Abstract
Background
During synchronised mechanical ventilation, positive airway pressure and spontaneous inspiration coincide. If synchronous ventilation is provoked, adequate gas exchange should be achieved at lower peak airway pressures, potentially reducing baro/volutrauma, air leak and bronchopulmonary dysplasia. Synchronous ventilation can potentially be achieved by manipulation of rate and inspiratory time during conventional ventilation and employment of patient‐triggered ventilation.
Objectives
To compare the efficacy of:
(i) synchronised mechanical ventilation, delivered as high‐frequency positive pressure ventilation (HFPPV) or patient‐triggered ventilation (assist control ventilation (ACV) and synchronous intermittent mandatory ventilation (SIMV)), with conventional ventilation or high‐frequency oscillation (HFO);
(ii) different types of triggered ventilation (ACV, SIMV, pressure‐regulated volume control ventilation (PRVCV), SIMV with pressure support (PS) and pressure support ventilation (PSV)).
Search methods
We used the standard search strategy of the Cochrane Neonatal Review group to search the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 5), MEDLINE via PubMed (1966 to June 5 2016), EMBASE (1980 to June 5 2016), and CINAHL (1982 to June 5 2016). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised or quasi‐randomised clinical trials comparing synchronised ventilation delivered as HFPPV to CMV, or ACV/SIMV to CMV or HFO in neonates. Randomised trials comparing different triggered ventilation modes (ACV, SIMV, SIMV plus PS, PRVCV and PSV) in neonates.
Data collection and analysis
Data were collected regarding clinical outcomes including mortality, air leaks (pneumothorax or pulmonary interstitial emphysema (PIE)), severe intraventricular haemorrhage (grades 3 and 4), bronchopulmonary dysplasia (BPD) (oxygen dependency beyond 28 days), moderate/severe BPD (oxygen/respiratory support dependency beyond 36 weeks' postmenstrual age (PMA) and duration of weaning/ventilation.
Eight comparisons were made: (i) HFPPV versus CMV; (ii) ACV/SIMV versus CMV; (iii) SIMV or SIMV + PS versus HFO; iv) ACV versus SIMV; (v) SIMV plus PS versus SIMV; vi) SIMV versus PRVCV; vii) SIMV vs PSV; viii) ACV versus PSV. Data analysis was conducted using relative risk for categorical outcomes, mean difference for outcomes measured on a continuous scale.
Main results
Twenty‐two studies are included in this review. The meta‐analysis demonstrates that HFPPV compared to CMV was associated with a reduction in the risk of air leak (typical relative risk (RR) for pneumothorax was 0.69, 95% confidence interval (CI) 0.51 to 0.93). ACV/SIMV compared to CMV was associated with a shorter duration of ventilation (mean difference (MD) −38.3 hours, 95% CI −53.90 to −22.69). SIMV or SIMV + PS was associated with a greater risk of moderate/severe BPD compared to HFO (RR 1.33, 95% CI 1.07 to 1.65) and a longer duration of mechanical ventilation compared to HFO (MD 1.89 days, 95% CI 1.04 to 2.74).
ACV compared to SIMV was associated with a trend to a shorter duration of weaning (MD −42.38 hours, 95% CI −94.35 to 9.60). Neither HFPPV nor triggered ventilation was associated with a significant reduction in the incidence of BPD. There was a non‐significant trend towards a lower mortality rate using HFPPV versus CMV and a non‐significant trend towards a higher mortality rate using triggered ventilation versus CMV. No disadvantage of HFPPV or triggered ventilation was noted regarding other outcomes.
Authors' conclusions
Compared to conventional ventilation, benefit is demonstrated for both HFPPV and triggered ventilation with regard to a reduction in air leak and a shorter duration of ventilation, respectively. In none of the trials was complex respiratory monitoring undertaken and thus it is not possible to conclude that the mechanism of producing those benefits is by provocation of synchronised ventilation. Triggered ventilation in the form of SIMV ± PS resulted in a greater risk of BPD and duration of ventilation compared to HFO. Optimisation of trigger and ventilator design with respect to respiratory diagnosis is encouraged before embarking on further trials. It is essential that newer forms of triggered ventilation are tested in randomised trials that are adequately powered to assess long‐term outcomes before they are incorporated into routine clinical practice.
Plain language summary
Synchronised mechanical ventilation for respiratory support in newborn infants
The majority of newborn babies in need of mechanical assistance to support them also breathe to some degree. If the baby's attempts to breathe are synchronised with the mechanical breaths from the ventilator, less pressure may be needed. This could reduce the chance of air leak or variations in blood flow to the brain. The review of trials found, when compared to conventional mechanical ventilation (CMV), high‐frequency positive pressure ventilation (HFPPV) reduced the risk of air leak and triggered ventilation was associated with a shorter duration of ventilation. Compared to high‐frequency oscillation, however, certain triggered modes of ventilation resulted in a greater risk of moderate to severe chronic lung disease and a longer duration of ventilation. Newer forms of triggered ventilation have only been evaluated in small randomised trials and have not been demonstrated to have advantages in important clinical outcomes.
Background
Description of the condition
The majority of neonates breathe during mechanical ventilation. Those that actively exhale against positive pressure inflation develop pneumothoraces; whereas if positive pressure inflation and spontaneous inspiration coincide (synchrony), oxygenation and carbon dioxide elimination improve. If synchrony occurs, it should be possible to achieve adequate gas exchange at lower inflating pressures, reducing barotrauma, a known risk factor for bronchopulmonary dysplasia. Active expiration is more common if infants have non‐compliant lungs and are ventilated with long inspiratory times or relatively slow ventilator rates (30 to 40 bpm), or both. Increasing ventilator rate and reducing inspiratory time, mimicking more closely the preterm infant's respiratory pattern, has been shown in a proportion of infants to stop them actively expiring.
Description of the intervention
Synchronised ventilation can be achieved by HFPPV during which the ventilator rate more closely resembles the spontaneous respiratory rate of very prematurely born infants, and this is more likely to be associated with synchronous ventilation. It can also be achieved by PTV (ACV, SIMV, PRVCV or PSV), during which the infant's respiratory efforts exceeding a critical level trigger a positive pressure inflation. In SIMV only a preset number of the infant's respiratory efforts trigger positive pressure inflations.
How the intervention might work
During mechanical ventilation, infants actively exhale against positive pressure inflation and develop pneumothoraces. Theoretically, by increasing synchronous ventilation, either HFPPV or PTV could reduce air leaks and associated intraventricular haemorrhage and BPD. Indeed, 'triggered ventilation' compared to conventional mechanical ventilation (CMV) has been shown to improve tidal volume (Jarreau 1996) and oxygenation (Cleary 1995) and reduce blood pressure fluctuations (Hummler 1996). Synchronised mechanical ventilation might also reduce baro trauma and hence BPD.
Why it is important to do this review
The aim of this review was to determine whether HFPPV or triggered ventilation were associated with positive outcomes for prematurely born neonates. This review updates the existing review of synchronised ventilation which was published in the Cochrane Database of Systematic Reviews Issue 1, 2008 (Greenough 2008).
Objectives
To compare the efficacy of:
(i) synchronised mechanical ventilation, delivered as high‐frequency positive pressure ventilation (HFPPV) or patient‐triggered ventilation (assist control ventilation (ACV) and synchronous intermittent mandatory ventilation (SIMV)), with conventional ventilation or high‐frequency oscillation (HFO);
(ii) different types of triggered ventilation (ACV, SIMV, pressure‐regulated volume control ventilation (PRVCV), SIMV with pressure support (PS) and pressure support ventilation (PSV))
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised clinical trials comparing the use of synchronised ventilation (HFPPV or patient‐triggered ventilation) to conventional ventilation or high‐frequency oscillation, and randomised trials of different modes of triggered ventilation in neonates were considered for this review.
Types of participants
Neonates (less than four weeks of age) requiring assisted ventilation.
Types of interventions
We considered two forms of ventilation likely to induce synchrony:
high‐frequency positive pressure ventilation (HFPPV, ventilator rates ≥ 60 bpm); and triggered ventilation.
Triggered ventilation was divided into:
assist control ventilation (ACV), otherwise known as synchronous intermittent positive pressure ventilation (SIPPV), the infant being able to trigger a positive pressure inflation with each breath;
synchronised intermittent mandatory ventilation (SIMV), the infant being able to trigger only a pre‐set number of positive pressure inflations;
pressure‐regulated volume control ventilation (PRVCV), a synchronised pressure‐limited assist control mode that sequentially varies the delivered pressure to approximate a target inspiratory tidal volume;
pressure support ventilation, the initiation and end of inflation triggered by the infant's respiratory effort;
SIMV with pressure support (PS); PS assists every additional spontaneous breath beyond the set SIMV rate.
These modes of ventilation were compared to non‐synchronised ventilation either in the form of conventional ventilation (CMV), which for the purpose of this review is defined as pressure pre‐set, time‐limited ventilation delivered at rates of fewer than 60 bpm, or high‐frequency oscillatory ventilation (HFO).
Infants were randomly allocated to receive one or other forms of ventilation (except in Heicher 1981 when alternate allocation was used):
HFPPV versus CMV;
ACV or SIMV versus CMV;
SIMV or SIMV + PS versus HFO.
Triggered modes of ventilation were compared:
ACV versus SIMV;
SIMV plus PS versus SIMV;
SIMV versus PRVCV;
SIMV versus PSV;
ACV versus PSV.
Types of outcome measures
Primary outcomes
Data regarding clinical outcomes included:
mortality;
air leaks (pneumothorax or pulmonary interstitial emphysema (PIE));
severe intraventricular haemorrhage (grades 3 and 4);
bronchopulmonary dysplasia (BPD) (oxygen dependency beyond 28 days);
moderate/severe BPD (oxygen dependency or respiratory support dependency (or both) at 36 weeks' postmenstrual age);
duration of weaning/ventilation.
Search methods for identification of studies
Electronic searches
For the 2016 update we used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal Group search strategy for specialized register).
We conducted a comprehensive search including: the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 5) in The Cochrane Library; MEDLINE via PubMed (1996 to June 5 2016); EMBASE (1980 to June 5 2016); and CINAHL (1982 to June 5 2016) using the following search terms: (mechanical ventilation[MeSH] OR respiration, artificial[MeSH] OR mechanical ventilation OR triggered ventilation OR SIMV), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database). We did not apply language restrictions. We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform www.whoint/ictrp/search/en/; and the ISRCTN Registry).
For the previous version of this review we searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, 2008); Oxford Database of Perinatal Trials; MEDLINE from 1966 to 2008; and EMBASE 1996 to 2008 (MeSH terms: mechanical ventilation; triggered ventilation; artificial respiration; newborn infant).
Searching other resources
We searched previous reviews, abstracts, symposia proceedings as well as conducting handsearches of journals in the English language and establishing contact with expert informants.
Data collection and analysis
Three of the review authors (AS, TR, VM) identified trials that might be included. Each trial was then assessed independently by each review author who completed data collection forms that the review authors had previously agreed upon. The results were then compared and if there was disagreement a fourth and fifth review author (AG, ADM) assessed the results independently. For each included trial, we collected information regarding the method of randomisation, blinding, stratification, number of centres participating in the study, trial inclusion and exclusion criteria and sample size. We also collected demographic data of the trial participants (e.g. gestational age, birth weight, postnatal age, primary diagnosis). We analysed information on clinical outcomes including death, air leaks, intraventricular haemorrhage, chronic lung disease, duration of ventilation or weaning, ventilation mode failure and extubation failure. The denominator for each outcome was the number randomised. In the meta‐analyses involving comparison with CMV or HFO, either HFPPV or ACV/SIMV was designated the experimental therapy. In the meta‐analyses of ACV or PRVCV versus SIMV, then ACV or PRVCV was designated the experimental therapy. In the meta‐analyses of SIMV with PS versus SIMV, SIMV with PS was designated the experimental therapy, and in the meta‐analysis of ACV versus PSV, ACV was designated the experimental therapy.
Results
Description of studies
Included studies
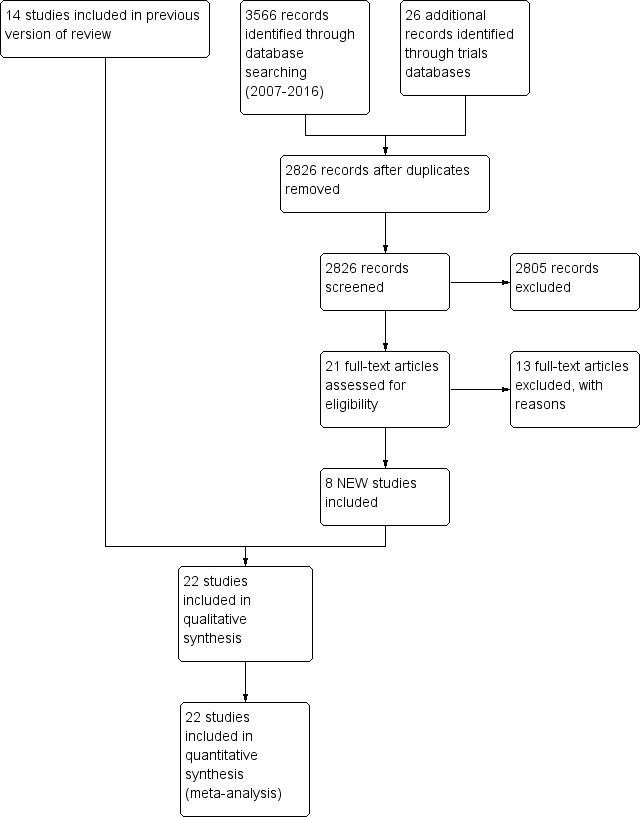
We identified twenty‐two studies for inclusion in this review (see Figure 1).
1.
Study flow diagram: review update
1. HFPPV versus CMV (Heicher 1981; OCTAVE 1991; Pohlandt 1992; Amini 2013).
2. ACV/SIMV versus CMV: Chan 1993 (ACV versus CMV); Donn 1994 (SIMV versus CMV); Bernstein 1996 (SIMV versus CMV); Chen 1997 (SIMV versus CMV); Baumer 2000 (ACV versus CMV); Beresford 2000 (ACV versus CMV); Liu 2011 (ACV versus CMV).
3. SIMV or SIMV + PS versus HFO (Courtney 2002a; Craft 2003a; Singh 2012; Sun 2014)
4. ACV versus SIMV (Chan 1994; Dimitriou 1995a; Dimitriou 1995b)
5. SIMV plus PS versus SIMV (Reyes 2006)
6. SIMV versus PRVCV (D'Angio 2005)
7. SIMV versus PSV (Erdemir 2014)
8. ACV versus PSV (Patel 2012)
Pohlandt 1992, Chan 1993, Chan 1994, Dimitriou 1995a, Dimitriou 1995b, Baumer 2000 and Beresford 2000 included only preterm infants. Donn 1994 included preterm infants with a birth weight between 1100 grams and 1500 grams. Courtney 2002a included infants with a birth weight between 601 grams and 1200 grams. Reyes 2006 included preterm infants with a birth weight of 500 grams to 1000 grams. Singh 2012 included infants with a birth weight of greater than 750 grams. D'Angio 2005 included preterm infants with a birth weight of 500 grams to 1249 grams. Erdemir 2014 and Sun 2014 included preterm infants with birth weight less than 1500 grams. Craft 2003a included preterm infants with birth weight less than 1000 grams. Bernstein 1996, Heicher 1981 and OCTAVE 1991 studied mainly premature neonates. Chen 1997 included term and preterm infants but analysed the groups separately; only the results from the 63 infants with RDS are included in the meta‐analysis. Amini 2013 does not make it clear whether term as well as preterm infants were included. Patel 2012 included infants of any gestation who were less than 14 days old.
Information regarding use of antenatal steroids was given only in Baumer 2000, Beresford 2000, Courtney 2002a, Craft 2003a, D'Angio 2005, Reyes 2006, Patel 2012, Singh 2012, Erdemir 2014 and Sun 2014. Data regarding surfactant usage were available in the trials of Donn 1994, Chan 1994, Dimitriou 1995a, Dimitriou 1995b, Bernstein 1996, Chen 1997, Baumer 2000, Beresford 2000, Courtney 2002a, Craft 2003a, D'Angio 2005, Reyes 2006, Patel 2012, Singh 2012, Amini 2013, Erdemir 2014 and Sun 2014. The age at entry varied between studies; the trials of Chan 1993, Chan 1994, Dimitriou 1995a, Dimitriou 1995b, Reyes 2006, Patel 2012 and Erdemir 2014 were weaning studies. Data for the duration of ventilation or weaning were obtained by personal communication with the investigators for Chan 1993, Chan 1994, Dimitriou 1995a, Dimitriou 1995b, Baumer 2000 and Beresford 2000.
Excluded studies
Seventy‐two additional studies were detected, but were found to be not eligible for inclusion in this review (see table Characteristics of excluded studies).
Risk of bias in included studies
All the studies but one were randomised; Heicher 1981 used alternate allocation. The method of randomisation is reported in all but one trial (Chen 1997). Certain outcomes were only available in some of the trials and therefore only presented for the subgroups in which they were reported for at least two trials, except for the trials assessing PSV, PRVCV and SIMV plus PS.
HFPPV versus CMV: In Heicher 1981 and OCTAVE 1991 analysis was by intention to treat, but in Pohlandt 1992 infants not ventilated strictly according to the technique to which they were randomised were excluded from the analysis.
SIMV/ACV versus CMV: Analysis was according to intention to treat in Chan 1993 and Baumer 2000; in Bernstein 1996, 6% of infants erroneously randomised because of problems including seizures, non‐viability and birth weight of less than 500 grams were excluded from the analysis.
SIMV or SIMV + PS versus HFO: Analysis was according to intention to treat in Courtney 2002a and Sun 2014. In Craft 2003a an ad‐hoc interim analysis was performed due to declining recruitment and the study was curtailed.
ACV versus SIMV: Dimitriou 1995 is presented as two studies (Dimitriou 1995a; Dimitriou 1995b), as two separate consecutive randomised trials were performed in which ACV was compared to two different methods of delivering SIMV. In Chan 1994, Dimitriou 1995a and Dimitriou 1995b weaning failure was defined as no reduction in ventilator settings over a 24 or 48 hour period respectively; extubation failure was defined as a requirement for re‐intubation using standardised criteria within a 48 hour period.
SIMV plus PS versus SIMV: In Reyes 2006 analysis was by intention to treat; five infants who were randomised met exclusion criteria and their results were not included in the analysis.
PRVCV versus SIMV: In D'Angio 2005 analysis was by intention to treat; one infant did not receive the allocated intervention.
SIMV vs PSV: In Erdemir 2014 weaning and extubation was performed according to standardised criteria. Analysis was not stated to be by intention to treat; however there were no failures of weaning reported in either arm.
ACV vs PSV: In Patel 2012 analysis was by intention to treat. Weaning was by standardised criteria.
Effects of interventions
HFPPV versus CMV (comparison 1)
Death (Analysis 1.1): Four trials reported this outcome (Heicher 1981; OCTAVE 1991; Pohlandt 1992; Amini 2013). None demonstrated a significant effect. The meta‐analysis indicates a trend towards reduction in death rate using HFPPV but this does not reach statistical significance (relative risk (RR) 0.78, 95% confidence interval (CI) 0.61 to 1.00).
1.1. Analysis.

Comparison 1: HFPPV vs CMV, Outcome 1: Death
Air leaks (Analysis 1.2 and Analysis 1.3): Three trials reported pneumothorax as an outcome (Heicher 1981; OCTAVE 1991; Pohlandt 1992); one trial reported a significant effect, with a lower rate of pneumothorax in the HFPPV group (Heicher 1981). The meta‐analysis supports a significant reduction in the risk of pneumothorax (typical RR for pneumothorax was 0.69, 95% CI 0.51 to 0.93). Pohlandt 1992 reported a significant reduction in PIE in the HFPPV group (RR 0.68, 95% CI 0.49 to 0.94). Amini 2013 reported total air leaks as outcome variable, with a non‐significant trend favouring HFPPV (RR 0.25, 95% CI 0.06 to 1.08).
1.2. Analysis.

Comparison 1: HFPPV vs CMV, Outcome 2: Air leaks
1.3. Analysis.

Comparison 1: HFPPV vs CMV, Outcome 3: Total air leak
BPD (oxygen dependency at 28 days) (Analysis 1.4): Four trials reported this outcome (Heicher 1981; OCTAVE 1991; Pohlandt 1992; Amini 2013). None demonstrated a significant effect. The meta‐analysis found no evidence of effect.
1.4. Analysis.

Comparison 1: HFPPV vs CMV, Outcome 4: BPD (oxygen dependency at 28 days)
IVH (Analysis 1.5): One trial reported this outcome, with a significantly lower rate of IVH in the HFPPV group (RR 0.13, 95% CI 0.02 to 0.94) (Amini 2013).
1.5. Analysis.

Comparison 1: HFPPV vs CMV, Outcome 5: IVH
Other outcomes: The incidence of PDA post randomisation was given in two trials (Heicher 1981; Pohlandt 1992); in neither did it differ significantly.
ACV/SIMV versus CMV (comparison 2)
Death (Analysis 2.1): Six trials reported this outcome (Donn 1994; Bernstein 1996; Chen 1997; Baumer 2000; Beresford 2000; Liu 2011). None demonstrated a significant effect. The meta‐analysis indicates a trend towards an increase in death rate using ACV/SIMV but this does not reach statistical significance (typical RR 1.17, 95% CI 0.94 to 1.47).
2.1. Analysis.

Comparison 2: ACV/SIMV vs CMV, Outcome 1: Death
Air leaks (Analysis 2.2): Seven trials reported this outcome (Chan 1993; Donn 1994; Bernstein 1996; Chen 1997; Baumer 2000; Beresford 2000; Liu 2011). None demonstrated a significant effect. The meta‐analysis found no evidence of effect.
2.2. Analysis.

Comparison 2: ACV/SIMV vs CMV, Outcome 2: Air leaks
Duration of ventilation (hours) (Analysis 2.3): Five trials reported this outcome (Donn 1994; Chen 1997; Baumer 2000; Beresford 2000; Liu 2011). Bernstein 1996 also reported the duration of ventilation, but the data are presented as the median and 95% CIs (SIMV 103 hours, 95% CI 94 to 118 versus CMV 120 hours, 95% CI 101 to 142), and the results, therefore, could not be meta‐analysed in the relevant Outcome (2.3). A significantly shorter duration of ventilation was noted in Chen's study (Chen 1997) and Donn's study (Donn 1994). The meta‐analysis of the five studies supported a significant reduction in ventilation duration (MD −38.30 hours, 95% CI −53.90 to −22.69). Chan 1993 reported the duration of weaning: ACV mean 39 hours, SD 45 versus CMV mean 65 hours, SD 75 (the results are not presented in Outcome 2.3).
2.3. Analysis.

Comparison 2: ACV/SIMV vs CMV, Outcome 3: Duration of ventilation (hours)
Extubation failure (Analysis 2.4): Four trials reported this outcome (Chan 1993; Donn 1994; Chen 1997; Baumer 2000). One trial reported a significant effect in favour of ACV/SIMV (Chen 1997). The meta‐analysis of the results of the four trials, however, did not demonstrate a significant effect, the typical RR being 0.93 (95% CI 0.68 to 1.28).
2.4. Analysis.

Comparison 2: ACV/SIMV vs CMV, Outcome 4: Extubation failure
Severe IVH (Analysis 2.5): Six trials reported this outcome (Donn 1994; Bernstein 1996; Chen 1997; Baumer 2000; Beresford 2000; Liu 2011). None demonstrated a significant effect. The meta‐analysis found no evidence of effect.
2.5. Analysis.

Comparison 2: ACV/SIMV vs CMV, Outcome 5: Severe IVH
BPD (oxygen dependency at 28 days) (Analysis 2.6): Four trials reported this outcome (Baumer 2000; Bernstein 1996; Chen 1997; Donn 1994). None demonstrated a significant effect. The meta‐analysis found no evidence of effect.
2.6. Analysis.

Comparison 2: ACV/SIMV vs CMV, Outcome 6: BPD (oxygen dependency at 28 days)
Moderate/severe BPD (Oxygen dependency at 36 weeks postmenstrual age) (Analysis 2.7): Two trials reported this outcome (Baumer 2000; Beresford 2000). Neither demonstrated a significant effect. The meta‐analysis found no evidence of effect.
2.7. Analysis.

Comparison 2: ACV/SIMV vs CMV, Outcome 7: Moderate/Severe BPD (oxygen dependent at 36 weeks PCA)
Other outcomes: In two trials the incidence of PDA is given post randomisation (Chen 1997; Beresford 2000). In one trial only the incidence of PDA requiring indomethacin and/or ligation was higher in the conventional group for both survivors (P < 0.05) and the whole population of infants (P < 0.05) (Beresford 2000).
SIMV OR SIMV + PS versus HFO (comparison 3)
Death (Analysis 3.1): Four trials of SIMV OR SIMV + PS VS HFO reported this outcome (Courtney 2002a; Craft 2003a; Singh 2012; Sun 2014). Sun 2014 reported a significant effect in favour of HFO (RR 3.21, 95% CI 1.07 to 9.67). No other trials reported a difference in this outcome, and the meta‐analysis found no evidence of effect.
3.1. Analysis.

Comparison 3: SIMV or SIMV + PS vs HFOV, Outcome 1: Death
BPD: oxygen dependency at 28 days (Analysis 3.2): One trial reported this outcome with no significant effect (Singh 2012).
3.2. Analysis.

Comparison 3: SIMV or SIMV + PS vs HFOV, Outcome 2: BPD: oxygen requirement at 28 days
Moderate/Severe BPD (Analysis 3.3): Three trials reported this outcome (Courtney 2002a; Craft 2003a; Sun 2014). Sun 2014 reported a significant effect in favour of HFO (RR 2.24, 95% CI 1.20 to 4.18) and Courtney 2002a reported a trend in favour of HFO (RR 1.24, 95% CI 0.96 to 1.60). The meta‐analysis showed a significant effect in favour of HFO (RR 1.33, 95% CI 1.07 to 1.65).
3.3. Analysis.

Comparison 3: SIMV or SIMV + PS vs HFOV, Outcome 3: Moderate/ Severe BPD: Oxygen requirement at 36 weeks PCA
Death or BPD (Analysis 3.4): Only one study reported this combined outcome, with a significant effect in favour of HFO (RR 2.38, 95% CI 1.41 to 4.03) (Sun 2014).
3.4. Analysis.

Comparison 3: SIMV or SIMV + PS vs HFOV, Outcome 4: Death or BPD
Duration of mechanical ventilation (Analysis 3.5): Two trials reported this outcome with both showing a significant effect in favour of HFO (Singh 2012; Sun 2014). The meta‐analysis demonstrated a significant effect in favour of HFO (Mean difference 1.89 days, 95% CI 1.04 to 2.74).
3.5. Analysis.

Comparison 3: SIMV or SIMV + PS vs HFOV, Outcome 5: Duration of mechanical ventilation
Air Leaks (Analysis 3.6): One trial reported PIE as an outcome and showed a significant decrease of this outcome in the SIMV group (RR 0.66, CI 0.44 to 0.99) (Courtney 2002a). Three trials reported pneumothorax as an outcome with no significant difference (Courtney 2002a; Craft 2003a; Sun 2014).
3.6. Analysis.

Comparison 3: SIMV or SIMV + PS vs HFOV, Outcome 6: Air leaks
IVH Grade 3 or 4 (Analysis 3.7): Four trials reported this outcome (Courtney 2002a; Craft 2003a; Singh 2012; Sun 2014). No trial found a significant difference.
3.7. Analysis.

Comparison 3: SIMV or SIMV + PS vs HFOV, Outcome 7: IVH Grade 3/4
ACV versus SIMV (comparison 4)
Duration of weaning (hours) (Analysis 4.1): Three trials of ACV versus SIMV reported this outcome (Chan 1994; Dimitriou 1995a; Dimitriou 1995b). None demonstrated a significant effect. In all three, however, the duration of weaning tended to be shorter in infants supported by ACV rather than SIMV. The meta‐analysis supported a trend in this direction which, however, did not reach statistical significance.
4.1. Analysis.

Comparison 4: ACV vs SIMV, Outcome 1: Duration of weaning (hours)
Weaning failure (Analysis 4.2): Three trials of ACV versus SIMV reported this outcome (Chan 1994; Dimitriou 1995a; Dimitriou 1995b). None demonstrated a significant effect. The meta‐analysis found no evidence of effect.
4.2. Analysis.

Comparison 4: ACV vs SIMV, Outcome 2: Weaning failure
Extubation failure (Analysis 4.3): Three trials of ACV versus SIMV (Chan 1994; Dimitriou 1995a; Dimitriou 1995b) reported this outcome. None demonstrated a significant effect. The meta‐analysis found no evidence of effect.
4.3. Analysis.

Comparison 4: ACV vs SIMV, Outcome 3: Extubation failure
Air leaks (Analysis 4.4): Three trials of ACV versus SIMV (Chan 1994; Dimitriou 1995a; Dimitriou 1995b) reported this outcome. None demonstrated a significant effect. The meta‐analysis found no evidence of effect.
4.4. Analysis.

Comparison 4: ACV vs SIMV, Outcome 4: Air leaks
SIMV PLUS PS versus SIMV (comparison 5)
Only Reyes 2006 reports on SIMV plus PS compared to SIMV alone.
Death (Analysis 5.1): There was no significant effect with regard to death at 28 days or death prior to discharge.
5.1. Analysis.

Comparison 5: PS + SIMV versus SIMV, Outcome 1: Death
Air leaks (Analysis 5.2): The occurrence of PIE and pneumothorax are reported separately; there were no significant differences in either outcome.
5.2. Analysis.

Comparison 5: PS + SIMV versus SIMV, Outcome 2: Air leaks
BPD, oxygen dependency at 28 days (Outcome 5.3.1): There was no significant effect.
Moderate/severe BPD, oxygen dependency at 36 weeks postmenstrual age (Outcome 5.3.2): There was no overall significant effect.
Severe IVH (Grade III and IV) (Analysis 5.4): There was no significant effect.
5.4. Analysis.

Comparison 5: PS + SIMV versus SIMV, Outcome 4: Severe IVH (grade III and IV)
Other outcomes: There was no significant effect re PDA. Days on mechanical ventilation and supplementary oxygen did not differ by ventilation status, but in the subgroup of infants with birth weight of 700 grams to 1000 grams, the days of supplementary oxygen were lower in the SIMV plus PS group (P = 0.034).
PRVCV versus SIMV (comparison 6)
Death prior to discharge (Analysis 6.1): One trial (D'Angio 2005) reported this with no significant difference between intervention.
6.1. Analysis.

Comparison 6: PRVCV vs SIMV, Outcome 1: Death prior to discharge
BPD, oxygen requirement at 36 weeks' postmenstrual age in survivors (Analysis 6.2): One trial reported this with no significant difference between intervention (D'Angio 2005).
6.2. Analysis.

Comparison 6: PRVCV vs SIMV, Outcome 2: BPD: oxygen requirement at 36 weeks in survivors
Airleak (PIE (Outcome 6.3.1) or Pneumothorax (Outcome 6.3.2)): In the PRVCV versus SIMV trial there were no significant effects with regard to either pneumothorax or PIE (D'Angio 2005).
Severe IVH (Analysis 6.4): One trial reported this outcome with no significant difference between intervention (D'Angio 2005).
6.4. Analysis.

Comparison 6: PRVCV vs SIMV, Outcome 4: Severe IVH
SIMV versus PSV (comparison 7)
Duration of weaning (Analysis 7.1): One trial reported this outcome (Erdemir 2014). There was no significant difference.
7.1. Analysis.

Comparison 7: PSV vs SIMV, Outcome 1: Duration of weaning
Extubation failure (Analysis 7.2): One trial reported this outcome (Erdemir 2014). There was no significant difference.
7.2. Analysis.

Comparison 7: PSV vs SIMV, Outcome 2: Extubation failure
Air leaks (total) (Analysis 7.3): One trial reported this outcome (Erdemir 2014). There was no significant difference.
7.3. Analysis.

Comparison 7: PSV vs SIMV, Outcome 3: Air leaks (total)
Moderate/Severe BPD; Oxygen requirement at 36 weeks postmenstrual age (Analysis 7.4): One trial reported this outcome (Erdemir 2014). There was no significant difference.
7.4. Analysis.

Comparison 7: PSV vs SIMV, Outcome 4: Moderate/Severe BPD: oxygen requirement at 36 weeks PCA
ACV versus PSV (comparison 8)
Duration of weaning (Outcome 8.1): One trial reported this outcome (Patel 2012). The data were reported as median and range. No significant difference was reported.
Discussion
Physiological studies have demonstrated in prematurely born neonates that both high‐frequency positive pressure ventilation and triggered ventilation are more likely to provoke a synchronous respiratory interaction; that is, the infant's inspiratory efforts coincide with positive pressure inflations. When compared to CMV, those ventilation modes were shown to be associated with improved ventilation. Unfortunately, in none of the subsequent randomised trials is it reported whether synchronous ventilation was achieved and few outcome measures are consistently reported in all relevant trials. Nevertheless, the meta‐analyses demonstrate a significant decrease in air leak and a shorter duration of ventilation with HFPPV and triggered ventilation respectively. However, no significant effect on the incidence of BPD or death has been shown using either HFPPV or trigger ventilation modes.
No deleterious effects of those two ventilatory modes were highlighted. Some positive effects have been demonstrated of the newer triggered modes PRVCV and SIMV plus PS, but both modes have each only been examined in one randomised trial; further trials are required which incorporate long‐term outcomes.
A meta‐analysis of the studies comparing SIMV or SIMV + PS with HFO show a significant decrease in moderate or severe BPD, and significantly shorter duration of mechanical ventilation with HFO. Whether other trigger modes such as ACV have similar results to HFO requires investigating.
Authors' conclusions
Implications for practice.
Comparative trials of assisted ventilation in neonates demonstrate that, compared to CMV, HFPPV is associated with a reduced risk of air leak and triggered ventilation with a shorter duration of ventilation. On clinical grounds, those ventilatory modes would seem preferable for preterm neonates to 'conventional' ventilation delivered at rates of less than 60 bpm. In addition, HFO resulted in a reduced risk of BPD and duration of ventilation compared to SIMV with or without pressure support. It is important to determine if other triggered modes such as ACV have similar poor results compared to HFO.
Comparative trials demonstrated that in preterm infants in the recovery stage of respiratory distress, ACV compared to SIMV is associated with a shorter duration of ventilation; thus, ACV would seem the more desirable mode of weaning for preterm neonates. There are insufficient randomised trials of PRVCV or pressure support versus SIMV to make any conclusions to their efficacy with regard to long‐term efficacy.
Implications for research.
Further trials are encouraged to assess whether ventilation modes likely to provoke synchronous ventilation will have other benefits and whether the mechanism of such effects is by provoking synchrony. Optimisation of trigger and ventilator performance with respect to respiratory diagnosis is essential. Randomised trials of the newer triggered modes with long‐term outcomes are essential to determining their efficacy.
What's new
| Date | Event | Description |
|---|---|---|
| 7 July 2020 | Amended | We have corrected the Declaration of interest section. |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 1, 1998
| Date | Event | Description |
|---|---|---|
| 22 August 2016 | Amended | Author order corrected |
| 22 September 2015 | New citation required and conclusions have changed | Conclusions updated. |
| 24 July 2015 | New search has been performed | Seven additional studies incorporated, with new comparisons. |
| 21 May 2012 | New search has been performed | This review updates the existing review of "Synchronized mechanical ventilation for respiratory support in newborn infants" published in The Cochrane Library, Issue 4, 2008 (Greenough 2008). |
| 21 May 2012 | New citation required but conclusions have not changed | One additional study was identified as eligible for inclusion and additional studies were designated as "excluded studies". |
| 31 August 2007 | New citation required and conclusions have changed | Substantive amendment |
| 31 August 2007 | New search has been performed | This review updates the existing review of "Synchronized mechanical ventilation for respiratory support in newborn infants" published in The Cochrane Library, Issue 3, 2004 (Greenough 2004). Two additional studies were identified as eligible for inclusion and additional studies were designated as "excluded studies". Two further triggered modes are included: pressure support and pressure regulated volume control ventilation have been included. |
Acknowledgements
Dr. Murthy was supported by the Guy's and St Thomas' Charity Fund.
Appendices
Appendix 1. Standard search methodology
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
EMBASE: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
The Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Data and analyses
Comparison 1. HFPPV vs CMV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Death | 4 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.61, 1.00] |
| 1.2 Air leaks | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.2.1 Pneumothorax | 3 | 585 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.51, 0.93] |
| 1.2.2 Pulmonary interstitial emphysema | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.49, 0.94] |
| 1.3 Total air leak | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 1.08] |
| 1.4 BPD (oxygen dependency at 28 days) | 4 | 647 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.77, 1.46] |
| 1.5 IVH | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.12 [0.02, 0.94] |
Comparison 2. ACV/SIMV vs CMV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Death | 6 | 1790 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.94, 1.47] |
| 2.2 Air leaks | 7 | 1830 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.76, 1.27] |
| 2.3 Duration of ventilation (hours) | 5 | 1463 | Mean Difference (IV, Fixed, 95% CI) | ‐38.30 [‐53.90, ‐22.69] |
| 2.4 Extubation failure | 4 | 1056 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.68, 1.28] |
| 2.5 Severe IVH | 6 | 1790 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.73, 1.40] |
| 2.6 BPD (oxygen dependency at 28 days) | 4 | 805 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.75, 1.12] |
| 2.7 Moderate/Severe BPD (oxygen dependent at 36 weeks PCA) | 2 | 1310 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.75, 1.08] |
Comparison 3. SIMV or SIMV + PS vs HFOV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Death | 4 | 996 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.91, 1.71] |
| 3.2 BPD: oxygen requirement at 28 days | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.21 [0.37, 27.83] |
| 3.3 Moderate/ Severe BPD: Oxygen requirement at 36 weeks PCA | 3 | 869 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.07, 1.65] |
| 3.4 Death or BPD | 1 | 356 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [1.41, 4.03] |
| 3.5 Duration of mechanical ventilation | 2 | 466 | Mean Difference (IV, Fixed, 95% CI) | 1.89 [1.04, 2.74] |
| 3.6 Air leaks | 3 | 1398 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.70, 1.19] |
| 3.6.1 Pulmonary interstitial emphysema | 1 | 498 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.44, 0.99] |
| 3.6.2 Pneumothorax | 3 | 900 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.82, 1.64] |
| 3.7 IVH Grade 3/4 | 4 | 1010 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.71, 1.24] |
Comparison 4. ACV vs SIMV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Duration of weaning (hours) | 3 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐42.38 [‐94.35, 9.60] |
| 4.2 Weaning failure | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.31, 1.93] |
| 4.3 Extubation failure | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.37, 2.67] |
| 4.4 Air leaks | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.23, 2.83] |
| 4.4.1 Total air leaks | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.23, 2.83] |
Comparison 5. PS + SIMV versus SIMV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Death | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1.1 Death during first 28 days | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.08, 2.01] |
| 5.1.2 Death prior to discharge | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.31, 2.43] |
| 5.2 Air leaks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.2.1 Pneumothorax | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 5.2.2 Pulmonary interstitial emphysema | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.25, 2.15] |
| 5.3 BPD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.3.1 BPD (oxygen dependency at 28 days) | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.84, 1.23] |
| 5.3.2 Moderate/Severe BPD (oxygen dependency at 36 weeks PMA) | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.42, 1.18] |
| 5.4 Severe IVH (grade III and IV) | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.41, 2.08] |
5.3. Analysis.

Comparison 5: PS + SIMV versus SIMV, Outcome 3: BPD
Comparison 6. PRVCV vs SIMV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Death prior to discharge | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.50, 2.11] |
| 6.2 BPD: oxygen requirement at 36 weeks in survivors | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.55, 1.27] |
| 6.3 Air leak | 1 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.51, 2.13] |
| 6.3.1 PIE | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.56, 4.91] |
| 6.3.2 Pneumothorax | 1 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.26, 1.88] |
| 6.4 Severe IVH | 1 | 203 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.29, 1.58] |
6.3. Analysis.

Comparison 6: PRVCV vs SIMV, Outcome 3: Air leak
Comparison 7. PSV vs SIMV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Duration of weaning | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐11.30 [‐26.53, 3.93] |
| 7.2 Extubation failure | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.57, 2.07] |
| 7.3 Air leaks (total) | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.00] |
| 7.4 Moderate/Severe BPD: oxygen requirement at 36 weeks PCA | 1 | 60 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.46, 2.17] |
Comparison 8. ACV versus PSV.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Duration of weaning | 1 | Other data | No numeric data |
8.1. Analysis.
Comparison 8: ACV versus PSV, Outcome 1: Duration of weaning
| Duration of weaning | ||||
| Study | Participants | Duration of weaning ACV (Median (range)) | Duration of weaning PSV (Median(range)) | Significance |
| Patel 2012 | 36 | 34 (7‐100) | 27 (10‐169) | p=0.88 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Amini 2013.
| Study characteristics | ||
| Methods | Randomised Single centre trial Randomisation method: block randomisation Blinding of randomisation: unclear Blinding of intervention: no Blinding of outcome measurement: no Complete follow‐up: yes |
|
| Participants | All neonates admitted to NICU with respiratory failure requiring mechanical ventilation (respiratory failure and gestational age not defined). All infants received surfactant. Exclusion criteria include: complex congenital heart disease, genetic syndromes, major anomalies, hypoxic ischaemic encephalopathy or birth asphyxia. HFPPV 31; CMV 31 |
|
| Interventions | HFPPV or CMV | |
| Outcomes | No primary outcome stated. Outcomes reported were IVH, air leak, mortality, treatment failure, duration of mechanical ventilation, number of doses of surfactant administered, oxygen requirement at 28 days, PVL | |
| Notes | Ventilator types: Bearcub 750 used for both interventions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Block randomisation"; no further details given |
| Allocation concealment (selection bias) | Low risk | "Block randomisation"; no further details given. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Clinician aware of intervention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Clinicians likely to be aware of intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up of recruited infants |
Baumer 2000.
| Study characteristics | ||
| Methods | Randomised
Multicentre trial
Randomissation method: randomly allocated by telephone
Stratified by centre. Within each centre, randomisation in blocks to ensure a similar distribution of babies in each arm of the study Blinding of randomisation: yes Blinding of intervention: no Complete follow‐up: no Blinding of outcome measurement: no |
|
| Participants | Gestational age < 32 weeks. Assisted ventilation within 72 hours of birth Not ventilated for more than 6 hours at randomisation RDS Exclusion: major congenital malformation or inhalational pneumonitis Sample size: 924 PTV: 465 CMV: 459 | |
| Interventions | PTV vs CMV | |
| Outcomes | Primary: Hospital mortality or need for oxygen treatment at 36 weeks of gestation; pneumothorax cerebral ultrasound abnormality nearest to 36 weeks of gestation; duration of ventilation in survivors | |
| Notes | Ventilator types: PTV: SLE 2000 (airway pressure trigger) Draeger baby log 8000 (airway flow trigger) CMV: SLE 2000, Draeger Babylog, Sechrist 423 of those randomised to PTV and 422 infants randomised to CMV had cranial ultrasound examination | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly allocated by telephone" |
| Allocation concealment (selection bias) | Low risk | "Within each centre, randomisation was performed in blocks" Probably done |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Form of ventilation to which they were assigned from birth to final extubation" Comment: no blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Clinicians were allowed the discretion to change the baby from the assigned mode of ventilation" Comment: no blinding as clinicians aware of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: clinicians likely to be aware of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome for death (912/924); pneumothorax(922/924); cranial USS (848/924) |
Beresford 2000.
| Study characteristics | ||
| Methods | Randomised Multicentre trial Randomisation method: computer generated sequence hidden in sequentially numbered, opaque envelopes Stratified by BW Blinding of randomisation: yes Blinding of intervention: no Complete follow‐up: no Blinding of outcome measurement: no | |
| Participants | Birth weight 1000 to 2000 grams Assisted ventilation within 24 hours of birth RDS Exclusion: major malformations, congenital heart disease, MAS Sample size: 386 PTV: 193 CMV: 193 | |
| Interventions | PTV vs CMV | |
| Outcomes | Primary: Incidence of CLD Secondary: Death Pneumothorax IVH Cystic PVL Shunt insertion | |
| Notes | Ventilator types: SLE 2000 (airway pressure trigger) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated sequence". |
| Allocation concealment (selection bias) | Low risk | "Hidden in sequentially numbered, sealed, opaque envelopes". |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Study design was such as to preclude crossover of treatment strategy". Comment: clinician aware of the mode of intervention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: clinicians aware of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: clinicians aware of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: complete data present |
Bernstein 1996.
| Study characteristics | ||
| Methods | Randomised
Multicenter trial
Intention‐to‐treat basis
Randomisation method: sealed, opaque envelopes. Stratified by BW Blinding of randomisation: yes Blinding of intervention: no Complete follow‐up: no Blinding of outcome measurement: no |
|
| Participants | BW > 500 grams Assisted ventilation Age < 36 hours RDS, congenital pneumonia, MAS CxR with abnormal lung parenchyma, FiO₂ > 0.4 (all BW) and MAP > 7 cmH₂O (for infants with BW > 1250 grams). Duration of CMV prior randomisation < 12 hours, spontaneous breathing rate > 20 bmp and indwelling arterial line Exclusion: infants with air leak, seizures, IVH grade III or IV, neuromuscular disease affecting respiration, major malformations including chromosomal abnormalities, CDH, CHD (except PDA), lung hypoplasia, septic shock or severe skin disease Sample size: 350* SIMV: 178 (167 analysed) CMV: 172 (160 analysed) *23 excluded post randomisation | |
| Interventions | SIMV vs CMV | |
| Outcomes | Primary: Acute effect on oxygenation Sedative/analgesic drug requirements Duration of ventilation Air leaks Secondary: Severe IVH Death Need for pharmacological paralysis ECMO or long‐term supplemental oxygen The age at which infants undergoing long‐term ventilation (> 14 days) regained their BW | |
| Notes | Ventilator types: Infant Star with Star Sync module (abdominal movement monitor) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation schedules were generated for each centre by computer" |
| Allocation concealment (selection bias) | Low risk | "Sequenfial, opaque, sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Intention‐to‐treat protocol" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: clinicians aware of intervention (intention‐to‐treat protocol) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Data were collected prospectively" Comment: clinicians probably aware of intervention (intention‐to‐treat protocol) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome for all participants reported |
Chan 1993.
| Study characteristics | ||
| Methods | Randomised Single centre trial Randomisation method: sealed, opaque envelopes Blinding of randomisation: yes Blinding of intervention: no Complete follow‐up: no Blinding of outcome measurement: no | |
| Participants | Gestational age < 36 weeks. Age: 1 to 21 days. RDS. In the recovery stage of the respiratory disease (at 40 bpm) Sample size: 40 PTV: 20 CMV: 20 | |
| Interventions | PTV vs CMV | |
| Outcomes | Primary: Hours of ventilation from entering the study until first extubation (weaning) Secondary: Number of infants who failed weaning Number of infants who failed extubation | |
| Notes | Ventilator types: SLE 2000 (airway pressure trigger), Sechrist IV‐100B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Sealed, opaque envelopes" |
| Allocation concealment (selection bias) | Low risk | Not revealed to clinician |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "protocol for weaning from ventilation was similar in both groups" Comment: clinicians not blinded to the method of weaning. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote:"not possible to "blind" the clinicians" Comment: different ventilators used for the two study groups |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: clinicians aware of intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome for all participants reported |
Chan 1994.
| Study characteristics | ||
| Methods | Randomised Single centre trial Randomisation method: sealed, opaque envelopes Blinding of randomisation: yes Blinding of intervention: no Complete follow‐up: no Blinding of outcome measurement: no | |
| Participants | GA < 35 weeks Age < 1 to 23 days Weaning – loaded with aminophylline Exclusion: apnoea, failure to trigger Sample size: 40 SIMV: 20 CMV: 20 | |
| Interventions | PTV vs SIMV | |
| Outcomes | Primary: Duration of weaning Secondary: Number of infants who failed weaning Number of infants who failed extubation | |
| Notes | Ventilator types: SLE 2000 (airway pressure trigger) CMV | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised" By sealed opaque envelope |
| Allocation concealment (selection bias) | Low risk | "Consecutively drawing cards from sealed envelope" Comment: clinicians blinded to allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "protocol for weaning from ventilation was similar in both groups" Comment: clinicians aware of intervention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: clinicians aware of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: clinicians aware of intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome for all participants reported |
Chen 1997.
| Study characteristics | ||
| Methods | Randomised Single centre trial Blinding of randomisation: not stated Blinding of intervention: no Complete follow‐up: no Blinding of outcome measurement: no | |
| Participants | BW < 1.75 kg, GA < 34 weeks and RDS Assisted ventilation Exclusion: congenital malformation, inherited metabolic abnormalities, sepsis, treatment with muscle relaxants Sample size: 77 SIMV: 38 CMV: 39 RDS sample size: 62 SIMV: 31 CMV: 31 MAS sample size: 15 SIMV: 7 CMV: 8 Term infants (MAS) excluded from the analysis | |
| Interventions | SIMV vs CMV | |
| Outcomes | Primary: Duration of ventilation Need of reintubation Air leaks PDA IVH Secondary: BPD ROP Death |
|
| Notes | Ventilator types: Infant Star with Star sync module (airflow trigger) (CMV) Bear Cub | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised" |
| Allocation concealment (selection bias) | Unclear risk | Comment: the evaluator was unaware of the treatment status of the patients |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: not possible to assess |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not possible to assess |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: not possible to assess |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome for all participants reported |
Courtney 2002a.
| Study characteristics | ||
| Methods | Randomised controlled multicentre study randomised by off‐site clinical coordination centre |
|
| Participants | BW 601 to 1200 grams appropriately developed for gestational age < 4 hours of age and expected to require ventilation for at least 24 hours Exclusion: if Apgar at 5 minutes < 4; a base deficit of 15 or more prior to study; severe hypotension; chromosomal or genetic abnormalities; congenital heart disease or known neuromuscular disease |
|
| Interventions | HFO with Sensormedics 3100a or SIMV with either VIP Bird, Babylog 8000, Bear Cub with neonatal monitoring or Bear Cub 750vs | |
| Outcomes | Primary outcome: death or BPD (oxygen requirement at 36 weeks) successful extubation IVH, PVL, pneumothorax, PIE, pulmonary haemorrhage, bacteraemia, PDA, NEC, ROP |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by off‐site clinical coordination centre. Probably done |
| Allocation concealment (selection bias) | Low risk | Off‐site allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of clinicians: different ventilators used for different arms |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding possible as above |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Clinicians likely to be aware of intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10 infants from HFOV and 4 from SIMV withdrawn – data analysed until point of withdrawal |
Craft 2003a.
| Study characteristics | ||
| Methods | Multicentre randomised controlled trial Randomised using random number sequence with assignments in opaque sealed envelopes Block randomisation (units of 10) crossover to alternative method in event of failure – analysis by intention to treat |
|
| Participants | 23 to 34 weeks gestation weighing < 1000 grams requiring mechanical ventilation No exclusion criteria stated Grouped 500 to 750 g and 750 to 1000 grams – data combined for meta‐analysis |
|
| Interventions | SIMV or high‐frequency flow interruption (HFFI) | |
| Outcomes | Days of mechanical ventilation Days of CPAP days of oxygen requirement BPD (Oxygen requirement at 36 weeks) Airleak PDA Grade 3 or 4 IVH Grade 3 or 4 ROP Death |
|
| Notes | Infant Star ventilator. Graseby capsule used for synchronisation. Extubation when rate reduced to 8 to 12 bpm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Infants were randomly assigned by a sealed opaque envelope, with a previously generated random number sequence" |
| Allocation concealment (selection bias) | Low risk | Clinicians blinded to allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not possible to blind clinician to treatment arm |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind clinician to treatment arm |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors likely aware of treatment arm |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition unclear. Study terminated at ad‐hoc interim analysis |
D'Angio 2005.
| Study characteristics | ||
| Methods | Randomised Single centre trial Randomisation method: block randomisation scheme by one of the investigators Blinding of randomisation: yes Blinding of intervention: no Complete follow‐up: no Blinding of outcome measurement: no | |
| Participants | Ventilated infants BW of 500 to 1249 grams Less than six hours of age Gestational age > 24 weeks Sample size: 212 PRVCV: 104 SIMV: 108 | |
| Interventions | PRVCV vs SIMV | |
| Outcomes | Primary: proportion of infants alive and extubated at 14 days Secondary: death Moderate/severe BPD Air leaks Severe IVH (grades 3 and 4) | |
| Notes | Ventilator type: Servo 300, infants who required slow rates > 40 bpm (maximum for the Servo 300) were transferred to the BIRD VIP ventilator | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Quote: "randomly assigned " Comment: probably done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Comment: probably done (Failure and Weaning protocol followed) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: clinicians likely to be aware of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: clinicians likely to be aware of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: respiratory outcomes for all participants reported |
Dimitriou 1995a.
| Study characteristics | ||
| Methods | Randomised Single centre trial Randomisation method: sealed, opaque envelopes Blinding of randomisation: yes Blinding of intervention: no Complete follow‐up: no Blinding of outcome measurement: no | |
| Participants | GA < 35 weeks Age < 15 days Weaning – loaded with aminophylline Exclusion: apnoea, failure to trigger Sample size: 40 PTV: 20 SIMV: 20 | |
| Interventions | PTV vs SIMV | |
| Outcomes | Primary: Duration of weaning Secondary: Number of infants who failed weaning Number of infants who failed extubation | |
| Notes | Ventilator types: SLE 2000 (airway pressure trigger) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random selection" |
| Allocation concealment (selection bias) | Low risk | "Drawing a card" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Done as protocol followed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: clinicians likely to be aware of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome of all trial participants reported. |
Dimitriou 1995b.
| Study characteristics | ||
| Methods | Randomised. Single centre trial Randomisation method: sealed, opaque envelopes Blinding of randomisation: yes Blinding of intervention: no Complete follow‐up: no Blinding of outcome measurement: no | |
| Participants | GA < 35 weeks Age < 15 days Weaning – loaded with aminophylline Exclusion: apnoea, failure to trigger Sample size: 40 PTV: 20 SIMV: 20 | |
| Interventions | PTV vs SIMV | |
| Outcomes | Primary: Duration of weaning Secondary: Number of infants who failed weaning Number of infants who failed extubation | |
| Notes | Ventilator types: SLE 2000 (airway pressure trigger) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Random selection" |
| Allocation concealment (selection bias) | Low risk | "Drawing a card" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Done as protocol followed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: clinicians likely to be aware of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: outcome of all trial participants reported. |
Donn 1994.
| Study characteristics | ||
| Methods | Randomised Single centre trial Randomisation method: lottery (sampling without replacement) Blinding of randomisation: not reported Blinding of intervention: no Complete follow‐up: no Blinding of outcome measurement: no | |
| Participants | Preterm infants. BW between 1.1 to 1.5 kg RDS, SRT Sample size: 30 PTV: 15 CMV: 15 | |
| Interventions | PTV vs CMV | |
| Outcomes | Primary: Duration of ventilation Secondary: Air leaks IVH CLD | |
| Notes | Ventilator types: PTV V.I.P. BIRD (airflow trigger) CMV Sechrist IV‐100B, V.I.P. BIRD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised" |
| Allocation concealment (selection bias) | Low risk | "lottery (sampling without replacement)" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not possible to assess |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcome of all trial participants reported |
Erdemir 2014.
| Study characteristics | ||
| Methods | Randomised Single centre trial Sealed envelope randomisation |
|
| Participants | 60 prematurely born infants 30 SIMV 30 gestation < 33 weeks or birth weight < 1500 grams requiring mechanical ventilation for RDS Exclusion criteria: admission after 6 hours of age congenital cardiac, respiratory or CNS malformation congenital metabolic disease congenital pneumonia or sepsis perinatal asphyxia leak around ET tube of < 20% |
|
| Interventions | Received surfactant placed on PTV, then randomised to SIMV or PSV + VG when FiO₂ < 0.4, RR < 60, PIP 16 cmH₂O, PEEP 4 cmH₂O with adequate blood gases | |
| Outcomes | Duration of weaning time to extubation PIP, MAP, Vt, RR at start, during and at end of weaning |
|
| Notes | 58 recruited, 45 reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Sealed envelope randomisation": sequence generation unclear |
| Allocation concealment (selection bias) | Low risk | "Sealed envelope randomisation" |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcomes reported for all participants |
Heicher 1981.
| Study characteristics | ||
| Methods | Quasi‐randomised Single centre trial Patients alternatively assigned to one of the two study ventilatory modes Blinding of randomisation: no Blinding of intervention: no Complete follow‐up: no Blinding of outcome measurement: no | |
| Participants | Birth weight > 750 grams. No gross anomalies. Abnormal lung fields on chest radiograph. Respiratory distress syndrome, pneumonia Exclusion: infants with chromosomal abnormalities or meconium aspiration Sample size: 102 Rapid rates: 51 Slow rates: 51 | |
| Interventions | HFPPV. Rapid rates (60 bpm) with IT: 0.5 sec versus slow rates (20 to 40 bpm) with IT: 1sec | |
| Outcomes | Clinical improvement Need for pharmacological paralysis Hours of assisted ventilation Hours of FiO₂ > 0.6. CLD Mortality |
|
| Notes | Ventilator types: Baby Bird, Bird Co | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "were alternately assigned" Comment: not randomised |
| Allocation concealment (selection bias) | High risk | Comment: assigned not randomised |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote:"remained constant throughout the study period" Comment: probably done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcomes reported for all trial participants |
Liu 2011.
| Study characteristics | ||
| Methods | Randomised trial Single centre Randomisation method: random number table Blinding of randomisation: unclear Blinding of intervention: no Complete follow‐up: yes Blinding of outcome measurement: not clear |
|
| Participants | GA < 35 weeks Mechanical ventilation RDS Age < 12 hours old Arterial blood gas pH < 7.25; PaO₂ < 50mmHg; PaCO₂ > 50 mmHg PaO₂/FiO₂ ≤ 250 mmHg; a/APO₂ ≤ 0.22 Exclusion criteria: congenital lung abnormalities, pulmonary haemorrhage, pneumothorax, congenital pneumonia, meconium aspiration, wet lung, complex congenital heart disease, grade 3/4 intracranial haemorrhage Sample size: 84 SIPPV + VG: 31 CMV: 30 HFOV: 23 |
|
| Interventions | SIPPV +VG vs CMV vs HFOV | |
| Outcomes | Primary: Duration of mechanical ventilation Oxygenation status Secondary: Death Air leak Ventilation associated pneumonia Intraventricular haemorrhage (Grade 3/4) |
|
| Notes | Ventilator types: Babylog 8000 plus, Sensormedics 3100 A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: random number table used |
| Allocation concealment (selection bias) | High risk | Comment: probably not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | Comment: probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcomes of all participants reported. |
OCTAVE 1991.
| Study characteristics | ||
| Methods | Randomised Multicentre trial Randomisation method: sealed, opaque envelopes Blinding of randomisation: yes Blinding of intervention: no Complete follow‐up: yes Blinding of outcome measurement: no | |
| Participants | Age < 72 hours. Assisted ventilation Exclusion: meconium aspiration Sample size: 346 HFPPV: 174 CMV: 172 | |
| Interventions | HFPPV (60 bpm) versus CMV (20 to 40 bpm) | |
| Outcomes | Incidence of pneumothorax Incidence and severity of CLD Mortality Neurodevelopmental outcome | |
| Notes | Ventilator type: Sechrist IV 100B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random assignment" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "sealed,opaque, serially numbered envelope" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: clinicians probably aware of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: clinicians probably aware of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: short‐term outcome reported for all participants |
Patel 2012.
| Study characteristics | ||
| Methods | Randomised Single centre study Randomisation: sequential opaque sealed envelopes with contents generated by random number table Randomised at initiation of weaning ventilation |
|
| Participants | Ventilated, less than 14 days old excluding: congenital heart disease or HIE Evaluation of weaning: randomised when FiO₂ < 0.4; PIP ≤ 20 cmH₂O if ≥ 29 weeks' gestation; ≤ 17 cmH₂O if between 26 and 29 weeks' gestation; or ≤ 15 cmH₂O if ≤ 26 weeks' gestation 18 ACV, 18 PSV |
|
| Interventions | ACV Vs PSV (backup 40 bpm, trigger 0.6 to 1.0 litre/min) | |
| Outcomes | Duration of weaning, time to extubation | |
| Notes | All infants ventilated with SLE5000 Data in paper expressed as median and range |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised using a sequential opaque sealed envelope system, the contents having been determined by random number table generation": low risk |
| Allocation concealment (selection bias) | Low risk | As above; block allocation with six in each block |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No blinding of clinician. Analysis by intention to treat |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not all short term outcomes measured at all time points, but relevant outcomes reported for all participants |
Pohlandt 1992.
| Study characteristics | ||
| Methods | Randomised (with stratification for gestational age) Method of randomisation: random number table. Blinding of randomisation: not reported Blinding of intervention: no Complete follow‐up: no Blinding of outcome measurement: no | |
| Participants | Gestational age < 32 weeks Assisted ventilation Supplemental FiO₂ > 0.4 Sample size: 181 HFPPV: 91 CMV: 90 | |
| Interventions | HFPPV (60 bpm with IT: 0.3 sec) vs CMV (30 to 40 bpm with IT: 1 sec) | |
| Outcomes | Incidence of extra‐alveolar air leaks Mortality |
|
| Notes | Ventilator types: AIV Loosco MKII, Biomed MVP 10, Babylog‐Draeger, Sechrist IV‐100B, Siemens Servo‐B and Servo‐C, Stephan 181 infants were enrolled into the study, but only 137 fulfilled the criteria and their results were analysed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "random number table" Comment: probably not followed |
| Allocation concealment (selection bias) | High risk | Comment: probably not done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: clinicians probably aware of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: primary outcomes of all participants reported |
Reyes 2006.
| Study characteristics | ||
| Methods | Randomised Single centre Randomisation method: sequential Sealed opaque envelopes from a computer‐generated randomised list Blinding of randomisation: no Complete follow‐up: yes Blinding of outcome measurement: no | |
| Participants | Birth weight 500 to 1000 grams appropriate birth weight for gestational age Mechanical ventilation requirement < 12 hours after birth until 7 days Exclusion: congenital anomalies; neuromuscular disease; lung hypoplasia; congenital heart disease; hypotension requiring intravenous medication; PIE or pneumothorax; required HFOV > 24 hours; received sedation or muscle relaxation Sample size: 107 53: SIMV plus PS 54: SIMV | |
| Interventions | SIMV plus PS versus SIMV | |
| Outcomes | Primary: Proportion of infants requiring supplementary oxygen at 28 days Secondary: Death Air leaks BPD IVH (Grades III and IV) (Grade III and IVH) | |
| Notes | Ventilator type: Pressure‐limited flow triggered VIP ventilator | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Block randomisation" Comment: probably done |
| Allocation concealment (selection bias) | High risk | Comment: probably not followed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "study protocol was actively followed" Comment: probably done |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Caregivers were not blinded to the assigned modality" Comment: not followed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: clinicians probably aware of the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: outcomes of all trial participants reported |
Singh 2012.
| Study characteristics | ||
| Methods | Randomised controlled single centre study Randomisation: web‐based random number generator generated allocation sequence, which was placed in sequential sealed opaque envelopes |
|
| Participants | preterm neonates requiring mechanical ventilation excluding: BW < 750 grams; major congenital anomaly; perinatal asphyxia; shock; prior air leak If did not undergo 24 hours of ventilation the patient was excluded from analysis 66 HFO, 84 SIMV |
|
| Interventions | SIMV or HFOV | |
| Outcomes | FiO₂, MAP, OI at 1, 6 and 24 hours Duration of ventilation Oxygen dependency at 28 days Hospital stay Survival IVH, PVL, PDA, ROP, pulmonary haemorrhage, ventilator associated pneumonia |
|
| Notes | SIMV delivered with SLE2000, HFOV with Draeger Babylog 8000 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Simple randomisation using a web‐based random number generator ": probably done |
| Allocation concealment (selection bias) | Low risk | "slip of paper bearing the intervention was kept in serially numbered opaque sealed envelopes": probably done |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | HFOV arm 17 died/left study; SIMV 23 died/left study |
Sun 2014.
| Study characteristics | ||
| Methods | Randomised single centre study computer‐generated randomisation after consent stratified by sex and gestational age |
|
| Participants | admitted to NICU with gestational age < 32 weeks; birth weight < 1500 grams; requiring mechanical ventilation for respiratory distress syndrome with PaO₂/FiO₂ < 200 mmHg exclusion: genetic metabolic diseases; congenital abnormalities; pneumothorax or grade III or IV IVH prior to randomisation randomised: 336 — 184 to SIMV, 182 to HFOV |
|
| Interventions | SIMV + PS vs HFOV | |
| Outcomes | Primary outcome: mortality and BPD Secondary outcomes: days of mechanical ventilation; hospital stay; surfactant requirement; ROP; pulmonary haemorrhage; PDA; NEC; pneumothorax. Moderate or severe disability at 18 months |
|
| Notes | SLE5000 and Servo‐i ventilators not analysed: 41 in SIMV, 37 in HFOV group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation plan": probably done |
| Allocation concealment (selection bias) | Low risk | Randomisation stratified by centre, by sex and gestational age using variable block size block randomisation: probably done |
| Blinding (performance bias and detection bias) All outcomes | High risk | Different ventilators used, no blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different ventilators used, no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Different ventilators used, no blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High risk for long‐term outcomes with approximately 30% lost to follow‐up Low risk for short‐term outcomes |
ACV: assist control ventilation BPD: bronchopulmonary dysplasia BW: birth weight CDH: congenital diaphragmatic hernia CHD: congenital heart disease CLD: chronic lung disease CMV: conventional mechanical ventilation CPAP: continuous positive airway pressure ECMO: extracorporeal membrane oxygenation GA: gestational age HFO: high‐frequency oscillation HFOV: high frequency oscillatory ventilation HFPPV: high‐frequency positive pressure ventilation IT: inspiratory time IVH: intraventricular haemorrhage MAP: mean airway pressure MAS: meconium aspiration syndrome NEC: necrotising enterocolitis OI: oxygenation index PDA: patent ductus arteriosus PIE: pulmonary interstitial emphysema PIP: peak inspiratory pressure PRVCV: pressure‐regulated volume control ventilation PS: pressure support PTV: patient‐triggered ventilation PVL: periventricular leukomalacia RDS: respiratory distress syndrome ROP: retinopathy of prematurity SIMV: synchronised intermittent mandatory ventilation SRT: surfactant replacement therapy VG: volume guarantee
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abd El‐Moneim 2005 | Short‐term cross over study |
| Abubakar 2005 | Randomised short‐term cross over study |
| Amitay 1993 | Not assigned respiratory support mode by randomisation |
| Bernstein 1993 | Not assigned respiratory support mode by randomisation |
| Bitondo 2012 | Adult crossover study of PSV controlled automatically or by clinician |
| Chan 1993b | Not assigned respiratory support mode by randomisation |
| Cheema 2001 | Short‐term comparison of volume guarantee synchronised ventilation to PTV or SIMV |
| Clavieras 2013 | Adult study comparing methods of pressure support |
| Cleary 1995 | Acute effects of synchronised ventilation |
| Dani 2006 | Short‐term randomised comparison of PSV with VG to HFOV |
| de la Oliva 2012 | Non‐randomised cross‐over study in the paediatric population |
| De Luca 2009 | Cross over trial comparing ACV in time‐cycled and flow‐cycled modality |
| deBoer 1993 | Not assigned respiratory support mode by randomisation |
| Dimitriou 1998 | Comparison of triggering devices |
| Duman 2012 | Comparison of volume targeting vs pressure limited ventilation in one mode of triggered ventilation |
| Durand 2001 | Randomised pilot study comparing HFOV to SIMV |
| Estay 2009 | Assess the effects of flow sensor dead space during SIMV |
| Firme 2005 | Randomised short‐term cross‐over study |
| Friedlich 1999 | Nasopharyngeal SIMV versus CPAP |
| Greenough 1986 | Not assigned respiratory support mode by randomisation |
| Greenough 1987a | Not assigned respiratory support mode by randomisation |
| Greenough 1987b | Not assigned respiratory support mode by randomisation |
| Greenough 1988a | Not assigned respiratory support mode by randomisation |
| Greenough 1988b | Not assigned respiratory support mode by randomisation |
| Greenough 1991 | Not assigned respiratory support mode by randomisation |
| Gupta 2008 | Quasi‐experimental cross‐over study |
| Guthrie 2005 | Randomised short‐term comparison of SIMV and mandatory minute volume |
| Guven 2013 | Comparison of volume guarantee versus pressure‐limited ventilation in one mode of triggered ventilation |
| Herrera 2002 | Acute effects of volume‐guaranteed SIMV |
| Hird 1990a | Not assigned respiratory support mode by randomisation |
| Hird 1990b | Not assigned respiratory support mode by randomisation |
| Hird 1991a | Not assigned respiratory support mode by randomisation |
| Hird 1991b | Not assigned respiratory support mode by randomisation |
| Hird 1991c | Not assigned respiratory support mode by randomisation |
| Hird 1991d | Not assigned respiratory support mode by randomisation |
| Hummler 1996 | Acute effects of synchronised ventilation |
| Hummler 1997 | Acute effects of mechanical ventilation |
| Hummler 2006 | Randomised short‐term cross‐over study |
| Jaber 2005 | Randomised short‐term cross‐over comparison of PSV and volume support ventilation |
| Jarreau 1996 | Acute outcome of synchronised ventilation |
| John 1994 | Comparison of triggering devices |
| Kapasi 2001 | Short‐term randomised comparison of ventilation modes |
| Keszler 2004 | Short‐term randomised comparison of ventilation modes with ACV with or without volume guarantee |
| Laubscher 1997 | Comparison of triggering devices |
| Lista 2006 | Acute effects of different levels of volume targeting during synchronised intermittent positive pressure ventilation within the context of a randomised trial |
| Luyt 2001 | Acute effects of PTV and conventional ventilation compared within the context of a randomised trial |
| McCallion 2007 | Observational study studying the effects of spontaneous breathing, triggered and untriggered inflations |
| Migliori 2003 | Non randomised comparison of pressure support synchronised ventilation and SIMV |
| Mitchell 1989 | Not assigned respiratory support mode by randomisation |
| Mizuno 1994 | Not assigned respiratory support mode by randomisation |
| Moretti 1999 | Nasal SIPPV to nasal CPAP |
| Mrozek 2000 | Randomised comparison (short‐term) of volume‐targeted synchronised ventilation and CMV |
| Nacoti 2012 | Paediatric study comparing PSV to PSV with sighs. Non‐randomised. |
| Nafday 2005 | Randomised comparison (short‐term) of pressure support with volume guarantee |
| Nakae 1998 | Comparison of triggering devices |
| Nikischin 1996 | Comparison of triggering devices |
| Nishimura 1995 | Comparison of triggering devices |
| Olsen 2002 | Cross‐over trial comparing pressure support with volume guarantee synchronised ventilation to SIMV |
| Osorio 2005 | Cross‐over short‐term comparison of SIMV with or without pressure support |
| Patel 2009 | Cross‐over trial comparing SIMV and SIMV with pressure support |
| Polimeni 2006 | Short‐term cross‐over study |
| Scopesi 2007 | Short‐term cross‐over study |
| Servant 1992 | Not assigned respiratory support mode by randomisation |
| Smith 1997 | Acute outcome of synchronised ventilation |
| Takeuchi 1994 | Not assigned respiratory support mode by randomisation |
| Thiagarajan 2004 | Comparison of triggering devices |
| Upton 1990 | Not assigned respiratory support mode by randomisation |
| Vishveshwara 1991 | Not assigned respiratory support mode by randomisation |
| Wheeler 2012 | Randomised cross‐over trial to study the effects of differing back up rates within one mode of triggered ventilation |
Contributions of authors
Professor Anne Greenough and Professor Anthony Milner have co‐authored all issues of this review.
Dr. Murthy coauthored this 2012 review update.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
AG received no grants or speaker fees for the three years prior to the publication of this review.
TER has no interest to declare
AS has no interest to declare
VM has no interest to declare
ADM has no interest to declare
Edited (no change to conclusions)
References
References to studies included in this review
Amini 2013 {published data only}
- Amini E, Nayeri FS, Hemati A, Esmaeilinia T, Nili F, Dalili H, et al. Comparison of High Frequency Positive Pressure Mechanical Ventilation (HFPPV) With Conventional Method in the Treatment of Neonatal Respiratory Failure. Iranian Red Crescent Medical Journal 2013;15(3):183-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baumer 2000 {published data only}
- Baumer JH. International randomised controlled trial of patient triggered ventilation in neonatal respiratory distress syndrome. Archives of Disease in Childhood 2000;82(1):F5-F10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Beresford 2000 {published data only}
- Beresford MW, Shaw NJ, Manning D. Randomised controlled trial of patient triggered and conventional fast rate ventilation in neonatal respiratory distress syndrome. Archives of Disease in Childhood 2000;82(1):F14-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bernstein 1996 {published data only}
- Bernstein G, Mannino FL, Heldt GP, Callahan JD, Bull DH, Sola A, et al. Randomized multicenter trial comparing synchronized and conventional intermittent mandatory ventilation in neonates. Journal of Pediatrics 1996;128(4):453-63. [DOI] [PubMed] [Google Scholar]
Chan 1993 {published data only}
- Chan V, Greenough A. Randomised controlled trial of weaning by patient triggered ventilation or conventional ventilation. European Journal of Pediatrics 1993;152(1):51-4. [DOI] [PubMed] [Google Scholar]
Chan 1994 {published data only}
- Chan V, Greenough A. Comparison of weaning by patient triggered ventilation or synchronous mandatory intermittent ventilation. Acta Paediatrica 1994;83(3):335-7. [DOI] [PubMed] [Google Scholar]
Chen 1997 {published data only}
- Chen J-Y, Ling U-P, Chen J-H. Comparison of synchronized and conventional intermittent mandatory ventilation in neonates. Acta Paediatrica Japonica 1997;39(5):578-83. [DOI] [PubMed] [Google Scholar]
Courtney 2002a {published data only}
- Courtney SE, Durand DJ, Asselin JM, Hudak ML, Aschner JL, Shoemaker CT. High-Frequency Oscillatory ventilation versus conventional mechanical ventilation for very-low birth-weight infants. New England Journal of Medicine 2002;347(9):643-52. [PMID: ] [DOI] [PubMed] [Google Scholar]
Craft 2003a {published data only}
- Craft AP, Bhandari V, Finer NN. The sy-fi study: a randomized prospective trial of synchronized intermittent mandatory ventilation versus a high-frequency flow interrupter in infants less than 1000 g. Journal of Perinatology 2003;23(1):14-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
D'Angio 2005 {published data only}
- D'Angio CT, Chess PR, Kovacs SJ, Sinkin RA, Phelps DL, Kendig JW, et al. Pressure-regulated volume control ventilation vs synchronized intermittent mandatory ventilation for very low birthweight infants. Archives of Pediatric and Adolescent Medicine 2005;159(9):868-75. [DOI] [PubMed] [Google Scholar]
Dimitriou 1995a {published data only}
- Dimitriou G, Greenough A, Giffin FJ, Chan V. Synchronous intermittent mandatory ventilation modes versus patient triggered ventilation during weaning. Archives of Disease in Childhood 1995;72(3):F188-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dimitriou 1995b {published data only}
- Dimitriou G, Greenough A, Giffin FJ, Chan V. Synchronous intermittent mandatory ventilation modes versus patient triggered ventilation during weaning. Archives of Disease in Childhood 1995;72(3):F188-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donn 1994 {published data only}
- Donn SM, Nicks JJ, Becker MA. Flow-synchronized ventilation of preterm infants with respiratory distress syndrome. Journal of Perinatology 1994;14(2):90-4. [PubMed] [Google Scholar]
Erdemir 2014 {published data only}
- Erdemir A, Kahramaner Z, Turkoglu E, Cosar H, Sutcuoglu S, Ozer EA. Effects of synchronized intermittent mandatory ventilation versus pressure support plus volume guarantee ventilation in the weaning phase of preterm infants*. Pediatric Critical Care Medicine 2014;15(3):236-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Heicher 1981 {published data only}
- Heicher DA, Kasting DS, Harrod JR. Prospective clinical comparison of two methods for mechanical ventilation of neonates: rapid rate and short inspiratory time versus slow rate and long inspiratory time. Journal of Pediatrics 1981;98(6):957-61. [DOI] [PubMed] [Google Scholar]
Liu 2011 {published data only}
- Liu CQ, Cui Z, Xia YF, Ma Li, Fan LL. Randomized controlled study of targeted tidal volume ventilation for treatment of severe neonatal respiratory distress syndrome [Chinese]. Zhongguo Dang Dai Er Ke Za Zhi 2011;13(9):696-9. [PMID: ] [PubMed] [Google Scholar]
OCTAVE 1991 {published data only}
- Oxford Region Controlled Trial of Artificial Ventilation (OCTAVE) Study Group. Multicentre randomised controlled trial of high against low frequency positive pressure ventilation. Archives of Disease in Childhood 1991;66(7 Spec No):770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patel 2012 {published data only}
- Patel DS, Murthy V, Hannam S, Lee S, Rafferty GF, Greenough A. Randomised weaning trial comparing assist control to pressure support ventilation. Archives of Disease in Childhood. Fetal and Neonatal Edition 2012;97(6):F429-33. [PMID: ] [DOI] [PubMed] [Google Scholar]
Pohlandt 1992 {published data only}
- Pohlandt F, Saule H, Schrîder H, Leonhardt A, Hîrnchen H, Wolff C, Bernsau U, Oppermann H-C. Decreased incidence of extra-alveolar air leakage or death prior to air leakage in high versus low rate positive pressure ventilation: results of a randomised seven-centre trial in preterm infants. European Journal of Pediatrics 1992;151(12):904-9. [DOI] [PubMed] [Google Scholar]
Reyes 2006 {published data only}
- Reyes ZC, Claure N, Tauscher MK, D'Ugard C, Vanbuskirk S, Bancalari E. Randomized, controlled trial comparing synchronized intermittent mandatory ventilation and synchronized intermittent mandatory ventilation plus pressure support in preterm infants. Pediatrics 2006;118(4):1409-17. [DOI] [PubMed] [Google Scholar]
Singh 2012 {published data only}
- Singh S N, Malik G K, Prashanth G P, Singh A, Kumar M. High frequency oscillatory ventilation versus synchronized intermittent mandatory ventilation in preterm neonates with hyaline membrane disease: a randomized controlled trial. Indian Journal of Pediatrics 2012;49(5):405-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sun 2014 {published data only}
- Sun H, Cheng R, Kang W, Xiong H, Zhou C, Zhang Y, et al. High-frequency oscillatory ventilation versus synchronized intermittent mandatory ventilation plus pressure support in preterm infants with severe respiratory distress syndrome. Respiratory Care 2014;59(2):159-69. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abd El‐Moneim 2005 {published data only}
- Abd El-Moneim ES, Fuerste HO, Krueger M, Elmagd AA, Brandis M, Schulte-Moenting J, et al. Pressure support ventilation combined with volume guarantee versus synchronized intermittent mandatory ventilation: a pilot crossover trial in premature infants in their weaning phase. Critical Care Medicine 2005;6(3):286-92. [DOI] [PubMed] [Google Scholar]
Abubakar 2005 {published data only}
- Abubakar K, Keszler M. Effect of volume guarantee combined with assist/control vs synchronized intermittent mandatory ventilation. Journal of Perinatology 2005;25(10):638-42. [DOI] [PubMed] [Google Scholar]
Amitay 1993 {published data only}
- Amitay M, Etches PC, Finer NN, Maidens JM. Synchronous mechanical ventilation of the neonate with respiratory disease. Critical Care Medicine 1993;21(1):118-24. [DOI] [PubMed] [Google Scholar]
Bernstein 1993 {published data only}
- Bernstein G, Cleary JP, Heldt GP, Rosas JF, Schellenberg l, Mannino FL. Response time and reliability of three neonatal patient triggered ventilators. American Review of Respiratory Disease 1993;148(2):358-64. [DOI] [PubMed] [Google Scholar]
Bitondo 2012 {published data only}
- Bitondo M M, Aguirre-Bermeo H M, Moccaldo A, De Santis P, Bernini V, Tersali A, et al. Patient-ventilator asynchrony during conventional or automated pressure support ventilation in difficult-to-wean patients. Critical Care 2012;16(Suppl 1):P126. [DOI: 10.1186/cc10733] [DOI] [Google Scholar]
Chan 1993b {published data only}
- Chan V, Greenough A. Neonatal patient triggered ventilators. Performance in acute and chronic lung disease. Br J Int Care 1993;3:216-9. [Google Scholar]
Cheema 2001 {published data only}
- Cheema IU, Ahluwalia JS. Feasibility of tidal volume-guided ventilation in newborn infants: a randomized, crossover trial using the volume guarantee modality. Pediatrics 2001;107:1323-8. [DOI] [PubMed] [Google Scholar]
Clavieras 2013 {published data only}
- Clavieras N, Wysocki M, Coisel Y, Galia F, Conseil M, Chanques G, et al. Prospective randomized crossover study of a new closed-loop control system versus pressure support during weaning from mechanical ventilation. Anesthesiology 2013;119(3):631-41. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cleary 1995 {published data only}
- Cleary JP, Bernstein G, Mannino FL, Heldt BP. Improved oxygenation during synchronized intermittent mandatory ventilation in neonates with respiratory distress syndrome: a randomized, crossover study. Journal of Pediatrics 1995;126(3):407-11. [DOI] [PubMed] [Google Scholar]
Dani 2006 {published data only}
- Dani C, Bertini G, Pezzati M, Filippi L, Pratesi S, Caviglioli C, et al. Effects of pressure support ventilation plus volume guarantee vs high-frequency oscillatory ventilation on lung inflammation in preterm infants. Pediatric Pulmonology 2006;41(3):242-9. [DOI] [PubMed] [Google Scholar]
deBoer 1993 {published data only}
- Boer RC, Jones A, Ward PS, Baumer JH. Long term trigger ventilation in neonatal respiratory distress syndrome. Archives of Disease in Childhood 1993;68:308-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
de la Oliva 2012 {published data only}
- la Oliva P, Schuffelmann C, Gomez-Zamora A, Villar J, Kacmarek R M. Asynchrony, neural drive, ventilatory variability and COMFORT: NAVA versus pressure support in pediatric patients. A non-randomized cross-over trial. Intensive Care Med 2012;38(5):838-46. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Luca 2009 {published data only}
- De Luca D, Conti G, Piastra M, Paolillo PM. Flow-cycled versus time-cycled sIPPV in preterm babies with RDS: a breath-to-breath randomised cross-over trial. Archives of Disease in Childhood. Fetal and Neonatal edition 2009;94(6):F397-401. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dimitriou 1998 {published data only}
- Dimitriou G, Greenough A, Lauscher B, Yamaguchi N. Comparison of airway pressure triggered and airflow triggered ventilation in immature infants. Acta Paediatrica 1998;87(12):1256-60. [DOI] [PubMed] [Google Scholar]
Duman 2012 {published data only}
- Duman N, Tuzun F, Sutcuoglu S, Yesilirmak C D, Kumral A, Ozkan H. Impact of volume guarantee on synchronized ventilation in preterm infants: a randomized controlled trial. Intensive Care Med 2012;38(8):1358-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Durand 2001 {published data only}
- Durand DJ, Asselin JM, Hudak ML, Aschner JL, McArtor RD, Cleary JP, et al. Early high-frequency oscillatory ventilation versus synchronized intermittent mandatory ventilation in very low birth weight infants: a pilot study of two ventilation protocols. Journal of Perinatology 2001;21(4):221-9. [DOI] [PubMed] [Google Scholar]
Estay 2009 {published data only}
- Estay A, Claure N, D'Ugard C, Organero R, Bancalari E. Effects of instrumental dead space reduction during weaning from synchronized ventilation in preterm infants. Journal of Perinatology 2010;30(7):479-83. [PMID: ] [DOI] [PubMed] [Google Scholar]
Firme 2005 {published data only}
- Firme SR, McEvoy CT, Alconcel C, Tanner J, Durand M. Episodes of hypoxemia during synchronized intermittent mandatory ventilation in ventilator-dependent very low birth weight infants. Pediatric Pulmonology 2005;40(1):9-14. [DOI] [PubMed] [Google Scholar]
Friedlich 1999 {published data only}
- Friedlich P, Lecart C, Posen R, Ramicone E, Chan L, Ramanathan R. A randomized trial of nasopharyngeal synchronized intermittent mandatory ventilation versus nasopharyngeal continuous positive airway pressure in very low birthweight infants after extubation. Journal of Perinatology 1999;19(6 Pt 1):413-18. [DOI] [PubMed] [Google Scholar]
Greenough 1986 {published data only}
- Greenough A, Morley CJ, Pool J. Fighting the ventilator - are fast rates an effective alternative to paralysis? Early Human Development 1986;13(2):189-94. [DOI] [PubMed] [Google Scholar]
Greenough 1987a {published data only}
- Greenough A, Pool J, Greenall F, Morley CJ, Gamsu H. Comparison of different rates of artificial ventilation in preterm neonates with the respiratory distress syndrome. Acta Paediatrica Scandinavica 1987;76(5):706-12. [DOI] [PubMed] [Google Scholar]
Greenough 1987b {published data only}
- Greenough A, Greenall F, Gamsu H. Synchronous respiration - which ventilator rate is best? Acta Paediatrica Scandinavica 1987;76(5):713-18. [DOI] [PubMed] [Google Scholar]
Greenough 1988a {published data only}
- Greenough A, Greenall F. Patient triggered ventilation in premature neonates. Archives of Disease in Childhood 1988;63:77-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenough 1988b {published data only}
- Greenough A, Pool J. Neonatal patient triggered ventilation. Archives of Disease in Childhood 1988;63:394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenough 1991 {published data only}
- Greenough A, Hird MF, Chan V. Airway pressure triggered ventilation for preterm neonates. Journal of Perinatal Medicine 1991;19(6):471-6. [DOI] [PubMed] [Google Scholar]
Gupta 2008 {published data only}
- Gupta S, Sinha SK, Donn SM. The effect of two levels of pressure support ventilation on tidal volume delivery and minute ventilation in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2009;94(2):F80-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Guthrie 2005 {published data only}
- Guthrie SO, Lynn C, Lafleur BJ, Donn SM, Walsh WF. A crossover analysis of mandatory minute ventilation compared to synchronized intermittent mandatory ventilation in neonates. Journal of Perinatology 2005;25(10):643-6. [DOI] [PubMed] [Google Scholar]
Guven 2013 {published data only}
- Guven S, Bozdag S, Saner H, Cetinkaya M, Yazar A S, Erguven M. Early neonatal outcomes of volume guaranteed ventilation in preterm infants with respiratory distress syndrome. J Matern Fetal Neonatal Med 2013;26(4):396-401. [PMID: 23039373 ] [DOI] [PubMed] [Google Scholar]
Herrera 2002 {published data only}
- Herrera CM, Gerhardt T, Claure N, Everett R, Musante G, Thomas C, et al. Effects of volume-guaranteed synchronized intermittent mandatory ventilation in preterm infants recovering from respiratory failure. Pediatrics 2002;110(3):529-33. [DOI] [PubMed] [Google Scholar]
Hird 1990a {published data only}
- Hird MF, Greenough A. Causes of failure of neonatal patient triggered ventilation. Early Human Development 1990;23(2):101-8. [DOI] [PubMed] [Google Scholar]
Hird 1990b {published data only}
- Hird MF, Greenough A. Gestational age: an important influence on the success of patient triggered ventilation. Clinical Physics and Physiological Measurement 1990;11(4):307-12. [DOI] [PubMed] [Google Scholar]
Hird 1991a {published data only}
- Hird MF, Greenough A. Randomised trial of patient triggered ventilation versus high frequency positive pressure ventilation in acute respiratory distress. Journal of Perinatal Medicine 1991;19(5):379-84 (listed in Wallach EE (ed) Current Opinion in Obstetrics & Gynecology, February 1993). [DOI] [PubMed] [Google Scholar]
Hird 1991b {published data only}
- Hird MF, Greenough A. Patient triggered ventilation in chronically ventilator-dependent infants. European Journal of Pediatrics 1991;150(10):732-4. [DOI] [PubMed] [Google Scholar]
Hird 1991c {published data only}
- Hird MF, Greenough A. Patient triggered ventilation using a flow triggered system. Archives of Disease in Childhood 1991;66(10 Spec No):1140-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hird 1991d {published data only}
- Hird MF, Greenough A. Comparison of triggering systems for neonatal patient triggered ventilation. Archives of Disease in Childhood 1991;66(4 Spec No):426-8 (abstracted in Clinical Digest Series - Pediatrics/Neonatology, Northbrook IL, USA). [DOI] [PMC free article] [PubMed] [Google Scholar]
Hummler 1996 {published data only}
- Hummler H, Gerhardt T, Gonzalez A, Claure N, Everett R, Bancalari E. Influence of different methods of synchronized mechanical ventilation on ventilation, gas exchange, patient effort and blood pressure fluctuations in premature neonates. Pediatric Pulmonology 1996;22(5):305-13. [DOI] [PubMed] [Google Scholar]
Hummler 1997 {published data only}
- Hummler H, Gerhardt T, Gonzalez A, Claure N, Everett R, Bancalari E. Increased incidence of sighs (augmented inspiratory eforts) during synchronized intermittent mandatory ventilation (SIMV) in preterm neonates. Pediatric Pulmonology 1997;24(3):195-203. [DOI] [PubMed] [Google Scholar]
Hummler 2006 {published data only}
- Hummler H, Engelmann A, Pohlandt F, Franz AR. Volume-controlled intermittent mandatory ventilation in preterm infants with hypoxemic episodes. Intensive Care Medicine 2006;32(4):577-84. [DOI] [PubMed] [Google Scholar]
Jaber 2005 {published data only}
- Jaber S, Delay JM, Matecki S, Sebbane M, Eledjam JJ, Brochard L. Volume-guaranteed pressure-support ventilation facing acute changes in ventilatory demand. Intensive Care Medicine 2005;31(9):1181-8. [DOI] [PubMed] [Google Scholar]
Jarreau 1996 {published data only}
- Jarreau P-H, Moriette G, Mussat P, Mariette C, Mohanna A, Harf A, et al. Patient-triggered ventilation decreases the work of breathing in neonates. American Journal of Respiratory and Critical Care Medicine 1996;153(3):1176-81. [DOI] [PubMed] [Google Scholar]
John 1994 {published data only}
- John J, Bjîrklung LJ, Svenningsen NW, Jonson B. Airway and body surface sensors for triggering in neonatal ventilation. Acta Paediatrica 1994;83(9):903-9. [DOI] [PubMed] [Google Scholar]
Kapasi 2001 {published data only}
- Kapasi M, Fujino Y, Kirmse M, Catlin EA, Kacmarek RM. Effort and work of breathing in neonates during assisted patient triggered ventilation. Pediatric Critical Care Medicine 2001;2:9-16. [DOI] [PubMed] [Google Scholar]
Keszler 2004 {published data only}
- Keszler M, Abubakar K. Volume guarantee: stability of tidal volume and incidence of hypocarbia. Pediatric Pulmonology 2004;38(3):240-5. [DOI] [PubMed] [Google Scholar]
Laubscher 1997 {published data only}
- Laubscher B Greenough A, Kavadia V. Comparison of body surface and airway triggered ventilation in extremely premature infants. Acta Paediatrica 1997;86(1):102-4. [DOI] [PubMed] [Google Scholar]
Lista 2006 {published data only}
- Lista G, Castoldi F, Fontana P, Reali R, Reggiani A, Bianchi S, et al. Lung inflammation in preterm infants with respiratory distress syndrome: effects of ventilation with different tidal volumes. Pediatric Pulmonology 2006;41(4):357-63. [DOI] [PubMed] [Google Scholar]
Luyt 2001 {published data only}
- Luyt K, Wright D, Baumer JH. Randomised study comparing extent of hypocarbia in preterm infants during conventional and patient triggered ventilation. Archives of Disease in Childhood. Fetal and Neonatal edition 2001;84(1):F14-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCallion 2007 {published data only}
- McCallion N, Lau R, Morley CJ, Dargaville PA. Neonatal volume guarantee ventilation: effects of spontaneous breathing, triggered and untriggered inflations. Archives of Disease in Childhood. Fetal and Neonatal Edition 2008;93(1):F36-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Migliori 2003 {published data only}
- Migliori C, Cavazza A, Motta M, Chirico G. Effect on respiratory function of pressure support ventilation versus synchronised intermittent mandatory ventilation in preterm infants. Pediatric Pulmonology 2003;35(5):364-7. [DOI] [PubMed] [Google Scholar]
Mitchell 1989 {published data only}
- Mitchell A, Greenough A, Hird MF. Limitations of neonatal patient triggered ventilation. Archives of Disease in Childhood 1989;64:924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mizuno 1994 {published data only}
- Mizuno K, Takeuchi T, Itabashi K, Okuyama K. Efficacy of synchronized IMV on weaning neonates from the ventilator. Acta Paediatrica Japonica 1994;36(2):162-6. [DOI] [PubMed] [Google Scholar]
Moretti 1999 {published data only}
- Moretti C, Gizzi C, Papoff P, Lampariello S, Capoferri M, Calcagnini G, Bucci G. Comparing the effects of nasal synchronized intermittent positive pressure ventilation (nSIPPV) and nasal continuous positive airway pressure (nCPAP) after extubation in very low birth weight infants. Early Human Development 1999;56(2–3):167–77. [DOI] [PubMed] [Google Scholar]
Mrozek 2000 {published data only}
- Mrozek JD, Bendel-Stenzel EM, Meyers PA, Bing DR, Connett JE, Mammel MC. Randomized controlled trial of volume-targeted synchronized ventilation and conventional intermittent mandatory ventilation following initial exogenous surfactant therapy. Pediatric Pulmonology 2000;29(1):11-8. [DOI] [PubMed] [Google Scholar]
Nacoti 2012 {published data only}
- Nacoti M, Spagnolli E, Bonanomi E, Barbanti C, Cereda M, Fumagalli R. Sigh improves gas exchange and respiratory mechanics in children undergoing pressure support after major surgery. Minerva Anestesiologica 2012;78(8):920-9. [PMID: ] [PubMed] [Google Scholar]
Nafday 2005 {published data only}
- Nafday SM, Green RS, Lin J, Brion LP, Ochshorn I, Holzman IR. Is there an advantage of using pressure support ventilation with volume guarantee in the initial management of preterm infants with respiratory distress syndrome? A pilot study. Journal of Perinatology 2005;25(3):193-7. [DOI] [PubMed] [Google Scholar]
Nakae 1998 {published data only}
- Nakae Y, Yamakage M, Horikawa D, Aimono M, Tamiya K, Namiki A. Triggering delay time and work of breathing in three paediatric patient triggered ventilators. Canadian Journal of Anaesthesia 1998;45(3):261-5. [DOI] [PubMed] [Google Scholar]
Nikischin 1996 {published data only}
- Nikischin W, Gerhardt T, Everett R, Gonzalez A, Hummler H, Bancalari E. Patient triggered ventilation: a comparison of tidal volume and chest wall and abdominal motion as trigger signals. Pediatric Pulmonology 1996;22(1):28-34. [DOI] [PubMed] [Google Scholar]
Nishimura 1995 {published data only}
- Nishimura M, Hess D, Kacmarek RM. The response of flow-triggered infant ventilators. American Journal of Respiratory and Critical Care Medicine 1995;152(6 Pt 1):1901-9. [DOI] [PubMed] [Google Scholar]
Olsen 2002 {published data only}
- Olsen SL, Thibeault DW, Truog WE. Crossover trial comparing pressure support with synchronized intermittent mandatory ventilation. Journal of Perinatology 2002;22(6):461-6. [DOI] [PubMed] [Google Scholar]
Osorio 2005 {published data only}
- Osorio W, Claure N, D'Ugard C, Athavale K, Bancalari E. Effects of pressure support during an acute reduction of synchronized intermittent mandatory ventilation in preterm infants. Journal of Perinatology 2005;25(6):412-6. [DOI] [PubMed] [Google Scholar]
Patel 2009 {published data only}
- Patel DS, Rafferty GF, Lee S, Hannam S, Greenough A. Work of breathing during SIMV with and without pressure support. Archives of Disease in Childhood 2009;94(6):434-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Polimeni 2006 {published data only}
- Polimeni V, Claure N, D'Ugard C, Bancalari E. Effects of volume-targeted synchronized intermittent mandatory ventilation on spontaneous episodes of hypoxemia in preterm infants. Biology of the Neonate 2006;89(1):50-5. [DOI] [PubMed] [Google Scholar]
Scopesi 2007 {published data only}
- Scopesi F, Calevo MG, Rolfe P, Arioni C, Traggiai C, Risso FM, et al. Volume targeted ventilation (volume guarantee) in the weaning phase of premature newborn infants. Paediatric Pulmonology 2007;42(10):864-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Servant 1992 {published data only}
- Servant GM, Nicks JJ, Donn SM, Bandy KP, Lathrop C, Dechert RE. Feasibility of applying flow-synchronized ventilation to very low birthweight infants. Respiratory Care 1992;37:249-53. [Google Scholar]
Smith 1997 {published data only}
- Smith KM, Walig TM, Bing DR, Georgieff MK, Boros SJ, Mammel MC. Lower respiratory rates without decreases in oxygen consumption during neonatal synchronized intermittent mandatory ventilation. Intensive Care Medicine 1997;23(4):463-8. [DOI] [PubMed] [Google Scholar]
Takeuchi 1994 {published data only}
- Takeuchi MK, Itabashi K, Okuyama K. Efficacy of synchronized IMV on weaning neonates from the ventilator. Acta Paediatrica Japonica 1994;36(2):162-6. [DOI] [PubMed] [Google Scholar]
Thiagarajan 2004 {published data only}
- Thiagarajan RR, Coleman DM, Bratton SL, Watson RS, Martin LD. Inspiratory work of breathing is not decreased by flow-triggered sensing during spontaneous breathing in children receiving mechanical ventilation: a preliminary report. Pediatric Critical Care Medicine 2004;5(4):375-8. [DOI] [PubMed] [Google Scholar]
Upton 1990 {published data only}
- Upton CJ, Milner AD, Stokes GM. The effect of changes in inspiratory time on neonatal triggered ventilation. European Journal of Pediatrics 1990;149(9):648-50. [DOI] [PubMed] [Google Scholar]
Vishveshwara 1991 {published data only}
- Vishveshwara N, Freeman B, Peck M, Caliwag N, Shook S, Rajani KB. Patient triggered synchronized assisted ventilation of newborns: report of a preliminary study and three years' experience. Journal of Perinatology 1991;11(4):347-54. [PubMed] [Google Scholar]
Wheeler 2012 {published data only}
- Wheeler KI, Morley CJ, Hooper SB, Davis PG. Lower back-up rates improve ventilator triggering during assist-control ventilation: a randomized crossover trial. Journal of Perinatology 2012;32(2):111-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Greenough 1998
- Greenough A, Milner AD, Dimitriou G. Synchronized mechanical ventilation for respiratory support in newborn infants. Cochrane Database of Systematic Reviews 1998, Issue 1. [DOI: 10.1002/14651858.CD000456] [DOI] [PubMed] [Google Scholar]
Greenough 2001
- Greenough A, Milner AD, Dimitriou G. Synchronized mechanical ventilation for respiratory support in newborn infants. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD000456] [DOI] [PubMed] [Google Scholar]
Greenough 2004
- Greenough A, Milner AD, Dimitriou G. Synchronized mechanical ventilation for respiratory support in newborn infants. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD000456.pub2] [DOI] [PubMed] [Google Scholar]
Greenough 2008
- Greenough A, Dimitriou G, Prendergast M, Milner AD. Synchronized mechanical ventilation for respiratory supporting newborn infants. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD000456.pub3] [DOI] [PubMed] [Google Scholar]


