Abstract
Background
Associations between nursing home residents’ oral health status and quality of life, respiratory tract infections, and nutritional status have been reported. Educational interventions for nurses or residents, or both, focusing on knowledge and skills related to oral health management may have the potential to improve residents’ oral health.
Objectives
To assess the effects of oral health educational interventions for nursing home staff or residents, or both, to maintain or improve the oral health of nursing home residents.
Search methods
We searched the Cochrane Oral Health Trials Register (to 18 January 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2015, Issue 12), MEDLINE Ovid (1946 to 18 January 2016), Embase Ovid (1980 to 18 January 2016), CINAHL EBSCO (1937 to 18 January 2016), and Web of Science Conference Proceedings (1990 to 18 January 2016). We searched ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform for ongoing trials to 18 January 2016. In addition, we searched reference lists of identified articles and contacted experts in the field. We placed no restrictions on language or date of publication when searching the electronic databases.
Selection criteria
Randomised controlled trials (RCTs) and cluster‐RCTs comparing oral health educational programmes for nursing staff or residents, or both with usual care or any other oral healthcare intervention.
Data collection and analysis
Two review authors independently screened articles retrieved from the searches for relevance, extracted data from included studies, assessed risk of bias for each included study, and evaluated the overall quality of the evidence. We retrieved data about the development and evaluation processes of complex interventions on the basis of the Criteria for Reporting the Development and Evaluation of Complex Interventions in healthcare: revised guideline (CReDECI 2). We contacted authors of relevant studies for additional information.
Main results
We included nine RCTs involving 3253 nursing home residents in this review; seven of these trials used cluster randomisation. The mean resident age ranged from 78 to 86 years across studies, and most participants were women (more than 66% in all studies). The proportion of residents with dental protheses ranged from 62% to 87%, and the proportion of edentulous residents ranged from 32% to 90% across studies.
Eight studies compared educational interventions with information and practical components versus (optimised) usual care, while the ninth study compared educational interventions with information only versus usual care. All interventions included educational sessions on oral health for nursing staff (five trials) or for both staff and residents (four trials), and used more than one active component. Follow‐up of included studies ranged from three months to five years.
No study showed overall low risk of bias. Four studies had a high risk of bias, and the other five studies were at unclear risk of bias.
None of the trials assessed our predefined primary outcomes 'oral health' and 'oral health‐related quality of life'. All trials assessed our third primary outcome, 'dental or denture plaque'. Meta‐analyses showed no evidence of a difference between interventions and usual care for dental plaque (mean difference ‐0.04, 95% confidence interval (CI) ‐0.26 to 0.17; six trials; 437 participants; low quality evidence) or denture plaque (standardised mean difference ‐0.60, 95% CI ‐1.25 to 0.05; five trials; 816 participants; low quality evidence). None of the studies assessed adverse events of the intervention.
Authors' conclusions
We found insufficient evidence to draw robust conclusions about the effects of oral health educational interventions for nursing home staff and residents. We did not find evidence of meaningful effects of educational interventions on any measure of residents' oral health; however, the quality of the available evidence is low. More adequately powered and high‐quality studies using relevant outcome measures are needed.
Plain language summary
Education for nursing home staff and/or residents to improve residents' oral health
Review question
We reviewed the evidence about the effectiveness of oral health education for nursing staff or nursing home residents compared to usual care for improving residents' oral health.
Background
Nursing home residents are often unable to carry out proper oral care, which is an important factor in maintaining the health of the mouth, teeth, and gums. Nursing home staff may not be prepared to provide adequate care. Therefore, oral health care education for residents and/or nursing staff may be one strategy to improve this situation.
Study characteristics
We searched for relevant studies that had been conducted up until January 2016 and identified nine trials involving a total of 3253 nursing home residents. The average age of residents across the studies ranged from 78 to 86 years. In all of the studies most of the people taking part had dentures (between 62% and 87%).
The trials evaluated a variety of approaches including educational programmes, skills training, and written information material. Topics included dental issues that were particularly relevant for older people such as care of dentures and covered dental and oral diseases, prevention of oral diseases, dental hygiene tools, and oral health care guidelines. The length of the trials ranged from three months to five years.
Key results
We could not identify a clear benefit of training of nurses and/or residents on residents' dental health as assessed by dental and denture plaque. No study assessed oral health, oral health‐related quality of life or adverse events. As education programmes were not fully described, results do not allow for clear conclusions about the effectiveness or potential harm of specific oral health education interventions in nursing homes.
Quality of the evidence
Overall, there was a low quality of information from the studies regarding all of the results. We conclude that there is a need for clinical trials to investigate the advantages and harms of oral health educational programmes in nursing homes.
Summary of findings
Summary of findings for the main comparison. Oral health education for nursing home staff and residents (with information and practical components) compared to usual care.
| Oral health education for nursing home staff and residents (with information and practical components) compared to usual care | ||||||
|
Patient or population: nursing home residents Settings: nursing homes Intervention: oral health education (with information and practical components) Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Oral health education (with information and practical components) | |||||
| Oral health‐related quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not reported |
| Oral health | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome not reported |
|
Dental plaque Plaque Index, Oral Hygiene Index, Geriatric Simplified Debris Index (all scales scoring 0 to 3) Follow‐up: 3 to 60 months |
Mean dental plaque scores in control groups ranged from 0.29 to 2.18 (0 to 3 scale) | Mean difference 0.04 lower (95% CI 0.26 lower to 0.17 higher) |
See comment | 437 (6 RCTs) | ⊕⊕⊝⊝ low1,2 | Information from 2 further studies was not available for meta‐analysis. A lower score indicates less plaque. |
|
Denture plaque Denture Plaque Index (scoring 0 to 3) and method of Augsburger (scoring 0 to 4) Follow‐up: 3 to 60 months |
See comment | Standardised mean difference
0.60 lower (95% CI 1.25 lower to 0.05 higher) |
See comment | 816 (5 RCTs) | ⊕⊕⊝⊝ low1,2 | Different outcome scales used Information from another study was not available for meta‐analysis. |
|
Gingivitis Gingival Bleeding Index and method of Suomi Follow‐up: 3 to 6 months |
245 (3 RCTs) |
⊕⊕⊝⊝ low1,3 | No meta‐analysis (data not comparable). 3 studies at unclear risk of bias showed inconsistent results. |
|||
|
Denture‐induced stomatitis Method of Budtz‐Jørgensen Follow‐up: 6 to 18 months |
417 (2 RCTs) |
⊕⊕⊝⊝ low1,3 | No meta‐analysis (data not comparable). 2 studies at unclear, in Frenkel 2001, or high risk of bias, in Mojon 1998, showed no apparent differences at short‐term, in Frenkel 2001, or long‐term follow‐up (Mojon 1998). |
|||
|
Caries/root caries Follow‐up: 6 to 18 months |
178 (2 RCTs) |
⊕⊕⊝⊝ low1,3 | 2 studies at unclear, in Frenkel 2001, or high risk of bias, in Mojon 1998, showed no apparent differences at short‐term, in Frenkel 2001, or long‐term follow‐up (Mojon 1998). | |||
| CI: confidence interval; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one level due to serious risk of bias. 2Downgraded one level due to inconsistency (heterogeneity): I2= 66% and 92%. 3Downgraded one level due to inconsistency (clinical heterogeneity).
Background
Description of the condition
The demographic shift in high‐income countries as people live longer has important implications for healthcare services. There are and will be more elderly people with important morbidity and care dependency (Branca 2009), and an increasing number of nursing home residents. Whereas, until recently, most nursing home residents were provided with dentures, now a larger number of residents retain a considerable number of natural teeth (Hopcraft 2012; Porter 2015; Samson 2008). Declining rates of edentulism (having no teeth) have important implications for oral health and oral health management of these residents, as a greater proportion will have teeth at risk of dental caries and periodontal disease. Additionally, high‐quality and complex dental prostheses (i.e. implants, bridges, and removable dental prostheses) must be maintained. Nursing home residents are often unable to carry out adequate oral care, such as optimal removal of dental plaque. Continuous preventive and curative oral health care provided by trained staff therefore seems increasingly important. In nursing homes, nursing staff and other caregivers play a crucial role in the provision of daily oral health care (Porter 2015), as well as in initiating and organising routine prophylaxis and treatment by dental professionals (Nitschke 2010).
In the past, it was reported that nursing staff frequently do not acknowledge the importance of oral health and lack knowledge about how to achieve it (Glassman 1994). Some more recent studies have suggested knowledge about oral hygiene is still inadequate among nursing staff (Jablonski 2009; Wardh 2012), and that there is a large discrepancy between the proposed need for assistance with daily oral hygiene among nursing home residents and the actual assistance provided by nursing staff (Forsell 2009). In addition to dependence, some people with dementia exhibit unco‐operative or disruptive behaviour during helping interactions. These behaviours range from mild (e.g. turning the head away) to extreme resistance (e.g. hitting the nursing staff) (Jablonski 2009). This so‐called care‐resistant behaviour is a common phenomenon encountered by nursing staff during the provision of oral care (Frenkel 1999; Jablonski 2011).
Measures of poor oral health (e.g. gingivitis, caries, and chewing difficulties), along with discomfort and pain, have been reported for nursing home residents in different countries (De Visschere 2006; Gluhak 2010; Hopcraft 2012; Simunković 2005; Wyatt 2002). Dental plaque generally leads to an increased risk for dental caries, gingivitis, and periodontitis, as well as other infections in the oral cavity.
In addition, poor oral hygiene and poor dental status among elderly people are associated with reduced quality of life (Porter 2015; Walker 2007). Elderly people in day care have shown considerably higher levels of oral health problems compared to community‐dwelling elderly (Walker 2007), due to difficulties in maintaining a sufficient level of oral hygiene and in accessing professional dental care (Forsell 2009). Two‐thirds of elderly people living in residential facilities use dental services only in the case of dental problems and on demand (De Baat 1993; Isaksson 2007). In a British study, 70% of residents had not seen a dentist for more than five years (Frenkel 2000).
The accumulation of micro‐organisms on teeth and denture surfaces, caused by lack of oral hygiene, may influence residents' general health by causing pneumonia, arteriosclerosis, and infection‐related disorders (Azarpazhooh 2006; Desvarieux 2003; Scannapieco 2003; Shay 2002). Evidence from randomised controlled trials (RCTs) suggests positive preventive effects of oral care on respiratory tract infection and pneumonia in nursing home residents (El‐Rabbany 2015; Sjögren 2008).
Furthermore, poor oral health has been associated with involuntary weight loss (Mojon 1999; Sheiham 2001). Institutionalised elderly people who suffer from oral discomfort, problems with chewing or swallowing, compromised dentition, or poorly fitted dentures have a higher incidence of nutritional deficiency (Saarela 2014; Saunders 2007).
Consequently, interventions to improve oral health seem warranted, as they possibly yield a number of positive effects including improved nutritional status and health‐related quality of life (Naito 2010).
Description of the intervention
In the context of this review, we have defined oral health‐related educational interventions as programmes that facilitate knowledge and skills acquisition in nursing home staff and/or residents to maintain or improve oral health, using a variety of formats (e.g. lectures, demonstrations, practical education). Programmes may target nursing home residents, staff, or both and may be provided to individuals or groups.
Interventions are usually complex, consisting of several different components. Three commonly included components included are:
theoretical education for staff or residents, or both aiming at improving knowledge of oral health and oral health care;
staff training (practical education) on examination and cleansing of the mouth or dentures, or both of residents; and
oral hygiene skills training for residents.
How the intervention might work
In general, the goal of educational interventions is to improve oral and dental health of residents by increasing knowledge and skill levels of nursing home staff or nursing home residents, or both.
Interventions targeting nursing home residents, especially oral hygiene skills training, may foster the ability to self perform dental care and thus decrease the need for assistance. There may also be direct effects of education on residents' attitudes as well as on quality of life.
Interventions targeting nursing home staff will strengthen staff expertise in oral care for residents. Furthermore, staff attitude and self efficacy will be influenced, leading (together with improved knowledge and skills) to improved oral health‐related behaviour and, as a consequence, improved oral health outcomes for residents.
Why it is important to do this review
The Cochrane Oral Health Group undertook an extensive prioritisation exercise in 2014 to identify a core portfolio of titles that were the most clinically important ones to maintain on the Cochrane Library (Worthington 2015). This review was identified as a priority title by the dental public health expert panel (Cochrane OHG priority review portfolio).
At the time of protocol preparation, there were no systematic reviews available summarising the effects of providing oral healthcare education to nursing home staff and/or residents. In 2014, a systematic review on oral healthcare education was published, which included two RCTs, one non‐randomised study, and three cross‐sectional studies (De Lugt‐Lustig 2014). The authors concluded that oral healthcare education for nursing staff may have a positive effect on attitudes and oral healthcare knowledge of nurses in nursing homes and on oral hygiene of residents. Meta‐analyses by Wang 2015 including one RCT and four before‐and‐after studies also found limited evidence that education for caregivers is effective in improving oral health. However, both reviews only focused on educational interventions for nursing staff or caregivers. De Lugt‐Lustig 2014 disregarded any educational interventions considering nursing home residents as recipients, whereas Wang 2015 excluded education for residents only. In addition, a further systematic review, Weening‐Verbree 2013, which included a broad range of study designs, concluded that knowledge, self efficacy, and facilitation of behaviour are determinants that are used in successful implementation strategies. Taking into account the heterogeneity of studies, the authors were not able to recommend a single strategy or combination of strategies for improving oral health in institutionalised elderly. Furthermore, they highlighted the importance of considering contextual factors (e.g. setting) when choosing implementation strategies. A high‐quality systematic review considering the growing number of RCTs published in recent years and describing the components of interventions was therefore warranted. This systematic review may impact the implementation of different approaches and trigger the development of new interventions on the basis of current best evidence.
Objectives
To assess the effects of oral health educational interventions for nursing home staff or residents, or both, to maintain or improve residents’ oral health.
To describe the components of the complex interventions used in the included studies.
Methods
Criteria for considering studies for this review
Types of studies
We included individually randomised controlled trials (RCTs) or cluster‐RCTs with a follow‐up period of at least two weeks including groups of nursing home staff or residents, or both allocated either to:
a programme aiming to maintain or improve oral health of nursing home residents by one or more oral health educational and/or oral hygiene promotion interventions (the intervention group); or
(optimised) regular dental care or any other oral healthcare intervention (the control group). Optimised care means that the control group receives an additional component in addition to usual standardised care, e.g. provision of dental care auxiliaries or informative presentations.
We considered additional publications on development and piloting of complex interventions as well as process evaluations, without focusing on specific study designs, in order to capture single components of the complex interventions and contextual factors.
Types of participants
We considered male and female residents living in facilities providing supervision or nursing care for the elderly (e.g. nursing homes or long‐term care facilities) for inclusion. The majority of residents in a study had to be over the age of 64 years, or the mean age had to be at least 65 years. We also included nursing staff working in these facilities or a combination of residents and nursing staff. We included all participants involved in the primary studies (i.e. irrespective of residents' oral health status or qualifications of the nursing staff).
Types of interventions
We included any trial of an intervention or group of interventions where participants or clusters were allocated to receive an oral health education programme versus (optimised) usual oral health care, or any other oral healthcare intervention.
Oral health education programmes include either direct‐to‐staff programmes, direct‐to‐resident programmes (e.g. oral hygiene promotion or skills training), or a combination of both. We expected approaches to vary, for example in terms of frequency, length, and content, with the educational content likely to include some or all of the following: oral health, oral diseases and impact on general health, diet, oral hygiene measures, best oral care practices for elderly people with natural dentition and dentures, oral hygiene promotion, and skills training.
We expected some interventions to be designed as complex interventions comprising more than one of the components outlined above. Following the 'framework for design and evaluation of complex interventions', it may not be possible to extract the effective or ineffective components of the interventions (Campbell 2000; Craig 2008), but components of included programmes were collected and described in detail as suggested by Lenz 2007.
We considered interventions without educational or oral hygiene promotion components only as controls. In addition, we considered sole organisational interventions aiming to change organisational policies, for example through introduction of practice guidelines, oral health co‐ordinators, regular visits by a dentist or dental hygienist for professional oral care and examination, or regular visits of residents in dental surgeries, only as controls. The same applied to exclusive oral hygiene interventions (e.g. the provision of chemical topical interventions and mechanical auxiliaries).
Types of outcome measures
Primary outcomes
Oral health‐related quality of life: measured by instruments such as Oral Health Impact Profile (OHIP) or OHIP‐14 (Slade 1994; Slade 1997), Geriatric/General Oral Health Assessment Index (GOHAI) (Atchison 1990), Dental Impact Profile (DIP) (Strauss 1993).
Oral health: oral health was defined as a functional, structural, aesthetic, physiologic, and psychosocial state of well‐being assessed by an instrument such as Brief Oral Health Status Examination (BOHSE) or Oral Health Assessment Tool (OHAT) that covers different oral hygiene categories (Chalmers 2005; Kayser‐Jones 1995).
-
Dental health: dental health comprises only one aspect of oral health, such as:
dental or denture plaque, or both: measured by plaque scores and denture cleanliness scores (scales) such as Plaque Index (Silness 1964), Mucosal‐Plaque Index (MPS) (Henriksen 1999), or Denture Plaque Index (Wefers 1999);
gingivitis or denture‐induced stomatitis: measured by instruments such as Gingival Bleeding Index (GBI) (Ainamo 1975), Gingival Index (Löe 1967), or Community Periodontal Index (Benigeri 2000); or
caries, incidence of new caries.
Secondary outcomes
Nutritional status (e.g. body weight, Body Mass Index (BMI)).
Incidence of respiratory diseases and pneumonia.
Adverse effects of the interventions.
Intermediate outcomes
Oral health‐related knowledge of staff or residents, or both: measured by any instruments used in the included studies (e.g. questionnaire or interview).
Oral health‐related attitude and behaviour of staff or residents, or both: measured by any instruments used in the included studies (e.g. questionnaire or interview).
Search methods for identification of studies
We developed detailed search strategies for each database to identify studies for inclusion in the review. These were based on the search strategy developed for MEDLINE Ovid but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free‐text terms and was linked with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying RCTs in MEDLINE: sensitivity‐maximising version (2008 revision) as referenced in Section 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2011). Details of the MEDLINE search are provided in Appendix 3. The searches of EMBASE and CINAHL were linked to Cochrane Oral Health's filters for identifying RCTs.
Electronic searches
We searched the following electronic databases:
Cochrane Oral Health Trials Register (to 18 January 2016) (see Appendix 1);
Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 12) in the Cochrane Library (searched 18 January 2016) (see Appendix 2);
MEDLINE Ovid (1946 to 18 January 2016) (see Appendix 3);
Embase Ovid (1980 to 18 January 2016) (see Appendix 4);
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; 1937 to 18 January 2016) (see Appendix 5);
Web of Science Conference Proceedings (1990 to 18 January 2016) (see Appendix 6).
We placed no restrictions on language or date of publication when searching the electronic databases.
Searching other resources
We searched the following databases for ongoing trials:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 18 January 2016) (see Appendix 7);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 18 January 2016) (see Appendix 8).
We examined the reference lists of published reviews and contacted experts in the field to identify unpublished or ongoing studies. We did not perform a separate search for adverse effects of interventions, considering the adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Two review authors (MA and RK) independently screened the titles and abstracts of citations identified by the search and independently assessed articles for inclusion according to the above criteria. We resolved disagreements by discussion.
Data extraction and management
Pairs of review authors (MA, RK, SK) independently extracted data using a piloted standardised data collection sheet. These data were entered into Review Manager 5 software (RevMan 2014). The review authors were not blinded to authors of included studies. The two review authors resolved disagreements by discussion or, if necessary, by consulting a third review author in order to reach consensus. Data were sought per participant or randomised cluster (nursing home) on all outcome measures of interest for all assessment points (including baseline). We extracted data for characteristics of participants, baseline data, interventions, duration of intervention, length of follow‐up, outcome measures, and adverse events.
We retrieved data on research processes of the development, piloting, and evaluation of the complex interventions on the basis of Criteria for Reporting the Development and Evaluation of Complex Interventions in healthcare: revised guideline (CReDECI 2) (Möhler 2015). Criteria comprise developmental details like the description and intensity of the components, feasibility, and piloting, as well as evaluation of the complex intervention. We also extracted data on the fidelity of the intervention implementation.
If, as was frequently the case, no sufficient information on the above‐mentioned issues was available from included publications, we attempted to obtain additional information by contacting authors of the primary studies (e.g. by asking about related publications). Following earlier suggestions (Lenz 2007), we performed additional extra searches for publications related to included studies.
Assessment of risk of bias in included studies
Our assessment of risk of bias followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two review authors (MA, RK) independently assessed and scored studies in order to identify any potential sources of systematic bias through selection bias, performance bias, attrition bias, or detection bias as recommended in the Cochrane Handbook addressing six domains (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and other issues). We assigned a judgement concerning the related risk of bias for each domain, either 'low risk', 'high risk', or where insufficient information was available to make a judgement, 'unclear risk'. In addition, we assessed recruitment bias for cluster‐randomised trials, that is whether individuals were recruited after clusters had been randomised. We completed a 'Risk of bias' table for each included study and presented the results graphically.
Measures of treatment effect
For continuous data (e.g. plaque indices), we used the mean difference (MD) and its corresponding 95% confidence interval (CI) if the same instrument was used, or the standardised mean difference (SMD) if different instruments were used for the same outcome measure. For dichotomous data (e.g. incidence of respiratory disease), we used risk ratio (RR). We sought and recorded the absolute numbers in each group and the numbers experiencing the outcome of interest.
Unit of analysis issues
For each study we considered whether groups of individuals were randomised in clusters or individually, whether individuals underwent more than one intervention, and whether there were multiple observation times for the same outcome. As results from more than one time point per study cannot be combined in meta‐analysis, depending on the reported follow‐up periods of included studies, we performed subgroup analyses for studies with short‐term and long‐term follow‐up (Higgins 2011), only using the longest. For cluster RCTs we checked whether results were cluster‐adjusted. For combined meta‐analyses of individual and cluster‐RCTs, we estimated 'effective sample sizes' for cluster‐RCTs as described in the Cochrane Handbook for Systematic Reviews of Interventions (Sections 16.3.4 to 16.3.7) (Higgins 2011). We aimed to use intraclass correlation coefficients (ICC) as reported in included studies. As no ICCs were reported in included studies, or values seemed unrealistically high, we considered a conservative estimate of ICC = 0.05 based on experience from studies on caries (personal communication with Cochrane Oral Health).
Dealing with missing data
Where data were missing from the published report of a trial, we contacted authors to obtain the data and to clarify any uncertainties. Analyses included only available data (ignoring missing data). However, we used methods for estimating missing standard deviations if necessary (Higgins 2011). Otherwise, we did not undertake any imputation techniques or use statistical methods to account for missing data.
Assessment of heterogeneity
We assessed heterogeneity statistically and quantified it among trials included in each analysis using the I² statistic. We followed the guidance of the Cochrane Handbook to interpret the I² statistic, with I² greater than 50% representing substantial heterogeneity and greater than 75% representing considerable heterogeneity (Higgins 2011). To assess clinical heterogeneity, we analysed all studies in terms of interventions, participants, and outcomes.
Assessment of reporting biases
In order to minimise the risk of publication bias, we performed comprehensive searches in multiple databases, including searching for unpublished studies. Due to the small number of included trials, we did not use a funnel plot to assess the likelihood of publication bias.
Data synthesis
We grouped studies according to interventions. For each intervention type, we performed meta‐analyses if we considered the studies to be reasonably clinically and methodologically homogeneous. We used random‐effects models for all analyses.
Subgroup analysis and investigation of heterogeneity
We conducted the following subgroup analyses for the outcomes dental and denture plaque:
intervention recipient (nursing staff only versus nursing staff and residents);
follow‐up period (short term versus long term);
education in the context of guideline implementation versus education without guideline implementation.
Sensitivity analysis
We performed no sensitivity analyses.
Presentation of main results
We have displayed results in a 'Summary of findings' table for the primary outcomes, using GRADEpro software (GRADE 2014), according to the methods outlined in the Cochrane Handbook (Section 11.5) (Higgins 2011). We assessed the quality of the evidence with reference to overall risk of bias of included studies, directness of the evidence, consistency of results, and precision of estimates. We categorised the quality of the evidence for each of the primary outcomes as high, moderate, low, or very low.
Results
Description of studies
See Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
Results of the search
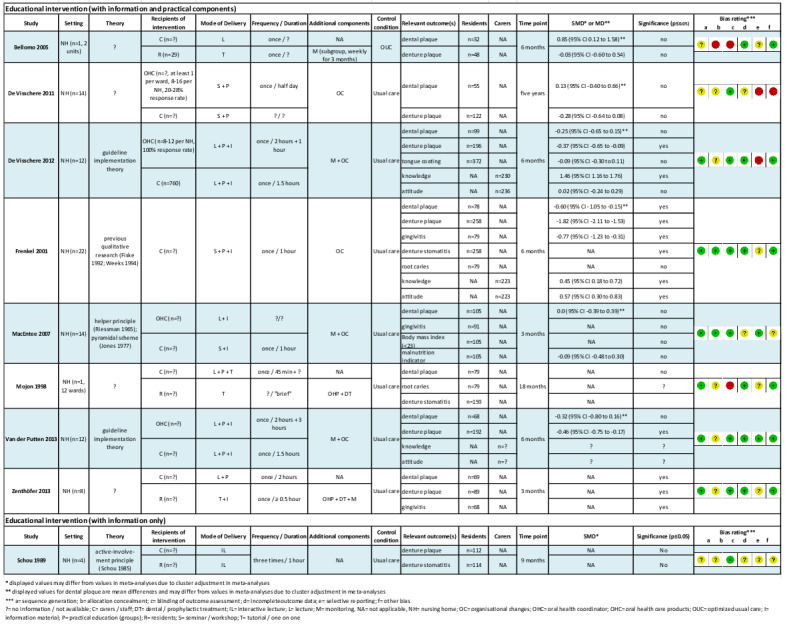
We screened a total of 1454 abstracts for inclusion (Table 2; Figure 1), and assessed 37 publications in full text. Eighteen publications reporting nine trials fulfilled the eligibility criteria (Bellomo 2005; De Visschere 2011; De Visschere 2012; Frenkel 2001; MacEntee 2007; Mojon 1998; Schou 1989; Van der Putten 2013; Zenthöfer 2013).
1. Search results.
| Database | First search date | Records retrieved (excl. duplicates) | Top‐up search | Records retrieved (excl. duplicates) | Top‐up search | Records retrieved (excl. duplicates) |
| Cochrane Oral Health Trials' Register | 31 July 2013 | 567 | 27 January 2015 | 123 | 18 January 2016 | 51 |
| CENTRAL via the Cochrane Library | 31 July 2013 | 164 | 27 January 2015 | 29 | 18 January 2016 | 10 |
| MEDLINE via OVID | 31 July 2013 | 509 (with RCT filter) | 27 January 2015 | 42 (with RCT filter) | 18 January 2016 | 33 (with filter) |
| Embase via OVID | 31 July 2013 | 214 (with RCT filter) | 27 January 2015 | 51 (with RCT filter) | 18 January 2016 | 18 (with filter) |
| CINAHL via EBSCO | 31 July 2013 | 94 (with RCT filter) | 27 January 2015 | 11 (with RCT filter) | 18 January 2016 | 8 (with filter) |
| Web of Science Conference Proceedings | 31 July 2013 | 151 | 27 January 2015 | 6 | 18 January 2016 | 3 |
| ClinicalTrials.gov | 31 July 2013 | 22 | 27 January 2015 | 23 | 18 January 2016 | 24 |
| WHO International Clinical Trials Registry Platform | Not performed | ‐ | 27 January 2015 | 3 | 18 January 2016 | 0 |
RCT: randomised controlled trial WHO: World Health Organization
1.
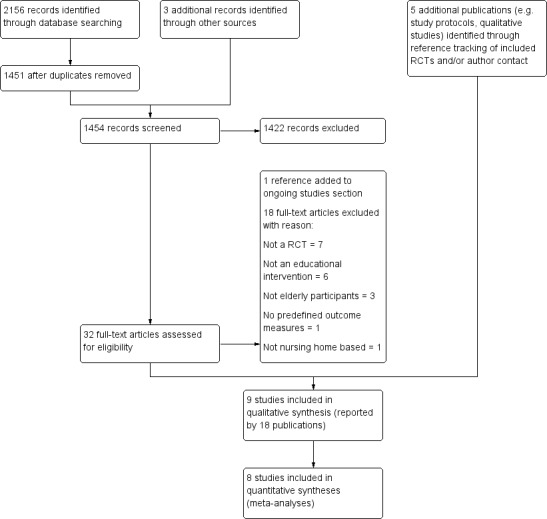
Study flow diagram
Included studies
We included two individual RCTs, Bellomo 2005 and Zenthöfer 2013, and seven cluster‐RCTs (De Visschere 2011; De Visschere 2012; Frenkel 2001; MacEntee 2007; Mojon 1998; Schou 1989; Van der Putten 2013), with a total of 3253 nursing home residents as participants.
Funding sources
All but two studies stated information about any kind of funding, for example financial or material support (Bellomo 2005; Schou 1989). One of these studies stated that they received funding for data collection from a commercial source (De Visschere 2011). One study received support from a commercial source in the form of free oral healthcare material (Zenthöfer 2013). Two studies obtained grants from non‐commercial sources (Frenkel 2001; MacEntee 2007). The remaining three studies received grants from non‐commercial sources as well as free oral hygiene products from commercial sources (De Visschere 2012; Mojon 1998; Van der Putten 2013).
Setting
All studies were based in nursing homes. However, in Schou 1989, the included institutions were described as "institutions for elderly". MacEntee 2007 only recruited intermediate‐care residents. In Zenthöfer 2013, participants were recruited from institutions for elderly (including assisted accommodation). All of the studies except MacEntee 2007 were conducted in Europe.
Recipients of the educational intervention
In five studies, the target groups of the educational interventions were nursing home staff (De Visschere 2011; De Visschere 2012; Frenkel 2001; MacEntee 2007; Van der Putten 2013). More precisely, two studies aimed to include solely nursing staff (Frenkel 2001; MacEntee 2007), and three studies focused on managerial and nursing staff (De Visschere 2011; De Visschere 2012; Van der Putten 2013). Four studies examined interventions that comprised components for both nursing home staff and residents (Bellomo 2005; Mojon 1998; Schou 1989; Zenthöfer 2013).
The mean resident age ranged from 78.3 to 86.0 years across studies. Most participants were women (more than 66% in all studies). The proportion of residents with dental prostheses ranged from 62.1% to 87.3% across studies. Mean number of natural teeth varied between 4.7 and 17.4 at baseline, and the proportion of edentulous residents had a wide range, from 32.4% to 89.9% (see Characteristics of included studies). Two studies reported information about cognitive status or mental health, or both (De Visschere 2012; Van der Putten 2013). In four studies, cognitive impairment or dementia, or both was an exclusion criterion (Frenkel 2001; MacEntee 2007; Mojon 1998; Zenthöfer 2013). In De Visschere 2012, cognitive impairment tested by the Mini Mental State Examination (MMSE less than 26) was present in 86.5% of the sample. In Van der Putten 2013, 47% of participants in the intervention group and 58% in the control group had a diagnosis of dementia.
Some information on characteristics of participating nursing staff was reported in all studies except Bellomo 2005 and Schou 1989. The two studies examining knowledge and attitudes of nursing staff reported the most detailed description of the characteristics of the employed nursing staff (De Visschere 2012; Frenkel 2001). In both studies, approximately 95% of nursing staff were female. In Frenkel 2001, the median length of work experience was four years, and about 67% reported attending a dentist at least once a year. In De Visschere 2012, nursing staff had been employed for a mean of 13.5 years; 62% were nurses and 38% auxiliary nurses. About 78% reported attending a dentist at least once a year. In addition, this study provided detailed information about the amount and numbers of staff members with previous education or skills training in oral health care. Five studies included nurses as well as nurse assistants (De Visschere 2011; De Visschere 2012; Mojon 1998; Van der Putten 2013; Zenthöfer 2013). Five studies reported the number or proportion of intervention group staff attending the educational sessions. In Frenkel 2001, 65.6% of caregivers attended the educational session. In MacEntee 2007, the proportion of care‐aides attending the educational session in the intervention group and control group was 15% and 22%, respectively. De Visschere 2012 reported a 100% response rate (8 to 12 people per home). In Mojon 1998, the education course was attended by 76% of the caregivers. In Zenthöfer 2013, the number ranged from 20 to 40 people per nursing home.
Duration of follow‐up
Follow‐up for six studies ranged from three months, in Bellomo 2005, MacEntee 2007, and Zenthöfer 2013, to six months (De Visschere 2012; Frenkel 2001; Van der Putten 2013); we categorised this as short term. Follow‐up for three studies ranged from nine months, in Schou 1989, to five years (De Visschere 2011); we categorised this as long term.
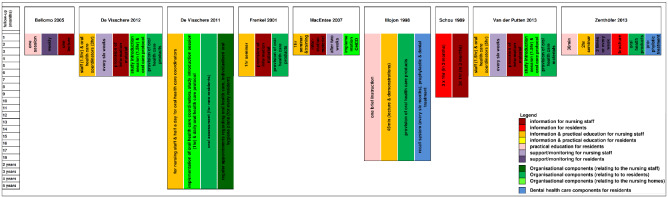
Description of interventions
Studies differed in terms of intervention components as well as target groups. In all studies, the oral health educational intervention consisted of more than one component (see Figure 2). In all but one study (Bellomo 2005), nursing staff participated in one or more educational sessions. In eight studies, the intervention comprised education including theoretical information and skills training (Bellomo 2005; De Visschere 2011; De Visschere 2012; Frenkel 2001; MacEntee 2007; Mojon 1998; Van der Putten 2013; Zenthöfer 2013). In one study (Schou 1989), the educational intervention focused solely on theoretical information.
2.
Intervention components
Educational interventions with information and practical components (n = 8)
In Bellomo 2005, the long‐term care home residents received occupational therapy instruction on tooth and denture brushing. In addition, a subgroup of residents needing assistance were monitored weekly and, if necessary, re‐educated by an occupational therapist. De Visschere 2011 implemented a daily oral hygiene protocol. The implementation process included a half‐day theoretical and practical educational session for oral health co‐ordinators who had to educate the other nursing staff and prepare individualised oral hygiene plans for all residents. In two studies, the supervised implementation of an oral health guideline for older people in long‐term care institutions included theoretical and practical education sessions ranging from one to two hours for several groups of nursing home staff, using a train‐the‐trainer concept, and a daily oral healthcare protocol (De Visschere 2012; Van der Putten 2013). In Frenkel 2001, care assistants were educated by an experienced health promoter in a one‐hour session that consisted of a theoretical section on the role of plaque, oral diseases and a practical section with oral hygiene skills training. MacEntee 2007 used a "pyramidal education" approach to train the nursing staff of the intervention homes. Initially, a permanent member of the nursing staff was trained and installed as a nursing educator, who then trained the care‐aides in a single one‐hour seminar, which included a theoretical section (photographs and texts) and demonstrations of how to examine and clean the mouth. In addition, the nurse educators had to manage the oral health care provided by the care‐aides and were supported by the dental hygienist on request. In Mojon 1998, nursing staff took part in an interactive lecture (45 minutes) with slides and demonstration of brushing techniques. Every nurse or nurse aide received personalised oral hygiene instructions for their residents from a dental hygienist. Also, residents received brief (personalised) oral hygiene instructions from a dental hygienist. In addition, for residents, dental or prophylactic treatment including scaling at the start of the study and the implementation of a recall system were applied in the intervention group. In Zenthöfer 2013, the basic intervention for the three intervention groups consisted of a two‐hour session (PowerPoint presentation, a film) with dental demonstration models to train nursing staff. Residents received instruction and training on oral hygiene skills according to their specific needs. The residents of two of the three intervention groups were supported either by a dentist (re‐instruction and motivation after four and eight weeks) or by caregivers (provision of help twice a week using a standardised procedure). In addition, professional cleaning of dentures and teeth was performed for all intervention groups at the beginning.
Additional informational material on oral health and hygiene was provided to trained staff in four studies (De Visschere 2012; Frenkel 2001; MacEntee 2007; Van der Putten 2013) and to residents in one study (Zenthöfer 2013). Toothbrushes and/or other oral healthcare products were provided for intervention group residents in four studies (De Visschere 2012; Frenkel 2001; Mojon 1998; Van der Putten 2013).
Educational interventions with information only (n = 1)
Schou 1989 only used education without further practical components. Nursing staff and residents took part in a dental health education programme using the "active involvement principle". Each intervention group (one for residents only, one for nursing staff only, and a combined one) met three times for one hour in a monthly interval to talk about dental health and individual experiences with oral health prevention procedures. Sessions were conducted by a dental hygienist who planned the content of the sessions in co‐operation with the participants.
Theoretical basis for intervention
Five studies provided at least some information on the interventions’ theoretical background. MacEntee 2007 based the intervention on a "pyramidal scheme" that evolved from the "helper principle" (Jones 1977; Riessman 1965). Schou 1989 used the "active involvement principle" for the dental education programme, which had been examined with positive results in another population (Schou 1985). Frenkel 2001 did not mention underlying theories, but based the intervention on previous research and her own qualitative study (Frenkel 1999). Two studies used a guideline implementation theory (De Visschere 2012; Van der Putten 2013). In four studies, no theoretical basis was mentioned (Bellomo 2005; De Visschere 2011; Mojon 1998; Zenthöfer 2013).
Content
Instructions and training of oral hygiene skills for residents included brushing techniques (tooth and dentures) (Bellomo 2005; Mojon 1998; Zenthöfer 2013), and in some cases also handling of tooth/interdental space brushes, and advice on other auxiliaries, for example mouth rinses (Zenthöfer 2013). Training or demonstrations for nursing staff covered the same aspects (De Visschere 2011; De Visschere 2012; Frenkel 2001; MacEntee 2007; Van der Putten 2013).
The theoretical sessions covered the following topics:
specifics of gerodontology/geriatric dentistry (e.g. MacEntee 2007; Zenthöfer 2013);
dental and oral diseases (e.g. Frenkel 2001; MacEntee 2007; Zenthöfer 2013);
prevention of oral diseases (e.g. Mojon 1998);
dental hygiene auxiliaries and handling (e.g. Frenkel 2001; MacEntee 2007; Mojon 1998; Zenthöfer 2013);
handling of removable dentures (e.g. Zenthöfer 2013);
oral healthcare guideline or daily oral healthcare protocol, or both (De Visschere 2011; De Visschere 2012; Van der Putten 2013).
Educators
The educational sessions for nursing staff were given by dentists (Zenthöfer 2013), dental hygienists (MacEntee 2007; Mojon 1998; Schou 1989), trained nursing staff (so‐called oral health co‐ordinators, ward oral healthcare organisers, nurse educators) (De Visschere 2011; De Visschere 2012; MacEntee 2007; Van der Putten 2013), and health promoters (Frenkel 2001). In studies that used a pyramidal or train‐the‐trainer concept, a combination of the aforementioned professions was involved in the educational components (De Visschere 2011; De Visschere 2012; MacEntee 2007; Van der Putten 2013).
Oral hygiene education or skills training for residents was provided by different professions including dental hygienists and occupational therapists (Bellomo 2005; Schou 1989; Zenthöfer 2013). Instructions on oral hygiene to nursing home residents were provided individually (Mojon 1998; Zenthöfer 2013).
Presentation mode
Seven studies made use of more than one presentation mode for educational sessions. Four studies combined theoretical and practical sections for the educational sessions (Frenkel 2001; MacEntee 2007; Mojon 1998; Zenthöfer 2013). Three studies used a combination of oral presentations, lectures, PowerPoint presentations, and practical sessions of differing length (De Visschere 2011; De Visschere 2012; Van der Putten 2013).
Duration and frequency
Not all studies specified the duration and frequency of the educational interventions or their different components.
Zenthöfer 2013 reported the duration of oral health instructions or skills training for nursing home residents as 30 minutes. In Schou 1989, oral health education for residents comprised three one‐hour sessions at monthly intervals. Re‐instruction or re‐education for residents took place weekly in Bellomo 2005. The frequency in Zenthöfer 2013 varied between the two intervention groups: residents received re‐instruction twice a week or after four and eight weeks.
The reported session duration for nursing staff ranged from 45 minutes, in Mojon 1998, to three hours (Schou 1989). The length for the training sessions for oral health co‐ordinators (train‐the‐trainer) varied from three to five hours and was not reported in two studies. The oral health co‐ordinators were supported by a dental hygienist two weeks after study start, in MacEntee 2007, or every six weeks (De Visschere 2012; Van der Putten 2013).
Control groups
One study included a control intervention that was similar in time and effort to the active intervention (Bellomo 2005). In MacEntee 2007, the control group received the same seminar (with photographs, texts, demonstrations), but education was provided by the dental hygienist. In the other studies (De Visschere 2011; De Visschere 2012; Frenkel 2001; Mojon 1998; Schou 1989; Van der Putten 2013; Zenthöfer 2013), no intervention was offered to the control group. Four studies specified usual care. In two of these studies, usual care implied oral health care according to the unsupervised implementation of a guideline (De Visschere 2012; Van der Putten 2013). De Visschere 2011 used a control group with residents receiving usual oral care in control homes and another group with residents living in intervention homes, but who did not receive the intervention, and in Mojon 1998, usual care meant that prior to the study no oral hygiene care was provided by the caregivers on a regular basis; cleaning of the teeth only took place if requested by the dentist; and oral care was provided by a dentist only at request of the resident, family, or caregivers.
Measured outcomes
Oral health‐related quality of life
None of the studies reported this outcome.
Oral health
None of the studies reported this outcome.
Dental health
This includes dental or denture plaque, inflammation of oral mucosa, and caries. The measures assessed and instruments used are shown in Table 3.
2. Summary of dental health outcomes assessed.
| Dental plaque | Denture plaque | Gingivitis | Caries | |
| Bellomo 2005 | PI | Index for plaque accumulation (Denture Plaque Index) by Ambjørnsen 1982 | Not assessed | Not assessed |
| De Visschere 2011 | PI (PO) | Method of Augsburger 1982 (PO) | Not assessed | Not assessed |
| De Visschere 2012 | PI (PO) | Method of Augsburger 1982 (PO) | Not assessed | Not assessed |
| Frenkel 2001 | OHI‐S (PO) | Method of Augsburger 1982 (PO) | Gingivitis (Suomi 1968) (PO) Denture‐induced stomatitis (Budtz‐Jørgensen 1970) (PO) |
Root caries* (SO) |
| MacEntee 2007 | GDI‐S (PO) | Not assessed | GBI* (PO) | Not assessed |
| Mojon 1998 | PI (PO) | Not assessed | Denture‐induced stomatitis (Budtz‐Jørgensen 1970) (PO) | Caries* (PO) Root caries* (PO) |
| Schou 1989 | Not assessed | Index for plaque accumulation (Denture Plaque Index) by Ambjørnsen 1982 (PO) | Denture‐induced stomatitis (Budtz‐Jørgensen 1970) (PO) | Not assessed |
| Van der Putten 2013 | PI (PO) | Method of Augsburger 1982 (PO) | Not assessed | Not assessed |
| Zenthöfer 2013 | Plaque‐control record* (PO) | DHI* (PO) | GBI* (PO) | Not assessed |
| PO: primary outcome measure; SO: secondary outcome measure. DHI: Denture Hygiene Index; GBI: Gingival Bleeding Index; GDI‐S: Geriatric Simplified Debris Index; OHI‐S: Simplified Oral Hygiene Index; PI: Plaque Index | ||||
*Dichotomously assessed.
All studies except Schou 1989 provided information on assessment of dental plaque. Seven studies used a four‐point (0 to 3) plaque scale based on the Plaque Index described by Silness 1964 (Bellomo 2005; De Visschere 2011; De Visschere 2012; Mojon 1998; Van der Putten 2013), or the (simplified) Oral Hygiene Index (OHI‐S) by Greene 1964 (Frenkel 2001; MacEntee 2007). Six studies reported mean dental plaque, and one median dental plaque (Mojon 1998). In Zenthöfer 2013, dental plaque was assessed dichotomously using the "plaque‐control record". We were able to meta‐analyse up to six studies on dental plaque depending on subgroups (Bellomo 2005; De Visschere 2011; De Visschere 2012; Frenkel 2001; MacEntee 2007; Van der Putten 2013).
Seven studies reported on denture plaque (Bellomo 2005; De Visschere 2011; De Visschere 2012; Frenkel 2001; Schou 1989; Van der Putten 2013; Zenthöfer 2013). Denture plaque was assessed in four studies using a five‐point scale (0 to 4) described by Augsburger 1982 (De Visschere 2011; De Visschere 2012; Van der Putten 2013), and in two studies using a four‐point scale (0 to 3) described by Ambjørnsen 1982 (Bellomo 2005; Schou 1989). A further study used the Denture Hygiene Index to assess presence or absence of denture plaque (Zenthöfer 2013). The authors were unable to provide usable data, so we were unable to include results in meta‐analyses (Zenthöfer 2013). We included data from five studies in meta‐analyses comparing educational interventions including information and practical components with (optimised) usual care (Bellomo 2005; De Visschere 2011; De Visschere 2012; Frenkel 2001; Van der Putten 2013).
Five studies reported data on inflammation of oral mucosa such as gingivitis, in Frenkel 2001, MacEntee 2007, and Zenthöfer 2013, or denture stomatitis (Frenkel 2001; Mojon 1998; Schou 1989). Two studies assessed gingivitis dichotomously applying the Gingival Bleeding Index (MacEntee 2007; Zenthöfer 2013). Mean gingivitis scores were reported by Frenkel 2001 using the method of Suomi 1968. Denture stomatitis was classified and scored according to the erythema severity (Frenkel 2001; Mojon 1998; Schou 1989). Due to the fact that the studies used different outcomes and measures for inflammation of oral mucosa, we were unable to use the data for meta‐analyses.
Two studies reported data on root caries (Frenkel 2001; Mojon 1998). In Frenkel 2001, root caries were assessed on a binary scale as absent/present and reported as median score. Mojon 1998 reported active root caries as percentage of the total number of natural teeth.
Nutritional status
Only one study reported clinical measures of nutritional status (MacEntee 2007). The BMI was determined for every resident and binarily coded, with a score of less than 23 suggesting undernourishment. The Malnutrition Indicator Score (range 0 to 30, higher scores indicate better nourishment) as part of the Mini Nutritional Assessment was assessed, and mean scores were reported.
Incidence of respiratory diseases and pneumonia
None of the studies reported this outcome.
Adverse effects
None of the studies reported this outcome.
Oral health‐related knowledge
Two studies reported oral health‐related knowledge of nursing staff (De Visschere 2012; Frenkel 2001), assessed by self administered questionnaires reporting true/false responses to 26 statements, in Frenkel 2001, or 15 statements, in De Visschere 2012. The authors of a further study confirmed that this outcome was measured, but data are currently unavailable (Van der Putten 2013). We were able to combine data of two studies on staff knowledge (De Visschere 2012; Frenkel 2001). None of the studies reported oral health‐related knowledge of residents.
Oral health‐related attitude
Three studies assessed oral health‐related attitude of nursing staff (De Visschere 2012; Frenkel 2001; Van der Putten 2013). Self administered questionnaires were applied with four statements using a three‐point Likert scale (De Visschere 2012; Van der Putten 2013), or with 25 statements using a five‐point Likert scale (Frenkel 2001). We were able to combine the data of two studies (De Visschere 2012; Frenkel 2001), with data from the third study currently unavailable (Van der Putten 2013). No study reported oral health‐related attitude of residents.
Oral health‐related behaviour
One study assessed oral health‐related behaviour of residents (Bellomo 2005). An in‐depth structured interview with the residents was taken to obtain information about toothbrushing habits and used dental hygiene auxiliaries.
Excluded studies
We excluded 18 studies: seven were non‐randomised; six had no educational component; three included students or adults; one was not nursing home based; and one assessed no predefined outcomes (see Characteristics of excluded studies; Figure 1).
Ongoing studies
We identified one ongoing study assessing the effects of an intervention combining "best mouth care practices" with behavioural techniques (Jablonski 2011). Participants are nursing home residents with any kind of diagnosed dementia. The primary outcome measure is the reduction in care‐resistant behaviour. Oral health using the "Oral Health Assessment Tool" is assessed as the secondary outcome.
Risk of bias in included studies
We contacted the first or senior authors of all studies and asked them to provide further information on methodological details not reported in the publications. All authors responded to our requests, with all but one author being able to provide further information.
Allocation
Random sequence generation
Three studies described an adequate method of random sequence generation (De Visschere 2012; Frenkel 2001; Zenthöfer 2013). The authors of two studies responded to our requests for further information (MacEntee 2007; Mojon 1998), which clarified that their methods were adequate. In addition, random sequence generation was probably done in one study since the investigators described an adequate method for another study based on one protocol (Van der Putten 2013). We therefore assessed six studies as being at low risk of bias for this domain. The remaining three studies only stated that participants were randomised but did not describe their methods, and so were assessed as being at unclear risk of bias for this domain (Bellomo 2005; De Visschere 2011; Schou 1989).
Allocation concealment
Two studies provided details of how the random sequence was concealed from those involved in the study (Frenkel 2001; MacEntee 2007). One author stated on request that allocation was not concealed, and so we assessed the study as at high risk of bias for this domain (Bellomo 2005). The remaining six studies did not mention any methods used to conceal the random sequence, and we therefore assessed them as being at unclear risk of bias (De Visschere 2011; De Visschere 2012; Mojon 1998; Schou 1989; Van der Putten 2013; Zenthöfer 2013).
Blinding
Blinding of participants and personnel (performance bias)
In educational interventions, blinding of participants is often not possible, but the relevance of lack of blinding varies according to circumstances. Lack of blinding of study participants or staff, or both, may affect outcomes (Higgins 2011, Chapter 8.4.2), especially if outcomes are self reported, as for example self assessed oral health‐related quality of life. Only one study reported blinding of participants (MacEntee 2007). We judged all nine studies to be at low risk of performance bias, given that we considered the influence of residents' and staff awareness on oral health behaviour to be negligible.
Blinding of outcome assessment (detection bias)
Seven studies reported blinded outcome assessment, and we rated them as at low risk of bias for this domain (De Visschere 2011; De Visschere 2012; Frenkel 2001; MacEntee 2007; Schou 1989; Van der Putten 2013; Zenthöfer 2013). We assessed two studies as being at high risk of bias (Bellomo 2005; Mojon 1998): in one study, the author stated on request that the outcome assessor was not blinded (Bellomo 2005), and in the other study, blinding was not possible for practical reasons (Mojon 1998).
Incomplete outcome data
We assessed six studies as being at low risk of bias for this domain because attrition was low considering the special characteristics of the study population (especially high morbidity), and was roughly equal between groups (Bellomo 2005; De Visschere 2012; Frenkel 2001; Mojon 1998; Van der Putten 2013; Zenthöfer 2013). We assessed three studies as at unclear risk (De Visschere 2011; MacEntee 2007; Schou 1989).
Selective reporting
We assessed two studies as being at low risk of selective reporting bias as we could detect no obvious problems (MacEntee 2007; Van der Putten 2013). However, reports of the preplanned outcomes oral health‐related knowledge and attitude are still under preparation (Van der Putten 2013). Data reported by De Visschere 2012 differed to some extent from the data in the trial registration (tongue coating was not preplanned), and results of De Visschere 2011 were not available for all preplanned outcome periods. We rated both studies as having a high risk of bias. We rated the remaining five studies as having an unclear risk of bias due to unavailable protocols or trial registrations (Bellomo 2005; Frenkel 2001; Mojon 1998; Schou 1989; Zenthöfer 2013).
Other potential sources of bias
We assessed the trials for other potential sources of bias such as recruitment bias in cluster‐randomised trials or contamination.
Seven of the included trials were cluster‐randomised trials, using nursing homes as the unit of randomisation. In one trial, it was clear that nursing homes residents gave informed consent or were examined at baseline after randomisation (De Visschere 2011), resulting in a high risk of bias. In addition, there was potential contamination between groups in this study (De Visschere 2011). Four trials were at low risk of bias (De Visschere 2012; Frenkel 2001; Mojon 1998; Van der Putten 2013), and the other trials provided insufficient information, resulting in a judgement of unclear risk of bias (MacEntee 2007; Schou 1989).
Overall risk of bias
No study showed overall low risk of bias. Four studies had a high risk of bias (Bellomo 2005; De Visschere 2011; De Visschere 2012; Mojon 1998). The other five studies were at unclear risk of bias (Frenkel 2001; MacEntee 2007; Schou 1989; Van der Putten 2013; Zenthöfer 2013). See Figure 3; Figure 4.
3.
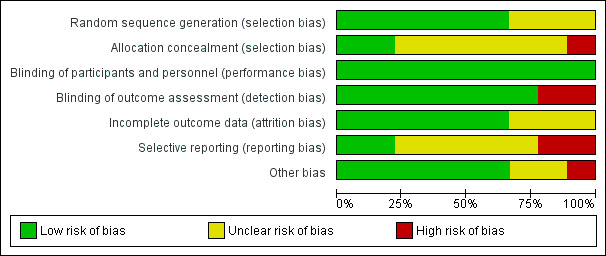
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
4.
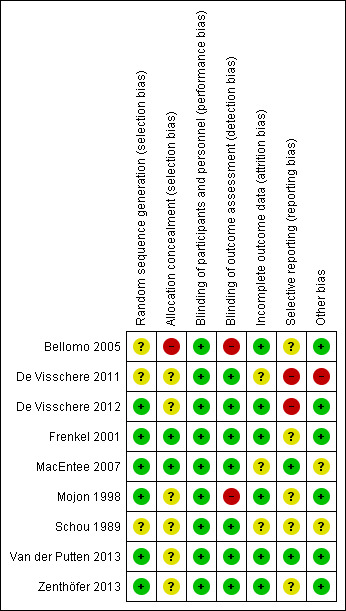
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Effects of interventions
See: Table 1
5.
Summary of included studies
Comparison 1: Educational intervention (with information and practical components) versus (optimised) usual care
Six out of eight trials for this comparison presented data suitable for inclusion in meta‐analyses. First, we have presented our analysis comprising the primary outcomes dental plaque and denture plaque. We have presented subgroup analysis for:
intervention recipient (nursing staff only versus nursing staff and residents);
follow‐up period (short term versus long term);
education in the context of guideline implementation versus education without guideline implementation (limited to short‐term follow‐up).
We have presented results for gingivitis, denture‐induced stomatitis, caries, secondary outcomes, and oral health‐related behaviour in a narrative form.
Primary outcomes
Oral health‐related quality of life
Not measured in the included studies.
Oral health
Not masured in the included studies.
Dental plaque
We meta‐analysed six studies (Bellomo 2005; De Visschere 2011; De Visschere 2012; Frenkel 2001; MacEntee 2007; Van der Putten 2013), three at high and three at unclear risk of bias, involving 437 nursing home residents. Dental plaque scores did not differ between residents who were cared for by nursing staff educated in oral health and than those receiving usual care (mean difference (MD) ‐0.04, 95% confidence interval (CI) ‐0.26 to 0.17; P = 0.71; effective sample size N = 353) (Analysis 1.1). There were no apparent subgroup differences for follow‐up periods (Analysis 1.2) or education in context of guideline implementation (Analysis 1.3). The subgroup analysis for educational intervention recipients showed a positive result in favour of interventions providing education for nursing staff only compared to education for staff and residents (P = 0.002), but results should be viewed with caution as there was only one study in the second category (Analysis 1.1).
1.1. Analysis.
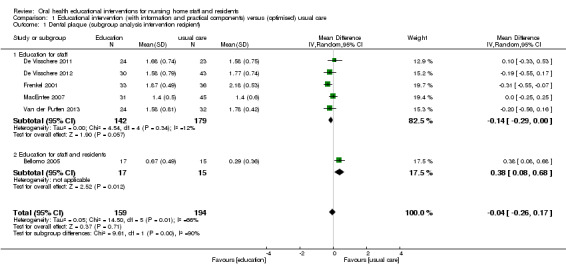
Comparison 1 Educational intervention (with information and practical components) versus (optimised) usual care, Outcome 1 Dental plaque (subgroup analysis intervention recipient).
1.2. Analysis.
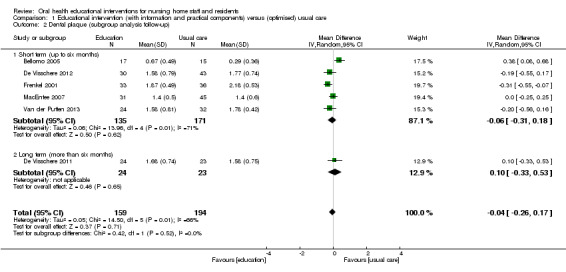
Comparison 1 Educational intervention (with information and practical components) versus (optimised) usual care, Outcome 2 Dental plaque (subgroup analysis follow‐up).
1.3. Analysis.
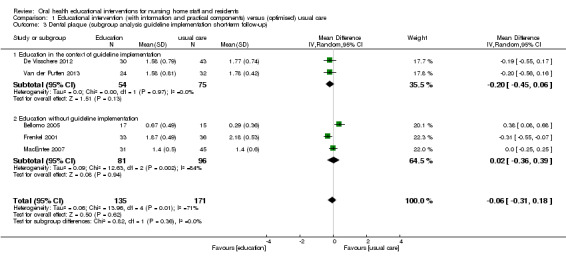
Comparison 1 Educational intervention (with information and practical components) versus (optimised) usual care, Outcome 3 Dental plaque (subgroup analysis guideline implementation short‐term follow‐up).
We were unable to include two studies in this meta‐analysis (Mojon 1998; Zenthöfer 2013), which had high and unclear risk of bias, and involved 148 participants. In Mojon 1998, there was an increase in the median dental plaque scores for both groups (0.06 in the intervention group, 0.25 in the control group), a non‐significant difference (P = 0.06). In Zenthöfer 2013, dental plaque was assessed dichotomously, demonstrating lower mean percentages of plaque‐positive dental sites in three intervention groups compared to usual care (no P values reported).
Denture plaque
We combined five studies, involving 816 nursing home residents, in a meta‐analysis. Three of the studies were at high risk of bias (Bellomo 2005; De Visschere 2011; De Visschere 2012), and two were at unclear risk of bias (Frenkel 2001; Van der Putten 2013). Denture plaque scores were not lower for residents who were cared for by nursing staff educated in oral health compared to residents receiving usual care (standardised mean difference (SMD) ‐0.60, 95% CI ‐1.25 to 0.05; P = 0.07; effective sample size N = 524) (Analysis 1.4). Subgroup analyses for educational intervention recipient, follow‐up period, and education in the context of guideline implementation showed no significant differences (Analysis 1.4; Analysis 1.5; Analysis 1.6).
1.4. Analysis.
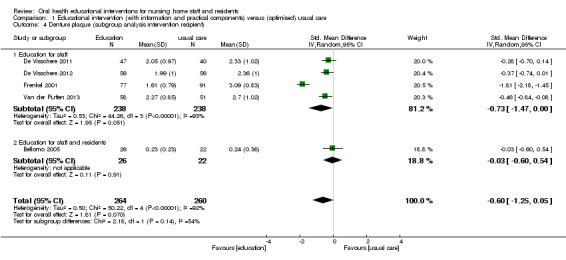
Comparison 1 Educational intervention (with information and practical components) versus (optimised) usual care, Outcome 4 Denture plaque (subgroup analysis intervention recipient).
1.5. Analysis.
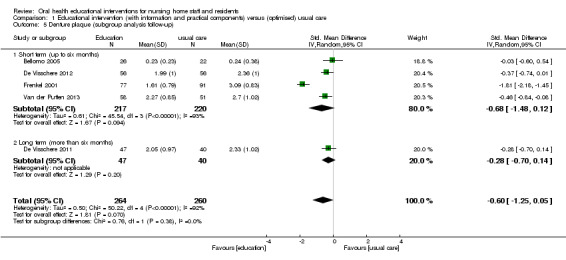
Comparison 1 Educational intervention (with information and practical components) versus (optimised) usual care, Outcome 5 Denture plaque (subgroup analysis follow‐up).
1.6. Analysis.
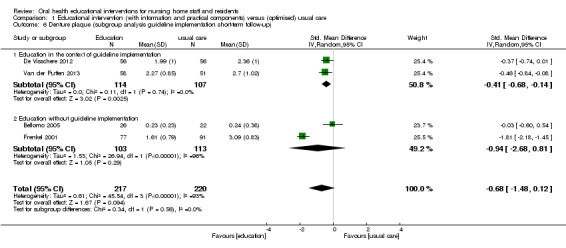
Comparison 1 Educational intervention (with information and practical components) versus (optimised) usual care, Outcome 6 Denture plaque (subgroup analysis guideline implementation short‐term follow‐up).
Zenthöfer 2013, which included 89 participants and was at unclear risk of bias, did not provide adequate data for meta‐analysis. Denture plaque was assessed dichotomously, and trial authors reported a lower mean percentage of plaque‐positive denture sites in intervention groups compared to usual care (no P values reported).
Gingivitis
Three studies with short‐term follow‐up (Frenkel 2001; MacEntee 2007; Zenthöfer 2013), all at unclear risk of bias, assessed gingivitis in residents, but we were unable to pool the data. Frenkel 2001 analysed 79 residents and showed a statistically significant difference in mean gingivitis score in favour of the intervention group (adjusted MD ‐0.28, 95% CI ‐0.42 to ‐0.15; P < 0.001). In Zenthöfer 2013, which analysed 68 residents, the mean values for the Gingival Bleeding Index were lower in intervention groups one (8.5, standard deviation (SD) 11.9), two (7.4, SD 8.7), and three (20.7, SD 23.9) compared to the control group (21.8, SD 27.5) (P values not reported/not calculated). MacEntee 2007 analysed 98 residents and showed no significantly different scores for gingivitis between intervention and control (MD ‐0.2, 95% CI ‐7.3 to 7.0; P = 0.48).
Denture‐induced stomatitis
Frenkel 2001, which involved 258 residents and was at unclear risk of bias, assessed severity of denture‐induced stomatitis. Stomatitis was reduced in the intervention group at six months' follow‐up compared to the control group (P < 0.0001). Mojon 1998, with 159 residents, assessed the incidence of mucosal and other lesions. There was no significant difference between groups for incidence of denture stomatitis (37% versus 42.2%; P value not reported) or other lesions (18.5% versus 31.6%; P = 0.06), but for incidence of glossitis, the result was in favour of the intervention group (4.9% versus 25%; P = 0.005).
Caries and root caries
Two studies at unclear and high risk of bias assessed caries or root caries (Frenkel 2001; Mojon 1998). The individual studies showed no significant differences between intervention groups and control groups at both short‐term and long‐term follow‐up.
Secondary outcomes
None of the included studies assessed incidence of respiratory diseases, pneumonia, or adverse effects. Only one study assessed nutritional status (BMI and Malnutrition Indicator Score), which had short‐term follow‐up and an unclear risk of bias (MacEntee 2007). There was no benefit related to BMI scores less than 23 for residents receiving the intervention (odds ratio 1.0, 95% CI 0.3 to 3.1; P = 0.49). The mean difference of Malnutrition Indicator Score (range 0 to 30) was ‐1.1 (95% CI ‐2.9 to 0.7; P = 0.11).
Intermediate outcomes
Oral health‐related knowledge
We combined two studies at high and unclear risk of bias involving 453 members of nursing staff in a meta‐analysis (De Visschere 2012; Frenkel 2001). A non‐significant difference in knowledge was reported for nursing staff receiving educational interventions compared to those without education (SMD 0.94, 95% CI ‐0.04 to 1.92; P = 0.06) (Analysis 1.7).
1.7. Analysis.
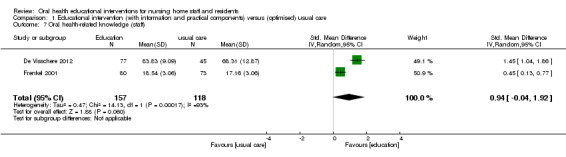
Comparison 1 Educational intervention (with information and practical components) versus (optimised) usual care, Outcome 7 Oral health‐related knowledge (staff).
Oral health‐related attitude
We combined two studies involving 459 members of nursing staff in a meta‐analysis (De Visschere 2012; Frenkel 2001). A non‐significant difference in attitude was reported for nursing staff receiving educational interventions compared to those without education (SMD 0.30, 95% CI ‐0.23 to 0.83; P = 0.27) (Analysis 1.8).
1.8. Analysis.
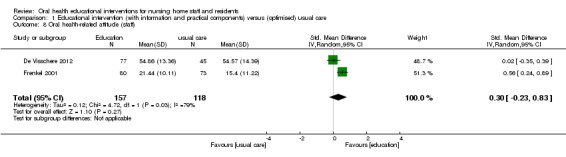
Comparison 1 Educational intervention (with information and practical components) versus (optimised) usual care, Outcome 8 Oral health‐related attitude (staff).
Oral‐health related behaviour
One study assessed oral health‐related behaviour of residents (Bellomo 2005). The 60 residents were interviewed about their toothbrushing habits. Use of toothbrushes and the frequency of brushing during the study period were comparable in both groups.
Comparison 2: Educational intervention (information only) versus usual care
One study (Schou 1989), at unclear risk of bias and analysing 114 residents, showed that there is insufficient evidence to determine whether or not denture plaque scores are lower when educating residents or nursing staff without practical training. There was no difference in the prevalence of denture stomatitis between the groups. The number of residents in the intervention groups who reported correct denture cleaning habits increased, but not significantly compared to baseline (baseline N = 28; follow‐up N = 38). The study did not assess any other outcomes of this review.
Reporting of complex interventions (CReDECI 2)
Findings for the reporting quality of complex interventions, using the CReDECI 2 checklist (Möhler 2015), are described in Table 4. Information about the development stage (four items) was rarely provided, as most studies failed to report reasons for selection of intervention components/essential function (item 2) or intended interactions between components (item 3). Five studies provided at least some information about the intervention's underlying theoretical basis (item 1) (De Visschere 2012; Frenkel 2001; MacEntee 2007; Schou 1989; Van der Putten 2013). Only MacEntee 2007 and Schou 1989 considered item 2. None of the studies reported consideration of context characteristics in intervention modelling (item 4). Only three studies reported information on the feasibility and piloting stage (item 5) (Frenkel 2001; MacEntee 2007; Schou 1989). A description of the strategy for delivering the intervention (item 7) was reported in all but one study (De Visschere 2011). Six studies described the materials or tools used for the intervention delivery (item 8) (De Visschere 2012; Frenkel 2001; MacEntee 2007; Mojon 1998; Van der Putten 2013; Zenthöfer 2013). In two studies, the process evaluation (item 10) was planned a priori (De Visschere 2012; Van der Putten 2013), and in another two studies qualitative methods for identification of facilitators and barriers were mentioned (De Visschere 2011; MacEntee 2007). A description of costs or required resources for the delivery of the interventions was only provided in Frenkel 2001. Overall, most studies reported insufficient information on processes (Figure 6).
3. Evaluation of the included studies using the CReDECI 2 checklist.
| Bellomo 2005 | De Visschere 2011 | De Visschere 2012 | Frenkel 2001 | MacEntee 2007 | Mojon 1998 | Schou 1989 | Van der Putten 2013 | Zenthöfer 2013 | |
| Item 1: Description of the intervention’s underlying theoretical basis | No | No | Yes, guideline implementation theory; p. e97 & p. 2 | (Yes), qualitative data from previous research; p. 92 | Yes, pyramidal scheme (Jones 1977); p. 26 (Ref. 30‐35) | No | Yes, "active involvement principle"; (Ref. 14) | Yes, guideline implementation theory; p. 1144 & p. 2 | No |
| Item 2: Description of all intervention components, including the reasons for their selection as well as their aims/essential functions | No | No | No | No | Yes, p. 26 | No | Yes, p. 3 (& Ref. 14) | No | No |
| Item 3: Illustration of any intended interactions between different components | No | No | (Yes, p. e98) | No | Yes, p. 26 & 28 | No | No | (Yes), p. 1146 | No |
| Item 4: Description and consideration of the context’s characteristics in intervention modelling | No | No | No | No | No | No | No | No | No |
| Item 5: Description of the pilot test and its impact on the definite intervention | No | No | No | (Yes), p. 92 | (Yes), research proposal (provided by the author on request) | No | Yes, Ref. 14 (modified intervention) | No | No |
| Item 6: Description of the control condition (comparator) and reasons for the selection | Yes, p. 26 | No | Yes, p. e97 | No | Yes, p. 28 | Yes, p. 828 | No | Yes, p. 1146 | No |
| Item 7: Description of the strategy for delivering the intervention within the study context | Yes, p. 26 | No | Yes, p. e98 | Yes, p. 290 & 92 | Yes, p. 27‐28 | Yes, p. ?? | Yes, p. 3 | Yes, p. 1146 | Yes, p. 262‐263 |
| Item 8: Description of all materials or tools used in the delivery of the intervention | No | No | Yes, p. e98 | Yes, p. 92 | Yes, p. 27‐28 (footnotes 2+3; restructured training material: bcdental.org/YourDentalHealth/YourDentalHealth.aspx?id=9974) |
Yes, p. 143 | No | Yes, p. 1146 | Yes, p. 263 & Ref. 19 |
| Item 9: Description of fidelity of the delivery process compared to the study protocol | No | (Yes), p. 419 | No | No | Yes, p. 30 | Yes, p. 830 | No | No | No |
| Item 10: Description of a process evaluation and its underlying theoretical basis | No | (Yes), p. 423 qualitative approach/publication mentioned | Yes, p. 5 | No | (Yes), research proposal MacEntee 2007 | No | No | Yes, p. 5 | No |
| Item 11: Description of internal facilitators and barriers potentially influencing the delivery of the intervention as revealed by the process evaluation | No | Yes, p. 423 (findings published in an additional publication p. 115‐122) | Yes, p. e102 results of process evaluation scantly mentioned (more information in a second publication p. 115‐122) | No | No (planned, but unfinished/unpublished) | No | No | No (publication in preparation) | No |
| Item 12: Description of external conditions or factors occurring during the study which might have influenced the delivery of the intervention or mode of action (how it works) | No | No | No | No | Yes, p. 31‐32 | Yes, p. 147 | No | No | No |
| Item 13: Description of costs or required resources for the delivery of the intervention | No | No | No | Yes, tab 4 on p. 294 | No | No | No | No | No |
6.
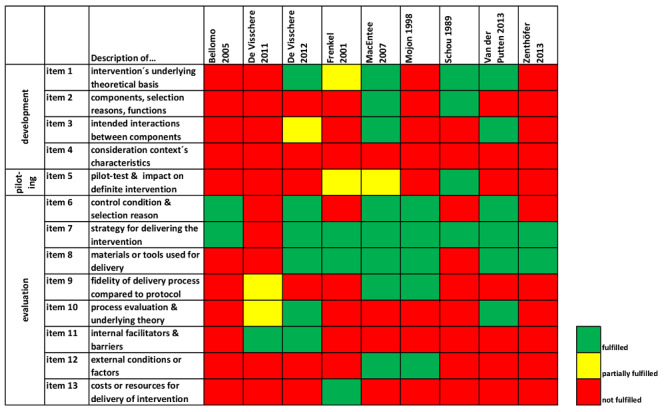
Summary of CReDECI 2: review authors' judgements of fulfilment of CReDECI 2 items for each included study
Discussion
Summary of main results
We have summarised results from nine RCTs, involving 3253 nursing home residents, investigating the effects of oral health educational interventions for nursing staff or nursing home residents, or both. All interventions were complex interventions that used more than one active component. We retrieved process measures and details of the development and evaluation of the complex interventions used, if available, using the CReDECI 2 criteria. The number of intervention components differed markedly between studies (Figure 2), but all interventions included theoretical or practical education sessions, or both, on oral health for nursing staff as one of the main intervention components. We categorised the interventions into two groups: educational intervention with information and practical components or educational intervention with information only. All trials aimed at improving different measures of dental health. Some were additionally concerned with increasing knowledge or improving attitude (De Visschere 2012; Frenkel 2001; Van der Putten 2013). Control interventions differed between studies, ranging from "usual care" to control interventions comparable to the active intervention in terms of time and effort spent.
None of the included studies assessed our primary outcomes oral health‐related quality of life and oral health. All studies assessed our third primary outcome dental health, although the outcome measures varied between studies (see Table 3). We found no clear beneficial effect on any dental health measure, and quality of the evidence was generally low.
None of the included studies assessed our secondary outcomes incidence of respiratory diseases, incidence of pneumonia, and adverse effects. Nutritional status (BMI and Malnutrition Indicator Score) was only assessed in MacEntee 2007, which reported no differences between the groups.
In summary, important uncertainties remain. For example, surrogate measures were reported most often, while patient‐relevant measures such as quality of life and pneumonia were not assessed. Moreover, there was no information about adverse events and costs. Due to insufficient reporting of interventions, we are unable to reach strong conclusions about the overall effects or the effects of components of the complex interventions used.
Overall completeness and applicability of evidence
This review included nine individual and cluster‐RCTs, which were conducted in six high‐income countries: the Netherlands, Belgium, Germany, the UK, Switzerland, and Canada. We did not identify any studies conducted in low‐income countries. This limits the applicability of our results, since evidence from a small number of countries cannot be directly applied to other countries with different healthcare systems.
Length of treatment effects (sustainability)
The majority of trials (six RCTs) presented data on short‐term effects (three or six months). This seems reasonable for outcomes like plaque and oral inflammation. However, it can be considered inadequate for other outcomes such as caries, and possibly knowledge and attitude. Sustainable effects on knowledge and attitudes for nursing staff are probably needed to allow for behavioural changes in oral health care. Studies should therefore measure outcomes in the longer term.
Type of outcomes
We would argue that the most important goal of the investigated interventions was to improve the well‐being of nursing home residents, as well as their oral and general health. However, none of the included studies reported data on oral health or oral health‐related quality of life. The reported outcomes (e.g. plaque, gingivitis) are important measures of dental health, but may not represent the most important outcomes of interventions to improve oral health in nursing home residents. Still, they can be seen as important surrogate parameters and as adequate indicators for future tooth survival and corresponding well‐being (Durham 2013; Mack 2005; Tonetti 2015), especially as in nursing home residents, treatment effects on tooth survival might be hard to establish due to the length of time until tooth loss and limited life expectancy of residents. Apart from directly related outcomes, nutritional status of residents has been related to oral and dental health (Naito 2010), but only one study assessed this outcome, with inconclusive results. None of the included studies assessed care‐resistant behaviour, although this has been described as an important factor in oral healthcare delivery (Frenkel 1999). Here, results from ongoing study Jablonski 2011 may provide meaningful data in the future.
Type of participants
The included studies comprised a variety of nursing staff and nursing home residents. Nursing staff varied in terms of qualifications and work experience. Nursing home residents varied in terms of oral health status, care dependency, and cognitive status. Some studies excluded residents with dementia, in Zenthöfer 2013, or cognitive impairment (Frenkel 2001; Mojon 1998). In addition, two studies excluded residents with "care‐resistant behaviour" (De Visschere 2012; Van der Putten 2013). Since cognitive impairment is an important factor for nursing home admissions, and rates of residents with dementia regularly exceed 50% (Seitz 2010), this should be considered when judging the generalisability of results. Also, severe cognitive impairment is often a cause of resistance of the residents to oral healthcare activities (Forsell 2011). Excluding these residents may therefore lead to overestimation of treatment effects.
Type of intervention
Oral health education programmes are complex interventions with multiple components that are implemented in complex environments with, for example, different dental and/or organisational conditions and prerequisites. Hence, interpretation of results is difficult. As would be expected for a review of complex educational interventions, outcomes could not be clearly associated with single components since education programmes varied markedly. For example, the duration of interventions has an impact on dental health outcomes, and the same applies to other sources of heterogeneity, for example intensity and components of interventions and mode of delivery. Here, educators delivering the intervention were from a range of professional backgrounds (dentists, dental hygienists, trained nursing staff, health promoters). In most studies, the educators were not members of the established nursing staff, except nurses who were trained as oral healthcare co‐ordinators. In general, reporting of the complex interventions as recommended by CReDECI 2 was poor. For example, it was not reported where nurses received training by a trained oral healthcare co‐ordinator or numbers of participating nurses and nurse assistants, and there was no information about the direct effects of the education programmes. Control interventions differed markedly with a number of alternative interventions used as controls, which might have masked a possible favourable effect of interventions.
The important role of an underlying theory and a systematic approach to developing behaviour change interventions has been disregarded by most intervention developers, or at least there is little information on this important aspect. In the future, it is crucial to use specified language when reporting content, techniques, and underlying theoretical frameworks of complex interventions, for example applying the suggestions of Michie and colleagues, who have highlighted the important issues concerning behaviour change intervention development and research (Michie 2009), and have suggested approaches for adequately designing such interventions (Michie 2011).
Quality of the evidence
The overall quality of the evidence is reported in Table 1.
Educational intervention (with information and practical components) versus usual care
The quality of the evidence included in this comparison limits the confidence with which we can draw conclusions about the efficacy of educational interventions on oral health of nursing home residents. No study included in this comparison was at low risk of bias; we assessed four studies as at unclear risk of bias and four as at high risk of bias. We considered these 'Risk of bias' issues in conjunction with the fact that meta‐analyses showed statistically significant heterogeneity (I² = 60% and 92%). We downgraded the evidence due to serious risk of bias and inconsistency, which resulted in our rating the evidence as low quality for all assessed outcomes. Low‐quality evidence means that future research in these areas is very likely to have an impact on our confidence in the estimate of effect and likely to change the estimate. In addition, it is very concerning that there was no evidence on our primary outcome of oral health‐related quality of life and some of the secondary outcomes of this review. These outcomes are likely to be important to nursing home residents and caregivers.
Educational interventions with information only versus usual care
There was insufficient evidence from only one study with unclear risk of bias and low quality evidence on the effects of educational interventions with information only, not allowing any conclusions.
Potential biases in the review process
Due to our comprehensive search strategy, we are confident that publication bias is unlikely. Two review authors independently performed selection of studies and data extraction, and analyses were undertaken and differences were resolved by consensus and by consulting a third review author when necessary.
Agreements and disagreements with other studies or reviews
The results of our meta‐analyses (of RCTs) differ from two recent systematic reviews and meta‐analyses (De Lugt‐Lustig 2014; Wang 2015). Both reviews concluded that oral health education for nurses and informal caregivers may yield positive effects on residents' oral health. However, there are some differences between these reviews and the present review regarding methodological approaches used. Selection criteria differed for study design, time period, and target participants. We only included RCTs and cluster‐RCTs, whereas De Lugt‐Lustig 2014 and Wang 2015 additionally included before‐and‐after studies, cross‐sectional studies, and non‐randomised studies. The literature search was limited to articles since 1990 and disregarded publications like doctoral theses or unpublished studies (De Lugt‐Lustig 2014). De Lugt‐Lustig 2014 disregarded any educational interventions considering nursing home residents as recipients, whereas Wang 2015 excluded education for residents only. Differing from the other reviews, our review presents data on the fidelity of the intervention implementation (using CReDECI 2), and we contacted the first and/or senior author of included studies to obtain detailed information about the interventions and missing data.
A further systematic review including a broad range of study designs indicated that knowledge, self efficacy, and facilitation of behaviour are determinants that are used in successful implementation strategies (Weening‐Verbree 2013). In line with our results, the authors were not able to recommend a single strategy or combination of strategies for improving oral health in institutionalised elderly people.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence to draw robust conclusions about the effects of oral health educational interventions for nursing home staff and residents in addition to usual care. Eligible studies did not provide evidence of the effectiveness of educational interventions on any measure of residents' oral health or nurses' oral health knowledge and attitude. Moreover, the small number of available studies and important heterogeneity among studies do not allow for clear practice recommendations. Information on many aspects that seem relevant for nursing homes to guide decision making about implementation of such interventions cannot be drawn from the available studies. For example, important resident‐related outcomes such as oral health‐related quality of life and nutritional status were not or were rarely assessed. Important intermediate measures, for example, staff knowledge and attitude, were also rarely assessed.
Importantly, due to marked clinical heterogeneity of the interventions, it is not possible to reliably determine the effectiveness of specific educational programmes or intervention components. Reporting of the development, piloting, and evaluation processes for the complex interventions was insufficient. Facilitators, barriers, and resources of the different interventions therefore remain unknown, but are clearly relevant for decisions on implementation. Based on the available evidence, it seems impossible to make recommendations about the extent and content of educational programmes as well as favoured educators (e.g. nurses versus dentists) and participants (e.g. nurses versus residents).
It is therefore unclear at present which kind of oral healthcare education programme should be implemented and if nursing homes can expect positive effects from the implementation of such a programme.
Implications for research.
There is a need for further RCTs to determine whether oral health educational interventions for nursing home staff and residents are effective, especially on residents’ oral health‐related quality of life and oral health, as well as residents’ and staff knowledge and attitude. Future trials need to be rigorous in design and delivery with adequate reporting including detailed descriptions of participants, setting, intervention and control conditions.
Nursing home residents differ in terms of personal and medical characteristics, for example, oral health status, care dependency, cognitive status, and medical diagnoses. Nursing homes and/or wards and staff also differ. Nurses mainly taking care of elderly with high levels of care dependency or who are cognitively impaired may need a somewhat different educational content/intervention. Researchers should consider (and describe) these characteristics in future cluster‐RCTs. In addition, subgroup analyses, for example of residents with dementia or different levels of cognitive impairment, are needed, as has been planned in an ongoing study (Jablonski 2011).
Most included programmes comprised singular or short‐term interventions, and outcomes were usually assessed in the short term. Future studies could therefore implement interventions with a higher frequency of education sessions or continuous provision of oral healthcare education for nursing staff and aim for longer follow‐up periods.
Ideally, control interventions should be comparable to experimental interventions in terms of duration and effort for participants to allow for successful blinding and reduce the risk of a Hawthorne effect. As for experimental interventions, control interventions as well as 'usual care' should be described in detail.
Outcome measures should be determined by established and validated measurement methods.
Interventions should be rigorously developed and reported following recommendations for complex interventions (Craig 2008). Michie 2009 has called for better reporting of complex behaviour change interventions, and for authors and reviewers to use reporting guidelines to ensure this. This should include established reporting guidelines that cover different development and evaluation stages, for example the CONSORT statement and, if appropriate, extensions, as well as the whole process of developing and evaluating complex interventions, for example using CReDECI 2 (Möhler 2015). Furthermore, as categorisation of behavioural interventions is problematic due to inconsistent use of descriptive language and may cause problems in replication and implementation (Abraham 2008), interventions should be reported in more detail. for example using the Template for Intervention Description and Replication (TIDieR) criteria (Hoffmann 2014). In addition, Michie 2011 pointed out that behaviour change interventions in particular need to be developed using an underlying theory and systematic and rigorous methods, suggesting methods for characterising and designing behaviour change interventions (e.g. the "behaviour change wheel"), which can be used to design new interventions. However, in our review we showed that although eight of the studies reported at least some information about the strategy of delivering the intervention, and six of nine studies reported information about tools and materials used for delivery, a more detailed description and more easily available curricula and manuals are warranted to ensure transparency, replicability, and integrity of the complex interventions used.
Acknowledgements
We wish to acknowledge the contribution to the protocol of consumer reviewer Johannes van Dijk (gerontopsychiatric nurse and trainer in dementia care mapping, Hamburg, Germany) and statistical advice and support of statistician Burkhard Haastert (mediStatistica, Neuenrade, Germany). We wish to thank Anne Littlewood (Cochrane Oral Health Group) for her assistance with literature searching and Helen Worthington, Jan Clarkson, Laura MacDonald, and Phil Riley (Cochrane Oral Health) for their help with the preparation of this review. We also thank Lucy O'Malley, Debbie Bonetti, Catherine Waldron, and Linet Weening for their helpful comments on drafts of this review and Lisa Winer for copy editing the final draft.
Appendices
Appendix 1. Cochrane Oral Health Trials Register search strategy
#1 ((home* and (nurs* or elder* or old* or care or "assisted living" or convalescen* or rest* or retir* or resident* or "long stay" or longstay or "long term"))) AND (INREGISTER) #2 ((facilit* and (nurs* or elder* or old* or care or "assisted living" or convalescen* or rest* or retir* or resident* or "long stay" or longstay or "long term"))) AND (INREGISTER) #3 ((institut* and (nurs* or elder* or old* or care or "assisted living" or convalescen* or rest* or retir* or resident* or "long stay" or longstay or "long term"))) AND (INREGISTER) #4 ((residenc* and (nurs* or elder* or old* or care or "assisted living" or convalescen* or rest* or retir* or resident* or "long stay" or longstay or "long term"))) AND (INREGISTER) #5 (((dental or tooth or teeth or enamel or root*) AND (decay* or caries or carious or "white spot*" or plaque or reminerali* or deminerali* or erosion* or abrasion* or wear))) AND (INREGISTER) #6 ((denture* and (clean* or clens*))) AND (INREGISTER) #7 ((periodont* or gingivi* or gingiva*)) AND (INREGISTER) #8 ((stomatitis or "mouth ulcer*" or "oral ulcer*" or (oral and candidi*) or (mouth* and candidi*) or "aphthous ulcer*" or (aphthae and ulcer*) or (mucositis and mouth*) or (mucositis and oral) or xerostomi* or "dry mouth*")) AND (INREGISTER) #9 (((oral and health*) or (mouth* and health*) or (dental and health*))) AND (INREGISTER) #10 ((halitosis or "mouth odour*" or "mouth odor*" or "mouth malodour*" or "mouth malodor*" or "oral odour*" or "oral odor*" or "oral malodour*" or "oral malodor*" or (breath and odour*) or (breath and odor*) or (breath and malodour*) or (breath and malodor*))) AND (INREGISTER) #11 ((("oral cancer*" or (gingiv* or mouth* or lip or lips or tongue* or "salivary gland*" or palat* or parotid* or sublingual or submandibular)) AND (cancer* or carcinoma* or neoplasm* or tumour* or tumor* or lesion* or malignan*))) AND (INREGISTER) #12 ((leukoplak* or "hairy tongue*")) AND (INREGISTER) #13 (("oral hygiene" or (mouth and care) or (dental and care) or (care and teeth) or (mouth and hygiene) or (plaque and control*) or (plaque and remov*))) AND (INREGISTER) #14 ((toothbrush* or tooth‐brush* or toothpaste* or dentifrice* or mouthwash* or mouth‐wash* or mouthrinse* or mouth‐rinse* or fluoride*)) AND (INREGISTER) #15 ((floss* or "interdental brush*" or "inter‐dental brush*" or (tooth and clean*) or (teeth and clean*) or (denture* and hygien*) or (denture* and clean*) or (tongue* and scrap*) or (tongue* and brush*) or (chewing and stick*) or (chewing and gum*))) AND (INREGISTER) #16 (((oral and care) or (oral and "self care"))) AND (INREGISTER) #17 ((instruct* or advis* or advice or educat* or promot* or teach* or train*)) AND (INREGISTER) #18 (((demonstrat* and toothbrush*) or (demonstrat* and "tooth brush*") or (demonstrat* and tooth‐brush*) or (demonstrat* and floss*) or (demonstrat* and "interdental brush*") or (demonstrat* and "inter‐dental brush*") or (demonstrat* and "interdental clean*") or (demonstrat* and "inter‐dental clean*") or (demonstrat* and woodstick*) or (demonstrat* and wood‐stick*) or (demonstrat* and "wood stick*"))) AND (INREGISTER) #19 (((supervis* and toothbrush*) or (supervis* and "tooth brush*") or (supervis* and tooth‐brush*) or (supervis* and floss*) or (supervis* and "interdental brush*") or (supervis* and "inter‐dental brush*") or (supervis* and "interdental clean*") or (supervis* and "inter‐dental clean*") or (supervis* and woodstick*) or (supervis* and wood‐stick*) or (supervis* and "wood stick*"))) AND (INREGISTER) #20 ((demonstrat* and (dentur* and (clean* or clens*)))) AND (INREGISTER) #21 ((supervis* and (dentur* and (clean* or clens*)))) AND (INREGISTER) #22 ((lectur* or seminar* or presentation* or program* or session* or tutorial* or video* or audio* or DVD* or online or podcast* or vodcast*)) AND (INREGISTER) #23 ((leaflet* or manual* or book* or pamphlet* or brochure*)) AND (INREGISTER) #24 (#1 or #2 or #3 or #4) AND (INREGISTER) #25 (#5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16) AND (INREGISTER) #26 (#17 or #18 or #19 or #20 or #21 or #22 or #23) AND (INREGISTER) #27 (#24 and #25 and #26) AND (INREGISTER)
Appendix 2. Cochrane Central Register of Controlled Clinical Trials (CENTRAL) search strategy
#1 [mh "Nursing home"] #2 [mh ^"Homes for the aged"] #3 (home* and (nurs* or elder* or old* or care or "assisted living" or convalescen* or rest* or retir* or resident* or "long stay" or longstay or "long term")):ti,ab #4 (facilit* and (nurs* or elder* or old* or care or "assisted living" or convalescen* or rest* or retir* or resident* or "long stay" or longstay or "long term")):ti,ab #5 (institut* and (nurs* or elder* or old* or care or "assisted living" or convalescen* or rest* or retir* or resident* or "long stay" or longstay or "long term")):ti,ab #6 (residenc* and (nurs* or elder* or old* or care or "assisted living" or convalescen* or rest* or retir or "long stay" or longstay or "long term")):ti,ab #7 #1 or #2 or #3 or #4 or #5 or #6 #8 [mh ^"Oral health"] #9 [mh "Stomatognathic diseases"] #10 [mh ^Halitosis] #11 ((dental or tooth or teeth or enamel or root*) and (decay* or caries or carious or "white spot*" or plaque or reminerali* or deminerali* or erosion* or abrasion* or wear*)) #12 (denture* and (clean* or clens*)):ti,ab #13 (periodont* or gingivi* or gingiva*):ti,ab #14 (stomatitis or "mouth ulcer*" or "oral ulcer*" or (oral near/5 candidi*) or (mouth* near/5 candidi*) or "aphthous ulcer*" or (aphthae near/3 ulcer*) or (mucositis near/5 mouth*) or (mucositis near/5 oral) or xerostomi* or "dry mouth*"):ti,ab #15 ((oral near/5 health*) or (mouth near/5 health*) or (dental near/5 health*)):ti,ab #16 (halitosis or "mouth odour*" or "mouth odor*" or "mouth malodour*" or "mouth malodor*" or "oral odour*" or "oral odor*" or "oral malodour*" or "oral malodor*" or (breath near/5 odour*) or (breath near/5 odor*) or (breath near/5 malodour*) or (breath near/5 malodor)):ti,ab #17 [mh "Mouth neoplasms"] #18 (("oral cancer*" or (gingiv* or mouth* or lip or lips or tongue* or "salivary gland*" or palat* or parotid* or sublingual or submandibular)) and (cancer* or carcinoma* or neoplasm* or tumour* or tumor* or lesion* or malignan*)):ti,ab #19 leukoplak*:ti,ab #20 "hairy tongue$":ti,ab #21 [mh ^"Oral hygiene"] #22 [mh Mouthwashes] #23 [mh Dentifrices] #24 ("oral hygiene" or (mouth* near/3 care) or (dental near/3 care) or (care near/3 teeth) or (mouth* near/3 hygiene) or (plaque near/3 control*) or (plaque near/3 remov*)):ti,ab #25 (toothbrush* or tooth‐brush* or toothpaste* or dentifrice* or mouthwash* or mouth‐wash* or mouthrinse* or mouth‐rinse* or fluoride*):ti,ab #26 (floss* or "interdental brush*" or "inter‐dental brush*" or (tooth near/5 clean*) or (teeth near/5 clean*) or (denture* near/5 hygien*) or (denture* near/5 clean*) or (tongue* near/5 scrap*) or (tongue* near/5 brush*) or (chewing near/5 stick*) or (chewing near/5 gum)):ti,ab #27 ((oral near/3 care) or (oral near/3 "self care")):ti,ab #28 #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 #29 [mh ^"Health education, dental"] #30 [mh "Health promotion"] #31 (instruct* or advis* or advice or educat* or promot* or teach* or train*):ti,ab #32 ((demonstrat* near/5 toothbrush*) or (demonstrat* near/5 "tooth brush*") or (demonstrat* near/5 tooth‐brush*) or (demonstrat* near/5 floss*) or (demonstrat* near/5 "interdental brush*") or (demonstrat* near/5 "inter‐dental brush*") or (demonstrat* near/5 "interdental clean*") or (demonstrat* near/5 "inter‐dental clean*") or (demonstrat* near/5 woodstick*) or (demonstrat* near/5 wood‐stick*) or (demonstrat* near/5 "wood stick*")):ti,ab #33 ((supervis* near/5 toothbrush*) or (supervis* near/5 "tooth brush*") or (supervis* near/5 tooth‐brush*) or (supervis* near/5 floss*) or (supervis* near/5 "interdental brush*") or (supervis* near/5 "inter‐dental brush*") or (supervis* near/5 "interdental clean*") or (supervis* near/5 "inter‐dental clean*") or (supervis* near/5 woodstick*) or (supervis* near/5 wood‐stick*) or (supervis* near/5 "wood stick*")):ti,ab #34 (demonstrat* near/5 (dentur* near/3 (clean* or clens*))):ti,ab #35 (supervis* near/5 (dentur* near/3 (clean* or clens*))):ti,ab #36 (lectur* or seminar* or presentation* or program* or session* or tutorial* or video* or audio* or DVD* or online or podcast* or vodcast*):ti,ab #37 (leaflet* or manual* or book* or pamphlet* or brochure*):ti,ab #38 #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 #39 #7 and #28 and #38
Appendix 3. MEDLINE Ovid search strategy
1. exp Nursing home/ 2. Homes for the aged/ 3. (home$ adj5 (nurs$ or elder$ or old$ or care or "assisted living" or convalescen$ or rest$ or retir$ or resident$ or "long stay" or longstay or "long term")).mp. 4. (facilit$ adj5 (nurs$ or elder$ or old$ or care or "assisted living" or convalescen$ or rest$ or retir$ or resident$ or "long stay" or longstay or "long term")).mp. 5. (institut$ adj5 (nurs$ or elder$ or old$ or care or "assisted living" or convalescen$ or rest$ or retir$ or resident$ or "long stay" or longstay or "long term")).mp. 6. (residenc$ adj5 (nurs$ or elder$ or old$ or care or "assisted living" or convalescen$ or rest$ or retir$ or "long stay" or longstay or "long term")).mp. 7. or/1‐6 8. Oral health/ 9. exp Stomatognathic diseases/ 10. Halitosis/ 11. ((dental or tooth or teeth or enamel or root$) and (decay$ or caries or carious or "white spot$" or plaque or reminerali$ or deminerali$ or erosion$ or abrasion$ or wear)).mp. 12. (denture$ and (clean$ or clens$)).mp. 13. (periodont$ or gingivi$ or gingiva$).mp. 14. (stomatitis or "mouth ulcer$" or "oral ulcer$" or (oral adj5 candidi$) or (mouth$ adj5 candidi$) or "aphthous ulcer$" or (aphthae adj3 ulcer$) or (mucositis adj5 mouth$) or (mucositis adj5 oral) or xerostomi$ or "dry mouth$").mp. 15. ((oral adj5 health$) or (mouth adj5 health$) or (dental adj5 health$)).mp. 16. (halitosis or "mouth odour$" or "mouth odor$" or "mouth malodour$" or "mouth malodor$" or "oral malodour$" or "oral malodor$" or (breath adj5 malodour$) or (breath adj5 malodor$) or (breath adj5 odour$) or (breath adj5 odor$)).mp. 17. exp Mouth neoplasms/ 18. (("oral cancer$" or (gingiv$or mouth or lip or lips or tongue$ or "salivary gland$" or palat$ or parotid$ or sublingual or submandibular)) and (cancer$ or carcinoma$ or neoplasm$ or tumour$ or tumor$ or lesion$ or malignan$)).mp. 19. leukoplak$.mp. 20. "hairy tongue$".mp. 21. exp Oral hygiene/ 22. exp Mouthwashes/ 23. exp Dentifrices/ 24. ("oral hygiene" or (mouth$ adj3 care) or (dental adj3 care) or (care adj3 teeth) or (mouth$ adj3 hygiene) or (plaque adj3 control$) or (plaque adj3 remov$)).mp. 25. (toothbrush$ or tooth‐brush$ or toothpaste$ or dentifrice$ or mouthwash$ or mouth‐wash$ or mouthrinse$ or mouth‐rinse$ or fluoride$).mp. 26. (floss$ or "interdental brush$" or "inter‐dental brush$" or (tooth adj5 clean$) or (teeth adj5 clean$) or (denture$ adj5 hygien$) or (denture$ adj5 clean$) or (tongue$ adj5 scrap$) or (tongue$ adj5 brush$) or (chewing adj5 stick$) or (chewing adj5 gum$)).mp. 27. ((oral adj3 care) or (oral adj3 "self care")).mp. 28. or/8‐27 29. Health education, dental/ 30. exp Health promotion/ 31. (instruct$ or advis$ or advice or educat$ or promot$ or teach$ or train$).mp. 32. ((demonstrat$ adj5 toothbrush$) or (demonstrat$ adj5 "tooth brush$") or (demonstrat$ adj5 tooth‐brush$) or (demonstrat$ adj5 floss$) or (demonstrat$ adj5 "interdental brush$") or (demonstrat$ adj5 "inter‐dental brush$") or (demonstrat$ adj5 "interdental clean$") or (demonstrat$ adj5 "inter‐dental clean$") or (demonstrat$ adj5 wood‐stick$) or (demonstrat$ adj5 woodstick$) or (demonstrat$ adj5 "wood stick$")).mp. 33. (demonstrat$ adj5 (denture$ adj3 (clean$ or clens$))).mp. 34. (supervis$ adj5 (denture$ adj3 (clean$ or clens$))).mp. 35. ((supervis$ adj5 toothbrush$) or (supervis$ adj5 "tooth brush$") or (supervis$ adj5 tooth‐brush$) or (supervis$ adj5 floss$) or (supervis$ adj5 "interdental brush$") or (supervis$ adj5 "inter‐dental brush$") or (supervis$ adj5 "interdental clean$") or (supervis$ adj5 "inter‐dental clean$") or (supervis$ adj5 wood‐stick$) or (supervis$ adj5 woodstick$) or (supervis$ adj5 "wood stick$")).mp. 36. (lecture$ or seminar$ or presentation$ or session$ or tutorial$ or video or audio or DVD or online or podcast$ or vodcast$).mp. 37. (leaflet$ or manual$ or book$ or pamphlet$ or brochure$).mp. 38. or/29‐37 39. 7 and 28 and 38 The search was linked with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0, updated March 2011 (Lefebvre 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 4. Embase Ovid search strategy
1. Nursing home/ 2. Home for the aged/ 3. (home$ adj5 (nurs$ or elder$ or old$ or care or "assisted living" or convalescen$ or rest$ or retir$ or resident$ or "long stay" or longstay or "long term")).mp. 4. (facilit$ adj5 (nurs$ or elder$ or old$ or care or "assisted living" or convalescen$ or rest$ or retir$ or resident$ or "long stay" or longstay or "long term")).mp. 5. (institut$ adj5 (nurs$ or elder$ or old$ or care or "assisted living" or convalescen$ or rest$ or retir$ or resident$ or "long stay" or longstay or "long term")).mp. 6. (residenc$ adj5 (nurs$ or elder$ or old$ or care or "assisted living" or convalescen$ or rest$ or retir$ or "long stay" or longstay or "long term")).mp. 7. or/1‐6 8. exp Mouth disease/ 9. Halitosis/ 10. ((dental or tooth or teeth or enamel or root$) and (decay$ or caries or carious or "white spot$" or plaque or reminerali$ or deminerali$ or erosion$ or abrasion$ or wear)).mp. 11. (denture$ and (clean$ or clens$)).mp. 12. (periodont$ or gingivi$ or gingiva$).mp. 13. (stomatitis or "mouth ulcer$" or "oral ulcer$" or (oral adj5 candidi$) or (mouth$ adj5 candidi$) or "aphthous ulcer$" or (aphthae adj3 ulcer$) or (mucositis adj5 mouth$) or (mucositis adj5 oral) or xerostomi$ or "dry mouth$").mp. 14. ((oral adj5 health$) or (mouth adj5 health$) or (dental adj5 health$)).mp. 15. (halitosis or "mouth odour$" or "mouth odor$" or "mouth malodour$" or "mouth malodor$" or "oral odour$" or "oral odor$" or "oral malodour$" or "oral malodor$" or (breath adj5 malodour$) or (breath adj5 malodor$) or (breath adj5 odour$) or (breath adj5 odor$)).mp. 16. exp Mouth tumor/ 17. (("oral cancer$" or (gingiv$ or mouth$ or lip or lips or tongue$ or "salivary gland$" or palat$ or parotid$ or sublingual or submandibular)) and (cancer$ or carcinoma$ or neoplasm$ or tumour$ or tumor$ or lesion$ or malignan$)).mp. 18. leukoplak$.mp. 19. "hairy tongue$".mp. 20. exp Mouth hygiene/˜ 21. Mouthwash/ 22. Toothpaste/ 23. ("oral hygiene" or (mouth$ adj3 care) or (dental adj3 care) or (care adj3 teeth) or (mouth$ adj3 hygiene) or (plaque adj3 control$) or (plaque adj3 remov$)).mp. 24. (toothbrush$ or tooth‐brush$ or toothpaste$ or dentifrice$ or mouthwash$ or mouth‐wash$ or mouthrinse$ or mouth‐rinse$ or fluoride$).mp. 25. (floss$ or "interdental brush$" or "inter‐dental brush$" or (tooth adj5 clean$) or (teeth adj5 clean$) or (denture$ adj5 hygien$) or (denture$ adj5 clean$) or (tongue$ adj5 scrap$) or (tongue$ adj5 brush$) or (chewing adj5 stick$) or (chewing adj5 gum$)).mp. 26. ((oral adj3 care) or (oral adj3 "self care")).mp. 27. or/8‐26 28. Dental health education/ 29. Health promotion/ 30. (instruct$ or advis$ or advice or educat$ or promot$ or teach$ or train$).mp. 31. ((demonstrat$ adj5 toothbrush$) or (demonstrat$ adj5 "tooth brush$") or (demonstrat$ adj5 tooth‐brush$) or (demonstrat$ adj5 floss$) or (demonstrat$ adj5 "interdental brush$") or (demonstrat$ adj5 "inter‐dental brush$") or (demonstrat$ adj5 "interdental clean$") or (demonstrat$ adj5 "inter‐dental clean$") or (demonstrat$ adj5 wood‐stick$) or (demonstrat$ adj5 woodstick$) or (demonstrat$ adj5 "wood stick$")).mp. 32. (demonstrat$ adj5 (denture$ adj3 (clean$ or clens$))).mp. 33. (supervis$ adj5 (denture$ adj3 (clean$ or clens$))).mp. 34. ((supervis$ adj5 toothbrush$) or (supervis$ adj5 "tooth brush$") or (supervis$ adj5 tooth‐brush$) or (supervis$ adj5 floss$) or (supervis$ adj5 "interdental brush$") or (supervis$ adj5 "inter‐dental brush$") or (supervis$ adj5 "interdental clean$") or (supervis$ adj5 "inter‐dental clean$") or (supervis$ adj5 wood‐stick$) or (supervis$ adj5 woodstick$) or (supervis$ adj5 "wood stick$")).mp. 35. (lecture$ or seminar$ or presentation$ or program$ or session$ or tutorial$ or video or audio or DVD or online or podcast$ or vodcast$).mp. 36. (leaflet$ or manual$ or book$ or pamphlet$ or brochure$).mp. 37. or/28‐36 38. 7 and 27 and 37
The above subject search was linked to Cochrane Oral Health's filter for identifying RCTs in Embase Ovid:
1. random$.ti,ab. 2. factorial$.ti,ab. 3. (crossover$ or cross over$ or cross‐over$).ti,ab. 4. placebo$.ti,ab. 5. (doubl$ adj blind$).ti,ab. 6. (singl$ adj blind$).ti,ab. 7. assign$.ti,ab. 8. allocat$.ti,ab. 9. volunteer$.ti,ab. 10. CROSSOVER PROCEDURE.sh. 11. DOUBLE‐BLIND PROCEDURE.sh. 12. RANDOMIZED CONTROLLED TRIAL.sh. 13. SINGLE BLIND PROCEDURE.sh. 14. or/1‐13 15. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 16. 14 NOT 15
Appendix 5. CINAHL EBSCO search strategy
S1 (MH "Nursing Homes+”) S2 (MH "Nursing Home Patients") S3 (home* N5 nurs*) or (home* N5 elder*) or (home* N5 old*) or (home* N5 care*) or (home* N5 "assisted living") or (home* N5 convalescen*) or (home* N5 rest*) or (home* N5 retir*) or (home* N5 resident*) or (home* N5 "long stay") or (home* N5 longstay) or (home* N5 "long term") S4 (facilit* N5 nurs*) or (facilit* N5 elder*) or (facilit* N5 old*) or (facilit* N5 care*) or (facilit* N5 "assisted living") or (facilit* N5 convalescen*) or (facilit* N5 rest*) or (facilit* N5 retir*) or (facilit* N5 resident*) or (facilit* N5 "long stay") or (facilit* N5 longstay) or (facilit* N5 "long term") S5 (institut* N5 nurs*) or (institut* N5 elder*) or (institut* N5 old*) or (institut* N5 care*) or (institut* N5 "assisted living") or (institut* N5 convalescen*) or (institut* N5 rest*) or (institut* N5 retir*) or (institut* N5 resident*) or (institut* N5 "long stay") or (institut* N5 longstay) or (institut* N5 "long term") S6 (residenc* N5 nurs*) or (residenc* N5 elder*) or (residenc* N5 old*) or (residenc* N5 care*) or (residenc* N5 "assisted living") or (residenc* N5 convalescen*) or (residenc* N5 rest*) or (residenc* N5 retir*) or (residenc* N5 "long stay") or (residenc* N5 longstay) or (residenc* N5 "long term") S7 S1 or S2 or S3 or S4 or S5 or S6 S8 (MH "Oral health") S9 (MH "Stomatognathic diseases+") S10 (MH Halitosis) S11 ((dental or tooth or enamel or root*) AND (decay* or caries or carious or "white spot*" or plaque or remineral* or demineral* or erosion* or abrasion* or wear)) S12 (denture* and (clean* or clens*)) S13 (periodont* or gingivi* or gingiva*) S14 (stomatitis or "mouth ulcer*" or "oral ulcer*" or (oral N5 candidi*) or (mouth N5 candidi*) or "aphthous ulcer*" or (aphthae N3 ulcer*) or (mucositis N5 mouth*) or (mucositis N5 oral) or xerostomi* or "dry mouth*") S15 ((oral N5 health*) or (mouth N5 health*) or (dental N5 health*)) S16 (halitosis or "mouth odour*" or "mouth odor*" or "mouth malodour*" or "mouth malodor*" or "oral odour*" or "oral odor*" or "oral malodour*" or "oral malodor*" or (breath N5 odour*) or (breath N5 odor*) or (breath N5 malodour*) or (breath N5 malodor)) S17 (MH "Mouth neoplasms+") S18 (("oral cancer*" or (gingiv* or mouth* or lip or lips or tongue* or "salivary gland*" or palat* or parotid* or sublingual or submandibular)) and (cancer* or carcinoma* or neoplasm* or tumour* or tumor* or lesion* or malignan*)) S19 leukoplak* S20 “hairy tongue*” S21 (MH "Oral hygiene+") S22 (MH Mouthwashes+) S23 (MH Dentifrices+) S24 ("oral hygiene" or (mouth* N3 care) or (dental N3 care) or (care N3 teeth) or (mouth* N3 hygiene) or (plaque N3 control*) or (plaque N3 remov*)) S25 (toothbrush* or tooth‐brush* or toothpaste* or dentifrice* or mouthwash* or mouth‐wash* or mouthrinse* or mouth‐rinse* or fluoride*) S26 (floss* or "interdental brush*" or "inter‐dental brush*" or (tooth N5 clean*) or (teeth N5 clean*) or (denture* N5 hygien*) or (denture* N5 clean*) or (tongue* N5 scrap*) or (tongue* N5 brush*) or (chewing N5 stick*) or (chewing N5 gum)) S27 ((oral N3 care) or (oral N3 "self care")) S28 S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 S29 (MH "Dental health education") S30 (MH "Health promotion") S31 (instruct* or advis* or advice or educat* or promot* or teach* or train*) S32 ((demonstrat* N5 toothbrush*) or (demonstrat* N5 "tooth brush*") or (demonstrat* N5 tooth‐brush*) or (demonstrat* N5 floss*) or (demonstrat* N5 "interdental brush*") or (demonstrat* N5 "inter‐dental brush*") or (demonstrat* N5 "interdental clean*") or (demonstrat* N5 "inter‐dental clean*") or (demonstrat* N5 woodstick*) or (demonstrat* N5 wood‐stick*) or (demonstrat* N5 "wood stick*")) S33 ((supervis* N5 toothbrush*) or (supervis* N5 "tooth brush*") or (supervis* N5 tooth‐brush*) or (supervis* N5 floss*) or (supervis* N5 "interdental brush*") or (supervis* N5 "inter‐dental brush*") or (supervis* N5 "interdental clean*") or (supervis* N5 "inter‐dental clean*") or (supervis* N5 woodstick*) or (supervis* N5 wood‐stick*) or (supervis* N5 "wood stick*")) S34 (demonstrat* N5 (dentur* N3 (clean* or clens*))) S35 (supervis* N5 (dentur* N3 (clean* or clens*))) S36 (lectur* or seminar* or presentation* or program* or session* or tutorial* or video* or audio* or DVD* or online or podcast* or vodcast*) S37 (leaflet* or manual* or book* or pamphlet* or brochure*) S38 S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 S39 S7 and S28 and S38
The above subject search was linked to Cochrane Oral Health's filter for identifying RCTs in CINAHL EBSCO:
S1 MH Random Assignment or MH Single‐blind Studies or MH Double‐blind Studies or MH Triple‐blind Studies or MH Crossover design or MH Factorial Design S2 TI ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or AB ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") or SU ("multicentre study" or "multicenter study" or "multi‐centre study" or "multi‐center study") S3 TI random* or AB random* S4 AB "latin square" or TI "latin square" S5 TI (crossover or cross‐over) or AB (crossover or cross‐over) or SU (crossover or cross‐over) S6 MH Placebos S7 AB (singl* or doubl* or trebl* or tripl*) or TI (singl* or doubl* or trebl* or tripl*) S8 TI blind* or AB mask* or AB blind* or TI mask* S9 S7 and S8 S10 TI Placebo* or AB Placebo* or SU Placebo* S11 MH Clinical Trials S12 TI (Clinical AND Trial) or AB (Clinical AND Trial) or SU (Clinical AND Trial) S13 S1 or S2 or S3 or S4 or S5 or S6 or S9 or S10 or S11 or S12
Appendix 6. Web of Science Conference Proceedings search strategy
#1 TS=(home* or facilit* or institut* or residenc*) #2 TS=(oral and (hygiene or health)) #3 TS=((dental or tooth or teeth or enamel or root) AND (caries or decay* or carious or "white spot*" or plaque or deminerali* or reminerali* or erosion* or abrasion* or wear)) #4 TS=(denture* and (clean* or clens*)) #5 TS=(periodont* or gingiva* or gingivi*) #6 TS=(stomatitis or halitosis) #7 TS=((oral or mouth or dental) AND (ulcer* or candidi or mucositis or health or odour* or odor* or malodour* or malodor or cancer or carcinoma* or neoplasm* or tumor* or tumour* or lesion* or malignan*)) #8 TS=(xerostomi* or "dry mouth*") #9 TS=((aphthous or aphthae) and ulcer*) #10 TS=(breath* AND (odor* or odour* or malodour* or malodor*)) #11 TS=(leukoplak* or "hairy tongue*") #12 TS=(toothbrush* or tooth‐brush* or toothpaste* or dentifrice* or mouthwash* or mouth‐wash* or mouthrinse* or mouth‐rinse* or fluoride* or floss* or "interdental brush*" or "inter‐dental brush*" or "interdental clean*" or "inter‐dental clean*" or (tooth and clean*) or (teeth and clean*) or (mouth and health) or (mouth and care) or (mouth and hygiene) or (denture* and hygiene) or (tongue* and scrap*) or (tongue* and brush*) or (chewing and stick*) or (chewing and gum*)) #13 #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 #14 TS=(instruct* or advis* or advice or educat* or promot* or teach* or train* or demonstrat* or supervis*) #15 TS=(lecture* or seminar* or presentation* or session* or program* or tutorial* or video* or audio* or DVD* or online or podcast* or vodcast*) #16 TS=(leaflet* or manual* or book* or pamphlet* or brochure*) #17 #14 or #15 or #16 #18 #1 and #13 and #17 #19 TS=(random* or trial* or placebo* or group*) #20 #18 and #19
Appendix 7. US National Institutes of Health Trials Register (ClinicalTrials.gov) search strategy
A series of keyword searches was performed:
home and oral hygiene and education facility and oral hygiene and education residence and oral hygiene and education institution and oral hygiene and education home and oral hygiene and instruction facility and oral hygiene and instruction residence and oral hygiene and instruction institution and oral hygiene and instruction home and oral hygiene and advice facility and oral hygiene and advice residence and oral hygiene and advice institution and oral hygiene and advice home and oral hygiene and teach facility and oral hygiene and teach residence and oral hygiene and teach institution and oral hygiene and teach home and oral hygiene and train facility and oral hygiene and train residence and oral hygiene and train institution and oral hygiene and train home and oral hygiene and program facility and oral hygiene and program residence and oral hygiene and program institution and oral hygiene and program home and oral hygiene and programme facility and oral hygiene and programme residence and oral hygiene and programme institution and oral hygiene and programme
Appendix 8. WHO International Clinical Trials Registry Platform search strategy
A series of keyword searches was performed:
home and “oral hygiene” and education facility and “oral hygiene” and education residence and “oral hygiene” and education institution and “oral hygiene” and education home and “oral hygiene” and instruction facility and “oral hygiene” and instruction residence and “oral hygiene” and instruction institution and “oral hygiene” and instruction home and “oral hygiene” and advice facility and “oral hygiene” and advice residence and “oral hygiene” and advice institution and “oral hygiene” and advice home and “oral hygiene” and teach facility and “oral hygiene” and teach residence and “oral hygiene” and teach institution and “oral hygiene” and teach home and “oral hygiene” and train facility and “oral hygiene” and train residence and “oral hygiene” and train institution and “oral hygiene” and train home and “oral hygiene” and program facility and “oral hygiene” and program residence and “oral hygiene” and program institution and “oral hygiene” and program home and “oral hygiene” and programme facility and “oral hygiene” and programme residence and “oral hygiene” and programme institution and “oral hygiene” and programme
Data and analyses
Comparison 1. Educational intervention (with information and practical components) versus (optimised) usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dental plaque (subgroup analysis intervention recipient) | 6 | 353 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.26, 0.17] |
| 1.1 Education for staff | 5 | 321 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.29, 0.00] |
| 1.2 Education for staff and residents | 1 | 32 | Mean Difference (IV, Random, 95% CI) | 0.38 [0.08, 0.68] |
| 2 Dental plaque (subgroup analysis follow‐up) | 6 | 353 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.26, 0.17] |
| 2.1 Short term (up to six months) | 5 | 306 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.31, 0.18] |
| 2.2 Long term (more than six months) | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.33, 0.53] |
| 3 Dental plaque (subgroup analysis guideline implementation short‐term follow‐up) | 5 | 306 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.31, 0.18] |
| 3.1 Education in the context of guideline implementation | 2 | 129 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.45, 0.06] |
| 3.2 Education without guideline implementation | 3 | 177 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.36, 0.39] |
| 4 Denture plaque (subgroup analysis intervention recipient) | 5 | 524 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.25, 0.05] |
| 4.1 Education for staff | 4 | 476 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.73 [‐1.47, 0.00] |
| 4.2 Education for staff and residents | 1 | 48 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.60, 0.54] |
| 5 Denture plaque (subgroup analysis follow‐up) | 5 | 524 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐1.25, 0.05] |
| 5.1 Short term (up to six months) | 4 | 437 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.48, 0.12] |
| 5.2 Long term (more than six months) | 1 | 87 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.70, 0.14] |
| 6 Denture plaque (subgroup analysis guideline implementation short‐term follow‐up) | 4 | 437 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.48, 0.12] |
| 6.1 Education in the context of guideline implementation | 2 | 221 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.68, ‐0.14] |
| 6.2 Education without guideline implementation | 2 | 216 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.94 [‐2.68, 0.81] |
| 7 Oral health‐related knowledge (staff) | 2 | 275 | Std. Mean Difference (IV, Random, 95% CI) | 0.94 [‐0.04, 1.92] |
| 8 Oral health‐related attitude (staff) | 2 | 275 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.23, 0.83] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bellomo 2005.
| Methods | Randomised controlled trial, 2 arms (each with 2 subgroups: independent and assisted) Study period: not reported Planned follow‐up period: 6 months |
|
| Participants | Country: Switzerland Setting: long‐term care home Target audience for intervention: long‐term care home residents Number of randomised participants (long‐term care home residents): 61 Number of analysed participants (long‐term care home residents): 61 Age, mean, years: 85.4 in IG, 86.8 in CG (range for both groups 72 to 97 years) Female: 73.3% in IG, 71% in CG Edentulous: 36.7% in IG, 35.6% in CG Residents with dental prosthesis: 80% (of all participants) Inclusion criteria: residents of the long‐term care home (irrespective of state of (oral) health) Exclusion criteria: n/a |
|
| Interventions |
Intervention group 2 subgroups (one with independent residents (IG1) and one with residents who are in need of assistance (IG2)): Instruction/skills training:
Education:
Control group 2 subgroups (one with independent residents (CG1) and one with residents who are in need of assistance (CG2))
Education:
Co‐interventions n/a |
|
| Outcomes |
Primary outcomes Oral hygiene measures, level of autonomy (brushing), and residents' oral health‐related behaviour were assessed at baseline, 3 and 6 months after start of the study
|
|
| Funding | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information in the paper (correspondence: "randomisation was done by the nursing home via the patient allocation list (availability of rooms) in either one building or the other. no computer sequence generation etc.") |
| Allocation concealment (selection bias) | High risk | Insufficient information in the paper (correspondence: "no concealment") |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Groups corresponded to 2 separate buildings, which assured blinding of participants to the other study arm. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Insufficient information in the paper (correspondence: "outcome assessor was not blinded") |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two participants passed away during the experimental period and were excluded from analysis." (study group affiliation unclear) |
| Selective reporting (reporting bias) | Unclear risk | Correspondence: protocol not available, no trial registration |
| Other bias | Low risk | None apparent |
De Visschere 2011.
| Methods | Cluster‐randomised controlled trial, 3 arms Study period: 2003 to 2008 Planned follow‐up period: 5 years |
|
| Participants | Country: Belgium (Flanders) Setting: 14 nursing homes (selected out of 36 nursing homes using stratified cluster sampling) Target audience of intervention: nursing staff (nurses, nursing assistants, and nurse aides) Number of randomised participants (nursing home residents): 1393 Number of analysed participants (nursing home residents): 70 to 214 depending on outcome and measurement point Age, mean, years (SD): 84.93 (6.97) in IG, 86.0 (7.36) in CG1, 83.18 (8.63) in CG2 Female: 73.9% in IG, 77.6% in CG1, 74.4% in CG2 Edentulous residents: 67.8% in IG, 67.9% in CG1, 68.5% in CG2 Residents with dental prosthesis: 77% (all groups) Inclusion criteria: all residents eligible Exclusion criteria: n/a |
|
| Interventions |
Intervention group Implementation of an oral hygiene protocol including multiple components: Education/skills training:
Additional components:
Control group CG1: usual oral hygiene CG2: complex intervention was implemented in the nursing home, but residents did not receive intervention Co‐interventions n/a |
|
| Outcomes |
Primary outcomes Oral hygiene measures were assessed at baseline, and every year after the start of the study for a period of 5 years.
|
|
| Funding | GABA International (supported data collection) | |
| Notes | Results/data for primary outcomes only available for baseline, 2 years and 5 years after the start of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly selected" Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants and personnel or incomplete blinding, but the review authors judge that the assessed outcomes are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "examiners were blind as to which residents were included in the intervention or not" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Correspondence: "every year residents were randomly selected using stratified cluster sampling for outcome assessment (at least 20% residents per nursing home)" Comment: number of residents per assessment period (every year) unclear |
| Selective reporting (reporting bias) | High risk | No protocol found; results/data for primary outcomes only available for baseline, 2 years and 5 years after the start of the study. |
| Other bias | High risk | Possible contamination between groups, and high risk of recruitment bias (selection/recruitment of participants after randomisation) |
De Visschere 2012.
| Methods | Cluster‐randomised controlled trial, 2 arms Study period: spring to winter 2009 Planned follow‐up period: 6 months |
|
| Participants | Country: Belgium (Flanders) Setting: 12 nursing homes (with somatic and psychogeriatric residents) Target audience for the intervention: nursing home staff Number of randomised participants (nursing home residents; nursing staff): 373; 760 Number of analysed participants (nursing home residents; nursing staff): 297; 259 Age, mean, years (SD): 84.5 (8.5) in IG, 84.9 (7.6) in CG Female: 68.4% in IG, 78.0% in CG Edentulous residents: 55% in IG, 54.3% in CG Residents with dental prosthesis: 72.3% in IG, 69.1% in CG Inclusion criteria: having teeth and/or (removable) partial or complete dentures; physically suitable for examination; expected to be residing in the care home during the entire 6‐month period Exclusion criteria: residents in day care, in short‐term residency, in coma, in palliative care or terminally ill, using a denture adhesive, expressing verbal or physical resistiveness before or during an oral examination |
|
| Interventions |
Intervention group Supervised implementation of the "Oral health care Guideline for Older people in Long‐term care Institutions (OGOLI)" including multiple components: Education/skills training:
Information material:
Additional components:
Control group Usual oral health care according to the no‐supervised implemented guideline (since 2007 Dutch guideline OGOLI) The intervention was implemented in the control nursing homes after completion of data collection. Co‐interventions (for the intervention group)
|
|
| Outcomes |
Primary outcomes Oral hygiene measures were assessed at baseline and 6 months after the start of the study.
Oral health‐related knowledge and attitude were assessed at baseline and 6 months after the start of the study.
|
|
| Funding | Research project is funded by Open Ankh, Soest and Stichting De Opbouw, Utrecht, the Netherlands. Oral healthcare products were provided free by GABA International, Eureka Pharma Belgium, Oral‐B Belgium, and Johnson & Johnson consumer S.A/N.V. |
|
| Notes | Questionnaire (included 3 parts: personal items, attitude statements, knowledge statements): The reliability of the attitude part of the questionnaire was measured by a test–retest procedure in a comparable nursing home not involved in the study. Content and construct validity (knowledge part) was assessed by experts in the field of gerodontology including 1 dental hygienist and 3 dentists. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomly allocated to the intervention (n = 6) or the control group (n = 6) using computer‐aided tools" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants and personnel or incomplete blinding, but the review authors judge that the assessed outcomes are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The examiners were masked, they did not know whether a nursing home was allocated to the intervention or the control group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (De Visschere, clinical measures of nursing home residents): "main reasons for loss to follow‐up: death (35%), administrative errors (30%), address change, move or absence (15%), hospitalisation or sickness (9%) or refusals (9%). There were no differences in loss to follow‐up between those randomised to the intervention group and to the control group." Quote (Janssens, knowledge of nursing staff): baseline N = 760; "259 (34%) respondents were found, 165 belonging to the intervention group and 94 to the control group." "All respondents were analysed in the groups to which they were originally allocated (intention to treat analysis). There were no significant differences between the respondents (n = 259) and non‐respondents (n = 392) for the explanatory and outcome variables." |
| Selective reporting (reporting bias) | High risk | Outcomes reported as planned in the study protocol. Tongue coating as outcome not preplanned. |
| Other bias | Low risk | None apparent |
Frenkel 2001.
| Methods | Cluster‐randomised controlled trial, 2 arms Study period: not reported Planned follow‐up period: 6 months |
|
| Participants | Country: UK Setting: 22 nursing homes (with 20 to 40 beds) Target audience for intervention: nursing staff Number of randomised participants (nursing home residents; nursing staff): 412; 322 Number of analysed participants (nursing home residents; nursing staff): 337; 232 Age, mean, years (SD): 84.9 (8.2) in IG, 84.0 (8.3) in CG Female: 81.1% in IG, 75.8% in CG Edentulous: 75.1% in IG, 67.8% in CG Residents with dental prosthesis: 80.6% in IG, 80.1% in CG Inclusion criteria: residents who either wore dentures and/or had one or more natural teeth, and whose general health permitted oral examination to take place Exclusion criteria: residents with significant cognitive impairment |
|
| Interventions |
Intervention group Education/skills training:
Information material:
Control group Usual care (health education programme was delivered after completion of data collection) Co‐interventions
|
|
| Outcomes |
Primary outcomes Oral hygiene measures were assessed at baseline, 1 month and 6 months after education.
Caregiver oral health knowledge and attitudes were assessed at baseline, 1 month and 6 months after education.
Secondary outcomes The secondary outcome variables were recorded on a binary scale (absent/present).
|
|
| Funding | Grants from the NHS Executive South West (Research and Development Directorate) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Block randomisation (block size 4) was performed using a table of random numbers by an independent researcher not involved in data collection or delivery of the intervention." |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation was communicated directly and solely to the Health Promoter, and kept by her in a secure location during the trial. Staff in all participating nursing homes were asked to conceal their group allocation from the examiner." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants and personnel or incomplete blinding, but the review authors judge that the assessed outcomes are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "single‐blinded" and "examiner blindness" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Some indication of completeness of follow‐up except for dental plaque measure (some teeth could not be scored) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | None apparent |
MacEntee 2007.
| Methods | Cluster‐randomised controlled trial, 2 arms Study period: not reported Planned follow‐up: 3 months |
|
| Participants | Country: Canada Setting: 14 long‐term care facilities Target audience for intervention: care‐aides Number of randomised participants (nursing home residents with intermediate care): 127 Number of analysed participants (nursing home residents with intermediate care): 113 Age, mean, years: 78.3 in IG, 79.9 in CG Female: not reported Edentulous: excluded Residents with dental prosthesis: not reported Inclusion criteria: residents receiving intermediate care, with natural teeth, and cognitively and physically suitable for a clinical examination of the mouth Exclusion criteria: not explicitly stated |
|
| Interventions |
Intervention group Pyramidal education consisting of the following. Education/skills training:
Information material:
Additional components:
Control group Usual routine (comprises the same 1‐hour seminar with photographs, texts, and demonstrations but delivered directly to the care‐aides by the dental hygienist. No additional information or support.) Co‐interventions n/a |
|
| Outcomes |
Primary outcomes Oral hygiene measures were assessed at baseline and 3 months after the seminar.
Secondary outcomes Clinical measures of dietary nourishment and masticatory potential were assessed at baseline and 3 months after the seminar.
Additional outcomes: psychosocial outcomes were assessed using qualitative research methods (questionnaires, interviews). |
|
| Funding | BC Medical Service Foundation Grant | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised block design"; correspondence: "generated from a programme by a research assistant" |
| Allocation concealment (selection bias) | Low risk | Quote: "A person not involved in the education or analysis of results performed the random selections and assignments, and broke the code when all data were collected." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double‐blind.... Neither the examiner nor the residents knew the intervention assignments." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The examiner or assistant did not know the educational method assigned to the facilities, nor did they know the results from the baseline examinations when examining the residents 3 months later." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data for outcomes (gingival bleeding score, tab. 3) were not collected from all participants due to some participants being unable or unwilling to co‐operate fully. Losses to follow‐up comparable in intervention (N = 8) and control group (N = 6). |
| Selective reporting (reporting bias) | Low risk | Insufficient information in published study report, additional data provided on request. Results of the psychosocial aspects not published/not available. |
| Other bias | Unclear risk | Possible recruitment bias (insufficient information) |
Mojon 1998.
| Methods | Cluster‐randomised controlled trial, 2 arms Study period: not reported Planned follow‐up period: 18 months |
|
| Participants | Country: Switzerland Setting: 1 long‐term care facility (12 wards) Target audience for intervention: nursing staff (nurses and nurse aides) and residents Number of randomised participants (nursing home residents): 237 Number of analysed participants (nursing home residents): 159 Age, mean, years: 83.5 in IG, 84.6 in CG Female: 67% in IG, 69% in CG Edentulous: not reported Residents with dental prosthesis: 62.1% Inclusion criteria: residents Exclusion criteria: residents who could not provide informed consent (cognitive impairment) |
|
| Interventions |
Intervention group Education/skills training:
Control group Usual care (prior to the study no oral hygiene care was provided by the caregivers on a regular basis) Cleaning of the teeth only took place if requested by the dentist and oral care by a dentist only at request of the resident, family, or caregivers. Co‐interventions
|
|
| Outcomes |
Primary outcomes Oral hygiene measures and infections were assessed at baseline and 18 months later.
|
|
| Funding | The research project was supported by the Swiss National Foundation for Research. Oral healthcare products were donated by GABA AG, Mibelle AG Cosmetics, and Beecham Markenartikel AG. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Correspondence: "random number table" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants and personnel or incomplete blinding, but the review authors judge that the assessed outcomes are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The dentist who performed the follow‐up examination did not have access to the baseline examination form and he was not informed in which ward the resident was living." However, "it was not possible, for practical reasons, to prevent systematically the dentist in charge of the follow‐up examinations to know the ward in which the residents were living." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up (Budtz‐Jorgensen): "40 residents in the intervention group and 38 in the control group" (reason: all died) Lost to follow‐up (Mojon): "18 died during the study period, 10 became edentulous, and 2 were terminally ill at the time of the second examination. Among the 86 remaining residents, 7 refused the oral examination and 23 refused the bacterial sampling." (group allocation unclear) |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | None apparent |
Schou 1989.
| Methods | Cluster‐randomised controlled trial, 4 arms Study period: January 1985 to September 1985 Planned follow‐up period: 9 months |
|
| Participants | Country: Scotland, UK Setting: 4 institutions for the elderly Target audience for the intervention: nursing staff or residents, or both Number of randomised participants (residents): 201 Number of analysed participants (residents): 112 to 140 (depending on the outcome measure) Age, mean, years (range): 82 (48 to 99) for all study participants Female: not reported Edentulous: 89.9% of all study participants Residents with dental prosthesis: not reported Inclusion criteria: not reported Exclusion criteria: n/a |
|
| Interventions |
Intervention group 3 groups with a dental health education programme utilising the active involvement principle targeted at different audiences Education:
Control group Usual care (no educational programme) Co‐interventions n/a |
|
| Outcomes |
Primary outcomes Oral hygiene measures and oral health‐related behaviour were assessed at baseline and 2 months after termination of the programme (9 months after the start).
|
|
| Funding | Not reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The four institutions were (...) randomly allocated to a control group or one of the three programmes" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants and personnel or incomplete blinding, but the review authors judge that the assessed outcomes are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "dentist who examined all the subjects and who was blind to the individual's affiliation to experimental respective control groups." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Unclear risk | Possible recruitment bias (insufficient information) |
Van der Putten 2013.
| Methods | Cluster‐randomised controlled trial, 2 arms Study period: not reported Planned follow‐up period: 6 months |
|
| Participants | Country: Netherlands Setting: 12 nursing homes Target audience for the intervention: nursing home staff Number of randomised participants (nursing home residents): 343 Number of analysed participants (nursing home residents): 232 Age, mean, years (SD): 80.4 (9.4) in IG, 80.7 (10.9) in CG Female: 66% in IG, 69% in CG Edentulous: 70% in IG, 62% in CG Residents with dental prosthesis: 84% in IG, 75% in CG Inclusion criteria: having teeth and/or (removable) partial or complete dentures; physically suitable for examination; expected to be residing in the care home during the entire 6‐month period Exclusion criteria: residents in day care, in short‐term residency (on a rehabilitation ward, residing 2 weeks to 3 months = less than 6 months on this ward), in coma, in palliative care or terminally ill, using a denture adhesive, expressing verbal or physical resistiveness before or during an oral examination |
|
| Interventions |
Intervention group Supervised implementation of the "Oral health care Guideline for Older people in Long‐term care Institutions (OGOLI)" including multiple components: Education/skills training:
Information material:
Additional components:
Control group Usual oral health care according to the no‐supervised implemented guideline (since 2007 Dutch guideline OGOLI) Co‐interventions (for the intervention group)
|
|
| Outcomes |
Primary outcomes Oral hygiene measures were assessed at baseline and 6 months after the start of the study.
Planned outcomes
|
|
| Funding | Research project is funded by Open Ankh Foundation, Utrecht; The Opbouw Foundation, Utrecht; ZonMw, The Hague; Vereniging Zonnehuis, Amstelveen; Stichting Wetenschapsbevordering Verpleeghuiszorg, Amsterdam; MarkTwo Communications, Leusden und Fonds NutsOhra, Amsterdam. Oral healthcare products were provided free by GABA International, NoviaCura, and Johnson & Johnson. |
|
| Notes | Publication of oral health‐related knowledge and attitude in preparation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "allocated randomly" Comment: Probably done, since another report from the same investigators (one study protocol for both trials) described use of "computer‐aided tools" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants and personnel or incomplete blinding, but the review authors judge that the assessed outcomes are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "examiners were blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Over the course of the trial, 111 of the residents (32%) were lost to follow‐up, 62 (35%) in the intervention group and 49 (29.5%) in the control group. There were no significant differences in loss to follow‐up between the intervention and the control group (Chi‐square, p=0.18). The main reasons for loss to follow‐up were: deceased (66%), administrative error (7%), moved to another care home or otherwise absent (8%), intermediate disease (14%) or refusal (5%). There were also no statistically significant differences in residents’ personal and medical characteristics and dental and denture plaque scores at baseline between residents who completed the study and those who did not." |
| Selective reporting (reporting bias) | Low risk | Additional outcomes planned in the protocol (knowledge and attitude of nursing staff), paper in preparation for knowledge and attitude results (correspondence) |
| Other bias | Low risk | None apparent |
Zenthöfer 2013.
| Methods | Randomised controlled trial, 4 arms Study period: not reported Planned follow‐up period: 12 weeks (re‐evaluation after 3 years for IG) |
|
| Participants | Country: Germany (South‐West Germany) Setting: 8 institutions for elderly people (nursing homes including assisted accommodation) Target audience for intervention: nursing home residents and caregiver staff Number of randomised participants (nursing home residents): 106 Number of analysed participants (nursing home residents): 102 Age, mean, years: 81.5 in IG1, 82.6 in IG2, 79.5 in IG3, 79.4 in CG (mean age (SD), years, range for all study participants: 80.8 (7.45), 49 to 95) Female: 80.8% in IG1, 85.2% in IG2, 80.8% in IG3, 65.2% in CG (78.4% of all participants) Edentulous residents: 32.4% (all groups) Residents with dental prosthesis: 87.3% (all groups) Inclusion criteria: residents at care level 1 or with no care level (Germany has care insurance for financial support of people who need care. Care levels are numbered from 0 to 3, which increase with people’s need for care. To receive financial help at care level 1, a person must have a need for care of a minimum of 90 min per day, of which 45 min must be basic care, e.g. personal hygiene and nutrition.) Exclusion criteria: residents with dementia, severe infectious diseases, care level 2, or care level 3 |
|
| Interventions |
Intervention group(s) 3 groups with multiple interventions. Basic intervention for all 3 intervention groups: Education/instruction:
Information material:
Additional interventions:
Control group Usual oral hygiene Co‐interventions (for residents in the 3 intervention groups)
|
|
| Outcomes |
Primary outcomes Oral hygiene measures were assessed at baseline and 2 weeks, 6 weeks, 12 weeks, and 3 years after the intervention.
|
|
| Funding | Mouth rinses and toothpaste were provided free by GABA GmbH, Germany. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The principal investigator assigned group membership by lot" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Principal investigator gave the information to the second study clinician" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding of participants and personnel or incomplete blinding, but the review authors judge that the assessed outcomes are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Baseline and recall examinations (...) were performed without knowledge of the group to which the participant belonged (single‐blinded)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants (3.7%) were lost to follow‐up after 12 weeks (1 died, 1 was no longer in the home, 1 had to stay in hospital, and 1 no longer wished to participate). |
| Selective reporting (reporting bias) | Unclear risk | No protocol found |
| Other bias | Low risk | None apparent |
CG: control group IG: intervention group SD: standard deviation WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avenali 2011 | Participant age (mean age below 42 years) |
| Beck 210 | Not primarily an educational intervention |
| Czarkowski 2013 | Not a randomised controlled trial |
| Davies 1990 | Not a randomised controlled trial |
| Gammack 2009 | Not a randomised controlled trial |
| Jönsson 2012 | Not nursing home based |
| Lange 2000 | Participant age (ranging from 32 to 64 years) |
| Nicol 2005 | Not a randomised controlled trial |
| Park 2011 | Not a randomised controlled trial |
| Park 2014 | No predefined outcome measures |
| Paulsson 1998 | Not a randomised controlled trial |
| Quagliarello 2009 | No educational intervention |
| Simons 2000 | Not a randomised controlled trial |
| Sniehotta 2007 | Participants were students |
| Tan 2010 | Not primarily an educational intervention |
| Vandamme 2006 | No educational intervention |
| Wyatt 2009 | No educational intervention |
| Yonezawa 2003 | No educational intervention |
Characteristics of ongoing studies [ordered by study ID]
Jablonski 2011.
| Trial name or title | Reducing care‐resistant behaviors during oral hygiene in persons with dementia |
| Methods | Randomised controlled trial Estimated study completion date: April 2015 Study completion date: November 2015 |
| Participants | Country: Pennsylvania, USA Inclusion criteria: Nursing home residents (English‐speaking, age 65 or older, documented diagnosis of dementia, Alzheimer's disease, vascular dementia, or Lewy body dementia, identified by nursing home staff as resistant to mouth care, at least 2 adjacent teeth and/or daily wearing of at least 1 denture plate, the ability to hold a toothbrush, the ability to move his or her hand to his or her mouth) Estimated enrolment: 80 |
| Interventions | Behavioural: "Managing Oral Hygiene Using Threat Reduction (MOUTh)" The intervention combines best mouth care practices with a constellation of behavioural techniques that reduce threat perception and thereby prevent or de‐escalate care‐resistant behaviour. |
| Outcomes | Primary outcome measures: Reduction in care‐resistant behaviour (time frame: 4 weeks). Care‐resistant behavior will be measured using a refinement of the Resistiveness to Care Scale (specifically for use with people with dementia). Secondary outcome measures: Oral health (time frame: 4 weeks) will be measured as the total score obtained from the Oral Health Assessment Tool. |
| Starting date | April 2011 |
| Contact information | Principal Investigator: Rita A Jablonski, Penn State University |
| Notes | ClinicalTrials.gov identifier: NCT01363258 |
Differences between protocol and review
In addition to the resources outlined in the protocol, we also searched Web of Science Conference Proceedings and the World Health Organization Clinical Trials Registry Platform.
We used the revised reporting guideline CReDECI 2 instead of the original CReDECI criteria to retrieve data on process evaluation of the complex interventions (Möhler 2012).
In the case of continuous data as measures of treatment effect, we used mean differences or standardised mean differences between study groups at follow‐up instead of using differences in mean change from baseline.
In the Unit of analysis issues section, we planned to use intraclass correlation coefficients from included studies to calculate effective sample sizes. As there were no or unrealistic values, we used a conservative estimate based on Cochrane Oral Health experience of studies on caries in nursing home residents.
We did not use imputation techniques to account for missing data, as missing data were mostly equally distributed between groups, and numbers seemed reasonable for a population of nursing home residents.
To account for heterogeneity and apparent problems in synthesising complex interventions, we had originally planned to only conduct meta‐analysis for the same intervention (main trial and replication trials). After advice from Cochrane Oral Health, we did not pursue the initial plan and conducted a number of meta‐analyses on heterogeneous trials.
In the Subgroup analysis and investigation of heterogeneity section, we stated in the protocol that we would conduct subgroup analyses for study design (cluster randomised versus individual randomised participants) and type of control intervention (usual care versus active control). Due to the small number of studies, we were unable to conduct all predefined subgroup analyses, but instead conducted two additional subgroup analyses: follow‐up period (short term versus long term) and education in the context of guideline implementation versus education without guideline implementation.
Due to a lack of studies with low risk of bias, we did not perform sensitivity analyses based on risk of bias.
Contributions of authors
Martina Albrecht (MA) initially planned the review and wrote the protocol with important contribution from Sascha Köpke (SK). MA, SK, and Ramona Kupfer (RK) extracted and interpreted the data. MA wrote the review with important contribution from SK. RK, Daniel Reissmann (DR), and Ingrid Mühlhauser (IM) substantially commented on the draft versions.
Sources of support
Internal sources
Unit of Health Sciences and Education, University of Hamburg, Germany.
Nursing Research Group, Institute for Social Medicine, University of Lübeck, Germany.
External sources
Ministry of Education and Research, Germany.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
-
Cochrane Oral Health Global Alliance, UK.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (ohg.cochrane.org/partnerships‐alliances). Contributors over the last year have been: British Association for the Study of Community Dentistry, UK; British Society of Paediatric Dentistry, UK; Centre for Dental Education and Research at All India Institute of Medical Sciences, India; National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; NHS Education for Scotland, UK
Declarations of interest
Martina Albrecht: none known Ramona Kupfer: none known Daniel R Reissmann: none known Ingrid Mühlhauser: none known Sascha Köpke: none known
New
References
References to studies included in this review
Bellomo 2005 {published data only}
- Bellomo F. L´utilisation de l´ergothérapie comme technique de guidage pour la prise en charge de l´hygiène bucco‐dentaire des personnes agèes institutionnalisèes. [Thèse de doctorat]. Univ. Genève, 2006. [Google Scholar]
- Bellomo F, Preux F, Chung JP, Julien N, Budtz‐Jørgensen E, Müller F. The advantages of occupational therapy in oral hygiene measures for institutionalised elderly adults. Gerodontology 2005;22:24‐31. [DOI] [PubMed] [Google Scholar]
- Müller F. On article "Advantages of occupational therapy in oral hygiene measures for institutionalised elderly adults" [personal communication]. Email to M Albrecht 1 September 2014. [DOI] [PubMed]
De Visschere 2011 {published data only}
- Visschere L. Information about studies on "oral hygiene protocols in nursing homes" [personal communication]. Email to M Albrecht 28 November 2014.
- Visschere L, Baat C, Meyer L, Putten GJ, Peeters B, Söderfeldt B, et al. The integration of oral health care into day‐to‐day care in nursing homes: a qualitative study. Gerodontology 2015;32(2):115‐22. [DOI] [PubMed] [Google Scholar]
- Visschere L, Baat C, Schols JMGA, Deschepper E, Vanobbergen J. Evaluation of the implementation of an 'oral hygiene protocol' in nursing homes: a 5‐year longitudinal study. Community Dentistry and Oral Epidemiology 2011;39:416‐25. [DOI] [PubMed] [Google Scholar]
De Visschere 2012 {published data only}
- Visschere L. [Information about studies on "oral hygiene protocols in nursing homes" [personal communication]]. Email to M Albrecht 28 November 2014.
- Visschere L, Baat C, Meyer L, Putten GJ, Peeters B, Söderfeldt B, et al. The integration of oral health care into day‐to‐day care in nursing homes: a qualitative study. Gerodontology 2015;32(2):115‐22. [DOI] [PubMed] [Google Scholar]
- Visschere L, Schols J, Putten GJ, Baat C, Vanobbergen J. Effect evaluation of a supervised versus non‐supervised implementation of an oral health care guideline in nursing homes: a cluster randomised controlled clinical trial. Gerodontology 2012;29:e96‐106. [DOI] [PubMed] [Google Scholar]
- Janssens B, Visschere L, Putten GJ, Lugt‐Lustig K, Schols J, Vanobbergen J. Effect of an oral healthcare protocol in nursing homes on care staffs' knowledge and attitude towards oral health care: a cluster‐randomised controlled trial. Gerodontology 2014;33(2):275‐86. [DOI: 10.1111/ger.12164] [DOI] [PubMed] [Google Scholar]
- Putten GJ, Visschere L, Schols J, Baat C, Vanobbergen J. Supervised versus non‐supervised implementation of an oral health care guideline in (residential) care homes: a cluster randomized controlled trial. BMC Oral Health 2010;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frenkel 2001 {published data only}
- Frenkel HF. A health education intervention to improve oral health among institutionalised elderly people: a randomised controlled trial [Dissertation]. University of Bristol, 1998. [Google Scholar]
- Frenkel HF, Harvey I, Needs KM. Oral health care education and its effect on caregivers' knowledge and attitudes: a randomised controlled trial. Community Dentistry and Oral Epidemiology 2002;30:91‐100. [DOI] [PubMed] [Google Scholar]
- Frenkel HF, Harvey I, Newcombe RG. Improving oral health in institutionalised elderly people by educating caregivers: a randomised controlled trial. Community Dentistry and Oral Epidemiology 2001;29:289‐97. [DOI] [PubMed] [Google Scholar]
- Newcombe RG. Information about the trial 'Improving oral health in institutionalised elderly people by educating caregivers: a randomised controlled trial' [personal communication]. Email to M Albrecht 3 February 2016. [DOI] [PubMed]
MacEntee 2007 {published data only}
- MacEntee M. Information about the trial 'Provision of mouth care in LTC facilities: an educational trial' and training material [personal communication]. Email to M Albrecht 15 January 2015.
- MacEntee MI, Wyatt CCL, Beattie BL, Paterson B, Levy‐Milne R, McCandless L, et al. Provision of mouth‐care in long‐term care facilities: an educational trial. Community Dentistry and Oral Epidemiology 2007;35:25‐34. [DOI] [PubMed] [Google Scholar]
- MacEntee MI, Wyatt CCL, Paterson B, Kazanjian A, Milne RL, Beattie L, et al. Impact of in‐house education on oral and general health of elders in long‐term care facilities ‐ research proposal. 2000.
Mojon 1998 {published data only}
- Budtz‐Jørgensen E, Mojon E, Rentsch A, Deslauriers N. Effects of an oral health program on the occurrence of oral candidosis in a long‐term care facility. Community Dentistry and Oral Epidemiology 2000;28:141‐9. [DOI] [PubMed] [Google Scholar]
- Mojon P. Randomization ‐ used methods of sequence generation [personal communication]. Email to M Albrecht 7 May 2014.
- Mojon P, Rentsch A, Budtz‐Jørgensen E, Baehni PC. Effects of an oral health program on selected clinical parameters and salivary bacteria in a long‐term care facility. European Journal of Oral Sciences 1998;106:827‐34. [DOI] [PubMed] [Google Scholar]
Schou 1989 {published data only}
- Schou L. Active‐involvement principle in dental health education. Community Dentistry and Oral Epidemiology 1985;13:128‐32. [DOI] [PubMed] [Google Scholar]
- Schou L. Article 'Oral health promotion for institutionalised elderly' [personal communication]. Email to: M Albrecht 20 January 2015.
- Schou L, Wight C, Clemson N, Douglas S, Clark C. Oral health promotion for institutionalised elderly. Dental Health Education 1989;17:2‐6. [DOI] [PubMed] [Google Scholar]
Van der Putten 2013 {published data only}
- Putten GJ. Information about the study 'Effectiveness of supervised implementation of an oral health care guideline in care homes: a single‐blinded cluster randomized controlled trial' [personal communication]. Email to M Albrecht 7 January 2015.
- Putten GJ, Mulder J, Baat C, Visschere L, Vanobbergen J, Schols J. Effectiveness of supervised implementation of an oral health care guideline in care homes; a single‐blinded cluster randomized controlled trial. Clinical Oral Investigations 2013;17:1143‐53. [DOI] [PubMed] [Google Scholar]
- Putten GJ, Visschere L, Schols J, Baat C, Vanobbergen J. Supervised versus non‐supervised implementation of an oral health care guideline in (residential) care homes: a cluster randomized controlled trial. BMC Oral Health 2010;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zenthöfer 2013 {published data only}
- Zenthöfer A. [Reply to request on article 'Improving oral hygiene in the long‐term care of the elderly ‐ a RCT' [personal communication]]. Email to: M Albrecht 27 November 2014.
- Zenthöfer A, Dieke R, Dieke A, Wege KC, Rammelsberg P, Hassel AJ. Improving oral hygiene in the long‐term care of the elderly ‐ a RCT. Community Dentistry and Oral Epidemiology 2013;41:261‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Avenali 2011 {published data only}
- Avenali L, Guerra F, Cipriano L, Corridore D, Ottolenghi L. Diasbled patients and oral health in Rome, Italy: long‐term evaluation of educational initiatives. Annali di Stomatologia 2011;2:25‐30. [PMC free article] [PubMed] [Google Scholar]
Beck 210 {published data only}
- Beck AM, Damkjaer K, Sørbye LW. Physical and social functional abilities seem to be maintained by a multifaceted randomized controlled nutritional intervention among old (>65 years) Danish nursing home residents. Archives of Gerontology and Geriatrics 2010;50:351‐5. [DOI] [PubMed] [Google Scholar]
Czarkowski 2013 {published data only}
- Czarkowski G, Allroggen S, Köster‐Schmidt A, Bausback‐Schomakers S, Frank M, Heudorf U. Oral health hygiene education programme for nursing personnel to improve oral health of residents in long‐term care facilities 2010 in Frankfurt/Main, Germany [Schulung von Pflegepersonal in Altenpflegeheimen zur Verbesserung der Mundhygiene bei den Bewohnern – Interventionsstudie in Frankfurt am Main 2010]. Gesundheitswesen 2013;75:368‐75. [DOI] [PubMed] [Google Scholar]
Davies 1990 {published data only}
- Davies KW, Whittle JG. Dental health education: training of home carers of mentally handicapped adults. Community Dental Health 1990;7:193‐7. [PubMed] [Google Scholar]
Gammack 2009 {published data only}
- Gammack JK, Pulisetty S. Nursing education and improvement in oral care delivery in long‐term care. Journal of the American Medical Directors Association 2009;10:658‐61. [DOI] [PubMed] [Google Scholar]
Jönsson 2012 {published data only}
- Jönsson B, Öhrn K, Lindberg P, Oscarson N. Cost‐effectiveness of an individually tailored oral health educational programme based on cognitive behavioural strategies in non‐surgical periodontal treatment. Journal of Clinical Periodontology 2012;39:659‐65. [DOI] [PubMed] [Google Scholar]
Lange 2000 {published data only}
- Lange B, Cook C, Dunning D, Froeschle ML, Kent D. Improving the oral hygiene of institutionalized mentally retarded clients. Journal of Dental Hygiene 2000;74:205‐9. [PubMed] [Google Scholar]
Nicol 2005 {published data only}
- Nicol R, Sweeney MP, McHugh S, Bagg J. Effectiveness of health care worker training on the oral health of elderly residents of nursing homes. Community Dentistry and Oral Epidemiology 2005;33:115‐24. [DOI] [PubMed] [Google Scholar]
Park 2011 {published data only}
- Park MS, Choi‐Kwon S. The effects of oral care education on caregivers' knowledge, attitude & behavior towards oral hygiene for elderly residents in a nursing home [구강간호교육이 노인요양시설 돌봄제공자의 구강간호 지식, 태도 및 행위와 재원노인의 구강위생에 미치는 효과]. Journal of Korean Academy of Nursing 2011;41:684‐93. [DOI] [PubMed] [Google Scholar]
Park 2014 {published data only}
- Park YH, Chang H. Effect of a health coaching self‐management program for older adults with multimorbidity in nursing homes. Patient Preference and Adherence 2014;8:959‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paulsson 1998 {published data only}
- Paulsson G, Fridlund B, Holmen A, Hyg O, Nederfors T. Evaluation of an oral health education program for nursing personnel in special housing facilities for the elderly. Special Care in Dentistry 1998;18:234‐42. [DOI] [PubMed] [Google Scholar]
Quagliarello 2009 {published data only}
- Quagliarello V, Juthani‐Mehta M, Ginter S, Towle V, Allore H, Tinetti M. Pilot testing of intervention protocols to prevent pneumonia in nursing home residents. Journal of the American Geriatrics Society 2009;57:1226‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simons 2000 {published data only}
- Simons D, Baker P, Jones B, Kidd EAM, Beighton D. An evaluation of an oral health training programme for carers of the elderly in residential homes. British Dental Journal 2000;188:206‐10. [DOI] [PubMed] [Google Scholar]
Sniehotta 2007 {published data only}
- Sniehotta FF, Araujo Soares V, Dombrowski SU. Randomized controlled trial of a one‐minute intervention changing oral self‐care behavior. Journal of Dental Research 2007;86:641‐5. [DOI] [PubMed] [Google Scholar]
Tan 2010 {published data only}
- Tan HP, Lo ECM, Dyson JE, Corbet EF. A randomized trial on root caries prevention in elders. Journal of Dental Research 2010;89:1086‐90. [DOI] [PubMed] [Google Scholar]
Vandamme 2006 {published data only}
- Vandamme K, Opdebeeck H, Naert I. Pathways in multidisciplinary oral health care as a tool to improve clinical performance. International Journal of Prosthodontics 2006;19:227‐35. [PubMed] [Google Scholar]
Wyatt 2009 {published data only}
- Wyatt CCL. A 5‐year follow‐up of older adults residing in long‐term care facilities: utilisation of a comprehensive dental programme. Gerodontology 2009;26:282‐90. [DOI] [PubMed] [Google Scholar]
Yonezawa 2003 {published data only}
- Yonezawa H, Takasaki K, Teraoka K, Asaka T, Sata C, Tsuchiya K. Effects of tongue and oral mucosa cleaning on oral Candida species and production of volatile sulfur compounds in the elderly in a nursing home. Journal of Medical and Dental Sciences 2003;50:1‐8. [PubMed] [Google Scholar]
References to ongoing studies
Jablonski 2011 {published data only}
- Jablonski RA, Kolanowski A, Therrien B, Mahoney EK, Kassab C, Lesli DL. Reducing care‐resistant behaviors during oral hygiene in persons with dementia. BMC Oral Health 2011;11:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abraham 2008
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychology 2008;27(3):379‐87. [DOI] [PubMed] [Google Scholar]
Ainamo 1975
- Ainamo J, Bay I. Problems and proposals for recording gingivitis and plaque. International Dental Journal 1975;25(4):229‐35. [PubMed] [Google Scholar]
Ambjørnsen 1982
- Ambjørnsen E, Valderhaug J, Norheim PW, Fløystrand F. Assessment of an additive index for plaque accumulation on complete maxillary dentures. Acta Odontologica Scandinavica 1982;40:203‐8. [DOI] [PubMed] [Google Scholar]
Atchison 1990
- Atchison KA, Dolan TA. Development of the Geriatric Oral Health Assessment Index. Journal of Dental Education 1990;54(11):680‐7. [PubMed] [Google Scholar]
Augsburger 1982
- Augsburger RH, Elahi JM. Evaluation of seven proprietary denture cleansers. Journal of Prosthetic Dentistry 1982;47:356‐9. [DOI] [PubMed] [Google Scholar]
Azarpazhooh 2006
- Azarpazhooh A, Leake JL. Systematic review of the association between respiratory diseases and oral health. Journal of Periodontology 2006;77(9):1465‐82. [DOI] [PubMed] [Google Scholar]
Benigeri 2000
- Benigeri M, Brodeur JM, Payette M, Charbonneau A, Ismail AI. Community periodontal index of treatment needs and prevalence of periodontal conditions. Journal of Clinical Periodontology 2000;27(5):308‐12. [DOI] [PubMed] [Google Scholar]
Branca 2009
- Branca S, Bennati E, Ferlito L, Spallina G, Cardillo E, Malaguarnera M, et al. The health‐care in the extreme longevity. Archives of Gerontology and Geriatrics 2009;49(1):32‐4. [DOI] [PubMed] [Google Scholar]
Budtz‐Jørgensen 1970
- Budtz‐Jørgensen E, Bertram U. Denture stomatitis. I. The etiology in relation to trauma and infection. Acta Odontologica Scandinavica 1970;28:71‐92. [DOI] [PubMed] [Google Scholar]
Budtz‐Jørgensen 1978
- Budtz‐Jørgensen E. Clinical aspects of Candida infection in denture wearers. Journal of the American Dental Association 1978;96:474‐9. [DOI] [PubMed] [Google Scholar]
Campbell 2000
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321(7262):694‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chalmers 2005
- Chalmers JM, King PL, Spencer AJ, Wright FA, Carter KD. The oral health assessment tool ‐ validity and reliability. Australian Dental Journal 2005;50(3):191‐9. [DOI] [PubMed] [Google Scholar]
Craig 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Baat 1993
- Baat C, Bruins H, Rossum G, Kalk W. Oral health care for nursing home residents in the Netherlands ‐ a national survey. Community Dentistry and Oral Epidemiology 1993;21(4):240‐2. [DOI] [PubMed] [Google Scholar]
De Lugt‐Lustig 2014
- Lugt‐Lustig KHME, Vanobbergen JNO, Putten GJ, Visschere LMJ, Schols JMGA, Baat C. Effect of oral healthcare education on knowledge, attitude and skills of care home nurses: a systematic literature review. Community Dentistry and Oral Epidemiology 2014;42:88‐96. [DOI] [PubMed] [Google Scholar]
De Visschere 2006
- Visschere LM, Grooten L, Theuniers G, Vanobbergen JN. Oral hygiene of elderly people in long‐term care institutions – a cross‐sectional study. Gerodontology 2006;23(4):195‐204. [DOI] [PubMed] [Google Scholar]
Desvarieux 2003
- Desvarieux M, Demmer RT, Rundek T, Boden‐Albala B, Jacobs DR Jr, Papapanou PN, et al. Relationship between periodontal disease, tooth loss, and carotid artery plaque: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Stroke 2003;34(9):2120‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Durham 2013
- Durham J, Fraser HM, McCracken GI, Stone KM, John MT, Preshaw PM. Impact of periodontitis on oral health‐related quality of life. Journal of Dentistry 2013;41:370‐6. [DOI] [PubMed] [Google Scholar]
El‐Rabbany 2015
- El‐Rabbany M, Zaghlol N, Bhandari M, Azarpazhooh A. Prophylactic oral health procedures to prevent hospital‐acquired and ventilator‐associated pneumonia: a systematic review. International Journal of Nursing Studies 2015;52:452‐64. [DOI] [PubMed] [Google Scholar]
Forsell 2009
- Forsell M, Sjögren P, Johansson O. Need of assistance with daily oral hygiene measures among nursing home resident elderly versus the actual assistance received from the staff. The Open Dentistry Journal 2009;3:241‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forsell 2011
- Forsell M, Sjogren P, Kullberg E, Johansson O, Wedel P, Herbst B, et al. Attitudes and perceptions towards oral hygiene tasks among geriatric nursing home staff. International Journal of Dental Hygiene 2011;9:199‐203. [DOI] [PubMed] [Google Scholar]
Frenkel 1999
- Frenkel HF. Behind the screens: care staff observations on delivery of oral health care in nursing homes. Gerodontology 1999;16(2):75‐80. [DOI] [PubMed] [Google Scholar]
Frenkel 2000
- Frenkel H, Harvey I, Newcombe RG. Oral health care among nursing home residents in Avon. Gerodontology 2000;17(1):33‐8. [DOI] [PubMed] [Google Scholar]
Glassman 1994
- Glassman P, Miller C, Woznick T, Jones C. A preventive dentistry training programme for caretakers of persons with disabilities residing in community residential facilities. Special Care in Dentistry 1994;14(4):137‐43. [DOI] [PubMed] [Google Scholar]
Gluhak 2010
- Gluhak C, Arnetzl GV, Kirmeier R, Jakse N, Arnetzl G. Oral status among seniors in nine nursing homes in Styria, Austria. Gerodontology 2010;27(1):47‐52. [DOI] [PubMed] [Google Scholar]
GRADE 2014 [Computer program]
- McMaster University. GRADEpro. www.gradepro.org. McMaster University, 2014.
Greene 1964
- Greene JC, Vermillion JR. The simplified oral hygiene index. Journal of the American Dental Association 1964;68:25‐31. [DOI] [PubMed] [Google Scholar]
Greene 1967
- Greene JC. The Oral Hygiene Index: development and uses. Journal of Periodontology 1967;38:625‐37. [PubMed] [Google Scholar]
Henriksen 1999
- Henriksen BM, Ambjørnsen E, Axéll TE. Evaluation of a mucosal‐plaque index (MPS) designed to assess oral care in groups of elderly. Special Care in Dentistry 1999;19(4):154‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. [DOI] [PubMed] [Google Scholar]
Hopcraft 2012
- Hopcraft MS, Morgan MV, Satur JG, Wright FA. Edentulism and dental caries in Victorian nursing homes. Gerodontology 2012;29(2):e512‐9. [DOI] [PubMed] [Google Scholar]
Isaksson 2007
- Isaksson R, Söderfeldt B. Oral status and treatment needs among elderly within municipal long‐term care 2002‐2004. Swedish Dental Journal 2007;31(1):45‐52. [PubMed] [Google Scholar]
Jablonski 2009
- Jablonski RA, Munro CL, Grap MJ, Schubert CM, Ligon M, Spigelmyer P. Mouth care in nursing homes: knowledge, beliefs, and practices of nursing assistants. Geriatric Nursing 2009;30(2):99‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1977
- Jones F, Fremouw W, Carples S. Pyramid training of elementary school teachers to use classroom management skills package. Journal of Applied Behavior Analysis 1977;10:239‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kayser‐Jones 1995
- Kayser‐Jones J, Bird WF, Paul SM, Long L, Schell ES. An instrument to assess the oral health status of nursing home residents. The Gerontologist 1995;35(6):814‐24. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lenz 2007
- Lenz M, Steckelberg A, Richter B, Mühlhauser I. Meta‐analysis does not allow appraisal of complex interventions in diabetes and hypertension self‐management: a methodological review. Diabetologia 2007;50(7):1375‐83. [DOI] [PubMed] [Google Scholar]
Löe 1967
- Löe H. The Gingival Index, the Plaque Index and the Retention Index Systems. Journal of Periodontology 1967;38(6):610‐6. [DOI] [PubMed] [Google Scholar]
Mack 2005
- Mack F, Schwahn C, Feine JS, Mundt T, Bernhardt O, John U, et al. The impact of tooth loss on general health related to quality of life among elderly Pomeranians: results from the study of health in Pomerania (SHIP‐O). International Journal of Prosthodontics 2005;18:414‐9. [PubMed] [Google Scholar]
Michie 2009
- Michie S, Fixsen D, Grimshaw JM, Eccles MP. Specifying and reporting complex behaviour change interventions: the need for a scientific method. Implementation Science 2009;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michie 2011
- Michie S, Stralen MM, West R. The behaviour change wheel: A new method for characterising and designing behaviour change interventions. Implementation Science 2011;6:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mojon 1999
- Mojon P, Budtz‐Jørgensen E, Rapin CH. Relationship between oral health and nutrition in very old people. Age and Ageing 1999;28(5):463‐8. [DOI] [PubMed] [Google Scholar]
Möhler 2012
- Möhler R, Bartoszek G, Köpke S, Meyer G. Proposed criteria for reporting the development and evaluation of complex interventions in healthcare (CReDECI): guideline development. International Journal of Nursing Studies 2012;49(1):40‐6. [DOI] [PubMed] [Google Scholar]
Möhler 2015
- Möhler R, Köpke S, Meyer G. Criteria for Reporting the Development and Evaluation of Complex Interventions in healthcare ‐ revised guideline (CReDECI 2). Trials 2015;16:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naito 2010
- Naito M, Kato T, Fujii W, Ozeki M, Yokoyama M, Hamajima N, et al. Effects of dental treatment on the quality of life and activities of daily living in institutionalized elderly in Japan. Archives of Gerontology and Geriatrics 2010;50(1):65‐8. [DOI] [PubMed] [Google Scholar]
Nitschke 2010
- Nitschke I, Majdani M, Sobotta BA, Reiber T, Hopfenmüller W. Dental care of frail older people and those caring for them. Journal of Clinical Nursing 2010;19(13‐14):1882‐90. [DOI] [PubMed] [Google Scholar]
O'Leary 1972
- O'Leary TJ, Drake RB, Naylor JE. The plaque control record. Journal of Periodontology 1972;43:38. [DOI] [PubMed] [Google Scholar]
Porter 2015
- Porter J, Ntoula A, Read A, Murdoch M, Ola D, Tsakos G. The impact of oral health on the quality of life of nursing home residents. Health and Quality of Life Outcomes 2015;13:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014
- Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riessman 1965
- Riessman F. The helper therapy principle. Social Work 1965;10:27‐32. [Google Scholar]
Saarela 2014
- Saarela RKT, Soini H, Hiltunen K, Muurinen S, Suominen M, Pitkala K. Dentition status, malnutrition and mortality among older service housing residents. Journal of Nutrition, Health and Aging 18;1:34‐8. [DOI] [PubMed] [Google Scholar]
Samson 2008
- Samson H, Strand GV, Haugejorden O. Change in oral health status among institutionalized Norwegian elderly over a period of 16 years. Acta Odontologica Scandinavica 2008;66(6):368‐73. [DOI] [PubMed] [Google Scholar]
Saunders 2007
- Saunders MJ, Stattmiller SP, Kirk KM. Oral health issues in the nutrition of institutionalized elders. Journal of Nutrition for the Elderly 2007;26(3‐4):39‐58. [DOI] [PubMed] [Google Scholar]
Scannapieco 2003
- Scannapieco FA, Bush RB, Paju S. Association between periodontal disease and risk for nosocomial bacterial pneumonia and chronic obstructive pulmonary disease. A systematic review. Annals of Periodontology 2003;8(1):54‐69. [DOI] [PubMed] [Google Scholar]
Schou 1985
- Schou L. Active‐involvement principle in dental health education. Community Dentistry and Oral Epidemiology 1985;13:128‐32. [DOI] [PubMed] [Google Scholar]
Seitz 2010
- Seitz D, Purandare N, Conn D. Prevalence of psychiatric disorders among older adults in long‐term care homes: a systematic review. International Psychogeriatrics/IPA 2010;22(7):1025‐39. [DOI] [PubMed] [Google Scholar]
Shay 2002
- Shay K. Infectious complications of dental and periodontal diseases in the elderly population. Clinical Infectious Diseases 2002;34(9):1215–23. [DOI] [PubMed] [Google Scholar]
Sheiham 2001
- Sheiham A, Steele JG, Marcenes W, Lowe C, Finch S, Bates CJ, et al. The relationship among dental status, nutrient intake, and nutritional status in older people. Journal of Dental Research 2001;80(2):408‐13. [DOI] [PubMed] [Google Scholar]
Silness 1964
- Silness J, Löe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontologica Scandinavica 1964;22:121‐35. [DOI] [PubMed] [Google Scholar]
Simunković 2005
- Simunković SK, Boras VV, Pandurić J, Zilić IA. Oral health among institutionalised elderly in Zagreb, Croatia. Gerodontology 2005;22(4):238‐41. [DOI] [PubMed] [Google Scholar]
Sjögren 2008
- Sjögren P, Nilsson E, Forsell M, Johansson O, Hoogstraate J. A systematic review of the preventive effect of oral hygiene on pneumonia and respiratory tract infection in elderly people in hospitals and nursing homes: effect estimates and methodological quality of randomized controlled trials. Journal of the American Geriatrics Society 2008;56(11):2124‐30. [DOI] [PubMed] [Google Scholar]
Slade 1994
- Slade GD, Spencer AJ. Development and evaluation of the Oral Health Impact Profile. Community Dental Health 1994;11(1):3‐11. [PubMed] [Google Scholar]
Slade 1997
- Slade GD. Derivation and validation of a short‐form oral health impact profile. Community Dentistry and Oral Epidemiology 1997;25(4):284‐90. [DOI] [PubMed] [Google Scholar]
Strauss 1993
- Strauss RP, Hunt RJ. Understanding the value of teeth to older adults: influences on the quality of life. Journal of the American Dental Association 1993;124(1):105‐10. [DOI] [PubMed] [Google Scholar]
Suomi 1968
- Suomi JD, Barbano JP. Patterns of gingivitis. Journal of Periodontology 1968;39:71‐4. [DOI] [PubMed] [Google Scholar]
Tonetti 2015
- Tonetti MS, Eickholz P, Loos BG, Papapanou P, Velden U, Armitage G, et al. Principles in prevention of periodontal diseases. Journal of Clinical Periodontology 2015;42(Suppl 16):S5‐11. [DOI: 10.1111/jcpe.12368] [DOI] [PubMed] [Google Scholar]
Walker 2007
- Walker RJ, Kiyak HA. The impact of providing dental services to frail older adults: perceptions of elders in adult day health centers. Special Care in Dentistry 2007;27(4):139‐43. [DOI] [PubMed] [Google Scholar]
Wang 2015
- Wang TF, Huang CM, Chou C, Yu S. Effect of oral health education programs for caregivers on oral hygiene of the elderly: A systemic review and meta‐analysis. International Journal of Nursing Studies 2015;52(6):1090‐6. [DOI: 10.1016/j.ijnurstu.2015.01.015] [DOI] [PubMed] [Google Scholar]
Wardh 2012
- Wårdh I, Jonsson M, Wikström M. Attitudes to and knowledge about oral health care among nursing home personnel ‐ an area in need of improvement. Gerodontology 2012;29(2):e787‐92. [DOI] [PubMed] [Google Scholar]
Weening‐Verbree 2013
- Weening‐Verbree L, Huisman‐de Waal G, Dusseldorp L, Acterberg T, Schoonhoven L. Oral health care in older people in long term care facilities: a systematic review of implementation strategies. International Journal of Nursing Studies 2013;50:569‐82. [DOI] [PubMed] [Google Scholar]
Wefers 1999
- Wefers KP. The 'Denture Hygiene Index' (DHI). Dental Forum 1999;1:13‐5. [Google Scholar]
Winkel 2003
- Winkel EG, Roldán S, Winkelhoff AJ, Herrera D, Sanz M. Clinical effects of a new mouthrinse containing chlorhexidine, cetylpyridinium chloride and zinc‐lactate on oral halitosis. A dual‐center, double‐blind placebo‐controlled study. Journal of Clinical Periodontology 2003;30:300‐6. [DOI] [PubMed] [Google Scholar]
Worthington 2015
- Worthington H, Clarkson J, Weldon J. Priority oral health research identification for clinical decision‐making. Evidence‐based Dentistry 2015;16(3):69‐71. [DOI] [PubMed] [Google Scholar]
Wyatt 2002
- Wyatt CC. Elderly Canadians residing in long‐term care hospitals: Part II. Dental caries status. Journal of the Canadian Dental Association 2002;68(6):359‐63. [PubMed] [Google Scholar]