Abstract
Background
The treatment of brain metastasis is generally palliative since most patients have uncontrollable systemic cancer. Historically, whole brain radiation therapy (WBRT) has been the treatment of choice, although more recently focused radiation therapy e.g. stereotactic radiosurgery (SRS) has developed a role in selected patients. In certain circumstances, such as single brain metastasis, death may be more likely from brain involvement than systemic disease. In this group surgical resection has been proposed to relieve symptoms and prolong survival.
Objectives
To assess the clinical effectiveness of surgical resection plus WBRT versus WBRT alone in the treatment of patients with single brain metastasis.
Search methods
The following databases were part of a systematic literature search: Cochrane Central Register of Controlled Trials (CENTRAL Issue 2, 2010), MEDLINE, EMBASE, CancerLit, Biosis and the Science Citation Index. References of identified studies were hand searched, as were the Journal of Neuro‐Oncology and Neuro‐Oncology, including all conference abstracts. Specialists in neuro‐oncology were contacted for further information. The searches for MEDLINE and EMBASE were updated in October 2007 and December 2010.
Selection criteria
Randomised controlled trials (RCTs) comparing surgery and WBRT with WBRT alone in patients of all ages with proven or suspected single brain metastasis.
Data collection and analysis
Two review authors independently assessed the search results for relevance, undertook critical appraisal according to known guidelines and extracted data using a pre‐specified pro‐forma.
Main results
Three RCTs were identified enrolling 195 patients in total. No significant difference in survival was found (hazard ratio (HR) 0.72, 95% CI 0.34 to 1.55, P = 0.40) although there was heterogeneity between trials (I2 = 83%). One trial found surgery and WBRT increased the duration of Functionally Independent Survival (FIS) (HR 0.42, 95% CI 0.22 to 0.82, P = 0.01). There was some indication that surgery and WBRT might reduce the risk of deaths due to neurological cause (relative risk (RR) 0.68, 95% CI 0.43 to 1.09, P = 0.11). The risk of adverse events was not statistically proven to be different between arms although actual event numbers were higher in the surgery arm.
Authors' conclusions
Surgery and WBRT may improve FIS but not overall survival. It may also reduce the proportion of deaths due to neurological cause. All these results were in a highly selected group of patients. Patients undergoing surgery were not reported to have any higher risk of adverse events than patients who only had WBRT.
Plain language summary
Surgery and whole brain radiation therapy versus whole brain radiation therapy alone for single brain metastases
For patients with single brain metastasis there is good evidence from randomised controlled trials (RCTs) that surgery in addition to whole brain radiation therapy (WBRT) does not improve overall survival.Treatment of brain metastasis is usually palliative although in selected patients ‐ particularly those with only a single metastasis to the brain ‐ surgery could be considered. This review analysed the evidence from three RCTs, enrolling a select group of patients, and found that the combination of surgery and WBRT did not improve overall survival compared with WBRT alone. The addition of surgery may improve the length of time patients remained independent from others for support and there is a suggestion it may also reduce the risk of death due to neurological causes. Patients undergoing surgery were not reported have a higher risk of adverse events than patients who only had WBRT. Decisions on the treatment for an individual patient are best made as part of a multidisciplinary team.
Background
Description of the condition
Brain metastasis are cancers that spread to the brain from a primary site outside of the brain. Metastasis are the most frequent type of brain malignancy, comprising around 50% of all brain tumours, and have an incidence of 14 cases per 100,000 population a year (Counsell 1998). They occur in between 20 to 40% of those with cancer, as determined at autopsy (Posner 1978), with the most common primary sites being lung and breast. Patients usually present with a short history of focal neurological symptoms, symptoms of raised intracranial pressure, or seizures. Symptoms vary depending on the site, size and number of metastasis. Previously around 50% of brain metastases were thought to be single (Delattre 1988) although advances in brain imaging suggest this figure is now closer to 30% (Schaeffer 1996).
Generally, the treatment is palliative, as most patients have uncontrollable cancer outside the brain. Steroids frequently cause a resolution or improvement of symptoms but side effects can be problematic and without further treatment neurological symptoms will recur (Kaal 2004). Median survival in patients having no treatment after diagnosis of brain metastases is one month, with most patients dying from their neurological disease. For patients taking steroids it is two months, and for people taking steroids and undergoing WBRT it is three to six months (Cairncross 1980).
Description of the intervention
Whole brain radiation therapy (WBRT) has historically been the accepted palliative treatment of choice with potential benefits including relief of symptoms and longer survival (Barker II 2005). Patients who respond to radiation are usually less than 60 years old, have a good Karnofsky Performance Score (KPS) (greater than 70), have radiosensitive primary tumours and controlled primary disease with metastatic spread confined to the brain (Diener‐West 1989). The optimal dose fractionation schedule for treatment of brain metastases remains uncertain and varies widely (Tsao 2009).
Focal radiotherapy techniques, such as stereotactic radiosurgery (SRS), have been developed that focus higher radiotherapy doses on the metastasis but with less damage to surrounding brain than WBRT (Patil 2010). This has been proposed to provide increased local control and potentially fewer long‐term cognitive side‐effects (Andrews 2004).
Surgical resection involves a craniotomy and surgical excision of the lesion. It is commonly performed under general anaesthesia although can be performed 'awake' when cortical stimulation is justified to identify eloquent tissue. The lesion is removed using microsurgical techniques or macroscopically. Post‐operatively the patient will recover in more intensively monitored environments initially prior to discharge. Most patients will not be suitable for surgery because of multiple lesions, a surgically inaccessible lesion location, active primary disease, or co‐morbidity.
Following surgery or SRS, WBRT is usually recommended to prevent local recurrence and target any micro‐metastases not detected on initial imaging; this management is based on the findings of a single RCT that found those undergoing WBRT after surgery have a reduced local recurrence rate compared with those who had surgery alone (Patchell 1998). Previosuly, symptomatic radiation induced dementia was previously believed to be rare with modern dosing schedules, with one chart‐review study revealing a frequency of less than 5% (DeAngelis 1989). Recent studies have increasingly recognised the cognitive effects of WBRT, particularly with an increase in survival times, and questioned the routine use of WBRT after surgery or SRS (Aoyama 2006; Chang 2009; Roos 2006; Soffieti 2010).
How the intervention might work
Surgical resection provides its main benefits through direct removal of the targeted mass lesion. It has been proposed to help maintain a patients quality of life (QoL), prevent death directly from the metastasis and prolong survival. In patients with raised intra‐cranial pressure surgery can be life saving and bring immediate relief of symptoms. Surgery also provides the opportunity to assess the histology of the lesion as some lesions thought to be metastasis may have an alternative origin requiring different treatment (e.g. brain abscess). The potential benefits of surgical resection must be balanced against the risks of post‐operative morbidity and mortality.
Whole brain radiation therapy (WBRT) is designed to treat identified metastasis as well as prophylaxis against any 'micro‐metastasis' not detected on pre‐intervention imaging. Radiation therapy in general is aimed at inducing terminal damage to the DNA of neoplastic cells. It is administered on an out‐patient basis for five days a week over two weeks.
Why it is important to do this review
Although there are in theory advantages to having a metastasis surgically removed there is also a definite risk involved with undergoing surgery. There are no systematic reviews or meta‐analyses in this area and the choice of treatment is controversial. In order to improve patient outcomes maximise the use of resources it is necessary to have a clear description of the potential risks and benefits of surgery in treating patients with single brain metastasis.
Objectives
To assess if surgical resection followed by WBRT holds any clinical advantage over WBRT alone in the treatment of single brain metastasis.
Methods
Criteria for considering studies for this review
Types of studies
RCTs meeting the selection criteria (Criteria for considering studies for this review). External signs of each surgery are usually clinically obvious meaning blinding is difficult and was not an inclusion criteria. Foreign language journals were eligible for inclusion.
Types of participants
Patients with proven systemic cancer (i.e. primary site confirmed by histology) and a suspected single brain metastasis (on imaging and clinical findings) were included. Imaging had to include at least CT although ideally contrast enhanced MRI to optimise sensitivity for detecting multiple metastasis. Additional imaging modalities (e.g. positron emission tomography or magnetic resonance spectroscopy) were not mandatory. The brain metastasis did not have to be histologically proven pre‐hoc (e.g. by biopsy). Patients should have been stratified by age, performance status, primary tumour and extent of systemic disease as these are the strongest prognostic factors (Noordijk 1994). Performance status could be recorded using the KPS (Karnofsky 1948) or the World Health Organisation Score (WHO/ECOG) (WHO 1982).
Types of interventions
Surgical resection and WBRT versus WBRT alone, defined as:
Surgical resection: all procedures where the pre‐operative aim was to remove more tissue than is necessary for diagnosis. The technique normally involved general anaesthesia, craniotomy and attempted total macroscopic microsurgical resection of the lesion. All aids to achieving surgical resection ‐ including neuro‐navigation, 5‐ALA/Gliloan guided resection, awake craniotomy, Sonowand and intra‐operative MRI ‐ were eligible for inclusion. Extent of resection was often graded as either total macroscopic, partial or de‐bulking; all of which were eligible for inclusion. Assessment of complete resection could have been made by the surgeons operating opinion but ideally by early post‐operative imaging (Hensen 2008).
WBRT: conventional whole brain radiotherapy fractionation schedules were 3000 cGy in 10 fractions or 2000 cGy in 5 fractions. There is currently no evidence that alternative schedules or administration of radiosensitising agents improves patient outcomes (Tsao 2009).
Stereotactic Radiosurgery (SRS): techniques such as LINAC, Gamma Knife, Cyberknife or proton beam were not eligible for inclusion in either arm of this review.
Post‐operative care: was decided by the individual attending physician on an individual patient basis in light of the absence of specific evidence based therapies at this stage.
Types of outcome measures
Primary outcomes
Survival: was the length of time (in days, weeks or months) from randomisation to death.
Secondary outcomes
Time to progression (TTP)/progression free survival (PFS): open and thorough criteria were used to define recurrence according to clinical symptoms, imaging or increasing steroid therapy (Wen 2010).
Quality of Life (QoL): measured using a reliable and objective grading measure, for example the EORTC QLQC30/BN‐20 and FACT‐BrS (Mauer 2008).
Functionally independent survival (FIS): time to KPS less than 70 or other grading system from randomisation.
Neurological death: whether a person died primarily from their intracerebral metastasis rather than fulminant systemic disease. A neurological death was also one where both systemic and brain metastasis were active at the time of death, as this would be a failure of the brain treatment. Death from neurological cause also involved acute deterioration (from either rapid growth of the metastasis, haemorrhage, invasion of local structure or decompensation of raised intra‐cerebral pressure) or more gradual tumour expansion and deterioration of neurologic function. Care was taken to try and distinguish any contribution that treatment related morbidity may have contributed.
Symptom control: improvement of symptoms, or a prolonged maintenance of symptoms without deterioration.
Adverse Events (AE): nature, as defined using MedDRA (Medical Dictionary for Regulatory Authorities) criteria, and timing (MedDRA 2008). Examples included: haematoma, wound complications, infection (and site), CSF leak, oedema, seizures and general medical complications. Further procedures required for complications should have been noted. Both the total number of complications and complications per patient should have been stated.
Mortality: cause specific immediately following procedure and at 30 days
Search methods for identification of studies
The following databases were part of a systematic literature search: Cochrane Central Register of Controlled Trials (CENTRAL Issue 4, 2010), MEDLINE, EMBASE, CancerLit, Biosis and the Science Citation Index. References of identified studies were hand searched, as was the Journal of Neuro to Oncology over the previous 10 years and Neuro to Oncology over the past 2 years, including all conference abstracts. Specialists in neuro to oncology were also contacted. The searches for MEDLINE and EMBASE were updated in October 2007 and December 2010.
Electronic searches
The same principle was used to search each database. Firstly, the terms and phrases identifying all the randomised controlled trials (RCTs) were combined using the Boolean "OR". Secondly, all the terms and phrases describing the disease of interest, namely cerebral metastasis, were combined with "OR". Thirdly, terms describing the intervention of interest i.e. surgical resection or whole brain radiation therapy was also combined with "OR". Items which fulfilled all three criteria were identified by linking the results of these searches with the Boolean 'AND' operator and the results displayed. Wild cards and truncation symbols were used to ensure terms with alternative spelling and/or endings were not missed. MeSH headings were exploded. The full search strategies are described in the Appendices.
Searching other resources
Reference searching
The references of all identified studies were searched for additional trials.
Hand searching
A hand search of the Journal of Neuro‐Oncology and Neuro‐oncology were undertaken in order to identify trials that may not have been picked up by the electronic database searches. This included searching of all conference abstracts published in the journals.
Personal communication
Neuro‐oncology experts were contacted to see if they were aware of any published, unpublished, pending or recently commenced trials. These people included:
Michael Brada, London, UK: Chairman of National Cancer Research Institute Brain Tumour Section.
Martin J van den Bent, Chairman of the European Organisation for Research Trials in Cancer (EORTC) Brain Tumour Section, Netherlands
The following RCT primary authors were also contacted:
Charles J Vecht (Utrecht, Netherlands: Chairman of the EORTC Brain Tumour Group and author of one of the already identified papers).
Arlan H Mintz (Hamilton, Canada: author of one of the identified papers)
Roy A Patchell (Kentucky, USA: author of one of the identified papers)
In addition for the 2010 update the following experts were contacted:
Michael Weller (University Hospital Zurich, Switzerland),
Wolfgang Wick (Heidelberg, Germany)
Susan Short (University College Hospital, London)
Roger Stupp (University of Lausanne, Switzerland).
Data collection and analysis
Selection of studies
Identification of studies was made in two stages. Abstracts returned by the original search were examined independently by two review authors (MGH & RG) and screened to see if they met the inclusion criteria. Next, full texts of the selected references were obtained and likewise examined. At all times any disagreements were resolved through discussion. If sufficient data were not available for assessment of a trial the relevant trial authors were contacted.
Data extraction and management
For included studies, two review authors (MGH & RG) independently abstracted data on characteristics of patients, interventions, study quality, endpoints and deviations from protocol using a pre‐specified form designed to complete the information required for the table of characteristics of included studies (Table 1; Table 2). Differences were reconciled by discussion or by consultation with a third review author.
1. Internal Validity.
| Characteristic | Patchell 1990 | Vecht 1993 | Mintz 1996 |
| Power calculation? | Yes (but too optimistic) | No | Yes |
| Proper randomisation? | Yes | Yes | Yes |
| Groups similar at baseline? | Yes | Yes | No |
| Blinding? | No | No | No |
| Eligibility criteria stated? | Yes | Yes | Yes |
| Objective outcome measures? | Survival: Yes. Others: No | Survival: Yes. Others: No | Survival: Yes. Others: No |
| Analysis on an ITT basis? | No | No | Yes |
| All patients accounted for? | Yes | Yes | Yes |
| Withdrawals specified? | Yes | Yes | Yes |
| Withdrawal reasons given? | Yes | Yes | Yes |
| Conflict of interest? | No | No | No |
2. External Validity.
| Characteristic | Patchell 1990 | Vecht 1993 | Mintz 1996 |
| Age (mean and range) | Surgery: 59 (44 to 74), WBRT: 60 (49 to 74) | Surgery: 59 (30 to 75), WBRT: 60 (32 to 78) | Surgery 58 (SD 9.9), WBRT 59 (SD 9.0) |
| Sex (M:F) | Surgery 18:7, WBRT 14:9 | Surgery 15:17, WBRT 18:13 | Surgery 22:21, WBRT 24:17 |
| KPS (mean and range) | Surgery: 90 (70 to 100), WBRT: 90 (70 to 100) | (WHO) Surgery: 0 = 3, 1 = 21, 2 = 8. WBRT 0 = 4, 1 = 18, 2 = 9 | Surgery = 67%, WBRT = 80% |
| Extra‐cranial metastasis | Surgery: 36%, WBRT: 37% | Surgery: 30%, WBRT: 30% | Surgery = 49%, WBRT = 41% |
| Extent of surgery | Complete in all cases (CT day 2 to 5) | Not assessed | 95% complete |
Participants
For each trial, data on the number of patients randomised, analysed and excluded from the investigator's analyses was extracted. The number of patients censored, due to either incomplete follow up, loss to follow up or competing event, were noted. The minimum and maximum follow up length was also noted for use in calculating hazard ratios (HRs).
Interventions
Actual numbers of participants undergoing treatments were assessed, taking into account those with protocol violations, where published. Data on the proportion of patients in the research treatment and control arms who completed radiotherapy as planned, did not start radiotherapy and who experienced delay were also extracted. Details of total dose and fractionation of WBRT were compared. The number of patients undergoing further therapy and the type were noted, and any implications discussed. Duration of follow up, and ascertainment of morbidity and neurological cause of death were also noted.
Assessment of risk of bias in included studies
Trials deemed relevant were critically appraised according to a checklist (Fowkes 1991) and the criteria reported in the NHS Centre for Reviews and Dissemination report (CRD 2009). Tables were constructed to summarise internal and external validity (Juni 2001). Trials were allocated according to risk of bias as described in the Cochrane Handbook (Higgins 2009). Critical appraisal was performed by two independent review authors (MGH & RG). Any disputes were resolved through discussion.
Measures of treatment effect
Time to event data (survival, TTP/PFS, FIS): the log hazard ratio (logHR)and its standard error were abstracted from trial reports. If these were not reported, we digitised electronic versions of the published Kaplan‐Meier survival curves using Adobe Photoshop, noted the minimum and maximum follow to up and hence estimated the logHR and its standard error using Parmar's methods (Parmar 1998). These calculations were performed independently by two review authors (MGH & HD) using an Excel spreadsheet and a specially written program in Stata 921 (Stata 2005), which were validated using data presented in Table V of Parmar 1998.
Continuous outcomes (QoL and symptoms): the final value and standard deviation of the outcome of interest in each treatment arm at the end of the follow‐up was abstracted.
Dichotomous outcomes (neurological death, adverse events and mortality): the number of patients in each treatment arm who experienced the outcome of interest was abstracted in order to estimate a relative risk (RR).
For continuous and dichotomous data we abstracted the number of patients assessed at endpoint was abstracted.
Where possible all data abstracted was that pertaining to an intention to treat (ITT) analysis.
Unit of analysis issues
If the HR and its variance were not presented we attempted to abstract the data required to estimate them (Parmar 1998).
Dealing with missing data
In the case of missing data required for the review outcomes the study authors were contacted.
Assessment of heterogeneity
Heterogeneity between studies was assessed by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials which could not be ascribed to sampling variation (Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001).
Assessment of reporting biases
We intended to construct a funnel plot of treatment effect versus precision in order to investigate the likelihood of publication bias. If these plots suggested that treatment effects may not be sampled from a symmetric distribution, as assumed by the random effects model, further meta to analyses would be performed using the fixed effects models.
Data synthesis
Integration of data into RevMan 5.0.25 was performed by a single author (MGH).
Time to event data: HRs and variance were pooled using the generic inverse variance function of RevMan 5.0.25
Continuous outcomes: we pooled the weighted mean differences between the treatment arms at the end of the follow‐up using the mean difference method if all trials have measured the outcome on the same scale or used the standardised mean difference method.
Dichotomous outcomes: the RR for each study was calculated and then all studies were pooled
Random effects models were used for all meta‐analyses (Der Simonian 1986).
Subgroup analysis and investigation of heterogeneity
In light of the known benefits of chemotherapy in primary disease we planned to assign trials including chemotherapy to a separate subgroup analysis.
A funnel plot of treatment effect versus prevision with the data from all studies included was proposed if sufficient studies were identified in order to investigate the likelihood of publication bias
Sensitivity analysis
Studies that included objective blinded early post‐operative MRI in their assessment of extent of resection were subjected to a subsequent sensitivity analysis.
Results
Description of studies
Results of the search
The original search strategy revealed 860 results out of which three RCTs were identified as meeting the inclusion criteria (Mintz 1996; Patchell 1990; Vecht 1993). All three studies were deemed to be at low risk of bias and were sufficiently similar in design characteristics to warrant combination of results in meta‐analysis. See table of included studies and Table 2 for full details of each study.
The update on 21st December 2010 identified 176 references in MEDLINE, 376 references in EMBASE and 27 references in CENTRAL. No new studies were identified for inclusion.
Included studies
The first study by was a single institution RCT set in Kentucky, USA (Patchell 1990). It randomised 48 patients to confirmatory brain tumour biopsy followed by WBRT or surgical resection of metastasis and WBRT. Randomisation was by computer generated random numbers after stratification for prognostic factors but outcome assessors were not blinded. It included mainly young (mean age 60 years) and fit subjects (mean KPS = 90). All participants underwent pre‐operative MRI whilst those in the radiotherapy arm also underwent biopsy. Outcome measures included survival, functional independence, radiographic changes in tumour size, time to recurrence and cause of death. The logHRs for survival and FIS and their standard errors, as estimated by Cox regression, were reported to us by the trial's lead statistician.
The second study was multi‐centre RCT set in the Netherlands. It randomised 63 patients to surgical resection followed by WBRT versus WBRT alone (Vecht 1993). Randomisation was performed centrally by telephone in blocks after stratification for prognostic factors but outcome assessors were not blinded. The participants were mainly young (mean age 60 years) but slightly less fit (WHO score of 2 or less) than in the previous study. Only CT imaging had to be performed and biopsy was not mandatory in the radiotherapy only group. The minimum and maximum duration of follow to up were assumed to be 1 and 70 months respectively. Outcome measures included survival, functionally independent survival and cause of death. The log (HR) and its standard error were estimated from the published survival curves (Parmar 1998).
The final study was a multi‐centre RCT set in Canada. It randomised 84 patients to surgical resection followed by WBRT versus WBRT alone (Mintz 1996). Randomisation was by central telephone randomisation after stratification for prognostic factors but outcome assessors were not blinded. This patients were mainly young (median age 59 years) but entry criteria allowed less fit patients (KPS of 50 or more) than in the previous two studies. Only CT imaging was required and biopsy was only undertaken when the diagnosis was in doubt. Outcome measures were survival, cause of death, functional status (by KPS) and QOL (using Spitzer QOL index), and surgical complications within 30 days. The minimum and maximum duration of follow to up were assumed to be 0 and 34 months respectively. The logHR and its standard error were estimated from the published survival curves (Parmar 1998).
Excluded studies
A single RCT was identified for possible inclusion (Noordijk 1994) but on retrieval of the full article it was noted that it was a review of prognostic factors in a previously reported RCT (Vecht 1993) rather than unique data in its own right.
Risk of bias in included studies
Allocation
All studies included adequate methods of randomising patients. In the trial by Patchell (Patchell 1990), selection of cases for randomisation was by a single neurosurgeon, which may have resulted in inclusion of only those most suitable for surgery. However, there was no obvious discrepancy in any prognostic factors between the groups. In the trial by Mintz (Mintz 1996), it is not clear the reasons why so many people who were suitable for inclusion in the trial refused randomisation when compared with the other trials. There is the possibility then that the group randomised in the Mintz trial is not representative of all those eligible.
Blinding
No studies were blinded. This is likely due to the obvious clinical signs of those who underwent craniotomy which would make blinding difficult and probably unreliable. For these reasons blinding was not a strict pre‐requisite for inclusion into this review but nevertheless it is a source of potential bias for all secondary outcomes but not for the primary outcome of survival.
Incomplete outcome data
Only the Mintz study provided an ITT analysis (Mintz 1996). In all studies, however, withdrawals and reasons were given for all participants.
Selective reporting
As it was not possible to blind clinicians about the treatment allocation after the intervention, there may be bias arising in the determination of neurological death and the diagnosis of adverse effects. Reporting of adverse effects is subjective, and there may be a systematic bias depending on the speciality of the attending clinician. Many criteria are available to improve the objectivity of these diagnoses, but unfortunately none were explicitly noted as being used in the above trials.
Other potential sources of bias
Many patients in each group received further therapy, for example surgery or steroids. However, there did not appear to be a clear bias to more intensive follow up therapy in either arm of any of the trials. There was no obvious bias in the censoring of individuals in any of the trials.
In each trial, patients were followed up for monthly intervals up to 6 months, and for longer intervals thereafter (2 to 3 months). It is therefore not known when within this time interval an event occurred, particularly a reduction in FIS. This represents a significant time interval, especially considering the short time to recurrence of these outcomes in the first instance.
Effects of interventions
The three RCTs included a total of 195 subjects (Mintz 1996; Patchell 1990; Vecht 1993).
Overall survival
The analysis did not demonstrate a statistically significant difference in survival between the two treatments (HR 0.72, 95% CI 0.34 to 1.55, P = 0.40). There was substantial heterogeneity between the trials (I2 = 83%); the trials by Patchell and Vecht both reported better survival in those undergoing surgery and WBRT while that by Mintz reported better survival in patients receiving only WBRT.
Functionally independent survival
Only one trial could be included in the analysis (Patchell 1990); the other trials did not include sufficient data in order to calculate the necessary HR and variance. The trial by Vecht did not report FIS for the entire trial population while the trial by Mintz did not present its results graphically. The Patchell trial found that those treated by surgery and WBRT maintained their functional independence longer than those treated by WBRT alone (HR 0.42, 95% CI 0.22 to 0.82, P = 0.01).
Neurological death
There was a trend that those treated by surgery were less likely to die from neurological causes (RR 0.68, 95% CI 0.43 to 1.09, P = 0.11). No statistical heterogeneity was found between trials (I2 = 0%).
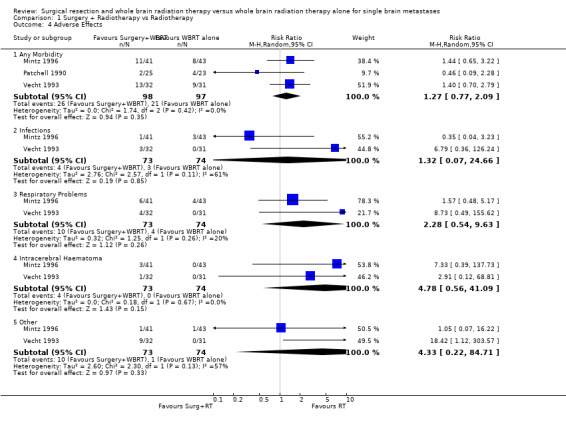
Adverse events
The reports of adverse events were difficult to interpret as more than one adverse event was reported for some patients and this was not clearly described in the included RCTs. The statistical analysis did not allow for the clustering of events within patients; correct allowance for this would widen the CIs. Without allowing for this the results do not demonstrate that either treatment was more likely to cause adverse events (RR 1.27, 95% CI = 0.77 to 2.09, P =0.35). Mortality at 30 days was also similar in both arms of each of the trials. No statistical heterogeneity between these findings was identified (I2= 0%). Individual subcategories did not demonstrate that any one type of complication was significantly more likely in one group than the other.
The Patchell study did not report adverse effects by category. The Vecht study reported complications in the surgical arm as: respiratory problems (4), intracerebral haematoma (1), infectious disease (3) and other complications (9). These were in 13 patients and serious in 4. The Mintz study reported: infections (surgery 1 versus WBRT 1); haematoma (surgery 3 versus WBRT 0); other (surgery 7 versus WBRT 7). The reporting of adverse events was not structured with clear definitions and may have been biased by informal reporting.
A funnel plot on included studies was constructed (Figure 1).
1.
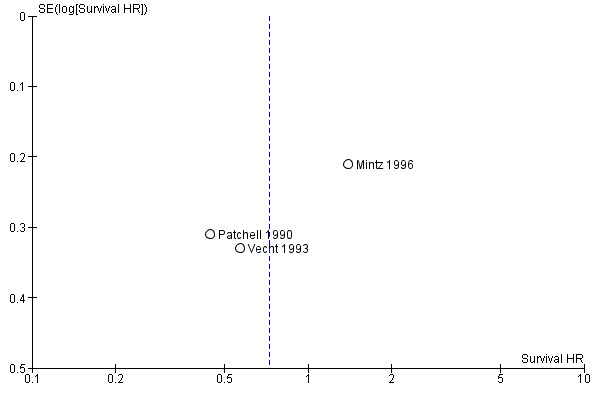
Funnel plot survival
Discussion
Summary of main results
Although no statistically significant difference between surgery plus WBRT and WBRT alone was found in the meta to analysis, the trials by Patchell and Vecht were in favour of surgery (Patchell 1990; Vecht 1993), while that by Mintz was in favour of WBRT alone (Mintz 1996). The key differences between the trials are the decreased survival of those in WBRT alone in the trial by Patchell, and the decreased survival for those treated by surgery and WBRT in the trial by Mintz. Two possible reasons may account for the findings in the Patchell trial. Firstly, the majority of their patients had non‐small cell lung cancer, a highly radio‐resistant tumour, which would not be expected to respond to WBRT particularly well. Secondly, there may be a selection bias, as patients were selected for surgery in a specialist unit by a single neurosurgeon. This may have resulted in randomisation of only patients who were particularly suited to surgery. In the Mintz trial, the key issue is why patients who underwent surgery had a poorer survival than those who did not. The entry criteria allowed patients with a poorer KPS and a larger percentage had extracranial metastases (Table 1). Patients with a lower KPS and extracranial disease are known to have a poorer survival hence the potential benefits of surgery may not be applicable in this more ill population. The radiotherapy group had a considerably longer time between primary tumour diagnosis and metastasis than the surgery group, possibly reflecting less aggressive disease, which is known to confer a better survival (Patchell 1990). Furthermore, the entry criteria did not specify a minimum life expectancy of six months, as in the other two trials. The patients examined by Mintz, having in general a lower life expectancy and more active disease, would seem less likely to benefit from surgery (Mintz 1996). It could be that in this group of patients the surgical approach does not provide any improvement in survival, but in a more selected group (examined in the other two trials) surgery may increase survival.
Overall completeness and applicability of evidence
Although much focus is put on the benefit in survival times, equally important is the benefit to patients' QoL, as few patients were cured overall. No trial directly examined QoL, which is a major shortcoming; FIS was examined in only one trial although KPS is known to correlate poorly with patients' own perception of QoL. The one trial did find an improvement in FIS with surgery (Patchell 1990). In addition the trial by Vecht found an improvement in FIS in patients treated by surgery who had stable extra‐cranial disease (Vecht 1993), although the trial by Mintz found little difference between arms in the stable extra‐cranial disease subgroup (Mintz 1996). It has been noted that patients in general maintain their functional independence until a few months before death after both approaches, and that after surgery there is a trend for the patients' KPS score to improve, although this is mainly in those who have a high score already (Vecht 1993). It would be of benefit for further trials to clarify exactly the nature of this benefit in QoL, especially in regard to a reduction in the dose of steroids (Macdonald 1990).
Quality of the evidence
In all trials the number of patients was very small (84, 48 and 63 in the trials of Mintz 1996; Patchell 1990; Vecht 1993 respectively), due in part to the highly selected nature of cases. In the trial by Patchell, sample size was calculated with a highly optimistic end point in mind, derived from a previous non‐randomised study (Patchell 1986). In the trial by Vecht, the slow rate of entry of participants meant it took over six years to accrue all their data (Vecht 1993). This opens up questions as to the standardisation of treatment during this time. The trial by Mintz found 143 capable of being randomised, although only 84 consented to trial randomisation (Mintz 1996). This demonstrates that it is possible to generate much larger numbers, but only in a large multi to institution study.
Potential biases in the review process
This review only included the results of highly selected series of patients and the findings are not necessarily applicable to a larger cohort of those with brain metastasis. It is important to note that there are many other important indications for operating on metastases, for example raised intra‐cerebral pressure or obstructive hydrocephalus. There may also be other benefits to surgery which were not examined in any of the three RCTs, such as reducing steroid doses, improving control during WBRT and improving some neurological symptoms. In the case of a single brain metastasis without an obvious primary site, the case for resection is well established for histological confirmation. Authors have suggested that for certain radio‐resistant tumours, such as non‐small cell lung cancer, surgery should always be considered in the management (Patchell 1990).
Agreements and disagreements with other studies or reviews
Another systematic review and meta‐analysis has been published (Mintz 2007). This review heavily cites this Cochrane review and the conclusions are appropriately concordant. Another systematic review and evidence based practice guideline has been published (Gaspar 2010). This review stated that surgical resection plus WBRT for single brain metastasis is an effective treatment based on the findings of the three known RCTs also included in this Cochrane review. Only a descriptive analysis of the RCTs was performed and the omission of a meta‐analysis is a disappointing shortcoming of their review. We have shown that the correct interpretation of the three RCTs is critically dependent on the meta‐analysis, which reveals that surgery does not lead to a longer survival time compared to WBRT alone, necessitating that our conclusions surpass the findings in their review. Other non‐systematic review articles in the field have broadly recommended surgery for single brain metastasis based on the findings of the two selected RCTs that are in favour of surgery (Ewend 2005). This Cochrane review offers a higher level of evidence due to it's meta‐analysis, thorough discussion of individual RCTs, and transparent methodology aimed at producing un‐biased conclusions.
Authors' conclusions
Implications for practice.
It is difficult to advise either patients or colleagues on the basis of evidence from such small studies. It is important to note that these results were obtained in a highly selected group of patients ‐ under close follow‐up and receiving further active therapy in many cases ‐ who are not necessarily representative of the majority of those with single brain metastasis. In this group, the surgical approach did not improve OS. Surgery may reduce the number of deaths due to neurological cause, while one trial has suggested an increase in the duration of a patients FIS. Adverse events were similar in each group whilst QoL was not directly examined. Those most likely to benefit from surgery are of young age, have good neurological function, and controlled primary disease (Noordijk 1994). Careful attention to prognostic factors will see only those who have the most to gain from surgery, while those who are less well will avoid unnecessary risks and morbidity. It must not be forgotten that the overall outlook for patients at two years is dismally poor with either intervention and death is commonly due to systemic disease. Currently, the management for the majority of those with single brain metastasis will be WBRT alone, due to active systemic disease and other co‐morbidity. Decisions of the most appropriate treatment for an individual patient should be made at an MDT meeting in line with NICE guidance (NICE 2006).
Implications for research.
Further trials in this area would help increase the robustness of the data both by answering methodological shortcomings and recruiting greater numbers. Follow‐up should be longer and at patients examined at closer intervals. Statistically, the reporting in trials of HRs and their variance would improve the accuracy in reading data from Kaplan‐Meier plots. These results could be combined in future updates of this review to provide an up‐to‐date conclusion on management of single brain metastasis.
Recently, much attention has been given to focal radiotherapy techniques, known as Stereotactic Radiosurgery (SRS). A meta‐analysis (Stafinski 2006) and a Cochrane review (Patil 2010) of three RCTS (Andrews 2004; Chougle 2000; Kondziolka 1999) comparing SRS and WBRT versus WBRT alone both found that combination therapy improved survival in only those with single but not multiple brain metastasis. A single RCT has examined surgery and WBRT in comparison with SRS and found, after terminating prematurely, that survival and local control is comparable but distant recurrences more common with SRS alone (Muacevic 2008); another similar RCT is on‐going (Roos 2008). In the future the management of single brain metastasis is likely to focus on surgery versus SRS and the role of WBRT (Aoyama 2006; Chang 2009; Roos 2006; Soffieti 2010).
What's new
| Date | Event | Description |
|---|---|---|
| 17 July 2018 | Amended | Next stage expected date amended. |
| 24 May 2018 | Review declared as stable | This review is currently not a priority topic area. |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 29 December 2010 | New search has been performed | Search strategies amended and updated. Text reviewed but conclusions unchanged. No new RCTs included. |
| 9 October 2007 | New search has been performed | Searches were re‐run on 10 October 2007. No additional trials were identified. |
| 11 July 2004 | New citation required and conclusions have changed | Substantive amendment |
Notes
Nil.
Acknowledgements
Richard Kryscio (lead statistician for the Patchell 1990 trial) for supplying additional data.
MGH acknowledges receipt of a Cochrane Gynaecological Cancer Review Group Grant for the update of this review in 2007.
Jane Hayes of the Cochrane Gynaecological Cancer Review Group in Bath for updating the search strategy for the 2010 update.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Brain Neoplasms explode all trees #2 (brain or cerebral or intracranial or intracerebral) near/5 (metasta* or cancer* or tumor* or tumour* or neoplas* or carcinoma* or malignan*) #3 (#1 OR #2) #4 MeSH descriptor Radiotherapy explode all trees #5 Any MeSH descriptor with qualifier: RT #6 radiotherap* #7 radiation #8 irradiation #9 WBRT #10 (#4 OR #5 OR #6 OR #7 OR #8 OR #9) #11 MeSH descriptor Neurosurgical Procedures explode all trees #12 Any MeSH descriptor with qualifier: SU #13 neurosurg* #14 surg* #15 (#11 OR #12 OR #13 OR #14) #16 (#3 AND #10 AND #15) #17 (#16), from 2007 to 2010
Appendix 2. MEDLINE search strategy
1 exp Brain Neoplasms/ 2 ((brain or cerebral or intracranial or intracerebral) adj5 (metasta* or cancer* or tumor* or tumour* or neoplas* or carcinoma* or malignan*)) 3 1 or 2 4 exp Radiotherapy/ 5 radiotherapy.fs. 6 radiotherap*.mp. 7 radiation.mp. 8 irradiation.mp. 9 WBRT.mp. 10 4 or 5 or 6 or 7 or 8 or 9 11 exp Neurosurgical Procedures/ 12 surgery.fs. 13 neurosurg*.mp. 14 surg*.mp. 15 11 or 12 or 13 or 14 16 3 and 10 and 15 17 randomized controlled trial.pt. 18 controlled clinical trial.pt. 19 randomized.ab. 20 placebo.ab. 21 clinical trials as topic.sh. 22 randomly.ab. 23 trial.ti. 24 17 or 18 or 19 or 20 or 21 or 22 or 23 25 16 and 24 26 limit 25 to yr="2007 ‐ 2010"
key: mp=title, original title, abstract, name of substance word, subject heading word, unique identifier, pt=publication type, fs=floating subheading, ab=abstract, ti=title, sh=subject heading
Appendix 3. EMBASE search strategy
1 exp brain tumor/ 2 ((brain or cerebral or intracranial or intracerebral) adj5 (metasta* or cancer* or tumor* or tumour* or neoplas* or carcinoma* or malignan*)).mp. 3 1 or 2 4 exp radiotherapy/ 5 cancer radiotherapy/ 6 rt.fs. 7 radiotherap*.mp. 8 radiation.mp. 9 irradiation.mp. 10 WBRT.mp. 11 4 or 5 or 6 or 7 or 8 or 9 or 10 12 exp neurosurgery/ 13 su.fs. 14 neurosurg*.mp. 15 surg*.mp. 16 12 or 13 or 14 or 15 17 3 and 11 and 16 18 crossover procedure/ 19 double blind procedure/ 20 randomized controlled trial/ 21 single blind procedure/ 22 random*.mp. 23 factorial*.mp. 24 crossover*.mp. 25 cross over*.mp. 26 cross‐over*.mp. 27 placebo*.mp. 28 (doubl* adj blind*).mp. 29 (singl* adj blind*).mp. 30 assign*.mp. 31 allocat*.mp. 32 volunteer*.mp. 33 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 34 17 and 33 35 limit 34 to yr="2007 ‐ 2010"
key mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, fs=floating subheading
Appendix 4. Biosis previews search strategy
Words or phrases in the Title, Subjects or Abstract were searched. 1. randomi?ed & control* & trial 2. control* & clinical & trial 3. random* & allocat* 4. double & (blind* , mask*) 5. single & (blind* , mask*) 6. clinical & trial 7. control & group 8. control* & trial 9. clinical & study 10. control* & study 11. OR/1‐10 12. brain & tumo*r 13. brain & neoplasm 14. brain & cancer 15. metastas?s 16. secondar* 17. tumor [Major Concept] 18. OR/12‐17 19. neurosurg* 20. combined & modality & therapy 21. stereota* & biopsy 22. biopsy & resection 23. surg* & treatment 24. OR/19‐23 25. radiation therap 26. radiotherapy 27. irradiation 28. OR/25‐27 29. 24 AND 28 30. 18 AND 29 31. 11 AND 30
Appendix 5. Science Citation Index search strategy
A similar search strategy to the one for BIOSIS was used. Searches were made in the title, keyword or abstract. Unlike BIOSIS, there was no "major concepts" search facility. The differences were as follows: (1) "tumo*" was used in place of "tumo?r" (2) "central & nervous & system & tumo*" and "central & nervous & neoplasm" were two additional searches (3) "extent & resection" was used in place of "extent of resection"
Data and analyses
Comparison 1. Surgery + Radiotherapy vs Radiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival | 3 | Survival HR (Random, 95% CI) | 0.72 [0.34, 1.55] | |
| 2 Functional Independent Surival | 1 | HR (Random, 95% CI) | 0.42 [0.22, 0.82] | |
| 3 Neurological Death | 3 | 185 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.43, 1.09] |
| 4 Adverse Effects | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Any Morbidity | 3 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.77, 2.09] |
| 4.2 Infections | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.07, 24.66] |
| 4.3 Respiratory Problems | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 2.28 [0.54, 9.63] |
| 4.4 Intracerebral Haematoma | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 4.78 [0.56, 41.09] |
| 4.5 Other | 2 | 147 | Risk Ratio (M‐H, Random, 95% CI) | 4.33 [0.22, 84.71] |
1.1. Analysis.
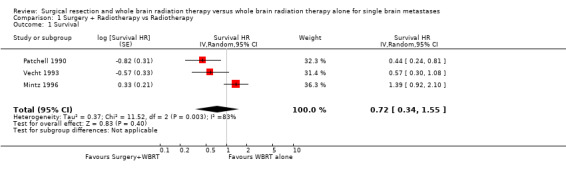
Comparison 1 Surgery + Radiotherapy vs Radiotherapy, Outcome 1 Survival.
1.2. Analysis.
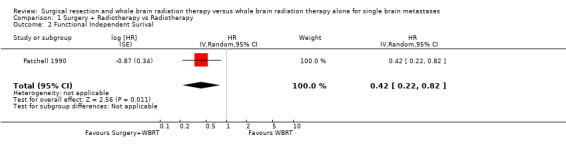
Comparison 1 Surgery + Radiotherapy vs Radiotherapy, Outcome 2 Functional Independent Surival.
1.3. Analysis.
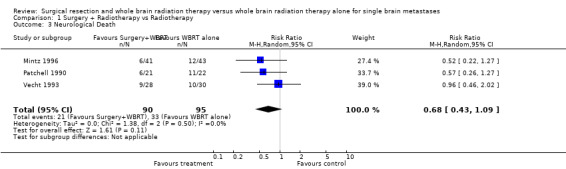
Comparison 1 Surgery + Radiotherapy vs Radiotherapy, Outcome 3 Neurological Death.
1.4. Analysis.
Comparison 1 Surgery + Radiotherapy vs Radiotherapy, Outcome 4 Adverse Effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Mintz 1996.
| Methods | Randomized Controlled Study. Multicentre Central telephone randomization Stratified by: Type (lung:other) Size (< 3cm: >= 3cm) Extent of primary (no evidence of primary, localised primary disease, localised + extracerebral) | |
| Participants | Inclusion Criteria: Single intracerebral metastasis Age < 80yrs Histologically verified cancer in last 5 years Karnofsky >= 50. Exclusion Criteria: Brain stem/basal ganglia tumours Underlying medical illness that would preclude adequate follow‐up Meningeal metastases Previous cranial RT Immediate resection required Radiosensitive systemic tumours (e.g. SCLC, lymphoma, leukaemia, skin cancer other than melanoma) | |
| Interventions | surgery plus radiation vs radiation alone WBRT was 3000 cGy therapy over 2 weeks ( (300cGy x 10 fractions). Surgery was aimed at macro‐scopical excision, with post‐operative CT assessment. | |
| Outcomes | Follow up monthly for first 6 months and every 3 months thereafter; examiner not defined. Follow up consisted of history, physical examination, Karnofsky performance status and Spitzer quality of life index, as well as CT scanning. Outcome measures were; 1. survival, with cause of death (either neurological, systemic or combined) 2. Functional status and quality of life, with functionally independent survival defined as a Karnofsky < 70). 3. Treatment complications, for surgery (wound infection, venous thrombosis, pulmonary embolism, myocardial ischaemia and pneumonia) and for radiation (radiation necrosis). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
Patchell 1990.
| Methods | Randomized Controlled Study. Single centre "computer generated random numbers" Non‐blinded Stratified by: Location (supratentorial:infratentorial) Type (lung: other) Extent of primary (no tumour: stable: progressive) | |
| Participants | Inclusion Criteria: Single intracerebral metastasis Age >= 18 years Histology of cancer out with the central nervous system Karnofsky >= 70. Exclusion Criteria: Resection not feasible Meningeal Metastases Previous cranial RT Immediate resection required Radiosensitive systemic tumours (e.g SCLC, lymphoma, leukaemia, multiple myeloma, germ cell tumour) | |
| Interventions | "Resection + cranial radiation" vs "cranial radiation" (but patients with supratentorial tumours randomised to "cranial radiation" were stereotactically biopsied prior to radiation, infratentorial tumours were not biopsied). 56 patients satisfied inclusion and exclusion criteria (Oct 85 ‐ Dec 88) 2 patients declined 54 patients randomised. 6 excluded because histology not metastasis (e.g. 2GBM, 1 LGA, 2 abscesses, 1 inflammatory reaction) 25 randomised to "resection + radiation" vs 23 to "radiation only" Radiation dose 36Gy, 3fractions/day over 12 days | |
| Outcomes | Follow‐up every 3 months by neurological exam and imaging(CT/MRI). Outcome measures: Death (30 days from surgery; neurological death; non‐neurological death) Length of survival Time to recurrence (by imaging) Clinical improvement (change in Karnofsky) Morbidity (Karnofsky 30 days post‐treatment < pre‐treatment) "Quality of Life" (length of time Karnofsky >= 70) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
Vecht 1993.
| Methods | Randomized Controlled Study. Multicentre Central telephone randomization Non‐blinded Stratified by: Centre (block sizes of 4) Type (lung: other) Extent of primary (no tumour/stable: progressive) | |
| Participants | Inclusion criteria: age >= 18 years, histologically verified extracranial malignancy, apparent presence of single brain metastasis as documented on CT, Karnofsky <= 70 or WHO scale < 2 with neurological function <=, life expectancy < 6 months, fit for treatment and informed consent. Exclusion criteria were small cell lung cancer, malignant lymphoma, and documented or suspected meningeal disease or intracranial tumour deposits other than a single parenchymal brain metastasis. | |
| Interventions | "neurosurgical excision plus radiotherapy" vs "radiotherapy alone". Surgery was by macro‐scopical excision. Radiotherapy was a total of 40Gy in 2 weeks, 2 fractions per day. 66 patients were randomised between January 1st 1985 and January 1st 1991. 2 were excluded due to challenge of their diagnosis, while 1 was excluded due to delay in treatment initiation. Of the remaining 63, 32 were randomised to the surgical arm and 31 to the radiotherapy alone arm. | |
| Outcomes | Patients were seen once a month during the first 6 months, then every 2 months thereafter. Data recorded were WHO status, neurological functional scale, neurological examination, and patients residence. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
GBM: glioblastoma multiforme LGA: low grade astrocytoma RT: radiotherapy SCLC: small cell lung cancer WBRT: whole brain radiation therapy WHO: World Health Organisation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Noordijk 1994 | This is a subsequent report on the same trial as reported by Vecht 1993. The paper more specifically looks at the possible effects of age and extracranial tumour activity on magnitude of difference between the randomised groups. |
Differences between protocol and review
Nil.
Contributions of authors
MGH performed the statistical analysis, helped in the text of the review and was involved in the editing process.
RG performed the literature search, analysis, helped write the text of the review, and oversaw the final draft.
Dr Walker assisted in the literature search strategy was involved in discussions about the analysis and helped in the text of the review.
HD supervised the statistical analysis, and was involved in the editing of the final draft.
Sources of support
Internal sources
MGH was the recipient of a Cochrane Gynaecological Cancer Review Group Grant, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Mintz 1996 {published data only}
- Mintz AH, Kestle J, Rathbone MP, Gaspar L, Hugenholtz H, Fisher B, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with single intracerebral metastasis. Cancer 1996;78(7):1470‐6. [DOI] [PubMed] [Google Scholar]
Patchell 1990 {published and unpublished data}
- Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. New England Journal of Medicine 1990;322(8):494‐500. [DOI] [PubMed] [Google Scholar]
Vecht 1993 {published data only}
- Vecht CJ, Haaxma‐Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery?. Annals of Neurology 1993;33(6):583‐90. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Noordijk 1994 {published data only}
- Noordijk EM, Vecht CJ, Haaxma‐Reiche H, Padberg GW, Voormolen JHC, Hoekstra FH, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. International Journal of Radiation Oncology, Biology, Physics 1994;29(4):711‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Andrews 2004
- Andrews DW, Scott CB, Sperduto PW, Flanders AE, Gaspar LE, Schell MC, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet 2004;363:1665‐72. [DOI] [PubMed] [Google Scholar]
Aoyama 2006
- Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, et al. Stereotactic radiosurgery plus whole‐brain radiation therapy versus stereotactic radiosurgery alone for treatment of brain metastases: A randomized controlled trial. JAMA 2006;295(21):2483‐91. [DOI] [PubMed] [Google Scholar]
Barker II 2005
- Barker FG II. Surgical and Radiosurgical Management of Brain Metastases. Surgical Clinics of North America 2005;85:329‐45. [DOI] [PubMed] [Google Scholar]
Cairncross 1980
- Cairncross JG, Kim J‐H, Posner JB. Radiation Therapy for brain metastases. Annals of Neurology 1980;7:529‐41. [DOI] [PubMed] [Google Scholar]
Chang 2009
- Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole‐brain irradiation: a randomised controlled trial.. Lancet Oncology 2009;10(11):1037‐44. [DOI] [PubMed] [Google Scholar]
Chougle 2000
- Chougle PB, Burton‐Williams M, Zheng SZ, Ponte B, Noren G, Alderson L. Randomised treatment of brain metastasis with gamma knife radiosurgery, whole brain radiotherapy or both. International Journal of Radiation Oncology, Biology and Physics 2000;48(3 (supplement)):114. [Google Scholar]
Counsell 1998
- Counsell C, Grant R. Incidence studies of primary and secondary intracranial tumors: A systematic review of their methodology and results. Journal of Neuro‐Oncology 1998;37:241‐50. [DOI] [PubMed] [Google Scholar]
CRD 2009
- Centre for Reviews and Dissemintation. Systematic Reviews: CRD's guidance for undertaking reviews in health care. York: York Publishing Services Limited, 2009. [Google Scholar]
DeAngelis 1989
- DeAngelis LM, Delattre JY, Posner JB. Radiation‐induced dementia in patients cured of brain metastases. Neurology 1989;39:789–96. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Review in Health Care: Meta‐analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
Delattre 1988
- Delattre JY, Krol G, Thaler HT, Posner JB. Distribution of brain metastases. Archives of Neurology 1988;45:741‐4. [DOI] [PubMed] [Google Scholar]
Der Simonian 1986
- Simonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Diener‐West 1989
- Diener‐West M, Dobbins TW, Phillips TL, Nelson DF. Identification of an optimal subgroup for treatment evaluation of patients with brain metastasis using RTOG study 7916. International Journal of Radiation Oncology, Biology, Physics 1989;16(3):669‐73. [DOI] [PubMed] [Google Scholar]
Ewend 2005
- Ewend MG, Elbabaa S, Carey LA. Current treatment paradigms for the management of patients with brain metastasis. Neurosurgery 2005;57:S4‐66 ‐ S4‐77. [DOI] [PubMed] [Google Scholar]
Fowkes 1991
- Fowkes FGR, Fulton PM. Critical appraisal of published research: introductory guidelines. BMJ 1991;302:1136‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaspar 2010
- Gaspar LE, Mehta MP, Patchell RA, Burri SH, Robinson PD, et al. The role of whole brain radiation therapy in the management of newly diagnosed brain metastasis: a systematic review and evidence‐based clinical practice guideline. Journal of Neuro‐oncology 2010;96:17‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hensen 2008
- Hensen JW, Ulmer S, Harris GJ. Brain tumour imaging in clinical trials. American Journal of Neuroradiology 2008;29:419‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane.org.
Juni 2001
- Juni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. BMJ 2001;323:42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaal 2004
- Kaal ECA, Vecht CJ. The management of brain edema in brain tumour. Current Opinion in Oncology 2004;16:593‐600. [DOI] [PubMed] [Google Scholar]
Karnofsky 1948
- Karnofksy DA. The use of nitrogen mustards in the palliative treatment of carcinoma. Cancer 1948;1:634‐56. [Google Scholar]
Kondziolka 1999
- Kondziolka D, Patel A, Lundsford LD, Kassam A, Flickinger JC. Stereotactic radiosurgery plus whole brain radiotherapy versus radiotherapy alone for patients with multiple brain metastasis. International Journal of Radiation Oncology, Biology and Physics 1999;45(2):427‐34. [DOI] [PubMed] [Google Scholar]
Macdonald 1990
- MacDonald DR, Cairncross JG. Surgery for single brain metastasis. New England Journal of Medicine 1990;323(2):132‐3. [DOI] [PubMed] [Google Scholar]
Mauer 2008
- Mauer ME, Bottomley A, Taphoorn MJB. Evaluating health‐related quality of life and symptom burden in brain tumour patients: instruments for use in clinical trials and clinical practice. Current Opinion in Neurology 2008;21:741‐53. [DOI] [PubMed] [Google Scholar]
MedDRA 2008
- Medical Dictionary for Regulatory Authorities. http://www.meddramsso.com/ 2008. [http://www.meddramsso.com/MSSOWeb/index.htm]
Mintz 2007
- Mintz A. Perry J. Spithoff K. Chambers A. Laperriere N. Management of single brain metastasis: A practice guideline. Current Oncology 2007;14(4):131‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muacevic 2008
- Muacevic A. Wowra B. Siefert A. Tonn JC. Steiger HJ. Kreth FW. Microsurgery plus whole brain irradiation versus Gamma Knife surgery alone for treatment of single metastases to the brain: a randomized controlled multi‐centre phase III trial.. Journal of Neuro‐oncology 2008;87(3):299‐307. [DOI] [PubMed] [Google Scholar]
NICE 2006
- Improving outcomes for people with brain and other CNS tumours ‐ the manual June 2006. National Institute for Health and Clinical Excellence (NICE): http://www.nice.org.uk/nicemedia/pdf/CSG_brain_manual.pdf. [http://www.nice.org.uk/guidance/index.jsp?action=download&o=28963]
Parmar 1998
- Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature of endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Patchell 1986
- Patchell RA, Cirrincione C, Thaler HT, Galicich JH, Ki JH, Posner JB. Single brain metastasis: surgery plus radiation or radiation alone. New England Journal of Medicine 1986;36:447‐53. [DOI] [PubMed] [Google Scholar]
Patchell 1998
- Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998;280(17):1485‐9. [DOI] [PubMed] [Google Scholar]
Patil 2010
- Patil CG, Pricola K, Garg SK, Bryant A, Black KL. Whole brain radiation therapy (WBRT) alone versus WBRT and radiosurgery for the treatment of brain metastases.. Cochrane Database of Systematic Reviews 2010, Issue 6. Art. No.: CD006121.. [DOI: 10.1002/14651858.CD006121.pub2.] [DOI] [PubMed] [Google Scholar]
Posner 1978
- Posner JB, Chernik NL. Intracranial metastasis from systemic cancer. Archives of Neurology 1978;19:579‐92. [PubMed] [Google Scholar]
Roos 2006
- Roos DE, Wirth A, Burmeister BH, Spry NA, Drummond KJ, Beresford JA, et al. Whole brain irradiation following surgery or radiosurgery for solitary brain metastases: Mature results of a prematurely closed randomized Trans‐Tasman Radiation Oncology Group trial (TROG 98.05). Radiotherapy and Oncology 2006;80(3):318‐22. [DOI] [PubMed] [Google Scholar]
Roos 2008
- Roos D. A Trial Comparing Radiosurgery with Surgery for Solitary Brain Metastasis. National Cancer Institute 2008. [http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=442357&version=patient&protocolsearchid=4142948]
Schaeffer 1996
- Schaeffer PW, Budzik RF, Gonzalez RG. Imaging of cerebral metastases. Neurosurgical Clinics of North America 1996;7:393‐423. [PubMed] [Google Scholar]
Soffieti 2010
- Soffietti R, Mueller RP, Abacioglu MU, Villa S, Fauchon F, Baumert B, et al. Quality‐of‐life results of an EORTC phase III randomized trial of adjuvant whole‐brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases of solid tumors. Journal of Clinical Oncology 2010;28(15):9036. [Google Scholar]
Stafinski 2006
- Stafinski T, Jhangri G, Yan E, Menon D. Effectiveness of stereotactic radiosurgery alone or in combination with whole brain radiotherapy compared to conventional surgery and/or whole brain radiotherapy for the treatment of one or more brain metastases: A systematic review and meta‐analysis.. Cancer Treatment Review 2006;32(3):203‐13. [DOI] [PubMed] [Google Scholar]
Stata 2005 [Computer program]
- StataCorp LP. Stata Statistical Software. Version Release 9. College Station, TX: StataCorp LP, 2005.
Tsao 2009
- Tsao MN, Lloyd N, Wong R, Chow E, Rakovitch E, Laperriere N. Whole brain radiotherapy for the treatment of multiple brain metastases. Cochrane Database of Systematic Reviews 2009, Issue Issue 3. Art. No.: CD003869.. [DOI: 10.1002/14651858.CD003869.pub2.] [DOI] [PubMed] [Google Scholar]
Wen 2010
- Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, et al. Updated response assessment criteria in high grade gliomas: response assessment in neuro‐oncology working group. Journal of Clinical Oncology 2010;28(11):1963‐72. [DOI] [PubMed] [Google Scholar]
WHO 1982
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the eastern cooperative oncology group. American Journal of Clinical Oncology 1982;5:649‐55. [PubMed] [Google Scholar]


