Abstract
Background
This review updates part of an earlier Cochrane review on 'Pregabalin for acute and chronic pain in adults' (Moore 2009), and considers only fibromyalgia pain.
Antiepileptic drugs have been used in pain management since the 1960s. Pregabalin is an antiepileptic drug also used in management of chronic pain conditions, including fibromyalgia. Pain response with pregabalin is associated with major benefits for other symptoms, and improved quality of life and function in people with chronic painful conditions.
Objectives
To assess the analgesic efficacy and adverse events of pregabalin for pain in fibromyalgia in adults, compared with placebo or any active comparator.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, and EMBASE for randomised controlled trials from inception to May 2009 for the original review and to 16 March 2016 for this update. We also searched the reference lists of retrieved studies and reviews, and online clinical trial registries.
Selection criteria
We included randomised, double‐blind trials of eight weeks' duration or longer, comparing pregabalin with placebo or another active treatment for relief of pain in fibromyalgia, and reporting on the analgesic effect of pregabalin, with subjective pain assessment by the participant.
Data collection and analysis
Two review authors independently extracted data and assessed trial quality and potential bias. Primary outcomes were participants with moderate pain relief (at least 30% pain relief over baseline or much or very much improved on Patient Global Impression of Change scale (PGIC)) or substantial pain relief (at least 50% pain relief over baseline or very much improved on PGIC). Where pooled analysis was possible, we used dichotomous data to calculate risk ratio and number needed to treat (NNT), using standard methods. We assessed the quality of the evidence using GRADE (Grading of Recommendations Assessment, Development and Evaluation) and created 'Summary of findings' tables.
Main results
Our searches identified two new published studies with classic design, and one new published study with an enriched enrolment randomised withdrawal (EERW) design.
We included eight studies. Five (3283 participants) had a classic design in which participants were randomised at the start of the study to pregabalin (150, 300, 450, or 600 mg daily) or placebo, with assessment after 8 to 13 weeks of stable treatment. No studies included active comparators. Studies had low risk of bias, except that the last observation carried forward (LOCF) imputation method used in analyses of the primary outcomes could overestimate treatment effect.
Pregabalin increased the number of participants experiencing substantial benefit (at least 50% pain intensity reduction after 12 or 13 weeks' stable treatment (450 mg: RR 1.8, 95% CI 1.4 to 2.1, 1874 participants, 5 studies, high quality evidence)). Substantial benefit with pregabalin 300 to 600 mg was experienced by about 14% of participants with placebo, but about 9% more with pregabalin 300 to 600 mg (22% to 24%) (high quality evidence). Pregabalin increased the number of participants experiencing moderate benefit (at least 30% pain intensity reduction after 12 or 13 weeks' stable treatment) (450 mg: RR 1.5, 95% CI (1.3 to 1.7), 1874 participants, 5 studies, high quality evidence). Moderate benefit with pregabalin 300 to 600 mg was experienced by about 28% of participants with placebo, but about 11% more with pregabalin 300 to 600 mg (39% to 43%) (high quality evidence). A similar magnitude of effect was found using PGIC of 'very much improved' and 'much or very much improved'. NNTs for these outcomes ranged between 7 and 14 (high quality evidence).
A small study (177 participants) compared nightly with twice‐daily pregabalin, and concluded there was no difference in effect.
Two studies (1492 participants began initial dose titration, 687 participants randomised) had an EERW design in which those with good pain relief after titration were randomised, double blind, to continuing the effective dose (300 to 600 mg pregabalin daily) or a short down‐titration to placebo for 13 or 26 weeks. We calculated the outcome of maintained therapeutic response (MTR) without withdrawal, equivalent to a moderate benefit. Of those randomised, 40% had MTR with pregabalin and 20% with placebo (high quality evidence). The NNT was 5, but normalised to the starting population tested it was 12. About 10% of the initial population would have achieved the MTR outcome, similar to the result from studies of classic design. MTR had no imputation concerns.
The majority (70% to 90%) of participants in all treatment groups experienced adverse events. Specific adverse events were more common with pregabalin than placebo, in particular dizziness, somnolence, weight gain, and peripheral oedema, with number needed to harm of 3.7, 7.4, 18, and 19 respectively for all doses combined (high quality evidence). Serious adverse events did not differ between active treatment groups and placebo (very low quality evidence). Withdrawals for any reason were more common with pregabalin than placebo only with the 600 mg dose in studies of classic design. Withdrawals due to adverse events were about 10% higher with pregabalin than placebo, but withdrawals due to lack of efficacy were about 6% lower (high quality evidence).
Authors' conclusions
Pregabalin 300 to 600 mg produces a major reduction in pain intensity over 12 to 26 weeks with tolerable adverse events for a small proportion of people (about 10% more than placebo) with moderate or severe pain due to fibromyalgia. The degree of pain relief is known to be accompanied by improvements in other symptoms, quality of life, and function. These results are similar to other effective medicines in fibromyalgia (milnacipran, duloxetine).
Plain language summary
Pregabalin for treating fibromyalgia pain in adults
Bottom line
We found high quality evidence that pregabalin at daily doses of 300 to 600 mg produces a large fall in pain in about 1 in 10 people with moderate or severe pain from fibromyalgia. Pain reduction comes with improvements in other symptoms, in quality of life, and in ability to function.
Background
Fibromyalgia is characterised by persistent, widespread pain and tenderness, sleep problems, and fatigue. Common pain‐relieving medicines such as paracetamol and ibuprofen are not usually considered effective. Medicines used to treat epilepsy or depression can be effective in some people with fibromyalgia and other forms of chronic (persistent, long‐lasting) pain where there may be nerve damage. Pregabalin is an antiepileptic licensed to treat fibromyalgia in some parts of the world, in particular the USA.
This review is an update of one originally published in 2009, which examined the effects of pregabalin on all types of pain. In this review we have only examined fibromyalgia pain. The earlier review showed that pregabalin worked in a small proportion of people with fibromyalgia. This is the same as all other fibromyalgia treatments to date, and for chronic pain conditions generally. Our definition of 'worked' involved both a high level of pain relief and being able to take the medication over a longer period without intolerable side effects.
Study characteristics
We searched scientific databases for studies that looked at the effects of pregabalin in adults with fibromyalgia who had moderate or severe pain. The treatment had to last at least eight weeks. The evidence is current to March 2016.
Eight studies satisfied the inclusion criteria, including three new studies for this update. Five studies randomised 3283 participants to immediate treatment with pregabalin or placebo. Two studies identified 687 out of 1492 participants who had a good pain response and could take the medicine, and then randomised them to continued treatment with pregabalin or placebo. Study quality was good. One other had no useful data.
Key results
High quality evidence showed that 1 in 10 people with moderate or severe fibromyalgia pain reported a large fall in pain by a third to a half over 12 to 26 weeks. This is an outcome that people with fibromyalgia consider to be useful. The dose of pregabalin was 300 to 600 mg daily.
Side effects occurred in 8 or 9 people in 10, often while adjusting to the medicine. Particular side effects were dizziness (affecting 1 in 4 participants), drowsiness (1 in 7), weight gain (1 in 18), and peripheral oedema (1 in 19) (high quality evidence). Serious side effects were no more common with pregabalin than with placebo, affecting 1 or 2 in 100. About 1 in 10 more participants taking pregabalin withdrew from the study because of side effects, and 1 in 17 fewer withdrew because the medicine was not working.
Quality of the evidence
The evidence was mostly of high quality, which means we are very confident that the true effect lies close to that of the estimate of the effect in this review. Concern about how information was handled when people left the studies was offset by other information showing that results were not impacted by this to any important degree.
Summary of findings
Background
This protocol is based on a template for reviews of drugs used to relieve fibromyalgia. The aim is for all reviews to use the same methods, based on new criteria for what constitutes reliable evidence in chronic pain (Moore 2010a; Appendix 1).
The earlier review of 'Pregabalin for acute and chronic pain in adults' has been split into separate titles (Moore 2009). This review looked at pregabalin for pain in fibromyalgia, and a separate review will look at pregabalin for neuropathic pain. These new titles will serve to update the original.
Description of the condition
Fibromyalgia symptoms can be assessed by self‐report of the patient using the fibromyalgia criteria and severity scales for clinical and epidemiological studies, a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia (so‐called Fibromyalgia Symptom Questionnaire) (Wolfe 2011). Fibromyalgia was previously defined by the American College of Rheumatology (ACR) 1990 classification criteria as widespread pain lasting for longer than three months with tenderness on palpation at 11 or more of 18 specified tender points (Wolfe 1990). For a clinical diagnosis, the ACR 1990 classification criteria and the ACR 2010 preliminary diagnostic criteria can both be used (Wolfe 1990; Wolfe 2010). Lacking a specific laboratory test, diagnosis is established by a history of the key symptoms and the exclusion of somatic diseases sufficiently explaining the key symptoms (Wolfe 2010). The indexing of fibromyalgia within the International Classification of Diseases is under debate. While some rheumatologists have thought of it as a specific pain disorder and central sensitivity syndrome (Clauw 2014; Yunus 2008), recent research points at small fibre pathology in a subgroup of fibromyalgia patients that may be of pathophysiological importance (Oaklander 2013; Üçeyler 2013a) though this is regarded as speculative. In psychiatry and psychosomatic medicine, fibromyalgia symptoms are categorised as a functional somatic syndrome, a bodily distress syndrome, a somatic physical symptom disorder, or a somatoform disorder (Häuser 2014).
Fibromyalgia is a heterogenous condition. The definite aetiology (causes) of this syndrome remains unknown. A model of interacting biological and psychosocial variables in the predisposition, triggering, and development of the chronicity of fibromyalgia symptoms has been suggested (Sommer 2012). Depression (Forseth 1999), genetics (Arnold 2013; Lee 2012), obesity combined with physical inactivity (Mork 2010), physical and sexual abuse in childhood (Häuser 2011), sleep problems (Mork 2010), and smoking (Choi 2010), predict future development of fibromyalgia. Psychosocial stress (working place and family conflicts) and physical stress (infections, surgery, accidents) might trigger the onset of chronic widespread pain and fatigue (Clauw 2014; Sommer 2012). Depression and post‐traumatic stress disorder worsen fibromyalgia symptoms (Häuser 2013a; Lange 2010).
Several factors are associated with the pathophysiology (functional changes associated with or resulting from disease) of fibromyalgia, but the relationship is unclear. The functional changes include alteration of sensory processing in the brain, reduced reactivity of the hypothalamus‐pituitary‐adrenal axis to stress, increased pro‐inflammatory and reduced anti‐inflammatory cytokine profiles (produced by cells involved in inflammation), disturbances in neurotransmitters such as dopamine and serotonin, and pathology (Oaklander 2013; Sommer 2012; Üçeyler 2013a). Prolonged exposure to stress, as outlined above, may contribute to these functional changes in predisposed individuals (Bradley 2009). There are similarities to, and differences from, neuropathic pain (Koroschetz 2011).
Patients often report high disability levels and poor quality of life along with extensive use of medical care (Häuser 2015). Many people with fibromyalgia are significantly disabled, and experience moderate or severe pain for many years (Bennett 2007). Chronic painful conditions comprised five of the 11 top‐ranking conditions for years lived with disability in 2010 (Vos 2012), and are responsible for considerable loss of quality of life, and employment, and increased health costs (Moore 2014a).
Fibromyalgia is common. One component of fibromyalgia, chronic widespread pain, is not only associated with other symptoms such as poor sleep, fatigue, and depression (Wolfe 2014), but also estimated to affect 11% of the general population (Mansfield 2016). Numerous studies have investigated prevalence of fibromyalgia in different settings and countries. A review gives a global mean prevalence of potential cases of fibromyalgia of 2.7% (range 0.4% to 9.3%), with a mean in the Americas of 3.1%, in Europe of 2.5%, and in Asia of 1.7% (Queiroz 2013). Changes in diagnostic criteria do not appear to have significantly affected estimates of prevalence (Wolfe 2013). A survey using a modification of the 2010 ACR criteria found a prevalence of 1.8% in a large US survey, but 73% of these were given a different diagnosis by their physician (Walitt 2015). Estimates of prevalence in specific populations vary greatly, but have been reported to be as high as 9% in female textile workers in Turkey and 10% in metal workers in Brazil (59% in those with repetitive strain injury; Queiroz 2013). Women are more frequently diagnosed with the disorder when the 1990 ACR criteria are used for clinical surveys. Using these criteria, the women‐to‐men ratio has ranged from 8:1 to 30:1 in patients who were studied in clinical institutions and surveys. However, with criteria that do not use tender point examination, the sex ratio can be close to equal. The sex ratio has ranged from 4:1 to 1:1 in studies that were conducted in the general population using the research criteria for fibromyalgia (Häuser 2015; Queiroz 2013).
Fibromyalgia pain is known to be difficult to treat effectively, with only a minority of individuals experiencing a clinically relevant benefit from any intervention. Recent evidence‐based guidelines recommend a multidisciplinary approach, with pharmacological treatment being combined with physical or cognitive training, or both. Interventions aim to reduce the key symptoms of fibromyalgia (pain, sleep problems, fatigue) and the associated symptoms (depression, disability) and to improve daily functioning (Eich 2012; Fitzcharles 2013). Conventional analgesics are usually not effective. Patients are often offered treatment with antidepressants like serotonin and noradrenaline reuptake inhibitors (Häuser 2013b; Lunn 2014), tricyclic agents such as amitriptyline (Moore 2012a), or anticonvulsants like gabapentin or pregabalin (Moore 2014b; Wiffen 2013; Üçeyler 2013b). The proportion of patients who achieve worthwhile pain relief (typically at least 50% reduction in pain intensity) is small (Moore 2013b), and generally reaches only 10% to 25% more than with placebo, with numbers needed to treat (NNTs) between 9.8 and 14 in fibromyalgia (Wiffen 2013), somewhat higher (worse) than for neuropathic pain (Kalso 2013; Wiffen 2013). Those who do experience good levels of pain relief, however, also benefit from substantial reductions in other symptoms, such as fatigue, function, sleep, depression, anxiety, and ability to work, with significant improvement in quality of life (Moore 2010c; Straube 2011). Fibromyalgia is not particularly different from other chronic pain with regard to a small proportion of trial participants having a good response to analgesic treatment (Moore 2013b).
Description of the intervention
Pregabalin is marketed under different trademarks worldwide and is also manufactured in combination with vitamin B12; we have not included the combination in this review. Pregabalin is licensed for the treatment of epilepsy, generalised anxiety disorder, and peripheral and central neuropathic pain in adults; in the USA and some other jurisdictions it is additionally licensed for treatment of fibromyalgia. Guidance suggests that pregabalin treatment can be started at a dose of 150 mg per day. Based on individual patient response and tolerability, the dosage may be increased to 300 mg per day after an interval of three to seven days and, if needed, to a maximum dose of 600 mg per day after an additional seven‐day interval (EMC 2016). Pregabalin is approved for fibromyalgia in the USA and Canada, and in a number of countries in South America, the Middle East, and Asia.
A particular issue with pregabalin is that of dose and dose escalation, with different upper limits used in different trials. In standard clinical trials in neuropathic pain there is known to be a significant dose‐response (Straube 2008). Another issue is the possible impact of enriched enrolment on the efficacy reported in trials, although the rather small amount of partial enrichment that could occur in most trials is without any significant effect (Straube 2008). More interesting is the publication of the results of trials with a complete enrichment, randomised withdrawal (EERW) design in fibromyalgia. These offer a potential challenge to interpretation of clinical trial data (McQuay 2008; Moore 2015).
How the intervention might work
Pregabalin has a mechanism of action similar to gabapentin, binding to calcium channels and reducing calcium influx as well as influencing GABAergic neurotransmission (Taylor 2007). This mode of action confers antiepileptic, analgesic, and anxiolytic effects. It is more potent than gabapentin due to a higher affinity for calcium channels and is therefore used at lower doses, with substantial differences in gastrointestinal absorption (Bockbrader 2010). The dosing regimen for pregabalin is two times daily.
Why it is important to do this review
Pregabalin can be used to treat pain associated with fibromyalgia in adults, and is licensed for this condition in the USA and some other countries, but not in Europe.
In addition, the standards used to assess evidence in chronic pain trials have changed substantially, with particular attention being paid to trial duration, withdrawals, and statistical imputation following withdrawal, all of which can substantially alter estimates of efficacy. The most important change is the move from using average pain scores, or average change in pain scores, to the number of people who have a large decrease in pain (by at least 50% or 30%) and who continue in treatment, ideally in trials of 8 to 12 weeks or longer. Pain intensity reduction of 50% or more has been shown to correlate with improvements in comorbid symptoms, function, and health‐related quality of life generally for acute and chronic pain (Moore 2013a), and specifically for fibromyalgia (Moore 2010b). These standards are set out in the reference guide for pain studies (PaPaS 2012).
This Cochrane review assessed the evidence using methods that make both statistical and clinical sense, and used developing criteria for what constitutes reliable evidence in chronic pain (Moore 2010a). The studies included and analysed needed to meet a minimum of reporting quality (blinding, randomisation), validity (duration, dose and timing, diagnosis, outcomes, etc.), and size (ideally at least 500 participants in a comparison in which the NNT is 4 or above (Moore 1998)). This approach sets high standards and marks a departure from how reviews were conducted previously. The use of unbiased studies reduces the chances of overestimating treatment effects (Mills 2015).
Objectives
To assess the analgesic efficacy and adverse events of pregabalin for pain in fibromyalgia in adults, compared with placebo or any active comparator.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) with double‐blind assessment of participant outcomes and a duration of eight weeks or longer. We required full journal publication, with the exception of online clinical trial results summaries of otherwise unpublished clinical trials, and abstracts with sufficient data for analysis. We did not include short abstracts (usually meeting reports). We excluded studies that were non‐randomised, studies of experimental pain, case reports, and clinical observations.
Trials had to have at least 20 participants per treatment arm. This is based on growing evidence of bias in small studies (Dechartres 2013; Dechartres 2014; Moore 1998).
Types of participants
Studies included adult participants aged 18 years and above, with pain due to fibromyalgia, diagnosed using the 1990 or 2010 criteria (Wolfe 1990; Wolfe 2010).
Types of interventions
Pregabalin at any dose, by any route, administered for the relief of fibromyalgia pain, and compared to placebo or any active comparator.
Types of outcome measures
We anticipated that studies would use a variety of outcome measures, with the majority of studies using a visual analogue scale (VAS) for pain intensity or pain relief, or both. We were particularly interested in Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) definitions for moderate and substantial benefit in chronic pain studies (Dworkin 2008). These are defined as:
at least 30% pain relief over baseline (moderate);
at least 50% pain relief over baseline (substantial);
much or very much improved on Patient Global Impression of Change scale (PGIC) (moderate);
very much improved on PGIC (substantial).
These outcomes are analytically relevant, concentrating as they do on dichotomous outcomes where pain responses do not follow a normal (Gaussian) distribution. They are also clinically relevant; people with chronic pain desire high levels of pain relief, ideally more than 50%, and ideally no worse than mild pain (Moore 2013a; O'Brien 2010).
Primary outcomes
Participant‐reported pain relief of 30% or greater.
Participant‐reported pain relief of 50% or greater.
PGIC much or very much improved.
PGIC very much improved.
Secondary outcomes
Any pain‐related outcome indicating some improvement.
Withdrawals due to lack of efficacy, adverse events, and for any cause.
Participants experiencing any adverse event.
Participants experiencing any serious adverse event. Serious adverse events typically include any untoward medical occurrence or effect that at any dose results in death, is life‐threatening, requires hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability or incapacity, is a congenital anomaly or birth defect, is an 'important medical event' that may jeopardise the patient, or may require an intervention to prevent one of the above characteristics or consequences.
Specific adverse events, particularly somnolence and dizziness.
Search methods for identification of studies
Electronic searches
We searched the following databases, without language restrictions.
Cochrane Central Register of Controlled Trials (CENTRAL, via the Cochrane Register of Studies Online database (CRSO)) to 16 March 2016.
MEDLINE (via Ovid) from 1946 to 16 March 2016.
EMBASE (via Ovid) from 1974 to 16 March 2016.
The search strategies for CENTRAL, MEDLINE, and EMBASE are in Appendix 2, Appendix 3, and Appendix 4.
Searching other resources
We reviewed the bibliographies of any RCTs identified and review articles, and searched clinical trial databases (ClinicalTrials.gov (ClinicalTrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/)) to identify additional published or unpublished data. We did not contact investigators or study sponsors for this update.
Data collection and analysis
Selection of studies
Two review authors read the abstract of each study identified by the search, eliminated studies that clearly did not satisfy the inclusion criteria, and obtained full copies of the remaining studies. The same review authors then independently read these studies to determine eligibility; any disagreements or uncertainty were settled by discussion, with a third review author if necessary. Studies were not anonymised in any way before assessment. We have included a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart (Figure 1).
1.
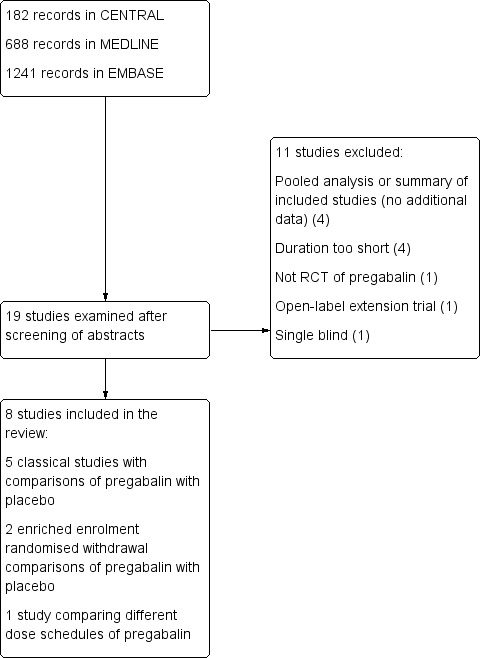
Study flow diagram.
Data extraction and management
Two review authors independently extracted data using a standard form and checked these for agreement before entry into Review Manager 5 (RevMan 2014), or any other analysis tool. We included information about the pain condition and number of participants treated, drug and dosing regimen, study design (placebo or active control, standard design or EERW), study duration and follow‐up, analgesic outcome measures and results, withdrawals, and adverse events (participants experiencing any adverse event, or serious adverse event).
Assessment of risk of bias in included studies
We used the Oxford Quality Score as the basis for inclusion (Jadad 1996), limiting inclusion to studies that were randomised and double blind as a minimum.
Two review authors independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Chapter 8, Higgins 2011), and adapted from those used by the Cochrane Pregnancy and Childbirth Group, resolving any disagreements by discussion. We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, random number table; computer random number generator); unclear risk of bias (when the method used to generate the sequence is not clearly stated). We excluded studies that used a non‐random process (odd or even date of birth; hospital or clinic record number) and were therefore at a high risk of bias.
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (telephone or central randomisation; consecutively numbered, sealed, opaque envelopes); unclear risk of bias (when method not clearly stated). We excluded studies that did not conceal allocation and were therefore at a high risk of bias (open list).
Blinding of participants and personnel, and outcome assessment (checking for possible performance bias and detection bias). We assessed the methods used to blind study participants, personnel and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, identical tablets, matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how this was achieved). We excluded studies that were not double blind and therefore at a high risk of bias.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk of bias (fewer than 10% of participants did not complete the study or used 'baseline observation carried forward' analysis, or both); unclear risk of bias (used 'last observation carried forward' analysis); or high risk of bias (used 'completer' analysis).
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (200 participants or more per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); or high risk of bias (fewer than 50 participants per treatment arm).
Measures of treatment effect
We calculated NNTs as the reciprocal of the absolute risk reduction (McQuay 1998). For unwanted effects, the NNT becomes the number needed to harm (NNH) and is calculated in the same manner. We used dichotomous data to calculate risk ratio with 95% confidence intervals using a fixed‐effect model unless we found significant statistical heterogeneity (see below). We did not use continuous data in analyses.
We have used the following terms to describe adverse outcomes in terms of harm or prevention of harm.
When significantly fewer adverse outcomes occurred with treatment than with control (placebo or active), we used the term 'number needed to treat to prevent' one event (NNTp).
When significantly more adverse outcomes occurred with treatment compared with control (placebo or active), we used the term 'number needed to harm' (NNH).
Unit of analysis issues
We accepted randomisation to individual participant only. In the event of a study having more than one active treatment arm in which data were not combined for analysis, we planned to split the control treatment arm between active treatment arms.
Dealing with missing data
We have used intention‐to‐treat (ITT) analysis where the ITT population consists of participants who were randomised, took at least one dose of the assigned study medication, and provided at least one post‐baseline assessment. We assigned missing participants zero improvement wherever possible.
Assessment of heterogeneity
We dealt with clinical heterogeneity by combining studies that examined similar conditions. We assessed statistical heterogeneity visually (L'Abbé 1987), and by using the I² statistic. When the I² value was greater than 50%, we considered possible reasons for this for in studies of classic design.
Assessment of reporting biases
The aim of this review was to use dichotomous outcomes of known utility and of value to patients (Hoffman 2010; Moore 2010b; Moore 2010c; Moore 2010d; Moore 2013a). The review did not depend on what the authors of the original studies chose to report or not, though clearly difficulties would arise if studies failed to report relevant dichotomous results. We extracted and used continuous data, which probably reflect efficacy and utility poorly, where useful for illustrative purposes only.
We planned to assess publication bias using a method designed to detect the amount of unpublished data with a null effect required to make any result clinically irrelevant (usually taken to mean a NNT of 10 or higher) (Moore 2008), but this was not possible with low effect sizes.
Data synthesis
We planned to use a fixed‐effect model for meta‐analysis. We would have used a random‐effects model if we had found significant clinical heterogeneity and considered it appropriate to combine studies.
We analysed data for each painful condition in three tiers, according to outcome and freedom from known sources of bias.
The first tier used data meeting current best standards, where studies report the outcome of at least 50% pain intensity reduction over baseline (or its equivalent), without the use of last observation carried forward (LOCF) or other imputation method for dropouts, report an ITT analysis, last eight or more weeks, have a parallel‐group design, and have at least 200 participants (preferably at least 400) in the comparison (Moore 1998; Moore 2010a; Moore 2012a; Moore 2012b). We have reported these top‐tier results first.
The second tier used data from at least 200 participants but where one or more of the first‐tier conditions above was not met (reporting at least 30% pain intensity reduction (or equivalent), using LOCF or a completer analysis, or lasting four to eight weeks).
The third tier of evidence related to data from fewer than 200 participants, or where significant problems were expected because, for example, there was major heterogeneity between studies, or where there were shortcomings in allocation concealment, attrition, or incomplete outcome data. For this third tier of evidence, no data synthesis is reasonable and may be misleading, but an indication of beneficial effects might be possible.
Quality of the evidence
We have used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system to assess the quality of the evidence related to the key outcomes listed in Types of outcome measures, as appropriate (Appendix 5; Section 12.2, Higgins 2011). Two review authors independently rated the quality of evidence for each outcome.
We paid particular attention to:
inconsistency, where point estimates vary widely across studies, or confidence intervals of studies show minimal or no overlap (Guyatt 2011);
potential for publication bias, based on the amount of unpublished data required to make the result clinically irrelevant (Moore 2008).
In addition, there may be circumstances where the overall rating for a particular outcome needs to be adjusted as recommended by GRADE guidelines (Guyatt 2013a). For example, if there are so few data that the results are highly susceptible to the random play of chance, or if a studies use LOCF imputation in circumstances where there are substantial differences in adverse event withdrawals, one would have no confidence in the result, and would need to downgrade the quality of the evidence by three levels, to very low quality. In circumstances where no data were reported for an outcome, we would report the level of evidence as very low quality (Guyatt 2013b).
'Summary of findings' table
We have included 'Summary of findings' tables as set out in the Cochrane Pain, Palliative and Supportive Care Group author guide (PaPaS 2012), and recommended in the Cochrane Handbook (Chapter 11, Higgins 2011). The tables include, where possible, outcomes of at least 50% (substantial benefit) and at least 30% pain intensity reduction or maintenance of therapeutic response (MTR) (moderate benefit), PGIC (possibly at least substantial improvement and at least moderate improvement), withdrawals due to lack of efficacy, withdrawals due to adverse events, serious adverse events, and death (a particular serious adverse event).
Subgroup analysis and investigation of heterogeneity
We did not plan subgroup analyses since our experience based on previous reviews indicates that there would be inadequate information for meaningful subgroup analysis beyond dose, for example information on age or severity of pain is not usually recorded separately.
Sensitivity analysis
We did not plan any sensitivity analysis.
Results
Description of studies
The original review included five studies on fibromyalgia. Four were full publications (Arnold 2008; Crofford 2005; Crofford 2008; Mease 2008), and one was a PhRMA Web Synopsis (A0081100 2008). This trial was registered as NCT00333866 and was subsequently published (Pauer 2011).
Results of the search
The flow diagram of the search results is shown in Figure 1. We examined 19 articles for possible inclusion, which included the five identified in the original review.
We also identified three ongoing studies in ClinicalTrials.gov (NCT01387607; NCT02146430; NCT02187159).
Included studies
We included eight studies in this review.
We included five studies (3283 participants) in the main analysis using a 'classical' clinical trial design where participants were randomised at the start of the trial to pregabalin or placebo using fixed‐dose titration strategies (Arnold 2008; Crofford 2005; Mease 2008; Ohta 2012; Pauer 2011). One other study compared a single nightly dose of pregabalin (morning dose placebo) with twice‐daily dosing in 177 participants (Nasser 2014), and did not contribute to any analyses.
We have also reported on two studies using an enriched enrolment randomised withdrawal (EERW) design, in which participants underwent titration to effect and tolerance with pregabalin, and those successfully reaching those targets were then randomised to continued pregabalin or phased withdrawal to placebo (Arnold 2014; Crofford 2008). In these studies 687 participants entered the randomised withdrawal phase.
The majority of participants were women (89% to 95%) and white (76% to 96%), and the mean age was 47 to 50 years. All participants had fibromyalgia diagnosed according to the American College of Rheumatology (ACR) 1990 criteria (Wolfe 1990). Full details are in the Characteristics of included studies table. Where mentioned, the duration of fibromyalgia symptoms averaged about four years; baseline pain intensity averaged between 6.5 and 7.8/10, equivalent to severe pain (Collins 1997).
Studies were generally carried out over a duration of two to three months. Of the six classic studies, two lasted eight weeks (Crofford 2005; Nasser 2014), and the others lasted a total of 13 or 14 weeks, generally with 12 weeks on stable doses. The two EERW studies had double‐blind treatment periods after randomisation lasting 13 weeks, in Arnold 2014, or 26 weeks, in Crofford 2008.
All of the studies of classic design used LOCF imputation for missing data, including when participants withdrew from treatment. The two EERW studies reported an outcome of loss of therapeutic effect, which was without imputation.
Excluded studies
We excluded 11 studies (12 reports) from the search (Arnold 2012; Arnold 2015; Byon 2010; Emir 2010; NCT00760474; NCT01268631; NCT01904097; Ohta 2013; Ramzy 2016; Roth 2012; Russell 2009). Reasons for exclusion are in the Characteristics of excluded studies table. The earlier review identified one study, Arnold 2013, in ClinicalTrials.gov, which has since been published as Arnold 2015.
Risk of bias in included studies
Figure 2 shows the overall 'Risk of bias' assessment of included studies. 'Risk of bias' assessments for each criterion for each study are given in the Characteristics of included studies table and Figure 3. All included studies had Oxford Quality Scores of 3/5 to 5/5.
2.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
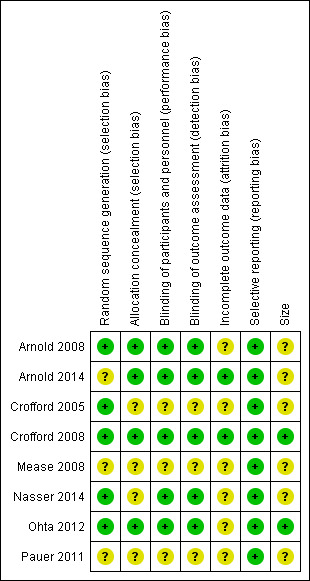
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All of the studies were randomised, but only five adequately described the method used to generate the random sequence (Arnold 2008; Crofford 2005; Crofford 2008; Nasser 2014; Ohta 2012). Arnold 2008, Arnold 2014, Crofford 2008, and Ohta 2012 adequately described the methods used to ensure that allocation of participants to treatment groups was concealed. The remaining four studies did not report the method used (Crofford 2005; Mease 2008; Nasser 2014; Pauer 2011).
Blinding
All studies were described as double blind, but Crofford 2005, Mease 2008, and Pauer 2011 did not describe the methods used to ensure that participants and interacting investigators were unable to differentiate between the treatment and control tablets. The other five studies provided adequate information.
Incomplete outcome data
Five studies used LOCF to impute data for participants who withdrew for any reason in analyses of individual pain outcomes (Arnold 2008; Crofford 2005; Mease 2008; Ohta 2012; Pauer 2011). Nasser 2014 did not provide any information on the imputation method for missing data. Two studies did not use imputation (Arnold 2014; Crofford 2008).
Selective reporting
All studies reported the outcomes specified in the methods.
Other potential sources of bias
Only two studies had more than 200 participants in individual trial arms (Crofford 2008; Ohta 2012), and none had fewer than 50 participants in a trial arm.
Effects of interventions
Summary of findings for the main comparison. Pregabalin compared with placebo for fibromyalgia in studies of classic design.
| Pregabalin compared with placebo for fibromyalgia in studies of classic design | ||||||
|
Patient or population: adults with fibromyalgia enrolled in studies of classic design Settings: community Intervention: pregabalin 450 mg Comparison: placebo | ||||||
|
Outcomes (follow up: 8‐14 weeks) |
Probable outcome with pregabalin | Probable outcome with placebo |
RR, NNT, NNH, NNTp (95% CI) |
No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| ‘Substantial benefit’ (at least 50% reduction in pain) | 240 per 1000 | 140 per 1000 | RR 1.8 (1.4 to 2.1) NNT 9.7 (7.2 to 15) |
1874 participants (5 studies) |
High quality | 1 |
| ‘Substantial benefit’ (PGIC very much improved) | 170 per 1000 | 90 per 1000 | RR 1.9 (1.5 to 2.4) NNT 12 (9.0 to 20) |
1869 participants (5 studies) |
High quality | 1 |
| ‘Moderate benefit’ (at least 30% reduction in pain) | 430 per 1000 | 290 per 1000 | RR 1.5 (1.3 to 1.7) NNT 7.2 (5.5 to 10) |
1874 participants (5 studies) |
High quality | 1 |
| ‘Moderate benefit’ (PGIC much or very much improved) | 360 per 1000 | 270 per 1000 | RR 1.3 (1.2 to 1.5) NNT 11 (7.8 to 22) |
1869 participants (5 studies) |
High quality | 1 |
| Withdrawals due to lack of efficacy | 40 per 1000 | 100 per 1000 | RR 0.4 (0.2 to 0.5) NNTp 15 (11 to 24) |
1874 participants (5 studies) |
High quality | Large number of participants and events, clearly reported |
| Withdrawals due to adverse events | 170 per 1000 | 90 per 1000 | RR 2.0 (1.6 to 2.6) NNH 11 (8.4 to 17) |
1874 participants (5 studies) |
High quality | Large number of participants and events, clearly reported |
| Serious adverse events | 21 per 1000 | 11 per 1000 | RR 1.9 (0.8 to 4.6) | 1238 participants (3 studies) |
Very low quality | Downgraded 3 levels due to small numbers of events. Number of events too small to make any reliable judgement about differences between pregabalin and placebo |
| Death | No deaths recorded at any dose of pregabalin or placebo in any study | Not calculated | 3460 participants (6 studies) |
Very low quality | No data because no events were reported | |
| BOCF: baseline observation carried forward; CI: confidence interval; LOCF: last observation carried forward; NNH: number needed to harm; NNT: number needed to treat; NNTp: number needed to treat to prevent; PGIC: Patient Global Impression of Change; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1: We decided not to downgrade for LOCF imputation because LOCF NNT and BOCF NNT similar in magnitude with no clinical implications in the differences
Summary of findings 2. Pregabalin compared with placebo for fibromyalgia in enriched enrolment randomised withdrawal (EERW) studies.
| Pregabalin compared with placebo for fibromyalgia in enriched enrolment randomised withdrawal (EERW) studies | ||||||
|
Patient or population: adults with fibromyalgia enrolled in EERW studies Settings: community Intervention: pregabalin 300 to 600 mg Comparison: placebo | ||||||
|
Outcomes (follow up: 13‐26 weeks) |
Probable outcome with pregabalin | Probable outcome with placebo |
RR, NNT, NNH (95% CI) |
No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Maintenance of initial therapeutic response of at least 30% pain intensity reduction over baseline and continued treatment at 13 or 26 weeks (equivalent to 'moderate benefit') |
400 per 1000 | 200 per 1000 | RR 1.9 (1.5 to 2.4) NNT 5.3 (3.9 to 8.2) |
687 participants (2 studies) |
High quality | Fully defined outcomes, without imputation. Consistent effects in both studies. Note that the population in these studies was enriched (46% of initial enrolled) |
| Participants experiencing at least 1 adverse event | 650 per 1000 | 490 per 1000 | RR 1.3 (1.2 to 1.5) NNH 6.2 (4.3 to 11) |
687 participants (2 studies) |
High quality | Adequate numbers of participants and events |
| Serious adverse events | 23 per 1000 | 6 per 1000 | Not calculated | 687 participants (2 studies) |
Very low quality | Small numbers of events |
| Death | 1 death | 1 death | Not calculated | 687 participants (2 studies) |
Very low quality | Small numbers of events |
| CI: confidence interval; NNH: number needed to harm; NNT: number needed to treat; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Details of results in individual studies are in Appendix 6. The analyses are necessarily different for classic study designs and EERW designs, so the format of the analyses is for efficacy and harm to be analysed for the classic study design and EERW design separately.
1. Classic study design
Efficacy in studies of classic design
All studies of classic design used LOCF imputation, so despite good trial design and large numbers, we classified them as second‐tier evidence. We assessed the evidence as high quality because studies were randomised and double blind, had large numbers of participants, and were consistent; this was downgraded because of the use of LOCF imputation, but upgraded because the LOCF NNT estimations were similar to non‐imputed analyses from individual patient data analyses from the same studies (see Table 1) (Straube 2010a).
One small study showed no benefit of nightly over twice‐daily pregabalin dosing (Nasser 2014).
Various doses of pregabalin were compared with placebo in classic studies (Arnold 2008; Crofford 2005; Mease 2008; Ohta 2012; Pauer 2011. In this analysis we have chosen to include a single study in which 150 mg pregabalin was used, because it had more than 200 participants in the comparison and examined a general dose‐response relationship. Efficacy outcomes are provided in the table Summary of results A below.
Pregabalin 150 mg daily versus placebo
At least 30% pain intensity reduction over baseline (moderate benefit)
One study (263 participants) reported at least 30% improvement from baseline pain intensity (Crofford 2005).
The proportion of participants with ≥ 30% pain relief with pregabalin 150 mg was 31% (41/132).
The proportion of participants with ≥ 30% pain relief with placebo was 27% (36/131).
The risk ratio (RR) for pregabalin compared with placebo was 1.1 (95% confidence interval (CI) 0.78 to 1.7) (Analysis 1.1). NNT was not calculated.
1.1. Analysis.
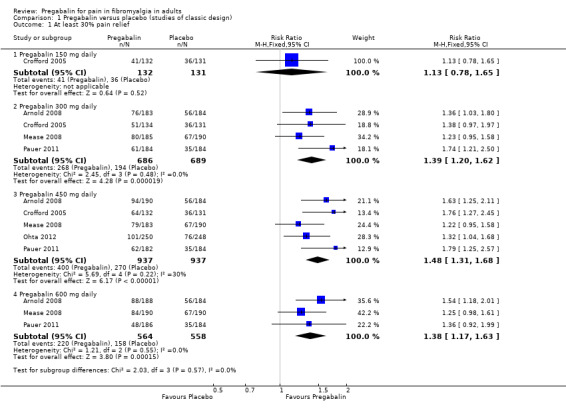
Comparison 1 Pregabalin versus placebo (studies of classic design), Outcome 1 At least 30% pain relief.
At least 50% pain intensity reduction over baseline (substantial benefit)
One study (263 participants) reported at least 50% improvement from baseline pain intensity (Crofford 2005).
The proportion of participants with ≥ 50% pain relief with pregabalin 150 mg was 13% (17/132).
The proportion of participants with ≥ 50% pain relief with placebo was 13% (17/131).
The RR for pregabalin compared with placebo was 0.99 (95% CI 0.53 to 1.9) (Analysis 1.2; Figure 4). NNT was not calculated.
1.2. Analysis.
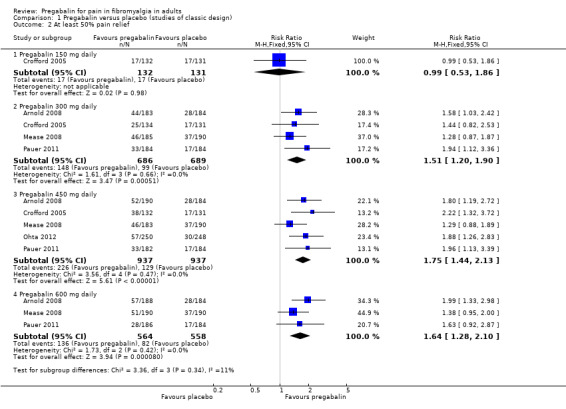
Comparison 1 Pregabalin versus placebo (studies of classic design), Outcome 2 At least 50% pain relief.
4.
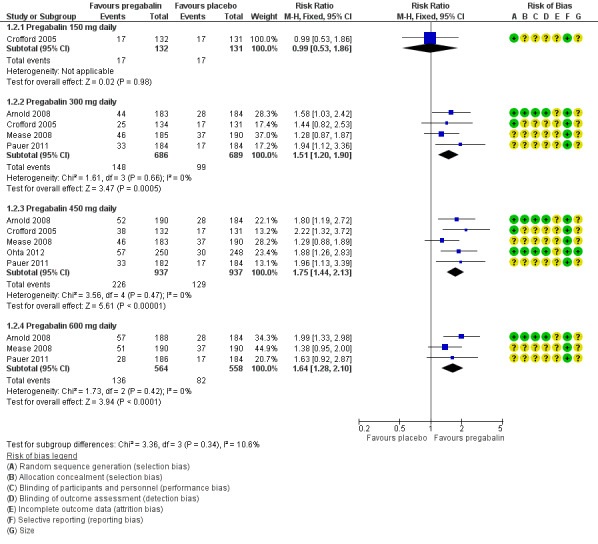
Forest plot of comparison: 1 Pregabalin versus placebo, outcome: 1.2 At least 50% pain relief.
PGIC 'much or very much improved' (moderate benefit)
One study (263 participants) reported PGIC of much or very much improved (Crofford 2005).
The proportion of participants with PGIC of much or very much improved with pregabalin 150 mg was 32% (42/132).
The proportion of participants with PGIC of much or very much improved with placebo was 26% (34/131).
The RR for pregabalin compared with placebo was 1.2 (95% CI 0.84 to 1.8) (Analysis 1.3; Figure 5). NNT was not calculated.
1.3. Analysis.
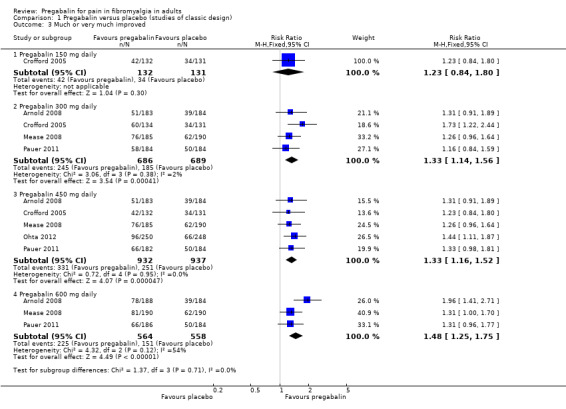
Comparison 1 Pregabalin versus placebo (studies of classic design), Outcome 3 Much or very much improved.
5.
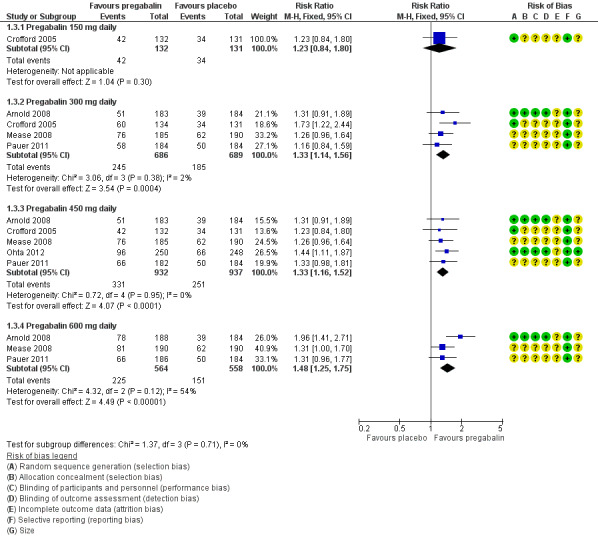
Forest plot of comparison: 1 Pregabalin versus placebo, outcome: 1.3 Much or very much improved.
PGIC 'very much improved' (substantial benefit)
There were no data for this outcome.
Pregabalin 300 mg daily versus placebo
At least 30% pain intensity reduction over baseline (moderate benefit)
Four studies (1375 participants) reported at least 30% pain relief (Arnold 2008; Crofford 2005; Mease 2008; Pauer 2011).
The proportion of participants with ≥ 30% pain relief with pregabalin 300 mg was 39% (268/686).
The proportion of participants with ≥ 30% pain relief with placebo was 28% (194/689)
The RR for pregabalin compared with placebo was 1.4 (95% CI 1.2 to 1.6) (Analysis 1.1), and the NNT was 9.2 (6.3 to 17) for at least moderate pain relief.
At least 50% pain intensity reduction over baseline (substantial benefit)
Four studies (1375 participants) reported at least 50% pain relief (Arnold 2008; Crofford 2005; Mease 2008; Pauer 2011).
The proportion of participants with ≥ 50% pain relief with pregabalin 300 mg was 22% (148/686).
The proportion of participants with ≥ 50% pain relief with placebo was 14% (99/689).
The RR for pregabalin compared with placebo was 1.5 (95% CI 1.2 to 1.9) (Analysis 1.2; Figure 4), and the NNT was 14 (8.9 to 32) for at least substantial pain relief.
PGIC 'much or very much improved' (moderate benefit)
Four studies (1375 participants) reported PGIC of much or very much improved (Arnold 2008; Crofford 2005; Mease 2008; Pauer 2011).
The proportion of participants with PGIC of much or very much improved with pregabalin 300 mg was 36% (245/686).
The proportion of participants with PGIC of much or very much improved with placebo was 27% (185/689).
The RR for pregabalin compared with placebo was 1.3 (95% CI 1.1 to 1.6) (Analysis 1.3; Figure 5), and the NNT was 11 (7.3 to 25) for moderate benefit.
PGIC 'very much improved' (substantial benefit)
Four studies (1375 participants) reported PGIC of very much improved (Arnold 2008; Crofford 2005; Mease 2008; Pauer 2011).
The proportion of participants with PGIC of very much improved with pregabalin 300 mg was 17% (115/686).
The proportion of participants with PGIC of very much improved with placebo was 10% (72/689).
The RR for pregabalin compared with placebo was 1.6 (95% CI 1.2 to 2.1) (Analysis 1.4), and the NNT was 16 (10 to 37) for substantial benefit.
1.4. Analysis.
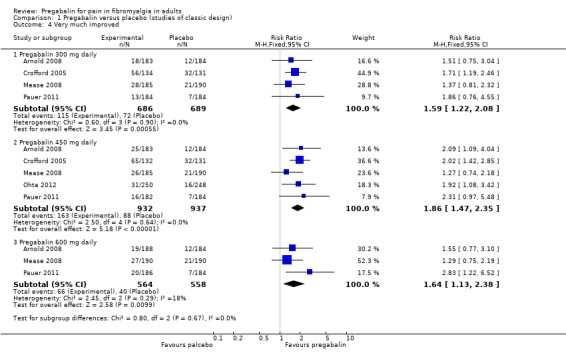
Comparison 1 Pregabalin versus placebo (studies of classic design), Outcome 4 Very much improved.
Pregabalin 450 mg daily versus placebo
Ohta 2012 reported combined results for 300 mg and 450 mg daily. The results are analysed as 450 mg daily because the majority of participants received that dose (59/250 received 300 mg daily and 178/250 received 450 mg daily).
At least 30% pain intensity reduction over baseline (moderate benefit)
Five studies (1874 participants) reported at least 30% pain relief (Arnold 2008; Crofford 2005; Mease 2008; Ohta 2012; Pauer 2011).
The proportion of participants with ≥ 30% pain relief with pregabalin 450 mg was 43% (400/937).
The proportion of participants with ≥ 30% pain relief with placebo was 29% (270/937)
The RR for pregabalin compared with placebo was 1.5 (95% CI 1.3 to 1.7) (Analysis 1.1), and the NNT was 7.2 (5.5 to 10) for at least moderate pain relief.
At least 50% pain intensity reduction over baseline (substantial benefit)
Five studies (1874 participants) reported at least 50% pain relief (Arnold 2008; Crofford 2005; Mease 2008; Ohta 2012; Pauer 2011).
The proportion of participants with ≥ 50% pain relief with pregabalin 450 mg was 24% (226/937).
The proportion of participants with ≥ 50% pain relief with placebo was 14% (129/937).
The RR for pregabalin compared with placebo was 1.8 (95% CI 1.4 to 2.1) (Analysis 1.2; Figure 4), and the NNT was 9.7 (7.2 to 15) for at least substantial pain relief.
PGIC 'much or very much improved' (moderate benefit)
Five studies (1869 participants) reported PGIC of much or very much improved (Arnold 2008; Crofford 2005; Mease 2008; Ohta 2012; Pauer 2011).
The proportion of participants with PGIC of much or very much improved with pregabalin 450 mg was 36% (331/932).
The proportion of participants with PGIC of much or very much improved with placebo was 27% (251/937).
The RR for pregabalin compared with placebo was 1.3 (95% CI 1.2 to 1.5) (Analysis 1.3; Figure 5), and the NNT was 11 (7.8 to 22) for moderate benefit.
PGIC 'very much improved' (substantial benefit)
Five studies (1869 participants) reported PGIC of very much improved (Arnold 2008; Crofford 2005; Mease 2008; Ohta 2012; Pauer 2011).
The proportion of participants with PGIC of very much improved with pregabalin 450 mg was 17% (163/932).
The proportion of participants with PGIC of very much improved with placebo was 9% (88/937).
The RR for pregabalin compared with placebo was 1.9 (95% CI 1.5 to 2.4) (Analysis 1.4), and the NNT was 12 (9.0 to 20) for substantial benefit.
Pregabalin 600 mg daily versus placebo
At least 30% pain intensity reduction over baseline (moderate benefit)
Three studies (1122 participants) reported at least 30% pain relief (Arnold 2008; Mease 2008; Pauer 2011).
The proportion of participants with ≥ 30% pain relief with pregabalin 600 mg was 39% (220/564).
The proportion of participants with ≥ 30% pain relief with placebo was 28% (158/558)
The RR for pregabalin compared with placebo was 1.4 (95% CI 1.2 to 1.6) (Analysis 1.1), and the NNT was 9.4 (6.2 to 19) for at least moderate pain relief.
At least 50% pain intensity reduction over baseline (substantial benefit)
Three studies (1122 participants) reported at least 50% pain relief (Arnold 2008; Mease 2008; Pauer 2011).
The proportion of participants with ≥ 50% pain relief with pregabalin 600 mg was 24% (136/564).
The proportion of participants with ≥ 50% pain relief with placebo was 15% (82/558).
The RR for pregabalin compared with placebo was 1.6 (95% CI 1.3 to 2.1) (Analysis 1.2; Figure 4), and the NNT was 11 (7.1 to 21) for at least substantial pain relief.
PGIC 'much or very much improved' (moderate benefit)
Three studies (1122 participants) reported PGIC of much or very much improved (Arnold 2008; Mease 2008; Pauer 2011).
The proportion of participants with PGIC of much or very much improved with pregabalin 600 mg was 40% (225/564).
The proportion of participants with PGIC of much or very much improved with placebo was 27% (151/558).
The RR for pregabalin compared with placebo was 1.5 (95% CI 1.3 to 1.8) (Analysis 1.3; Figure 5), and the NNT was 7.8 (5.5 to 14) for moderate benefit.
PGIC 'very much improved' (substantial benefit)
Three studies (1122 participants) reported PGIC of very much improved (Arnold 2008; Mease 2008; Pauer 2011).
The proportion of participants with PGIC of very much improved with pregabalin 600 mg was 12% (66/564).
The proportion of participants with PGIC of very much improved with placebo was 7% (40/558).
The RR for pregabalin compared with placebo was 1.6 (95% CI 1.1 to 2.4) (Analysis 1.4), and the NNT was 22 (13 to 89) for substantial benefit.
| Summary of results A: Efficacy outcomes with different doses of pregabalin in fibromyalgia | ||||||
| Outcome ‐ daily dose | Number of | Per cent with outcome | Relative benefit (95% CI) | NNT (95% CI) | ||
| Studies | Participants | Pregabalin | Placebo | |||
| At least 30% pain intensity reduction over baseline (moderate benefit) | ||||||
| 150 mg* | 1 | 263 | 31 | 27 | 1.1 (0.8 to 1.7) | Not calculated |
| 300 mg | 4 | 1375 | 39 | 28 | 1.4 (1.2 to 1.6) | 9.2 (6.3 to 17) |
| 450 mg | 5 | 1874 | 43 | 29 | 1.5 (1.3 to 1.7) | 7.2 (5.5 to 10) |
| 600 mg | 3 | 1122 | 39 | 28 | 1.4 (1.2 to 1.6) | 9.4 (6.2 to 19) |
| At least 50% pain intensity reduction over baseline (substantial benefit) | ||||||
| 150 mg* | 1 | 263 | 13 | 13 | 0.99 (0.5 to 1.9) | Not calculated |
| 300 mg | 4 | 1375 | 22 | 14 | 1.5 (1.2 to 1.9) | 14 (8.9 to 32) |
| 450 mg | 5 | 1874 | 24 | 14 | 1.8 (1.4 to 2.1) | 9.7 (7.2 to 15) |
| 600 mg | 3 | 1122 | 24 | 15 | 1.6 (1.3 to 2.1) | 11 (7.1 to 21) |
| PGIC much or very much improved (moderate benefit) | ||||||
| 150 mg* | 1 | 263 | 32 | 26 | 1.2 (0.8 to 1.8) | Not calculated |
| 300 mg | 4 | 1375 | 36 | 27 | 1.3 (1.1 to 1.6) | 11 (7.3 to 25) |
| 450 mg | 5 | 1869 | 36 | 27 | 1.3 (1.2 to 1.5) | 11 (7.8 to 22) |
| 600 mg | 3 | 1122 | 40 | 27 | 1.5 (1.3 to 1.8) | 7.8 (5.5 to 14) |
| PGIC very much improved (substantial benefit) | ||||||
| 300 mg | 4 | 1375 | 17 | 10 | 1.6 (1.2 to 2.1) | 16 (10 to 37) |
| 450 mg | 5 | 1869 | 17 | 9 | 1.9 (1.5 to 2.4) | 12 (9.0 to 20) |
| 600 mg | 3 | 1122 | 12 | 7 | 1.6 (1.1 to 2.4) | 22 (13 to 89) |
| *Information from a single study, included to examine a general dose‐response relationship and should be interpreted with caution. CI: confidence interval; NNT: number needed to treat; PGIC: Patient Global Impression of Change | ||||||
All of these analyses demonstrated a high degree of consistency between studies (Figure 4, Figure 5), with generally low I² values.
As we had good reporting of these efficacy outcomes of substantial and moderate benefit, we did not need to perform an analysis for any pain‐related improvement.
Withdrawals
All studies in the main analysis provided data on withdrawals over the study period for all causes, due to lack of efficacy, and due to adverse events (Arnold 2008; Crofford 2005; Mease 2008; Nasser 2014; Ohta 2012; Pauer 2011). We assessed the evidence as high quality because studies were randomised and double blind, and had large numbers of participants.
Nasser 2014 reported no significant difference in withdrawal rates between once‐nightly administration and twice‐daily administration of pregabalin.
Withdrawal outcomes are provided in the table Summary of results B below.
All‐cause withdrawals
The analysis for all‐cause withdrawals is shown in Analysis 1.5.
1.5. Analysis.
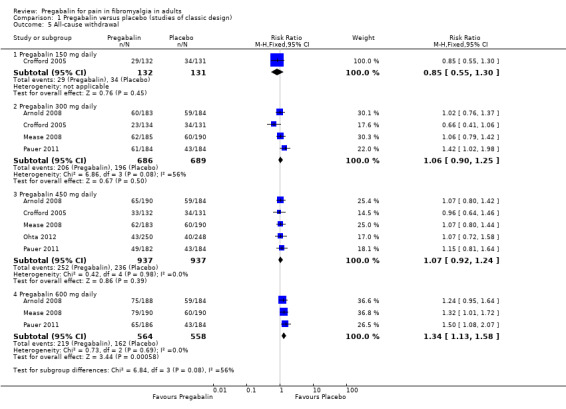
Comparison 1 Pregabalin versus placebo (studies of classic design), Outcome 5 All‐cause withdrawal.
Pregabalin 150 mg: 22% (29/132); placebo 26% (34/131). The RR was 0.85 (95% CI 0.55 to 1.3).
Pregabalin 300 mg: 30% (206/686); placebo 28% (196/689). The RR was 1.1 (95% CI 0.90 to 1.3).
Pregabalin 450 mg: 27% (252/937); placebo 30% (236/937). The RR was 1.1 (95% CI 0.92 to 1.2).
Pregabalin 600 mg: 39% (219/564); placebo 29% (162/558). The RR was 1.3 (95% CI 1.1 to 1.6), and the NNH was 10 (6.5 to 23).
Adverse event withdrawals
The analysis for adverse event withdrawals is shown in Analysis 1.6.
1.6. Analysis.
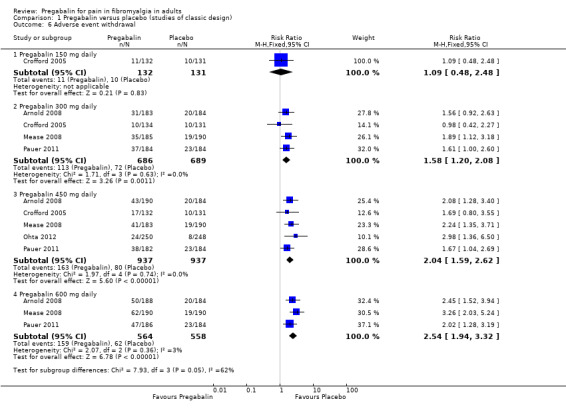
Comparison 1 Pregabalin versus placebo (studies of classic design), Outcome 6 Adverse event withdrawal.
Pregabalin 150 mg: 8% (11/132); placebo 8% (10/131). The RR was 1.1 (95% CI 0.48 to 2.5).
Pregabalin 300 mg: 16% (113/686); placebo 10% (72/689). The RR was 1.6 (95% CI 1.2 to 2.1), and the NNH was 17 (10 to 41).
Pregabalin 450 mg: 17% (163/937); placebo 9% (80/937). The RR was 2.0 (95% CI 1.6 to 2.6), and the NNH was 11 (8.4 to 17).
Pregabalin 600 mg: 28% (159/564); placebo 11% (62/568). The RR was 2.5 (95% CI 1.9 to 3.3), and the NNH was 5.9 (4.6 to 8).
Lack of efficacy withdrawals
The analysis for lack of efficacy withdrawals is shown in Analysis 1.7.
1.7. Analysis.

Comparison 1 Pregabalin versus placebo (studies of classic design), Outcome 7 Lack of efficacy withdrawal.
Pregabalin 150 mg: 9% (12/132); placebo 14% (13/131). The RR was 0.66 (95% CI 0.33 to 1.3).
Pregabalin 300 mg: 4% (29/686); placebo 10% (68/689). The RR was 0.43 (95% CI 0.28 to 0.65), and the NNTp was 18 (12 to 34).
Pregabalin 450 mg: 4% (33/937); placebo 10% (94/937). The RR was 0.35 (95% CI 0.24 to 0.52), and the NNTp was 15 (11 to 24).
Pregabalin 600 mg: 2% (14/564); placebo 9% (50/568). The RR was 0.28 (95% CI 0.15 to 0.50), and the NNTp was 15 (11 to 26).
| Summary of results B: Withdrawal with different doses of pregabalin in fibromyalgia | ||||||
| Outcome ‐ daily dose | Number of | Per cent with outcome | Relative risk (95% CI) | NNH (95% CI) | ||
| Studies | Participants | Pregabalin | Placebo | |||
| All‐cause withdrawals | ||||||
| 150 mg | 1 | 263 | 22 | 26 | 0.85 (0.55 to 1.3) | Not calculated |
| 300 mg | 4 | 1375 | 30 | 28 | 1.1 (0.90 to 1.3) | Not calculated |
| 450 mg | 5 | 1874 | 27 | 30 | 1.1 (0.92 to 1.2) | Not calculated |
| 600 mg | 3 | 1122 | 39 | 29 | 1.3 (1.1 to 1.6) | 10 (6.5 to 23) |
| Adverse event withdrawals | ||||||
| 150 mg | 1 | 263 | 8 | 8 | 1.1 (0.48 to 2.5) | Not calculated |
| 300 mg | 4 | 1375 | 16 | 10 | 1.6 (1.2 to 2.1) | 17 (10 to 41) |
| 450 mg | 5 | 1874 | 17 | 9 | 2.0 (1.6 to 2.6) | 11 (8.4 to 17) |
| 600 mg | 3 | 1122 | 28 | 11 | 2.5 (1.9 to 3.3) | 5.9 (4.6 to 8) |
| Lack of efficacy withdrawals | NNTp (95% CI) | |||||
| 150 mg | 1 | 263 | 9 | 14 | 0.66 (0.33 to 1.3) | Not calculated |
| 300 mg | 4 | 1375 | 4 | 10 | 0.43 (0.28 to 0.65) | 18 (12 to 34) |
| 450 mg | 5 | 1874 | 4 | 10 | 0.35 (0.24 to 0.52) | 15 (11 to 24) |
| 600 mg | 3 | 1122 | 2 | 9 | 0.28 (0.15 to 0.50) | 15 (11 to 26) |
| CI: confidence interval; NNH: number needed to harm; NNTp: number needed to treat to prevent | ||||||
Adverse events in studies of classic design
All the randomised studies of classic design provided some information on participants experiencing adverse events over the study period, which was collected from spontaneous reports and clinical observation and evaluation. Ohta 2012 reported combined results for 300 mg and 450 mg daily. The results are analysed as 450 mg daily because the majority of participants received that dose.
Nasser 2014 did not provide data suitable for pooled analysis. It reported no significant difference in adverse events with different dosing regimens.
Adverse event outcomes are provided in the table Summary of results C below.
Participants with at least one adverse event
Five studies provided data on the number of participants reporting one or more adverse events with different dose regimens over the period of the study (8 to 14 weeks) (Arnold 2008; Crofford 2005; Mease 2008; Ohta 2012; Pauer 2011). We assessed the evidence as high quality because studies were randomised and double blind, and had large numbers of participants.
The majority of participants reported at least one adverse event over the period, including 77% to 73% of those participants receiving placebo, and the adverse event frequency increased with dose escalation (Analysis 1.8).
1.8. Analysis.
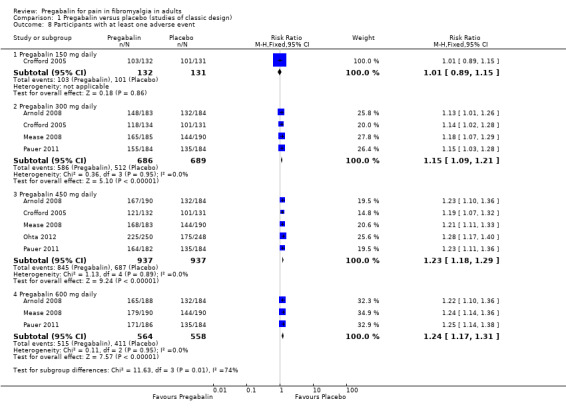
Comparison 1 Pregabalin versus placebo (studies of classic design), Outcome 8 Participants with at least one adverse event.
One study provided data for 150 mg pregabalin versus placebo (Crofford 2005); 78% with active treatment experienced at least one adverse event, compared to 77% on placebo. The RR was 1.01 (95% CI 0.89 to 1.2).
Four studies provided data for 300 mg pregabalin versus placebo (Arnold 2008; Crofford 2005; Mease 2008; Pauer 2011); 85% with active treatment experienced at least one adverse event, compared to 74% on placebo. The RR was 1.2 (95% CI 1.1 to 1.2), and the NNH was 9.0 (6.5 to 15).
Five studies provided data for 450 mg pregabalin versus placebo (Arnold 2008; Crofford 2005; Mease 2008; Ohta 2012; Pauer 2011); 90% with active treatment experienced at least one adverse event, compared to 73% on placebo. The RR was 1.2 (95% CI 1.18 to 1.3), and the NNH was 5.9 (4.9 to 7.4).
Three studies provided data for 600 mg pregabalin versus placebo (Arnold 2008; Mease 2008; Pauer 2011); 91% with active treatment experienced at least one adverse event, compared to 73% on placebo. The RR was 1.2 (95% CI 1.17 to 1.3), and the NNH was 5.7 (4.6 to 7.5).
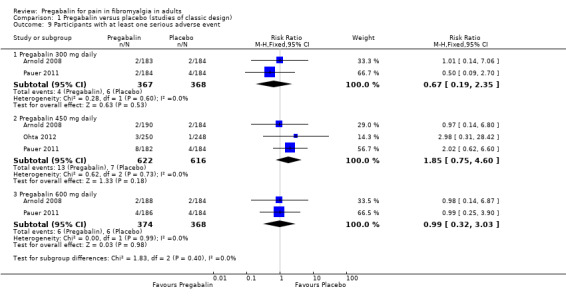
Serious adverse events
Three studies provided data on participants experiencing a serious adverse event (Arnold 2008; Ohta 2012; Pauer 2011). We assessed the evidence as very low quality because while studies were randomised and double blind, the incidence of serious adverse events was low, and so the number of events for analysis was small, meaning that random play of chance might have affected the result.
The incidence rate for pregabalin at various doses with corresponding placebo follows.
Pregabalin 300 mg: 1.1% (4/367); placebo: 1.6% (6/368).
Pregabalin 450 mg: 2.1% (13/622); placebo: 1.1% (7/616).
Pregabalin 600 mg: 1.6% (6/374); placebo: 1.6% (6/368).
There were no significant differences between either dose of pregabalin and corresponding placebo or between the three doses of pregabalin (Analysis 1.9).
1.9. Analysis.
Comparison 1 Pregabalin versus placebo (studies of classic design), Outcome 9 Participants with at least one serious adverse event.
Death
No deaths were reported in the six studies with a classic design comparing pregabalin with placebo, or comparing two different pregabalin dosing regimens.
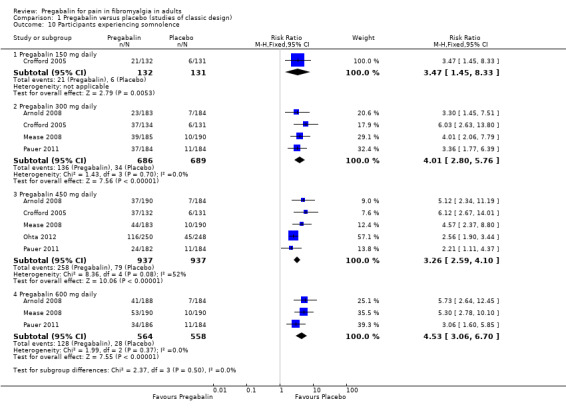
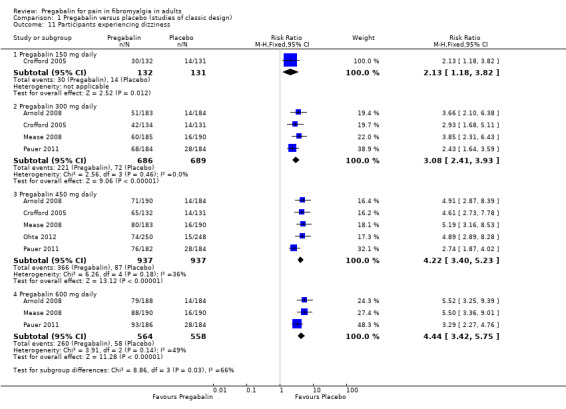
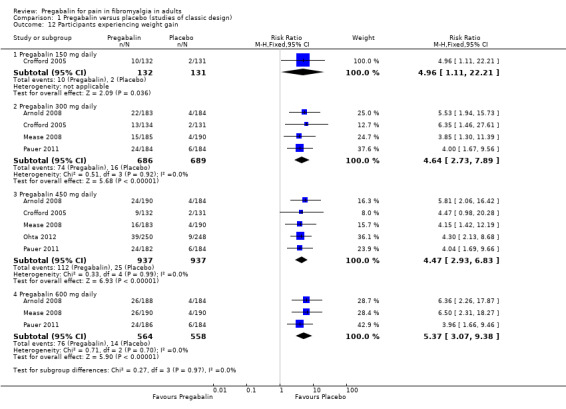
Specific adverse events
Five RCTs provided data for numbers of participants experiencing specific adverse events (Arnold 2008; Crofford 2005; Mease 2008; Ohta 2012; Pauer 2011). Rates for each event are presented below for individual doses and all doses combined.
The combined results for somnolence, dizziness, weight gain, and peripheral oedema showed a significant difference from placebo (Analysis 1.10; Analysis 1.11; Analysis 1.12; Analysis 1.13).
1.10. Analysis.
Comparison 1 Pregabalin versus placebo (studies of classic design), Outcome 10 Participants experiencing somnolence.
1.11. Analysis.
Comparison 1 Pregabalin versus placebo (studies of classic design), Outcome 11 Participants experiencing dizziness.
1.12. Analysis.
Comparison 1 Pregabalin versus placebo (studies of classic design), Outcome 12 Participants experiencing weight gain.
1.13. Analysis.
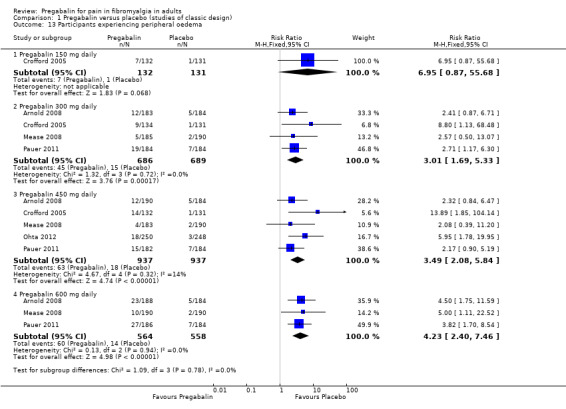
Comparison 1 Pregabalin versus placebo (studies of classic design), Outcome 13 Participants experiencing peripheral oedema.
The results for somnolence, dizziness, weight gain, and peripheral oedema for different doses showed that higher doses of pregabalin produced higher adverse event rates, with consequent lower (worse) NNH values (Analysis 1.10; Analysis 1.11; Analysis 1.12; Analysis 1.13).
| Summary of results C: Adverse events with different doses of pregabalin in fibromyalgia | ||||||
| Outcome ‐ daily dose | Number of | Per cent with outcome | Relative risk (95% CI) | NNH (95% CI) | ||
| Studies | Participants | Pregabalin | Placebo | |||
| Any adverse event | ||||||
| 150 mg* | 1 | 263 | 78 | 77 | 1.01 (0.89 to 1.2) | Not calculated |
| 300 mg | 4 | 1275 | 85 | 74 | 1.2 (1.1 to 1.2) | 9.0 (6.5 to 15) |
| 450 mg | 5 | 1874 | 90 | 73 | 1.2 (1.18 to 1.3) | 5.9 (4.9 to 7.4) |
| 600 mg | 3 | 1122 | 91 | 74 | 1.2 (1.17 to 1.3) | 5.7 (4.6 to 7.5) |
| Serious adverse event | ||||||
| 300 mg | 2 | 735 | 1 | 2 | 0.7 (0.2 to 2.4) | Not calculated |
| 450 mg | 3 | 1238 | 2 | 1 | 1.9 (0.8 to 4.6) | Not calculated |
| 600 mg | 2 | 742 | 2 | 2 | 1.0 (0.3 to 3.0) | Not calculated |
| Specific adverse events ‐ 150 mg | ||||||
| Somnolence* | 1 | 263 | 16 | 5 | 3.5 (1.5 to 8.3) | Not calculated |
| Dizziness* | 1 | 263 | 23 | 11 | 2.1 (1.2 to 3.8) | Not calculated |
| Weight gain* | 1 | 263 | 8 | 2 | 5.0 (1.1 to 22) | Not calculated |
| Peripheral oedema* | 1 | 263 | 5 | 1 | 7.0 (0.9 to 56) | Not calculated |
| Specific adverse events ‐ 300 mg | ||||||
| Somnolence | 4 | 1375 | 20 | 5 | 4.0 (2.8 to 5.8) | 6.7 (5.5 to 8.7) |
| Dizziness | 4 | 1375 | 32 | 10 | 3.1 (2.4 to 3.9) | 4.6 (3.9 to 5.7) |
| Weight gain | 4 | 1375 | 11 | 2 | 4.6 (2.7 to 7.9) | 12 (9.1 to 17) |
| Peripheral oedema | 4 | 1375 | 7 | 2 | 3.0 (1.7 to 5.3) | 23 (15 to 45) |
| Specific adverse events ‐ 450 mg | ||||||
| Somnolence | 5 | 1874 | 28 | 8 | 3.3 (2.6 to 4.1) | 5.2 (4.5 to 6.4) |
| Dizziness | 5 | 1874 | 39 | 9 | 4.2 (3.4 to 5.2) | 3.4 (3 to 3.8) |
| Weight gain | 5 | 1874 | 12 | 3 | 4.5 (2.9 to 6.8) | 11 (8.6 to 14) |
| Peripheral oedema | 5 | 1874 | 7 | 2 | 3.5 (2.1 to 5.8) | 21 (15 to 34) |
| Specific adverse events ‐ 600 mg | ||||||
| Somnolence | 3 | 1122 | 23 | 5 | 4.5 (3.1 to 6.7) | 5.7 (4.6 to 7.3) |
| Dizziness | 3 | 1122 | 46 | 10 | 4.4 (3.4 to 5.8) | 2.8 (2.5 to 3.2) |
| Weight gain | 3 | 1122 | 13 | 3 | 5.4 (3.1 to 9.4) | 9.1 (7.1 to 13) |
| Peripheral oedema | 3 | 1122 | 11 | 3 | 4.2 (2.4 to 7.5) | 12 (9.1 to 19) |
| Specific adverse events ‐ all doses combined | ||||||
| Somnolence | 5 | 3256 | 23 | 10 | 2.4 (1.9 to 3) | 7.4 (6.1 to 9.2) |
| Dizziness | 5 | 3256 | 38 | 11 | 3.5 (2.3 to 4.3) | 3.7 (3.3 to 4.2) |
| Weight gain | 5 | 3256 | 9 | 3 | 2.8 (1.8 to 4.1) | 18 (14 to 26) |
| Peripheral oedema | 5 | 3256 | 8 | 2 | 3.4 (2.1 to 5.5) | 19 (14 to 26) |
| *Information from a single study, included to examine a general dose‐response relationship and should be interpreted with caution. CI: confidence interval; NNH: number needed to harm | ||||||
2. Enriched enrolment randomised withdrawal study designs
Both EERW studies had a similar overall design. Both screened participants for eligibility, as would occur with a classical design. Participants were then given single (participant)‐blind pregabalin at increasing doses over three weeks, to determine the dose giving maximum benefit with tolerable adverse events, and then maintained on that dose for a further three weeks. Those who met criteria for an adequate response were at that point randomised to double‐blind treatment with continued pregabalin at the maintenance dose, or a phased dose reduction to placebo over a week, with treatment continuing for up to 13 weeks, in Arnold 2014, or 26 weeks, in Crofford 2008 (see Table 2).
Dosing was different between the studies. Arnold 2014 used pregabalin controlled release (CR) at doses of 330 to 495 mg per day. Crofford 2008 used fixed doses of 300, 450, and 600 mg per day.
In both studies a high‐level response was required to enter the double‐blind randomised phase. To continue in the study, both required at least 50% reduction in pain relative to baseline as measured using the daily pain diary. Crofford 2008 also required participants to have overall improvement on the PGIC scale of "much improved" or "very much improved", with the criteria met at weeks four, five, and six.
The primary outcome in both studies was the loss of therapeutic response, which was defined differently. Arnold 2014 used a definition of less than 30% pain reduction relative to the single‐blind baseline (based on daily pain diary), or patient discontinuation because of lack of efficacy or adverse events in the double‐blind phase. Crofford 2008 used less than 30% reduction in pain visual analogue scale score relative to open‐label baseline value at two consecutive visits in the double‐blind phase or worsening of fibromyalgia symptoms necessitating alternate treatment; this definition did not include withdrawals due to lack of efficacy or adverse events.
We have therefore chosen to extract an outcome similar to Arnold 2014 from both studies, but to make the outcome positive: the maintenance of therapeutic response (MTR). Participants fulfilling this outcome would have at least 30% pain intensity reduction over baseline AND continue treatment, with any adverse events being of at least tolerable intensity (withdrawal for any cause being a treatment failure). This allowed efficacy results from both EERW studies to be combined, and to be compared with the similar outcome of moderate benefit used in classical design studies.
EERW study participant flows
In order to understand the results of EERW studies it is necessary to understand the participant flows. We have therefore combined the patient flow data from Arnold 2014 and Crofford 2008 and simplified it to provide a background.
Number of participants screened and entered into the dose titration phase: 1492 (100% of original).
Number discontinued in dose titration phase: 509; number completing dose titration: 983 (66%).
Number with inadequate therapeutic response to proceed: 295; number entering randomised double‐blind phase: 688 (46%).
Number completing randomised double‐blind phase (pregabalin and placebo) with MTR: 208 (14%).
One difference between a randomised withdrawal study and a classic design is the complete enrichment of the double‐blind phase, which is limited only to responders (Straube 2008). In this case, only 46% of the participants who would have entered a classically designed study have been randomised.
Efficacy in EERW studies
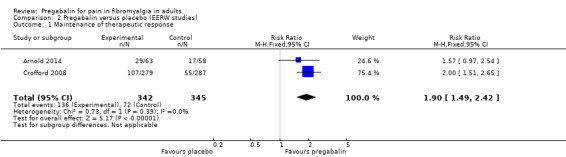
Maintenance of therapeutic effect
Because no imputation was used, we regarded this evidence as first‐tier evidence. We assessed the evidence as high quality because studies were randomised and double blind, had large numbers of participants, and were consistent.
Two studies with 687 participants reported results equivalent to 'moderate benefit': MTR of at least 30% pain intensity reduction over baseline and continued treatment at 13 or 26 weeks (Arnold 2014; Crofford 2008).
The proportion of participants with MTR with pregabalin was 40% (136/342).
The proportion of participants with MTR with placebo was 20% (72/345).
The RR for pregabalin compared with placebo was 1.9 (95% CI 1.5 to 2.4) (Analysis 2.1), and the NNT was 5.3 (3.9 to 8.2) for MTR.
2.1. Analysis.
Comparison 2 Pregabalin versus placebo (EERW studies), Outcome 1 Maintenance of therapeutic response.
Other pain outcomes were not relevant to the EERW trial design, or were not reported.
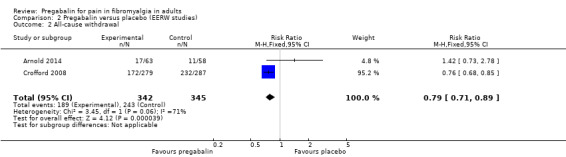
Withdrawals during the randomised double‐blind phase of EERW studies
Two studies with 687 participants reported the number of participants withdrawing for any reason during the double‐blind period over 13 or 26 weeks (Arnold 2014; Crofford 2008). We assessed the evidence as high quality because studies were randomised and double blind, had large numbers of participants, and were consistent.
The proportion of participants withdrawing with pregabalin was 55% (189/342).
The proportion of participants withdrawing with placebo was 70% (243/345).
The RR for pregabalin compared with placebo was 0.79 (95% CI 0.71 to 0.89) (Analysis 2.2), and the NNTp was 6.6 (4.5 to 12).
2.2. Analysis.
Comparison 2 Pregabalin versus placebo (EERW studies), Outcome 2 All‐cause withdrawal.
The I² value for this analysis was 71%, likely because one of the studies was small (121 participants in comparison) and highly susceptible to random chance. The point estimate for this study had very wide confidence intervals.
Adverse events in the randomised double‐blind phase of EERW studies
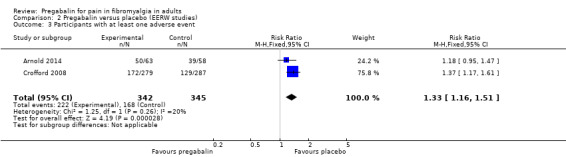
Participants with at least one adverse event
Two studies with 687 participants reported the number of participants experiencing adverse events during the double‐blind period over 13 or 26 weeks (Arnold 2014; Crofford 2008). We assessed the evidence as high quality because studies were randomised and double blind, had large numbers of participants, and were consistent.
The proportion of participants experiencing an adverse event with pregabalin was 65% (222/342).
The proportion of participants experiencing an adverse event with placebo was 49% (168/345).
The RR for pregabalin compared with placebo was 1.3 (95% CI 1.2 to 1.5) (Analysis 2.3), and the NNH was 6.2 (4.3 to 11).
2.3. Analysis.
Comparison 2 Pregabalin versus placebo (EERW studies), Outcome 3 Participants with at least one adverse event.
Serious adverse events
Serious adverse events were uncommon in the double‐blind phase. In the two studies combined, 8/342 (2.3%) experienced a serious adverse event with pregabalin, compared with 2/345 (0.6%) with placebo. We assessed the evidence as very low quality because of the small number of events.
Death
Crofford 2008 reported that two participants died during the trial, one while taking placebo and the other after pregabalin use had ended. Neither was considered associated with the study drug. We assessed the evidence as very low quality because of the small number of events.
Adverse events in the titration phase of EERW studies
At least one adverse event was experienced by 82% (1216/1492) participants during the initial titration with pregabalin, and 250 participants withdrew because of adverse events. Thirteen serious adverse events were reported, a rate of 0.9%.
Discussion
Summary of main results
Participants in these studies all had moderate or severe pain of fibromyalgia, often long‐lasting with an initial average pain score of about 7/10, and an average duration of symptoms of four years. The primary outcomes were 'substantial' pain relief, ideally a reduction in pain intensity by 50% or more, and 'moderate' pain relief, a reduction by 30% or more, and sustained over the duration of the trial, typically three months. These outcomes are judged as desirable by people with pain (Moore 2013a).
Classical trial designs
Five large studies involving 3283 participants compared pregabalin with placebo.
The ideal would have been for this pain reduction outcome to be combined with that of tolerability, where withdrawal for any reason would be regarded as treatment failure; most withdrawals were due to adverse events or lack of efficacy. Results in all of the trials of classic design used a LOCF imputation, where pain measures at withdrawal were counted as if they had been scored at the end of the trial. This form of imputation has the potential to overestimate the treatment effect by a large amount (Moore 2012b). Overestimation is more likely when adverse event withdrawals are greater with active than placebo. As this was the case for pregabalin, we could not judge the evidence as first tier, and so classified efficacy results from studies of classic design as second‐tier evidence.
There was little evidence for pregabalin 150 mg, coming from a single study indicating that this dose was no better than placebo. For pregabalin doses of 300 to 600 mg daily, the NNT for substantial benefit outcomes were different: at least 50% pain intensity reduction was about 10, and for PGIC very much improved was about 20. There was more agreement for moderate‐benefit outcomes: at least 30% pain intensity reduction was about 9, and for PGIC much or very much improved was about 11. There was no pronounced dose response for efficacy, though the better results tended to come from 450 mg daily. The results showed that a very significant degree of pain benefit was achieved by about 10% more people with pregabalin 300 to 600 mg daily than with placebo. The importance of the benefit is discussed below.
Participants tended to report more adverse events with higher doses of pregabalin, giving lower (worse) NNH values with the higher doses. It was common for participants to report an adverse event in these long‐duration studies. Around three‐quarters of participants taking placebo reported an adverse event, though this was higher at up to 90% with pregabalin. Somnolence, dizziness, weight gain, and peripheral oedema were amongst the most common adverse events, with around a quarter of participants affected by dizziness, for example. Serious adverse events were no more common with pregabalin than placebo, and were in the range of 1% to 2%.
Withdrawals because of adverse events were more common with higher doses of pregabalin, by 6% to 17% more than with placebo over the range 300 to 600 mg daily. By contrast, lack of efficacy withdrawals were around 6% lower with pregabalin.
EERW trial designs
Two large studies involving 1492 participants compared pregabalin with placebo. Of these participants, 687 achieved adequate pain intensity reduction with an initial pregabalin titration and were able to tolerate any adverse events; they were then randomised to continuing the effective dose of pregabalin, or tapered withdrawal to placebo in a double‐blind manner.
In order to combine the two studies and make a comparison with studies of classic design, we used the outcome of maintenance of therapeutic response of at least 30% pain intensity reduction over baseline and continued treatment at 13 or 26 weeks without withdrawal. As there was no imputation, we regarded this evidence as first tier.
The outcome of maintenance of therapeutic response is equivalent to a 'moderate benefit' and was achieved by 20% more participants taking pregabalin than taking placebo, giving a NNT of about 5. This was 20% of the randomised population, which was only half of the initial population for the studies, so the NNT of 5 would be comparable to a NNT of 10 in a trial of classic design, close to what was found.
About 15% more participants experienced an adverse event in the double‐blind phase with pregabalin than with placebo. Serious adverse events were rare, and two deaths were not likely to have been connected with the drugs tested.
Importance of outcomes in fibromyalgia
Four of the five studies of classic design included in this review have previously been the subject of individual patient level analyses, and for pregabalin we are in the unique situation of having individual patient level analyses for both pain response and the link between pain and other important outcomes in fibromyalgia. These analyses have shown that with greater degrees of pain relief there is a graded response for other symptoms, such as sleep, depression, impact of fibromyalgia, quality of life, functional ability, ability to work, and interference with work (Moore 2010b; Straube 2011).
Achieving a pain outcome of at least 30% or at least 50% pain intensity reduction with ongoing therapy (no withdrawal) comes with major improvements in all these other outcomes, so that for many of these patients substantial pain benefit (at least 50% pain intensity reduction) was associated with normalisation of these other symptoms.
This finding is possibly unique for fibromyalgia, and comes from an analysis of these four studies that is without imputation, and provides efficacy results for responders with both pain relief and ability to keep taking the medication (Straube 2010a).
As yet unpublished analysis of the pregabalin trials indicates that if pain relief is not achieved by about six weeks, then it is highly unlikely good pain relief will be achieved with continued treatment.
Overall completeness and applicability of evidence
The included studies were of insufficient duration to determine long‐term use, but at least one of the EERW studies had a 26‐week (six‐month) duration, demonstrating largely unchanged responses over the longer term (Crofford 2008). In addition, extension studies have demonstrated tolerability and efficacy for up to one year (Arnold 2012; Ohta 2013).
Study participants were typical of people with fibromyalgia, being mainly women in their 50s, with a reliable diagnosis of fibromyalgia, with moderate or severe pain and functional disability. There is no reason to suspect that results of these studies would not be applicable to the majority of people with fibromyalgia, although more comorbidities are likely in the general population. We excluded one study because it had a duration of less than eight weeks (Arnold 2015). In this cross‐over study conducted in 197 people with fibromyalgia and comorbid depression taking concurrent antidepressant medicines, addition of pregabalin at 300 mg or 450 mg daily significantly improved fibromyalgia pain and other symptoms.
Data from many of the studies of classic design in this review have been the subject of independent individual patient level analyses that linked pain benefit to benefits in a wide range of other concomitant symptoms, including quality of life and work (Moore 2010b; Straube 2011). Good pain relief was associated with major improvements in all of these, in common with other findings in acute and chronic pain conditions (Conaghan 2015; Moore 2013a).
However, it is worth noting that the studies often excluded people with depression, which is commonly associated with fibromyalgia. For example, the longest‐duration study excluded people "with severe depression or other severe psychiatric or severe medical conditions that made entry into a clinical trial inappropriate in the judgment of the investigator" (Crofford 2008). Others excluded people with "clinically significant or unstable medical or psychological conditions" (Mease 2008). Trials were exclusively of pregabalin monotherapy where other therapies were discontinued, which may well be rather different from what occurs in everyday clinical practice. While it could be argued that exclusions were sensible in the context of a clinical trial, it also means that results may not be unthinkingly extrapolated to all people presenting with fibromyalgia.
Objections to the clinical trial evidence include the populations studied. The trials generally exclude people with mental illness, concomitant medical/rheumatic conditions, men, and people without the ability to participate in clinical trials. In clinical practice, long‐term use of medications is unusual, with only about 20% using medicines (including pregabalin) for longer than a year (Kim 2013). Benefits are not apparent in longitudinal studies, but that might reflect treatment continuing in the absence of benefit (Wolfe 2012).
Quality of the evidence
All of the studies in the review were described as randomised and double blind, had Oxford Quality Scores of 3/5 or above, and not at high risk of bias for any item. Studies lasted for eight weeks or longer, and reported clinically useful outcomes, making them valid. Diagnostic criteria for inclusion were reasonable, using appropriate definitions and duration of pain. Participants had to have had initial pain of at least moderate intensity, meaning that studies would be sensitive enough to measure any analgesic effect. Sample sizes were moderate or large, minimising the effects of random chance and small study bias.
The one major problem was the use of LOCF imputation for missing data in a situation where around 10% more participants withdrew because of adverse events with pregabalin compared with placebo. This is known to give rise to overestimation of treatment effects (Moore 2012b). The potential effects of imputation method for pregabalin in fibromyalgia can be seen in Summary of results D below, which compares meta‐analyses on essentially the same data sets carried out where LOCF data were available, or where non‐imputed responder analyses were possible where withdrawal was considered to be treatment failure.
| Summary of results D: Comparison of pregabalin efficacy in different meta‐analyses, using different imputation methods | ||||||
| Review | Dose | Participants in comparisons | NNT for ≥ 30% PIR | NNT for ≥ 50% PIR | NNT for PGIC much or very much improved | NNT for PGIC very much improved |
| Trials of classic design with LOCF imputation | ||||||
| This review | 300 mg | 1375 | 9.2 (6.3 to 17) | 14 (8.9 to 32) | 11 (7.3 to 25) | 16 (10 to 37) |
| 450 mg | 1874 | 7.2 (5.5 to 10) | 9.7 (7.2 to 15) | 11 (7.8 to 22) | 12 (9.0 to 20) | |
| 600 mg | 1122 | 9.4 (6.2 to 19) | 11 (7.1 to 21) | 7.8 (5.5 to 14) | 22 (13 to 89) | |
| 2009 review | 300 mg | 1374 | 9.2 (6.3 to 17) | 14 (9.0 to 33) | 11 (7.3 to 26) | 16 (9.9 to 37) |
| 450 mg | 1376 | 6.6 (5.0 to 9.8) | 9.8 (7.0 to 16) | 6.8 (5.1 to 10) | 11 (7.9 to 20) | |
| 600 mg | 1122 | 9.1 (6.1 to 18) | 11 (7.1 to 21) | 7.7 (5.4 to 13) | 21 (12 to 83) | |
|
Straube 2010b Review of clinical trial reports |
300 mg | 1374 | 9.2 (6.1 to 19) | 14 (9.0 to 33) | 12 (7.4 to 27) | Not available |
| 450 mg | 1376 | 7.1 (5.1 to 12) | 9.9 (7.0 to 17) | 6.9 (5.2 to 10) | Not available | |
| 600 mg | 1122 | 8.8 (5.9 to 17) | 11 (7.1 to 21) | 7.8 (5.5 to 14) | Not available | |
| Studies or analyses with no imputation | ||||||
| This review EERW study design |
300 to 600 mg | 687 | 11.5 (8.5 to 18)* | –– | –– | –– |
|
Straube 2010a Responder analysis |
300 mg | 1374 | Not calculated | 22 (11 to 870) | 14 (8.5 to 44) | 26 (15 to > 100) |
| 450 mg | 1376 | 11 (6.9 to 29) | 16 (9.3 to 59) | 8.9 (6.2 to 16) | 24 (14 to 80) | |
| 600 mg | 1122 | Not calculated | 13 (8.1 to 31) | 13 (7.7 to 41) | Not calculated | |
| *The NNT for the EERW design study is normalised to the starting population by multiplying NNT values obtained in the randomised double‐blind phase by 1/0.46 to correct for the fact that only 46% of the initial population were able to enter the randomised phase, and withdrew beforehand or had insufficient pain response. EERW: enriched enrolment randomised withdrawal; LOCF: last observation carried forward; NNT: number needed to treat; PGIC: Patient Global Impression of Change; PIR: pain intensity reduction | ||||||
At least three meta‐analyses, this review, a previous version of this review (Moore 2009), and a meta‐analysis based on company clinical trial reports, Straube 2010b, have used substantially similar sources of data from four or five of the studies of classic design that compare pregabalin with placebo in fibromyalgia. The results in Summary of results D above show that they have come to essentially the same magnitude of effect size, with virtually identical NNT values for particular outcomes.
By contrast, a responder analysis based on individual patient level data, where a responder was defined as both having the response and NOT withdrawing, produced numerically higher (worse) values for NNT for each of the outcomes. Moreover, if the results of the EERW meta‐analysis in this review are normalised to the starting population for the studies, then the calculated NNT for comparison with classical study designs is, at 11, the same as that for the same outcome in the responder analysis (Straube 2010b), but also numerically higher than equivalent NNTs in studies of classic design.
However, there is also substantial agreement between all of these different analyses. None claims exceptional efficacy. All demonstrate modest efficacy, with perhaps 10% more people having a moderate or substantial benefit with pregabalin who would not have done so with placebo. Moreover, this result occurred at three months after treatment began, and where we know that good pain response is associated with substantial improvements with a range of other important outcomes such as sleep, depression, quality of life, and work.
In view of the overall quality and size of studies, consistency of effect between imputation methods, and consistency between studies, we considered the overall GRADE rating for efficacy to be high quality, meaning that further research is very unlikely to change our confidence in the estimate of effect. For serious adverse events and deaths, where there were few events, results could be radically changed by additional data, so we gave these lower GRADE ratings.
Potential biases in the review process
We know of no potential biases in the review process. We had planned to calculate the number of participants who would need to be in trials with zero effect (risk ratio of 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level (Moore 2008), but this method is not applicable with low effect sizes.
Agreements and disagreements with other studies or reviews
The results in this review are in substantial agreement with the previous version (Moore 2009), and two other reviews that also examined four of the five classical studies (Straube 2010a; Straube 2010b), though none of these reviews were able to perform a pooled analysis of the EERW studies. A review using three published studies with LOCF imputation reported very similar NNT values to the LOCF analyses in other reviews (Tzellos 2010). Another review used the same four studies, but pooled doses, and also concluded that pregabalin was better than placebo for reducing pain (Häuser 2010). The evidence base for pregabalin in fibromyalgia was regarded as strong in a guideline for fibromyalgia treatment developed by a group of German associations (Häuser 2008). Pregabalin is also recommended by the European League Against Rheumatism (Macfarlane 2016).
It is also worth noting that the magnitude of response to pregabalin in fibromyalgia for long‐term outcomes of moderate or substantial improvement is similar to that seen for both milnacipran (NNTs 6 to 10; Cording 2015) and duloxetine, whether a LOCF imputation was used (NNT 8; Lunn 2014), or true responder definition (NNT 9; Moore 2014c).
Authors' conclusions
Implications for practice.
For people with fibromyalgia
Pregabalin offers good pain relief to only a minority of people with fibromyalgia; it will not work for most people. This is also the case for other treatments for fibromyalgia, such as duloxetine and milnacipran. These treatments provide moderate or substantial pain benefit to about 10% more people than does placebo. The good news is that pain relief comes together with improved sleep, relief of any depression, less impact on life, and improved quality of life and ability to work.
People who take pregabalin are likely to experience adverse events, which may be troublesome. Pregabalin is not licensed to treat fibromyalgia in many countries, but has been licensed by the US Food and Drug Administration and a number of other countries worldwide.
For clinicians
Pregabalin offers good pain relief to only a minority of people with fibromyalgia; it will not work for most people. This is also the case for other treatments for fibromyalgia, such as duloxetine and milnacipran, which are the only other drug treatments with good evidence of efficacy in fibromyalgia. Pregabalin is not licensed to treat fibromyalgia in many countries.
Since relatively few participants achieve a worthwhile response with pregabalin, it is important to establish switching rules, so that when someone does not respond within a specified time, they can be switched to an alternative treatment. Emerging evidence indicates that the lack of any worthwhile pain relief within four to six weeks means that no pain relief is likely in the longer term. Stopping or switching rules will reduce the number of participants exposed to adverse events in the absence of benefit.
The available evidence shows that there is no difference between nighttime dosing and divided, twice‐daily dosing.
Because adverse events are common, dosing in clinical practice often follows the following general principles to generate maximum likelihood of benefit and minimal likelihood of early withdrawal.
Start with low doses. For those patients who work, changes might be made over the weekend, with five to seven days on the new dose before any further increase.
Giving the drug at bedtime may increase the benefit and decrease side effects.
Adverse effects are common, so carefully balance these to analgesia.
Do not expect any major pain relief until 150 mg is reached.
While some patients may achieve good relief with lower doses, it is likely that 450 mg daily will provide the most effective balance between benefit and adverse events for most patients.
For policymakers
Since no single treatment is effective in a majority of individuals with fibromyalgia, this relatively small number who benefit may be considered worthwhile, particularly if switching rules are in place.
For funders
Since no single treatment is effective in a majority of individuals with fibromyalgia, this relatively small number who benefit may be considered worthwhile, particularly if switching rules are in place. The magnitude of benefit in those people who do respond is worthwhile, and it extends to major improvement in quality of life, function, and ability to work. This probably makes successful treatment of fibromyalgia cost‐effective, as people with moderate or severe chronic pain consume much greater health service and non‐health service resources than those with well‐treated pain.
Implications for research.
General
Because the trials of classic design in this review used the last‐observation‐carried‐forward (LOCF) imputation method for study withdrawals, post‐hoc individual participant level analyses using baseline observation carried forward (BOCF) would be regarded as appropriate to strengthen the findings, especially if the pain reduction was linked to improved quality of life and function. For pregabalin we are in the unique situation of having individual patient level analyses for both pain response and the link between pain and other important outcomes in fibromyalgia.
However, there is a gap between the dosing regimens used in the clinical trials, where fairly rapid dose elevations are made over a few weeks, and clinical practice, where dosing increases can be quite slow. Practical research about the most effective use of medicines known to be effective in only a small proportion of patients could be very important. Indeed, situations could be envisaged where the degree of recruitment to successful treatment might have a major impact on treating a very difficult, debilitating, and costly condition.
Design
The design of trials was adequate, but reporting of clinically relevant outcomes using appropriate imputation for withdrawal would improve the relevance of the findings for clinical practice. The use of EERW designs for comparison with classic trial designs indicates that good‐quality EERW designs of long duration may be appropriate for fibromyalgia.
No immediate design for testing initial dosing regimens presents itself.
Measurement (endpoints)
Assessment of fibromyalgia symptoms should be based on dichotomous participant‐reported outcomes of proven clinical utility. The endpoint used in this review, of maintenance of therapeutic response without withdrawal, might be more clearly stated in trial reports, and used as a primary outcome in future trials, including pragmatic trials of dosing regimens.
Comparison between active treatments
Studies involving other treatments, including non‐pharmacological interventions, may be valuable in this context. A multicomponent approach reflects current practice.
What's new
| Date | Event | Description |
|---|---|---|
| 30 September 2016 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 7, 2015 Review first published: Issue 9, 2016
| Date | Event | Description |
|---|---|---|
| 12 January 2017 | Amended | Minor typo corrected: 'x pathology' changed to 'pathology' in Description of the condition. |
Notes
A new search within two years is not likely to identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
The protocol was developed in collaboration with the Cochrane Musculoskeletal Group, the Cochrane Neuromuscular Diseases Group, and the Cochrane Pain, Palliative and Supportive Care Group, and followed an agreed template for fibromyalgia. The editorial process was managed by the Cochrane Pain, Palliative and Supportive Care Group.
Institutional support is provided by the Oxford Pain Relief Trust.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group.
Disclaimer: The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. Methodological considerations for chronic pain
There have been several recent changes in how the efficacy of conventional and unconventional treatments is assessed in chronic painful conditions. The outcomes are now better defined, particularly with new criteria for what constitutes moderate or substantial benefit (Dworkin 2008); older trials may only report participants with 'any improvement'. Newer trials tend to be larger, avoiding problems from the random play of chance. Newer trials also tend to be of longer duration, up to 12 weeks, and provide a more rigorous and valid assessment of efficacy in chronic conditions. New standards have evolved for assessing efficacy in neuropathic pain, and we are now applying stricter criteria for the inclusion of trials and assessment of outcomes, and are more aware of problems that may affect our overall assessment. A summary of some of the recent insights that must be considered in this new review follows.
Pain results tend to have a U‐shaped distribution rather than a bell‐shaped distribution. This is true in acute pain (Moore 2011a; Moore 2011b), back pain (Moore 2010d), and arthritis (Moore 2010c), as well as in fibromyalgia (Straube 2010a); in all cases average results usually describe the experience of almost no one in the trial. Data expressed as averages are potentially misleading, unless they can be proven to be suitable.
As a consequence, we have to depend on dichotomous results (the individual either has or does not have the outcome) usually from pain changes or patient global assessments. The Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) group has helped with their definitions of minimal, moderate, and substantial improvement (Dworkin 2008). In arthritis, trials of less than 12 weeks' duration, and especially those shorter than eight weeks, overestimate the effect of treatment (Moore 2010c); the effect is particularly strong for less effective analgesics, and this may also be relevant in neuropathic‐type pain.
The proportion of patients with at least moderate benefit can be small, even with an effective medicine, falling from 60% with an effective medicine in arthritis to 30% in fibromyalgia (Moore 2009; Moore 2010c; Moore 2013b; Moore 2014c; Straube 2008; Sultan 2008). A Cochrane review of pregabalin in neuropathic pain and fibromyalgia demonstrated different response rates for different types of chronic pain (higher in diabetic neuropathy and postherpetic neuralgia and lower in central pain and fibromyalgia) (Moore 2009). This indicates that different neuropathic pain conditions should be treated separately from one another, and that pooling should not be done unless there are good grounds for doing so.
Individual patient analyses indicate that patients who get good pain relief (moderate or better) have major benefits in many other outcomes, affecting quality of life in a significant way (Moore 2010b; Moore 2014a).
Imputation methods such as last observation carried forward (LOCF), used when participants withdraw from clinical trials, can overstate drug efficacy, especially when adverse event withdrawals with drug are greater than those with placebo (Moore 2012b).
Appendix 2. Search strategy for CENTRAL (via CRSO)
MESH DESCRIPTOR Pain EXPLODE ALL TREES (32202)
MESH DESCRIPTOR Somatosensory Disorders EXPLODE ALL TREES (778)
MESH DESCRIPTOR Fibromyalgia (614)
MESH DESCRIPTOR Myofascial Pain Syndromes EXPLODE ALL TREES (373)
MESH DESCRIPTOR Polymyalgia Rheumatica EXPLODE ALL TREES (44)
((pain* or discomfort*) and (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)):TI,AB,KY (21303)
(fibromyalgi* or fibrosti* or FM or FMS):TI,AB,KY (2139)
((neur* or nerv*) and (compress* or damag*)):TI,AB,KY (2286)
1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 (49957)
MESH DESCRIPTOR pregabalin EXPLODE ALL TREES (258)
lyrica:TI,AB,KY (13)
10 or 11 (267)
9 and 12 (182)
Appendix 3. Search strategy for MEDLINE (via Ovid)
exp PAIN/ (325527)
exp SOMATOSENSORY DISORDERS/ (17815)
FIBROMYALGIA/ (6858)
exp MYOFASCIAL PAIN SYNDROMES/ (6007)
POLYMYALGIA RHEUMATICA/ (2246)
((pain* or discomfort*) adj10 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)).mp. (68934)
(fibromyalgi* or fibrosti* or FM or FMS).mp. (21973)
1 or 2 or 3 or 4 or 5 or 6 or 7 (382871)
pregabalin.mp. (1266)
Lyrica.mp. (65)
9 or 10 (1273)
randomized controlled trial.pt. (409198)
controlled clinical trial.pt. (90242)
randomized.ab. (306265)
placebo.ab. (156066)
drug therapy.fs. (1829870)
randomly.ab. (216534)
trial.ab. (315943)
groups.ab. (1370026)
12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 (3471873)
8 and 11 and 20 (688)
Appendix 4. Search strategy for EMBASE (via Ovid)
exp PAIN/ (964426)
exp SOMATOSENSORY DISORDERS/ (73535)
FIBROMYALGIA/ (15344)
exp MYOFASCIAL PAIN SYNDROMES/ (6916)
POLYMYALGIA RHEUMATICA/ (3408)
((pain* or discomfort*) adj10 (central or complex or rheumat* or muscl* or muscul* or myofasci* or nerv* or neuralg* or neuropath*)).mp. (133427)
(fibromyalgi* or fibrosti* or FM or FMS).mp. (35108)
1 or 2 or 3 or 4 or 5 or 6 or 7 (1039981)
exp Pregabalin/ (9173)
Lyrica.mp. (883)
9 or 10 (9179)
crossover‐procedure/ (46381)
double‐blind procedure/ (129344)
randomized controlled trial/ (397653)
(random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or (doubl* adj blind*) or assign* or allocat*).tw. (1426945)
12 or 13 or 14 or 15 (1511078)
8 and 11 and 16 (1241)
Appendix 5. GRADE: criteria for assigning grade of evidence
The GRADE system uses the following criteria for assigning a quality level to a body of evidence (Chapter 12, Higgins 2011).
High: randomised trials; or double‐upgraded observational studies.
Moderate: downgraded randomised trials; or upgraded observational studies.
Low: double‐downgraded randomised trials; or observational studies.
Very low: triple‐downgraded randomised trials; or downgraded observational studies; or case series/case reports.
Factors that may decrease the quality level of a body of evidence are:
limitations in the design and implementation of available studies suggesting high likelihood of bias;
indirectness of evidence (indirect population, intervention, control, outcomes);
unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
imprecision of results (wide confidence intervals);
high probability of publication bias.
Factors that may increase the quality level of a body of evidence are:
large magnitude of effect;
all plausible confounding would reduce a demonstrated effect or suggest a spurious effect when results show no effect;
dose‐response gradient.
Appendix 6. Outcome results from individual studies
| Study | Efficacy | Withdrawals | At least one AE or a serious AE | Specific AE |
| Classic study design | ||||
| Arnold 2008 NCT00230776 | PGIC much improved: Pla = 27/184, P300 = 33/183, P450 = 55/190, P600 = 59/188 PGIC very much improved: Pla = 12/184, P300 = 18/183, P450 = 25/190, P600 = 19/188 PGIC much worse: Pla = 11/184, P300 = 13/183, P450 = 9/190, P600 = 14/188 PGIC very much worse: Pla = 11/184, P300 = 3/183, P450 = 2/190, P600 = 3/188 ≥ 30% pain improvement: Pla = 56/184, P300 = 76/183, P450 = 94/190, P600 = 88/188 ≥ 50% pain improvement: Pla = 28/184, P300 = 44/183, P450 = 52/190, P600 = 57/188 | Pla: AC = 59/184 AE = 20/184 LOE = 20/184 P300: AC = 60/183 AE = 31/183 LOE = 6/183 P450: AC = 65/190 AE = 43/190 LOE = 6/190 P600: AC = 75/188 AE = 50/188 LOE = 8/188 | ≥ 1 AE:
Pla = 132/184
P300 = 148/183 P450 = 167/190 P600 = 165/188 SAE: Pla = 2/184 P300 = 2/183 P450 = 2/190 P600 = 2/188 |
Not clear if participant numbers or event numbers (assumed participant numbers) Top 6 AE by frequency: Dizziness Pla = 14/184, P300 = 51/183, P450 = 71/190, P600 = 79/188 Somnolence Pla = 7/184, P300 = 23/183, P450 = 37/190, P600 = 41/188 Weight gain Pla = 4/184, P300 = 22/183, P450 = 24/190, P600 = 26/188 Headache Pla = 19/184, P300 = 14/183, P450 = 24/190, P600 = 14/188 Peripheral oedema Pla = 5/184, P300 = 12/183, P450 = 12/190, P600 = 23/188 Fatigue Pla = 8/184, P300 = 151/183, P450 = 11/190, P600 = 17/188 |
| Crofford 2005 | PGIC much or very much improved: Pla = 34/131, P150 = 42/132, P300 = 60/134, P450 = 69/132 PGIC very much improved: Pla = 32/131, P150 = 42/132, P300 = 58/134, P450 = 65/132 ≥ 30% pain improvement: Pla = 36/131, P150 = 41/132, P300 = 51/134, P450 = 64/132 ≥ 50% pain improvement: Pla = 17/131, P150 = 17/132, P300 = 25/134, P450 = 38/132 | Pla: AC = 34/131 AE = 10/131 LOE = 18/131 P150: AC = 29/132 AE = 11/132 LOE = 12/132 P300: AC = 23/134 AE = 10/134 LOE = 6/134 P450: AC = 33/132 AE = 17/132 LOE = 8/132 | ≥ 1 AE: Pla = 101/131 P150 = 103/132 P300 = 118/134 P450 = 121/132 | Top 6 AE by frequency: Dizziness Pla = 14/131, P150 = 30/132, P300 = 42/134, P450 = 65/132 Somnolence Pla = 6/131, P150 = 21/132, P300 = 37/134, P450 = 37/132 Weight gain Pla = 2/131, P150 = 10/132, P300 = 13/134, P450 = 9/132 Headache Pla = 25/131, P150 = 16/132, P300 = 20/134, P450 = 17/132 Dry mouth Pla = 2/131, P150 = 9/132, P300 = 8/134, P450 = 17/132 Peripheral oedema Pla = 1/131, P150 = 7/132, P300 = 9/134, P450 = 14/132 Infection Pla = 22/131, P150 = 11/132, P300 = 13/134, P450 = 13/132 |
| Mease 2008 NCT00645398 | PGIC much improved: Pla = 41/190, P300 = 48/185, P450 = 45/183, P600 = 54/190 PGIC very much improved: Pla = 21/190, P300 = 28/185, P450 = 26/183, P600 = 27/190 PGIC much worse: Pla = 19/190, P300 = 11/185, P450 = 12/183, P600 = 8/190 PGIC very much worse: Pla = 6/190, P300 = 4/185, P450 = 7/183, P600 = 1/190 | Pla: AC = 60/190 AE = 19/190 LOE = 22/190 P300: AC = 62/185 AE = 35/185 LOE = 9/185 P450: AC = 62/183 AE = 41/183 LOE = 6/183 P600: AC = 79/190 AE = 62/190 LOE = 3/190 | ≥ 1 AE:
Pla = 144/190
P300 = 165/185 P450 = 168/183 P600 = 179/190 |
Top 6 AE by frequency: Dizziness Pla = 16/190, P300 = 60/185, P450 = 80/183, P600 = 88/190 Somnolence Pla = 10/190, P300 = 39/185, P450 = 44/183, P600 = 53/190 Weight gain Pla = 4/190, P300 = 15/185, P450 = 16/183, P600 = 26/190 Headache Pla = 12/190, P300 = 15/185, P450 = 17/183, P600 = 15/190 Dry mouth Pla = 4/190, P300 = 14/185, P450 = 19/183, P600 = 20/190 Peripheral oedema Pla = 2/190, P300 = 5/185, P450 = 4/183, P600 = 10/190 Nausea Pla = 11/190, P300 = 9/185, P450 = 8/183, P600 = 20/190 Amblyopia (blurred vision) Pla = 3/190, P300 = 12/185, P450 = 12/183, P600 = 17/190 |
| Nasser 2014 NCT01226667 | ≥ 30% pain improvement: ON = 41/89 TD = 35/88 ≥ 50% pain improvement: ON = 27/89 TD = 25/88 Moderately better + better + great deal better: ON = 48/89 TD = 45/88 Better + great deal better: ON = 26/89 TD = 24/88 | ON: AC = 29/89 AE = 21/89 LOE = 1/89 TD: AC = 24/88 AE = 20/88 LOE = 1/88 | ≥ 1 AE: ON = 62/89 TD = 74/88 | No significant difference in AE Dizziness TD = 18/88, ON = 18/89 Somnolence TD = 8/88, ON = 4/89 Headache TD = 6/88, ON = 7/89 Blurred vision TD = 7/88, ON = 8/89 Fatigue TD = 10/88, ON = 14/89 Oedema TD = 11/88, ON = 7/89 Cognitive TD = 14/88, ON = 13/89 Nausea TD = 3/88, ON = 7/89 Euphoria TD = 5/88, ON = 1/89 Vertigo TD = 8/88, ON = 3/89 |
| Ohta 2012 NCT00830167 | PGIC much improved: Pla = 50/248, P300/450 = 65/250 PGIC very much improved: Pla = 16/248, P300/450 = 31/250 PGIC much worse: Pla = 13/248, P300/450 = 13/250 PGIC very much worse: P = 6/287, P300/450 = 4/250 ≥ 30% pain improvement: P = 76/248, P300/450 = 101/250 ≥ 50% pain improvement: P = 30/248, P300/450 = 57/250 | Pla: AC = 40/248 AE = 6/248 LOE = 26/250 P300/450: AC = 43/250 AE = 19/248 LOE = 10/248 | ≥ 1 AE: Pla = 175/248, P300/450 = 225/250 SAE: Pla = 1/248, P300/450 = 3/250 | Somnolence Pla = 45/248, P300/450 = 116/250 Dizziness Pla = 15/248, P300/450 = 74/250 Nasopharyngitis Pla = 45/248, P300/450 = 45/250 Increased weight Pla = 9/248, P300/450 = 39/250 Constipation Pla = 17/248, P300/450 = 36/250 Feeling abnormal Pla = 3/248, P300/450 = 20/250 Peripheral oedema Pla = 3/248, P300/450 = 18/250 Headache Pla = 15/248, P300/450 = 15/250 Blurred vision Pla = 3/248, P300/450 = 13/250 |
| Pauer 2011 NCT00333866 | PGIC much improved: Pla = 43/184, P300 = 45/184, P450 = 50/182, P600 = 46/186 PGIC very much improved: Pla = 7/184, P300 = 13/184, P450 = 16/182, P600 = 20/186 PGIC much worse: Pla = 17/184, P300 = 14/184, P450 = 8/182, P600 = 10/186 PGIC very much worse: Pla = 3/184, P300 = 5/184, P450 = 2/182, P600 = 3/186 ≥ 30% pain improvement: Pla = 35/184, P300 = 61/184, P450 = 62/182, P600 = 48/186 ≥ 50% pain improvement: Pla = 17/184, P300 = 33/184, P450 = 33/182, P600 = 28/186 | Pla: AC = 43/184 AE = 23/184 LOE = 8/184 P300: AC = 61/184 AE = 37/184 LOE = 6/184 P450: AC = 65/182 AE = 38/182 LOE = 3/182 P600: AC = 43/186 AE = 47/186 LOE = 5/186 | ≥ 1 AE: Pla = 135/184, P300 = 155/184, P450 = 164/182, P600 = 171/186 SAE: Pla = 4/184, P300 = 2/184, P450 = 8/182, P600 = 4/186 | Top 6 AE by frequency: Dizziness Pla = 28/184, P300 = 68/184, P450 = 76/182, P600 = 93/186 Somnolence Pla = 11/184, P300 = 37/184, P450 = 24/182, P600 = 34/186 Weight increase Pla = 6/184, P300 = 24/184, P450 = 24/182, P600 = 24/186 Peripheral oedema Pla = 7/184, P300 = 19/184, P450 = 15/182, P600 = 27/186 Dry mouth Pla = 4/184, P300 = 16/184, P450 = 20/182, P600 = 20/186 Fatigue Pla = 15/184, P300 = 14/184, P450 = 26/182, P600 = 17/186 |
| Enriched enrolment randomised withdrawal (EERW) study design | ||||
| Arnold 2014 NCT01271933 | Pla: AC = 11/58 AE = 0/58 P330‐495 CR: AC = 17/63 AE = 3/63 | –– | ≥ 1 AE: Pla = 39/58 P330‐495 CR = 50/63 SAE: Pla = 0/58 P330‐495 CR = 2/63 | Dizziness Pla = 13/58, P330‐495 CR = 12/63 Somnolence Pla = 6/58, P330‐495 CR = 6/63 Weight gain Pla = 6/58, P330‐495 CR = 4/63 Headache Pla = 3/58, P330‐495 CR = 2/63 Fatigue Pla = 5/58, P330‐495 CR = 1/63 Nausea Pla = 5/58, P330‐495 CR = 1/63 Peripheral oedema Pla = 11/58, P330‐495 CR = 5/63 Vision blurred Pla = 4/58, P330‐495 CR = 0/63 Dry mouth Pla = 3/58, P330‐495 CR = 6/63 Insomnia Pla = 7/58, P330‐495 CR = 1/63 Disturbance in attention Pla = 1/58, P330‐495 CR = 1/63 Nasopharyngitis Pla = 1/58, P330‐495 CR = 3/63 |
| Crofford 2008 | Pla: AC = 232/287 AE = 20/287 P300: AC = 30/63 AE = 12/63 P450: AC = 24/73 AE = 13/73 P600: AC = 53/143 AE = 22/143 | –– | ≥ 1 AE: Pla = 129/287 P300 = 37/63 P450 = 46/73 P600 = 89/143 SAE: Pla = 3/287 P300 = 1/63 P450 = 0/73 P600 = 7/143 Deaths: 2 | Participant no.: Top 6 AE by frequency: Insomnia Pla = 18/287, P300 = 5/63, P450 = 2/73, P600 = 9/143 Nausea Pla = 13/287, P300 = 4/63, P450 = 5/73, P600 = 5/143 Anxiety Pla = 5/287, P300 = 3/63, P450 = 3/73, P600 = 8/143 Arthralgia Pla = 5/287, P300 = 3/63, P450 = 5/73, P600 = 6/143 Sinusitis Pla = 8/287, P300 = 1/63, P450 = 4/73, P600 = 9/143 Influenza Pla = 3/287, P300 = 3/63, P450 = 3/73, P600 = 7/143 Weight gain Pla = 1/287, P300 = 1/63, P450 = 3/73, P600 = 6/143 Peripheral oedema: Pla = 2/287, P300 = 2/63, P450 = 3/73, P600 = 3/143 No information on dizziness and somnolence because patients with these AE at the end of open label treatment were censored. |
| AC: all cause; AE: adverse event; CR: controlled release; LOE: lack of efficacy; ON: once nightly; P[number]: pregabalin[dose in mg]; PGIC: Patient Global Impression of Change; Pla: placebo; SAE: serious adverse event; TD: twice daily | ||||
Data and analyses
Comparison 1. Pregabalin versus placebo (studies of classic design).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 At least 30% pain relief | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Pregabalin 150 mg daily | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.78, 1.65] |
| 1.2 Pregabalin 300 mg daily | 4 | 1375 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [1.20, 1.62] |
| 1.3 Pregabalin 450 mg daily | 5 | 1874 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.31, 1.68] |
| 1.4 Pregabalin 600 mg daily | 3 | 1122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.17, 1.63] |
| 2 At least 50% pain relief | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Pregabalin 150 mg daily | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.53, 1.86] |
| 2.2 Pregabalin 300 mg daily | 4 | 1375 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.51 [1.20, 1.90] |
| 2.3 Pregabalin 450 mg daily | 5 | 1874 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.44, 2.13] |
| 2.4 Pregabalin 600 mg daily | 3 | 1122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.28, 2.10] |
| 3 Much or very much improved | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Pregabalin 150 mg daily | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.84, 1.80] |
| 3.2 Pregabalin 300 mg daily | 4 | 1375 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.14, 1.56] |
| 3.3 Pregabalin 450 mg daily | 5 | 1869 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.16, 1.52] |
| 3.4 Pregabalin 600 mg daily | 3 | 1122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [1.25, 1.75] |
| 4 Very much improved | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Pregabalin 300 mg daily | 4 | 1375 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.59 [1.22, 2.08] |
| 4.2 Pregabalin 450 mg daily | 5 | 1869 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.47, 2.35] |
| 4.3 Pregabalin 600 mg daily | 3 | 1122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.13, 2.38] |
| 5 All‐cause withdrawal | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Pregabalin 150 mg daily | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.55, 1.30] |
| 5.2 Pregabalin 300 mg daily | 4 | 1375 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.90, 1.25] |
| 5.3 Pregabalin 450 mg daily | 5 | 1874 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.24] |
| 5.4 Pregabalin 600 mg daily | 3 | 1122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [1.13, 1.58] |
| 6 Adverse event withdrawal | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Pregabalin 150 mg daily | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.48, 2.48] |
| 6.2 Pregabalin 300 mg daily | 4 | 1375 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.20, 2.08] |
| 6.3 Pregabalin 450 mg daily | 5 | 1874 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [1.59, 2.62] |
| 6.4 Pregabalin 600 mg daily | 3 | 1122 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [1.94, 3.32] |
| 7 Lack of efficacy withdrawal | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Pregabalin 150 mg daily | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.33, 1.32] |
| 7.2 Pregabalin 300 mg daily | 4 | 1375 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.28, 0.65] |
| 7.3 Pregabalin 450 mg daily | 5 | 1874 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.24, 0.52] |
| 7.4 Pregabalin 600 mg daily | 3 | 1122 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.15, 0.50] |
| 8 Participants with at least one adverse event | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Pregabalin 150 mg daily | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.89, 1.15] |
| 8.2 Pregabalin 300 mg daily | 4 | 1375 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.09, 1.21] |
| 8.3 Pregabalin 450 mg daily | 5 | 1874 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.18, 1.29] |
| 8.4 Pregabalin 600 mg daily | 3 | 1122 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [1.17, 1.31] |
| 9 Participants with at least one serious adverse event | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Pregabalin 300 mg daily | 2 | 735 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.19, 2.35] |
| 9.2 Pregabalin 450 mg daily | 3 | 1238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.75, 4.60] |
| 9.3 Pregabalin 600 mg daily | 2 | 742 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.32, 3.03] |
| 10 Participants experiencing somnolence | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Pregabalin 150 mg daily | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.47 [1.45, 8.33] |
| 10.2 Pregabalin 300 mg daily | 4 | 1375 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.01 [2.80, 5.76] |
| 10.3 Pregabalin 450 mg daily | 5 | 1874 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [2.59, 4.10] |
| 10.4 Pregabalin 600 mg daily | 3 | 1122 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.53 [3.06, 6.70] |
| 11 Participants experiencing dizziness | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Pregabalin 150 mg daily | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [1.18, 3.82] |
| 11.2 Pregabalin 300 mg daily | 4 | 1375 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [2.41, 3.93] |
| 11.3 Pregabalin 450 mg daily | 5 | 1874 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.22 [3.40, 5.23] |
| 11.4 Pregabalin 600 mg daily | 3 | 1122 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.44 [3.42, 5.75] |
| 12 Participants experiencing weight gain | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Pregabalin 150 mg daily | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.96 [1.11, 22.21] |
| 12.2 Pregabalin 300 mg daily | 4 | 1375 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.64 [2.73, 7.89] |
| 12.3 Pregabalin 450 mg daily | 5 | 1874 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.47 [2.93, 6.83] |
| 12.4 Pregabalin 600 mg daily | 3 | 1122 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.37 [3.07, 9.38] |
| 13 Participants experiencing peripheral oedema | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Pregabalin 150 mg daily | 1 | 263 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.95 [0.87, 55.68] |
| 13.2 Pregabalin 300 mg daily | 4 | 1375 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [1.69, 5.33] |
| 13.3 Pregabalin 450 mg daily | 5 | 1874 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.49 [2.08, 5.84] |
| 13.4 Pregabalin 600 mg daily | 3 | 1122 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.23 [2.40, 7.46] |
Comparison 2. Pregabalin versus placebo (EERW studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maintenance of therapeutic response | 2 | 687 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [1.49, 2.42] |
| 2 All‐cause withdrawal | 2 | 687 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.71, 0.89] |
| 3 Participants with at least one adverse event | 2 | 687 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.16, 1.51] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arnold 2008.
| Methods | 14‐week randomised, placebo‐controlled, double‐blind, parallel‐group trial | |
| Participants | Fibromyalgia according to ACR classification and pain of at least 40/100 mm and pain score ≥ 4 on 11‐point numerical rating scale in week before randomisation N = 750 (745 analysed) Mean age 50 years, 95% female, 91% white Baseline pain: 6.7/10 |
|
| Interventions | 1‐week single‐blinded placebo run‐in phase, 2‐week double‐blinded dose escalation phase, 12‐week fixed dose Pregabalin 300 mg daily, n = 183 Pregabalin 450 mg daily, n = 190 Pregabalin 600 mg daily, n = 188 Placebo daily, n = 184 |
|
| Outcomes | Proportion of participants with ≥ 30% or ≥ 50% decrease in mean pain score between endpoint and baseline PGIC (7‐point scale from very much improved to very much worse) Adverse events Withdrawals |
|
| Notes | Pfizer Global Research and Development sponsored. LOCF used to account for missing data and withdrawals Oxford Quality Score: R2, DB2, W1. Total = 5/5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random assignment to treatment regimens used a 1:1:1:1 ratio according to a computer generated pseudo‐random code using the method of random permuted blocks" |
| Allocation concealment (selection bias) | Low risk | "Random assignment was managed by a tele‐randomisation system" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To maintain the blinding, all doses of pregabalin and placebo were packaged using identical encapsulation" "were identical in appearance and taste from which they took 1 capsule twice a day" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "To maintain the blinding, all doses of pregabalin and placebo were packaged using identical encapsulation"; "were identical in appearance and taste from which they took 1 capsule twice a day" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF imputation |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Size | Unclear risk | 50 to 199 participants per treatment arm (183 to 190) |
Arnold 2014.
| Methods | 13‐week multicentre, controlled‐release, double‐blind, placebo‐controlled, EERW trial | |
| Participants | Fibromyalgia according to ACR classification and pain diary score ≥ 4 on 11‐point numerical rating scale N = 441 entered titration phase, 121 randomised to double‐blind phase Mean age 50 years, 91% female, 90% white Baseline mean pain score: 6.8/10 |
|
| Interventions | 4 phases: 1) baseline (1 week), 2) single‐blind (participants were blinded) treatment (6 weeks), 3) double‐blind treatment (13 weeks), and 4) a double‐blind taper period (1 week) Pregabalin controlled release 330 to 495 mg daily, n = 63 Placebo, n = 58 |
|
| Outcomes | PI on an 11‐point numerical rating scale, using daily pain diary Adverse events Withdrawals |
|
| Notes | Pfizer sponsored Oxford Quality Score: R2, DB2, W1. Total = 5/5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described, "randomly assigned" |
| Allocation concealment (selection bias) | Low risk | "telephone using the Interactive Voice Recognition System (IVRS)" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated to be double blind. Used a matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated to be double blind. Used a matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Size | Unclear risk | 50 to 199 participants per treatment arm (58, 63) |
Crofford 2005.
| Methods | 8‐week multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group trial | |
| Participants | Fibromyalgia according to ACR classification. Pain of at least 40/100 mm and pain diary score ≥ 4 on 11‐point numerical rating scale in week before randomisation N = 529 Mean age 49 years, 92% female, 93% white Baseline mean pain score: 7/10 |
|
| Interventions | 8‐week fixed dose (except for pregabalin 450 mg/day who received 300 mg/day for the first 3 days, and then 450 mg/day) Pregabalin 150 mg daily, n = 132 Pregabalin 300 mg daily, n = 134 Pregabalin 450 mg daily, n = 132 Placebo daily, n = 131 |
|
| Outcomes | Proportion of participants with ≥ 30% or ≥ 50% decrease in mean pain score between endpoint and baseline PGIC (7‐point scale from very much improved to very much worse) Adverse events Withdrawals |
|
| Notes | Pfizer Global Research and Development sponsored. LOCF used to account for missing data and participant withdrawn Oxford Quality Score: R2, DB1, W1. Total = 4/5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was by computer‐generated code using a block size of 8" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double blind, no details of method used |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double blind, no details of method used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF imputation |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Size | Unclear risk | 50 to 199 participants per treatment arm (131 to 134) |
Crofford 2008.
| Methods | 26‐week randomised, double‐blind, placebo‐controlled, parallel‐group, EERW trial. Participants initially screened for response (≥ 50% decrease in pain and PGIC of much or very much improved). Responders to initial titration selected for randomisation to placebo or continued use of tolerated effective dose | |
| Participants | Fibromyalgia according to ACR classification and pain of at least 40/100 mm in week before randomisation, with 6 months of follow‐up N = 1051 entered open‐label phase (6 weeks); 566 randomised to double‐blind phase (26 weeks) Mean age 49 years, 93.5% female, 90% white Baseline mean pain: 78/100 |
|
| Interventions | Pregabalin titrated to a maximum of 600 mg daily, n = 279 (300 mg = 63; 450 mg = 73; 600 mg = 143) Placebo daily, n = 287 |
|
| Outcomes | Loss of therapeutic response (worsening of pain or other symptoms, pain reduction less than 30% of baseline on several occasions, withdrawal) measured in days, converted to maintenance of therapeutic response for analysis Adverse events Withdrawals |
|
| Notes | Pfizer Global Research and Development sponsored Oxford Quality Score: R2, DB2, W1. Total = 5/5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A tele‐randomisation system randomised responders to either matching placebo or optimal open label dosage of pregabalin (1:1)" Review authors judged this low risk, assuming computer generation |
| Allocation concealment (selection bias) | Low risk | Tele‐randomisation system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "matching placebo or optimal open‐label dosage of pregabalin" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "matching placebo or optimal open‐label dosage of pregabalin" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Size | Low risk | > 200 participants per treatment arm |
Mease 2008.
| Methods | 12‐week multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group trial | |
| Participants | Fibromyalgia according to ACR classification and pain of at least 40/100 mm in week before randomisation N = 748 Mean age 49 years, 94% female, 90% white Baseline mean pain: 7.1/10 |
|
| Interventions | 1‐week dose escalation (all participants started at 150 mg), 12 weeks with fixed dose Pregabalin 300 mg daily, n = 185 Pregabalin 450 mg daily, n = 183 Pregabalin 600 mg daily, n = 190 Placebo daily, n = 190 |
|
| Outcomes | Proportion of participants with ≥ 50% decrease in mean pain score PGIC (7‐point scale from very much improved to very much worse) between endpoint and baseline Adverse events Withdrawals |
|
| Notes | Pfizer sponsored. LOCF used to account for missing data and participant withdrawn Oxford Quality Score: R2, DB2, W1. Total = 5/5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised. Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Stated to be double blind, method not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Stated to be double blind, method not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF imputation |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Size | Unclear risk | 50 to 199 participants per treatment arm (183 to 190) |
Nasser 2014.
| Methods | 8‐week multicentre, randomised, double‐blind comparison of 2 dosing schedules | |
| Participants | Females with fibromyalgia according to ACR classification and pain diary score ≥ 4 on 11‐point numerical rating scale N = 177 Mean age 50 years, 96% white Baseline mean pain: 7.1/10 |
|
| Interventions | 300 mg dose taken once nightly (placebo in the morning) or as a divided dose, twice daily Once nightly (week 1: 75 mg; week 2: 150 mg; week 3: 225 mg; weeks 4 to 8: 300 mg; week 9: taper dose, n = 89) Twice daily (week 1: 75 mg x 2; week 2 to 8: 150 mg x 2; week 9: taper dose, n = 88) | |
| Outcomes | Proportion of participants with ≥ 30% or ≥ 50% decrease in mean pain score between endpoint and baseline PGIC (7‐point scale from very much improved to very much worse) Adverse events Withdrawals |
|
| Notes | Pfizer sponsored Oxford Quality Score: R2, DB2, W1. Total = 4/5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomised using a random number generator to assign patients to either group" |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated to be double blind, placebo replaced morning dose for nightly dosing (judged as low risk) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated to be double blind, placebo replaced morning dose for nightly dosing (judged as low risk) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF imputation |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Size | Unclear risk | 50 to 199 participants per treatment arm (88 and 89) |
Ohta 2012.
| Methods | 12‐week multicentre, randomised, double‐blind, placebo‐controlled trial | |
| Participants | Japanese participants with fibromyalgia according to ACR classification. Pain of ≥ 40/100 mm and pain diary score ≥ 4 on 11‐point numerical rating scale before randomisation N = 498 Mean age 48 years, 89% female Baseline mean pain: 6.5/10 |
|
| Interventions | 4 phases: 1‐week single‐blind run‐in period, 3‐week dose escalation, 12‐week fixed dose at 300 or 450 mg, 1‐week taper phase Pregabalin all doses, n = 250 Placebo, n = 248 |
|
| Outcomes | Proportion of participants with ≥ 30% or ≥ 50% decrease in mean pain score PGIC (7‐point scale from very much improved to very much worse) between endpoint and baseline Adverse events Withdrawals |
|
| Notes | Pfizer sponsored Oxford Quality Score: R2, DB2, W1. Total = 5/5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation control system (IMPALA), which provided subject randomisation numbers" |
| Allocation concealment (selection bias) | Low risk | "Randomisation control system (IMPALA), which provided subject randomisation numbers". Review authors judged this as low risk, assuming remote allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Pregabalin and placebo capsules were prescribed by the investigator using blinded drug numbers issued by IMPALA" "identical placebo" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Pregabalin and placebo capsules were prescribed by the investigator using blinded drug numbers issued by IMPALA" "identical placebo" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF imputation |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Size | Low risk | > 200 participants per treatment arm (248 and 250) |
Pauer 2011.
| Methods | 14‐week multicentre, randomised, double‐blind, placebo‐controlled trial | |
| Participants | Fibromyalgia according to ACR classification. Pain ≥ 40/100 mm and pain diary score ≥ 4 on 11‐point numerical rating scale in week before randomisation N = 747 Mean age 49 years, 91% female, 76% white Baseline mean pain score: 6.7/10 |
|
| Interventions | 1‐week placebo run‐in phase, 2‐week randomised dose escalation phase, 12‐week fixed‐dose phase Placebo, n = 184 Pregabalin 300 mg, n = 184 Pregabalin 450 mg, n = 182 Pregabalin 600 mg, n = 186 |
|
| Outcomes | Proportion of participants with ≥ 30% or ≥ 50% decrease in mean pain score between endpoint and baseline PGIC (7‐point scale from very much improved to very much worse) Adverse events Withdrawals |
|
| Notes | Pfizer Global Research and Development sponsored. LOCF used to account for missing data and participant withdrawn Oxford Quality Score: R1, DB1, W1. Total = 3/5 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised. Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Stated to be double blind. Method not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Stated to be double blind. Method not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF imputation |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
| Size | Unclear risk | 50 to 199 participants per treatment arm (182 to 186) |
ACR: American College of Rheumatology; DB: double‐blind; EERW: enriched enrolment randomised withdrawal; LOCF: last observation carried forward; N: number of participants in study; n: number of participants in treatment arm; PGIC: Patient Global Impression of Change; PI: pain intensity; R: randomised; W: withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arnold 2012 | Pooled analysis |
| Arnold 2015 | Cross‐over: 3‐week escalation, 3‐week fixed dose, 2‐week taper/placebo washout period |
| Byon 2010 | Summary |
| Emir 2010 | Summary of 4 RCTs |
| NCT00760474 | 4‐week study only |
| NCT01268631 | Cross‐over 2 x 2 weeks |
| NCT01904097 | Single blind |
| Ohta 2013 | Non‐randomised, open‐label extension trial |
| Ramzy 2016 | Participants already taking pregabalin randomised to 1 of 3 antidepressants |
| Roth 2012 | 4‐week study on sleep maintenance in people with fibromyalgia |
| Russell 2009 | Summary of 2 RCTs |
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT01387607.
| Trial name or title | A 14‐week, randomized, double‐blind placebo‐controlled study for pregabalin in subjects with fibromyalgia |
| Methods | 14‐week randomised, parallel, double‐blind, placebo‐controlled study |
| Participants | Estimated: 324 Fibromylagia (ACR), pain intensity ≥ 40/100 Men and women over 18 years |
| Interventions | Pregabalin capsule, 300 to 450 mg/day, twice daily Placebo, twice daily |
| Outcomes | Relief of pain associated with fibromyalgia by comparing with the baseline, the difference for endpoint mean pain score (the mean of the last 7 pain diary entries) in the double‐blind treatment period between pregabalin and placebo groups Fibromyalgia symptoms Adverse events Withdrawals |
| Starting date | February 2012 |
| Contact information | Pfizer |
| Notes | Recruiting, estimated completion date: November 2016 Record verified February 2016 |
NCT02146430.
| Trial name or title | A randomized, double‐blind, placebo‐ and active‐controlled study of DS‐5565 for treatment of pain associated with fibromyalgia |
| Methods | 13‐week randomised, parallel, double‐blind, placebo‐ and active‐controlled study |
| Participants | N = 1294 Fibromylagia (ACR), pain intensity ≥ 40/100 Men and women over 18 years |
| Interventions | DS‐5565 (mirogabalin) 15 mg tablet, once daily DS‐5565 (mirogabalin) 15 mg tablet, twice daily Pregabalin 150 mg capsule, twice daily Placebo tablet matching DS‐5565 tablet Placebo capsule matching pregabalin capsule Participants take half daily dose in first week |
| Outcomes | ≥ 30% and ≥ 50% responders at 13 weeks PGIC Fibromyalgia symptoms Adverse events Withdrawals |
| Starting date | May 2014 |
| Contact information | Daiichi Sankyo Inc, Domenico Merante, MD |
| Notes | Estimated completion date: February 2017 Record verified March 2016 |
NCT02187159.
| Trial name or title | A randomized, double‐blind, placebo‐ and active‐controlled study of DS‐5565 for treatment of pain associated with fibromyalgia |
| Methods | 13‐week randomised, parallel, double‐blind, placebo‐ and active‐controlled study |
| Participants | Estimated 1270 Fibromylagia (ACR), pain intensity ≥ 40/100 Men and women over 18 years |
| Interventions | DS‐5565 15 mg tablet 150 mg pregabalin capsule Placebo tablet matching DS‐5565 tablet Placebo capsule matching pregabalin capsule 75 mg pregabalin capsule |
| Outcomes | Change in weekly average daily pain score, DS‐5565 versus placebo |
| Starting date | October 2014 |
| Contact information | Daiichi Sankyo Inc, Domenico Merante, MD |
| Notes | Estimated completion date: March 2017 |
ACR: American College of Rheumatology; PGIC: Patient Global Impression of Change
Differences between protocol and review
Minor differences exist between protocol and review, mainly relating to the improvements in knowledge that have occurred, for example studies had to have at least 20 participants per treatment arm to be included, and we used GRADE to assess the quality of the evidence and included 'Summary of findings' tables.
For the EERW trials, we used the outcome 'maintenance of therapeutic response' (MTR), which was not in the protocol, but is equivalent to 'moderate benefit'.
Contributions of authors
MC and PW carried out searches and selected studies for inclusion. RAM and SD carried out data extraction, assessed risk of bias, and carried out analyses. All authors were involved in writing the final review. All authors will be involved in any future updates.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
-
The National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant: 13/89/29 ‐ Addressing the unmet need of chronic pain: providing the evidence for treatments of pain
Declarations of interest
SD: none known.
MC: none known.
PW: none known.
SL: none known; SL is a specialist pain physician and manages patients with fibromyalgia.
TP: none known; TP is a specialist pain physician and manages patients with fibromyalgia.
RAM has received grant support from RB relating to individual patient level analyses of trial data on ibuprofen in acute pain and the effects of food on drug absorption of analgesics (2013), and from Grünenthal relating to individual patient level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015).
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Arnold 2008 {published data only}
- Arnold LM, Russell IJ, Diri EW, Duan WR, Young JP Jr, Sharma U, et al. A 14‐week, randomized, double‐blinded, placebo‐controlled monotherapy trial of pregabalin in patients with fibromyalgia. Journal of Pain 2008;9(9):792‐805. [CTG: NCT00230776; DOI: 10.1016/j.jpain.2008.03.013] [DOI] [PubMed] [Google Scholar]
Arnold 2014 {published data only}
- Arnold LM, Arsenault P, Huffman C, Patrick JL, Messig M, Chew ML, et al. Once daily controlled‐release pregabalin in the treatment of patients with fibromyalgia: a phase III, double‐blind, randomized withdrawal, placebo‐controlled study. Current Medical Research and Opinion 2014;30(10):2069‐83. [CTG: NCT01271933; DOI: 10.1185/03007995.2014.928275] [DOI] [PubMed] [Google Scholar]
Crofford 2005 {published data only}
- Crofford LJ, Rowbotham MC, Mease PJ, Russell IJ, Dworkin RH, Pregabalin 1008‐105 Study Group, et al. Pregabalin for the treatment of fibromyalgia syndrome: results of a randomized, double‐blind, placebo‐controlled trial. Arthritis and Rheumatism 2005;52(4):1264‐73. [DOI: 10.1002/art.20983] [DOI] [PubMed] [Google Scholar]
Crofford 2008 {published data only}
- Crofford LJ, Mease PJ, Simpson SL, Young JP Jr, Martin SA, Haig GM, et al. Fibromyalgia relapse evaluation and efficacy for durability of meaningful relief (FREEDOM): a 6‐month, double‐blind, placebo‐controlled trial with pregabalin. Pain 2008;136(3):419‐31. [DOI: 10.1016/j.pain.2008.02.027] [DOI] [PubMed] [Google Scholar]
- Pauer L, Atkinson G, Murphy TK, Petersel D, Zeiher B. Long‐term maintenance of response across multiple fibromyalgia symptom domains in a randomized withdrawal study of pregabalin. Clinical Journal of Pain 2012;28(7):609‐14. [CTG: NCT00151489; DOI: 10.1097/AJP.0b013e31823dd315] [DOI] [PubMed] [Google Scholar]
Mease 2008 {published data only}
- Mease PJ, Russell IJ, Arnold LM, Florian H, Young JP Jr, Martin SA, et al. A randomized, double‐blind, placebo‐controlled, phase III trial of pregabalin in the treatment of patients with fibromyalgia. Journal of Rheumatology 2008;35(3):502‐14. [CTG: NCT00645398] [PubMed] [Google Scholar]
Nasser 2014 {published data only}
- Nasser K, Kivitz AJ, Maricic MJ, Silver DS, Silverman SL. Twice daily versus once nightly dosing of pregabalin for fibromyalgia: a double‐blind randomized clinical trial of efficacy and safety. Arthritis Care and Research 2014;66(2):293‐300. [CTG: NCT01226667; DOI: 10.1002/acr.22111] [DOI] [PubMed] [Google Scholar]
Ohta 2012 {published data only}
- Ohta H, Oka H, Usui C, Ohkura M, Suzuki M, Nishioka K. A randomized, double‐blind, multicenter, placebo‐controlled phase III trial to evaluate the efficacy and safety of pregabalin in Japanese patients with fibromyalgia. Arthritis Research and Therapy 2012;14(5):R217. [CTG: NCT00830167; DOI: 10.1186/ar4056] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pauer 2011 {published data only}
- A 14‐week, randomized, double‐blind, placebo‐controlled trial of pregabalin twice daily in patients with fibromyalgia. PhRMA Web Synopsis 7 March 2008.
- Pauer L, Winkelmann A, Arsenault P, Jespersen A, Whelan L, Atkinson G, et al. An international, randomized, double‐blind, placebo‐controlled, phase III trial of pregabalin monotherapy in treatment of patients with fibromyalgia. Journal of Rheumatology 2011;38(12):2643‐52. [CTG: NCT00333866; DOI: 10.3899/jrheum.110569] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arnold 2012 {published data only}
- Arnold LM, Emir B, Murphy TK, Zeiher BG, Pauer L, Scott G, et al. Safety profile and tolerability of up to 1 year of pregabalin treatment in 3 open‐label extension studies in patients with fibromyalgia. Clinical Therapeutics 2012;34(5):1092‐102. [DOI: 10.1016/j.clinthera.2012.03.003] [DOI] [PubMed] [Google Scholar]
Arnold 2015 {published data only}
- Arnold LM, Sarzi‐Puttini P, Arsenault P, Khan T, Bhadra Brown P, Clair A, et al. Efficacy and safety of pregabalin in patients with fibromyalgia and comorbid depression taking concurrent antidepressant medication: a randomized, placebo‐controlled study. Journal of Rheumatology 2015;42(7):1237‐44. [DOI: 10.3899/jrheum.141196] [DOI] [PubMed] [Google Scholar]
- NCT01432236. A phase 3b multicenter, double‐blind, randomized, placebo‐controlled, 2‐way crossover study of pregabalin in the treatment of fibromyalgia with concurrent antidepressant therapy for comorbid depression. clinicaltrials.gov/ct2/show/NCT01432236 (accessed 16 March 2016).
Byon 2010 {published data only}
- Byon W, Ouellet D, Chew M, Ito K, Burger P, Pauer L, et al. Exposure‐response analyses of the effects of pregabalin in patients with fibromyalgia using daily pain scores and patient global impression of change. Journal of Clinical Pharmacology 2010;50(7):803‐15. [DOI: 10.1177/0091270009352187] [DOI] [PubMed] [Google Scholar]
Emir 2010 {published data only}
- Emir B, Murphy TK, Petersel DL, Whalen E. Treatment response to pregabalin in fibromyalgia pain: effect of patient baseline characteristics. Expert Opinion on Pharmacotherapy 2010;11(14):2275‐80. [DOI: 10.1517/14656566.2010.509717] [DOI] [PubMed] [Google Scholar]
NCT00760474 {unpublished data only}
- NCT00760474. A double‐blind, placebo‐controlled cross‐over study in fibromyalgia subjects to examine effects of pregabalin on brain response to mechanical pain as assessed by functional magnetic resonance imaging, proton magnetic resonance spectroscopy and subjective ratings. clinicaltrials.gov/ct2/show/NCT00760474 (accessed 16 March 2016).
NCT01268631 {unpublished data only}
- NCT01268631. Mechanism‐based choice of therapy: can treatments success in fibromyalgia patients be coupled to psychophysical pain modulation profile?. clinicaltrials.gov/ct2/show/NCT01268631 (accessed 16 March 2016).
NCT01904097 {unpublished data only}
- NCT01904097. Study of the brain with optic functional neuroimaging in patients with chronic pain using transcranial direct current stimulation. clinicaltrials.gov/ct2/show/NCT01904097 (accessed 16 March 2016).
Ohta 2013 {published data only}
- Ohta H, Oka H, Usui C, Ohkura M, Suzuki M, Nishioka K. An open‐label long‐term phase III extension trial to evaluate the safety and efficacy of pregabalin in Japanese patients with fibromyalgia. Modern Rheumatology/the Japan Rheumatism Association 2013;23(6):1108‐15. [DOI: 10.1007/s10165-012-0803-x] [DOI] [PubMed] [Google Scholar]
Ramzy 2016 {published data only}
- Ramzy EA. Comparative efficacy of newer antidepressants in combination with pregabalin for fibromyalgia syndrome: a controlled, randomized study. Pain Practice 2016;Feb 19. [DOI: 10.1111/papr.12409] [DOI] [PubMed] [Google Scholar]
Roth 2012 {published data only}
- Roth T, Lankford DA, Bhadra P, Whalen E, Resnick EM. Effect of pregabalin on sleep in patients with fibromyalgia and sleep maintenance disturbance: a randomized, placebo‐controlled, 2‐way crossover polysomnography study. Arthritis Care and Research 2012;64(4):597‐606. [DOI: 10.1002/acr.21595] [DOI] [PubMed] [Google Scholar]
Russell 2009 {published data only}
- Russell IJ, Crofford LJ, Leon T, Cappelleri JC, Bushmakin AG, Whalen E, et al. The effects of pregabalin on sleep disturbance symptoms among individuals with fibromyalgia syndrome. Sleep Medicine 2009;10(6):604‐10. [DOI: 10.1016/j.sleep.2009.01.009] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01387607 {unpublished data only}
- NCT01387607. A 14‐week, randomized, double‐blind placebo‐controlled study for pregabalin in patients with fibromyalgia. clinicaltrials.gov/ct2/show/NCT01387607 (accessed 16 March 2016). [A0081241]
NCT02146430 {unpublished data only}
- NCT02146430. A randomized, double‐blind, placebo‐ and active‐controlled study of DS‐5565 for treatment of pain associated with fibromyalgia. clinicaltrials.gov/ct2/show/NCT02146430 (accessed 16 March 2016). [Sponsor ID: DS5565‐A‐E309]
NCT02187159 {unpublished data only}
- NCT02187159. A randomized, double‐blind, placebo‐ and active‐controlled study of DS‐5565 for treatment of pain associated with fibromyalgia. clinicaltrials.gov/ct2/show/NCT02187159 (accessed 16 March 2016). [Sponsor ID: DS5565‐A‐E311]
Additional references
Arnold 2013
- Arnold LM, Fan J, Russell IJ, Yunus MB, Khan MA, Kushner I, et al. The fibromyalgia family study: a genome‐wide linkage scan study. Arthritis and Rheumatism 2013;65(4):1122‐8. [DOI: 10.1002/art.37842] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 2007
- Bennett RM, Jones J, Turk DC, Russell IJ, Matallana L. An internet survey of 2,596 people with fibromyalgia. BMC Musculoskeletal Disorders 2007;8:27. [DOI: 10.1186/1471-2474-8-27] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bockbrader 2010
- Bockbrader HN, Wesche D, Miller R, Chapel S, Janiczek N, Burger P. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clinical Pharmacokinetics 2010;49(10):661‐9. [DOI: 10.2165/11536200-000000000-00000] [DOI] [PubMed] [Google Scholar]
Bradley 2009
- Bradley LA. Pathophysiology of fibromyalgia. The American Journal of Medicine 2009;122:S22‐30. [DOI: 10.1016/j.amjmed.2009.09.008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choi 2010
- Choi CJ, Knutsen R, Oda K, Fraser GE, Knutsen SF. The association between incident self‐reported fibromyalgia and nonpsychiatric factors: 25‐years follow‐up of the Adventist Health Study. Journal of Pain 2010;11:994‐1003. [DOI: 10.1016/j.jpain.2010.01.267] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clauw 2014
- Clauw DJ. Fibromyalgia: a clinical review. JAMA 2014;311:1547‐55. [DOI: 10.1001/jama.2014.3266] [DOI] [PubMed] [Google Scholar]
Collins 1997
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres?. Pain 1997;72(1‐2):95‐7. [DOI: 10.1016/S0304-3959(97)00005-5] [DOI] [PubMed] [Google Scholar]
Conaghan 2015
- Conaghan PG, Peloso PM, Everett SV, Rajagopalan S, Black CM, Mavros P, et al. Inadequate pain relief and large functional loss among patients with knee osteoarthritis: evidence from a prospective multinational longitudinal study of osteoarthritis real‐world therapies. Rheumatology (Oxford) 2015;54(2):270‐7. [DOI: 10.1093/rheumatology/keu332] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cording 2015
- Cording M, Derry S, Phillips T, Moore RA, Wiffen PJ. Milnacipran for pain in fibromyalgia in adults. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD008244.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dechartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta‐epidemiological study. BMJ 2013;346:f2304. [DOI: 10.1136/bmj.f2304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dechartres 2014
- Dechartres A, Altman DG, Trinquart L, Boutron I, Ravaud P. Association between analytic strategy and estimates of treatment outcomes in meta‐analyses. JAMA 2014;312(6):623‐30. [DOI: 10.1001/jama.2014.8166] [DOI] [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI: 10.1016/j.jpain.2007.09.005] [DOI] [PubMed] [Google Scholar]
Eich 2012
- Eich W, Häuser W, Arnold B, Bernardy K, Brückle W, Eidmann U, et al. Fibromyalgia syndrome. General principles and coordination of clinical care and patient education. Schmerz 2012;26:268‐75. [DOI: 10.1007/s00482-012-1167-z] [DOI] [PubMed] [Google Scholar]
EMC 2016
- Pregabalin. Electronic Medicines Compendium. www.medicines.org.uk/emc/medicine/14651 (accessed 16 March 2016).
Fitzcharles 2013
- Fitzcharles MA, Ste‐Marie PA, Goldenberg DL, Pereira JX, Abbey S, Choinière M, et al. 2012 Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome: executive summary. Pain Research Management 2013;18:119‐26. [PUBMED: 23748251] [DOI] [PMC free article] [PubMed] [Google Scholar]
Forseth 1999
- Forseth KO, Husby G, Gran JT, Førre O. Prognostic factors for the development of fibromyalgia in women with self‐reported musculoskeletal pain. A prospective study. Journal of Rheumatology 1999;26:2458‐67. [PUBMED: 10555910] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence – inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [DOI: 10.1016/j.jclinepi.2011.03.017] [DOI] [PubMed] [Google Scholar]
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso‐Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151‐7. [DOI: 10.1016/j.jclinepi.2012.01.006] [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines: 12. Preparing summary of findings tables‐binary outcomes. Journal of Clinical Epidemiology 2013;66(2):158‐72. [DOI: 10.1016/j.jclinepi.2012.01.012] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 Available at www.cochrane‐handbook.org.
Hoffman 2010
- Hoffman DL, Sadosky A, Dukes EM, Alvir J. How do changes in pain severity levels correspond to changes in health status and function in patients with painful diabetic peripheral neuropathy?. Pain 2010;149(2):194‐201. [DOI: 10.1016/j.pain.2009.09.017] [DOI] [PubMed] [Google Scholar]
Häuser 2008
- Häuser W, Arnold B, Eich W, Felde E, Flügge C, Henningsen P, et al. 13. Management of fibromyalgia syndrome ‐ an interdisciplinary evidence‐based guideline. German Medical Science 2008;6:Doc 14. [PUBMED: 19675740] [PMC free article] [PubMed] [Google Scholar]
Häuser 2010
- Häuser W, Petzke F, Sommer C. Comparative efficacy and harms of duloxetine, milnacipran, and pregabalin in fibromyalgia syndrome. Journal of Pain 2010;11(6):505‐21. [DOI: 10.1016/j.jpain.2010.01.002] [DOI] [PubMed] [Google Scholar]
Häuser 2011
- Häuser W, Kosseva M, Üceyler N, Klose P, Sommer C. Emotional, physical, and sexual abuse in fibromyalgia syndrome: a systematic review with meta‐analysis. Arthritis Care & Research 2011;63:808‐20. [DOI: 10.1002/acr.20328] [DOI] [PubMed] [Google Scholar]
Häuser 2013a
- Häuser W, Galek A, Erbslöh‐Möller B, Köllner V, Kühn‐Becker H, Langhorst J, et al. Posttraumatic stress disorder in fibromyalgia syndrome: prevalence, temporal relationship between posttraumatic stress and fibromyalgia symptoms, and impact on clinical outcome. Pain 2013;154:1216‐23. [DOI: 10.1016/j.pain.2013.03.034] [DOI] [PubMed] [Google Scholar]
Häuser 2013b
- Häuser W, Urrútia G, Tort S, Uçeyler N, Walitt B. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD010292] [DOI] [PubMed] [Google Scholar]
Häuser 2014
- Häuser W, Henningsen P. Fibromyalgia syndrome ‐ a somaform disorder?. European Journal of Pain 2014;18:1052‐9. [DOI: 10.1002/j.1532-2149.2014.00453.x] [DOI] [PubMed] [Google Scholar]
Häuser 2015
- Häuser W, Ablin J, Fitzcharles MA, Littlejohn G, Luciano JV, Usui C, et al. Fibromyalgia. Nature Reviews Disease Primers 2015;1:Article number: 15022. [DOI: 10.1038/nrdp.2015.22] [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI: ] [DOI] [PubMed] [Google Scholar]
Kalso 2013
- Kalso E, Aldington DJ, Moore RA. Drugs for neuropathic pain. BMJ 2013;347:f7339. [DOI: 10.1136/bmj.f7339] [DOI] [PubMed] [Google Scholar]
Kim 2013
- Kim SC, Landon JE, Solomon DH. Clinical characteristics and medication uses among fibromyalgia patients newly prescribed amitriptyline, duloxetine, gabapentin, or pregabalin. Arthritis Care and Research 2013;65(11):1813‐9. [DOI: 10.1002/j.1532-2149.2012.00234.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koroschetz 2011
- Koroschetz J, Rehm SE, Gockel U, Brosz M, Freynhagen R, Tölle TR, et al. Fibromyalgia and neuropathic pain ‐ differences and similarities. A comparison of 3057 patients with diabetic painful neuropathy and fibromyalgia. BMC Neurology 2011;11:55. [DOI: 10.1186/1471-2377-11-55] [DOI] [PMC free article] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107(2):224‐33. [DOI: 10.7326/0003-4819-107-2-224] [DOI] [PubMed] [Google Scholar]
Lange 2010
- Lange M, Petermann F. Influence of depression on fibromyalgia: A systematic review. Schmerz 2010;24:326‐33. [DOI: 10.1007/s00482-010-0937-8] [DOI] [PubMed] [Google Scholar]
Lee 2012
- Lee YH, Choi SJ, Ji JD, Song GG. Candidate gene studies of fibromyalgia: a systematic review and meta‐analysis. Rheumatology International 2012;32:417‐26. [DOI: 10.1007/s00296-010-1678-9] [DOI] [PubMed] [Google Scholar]
Lunn 2014
- Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD007115.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macfarlane 2016
- Macfarlane GJ, Kronisch C, Dean LE, Atzeni F, Häuser W, Fluß E, et al. EULAR revised recommendations for the management of fibromyalgia. Annals of the Rheumatic Diseases 2016;Epub ahead of print. [DOI: 10.1136/annrheumdis-2016-209724] [DOI] [PubMed] [Google Scholar]
Mansfield 2016
- Mansfield KE, Sim J, Jordan JL, Jordan KP. A systematic review and meta‐analysis of the prevalence of chronic widespread pain in the general population. Pain 2016;157(1):55‐64. [DOI: 10.1097/j.pain.0000000000000314] [DOI] [PMC free article] [PubMed] [Google Scholar]
McQuay 1998
- McQuay H, Moore R. An Evidence‐based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [ISBN: 0‐19‐262718‐X] [Google Scholar]
McQuay 2008
- McQuay HJ, Derry S, Moore RA, Poulain P, Legout V. Enriched enrolment with randomised withdrawal (EERW): Time for a new look at clinical trial design in chronic pain. Pain 2008;135(3):217‐20. [DOI: 10.1016/j.pain.2008.01.014] [DOI] [PubMed] [Google Scholar]
Mills 2015
- Mills EJ, Ayers D, Chou R, Thorlund K. Are current standards of reporting quality for clinical trials sufficient in addressing important sources of bias?. Contemporary Clinical Trials 2015;45(Pt A):2‐7. [DOI: 10.1016/j.cct.2015.07.019] [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978–0–931092–69–5] [Google Scholar]
Moore 2010a
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: 10.1016/j.pain.2010.05.011] [DOI] [PubMed] [Google Scholar]
Moore 2010b
- Moore RA, Straube S, Paine J, Phillips CJ, Derry S, McQuay HJ. Fibromyalgia: moderate and substantial pain intensity reduction predicts improvement in other outcomes and substantial quality of life gain. Pain 2010;149(2):360‐4. [DOI: 10.1016/j.pain.2010.02.039] [DOI] [PubMed] [Google Scholar]
Moore 2010c
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta‐analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374‐9. [DOI: 10.1136/ard.2009.107805] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010d
- Moore RA, Smugar SS, Wang H, Peloso PM, Gammaitoni A. Numbers‐needed‐to‐treat analyses ‐ do timing, dropouts, and outcome matter? Pooled analysis of two randomized, placebo‐controlled chronic low back pain trials. Pain 2010;151(3):592‐7. [DOI: 10.1016/j.pain.2010.07.013] [DOI] [PubMed] [Google Scholar]
Moore 2011a
- Moore RA, Straube S, Paine J, Derry S, McQuay HJ. Minimum efficacy criteria for comparisons between treatments using individual patient meta‐analysis of acute pain trials: examples of etoricoxib, paracetamol, ibuprofen, and ibuprofen/paracetamol combinations after third molar extraction. Pain 2011;152(5):982‐9. [DOI: 10.1016/j.pain.2010.11.030] [DOI] [PubMed] [Google Scholar]
Moore 2011b
- Moore RA, Mhuircheartaigh RJ, Derry S, McQuay HJ. Mean analgesic consumption is inappropriate for testing analgesic efficacy in post‐operative pain: analysis and alternative suggestion. European Journal of Anaesthesiology 2011;28(6):427‐32. [DOI: 10.1097/EJA.0b013e328343c569] [DOI] [PubMed] [Google Scholar]
Moore 2012a
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD008242.pub2] [DOI] [PubMed] [Google Scholar]
Moore 2012b
- Moore RA, Straube S, Eccleston C, Derry S, Aldington D, Wiffen P, et al. Estimate at your peril: imputation methods for patient withdrawal can bias efficacy outcomes in chronic pain trials using responder analyses. Pain 2012;153(2):265‐8. [DOI: 10.1016/j.pain.2011.10.004] [DOI] [PubMed] [Google Scholar]
Moore 2013a
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400‐12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Moore 2013b
- Moore A, Derry S, Eccleston C, Kalso E. Expect analgesic failure; pursue analgesic success. BMJ 2013;346:f2690. [DOI: 10.1136/bmj.f2690] [DOI] [PubMed] [Google Scholar]
Moore 2014a
- Moore RA, Derry S, Taylor RS, Straube S, Phillips CJ. The costs and consequences of adequately managed chronic non‐cancer pain and chronic neuropathic pain. Pain Practice 2014;14(1):79‐94. [DOI: 10.1111/papr.12050] [DOI] [PubMed] [Google Scholar]
Moore 2014b
- Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD007938.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2014c
- Moore RA, Cai N, Skljarevski V, Tölle TR. Duloxetine use in chronic painful conditions ‐ individual patient data responder analysis. European Journal of Pain 2014;18(1):67‐75. [DOI: 10.1002/j.1532-2149.2013.00341.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2015
Mork 2010
- Mork PJ, Vasseljen O, Nilsen TI. Association between physical exercise, body mass index, and risk of fibromyalgia: longitudinal data from the Norwegian Nord‐Trøndelag Health Study. Arthritis Care & Research 2010;62:611‐7. [DOI: 10.1002/acr.20118] [DOI] [PubMed] [Google Scholar]
O'Brien 2010
- O'Brien EM, Staud RM, Hassinger AD, McCulloch RC, Craggs JG, Atchison JW, et al. Patient‐centered perspective on treatment outcomes in chronic pain. Pain Medicine 2010;11(1):6‐15. [DOI: 10.1111/j.1526-4637.2009.00685.x] [DOI] [PubMed] [Google Scholar]
Oaklander 2013
- Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small‐fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain 2013;154:2310‐6. [DOI: 10.1016/j.pain.2013.06.001] [DOI] [PMC free article] [PubMed] [Google Scholar]
PaPaS 2012
- PaPaS author and referee guidance. papas.cochrane.org/papas‐documents (accessed 16 March 2016).
Queiroz 2013
- Queiroz LP. Worldwide epidemiology of fibromyalgia. Current Pain and Headache Reports 2013;17(8):356. [DOI: 10.1007/s11916-013-0356-5] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sommer 2012
- Sommer C, Häuser W, Burgmer M, Engelhardt R, Gerhold K, Petzke F, et al. Etiology and pathophysiology of fibromyalgia syndrome. Schmerz 2012;26:259‐67. [DOI: 10.1007/s00482-012-1174-0] [DOI] [PubMed] [Google Scholar]
Straube 2008
- Straube S, Derry S, McQuay HJ, Moore RA. Enriched enrollment: definition and effects of enrichment and dose in trials of pregabalin and gabapentin in neuropathic pain. A systematic review. British Journal of Clinical Pharmacology 2008;66(2):266‐75. [DOI: 10.1111/j.1365-2125.2008.03200.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Straube 2010a
- Straube S, Derry S, Moore RA, Paine J, McQuay HJ. Pregabalin in fibromyalgia ‐ responder analysis from individual patient data. BMC Musculoskeletal Disorders 2010;11:150. [DOI: 10.1186/1471-2474-11-150] [DOI] [PMC free article] [PubMed] [Google Scholar]
Straube 2010b
- Straube S, Derry S, Moore RA, McQuay HJ. Pregabalin in fibromyalgia: meta‐analysis of efficacy and safety from company clinical trial reports. Rheumatology (Oxford) 2010;49(4):706‐15. [DOI: 10.1093/rheumatology/kep432] [DOI] [PubMed] [Google Scholar]
Straube 2011
- Straube S, Moore RA, Paine J, Derry S, Phillips CJ, Hallier E, et al. Interference with work in fibromyalgia: effect of treatment with pregabalin and relation to pain response. BMC Musculoskeletal Disorders 2011;12:125. [DOI: 10.1186/1471-2474-12-125] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sultan 2008
- Sultan A, Gaskell H, Derry S, Moore RA. Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurology 2008;8:29. [DOI: 10.1186/1471-2377-8-29] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2007
- Taylor CP, Angelotti T, Fauman E. Pharmacology and mechanism of action of pregabalin: the calcium channel alpha2‐delta (alpha2‐delta) subunit as a target for antiepileptic drug discovery. Epilepsy Research 2007;73(2):137‐50. [DOI: 10.1016/j.eplepsyres.2006.09.008] [DOI] [PubMed] [Google Scholar]
Tzellos 2010
- Tzellos TG, Toulis KA, Goulis DG, Papazisis G, Zampeli VA, Vakfari A, et al. Gabapentin and pregabalin in the treatment of fibromyalgia: a systematic review and a meta‐analysis. Journal of Clinical Pharmacology and Therapeutics 2010;35(6):239‐56. [DOI: 10.1111/j.1365-2710.2009.01144.x] [DOI] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. The Lancet 2012;380(9859):2163‐96. [DOI: 10.1016/S0140-6736(12)61729-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walitt 2015
- Walitt B, Nahin RL, Katz RS, Bergman MJ, Wolfe F. The prevalence and characteristics of fibromyalgia in the 2012 National Health Interview Survey. PLoS One 2015;10(9):e0138024. [DOI: 10.1371/journal.pone.0138024] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiffen 2013
- Wiffen PJ, Derry S, Moore RA, Aldington D, Cole P, Rice ASC, et al. Antiepileptic drugs for neuropathic pain and fibromyalgia ‐ an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD010567.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolfe 1990
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and Rheumatism 1990;33(2):160‐72. [DOI: 10.1002/art.1780330203] [DOI] [PubMed] [Google Scholar]
Wolfe 2010
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care and Research 2010;62(5):600‐10. [DOI: 10.1002/acr.20140] [DOI] [PubMed] [Google Scholar]
Wolfe 2011
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RS, et al. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. Journal of Rheumatology 2011;38:1113‐22. [DOI: 10.3899/jrheum.100594] [DOI] [PubMed] [Google Scholar]
Wolfe 2012
- Wolfe F, Walitt BT, Katz RS, Lee YC, Michaud KD, Häuser W. Longitudinal patterns of analgesic and central acting drug use and associated effectiveness in fibromyalgia. European Journal of Pain 2013;17(4):581‐6. [DOI: 10.1002/j.1532-2149.2012.00234.x] [DOI] [PubMed] [Google Scholar]
Wolfe 2013
- Wolfe F, Brähler E, Hinz A, Häuser W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general population. Arthritis Care and Research 2013;645(5):777‐85. [DOI: 10.1002/acr.21931] [DOI] [PubMed] [Google Scholar]
Wolfe 2014
- Wolfe F, Walitt BT, Häuser W. What is fibromyalgia, how is it diagnosed and what does it really mean?. Arthritis Care and Research 2014;66(7):969‐71. [DOI: 10.1002/acr.22207] [DOI] [PubMed] [Google Scholar]
Yunus 2008
- Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Seminars in Arthritis and Rheumatism 2008;37:339‐52. [DOI: 10.1016/j.semarthrit.2007.09.003] [DOI] [PubMed] [Google Scholar]
Üçeyler 2013a
- Üçeyler N, Zeller D, Kahn AK, Kewenig S, Kittel‐Schneider S, Schmid A, et al. Small fibre pathology in patients with fibromyalgia syndrome. Brain 2013;136:e247. [DOI: 10.1093/brain/awt053] [DOI] [PubMed] [Google Scholar]
Üçeyler 2013b
- Üçeyler N, Sommer C, Walitt B, Häuser W. Anticonvulsants for fibromyalgia. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD010782] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Moore 2009
- Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007076.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


