Abstract
Background
Ovarian cancer is the sixth most common cancer among women. In addition to diagnosis and staging, primary surgery is performed to achieve optimal cytoreduction (surgical efforts aimed at removing the bulk of the tumour) as the amount of residual tumour is one of the most important prognostic factors for survival of women with epithelial ovarian cancer. An optimal outcome of cytoreductive surgery remains a subject of controversy to many practising gynae‐oncologists. The Gynaecologic Oncology group (GOG) currently defines 'optimal' as having residual tumour nodules each measuring 1 cm or less in maximum diameter, with complete cytoreduction (microscopic disease) being the ideal surgical outcome. Although the size of residual tumour masses after surgery has been shown to be an important prognostic factor for advanced ovarian cancer, it is unclear whether it is the surgical procedure that is directly responsible for the superior outcome that is associated with less residual disease.
Objectives
To evaluate the effectiveness and safety of optimal primary cytoreductive surgery for women with surgically staged advanced epithelial ovarian cancer (stages III and IV).
To assess the impact of various residual tumour sizes, over a range between zero and 2 cm, on overall survival.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 3) and the Cochrane Gynaecological Cancer Review Group Trials Register, MEDLINE and EMBASE (up to August 2010). We also searched registers of clinical trials, abstracts of scientific meetings, reference lists of included studies and contacted experts in the field.
Selection criteria
Retrospective data on residual disease from randomised controlled trials (RCTs) or prospective and retrospective observational studies which included a multivariate analysis of 100 or more adult women with surgically staged advanced epithelial ovarian cancer and who underwent primary cytoreductive surgery followed by adjuvant platinum‐based chemotherapy. We only included studies that defined optimal cytoreduction as surgery leading to residual tumours with a maximum diameter of any threshold up to 2 cm.
Data collection and analysis
Two review authors independently abstracted data and assessed risk of bias. Where possible, the data were synthesised in a meta‐analysis.
Main results
There were no RCTs or prospective non‐RCTs identified that were designed to evaluate the effectiveness of surgery when performed as a primary procedure in advanced stage ovarian cancer.
We found 11 retrospective studies that included a multivariate analysis that met our inclusion criteria. Analyses showed the prognostic importance of complete cytoreduction, where the residual disease was microscopic that is no visible disease, as overall (OS) and progression‐free survival (PFS) were significantly prolonged in these groups of women. PFS was not reported in all of the studies but was sufficiently documented to allow firm conclusions to be drawn.
When we compared suboptimal (> 1 cm) versus optimal (< 1 cm) cytoreduction the survival estimates were attenuated but remained statistically significant in favour of the lower volume disease group There was no significant difference in OS and only a borderline difference in PFS when residual disease of > 2 cm and < 2 cm were compared (hazard ratio (HR) 1.65, 95% CI 0.82 to 3.31; and HR 1.27, 95% CI 1.00 to 1.61, P = 0.05 for OS and PFS respectively).
There was a high risk of bias due to the retrospective nature of these studies where, despite statistical adjustment for important prognostic factors, selection bias was still likely to be of particular concern.
Adverse events, quality of life (QoL) and cost‐effectiveness were not reported by treatment arm or to a satisfactory level in any of the studies.
Authors' conclusions
During primary surgery for advanced stage epithelial ovarian cancer all attempts should be made to achieve complete cytoreduction. When this is not achievable, the surgical goal should be optimal (< 1 cm) residual disease. Due to the high risk of bias in the current evidence, randomised controlled trials should be performed to determine whether it is the surgical intervention or patient‐related and disease‐related factors that are associated with the improved survival in these groups of women. The findings of this review that women with residual disease < 1 cm still do better than women with residual disease > 1 cm should prompt the surgical community to retain this category and consider re‐defining it as 'near optimal' cytoreduction, reserving the term 'suboptimal' cytoreduction to cases where the residual disease is > 1 cm (optimal/near optimal/suboptimal instead of complete/optimal/suboptimal).
Keywords: Adolescent; Adult; Aged; Female; Humans; Middle Aged; Young Adult; Carcinoma, Ovarian Epithelial; Neoplasm, Residual; Neoplasms, Glandular and Epithelial; Neoplasms, Glandular and Epithelial/mortality; Neoplasms, Glandular and Epithelial/pathology; Neoplasms, Glandular and Epithelial/surgery; Ovarian Neoplasms; Ovarian Neoplasms/mortality; Ovarian Neoplasms/pathology; Ovarian Neoplasms/surgery; Retrospective Studies; Survival Analysis; Tumor Burden
Plain language summary
Clear survival benefit is achieved if all or most (< 1 cm remaining) of the tumour after primary surgical treatment for advanced epithelial ovarian cancer is removed
Ovarian cancer is a cancerous growth arising from different parts of the ovary. It is the sixth most common cancer among women. Most ovarian cancers are classified as epithelial. Ovarian epithelial cancer is a disease in which malignant (cancer) cells form in the tissue covering the ovary and most cases are epithelial. Primary surgery is performed to achieve optimal cytoreduction (surgical efforts aiming at removing the bulk of the tumour) as the amount of tumour that remains after surgery (residual disease) is one of the most important factors that is taken into account when determining a prognosis (prognostic factor) for survival of epithelial ovarian cancer. Optimal cytoreductive surgery remains a subject of controversy to many practising obstetric gynaecologists who specialise in the diagnosis and treatment of women with cancer of the reproductive organs (gynae‐oncologists). The Gynaecologic Oncology Group (GOG) currently defines 'optimal' as having a small aggregation of remaining cancer cells after surgery (residual tumour nodules) each measuring 1 cm or less in maximum diameter, with complete cytoreduction (microscopic disease) being the ideal surgical outcome. Although the size of residual tumour masses after surgery has been shown to be an important prognostic factor for advanced ovarian cancer, there is limited evidence to support the conclusion that the surgical procedure is directly responsible for the superior outcome associated with less residual disease. This review assessed overall and progression‐free survival of optimal primary cytoreductive surgery for women with advanced epithelial ovarian cancer (stages III and IV). We found 11 retrospective studies that included more than 100 women and used a multivariate analysis (used statistical adjustment for important prognostic factors) and met our inclusion criteria. Analyses showed the prognostic importance of complete cytoreduction, where the residual disease is microscopic with no visible disease, as overall (OS) and progression‐free survival (PFS) were significantly prolonged in these groups of women. PFS was not reported in all of the studies but was sufficiently documented to allow firm conclusions to be drawn. When we compared suboptimal (> 1 cm) versus optimal (< 1 cm) cytoreduction the survival estimates were attenuated but remained statistically significant in favour of the lower volume disease group, but there was no significant difference in OS and only a borderline difference in PFS when residual disease of > 2 cm and < 2 cm were compared. There was a high risk of bias due to the retrospective nature of these studies. Adverse events, quality of life (QoL) and cost‐effectiveness were not reported by treatment arm or to a satisfactory level in any of the studies. During primary surgery for advanced stage epithelial ovarian cancer, all attempts should be made to achieve complete cytoreduction. When this is not achievable, the surgical goal should be optimal (< 1 cm) residual disease. Due to the high risk of bias in the current evidence, randomised controlled trials should be performed to determine whether it is the surgical intervention or patient‐related and disease‐related factors that are associated with the improved survival in these groups of women.
Background
Description of the condition
Ovarian cancer is the sixth most common cancer among women (GLOBOCAN 2002). Worldwide there are more than 200,000 new cases of ovarian cancer each year, accounting for around 4% of all cancers diagnosed in women. A woman's risk of developing cancer of the ovary by age 75 years varies between countries, ranging from 0.5% to 1.6%, corresponding to an age‐standardised rate of 5 to 14 cases per year in 100,000 women (IARC 2002). More than 90% of ovarian cancers are surface epithelial tumours as they arise from the surface covering the ovary or the lining of ovarian cysts (Quirk 2005).
The spread of the disease is described using the International Federation of Gynecology and Obstetrics (FIGO) staging, where stage I disease is confined to the ovaries; stage II disease is confined to the true pelvis, stage III disease is an abdominal disease where there is spread to the lining (peritoneum) of the abdominal cavity outside the pelvis or regional lymph glands spread, or both, whilst stage IV disease is a disease with spread to distant organs such as the chest or liver (Benedet 2000). Stage I and II tumours are considered to be early disease, while stages III and IV represent late or advanced disease. In Europe, just over a third of women with ovarian cancer are alive five years after diagnosis (EUROCARE 2003), largely because most women with ovarian cancer are diagnosed when the cancer is already at an advanced stage (Aletti 2006; Jemal 2008).
Description of the intervention
Surgery and chemotherapy are the mainstay of treatment. In early stage disease (FIGO stage I‐II) surgery will cure most women (Trimbos 2003). However, around 75% of women present with advanced disease (FIGO stage III/IV) when surgery alone cannot be curative (Fader 2007). Primary surgery is performed to achieve optimal cytoreduction as the amount of residual tumour is one of the most important prognostic factors for survival of epithelial ovarian cancer (Bristow 2002; Griffiths 1975; Hoskins 1994). After surgery, most patients now receive platinum‐based chemotherapy (Bristow 2002).
The terms cytoreductive and debulking surgery are used interchangeably to indicate surgical efforts aimed at removing the bulk of the tumour. Complete cytoreduction is achieved when there is no visible tumour left after surgery. The term 'optimal cytoreduction' has been variably defined as referring to a maximal diameter of residual tumour of 0 to 2 cm. The Gynaecologic Oncology Group (GOG) currently defines optimal as having residual tumour nodules each measuring 1 cm or less in maximum diameter (Fader 2007). Alternatively, optimal cytoreduction has been defined as no residual tumour load (Colombo 2006; Vergote 1998; Vergote 2003). No residual tumour has also been described as 'complete cytoreduction' and has been shown to result in better survival than suboptimal cytoreduction and to be a better predictor of survival than the extent of metastatic disease present before surgery (Eisenkop 1998; Eisenkop 2003).
Optimal or complete cytoreduction for the majority of patients is a reasonable goal (Eisenkop 1998; Eisenkop 2003), especially because the success of postoperative chemotherapy correlates with lower residual tumour volume. Two studies (Bristow 2002; Von Georgi 2003) demonstrated better survival for patients undergoing cytoreduction to less than 0.5 cm or no gross remaining disease. Their study showed that each 10% increase in maximal cytoreduction was associated with a 5.5% increase in median survival time. On the other hand, Vergote 1998 demonstrated significant differences in survival based on an estimation of the number of grams of residual tumour as women with residual tumour less than 1 g after surgery had significantly better median survival (30 months) than women with more than 10 g of residual tumour (12 months). In an analysis of 433 patients with stage III and IV ovarian cancer who underwent primary cytoreduction, Stoeckle 2004found that the number of residual nodules rather than their size was predictive of outcome.
Additionally, it has been shown that if surgery is performed by physicians with training in gynaecological oncology, patients tend to survive longer than if surgery is performed by general surgeons or generalist gynaecologists (Paulsen 2006). However, optimal cytoreduction must not be considered separately from the possible morbidity consequent to debulking surgery and the subsequent quality of life (QoL) (Deffieux 2006). Postoperative mortality following debulking surgery for ovarian cancer has been reported to range from 1% (Venesmaa 1992) to 6% (Vergote 1998). Major surgical complications include haemorrhage, thromboembolic disease, infection, myocardial infarction, bowel obstruction, visceral injuries, fistulae and wound breakdown (Sharma 2005).
Health economic evaluation of radical surgery and the management of associated morbidities and complications including length of surgical procedure, prolonged hospital admission and high dependency unit (HDU) or intensive care unit (ICU) support also need to be evaluated to justify any potential benefit in outcome survival.
Why it is important to do this review
Optimal cytoreductive surgery remains a subject of controversy to many practising gynae‐oncologists. A survey of the Society of Gynecologic Oncologists (SGO) revealed that 12% of respondents defined optimal cytoreductive surgery as no residual tumour, whereas 14%, 61% and 13% used residual disease thresholds of 0.5 cm, 1 cm, and between 1.5 cm and 2.0 cm respectively (Eisenkop 2001).
Although the size of residual tumour masses after surgery has been shown to be an important prognostic factor for advanced ovarian cancer, there is limited evidence to support the conclusion that the surgical procedure is directly responsible for the superior outcome associated with less residual disease (Girling 1996; Hunter 1992). Many factors influence a surgeon's ability to remove most visible tumour (Markman 2007). The ability to perform optimal cytoreduction may be more feasible in patients with biologically less aggressive tumours (Covens 2000; Hoskins 1992) that are destined to have more favourable outcomes. The results of an earlier meta‐analysis on cytoreductive surgery (Hunter 1992) might have been flawed, not only due to the absence of clear definitions but also due to the combined effects of subsequent chemotherapy (Munstedt 2004).
The benefits of primary surgical cytoreduction in ovarian cancer have not been defined through well designed and conducted prospective phase III trials (Covens 2000; Markman 2007). The role of primary cytoreductive surgery in Stage IV ovarian cancer is even more controversial (Colombo 2006; Vergote 2003).
Objectives
To evaluate the effectiveness, safety and cost‐effectiveness of optimal primary cytoreductive surgery for women with surgically staged advanced epithelial ovarian cancer (stages III and IV)
To assess the impact of various residual tumour sizes, over a range between zero and 2 cm, on overall survival
Methods
Criteria for considering studies for this review
Types of studies
As it is not ethically possible to assign patients to cytoreductive surgery which is not optimal, the review was based on retrospective and prospective studies rather than randomised controlled trials. We only included data from randomised controlled trials (RCTs), prospective and retrospective cohort studies and unselected case series of 100 or more patients which included concurrent comparison groups. Data collected from RCTs were retrospective as groups of women were randomised to various chemotherapy protocols after primary surgery and the surgical outcome was categorised as complete (microscopic or no visible disease), optimal and suboptimal based on the maximum size of postoperative residual disease.
Case‐control studies, studies that did not have concurrent comparison groups and case series of fewer than 100 patients were excluded.
In order to minimise selection bias, we included only studies that used statistical adjustment for baseline case mix using multivariable analyses (for example age, stage, grade).
Types of participants
Adult women (over 18 years of age) with surgically staged advanced epithelial ovarian cancer (FIGO stage III/IV) who had confirmed histological diagnoses. Women with other concurrent malignancies were excluded.
Types of interventions
Intervention: primary optimal cytoreductive surgery followed by adjuvant platinum‐based chemotherapy. We only included studies that defined optimal cytoreduction as surgery leading to residual tumours with a maximum diameter of any threshold up to 2 cm. Patients who received chemotherapy prior to surgery were excluded.
Comparison: women who had primary surgery resulting in residual disease which did not meet the criteria specified in the study as optimal, followed by adjuvant platinum‐based chemotherapy.
Types of outcome measures
Primary outcomes
Overall survival: survival until death from all causes. Survival was assessed from the time when women were enrolled in the study.
Secondary outcomes
Progression‐free survival.
Quality of life (QoL), measured using a scale that has been validated through reporting of norms in a peer‐reviewed publication.
Cost‐effectiveness.
-
Adverse events, for example:
direct surgical morbidity (e.g. injury to bladder, ureter, vascular, small bowel or colon), presence of and complications from adhesions, febrile morbidity, intestinal obstruction, haematoma, local infection and fistulae);
surgically‐related systemic morbidity including chest infection, thrombo‐embolic events (deep vein thrombosis and pulmonary embolism), cardiac events (cardiac ischemias and cardiac failure), cerebrovascular accident;
delayed discharge or delayed adjuvant chemotherapy treatment, unscheduled re‐admission.
Search methods for identification of studies
Papers in all languages were sought and translations carried out when necessary.
Electronic searches
The following electronic databases were searched:
Cochrane Gynaecological Cancer Collaborative Review Group Trials Register;
Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 3);
MEDLINE (to August 2010);
EMBASE (to August 2010).
For MEDLINE, EMBASE and CENTRAL, search strategies based on the terms related to the review topic are presented in Appendix 1, Appendix 2 and Appendix 3 respectively. For databases other than MEDLINE, the search strategy was adapted accordingly. Databases were searched from 1950 until August 2010.
All relevant articles found were identified on PubMed and, using the 'related articles' feature, a further search was carried out for newly published articles.
Searching other resources
Unpublished and grey literature
Metaregister, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov and www.cancer.gov/clinicaltrials were searched for ongoing trials.
Handsearching
The citation list of relevant publications, abstracts of scientific meetings and of included studies were checked through handsearching, and experts in the field were contacted to identify further reports trials. Reports of conferences were handsearched in the following sources.
Gynecologic Oncology (Annual Meeting of the American Society of Gynecologic Oncologist).
International Journal of Gynecological Cancer (Annual Meeting of the International Gynecologic Cancer Society).
British Journal of Cancer.
British Cancer Research Meeting.
Annual Meeting of European Society of Medical Oncology (ESMO).
Annual Meeting of the American Society of Clinical Oncology (ASCO).
Correspondence
Authors of relevant trials were contacted to ask if they knew of further data, which may or may not have been published.
Data collection and analysis
Selection of studies
All titles and abstracts retrieved by electronic searching were downloaded to the reference management database Endnote, duplicates were removed and the remaining references were examined by two review authors (AE, MH) independently. Those studies which clearly did not meet the inclusion criteria were excluded and copies of the full text of potentially relevant references were obtained. The eligibility of retrieved papers was assessed independently by two review authors (AE, MH). Disagreements were resolved by discussion between the two review authors or, where necessary, by appeal to a third review author (AB). Reasons for exclusion were documented.
Data extraction and management
For included studies, data were extracted as recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (2008). This included data on the following.
Author, year of publication and journal citation (including language).
Country.
Setting.
Inclusion and exclusion criteria.
Study design, methodology.
-
Study population:
total number enrolled in each group;
patient characteristics;
age;
co‐morbidities.
-
Ovarian cancer details at diagnosis:
FIGO stage (III or IV);
histological cell type;
preoperative tumour volume;
ascites (large or small volume);
tumour grade;
extent of disease.
-
Intervention details:
details of primary optimal cytoreductive surgery;
-
details of adjuvant platinum based chemotherapy
dose,
cycle length;
type of surgeon (gynae‐oncologist, gynaecologist, general surgeon);
experience of surgeon;
type of surgery (ultra‐radical or standard).
Risk of bias in study (see below).
Duration of follow‐up.
Outcomes: see above.
Data on outcomes were extracted as below.
For time to event data (survival and progression‐free survival), we extracted the log of the hazard ratio (log(HR)) and its standard error from trial reports; if these were not reported, we attempted to estimate the log (HR) and its standard error using the methods of Parmar 1998.
Where possible, all data extracted were those relevant to an intention‐to‐treat analysis in which participants were analysed in the groups to which they were assigned.
The time points at which outcomes were collected and reported were noted.
Data were abstracted independently by two review authors (AE, MH) onto a data abstraction form specially designed for the review. Differences between review authors were resolved by discussion or by appeal to a third review author (AB), when necessary.
Assessment of risk of bias in included studies
Risk of bias in the included studies was assessed on the basis of the following criteria.
Blinding
We coded the adequacy of blinding of participants and outcome assessors as:
yes;
no;
unclear.
Loss to follow‐up
We recorded the proportion of participants whose outcomes were not reported at the end of the study.
We coded loss to follow‐up as:
yes, if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms;
unclear, if loss to follow‐up was not reported;
no, if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms.
Cohort selection
Was the cohort studied representative of women with advanced epithelial ovarian cancer?
Yes, if representative of women with advanced epithelial ovarian cancer.
No, if a group of patients was selected.
Unclear, if selection of the group was not described.
Comparability of treatment groups
Were differences between the two groups controlled for, in particular with reference to age, FIGO stage (proportion of patients with stage III and IV disease), histology, type of surgeon, preoperative tumour volume, large volume ascites (more than one litre)?
Yes, if at least two of these characteristics were reported and any reported differences were controlled for.
No, if the two groups differed and differences were not controlled for.
Unclear, if fewer than two of these characteristics were reported even if there were no other differences between the groups and other characteristics had been controlled for.
Selective reporting of outcomes
Were reports of the study free of suggestion of selective outcome reporting?
Yes, if it was deemed that the study was free of selective outcome reporting e.g. study adheres to protocol.
No, if there was evidence of selective outcome reporting.
Unclear, if it is not obvious whether or not outcomes were selectively reported.
Other potential threats to validity
Was the study apparently free of other problems that could put it at a high risk of bias?
Yes.
No.
Unclear.
The risk of bias tool was applied independently by two review authors (AE, MH) and differences resolved by discussion or by appeal to a third review author (AB). Results are summarised in both a risk of bias graph and a risk of bias summary. Results of meta‐analyses were interpreted in light of the findings with respect to risk of bias.
Measures of treatment effect
We used the following measures of the effect of treatment.
For time to event (overall and progression‐free survival) data, we used the hazard ratio, where possible.
Dealing with missing data
We did not impute missing outcome data for any of the outcomes.
Assessment of heterogeneity
Heterogeneity between studies was assessed by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials which cannot be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001) and, where possible, by subgroup analyses (see below). If there was evidence of substantial heterogeneity, the possible reasons for this were investigated and reported.
Assessment of reporting biases
We did not produce a funnel plot to assess the potential for small study effects since there were only six trials in the largest meta‐analysis, which assessed overall survival in women with residual disease < 1 cm compared to women with microscopic disease.
Data synthesis
If sufficient clinically similar studies were available, their adjusted results were pooled in meta‐analyses.
For time to event data, hazard ratios were pooled using the generic inverse variance facility of RevMan 5.
Random‐effects models with inverse variance weighting were used for all meta‐analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
We examined studies that defined optimal (the maximum diameter of residual tumour at the end of primary surgery) as being microscopic disease, < 1 cm and < 2 cm separately. In addition subgroup analyses were performed grouping studies by:
FIGO stage (previously stated as being by stage III and IV, but we subgrouped studies by stage III, IIIC, IV and all advanced stages if studies included all advanced cases together).
Factors such as age, grade, length of follow‐up, type and experience of surgeon and type of surgery were considered in the interpretation of any heterogeneity.
Results
Description of studies
Results of the search
The search strategy identified 1274 unique references. The title and abstract screening of these references identified 84 studies as potentially eligible for the review. The full text screening of the 84 studies excluded 73 for the reasons described in the table Characteristics of excluded studies. The remaining 11 studies met our inclusion criteria and are described in the table Characteristics of included studies.
Searches of the grey literature did not identify any additional relevant trials.
There were four randomised controlled trials (Redman et al; Rose et al; Van der Burg et al; Vergote et al) evaluating the effectiveness of surgery in advanced stage epithelial ovarian cancer. However, all four of these trials were excluded as they were designed to evaluate the benefits of surgery (interval debulking surgery) after an induction period with chemotherapy treatment; three of these four studies were where the surgery was performed as a secondary procedure after primary surgery and have been evaluated in a separate Cochrane review.
Included studies
The 11 included studies (Akahira 2001; Aletti 2006; Chan 2003; Chi 2001; Chi 2006; Eisenkop 2003; McGuire 1995; Salani 2007; Van Geene 1996; Winter 2007; Winter 2008) assessed a total of 4735 women (3844 were stage III and 891 were stage IV).
Two studies reported exclusively on patients with stage IV epithelial ovarian cancer (EOC) (Akahira 2001; Winter 2008) and included 225 and 360 stage IV patients respectively.
Three studies reported exclusively on patients with stage IIIC EOC (Aletti 2006; Chi 2006; Eisenkop 2003); the Winter 2007 study reported patients with stage IIIA‐C disease; whilst five studies reported on both stage III and IV EOC (Chan 2003; Chi 2001; McGuire 1995; Salani 2007; Van Geene 1996). The number of patients with stage IV disease included in the latter six studies varied from 20 (Chan 2003) to 153 (McGuire 1995).
The number of patients included in all studies varied from 104 patients in the Chan 2003 study to 1895 patients in the Winter 2007 study. The latter included the largest number of patients as it included patients from six different Gynecologic Oncology Group (GOG) trials; hence it contributed 40% of patients included in this review.
For a summary of the total number of women included in each study as well as stage and residual disease details see Table 1.
1. Summary of stage and residual disease in included studies.
| Study | No. | Stage | RD used as optimal | Optimal No. (%) |
Suboptimal No. (%) |
|
| IIII No.(%) | IV No. (%) | |||||
| Akahira 2001 | 225 | 0 | 225 | <2 cm | 70 (31.1) | 155 (68.9) |
| Aletti 2006 | 194 | 194 | 0 | <1 cm | Microscopic: 46 (23.7) <1cm: 22 (43.8) Total: 68 (67.5) |
63 (32.4) |
| Chan 2003 | 104 | 94 (80.8) | 20 (19.2) | <1 cm | 71 (68.3) | 33 (31.7) |
| Chi 2001 | 282 | 216 (77) | 66 (23) | <1 cm | 71 *(25.3) | 210 (74.7) |
| Chi 2006 | 465 | 465 | 0 | <1 cm | Microscopic: 67 (14.4) <1cm: 169 (36.4) Total: 236(50.8) |
229 (49.2) |
| Eisenkop 2003 | 408 | 408 | 0 | 0 cm | Microscopic: 351 (86) < 1 cm: 41 (10) Total: 392 (96) |
16 (4) |
| McGuire 1995 | 458 | 305 (67) | 153 (33) | All sub‐optimal | 1‐2 cm: 85 (18.6) |
> 2cm: 373 (81.4) |
| Salani 2007 | 125 | 97 (78) | 28 (22) | 0 cm | Microscopic: 39 (31.2) < 1 cm: 63 (50.4) Total: 102 (81.6) |
23 (18.4) |
| Van Geene 1996 | 219 | 180 (82) | 39 (18) | <2 cm | <2 cm: 92 (42) | > 2cm: 127 (58) |
| Winter 2007 | 1895 | 1895 | 0 | 0 cm | Microscopic: 437 (23.1) <1 cm: 791 (41.7) Total: 1228 (64.8) |
>1 cm: 667 (35.2) |
| Winter 2008 | 360 | 0 | 360 | 0 cm | Microscopic: 29 (8) < 1 cm: 79 (22) Total: 108 (30) |
252 (70) |
* of 281 patients as reported by the authors
Design
Retrospective studies comprised six out of the 11 included studies (Akahira 2001; Aletti 2006; Chan 2003; Chi 2001; Chi 2006; Salani 2007).
Two studies were prospective cohort studies (Eisenkop 2003; Van Geene 1996).
The Winter 2007 and Winter 2008 studies were a retrospective analysis of six and four randomised controlled trials of various chemotherapy protocols respectively. The Winter 2007 study reported on patients with stage III EOC and the Winter 2008 reported on patients with stage IV EOC. The former included patients from GOG protocols 111, 114, 132, 152, 158 and 172 (Markman, 2001; McGuire 1996; Muggia, 2000; Rose 2004; Ozols, 2003; Armstrong, 2006) and the latter included patients from GOG protocols 111, 132, 152 and 162 (McGuire 1996; Muggia, 2000; Rose 2004; Spriggs 2007). Likewise the McGuire 1995 study was a retrospective analysis of a randomised controlled trial of two different chemotherapy protocols.
Participant characteristics
Nine studies were conducted in the USA ( Aletti 2006; Chan 2003; Chi 2001; Chi 2006; Eisenkop 2003; McGuire 1995; Salani 2007; Winter 2007; Winter 2008), whilst the Van Geene 1996 study was conducted in the UK and the Akahira 2001 study was conducted in 24 centres in Japan.
The median age reported for patients with advanced EOC varied between 54 to 64 years with the range between 16 to 91 years.
Intervention details
Patients in all the studies included in this review were treated by primary cytoreductive surgery followed by platinum‐based adjuvant chemotherapy. All patients were confirmed histologically to have invasive epithelial ovarian cancer.
The speciality of the surgeon who performed primary cytoreduction (for example, general surgeon, gynaecologic surgeon or specialist gynaecologic oncology surgeon) was not reported in seven of the included studies (Akahira 2001; Aletti 2006; McGuire 1995; Salani 2007; Van Geene 1996; Winter 2007; Winter 2008) whereas specialist gynaecologic oncology surgeons undertook the primary cytoreduction procedure in four studies (Chan 2003; Chi 2001; Chi 2006; Eisenkop 2003).
The mean duration of primary cytoreductive surgery was reported to be 210 minutes (range 40 to 480 min) in Aletti 2006. Similarly the median duration of primary cytoreductive surgery was reported to be 194 minutes (range 60 to 750 min) and 180 minutes (range 55 to 480 min) in the Chi 2006 and Eisenkop 2003 studies respectively. All three studies reported on patients with stage IIIC disease. On the other hand, the Akahira 2001 study reported on patients with stage IV disease and the median duration of primary cytoreductive surgery was found to be 240 minutes (range 40 to 780 min).
The duration of the surgery was not reported in the remaining seven studies (Chan 2003; Chi 2001; McGuire 1995; Salani 2007; Van Geene 1996; Winter 2007; Winter 2008).
The median estimated operative blood loss was 500 ml (range 20 to 7500 ml); 850 ml (range 30 to 5000 ml) and 1085 ml (range 40 to 11,000 ml) in the Chi 2006; Eisenkop 2003; Akahira 2001 studies respectively. In the latter study, blood transfusion was given to 112 patients (50%).
Only two studies reported on the length of hospital stay (LHS) (Chi 2006; Eisenkop 2003) and the median LHS was 10 days, with a range of 0 to 59 and 0 to 93 respectively.
Postoperative mortality within 30 days of primary cytoreductive surgery was reported to be 1.5%, 2.83%, 0.6% and 2.5% in the Aletti 2006; Chi 2001; Chi 2006; Eisenkop 2003 studies respectively. Salani 2007 reported on the major postoperative complication rate (29.4%) and postoperative mortality rate (1.9%) only in the subgroup of patients who achieved optimal cytoreduction (defined as residual disease < 1 cm). In these five studies 26 out of 1451 patients died (mean postoperative mortality of 1.8%, 95% CI 0.36 to 3.2%).
Postoperative mortality and morbidity were not reported in five studies (Chan 2003; McGuire 1995; Van Geene 1996; Winter 2007; Winter 2008).
In one study (Salani 2007), women who achieved optimal cytoreduction (defined as residual disease < 1 cm) had higher major postoperative morbidity (16/34) (47.1%, 95% CI 29.8 to 64.9%) when multiple bowel resection (two or more) was performed as a part of the primary cytoreductive surgery compared to 14/68 (20.6%, 95% CI 11.7 to 32.1%) when one or no bowel resection was performed (P < 0.01).
Two studies used a postoperative residual disease cutoff of < 2 cm to define an optimal surgical outcome (Akahira 2001; Van Geene 1996). Only one study considered that an optimal outcome was achieved only if no visible disease was left behind at the conclusion of primary cytoreductive surgery (Eisenkop 2003). This is sometimes called complete cytoreduction. Four studies used a postoperative residual disease cutoff of < 1 cm to define an optimal surgical outcome (Aletti 2006; Chan 2003; Chan 2003; Salani 2007). The remaining four studies did not define what is considered optimal in the study methodology (Chi 2001; Chi 2006; Winter 2007; Winter 2008) but analysed the outcome by a range of postoperative residual disease.
The rate of complete cytoreduction (microscopic residual disease) was reported in six studies (Aletti 2006; Chi 2006; Eisenkop 2003; Salani 2007; Winter 2007; Winter 2008). it was achieved in 969 out of 3447 patients (28.1%) with the lowest complete cytoreduction rate reported by Chi 2006 and the highest complete cytoreduction rate (86%) reported by Eisenkop 2003.
Postoperative residual disease (RD) < 1 cm was achieved in 2276 out of 3832 patients (59.4%) as calculated from eight studies (Aletti 2006; Chan 2003; Chi 2001; Chi 2006; Eisenkop 2003; Salani 2007; Winter 2007; Winter 2008). The lowest rate for RD < 1 cm was 25.3% (71/281) in the Chi 2001 study and the highest was 96% (392/408) in the Eisenkop 2003 study.
In seven studies all patients received postoperative platinum‐based chemotherapy (Aletti 2006; Chan 2003; Eisenkop 2003; McGuire 1995; Van Geene 1996; Winter 2007; Winter 2008). In the remaining four studies (Akahira 2001; Chi 2001; Chi 2006; Salani 2007) the majority of patients (95.1%, 96%, 97%, 98.4% respectively) received postoperative platinum‐based chemotherapy. The main reason for not receiving postoperative chemotherapy was postoperative death within 30 days of surgery and absent patient records (Chi 2001; Salani 2007). Other reasons for not receiving postoperative chemotherapy or receiving non‐platinum based chemotherapy were poorly reported.
Six studies reported the survival outcome for the complete cytoreduction group, that is patients left with microscopic residual disease (Aletti 2006; Chi 2006; Eisenkop 2003; Salani 2007; Winter 2007; Winter 2008).
Outcomes
The median duration of follow‐up varied from 28 months (Winter 2008) to 47.5 months (Akahira 2001) with a range between 1 and 199 months (Chi 2006). The duration of follow‐up was not reported in two studies (McGuire 1995; Van Geene 1996).
All 11 studies reported overall survival and the trials of Winter 2007 and Winter 2008 reported progression‐free survival and used appropriate statistical techniques (hazard ratios to correctly allow for censoring). Prognostic factors were adjusted for in the analysis of survival outcomes in each study using Cox regression.
The hazard ratio in the Akahira 2001 study was adjusted for: residual disease, histology and performance status.
The hazard ratio in the Aletti 2006 study was adjusted for: residual disease, age, American Society of Anesthesiology (ASA) score, histological grade, operative time and aggressive surgery.
The hazard ratio in the Chan 2003 study was adjusted for: residual disease, age (older versus younger), stage (IV versus III) and performance status (1 to 2 versus 0).
The hazard ratio in the Chi 2001 study was adjusted for: residual disease, age, stage (IIIC and IV versus IIIA/IIIB) and ascites (yes versus no).
The hazard ratio in the Chi 2006 study was adjusted for: residual disease, age and ascites.
The hazard ratio in the Eisenkop 2003 study was adjusted for: residual disease and sum of rankings (a numerical ranking system was devised to reflect the continuum of progressively extensive tumour involvement for five anatomic regions).
The hazard ratio in the McGuire 1995 study was adjusted for: residual disease, age, GOG performance status, histological subtype, stage or residual disease and measurable disease.
The hazard ratio in the Salani 2007 study was adjusted for: residual disease and other covariates having some prognostic value in univariate analyses but it has not reported which ones were significant. Hence the hazard ratio may have been adjusted for any of the following variables: number of bowel resections, age, stage and ascites.
The hazard ratio in the Van Geene 1996 study was adjusted for: residual disease, performance status and pattern of spread.
The hazard ratio in the Winter 2007 study was adjusted for: residual disease, age (discrete), race, GOG performance status, histology and tumour grade.
The hazard ratio in the Winter 2008 study was adjusted for: residual disease, histology and stage IV disease site.
For the distribution of these factors at baseline for each trial and by treatment arm see the table Characteristics of included studies.
Adverse events and QoL were not reported by treatment arm or to a satisfactory level in any of the studies.
Excluded studies
Seventy‐four references were excluded after obtaining the full text, for the following primary reasons.
Thirty‐four references (Alphs 2006; Andersen Soegaard 2005; Benedetti‐Panici 1996; Bristow 1999; Cai 2007; Colozza 1997; Del Campo 1994; Gao 2001; Gershenson 1989; Gershenson 1995; Grem 1991; Hainsworth 1990; Hakes 1992; Hamid 2002; Hardy 1991; Hoskins 1996; Kaern 2005; Kirmani 1994; Kristensen 1995; Lorusso 1998; Malik 1998; Marchetti 1993; Ngan 1989; Palmer 1992; Redman 1986; Shapiro 1998; Strauss 1996; Sutton 1989; Tay 1996; Taylor 1994; Vallejos 1997; Willemse 1992; Wils 1990; Zang 1999) were excluded because they did not include at least 100 patients with advanced epithelial ovarian cancer.
Six studies (Alberts 1996; Bertelsen 1990; Brinkhuis 1996a; Piver 1991; Sessa 1991; Wimberger 2007) either did not report multivariate analyses or did not include residual disease as a variable.
Thirteen studies (Alberts 1993; Bertelsen 1993; Brinkhuis 1996b; Conte 1991; Conte 1996; Creasman 1990; Gershenson 1992; Hoskins 1992; Hoskins 1997; Itamochi 2002; Uyar 2005; Wadler 1996; Warwick 1995) did not report survival by residual disease.
Non‐platinum based chemotherapy was given to a proportion of patients in four studies (Barda 2004; Bonnefoi 1999; de Oliviera 1990; Tingulstad 2003) and chemotherapy data were absent in the Bailey 2006 study. Patients received preoperative chemotherapy in two studies (Shinozuka 1999; Sun 2000).
Two studies (Todo 2003; Van Der Burg 1996) included patients who received neoadjuvant chemotherapy and interval debulking surgery.
Likewise, the Vergote 2010 study was excluded as it included 57 patients (17%) who received neoadjuvant chemotherapy and underwent interval debulking surgery in the primary debulking arm. Reported complications in the primary debulking arm included 24 patients who had side effects or complications typical of chemotherapy e.g., taxol allergy, neutropenic sepsis, neuropathy.
Six studies (Crawford 2005; di Re 1996; Geisler 2004; Skarlos 1996; Takano 2006; Takano 2007) included patients with early stage disease and it was not possible to distinguish between early and advanced stage participants. The Le 1997 study did not report the survival data from the stage IIIC and IV subgroup and the authors no longer had access to this data.
Two studies (Baker 1994; Omura 1989) reported a HR for overall survival but did not include the corresponding 95% confidence interval, SE (lnHR) or exact P value.
The trial of Rose 2004 reported on outcomes after secondary debulking surgery. However, the trial statistician (Dr Mark Brady) of the included study of Winter 2007 alerted us to the results of GOG 152, which reported by residual disease after primary cytoreductive surgery.
The Yamamoto 2007 study included 67 selected patients with rare histological subtypes.
For further details of all the excluded studies see the Characteristics of excluded studies table.
Risk of bias in included studies
Although the included studies were a combination of RCTs, prospective and retrospective studies, the comparison of residual disease was retrospective in nature and consequently all studies were at high risk of bias. At most, they only satisfied two of the seven criteria (see Figure 1; Figure 2).
1.
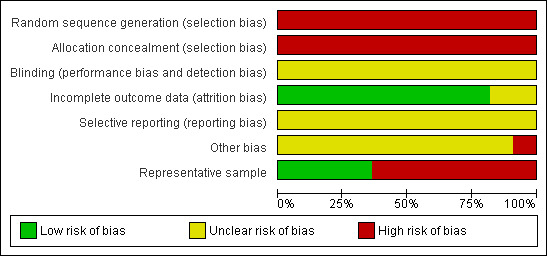
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
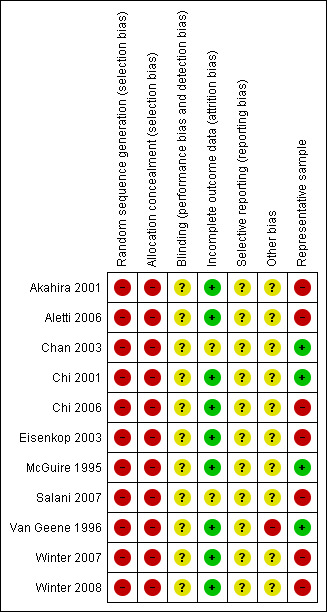
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
The methods of sequence generation and allocation of concealment were not applicable to the retrospective studies included in the review so these individual items were flagged as being at high risk of bias for all studies. Blinding of the outcome assessor was not reported in any of the studies and it was unclear whether there had been selective reporting of outcomes in all of the studies. There was insufficient information to make a judgement on whether any additional risk factor for bias existed, apart from the Van Geene 1996 study where it was unclear whether no residual disease was included in the less than 2 cm residual disease group in the analysis as it had been in the baseline tables. All but two of the studies (Chan 2003; Salani 2007) assessed an adequate proportion of their recruited women as most eligible women were assessed at the endpoint for all outcomes. Only four studies (Chan 2003; Chi 2001; McGuire 1995; Van Geene 1996) appeared to include a representative sample of women with advanced epithelial ovarian cancer.
Effects of interventions
Meta‐analyses of survival are based on hazard ratios (HRs) that were adjusted for prognostic variables (see Included studies for full details).
Where possible meta analyses subgrouped studies by FIGO stage (stage III, IIIC, IV and all advanced stages, if studies included all advanced cases together). The results of these subgroup analyses were robust to the findings of the overall pooled estimate for all comparisons so the results of each subgroup are not discussed in this section (see Analysis 1.1 to Analysis 10.2).
1.1. Analysis.
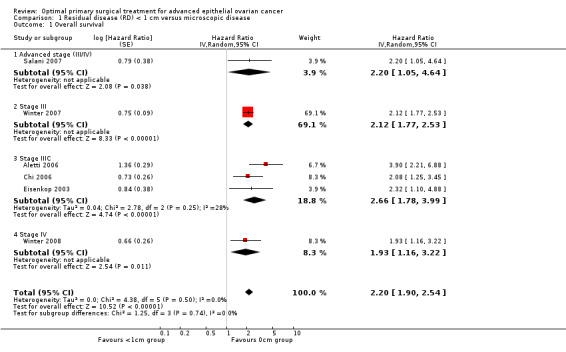
Comparison 1 Residual disease (RD) < 1 cm versus microscopic disease, Outcome 1 Overall survival.
10.2. Analysis.
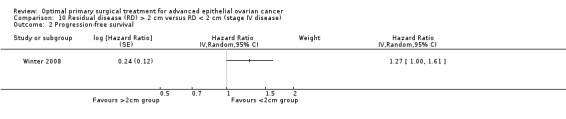
Comparison 10 Residual disease (RD) > 2 cm versus RD < 2 cm (stage IV disease), Outcome 2 Progression‐free survival.
Overall survival (risk of death from all causes)
Residual disease < 1 cm versus microscopic disease
Meta‐analysis of six studies (Aletti 2006; Chi 2006; Eisenkop 2003; Salani 2007; Winter 2007; Winter 2008), assessing 3447 participants, found that women who were optimally debulked (RD < 1 cm) after primary surgery had more than twice the risk of death compared to women with only microscopic disease (HR 2.20, 95% CI 1.90 to 2.54). The percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance) was not important (I2 = 0%).
Residual disease 1 to 2 cm versus microscopic disease
The Aletti 2006 study, which included only patients with stage IIIC disease, found that women who had residual disease between 1 and 2 cm after primary surgery had more than six times the risk of death compared to women with only microscopic disease (HR 6.23, 95% CI 3.14 to 12.38).
Residual disease > 1 cm versus microscopic disease
Meta‐analysis of four studies (Chi 2006; Eisenkop 2003; Salani 2007; Winter 2007), assessing 2893 participants, found that women who were suboptimally debulked (RD > 1 cm) after primary surgery had more than three times the risk of death compared to women with only microscopic disease (HR 3.16, 95% CI 2.26 to 4.41). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance may represent modest heterogeneity (I2 = 54%).
Residual disease > 2 cm versus microscopic disease
The Aletti 2006 study, which included only patients with stage IIIC disease, found that women who were suboptimally debulked (RD > 2 cm) after primary surgery had more than 12 times the risk of death compared to women with only microscopic disease (HR 12.94, 95% CI 6.91 to 24.22).
Residual disease 1 to 5 cm versus microscopic disease
The Winter 2008 study, which included only patients with stage IV disease, found that women who had residual disease between 1 and 5 cm after primary surgery had a statistically significant greater risk of death compared to women with only microscopic disease (HR 1.82, 95% CI 1.14 to 2.92).
Residual disease > 5 cm versus microscopic disease
The Winter 2008 study, which included only patients with stage IV disease, found that women who had residual disease > 5 cm after primary surgery had more than two and a half times the risk of death compared to women with only microscopic disease (HR 2.72, 95% CI 1.67 to 4.44).
Residual disease > 1 cm versus residual disease < 1 cm
Meta‐analysis of two studies (Chan 2003; Winter 2008), assessing 464participants, found that women who were suboptimally debulked (RD > 1 cm) after primary surgery had a statistically significant greater risk of death compared to women who were optimally debulked (RD < 1 cm) (HR 1.36, 95% CI 1.10 to 1.68). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance is not important (I2 = 0%).
Residual disease 1 to 2 cm versus residual disease < 1 cm
The trial of Chi 2001 found that women who had residual disease between 1 and 2 cm after primary surgery had a statistically significant greater risk of death compared to women who were optimally debulked to postoperative RD < 1 cm (HR 1.70, 95% CI 1.11 to 2.60).
Residual disease > 2 cm versus residual disease < 1 cm
The Chi 2001 study found that women who were suboptimally debulked (RD > 2 cm) after primary surgery had twice the risk of death compared to women who were optimally debulked to postoperative RD < 1 cm (HR 2.00, 95% CI 1.36 to 2.94).
Residual disease > 2 cm versus residual disease < 2 cm
Meta‐analysis of two studies (Akahira 2001; Winter 2008), which included only patients with stage IV disease, and assessed 585 participants found no statistically significant difference in the risk of death between women who were suboptimally debulked (RD > 2 cm) after primary surgery and those who were debulked to < 2 cm (HR 1.65, 95% CI 0.82 to 3.31). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance alone may represent considerable heterogeneity (I2 = 91%). The two studies were inconsistent: the Akahira 2001 study reported a large and significant survival difference in favour of debulking to less than 2 cm, whereas Winter 2008 found no significant difference in survival.
Progression‐free survival (risk of disease progression)
Residual disease < 1 cm versus microscopic disease
Meta‐analysis of two studies (Winter 2007; Winter 2008), assessing 2255 participants, found that women who were optimally debulked (RD < 1 cm) after primary surgery had almost twice the risk of disease progression compared to women with only microscopic disease (HR 1.96, 95% CI 1.72 to 2.23). The percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance) is not important (I2 = 0%).
Residual disease > 1 cm versus microscopic disease
The Winter 2007 study, which included only patients with stage III disease, found that women who were suboptimally debulked (RD > 1 cm) after primary surgery had more than twice the risk of disease progression compared to women with only microscopic disease (HR 2.36, 95% CI 2.06 to 2.71).
Residual disease 1 to 5 cm versus microscopic disease
The Winter 2008 study, which included only patients with stage IV disease, found that women who had residual disease between 1 cm and 5 cm after primary surgery had more than twice the risk of disease progression compared to women with only microscopic disease (HR 2.16, 95% CI 1.38 to 3.39).
Residual disease > 5 cm versus microscopic disease
The Winter 2008 study, which included only patients with stage IV disease, found that women who had residual disease between 1 cm and 5 cm after primary surgery had a statistically significant greater risk of disease progression compared to women with only microscopic disease (HR 1.49, 95% CI 1.16 to 1.92).
Residual disease > 1 cm versus residual disease < 1 cm
The Winter 2008 study, which included only patients with stage IV disease, found that women who were suboptimally debulked (RD > 1 cm) after primary surgery had a statistically significant greater risk of disease progression compared to women who were optimally debulked to postoperative RD < 1 cm (HR 1.30, 95% CI 1.03 to 1.64).
Residual disease > 2 cm versus residual disease < 2 cm
The Winter 2008 study, which included only patients with stage IV disease, found that women who were suboptimally debulked (RD > 2 cm) after primary surgery had a (borderline) statistically significant greater risk of disease progression compared to those who were debulked to < 2 cm (HR 1.27, 95% CI 1.00 to 1.61).
Discussion
Summary of main results
Although the included studies were a combination of RCTs, prospective and retrospective studies, the comparison of residual disease was retrospective in nature.
We found 11 studies reporting retrospective analyses of residual disease that met our inclusion criteria. These studies assessed survival after primary cytoreductive surgery followed by adjuvant platinum‐based chemotherapy in women with advanced epithelial ovarian cancer.
Meta and single study analyses clearly show the prognostic importance of complete cytoreduction (microscopic disease only with no visible disease) as overall and progression‐free survival were significantly prolonged in these groups of women (most studies showed a large statistically significant greater risk of death in all residual disease groups compared to microscopic disease). Progression‐free survival was not reported in all of the studies but was sufficiently documented to allow firm conclusions to be drawn. The fact that all of the studies included at least 100 women and used statistical adjustment for important prognostic factors increased the level of certainty in the estimates, despite the fact that the review was restricted to retrospective studies, prospective studies and retrospective analysis of randomised controlled studies.
When we compared suboptimal (> 1 cm) versus optimal (<1cm) cytoreduction the estimates were attenuated compared to the microscopic disease comparisons. All analyses showed a survival benefit in women who had been optimally debulked when this was defined as residual disease less than 1 cm (HR 1.36, 95% CI 1.10 to 1.68; HR 1.70, 95% CI 1.11 to 2.60; and HR 2.00, 95% CI 1.36 to 2.94 for OS for the comparisons > 1 cm versus <1 cm, 1 to 2 cm versus < 1 cm, and > 2 cm versus < 1 cm respectively and HR 1.30, 95% CI 1.03 to 1.64 for PFS for the > 1 cm versus <1 cm comparison; but there was no statistically significant difference in OS for residual disease of greater than and less than 2 cm (HR 1.65, 95% CI 0.82 to 3.31). In one study (Winter 2008), which included only patients with stage IV disease, there was a (borderline) statistically significant greater risk of disease progression in the > 2 cm group compared to the < 2 cm group (HR 1.27, 95% CI 1.00 to 1.61, P = 0.05).
Adverse events, QoL and economic evaluation were not reported by treatment arm or to a satisfactory level in any of the studies. QoL may be of additional importance to women who present at an advanced stage and have obvious physical limitations to their life after developing the disease and as a result of the effects of receiving treatment. We did not find many studies that compared our specified optimal and suboptimal categories. Only two studies compared residual disease greater and less than 1 cm or 2 cm (see above), where most studies emphasised the importance of making every effort to try and reduce the tumour to microscopic disease.
Overall completeness and applicability of evidence
The evidence from this review indicates that complete (no visible residual disease) and optimal (residual disease < 1 cm) primary surgical cytoreduction is associated with prolonged survival in advanced epithelial ovarian cancer. Although the findings do not enable us to determine whether it is a direct effect of the surgical intervention that women with complete cytoreduction do better, every effort should be made to reduce the tumour to microscopic disease. Where this is considered not achievable, attempts should be made to obtain optimal cytoreduction, defined as residual disease less than 1 cm. Residual disease, defined as being less than 2 cm, did not appear to have a significant survival benefit when compared to residual disease greater than 2 cm, but little evidence was available. In selected cases where it appears preoperatively that complete or optimal cytoreduction is not achievable at primary surgery, neoadjuvant chemotherapy followed by interval debulking surgery could be considered as neoadjuvant chemotherapy followed by interval debulking surgery in bulky stage III and stage IV disease and was not inferior to primary surgery in a recent study (Vergote 2010). The predictability of surgical outcome (for example residual disease) is an area of controversy and clinical ambiguity.
The criteria for assignment of patients to primary surgery were selective in most cases so statistical adjustment was necessary to minimise bias. The review benefited from having restrictive inclusion criteria. By only including studies with more than 100 women, satisfactory conclusions could be made in all of the multivariate analyses as the number of women in each study was adequate.
We were unable to report on quality of life (QoL), adverse event outcomes or cost‐effectiveness. None of the included studies had QoL assessments as a component of the studies. Treatment‐related morbidity very often degrades the quality of the time that patients live, which is especially important after the completion of treatment for advanced cancer where patients have poor prognosis and will want to enjoy a comfortable standard of living during their final months. However, this needs to be considered in the context of the findings from this review in that women in whom complete cytoreduction is achieved have a much better survival (median survival in microscopic group was 71.9 months in the Winter 2007 study, which included the largest analysis in the review), suggesting that the potential benefits of prolonging survival may outweigh the disadvantages of any short‐term morbidities associated with the surgical procedure.
Quality of the evidence
The 11 studies that met our inclusion criteria included retrospective analyses and were all at a high risk of bias. As the surgical efforts may vary with age, performance status and intraoperative events or complications, which were not thoroughly reported, we included only sufficiently large studies that controlled for various co‐factors using multivariate analysis in order to reduce the possibility of selection bias. The exact reasons for performing one type of surgery over another were not well documented and it was likely that women in generally poor health would be subjected to less aggressive surgery and thus would be more likely to have larger residual disease. This would most likely result in poorer survival, although we applied strict inclusion criteria and included studies that used statistical adjustment (see above). The studies reported adjusted hazard ratio estimates using Cox proportional hazards models. A hazard ratio is the best statistic to summarise the difference in risk between two intervention groups over the duration of a study when there is 'censoring', that is the time to death (or disease progression) is unknown for some women as they are still alive (or disease free) at the end of the trial. All studies were at high risk of bias as they, at most, only satisfied two of the criteria used to assess risk of bias. Many of the individual risk of bias items could not be scored as having low risk of bias given the fact that only non‐randomised designs were identified; and we were cautious when deciding whether studies were selectively reported or whether any additional source of bias may have been present and scored these items as being unclear. The predominant source of selection bias is based on the view that tumours that are biologically less aggressive may be more amenable to surgical cytoreduction. The confounding influences of 'cause and effect' or 'association' can only be determined through well designed RCTs that minimise the effects of patient‐related and disease‐related factors on outcome survival. None were identified during this systematic review. This is in addition to the potential biases associated with the current method or practice of determining cytoreductive outcome, which is largely a subjective and non‐systematic assessment by the surgeon at the end of the surgical procedure (Chi 2007).
Potential biases in the review process
A comprehensive search was performed, including a thorough search of the grey literature, and all studies were sifted and data extracted by two review authors working independently. We were not restrictive in our inclusion criteria with regards to types of studies as we included non‐randomised studies with concurrent comparison groups that used multivariate analyses. We attempted to ensure that we did not overlook any relevant evidence by searching a wide range of reasonable quality non‐randomised study designs (case‐control studies, studies that did not have concurrent comparison groups and case series of fewer than 100 patients were excluded).
A significant threat to the validity of the review is likely to be publication bias, that is studies that did not find the treatment to have been effective may not have been published. We found an insufficient number of studies that met the inclusion criteria to adequately assess this possibility.
Agreements and disagreements with other studies or reviews
The results of this review are consistent with the previously published review by Bristow 2002, which showed a direct correlation between degree of cytoreduction and survival so that for each 10% increase in maximal cytoreduction there was a 5.5% increase in median survival time. The results are also consistent with previously published recommendations of national and international organisations and societies relating to surgical practices in the management of advanced stage epithelial ovarian cancer, and the increasing attempts of practising gynaecological oncologists to achieve complete cytoreduction. The increasing realisation of the significantly better survival outcomes associated with complete cytoreduction has resulted in the consideration of re‐defining the term 'optimal cytoreduction' by the Gynaecological Cancer Inter‐Group (GCIG), from its current definition of < 1 cm residual disease to no visible residual disease (microscopic disease only).
A system of classification of completeness of cytoreduction akin to that used in peritoneal carcinomatosis (Cotte 2010; Sugarbaker 2009) may offer significant advantages for practice in surgery for advanced ovarian cancer. It provides a readily workable and reproducible measure of surgical outcome and provides for sensible comparisons of practice between centres offering this service. None the less, the current terminology of complete, optimal and incomplete cytoreduction is ingrained into practice and the literature base.
If the term optimal cytoreduction is to be used solely for the group where there is no visible residual disease, the findings of this review that women with residual disease < 1 cm still do better than women with residual disease > 1 cm should prompt the surgical community to retain this category and consider re‐defining it as 'near optimal' cytoreduction, reserving the term 'suboptimal' cytoreduction to cases where the residual disease is > 1 cm (optimal, near optimal, suboptimal instead of complete, optimal, suboptimal). Interestingly, a recent commentary made similar suggestions but using the terms complete, minimal and gross (Zapardiel 2011).
Although not investigated in this review, the results are also consistent with the finding that complete and optimal cytoreduction are associated with improved survival outcomes when performed after treatment with neoadjuvant chemotherapy, that is interval debulking surgery (Vergote 2010).
Authors' conclusions
Implications for practice.
At primary surgery for advanced stage epithelial ovarian cancer, all attempts should be made to achieve complete cytoreduction. When this is not achievable, the surgical goal should be optimal (< 1 cm) residual disease. In selected cases where it appears preoperatively that complete or optimal cytoreduction is not achievable at primary surgery, neoadjuvant chemotherapy followed by interval debulking surgery (delayed primary surgery) could be considered as neoadjuvant chemotherapy followed by interval debulking surgery in bulky stage III and stage IV disease and was not inferior to primary surgery in recent findings. However it is acknowledged that there is considerable variation in achieving complete or optimal cytoreduction between different surgeons and centres. Predicting the achievement of complete or optimal cytoreduction prior to surgery will be dependent on this variation, resulting in difficulties in developing models of prediction.
Maximal surgical efforts remain a key determinant of survival outcome in advanced ovarian cancer. Whether surgery is the primary treatment or is after neoadjuvant chemotherapy, the surgical goal should be to completely remove all gross disease, although residual disease of less than 1 cm should still be regarded as a favourable outcome.
Implications for research.
As the results of this review have identified a strong association between the achievement of complete and optimal cytoreduction and improved survival outcomes, it would be considered inappropriate to randomise women in whom complete or optimal cytoreduction is achievable to a surgical intervention where suboptimal cytoreduction or no surgery is performed. Instead, future research should focus on investigations that determine whether increasing attempts at achieving complete cytoreduction have a direct effect in improving survival outcomes using methodologies and trial designs that reduce or eliminate confounding effects such as the patient's performance status, disease spread and tumour biology, and that may have influenced the outcomes of this review as a result of the high risk of bias.
Such investigations are considered feasible as the current rates of complete cytoreduction in many centres in the UK and other countries are estimated to be well below 50%. It would be possible therefore to recruit patients in whom primary surgery has been performed when, by using their standard surgical approach, complete cytoreduction has not been achieved and to randomise them intraoperatively to no further surgery versus further surgical attempts at the time of the primary surgery. Further surgery would remove more disease thereby achieving greater cytoreduction and a higher proportion of cases where the end result of the surgical intervention is optimal or complete cytoreduction.
Alternatively, as there are significant disparities between surgeons and centres in their optimal and complete cytoreduction rates, one could consider randomising patients managed by surgeons and centres with a low complete or optimal cytoreduction rate to surgeons and centres that are more inclined to or capable of achieving complete or optimal cytoreduction.
The performance of ultra‐radical or extensive radical surgery can also be investigated by classifying or grading the performance of this intervention and quantifying the degree to which it is performed in the achievement of complete or optimal cytoreduction. Other pragmatic designs, including cluster randomisation, would also be feasible. The increasing practice of offering neoadjuvant chemotherapy followed by interval debulking surgery should not complicate the performance of these trials, by including stratification of this factor within the study design.
Greater emphasis should also be made in future studies to investigate QoL parameters, health economic analyses and adverse effects and complications of the surgery as there are significant deficiencies in previous studies in evaluating these outcome measures. Such investigations should be given high priority as this systematic review has identified significantly large differences in survival outcomes between cases that are suboptimally cytoreduced and cases where complete cytoreduction is achieved.
Also, the complete cytoreduction rate in many countries remains low, suggesting that a maximal attempt to achieve complete cytoreduction is currently not being performed by the majority of practising gynaecological oncologists. It is presumed that the variation in surgical practices and the achievement of complete cytoreduction between gynaecological oncologists are the result of the deficiencies in the current evidence and the selection bias associated with the retrospective studies, which would be addressed by the performance of a RCT.
What's new
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 8, 2011
| Date | Event | Description |
|---|---|---|
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
Acknowledgements
We thank Chris Williams for clinical and editorial advice, Jane Hayes and Anne Oestmann for designing the search strategy and Gail Quinn and Clare Jess for their contribution to the editorial process. We also thank Heather Dickinson for many helpful suggestions and being a co‐author on the protocol.
Appendices
Appendix 1. MEDLINE search strategy
MEDLINE Ovid 1950 to July Week 3, 2010
exp Ovarian Neoplasms/
(ovar* adj5 cancer*).mp.
(ovar* adj5 neoplas*).mp.
(ovar* adj5 carcinom*).mp.
(ovar* adj5 malignan*).mp.
(ovar* adj5 tumor*).mp.
(ovar* adj5 tumour*).mp.
1 or 2 or 3 or 4 or 5 or 6 or 7
exp Surgical Procedures, Operative/
surg*.mp.
"surgery".fs.
9 or 10 or 11
debulk*.mp.
cytoreduc*.mp.
13 or 14
8 and 12 and 15
"randomized controlled trial".pt.
"controlled clinical trial".pt.
random*.mp.
trial*.mp.
group*.mp.
exp Cohort Studies/
cohort*.mp.
series.mp.
17 or 18 or 19 or 20 or 21 or 22 or 23 or 24
16 and 25
Animals/
Humans/
27 not (27 and 28)
26 not 29
key: mp=title, original title, abstract, name of substance word, subject heading word, fs= floating subheading, pt=publication type
Appendix 2. EMBASE search strategy
EMBASE Ovid 1980 to Week 30, 2010
exp Ovary Tumor/
(ovar* adj5 cancer*).mp.
(ovar* adj5 neoplas*).mp. [
(ovar* adj5 carcinom*).mp.
(ovar* adj5 malignan*).mp.
(ovar* adj5 tumor*).mp.]
(ovar* adj5 tumour*).mp.
1 or 2 or 3 or 4 or 5 or 6 or 7
exp Surgery/
surg*.mp.
su.fs.
9 or 10 or 11
debulk*.mp.
cytoreduc*.mp.
13 or 14
8 and 12 and 15
exp Controlled Clinical Trial/
random*.mp.
trial*.mp.
group*.mp.
exp Cohort Analysis/
cohort*.mp.
series.mp.
17 or 18 or 19 or 20 or 21 or 22 or 23
16 and 24
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name, fs=floating subheading
Appendix 3. CENTRAL search strategy
CENTRAL Issue 3, 2010
MeSH descriptor Ovarian Neoplasms explode all trees
ovar* near/5 cancer*
ovar* near/5 neoplas*
ovar* near/5 carcinom*
ovar* near/5 malignan*
ovar* near/5 tumor*
ovar* near/5 tumour*
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7)
MeSH descriptor Surgical Procedures, Operative explode all trees
surg*
Any MeSH descriptor with qualifier: SU
(#9 OR #10 OR #11)
debulk*
cytoreduc*
(#13 OR #14)
(#8 AND #12 AND #15)
Data and analyses
Comparison 1. Residual disease (RD) < 1 cm versus microscopic disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 6 | Hazard Ratio (Random, 95% CI) | 2.20 [1.90, 2.54] | |
| 1.1 Advanced stage (III/IV) | 1 | Hazard Ratio (Random, 95% CI) | 2.20 [1.05, 4.64] | |
| 1.2 Stage III | 1 | Hazard Ratio (Random, 95% CI) | 2.12 [1.77, 2.53] | |
| 1.3 Stage IIIC | 3 | Hazard Ratio (Random, 95% CI) | 2.66 [1.78, 3.99] | |
| 1.4 Stage IV | 1 | Hazard Ratio (Random, 95% CI) | 1.93 [1.16, 3.22] | |
| 2 Progression‐free survival | 2 | Hazard Ratio (Random, 95% CI) | 1.96 [1.72, 2.23] | |
| 2.1 Stage III | 1 | Hazard Ratio (Random, 95% CI) | 1.95 [1.70, 2.24] | |
| 2.2 Stage IV | 1 | Hazard Ratio (Random, 95% CI) | 1.99 [1.25, 3.19] |
1.2. Analysis.
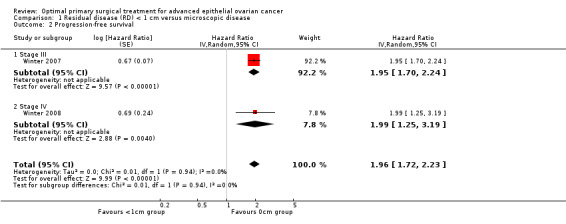
Comparison 1 Residual disease (RD) < 1 cm versus microscopic disease, Outcome 2 Progression‐free survival.
Comparison 2. Residual disease (RD) 1 ‐ 2 cm versus microscopic disease (stage IIIC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only |
2.1. Analysis.
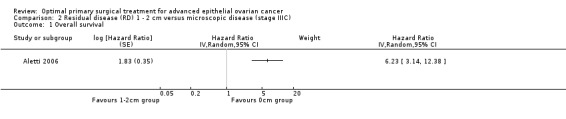
Comparison 2 Residual disease (RD) 1 ‐ 2 cm versus microscopic disease (stage IIIC), Outcome 1 Overall survival.
Comparison 3. Residual disease (RD) > 1 cm versus microscopic disease.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 4 | Hazard Ratio (Random, 95% CI) | 3.16 [2.26, 4.41] | |
| 1.1 Advanced stage (III/IV) | 1 | Hazard Ratio (Random, 95% CI) | 5.87 [2.68, 12.86] | |
| 1.2 Stage III | 1 | Hazard Ratio (Random, 95% CI) | 2.46 [2.06, 2.93] | |
| 1.3 Stage IIIC | 2 | Hazard Ratio (Random, 95% CI) | 3.36 [2.33, 4.84] | |
| 2 Progression‐free survival (stage III) | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only |
3.1. Analysis.

Comparison 3 Residual disease (RD) > 1 cm versus microscopic disease, Outcome 1 Overall survival.
3.2. Analysis.
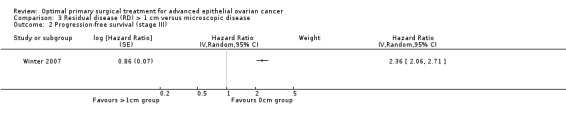
Comparison 3 Residual disease (RD) > 1 cm versus microscopic disease, Outcome 2 Progression‐free survival (stage III).
Comparison 4. Residual disease (RD) > 2cm versus microscopic disease (stage IIIC).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only |
4.1. Analysis.

Comparison 4 Residual disease (RD) > 2cm versus microscopic disease (stage IIIC), Outcome 1 Overall survival.
Comparison 5. Residual disease (RD) 1 ‐ 5 cm versus microscopic disease (stage IV disease).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2 Progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only |
5.1. Analysis.
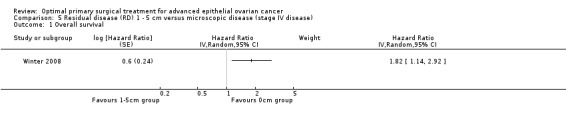
Comparison 5 Residual disease (RD) 1 ‐ 5 cm versus microscopic disease (stage IV disease), Outcome 1 Overall survival.
5.2. Analysis.
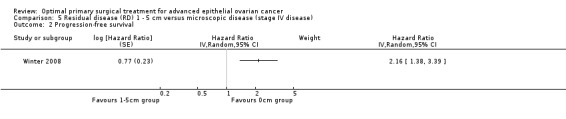
Comparison 5 Residual disease (RD) 1 ‐ 5 cm versus microscopic disease (stage IV disease), Outcome 2 Progression‐free survival.
Comparison 6. Residual disease (RD) > 5 cm versus microscopic disease (stage IV disease).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2 Progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only |
6.1. Analysis.
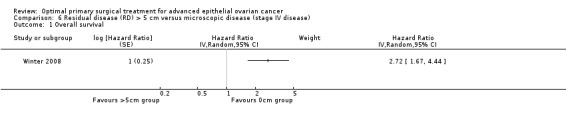
Comparison 6 Residual disease (RD) > 5 cm versus microscopic disease (stage IV disease), Outcome 1 Overall survival.
6.2. Analysis.
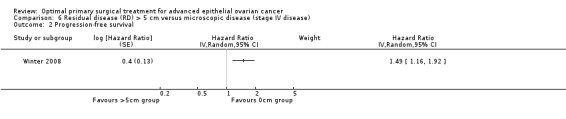
Comparison 6 Residual disease (RD) > 5 cm versus microscopic disease (stage IV disease), Outcome 2 Progression‐free survival.
Comparison 7. Residual disease (RD) > 1 cm versus RD < 1 cm.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 2 | Hazard Ratio (Random, 95% CI) | 1.36 [1.10, 1.68] | |
| 1.1 Advanced stage (III/IV) | 1 | Hazard Ratio (Random, 95% CI) | 1.67 [1.02, 2.72] | |
| 1.2 Stage IV | 1 | Hazard Ratio (Random, 95% CI) | 1.30 [1.03, 1.64] | |
| 2 Progression‐free survival (stage IV) | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only |
7.1. Analysis.
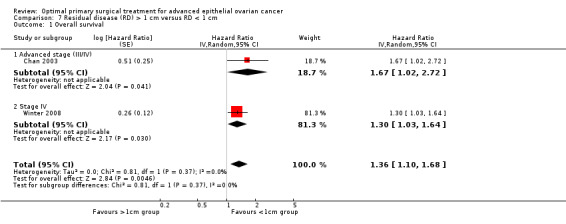
Comparison 7 Residual disease (RD) > 1 cm versus RD < 1 cm, Outcome 1 Overall survival.
7.2. Analysis.
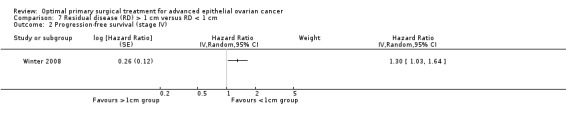
Comparison 7 Residual disease (RD) > 1 cm versus RD < 1 cm, Outcome 2 Progression‐free survival (stage IV).
Comparison 8. Residual disease (RD) 1 ‐ 2 cm versus RD < 1 cm.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only |
8.1. Analysis.
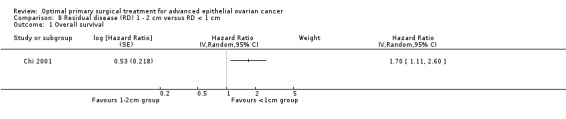
Comparison 8 Residual disease (RD) 1 ‐ 2 cm versus RD < 1 cm, Outcome 1 Overall survival.
Comparison 9. Residual disease (RD) > 2cm versus RD < 1 cm.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only |
9.1. Analysis.
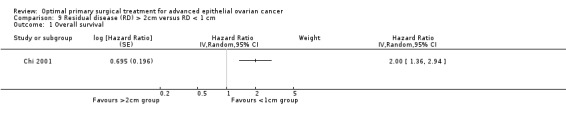
Comparison 9 Residual disease (RD) > 2cm versus RD < 1 cm, Outcome 1 Overall survival.
Comparison 10. Residual disease (RD) > 2 cm versus RD < 2 cm (stage IV disease).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 2 | Hazard Ratio (Random, 95% CI) | 1.65 [0.82, 3.31] | |
| 2 Progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only |
10.1. Analysis.
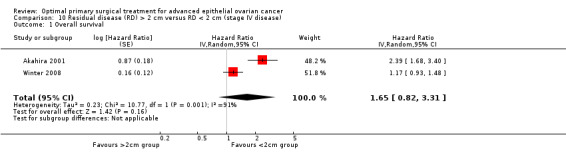
Comparison 10 Residual disease (RD) > 2 cm versus RD < 2 cm (stage IV disease), Outcome 1 Overall survival.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akahira 2001.
| Methods | Multicentre retrospective analysis: 24 Japanese institutions received questionnaires regarding stage IV epithelial ovarian cancer patients. |
|
| Participants | 225 women with stage IV ovarian cancer whose disease had been confirmed by exploration and only patients with complete medical records were included. Stage IV disease was defined according to FIGO. Only patients who underwent an initial attempt at surgical debulking were analysed. The median age in the study was 54 years (range: 26 to 85 years). All 225 women had FIGO stage IV disease. Histological cell type: Serous: 136 (60.5%), Mucinous: 16 (7%), Clear cell 26 (11.5%), Endometrioid 27 (12%), Transitional 4 (2%), Undifferentiated 12 (5%), Other 4 (2%). Extent of disease: Pleural effusion: 89 (39.5%), Liver: 34 (15%), Lung: 8 (3.5%), Lymph node: 44 (19.5%), Other: 15 (6.5%), Multiple sites: 35 (15%). Performance status: 0: 26 (11%), 1: 76 (34%), 2: 49 (22%), 3: 67 (30%), 4: 7 (3%). |
|
| Interventions |
Intervention group: Optimal cytoreduction was defined as no gross residual tumour greater than 2 cm in diameter. Comparison group: Suboptimal cytoreduction was defined as any gross residual disease remaining greater than 2 cm in diameter. |
|
| Outcomes | Overall survival: HR adjusted for histology and performance status:
Adverse events; Median blood loss, blood transfusions. |
|
| Notes | There were 70 women (31.1%) in optimal group and 155 (68.9%) in suboptimal group. The median patient follow‐up time was 47.5 months (range: 13 to 112 months). The median survival for all patients with stage IV ovarian cancer was 20 months, with an estimated 5‐year survival rate of 19.6%. Mean survival in optimal group was 32 months and 16 months in suboptimal group (P<0.0001). MV analysis included the histology and performance status as covariates in the model. The median duration of the debulking surgery was 240 minutes (range: 40 to 780 mins). The median estimated blood loss was 1085 ml (range 40 to 11,000 ml), and 112 patients (50%) received blood transfusions intra‐ and postoperatively. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were not randomised |
| Allocation concealment (selection bias) | High risk | Concealment of allocation irrelevant to this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 225/225 (100%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Representative sample | High risk | Only patients with FIGO stage IV disease were included in the study |
Aletti 2006.
| Methods | Retrospective cohort study of consecutive patients identified from surgical records. | |
| Participants | Patients with FIGO stage IIIC ovarian cancer, where disease status was extracted from surgical exploration notes. The mean and median age at study entry was 64.4 and 64 years respectively (range: 24‐87). All women presented with FIGO stage IIIC ‐ 194 (100%). Tumor cell type: Serous 126 (64.9%), Mucinous: 4 (2.1%), Endometrioid: 18 (9.3%), Clear cell: 7 (3.6%), Mixed: 17 (8.8%), Seroanaplastic: 17 (8.8%), Mullerian origin: 2 (1%). Tumor grade: 1: 1 (0.5%), 2: 13 (6.7%), 3: 180 (92.8%). ASA score: 1: 7 (3.6%), 2: 87 (44.8%), 3: 88 (45.4%), 4: 7 (3.6%), Unknown: 5 (2.6%). Ascites: Mean: 2076 ml, Median 1000 ml, (Range: 0‐12,000 ml). Extent of disease: carcinomatosis: 144 (74.2%), diaphragm involvement: 137 (70.6), mesentery: 138 (71.1), cul‐de‐sac: 163 (84), omentum 168: (86.6), Ascites 160: (82.5). |
|
| Interventions | Residual disease was noted as follows:
Optimal cytoreduction was defined as residual disease <1 cm. All patients were scheduled for treatment with first‐line postoperative platinum‐based chemotherapy (paclitaxel or cyclophosphamide for 6 to 8 courses, every 3 to 4 weeks). |
|
| Outcomes |
|
|
| Notes | Median length of follow‐up: 2.7 years. Mean length of follow‐up: 3.5 years (range, 0.02‐10.5 years). 5‐year disease specific death rate: Optimal group: 70/131 (53.4%); Suboptimal group: 56/63 (88.9%). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Retrospective study |
| Allocation concealment (selection bias) | High risk | Concealment of allocation irrelevant to this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 194/194 (100%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Representative sample | High risk | “Only patients with FIGO stage IIIC OC were included in the study” |
Chan 2003.
| Methods | Retrospective cohort study. | |
| Participants | All consecutive cases of advanced‐stage epithelial ovarian carcinoma diagnosed in younger women (range 22 ‐ 45 years) were identified from tumour registry databases and a comparable group of 52 women who averaged 21 years older (range 46‐85 years) was selected as controls. One‐to‐one matching from the same database was performed based on the date of diagnosis and stage of disease during the same period in the same institution. Thus, the controls were similarly distributed across 17 years. The mean age at study entry was 50.5 years with a range between 22 and 85 years (40 (SD=5.7) and 61 years (SD=8.7) for younger and older women respectively). 5 (4.8%) women had FIGO stage IIIA, 5 (4.8%) had stage IIIB, 74 (71.1%) women had stage IIIC and 20 (19.2%) had stage IV disease. Tumor cell type: Papillary serous 72 (63.16%), Mucinous: 3 (2.63%), Endometrioid: 17 (14.9%), Clear cell: 1 (0.88%), Small cell: 3 (2.63%), Undifferentiated: 8 (7%). Tumour grade: 1: 8 (7%), 2: 24 (21.1%), 3: 72 (63.2%). Performance status: 0: 65 (57%), 1‐2: 35 (30.7%), Unknown: 4 (3.51%). |
|
| Interventions | Residual disease was noted as follows:
Patients were divided into optimal (less than 1 cm) and suboptimal (1 cm or more) groups based on residual disease after initial surgery. Optimal debulking was achieved in 36 (69%) and 35 (67%) patients in younger in older groups respectively. All patients received either a platinum/paclitaxel or a platinum/cyclophosphamide regimen for primary chemotherapy and women who underwent neoadjuvant chemotherapy with interval debulking were removed from the study. Gynecology oncologists from our academic institution surgically staged all patients. |
|
| Outcomes | A multivariable analysis which included older versus younger age, stage (IV vs. III), performance status (1‐2 vs. 0) and residual disease (suboptimal vs. optimal) was performed to evaluate all factors that were significant in the univariable analysis. Overall survival: HR adjusted for prognostic categories (see above):
|
|
| Notes | The median follow‐up after surgery was 33 months (range 6‐142 months). ‐Five year survival: of Younger and Older Patients: Optimal: 59% and 21% in young and old patients respectively, Suboptimal: 28% and 22% in young and old patients respectively. Median Survival: Optimal: 66 months and 45 in young and old patients respectively, Suboptimal: 37 and 19 months in young and old patients respectively, P=0.003. Other variables in Cox model: Older versus younger age (HR 1.82; 95% CI 1.09 to 3.05), stage IV versus stage III disease (HR 3.00; 95% CI 1.71 to 5.25), performance status 1 to 2 versus 0 (HR 1.89; 95% CI 1.13 to 3.15). Despite the higher prevalence of poorly differentiated tumours in the older group, tumour grade (3 versus 1 to 2) was not an important prognostic factor in multivariable analysis (HR 1.06; 95% CI 0.57 to 1.97). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were not randomised |
| Allocation concealment (selection bias) | High risk | Concealment of allocation irrelevant to this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Representative sample | Low risk | All patients were stage III and IV |
Chi 2001.
| Methods | Retrospective cohort study. | |
| Participants | 282 patients with stage III and IV epithelial ovarian cancer. Patients with ovarian tumours of low‐malignant potential were excluded from this study. All patients were treated between 1987 and 1994 at Memorial Sloan‐Kettering Cancer Center (MSKCC). The median age at study entry was 59 years with a range between 22 and 87 years. 22 (8%) women had FIGO stage IIIA/IIIB, 194 (69%) had stage IIIC and 66 (23%) had stage IV disease. Tumor cell type: Serous 199 (71%), Endometrioid: 46 (16%), Clear cell: 19 (7%), Mucinous: 10 (4%), Mixed: 8 (3%). Tumour grade: 1: 13 (5%), 2: 69 (24%), 3: 184 (65%). Ascites: Yes: 238 (84%), No: 43 (15%), Unknown: 1 (1%). |
|
| Interventions | Patients were treated with primary surgery followed by chemotherapy. Type of surgeon Residual disease was noted as follows:
The following types of chemotherapy were given to women in the study: Cisplatin/cyclophosphamide: 143 (51%), Carboplatin/cyclophosphamide: 65 (23%), Carboplatin/paclitaxel: 31 (11%), Cisplatin/paclitaxel 24 (8%), Carboplatin: 7 (3%), Cisplatin 1 (<1%), None or unknown 10 (4%). Gynecology oncologists from our academic institution surgically staged all patients. |
|
| Outcomes | A multivariable analysis which included age, stage (IIIC and IV vs. IIIA/IIIB), ascites (yes vs. no) and residual disease (1‐2cm and >2cm vs. <1cm) was performed to evaluate important prognostic factors. Overall survival: HR adjusted for prognostic categories (see above):
Direct surgical morbidity 8 patients (2.83%) died within 1 month of surgery. |
|
| Notes | Of the 295 patients who were treated for FIGO stage III and IV epithelial ovarian cancer at this centre over the period of the study, 13 (5%) were lost to follow‐up, and the remaining 282 form the study group for this analysis. Median follow‐up in the study was 32 months (range: 1‐139 mo). The chemotherapy was platinum based and when patients who had initially had single agent therapy or combinations with cyclophosphamide recurred they were often given paclitaxel. Suvival was calculated as the number of months from initial surgery to death or the date of last follow‐up. 214 of the 282 (76%) patients were dead from disease or other causes at the time of census. Multivariate analysis: Only patient age at diagnosis (p=0.001), presence of ascites (P=0.001) and the size of residual disease after primary cytoreductive surgery {1cm vs 1‐2cm vs. >2cm (p=0.02 and 0.001, respectively)} retained prognostic significance. Kaplan‐Meier curve Patients with no more than 1cm of residual disease after primary surgery have a 5‐year survival of 50% and a median survival of 55 months. There is no statistically significant difference in survival between those patients with 1 to 2 cm of residual disease and those with greater than 2 cm residual (P=0.40). this combined group of patients have a 5‐year survival of 22% with a median survival of 28 months. Impact of residual tumour volume for FIGO stage III A subgroup analysis of the 216 patients with stage III disease was done to examine the impact of size of residual disease on survival. 56 of these patients had up to 1 cm of residual disease and had 5‐year survival of 50% and median survival of 56 months. 73 of these patients had between 1 and 2 cm of residual disease and had 5‐year survival of 28% and median survival of 31 months. 87 of these patients had greater than 2 cm of residual disease after surgery and had 5‐year survival of 21% and a median survival of 28 months. The differences in survival are statistically significant between the patients with up to 1 cm of residual disease and the patients in the other 2 groups (P=0.001). There is no statistically significant difference in survival between the patients who had more than 1 cm residual disease. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were not randomised |
| Allocation concealment (selection bias) | High risk | Concealment of allocation is irrelevant to this retrospective study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 282/295 (96%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Representative sample | Low risk | Study included women with advanced (stage III and IV) epithelial ovarian cancer and reported important prognostic factors |
Chi 2006.
| Methods | Retrospective study. | |
| Participants | Patients with stage IIIC epithelial ovarian cancer. The median age at study entry was 60 years (range: 22‐87). All women presented with FIGO stage IIIC ‐ 465 (100%). Tumour cell type: Serous 331 (72%), Endometrioid: 57 (12%), Clear cell: 22 (5%), Mixed: 53 (11%). Tumour grade: 1: 13 (3%), 2: 90 (19%), 3: 339 (73%), Unknown: 23 (5%). Ascites: Median 1,600 ml, (Range: 0‐17,000 ml), Presence of ascites (N=429): No= 58 (14%); Yes= 371 (86%). |
|
| Interventions | Type of surgeon: Gynecologic oncologist. Options for residual disease on the standardised operative form were as follows:
Optimal is defined in 2 ways as no residual disease and <1cm, suboptimal defined as >1cm. Postoperative chemotherapy records were available in 440/465 (95%) patients. Of these 440 patients, 426 (97%) were treated with primary platinum‐based systemic chemotherapy with the intent to treat with at least 6 cycles. |
|
| Outcomes | Three patients (0.6%) died within 30 days of surgery. Overall survival: HR adjusted for age and ascites using Cox model: <1cm vs 0cm HR 2.07 (95% CI 1.23 to 3.46); >1cm vs 0cm HR 3.70 (95% CI 2.27 to 6.04). |
|
| Notes | Median follow‐up: 38 months (range:1‐199 mo). 17 year death rate: Optimal group: 105/236; Suboptimal group: 188/229. Median overall survival in relation to the 5 residual disease categories was: No gross residual disease: 106 months; gross <0.5 cm: 66 months; 0.6 to 1.0 cm: 48 months; 1 to 2 cm: 33 months; and >2 cm: 34 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Retrospective study |
| Allocation concealment (selection bias) | High risk | Concealment of allocation irrelevant to this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 465/465 (100%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists. |
| Representative sample | High risk | "Patients with stage IIIC epithelial ovarian cancer who underwent primary cytoreductive surgery at our institution" |
Eisenkop 2003.
| Methods | This is a prospective study of patients with FIGO stage IIIC ovarian treated with primary cytoreductive surgery followed by platinum based chemotherapy between 1990 and 2002 at a single North American institution. | |
| Participants | 408 consecutive women presenting with stage IIIC epithelial ovarian cancer form the study group. The median age at study entry was 62.8 years (range: 24‐91). All women presented with FIGO stage IIIC epithelial ovarian cancer ‐ 408 (100%). Tumour cell type: Serous: 239 (58.5%), Unspecified adenocarcinoma: 98 (24%), Endometrioid: 32 (8%), Clear cell: 10 (2.5%), Mucinous: 18 (4.5%), Mixed: 9 (2%), Transitional cell: 2 (0.5%). Tumour grade: 1: 21 (5%), 2: 82 (20%), 3: 304 (75%), Unspecified: 1 woman. Volume of ascites: None: 20 (5%), <1000ml: 114(28%), >1000ml: 249(61%), Not recorded: 24(6%). GOG Performance score: 0: 17 (4%), 1: 88 (21.5%), 2: 177 (43.5%), 3: 59 (14.5%), 4: 2 (0.5%), Unspecified: 65 (16%). Preoperative tumour volume: Location of the largest metastases: Omentum and adjacent structures: 228 (56%), Pelvis: 102 (25%), Retroperitonal lymph nodes: 34 (8%), Diaphragm: 12 (3%), Other (Large bowel, small bowel, mesentery, etc): 32 (8%); Largest metastatic disease: <10cm: 104 (26%), >10cm: 302 (74%). |
|
| Interventions | Residual disease was noted as follows:
Surgery was undertaken by a gynaecological oncologist and disease was assessed intraoperatively in each of the following 5 regions: the left and right upper abdominal quadrants, the pelvis, the retroperitoneum and the central abdomen. A specifically defined numerical rank of 0‐3 was assigned to each of the 5 regions and the ranks for each of the 5 regions were summed to give a total score before cytoreduction. Optimal cytoreduction was defined as complete cytoreduction with no visible residual disease. The authors have previously described in other publications how this can be achieved at different anatomical sites but recourse to bowel resection was routine as was pelvic and para‐aortic nodal dissection. Post operative chemotherapy was platinum based: cisplatin (50‐100mg/m2) or carboplatin (300‐400mg/m2) given in combination therapy with either cyclophosphamide or paclitaxel every 3 weeks for a planned 6 to 8 cycles. |
|
| Outcomes | Overall survival: HR adjusted for sum of rankings (a numerical ranking system was devised to reflect the continuum of progressively extensive tumour involvement for five anatomic regions) using Cox model: <1cm vs 0cm HR =2.32 (95% CI: 1.20 to 5.37); >1cm vs 0cm HR =2.98 (95% CI: 1.74 to 5.23). Direct surgical morbidity and mortality Postoperative mortality occurred in 10 (2.5%) patients. Other morbidity including surgically related systemic morbidity such as chest infection, thromboembolic disease and cardiovascular events have not been reported. Recovery The median length of hospital stay was 10 days. |
|
| Notes | The median follow‐up interval was 32.8 months. Suvival was measured in months from the date of primary surgery to the time of death or last follow‐up appointment using life table analysis. Survival outcomes were analysed based on the numerical ranking of disease in each anatomical region, the sum of the ranking and the cytoreductive outcome. The median survival was 58.2 months (24‐91%) and the estimated 5‐year survival was 49%. Ranking of disease load 349 (85.5%) of patients had ranking in all 5 designated regions. Ranking was not possible in the rest because lymph node dissection was deferred in 48 patients (12%) or the pattern of spread was inconsistent with ranking criteria in 16 patients (4%). On univariate analysis, categorisation of the sum of ranking scores ( 0‐5 vs 6‐10, vs ≥11), as well as ranking in the left upper abdominal quadrant and in the central abdomen were statistically important determinants of survival. Univariate analysis showed that any rank score over zero (any disease) in the left upper abdominal quadrant (P = 0.01) and in the central abdominal region (P=0.04) adversely affected survival. An effect of the anatomical site of disease on survival was not confirmed on multivariate analysis. On multivariate analysis, survival was most influenced by the completeness of cytoreduction (P = 0.001), and less influenced by the categorised sum of rankings (P=0.05). This study demonstrates that high rates of complete cytoreduction can be achieved within dedicated teams with suitable training. The independent effect of completeness of cytoreduction on survival is confirmed though the median length of follow‐up in the report is modest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were not randomised |
| Allocation concealment (selection bias) | High risk | Concealment is not relevant to this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 408/408 (100%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Representative sample | High risk | "Between 1990 and 2002, consecutive patients with stage IIIC ovarian cancer having primary cytoreductive surgery were eligible" |
McGuire 1995.
| Methods | Retrospective analysis of a prospective randomised controlled trial comparing different chemotherapy dosing schedules. It aimed to determine the importance of chemotherapy dose intensity on survival, progression free survival (PFS), and response. This was not a trial of surgery but the report allows a comparison of survival outcomes for subgroups women with stage III ovarian cancer who have had <2 cm or ≥ 2cm of residual disease following surgery and therefore is relevant to this review. | |
| Participants | 458 women with FIGO stage III and IV epithelial ovarian cancer were recruited. These were women who had more than 1 cm residual disease following initial surgery. 27 women were ineligible: incorrect stage (n=5), incorrect primary tumour (n=9), incorrect cell type (n=7), history of prior malignancy (n=3), prior chemotherapy (n=1) and other (n=2). Women with borderline ovarian tumours (low malignant potential) were excluded. Recruitment was from December 1986 to April 1990 and all patients had undergone a surgical procedure. The median age at study entry was 60 years (range: 20‐83). 305 (67%) and 153 (33%) women had FIGO stage III and IV disease respectively. Tumour cell type: Serous 312 (68.1%), Endometrioid: 64 (14%), Mucinous; 12 (2.6%), Clear cell: 12 (2.6%), other: 58 (12.7%). Tumour grade: 1: 26 (9%), 2: 114 (39%), 3: 152 (52%), Not specified 2 (1%). GOG score: 0: 150 (32.8), 2: 213 (46.5%), 3: 95 (20.7%). |
|
| Interventions | Residual disease was noted as follows:
Definition of optimal surgery: All patients were suboptimally cytoreduced with >1cm of residual disease. Chemotherapy: Two trial arms with patients receiving either standard chemotherapy: cyclophosphamide 500mg/m2 and cisplatin 50mg/m2 intravenously every 3 weeks for 8 courses OR intense chemotherapy: cyclophosphamide 1000mg/m2 and cisplatin 100mg/m2 intravenously every 3 weeks for four courses. Dose modification was rigidly controlled to maintain intensity. |
|
| Outcomes | Overall survival and progression‐free survival: HR adjusted for age, GOG performance status, histological sub‐type, stage/residual disease and measurable disease using Cox model: III, ≥ 2cm vs III, 1‐2cm: HR=1.91; IV, 1‐2cm vs III, 1‐2cm: HR=1.89; IV, ≥ 2cm vs III, 1‐2cm: HR=2.29. Overall and progression‐free survival (PFS) were measured from the date of randomisation. All eligible patients were included in the analysis of outcomes. All causes of death were used to calculate survival, and the estimates were based on Kaplan Meier procedures. |
|
| Notes | Mean and median length of follow‐up were not reported. Since this trial was a trial of chemotherapeutic regimens, the randomisation did not aim to compare the effect of different degrees of surgical debulking. The findings borne out on multivariate analysis are similar to those in retrospective and cohort studies. The prospective nature of this study has however facilitated the collection of a fairly complete data set and gives this work some authority. Other variables in Cox model Age (years): reference group: women aged less than 55 years (P=0.47): 55‐65: HR=1.08; >65: HR=1.38. GOG performance status: reference group: GOG 0 (P=0.009) 1: HR=1.26, 2: HR=1.56. Histological subtype: reference group: Serous adenocarcinoma (P<0.001): Endometrioid: HR=0.951, Mucinous: HR=8.31, Clear cell: HR=1.79, Other: HR=0.84. Measurable disease: reference group: No: (P=0.01) Yes: HR=1.43. From the study both advancing age and worsening performance status were associated with poorer survival. In addition, mucinous histology is associated with an 8.3 times greater death rate than serous histology (P<0.001). The study shows residual disease after surgery impacts on survival. Even in suboptimal cytoreduction (residual disease greater than 1cm), women with stage III disease and residual disease diameter less than 2cm exhibited lower death rates than either those with stage III diease and residual disease diameter of ≥ 2cm, or those with stage IV disease. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | This was not a randomised trial of surgery |
| Allocation concealment (selection bias) | High risk | Concealment is not relevant to this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 458/458 (100%) 485 were included in the study, but the authors specified the number of patients who were excluded and the reasons for their exclusion |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Representative sample | Low risk | 458 women with FIGO stage III and IV epithelial ovarian cancer were recruited |
Salani 2007.
| Methods | Labelled as a case‐control study but for the scope of this review it was effectively a retrospective cohort study. All patients with pathologically confirmed Stage IIIC or IV epithelial ovarian cancer undergoing primary surgery at John Hopkins Medica Institutions (JHMI) between October 1997 and February 2006 were retrospectively identified from institutional tumour registry databases. | |
| Participants | 125 patients with pathologically confirmed Stage IIIC or IV epithelial ovarian cancer undergoing primary surgery. Patients were excluded from the study for the following: histology consistent with low‐grade, borderline, carcinosarcoma or non‐epithelial carcinoma, or the administration of neoadjuvant chemotherapy (treatment with chemotherapy agents prior to surgical cytoreductive surgery). Median age at study entry ranged between 62 and 66 in the three surgical groups. 97 (78%) and 28 (22%) women had FIGO stage III and IV disease respectively. Median ascites was 2000, 550, 1250ml in the multiple bowel resection, the one or less resection and the suboptimal groups respectively. |
|
| Interventions | Residual disease was classified as follows:
Patients undergoing optimal primary cytoreductive surgery, defined as residual disease <1 cm, with two or more bowel resections were identified as cases (n=34). Two control groups were utilised for comparison. The first control group was selected as patients undergoing optimal primary cytoreductive surgery with one or less bowel resections, matched at a ratio of two controls to each case by age and stage (n=68). The second control group of patients consisted of patients undergoing primary cytoreductive surgery with suboptimal residual disease, defined as greater than 1 cm, irrespective of the number of bowel resections (n=23). Individual subject data were collected retrospectively from inpatient and ambulatory medical records. Surgical and pathology reports from the primary surgery were reviewed in all cases. |
|
| Outcomes | Overall survival: HR adjusted for covariates having some prognostic value in univariate analyses but it was not reported which ones were significant. Hence the HR may have adjusted for any of the following variables: number of bowel resections, age, stage and ascites. <1cm (optimal) vs 0cm (complete): HR=2.2 (95% CI 1.08 to 4.44), P=0.03; >1cm (suboptimal) vs 0cm: HR=5.9 (95% CI 2.7 to 13.0), P<0.0001. |
|
| Notes | All patients received adjuvant chemotherapy with combination platinum‐based and taxane chemotherapy, excluding two patients who suffered postoperative mortality. The median follow‐up of surviving patients was 19.3 months from the time of surgery and the median survival was 37 months for all patients with optimal residual. The authors reported 28 women were optimally debulked in the 0‐1 bowel resection group but this was in fact 38 as this corresponded to 55.9% quoted in table 2 on page 496 (38/68). Patients with optimal cytoreduction who underwent ≥2 bowel resections experienced a higher median EBL, 700 mL, compared to a median EBL of 500 mL for patients who underwent one or no bowel resections (p=0.01). Length of hospital stay was also significantly longer in patients who underwent multiple bowel resections compared to patients who underwent one or no bowel resections, median LOS of 10 days compared to 7 days, respectively (P=0.01). Major postoperative complications occurred in 30 (29.4%) of the 102 patients undergoing optimal cytoreductive surgery There were 14/68 complications in the group undergoing 1 or no bowel resection: 20.6% (95% CI 11.7% 32.1%), compared to 16/34 in the group undergoing two bowel resections: 47.1% (95% CI 29.8%, 64.9%, P=0.01). This included two postoperative deaths; one in an elderly patient who developed refractory sepsis and another in a patient who developed a massive pulmonary embolism. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were not randomised |
| Allocation concealment (selection bias) | High risk | Concealment of allocation irrelevant to this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Representative sample | High risk | 125 patients with pathologically confirmed Stage IIIC or IV epithelial ovarian cancer undergoing primary surgery. Patients with stage IIIA and IIIB were excluded |
Van Geene 1996.
| Methods | Prospective cohort study: The two groups were defined from data collected prospectively at laparotomy. All patients with ovarian cancer referred to the departments of gynaecological oncology at two hospitals between 1981‐1989 were entered into prospective surgical studies. |
|
| Participants | During the 8‐year period in the study a total of 256 patients with previously untreated primary EOC were referred for consideration of surgery and chemotherapy. Thirty‐seven patients with stage II disease were excluded from this analysis leaving 219 patients with stage III‐IV disease to form the basis of the study. Median age at study entry was 57 years (range 24‐75 yrs). 180 (82%) and 39 (18%) women had FIGO stage III and IV disease respectively. Histological cell type was as follows: serous: 134 (61%), endometrioid: 34 (15%), mucinous: 32 (15%), clear cell: 7 (3%), undifferentiated: 12 (6%). 50 (25%) women had tumour grade classified as being well, 68 (34%) had grade as moderate, 75 (37%) had poor grade and in 9 (4%) women the grade was unknown. 101 (46%) women had GOG performance status 0, 94 (43%) had status 1, 23 (10.5%) women had status 2 and for 1 (0.5%) woman their status was unknown. Mode of spread was as follows: Bulky: 100 (46%), spreading: 119 (54%). |
|
| Interventions | Reported categories for residual disease were as follows:
All patients received cis‐platinum containing chemotherapy at the dose of 75 mg/m2 up to a total of six courses depending on response and toxicity. |
|
| Outcomes | Overall survival: HR adjusted for performance status and pattern of spread using Cox model: >2cm vs <2cm: HR=1.83, P<0.0001. We requested the exact P value and 95% CI from the study authors but the data were no longer available. Table 4 is confusing as no residual disease and less than 2cm residual disease was compared to >2cm. This was grouped in table 2. |
|
| Notes | The two groups were defined from data collected prospectively at laparotomy. Patients with small volume (≤ 0.5 cm) but widespread disease(> 10 metastatic nodules) were assigned to the seedling group and patients with large volume disease (> 0.5 cm) spread outside the pelvis were assigned to the bulky disease group. Optimal debulking, ie residual disease less than 2 cms, was achieved in 92 (42%) of the patients with similar rates between the two groups (P = 0.09). Complete macroscopic clearance was achieved in only 15 patients, all of which were in the bulky spread group. Complete macroscopic clearance was achieved in only 15 patients, all of which were in the bulky spread group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Patients were not randomised |
| Allocation concealment (selection bias) | High risk | Concealment of allocation irrelevant to this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 218/219 (99.5%) for overall survival and appropriate statistical techniques were used to account for censoring |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | >2cm vs <2cm: HR=1.83, P<0.0001 Table 4 is confusing as no residual disease and less than 2cm residual disease was compared to >2cm. This was grouped in table 2 |
| Representative sample | Low risk | Thirty‐seven patients with stage II disease were excluded from this analysis leaving 219 patients with stage III‐IV disease to form the basis of the study |
Winter 2007.
| Methods | The current study was a retrospective review of data from patients treated with platinum and paclitaxel combination chemotherapy on one of six prospective randomised clinical trials conducted by GOG: protocols 111, 114, 132, 152, 158, and 172. GOG 111: included suboptimal (> 1cm RD) stage III/IV EOC (ELIGIBLE PATIENTS =123). GOG 114: included optimal (< 1cm RD) stage III EOC (ELIGIBLE PATIENTS =226). GOG 132: included suboptimal (> 1cm RD) stage III/IV EOC (ELIGIBLE PATIENTS =147). GOG 152: included suboptimal (1‐cm residual), stage III EOC (ELIGIBLE PATIENTS =397). GOG 158: included suboptimal (> 1cm RD) stage III EOC (ELIGIBLE PATIENTS =792). GOG 172: included optimal (≤ 1cm RD) stage III EOC/PSPC (ELIGIBLE PATIENTS =210). |
|
| Participants | Data from 1895 patients with stage III invasive EOC who underwent primary surgical cytoreduction followed by paclitaxel/platinum chemotherapy, while participating in one of six GOG clinical trials, was analysed for the present study. The median age was 57 years (range 16 to 86 years). All 1895 women had FIGO stage III. Histological cell type was as follows: serous: 1392 (73.5%), endometrioid: 166 (8.8%), mucinous: 34 (1.8%), mixed Epithelial: 142 (7.5%), adenocarcinoma unspecified: 49 (2.6%), clear cell: 62 (3.3%), undifferentiated: 26 (1.4%), other: 24 (1.3%). 179 (9.5%) women had tumour grade 1, 719 (37.9%) had grade 2 and 997 (52.6%) women had tumour grade 3. Tumor grade details: 1: 179 (9.5%), 2: 719 (37.9%), 3: 997 (52.6%). Ethnicity details: White: 1669 (88.1%), African‐American: 111 (5.9%), Other: 115 (6.1%). |
|
| Interventions | Reported categories for residual disease were as follows:
Optimal was not defined, yet patients were divided into three groups for analysis, based on residual disease status (as above). The following chemotherapy schedules were given in the 6 trials:
|
|
| Outcomes | Overall survival and progression‐free survival: HR adjusted for age (discrete), race, GOG performance status, histology and tumour grade using Cox model: 0.1‐1cm vs 0cm (microscopic): HR=2.11 (95% CI 1.78 to 2.49), P<0.001 and HR=1.96 (95% CI 1.70 to 2.26), P<0.001 for OS and PFS respectively. >1cm vs 0cm (microscopic): HR=2.47 (95% CI 2.09 to 2.92), P<0.001 and HR=2.36 (95% CI 2.04 to 2.73), P<0.001 for OS and PFS respectively. |
|
| Notes | One thousand five hundred and five recurrences and 1323 deaths were identified during a median follow‐up period of 43 months: The median PFS was 17.1 months (95% CI: 16.4 to 17.8 months), The median OS was 45.3 months (95% CI: 43.0 to 47.7 months). PFS for disease residual: Microscopic: N= 437, PFS was 33.0 months, 0.1‐1.0 cm: N= 791, PFS) was 16.8 months, >1.0 cm: N= 667, PFS was 14.1 months, P < 0.001. OS for disease residual: Microscopic: N= 437, OS was 71.9 mo, 0.1‐1.0 cm: N= 791, OS was 42.4 mo, >1.0 cm: N= 667, OS was 35.0 mo, P < 0.001. Increasing age was associated with decreased PFS and OS. Median PFS and OS were shorter for patients with a performance status (PS) of 1 or 2 when compared with those with a PS of 0. No difference in median PFS was evident between PS 1 and PS 2 patients, whereas the difference in median OS between the same groups was observed. Based on tumour histology, patients with endometrioid histology had improved clinical outcomes compared with those with serous tumours. Patients with mucinous or clear‐cell tumours had decreased PFS and OS. Patients with mucinous cell type had a median OS of only 15 months compared with 24, 45, and 56 months for clear‐cell, serous, and endometrioid cell types, respectively. Patients with microscopic residual disease had the longest PFS and OS 33 and 72 months, respectively compared with patients with any gross residual disease. The differences in median PFS and OS between the 0.1 to 1.0 cm and > 1.0 cm residual disease groups were also evident, albeit small (3 months in median PFS and 7 months in median OS). Patients with grade 2 or 3 tumours were associated with decreased PFS and OS. Race was not significantly associated with PFS or OS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Retrospective study |
| Allocation concealment (selection bias) | High risk | Concealment of allocation irrelevant to this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 1,895/1,895 (100%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Representative sample | High risk | All patients had stage III disease |
Winter 2008.
| Methods | Retrospective review of 4 RCTs. The current study was a retrospective review of data from patients with stage IV EOC treated with platinum and paclitaxel combination chemotherapy on one of four prospective randomised clinical trials conducted by the GOG: protocols 111, 132, 152, and 162. | |
| Participants | 360 patients with stage IV invasive EOC who underwent primary surgical cytoreduction followed by paclitaxel/platinum chemotherapy while participating in one of four GOG clinical trials. The median age of patients was 59 years (range, 24 to 86 years). 317 (88%) women were white, 28 (8%) were black and 15 (4%) were of other ethnic origin. 97 (27%) had GOG performance status 0, 203 (56%) had status 1 and 60 (17%) had status 2. 24 (7%) women had tumour grade 1, 112 (31%) grade 2 and 224 (62%) had grade 3 disease. Histology was as follows: Serous 268 (74.5%) Endometrioid 28 (8%), Mucinous 7 (2%), Clear cell 12 (3%), Adenocarcinoma unspecified 9 (2.5%), Mixed epithelial 22 (6%), Undifferentiated 9 (2.5%), Other 5 (1.5%). The median residual tumour size was 3 cm (Range 0.0‐40.0). Stage IV disease site was as follows: Distant: 45 (12.5%), Parenchymal liver: 64 (17.75%), Pleural effusion: 172 (47.75%), Subcutaneous: 32 (9%), others: 3 (1%), multiple sites: 44 (12%). |
|
| Interventions | The maximum diameter of residual tumour that was used to define optimal cytoreduction: 1 cm (in original RCTs). All 4 RCTs included suboptimal disease (> 1cm). Residual disease was noted as follows:
Optimal cytoreduction was defined as residual disease <1 cm and a sensitivity analysis was performed defining residual disease as <2cm. All patients were treated with primary surgical cytoreduction and six cycles of a 24‐hour infusion of intravenous paclitaxel 135 mg/m2, followed by intravenous cisplatin 75 mg/m2. |
|
| Outcomes |
|
|
| Notes | The median length of follow‐up was 28 months. When evaluating the association of clinicopathologic factors with residual disease status, there was no difference between the residual disease groups and demographic, clinical,and pathologic factors. Stage IV site did not seem to have significant association with residual disease group distributions. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Retrospective study |
| Allocation concealment (selection bias) | High risk | Concealment of allocation irrelevant to this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 320/320 (100%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Representative sample | High risk | All stage were stage IV disease |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alberts 1993 | No survival analysis by RD as all patients had suboptimal surgery (defined as more than 2 cm). |
| Alberts 1996 | No multi‐variate analysis data. |
| Alphs 2006 | Included only 78 patients, 8 patients we're early stage and 9 patients received NAC. |
| Andersen Soegaard 2005 | This study included only 83 patients, of which 66 received platinum‐based chemotherapy. No multivariate analysis was performed. |
| Bailey 2006 | Chemotherapy data are absent. |
| Baker 1994 | 95% CI or SE (HR) are not reported and the HR point estimate for OS of 1.66 across all categories and it is not clear if the <1cm category was used as the reference group when compared to both 1‐2cm and >2cm residual disease. |
| Barda 2004 | 27.3% of OvCa received non‐platinum chemotherapy. |
| Benedetti‐Panici 1996 | Included only 66 patients and stage IIb, No survival data per RD. Also included NAC/IDS. |
| Bertelsen 1990 | Study does not include a multivariate analysis. |
| Bertelsen 1993 | No survival data per residual disease. |
| Bonnefoi 1999 | 38 patients had NAC and 27 patients had non‐platinum chemotherapy. |
| Brinkhuis 1996a | No direct comparison by size of residual disease and there is no multivariate analysis. |
| Brinkhuis 1996b | 1 group of patients did not receive platinum chemotherapy except at progression. Survival data per RD is reported for all patients collectively. |
| Bristow 1999 | Included only 84 patients. |
| Cai 2007 | Included 95 patients. We suspect that IDS cases were included. |
| Colozza 1997 | Included only 39 patients. |
| Conte 1991 | No survival data per residual disease. |
| Conte 1996 | There is no optimal group. No survival data per residual disease. |
| Crawford 2005 | 18% of the cases were stage IC and II. |
| Creasman 1990 | All cases were sub‐optimal, defined as RD greater than 1 cm, no analysis by RD. |
| de Oliviera 1990 | 1 arm did not receive platinum‐based chemotherapy. |
| Del Campo 1994 | Included only 91 patients. |
| di Re 1996 | 14 patients had borderline tumours. Also included stage II cases. Before 1979, patients received non‐platinum chemotherapy. |
| Gao 2001 | Only 31 cases. |
| Geisler 2004 | 24 patients were Stage I and II. |
| Gershenson 1989 | Included only 50 patients. |
| Gershenson 1992 | All patients were optimal, defined as RD less than 2 cm. No further analysis of survival by RD. |
| Gershenson 1995 | Included only 51 patients. |
| Grem 1991 | Included only 43 patients. |
| Hainsworth 1990 | Included only 25 patients. |
| Hakes 1992 | Included only 78 patients. |
| Hamid 2002 | Only included 62 patients. |
| Hardy 1991 | Included only 30 Stage IV patients. |
| Hoskins 1992 | All patients are optimal i.e. less than 1 cm. Survival data is per preoperative disease volume rather than RD. |
| Hoskins 1996 | Included only 29 patients. |
| Hoskins 1997 | No survival by residual disease. |
| Itamochi 2002 | Optimal surgery i.e. size of RD is not properly defined. |
| Kaern 2005 | Included only 31 stage III patients with no control group having RD more than 1 cm. |
| Kirmani 1994 | Included only 29 patients. |
| Kristensen 1995 | Included only 27 patients. |
| Le 1997 | Data for stage IIIC and IV subgroup was not reported and authors no longer had access to this data. |
| Lorusso 1998 | Included only 34 patients. |
| Malik 1998 | Included only 21 patients. |
| Marchetti 1993 | Included only 70 patients. |
| Ngan 1989 | Contained 65 patients only and 15 patients were excluded, so only 50 patients. |
| Omura 1989 | 95% CIs and P values from Cox model in adjusted estimates are not reported. Can not use Parmar's methods given the number of deaths and log rank p value as we need the unadjusted estimate. |
| Palmer 1992 | Included only 70 patients. |
| Piver 1991 | 43 patients did not receive platinum‐based chemotherapy. No multivariate analysis. |
| Redman 1986 | Included 89 patients, 11 of whom initially did not receive platinum chemotherapy. |
| Rose 2004 | Reported on outcome after ''secondary'' debulking surgery. However Winter et al., 2007 included the results of GOG 152 by residual diease after primary cytoreductive surgery. This has been confirmed through personal communication with GOG statistician (Dr Mark Brady). |
| Sessa 1991 | No multivariate analysis performed. |
| Shapiro 1998 | Included only 26 patients. |
| Shinozuka 1999 | Some patients received preoperative chemotherapy. |
| Skarlos 1996 | Included patients with stage IIC. |
| Strauss 1996 | Included 42 patients only. |
| Sun 2000 | Patients who did not receive preoperative chemotherapy are only 76. Nature of chemotherapy received not clear. |
| Sutton 1989 | Included only 56 patients. |
| Takano 2006 | Most patients were early stage disease which can not be separated from late staged cases. |
| Takano 2007 | Included early stage disease (Stage IC and II) which can not be separated from late staged cases. |
| Tay 1996 | Included 62 patients only. Did not include survival data per optimal versus suboptimal. |
| Taylor 1994 | Included only 64 patients. |
| Tingulstad 2003 | 6 patients did not receive chemotherapy and 6 patients received non‐platinum chemotherapy. |
| Todo 2003 | Included patients who had NEC/IDS. |
| Uyar 2005 | 18 patients were stage I and II. No survival data per RD. |
| Vallejos 1997 | Included only 30 patients. |
| Van Der Burg 1996 | Reported results per residual disease after NAC/IDS. |
| Vergote 2010 | In the primary debulking arm, 57 patients (17%) received neoadjuvant chemotherapy and underwent interval debulking surgery. Complication in the primary debulking arm included 24 patients who had side effects or complications typical of chemotherapy e.g. taxol allergy, neutropenic sepsis, neuropathy (supplementary appendix, page 38). |
| Wadler 1996 | Survival reported per residual disease in all patients including 118 who received non‐platinum chemotherapy. |
| Warwick 1995 | 31 patients were stage II. No survival data per RD. |
| Willemse 1992 | Included only 76 patients. |
| Wils 1990 | Included only 88 patients. |
| Wimberger 2007 | Multivariate analyses did not include residual disease and the study also included women with stage IIB and IIC disease. we attempted to contact the authors for further information but at time of submission of the review there had been no correspondence. |
| Yamamoto 2007 | Included 67 ''selected'' patients with rare histological subtype. |
| Zang 1999 | Included only 71 patients and 31 of them received neoadjuvant chemotherapy. |
Differences between protocol and review
We added the following study constraint in the types of studies section, as it was apparent that selection bias would have considerably distorted results.
In order to minimise selection bias, we included only studies that used statistical adjustment for baseline case mix using multivariable analyses (for example, age, stage, grade).
We removed discussion of unadjusted results from the data synthesis, subgroup analysis and investigation of heterogeneity and sensitivity analysis sections as we do not plan to use unadjusted results in future updates, due to the risk of selection bias.
We also only included data from randomised controlled trials (RCTs), prospective and retrospective cohort studies and unselected case series of 100 or more patients that included concurrent comparison groups. We had initially stated that all studies meeting the inclusion criteria which allocated 30 or more women would be included, but many small studies were of inadequate quality so we added this constraint (> 100 in the study) to ensure higher quality evidence.
We did not find any relevant ongoing trials or active trial groups, so we did not make any contacts. The following sentence in the 'Unpublished and Grey literature' subsection in the 'searching other resources' part of the methods was removed:
"Searching other resources
"The main investigators of any relevant ongoing trials will be contacted for further information, as will any major co‐operative trials groups active in this area."
Adverse events, quality of life or other continuous outcomes were not reported in any of the studies so sections in the review which discussed the handling of dichotomous and continuous data were removed, as they were unnecessary:
"Data extraction and management
For dichotomous outcomes (e.g. adverse events), we will extract the number of patients in each group who experience the outcome of interest and the number of patients assessed at endpoint, in order to estimate a risk ratio.
For continuous outcomes (e.g. quality of life measures), we will extract the final value and standard deviation of the outcome of interest and the number of patients assessed at endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference between treatment arms and its standard error.
Measures of treatment effect
For dichotomous outcomes (e.g. adverse events, or time‐to‐event data if it is not possible to use a hazard ratio), we will use the risk ratio.
For continuous outcomes, we will use the mean difference between treatment arms if all trials measured the outcome on the same scale, otherwise standardised mean differences will be used.
Data synthesis
For any dichotomous outcomes, the risk ratio will be calculated for each study and these will then be pooled.
For continuous outcomes, the mean differences (or standardised mean differences) between the treatment arms at the end of follow‐up will be pooled."
We did not produce a funnel plot to assess the potential for small study effects since there were only six trials in the largest meta‐analysis, which assessed overall survival in women with residual disease < 1 cm compared to women with microscopic disease. The following paragraph on reporting biases was removed:
"Assessment of reporting biases
Funnel plots corresponding to meta‐analysis of the primary outcome will be examined to assess the potential for small study effects. When there is evidence of small‐study effects, publication bias will be considered as only one of a number of possible explanations. If these plots suggest that treatment effects may not be sampled from a symmetric distribution, as assumed by the random effects model, sensitivity analyses will be performed using fixed effects models."
The review was restricted to studies that were at high risk of bias and we modified the inclusion criteria to include only studies that used multivariate analyses (see above) so we did not carry out sensitivity analyses. We had specified the following in the protocol:
"Sensitivity analysis
Sensitivity analyses will be performed (i) excluding studies at high risk of bias and (ii) using unadjusted results."
Contributions of authors
AE, MH and RN drafted the clinical sections of the review; BWR helped with data extraction and contributed to clinical sections of the review; AB drafted the methodological sections of the review. All authors agreed the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Department of Health, UK.
NHS Cochrane Collaboration programme Grant Scheme CPG‐506
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Akahira 2001 {published data only}
- Akahira JI, Yoshikawa H, Shimizu Y, Tsunematsu R, Hirakawa T, Kuramoto H. Prognostic factors of stage IV epithelial ovarian cancer: a multicenter retrospective study. Gynecologic Oncology 2001;81(3):398‐403. [DOI] [PubMed] [Google Scholar]
Aletti 2006 {published data only}
- Aletti GD, Dowdy SC, Gostout BS, Jones MB, Stanhope CR, Wilson TO, et al. Aggressive surgical effort and improved survival in advanced‐stage ovarian cancer. Obstetrics and Gynecology 2006;107:77–85. [DOI] [PubMed] [Google Scholar]
- Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Relationship among surgical complexity, short‐term morbidity, and overall survival in primary surgery for advanced ovarian cancer. American Journal of Obstetrics and Gynecology 2007;197(6):1‐7. [DOI] [PubMed] [Google Scholar]
- Aletti GD, Dowdy SC, Podratz KC, Cliby WA. Surgical treatment of diaphragm disease correlates with improved survival in optimally debulked advanced stage ovarian cancer. Gynecologic Oncology 2006;100(2):283‐7. [DOI] [PubMed] [Google Scholar]
- Aletti GD, Podratz KC, Jones MB, Cliby WA. Role of rectosigmoidectomy and stripping of pelvic peritoneum in outcomes of patients with advanced ovarian cancer. Journal of the American College of Surgeons 2006;203(4):521‐6. [DOI] [PubMed] [Google Scholar]
Chan 2003 {published data only}
- Chan JK, Loizzi V, Lin YG, Osann K, Brewster WR, DiSaia PJ. Stages III and IV invasive epithelial ovarian carcinoma in younger versus older women: What prognostic factors are important?. Obstetrics and Gynecology 2003;102(1):156‐61. [DOI] [PubMed] [Google Scholar]
Chi 2001 {published data only}
- Chi DS, Liao JB, Leon LF, Venkatraman ES, Hensley ML, Bhaskaran D, et al. Identification of prognostic factors in advanced epithelial ovarian carcinoma. Gynecologic Oncology 2001;82(3):532‐7. [DOI] [PubMed] [Google Scholar]
Chi 2006 {published data only}
- Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu‐Rustum NR, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)?. Gynecologic Oncology 2006;103:559–64. [DOI] [PubMed] [Google Scholar]
- Eisenhauer EL, Abu‐Rustum NR, Sonoda Y, Aghajanian C, Barakat RR, Chi DS. The effect of maximal surgical cytoreduction on sensitivity to platinum‐taxane chemotherapy and subsequent survival in patients with advanced ovarian cancer. Gynecologic Oncology 2008;108(2):276‐81. [DOI] [PubMed] [Google Scholar]
Eisenkop 2003 {published data only}
- Eisenkop SM, Friedman RL, Wang HJ. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: A prospective study. Gynecologic Oncology 1998;69(2):103‐8. [DOI] [PubMed] [Google Scholar]
- Eisenkop SM, Spirtos NM, Friedman RL, Lin WC, Pisani AL, Perticucci S. Relative influences of tumor volume before surgery and the cytoreductive outcome on survival for patients with advanced ovarian cancer: a prospective study. Gynecologic Oncology 2003;90(2):390‐6. [DOI] [PubMed] [Google Scholar]
McGuire 1995 {published data only}
- Hoskins WJ, McGuire WP, Brady MF, Homesley HD, Creasman WT, Berman M, et al. The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial ovarian carcinoma. American Journal of Obstetrics and Gynecology 1994;170(4):974‐80. [DOI] [PubMed] [Google Scholar]
- McGuire WP, Hoskins WJ, Brady MF, Homesley HD, Creasman WT, Berman ML, et al. Assessment of dose‐intensive therapy in suboptimally debulked ovarian cancer: A Gynecologic Oncology Group Study. Journal of Clinical Oncology 1995;13:1589‐99. [DOI] [PubMed] [Google Scholar]
Salani 2007 {published data only}
- Salani R, Zahurak ML, Santillan A, Giuntoli RL, Bristow RE. Survival impact of multiple bowel resections in patients undergoing primary cytoreductive surgery for advanced ovarian cancer: A case‐control study. Gynecologic Oncology 2007;107(3):495‐9. [DOI] [PubMed] [Google Scholar]
Van Geene 1996 {published data only}
- Geene P, Varma R, Dunn J, Chan KK, Luesley DM. The prognostic significance of intraperitoneal growth characteristics in epithelial ovarian carcinoma. International Journal of Gynecological Cancer 1996;6(3):219‐24. [Google Scholar]
Winter 2007 {published data only}
- Armstrong DK, Bundy BW, LariHuang HQ, Baergen RL, Shashikant Copeland LJ, Walker JL, Burger RA, the Gynecologic Oncology Group. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. New England Journal of Medicine 2006;354(1):34‐43. [DOI] [PubMed] [Google Scholar]
- Markman M, Bundy BM, Alberts DS, Fowler JM, Clark‐Pearson DL, Carson LF, et al. Phase III Trial of Standard‐Dose Intravenous Cisplatin Plus Paclitaxel Versus Moderately High‐Dose Carboplatin Followed by Intravenous Paclitaxel and Intraperitoneal Cisplatin in Small‐Volume Stage III Ovarian Carcinoma: An Intergroup Study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. Journal of Clinical Oncology 2001;19(4):1001‐07. [DOI] [PubMed] [Google Scholar]
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and Cisplatin Compared with Paclitaxel and Cisplatin in Patients with Stage III and Stage IV Ovarian Cancer. New England Journal of Medicine 1996;334(1):1‐6. [DOI] [PubMed] [Google Scholar]
- Muggia FM, Braly PS, Brady MF, Sutton GN, Theodore HL, Samuel LA, Ronald D.Kucera, Paul R.Small, James M. Phase III Randomized Study of Cisplatin Versus Paclitaxel Versus Cisplatin and Paclitaxel in Patients With Suboptimal Stage III or IV Ovarian Cancer: A Gynecologic Oncology Group Study. Journal of Clinical Oncology 2000;18(1):106‐115. [DOI] [PubMed] [Google Scholar]
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke‐Pearson DB, Robert AM, et al. Phase III Trial of Carboplatin and Paclitaxel Compared With Cisplatin and Paclitaxel in Patients With Optimally Resected Stage III Ovarian Cancer: A Gynecologic Oncology Group Study. Journal of Clinical Oncology 2003;21(17):3194‐3200. [DOI] [PubMed] [Google Scholar]
- Rose PG, Nerenstone S, Brady MF, Clarke‐Pearson D, Olt G, Rubin SC, et al. Secondary Surgical Cytoreduction for Advanced Ovarian Carcinoma. New England Journal of Medicine 2004;351(24):2489‐97. [DOI] [PubMed] [Google Scholar]
- Winter WE, Maxwell III GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic Factors for Stage III Epithelial Ovarian Cancer: A Gynecologic Oncology Group Study. Journal of Clinical Oncology 2007;25(24):3621‐27. [DOI] [PubMed] [Google Scholar]
Winter 2008 {published data only}
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and Cisplatin Compared with Paclitaxel and Cisplatin in Patients with Stage III and Stage IV Ovarian Cancer. New England Journal of Medicine 1996;334(1):1‐6. [DOI] [PubMed] [Google Scholar]
- Muggia FM, Braly PS, Brady MF, Sutton GN, Theodore HL, Samuel LA, et al. Phase III Randomized Study of Cisplatin Versus Paclitaxel Versus Cisplatin and Paclitaxel in Patients With Suboptimal Stage III or IV Ovarian Cancer: A Gynecologic Oncology Group Study. Journal of Clinical Oncology 2000;18(1):106‐115. [DOI] [PubMed] [Google Scholar]
- Rose PG, Nerenstone S, Brady MF, Clarke‐Pearson D, Olt G, Rubin SC, et al. Secondary Surgical Cytoreduction for Advanced Ovarian Carcinoma. New England Journal of Medicine 2004;351(24):2489‐97. [DOI] [PubMed] [Google Scholar]
- Spriggs DR, Brady MF, Vaccarello L, Clarke‐Pearson DL, Burger RA, Mannel R, et al. Phase III randomized trial of intravenous cisplatin plus a 24‐ or 96‐hour infusion of paclitaxel in epithelial ovarian cancer: a Gynecologic Oncology Group Study.. Journal of Clinical Oncology 2007;25(28):4466‐71. [DOI] [PubMed] [Google Scholar]
- Winter WE, Maxwell GL, Tian C, Sundborg MJ, Rose GS, Rose PG, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 2008;26(1):83‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alberts 1993 {published data only}
- Alberts DS, Dahlberg S, Green SJ, Garcia D, Hannigan EV, O'Toole R, et al. Analysis of patient age as an independent prognostic factor for survival in a phase III study of cisplatin‐cyclophosphamide versus carboplatin‐cyclophosphamide in stages III (suboptimal) and IV ovarian cancer. A Southwest Oncology Group study.. Cancer 1993;71(2 Suppl):618‐627. [DOI] [PubMed] [Google Scholar]
Alberts 1996 {published data only}
- Alberts DS, Liu PY, Hannigan EEV, O'Toole RW, Stephen DY, James AF, et al. Intraperitoneal Cisplatin plus Intravenous Cyclophosphamide versus Intravenous Cisplatin plus Intravenous Cyclophosphamide for Stage III Ovarian Cancer. New England Journal of Medicine 1996;335(26):1950‐55. [DOI] [PubMed] [Google Scholar]
Alphs 2006 {published data only}
- Alphs HH, Zahurak ML, Bristow RE, Diaz‐Montes TP. Predictors of surgical outcome and survival among elderly women diagnosed with ovarian and primary peritoneal cancer. Gynecologic Oncology 2006;103(3):1048‐53. [DOI] [PubMed] [Google Scholar]
Andersen Soegaard 2005 {published data only}
- Soegaard Andersen E, Knudsen A, Svarrer T, Lund B, Nielsen K, Grove A, et al. The results of treatment of epithelial ovarian cancer after centralisation of primary surgery. Results from North Jutland, Denmark. Gynecologic Oncology 2005;99(3):552‐6. [DOI] [PubMed] [Google Scholar]
Bailey 2006 {published data only}
- Bailey J, Murdoch J, Anderson R, Weeks J, Foy C. Stage III and IV ovarian cancer in the South West of England: five‐year outcome analysis for cases treated in 1998. International Journal of Gynecological Cancer 2006;1:25‐29. [DOI] [PubMed] [Google Scholar]
Baker 1994 {published data only}
- Baker TR, Piver MS, Hempling RE. Long term survival by cytoreductive surgery to less than 1 cm, induction weekly cisplatin and monthly cisplatin, doxorubicin, and cyclophosphamide therapy in advanced ovarian adenocarcinoma. Cancer 1994;74(2):656‐63. [DOI] [PubMed] [Google Scholar]
Barda 2004 {published data only}
- Barda G, Menczer J, Chetrit A, Lubin F, Beck D, Piura B, et al. Comparison between primary peritoneal and epithelial ovarian carcinoma: A population‐based study. American Journal of Obstetrics and Gynecology 2004;190(4):1039‐45. [DOI] [PubMed] [Google Scholar]
Benedetti‐Panici 1996 {published data only}
- Benedetti‐Panici P, Maneschi F, Scambia G, Cutillo G, Greggi S, Mancuso S. The pelvic retroperitoneal approach in the treatment of advanced ovarian carcinoma. Obstetrics & Gynecology 1996;87(4):532‐38. [DOI] [PubMed] [Google Scholar]
Bertelsen 1990 {published data only}
- Bertelsen K. Tumor reduction surgery and long‐term survival in advanced ovarian cancer: a DACOVA study. Gynecologic Oncology 1990;38(2):203‐9. [DOI] [PubMed] [Google Scholar]
Bertelsen 1993 {published data only}
- Bertelsen K, Jakobsen A, Strøyer J, Nielsen K, Sandberg E, Andersen JE, et al. A Prospective Randomized Comparison of 6 and 12 Cycles of Cyclophosphamide, Adriamycin, and Cisplatin in Advanced Epithelial Ovarian Cancer: A Danish Ovarian Study Group Trial (DACOVA). Gynecologic Oncology 1993;49:30‐36. [DOI] [PubMed] [Google Scholar]
Bonnefoi 1999 {published data only}
- Bonnefoi H, A'Hern RP, Fisher C, Macfarlane V, Barton D, Blake P, et al. Natural history of stage IV epithelial ovarian cancer.[see comment]. Journal of Clinical Oncology 1999;17(3):767‐75. [DOI] [PubMed] [Google Scholar]
Brinkhuis 1996a {published data only}
- Brinkhuis M, Baak J P A, Diest P J, Lund B, Wils J. In Dutch and Danish patients with FIGO III ovarian carcinoma, geographic survival differences are associated with differences in quantitative pathologic features. International Journal of Gynecological Cancer 1996;6(2):108‐14. [Google Scholar]
Brinkhuis 1996b {published data only}
- Brinkhuis M, Lund B, Meijer GA, Baak JPA. Quantitative pathological variables as prognostic factors for overall survival in Danish patients with FIGO stage III ovarian cancer. International Journal of Gynecological Cancer 1996;6(3):168‐174. [Google Scholar]
Bristow 1999 {published data only}
- Bristow RE, Montz FJ, Lagasse LD, Leuchter RS, Karlan BY. Survival impact of surgical cytoreduction in stage IV epithelial ovarian cancer. Gynecologic Oncology 1999;72(3):278‐87. [DOI] [PubMed] [Google Scholar]
Cai 2007 {published data only}
- Cai HB, Zhou YF, Chen HZ, Hou HY. The Role of Bowel Surgery with Cytoreduction for Epithelial Ovarian Cancer. Clinical Oncology 2007;19(10):757‐762. [DOI] [PubMed] [Google Scholar]
Colozza 1997 {published data only}
- Colozza M, Mosconi AM, Gori S, Belsanti V, Basurto C, Angelis V, et al. Long‐term results in patients with advanced epithelial ovarian carcinoma treated with a combination of cisplatin, doxorubicin, and cyclophosphamide. American Journal of Clinical Oncology 1997;20(5):522‐26. [DOI] [PubMed] [Google Scholar]
Conte 1991 {published data only}
- Conte PF, Bruzzone M, Carnino F, Chiara S, Donadio M, Facchini V, et al. Carboplatin, doxorubicin, and cyclophosphamide versus cisplatin, doxorubicin, and cyclophosphamide: a randomized trial in stage III‐IV epithelial ovarian carcinoma. Journal of Clinical Oncology 1991;9(4):658‐63. [DOI] [PubMed] [Google Scholar]
Conte 1996 {published data only}
- Conte PF, Bruzzone M, Carnino F, Gadducci A, Algeri R, Bellini A, et al. High‐dose versus low‐dose cisplatin in combination with cyclophosphamide and epidoxorubicin in suboptimal ovarian cancer: a randomized study of the Gruppo Oncologico Nord‐Ovest. Journal of Clinical Oncology 1996;14(2):351‐56. [DOI] [PubMed] [Google Scholar]
Crawford 2005 {published data only}
- Crawford SC, Vasey PA, Paul J, Hay A, Davis JA, Kaye SB. Does aggressive surgery only benefit patients with less advanced ovarian cancer? Results from an international comparison within the SCOTROC‐1 trial. Journal of Clinical Oncology 2005;23(34):8802‐11. [DOI] [PubMed] [Google Scholar]
Creasman 1990 {published data only}
- Creasman WT, Omura GA, Brady MF, Yordan E, DiSaia PJ, Beecham J. A randomized trial of cyclophosphamide, doxorubicin, and cisplatin with or without bacillus Calmette‐Guerin in patients with suboptimal stage III and IV ovarian cancer: A Gynecologic Oncology Group Study. Gynecologic Oncology 1990;39(3):239‐43. [DOI] [PubMed] [Google Scholar]
de Oliviera 1990 {published data only}
- Oliveira CF, Lacave AJ, Villani C, Wolff JP, Re F, Namer M. Randomized comparison of cyclophosphamide, doxorubicin and cisplatin (CAP) versus cyclophosphamide and doxorubicin (CA) for the treatment of advanced ovarian cancer (ADOVCA). A EORTC Gynecological Cancer Cooperative Group Study.. European Journal of Gynaecologic Oncology 1990;11(5):323‐30. [PubMed] [Google Scholar]
Del Campo 1994 {published data only}
- Del Campo, Jose M.Felip, EnriquetaRubio, DiegoVidal, RosoBermejo, BegoñaColomer, RamonZanon, Vicente. Long‐Term Survival in Advanced Ovarian Cancer after Cytoreduction and Chemotherapy Treatment. Gynecologic Oncology 1994;53(1):27‐32. [DOI] [PubMed] [Google Scholar]
di Re 1996 {published data only}
- Re F, Baiocchi G, Fontanelli R, Grosso G, Cobellis L, Raspagliesi F, et al. Systematic pelvic and paraaortic lymphadenectomy for advanced ovarian cancer: prognostic significance of node metastases. Gynecologic Oncology 1996;62(3):360‐65. [DOI] [PubMed] [Google Scholar]
Gao 2001 {published data only}
- Gao J, Zheng A, Chen W, Peng Z, Cao Z. [A study of prognostic factors of stage IV epithelial ovarian cancer]. [Chinese]. Hua Hsi i Ko Ta Hsueh Hsueh Pao [Journal of West China University of Medical Sciences] 2001;32(2):309‐312. [PubMed] [Google Scholar]
Geisler 2004 {published data only}
- Geisler JP, Tammela JE, Manahan KJ, Geisler HE, Miller GA, Zhou Z, et al. HSP27 in patients with ovarian carcinoma: still an independent prognostic indicator at 60 months follow‐up. European Journal of Gynaecological Oncology 2004;25(2):165‐168. [PubMed] [Google Scholar]
Gershenson 1989 {published data only}
- Gershenson DM, Wharton J, Taylor C, Stringer LJ, Edwards CA, Creighton L, et al. Treatment of advanced epithelial ovarian cancer with cisplatin and cyclophosphamide. Gynecologic Oncology 1989;32(3):336‐341. [DOI] [PubMed] [Google Scholar]
Gershenson 1992 {published data only}
- Gershenson DM, Mitchell MF, Atkinson N, Elvio G, Kavanagh J, Burke M, et al. The effect of prolonged cisplatin‐based chemotherapy on progression‐free survival in patients with optimal epithelial ovarian cancer: "Maintenance" therapy reconsidered. Gynecologic Oncology 1992;47(1):7‐13. [DOI] [PubMed] [Google Scholar]
Gershenson 1995 {published data only}
- Gershenson DM, Morris M, Burke TW, Levenback C, Kavanagh JJ, Fromm GL. Combined Cisplatin and Carboplatin Chemotherapy for Treatment of Advanced Epithelial Ovarian Cancer. Gynecologic Oncology 1995;58(3):349‐355. [DOI] [PubMed] [Google Scholar]
Grem 1991 {published data only}
- Grem J, O'Dwyer P, Elson P, Simon N, Trump D, Frontiera M, et al. Cisplatin, carboplatin, and cyclophosphamide combination chemotherapy in advanced‐stage ovarian carcinoma: an Eastern Cooperative Oncology Group pilot study. Journal of Clinical Oncology 1989;9(10):1793‐1800. [DOI] [PubMed] [Google Scholar]
Hainsworth 1990 {published data only}
- Hainsworth JD, Burnett LS, Jones HW 3rd, Grosh WW, Johnson DH, Greco FA. High‐dose cisplatin combination chemotherapy in the treatment of advanced epithelial ovarian carcinoma. Journal of Clinical Oncology 1990;8(3):502‐508. [DOI] [PubMed] [Google Scholar]
Hakes 1992 {published data only}
- Hakes TB, Chalas E, Hoskins WJ, Jones WB, Markman M, Rubin SC, et al. Randomized prospective trial of 5 versus 10 cycles of cyclophosphamide, doxorubicin, and cisplatin in advanced ovarian carcinoma. Gynecologic Oncology 1992;45(3):284‐289. [DOI] [PubMed] [Google Scholar]
Hamid 2002 {published data only}
- Hamid D, Rohr S, Baldauf JJ, Ritter J, Kurtz E, Dufour P, et al. Interest of intestinal resection for treatment of advanced ovarian carcinoma [Intérét des exéréses digestives dans le traitement des cancers écolués de l'ovaire]. Annales de Chirurgie 2002;127(1):40‐47. [DOI] [PubMed] [Google Scholar]
Hardy 1991 {published data only}
- Hardy JR, Wiltshaw E, Blake PR, Harper P, Slevin M, Perren TJ, et al. Cisplatin and carboplatin in combination for the treatment of stage IV ovarian carcinoma. Annals of Oncology 1991;2(2):131‐136. [DOI] [PubMed] [Google Scholar]
Hoskins 1992 {published data only}
- Hoskins WJ, Bundy BN, Thigpen JT, Omura GA. The influence of cytoreductive surgery on recurrence‐free interval and survival in small‐volume stage III epithelial ovarian cancer: a Gynecologic Oncology Group study. Gynecologic Oncology 1992;47(2):159‐66. [DOI] [PubMed] [Google Scholar]
Hoskins 1996 {published data only}
- Hoskins PJ, Swenerton KD, Pike JA, McMurtrie EM, Lee N. "MECCA": A Developmental, Dose‐Intensive, Non‐Cross‐Resistant Platinum‐Based Chemotherapy for Advanced Ovarian Cancer. Gynecologic Oncology 1996;63(3):345‐351. [DOI] [PubMed] [Google Scholar]
Hoskins 1997 {published data only}
- Hoskins WJ, McGuire WP, Brady, MF, Kucera PR, Partridge EE, Look KY, et al. Combination paclitaxel (Taxol(R))‐cisplatin vs cyclophosphamide‐cisplatin as primary therapy in patients with suboptimally debulked advanced ovarian cancer. International Journal of Gynecological Cancer 1997;7:9‐13. [Google Scholar]
Itamochi 2002 {published data only}
- Itamochi H, Kigawa J, Sugiyama T, Kikuchi Y, Suzuki M, Terakawa N. Low proliferation activity may be associated with chemoresistance in clear cell carcinoma of the ovary. Obstetrics & Gynecology 2002;100(2):281‐287. [DOI] [PubMed] [Google Scholar]
Kaern 2005 {published data only}
- Kaern J, Aghmesheh M, Nesland JM, Danielsen HE, Sandstad B, Friedlander M, et al. Prognostic factors in ovarian carcinoma stage III patients. Can biomarkers improve the prediction of short‐ and long‐term survivors?. International Journal of Gynecological Cancer 2005;15(6):1014‐22. [DOI] [PubMed] [Google Scholar]
Kirmani 1994 {published data only}
- Kirmani S, Braly PS, McClay EF, Saltzstein SL, Plaxe SC, Kim S, et al. A Comparison of Intravenous versus Intraperitoneal Chemotherapy for the Initial Treatment of Ovarian Cancer. Gynecologic Oncology 1994;54(3):338‐344. [DOI] [PubMed] [Google Scholar]
Kristensen 1995 {published data only}
- Kristensen GB, Baekelandt M, Vergote IB, Trope C. A phase II study of carboplatin and hexamethylmelamine as induction chemotherapy in advanced epithelial ovarian carcinoma. European Journal of Cancer 1995;31(11):1778‐80. [DOI] [PubMed] [Google Scholar]
Le 1997 {published data only}
- Le T, Krepart GV, Lotocki R J, Heywood MS. Does debulking surgery improve survival in biologically aggressive ovarian carcinoma?. Gynecologic Oncology 1997;67(2):208‐14. [DOI] [PubMed] [Google Scholar]
Lorusso 1998 {published data only}
- Lorusso V, Leone B, Vagno G, Manzione L, Palmeri S, Vallejo C. Combined Carboplatin plus Ifosfamide and Cisplatin in Patients with Advanced Ovarian Carcinoma. A Phase I‐II Study. Gynecologic Oncology 1998;68(2):172‐177. [DOI] [PubMed] [Google Scholar]
Malik 1998 {published data only}
- Malik IM, Khan ZK, Khan WA, Hussain M, Moid I, Rizvi J. Continuous infusion of ifosfamide and cisplatin as first‐line therapy of patients with suboptimally debulked stage III–IV epithelial ovarian cancer. International Journal of Gynecological Cancer 1998;8(2):138‐143. [Google Scholar]
Marchetti 1993 {published data only}
- Marchetti DL, Lele SB, Priore RL, McPhee ME, Hreshchyshyn MM. Treatment of Advanced Ovarian Carcinoma in the Elderly. Gynecologic Oncology 1993;49(1):86‐91. [DOI] [PubMed] [Google Scholar]
Ngan 1989 {published data only}
- Ngan HY, Choo YC, Cheung M, Wong LC, Ma HK, Collins R. A Randomized Study of High‐Dose versus Low‐Dose cis‐Platinum Combined with Cyclophosphamide in the Treatment of Advanced Ovarian Cancer. Chemotherapy 1989;35(3):221‐227. [DOI] [PubMed] [Google Scholar]
Omura 1989 {published data only}
- Omura GA, Bundy BN, Berek JS, Curry S, Delgado G Mortel R. Randomized trial of cyclophosphamide plus cisplatin with or without doxorubicin in ovarian carcinoma: a Gynecologic Oncology Group Study. Journal of Clinical Oncology 1989;7(4):457‐465. [DOI] [PubMed] [Google Scholar]
Palmer 1992 {published data only}
- Palmer MC, Shepert E, Schepansky A, MacLean GD. Novel, dose intensive, single‐agent cisplatin in the first‐line management of advanced stage ovarian cancer. International Journal of Gynecological Cancer 1992;2(6):301‐306. [DOI] [PubMed] [Google Scholar]
Piver 1991 {published data only}
- River MS, Fanning J, Sprance HE. Five‐year survival for cisplatin‐based chemotherapy versus single‐agent melphalan in patients with advanced ovarian cancer and optimal debulking surgery. Journal of Surgical Oncology 1991;48(1):39‐44. [DOI] [PubMed] [Google Scholar]
Redman 1986 {published data only}
- Redman JR, Petroni GR, Saigo PE, Geller NL, Hakes TB. Prognostic factors in advanced ovarian carcinoma. Journal of Clinical Oncology 1986;4(4):515‐523. [DOI] [PubMed] [Google Scholar]
Rose 2004 {published data only}
- Rose PG, Nerenstone S, Brady MF, Clarke‐Pearson D, Olt G, Rubin SC, et al and the Gynecologic Oncology Group. Secondary Surgical Cytoreduction for Advanced Ovarian Carcinoma. New England Journal of Medicine 2004;351(24):2489‐97. [DOI] [PubMed] [Google Scholar]
Sessa 1991 {published data only}
- Sessa C, Colombo N, Bolis G, Marsoni S, Mangioni C. Randomized comparison of hexamethylmelamine, adriamycin, cyclophosphamide (HAC) vs. cisplatin, adriamycin, cyclophosphamide (PAC) in advanced ovarian cancer: long‐term results.. Cancer Treatment Reviews 1991;18(Mar;18 Suppl A):37‐46. [DOI] [PubMed] [Google Scholar]
Shapiro 1998 {published data only}
- Shapiro JD, Rothenberg ML, Sarosy, GA, Steinberg SM, Adamo DO, Reed E, et al. Dose intensive combination platinum and cyclophosphamide in the treatment of patients with advanced untreated epithelial ovarian cancer. Cancer 1998;83(9):1980‐88. [PubMed] [Google Scholar]
Shinozuka 1999 {published data only}
- Shinozuka T, Miyamoto T, Muramatsu T, Hirasawa T, Murakami M, Makino T, et al. High dose chemotherapy with autologous stem cell support in the treatment of patients with ovarian carcinoma: Long term results for 105 patients. Cancer 1999;85(7):1555‐64. [DOI] [PubMed] [Google Scholar]
Skarlos 1996 {published data only}
- Skarlos DV, Aravantinos G, Kosmidis P, Pavlidis N, Gennatas K, Beer M, et al. Carboplatin alone compared with its combination with epirubicin and cyclophosphamide in untreated advanced epithelial ovarian cancer: a hellenic co‐operative oncology group study. European Journal of Cancer 1996;32(3):421‐28. [DOI] [PubMed] [Google Scholar]
Strauss 1996 {published data only}
- Strauss G, Lund B, Hansen M, Hansen OP, Hansen HH. Combined high‐dose platinum and etoposide in previously untreated ovarian cancer patients with residual disease. International Journal of Gynecological Cancer 1996;6(5):410‐414. [Google Scholar]
Sun 2000 {published data only}
- Sun T, Feng Y, ZhuY, Zheng Y. Therapeutic strategy in the management of stage II ‐ IV epithelial ovarian carcinoma. Chinese Medical Journal 2000;113(7):625‐627. [PubMed] [Google Scholar]
Sutton 1989 {published data only}
- Sutton GP, Stehman FB, Einhorn LH, Roth LM, Blessing JA, Ehrlich CE. Ten‐year follow‐up of patients receiving cisplatin, doxorubicin, and cyclophosphamide chemotherapy for advanced epithelial ovarian carcinoma. Journal of Clinical Oncology 1989;7:223‐229. [DOI] [PubMed] [Google Scholar]
Takano 2006 {published data only}
- Takano MJ, Kikuchi Y, Yaegashi N, Suzuki M, Tsuda H, Sagae S, et al. Adjuvant chemotherapy with irinotecan hydrochloride and cisplatin for clear cell carcinoma of the ovary. Oncology Reports 2006;16(6):1301‐1306. [PubMed] [Google Scholar]
Takano 2007 {published data only}
- Takano M, Sugiyama T, Yaegashi N, .Suzuki M, Sagae S, Udagawa, et al. Progression‐free survival and overall survival of patients with clear cell carcinoma of the ovary treated with paclitaxel‐carboplatin or irinotecan‐cisplatin: Retrospective analysis. International Journal of Clinical Oncology 2007;12(4):256‐260. [DOI] [PubMed] [Google Scholar]
Tay 1996 {published data only}
- Tay SK, Chang TC. An evaluation of the policy of routine treatment of advanced epithelial ovarian carcinoma by debulking surgery and combined platinum‐cyclophosphamide chemotherapy in Singapore. International Journal of Gynecological Cancer 1996;6(1):44‐48. [Google Scholar]
Taylor 1994 {published data only}
- Taylor AE, Wiltshaw E, Gore ME, Fryatt I, Fisher C. Long‐term follow‐up of the first randomized study of cisplatin versus carboplatin for advanced epithelial ovarian cancer. Journal Of Clinical Oncology 1994;12(10):2066‐70. [DOI] [PubMed] [Google Scholar]
Tingulstad 2003 {published data only}
- Tingulstad S, Skjeldestad FE, Hagen B. The effect of centralization of primary surgery on survival in ovarian cancer patients. Obstetrics and Gynecology 2003;102(3):499‐505. [DOI] [PubMed] [Google Scholar]
Todo 2003 {published data only}
- Todo Y, Sakuragi N, Oikawa M, Negishi H, Yamamoto R, Yoshiaki K, et al. Cytoreductive surgery combined with organ resection for advanced ovarian carcinoma. International Journal of Clinical Oncology 2003;8(2):90‐96. [DOI] [PubMed] [Google Scholar]
Uyar 2005 {published data only}
- Uyar D, Frasure HE, Markman M, Gruenigen VE. Treatment patterns by decade of life in elderly women ([greater‐than or equal to]70 years of age) with ovarian cancer. Gynecologic Oncology 2005;98(3):403‐408. [DOI] [PubMed] [Google Scholar]
Vallejos 1997 {published data only}
- Vallejos C, Solidoro A, Castellano C, Barriga C, Galdos O, Casanova R, et al. Ifosfamide plus Cisplatin as Primary Chemotherapy of Advanced Ovarian Cancer. Gynecologic Oncology 1997;67(2):168‐171. [DOI] [PubMed] [Google Scholar]
Van Der Burg 1996 {published data only}
- Burg MEL, Lent M, Buyse M, Kobierska A, Maggioni A, Favalli G, et al. The role of intervention debulking surgery in advanced epithelial ovarian cancer: an EORTC Gynecological Cancer Cooperative Group study. International Journal of Gynecological Cancer 1996;6:30‐38. [Google Scholar]
Vergote 2010 {published and unpublished data}
- Vergote I, Trope M, Claes G, Amant F, Kristensen GB, Ehlen T, et al. Neoadjuvant Chemotherapy or Primary Surgery in Stage IIIC or IV Ovarian Cancer. New England Journal of Medicine 2010;363(10):943‐53. [DOI] [PubMed] [Google Scholar]
Wadler 1996 {published data only}
- Wadler S, Yeap B, Vogl S, Carbone P. Randomized trial of initial therapy with melphalan versus cisplatin‐based combination chemotherapy in patients with advanced ovarian carcinoma: Initial and long term results ‐ Eastern Cooperative Oncology Group study E2878. Cancer 1996;77(4):733‐742. [PubMed] [Google Scholar]
Warwick 1995 {published data only}
- Warwick J, Kehoe S, Earl H, Luesley D, Redman C, Chan KK. Long‐term follow‐up of patients with advanced ovarian cancer treated in randomised clinical trials. British Journal of Cancer 1995;72(6):1513‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Willemse 1992 {published data only}
- Willemse PHB, Vries EGE, Kloppenburg M, Fontein DL, Aalders JG, Boonstra H, et al. Carboplatin with cyclophosphamide in patients with advanced ovarian cancer: an efficacy and quality‐adjusted survival analysis. International Journal of Gynecological Cancer 1992;2(5):236‐243. [DOI] [PubMed] [Google Scholar]
Wils 1990 {published data only}
- Wils JA. Long‐term follow‐up of patients with advanced ovarian carcinoma treated with debulking surgery and chemotherapy consisting of cisplatin, doxorubicin, and cyclophosphamide. Gynecologic Oncology Group of the Comprehensive Cancer Center. Oncology 1990;47(2):115‐120. [DOI] [PubMed] [Google Scholar]
Wimberger 2007 {published data only}
- Wimberger P, Lehmann N, Kimmig R, Burges A, Meier W, Du Bois A. Prognostic factors for complete debulking in advanced ovarian cancer and its impact on survival. An exploratory analysis of a prospectively randomized phase III study of the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO‐OVAR). Gynecologic Oncology 2007;106(1):69‐74. [DOI] [PubMed] [Google Scholar]
Yamamoto 2007 {published data only}
- Yamamoto S, Tsuda H, Yoshikawa T, Kudoh K, Kita T, et al. Clear cell adenocarcinoma associated with clear cell adenofibromatous components: A subgroup of ovarian clear cell adenocarcinoma with distinct clinicopathologic characteristics. American Journal of Surgical Pathology 2007;31(7):999‐1006. [DOI] [PubMed] [Google Scholar]
Zang 1999 {published data only}
- Zang RY, Zhang ZY, Cai SM, Li ZT, Chen J, et al. Cytoreductive surgery for stage IV epithelial ovarian cancer. Journal of Experimental & Clinical Cancer Research 1999;18(4):449‐54. [PubMed] [Google Scholar]
Additional references
Armstrong, 2006
- Armstrong DK, Bundy BW, LariHuang HQ, Baergen RL, Shashikant C, Walker LJ, et al. Intraperitoneal Cisplatin and Paclitaxel in Ovarian Cancer. New England Journal of Medicine 2006;354(1):34‐43. [DOI] [PubMed] [Google Scholar]
Benedet 2000
- Benedet JL, Bender H, Jones H, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers FIGO Committee on Gynecologic Oncology. International Journal of Gynaecology and Obstetrics 2000;70:209‐262. [PubMed] [Google Scholar]
Bristow 2002
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival Effect of Maximal Cytoreductive Surgery for Advanced Ovarian Carcinoma During the Platinum Era: A Meta‐Analysis. Journal of Clinical Oncology 2002;20 (5):1248‐59. [DOI] [PubMed] [Google Scholar]
Chi 2007
- Chi DS, Ramirez PT, Teitcher JB, Mironov S, Sarasohn DM, Lyer RB. Prospective study of the correlation between postoperative computed tomography scan and primary surgeon assessment in patients with advanced ovarian, tubal, and peritoneal carcinoma reported to have undergone primary surgical cytoreduction to residual disease 1 cm or less. Journal of Clinical Oncology 2007;25:4946‐51. [DOI] [PubMed] [Google Scholar]
Colombo 2006
- Colombo N, Gorp T, Parma G, Amant F, Gatta G, Sessa C, Vergote I. Ovarian cancer. Critical Reviews in Oncology/Hematology 2006;60 (2):159‐179. [DOI] [PubMed] [Google Scholar]
Cotte 2010
- Cotte E, Passot G, Gilly F‐N, Glehen O. Selection of patients and staging of peritoneal surface malignancies. World Journal of Gastrointestinal Oncology 2010;2(1):31‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covens 2000
- Covens AL. A Critique of Surgical Cytoreduction in Advanced Ovarian Cancer. Gynecologic Oncology 2000;78:269‐74. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG (eds). Systematic Reviews in Health Care: Meta‐Analysis in Context (2nd edition). London: BMJ Publication Group, 2001. [Google Scholar]
Deffieux 2006
- Deffieux X, Castaigne D, Pomel C. Role of laparoscopy to evaluate candidates for complete cytoreduction in advanced stages of epithelial ovarian cancer. International Journal of Gynecological Cancer 2006;16 (s1):35‐40. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Eisenkop 1998
- Eisenkop SM, Friedman RL, Wang HJ. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced ovarian cancer: a prospective study. Gynecologic Oncology 1998;69:103‐108. [DOI] [PubMed] [Google Scholar]
Eisenkop 2001
- Eisenkop SM, Spirtos NM. Procedures required to accomplish complete cytoreduction in ovarian cancer: is there a correlation with "biological aggressiveness" and survival?. Gynecolpogic Oncology 2001;82:435‐41. [DOI] [PubMed] [Google Scholar]
EUROCARE 2003
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, Grosclaude P, Hédelin G, Matsuda T, Møller H, Möller T, Verdecchia A, Capocaccia R, Gatta G, Micheli A, Santaquilani M, Roazzi P, Lisi D, and the EUROCARE Working Group. EUROCARE‐3: survival of cancer patients diagnosed 1990‐94 ‐ results and commentary. Annals of Oncology 2003;14 (Supplement 5):v61‐v118. [DOI] [PubMed] [Google Scholar]
Fader 2007
- Fader AN, Rose PG. Role of Surgery in Ovarian Carcinoma. Journal of Clinical Oncology 2007;25 (20):2873‐83. [DOI] [PubMed] [Google Scholar]
Girling 1996
- Girling JC, Soutter WP. Cytoreductive surgery in the primary management of advanced epithelial ovarian carcinoma: A topic for debate. International Journal of Gynecological Cancer 1996;1:81‐84. [Google Scholar]
GLOBOCAN 2002
- Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2002. 'Cancer Incidence, Mortality and Prevalence Worldwide. IARC CancerBase No. 5, version 2.0. IARCPress, Lyon 2004.
Griffiths 1975
- Griffiths. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Journal of the National Cancer Institute Monographs 1975;42:1014. [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoskins 1994
- Hoskins WJ, McGuire WP, Brady MF, Homesley HD, Creasman WT, Berman M, et al. 'The effect of diameter of largest residual disease on survival after primary cytoreductive surgery in patients with suboptimal residual epithelial epithelial ovarian carcinoma: a Gynecologic Oncology Group study'. American Journal of Obstetrics and Gynecology 1994;170:974‐980. [DOI] [PubMed] [Google Scholar]
Hunter 1992
- Hunter RW, Alexander ND, Soutter WP. 'Meta‐analysis of surgery in advanced ovarian carcinoma: Is maximum cytoreductive surgery an independent determinant of prognosis?'. American Journal of Obstetrics and Gynecology 1992;166:504‐511. [DOI] [PubMed] [Google Scholar]
IARC 2002
- Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer Incidence in Five Continents. IARC Scientific Publication No. 155,Lyon 2002; Vol. Volume VIII.
Jemal 2008
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer Statistics. CA: A Cancer Journal for Clinicians 2008;58:71‐96. [DOI] [PubMed] [Google Scholar]
Markman 2007
- Markman M. 'Concept of Optimal Surgical Cytoreduction in Advanced Ovarian Cancer: A Brief Critique and a Call for Action'. Journal of Clinical Oncology 2007;25 (27):4168‐70. [DOI] [PubMed] [Google Scholar]
Markman, 2001
- Markman M, Bundy BN, Alberts DS, Fowler JM, Clark‐Pearson DL, Carson LF, et al. Phase III Trial of Standard‐Dose Intravenous Cisplatin Plus Paclitaxel Versus Moderately High‐Dose Carboplatin Followed by Intravenous Paclitaxel and Intraperitoneal Cisplatin in Small‐Volume Stage III Ovarian Carcinoma: An Intergroup Study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. Journal of Clinical Oncology 2001;19(4):1001‐07. [DOI] [PubMed] [Google Scholar]
McGuire 1996
- McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, et al. Cyclophosphamide and Cisplatin Compared with Paclitaxel and Cisplatin in Patients with Stage III and Stage IV Ovarian Cancer. New England Journal of Medicine 1996;334(1):1‐6. [DOI] [PubMed] [Google Scholar]
Muggia, 2000
- Muggia FM, Braly PS, Brady MF, Sutton GN, Theodore HL, Samuel AL, et al. Phase III Randomized Study of Cisplatin Versus Paclitaxel Versus Cisplatin and Paclitaxel in Patients With Suboptimal Stage III or IV Ovarian Cancer: A Gynecologic Oncology Group Study. Journal of Clinical Oncology 2000;18(1):106‐115. [DOI] [PubMed] [Google Scholar]
Munstedt 2004
- Munstedt K, Franke FE. Role of primary surgery in advanced ovarian cancer. World Journal of Surgical oncology 2004;2 (1):32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ozols, 2003
- Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke‐Pearson DB, Mannel RA, et al. Phase III Trial of Carboplatin and Paclitaxel Compared With Cisplatin and Paclitaxel in Patients With Optimally Resected Stage III Ovarian Cancer: A Gynecologic Oncology Group Study. Journal of Clinical Oncology 2003;21(17):3194‐3200. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Paulsen 2006
- Paulsen T, Kjaerheim K, Kaern J, Tretli S, Trope C. Improved short‐term survival for advanced ovarian, tubal, and peritoneal cancer patients operated at teaching hospitals. International Journal of Gynecological Cancer 2006;16 (s1):11‐17. [DOI] [PubMed] [Google Scholar]
Quirk 2005
- Quirk JT, Natarajan N, Mettlin CJ. Age‐specific ovarian cancer incidence rate patterns in the United States. Gynecologic Oncology 2005;99(1):248‐250. [DOI] [PubMed] [Google Scholar]
Sharma 2005
- Sharma S, Driscoll DBA, Odunsi K, Venkatadri A, Lele S. Safety and efficacy of cytoreductive surgery for epithelial ovarian cancer in elderly and high‐risk surgical patients. American Journal of Obstetrics & Gynecology December 2005;193(6):2077‐82. [DOI] [PubMed] [Google Scholar]
Spriggs 2007
- Spriggs DR, Brady M, Vaccarello L, Clarke‐Pearson DL, Burger RA, Mannel R, et al. Phase III randomized trial of intravenous cisplatin plus a 24‐ or 96‐hour infusion of paclitaxel in epithelial ovarian cancer: a Gynecologic Oncology Group Study.. Journal of Clinical Oncology 2007;25(28):4466‐71. [DOI] [PubMed] [Google Scholar]
Stoeckle 2004
- Stoeckle E, Paravis P, Floquet A. 'Number of residual nodules, better than size, defines optimal surgery in advanced epithelial ovarian cancer'. International Journal of Gynecological Cancer 2004;14:779‐87. [DOI] [PubMed] [Google Scholar]
Sugarbaker 2009
- Sugarbaker PH. Cytoreductive surgery and perioperative intraperitoneal chemotherapy for the treatment of advanced primary and recurrent ovarian cancer. Current opinion in obstetrics and Gynecology 2009;21:15‐24. [DOI] [PubMed] [Google Scholar]
Trimbos 2003
- Trimbos JB, Vergote I, Bolis G, Vermorken JB, Mangioni C, Madronal C, et al. 'Impact of adjuvant chemotherapy and surgical staging in early‐stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer ‐ Adjuvant Chemotherapy in Ovarian Neoplasm Trial'. Journal of the National Cancer Institute 2003;95:113‐25. [PubMed] [Google Scholar]
Venesmaa 1992
- Venesmaa P, Ylikorkala O. Morbidity and mortality associated with primary and repeat operations for ovarian cancer. Obstetrics and Gynecology 1992;79:168‐172. [PubMed] [Google Scholar]
Vergote 1998
- Vergote I, Wever I, Tjalma W, Gramberen M, Decloedt J, Dam P. Neoadjuvant Chemotherapy or Primary Debulking Surgery in Advanced Ovarian Carcinoma: A Retrospective Analysis of 285 Patients. Gynecologic Oncology 1998;71 (3):431‐436. [DOI] [PubMed] [Google Scholar]
Vergote 2003
- Vergote I, Trimbos BJ. Treatment of patients with early epithelial ovarian cancer. Current Opinion in Oncology 2003;15 (6):452‐5. [DOI] [PubMed] [Google Scholar]
Von Georgi 2003
- Georgi R, Franke FE, Munstedt K. The influence of tumor biology, surgery, and postoperative therapy on patient prognosis in advanced ovarian carcinomas. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2003;111:189‐196. [DOI] [PubMed] [Google Scholar]
Zapardiel 2011
- Zapardiel I, Morrow CP. New terminology for cytoreduction in advanced ovarian cancer. The Lancet 2011;12:214. [DOI] [PubMed] [Google Scholar]


