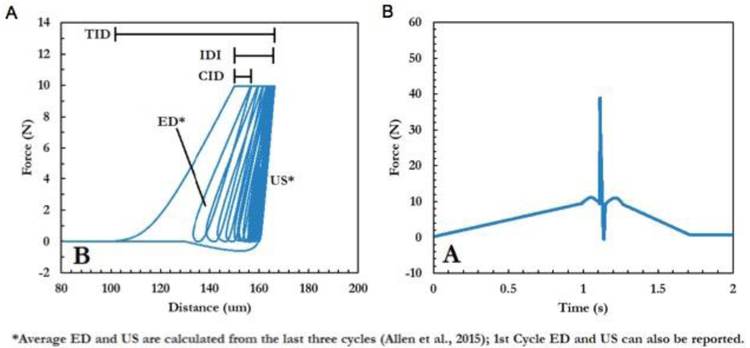
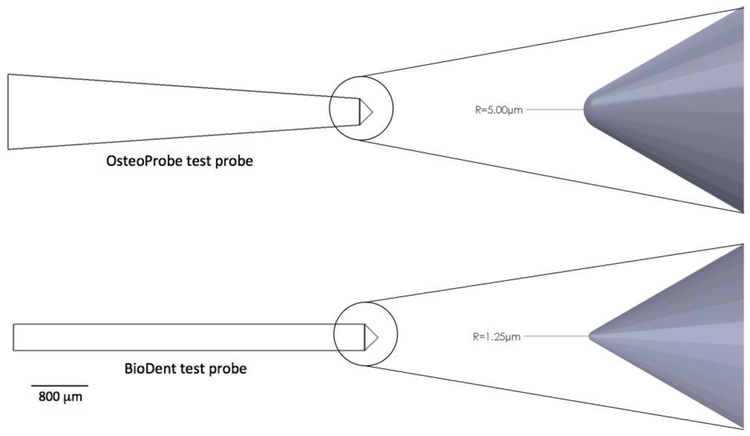
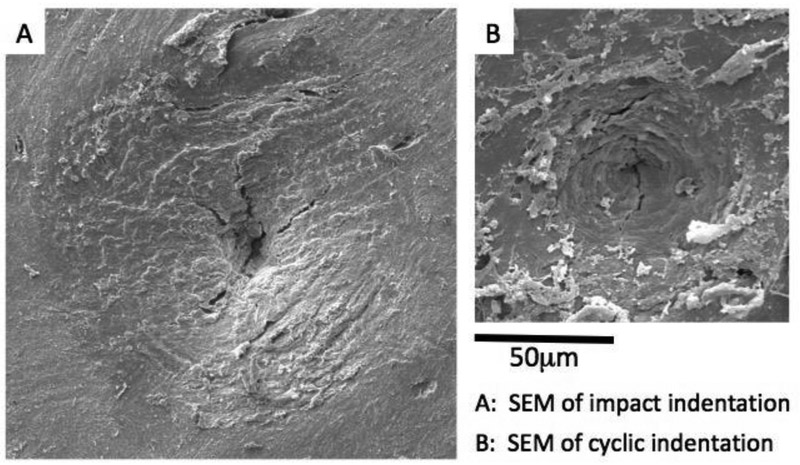
BioDent is a cyclic microindentation device utilizing dual probe assembly consisting of an outer reference probe and an inner test probe. The outer reference probe penetrates the skin and soft tissues and rests on the bone surface while the test probe engages and conducts measures on the bone tissue surface. This process can be done either by hand or through a mount. The 90° cono-spherical inner test probe (375 μm diameter, 2.5 μm tip radius) then engages until the user-defined load force is achieved (between 2 and 10 N), then retracts. This occurs cyclically up to 20 times, depending on the desired number of cycles. The waveform is such that loading, peak force dwell, and unloading are each 1/3 of the cycle 30. The system displays the force-displacement curve on the screen as testing occurs, and outputs a set of result parameters at the end of the test. These include 1st ID (indentation distance of the first cycle), TID (the total distance indented), IDI (indentation distance increase between first and last cycle), creep ID (distance increase during 1st cycle constant peak force), US (unloading slope), and energy dissipated (area inside force-displacement curve) 31 (Figure 2A). In contrast, the OsteoProbe is an impact indentation device with no outer reference probe. The test probe (375 μm tip diameter, <10 μm tip radius) is pressed onto the bone surface with handheld force. When an applied force of 10 N is achieved, a spring mechanism activates and applies an additional 30 N force over 2.5 ms (Figure 2B). After the completion of the single-cycle indentation, the system outputs a dimensionless parameter, bone mineral strength index (BMSi). BMSi is defined as 100 multiplied by the indentation distance of the test (μm), divided by an assumed PMMA indentation distance of 150 μm. After the completion of all testing on a specimen (typically 5-10 indents 32), the user indents an actual PMMA block for calibration and the final BMSi values are calculated. Since the BioDent is a cyclic microindentation device and OsteoProbe utilizes impact microindentation, the two devices are inherently different and may measure different behaviors of bone 33. It is worth noting that a higher IDI generally reflects poor resistance to indentation, while a higher BMSi suggests relatively higher resistance against indentation. The peak force of OsteoProbe can be up to 20 times greater than BioDent, and the load rate is up to 30,000 times greater. Additionally, there are differences in the way the two devices penetrate the encasing soft tissue (Figure 3). With the BioDent, three probe assembly types are available (BP1, 2, and 3) with differing reference probe geometries. The BP1 is meant for penetration and scrape removal of soft tissue, whereas the BP2 and BP3 are meant for ex vivo large and small bones respectively. In contrast, the OsteoProbe punctures soft tissues with the 10 N initial force with the testing probe before engaging the bone tissue with the additional 30 N. Both RPI devices create indentations that contain microcracks that emanate from the tip and contain a combination of elastic, plastic, and damage processes (Figure 4).
Figure 2:
Typically data and graphs displayed by from the (A) cyclic RPI (BioDent) and (B) impact RPI (OsteoProbe). Cyclic RPI allows the computation of a number of parameters based on the characteristics of the force-displace curve, while impact RPI only computes the Bone Materials Strength index, a normalized measure of indentation depth resulting from the approximately 40N indentation force.
Figure 3:
A comparison of test probes from the OsteoProbe impact microindentation and the BioDent cyclic microindentation systems. While the OsteoProbe does not have a reference probe, the outer reference probe for the BioDent is not shown here.
Figure 4:
Scanning electron micrographs of indented regions created by (A) impact indentation (OsteoProbe) and (B) cyclic indentation (BioDent) on an unprepared human bone surface. Both RPI methods result in damage including cracks and plasticity indicating the complex processes at the material level during the indentations (Images courtesy of Active Life Scientific).
2.2.1. OsteoProbe Studies
In recent years, clinical studies have been conducted almost exclusively with the OsteoProbe rather than the BioDent. This is unsurprising given the OsteoProbe is more user-friendly and perhaps more suitable for clinical implementation. A recent paper studied a group of 20 inactive but otherwise healthy postmenopausal women, before and after a short-term jumping exercise intervention 34. For three months, the women were asked to jump on the same leg according to a prescribed exercise regimen. At both time points, DXA of the hip, femoral neck, and lumbar spine were conducted for aBMD measurement. HR-pQCT was conducted on the tibiae for quantitative morphometry, and BMSi was measured by OsteoProbe. The study found after three months, there were no changes in aBMD or microarchitecture at any site, but a significant 7% increase in BMSi in the intervention leg. Interestingly, BMSi change was also negatively correlated with lumbar spine aBMD, suggesting that those with lower bone mineral density may be more responsive to short-term, high-impact exercise. Another study recruited postmenopausal women with history of distal radius fracture, hip fracture, and no fracture 35, and found that BMSi was significantly lower in the distal radius fracture group, even after adjustment for age, body mass, and femoral neck BMD. Though the unadjusted BMSi difference was significant, it was small (74.4 ± 8.8 for fracture vs. 77.4 ± 8.8 for controls), suggesting small differences in BMSi may be important for this fracture site. Conversely, the study found no significant differences in BMSi between the hip fracture and control group, and notes no study has found such a difference.
Older women also suffer from increased incidence of vertebral fracture, so the use of OsteoProbe in predicting vertebral fracture risk has also become an area of interest. One recent paper performed tibial OsteoProbe indentation on a very large group of older women (n = 472, age 75-80) and found BMSi, radial-, and tibial- cortical porosity does not differential those who had history of prevalent vertebral fracture 36. In contrast, another study showed BMSi is reduced in patients that suffered from fractures independently of the fracture site 37. Another study found neither BMSi nor lumbar aBMD were significantly different between non-osteoporotic vertebral fracture and non-fracture groups 38. In contrast, Sosa and colleagues found a significant difference in BMSi and lumbar aBMD between control and osteoporotic (lumbar T-score < −2.5) vertebral fracture groups 39. The discordance in these findings merits cautious interpretation and underscores that the etiology of these fractures are complex and a single measurement may not discern the fracture and non-fracture populations. More work is needed to determine the fundamental mechanisms responsible for the differences in these studies, and whether OsteoProbe is appropriate for fracture prediction at distant skeletal sites.
Since some therapies such as anti-inflammatory glucocorticoids may increase bone fragility before any changes in BMD can be detected 40, the ability to monitor the changes in bone tissue mechanical behavior could provide valuable feedback in the treatment administration. Indeed, one of the first studies to use OsteoProbe for drug monitoring studied patients receiving intervention for glucocorticoid-induced osteoporosis 40. Patients received calcium and Vitamin D, as well as either teriparatide (for severe osteoporosis), denosumab, risedronate, or no additional drug. Patients on teriparatide, denosumab, and risedronate had significantly increased BMSi by 20 weeks. Conversely, patients on only calcium and Vitamin D had a BMSi that decreased significantly by 11.4% at 7 weeks, were subsequently switched to bisphosphonate intervention. Although limited in its relatively small cohort size and uncontrolled patients’ inflammatory diseases and initial lumbar and femoral neck aBMDs, this study was the first to demonstrate the feasibility of using the Osteoprobe to monitor the effects of drug therapies on bone tissue quality. In another study, BMSi is significantly lower in postmenopausal women who suffered a fracture while receiving oral bisphosphonate treatment, compared to those who did not suffer a fracture 41. This difference remained significant even after adjustment for age, gender, treatment time, and lumbar spine BMD.
Finally, OsteoProbe has been utilized in cohorts with diseases where bone quality may be compromised. In particular, individuals with Type 2 Diabetes (T2D) mellitus has been shown to exhibit compromised BMSi despite normal or higher-than-normal BMD and favorable microarchitecture across multiple studies 42-44. Though the mechanisms driving reduced BMSi in diabetics are unclear, the changes BMSi has been associated with the accumulation of advanced glycation end products (AGEs) in human bone 33 and may be the putative mechanism for the fragility observed in T2D. BMSi has also been reported in other clinical morbidities, including chronic kidney disease and transplantation 45, acromegaly 46, Paget’s disease 47, Camurati-Engelmann disease, Type 1 Gaucher disease 48, and obesity 49, with BMSi observed to be lower in the respective pathological cohorts.
2.2.2. BioDent Studies
As BioDent was the first commercial RPI instrument, it was also first utilized in clinical studies. In the initial clinical study 30, Diez-Perez and colleagues studied a group of 27 women who suffered fracture requiring hospitalization (25 hip fractures, 2 multi-vertebral fractures), as well as 8 sex-matched controls. After adjustment for age, IDI was significantly greater in the fracture group than controls. A second clinical study 50 probed possible relationships between long-term bisphosphonate treatment and atypical femoral fracture. Four groups of patients were studied: controls with no fracture history, long-term bisphosphonate patients with no fracture, controls with history of typical fracture, and long-term bisphosphonate patients with fracture. The study found TID and IDI were different between fracture and non-fracture groups, but did not detect differences due to bisphosphonates use alone.
A number of cadaver studies have been performed with BioDent RPI. One study found that IDI was correlated with femoral neck strength IDI (r = −0.478), and utilizing aBMD and IDI together in a multivariate model significantly increased the predictive ability of bone strength (r = 0.883) than either measurement alone 51. BioDent was also capable of monitoring the effects of in vitro aminoguanidine and pyridoxamine incubation on ex vivo bone tissue mechanical behavior 52. In another application, IDI was used to predict maximum screw torque in fracture fixation 53.
2.2.3. Studies Comparing OsteoProbe, BioDent, and Whole-Bone Behavior
To our knowledge, there have been no clinical studies that have directly compared both the BioDent and OsteoProbe measurements in humans at a population scale. However, cadaver studies provide the opportunity to compare the BioDent and Osteoprobe with other mechanical testing modalities 33,54,55. Karim and co-authors suggest BioDent measures creep or fatigue crack growth from cyclic loading, whereas OsteoProbe is sensitive to energy dissipated from rapid loading 54. Although Biodent and Osteoprobe measurements appear to be correlated in some cases, these measures appear to be differentially sensitive to various aspects of bone composition. For example, Uppuganti and colleagues 55 found cyclic RPI to be sensitive to tissue mineral density (r = −0.89), and OsteoProbe to be sensitive to cortical porosity (r = −0.90; Table 2). Abraham et al 33 found that BMSi was more strongly correlated with advanced glycation end product (AGEs) content (r = −0.613) than IDI (r = 0.281). In the same study, IDI and BMSi correlated with tissue mineral density roughly to the same degree (r = −0.390; r = 0.430, respectively). Uppuganti et al. have also found correlations between quasi-static apparent level mechanical behavior in bending to be correlated with BMSi 55, while Abraham et al. 33 did not 33 (Table 3). Yet whole bone mechanical behavior appears to be correlated with IDI 51, and Granke et al. 56 found moderate correlations between IDI and femoral cortical beam bending parameters. Granke et al. 57 found only a weak correlation between IDI and crack initiation toughness at the tissue-level in prepared cortical beams.
Table 2.
Studies relating RPI and bone compositional and microstructural parameters. N.S. denotes not significant.
| Study | Compositional Parameter | Relationship to IDI (R-value) | Relationship to BMSi (R-value) |
|---|---|---|---|
| Abraham et al., 2016 | AGEs content | +0.281 | −0.613 |
| Tissue mineral density | −0.390 | +0.430 | |
| Cortical porosity | +0.290 | −0.299 | |
| Uppuganti et al., 2017 | Tissue mineral density (distal radius) | n.s. | −0.89 |
| Cortical porosity (distal radius) | n.s. | −0.90 | |
| Cortical porosity (proximal humerous) | n.s. | −0.70 |
Table 3.
Correlations relating RPI and tissue-level/whole-bone mechanical behavior. N.S. denotes not significant.
| Study | Mechanical Parameter | Relationship to IDI (R-value) | Relationship to BMSi (R-value) |
|---|---|---|---|
| Granke et al., 2014 | Femoral mid-shaft cortical beam 3-pt. bending ultimate stress | −0.497 | - |
| Femoral mid-shaft cortical beam 3-pt. bending toughness to failure | −0.494 | - | |
| Granke et al., 2015 | Femoral mid-shaft cortical beam crack initiation toughness | −0.26 | |
| Abraham et al., 2015 | Femoral neck failure load | −0.478 | - |
| Uppuganti et al., 2017 | Tissue 3-pt. bending strength | - | +0.739 |
| Tissue yield strength | - | +0.745 | |
| Tissue modulus | - | +0.722 | |
| Tissue toughness | - | n.s. |
3. Future Directions and Conclusion
In contrast to traditional indentation approaches, reference point indentation applies loads at a much higher rate, and this adds a degree of complexity in the material response of the bone tissue. As bone is a viscoelastic material 58-61, the quasi-static indentation approaches are designed to reduce the time-dependence of the intricately woven elastic-plastic processes and damage mechanisms invoked during testing 62. The simplicity of use in RPI approaches concomitantly increases the phenomenological turbulence of the bone tissue in response to the mechanical loading. It thus perhaps is not surprising that studies that seek to reconcile RPI-derived measurements with quasi-static mechanical testing find varying degrees of correlation – and none in some cases. Tests such as tension and compression come with the luxurious simplicity of being able to compartmentalize the elastic and plastic response on a stress-strain curve, yet these processes are occurring simultaneously during indentation. To confound matters further, the high loading rate of the OsteoProbe invokes a substantial time-dependent hardening response that can only be effectively probed by dynamic mechanical analyses over loading frequencies spanning several orders of magnitude 61. Finite element modeling studies help to elucidate the specific regional mechanisms and the constitutive behavior that occur in the dynamic reference point indentation measurements processes 63,64, but the development of these models require a clearer understanding of the hierarchical composition of material behavior at the relevant length scales.
Whether or not a significant change in BMSi between populations is meaningful remains an area of active investigation. Although tracking specific adverse clinical events prospectively (i.e. fracture, etc.) would be most informative of the predictive nature of BMSi, this is not always feasible and would be a resource-intensive endeavor. Additionally, there is a need to improve the understanding of the specific compositional factors that most strongly influence BioDent and OsteoProbe. Moreover, it appears that the two devices measure different aspects of bone mechanical behavior, so neither device will entirely capture all changes in bone tissue material quality. As a practical matter, understanding the relationship between composition and indentation-measured mechanical behavior will better identify the diseases populations that are mostly likely to benefit from these tools. A more fundamental understanding of RPI is also important if the technology is to gain wider adoption. Even as RPI’s distance-based parameters applied in a dynamic manner may not fully recapitulate the parameters obtained from carefully controlled, laboratory-based quasi-static mechanical testing, RPI has enabled clinical measurements of bone tissue mechanical behavior, where otherwise none could be done at all (save for biopsies). It is likely that the optimal clinical value of RPI will be achieved in combination with radiographic imaging, particularly when disparities between BMD and fracture risk are suspected. Clinically, these measures should be carefully interpreted in the context of the pathology and the patient as multiple compositional factors can contribute to changes in measurements across populations. Moreover, it is to be cautioned that measurements such as those determined by RPI are intended to reflect only one aspect of bone health.
Table 1.
Features of BioDent and OsteoProbe RPI.
| Feature | BioDent | OsteoProbe |
|---|---|---|
| Method | Handheld or Mounted | Handheld |
| Reference Probe | Type BP1, BP2, or BP3 | None |
| Load Force | 2-10 N | 40 N |
| Load Rate | 4 N/s – 300N/s (customizable) | 120,000 N/s |
| Cycles | Multiple | One |
| Output Parameters | 1st ID, TID, IDI, creep ID, US, energy dissipated | BMSi |
Acknowledgments
Funding: The National Institutes of Health P30 AR057235, K01AR069116, and R21AR069804.
Grant Support: The National Institutes of Health P30 AR057235, K01AR069116, and R21AR069804.
Footnotes
Conflict of Interest: S.Y. Tang is an uncompensated scientific advisor to Active Life Scientific.
Ethical approval : Not applicable.
Informed consent: Not applicable.
Disclosure: S.Y. Tang is an uncompensated scientific advisor to Active Life Scientific.
4. REFERENCES
- 1.Zimmermann EA, Schaible E, Bale H, et al. Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proceedings of the National Academy of Sciences of the United States of America 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hernandez CJ, van der Meulen MC. Understanding Bone Strength Is Not Enough. J Bone Miner Res 2017; 32(6): 1157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings SR, Bates D, Black DM. Clinical use of bone densitometry: scientific review. JAMA 2002; 288(15): 1889–97. [DOI] [PubMed] [Google Scholar]
- 4.Donnelly E. Methods for Assessing Bone Quality: A Review. Clinical orthopaedics and related research 2010; 469(8): 2128–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nyman JS, Granke M, Singleton RC, Pharr GM. Tissue-Level Mechanical Properties of Bone Contributing to Fracture Risk. Current osteoporosis reports 2016; 14(4): 138–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boskey AL, Wright TM, Blank RD. Collagen and bone strength. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 1999; 14(3): 330–5. [DOI] [PubMed] [Google Scholar]
- 7.Keaveny TM, Morgan EF, Niebur GL, Yeh OC. Biomechanics of trabecular bone. Annual review of biomedical engineering 2001; 3: 307–33. [DOI] [PubMed] [Google Scholar]
- 8.Morris MD, Mandair GS. Raman assessment of bone quality. Clinical orthopaedics and related research 2011; 469(8): 2160–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manhard MK, Uppuganti S, Granke M, Gochberg DF, Nyman JS, Does MD. MRI-derived bound and pore water concentrations as predictors of fracture resistance. Bone 2016; 87: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sroga GE, Vashishth D. Effects of Bone Matrix Proteins on Fracture and Fragility in Osteoporosis. Current osteoporosis reports 2012; 10(2): 141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reilly DT, Burstein AH, Frankel VH. The elastic modulus for bone. Journal of Biomechanics 1974; 7(3): 271–5. [DOI] [PubMed] [Google Scholar]
- 12.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Calcified tissue international 1993; 14(4): 595–608. [DOI] [PubMed] [Google Scholar]
- 13.Vashishth D, Behiri JC, Bonfield W. Crack growth resistance in cortical bone: concept of microcrack toughening. Journal of Biomechanics 1997; 30(8): 763–9. [DOI] [PubMed] [Google Scholar]
- 14.Zysset PK. Indentation of bone tissue: a short review. Osteoporos Int 2009; 20(6): 1049–55. [DOI] [PubMed] [Google Scholar]
- 15.Tomanik M, Nikodem A, Filipiak J. Microhardness of human cancellous bone tissue in progressive hip osteoarthritis. J Mech Behav Biomed Mater 2016; 64: 86–93. [DOI] [PubMed] [Google Scholar]
- 16.Boivin G, Bala Y, Doublier A, et al. The role of mineralization and organic matrix in the microhardness of bone tissue from controls and osteoporotic patients. Bone 2008; 43(3): 532–8. [DOI] [PubMed] [Google Scholar]
- 17.Huja SS, Beck FM, Thurman DT. Indentation properties of young and old osteons. Calcified tissue international 2006; 78(6): 392–7. [DOI] [PubMed] [Google Scholar]
- 18.Oliver WC, Pharr GM. Measurement of hardness and elastic modulus by instrumented indentation: Advances in understanding and refinements to methodology. Journal of Materials Research 2004; 19(1): 3–20. [Google Scholar]
- 19.Ferguson VL. Deformation partitioning provides insight into elastic, plastic, and viscous contributions to bone material behavior. Journal of the mechanical behavior of biomedical materials 2009; 2(4): 364–74. [DOI] [PubMed] [Google Scholar]
- 20.Hoffler CE, Guo XE, Zysset PK, Goldstein SA. An application of nanoindentation technique to measure bone tissue Lamellae properties. J Biomech Eng 2005; 127(7): 1046–53. [DOI] [PubMed] [Google Scholar]
- 21.Lloyd AA, Gludovatz B, Riedel C, et al. Atypical fracture with long-term bisphosphonate therapy is associated with altered cortical composition and reduced fracture resistance. Proceedings of the National Academy of Sciences 2017; 114(33): 8722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duboeuf F, Burt-Pichat B, Farlay D, Suy P, Truy E, Boivin G. Bone quality and biomechanical function: a lesson from human ossicles. Bone 2015; 73: 105–10. [DOI] [PubMed] [Google Scholar]
- 23.Bridges D, Randall C, Hansma PK. A new device for performing reference point indentation without a reference probe. The Review of scientific instruments 2012; 83(4): 044301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansma P, Turner P, Fantner G. Bone diagnostic instrument. Review of Scientific Instruments 2006. [Google Scholar]
- 25.Hansma P, Yu H, Schultz D, et al. The tissue diagnostic instrument. The Review of scientific instruments 2009; 80(5): 054303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang SY, Mathews P, Randall C, et al. In situ Materials Characterization using the Tissue Diagnostic Instrument. Polymer Testing 2010; 29(2): 159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bridges D, Randall C, Hansma PK. A new device for performing reference point indentation without a reference probe. Rev Sci Instrum 2012; 83(4): 044301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansma P, Turner P, Drake B, et al. The bone diagnostic instrument II: indentation distance increase. Rev Sci Instrum 2008; 79(6): 064303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herrera S, Diez-Perez A. Clinical experience with microindentation in vivo in humans. Bone 2017; 95: 175–82. [DOI] [PubMed] [Google Scholar]
- 30.Diez-Perez A, Guerri R, Nogues X, et al. Microindentation for in vivo measurement of bone tissue mechanical properties in humans. J Bone Miner Res 2010; 25(8): 1877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen MR, McNerny EM, Organ JM, Wallace JM. True Gold or Pyrite: A Review of Reference Point Indentation for Assessing Bone Mechanical Properties In Vivo. J Bone Miner Res 2015; 30(9): 1539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diez-Perez A, Bouxsein ML, Eriksen EF, et al. Technical note: Recommendations for a standard procedure to assess cortical bone at the tissue-level in vivo using impact microindentation. Bone Rep 2016; 5: 181–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abraham AC, Agarwalla A, Yadavalli A, Liu JY, Tang SY. Microstructural and compositional contributions towards the mechanical behavior of aging human bone measured by cyclic and impact reference point indentation. Bone 2016; 87: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sundh D, Nilsson M, Zoulakis M, et al. High-Impact Mechanical Loading Increases Bone Material Strength in Postmenopausal Women-A 3-Month Intervention Study. J Bone Miner Res 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rozental TD, Walley KC, Demissie S, et al. Bone Material Strength Index as Measured by Impact Microindentation in Postmenopausal Women With Distal Radius and Hip Fractures. J Bone Miner Res 2018; 33(4): 621–6. [DOI] [PubMed] [Google Scholar]
- 36.Johansson L, Sundh D, Zoulakis M, et al. The Prevalence of Vertebral Fractures Is Associated With Reduced Hip Bone Density and Inferior Peripheral Appendicular Volumetric Bone Density and Structure in Older Women. J Bone Miner Res 2018; 33(2): 250–60. [DOI] [PubMed] [Google Scholar]
- 37.Malgo F, Hamdy NAT, Papapoulos SE, Appelman-Dijkstra NM. Bone material strength index as measured by impact microindentation is low in patients with fractures irrespective of fracture site. Osteoporos Int 2017; 28(8): 2433–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rudang R, Zoulakis M, Sundh D, et al. Bone material strength is associated with areal BMD but not with prevalent fractures in older women. Osteoporos Int 2016; 27(4): 1585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sosa DD, Eriksen EF. Reduced Bone Material Strength is Associated with Increased Risk and Severity of Osteoporotic Fractures. An Impact Microindentation Study. Calcif Tissue Int 2017; 101(1): 34–42. [DOI] [PubMed] [Google Scholar]
- 40.Mellibovsky L, Prieto-Alhambra D, Mellibovsky F, et al. Bone Tissue Properties Measurement by Reference Point Indentation in Glucocorticoid-Induced Osteoporosis. J Bone Miner Res 2015; 30(9): 1651–6. [DOI] [PubMed] [Google Scholar]
- 41.Nogues X, Prieto-Alhambra D, Guerri-Fernandez R, et al. Fracture during oral bisphosphonate therapy is associated with deteriorated bone material strength index. Bone 2017; 103: 64–9. [DOI] [PubMed] [Google Scholar]
- 42.Nilsson AG, Sundh D, Johansson L, et al. Type 2 Diabetes Mellitus Is Associated With Better Bone Microarchitecture But Lower Bone Material Strength and Poorer Physical Function in Elderly Women: A Population-Based Study. J Bone Miner Res 2017; 32(5): 1062–71. [DOI] [PubMed] [Google Scholar]
- 43.Furst JR, Bandeira LC, Fan WW, et al. Advanced Glycation Endproducts and Bone Material Strength in Type 2 Diabetes. J Clin Endocrinol Metab 2016; 101(6): 2502–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farr JN, Drake MT, Amin S, Melton LJ 3rd, McCready LK, Khosla S. In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res 2014; 29(4): 787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perez-Saez MJ, Herrera S, Prieto-Alhambra D, et al. Bone density, microarchitecture, and material strength in chronic kidney disease patients at the time of kidney transplantation. Osteoporos Int 2017; 28(9): 2723–7. [DOI] [PubMed] [Google Scholar]
- 46.Malgo F, Hamdy NA, Rabelink TJ, et al. Bone material strength index as measured by impact microindentation is altered in patients with acromegaly. Eur J Endocrinol 2017; 176(3): 339–47. [DOI] [PubMed] [Google Scholar]
- 47.Malgo F, Hamdy NA, Papapoulos SE, Appelman-Dijkstra NM. Impact Microindentation: Consistency of Serial Measurements and Alterations in Patients With Paget's Disease of the Tibia. J Bone Miner Res 2017; 32(12): 2375–80. [DOI] [PubMed] [Google Scholar]
- 48.Herrera S, Perez-Lopez J, Molto-Abad M, et al. Assessment of Bone Health in Patients With Type 1 Gaucher Disease Using Impact Microindentation. J Bone Miner Res 2017; 32(7): 1575–81. [DOI] [PubMed] [Google Scholar]
- 49.Sundh D, Rudang R, Zoulakis M, Nilsson AG, Darelid A, Lorentzon M. A High Amount of Local Adipose Tissue Is Associated With High Cortical Porosity and Low Bone Material Strength in Older Women. J Bone Miner Res 2016; 31(4): 749–57. [DOI] [PubMed] [Google Scholar]
- 50.Guerri-Fernandez RC, Nogues X, Quesada Gomez JM, et al. Microindentation for in vivo measurement of bone tissue material properties in atypical femoral fracture patients and controls. J Bone Miner Res 2013; 28(1): 162–8. [DOI] [PubMed] [Google Scholar]
- 51.Abraham AC, Agarwalla A, Yadavalli A, McAndrew C, Liu JY, Tang SY. Multiscale Predictors of Femoral Neck In Situ Strength in Aging Women: Contributions of BMD, Cortical Porosity, Reference Point Indentation, and Nonenzymatic Glycation. J Bone Miner Res 2015; 30(12): 2207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abar O, Dharmar S, Tang SY. The effect of aminoguanidine (AG) and pyridoxamine (PM) on ageing human cortical bone. Bone Joint Res 2018; 7(1): 105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McAndrew CM, Agarwalla A, Abraham AC, Feuchtbaum E, Ricci WM, Tang SY. Local bone quality measurements correlates with maximum screw torque at the femoral diaphysis. Clin Biomech (Bristol, Avon) 2018; 52: 95–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karim L, Van Vliet M, Bouxsein ML. Comparison of cyclic and impact-based reference point indentation measurements in human cadaveric tibia. Bone 2018; 106: 90–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Uppuganti S, Granke M, Manhard MK, et al. Differences in sensitivity to microstructure between cyclic- and impact-based microindentation of human cortical bone. J Orthop Res 2017; 35(7): 1442–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Granke M, Coulmier A, Uppuganti S, Gaddy JA, Does MD, Nyman JS. Insights into reference point indentation involving human cortical bone: sensitivity to tissue anisotropy and mechanical behavior. J Mech Behav Biomed Mater 2014; 37: 174–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Granke M, Makowski AJ, Uppuganti S, Does MD, Nyman JS. Identifying Novel Clinical Surrogates to Assess Human Bone Fracture Toughness. J Bone Miner Res 2015; 30(7): 1290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lakes RS, Katz JL. Viscoelastic properties of wet cortical bone--II. Relaxation mechanisms. Journal of Biomechanics 1979; 12(9): 679–87. [DOI] [PubMed] [Google Scholar]
- 59.Lakes RS, Katz JL. Viscoelastic properties of wet cortical bone--III. A non-linear constitutive equation. Journal of Biomechanics 1979; 12(9): 689–98. [DOI] [PubMed] [Google Scholar]
- 60.Lakes RS, Katz JL, Sternstein SS. Viscoelastic properties of wet cortical bone--I. Torsional and biaxial studies. Journal of Biomechanics 1979; 12(9): 657–78. [DOI] [PubMed] [Google Scholar]
- 61.Buechner PM, Lakes RS. Size effects in the elasticity and viscoelasticity of bone. Biomechanics and modeling in mechanobiology 2003; 1(4): 295–301. [DOI] [PubMed] [Google Scholar]
- 62.Fan Z, Rho J-Y. Effects of viscoelasticity and time-dependent plasticity on nanoindentation measurements of human cortical bone. Journal of biomedical materials research Part A 2003; 67(1): 208–14. [DOI] [PubMed] [Google Scholar]
- 63.Hoffseth K, Randall C, Hansma P, Yang HT. Study of indentation of a sample equine bone using finite element simulation and single cycle reference point indentation. J Mech Behav Biomed Mater 2015; 42: 282–91. [DOI] [PubMed] [Google Scholar]
- 64.Idkaidek A, Agarwal V, Jasiuk I. Finite element simulation of Reference Point Indentation on bone. J Mech Behav Biomed Mater 2017; 65: 574–83. [DOI] [PubMed] [Google Scholar]