Abstract
Background
Anthracyclines are among the most effective chemotherapeutic agents in the treatment of numerous malignancies. Unfortunately, their use is limited by a dose‐dependent cardiotoxicity. In an effort to prevent this cardiotoxicity, different cardioprotective agents have been studied.
Objectives
The objective of this review was to assess the efficacy of different cardioprotective agents in preventing heart damage in cancer patients treated with anthracyclines.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 10), MEDLINE (1966 to November 2010) and EMBASE (1980 to November 2010) databases. In addition, we handsearched reference lists, conference proceedings of the International Society of Paediatric Oncology (SIOP) and American Society of Clinical Oncology (ASCO) meetings (1998 to 2010) and ongoing trials registers.
Selection criteria
Randomised controlled trials (RCTs) in which any cardioprotective agent was compared to no additional therapy or placebo in cancer patients (children and adults) receiving anthracyclines.
Data collection and analysis
Two review authors independently performed the study selection, risk of bias assessment and data extraction including adverse effects.
Main results
We identified RCTs for the eight cardioprotective agents N‐acetylcysteine, phenethylamines, coenzyme Q10, a combination of vitamins E and C and N‐acetylcysteine, L‐carnitine, carvedilol, amifostine and dexrazoxane (mostly for adults with advanced breast cancer). All studies had methodological limitations and for the first seven agents there were too few studies to allow pooling of results. None of the individual studies showed a cardioprotective effect. The 10 included studies on dexrazoxane enrolled 1619 patients. The meta‐analysis for dexrazoxane showed a statistically significant benefit in favour of dexrazoxane for the occurrence of heart failure (risk ratio (RR) 0.29, 95% CI 0.20 to 0.41). No evidence was found for a difference in response rate or survival between the dexrazoxane and control groups. The results for adverse effects were ambiguous. No significant difference in the occurrence of secondary malignancies was identified.
Authors' conclusions
No definitive conclusions can be made about the efficacy of cardioprotective agents for which pooling of results was impossible. Dexrazoxane prevents heart damage and no evidence for a difference in response rate or survival between the dexrazoxane and control groups was identified. The evidence available did not allow us to reach any definite conclusions about adverse effects. We conclude that if the risk of cardiac damage is expected to be high, it might be justified to use dexrazoxane in patients with cancer treated with anthracyclines. However, clinicians should weigh the cardioprotective effect of dexrazoxane against the possible risk of adverse effects for each individual patient.
Keywords: Humans; Anthracyclines; Anthracyclines/adverse effects; Antibiotics, Antineoplastic; Antibiotics, Antineoplastic/adverse effects; Cardiotonic Agents; Cardiotonic Agents/therapeutic use; Cytoprotection; Heart Diseases; Heart Diseases/chemically induced; Heart Diseases/prevention & control; Neoplasms; Neoplasms/drug therapy; Randomized Controlled Trials as Topic; Razoxane; Razoxane/therapeutic use
Plain language summary
Drugs to prevent heart damage in cancer patients receiving anthracyclines
Anthracyclines are among the most effective chemotherapy treatments available for various types of cancer. However, there is a risk of damage to the heart (cardiotoxicity) depending on the cumulative dose. Certain drugs might prevent this damage, but for many of these drugs, the review authors found no high quality evidence about whether they were effective in protecting the heart and they were unable to draw conclusions. For dexrazoxane, the review authors found 10 studies enrolling over 1600 patients. These studies provided evidence that dexrazoxane prevented heart damage without interfering with the anti‐tumour effects of anthracycline treatment. Patients who got dexrazoxane with their anthracycline treatment had about one third of the risk of heart failure compared to patients who got anthracyclines without dexrazoxane. Dexrazoxane had no effect on survival. We can't be sure about whether it had any undesirable side effects.
Background
Anthracyclines, that is doxorubicin, epirubicin and daunorubicin, are among the most effective drugs used in chemotherapy for cancer patients. They are widely used to treat solid tumours and leukaemia in both adults and children. Their use is, however, limited because they often cause damage to the heart, especially if the patient is given a high dose (Bonadonna 1969; Lefrak 1973).
We do not understand exactly how anthracyclines cause heart damage. It may be because of lipid peroxidation and the generation of free radicals by anthracycline‐iron complexes. The heart is particularly vulnerable to injury from free radicals because it has a lower level of protective enzymes such as superoxide dismutase than other tissues (Keizer 1990; Myers 1998). The damage to heart cells may eventually lead to irreversible heart failure.
Heart damage after anthracycline therapy can be divided into early and late cardiotoxicity. Early cardiotoxicity refers to heart damage that develops during anthracycline therapy or in the first year after completion; late cardiotoxicity refers to heart damage that only becomes evident at least one year after the completion of anthracycline therapy (Shan 1996). The risk of developing heart failure remains a lifelong threat, especially to children who have a long life‐expectancy after successful treatment for cancer.
Heart damage can occur as either subclinical or clinical cardiotoxicity. The term subclinical cardiotoxicity is used to describe various cardiac abnormalities, diagnosed with different diagnostic methods in patients without symptoms. Clinical cardiotoxicity is defined on the basis of symptoms of clinical heart failure that are confirmed by an abnormal diagnostic test. In the end stage of clinical heart failure, heart transplantation is the only remaining treatment option.
There is a wide variation in the reported frequency of both clinical and subclinical cardiotoxicity after anthracycline therapy. In children, the prevalence of subclinical heart failure at a median of 6.4 years after treatment has been reported to be more than 57% (Kremer 2002a) and the incidence of clinical heart failure is as high as 16% 0.9 to 4.8 years after treatment (Kremer 2002b). Part of this variation can be explained by the type of anthracycline used, the total anthracycline dose and the peak anthracycline dose. Some of the variation may be explained by different definitions of heart failure and different ways of assessing it. Further variation may be explained by additional risk factors for developing heart damage such as radiation therapy involving the heart region; type of tumour; exposure to cyclophosphamide, iphosphamide or amsacrine; female sex; age (children and elderly people have a higher risk); and existing heart disease.
Clinicians face a clinical dilemma as they balance the efficacy of longer duration of therapy against the cardiotoxicity associated with higher cumulative doses of anthracyclines. In an effort to prevent or reduce this cardiotoxicity, extensive research has been devoted to the identification of methods or drugs capable of ameliorating the toxicity. Several less cardiotoxic anthracycline analogs have been developed, including liposomal anthracyclines (Batist 2001; Muggia 1991; Muggia 1997; Van Dalen 2010), and the cumulative and peak doses of anthracycline therapy have been reduced (Legha 1982a; Lipshultz 1998; Von Hoff 1979; Van Dalen 2009). Despite these efforts, cardiotoxicity remains a problem.
A different approach to reducing anthracycline‐induced heart damage is the use of cardioprotective agents, of which dexrazoxane (Cardioxane, ICRF‐187; Zinecard, ADR‐529) is the most widely investigated drug (Swain 1997a(088001); Wexler 1996). Other drugs like L‐carnitine, probucol, coenzyme Q10, n‐acetylcysteine, vitamin E, digoxin, enalapril, phenethylamines, deferoxamine, ethylenediaminetetraacetic acid (EDTA), superoxide dismutase and monohydroxyethylrutoside are less well investigated; however cardioprotective effects have been reported (De Leonardis 1985; Elihu 1998; Garbrecht 1986; Guthrie 1977; Iarussi 1994; Kawasaki 1992; Legha 1982b; Silber 2001; Singal 1995; Unverferth 1983a; Van Acker 2000).
An important question regarding any cardioprotective intervention during anthracycline therapy is whether the cardioprotective drug can decrease the heart damage caused by anthracyclines without reducing the anti‐tumour efficacy and without negative effects on toxicities other than cardiac damage, such as alopecia, nausea, vomiting, stomatitis, diarrhoea, fatigue, anaemia, leukopenia and thrombocytopenia.
This is the second update of the systematic review on cardioprotective interventions during anthracycline therapy. Since performing the first update of this review, new evidence on the cardioprotective drugs has became available. All new evidence is included in this update.
Objectives
Primary objective
To ascertain the efficacy of any cardioprotective agent to prevent heart damage in patients with cancer treated with anthracyclines when compared to placebo or no additional treatment
Secondary objectives
To determine possible effects of these cardioprotective interventions on the anti‐tumour efficacy of anthracyclines
To determine possible effects of these cardioprotective interventions on anthracycline toxicities other than cardiac damage
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Patients with cancer (both adults and children) who received anthracycline chemotherapy.
Types of interventions
Intervention: anthracycline therapy together with a cardioprotective agent.
Control: anthracycline therapy with or without a placebo.
In the design of the study, it should have been the intention to treat both the intervention and control groups with the same cumulative anthracycline dose; the median or mean cumulative anthracycline dose actually received should not have differed between treatment groups by 100 mg/m2 of body surface area or more. Chemotherapy other than anthracyclines and radiotherapy involving the heart region should have been the same in both treatment groups.
Types of outcome measures
Primary outcomes
Heart failure, that is clinical (as defined by the authors) or subclinical heart failure (defined as either histological abnormalities scored by the Billingham score on endomyocardial biopsy or abnormalities in cardiac function measured by echocardiography or radionuclide ventriculography).
Secondary outcomes
These included potential adverse effects of cardioprotective interventions on:
response (defined as the number of complete and partial remissions);
overall survival (OS);
progression‐free survival (PFS);
quality of life (QoL);
toxicities other than cardiac damage (such as alopecia, nausea, vomiting, stomatitis, diarrhoea, fatigue, anaemia, leukopenia, thrombocytopenia).
Search methods for identification of studies
Electronic searches
See: Gynaecological Cancer Review Group methods used in reviews.
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 10), MEDLINE (PubMed) (from 1966 to November 2010) and EMBASE (Ovid) (from 1980 to November 2010) databases were searched. The search strategies for the different electronic databases (using a combination of controlled vocabulary and text word terms) are detailed in the appendices (Appendix 1, Appendix 2, Appendix 3).
Searching other resources
Information about trials not listed in CENTRAL, MEDLINE or EMBASE, either published or unpublished, was located by searching the reference lists of relevant articles and review articles. In addition, the conference proceedings of the International Society for Paediatric Oncology (SIOP) and the American Society of Clinical Oncology (ASCO) were searched from 1998 to 2010 for cardioprotective interventions included in the original review; and from 2003 to 2010 for newly included (since the first update) cardioprotective interventions. We searched for ongoing trials by scanning the ISRCTN register and the National Institute of Health register (www.controlled‐trials.com) (both screened November 2010). No language restriction was imposed.
Data collection and analysis
Selection of studies
After performing the search strategy described previously, identification of studies meeting the inclusion criteria was undertaken independently by two review authors. Any study seemingly meeting the inclusion criteria based on the title, abstract, or both, was obtained in full for closer inspection. Discrepancies were resolved by discussion. No arbitration by the contact editor was needed.
Data extraction and management
Data extraction was performed independently by two review authors using standardised forms. The characteristics of the participants (for example age, type of malignancy, stage of disease), interventions (for example route of delivery, dose, timing), outcome measures and length of follow up were extracted. To inform interpretation of the findings, the similarity of the experimental groups at baseline regarding the most important prognostic indicators (that is age, prior cardiotoxic therapy, prior cardiac dysfunction and stage of disease) was assessed. Discrepancies between review authors were solved by discussion. No arbitration by the contact editor was needed.
Assessment of risk of bias in included studies
The risk of bias in the included trials was assessed independently by two review authors according to the following criteria: concealment of treatment allocation, blinding of care providers, blinding of patients, blinding of outcome assessors (in the updates we assessed this item for each outcome separately), and completeness of follow up (in the updates we assessed this item for each outcome separately). See additional Table 1 for a description of the criteria used. Allocation concealment was assessed using the scale set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2006). Discrepancies between review authors were resolved by discussion. No arbitration by the contact editor was needed.
1. Criteria list for the risk of bias assessment in included studies.
| Item ID | Description | Implementation |
| Patient selection | Note: all criteria were scored yes (+), no (‐) or unclear (?) | |
| a | Was the treatment allocation concealed? | Allocation must have been performed by a person not responsible for determining eligibility of patients for inclusion. |
| Interventions | ||
| b | Was the care provider blinded to the intervention? | Adequate information about blinding must have been provided. |
| c | Was the patient blinded to the intervention? | Adequate information about blinding must have been provided. |
| Outcome measurements (for each outcome separately) | ||
| d | Was the outcome assessor blinded to the intervention? | Adequate information about blinding must have been provided. |
| e | Were patients lost to follow‐up described and acceptable? | For each outcome measure the number of evaluated patients must be mentioned. If the percentage of loss‐to‐follow‐up does not exceed 20% and does not lead to substantial bias, a yes is scored. |
Data synthesis
The data were entered into RevMan and analysed according to the guidelines of the Cochrane Handbook (Higgins 2006). Dichotomous outcomes were related to risk using the risk ratio (RR). If possible, data were extracted by allocated group, irrespective of compliance with the allocated intervention, in order to allow an intention‐to‐treat analysis. It was stated if this was not possible. Heterogeneity was assessed both by visual inspection of forest plots and by a formal statistical test for heterogeneity, that is the I2 statistic (I2 > 50% was considered to represent substantial heterogeneity) (Higgins 2006). If no substantial heterogeneity was detected, a fixed‐effect model was used for the estimation of treatment effects; otherwise we used the random‐effects model. All results are presented with the corresponding 95% confidence interval (CI).
A meta‐analysis was performed for each cardioprotective intervention for which two or more studies were identified. Interventions for which fewer studies were identified were summarised descriptively. For outcomes where only one study was available we were unable to calculate a RR if one of the treatment groups experienced no events and the Fischer's exact test was used instead (in statcalc.exe). Subgroup analyses were not performed. For PFS and OS, we used the generic inverse variance function of RevMan to combine logs of the hazard ratios (HRs).
Parmar's method was used to extract the log of the HR and its standard error (SE) from survival curves (Parmar 1998) for the studies of Marty 2006 and Speyer 1992. We digitised the published Kaplan‐Meier survival curves and noted the minimum and maximum duration of follow up, which are required for Parmar's method. We performed the required calculations in Stata 9, using a specially written program, which yielded the reported log(HR) and variance when used on the data presented in table V of Parmar 1998. For the study of Swain (Swain 1997a(088001); Swain 1997a(088006)), calculations were performed in an Excel spreadsheet.
The risk of bias in the studies included in the analyses was taken into account in the interpretation of the results of the review. For all outcomes for which pooling was possible we performed sensitivity analyses for all risk of bias criteria separately. We excluded the studies with a high risk of bias and the studies for which the risk of bias was unclear to compare the results of the studies with a low risk of bias with the results of all available studies.
Results
Description of studies
We assessed the search results for CENTRAL (175 references: 48 identified in the first update (April 2007); four identified in the second update (November 2010)), MEDLINE (1129 references: 240 identified in the first update (April 2007); 57 identified in the second update (November 2010)) and EMBASE (2729 references: 1606 identified in the first update (April 2007); 162 identified in the second update (November 2010)). We included a total of 19 articles which fulfilled all the criteria for including studies in this review: one addressed N‐acetylcysteine; two addressed phenethylamines; one addressed coenzyme Q10; one addressed the combination of vitamin E, vitamin C and N‐acetylcysteine; one addressed L‐carnitine (new in the first update); one addressed carvedilol (new in the first update); one addressed amifostine (new in the second update); and 11 addressed dexrazoxane (three new in the first update; three new in the second update). One of the articles addressing dexrazoxane provided the results of two RCTs (Swain 1997a(088001); Swain 1997a(088006)) and two articles (Barry 2008; Lipshultz 2010) provided long‐term follow‐up data of a RCT addressing dexrazoxane (Lipshultz 2004); therefore the total number of identified RCTs was 18 (that is 10 for dexrazoxane and 8 for other cardioprotective interventions) (see Characteristics of included studies table).
Twenty‐five articles were excluded for reasons described in the Characteristics of excluded studies table. Two studies did not provide enough information to assess eligibility for this review and we did not succeed in contacting the authors. These studies are included in the Characteristics of studies awaiting classification table. The remaining 3987 articles were excluded since they were not RCTs, were laboratory studies, were animal studies, or did not have heart failure as an outcome measure.
By scanning the reference lists of relevant articles and reviews no additional studies could be included in the review. However, we identified the abstracts of two studies addressing dexrazoxane. These studies have not been published yet and are awaiting further classification (see Characteristics of studies awaiting classification table). We also identified one ongoing trial addressing dexrazoxane, which is described in the Characteristics of ongoing studies table. Three studies were added to the Characteristics of excluded studies table.
We identified abstracts of four studies addressing dexrazoxane and an abstract of one study addressing amifostine by scanning the conference proceedings of SIOP and ASCO. These studies have not been published yet and are awaiting further classification (see Characteristics of studies awaiting classification table).
By scanning the ongoing trials databases we identified six ongoing trials, three addressing dexrazoxane, one addressing L‐carnitine, one addressing valsartan and one addressing the ACE‐inhibitor enalapril (see Characteristics of ongoing studies table).
We also checked (November 2010) if new information was available on the studies added to the Characteristics of ongoing studies table and the Characteristics of studies awaiting classification table in the original version and the first update of this review. Data on cardiac outcomes of one of the ongoing studies had been published and was identified in the search of the electronic databases (Schwartz 2009). One of the studies in the Characteristics of studies awaiting classification table had been published in full text and was identified in the search of the electronic databases (Gallegos‐Castorena 2007). These studies have been moved to the Characteristics of included studies table.
The characteristics of the included studies are summarised below and their baseline characteristics are described in the Characteristics of included studies table.
For the following possible cardioprotective interventions we were not able to include RCTs: probucol, vitamin E alone, digoxin, ACE‐inhibitors, deferoxamine, EDTA, superoxide dismutase, monohydroxyethylrutoside, vitamin C alone, guanidines, cytochromes, vitamin A, sildenafil, selenium, glutathione, valsartan, and trimetazidine.
N‐acetylcysteine
One study addressed N‐acetylcysteine (Myers 1983). This study included 54 adults (24 in the intervention group and 30 in the control group) with a solid tumour and who were treated with doxorubicin. However, five patients in the intervention group stopped N‐acetylcysteine due to nausea. It was unclear if patients in the intervention and control groups received similar cumulative doses of anthracycline.
Phenethylamines
Two studies addressed phenethylamines (Kraft 1990; Milei 1987).
Milei et al investigated the effect of prenylamine versus placebo (Milei 1987). This study included 36 adults (18 in both the intervention and control groups) with a solid tumour and who were treated with doxorubicin. Ten patients (five in each group) were withdrawn because they died prior to undergoing the final evaluation. It was unclear if patients in the intervention and control groups received similar cumulative anthracycline doses.
Kraft et al investigated the effect of verapamil versus no cardioprotective intervention (Kraft 1990). This study included 64 adults (30 in the intervention group and 34 in the control group) with leukaemia, who were treated with daunorubicin. Only 30 (13 in the intervention group and 17 in the control group) randomised patients were evaluated. It was unclear if patients in the intervention and control groups received similar cumulative anthracycline doses.
Coenzyme Q10
One study addressed coenzyme Q10 (Iarussi 1994). This study included 20 children (10 in both the intervention and control groups) with either a solid tumour or leukaemia, who were all treated with doxorubicin and in some cases also daunorubicin. The cumulative anthracycline dose that patients received was similar in the intervention and control group.
Combination of vitamin E, vitamin C and N‐acetylcysteine
One study addressed a combination of vitamin E, vitamin C and N‐acetylcysteine (Wagdi 1995). This study included 17 adults but three patients were lost to follow up (one refused treatment and two refused control visits). It was unclear to which group these patients were randomised. Therefore, data were available on six patients in the intervention group and eight in the control group. Patients were diagnosed with a solid tumour and treated with doxorubicin. The cumulative anthracycline dose that patients received was comparable between the intervention and control groups.
L‐carnitine
One study, identified in the first update of this review, addressed L‐carnitine (Waldner 2006). This study included 40 adults (20 in both the intervention and control group) with a solid tumour, who were treated with doxorubicin. It was unclear if patients in the intervention and control groups received similar cumulative anthracycline doses.
Carvedilol
One study, identified in the first update of this review, addressed carvedilol (Kalay 2006). This study included 50 adults (25 in both the intervention and control group). For seven patients it was not clear if they had a solid tumour or a haematological malignancy, but the 43 other patients were diagnosed with a solid tumour. They were treated with doxorubicin or epirubicin. The cumulative anthracycline dose that patients received was comparable between the intervention and control groups.
Amifostine
One study, identified in the second update, addressed amifostine (Gallegos‐Castorena 2007). This study included 28 children (15 in the intervention group and 13 in the control group) with osteosarcoma, who were treated with doxorubicin. It was unclear if patients in the intervention and control groups received similar cumulative anthracycline doses.
Dexrazoxane
Ten RCTs addressed dexrazoxane (Galetta 2005; Lipshultz 2004; Lopez 1998; Marty 2006; Speyer 1992; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996; Wexler 1996; one RCT (Schwartz 2009) was identified in the second update of this review). The total number of patients was 1619 (799 in the dexrazoxane group and 820 in the control group). In eight studies the control group did not receive a cardioprotective intervention (n = 535); in two studies (Swain 1997a(088001); Swain 1997a(088006)) the control group received a placebo (n = 285). Six studies included adult patients (Galetta 2005; Marty 2006; Speyer 1992; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996); three studies included both children and adults (Lopez 1998; Schwartz 2009; Wexler 1996); and one study included solely children (Lipshultz 2004). In nine studies patients were diagnosed with a solid tumour; the majority of the patients included in these studies were adults with advanced breast cancer. In one study the patients were diagnosed with leukaemia (Lipshultz 2004). In six studies patients were treated with doxorubicin (Lipshultz 2004; Speyer 1992; Swain 1997a(088001); Swain 1997a(088006); Wexler 1996); in three studies with epirubicin (Galetta 2005; Lopez 1998; Schwartz 2009; Venturini 1996); and in one study patients were treated with either epirubicin or doxorubicin (Marty 2006). The ratio of study drug to anthracycline dose varied between studies was either 6.25:1, 10:1 or 20:1. In five studies it was unclear if patients in the intervention and control groups received similar cumulative anthracycline doses (Galetta 2005; Lipshultz 2004; Swain 1997a(088001); Swain 1997a(088006); Schwartz 2009); in three studies patients in the intervention and control groups received comparable cumulative anthracycline doses (Lopez 1998; Marty 2006; Venturini 1996); and in two studies patients in the dexrazoxane group received a higher cumulative anthracycline dose (100 mg/m2 or more) than patients in the control group (Speyer 1992; Wexler 1996).
Risk of bias in included studies
See additional Table 1 for the list of criteria for the assessment of risk of bias. See additional Table 2 for the exact scores per included study.
2. Risk of bias assessment in included studies.
| Study | a | b | c | d | e | Intervention |
| Myers 1983 | ? | ‐ | ‐ | Clinical heart failure: ?; response rate: ?; adverse effects: ? | Clinical heart failure: +; response rate: +; adverse effects: + | N‐acetylcysteine |
| Iarussi 1994 | ? | ‐ | ‐ | subclinical heart failure: ? | subclinical heart failure: ? | Coenzyme Q10 |
| Wagdi 1995 | ? | + | + | subclinical heart failure: + | subclinical heart failure: + | Combination of vitamin E, vitamin C and N‐acetylcysteine |
| Milei 1987 | ? | ? | + | Clinical heart failure: + | Clinical heart failure: ‐ | Phenethylamines |
| Kraft 1990 | ? | ‐ | ‐ | Clinical heart failure: + | Clinical heart failure: ‐ | Phenethylamines |
| Venturini 1996 | + | ‐ | ‐ | Clinical heart failure: +; subclinical heart failure: +; response rate: ? | Clinical heart failure: +; subclinical heart failure: +; response rate: + | Dexrazoxane |
| Lopez 1998 | ? | ‐ | ‐ | Clinical heart failure: ?; subclinical heart failure: ?; response rate: ?; adverse effects: ? | Clinical heart failure: +; subclinical heart failure: +; response rate: +; adverse effects: + | Dexrazoxane |
| Swain 1997 (088001) | + | + | + | Clinical heart failure: +; subclinical heart failure: +; response rate: +; PFS: +; adverse effects: ? | Clinical heart failure: +; subclinical heart failure: +; response rate: +; PFS: +; OS: +; adverse effects: + | Dexrazoxane |
| Swain 1997 (088006) | + | + | + | Clinical heart failure: +; subclinical heart failure: +; response rate: +; PFS: +; adverse effects: ? | Clinical heart failure: +; subclinical heart failure: +; response rate: ‐; PFS: +; OS: +; adverse effects: ? | Dexrazoxane |
| Speyer 1992 | ? | ‐ | ‐ | Clinical heart failure: +; subclinical heart failure: +; response rate: ?; PFS: ?; adverse effects: ? | Clinical heart failure: ?; subclinical heart failure: ?; response rate: +; PFS: +; OS: +; adverse effects: + | Dexrazoxane |
| Wexler 1996 | + | ‐ | ‐ | Clinical heart failure: ?; subclinical heart failure: +; response rate: + | Clinical heart failure: +; subclinical heart failure: +; response rate: + | Dexrazoxane |
| Lipshultz 2004 | + | ‐ | ‐ | Clinical heart failure: ? (for long‐term cardiac follow‐up: +); response rate: ?; long‐term follow‐up data adverse effects: ? | Clinical heart failure: ? (for long‐term cardiac follow‐up: ‐); response rate: +; long‐term follow‐up data adverse effects: + | Dexrazoxane |
| Galetta 2005 | ? | ‐ | ‐ | Subclinical heart failure: ? | Subclinical heart failure: + | Dexrazoxane |
| Marty 2006 | + | ‐ | ‐ | Clinical heart failure: +; subclinical heart failure: +; response rate: ?; PFS: ?; adverse effects: ? | Clinical heart failure: +; subclinical heart failure: +; response rate: +; PFS: +; OS: +; adverse effects: + | Dexrazoxane |
| Schwartz 2009 | ? | ? | ? | Clinical heart failure: ?; response rate: ?; adverse effects: ? | Clinical heart failure: +; response rate: +; adverse effects: + | Dexrazoxane |
| Waldner 2006 | ? | ? | + | Clinical heart failure: ?; quality of life: ? | Clinical heart failure: ?; quality of life: ?; OS: ? | L‐carnitine |
| Kalay 2006 | ? | ‐ | + | Clinical heart failure: ?; subclinical heart failure: + | Clinical heart failure: ?; subclinical heart failure: ? | Carvedilol |
| Gallegos‐Castorena 2007 | ? | ? | ? | Clinical heart failure: ?; subclinical heart failure: ?; response rate: ?; adverse effects: ? | Clinical heart failure: +; subclinical heart failure: +; response rate: +; adverse effects: + | Amifostine |
N‐acetylcysteine
It was not reported if concealed treatment allocation was applied. Neither care providers nor patients were blinded to treatment. For clinical heart failure, response rate and adverse effects, it was unclear if the outcome assessor was blinded to treatment. The number of patients lost to follow up was described and was acceptable for all evaluated outcomes.
Phenethylamines
In both studies it was unclear if concealed treatment allocation was applied. In the study of Kraft 1990 neither care providers nor patients were blinded to treatment. In the study of Milei 1987 patients were blinded to treatment, whereas for care providers this was unclear. For clinical heart failure, outcome assessors were blinded to treatment in both studies. In both studies patients lost to follow up were described, but the number was unacceptable.
Coenzyme Q10
It was not reported if concealed treatment allocation was applied. Neither the care provider nor patients were blinded to treatment. For subclinical heart failure, it was unclear if the outcome assessor was blinded to treatment. It was also unclear if patients lost to follow up were described and the number acceptable.
Combination of vitamin E, vitamin C and N‐acetylcysteine
It was not reported if concealed treatment allocation was applied. Both care providers and patients were blinded to treatment. For subclinical heart failure, the outcome assessor was blinded to treatment; patients lost to follow up were described and the number was acceptable.
L‐carnitine
It was not reported if concealed treatment allocation was applied. It was unclear if the care provider was blinded to treatment but patients were blinded. For blinding of the outcome assessor we scored each different outcome, with the exception of OS since blinding was not relevant for that outcome. For both clinical heart failure and QoL it was unclear if the outcome assessor was blinded to treatment. For all evaluated outcomes (that is clinical heart failure, QoL and OS) it was unclear if patients lost to follow up were described and the number was acceptable.
Carvedilol
It was not reported if concealed treatment allocation was applied. The care providers were not blinded to treatment whereas the patients were. For clinical heart failure, it was unclear if the outcome assessor was blinded to treatment but for subclinical heart failure they were. For both clinical and subclinical heart failure it was unclear if patients lost to follow up were described and the number was acceptable.
Amifostine
It was not reported if concealed treatment allocation was applied. It was unclear if the care provider and patients were blinded to treatment. For clinical heart failure, subclinical heart failure, response rate and adverse effects it was unclear if the outcome assessor was blinded to treatment. Patients lost to follow up were described and the number was acceptable for all evaluated outcomes.
Dexrazoxane
Six studies applied concealed treatment allocation, whereas four studies did not report concealed treatment allocation. Both care providers and patients were blinded to treatment in two studies; in seven studies they were not blinded and in one study this was unclear. For blinding of the outcome assessor we scored each different outcome with the exception of OS, since for that outcome blinding was not relevant. Nine studies evaluated clinical heart failure: in five the outcome assessor was blinded to treatment, whereas in four this was unclear. Eight studies evaluated subclinical heart failure: in six the outcome assessor was blinded to treatment, whereas in two this was unclear. Nine studies evaluated response rate: in three the outcome assessor was blinded to treatment, whereas in six this was unclear. Four studies evaluated PFS: in two the outcome assessor was blinded to treatment, whereas in two this was unclear. Seven studies evaluated adverse effects: in all studies it was unclear if the outcome assessor was blinded to treatment. Patients lost to follow up were also scored for each different outcome. For clinical heart failure patients lost to follow up were described and acceptable in seven of the nine studies evaluating this outcome, whereas in two studies this was unclear. For subclinical heart failure, patients lost to follow up were described and acceptable in seven of the eight studies describing this outcome, whereas in one study this was unclear. For response rate, patients lost to follow up were described and acceptable in eight of the nine studies describing this outcome, whereas in one study this was unacceptable. For both PFS and OS, patients lost to follow up were described and acceptable in all four studies evaluating this outcome. For adverse effects, patients lost to follow up were described and acceptable in six of the seven studies evaluating this outcome, whereas in one study this was unclear.
In conclusion, the risk of bias in the included studies varied and bias could not be ruled out in the following percentage of included studies: selection bias (based on concealment of allocation) 40%; performance bias (based on blinding of the care provider and patient) 80%; detection bias (based on blinding of the outcome assessor) 44% for clinical heart failure (based on the original data of Lipshultz 2004; using the long‐term follow‐up data this would be 33%), 25% for subclinical heart failure, 67% for response rate, 50% for PFS, and 100% for adverse effects; and finally attrition bias (based on the completeness of follow up) 22% for clinical heart failure, 13% for subclinical heart failure, 11% for response rate, 0% for both PFS and OS, and 14% for adverse effects.
Effects of interventions
Not all articles allowed data extraction for all endpoints (see Characteristics of included studies table for a more detailed description of the extractable endpoints in each article).
N‐acetylcysteine
Since only one study addressed N‐acetylcysteine, pooling of results was not possible. We therefore provide descriptive results for this study. All the RR, 95% CI and P values mentioned below were calculated in RevMan, with the exception of the Fischer's exact test P value.
Heart failure only included cases of clinical heart failure. Three of the 24 (12.5%) patients treated with N‐acetylcysteine developed clinical heart failure, as did 3 of the 30 (10%) control patients (RR 1.25, 95% CI 0.28 to 5.64, P = 0.77).
The response rate in the intervention group was 16.7% (4/24 patients) and in the control group it was 6.7% (2/30 patients) with a RR of 2.50 (95% CI 0.50 to 12.51, P = 0.26). These patients had no evaluable disease or partial remission; we assumed that the patients with no evaluable disease were in complete remission.
With regard to adverse effects other than cardiac damage, the major difference between the intervention and control groups was the presence of diarrhoea in the group receiving N‐acetylcysteine (7/24 (29%) versus 0/30 (0%) control patients (Fischer's exact test P = 0.002); we were unable to calculate a RR for this comparison because one group experienced no events. Nausea occurred in 7/24 patients (29%) in the intervention group versus 6/30 patients (20%) in the control group (RR 1.46, 95% CI 0.56 to 3.77, P = 0.44), but in the intervention group it was less severe. Alopecia occurred in 12/24 patients (50%) in the intervention group versus 9/30 patients (30%) in the control group (RR 1.67, 95% CI 0.85 to 3.28, P = 0.14). In three patients (12.5%) in the intervention group, an erythematous flare developed at sites of previous venepuncture (Fischer's exact test P = 0.08); we were unable to calculate a RR for this comparison because one group experienced no events. There were no differences in the occurrence of mucositis, leukopenia, thrombocytopenia and low haemoglobin level (defined as less than eight) between the two groups.
Phenethylamines
Pooling of results of the two RCTs evaluating phenethylamines was not possible since the definitions used to describe heart failure were not compatible (see Characteristics of included studies table). We therefore provide descriptive results for these studies. All the RR, 95% CI and P values mentioned below were calculated in RevMan, with the exception of the Fischer's exact test P value.
In both studies heart failure included only cases of clinical heart failure. In the study of Milei 1987 no patients (0%) in the intervention group and two (11%) patients in the control group developed heart failure (Fischer's exact test P = 0.49); we were unable to calculate a RR for this comparison because one group experienced no events. In the study of Kraft 1990 no patients (0%) in the intervention group and one patient (3%) in the control group developed heart failure (Fischer's exact test P = 1); we were unable to calculate a RR for this comparison because one group experienced no events.
Coenzyme Q10
Since only one study addressed coenzyme Q10, pooling of results was not possible. We therefore provide descriptive results for this study.
Heart failure included only cases of subclinical heart failure. In both the intervention and the control group none of the patients developed subclinical heart failure.
Combination of vitamin E, vitamin C and N‐acetylcysteine
Since only one study addressed the combination of vitamin E, vitamin C and N‐acetylcysteine, pooling of results was not possible. We therefore provide descriptive results for this study. All the RR, 95% CI and P values mentioned below were calculated in RevMan.
Heart failure included only cases of subclinical heart failure. One patient (16.7%) in the intervention group and four patients (50%) in the control group developed subclinical heart failure (RR 0.33, 95% CI 0.05 to 2.27, P = 0.26).
L‐carnitine
Since only one study addressed L‐carnitine, pooling of results was not possible. We therefore provide descriptive results for this study.
Heart failure included only cases of clinical heart failure. In both the intervention and control groups, none of the patients developed clinical heart failure. No significant differences in QoL (according to a standardised questionnaire by Tuchler 1992 and Hofmann 1993) or OS were identified.
Carvedilol
Since only one study addressed carvedilol, pooling of results was not possible. We therefore provide descriptive results for this study. All the RR, 95% CI and P values mentioned below were calculated in RevMan, with the exception of the Fischer's exact test P value.
None of the patients in the intervention group and one patient (4%) in the control group developed clinical heart failure (Fischer's exact test P = 1); we were unable to calculate a RR for this comparison because one group experienced no events. One patient (4%) in the intervention group and five patients (20%) in the control group developed heart failure (that is clinical and subclinical heart failure combined) (RR 0.20, 95% CI 0.03 to 1.59, P = 0.13).
Amifostine
Since only one study addressed amifostine, pooling of results was not possible. We therefore provide descriptive results for this study. All the RR, 95% CI and P values mentioned below were calculated in RevMan, with the exception of the Fischer's exact test P value.
None of the patients in this study developed clinical heart failure. None of the patients in the intervention group and two patients in the control group (15.4%) developed subclinical heart failure (Fischer's exact test for heart failure (that is clinical and subclinical heart failure combined) P = 0.21); we were unable to calculate a RR for this comparison because one group experienced no events.
The response rate in the intervention group was 93.3% (14/15 patients) and in the control group it was 58.3% (7/12 patients; for one patient no histological examination was available) with a RR of 1.60 (95% CI 0.97 to 2.63, P = 0.06).
With regard to adverse effects other than cardiac damage (grade 3 or higher), the major difference between the intervention and control groups was the presence of vomiting in the group receiving amifostine (15/14 (100%) versus 1/13 (7.7%) of the control patients), RR of 9.04 (95% CI 1.99 to 41.12, P = 0.004). Renal toxicity occurred in 0/15 patients (0%) in the intervention group and 2/13 patients (15.4%) in the control group (Fischer's exact test P = 0.21); we were unable to calculate a RR for this comparison because one group experienced no events). Audiological toxicity occurred in none of the study patients.
Dexrazoxane
Clinical heart failure
We could collect data on clinical heart failure from eight trials with a total of 1561 patients (Lipshultz 2004; Lopez 1998; Marty 2006; Schwartz 2009; Speyer 1992; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996) (see Figure 1). There were 11 cases of clinical heart failure among 769 patients randomised to dexrazoxane and 69 among 792 randomised to the control group. In one study there were no cases of clinical heart failure in either treatment group (Lipshultz 2004) and, therefore, the results of this study are not estimable for the meta‐analysis of the RR. The meta‐analysis showed a benefit in favour of dexrazoxane use (RR 0.18, 95% CI 0.10 to 0.32, P < 0.00001). No substantial heterogeneity was detected (I² = 0%).
1.
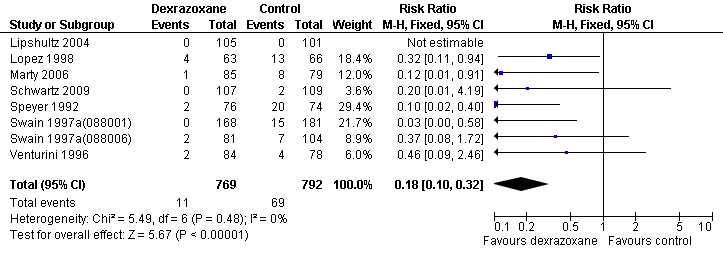
Forest plot of comparison: 1 Dexrazoxane versus no dexrazoxane / placebo, outcome: 1.1 Clinical heart failure.
We excluded the study of Wexler 1996 from this analysis since in this study it was not possible to separate cases of clinical and subclinical heart failure. In the study of Galetta 2005 no information on the occurrence of clinical heart failure was provided.
Long‐term follow‐up data of the study of Lipshultz 2004 (Lipshultz 2010) have been published on 134 of 205 randomised patients (68 of the 105 patients in the dexrazoxane group and 66 of the 100 patients in the control group). The median follow up in the dexrazoxane group was 6.2 years (range 3 to 7.7 years) and the median follow up in the control group was 5.7 years (range 2.8 to 7.6 years). There were still no cases of clinical heart failure in either treatment group.
Heart failure (that is clinical and subclinical heart failure combined)
Data on heart failure could be extracted from five trials with a total of 643 patients (Lopez 1998; Marty 2006; Speyer 1992; Venturini 1996; Wexler 1996) (see Figure 2). There were 34 cases of heart failure among 328 patients randomised to dexrazoxane and 113 among 315 randomised to the control group. Since in the study from Wexler 1996 it was not possible to separate cases of clinical and subclinical heart failure, it is not possible to give the exact number of patients with subclinical heart failure that were included in this meta‐analysis. However, at least 21 of the 34 patients with heart failure in the dexrazoxane group and at least 58 of the 118 patients with heart failure in the control group suffered from subclinical heart failure. The meta‐analysis showed a benefit in favour of dexrazoxane use (RR 0.29, 95% CI 0.20 to 0.41, P < 0.00001). No heterogeneity was detected (I² = 0%).
2.
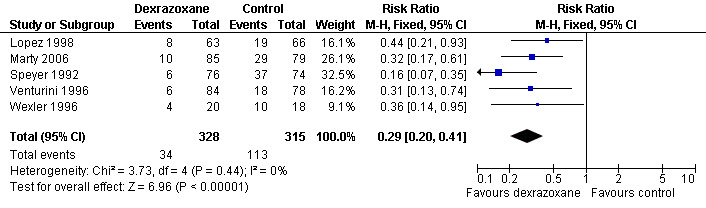
Forest plot of comparison: 1 Dexrazoxane versus no dexrazoxane / placebo, outcome: 1.2 Heart failure (i.e. clinical and subclinical heart failure combined).
We excluded the studies of Swain (Swain 1997a(088001); Swain 1997a(088006)) from this analysis since the definition of subclinical heart failure used in these studies was too different from the definitions used in the other studies. We excluded the study of Galetta 2005, since in this study clinical heart failure was not evaluated and therefore the results included only cases of subclinical heart failure. We excluded the study of Schwartz 2009 since in this study subclinical heart failure was not addressed and therefore the results only include cases of clinical heart failure. In the study of Lipshultz 2004 (including the long‐term follow‐up data) the necessary information on the occurrence of subclinical heart failure was not provided. It should be noted that patients from the studies of Marty 2006; Lopez 1998; Speyer 1992 and Venturini 1996 who suffered from clinical heart failure were also included in the meta‐analysis of clinical heart failure as mentioned above.
Response rate
Data on response rate (defined as the number of patients in complete and partial remission) could be extracted from six trials with a total of 1021 patients (Lopez 1998; Marty 2006; Speyer 1992; Swain 1997a(088001); Swain 1997a(088006); Venturini 1996) (see Figure 3). These trials used comparable criteria to assess tumour response. From the studies of Swain (Swain 1997a(088001); Swain 1997a(088006)) only patients with evaluable disease were included (for study 088001: 141 in the dexrazoxane group and 152 in the control group; for study 088006: 54 in the dexrazoxane group and 69 in the control group). There were 223 complete and partial responses among 503 patients randomised to dexrazoxane and 260 among 518 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 0.89, 95% CI 0.78 to 1.02, P = 0.08). No heterogeneity was detected (I² = 0%).
3.
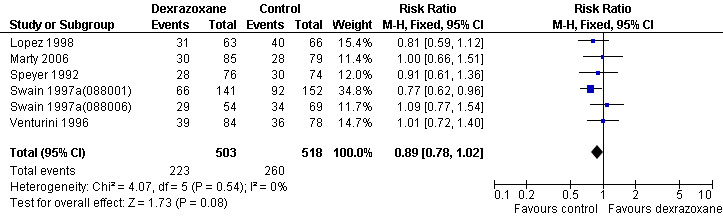
Forest plot of comparison: 1 Dexrazoxane versus no dexrazoxane / placebo, outcome: 1.3 Response rate.
Two studies (Lopez 1998; Speyer 1992) mentioned that the response rate was determined by at least two observers. We excluded the study of Wexler 1996 from this analysis since it was impossible to separate the three non‐randomised patients from the randomised patients in the dexrazoxane group. We excluded the study of Lipshultz 2004 and Schwartz 2009 since partial remission was not mentioned and therefore the results only included complete remissions. In the study of Galetta 2005 no information on response rate was provided.
Please note that due to the nature of this measurement (that is the number of patients with a remission) a high event rate is favourable. Therefore, in the figure of this analysis 'favours control' is on the left and 'favours dexrazoxane' is on the right, as opposed to the figures for the other analyses.
Survival
Data on survival could be extracted from four trials with a total of 848 patients (Marty 2006; Speyer 1992; Swain 1997a(088001); Swain 1997a(088006)). Two studies (Swain 1997a(088001); Swain 1997a(088006)) presented HRs with 95% CIs and the other studies (Marty 2006; Speyer 1992) provided survival curves. Results of the individual studies are shown in Table 3. No statistically significant differences between the treatment arms were found. For PFS the meta‐analysis showed no significant difference between the dexrazoxane and control groups (HR 1.01, 95% CI 0.86 to 1.18, P = 0.89) (see Figure 4). However, unexplained heterogeneity was detected (I² = 68%). Using a random‐effects model confirmed the findings of no significant difference between treatment groups (RR 0.97, 95% CI 0.73 to 1.29, P = 0.84) (analysis not shown). For OS the meta‐analysis also showed no significant difference between the dexrazoxane and the control groups (HR 1.04, 95% CI 0.88 to 1.23, P = 0.65) (see Figure 5). No heterogeneity was detected (I² = 0%).
3. Survival: dexrazoxane versus control treatment.
| Study | Median progression free survival | Median overall survival |
| Speyer 1992 | 10.1 months versus 9.4 months | 18.3 months versus 16.7 months |
| Swain 1997a (088001) | 254 days versus 260 days | 598 days versus 551 days |
| Swain 1997a (088006) | 233 days versus 249 days | 458 days versus 553 days |
| Marty 2006 | 7.8 months versus 7 months | 13.5 months versus 16 months |
4.
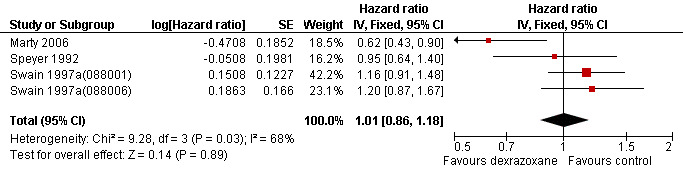
Forest plot of comparison: 1 Dexrazoxane versus no dexrazoxane / placebo, outcome: 1.4 Progression‐free survival.
5.
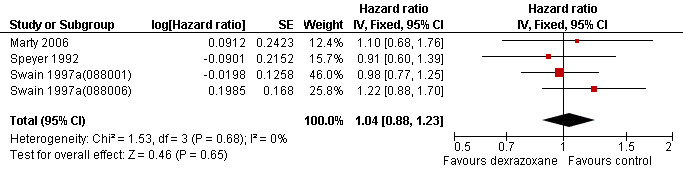
Forest plot of comparison: 1 Dexrazoxane versus no dexrazoxane / placebo, outcome: 1.5 Overall survival.
We excluded the study of Venturini 1996 from this analysis since it did not include the two patients who did not receive any chemotherapy in the evaluation of survival. We excluded the study of Wexler 1996 from this analysis since it was impossible to separate the three non‐randomised patients from the randomised patients in the dexrazoxane group. We excluded the study of Lopez 1998 from this analysis since we were not able to reliably extract data needed to use Parmar's method for the assessment of survival for this study. However, none of the excluded studies showed statistically significant differences between the treatment arms. In the studies of Galetta 2005, Lipshultz 2004 and Schwartz 2009 no information on PFS and OS was provided.
Adverse effects
Data on adverse effects could be extracted from seven RCTs: Lopez 1998 used the World Health Organization (WHO) criteria (Miller 1981); Swain 1997a(088001) and Swain 1997a(088006) used the Eastern Cooperative Oncology Group (ECOG) criteria (Oken 1982); Marty 2006 and Schwartz 2009 used the common toxicity (CTC) criteria (version 2). In the study of Speyer 1992, no references on which the grading of the adverse effects was based were mentioned. It did provide the definitions of the different adverse effects used in the study but they were not comparable to the WHO, ECOG or CTC criteria. In the study of Lipshultz 2004 no definitions were provided.
Since all patients receiving chemotherapy will suffer from side effects, we decided to analyse only the severe and life threatening effects. For studies using the ECOG, WHO or CTC criteria, we defined this as grade 3 (severe) or grade 4 (life‐threatening); for the study of Speyer 1992 we excluded the two lowest grades reported.
It was possible to perform meta‐analyses for adverse effects for which more than one RCT was available. For adverse effects for which only one RCT was available we provide descriptive results (all the RR, 95% CI and P values mentioned below are calculated in RevMan with the fixed‐effect model, unless stated otherwise). It was not clear what the timing and frequency of the evaluation of the side effects was in the different studies.
We excluded the study of Wexler 1996 from this analysis since this study did not report the number of patients having suffered an adverse effect. We excluded the study of Venturini 1996 from this analysis since it did not include the two patients who did not receive any chemotherapy in the evaluation of adverse effects. In the studies of Galetta 2005 no information on adverse effects was provided.
Thrombocytopenia grade 3 or 4
Data could be extracted from two trials with a total of 293 patients (Lopez 1998; Marty 2006). These trials used comparable criteria. There were 11 cases among 148 patients randomised to dexrazoxane and 11 among 145 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 1.04, 95% CI 0.49 to 2.21, P = 0.93). No heterogeneity was detected (I² = 0%).
Abnormal platelet count at nadir grade 3 or 4
Data could be extracted from two trials with a total of 534 patients (Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were 21 cases among 249 patients randomised to dexrazoxane and 26 among 285 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 0.92, 95% CI 0.53 to 1.59, P = 0.76). No substantial heterogeneity was detected (I² = 32%).
Abnormal platelet count at recovery grade 3 or 4
Data could be extracted from two trials with a total of 534 patients (Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were 2 cases among 249 patients randomised to dexrazoxane and 3 among 285 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 0.81, 95% CI 0.16 to 4.15, P = 0.80). No heterogeneity was detected (I² = 0%).
Neutropenia grade 3 or 4
Data could be extracted from two trials with a total of 293 patients (Lopez 1998; Marty 2006). These trials used comparable criteria. There were 91 cases among 148 patients randomised to dexrazoxane and 88 among 145 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 1.04, 95% CI 0.90 to 1.21, P = 0.60). No heterogeneity was detected (I² = 0%).
Abnormal granulocyte count at nadir grade 3 or 4
Data could be extracted from two trials with a total of 534 patients (Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were 221 cases among 249 patients randomised to dexrazoxane and 244 among 285 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 1.04, 95% CI 0.97 to 1.11, P = 0.26). No substantial heterogeneity was detected (I² = 33%).
Abnormal granulocyte count at recovery grade 3 or 4
Data could be extracted from two trials with a total of 534 patients (Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were 42 cases among 249 patients randomised to dexrazoxane and 57 among 285 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 0.85, 95% CI 0.59 to 1.21, P = 0.36). No heterogeneity was detected (I² = 0%).
Abnormal white blood cell count at nadir grade 3 or 4
Data could be extracted from two trials with a total of 534 patients (Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were 195 cases among 249 patients randomised to dexrazoxane and 193 among 285 randomised to the control group. The meta‐analysis showed a significant difference in favour of the control treatment (RR 1.16, 95% CI 1.05 to 1.29, P = 0.005). No heterogeneity was detected (I² = 0%).
Abnormal white blood cell count at recovery grade 3 or 4
Data could be extracted from two trials with a total of 534 patients (Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were 14 cases among 249 patients randomised to dexrazoxane and 23 among 285 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 0.69, 95% CI 0.36 to 1.31, P = 0.26). No heterogeneity was detected (I² = 0%).
Anaemia grade 3 or 4
Data could be extracted from three trials with a total of 509 patients (Lopez 1998; Marty 2006; Schwartz 2009). These trials used comparable criteria. There were 84 cases among 255 patients randomised to dexrazoxane and 61 among 254 randomised to the control group. The meta‐analysis showed a significant difference in favour of the control treatment (RR 1.40, 95% CI 1.08 to 1.81, P = 0.01). No heterogeneity was detected (I² = 0%).
Stomatitis grade 3 or 4
Data could be extracted from four trials with a total of 914 patients (Marty 2006; Schwartz 2009; Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were 45 cases among 441 patients randomised to dexrazoxane and 56 among 473 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 0.85, 95% CI 0.60 to 1.21, P = 0.38). No heterogeneity was detected (I² = 0%).
The study of Lopez 1998 was excluded from this analysis since the criteria used for diagnosis of stomatitis were not comparable with those used by Marty 2006; Swain 1997a(088001) and Swain 1997a(088006) (see additional Table 4).
4. Adverse effects: dexrazoxane versus control treatment.
| Adverse effect | Study | Definition | % of index patients | % of controls | Risk Ratio / Relative Risk (95%CI) | P value |
| Asthenia grade 3 or 4 | Marty 2006 | CTC criteria | 2 | 3 | 0.93 [0.13, 6.44] | 0.94 |
| Fatigue grade 3 or 4 | Marty 2006 | CTC criteria | 3 | 1 | 2.79 [0.30, 26.25] | 0.37 |
| Bone pain grade 3 or 4 | Marty 2006 | CTC criteria | 0 | 5 | We were unable to calculate a RR because one group experienced no events | 0.05*** |
| Pyrexia grade 3 or 4 | Marty 2006 | CTC criteria | 2 | 0 | We were unable to calculate a RR because one group experienced no events | 0.50*** |
| Febrile bone marrow aplasia grade 3 or 4 | Marty 2006 | CTC criteria | 5 | 1 | 3.72 [0.42, 32.55] | 0.24 |
| Leukopenia grade 3 or 4 | Marty 2006 | CTC criteria | 20 | 18 | 1.13 [0.60, 2.14] | 0.71 |
| Febrile neutropenia grade 3 or 4 | Marty 2006 | CTC criteria | 18 | 14 | 1.27 [0.62, 2.59] | 0.52 |
| Absolute neutrophil count grade 3 or 4 | Schwartz 2009 | CTC criteria | 94 | 85 | 1.10 [1.0, 1.20] | 0.05 |
| Platelets grade 3 or 4 | Schwartz 2009 | CTC criteria | 72 | 29 | 2.45 [1.79, 3.36] | < 0.00001 |
| Thrombosis grade 3 or 4 | Schwartz 2009 | CTC criteria | 4 | 1 | 4.07 [0.46, 35.87] | 0.21 |
| Constipation grade 3 or 4 | Marty 2006 | CTC criteria | 1 | 0 | We were unable to calculate a RR because one group experienced no events | 1*** |
| Diarrhoea grade 3 or 4 | Marty 2006 | CTC criteria | 1 | 1 | 0.93 [0.06, 14.61] | 0.96 |
| Stomatitis grade 3 or 4 | Lopez 1998 | WHO criteria | 10 | 15 | 0.63 [0.24, 1.63] | 0.34 |
| Stomatitis: ulcers can eat | Speyer 1992 | No references provided | 13 | 15 | 0.89 [0.40, 1.96] | 0.76 |
| Stomatitis: ulcers cannot eat | Speyer 1992 | No references provided | 4 | 9 | 0.42 [0.11, 1.55] | 0.19 |
| Mucosal inflammation grade 3 or 4 | Marty 2006 | CTC criteria | 0 | 1 | We were unable to calculate a RR because one group experienced no events | 0.48*** |
| Typhilitis grade 3 or 4 | Schwartz 2009 | CTC criteria | 3 | 8 | 0.34 [0.09, 1.22] | 0.10 |
| Nausea and vomiting grade 3 or 4 | Lopez 1998 | WHO criteria | 5 | 15 | 0.31 [0.09, 1.09] | 0.07 |
| Nausea and vomiting grade 3 or 4 | Schwartz 2009 | CTC criteria | 9 | 9 | 1.02 [0.44, 2.35] | 0.97 |
| Nausea and vomiting: controllable | Speyer 1992 | No references provided | 61 | 57 | 1.07 [0.81, 1.40] | 0.64 |
| Nausea and vomiting: vomiting intractable | Speyer 1992 | No references provided | 3 | 7 | 0.39 [0.08, 1.95] | 0.25 |
| Death due to toxicity | Speyer 1992 | No references provided | 3 | 7 | 0.39 [0.08, 1.95] | 0.25 |
| Alopecia: severe | Speyer 1992 | No references provided | 91 | 89 | 1.02 [0.91, 1.13] | 0.74 |
| Fever: with positive blood cultures | Speyer 1992 | No references provided | 3 | 4 | 0.65 [0.11, 3.77] | 0.63 |
| Fever: with positive other cultures | Speyer 1992 | No references provided | 5 | 3 | 1.95 [0.37, 10.31] | 0.43 |
| Sepsis grade 3 or 4 | Schwartz 2009 | CTC criteria | 17 | 8 | 2.04 [0.96, 4.33] | 0.06 |
| Infection, not otherwise specified/unknown grade 3 or 4 | Schwartz 2009 | CTC criteria | 70 | 44 | 1.59 [1.25, 2.03] | 0.0002 |
| Pulmonary grade 3 or 4* | Schwartz 2009 | CTC criteria | 12 | 3 | 4.41 [1.29, 15.05] | 0.02 |
| Peripheral nervous system grade 3 or 4 | Schwartz 2009 | CTC criteria | 2 | 3 | 0.68 [0.12, 3.98] | 0.67 |
| Central nervous system grade 3 or 4** | Schwartz 2009 | CTC criteria | 1 | 0 | We were unable to calculate a RR because one group experienced no events | 0.50*** |
| Allergic reaction grade 3 or 4 | Schwartz 2009 | CTC criteria | 7 | 2 | 3.57 [0.76, 16.78] | 0.11 |
* includes diffusion capacity for carbon monoxide, vital capacity, pulmonary/functional and oxygen saturation
** central nervous system includes mood, cortical and cerebellar
*** Fischer's exact
CTC: Common Toxicity Criteria
WHO: World Health Organisation
CI: confidence interval
Nausea grade 3 or 4
Data could be extracted from three trials with a total of 698 patients (Marty 2006; Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were 46 cases among 334 patients randomised to dexrazoxane and 77 among 364 randomised to the control group. The meta‐analysis showed a significant difference in favour of dexrazoxane (RR 0.68, 95% CI 0.49 to 0.94, P = 0.02). No heterogeneity was detected (I² = 0%).
Vomiting grade 3 or 4
Data could be extracted from three trials with a total of 698 patients (Marty 2006; Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were 42 cases among 334 patients randomised to dexrazoxane and 60 among 364 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 0.79, 95% CI 0.55 to 1.14, P = 0.20). However, unexplained heterogeneity was detected (I² = 54%). The use of a random‐effects model confirmed the findings of no significant difference between treatment groups (RR 0.71, 95% CI 0.37 to 1.39, P = 0.32) (analysis not shown).
Neurotoxicity grade 3 or 4
Data could be extracted from two trials with a total of 534 patients (Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were two cases among 249 patients randomised to dexrazoxane and five among 285 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 0.53, 95% CI 0.12 to 2.26, P = 0.39). However, unexplained heterogeneity was detected (I² = 63%). Using a random‐effects model confirmed the findings of no significant difference between treatment groups (RR 0.62, 95% CI 0.03 to 13.45, P = 0.76) (analysis not shown).
Pain on injection grade 3 or 4
Data could be extracted from two trials with a total of 534 patients (Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were four cases among 249 patients randomised to dexrazoxane and three among 285 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 1.51, 95% CI 0.34 to 6.72, P = 0.59). No heterogeneity was detected (I² = 0%).
Anorexia grade 3 or 4
Data could be extracted from two trials with a total of 534 patients (Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were 23 cases among 249 patients randomised to dexrazoxane and 27 among 285 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 0.97, 95% CI 0.57 to 1.64, P = 0.90). No heterogeneity was detected (I² = 0%).
Alopecia grade 3 or 4
Data could be extracted from three trials with a total of 698 patients (Marty 2006; Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were 227 cases among 334 patients randomised to dexrazoxane and 251 among 364 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 1.02, 95% CI 0.94 to 1.11, P = 0.62). No heterogeneity was detected (I² = 0%).
Phlebitis grade 3 or 4
Data could be extracted from two trials with a total of 534 patients (Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were four cases among 249 patients randomised to dexrazoxane and three among 285 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 1.54, 95% CI 0.35 to 6.75, P = 0.56). No heterogeneity was detected (I² = 0%).
Diarrhoea grade 3 or 4
Data could be extracted from two trials with a total of 534 patients (Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were 10 cases among 249 patients randomised to dexrazoxane and 10 among 285 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 1.17, 95% CI 0.49 to 2.79, P = 0.73). No substantial heterogeneity was detected (I² = 26%).
The study of Marty 2006 was excluded from this analysis since the criteria used for diagnosis of diarrhoea were not comparable with those used by Swain 1997a(088001) and Swain 1997a(088006) (see additional Table 4).
Fever grade 3 or 4
Data could be extracted from two trials with a total of 534 patients (Swain 1997a(088001); Swain 1997a(088006)). These trials used comparable criteria. There were 25 cases among 249 patients randomised to dexrazoxane and 20 among 285 randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 1.44, 95% CI 0.81 to 2.53, P = 0.21). No heterogeneity was detected (I² = 0%).
The study of Marty 2006 was excluded from this analysis since the criteria used for diagnosis of fever were not comparable with those used by Swain 1997a(088001) and Swain 1997a(088006) (see additional Table 4).
Secondary malignant disease
Data could be extracted from two trials with a total of 421 patients (Lipshultz 2004 (that is Barry 2008); Schwartz 2009). There were three cases of secondary malignant disease among 212 patients randomised to dexrazoxane and two among 209 patients randomised to the control group. The meta‐analysis showed no significant difference between the treatment groups (RR 1.39, 95% CI 0.28 to 6.90, P = 0.69). No substantial heterogeneity was detected (I² = 23%).
The secondary tumours in the dexrazoxane group were two cases of acute myeloid leukemia (AML) and one osteosarcoma; the secondary tumours in the control group were one case of AML and one melanoma (located outside the cranial radiation field).
Non‐pooled adverse effects
For adverse effects for which only one RCT was available, see additional Table 4 for descriptive results. For three adverse effects grade 3 or 4 (that is platelets, infection not otherwise specified or unknown and pulmonary) a significant difference in favour of the control group was identified. For two adverse effects grade 3 or 4 (that is absolute neutrophil count and sepsis) a borderline significant difference in favour of the control group was identified (P = 0.05 and P = 0.06 respectively). For one adverse effect grade 3 or 4 (that is bone pain) a borderline significant difference in favour of the dexrazoxane group was identified (Fischer's exact test P = 0.05). For the other adverse effects no significant difference between the treatment groups was observed.
Quality of life (QoL)
None of the studies evaluated QoL.
Subgroup analyses
Subgroup analyses for children versus adults and leukaemias versus solid tumours were not performed. Only one of the included studies evaluated the effect of dexrazoxane in children only (Lipshultz 2004). Three studies included both adults and children but data could not be separated for adults and children (Lopez 1998; Schwartz 2009; Wexler 1996). Only one of the included studies evaluated the effect of dexrazoxane in patients treated with leukaemia (Lipshultz 2004), in all other studies patients were diagnosed with a solid tumour.
Sensitivity analyses for the risk of bias criteria
The results of the sensitivity analyses were consistent among the trials and did not differ from the overall analyses for all meta‐analyses except for secondary malignant disease. When only including the study with a low risk of selection bias (based on allocation concealment) the point estimate (RR) changed from 1.39 (that is favours control treatment) to 0.32 (favours dexrazoxane). Both the sensitivity and the overall analyses did not show a statistically significant difference between treatment groups and the 95% CIs overlapped.
Discussion
Heart damage due to anthracycline chemotherapy is a considerable, serious problem. It reduces QoL and can even cause premature death. Also, when heart damage occurs during therapy the maximum cumulative dose of anthracyclines needs to be limited and as a result the efficacy of anthracycline chemotherapy will be reduced. This is the second update of the systematic review evaluating the existing evidence on all known possibly cardioprotective agents.
We identified RCTs for N‐acetylcysteine, phenethylamines, coenzyme Q10, the combination of vitamin E, vitamin C and N‐acetylcysteine, L‐carnitine, carvedilol, amifostine and dexrazoxane. For the other possible cardioprotective interventions included in our search no RCTs were found. Non‐randomised studies and case reports have been published for some of these interventions. However, due to the high risk of bias associated with these study designs, they were not included in this systematic review and no conclusions can be made about the efficacy of these interventions in preventing heart damage in patients treated with anthracyclines.
For N‐acetylcysteine, phenethylamines, coenzyme Q10, the combination of vitamin E, vitamin C and N‐acetylcysteine, L‐carnitine, carvedilol and amifostine, pooling of results was not possible either because only one RCT was available or (where two RCTs were identified) because the definitions used to describe heart failure were not comparable. All trials had methodological limitations so the presence of bias cannot be ruled out. None of the included RCTs showed a statistically significant difference in the occurrence of heart failure. The reason why no significant difference between the treatment groups was identified in these studies could be due to the fact that the number of patients included in these studies was too small to detect a difference between the treatment groups (that is low power). Also, anthracycline‐induced cardiotoxicity is dose‐dependent and in some of the studies patients received a relatively low cumulative anthracycline dose. Presently, no definitive conclusions can be made about the efficacy of these cardioprotective interventions in preventing heart damage in patients treated with anthracyclines.
For dexrazoxane, 10 RCTs were identified. It should be emphasised that the majority of the patients included in these studies were adults with advanced breast cancer. Subgroup analyses for children versus adults and leukaemias versus solid tumours were not possible.
Our meta‐analysis showed a statistically significant benefit in favour of the use of dexrazoxane for the occurrence of both clinical heart failure and clinical and subclinical heart failure combined (RR 0.18, 95% CI 0.10 to 0.32, P < 0.0001 (eight trials, including long‐term follow‐up data of Lipshultz 2004) and RR 0.29, 95% CI 0.20 to 0.41, P < 0.0001 (five trials), respectively). However, an important question regarding any cardioprotective intervention during anthracycline therapy is whether the cardioprotective drug could decrease the heart damage by anthracyclines without reducing the anti‐tumour efficacy and without negative effects on toxicities other than cardiac damage. In the original version of this review (Van Dalen 2005) there was some suggestion that patients treated with dexrazoxane might have a lower response rate (RR 0.88, 95% CI 0.77 to 1.01, P = 0.06 (five trials)). However, in the updated meta‐analysis this was not confirmed. No statistically significant difference in response rate between the dexrazoxane and control group was found (RR 0.89, 95% CI 0.78 to 1.02, P = 0.08 (six trials)). Furthermore, the value of response rate for predicting survival is not clear (Odaimi 1987; Pierga 2001). In our meta‐analysis of both PFS and OS no significant difference between the dexrazoxane and control group was found, which included the individual study in which a difference in response rate was identified (Swain 1997a(088001)). However, in the meta‐analysis of PFS unexplained substantial heterogeneity was detected. For 20 adverse effects (grade 3 or 4) it was possible to perform a meta‐analysis (including either two, three or four trials). For thrombocytopenia, abnormal platelet count at nadir, abnormal platelet count at recovery, neutropenia, abnormal granulocyte count at nadir, abnormal granulocyte count at recovery, abnormal white blood cell count at recovery, stomatitis, pain on injection, anorexia, alopecia, phlebitis, diarrhoea, fever, vomiting, neurotoxicity and secondary malignant disease no significant differences between treatment groups were identified. However, in the meta‐analyses of both vomiting and neurotoxicity unexplained substantial heterogeneity was detected. For abnormal white blood cell count at nadir (RR 1.16, 95% CI 1.05 to 1.29, P = 0.005) and anaemia (RR 1.40, 95% CI 1.08 to 1.81, P = 0.01) there were significant differences in favour of the control group; as opposed to the first update of this review (Van Dalen 2008) where no significant differences were seen between treatment groups). For nausea a significant difference in favour of dexrazoxane was identified (RR 0.69, 95% CI 0.49 to 0.94, P = 0.02). For some adverse effects pooling was not possible (see additional Table 4). For three adverse effects grade 3 or 4 (that is platelets, infection not otherwise specified or unknown and pulmonary) a significant difference in favour of the control group was identified. For two adverse effects grade 3 or 4 (that is absolute neutrophil count and sepsis) a borderline significant difference in favour of the control group was identified (P = 0.05 and P = 0.06 respectively). For the other adverse effects no significant difference between the treatment groups was observed.
At the moment, despite its clear cardioprotective effects, dexrazoxane is not routinely used in clinical practice. This might be explained by the suspicion of interference with anti‐tumour efficacy (that is response rate and survival) and by the occurrence of secondary malignant disease. However, our meta‐analyses of anti‐tumour efficacy and secondary malignant disease showed no significant difference between patients who were treated with or without dexrazoxane. This latter finding was also identified in a recent publication (Van Dalen 2011), a meta‐analysis including three of the four randomised trials available on secondary malignancies after dexrazoxane (Barry 2008 included in this review; and Tebbi 2007, this study includes data on two RCTs including the one by Schwartz 2009 which was eligible for inclusion in our review), which did not show a significant difference in the occurrence of secondary malignancies between children treated with or without dexrazoxane (RR 1.16, 95% CI 0.06 to 22.17, P = 0.92; eight secondary malignancies in the dexrazoxane group and four in the control group). One other trial did not provide enough information to be included in the meta‐analysis but showed no statistically significant difference in the 5‐year and 10‐year cumulative incidence of secondary malignancies between treatment groups (Salzer 2010; this study did not provide data on cardiotoxicity and thus was not eligible for inclusion in our review).
In three of the 10 studies patients in the intervention and control groups received comparable cumulative anthracycline doses. In two studies patients in the dexrazoxane group received a higher cumulative anthracycline dose (100 mg/m2 or more) than patients in the control group. So despite a higher cumulative anthracycline dose received in the dexrazoxane group there was still a significantly lower rate of cardiotoxicity. In five studies it was unclear if patients in the intervention and control groups received similar cumulative anthracycline doses. If patients in the control group received a higher cumulative anthracycline dose than patients treated with dexrazoxane this could have led to an overestimation of the cardioprotective effect of dexrazoxane (and vice versa). This uncertainty should also be kept in mind when interpreting the results of the secondary outcomes (response rate, survival and adverse effects).
The risk of bias in the included studies varied. In many studies bias could not be ruled out due to lack of reporting. However, at the moment this is the best available evidence on RCTs evaluating dexrazoxane.
In the 10 included studies different ratios of dexrazoxane to anthracyclines were used. We did not analyse the effect of these different ratios on the outcomes.
It should be kept in mind that the inclusion of studies for this systematic review was limited to RCTs describing cardiotoxicity and, as a result, the analyses of response rate, survival, adverse effects and QoL were possibly based on only a subgroup of trials comparing cardioprotective interventions with a control group.
We are awaiting (additional) results of the currently ongoing studies and the studies on the use of the following cardioprotective agents, which await further classification: dexrazoxane (n = 9), valsartan (n = 1), L‐carnitine (n = 1), enalapril (n = 1), telmirsartan (n = 1) and the combination hydroprednisone and gluthatione (n = 1).
Authors' conclusions
Implications for practice.
Probucol, vitamin E alone, vitamin C alone, ACE‐inhibitors, EDTA, deferoxamine, vitamin A, superoxide dismutase, monohydroxyethylrutoside, guanidines, cytochromes, selenium, sildenafil, trimetazidine, digoxin, valsartan and glutathione
For these cardioprotective interventions no RCTs were identified and so no conclusions can be made about their efficacy in preventing heart damage in patients treated with anthracyclines. Based on the currently available evidence, we are not able to give recommendations for clinical practice.
N‐acetylcysteine, phenethylamines, coenzyme Q10, a combination of vitamin E, vitamin C and N‐acetylcysteine, L‐carnitine, carvedilol and amifostine
For these cardioprotective interventions pooling was not possible, so no high quality evidence was available and therefore no definitive conclusions can be made about their efficacy in preventing heart damage in patients treated with anthracyclines. Based on the currently available evidence, we are not able to give recommendations for clinical practice.
Dexrazoxane
Our meta‐analysis clearly showed the efficacy of dexrazoxane in preventing heart damage in patients treated with anthracyclines. No evidence of a lower response rate or a negative effect on PFS and OS was identified. The results for adverse effects are ambiguous. No significant difference in the occurrence of secondary malignant disease was identified. It should be emphasised that the majority of the patients included in these studies were adults with advanced breast cancer.
We conclude that if the risk of cardiac damage is expected to be high, it might be justified to use dexrazoxane in patients with cancer treated with anthracyclines. However, clinicians should weigh the cardioprotective effect of dexrazoxane against the possible risk of adverse effects including secondary malignancies for each individual patient.
Implications for research.
Probucol, vitamin E alone, vitamin C alone, ACE‐inhibitors, EDTA, deferoxamine, vitamin A, superoxide dismutase, monohydroxyethylrutoside, guanidines, cytochromes, selenium, sildenafil, trimetazidine, digoxin, valsartan and glutathione
No RCTs were identified for these cardioprotective interventions. Therefore, before definitive conclusions can be made about their efficacy in preventing heart damage in patients treated with anthracyclines, high quality RCTs need to be undertaken. These RCTs should be performed in homogeneous study populations treated for either a haematological malignancy or a solid tumour, with a long‐term follow up using valid outcome definitions (including cardiotoxicity, anti‐tumour efficacy, survival and adverse effects). Also, since data obtained in adults cannot be extrapolated to children, they should be evaluated in children. The number of included patients should be sufficient to obtain the power needed for the results to be reliable.
N‐acetylcysteine, phenethylamines, coenzyme Q10, a combination of vitamin E, vitamin C and N‐acetylcysteine, L‐carnitine, carvedilol and amifostine
Few RCTs were identified for these cardioprotective interventions. Therefore, before definitive conclusions can be made about their efficacy in preventing heart damage in patients treated with anthracyclines, high quality RCTs need to be undertaken. These RCTs should be performed in homogeneous study populations treated for either a haematological malignancy or a solid tumour, with a long‐term follow up using valid outcome definitions (including cardiotoxicity, anti‐tumour efficacy, survival and adverse effects). Also, since data obtained in adults cannot be extrapolated to children, they should be evaluated (further) in children. The number of included patients should be sufficient to obtain the power needed for the results to be reliable.
Dexrazoxane
Future trials on dexrazoxane should be performed in homogeneous study populations treated for either a haematological malignancy or a solid tumour, with a long‐term follow up using valid outcome definitions (including cardiotoxicity, anti‐tumour efficacy, survival and adverse effects). Since there is only a small amount of data for children, and also because data obtained in adults cannot be extrapolated to children, dexrazoxane should be further evaluated in children. The number of included patients should be sufficient to obtain the power needed for the results to be reliable. We are awaiting the results of the ongoing studies currently being performed in children. The performance of an individual patient data analysis is another possibility to assess the effect of dexrazoxane on survival.
What's new
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 24 February 2015 | Amended | Contact details updated. |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
| 26 February 2014 | Amended | Contact details updated. |
| 9 May 2011 | New search has been performed | The search for eligible studies was updated to November 2010. |
| 9 May 2011 | New citation required and conclusions have changed | Summary of most important changes in results of this second update when compared to the first update of this review: We identified a new randomised controlled trial (RCT) on the use of amifostine (no eligible data on amifostine were available before). Also, we identified a new RCT on the use of dexrazoxane and long‐term follow‐up data of an already included RCT on dexrazoxane. Finally, we identified a new ongoing trial (on enalapril maleate) and two new trials awaiting assessment (on telmirsartan and the combination of hydroprednisone and gluthatione). Again, only for dexrazoxane pooling of results was possible and for the occurrence of cardiotoxicity, response rate and survival the conclusions did not change. More information on adverse effects became available including secondary malignant disease. |
| 19 August 2008 | Amended | Converted to new review format. |
| 18 February 2008 | New citation required and conclusions have changed | Substantive amendment |
| 10 July 2007 | Amended | New studies found and included or excluded: 01/04/07 Conclusions changed: 10/07/07 Summary of most important changes in results of the update when compared to the original review: as opposed to the original review, there was no evidence for a lesser tumour response rate with the use of dexrazoxane. For adverse effects now pooling of results was possible: only for one adverse effect (abnormal white blood cell count at nadir) a difference in favour of the control group was identified. The search for eligible studies was updated to April 2007 using an updated search strategy and including eight new possible cardioprotective agents. And as opposed to the original review, for the update we searched in ongoing trials databases. Instead of pooling results when three or more randomised controlled trials (RCTs) were available, we now pooled results of two or more RCTs. Instead of focusing only on the primary outcome (heart failure) when assessing the quality of included studies, we now assessed the quality criteria blinding of the outcome assessor and completeness of follow‐up for all outcomes separately. Prior cardiac dysfunction was added as a baseline characteristic. Sex, age per treatment group, anthracycline peak dose, anthracycline infusion duration, cumulative anthracycline doses in the intervention and control groups, and a description of other chemotherapy and / or radiotherapy in the study protocol were added to the table of included studies. Five new RCTs were included: one addressing L‐carnitine, one addressing carvedilol and three additional ones addressing dexrazoxane. We also identified six ongoing studies and seven studies awaiting assessment evaluating different cardioprotective agents; characteristics of these trials are provided. Again, only for dexrazoxane pooling of results was possible and for the occurrence of cardiotoxicity and survival the conclusions did not change. As opposed to the original review, now there was no evidence for a lesser tumour response rate with the use of dexrazoxane. For adverse effects now pooling of results was possible: only for one adverse effect (abnormal white blood cell count at nadir) a difference in favour of the control group was identified. We conclude that if the risk of cardiac damage is expected to be high, it might be justified to use dexrazoxane in patients with cancer treated with anthracyclines. However, for each individual patient clinicians should weigh the cardioprotective effect of dexrazoxane against the possible risk of adverse effects. |
Acknowledgements
We would like to thank Rob Scholten, Marianne van de Wetering, Jan Tijssen, Piet Bakker, Heleen van der Pal and the Cochrane Gynaecological Cancer Review Group Editorial office for their advice and support and Anne Blaes for providing additional information on her ongoing study. Also, we would like to thank Marcello Dinisio for translating an Italian article, Leon Bax for translating a Japanese article and Alexey Nabatov for translating Russian articles. We are grateful to Edith LeClercq for running the search for the second update in CENTRAL, MEDLINE and EMBASE and providing us with the titles and abstracts. Finally, we would like to thank our funders for the financial support which made it possible to perform this systematic review.
For the studies of Speyer 1992 and both studies of Swain 1997, the hazard ratio and associated statistics were initially calculated using an Excel spreadsheet provided by Dr MG Hart of the Department of Clinical Neurosciences, Western General Hospital, Edinburgh, United Kingdom. For the update of this review, the necessary data for the survival analyses were (re)calculated using a spreadsheet developed by Heather Dickinson.
Appendices
Appendix 1. Search strategy for Cochrane Central Register of Controlled Trials (CENTRAL)
(1) For the different cardioprotective interventions we used the following subject headings and text words:
(dexrazoxane OR cardioxane OR zinecard OR ADR‐529 OR ICRF‐187 OR razoxane OR piperazines OR dexrazoxan* OR cardioxan* OR zinecar* OR ADR‐5* OR ICRF* OR razoxan* OR piperazin*) OR (carvedilol OR carvedil*) OR (ascorbic acid OR vitamin C OR ascorbic ac*) OR (vitamin a OR tretinoin OR retinoic acid OR carotenoids OR retinoids OR retino* OR tretinoi* OR carotenoi*) OR (trimetazidine OR vastarel OR idaptan OR vasartel OR trimetazid* OR piperazines OR piperazin*) OR (glutathione OR glutathione disulfide OR S‐nitrosoglutathione OR glutathion*) OR (coenzymes OR coenzym* OR coenzyme Q10 OR ubiquinone OR ubiquinone Q10 OR CoQ10 OR CoQ 10) OR (ethylenediaminetetraacetic acid OR edetic acid OR EDTA OR edetic*) OR (acetylcysteine OR N‐acetylcysteine OR acetylcyst* OR N‐acetylcyst*) OR (hydroxyethylrutoside OR frederine OR frederin* OR hydroxyethylrutos*) OR (deferoxamine OR desferal OR desferrioxamine OR deferoxam* OR desfer* OR desferrioxam*) OR (digoxin OR digitalis OR digitalis glycosides OR digitalis glycosid* OR digox*) OR (amifostine OR aminopropylaminoethylthiophosphoric acid OR APAETP OR amifostin*) OR (vitamin E OR alpha‐tocopherol OR tocopherols OR tocotrienols OR tocotrien* OR tocopherol* OR alpha‐tocopher*) OR (phenethylamines OR phenethylam* OR verapamil OR verapam* OR prenylamine OR prenylam*) OR (valsartan OR valsart* OR angiotensin II receptor antagonist) OR (angiotensin‐converting enzyme inhibitors OR enalapril OR angiotensin‐converting enzyme antagonists OR renitec OR ACE inhibitor* OR angiotensin‐converting enzyme inhibitor* OR enalapri* OR angiotensin‐converting enzyme antagonist*) OR (carnitine OR l‐carnitine OR carnit*) OR (superoxide dismutase OR superoxide dismut*) OR (guanidines OR guanidi* OR metaiodobenzylguanidi*) OR (probucol OR probuc*) OR (cytochromes OR cytochrom*) OR (sildenafil OR sildenafil citrate OR viagra OR sildenaf*) OR (selenium OR seleni*) in Clinical Trials
(2) For anthracyclines we used the following subject headings and text words:
(anthracyclines OR anthracycline antibiotics OR doxorubicin OR adriamycin OR epirubicin OR idarubicin OR daunorubicin OR rubidomycin OR daunoxome OR myocet OR caelyx OR doxil) in Clinical Trials
(3) For cardiotoxicity we included the following subject headings and text words:
(heart OR heart disease OR heart diseases OR cardiac disease OR cardiac diseases OR cardiotoxicity OR cardiomyopathy OR cardiomyopathies OR heart failure OR congestive heart failure OR ventricular dysfunction) in Clinical Trials
Searches were combined as (1) AND (2) AND (3).
These search strategies were used for both updates of this review; for the original version a slightly different search strategy was used based on the original MEDLINE/PubMed strategy as presented in Appendix 2.
Appendix 2. Search strategy for MEDLINE (PubMed)
(1) For the different cardioprotective interventions we used the following subject headings and text words:
Dexrazoxane: (dexrazoxane OR cardioxane OR ADR‐529 OR ICRF‐187 OR zinecard OR razoxane OR piperazines OR dexrazoxan* OR cardioxan* OR ADR‐5* OR ICRF* OR zinecar* OR razoxan* OR piperazin*).
L‐carnitine: (carnitine OR carnit*).
Probucol: (probucol OR probuc*).
Coenzyme Q10: (coenzymes OR coenzyme Q10 OR coenzym* OR ubiquinone Q10 OR CoQ10 OR CoQ 10). For the original search (August 2002) we used (coenzymes OR coenzyme Q10 OR coenzym*).
N‐acetylcysteine: (acetylcysteine OR acetylcyst* OR NAC OR N‐acetylcysteine OR N‐acetylcyst*). For the original search (August 2002) we used (acetylcysteine OR acetylcyst*).
Vitamin E: (vitamin E OR alpha‐tocopherol OR tocopherols OR alpha‐tocopher* OR tocopherol* OR tocotrienols OR tocotrien*). For the original search (August 2002) we used (vitamin E OR alpha‐tocopherol OR tocopherols OR alpha‐tocopher* OR tocopher*).
Digoxin: (digoxin OR digitalis glycosides OR digitalis OR digox* OR digitalis glycosid*).
Angiotensin‐converting enzyme inhibitors: (angiotensin‐converting enzyme inhibitors OR angiotensin‐converting enzyme inhibitor* OR ACE inhibitors OR enalapril OR enalapri* OR angiotensin converting enzyme antagonist* OR renitec). For the original search (August 2002) we used (angiotensin‐converting enzyme inhibitors OR angiotensin‐converting enzyme inhibitor* OR ACE inhibitors OR enalapril OR enalapri*).
Phenetylamines: (phenetylamines OR phenetylam* OR verapamil OR verapam* OR prenylamine OR prenylam*).
Deferoxamine: (deferoxamine OR deferoxam* OR desferal OR desfer* OR desferrioxamine OR desferrioxam*).
Ethylenediaminetetraacetic acid (EDTA): (edetic acid OR EDTA OR edetic* OR ethylenediaminetetraacetic acid). For the original search (August 2002) we used (edetic acid OR EDTA OR edetic*).
Superoxide dismutase: (superoxide dismutase OR superoxide dismut*).
Monohydroxyethylrutoside: (hydroxyethylrutoside OR hydroxyethylrutos* OR frederine OR frederin*).
Vitamin C: (vitamin C OR ascorbic acid OR ascorbic ac*).
Guanidines: (guanidines OR guanidi* OR metaiodobenzylguanidine OR metaiodobenzylguanidi*).
Cytochromes: (cytochromes OR cytochrom*).
The cardioprotective interventions stated below were added in the updates of this review:
Vitamin A: (vitamin A OR retinol OR tretinoin OR retinoic acid OR vitamin A acid OR carotenoids OR retinoids OR retinoi* OR tretinoi* OR carotenoi*).
Sildenafil: (sildenafil OR sildenafil citrate OR viagra OR sildenaf*).
Selenium: (selenium OR selen*).
Glutathione: (glutathione OR glutathione disulfide OR S‐nitrosoglutathione OR glutathion*).
Valsartan: (valsartan OR valsart* OR angiotension II receptor antagonist).
Carvedilol: (carvedilol OR carvedil*).
Trimetazidine: (trimetazidine OR vastarel OR idaptan OR vasartel OR trimetazid* OR piperazines OR piperazin*).
Amifostine: (amifostine OR amifostin* OR aminopropylaminoethylthiophosphoric acid OR APAETP).
(2) For anthracyclines we used the following subject headings and text words:
(anthracyclines OR anthracyclin* OR anthracycline antibiotics OR antibiotics, anthracycline OR 4‐demethoxydaunorubicin OR 4 demethoxydaunorubicin OR 4‐desmethoxydaunorubicin OR 4 desmethoxydaunorubicin OR IMI 30 OR IMI30 OR IMI‐30 OR idarubicin hydrochloride OR hydrochloride, idarubicin OR NSC 256439 OR NSC‐256439 OR NSC256439 OR idarubicin OR idarubic* OR 4'‐epiadriamycin OR 4' epiadriamycin OR 4'‐epidoxorubicin OR 4' epidoxorubicin OR 4'‐epi‐doxorubicin OR 4' epi doxorubicin 4'‐epi‐adriamycin OR 4' epi adriamycin OR 4'‐epi‐DXR OR 4' epi DXR OR epirubicin hydrochloride OR hydrochloride, epirubicin OR farmorubicin OR IMI‐28 OR IMI 28 OR IMI28 OR NSC 256942 OR NSC‐256942 OR NSC256942 OR epirubicin OR epirubic* OR adriablastine OR adriblastin OR adriablastin OR adriamycin OR DOX‐SL OR DOX SL OR doxorubicin hydrochloride OR hydrochloride doxorubicin OR doxorubic* OR adriamyc* OR dauno‐rubidomycine OR dauno rubidomycin OR rubidomycin OR rubomycin OR daunomycin OR cerubidine OR daunoblastin OR daunoblastine OR daunorubicin hydrochloride OR hydrochloride, daunorubicin OR daunorubic* OR rubidomyc* OR NSC‐82151 OR NSC 82151 OR NSC82151 OR daunoxome OR daunosom* OR doxil OR caelyx OR liposomal doxorubicin OR doxorubicin, liposomal OR myocet OR doxorubicin OR daunorubicin). For the original search (August 2002) we used (anthracyclines OR antibiotic, anthracycline OR anthracyclin* OR doxorubicin OR doxorubic* OR adriamycin OR adriamyc* OR daunorubicin OR daunorubic* OR epirubicin OR epirubic*).
(3) For cardiotoxicity we included the following subject headings and text words in the updates of this review:
(heart OR heart diseases OR heart disease OR disease, heart OR diseases, heart OR cardiac diseases OR cardiac disease OR diseases, cardiac OR disease, cardiac OR cardiotoxicity OR cardiomyopathy OR heart failure, congestive OR heart failure OR cardiomyopathy, congestive OR ventricular dysfunction OR ventricular dysfunction, left OR ventricular dysfunction, right).
(4) For the methodological search we used the highly sensitive search strategy for identifying reports of randomised controlled trials (sensitivity‐maximizing version) (Higgins 2008). For the original search (August 2002) and for the first update (April 2007) we used the highly sensitive search strategy for identifying reports of randomised controlled trials (all phases) as described in the Cochrane Handbook (Higgins 2006).
For each cardioprotective intervention, searches were combined as (1) AND (2) AND (3) AND (4).
Appendix 3. Search strategy for EMBASE (Ovid)
(1) For the different cardioprotective interventions we used the following subject headings and text words:
dexrazoxane.mp. or exp Razoxane/ or cardioxane.mp. or ICRF‐187.mp. or ADR‐529.mp. or zinecard.mp. or piperazines.mp. or exp Piperazine Derivative/ or (piperazin$ or dexrazoxan$ or cardioxan$ or razoxan$ or zinecar$ or ICRF$ or ADR‐5$).mp. or vitamin A.mp. or exp RETINOL/ or retinoic acid.mp. or exp Retinoic Acid/ or retinol.mp. or tretinoin.mp. or vitamin a acid.mp. or carotenoids.mp. or exp Carotenoid/ or retinoids.mp. or exp Retinoid/ or (retino$ or Tretinoi$ or carotenoi$).mp. or trimetazidine.mp. or exp TRIMETAZIDINE/ or trimethazidine.mp. or vastarel.mp. or trimetazid$.mp. or L‐carnitine.mp. or exp Carnitine/ or carnit$.mp. or superoxide dismutase.mp. or exp Superoxide Dismutase/ or superoxide dismut$.mp. or ACE inhibitor.mp. or angiotensin‐converting enzyme inhibitor.mp. or angiotensin‐converting enzyme antagonist.mp. or Enalapril/ or renitec.mp. or (angiotensin converting enzyme antagonist$ or enalapri$ or angiotensin‐converting enzyme inhibitor$).mp. or amifostine.mp. or exp AMIFOSTINE/ or APAETP.mp. or aminopropylaminoethylthiophosphoric acid.mp. or amifostin$.mp. or carvedilol.mp. or exp CARVEDILOL/ or carvedil$.mp. or exp DEFEROXAMINE MESYLATE/ or exp DEFEROXAMINE/ or deferoxamine.mp. or desferal.mp. or desferrioxamine.mp. or (desferrioxam$ or desfer$ or desferoxam$).mp. or digoxin.mp. or exp DIGOXIN/ or exp DIGITALIS INTOXICATION/ or exp DIGITALIS/ or DIGITALIS GLYCOSIDE/ or digitalis.mp. or (digitalis glycosides or dogox$ or digitalis glycosid$).mp. or edetic acid.mp. or exp Edetic Acid/ or (EDTA or ethylenediaminetetraacetic acid or edetic$).mp. or exp GLUTATHIONE DERIVATIVE/ or exp GLUTATHIONE DISULFIDE/ or exp GLUTATHIONE/ or glutathione.mp. or glutathion$.mp. or s‐nitrosoglutathione.mp. or exp S Nitrosoglutathione/ or guanidines.mp. or exp Guanidine Derivative/ or metaiodobenzylguanidine.mp. or exp "(3 Iodobenzyl)Guanidine"/ or guanidi$.mp. or hydroxyethylrutoside.mp. or exp Monoxerutin/ or (frederine or frederin$ or monoxerut$ or hydroxyethylrutos$).mp. or n‐acetylcysteine.mp. or exp Acetylcysteine/ or ACETYLCYSTEINE DERIVATIVE/ or (acetylcyst$ or N‐acetylcyst$).mp. or phenetylamines.mp. or exp Phenethylamine/ or prenylamine.mp. or exp PRENYLAMINE/ or exp VERAPAMIL/ or verapamil.mp. or exp VERAPAMIL DERIVATIVE/ or (phenetylam$ or verapam$ or prenylam$).mp. or exp PROBUCOL/ or probucol.mp. or probuc$.mp. or exp SELENIUM DERIVATIVE/ or exp SELENIUM/ or selenium.mp. or seleni$.mp. or exp VALSARTAN/ or valsartan.mp. or exp Angiotensin 2 Receptor Antagonist/ or angiotensin II receptor antagonist.mp. or exp Angiotensin II Antagonist/ or (angiotensin II inhibitor or valsart$).mp. or ascorbic acid.mp. or exp Ascorbic Acid/ or vitamin c.mp. or ascorbic ac$.mp. or vitamin E.mp. or alpha tocopherol.mp. or exp Alpha Tocopherol/ or tocopherols.mp. or exp Tocopherol/ or tocotrienols.mp. or exp Alpha Tocotrienol/ or (tocotrien$ or alpha tocopher$ or tocopherol$).mp. or coenzymes.mp. or exp Coenzyme/ or coenzyme Q10.mp. or exp Ubidecarenone/ or ubiquinone.mp. or exp UBIQUINONE DERIVATIVE/ or exp UBIQUINONE/ or (ubiquinone Q10 or CoQ10).mp. or cytochromes.mp. or exp Cytochrome/ or cytochrom$.mp. or sildenafil.mp. or exp SILDENAFIL/ or viagra.mp.
(2) For anthracyclines we used the following subject headings and text words:
exp ANTHRACYCLINE ANTIBIOTIC AGENT/ or exp ANTHRACYCLINE/ or exp ANTHRACYCLINE DERIVATIVE/ or (anthracycline or anthracyclines).mp. or anthracyclin$.mp. or doxorubicin.mp. or exp DOXORUBICIN DERIVATIVE/ or exp DOXORUBICIN/ or adriamycin.mp. or exp DAUNORUBICIN DERIVATIVE/ or daunorubicin.mp. or exp DAUNORUBICIN/ or rubidomycin.mp. or epirubicin.mp. or exp EPIRUBICIN/ or exp IDARUBICIN DERIVATIVE/ or exp IDARUBICIN/ or idarubicin.mp. or (doxorubic$ or adriamyc$ or daunorubic$ or rubidomyc$ or epirubic$ or idarubic$).mp. or (daunoxome or doxil or caelyx or myocet).mp.
(3) For cardiotoxicity we included the following subject headings and text words:
left ventricular dysfunction.mp. or exp Heart Left Ventricle Failure/ or exp Heart/ or exp Heart Right Ventricle Failure/ or exp Echocardiography/ or right ventricular dysfunction.mp. or exp Heart Failure/ or echocardiography.mp. or ventricular dysfunction.mp. or heart failure.mp. or exp Heart Failure/ or congestive heart failure.mp. or exp Congestive Heart Failure/ or cardiomyopathy.mp. or exp CARDIOMYOPATHY/ or exp CONGESTIVE CARDIOMYOPATHY/ or cardiotoxicity.mp. or exp CARDIOTOXICITY/ or heart disease.mp. or exp Heart Disease/ or cardiac disease.mp.
(4) For the methodological search we used the following subject headings and text words:
For the first update: Randomized Controlled Trial/ or Clinical Trial/ or random allocation.mp. or exp Randomization/ or Double Blind Procedure/ or Single Blind Procedure/ or Clinical Trial/ OR Controlled study/ or placebo.mp. or exp PLACEBO/ or placebo$.mp. or random$.mp. or comparative study.mp. or exp Comparative Study/ or prospective study.mp. or exp Prospective Study/ or research design.mp. or evaluation studies.mp. or follow‐up studies.mp. or (clinical trial or ((singl$ or doubl$ or tripl$ or trebl$) and (mask$ or blind))).mp. or (control$ or prospectiv$ or volunteer$).mp.
For the second update: (Randomized Controlled Trial/ or Controlled Clinical Trial/ or randomized.ti,ab. or placebo.ti,ab. or randomly.ti,ab. or trial.ti,ab. or groups.ti,ab. or drug therapy.sh.) and Human/
Searches were combined as (1) AND (2) AND (3) AND (4).
These search strategies were used for both updates of this review; for the original version a slightly different search strategy was used based on the original MEDLINE/PubMed strategy as presented in Appendix 2.
[mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]; [ti,ab=title, abstract]; [sh=subject heading]
Data and analyses
Comparison 1. Dexrazoxane versus no dexrazoxane or placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Clinical heart failure | 8 | 1561 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.10, 0.32] |
| 2 Heart failure (i.e. clinical and subclinical heart failure combined) | 5 | 643 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.20, 0.41] |
| 3 Response rate | 6 | 1021 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.78, 1.02] |
| 4 Progression‐free survival | 4 | Hazard ratio (Fixed, 95% CI) | 1.01 [0.86, 1.18] | |
| 5 Overall survival | 4 | Hazard ratio (Fixed, 95% CI) | 1.04 [0.88, 1.23] | |
| 6 Adverse effects: thrombocytopenia grade 3 or 4 | 2 | 293 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.49, 2.21] |
| 7 Adverse effects: abnormal platelet count at nadir grade 3 or 4 | 2 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.53, 1.59] |
| 8 Adverse effects: abnormal platelet count at recovery grade 3 or 4 | 2 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.16, 4.15] |
| 9 Adverse effects: neutropenia grade 3 or 4 | 2 | 293 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.90, 1.21] |
| 10 Adverse effects: abnormal granulocyte count at nadir grade 3 or 4 | 2 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.97, 1.11] |
| 11 Adverse effects: abnormal granulocyte count at recovery grade 3 or 4 | 2 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.59, 1.21] |
| 12 Adverse effects: abnormal white blood cell count at nadir grade 3 or 4 | 2 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.05, 1.29] |
| 13 Adverse effects: abnormal white blood cell count at recovery grade 3 or 4 | 2 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.36, 1.31] |
| 14 Adverse effects: anaemia grade 3 or 4 | 3 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [1.08, 1.81] |
| 15 Adverse effects: stomatitis grade 3 or 4 | 4 | 914 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.60, 1.21] |
| 16 Adverse effects: nausea grade 3 or 4 | 3 | 698 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.49, 0.94] |
| 17 Adverse effects: vomiting grade 3 or 4 | 3 | 698 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.55, 1.14] |
| 18 Adverse effects: neurotoxicity grade 3 or 4 | 2 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.12, 2.26] |
| 19 Adverse effects: pain on injection grade 3 or 4 | 2 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [0.34, 6.72] |
| 20 Adverse effects: anorexia grade 3 or 4 | 2 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.57, 1.64] |
| 21 Adverse effects: alopecia grade 3 or 4 | 3 | 698 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.94, 1.11] |
| 22 Adverse effects: phlebitis grade 3 or 4 | 2 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.35, 6.75] |
| 23 Adverse effects: diarrhoea grade 3 or 4 | 2 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.49, 2.79] |
| 24 Adverse effects: fever grade 3 or 4 | 2 | 534 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.81, 2.53] |
| 25 Adverse effects: secondary malignant disease | 2 | 421 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.28, 6.90] |
1.1. Analysis.
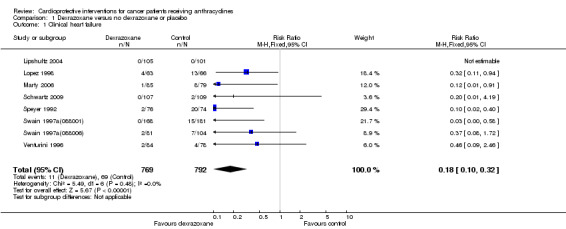
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 1 Clinical heart failure.
1.2. Analysis.
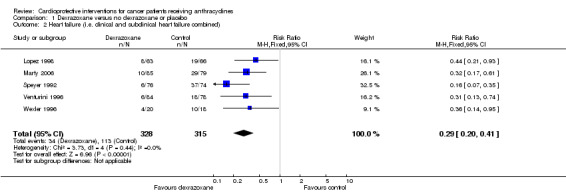
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 2 Heart failure (i.e. clinical and subclinical heart failure combined).
1.3. Analysis.
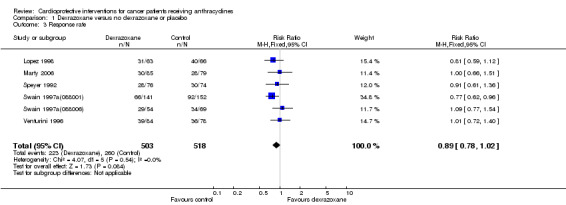
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 3 Response rate.
1.4. Analysis.
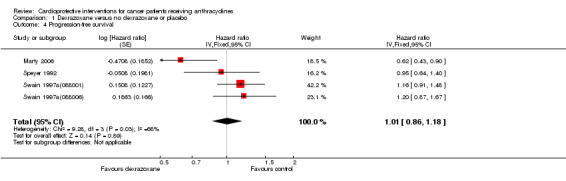
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 4 Progression‐free survival.
1.5. Analysis.
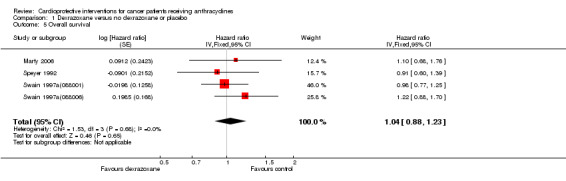
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 5 Overall survival.
1.6. Analysis.
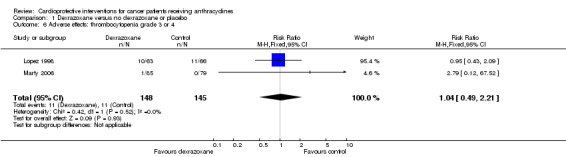
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 6 Adverse effects: thrombocytopenia grade 3 or 4.
1.7. Analysis.
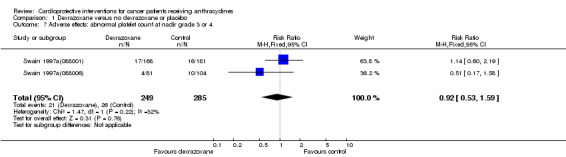
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 7 Adverse effects: abnormal platelet count at nadir grade 3 or 4.
1.8. Analysis.
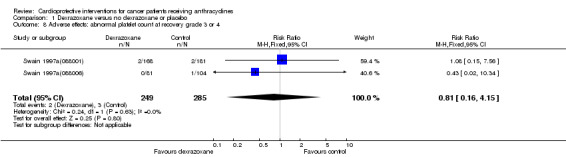
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 8 Adverse effects: abnormal platelet count at recovery grade 3 or 4.
1.9. Analysis.
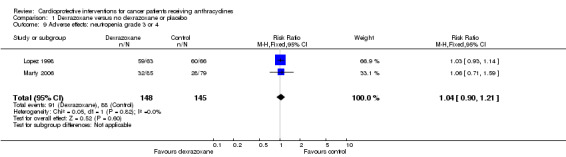
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 9 Adverse effects: neutropenia grade 3 or 4.
1.10. Analysis.
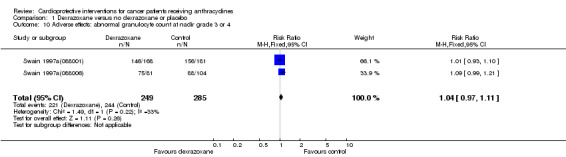
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 10 Adverse effects: abnormal granulocyte count at nadir grade 3 or 4.
1.11. Analysis.
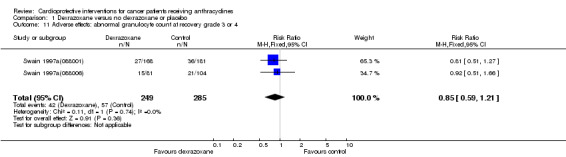
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 11 Adverse effects: abnormal granulocyte count at recovery grade 3 or 4.
1.12. Analysis.
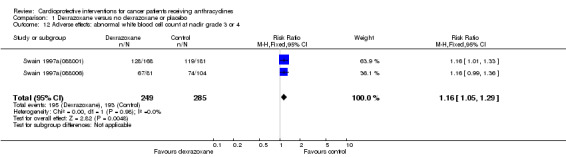
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 12 Adverse effects: abnormal white blood cell count at nadir grade 3 or 4.
1.13. Analysis.
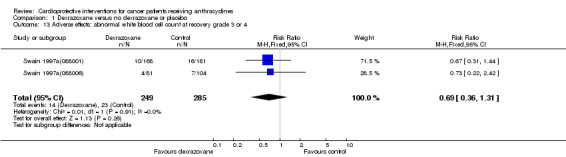
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 13 Adverse effects: abnormal white blood cell count at recovery grade 3 or 4.
1.14. Analysis.
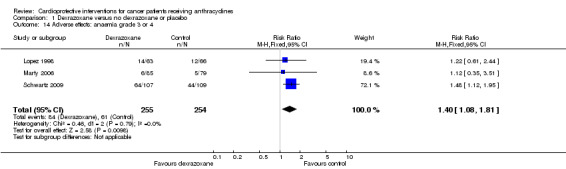
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 14 Adverse effects: anaemia grade 3 or 4.
1.15. Analysis.
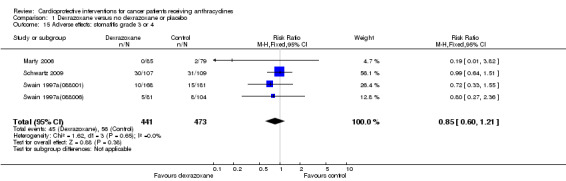
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 15 Adverse effects: stomatitis grade 3 or 4.
1.16. Analysis.
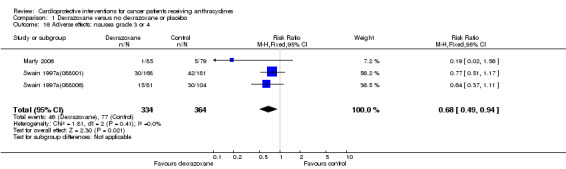
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 16 Adverse effects: nausea grade 3 or 4.
1.17. Analysis.
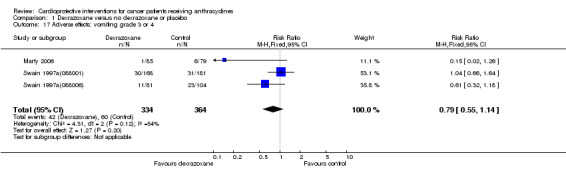
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 17 Adverse effects: vomiting grade 3 or 4.
1.18. Analysis.
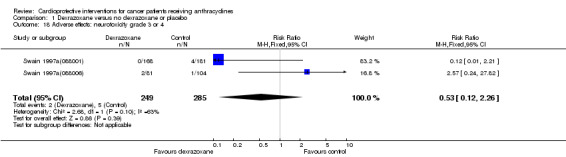
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 18 Adverse effects: neurotoxicity grade 3 or 4.
1.19. Analysis.

Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 19 Adverse effects: pain on injection grade 3 or 4.
1.20. Analysis.
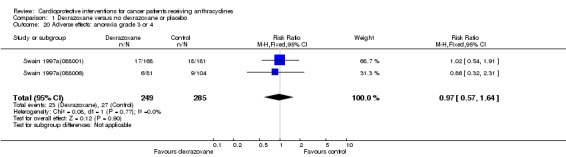
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 20 Adverse effects: anorexia grade 3 or 4.
1.21. Analysis.
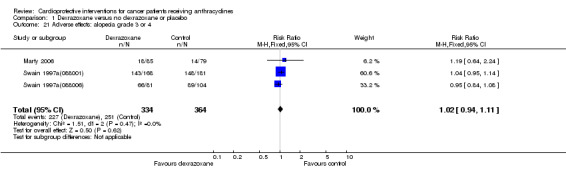
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 21 Adverse effects: alopecia grade 3 or 4.
1.22. Analysis.
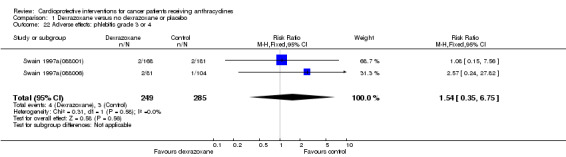
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 22 Adverse effects: phlebitis grade 3 or 4.
1.23. Analysis.
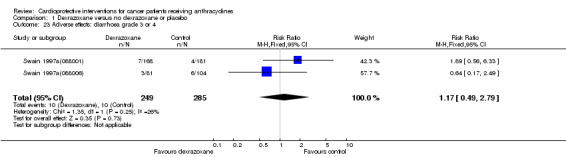
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 23 Adverse effects: diarrhoea grade 3 or 4.
1.24. Analysis.
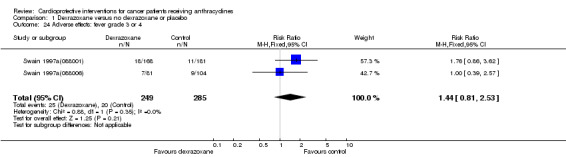
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 24 Adverse effects: fever grade 3 or 4.
1.25. Analysis.
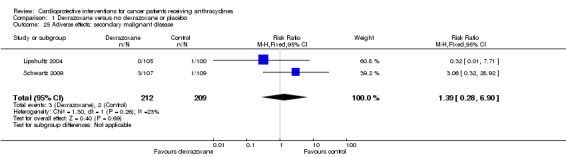
Comparison 1 Dexrazoxane versus no dexrazoxane or placebo, Outcome 25 Adverse effects: secondary malignant disease.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Galetta 2005.
| Methods | Method of randomisation not clear. | |
| Participants | 20 patients (median age 54 years (all < 60 years); 11 males and 9 females) with non‐Hodgkin lymphoma (stage 2, 3 or 4, but number of patients with each stage in the different treatment groups nm) treated with epirubicin (cumulative dose nm; peak dose (i.e. maximal dose received in 1 week) 40 mg/m2; bolus infusion), cyclophosphamide, etoposide, prednisolone, vincristine, methotrexate, aracytin and bleomycin. No prior anthracyclines. No prior cardiac radiotherapy. No prior cardiac dysfunction. | |
| Interventions | Dexrazoxane (10:1 ratio of study drug to epirubicin; IV infusion over 15 minutes immediately after epirubicin) (n = 10) versus no cardioprotective intervention (n = 10). | |
| Outcomes | Heart failure (subclinical heart failure defined as abnormalities in for example left ventricular diastolic diameter, posterior wall diastolic thickness and LVEF as measured by echocardiography; no further definitions were provided). | |
| Notes | Length of follow up nm. Age in intervention and control group nm. Cumulative anthracycline dose per treatment group nm. |
|
Gallegos‐Castorena 2007.
| Methods | Method of randomisation not clear. | |
| Participants | 28 children (mean age 11.6 years (range 7 to 15); 14 males and 14 females) with osteosarcoma (stage nm; in 5 patients metastatic disease) treated with doxorubicin (cumulative dose nm, but according to protocol patients should receive 150 mg/m2; peak dose (i.e. maximal dose received in one week) 75 mg/m2; infusion duration nm) and intra‐arterial cisplatin. No prior anthracyclines. No prior cardiac radiotherapy. Prior cardiac dysfunction nm. | |
| Interventions | Amifostine (740 mg/m2/dose (cumulative dose according to protocol 2960 mg/m2); IV infusion under sedation over 15 minutes immediately prior to each cisplatin dose) (n=15) versus no cardioprotective intervention (n=13). | |
| Outcomes | Heart failure (clinical heart failure and subclinical heart failure according to WHO criteria; it was stated that for the evaluated parameters they did not differ from the NCI system, i.e. grade 1‐2 is subclinical). Response rate (complete/good remission defined as >90% necrosis after tumorectomy; partial remission defined as 60‐90% necrosis after tumorectomy). Adverse effects (according to WHO criteria; it was stated that for the evaluated parameters they did not differ from the NCI system). |
|
| Notes | Length of follow up nm. Age in intervention and control group nm, but it was stated that the groups were not statistically different. Cumulative anthracycline dose per treatment group nm. |
|
Iarussi 1994.
| Methods | Method of randomisation not clear (block randomisation). | |
| Participants | 20 children (aged 1 to 15 years, sex nm) with acute lymphoblastic leukaemia or Non‐Hodgkin lymphoma (stage nm) treated with doxorubicin and patients with ALL also daunorubicin (cumulative dose 210‐270 mg/m2; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm (but no differences in echocardiographic parameters at baseline between the treatment groups). | |
| Interventions | Coenzyme Q10 (100 mg per os twice daily) (n=10) versus no cardioprotective intervention (n=10). | |
| Outcomes | Heart failure (subclinical heart failure defined as echocardiographic LVSF < 28%). | |
| Notes | Length of follow up nm. Mean age in intervention group: 5.6 years; mean age in control group: 5.1 years. Cumulative anthracycline dose in intervention group: 210‐270 mg/m2 (mean 240 mg/m2); cumulative anthracycline dose in control group: 210‐270 mg/m2 (mean 252 mg/m2). |
|
Kalay 2006.
| Methods | Method of randomisation not clear. | |
| Participants | 50 patients (age for all randomised patients nm: see notes; 43 females and 7 males) with breast cancer, lymphoma or other type of malignancy (stage nm) treated with therapy including adriamycin or epirubicin (cumulative dose for all randomised patients nm: see notes; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). No prior anthracyclines. No prior cardiac radiotherapy. No prior cardiac dysfunction. | |
| Interventions | Carvedilol (12.5 mg per os once daily) (n = 25) versus placebo (n = 25). | |
| Outcomes | Heart failure (clinical heart failure defined as decompensated heart failure; subclinical heart failure defined as echocardiographic LVEF < 50%). | |
| Notes | Length of follow up 6 months. Mean age in intervention group 46.8 years and mean age in control group 49 years. Cumulative doxorubicin and epirubicin dose in intervention group: 525.3 mg/m2 and 787.9 mg/m2; cumulative doxorubicin and epirubicin dose in control group: 513.6 mg/m2 and 770.4 mg/m2. |
|
Kraft 1990.
| Methods | Method of randomisation not clear. | |
| Participants | 64 patients (aged 19 to 59 years; 31 males and 33 females) with acute myeloid leukaemia (stage nm) treated with therapy including daunorubicin (cumulative dose circa 360‐540 mg/m2 according to protocol; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). No prior anthracycline therapy. No prior cardiac radiotherapy. Prior cardiac dysfunction nm (but no differences in pretreatment cardiologic findings between the treatment groups). | |
| Interventions | Verapamil (40 mg per os thrice daily) (n = 30) versus no cardioprotective intervention (n=34). | |
| Outcomes | Heart failure (clinical heart failure defined as NYHA class 4). | |
| Notes | Length of follow up nm. Mean age in both intervention and control group: 41.3 years. Cumulative anthracycline dose in intervention group: circa 360‐540 mg/m2; cumulative anthracycline dose in control group: circa 360‐540 mg/m2. |
|
Lipshultz 2004.
| Methods | Computer‐generated randomisation was performed centrally at the Quality Assurance Office for Clinical Trials (permuted block design with institutional balancing to ensure that a treatment imbalance within an institution was no greater than 3 patients). | |
| Participants | 206 children (age for all randomised patients nm: see notes; 120 boys and 86 girls) with high‐risk ALL treated with multiagent chemotherapy (including doxorubicin: see notes) and CNS irradiation. No prior anthracycline therapy. No prior cardiac radiotherapy. Part of the patients were diagnosed with prior cardiac dysfunction (by either echocardiography or the cardiac marker troponin T), but the exact number of patients was nm. | |
| Interventions | Dexrazoxane (10:1 ratio of study drug to doxorubicin; IV bolus up to 15 minutes immediately before doxorubicin) (n=105) versus no cardioprotective intervention (n=101). | |
| Outcomes | Heart failure (clinical heart failure defined as congestive heart failure or other symptomatic cardiac disease). Response rate (defined as the number of patients in complete remission; no definition of complete remission provided). Adverse effects (no definition provided). |
|
| Notes | Median length of follow up 2.7 years. Median age 7.5 years in intervention group and 7.3 years in control group. According to protocol patients in both treatment groups should have received a cumulative doxorubicin dose of 300 mg/m2 (peak dose (i.e. maximal dose received in 1 week) 30 mg/m2; infusion duration nm). Long‐term follow‐up data of this study have been published (Lipshultz 2010; Barry 2008). Both studies included 205 randomised patients (105 in the dexrazoxane group and 100 in the control group) as opposed to the original publication, which included 206 randomised patients. Lipshultz 2010 provided long‐term follow‐up data (median follow up in the dexrazoxane group 6.2 years; range 3 to 7.7 years and in the control group 5.7 years; range 2.8 to 7.6 years) on clinical heart failure for 134 of the 205 randomised patients, i.e. 68 (27 males and 41 females) of the 105 patients in the dexrazoxane group and 66 (30 males and 36 females) of the 100 patients in the control group. These were patients for which data were available after treatment completion. It was stated that children leaving the study did not differ in any clinical characteristic from those who stayed in the study. The median cumulative anthracycline dose in the dexrazoxane group was 300 mg/m2 (range 300 to 300 mg/m2) and in the control group it was also 300 mg/m2 (range 288 to 300 mg/m2) with an infusion duration up to 15 minutes (push or bolus). Barry 2008 provided long‐term follow‐up data (median follow up 6.2 years) on secondary malignant disease. |
|
Lopez 1998.
| Methods | Method of randomisation not clear. | |
| Participants | 129 patients (aged 14‐75 years; sex 29 males and 109 females) with metastatic breast cancer (n=95) or advanced soft tissue sarcoma (n=34) treated with epirubicin (cumulative dose for all randomised patients nm: see notes; peak dose (i.e. maximal dose received in 1 week) 160 mg/m2; bolus infusion). No prior anthracycline therapy. Prior cardiac radiotherapy possible in 18 patients in the dexrazoxane group and 13 patients in the control group (< 20 Gy on the heart). No prior cardiac dysfunction. | |
| Interventions | Dexrazoxane (1000 mg/m2 versus 160 mg/m2 epirubicin; IV infusion over 15 minutes 30 minutes before epirubicin) (n=63) versus no cardioprotective intervention (n=66). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as NYHA class 2,3 or 4; subclinical heart failure defined as a decrease in left ventricular ejection fraction as measured by MUGA to less than 45% or a decrease from baseline of >= 20% and no development of clinical heart failure later on). Response rate (according to standard WHO criteria: a 50% decrease (or 30% decrease in one diameter) was required for assessable disease). Adverse effects (according to WHO criteria). |
|
| Notes | Length of follow up nm. Median age in intervention group for breast cancer: 55 years and for soft tissue sarcoma: 55 years; median age in control group for breast cancer: 58 years and for soft tissue sarcoma: 51 years. Cumulative anthracycline dose in intervention group: median 960 mg/m2; cumulative anthracycline dose in control group: median 880 mg/m2. |
|
Marty 2006.
| Methods | Randomisation was performed centrally using a permuted block design, which was stratified by center and thus by type of anthracycline used and dose of dexrazoxane (open label study). | |
| Participants | 164 patients (median age 52 years (range 30‐76); all females) with advanced or metastatic breast cancer treated with either epirubicin or doxorubicin (cumulative dose: see notes; peak dose (i.e. maximal dose received in 1 week) see notes; infusion duration nm). Prior anthracycline therapy in both treatment groups (median cumulative dose similar in both: dexrazoxane group: a median cumulative doxorubicin dose of 290 mg/m2 (range 30‐650) in 46 patients and a median cumulative epirubicin dose of 421 mg/m2 (range 231‐599) in 42 patients; some patients were treated with both doxorubicin and epirubicin; control group: a median cumulative doxorubicin dose of 243 mg/m2 (range 60‐480) in 44 patients and a median cumulative epirubicin dose of 360 mg/m2 (range 94‐599) in 38 patients; some patients were treated with both doxorubicin and epirubicin). Prior cardiac radiotherapy was possible for 74 patients randomised to dexrazoxane and 62 patients in the control group (dose nm). No prior cardiac dysfunction. | |
| Interventions | Dexrazoxane (20:1 ratio of study drug to doxorubicin and 10:1 ratio to epirubicin; IV infusion over 15 minutes 30 minutes prior to anthracycline infusion) (n=85) versus no cardioprotective intervention (n=79). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as clinical signs of cardiac insufficiency (graded according to NYHA criteria); subclinical heart failure defined as 1) a reduction in LVEF by 10% absolute percentage points or more as measured by MUGA scan or 15% or more as measured by echocardiography, 2) a reduction in absolute LVEF as measured by echocardiography or MUGA scan to a value below 45%). Response rate (according to WHO criteria). Survival. Adverse effects (according to CTC criteria). |
|
| Notes | Length of follow up nm. Median age in intervention group 50 years; median age in control group 52 years. The cumulative anthracycline dose was calculated as all anthracyclines received during this study and prior to it using 50 mg/m2 epirubicin = 90 mg/m2 doxorubicin. The median cumulative anthracycline dose in the dexrazoxane group was 669 mg/m2 (range 247‐936); the median anthracycline peak dose (i.e. maximal dose received in 1 week) was 80 mg/m2 (range 37‐116). The median cumulative anthracycline dose in the control group was 608 mg/m2 (range 244‐900); the median anthracycline peak dose was 80 mg/m2 (40‐120). |
|
Milei 1987.
| Methods | Method of randomisation not clear (double‐blind trial). | |
| Participants | 36 patients (age and sex for all randomised patients nm: see notes) with solid tumours (stage nm) treated with doxorubicin (cumulative dose for all randomised patients nm: see notes; peak dose (i.e. maximal dose received in 1 week) 40‐50 mg/m2; infusion duration: nm) and either vincristine or cyclophosphamide. No prior anthracycline therapy. No prior cardiac radiotherapy. No prior cardiac dysfunction. | |
| Interventions | Prenylamine (200 mg per os once daily) (n=18) versus placebo (n=18). | |
| Outcomes | Heart failure (clinical heart failure defined as congestive cardiomyopathy). | |
| Notes | Length of follow up: 5‐9 months. Age in evaluable patients in intervention group (n=13): 49‐75 years; age in evaluable patients in control group (n=13): 45‐64 years. Sex in evaluable patients (n=26): 9 males and 17 females. Cumulative anthracycline dose in evaluable patients in intervention group (n=13): 150‐600 mg/m2; cumulative anthracycline dose in evaluable patients in control group (n=13): 160‐500 mg/m2. |
|
Myers 1983.
| Methods | Method of randomisation not clear (patients were matched for age, sex and disease status). | |
| Participants | 54 patients (age 18‐80 years; 26 males and 28 females) with breast cancer, nodular lymphoma, metastatic soft tissue sarcoma or other tumour (stage nm) treated with doxorubicin (cumulative dose nm; peak dose (i.e. maximal dose received in 1 week) 75 mg/m2; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy is possible (maximal 600 rad; number of patients nm). No prior cardiac dysfunction. | |
| Interventions | N‐acetylcysteine (5.5gm/m2 orally preceding the doxorubicin gift) (n=24) versus no cardioprotective intervention (n=30). | |
| Outcomes | Heart failure (clinical heart failure defined as congestive heart failure). Response rate (defined as partial remission and no evaluable disease with a duration greater than 4 months). Adverse effects (nausea and vomiting, alopecia, leukopenia (<1000), thrombocytopenia (<20000), haemoglobin < 8, diarrhoea, mucositis, erythematous flare at sites of previous venipunctures). |
|
| Notes | Length of follow up nm. Median age in intervention group: 53.5 years; median age in control group: 44 years. Cumulative anthracycline dose in intervention and control group nm. |
|
Schwartz 2009.
| Methods | Method of randomisation not clear. | |
| Participants | 216 children (mean age 14 years (range 4‐21); 140 males and 76 females) with intermediate or high risk Hodgkin lymphoma (stage IB n=1, stage II n=81, stage III n=52, stage IV n=70, stage unknown n=12) treated with multiagent chemotherapy including doxorubicin (cumulative dose nm (according to protocol 180 mg/m2 for patients with rapid early response and 300 mg/m2 for patients with slow early response; it was stated that there were virtually no dose reductions); peak dose (i.e. maximal dose received in 1 week) 60 mg/m2; infusion duration nm). Patients received 21 Gy of radiotherapy to mantle if it involved Hodgkin lymphoma; pericardial infusions, lung disease or pericardial involvement were treated with 10.5 Gy (no further information provided). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm. | |
| Interventions | Dexrazoxane (10:1 ratio of study drug to doxorubicin (see notes); IV infusion (infusion duration and timing in relation with doxorubicin nm)) (n=107) versus no cardioprotective intervention (n=109). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined according to NCI‐CTCv2.0 criteria). Response rate (complete remission defined as disappearance of active disease (gallium negative, 70% or more decrease in the sum of the products of the perpendicular diameters of measurable lesions and negative bone marrow or bone scan if initially positive). Adverse effects (according to NCI‐CTCv2.0 criteria). |
|
| Notes | Length of follow up nm (median follow‐up for patients without an event was 5.2 years). Age in intervention and control group nm. Cumulative anthracycline dose per treatment group nm. No significant difference in number of patients with rapid and slow early response between treatment groups identified (P=0.07). It was stated that dexrazoxane was also given on day 7 (besides on day 0 and 1 together with doxorubicin), we did not include this additional gift in the ratio of study drug to doxorubicin. |
|
Speyer 1992.
| Methods | Method of randomisation not clear (patients were stratified by prior adjuvant cyclophosphamide, methotrexate and 5FU and cardiac risk factors; blocks of 10 patients within each stratum). | |
| Participants | 150 patients (aged 27‐76 years; all females) with breast cancer (stage nm) treated with doxorubicin (cumulative dose range 25‐2150 mg/m2; peak dose (i.e. maximal dose received in 1 week) 50 mg/m2; infusion duration 5 to 10 minutes), 5FU and cyclophosphamide. No prior anthracycline therapy. Prior cardiac radiotherapy possible in 28 patients (14 in each treatment group). No prior cardiac dysfunction. | |
| Interventions | Dexrazoxane (20:1 ratio of study drug to doxorubicin; IV infusion over 15 minutes 30 minutes before doxorubicin) (n=76) versus no cardioprotective intervention (n=74). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as NYHA class 2,3 or 4 i.e. any signs and symptoms of clinical congestive heart failure; subclinical heart failure defined as NYHA class 1 i.e. a decrease in LVEF as measured by MUGA of ≥20% from baseline or a decrease in LVEF as measured by MUGA to < 45% or an endomyocardial biopsy score ≥2 according to the Billingham scale). Response rate (according to ECOG criteria). Survival. Adverse effects. |
|
| Notes | Not in all patients an endomyocardial biopsy was performed. Length of follow up nm. Age in intervention group: mean 55.5 years and median 58 years; age in control group: mean 56.2 years and median 58 years. Cumulative anthracycline dose in intervention group: mean 558 mg/m2 (range 50‐2150); cumulative anthracycline dose in control group: mean 407.4 mg/m2 (range 25‐950). |
|
Swain 1997a(088001).
| Methods | Block randomisation according to a prospectively prepared randomisation list (a separate list was prepared for each investigational site and within each site, the assignments were stratified relative to the presence or absence of cardiac risk factors and on the basis of measurable versus nonmeasurable disease). | |
| Participants | 349 patients (aged 25‐84 years; all females) with breast cancer (stage III or IV) treated with doxorubicin (cumulative dose <100‐2700 mg/m2; peak dose (i.e. maximal dose received in 1 week) 50 mg/m2; infusion duration nm), fluorouracil and cyclophosphamide. No prior anthracycline therapy. Prior cardiac radiotherapy in 20 patients in the dexrazoxane group and 14 patients in the control group (dose nm). No prior cardiac dysfunction. | |
| Interventions | Dexrazoxane (10:1 ratio of study drug to doxorubicin; slow IV push or rapid‐drip IV infusion between 15 and 30 minutes before doxorubicin) (n=168) versus placebo (n=181). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as 2 or more of the following: cardiomegaly established by radiography, basilar rales, S3 gallop or paroxysmal nocturnal dyspnoea, orthopnoea, or significant dyspnoea on exertion; subclinical heart failure defined as 1) decline in LVEF as measured by MUGA from baseline of ≥10% below the institution's LLN, 2) a decline in LVEF as measured by MUGA of at least 20% from baseline or 3) decline in LVEF as measured by MUGA to at least 5% below the institution's LLN). Response rate (according to ECOG criteria; see notes). Survival. Adverse effects (according to ECOG criteria). |
|
| Notes | Length of follow up: in the intervention group median 532 days (range 1‐1863); in the control group median 511 days (range 1‐1652). Median age in intervention group: 58 years; median age in control group: 56 years. Cumulative anthracycline dose in intervention and control group nm. |
|
Swain 1997a(088006).
| Methods | Block randomisation according to a prospectively prepared randomisation list (a separate list was prepared for each investigational site and within each site, the assignments were stratified relative to the presence or absence of cardiac risk factors and on the basis of measurable versus nonmeasurable disease). | |
| Participants | 185 patients (aged 23‐79 years; all females) with breast cancer (stage IIIB or IV) treated with doxorubicin (cumulative dose <100‐1750 mg/m2; peak dose (i.e. maximal dose received in 1 week) 50 mg/m2; infusion duration nm), fluorouracil and cyclophosphamide. No prior anthracycline therapy. Prior cardiac radiotherapy in 3 patients in the dexrazoxane group and 9 patients in the control group (dose nm). No prior cardiac dysfunction. | |
| Interventions | Dexrazoxane (10:1 ratio of study drug to doxorubicin; slow IV push or rapid‐drip IV infusion between 15 and 30 minutes before doxorubicin) (n=81) versus placebo (n=104). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as 2 or more of the following: cardiomegaly established by radiography, basilar rales, S3 gallop or paroxysmal nocturnal dyspnoea, orthopnoea, or significant dyspnoea on exertion; subclinical heart failure defined as 1) decline in LVEF as measured by MUGA from baseline of ≥10% below the institution's LLN, 2) a decline in LVEF as measured by MUGA of at least 20% from baseline or 3) decline in LVEF as measured by MUGA to at least 5% below the institution's LLN). Response rate (according to ECOG criteria; see notes). Survival. Adverse effects (according to ECOG criteria). |
|
| Notes | Length of follow up: in the intervention group 397 days (6‐1393); in the control group 517 days (range 29‐1429). Median age in intervention group: 56 years; median age in control group: 59.5 years. Cumulative anthracycline dose in intervention and control group nm. |
|
Venturini 1996.
| Methods | Randomisation was performed by a phone call to the study coordination center (patients were stratified before randomisation by institution and according to previously received adjuvant chemotherapy with anthracyclines). | |
| Participants | 162 patients (median age 57 years (range 32‐74); all females) with breast cancer (metastatic, locally advanced (IIIB) or inflammatory: comparable between treatment groups) treated with therapy including epirubicin (cumulative dose for all randomised patients range 0‐1440 mg/m2; peak dose (i.e. maximal dose received in 1 week) 60 or 120 mg/m2; infusion duration nm). Prior anthracycline therapy: yes (see notes). Prior cardiac radiotherapy: yes (see notes). No prior cardiac dysfunction. | |
| Interventions | Dexrazoxane (10:1 ratio of study drug to epirubicin; IV infusion over 15 minutes, beginning 30 minutes before epirubicin) (n=84) versus no cardioprotective intervention (n=78). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as NYHA class 2, 3 or 4; subclinical heart failure defined as LVEF as measured by MUGA < = 45% or > = 20 EF units as compared to baseline). Response rate (according to WHO criteria). |
|
| Notes | Length of follow up nm. Median age in intervention and control group: 57 years. Cumulative anthracycline dose in intervention group: mean 702 mg/m2 (range 0‐1440); cumulative anthracycline dose in control group: mean 713 mg/m2 (range 120‐1200). This included prior anthracycline therapy (doxorubicin versus epirubicin = 1:2). Prior cumulative anthracycline dose in intervention group (n=14): median 410 mg/m2 (range 180‐800); prior cumulative anthracycline dose in control group (n=11): median 360 mg/m2 (range 240‐600). Prior cardiac radiotherapy in 11 patients treated with dexrazoxane and in 13 control patients (dose nm). |
|
Wagdi 1995.
| Methods | Method of randomisation not clear. | |
| Participants | 17 patients (age and sex for all randomised patients nm: see notes) with Morbus Hodgkin, Non‐Hodgkin lymphoma or breast cancer (stage nm) treated with therapy including doxorubicin (cumulative dose for all randomised patients nm: see notes; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm. | |
| Interventions | Vitamin E (600 mg/day), vitamin C (1000 mg only on days when chemotherapy was applied), and N‐acetylcysteine (200 mg only on days when chemotherapy was applied) (n=6) versus placebo (n=8) | |
| Outcomes | Heart failure (subclinical heart failure defined as > 6% decrease in echocardiographic ejection fraction between start of and within 3 weeks of end of chemotherapy). | |
| Notes | Length of follow up maximal 3 weeks after end chemotherapy. Age in intervention group: 41 years; age in control group 31 years. Sex in available patients: 7 males and 7 females. Cumulative anthracycline dose in intervention group: median 143 mg/m2; cumulative anthracycline dose in control group: median 178 mg/m2. |
|
Waldner 2006.
| Methods | Method of randomisation not clear. | |
| Participants | 40 patients (age for all randomised patients nm; sex nm) with non‐Hodgkin lymphoma (stage nm) treated with doxorubicin (cumulative dose: see notes; peak dose (i.e. maximal dose received in 1 week) 100 mg/m2; infusion duration nm), cyclophosphamide, vincristine and prednisolone. Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction was present in 6 patients (3 in each group; no definition provided). | |
| Interventions | L‐carnitine (3 gram infusion before each chemotherapy cycle and 1 gram orally during the following 21 days) (n=20) versus placebo (n=20). | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as cardiac problems). Survival. Quality of life (according to a standardized questionnaire by Hofmann 1993 and Tuchler 1992). |
|
| Notes | Length of follow up 6 months. Median age 66 years in intervention group and 64 years in control group. According to protocol patients in both treatment groups should have received a cumulative doxorubicin dose of up to 600 mg/m2. |
|
Wexler 1996.
| Methods | Patients underwent a computer‐generated 1: 1 factorial randomisation (open‐label trial). | |
| Participants | 41 patients (aged 4‐24 years; 26 males and 15 females) with one of the Ewing's sarcoma family of tumors (stage comparable between treatment groups) treated with doxorubicin (cumulative dose for all randomised patients range 70‐410 mg/m2; peak dose (i.e. maximal dose received in 1 week) 50 or 70 mg/m2; infusion duration 15 minutes); 38 patients were randomised, whereas 3 patients received dexrazoxane without randomisation), vincristine, etoposide, cyclophosphamide and ifosfamide and if necessary, radiotherapy for local control. No prior anthracycline therapy. No prior cardiac radiotherapy. No prior cardiac dysfunction. | |
| Interventions | Dexrazoxane (20:1 ratio of study drug to doxorubicin; IV infusion over 15 minutes immediately before doxorubicin) (n=20) versus no cardioprotective intervention (n=18). | |
| Outcomes | Heart failure (defined as 1) evidence of clinical congestive heart failure, 2) a reduction in LVEF as measured by MUGA to < 45% or 3) a decrease in LVEF as measured by MUGA of > 20 percentage points from baseline). Response rate (according to ECOG criteria). |
|
| Notes | Length of follow up for all randomised patients: median potential 39 months (37 months for the intervention group; 40 months for the control group). Median age in intervention group: 18.5 years; median age in control group: 15.5 years. Cumulative anthracycline dose in the intervention group: median 410 mg/m2 (range 140‐410); cumulative anthracycline dose in the control group: median 310 mg/m2 (range 70‐410). |
|
ALL: acute lymphoblastic leukaemia
CNS: central nervous system
CTC: common toxicity criteria
ECOG: Eastern Cooperative Oncology Group
IV: intravenous
LLN: lower limit of normal
LVEF: left ventricular ejection fraction
LVSF: left ventricular shortening fraction
MUGA: multiple gated acquisition scan
nm: not mentioned
NYHA: New York Heart Association
WHO: World Health Organization
5FU: 5‐fluorouracil
NCI: National Cancer Institute
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bates 1997 | Duplicate publication of Swain 1997a(088001) and Swain 1997a(088006) |
| Batist 1985 | Unclear if patients were treated with anthracyclines, part of the patients were not randomised, no cardiac parameters mentioned |
| Cardinale 2006 | Not all patients were treated with anthracyclines and patients were only randomised when they had a high troponin I (cardiac marker) value, i.e. there was already some damage to the heart. The objective of this study was to prevent the occurrence of further cardiac damage not the initial damage |
| Cascinu 1995 | Cardiotoxicity was not evaluated (i.e. study was aimed at cisplatin neurotoxicity) |
| Judy 1984 | No randomised controlled trial |
| Massida 1997 | Cardiac function not measured by echocardiography or radionuclide ventriculography |
| Michelotti 2000 | Duplicate publication of Venturini 1996 |
| Moghrabi 2007 | Long‐term follow‐up data of Lipshultz 2004, but no information which was not included in the other long‐term follow‐up studies of this RCT was provided |
| Nakamae 2005 | No comparison of treatment with and without cardioprotective agent (i.e. valsartan) |
| Neto 2006 | No randomised controlled trial |
| Paiva 2005 | No randomised controlled trial |
| Piccinini 1987 | Animal study |
| Rosenfeld 1992 | Duplicate publication of Swain 1997a(088001) and Swain 1997a(088006) |
| Speyer 1988 | Duplicate publication of Speyer 1992 |
| Speyer 1990 | Duplicate publication of Speyer 1992 |
| Swain 1997b | No randomised controlled trial and overlap with Swain 1997a(088001) and Swain 1997a(088006) |
| Tallarico 2003 | No randomised controlled trial |
| Tebbi 2007 | The first publication of POG studies 9426 (NCT00002827/POG9426) and 9425 (Schwartz 2009), but the subject was secondary malignancies after dexrazoxane use. No results on cardiotoxicity were provided |
| Ten Bokkel 1990 | Duplicate publication of Ten Bokkel‐Huinink 1992 |
| Tonkin 1996 | Duplicate publication of Swain 1997a(088001) and Swain 1997a(088006) |
| Unverferth 1983a | Histological abnormalities on endomyocardial biopsy not scored with the Billingham score |
| Unverferth 1983b | Histological abnormalities on endomyocardial biopsy not scored with the Billingham score |
| Vici 1998 | Duplicate publication of Lopez 1998 |
| Wagdi 1996 | Duplicate publication of Wagdi 1995 |
| Weisberg 1992 | Duplicate publication of Swain 1997a(088001) and Swain 1997a(088006) |
| Weitzman 1980 | Number of patients with abnormal cardiac function not mentioned |
| Whittaker 1984 | Cardiac function not measured by echocardiography or radionuclide ventriculography and overlap with Whittaker 1987 |
| Whittaker 1987 | Number of patients in intervention and control group not mentioned |
Characteristics of studies awaiting assessment [ordered by study ID]
Cadeddu 2010.
| Methods | Method of randomisation not clear. |
| Participants | 49 patients (mean age 56 years; 12 males and 37 females) with a variety of solid cancers (stage I‐IV) treated with epirubicin‐based chemotherapy (all patients received the schedules cumulative dose of 400 mg/m2; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). No prior anthracycline therapy. No prior cardiac radiotherapy. No prior cardiac dysfunction. |
| Interventions | Telmirsartan (angiotensin II type 1 receptor blocker) (40 mg/day; n = 25) versus placebo (n = 24). |
| Outcomes | Cardiotoxicity and hypotension. |
| Notes | From the information published in this article it was unclear if the study is eligible for our review. We did not succeed in obtaining additional information from the authors. Number of patients with abnormal cardiac function was not mentioned. Length of follow up nm. Unclear if chemotherapy other than anthracyclines and radiotherapy involving the heart region is the same in both treatment groups. Mean age in intervention group 52.9 years and mean age in control group 53 years. |
Cardenas 2003.
| Methods | Method of randomisation not clear. |
| Participants | Children (number of children: see notes) (aged 1 to 18 years; sex nm) with cancer (type and stage nm) treated with anthracyclines (analogue nm; cumulative dose nm; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). No prior anthracycline therapy. No prior cardiac radiotherapy. Prior cardiac dysfunction nm. |
| Interventions | Dexrazoxane (ratio to anthracycline nm; timing in relation to anthracycline nm) versus no cardioprotective intervention (number of patients in each group nm). |
| Outcomes | Heart failure (subclinical heart failure defined as "a drop of the 20% as a basic value for FEV or that it was progressive" as measured by echocardiography): no significant difference between both groups. Number of patients that remained free of illness at 24 months: no significant difference between both groups. Adverse effects: no significant differences between both groups. |
| Notes | Unclear if this is an ongoing or completed study. Length of follow up nm. Cumulative anthracycline dose per treatment group nm. Age per treatment group nm. Number of included children is unclear (in methods n=50; in results n=52). |
De Berranger 2006.
| Methods | Method of randomisation not clear. |
| Participants | 16 children (median age 8.5 years; 9 boys and 7 girls) with acute leukaemia (11 AML; 5 ALL; stage nm) treated with doxorubicin containing therapy (cumulative dose 450 mg/m2 for AML and 310 mg/m2 for ALL; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm. |
| Interventions | Dexrazoxane (1 g for 50 mg of doxorubicin equivalent dose; timing in relation to anthracycline nm) (n=8) versus no cardioprotective intervention (n=8). |
| Outcomes | Heart failure (i.e. mean LVSF and mean wall stress before chemotherapy and 1 year after diagnosis): all values were comparable. Survival: no difference in disease‐free and overall survival. Adverse effects: 2 patients in the dexrazoxane group had severe hepatic toxicity (WHO criteria grade 3 or more); no other toxicity WHO criteria more than grade 1 observed. |
| Notes | Unclear if this is an ongoing or completed study. Median follow up 28.5 months. Cumulative anthracycline dose per treatment group nm. Age per treatment group nm. |
Feldmann 1992.
| Methods | Method of randomisation not clear. |
| Participants | 155 patients (median age 66 years; sex: see notes) with advanced small cell lung cancer treated with doxorubicin containing chemotherapy (cumulative dose nm; peak dose (i.e. maximal dose received in 1 week) 50 mg/m2; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm. |
| Interventions | Dexrazoxane (10:1 ratio of study drug to doxorubicin; within 30 minutes prior to doxorubicin by IV bolus) versus placebo (number of patients in each group nm). |
| Outcomes | Heart failure (defined as cardiac events): significant difference in favour of the dexrazoxane group. Other toxicities and response rate: level of significance not mentioned. |
| Notes | Unclear if this is an ongoing or completed study. Length of follow up nm. Cumulative anthracycline dose per treatment group nm. Median age in both treatment groups 66 years. 70% of dexrazoxane patients was male and 62% of the control patients. 105 patients were evaluable: 43 in the intervention group and 62 in the control group. |
Gu 2010.
| Methods | Method of randomisation not clear. |
| Participants | 102 patients (age nm; sex nm) with breast cancer (stage nm) receiving anthracycline‐based chemotherapy (cumulative dose nm; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm. |
| Interventions | Hydroprednisone and gluthatione by intravenous injection (n=52) versus no cardioprotective intervention (n=50). |
| Outcomes | The incidence rates of nausea and vomiting and rise of hepatic glutamate alanine aminotransferase in the hydroprednisone and glutathione group were significantly depressed as compared to those in the control group (P=0.003; P=0.001 respectively). There was no significant difference in aleucocytosis and electrocardiogram changes between the two groups (P>0.05). |
| Notes | This article is written in Chinese, but an English abstract was included in the publication. Information provided in this table is based on the abstract only; we did not succeed in obtaining additional information from the authors. Length of follow‐up nm. Unclear if cardiotoxicity as defined in the inclusion criteria of this review is evaluated in this study. Unclear if chemotherapy other than anthracyclines and radiotherapy involving the heart region is the same in both treatment groups. Cumulative anthracycline dose per treatment group nm. Age per treatment group nm. |
Jackowska 2003.
| Methods | Unclear if this is a randomised controlled trial. |
| Participants | 107 children (age nm; sex nm) with acute lymphoblastic leukaemia (stage nm) treated with either doxorubicin or daunorubicin containing therapy (cumulative dose 120 mg/m2; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm. |
| Interventions | Dexrazoxane (ratio to anthracycline nm; timing in relation to anthracycline nm) (n=43) versus no cardioprotective intervention (n=64). |
| Outcomes | Toxicities other than cardiotoxicity. |
| Notes | Unclear if this is an ongoing or completed study. Unclear if cardiotoxicity is evaluated in this study. Length of follow up nm. Cumulative anthracycline dose per treatment group nm. Age per treatment group nm. |
Saad El‐Din 2003.
| Methods | Method of randomisation not clear. |
| Participants | 46 children (age nm; sex nm) with standard risk acute lymphoblastic leukaemia treated with doxorubicin containing chemotherapy (cumulative dose nm; peak dose (i.e. maximal dose received in 1 week) nm; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy nm. Prior cardiac dysfunction nm. |
| Interventions | Dexrazoxane (15:1 ratio of study drug to doxorubicin; prior to doxorubicin) versus no cardioprotective intervention (number of patients in each group nm). |
| Outcomes | Heart failure (subclinical heart failure on among others echocardiography and MUGA scan; definitions nm): significant difference in favour of dexrazoxane. Response rate (defined as the number of patients in complete remission): no significant difference between both groups. Adverse effects (definition nm): similar in both groups. |
| Notes | Unclear if this is an ongoing or completed study. 41 out of 46 randomised patients were evaluated. Length of follow up nm. Cumulative anthracycline dose per treatment group nm. Age per treatment group nm. |
Ten Bokkel‐Huinink 1992.
| Methods | Method of randomisation not clear. |
| Participants | 112 patients (age: see notes; sex nm) with breast cancer (stage nm) treated with doxorubicin containing chemotherapy (cumulative dose nm; peak dose (i.e. maximal dose received in 1 week) 50 mg/m2; infusion duration nm). Prior anthracycline therapy nm. Prior cardiac radiotherapy and prior cardiac dysfunction well balanced between treatment groups (number of patients nm). |
| Interventions | Dexrazoxane (20:1 ratio of study drug to doxorubicin; within 30 minutes prior to doxorubicin) (n=57) versus no cardioprotective intervention (n=55). |
| Outcomes | Heart failure (clinical cardiomyopathy or subclinical heart failure as measured by gated pool ejection fraction; definitions nm): no significant difference between treatment groups. Response rate (complete and partial remission; definitions nm): not influenced by dexrazoxane. Adverse effects (definitions nm): slightly increased myelosuppression with dexrazoxane; no increase in other toxicities. |
| Notes | Unclear if this is an ongoing or completed study. Length of follow up nm. Cumulative anthracycline dose per treatment group nm. Mean age in intervention group 46 years; mean age in control group 45 years. |
ALL: acute lymphoblastic leukaemia
AML: acute myeloid leukaemia
FEV: ejection fraction
LVSF: left ventricular shortening fraction
MUGA: multiple gated acquisition scan
nm: not mentioned
WHO: World Health Organization
Characteristics of ongoing studies [ordered by study ID]
NCT00002827/POG9426.
| Trial name or title | Phase III randomised study of response‐dependent therapy with doxorubicin/bleomycin/vincristine/etoposide (DBVE) with versus without dexrazoxane followed by low‐dose involved‐field radiotherapy for newly diagnosed stage IA/IIA/IIIA1 childhood Hodgkin's disease |
| Methods | Randomised controlled trial |
| Participants | Children with stage IA/IIA/IIIA1 Hodgkin's disease (maximal age 21 years) |
| Interventions | Dexrazoxane versus no cardioprotective intervention |
| Outcomes | Cardiac toxicity, pulmonary toxicity, tumour response, event‐free survival |
| Starting date | May 1999 |
| Contact information | Study chairs Sharon B Murphy and Michael A Weiner |
| Notes | ‐ |
NCT00016276.
| Trial name or title | Phase III randomised study of doxorubicin and cyclophosphamide with or without dexrazoxane followed by paclitaxel with or without trastuzumab (herceptin) followed by surgery and radiotherapy with or without trastuzumab in women with HER‐2+ stage IIIA or IIIB or regional stage IV breast cancer |
| Methods | Randomised controlled trial |
| Participants | Women with HER‐2+ stage IIIA or IIIB or regional stage IV breast cancer (minimal age 18 years) |
| Interventions | Dexrazoxane versus no cardioprotective intervention |
| Outcomes | Cardiac toxicity, tumour response, survival, other toxicities |
| Starting date | May 2001 |
| Contact information | Study chair Mark L Graham |
| Notes | ‐ |
NCT00162955.
| Trial name or title | The multi‐centers trial for patients with Non‐Hodgkin's lymphoma to assess the protective effect of valsartan on chronic cardiotoxicity induced by CHOP |
| Methods | Randomised controlled trial |
| Participants | Patients with non‐Hodgkin's lymphoma (minimal age 15 years; maximal age 70 years) |
| Interventions | Valsartan versus no cardioprotective intervention |
| Outcomes | Cardiac events |
| Starting date | May 2004 |
| Contact information | Principal investigator Hirohisa Nakamae |
| Notes | ‐ |
NCT00247975.
| Trial name or title | Primary prevention of anthracycline‐induced cardiotoxicity with L‐carnitine in patients with breast cancer (PPACC) ‐ pilot study |
| Methods | Randomised controlled trial |
| Participants | Women with stage I/II/III breast cancer (minimal age 18 years) |
| Interventions | L‐carnitine versus no cardioprotective intervention (see notes) |
| Outcomes | Cardiac toxicity, other toxicities |
| Starting date | November 2005 |
| Contact information | Principal investigator Benjamin JW Chow |
| Notes | In another reference to this trial (on www.clinicaltrials.gov) it was mentioned that L‐carnitine was compared to placebo |
NCT00895414.
| Trial name or title | Enalapril maleate and doxorubicin hydrochloride in treating women with breast cancer |
| Methods | Randomised controlled trial |
| Participants | Women with breast cancer (minimal age 18 years) |
| Interventions | Enalapril maleate versus no cardioprotective intervention (patients are their own controls) |
| Outcomes | Cardiac toxicity (clinical heart failure and echocardiographic abnormalities) |
| Starting date | April 2009 |
| Contact information | Principal investigator Anne Blaes |
| Notes | We received additional information from the principal investigator, which is included in this table |
NCT01230983/POG9404.
| Trial name or title | Intensive treatment for T‐cell acute lymphoblastic leukaemia and advanced stage lymphoblastic non‐Hodgkin's lymphoma: a Pediatric Oncology Group Phase III study |
| Methods | Randomised controlled trial |
| Participants | Children with T‐cell acute lymphoblastic leukaemia or advanced stage lymphoblastic non‐Hodgkin lymphoma (maximal age 21 years) |
| Interventions | Dexrazoxane versus no cardioprotective intervention |
| Outcomes | Cardiac toxicity |
| Starting date | Not mentioned |
| Contact information | Study chair Barbara L Asselin |
| Notes | ‐ |
CHOP: cyclophosphamide, doxorubicin, prednisone and vincristine
Contributions of authors
Elvira van Dalen designed the study and wrote the protocol. She developed the search strategy and ran the searches in the three electronic databases for the original version and the first update of this review. She searched for unpublished studies and identified the studies meeting the inclusion criteria. She performed the data‐extraction and risk of bias assessment of the included studies. She analysed the data and interpreted the results. She wrote and revised the manuscript.
Leontien Kremer designed the study and critically reviewed the protocol. She identified the studies meeting the inclusion criteria and performed the data‐extraction and risk of bias assessment of the included studies. She contributed to the data‐analysis and the interpretation of the results. She critically reviewed the manuscript.
Huib Caron critically reviewed the protocol. He contributed to the data‐analysis and the interpretation of the results. He critically reviewed the manuscript.
Heather Dickinson critically reviewed the protocol. She contributed to the data‐analysis and the interpretation of the results. She critically reviewed the manuscript.
All authors approved the final version.
Sources of support
Internal sources
Dutch Cochrane Centre, Netherlands.
External sources
Foundation of Pediatric Cancer Research (SKK) Amsterdam, Netherlands.
Jacques H. de Jong Foundation, Netherlands.
Knowledge and Research Center for Alternative Medicine (ViFAB) / Danish Cancer Society, Denmark.
Stichting Kinderen Kankervrij (KiKa), Netherlands.
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Galetta 2005 {published data only}
- Galetta F, Franzoni F, Cervetti G, Cecconi N, Carpi A, Petrini M, et al. Effect of epirubicin‐based chemotherapy and dexrazoxane supplementation on QT dispersion in non‐Hodgkin lymphoma patients. Biomedicine & Pharmacotherapy 2005;59:541‐4. [DOI] [PubMed] [Google Scholar]
Gallegos‐Castorena 2007 {published data only}
- Gallegos‐Castorena S, Martínez‐Avalos A, Mohar‐Betancourt A, Guerrero‐Avendaño G, Zapata‐Tarrés M, Medina‐Sansón A. Toxicity prevention with amifostine in pediatric osteosarcoma patients treated with cisplatin and doxorubicin. Pediatric Hematology and Oncology 2007;24:403‐8. [DOI] [PubMed] [Google Scholar]
Iarussi 1994 {published data only}
- Iarussi D, Auricchio U, Agretto A, Murano A, Giuliano M, Casale F, et al. Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: control study in children with acute lymphoblastic leukemia and non‐hodgkin lymphoma. Molecular Aspects of Medicine 1994;15 Suppl:207‐12. [DOI] [PubMed] [Google Scholar]
Kalay 2006 {published data only}
- Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, et al. Protective effects of carvedilol against anthracycline‐induced cardiomyopathy. Journal of the American College of Cardiology 2006;48:2258‐62. [DOI] [PubMed] [Google Scholar]
Kraft 1990 {published data only}
- Kraft J, Grille W, Appelt M, Hossfeld DK, Eichelbaum M, Koslowski B, et al. Effects of verapamil on anthracycline‐induced cardiomyopathy: preliminary results of a prospective multicenter trial. Haematology and Blood Transfusion 1990;33:566‐70. [DOI] [PubMed] [Google Scholar]
Lipshultz 2004 {published data only}
- Barry EV, Vrooman LM, Dahlberg SE, Neuberg DS, Asselin BL, Athale UH, et al. Absence of secondary malignant neoplasms in children with high‐risk acute lymphoblastic leukemia treated with dexrazoxane. Journal of Clinical Oncology 2008;26:1106‐11. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Rifai N, Dalton VM, Levy DE, Silverman LB, Lipsitz SR, et al. The effect of dexrazoxane on myocardial injury in doxorubicin‐treated children with acute lymphoblastic leukemia. The New England Journal of Medicine 2004;351:145‐53. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin‐treated children with high‐risk acute lymphoblastic leukaemia: long‐term follow‐up of a prospective, randomised, multicentre trial. Lancet Oncology 2010;11:950‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 1998 {published data only}
- Lopez M, Vici P, Lauro L, Conti F, Paoletti G, Ferraironi A, et al. Randomized prospective clinical trial of high‐dose epirubicin and dexrazoxane in patients with advanced breast cancer and soft tissue sarcomas. Journal of Clinical Oncology 1998;16:86‐92. [DOI] [PubMed] [Google Scholar]
Marty 2006 {published data only}
- Marty M, Espie M, Llombart A, Monnier A, Rapoport BL, Stahalova V. Multicenter randomized phase III study of the cardioprotective effect of dexrazoxane (Cardioxane) in advanced/metastatic breast cancer patients treated with anthracycline‐based chemotherapy. Annals of Oncology 2006;17:614‐22. [DOI] [PubMed] [Google Scholar]
Milei 1987 {published data only}
- Milei J, Marantz A, Ale J, Vazquez A, Buceta JE. Prevention of adriamycin‐induced cardiotoxicity by prenylamine: a pilot double blind study. Cancer Drug Delivery 1987;4:129‐36. [DOI] [PubMed] [Google Scholar]
Myers 1983 {published data only}
- Myers C, Bonow R, Palmeri S, Jenkins J, Corden B, Locker G, et al. A randomized controlled trial assessing the prevention of doxorubicin cardiomyopathy by N‐acetylcysteine. Seminars in Oncology 1983;10 Suppl 1(1):53‐5. [PubMed] [Google Scholar]
Schwartz 2009 {published data only}
- Schwartz CL, Constine LS, Villaluna D, London WB, Hutchison RE, Sposto R, et al. A risk‐adapted, response‐based approach using ABVE‐PC for children and adolescents with intermediate‐ and high‐risk Hodgkin lymphoma: the results of P9425. Blood 2009;114:2051‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Speyer 1992 {published data only}
- Speyer JL, Green MD, Zeleniuch‐Jacquotte A, Wernz JC, Rey M, Sanger J, et al. ICRF‐187 permits longer treatment with doxorubicin in women with breast cancer. Journal of Clinical Oncology 1992;10:117‐27. [DOI] [PubMed] [Google Scholar]
Swain 1997a(088001) {published data only}
- Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, et al. Cardioprotection with dexrazoxane for doxorubicin‐containing therapy in advanced breast cancer. Journal of Clinical Oncology 1997;15:1318‐32. [DOI] [PubMed] [Google Scholar]
Swain 1997a(088006) {published data only}
- Swain SM, Whaley FS, Gerber MC, Weisberg S, York M, Spicer D, et al. Cardioprotection with dexrazoxane for doxorubicin‐containing therapy in advanced breast cancer. Journal of Clinical Oncology 1997;15:1318‐32. [DOI] [PubMed] [Google Scholar]
Venturini 1996 {published data only}
- Venturini M, Michelotti A, Mastro L, Gallo L, Carnino F, Garrone O, et al. Multicenter randomized controlled clinical trial to evaluate cardioprotection of dexrazoxane versus no cardioprotection in women receiving epirubicin chemotherapy for advanced breast cancer. Journal of Clinical Oncology 1996;14:3112‐20. [DOI] [PubMed] [Google Scholar]
Wagdi 1995 {published data only}
- Wagdi PH, Rouvinez G, Fluri M, Aeschbacher B, Thoni A, Schefer H, et al. Cardioprotection during chemo‐and radiotherapy of malignant diseases ‐ an echocardiographic pilot study [Kardioprotektion bei chemo‐und radiotherapie fur maligne erkrankungen ‐ eine echokardiographische pilotstudie]. Schweizerische Rundschau fur Medizin (Praxis) 1995;84:1220‐3. [PubMed] [Google Scholar]
Waldner 2006 {published data only}
- Waldner R, Laschan C, Lohninger A, Gessner M, Tuchler H, Huemer M, et al. Effects of doxorubicin‐containing chemotherapy and a combination with L‐carnitine on oxidative metabolism in patients with non‐Hodgkin lymphoma. Journal of Cancer Research and Clinical Oncology 2006;132:121‐8. [DOI] [PubMed] [Google Scholar]
Wexler 1996 {published data only}
- Wexler LH, Andrich MP, Venzon D, Berg SL, Weaver‐McClure L, Chen CC, et al. Randomized trial of the cardioprotective agent ICRF‐187 in pediatric sarcoma patients treated with doxorubicin. Journal of Clinical Oncology 1996;14:362‐72. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bates 1997 {published data only}
- Bates M, Lieu D, Zagari M, Spiers A, Williamson T. A pharmacoeconomic evaluation of the use of dexrazoxane in preventing anthracycline‐induced cardiotoxicity in patients with stage IIIB or IV metastatic breast cancer. Clinical Therapeutics 1997;19:167‐84. [DOI] [PubMed] [Google Scholar]
Batist 1985 {published data only}
- Batist G, Norton J, Katki AG, Wagman L, Ferrans VJ, Maher M, et al. Cardiac and red blood cell glutathione peroxidase: results of a prospective randomized trial in patients on total parenteral nutrition. Cancer Research 1985;45:5900‐3. [PubMed] [Google Scholar]
Cardinale 2006 {published data only}
- Cardinale D, Colombo A, Sandri MT, Lamantia G, Colombo N, Civelli M, et al. Prevention of high‐dose chemotherapy‐induced cardiotoxicity in high‐risk patients by angiotensin‐converting enzyme inhibition. Circulation 2006;114:2474‐81. [DOI] [PubMed] [Google Scholar]
Cascinu 1995 {published data only}
- Cascinu S, Cordella L, Ferro E, Fronzoni M, Catalano G. Neuroprotective effect of reduced glutathione on cisplatin‐based chemotherapy in advanced gastric cancer: a randomized double‐blind placebo‐controlled trial. Journal of Clinical Oncology 1995;13:26‐32. [DOI] [PubMed] [Google Scholar]
Judy 1984 {published data only}
- Judy WV, Hall JH, Dugan W, Toth PD, Folkers K. Coenzyme Q10 reduction of adriamycin cardiotoxicity. In: Folkers K, Yamamura Y editor(s). Biomedical and clinical aspects of coenzyme Q. Vol. 4, Amsterdam: Elsevier / North‐Holland Biomedical Press, 1984:231‐41. [Google Scholar]
Massida 1997 {published data only}
- Massidda B, Fenu MA, Ionta MT, Tronci M, Foddi MR, Montaldo C, et al. Early detection of the anthracycline‐induced cardiotoxicity. A non‐invasive haemodynamic study. Anticancer Research 1997;17:663‐8. [PubMed] [Google Scholar]
Michelotti 2000 {published data only}
- Michelotti A, Venturini M, Tibaldi C, Bengala C, Gallo L, Carnino F, et al. Single agent epirubicin as first line chemotherapy for metastatic breast cancer patients. Breast Cancer Research and Treatment 2000;59:133‐9. [DOI] [PubMed] [Google Scholar]
Moghrabi 2007 {published data only}
- Moghrabi A, Levy DE, Asselin B, Barr R, Clavell L, Hurwitz C, et al. Results of the Dana‐Farber Cancer Institute ALL Consortium Protocol 95‐01 for children with acute lymphoblastic leukemia. Blood 2007;103:896‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakamae 2005 {published data only}
- Nakamae H, Tsumura K, Terada Y, Nakane T, Nakamae M, Ohta K, et al. Notable effects of angiotensin II receptor blocker, valsartan, on acute cardiotoxic changes after standard chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisolone. Cancer 2005;104:2492‐8. [DOI] [PubMed] [Google Scholar]
Neto 2006 {published data only}
- Neto RPDM, Petrilli AS, Silva CMC, Campos Filho O, Oporto VM, Gomes LDFG, et al. Left ventricular systolic function assessed by echocardiography in children and adolescents with osteosarcoma treated with doxorubicin alone or in combination with dexrazoxane. Arquivos Brasileiros de Cardiologia 2006;87:699‐706. [DOI] [PubMed] [Google Scholar]
Paiva 2005 {published data only}
- Paiva MG, Petrilli AS, Moises VA, Macedo CR, Tanaka C, Campos O. Cardioprotective effect of dexrazoxane during treatment with doxorubicin: a study using low‐dose dobutamine stress echocardiography. Pediatric Blood and Cancer 2005;45:902‐8. [DOI] [PubMed] [Google Scholar]
Piccinini 1987 {published data only}
- Piccinini F, Monti E, Paracchini L. Protective effect of trimetazidine on the cardiotoxicity of doxorubicin. Concours Medical 1987;109:3479‐83. [Google Scholar]
Rosenfeld 1992 {published data only}
- Rosenfeld CS, Weisberg SR, York RM, Jones SE, Spicer DV, Khojasteh A, et al. Prevention of adriamycin cardiomyopathy with dexrazoxane (ADR‐529, ICRF‐187). Proceedings of ASCO 1992;11:62. [Google Scholar]
Speyer 1988 {published data only}
- Speyer JL, Green MD, Kramer E, Rey M, Sanger J, Ward C, et al. Protective effect of the bispiperazinedione ICRF‐187 against doxorubicin‐induced cardiac toxicity in women with advanced breast cancer. The New England Journal of Medicine 1988;319:745‐52. [DOI] [PubMed] [Google Scholar]
Speyer 1990 {published data only}
- Speyer JL, Green MD, Sanger J, Zeleniuch‐Jacquotte A, Kramer E, Rey M, et al. A prospective randomized trial of ICRF‐187 for prevention of cumulative doxorubicin‐induced cardiac toxicity in women with breast cancer. Cancer Treatment Reviews 1990;17:161‐3. [DOI] [PubMed] [Google Scholar]
Swain 1997b {published data only}
- Swain SM, Whaley FS, Gerber MC, Ewer MS, Bianchine JR, Gams RA. Delayed administration of dexrazoxane provides cardioprotection for patients with advanced breast cancer treated with doxorubicin‐containing therapy. Journal of Clinical Oncology 1997;15:1333‐40. [DOI] [PubMed] [Google Scholar]
Tallarico 2003 {published data only}
- Tallarico D, Rizzo V, Maio F, Petretto F, Bianco G, Placanica G, et al. Myocardial cytoprotection by trimetazidine against anthracycline‐induced cardiotoxicity in anticancer chemotherapy. Angiology 2003;54:219‐27. [DOI] [PubMed] [Google Scholar]
Tebbi 2007 {published data only}
- Tebbi CK, London WB, Friedman F, Villaluna D, Alarcon PA, Constine LS, et al. Dexrazoxane‐associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. Journal of Clinical Oncology 2007;25:493‐500. [DOI] [PubMed] [Google Scholar]
Ten Bokkel 1990 {published data only}
- Bokkel‐Huinink WW, Rodenhuis S, Schreuder JE, Dubbelman R, Bierhorst F, Tinteren H, et al. ICRF 187 protects against doxorubicin induced cardiomyopathy. Annals of Oncology 1990;1:106. [Google Scholar]
Tonkin 1996 {published data only}
- Tonkin K, Bates M, Lieu D, Arundell E, Williamson TY, Zagari M. Dexrazoxane cardioprotection for patients receiving FAC chemotherapy: a pharmacoeconomic evaluation. Annals of Cancer Control Research 1996;6:458‐73. [PubMed] [Google Scholar]
Unverferth 1983a {published data only}
- Unverferth DV, Fertel RH, Balcerzak SP, Magorien RD, O'Dorisio MS. N‐Acetylcysteine prevents the doxorubicin‐induced decrease of cyclic GMP. Seminars in Oncology 1983;10 Suppl 1(1):49‐52. [PubMed] [Google Scholar]
Unverferth 1983b {published data only}
- Unverferth DV, Jagadeesh JM, Unverferth BJ, Magorien RD, Leier CV, Balcerzak SP. Attempt to prevent doxorubicin‐induced acute human myocardial morphologic damage with acetylcysteine. Journal of the National Cancer Institute 1983;71:917‐20. [PubMed] [Google Scholar]
Vici 1998 {published data only}
- Vici P, Ferraironi A, Lauro L, Carpano S, Conti F, Belli F, et al. Dexrazoxane cardioprotection in advanced breast cancer patients undergoing high‐dose epirubicin treatment. La Clinica Terapeutica 1998;149:15‐20. [PubMed] [Google Scholar]
Wagdi 1996 {published data only}
- Wagdi P, Fluri M, Aeschbacher B, Fikrle A, Meier B. Cardioprotection in patients undergoing chemo‐and/or radiotherapy for neoplastic disease: a pilot study. Japanese Heart Journal 1996;37:353‐9. [DOI] [PubMed] [Google Scholar]
Weisberg 1992 {published data only}
- Weisberg SR, Rosenfeld CS, York RM, Jones SE, Spicer DV, Khojasteh A, et al. Dexrazoxane (ADR‐529, ICRF‐187, Zinecard) protects against doxorubicin induced chronic cardiotoxicity. Proceedings of ASCO 1992;11:91. [Google Scholar]
Weitzman 1980 {published data only}
- Weitzman SA, Lorell B, Carey RW, Kaufman S, Stossel TP. Prospective study of tocopherol prophylaxis for anthracycline cardiac toxicity. Current Therapeutic Research 1980;28:682‐6. [Google Scholar]
Whittaker 1984 {published data only}
- Whittaker JA, Al‐Ismail SAD. Effect of digoxin and vitamin E in preventing cardiac damage caused by doxorubicin in acute myeloid leukaemia. British Medical Journal 1984;288:283‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whittaker 1987 {published data only}
- Whittaker JA, Al‐Ismail SAD, Nicholson DM, Saour JN. Doxorubicin cardiotoxicity: attempts at prevention with digoxin and alpha‐tocopherol (vitamin E). Clinical Trials Journal 1987;24 Suppl 1:45‐55. [Google Scholar]
References to studies awaiting assessment
Cadeddu 2010 {published data only (unpublished sought but not used)}
- Cadeddu C, Piras A, Mantovani G, Deidda M, Dessì M, Madeddu C, et al. Protective effects of the angiotensin II receptor blocker telmisartan on epirubicin‐induced inflammation, oxidative stress, and early ventricular impairment. American Heart Journal 2010;160:487.e1‐7. [DOI] [PubMed] [Google Scholar]
Cardenas 2003 {published data only}
- Cardenas R, Mercado CG, Rivela LR, Calderon EC. Effectiveness and safety of dexrazoxane as a cardioprotector in Mexican children with cancer. Medical and Pediatric Oncology 2003;41:PL001. [Google Scholar]
De Berranger 2006 {published data only}
- Berranger E. Results of randomized study on dexrazoxane administration in children with de novo acute leukemia. ASCO Annual Meeting (abstract number 9037). 2006.
Feldmann 1992 {published data only}
- Feldmann JE, Jones SE, Weisberg SR, Gandars DR, Lyman GH, York RM, et al. Advanced small cell lung cancer treated with CAV (cyclophosphamide + adriamycin + vincristine) chemotherapy and the cardioprotective agent dexrazoxane (ADR‐529, ICRF‐187, Zinecard). ASCO Annual Meeting (abstract number 993). 1992.
Gu 2010 {published data only (unpublished sought but not used)}
- Gu H‐S, Li J‐Y, Zhang W‐H, Zhang Y, Jia S, Fu Y. Clinical observation of hydroprednisone and glutathione in chemotherapy of breast cancer. Chinese Journal of Cancer Prevention and Treatment 2010;17:131‐3. [Google Scholar]
Jackowska 2003 {published data only}
- Jackowska T, Rokicka‐Milewska R, Matysiak M, Balwierz W, Kowalczyk J, Wachowiak J, et al. Assessment of dexrazoxane tolerance in leukemic children receiving anthracyclines. Medical and Pediatric Oncology 2003;41:PD003. [Google Scholar]
Saad El‐Din 2003 {published data only}
- Saad El‐Din I, Saban K, Abdel Rahman K, Gabaly M. A randomized clinical trial to evaluate cardioprotective effect of ICRF‐187 in children with acute lymphoblastic leukemia. Medical and Pediatric Oncology 2003;41:O109. [Google Scholar]
Ten Bokkel‐Huinink 1992 {published data only}
- Bokkel‐Huinink WW, Schreuder JE, Dubbelman R, Bierhorst F, Tinteren H, Dalesio O, et al. ICRF 187 protects against doxorubicin induced cardiomyopathy. Annals of Oncology (abstract number 221) 1992:114. [Google Scholar]
References to ongoing studies
NCT00002827/POG9426 {published data only}
- Phase III randomised study of response‐dependent therapy with doxorubicin/bleomycin/vincristine/etoposide (DBVE) with versus without dexrazoxane followed by low‐dose involved‐field radiotherapy for newly diagnosed stage IA/IIA/IIIA1 childhood Hodgkin's disease. www.controlled‐trials.com.
NCT00016276 {published data only}
- Phase III randomised study of doxorubicin and cyclophosphamide with or without dexrazoxane followed by paclitaxel with or without trastuzumab (herceptin) followed by surgery and radiotherapy with or without trastuzumab in women with HER‐2+ stage IIIA or IIIB or regional stage IV breast cancer. www.controlled‐trials.com.
NCT00162955 {published data only}
- The multi‐centers trial for patients with Non‐Hodgkin's lymphoma to assess the protective effect of valsartan on chronic cardiotoxicity induced by CHOP. www.controlled‐trials.com.
NCT00247975 {published data only}
- Primary prevention of anthracycline‐induced cardiotoxicity with L‐carnitine in patients with breast cancer (PPACC)‐pilot study. www.controlled‐trials.com.
NCT00895414 {published and unpublished data}
- Enalapril maleate and doxorubicin hydrochloride in treating women with breast cancer. www.controlled‐trials.com.
NCT01230983/POG9404 {published data only}
- Intensive treatment for T‐cell acute lymphoblastic leukemia and advanced stage lymphoblastic Non‐Hodgkin's lymphoma: a Pediatric Oncology Group Phase III study. www.controlled‐trials.com.
Additional references
Barry 2008
- Barry EV, Vrooman LM, Dahlberg SE, Neuberg DS, Asselin BL, Athale UH, et al. Absence of secondary malignant neoplasms in children with high‐risk acute lymphoblastic leukemia treated with dexrazoxane. Journal of Clinical Oncology 2008;26:1106‐11. [DOI] [PubMed] [Google Scholar]
Batist 2001
- Batist G, Ramakriskan G, Sekhar Rao C, Chandrasekharan A, Gutheil J, Guthrie T, et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome‐encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized multicenter trial of metastatic breast cancer. Journal of Clinical Oncology 2001;19:1444‐54. [DOI] [PubMed] [Google Scholar]
Bonadonna 1969
- Bonadonna G, Morfandini S. Cardiac toxicity of daunorubicin. Lancet 1969;1:837. [PubMed] [Google Scholar]
De Leonardis 1985
- Leonardis V, Neri B, Bacalli S, Cinelli P. Reduction of cardiac toxicity of anthracyclines by L‐Carnitine: preliminary overview of clinical data. International Journal of Clinical Pharmacology Research 1985;5:137‐42. [PubMed] [Google Scholar]
Elihu 1998
- Elihu N, Anandasbapathy S, Frishman WH. Chelation therapy in cardiovascular disease: ethylenediaminetetraacetic acid, deferoxamine and dexrazoxane. Journal of Clinical Pharmacology 1998;38:101‐5. [DOI] [PubMed] [Google Scholar]
Garbrecht 1986
- Garbrecht M, Müllerleile U. Verapamil in the prevention of adriamycin‐induced cardiomyopathy. Klinische Wochenschrift 1986;64 Suppl VII:132‐4. [PubMed] [Google Scholar]
Guthrie 1977
- Guthrie D, Gibson AL. Doxorubicin cardiotoxicity: possible role of digoxin in its prevention. British Medical Journal 1977;2:1447‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated September 2006]. John Wiley & Sons, Ltd, In: The Cochrane Library, Issue 4, 2006. Chichester, UK. [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org, 2008. [Google Scholar]
Hofmann 1993
- Hofmann S, Tuchler H, Bernhart M, Stacher A, Lutz D. A questionnaire suitable for general practice for detection of the health status and quality of life of patients with hemato‐oncologic diseases: psychometric properties. The study G "quality of life" of the International Society for Chemo‐and Immunotherapy (I.G.C.I.). Wiener Klinische Wochenschrift 1993;105:277‐83. [PubMed] [Google Scholar]
Kawasaki 1992
- Kawasaki S, Akiyama S, Kurokawa T, Kataoka M, Dohmitsu K, Kondoh K, et al. Polyoxyethylene‐modified superoxide dismutase reduces side effects of adriamycin and mitomycin C. Japanese Journal of Cancer Research 1992;83:899‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keizer 1990
- Keizer HG, Pinedo HM, Schuurhuis GJ, Joenje H. Doxorubicin (adriamycin): a critical review of free radical‐dependent mechanisms of cytotoxicity. Pharmacology and Therapeutics 1990;47:219‐31. [DOI] [PubMed] [Google Scholar]
Kremer 2002a
- Kremer LCM, Pal HJH, Offringa M, Dalen EC, Voute PA. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Annals of Oncology 2002;13:819‐29. [DOI] [PubMed] [Google Scholar]
Kremer 2002b
- Kremer LCM, Dalen EC, Offringa M, Voute PA. Frequency and risk factors of anthracycline‐induced heart failure in children: a systematic review. Annals of Oncology 2002;13:503‐12. [DOI] [PubMed] [Google Scholar]
Lefrak 1973
- Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973;32:302‐14. [DOI] [PubMed] [Google Scholar]
Legha 1982a
- Legha SS, Benjamin RS, Mackay B, Ewer M, Wallace S, Valdivieso M, et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Annals of Internal Medicine 1982;96:133‐9. [DOI] [PubMed] [Google Scholar]
Legha 1982b
- Legha SS, Wang Y, Mackay B, Ewer M, Hortobagyi GN, Benjamin RS, et al. Clinical and pharmacologic investigation of the effects of a‐tocopherol on adriamycin cardiotoxicity. Annals of the New York Academy of Sciences 1982;393:411‐8. [DOI] [PubMed] [Google Scholar]
Lipshultz 1998
- Lipshultz SE, Sallan SE, Giantris AL, Lipsitz SR, Dalton V, Colan SD. 48 hour continuous doxorubicin infusion is not cardioprotective in children assessed 18 months later: the DFCI 91001 ALL protocol. Proceedings of the American Society of Clinical Oncology 1998;17:528a. [Google Scholar]
Lipshultz 2010
- Lipshultz SE, Scully RE, Lipsitz SR, Sallan SE, Silverman LB, Miller TL, et al. Assessment of dexrazoxane as a cardioprotectant in doxorubicin‐treated children with high‐risk acute lymphoblastic leukaemia: long‐term follow‐up of a prospective, randomised, multicentre trial. Lancet Oncology 2010;11:950‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 1981
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207‐14. [DOI] [PubMed] [Google Scholar]
Muggia 1991
- Muggia FM, Green MD. New anthracycline antitumor antibiotics. Critical Reviews in Oncology/Hematology 1991;11:43‐64. [DOI] [PubMed] [Google Scholar]
Muggia 1997
- Muggia FM, Hainsworth JD, Jeffers S, Miller P, Groshen S, Tan M, et al. Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. Journal of Clinical Oncology 1997;15:987‐93. [DOI] [PubMed] [Google Scholar]
Myers 1998
- Myers C. The role of iron in doxorubicin‐induced cardiomyopathy. Seminars in Oncology 1998;25 Suppl 10:10‐4. [PubMed] [Google Scholar]
Odaimi 1987
- Odaimi M, Ajani J. High‐dose chemotherapy. American Journal of Clinical Oncology 1987;10:123‐32. [DOI] [PubMed] [Google Scholar]
Oken 1982
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American Journal of Clinical Oncology 1982;5:649‐55. [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Pierga 2001
- Pierga JY, Robain M, Jouve M, Asselain B, Dieras V, Beuzeboc P, et al. Response to chemotherapy is a major parameter‐influencing long‐term survival of metastatic breast cancer patients. Annals of Oncology 2001;12:231‐7. [DOI] [PubMed] [Google Scholar]
Salzer 2010
- Salzer WL, Devidas M, Carroll WL, Winick N, Pullen J, Hunger SP, et al. Long‐term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984‐2001: a report from the children's oncology group. Leukemia 2010;24(2):355‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shan 1996
- Shan K, Lincoff AM, Young JB. Anthracycline‐induced cardiotoxicity. Annals of Internal Medicine 1996;125:47‐58. [DOI] [PubMed] [Google Scholar]
Silber 2001
- Silber JH, Cnaan A, Clark BJ, Paridon SM, Chin AJ, Rychik J, et al. Design and baseline characteristics for the ACE inhibitor after anthracycline (AAA) study of cardiac dysfunction in long‐term pediatric cancer survivors. American Heart Journal 2001;142:577‐85. [DOI] [PubMed] [Google Scholar]
Singal 1995
- Singal PK, Siveski‐Iliskovic N, Hill M, Thomas TP, Li T. Combination therapy with probucol prevents adriamycin‐induced cardiomyopathy. Journal of Molecular and Cellular Cardiology 1995;27:1055‐62. [DOI] [PubMed] [Google Scholar]
Tuchler 1992
- Tuchler H, Hofmann S, Bernhart M, Brugiatelli M, Chrobak L, Franke A, et al. A short multilingual quality of life questionnaire ‐ practicability, reliability and interlingual homogeneity. Quality of Life Research 1992;1:107‐17. [DOI] [PubMed] [Google Scholar]
Van Acker 2000
- Acker FAA, Acker SABE, Kramer K, Haenen GR, Bast A, Vijgh WJ. 7‐monohydroxyethylrutoside protects against chronic doxorubicin‐induced cardiotoxicity when administered only once per week. Clinical Cancer Research 2000;6:1337‐41. [PubMed] [Google Scholar]
Van Dalen 2009
- Dalen EC, Pal HJ, Caron HN, Kremer LC. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD005008.pub2] [DOI] [Google Scholar]
Van Dalen 2010
- Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD005006.pub3] [DOI] [PubMed] [Google Scholar]
Van Dalen 2011
- Dalen EC, Berg H, Raphaël MF, Caron HN, Kremer LC. Should anthracyclines and dexrazoxane be used for children with cancer?. Lancet Oncology 2011;12(1):12‐3. [DOI] [PubMed] [Google Scholar]
Von Hoff 1979
- Hoff DD, Layard MW, Basa P, Davis HL Jr, Hoff AL, Rozencweig M, et al. Risk factors for doxorubicin‐induced congestive heart failure. Annals of Internal Medicine 1979;91:710‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Van Dalen 2005
- Dalen EC, Caron HN, Dickinson HO, Kremer LCM. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003917.pub3] [DOI] [PubMed] [Google Scholar]
Van Dalen 2008
- Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD003917.pub3] [DOI] [PubMed] [Google Scholar]


