Abstract
Background
Hydatidiform mole (HM), also called a molar pregnancy, is characterised by an overgrowth of foetal chorionic tissue within the uterus. HMs may be partial (PM) or complete (CM) depending on their gross appearance, histopathology and karyotype. PMs usually have a triploid karyotype, derived from maternal and paternal origins, whereas CMs are diploid and have paternal origins only. Most women with HM can be cured by evacuation of retained products of conception (ERPC) and their fertility preserved. However, in some women the growth persists and develops into gestational trophoblastic neoplasia (GTN), a malignant form of the disease that requires treatment with chemotherapy. CMs have a higher rate of malignant transformation than PMs. It may be possible to reduce the risk of GTN in women with HM by administering prophylactic chemotherapy (P‐Chem). However, P‐Chem given before or after evacuation of HM to prevent malignant sequelae remains controversial, as the risks and benefits of this practice are unclear.
Objectives
To systematically review the evidence for the effectiveness and safety of P‐Chem to prevent GTN in women with a molar pregnancy.
Search methods
We performed electronic searches in the Cochrane Gynaecological Cancer Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 2, 2012), MEDLINE (1946 to February week 4, 2012) and EMBASE (1980 to week 9, 2012). The search strategy was developed using free text and medical subject headings (MESH). We handsearched reference lists of relevant literature to identify additional studies.
Selection criteria
We included randomised controlled trials (RCTs) of P‐Chem for HM.
Data collection and analysis
Two review authors independently assessed studies for inclusion in the review and extracted data using a specifically designed data collection form. Meta‐analyses were performed by pooling data from individual trials using RevMan 5.1 software.
Main results
We included three RCTs with a combined total of 613 participants. One study compared prophylactic dactinomycin to no prophylaxis (60 participants); the other two studies compared prophylactic methotrexate to no prophylaxis (420 and 133 participants). All participants were diagnosed with CMs. We considered the latter two studies to be of poor methodological quality.
P‐Chem reduced the risk of GTN occurring in women following a CM (3 studies, 550 participants; RR 0.37; 95% confidence interval (CI) 0.24 to 0.57; I2 = 0%; P < 0.00001), However, owing to the poor quality of two of the included studies, we performed sensitivity analyses excluding these two studies. This left only one small study of high‐risk women to contribute data for this primary outcome (59 participants; RR 0.28; 95% CI 0.10 to 0.73; P = 0.01), therefore we consider this evidence to be of a low quality.
The time to diagnosis was longer in the P‐Chem group than the control group (2 studies, 33 participants; mean difference (MD) 28.72; 95% CI 13.19 to 44.24; P = 0.0003) and the P‐Chem group required more courses to cure subsequent GTN (1 poor‐quality study, 14 participants; MD 1.10; 95% CI 0.52 to 1.68; P = 0.0002). We consider this evidence to be of a low to very low quality for similar reasons to those listed above.
There were insufficient data to perform meta‐analyses for toxicity, overall survival, drug resistance and reproductive outcomes.
Authors' conclusions
P‐Chem may reduce the risk of progression to GTN in women with CMs who are at a high risk of malignant transformation; however, current evidence in favour of P‐Chem is limited by the poor methodological quality and small size of the included studies. As P‐Chem may increase drug resistance, delay treatment of GTN and expose women unnecessarily to toxic side effects, this practice cannot currently be recommended.
Keywords: Female, Humans, Pregnancy, Antineoplastic Agents, Antineoplastic Agents/therapeutic use, Dactinomycin, Dactinomycin/therapeutic use, Gestational Trophoblastic Disease, Gestational Trophoblastic Disease/prevention & control, Hydatidiform Mole, Hydatidiform Mole/drug therapy, Methotrexate, Methotrexate/therapeutic use, Randomized Controlled Trials as Topic
Prophylactic (preventive) chemotherapy for hydatidiform mole (molar pregnancy) to prevent cancerous growth later
A molar pregnancy (hydatidiform mole) develops following an abnormal process of conception, whereby placental tissue overgrows inside the womb (uterus). Molar pregnancies are classified as complete (CM) or partial (PM) based on their appearance (gross and microscopic), and their chromosome pattern. Moles are usually suspected at the early pregnancy scan and women often present with bleeding, similar to a miscarriage. The molar tissue is removed by evacuation of retained products of conception (ERPC), also known as dilatation and curettage (D&C) and women generally make a full recovery. However, some women go on to develop a cancer in the womb (about 1 in every 5 women with a CM and 1 in 200 with a PM). Women are generally at a higher risk of getting this cancer, which is known as gestational trophoblastic neoplasia (GTN), if they are over 40 years old, have a large increase in the size of the womb, have large cysts in the ovaries or have high initial levels of β‐human chorionic gonadotrophin (hCG) (the pregnancy hormone) in their blood. Although treatment of the cancer with chemotherapy (anti‐cancer drugs) is almost always effective, it has been suggested that routinely giving women anti‐cancer drugs (P‐Chem) before or after the removal the molar tissue may reduce the risk of the cancerous tissue developing.
By doing this review, we tried to assess the benefits and risks of giving P‐Chem to women with molar pregnancies, before or after ERPC. We found three randomised studies involving a total of 613 women. Two studies tested methotrexate in all women with a CM and one study tested dactinomycin in women with a CM who were at a high risk of getting GTN. The two methotrexate studies are older studies that used relatively poor research methods, therefore their findings cannot be relied upon. Overall the review findings suggest that P‐Chem reduces the number of women developing cancer after molar pregnancy; however, this is probably only be true for women with high‐risk moles. In addition, P‐Chem might make the time to diagnosing the cancer longer and might increase the number of anti‐cancer treatments needed to cure the cancer if it develops. We were unable to assess the short‐ and long‐term side‐effects of P‐Chem in this review because there was not enough available data; however, we are concerned that the five‐ and eight‐day courses of P‐Chem used by researchers in these studies are too toxic to be given to women routinely.
Currently there is insufficient evidence to support giving anti‐cancer drugs to women with molar pregnancies. However, GTN is almost always cured with modern care and P‐Chem for molar pregnancy would only reduce the risk of needing full‐scale chemotherapy, but would not remove that risk. In addition, it would not change the need for careful monitoring and follow‐up of women with hydatidiform moles.
Summary of findings
Summary of findings for the main comparison.
| Prophylactic chemotherapy compared with no prophylactic chemotherapy for hydatidiform mole | ||||||
|
Patient or population: women with a molar pregnancy Settings: inpatient Intervention: methotrexate or dactinomycin Comparison: placebo or no prophylaxis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No prophylaxis | P‐Chem | |||||
|
Incidence of GTN (including low‐quality studies) |
Mixed‐risk population | RR 0.37 (0.24 to 0.57) | 550 women (3 studies) | ⊕⊕⊝⊝ low | The NNTB to prevent 1 woman developing GTN after evacuation of HM was 6 (95% CI 5 to 10). We downgraded this evidence because this meta‐analysis included 2 studies that we considered to be of poor methodological quality | |
| 254 per 1000 | 94 per 1000 (61 to 145) | |||||
| High‐risk population |
RR 0.29 (0.14 to 0.60) |
99 women (2 studies) |
⊕⊕⊝⊝ low | The NNTB for women with high‐risk HM was 3 (95% CI 2 to 5). We downgraded this evidence because the meta‐analysis included 2 small studies, 1 of which was of a poor methodological quality | ||
| 490 per 1000 | 142 per 1000 (69 to 294) | |||||
|
Incidence of GTN (excluding low‐quality studies) |
High‐risk population | RR 0.28 (0.10 to 0.73) | 59 women (1 study) |
⊕⊕⊝⊝ low | The NNTB to prevent 1 woman developing GTN after evacuation of high‐risk HM was 3 (95% CI 2 to 20). We downgraded this evidence because only 1 small study (Limpongsanurak 2001) contributed data, giving an imprecise result | |
| 500 per 1000 | 140 per 1000 (50 to 365) | |||||
|
Time to GTN diagnosis (days) |
The mean time to GTN diagnosis ranged across control groups from 35.7 days to 59.5 days | The mean time to GTN diagnosis in the intervention groups was 65.5 days to 81.8 days (higher) | MD 28.72 (13.19 to 44.24) | 33 women (2 studies) | ⊕⊕⊝⊝ low | We downgraded this evidence because the meta‐analysis included 1 study of poor methodological quality (Kim 1986). When this study was excluded, the results of the remaining study (Limpongsanurak 2001; 19 women) were: MD 22.30; 95% CI ‐9.05 to 53.65 |
| Number of courses of chemotherapy to cure | The mean number of courses of chemotherapy required to cure subsequent GTN was 1.4 courses (10 women) | The mean number of courses of chemotherapy required to cure subsequent GTN was 2.5 courses (4 women) |
MD 1.10 (0.52 to 1.68) |
14 women (1 study) | ⊕⊝⊝⊝ very low | This analysis only included 1 study (Kim 1986) that we considered to be of a poor methodological quality |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HM: hydatidiform mole; NNTB: number needed to treat for an additional beneficial outcome; RR: risk ratio; MD: mean difference; GTN: gestational trophoblastic neoplasia. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
The assumed risk for the mixed‐risk population was calculated by using the weighted mean risk across the control group for this outcome. The assumed risk for the high‐risk population was based on the control group of Limpongsanurak 2001, which was the only study to evaluate a high‐risk population only.
Background
Description of the condition
Gestational trophoblastic disease (GTD) is a spectrum of disease characterised by an autonomous overgrowth of foetal chorionic tissue or trophoblast. Hydatidiform mole (HM) is the most common and benign form of the disease. The prevalence of HM is the highest in Asia, with rates ranging from 1 to 2 per 1000 pregnancies in Japan and China (Palmer 1994; Song 1987; Takeuchi 1987) to 12 per 1000 pregnancies in Indonesia, India and Turkey (Aziz 1984; Gul 1997; Steigrad 2003). In North America and Europe, the incidence is reported to be lower, at 0.5 to 1 per 1000 pregnancies (Lee 2009; Steigrad 2003). The incidence has been reported to vary with race (Tham 2003), maternal age (Parazzini 1986), parity (Bagshawe 1986) and diet (Berkowitz 1985; Parazzini 1988). Variations in prevalence may be because of differences in reporting between hospital‐based and population‐based data or in the availability of central pathology review (Seckl 2010; Smith 2003).
HMs are categorised as partial (PM) or complete moles (CM) based on their gross morphology, histopathology and karyotype. CMs usually occur when a duplicated haploid sperm fertilises an anucleate or 'empty' ovum; the ensuing diploid product (usually 46 XX) is therefore paternally derived (Fisher 2009). PMs are usually triploid (69XXX, 69XXY or 69XYY), with two sets of paternal haploid genes and one set of maternal haploid genes, and occur when two sperm fertilise one ovum (Fisher 2009). With CMs there is no evidence of foetal tissue; however, with PM an embryo or foetus frequently dies in early pregnancy and foetal tissue and blood cells may be identified in 20% and 50% of PM specimens, respectively (Sebire 2009).
HMs usually present with vaginal bleeding. Associated features (including excessive uterine enlargement, theca lutein ovarian cysts, hyperemesis, pre‐eclampsia and hyperthyroidism) are more common in CMs; however, they occur less frequently as the routine use of ultrasound has led to earlier diagnosis (Seckl 2010). The management of PMs and CMs is similar (Berkowitz 2009a; Berkowitz 2009b). For women who want to preserve their fertility, an evacuation of the retained products of conception (ERPC) is performed, ideally by suction curettage, to remove all trophoblastic tissue completely (Seckl 2010). Most women are cured in this way; however, in some women HM persists and becomes malignant (gestational trophoblastic neoplasia (GTN)), requiring treatment with chemotherapy. In Japan, second ERPCs are performed routinely for HMs within one week of the initial ERPC, to ensure that there is no residual molar tissue in the uterus (Sasaki 2009). Second ERPCs may reduce the risk of GTN; however, to our knowledge, there is currently insufficient evidence to support this routine practice. Hysterectomy may reduce the risk of GTN by up to 10% (Bahar 1989; Curry 1975) and is an option for women who do not wish to retain their fertility or who experience life‐threatening bleeding at the time of evacuation; however, it does not avoid the need for subsequent monitoring or chemotherapy (Tidy 2009).
Transformation to GTN is considered to have occurred when trophoblastic activity remains following evacuation, as shown by a plateau or rise in serial β‐human chorionic gonadotrophin (hCG) levels, raised hCG levels six months after evacuation or if the histopathological examination indicates choriocarcinoma (Kohorn 2009). The risk of developing GTN is reported to be 16% to 20% in women with CM (Berkowitz 1995; Curry 1975; Felemban 1998; Seckl 2009) and 0.5% to 1% in women with PM (Bagshawe 1990; Seckl 2009). In the UK, this translates to a GTN transformation rate of approximately 8% of all molar pregnancies (Seckl 2009). Thresholds for treating persistent GTD differ by region with, for example, more than twice as many women in the US (20%) receiving chemotherapy for persistent GTD than in the UK (Hancock 2009).
HMs may be categorised as being at a low or high risk of malignant transformation based on criteria first introduced by Bagshawe 1976 (Table 3; Berkowitz 1987). Women with high‐risk HMs have more than one of the following characteristics: an initial serum β‐hCG more than 100,000 mIU/mL, uterine size larger than gestational age, theca lutein cysts more than 6 cm in diameter, maternal age over 40 years, and other associated medical and epidemiological factors, including previous GTD, hyperthyroidism and trophoblastic embolisation (Berkowitz 1995). Approximately 30% to 50% of high‐risk HMs will progress to GTN (Goldstein 1981; Limpongsanurak 2001; Uberti 2009).
Table 1.
Risk scoring system for the prediction of GTN in women with molar pregnancy*
| Prognostic factor | Score | |||
| 0 | 1 | 2 | 3 | |
| U/S diagnosis | Partial | Complete | Recurrent | |
| Uterine size for GA (months) | not more than 1 | > 1 | > 2 | > 3 |
| hCG level (mIU/mL) | < 50,000 | > 50,000 to < 100,000 | > 100,000 to < 1,000,000 | > 1,000,000 |
| Diameter of theca lutein cysts (cm) | ‐ | < 6 | < 6 to < 10 | > 10 |
| Patient age (years) | ‐ | < 20 | ≥ 40 | > 50 |
| Medical complications** | ‐ | ≥ 1 | ‐ | ‐ |
*From Berkowitz 1987
Low risk is defined as a score of < 4; high risk is defined as a score ≥ 4
U/S: ultrasound; GA: gestational age, hCG: β‐human chorionic gonadotrophin.
** hyperemesis, hyperthyroidism, pre‐eclampsia, trophoblastic embolisation, disseminated intravascular coagulation.
GTN, which may also follow a 'normal' pregnancy, ectopic or miscarriage, is classified as low or high risk using a modified World Health Organization (WHO) scoring system adapted by the International Federation of Gynaecology and Obstetrics (FIGO 2009). Low‐risk GTN accounts for 95% of cases in the UK and has a cure rate of almost 100% (Seckl 2010). High‐risk GTN has a cure rate of between 80% and 90%; these lesions require combination chemotherapy regimens and frequently develop drug resistance (Goldstein 2012).
Description of the intervention
Methotrexate was first reported to be active against trophoblastic tissue in the mid‐1950s (Hertz 1956). Since then, GTN has been shown to be a highly chemosensitive disease, with various chemotherapeutic agents achieving good rates of cure. All women with 'low‐risk' GTN and approximately 80% to 90% of women with 'high‐risk' GTN will be cured following treatment with one or more chemotherapy regimens (Seckl 2010; Goldstein 2012). Since chemotherapy drugs are associated with various toxic effects, most commonly myelotoxicity, gastrointestinal toxicity, stomatitis and alopecia, the chemotherapeutic aim when treating GTN is to provide the most effective treatment with the least toxicity. Methotrexate and dactinomycin are considered to be relatively safe agents that are commonly administered as first‐line chemotherapy for GTN, alone or in combination with other agents (Alazzam 2012). They have not been shown to be associated with adverse reproductive outcomes, ovarian failure or second tumours (Goldstein 1995).
The use of prophylactic chemotherapy (P‐Chem) in women with molar pregnancy was first described in 1966 (Lewis 1966). Since then, studies of dactinomycin and methotrexate administered before, during or after evacuation of a molar pregnancy have reported encouraging results (see Table 4). Several studies have found a significant reduction in GTN for high‐risk HMs only (Kim 1986; Fasoli 1982; Park 1996). Various dosing schedules have been described including five‐day dactinomycin (Goldstein 1974; Goldstein 1981; Limpongsanurak 2001; Park 1996), eight‐day methotrexate‐folinic acid (Goldstein 1971; Kim 1986; Park 1996) and single‐dose dactinomycin (Uberti 2006; Uberti 2009).
Table 2.
Comparative studies of P‐Chem for hydatidiform mole
| Study | Design | Participants (P‐Chem) |
Participants (control/no P‐Chem) |
Intervention | Rate of GTN (P‐Chem) | Rate of GTN (control) | Comments |
| Koga 1968* | Case‐control | 107 women (HM) | 42 women (HM) | Methotrexate 10 mg/day PO x 7 days given within 3 weeks of ERPC | 2/107 (2%) | 4/42 (10%) | No choriocarcinoma observed in the P‐Chem group vs. 3/42 in the control group. Toxic side effects occurred in 84/107 women, including stomatitis (34/107) and myelosuppression (22/107) |
| Goldstein 1971 | Prospective case‐control | 73 women (CM) | 116 women (CM) | 3 intervention arms: methotrexate 0.3 mg/kg/day x 5 days (20 women); or dactinomycin 9‐12 μg/kg/day x 5 days (53 women); ERPC on day 3 | 6/73 (8%) |
23/116 (20%) | No metastatic disease observed in the P‐Chem groups. P‐Chem well tolerated with minor side effects |
| Goldstein 1974 | Prospective case‐control | 100 women (HM) | 100 women (HM) | Dactinomycin 12 μg/kg/day x 5 days. ERPC on day 3 | 2/100 (2%) |
16/100 (16%) |
No metastatic disease observed in the P‐Chem group vs. 4/100 in the control group (4%). Reversible alopecia occurred in 32% of the P‐Chem group. No serious toxic reactions |
| Goldstein 1981 | Prospective case‐control | 174 women (CM) | 858 women (CM) | Dactinomycin 12 μg/kg/day x 5 days. ERPC on day 3 | 10/247 (4%) |
160/858 (19%) | No metastatic disease observed in the P‐Chem group vs. 34/858 (4%) in the control group. This report includes data from Goldstein 1974 |
| Fasoli 1982 | Retrospective case‐control | 104 women (92% CM) | 250 women (CM) | Methotrexate 10 mg/day PO x 5 days every 3 weeks for 3 cycles | 3/104 (3%) |
23/250 (9%) |
Significantly fewer high‐risk women in the P‐Chem group (1/47) vs. the control group (18/126) developed GTN (2% vs. 14%; P < 0.05). 2 women had severe myelosuppression and 1 had severe alopecia |
| Kashimura 1986* | RCT (?) | 293 women (CM) | 127 women (CM) | Methotrexate 10 mg/day (IM or PO) for 7 days, within 3 weeks of evacuation | 22/293 (7%) |
23/127 (18%) |
There were 5 cases of metastatic disease in each group (1.7% vs. 3.9%, respectively) 27.3% of the P‐Chem group experienced drug‐related side effects including stomatitis (10.3%), nausea/vomiting (6.8%) and leukopenia (4.4%). However none were reported to be severe |
| Kim 1986 | RCT | 39/71 women (CM; 18/31 low‐risk and 21/40 high‐risk women) | 32 women (CM) | Methotrexate 1.0 mg/kg/day IM (days 1, 3, 5, 7) and citrovorum factor rescue 0.1 mg/kg/day IM (days 2, 4, 6, 8). ERPC on day 3 | 4/39 (10%) | 10/32 (31%) | Significantly fewer high‐risk women in the P‐Chem group (14%) vs. the control group (47%) developed GTN. There was no significant difference in the GTN rates of low‐risk women between groups |
| Park 1996 | Retrospective case‐control | 52 women (14 low‐risk, 21 medium‐risk and 17 high‐risk HM) | 88 women (38 low‐risk, 25 medium‐risk and 25 high‐risk HM) |
Methotrexate 1 mg/kg (days 1, 3, 5, 7) and citrovorum factor (0.1 mg/kg (days 2, 4, 6, 8); or dactinomycin 12 μg/kg/day x 5 days started at the time of ERPC | 8/52 (15.4%) |
28/88 (31.8%) | Significantly fewer high‐risk women in the P‐Chem group (7/17) vs. the control group (22/25) developed GTN (41% vs. 88%; P < 0.01). There was no significant difference in the GTN rates in low‐ and medium‐risk women between groups. The time to achieve normal hCG levels was shorter in high‐risk women in the P‐Chem group |
| Limpongsanurak 2001* | Double‐blind RCT | 30 women (high‐risk CM) | 30 women (high risk CM) | Dactinomycin 10 µg/kg for 5 days, within 1 week after ERPC and histology | 4/29 (15.4%) |
15/30 (50%) |
Mild, reversible side effects reported including stomatitis (10%), nausea/vomiting (10%), oral ulcers (3.3%) and hair loss (13.3%) ‐ all grade 1 except for 2 women with grade 2 patchy alopecia |
| Uberti 2006 | Retrospective case‐control | 29 adolescents (high‐risk CM) |
31 adolescents (high‐risk CM) |
Dactinomycin 1.25 mg/m2 IV given 1 hour before ERPC | 2/29 (6.9%) |
9/31 (29%) |
Mean risk scores and hCG levels were significantly higher and gestational age was significantly lower in the P‐Chem group than the control group. Mild and transient side effects included hepatotoxicity (10%) and mild alopecia (6.8%) |
| Uberti 2009 | Retrospective case‐control | 163 women (high risk, > 90% CM) |
102 women (high risk, > 90% CM) |
Dactinomycin 1.25 mg/m2 IV given 1 hour before ERPC | 30/163 (18.4%) | 35/102 (34.3%) | Mild and transient side effects including nausea (8%), raised liver enzymes (3.7%), stomatitis (3.1%), rash (2.4%) diarrhoea (2.4%), alopecia (1.2%) and neutropenia (0.6%) were seen in 21% of the P‐Chem group. Time to GTN diagnosis, subsequent drug resistance and the number of chemotherapy course to cure was similar in the 2 groups |
* Three studies administered P‐Chem after ERPC including Koga 1968, Kashimura 1986 and Limpongsanurak 2001.
CM; complete mole; ERPC: evacuation of retained products of conception; GTN: gestational trophoblastic neoplasia; HM: hydatidiform mole; IM: intramuscular; IV: intravenous; P‐Chem; prophylactic chemotherapy; PO: per os; RCT: randomised controlled trial.
How the intervention might work
As GTN is a highly chemosensitive disease, prophylaxis with chemotherapy agents that have known activity against trophoblast tumour cells may prevent progression to GTN. The use of P‐Chem has been based on an assumption that the development of GTN is pre‐determined, that metastatic GTN spreads via the bloodstream and that high serum levels of cytotoxic agents around the time of evacuation should reduce the ability of the trophoblast cells to invade or metastasise (Goldstein 1995).
P‐Chem may be particularly useful in women with high‐risk CMs, who have poor access to health care, for whom hormonal follow‐up is not available, or where poor compliance may be an issue (Limpongsanurak 2001; Uberti 2006; Berkowitz 2009a). In Latin America, loss to follow‐up may be as high as 44% in some areas; hence numerous referral centres in this region are reported to use P‐Chem (Charry 2009). However, the use of P‐Chem exposes women unnecessarily to toxic side effects (Kaye 2002 and Ratnam 1971 have reported toxicity‐related deaths with methotrexate prophylaxis), may lead to inadequate follow‐up, and incompletely protects women against persistent tumour (Goldstein 1995; Hancock 2009). Furthermore, P‐Chem may favour the development of drug resistance (Kim 1986) and delay the time to effective treatment, thereby having adverse effects on survival.
Why it is important to do this review
P‐Chem for high‐risk HM appears to be routine clinical practice in some regions of the world (Charry 2009). Although several studies have been reported, it remains unclear whether P‐Chem, which may be associated with substantial toxicity, will prevent malignant transformation of HM. Furthermore, if benefits to P‐Chem exist, it is not clear which drug regimen might have the best effectiveness‐to‐toxicity ratio. We undertook this review in an attempt to clarify the benefits and risks associated with P‐Chem for HM.
Objectives
To evaluate the effectiveness and safety of P‐Chem for the prevention of GTN in women with molar pregnancy.
To investigate whether any subgroup of women with HM may benefit more from P‐Chem than others.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Inclusions
All women diagnosed with HM.
Exclusions
Women who were diagnosed with other types of GTD such as invasive moles, choriocarcinoma and placental site trophoblastic tumour (PSTT).
Types of interventions
P‐Chem compared with no or other treatments (e.g. placebo or analgesic drugs).
Types of outcome measures
Primary outcomes
Incidence of GTN (invasive mole, choriocarcinoma, PSTT and epithelioid trophoblastic tumour (ETT).
Secondary outcomes
Drug toxicity, including myelotoxicity, gastrointestinal toxicity, stomatitis and alopecia.
Overall survival (more than five years).
Time to negative conversion of serum or urine β‐hCG.
Time to GTN diagnosis.
Incidence and nature of subsequent pregnancies.
Quality of life (QoL).
Search methods for identification of studies
Electronic searches
We searched the MEDLINE (1946 to February 2012) (Appendix 1), EMBASE (1980 to September 2012) (Appendix 2) and the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 2, 2012) (Appendix 3). No language restriction was applied.
Searching other resources
All relevant articles were identified on PubMed, and, using the 'related articles' feature, a further search was carried out for newly published articles. The reference lists from identified published trials were handsearched for further clinical trials. Papers in all languages were sought and translated as necessary. We searched the metaRegister of Controlled Trials (mRCT) and the National Research Register (NRR) archive for ongoing trials.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database, duplicates were removed and the remaining references were examined by three review authors (JF, FH) independently. Those studies that clearly did not meet the inclusion criteria were excluded and copies of the full text of potentially relevant references were obtained. The eligibility of retrieved papers was assessed independently by three review authors (JF, LX, TL). We documented the reasons for exclusion.
Data extraction and management
For included studies, data on patient characteristics; number recruited to each arm; number excluded from analysis; type of intervention; proportion of participants who received all, part or none of the intended treatment; methods of randomisation, blinding and allocation concealment; length of follow‐up and data on outcome were extracted independently by three review authors (JF, HC, TL). Differences between review authors were resolved by discussion or by appeal to a forth review author (LH, FF or TW) if necessary.
Assessment of risk of bias in included studies
We used the Cochrane Collaboration's tool for assessing risk of bias (Higgins 2011). Three review authors (JF, LX, TL) independently assessed the risk of bias within each included study based on the following six domains, with review authors' judgements presented as answers of 'Yes' (low risk of bias); 'No' (high risk of bias), and 'Unclear' (uncertain risk of bias).
Selection bias: random sequence generation and allocation concealment.
Performance bias: blinding of participants and personnel (women and treatment providers).
Detection bias: blinding of outcome assessment.
Attrition bias: incomplete outcome data. We considered studies to be at a high risk of bias if more than 20% of women were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms.
Reporting bias: selective reporting of outcomes.
Other possible sources of bias.
Assessment of heterogeneity
Random‐effects models were used for all meta‐analyses (DerSimonian 1986). Heterogeneity between studies was assessed by visual inspection of forest plots, by estimation of the I2 statistic, which summarises the percentage heterogeneity between trials that cannot be ascribed to sampling variation, and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). A level of I2 of less than 25% was considered as low level heterogeneity, 25% to 50% as moderate level, and higher than 50% as substantial heterogeneity (Higgins 2003). If there was evidence of substantial heterogeneity, the possible reasons for this were investigated and reported.
Data synthesis
Meta‐analysis was carried out using the Review Manager 5.1 software (RevMan 2011). We used random‐effects models for all meta‐analyses (DerSimonian 1986). For dichotomous outcomes, we calculated risk ratios (RR) and associated 95% confidence intervals (CIs). For continuous outcomes we pooled the mean differences (MD) between the treatment arms where trials measured the outcome on the same scale.
Subgroup analysis and investigation of heterogeneity
We subgrouped women by the type of chemotherapy agent (i.e. methotrexate and dactinomycin). We had planned to perform other subgroup analyses, including subgroups of women at a low and high risk of GTN, and according to drug regimens; however, this was not possible since these data were not reported in the included trials.
Sensitivity analysis
We performed sensitivity analysis to assess the robustness of the meta‐analyses by comparing the results using all trials and then excluding trials of lower methodological quality or those considered to be at a higher risk of bias.
Results
Description of studies
Results of the search
From the search, we identified and screened 133 citations, retrieving the full text of 86 citations that we considered potentially eligible for inclusion in this review. We excluded 79 out of these 86 papers. Of the remaining seven reports, we excluded four on the basis that they were not RCTs (Geng 2011; Uberti 2006; Uberti 2009 ‐ two papers), and included the remaining three studies (Kashimura 1986; Kim 1986; Limpongsanurak 2001) (Figure 1).
Figure 1.
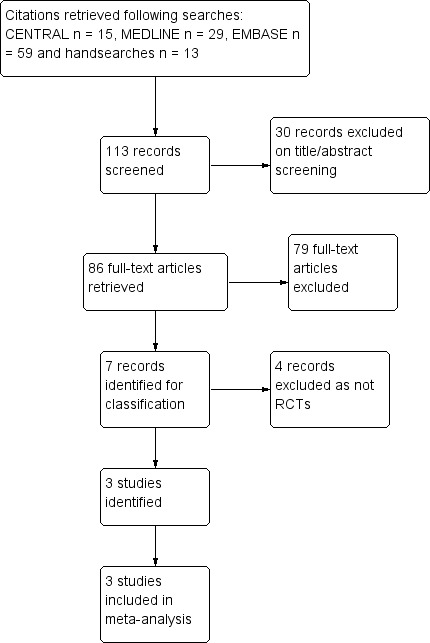
Study flow diagram.
Included studies
We included three studies with a total of 613 participants (Kashimura 1986; Kim 1986; Limpongsanurak 2001). Two studies used prophylactic methotrexate and one trial used prophylactic dactinomycin. The number of evaluable participants in the three studies was 550. See Characteristics of included studies.
Kashimura 1986 'randomly selected' 420 women with low‐ or high‐risk CM to receive one course of prophylactic methotrexate (10 mg daily for seven days) within three weeks of ERPC, or no prophylaxis, and evaluated subsequent rates of GTN in the two groups.
Kim 1986 randomised 133 women with low‐ or high‐risk CM to prophylactic methotrexate or no prophylaxis. Only 71 out of 133 women completed this trial and were included in the analyses (39 in the treatment group and 32 in the untreated group). The intervention group (18 out of 31 low‐risk women and 21 out of 40 high‐risk women) received one course of methotrexate with citrovorum rescue factor (methotrexate 1.0 mg/kg/day intramuscular (IM) on days 1, 3, 5 and 7; citrovorum rescue factor 0.1 mg/kg/day IM on days 2, 4, 6 and 8); the control group received no treatment. The ERPC in the intervention group was done on the third or fourth day of P‐Chem.
Limpongsanurak 2001 randomised 60 women with high‐risk CM to dactinomycin prophylaxis (10 μg/kg body weight daily for 5 days; 30 women) or no prophylaxis (30 women) within one week after ERPC. One woman was lost to follow‐up.
Excluded studies
We excluded three retrospective studies (Geng 2011; Uberti 2006; Uberti 2009 ‐ two reports). See Characteristics of excluded studies.
Risk of bias in included studies
We assessed the risk of bias of the three included RCTs using the Cochrane Collaboration's 'Risk of bias' tool, see Figure 2. Overall, we consider the older studies of Kashimura 1986 and Kim 1986 to be at a high risk of bias and Limpongsanurak 2001 to be at a low risk of bias. Dr. Limpongsanurak provided us with additional methodological details for Limpongsanurak 2001 via e‐mail. Although we attempted to contact the other authors by e‐mail for more details, we were unsuccessful as we had no contact details for the authors of Kim 1986, and received no reply to our queries from Dr. Kashimura (Kashimura 1986).
Figure 2.
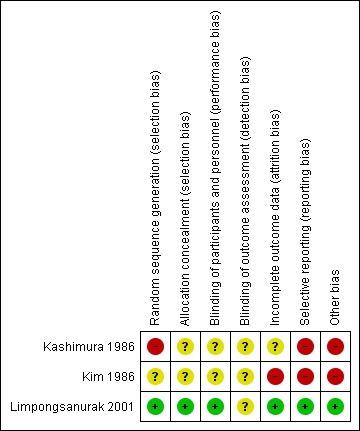
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Limpongsanurak 2001 used lot‐drawing to randomise women and sealed opaque envelopes to conceal random group allocations; we assessed these methods to be at a low risk of bias. We assessed the randomisation and allocation methods used in Kim 1986 to be of unclear risk of bias and assessed the random sequence generation in Kashimura 1986 to be at a high risk of bias. In the latter report, participants were 'selected at random', which suggests that participant selection in this study may not have been a truly random process. Furthermore, no randomisation ratio was described and yet the intervention and control groups were very different sizes (293 women versus 127 women).
Blinding
The only trial that reported blinding was Limpongsanurak 2001. Although precise details were not reported, this trial was described as 'double‐blind' as control participants received a similar‐looking intravenous (IV) solution with analgesic drugs for five days, and neither the participant nor the attending doctor knew to which group the participant had been allocated. It is unclear whether outcome assessment was also blind.
Incomplete outcome data
We assessed Kim 1986 as being at a high risk of attrition bias as 62 out of 133 women were excluded from the analyses owing to loss to follow‐up (36 women), insufficient length of follow‐up (7 women) and hysterectomy (19 women). Of the 60 women in Limpongsanurak 2001, one woman in the P‐Chem group was lost to follow‐up one month after treatment and was not included in the main analyses. Kashimura 1986 reported complete data sets for the main outcomes.
Selective reporting
All pre‐specified outcomes were reported for Kim 1986 and Limpongsanurak 2001. Kashimura 1986 failed to report baseline characteristics of the two study groups that could represent reporting bias, especially since there were proportionally more women aged 40 years and over in the control group.
Other potential sources of bias
In Kashimura 1986, more women in the control group were 40 years old and over (22% in control group versus 11% in P‐Chem group). The older women were more likely to progress to GTN (39% versus 12%). This may have biased the results in favour of the P‐Chem group.
Limpongsanurak 2001 included women with high‐risk CM only, whereas Kim 1986 and Kashimura 1986 included women with low‐ and high‐risk CM. Since Kim 1986 showed that P‐Chem was not beneficial to women with low‐risk CM, by including these women the meta‐analysis results may be biased in the direction of the control arm.
Limpongsanurak 2001 and Kashimura 1986 gave P‐Chem after ERPC whereas Kim 1986 started P‐Chem before the ERPC. If treatment was commenced before ERPC, this trial may have included women with PM and hydropic abortion that would otherwise have been excluded following histological diagnosis of evacuation products. It is unclear whether women with PM or hydropic abortion were present in the same numbers within the allocated groups.
Kashimura 1986 and Kim 1986 were older studies that took place before rigorous RCT guidelines were in place and therefore are lacking in methodological quality. It is not possible to determine whether these were true RCTs so we have assumed that they were but considered them to be at a high risk of bias overall; therefore we have performed sensitivity analysis and downgraded the results accordingly.
Effects of interventions
See: Table 1
Incidence of GTN
Incidence of GTN (overall)
P‐Chem was associated with a significant reduction in the incidence of GTN compared with the control group (3 studies; 550 participants; 30 out of 361 versus 48 out of 189; RR 0.37; 95% CI 0.24 to 0.57; I2 = 0%; P < 0.00001; Analysis 1.1) with no significant difference between methotrexate and dactinomycin prophylaxis subgroups.
Analysis 1.1.
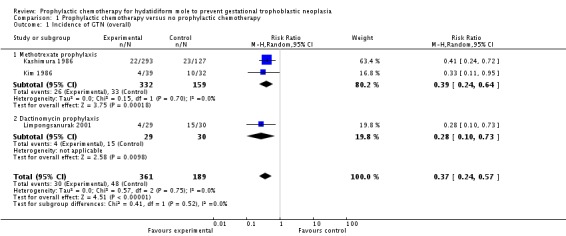
Comparison 1 Prophylactic chemotherapy versus no prophylactic chemotherapy, Outcome 1 Incidence of GTN (overall).
We performed sensitivity analysis for this outcome as two of the included studies were at a high risk of bias. When these two studies (both in the methotrexate subgroup) were excluded, only one trial remained (59 participants; 4 out of 29 versus 15 out of 30; RR 0.28; 95% CI 0.10 to 0.73; P = 0.01); therefore we consider this evidence to be of a low quality.
Incidence of GTN (high‐risk HM only)
When only high‐risk women were included, P‐Chem was similarly associated with a significant reduction in the incidence of GTN compared with the control group (2 studies; 99 participants; 7 out of 50 versus 24 out of 49; RR 0.29; 95% CI 0.14 to 0.60; I2 = 0%; P = 0.001; Analysis 1.2). Sensitivity analysis gave the same results as above when the only well‐conducted trial was included (59 participants; 4 out of 29 versus 15 out of 30; RR 0.28; 95% CI 0.10 to 0.73; P = 0.01).
Analysis 1.2.
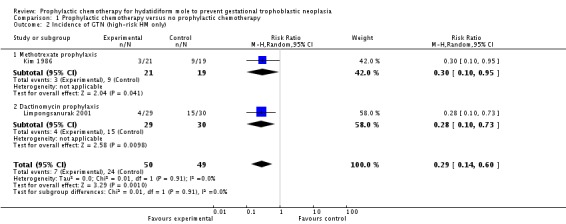
Comparison 1 Prophylactic chemotherapy versus no prophylactic chemotherapy, Outcome 2 Incidence of GTN (high‐risk HM only).
Invasive mole and choriocarcinoma
There were insufficient data from these trials to analyse the rates of invasive mole and choriocarcinoma in the study groups. Limpongsanurak 2001 utilised a prognostic scoring system to diagnose GTN (not histology) and secondary histology was known in only three participants, two of whom underwent hysterectomy for excessive bleeding. Kashimura 1986 diagnosed 27 out of 45 cases of GTN histologically and 18 out of 45 by a Japanese prognostic scoring system. Four of these participants had choriocarcinoma (two in each study group) and 41 were considered to have invasive mole. Kim 1986 diagnosed most cases of GTN based on persistent or rising hCG levels, persistent or recurrent uterine haemorrhage, or clinical/histological evidence of metastases, and did not distinguish between invasive mole and choriocarcinoma.
Time to GTN diagnosis
Two studies reported this outcome for 33 participants who developed GTN (Kim 1986; Limpongsanurak 2001). The time to GTN diagnosis was significantly longer in the P‐Chem group compared with the control group (MD 28.72 days; 95% CI 13.9 to 44.24; I2 = 0%; P = 0.0003; Analysis 1.3). When we excluded Kim 1986 from the sensitivity analysis, results for the one remaining study were similar but not significant (19 participants; MD 22.30 days; 95% CI to 9.05 to 53.65; P = 0.16).
Analysis 1.3.
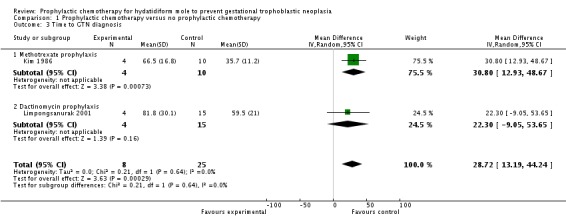
Comparison 1 Prophylactic chemotherapy versus no prophylactic chemotherapy, Outcome 3 Time to GTN diagnosis.
Toxicity
None of the studies reported adverse effects in the control groups and we have not imputed these data. Limpongsanurak 2001 (five‐day dactinomycin) only reported side effects as percentages, including stomatitis (10%), nausea or vomiting (10%), oral ulcers (3.3%) and hair loss (13.3%). All adverse effects in this study were grade 1 except for two women with patchy alopecia (grade 2). The other two studies reported the following:
Kashimura 1986 (239 women): stomatitis (10.3%), nausea or vomiting (6.8%) and leukopenia (4.4%);
Kim 1986 (39 women): epithelial (5.1%), hepatic (7.7%), haematological (7.7%) and neuromuscular (2.6%) toxicity.
In these latter two studies, grades of toxicity were not reported. Both reports state that there were no severe complications or drug‐related deaths.
Courses of chemotherapy
Only one poor‐quality study reported this outcome. Women in the methotrexate prophylaxis group needed more subsequent chemotherapy courses for GTN treatment than the control group (14 women; MD 1.10; 95% CI 0.52 to 1.68; P = 0.0002).
Survival
There were no deaths during the follow‐up periods in Kim 1986 and Limpongsanurak 2001; however, Kashimura 1986 reported three deaths from metastatic disease that occurred two to 12 years after ERPC, including two women in P‐Chem group and one in the control group.
Subsequent pregnancies
Limpongsanurak 2001 did not report subsequent pregnancy rates. Women in Kim 1986 were given contraception for one year after ERPC and subsequent pregnancy rates were assessed. Out of 51 women who attempted to become pregnant, 40 had 44 pregnancies including 24 out of 29 in the P‐Chem group and 16 out of 22 in the control group. The rates of full‐term pregnancies were reported as being similar in the two groups (25 out of 27 (93%) in the P‐Chem group versus 16 out of 17 (94%) in the control group).
Kashimura 1986 obtained these data for 101 out of 420 participants only (24%): 74 out of 112 (67%) subsequent pregnancies were normal full‐term pregnancies in the P‐Chem group compared with 19 out of 31 (61%) in the control group. The induced abortion rate was 22% of pregnancies in the P‐Chem group and 13% in the control group.
It was not possible to perform a meta‐analysis of these very limited data.
Discussion
Summary of main results
In a limited meta‐analysis of three studies that included low‐ and high‐risk molar pregnancies, P‐Chem reduced the incidence of subsequent GTN by approximately two‐thirds overall. On sensitivity analysis, where the only satisfactory study (high‐risk women only) contributed data, the results showed a similar effect but with a wider CI. (See Table 1). The interpretation of these results was influenced by the poor methodological quality of the two older included studies, and the small number of participants in the only other study. We therefore consider this evidence to be of low quality.
The diagnosis of GTN occurred approximately one month later in the P‐Chem group compared with the control group in the meta‐analysis of two studies that reported this outcome. The P‐Chem group needed approximately one extra course of chemotherapy than the control group to achieve a cure in the participants whose disease progressed to GTN. We consider this evidence to be of a very low quality for similar reasons as those given above.
Data on toxicity were insufficient for meta‐analysis; however, it was reported that no participants in any of the studies experienced severe drug‐related complications or deaths.
Overall completeness and applicability of evidence
We consider this evidence to be incomplete and not widely applicable:
Incidence of GTN
This limited evidence in favour of P‐Chem may apply to women with high‐risk CM only. Kim 1986 found that P‐Chem did not benefit women with low‐risk HM and some non‐randomised studies have drawn similar conclusions (Fasoli 1982; Park 1996). This is probably why the more recent studies have excluded low‐risk women (e.g. Limpongsanurak 2001; Uberti 2009). Furthermore, the incidence of GTN in the high‐risk control groups of included studies was high, at 47% and 50% for Kim 1986 and Limpongsanurak 2001, respectively. This may reflect regional differences in the rates of CM transformation or the selection/diagnostic criteria applied, and needs further clarification.
Only two chemotherapeutic agents, namely, methotrexate and actinomycin D, were investigated. None of the included studies evaluated the less toxic single‐dose regimens of these agents that have been shown to be useful to treat low‐risk GTN (Alazzam 2012). One RCT of single‐dose dactinomycin for P‐Chem was proposed by Goldstein 1995, but has never been conducted. Uberti 2006 and Uberti 2009 report the results of two retrospective studies of single‐dose dactinomycin administered before evacuation of high‐risk molar pregnancies, showing a reduction in post‐molar GTN of 76% and 46%, respectively, with minimal adverse effects. Since this low‐dose dactinomycin regimen appears to be in practice (personal communication with Dr Uberti) it should be evaluated in an RCT.
Toxicity
Toxicity was not rigorously reported in the included studies and meta‐analyses of these data were not possible. When used to treat low‐risk GTN, five‐day dactinomycin and five‐ and eight‐day methotrexate regimens have been associated with severe adverse effects including myelosuppression, hepatotoxicity and alopecia (dactinomycin); hence there is a move towards less toxic chemotherapy regimens for the treatment of low‐risk GTN (Alazzam 2012). For this reason, the more toxic five‐ and eight‐day regimens that have historically been used in studies of P‐Chem (Table 4) are unlikely to be favoured as prophylaxis for HM.
Survival
Only one study (Kashimura 1986) reported long‐term follow‐up, with three deaths occurring from metastatic disease between two and 12 years after ERPC (two in the P‐Chem group and one in the control group); neither Kim 1986 nor Limpongsanurak 2001 assessed the impact of P‐Chem on long‐term overall survival, hence the evidence for this outcome is incomplete. Longer follow‐up of participants is needed in any future studies of P‐Chem interventions for HM.
Subsequent pregnancy and QoL
Although subsequent pregnancies were reported by Kashimura 1986 and Kim 1986, these data were incomplete (owing to high attrition) and it was not possible to draw any conclusions. Kim 1986 reported that 78.4% of their participants experienced at least one pregnancy after one year of contraception during the follow‐up period and the frequency of full‐term delivery was 92.6% and 94.1%, for the treatment and control groups, respectively. In Kashimura 1986, comparable rates of secondary infertility and a similar time to first menstruation after ERPC were reported in the P‐Chem and control groups. Neither Kim 1986 nor Limpongsanurak 2001 assessed the impact of P‐Chem on long‐term ovarian function or QoL. However, these chemotherapy agents have been used extensively to treat GTN over several decades and have not been shown to adversely affect reproductive outcomes, ovarian function or to be associated with second tumours (Garner 2002; Garrett 2008; Goldstein 1995; Uberti 2006; Uberti 2009).
Uberti 2009 has suggested that P‐Chem reduces the emotional complications associated with HM but we were unable to corroborate this owing to a lack of QoL data.
Drug resistance
Drug resistance may occur following P‐Chem, as the agents used for P‐Chem are also used as first‐line treatment for GTN. Only one included study compared the number of courses of chemotherapy required to treat subsequent GTN (Kim 1986). These investigators found that women in the P‐Chem group (4 women) required more courses than women in the control group (10 women), suggesting that P‐Chem may increase resistance to subsequent chemotherapy. Owing to the poor methodological quality of the Kim 1986 study and the small number of participants concerned, we are very uncertain of this estimate of effect (Table 1). To prevent resistance to treatment it has been suggested that an alternative agent be used for the treatment of persistent disease (Goldstein 1995). Drug resistance could not be adequately evaluated in this review and warrants further investigation.
Time to GTN diagnosis
The time interval from the index pregnancy to the diagnosis of GTN is considered to be a risk factor for the development of GTN and is included in the Modified WHO Prognostic Scoring System (FIGO 2009). Again, extremely limited evidence from Kim 1986 suggests that P‐Chem may delay the time to diagnosis of GTN. It is unclear whether such a delay might potentially lead to up‐scoring of GTN lesions from low to high risk. Investigators of some retrospective studies of single‐dose dactinomycin found no difference in the time to GTN diagnosis among their participants (Uberti 2006; Uberti 2009). Thus further research is needed to clarify the impact of low‐dose P‐Chem on the time to diagnosis and subsequent GTN risk scores.
Timing of ERPC
Most studies of P‐Chem to date have administered P‐Chem before performing the ERPC with the exception of Limpongsanurak 2001, Kashimura 1986 and Koga 1968, where participants received their P‐Chem within one week and three weeks of the ERPC. Only Limpongsanurak 2001 included participants based on a histological diagnosis. Goldstein 1971 was the first to describe performing the ERPC on P‐Chem day three (see Table 4) and this practice was included in several subsequent studies, including Kim 1986. Administering P‐Chem before ERPC may theoretically be more effective in preventing haematogenous spread of the molar tissue during the procedure. Since the incidence of GTN in the high‐risk groups of Kim 1986 and Limpongsanurak 2001 were similar, it would appear that administering P‐Chem before ERPC may not confer any additional benefit. Treating women with P‐Chem before evacuation carries the inherent risk of over‐treating women, as 10% of cases that are thought to be molar on ultrasound may turn out to be non‐molar hydropic abortions (Fowler 2006). Thus we propose that, in any future studies of P‐Chem, the intervention is administered after ERPC, and following a histological diagnosis of CM.
Quality of the evidence
We consider this evidence to be of a low to very low quality. This conclusion is based on our assessment that two of the included studies were of poor methodological quality and at a high risk of bias (See Risk of bias in included studies); the third study was of a good quality but consisted of only 60 participants. Kashimura 1986 and Kim 1986 are older studies that took place before rigorous RCT guidelines were in place.
With reference to the evidence for high‐risk CMs only, we calculated that the number needed to treat for an additional beneficial outcome (NNTB) to prevent one woman with high‐risk CM developing GTN was 3, with a 95% CI of 2 to 20 women; this wide CI illustrates the uncertainty concerning this evidence. Therefore, we believe that further research is very likely to have an important impact on our confidence in the estimate of effects, and is likely to change the estimates.
With regard to the number of courses of chemotherapy required to cure post‐molar GTN, we are very uncertain about this estimate of effect (see Table 1).
Potential biases in the review process
We included all identified RCTs in this review, including two older studies of poor methodological quality. We rigorously debated the merits of including these weaker studies as, in so doing, we might have biased the results in favour of P‐Chem. In particular, Kashimura 1986 describes its study design as 'prospective', with participants 'selected at random'. While some authors have interpreted this study design as an RCT (Limpongsanurak 2001), it has also been referred to as a retrospective study (Goldstein 1995). We were unsuccessful in making contact with the investigators of either Kim 1986 or Kashimura 1986 and therefore it was not possible to determine whether these were true RCTs. We decided to include them in our meta‐analyses. This may seem controversial; however, we performed sensitivity analyses and downgraded the meta‐analyses results accordingly. Sensitivity analysis of the main outcome produced similar findings when these studies were excluded. Furthermore, these weaker studies included low‐ and high‐risk women in their sample, which may have biased the results in the direction of the control group, as P‐Chem was found in these studies to have little benefit for women with a low risk of developing GTN.
Agreements and disagreements with other studies or reviews
Most recent studies of P‐Chem have been conducted in Asia and South America. This may be indicative of higher prevalences of GTD, limited health resources or lower rates of follow‐up experienced by in these regions. However, concerns regarding the exposure of women to unnecessary side effects (Goldstein 1995) may have played a role in the lack of contributing data from centres in North America and Europe.
Most recently, investigators in Brazil conducted two retrospective case‐control studies and reported a significant reduction in the rate of GTN transformation with the use of a single bolus dose of dactinomycin before evacuation for high‐risk HM, with minimal side effects (see Table 4; Uberti 2006; Uberti 2009). Baseline risk scores and hCG levels were significantly greater in the P‐Chem arm of the latter study, yet the incidence of GTN was significantly lower in the P‐Chem group compared with the control group. These studies suggest that low‐dose dactinomycin may have an improved effectiveness‐to‐toxicity ratio and, hence, greater general acceptability as a P‐Chem regimen.
It has been argued that because of the excellent primary cure rates among women with GTN, most doctors prefer to monitor hCG levels in women following HM, rather than administer P‐Chem (Hurteau 2003). In addition, even if prophylaxis reduces the risk of GTN, women who are given prophylaxis would still require the same monitoring and follow‐up as those who are not. However, P‐Chem this may reduce emotional costs for affected women, as well as operating costs for institutions (Uberti 2009). In Uberti 2009, the number of women with high‐risk HM who needed to be treated (NNTB) to prevent one case of GTN was seven; at this rate, P‐Chem would apparently result in substantial cost savings to their GTD centre. Following a personal communication with Dr Uberti, we understand that this regimen is already in clinical practice in this GTD Centre in Brazil. The emotional (QoL) and cost implications of P‐Chem versus no P‐Chem could not be evaluated in this review,
P‐Chem may not be the only method of reducing the incidence of GTN. In Japan, second ERPCs are performed within one week of the first ERPC following histological confirmation of HM (Sasaki 2009), and, in Indonesia, women with HM are given vitamin A supplementation (personal communication; Andrijono 2010). In one double‐blind RCT conducted in Indonesia, vitamin A prophylaxis was compared with an identical placebo in women with CMs (Andrijono 2010). The theoretical basis for this intervention was that vitamin A has been shown to cause trophoblastic cells to undergo apoptosis (Andrijono 2010). Investigators reported that only 2 out of 30 women (6.7%) with CM who received 200,000 IU of vitamin A per day progressed to GTN compared with 10 out of 35 in the placebo group (28.6%) (P = 0.029). Side effects appeared to have been minimal. The proportion of high‐risk CMs and the median duration of prophylaxis were not reported; however, these results are encouraging and warrant further research into vitamin A supplementation in women with HM.
Authors' conclusions
P‐Chem may reduce the risk of progression to GTN in women with CMs who are at a high risk of malignant transformation. However, the five and eight‐day methotrexate and dactinomycin regimens studied in this review were too toxic for routine use, may delay the time to GTN diagnosis and may lead to subsequent drug resistance. The current evidence in favour of P‐Chem is limited by the small numbers and poor methodological quality of available RCTs in this field. Hence there is currently insufficient evidence to support the use of P‐Chem in clinical practice.
One well‐conducted RCT of single‐dose dactinomycin compared with placebo for high‐risk CM may be appropriate in countries with limited healthcare resources and where follow‐up and hCG surveillance are more difficult. Baseline histology should be reported and outcomes should include time to GTN diagnosis, HM and GTN risk scores at diagnosis, the number of courses to cure, long‐term survival, QoL, subsequent pregnancies and an economic evaluation of the intervention groups over time.
Other RCTs that may be helpful include:
vitamin A studies (e.g. a 200,000 IU bolus or monthly) versus placebo, in women with a histological diagnosis of HM;
second ERPC versus no additional (routine) ERPC in women with a histological diagnosis of HM. (There is currently a pilot Phase II study [GOG‐0242] underway to investigate the effect of a second ERPC in women with low‐risk GTN on the frequency of surgical cure and the rate of persistent GTN (GOG 0242)).
Acknowledgements
We would like to thank:
Clare Jess, Gail Quinn, Jo Morrison and the staff of the Cochrane Gynaecological Cancer Review Group for their advice and administrative support throughout the review process;
Library staff at the Royal United Hospital, Bath, UK, for their assistance with the sourcing of articles;
Donald Goldstein for responding to reprint requests;
Elza Uberti for providing copies of Uberti 2006 and Uberti 2009 and responding to e‐mailed queries;
Andri Andrijono for responding to e‐mailed queries.
This review received methodological and statistical support as part of the 10/4001/12 NIHR Cochrane Programme Grant Scheme ‐ Optimising Care, Diagnosis and Treatment Pathways to Ensure Cost Effectiveness and Best Practice in Gynaecological Cancer: Improving Evidence for the NHS
Appendices
Appendix 1. MEDLINE (Ovid Web) search strategy
1 Gestational Trophoblastic Disease/ 2 exp Hydatidiform Mole/ 3 (hydatid* adj2 mole*).mp. 4 (molar adj2 pregnanc*).mp. 5 1 or 2 or 3 or 4 6 exp Antineoplastic Agents/ 7 Antineoplastic Combined Chemotherapy Protocols/ 8 Chemoprevention/ 9 (chemotherap* or chemoprophyla* or chemoprevention).mp. 10 (methotrexate or arnethopterin or dactinomycin or actinomycin D or fluorouracil or etoposide).mp. 11 6 or 7 or 8 or 9 or 10 12 5 and 11 13 randomized controlled trial.pt. 14 controlled clinical trial.pt. 15 randomized.ab. 16 placebo.ab. 17 clinical trials as topic.sh. 18 randomly.ab. 19 trial.ti. 20 13 or 14 or 15 or 16 or 17 or 18 or 19 21 12 and 20 22 exp animals/ not humans.sh. 23 21 not 22
Appendix 2. EMBASE search strategy
1 trophoblastic tumor/ 2 hydatidiform mole/ 3 (hydatid* adj2 mole*).mp. 4 (molar adj2 pregnanc*).mp. 5 1 or 2 or 3 or 4 6 exp chemotherapy/ 7 exp antineoplastic agent/ 8 chemoprophylaxis/ 9 (chemotherap* or chemoprophyla* or chemoprevention).mp. 10 (methotrexate or arnethopterin or dactinomycin or actinomycin D or fluorouracil or etoposide).mp. 11 6 or 7 or 8 or 9 or 10 12 5 and 11 13 crossover procedure/ 14 double‐blind procedure/ 15 randomized controlled trial/ 16 single‐blind procedure/ 17 random*.mp. 18 factorial*.mp. 19 (crossover* or cross over* or cross‐over*).mp. 20 placebo*.mp. 21 (double* adj blind*).mp. 22 (singl* adj blind*).mp. 23 assign*.mp. 24 allocat*.mp. 25 volunteer*.mp. 26 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 27 12 and 26 28 (exp Animal/ or Nonhuman/ or exp Animal Experiment/) not Human/ 29 27 not 28
Appendix 3. CENTRAL search strategy
#1 MeSH descriptor Gestational Trophoblastic Disease, this term only #2 MeSH descriptor Hydatidiform Mole explode all trees #3 hydatid* near/2 mole* #4 molar near/2 pregnanc* #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Antineoplastic Agents explode all trees #7 MeSH descriptor Antineoplastic Combined Chemotherapy Protocols, this term only #8 MeSH descriptor Chemoprevention, this term only #9 (chemotherap* or chemoprophyla* or chemoprevention) #10 (methotrexate or arnethopterin or dactinomycin or actinomycin D or fluorouracil or etoposide) #11 (#6 OR #7 OR #8 OR #9 OR #10) #12 (#5 AND #11)
Data and analyses
Comparison 1.
Prophylactic chemotherapy versus no prophylactic chemotherapy
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of GTN (overall) | 3 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.24, 0.57] |
| 1.1 Methotrexate prophylaxis | 2 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.24, 0.64] |
| 1.2 Dactinomycin prophylaxis | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.10, 0.73] |
| 2 Incidence of GTN (high‐risk HM only) | 2 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.14, 0.60] |
| 2.1 Methotrexate prophylaxis | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.10, 0.95] |
| 2.2 Dactinomycin prophylaxis | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.10, 0.73] |
| 3 Time to GTN diagnosis | 2 | 33 | Mean Difference (IV, Random, 95% CI) | 28.72 [13.19, 44.24] |
| 3.1 Methotrexate prophylaxis | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 30.80 [12.93, 48.67] |
| 3.2 Dactinomycin prophylaxis | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 22.30 [‐9.05, 53.65] |
| 4 Number of courses of chemotherapy to cure | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Methotrexate prophylaxis | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 1.1 [0.52, 1.68] |
| 5 Mortality rate | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
Analysis 1.4.
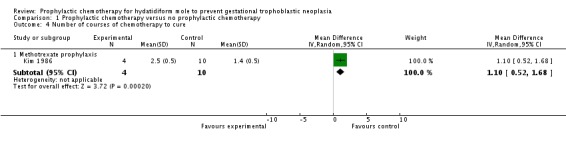
Comparison 1 Prophylactic chemotherapy versus no prophylactic chemotherapy, Outcome 4 Number of courses of chemotherapy to cure.
Analysis 1.5.
Comparison 1 Prophylactic chemotherapy versus no prophylactic chemotherapy, Outcome 5 Mortality rate.
What's new
Last assessed as up‐to‐date: 10 August 2012.
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
History
Protocol first published: Issue 3, 2008 Review first published: Issue 10, 2012
| Date | Event | Description |
|---|---|---|
| 1 April 2015 | Amended | Contact details updated. |
| 24 February 2015 | Amended | Contact details updated. |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
Differences between protocol and review
None.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Kashimura 1986
| Methods | RCT conducted in Japan. Participants recruited between 1963 and 1977. Stated as a prospective study with participants 'selected at random' to receive prophylaxis This may not be a true RCT | |
| Participants | 420 women with molar pregnancy (low and high risk) Excluded women who were referred longer than 3 weeks after evacuation, those who had received other drugs for prophylaxis (see 'Risk of bias' table below), women who had undergone hysterectomy and women diagnosed as having partial mole or hydropic degeneration |
|
| Interventions | Arm 1: methotrexate 10 mg daily (IM or oral) for 7 days, within 3 weeks of evacuation (293 women) Arm 2: no P‐Chem (127 women) Women were followed up weekly with urine hCG measurements |
|
| Outcomes | GTN diagnosed by histology or Ishizuka score (a risk rating system used in Japan); side effects and subsequent pregnancy | |
| Notes | 5‐ to 15‐year follow‐up reported. Time to invasive mole diagnosis was 56.8 days in P‐Chem group and 42.7 days in control group (SD not given; P = 0.6). No attrition occurred for primary outcomes. Only reported adverse effects in the P‐Chem group: 27.3% experienced drug‐related side effects including stomatitis (10.3%), nausea/vomiting (6.8%) and leukopenia (4.4%). Grades of toxicity were not reported but the report states that there were no severe complications or drug‐related deaths. Baseline characteristics were not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | 'Selected at random' suggests that this was not a truly random process. No randomisation ratio was described and yet the 2 groups were very different sizes (293 vs. 127). The authors stated that 39 patients who received other drugs besides methotrexate were excluded from the study; this also suggests a non‐random study design |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Attrition was low for the main outcomes but high for long‐term outcomes such as subsequent pregnancies |
| Selective reporting (reporting bias) | High risk | Baseline characteristics of the 2 groups were not compared/reported |
| Other bias | High risk | More women in the control group were ≥ 40 years old (22% vs. 11%) and progressed to GTN (39% vs. 12%) |
Kim 1986
| Methods | RCT conducted in Korea. Participants recruited between 1978 and 1984 | |
| Participants | 133 women with complete hydatidiform mole (both high and low risk) were randomised into 2 groups, but 62 were excluded (36 lost to follow‐up, 7 had 'insufficient length of follow‐up' and 19 had hysterectomy) and only 71 completed this trial (39 in the treatment group and 32 in the untreated group) | |
| Interventions | Arm 1: methotrexate 1.0 mg/kg/day IM on days 1, 3, 5 and 7 and citrovorum factor rescue 0.1 mg/kg/day IM on days 2, 4, 6 and 8 (39/71 women including 18/31 low‐risk and 21/40 high‐risk women). ERPC was done on the third or fourth day of P‐Chem Arm 2: no treatment other than ERPC (32 women including 13/31 low‐risk and 19/40 high‐risk women) |
|
| Outcomes | Efficacy: incidence of GTN Adverse effects: incidence of gastrointestinal toxicity, myelotoxicity, epithelial toxicity including rash, hair loss and mouth ulcers The number of courses required to achieve remission in cases of GTN Time to GTN diagnosis Subsequent pregnancy |
|
| Notes | Baseline characteristics were similar between the groups, including the proportion of low‐ and high‐risk lesions. ERPC was done on the third or fourth day of P‐Chem. Women were followed up weekly for until hCG was normal for 3 consecutive weeks, then monthly for 6 months, then bimonthly for 6 months, then every 6 months. The mean duration of follow‐up was 19 months (SD 9.7; range 6 to 50). All women were in complete remission at study closure Pregnancy rates after molar pregnancy were similar between the 2 groups (93% vs. 94%) P‐Chem had little effect on the rate of subsequent GTN in the low‐risk group; only 2/31 low‐risk women developed GTN (1 women in each study group) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Of 133 women treated, 62 were excluded from the study (36 were lost to follow‐up, 7 had insufficient length of follow‐up and 19 had a hysterectomy). Therefore the outcome data were extracted from the 71 women (39 in the treatment group and 32 in the untreated group) |
| Selective reporting (reporting bias) | High risk | All the pre‐specified outcomes were reported. However, certain women were excluded from the analyses (those who underwent hysterectomy and those with insufficient follow‐up) therefore the analyses were not by intention‐to‐treat |
| Other bias | High risk | It is unclear on what basis the participants were initially diagnosed as having CM. If prophylaxis was given based on a clinical diagnosis before ERPC, this may have resulted in women with hydropic degeneration or PM being included in the study |
Limpongsanurak 2001
| Methods | RCT conducted in Thailand. Participants were recruited between 1989 and 1994 | |
| Participants | Women diagnosed with high‐risk CM (with histological diagnosis) within 1 week of evacuation of molar tissue. Women were considered 'high risk' if they had at least 1 of the following characteristics: initial serum hCG > 100,000 mIU/mL; uterine size larger than dates; theca lutein cysts > 6 cm; age > 40 years; or associated medical and epidemiological factors including previous GTD, toxaemia, hyperthyroidism, trophoblast embolisation or disseminated intravascular coagulation 60 participants were randomised into 2 groups (30:30) |
|
| Interventions | Arm 1: IV actinomycin D (10 µg/kg) for 5 days, within 1 week of evacuation of molar tissue Arm 2: IV fluids and analgesic drugs for 5 days within 1 week of evacuation of molar tissue |
|
| Outcomes | Efficacy: incidence of GTN Adverse effects: incidence of gastrointestinal toxicity, myelotoxicity, hair loss, mouth ulcers Time to diagnosis of GTN |
|
| Notes | The gestational age at diagnosis of HM was 13.8±3.0 weeks in the intervention group and 13.6±4.2 weeks in the control group. Women were followed up for 1 year with hCG assays every 2 weeks for 3 months, then monthly for 3 months, then every 2 months up to 1 year The diagnosis of GTN was made in all women in the P‐Chem group (4/4) and 12/15 women in the control group according to the following criteria: rising hCG levels for 2 weeks or a plateau for 3 weeks; persistent or recurrent vaginal bleeding with detectable hCG levels; clinical or histological evidence of invasive mole, choriocarcinoma or metastases with persistently high or rising hCG values. Histology was obtained for 3 participants 2 out of 4 women in the P‐Chem group and 3 out of 15 in the control group were lost to follow‐up after diagnosis of GTN, therefore 5 women with GTN received no subsequent treatment and data were insufficient to compare the number of chemotherapy courses received in each group Side effects were reported as percentages and only recorded for the P‐Chem group, as follows: stomatitis (10%), nausea/vomiting (10%), oral ulcers (3.3%) and hair loss (13.3%). All adverse effects were grade 1 except for 2 patients with patchy alopecia (grade 2) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was generated by lot‐drawing (information obtained by e‐mail correspondence with Dr Limpongsanurak) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes (information obtained by e‐mail correspondence with Dr Limpongsanurak) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as double‐blind, but the details of outcome assessment are unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman in the P‐Chem group was lost to follow‐up 1 month after treatment and not included in the primary outcome analysis |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported |
| Other bias | Low risk | Baseline characteristics and risk factors for disease progression were similar between the groups |
CM: complete mole; ERPC: evacuation of retained products of conception; GTD: gestational trophoblastic disease; GTN: gestational trophoblastic neoplasia; hCG: β‐human chorionic gonadotrophin; IM: intramuscular; IV: intravenous; P‐Chem: prophylactic chemotherapy; RCT: randomised controlled trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Geng 2011 | A retrospective study evaluating characteristics and outcomes for 23 women with high‐risk HM who received prophylactic chemotherapy (5‐FU or dactinomycin) |
| Uberti 2006 | A retrospective study evaluating a bolus dose of dactinomycin for prevention of persistent GTD in 29 adolescents with high‐risk molar pregnancy compared with a similar control group of 31 adolescents |
| Uberti 2009 | A retrospective study evaluating the effect of a bolus dose of dactinomycin, given 1 hour before ERPC to women with high‐risk HM, on the rate of malignant transformation to GTN |
5‐FU: 5‐fluorouracil; ERPC: evacuation of retained products of conception; GTD: gestational trophoblastic disease; GTN: gestational trophoblastic neoplasia; HM: hydatidiform mole.
Characteristics of ongoing studies [ordered by study ID]
GOG 0242
| Trial name or title | Second Curettage in Treating Patients With Persistent Non‐Metastatic Gestational Trophoblastic Tumor |
| Methods | Mutlicentre Phase II study (NCT00521118) |
| Participants | Women with histologically confirmed gestational trophoblastic neoplasia (GTN) (complete or partial hydatidiform mole) with no histologically confirmed choriocarcinoma, placental site trophoblastic tumor (PSTT), or epithelioid trophoblastic tumour (ETT) on the first curettage. Persistent, low‐risk disease (based on FIGO/WHO 2002 staging and risk scoring criteria), as defined by 1 of the following criteria: Less than 10% decline in beta‐human chorionic gonadotropin (hCG) levels, based on four consecutive measurements over a 3‐week period (plateau); Greater than 20% rise in beta‐hCG levels, based on three consecutive measurements over a 2‐week period; Beta‐hCG level remains elevated above normal for ≥ 6 months. WHO risk score ≤ 6. Must have a clinically significant elevated beta‐hCG level of > 20 miu/mL. No evidence of metastatic disease beyond the uterus by pelvic examination, pelvic ultrasound, and chest x‐ray. No previously treated, persistent or recurrent GTN (same gestation) that have been treated with chemotherapy |
| Interventions | Patients undergo a second curettage rather than standard treatment (immediate chemotherapy). Patients whose disease has transformed into choriocarcinoma, placental site trophoblastic tumour, or epithelioid trophoblastic tumour (histologically diagnosed at the second curettage) are removed from the study. All other patients undergo weekly beta‐human chorionic gonadotropin (hCG) testing beginning 14 days after the second curettage and continuing until the beta‐hCG level is normal. Patients then undergo further beta‐HCG testing weekly for 4 weeks and then monthly for 5 months. If the level does not regress to normal, or rises, or if metastatic disease is identified, the patient is removed from the study. |
| Outcomes | Frequency of surgical cure, defined a normal beta‐human chorionic gonadotropin (hCG) level documented for 6 consecutive months AND no chemotherapy. Development of choriocarcinoma, placental site trophoblastic tumour (PSTT), or epithelioid trophoblastic tumour (ETT) histologically diagnosed at second curettage. Development of “second persistent” disease, defined as failure to achieve or maintain a normal assay, or a plateau, or a rise in the assay level after second curettage. Frequency and severity of adverse effects of second curettage, specifically uterine operative injury, haemorrhage, and infection (pelvis, fallopian tubes, and ovaries), as assessed by CTCAE version 3.0. |
| Starting date | October 2007 |
| Contact information | Philip J. DiSaia, Gynecologic Oncology Group |
| Notes |
Contributions of authors
Jing Fu, Fan He: protocol development, methodological quality assessment, retrieval of papers, data extraction, data analysis and writing the review.
Lingxia Xie, Hengxi Chen: protocol development, methodological quality assessment, data extraction and revision of the review.
Fang Fang, Taixiang Wu, Hu Lina: protocol development, content expert and revision of the review.
Tess Lawrie: methodology, data extraction, data analysis and writing.
Sources of support
Internal sources
West China Secondary Hospital, Sichuan University, China.
External sources
This review received methodological and statistical support as part of the 10/4001/12 NIHR Cochrane Programme Grant Scheme ‐ Optimising care, diagnosis and treatment pathways to ensure cost effectiveness and best practice in gynaecological cancer: improving evidence for the NHS, UK.
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
- Kashimura Y, Kashimura M, Sugimori H, Tsukamoto N, Matsuyama T, Matsukuma K, et al. Prophylactic chemotherapy for hydatidiform mole: five to 15 years follow up. Cancer 1986;58:624‐9. [DOI] [PubMed] [Google Scholar]
- Kim DS, Moon H, Kim KT, Moon YJ, Hwang YY. Effects of prophylactic chemotherapy for persistent trophoblastic disease in patients with complete hydatidiform mole. Obstetrics and Gynecology 1986;67:690‐4. [DOI] [PubMed] [Google Scholar]
- Limpongsanurak S. Prophylactic actinomycin D for high‐risk complete hydatidiform mole. The Journal of Reproductive Medicine 2001;46:110‐6. [PubMed] [Google Scholar]
References to studies excluded from this review
- Geng S, Feng FZ, Xiang Y, Wan XR, Zhou Y, Zhonghua Fu, et al. Analysis of prophylactic chemotherapy outcome and clinical characteristics in patients of high‐risk hydatidiform mole. Zhonghua Fu Chan Ke Za Zhi 2011;46(1):24‐7. [Chinese] [PubMed] [Google Scholar]
- Uberti EMH, Diestel MCF, Guimaraes FE, Napoli G, Schmid H. Single‐dose actinomycin D: efficacy in the prophylaxis of post molar gestational trophoblastic neoplasia in adolescents with high‐risk hydatidiform mole. Gynecologic Oncology 2006;102:325‐32. [DOI] [PubMed] [Google Scholar]
- Uberti EMH, Fajardo MC, Ferreira SVVR, Pereira MV, Seger RC, Moreira MAR, et al. Reproductive outcome after discharge of patients with high‐risk hydatidiform mole with or without use of one bolus dose of actinomycin D, as prophylactic chemotherapy, during the uterine evacuation of molar pregnancy. Gynecologic Oncology 2009;115:476‐81. [DOI] [PubMed] [Google Scholar]; Uberti EMH, Fajardo MC, Cunha AGV, Rosa MW, Ayub ACK, Graudenz MS, et al. Prevention of post molar gestational trophoblastic neoplasia using prophylactic single bolus dose of actinomycin D in high‐risk hydatidiform mole: a simple, effective, secure and low‐cost approach without adverse effects on compliance to general follow‐up or subsequent treatment. Gynecologic Oncology 2009;114:299‐305. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Gynecologic Oncology Group. Second Curettage in Treating Patients With Persistent Non‐Metastatic Gestational Trophoblastic Tumor. NCT00521118. http://clinicaltrials.gov/ct2/show/NCT00521118?term=GOG‐0242&rank=1.
Additional references
- Alazzam M, Tidy J, Hancock BW, Osborne R, Lawrie T. First line chemotherapy for low risk gestational trophoblastic neoplasia. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD007102.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrijono M, Muhilal M. Prevention of post‐mole malignant trophoblastic disease with vitamin A. Asian Pacific Journal of Cancer Prevention 2010;11:567‐70. [PubMed] [Google Scholar]
- Aziz MF, Kampono N, Moegni EM. Epidemiology of gestational trophoblastic neoplasm at the Dr.Cipto Mandunkusumo Hospital, Jakarta, Indonesia. Advances in Experimental Medicine and Biology 1984;176:165‐75. [DOI] [PubMed] [Google Scholar]
- Bagshawe KD. Risk and prognostic factors in trophoblastic neoplasia. Cancer 1976;38:1373‐85. [DOI] [PubMed] [Google Scholar]
- Bagshawe KD, Dent J, Webb J. Hydatidiform mole in England and Wales 1973‐1983. Lancet 1986;2:673‐7. [DOI] [PubMed] [Google Scholar]
- Bagshawe KD, Lawler SD, Paradinas FJ, Dent J. Gestational trophoblastic tumours following initial diagnosis of partial hydatidiform mole. Lancet 1990;335:1074‐6. [DOI] [PubMed] [Google Scholar]
- Bahar AM, Ashneh MS, Senthilsclvan A. Hydatidiform mole in the elderly: hysterectomy or evacuation. Obstetrics and Gynaecology 1989;29:233‐8. [DOI] [PubMed] [Google Scholar]
- Berkowitz RS, Goldstein DP, Bernstein MR. Natural history of partial molar pregnancy. Obstetrics and Gynecology 1985;66:677‐81. [PubMed] [Google Scholar]
- Berkowitz RS, Goldstein DP, Dubeshter B, Bernstein M. Management of complete molar pregnancy. Journal of Reproductive Medicine 1987;32:634‐9. [PubMed] [Google Scholar]
- Berkowitz RS, Goldstein DP. Gestational trophoblastic disease. Cancer 1995;76:2079‐85. [DOI] [PubMed] [Google Scholar]
- Berkowitz RS, Goldstein DP. Chapter 9: presentation and management of molar pregnancy. In: Hancock BW, Seckl MJ, Berkowitz RS, Cole LA editor(s). Gestational Trophoblastic Disease. 3rd Edition. London: International Society for the Study of Trophoblastic Disease, 2009:249‐76. www.isstd.org/isstd/chapter09_files/GTD3RDCH09.pdf (accessed 21 August 2012). [Google Scholar]
- Berkowitz RS, Goldstein DP. Current management of gestational trophoblastic diseases. Gynecologic Oncology 2009;112:654‐62. [DOI] [PubMed] [Google Scholar]
- Charry RC. Chapter 16: presentation and management of molar pregnancy and gestational trophoblastic neoplasia in Latin America. In: Hancock BW, Seckl MJ, Berkowitz RS, Cole LA editor(s). Gestational Trophoblastic Disease. 3rd Edition. London: International Society for the Study of Trophoblastic Diseases, 2009:407‐19. www.isstd.org/isstd/chapter16.html (accessed 21 August 2012). [Google Scholar]
- Curry SL, Hammond CB, Tyrey L, Creasman WT, Parker RT. Hydatidiform mole: diagnosis, management, and long‐term follow up of 347 patients. Obstetrics and Gynecology 1975;45:1‐8. [PubMed] [Google Scholar]
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
- Fasoli M, Ratti F, Francheschi S, Vecchia C, Pecorelli S, Mangioni C. Management of gestational trophoblastic disease: results of a cooperative study. Obstetrics and Gynecology 1982;60(2):205‐9. [PubMed] [Google Scholar]
- Felemban AA, Bakri YN, Alkharif HA, Altuwaijri SM, Shalhoub J, Berkowitz RS. Complete molar pregnancy clinical trends at King Fahad Hospital, Riyadh, Kingdom of Saudi Arabia. The Journal of Reproductive Medicine 1998;43(1):11‐3. [PubMed] [Google Scholar]
- FIGO Committee on Gynecologic Oncology. Current FIGO staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia. International Journal of Gynecology and Obstetrics 2009;105:3‐4. [DOI] [PubMed] [Google Scholar]
- Fisher R. Chapter 2: genetics. In: Hancock BW, Seckl M, Berkowitz RS, Cole LA editor(s). Gestational Trophoblastic Disease. 3rd Edition. London: International Society for the Study of Trophoblastic Diseases, 2009:6‐48. www.isstd.org/isstd/chapter02.html (accessed 21 August 2012). [Google Scholar]
- Fowler DJ, Lindsay I, Seckl MJ, Sebire NJ. Routine pre‐evacuation ultrasound diagnosis of hydatidiform mole: experience of more than 1000 cases from a regional referral center. Ultrasound in Obstetrics and Gynecology 2006;27:56‐60. [DOI] [PubMed] [Google Scholar]
- Garner EI, Lipson E, Bernstein MR. Subsequent pregnancy experience in patients with molar pregnancy and gestational trophoblastic tumor. Journal of Reproductive Medicine 2002;47(5):380‐6. [PubMed] [Google Scholar]
- Garrett LA, Garner EIO, Feltmate CM. Subsequent pregnancy outcomes in patients with molar pregnancy and persistent gestational trophoblastic neoplasia. Journal of Reproductive Medicine 2008;53:481‐6. [PubMed] [Google Scholar]
- Goldstein DP. Prophylactic chemotherapy of patients with molar pregnancy. Obstetrics and Gynecology 1971;38(6):817‐22. [PubMed] [Google Scholar]
- Goldstein DP. Prevention of gestational trophoblastic disease by use of actinomycin D in molar pregnancies. Obstetrics and Gynecology 1974;43:475‐9. [PubMed] [Google Scholar]
- Goldstein DP, Berkowitz RS, Bernstein MR. Management of molar pregnancy. Journal of Reproductive Medicine 1981;26:208‐12. [PubMed] [Google Scholar]
- Goldstein DP, Berkowitz RS. Prophylactic chemotherapy of complete molar pregnancy. Seminars in Oncology 1995;22(2):157‐60. [PubMed] [Google Scholar]
- Goldstein DP, Berkowitz RS. Current management of gestational trophoblastic neoplasia. Hematology and Oncology Clinics of North America 2012;26:111‐131. [DOI] [PubMed] [Google Scholar]
- Gul T, Yilmazturk A. A review of trophoblastic diseases at the Medical School of Dicle University. European Journal of Obstetrics, Gynaecology and Reproductive Biology 1997;74:37‐40. [DOI] [PubMed] [Google Scholar]
- Hancock BW. Chapter 19: difference in management and treatment: a critical appraisal. In: Hancock BW, Seckl MJ, Berkowitz, Cole LA editor(s). IGestational Trophoblastic Disease. 3rd Edition. London: International Society for the Study of Trophoblastic Diseases, 2009:447‐59. www.isstd.org/isstd/chapter19.html (accessed 21 August 2012). [Google Scholar]
- Hertz R, Li MC, Spencer DB. Effect of methotrexate therapy upon choriocarcinoma and chorioadenoma. Proceedings of the Society of Experimental Biology and Medicine 1956;93(2):361‐6. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Hurteau J. Gestational trophoblastic disease: management of hydatidiform mole. Clinical Obstetrics and Gynecology 2003;46(3):557‐69. [DOI] [PubMed] [Google Scholar]
- Kaye DD. Gestational trophoblastic disease following complete hydatidiform mole in Mulago Hospital, Kampala, Uganda. African Health Sciences 2002;2(2):47‐51. [PMC free article] [PubMed] [Google Scholar]
- Koga K, Maeda K. Prophylactic chemotherapy with Amethopterin for prevention of choriocarcinoma following removal of hydatidiform mole. American Journal of Obstetrics and Gynecology 1968;100(2):270‐5. [Google Scholar]
- Kohorn EI. Chapter 7: the FIGO 2000 staging and risk factor scoring system for gestational trophoblastic neoplasia: a critical analysis. In: Hancock BW, Seckl MJ, Berkowitz RS, Cole LA editor(s). Gestational Trophoblastic Neoplasia. 3rd Edition. London: International Society for the Study of Trophoblastic Disease, 2009:205‐15. www.isstd.org/isstd/chapter07.html (accessed 21 August 2012). [Google Scholar]
- Lee C, Smith HO, Kim SJ. Chapter 3: epidemiology. In: Hancock BW, Newlands ES, Berkowitz RS, Cole LA editor(s). Gestational Trophoblastic Diseases. 3rd Edition. London: International Society for the Study of Trophoblastic Diseases, 2009:49‐96. www.isstd.org/isstd/chapter03.html (accessed 21 August 2012). [Google Scholar]
- Lewis JL, Gore H, Hertig AT. Treatment of trophoblastic neoplasms. With rationale for the use of adjunctive chemotherapy at the time of indicated operation. American Journal of Obstetrics and Gynaecology 1966;98:716. [PubMed] [Google Scholar]
- Palmer JR. Advances in the epidemiology of gestational trophoblastic disease. Journal of Reproductive Medicine 1994;39:155. [PubMed] [Google Scholar]
- Parazzini F, Vecchia LL. Pampollona S. Parental age and risk of complete and partial hydatidiform mole. British Journal of Obstetrics and Gynaecology 1986;93:582‐5. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Vecchia C, Mangili G, Caminiti C, Negri E, Cecchetti G, et al. Dietary factors and risk of trophoblastic disease. American Journal of Obstetrics and Gynecology 1988;158(1):93‐9. [DOI] [PubMed] [Google Scholar]
- Park TK, Kim SN, Lee SK. Analysis of risk factors for postmolar trophoblastic disease: categorization of risk factors and effect of prophylactic chemotherapy. Yonsei Medical Journal 1996;37(6):412‐9. [DOI] [PubMed] [Google Scholar]
- Ratnam SS, Teoh ES, Dawood MY. Methotrexate for prophylaxis of choriocarcinoma. American Journal of Obstetrics and Gynecology 1971;111:1021. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
- Sasaki S, Sasaki Y. Chapter 15: clinical management of trophoblastic diseases in Japan. In: Hancock BW, Seckl MJ, Berkowitz RS, Cole LA editor(s). Gestational Trophoblastic Disease. 3rd Edition. London: International Society for the Study of Trophoblastic Diseases, 2009:398‐406. www.isstd.org/isstd/chapter15.html (accessed 21 August 2012). [Google Scholar]
- Sebire NJ, Lindsay I, Paradinas F. Chapter 4: pathology. In: Hancock BW, Seckl MJ, Berkowitz RS, Cole LA editor(s). Gestational Trophoblastic Disease. 3rd Edition. London: International Society for the Study of Trophoblastic Diseases, 2009:97‐147. www.isstd.org/isstd/chapter04.html (21 August 2012). [Google Scholar]
- Seckl MJ. Chapter 10: presentation and management of persistent gestational trophoblastic disease (GTD) and gestational trophoblastic tumours/neoplasia (GTT/GTN) in the United Kingdom. In: Hancock BW, Seckl MJ, Berkowitz RS, Cole LA editor(s). Gestational Trophoblastic Disease. 3rd Edition. London: International Society for the Study of Trophoblastic Diseases, 2009:277‐98. www.isstd.org/isstd/chapter10.html (accessed 21 August 2012). [Google Scholar]
- Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet 2010;376:717‐29. [DOI] [PubMed] [Google Scholar]
- Smith HO. Gestational trophoblastic disease epidemiology and trends. Clinical Obstetrics and Gynecology 2003;46(3):541‐56. [DOI] [PubMed] [Google Scholar]
- Song H, Wu P. Hydatidiform mole in China: a preliminary survey of incidence of more than three million women. Bulletin of the World Health Organization 1987;65:507‐11. [PMC free article] [PubMed] [Google Scholar]
- Steigrad SJ. Epidemiology of gestational trophoblastic diseases. Best Practice and Research Clinical Obstetrics and Gynaecology 2003;17:837‐47. [DOI] [PubMed] [Google Scholar]
- Takeuchi S. Incidence of gestational trophoblastic disease by regional registration in Japan. Human Reproduction 1987;2:729‐34. [DOI] [PubMed] [Google Scholar]
- Tham B, Everard J, Tidy J, Drew D, Hancock B. Gestational trophoblastic disease in the Asian population of Northern England and North Wales. British Journal of Obstetrics and Gynaecology 2003;110:555‐9. [PubMed] [Google Scholar]
- Tidy J. Chapter 18: the role of surgery in the management of gestational trophoblastic disease. In: Hancock BW, Seckl MJ, Berkowitz RS, Cole LA editor(s). Gestational Trophoblastic Disease. 3rd Edition. London: International Society for the Study of Trophoblastic Diseases, 2009:430‐46. www.isstd.org/isstd/chapter18.html (accessed 21 August 2012). [Google Scholar]


