Abstract
Background
This review update has been managed by both the Childhood Cancer and Cochrane Gynaecological, Neuro-oncology and Orphan Cancer Groups.
The use of anthracycline chemotherapy is limited by the occurrence of cardiotoxicity. To prevent this cardiotoxicity, different anthracycline dosage schedules have been studied.
Objectives
To determine the occurrence of cardiotoxicity with the use of different anthracycline dosage schedules (that is peak doses and infusion durations) in people with cancer.
Search methods
We searched the databases of the Cochrane Register of Controlled Trials (CENTRAL) (the Cochrane Library, Issue 11, 2015), MEDLINE (1966 to December 2015), and EMBASE (1980 to December 2015). We also searched reference lists of relevant articles, conference proceedings, experts in the field, and ongoing trials databases.
Selection criteria
Randomised controlled trials (RCTs) in which different anthracycline dosage schedules were compared in people with cancer (children and adults).
Data collection and analysis
Two review authors independently performed the study selection, the 'Risk of bias' assessment, and data extraction. We performed analyses according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions.
Main results
We identified 11 studies: 7 evaluated different infusion durations (803 participants), and 4 evaluated different peak doses (5280 participants). Seven studies were RCTs addressing different anthracycline infusion durations; we identified long‐term follow‐up data for one of the trials in this update. The meta‐analysis showed a statistically significant lower rate of clinical heart failure with an infusion duration of six hours or longer as compared to a shorter infusion duration (risk ratio (RR) 0.27; 95% confidence interval 0.09 to 0.81; 5 studies; 557 participants). The majority of participants included in these studies were adults with different solid tumours. For different anthracycline peak doses, we identified two RCTs addressing a doxorubicin peak dose of less than 60 mg/m2 versus 60 mg/m2 or more, one RCT addressing a liposomal doxorubicin peak dose of 25 mg/m2 versus 50 mg/m2, and one RCT addressing an epirubicin peak dose of 83 mg/m2 versus 110 mg/m2. A significant difference in the occurrence of clinical heart failure was identified in none of the studies. The participants included in these studies were adults with different solid tumours. High or unclear 'Risk of bias' issues were present in all studies.
Authors' conclusions
An anthracycline infusion duration of six hours or longer reduces the risk of clinical heart failure, and it seems to reduce the risk of subclinical cardiac damage. Since there is only a small amount of data for children and data obtained in adults cannot be extrapolated to children, different anthracycline infusion durations should be evaluated further in children.
We identified no significant difference in the occurrence of clinical heart failure in participants treated with a doxorubicin peak dose of less than 60 mg/m2 or 60 mg/m2 or more. Only one RCT was available for the other identified peak doses, so we can make no definitive conclusions about the occurrence of cardiotoxicity. More high‐quality research is needed, both in children and adults and in leukaemias and solid tumours.
Keywords: Adult; Child; Humans; Anthracyclines; Anthracyclines/administration & dosage; Anthracyclines/adverse effects; Antibiotics, Antineoplastic; Antibiotics, Antineoplastic/administration & dosage; Antibiotics, Antineoplastic/adverse effects; Cardiac Output, Low; Cardiac Output, Low/chemically induced; Cardiac Output, Low/prevention & control; Heart; Heart/drug effects; Heart Diseases; Heart Diseases/chemically induced; Neoplasms; Neoplasms/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Different dosage schedules for reducing damage to the heart in people with cancer receiving anthracycline chemotherapy
Review question We reviewed the evidence of different anthracycline dosage schedules to cause damage to the heart in people with cancer of all ages receiving anthracycline chemotherapy. We also looked at tumour response, participant survival, adverse effects other than damage to the heart, and quality of life.
Background Anthracyclines are one of the most effective treatments for various types of cancer. Unfortunately, there is a risk of heart damage depending on the total dose a patient has received. In an effort to prevent heart damage, different anthracycline dosage schedules such as different infusion durations or different individual peak doses (the maximal dose received in one week) are being used.
Study characteristics The evidence is current to December 2015.
We found 11 studies: 7 evaluated different infusion durations (803 participants), and 4 evaluated different peak doses (5280 participants). Participants had different types of cancer.
Key results For the use of different anthracycline infusion durations, the authors found that an anthracycline infusion duration of six hours or longer reduces the risk of clinical heart failure (for example shortness of breath or leg oedema), and it seems to reduce the risk of subclinical heart failure (that is heart damage diagnosed for example by an echocardiography in people without symptoms). Only a small amount of data was available for children and individuals with leukaemia, since most studies evaluating different anthracycline infusion durations were performed in adults with solid tumours.
Based on the currently available evidence, we are not able to favour either a doxorubicin peak dose of less than 60 mg/m2 or 60 mg/m2 or more. There was not enough high‐quality evidence available for the use of other anthracycline peak doses to be able to draw conclusions. No data were available for children and individuals with leukaemia.
Further high‐quality research is needed.
Quality of the evidence All studies had problems relating to quality of the evidence.
Background
Anthracyclines are among the most effective drugs used in chemotherapy for people with cancer and are widely used to treat both solid tumours and leukaemia in both adults and children. However, their use is limited because they often cause damage to the heart, especially if given high doses (Bonadonna 1969; Lefrak 1973; Van Dalen 2006b; Van der Pal 2012).
Heart damage after anthracycline therapy can be divided into early and late cardiotoxicity according to the time of presentation: early cardiotoxicity refers to heart damage that develops during anthracycline therapy or in the first year after its completion, and late cardiotoxicity manifests itself at least one year after the completion of anthracycline therapy (Shan 1996). The risk of developing heart failure remains a lifelong threat, especially for children and young adults, who have a long life expectancy after successful treatment for cancer. The risk of developing clinical heart failure 20 years after anthracycline therapy for childhood cancer is estimated to be approximately 5.5%, and 9.8% if treated with doses of 300 mg/m2 or more (Van Dalen 2006b).
Heart damage can occur as either subclinical cardiotoxicity or clinical cardiotoxicity. The term subclinical cardiotoxicity is used to describe various cardiac abnormalities, diagnosed with different diagnostic methods in patients without symptoms. Examples are histological abnormalities according to the Billingham score (Billingham 1978), or abnormalities in cardiac function measured by echocardiography or radionuclide ventriculography. Clinical cardiotoxicity is defined on the basis of symptoms of clinical heart failure and confirmed by an abnormal diagnostic test. In end‐stage clinical heart failure, heart transplantation is the only remaining option to avoid cardiac death.
The reported frequency of both clinical and subclinical cardiotoxicity after anthracycline therapy varies widely. In children, the prevalence of subclinical cardiac damage has been reported to be more than 57% at a median of 6.4 years after treatment (Kremer 2002a), and the incidence of clinical heart failure as high as 16% 0.9 to 4.8 years after treatment (Kremer 2002b). In adults, the prevalence of subclinical cardiac damage has been reported to be 36% during anthracycline therapy (Nousiainen 2002), and the incidence of clinical heart failure 30% at a median of 37 months after treatment (Meinardi 2002). However, we did not perform systematic reviews on the frequency of anthracycline‐induced cardiotoxicity in adults. Part of this variation can be explained by the type of anthracycline used, the total anthracycline dose, and the presence of additional risk factors for developing heart damage, such as radiation therapy involving the heart region, type of tumour, exposure to cyclophosphamide, mitoxantrone, iphosphamide, amsacrine, trastuzumab or taxanes, or the presence of pre‐existing heart disease. There also seems to be a higher risk for females, children, and elderly people (Kremer 2002b; Ng 2006; Simbre 2005; Van Dalen 2004).
Clinicians confront a clinical dilemma as they balance the anti‐tumour efficacy of anthracyclines against the associated cardiotoxicity. In an effort to prevent or reduce this toxicity, extensive research has been devoted to the identification of methods or drugs capable of ameliorating this toxicity, such as different anthracycline derivates (for example doxorubicin, daunorubicin, epirubicin, and liposomal preparations) (Batist 2001; Muggia 1991; Van Dalen 2010), cardioprotective agents (for example dexrazoxane) (Van Dalen 2011), or omitting anthracyclines altogether (Van Dalen 2014). A different approach is the use of less cardiotoxic dosage schedules for anthracycline chemotherapy, that is peak anthracycline doses and duration of infusion of anthracycline therapy (Legha 1982; Lipshultz 2002).
An important question regarding any anthracycline dosage schedule is whether it has a lower cardiotoxic effect without reducing the anti‐tumour efficacy and without negative effects on toxicities other than cardiac damage, such as alopecia, nausea, vomiting, stomatitis, diarrhoea, fatigue, anaemia, leukopenia, and thrombocytopenia.
This is the second update of the first systematic review on the cardiotoxicity of different anthracycline dosage schedules (Van Dalen 2006; Van Dalen 2009). Since we performed the first update, long‐term follow‐up data on the use of different anthracycline infusion durations in children with acute lymphoblastic leukaemia have become available. We have included all new evidence in this update.
Objectives
To determine the occurrence of cardiotoxicity with the use of different anthracycline dosage schedules (that is peak doses and infusion durations) in people with cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials comparing the occurrence of heart damage with the use of any type of dosage schedule of anthracycline chemotherapy with another type of dosage schedule.
Types of participants
People with cancer (both adults and children) who received anthracycline chemotherapy.
Types of interventions
The same anthracycline derivative with the same (cumulative) anthracycline dose but with different types of dosage schedules, that is different peak dose (defined as the maximal dose received in one week) or different infusion duration. For the same cumulative anthracycline dose, we used the following definition: in the design of the study it should have been the intention to treat both the intervention and control group with the same cumulative anthracycline dose, and the difference in the actually received median or mean cumulative anthracycline dose between both treatment groups should not differ by more than 100 mg/m2 of body surface area. Chemotherapy other than anthracyclines and radiotherapy involving the heart region should be the same in both treatment groups.
Types of outcome measures
Primary outcomes
Heart failure, that is clinical heart failure or subclinical cardiac damage, or both (definitions: clinical heart failure as defined by the authors; subclinical cardiac damage defined as either histological abnormalities according to the Billingham score on myocardial biopsy (Billingham 1978), or abnormalities in cardiac function measured by echocardiography or radionuclide ventriculography). If possible, we assessed both early and late cardiotoxicity (early cardiotoxicity refers to heart damage that develops during anthracycline therapy or in the first year after its completion, and late cardiotoxicity manifests itself at least one year after the completion of anthracycline therapy).
Secondary outcomes
Potential adverse effects of the different types of anthracycline dosage schedules on:
tumour response;
participant survival (progression‐free survival and overall survival);
toxicities other than cardiac damage;
quality of life.
Search methods for identification of studies
Electronic searches
We searched the electronic databases of the Cochrane Central Register of Controlled Trials (CENTRAL) (Cochrane Library, Issue 11, 2015), MEDLINE/PubMed (from 1966 to 9 December 2015), and EMBASE/Ovid (from 1980 to 9 December 2015). The search strategies for the different databases (using a combination of subject headings and text word terms) are stated in Appendix 1, Appendix 1, and Appendix 2. For the updates of this review (the current one and the first one in November 2008), we adapted the search strategies used in the original search (until June 2004). The exact changes are stated in the Appendices.
Searching other resources
We located information about trials not registered in CENTRAL, MEDLINE, or EMBASE, either published or unpublished, by searching the reference lists of relevant articles and reviews. In addition, we also searched the conference proceedings of the International Society of Paediatric Oncology (SIOP) and the American Society of Clinical Oncology (ASCO) from 2000 to 2015. We searched for ongoing trials by scanning the ISRCTN register and ClinicalTrials.gov (both screened 27 December 2015). We asked experts in the field for potentially relevant articles. We imposed no language restriction.
Data collection and analysis
Selection of studies
Two review authors (EvD, HvdP), after performing the search strategy described previously, independently undertook identification of studies meeting the inclusion criteria. We resolved any discrepancies by consensus. We did not require any third‐party arbitration. Any study seemingly meeting the inclusion criteria on grounds of the title or abstract, or both, was obtained in full for closer inspection.
Data extraction and management
Two review authors (EvD, HvdP) independently extracted the data using standardised forms. We extracted data of the characteristics of participants (such as age, sex, type of malignancy), interventions (such as cumulative anthracycline dose, peak dose, infusion duration), outcome measures, and length of follow‐up. We resolved any discrepancies by consensus. We did not require any third‐party arbitration.
Assessment of risk of bias in included studies
Two review authors (EvD, HvdP, LK) independently assessed the risk of bias in included studies. We assessed the risk of bias as described in the module of Cochrane Childhood Cancer (Kremer 2014), which is based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed the following items:
random sequence generation (selection bias);
allocation concealment (selection bias);
blinding of participants and personnel (performance bias);
blinding of outcome assessment (detection bias; for each outcome separately);
incomplete outcome data (attrition bias; for each outcome separately);
selective reporting (reporting bias); and
other potential sources of bias.
We resolved any discrepancies by consensus. We did not require any third‐party arbitration.
Data synthesis
We entered data into Review Manager 2014 and analysed the data according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). Dichotomous variables were related to risk using the risk ratio. If possible, we extracted data by allocation intervention, irrespective of compliance with the allocated intervention, in order to allow an intention‐to‐treat analysis. If this was not possible, we stated this. We assessed heterogeneity by both visual inspection of the forest plots and by a formal statistical test for heterogeneity, that is the I2 statistic (I2 greater than 50% was considered substantial heterogeneity). If there was evidence of substantial heterogeneity, we reported this. We used a random‐effects model throughout the review. We presented all the results with the corresponding 95% confidence interval. For survival, we used the generic inverse variance function of Review Manager 2014 to combine logs of the hazard ratios (HRs). Where necessary, we used Parmar's method to extract the log of the HR and its standard error (Parmar 1998). We otherwise summarised survival qualitatively. For different anthracycline infusion durations, we used six hours as a cut‐off point (that is greater than or equal to six hours versus less than six hours). In the protocol we stated that we would analyse different anthracycline peak doses as high (greater than or equal to 50 mg/m2) versus low doses (less than 50 mg/m2) received in one week. However, if we would have applied this definition to the included studies, pooling would not have been possible. Therefore, keeping in mind that any cut‐off point would be arbitrary, we decided to define a low peak dose as less than 60 mg/m2 of the same anthracycline derivative in one week and a high peak dose as greater than or equal to 60 mg/m2 of the same anthracycline derivative in one week. Outcomes for which we could extract data from only one trial were summarised qualitatively. We took into account the risk of bias of included studies in the analyses and interpretation of the results of the review. For all outcomes for which pooling was possible, we performed sensitivity analyses for all 'Risk of bias' criteria separately. We excluded the low‐quality studies and the studies for which the quality was unclear and compared the results of the good‐quality studies with the results of all available studies. It was our intention to perform subgroup analyses for children and adults and leukaemias and solid tumours, but unfortunately this was not possible (see Results for reasons).
Results
Description of studies
Results of the search
After searching the electronic databases CENTRAL, MEDLINE/PubMed, and EMBASE/Ovid (4200 references, of which 1117 were identified in the current update), we included a total of nine articles that fulfilled all the criteria for considering studies for this review (one of these articles we identified in the current update and provided long‐term follow‐up data of an earlier included RCT (Lipshultz 2002), so the total number of included studies identified in the electronic database search was eight; one of these studies was included after receiving additional information from the first author (Heidenreich 2004)).
We excluded 44 articles for reasons described in the Characteristics of excluded studies table. We excluded one conference abstract after identification of the full‐text manuscript (Advani 2014; Advani 2015), making the total number of excluded articles 45. We excluded the remaining 4147 articles since they were not randomised controlled trials (RCTs), were laboratory studies, were animal studies, did not include people with cancer, did not describe anthracycline therapy with different dosage schedules, the cumulative anthracycline doses differed between intervention and control group, chemotherapy other than anthracyclines and/or radiotherapy involving the heart region differed between treatment groups, and/or did not have heart failure as an outcome measure.
We scanned reference lists of relevant articles and reviews and identified an additional 11 studies (none new in the current update), of which three fulfilled all criteria for considering studies for this review, whereas the other eight did not (see Characteristics of excluded studies table).
We scanned conference proceedings and ongoing‐trials databases and identified an additional four studies (none new in the current update). However, three did not fulfil all criteria for considering studies for this review, and we therefore added them to the Characteristics of excluded studies table. We identified one study that has not been published and is awaiting further assessment (Ruiz 2006; see Characteristics of studies awaiting classification table).
We contacted experts in the field, who provided us with two additional studies (none new in the current update), which did not fulfil all criteria for considering studies for this review, and we added them to the Characteristics of excluded studies table.
The total number of identified RCTs was therefore 11 (described in 12 manuscripts; see Figure 1). Seven trials addressed different anthracycline infusion durations, and four trials addressed different anthracycline peak doses (see Characteristics of included studies).
1.
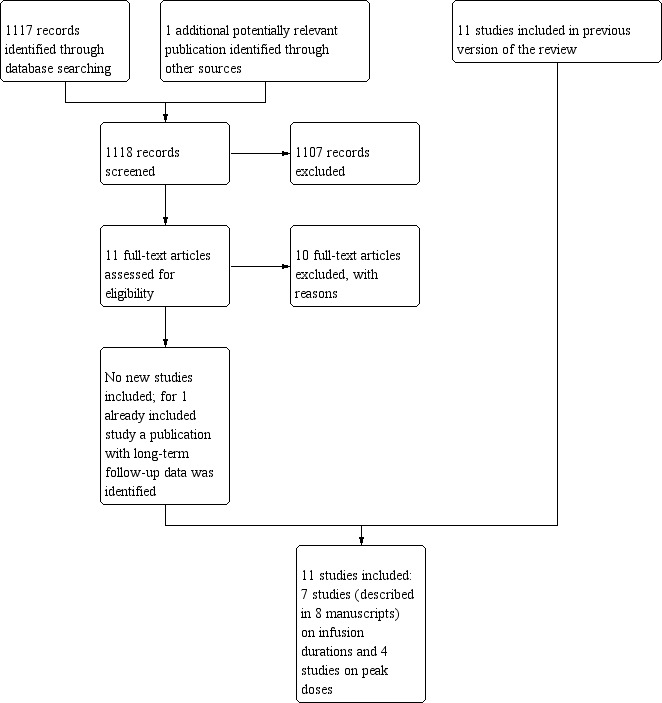
Study flow diagram.
Included studies
Description of studies addressing different anthracycline infusion durations
Seven trials with a total of 803 participants addressed different anthracycline infusion durations (Casper 1991; Escherich 2007; Hortobagyi 1989; Lipshultz 2002; Shapira 1990; Steinherz 1993; Zalupski 1991). For 779 participants, it was clear to which treatment group they were randomised: 384 participants were randomised to an infusion duration of less than six hours, whereas 395 participants were randomised to an infusion duration of six hours or more. It was not documented to which group the other 24 participants were randomised (from the primary study of Lipshultz 2002). Three studies included children (Escherich 2007; Lipshultz 2002; Steinherz 1993), three studies included adults (Casper 1991; Hortobagyi 1989; Zalupski 1991), and the remaining study did not mention the age of the participants (Shapira 1990). However, since participants in this study had either breast or ovarian cancer, it is likely that they were adults. In four studies, participants were treated with doxorubicin (Casper 1991; Lipshultz 2002; Shapira 1990; Zalupski 1991), in two studies with daunorubicin (Escherich 2007; Steinherz 1993), and in one study with epirubicin (Hortobagyi 1989). There were no important differences in cumulative anthracycline doses received in both treatment arms of the different RCTs. In three studies, participants were diagnosed with leukaemia (Escherich 2007; Lipshultz 2002; Steinherz 1993), in two studies with soft tissue sarcoma (Casper 1991; Zalupski 1991), in one study with breast cancer (Hortobagyi 1989), and in one study with either breast cancer or ovarian cancer (Shapira 1990). In two studies, the follow‐up of at least part of the included participants was more than one year (Lipshultz 2002; Steinherz 1993); it is therefore possible that these studies included cases of both early and late cardiotoxicity. In one study, the follow‐up was only seven days; after the first daunorubicin administration, all children received additional daunorubicin with an infusion duration of one hour, so only data for the first seven days were eligible for this review, and only early acute cardiotoxicity could be evaluated in this study (Escherich 2007). In the other studies, the length of follow‐up was not documented, and as a result we do not know if the cases of cardiotoxicity in these studies are early or late. However, given that most people included in these trials had advanced or metastatic disease and the associated effect on survival duration, we presumed that cases of heart failure in these trials were early cardiotoxicity.
It should be noted that in Lipshultz 2012, the long‐term follow‐up study of Lipshultz 2002, it was stated that the bolus infusion was given within 15 minutes, instead of the 1‐hour infusion duration documented in the primary publication of this study. The authors provided the following clarification: "all infusions were less than 1 hour and basically this was less than 15 minutes".
Description of studies addressing different anthracycline peak doses
Two trials with a total of 4146 adult participants with breast cancer compared a doxorubicin peak dose of less than 60 mg/m2 (2103 participants) with a doxorubicin peak dose of 60 mg/m2 or more (2043 participants) (Budman 1998; Linden 2007). There were no important differences in cumulative anthracycline doses received in both treatment arms of the different RCTs. In one of the trials, the length of follow‐up was more than one year for all participants (Budman 1998); it is therefore possible that this study included cases of both early and late cardiotoxicity. In the other trial, the length of follow‐up was not documented, and as a result we do not know if the cases of cardiotoxicity in this study are early or late (Linden 2007). However, given that the median follow‐up of participants still alive at the time of analysis was 7.2 years, it is possible that this study included cases of both early and late cardiotoxicity.
One trial including 48 adults with prostate cancer compared liposomal doxorubicin (Caelyx) peak doses of 25 mg/m2 (22 participants) and 50 mg/m2 (26 participants) (Heidenreich 2004). There were no important differences in cumulative anthracycline doses received in both treatment arms. The mean follow‐up was 42 months; it is therefore possible that this study included cases of both early and late cardiotoxicity.
One trial including 1086 adults with breast cancer compared epirubicin peak doses of 83 mg/m2 (535 participants) and 110 mg/m2 (551 participants) (Fountzilas 2008). There were no important differences in cumulative anthracycline doses received in both treatment arms. The median follow‐up was 40 months; it is therefore possible that this study included cases of both early and late cardiotoxicity.
Risk of bias in included studies
See the 'Risk of bias' section of the Characteristics of included studies table and Figure 2 for the exact scores and the support for the judgements per included study.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
For evaluating selection bias, we have assessed the random sequence generation and the allocation concealment.
Of the seven studies addressing different anthracycline infusion durations, two studies had a concealed treatment allocation, while the presence of random sequence generation was unclear (Lipshultz 2002; Zalupski 1991), in one study both random sequence generation and allocation concealment were incorrectly performed (Shapira 1990), and four studies did not specify the random sequence generation and the allocation concealment (Casper 1991; Escherich 2007; Hortobagyi 1989; Steinherz 1993).
Four studies addressed different anthracycline peak doses. Two studies compared a doxorubicin peak dose of less than 60 mg/m2 with a doxorubicin peak dose of 60 mg/m2 or more (Budman 1998; Linden 2007). Both trials did not specify the random sequence generation and the allocation concealment. One trial compared liposomal doxorubicin (Caelyx) peak doses of 25 mg/m2 and 50 mg/m2 (Heidenreich 2004). It did not specify the random sequence generation and the allocation concealment. One trial compared epirubicin peak doses of 83 mg/m2 and 110 mg/m2 (Fountzilas 2008). This trial had a concealed treatment allocation, but the presence of random sequence generation was unclear.
Blinding
Performance bias
For evaluating performance bias, we assessed the blinding of participants and personnel.
Of the seven studies addressing different anthracycline infusion durations, in one study participants and treating physicians were not blinded (Lipshultz 2002). In the other six studies this was unclear (Casper 1991; Escherich 2007; Hortobagyi 1989; Shapira 1990; Steinherz 1993; Zalupski 1991), however, since participants in both treatment groups received their anthracycline therapy with different infusion durations and with different catheters or pump devices, participants and personnel were probably not blinded.
Four studies addressed different anthracycline peak doses. Two studies compared a doxorubicin peak dose of less than 60 mg/m2 with a doxorubicin peak dose of 60 mg/m2 or more (Budman 1998; Linden 2007). In both trials it was unclear if participants and personnel were blinded to treatment. However, since participants in both treatment groups received their anthracycline therapy with different peak doses, and as a result different treatment durations, this was most likely not the case. The same was true for the trial comparing liposomal doxorubicin (Caelyx) peak doses of 25 mg/m2 and 50 mg/m2 (Heidenreich 2004), and the trial comparing epirubicin peak doses of 83 mg/m2 and 110 mg/m2 (Fountzilas 2008).
Detection bias
For evaluating detection bias, we assessed the blinding of outcome assessors for all separate outcomes.
Of the seven studies addressing different anthracycline infusion durations, six studies evaluated clinical heart failure; in all six studies it was unclear if the outcome assessors were blinded to treatment (Casper 1991; Escherich 2007; Hortobagyi 1989; Lipshultz 2002; Shapira 1990; Zalupski 1991). All seven studies evaluated subclinical heart failure (as a dichotomous or continuous outcome, or both); in one study outcome assessors were blinded to treatment (Lipshultz 2002), while in the other six studies this was unclear (Casper 1991; Escherich 2007; Hortobagyi 1989; Shapira 1990; Steinherz 1993; Zalupski 1991). For the assessment of response rate, it was unclear if the outcome assessor was blinded to treatment in all three studies evaluating this outcome (Escherich 2007; Hortobagyi 1989; Zalupski 1991). The same was true for all three studies evaluating overall survival, but since this item is not applicable for overall survival, we judged this as a low risk of bias (Casper 1991; Hortobagyi 1989; Zalupski 1991). For the assessment of adverse effects other than cardiac damage, it was unclear if the outcome assessor was blinded to treatment in the one study evaluating this outcome (Shapira 1990). None of the studies evaluated PFS and quality of life.
Four studies addressed different anthracycline peak doses. Two studies compared a doxorubicin peak dose of less than 60 mg/m2 with a doxorubicin peak dose of 60 mg/m2 or more (Budman 1998; Linden 2007). For clinical heart failure and adverse effects other than cardiac damage, it was unclear if the outcome assessor was blinded to treatment in both trials. The same was true for overall survival, but since this item is not applicable for OS, we judged this as a low risk of bias. Other outcomes were not addressed. One trial compared liposomal doxorubicin (Caelyx) peak doses of 25 mg/m2 and 50 mg/m2 (Heidenreich 2004). For all outcomes (that is clinical heart failure, subclinical heart failure, response rate, adverse effects other than cardiac damage, and QoL) it was unclear if the outcome assessor was blinded to treatment. Other outcomes were not addressed. One trial compared epirubicin peak doses of 83 mg/m2 and 110 mg/m2 (Fountzilas 2008). For both clinical heart failure and adverse effects other than cardiac damage, it was unclear if the outcome assessor was blinded to treatment. Other outcomes were not addressed.
Incomplete outcome data
For evaluating attrition bias, we assessed incomplete outcome data for all separate outcomes.
Of the seven studies addressing different anthracycline infusion durations, six studies evaluated clinical heart failure: in three studies the risk of attrition bias was low (Hortobagyi 1989; Shapira 1990; Zalupski 1991), in two the risk of attrition bias was high (Escherich 2007; Lipshultz 2002), and in the other study this was unclear (Casper 1991). All seven studies evaluated subclinical heart failure (as a dichotomous or continuous outcome, or both); in four studies the risk of attrition bias was low (Hortobagyi 1989; Shapira 1990; Steinherz 1993; Zalupski 1991), while in three studies the risk of attrition bias was high (Casper 1991; Escherich 2007; Lipshultz 2002). Three studies evaluated response rate, of which two had a low risk of attrition bias (Hortobagyi 1989; Zalupski 1991), and one had a high risk of attrition bias (Escherich 2007). Three studies evaluated OS, and in all of them the risk of attrition bias was low (Casper 1991; Hortobagyi 1989; Zalupski 1991). For the assessment of adverse effects other than cardiac damage, in the one study evaluating this outcome the risk of attrition bias was low (Shapira 1990). None of the studies evaluated PFS and quality of life.
Four studies addressed different anthracycline peak doses. Two studies compared a doxorubicin peak dose of less than 60 mg/m2 with a doxorubicin peak dose of 60 mg/m2 or more (Budman 1998; Linden 2007). For clinical heart failure the risk of attrition bias was low in both trials. For adverse effects other than cardiac damage the risk of attrition bias was low in one trial (Linden 2007), whereas in the other trial this was unclear (Budman 1998). For OS the risk of attrition bias was unclear in both trials. Other outcomes were not addressed. One trial compared liposomal doxorubicin (Caelyx) peak doses of 25 mg/m2 and 50 mg/m2 (Heidenreich 2004). For clinical heart failure, subclinical heart failure, response rate and adverse effects other than cardiac damage the risk of attrition bias was low, whereas for QoL this was unclear. Other outcomes were not addressed. One trial compared epirubicin peak doses of 83 mg/m2 and 110 mg/m2 (Fountzilas 2008). For both clinical heart failure and adverse effects other than cardiac damage the risk of attrition bias was unclear. Other outcomes were not addressed.
Selective reporting
For evaluating reporting bias, we assessed selective reporting.
Of the seven studies addressing different anthracycline infusion durations, in three studies we judged the risk of reporting bias to be low (Casper 1991; Hortobagyi 1989; Zalupski 1991), while in four we judged it to be high (Escherich 2007; Lipshultz 2002; Shapira 1990; Steinherz 1993).
Four studies addressed different anthracycline peak doses. Two studies compared a doxorubicin peak dose of less than 60 mg/m2 with a doxorubicin peak dose of 60 mg/m2 or more (Budman 1998; Linden 2007). In both trials the risk of reporting bias was low. The risk of reporting bias was high in both the trial comparing liposomal doxorubicin (Caelyx) peak doses of 25 mg/m2 and 50 mg/m2 (Heidenreich 2004), and the trial comparing epirubicin peak doses of 83 mg/m2 and 110 mg/m2 (Fountzilas 2008).
Other potential sources of bias
For evaluating other potential sources of bias, we assessed the following items: baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex, and/or prior cardiac dysfunction) and difference in length of follow‐up between treatment arms.
Of the seven studies addressing different anthracycline infusion durations, in one the risk of other bias was high (Hortobagyi 1989), while in the six other studies this was unclear (Casper 1991; Escherich 2007; Lipshultz 2002; Shapira 1990; Steinherz 1993; Zalupski 1991). For a more detailed description of all different items, see the 'Risk of bias' section of the Characteristics of included studies table.
Four studies addressed different anthracycline peak doses. Two studies compared a doxorubicin peak dose of less than 60 mg/m2 with a doxorubicin peak dose of 60 mg/m2 or more (Budman 1998; Linden 2007), one trial compared liposomal doxorubicin (Caelyx) peak doses of 25 mg/m2 and 50 mg/m2 (Heidenreich 2004), and one trial compared epirubicin peak doses of 83 mg/m2 and 110 mg/m2 (Fountzilas 2008). In all trials the risk of other bias was unclear. For a more detailed description of all different items, see the 'Risk of bias' section of the Characteristics of included studies table.
Effects of interventions
Different anthracycline infusion durations (i.e. greater than or equal to six hours versus less than six hours)
Not all articles allowed data extraction for all outcomes (see Characteristics of included studies for a more detailed description of the extractable outcomes of each study).
Clinical heart failure
We could collect data on clinical heart failure from 6 trials with a total of 735 participants (Casper 1991; Escherich 2007; Hortobagyi 1989; Lipshultz 2002; Shapira 1990; Zalupski 1991). However, since the eligible follow‐up in the study of Escherich 2007 was very short (that is only seven days), we felt it was inappropriate to include the data on clinical heart failure from this study in the pooled analyses. We therefore provide only descriptive results from this study: none of the participants included in Escherich 2007 developed clinical heart failure within seven days after the start of treatment.
The other five trials included a total of 557 participants. There were 19 cases of clinical heart failure among 277 participants randomised to an infusion duration of less than 6 hours and 4 cases among 280 participants randomised to an infusion duration of 6 hours or more. In one study there were no cases of clinical heart failure in both treatment groups (Lipshultz 2002), therefore the results of this study are not estimable for the meta‐analysis of the RR. The meta‐analysis showed a statistically significant lower rate of clinical heart failure with an infusion duration of six hours or longer as compared to a shorter infusion duration (RR 0.27, 95% confidence interval (CI) 0.09 to 0.81; P = 0.02) (Analysis 1.1). We detected no substantial heterogeneity (I2 = 2%).
1.1. Analysis.

Comparison 1: Infusion duration less than 6 hours versus infusion duration 6 hours or more, Outcome 1: Clinical heart failure
Please note that for the study of Lipshultz 2002 it was not possible to perform an intention‐to‐treat analysis (see Characteristics of included studies). Participants included in the meta‐analysis were all adults diagnosed with a solid tumour. As the length of follow‐up was not documented, we do not know if the cases of clinical heart failure included in the meta‐analysis were early or late. However, given that most people included in these trials had advanced or metastatic disease and the associated effect on survival duration, we presume that the cases of clinical heart failure in this meta‐analysis were early cardiotoxicity.
We excluded the study of Steinherz 1993 from this analysis, since it did not report clinical heart failure.
Long‐term follow‐up data of Lipshultz 2002 have been published on 92 of the 240 participants (N = 43 in the bolus group and N = 49 in the continuous infusion group) (Lipshultz 2012). The median length of follow‐up was 8 years with a range of 3 to 13 years (8.3 years in the bolus group and 8.2 years in the continuous infusion group). Again, there were no cases of clinical heart failure in both treatment groups.
Clinical and subclinical heart failure combined
We could extract data on clinical and subclinical heart failure combined from five trials, however we did not pool results because the definitions of subclinical heart failure used in the different trials were too diverse (see Characteristics of included studies: Casper 1991; Escherich 2007; Hortobagyi 1989; Shapira 1990; Zalupski 1991). We therefore provide descriptive results for these studies.
In four of the five studies, participants were adults diagnosed with a solid tumour (Casper 1991; Hortobagyi 1989; Shapira 1990; Zalupski 1991). As the length of follow‐up was not documented, we do not know if the cases of heart failure included in the analyses were early or late. However, given that most participants included in these trials had advanced or metastatic disease and the associated effect on survival duration, we presume that the cases of heart failure in the analyses were early cardiotoxicity. In one of the five studies, participants were children with leukaemia (Escherich 2007). As the eligible follow‐up duration of this study was seven days, we could only evaluate early cardiotoxicity.
In two out of five studies, a statistically significant difference in favour of participants randomised to an infusion duration of six hours or more was identified (Shapira 1990; Zalupski 1991), while in the other studies no significant differences were found (Analysis 1.2). Since in the study of Escherich 2007 no participants developed clinical or subclinical heart failure, we did not include a figure for this study.
1.2. Analysis.

Comparison 1: Infusion duration less than 6 hours versus infusion duration 6 hours or more, Outcome 2: (Sub)clinical heart failure combined
We excluded the study of Lipshultz 2002 from this analysis (both the original study and the long‐term follow‐up study), as the number of participants that developed subclinical heart failure was not provided, and only cases of clinical heart failure were thus included in the results. We excluded the study of Steinherz 1993 because clinical heart failure was not evaluated, and therefore the results only included cases of subclinical heart failure.
For all studies, it should be noted that participants who suffered from clinical heart failure are also included in the meta‐analysis of clinical heart failure as mentioned above.
Subclinical heart failure described as a continuous outcome
We could collect data on subclinical heart failure described as a continuous outcome from three trials (Lipshultz 2002; Shapira 1990; Steinherz 1993), however we did not pool results because the definitions of subclinical heart failure used in the different trials were too diverse (see Characteristics of included studies). We therefore provide descriptive results of these studies. Two studies evaluated children diagnosed with leukaemia (Lipshultz 2002; Steinherz 1993), whereas the third study evaluated adults with a solid tumour.
Lipshultz 2002 provided the median Z score of different echocardiographic parameters (bolus group versus continuous infusion group): diastolic dimension (‐0.12 versus ‐0.23), wall thickness (‐0.32 versus ‐0.28), systolic dimension (0.85 versus 0.38), left ventricular shortening fraction (LVSF) (‐2.34 versus ‐1.77), and mass (‐0.65 versus ‐0.47). None of the differences were significant. Please note that only a small percentage of the randomised participants were evaluated for this outcome (21% to 26%). Long‐term follow‐up data of Lipshultz 2002 have been published on 92 of the 240 participants (N = 43 in the bolus group and N = 49 in the continuous infusion group) (Lipshultz 2012). The median length of follow‐up was 8 years with a range of 3 to 13 years (8.3 years in the bolus group and 8.2 years in the continuous infusion group). Again no significant differences were identified for different echocardiographic parameters at three, six, and eight years after tumour diagnosis: LVSF, left ventricular end diastolic posterior wall thickness, left ventricular mass, left ventricular end systolic dimension, and left ventricular end diastolic dimension. Not all participants were assessed at all three time points.
Shapira 1990 provided the mean fall in left ventricular ejection fraction (LVEF), which was 17% in the bolus group versus 4% in the continuous infusion group at a cumulative anthracycline dose of 300 mg/m2, and 21% in the bolus group versus 6% in the continuous infusion group at a cumulative anthracycline dose of 400 mg/m2. This difference between both groups is highly significant for both doses (P < 0.001).
Steinherz 1993 provided the median change in LVSF, which was ‐6.5 for the bolus group and +1 for the continuous infusion group. It was not stated if this is a significant difference.
Response rate
We could extract data on response rate from 2 trials with a total of 292 adult participants with a solid tumour (Hortobagyi 1989; Zalupski 1991). These trials used comparable criteria to assess tumour response (see Characteristics of included studies). There were 23 complete or partial responses among 143 participants randomised to an infusion duration of less than 6 hours, and 28 among 149 participants randomised to an infusion duration of 6 hours or more. The meta‐analysis showed no significant difference in the response rate between both treatment groups (RR 1.20, 95% CI 0.65 to 2.22; P = 0.56) (Analysis 1.3). We detected no substantial heterogeneity (I2 = 16%). None of the studies documented that the response rate was determined by at least two observers.
1.3. Analysis.

Comparison 1: Infusion duration less than 6 hours versus infusion duration 6 hours or more, Outcome 3: Response rate
The study of Escherich 2007 (178 children with leukaemia) did report the number of good and poor responses at day 7. As the definition of response rate was not comparable with the above studies, and also due to the short eligible follow‐up duration (that is seven days), we did not include the results of this study in the meta‐analysis. However, no statistically significant difference in response rate between both treatment groups was identified (RR 1.23, 95% CI 0.91 to 1.66; P = 0.18) (Analysis 1.3). It was not documented if the response rate was determined by at least two observers.
We excluded the studies of Casper 1991, Lipshultz 2002 (both the original study and the long‐term follow‐up study), Shapira 1990, and Steinherz 1993 from this analysis because none of these studies documented the response rate per treatment group.
Please note that due to the nature of this measurement (that is the number of participants with a remission), a high event rate is favourable; therefore, in the graph of this analysis (Analysis 1.3), 'favours less than six hours' is on the left, and 'favours greater than or equal to six hours' is on the right, as opposed to the graphs of the other analyses.
Survival
None of the seven included studies evaluated PFS of participants randomised to different infusion durations (only data from the first seven treatment days for the study of Escherich 2007 were eligible for this review; see Characteristics of included studies table).
We could extract data on OS from 2 trials with a total of 322 adults with a solid tumour (Casper 1991; Zalupski 1991). One study presented the hazard ratio (HR) with 95% CI (Zalupski 1991), and the other study provided a survival curve (Casper 1991). The meta‐analysis showed no significant difference between the treatment groups (HR 1.42, 95% CI 0.61 to 3.30; P = 0.42) (Analysis 1.4). However, we detected unexplained heterogeneity (I2 = 75.0%).
1.4. Analysis.

Comparison 1: Infusion duration less than 6 hours versus infusion duration 6 hours or more, Outcome 4: Overall survival
Please note that for the study of Casper 1991, it was not possible to perform an intention‐to‐treat analysis because the survival data were presented with a participant randomised to bolus therapy who actually received the drug by continuous infusion in the continuous infusion group.
We excluded the studies of Lipshultz 2002 (both the original study and the long‐term follow‐up study), Shapira 1990, and Steinherz 1993 from this analysis because none of these studies documented OS per treatment group. Lipshultz 2002 did mention the five‐year event‐free survival (EFS) for the different treatment groups, and no significant difference was identified (89% EFS in the short infusion group and 87.3% in the continuous infusion group; P = 0.50). Long‐term follow‐up data of Lipshultz 2002 have been published on 92 of the 240 participants (N = 43 in the bolus group and N = 49 in the continuous infusion group) (Lipshultz 2012). The median length of follow‐up was 8 years with a range of 3 to 13 years (8.3 years in the bolus group and 8.2 years in the continuous infusion group). Again, no significant difference in EFS between both treatment groups was identified (10‐year EFS 79% in the bolus group versus 83% in the continuous infusion group; P = 0.24).
We excluded the study of Hortobagyi 1989 from this analysis because we were not able to reliably extract data needed to use Parmar's method for the assessment of survival for this study. However, in the Hortobagyi 1989 study participants randomised to an infusion duration of less than 6 hours had a median survival of 7 months (range 1 to 19+ months), and participants randomised to an infusion duration of 6 hours or more had a median survival of 9 months (range 1 to 21 months). Escherich 2007 did not report overall survival.
Adverse effects other than cardiac damage
Since only one study including adults with a solid tumour provided adequate data on adverse effects other than cardiac damage (Shapira 1990), pooling of results was not possible. We therefore provide descriptive results for this study. All analyses were performed in Review Manager 2014 with the random‐effects model. Given that all participants receiving anthracycline chemotherapy will suffer from side effects, we decided to analyse only the severe and life‐threatening effects. We defined this as grade 3 or 4 toxicity. We could only evaluate fatal sepsis. One participant randomised to an infusion duration of less than six hours died as the result of a sepsis. No statistically significant difference was identified between the treatment arms (RR 3.00, 95% CI 0.13 to 70.92, P = 0.50).
Quality of life
None of the studies evaluated QoL.
Subgroup analyses
We did not perform subgroup analyses for children versus adults and leukaemias versus solid tumours. Only in the meta‐analysis of clinical heart failure was a study evaluating children with leukaemia included, but as none of the participants developed clinical heart failure, this study could not be included in the calculation of RR.
Sensitivity analyses for the used 'Risk of bias' criteria
The results of the sensitivity analyses for the 'Risk of bias' criteria were consistent among the trials and did not differ from the overall analyses.
Different anthracycline peak doses
Doxorubicin peak dose less than 60 mg/m2 versus greater than or equal to 60 mg/m2
Not all articles allowed data extraction for all outcomes (see Characteristics of included studies for a more detailed description of the extractable outcomes of each study).
Clinical heart failure
We could collect data on clinical heart failure from 2 trials with a total of 4146 adults diagnosed with breast cancer (Budman 1998; Linden 2007). There were 12 cases of clinical heart failure among 2103 participants randomised to a peak dose of less than 60 mg/m2 and 20 cases among 2043 participants randomised to a peak dose of 60 mg/m2 or more. The meta‐analysis showed no significant difference in the occurrence of clinical heart failure between both treatment groups (RR 0.65, 95% CI 0.23 to 1.88; P = 0.43) (Analysis 2.1). However, we detected unexplained heterogeneity (I2 = 47%).
2.1. Analysis.

Comparison 2: Doxorubicin peak dose less than 60 mg/m2 versus 60 mg/m2 or more, Outcome 1: Clinical heart failure
Please note that in one of the trials the length of follow‐up was more than one year in all participants (Budman 1998). It is therefore possible that this study included cases of both early and late clinical heart failure. In the other trial, the length of follow‐up was not documented, and as a result we do not know if the cases of clinical heart failure in this study are early or late (Linden 2007). However, given that the median follow‐up of participants still alive at the time of analysis was 7.2 years, it is possible that this study included cases of both early and late clinical heart failure.
Clinical and subclinical heart failure combined
In both trials, no information on subclinical heart failure was provided.
Subclinical heart failure described as a continuous outcome
In both trials, no information on subclinical heart failure was provided.
Response rate
In both trials, no information on response rate was provided.
Survival
In both trials, no information on PFS was provided.
We could extract data on OS from 2 trials with a total of 4146 adults diagnosed with breast cancer (Budman 1998; Linden 2007). One study provided the P value, the number of events on each treatment arm, and the randomisation ratio was 1:1 (Budman 1998); the other study provided the HR with 95% CI. The meta‐analysis showed no significant differences in overall survival between the treatment groups (HR 1.06, 95% CI 0.93 to 1.22; P = 0.39) (Analysis 2.2). We detected no heterogeneity (I2 = 0%).
2.2. Analysis.

Comparison 2: Doxorubicin peak dose less than 60 mg/m2 versus 60 mg/m2 or more, Outcome 2: Overall survival
Adverse effects other than cardiac damage
Given that all people receiving anthracycline chemotherapy will suffer from side effects, we decided to analyse only the severe and life‐threatening effects. We defined this as grade 3 or 4 toxicity. We evaluated the following adverse effects: treatment‐related death, granulocytopenia (grade 4), leukopenia (grade 4), thrombocytopenia (grade 4), diarrhoea (grade 3 or 4), dyspnoea (grade 3 or 4), infection (grade 3 or 4), malaise/fatigue/lethargy (grade 3 or 4), nausea (grade 3 or 4), stomatitis (grade 3 or 4), vomiting (grade 3 or 4), pharynx/esophagitis (grade 3 or 4), phlebitis/thrombosis/embolism (grade 3 or 4), fever without infection (grade 3 or 4), oedema (grade 3 or 4), and hypotension (grade 3 or 4) (Linden 2007), leukopenia grade 3 or 4 (i.e. fewer than 1900 cells/µl) and death attributable to chemotherapy (Budman 1998). However, since the trials did not use comparable definitions, it was not possible to perform a meta‐analysis. We therefore provide descriptive results (Analysis 2.3).
2.3. Analysis.

Comparison 2: Doxorubicin peak dose less than 60 mg/m2 versus 60 mg/m2 or more, Outcome 3: Adverse effects other than cardiac damage
For leukopenia grade 4 (RR 0.58, 95% CI 0.53 to 0.64; P < 0.00001), leukopenia grade 3 or 4 (RR 0.26, 95% CI 0.21 to 0.31; P < 0.00001), granulocytopenia (RR 0.67, 95% CI 0.61 to 0.73; P < 0.00001), thrombocytopenia (RR 0.45, 95% CI 0.34 to 0.59; P < 0.00001), diarrhoea (RR 0.34, 95% CI 0.19 to 0.60; P = 0.0002), dyspnoea (RR 0.51, 95% CI 0.28 to 0.93; P = 0.03), infection (RR 0.61, 95% CI 0.42 to 0.86; P = 0.006), malaise/fatigue/lethargy (RR 0.66, 95% CI 0.49 to 0.91; P = 0.01), and stomatitis (RR 0.40, 95% CI 0.27 to 0.61; P < 0.0001) a statistically significant difference in favour of participants treated with a peak dose of less than 60 mg/m2 was identified.
For vomiting (RR 1.31, 95% CI 1.07 to 1.59; P = 0.008) a statistically significant difference in favour of participants treated with a peak dose of 60 mg/m2 or more was identified.
For treatment‐related death (RR 0.19, 95% CI 0.01 to 3.99; P = 0.29), death attributable to chemotherapy (RR 0.34, 95% CI 0.01 to 8.26; P = 0.51), and nausea (RR 1.19, 95% CI 0.98 to 1.44; P = 0.08), no significant differences between the treatment groups were identified.
Due to insufficient data, we could not include pharynx/oesophagitis, phlebitis/thrombosis/embolism, fever without infection, oedema, and hypotension in the analyses. However, the article did not document if there was a statistically significant difference between both treatment groups for oedema and hypotension, while it identified a statistically significant difference in favour of participants treated with a peak dose of less than 60 mg/m2 for the other adverse effects.
Quality of life
No information on QoL was provided.
Subgroup analyses
As all participants included in the analyses were adults with a solid tumour, it was not possible to perform subgroup analyses for children versus adults and leukaemias versus solid tumours.
Sensitivity analyses for the used 'Risk of bias' criteria
The results of the sensitivity analyses for the 'Risk of bias' criteria were consistent among the trials and did not differ from the overall analyses.
Liposomal doxorubicin (Caelyx) peak dose 25 mg/m2 versus 50 mg/m2
One trial compared liposomal doxorubicin (Caelyx) peak doses of 25 mg/m2 and 50 mg/m2 (Heidenreich 2004). All 48 participants were adults with prostate cancer. Since there was only one trial, subgroup analyses and sensitivity analyses for the used 'Risk of bias' criteria were not applicable. See Characteristics of included studies for a more detailed description of the extractable outcomes.
Clinical heart failure
No participants in either treatment group developed clinical heart failure. The mean follow‐up was 42 months, therefore this study evaluated both early and late cardiotoxicity.
Clinical and subclinical heart failure combined
No participants in either treatment group developed clinical or subclinical heart failure. Again, since the mean follow‐up was 42 months, this study evaluated both early and late cardiotoxicity.
Subclinical heart failure described as a continuous outcome
Subclinical heart failure was not evaluated as a continuous outcome in this study.
Response rate
There were no objective palliative tumour responses (defined as a decrease in prostate‐specific antigen (PSA) levels of greater than or equal to 50%) among 22 participants randomised to a peak dose of 25 mg/m2, and there were 8 objective palliative tumour responses among 26 participants randomised to a peak dose of 50 mg/m2. The analysis showed a borderline significant difference in favour of participants treated with a peak dose of 50 mg/m2 (RR 0.07, 95% CI 0.00 to 1.13; P = 0.06) (Analysis 3.1). It was not documented if the response was determined by at least two observers.
3.1. Analysis.

Comparison 3: Liposomal doxorubicin (Caelyx) peak dose 25 mg/m2 versus 50 mg/m2, Outcome 1: Response rate (defined as objective palliative tumour response (i.e. decrease in PSA levels of >= 50%))
Please note that due to the nature of this measurement (that is the number of participants with an objective palliative tumour response), a high event rate is favourable; therefore, in the graph of this analysis (Analysis 3.1), 'favours 50 mg/m2' is on the left and 'favours 25 mg/m2' is on the right, as opposed to the graphs of the other analyses.
Survival
No information on PFS and OS was provided.
Adverse effects other than cardiac damage
Given that all participants receiving anthracycline chemotherapy will suffer from side effects, we decided to analyse only the severe and life‐threatening effects. We defined this as grade 3 or 4 toxicity. This study evaluated the following adverse effects: gastrointestinal toxicity (grade 3 or 4), tachycardia (grade 3 or 4), arrhythmia (grade 3 or 4), dyspnoea (grade 3 or 4), palmar‐plantar erythrodysesthesia (grade 3 or 4), hepatic toxicity (grade 3 or 4), leukopenia (grade 3 or 4), thrombocytopenia (grade 3 or 4), haemoglobin‐related toxicity (grade 3 or 4), biochemical toxicities (grade 4), and neurological toxicities (grade 3 or 4) (Analysis 3.2).
3.2. Analysis.

Comparison 3: Liposomal doxorubicin (Caelyx) peak dose 25 mg/m2 versus 50 mg/m2, Outcome 2: Adverse effects other than cardiac damage
For hepatic toxicity (RR 0.20, 95% CI 0.05 to 0.79; P = 0.02) a statistically significant difference in favour of participants treated with a peak dose of 25 mg/m2 was identified.
For tachycardia (RR 0.06, 95% CI 0.00 to 1.00; P = 0.05) and haemoglobin‐related toxicity (RR 0.07, 95% CI 0.00 to 1.13; P = 0.06) a borderline‐significant difference in favour of participants treated with a peak dose of 25 mg/m2 was identified.
For palmar‐plantar erythrodysesthesia (RR 5.91, 95% CI 1.45 to 24.16; P = 0.01) a statistically significant difference in favour of participants treated with a peak dose of 50 mg/m2 was identified.
For gastrointestinal toxicity (RR 0.17, 95% CI 0.01 to 3.08; P = 0.23), arrhythmia (RR 0.39, 95% CI 0.04 to 3.52; P = 0.40), dyspnoea (RR 0.47, 95% CI 0.10 to 2.20; P = 0.34), leukopenia (RR 0.24, 95% CI 0.03 to 1.87; P = 0.17), thrombocytopenia (RR 0.39, 95% CI 0.02 to 9.15; P = 0.56), biochemical toxicities (no participants in either treatment group), and neurological toxicities (no participants in either treatment group) no significant differences between the treatment groups were identified.
Quality of life
No significant differences between the treatment groups in QoLquality of life were identified (no further data available).
Epirubicin peak dose 110 mg/m2 versus 83 mg/m2
One trial compared epirubicin peak doses of 83 mg/m2 and 110 mg/m2 (Fountzilas 2008). All 1086 participants were adults with breast cancer. As there was only one trial, subgroup analyses and sensitivity analyses for the used 'Risk of bias' criteria were not applicable. See Characteristics of included studies for a more detailed description of the extractable outcomes.
Clinical heart failure
There was one case of clinical heart failure in both treatment groups. The analysis showed no significant difference between both treatment groups (RR 0.97, 95% CI 0.06 to 15.48; P = 0.98) (Analysis 4.1). The median follow‐up was 40 months, therefore it is possible that this study included cases of both early and late cardiotoxicity.
4.1. Analysis.

Comparison 4: Epirubicin peak dose 110 mg/m2 versus 83 mg/m2, Outcome 1: Clinical heart failure
Clinical and subclinical heart failure combined
No information on subclinical heart failure was provided.
Subclinical heart failure described as a continuous outcome
No information on subclinical heart failure was provided.
Response rate
No information on response rate was provided.
Survival
No information on PFS and OS was provided.
Adverse effects other than cardiac damage
Given that all participants receiving anthracycline chemotherapy will suffer from side effects, we decided to analyse only the severe and life‐threatening effects. We defined this as grade 3 or 4 toxicity. This study evaluated the following adverse effects: anaemia (grade 3 or 4), leukopenia (grade 3 or 4), neutropenia (grade 3 or 4), febrile neutropenia (grade 3 or 4), thrombocytopenia (grade 3 or 4), nausea/vomiting (grade 3 or 4), fatigue (grade 3 or 4), infection (grade 3 or 4), central nervous system (grade 3 or 4), pulmonary (grade 3 or 4), peripheral neuropathy (grade 3 or 4), hepatotoxicity (grade 3 or 4), hypersensitivity reactions (grade 3 or 4), mucositis (grade 3 or 4), pain (grade 3 or 4), arthralgias/myalgias (grade 3 or 4), and treatment‐related death (Analysis 4.2).
4.2. Analysis.

Comparison 4: Epirubicin peak dose 110 mg/m2 versus 83 mg/m2, Outcome 2: Adverse effects other than cardiac damage
For peripheral neuropathy (RR 4.50, 95% CI 2.37 to 8.54; P < 0.00001), hypersensitivity reactions (RR 3.88, 95% CI 1.71 to 8.82; P = 0.001), and arthralgias/myalgias (RR 3.88, 95% CI 1.31 to 11.54; P = 0.01) a statistically significant difference in favour of participants treated with a peak dose of 83 mg/m2 was identified.
For anaemia (RR 2.91, 95% CI 0.79 to 10.70; P = 0.11), leukopenia (RR 1.06, 95% CI 0.75 to 1.49; P = 0.75), neutropenia (RR 1.05, 95% CI 0.84 to 1.31; P = 0.68), febrile neutropenia (RR 0.78, 95% CI 0.47 to 1.31; P = 0.35), thrombocytopenia (RR 12.62, 95% CI 0.71 to 223.52; P = 0.08), nausea/vomiting (RR 0.91, 95% CI 0.45 to 1.82; P = 0.79), fatigue (RR 0.32, 95% CI 0.07 to 1.60; P = 0.17), infection (RR 0.79, 95% CI 0.48 to 1.31; P = 0.36), central nervous system (RR 2.91, 95% CI 0.12 to 71.35; P = 0.51), pulmonary (RR 2.91, 95% CI 0.12 to 71.35; P = 0.51), hepatotoxicity (RR 1.70, 95% CI 0.50 to 5.77; P = 0.40), mucositis (RR 1.05, 95% CI 0.48 to 2.28; P = 0.90), pain (RR 0.32, 95% CI 0.01 to 7.93; P = 0.49), and treatment‐related death (RR 2.91, 95% CI 0.12 to 71.35; P = 0.51) no significant differences between the treatment groups were identified.
Quality of life
No information on QoL was provided.
Discussion
Heart damage due to anthracycline chemotherapy is a considerable and serious problem. It reduces QoL and can even cause premature death. Also, when heart damage occurs during therapy, the maximum cumulative dose of anthracyclines needs to be limited resulting in reduced efficacy of anthracycline chemotherapy.
This is the second update of the first systematic review evaluating the existing evidence on different anthracycline dosage schedules (that is different infusion durations and different peak doses) for reducing cardiotoxicity. We included only RCTs as it is widely recognised that only this study design can obtain unbiased evidence on the use of anthracyclines, provided that the design and execution are adequate.
Different anthracycline infusion durations
We identified seven trials for different anthracycline infusion durations. These trials all compared a bolus infusion (up to a maximum of 1 hour; for the Lipshultz 2002 study the exact duration of bolus infusion was not clear, as in one publication it was stated to be 1 hour, while in another publication it was stated to be within 15 minutes; the authors provided the following clarification: "all infusions were less than 1 hour and basically this was less than 15 minutes") with a longer infusion duration (varying from 6 to 96 hours). Our meta‐analysis of five trials showed a statistically significant lower rate of clinical heart failure with an infusion duration of six hours or longer as compared to a shorter infusion duration (RR 0.27, 95% CI 0.09 to 0.81). This finding is supported by two out of five individual studies evaluating clinical and subclinical heart failure combined, which also showed a statistically significant lower rate of clinical and subclinical heart failure combined in participants randomised to an infusion duration of six hours or longer as compared to participants randomised to a shorter infusion duration. Also, one of the two individual studies evaluating the significance of the difference in subclinical heart failure as a continuous outcome between both treatment groups showed a significant difference in the mean fall of the left ventricular ejection fraction in favour of the infusion duration of six hours or longer. Another study did not mention the significance of the difference.
However, an important question regarding any cardioprotective intervention during anthracycline therapy is whether the intervention could selectively decrease the heart damage by anthracyclines without reducing the antitumour efficacy (that is tumour response and patient survival) and without negative effects on QoL and toxicities other than cardiac damage. Our meta‐analysis of two trials for response rate showed no significant difference between both treatment groups. One study evaluated the number of good responses at day seven and identified no significant difference between the treatment groups. Also no statistically significant difference in OS was found between the treatment groups in our meta‐analysis of two trials, but please note that there was unexplained heterogeneity. No data on PFS was available. This review does not allow for any conclusions regarding adverse effects other than cardiac damage and QoL in participants treated with different anthracycline infusion durations.
It should be kept in mind that the inclusion of studies for this systematic review was limited to RCTs describing cardiotoxicity, and as a result, the analyses of response rate, survival, adverse effects other than cardiac damage, and QoL were possibly based on only a subgroup of trials comparing different anthracycline infusion durations.
It should be noted that 'no evidence of effect', as identified in this review, is not the same as 'evidence of no effect'. The reason that some studies did not identify a significant difference between both study groups could be due to the fact that the number of participants included in these studies was too small to detect a difference between the treatment groups (that is low power). Furthermore, the length of follow‐up could have been too short to detect a significant difference between the treatment groups. This is especially true for the study by Escherich 2007, in which the eligible follow‐up was only seven days.
It should be emphasised that the majority of participants included in these studies were adults with different solid tumours. Subgroup analyses for children versus adults and leukaemias versus solid tumours were not possible. We could not include children with leukaemia in the performed meta‐analyses, but included them in the descriptive results of non‐pooled studies. Two studies with children diagnosed with leukaemia evaluated clinical heart failure (Escherich 2007; Lipshultz 2002), and identified no differences (however, as mentioned above, one of these studies had a very short follow‐up of only seven days). Long‐term follow‐up data (median length of follow‐up 8 years with a range of 3 to 13 years) of Lipshultz 2002 showed the same result. Three studies with children diagnosed with leukaemia evaluated subclinical heart failure, of which two studies assessed the significance of the difference between both treatment groups (Escherich 2007; Lipshultz 2002). Again, no differences were identified; the long‐term follow‐up data of Lipshultz 2002 did not change these results. The third study suggested a decrease in left ventricular shortening fraction in children treated with bolus infusion (Steinherz 1993). One study with children diagnosed with leukaemia evaluated response rate (Escherich 2007). This study identified no difference in response, but it should be noted that the follow‐up was only seven days. We could obtain no information regarding PFS and OS, adverse effects other than cardiac damage, and QoL for paediatric participants. However, Lipshultz 2002 did mention the five‐year event‐free survival for the different treatment groups, and no significant difference was identified (89% event‐free survival in the short infusion group and 87.3% in the continuous infusion group; P = 0.50). The same was true for the long‐term follow‐up data: 10‐year event‐free survival 79% in the bolus group versus 83% in the continuous infusion group; P = 0.24.
Regarding early and late cardiotoxicity, we must conclude the following. Only two studies in children documented that the follow‐up of at least part of the included participants was more than one year (Lipshultz 2002; Steinherz 1993). It is therefore possible that these studies included cases of both early and late cardiotoxicity. One study in children had a follow‐up of only seven days, so in this study we could evaluate only acute cardiotoxicity (Escherich 2007). All participants in the long‐term follow‐up study of Lipshultz 2002 had a follow‐up of at least three years, so in this study we could evaluate late cardiotoxicity. The other studies did not document the length of follow‐up, and as a result we do not know if the cases of cardiotoxicity were early or late. However, given that most participants included in these trials had advanced or metastatic disease and the associated effect on survival duration, we presume that cases of heart failure in these trials were early cardiotoxicity.
As described earlier, the risk of bias in the included studies varied; in many studies we could not rule out bias due to lack of reporting. However, currently this is the best available evidence of RCTs evaluating different anthracycline infusion durations.
Furthermore, the included RCTs used three different anthracycline derivatives (doxorubicin, epirubicin, and daunorubicin), and we assumed that they all behaved similarly. We therefore combined them in the meta‐analyses.
Different anthracycline peak doses
We identified two trials for different doxorubicin peak doses. These trials compared a doxorubicin peak dose of less than 60 mg/m2 with a doxorubicin peak dose of 60 mg/m2 or more (that is 40 versus 60 mg/m2 or 54 versus 81 mg/m2). Our meta‐analysis showed no significant difference in the occurrence of clinical heart failure between the treatment groups, but please note that there was unexplained heterogeneity. No information on subclinical heart failure was provided, and therefore no conclusions can be made regarding this outcome. Again, an important question regarding any cardioprotective intervention during anthracycline therapy is whether the intervention could selectively decrease the heart damage by anthracyclines without reducing the antitumour efficacy and without negative effects on QoL and toxicities other than cardiac damage. Our meta‐analysis showed no significant difference in OS between the treatment groups. With regard to adverse effects other than cardiac damage, pooling of results was not possible. In the individual studies the results were not unambiguous: for most evaluated adverse effects a significant difference in favour of participants treated with a peak dose of less than 60 mg/m2 was identified, but for others either no difference between the treatment groups was identified or there was a significant difference in favour of participants treated with a peak dose of 60 mg/m2 or more. No information on response rate, PFS and QoL was provided, and therefore no conclusions can be made regarding these outcomes.
In the study evaluating 25 mg/m2 versus 50 mg/m2 liposomal doxorubicin (Caelyx), no significant difference between the treatment groups was identified for both clinical and subclinical heart failure. Again, an important question regarding any cardioprotective intervention during anthracycline therapy is whether the intervention could selectively decrease the heart damage by anthracyclines without reducing the antitumour efficacy and without negative effects on QoL and toxicities other than cardiac damage. For response rate (defined as a reduction in serum prostate‐specific antigen (PSA) levels by greater than or equal to 50% relative to baseline, with this reduction persisting for greater than or equal to four weeks and being accompanied by stabilisation or improvement in the participant's performance status) a borderline‐significant difference in favour of participants treated with a peak dose of 50 mg/m2 was identified (RR 0.07, 95% CI 0.00 to 1.13; P = 0.05). However, the significance of PSA reduction as a surrogate marker for response and survival must be considered with caution. The clinical relevance of decreased PSA levels remains subject to debate (Millikan 2003; Verbel 2002). With regard to adverse effects other than cardiac damage, the results were not unambiguous: for most evaluated adverse effects no significant difference between both treatment groups was identified, but for others either a significant difference in favour of participants treated with a peak dose of 25 mg/m2 or 50 mg/m2 was shown. No significant difference in the QoL between the treatment groups was identified. No information on PFS and OS was provided, and therefore no conclusions can be made regarding these outcomes.
In the study evaluating 83 mg/m2 versus 110 mg/m2 epirubicin, no significant difference between the treatment groups was identified for clinical heart failure. No information on subclinical heart failure was provided, and therefore no conclusions can be made regarding this outcome. Again, an important question regarding any cardioprotective intervention during anthracycline therapy is whether the intervention could selectively decrease the heart damage by anthracyclines without reducing the antitumour efficacy and without negative effects on QoL and toxicities other than cardiac damage. With regard to adverse effects other than cardiac damage, the results were not unambiguous: for most evaluated adverse effects no significant difference between the treatment groups was identified, whereas for some a significant difference in favour of participants treated with a peak dose of 83 mg/m2 was identified. No information on response rate, PFS and OS and QoL was provided, and therefore no conclusions can be made regarding these outcomes.
It should be kept in mind that the inclusion of studies for this systematic review was limited to RCTs describing cardiotoxicity, and as a result, the analyses of response rate, survival, adverse effects other than cardiac damage, and QoL were possibly based on only a subgroup of trials comparing different anthracycline peak doses.
It should be noted that 'no evidence of effect', as identified in this review, is not the same as 'evidence of no effect'. The reason that some studies did not identify a significant difference between both study groups could be due to the fact that the number of participants included in these studies was too small to detect a difference between the treatment groups (that is low power). Furthermore, the length of follow‐up could have been too short to detect a significant difference between the treatment groups.
It should be emphasised that all participants included in the studies evaluating different anthracycline peak doses were adults with different solid tumours. As a result, subgroup analyses for children versus adults and leukaemia versus solid tumours were not possible.
Regarding early and late cardiotoxicity, we must conclude the following. In three studies, the follow‐up of at least part of the included participants was more than one year (Budman 1998; Fountzilas 2008; Heidenreich 2004). It is therefore possible that these studies included cases of both early and late cardiotoxicity. The fourth study did not mention the length of follow‐up, and as a result we do not know if the cases of cardiotoxicity in this studies were early or late (Linden 2007). However, given that the median follow‐up of participants still alive at the time of analysis was 7.2 years, it is possible that this study included cases of both early and late cardiotoxicity.
As described earlier, the risk of bias in the included studies varied; in many studies we could not rule out bias due to lack of reporting. However, currently this is the best available evidence of RCTs evaluating different anthracycline peak doses.
We identified eligible RCTs for only for a limited number of anthracycline peak doses. We found no appropriate studies for other combinations, and therefore can make no conclusions regarding the use of these combinations in preventing anthracycline‐induced heart failure in participants treated with anthracyclines.
Also, more definitions of anthracycline peak doses than the one we used in this systematic review (that is the maximal anthracycline dose received in one week) are possible, for example single‐dose infusion versus consecutive divided daily doses. To evaluate all the available evidence on the exact role of other kinds of peak doses on the occurrence of anthracycline‐induced cardiotoxicity in adults and children, one or more new (Cochrane) systematic reviews can be initiated. These systematic reviews should focus not only on anthracycline‐induced cardiotoxicity, but also on antitumour efficacy, adverse effects other than cardiac damage, and QoL.
We are awaiting the full‐text publication of the trial currently awaiting assessment (Ruiz 2006); from the currently available data it is unclear if this study is eligible for inclusion in this review.
Authors' conclusions
Implications for practice.
Different anthracycline infusion durations
For different anthracycline infusion durations, our meta‐analysis clearly showed that an anthracycline infusion duration of six hours or longer reduces the risk of clinical heart failure, and in individual studies it seems to reduce the risk of subclinical heart failure. There is no evidence suggesting a difference in response rate and survival between the treatment groups. We can make no conclusions regarding adverse effects other than cardiac damage and QoL. It should be emphasised that the majority of the participants included in these studies were adults with advanced solid tumours. We found no new studies since the last version of this review.
We conclude that if the risk of cardiac damage is expected to be high or if it is necessary to administer a higher cumulative anthracycline dose, it might be justified to use an infusion duration of six hours or longer in people with cancer treated with anthracyclines. However, clinicians should weigh the cardioprotective effect of a longer infusion duration against the uncertain risk of a reduced antitumour efficacy and adverse effects other than cardiac damage for each individual patient. We found no new studies since the last version of this review.
Different anthracycline peak doses
For different doxorubicin peak doses (that is less than 60 mg/m2 versus 60 mg/m2 or more), our meta‐analysis showed no significant difference in the occurrence of clinical heart failure between the treatment groups. There is no evidence suggesting a difference in overall survival between the treatment groups. We could not pool the results of adverse effects other than cardiac damage, but they were not unambiguous in the individual studies. No information on subclinical heart failure, response rate, PFS and QoL was provided. It should be emphasised that all participants included in these studies were adults with solid tumours. Based on the currently available evidence, we are not able to favour a doxorubicin peak dose of either less than 60 mg/m2 or 60 mg/m2 or more.
Since we identified only one RCT evaluating a liposomal doxorubicin (Caelyx) peak dose of 25 mg/m2 versus 50 mg/m2, we can make no definitive conclusions regarding this peak dose. No significant differences between the treatment groups for both clinical and subclinical heart failure were identified. There was a borderline‐significant difference in response rate in favour of participants treated with a peak dose of 50 mg/m2. No significant difference between the treatment groups for QoL was shown. However, these findings should be confirmed in other RCTs. The results of adverse effects other than cardiac damage were not unambiguous. No information on PFS and OS was provided. It should be emphasised that the all participants included in these studies were adults with solid tumours. Based on the currently available evidence, we are not able to make recommendations for clinical practice.
Since we identified only one RCT evaluating an epirubicin peak dose of 83 mg/m2 versus 110 mg/m2, we can make no definitive conclusions regarding this peak dose. No significant difference between the treatment groups for clinical heart failure was identified. However, this finding should be confirmed in other RCTs. The results of adverse effects other than cardiac damage were not unambiguous. No information on subclinical heart failure, response rate, PFS and QoL was provided. It should be emphasised that all the participants included in these studies were adults with solid tumours. Based on the currently available evidence, we are not able to make recommendations for clinical practice.
As no high‐quality evidence was available for other combinations of anthracycline peak doses, we can make no conclusions about the efficacy of different peak doses in preventing heart damage in people treated with anthracyclines. Based on the currently available evidence, we are not able to make recommendations for clinical practice.
Implications for research.
Different anthracycline infusion durations
Since there is only a small amount of data for children and also because data obtained in adults cannot be extrapolated to children, different anthracycline infusion durations should be evaluated further in children. Future RCTs should be performed in homogeneous study populations treated for either a haematological malignancy or a solid tumour using valid outcome definitions (including cardiotoxicity, response rate, survival, and adverse effects other than cardiac damage). The follow‐up should be long enough to identify late‐onset cardiotoxic effects. The number of included participants should be sufficient to obtain the power needed for the results to be reliable. Also, it will be very interesting to examine long‐term follow‐up data from the already performed RCTs.
Different anthracycline peak doses
Future RCTs should be performed in homogeneous study populations treated for either a haematological malignancy or a solid tumour using valid outcome definitions (including cardiotoxicity, response rate, survival, and adverse effects other than cardiac damage). The follow‐up should be long enough to identify late‐onset cardiotoxic effects. The number of included participants should be sufficient to obtain the power needed for the results to be reliable. Since no data for children are available and data obtained in adults cannot be extrapolated to children, different anthracycline peak doses should also be evaluated in children. Also, it will be very interesting to examine long‐term follow‐up data from the already performed RCTs.
What's new
| Date | Event | Description |
|---|---|---|
| 8 June 2020 | Review declared as stable | This Cochrane review has had low usage and is currently not a priority for updating. |
History
Protocol first published: Issue 3, 2004 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 20 January 2016 | New citation required but conclusions have not changed | Summary of most important changes in the update: The search for eligible studies was updated to December 2015. We identified long‐term follow‐up data of one of the earlier included RCTs on different infusion durations in children with acute lymphoblastic leukaemia: with a longer follow‐up none of the assessed outcomes changed. However, more data on the risk of bias became available, changing the risk of performance bias from unclear to high. For the 'Risk of bias' assessment we used the most recent recommendations of the Cochrane Childhood Cancer, which are based on the Cochrane Handbook for Systematic Reviews of Interventions. All publications (including those already included in earlier versions of the review) were scored using the new 'Risk of bias' criteria. |
| 9 December 2015 | New search has been performed | The search for eligible studies was updated to December 2015. |
| 11 May 2009 | New citation required and conclusions have changed | Summary of most important changes in the update: The search for eligible studies was updated to November 2008 using an updated search strategy. Five new RCTs were included: one addressing anthracycline infusion duration and four assessing anthracycline peak doses. We also identified a study awaiting classification (addressing anthracycline peak doses); characteristics of this trial are provided. As opposed to the original review, in which no trials addressing anthracycline peak doses were identified, now there was evidence available for different combinations of anthracycline peak doses. For different anthracycline infusion durations the conclusions did not change. We did identify an error in the original analysis of response rate of different anthracycline infusion durations. The effect estimate changed from a risk ratio of 0.83 (95% CI 0.45 to 1.54; P = 0.6) to a risk ratio of 1.20 (95% CI 0.65 to 2.22; P = 0.6). However, the overall conclusion did not change. |
| 14 October 2008 | Amended | Converted to new review format. |
| 27 June 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank Edith Leclercq, the Trials Search Co‐ordinator of Cochrane Childhood Cancer, for running the search strategy for the updates in the different databases and providing us with the titles and abstracts of the searches. We thank Jorrit van As for the identification of studies in the top‐up search performed in December 2015. We would also like to thank Prof Dr Axel Heidenreich and Prof Dr Steven Lipshultz for providing additional information regarding their studies. Huib Caron was a co‐author of the protocol for this systematic review, the original version, and the first update, and we thank him for his valuable input. Finally, we would like to thank Stichting Kinderen Kankervrij (KiKa), the Netherlands for the financial support that made it possible to perform this update; the editorial base of the Cochrane Childhood Cancer Group is funded by Stichting Kinderen Kankervrij (KiKa). We calculated the hazard ratio and associated statistics for survival analyses using an Excel spreadsheet developed by Matthew Sydes and Jayne Tierney of the Medical Research Council Clinical Trials Unit, London, United Kingdom. We also thank Jo Morrison, Gail Quinn and Clare Jess for their contribution to the editorial process.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Search strategy for EMBASE/Ovid
For anthracycline chemotherapy the following subject headings and text words were used (in the original version of the review):
exp doxorubicin derivative/ or exp doxorubicin/ or exp daunorubicin derivative/ or exp daunorubicin/ or exp epirubicin/ or exp idarubicin/ or anthracycline antibiotic agent/ or anthracycline/ or exp anthracycline derivative/
For the updates we added the following to the search: or anthracyclines.mp or anthracyclin$.mp or anthracycline antibiotics.mp or doxorubicin.mp or adriablastin$.mp or adriblastin$.mp or adriamycin.mp or doxorubicin hydrochloride.mp or doxorubic$.mp or adriamyc$.mp or doxil.mp or caelyx.mp or myocet.mp or liposomal doxorubicin.mp or exp idarubicin derivative/ or idarubicin.mp or (4 demethoxydaunomycin or 4 demethoxydaunorubicin or 4 desmethoxydaunomycin or 4 desmethoxydaunorubicin).mp or idarubicin hydrochloride.mp or idarubic$.mp or epirubicin.mp or (epirubic$ or farmorubicin$).mp or (epirubicin hydrochloride or 4' epirubicin or epidoxorubicin or 4' epidoxorubicin or epiadriamycin or 4' epiadriamycin or 4 epiadriamycin).mp or daunorubicin.mp or daunorubic$.mp or (rubidomycin or rubomycin).mp or (cerubidin$ or daunoblastin$ or rubidomyc$ or daunoxome or daunosom$ or daunomycin or daunorubimycin or daunorubidomycin or daunorubicin hydrochloride or daunomycin hydrochloride).mp
For the different dosage schedules the following subject headings and text words were used (in the original version of the review):
(administration and dosage).mp or exp drug administration/ or exp intravenous drug administration/ or peak dose.mp or infusion duration.mp
For the updates we added the following to the search: or drug administration schedule.mp or exp drug administration/ or drug administration route.mp or exp drug administration route/ or drug administration routes.mp or drug administration method.mp or drug administration schedule.mp or drug administration schedules.mp or intravenous drug administration.mp or cumulative.mp or dosage.mp
For heart damage the following subject headings and text words were used (in the original version of the review):
exp heart ventricle failure/ or exp heart left ventricle function/ or exp congestive heart failure/ or exp heart right ventricle failure/ or exp heart failure/ or exp heart/ or exp forward heart failure/ or exp heart right ventricle function/ or exp heart disease/ or exp cardiotoxicity/ or exp cardiomyopathy/ or exp congestive cardiomyopathy/ or exp heart ventricle function/ or exp congestive heart failure/
For the updates we added the following to the search: or heart.mp or heart ejection fraction/ or exp heart right ventricle ejection fraction/ or exp heart function/ or exp forward heart failure/ or exp heart function test/ or exp heart left ventricle failure/ or exp heart ventriculography/ or exp heart left ventricle ejection fraction/ or congestive heart failure.mp or cardiomyopathy.mp or cardiotoxicity.mp or heart disease.mp or cardiac disease.mp or heart failure.mp or ventricular dysfunction.mp or shortening fraction.mp or ejection fraction.mp or (MUGA or LVEF or LVSF).mp or echocardiography.mp or exp echocardiography/ or radionuclide angiography.mp or radionuclide ventriculography.mp or exp radioisotope ventriculography/ or gated blood‐pool imaging.mp or endomyocardial biopsy.mp or exp heart muscle biopsy/ or angiocardiography.mp or exp angiocardiography/ or blood pool scintigraphy.mp or (cardiotox$ or cardiomyop$ or echocardiogr$ or ventriculogr$ or scintigr$).mp
For randomised controlled trials the following subject headings and text words were used (in the original version of the review; based on the highly sensitive search strategy for identifying reports of randomised controlled trials as described in the Cochrane Handbook (Higgins 2005)):
Randomized controlled trial/ or clinical trial/ or exp clinical trial/ or exp randomization/ or exp controlled study/ or double blind procedure/ or single blind procedure/ or exp placebo/ or exp comparative study/ or exp prospective study/
For the updates we used the following strategy (based on the highly sensitive search strategy for identifying reports of randomised controlled trials as described in the Cochrane Handbook (Higgins 2008)): (randomized controlled trial/ or controlled clinical trial/ or randomized.ti,ab or placebo.ti,ab or randomly.ti,ab or trial.ti,ab or groups.ti,ab or drug therapy.sh) and human/
The above described searches for anthracycline chemotherapy, dosage schedules, heart damage and randomised trials were combined.
[mp = title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name]; [ti,ab = title, abstract]; [sh = subject heading]
Appendix 2. Search strategy for the Cochrane Central Register of Controlled Trials (CENTRAL)
For anthracycline chemotherapy the following subject headings and text words were used (in the original version of the review):
Anthracyclines or adriamycin or epirubicin or daunorubicin or idarubicin or doxorubicin or anthracyclin* or doxorubic* or idarubic* or epirubic* or daunorubic* or epiadriamycin or adriamyc* or rubidomyc* or doxil or daunoxome or antibiotics anthracycline or (anthracycline next antibiotic)
For the updates we added the following to the search: or anthracycline antibiotics or farmorubicin* or rubidomycin or cerubidin* or caelyx or myocet
For the different dosage schedules the following subject headings and text words were used (in the original version of the review):
Drug administration schedule or infusions intravenous or dosage or (peak next dose) or (drug next administration next schedule)
For the updates we added the following to the search: or (administration and dosage) or drug administration schedules or cumulative or peak or infusion duration
For heart damage the following subject headings and text words were used (in the original version of the review):
Heart or heart diseases or ventricular dysfunction or (heart next diseases) or heart* or (heart next disease*) or (cardiac next disease*) or cardiomyopathy or cardiotoxicity or (heart next failure) or (cardiac next failure) or (congestive next heart next failure)
For the updates we added the following to the search: or cardiotox* or cardiomyopathies or cardiomyop* or shortening fraction or ejection fraction or LVSF or LVEF or echocardiography or echocardiogr* or radionuclide angiography or radionuclide ventriculography or ventriculogr* or MUGA or gated blood‐pool imaging or angiocardiography or endomyocardial biopsy or first pass ventriculography
The above described searches for anthracycline chemotherapy, dosage schedules and heart damage were combined.
Appendix 3. Search strategy for MEDLINE/PubMed
For anthracycline chemotherapy the following subject headings and text words were used (in the original version of the review):
anthracyclines OR anthracyclin* OR anthracycline antibiotics OR antibiotics, anthracycline OR 4‐demethoxydaunorubicin OR 4 demethoxydaunorubicin OR 4‐desmethoxydaunorubicin OR 4 desmethoxydaunorubicin OR IMI 30 OR IMI30 OR IMI‐30 OR idarubicin hydrochloride OR hydrochloride, idarubicin OR NSC 256439 OR NSC‐256439 OR NSC256439 OR idarubicin OR idarubic* OR 4'‐epiadriamycin OR 4' epiadriamycin OR 4'‐epidoxorubicin OR 4' epidoxorubicin OR 4'‐epi‐doxorubicin OR 4' epi doxorubicin OR 4'‐epi‐adriamycin OR 4' epi adriamycin OR 4'‐epi‐DXR OR 4' epi DXR OR epirubicin hydrochloride OR hydrochloride, epirubicin OR farmorubicin OR IMI‐28 OR IMI 28 OR IMI28 OR NSC 256942 OR NSC‐256942 OR NSC256942 OR epirubicin OR epirubic* OR adriablastine OR adriblastin OR adriablastin OR adriamycin OR DOX‐SL OR DOX SL OR DOXSL OR doxorubicin hydrochloride OR hydrochloride, doxorubicin OR doxorubic* OR adriamyc* OR dauno‐rubidomycine OR dauno rubidomycin OR rubidomycin OR rubomycin OR daunomycin OR cerubidine OR daunoblastin OR daunoblastine OR daunorubicin hydrochloride OR hydrochloride, daunorubicin OR daunorubic* OR rubidomyc* OR NSC‐82151 OR NSC 82151 OR NSC82151 OR daunoxome OR daunosom* OR doxil OR caelyx OR liposomal doxorubicin OR doxorubicin, liposomal.
For the updates we changed farmorubicin into farmorubicin*, adriblastin into adriblastin* and dauno rubidomycin into dauno rubidomycine; we added myocet, daunorubicin and doxorubicin (using OR) to the search.
For the different dosage schedules the following subject headings and text words were used (in both the original version of the review and the updates):
administration and dosage OR administration schedule, drug OR administration schedules, drug OR drug administration schedules OR schedule, drug administration OR schedules, drug administration OR drug administration schedule OR cumulative OR peak OR infusion duration OR dosage.
For heart damage the following subject headings and text words were used (in the original version of the review):
heart OR heart diseases OR heart disease OR disease, heart OR diseases, heart OR cardiac diseases OR cardiac disease OR diseases, cardiac OR disease, cardiac OR cardiotoxicity OR cardiomyopathy OR heart failure, congestive OR heart failure OR cardiomyopathy, congestive OR ventricular dysfunction OR ventricular dysfunction, left OR ventricular dysfunction, right.
For the updates we added the following to the search: OR shortening fraction OR ejection fraction OR echocardiography OR radionuclide angiography OR radionuclide ventriculography OR ventriculography, radionuclide OR gated blood‐pool imaging OR blood pool scintigraphy OR gated radionuclide ventriculography OR ventriculography, first pass OR cardiotox* OR cardiomyop* OR echocardiogr* OR ventriculogr* OR scintigr* OR MUGA OR LVEF OR LVSF OR endomyocardial biopsy OR angiocardiography OR cardiomyopathies.
The above described searches for anthracycline chemotherapy, dosage schedules and heart damage were combined. Finally, the results of this search were combined with the highly sensitive search strategy for identifying reports of randomised controlled trials as described in the Cochrane Handbook (for the original review: Higgins 2005 (all phases); for the updates: Higgins 2008 (sensitivity‐maximizing version)).
Data and analyses
Comparison 1. Infusion duration less than 6 hours versus infusion duration 6 hours or more.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Clinical heart failure | 5 | 557 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.09, 0.81] |
| 1.2 (Sub)clinical heart failure combined | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.2.1 (Sub)clinical heart failure combined (subclinical defined as >=10% decrease in LVEF) | 1 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.46, 1.26] |
| 1.2.2 (Sub)clinical heart failure combined (subclinical defined as >=15% decrease in LVEF) | 1 | 52 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.03, 2.78] |
| 1.2.3 (Sub)clinical heart failure combined (subclinical defined as a fall in LVEF of > 20%) | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.00, 0.60] |
| 1.2.4 (Sub)clinical heart failure combined (subclinical defined as a decrease in LVEF) | 1 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.15, 0.90] |
| 1.3 Response rate | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.3.1 Response rate (defined as complete or partial remission) | 2 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.65, 2.22] |
| 1.3.2 Response rate (defined as good response) | 1 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.91, 1.66] |
| 1.4 Overall survival | 2 | 322 | Hazard Ratio (IV, Random, 95% CI) | 1.42 [0.61, 3.30] |
Comparison 2. Doxorubicin peak dose less than 60 mg/m2 versus 60 mg/m2 or more.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Clinical heart failure | 2 | 4146 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.23, 1.88] |
| 2.2 Overall survival | 2 | 4146 | Hazard Ratio (IV, Random, 95% CI) | 1.06 [0.93, 1.22] |
| 2.3 Adverse effects other than cardiac damage | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3.1 Treatment‐related death | 1 | 3114 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.99] |
| 2.3.2 Death attributable to chemotherapy | 1 | 1032 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 2.3.3 Leukopenia grade 4 | 1 | 3114 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.53, 0.64] |
| 2.3.4 Leukopenia grade 3 or 4 | 1 | 1032 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.21, 0.31] |
| 2.3.5 Granulocytopenia grade 4 | 1 | 3114 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.61, 0.73] |
| 2.3.6 Thrombocytopenia grade 4 | 1 | 3114 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.34, 0.59] |
| 2.3.7 Diarrhoea grade 3 or 4 | 1 | 3114 | Risk Ratio (M‐H, Random, 95% CI) | 0.34 [0.19, 0.60] |
| 2.3.8 Dyspnoea grade 3 or 4 | 1 | 3114 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.28, 0.93] |
| 2.3.9 Infection grade 3 or 4 | 1 | 3114 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.42, 0.86] |
| 2.3.10 Malaise/fatigue/lethargy grade 3 or 4 | 1 | 3114 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.49, 0.91] |
| 2.3.11 Nausea grade 3 or 4 | 1 | 3114 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.98, 1.44] |
| 2.3.12 Stomatitis grade 3 or 4 | 1 | 3114 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.27, 0.61] |
| 2.3.13 Vomiting grade 3 or 4 | 1 | 3114 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [1.07, 1.59] |
Comparison 3. Liposomal doxorubicin (Caelyx) peak dose 25 mg/m2 versus 50 mg/m2.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Response rate (defined as objective palliative tumour response (i.e. decrease in PSA levels of >= 50%)) | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.13] |
| 3.2 Adverse effects other than cardiac damage | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.2.1 Gastrointestinal toxicity grade 3 or 4 | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.01, 3.08] |
| 3.2.2 Tachycardia grade 3 or 4 | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.00, 1.00] |
| 3.2.3 Arrhythmia grade 3 or 4 | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.04, 3.52] |
| 3.2.4 Dyspnoea grade 3 or 4 | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.10, 2.20] |
| 3.2.5 Palmar‐plantar erythrodysesthesia grade 3 or 4 | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 5.91 [1.45, 24.16] |
| 3.2.6 Hepatic toxicity grade 3 or 4 | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.05, 0.79] |
| 3.2.7 Leukopenia grade 3 or 4 | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.24 [0.03, 1.87] |
| 3.2.8 Thrombocytopenia grade 3 or 4 | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.02, 9.15] |
| 3.2.9 Haemoglobin‐related toxicity grade 3 or 4 | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.13] |
Comparison 4. Epirubicin peak dose 110 mg/m2 versus 83 mg/m2.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Clinical heart failure | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.06, 15.48] |
| 4.2 Adverse effects other than cardiac damage | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.2.1 Anaemia grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 2.91 [0.79, 10.70] |
| 4.2.2 Leukopenia grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.75, 1.49] |
| 4.2.3 Neutropenia grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.84, 1.31] |
| 4.2.4 Febrile neutropenia grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.47, 1.31] |
| 4.2.5 Thrombocytopenia grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 12.62 [0.71, 223.52] |
| 4.2.6 Nausea/vomiting grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.45, 1.82] |
| 4.2.7 Fatigue grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.07, 1.60] |
| 4.2.8 Infection grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.48, 1.31] |
| 4.2.9 Central nervous system grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 2.91 [0.12, 71.35] |
| 4.2.10 Pulmonary grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 2.91 [0.12, 71.35] |
| 4.2.11 Peripheral neuropathy grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 4.50 [2.37, 8.54] |
| 4.2.12 Hepatotoxicity grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.50, 5.77] |
| 4.2.13 Hypersensitivity reactions grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 3.88 [1.71, 8.82] |
| 4.2.14 Mucositis grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.48, 2.28] |
| 4.2.15 Pain grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.93] |
| 4.2.16 Arthralgias/myalgias grade 3 or 4 | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 3.88 [1.31, 11.54] |
| 4.2.17 Treatment‐related death | 1 | 1086 | Risk Ratio (M‐H, Random, 95% CI) | 2.91 [0.12, 71.35] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Budman 1998.
| Study characteristics | ||
| Methods | Method of randomisation not clear (stratified according to the type of primary surgery (mastectomy or lumpectomy), number of involved axillary lymph nodes (1 to 3, 4 to 9, or 10 or more), menopausal status (premenopausal or perimenopausal/postmenopausal), and estrogen receptor status (negative or positive)) | |
| Participants | 1032 women (median age 48 and 49 years in the peak dose 60 mg/m2 and 40 mg/m2 respectively) with unilateral stage II adenocarcinoma of the breast (T1N1M0/T2N1M0) treated with doxorubicin, cyclophosphamide, and 5‐fluorouracil. Also, if a lumpectomy was performed, women received radiotherapy of the entire breast (5040 cGy and a 1504 cGy boost on the area of the excision); mastectomy participants received no irradiation (the majority of women in both groups received a mastectomy, but exact numbers nm; location of the tumour nm). No prior anthracycline therapy; no prior cardiac radiotherapy; no prior cardiac dysfunction | |
| Interventions | Doxorubicin (infusion duration nm) with a peak dose of either 60 mg/m2 (N = 519; cumulative anthracycline dose nm; the planned cumulative dose was 240 mg/m2) or 40 mg/m2 (N = 513; cumulative anthracycline dose nm; the planned cumulative dose was 240 mg/m2) | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as CALGB grade 3 to 5) OS (defined as time from study entry to death from any cause) Adverse effects other than cardiac damage (according to CALGB criteria) |
|
| Notes | Some of the data presented in this table were obtained from an earlier article describing this study (Wood 1994). This article was excluded from the original version of the review because it was unknown if women in both treatment groups received the same cumulative anthracycline dose. In Budman 1998 it was stated that both treatment regimens delivered the same cumulative dose (even though the exact cumulative anthracycline dose is still not documented). There was a third treatment group in this study, i.e. a doxorubicin peak dose of 30 mg/m2. However, women in this group were excluded from this review because the cumulative doses of doxorubicin, cyclophosphamide, and 5‐fluorouracil were lower than the other treatment groups. Length of follow‐up nm (including the women in the third treatment group who were excluded from this review, the median follow‐up was 9 years; range 3.5 to 12.8 years). The study was supported in part by different Public Health Service grants from the National Cancer Institute, National Institutes of Health, Department of Health and Human Services, but no information on the influence of funders was provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided, although due to the nature of the interventions, this was most likely not the case |
| Blinding of outcome assessment (detection bias): clinical heart failure | Unclear risk | No information on blinding of outcome assessors was provided for clinical heart failure |
| Blinding of outcome assessment (detection bias): overall survival | Low risk | No information on blinding of outcome assessors was provided, but since this is not applicable for overall survival, we judged this as a low risk of bias. |
| Blinding of outcome assessment (detection bias): adverse effects other than cardiac damage | Unclear risk | No information on blinding of outcome assessors was provided for adverse effects other than cardiac damage |
| Incomplete outcome data (attrition bias): clinical heart failure | Low risk | Almost all women (99.1%) were included in the analysis of clinical heart failure |
| Incomplete outcome data (attrition bias): overall survival | Unclear risk | Not documented how many women were included in the analysis of overall survival |
| Incomplete outcome data (attrition bias): adverse effects other than cardiac damage | Unclear risk | Not documented how many women were included in the analysis of adverse effects other than cardiac damage |
| Selective reporting (reporting bias) | Low risk | There was no reference to a protocol provided in the manuscript (and we did not search for it), but all expected outcomes were reported |
| Other bias | Unclear risk |
Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex, and/or prior cardiac dysfunction): no (all items were balanced between treatment groups) Difference in length of follow‐up between treatment arms: unclear (not reported) |
Casper 1991.
| Study characteristics | ||
| Methods | Method of randomisation not clear (stratified according to presence or absence of microscopically positive margins) | |
| Participants | 82 participants (aged 18 to 87 years; 39 women and 44 men) with high‐grade non‐metastatic soft tissue sarcoma treated with doxorubicin. No prior anthracycline therapy; prior cardiac radiotherapy possible for 2 participants in the bolus group and 3 participants in the continuous infusion group; no prior cardiac dysfunction | |
| Interventions | Doxorubicin (peak dose 60 mg/m2) every 3 weeks for a total of 9 cycles with either bolus (5 to 10 min) infusion (N = 39; median cumulative dose 420 mg/m2; range 60 to 540 mg/m2) or continuous infusion (72 h) (N = 43; median cumulative dose nm; range 120 to 540 mg/m2) | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as congestive heart failure; subclinical heart failure defined as a 10% or more decrease in LVEF at rest as measured by radionuclide cineangiograms) OS (definition nm) |
|
| Notes | One participant randomised to bolus therapy actually received the drug by continuous infusion. We performed an intention‐to‐treat analysis, but the data presented in this table are for 38 participants in the bolus and 44 participants in the continuous infusion group. One participant in the bolus group and 3 participants in the continuous infusion group never received treatment Length of follow‐up nm The study was supported by a grant from the National Institutes of Health, but no information on the influence of funders was provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided, although due to the nature of the interventions, this was most likely not the case |
| Blinding of outcome assessment (detection bias): clinical heart failure | Unclear risk | No information on blinding of outcome assessors was provided for clinical heart failure. |
| Blinding of outcome assessment (detection bias): subclinical heart failure (dichotomous and/or continuous) | Unclear risk | No information on blinding of outcome assessors was provided for subclinical heart failure |
| Blinding of outcome assessment (detection bias): overall survival | Low risk | No information on blinding of outcome assessors was provided, but since this is not applicable for overall survival, we judged this as a low risk of bias |
| Incomplete outcome data (attrition bias): clinical heart failure | Unclear risk | Not documented how many participants were included in the analysis of clinical heart failure |
| Incomplete outcome data (attrition bias): subclinical heart failure (dichotomous and/or continuous) | High risk | Only 84.1% of participants were included in the analysis |
| Incomplete outcome data (attrition bias): overall survival | Low risk | All participants were included in the analysis |
| Selective reporting (reporting bias) | Low risk | There was no reference to a protocol provided in the manuscript (and we did not search for it), but all expected outcomes were reported |
| Other bias | Unclear risk |
Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex, and/or prior cardiac dysfunction): unclear (unclear if prior cardiotoxic treatment was balanced between treatment groups; all other items were balanced between treatment groups) Difference in length of follow‐up between treatment arms: unclear (not reported) |
Escherich 2007.
| Study characteristics | ||
| Methods | Method of randomisation not clear (stratified according to white blood cell count < 25/nl or >= 25/nl) | |
| Participants | 178 children (of which 101 children were evaluable; these evaluable children were aged 1.1 to 17.9 years; 60 boys and 41 girls) with low‐ or high‐risk B‐precursor ALL or T‐ALL treated with a multidrug regimen including daunorubicin. No prior anthracycline therapy; no prior cardiac radiotherapy; prior cardiac dysfunction nm. | |
| Interventions | Daunorubicin (peak dose 36 mg/m2) on day 1 with either bolus (1 hour) infusion (N = 85; cumulative anthracycline dose 36 mg/m2 on day 7; see notes) or continuous (24 hours) infusion (N = 93; cumulative anthracycline dose 36 mg/m2 on day 7; see notes) | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as clinical signs of cardiac insufficiency; subclinical heart failure defined as LVSF < 25%) Response rate (i.e. good response defined as an absolute blast cell count < 1000/µl at day 7) |
|
| Notes | Only 101 of the 178 children were evaluable for in‐vivo cell kill; the other 77 children (42 in the 1‐hour infusion group and 35 in the 24‐hours infusion group) had incomplete data or insufficient smears. However, we performed an intention‐to‐treat analysis. After the first daunorubicin administration, all children received additional daunorubicin with an infusion duration of 1 hour. Therefore, only data for the first 7 days are eligible for this review. Length of follow‐up 7 days. The study was supported in part by Fördergemeinschaft Kinderkrebszentrum Hamburg e.V. and Elterninitiative Kinderkrebsklinik Düsseldorf e.V., but no information on the influence of funders was provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided, although due to the nature of the interventions, this was most likely not the case |
| Blinding of outcome assessment (detection bias): clinical heart failure | Unclear risk | No information on blinding of outcome assessors was provided for clinical heart failure |
| Blinding of outcome assessment (detection bias): subclinical heart failure (dichotomous and/or continuous) | Unclear risk | No information on blinding of outcome assessors was provided for subclinical heart failure |
| Blinding of outcome assessment (detection bias): tumour response | Unclear risk | No information on blinding of outcome assessors was provided for tumour response |
| Incomplete outcome data (attrition bias): clinical heart failure | High risk | 43% of children lost to follow‐up |
| Incomplete outcome data (attrition bias): subclinical heart failure (dichotomous and/or continuous) | High risk | 43% of children lost to follow‐up |
| Incomplete outcome data (attrition bias): tumour response | High risk | 43% of children lost to follow‐up |
| Selective reporting (reporting bias) | High risk | There was no reference to a protocol provided in the manuscript (and we did not search for it), but not all expected outcomes were reported in a useful manner |
| Other bias | Unclear risk |
Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex, and/or prior cardiac dysfunction): unclear (unclear if prior cardiac dysfunction was balanced between treatment groups; the other items were balanced between treatment groups) Difference in length of follow‐up between treatment arms: no |
Fountzilas 2008.
| Study characteristics | ||
| Methods | Randomisations were performed at the HeCOG Data Office (balanced by centre and stratified according to menopausal status (premenopausal versus postmenopausal), hormonal receptor status (positive versus negative), and number of positive nodes (1 to 3 versus 4 or more) | |
| Participants | 1086 women (aged 22 to 79 years) with non‐metastatic node‐positive epithelial breast cancer (T1‐4/N1‐2/M0) treated with epirubicin, paclitaxel, cyclophosphamide, methotrexate, and fluorouracil. Also, radiotherapy was mandatory for all women with breast‐conserving surgery (35% of women in both treatment groups) or for those with 4 or more positive lymph nodes (52% of women in the high peak dose group and 51% of women in the low peak dose group), and/or tumour size 5 cm or larger (irrespective of the initial operation type; 11% of women in both treatment groups). Radiation dose was 50 to 55 Gy on the entire breast or chest wall followed by a 10 to 15 Gy boost on the area where the tumour was initially located (Fountzilas 2005). Location of the tumour was nm. Prior anthracycline therapy nm; prior cardiac radiotherapy nm; no prior cardiac dysfunction | |
| Interventions | Epirubicin (infusion duration nm) with a peak dose of either 110 mg/m2 (N = 551; cumulative anthracycline dose nm; the planned cumulative dose was 330 mg/m2) or 83 mg/m2 (N = 535; cumulative anthracycline dose nm; the planned cumulative dose was 332 mg/m2) | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as mild congestive heart failure responsive to therapy (WHO grade 3)) Adverse effects other than cardiac damage (according to WHO criteria) |
|
| Notes | The data presented in this table are for the 1063 out of 1086 women (540 out of 551 women in the high peak dose group and 523 out of 535 in the low peak dose group); 14 women were excluded because they never started therapy and 9 women had incomplete treatment and toxicity data. However, we performed an intention‐to‐treat analysis. Although the cumulative anthracycline doses women in both treatment groups received were not documented, the authors of this study have stated that the median cumulative doses of all drugs were almost identical in both groups. Median length of follow‐up 40 months. No funding documented. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that randomisation was performed at the HeCOG Data Office, but no further information on the method of randomisation was provided |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed at the HeCOG Data Office |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided, although due to the nature of the interventions, this was most likely not the case |
| Blinding of outcome assessment (detection bias): clinical heart failure | Unclear risk | No information on blinding of outcome assessors was provided for clinical heart failure |
| Blinding of outcome assessment (detection bias): adverse effects other than cardiac damage | Unclear risk | No information on blinding of outcome assessors was provided for adverse effects other than cardiac damage |
| Incomplete outcome data (attrition bias): clinical heart failure | Unclear risk | It was not documented in how many women clinical heart failure was assessed; at least 2.1% not analysed |
| Incomplete outcome data (attrition bias): adverse effects other than cardiac damage | Unclear risk | It was not documented in how many women adverse effects other than cardiac damage were assessed; at least 2.1% not analysed |
| Selective reporting (reporting bias) | High risk | There was no reference to a protocol provided in the manuscript (and we did not search for it), but not all expected outcomes were reported in a useful manner |
| Other bias | Unclear risk |
Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex, and/or prior cardiac dysfunction): unclear (unclear if prior cardiotoxic treatment was balanced between treatment groups; the other items were balanced between treatment groups) Difference in length of follow‐up between treatment arms: unclear (not reported) |
Heidenreich 2004.
| Study characteristics | ||
| Methods | Method of randomisation not clear | |
| Participants | 48 men (aged 58 to 79 years) with metastatic hormone‐refractory prostate carcinoma treated with liposomal doxorubicin (Caelyx). No prior anthracycline therapy; prior cardiac radiotherapy nm; no prior cardiac dysfunction | |
| Interventions | Liposomal doxorubicin (Caelyx; 1‐hour infusion) with a peak dose of either 25 mg/m2 (N = 22; cumulative anthracycline dose 323.5 mg per man; range 50 to 600 mg per man) or 50 mg/m2 (N = 26; cumulative anthracycline dose 416.13 mg per man; range 100 to 1200 mg per man) | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as congestive heart failure; subclinical heart failure defined as LVEF < 40% on echocardiography). Response rate (i.e. objective palliative response rate defined as a reduction in serum PSA levels by >= 50% relative to baseline, with this reduction persisting for >= 4 weeks and accompanied by stabilisation or improvement in the man's performance status). Quality of life (according to the 30‐item European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire). Adverse effects other than cardiac damage (according to the National Cancer Institute of Canada/CALGB grading system). |
|
| Notes | Mean length of follow‐up 42 months. Anthracycline doses were not available as mg/m2. Some of the information provided in this table was not included in the article, but was provided by the author upon our request. No funding documented. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided, although due to the nature of the interventions, this was most likely not the case |
| Blinding of outcome assessment (detection bias): clinical heart failure | Unclear risk | No information on blinding of outcome assessors was provided for clinical heart failure |
| Blinding of outcome assessment (detection bias): subclinical heart failure (dichotomous and/or continuous) | Unclear risk | No information on blinding of outcome assessors was provided for subclinical heart failure |
| Blinding of outcome assessment (detection bias): tumour response | Unclear risk | No information on blinding of outcome assessors was provided for tumour response |
| Blinding of outcome assessment (detection bias): adverse effects other than cardiac damage | Unclear risk | No information on blinding of outcome assessors was provided for adverse effects other than cardiac damage |
| Blinding of outcome assessment (detection bias): quality of life | Unclear risk | No information on blinding of outcome assessors was provided for quality of life |
| Incomplete outcome data (attrition bias): clinical heart failure | Low risk | All men were included in the analysis |
| Incomplete outcome data (attrition bias): subclinical heart failure (dichotomous and/or continuous) | Low risk | All men were included in the analysis |
| Incomplete outcome data (attrition bias): tumour response | Low risk | Almost all men (96%) were included in the analysis of clinical heart failure |
| Incomplete outcome data (attrition bias): adverse effects other than cardiac damage | Low risk | All men were included in the analysis |
| Incomplete outcome data (attrition bias): quality of life | Unclear risk | It was not documented in how many men quality of life was assessed |
| Selective reporting (reporting bias) | High risk | There was no reference to a protocol provided in the manuscript (and we did not search for it), but not all expected outcomes were reported in a useful manner |
| Other bias | Unclear risk |
Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex, and/or prior cardiac dysfunction): unclear (unclear if prior cardiotoxic treatment was balanced between treatment groups; the other items were balanced between treatment groups) Difference in length of follow‐up between treatment arms: unclear (not reported) |
Hortobagyi 1989.
| Study characteristics | ||
| Methods | Method of randomisation not clear (stratified according to performance status, number of organ sites involved by metastases, cumulative dose of prior anthracyclines, and whether prior anthracycline therapy had been given as postoperative adjuvant or as palliative treatment for metastatic disease) | |
| Participants | 52 women (aged 28 to 74 years) with progressive metastatic breast cancer treated with epirubicin. Prior anthracycline therapy in 21 women in the continuous infusion group and 12 women in the bolus infusion group; for 2 women in the bolus infusion group it was unclear; cumulative dose of prior anthracycline therapy nm. Prior cardiac radiotherapy nm; prior cardiac dysfunction possible (number of participants nm) | |
| Interventions | Epirubicin (peak dose 90 mg/m2) with either bolus (15 minutes) infusion (N = 25; median cumulative anthracycline dose including previous therapy 540 mg/m2; range 90 to 1055 mg/m2) or continuous (48 hours) infusion (N = 27; median cumulative anthracycline dose including previous therapy 630 mg/m2; range 110 to 1420 mg/m2) | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as congestive heart failure; subclinical heart failure defined as a 15% or more decrease in LVEF as measured by cardiac scan or echocardiography). Tumour response (i.e. CR defined as disappearance of all clinical evidence of active tumour including symptoms and signs for a minimum of 4 weeks; PR defined as a greater than 50% decrease in the sum of the products of the longest perpendicular diameters of measurable lesions for at least 4 weeks. Simultaneous increase in the size of any lesion or the appearance of any new lesions was not permitted). Survival (OS was defined as survival from the initiation of present drug therapy). |
|
| Notes | Length of follow‐up nm. The study was supported in part by a grant‐in‐aid from Farmitalia, but no information on the influence of funders was provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided, although, due to the nature of the interventions, this was most likely not the case |
| Blinding of outcome assessment (detection bias): clinical heart failure | Unclear risk | No information on blinding of outcome assessors was provided for clinical heart failure |
| Blinding of outcome assessment (detection bias): subclinical heart failure (dichotomous and/or continuous) | Unclear risk | No information on blinding of outcome assessors was provided for subclinical heart failure |
| Blinding of outcome assessment (detection bias): tumour response | Unclear risk | No information on blinding of outcome assessors was provided for tumour response |
| Blinding of outcome assessment (detection bias): overall survival | Low risk | No information on blinding of outcome assessors was provided, but since this is not applicable for overall survival we judged this as a low risk of bias |
| Incomplete outcome data (attrition bias): clinical heart failure | Low risk | Almost all participants (96.2%) were included in the analysis |
| Incomplete outcome data (attrition bias): subclinical heart failure (dichotomous and/or continuous) | Low risk | Almost all participants (96.2%) were included in the analysis |
| Incomplete outcome data (attrition bias): tumour response | Low risk | Almost all participants (96.2%) were included in the analysis |
| Incomplete outcome data (attrition bias): overall survival | Low risk | Almost all participants (96.2%) were included in the analysis |
| Selective reporting (reporting bias) | Low risk | There was no reference to a protocol provided in the manuscript (and we did not search for it), but all expected outcomes were reported |
| Other bias | High risk |
Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex, and/or prior cardiac dysfunction): high (prior anthracycline use was not balanced between treatment arms; unclear if prior cardiac radiotherapy and prior cardiac dysfunction were balanced between treatment groups; all other items were balanced between treatment groups) Difference in length of follow‐up between treatment arms: no |
Linden 2007.
| Study characteristics | ||
| Methods | Method of randomisation not clear (no stratification factors were used due to large sample size) | |
| Participants | 3114 women (aged 21.9 to 76.9 years) with high‐risk stage I or II breast cancer treated with doxorubicin and cyclophosphamide. Also, if less than a mastectomy was performed, women received external beam radiation therapy (39% of the women in the low peak dose group and 38% of women in the high peak dose group; location of the tumour nm; dose nm). No prior anthracycline therapy; no prior cardiac radiotherapy; no prior cardiac dysfunction | |
| Interventions | Doxorubicin (infusion duration nm) with a peak dose of either 54 mg/m2 (N = 1590; cumulative anthracycline dose nm; the planned cumulative dose was 324 mg/m2) or 81 mg/m2 (N = 1524; cumulative dose nm; the planned cumulative dose was 324 mg/m2) | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as congestive heart failure grade 3 or 4 according to SWOG criteria) OS (defined as time from registration to time of death due to any cause) Adverse effects other than cardiac damage (according to SWOG criteria) |
|
| Notes | Although the cumulative anthracycline doses women in both treatment groups received were not documented, the authors of this study have stated that women in both groups received identical total doses of chemotherapeutic agents. Length of follow‐up nm (median follow‐up for women still alive at the time of analysis is 7.2 years). The study was supported in part by the US Public Health Service Cooperative Agreement grants, but no information on the influence of funders was provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided, although due to the nature of the interventions, this was most likely not the case |
| Blinding of outcome assessment (detection bias): clinical heart failure | Unclear risk | No information on blinding of outcome assessors was provided for clinical heart failure |
| Blinding of outcome assessment (detection bias): overall survival | Low risk | No information on blinding of outcome assessors was provided, but since this is not applicable for overall survival we judged this as a low risk of bias |
| Blinding of outcome assessment (detection bias): adverse effects other than cardiac damage | Unclear risk | No information on blinding of outcome assessors was provided for adverse effects other than cardiac damage |
| Incomplete outcome data (attrition bias): clinical heart failure | Low risk | Almost all women (99.3%) were included in the analysis of clinical heart failure |
| Incomplete outcome data (attrition bias): overall survival | Unclear risk | Not documented how many women were included in the analysis of overall survival |
| Incomplete outcome data (attrition bias): adverse effects other than cardiac damage | Low risk | Almost all women (99.3%) were included in the analysis of adverse effects other than cardiac damage |
| Selective reporting (reporting bias) | Low risk | There was no reference to a protocol provided in the manuscript (and we did not search for it), but all expected outcomes were reported |
| Other bias | Unclear risk |
Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex, and/or prior cardiac dysfunction): no (all items were balanced between treatment groups) Difference in length of follow‐up between treatment arms: unclear (not reported) |
Lipshultz 2002.
| Study characteristics | ||
| Methods | Randomisations were performed centrally; in Lipshultz 2012 it was reported that a permuted‐block algorithm stratified by institution was used | |
| Participants | 145 children who had at least 1 follow‐up echocardiogram obtained before 1 April 1997 out of an RCT with 240 participants (Silverman 2001) (age 0.4 to 17.9 years; 53 girls and 68 boys) with high risk ALL treated with doxorubicin (all children received 30 mg/m2 doxorubicin on each of 2 days as a bolus infusion during induction therapy; this information was only reported in the long‐term follow‐up study), steroids, cytarabine, vincristine, methotrexate, 6‐MP, and asparaginase. No prior anthracycline therapy; no prior cardiac radiotherapy; no prior clinical cardiac dysfunction, in both groups prior asymptomatic cardiac dysfunction on echocardiography present (number of children nm) | |
| Interventions | Doxorubicin (peak dose 30 mg/m2) every 3 weeks with either bolus (less than 1 hour; see notes) infusion (N = 64; median cumulative anthracycline dose 336 mg/m2; range 228 to 360 mg/m2) or continuous (48 hours) infusion (N = 57; median cumulative anthracycline dose 340 mg/m2; range 222 to 360 mg/m2) | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as congestive heart failure; subclinical heart failure defined as median fall in left ventricular characteristics) | |
| Notes | The data presented in this table are for the 121 of the 145 children who had an echocardiogram of good quality. It was not documented to which group the 24 excluded children were randomised, so it was not possible to perform an intention‐to‐treat analysis. Median follow‐up was 1.5 years (range 0 to 4.7 years). Long‐term follow‐up data of this study have been published on 92 participants (N = 43 in bolus group and N = 49 in the continuous infusion group) who had at least 1 follow‐up echocardiogram at least 3 years after infusion duration assignment, had a baseline echocardiogram, and were in continuous complete remission (Lipshultz 2012). Median age at diagnosis was 4.6 years (range 1.6 to 16.2 years) in the bolus group and 3.7 years (range 0.7 to 16.9 years) in the continuous infusion group. It should be noted that in Lipshultz 2012 it was stated that a bolus infusion was given within 15 minutes instead of a 1‐hour infusion duration documented in the primary publication of this study (Lipshultz 2002). The authors provided the following clarification: "all infusions were less than 1 hour and basically this was less than 15 minutes". The median length of follow‐up was 8 years with a range of 3 to 13 years (8.3 years in the bolus group and 8.2 years in the continuous infusion group). Results of baseline echocardiograms were not reported, so it is unclear if there were children with prior cardiac dysfunction. The median cumulative doxorubicin dose in the bolus group was 342 mg/m2 (range 196 to 360 mg/m2); in the continuous infusion group it was 352 mg/m2 (range 204 to 360 mg/m2). The study was supported in part by National Institutes of Health grants, Children's Cardiomyopathy Foundation, Women's Cancer Association, Lance Armstrong Foundation, STOP Children's Cancer Foundation, Scott Howard Fund, and the Michael Garil Fund, but no information on the influence of funders was provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Allocation concealment (selection bias) | Low risk | Randomisations were performed centrally |
| Blinding of participants and personnel (performance bias) | High risk | Participants and treating physicians were not blinded |
| Blinding of outcome assessment (detection bias): clinical heart failure | Unclear risk | No information on blinding of outcome assessors was provided for clinical heart failure |
| Blinding of outcome assessment (detection bias): subclinical heart failure (dichotomous and/or continuous) | Low risk | The outcome assessors of subclinical heart failure were blinded |
| Incomplete outcome data (attrition bias): clinical heart failure | High risk | Only 50.4% of children were included in the analysis |
| Incomplete outcome data (attrition bias): subclinical heart failure (dichotomous and/or continuous) | High risk | Less than 38% of children evaluated for the different cardiac parameters |
| Selective reporting (reporting bias) | High risk | There was no reference to a protocol provided in the manuscript (and we did not search for it), but not all expected outcomes were reported in a useful manner |
| Other bias | Unclear risk |
Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex, and/or prior cardiac dysfunction): unclear (unclear if prior cardiac dysfunction was balanced between treatment groups; the other items were balanced between treatment groups) Difference in length of follow‐up between treatment arms: no |
Shapira 1990.
| Study characteristics | ||
| Methods | Randomisations were performed according to the last digit of the national identification number | |
| Participants | 62 women (age nm) with stage III or stage IV breast cancer (N = 36) or ovarian cancer (N = 26) treated with doxorubicin, cyclophosphamide, and either 5‐FU (breast cancer) or cisplatin (ovarian cancer). No prior anthracycline therapy; prior cardiac radiotherapy possible for 1 woman in the short infusion group and 3 women in the prolonged infusion group; no prior cardiac dysfunction | |
| Interventions | Doxorubicin (peak dose 50 mg/m2) every 3 weeks with either short infusion (15 to 20 minutes) (N = 31; mean cumulative dose 410 mg/m2; range 200 to 550 mg/m2) or prolonged infusion (6 hours) (N = 31; mean cumulative dose 428 mg/m2; range 250 to 600 mg/m2) | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as symptoms of congestive heart failure; subclinical heart failure defined as a fall in LVEF of more than 20% as measured by gated pool radionuclide angiography and defined as the mean fall in LVEF) Adverse effects other than cardiac damage (according to SWOG criteria) |
|
| Notes | Length of follow‐up nm No funding documented |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomisations were performed according to the last digit of the national identification number |
| Allocation concealment (selection bias) | High risk | Randomisations were performed according to the last digit of the national identification number |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided, although due to the nature of the interventions, this was most likely not the case |
| Blinding of outcome assessment (detection bias): clinical heart failure | Unclear risk | No information on blinding of outcome assessors was provided for clinical heart failure |
| Blinding of outcome assessment (detection bias): subclinical heart failure (dichotomous and/or continuous) | Unclear risk | No information on blinding of outcome assessors was provided for subclinical heart failure |
| Blinding of outcome assessment (detection bias): adverse effects other than cardiac damage | Unclear risk | No information on blinding of outcome assessors was provided for adverse effects other than cardiac damage |
| Incomplete outcome data (attrition bias): clinical heart failure | Low risk | Almost all women (93.5%) were included in the analysis of clinical heart failure |
| Incomplete outcome data (attrition bias): subclinical heart failure (dichotomous and/or continuous) | Low risk | Almost all women (93.5%) were included in the analysis of subclinical heart failure |
| Incomplete outcome data (attrition bias): adverse effects other than cardiac damage | Low risk | Almost all women (93.5%) were included in the analysis of adverse effects other than cardiac damage |
| Selective reporting (reporting bias) | High risk | There was no reference to a protocol provided in the manuscript (and we did not search for it), but not all expected outcomes were reported in a useful manner |
| Other bias | Unclear risk |
Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex, and/or prior cardiac dysfunction): unclear (unclear if age and prior cardiotoxic treatment were balanced between treatment groups; the other items were balanced between treatment groups) Difference in length of follow‐up between treatment arms: unclear (not reported) |
Steinherz 1993.
| Study characteristics | ||
| Methods | Method of randomisation not clear (stratified according to risk group, degree of leukocyte count elevation, age, FAB morphology, and presence or absence of lymphoma syndrome) | |
| Participants | 44 participants (aged 1 to 19 years; median 7 years; 11 girls and 33 boys) with ALL (31 high risk and 13 average risk) treated with daunorubicin, cytosine arabinoside, cyclophosphamide, vincristine, prednisone, L‐asparaginase, methotrexate, 6‐MP, thioguanine, and sometimes spinal (12 Gy for participants with CNS disease at diagnosis; N = 3, nm in which treatment group) and /or cranial irradiation. No prior anthracycline therapy; no prior cardiac radiotherapy; prior cardiac dysfunction nm | |
| Interventions | Daunorubicin (peak dose 120 mg/m2) with either bolus (push) infusion (N = 22; median cumulative dose 360 mg/m2 (range 120 to 585 mg/m2) for 18 participants with an echocardiogram) or continuous (48 hours) infusion (N = 22; median cumulative dose 400 mg/m2 (range 120 to 558 mg/m2) for 18 participants with an echocardiogram) | |
| Outcomes | Heart failure (i.e. subclinical heart failure defined as a LVSF of less than 29% or a 10% unit or more decrease from baseline to 29% (borderline function) or median change in LVSF as measured by echocardiography) | |
| Notes | Median length of follow‐up 54+ months (minimal 25+ months) No funding documented |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Allocation concealment (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided, although due to the nature of the interventions, this was most likely not the case |
| Blinding of outcome assessment (detection bias): subclinical heart failure (dichotomous and/or continuous) | Unclear risk | No information on blinding of outcome assessors was provided for subclinical heart failure |
| Incomplete outcome data (attrition bias): subclinical heart failure (dichotomous and/or continuous) | Low risk | All participants were included in the analysis |
| Selective reporting (reporting bias) | High risk | There was no reference to a protocol provided in the manuscript (and we did not search for it), but not all expected outcomes were reported in a useful manner |
| Other bias | Unclear risk |
Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex, and/or prior cardiac dysfunction): unclear (unclear if age, sex, and prior cardiac dysfunction were balanced between treatment groups; no prior cardiotoxic treatment) Difference in length of follow‐up between treatment arms: unclear (not reported) |
Zalupski 1991.
| Study characteristics | ||
| Methods | Randomisation was performed through the SWOG statistical centre (not stratified) | |
| Participants | 240 participants (aged 17 to 83 years; 121 women and 119 men) with metastatic soft tissue sarcoma treated with doxorubicin and dacarbazine. No prior anthracycline therapy; prior cardiac radiotherapy possible for 36 participants in bolus group and 31 participants in continuous infusion group; no prior cardiac dysfunction | |
| Interventions | Doxorubicin (60 mg/m2) repeated at 21‐day intervals by either bolus (N = 118; median cumulative dose 240 mg/m2) or continuous (96 hours) infusion (N = 122; median cumulative dose 221 mg/m2) | |
| Outcomes | Heart failure (i.e. clinical heart failure defined as drug‐related cardiac death and clinical cardiac events; subclinical heart failure defined as a decrease in LVEF as measured by non‐invasive testing. It was not documented what the exact method of non‐invasive testing was). Tumour response (i.e. CR defined as disappearance of all clinical evidence of tumour for a minimum of 4 weeks; PR defined as a 50% or greater reduction in the sum of the products of the perpendicular diameters of all measured lesions, no simultaneous increase in the size of any lesion could occur and no new lesions could occur. The response had to be maintained for at least 4 weeks). Survival (OS was defined as measured from the time of randomisation to death). |
|
| Notes | One participant randomised to the continuous infusion group received bolus infusion by mistake, and only 233 started therapy. However, we performed an intention‐to‐treat analysis. Length of follow‐up nm. The study was supported in part by different Public Health Service Cooperative Agreement grants awarded by the National Cancer Institute, National Institutes of Health, and Department of Health and Human Services, but no information on the influence of funders was provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that this was a randomised study, but no further information on the methods of randomisation was provided |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed through the SWOG statistical centre |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information on blinding of participants and personnel was provided, although due to the nature of the interventions, this was most likely not the case |
| Blinding of outcome assessment (detection bias): clinical heart failure | Unclear risk | No information on blinding of outcome assessors was provided for clinical heart failure |
| Blinding of outcome assessment (detection bias): subclinical heart failure (dichotomous and/or continuous) | Unclear risk | No information on blinding of outcome assessors was provided for subclinical heart failure |
| Blinding of outcome assessment (detection bias): tumour response | Unclear risk | No information on blinding of outcome assessors was provided for tumour response |
| Blinding of outcome assessment (detection bias): overall survival | Low risk | No information on blinding of outcome assessors was provided, but since this is not applicable for overall survival we judged this as a low risk of bias |
| Incomplete outcome data (attrition bias): clinical heart failure | Low risk | Almost all participants (97.1%) were included in the analysis |
| Incomplete outcome data (attrition bias): subclinical heart failure (dichotomous and/or continuous) | Low risk | Almost all participants (97.1%) were included in the analysis |
| Incomplete outcome data (attrition bias): tumour response | Low risk | Almost all participants (97.1%) were included in the analysis |
| Incomplete outcome data (attrition bias): overall survival | Low risk | Almost all participants (97.1%) were included in the analysis |
| Selective reporting (reporting bias) | Low risk | There was no reference to a protocol provided in the manuscript (and we did not search for it), but all expected outcomes were reported |
| Other bias | Unclear risk |
Baseline imbalance between treatment arms related to outcome (prior cardiotoxic treatment, age, sex, and/or prior cardiac dysfunction): unclear (unclear if prior cardiac radiotherapy and anthracycline was balanced between treatment arms; all other items were balanced between treatment groups) Difference in length of follow‐up between treatment arms: unclear (not reported) |
5‐FU = 5‐fluorouracil 6‐MP = 6‐mercaptopurine ALL = acute lymphoblastic leukaemia CALGB = Cancer and Leukemia Group B CNS = central nervous system CR = complete remission FAB = French American British LVEF = left ventricular ejection fraction LVSF = left ventricular shortening fraction HeCOG = Hellenic Cooperative Oncology Group nm = not documented OS = overall survival PR = partial remission PSA = prostate‐specific antigen RCT = randomised controlled trial SWOG = Southwest Oncology Group T‐ALL = T‐cell acute lymphoblastic leukaemia WHO = World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adam 1994 | No randomised controlled trial. |
| Advani 2014 | Conference proceeding of Advani 2015. |
| Advani 2015 | Study does not evaluate different anthracycline dosage schedules. |
| Alba 2004 | Difference in chemotherapy other than anthracyclines between intervention and control group. |
| Bastholt 1996 | Cumulative anthracycline dosis of intervention and control group not mentioned. |
| Berchem 1996 | No randomised controlled trial. |
| Berrak 2001 | No randomised controlled trial. |
| Blomqvist 1993 | Heart failure not mentioned. |
| Budd 2015 | Difference in chemotherapy other than anthracyclines between intervention and control group. |
| Buzdar 2007 | No randomised controlled trial. |
| Carmo‐Pereira 1987 | Difference in actually received cumulative anthracycline dosis between intervention and control group. |
| Carrio 1993 | No randomised controlled trial. |
| Creutzig 2007 | Article describes two studies: one is no randomised controlled trial; one does not evaluate different anthracycline dosage schedules, but different anthracycline derivates. |
| Ditsch 2012 | Difference in therapy other than anthracyclines between intervention and control group; difference in cumulative anthracycline dosis between intervention and control group. |
| Dorup 2004 | No randomised controlled trial. |
| Ehrlich 1979 | Difference in chemotherapy other than anthracyclines between intervention and control group; duplicate publication of Sutton 1989. |
| Eksborg 1997 | Number of patients with heart failure not mentioned. |
| Ewer 1998 | No randomised controlled trial. |
| Gabizon 2008 | Cumulative anthracycline dosis of intervention and control group not mentioned; cardiotoxicity not stated for patients with different anthracycline peak doses; pharmacokinetics study. |
| Gupta 2003 | No randomised controlled trial. |
| Habeshaw 1991 | Difference in cumulative anthracycline dosis between intervention and control group |
| Henderson 2003 | Difference in cumulative anthracycline dosis between intervention and control group. |
| Hochster 1985 | No randomised controlled trial. |
| Hoeltgen 1983 | Difference in cumulative anthracycline dosis between intervention and control group. |
| Horacek 2010 | Difference in therapy other than anthracyclines between intervention and control group. |
| Hubert 2000 | Difference in cumulative anthracycline dosis between intervention and control group; similar anthracycline peak dosis and infusion duration between intervention and control group. |
| Hunault‐Berger 2001 | Cumulative anthracycline dosis of intervention and control group not mentioned. |
| Irwin 1980 | Difference in chemotherapy other than anthracyclines between intervention and control group. |
| ISRCTN 83324925 | Ongoing trial which does not contain unconfounded information on anthracycline cardiotoxicity; difference in chemotherapy other than anthracyclines between intervention and control group. |
| Kilickap 2007 | No randomised controlled trial. |
| Kinoshita 2004 | Similar anthracycline peak dosis between intervention and control group; anthracycline infusion duration not mentioned; difference in cumulative anthracycline dosis between intervention and control group. |
| Krupicka 2002 | Difference in cumulative anthracycline dosis between intervention and control group. |
| Lalisang 1997 | Difference in cumulative anthracycline dosis between intervention and control group; dose‐finding study. |
| Levitt 2004 | No randomised controlled trial. |
| Lippens 1987 | No randomised controlled trial. |
| Luck (study A) 1997 | No randomised controlled trial; duplicate publication of Luck (study B) 1997. |
| Luck (study B) 1997 | No randomised controlled trial; duplicate publication of Luck (study A) 1997. |
| Magné 2009 | No randomised controlled trial. |
| Marschner 1994 | Difference in cumulative anthracycline dosis between intervention and control group. |
| Miller 1999 | Heart failure not mentioned. |
| Moebus 2010 | Difference in cumulative anthracycline dosis between intervention and control group. |
| Nemoto 1987 | Similar anthracycline peak dosis and infusion duration between intervention and control group. |
| Nielsen 1998 | Similar anthracycline peak dosis and infusion duration between intervention and control group. Only a part of the patients received the same cumulative anthracycline dosis and no separate results were given for these patients; the investigators were not able to provide this information. |
| Nuzzo 2011 | Difference in cumulative anthracycline dosis between intervention and control group. |
| O'Bryan 1977 | Difference in planned cumulative anthracycline dosis between intervention and control group. |
| Ohmachi 2011 | Difference in therapy other than anthracyclines between intervention and control group; similar anthracycline peak dosis between intervention and control group; anthracycline infusion duration not mentioned. |
| Rubin 1980 | Difference in chemotherapy other than anthracyclines between intervention and control group. |
| Stapleton 2007 | No randomised controlled trial. |
| Sutton 1989 | Difference in chemotherapy other than anthracyclines between intervention and control group; duplicate publication of Ehrlich 1979. |
| Swain 2003 | No randomised controlled trial. |
| SWOG S0221 | Difference in chemotherapy other than anthracyclines between intervention and control group; ongoing study. |
| Torti 1983 | No randomised controlled trial. |
| Umsawasdi 1989 | Difference in both anthracycline peak dosis and infusion duration between intervention and control group. |
| Valdivieso 1984 | Number of patients with abnormal cardiac function not mentioned. |
| Watanabe 2011 | Difference in therapy other than anthracyclines between intervention and control group; similar anthracycline peak dosis between intervention and control group; anthracycline infusion duration not mentioned. |
| Wood 1994 | Cumulative anthracycline dosis of intervention and control group not mentioned. |
| Woodward 2003 | No randomised controlled trial. |
| Yates 1982 | Difference in cumulative anthracycline dosis between intervention and control group. |
Characteristics of studies awaiting classification [ordered by study ID]
Ruiz 2006.
| Methods | Method of randomisation unclear |
| Participants | 11 participants (median age 50 years; sex nm) with metastatic breast cancer treated with pegylated liposomal doxorubicin. Prior anthracycline therapy nm; prior cardiac radiotherapy possible for 4 participants (number of participants in each treatment group nm); no prior cardiac dysfunction |
| Interventions | Pegylated liposomal doxorubicin with a peak dose of either 50 mg/m2 or 60 mg/m2 (infusion duration nm; number of participants in each treatment group nm; cumulative anthracycline dose nm) |
| Outcomes | Response rate: 1/4 evaluable participants in the 50 mg/m2 group achieved a partial response (definition nm) as did 2/4 evaluable participants in the 60 mg/m2 group. PFS (definition nm): median time to progression was 104 days in the 50 mg/m2 group and 168 days in the 60 mg/m2 group. Toxicity: not presented for each treatment group separately; unclear if cardiotoxicity has been evaluated. |
| Notes | Not all randomised participants were evaluated. Median length of follow‐up was 9.2 months. This study has not been published in full text (29 December 2015); from the currently available data it is unclear if this study is eligible for inclusion in this review |
nm: not mentioned PFS: progression‐free survival
Differences between protocol and review
We stated in the protocol that we would analyse different anthracycline peak doses as high (greater than or equal to 50 mg/m2) versus low (less than 50 mg/m2) doses received in one week. However, if we would have applied this definition to the included studies, pooling would not have been possible. Therefore, keeping in mind that any cut‐off point is arbitrary, we decided to define a low peak dose as less than 60 mg/m2 and a high peak dose as greater than or equal to 60 mg/m2.
For the second update we used the most recent recommendations of Cochrane Childhood Cancer for the assessment of risk of bias in the included studies, which are based on the Cochrane Handbook for Systematic Reviews of Interventions. All publications (including those already included in earlier versions of the review) were scored using the new 'Risk of bias' items.
Contributions of authors
Elvira C van Dalen designed the study and wrote the review. She developed the search strategy and undertook the searches in the different electronic databases for the original review. She searched for unpublished and ongoing studies and identified the studies meeting the inclusion criteria. She performed the data extraction and 'Risk of bias' assessment of the included studies. She analysed the data and interpreted the results. She wrote and revised the manuscript.
Helena JH van der Pal identified studies meeting the inclusion criteria. She performed the data extraction and 'Risk of bias' assessment of the included studies. She contributed to the data analysis and the interpretation of the results. She critically reviewed the manuscript.
Leontien CM Kremer designed the study. She contributed to the 'Risk of bias' assessment, data analysis, and the interpretation of the results. She critically reviewed the manuscript.
All review authors approved the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
Stichting Kinderen Kankervrij (KiKa), Netherlands
Declarations of interest
Elvira C van Dalen: None known.
Helena JH van der Pal: None known.
Leontien CM Kremer: None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Budman 1998 {published data only}
- Budman DR, Berry DA, Cirrincione CT, Henderson IC, Wood WC, Weiss RB et al. Dose and dose intensity as determinants of outcome in the adjuvant treatment of breast cancer. The Cancer and Leukemia Group B. Journal of the National Cancer Institute 1998;90:1205-11. [DOI] [PubMed] [Google Scholar]
Casper 1991 {published data only}
- Casper ES, Gaynor JJ, Hajdu SI, Magill GB, Tan C, Friedrich C et al. A prospective randomized trial of adjuvant chemotherapy with bolus versus continuous infusion of doxorubicin in patients with high-grade extremity soft tissue sarcoma and an analysis of prognostic factors. Cancer 1991;68:1221-9. [DOI] [PubMed] [Google Scholar]
Escherich 2007 {published data only}
- Escherich G, Göbel U, Jorch N, Spaar HJ, Janka-Schaub GE. Daunorubicin-induced cell kill with 1-hour versus 24-hour infusions: a randomized comparison in children with newly diagnosed acute lymphoblastic leukemia. Klinische Pädiatrie 2007;219:134-8. [DOI] [PubMed] [Google Scholar]
Fountzilas 2008 {published data only}
- Fountzilas G, Dafni U, Gogas H, Linardou H, Kalofonos HP, Briasoulis E et al. Postoperative dose-dense sequential chemotherapy with epirubicin, paclitaxel and CMF in patients with high-risk breast cancer: safety analysis of the Hellenic Cooperative Oncology Group randomized phase III trial HE 10/00. Annals of Oncology 2008;19:853-60. [DOI] [PubMed] [Google Scholar]
Heidenreich 2004 {published and unpublished data}
- Heidenreich A, Sommer F, Ohlmann CH, Schrader AJ, Olbert P, Goecke J et al. Prospective randomized phase II trial of pegylated doxorubicin in the management of symptomatic hormone-refractory prostate carcinoma. Cancer 2004;101:948-56. [DOI] [PubMed] [Google Scholar]
Hortobagyi 1989 {published data only}
- Hortobagyi GN, Yap H, Kau SW, Fraschini G, Ewer MS, Chawla SP et al. A comparative study of doxorubicin and epirubicin in patients with metastatic breast cancer. American Journal of Clinical Oncology 1989;12:57-62. [DOI] [PubMed] [Google Scholar]
Linden 2007 {published data only}
- Linden HM, Haskell CM, Green SJ, Osborne CK, Sledge GW Jr, Shapiro CL et al. Sequenced compared with simultaneous anthracycline and cyclophosphamide in high-risk stage I and II breast cancer: final analysis from INT-0137 (S9313). Journal of Clinical Oncology 2007;25:656-61. [DOI] [PubMed] [Google Scholar]
Lipshultz 2002 {published and unpublished data}
- *.Lipshultz SE, Giantris AL, Lipsitz SR, Kimball Dalton V, Asselin BL, Barr RD et al. Doxorubicin administration by continuous infusion is not cardioprotective: the Dana-Farber 91-01 acute lymphoblastic leukemia protocol. Journal of Clinical Oncology 2002;20:1677-82. [DOI] [PubMed] [Google Scholar]
- Lipshultz SE, Miller TL, Lipsitz SR, Neuberg DS, Dahlberg SE, Colan SD et al. Continuous versus bolus infusion of doxorubicin in children with ALL: long-term cardiac outcomes. Pediatrics 2012;130(6):1003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shapira 1990 {published data only}
- Shapira J, Gotfried M, Lishner M, Ravid M. Reduced cardiotoxicity of doxorubicin by a 6-hour infusion regimen: a prospective randomized evaluation. Cancer 1990;65:870-3. [DOI] [PubMed] [Google Scholar]
Steinherz 1993 {published data only}
- Steinherz PG, Redner A, Steinherz L, Meyers P, Tan C, Heller G. Development of a new intensive therapy for acute lymphoblastic leukemia in children at increased risk of early relapse. Cancer 1993;72:3120-30. [DOI] [PubMed] [Google Scholar]
Zalupski 1991 {published data only}
- Zalupski M, Metch B, Balcerzak S, Fletcher WS, Chapman R, Bonnet JD et al. Phase III comparison of doxorubicin and dacarbazine given by bolus versus infusion in patients with soft-tissue sarcomas: a Southwest Oncology Group Study. Journal of the National Cancer Institute 1991;83:926-32. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adam 1994 {published data only}
- Adam Z, Elbl L, Vorlicek J, Hajek R, Tomiska M, Hejlova N et al. Lower cardiotoxicity of adriamycin during continuous administration to patients with refractory multiple myeloma treated with cyclophosphamide, vincristine, adriamycin and dexamethasone. Vnitrni Lekarstvi 1994;40:506-12. [PubMed] [Google Scholar]
Advani 2014 {published data only}
- Advani PP, Ballman KV, Dockter TJ, Colon-Otero G, Perez EA. Long-term cardiac safety analysis of NCCTG (Alliance) N9831 adjuvant trastuzumab (H) trial (abstract 603). In: Journal of Clinical Oncology. suppl edition. Vol. 32. 2014:5s. [DOI] [PMC free article] [PubMed]
Advani 2015 {published data only}
- Advani PP, Ballman KV, Dockter TJ, Colon-Otero G, Perez EA. Long-term cardiac safety analysis of NCCTG N9831 (Alliance) Adjuvant Trastuzumab Trial. Journal of Clinical Oncology 2015;pii: JCO.2015.61.8413:Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alba 2004 {published data only}
- Alba E, Martin M, Ramos M, Adrover E, Balil A, Jara C et al. Multicenter randomized trial comparing sequential with concomitant administration of doxorubicin and docetaxel as first-line treatment of metastatic breast cancer: a Spanish Breast Cancer Research Group (GEICAM-9903) phase III study. Journal of Clinical Oncology 2004;22:2587-93. [DOI] [PubMed] [Google Scholar]
Bastholt 1996 {published data only}
- Bastholt L, Dalmark M, Gjedde SB, Pfeiffer P, Pedersen D, Sandberg E et al. Dose-response relationship of epirubicin in the treatment of postmenopausal patients with metastatic breast cancer: a randomized study of epirubicin at four different dose levels performed by the Danish Breast Cancer Cooperative Group. Journal of Clinical Oncology 1996;14:1146-55. [DOI] [PubMed] [Google Scholar]
Berchem 1996 {published data only}
- Berchem GJ, Ries F, Hanfelt J, Duhem C, Keipes M, Delagardelle C et al. Epirubicin cardiotoxicity: a study comparing low- with high-dose intensity weekly schedules. Support Care Cancer 1996;4:308-12. [DOI] [PubMed] [Google Scholar]
Berrak 2001 {published data only}
- Berrak SG, Ewer MS, Jaffe N, Pearson P, Ried H, Zietz HA et al. Doxorubicin cardiotoxicity in children: reduced incidence of cardiac dysfunction associated with continuous infusion schedules. Oncology Reports 2001;8:611-4. [DOI] [PubMed] [Google Scholar]
Blomqvist 1993 {published data only}
- Blomqvist C, Elomaa I, Rissanen P, Hietanen P, Nevasaari K, Helle L. Influence of treatment schedule on toxicity and efficacy of cyclophosphamide, epirubicin, and fluorouracil in metastatic breast cancer: a randomized trial comparing weekly and every-4-week administration. Journal of Clinical Oncology 1993;11:467-73. [DOI] [PubMed] [Google Scholar]
Budd 2015 {published data only}
- Budd GT, Barlow WE, Moore HC, Hobday TJ, Stewart JA, Isaacs C et al. SWOG S0221: a phase III trial comparing chemotherapy schedules in high-risk early-stage breast cancer. Journal of Clinical Oncology 2015;33(1):58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buzdar 2007 {published data only}
- Buzdar AU. Adjuvant chemotherapy for high-risk operable breast cancer. Journal of Clinical Oncology 2007;25:1642-4. [DOI] [PubMed] [Google Scholar]
Carmo‐Pereira 1987 {published data only}
- Carmo-Pereira J, Oliviera Costa F, Henriques E, Godinho F, Cantinho-Lopes MG, Sales-Luis A et al. A comparison of two doses of adriamycin in the primary chemotherapy of disseminated breast carcinoma. British Journal of Cancer 1987;56:471-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrio 1993 {published data only}
- Carrio I, Lopez-Pousa A, Estorch M, Duncker D, Berna L, Torres G et al. Detection of doxorubicin cardiotoxicity in patients with sarcomas by indium-111-antimyosin monoclonal antibody studies. Journal of Nuclear Medicine 1993;34:1503-7. [PubMed] [Google Scholar]
Creutzig 2007 {published data only}
- Creutzig U, Diekamp S, Zimmermann M, Reinhardt D. Longitudinal evaluation of early and late anthracycline cardiotoxicity in children with AML. Pediatric Blood and Cancer 2007;48:651-62. [DOI] [PubMed] [Google Scholar]
Ditsch 2012 {published data only}
- Ditsch N, Vodermaier A, Hinke A, Burghardt S, Lenhard M, Löhrs B et al. Dose-dense intensified sequential versus conventionally-dosed anthracycline and taxane-containing neoadjuvant therapy in patients with inflammatory breast cancer. Anticancer Research 2012;32(8):3539-45. [PubMed] [Google Scholar]
Dorup 2004 {published data only}
- Dorup I, Levitt G, Sullivan I, Sorensen K. Prospective longitudinal assessment of late anthracycline cardiotoxicity after childhood cancer: the role of diastolic function. Heart 2004;90:1214-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ehrlich 1979 {published data only}
- Ehrlich CE, Einhorn L, Williams SD, Morgan J. Chemotherapy for stage III-IV epithelial ovarian cancer with cis-dichlorodiammineplatinum(II), adriamycin, and cyclophosphamide: a preliminary report. Cancer Treatment Reports 1979;63:281-8. [PubMed] [Google Scholar]
Eksborg 1997 {published data only}
- Eksborg S, Bjorkholm M, Hast R, Fagerlund E. Plasma pharmacokinetics of idarubicin and its 13-dihydro metabolite - a comparison of bolus versus 2 h infusion during a 3 day course. Anti-Cancer Drugs 1997;8:42-7. [DOI] [PubMed] [Google Scholar]
Ewer 1998 {published data only}
- Ewer MS, Jaffe N, Ried H, Zietz HA, Benjamin RS. Doxorubicin cardiotoxicity in children: comparison of consecutive divided daily dose administration schedule with single dose (rapid) infusion administration. Medical and Pediatric Oncology 1998;31:512-5. [DOI] [PubMed] [Google Scholar]
Gabizon 2008 {published data only}
- Gabizon A, Isacson R, Rosengarten O, Tzemach D, Shmeeda H, Sapir R. An open-label study to evaluate dose and cycle dependence of the pharmacokinetics of pegylated liposomal doxorubicin. Cancer Chemotherapy and Pharmacology 2008;61:695-702. [DOI] [PubMed] [Google Scholar]
Gupta 2003 {published data only}
- Gupta M, Steinherz PG, Cheung NK, Steinherz L. Late cardiotoxicity after bolus versus infusion anthracycline therapy for childhood cancers. Medical and Pediatric Oncology 2003;40:343-7. [DOI] [PubMed] [Google Scholar]
Habeshaw 1991 {published data only}
- Habeshaw T, Paul J, Jones R, Stallard S, Kaye SB, Soukop M et al. Epirubicin at two dose levels with prednisolone as treatment for advanced breast cancer: the results of a randomized trial. Journal of Clinical Oncology 1991;9:295-304. [DOI] [PubMed] [Google Scholar]
Henderson 2003 {published data only}
- Henderson IC, Berry DA, Demetri GD, Cirrincione CT, Goldstein LJ, Martino S et al. Improved outcomes from adding sequential Paclitaxel but not from escalating Doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. Journal of Clinical Oncology 2003;21:976-83. [DOI] [PubMed] [Google Scholar]
Hochster 1985 {published data only}
- Hochster HS, Green MD, Speyer J, Fazzini E, Blum R, Muggia FM. 4'epidoxorubicin (epirubicin): activity in hepatocellular carcinoma. Journal of Clinical Oncology 1985;3:1535-40. [DOI] [PubMed] [Google Scholar]
Hoeltgen 1983 {published data only}
- Hoeltgen TM, MacIntyre JM, Perlia CP, Lagakos SW, Stolbach LL, Bennett JM. Adriamycin and cytoxan in the treatment of inoperable lung cancer. Cancer 1983;51:2005-12. [DOI] [PubMed] [Google Scholar]
Horacek 2010 {published data only}
- Horacek JM, Vasatova M, Tichy M, Pudil R, Jebavy L, Maly J. The use of cardiac biomarkers in detection of cardiotoxicity associated with conventional and high-dose chemotherapy for acute leukemia. Experimental Oncology 2010;32(2):97-9. [PubMed] [Google Scholar]
Hubert 2000 {published data only}
- Hubert A, Lyass O, Pode D, Gabizon A. Doxil (Caelyx): an exploratory study with pharmacokinetics in patients with hormone-refractory prostate cancer. Anti-Cancer Drugs 2000;11:123-7. [DOI] [PubMed] [Google Scholar]
Hunault‐Berger 2001 {published data only}
- Hunault-Berger M, Milpied N, Bernard M, Jouet JP, Delain M, Desablens B et al. Daunorubicin continuous infusion induces more toxicity than bolus infusion in acute lymphoblastic leukemia induction regimen: a randomized study. Leukemia 2001;15:898-902. [DOI] [PubMed] [Google Scholar]
Irwin 1980 {published data only}
- Irwin LE, Chlebowski RT, Weiner JM, Reynolds R, Pugh RP, Ryden VMJ et al. Randomized comparison of two combination chemotherapy regimens containing doxorubicin in patients with metastatic breast cancer: a Western Cancer Study Group trial. Cancer Treatment Reports 1980;64:981-4. [PubMed] [Google Scholar]
ISRCTN 83324925 {published and unpublished data}
- ISRCTN 83324925. A randomized comparative trial of infusional ECF versus conventional FEC as adjuvant chemotherapy in patients with poor prognosis breast cancer. http://www.isrctn.com/ISRCTN83324925 (accessed 24 March 2005).
Kilickap 2007 {published data only}
- Kilickap S, Barista I, Akgul E, Aytemir K, Aksoy S, Tekuzman AG. Early and late arrhythmogenic effects of doxorubicin. Southern Medical Journal 2007;100:262-5. [DOI] [PubMed] [Google Scholar]
Kinoshita 2004 {published data only}
- Kinoshita T, Hotta T, Tobinai K, Kobayashi T, Ishizuka N, Tomonaga M et al. A randomized controlled trial investigating the survival benefit of dose-intensified multidrug combination chemotherapy (LSG9) for intermediate- or high-grade non-Hodgkin's lymphoma: Japan Clinical Oncology Group Study 9002. International Journal of Hematology 2004;80:341-50. [DOI] [PubMed] [Google Scholar]
Krupicka 2002 {published data only}
- Krupicka J, Markova J, Pohlreich D, Kozak T, Linkova H, Diehl V. Echocardiographic evaluation of acute cardiotoxicity in the treatment of Hodgkin disease according to the German Hodgkin's Lymphoma Study Group. Leukemia & Lymphoma 2002;43:2325-9. [PubMed] [Google Scholar]
Lalisang 1997 {published data only}
- Lalisang RI, Wils JA, Nortier HW, Burghouts JT, Hupperets PS, Erdkamp FL et al. Comparative study of dose escalation versus interval reduction to obtain dose-intensification of epirubicin and cyclophosphamide with granulocyte colony-stimulating factor in advanced breast cancer. Journal of Clinical Oncology 1997;15:1367-76. [DOI] [PubMed] [Google Scholar]
Levitt 2004 {published data only}
- Levitt GA, Dorup I, Sorensen K, Sullivan I. Does anthracycline administration by infusion in children affect late cardiotoxicity? British Journal of Haematology 2004;124:463-8. [DOI] [PubMed] [Google Scholar]
Lippens 1987 {published data only}
- Lippens RJJ, Van Lier HJJ, Zwagemakers JFC. Tolerance of 24-hour infusions of low-and high-dose bolus injections of adriamycin in children. Pediatric Hematology and Oncology 1987;4:189-97. [DOI] [PubMed] [Google Scholar]
Luck (study A) 1997 {published data only}
- Luck HJ, Du Bois A, Thomssen C, Lisboa B, Untch M, Kohler G et al. A Phase II study. Paclitaxel and epirubicin as first-line therapy for patients with metastatic breast cancer. Oncology 1997;11:34-7. [PubMed] [Google Scholar]
Luck (study B) 1997 {published data only}
- Luck HJ, Thomssen C, Du Bois A, Untch M, Lisboa M, Kohler G et al. Phase II study of paclitaxel and epirubicin as first-line therapy in patients with metastatic breast cancer. Seminars in Oncology 1997;24:S17-35-S17-39. [PubMed] [Google Scholar]
Magné 2009 {published data only}
- Magné N, Castadot P, Chargari C, Di Leo A, Philippson C, Van Houtte P. Special focus on cardiac toxicity of different sequences of adjuvant doxorubicin/docetaxel/CMF regimens combined with radiotherapy in breast cancer patients. Radiotherapy and Oncology 2009;90(1):116-21. [DOI] [PubMed] [Google Scholar]
Marschner 1994 {published data only}
- Marschner N, Kreienberg R, Souchon R, Rath U, Eggeling B, Voigtmann R et al. Evaluation of the importance and relevance of dose intensity using epirubicin and cyclophosphamide in metastatic breast cancer: interim analysis of a prospective randomized trial. Seminars in Oncology 1994;21:10-6. [PubMed] [Google Scholar]
Miller 1999 {published data only (unpublished sought but not used)}
- Miller KD, McCaskill-Stevens W, Sisk J, Loesch DM, Monaco F, Seshadri R et al. Combination versus sequential doxorubicin and docetaxel as primary chemotherapy for breast cancer: A randomized pilot trial of the Hoosier Oncology Group. Journal of Clinical Oncology 1999;17:3033-7. [DOI] [PubMed] [Google Scholar]
Moebus 2010 {published data only}
- Moebus V, Jackisch C, Lueck HJ, du Bois A, Thomssen C, Kurbacher C et al. Intense dose-dense sequential chemotherapy with epirubicin, paclitaxel, and cyclophosphamide compared with conventionally scheduled chemotherapy in high-risk primary breast cancer: mature results of an AGO phase III study. Journal of Clinical Oncology 2010;28(17):2874-80. [DOI] [PubMed] [Google Scholar]
Nemoto 1987 {published data only}
- Nemoto T, Rosner D, Diaz R, Dao T, Sponzo R, Cunningham T et al. Combination chemotherapy for metastatic breast cancer, comparison of multiple drug therapy with 5-flourouracil, cytoxan and prednisone with adriamycin or adrenalectomy. Cancer 1978;41:2073-7. [DOI] [PubMed] [Google Scholar]
Nielsen 1998 {published and unpublished data}
- Nielsen OS, Dombernowsky P, Mouridsen H, Crowther D, Verweij J, Buesa J et al. High-dose epirubicin is not an alternative to standard-dose doxorubicin in the treatment of advanced soft tissue sarcomas. A study of the EORTC Soft Tissue and Bone Sarcoma Group. British Journal of Cancer 1998;78:1634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nuzzo 2011 {published data only}
- Nuzzo F, Morabito A, Gravina A, Di Rella F, Landi G, Pacilio C et al. Effects on quality of life of weekly docetaxel-based chemotherapy in patients with locally advanced or metastatic breast cancer: results of a single-centre randomized phase 3 trial. BMC Cancer 2011;11:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Bryan 1977 {published data only}
- O'Bryan RM, Baker LH, Gottlieb JE, Rivkin SE, Balcerzak SP, Grumet GN et al. Dose response evaluation of adriamycin in human neoplasia. Cancer 1977;39:1940-8. [DOI] [PubMed] [Google Scholar]
Ohmachi 2011 {published data only}
- Ohmachi K, Tobinai K, Kobayashi Y, Itoh K, Nakata M, Shibata T et al. Phase III trial of CHOP-21 versus CHOP-14 for aggressive non-Hodgkin's lymphoma: final results of the Japan Clinical Oncology Group Study, JCOG 9809. Annals of Oncology 2011;22(6):1382-91. [DOI] [PubMed] [Google Scholar]
Rubin 1980 {published data only}
- Rubin J, Decker DA, Ahmann DL, Eagan RT, Ingle JN, Hahn R. An evaluation of two schedules of VP-16 and adriamycin in patients with advanced breast cancer. Oncology 1980;37:149-51. [DOI] [PubMed] [Google Scholar]
Stapleton 2007 {published data only}
- Stapleton GE, Stapleton SL, Martinez A, Ayres NA, Kovalchin JP, Bezold LI et al. Evaluation of longitudinal ventricular function with tissue Doppler echocardiography in children treated with anthracyclines. Journal of the American Society of Echocardiography 2007;20:492-7. [DOI] [PubMed] [Google Scholar]
Sutton 1989 {published data only}
- Sutton GP, Stehman FB, Einhorn LH, Roth LM, Blessing JA, Ehrlich CE. Ten-year follow-up of patients receiving cisplatin, doxorubicin, and cyclophosphamide chemotherapy for advanced epithelial ovarian carcinoma. Journal of Clinical Oncology 1989;7:223-9. [DOI] [PubMed] [Google Scholar]
Swain 2003 {published data only}
- Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin, a retrospective analysis of three trials. Cancer 2003;97:2869-79. [DOI] [PubMed] [Google Scholar]
SWOG S0221 {published data only}
- Southwest Oncology Group (SWOG). S0221 Adjuvant Doxorubicin, Cyclophosphamide, and Paclitaxel in Treating Patients With Breast Cancer. http://www.kccop.org/breast/swog0221.htm (accessed 7 May 2009) .
Torti 1983 {published data only}
- Torti FM, Bristow MR, Howes AE, Aston D, Stockdale FE, Carter SK et al. Reduced cardiotoxicity of doxorubicin delivered on a weekly schedule, assessment by endomyocardial biopsy. Annals of Internal Medicine 1983;99:745-9. [DOI] [PubMed] [Google Scholar]
Umsawasdi 1989 {published data only}
- Umsawasdi T, Valdivieso M, Booser DJ, Barkley HT, Ewer M, MacKay B et al. Weekly doxorubicin every 3 weeks in cyclophosphamide, doxorubicin and cisplatin chemotherapy for non-small cell lung cancer. Cancer 1989;64:1995-2000. [DOI] [PubMed] [Google Scholar]
Valdivieso 1984 {published data only}
- Valdivieso M, Burgess MA, Ewer MS, Mackay B, Wallace S, Benjamin RS et al. Increased therapeutic index of weekly doxorubicin in the therapy of non-small cell lung cancer: a prospective, randomized study. Journal of Clinical Oncology 1984;2:207-14. [DOI] [PubMed] [Google Scholar]
Watanabe 2011 {published data only}
- Watanabe T, Tobinai K, Shibata T, Tsukasaki K, Morishima Y, Maseki N et al. Phase II/III study of R-CHOP-21 versus R-CHOP-14 for untreated indolent B-cell non-Hodgkin's lymphoma: JCOG 0203 trial. Journal of Clinical Oncology 2011;29(30):3990-8. [DOI] [PubMed] [Google Scholar]
Wood 1994 {published data only}
- Wood WC, Budman DR, Korzun AH, Cooper MR, Younger J, Hart RD et al. Dose and dose intensity of adjuvant chemotherapy for stage II, node-positive breast carcinoma. The New England Journal of Medicine 1994;330:1253-9. [DOI] [PubMed] [Google Scholar]
Woodward 2003 {published data only}
- Woodward WA, Strom EA, McNeese MD, Perkins GH, Outlaw EL, Hortobagyi GN et al. Cardiovascular death and second non-breast cancer malignancy after postmastectomy radiation and doxorubicin-based chemotherapy. International Journal of Radiation Oncology, Biology, Physics 2003;57:3270335. [DOI] [PubMed] [Google Scholar]
Yates 1982 {published data only}
- Yates J, Glidewell O, Wiernik P, Cooper MR, Steinberg D, Dosik H et al. Cytosine arabinoside with daunorubicin or adriamycin for therapy of acute myelocytic leukemia: a CALGB study. Blood 1982;60:454-62. [PubMed] [Google Scholar]
References to studies awaiting assessment
Ruiz 2006 {published data only}
- Ruiz M, Bayo J, Moreno Nogueira JA, Dorta J, Casas A, Morales M. Randomized open label phase II study of pegylated liposomal doxorubicine (PLD) four or six-week scheduled in metastatic breast cancer (MBC) patients (p). In: Journal of Clinical Oncology (Meeting Abstracts). Vol. 24. 2006:18s; abstract 10751.
Additional references
Batist 2001
- Batist G, Ramakriskan G, Rao CS, Chandrasekharan A, Gutheil J, Guthrie T et al. Reduced cardiotoxicity and preserved antitumor efficacy of liposome-encapsulated doxorubicin and cyclophosphamide compared with conventional doxorubicin and cyclophosphamide in a randomized multicenter trial of metastatic breast cancer. Journal of Clinical Oncology 2001;19:1444-54. [DOI] [PubMed] [Google Scholar]
Billingham 1978
- Billingham ME, Mason JW, Bristow MR, Daniels JR. Anthracycline cardiomyopathy monitored by morphologic changes. Cancer Treatment Reports 1978;62:865-72. [PubMed] [Google Scholar]
Bonadonna 1969
- Bonadonna G, Monfardini S. Cardiac toxicity of daunorubicin. The Lancet 1969;1:837. [PubMed] [Google Scholar]
Fountzilas 2005
- Fountzilas G, Skarlos D, Dafni U, Gogas H, Briasoulis E, Pectasides D et al. Postoperative dose-dense sequential chemotherapy with epirubicin, followed by CMF with or without paclitaxel, in patients with high-risk operable breast cancer: a randomized phase III study conducted by the Hellenic Cooperative Oncology Group. Annals of Oncology 2005;16:1762-71. [DOI] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews on Interventions Version 4.2.5 [updated May 2005]. John Wiley & Sons Ltd, Chichester (UK).
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Kremer 2002a
- Kremer LC, Pal HJ, Offringa M, Dalen EC, Voûte PA. Frequency and risk factors of subclinical cardiotoxicity after anthracycline therapy in children: a systematic review. Annals of Oncology 2002;13:819-29. [DOI] [PubMed] [Google Scholar]
Kremer 2002b
- Kremer LC, Dalen EC, Offringa M, Voûte PA. Frequency and risk factors of anthracycline-induced clinical heart failure in children: a systematic review. Annals of Oncology 2002;13:503-12. [DOI] [PubMed] [Google Scholar]
Kremer 2014
- Kremer LCM, Leclercq E, Dalen EC. Cochrane Childhood Cancer. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). About The Cochrane Collaboration 2014, issue 12. Art. No.: CHILDCA.
Lefrak 1973
- Lefrak EA, Pitha J, Rosenheim S, Gottlieb JA. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973;32:302-14. [DOI] [PubMed] [Google Scholar]
Legha 1982
- Legha SS, Benjamin RS, Mackay B, Ewer M, Wallace S, Valdivieso M et al. Reduction of doxorubicin cardiotoxicity by prolonged continuous intravenous infusion. Annals of Internal Medicine 1982;96:133-9. [DOI] [PubMed] [Google Scholar]
Lipshultz 2012
- Lipshultz SE, Miller TL, Lipsitz SR, Neuberg DS, Dahlberg SE, Colan SD et al. Continuous versus bolus infusion of doxorubicin in children with ALL: long-term cardiac outcomes. Pediatrics 2012;130(6):1003-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meinardi 2002
- Meinardi MT, Van der Graaf WTA, Gietema JA, Van den Berg MP, Sleijfer DT, De Vries EGE et al. Evaluation of long term cardiotoxicity after epirubicin containing adjuvant chemotherapy and locoregional radiotherapy for breast cancer using various detection techniques. Heart 2002;88:81-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Millikan 2003
- Millikan R, Thall PF, Lee SJ, Jones D, Cannon MW, Kuebler JP et al. Randomized, multicenter, phase II trial of two multicomponent regimens in androgen-independent prostate cancer. Journal of Clinical Oncology 2003;21:878-83. [DOI] [PubMed] [Google Scholar]
Muggia 1991
- Muggia FM, Green MD. New anthracycline antitumor antibiotics. Critical Reviews in Oncology/Hematology 1991;11:43-64. [DOI] [PubMed] [Google Scholar]
Ng 2006
- Ng R, Better N, Green MD. Anticancer agents and cardiotoxicity. Seminars in Oncology 2006;33:2-14. [DOI] [PubMed] [Google Scholar]
Nousiainen 2002
- Nousiainen T, Jantunen E, Vanninen E, Hartikainen J. Early decline in left ventricular ejection fraction predicts doxorubicin cardiotoxicity in lymphoma patients. British Journal of Cancer 2002;86:1697-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815-34. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Shan 1996
- Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Annals of Internal Medicine 1996;125:47-58. [DOI] [PubMed] [Google Scholar]
Silverman 2001
- Silverman LB, Gelber RD, Kimball Dalton V, Asselin BL, Barr RD, Clavell LA et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana-Farber Consortium Protocol 91-01. Blood 2001;97:1211-8. [DOI] [PubMed] [Google Scholar]
Simbre 2005
- Simbre II VC, Duffy SA, Dadlani GH, Miller TL, Lipshultz SE. Cardiotoxicity of cancer chemotherapy, implications for children. Pediatric Drugs 2005;7:187-202. [DOI] [PubMed] [Google Scholar]
Van Dalen 2004
- Van Dalen EC, Pal HJ, Bakker PJ, Caron HN, Kremer LC. Cumulative incidence and risk factors of mitoxantrone-induced cardiotoxicity in children: a systematic review. European Journal of Cancer 2004;40(5):643-52. [DOI] [PubMed] [Google Scholar]
Van Dalen 2006b
- Van Dalen EC, Pal HJ, Kok WE, Caron HN, Kremer LC. Clinical heart failure in a cohort of children treated with anthracyclines: a long-term follow-up study. European Journal of Cancer 2006;42(18):3191-8. [DOI] [PubMed] [Google Scholar]
Van Dalen 2010
- Van Dalen EC, Michiels EM, Caron HN, Kremer LC. Different anthracycline derivates for reducing cardiotoxicity in cancer patients. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD005006.pub3] [DOI] [PubMed] [Google Scholar]
Van Dalen 2011
- Van Dalen EC, Caron HN, Dickinson HO, Kremer LC. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD003917.pub4] [DOI] [Google Scholar]
Van Dalen 2014
- Van Dalen EC, Raphaël MF, Caron HN, Kremer LCM. Treatment including anthracyclines versus treatment not including anthracyclines for childhood cancer. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD006647.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Pal 2012
- Van der Pal HJ, Dalen EC, Delden E, Dijk IW, Kok WE, Geskus RB et al. High risk of symptomatic cardiac events in childhood cancer survivors. Journal of Clinical Oncology 2012;30(13):1429-37. [DOI] [PubMed] [Google Scholar]
Verbel 2002
- Verbel DA, Heller G, Kelly WK, Scher HI. Quantifying the amount of variation in survival explained by prostate-specific antigen. Clinical Cancer Research 2002;8:2576-9. [PubMed] [Google Scholar]
References to other published versions of this review
Van Dalen 2006
- Van Dalen EC, Van der Pal HJH, Caron HN, Kremer LCM. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD005008.pub2] [DOI] [PubMed] [Google Scholar]
Van Dalen 2009
- Van Dalen EC, Pal HJH, Caron HN, Kremer LCM. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD005008.pub3] [DOI] [Google Scholar]


