Abstract
Background
Gestational diabetes mellitus (GDM) is any degree of glucose intolerance that first presents and is recognised during pregnancy and usually resolves after the birth of the baby. GDM is associated with increased short‐ and long‐term morbidity for the mother and her baby. Treatment usually includes lifestyle modification and/or pharmacological therapy (oral antidiabetic agents or insulin) with the aim to maintain treatment targets for blood glucose concentrations. Finding novel treatment agents which are effective, acceptable and safe for the mother and her baby are important. One such emerging potential intervention is myo‐inositol which is an isomer of inositol and occurs endogenously and is found in natural dietary sources such as fruits, vegetables, nuts and cereals.
Objectives
To assess if dietary supplementation with myo‐inositol during pregnancy is safe and effective, for the mother and fetus, in treating gestational diabetes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (30 April 2016), ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (7 April 2016), and reference lists of retrieved studies.
Selection criteria
All published and unpublished randomised controlled trials or cluster‐randomised controlled trials reporting on the use of myo‐inositol compared with placebo, no treatment or another intervention for the treatment of women with gestational diabetes. Quasi‐randomised and cross‐over studies are not eligible for inclusion. Women with pre‐existing diabetes were excluded.
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. For key outcomes (where data were available), we assessed the quality of the evidence using the GRADE approach.
Main results
We included two studies (142 women and infants), both were conducted in women in Italy and compared myo‐inositol with a placebo control.
None of the maternal primary outcomes pre‐specified for this review were reported in the included studies: hypertensive disorders of pregnancy; caesarean section; development of subsequent type 2 diabetes mellitus. No data were reported for the majority of this review's maternal secondary outcomes. We could only perform meta‐analysis for two secondary outcomes: fasting oral glucose tolerance test and additional pharmacological treatment. All other results are based on data from single studies. Overall, the risk of bias of the included studies was judged to be unclear due to lack of key methodological information.
There was no evidence of a difference between treatment groups in need for additional pharmacotherapy or weight gain during pregnancy, although myo‐inositol was associated with a lower body mass index (BMI) change (mean difference (MD) ‐1.50 kg/m2; 95% confidence interval (CI) ‐2.35 to ‐0.65; one trial, n = 73). Myo‐inositol was associated with a reduction in the fasting blood glucose concentration at the end of treatment (MD ‐0.47 mmol/L; 95% CI ‐0.59 to ‐0.35; two trials, n = 142 women) compared with the control group. One small trial reported that myo‐inositol was associated with a reduction in one‐hour post‐prandial blood glucose concentration at the end of treatment (MD ‐0.90 mmol/L; 95% CI ‐1.73 to ‐0.07; one trial, n = 73 women) compared with the control group. There was no difference between groups for the two‐hour post‐prandial blood glucose concentrations between groups (MD ‐0.70 mmol/L; 95% CI ‐1.46 to 0.06; one trial, n = 73 women). The one‐hour and two‐hour blood glucose concentrations show evidence of imprecision associated with wide CIs and small sample size.
For the infant, there was no evidence of a difference in the risk for being born large‐for‐gestational age between the myo‐inositol and the control group (risk ratio (RR) 0.36; 95% CI 0.02 to 8.58; one trial, n = 73 infants; low‐quality evidence). The evidence was downgraded due to imprecision. This review's other primary outcomes were not reported in the included trials: perinatal mortality (stillbirth and neonatal mortality); mortality of morbidity composite (as defined by the trials); neurosensory disability. Infants in the myo‐inositol group were less likely to have neonatal hypoglycaemia compared with the placebo group (RR 0.05; 95% CI 0.00 to 0.85; one study, n = 73 infants; low‐quality evidence). There is evidence of imprecision for this outcome with low event rates and small sample size. There was no evidence of a difference between treatment and placebo groups for preterm birth or birthweight. Myo‐inositol was associated with a later gestational age at birth compared with the placebo group (MD 2.10 weeks; 95% CI 1.27 to 2.93; one trial, n = 73 infants). No data were reported for any of the other neonatal outcomes for this review.
No long‐term outcomes were reported for the mother, infant as a child, infant as an adult, or health service outcomes.
Authors' conclusions
There are insufficient data to evaluate the effect of myo‐inositol for the treatment of gestational diabetes, with no data to examine the majority of outcomes in this review. There do not appear to be any benefits for the infant associated with exposure to myo‐inositol such as reduced risk of being born large‐for‐gestational age. Although the risk of neonatal hypoglycaemia is reduced for the myo‐inositol group, there is evidence of imprecision. Evidence from two studies suggested that myo‐inositol was associated with a reduced change in maternal BMI and fasting blood sugar concentration compared with placebo. There is a lack of reporting of the clinically meaningful outcomes pre‐specified for this review.
Uncertainty of the effectiveness of myo‐inositol as a treatment for GDM for key maternal and infant outcomes remains and further high‐ quality trials with appropriate sample sizes are required to further investigate the role of myo‐inositol as a treatment or co‐treatment for women with gestational diabetes. Future trials should report on the core outcomes for GDM identified in the methods section of this review. Participants of varying ethnicities and with varying risk factors for GDM should be included in future trials. In addition, further trials of myo‐inositol for the treatment of GDM should explore the optimal dose, frequency and timing of supplementation, report on adverse effects and assess the long‐ term effects of this intervention. Economic analysis or health service use and costs should also be included.
Plain language summary
Does taking a supplement of myo‐inositol work as an effective treatment for women who develop diabetes during pregnancy?
What is the issue?
During pregnancy the mother develops resistance to insulin and the uptake of glucose from the blood is reduced to ensure the baby has a consistent supply of glucose. The mother has to produce extra insulin to keep her blood glucose levels under control or she is at risk of developing gestational diabetes mellitus (GDM). GDM is diabetes that occurs during pregnancy and resolves after the birth of the baby. It is an increasing problem around the world, causing both long‐ and short‐term complications for the mother and her baby. Women with GDM are at greater risk of developing high blood pressure and having a caesarean section for the birth. Their babies can grow large for their gestational age, which increases the likelihood of having an injury at birth such as broken bones or a shoulder becoming stuck. In the long term both the mother and her child are at increased risk of developing type 2 diabetes.
Why is this important?
Dietary and lifestyle counselling is the first line of treatment for women with GDM. An oral hypoglycaemic drug or insulin therapy is recommended for the women who are still unable to maintain target blood glucose levels. Finding a treatment that controls the mother’s blood sugar levels without harming the mother or her baby is important. Myo‐inositol is a natural form of inositol that is found in fruits, vegetables, nuts and cereals. It is a simple carbohydrate nutrient the body requires for many cell functions. Myo‐inositol is available as a dietary supplement, in water‐soluble powder form or as capsules.
What evidence did we find?
We searched for evidence in April 2016 and identified two randomised controlled studies (involving 142 women and their babies). Both studies were conducted in Italy (and were judged to be at an unclear risk of bias). The women were diagnosed with GDM at 12 to 13 weeks' gestation in one study and at 26 weeks' gestation in the other. The findings from these trials suggested that myo‐inositol can reduce fasting blood glucose levels. The need for supplementary insulin was not clearly different between the women receiving myo‐inositol and the control groups. One of the studies showed reduced glucose levels at one hour after a meal (one study, 73 women) There was no evidence to suggest that the babies were at reduced risk of being born large‐for‐gestational age (one study, 73 infants). Myo‐inositol appeared to reduce the risk of the baby having low blood sugar levels at birth and being born at a later gestational age, although the evidence was of low quality. Many of the infant and maternal outcomes identified as being of interest for this review were not reported in the included studies ‐ these included: high blood pressure during the pregnancy, caesarean section, the development of type 2 diabetes (maternal), and the number of babies who died or were unwell, or the number of babies with neurosensory disability. No long‐term outcomes were reported for the mother, infant as a child, infant as an adult or health service outcomes.
What does this mean?
Because of the limited number of studies reporting on myo‐inositol for the treatment of women with GDM, lack of data on the outcomes of importance for this review and the low‐quality evidence based on two small studies, we cannot be certain if myo‐inositol is useful as a treatment intervention for women with GDM. The available evidence is insufficient to support the use of myo‐inositol. Further high‐quality trials with large sample sizes are required to investigate the role of myo‐inositol as a treatment or a co‐treatment for women with gestational diabetes.
Summary of findings
Summary of findings for the main comparison. Myo‐inositol versus placebo for the treatment of gestational diabetes ‐ Neonatal outcomes.
| Myo‐inositol versus placebo for the treatment of gestational diabetes ‐ Neonatal outcomes | ||||||
|
Setting: women were out‐patients attending a high risk pregnancy unit at hospitals in Italy. Patient or population: pregnant women with gestational diabetes Intervention: 4 g myo‐inositol + 400 mcg folic acid orally per day (dosage regimen not stated), and exercise and dietary advice (n = 36) Comparison: placebo 400 mcg folic acid orally per day, and exercise and dietary advice (n = 39) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with Placebo | Risk with Myo‐inositol | |||||
| Large‐for‐gestational age | 26 per 1000 | 9 per 1000 (1 to 226) | RR 0.36 (0.02 to 8.58) | 73 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | Low event rates and small sample size indicate imprecision. 0/35 events in myo‐inositol group and 1/38 events in the placebo group. |
| Perinatal mortality | not estimable | (0 studies) | ‐ | No data were reported for this outcome. | ||
| Neonatal hypoglycaemia | 263 per 1000 | 13 per 1000 (0 to 224) | RR 0.05 (0.00 to 0.85) | 73 (1 RCT) | ⊕⊕⊝⊝ LOW 1 | Low event rates and small sample size indicate imprecision. 0/35 events in myo‐inositol group and 10/38 events in the placebo group. |
| Composite outcome of serious neonatal outcomes | not estimable | (0 studies) | ‐ | No data were reported for this outcome. | ||
| Childhood/adulthood adiposity | not estimable | (0 studies) | ‐ | No data were reported for this outcome. | ||
| Childhood/adulthood diabetes | not estimable | (0 studies) | ‐ | No data were reported for this outcome. | ||
| Neurosensory disability | not estimable | (0 studies) | ‐ | No data were reported for this outcome. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Small sample size, single trial and low events indicate imprecision ‐ downgraded ‐ 2 levels
Summary of findings 2. Myo‐inositol versus placebo for the treatment of gestational diabetes ‐ Maternal outcomes.
| Myo‐inositol versus placebo for the treatment of gestational diabetes ‐ Maternal outcomes | ||||||
|
Setting: women were out‐patients attending a high risk pregnancy unit at a hospital in Italy. Patient or population: pregnant women with gestational diabetes Intervention: 4 g myo‐inositol + 400 mcg folic acid orally per day (dosage regimen not stated), and exercise and dietary advice (n = 36) Comparison: placebo 400 mcg folic acid orally per day, and exercise and dietary advice (n = 39) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with Placebo | Risk with Myo‐inositol | |||||
| Hypertensive disorders of pregnancy | not estimable | (0 studies) | ‐ | No data were reported for this outcome. | ||
| Caesarean section | not estimable | (0 studies) | ‐ | No data were reported for this outcome. | ||
| Induction of labour | not estimable | (0 studies) | ‐ | No data were reported for this outcome. | ||
| Perineal trauma | not estimable | (0 studies) | ‐ | No data were reported for this outcome. | ||
| Postnatal weight retention/return to pre‐pregnancy weight | not estimable | (0 studies) | ‐ | No data were reported for this outcome. | ||
| Postnatal depression | not estimable | (0 studies) | ‐ | No data were reported for this outcome. | ||
| Development of subsequent type 2 diabetes mellitus | not estimable | (0 studies) | ‐ | No data were reported for this outcome. | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
Background
Description of the condition
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance with onset or first recognition during pregnancy (Alberti 1998). During pregnancy, the placenta releases hormones that increase maternal insulin resistance to ensure a consistent supply of glucose to the growing fetus (McCance 2011). In compensation for this, the maternal pancreas secretes more insulin to maintain glycaemic control. Gestational diabetes occurs when this compensatory mechanism malfunctions and there is not enough insulin for appropriate glucose metabolism (McCurdy 2010). This results in increased maternal blood glucose concentrations, or hyperglycaemia, and an increased amount of glucose crossing the placenta, over‐nourishing the fetus (McCurdy 2010).
The diagnosis of GDM is usually made using an oral glucose tolerance test (OGTT) between 24 to 28 weeks' gestation. The diagnostic criteria vary from country to country (ACOG 2013; ADA 2008; CDA 2008; IADPSG 2010; Nankervis 2013; NICE 2015; New Zealand Ministry of Health 2014) (Table 3), making overall estimates of prevalence difficult, but GDM is thought to affect between 1% and 24% of pregnancies (Ferrara 2007). GDM can have significant adverse effects on the immediate and long‐term health of the mother and baby (The HAPO Study Cooperative Research Group 2008; Wang 2013; Wendland 2012), and the rising global incidence of GDM is exposing more women and infants to the increased risk of these short‐ and long‐term health complications (Ferrara 2007).
1. Diagnostic criteria for GDM.
| Organisation | Testing schedule |
Abnormal values required for diagnosis of GDM |
Threshold equal to or greater than | |||
| Fasting | 1 hour | 2 hours | 3 hours | |||
| Australasian Diabetes in Pregnancy Society (Nankervis 2013) | OGTT | 1 | 5.1 mmol/L |
10.0 mmol/L |
8.5 mmol/L |
– |
| American Diabetes Association (ADA 2008) | 75 g OGTT | 1 | 5.3 mmol/L |
10.0 mmol/L |
8.6 mmol/L |
– |
| American College of Obstetricians and Gynecologists* (ACOG 2013) |
Either Carpenter & Coustan 100 g OGTT |
2 | 5.3 mmol/L |
10.0 mmol/L |
8.6 mmol/L |
7.8 mmol/L |
| or NDDG 100 g OGTT |
2 | 5.8 mmol/L |
10.6 mmol/L |
9.2 mmol/L |
8.0 mmol/L |
|
| International Association of Diabetes and Pregnancy Study Groups (IADPSG 2010) | 75 g OGTT | 1 | 5.1 mmol/L |
10.0 mmol/L |
8.5 mmol/L |
– |
| World Health Organization (Alberti 1998) National Institute for Health and Care Excellence (NICE 2015) |
75 g OGTT | 1 | 7.0 mmol/L |
– | 11.1 mmol/L | – |
| Canadian Diabetes Association (CDA 2008) | 75 g OGTT | 2 | 5.3 mmol/L |
10.6 mmol/L |
8.9 mmol/L |
– |
| New Zealand Ministry of Health (New Zealand Ministry of Health 2014) | 75 g OGTT | 1 | 5.5 mmol/L |
– | 9.0 mmol/L |
– |
OGTT: oral glucose tolerance test
* American College of Obstetricians and Gynecologists stipulates that either the Carpenter and Coustan or the National Diabetes Data Group (NDDG) thresholds are appropriate to use.
Women with GDM are at greater risk of developing pre‐eclampsia and undergoing a caesarean section (Alwan 2009), while long term, over half will go on to develop type 2 diabetes within 10 years (Kim 2002). Infants of mothers with GDM can grow disproportionately large for their gestational age (Catalano 2003). This in turn increases the likelihood of traumatic birth or shoulder dystocia (when the shoulders become stuck in the birth canal) leading to birth injuries such as bone fracture or nerve palsy (Crowther 2005; Landon 2009). Additional risks for the immediate health of the infant include respiratory distress syndrome, jaundice, hypoglycaemia, and admission to the neonatal intensive care unit (Crowther 2005; Landon 2009). In the long term, babies born to mothers with GDM have a high likelihood of being obese as children and adults, and are at increased risk of metabolic syndrome and subsequently developing diabetes, perpetuating the cycle of poor health outcomes (Boney 2005; West 2011). Identification of effective treatment measures for GDM is of great importance.
Description of the intervention
Both non‐pharmacological and pharmacological interventions are used to treat gestational diabetes. Currently, dietary and lifestyle counselling is the first line of treatment for women with GDM. Oral hypoglycaemics and/or insulin therapy are recommended for women with GDM who are unable to maintain glycaemic control with dietary and lifestyle interventions alone (Metzger 1998; NICE 2015; New Zealand Ministry of Health 2014).
Myo‐inositol has been identified as a potentially new and novel treatment for GDM (Croze 2013). Myo‐inositol is one of the nine isomers (forms) of inositol, a simple carbohydrate and nutrient the body requires for many cell functions (Croze 2013). Common dietary sources of inositol include fresh fruit and vegetables, cereals, legumes and nuts (Clements 1980). Myo‐inositol is available as a dietary supplement, in water‐soluble powder form or as capsules.
Myo‐inositol has been used therapeutically in a number of settings. In women with polycystic ovarian syndrome (PCOS), myo‐inositol supplementation has been associated with an improvement in insulin sensitivity, and ovulatory function (Genazzani 2008; Minozzi 2008; Papaleo 2007). Myo‐inositol has been found to assist the normal production of thyroid hormone in patients with autoimmune thyroiditis (chronic inflammation of the thyroid gland) (Nordio 2013), and is associated with successful treatment of premenstrual dysphoric disorder, a mood disorder disrupting the social and/or occupational life of affected women (Carlomagno 2011). Increased numbers and quality of oocytes (immature eggs within the ovary) in women undergoing in vitro fertilisation treatment for a previous history of infertility have also been reported following myo‐inositol supplementation (Unfer 2011).
A retrospective review of 46 pregnant women treated with myo‐inositol compared to 37 controls, described myo‐inositol as safe during the pre‐pregnancy and early pregnancy period, when used in insulin‐resistant conditions, with no reported side effects (D'Anna 2012).
How the intervention might work
The way insulin functions within the body is complex and still not fully understood (Cohen 2006; Croze 2013). Insulin has been shown to use second messengers to help transmit signals from insulin to its target cells. Second messengers increase the speed and strength of insulins message within its target cells (Croze 2013). Myo‐inositol has been identified as a second messenger of insulin improving the body's sensitivity to the effects of insulin (Larner 2010; Saltiel 1990).
Why it is important to do this review
A Cochrane systematic review has found some limited evidence to suggest that myo‐inositol may be effective at preventing the onset of GDM (Crawford 2015). Given the beneficial effects observed on insulin sensitivity, myo‐inositol may be useful as a treatment for women with gestational diabetes.
Objectives
To assess if dietary supplementation with myo‐inositol during pregnancy is safe and effective, for the mother and fetus, in treating gestational diabetes.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished randomised controlled trials and cluster‐randomised trials, including conference abstracts, assessing the effects of myo‐inositol for the treatment of gestational diabetes mellitus (GDM). Quasi‐randomised trials and cross‐over trials are not eligible for inclusion in this review.
Types of participants
Pregnant women with a diagnosis of GDM (as defined by trialists). Women with pre‐existing type 1 or type 2 diabetes were excluded.
Types of interventions
The intervention includes administration of any dose of myo‐inositol, alone or in a combination preparation, after diagnosis of GDM in pregnancy, for the treatment of GDM. We included studies where the intervention is compared with those who received no treatment, placebo or another intervention.
Types of outcome measures
This review uses a core set of outcomes developed through consensus by a group of authors who have written other Cochrane reviews on prevention of and treatments for women with GDM. This was done to improve consistency between reviews relating to women with GDM.
Primary outcomes
Maternal outcomes
Hypertensive disorders of pregnancy (including pre‐eclampsia, eclampsia, pregnancy‐induced hypertension)
Caesarean section
Development of subsequent type 2 diabetes mellitus (timeframe of long‐term follow‐up defined by trialists)
Neonatal outcomes
Large‐for‐gestational age (birthweight greater than the 90th centile; or as defined by individual trials)
Perinatal mortality (stillbirth and neonatal mortality)
Mortality or morbidity composite (variously defined by trials, e.g. infant death, shoulder dystocia, bone fracture or nerve palsy)
Neurosensory disability
Secondary outcomes
Maternal outcomes
Induction of labour
Perineal trauma
Placental abruption
Postpartum haemorrhage
Postpartum infection
Weight gain during pregnancy
Adherence to the intervention (as defined by trialists)
Behaviour changes associated with the intervention (as defined by trialists)
Relevant biomarker changes associated with the intervention (e.g. adiponectin, free fatty acids, triglycerides, high‐density lipoproteins, low‐density lipoproteins, insulin)
Breastfeeding (e.g. at discharge, six weeks postpartum)
Use of additional pharmacotherapy
Glycaemic control during/end of treatment (as defined by trialists)
Maternal hypoglycaemia
Maternal mortality
Sense of well‐being and quality of life
Views of the intervention
Long‐term maternal outcomes
Postnatal depression
Postnatal weight retention or return to pre‐pregnancy weight
Body mass index (BMI)
GDM in a subsequent pregnancy
Type 1 diabetes mellitus
Impaired glucose tolerance
Cardiovascular health (as defined by trialists, including blood pressure, hypertension, cardiovascular disease, metabolic syndrome)
Neonatal/Infant outcomes
Stillbirth
Neonatal mortality
Gestational age at birth
Preterm birth (less than 37 weeks' gestation and less than 32 weeks' gestation)
Apgar score (less than seven at five minutes)
Macrosomia
Small‐for‐gestational age
Birthweight and z‐score
Head circumference and z‐score
Length and z‐score
Ponderal index
Adiposity (as defined by trialists)
Shoulder dystocia
Bone fracture
Nerve palsy
Respiratory distress syndrome
Hypoglycaemia (as defined by trialists)
Hyperbilirubinaemia
Neonatal hypocalcaemia
Polycythaemia (increase in the number of red blood cells)
Relevant biomarker changes associated with the intervention (e.g. cord C‐peptide, cord insulin)
Later childhood outcomes
Weight and z‐scores
Height and z‐scores
Head circumference and z‐scores
Adiposity (e.g. as measured by BMI, skinfold thickness)
Blood pressure
Type 1 diabetes mellitus
Type 2 diabetes mellitus
Impaired glucose tolerance
Dyslipidaemia or metabolic syndrome
Educational achievement
Adulthood outcomes
Weight
Height
Adiposity (e.g. as measured by BMI, skinfold thickness)
Cardiovascular health (as defined by trialists, including blood pressure, hypertension, cardiovascular disease, metabolic syndrome)
Type 1 diabetes mellitus
Type 2 diabetes mellitus
Impaired glucose tolerance
Dyslipidaemia or metabolic syndrome
Employment, education and social status/achievement
Health services cost
Number of hospital or health professional visits (e.g. midwife, obstetrician, physician, dietitian, diabetic nurse)
Number of antenatal visits or admissions
Length of antenatal stay
Neonatal intensive care unit admission
Length of postnatal stay (mother)
Length of postnatal stay (baby)
Costs to families associated with the management provided
Costs associated with the intervention (as defined by trialists)
Cost of maternal care
Cost of offspring care
Search methods for identification of studies
The following methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting their Information Specialist (30 April 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For the full search methods used to populate the Pregnancy and Childbirth Group’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth Group review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (see Appendix 1 for search terms) (searched 7 April 2016).
Searching other resources
We searched reference lists of retrieved studies. We did not apply any language or date restrictions.
Data collection and analysis
Selection of studies
Two review authors independently assessed for inclusion all the potential studies we identified as a result of the search strategy (JB, TC). There were no disagreements. A study flow diagram was produced to map out the number of records identified, included and excluded (Figure 1).
1.
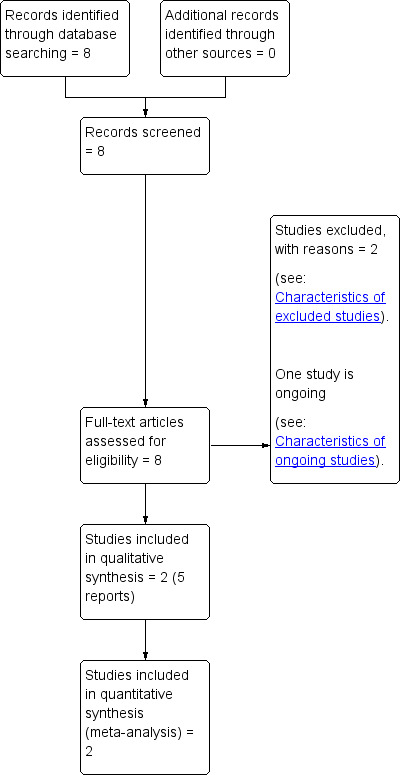
Study flow diagram.
Data extraction and management
We designed a form to extract data based on the Cochrane Pregnancy and Childbirth Group's data extraction form. For eligible studies, two review authors extracted the data (JB, TC or JA) using the agreed form. There were no discrepancies that needed to be resolved. We entered data into Review Manager software (RevMan 2014) and checked for accuracy. Where information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias (JB, TC or JA) for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We did not have any disagreements that required discussion with a third author.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include any missing data in the analyses.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. We planned to explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessing the quality of the body of evidence using the GRADE approach
The quality of the evidence was assessed using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes. We selected up to a maximum of seven outcomes for the mother and seven for the infant covering both short‐ and long‐term outcomes for the main comparisons.
Maternal
Hypertensive disorders of pregnancy (including pre‐eclampsia, eclampsia, pregnancy‐induced hypertension)
Caesarean section
Induction of labour
Perineal trauma
Postnatal weight retention or return to pre‐pregnancy weight
Postnatal depression
Development of subsequent type 2 diabetes mellitus
Offspring (infant, child, adult)
Large‐for‐gestational age
Perinatal mortality (stillbirth and neonatal mortality)
Composite of serious neonatal outcomes
Neonatal hypoglycaemia (variously defined)
Offspring adiposity (e.g. as measured by BMI, skinfold thickness)
Offspring diabetes
Neurosensory disability
We used the GRADEpro Guideline Development Tool to import data from Review Manager 5.3 (RevMan 2014) in order to create 'Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes will be produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we have presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference with 95% confidence intervals if outcomes were measured in the same way between trials.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials. In future updates of this review where cluster‐randomised trials are identified, they will be included in the analyses along with individually‐randomised trials. We will make adjustments using the methods described in the Handbook [Section 16.3.4 or 16.3.6] (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. We will consider it reasonable to combine the results from both cluster‐randomised trials and individually‐randomised trials if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
Multiple pregnancy
None of the included trials included women with a multiple pregnancy. We acknowledge that there may be unit of analysis issues that arise when the women randomised have a multiple pregnancy. In future updates of this review, if trials with multiple pregnancies are included, we will present maternal data as per woman randomised and neonatal data per infant.
Multiple‐arm studies
No multiple arm trials were identified. In future updates, where a trial has multiple intervention arms we will avoid 'double counting' of participants by combining groups to create a single pair‐wise comparison, if possible. Where this is not possible, we will split the 'shared' group into two or more groups with smaller sample size and include two or more (reasonably independent) comparisons.
Dealing with missing data
For included studies, we noted levels of attrition. There were no trials with more than 20% attrition. In future updates of this review, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if an I² was greater than 30% and either a Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
Only two trials were included in the meta‐analysis. If in future updates there are 10 or more studies included in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies are estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. Where there was clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we use random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
There were insufficient trials to be able to explore subgroup analyses. In future updates, if there are sufficient data and if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses.
Obese women versus non‐obese women
Polycystic ovary syndrome (PCOS) women versus non‐PCOS women
Myo‐inositol alone versus as a co‐intervention
Dosage (high versus low)
Type of preparation (e.g. powder versus gel capsules)
Study design (individually‐randomised trials versus cluster‐randomised trials, if cluster‐randomised trials are identified)
The following primary outcomes will be used in subgroup analysis.
Maternal outcomes
Hypertensive disorders of pregnancy (e.g. pre‐eclampsia, eclampsia, pregnancy‐induced hypertension)
Caesarean section
Development of subsequent type 2 diabetes mellitus
Neonatal outcomes
Large‐for‐gestational age
Perinatal mortality (stillbirth and neonatal mortality)
Composite of serious neonatal outcomes
Neurosensory disability
In future updates of this review, we will assess subgroup differences, where appropriate, by interaction tests available within RevMan (RevMan 2014). We will report the results of subgroup analyses quoting the Chi2 statistic and P value, and the interaction test I² value.
Sensitivity analysis
Planned sensitivity analysis was not carried out due to insufficient data. In future updates of this review, where there is evidence of significant heterogeneity, we will explore this by using the quality of the included trials for the primary outcomes. Trials that have a low risk of bias for allocation concealment will be compared with those judged to be of unclear or high risk of bias.
Results
Description of studies
Results of the search
See: (Figure 1).
The search retrieved eight relevant reports of five studies.
Two studies met the inclusion criteria for this review (Corrado 2011; Matarrelli 2013) (See Characteristics of included studies). Two studies were excluded (Malvasi 2014; Valent 2014) (See Characteristics of excluded studies). One ongoing study was identified (Vitacolonna 2014) (See Characteristics of ongoing studies). This four‐arm study plans to recruit 80 pregnant women with gestational diabetes. The interventions include myo‐inositol 2000 mg twice a day; d‐chiro‐inositol 250 mg twice a day; myo‐inositol 550 mg plus d‐chiro‐inositol 13.8 mg twice a day and the control group will receive folic acid 400 mcg/day. In subsequent updates of this review we will look to see if data have been published that relate to this trial.
Included studies
Two trials met the eligibility criteria for inclusion in this review (Corrado 2011; Matarrelli 2013) (See Characteristics of included studies). Both trials were individually‐randomised controlled trials including a total of 142 women. Corrado 2011 randomised 84 women and included 69 in the final analysis. Matarrelli 2013 randomised 75 women, 73 of whom were included in the final analysis.
Population
All participants were pregnant women recruited between 2008 and 2011. Diagnosis of gestational diabetes mellitus (GDM) was between 24 and 28 weeks by American Diabetes Association criteria (ADA 2008) in Corrado 2011, or in the first or early second trimester by International Association of Diabetes and Pregnancy Study Groups criteria (IADPSG 2010) in Matarrelli 2013 (Table 4). All the participants in Corrado 2011 were reported to be "Caucasian". The ethnicity of the participants in Matarrelli 2013 was not reported. The women in Matarrelli 2013 had a slightly higher mean age (mean age 33.0 years intervention group; 33.8 years control group), compared to the women in Corrado 2011 (mean age 28.7 years intervention group; 28.4 years control group). Primiparious and multiparous women were included in both trials with approximately even proportions of both. Pre‐pregnancy BMI was similar between groups in both trials (Corrado 2011 mean BMI intervention group 25.1 kg/m2; 24.2 kg/m2 control group; Matarrelli 2013 mean BMI 23.5 mg/kg2 intervention group; 24.7 mg/kg2 control group). An exclusion criteria of Matarrelli 2013 was BMI greater than 35 mg/kg2. Both trials used ADA 2008 treatment targets, reported in Table 5.
2. Diagnostic criteria used in included trials.
| Study ID | Timing | Diagnosis | Criteria |
| Corrado 2011 | 24 to 28 weeks' | At least 2 abnormal plasma glucose values following 100 g OGTT Fasting: ≥ 5.3 mmol/L (≥ 95 mg/dL) 1 hour: ≥ 10.0 mmol/L (≥ 180 mg/dL) 2 hour: ≥ 8.6 mmol/L (≥ 155 mg/dL) 3 hour: ≥ 7.8 mmol/L (≥ 140 mg/dL) |
(ADA 2008) |
| Matarrelli 2013 | 24 to 28 weeks' | One or more abnormal blood glucose values following 75 g OGTT Fasting: > 5.1 mmol/L (> 92 mg/dL) 1 hour: > 10.0 mmol/L (> 180 mg/dL) 2 hour: > 8.5 mmol/L (> 153 mg/dL) |
(IADPSG 2010) |
3. ADA 2008 Treatment targets used in included trials.
| Fasting | 1‐hour post‐prandial | 2‐hour post‐prandial |
| < 5.8 mmol/L (< 105 mg/dL) | Either < 8.6 mmol/L (< 155 mg/dL) |
Or < 7.2 mmol/L (< 130 mg/dL) |
Setting
Both trials were conducted in Obstetric and Gynecology Departments of University Hospitals in two regions of Southern Italy.
Intervention
Each of the trials used a total of 4000 mg myo‐inositol plus 400 mcg folic acid per day in divided doses as the intervention, and 400 mcg of folic acid as the placebo. Women were supplemented for eight weeks in Corrado 2011. The duration of supplementation was not stated in Matarrelli 2013.
Outcomes
The outcomes of this review were poorly reported in the two included trials. None of the maternal primary outcomes of this review were reported (hypertensive disorders of pregnancy; development of subsequent type 2 diabetes mellitus). For the infant, large‐for‐gestational age was reported in one trial (Matarrelli 2013). None of the other primary infant outcomes for this review were reported in the included trials (perinatal mortality; mortality or morbidity composite; neurosensory disability).
For maternal secondary outcomes, only weight gain during pregnancy (Corrado 2011), BMI increase (Corrado 2011; Matarrelli 2013), need for insulin therapy (Corrado 2011; Matarrelli 2013) and glycaemic control (Corrado 2011; Matarrelli 2013) were reported. None of the other pre‐specified maternal outcomes or long‐term maternal outcomes were reported in the included trials. For neonatal secondary outcomes, only gestational age at birth (Matarrelli 2013), preterm birth (Corrado 2011), birthweight (Matarrelli 2013) and neonatal hypoglycaemia (Matarrelli 2013) were reported. None of the other pre‐specified neonatal outcomes or long‐term outcomes for childhood or adulthood were reported in the included trials.
Excluded studies
Two trials identified in the search were excluded from this review (Malvasi 2014; Valent 2014).
Malvasi 2014 assessed the use of myo‐inositol supplementation in preventing the onset of GDM and is included in the Cochrane review 'Antenatal dietary supplementation with myo‐inositol in women during pregnancy for preventing gestational diabetes' (Crawford 2015). Valent 2014 is a trial registration of a prospective cohort pilot study examining the pharmaco‐dynamics of myo‐inositol in pregnancy.
Risk of bias in included studies
See Figure 2; Figure 3 for a summary of the risk of bias of included studies.
2.
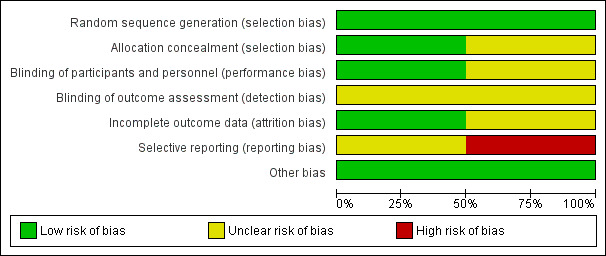
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
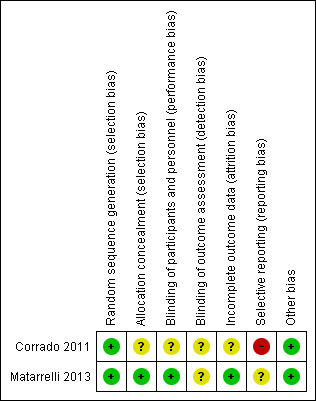
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Both trials (Corrado 2011; Matarrelli 2013) used computer‐generated randomisation and were judged to be at low risk of selection bias. Matarrelli 2013 used a central pharmacy to prepare identical looking sealed sachets, numbered according to the computer‐generated randomisation scheme. This was judged to be at low risk of bias for allocation concealment. No details of allocation concealment method were provided in Corrado 2011, and the risk of bias was judged to be unclear.
Blinding
Matarrelli 2013 described blinding of participants and the healthcare providers caring for them and was judged to be at low risk of performance bias. Blinding was not undertaken in Corrado 2011 as this was an open‐label trial. It was assessed as having an unclear risk of performance bias.
Neither trial provided sufficient detail on blinding of outcome assessors and were both judged to have an unclear risk of detection bias. However, in Corrado 2011 the primary outcome was objective measurement of laboratory results which would not be influenced by knowledge of group allocation.
Incomplete outcome data
Corrado 2011 randomised 84 women but analysis was conducted on only 69 women (four from the study group and 11 in the control group were excluded post randomisation). This was assessed as having an unclear risk of attrition bias. One woman from each group withdrew from Matarrelli 2013 (reason not provided) was judged to have a low risk of attrition bias.
Selective reporting
Corrado 2011 was judged to be of high risk of reporting bias as although the pre‐specified maternal outcomes are reported, there are no other pregnancy or birth outcomes specified or reported. No neonatal outcomes are reported despite birthweight being listed in the protocol on ClinicalTrials.gov (NCT0073448).
Matarrelli 2013 was judged to be of unclear risk of bias. They reported on their pre‐specified maternal and infant outcomes. Neonatal hypoglycaemia was not a pre‐specified outcome, but results were provided.
Other potential sources of bias
Both Corrado 2011 and Matarrelli 2013 were judged to be of low risk for other sources of bias and baseline characteristics were reported to be similar between intervention and control groups.
Effects of interventions
1.0 Myo‐inositol versus placebo
Primary outcomes
Maternal outcomes
None of the maternal primary outcomes of this review were reported in the two included trials (hypertensive disorders of pregnancy; caesarean section; development of subsequent type 2 diabetes mellitus).
Neonatal outcomes
Large‐for‐gestational age was reported in one trial (Matarrelli 2013). There were no events recorded in the 35 infants in the myo‐inositol group and one event of the 38 infants in the control group. There was no clear evidence of a difference in the risk for being born large‐for‐gestational age between the myo‐inositol and the control group (risk ratio (RR) 0.36; 95% confidence interval (CI) 0.02 to 8.58; one trial, n = 73 infants; Analysis 1.1). There is evidence of imprecision with wide CIs, small sample size and low event rates. Using GRADE methodology, we judged this to below‐quality evidence due to imprecision. For infants whose mothers had been in the placebo group, the chance of being born large‐for‐gestational age was 3%; if the mother was in the myo‐inositol group, the chance could have ranged from 0% to 23% (Table 1).
1.1. Analysis.
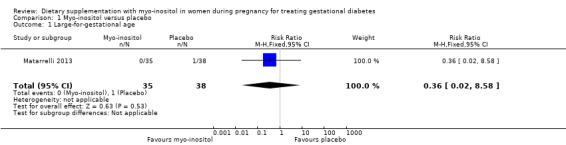
Comparison 1 Myo‐inositol versus placebo, Outcome 1 Large‐for‐gestational age.
No data were reported for the other primary neonatal outcomes of this review (perinatal mortality; mortality or morbidity composite; neurosensory disability).
Secondary outcomes
Maternal outcomes
Weight gain during pregnancy was reported in one trial (Corrado 2011), and one trial reported on BMI change during pregnancy (Matarrelli 2013). There was no clear evidence of a difference in weight gain during pregnancy between the myo‐inositol and the control group (mean difference (MD) ‐0.50 kg; 95% CI ‐3.25 to 2.25; one trial, n = 69 women; Analysis 1.2). There is evidence of imprecision with wide CIs and small sample size. The Matarrelli 2013 trial reported that myo‐inositol was associated with a reduction in BMI during pregnancy compared with the control group (MD ‐1.50 kg/m2; 95% CI ‐2.35 to ‐0.65; one trial, n = 73 women; Analysis 1.3).
1.2. Analysis.
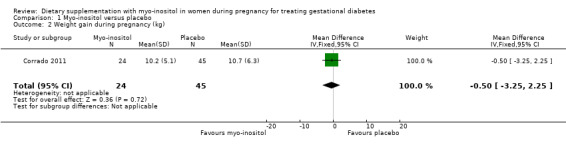
Comparison 1 Myo‐inositol versus placebo, Outcome 2 Weight gain during pregnancy (kg).
1.3. Analysis.
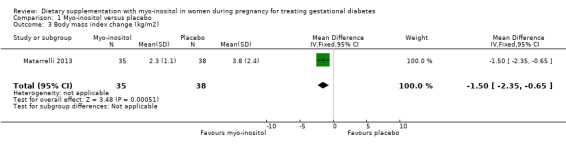
Comparison 1 Myo‐inositol versus placebo, Outcome 3 Body mass index change (kg/m2).
Use of additional pharmacotherapy was reported in both of the included studies (Corrado 2011; Matarrelli 2013). There was no clear evidence of a difference in the need for supplementary insulin between the myo‐inositol (4/63) and the control group (17/94) (average RR 0.37; 95% CI 0.08 to 1.73; two trials, n = 157 women; random‐effects I2 = 45%, Tau2 = 0.61; Analysis 1.4). There is evidence of imprecision with wide CIs, small sample size and low event rates.
1.4. Analysis.
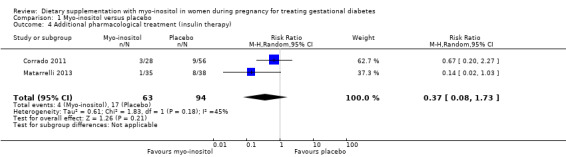
Comparison 1 Myo‐inositol versus placebo, Outcome 4 Additional pharmacological treatment (insulin therapy).
Glycaemic control during/end of treatment. Both trials (Corrado 2011; Matarrelli 2013) reported on fasting blood glucose concentrations at the end of treatment. Myo‐inositol treatment was associated a reduction in fasting blood glucose concentration at the end of treatment (MD ‐0.47 mmol/L; 95% CI ‐0.59 to ‐0.35; two trials, n = 142 women; Analysis 1.5) compared with the control group. The Matarrelli 2013 trial reported that myo‐inositol was associated with a reduction in one‐hour post‐prandial blood glucose concentrations at the end of treatment (MD ‐0.90 mmol/L; 95% CI ‐1.73 to ‐0.07; one trial, n = 73 women; Analysis 1.6) compared with the control group. There was no clear difference between groups for two‐hour post‐prandial blood glucose concentrations, although the same trend towards reduced blood glucose concentrations is observed in the myo‐inositol group (MD ‐0.70 mmol/L; 95% CI ‐1.46 to 0.06; one trial, n = 73 women; Analysis 1.7). The one‐hour and two‐hour blood glucose concentrations show evidence of imprecision associated with wide CIs and small sample size.
1.5. Analysis.
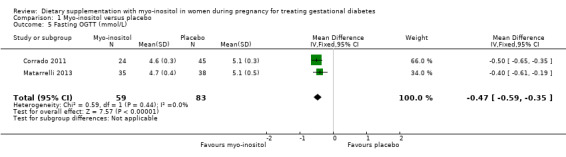
Comparison 1 Myo‐inositol versus placebo, Outcome 5 Fasting OGTT (mmol/L).
1.6. Analysis.
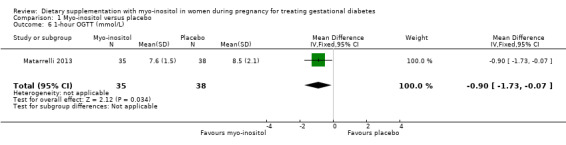
Comparison 1 Myo‐inositol versus placebo, Outcome 6 1‐hour OGTT (mmol/L).
1.7. Analysis.
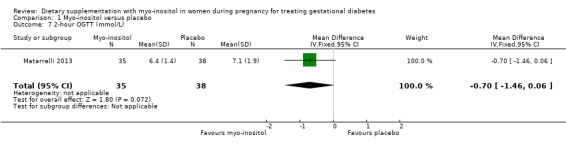
Comparison 1 Myo‐inositol versus placebo, Outcome 7 2‐hour OGTT (mmol/L).
No data were reported for any of the other maternal secondary outcomes of this review (placental abruption; induction of labour; perineal trauma; postpartum haemorrhage; postpartum infection; adherence to the intervention; behaviour changes associated with the intervention; relevant biomarker changes associated with the intervention; sense of well‐being and quality of life; views of the intervention; breastfeeding; maternal hypoglycaemia; maternal mortality).
Long‐term maternal outcomes
No data were reported for any of the long‐term maternal outcomes pre‐specified in this review (postnatal depression; postnatal weight retention or return to pre‐pregnancy weight; body mass index; GDM in a subsequent pregnancy; type I diabetes mellitus; impaired glucose tolerance; cardiovascular health (as defined by trialists).
Neonatal outcomes
Gestational age at birth was reported in one study (Matarrelli 2013). Treatment with myo‐inositol was associated with being born at a later gestational age than the placebo group (MD 2.10 weeks; 95% CI 1.27 to 2.93; one trial, n = 73 infants; Analysis 1.8).
1.8. Analysis.
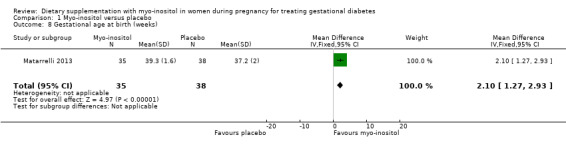
Comparison 1 Myo‐inositol versus placebo, Outcome 8 Gestational age at birth (weeks).
Preterm birth was reported in one study (Corrado 2011). There was no evidence of a clear difference between groups for the risk of preterm birth (< 37 weeks) (RR 1.00; 95% CI 0.09 to 10.56; one study, n = 84 infants; Analysis 1.9). Caution is advised in interpreting these results due to imprecision with low event rates, small sample size and wide CIs crossing the line of no effect being observed.
1.9. Analysis.
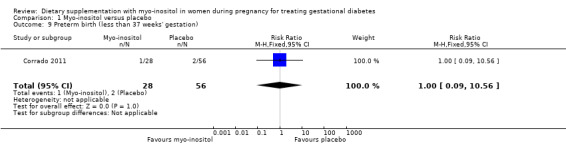
Comparison 1 Myo‐inositol versus placebo, Outcome 9 Preterm birth (less than 37 weeks' gestation).
Birthweight was reported in one study (Matarrelli 2013). There was no evidence of a clear difference between the myo‐inositol and the placebo groups for birthweight (MD 16.00 g; 95% CI ‐209.72 to 241.72; one study, n = 73 infants; Analysis 1.10). There is evidence of imprecision with wide CIs crossing the line of no effect.
1.10. Analysis.
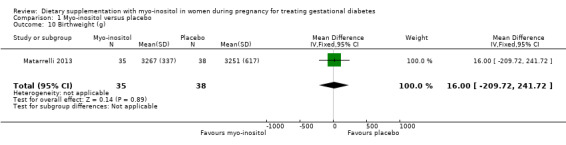
Comparison 1 Myo‐inositol versus placebo, Outcome 10 Birthweight (g).
Neonatal hypoglycaemia was reported in one study (Matarrelli 2013). Maternal treatment with myo‐inositol was associated with a reduced risk of neonatal hypoglycaemia compared with placebo (RR 0.05; 95% CI 0.00 to 0.85; one study, n = 73 infants; Analysis 1.11). There is evidence of imprecision for this outcome with low event rates and small sample size. Using GRADE methodology, we judged this to be low‐quality evidence due to imprecision. For infants whose mothers were in the placebo group, the chance of neonatal hypoglycaemia was 26%; if the infants' mothers had been in the myo‐inositol group, the chance would have ranged from 0% to 22%.
1.11. Analysis.
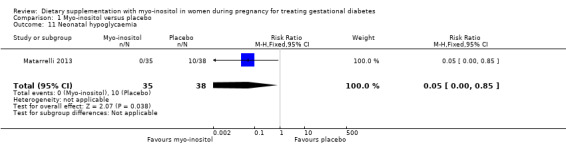
Comparison 1 Myo‐inositol versus placebo, Outcome 11 Neonatal hypoglycaemia.
No data were reported for any of the other neonatal secondary outcomes of this review (stillbirth; neonatal mortality; Apgar score (less than seven at five minutes); macrosomia; small‐for‐gestational age; birthweight z‐score; head circumference and z‐score; length and z‐score; ponderal index; adiposity; shoulder dystocia; bone fracture; nerve palsy; respiratory distress syndrome; hyperbilirubinaemia; neonatal hypocalcaemia; polycythaemia; relevant biomarker changes associated with the intervention.
Later childhood outcomes
No data were reported for any of the long‐term outcomes of the neonate as a child for this review (weight and z‐scores; height and z‐scores; head circumference and z‐scores; adiposity; blood pressure; type I or type 2 diabetes mellitus; impaired glucose tolerance; dyslipidaemia or metabolic syndrome; educational achievement).
Adulthood outcomes
No data were reported for any of the long‐term outcomes of the neonate as an adult for this review (weight; height; adiposity; cardiovascular health; type I or type 2 diabetes mellitus; impaired glucose tolerance; dyslipidaemia or metabolic syndrome; employment, education and social status/achievement).
Health service outcomes
No data were reported for any of the health service outcomes pre‐specified for this review (number of hospital or health professional visits; number of antenatal visits or admissions; length of antenatal stay; neonatal intensive care unit admission; length of postnatal stay (mother); length of postnatal stay (baby); costs to families associated with the management provided; costs associated with the intervention; cost of maternal care; cost of offspring care).
Discussion
Summary of main results
This review includes two small trials (involving 142 women and their babies).
None of the maternal primary outcomes for this review were reported in the included studies. There was no clear evidence for a difference between those receiving myo‐inositol and those receiving placebo for weight gain during pregnancy or for the need for additional pharmacological therapy. There was limited evidence from two trials (142 women) that suggested that myo‐inositol was associated with a reduced fasting blood glucose concentration compared to placebo. There was no evidence of a difference between groups for the two‐hour postprandial blood glucose concentration in one study of 73 women. The clinical significance of the maternal blood glucose outcomes is not clear. No other maternal outcomes for this review were reported.
For the infant, there was no evidence of a difference between myo‐inositol and placebo groups for the risk of being born large‐for‐gestational age. None of the other infant primary outcomes were reported in the included studies. There was no evidence of a difference between myo‐inositol and placebo groups for the risk of preterm birth or birthweight. One study of 73 infants reported that maternal treatment with myo‐inositol was associated with a reduced risk of neonatal hypoglycaemia and with being born at a later gestational age. This is of potential clinical importance if these results can be confirmed in further studies. No other neonatal outcomes for this review were reported.
Overall completeness and applicability of evidence
The evidence is limited in terms of the number of studies available (n = 2), and in the lack of data reported for the majority of outcomes pre‐specified for this review.
Quality of the evidence
Overall, the risk of bias of the included studies was judged to be unclear due to lack of key methodological information.
The quality of the evidence, where it was able to be judged was low. Downgrading of the quality of evidence was mainly due to imprecision (small sample size, single trial and low events) (Table 1; Table 2). None of the maternal outcomes selected for judgement using GRADE had data reported. For the neonatal outcomes selected for GRADE only large‐for‐gestational age and neonatal hypoglycaemia had data reported.
Potential biases in the review process
We conducted a thorough search of multiple databases including trial registries and sought both published and unpublished reports without restrictions in language or date of publication. Identification, data extraction and data entry were performed independently by two review authors.
Agreements and disagreements with other studies or reviews
Minimal literature was found on the use of myo‐inositol for the treatment of gestational diabetes mellitus (GDM). One non‐randomised pilot study (n = 60) (Lubin 2016) suggested that in women with GDM who were not well‐controlled with diet alone, myo‐inositol appeared to be a safe first‐line treatment with no adverse side effects.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence on the use of myo‐inositol for the treatment of gestational diabetes mellitus (GDM) to support its use. Uncertainty remains around adverse effects, as these outcomes are not reported in the included trials.
Implications for research.
There is uncertainty regarding the use of myo‐inositol for the treatment of GDM. This is mainly due to insufficient data and lack of reporting of clinically meaningful outcomes for women with GDM and their babies. Further trials are required that look at the role of myo‐inositol for the treatment or co‐treatment of GDM that report on the core outcomes for GDM identified in the methods section of this review. Participants of varying ethnicities and with varying risk factors for GDM should be included in future trials. In addition, further trials of myo‐inositol for the treatment of GDM should explore the optimal dose, frequency and timing of supplementation, report on adverse effects and assess the long‐term effects of this intervention. Economic analysis or health service use and costs should also be included.
Acknowledgements
Portions of the methods section of this review are based on a standard template used by the Cochrane Pregnancy and Childbirth Review Group. Outcomes may be similar to other Cochrane reviews on treatment for gestational diabetes due to the attempt to have consistency across all protocols and reviews on this condition.
This review came about whilst work was being conducted on the Cochrane systematic review 'Antenatal dietary supplementation with myo‐inositol in women during pregnancy for preventing gestational diabetes' (Crawford 2015). It was recognised that studies were excluded from that review on the basis that they used myo‐inositol for the treatment of gestational diabetes mellitus, rather than for prevention. This accounts for the similarities between certain aspects of this review and the 'Antenatal dietary supplementation with myo‐inositol in women during pregnancy for preventing gestational diabetes' review.
We acknowledge the support from the Cochrane Pregnancy and Childbirth Review Group editorial team in Liverpool, the Australian and New Zealand Satellite of the Cochrane Pregnancy and Childbirth Review Group (funded by NHMRC) and the Liggins Institute, University of Auckland, New Zealand.
As part of the pre‐publication editorial process, this review has been commented on by four peers (an editor and three referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Search terms for ClinicalTrials.gov and ICTRP
gestational diabetes AND myoinositol
gestational diabetes AND myo‐inositol
gestational diabetes AND inositol
gdm AND myoinositol
gdm AND myo‐inositol
gdm AND inositol
Data and analyses
Comparison 1. Myo‐inositol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Large‐for‐gestational age | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.02, 8.58] |
| 2 Weight gain during pregnancy (kg) | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐3.25, 2.25] |
| 3 Body mass index change (kg/m2) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐1.5 [‐2.35, ‐0.65] |
| 4 Additional pharmacological treatment (insulin therapy) | 2 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.08, 1.73] |
| 5 Fasting OGTT (mmol/L) | 2 | 142 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐0.59, ‐0.35] |
| 6 1‐hour OGTT (mmol/L) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.73, ‐0.07] |
| 7 2‐hour OGTT (mmol/L) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.46, 0.06] |
| 8 Gestational age at birth (weeks) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [1.27, 2.93] |
| 9 Preterm birth (less than 37 weeks' gestation) | 1 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.09, 10.56] |
| 10 Birthweight (g) | 1 | 73 | Mean Difference (IV, Fixed, 95% CI) | 16.0 [‐209.72, 241.72] |
| 11 Neonatal hypoglycaemia | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.85] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Corrado 2011.
| Methods | Type of study: randomised controlled trial, parallel, single centre, 1:2 randomisation ratio. | |
| Participants | 84 women from Italy. Eligibility criteria: women diagnosed with gestational diabetes according to the ADA 2008 criteria (ADA 2008). Exclusion criteria: not stated. Location: Department of Obstetrics and Gynecology, Policlinico Universitario, Messina, Italy. Timeframe: April 2008 to September 2009. |
|
| Interventions | Intervention: 4 g myo‐inositol + 400 mcg folic acid orally per day (2 g myo‐inositol and 200 mcg folic acid twice daily), and diet treatment according to American Diabetes Association recommendations (ADA 2008) (n = 28). Comparison: 400 mcg folic acid orally per day, and diet treatment according to American Diabetes Association recommendations (ADA 2008) (n = 56). |
|
| Outcomes | Primary: homeostasis model assessment of insulin resistance (HOMA‐IR). Secondary necessity for insulin therapy, altered glycaemic values, gestational age at birth, maternal BMI increase, fetal biometry percentiles at ultrasound (abdominal circumference, biparietal diameter, head circumference, femur length), occurrence of polyhydramnios, route of delivery, preterm birth, gestational weight gain. |
|
| Notes | Sample size calculation: yes. Intention‐to‐treat analysis: no. Funding: not stated. No conflicts of interest were declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computerised randomization." |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Trial was open‐label and blinding was not described. However, the primary outcome was objective measurement of laboratory results which would not be influenced by knowledge of group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 women from the study group and 9 in the control group required insulin treatment for lack of glycaemic control and were excluded post randomisation. 1 women in the study group and 2 in the control group were excluded post randomisation because they delivered prematurely before 35 weeks' gestation. Intention‐to‐treat analysis not conducted. |
| Selective reporting (reporting bias) | High risk | Prespecified maternal outcomes are reported on. No other pregnancy or birth outcomes are specified or reported on. No neonatal outcomes are reported despite birthweight being listed in the protocol on ClinicalTrials.gov (NCT00734448). |
| Other bias | Low risk | Appears free of other bias. |
Matarrelli 2013.
| Methods | Type of study: randomised controlled trial, parallel, single centre. | |
| Participants | 75 women from Italy. Eligibility criteria: women with singleton pregnancy, elevated fasting glucose (≥ 5.1 mmol/L and ≤ 7.0mmol/L according to National Guidelines based on IADPSG 2010 criteria). Exclusion criteria: pre‐gestational obesity (BMI > 35 kg/m2), refusal to participate. Location: High Risk Pregnancy Unit, Hospital of University "Gabriele d'Annunzio", Chieti, Italy. Timeframe: August 2010 to April 2011. |
|
| Interventions | Intervention: 4 g myo‐inositol + 400 mcg folic acid orally per day (dosage regimen not stated), and exercise and dietary advice (n = 36). Comparison: 400 mcg folic acid orally per day, and exercise and dietary advice (n = 39). |
|
| Outcomes | Primary: abnormal 75 g 2 hours OGTT at 24‐28 weeks' gestation (IADPSG 2010 criteria). Secondary: necessity for insulin therapy, altered glycaemic values, gestational age at birth, maternal BMI increase, fetal biometry percentiles at ultrasound (abdominal circumference, biparietal diameter, head circumference, femur length), occurrence of polyhydramnios, route of delivery. |
|
| Notes | Sample size calculation: yes based on OGTT at 24‐28 weeks. Intention‐to‐treat analysis: yes. Funding: materials were provided by Italfarmaco SpA, but they did not have access to the data or the results. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated random sampling method was used." |
| Allocation concealment (selection bias) | Low risk | Identical looking sachets. The pharmacist sealed and randomly numbered the sachets according to the computer‐generated scheme and was the sole healthcare provider to have access to these data until blinding was broken. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both patients and healthcare providers with whom they had contact were blinded to the meaning of the allotment numbers. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided on blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intervention group n = 1 withdrawal. Control group n = 1 withdrawal. |
| Selective reporting (reporting bias) | Unclear risk | All prespecified outcomes were reported on. Neonatal hypoglycaemia was not a prespecified outcome, but results were provided. |
| Other bias | Low risk | Baseline characteristics were similar. |
ADA: American Diabetes Association BMI: body mass index g: grams IADPSG: International Association of Diabetes and Pregnancy Study Groups kg/m2: kilogram per metre squared mcg: micrograms mmol/L: millimoles per litreOGTT: oral glucose tolerance test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Malvasi 2014 | Trial conducted in healthy pregnant women using a combination of myo‐inositol, D‐chiro‐inositol, manganese and folic acid to prevent the development of gestational diabetes, rather than as a treatment. |
| Valent 2014 | Ongoing prospective cohort pilot study, examining pharmacokinetics of myo‐inositol in pregnancy. Not a randomised study. |
Characteristics of ongoing studies [ordered by study ID]
Vitacolonna 2014.
| Trial name or title | Inositol stereoisomers to treat gestational diabetes. |
| Methods | Randomised, interventional, parallel assignment, efficacy study. |
| Participants | 80 women to be enrolled between 24 to 28 weeks' gestation, following diagnosis with gestational diabetes mellitus. Inclusion: gestational diabetes diagnosed between 24 to 28 weeks' gestation, Caucasian pregnant women. Exclusion: pre‐pregnancy diabetes, non‐singleton pregnancy. |
| Interventions | Subgroup A: folic acid 400 mcg/day. Subgroup B: myo‐inositol 2000 mg twice a day. Subgroup C: d‐chiro‐inositol 250 mg twice a day. Subgroup D: myo‐inositol 550 mg plus d‐chiro‐inositol 13.8 mg twice a day. |
| Outcomes | Primary: Insulin resistance level evaluated by homeostasis model assessment of insulin resistance Secondary: Hypertensive disorders Caesarean section Need for insulin therapy/insulin dosage Lipid profile Macrosomia Neonatal hypoglycaemia Jaundice requiring phototherapy |
| Starting date | April 2014 (not yet recruiting according to ClinicalTrials.gov). |
| Contact information | Ester Vitacolonna, Universita degli Studi 'G. d'Annunzio" chieti e Pescara, Italy. |
| Notes | Clinicaltrials.gov Identifier: NCT02097069. |
Differences between protocol and review
There are no major differences between our published protocol (Crawford 2016) and the full review.
Contributions of authors
Julie Brown (JB) is guarantor for this review. Julie Brown (JB) is the contact person for this review.
All authors (TC, JB, J Alsweiler and CA Crowther) contributed to the preparation of this review.
Julie Brown conceived the review, screened retrieved papers against eligibility criteria, appraised quality of papers, extracted data from papers, checked data in RevMan, analysed and interpreted data, providing a methodological perspective, wrote the first draft of the review and contributed to subsequent versions of the review following feedback from other authors
Tineke Crawford screened search results, retrieved relevant papers, screened retrieved papers against eligibility criteria, appraised quality of papers, extracted data from papers, wrote to authors of papers for additional information, entered data into RevMan, analysed and interpreted data, and assisted in the incorporation of feedback into subsequent versions of the review.
Jane Alsweiler appraised the quality of papers, extracted data from papers, analysed and interpreted data, providing a neonatal clinical perspective, and contributed to versions of the review.
Caroline Crowther provided obstetric and methodological expertise, interpreted the data, and commented on versions of the review including the final version.
Sources of support
Internal sources
-
Liggins Institute, University of Auckland, New Zealand.
- Infrastructure to support the preparation of this protocol is from the Liggins Institute, University of Auckland, New Zealand
External sources
-
The Australasian Satellite of the Cochrane Pregnancy and Childbirth Review Group (funded by the NHMRC). Adelaide, Australia.
(incorporating the New Zealand Branch)
Declarations of interest
Tineke J Crawford: none known
Caroline A Crowther: none known
Jane Alsweiler: none known
Julie Brown: none known
New
References
References to studies included in this review
Corrado 2011 {published data only}
- Corrado F, D'Anna R, Vieste G, Giordano D, Pintaudi B, Santamaria A, et al. The effect of myoinositol supplementation on insulin resistance in patients with gestational diabetes. Diabetic Medicine 2011;28(8):972‐5. [DOI] [PubMed] [Google Scholar]
- NCT00734448. Myo‐inositol administration in gestational diabetes. clinicaltrials.gov/show/NCT00734448 Date first received: 16 April 2008.
Matarrelli 2013 {published data only}
- Matarrelli B, Vitacolonna E, D'Angelo M, Pavone G, Celentano C. Early treatment of gestational diabetes and obstetric adverse outcome prevention. Ultrasound in Obstetrics & Gynecology 2011;38(Suppl 1):54. [Google Scholar]
- Matarrelli B, Vitacolonna E, D'angelo M, Pavone G, Mattei PA, Liberati M, et al. Effect of dietary myo‐inositol supplementation in pregnancy on the incidence of maternal gestational diabetes mellitus and fetal outcomes: a randomized controlled trial. Journal of Maternal‐Fetal & Neonatal Medicine 2013;26(10):967‐72. [DOI] [PubMed] [Google Scholar]
- NCT01342874. Improved outcomes associated with inositol dietary supplementation in women with gestational diabetes mellitus (inositol). clinicaltrials.gov/show/NCT01342874 Dare first received: 19 April 2011.
References to studies excluded from this review
Malvasi 2014 {published data only}
- Malvasi A, Casciaro F, Minervini MM, Kosmas I, Mynbaev OA, Pacella E, et al. Myo‐inositol, D‐chiro‐inositol, folic acid and manganese in second trimester of pregnancy: a preliminary investigation. European Review for Medical and Pharmacological Sciences 2014;18(2):270‐4. [PubMed] [Google Scholar]
Valent 2014 {unpublished data only}
- NCT02149992. Glycaemic impact of myo‐inositol in pregnancy. clinicaltrials.gov/show/NCT02149992 Date first received: 25 May 2014.
References to ongoing studies
Vitacolonna 2014 {published data only}
- NCT02097069. Inositol stereoisomers to treat gestational diabetes. clinicaltrials.gov/show/NCT02097069 Date first received: 27 February 2014.
Additional references
ACOG 2013
- American College of Obstetricians and Gynecologists. Gestational Diabetes Mellitus: Practice Bulletin Number 137. Obstetrics and Gynecology 2013;122(2 Pt 1):406‐16. [DOI] [PubMed] [Google Scholar]
ADA 2008
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care 2008;31(Suppl 1):S55‐S60. [DOI] [PubMed] [Google Scholar]
Alberti 1998
- Alberti K, Zimmet P. Definition, diagnosis and classification of diabetes mellitus. Part 1: Diagnosis and classification of diabetes mellitus: World Health Organization Report. Diabetic Medicine 1998;15(7):539‐53. [DOI] [PubMed] [Google Scholar]
Alwan 2009
- Alwan N, Tuffnell D, West J. Treatments for gestational diabetes. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD003395.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boney 2005
- Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115(3):e290‐6. [DOI] [PubMed] [Google Scholar]
Carlomagno 2011
- Carlomagno G, Unfer V, Buffo S, D'Ambrosio F. Myo‐inositol in the treatment of premenstrual dysphoric disorder. Human Psychopharmacology: Clinical and Experimental 2011;26(7):526‐30. [DOI] [PubMed] [Google Scholar]
Catalano 2003
- Catalano P, Thomas A, Huston‐Presley L, Amini S. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. American Journal of Obstetrics and Gynecology 2003;189(6):1698‐704. [DOI] [PubMed] [Google Scholar]
CDA 2008
- Canadian Diabetes Association. Clinical practice guidelines for the prevention and management of diabetes in Canada. Canadian Journal of Diabetes 2008;32(Suppl 1):S1‐S201. [DOI] [PubMed] [Google Scholar]
Clements 1980
- Clements RS, Darnell B. Myo‐inositol content of common foods: development of a high myo‐inositol diet. American Journal of Clinical Nutrition 1980;33:1954‐67. [DOI] [PubMed] [Google Scholar]
Cohen 2006
- Cohen P. The twentieth century struggle to decipher insulin signalling. Nature Reviews. Molecular Cell Biology 2006;7:867‐73. [DOI] [PubMed] [Google Scholar]
Crawford 2015
- Crawford TJ, Crowther CA, Alsweiler J, Brown J. Antenatal dietary supplementation with myo‐inositol in women during pregnancy for preventing gestational diabetes. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD011507.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Crowther 2005
- Crowther C, Hiller J, Moss J, McPhee A, Jeffries W, Robinson J. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine 2005;352:2477‐86. [DOI] [PubMed] [Google Scholar]
Croze 2013
- Croze ML, Soulage CO. Potential role and therapeutic interests of myo‐inositol in metabolic diseases. Biochimie 2013;95(10):1811‐27. [DOI] [PubMed] [Google Scholar]
D'Anna 2012
- D'Anna R, Bendetto V, Rizzo P, Raffone E, Interdanto ML, Corado F, et al. Myo‐inositol may prevent gestational diabetes in PCOS women. Gynecological Endocrinology 2012;28(6):440‐2. [DOI] [PubMed] [Google Scholar]
Ferrara 2007
- Ferrara A. Increasing prevalence of gestational diabetes mellitus. Diabetes Care 2007;30(Suppl 2):S141‐S146. [DOI] [PubMed] [Google Scholar]
Genazzani 2008
- Genazzani AD, Lanzoni C, Ricchieri F, Jasonni VM. Myo‐inositol administration positively affects hyperinsulinemia and hormonal parameters in overweight patients with polycystic ovarian syndrome. Gynecological Endocrinology 2008;24:139‐44. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
IADPSG 2010
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33(3):676‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2002
- Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25:1862‐8. [DOI] [PubMed] [Google Scholar]
Landon 2009
- Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicentre, randomized trial of treatment for mild gestational diabetes. New England Journal of Medicine 2009;361(14):1339‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larner 2010
- Larner J, Brautigan DL, Thorner MO. D‐chiro‐inositol glycans in insulin signaling and insulin resistance. Molecular Medicine 2010;16(11‐12):543‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lubin 2016
McCance 2011
- McCance DR. Pregnancy and diabetes. Best Practice & Research Clinical Endocrinology & Metabolism 2011;25:945‐58. [DOI] [PubMed] [Google Scholar]
McCurdy 2010
- McCurdy C, Friedman J. Mechanisms underlying insulin resistance in human pregnancy and gestational diabetes mellitus. In: Kim K, Ferrara A editor(s). Gestational Diabetes During and After Pregnancy. London: Springer, 2010:125‐38. [Google Scholar]
Metzger 1998
- Metzger BE, Coustan DR. Proceedings of the Fourth International Workshop‐Conference on Gestational Diabetes Mellitus. Diabetes Care 1998;21 (Suppl. 2):B1‐B167. [PubMed] [Google Scholar]
Minozzi 2008
- Minozzi M, D'Andrea G, Unfer V. Treatment of hirsutism with myo‐inositol: a prospective clinical study. Reproductive Biomedicine Online 2008;17(4):579‐82. [DOI] [PubMed] [Google Scholar]
Nankervis 2013
- Nankervis A, McIntyre HD, Moses R, Ross GP, Callaway L, Porter C, et al. for the Australasian Diabetes in Pregnancy Society (ADIPS). Consensus Guidelines for the Testing and Diagnosis of Gestational Diabetes Mellitus in Australia. URL: http://adips.org/downloads/ADIPSConsensusGuidelinesGDM‐03.05.13VersionACCEPTEDFINAL.pdf (accessed 03 July 2015).
NCT00734448
- NCT00734448. Myo‐inositol administration in gestational diabetes. clinicaltrials.gov/show/NCT00734448 Date first received: 16 April 2008.
New Zealand Ministry of Health 2014
- New Zealand Ministry of Health. Screening, Diagnosis and Management of Gestational Diabetes in New Zealand: A Clinical Practice Guideline. Wellington, New Zealand: Ministry of Health, 2014. [Google Scholar]
NICE 2015
- National Institute for Health and Care Excellence. Diabetes in Pregnancy: Management of Diabetes and its Complications from Pre‐conception to the Postnatal Period. Manchester: NICE, 2015. [PubMed] [Google Scholar]
Nordio 2013
- Nordio M, Pajalich R. Combined treatment with Myo‐inositol and selenium ensures euthyroidism in subclinical hypothyroidism patients with autoimmune thyroiditis. Journal of Thyroid Research 2013;2013:Article ID 424163. [DOI: 10.1155/2013/424163] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papaleo 2007
- Papaleo E, Unfer V, Baillargeon JP, Santis L, Fusi F, Brigante C, et al. Myoinositol in patients with polycystic ovarian syndrome: a novel method for ovulation induction. Gynecological Endocrinology 2007;23:700‐3. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Saltiel 1990
- Saltiel AR. Second messengers of insulin action. Diabetes Care 1990;13(3):244‐56. [DOI] [PubMed] [Google Scholar]
The HAPO Study Cooperative Research Group 2008
- The HAPO Study Cooperative Research Group. Hyperglycaemia and adverse pregnancy outcomes. New England Journal of Medicine 2008;358(19):1991‐2002. [DOI] [PubMed] [Google Scholar]
Unfer 2011
- Unfer V, Raffone E, Rizzo P, Buffo S. Effect of a supplementation with myo‐inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecological Endocrinology 2011;27:857‐61. [DOI] [PubMed] [Google Scholar]
Wang 2013
- Wang Z, Kanguru L, Hussein J, Fitzmaurice A, Ritchie K. Incidence of adverse outcomes associated with gestational diabetes mellitus in low‐ and middle‐income countries. International Journal of Gynecology and Obstetrics 2013;121:14‐9. [DOI] [PubMed] [Google Scholar]
Wendland 2012
- Wendland E, Torloni M, Falavigna M, Trujilo J, Dose MA, Campos MA, et al. Gestational diabetes and pregnancy outcomes: a systematic review of the World Health Organization (WHO) and the International Association of Diabetes and Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy and Childbirth 2012;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2011
- West NA, Crume TL, Maligie MA, Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia 2011;54(3):504‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Crawford 2016
- Crawford TJ, Crowther CA, Alsweiler J, Brown J. Dietary supplementation with myo‐inositol in women during pregnancy for treating gestational diabetes. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD012048] [DOI] [PMC free article] [PubMed] [Google Scholar]


