Abstract
Background
Thymic carcinoma or advanced thymoma is a rare cancer of the thymus gland that tends to be aggressive and infiltrate neighbouring organs, making total resection very difficult. Induction or adjuvant chemotherapy, or both, are often used in a multimodality approach to treat people affected by this condition, but the effectiveness of chemotherapy for thymic carcinoma or advanced thymoma remains uncertain.
Objectives
To assess the role of chemotherapy in adults with thymic carcinoma or advanced thymoma.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2012, Issue 7), MEDLINE (accessed via Ovid from 1966 to July 2012), EMBASE (accessed via Ovid, from 1980 to July 2012), Latin American and Caribbean Literature on Health Sciences (LILACS), the Chinese Biological Medicine Database (CBM, 1978 to July 2012), China National Knowledge Infrastructure (CNKI, 1980 to July 2012) and the Chinese scientific periodical database VIP Information (VIP, 1989 to July 2012). There was no language restriction in searching for studies.
Selection criteria
We planned to include randomised controlled trials (RCTs) of trials using chemotherapy (either single‐agent or combination chemotherapy plus surgery, radiotherapy or not) for thymic carcinoma and/or advanced thymoma. We planned to include all adults (aged 18 years and over) diagnosed with thymic carcinoma and/or with Masaoka stage III or IV thymic tumours. The intended primary outcomes were overall survival (OS) and progression‐free survival (PFS).
Data collection and analysis
Two review authors independently evaluated the search results according to the inclusion and exclusion criteria. There were no studies identified for inclusion and therefore no data extraction was completed.
Main results
No RCTs were eligible for inclusion in this review. We report details of excluded prospective studies in an additional table and try to provide some useful evidence regarding current practice.
Authors' conclusions
There were no RCTs eligible for inclusion in this review. In current practice the most common regimen for adult patients with thymic carcinoma or advanced thymoma is cisplatin‐based chemotherapy. Considering the condition is rare, it is suggested that an international group is set up to organise and evaluate prospective collection of data from cohorts of patients to inform current clinical practice.
Plain language summary
Chemotherapy (drug treatment) for inoperable thymic cancer in adults
Question: Which type and when should chemotherapy (drug treatment) be given to people with thymic carcinoma or advanced thymoma?
Background: Thymic cancers are rare tumours arising in the thymus gland behind the breastbone in the chest cavity. For people with advanced‐stage thymic tumours complete surgical resection is normally not possible and the only treatment option is combined chemotherapy. This review aimed to assess the role of chemotherapy in people with advanced thymic tumours.
Main findings: We did not identify any suitable clinical trials for inclusion in this review from our search (up to July 2012). We have reported details of prospective studies that are not suitable for inclusion in the review, which provide some useful evidence about current clinical practice.
Quality of the evidence: While various treatment options are used, cisplatin‐based chemotherapy is currently the usual regimen of choice, however this is not supported by good‐quality trials.
Background
Description of the condition
Thymic carcinoma or advanced thymoma (stage III and IV thymomas according to the Masaoka system) (Masaoka 1981) is a rare cancer of the thymus gland. The incidence of tumours of thymus is about one to five per million population per year (Travis 2004). It is an invasive mediastinal epithelial neoplasm that arises from the epithelial cells in the thymus gland and often has locoregional invasion or metastasises after diagnosis. These tumours tend to be aggressive and infiltrate neighbouring organs, which makes total resection very difficult. Thymic carcinoma has a poor prognosis compared with thymoma (Rosai 1999; Travis 2004).
The World Heath Organization (WHO) classification (Travis 2004) divides thymic cancer into nine different types and the Masaoka staging system has been used for clinical staging for many years (Kondo 2003; Masaoka 1981; Yano 2008). Tumours of the thymus comprise neoplasms which are assumed to arise from or differentiate towards thymic cellular constituents, including thymic epithelial tumours (thymomas, thymic carcinomas, neuroendocrine tumours), germ cell tumours, and lymphoid and mesenchymal tumours. For the differential diagnosis of thymoma and thymic carcinoma see Appendix 1 (Travis 2004). Prognostic factors for survival include clinical stage at diagnosis and tumour resectability (Kondo 2003; Yano 2008). The median survival of people with thymic cancer is approximately 24 to 49 months with a five‐year survival average of 30% to 50% (Eng 2004; Giaccone 2005; Yano 2008).
Tumours of the thymus are rare human neoplasms and account for less than 1% of all adult cancers, with an incidence rate of one to five per million population per year and thymomas are the most frequent thymic tumours in adults (Travis 2004). Before the 1970s, thymic cancer was not recognised as an entity separate from thymoma, however the incidence of thymic cancer has increased in recent years, which may in part be due to the more precise WHO classification (Giaccone 2005; Travis 2004). There are currently no precise epidemiological data for thymic cancer.
The aetiology of thymic cancer is largely unknown and the biology is complex (Travis 2004). The more aggressive nature and poorer prognosis of these carcinomas suggest that they are distinct from thymomas, but cases of coexistence and even apparent devolution of the former from the latter have been noted and may suggest that they are merely different stages of the spectrum of thymic epithelial neoplasia (Chung 2000).
People with thymic cancer are commonly asymptomatic and a diagnosis is only made once the tumour reaches a large size. People usually present with one or more of the following symptoms: cough, shortness of breath, chest pain, dyspnoea or a lump. Thymic cancers are usually identified by chest X‐ray and computerised tomography (CT) scan and diagnosis is confirmed by histological pathology. Thymomas often manifest clinically by causing autoimmune diseases, in particular myasthenia gravis.
Thymic cancers are aggressive and highly lethal tumours. People usually present in an advanced stage and a multimodality treatment approach may be appropriate with the aim of improving survival. Several authors have reported that a multidisciplinary approach involving surgery, radiotherapy and chemotherapy is beneficial (Geffen 2001; Lin 2005; Yokoi 2007).
Surgery is the primary treatment option for thymic malignancies, with complete resection resulting in the best prognosis (Giaccone 2005; Kondo 2003; Lin 2005; Yano 2008). In addition to surgery, radiation therapy, either alone or in combination with chemotherapy, may be given. Radiotherapy is used to control local lesions of unresectable or incompletely resected thymic cancer. It also plays an important role in reducing local recurrence as an adjuvant therapy after complete resection (Tetsuo 2004). Whether adjuvant radiotherapy should be given after resection remains controversial. Most clinicians recommend radiotherapy but a few studies have shown no significant difference in survival between surgery alone and surgery with radiotherapy (Kondo 2003; Liu 2002). Adjuvant chemotherapy has been considered for cases of unresectable, recurrent or metastatic thymoma, as well as for those who have undergone subtotal resection.
One guideline on management of thymoma recommended: 1) surgery (complete and incomplete resections); 2) chemotherapy (cisplatin‐based therapy alone or in combination; octreotide alone or with a corticosteroid; 3) radiotherapy; 4. concurrent chemotherapy plus radiotherapy; 5) surgery plus radiotherapy or chemotherapy; 6) sequencing of multimodality therapy but it excluded thymic carcinoma and carcinoid tumours (Falkson 2008).
Several reports of thymic cancer and advanced thymoma have demonstrated an objective response with cisplatin‐based combination chemotherapy (Kitami 2001; Lin 2005; Loehrer 1994; Loehrer 2001; Weide 1993). Chemotherapy plays an important role in both primary and relapsed stage IV thymic cancer in terms of prolonging the disease‐free survival and median survival of people with lymphoepithelioma‐like or squamous cell histology types (Lin 2005).
Description of the intervention
Advanced thymoma and thymic cancer are not usually managed by surgical resection or radiotherapy alone. An important step in the management of these tumours is the introduction of systemic or regional chemotherapy or a multimodality treatment that includes cisplatin‐based chemotherapy, surgery and adjuvant chemoradiotherapy. The multimodality approach has shown benefits in terms of the resectability rate and the survival of advanced‐stage thymic tumour patients (Lucchi 2005; Shin 1998).
Chemotherapy has been widely utilised in unresectable and resectable thymoma and thymic cancer. The aim of pre‐operative chemotherapy (neoadjuvant or induction chemotherapy) is to reduce the bulk of the tumour, shrink its size and increase the possibility of surgical resection. Adjuvant chemotherapy is given to reduce the chance of recurrence and metastasis and prolong survival. Thymic cancer and advanced thymoma are rare and currently there is no standardised treatment regimen in terms of dose and cycle of chemotherapy. Some cases of advanced thymoma and thymic cancer have been reported to respond to several different chemotherapeutic regimens, including CAP (cisplatin, doxorubicin and cyclophosphamide) (Loehrer 1994), VIP (etoposide, ifosfamide and cisplatin) (Loehrer 2001) and modified ADOC therapy (adriamycin, nedaplatin, cyclophosphamide and vincristine) (Kitami 2001). Other therapeutic regimens, including PVB (cisplatin, vinblastine, bleomycin) and CHOP‐E (vincristine, cyclophosphamide, adriamycin, prednisolone and etoposide), have not been found to be effective in people with thymic cancer (Kitami 2001). The role of systemic chemotherapy and the optimal regimen remain uncertain. Cisplatin‐based chemotherapy is mostly advocated for either palliative or adjuvant treatment. For palliative or pre‐operative chemotherapy, there are six combinations of chemotherapy regimens:
cisplatin, doxorubicin and cyclophosphamide (CAP) combination therapy;
CAP with prednisone;
cisplatin, doxorubicin, vincristine and cyclophosphamide (ADOC);
cisplatin and etoposide (PE);
combined ifosfamide, cisplatin and etoposide (VIP); and
carboplatin/paclitaxel for first‐line therapy.
For second‐line chemotherapy there are seven single therapeutic agents (etoposide, ifosfamide, pemetrexed, octreotide/prednisone, 5‐fluorouracil/leucovorin, gemcitabine and paclitaxel) used for thymic malignancies (Hernandez‐Ilizaliturri 2004; ).
How the intervention might work
Chemotherapy in the pre‐operative setting may improve prognosis by enabling a tumour to be resected and reduce the incidence of pleural and systemic relapses. Only a few studies have reported on the treatment of thymic cancer and advanced thymoma. Overall response rates range from 20% to 75% and occasional complete response to chemotherapy has been reported (Kitami 2001; Lin 2005; Loehrer 2001). These chemotherapy regimens may also be utilised for adjuvant treatment alone or combined with radiotherapy. Evidence shows that the median survival time of trimodality treatments (surgery, radiotherapy, chemotherapy) ranges from 11 to 39 months (Hernandez‐Ilizaliturri 2004; Hsu 1994; Kitami 2001; Lin 2005; Loehrer 2001; Zhang 2007).
Why it is important to do this review
The role of chemotherapy in thymic cancer and advanced thymoma is unclear and based on current studies the clinical response is variable. Some reports show that chemotherapy appears to have a significant prognostic effect on thymic cancer and advanced thymoma (Hernandez‐Ilizaliturri 2004; Kitami 2001; Lin 2005; Loehrer 2001; Zhang 2007) while others have found either an unclear or no advantage (Ji 2006; Kondo 2003; Nakamura 2000). For people with an undifferentiated histology, multidisciplinary treatment or chemotherapy might not be helpful in either primary or relapsed stage IV thymic cancer (Lin 2005).
Many studies have shown significant benefits of multimodality treatments. Nevertheless, which combination of treatments is most beneficial has still not been established. In particular, the clinical benefit of chemotherapy in palliative, neoadjuvant or adjuvant treatment of thymic cancer is unclear. To date, a systematic review of treatment of thymic cancer and advanced thymoma has not been published, so it is necessary to summarise the current evidence to investigate the effectiveness, toxicity and effects on quality of life of chemotherapy as a treatment for thymic cancer and advanced thymoma in adults.
Objectives
To assess the role of chemotherapy in adults with thymic carcinoma or advanced thymoma.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs).
Types of participants
We planned to include all adults (aged 18 years and over) diagnosed with thymic carcinoma and/or with Masaoka stage III or IV thymic tumours. There were no limitations with regard to gender or racial characteristics.
The criteria for diagnosis were:
physical examination and history;
chest X‐ray or CT (first time) scan, or both;
pathological histology to confirm thymic cancer type using the WHO criteria (Travis 2004).
Types of interventions
We planned to include chemotherapy (either single‐agent or combination chemotherapy plus surgery, radiotherapy or not) for thymic carcinoma and/or advanced thymoma.
Types of outcome measures
Primary outcomes
The primary outcome was overall survival (OS), defined as survival until death from all causes. Survival time was assessed in months from the time of randomisation of participants or enrolment in the study.
Progression‐free survival (PFS).
Secondary outcomes
Response rate: the criteria to determine objective tumour response were according to Response Evaluation Criteria in Solid Tumour (RECIST) version 1.1 (Eisenhauer 2009).
Adverse events: grades of toxicity classified according to the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 criteria (CTCAE 2006) and grouped as follows:
haematological (anaemia, neutropenia, liver dysfunction);
gastrointestinal (dry mouth, dysphagia (difficulty swallowing), nausea, vomiting, diarrhoea);
respiratory (adult respiratory distress syndrome (ARDS), cough, shortness of breath, obstruction/stenosis of airway, pleural effusion);
dermatology/skin (stomatitis, mucositis, alopecia, allergy, hair loss/alopecia, rash);
neurological (peripheral and central);
infection;
cardiac;
constitutional symptoms (fatigue, fever, hypothermia).
3. Quality of life (QoL), measured by a validated scale.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2012, Issue 7) (Appendix 2), MEDLINE (accessed via Ovid from 1966 to July 2012) (Appendix 3), EMBASE (accessed via Ovid from 1980 to July 2012) (Appendix 4), Latin American and Caribbean Literature on Health Sciences (LILACS) (Appendix 5), the Chinese Biological Medicine Database (CBM, 1978 to July 2012) (Appendix 6), China National Knowledge Infrastructure (CNKI, 1980 to July 2012) (Appendix 7) and the Chinese scientific periodical database VIP Information (VIP, 1989 to July 2012) (Appendix 8).
The search strategies used were executed by the author team.
There was no language restriction in searching for studies.
Searching other resources
We conducted searches for ongoing trials in the metaRegister of Controlled Trials (http://www.controlled-trials.com/mrct/), WHO International Clinical Trial Registration Platform search portal (http://www.who.int/trialsearch/), International Standard Randomised Controlled Trial Number Register (ISRCTN), Physician Data Query (http://www.nci.nih.gov), http://www.clinicaltrials.gov, http://www.cancer.gov/clinicaltrials, International Guideline Library (www.g-i-n.net/library/international-guidelines-library/) etc. We also searched bibliographies of relevant studies and guidelines for possible references to additional trials and communicated with corresponding authors and clinical experts where possible to enquire about other published or unpublished relevant studies.
Data collection and analysis
Selection of studies
We downloaded all the citations retrieved by electronic searching to a reference management database (EndNote X2) and removed duplicates and records where no abstracts were available. We included studies presented only in abstract form or letter. For those studies not published in English we arranged translation.
Two review authors independently evaluated the search results according to the inclusion and exclusion criteria. We classified the abstracts as: (a) definitely include, (b) unsure and (c) definitely exclude. Any disagreements were resolved by discussion.
We obtained full copies of those citations classified as (a) or (b). Both review authors also worked independently to determine which studies met the inclusion criteria and classified the studies as: (1) included, (2) awaiting assessment or (3) excluded. We documented the concordance between the two review authors and disagreements were resolved by the third review author. We contacted the authors of studies classified as (2) for further clarification. We excluded studies identified as (3) and described the relevant reasons in the Characteristics of excluded studies table.
Data extraction and management
We had planned to extract study general information (author, year, journal citation, language, country, setting) and methodological characteristics (study design, characteristics of participants (inclusion criteria, age, Masaoka stage, WHO histological cell type if possible, co‐morbidity, previous treatment), interventions, risk of bias, duration of follow‐up and main outcomes, etc.), but no studies met the inclusion criteria and therefore no data extraction was completed.
Assessment of risk of bias in included studies
We had planned to assess risk of bias for RCTs using The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011) using the following criteria:
1. Sequence generation (method of randomisation)
Yes: if the allocation sequence was generated by a computer; referring to a random number table; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots.
No: if a system involving dates, names or admittance numbers was used for the allocation of participants. This would also include those studies involving non‐random approaches, for example allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; and by availability of the intervention.
Unclear: if the trial was described as randomised, but the allocation sequence generation was insufficient.
2. Allocation concealment (selection bias)
Yes: if the allocation of participants involved a central independent unit, on‐site locked computer, identically appearing numbered containers prepared by an independent investigator, or serially numbered, sealed and opaque envelopes.
No: if the allocation sequence was known by the investigators or participants who could possibly foresee assignments and thus introduce selection bias, such as using an open random allocation schedule; assignment envelopes were used without appropriate safeguards; alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure.
Unclear: if the trial was described as using allocation concealment, but the method used was not described or information was insufficient to permit a judgement of 'Yes' or 'No'.
3. Masking (blinding) of participants, treatment providers and outcome assessors (detection bias)
Yes (adequate): if the outcome assessment was masked and the non‐masking of others was unlikely to introduce bias.
No (inadequate): if there was no masking or incomplete masking, and the outcome or the outcome measurement was likely to be influenced by lack of masking.
Unclear: if there was insufficient information to permit judgement of 'Yes' or 'No', or the study did not address this outcome.
4. Incomplete outcome data addressed
We assessed each main outcome for information on the number of participants lost to follow‐up, whether this was less than 20% and the reasons:
Yes: if it was specified that there were no drop‐outs or withdrawals; reasons for missing outcome data were unlikely to be related to true outcome; missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups; missing data have been imputed using appropriate methods; the missing outcomes were not enough to induce a clinically relevant impact on the intervention effect estimate.
No: if the number or reasons for missing outcome data were likely to be related to true outcome; the proportion of missing outcomes with observed event risk was enough to induce clinically relevant bias in intervention effect estimate; drop‐outs and withdrawals were not described.
Unclear: insufficient reporting of attrition/exclusions to permit judgement of 'Yes' or 'No' or the study does not address this outcome.
5. Selective outcome reporting
Yes: study protocol is available and all of the study's pre‐specified (primary and secondary) outcomes have been reported in the pre‐specified way; study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified.
No: not all of the study's pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely; the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear: insufficient information to permit judgement of 'Yes' or 'No'.
6. Other potential threats to validity
Yes: the study appears to be free of other sources of bias.
No: had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal stopping rule); extreme baseline imbalance; has been claimed to have been fraudulent.
Unclear: insufficient information to assess whether an important risk of bias exists; or insufficient rationale or evidence that an identified problem introduced bias.
Two review authors independently performed quality assessment and disagreements were resolved by a third party. We graded each of the parameters as:
A: adequate or yes (low risk of bias);
B: unclear or not reported; or
C: inadequate or no (high risk of bias).
Assessment of reporting biases
As no studies were available for inclusion, we did not use funnel plots (effect size against standard error) to assess potential bias such as publication bias.
Data synthesis
As no studies were available for inclusion no data analysis could be completed. However, we have narratively described relevant prospect studies in the excluded studies table (Characteristics of excluded studies).
Sensitivity analysis
As no studies were included, this was not required.
Results
Description of studies
Results of the search
The results of the searches identified a total of 5778 references (MEDLINE 2641, EMBASE 2733, CENTRAL 14, CBM 134, CNKI 228 and 28 from other sources). We imported these into a reference management database (EndNote) and removed 706 duplicate records. Two review authors (ML Wei, DY Kang) independently screened the remaining 5072 references, excluded 5020 reports not related to the review topic and obtained full‐text copies of 52 potentially relevant reports (Figure 1). We were unable to identify any RCTs for inclusion.
1.
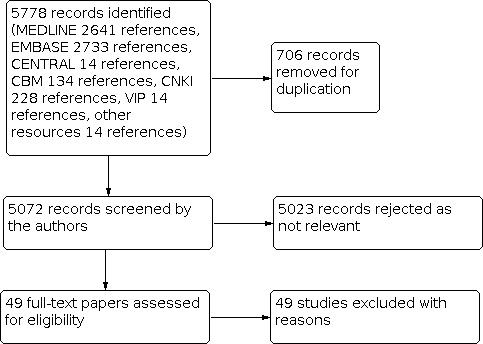
Searching flow diagram
Included studies
No studies were identified for inclusion.
Excluded studies
Forty‐nine studies were excluded. These are reported in Characteristics of excluded studies. Of these 14 were identified as prospective studies and from these we have documented the main treatments in current practice in Table 1 ('Characteristics of excluded prospective studies').
1. Characteristics of excluded prospective studies.
| Study ID | Study place | Study year | Study method | Participants | Interventions | Overall survival | Progression‐free survival (PFS) months | Adverse events |
| Bretti 2004 | Torino, Italy | 1989 to 2001 | A multimodal approach study, prospective | 63 (37M, 26F) Age: 51 (17 to 84) Stage III/IV: 43/20 thymic carcinoma 4 |
Surgery (30) versus Neoadjuvant plus surgery (33, included preoperative radiotherapy in the first 8 (30 Gy was administered in 15 fractions over a period of 3 weeks) and chemotherapy in the remaining 25). Of them, radical resection 32 (20 patients ab initio (all stage III) and in 12 patients after neoadjuvant treatment (eight stage III and four stage IVa) Chemotherapy: ADOC: 18 participants; CDDP plus VP: 16 participants |
As of December 2001, a total of 21 patients (33.3%) were alive and disease free. Median survival 142.1 months (0 to 308) in stage III, 45.9 months (21.0 to 70.8) in stage IV |
Neoadjuvant plus surgery: median 56.9 (19.2 to 94.5), slightly lower than those radically resected | 40 (63.5%) had progressed and 29 (46.0%) had died.6 died of causes other than progressive disease: 2 of leukopenia (3 and 5 years after surgery and radiotherapy), 1 of squamous cell bronchial carcinoma (12 years after surgery and radiation therapy), 1 of radiation‐induced pneumonitis, 1 of congestive heart failure (5 years after the completion of chemotherapy) (4 cycles of ADOC scheme, surgery and radiotherapy), 1 of hepatic toxicity following chemotherapy administered for recurrent disease (4 years after surgery and radiation therapy) |
| Cardillo 2010 | Rome, Italy | 1991 to 2007 | Retrospective study and prospective data collection | 61 (44M, 17F) Age: 45.7 ± 12.5 (14 to 72) Stage III/IV: 34/27 thymic carcinoma 10 |
A. IC plus surgery (31, stage III/IV: 18/13) B. Surgery only (30, stage III/IV: 16/14) Induction chemotherapy: CAP plus prednisone |
The univariate analysis showed the 10‐year survival rate, respectively, of 57.9% in group A and 38.1% in group B (P = 0.03); 59.8% in stage III and 28.2% in stage IVa (P = 0.02); 48.8% in R0 resection and 36.5% in R1 resection (P = 0.04). | Not reported | 6 participants in IC group and 7 participants in surgery group died of disease 9 participants had major non‐lethal complications (1 pulmonary embolism, 1 postoperative bleeding, 2 pulmonary infections and 5 wound infections), which resolved with conservative treatment. 1 adult had respiratory distress syndrome after surgical resection and required prolonged hospitalisation |
| Giaccone 1996 | 7 European institutions | 1985 to 1991 | Phase II study | 16 (10M, 6F) Age: 45 (20 to 67) |
Chemotherapy: PE | Survival at 5, 7 years was 50%, 42% respectively 2 participants who did not have signs of major tumour regression on chemotherapy never progressed after a continuous follow‐up period of more than 8 and 9 years |
Median PFS 2.2 years, with 48%, 38% and 26% of participants at 3, 5 and 7 years | 9 deaths The toxicity was tolerable Grade III‐IV: leukopenia 51%, nausea and vomiting 81% and alopecia 69% |
| Kim 2004 | Texas, US | 1990 to 2000 | Phase II study: multidisciplinary approach for unresectable thymomas (Abstract) | 22 (9M, 13F) Age: 47 (25 to 70) Stage III/IV: 11/11 |
Induction chemotherapy surgical resection, radiation therapy and consolidation chemotherapy Chemotherapy: CAP plus prednisone |
5‐year OS: 95% 10‐year OS: 79% |
PFS was the same: 77% (95% CI 0.58 to 1.0) at 5 years and 7 years. 18/19 completing multidisciplinary approach were disease‐free | Myelosuppression. 9 participants grade III/IV neutropenia Non‐haematologic: fatigue, nausea, vomiting, and decreased appetite. 1 acute respiratory distress syndrome after resection |
| Kunitoh 2009 | Tokyo, Japan | 1997 to 2004 | A prospective phase II trial for stage IV | 30 (16M, 14F) Age: 47.5 (29 to 69) Stage III/IV: 0/30 |
Chemotherapy: CODE | 5‐year OS: 65% | Median PFS: 0.79 years (95% CI 0.52 to 1.40), PFS at 2 years was 15% | Haematological: well tolerated with no deaths due to toxicity, although 70% of cases experienced grade IV neutropenia; this was generally transient and rarely complicated by infection/fever. 26/27 participants had tumour relapse |
| Kunitoh 2010 | 8 institutions, Japan | 1997 to 2005 | A prospective phase II trial for stage III | 23 (17M, 6F) Age: 56 (28 to 70) Stage III/IV: 23/0 |
Chemotherapy: CODE | 5‐year OS: 85% 8‐year OS: 69% |
21 eligible cases, median PFS was 4.5 years (95% CI 2.3 to the upper limit not calculable years), PFS at 2, 5 and 8 years was 80%, 43% (95% CI 21% to 63%) and 32%, respectively 5‐ and 8‐year PFS for those who underwent resection was 46% and 36% for those with surgical resection and 39% and 26% for those without, respectively |
Haematological: half experienced grade 4 neutropenia, generally transient and complicated by infection in only 1 case. Substantial anaemia was frequently observed. No deaths related to toxicity. The toxicity was evaluated according to the Japan criteria |
| Loehrer 1994 | ECOG, US | 1983 to 1992 | A prospective mult‐institutional trial | 30 (16M, 14F) Age: 49 (26 to 74) Thymoma 29, thymic carcinoma 1 |
Chemotherapy: CAP 31 entered, 30 assessable and eligible | 5‐year survival rate 32% ± 11.7%, median survival 37.7 months (range, 2 to 91.9 plus), 15/30 alive at analysis | 10 participants stable disease | Time to treatment failure 18.4 months (range 0.8 to 91.9 plus) Primary toxicities were alopecia, mild haematologic, fever associated with neutropenia. ECOG grade III: 12 participants, grade IV: 4 participants |
| Loehrer 2001 | ECOG, US | 1995 to 1997 | A prospective inter‐group trial | 34, 28 analysis (17M, 11F) Age: 55 (20 to 76) Stage III/IV: 6/22 thymic carcinoma 8 |
Chemotherapy: VIP | 15/28 alive, median survival 31.6 months (range, 12.8 to 52.3). 7/8 participants with thymic carcinoma and 6/20 participants with thymoma died to date. 2‐year survival rates was 70% | Not reported | Majority of grade 3 and grade 4 toxicities were haematologic; 28 episodes of grade 4 haematologic toxicities occurred in 16 participants |
| Lucchi 2005 | Pisa, Italy | 1976 to 2003 | Prospective and retrospective study | 56 (31M, 25F) Age: 53.3 (25 to 80) |
Induction chemotherapy plus surgery (36) (after 1989) versus surgery (20) (before 1989) Chemotherapy: PE plus epirubicin |
Overall median survival time 113.2 months, 10‐year survival rates were 48% and 45.7% for stage III and IVA thymomas, respectively (difference not significant) | 34 participants were still alive (31 disease‐free), whereas 22 died (2 disease‐free) | Of the remaining 13 participants, 3 had liver metastases, 2 bone metastases, 5 mediastinal relapse, 3 multiple metastases. Common non‐hematological toxicities were alopecia and nausea/vomiting, usually mild or moderate. No epirubicin‐related cardiotoxicity was recorded during treatment. 2 participants died of thymomas for multiple metastases |
| Loehrer 2004 | ECOG, US | 1998 to 2000 | Phase II study | 42 participants entered, 38 assessable (1 inconclusive histology; 3 negative octreotide CT scan) Thymoma: 32, thymic carcinoma or thymic carcinoid: 6 |
A. Octreotide alone, 0.5 mg, for those in a complete or partial remission (17 participants) versus B. Octreotide 0.5 mg* 2 courses plus prednisone 0.6 mg/kg orally four times daily, for participants with stable disease (21 participants) Duration: max 1 year (12 cycles), those with progressive disease were removed; no dose modifications permitted. Participants with serious infection secondary to immunosuppression from prednisone were removed |
25/38 alive OS A. 9/17 (52.9%) (95% CI 27.8 to 77%) versus B. 16/21 (76.2%) (95% CI 52.8 to 92.8%) 2‐year survival 75.7% |
33/38 (86.8%) progression, 16/17 (94.1%) in A (median 2.0) versus 17/21 (81.0%) in B (median 9.2); the difference was statistically significant (P = 0.039) PFS for thymoma: 8.8 months (95% CI 3.7 to 12.3 months) versus for thymic carcinoma: 4.8 months (95% CI 1.9 to 9.5 months) |
8 participants grade 4 or 5 toxicity A. 3 participants grade 4 toxicity, acidosis, hyperglycaemia, hypocalcaemia, hypoglycaemia, dyspnoea, anaemia, leukopenia, elevated bilirubin, AST or ALT, and elevated creatinine B. 1 participant had a lethal toxicity secondary to a grade 5 infection without neutropenia; 4 participants had grade 4 toxicity of a similar toxicity as those octreotide alone |
| Palmieri 2010 | 6 centres, Italy | 2005 to 2008 | Phase II study | 15 (10M, 5F) Stage IV: 15 Thymic carcinoma: 3 |
CAP to GEM: oral capecitabine (650 mg/mq twice daily on days 1 to 14) and IV gemcitabine (1000 mg/mq on days 1 and 8) every 3 weeks (first cycle). If an objective partial response or stable disease, the participants could receive additional cycles until disease progression. After disease progression, 11 received supportive care, 2 participants with paclitaxel and docetaxel, respectively Duration: 105 cycles, median cycles 6, range 3 to 9 |
2‐year survival 10/15 (67%) | PFS: 11 months (95% CI 3 to 17) and 6 months (95% CI 3 to 11), respectively | No toxic deaths occurred. Grade 1–2 nausea/vomiting, diarrhoea, alopecia and hand‐foot syndrome were the most common non to haematologic toxic effects. The most common grade 3 haematologic toxicity was neutropenia (3) (20%) and anaemia (2) (13%); and grade 3 diarrhoea (1) (6.7%) |
| Rea 2011 | Padova, Italy | 1980 to 2008 | Multimodality treatment for advanced thymic tumours in 1 institution, a long‐term outcome study | 75 (32, 43) Age: 23 to 77 Stage III/IV: 51/24 |
Resectable(37) versus unresectable(38) Induction chemotherapy: 38 26 (68.4%) remission, 12 (31.6%) stable Regimen: ADOC Surgery Adjuvant treatment |
5‐year OS: 70% 10‐year OS: 57% |
33 (44%) died (8 without evidence of disease) and 42 (56%) were alive (10 with recurrence of disease) | No perioperative mortality occurred. Major complications were observed in 4 (5.3%): wound infection with sternal dehiscence, bronchopleural fistula after pneumonectomy, pneumonia and respiratory insufficiency in 1 with myasthenia gravis and phrenic nerve resection. Chemotherapy was well tolerated with no episodes of major toxicity. 21/61 (34.4%) recurrence |
| Shin 1998 | Houston, US | 1990 to 1996 | Prospective cohort study | 13, 12 assessed (5, 7) Age: 39.6 (23 to 66) Stage III/IV: 4/8 |
Induction chemotherapy: 13 CAP plus prednisone Surgery: 11 Radiotherapy: 12 Consolidation chemotherapy: 11 Regimen: 80% doses of cyclophosphamide, doxorubicin and cisplatin and 100% dose of prednisone Duration: repeated every 3 weeks for 3 courses |
7‐year OS: 100% | 10 participants remained disease‐free at a median follow‐up at 43 months (disease‐free survival at 7 years 73%) | Myelosuppression |
| Venuta 2003 | Rome, Italy | 1989 to 2002 | Prospective cases series with multimodality treatment | 45 (29M, 16F) Age: 50 ± 13 Stage III/IV: 45/0 thymic carcinoma 11 |
Chemotherapy: tumours that were not considered radically resectable underwent biopsy and induction chemotherapy Surgery: 45 Adjuvant chemoradiotherapy: 45 Induction chemotherapy: 8, PE plus epirubicin |
10‐year survival 78%, whereas the cumulative disease‐free survival was 53%. Survival for participants receiving induction chemotherapy was 90% versus 71% for participants undergoing primary surgery (P = 0.2) | During follow‐up 9 participants (20%) had tumour recurrence at a mean of 50 ± 42 months after surgery. 4 previously received induction chemotherapy | Major complications after surgery in 3 participants (6.7%) included sternal dehiscence, pulmonary embolism and recurrent bilateral pleural effusions. 5 participants died of their disease at mean of 30 ± 22 months after operation, 3 died from causes not related to the tumour at a mean follow‐up 29 ± 24 months after the operation. 4 participants with recurrence were still alive |
ADOC: cisplatin, doxorubicin, vincristine, cyclophosphamide CAP: cisplatin, doxorubicin and cyclophosphamide CI: confidence interval CODE: cisplatin, vincristine, doxorubicin and etoposide IC: induction chemotherapy IV: intravenous OS: overall survival PE: cisplatin and etoposide PFS: progression‐free survival VIP: etoposide, ifosfamide and cisplatin CDDP plus VP: cisplatin and etoposide CT: computerized tomography ALT: alanine transaminase AST: aspartate aminotransferase
Risk of bias in included studies
As no studies were included, this could not be completed.
Effects of interventions
As no studies were included, this section could not be completed.
Discussion
Summary of main results
There were no randomised controlled trials (RCTs) available for inclusion in this review. We have reported details of the prospective studies identified in Table 1, which provides some useful evidence of current clinical practice.
Among the 52 excluded studies, 14 were prospective studies from 1983 to 2009 which included 526 participants between 16 and 75 years old in Italy, the US, Europe (multicentre) and Japan (Bretti 2004; Cardillo 2010; Giaccone 1996; Kim 2004; Kunitoh 2009; Kunitoh 2010; Loehrer 1994; Loehrer 2001; Loehrer 2004; Lucchi 2005, Lucchi 2006; Palmieri 2010; Rea 2011; Shin 1998; Venuta 2003); six of these were phase II trials (Giaccone 1996; Kim 2004; Kunitoh 2009; Kunitoh 2010; Loehrer 2004,; Palmieri 2010). Three studies (Cardillo 2010; Lucchi 2005; Rea 2011) described both prospective and retrospective methodology.
Cisplatin‐based combination chemotherapy was adopted in 12 prospective studies including 525 participants (Bretti 2004; Cardillo 2010; Giaccone 1996; Kim 2004; Kunitoh 2009; Kunitoh 2010; Loehrer 2001; Loehrer 1994; Lucchi 2005; Rea 2011; Shin 1998; Venuta 2003). Only two trials (Loehrer 2004; Palmieri 2010) with a total of 57 patients used non‐cisplatin regimens (octreotide with or without prednisone and capecitabine plus gemcitabine). Two prospective studies in the US (Kim 2004; Shin 1998) used CAP (cisplatin, doxorubicin and cyclophosphamide): one study included 13 patients with unresectable malignant thymomas and seven‐year survival was 100% (Shin 1998); the second included 22 unresectable thymoma patients and 10‐year overall survival was 79% (Kim 2004).
There was some variation in the treatment regimens: a dose intensity of 20 mg/m2 to 100mg/m2 per day was given in three to four cycles at about 21‐day intervals. The regimens of combined cisplatin chemotherapy included: cisplatin, doxorubicin, vincristine, cyclophosphamide (ADOC) (Bretti 2004; Rea 2011); cisplatin, doxorubicin or cyclophosphamide (CAP) plus prednisone (Cardillo 2010; Kim 2004; Shin 1998); CAP without prednisone (Loehrer 1994); etoposide (PE) with or without epirubicin (Giaccone 1996; Lucchi 2005; Venuta 2003); cisplatin, ifosfamide and etoposide (VIP) (Loehrer 2001); cisplatin, vincristine, doxorubicin and etoposide (CODE) (Kunitoh 2009Kunitoh 2010). The other regimens without cisplatin included octreotide alone or plus prednisone (Loehrer 2004), and capecitabine and gemcitabine (Palmieri 2010). Seven prospective studies with 335 participants adopted neoadjuvant chemotherapy for participants with advanced thymoma (Bretti 2004; Cardillo 2010; Kim 2004; Lucchi 2005; Rea 2011; Shin 1998; Venuta 2003); those who were judged sufficiently down‐staged received radical surgical resection.
Overall completeness and applicability of evidence
The lack of RCTs means that current treatment options are based on expertise and experience.
In the wider literature there is limited evidence that a multimodality approach including neoadjuvant chemotherapy may have some benefit for participants with inoperable thymoma and thymic carcinoma. Cisplatin‐based chemotherapy seems to be the treatment of choice.
This review may not be comprehensive but may give an indication of current practice that could help inform patients and practitioners. As these conditions are rare and progress relatively slowly, it seems unlikely that a RCT will be done as there would be insufficient patients for a meaningful comparison.
Authors' conclusions
Implications for practice.
Without evidence from randomised controlled trials, treatment of thymic carcinoma and advanced thymoma is based on expert opinion or experience. Cisplatin‐based chemotherapy is the usual regimen of choice in current practice.
Implications for research.
Considering this condition is rare, it is suggested that an international group is set up to organise and evaluate prospective collection of long‐term data from cohorts of patients to inform current clinical practice. Cisplatin‐based chemotherapy plus prednisone may warrant additional investigation.
What's new
| Date | Event | Description |
|---|---|---|
| 20 October 2022 | Amended | Update to acknowledgements section |
History
Protocol first published: Issue 7, 2010 Review first published: Issue 8, 2013
| Date | Event | Description |
|---|---|---|
| 22 September 2022 | Review declared as stable | No additional studies expected and not currently a priority topic area. |
| 22 September 2022 | Amended | The literature search was extended until 12 April 2022. After title and abstract screening 16 papers were identified for full text assessment. None of these were found to meet the inclusion criteria see Appendix 9. |
| 27 March 2014 | Amended | Contact details updated. |
Acknowledgements
We thank the Cochrane Gynaecological Cancer and Orphan Cancer Review Group editorial team, especially Clare Jess and Gail Quinn for their contributions to the editorial process, and Jane Hayes for helping design the original search search strategy and Jo Platt for running the update searches in 2022. We also thank Lesley Smith, statistician, for her guidance on non‐randomised studies, useful comments and comprehensive additions to the review. We thank Phil Wiffen who supported the methodological writing of the review. Many thanks Dr Chris Williams, the previous co‐ordinating Editor, for his comments and useful suggestions during the original review development. Thanks to Dr Ming Yang, Dr Weidong Chen and Mr Xin Mu, etc. for help with additional reference searching and location. Special thanks to the peer review comments from Dr Martie Muller, Dr Tess Lawrie, Dr Tom Kenny, Dr F Grassin and Dr Kathie Godfrey. Thanks to Jun Xia and Jenny Bellorini (Copy‐Editor) for their suggestions on the review. Also, thanks to Jifan Fang and Biao Yang from West China Hospital, Sichuan University for documenting study assessment for the amendment in 2022.
Appendices
Appendix 1. The relationship between thymic carcinoma and thymoma
| Feature | Thymoma | Thymic carcinoma |
| Organotypic (thymus‐like) histological features | Almost always present (lobular pattern, perivascular spaces, immature, TdT+/CD1a+/CD99+ T to cells) | None or abortive |
| CD5, CD70 and CD117 expression in epithelial cells | No | Frequent (~ 60%) |
| Invasion | Variable | Almost always |
| Myasthenia gravis | Variable: 10% to 80% | No |
| Other autoimmune diseases | Common | Rare |
| Clinical behaviour | Often curable by surgery; metastases are rare Usually long survival due to indolent clinical course | Often unresectable; metastases are frequent Often short survival due to progressive disease |
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor Thymus Neoplasms explode all trees #2 MeSH descriptor Thymoma explode all trees #3 (thymic or thymus) near/5 (neoplas* or cancer* or carcinom* or tumor* or tumour* or malignan*) #4 thymoma* #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Antineoplastic Agents explode all trees #7 MeSH descriptor Antineoplastic Combined Chemotherapy Protocols explode all trees #8 MeSH descriptor Chemotherapy, Adjuvant explode all trees #9 chemotherap* or chemoradi* or radiochemotherap* #10 cisplatin #11 doxorubicin #12 cyclophosphamide #13 prednisone #14 vincristine #15 etoposide #16 ifosfamide #17 octreotide #18 leucovorin #19 gemcitabine #20 paclitaxel #21 fluorouracil #22 carboplatin #23 pemetrexed #24 (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23) #25 (#5 AND #24)
Appendix 3. MEDLINE search strategy
1 exp Thymus Neoplasms/ 2 exp Thymoma/ 3 (thym* adj5 (neoplas* or cancer* or carcinom* or tumor* or tumour* or malignan*)).mp. 4 thymoma*.mp. 5 1 or 2 or 3 or 4 6 exp Antineoplastic Agents/ 7 exp Antineoplastic Combined Chemotherapy Protocols/ 8 Chemotherapy, Adjuvant/ 9 (chemotherap* or chemoradi* or radiochemotherap*).mp. 10 cisplatin.mp. 11 doxorubicin.mp. 12 cyclophosphamide.mp. 13 prednisone.mp. 14 vincristine.mp. 15 etoposide.mp. 16 ifosfamide.mp. 17 octreotide.mp. 18 leucovorin.mp. 19 gemcitabine.mp. 20 paclitaxel.mp. 21 fluorouracil.mp. 22 carboplatin.mp. 23 pemetrexed.mp. 24 or/6 to 23 25 5 and 24 26 (animals not (humans and animals)).sh. 27 25 not 26
Key: mp = title, original title, abstract, name of substance word, subject heading word, unique identifier fs = floating subheading sh = subject heading
Appendix 4. EMBASE search strategy
1 exp thymoma/ 2 ((thymic or thymus) adj5 (neoplas* or cancer* or carcinom* or tumor* or tumour* or malignan*)).mp. 3 thymoma*.mp. 4 1 or 2 or 3 5 exp antineoplastic agent/ 6 exp chemotherapy/ 7 (chemotherap* or chemoradi* or radiochemotherap).mp. 8 cisplatin.mp. 9 doxorubicin.mp. 10 cyclophosphamide.mp. 11 prednisone.mp. 12 vincristine.mp. 13 etoposide.mp. 14 ifosfamide.mp. 15 octreotide.mp. 16 leucovorin.mp. 17 gemcitabine.mp. 18 paclitaxel.mp. 19 fluorouracil.mp. 20 carboplatin.mp. 21 pemetrexed.mp. 22 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 23 4 and 22 24 limit 23 to human
key: mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name
Appendix 5. LILACS search strategy
thymoma or thymic tumor or thymic carcinoma in (subject)
Appendix 6. Chinese Biological Medicine Database search strategy
(thymoma or thymic carcinoma) and chemotherapy
Appendix 7. China National Knowledge Infrastructure search strategy
(thymic carcinoma or thymoma) and chemotherapy in key words
Appendix 8. Chinese scientific periodical database of VIP Information search strategy
(thymic carcinoma or thymoma) and chemotherapy in key words or title
Appendix 9. Studies excluded for amendment 2022
| [1] | Hu X, Zhu H, Feng Y, et al. 117TiP A phase II study of toripalimab combined with paclitaxel/carboplatin for the first‐line treatment of advanced thymic carcinoma. Annals of Oncology. Conference: ESMO Immuno‐Oncology Congress 2021. Virtual, Online. 32 (Supplement 7) (pp S1426), 2021. |
| [2] | Koizumi T, Agatsuma T, Tateishi K, et al. Combination chemotherapy with doxorubicin, vincristine, cyclophosphamide, and platinum compounds for advanced thymic carcinoma. Journal of Thoracic Oncology. Conference: 5th Asia Pacific Lung Cancer Conference, APLCC and 3rd International Thymic Malignancy Interest Group, ITMIG Annual Meeting. Fukuoka Japan. Conference Publication: 7 (11 SUPPL. 5) (pp S423), 2012. |
| [3] | Toyozawa R, Hirai H, Inamasu E, et al. Effectiveness of amrubicin for second‐line or more chemotherapy for patients with recurrent thymic carcinoma [M]. Journal of Thoracic Oncology. Conference: 5th Asia Pacific Lung Cancer Conference, APLCC and 3rd International Thymic Malignancy Interest Group, ITMIG Annual Meeting. Fukuoka Japan. Conference Publication: 7 (11 SUPPL. 5) (pp S424), 2012. |
| [4] | Xu J P, Hao X Z, Zhang X R, et al. Efficacy and safety of the combination of paclitaxel and platinum in advanced thymic carcinoma. Thoracic Cancer. 7(2) (pp 222‐5), 2016. |
| [5] | Wang Y, Nie J, Dai L, et al. Efficacy and toxicities of gemcitabine and cisplatin combined with endostar in advanced thymoma and thymic carcinoma. Thoracic Cancer. 10(1) (pp 17‐23), 2019. |
| [6] | Uchibori A, Kato D, Takeda N, et al. EP1.15‐20 Good Control by Re‐Administration of Carboplatin and Paclitaxel Against Unresectable Thymic Carcinoma. Journal of Thoracic Oncology. Conference: IASLC 2019 World Conference on Lung Cancer (WCLC). Barcelona Spain. 14(10 Supplement) (pp S1060), 2019. |
| [7] | Hirai F, Yamanaka T, Taguchi K, et.al. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Annals of Oncology, 2015, 26(2): 363‐8. |
| [8] | Kogure Y, Hirai F, Yamanaka T, et al. A multicenter prospective study of carboplatin and paclitaxel for advanced thymic carcinoma: West Japan oncology group 4207l. Journal of Thoracic Oncology. Conference: 4th International Thymic Malignancy Interest Group Annual Meeting, ITMIG 2013. Bethesda, MD United States. Conference Publication: (8(SUPPL. 1) (pp 22), 2013. |
| [9] | Kawashima Y, Inoue A, Sugawara S, et al. Phase II study of amrubicin (AMR) and carboplatin (CBDCA) for invasive thymoma (IT) and thymic carcinoma: NJLCG0803 [M]. Journal of Clinical Oncology. Conference: 2013 Annual Meeting of the American Society of Clinical Oncology, ASCO. Chicago, IL United States. Conference Publication: 31(15 SUPPL. 1) (no pagination), 2013. |
| [10] | Inoue A, Sugawara S, Harada M, et al. Phase II study of amrubicin combined with carboplatin for thymic carcinoma and invasive thymoma north Japan lung cancer group study 0803. Journal of Thoracic Oncology. 9(12) (pp 1805‐9), 2014. |
| [11] | Zucali P A, De Pas T, Palmieri G, et al. Phase II study of everolimus in patients with thymoma and thymic carcinoma previously treated with cisplatin‐based chemotherapy. Journal of Clinical Oncology. 36(4) (pp 342‐9), 2018. |
| [12] | Gbolahan O B, Porter R F, Salter J T, et al. A Phase II Study of Pemetrexed in Patients with Recurrent Thymoma and Thymic Carcinoma. Journal of Thoracic Oncology. 13(12) (pp 1940‐8), 2018. |
| [13] | Huang J, Raz D, Cristea M, et al. Phase II trial of cetuximab and chemotherapy followed by surgical resection for locally advanced thymoma. Journal of Thoracic Oncology. Conference: 18th World Conference on Lung Cancer of the International Association for the Study of Lung Cancer, IASLC 2017. Yokohama Japan. 12(11 Supplement 2) (pp S1749), 2017. |
| [14] | JPRN U. Phase II trial of S‐1 treatment as palliative‐intent chemotherapy for previously treated advanced thymic carcinoma. https://trialsearch.who.int/Trial2.aspx?TrialID=JPRN‐UMIN000010736. 2013. |
| [15] | Hellyer JA, Gubens MA, Cunanan KM, et al. Phase II trial of single agent amrubicin in patients with previously treated advanced thymic malignancies. Lung Cancer 2019, 137: 71‐5 |
| [16] | Wang Junjie, Duan Renhui. Short‐term clinical effect observation of gemcitabine combined with oxaliplatin in the treatment of recurrent or metastatic thymic carcinoma. Modern Medicine, 2014, (1): 51‐3. |
| [17] | Du Haixia. Efficacy observation of gemcitabine combined with platinum drugs in the treatment of advanced thymic carcinoma. China Health Industry 2013, 22 vo 10): 74‐6. |
| [18] | Zhang Yuming. Clinical study of three‐dimensional conformal radiation therapy combined with etoposide cisplatin regimen in the treatment of advanced thymic carcinoma. Journal of Practical Medical Technology, 2021, 28(3): 393‐5. |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bonomi 1993 | Did not meet the review criteria: phase II trial of 24 patients treated with cisplatin in Eastern Cooperative Oncology Group for 4 years |
| Bretti 2004 | Did not meet the review criteria: not a RCT |
| Cardillo 2010 | Did not meet the review criteria: not a RCT |
| Cowen 1995 | Did not meet the review criteria: not a RCT. The primary treatment for all participants was irradiation and we were unable to extract any effect data for the 74 participants (50%) who received CAP chemotherapy as part of the treatment |
| Daniele 2003 | Did not meet the review criteria: 2 cases reported and reviewed |
| Evans 2005 | Did not meet the review criteria: review |
| Falkson 2009 | Did not meet the review criteria: review |
| Fan 2008 | Did not meet the review criteria: retrospective survival analysis |
| Fornasiero 1990 | Duplication of Fornasiero 1991 |
| Fornasiero 1991 | Did not meet the review criteria: 13‐year retrospective study of 37 patients in Padova, Italy from 1977 to 1990 |
| Fujimura 1987 | Did not meet the review criteria: surgical treatment |
| Giaccone 1996 | Did not meet the review criteria: phase II study |
| Giaccone 2000 | Did not meet the review criteria: review |
| Giaccone 2006 | Did not meet the review criteria: 29 cases |
| Girard 2009 | Did not meet the review criteria |
| Grassin 2010 | Did not meet the review criteria: not a RCT |
| Gripp 1998 | Did not meet the review criteria: treatment did not include chemotherapy |
| Huang 2008 | Did not meet the review criteria: editorial comment |
| Huang 2009 | Did not meet the review criteria: surgical intervention |
| Kim 2004 | Did not meet the review criteria: phase II study |
| Kim 2008 | Did not meet the review criteria: survival analysis |
| Koizumi 2002 | Did not meet the review criteria: 8 participants with thymic carcinoma |
| Kong 2005 | Did not meet the review criteria: survival analysis. The authors retrospectively reviewed the medical records of 49 participants with thymic carcinoma and 6 of them developed brain metastasis |
| Kunitoh 2009 | Did not meet the review criteria: prospective phase II trial |
| Kunitoh 2010 | Did not meet the review criteria: prospective phase II trial |
| Kurup 2004 | Did not meet the review criteria: review article |
| Lee 2009 | Did not meet the review criteria: retrospective cases series (60) analysis of thymic carcinoma; a 20‐year experience at a single institution in Seoul, Korea from 1986 to 2005 |
| Loehrer 1994 | Did not meet the review criteria: prospective multi‐institutional trial |
| Loehrer 2001 | Did not meet the review criteria: prospective inter‐group trial |
| Loehrer 2004 | Did not meet the review criteria: prospective phase II trial |
| Lucchi 2005 | Did not meet the review criteria: prospective and retrospective study |
| Lucchi 2006 | Did not meet the review criteria: prospective study of 30 patients with neoadjuvant chemotherapy in Pisa, Italy from 1989 to 2004 |
| Luo 2004 | Did not meet the review criteria: comparative analysis of 40 cases (prospective and retrospective) in Shanghai, China from 1995 to 2002 |
| Myojin 2000 | Did not meet the review criteria: no chemotherapy |
| Oberg 2009 | Did not meet the review criteria: guideline |
| Palmieri 2010 | Did not meet the review criteria: open‐label, non‐randomised, prospective, phase II study |
| Rajan 2008 | Did not meet the review criteria: review |
| Rajan 2011 | Did not meet the review criteria: review |
| Rea 2011 | Did not meet the review criteria: not a RCT. Multimodality treatment for advanced thymic tumours in 1 institution; a long‐term outcome study |
| Shin 1998 | Did not meet the review criteria: prospective cohort study |
| Ströbel 2004 | Did not meet the review criteria: survival analysis |
| Venuta 1997 | Same study as Venuta 2003 |
| Venuta 2003 | Did not meet the review criteria: prospective case series (45) with multimodality treatment |
| White 1990 | Did not meet the review criteria: review |
| Wright 2005 | Did not meet the review criteria |
| Xu 2009 | Did not meet the review criteria: 15 cases |
| Yano 2005 | Did not meet the review criteria: no details of chemotherapy |
| Yokoi 2007 | Did not meet the review criteria: 17 cases with CAMP (cisplatin, doxorubicin, methyl to prednisolone 1000 mg/d 1 to 4, 500 mg/d 5 to 6) and 10‐year overall survival > 80.7% |
| Zhu 2009 | Did not meet the review criteria: reported using randomisation, but after contacting the author we judged it was not a real RCT |
RCT: randomised controlled trial
Differences between protocol and review
As an additional search resource, we added the International Guidelines Library (www.g-i-n.net/library).
Contributions of authors
ML Wei performed previous work, conceived the review idea and drafted the review. ML Wei and YM Mu designed and co‐ordinated the review. ML Wei, DY Kang and M Qiu wrote the review. DY Kang provided support with statistical methods. L Gu and ZY Liao provided clinical comments on the review. General advice was provided by YM Mu.
Sources of support
Internal sources
Chinese Cochrane Centre, Chinese Centre of Evidence‐based Medicine, West China Hospital of Sichuan University, China
External sources
No sources of support provided
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies excluded from this review
Bonomi 1993 {published data only}
- Bonomi PD, Finkelstein D, Aisner S, Ettinger D. EST 2582 phase II trial of cisplatin in metastatic or recurrent thymoma. American Journal of Clinical Oncology 1993;16(4):342-5. [DOI] [PubMed] [Google Scholar]
Bretti 2004 {published data only}
- Bretti S, Berruti A, Loddo C, Sperone P, Casadio C, Tessa M, et al. Multimodal management of stages III-IVa malignant thymoma. Lung Cancer 2004;44(1):69-77. [DOI] [PubMed] [Google Scholar]
Cardillo 2010 {published data only}
- Cardillo G, Carleo F, Giunti R, Lopergolo MG, Salvadori L, De Massimi AR, et al. Predictors of survival in patients with locally advanced thymoma (Masaoka stage III and IVa). European Journal of Cardio-thoracic Surgery 2010;37:819-23. [DOI] [PubMed] [Google Scholar]
Cowen 1995 {published data only}
- Cowen D, Richaud P, Mornex F, Bachelot T, Jung GM, Mirabel X, et al. Thymoma: results of a multicentric retrospective series of 149 non-metastatic irradiated patients and review of the literature. FNCLCC trialists. Fédération Nationale des Centres de Lutte Contre le Cancer. Radiotherapy and Oncology 1995;34(1):9-16. [DOI] [PubMed] [Google Scholar]
Daniele 2003 {published data only}
- Daniele O, Fornasiero A. Ifosfamide in thymic neoplasms. Oncology 2003;65 Suppl 2:44-5. [DOI] [PubMed] [Google Scholar]
Evans 2005 {published data only}
- Evans TL, Lynch TJ. Role of chemotherapy in the management of advanced thymic tumors. Seminars in Thoracic and Cardiovascular Surgery 2005;17:41-50. [DOI] [PubMed] [Google Scholar]
Falkson 2009 {published data only}
- Falkson CB, Bezjak A, Darling G, Gregg R, Malthaner R, Maziak DE, et al. The management of thymoma: a systematic review and practice guideline. Journal of Thoracic Oncology 2009;4(7):911-9. [DOI] [PubMed] [Google Scholar]
Fan 2008 {published data only}
- Fan RT, Wang JM, Zhang HZ, Guo YN, Gu H. Clinical analysis of 45 patients with thymic carcinoma. Chinese Journal of Clinical Oncology 2008;35(9):488-93. [Google Scholar]
Fornasiero 1990 {published data only}
- Fornasiero A, Daniele O, Ghiotto C, Sartori F, Rea F, Piazza M, et al. Chemotherapy of invasive thymoma. Journal of Clinical Oncology 1990;8(8):1419-23. [DOI] [PubMed] [Google Scholar]
Fornasiero 1991 {published data only}
- Fornasiero A, Daniele O, Ghiotto C, Piazza M, Fiore-Donati L, Calabró F, et al. Chemotherapy for invasive thymoma. A 13-year experience. Cancer 1991;68(1):30-3. [DOI] [PubMed] [Google Scholar]
Fujimura 1987 {published data only}
- Fujimura ST, Kondo T, Handa M, Shiraishi Y, Tamahashi N, Nakada T. Results of surgical treatment for thymoma based on 66 patients. Journal of Thoracic and Cardiovascular Surgery 1987;93(5):708-14. [PubMed] [Google Scholar]
Giaccone 1996 {published data only}
- Giaccone G, Ardizzoni A, Kirkpatrick A, Clerico M, Sahmoud T, Zandwijk NV. Cisplatin and etoposide combination chemotherapy for locally advanced or metastatic thymoma: a phase II study of the European Organization for Research and Treatment of Cancer Lung Cancer Cooperative Group. Journal of Clinical Oncology 1996;14(3):814-20. [DOI] [PubMed] [Google Scholar]
Giaccone 2000 {published data only}
- Giaccone G. Treatment of thymoma and thymic carcinoma. Annals of Oncology 2000;11(Suppl 3):245-6. [DOI] [PubMed] [Google Scholar]
Giaccone 2006 {published data only}
- Giaccone G, Wilmink H, Paul MA, Valk P. Systemic treatment of malignant thymoma: a decade experience at a single institution. American Journal of Clinical Oncology 2006;29:336-44. [DOI] [PubMed] [Google Scholar]
Girard 2009 {published data only}
- Girard N, Mornex F, Van Houtte P, Cordier JF, Schil P. Thymoma: A focus on current therapeutic management. Journal of Thoracic Oncology 2009;4(1):119-26. [DOI] [PubMed] [Google Scholar]
Grassin 2010 {published data only}
Gripp 1998 {published data only}
- Gripp S, Hilgers K, Wurm R, Schmitt G. Thymoma: prognostic factors and treatment outcomes. Cancer 1998;83(8):1495-503. [PubMed] [Google Scholar]
Huang 2008 {published data only}
- Huang J, Riely GJ, Rosenzweig KE, Rusch VW. Multimodality therapy for locally advanced thymomas: state of the art or investigational therapy? Annals of Thoracic Surgery 2008;85(2):365-7. [DOI] [PubMed] [Google Scholar]
Huang 2009 {published data only}
- Huang J, Rizk NP, Travis WD, Riely GJ, Park BJ, Bains MS, et al. Comparison of patterns of relapse in thymic carcinoma and thymoma. Journal of Thoracic and Cardiovascular Surgery 2009;138(1):26-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2004 {published data only}
- Kim ES, Putnam JB, Komaki R, Walsh GL, Ro JY, Shin HJ, et al. Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report. Lung Cancer 2004;44(3):369-79. [DOI] [PubMed] [Google Scholar]
Kim 2008 {published data only}
- Kim BK, Cho BC, Choi HJ, Sohn JH, Park MS, Chang J, et al. A single institutional experience of surgically resected thymic epithelial tumors over 10 years: clinical outcomes and clinicopathologic features. Oncology Reports 2008;19(6):1525-31. [PubMed] [Google Scholar]
Koizumi 2002 {published data only}
- Koizumi T, Takabayashi Y, Yamagishi S, Tsushima K, Takamizawa A, Tsukadaira A, et al. Chemotherapy for advanced thymic carcinoma: clinical response to cisplatin, doxorubicin, vincristine, and cyclophosphamide (ADOC chemotherapy). American Journal of Clinical Oncology 2002;25(3):266-8. [DOI] [PubMed] [Google Scholar]
Kong 2005 {published data only}
- Kong DS, Lee JI, Nam DH, Park K, Suh YL. Cerebral involvement of metastatic thymic carcinoma. Journal of Neuro-Oncology 2005;75(2):143-7. [DOI] [PubMed] [Google Scholar]
Kunitoh 2009 {published data only}
- Kunitoh H, Tamura T, Shibata T, Nakagawa K, Takeda K, Nishiwaki Y, et al, JCOG Lung Cancer Study Group, Tokyo, Japan. A phase-II trial of dose-dense chemotherapy in patients with disseminated thymoma: Report of a Japan Clinical Oncology Group trial (JCOG 9605). British Journal of Cancer 2009;101(9):1549-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunitoh 2010 {published data only}
- Kunitoh H, Tamura T, Shibata T, Takeda K, Katakami N, Nakagawa K, et al. A phase II trial of dose-dense chemotherapy, followed by surgical resection and/or thoracic radiotherapy, in locally advanced thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9606). British Journal of Cancer 2010;103:6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kurup 2004 {published data only}
- Kurup A, Loehrer PJ Sr. Thymoma and thymic carcinoma: therapeutic approaches. Clinical Lung Cancer 2004;6(1):28-32. [DOI] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee CY, Bae MK, Park IK, Kim DJ, Lee JG, Chung KY. Early Masaoka stage and complete resection is important for prognosis of thymic carcinoma: a 20-year experience at a single institution. European Journal of Cardio-Thoracic Surgery 2009;36(1):159-63. [DOI] [PubMed] [Google Scholar]
Loehrer 1994 {published data only}
- Loehrer PJ Sr, Kim K, Aisner SC, Livingston R, Einhorn LH, Johnson D, et al. Cisplatin plus doxorubicin plus cyclophosphamide in metastatic or recurrent thymoma: final results of an intergroup trial. Journal of Clinical Oncology 1994;12(6):1164-8. [DOI] [PubMed] [Google Scholar]
Loehrer 2001 {published data only}
- Loehrer PJ, Jiroutek M, Aisner S, Aisner J, Green M, Thomas CR, et al. Combined etoposide, ifosfamide, and cisplatin in the treatment of patients with advanced thymoma and thymic carcinoma: an intergroup trial. Cancer 2001;91(11):2010-5. [PubMed] [Google Scholar]
Loehrer 2004 {published data only}
- Loehrer PJ, Wang W, Johnson DH, Ettinger DS. Octreotide alone or with prednisone in patients with advanced thymoma and thymic carcinoma: an Eastern Cooperative Oncology Group phase II trial. Journal of Clinical Oncology 2004;22(2):293-9. [DOI] [PubMed] [Google Scholar]
Lucchi 2005 {published data only}
- Lucchi M, Ambrogi MC, Duranti L, Basolo F, Fontanini G, Angeletti CA, et al. Advanced stage thymomas and thymic carcinomas: results of multimodality treatments. Annals of Thoracic Surgery 2005;79(6):1840-4. [DOI] [PubMed] [Google Scholar]
Lucchi 2006 {published data only}
- Lucchi M, Melfi F, Dini P, Basolo F, Viti A, Givigliano F, et al. Neoadjuvant chemotherapy for stage III and IVA thymomas: a single-institution experience with a long follow-up. Journal of Thoracic Oncology 2006;1(4):308-13. [PubMed] [Google Scholar]
Luo 2004 {published data only}
- Luo QQ, Zhou YZ, Chen WH, Huang G, Yang J, Zhao XQ, et al. The reduction chemotherapy to the gigantic thymic malignant tumor before surgical treatment. Chinese Journal of Clinical Oncology 2004;31(2):92-4. [Google Scholar]
Myojin 2000 {published data only}
- Myojin M, Choi NC, Wright CD, Wain JC, Harris N, Hug EB, et al. Stage III thymoma: pattern of failure after surgery and postoperative radiotherapy and its implication for future study. International Journal of Radiation Oncology, Biology, Physics 2000;46(4):927-33. [DOI] [PubMed] [Google Scholar]
Oberg 2009 {published data only}
- Oberg K, Hellman P, Kwekkeboom D, Jelic S, ESMO Guidelines Working Group. Neuroendocrine bronchial and thymic tumors: ESMO clinical recommendation for diagnosis, treatment and follow-up. Annals of Oncology 2009;20(Suppl 4):147-9. [DOI] [PubMed] [Google Scholar]
Palmieri 2010 {published data only}
- Palmieri G, Merola G, Federico P, Petillo L, Marino M, Ceribelli, et al. Preliminary results of phase II study of capecitabine and gemcitabine (CAP-GEM) in patients with metastatic pretreated thymic epithelial tumors (TETs). Annals of Oncology 2010;21:1168-72. [DOI] [PubMed] [Google Scholar]
Rajan 2008 {published data only}
- Rajan A, Giaccone G. Treatment of advanced thymoma and thymic carcinoma. Current Treatment Options in Oncology 2008;9(4-6):277-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rajan 2011 {published data only}
- Rajan A, Giaccone G. Chemotherapy for thymic tumors: induction, consolidation, palliation. Thoracic Surgery Clinics 2011;21(1):107-14. [DOI] [PubMed] [Google Scholar]
Rea 2011 {published data only}
- Rea F, Marulli G, Di Chiara F, Schiavon M, Perissinotto E, Breda C, et al. Multidisciplinary approach for advanced stage thymic tumors: long-term outcome. Lung Cancer 2011;72(1):68-72. [DOI] [PubMed] [Google Scholar]
Shin 1998 {published data only}
- Shin DM, Walsh GL, Komaki R, Putnam JB, Nesbitt J, Ro JY, et al. A multidisciplinary approach to therapy for unresectable malignant thymoma. Annals of Internal Medicine 1998;129(2):100-4. [DOI] [PubMed] [Google Scholar]
Ströbel 2004 {published data only}
- Ströbel P, Bauer A, Puppe B, Kraushaar T, Krein A, Toyka K, et al. Tumor recurrence and survival in patients treated for thymomas and thymic squamous cell carcinomas: a retrospective analysis. Journal of Clinical Oncology 2004;22(8):1501-9. [DOI] [PubMed] [Google Scholar]
Venuta 1997 {published data only}
- Venuta F, Rendina EA, Longo F, Giacomo TD, Anile M, Mercadante E, et al. Long-term outcome after multimodality treatment for stage III thymic tumors. Annals of Thoracic Surgery 2003;76:1866-72. [DOI] [PubMed] [Google Scholar]
Venuta 2003 {published data only}
- Venuta F, Rendina EA, Longo F, De Giacomo T, Anile M, Mercadante E, et al. Long- term outcome after multimodality treatment for stage III thymic tumors. Annals of Thoracic Surgery 2003;76(6):1866-72. [DOI] [PubMed] [Google Scholar]
White 1990 {published data only}
- White L, Grossmann R, Tobias VH. Effective chemotherapy for invasive thymoma and thymic carcinoma: review of the literature. Cancer Journal 1990;3(2):110-2. [Google Scholar]
Wright 2005 {published data only}
- Wright CD, Wain JC, Wong DR, Donahue DM, Gaissert HA, Grillo HC, et al. Predictors of recurrence in thymic tumors: Importance of invasion, World Health Organization histology, and size. Journal of Thoracic and Cardiovascular Surgery 2005;130(5):1413-21. [DOI] [PubMed] [Google Scholar]
Xu 2009 {published data only}
- Xu JP, Zhang XR, Hao XZ, Li JL. Gemcitabine combined with cisplatin/carboplatin in treatment of advanced thymic carcinoma. World Clinical Drugs 2009;30(6):358-60. [Google Scholar]
Yano 2005 {published data only}
- Yano M, Fujii Y. Thymoma in elderly patients. Japanese Journal of Thoracic Surgery 2005;58(8 Suppl):739-44. [PubMed] [Google Scholar]
Yokoi 2007 {published data only}
- Yokoi K, Matsuguma H, Nakahara R, Kondo T, Kamiyama Y, Mori K, et al. Multidisciplinary treatment for advanced invasive thymoma with cisplatin, doxorubicin, and methylprednisolone. Journal of Thoracic Oncology 2007;2(1):73-8. [DOI] [PubMed] [Google Scholar]
Zhu 2009 {published data only}
- Zhu WY, Han JQ. 3 Dimensional Conformal Radiotherapy Combined with Chemotherapy for Thymoma [Thesis]. Shandong University, 2009. [Google Scholar]
Additional references
Chung 2000
- Chung DA. Thymic carcinoma - analysis of nineteen clinicopathological studies. Thoracic and Cardiovascular Surgeon 2000;48:114-9. [DOI] [PubMed] [Google Scholar]
CTCAE 2006
- Common Terminology Criteria for Adverse Events (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf 9 August 2006.
Eisenhauer 2009
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45:228-47. [DOI] [PubMed] [Google Scholar]
Eng 2004
Falkson 2008
- Falkson C, Bezjak A, Darling G, Gregg R, Malthaner R, Maziak D, et al. The management of thymoma: guideline recommendations. Toronto (ON): Cancer Care Ontario Program in Evidence-based Care 2008 Sep 26. 41 p;(Evidence-based series; no. 7-11).
Geffen 2001
- Geffen DB, Benharroch D, Yellin A, Ariad S, Or R, Cohen Y. Multimodal treatment of metastatic thymic carcinoma including high-dose chemotherapy with autologous stem cell transplantation [case report]. American Journal of Clinical Oncology 2001;24(6):566-9. [DOI] [PubMed] [Google Scholar]
Giaccone 2005
- Giuseppe G. Treatment of malignant thymoma. Current Opinion in Oncology 2005;17:140-6. [DOI] [PubMed] [Google Scholar]
Hernandez‐Ilizaliturri 2004
- Hernandez-Ilizaliturri FJ, Tan D, Cipolla D, Connolly G, Debb G, Ramnath N. Multimodality therapy for thymic carcinoma (TCA): results of a 30-year single-institution experience. American Journal of Clinical Oncology 2004;27(2):127. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org..
Hsu 1994
- Hsu CP. Thymic carcinoma. Ten year's experience in twenty patients. Journal of Thoracic and Cardiovascular Surgery 1994;107:615-20. [PubMed] [Google Scholar]
Ji 2006
- Ji W, Feng QF, Zhou ZM, Wang M, Chen DF, Zhang HX, et al. Analysis of treatment and prognosis of 73 thymic carcinoma patients. Chinese Journal of Radiation Oncology 2006;15(2):97-100. [Google Scholar]
Kitami 2001
- Kitami A, Suzuki T, Kamio Y, Suzuki S. Chemotherapy of thymic carcinoma: analysis of seven cases and review of the literature. Japanese Journal of Clinical Oncology 2001;31(12):601-4. [DOI] [PubMed] [Google Scholar]
Kondo 2003
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1320 patients from Japan. Annals of Thoracic Surgery 2003;76:878-84. [DOI] [PubMed] [Google Scholar]
Lin 2005
- Lin JT, Wei-Shu W, Yen CC, Liu JH, Chen PM, Chiou TJ. Stage IV thymic carcinoma: a study of 20 patients. American Journal of the Medical Sciences 2005;330(4):172-5. [DOI] [PubMed] [Google Scholar]
Liu 2002
- Liu HC, Hsu WH, Chen YJ, Chan YJ, Wu YC, Huang BS, et al. Primary thymic carcinoma. Annals of Thoracic Surgery 2002;73:1076-81. [DOI] [PubMed] [Google Scholar]
Masaoka 1981
- Masaoka A, Monden Y, Nakahara K, Tanioka T. Follow-up study of thymoma with special reference to their clinical stages. Cancer 1981;48:2485-92. [DOI] [PubMed] [Google Scholar]
Nakamura 2000
- Nakamura Y, Kunitoh H, Kubota K. Platinum-based chemotherapy with or without thoracic radiation therapy in patients with thymic carcinoma. Japanese Journal of Clinical Oncology 2000;30(9):385-8. [DOI] [PubMed] [Google Scholar]
Rosai 1999
- Rosai J. Histological typing of tumors of the thymus. 2nd edition. New York: Springer-Verlag, 1999. [Google Scholar]
Tetsuo 2004
- Tetsuo N, Yoshio T, Keiko H, Hiroyuki K, Masumi N, Hiroyuki H, et al. The role of radiotherapy for thymic carcinoma. Japanese Journal of Clinical Oncology 2004;34(12):722-6. [DOI] [PubMed] [Google Scholar]
Travis 2004
- Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC (Eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC Press, 2004. [Google Scholar]
Weide 1993
- Weide L, Ulbright T, Loehrer P, Williams S. Thymic carcinoma: a distinct clinical entity responsive to chemotherapy. Cancer 1993;71(4):1219-23. [DOI] [PubMed] [Google Scholar]
Yano 2008
- Yano M, Sasaki H, Yokoyama T, Yukiue H, Kawano O, Suzuki S, et al. Thymic carcinoma: 30 cases at a single institution. Journal of Thoracic Oncology 2008;3(3):265-9. [DOI] [PubMed] [Google Scholar]
Zhang 2007
- Zhang J, Huang C, Zhu K. Analysis of clinical characters and prognosis of 32 thymic carcinoma. Jiangxi Yiyao 2007;42(11):994-5. [Google Scholar]


