Abstract
Background
Systemic fungal infection is considered to be an important cause of morbidity and mortality in cancer patients, particularly those with neutropenia. Antifungal drugs are often given prophylactically, or empirically to patients with persistent fever.
Objectives
To compare the effect of fluconazole and amphotericin B on morbidity and mortality in patients with cancer complicated by neutropenia.
Search methods
We searched PubMed from 1966 to 7 July 2014 and the reference lists of identified articles.
Selection criteria
Randomised clinical trials comparing fluconazole with amphotericin B.
Data collection and analysis
The two review authors independently assessed trial eligibility and risk of bias, and abstracted data.
Main results
Seventeen trials (3798 patients, 381 deaths) were included. In two large three‐armed trials, results for amphotericin B were combined with results for nystatin in a 'polyene' group. Because nystatin is an ineffective drug in these circumstances, this approach creates a bias in favour of fluconazole. Furthermore, most patients were randomised to oral amphotericin B, which is poorly absorbed and poorly documented. There was overlap among the 'polyene' trials but we were unable to obtain any information from the trial authors or from Pfizer, the manufacturer of fluconazole, to clarify these issues. There were no significant differences in effect between fluconazole and amphotericin B, but the confidence intervals were wide. More patients dropped out of the study when they received amphotericin B, but as none of the trials were blinded decisions on premature interruption of therapy could have been biased. Furthermore, amphotericin B was not given under optimal circumstances, with premedication to reduce infusion‐related toxicity, slow infusion, and with fluid, potassium and magnesium supplements to prevent nephrotoxicity. The major harms were hepatic impairment and gastrointestinal adverse effects with fluconazole and infusion‐related toxicity, renal impairment and gastrointestinal adverse effects with amphotericin B. For the 2011 and 2014 updates no additional trials were identified for inclusion.
Authors' conclusions
Amphotericin B has been disfavoured in several of the trials through their design or analysis, or both. Since intravenous amphotericin B is the only antifungal agent for which an effect on mortality has been shown, and since it is considerably cheaper than fluconazole, it should be the preferred agent.
Plain language summary
Prevention of fungal infections in cancer patients with amphotericin B or fluconazole
Cancer patients treated with chemotherapy or who receive a bone marrow transplant have an increased risk of acquiring fungal infections. Such infections can be life‐threatening. Antifungal drugs are therefore often given prophylactically to such patients, or when they have a fever. The review could not detect a difference in effect between amphotericin B and fluconazole but several of the trials were designed or analysed in a way that disfavoured amphotericin B, which is the only antifungal drug that has been shown to have an effect on mortality.
Background
Systemic fungal infection in cancer patients with neutropenia is associated with considerable morbidity and mortality (Verfaillie 1991). As it may be difficult to diagnose the infection with certainty, many clinicians use antifungal agents prophylactically in patients undergoing antileukaemic chemotherapy or bone marrow transplantation. In a review of placebo‐controlled trials in cancer patients with neutropenia the antifungal agents amphotericin B and fluconazole decreased the incidence of invasive fungal infection (Gøtzsche 2002a). In addition, there was an effect of amphotericin B on mortality.
In many centres amphotericin B is the drug of choice but it has a wide spectrum of adverse effects, including reversible nephrotoxicity. Fluconazole is better tolerated but has little or no activity against Candida glabrata, Candida krusei and Aspergillus, in contrast to amphotericin B (Working Party 1995). An additional problem is that the azoles are suspected of causing an increased incidence of bacterial infections (Gøtzsche 2002a; Viscoli 1994). Because of the uncertainty of these trade‐offs, we performed a review of trials that compared fluconazole and amphotericin B.
Objectives
To compare the effect of fluconazole and amphotericin B on morbidity and mortality in patients with cancer complicated by neutropenia.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials, irrespective of language, which compared fluconazole with amphotericin B in neutropenic cancer patients. Trials using allocation methods without concealment, such as allocation by birth date, were not accepted.
Types of participants
Patients with cancer complicated by neutropenia caused by chemotherapy or bone marrow transplantation. Studies solely concerned with the treatment or prevention of oral candidiasis were excluded as patients recruited for such studies rarely have neutropenia.
Types of interventions
Fluconazole or amphotericin B given intravenously or orally.
Types of outcome measures
Mortality, preferably after three months
Mortality ascribed to fungal infection
Invasive fungal infection (defined as positive blood culture, oesophageal candidiasis, lung infection or microscopically confirmed deep tissue involvement)
Colonisation
Use of additional (escape) antifungal therapy
Dropouts
Dropouts because of adverse effects
Other harms
Search methods for identification of studies
Electronic searches
We searched PubMed from 1966 to 7 July 2014 and the reference lists of identified articles.
The search strategy used is in Appendix 1. The search strategies have been developed and executed by the authors.
Searching other resources
This has not been carried out since 2007 as we have not found it worthwhile.
Data collection and analysis
Data extraction and management
Decisions on which trials to include and which variables to use when a number of options were available for the same outcome were made independently by the two review authors based on the methods sections of the trials. Details on diagnosis, drug, dose and duration of treatments, criteria for starting treatment, rules for use of additional (escape) antifungal therapy, length of follow‐up, randomisation and blinding methods, number of randomised patients, exclusions after randomisation, deaths, invasive fungal infections, colonisation, use of escape drug, total number of dropouts, dropouts because of adverse effects and other harms were independently extracted by both review authors. Differences in the data that were extracted were resolved by consensus.
We defined invasive fungal infection as a positive blood culture, oesophageal candidiasis, lung infection or microscopically confirmed deep tissue infection (Gøtzsche 2002a). We excluded cases of oropharyngeal and vulvovaginal candidiasis, skin infections, Candida in the urine and vaguely described infections.
We used PubMed and abstract books to obtain the authors' most recent addresses. All authors were asked to confirm the extracted information and to answer additional questions. We specifically asked for mortality data three months after study entry for all patients, including those the authors had excluded after the randomisation. We also sought details on the randomisation process, especially whether treatment allocation was concealed, for example central randomisation, sealed envelopes, or a code provided by a pharmacy or a company.
Data synthesis
The outcomes were weighted by the inverse variance; 95% confidence intervals (CI) are presented. Since heterogeneity of the studies was expected because of various designs; diagnoses; drugs, doses, routes of administration; and criteria for fungal invasion, colonisation and use of rescue drug, a random‐effects model was used. A fixed‐effect model analysis was preferred, however, if P value was greater than 0.10 for the test of heterogeneity (both overall and for each separate subgroup).
Results
Description of studies
We identified 20 potentially relevant trials and excluded three. In one, only 22 of the 41 patients had been randomised (Ellis 1995); another was a three‐armed trial of 96 patients where amphotericin B was only given every fourth day (published as an abstract, fungal disease not documented in any group) (Reed 1993); the third trial was stopped after 24 patients, when 11 of 12 patients on amphotericin B colloidal dispersion had experienced infusion‐related toxicity (no proven fungal infections occurred) (Timmers 2000).
The antifungal agent was given prophylactically in 10 trials (in conjunction with chemotherapy or bone marrow transplantation) (Akiyama 1993; Bodey 1994; Koh 2002; Menichetti 1994; Meunier 1991; Ninane 1994; Philpott‐Howard 1993; Powles 1990; Teshima 1994; Wolff 2000), empirically in six (to patients with persisting fever despite antibiotic treatment) (Anaissie 1996; Malik 1998; Marie 1993; Silling 1999; Viscoli 1996; Winston 2000) and as treatment in one (Lake 1996). The most common diseases were acute leukaemia in 10 trials (Akiyama 1993; Bodey 1994; Malik 1998; Marie 1993; Menichetti 1994; Meunier 1991; Ninane 1994; Philpott‐Howard 1993; Silling 1999; Winston 2000), bone marrow transplantation in five (Koh 2002; Powles 1990; Teshima 1994; Viscoli 1996; Wolff 2000), other cancers in one (Anaissie 1996) and cancer with oesophageal candidiasis in one (Lake 1996).
Three of the reports that we retrieved described large, three‐armed trials that had compared fluconazole, amphotericin B and nystatin, but had combined the outcomes for amphotericin B and nystatin in a 'polyene' group. To provide a reasonable range for the estimates of the effect of amphotericin B in the three‐armed 'polyene' trials, we performed two sets of analyses. In one we used the data as reported (unadjusted analysis), while in the other we assumed that nystatin was no better than placebo (adjusted analysis), which is what we found in separate meta‐analyses of nystatin trials (Gøtzsche 2002b; Johansen 1999). Since the combined 'polyene' group and the fluconazole group were of very similar size in the trials, we assumed that equal numbers of participants had been randomised to receive amphotericin B and nystatin. For the adjustments, we used the relative risks (RR) for amphotericin B versus placebo that we determined in our previous meta‐analysis (Johansen 1999). These RRs were 0.65 for mortality, 0.41 for invasive infection and 0.51 for colonisation. Therefore, we would expect 41 invasive infections in patients receiving amphotericin B for every 100 infections in patients receiving nystatin; that is the expected fraction of infections for amphotericin B in the 'polyene' trials is 41/141 = 0.29. Thus, for a study which reported seven infections in the 'polyene' group (Philpott‐Howard 1993), the expected number of infections in the amphotericin B subgroup would be 7 x 0.29 = 2.0. Doubling this number equals four infections for patients receiving amphotericin B, which is what we would have expected to find if all patients receiving 'polyenes' had received this drug.
The relationships between the trials and the centres contributing to them were unclear (Johansen 1999). One of the three‐armed 'polyene' trials was reported as a multicentre study of 536 patients, with Philpott‐Howard 1993 as first author. The trial report described that patients taking amphotericin B received 2 g/d and that the results from 50 patients at one of the centres had been published previously (by Rozenberg‐Arska 1991) (Philpott‐Howard 1993). However, these patients had received only 1600 mg/d orally. Furthermore, a third report by Finke 1990 (in Philpott‐Howard 1993) described 40 patients who received 800 mg/d orally. Finke noted that his study was part of a multicentre trial and referenced an abstract that had Brammer 1990 (in Philpott‐Howard 1993), identified as a Pfizer employee, as a co‐author. Brammer also co‐authored the paper by Philpott‐Howard 1993, which had not included Finke among the authors and did not reference his trial report, but it mentioned Finke as a member of the Multicentre Study Group. We therefore excluded Finke's trial assuming it was a duplicate publication. A fourth trial report of 248 patients who received 'at least 2g daily' listed Brammer as the only author. This report was an interim analysis but it was not referenced by newer reports. Since its methodology was very similar to that of the other trials and several of the investigators were the same as in the Philpott‐Howard 1993 report, we suspected that the paper by Brammer 1990 represented a premature account of the trial that had Philpott‐Howard 1993 as first author. When we first published our meta‐analysis in JAMA (Johansen 1999), we acknowledged the uncertainty but chose to represent the two reports by Brammer 1990 and Philpott‐Howard 1993 as two separate trials. However, we have now found an abstract by Schuler 1992 (in Philpott‐Howard 1993) with Brammer as co‐author that was published two years later than the primary paper by Brammer 1990 and which also compared fluconazole with a combined 'polyene' group in the same doses as in Philpott‐Howard 1993. The abstract described 18 centres and 6 countries, as in the paper by Philpott‐Howard 1993, and 538 patients compared to 536 patients in Philpott‐Howard 1993. We are now convinced that the papers by Brammer 1990 and Philpott‐Howard 1993 represent the same trial. However, whether or not the paper by Brammer 1990 is included as a separate trial would make a trivial difference in our results.
The fifth 'polyene' trial report seemed to describe a unique study (Ninane 1994), but it nevertheless reported that patients at one of the centres had received amphotericin B and nystatin simultaneously rather than in separate treatment arms. Furthermore, a more recent publication by Groll 1997 (Ninane 1994) described a trial of 50 patients who were randomised to receive fluconazole or nystatin. This trial was two‐armed even though the article mentioned that 18 patients 'were in part included in a previously reported multicentre study'; which was the three‐armed study by Ninane 1994.
Thus, in reality only two 'polyene' trials had been performed (Ninane 1994; Philpott‐Howard 1993). These findings raise the question of whether some single‐centre trials had not originally been part of a multicentre trial but had been combined in the 'polyene' multicentre trial reports.
For the 2011 and 2014 updates no additional trials were identified for inclusion.
Risk of bias in included studies
We adopted broad quality assessment criteria and considered the randomisation method concealed if central randomisation, sealed envelopes, a code provided by a pharmacy or a company was described, or if the generation of the allocation sequence was adequate (for example random numbers) and the trial was blinded. Treatment allocation was adequately concealed in seven trials (Akiyama 1993; Anaissie 1996; Bodey 1994; Malik 1998; Marie 1993; Teshima 1994; Viscoli 1996); none of the trials were blinded.
Duration of follow‐up was stated in a minority of the trials; it probably varied for different patients within the same study as some authors stated that the drugs had been given until the neutropenia had resolved and others that follow‐up for death continued until hospital discharge (see table of Included studies).
Effects of interventions
Seventeen trials (3798 patients, 381 deaths) were included in the analyses. There was no statistically significant difference in mortality between fluconazole and amphotericin B. When amphotericin B was given intravenously, and fluconazole intravenously or orally, the RR was 0.84 (95% confidence interval (CI) 0.67 to 1.05); when they were given orally it was 0.99 (95% CI 0.69 to 1.42). When oral fluconazole was compared with oral 'polyenes' (see Description of studies above), the RR was 0.83 (95% CI 0.43 to 1.59) in the adjusted analysis. The adjustment changed the number of deaths on amphotericin B from 24 to 19 in the only 'polyene' study which reported on deaths (Ninane 1994).
There was no significant difference in deaths ascribed to fungal infections between fluconazole and amphotericin B. The RR was 1.04 (95% CI 0.58 to 1.87) for intravenous therapy and 0.72 (95% CI 0.26 to 2.01) for oral treatment.
There was no significant difference in invasive fungal infections between fluconazole and amphotericin B. The RR was 0.88 (95% CI 0.66 to 1.18) for intravenous therapy and 1.02 (95% CI 0.52 to 1.99) for oral treatment. When oral fluconazole was compared with oral 'polyenes', the RR was 1.41 (95% CI 0.45 to 4.40) in the adjusted analysis. The adjustment changed the number of fungal invasions from seven to four in one trial (Philpott‐Howard 1993) whereas it was unchanged in the other trial in which only one invasive fungal infection was reported (Ninane 1994).
There was no significant difference in colonisation between fluconazole and amphotericin B. When both drugs were given intravenously the RR was 1.41 (95% CI 0.76 to 2.62), whereas the RR was 0.57 (95% CI 0.28 to 1.18) when the drugs were given orally. No adjustments were necessary for the 'polyene' studies because they did not report data on colonisation.
There was significant heterogeneity in the use of rescue drug and dropouts. There was no overall tendency for more patients in the fluconazole group to receive a rescue drug, but definitions and practices varied so much that the summary estimates were not particularly meaningful. There was no significant difference in the total number of dropouts in the two groups. However, more patients dropped out on amphotericin B in studies comparing oral administration of the drugs (RR 0.30, 95% CI 0.12 to 0.74), whereas more patients dropped out on fluconazole in a 'polyene' trial (RR 2.71, 95% CI 1.01 to 7.30) (we used the interim report by Brammer 1990 (in Philpott‐Howard 1993) for this analysis as no such data were reported in the full trial report (Philpott‐Howard 1993)). The number of dropouts because of adverse effects was higher with amphotericin B (RR 0.23, 95% CI 0.10 to 0.55 with intravenous drug and RR 0.14, 95% CI 0.03 to 0.60 with oral drug).
The major harms were hepatic impairment and gastrointestinal adverse effects with fluconazole and infusion‐related toxicity, renal impairment and gastrointestinal adverse effects with amphotericin B (Table 1). As none of the trials were blinded, and as the definitions of toxicity based on laboratory values were variable and sometimes lacked clinical relevance, it was not possible to provide a meaningful overview of the harms with the two drugs. The number of patients who were withdrawn from treatment with fluconazole because of increases in liver enzymes was not very different from the number withdrawn from amphotericin B because of increases in serum creatinine. It should be noted, however, that five patients treated with amphotericin B underwent haemodialysis.
1. Other harms.
| Trial | Experimental drug | Control | Number of patients | Harms |
| Anaissie 1996 | Fluconazole: 400 mg/d iv | Amphotericin B: 25‐50 mg/d iv | 75 versus 67 | Chills or fever: 0 versus 3; Hepatic toxicity: 2 (1 withdrawn) versus 3; Rash: 0 versus 2; Nausea, vomiting or diarrhoea: 0 versus 2 |
| Bodey 1994 | Fluconazole: 400 mg/d orally | Amphotericin B: 0.2 mg/kg/d iv | 41 versus 36 | Doubling of S‐creatinine: 0 versus 4 (1 haemodialysis); Liver enzyme increase: 1 (patient withdrawn) versus 0; Nausea, vomiting: 2 versus 4; Diarrhoea: 4 versus 4 |
| Koh 2002 | Fluconazole: 200 mg/d orally | Amphotericin B: 0,2 mg/kg/d iv | 100 versus 86 | Renal dysfunction: 0 versus 3 (withdrawn); Liver dysfunction: 3 (withdrawn) versus 0; Increase in S‐creatinine to more than 1.5 times baseline: 13 versus 18 |
| Lake 1996 | Fluconazole: 200 mg/d orally | Amphotericin B: 0.3 mg/kg/d iv | 16 versus 15 | Vomiting: 2 (1 withdrawn) versus 2; Rigors/fever: 0 versus 5 |
| Malik 1998 | Fluconazole: 400 mg/d orally | Amphotericin B: 0.5 mg/kg/d iv | 52 versus 48 | Chills and fever: 1 versus 4; Rash: 1 versus 0; Bronchospasm: 0 versus 2; Nephrotoxicity: 3 versus 9 (but defined as an increase in S‐creatinine that was only 50% of baseline values) |
| Marie 1993 | Fluconazole: 400 mg/d iv | Amphotericin B: 0.5 mg/kg/d iv | 65 versus 66 | Rigors: 18 versus 1; Fever: 12 versus 1; Liver enzyme increase: 2 (withdrawn) versus 0; Renal impairment: 0 versus 3 (withdrawn) |
| Silling 1999 | Fluconazole: 5.7 mg/kg/d iv | Amphotericin B: 0.75 mg/kg/d iv + 5‐flucytosine 150 mg/kg/d iv | 51 versus 47 | Side effects suspected to be drug‐related: 19.6% versus 97.6% [sic] of the patients |
| Viscoli 1996 | Fluconazole: 6 mg/kg/d iv | Amphotericin B: 0.8 mg/kg/d iv | 56 versus 56 | Chills or fever: 0 versus 42; Nephrotoxicity: 7 versus 10 (3 withdrawn, one of whom underwent haemodialysis); Liver enzyme increase: 6 versus 10; Rash: 2 versus 2 |
| Winston 2000 | Fluconazole: 400 mg/d iv | Amphotericin B: 0.5 mg/kg/d iv | 158 versus 159 | Chills: 1 versus 79 (3 withdrawn); Renal impairment: 1 versus 53 (7 withdrawn); Liver dysfunction: 2 (1 withdrawn) versus 1 (withdrawn); Rash: 7 versus 1: Diarrhoea: 4 versus 0 |
| Wolff 2000 | Fluconazole: 400 mg/d orally | Amphotericin B: 0.2 mg/kg/d iv | 196 versus 159 | Toxicity leading to treatment discontinuation: 1 (rash) versus 20 (13 renal, 3 underwent haemodialysis) |
| Akiyama 1993 | Fluconazole: 200 mg/d orally | Amphotericin B: 2.4 g/d orally | 40 versus 36 episodes | Data not reliable, 99% of the patients received fluconazole 'without any difficulty', but there were only 51 patients in total in the trial |
| Menichetti 1994 | Fluconazole: 150 mg/d orally | Amphotericin B: 2 g/d orally | 420 versus 400 | Gastrointestinal disturbances: 3 versus 28; Liver enzyme increases: 2 versus 0; Rash: 1 versus 0 |
| Meunier 1991 | Fluconazole: 200 mg/d orally | Amphotericin B: 400 mg/d orally + 30 mg/d sucking | 30 versus 29 | Altered liver function tests: 10 versus 8 |
| Powles 1990 | Fluconazole: 200 mg/d orally | Amphotericin B: 800 mg orally and 40 mg lozenges, and nystatin 1.2 MIU mouthwash daily | No data | No data |
| Teshima 1994 | Fluconazole: 400 mg/d orally | Amphotericin B: 2.4 g/d orally | 53 versus 55 | Article in Japanese |
| Ninane 1994 | Fluconazole: 3 mg/kg/d orally | Amphotericin B: 25 mg/kg/d orally and/or nystatin 4 MIO IU orally | 236 versus 249 ('polyenes') | Possible drug‐related side effects: 38 (8 withdrawn) versus 21 (3 withdrawn); Gastrointestinal effects: 27 versus 16 |
| Philpott‐Howard 1993 | Fluconazole: 50 mg/d orally | Amphotericin B: 2 g/d orally and/or nystatin 4 MIO IU orally | 269 versus 267 ('polyenes') | Skin reactions: 6 versus 2; GI effects: 7 versus 12; Biliary system: 2 versus 0; Generalized reaction: 1 versus 0; Change in hepatic function: '12‐15% of patients in each group'; Change in renal function: 'about 3%' in each group. Treatment withdrawn due to abnormal laboratory values: 10 versus 0 |
Discussion
Fluconazole and amphotericin B appeared to have similar efficacy. However, the confidence intervals were wide and amphotericin B was disfavoured in several of the largest trials through their design or analysis, or both. It is noteworthy that intravenous amphotericin B is the only antifungal agent for which an effect on mortality has been shown. (Gøtzsche 2002a). In the placebo‐controlled trials with fluconazole there was no tendency towards such an effect (RR 1.04, 95% CI 0.84 to 1.30) (Gøtzsche 2002a). The lack of effect on mortality in the placebo‐controlled trials could be related to the adverse effects of fluconazole. Fluconazole has been associated with an excess of graft‐versus‐host disease or organ failure in the two large studies that have been conducted in bone marrow transplant recipients (Goodman 1992; Slavin 1995). Furthermore, an increased incidence of bacteraemia has been reported not only with fluconazole but also with ketoconazole and itraconazole (Gøtzsche 2002a). This adverse effect could, therefore, be a class effect related specifically to azoles. More patients dropped out because of adverse effects while receiving amphotericin B, but it is uncertain to what degree fluconazole is better tolerated. Since none of the trials were blinded, decisions on premature termination of trial therapy could have been biased, for example relatively minor increases in serum creatinine could have led to withdrawal of amphotericin B. In two trials, for example, nephrotoxicity was defined as an increase in serum creatinine of 0.4 mg/dl (Winston 2000) and 0.5 mg/dl (Malik 1998), but the average value at baseline was 1.0 mg/dl (SD 0.4 mg/dl) in one of them (Malik 1998). In another trial nephrotoxicity was defined as repeated serum potassium levels below 3.5 mmol/l (Bodey 1994). The advantages of fluconazole may be smaller than indicated in our review if amphotericin B is administered under optimal circumstances. Effective premedication to reduce infusion‐related toxicity of amphotericin B was only described for two trials, and even in those premedication was not given with the first dose (Viscoli 1996; Winston 2000). Infusion‐related toxicity can also be reduced by infusing amphotericin B over four hours (Ellis 1992), and further reduced if the infusion time is 24 hours (Eriksson 2001). The drug was infused slowly, over four to six hours, in three trials (Bodey 1994; Marie 1993; Viscoli 1996). It seems worthwhile to supplement the therapy with fluid, potassium and magnesium to prevent nephrotoxicity. In a study where 32 patients received sufficient substitution to cover the losses induced by a mean dose of amphotericin B of 0.9 mg/kg/d over 20 days, no increase in serum creatinine was observed (Mayer 1999). In another study only one patient out of 78 on conventional amphotericin B who received potassium supplements experienced a doubling of serum creatinine (Nath 1999). None of the trials we reviewed provided such supplements.
In previous versions of this review, we did not include death attributed to fungal infection as an outcome measure. It is difficult to determine the cause of death in these severely ill patients and we therefore suspected that disease‐specific mortality would be unreliable. As we could not confirm this suspicion in a study of disease‐specific mortality (Due 2006), we have now included this outcome.
We experienced unexpected difficulties in obtaining responses to our requests for additional or clarifying information about the trials. The problems we encountered have been described in detail elsewhere (Johansen 1999). We did not succeed in getting any information from the investigators or Pfizer, the manufacturer of fluconazole, on the most pertinent issues: why oral amphotericin B was used, why the results for this drug were lumped together with those of an ineffective drug (Gøtzsche 2002b), and whether there was overlap between the different trials reports. Support from Pfizer was specifically mentioned in 10 of the trial reports. No sources of support from industry or public sources were declared in the other seven trial reports, but in two of these we found other descriptions of the trials that listed support from Pfizer (see table Characteristics of included studies). This fact raises a suspicion that Pfizer might also have been involved with some of the remaining five trials. Two of the authors indicated that the data were with the company and two indicated that they did not have access to their own data because of change of affiliation (Johansen 1999). Hence, we suggest that trial investigators should keep copies of their study data.
Oral or intravenous amphotericin B?
Most control group patients were given oral amphotericin B, which is poorly absorbed and poorly documented, and moreover is not recommended for prophylaxis (Working Party 1995). Although most fungal infections enter the body through the gastrointestinal tract, and oral amphotericin B eliminates fungi from the intestines in most patients (Bodey 1974; Meunier 1991), there is little documentation of its clinical effect when given orally; it would not be expected to be of much value once the fungi had found their way into the bloodstream. None of the seven trial reports using oral administration offered a rationale for this decision. This is in contrast to the placebo‐controlled trials of amphotericin B in which intravenous drug was given to most of the patients (Gøtzsche 2002a; Johansen 1999).
Biased and opaque reporting
We find it remarkable that the rationale for combining the results for amphotericin B and nystatin in a 'polyene' group was not explained in any of the many reports of the three‐armed trials. The relationship between these trials and the boundaries between them were obscure. For example, the dose of amphotericin B was not the same in various publications of the same trial. Furthermore, data from a two‐armed trial (Groll 1997) were used in a report of a three‐armed trial (Ninane 1994), which suggests that patients in the three‐armed trial may not have been truly randomised to three arms, although the trial was described as such.
When the trials were planned and conducted, nystatin was recognized as being an ineffective drug for invasive fungal infection. For example, Brammer, a Pfizer employee who was also the author of one of the 'polyene' trial reports (Philpott‐Howard 1993) that was the first published comparison of fluconazole and amphotericin B, wrote that "there has been no convincing evidence to demonstrate that (oral preparations of nystatin and amphotericin B) reduce the incidence of systemic invasion by Candida yeasts". Nystatin is almost insoluble and it is not recommended for use in cancer patients with neutropenia (Working Party 1995).
Since the effect of nystatin was similar to that of placebo when given to patients with severe immunodeficiency, such as those with cancer complicated by neutropenia (Johansen 1999; Gøtzsche 2002b), the 'polyene' trials seem to be biased in favour of fluconazole. Another indication that oral amphotericin B and nystatin are not the same is shown by the number of dropouts due to adverse effects. More patients dropped out while taking oral amphotericin B than fluconazole, whereas the opposite was noted for oral 'polyenes'; the difference between the two relative risks is significant (P value < 0.01, normal approximation).
The bias could be more pronounced than we found. For example, in a meta‐analysis presented at an international congress (Bow 1997), azoles (mainly fluconazole) were reported to be considerably and significantly better than 'polyenes', with odds ratios of 0.59 (95% CI 0.40 to 0.87) for reducing superficial fungal infection and 0.44 (95% CI 0.27 to 0.71) for reducing definite invasive infection. This meta‐analysis had a Pfizer associate among the authors, so it would have been possible to report the results for amphotericin B separately. We presented the problems we encountered in the trials sponsored by Pfizer at the same congress, at the same session, immediately after the presentation by Bow (Johansen 1997). We are therefore surprised that Bow later published a meta‐analysis (Bow 2002) with the same Pfizer associate as co‐author that included not only the two reports by Philpott‐Howard and Brammer as if they were two separate trials, which is very unlikely, but also the report by Rozenberg Arska which definitely was not a separate trial but a report from one of the centres that was included in the multicentre trial report (Philpott‐Howard 1993). Thus, although the sponsoring company was involved, this meta‐analysis included the same patients more than once. It also included at least one trial, by Schaison, that was not randomised (Gøtzsche 1997; Gøtzsche 2002a) although it stated that only randomised trials were included.
Another example of discrepant meta‐analyses has been encountered for a new antidepressant drug, where independent researchers did not find an advantage over existing drugs (P = 0.4) (Smith 1998; Song 1993;) in contrast to a meta‐analysis published by a company employee in response to the first meta‐analysis (P < 0.001) (Nakielny 1993). However, the second meta‐analysis contained fewer patients than the first, had less power, and included unpublished 'data on file', which are usually less favourable for new treatments than published ones (Hemminki 1980; Stern 1997). As illustrated by this example and by our own meta‐analysis, we believe readers should be sceptical about meta‐analyses and trials that have involved the company marketing the drug.
Our experiences with the fluconazole trials may create mixed feelings for the patients and may detract from their willingness to volunteer for clinical trials. Patients generally participate in clinical research for the benefit of future patients and they have a right to expect that the results can be accessed, trusted and openly discussed. It would be valuable for the patients if investigators, institutions and pharmaceutical companies were more helpful to those conducting meta‐analyses and also gave access to the trial data, which should be regarded as public, not private, property.
Authors' conclusions
Implications for practice.
The relative merits of fluconazole compared with amphotericin B cannot be judged based on the available trials. Intravenous amphotericin B should therefore be preferred for prophylactic or empirical antifungal therapy in cancer patients with neutropenia as it is the only antifungal agent for which an effect on mortality has been shown.
Implications for research.
A large trial, with several hundred deaths, that compares fluconazole with intravenous amphotericin B, either as standard drug or as a lipid soluble form to reduce the adverse effects, could be worthwhile. Given the many problems with the fluconazole trials performed previously, such a trial should be planned, executed and analysed independently of sponsors. To reduce the risk of biased meta‐analyses, we recommend that trial investigators keep copies of their data.
What's new
| Date | Event | Description |
|---|---|---|
| 8 March 2017 | Review declared as stable | Intervention superseded and is no longer in general use. |
History
Protocol first published: Issue 2, 1997 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 7 July 2014 | New search has been performed | Literature searches updated |
| 7 July 2014 | New citation required but conclusions have not changed | No new trials identified for inclusion |
| 14 September 2011 | New search has been performed | Review updated with new search details. No new studies were identified for inclusion. |
| 18 July 2011 | Amended | Searches re‐run July 2011. |
| 5 February 2008 | New citation required but conclusions have not changed | New studies sought but none found: 05/11/07. Two new outcomes added in November 2007 (death ascribed to fungal infection and harms). |
| 9 December 2004 | Amended | New studies found and included or excluded: 09/12/04 |
| 20 January 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank the following investigators for additional information on their trials: Dr Hideki Akiyama, Dr Gerald B Bodey, Dr V Lapierre, Dr Imtiaz Malik, Dr H Teshima, Dr Claudio Viscoli and Dr Drew J Winston. We are grateful for the translation of the Japanese article provided by Dr Hiroto Takada and the help from the German Cochrane Centre in locating German authors.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Gynaecological Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. PubMed search strategy
#1: random* OR control* OR blind* #2: nystatin OR amphotericin OR fluconazol* OR itraconazol* OR ketoconazol* OR miconazol* OR voriconazol* #3: bone‐marrow OR cancer* OR fungemia OR hematologic* OR malignan* OR neoplas* OR neutropeni* OR granulocytopeni* OR leukemi* OR lymphom* #4: #1 AND #2 AND #3.
Data and analyses
Comparison 1. Fluconazole versus amphotericin B.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death | 15 | 3151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.73, 1.05] |
| 1.1 I.v. or oral fluconazole vs i.v. amphotericin B | 10 | 1586 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.67, 1.05] |
| 1.2 Oral fluconazole vs oral amphotericin B | 4 | 1063 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.69, 1.42] |
| 1.3 Oral fluconazole vs oral "polyenes" | 1 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.43, 1.59] |
| 2 Death ascribed to fungal infection | 10 | 2279 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.57, 1.58] |
| 2.1 I.v. or oral fluconazole vs i.v. amphotericin B | 7 | 1324 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.58, 1.87] |
| 2.2 Oral fluconazole vs oral amphotericin B | 3 | 955 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.26, 2.01] |
| 3 Invasive infections | 15 | 3587 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.72, 1.21] |
| 3.1 I.v. or oral fluconazole vs i.v. amphotericin B | 9 | 1486 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.66, 1.18] |
| 3.2 Oral fluconazole vs oral amphotericin B | 4 | 1063 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.52, 1.99] |
| 3.3 Oral fluconazole vs oral "polyenes" | 2 | 1038 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.45, 4.40] |
| 4 Colonisation | 4 | 1132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.59, 1.47] |
| 4.1 I.v. or oral fluconazole vs i.v. amphotericin B | 2 | 204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.76, 2.62] |
| 4.2 Oral fluconazole vs oral amphotericin B | 2 | 928 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.28, 1.18] |
| 5 Use of rescue drug | 9 | 2616 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.80, 1.41] |
| 5.1 I.v. or oral fluconazole vs i.v. amphotericin B | 6 | 1201 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.76, 2.37] |
| 5.2 Oral fluconazole vs oral amphotericin B | 2 | 879 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.62, 1.06] |
| 5.3 Oral fluconazole vs oral "polyenes" | 1 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.76, 1.43] |
| 6 Dropouts | 8 | 1122 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.44, 1.29] |
| 6.1 I.v. or oral fluconazole vs i.v. amphotericin B | 4 | 631 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.69, 1.30] |
| 6.2 Oral fluconazole vs oral amphotericin B | 3 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.12, 0.74] |
| 6.3 Oral fluconazole vs oral "polyenes" | 1 | 248 | Risk Ratio (M‐H, Random, 95% CI) | 2.71 [1.01, 7.30] |
| 7 Dropouts because of adverse effects | 14 | 3489 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.14, 0.78] |
| 7.1 I.v. or oral fluconazole vs i.v. amphotericin B | 8 | 1388 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.10, 0.55] |
| 7.2 Oral fluconazole vs oral amphotericin B | 4 | 1063 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.03, 0.60] |
| 7.3 Oral fluconazole vs oral "polyenes" | 2 | 1038 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.51, 6.04] |
1.1. Analysis.
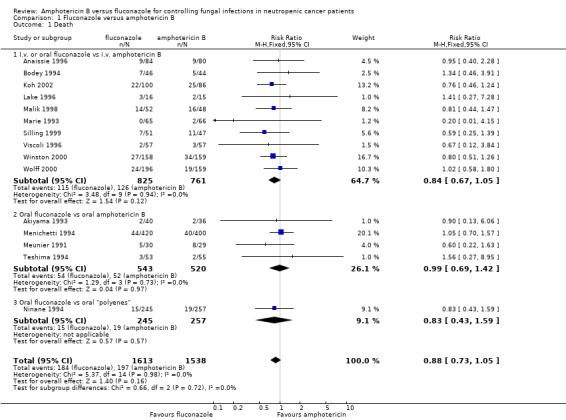
Comparison 1 Fluconazole versus amphotericin B, Outcome 1 Death.
1.2. Analysis.
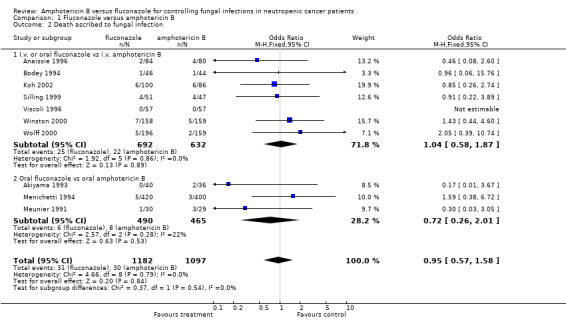
Comparison 1 Fluconazole versus amphotericin B, Outcome 2 Death ascribed to fungal infection.
1.3. Analysis.
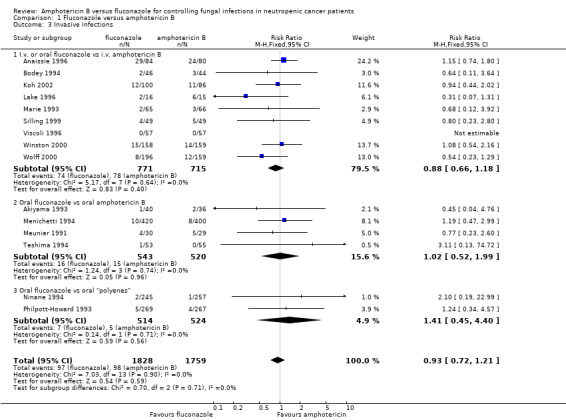
Comparison 1 Fluconazole versus amphotericin B, Outcome 3 Invasive infections.
1.4. Analysis.
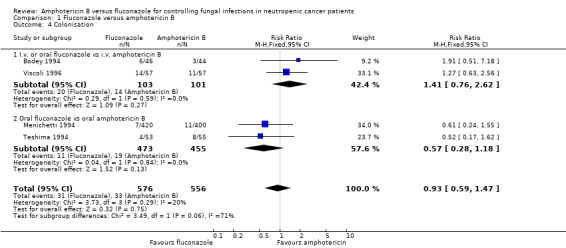
Comparison 1 Fluconazole versus amphotericin B, Outcome 4 Colonisation.
1.5. Analysis.
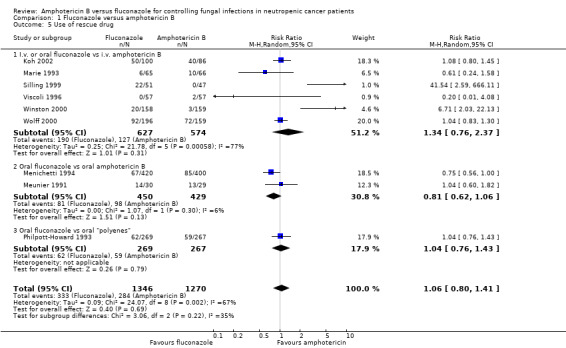
Comparison 1 Fluconazole versus amphotericin B, Outcome 5 Use of rescue drug.
1.6. Analysis.
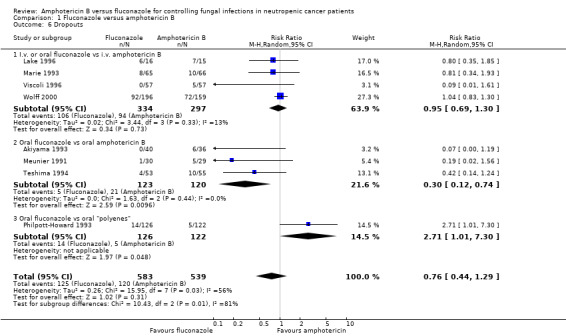
Comparison 1 Fluconazole versus amphotericin B, Outcome 6 Dropouts.
1.7. Analysis.
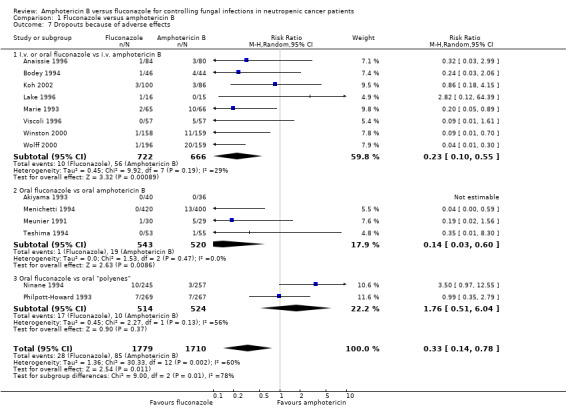
Comparison 1 Fluconazole versus amphotericin B, Outcome 7 Dropouts because of adverse effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akiyama 1993.
| Methods | Allocation concealment: sealed envelopes Blinding of study: no Support: Pfizer | |
| Participants | 51 participated total Excluded: NA Acute leukaemia, prophylactic | |
| Interventions | Fluconazole: 200 mg/d orally Amphotericin B: 2400 mg/d orally | |
| Outcomes | Death Infections Use of escape drug | |
| Notes | Follow‐up period (days): NA Days on fluconazole: 18 Days on amphotericin B: 17 Support: Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
Anaissie 1996.
| Methods | Allocation concealment: computer generated sealed consecutively numbered envelopes Blinding of study: no Support: Pfizer | |
| Participants | 164 participants total Excluded: 22 Other cancer, empirically | |
| Interventions | Fluconazole: 400 mg/d iv Amphotericin B: 25‐50 mg/d iv | |
| Outcomes | Death Infections Number of dropouts because of adverse effects | |
| Notes | Follow‐up period (days): NA Days on fluconazole: 11 Days on amphotericin B: 11 Support: Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
Bodey 1994.
| Methods | Allocation concealment: pharmacy Blinding of study: no Support: Pfizer | |
| Participants | 90 participated total Excluded: 13 Acute leukaemia, prophylactic | |
| Interventions | Fluconazole: 400 mg/d orally Amphotericin B: 0.2 mg/kg/d iv | |
| Outcomes | Death Infections Colonisation Use of escape drug Dropouts because of adverse effects | |
| Notes | Follow‐up period (days): 70 Days on fluconazole: 24 Days on amphotericin B: 19 Support: Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
Koh 2002.
| Methods | Allocation concealment: NA Blinding of study: no Support: none declared | |
| Participants | 186 participants total Excluded: none Bone marrow transplantation, prophylactic | |
| Interventions | Fluconazole: 200 mg/d orally Amphotericin B: 0.2 mg/kg/d iv, maximum dose 10 mg | |
| Outcomes | Death Infections Use of escape drug Dropouts because of adverse effects | |
| Notes | Follow‐up period (days): 100 Days on fluconazole 20. Days on amphotericin B: 20 Support: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
Lake 1996.
| Methods | Allocation concealment: NA Blinding of study: no Support: Pfizer (not apparent from the trial report, but was listed in a conference abstract) | |
| Participants | 31 participants total Excluded: 6 Oesophageal candidiasis, treatment | |
| Interventions | Fluconazole: 200 mg/d orally for 7 days Amphotericin B: 0.3 mg/kg/d iv | |
| Outcomes | Death Infections Dropouts because of adverse effects | |
| Notes | Follow‐up period (days): 4 weeks Days on fluconazole: 19 Days on amphotericin B: 12 Support: an author is from Pfizer; this is apparent from an abstract but is not mentioned in the final publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
Malik 1998.
| Methods | Allocation concealment: consecutive sealed envelopes Blinding of study: no Support: Pfizer | |
| Participants | 106 participants total Excluded: 6 Leukaemia, empirically | |
| Interventions | Fluconazole: 400 mg/d orally Amphotericin B: 0.5 mg/kg/d iv | |
| Outcomes | Death | |
| Notes | Follow‐up period (days): till resolution of neutropenia Days on fluconazole: NA Days on amphotericin B: NA Support: two authors from Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
Marie 1993.
| Methods | Allocation concealment: computer generated sealed envelopes Blinding of study: no Support: Pfizer | |
| Participants | 131 participants total Excluded: none Acute leukaemia, empirically | |
| Interventions | Fluconazole: 400 mg/d iv Amphotericin B: 0.5 mg/kg/d iv | |
| Outcomes | Death Infections Use of escape drug Dropouts because of adverse effects | |
| Notes | Follow‐up period (days): until 1 week after treatment cessation Days on fluconazole: 13.7 Days on amphotericin B: 14.7 Support: Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
Menichetti 1994.
| Methods | Allocation concealment: NA Blinding of study: no Support: Pfizer and Italian Medical Research Council | |
| Participants | 820 participated total Excluded 16 Acute leukaemia, prophylactic | |
| Interventions | Fluconazole: 150 mg/d orally Amphotericin B: 2000 mg/d orally | |
| Outcomes | Death Infections Colonisation Use of escape drug Dropouts because of adverse effects | |
| Notes | Follow‐up period (days): NA Days on fluconazole: 26 Days on amphotericin B: 25 Support: Pfizer and National Research Council | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
Meunier 1991.
| Methods | Allocation concealment: NA Blinding of study: no Support: Pfizer | |
| Participants | 59 participated total Excluded: NA Acute leukaemia, prophylactic | |
| Interventions | Fluconazole: 200 mg/d orally Amphotericin B: 400 mg/d orally + 30 mg/d sucking | |
| Outcomes | Death Infections Use of escape drug Dropouts because of adverse effects | |
| Notes | Follow‐up period (days): 60 Days on fluconazole: 16 Days on amphotericin B: 16.5 Support: Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
Ninane 1994.
| Methods | Allocation concealment: NA Blinding of study: no Support: Pfizer (not apparent from the trial report, but from another report describing the same trial, see Discussion) | |
| Participants | 502 participated total Excluded: NA Acute leukaemia, prophylactic | |
| Interventions | Fluconazole: 3 mg/kg/d orally Amphotericin B: 25 mg/kg/d orally and/or nystatin 4 MIO IU orally | |
| Outcomes | Death Infections Dropouts because of adverse effects | |
| Notes | Follow‐up period (days): 6 weeks Days on fluconazole: 27.8 Days on amphotericin B: NA Support: Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
Philpott‐Howard 1993.
| Methods | Allocation concealment: NA Blinding of study: no Support: Pfizer | |
| Participants | 536 participated total Excluded: 25 Acute leukaemia, prophylactic | |
| Interventions | Fluconazole: 50 mg/d orally Amphotericin B: 2000 mg/d orally and/or nystatin 4 MIO IU orally | |
| Outcomes | Infections Use of escape drug Dropouts because of adverse effects | |
| Notes | Follow‐up period (days): NA Days on fluconazole: 29.3 Days on amphotericin B: 31.3 Support: Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
Powles 1990.
| Methods | Allocation concealment: NA Blinding of study: no Support: none declared | |
| Participants | 100 participated total Excluded: NA Bone marrow transplantation, prophylactic | |
| Interventions | Fluconazole: 200 mg/d orally Amphotericin B: 800 mg/d orally and 10 mg lozenges 4 times daily, and nystatin 200,000 IU mouthwash 6 times daily | |
| Outcomes | Positive culture Use of escape drug | |
| Notes | Published as an abstract, no outcome data Support: NA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
Silling 1999.
| Methods | Allocation concealment: NA Blinding of study: NA Support: none declared | |
| Participants | 98 participated total Excluded: none Acute leukaemia, empirically | |
| Interventions | Fluconazole: 5.7 mg/kg/d iv. Amphotericin B: 0.75 mg/kg/d iv + 5‐flucytosine 150 mg/kg/d iv | |
| Outcomes | Death Infections Use of escape drug | |
| Notes | Follow‐up period (days): NA Days on fluconazole: NA Days on amphotericin B: NA Support: none declared Numerous problems, e.g. numbers of deaths described as 20, 19 and 18 in various publications; and number of randomised patients as 49 versus 49 and as 51 versus 47; no variables described under methods; one‐sided testing despite being a comparison of two active regimens | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Teshima 1994.
| Methods | Allocation concealment: sealed opaque envelopes Blinding of study: no Support: none declared | |
| Participants | 108 participated total Excluded: 3 Bone marrow transplantation, prophylactic | |
| Interventions | Fluconazole: 400 mg/d orally Amphotericin B: 2400 mg/d orally | |
| Outcomes | Death Infections Colonisation Dropouts because of adverse effects | |
| Notes | Follow‐up period (days): NA Days on fluconazole: 32.8 Days on amphotericin B: 33.4 Support: none declared | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
Viscoli 1996.
| Methods | Allocation concealment: computer generated, sealed envelopes Blinding of study: no Support: Pfizer and Italian Medical Research Council | |
| Participants | 114 participated total Excluded: 2 Bone marrow transplantation, empirically | |
| Interventions | Fluconazole: 6 mg/kg/d iv Amphotericin B: 0.8 mg/kg/d iv max 400 mg | |
| Outcomes | Death Infections Colonisation Use of escape drug Dropouts because of adverse effects | |
| Notes | Follow‐up period (days): 30 Days on fluconazole: 13 Days on amphotericin B: 10 Support: Pfizer and National Research Council | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
Winston 2000.
| Methods | Allocation concealment: computer generated, treatment assignment by pharmacy at each site Blinding of study: no Support: Pfizer | |
| Participants | 322 participated total Excluded: 5 Acute leukaemia, empirically | |
| Interventions | Fluconazole: 400 mg/d iv Amphotericin B: 0.5 mg/kg/d iv | |
| Outcomes | Death Infections Use of escape drug Dropouts because of adverse effects | |
| Notes | Follow‐up period (days): 30 Days on fluconazole: 8 Days on amphotericin B: 10 Support: Pfizer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
Wolff 2000.
| Methods | Allocation concealment: NA Blinding of study: no Support: none declared | |
| Participants | 355 participated total Excluded: none Bone marrow transplantation, prophylactic | |
| Interventions | Fluconazole: 400 mg/d orally Amphotericin B: 0.2 mg/kg/d iv | |
| Outcomes | Death Infections Colonisation Use of escape drug Dropouts because of adverse effects | |
| Notes | Follow‐up period (days): till hospital discharge (but only deaths during therapy are reported) Days on fluconazole: 13 Days on amphotericin B: 12 Support: none declared There was imbalance in number of patients in the two treatment arms in one centre; not clear how this was possible Colonisation data only for 73% of the patients; therefore not used in efficacy analysis. Three patients on amphotericin B underwent dialysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
NA: not available
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ellis 1995 | Only 22 of the 41 patients had been randomised |
| Reed 1993 | Only published as abstract, 96 patients randomised to fluconazole, amphotericin B or no treatment. No fungal disease was documented in any group, colonisation data insufficient for inclusion in the review |
| Timmers 2000 | Study stopped after 24 patients, 11 of 12 patients on amphotericin B colloidal dispersion (ABCD or Amphocil) experienced infusion‐related toxicity. No proven fungal infections occurred |
Differences between protocol and review
We have added harms and total number of dropouts, and also death ascribed to fungal infection as our research has shown that this outcome is reliable (Due 2006), contrary to our expectations.
Contributions of authors
HKJ wrote the draft meta‐analysis protocol; HKJ and PG contributed equally to selection of trials, data extraction and writing of the manuscript. Guarantors: both authors.
Sources of support
Internal sources
Rigshospitalet, Denmark.
External sources
JASCHA‐fonden, Denmark.
The Swedish Society of Medicine, Sweden.
Sygekassernes Helsefond, Denmark.
Nordic Council of Ministers, Denmark.
Declarations of interest
Helle Krogh Johansen ‐ nothing to declare Peter C Gøtzsche ‐ nothing to declare
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Akiyama 1993 {published and unpublished data}
- Akiyama H, Mori S, Tanikawa S, Sakamaki H, Onozawa Y. Fluconazole versus oral amphotericin B in preventing fungal infection in chemotherapy‐induced neutropenic patients with haematological malignancies. Mycoses 1993;36:373‐8. [DOI] [PubMed] [Google Scholar]
Anaissie 1996 {published data only}
- Anaissie EJ, Darouiche RO, Abi Said D, Uzun O, Mera J, Gentry LO, et al. Management of invasive candidal infections: results of a prospective, randomized, multicenter study of fluconazole versus amphotericin B and review of the literature. Clinical Infectious Diseases 1996;23:964‐72. [DOI] [PubMed] [Google Scholar]
Bodey 1994 {published and unpublished data}
- Bodey GP, Anaissie EJ, Elting LS, Estey E, O'Brien S, Kantarjian H. Antifungal prophylaxis during remission induction therapy for acute leukemia fluconazole versus intravenous amphotericin B. Cancer 1994;73:2099‐106. [DOI] [PubMed] [Google Scholar]
Koh 2002 {published data only}
- Koh LP, Kurup A, Goh YT, Fook‐Chong SM, Tan PH. Randomized trial of fluconazole versus low‐dose amphotericin B in prophylaxis against fungal infections in patients undergoing hematopoietic stem cell transplantation. American Journal of Hematology 2002;71(4):260‐7. [DOI] [PubMed] [Google Scholar]
Lake 1996 {published data only}
- Lake D, Kunzweiler J. A randomized trial of fluconazole vs. amphotericin B for the treatment of esophageal candidiasis in the adult cancer patient. Proceedings of the Annual Meeting of American Society for Clinical Oncology. 1991:344 (abstract 1222).
- Lake DE, Kunzweiler J, Beer M, Buell DN, Islam MZ. Fluconazole versus amphotericin B in the treatment of esophageal candidiasis in cancer patients. Chemotherapy 1996;42:308‐14. [DOI] [PubMed] [Google Scholar]
Malik 1998 {published and unpublished data}
- Malik IA, Moid I, Aziz Z, Khan S, Suleman M. A randomized comparison of fluconazole with amphotericin B as empiric anti‐fungal agents in cancer patients with prolonged fever and neutropenia. American Journal of Medicine 1998;105(6):478‐83. [DOI] [PubMed] [Google Scholar]
Marie 1993 {published and unpublished data}
- Lapierre V, Marie J‐P, Pico JL, Molina A, Andremont A, Lagrange P. Intravenous fluconazole (Flu) versus intravenous amphotericin B (AmB) in the management of febrile neutropenic patients: a multicentric randomized study. 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy, October 1992, Anaheim, California. 1992:214 (abstract 624).
- Marie JP, Lapierre V, Pico J, Vekhoff A, Molina L, Andremont A, et al. Etude multicentrique randomisée fluconazole iv versus amphotéricine B iv chez le patient neutropénique et fébrile. Cahiers d'Oncologie 1993;2:171‐3. [Google Scholar]
- Viscoli C. European studies of fluconazole (F) vs amphotericin B (AmB) in febrile neutropenic cancer patients. 7th European Congress of Clinical Microbiology and Infectious Diseases, March 26 to March 30, 1995, Vienna, Austria. 1995:14 (abstract 70).
Menichetti 1994 {published data only}
- Menichetti F, Favero A, Martino P, Bucaneve G, Micozzi A, D'Antonio D, et al. Preventing fungal infection in neutropenic patients with acute leukemia: fluconazole compared with oral amphotericin B. The GIMEMA Infection Program. Annals of Internal Medicine 1994;120:913‐8. [DOI] [PubMed] [Google Scholar]
Meunier 1991 {published data only}
- Meunier F, Aoun M, Janssens M, Dekoster C, Paesmans M. Chemoprophylaxis of fungal infections in granulocytopenic patients using fluconazole vs oral amphotericin B. Drug Investigation 1991;3:258‐65. [Google Scholar]
Ninane 1994 {published data only}
- Groll AH, Just‐Nuebling G, Kurz M, et al. Fluconazole versus nystatin in the prevention of candida infections in children and adolescents undergoing remission induction or consolidation chemotherapy for cancer. Journal of Antimicrobial Chemotherapy 1997;40:855‐62. [DOI] [PubMed] [Google Scholar]
- Ninane J. A multicentre study of fluconazole versus oral polyenes in the prevention of fungal infection in children with hematological or oncological malignancies. Multicentre Study Group. European Journal of Clinical Microbiology and Infectious Diseases 1994;13:330‐7. [DOI] [PubMed] [Google Scholar]
Philpott‐Howard 1993 {published data only}
- Brammer KW. Management of fungal infection in neutropenic patients with fluconazole. Haematology and Blood Transfusion 1990;33:546‐50. [DOI] [PubMed] [Google Scholar]
- Finke R. [Comparison of oral fluconazole and amphotericin B prophylaxis against fungal infections in the neutropenic phase of patients treated with antileukemic agents]. Mycoses 1990;33 Suppl 1:42‐54. [PubMed] [Google Scholar]
- Philpott‐Howard JN, Wade JJ, Mufti GJ, Brammer KW, Ehninger G. Randomized comparison of oral fluconazole versus oral polyenes for the prevention of fungal infection in patients at risk of neutropenia. Multicentre Study Group. Journal of Antimicrobial Chemotherapy 1993;31(6):973‐84. [DOI] [PubMed] [Google Scholar]
- Rozenberg‐Arska M, Dekker AW, Branger J, Verhoef J. A randomized study to compare oral fluconazole to amphotericin B in the prevention of fungal infections in patients with acute leukaemia. Journal of Antimicrobial Chemotherapy 1991;27(3):369‐76. [DOI] [PubMed] [Google Scholar]
- Schuler U, Ehninger G, Brammer K. Oral fluconazole (F) vs oral polyenes (P) as antimycotic prophylaxis in neutropenia: an European multicenter study. Annals of Hematology 1992;65:A120, abstract 356. [Google Scholar]
Powles 1990 {published data only}
- Milliken S, Gore M, Powles R, Harding M, Jameson B, Talbot D, et al. Fluconazole versus polyenes as oral anti‐fungal prophylaxis in autologous and allogeneic bone marrow recipients. Bone Marrow Transplantation 1989;4 Suppl 2:27 (abstract). [Google Scholar]
- Powles RL, Smith C, Treleaven J, Tiley C, Helenglass G. Antifungal prophylaxis with fluconazole in bone marrow transplants. Bone Marrow Transplantation 1990;5 Suppl 2:135, abstract 291. [Google Scholar]
Silling 1999 {published data only}
- Silling G, Fegeler W, Roos N, Essink M, Büchner T. Early empiric antifungal therapy of infections in neutropenic patients comparing fluconazole with amphotericin B/flucytosine. Mycoses 1999;42 Suppl 2:101‐4. [DOI] [PubMed] [Google Scholar]
- Silling G, Fegeler W, Roos N, Schomaker R, Essink M, Büchner T. Early empiric antifungal therapy of infections in neutropenic patients comparing fluconazole with amphotericin B/5‐fluorocytosine. Mycoses 1999;42(3):159. [DOI] [PubMed] [Google Scholar]
- Silling G, Fegeler W, Roos N, Schomaker R, Essink M, Büchner T. Early empiric antifungal therapy of infections in neutropenic patients with hematologic malignancies: fluconazole vs ampho B/5‐FC. Annals of Hematology 1999;78 Suppl 1:S2, abstract 8. [Google Scholar]
- Silling G, Fegeler W, Roos N, Schomaker R, Essink M, Büchner T. Fluconazole (FCA) versus amphotericin B/5‐flucytosine (ABF) for the treatment of febrile neutropenia. Annals of Hematology 1995;70 Suppl 1:A101, abstract 28. [Google Scholar]
- Silling‐Engelhardt G, Fegeler W, Roos N, Schomaker R, Essink M, Büchner T. Early empiric antifungal treatment of infections in neutropenic patients comparing fluconazole with amphotericin B/5‐flucytosine. Blood 1994;10 Suppl 1:306a. [Google Scholar]
- Silling‐Engelhardt G, Fegeler W, Roos N, Schwarz C, Essink M, Hiddeman W, et al. Interventional treatment of unexplained fever (FUO) and documented infections in neutropenic patients with hematologic malignancies: fluconazole versus ampho B/5‐FC. Annals of Hematology 1992;64:A110, abstract 147. [Google Scholar]
Teshima 1994 {published and unpublished data}
- Teshima H, Masaoka T, Hiraoka A, Miyazaki T, Imarmura M, Kitabayashi A. A randomised study to compare oral fluconazole with oral amphotericin B for suppression of fungal flora and prevention of fungal infection in bone marrow transplantation recipients. Drug Evaluation (Konnichi No Ishoku) 1994;7:535‐42. [Google Scholar]
Viscoli 1996 {published and unpublished data}
- Viscoli C, Castagnola E, Lint MT, Moroni C, Garaventa A, Rossi MR, et al. Fluconazole versus amphotericin B as empirical antifungal therapy of unexplained fever in granulocytopenic cancer patients: a pragmatic, multicentre, prospective and randomised clinical trial. European Journal of Cancer 1996;32A(5):814‐20. [DOI] [PubMed] [Google Scholar]
Winston 2000 {published and unpublished data}
- Winston DJ, Hathorn J, Schuster M. Fluconazole versus amphotericin B for empiric antifungal therapy of febrile neutropenic patients: results of a randomised multicenter trial. 20th International Congress of Chemotherapy, June 29 to July 3, 1997, Sydney, Australia. 1997:87 (abstract 3243).
- Winston DJ, Hathorn J, Schuster M. Fluconazole versus amphotericin B for empiric antifungal therapy of febrile neutropenic patients: results of a randomized multi‐center trial. 10th International Symposium on Infections in the Immunocomprised Host, June 21‐24, 1998, Davos, Switzerland. 1998:abstract no 112.
- Winston DJ, Hathorn JW, Schuster MG, Schiller GJ, Territo MC. A multicenter, randomized trial of fluconazole versus amphotericin B for empiric antifungal therapy of febrile neutropenic patients with cancer. American Journal of Medicine 2000;108:282‐9. [DOI] [PubMed] [Google Scholar]
Wolff 2000 {published data only}
- Wolff SN, Fay J, Stevens D, Herzig RH, Pohlman B, Bolwell B, et al. Fluconazole vs low‐dose amphotericin B for the prevention of fungal infections in patients undergoing bone marrow transplantation: a study of the North American Marrow Transplant Group. Bone Marrow Transplantation 2000;25(8):853‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ellis 1995 {published data only}
- Ellis ME, Halim MA, Spence D, Ernst P, Clink H, Kalin M, et al. Systemic amphotericin B versus fluconazole in the management of antibiotic resistant neutropenic fever ‐ preliminary observations from a pilot, exploratory study. Journal of Infection 1995;30:141‐6. [DOI] [PubMed] [Google Scholar]
Reed 1993 {published data only}
- Reed E, Rasmussen J, Roberson P, Armitage J, Bierman P, Vose J, et al. Interim analysis of fluconazole (flu), intermittent amphotericin (ampho) and no treatment (ntx) for prophylaxis of fungal disease in bone marrow transplant (BMT) and acute leukemia (AL) patients (pts). Proceedings of the Annual Meeting of American Society for Clinical Oncology. 1993:465 (abstract A1622).
Timmers 2000 {published data only}
- Timmers GJ, Zweegman S, Simoons Smit AM, Loenen AC, Touw D, Huijgens PC. Amphotericin B colloidal dispersion (Amphocil) vs fluconazole for the prevention of fungal infections in neutropenic patients: data of a prematurely stopped clinical trial. Bone Marrow Transplantation 2000;25(8):879‐84. [DOI] [PubMed] [Google Scholar]
Additional references
Bodey 1974
- Bodey GP, Rosenbaum B. Effect of prophylactic measures on the microbial flora of patients in protected environment units. Medicine 1974;53:209‐28. [DOI] [PubMed] [Google Scholar]
Bow 1997
- Bow EJ, Laverdiere M, Lussier N, Rotstein C, Ioannou S. Antifungal prophylaxis in neutropenic cancer patients ‐ a meta‐analysis. 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, September 28 to October 1, 1997, Toronto, Ontario, Canada. 1997:abstract no LM‐88.
Bow 2002
- Bow EJ, Laverdiere M, Lussier N, Rotstein C, Cheang MS, Ioannou S. Antifungal prophylaxis for severely neutropenic chemotherapy recipients: a meta analysis of randomized‐controlled clinical trials. Cancer 2002;94:3230‐46. [DOI] [PubMed] [Google Scholar]
Due 2006
- Due AK, Johansen HK, Gøtzsche PC. Fungal infection‐related mortality versus total mortality as an outcome in trials of antifungal agents. BioMed Central Medical Research Methodology 2006;6:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ellis 1992
- Ellis ME, al‐Hokail AA, Clink HM, Padmos MA, Ernst P, Spence DG, et al. Double‐blind randomized study of the effect of infusion rates on toxicity of amphotericin B. Antimicrobial Agents and Chemotherapy 1992;36:172‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eriksson 2001
- Eriksson U, Seifert B, Schaffner A. Comparison of effects of amphotericin B deoxycholate infused over 4 or 24 hours: randomised controlled trial. BMJ 2001;322(7286):579‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodman 1992
- Goodman JL, Winston DJ, Greenfield RA, Chandrasekar PH, Fox B, Kaizer H, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. New England Journal of Medicine 1992;326(13):845‐51. [DOI] [PubMed] [Google Scholar]
Groll 1997
- Groll AH, Just‐Nuebling G, Kurz M, Mueller C, Nowak‐Goettl U, Schwabe D, et al. Fluconazole versus nystatin in the prevention of candida infections in children and adolescents undergoing remission induction or consolidation chemotherapy for cancer. The Journal of Antimicrobial Chemotherapy 1997;40:855‐62. [DOI] [PubMed] [Google Scholar]
Gøtzsche 1997
- Gøtzsche PC, Johansen HK. Meta‐analysis of prophylactic or empirical antifungal treatment versus placebo or no treatment in patients with cancer complicated by neutropenia. BMJ 1997;314:1238‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gøtzsche 2002a
- Gøtzsche PC, Johansen HK. Routine versus selective antifungal administration for control of fungal infections in patients with cancer. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD000026] [DOI] [PubMed] [Google Scholar]
Gøtzsche 2002b
- Gøtzsche PC, Johansen HK. Nystatin prophylaxis and treatment in severely immunodepressed patients. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD002033] [DOI] [PubMed] [Google Scholar]
Hemminki 1980
- Hemminki E. Study of information submitted by drug companies to licensing authorities. BMJ 1980;280(6217):833‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johansen 1997
- Johansen HK, Gøtzsche PC. Systematic reviews of prophylactic or empirical antifungal therapy in cancer patients with neutropenia. 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, September 28 to October 1, 1997, Toronto, Ontario, Canada. 1997:abstract no LM‐89.
Mayer 1999
- Mayer J, Doubek M, Vorlicek J. Must we really fear toxicity of conventional amphotericin B in oncological patients?. Support Care Cancer 1999;7(1):51‐5. [DOI] [PubMed] [Google Scholar]
Nakielny 1993
- Nakielny J. Effective and acceptable treatment for depression (letter). BMJ 1993;306:1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nath 1999
- Nath CE, Shaw PJ, Gunning R, McLachlan AJ, Earl JW. Amphotericin B in children with malignant disease: a comparison of the toxicities and pharmacokinetics of amphotericin B administered in dextrose versus lipid emulsion. Antimicrobial Agents and Chemotherapy 1999;43(6):1417‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slavin 1995
- Slavin MA, Osborne B, Adams R, Levenstein MJ, Schoch HG, Feldman AR, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation ‐ a prospective, randomized, double‐blind study. Journal of Infectious Diseases 1995;171(6):1545‐52. [DOI] [PubMed] [Google Scholar]
Smith 1998
- Smith GD, Egger M. Unresolved issues and future developments. BMJ 1998;316:221‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 1993
- Song F, Freemantle N, Sheldon TA, House A, Watson P, Long A, et al. Selective serotonin reuptake inhibitors: meta‐analysis of efficacy and acceptability. BMJ 1993;306:683‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stern 1997
- Stern JM, Simes JR. Publication bias: evidence of delayed publication in a cohort study of clinical research projects. BMJ 1997;315:640‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verfaillie 1991
- Verfaillie C, Weisdorf D, Haake R, Hostetter M, Ramsay N, McGlave P. Candida infections in bone marrow transplant recipients. Bone Marrow Transplant 1991;8:177‐84. [PubMed] [Google Scholar]
Viscoli 1994
- Viscoli C, Bruzzi P, Castagnola E, Boni L, Calandra T, Gaya H, et al. Factors associated with bacteraemia in febrile, granulocytopenic cancer patients. European Journal of Cancer 1994;30A:430‐7. [DOI] [PubMed] [Google Scholar]
Working Party 1995
- Working Party of the British Society for Antimicrobial Chemotherapy. Antifungal drug chemotherapy. Antifungal drug susceptibility testing. Journal of Antimicrobial Chemotherapy 1995;36:899‐909. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Johansen 1999
- Johansen HK, Gøtzsche PC. Problems in the design and reporting of trials of antifungal agents encountered during meta‐analysis. JAMA 1999;282:1752‐9. [DOI] [PubMed] [Google Scholar]
Johansen 2000
- Johansen HK, Gøtzsche PC. Amphotericin B versus fluconazole for controlling fungal infections in neutropenic cancer patients. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD000239] [DOI] [PubMed] [Google Scholar]
Johansen 2002
- Johansen HK, Gøtzsche PC. Amphotericin B versus fluconazole for controlling fungal infections in neutropenic cancer patients. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD000239] [DOI] [PubMed] [Google Scholar]


