Abstract
Background
A huge clinical research database on adjuvant cancer treatment has verified improvements in breast cancer outcomes such as recurrence and mortality rates. On the other hand, adjuvant and neoadjuvant therapy with chemotherapy and radiotherapy impacts on quality of life due to substantial short‐ and long‐term side effects. A number of studies have evaluated the effect of exercise interventions on those side effects. This is an updated version of the original Cochrane review published in 2006. The original review identified some benefits of physical activity on physical fitness and the resulting capacity for performing activities of daily life. It also identified a lack of evidence for other outcomes, providing clear justification for an updated review.
Objectives
To assess the effect of aerobic or resistance exercise interventions during adjuvant treatment for breast cancer on treatment‐related side effects such as physical deterioration, fatigue, diminished quality of life, depression, and cognitive dysfunction.
Search methods
We carried out an updated search in the Cochrane Breast Cancer Group Specialised Register (30 March 2015), the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 2, 2015), MEDLINE (1966 to 30 March 2015), and EMBASE (1966 to 30 March 2015). We did not update the original searches in CINAHL (1982 to 2004), SPORTDiscus (1975 to 2004), PsycINFO (1872 to 2003), SIGLE (1880 to 2004), and ProQuest Digital Dissertations (1861 to 2004). We searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) and ClinicalTrials.gov for ongoing trials on 30 March 2015. We screened references in relevant reviews and published clinical trials.
Selection criteria
We included randomised controlled trials that examined aerobic or resistance exercise or both in women undergoing adjuvant treatment for breast cancer. Published and unpublished trials were eligible.
Data collection and analysis
Two review authors independently performed data extraction, assessed trials, and graded the methodological quality using Cochrane's 'Risk of bias' tool. Any disagreements were resolved through discussion or by consulting the third review author. We entered data into Review Manager for analysis. For outcomes assessed with a variety of instruments, we used the standardised mean difference (SMD) as a summary statistic for meta‐analysis; for those assessed with the same instrument, we used the mean difference (MD).
Main results
For this 2015 update we included a total of 32 studies with 2626 randomised women, 8 studies from the original search and 24 studies from the updated search. We found evidence that physical exercise during adjuvant treatment for breast cancer probably improves physical fitness (SMD 0.42, 95% confidence interval (CI) 0.25 to 0.59; 15 studies; 1310 women; moderate‐quality evidence) and slightly reduces fatigue (SMD ‐0.28, 95% CI ‐0.41 to ‐0.16; 19 studies; 1698 women; moderate‐quality evidence). Exercise may lead to little or no improvement in health‐related quality of life (MD 1.10, 95% CI ‐5.28 to 7.48; 1 study; 68 women; low‐quality evidence), a slight improvement in cancer site‐specific quality of life (MD 4.24, 95% CI ‐1.81 to 10.29; 4 studies; 262 women; low‐quality evidence), and an improvement in cognitive function (MD ‐11.55, 95% CI ‐22.06 to ‐1.05; 2 studies; 213 women; low‐quality evidence). Exercise probably leads to little or no difference in cancer‐specific quality of life (SMD 0.12, 95% CI 0.00 to 0.25; 12 studies; 1012 women; moderate‐quality evidence) and little or no difference in depression (SMD ‐0.15, 95% CI ‐0.30 to 0.01; 5 studies; 674 women; moderate‐quality evidence). Evidence for other outcomes ranged from low to moderate quality. Seven trials reported a very small number of adverse events.
Authors' conclusions
Exercise during adjuvant treatment for breast cancer can be regarded as a supportive self care intervention that probably results in less fatigue, improved physical fitness, and little or no difference in cancer‐specific quality of life and depression. Exercise may also slightly improve cancer site‐specific quality of life and cognitive function, while it may result in little or no difference in health‐related quality of life. This review is based on trials with a considerable degree of clinical heterogeneity regarding adjuvant cancer treatments and exercise interventions. Due to the difficulty of blinding exercise trials, all included trials were at high risk for performance bias. Furthermore, the majority of trials were at high risk for detection bias, largely due to most outcomes being self reported.
The findings of the updated review have enabled us to make a more precise conclusion that both aerobic and resistance exercise can be regarded as beneficial for individuals with adjuvant therapy‐related side effects. Further research is required to determine the optimal type, intensity, and timing of an exercise intervention. Furthermore, long‐term evaluation is required due to possible long‐term side effects of adjuvant treatment.
Keywords: Female; Humans; Exercise Therapy; Breast Neoplasms; Breast Neoplasms/psychology; Breast Neoplasms/therapy; Chemotherapy, Adjuvant; Chemotherapy, Adjuvant/adverse effects; Cognition; Depression; Depression/therapy; Fatigue; Fatigue/rehabilitation; Lymphedema; Lymphedema/etiology; Physical Fitness; Quality of Life; Radiotherapy, Adjuvant; Radiotherapy, Adjuvant/adverse effects; Randomized Controlled Trials as Topic; Weight Gain
Plain language summary
Exercise for women receiving chemotherapy or radiation therapy or both (adjuvant therapy) for breast cancer
What is the issue?
In the past, women receiving cancer treatment were usually advised to rest and avoid physical activity. But, we now know that too much rest and too little physical activity can lead to muscle wasting. This reduces women's physical fitness level and may limit their regular activities. Women also often have other side effects that can affect their daily lives, such as extreme tiredness (fatigue), depression, and reduced mental functioning, for example being able to remember things or keep focused.
Why does it matter?
The side effects of breast cancer treatment can interfere with daily activities and return to work. It is important to learn of ways to reduce these side effects.
We asked if physical exercise during chemotherapy or radiation therapy or both helped to reduce treatment side effects. Side effects studied included tiredness, depression, and reduced physical fitness and mental functioning. We also studied general effects such as health‐related, cancer‐specific, and cancer site‐specific quality of life. Questionnaires for cancer‐specific quality of life ask questions that are important for patients with cancer in general, for example about pain or nausea. Cancer site‐specific quality of life is measured with questionnaires that ask women with breast cancer about topics that are especially important to them, for example about breast symptoms or body image. We only included questionnaires that have been shown to be reliable.
We found 32 studies involving 2626 women. The included studies were published up through March 2015. Not all studies considered all of these potential side effects. Combining the results of these studies suggests that physical exercise probably improves physical fitness and slightly lessens fatigue. These studies also suggest that physical exercise probably results in little or no improvement in cancer‐specific quality of life and depression. Exercise may improve mental function and slightly improve cancer site‐specific quality of life, although the quality of the evidence was low for both of these outcomes. It may result in little or no improvement in health‐related quality of life, however the quality of evidence was low for this outcome. The quality of evidence may have been low because many of the studies did not have enough participants to observe small differences and because results may be biased due to people assessing the outcomes knowing which participants were in the control group.
Importantly, physical exercise did not harm most women. Very few women experienced discomfort or pain in their arms or legs.
What does this mean?
It appears that exercise during cancer treatment can help lessen fatigue and improve physical fitness. It probably results in little or no improvement in cancer‐specific quality of life and depression. It is unknown whether it helps for other side effects. At least nine current studies will help to answer the question if and how much exercise helps with the mentioned side effects and other side effects.
Summary of findings
Summary of findings for the main comparison. Exercise compared with control for women receiving adjuvant therapy for breast cancer.
| Exercise compared with control for women receiving adjuvant therapy for breast cancer | ||||
|
Population: women receiving adjuvant therapy (chemo‐ or radiotherapy or both) for breast cancer Settings: supervised or home based Intervention: aerobic or resistance exercise or a combination of both Comparison: control intervention (usual care or intervention that was not exercise, such as stretching) | ||||
| Outcomes | Relative effects* (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Exercise vs control | ||||
|
Physical fitness assessed with: 6‐ or 12‐minute walk test, peak oxygen uptake, and other scales (follow‐up: 18 weeks to 6 months) |
The mean physical fitness in the intervention group was 0.42 standard deviations higher (0.25 to 0.59 higher) | 1310 (15 RCTs) |
⊕⊕⊕⊝ moderate1 | SMD 0.42 (95% CI 0.25 to 0.59) |
|
Fatigue assessed with: FACIT‐F scale, (revised) Piper Fatigue Scale, Multidimensional Fatigue Inventory and other scales (follow‐up: 18 weeks to 6 months) |
The mean fatigue in the intervention group was 0.28 standard deviations lower (0.41 lower to 0.16 lower) | 1698 (19 RCTs) |
⊕⊕⊕⊝ moderate2 | SMD ‐0.28 (95% CI ‐0.41 to ‐0.16) |
|
Cancer‐specific quality of life assessed with: FACT‐G, EORTC QLQ‐C30 and other scales (follow‐up: 12 weeks to 6 months) |
The mean cancer‐specific quality of life in the intervention group was 0.12 standard deviations higher (0.00 to 0.25 higher) | 1012 (12 RCTs) |
⊕⊕⊕⊝ moderate3 | SMD 0.12 (95% CI 0.00 to 0.25) |
|
Health‐related quality of life assessed with EQ‐5D visual analogue scale (higher scores indicate higher quality of life, score range from 0 to 100) MID: 7 points (follow‐up: end of intervention) |
The mean health‐related quality of life in the intervention group was 1.10 points higher (5.28 lower to 7.48 higher) | 68 (1 RCT) |
⊕⊕⊝⊝ low4,5 | MD 1.10 (95% CI ‐5.28 to 7.48) |
|
Cancer site‐specific quality of life assessed with: FACT‐B (higher scores indicate better quality of life, score range from 0 to 144) MID: 7 to 8 points (follow‐up: end of intervention) |
The mean cancer site‐specific quality of life in the intervention group was 4.24 points higher (1.81 lower to 10.29 points higher) | 262 (4 RCTs) |
⊕⊕⊝⊝ low6,7 | MD 4.24 (95% CI ‐1.81 to 10.29) |
|
Depression assessed with: BDI, CES‐D (follow‐up: 6 months) |
The mean depression in the intervention group was 0.15 standard deviations lower (0.30 lower to 0.01 higher) | 674 (5 RCTs) |
⊕⊕⊕⊝ moderate8 | SMD ‐0.15 (95% CI ‐0.30 to 0.01) |
|
Cognitive function assessed with: Trail Making Test (less time in seconds needed for completing the test means less cognitive dysfunction) (follow‐up: end of intervention) |
The mean time needed for completing the test in the intervention group was 11.55 seconds less (22.06 seconds less to 1.05 seconds less) | 213 (2 RCTs) |
⊕⊕⊝⊝ low9,10 | MD ‐11.55 (95% CI ‐22.06 to ‐1.05) |
|
Lymphoedema assessed with: volumetric arm measurements and bioimpedance spectroscopy (follow‐up: 8 weeks) |
Assumed risk11:
85 per 1000
Corresponding risk: 60 per 1000 (30 to 123) |
436 (2 RCTs) |
⊕⊕⊝⊝ low12,13 | RR 0.71 (95% CI 0.35 to 1.45) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BDI: Beck Depression Inventory; CES‐D: Center for Epidemiological Studies‐Depression Scale; CI: confidence interval; FACIT‐F: Functional Assessment of Chronic Illness Therapy‐Fatigue Scale; FACT‐B: Functional Assessment of Cancer Therapy‐Breast; FACT‐G: Functional Assessment of Cancer Therapy‐General; MD: mean difference; MID: minimally important difference; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||
1Lack of blinding, low adherence and high or unclear contamination, several randomisation and many allocation concealment procedures were unclear, therefore we downgraded by one level. 2Lack of blinding, low adherence and high or unclear amount of contamination, many allocation concealment procedures were unclear, therefore we downgraded by one level. 3Lack of blinding, low adherence and high or unclear amount of contamination, and a high rate of incomplete outcome data, therefore we downgraded by one level. 4Lack of blinding, low adherence and high amount of contamination, high rate of incomplete outcome data, and group similarity at baseline was at high risk, therefore we downgraded by one level. 5Small number of participants and null effect and appreciable benefit included in the confidence interval for the mean difference: imprecision, therefore we further downgraded by one level. 6Lack of blinding, low adherence, a high or unclear amount of contamination in three of four trials in the meta‐analysis, two of four allocation concealment procedures were unclear, therefore we downgraded by one level. 7Small number of participants, wide confidence intervals for two of the four trials, and null effect and appreciable benefit included in the confidence interval for the mean difference: imprecision, therefore we further downgraded by one level. 8Lack of blinding, low adherence and unclear or high contamination, two published studies could not contribute to the meta‐analysis, and in one of those there were no changes in the depression scores in any of the groups, therefore we downgraded by one level. 9Lack of blinding, low and unclear adherence and unclear contamination, group similarity at baseline for one study was at high risk of bias, therefore we downgraded by one level. 10Small number of participants: imprecision, therefore we further downgraded by one level. 11Assumed risk based on the mean control group risk in the included studies. 12Lack of blinding, low adherence and unclear or high contamination, one of two allocation procedures was unclear, group similarity at baseline was at high risk of bias for one study, therefore we downgraded by one level. 13Small number of participants and null effect and appreciable harm and benefit included in the confidence interval for the risk ratio: imprecision, therefore we further downgraded by one level.
Background
Description of the condition
Breast cancer detection and management have undergone dramatic changes over the past three decades. Women are increasingly diagnosed with early‐stage disease, leaving them with treatment choices ranging from breast‐conserving options to mastectomy (Newman 2003). With the majority of breast cancers diagnosed at an early stage, treatment is focused on cure and the prevention of relapse due to micrometastatic disease. The mainstay of care is local therapy, consisting of mostly breast‐conserving surgery followed by radiotherapy. Adjuvant systemic therapy includes chemotherapy (cytotoxic agents) when there is an increased risk for systemic relapse and hormonal and/or antibody therapy (trastuzumab), depending on the expression of hormone and HER2/neu receptors.
Besides these major advances in managing both early and locally advanced breast cancer, women still have to deal with severe side effects and psychological distress during adjuvant therapy. This has a substantial impact on their quality of life. Side effects that appear with adjuvant cancer treatment differ depending on the mode of treatment, that is radiotherapy, chemotherapy, hormonal, or antibody therapy.
Radiotherapy is frequently associated with short‐term side effects such as fatigue and skin reactions, and relatively rare long‐term side effects including lymphoedema, cardiac and pulmonary toxicities, and secondary malignancy (Brown 2015). Chemotherapy is associated with short‐term side effects such as nausea, emesis, stomatitis, alopecia, myelosuppression, thromboembolism, myalgias, neuropathy, and fatigue. Long‐term side effects of chemotherapy are premature menopause, weight gain, fatigue, cardiac dysfunction, and cognitive dysfunction (Partridge 2001). Furthermore, people receiving radiotherapy or chemotherapy report anxiety and depression prior to, during, and after therapy due to treatment side effects (Spiegel 1997). Adjuvant hormonal therapy produces symptoms secondary to oestrogen withdrawal, such as hot flushes, bone demineralisation, and psychosexual effects (Rutqvist 2004). A particular concern with antibody therapy in combination with anthracycline chemotherapy is cardiac toxicity (Rayson 2008).
Description of the intervention
Although research is producing increasingly hopeful insights into the causes and cures for cancer, efforts to manage the side effects of adjuvant therapy have not kept apace (Patrick 2003). Exercise interventions may be effective in managing some of these side effects, such as fatigue, depression, and cognitive dysfunction.
How the intervention might work
Evidence concerning the natural progression of physical activity suggests that women with breast cancer significantly decrease physical activity and exercise from pre‐diagnosis to postdiagnosis (Irwin 2003). These decreases are associated with adjuvant cancer treatment; observed decreases in physical activity were greater among women who were treated with radiation and chemotherapy (50% decrease) compared with women who underwent surgery only (24% decrease) or who were treated with surgery and radiation only (23% decrease) (Irwin 2003).
The National Comprehensive Cancer Network defines cancer‐related fatigue as a "persistent, subjective sense of tiredness related to cancer or cancer treatment that interferes with usual functioning" (NCCN 2004). Fatigue results in substantial physical, psychosocial, cognitive, and socioeconomic consequences (Holley 2000). During and after adjuvant chemotherapy and radiotherapy the prevalence of fatigue is high and fluctuating (de Jong 2002; Jereczek‐Fossa 2001). Fatigue is also associated with factors such as depression, impaired quality of sleep, or pain (de Jong 2002).
The rationale supporting exercise interventions for cancer‐related fatigue is based on the proposition that the combined effects of a toxic treatment and a decreased level of activity during treatment result in a reduction in the capacity for physical performance. Patients must in turn use greater effort and expend more energy to perform daily activities, which leads to fatigue (NCCN 2004). Physical exercise training programmes may increase functional capacity, leading to reduced effort and decreased fatigue.
Women treated for breast cancer frequently experience higher levels of emotional distress than the general population (Spiegel 1997). The rationale for considering exercise as an intervention to reduce distress in women receiving adjuvant therapy for breast cancer is based upon literature that has demonstrated ameliorating effects of exercise on these problems. Results of studies with non‐cancer populations indicate that aerobic exercise training has antidepressant and anxiolytic effects and protects against harmful consequences of stress (Salmon 2001). There is evidence that cognitive dysfunction may also occur in women receiving adjuvant chemotherapy for breast cancer (O'Shaughnessy 2003; Rugo 2003; Tchen 2003). A meta‐analytic study conducted to examine the hypothesis that aerobic fitness training enhances the cognitive vitality of healthy but sedentary older adults indicated that fitness training has robust benefits for cognition (Colcombe 2003).
Why it is important to do this review
The majority of research focused on rehabilitation and health promotion in women who had completed cancer treatment. This review aims to evaluate the role of exercise in managing common side effects of adjuvant and neoadjuvant therapy for breast cancer. We conducted this review update to incorporate and analyse the increasing number of studies in women undergoing adjuvant treatment.
Objectives
To assess the effect of aerobic or resistance exercise interventions during adjuvant treatment for breast cancer on treatment‐related side effects such as physical deterioration, fatigue, diminished quality of life, depression, and cognitive dysfunction.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials of exercise training during adjuvant (including neoadjuvant) treatment (radiotherapy, chemotherapy) for women with non‐metastatic breast cancer.
Types of participants
We included studies involving women who were diagnosed with breast cancer stages I, II, and III and who were undergoing adjuvant (including neoadjuvant) chemotherapy, radiotherapy, or a combination concurrently with an exercise intervention in the active group.
Types of interventions
We included studies that assessed the effects of all forms of repeatedly performed aerobic or resistance exercise or both with programme duration of at least six weeks. To be included in this review, the exercise intervention had to coincide with the adjuvant treatment regimen rather than follow it. We excluded studies where the exercise intervention was part of a complex intervention (for example complete decongestive lymphatic therapy). We also excluded trials with interventions restricted to local muscular endurance (for example training of shoulders, back, or legs only) instead of including all major muscle groups or restricted to stretching exercises.
We included trials making the following comparisons:
exercise versus no exercise;
exercise versus other interventions (e.g. psychosocial interventions).
Types of outcome measures
Primary outcomes
physical fitness: objective tests measuring VO2 max or distance walked per time
fatigue: using a validated questionnaire such as Functional Assessment of Chronic Illness Therapy‐Fatigue (FACIT‐F)
quality of life (cancer‐specific quality of life, health‐related quality of life, cancer site‐specific quality of life): using a validated questionnaire such as Functional Assessment of Cancer Therapy‐General (FACT‐G), European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 36 (EORTC QLQ‐C30), Functional Assessment of Cancer Therapy‐Breast (FACT‐B)
depression: using a validated questionnaire such as Beck Depression Inventory (BDI)
cognitive function: using a validated test such as the Trail Making Test
Secondary outcomes
strength
other psychological distress outcomes
physical activity behaviour
multidimensional outcomes (e.g. pain)
harms
Search methods for identification of studies
Electronic searches
We searched the Cochrane Breast Cancer Group Specialised Register on 30 March 2015 (details of search strategies used by the group for the identification of studies and the procedure used to code references are outlined in the group's module at www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html). We extracted studies including the text words 'early', 'locally advanced', local recurrence', 'locoregional', 'exercise', and 'exercise therapy' on the Specialised Register for consideration.
Cochrane Central Register of Controlled Trials (CENTRAL) (via the Cochrane Library, Issue 2, 2015). See Appendix 1.
MEDLINE (via OvidSP) from 1966 until 30 March 2015. See Appendix 2.
EMBASE (via Embase.com) from 1966 until 30 March 2015. See Appendix 3.
The World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/Default.aspx) for all prospectively registered and ongoing trials on 30 March 2015. See Appendix 4.
ClinicalTrials.gov (clinicaltrials.gov/ct2/home) until 30 March 2015. See Appendix 5.
We did not update the searches in the original review in the following databases:
CINAHL (1982 to 2004)
SPORTDiscus (1975 to 2004)
PsycINFO (1872 to 2003)
SIGLE (1880 to 2004)
ProQuest Digital Dissertations (1861 to 2004)
Searching other resources
References from published studies
We screened references in relevant reviews and in published clinical trials for further trials.
Other
We consulted six experts in the field of cancer and exercise to identify additional trials. We applied no language restrictions.
Data collection and analysis
Selection of studies
Two review authors (either ACF and MHM or ACF and MM) independently reviewed the titles and abstracts of reports identified by the search and selected those that potentially fulfilled the inclusion criteria of this review. We retrieved these potentially relevant reports for more detailed evaluation. Both review authors then independently made a final selection of studies to be included in the review. A report was excluded according to the first criterion that it did not fulfil. We resolved disagreements by consensus, or if necessary by consulting a third person (MM or MHM) to reach a final decision.
Data extraction and management
Two review authors (ACF, MHM) independently extracted data (including study characteristics, study results, and point estimates together with measures of variability for selected outcome variables). We reviewed all discrepancies and achieved consensus through discussion, if necessary consulting a third person (MM) to reach a final decision. Where we found more than one publication for a study, we extracted data from all available publications if applicable. When a design publication and a results publication were available, we considered the results publication to be the primary reference. In cases where a doctoral dissertation was available, we considered this to be the primary reference for the study. In other cases, we considered the publication with the most relevant reported information for the review, especially regarding results, to be the primary reference.
Assessment of risk of bias in included studies
In the original version of the review, we assessed the included studies for quality using van Tulder methodological quality criteria. In the updated review, we used the Cochrane 'Risk of bias' tool (Higgins 2011). Two review authors (either ACF and MM or ACF and MHM) assessed the risk of bias of all included studies. This included assessment of sequence generation, allocation concealment, masking or blinding (of participants, researchers/healthcare providers, and outcome assessors), methods of addressing incomplete outcome data, selective reporting of outcomes, and other possible sources of bias including attrition from the exercise intervention. We graded each risk of bias parameter as high risk, low risk, or unclear risk based on recommendations for judging risk of bias provided in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For attrition bias, we judged studies to be at high risk when more than 20% of data were missing for short‐term follow‐up and more than 30% for long‐term follow‐up, as these are commonly used thresholds. We resolved disagreement through consensus, if necessary consulting a third review author (MHM or MM) for a final decision.
We used the last two rows in the 'Risk of bias' assessment tables to document reporting and amount of adherence and contamination in the exercise and control groups. If participants allocated to the exercise group do not exercise (non‐adherence), and at the same time participants allocated to the control group do exercise (contamination), the originally intended study groups are distorted into groups with participants who exercise and those who do not (moreover in unknown proportions). Effects may be underestimated as a result.
There are several bias issues inherent to exercise studies, that is blinding of participants and exercise supervising personnel is difficult or impossible, leading to high risk of performance bias in every study. It is therefore important to point out that the bias assessment for those items does not reflect a low quality of study design as such, but expresses the inevitable bias introduced by lack of blinding. Nevertheless, exercise intervention studies should be subjected to the same 'Risk of bias' assessment as other studies, for example drug intervention studies. A similar challenge applies to adherence and contamination in exercise studies. It is difficult to maximise exercise adherence, especially in a participant cohort with cancer, and a certain amount of contamination and imperfect adherence is to be expected. Confidence in the results might therefore be lowered, even if studies are well planned and reported.
When high risk of bias for a study was due to lack of blinding, contamination, and/or non‐adherence, we did not downgrade the quality of evidence for this alone. We only downgraded the evidence one level for risk of bias when other factors such as unclear allocation procedures or high attrition rates were present.
’Risk of bias’ tables for each study are presented in the Characteristics of included studies table and a summary of the risk of bias is presented.
Measures of treatment effect
Outcome measurements were presented as continuous data across included studies. As the first step, we extracted data on outcomes in the format in which they were reported. For selected outcomes we extracted group means for final values and change scores with the corresponding measures of variability such as standard deviations (SD) or confidence intervals (CI) and the number of participants on whom the outcome was assessed per group.
As a summary statistic for meta‐analysis of continuous outcomes, we either used the standardised mean difference (SMD) or the weighted mean difference (WMD). We chose the SMD in cases where different assessment instruments measuring the same construct were used across studies (for example for fatigue and physical fitness outcomes). We did not combine final values and change scores in meta‐analyses since the difference in standard deviation does not reflect "differences in measurement scale, but differences in the reliability of the measurements" (Deeks 2005).
We did not include data for outcomes assessed with subscales of questionnaires (for example physical functioning subscale of the 36‐Item Short Form Health Survey (SF‐36) or vitality subscale of the SF‐36, nausea item of the Symptom Checklist‐90 (SCL‐90)), because we only wanted to assess the respective full construct in this review as well as only include validated questionnaires, which often is not the case for subscales.
As a summary statistic for dichotomous outcomes we chose the risk ratio (RR). Lymphoedema was the only outcome that was analysed as a dichotomous outcome: two studies reported the number of participants with lymphoedema (Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel). For those outcomes with data available from only one study, we calculated and presented a summary statistic for this particular study.
Unit of analysis issues
Five studies were three‐arm trials (Courneya 2007 split into Courneya 2007 AET and Courneya 2007 RET; Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel; Schwartz 2007: Schwartz 2007 AET and Schwartz 2007 RET; Segal 2001: Segal 2001 SD and Segal 2001 SU; van Waart 2014: van Waart 2014 high and van Waart 2014 low), and they contributed to the meta‐analysis of physical fitness with two exercise groups. For all five studies, we incorporated both exercise arms into the meta‐analysis and allocated a control group to each of them (that is by halving the number of participants and events observed in the control group).
Dealing with missing data
Whenever possible, we tried to contact the investigators or sponsors of studies with missing data.
Assessment of heterogeneity
We used the random‐effects model to obtain the average effect of exercise because, in addition to the presence of random error, differences between exercise studies during adjuvant cancer treatment can also result from real differences between study populations, adjuvant cancer treatment, and the training stimulus. The random‐effects model considers these additional sources of between‐study variability as well as within‐study variability.
We evaluated inconsistency of results across studies using the I2 statistic, which describes the percentage of variability in the point estimates that is due to heterogeneity rather than sampling error (Higgins 2002). Following Higgins (Higgins 2003), we considered I2 values of 25% as indicating low heterogeneity, I2 values of 50% moderate heterogeneity, and I2 values of 75% large heterogeneity.
We also used visual assessment of forest plots; if no or small overlap of CIs for the results of individual studies was present, we assumed statistical heterogeneity.
Assessment of reporting biases
If we identified a sufficient number of studies (that is more than 10), we prepared funnel plots and visually examined them for signs of asymmetry to detect publication bias.
Data synthesis
We used the random‐effects model to obtain the average effect of exercise because, in addition to the presence of random error, differences between exercise studies during adjuvant cancer treatment can also result from real differences between study populations, adjuvant cancer treatment, and the training stimulus. The random‐effects model considers these additional sources of between‐study variability as well as within‐study variability.
We used the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach to assess the quality of the evidence, grading the following main outcomes for quality: physical fitness, fatigue, cancer‐specific quality of life, health‐related quality of life, cancer site‐specific quality of life, depression, cognitive function, and lymphoedema. We used GRADEproGDT software to develop the 'Summary of findings' table, and two review authors (either ACF and MM or ACF and MHM) graded the quality of the evidence for each outcome. We resolved disagreement by consensus, if necessary consulting a third review author (MHM or MM) for a final decision.
As blinding of participants and exercise supervising personnel is difficult or impossible, and as self reported outcomes inherently carry a high risk of detection bias, those items were assessed with a high risk of bias, but did not lead to downgrading unless there were additional high risks of bias (for example sequence generation and allocation concealment).
Subgroup analysis and investigation of heterogeneity
We did not conduct subgroup analyses.
If possible, in future updates we will consider conducting the following subgroup analyses: adjuvant treatment received, chemo‐ and radiotherapy or radiotherapy only, type of exercise intervention (aerobic or resistance exercise, self directed or supervised exercise).
Sensitivity analysis
Where important statistical inconsistency existed as measured by the I2 statistic, we conducted sensitivity analyses to assess the robustness of the review results by removing those studies that seemed to be estimating a different effect.
Results
Description of studies
Results of the search
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
In the original review published in 2006, we retrieved 32 full‐text references for more detailed evaluation after screening of 1612 potentially relevant references. From the 32 full‐text references, 22 publications were excluded, one trial was awaiting assessment (pending publication), and nine trials were included. We considered seven of the nine trials to be appropriate for inclusion in this updated review (Campbell 2005; Crowley 2003; Drouin 2002; MacVicar 1989; Mock 2004; Segal (Segal 2001 SD & Segal 2001 SU); Winningham 1988). We excluded two of these in this updated review because they were not randomised controlled trials (MacVicar 1986; Mock 1997). We included the trial that was awaiting assessment in the original review in this updated review (Battaglini 2004).
In this updated review, we retrieved a further 146 full‐text references following screening of 3297 titles and abstracts. From these 146 full‐text references we excluded 86 and identified 60 records as appropriate for inclusion in this review. Three records belonged to a study that had been included in the first version of this review, and 55 records related to 24 new studies. One study is awaiting assessment due to pending publication (Petrella 2012), and another study was due to be published after our analyses were finished (Lotzke 2016). For further details see Figure 1.
1.
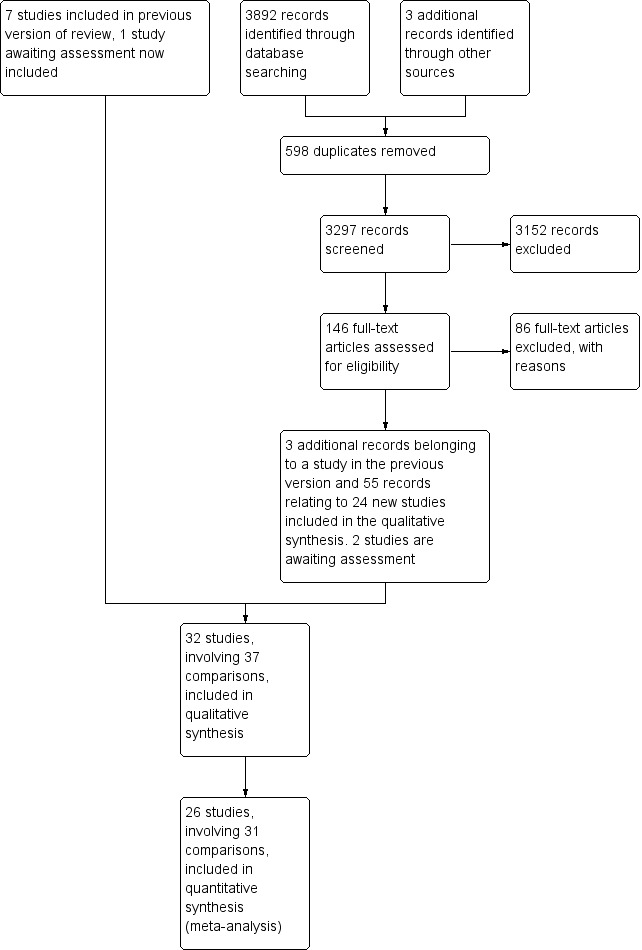
Study flow diagram.
Included studies
The eight studies from the original review and the 24 new studies amounted to a total of 32 studies (2626 participants) for inclusion in the review. Trial characteristics and outcomes are found in the Characteristics of included studies tables. We included only randomised controlled trials in this updated version of the review. Five of the included studies incorporated two separate exercise groups and are therefore entered twice for the purposes of statistical analysis (Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel; Schwartz 2007: Schwartz 2007 AET and Schwartz 2007 RET; Segal 2001: Segal 2001 SD and Segal 2001 SU; van Waart 2014: van Waart 2014 high and van Waart 2014 low).
Characteristics of participants
Women obtained different regimens of adjuvant treatment across these 32 exercise intervention studies: they received either chemotherapy or radiotherapy in one trial (Mock 2004); either chemotherapy or radiotherapy or a combination of the two in another 10 trials (Battaglini 2004; Cadmus 2007; Caldwell 2009; Campbell 2005; Eakin 2012; Haines 2010; Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel; Mutrie 2007; Perna 2010; Segal 2001: Segal 2001 SD and Segal 2001 SU), sequential chemo‐ and radiotherapy in one trial (Cornette 2013), chemotherapy only in nine trials (Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Crowley 2003; Ingram 2010; MacVicar 1989; Moros 2010; Schmidt 2014; Visovsky 2014; Winningham 1988; Yang 2011); neoadjuvant chemotherapy in two trials (Hornsby 2014; Rao 2012), neoadjuvant and adjuvant chemotherapy in one trial (Gokal 2013), and radiotherapy only in three trials (Drouin 2002; Reis 2013; Steindorf 2014). Five trials included women who were scheduled for chemotherapy, some of whom underwent radiation therapy as well (Dodd 2010; Husebo 2014; Schwartz 2007: Schwartz 2007 AET and Schwartz 2007 RET; Travier 2015; van Waart 2014: van Waart 2014 high and van Waart 2014 low).
Characteristics of the intervention
Mode of exercise differed across trials. Thirteen trials (Cadmus 2007; Dodd 2010; Drouin 2002; Gokal 2013; Hornsby 2014; Husebo 2014; MacVicar 1989; Mock 2004; Moros 2010; Reis 2013; Segal 2001: Segal 2001 SD and Segal 2001 SU; Winningham 1988; Yang 2011), and one of two intervention arms in two studies (Courneya 2007: Courneya 2007 AET; Schwartz 2007: Schwartz 2007 AET) tested aerobic exercise interventions, with three studies using cycle ergometer interval training (Hornsby 2014; MacVicar 1989; Winningham 1988), and six studies offering walking programmes (Drouin 2002; Gokal 2013; Husebo 2014; Mock 2004; Segal 2001: Segal 2001 SD and Segal 2001 SU; Yang 2011). Aerobic exercise also consisted of Nia exercise, in Reis 2013, and aerobic exercise self chosen by the participants in two studies, Cadmus 2007 and Dodd 2010, and in one intervention arm of one study (Schwartz 2007: Schwartz 2007 AET). Fifteen studies (Battaglini 2004; Caldwell 2009; Campbell 2005; Cornette 2013; Crowley 2003; Eakin 2012; Haines 2010; Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel; Husebo 2014; Ingram 2010; Moros 2010; Mutrie 2007; Perna 2010; Rao 2012; Travier 2015), and one of two intervention arms in one study (van Waart 2014: van Waart 2014 high) applied a combined aerobic‐resistance programme. Exercise was implemented as a supervised group exercise programme in seven studies (Battaglini 2004; Campbell 2005; Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; MacVicar 1989; Mutrie 2007; Travier 2015; Winningham 1988), and in one of two intervention arms in two studies (Segal 2001: Segal 2001 SU; van Waart 2014: van Waart 2014 high). Two studies tested resistance exercise interventions (Schmidt 2014; Steindorf 2014), as well as one intervention arm of two further studies (Courneya 2007: Courneya 2007 RET; Schwartz 2007: Schwartz 2007 RET). One study started with supervised resistance training at the hospital, followed by a combined self directed aerobic‐resistance programme (Cornette 2013).
Four studies used a stretching intervention as a comparison arm (Drouin 2002; Haines 2010; MacVicar 1989; Winningham 1988), two studies used progressive muscle relaxation as the comparison arm (Schmidt 2014; Steindorf 2014), and the remaining 26 studies compared an exercise intervention with no intervention.
Exercise interventions lasted six to seven weeks for women undergoing radiation treatment in two trials (Drouin 2002; Mock 2004), 10 weeks in two trials for women undergoing chemotherapy (MacVicar 1989; Winningham 1988), and 12 to 13 weeks in 11 trials (Caldwell 2009; Campbell 2005; Crowley 2003; Gokal 2013; Hornsby 2014; Mutrie 2007; Reis 2013; Schmidt 2014; Steindorf 2014; Visovsky 2014; Yang 2011). In nine trials, the exercise intervention lasted 18 to 32 weeks (Battaglini 2004; Cadmus 2007; Cornette 2013; Eakin 2012; Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel; Ingram 2010; Schwartz 2007: Schwartz 2007 AET and Schwartz 2007 RET; Segal 2001: Segal 2001 SD and Segal 2001 SU; Travier 2015).The longest intervention period was 52 weeks, in two trials (Dodd 2010; Haines 2010). Trials with shorter intervention periods (six to seven weeks) were those in which women received radiation treatment, which is of shorter duration than chemotherapy. In five trials (Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Husebo 2014; Mock 2004; Moros 2010; van Waart 2014: van Waart 2014 high and van Waart 2014 low), the exercise intervention was implemented to span the period of time from initiation to cessation of the woman's adjuvant therapy, and subsequently the intervention periods of women in the intervention arm of the trial varied in length (either six weeks with radiation treatment or three to six months with chemotherapy).
In 12 trials (Battaglini 2004; Campbell 2005; Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Hornsby 2014; MacVicar 1989; Moros 2010; Mutrie 2007; Rao 2012; Schmidt 2014; Steindorf 2014; Travier 2015; Winningham 1988), and in one of the two intervention arms in three trials (Hayes 2013: Hayes 2013 FtF; Segal 2001: Segal 2001 SU; van Waart 2014: van Waart 2014 low), the exercise intervention was supervised. Women's exercise was self directed in 15 trials (Cadmus 2007; Caldwell 2009; Crowley 2003; Dodd 2010; Drouin 2002; Eakin 2012; Gokal 2013; Haines 2010; Husebo 2014; Ingram 2010; Mock 2004; Reis 2013; Schwartz 2007: Schwartz 2007 AET and Schwartz 2007 RET; Visovsky 2014; Yang 2011), and in the second intervention arm of Hayes 2013 (Hayes 2013 Tel), Segal 2001 (Segal 2001 SD), and van Waart 2014 (van Waart 2014 low). Two studies started with supervised sessions, which were followed by self directed sessions in the home (Cornette 2013; Perna 2010). Two trials applied supervised, one‐on‐one sessions, Rao 2012 home based and Hornsby 2014 at the clinical institution.
Characteristics of the outcome measures
The most frequently assessed outcomes were physical fitness and fatigue, with 22 studies measuring physical fitness (Battaglini 2004; Caldwell 2009; Campbell 2005; Cornette 2013; Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Crowley 2003; Dodd 2010; Drouin 2002; Haines 2010; Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel; Hornsby 2014; Husebo 2014; MacVicar 1989; Mock 2004; Mutrie 2007; Reis 2013; Schmidt 2014; Schwartz 2007: Schwartz 2007 AET and Schwartz 2007 RET; Segal 2001: Segal 2001 SD and Segal 2001 SU; Steindorf 2014; Travier 2015; van Waart 2014: van Waart 2014 high and van Waart 2014 low), and 21 studies measuring fatigue (Battaglini 2004; Caldwell 2009; Campbell 2005; Cornette 2013; Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Crowley 2003; Dodd 2010; Drouin 2002; Eakin 2012; Gokal 2013; Haines 2010; Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel; Hornsby 2014; Husebo 2014; Mock 2004; Mutrie 2007; Reis 2013; Schmidt 2014; Steindorf 2014; Travier 2015; van Waart 2014: van Waart 2014 high and van Waart 2014 low). Other outcomes assessed were quality of life, strength, depression, anxiety, cognitive function, self esteem, mood disturbances, physical activity level, gait and balance, subjective upper body function, neuropathy symptoms, chemotherapy completion, shoulder mobility, arm morbidity, nausea relief, sleep disturbances, endocrine symptoms, and adverse effects. For detailed information on outcome measures see the Characteristics of included studies table.
Other study characteristics
Small sample size was common among the included studies. Sixteen studies randomised fewer than 50 women. Ten of the 32 studies randomised more than 50 women per group (Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Dodd 2010; Eakin 2012; Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel; Mock 2004; Mutrie 2007; Segal 2001: Segal 2001 SD and Segal 2001 SU; Steindorf 2014; Travier 2015; van Waart 2014: van Waart 2014 high and van Waart 2014 low). Sample sizes ranged from 10 to 242 women. The median sample size was 50 women, interquartile range (IQR) 22 to 124. Sample size was reported to be based on power calculations in 14 studies, and 12 of the 14 studies reached the target sample size.
Excluded studies
In the majority of cases, we excluded studies because the exercise intervention took place after the adjuvant treatment period. Other reasons were that studies were not randomised controlled trials, exercise was part of a complex intervention or no exercise intervention was implemented, the majority of participants were not women with breast cancer, or the exercise intervention had a duration of less than six weeks. Furthermore, we excluded studies assessing yoga or qigong because we regard both as a complex intervention. Some studies could not be characterised as controlled trials (they were study protocols or reviews). For a detailed description of the reasons for exclusion, see the Characteristics of excluded studies table. Note that this table contains not only clinical studies but also review articles that were part of our full‐text retrieval to confirm our decision to exclude studies when abstracts were ambiguous.
Risk of bias in included studies
We assessed the risk of bias for each included study and reported the judgements for the individual 'Risk of bias' domains in the ’Risk of bias’ table. We have presented these in the ’Risk of bias’ summary in Figure 2.
2.
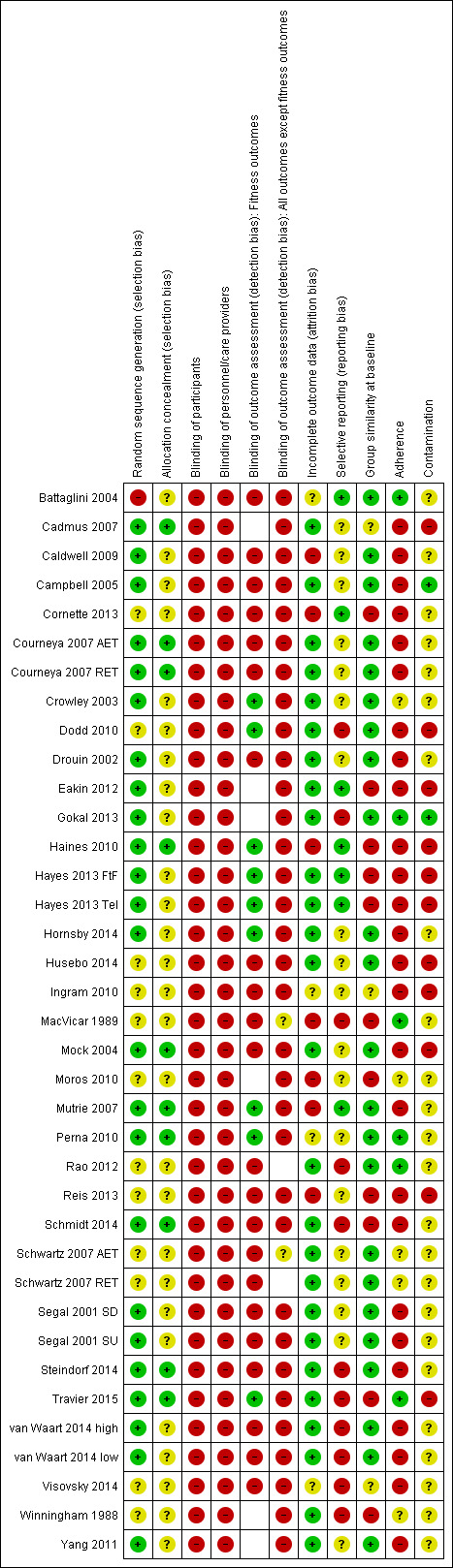
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Twenty trials were at a low risk of selection bias as they reported to have adequately generated their randomised sequence with a random component. One trial used a non‐random component to generate the sequence (Battaglini 2004), and was thus judged to be at a high risk of selection bias. We considered 11 trials to have an unclear risk of selection bias, largely because the generation of the random sequence was not described.
Allocation concealment
Nine trials adequately concealed allocation to the intervention so that participants and investigators could not foresee assignment to the study groups, and were thus judged to be at low risk of selection bias. Twenty‐three trials did not describe the method of allocation concealment or did not describe it in detail enough to allow for a definitive judgement, and were considered to have an unclear risk of selection bias.
Blinding
Blinding of participants and personnel
All trials included in this review were at high risk for performance bias because, owing to the nature of the intervention (exercise), it was not possible to blind the participants and the study personnel. Three studies mentioned a placebo group (Haines 2010; MacVicar 1989; Winningham 1988), in which women were instructed to do stretching in a similar setting to the exercise groups. But as knowledge about the difference between physical exercise and stretching is usually present in the population, we cannot assume that participants and personnel were unaware of being in the exercise or the stretching group.
Blinding of outcome assessors (detection bias)
Eight studies reported blinding of outcome assessors (Crowley 2003; Dodd 2010; Haines 2010; Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel; Hornsby 2014; Mutrie 2007; Perna 2010; Travier 2015). Blinding was performed for assessment of fitness outcomes, as well as for lymphoedema in one study (Hayes 2013), and for upper limb swelling and shoulder range of motion in another study (Haines 2010), with a low risk of bias for these outcomes, but not for the remaining self reported outcomes, for which risk of bias was high. Perna 2010 did not report the assessed fitness outcomes. In the remaining 24 studies, no information was given on blinding of outcome assessors, which we judged as lack of blinding and therefore high risk of bias for this item for all outcomes. In cases where no fitness outcomes or no self reported outcomes were measured in a trial, this appears as unclear risk of bias in the tables and as an empty cell in the ’Risk of bias’ summary.
Incomplete outcome data
Twenty‐three of the 32 studies reported to have analysed data according to the intention‐to‐treat (ITT) principle. Twenty of these 23 studies had low drop‐out rates or less than 20% missing data and were thus judged to be at low risk of attrition bias. Of the remaining three studies, one reported imbalanced drop‐out rates between the exercise group and the control group (Mutrie 2007), with almost twice as many dropped‐out participants in the intervention group (19 of 101) than in the control group (10 of 102); we therefore judged this study to be at high risk of attrition bias. The second study had more than 30% missing data (Cornette 2013). In spite of the trial authors undertaking an ITT analysis with imputation of missing data, we judged this study to be at high risk of attrition bias due to the amount of missing data. It remained unclear if there had been missing data in the third study, leading to a judgement of unclear risk of attrition bias (Visovsky 2014).
Five studies did not report if data were analysed by intention to treat. Three of these studies had more than 20% dropouts or missing data and were thus judged to be at high risk of attrition bias (Caldwell 2009; Haines 2010; Moros 2010). Another study did not report if there were missing data (Battaglini 2004), and was thus judged to be at an unclear risk of attrition bias. The remaining study had a low drop‐out rate (4 of 44 women) and was judged to be at low risk of attrition bias (Yang 2011).
We judged one study to be at high risk of attrition bias (Reis 2013), because it only reported a per‐protocol analysis of participants that adhered to the exercise intervention (12 of 22 women). In one study (Perna 2010), the numbers randomised to each arm as well as completion rates were unclear. The authors reported that they used regression modelling to impute missing values to conduct the analyses. We thus judged risk of attrition bias for this study as unclear and extracted no data. One study reported no outcome data as the study was closed early due to changes in the chemotherapy protocol (Ingram 2010); we judged this study to be at unclear risk of attrition bias. MacVicar 1989 undertook an analysis of 45 of 62 women; nine of the excluded women were reclassified to more advanced stages of disease than stage II during participation and not analysed for that reason. We judged this study to be at high risk of attrition bias.
Selective reporting
Six studies were at a low risk of reporting bias, as the studies had been registered prospectively or study protocols had been published and the prospectively registered outcomes were in line with the published ones. We considered 10 studies to be at high risk of reporting bias, because reporting of assessed outcomes in the final paper differed from entries in trial registries or study protocols, and no explanation was given. We considered 16 studies to be at an unclear risk for reporting bias, as no study protocol or design paper was available, and no trial registration had taken place; the information was therefore insufficient to judge this item for those studies.
Other potential sources of bias
Nineteen studies were at low risk of selection bias owing to adequate group similarity at baseline, three studies were at unclear risk for selection bias, and 10 studies were at high risk for selection bias, because group similarity at baseline was inadequate.
Adherence and contamination
Different approaches were used among the included studies to measure adherence, that is the level of exercise participation achieved once the woman had agreed to undertake it. Fifteen studies reported exercise levels in non‐exercising control groups (contamination) (Cadmus 2007; Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Crowley 2003; Dodd 2010; Eakin 2012; Gokal 2013; Haines 2010; Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel; Hornsby 2014; Husebo 2014; Mock 2004; Perna 2010; Reis 2013; Travier 2015; van Waart 2014: van Waart 2014 high and van Waart 2014 low). A high percentage of women (up to 70% in Dodd 2010) in the control groups reported to be regularly exercising or had a high level of activity. In a study with two exercise groups (Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel), the usual‐care group was more active than one of the exercise groups and as active as the other exercise group, according to the survey used.
In six studies, women adhered adequately to the exercise intervention, and in four studies this was unclear. In the remaining 22 studies, adherence to the exercise intervention was so low that we judged it to cause a high risk of bias. The amount of contamination was low in two studies, high in 10 studies, and unclear in the remaining 20 studies.
Effects of interventions
See: Table 1
Effectiveness of exercise programmes
Most trial authors reported study results as follow‐up values, which we pooled. When outcomes were assessed with different instruments, follow‐up values and change scores could not be pooled. We performed meta‐analyses for physical fitness, fatigue, cancer‐specific quality of life, cancer site‐specific quality of life, depression, cognitive function, strength, subjective upper body function, arm morbidity, anxiety, mood disturbance, self esteem, physical activity, gait and balance, and lymphoedema.
Studies that were included in the meta‐analysis for physical fitness predominantly either measured performance, for example distance walked in a given time, or maximum oxygen uptake. Studies that were included in the meta‐analysis for fatigue predominantly applied the Functional Assessment of Chronic Illness Therapy‐Fatigue (FACIT‐F) scale. Studies that were included in the meta‐analysis for cancer‐specific quality of life either used the Functional Assessment of Cancer Therapy‐General (FACT‐G) or the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 36 (EORTC QLQ‐C30) questionnaires. Studies that were included in the meta‐analysis for cancer site‐specific quality of life all used the Functional Assessment of Cancer Therapy‐Breast (FACT‐B) questionnaire. Studies that were included in the meta‐analysis for depression predominantly used the Center for Epidemiological Studies‐Depression scale (CES‐D). The two studies that were included in the meta‐analysis for cognitive function both used the Trail Making Test.
Primary outcomes
Physical fitness
Twelve studies applied tests of cardiorespiratory fitness (Battaglini 2004; Cornette 2013; Courneya 2007 (Courneya 2007 AET and Courneya 2007 RET); Crowley 2003; Dodd 2010; Drouin 2002; Hornsby 2014; MacVicar 1989; Schmidt 2014; Segal 2001 (Segal 2001 SD and Segal 2001 SU); Steindorf 2014; Travier 2015), eight studies assessed physical performance via timed walking distances (Caldwell 2009; Campbell 2005; Haines 2010; Husebo 2014; Mock 2004; Mutrie 2007; Reis 2013; Schwartz 2007 (Schwartz 2007 AET and Schwartz 2007 RET)), and two studies used other physical performance tests (Hayes 2013 (Hayes 2013 FtF and Hayes 2013 Tel); van Waart 2014 (van Waart 2014 high and van Waart 2014 low)). Hayes 2013 assessed heart rate at the end of test completion, and van Waart 2014 assessed endurance time in minutes.
Meta‐analysis was feasible for 15 of those 22 studies (1310 women) yielding 20 comparisons (Caldwell 2009; Campbell 2005; Cornette 2013; Courneya 2007 (Courneya 2007 AET and Courneya 2007 RET); Drouin 2002; Haines 2010; Hayes 2013 (Hayes 2013 FtF and Hayes 2013 Tel); Hornsby 2014; Husebo 2014; Mutrie 2007; Reis 2013; Schwartz 2007 (Schwartz 2007 AET and Schwartz 2007 RET); Segal 2001 (Segal 2001 SD and Segal 2001 SU); Travier 2015; van Waart 2014 (van Waart 2014 high and van Waart 2014 low)). The standardised mean difference (SMD) for the pooled data was 0.42 (95% confidence interval (CI) 0.25 to 0.59; I2 = 49%; Analysis 1.1; Figure 3). There was moderate heterogeneity with an I2 of 49%, which could be explained by the wide range of exercise interventions and outcome assessment protocols in the different studies.
1.1. Analysis.
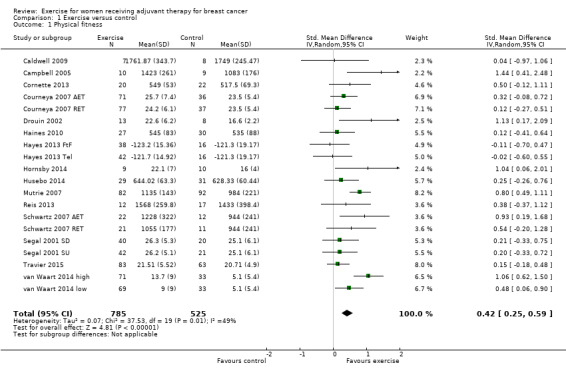
Comparison 1 Exercise versus control, Outcome 1 Physical fitness.
3.
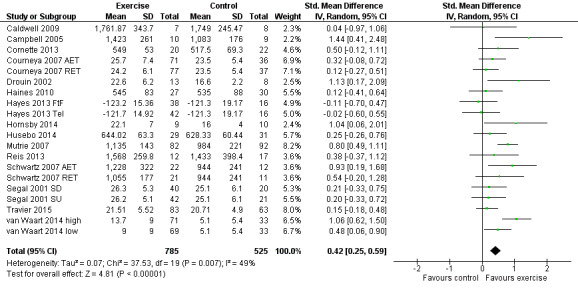
Forest plot of comparison: 1 Exercise versus control, outcome: 1.1 Physical fitness.
We could not transform data from two studies for meta‐analysis requirements (Crowley 2003; MacVicar 1989). Both studies reported small but statistically significant improvements. Another study reported no group differences in the ITT analysis for the 12‐minute walk test but presented no data for the ITT analysis (Mock 2004). Four studies reported neither data nor descriptive results for cardiorespiratory fitness. One study provided only a comparison of means without standard deviations for cardiorespiratory fitness data (Battaglini 2004).
We rated the result of a statistically significant improvement in physical fitness as moderate‐quality evidence due to lack of blinding, low adherence, and high or unclear contamination in most of the studies and because many randomisation and allocation procedures were unclear. Furthermore, there was a considerable number of women (390 in 4 studies) for whom no data were reported. There was no indication of publication bias from examination of the funnel plot for this outcome. See Table 1.
Fatigue
Meta‐analysis was possible for 19 studies (1698 women) yielding 22 comparisons (Battaglini 2004; Caldwell 2009; Campbell 2005; Cornette 2013; Courneya 2007 (Courneya 2007 AET and Courneya 2007 RET); Drouin 2002; Eakin 2012; Gokal 2013; Haines 2010; Hayes 2013 (Hayes 2013 FtF and Hayes 2013 Tel); Hornsby 2014; Husebo 2014; Mock 2004; Mutrie 2007; Reis 2013; Schmidt 2014; Steindorf 2014; Travier 2015; van Waart 2014 (van Waart 2014 high and van Waart 2014 low)). Several tools were used to measure fatigue: the FACIT‐F scale, the (revised) Piper Fatigue Scale, the Multidimensional Fatigue Inventory, the Schwartz Cancer Fatigue Scale, and the Fatigue Assessment Questionnaire and the Fatigue Quality List. The SMD between intervention and control was ‐0.28 (95% CI ‐0.41 to ‐0.16; I2 = 29%; Analysis 1.2; Figure 4), favouring the exercise group. Two studies did not report data for fatigue (Crowley 2003; Dodd 2010). Both studies reported that there were no statistically significant group differences. We assumed that their results would not have substantially influenced the pooled result because they involved only 22 and 119 women, respectively. We rated the result as moderate‐quality evidence due to lack of blinding, low adherence, and high or unclear contamination and because the allocation concealment procedures in many studies were unclear. The funnel plot was asymmetrical when Battaglini 2004 was included; otherwise there was no indication of publication bias from examination of the funnel plot for this outcome, and we did not downgrade for publication bias. See Table 1.
1.2. Analysis.
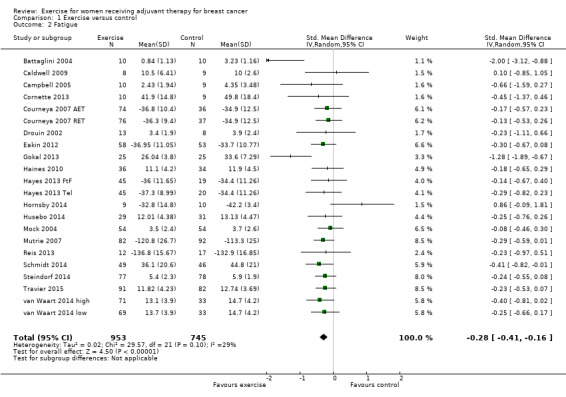
Comparison 1 Exercise versus control, Outcome 2 Fatigue.
4.
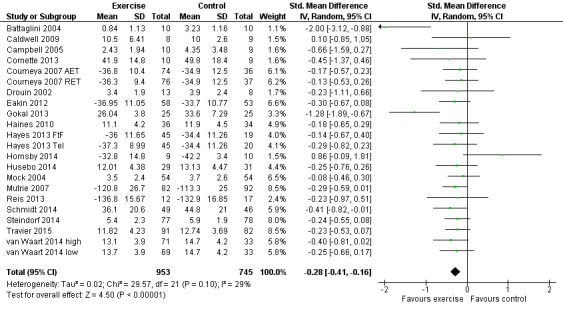
Forest plot of comparison: 1 Exercise versus control, outcome: 1.2 Fatigue.
Cancer‐specific quality of life
Sixteen studies examined effects of exercise on cancer‐specific quality of life (Cadmus 2007; Campbell 2005; Cornette 2013; Courneya 2007 (Courneya 2007 AET and Courneya 2007 RET); Dodd 2010; Haines 2010; Hornsby 2014; Moros 2010; Mutrie 2007; Reis 2013; Schmidt 2014; Segal 2001 (Segal 2001 SD and Segal 2001 SU); Steindorf 2014; Travier 2015; van Waart 2014 (van Waart 2014 high and van Waart 2014 low); Visovsky 2014). Cancer‐specific quality of life was measured with either the FACT‐G scale or the EORTC QLQ‐C30 questionnaire. Twelve studies (1012 women) reported final values, and one study reported change scores for this outcome (Campbell 2005). Meta‐analysis of the 12 studies reporting final values showed a SMD of 0.12 (95% CI 0.00 to 0.25; I2 = 0%; Analysis 1.3; Figure 5) (Cadmus 2007; Cornette 2013; Courneya 2007 (Courneya 2007 AET and Courneya 2007 RET); Haines 2010; Hornsby 2014; Moros 2010; Mutrie 2007; Reis 2013; Schmidt 2014; Steindorf 2014; Travier 2015; Visovsky 2014). The study reporting change scores found statistically significant differences between groups, favouring the exercise group, in cancer‐specific quality of life, using the FACT‐G (Campbell 2005).
1.3. Analysis.
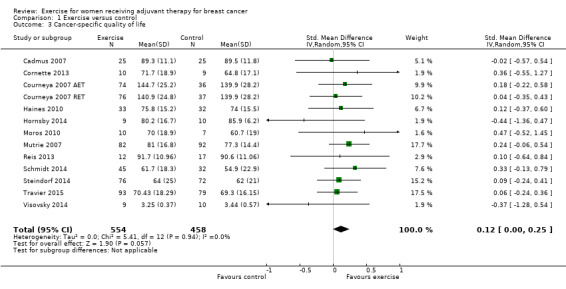
Comparison 1 Exercise versus control, Outcome 3 Cancer‐specific quality of life.
5.
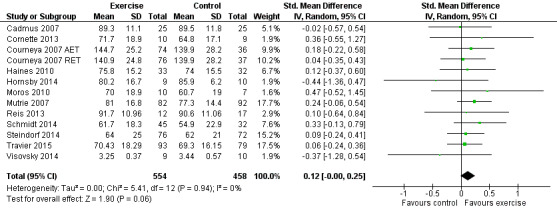
Forest plot of comparison: 1 Exercise versus control, outcome: 1.3 Cancer‐specific quality of life.
Segal 2001 (Segal 2001 SD and Segal 2001 SU) reported no significant differences between groups for cancer‐specific quality of life measured with FACT‐G, but reported no data. van Waart 2014 (van Waart 2014 high and van Waart 2014 low) assessed cancer‐specific quality of life with the EORTC QLQ‐C30, but reported no summary score and no score for global health, therefore we did not use data in the meta‐analysis. Results for the subscales were all in favour of the exercise groups, some reaching statistical significance and some not. One publication related to another study reported assessment of cancer‐specific quality of life with the Multidimensional Quality of Life scale, Cancer version (MQOLS‐Ca) (Dodd 2010), but neither data nor descriptive results were reported. Taken together, the reported results of studies that could not be included in the meta‐analysis seem to be in line with the pooled result. Results that were not reported concerned less than 10% of all women. We rated the result as moderate‐quality evidence due to lack of blinding, low adherence and unclear or high contamination, and a high rate of incomplete outcome data. We did not detect an indication of publication bias from the funnel plot. See Table 1.
Health‐related quality of life
Four studies examined generic health‐related quality of life (assessed via MOS 36‐Item Short Form Health Survey (MOS SF‐36)) (Cadmus 2007; Crowley 2003; Segal 2001 (Segal 2001 SD and Segal 2001 SU); Travier 2015). We did not perform a meta‐analysis because only data for subscales, but no data for physical and mental health summary measures, were presented. The studies did not find statistically significant differences between groups. Another study assessed generic health‐related quality of life via the EQ‐5D VAS (score range 0 to 100) and did not find statistically significant differences between groups: mean difference (MD) 1.10 (95% CI ‐5.28 to 7.48; Analysis 1.4) (Haines 2010). We rated this result as low‐quality evidence due to lack of blinding, low adherence and high contamination, a high rate of incomplete outcome data, and high risk of bias for group similarity at baseline. Additionally, we further downgraded for imprecision because of a small number of participants and because the null effect and an appreciable benefit were included in the confidence interval for the mean difference. With only one study extracted for the analysis, examination of the funnel plot for publication bias was not possible. We did not downgrade for publication bias. See Table 1.
1.4. Analysis.
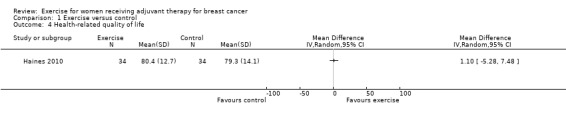
Comparison 1 Exercise versus control, Outcome 4 Health‐related quality of life.
Cancer site‐specific quality of life
Ten studies examined the effects of exercise on cancer site‐specific quality of life (Cadmus 2007; Campbell 2005; Eakin 2012; Haines 2010; Hayes 2013 (Hayes 2013 FtF and Hayes 2013 Tel); Hornsby 2014; Mutrie 2007; Schmidt 2014; Segal 2001 (Segal 2001 SD and Segal 2001 SU); Steindorf 2014). We could extract data for meta‐analysis from four studies (262 women) (Cadmus 2007; Campbell 2005; Hornsby 2014; Mutrie 2007), which had all used the FACT‐B questionnaire (score range 0 to 144): MD 4.24 (95% CI ‐1.81 to 10.29; I2 = 25%; Analysis 1.5). Three studies did not report a summary score of the EORTC QLQ‐BR23 questionnaire and were therefore not included in the meta‐analysis (Haines 2010; Schmidt 2014; Steindorf 2014). Segal 2001 (Segal 2001 SD and Segal 2001 SU ‐ 123 women) reported finding no significant differences between groups for cancer site‐specific quality of life measured with the FACT‐B questionnaire, but reported no data. We rated the result for cancer site‐specific quality of life as low‐quality evidence due to lack of blinding, low adherence, an unclear or high amount of contamination in three of the four studies in the meta‐analysis, and because two of four allocation concealment procedures were unclear. Furthermore, the number of women included in the meta‐analysis was small (n = 262), and the null effect as well as an appreciable benefit were included in the confidence interval for the mean difference, leading to further downgrading for imprecision. The four studies in the meta‐analysis were not sufficient for the examination of publication bias in the funnel plot, as at least 10 studies were considered a sufficient number. We did not downgrade for publication bias. See Table 1.
1.5. Analysis.
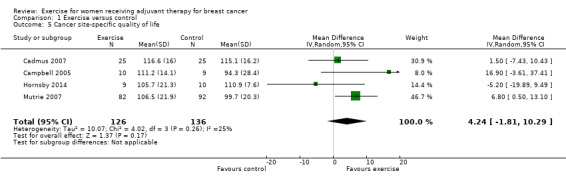
Comparison 1 Exercise versus control, Outcome 5 Cancer site‐specific quality of life.
Depression
Seven studies examined group differences for depression (Cadmus 2007; Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Dodd 2010; Mutrie 2007; Perna 2010; Schmidt 2014; Steindorf 2014). Meta‐analysis was possible for five studies (674 women) yielding six comparisons. Depression was assessed either with the Beck Depression Inventory (BDI) or with the Center for Epidemiological Studies‐Depression scale (CES‐D). The SMD between intervention and control was ‐0.15 (95% CI ‐0.30 to 0.01; I2 = 0%; Analysis 1.6; Figure 6). Two studies involving 51 and 119 women (Dodd 2010 and Perna 2010, respectively) did not report their data for depression, or it was not possible to extract data due to lack of information. Being borderline significant, their reported results might not necessarily alter that of the meta‐analysis, but they might contribute to a more or less clear inclusion of the null effect: one study reported finding a statistically significant effect in favour of the exercise group (Perna 2010), and the second study reported that depression scores did not change in any of the groups (Dodd 2010). We rated the result as moderate‐quality evidence due to lack of blinding, low adherence, and unclear or high contamination. The five studies in the meta‐analysis were not sufficient for the examination of publication bias in the funnel plot, as at least 10 studies were considered a sufficient number. We did not downgrade for publication bias. See Table 1.
1.6. Analysis.
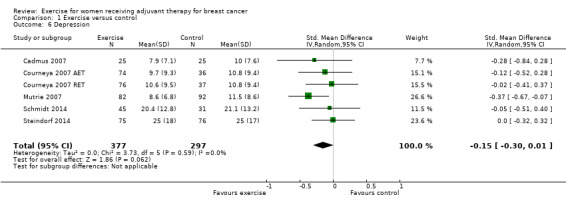
Comparison 1 Exercise versus control, Outcome 6 Depression.
6.
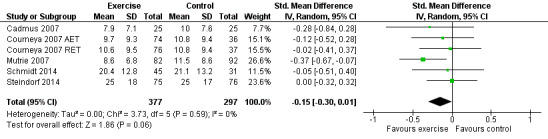
Forest plot of comparison: 1 Exercise versus control, outcome: 1.6 Depression.
Cognitive function
Two studies including a total of 213 women examined the effects of exercise on cognitive function with the Trail Making Test (Schmidt 2014; Steindorf 2014). The MD between intervention and control was ‐11.55 (95% CI ‐22.06 to ‐1.05; I2 = 0%; Analysis 1.7). Another study reported in their published study protocol that it aimed to assess cognitive function besides psychosocial well‐being, but the results paper did not mention the cognitive function outcome (Gokal 2013). Crowley 2003 reported having assessed attention performance with the Attention Functional Index, finding no statistically significant difference between groups. Data could not be extracted. Considering the relatively small number of women in the meta‐analysis, and missing data or no evidence of a difference for another 72 women outside the meta‐analysis, we can make a conclusion on the effect only under reservation. We rated the result as low‐quality evidence due to lack of blinding, low and unclear adherence and unclear contamination, and because group similarity at baseline for Schmidt 2014 was at high risk of bias. Schmidt 2014 reported a higher number of participants with depression, which often leads to impairment of cognitive function, in the control group. Our confidence in the result was lowered further because of imprecision (a small number of women). The two studies in the meta‐analysis were not sufficient for the examination of publication bias in the funnel plot as at least 10 studies were considered a sufficient number. We did not downgrade for publication bias. See Table 1.
1.7. Analysis.
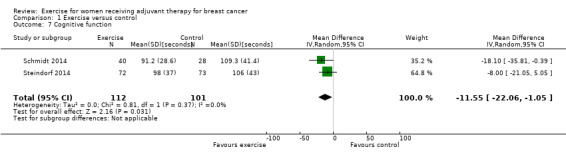
Comparison 1 Exercise versus control, Outcome 7 Cognitive function.
Secondary outcomes
Strength
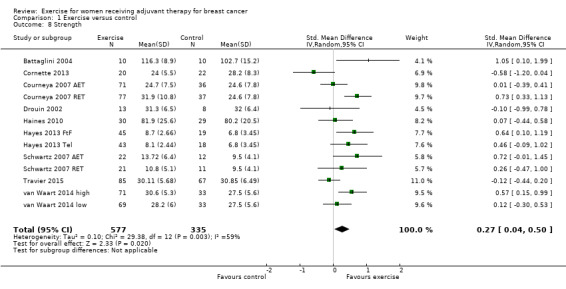
Fourteen studies reported assessment of changes in muscular strength (Battaglini 2004; Cornette 2013; Courneya 2007 (Courneya 2007 AET and Courneya 2007 RET); Crowley 2003; Drouin 2002; Haines 2010; Hayes 2013 (Hayes 2013 FtF and Hayes 2013 Tel); Ingram 2010; Schmidt 2014; Schwartz 2007 (Schwartz 2007 AET and Schwartz 2007 RET); Steindorf 2014; Travier 2015; van Waart 2014 (van Waart 2014 high and van Waart 2014 low); Visovsky 2014). We could extract data from nine studies yielding 13 comparisons for the meta‐analysis (Battaglini 2004; Cornette 2013; Courneya 2007 (Courneya 2007 AET and Courneya 2007 RET); Drouin 2002; Haines 2010; Hayes 2013 (Hayes 2013 FtF and Hayes 2013 Tel); Schwartz 2007 (Schwartz 2007 AET and Schwartz 2007 RET); Travier 2015; van Waart 2014 (van Waart 2014 high and van Waart 2014 low)), which showed a SMD of 0.27 (95% CI 0.04 to 0.50; I2 = 59%; 912 women; Analysis 1.8). The heterogeneity could be explained by a wide range of interventions and outcome assessment protocols.
1.8. Analysis.
Comparison 1 Exercise versus control, Outcome 8 Strength.
Two studies reported significant improvements in muscle strength (Schmidt 2014; Steindorf 2014), but did not report data for the results. One study reported finding no significant change in upper or lower body strength between the two groups across the study period (Crowley 2003), but did not report data for the results. One study measuring strength was finished early with no reported results (Ingram 2010), while another study did not report results about strength (Visovsky 2014).
We rated the result of a statistically significant improvement in strength as moderate‐quality evidence due to lack of blinding, low adherence and mostly unclear amount of contamination, and because many allocation procedures were unclear. We did not detect an indication of publication bias from the funnel plot.
The studies used different assessment protocols to measure muscular strength. Extracted data for the studies in the meta‐analysis is from the following assessment protocols: leg press strength (Cornette 2013; Haines 2010), grip strength (Drouin 2002; Travier 2015 ‐ right hand; van Waart 2014), overhead press, chest (Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Schwartz 2007: Schwartz 2007 AET and Schwartz 2007 RET), overall muscular strength (Battaglini 2004), and upper body function (strength and endurance) (Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel).
Subjective upper body function
Furthermore, two studies reported subjective upper body function measured with the Disability of Arm, Shoulder and Hand Questionnaire (DASH) (Eakin 2012; Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel). There was no statistically significant difference between groups in the meta‐analysis: MD ‐0.52 (95% ‐4.45 to 3.41; 231 women; Analysis 1.9). We rated the result as low‐quality evidence due to lack of blinding, low adherence and high contamination, and because the allocation concealment procedures were unclear. The group similarity at baseline was at high risk of bias as well. Furthermore, the number of participants was small, and confidence intervals were wide, raising concerns about imprecision, which further lowered our confidence in the result.
1.9. Analysis.
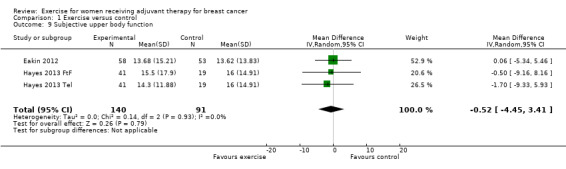
Comparison 1 Exercise versus control, Outcome 9 Subjective upper body function.
Shoulder mobility
Two studies measured shoulder range of motion (Haines 2010; Reis 2013), and one study reported a shoulder mobility score (Mutrie 2007). We could only extract data for the shoulder mobility score, and found a statistically significant difference between groups: MD 3.10 (95% CI 1.54 to 4.66; 174 women; 1 study; Analysis 1.10). Two further studies reported assessment of shoulder range of motion in their respective design papers, but did not mention the outcome in the final publications (Schmidt 2014; Steindorf 2014). We rated the result as low‐quality evidence due to lack of blinding, low adherence, and an unclear amount of contamination in the one study that reported results. The small number of participants and the lack of data reporting in four of five studies further lowered our confidence in the result.
1.10. Analysis.
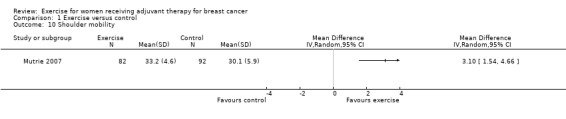
Comparison 1 Exercise versus control, Outcome 10 Shoulder mobility.
Arm morbidity
Two studies used the FACT‐B + 4 questionnaire, which for cancer site‐specific quality of life includes four questions in addition to the FACT‐B about arm morbidity and lymphoedema (higher scores mean less upper extremity impairment) (Eakin 2012; Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel). Meta‐analysis of the two studies yielding three comparisons showed a MD between groups of 1.11 (95% CI ‐4.07 to 6.29; I2 = 0%; 240 women; Analysis 1.11). We rated the result as low‐quality evidence due to lack of blinding, unclear allocation concealment, low adherence and high contamination, and imprecision (small number of participants).
1.11. Analysis.
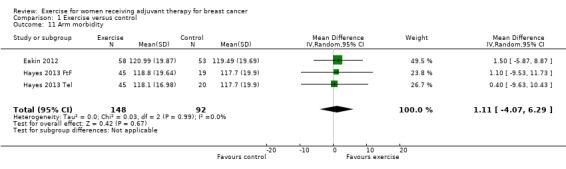
Comparison 1 Exercise versus control, Outcome 11 Arm morbidity.
Other psychological distress outcomes
Anxiety
Three studies assessed anxiety using the State‐Trait Anxiety Inventory (STAI) (Cadmus 2007; Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Eakin 2012); Eakin 2012 used the short form of the questionnaire, and Cadmus 2007 reported that they only used the state anxiety and not the trait anxiety scale. Cadmus 2007 found a small, not statistically significant effect. Meta‐analysis of the two studies using the whole questionnaire yielding three comparisons found no statistically significant difference between groups: MD ‐1.45 (95% CI ‐4.36 to 1.46; 331 women; Analysis 1.12) (Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Eakin 2012). We rated the result as low‐quality evidence due to lack of blinding, low adherence, and high or unclear contamination. The rather small number of participants further lowered our confidence in the results.
1.12. Analysis.
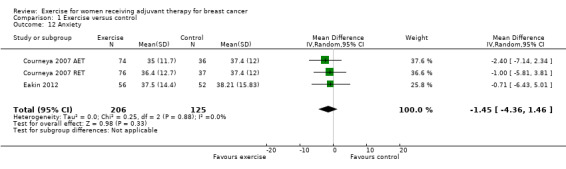
Comparison 1 Exercise versus control, Outcome 12 Anxiety.
Mood disturbances
Meta‐analysis of data from three small studies assessing mood disturbances showed a SMD of ‐1.00 (95% CI ‐1.40 to ‐0.60; I2 = 0%; 111 women; Analysis 1.13) (Drouin 2002; Gokal 2013; Yang 2011). One study used the long version of the Profile of Mood States (POMS) questionnaire (Drouin 2002), whereas the other two studies used the short form of the questionnaire (Gokal 2013; Yang 2011). We rated the statistically significant result of diminished mood disturbances as low‐quality evidence due to lack of blinding, low adherence and unclear contamination in two of the three studies, and because the allocation concealment procedures of the three studies were unclear. The small number of participants (n = 111) further lowered our confidence in the results.
1.13. Analysis.
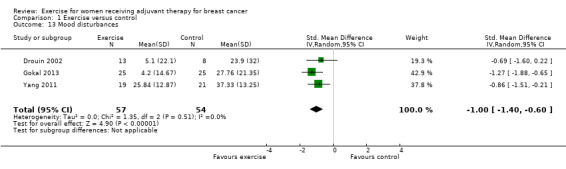
Comparison 1 Exercise versus control, Outcome 13 Mood disturbances.
Psychological distress
Another study used the General Health Questionnaire to assess psychological distress and did not find a statistically significant difference between groups: MD ‐1.47 (95% CI ‐9.38 to 6.44) (Moros 2010). One study measured negative and positive affects with the Positive and Negative Affect Schedule and found a statistically significant difference for positive affects but not for negative affects (positive affects: MD 4.10, 95% CI 1.38 to 6.82; negative affects: MD ‐2.10, 95% CI ‐4.18 to ‐0.02) (Mutrie 2007). Cadmus 2007 reported perceived stress and happiness; in this one study stress did not show a statistically significant difference (MD ‐3.10; 95% CI ‐6.63 to 0.43), and neither did happiness (MD ‐0.90; 95% CI ‐9.92 to 8.12).
Anxiety and Depression
Four studies used the Hospital Anxiety and Depression Scale to examine symptoms of depression and anxiety in one questionnaire (Cornette 2013; Gokal 2013; Travier 2015; van Waart 2014: van Waart 2014 high and van Waart 2014 low). Cornette 2013 reported final values for the summary score and found a statistically significant difference between groups: MD ‐6.10 (95% CI ‐9.65 to ‐2.55; 20 women; Analysis 1.14). The other three studies reported finding no statistically significant differences between groups for the summary score. van Waart 2014 did not report any data. Travier 2015 and Gokal 2013 additionally reported final values for the depression and anxiety subscales.
1.14. Analysis.
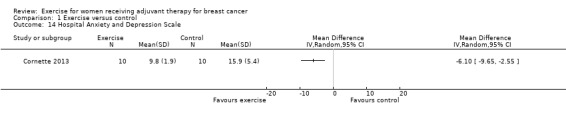
Comparison 1 Exercise versus control, Outcome 14 Hospital Anxiety and Depression Scale.
Due to the differences of the outcome assessment instruments and the differing utilisation of summary scores and subscales, we did not pool the data from the studies, apart from those using the POMS. We rated the results from the single studies as low‐quality evidence due to the high risk of bias in all of the studies and the small numbers of participants.
Sleep disturbances
Dodd 2010 and van Waart 2014 (van Waart 2014 high and van Waart 2014 low) reported sleep quality as an outcome, measured with the General Sleep Disturbance Scale and the Pittsburgh Sleep Quality Index respectively, but did not report results. Dodd 2010 reported only that no group differences were detected over time.
Self esteem
Three studies examined the effects of exercise on self esteem with the Rosenberg self‐esteem scale (Cadmus 2007; Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Gokal 2013). We found no statistically significant difference in the meta‐analysis: MD 1.69 (95% CI ‐0.01 to 3.39; 323 women; Analysis 1.15). The heterogeneity of 57% was introduced by Gokal 2013. Removal of Gokal 2013 in a sensitivity analysis lowered the heterogeneity to 0% with a MD of 0.97 (95% CI ‐0.28 to 2.21). We rated the result as low‐quality evidence due to lack of blinding, low adherence and high or unclear contamination in two studies, and unexplained heterogeneity.
1.15. Analysis.
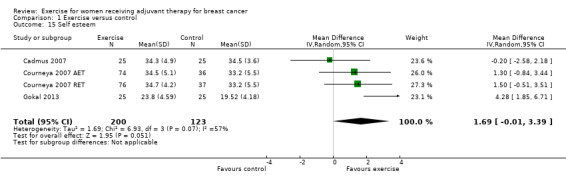
Comparison 1 Exercise versus control, Outcome 15 Self esteem.
Physical activity behaviour
Meta‐analysis of seven studies showed a SMD of 0.29 (95% CI 0.12 to 0.47; 549 women; Analysis 1.16) (Caldwell 2009; Cornette 2013; Eakin 2012; Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel; Husebo 2014; Mutrie 2007; Yang 2011). Six further studies also examined the effects of exercise on physical activity, but they did not report data that could be used for meta‐analysis (Cadmus 2007; Gokal 2013; Hornsby 2014; Mock 2004; Perna 2010; van Waart 2014: van Waart 2014 high and van Waart 2014 low). However, three of these studies reported that there were no statistically significant group differences for physical activity (Hornsby 2014; Mock 2004; van Waart 2014: van Waart 2014 high and van Waart 2014 low). On the other hand, Perna 2010 reported that there were significantly higher LTEQ (leisure time exercise questionnaire) scores in the intervention group than in controls.
1.16. Analysis.
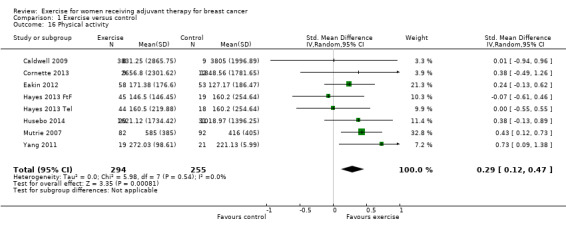
Comparison 1 Exercise versus control, Outcome 16 Physical activity.
We rated the result as moderate‐quality evidence due to lack of blinding, low adherence and high or unclear contamination, and because many (six of seven) allocation concealment procedures were unclear.
Multidimensional outcomes
Self efficacy
One study reported that confidence to exercise (self efficacy) increased significantly more in the intervention group than in the control group (Eakin 2012). We could not extract data. A second study reported that there were no statistically significant differences between groups for physical self efficacy (Crowley 2003). Another study reported assessment of self efficacy regarding the performance of physical activity in the design paper, but did not mention the outcome in the publication of study results (Travier 2015).
Functioning in daily life and return to work
One study reported that there were no statistically significant differences for functioning in daily life for either of the two exercise groups (van Waart 2014: van Waart 2014 high and van Waart 2014 low). Functioning in daily life was measured with the Impact on Participation and Autonomy (IPA) instrument. Another study reported assessment of perceived impact of the disease on participation and autonomy assessed with the same instrument (IPA) in the study design paper, but did not mention the outcome in the final publication (Travier 2015). The first study, van Waart 2014 (van Waart 2014 high and van Waart 2014 low), additionally used the study‐specific "Return to work questionnaire", and reported that at the end of the intervention a significantly greater number of participants in the exercise groups were working than in the usual‐care group, and that at follow‐up the intervention groups had significantly higher return‐to‐work rates than the usual‐care group and worked a significantly higher percentage of the pre‐illness hours on the job. We could not extract data.
Symptom severity and symptom interference
One small study used the Taiwanese version of the MD Anderson Symptom Inventory (MDASI‐T) to assess symptom severity and symptom interference with daily life and reached statistically significant results for both outcomes: symptom severity MD ‐1.49 (95% CI ‐2.36 to ‐0.62) and symptom interference MD ‐1.10 (95% CI ‐1.89 to ‐0.31) (Yang 2011). Another study used the Symptom Experience Scale, but did not report results (Visovsky 2014). We rated the results as low‐quality evidence due to lack of blinding, low adherence and unclear amount contamination, and because the allocation concealment procedure was unclear. The small number of participants (n = 40) further lowered our confidence in the results.
One study reported finding no statistically significant differences between groups for satisfaction with life (Campbell 2005). Another study developed a study‐specific functional wellness questionnaire, and did not find a statistically significant difference between groups (Crowley 2003).
Chemotherapy completion
Two studies assessed chemotherapy completion rates, but different outcome measures did not allow for meta‐analysis (Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; van Waart 2014: van Waart 2014 high and van Waart 2014 low). van Waart 2014 (van Waart 2014 high and van Waart 2014 low) reported how many women in each group required a dose adjustment of the chemotherapy and the average dose reduction among these women. Statistically significantly fewer women required a dose adjustment for the high‐intensity exercise group compared to the low‐intensity exercise and the control groups. There was also a statistically significant difference in the average dose reduction between the two exercise groups and the control group.
Courneya 2007 (Courneya 2007 AET and Courneya 2007 RET) assessed chemotherapy completion rate as the "average relative dose‐intensity (RDI) for the originally planned regimen based on standard formulas". The study reported the percentage of women in each group that received at least 85% of their planned RDI, and found no statistically significant differences between groups.
Other side effects relating to adjuvant cancer treatment: neuropathy symptoms, endocrine symptoms, pain, gait and balance, nausea
Neuropathic pain was measured with the neuropathic pain scale in one study (Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel), while another study, Visovsky 2014, measured neuropathy symptoms with the FACT‐Taxane scale in women treated with taxanes. Neither study reached statistically significant results: neuropathic pain (Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel): MD 3.64 (95% CI ‐1.32 to 8.60; 130 women; Analysis 1.17) and neuropathy symptoms (Visovsky 2014): MD ‐0.21 (95% CI ‐0.75 to 0.33; 19 women; Analysis 1.18). We rated these results as low‐quality evidence due to lack of blinding, unclear allocation concealment procedures, low or unclear adherence, and high or unclear contamination. Group similarity at baseline was unclear or at high risk of bias as well. Imprecision due to a small number of participants and wide confidence intervals further lowered our confidence in the results. Visovsky 2014 also reported assessment of cold thermal sensation and vibratory sensation in the trial registration, but did not report these outcomes in the results paper.
1.17. Analysis.
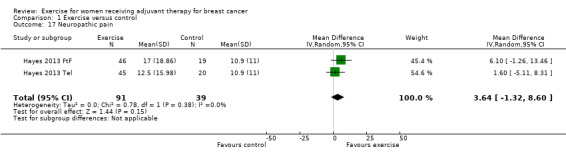
Comparison 1 Exercise versus control, Outcome 17 Neuropathic pain.
1.18. Analysis.
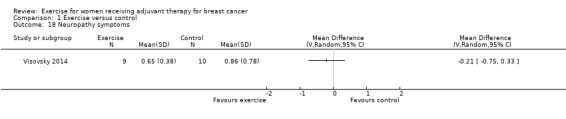
Comparison 1 Exercise versus control, Outcome 18 Neuropathy symptoms.
Hayes 2013 (Hayes 2013 FtF and Hayes 2013 Tel) measured menopausal symptoms with the Greene Climacteric Scale and found no group differences. No summary score was presented. Mutrie 2007 assessed endocrine symptoms with the FACT for endocrine symptoms (FACT‐ES): MD 1.30 (95% CI ‐1.49 to 4.09; 174 women; Analysis 1.19). We rated the result as low‐quality evidence due to lack of blinding, low adherence, and unclear contamination. The small number of participants in this one study reporting a summary score further lowered our confidence in the results.
1.19. Analysis.
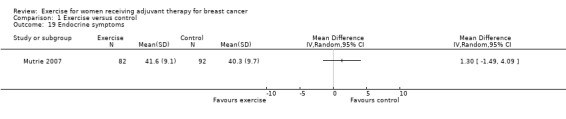
Comparison 1 Exercise versus control, Outcome 19 Endocrine symptoms.
Dodd 2010 reported pain measured with the Worst Pain Intensity Scale and found no statistically significant differences between groups. No data were reported.
Gait and balance was measured with the Timed Get‐up‐and‐Go Test in two studies (Caldwell 2009; Visovsky 2014), and with a step test in one study (Haines 2010). There was no statistically significant difference between groups in the meta‐analysis: SMD 0.10 (95% CI ‐0.25 to 0.46; 122 women; Analysis 1.20). We rated the result as low‐quality evidence due to lack of blinding, low and unclear adherence, and unclear and high contamination. Allocation concealment procedures were unclear in two studies. The small number of participants further lowered our confidence in the result.
1.20. Analysis.
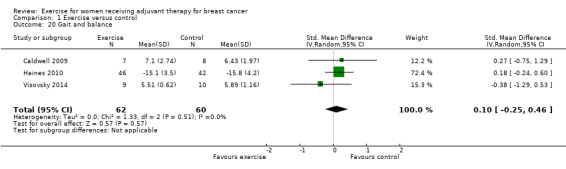
Comparison 1 Exercise versus control, Outcome 20 Gait and balance.
Two studies reported nausea as an outcome (Dodd 2010; Winningham 1988). Winningham 1988 assessed nausea with the Symptom Checklist‐90 (SCL‐90) item for nausea, whereas Dodd 2010 assessed nausea intensity with a subscale of a symptom checklist of 25 commonly experienced symptoms. We did not consider either of these to be valid methods of assessment and therefore did not report data here.
Harms
Twenty‐one studies assessed adverse effects due to exercise (Battaglini 2004; Cadmus 2007; Campbell 2005; Cornette 2013; Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Crowley 2003; Dodd 2010; Drouin 2002; Eakin 2012; Haines 2010;Hornsby 2014; Husebo 2014; Mock 2004; Moros 2010; Schmidt 2014; Schwartz 2007: Schwartz 2007 AET and Schwartz 2007 RET; Segal 2001: Segal 2001 SD and Segal 2001 SU; Steindorf 2014; Travier 2015; Visovsky 2014; Yang 2011). Seven studies observed adverse effects (Crowley 2003; Dodd 2010; Drouin 2002; Eakin 2012; Haines 2010; Hornsby 2014; Husebo 2014). Details of these adverse effects can be found in the Characteristics of included studies table. In general, adverse effects concerned only a very small number of women.
Seven studies described how relevant information on adverse effects was collected. In two studies this was done by the exercise trainers who supervised the intervention (Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Hornsby 2014). In one of these studies (Hornsby 2014), safety of the supervised aerobic exercise intervention was the primary outcome, and all adverse effects during aerobic training were monitored and reported on the participant case report forms. In one study with a telephone group (Eakin 2012), exercise physiologists recorded adverse effects after each call in case management folders; Husebo 2014 used biweekly telephone calls to monitor adverse effects. The participants in Haines 2010 were told to document adverse effects and accidental falls in a log book. Two studies used standardised questionnaires where participants recorded adverse effects (Schmidt 2014; Steindorf 2014). In one of these two studies (Steindorf 2014), adverse effects reported spontaneously by the participant or observed by therapists were also recorded. Many of the studies did not describe how relevant information was collected or whether surveillance of adverse effects was passive (spontaneously reported by participants) or active (based on structured questionnaires or interviews).
Lymphoedema
Two studies (Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel) systematically assessed the incidence of lymphoedema. Hayes 2013 (Hayes 2013 FtF and Hayes 2013 Tel) reported four objectively measured cases each in both exercise groups (face to face: n = 67 and telephone: n = 67) and six cases in the usual‐care group (n = 60). These numbers reported for Hayes 2013 (Hayes 2013 FtF and Hayes 2013 Tel) were for all women and not only women who exercised concurrently with their adjuvant treatment. Meta‐analysis of the two studies yielding four comparisons showed a risk ratio for lymphoedema of 0.71 (95% CI 0.35 to 1.45; 436 women; Analysis 1.21). Crowley 2003 reported lymphoedema as an adverse effect in one woman. Haines 2010 measured the circumference of upper limb segments and reported that changes in the circumference of upper limb segments favoured the intervention group.
1.21. Analysis.
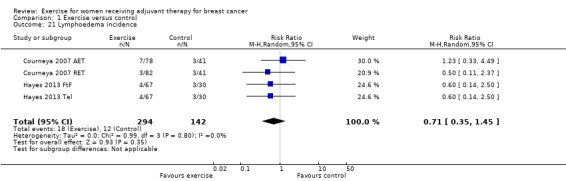
Comparison 1 Exercise versus control, Outcome 21 Lymphoedema incidence.
We rated the results as low‐quality evidence due to lack of blinding, low adherence and a high or unclear amount of contamination, the allocation concealment procedure was unclear in one of the two studies, and group similarity at baseline was at high risk of bias for one study. Furthermore, the null effect and appreciable harm and benefit were included in the confidence interval for the risk ratio. The two studies in the meta‐analysis were not sufficient for the examination of publication bias in the funnel plot as at least 10 studies were considered to be a sufficient number. We did not downgrade for publication bias. See Table 1.
Effectiveness and adverse effects during follow‐up
Several studies assessed effectiveness of exercise during adjuvant therapy after a follow‐up period of several months.
Five studies measured physical fitness 18 weeks, in Travier 2015, to six months after the intervention: SMD 0.26 (95% CI ‐0.06 to 0.57; 612 women; Analysis 2.1) (Cornette 2013; Husebo 2014; Mutrie 2007; Travier 2015; van Waart 2014: van Waart 2014 high and van Waart 2014 low). We rated the result as low‐quality evidence due to lack of blinding, low adherence, and unclear or high contamination. Additionally, randomisation and allocation concealment procedures were unclear in several studies. Heterogeneity with an I2 of 70% could be only partly explained by introduction of Travier 2015, which was the only study with a follow‐up period shorter than six months. Removal of the study in the sensitivity analysis still resulted in an I2 of 40% and a SMD of 0.38 (95% CI 0.121 to 0.63). It remained unclear why a shorter follow‐up period would result in a smaller effect for cardiorespiratory fitness. With regard to all of these studies, the large variation in effect and confidence intervals that did not overlap raised concerns about inconsistency, which further lowered our confidence in the result.
2.1. Analysis.
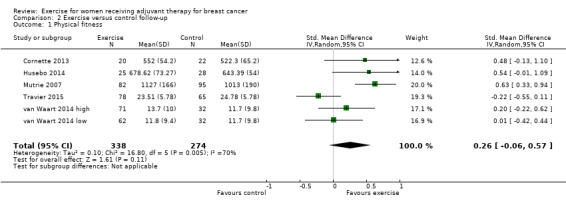
Comparison 2 Exercise versus control follow‐up, Outcome 1 Physical fitness.
Six studies assessed fatigue 18 weeks, in Travier 2015, to six months after the intervention period: SMD ‐0.21 (95% CI ‐0.35 to ‐0.07; 814 women; Analysis 2.2) (Cornette 2013; Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Husebo 2014; Mutrie 2007; Travier 2015; van Waart 2014: van Waart 2014 high and van Waart 2014 low). We rated the result as moderate‐quality evidence due to lack of blinding, low adherence, and unclear or high contamination. Furthermore, randomisation and allocation concealment procedures were unclear in several studies. A sensitivity analysis including only the five studies with a six‐month follow‐up period also showed a SMD between intervention and control of ‐0.21 (95% CI ‐0.37 to ‐0.05).
2.2. Analysis.
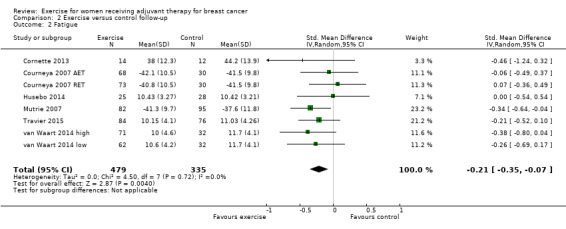
Comparison 2 Exercise versus control follow‐up, Outcome 2 Fatigue.
Six studies assessed cancer‐specific quality of life 12 weeks, in Visovsky 2014, to six months after the intervention: SMD 0.18 (95% CI 0.01 to 0.35; 583 women; Analysis 2.3) (Cornette 2013; Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Mutrie 2007; Travier 2015; van Waart 2014: van Waart 2014 high and van Waart 2014 low; Visovsky 2014). van Waart 2014 (van Waart 2014 high and van Waart 2014 low) only presented data for subscales and no summary score. We rated the result as moderate‐quality evidence due to lack of blinding, low adherence and unclear or high contamination, and because risk of attrition bias was high in two of the five studies in the meta‐analysis. Furthermore, randomisation and allocation concealment procedures were unclear in two studies. A sensitivity analysis including only the three studies with a six‐month follow‐up period showed a SMD between intervention and control of 0.25 (95% CI 0.04 to 0.45) (Cornette 2013; Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Mutrie 2007).
2.3. Analysis.
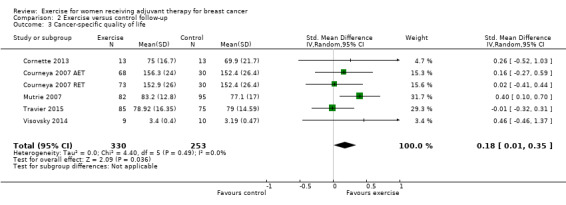
Comparison 2 Exercise versus control follow‐up, Outcome 3 Cancer‐specific quality of life.
Two studies assessed depression six months after the intervention period: SMD ‐0.27 (95% CI ‐0.48 to ‐0.06; 378 women; Analysis 2.4) (Courneya 2007: Courneya 2007 AET and Courneya 2007 RET; Mutrie 2007). We rated the result as moderate‐quality evidence due to lack of blinding, low adherence, and unclear contamination.
2.4. Analysis.
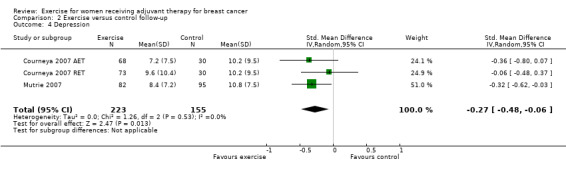
Comparison 2 Exercise versus control follow‐up, Outcome 4 Depression.
Three studies assessed strength at six months' follow‐up: SMD 0.00 (95% CI ‐0.30 to 0.30; 386 women; Analysis 2.5) (Cornette 2013; Travier 2015; van Waart 2014: van Waart 2014 high and van Waart 2014 low). The heterogeneity, with an I2 of 49%, was introduced by the high‐intensity group (resistance training) of van Waart 2014 high and was reduced to 0% when this study was removed in a sensitivity analysis (SMD ‐0.11; 95% CI ‐0.35 to 0.13); thus heterogeneity could be explained. We rated the result as low‐quality evidence due to lack of blinding, low adherence and unclear or high contamination, and because there was a high risk of selection bias in two of three studies. Furthermore, allocation concealment procedures were unclear in two studies. The wide confidence interval introduced uncertainty about the magnitude of the effect, and thus confidence in the result was lowered further.
2.5. Analysis.
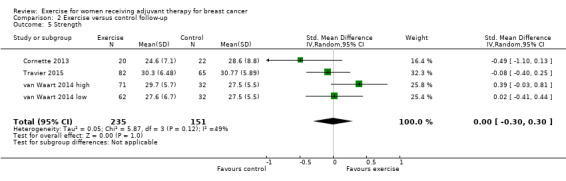
Comparison 2 Exercise versus control follow‐up, Outcome 5 Strength.
Three studies assessed physical activity at six months' follow‐up: SMD 0.28 (95% CI ‐0.05 to 0.61; 261 women; Analysis 2.6) (Cornette 2013; Husebo 2014; Mutrie 2007). We rated the result as low‐quality evidence due to lack of blinding, low adherence, and unclear or high contamination. Additionally, randomisation and allocation concealment procedures were unclear in two of the three studies and there was attrition bias in two studies. Furthermore, imprecision (rather small number of participants and null effect and appreciable benefit included in confidence interval for SMD) further lowered our confidence in the result.
2.6. Analysis.
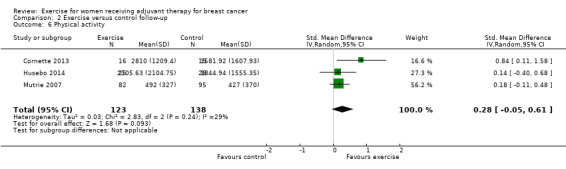
Comparison 2 Exercise versus control follow‐up, Outcome 6 Physical activity.
Courneya 2007 (Courneya 2007 AET and Courneya 2007 RET) also reported results for anxiety (MD ‐3.61; 95% CI ‐7.24 to 0.03; 201 women; Analysis 2.7) and self esteem (MD 1.20; 95% CI ‐0.41 to 2.81; 201 women; Analysis 2.8) six months after the intervention, and Mutrie 2007 reported results for endocrine symptoms (MD 1.30; 95% CI ‐1.65 to 4.25; 177 women; Analysis 2.9) and positive affects (MD ‐0.59; 95% CI ‐1.63 to 0.45) and negative affects (MD ‐1.70; 95% CI ‐3.62 to 0.22). One study also assessed neuropathy symptoms (MD ‐0.45; 95% CI ‐0.98 to 0.08; 19 women; Analysis 2.10) and gait and balance (MD ‐0.59; 95% CI ‐1.63 to 0.45; 19 women; Analysis 2.11) 12 weeks after the end of the intervention (Visovsky 2014). We rated all these results as low‐quality evidence due to the small number of women in the single studies, lack of blinding, low adherence, and unclear contamination. All 'Risk of bias' items for Visovsky 2014 were at high or unclear risk of bias.
2.7. Analysis.
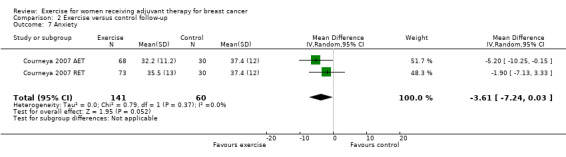
Comparison 2 Exercise versus control follow‐up, Outcome 7 Anxiety.
2.8. Analysis.
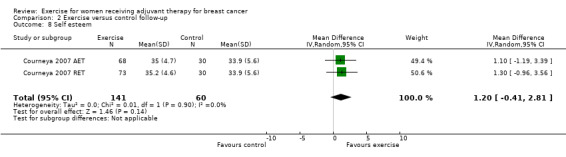
Comparison 2 Exercise versus control follow‐up, Outcome 8 Self esteem.
2.9. Analysis.
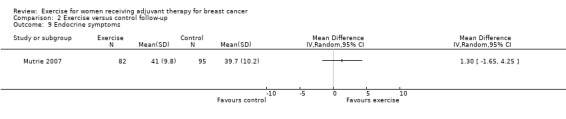
Comparison 2 Exercise versus control follow‐up, Outcome 9 Endocrine symptoms.
2.10. Analysis.
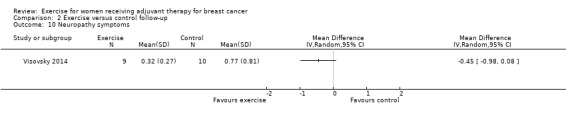
Comparison 2 Exercise versus control follow‐up, Outcome 10 Neuropathy symptoms.
2.11. Analysis.
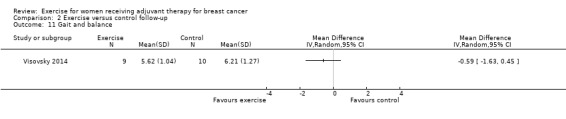
Comparison 2 Exercise versus control follow‐up, Outcome 11 Gait and balance.
One study with an intervention period of one year reported no significant differences for any outcome after one year, but did not report data (Dodd 2010).
One study reported long‐term follow‐up data for 18 months and five years in an additional paper from 2012 (Mutrie 2007), which was the longest follow‐up period of the included studies. For both follow‐up periods, less than 70% of the original participants were included in the analysis, therefore we did not present data here.
Lymphoedema
One study reported lymphoedema incidence eight weeks after the intervention (Hayes 2013: Hayes 2013 FtF and Hayes 2013 Tel). The numbers reported were for all women and not only women who exercised concurrently with their adjuvant treatment (risk ratio 0.79; 95% CI 0.37 to 1.69; 194 women; Analysis 2.12). Otherwise, harms or adverse effects were not reported after a follow‐up period.
2.12. Analysis.
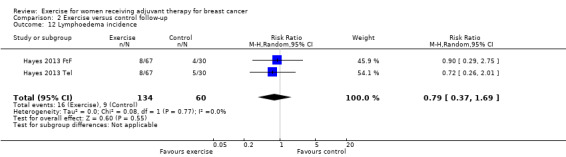
Comparison 2 Exercise versus control follow‐up, Outcome 12 Lymphoedema incidence.
Discussion
Summary of main results
Exercise during adjuvant treatment for breast cancer improves physical fitness and probably slightly reduces fatigue. It likely leads to little or no difference in depression and cancer‐specific quality of life. Women with breast cancer may benefit from exercise during adjuvant cancer treatment through improved cognitive function and slightly improved cancer site‐specific quality of life. Exercise may lead to little or no improvement in health‐related quality of life and may lower the risk of lymphoedema.
Exercise also probably slightly improves muscular strength and leads to a slightly higher amount of physical activity. Women with breast cancer who exercise during adjuvant treatment may experience fewer mood disturbances, and their shoulder mobility might be slightly improved. For other outcomes such as self esteem, exercise may lead to little or no difference. For some of the outcomes not all studies could be included in the meta‐analyses, therefore the results do not reflect the body of evidence as a whole. An improvement for several other outcomes is uncertain, mostly due to scarcity or lack of data.
Overall completeness and applicability of evidence
This review is based on studies with a considerable degree of clinical heterogeneity regarding adjuvant cancer treatment and exercise interventions. It remains to be explored whether differences in adjuvant cancer treatment and exercise intervention actually affect results. In spite of our comprehensive attempts to identify all relevant studies, we retrieved predominantly English language studies for inclusion in this review. This may reflect selective publication of English language studies with statistically significant findings. In addition, most of the included studies were primarily conducted among white women in high‐income countries, which makes generalisation of the results to different ethnic groups and countries questionable.
Type of intervention
Exercise interventions varied widely regarding implementation of aerobic or resistance exercise or a combination of both, supervised or home‐based, frequency, duration, and intensity. Furthermore, the reporting of the exercise intervention differed, and details were not always available.
As exercise as such can be seen as a complex intervention, the evaluation of exercise interventions is prone to the diverse challenges associated with evaluating complex interventions.
Key features of complex interventions (according to Craig 2008: the Medical Research Council guidance on complex interventions) applicable to exercise are the:
number of interacting components, e.g. exercise in a group versus alone; aerobic or resistance exercise; or the empathy of an exercise instructor;
number and difficulty of behaviours required by those delivering or receiving the intervention, e.g. adherence to the prescribed intensity of exercise or challenging participants to exercise at a higher heart rate;
number and variability of outcomes, e.g. fatigue, depression, anxiety with a range of scales to assess;
degree of flexibility or tailoring of the intervention permitted (non‐standardisation/reproducibility), e.g. tailoring the exercise intervention according to the motivation level of the group or to the current physical condition of the woman with breast cancer.
The aim of this review was to answer quite a broad review question, namely to assess the effect of exercise per se on several patient‐relevant outcomes for women undergoing adjuvant treatment for breast cancer, which is why we included exercise interventions delivered at diverse levels and with a variety of components. Based on this evidence, further steps could be taken to identify and differentiate all the interacting components of exercise interventions.
Type of control
Most studies reported usual care as the control intervention, but they often did not describe this in detail. As studies were conducted in different settings, home‐based or at the treatment clinic, and with different types of adjuvant treatment, variation in usual care in the included studies should be taken into account. Some studies offered control interventions in order to establish similar circumstances with regards to time and attention and, if applicable, group interaction.
Type of adjuvant treatment
Women were treated in some studies with radiation treatment only, chemotherapy only, or a combination of the two, resulting in large variation in the duration and frequency of adjuvant treatment as well as differences in possible side effects. We included studies with the latest treatment protocols as well as studies from the 1980s onward, meaning that not only treatment protocols but also drugs to treat side effects were used in many different ways in the included studies. Details on treatment protocols were provided in some, but not all studies, whereas drug treatment of side effects was most often not described.
Timing of outcome assessment and follow‐up
As it has been shown that, for example psychological distress of women, diminishes with time after diagnosis, timing of measuring outcomes can make a difference in the magnitude and direction of the effect of the intervention. The same may apply to other outcomes assessed in our review. Timing of outcome assessments was very heterogeneous in the included studies, which should be kept in mind when comparing different studies and interpreting results. Follow‐up ranged from no follow‐up to five years in one study. Analyses were only possible for a few studies with a maximum follow‐up period of six months, as either data reporting was poor or attrition rates were too high for studies with longer follow‐up periods. Apart from lymphoedema, which was reported after a short follow‐up period in one study, harms or adverse effects were not reported after a follow‐up period.
Reporting of outcome measures
A wide range of outcome measures was assessed across the studies, making it difficult to combine outcomes in meta‐analysis. Moreover, data reporting was often poor and did not provide estimates of effect size that could be pooled. Assessment and reporting of harms‐related data from exercise intervention studies during adjuvant cancer treatment also needs improvement. Future studies should apply so‐called core outcome sets to facilitate comparison and meta‐analysis (Gargon 2014).
Quality of the evidence
Quality of studies
Due to the nature of exercise as an intervention, blinding of participants and exercise supervisors is not possible. The precise effect of the absence of blinding on the magnitude and direction of the treatment effect is unclear, but constitutes a high risk for performance bias. Furthermore, many outcomes were self reported, which leads to a high inherent risk of detection bias, when blinding of participants is not possible. We decided not to downgrade studies for those 'Risk of bias' items alone. However, reasons for assigning studies a high risk of bias was never due only to lack of blinding, contamination, and/or non‐adherence, because other factors such as unclear allocation procedures or high attrition rates were present as well, leading to downgrading of the evidence one level for risk of bias. We included only randomised controlled studies in this version of the review, but 11 studies reported insufficient details on random sequence generation, and 23 provided insufficient details on allocation concealment.
Statistical power
Benefits of exercise interventions may be relatively small. Subsequently, the number of included participants should be great enough to allow for the detection of small differences between groups. The sample sizes in the included studies ranged from 5 to 91 in the intervention group.
Initial fitness level
The individual's level of fitness is an important factor to consider before determining the level of exercise intensity (ACSM 2000). According to the American College of Sports Medicine (ACSM 2000), deconditioned individuals may demonstrate increases in their cardiorespiratory fitness with exercise intensities at the lower end of the intensity continuum, whereas more fit individuals need to work at the higher end of the intensity continuum to improve fitness. A small number of studies limited participation to sedentary women; however, definitions of sedentary varied.
Adherence and contamination
For sedentary individuals, a change in personal health behaviour is required in order to take up regular exercise. Thus, any exercise intervention can additionally be evaluated according to the degree of behavioural change achieved in the intervention group; a lack of adherence can compromise the training stimulus as well as the sustainability of exercise behaviour. According to the American College of Sports Medicine (ACSM 2000), the art of exercise prescription is the "successful integration of exercise science with behavioral techniques that result in long‐term program compliance". Some studies applied theory‐based methods focused on changing behaviour. Adherence problems do not only arise in terms of participation in exercise sessions and frequency of sessions, but also in terms of the training intensity and duration actually achieved during each exercise session. Insufficient exercise intensity or duration may compromise the training stimulus as a whole. However, these two facets of the training stimulus were poorly evaluated and reported in many of the included studies.
Besides adherence, the extent to which the control group performs exercise (contamination) is a second critical component in exercise studies. Exercise contamination is rarely reported and often only when the exercise programme is home‐based. Furthermore, reports of adherence and contamination most often rely on self report by participants, which can lead to over‐reporting of adherence but also contamination.
Potential biases in the review process
Despite a well‐established methodology for conducting systematic reviews, subjective judgement is inevitable throughout the process. The main limitation of this review was the lack of sufficient information or data in many studies to make a clear judgement in various bias domains. Other limitations were the heterogeneity in the intervention delivered, adjuvant treatment, timing of outcome measurement, and the assessment and reporting of outcome measures with a wide range of outcome measures.
We did not systematically evaluate assessment instruments with regards to their strengths and weaknesses. However, we noted that for the outcome physical activity, the reported activity levels of women with breast cancer were surprisingly high in some studies. Three of seven studies in the meta‐analysis for physical activity used the International Physical Activity Questionnaire (IPAQ) (Caldwell 2009; Cornette 2013; Husebo 2014), which has been reported to lead to over‐reporting of physical activity (Lee 2011).
Mostly due to limited resources, we did not systematically assess the training stimulus in this version of the review as well as the baseline activity and fitness levels of participants and the application of behaviour change theories in the studies.
Agreements and disagreements with other studies or reviews
Another Cochrane systematic review assessed the effect of exercise during adjuvant therapy for cancer on quality of life (Mishra 2012), and another on fatigue (Cramp 2012). Both reviews included adults with different cancer diagnoses, not only breast cancer. Cramp 2012 also included studies evaluating the effect of exercise after adjuvant therapy, and Mishra 2012 included 10 studies with participants both during and after adjuvant therapy. Both reviews identified benefits on fatigue for participants with breast cancer, which is in agreement with the results of our review. Mishra 2012 reported that exercise interventions resulted in improvements in overall quality of life for all participants, but found no statistically significant difference for women with breast cancer. We did not perform a meta‐analysis for overall quality of life because only one study presented a summary measure, whereas the others only reported data for subscales. The single studies reporting that outcome did not result in a significant difference between groups.
Meneses‐Echavez 2015 reviewed the effect of supervised exercise during or after adjuvant therapy for breast cancer on cancer‐related fatigue, and found benefits as well.
Bourke 2014 published another Cochrane systematic review with the main goal of assessing the effects of interventions to promote exercise behaviour in sedentary people living with and beyond cancer. Eleven of 14 studies were conducted in women with breast cancer, but mostly in women who had finished adjuvant treatment. Interventions resulted in improvements in aerobic exercise tolerance at 8 to 12 weeks in intervention participants compared with controls. Aerobic exercise tolerance was also improved at six months. These findings are in agreement with our review, but participants differed with regard to cancer diagnosis and treatment status.
One systematic review assessed depression and anxiety in addition to fatigue and cancer‐specific quality of life in women with breast cancer undergoing adjuvant therapy (Carayol 2013). The authors reported that the exercise intervention led to statistically significant improvements for fatigue, cancer‐specific quality of life, and depression, while the decrease in anxiety was "borderline significant". Our results for both cancer‐specific quality of life and depression were close to showing a statistically significant difference between groups, favouring exercise, with a lower respectively upper limit of the confidence interval of 0.00 and 0.01. In three of the 17 included studies in the review by Carayol 2013, the intervention was yoga, which we excluded, regarding it as a complex intervention. For depression and anxiety, the authors pooled data from the Hamilton Anxiety and Depression Score with data from the Beck Depression Inventory and Center for Epidemiological Studies‐Depression scale, which we did not. Keeping in mind the similar but not identical inclusion criteria, the findings of this review are thus mostly in line with our review.
Authors' conclusions
Implications for practice.
Exercising while receiving adjuvant treatment for breast cancer is a feasible, supportive self care intervention. Based on current evidence, exercise probably slightly reduces fatigue and improves physical fitness. It likely leads to little or no difference in depression and cancer‐specific quality of life. Women with breast cancer may benefit from exercise during adjuvant cancer treatment through improved cognitive function and slightly improved cancer site‐specific quality of life. Exercise may lead to little or no improvement in health‐related quality of life. Muscular strength and physical activity are probably improved by exercising. Several further outcomes such as shoulder mobility showed slight improvements, and several such as self esteem showed little or no difference, but the quality of the evidence was low.
Exercise adherence during cancer treatment constitutes a challenge, and thus attempts to foster exercise participation might enhance effectiveness. For behaviour changes to occur (the adoption of regular exercise in this instance), it is essential that intervention programmes focus on underlying principles from theories about why people change their behaviours. The social cognitive theory appears to be a promising theoretical framework for promoting exercise behaviour in women with breast cancer (Pinto 2002; Rogers 2004; Rogers 2005). The key construct in the social cognitive theory is self efficacy. Exercise self efficacy can be described either as the confidence to overcome barriers to exercise or as confidence in the ability to perform certain exercise tasks. Self efficacy has proven to be an important correlate of exercise among women with breast cancer. Exercise self efficacy among women with breast cancer during cancer treatment is reported to be lowest when women are nauseated, tired, not interested, lacking time, and lacking exercise enjoyment (Rogers 2006).
Future exercise interventions should target the exercise barriers. Exercise enjoyment, for example, may be addressed through picking up recent trends in the field of fitness, such as Pilates, Nordic walking, Tai Chi, step aerobics, and dancing, of course adequately adjusted to the needs and limitations of the target group. Group exercise or partner‐assisted exercises may also increase exercise enjoyment. Time management may be addressed by exercise classes taking place in different locations, choosing venues that are accessible by public transport, and by scheduling classes at various times in the day and evening.
Implications for research.
At this stage there is still a lack of evidence for several relevant potential benefits of exercise as well as for harms. The increasing number of studies assessing the benefits and harms of exercise during adjuvant therapy is promising, but while study quality and reporting of studies have certainly improved since the first studies assessing this question, the quality of the evidence is still low for many outcomes. This is due in part to the difficulty of blinding participants and supervising personnel in studies with exercise as an intervention. For other factors that diminish the quality of evidence, such as lack of outcome assessor blinding, or the reporting of methodology and data, an improvement is feasible.
As described above, the actual training stimulus may substantially deviate from the assigned exercise regimen. In efficacy trials investigators need to ensure adherence to the intervention to determine whether exercise interventions in this population work. Exercise programmes should be designed to address exercise facilitators such as exercise enjoyment; this may be achieved by offering a variety of alternating exercise modes that assure an adequate training stimulus. Inclusion of sedentary participants only may be a way to deal with contamination issues, utilising the observation that physical activity and exercise decline during cancer treatment (Irwin 2003). While efforts can and should be made to maximise adherence and to minimise contamination, imperfect adherence and an amount of contamination is even to be expected in efficacy studies, lowering the confidence in the results. In effectiveness trials, we recommend that both adherence and contamination are reported as an outcome measure because poor adherence can render an efficacious intervention ineffective. To date, effectiveness trials are rare, but would be of additional value for this field of research.
Consensus of researchers on outcome measures for exercise studies involving women with breast cancer receiving adjuvant treatment is needed in order to facilitate interpretation and comparison of results across various interventions. The long‐term follow‐up of exercise interventions also requires attention because some side effects of adjuvant cancer treatment are long term, such as fatigue or deconditioning, and the effects of exercise themselves might have a long‐term component. Besides health‐related outcome measures, adherence and contamination as well as potential harms should be assessed and reported systematically. Reporting standards for harms should help to inform practitioners and the public on potential harms of exercise interventions during adjuvant cancer treatment (Ioannidis 2004).
Regarding recruitment difficulties and thus the problem of small sample sizes, multisite trials are advisable.
The seven ongoing studies and the two studies awaiting assessment identified during our search also have small sample sizes and assess a wide variety of outcomes with different outcome measures. The majority include interventions with aerobic exercise of moderate intensity and compare exercise to usual care, apart from one study awaiting assessment in which yoga is the control intervention (Lotzke 2016). One study is notable for its rather long intervention period of 12 months, a large estimated number of enrolment of 600 participants, and a planned follow‐up of 10 years, including relapse of breast cancer disease, breast cancer‐specific mortality, and overall mortality as secondary outcomes (NCT02240836).
Once the effectiveness of exercise ‐ even in widely varying frequency and intensity ‐ for women with breast cancer during adjuvant therapy for different outcomes has been established, the next step is to assess which frequency, intensity, and type of exercise (aerobic, resistance, combination) is most effective for which outcome. There are ongoing and published studies comparing different dose regimens to each other, but which we could not include in our review because they had no usual care or non‐exercising control group. The number of studies comparing exercise to not exercising is still much higher, and there are even still feasibility studies underway or currently being published, although feasibility of exercise studies has certainly been proven. The comparison of different dosages of exercise for different outcomes might be a question for several reviews of their own.
What's new
| Date | Event | Description |
|---|---|---|
| 22 September 2016 | Amended | Amended an author's affiliation |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 30 March 2015 | New citation required and conclusions have changed | 24 new studies added, with 2174 new participants. We added full 'Risk of bias' tables. Conclusions changed |
| 30 March 2015 | New search has been performed | Performed searches for new studies on 30 March 2015 |
| 9 September 2008 | Amended | Converted to new review format |
Acknowledgements
We would like to thank Melina Wilson, who rendered assistance in her role as Managing Editor of the Cochrane Breast Cancer Group and the Editorial Base of the Cochrane Breast Cancer Group. Moreover, we would like to thank the referees who provided valuable feedback during the peer review process and the authors of primary trials for additional information about their trials. Anke Schulz rendered support in statistics for this update version of the review. Juyoung Byun from the Korean Branch of the Australasian Cochrane Centre assisted with one Korean language publication. We also thank Karl‐Ludwig Resch for obtaining several dissertations and for help with data extraction and 'Risk of bias' assessment of one trial.
Appendices
Appendix 1. CENTRAL
#1 MeSH descriptor: [Breast Neoplasms] explode all trees #2 breast near cancer #3 breast near neoplasm* #4 breast near carcinoma* #5 breast near tumour* #6 breast near tumor* #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MeSH descriptor: [Exercise] explode all trees #9 MeSH descriptor: [Exercise Movement Techniques] explode all trees #10 MeSH descriptor: [Physical Education and Training] explode all trees #11 MeSH descriptor: [Exercise Therapy] explode all trees #12 MeSH descriptor: [Physical Fitness] explode all trees #13 exercise or exercise movement technique or exercise therapy or physical education or physical fitness or weight or training or strengthening endurance #14 #8 or #9 or #10 or #11 or #12 or #13 #15 #7 and #14
Appendix 2. MEDLINE search strategy (via OvidSP)
| # ▲ | Searches |
| 1 | randomized controlled trial.pt. |
| 2 | controlled clinical trial.pt. |
| 3 | randomized.ab. |
| 4 | placebo.ab. |
| 5 | Clinical Trials as Topic/ |
| 6 | randomly.ab. |
| 7 | trial.ti. |
| 8 | (crossover or cross‐over).tw. |
| 9 | Pragmatic Clinical Trials as Topic/ |
| 10 | pragmatic clinical trial.pt. |
| 11 | or/1‐10 |
| 12 | exp Breast Neoplasms/ |
| 13 | (breast adj6 cancer$).mp. |
| 14 | (breast adj6 neoplasm$).mp. |
| 15 | (breast adj6 carcinoma$).mp. |
| 16 | (breast adj6 tumour$).mp. |
| 17 | (breast adj6 tumor$).mp. |
| 18 | 12 or 13 or 14 or 15 or 16 or 17 |
| 19 | exp Exercise/ |
| 20 | exp Exercise Movement Techniques/ |
| 21 | exp Exercise Therapy/ |
| 22 | exercise.mp. |
| 23 | exp "Physical Education and Training"/ |
| 24 | physical‐education‐and‐training.mp. |
| 25 | exp Physical Fitness/ |
| 26 | physical fitness.mp. |
| 27 | Physical Exertion/ |
| 28 | exertion.mp. |
| 29 | exp Sports/ |
| 30 | sport$.mp. |
| 31 | physical activity.mp. |
| 32 | physical activities.mp. |
| 33 | exp Walking/ |
| 34 | walking.ti,ab. |
| 35 | exp Jogging/ |
| 36 | jogging.ti,ab. |
| 37 | exp Swimming/ |
| 38 | swimming.ti,ab. |
| 39 | exp Bicycling/ |
| 40 | bicycling.ti,ab. |
| 41 | cycling.ti,ab. |
| 42 | weight.ti,ab. |
| 43 | training.ti,ab. |
| 44 | muscle.ti,ab. |
| 45 | strengthening.ti,ab. |
| 46 | endurance.ti,ab. |
| 47 | 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 |
| 48 | adjuvant therapy.mp. |
| 49 | chemotherapy.mp. or exp Drug Therapy/ |
| 50 | radiotherapy.mp. or exp Radiotherapy/ |
| 51 | hormonal therapy.mp. |
| 52 | 48 or 49 or 50 or 51 |
| 53 | 11 and 18 and 47 and 52 |
| 54 | Animals/ not Humans/ |
| 55 | 53 not 54 |
Appendix 3. EMBASE search strategy (via Embase.com)
'breast cancer'/exp OR 'breast cancer'
'breast carcinoma'/exp
'breast neoplasm'
'breast tumour'
'breast tumor'/exp
#1 OR #2 OR #3 OR #4 OR #5
'exercise'/exp
exercise*
'exercise movement techniques'/exp OR 'exercise movement techniques'
'physical education training'
'physical education'/exp OR 'physical education'
'physical training'/exp OR 'physical training'
'physical fitness'/exp OR 'physical fitness'
'exercise therapy'/exp OR 'exercise therapy'
'exertion'/exp OR exertion
'sports'/exp OR sports
'walking'/exp OR walking
'jogging'/exp OR jogging
'swimming'/exp OR swimming
'cycling'/exp OR cycling
'bicycling'/exp OR bicycling
'endurance'/exp OR endurance
'weight'/exp OR weight
'training'/exp OR training
'muscle'/exp OR muscle
strengthening
'endurance'/exp OR endurance
#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR#25 OR #26 OR #27
'adjuvant therapy'/exp OR 'adjuvant therapy'
'chemotherapy'/exp OR chemotherapy
'radiotherapy'/exp OR radiotherapy
hormonal AND ('therapy'/exp OR therapy)
#29 OR #30 OR #31 OR #32
random* OR factorial* OR crossover* OR cross NEXT/1 over* OR placebo* OR (doubl* AND blind*) OR (singl* AND blind*) OR assign*OR allocat* OR volunteer* OR 'crossover procedure'/exp OR 'double blind procedure'/exp OR 'randomized controlled trial'/exp OR'single blind procedure'/exp
#6 AND #28 AND #33 AND #34
#35 NOT ([animals]/lim NOT [humans]/lim)
#36 AND [embase]/lim
Appendix 4. WHO ICTRP search strategies
Basic Searches:
1. Exercise for women receiving adjuvant therapy for breast cancer
2. Breast cancer AND exercise
Advanced Searches:
1. Title: Exercise for women receiving adjuvant therapy for breast cancer
Recruitment Status: ALL
2. Condition: breast cancer
Intervention: exercise
Recruitment Status: ALL
3. Condition: breast cancer
Intervention: exercise% OR exercise therap% OR physical education
Recruitment Status: ALL
Appendix 5. ClinicalTrials.gov search strategies
Basic Searches:
1. Exercise for women receiving adjuvant therapy for breast cancer
2. Breast cancer AND exercise
Advanced Searches:
1. Title: Exercise for women receiving adjuvant therapy for breast cancer
Recruitment: All studies
Study Results: All studies
Study Type: All studies
Gender: All Studies
2. Condition: breast cancer
Intervention: exercise OR exercises OR exercise therapy OR exercise therapies OR exercise movement technique OR exercise movement techniques OR physical education OR physical training OR physical fitness OR physical activity OR physical activities
Recruitment: All studies
Study Results: All studies
Study Type: All studies
Gender: All Studies
Data and analyses
Comparison 1. Exercise versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Physical fitness | 20 | 1310 | Std. Mean Difference (IV, Random, 95% CI) | 0.42 [0.25, 0.59] |
| 2 Fatigue | 22 | 1698 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.41, ‐0.16] |
| 3 Cancer‐specific quality of life | 13 | 1012 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.00, 0.25] |
| 4 Health‐related quality of life | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Cancer site‐specific quality of life | 4 | 262 | Mean Difference (IV, Random, 95% CI) | 4.24 [‐1.81, 10.29] |
| 6 Depression | 6 | 674 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.30, 0.01] |
| 7 Cognitive function | 2 | 213 | Mean Difference (IV, Random, 95% CI) | ‐11.55 [‐22.06, ‐1.05] |
| 8 Strength | 13 | 912 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.04, 0.50] |
| 9 Subjective upper body function | 3 | 231 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐4.45, 3.41] |
| 10 Shoulder mobility | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Arm morbidity | 3 | 240 | Mean Difference (IV, Random, 95% CI) | 1.11 [‐4.07, 6.29] |
| 12 Anxiety | 3 | 331 | Mean Difference (IV, Random, 95% CI) | ‐1.45 [‐4.36, 1.46] |
| 13 Mood disturbances | 3 | 111 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.40, ‐0.60] |
| 14 Hospital Anxiety and Depression Scale | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 15 Self esteem | 4 | 323 | Mean Difference (IV, Random, 95% CI) | 1.69 [‐0.01, 3.39] |
| 16 Physical activity | 8 | 549 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [0.12, 0.47] |
| 17 Neuropathic pain | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 3.64 [‐1.32, 8.60] |
| 18 Neuropathy symptoms | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 19 Endocrine symptoms | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20 Gait and balance | 3 | 122 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.25, 0.46] |
| 21 Lymphoedema incidence | 4 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.45] |
Comparison 2. Exercise versus control follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Physical fitness | 6 | 612 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.06, 0.57] |
| 2 Fatigue | 8 | 814 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.35, ‐0.07] |
| 3 Cancer‐specific quality of life | 6 | 583 | Std. Mean Difference (IV, Random, 95% CI) | 0.18 [0.01, 0.35] |
| 4 Depression | 3 | 378 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.48, ‐0.06] |
| 5 Strength | 4 | 386 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.30, 0.30] |
| 6 Physical activity | 3 | 261 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.05, 0.61] |
| 7 Anxiety | 2 | 201 | Mean Difference (IV, Random, 95% CI) | ‐3.61 [‐7.24, 0.03] |
| 8 Self esteem | 2 | 201 | Mean Difference (IV, Random, 95% CI) | 1.20 [‐0.41, 2.81] |
| 9 Endocrine symptoms | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 10 Neuropathy symptoms | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Gait and balance | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Lymphoedema incidence | 2 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.37, 1.69] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Battaglini 2004.
| Methods | RCT, 2 groups Study start and stop dates: not reported Length of intervention: 15 weeks Length of follow‐up: 5 to 7 weeks after the intervention |
|
| Participants | 20 breast cancer patients due to receive adjuvant therapy | |
| Interventions | Intervention (n = 10): Aerobic and resistance training at 40% to 60% maximum exercise capacity and stretching; 2/week, up to 60 minutes per session Control (n = 10): usual care |
|
| Outcomes |
Outcomes were measured at baseline and postintervention and at 3 time points during treatment Adverse events: "No cases of injury or any cancer treatment complications impeded subjects in the exercise group from completing the exercise protocol two times a week." |
|
| Notes | Funding: Grants obtained through the University of Northern Colorado (Dissertation), University of Northern Colorado, Sponsored Programs and Academic Research Center (2007) Conflicts of interest: The authors declared there was no potential conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Drawing of numbers (1 to 20) by the participants. Participants who drew even numbers were placed into the experimental group, while participants who drew odd numbers were placed into the control group |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There was no description of missing outcome data or attrition from the trial |
| Selective reporting (reporting bias) | Low risk | Dissertation with all assessed outcomes available |
| Group similarity at baseline | Low risk | No significant differences for weight, age, body fat, fitness (cardiorespiratory and strength) |
| Adherence | Low risk | "The adherence rate among all the subjects was 100%." All study participants completed the study protocol. One participant missed 1 week of exercise for reasons unrelated to the study |
| Contamination | Unclear risk | Not reported |
Cadmus 2007.
| Methods | RCT, 2 groups Study start and stop dates: March 2004 to July 2006 Length of intervention: 6 months Length of follow‐up: to end of the intervention |
|
| Participants | 50 newly diagnosed breast cancer patients, aged 35 to 75, who had not yet begun or had only recently begun adjuvant treatment (completed fewer than 2 weeks of radiation or 2 cycles of chemotherapy) | |
| Interventions | Intervention (n = 25): home‐based exercise program, type of exercise up to the women’s choice, weekly telephone calls, information, heart monitor, activity logs, 60% to 80% of predicted maximal heart rate, 5 days per week, 30 minutes, 120 sessions Control (n = 25): usual care | |
| Outcomes |
Outcomes were measured at baseline and 6 months Adverse events: none reported |
|
| Notes | Funding: Lance Armstrong Foundation, American Cancer Society, Susan G. Komen, National Institutes of Health Conflict of interest: None reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer program randomly assigned each study participant with equal probability to the exercise group or the usual‐care group |
| Allocation concealment (selection bias) | Low risk | The randomisation code for each participant was obtained by the principal investigator (who was not involved in recruitment or data collection) only after baseline measures for that woman had been completed, and staff conducting clinic visits did not have access to the randomisations program |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 women (10%) had missing 6‐month data (3 exercisers and 2 usual‐care group participants). All analyses were conducted according to the intention‐to‐treat principle. Baseline QoL values were carried forward for the 5 IMPACT study participants who had missing 6‐month data. Not reported for other outcomes |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not published prospectively |
| Group similarity at baseline | Unclear risk | "No differences between exercise and usual care groups at baseline. Exception: exercisers were more likely to receive lumpectomy than usual care group participants (P < 0.05)." |
| Adherence | High risk | Participants performed 144 (SD = 75) minutes of activity per week throughout the 6 months (range: 0 to 253). 64% met the goal of 150 min per week |
| Contamination | High risk | Women didn't have to be sedentary at baseline. 36% of IMPACT study control group participants reported no sports/recreational physical activity at 6‐month follow‐up; the remaining 64% of controls reported between 35 and 378 min/week of activity, with a median of 181 minutes. Obese cohort (mean BMI > 30 kg/m2) |
Caldwell 2009.
| Methods | RCT, 2 groups Study start and stop dates: not reported Length of intervention: 12 weeks Length of follow‐up: 2 weeks after last chemotherapy Study was discontinued due to peripheral neuropathy |
|
| Participants | 25 breast cancer patients undergoing neoadjuvant or adjuvant chemotherapy treatment | |
| Interventions | Intervention (n = 13): home‐based, low‐intensity level strength training and functional endurance regimen (strength training combined with walking) Control (n = 12): usual care |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Adverse events: Detailed adverse event data were not collected for participants in this study. Outcomes were measured at baseline and 2 weeks after the last chemotherapy treatment |
|
| Notes | Funding: None reported Conflict of interest: None reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation program |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants All outcomes | High risk | Not reported |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 participants were not able to start the study owing to changes in treatment plan and were not included in any analysis.
> 30% drop‐out Treatment of missing data was not described |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | No significant differences |
| Adherence | High risk | Once peripheral neuropathy developed, these participants (12 of 13) adopted a sporadic pattern of "walking only", which lasted for approximately 4 weeks before coming to an abrupt end. Participants in this study were not able to recall the times they exercised during the telephone contacts. The investigator was not able to measure adherence, which was defined as the number of sessions attended by each participant |
| Contamination | Unclear risk | Participants did not have to be sedentary |
Campbell 2005.
| Methods | RCT, 2 groups Study start and stop dates: not reported Length of intervention: 12 weeks Length of follow‐up: to end of the intervention |
|
| Participants | 22 breast cancer patients, after surgery, receiving chemotherapy or radiotherapy | |
| Interventions | Intervention (n = 12): supervised group exercise: aerobic and resistance training (walking, cycling, low‐level aerobics, muscle‐strengthening exercises, circuits), behaviour change communication, 60% to 75% HRmax, 10 to 20 min per session exercise, plus warm‐up, cool‐down, relaxation, 2 sessions per week Control (n = 10): no intervention |
|
| Outcomes |
Outcomes were measured at baseline and 12 weeks. Adverse events: "There were no adverse reactions to taking part in the exercise intervention" |
|
| Notes | Funding: Greater Glasgow NHS Trust Conflicts of interest: None reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated numbers, stratification by adjuvant cancer treatment |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self administered questionnaires were returned to researcher in sealed envelopes. Comment: high risk because items were self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out:
All participants: 3/22 (13.7%) No imputation of missing data |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | Similarity for the most important prognostic indicators |
| Adherence | High risk | Adherence: 70% of all sessions |
| Contamination | Low risk | Control group more physically active at baseline than intervention group (421 min vs 330 min). Controls remained at baseline level, whereas intervention group increased level. The self reported levels of physical activity at baseline for both groups and at follow‐up for the control group were similar to those found in sedentary populations |
Cornette 2013.
| Methods | RCT, 2 groups Study start and stop dates: Recruitment between June 2011 and June 2012 Length of intervention: 27 weeks Length of follow‐up: 27 weeks |
|
| Participants | 44 outpatient breast cancer patients with HER2‐negative status randomised, scheduled for adjuvant or neoadjuvant chemotherapy with 6 cycles (3FEC100+3 taxanes), followed by radiotherapy | |
| Interventions | Intervention (n = 22): aerobic and resistance training, 9 supervised sessions of resistance training and 72 unsupervised home‐based sessions (resistance and aerobic training): bicycle or walking 20 to 40 minutes 2/week, resistance training with resistance bands 1/week. Control (n = 22): adjuvant treatment only |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Outcomes were measured at time point: before start of chemotherapy, after 27 weeks of treatment, and after 27 weeks of follow‐up Adverse events: no adverse events were reported |
|
| Notes | Funding: Sponsored by Limoges University Hospital. Supported by a grant from the Ligue Contre le Cancer (19‐87) and the ALAIR‐AVD. Conflicts of interest: Not reported. Study registration: NCT01322412 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Block randomisations with variable block size, 1:1, no stratification, further details not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants All outcomes | High risk | Open study |
| Blinding of personnel/care providers All outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Open study |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Cardiorespiratory fitness and strength: 30/42 participants (68%) took part in all fitness evaluations. QoL, fatigue, psychological distress, physical activity: 19/42 (45.2%) participants in the analysis at the end of the intervention. ITT analysis for cardiorespiratory fitness (VO peak, 6‐MWT) and strength with imputation for missing values. "Several methods were tested and last observation carried forward (LOCF) was used." Otherwise no imputation for missing data |
| Selective reporting (reporting bias) | Low risk | Trial prospectively registered |
| Group similarity at baseline | High risk | Significant baseline differences in cardiorespiratory fitness and BMI |
| Adherence | High risk | 9 of 14 participants took part in more than 70% of the exercise program |
| Contamination | Unclear risk | The control group was not asked to abstain from physical activity |
Courneya 2007 AET.
| Methods | RCT, 3 groups Study start and stop dates: 2003 and 2005 Length of intervention: Duration of the chemotherapy, median 17 weeks (9 to 24 weeks) Length of follow‐up: 6 months for patient‐reported outcomes, for objectively measured outcomes 3 to 4 weeks after chemotherapy |
|
| Participants | 242 breast cancer patients initiating adjuvant chemotherapy | |
| Interventions |
Intervention group 1 (n = 78): 'Courneya AET' aerobic ‐ endurance exercise: cycle ergometer, treadmill, elliptical Intervention group 2 (n = 82): 'Courneya RET' muscular endurance exercise: weight machines (set with 9 exercises) Control group (n = 82): Usual care; women were asked not to initiate an exercise programme |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Patient‐rated outcomes were assessed at baseline (1 to 2 weeks after starting chemotherapy), midpoint (middle of chemotherapy), after the intervention (3 to 4 weeks after chemotherapy), and at the 6‐month follow‐up. Objectively measured outcomes were assessed at baseline and after intervention. Adverse events: "exercise did not cause adverse events" |
|
| Notes | Study description for Courneya AET (aerobic exercise training) and Courneya RET (resistance exercise training) Funding: Canadian Breast Cancer Research Alliance; the Canada Research Chairs Program, Research Team Grant from the National Cancer Institute of Canada with funds from the Canadian Cancer Society (CCS) and the NCIC/CCS Sociobehavioral Cancer Research Network, New Investigator Award from the Heart and Stroke Foundation of Canada; a New Investigator Award from the Canadian Institutes of Health Research and a Health Scholar Award from the Alberta Heritage Foundation for Medical Research; Canada Graduate Scholarship from the Canadian Institutes of Health Research and an Incentive Award from the Alberta Heritage Foundation for Medical Research. Conflict of interest: Authors declared no potential conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated program, stratification by centre and chemotherapy protocol |
| Allocation concealment (selection bias) | Low risk | “The allocation sequence was...concealed from the project directors at each site who assigned participants to groups” |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out:
All participants: 7.9% (19/242) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | No significant differences |
| Adherence | High risk | Aerobic exercise group: 72% sessions; 95.6% met duration; 87.2% met intensity Resistance exercise group: 68.2% sessions; 96.8% completed all 9 exercises; 96.9% completed 2 sets each; 94.5% completed 8 to 12 repetitions |
| Contamination | Unclear risk | Women were asked not to initiate an exercise programme. Otherwise not reported |
Courneya 2007 RET.
| Methods | RCT, 3 groups Study start and stop dates: 2003 and 2005 Length of intervention: Duration of the chemotherapy, median 17 weeks (9 to 24 weeks) Length of follow‐up: 6 months for patient‐reported outcomes, for objectively measured outcomes 3 to 4 weeks after chemotherapy |
|
| Participants | 242 breast cancer patients initiating adjuvant chemotherapy | |
| Interventions | Intervention group 1 (n = 78): 'Courneya AET' aerobic ‐ endurance exercise: cycle ergometer, treadmill, elliptical Intervention group 2 (n = 82): 'Courneya RET' muscular endurance exercise: weight machines (set with 9 exercises) Control group (n = 82): Usual care; women were asked not to initiate an exercise programme |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Patient‐rated outcomes were assessed at baseline (1 to 2 weeks after starting chemotherapy), midpoint (middle of chemotherapy), after the intervention (3 to 4 weeks after chemotherapy), and at the 6‐month follow‐up. Objectively measured outcomes were assessed at baseline and after intervention. Adverse events: "exercise did not cause adverse events" |
|
| Notes | Study description for Courneya AET (aerobic exercise training) and Courneya RET (resistance exercise training) Funding: Canadian Breast Cancer Research Alliance; the Canada Research Chairs Program, Research Team Grant from the National Cancer Institute of Canada with funds from the Canadian Cancer Society (CCS) and the NCIC/CCS Sociobehavioral Cancer Research Network, New Investigator Award from the Heart and Stroke Foundation of Canada; a New Investigator Award from the Canadian Institutes of Health Research and a Health Scholar Award from the Alberta Heritage Foundation for Medical Research; Canada Graduate Scholarship from the Canadian Institutes of Health Research and an Incentive Award from the Alberta Heritage Foundation for Medical Research. Conflict of interest: Authors declared no potential conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated program, stratification by centre and chemotherapy protocol |
| Allocation concealment (selection bias) | Low risk | “The allocation sequence was...concealed from the project directors at each site who assigned participants to groups” |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out:
All participants: 7.9% (19/242) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | No significant differences |
| Adherence | High risk | Aerobic exercise group: 72% sessions; 95.6% met duration; 87.2% met intensity Resistance exercise group: 68.2% sessions; 96.8% completed all 9 exercises; 96.9% completed 2 sets each; 94.5% completed 8 to 12 repetitions |
| Contamination | Unclear risk | Women were asked not to initiate an exercise programme. Otherwise not reported |
Crowley 2003.
| Methods | RCT, 2 groups Study start and stop dates: not reported Length of intervention: 13 weeks Length of follow‐up: to the end of the intervention |
|
| Participants | 22 breast cancer patients, stage I, II; after surgery, receiving adjuvant chemotherapy (doxorubicin (Adriamycin), cyclophosphamide (Cytoxan)), radiation therapy excluded | |
| Interventions | Intervention (n = 13): Aerobic training (walking) and resistance training (tubing), self directed, 60% of HRmax, 20 to 60 min per session, 3 to 5 d/week. Resistance training: 12 to 15 repetitions, approximately 20 minutes, 1 to 2 sets, 2 to 3 d/week, 13 weeks. Control (n = 9): Usual care, the same scheduled contact with the nurse researcher as the intervention group during weeks 1, 4, 7, 10, and 13, activity log |
|
| Outcomes |
Outcomes were measured at:
Adverse events: lymphoedema in 1 participant |
|
| Notes | Funding: Not reported Conflicts of interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table was utilised prior to the initiation of the study to randomise participants to 1 of the 2 groups. Consecutive numbers on the table were used with numbers ending in an even integer assigned to the exercise group, and numbers ending in an odd integer assigned to the comparison group. Each number was placed in an envelope that was then sealed. The outside of the envelope was then numbered in consecutive order |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope. Comment: it was not mentioned if the envelope was opaque |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | Low risk | "The study participant’s group assignment was blinded to the exercise physiologists performing the fitness testing at the week 13 appointment. Study participants were requested to not disclose which study group they had been randomized to." |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were reported for 2 participants for strength assessment. "A second strength was the adherence by both groups in maintaining activity logs and completing all study measures." |
| Selective reporting (reporting bias) | Unclear risk | Detailed results only available for cardiorespiratory fitness |
| Group similarity at baseline | Low risk | "The groups were balanced in terms of demographic and disease characteristics." |
| Adherence | Unclear risk | The nurse researcher used the logs of both groups to assess adherence to the structured exercise program. Adherence defined as completion of 80% of the individualised targeted endurance and strength exercise, frequency, duration, and intensity. The intervention group walked a mean of 113 minutes per week, as compared to 53 minutes by the comparison group. "The intervention group also demonstrated commitment to following the exercise intervention across the study period." |
| Contamination | Unclear risk | The intervention group walked a mean of 113 minutes per week, as compared to 53 minutes by the comparison group. Thus the intervention group was considered to be performing at a moderate level of activity per week, while the comparison group had a low level of activity over the study period. Study participants were asked to commit to not initiating participation in a formal exercise program during the study period. Continuation of an ongoing exercise regimen was acceptable |
Dodd 2010.
| Methods | RCT, 3 groups Study start and stop dates: 1999 to 2006 Length of intervention: 1 year Length of follow‐up: end of intervention |
|
| Participants | 119 participants randomised, majority with breast cancer (n = 112), but people with ovarian and colorectal cancer also included, undergoing chemotherapy, Karnofsky score > = 60. Excluded if they were having concurrent radiation therapy, and if pain intensity score greater than 3 | |
| Interventions |
|
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Outcomes were measured at time point: baseline (T1: the week before the second chemotherapy treatment), at the end of cancer treatment (T2: 4 to 6 months after T1), and at the end of the study (T3: approximately 1 year after the start of T1). Adverse events: hip pain, sciatica (n = 16), arm discomfort (n = 4), knee discomfort (n = 10), ankle discomfort (n = 3), and foot discomfort (n = 8) |
|
| Notes | Funding: National Cancer Institute (CA83316), and the Clinical Translational Research Institute, Clinical Research Center (CTSI‐CRC) (Dodd 2010) Conflicts of interest: The authors declared no potential conflicts of interest in 2 related publications |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as randomised, but method not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | Low risk | Cardiopulmonary exercise testing was performed in the exercise physiology lab by laboratory staff blinded to the participant’s group assignment |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Not reported for other outcomes. Comment: probably not done or self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out:
ITT, no imputation |
| Selective reporting (reporting bias) | High risk | Cardiopulmonary fitness was tested but not reported as an outcome. It was unclear if cardiorespiratory fitness was a predefined outcome or only measured to individualise the exercise prescription. In 1 of 5 related publications, a quality of life questionnaire was mentioned (MQOLS‐CA), but no results were reported in any publication. Outcomes were not reported completely, and could not be extracted for use in a meta‐analysis |
| Group similarity at baseline | Low risk | "The three groups of patients did not differ significantly in any of the demographic, disease, or treatment characteristics on entry into the study" |
| Adherence | High risk | Group 1 (exercise during and after adjuvant treatment) reported an adherence rate of 73% at T2 and 75.7% at T3 |
| Contamination | High risk | 44% of group 2 (exercise after adjuvant treatment) reported meeting ACSM 1998 guidelines (aerobic activities 3x/week, for 20‐minute duration, and at a moderate intensity) at T1, by T2 group 2 had decreased to 27%. 34% of group 3 (no exercise) reported meeting minimum criteria at T1, this decreased to 31% at T2. |
Drouin 2002.
| Methods | RCT, 2 groups Study start and stop dates: not reported Length of intervention: 7 weeks Length of follow‐up: end of intervention |
|
| Participants | 23 breast cancer patients, stages 0 to III; after surgery, receiving radiotherapy, sedentary | |
| Interventions | Intervention (n = 13): aerobic training (walking), self directed, 50% to 70% HRmax, 20 to 45 min per session, 3 to 5/week Control (n = 10): stretching, 3 to 5/week |
|
| Outcomes |
Outcomes were measured within 1 week prior to and within 1 week following a 7‐week radiation regimen. Adverse events: shoulder tendonitis and decreases in strength due to overtraining in 1 participant |
|
| Notes | Funding: grants from the Elsa U. Pardee Foundation in Midland, MI, USA, and the Max and Victoria Dreyfus Foundation in White Plains, NY, USA Conflict of interest: None reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants All outcomes | High risk | Not reported |
| Blinding of personnel/care providers All outcomes | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out:
All participants: 2/23 (8.7%) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | Similarity for the most important prognostic indicators |
| Adherence | High risk | Adherence defined as 21 minimum sessions out of 35 possible sessions Adherence per group:
|
| Contamination | Unclear risk | Obese cohort (mean BMI > 30 kg/m2). Otherwise not reported |
Eakin 2012.
| Methods | RCT, 2 groups Study start and stop dates: data were collected from April 2007 to April 2009 Length of intervention: 8 months Length of follow‐up: 8 weeks after end of the intervention |
|
| Participants | 142 non‐urban‐dwelling breast cancer patients, 111 underwent adjuvant therapy (chemotherapy, radiotherapy, or a combination) during the study intervention (unpublished data for these participants used in the analyses of this review) | |
| Interventions | Intervention (n = 58): home‐based, telephone‐delivered mixed (aerobic and resistance training) exercise Control (n = 53): usual care; did not receive any study exercise intervention‐related material until study completion |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Outcomes measures at 6 and 12 months Adverse events: muscle soreness in 2 participants and musculoskeletal injury in 1 participant |
|
| Notes | Funding: The National Breast Cancer Foundation (NBCF, Australia) and a Queensland Health Core Infrastructure grant funded the trial. EGE is supported by a National Health and Medical Research Council Senior Research Fellowship. SCH is supported by an Early Career Research Fellowship from the NBCF. Conflict of interest: The authors have no conflict of interest to disclose. Registered at: ACTRN12609000809235 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, unblocked sequence of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out all participants:
Retention was 97% at 6 months and 96% at 12 months. No imputation of missing data |
| Selective reporting (reporting bias) | Low risk | Registered prospectively |
| Group similarity at baseline | High risk | For 111 participants during adjuvant treatment: more with chemotherapy in the treatment group: 47/58 (81%) vs 36/53 (68%) For all participants: "There was no evidence of failure of randomization (i.e. all baseline group differences were P > 0.05), however there were some notable group differences (>=10%) in terms of income (<$52,000 per annum), receipt of radiotherapy at baseline, overall receipt of chemotherapy, surgery type and lymph node status." |
| Adherence | High risk | 41.2% at 6 months' postsurgery and 52.2% at 12 months' postsurgery met the criteria for aerobic activity. 45.6% at 6 months' postsurgery and 40.3% at 12 months' postsurgery met the criteria for strength. |
| Contamination | High risk | 30% to 40% of control group were active during the study period. They started out as being as active |
Gokal 2013.
| Methods | RCT, 2 groups Study start and stop dates: not reported Length of intervention: 12 weeks Length of follow‐up: end of intervention |
|
| Participants | 50 sedentary (< 30 minutes of moderate‐intensity exercise 5 times a week) breast cancer patients (stage I to III) during adjuvant or neoadjuvant chemotherapy. Recruited from outpatient clinics | |
| Interventions | Intervention (n = 25): home‐based, moderate‐intensity walking intervention (defined as walking at brisk pace) after 2 cycles of chemotherapy. 30 minutes of moderate‐intensity walking 5 times a week, encouraged to gradually increase walking duration from 10‐ to 30‐minute bouts through the course of the intervention. Control group (n = 25): usual care |
|
| Outcomes | Primary:
Secondary:
Outcomes measured at: midway through chemotherapy (pre‐intervention) and after the completion of chemotherapy (postintervention). All participants also completed outcome measures prior to receiving chemotherapy |
|
| Notes | Funding: Loughborough University as part of a PhD project.
Conflict of interest: There were no conflicts of interest to report. Study registration: ISRCTN50709297 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Block randomisation using four blocks was used to allocate patients into one of two groups by the researcher. Within each group of four patients, two were allocated to the intervention group and two to the control group; the allocation of groups within each block was random." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants All outcomes | High risk | There is no masking of participants or the research team |
| Blinding of personnel/care providers All outcomes | High risk | There is no masking of participants or the research team |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | There is no masking of participants or the research team |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants in the exercise group (20%) discontinued the intervention (4 due to hospitalisation and 1 due to medical difficulties), but completed all follow‐up measures and were included in the analysis. ITT analysis, no imputation described |
| Selective reporting (reporting bias) | High risk | Measures of cognitive function (mentioned in the design paper and study registration) were not reported in the paper |
| Group similarity at baseline | Low risk | There were no significant differences between groups in sociodemographic or treatment‐related variables. Using ITT, there were no significant between‐group differences in baseline measures of anxiety, depression, fatigue, self esteem, mood, or subjective ratings of physical activity. There was a small difference between groups in 2 subscales of mood: vigour and confusion |
| Adherence | Low risk | 20 (80%) out of the 25 participants randomised to the physical activity group adhered to the intervention and completed walking diaries. 5 participants discontinued participation within the first few weeks of the 12‐week intervention |
| Contamination | Low risk | Sedentary patients at baseline. Self report of perceived physical activity for control group: 23/25 moderately inactive to inactive |
Haines 2010.
| Methods | RCT, 2 groups Study start and stop dates: recruitment between May 2006 and September 2007 Length of intervention: 12 months Length of follow‐up: end of intervention |
|
| Participants | 89 breast cancer patients undergoing adjuvant therapy: chemotherapy, radiation, or a combination of both (more than 90% radiation) | |
| Interventions | Intervention group (n = 46): home‐based strength, balance, shoulder mobility, and cardiovascular endurance program. 36 minutes, of which 20 minutes were walking. Frequency not reported. Control group (n = 43): Static stretching, supine relaxation program following the Feldenkrais method |
|
| Outcomes | Primary outcome measure:
Secondary outcome measures:
Outcomes were measured at baseline, 3, 6, and 12 months Adverse events: 9 participants with musculoskeletal pain, 3 of which reported pain whilst performing exercises as a part of the intervention program and 1 as a part of the control program. 8 participants with 1 fall each, 1 of which was the result of an intervention group participant tripping on a tree stump whilst undertaking the walking program |
|
| Notes | Funding: Project grant from the Princess Alexandra Hospital Cancer Collaborative Group, National Health and Medical Research Council Career Development Award (606732) Conflict of interest: No conflicts of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to intervention or control groups using a computer‐generated randomisation sequence |
| Allocation concealment (selection bias) | Low risk | The randomisation sequence was entered into numbered, opaque, sealed envelopes by a study investigator and was held secure in an administration office separate from that of the investigators. Envelopes were only opened after completion of the initial assessment after which intervention or control programs were provided to participants according to the allocation sequence |
| Blinding of participants All outcomes | High risk | Sham intervention control group provided with what looked like an exercise program with an equivalent amount of supporting material. The video material was of similar content to that in the intervention program (though the actual exercises described differed). Comment: Participants would still have been aware if they were in the exercise group or the stretching group |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | Low risk | "blinded outcome assessment" |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intervention group: more than 30% of outcome data was missing at 6 months Control group: more than 30% of outcome data was missing at 6 months Handling of dropouts and missing data not reported |
| Selective reporting (reporting bias) | Low risk | The trial was registered prospectively with the Australia New Zealand Clinical Trials Registry (ACTRN12606000047594) |
| Group similarity at baseline | High risk | "Control group participants also appeared to be healthier at baseline." |
| Adherence | High risk | Participants were to document adherence in log books on a weekly basis and were asked about adherence in the last 2 weeks of 12 months. At the 12‐month review, 11 of 37 intervention group participants interviewed reported completing the strength/balance/shoulder mobility component of the program at least once in the past 2 weeks, whilst 7 reported completing it at least 3 times. The endurance component was completed at least once by 12, and at least 3 times by 7 |
| Contamination | High risk | In addition to the program provided, 25 out of 37 intervention group participants and 21 out of 36 control group participants had commenced other forms of exercise (e.g. walking, dancing, gymnasium, and aerobics) |
Hayes 2013 FtF.
| Methods | RCT, 3 groups Study start and stop dates: recruitment between October 2006 and June 2008 Length of intervention: 8 months Length of follow‐up: 8 weeks after end of the intervention |
|
| Participants | 194 breast cancer patients, of which 142 underwent adjuvant therapy concurrently with the exercise intervention (unpublished data for these patients used in the analyses of this review) | |
| Interventions | Intervention group 1: 'Hayes 2013 Tel' Telephone (n = 50) ‐ incorporating both aerobic and strength‐based exercises Intervention group 2: 'Hayes 2013FtF' Face to face (n = 51) ‐ incorporating both aerobic and strength‐based exercises Control group (n = 41): usual care |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Outcomes measured at: 6 months, 12 months |
|
| Notes | Funding: This research project was supported by the National Breast Cancer Foundation. The research positions of SH and EE are supported via an NBCF Early Career Research Fellowship and an NHMRC Senior Research Fellowship, respectively. Conflict of interest: Authors declare that they have no conflict of interest. Registered at ACTRN: 012606000233527 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Individually computer‐generated non‐blocked |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | Low risk | "assessors blinded to group allocation" |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | All outcomes except outcomes with clinical assessment: self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out: all participants
All participants: 14/194 (7.2%) Comment: For the 142 participants undergoing adjuvant therapy: More than 20% drop‐out for 3‐min step test (cardiorespiratory fitness) High risk for cardiorespiratory fitness ITT analysis, no imputation of data |
| Selective reporting (reporting bias) | Low risk | Registered prospectively |
| Group similarity at baseline | High risk | Slight imbalance in numbers, place of treatment (public vs private hospital), and rates of mastectomy between groups following randomisation. Rate of mastectomies higher in telephone group. This group has the biggest difference in QoL |
| Adherence | High risk | 25% did not meet the intervention goal at mid‐ or postintervention and did not increase their total physical activity by 30+ min (a priori deemed clinically relevant) between baseline and mid‐ or postintervention |
| Contamination | High risk | The Active Australia Survey showed that the usual‐care group was more active (more minutes per week) than the FtF group and as active as the Tel group at 6 months. At 12 months, the usual‐care group was more active than the FtF group and less active than the Tel group |
Hayes 2013 Tel.
| Methods | RCT, 3 groups Study start and stop dates: recruitment between October 2006 and June 2008 Length of intervention: 8 months Length of follow‐up: 8 weeks after end of the intervention |
|
| Participants | 194 breast cancer patients, of which 142 underwent adjuvant therapy concurrently with the exercise intervention (unpublished data for these patients used in the analyses of this review) | |
| Interventions |
Intervention group 1: 'Hayes 2013Tel' Telephone (n = 50) ‐ incorporating both aerobic and strength‐based exercises Intervention group 2: 'Hayes 2013 FtF' Face to face (n = 51) ‐ incorporating both aerobic and strength‐based exercises Control group (n = 41): usual care |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Outcomes measured at: 6 months, 12 months |
|
| Notes | Funding: This research project was supported by the National Breast Cancer Foundation. The research positions of SH and EE are supported via an NBCF Early Career Research Fellowship and an NHMRC Senior Research Fellowship, respectively. Conflict of interest: Authors declare that they have no conflict of interest. Registered at ACTRN: 012606000233527 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Individually computer‐generated non‐blocked |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | Low risk | "assessors blinded to group allocation" |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | All outcomes except outcomes with clinical assessment: self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out: all participants
All participants: 14/194 (7.2%) Comment: For the 142 participants undergoing adjuvant therapy: More than 20% drop‐out for 3‐min step test (cardiorespiratory fitness) High risk for cardiorespiratory fitness ITT analysis, no imputation of data |
| Selective reporting (reporting bias) | Low risk | Registered prospectively |
| Group similarity at baseline | High risk | Slight imbalance in numbers, place of treatment (public vs private hospital), and rates of mastectomy between groups following randomisation. Rate of mastectomies higher in telephone group. This group has the biggest difference in QoL |
| Adherence | High risk | 25% did not meet the intervention goal at mid‐ or postintervention and did not increase their total physical activity by 30+ min (a priori deemed clinically relevant) between baseline and mid‐ or postintervention |
| Contamination | High risk | The Active Australia Survey showed that the usual‐care group was more active (more minutes per week) than the FtF group and as active as the Tel group at 6 months. At 12 months, the usual‐care group was more active than the FtF group and less active than the Tel group |
Hornsby 2014.
| Methods | RCT, 2 groups Study start and stop dates: recruitment between March 2007 and January 2010 Length of intervention: 12 weeks Length of follow‐up: end of intervention |
|
| Participants | 20 breast cancer patients, stage IIB to IIIC operable breast cancer, neoadjuvant chemotherapy consisted of 4 cycles of doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2) every 3 weeks (i.e. 12 weeks in duration) Eligible if: Karnofsky performance status > 70 |
|
| Interventions | Intervention (n = 10): Aerobic training consisted of 3 one‐on‐one supervised cycle ergometry sessions per week on non‐consecutive days for 12 weeks. Control (n = 10): Neoadjuvant therapy only, participants were instructed to maintain their usual exercise levels throughout the duration of the study |
|
| Outcomes | Safety outcomes:
Efficacy outcomes:
Outcomes were measured at: CPET, echocardiogram, and self administered questionnaire were conducted at baseline and postintervention (12 weeks), whereas treatment‐related events were serially assessed across the study (i.e. baseline, 3, 6, 9, and 12 weeks). Exercise‐related events were monitored during CPET procedures and aerobic training sessions. Adverse event: unexplained leg pain in 1 participant |
|
| Notes | Funding: United States Department of Defense Breast Cancer Research Program of the Office of the Congressionally Directed Medical Research Programs – Ideas Award and funds from George and Susan Beischer (1 author). 1 author is supported by research grants from the National Cancer Institute (CA143254, CA142566, CA138634, CA133895, CA164751). Conflict of interest: The authors report no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated program (n = 10/group) |
| Allocation concealment (selection bias) | Unclear risk | The allocation sequence was concealed from the study co‐ordinator who assigned participants to groups |
| Blinding of participants All outcomes | High risk | It was not possible to blind participants or exercise staff to group assignment |
| Blinding of personnel/care providers All outcomes | High risk | It was not possible to blind participants or exercise staff to group assignment |
| Blinding of outcome assessment (detection bias) Fitness outcomes | Low risk | Study exercise physiologists conducting the baseline and postintervention (12 weeks) assessments were blinded to group assignment |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19/20 (95%) completed all study procedures. ITT, handling of 1 dropout not described |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | "The groups were balanced on all study outcomes at baseline." |
| Adherence | High risk | Overall attendance to planned exercise sessions was 82% (296 attended/360 prescribed; range 0 to 100%). Overall adherence to the planned exercise prescription was 66% (194 adhered sessions/296 attended). Adherence was calculated as the number of exercise sessions successfully completed (i.e. participant completed the exercise session at the planned duration and intensity) divided by the number of planned sessions attended |
| Contamination | Unclear risk | "There were no significant differences between groups for self‐reported exercise behavior." Otherwise not reported |
Husebo 2014.
| Methods | RCT, 2 groups Study start and stop dates: 2010 to 2012 Length of intervention: 17 weeks on average Length of follow‐up: 6 months |
|
| Participants | 67 breast cancer patients, stage I to III, surgically treated (mastectomy or lumpectomy), and allocated to adjuvant chemotherapy according to the national treatment guidelines of the Norwegian Breast Cancer Group | |
| Interventions | Intervention: Scheduled home‐based exercise intervention (n = 33), combined strength (resistance bands exercises 3 times a week) and aerobic (30 minutes of brisk walking daily) training Control group (n = 34): were advised to remain on their regular physical activity |
|
| Outcomes |
The study sample completed questionnaires and physical tests after surgery prior to chemotherapy (baseline), 18 to 24 weeks after baseline, and at the end of chemotherapy (Post1), and approximately 6 months after completing the chemotherapy regimen (Post2). Adverse events: 1 participant with knee discomfort and 1 participant with a syncope related to a secondary chronic condition |
|
| Notes | Funding: None reported. No conflict of interest declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The random assignment of subjects to the intervention group or to the control group was carried out by the use of concealed envelopes, drawn by the research assistant prior to the first data collection." |
| Allocation concealment (selection bias) | Unclear risk | "concealed envelopes" Comment: it was not mentioned if they were opaque |
| Blinding of participants All outcomes | High risk | Not reported |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Post1: immediately after chemo; Post2: 6 months' follow‐up 60 of 67 (89.6%) participants completed the data collection at time point 1. Comment: low risk. 52 of 67 (77.6%) participants completed the data collection at time point 2. Comment: high risk. ITT analysis for fatigue data. Per‐protocol analysis for physical activity (IPAQ) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | No significant differences in baseline values |
| Adherence | High risk | 17% adhered to the walking prescription of minimum 210 minutes/week of MVPA. 15% of the participants in the intervention group achieved the prescribed number of strength training (3/week) sessions. 58% met the general recommendations of 150 minutes/week of MVPA, and participants carried out approximately 2 sessions of resistance band exercises per week |
| Contamination | High risk | The control group had a mean exercise volume of 144 (SD 84) MVPA minutes per week, and 39% performed 150 minutes/week of MVPA or more. Data on exercise volume indicates that 48% of participants in both groups exercised according to the general recommended physical activity level or more. "there was a tendency of a significantly larger mean exercise volume in the intervention group compared to the control group (P = 0.051)" |
Ingram 2010.
| Methods | RCT, 2 groups, parallel‐group design Study start and stop dates: not reported Length of intervention: 24 weeks Length of follow‐up: study was closed early |
|
| Participants | 13 breast cancer patients due to commence adjuvant chemotherapy | |
| Interventions | Intervention (n = 8): home‐based combined aerobic and resistance exercise program, 30 to 45 min of aerobic exercise, at least 4 times per week. 8 resistance exercises, which included the arms, legs, and trunk, 3 times per week with enough resistance so that she tired after 6 to 12 repetitions. Control group (n = 5): usual care |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | No outcomes reported as study was closed early. Funding: Canadian Breast Cancer Research Alliance Developmental and Explanatory Grant #16542 Conflict of interest: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Not reported or self reported items Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study closed early |
| Selective reporting (reporting bias) | Unclear risk | No results reported because study closed early |
| Group similarity at baseline | Unclear risk | "No clinically meaningful or statistically significant differences between groups." |
| Adherence | High risk | Only 38% met all exercise targets |
| Contamination | High risk | "The women in the control group were unexpectedly quite active." Were asked not to begin a new exercise program |
MacVicar 1989.
| Methods | RCT, 3 groups, stratified by functional capacity Study start and stop dates: not reported Length of intervention: 10 to 12 weeks Length of follow‐up: end of intervention |
|
| Participants | 62 breast cancer patients, stage II; after surgery, receiving chemotherapy, entered the study; 45 patients were analysed for cardiorespiratory fitness; 24 patients (without placebo group and further patients excluded) were analysed for weight change and body composition |
|
| Interventions | Intervention (n = 18): aerobic training (cycling, interval training), 60% to 85% HRmax, 20 to 30 min per session, 3/week Control group 1 (n = 11): flexibility and stretching exercises ("placebo group") Control group 2 (n = 16): no intervention |
|
| Outcomes |
Outcomes measured at baseline and end of intervention |
|
| Notes | Funding: National Institutes of Health Grants RO1 NR 01078, National Center for Nursing Research and P 3OCA 16058 14, National Cancer Institute Conflict of interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated to be randomised, but method not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants All outcomes | High risk | Placebo group analysed for functional capacity did stretching exercises. Comment: participants would have been aware if they were in the exercise group or the stretching group |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | Unclear risk | No other relevant outcomes |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out rate for all participants: 17/62 (27.4%) 45 of 62 participants were analysed for functional capacity. Per‐protocol analysis (n = 45) of n = 62, who entered the study |
| Selective reporting (reporting bias) | High risk | It is unclear why data for only 2 groups was analysed for weight change and body composition |
| Group similarity at baseline | High risk | Educational status differed. Data for 17 women not shown for baseline |
| Adherence | Low risk | Adherence complete (missed sessions repeated) |
| Contamination | Unclear risk | "No subject participated in any other exercise or rehabilitation program during the 10 week data collection period." Comment: amount of physical activity outside of programs unclear |
Mock 2004.
| Methods | RCT, 2 groups Study start and stop dates: Potential participants were identified between 1998 and 2001 Length of intervention: 6 weeks to 6 months, depending on the duration of the adjuvant therapy Length of follow‐up: end of intervention |
|
| Participants | 119 breast cancer patients, stages 0 to III, after surgery, receiving chemotherapy or radiotherapy, sedentary | |
| Interventions | Intervention (n = 60): aerobic training (walking), self directed, 50% to 70% HRmax, 15 min per session, increased to 30 min as training progressed, 5 to 6/x week. Radiotherapy: 6 weeks exercise; chemotherapy: 3 to 6 months exercise. Control (n = 59): usual care |
|
| Outcomes |
Outcomes measured at: baseline and end of the adjuvant therapy/intervention |
|
| Notes | Funding: FIRE (Fatigue Initiative in Research and Education) multi‐institutional award from the Oncology Nursing Society Foundation Conflict of interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered, sealed, opaque envelopes, opened after baseline testing |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out:
All participants 11/119 (9.2%) Reported both ITT and per‐protocol analysis |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | No significant differences |
| Adherence | High risk | Not adherent: 15/54 (28%). 72% were adherent in the sense of the studies' definition (85% of minimum prescription) |
| Contamination | High risk | Contamination: 21/54 (39%) of the control group were exercising |
Moros 2010.
| Methods | RCT, 2 groups Study start and stop dates: not reported Length of intervention: 18 to 22 weeks Length of follow‐up: 10 to 15 days after end of intervention |
|
| Participants | 22 breast cancer patients, chemotherapy, not exercising regularly | |
| Interventions | Intervention group (n = 11): “dynamic aerobic exercise” adapted individually, 60‐minute sessions at 60% to 70% of maximum heart rate, 3/week Control group (n = 11): usual care |
|
| Outcomes |
Outcomes measured at time point: 10 to 15 days after end of intervention |
|
| Notes | Funding: not reported Conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised, but method not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out:
|
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | High risk | There were more obese women in the control group |
| Adherence | Unclear risk | Not described |
| Contamination | Unclear risk | Not described |
Mutrie 2007.
| Methods | RCT; 2 groups, stratification for hospital and treatment Study start and stop dates: recruitment from January 2004 to January 2005 Length of intervention: 12 weeks Length of follow‐up: 6 months |
|
| Participants | 203 breast cancer patients during treatment, chemo‐ or radiotherapy or both | |
| Interventions | Intervention (n = 101): supervised 12‐week group exercise 2 times/week, 45 minutes/session at moderate intensity (aerobic and strength). Participants encouraged to exercise 1x/week at home Control (n = 102): usual care |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Outcomes measured at time point: baseline, 12 weeks (intervention: 82, control: 92), and 6‐month follow‐up (intervention: 82, control: 95), 18 months and 5 years after the intervention |
|
| Notes | Funding: Cancer Research UK. One author was funded by the UK Medical Research Council. Conflict of interest: None declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised permuted blocks of length 4 and 6 |
| Allocation concealment (selection bias) | Low risk | “Randomisation was done by telephone to an interactive voice response system” |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | Low risk | “We took steps to blind the evaluation of outcomes by having questionnaire responses in sealed envelopes and ensuring that outcome measures were taken by researchers who were not involved in exercise classes” |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out: Intervention group:
Control group:
Comment: Differing rate between groups. Long term (18 months and 5 years >): 30% attrition ITT, no imputation of data |
| Selective reporting (reporting bias) | Low risk | Study protocol available |
| Group similarity at baseline | Low risk | "No obvious imbalances existed between study groups." Long term: Differences in baseline demographics between participants that did and did not return for follow‐up |
| Adherence | High risk | Participation in classes:
|
| Contamination | Unclear risk | Not reported |
Perna 2010.
| Methods | RCT, 2 groups Study start and stop dates: recruitment between April 2001 and July 2005 Length of intervention: 3 months Length of follow‐up: end of the intervention |
|
| Participants | 51 breast cancer patients, sedentary lifestyle (i.e. exercise less than 3/week for greater than 30 min/session in last 6 months); numbers in each group not reported. Many (44.1%) women received both radiation and chemotherapy, 26.5% received radiation only, 8.8% received chemotherapy only, and 20.6% received no adjuvant therapy | |
| Interventions | Intervention: individualised walking and resistance training program, 2 phases with a hospital‐based portion followed by a transition to home‐based exercise. Two 30‐min exercise adherence counselling sessions during the hospital‐based phase. Hospital based: 3 times per week for 4 weeks, aerobic: treadmill walking at 50% to 70% of GXT‐derived maximal heart rate (MHR). In subsequent weeks, duration was gradually increased by 5 min for a maximum of 40 min and a minimum of 30 min; intensity was increased to be within 70% to 85% of MHR according to participant comfort. Home based: instructed to walk 3 times per week, encouraged to walk every day for 30 minutes or more. Control: information control, 45 min session and informational brochure |
|
| Outcomes |
Outcomes measured at baseline and 3 months |
|
| Notes | Funding: National Cancer Institute (CAR0178801) and National Institutes of Health, General Clinical Research Grant (M01RR00533) Conflict of interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number sequence table |
| Allocation concealment (selection bias) | Low risk | Participant assignment to groups at enrolment was concealed from the project director |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | Low risk | Physicians monitoring graded exercise tests were blinded to participant group assignment. Similarly, a physical therapist or an exercise physiologist, blinded to participant assignment, performed strength assessments. Comment: No fitness outcomes reported in this paper |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers randomised to each arm are as well as completion rates are unclear. "we used regression modeling to impute missing values to conduct our analyses." Amount of missing data was not reported |
| Selective reporting (reporting bias) | Unclear risk | Further outcomes to be reported in another paper |
| Group similarity at baseline | Low risk | "found no significant differences with respect to demographic, cancer stage, treatment, and exercise characteristics." |
| Adherence | Low risk | Completed an average of 83% of their scheduled hospital‐based exercise sessions (M = 9.9, SD = 3.3 sessions); 76.9% completed all 12 sessions |
| Contamination | Unclear risk | Contamination not reported/GLTEQ scores increased from baseline by 32.7% in the control group |
Rao 2012.
| Methods | RCT, 2 groups Study start and stop dates: all participants were diagnosed and treated between March 2009 and April 2011 Length of intervention: 4 to 6 months (duration of chemotherapy) Length of follow‐up: a median follow‐up of 21.6 months |
|
| Participants | Women undergoing neoadjuvant chemotherapy for locally advanced, non‐metastatic breast cancer. Women had to have oestrogen receptor‐positive breast cancer, a BMI greater than 25, and a Karnofsky score > 80% |
|
| Interventions | Intervention (n = 5): home‐based exercise program, supervised, one on one, 3 times per week. Aerobic exercises and light weight lifting. Control (n = 5): usual care |
|
| Outcomes |
Outcomes measured before and after neoadjuvant chemotherapy |
|
| Notes | ClinicalTrials.gov (NCT01411787) Funding: Grant given by the Commercial Real Estate Women of Dallas Conflict of interest: None declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomization will occur by drawing cards entitled 'exercise' or 'control' from an envelope and then assigning the patient to this group." (ClinicalTrials.gov) |
| Allocation concealment (selection bias) | Unclear risk | An unlabeled envelope was opened by the research co‐ordinator to place the participant in either the control or boot camp arm of the study |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 10 women were randomised and completed all study parameters and were included in the analysis. All women were analysed in their respective group |
| Selective reporting (reporting bias) | High risk | "Improving fitness levels" not reported in results paper |
| Group similarity at baseline | Low risk | There were no statistically significant differences between groups with regard to tumour size, age, BMI, tumour grade, C‐peptide levels, or initial Ki‐67 |
| Adherence | Low risk | All 5 women in the exercise group completed > = 80% of the advised exercise sessions |
| Contamination | Unclear risk | Women were allowed to engage in their own exercise regimens and diet modifications. (ClinicalTrials.gov) BMI > 25, therefore "unlikely to have previously exercised" Comment: amount of physical activity not reported |
Reis 2013.
| Methods | RCT, 2 groups Study start and stop dates: November 2008 to January 2010 Length of intervention: 12 weeks Length of follow‐up: end of intervention |
|
| Participants | 41 breast cancer patients randomised, stage I to III, starting adjuvant radiotherapy. 26 participants (13 in each group) additionally received chemotherapy, 19 (10 in the exercise group and 9 in the control group) received hormone therapy, the latter "typically after chemotherapy and radiation therapy" | |
| Interventions | Intervention group (n = 22 randomised): Nia exercise ("cardiovascular and whole‐body conditioning program") 20 to 60 minutes 3 x per week for 12 weeks, and 3 meetings with principal investigator Control group (n = 19 randomised): usual care and 3 meetings with principal investigator, instructed to continue normal activities |
|
| Outcomes |
Outcomes were measured at start of radiation therapy, the completion of radiation therapy, and 6 weeks after completion of radiation therapy. Some participants received more than 6 weeks of radiotherapy |
|
| Notes | Funding: Not reported. Conflict of interest: "No financial relationships to disclose." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as "randomized", method not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop‐out (did not complete the 12‐week assessment):
Outcome data not reported for non‐adherent women in the exercise group. Per‐protocol analysis: The statistical analyses for fatigue, QoL, aerobic capacity, and shoulder flexibility compared the 12 women who practiced Nia to the 17 women randomised to the control group for whom data were collected at baseline, 6 weeks, and 12 weeks |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published, study not registered prospectively |
| Group similarity at baseline | High risk | "The two groups did not differ statistically in their demographics, although clinical differences appear to exist in age and employment, with the Nia group aged, on average, five years younger and more likely to be working full time than the control group." |
| Adherence | High risk | Assessed by reviewing participant logs. Logs were not uniformly maintained. Only 12 of 22 participants in the Nia group were adherent |
| Contamination | High risk | "66% of participants (n = 27) reported engaging in aerobic activity for at least three 20‐minute sessions per week prior to the cancer diagnosis. About 74% of those women (n = 20) continued to exercise during radiation therapy. No significant difference existed in the exercise history of the Nia group compared to the control group." Women in the control group reported engaging in aerobic exercise 0 to 41 times, or an average of almost 3 days per week (34 days in 6 weeks average) |
Schmidt 2014.
| Methods | RCT, 2 groups Study start and stop dates: April 2010 and August 2013 Length of intervention: 12 weeks Length of follow‐up: "Follow‐up data were also collected but not considered in the primary analyses" |
|
| Participants | 101 randomised patients with breast cancer under adjuvant chemotherapy | |
| Interventions | Intervention (n = 52): 12‐week supervised machine‐based progressive resistance training program, 60 minutes 2x/week, 3 sets, 8 to 12 repetitions at 60% to 80% of 1 repetition maximum (1‐RM) Control group (n = 49): supervised group‐based progressive muscle relaxation training according to Jacobson, 60 minutes 2x/week |
|
| Outcomes | Primary endpoint:
Secondary endpoints:
Outcomes measured during the first or second chemotherapy cycle pre‐intervention (baseline) and postintervention (week 13) |
|
| Notes | BEATE study, ClinicalTrials.gov registration: NCT01106820 Funding: German Cancer Research Center (DKFZ), Division of Preventive Oncology. Foundations: “Stiftung Leben mit Krebs” and “Manfred‐Lautenschlaeger‐Stiftung”. Conflict of interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomly allocated 1:1": predetermined lists with random block size, stratified by age and baseline physical fatigue level |
| Allocation concealment (selection bias) | Low risk | Allocation was performed by a biostatistician uninvolved in recruitment, based on predetermined lists with random block size, stratified by age and baseline physical fatigue level. Other study personnel did not have access to the randomisation lists |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Pre‐ and postintervention assessment of the primary endpoint was available in a total of 95 of 101 (94%) participants, 49 in exercise group and 46 in relaxation control group. "intent‐to‐treat‐basis". "As very few fatigue values were missing (3%), we performed complete‐case analyses" |
| Selective reporting (reporting bias) | High risk | Registered at ClinicalTrials.gov (NCT01106820), study protocol published, not all outcomes reported in this publication |
| Group similarity at baseline | High risk | Baseline characteristics, fatigue, and QoL were similarly distributed between both intervention groups, except for depression, which was significantly more common in the relaxation control group than in the exercise group. 2 in control group with metastasised cancer |
| Adherence | High risk | Median attendance was similar in both groups, with 17 out of 24 scheduled sessions attended (71%; interquartile range 11 to 22 in the exercise group and 11 to 23 in the relaxation control group) |
| Contamination | Unclear risk | Patients already participating in systematic intensive resistance or aerobic training (at least 1 hr twice/week) were excluded. Otherwise not reported |
Schwartz 2007 AET.
| Methods | RCT, parallel 3‐group design, stratified according to menopausal status (premenopausal or postmenopausal). Study start and stop dates: not reported Length of intervention: 6 months Length of follow‐up: end of intervention |
|
| Participants | 72 breast cancer patients, stages I to III (histologically confirmed); planning to begin chemotherapy with doxorubicin or methotrexate and receiving a glucocorticoid as part of the antiemetic regimen. Strenuous regular exercisers, that is women who exercised more than 250 minutes per week, were excluded |
|
| Interventions |
Intervention group 1 (n = 22): 'Schwartz AET' Aerobic exercise training (participant preferences); 77% weight‐bearing activities (walking/running), 15 to 30 minutes, 4 days per week Intervention group 2 (n = 21): 'Schwartz RET' Resistance exercise (Thera‐Band), 2 sets of 8 exercises (4 upper and 4 lower body) Control group (n = 23): usual care, women were instructed to continue usual activities, were not instructed to avoid exercise |
|
| Outcomes |
Outcomes measured at baseline and 6 months |
|
| Notes | Funding: Not reported Conflict of interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | Unclear risk | No other relevant outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out:
All participants: 8.3% (6/72) No data imputation |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | No significant differences |
| Adherence | Unclear risk | No adherence data available |
| Contamination | Unclear risk | Not reported. Control group not sedentary before cancer diagnosis |
Schwartz 2007 RET.
| Methods | RCT, parallel 3‐group design, stratified according to menopausal status (premenopausal or postmenopausal). Study start and stop dates: not reported Length of intervention: 6 months Length of follow‐up: end of intervention |
|
| Participants | 72 breast cancer patients, stages I to III (histologically confirmed); planning to begin chemotherapy with doxorubicin or methotrexate and receiving a glucocorticoid as part of the antiemetic regimen. Strenuous regular exercisers, that is women who exercised more than 250 minutes per week, were excluded |
|
| Interventions | Intervention group 1 (n = 22): 'Schwartz AET' Aerobic exercise training (participant preferences); 77% weight‐bearing activities (walking/running), 15 to 30 minutes, 4 days per week Intervention group 2 (n = 21): 'Schwartz RET' Resistance exercise (Thera‐Band), 2 sets of 8 exercises (4 upper and 4 lower body) Control group (n = 23): usual care, women were instructed to continue usual activities, were not instructed to avoid exercise |
|
| Outcomes |
Outcomes measured at baseline and 6 months |
|
| Notes | Funding: Not reported Conflict of interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported Comment: probably not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out:
All participants: 8.3% (6/72) No data imputation |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published |
| Group similarity at baseline | Low risk | No significant differences |
| Adherence | Unclear risk | No adherence data available |
| Contamination | Unclear risk | Not reported. Control group not sedentary before cancer diagnosis |
Segal 2001 SD.
| Methods | RCT, 3 groups Study start and stop dates: not reported Length of intervention: 26 weeks Length of follow‐up: end of intervention |
|
| Participants | 123 patients within 2 weeks of the initiation of their prescribed adjuvant therapy (radiotherapy, hormonal therapy, or chemotherapy). Patients receiving only alternative or dose‐intensive chemotherapy regimens were excluded | |
| Interventions |
Intervention group 1: 'Segal 2001 SD' self directed aerobic training (n = 40): progressive walking program at an exercise intensity of 50% to 60% of the predicted maximal oxygen uptake Intervention group 2: 'Segal 2001 SU' supervised training (n = 42): progressive walking program at an exercise intensity of 50% to 60% of the predicted maximal oxygen uptake Control group (n = 41): usual care |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Outcomes measured at baseline and 26 weeks |
|
| Notes | Funding: National Cancer Institute of Canada with funds from the Canadian Cancer Society (Grant in Aid of Research No. 7191) Conflict of interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Study co‐ordinator revealed group assignment after baseline testing. Method not described |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
End‐of‐intervention (26‐week) data were obtained for 99 participants (80.4%) ITT with imputation (most recent observed) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published prospectively. FACT‐G and FACT‐B: no detailed results, but stated that no significant differences |
| Group similarity at baseline | Low risk | "Baseline demographic, body weight, aerobic capacity, prior level of physical activity, and disease treatment characteristics of the subjects did not differ among the three groups. There were no baseline differences among groups for the eight SF‐36 scales" |
| Adherence | High risk | Adherence in intervention groups:
|
| Contamination | Unclear risk | Not reported |
Segal 2001 SU.
| Methods | RCT, 3 groups Study start and stop dates: not reported Length of intervention: 26 weeks Length of follow‐up: end of intervention |
|
| Participants | 123 patients within 2 weeks of the initiation of their prescribed adjuvant therapy (radiotherapy, hormonal therapy, or chemotherapy). Patients receiving only alternative or dose‐intensive chemotherapy regimens were excluded | |
| Interventions | Intervention group 1: 'Segal 2001 SD' self directed aerobic training (n = 40): progressive walking program at an exercise intensity of 50% to 60% of the predicted maximal oxygen uptake Intervention group 2: 'Segal 2001 SU' supervised training (n = 42): progressive walking program at an exercise intensity of 50% to 60% of the predicted maximal oxygen uptake Control group (n = 41): usual care |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Outcomes measured at baseline and 26 weeks |
|
| Notes | Funding: National Cancer Institute of Canada with funds from the Canadian Cancer Society (Grant in Aid of Research No. 7191) Conflict of interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Study co‐ordinator revealed group assignment after baseline testing. Method not described |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
End‐of‐intervention (26‐week) data were obtained for 99 participants (80.4%) ITT with imputation (most recent observed) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published prospectively. FACT‐G and FACT‐B: no detailed results, but stated that no significant differences |
| Group similarity at baseline | Low risk | "Baseline demographic, body weight, aerobic capacity, prior level of physical activity, and disease treatment characteristics of the subjects did not differ among the three groups. There were no baseline differences among groups for the eight SF‐36 scales" |
| Adherence | High risk | Adherence in intervention groups:
|
| Contamination | Unclear risk | Not reported |
Steindorf 2014.
| Methods | RCT, 2 groups Study start and stop dates: February 2011 and March 2013 Length of intervention: 12 weeks Length of follow‐up: end of intervention |
|
| Participants | 160 randomised participants with breast cancer (stage I to III after lumpectomy or mastectomy) undergoing adjuvant radiotherapy | |
| Interventions | Intervention (n = 80): supervised machine‐based progressive resistance training, 60 minutes, 2x/week, 3 sets, 8 to 12 repetitions at 60% to 80% of 1 repetition maximum Control (n = 80): supervised muscle relaxation training according to Jacobson, 60 minutes 2x/week |
|
| Outcomes | Primary endpoint:
Secondary endpoints:
Outcomes measured before start of radiotherapy (baseline, T0), postradiotherapy (week 7, T1), and postintervention (week 13, T2) |
|
| Notes | BEST study, registered at ClinicalTrials.gov (NCT01468766) Funding: Interdisciplinary Research Funding Program (intramural) of the National Center for Tumor Diseases (NCT), Heidelberg, Germany (grant number IFP project VI.1); foundations “Stiftung Leben mit Krebs” and the "Manfred‐Lautenschlaeger‐Stiftung” Conflict of interest: None declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Predetermined list generated with a blocked randomisation SAS procedure with a fixed block size, stratified by age and baseline physical fatigue level |
| Allocation concealment (selection bias) | Low risk | Allocation is done by the biometrician based on a predetermined list generated with a blocked randomisation SAS procedure with a fixed block size, stratified by age and baseline physical fatigue level. To prevent possible bias, study personnel involved in the recruitment and the baseline assessment do not have access to the randomisation lists and are not aware of the block size. Conversely, the biometrician does not have influence on the recruitment procedure |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Pre‐ and postintervention assessment of the primary endpoint was available for a total of 155 (97%) participants, 77 in the exercise group and 78 in the relaxation control group. Data were analysed on an ITT basis. As the number of missing fatigue values was very low (3%), we performed complete‐case analyses |
| Selective reporting (reporting bias) | High risk | Registered at ClinicalTrials.gov (NCT01468766), study protocol published. Not all outcomes reported in this publication |
| Group similarity at baseline | Low risk | Demographics and treatment characteristics did not differ significantly between both intervention groups. All primary and secondary outcome variables were equally distributed in exercise and relaxation control groups at baseline (all P > 0.05), except for the EORTC symptom dry mouth (P = 0.033) |
| Adherence | High risk | The median attended number out of 24 scheduled sessions was 19 in both groups (79%). (QR: 13 to 23, range 1 to 24) in exercise group and (QR: 12 to 22, range 0 to 24) in relaxation control group |
| Contamination | Unclear risk | Patients already participating in systematic intensive resistance or aerobic training (at least 1 hr twice/week) were excluded. Otherwise not reported |
Travier 2015.
| Methods | RCT, 2 groups Study start and stop dates: conducted between 2010 and 2013 Length of intervention: 18 weeks Length of follow‐up: 18 weeks |
|
| Participants | Patients with breast or colon cancer undergoing cancer treatment (204 breast cancer patients of 237 patients in total), intervention started 6 weeks postdiagnosis. Unpublished data (final values) for breast cancer patients used in the analyses of this review | |
| Interventions | Intervention (n = 102): supervised group exercise (aerobic and resistance) program based on Bandura’s social cognitive theory Control (n = 102): asked to maintain their habitual physical activity pattern |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Trial registration: Current Controlled Trials (ISRCTN43801571), Dutch Trial Register (NTR2138) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed computer‐generated randomisation, 1:1 ratio, stratified per age, adjuvant treatment, use of tissue expander, and hospital by sequential balancing |
| Allocation concealment (selection bias) | Low risk | "After the patient signed informed consent, the researcher (who was with the patient) called the data management department and provided the participants study number and the information necessary for stratification. The data manager then performed the randomization using a computer program and informed the researcher about the allocation (and also noted the allocation in the randomization log)." |
| Blinding of participants All outcomes | High risk | "not possible" |
| Blinding of personnel/care providers All outcomes | High risk | Not reported Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | Low risk | Outcome measures were assessed by researchers not involved with the participants |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out after 18 weeks:
Follow‐up: high risk Drop‐out after 36 weeks:
|
| Selective reporting (reporting bias) | High risk | Design paper published. EQ‐5D, IPA (Impact on Participation and Autonomy), self efficacy (items based on social cognitive theory) mentioned in the design paper, but not in the publication |
| Group similarity at baseline | High risk | "were comparable on most characteristics" Comment: More women in the intervention group were highly educated, had triple‐negative breast cancer, and were postmenopausal. Total physical activity levels tended to be higher in the control group |
| Adherence | Low risk | Participation in 83% (IQR 69% to 91%) of the classes. Reported to be physically active in 11 (IQR 6 to 14) of 18 weeks |
| Contamination | High risk | High level of physical activity reported by 56% of the controls at 18 weeks |
van Waart 2014 high.
| Methods | RCT, 3 groups Study start and stop dates: recruitment between March 2010 and December 2012 Length of intervention: approximately 20 weeks Length of follow‐up: 6 months |
|
| Participants | Patients with breast or colon cancer receiving adjuvant chemotherapy, 230 patients with breast cancer of 253 patients in total. Both intervention groups started exercising in the week of the first cycle of chemotherapy and continued until 3 weeks after the last cycle of chemotherapy. Mean length of chemotherapy 119.6 days (17 weeks) |
|
| Interventions | 1. Intervention group (n = 77): Onco‐Move, a relatively low‐intensity, home‐based, individualised, self managed physical activity program 2. Intervention group (n = 76): OnTrack, a relatively high‐intensity exercise program supervised by a physical therapist in an outpatient or general physical therapy practice setting 3. Control group (n = 77): usual care |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Outcomes assessed: before random assignment and start of chemotherapy (T0), at completion of chemotherapy (T1), and 6 months after completion of chemotherapy (T2) |
|
| Notes | Published protocol. Trial registration: The Netherlands Trial Register (NTR 2159) Funding: Supported by Alpe d'Huzes/Dutch Cancer Society Grant No. ALPE‐2009‐4299, the CZ Fund, Zilveren Kruis Achmea, and the Comprehensive Cancer Centre of the Netherlands. Conflicts of interest: 2 authors disclosed research funding by pharmaceutical companies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to Onco‐Move, OnTrack, or UC using the minimization method, which balanced groups with respect to age, primary diagnosis, treating hospital, and use of trastuzumab." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were available for 204 participants (89%) directly after chemotherapy, and for 196 (85%) at the 6‐month follow‐up. ITT without imputation |
| Selective reporting (reporting bias) | High risk | Differences between design paper and final publication (EQ‐5D, anthropometric measures and actigraph not mentioned in the final publication). Chemotherapy completion rate mentioned as outcome in the final publication, but not in the design paper |
| Group similarity at baseline | Low risk | Baseline characteristics were balanced across groups |
| Adherence | High risk | On average, participants in OnTrack attended 71% of the planned sessions. On the basis of the exercise diary, 48% of the OnTrack group and 55% of the Onco‐Move group followed the recommendations regarding daily activity levels at least 75% of the time |
| Contamination | Unclear risk | Not reported |
van Waart 2014 low.
| Methods | RCT, 3 groups Study start and stop dates: recruitment between March 2010 and December 2012 Length of intervention: approximately 20 weeks Length of follow‐up: 6 months |
|
| Participants | Patients with breast or colon cancer receiving adjuvant chemotherapy, 230 patients with breast cancer of 253 patients in total. Both intervention groups started exercising in the week of the first cycle of chemotherapy and continued until 3 weeks after the last cycle of chemotherapy. Mean length of chemotherapy 119.6 days (17 weeks) |
|
| Interventions |
1. Intervention group (n = 77): Onco‐Move, a relatively low‐intensity, home‐based, individualised, self managed physical activity program 2. Intervention group (n = 76): OnTrack, a relatively high‐intensity exercise program supervised by a physical therapist in an outpatient or general physical therapy practice setting 3. Control group (n = 77): usual care |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
Outcomes assessed: before random assignment and start of chemotherapy (T0), at completion of chemotherapy (T1), and 6 months after completion of chemotherapy (T2) |
|
| Notes | Published protocol. Trial registration: The Netherlands Trial Register (NTR 2159) Funding: Supported by Alpe d'Huzes/Dutch Cancer Society Grant No. ALPE‐2009‐4299, the CZ Fund, Zilveren Kruis Achmea, and the Comprehensive Cancer Centre of the Netherlands. Conflicts of interest: 2 authors disclosed research funding by pharmaceutical companies |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned to Onco‐Move, OnTrack, or UC using the minimization method, which balanced groups with respect to age, primary diagnosis, treating hospital, and use of trastuzumab." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were available for 204 participants (89%) directly after chemotherapy, and for 196 (85%) at the 6‐month follow‐up. ITT without imputation |
| Selective reporting (reporting bias) | High risk | Differences between design paper and final publication (EQ‐5D, anthropometric measures and actigraph not mentioned in the final publication). Chemotherapy completion rate mentioned as outcome in the final publication, but not in the design paper |
| Group similarity at baseline | Low risk | Baseline characteristics were balanced across groups |
| Adherence | High risk | On average, participants in OnTrack attended 71% of the planned sessions. On the basis of the exercise diary, 48% of the OnTrack group and 55% of the Onco‐Move group followed the recommendations regarding daily activity levels at least 75% of the time |
| Contamination | Unclear risk | Not reported |
Visovsky 2014.
| Methods | RCT, 2 groups Study start and stop dates: final enrolment completed August 2010, otherwise not reported Length of intervention: 12 weeks Length of follow‐up: 12 weeks' postintervention |
|
| Participants | Breast cancer patients receiving weekly paclitaxel for 2 months, randomisation after 4 cycles of doxorubicin and cyclophosphamide were completed, and prior to the first paclitaxel infusion | |
| Interventions | Intervention group (n = 9): home‐based aerobic (walking and progressive interval training) and resistance exercises for upper and lower extremities using resistance power bands. Brisk walking 5 to 7 days per week for the first 4 weeks, weeks 4 to 12 interval‐based workout consisting of light‐ to moderate‐intensity exercises performed for 30 minutes.
Strength training sessions: 3 times/week, initially: 1 to 2 sets of each exercise for 8 repetitions 1 to 2 times per week. From week 4, the number of sets was increased to 2 to 3 sets and 8 to 12 repetitions of each exercise per session. Control group (n = 10): breast cancer educational information, avoiding those topics related to exercise/physical activity in order to prevent contamination between groups. Educational sessions at the same intervals as intervention group |
|
| Outcomes |
Outcomes were assessed at baseline, 4 weeks, 12 weeks, 24 weeks. Adverse events: No injuries or falls were reported |
|
| Notes | Funding: This study was supported by a grant from the University of Nebraska Medical Center, Eppley Cancer Center, Dean’s Grant. Conflict of interest: None reported. Registered prospectively on ClinicalTrials.gov: NCT00869804 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was generated using sealed envelopes that were numbered and selected sequentially |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. Comment: not mentioned if they were opaque |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | The research nurses conducted all recruitment, data collection, and study interventions. The principal investigator provided ongoing supervision of the intervention |
| Blinding of outcome assessment (detection bias) Fitness outcomes | High risk | The research nurses conducted all recruitment, data collection, and study interventions. The principal investigator provided ongoing supervision of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts or missing data reported. All study participants were evaluated according to the randomisation schema regardless of completion of exercise sessions |
| Selective reporting (reporting bias) | High risk | Outcomes muscle strength, cold thermal sensation, and vibratory sensation, which were mentioned on ClinicalTrials.gov, were not reported. Results for symptom experience were not reported |
| Group similarity at baseline | Unclear risk | "No significant differences" |
| Adherence | High risk | Participants were given a diary to record both aerobic and resistance exercises. In order to objectively capture the aerobic exercise component, each exercise group participant was given a pedometer to be worn for each walking session throughout the entire 24‐week study. Mean walking time per week in minutes: 44.6 |
| Contamination | Unclear risk | Participants completed the Leisure Time Exercise (LTE) questionnaire at the baseline interview; "No significant differences" Participants assigned to the attention control group agreed not to begin a new exercise program or change their level of exercise during the course of the study. Level of exercise during study not reported |
Winningham 1988.
| Methods | RCT, 3 groups Study start and stop dates: not reported Length of intervention: 10 weeks Length of follow‐up: end of intervention |
|
| Participants | 42 breast cancer patients, after surgery, receiving chemotherapy, within the first 6 months of chemotherapy, but having had at least 3 treatments prior to entering the study program, not on doxorubicin, Karnofsky 60% to 100%. None of the participants received antiemetic medication | |
| Interventions | Intervention (n = 16): aerobic training (cycling, interval training), 60% to 85% HRmax, 20 to 30 min per session, 3/week, 10 weeks, supervised Control group 1 (n = 12): no intervention Control group 2 (n = 14): "placebo" mild stretching, conversation, 1/week, supervised |
|
| Outcomes |
Outcomes measured at baseline and end of intervention |
|
| Notes | Funding: Not reported Conflict of interest: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated as randomised. The generation of the random sequence was not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants All outcomes | High risk | Placebo group Comment: Patients in the placebo group would have been aware that they were not in the exercise group, but in the stretching group |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were accounted for |
| Selective reporting (reporting bias) | High risk | Participants answered all 90 items of the SCL‐90‐R. Hereby, "the investigators hoped to avoid sensitizing patients to awareness of any one symptom". Results for other items apart from nausea and somatisation part were not reported |
| Group similarity at baseline | High risk | "similar in age, height and body weight". Participants in control group were higher educated than those in exercise and placebo groups. Placebo group had more married women than exercise and placebo groups. |
| Adherence | Unclear risk | Not reported |
| Contamination | Unclear risk | Contamination not reported. Inclusion criterion: "not in an exercise program". Control participants were advised to notify project personnel if they began exercising on a regular basis either as part of a group or on their own |
Yang 2011.
| Methods | RCT, 2 groups Study start and stop dates: 2008 to 2009 Length of intervention: 12 weeks Length of follow‐up: end of intervention |
|
| Participants | 44 sedentary breast cancer patients randomised, 40 completed the study | |
| Interventions | Intervention group (n = 19): home‐based walking program, developed using the American College of Sports Medicine guidelines. Walking starting 2 to 3 days after each chemotherapy session, included 5 minutes warm‐up, 30 minutes brisk walking (60% to 80% of age‐adjusted HRmax), 5 minutes cool‐down Control group (n = 21): maintenance of their previous lifestyle for 12 weeks |
|
| Outcomes |
Outcomes measured at baseline, 6 and 12 weeks |
|
| Notes | Funding: Taipei Medical University Hospital (95TMU‐TMUH‐19), Taipei, Taiwan, Republic of China. Conflict of interest: None declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants All outcomes | High risk | Not done |
| Blinding of personnel/care providers All outcomes | High risk | Not reported. Comment: probably not done |
| Blinding of outcome assessment (detection bias) All outcomes except fitness outcomes | High risk | Self reported items |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out:
Total: 4/44 (9%) |
| Selective reporting (reporting bias) | Unclear risk | No study protocol published, study not registered prospectively |
| Group similarity at baseline | Low risk | The groups were generally balanced at baseline regarding demographic and disease‐related characteristics |
| Adherence | High risk | "About 20% did not completely adhere." Adherence was about 77% of the prescribed exercise sessions and 100% of the prescribed exercise intensity |
| Contamination | Unclear risk | Not reported |
6‐MWT: 6‐minute walk test ACSM: American College of Sports Medicine BDI: Beck Depression Inventory BMI: body mass index CES‐D: Center for Epidemiological Studies‐Depression Scale DASH: Disabilities of the Arm, Shoulder and Hand Questionnaire DEXA: dual‐energy X‐ray absorptiometry EORTC QLQ‐BR23: European Organisation for Research and Treatment of Cancer Breast Cancer‐Specific Quality of Life Questionnaire EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 36 FACIT‐F: Functional Assessment of Chronic Illness Therapy‐Fatigue Scale FACT‐An: Functional Assessment of Cancer Therapy‐Anaemia FACT‐B: Functional Assessment of Cancer Therapy‐Breast FACT‐ES: Functional Assessment of Cancer Therapy‐Endocrine Symptoms FACT‐G: Functional Assessment of Cancer Therapy‐General FACT‐Taxane: Functional Assessment of Cancer Therapy‐Taxane FQL: Fatigue Quality List GLTEQ: Godin Leisure‐Time Exercise Questionnaire GXT: Graded Exercise Test HADS: Hospital Anxiety and Depression Scale HRmax: maximum heart rate HRQoL: health‐related quality of life IPAQ: International Physical Activity Questionnaire IQR: interquartile range ITT: intention‐to‐treat L‐Dex: lymphoedema index MDASI‐T: Taiwanese version of the MD Anderson Symptom Inventory MET: metabolic equivalent of task MFI: Multidimensional Fatigue Inventory MOS SF‐36: Medical Outcomes Study 36‐Item Short Form Health Survey MQOLS‐CA: Multidimensional Quality of Life Scale‐Cancer MVPA: moderate to vigorous physical activity QoL: quality of life QR: Quartile Range PAL: physical activity level PANAS: Positive and Negative Affect Schedule PFS: Piper Fatigue Scale POMS: Profile of Mood States RCT: randomised controlled trial SD: standard deviation SF‐36: 36‐Item Short Form Health Survey SPAQ: Scottish Physical Activity Questionnaire SWLS: Satisfaction With Life Scale VO2 max: maximal oxygen uptake
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aaronson 2011 | Post‐treatment |
| Adamsen 2009 | Participants not predominantly breast cancer patients |
| Aghili 2007 | Duration of exercise intervention less than 6 weeks |
| Backman 2013 | Participants not predominantly breast cancer patients |
| Banasik 2011 | Post‐treatment |
| Banerjee 2007 | Yoga |
| Basen 2006 | Post‐treatment |
| Baumann 2009 | Not an RCT |
| Baumann 2011 | Not an RCT |
| Bower 2012 | Yoga |
| Burnham 2002 | Exercise intervention not concurrent with adjuvant cancer treatment |
| Cantarero‐Villanueva 2012 | Post‐treatment |
| Cantarero‐Villanueva 2013 | Post‐treatment |
| Carson 2009 | Post‐treatment |
| Charbonnier 2012 | Not an exercise intervention |
| Chetiyawardana 2004 | Radiotherapy ongoing for maximum 3 weeks |
| Courneya 2003a | Participants not predominantly breast cancer patients (40%), exercise as part of a complex intervention (group psychotherapy plus exercise) |
| Courneya 2003b | Exercise intervention not concurrent with adjuvant cancer treatment (< 50% under current hormone therapy) |
| Courneya 2006 | Participants were post‐treatment. Exercise intervention not concurrent with adjuvant cancer treatment |
| Culos‐Reed 2006 | Post‐treatment |
| Danhauer 2009 | Yoga |
| Demark‐Wahnefried 2002 | No clinical trial, protocol status, exercise as part of a complex intervention (diet and exercise‐based counselling program) |
| Demark‐Wahnefried 2003 | No clinical trial, design paper |
| Demark‐Wahnefried 2005 | Complex intervention |
| Demark‐Wahnefried 2006 | Complex intervention |
| Demark‐Wahnefried 2008 | Complex intervention |
| Dimeo 1999 | Participants not predominantly breast cancer patients |
| Duijts 2009 | Post‐treatment |
| Duijts 2012 | Post‐treatment |
| Emami 2012 | Post‐treatment |
| Ergun 2013 | Post‐treatment |
| Fairey 2003 | Exercise intervention not concurrent with adjuvant cancer treatment |
| Fairey 2005 | Post‐treatment |
| Fairey 2005a | Post‐treatment |
| Fernandez 2013 | Post‐treatment |
| Galantino 2010 | No exercise intervention |
| Given 2002 | Participants not predominantly breast cancer patients |
| Gomes 2011 | Not an exercise intervention |
| Gomez 2011 | Post‐treatment |
| Griffith 2009 | Participants not predominantly breast cancer patients |
| Hartmann 2013 | Not an RCT |
| Hatchett 2013 | Post‐treatment |
| Herrero 2006 | Post‐treatment |
| Ho 1986 | No exercise intervention |
| Huang 2014 | No health‐related outcome measure (adherence study) |
| Irwin 2008 | Post‐treatment |
| Irwin 2009 | Post‐treatment |
| Irwin 2009a | Post‐treatment |
| Janelsins 2011 | Post‐treatment |
| Kim 2006 | Complex intervention: exercise and stress management training |
| Kleine‐Tebbe 2006 | Data were not analysed to be used for a full publication (personal communication September 2013) |
| Kohler 2008 | Not an RCT |
| Kovacic 2011 | Yoga |
| Latikka 1997 | No clinical trial, review |
| Latka 2009 | Post‐treatment |
| Lauridsen 2005 | Exercise restricted to shoulder |
| Lee 2006 | Qigong |
| Ligibel 2006 | Post‐treatment |
| Ligibel 2008 | Post‐treatment |
| Ligibel 2009 | Post‐treatment |
| MacVicar 1986 | Not an RCT |
| Mamom 2012 | Duration of exercise intervention less than 6 weeks |
| Martin 2013 | Post‐treatment |
| Maryam 2010 | Not an RCT |
| McGuire 2011 | Post‐treatment |
| McKenzie 2003 | Intervention and adjuvant treatment not concurrent |
| Milecki 2013 | Radiotherapy ongoing for maximum 5 weeks |
| Moadel 2007 | Yoga |
| Mock 1994 | Exercise as part of a complex intervention (walking plus support group) |
| Mock 1997 | Not an RCT |
| Mock 2001 | Trial does not compare 2 groups as assigned by investigator |
| Mock 2002 | No exercise intervention |
| Moller 2013 | Participants not predominantly breast cancer patients |
| Mulero 2008 | Post‐treatment |
| Murtezani 2014 | Post‐treatment |
| Musanti 2012 | Post‐treatment |
| Mustian 2002 | No clinical trial, review |
| Mustian 2006 | Less than 6 weeks |
| Pickett 2002 | No health‐related outcome measure (adherence study) |
| Pinto 2003 | Exercise intervention not concurrent with adjuvant cancer treatment |
| Pinto 2008 | Post‐treatment |
| Pinto 2009 | Post‐treatment |
| Rabin 2006 | Post‐treatment |
| Raghavendra 2007 | Yoga |
| Rahnama 2010 | Post‐treatment |
| Rao 2008 | Yoga |
| Rao 2009 | Yoga |
| Rogers 2011 | Post‐treatment |
| Sandel 2005 | Not an exercise intervention |
| Schmitz 2005 | Post‐treatment |
| Schwartz 1999 | Trial does not compare 2 groups as assigned by investigator |
| Schwartz 2001 | Trial does not compare 2 groups as assigned by investigator |
| Scott 2013 | Post‐treatment |
| Segar 1998 | Exercise intervention not concurrent with adjuvant cancer treatment |
| Shaw 2003 | No clinical trial, protocol status, exercise as part of a complex intervention (calcium‐rich diet and exercise) |
| So 2006 | Not an RCT |
| Sprod 2012 | Post‐treatment |
| Stevinson 2009 | Commentary |
| Swenson 2009 | Complex intervention |
| Swenson 2010 | Complex intervention |
| Twiss 2009 | Post‐treatment |
| Vadiraja 2009 | Yoga |
| Vadiraja 2010 | Commentary |
| Vallance 2007 | Post‐treatment |
| Vallance 2008 | Post‐treatment |
| Vallance 2008a | Post‐treatment |
| Vincent 2013 | Not an RCT |
| Waltman 2010 | Post‐treatment |
| Wang 2010 | Exercise intervention does not coincide with adjuvant therapy at least 6 weeks |
| Wilkie 2003 | Participants not predominantly breast cancer patients (63% female), duration of intervention programme 4 weeks |
| Yeh 2006 | Qigong |
| Yuen 2007 | Post‐treatment |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Lotzke 2016.
| Methods | RCT, 2 groups |
| Participants | Women with stage I to III breast cancer during (neo‐)adjuvant therapy |
| Interventions | Weekly 60‐minute physical exercise session together with individual home‐based, self contained 20‐minute sessions twice a week. Control group: weekly 60‐minute Iyengar yoga session together with individual home‐based, self contained 20‐minute sessions twice a week |
| Outcomes |
|
| Notes |
Petrella 2012.
| Methods | RCT, 2 groups Study start and stop dates: not reported Length of intervention: 6 months Length of follow‐up: 6 months after the end of the intervention |
| Participants | Women who were within 4 to 12 weeks of surgery for stage I to III breast cancer and undergoing adjuvant chemotherapy |
| Interventions | Structured exercise program (6 months), aerobic and resistance training Control group: usual oncology care |
| Outcomes |
Outcomes assessed at: baseline and 3‐month intervals through 12 months |
| Notes | Abstract |
EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 36 FACT‐B: Functional Assessment of Cancer Therapy‐Breast RCT: randomised controlled trial SF‐36: 36‐Item Short Form Health Survey
Characteristics of ongoing studies [ordered by study ID]
ACTRN12614000051640.
| Trial name or title | Preventing ‘chemo‐brain’: Can exercise mitigate chemotherapy‐induced cognitive impairment in breast cancer patients? |
| Methods | RCT |
| Participants | Breast cancer patients scheduled to receive adjuvant chemotherapy with fluorouracil‐epirubicin‐cyclophosphamide‐docetaxel (FEC‐T) or docetaxel‐cyclophosphamide (TC) regimens, target sample size: 66 |
| Interventions | Combination of resistance (i.e. lifting weights) and aerobic exercise (e.g. walking, jogging, cycling), 2 times/week in an exercise clinic. moderate to high intensity (i.e. a perceived exertion of somewhat hard to hard) and will be relative to each participant's capabilities. Progressive exercise prescription, modified according to individual response. Approximately 60 min/session, small groups under the supervision of an accredited exercise physiologist. Length of program: will vary from 2.5 to 4 months according to the duration of the chemotherapy regimen Control group: usual care |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Starting date | June 2014 first participant enrolment |
| Contact information | Dr Prue Cormie ECU Health and Wellness Institute Edith Cowan University 270 Joondalup Drive Joondalup, WA 6027, Australia |
| Notes |
NCT01943695.
| Trial name or title | Aerobic training during or after adjuvant therapy |
| Methods | RCT, 4 arms |
| Participants | Sedentary breast cancer patients, estimated enrolment: 160 participants |
| Interventions |
|
| Outcomes | Primary:
Secondary:
|
| Starting date | January 2013 |
| Contact information | Lee W Jones Duke University Medical Center Durham, NC, USA, 27710 |
| Notes | Estimated study completion date: August 2017 |
NCT02117011.
| Trial name or title | Effects of a structured exercise program on cancer‐related fatigue in women receiving radiation therapy for breast cancer |
| Methods | RCT, estimated enrolment: 30 |
| Participants | African‐Americans undergoing radiation therapy for localised breast cancer. Sedentary, as defined as < 60 minutes of recreation or work requiring modest physical activity/week based on the 7‐day physical activity recall questionnaire. Having completed neo‐adjuvant or adjuvant chemotherapy |
| Interventions | 8‐week, moderate‐intensity aerobic exercise program Participants will be required to meet and maintain a goal of 75 min/week of aerobic exercise by using portable cycle ergometers, that is 15 min/day, 5 days/week |
| Outcomes | Fatigue: FACIT‐F Cancer‐specific quality of life: FACT‐G |
| Starting date | June 2013 |
| Contact information | |
| Notes | Estimated study completion date: December 2016 |
NCT02159157.
| Trial name or title | A randomized, controlled trial to determine the effects of an exercise intervention on physical activity during chemotherapy for patients with early stage breast cancer |
| Methods | RCT |
| Participants | Women or men with histologically confirmed breast cancer and no evidence of metastatic disease with a recommendation to begin chemotherapy within 4 weeks. Sedentary: participants must have a baseline activity level of < 150 minutes/wk of moderate to vigorous activity as calculated using the moderate to vigorous components of the Leisure‐Time Exercise Questionnaire for physical activity (completed during screening). Karnofsky performance status > or = to 80%. Estimated enrolment: 120 participants |
| Interventions | A physical therapist will design an exercise plan for each participant on the intervention arm. The participants randomised to the intervention arm will also receive phone calls to assist with tracking the study participant's exercise and motivating the study participant to adhere to the exercise prescription. Exercise prescription aimed at increasing physical activity by a minimum of 10 MET hours/week |
| Outcomes | Primary:
Secondary:
|
| Starting date | June 2014 |
| Contact information | Contact: Mary Chamberlin, MD Cancer.Research.Nurse@Dartmouth.edu |
| Notes | Estimated primary completion rate: June 2017 (Final data collection date for primary outcome measure) |
NCT02240836.
| Trial name or title | Energy Balance and Breast Cancer Aspects‐II (EBBA‐II) |
| Methods | RCT, 2 groups |
| Participants | Newly diagnosed breast cancer patients undergoing adjuvant therapy, DCIS grade 3, stage I + II breast cancer Estimated enrolment: 600 participants |
| Interventions | 12‐month exercise program comprised of strength and endurance training. Exercise groups supervised by experienced physiotherapists, the participants will attend the exercise groups for training 60 min, 2/week. Additionally, home‐based exercise for > = 120 minutes a week, aiming to perform a total of 240 minutes of exercise per week. The control group told to follow standard‐care regimen |
| Outcomes | Primary:
Secondary:
|
| Starting date | September 2014 |
| Contact information | Inger Thune inger.thune@uit.no |
| Notes | Estimated primary completion date: May 2016 Planned follow‐up: 10 years |
NCT02252991.
| Trial name or title | Adapted Physical Activity in Cancerology (APACAN) |
| Methods | RCT |
| Participants | Breast cancer patients receiving radio‐ or chemotherapy or both, estimated enrolment: 200 participants |
| Interventions | Physical activity, from 2 to 6 months Control group: physical activity after treatment |
| Outcomes | Fatigue: MFI QoL: EORTC QLQ‐C30 |
| Starting date | September 2014 |
| Contact information | Yves‐Jean.BIGNON@jp.fr |
| Notes | Estimated primary completion date: December 2016 (final data collection date for primary outcome measure) |
NCT02350582.
| Trial name or title | e‐CUIDACHEMO: Telerehabilitation During Chemotherapy in Breast Cancer |
| Methods | RCT |
| Participants | Breast cancer patients with internet access, estimated enrolment: 40 participants |
| Interventions | Resistance and endurance exercise via telerehabilitation |
| Outcomes | Primary:
Secondary:
|
| Starting date | April 2012 |
| Contact information | Manuel Arroyo‐Morales marroyo@ugr.es |
| Notes | Estimated study completion date: June 2015 |
6‐MWT: 6‐minute walk test DCIS: ductal carcinoma in situ EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire‐Core 36 FACIT‐F: Functional Assessment of Chronic Illness Therapy‐Fatigue Scale FACT‐G: Functional Assessment of Cancer Therapy‐General MET: metabolic equivalent of task MFI: Multidimensional Fatigue Inventory PFS: Piper Fatigue Scale QoL: quality of life RCT: randomised controlled trial
Differences between protocol and review
We included only randomised controlled trials in this updated version of the review, as opposed to the original version of the review, in which we also included controlled trials without a randomisation procedure. This was due to the increasing number of randomised trials.
We excluded hormonal therapy only as adjuvant therapy in this version of the review as we did not consider the side effects of hormonal therapy comparable in their severity to chemo‐ or radiotherapy or both. We did not include biological outcomes like immune function measured with T‐cells in this version of the review, as the focus of the review is on directly participant‐relevant outcomes. The same applied to morphological outcomes (changes in body weight or body composition).
We classified outcomes into primary and secondary outcomes in this version of the review.
We did not include data for outcomes assessed with subscales of questionnaires (for example physical functioning subscale of the SF‐36 or vitality subscale of the SF‐36, nausea item of the SCL‐90) in this version of the review.
We also assessed the quality of evidence for the main outcomes using the GRADE methodology and developed a 'Summary of findings' table. This ensures that the review complies with new Cochrane methodological standards.
Contributions of authors
ACF: handsearching and screening search results, study selection, data extraction, contacted experts for unpublished trials and trial investigators for additional data, methodological assessments, quantitative and qualitative synthesis of included studies, reporting.
MM: screening search results, study selection, methodological assessments, contributed to consensus finding when disagreement in study selection, data extraction or methodological assessments persisted between the other two review authors (ACF, MHM), quantitative and qualitative synthesis of included studies, manuscript review.
MHM: handsearching and screening search results, study selection, data extraction, methodological assessments, contributed to consensus finding when disagreement in study selection or methodological assessments persisted between the other two review authors (ACF, MM), quantitative and qualitative synthesis of included studies, reporting.
Declarations of interest
ACF, MM, MHM: None known.
Edited (no change to conclusions)
References
References to studies included in this review
Battaglini 2004 {published data only}
- Battaglini C, Bottaro M, Dennehy C, Barfoot D, Shields E, Kirk D, et al. The effects of resistance training on muscular strength and fatigue levels in breast cancer patients. Brazilian Journal of Sports Medicine 2006;12(3):139e‐44e. [Google Scholar]
- Battaglini CL. A randomized study on the effects of a prescribed exercise intervention on lean mass and fatigue changes in breast cancer patients during treatment [PhD thesis]. Greeley, USA.: University of Northern Colorado, 2004:197. [Google Scholar]
- Battaglini CL, Bottaro M, Dennehy C, Rae L, Shields E, Kirk D, et al. The effects of an individualized exercise intervention on body composition in breast cancer patients undergoing treatment. São Paulo Medical Journal 2007; Vol. 125, issue 1:22‐8. [DOI] [PMC free article] [PubMed]
- Battaglini CL, Mihalik JP, Bottaro M, Dennehy C, Petschauer MA, Hairston LS, et al. Effect of exercise on the caloric intake of breast cancer patients undergoing treatment. Brazilian Journal of Medical & Biological Research 2008;41(8):709‐15. [DOI] [PubMed] [Google Scholar]
Cadmus 2007 {published data only}
- Cadmus LA. Exercise and quality of life during and after treatment for breast cancer [PhD thesis]. New Haven, USA: Yale University, 2007. [Google Scholar]
- Cadmus LA, Chung G, Yu H, Salovey P, Irwin ML. Feasibility of institutional registry‐based recruitment for enrolling newly diagnosed breast cancer patients in an exercise trial. Journal of Physical Activity & Health 2011; Vol. 8, issue 7:955‐63. [DOI] [PubMed]
- Cadmus LA, Salovey P, Yu H, Chung G, Kasl S, Irwin ML. Exercise and quality of life during and after treatment for breast cancer: results of two randomized controlled trials. Psycho‐Oncology 2009;18(4):343‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caldwell 2009 {published data only}
- Caldwell MG. The effects of an endurance exercise regimen on cancer‐related fatigue and physical performance in women with breast cancer [PhD thesis]. Louisiana, USA: Louisiana State University, 2009. [Google Scholar]
Campbell 2005 {published and unpublished data}
- Campbell A, Mutrie N, White F, McGuire F, Kearney N. A pilot study of a supervised group exercise programme as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. European Journal of Oncology Nursing 2005;9(1):56‐63. [DOI] [PubMed] [Google Scholar]
Cornette 2013 {published data only}
- Cornette T. Effets de l’activité physique adaptée sur la fonction aérobie et la fatigue chez des patientes atteintes d’un cancer du sein en situation adjuvante et néo‐adjuvante [Adapted physical activity effect on aerobic function and fatigue in patients with breast cancer treated in adjuvant or neoadjuvant phase] [PhD thesis]. Limoges, France: University of Limoges, 2013. [Google Scholar]
- Cornette T, Vincent F, Antonini MT, Leobon S, Lavau‐Denes S, Tubiana N. Adapted physical activity effect on aerobic function and fatigue in patients with breast cancer treated in adjuvant or neoadjuvant phase (SAPA). European Journal of Cancer 2013;49:S434. [Google Scholar]
- Cornette T, Vincent F, Mandigout S, Antonini MT, Leobon S, Labrunie A, et al. Effects of home‐based exercise training on VO2 in breast cancer patients under adjuvant or neoadjuvant chemotherapy (sapa). European Journal of Physical and Rehabilitation Medicine 2015. [PubMed]
Courneya 2007 AET {published data only}
- Courneya KS, Friedenreich CM, Reid RD, Gelmon K, Mackey JR, Ladha AB, et al. Predictors of follow‐up exercise behavior 6 months after a randomized trial of exercise training during breast cancer chemotherapy. Breast Cancer Research & Treatment 2009;114(1):179‐87. [DOI] [PubMed] [Google Scholar]
- Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Moderators of the effects of exercise training in breast cancer patients receiving chemotherapy: a randomized controlled trial. Cancer 2008;112(8):1845‐53. [DOI] [PubMed] [Google Scholar]
- Courneya KS, McKenzie DC, Reid RD, Mackey JR, Gelmon K, Friedenreich CM, et al. Barriers to supervised exercise training in a randomized controlled trial of breast cancer patients receiving chemotherapy. Annals of Behavioral Medicine 2008;35(1):116‐22. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Reid RD, Friedenreich CM, Gelmon K, Proulx C, Vallance JK, et al. Understanding breast cancer patients' preference for two types of exercise training during chemotherapy in an unblinded randomized controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2008;5(52):9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courneya KS, Segal RJ, Gelmon K, Reid RD, Mackey JR, Friedenreich CM, et al. Predictors of supervised exercise adherence during breast cancer chemotherapy. Medicine & Science in Sports & Exercise 2008;40(6):1180‐7. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Segal RJ, Gelmon K, Reid RD, Mackey JR, Friedenreich CM, et al. Six‐month follow‐up of patient‐rated outcomes in a randomized controlled trial of exercise training during breast cancer chemotherapy. Cancer Epidemiology, Biomarkers & Prevention 2007;16(12):2572‐8. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Journal of Clinical Oncology 2007;25(28):4396‐404. [DOI] [PubMed] [Google Scholar]
- Dolan LB, Gelmon K, Courneya KS, MacKey JR, Segal RJ, Lane K, et al. Hemoglobin and aerobic fitness changes with supervised exercise training in breast cancer patients receiving chemotherapy. Cancer Epidemiology, Biomarkers & Prevention 2010;19(11):2826‐32. [DOI] [PubMed] [Google Scholar]
Courneya 2007 RET {published data only}
- Courneya KS, Friedenreich CM, Reid RD, Gelmon K, Mackey JR, Ladha AB, et al. Predictors of follow‐up exercise behavior 6 months after a randomized trial of exercise training during breast cancer chemotherapy. Breast Cancer Research & Treatment 2009;114(1):179‐87. [DOI] [PubMed] [Google Scholar]
- Courneya KS, McKenzie DC, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Moderators of the effects of exercise training in breast cancer patients receiving chemotherapy: a randomized controlled trial. Cancer 2008;112(8):1845‐53. [DOI] [PubMed] [Google Scholar]
- Courneya KS, McKenzie DC, Reid RD, Mackey JR, Gelmon K, Friedenreich CM, et al. Barriers to supervised exercise training in a randomized controlled trial of breast cancer patients receiving chemotherapy. Annals of Behavioral Medicine 2008;35(1):116‐22. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Reid RD, Friedenreich CM, Gelmon K, Proulx C, Vallance JK, et al. Understanding breast cancer patients' preference for two types of exercise training during chemotherapy in an unblinded randomized controlled trial. International Journal of Behavioral Nutrition and Physical Activity 2008;5:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courneya KS, Segal RJ, Gelmon K, Reid RD, Mackey JR, Friedenreich CM, et al. Predictors of supervised exercise adherence during breast cancer chemotherapy. Medicine & Science in Sports & Exercise 2008;40(6):1180‐7. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Segal RJ, Gelmon K, Reid RD, Mackey JR, Friedenreich CM, et al. Six‐month follow‐up of patient‐rated outcomes in a randomized controlled trial of exercise training during breast cancer chemotherapy. Cancer Epidemiology, Biomarkers & Prevention 2007;16(12):2572‐8. [DOI] [PubMed] [Google Scholar]
- Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Journal of Clinical Oncology 2007;25(28):4396‐404. [DOI] [PubMed] [Google Scholar]
- Dolan LB, Gelmon K, Courneya KS, MacKey JR, Segal RJ, Lane K, et al. Hemoglobin and aerobic fitness changes with supervised exercise training in breast cancer patients receiving chemotherapy. Cancer Epidemiology, Biomarkers & Prevention 2010;19(11):2826‐32. [DOI] [PubMed] [Google Scholar]
Crowley 2003 {published data only}
- Crowley SA. The effect of a structured exercise program on fatigue, strength, endurance, physical self‐efficacy, and functional wellness in women with early stage breast cancer [PhD thesis]. Ann Arbor, USA: University of Michigan, 2003:127. [Google Scholar]
Dodd 2010 {published data only}
- Chou FY, Dodd MJ, Paul SM. Timing and sustainability of an exercise intervention in women with breast cancer during and after cancer treatment. Oncology Nursing Forum 2012;39(1):91‐7. [DOI] [PubMed] [Google Scholar]
- DeNysschen CA, Brown JK, Cho MH, Dodd MJ. Nutritional symptom and body composition outcomes of aerobic exercise in women with breast cancer. Clinical Nursing Research 2011;20(1):29‐46. [DOI] [PubMed] [Google Scholar]
- Dodd MJ, Cho MH, Cooper BA, Miaskowski C. The effect of symptom clusters on functional status and quality of life in women with breast cancer. European Journal of Oncology Nursing 2010;14:101‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd MJ, Cho MH, Miaskowski C, Painter PL, Paul SM, Cooper BA, et al. A randomized controlled trial of home‐based exercise for cancer‐related fatigue in women during and after chemotherapy with or without radiation therapy. Cancer Nursing 2010;33(4):245‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Dodd MJ, Dibble SL, Abrams DI. Nausea at the end of adjuvant cancer treatment in relation to exercise during treatment in patients with breast cancer. Oncology Nursing Forum 2008;35(5):830‐5. [DOI] [PubMed] [Google Scholar]
- Wu HS, Dodd MJ, Cho MH. Patterns of fatigue and effect of exercise in patients receiving chemotherapy for breast cancer. Oncology Nursing Forum 2008;35(5):E90‐9. [Google Scholar]
Drouin 2002 {published data only}
- Drouin JS. Aerobic exercise training effects on physical function, fatigue and mood, immune status, and oxidative stress in subjects undergoing radiation treatment for breast cancer [PhD thesis]. Detroit, USA: Wayne State University, 2002:142. [Google Scholar]
- Drouin JS, Armstrong H, Krause S. Effects of aerobic exercise training on peak aerobic capacity, fatigue, and psychological factors during radiation for breast cancer. Rehabilitation Oncology 2005;23(1):11‐7. [Google Scholar]
- Drouin JS, Birk TJ, Wirth JC. Random control clinical trial on effects of aerobic exercise training on weight management during radiation treatment for breast cancer. Rehabilitation Oncology 2006;24(3):6‐10. [DOI] [PubMed] [Google Scholar]
- Drouin JS, Young TJ, Beeler J, Byrne K, Birk TJ, Hryniuk WM, et al. Random control clinical trial on the effects of aerobic exercise training on erythrocyte levels during radiation treatment for breast cancer. Cancer 2006;107(10):2490‐5. [DOI] [PubMed] [Google Scholar]
Eakin 2012 {published and unpublished data}
- Eakin EG, Lawler SP, Winkler EA, Hayes SC. A randomized trial of a telephone‐delivered exercise intervention for non‐urban dwelling women newly diagnosed with breast cancer: exercise for health. Annals of Behavioral Medicine 2012;43(2):229‐38. [DOI] [PubMed] [Google Scholar]
Gokal 2013 {published data only}
- Gokal K, Munir F, Wallis D, Ahmed S, Boiangiu I, Kancherla K. Can physical activity help to maintain cognitive functioning and psychosocial well‐being among breast cancer patients treated with chemotherapy? A randomised controlled trial: study protocol. BMC Public Health 2015;15:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokal K, Munir F, Wallis D, Ahmed S, Boiangiu I, Kancherla K. Physical activity intervention for cognitive & emotional functioning in breast cancer patients receiving chemotherapy. Psycho‐Oncology 2013;22:254. [Google Scholar]
- Gokal K, Wallis D, Ahmed S, Boiangiu I, Kancherla K, Munir F. Effects of a self‐managed home‐based walking intervention on psychosocial health outcomes for breast cancer patients receiving chemotherapy: a randomised controlled trial. Supportive Care in Cancer 2015. [DOI] [PubMed]
Haines 2010 {published data only}
- Haines TP, Sinnamon P, Wetzig NG, Lehman M, Walpole E, Pratt T, et al. Multimodal exercise improves quality of life of women being treated for breast cancer, but at what cost? Randomized trial with economic evaluation. Breast Cancer Research and Treatment 2010;124(1):163‐75. [DOI] [PubMed] [Google Scholar]
Hayes 2013 FtF {published and unpublished data}
- Hayes S, Rye S, Battistutta D, Yates P, Pyke C, Bashford J, et al. Design and implementation of the Exercise for Health trial ‐ a pragmatic exercise intervention for women with breast cancer. Contemporary Clinical Trials 2011; Vol. 32, issue 4:577‐85. [DOI] [PubMed]
- Hayes SC, Rye S, DiSipio T, Yates P, Bashford J, Pyke C. Exercise for health: A randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment‐related side effects following breast cancer. Breast Cancer Research and Treatment 2013;137(1):175‐86. [DOI] [PubMed] [Google Scholar]
Hayes 2013 Tel {published and unpublished data}
- Hayes S, Rye S, Battistutta D, Yates P, Pyke C, Bashford J, et al. Design and implementation of the Exercise for Health trial ‐ a pragmatic exercise intervention for women with breast cancer. Contemporary Clinical Trials 2011; Vol. 32, issue 4:577‐85. [DOI] [PubMed]
- Hayes SC, Rye S, DiSipio T, Yates P, Bashford J, Pyke C. Exercise for health: A randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment‐related side effects following breast cancer. Breast Cancer Research and Treatment 2013;137(1):175‐86. [DOI] [PubMed] [Google Scholar]
Hornsby 2014 {published data only}
- Hornsby WE, Douglas PS, West MJ, Kenjale AA, Lane AR, Schwitzer ER, et al. Safety and efficacy of aerobic training in operable breast cancer patients receiving neoadjuvant chemotherapy: A phase II randomized trial. Acta Oncologica 2014;53(1):65‐74. [DOI] [PubMed] [Google Scholar]
Husebo 2014 {published data only}
- Husebo AML, Dyrstad SM, Mjaaland I, Soreide JA, Bru E. Effects of scheduled exercise on cancer‐related fatigue in women with early breast cancer. The Scientific World Journal 2014;2014:Article ID 271828, 9 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ingram 2010 {published data only}
- Ingram C, Wessel J, Courneya KS. Evaluating the benefits of exercise for women receiving adjuvant therapy for breast cancer: research challenges. Canadian Oncology Nursing Journal 2010;20(2):96‐8. [PubMed] [Google Scholar]
- Ingram C, Wessel J, Courneya KS. Women's perceptions of home‐based exercise performed during adjuvant chemotherapy for breast cancer. European Journal of Oncology Nursing 2010;14(3):238‐43. [DOI] [PubMed] [Google Scholar]
MacVicar 1989 {published data only}
- MacVicar MG, Winningham ML, Nickel JL. Effects of aerobic interval training on cancer patients' functional capacity. Nursing Research 1989;38(6):348‐51. [PubMed] [Google Scholar]
- Winningham ML, MacVicar MG, Bondoc M, Anderson JI, Minton JP. Effect of aerobic exercise on body weight and composition in patients with breast cancer on adjuvant chemotherapy. Oncology Nursing Forum 1989;16:683‐9. [PubMed] [Google Scholar]
Mock 2004 {published data only}
- Mock V, Frangakis C, Davidson NE, Ropka ME, Pickett M, Poniatowski B, et al. Exercise manages fatigue during breast cancer treatment: A randomized controlled trial. Psycho‐Oncology 2005;14:464‐77. [DOI] [PubMed] [Google Scholar]
Moros 2010 {published data only}
Mutrie 2007 {published data only}
- Mutrie N, Campbell A, Barry S, Hefferon K, McConnachie A, Ritchie D, et al. Five‐year follow‐up of participants in a randomised controlled trial showing benefits from exercise for breast cancer survivors during adjuvant treatment. Are there lasting effects?. Journal of Cancer Survivorship 2012;6(4):420‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutrie N, Campbell AM, Whyte F, McConnachie A, Emslie C, Lee L, et al. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: pragmatic randomised controlled trial. BMJ 2007;334(7592):517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh L, Mutrie N, Campbell AM, Crawford JJ, Courneya KS. Effects of supervised exercise on motivational outcomes in breast cancer survivors at 5‐year follow‐up. European Journal of Oncology Nursing 2014;18(6):557‐63. [DOI] [PubMed] [Google Scholar]
Perna 2010 {published data only}
- Perna FM, Craft L, Freund KM, Skrinar G, Stone M, Kachnic L. The effect of a cognitive behavioral exercise intervention on clinical depression in a multiethnic sample of women with breast cancer: A randomized controlled trial. International Journal of Sport and Exercise Psychology 2010;8(1):36‐47. [Google Scholar]
Rao 2012 {published data only}
- Rao R, Cruz V, Peng Y, Harker‐Murray A, Haley BB, Zhao H, et al. Bootcamp during neoadjuvant chemotherapy for breast cancer: A randomized pilot trial. Breast Cancer: Basic and Clinical Research 2012; Vol. 6:39–46. [DOI] [PMC free article] [PubMed]
Reis 2013 {published data only}
- Reis D, Walsh ME, Young‐McCaughan S, Jones T. Effects of Nia exercise in women receiving radiation therapy for breast cancer. Oncology Nursing Forum 2013;40:E374‐81. [DOI] [PubMed] [Google Scholar]
Schmidt 2014 {published data only}
- Schmidt ME, Wiskemann J, Armbrust P, Schneeweiss A, Ulrich CM, Steindorf K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial. International Journal of Cancer 2014;137(2):417‐80. [DOI] [PubMed] [Google Scholar]
- Schmidt ME, Wiskemann J, Krakowski‐Roosen H, Knicker AJ, Habermann N, Schneeweiss A, et al. Progressive resistance versus relaxation training for breast cancer patients during adjuvant chemotherapy: design and rationale of a randomized controlled trial (BEATE study). Contemporary Clinical Trials 2013;34(1):117‐25. [DOI] [PubMed] [Google Scholar]
Schwartz 2007 AET {published data only}
- Schwartz AL, Winters‐Stone K, Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncology Nursing Forum 2007;34(3):627‐33. [DOI] [PubMed] [Google Scholar]
Schwartz 2007 RET {published data only}
- Schwartz AL, Winters‐Stone K, Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncology Nursing Forum 2007;34(3):627‐33. [DOI] [PubMed] [Google Scholar]
Segal 2001 SD {published and unpublished data}
- Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. Journal of Clinical Oncology 2001;19(3):657‐65. [DOI] [PubMed] [Google Scholar]
Segal 2001 SU {published and unpublished data}
- Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. Journal of Clinical Oncology 2001;19:657‐65. [DOI] [PubMed] [Google Scholar]
Steindorf 2014 {published data only}
- Potthoff K, Schmidt ME, Wiskemann J, Hof H, Klassen O, Habermann N, et al. Randomized controlled trial to evaluate the effects of progressive resistance training compared to progressive muscle relaxation in breast cancer patients undergoing adjuvant radiotherapy: the BEST study. BMC Cancer 2013; Vol. 13:162. [DOI] [PMC free article] [PubMed]
- Steindorf K, Schmidt ME, Klassen O, Ulrich CM, Oelmann J, Habermann N, et al. Randomized controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: results on cancer‐related fatigue and quality of life. Annals of Oncology 2014;25(11):2237‐43. [DOI] [PubMed] [Google Scholar]
Travier 2015 {published and unpublished data}
- May AM, Velthuis M, Travier N, Steins Bisschop CN, Wall E, Peeters P. Physical activity during cancer treatment (PACT) study: Short‐ and long‐term effects on fatigue of an 18‐week exercise intervention during adjuvant chemotherapy in patients with breast or colon cancer. Journal of Clinical Oncology 2014; Vol. 32, issue 15 suppl 1.
- Travier N, Velthuis MJ, Steins Bisschop CN, Buijs B, Monninkhof EM, Backx F, et al. Effects of an 18‐week exercise programme started early during breast cancer treatment: a randomised controlled trial. BMC Medicine 2015;13:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velthuis MJ, May AM, Koppejan‐Rensenbrink RA, Gijsen BC, Breda E, Wit GA, et al. Physical Activity during Cancer Treatment (PACT) Study: design of a randomised clinical trial. BMC Cancer 2010;10:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Waart 2014 high {published data only}
- Waart H, Stuiver MM, Sonke GS, Harten WH, Aaronson NK. Effect of low versus high intensity physical exercise during chemotherapy on physical fitness, fatigue and chemotherapy completion rates: Results of a randomized, controlled trial. Psycho‐Oncology 2014; Vol. 23:93.
- Waart H, Stuiver MM, Harten WH, Sonke GS, Aaronson NK. Design of the Physical exercise during Adjuvant Chemotherapy Effectiveness Study (PACES): A randomized controlled trial to evaluate effectiveness and cost‐effectiveness of physical exercise in improving physical fitness and reducing fatigue. BMC Cancer 2010;10:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Waart 2014 low {published data only}
- Waart H, Stuiver MM, Sonke GS, Harten WH, Aaronson NK. Effect of low versus high intensity physical exercise during chemotherapy on physical fitness, fatigue and chemotherapy completion rates: Results of a randomized, controlled trial. Psycho‐Oncology 2014; Vol. 23:93.
- Waart H, Stuiver MM, Harten WH, Sonke GS, Aaronson NK. Design of the Physical exercise during Adjuvant Chemotherapy Effectiveness Study (PACES): A randomized controlled trial to evaluate effectiveness and cost‐effectiveness of physical exercise in improving physical fitness and reducing fatigue. BMC Cancer 2010;10:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Visovsky 2014 {published and unpublished data}
- Visovsky C, Bovaird JA, Tofthagen C, Rice J. Heading Off Peripheral Neuropathy With Exercise: The HOPE Study. Nursing and Health 2014;2(6):115‐21. [Google Scholar]
Winningham 1988 {published data only}
- Winningham ML, MacVicar MG. The effect of aerobic exercise on patient reports of nausea. Oncology Nursing Forum 1988;15(4):447‐50. [PubMed] [Google Scholar]
Yang 2011 {published data only}
- Yang CY, Tsai JC, Huang YC, Lin CC. Effects of a home‐based walking program on perceived symptom and mood status in postoperative breast cancer women receiving adjuvant chemotherapy. Journal of Advanced Nursing 2011;67(1):158‐68. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aaronson 2011 {published data only}
- Aaronson TN, Duijts S, Beurden M, Hunter M, Oldenburg H. Cognitive behavioral therapy and physical exercise for climacteric symptoms in breast cancer patients experiencing treatment induced menopause: Final results of a multicenter randomized controlled trial. Psycho‐Oncology 2011;20:94‐5. [Google Scholar]
Adamsen 2009 {published data only}
- Adamsen L, Quist M, Andersen C, Moller T, Herrstedt J, Kronborg D, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: Randomised controlled trial. BMJ 2009;339(7726):895‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aghili 2007 {published data only}
- Aghili M, Farhan F, Rade M. A pilot study of the effects of programmed aerobic exercise on the severity of fatigue in cancer patients during external radiotherapy. European Journal of Oncology Nursing 2007;11(2):179‐82. [DOI] [PubMed] [Google Scholar]
Backman 2013 {published data only}
- Backman M, Wengstrom Y, Johansson B, Skoldengen I, Berglund A. Daily walking during adjuvant chemotherapy for patients with breast and colorectal cancer ‐ A randomized pilot study. European Journal of Cancer 2013;49:S397. [DOI] [PubMed] [Google Scholar]
Banasik 2011 {published data only}
- Banasik J, Williams H, Haberman M, Blank SE, Bendel R. Effect of Iyengar yoga practice on fatigue and diurnal salivary cortisol concentration in breast cancer survivors. Journal of the American Academy of Nurse Practitioners 2011;23(3):135‐42. [DOI] [PubMed] [Google Scholar]
Banerjee 2007 {published data only}
- Banerjee B, Vadiraj HS, Ram A, Rao R, Jayapal M, Gopinath KS, et al. Effects of an integrated yoga program in modulating psychological stress and radiation‐induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integrative Cancer Therapies 2007; Vol. 6, issue 3:242‐50. [DOI] [PubMed]
Basen 2006 {published data only}
- Basen EK, Taylor CL, Rosenblum C, Smith MA, Shinn EH, Greisinger A, et al. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Education and Counseling 2006;64:225‐34. [DOI] [PubMed] [Google Scholar]
Baumann 2009 {published data only}
- Baumann FT, Drosselmeyer N, Knicker A, Krakowski‐Roosen H, Schule K, Bloch W, et al. Effects of a 3‐month resistance training intervention on the cognitive abilities of breast cancer patients during chemotherapy. Deutsche Zeitschrift fur Onkologie 2009;41(2):70‐5. [Google Scholar]
Baumann 2011 {published data only}
- Baumann FT, Drosselmeyer N, Leskaroski A, Knicker A, Krakowski‐Roosen H, Zopf EM, et al. 12‐week resistance training with breast cancer patients during chemotherapy: Effects on cognitive abilities. Breast Care 2011;6(2):142‐3. [Google Scholar]
Bower 2012 {published data only}
- Bower JE, Garet D, Sternlieb B, Ganz PA, Irwin MR, Olmstead R, et al. Yoga for persistent fatigue in breast cancer survivors: A randomized controlled trial. Cancer 2012;118(15):3766‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burnham 2002 {published data only}
- Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Medicine & Science in Sports & Exercise 2002;34(12):1863‐7. [DOI] [PubMed] [Google Scholar]
Cantarero‐Villanueva 2012 {published data only}
- Cantarero‐Villanueva I, Fernandez‐Lao C, Fernandez‐De‐Las‐Penas C, Lopez‐Barajas IB, Del‐Moral‐Avila R, la‐Llave‐Rincon AI, et al. Effectiveness of water physical therapy on pain, pressure pain sensitivity, and myofascial trigger points in breast cancer survivors: a randomized, controlled clinical trial. Pain Medicine 2012;13(11):1509‐19. [DOI] [PubMed] [Google Scholar]
Cantarero‐Villanueva 2013 {published data only}
- Cantarero‐Villanueva I, Fernandez‐Lao C, Cuesta‐Vargas AI, Moral‐Avila R, Fernandez‐De‐Las‐Penas C, Arroyo‐Morales M. The effectiveness of a deep water aquatic exercise program in cancer‐related fatigue in breast cancer survivors: A randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2013;94(2):221‐30. [DOI] [PubMed] [Google Scholar]
Carson 2009 {published data only}
- Carson JW, Carson KM, Porter LS, Keefe FJ, Seewaldt VL. Yoga of Awareness program for menopausal symptoms in breast cancer survivors: results from a randomized trial. Supportive Care in Cancer 2009;17(10):1301‐9. [DOI] [PubMed] [Google Scholar]
Charbonnier 2012 {published data only}
- Charbonnier J, Levy A, Guichard JB, Garet M, Auberdiac P, Roche F, et al. Establishment of a pilot study of awareness retraining in physical activity in two selected populations of patients with breast cancer [French] [Mise en place d'une étude pilote de sensibilisation au rëentraînement à l'activité physique dans deux populations sélectionnées atteintes d'un cancer du sein]. Bulletin du Cancer 2012;99(7‐8):753‐9. [DOI] [PubMed] [Google Scholar]
Chetiyawardana 2004 {published data only}
- Chetiyawardana AD. A pilot study to investigate the effects on fitness and quality of life of an individualised exercise programme for breast cancer patients undergoing radiotherapy. http://www.controlled‐trials.com/ISRCTN26140710 (accessed 20 March 2015).
Courneya 2003a {published data only}
- Courneya KS, Friedenreich CM, Sela RA, Quinney HA, Rhodes RE, Handman M. The group psychotherapy and home‐based physical exercise (group‐hope) trial in cancer survivors: physical fitness and quality of life outcomes. Psycho‐Oncology 2003;12(4):357‐74. [DOI] [PubMed] [Google Scholar]
Courneya 2003b {published data only}
- Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. Journal of Clinical Oncology 2003;21(9):1660‐8. [DOI] [PubMed] [Google Scholar]
Courneya 2006 {published data only}
- Courneya KS, Jones LW, Mackey JR, Fairey AS. Exercise beliefs of breast cancer survivors before and after participation in a randomized controlled trial. International Journal of Behavioral Medicine 2006;13(3):259‐64. [DOI] [PubMed] [Google Scholar]
Culos‐Reed 2006 {published data only}
- Culos‐Reed SN, Carlson LE, Daroux LM, Hately‐Aldous S. A pilot study of yoga for breast cancer survivors: physical and psychological benefits. Psycho‐Oncology 2006;15(10):891‐7. [DOI] [PubMed] [Google Scholar]
Danhauer 2009 {published data only}
- Danhauer SC, Mihalko SL, Russell GB, Campbell CR, Felder L, Daley K, et al. Restorative yoga for women with breast cancer: findings from a randomized pilot study. Psycho‐Oncology 2009;18(4):360‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demark‐Wahnefried 2002 {published data only}
- Demark‐Wahnefried W. Randomized study of a diet and exercise‐based counseling program versus a standard counseling program for patients with early‐stage breast or prostate cancer. http://www.clinicaltrials.gov/ct/show/NCT00044980?order=128 (accessed 8 May 2002). [Ref Type: Unpublished Work]
Demark‐Wahnefried 2003 {published data only}
- Demark‐Wahnefried W, Morey MC, Clipp EC, Pieper CF, Snyder DC, Sloane R, et al. Leading the way in exercise and diet (Project LEAD): intervening to improve function among older breast and prostate cancer survivors. Controlled Clinical Trials 2003;24(2):206‐23. [DOI] [PubMed] [Google Scholar]
Demark‐Wahnefried 2005 {published data only}
- Demark‐Wahnefried W, Morey MC, Clipp EC, Snyder DC, Sloane R, Pieper F, et al. Results of Project LEAD (Leading the Way in Exercise and Diet) ‐ A trial testing an intervention of telephone‐counseling and mailed materials in improving physical functioning among older breast and prostate cancer survivors. Journal of Clinical Oncology 2005;23(16 Suppl):763S. [Google Scholar]
Demark‐Wahnefried 2006 {published data only}
- Demark‐Wahnefried W, Clipp EC, Morey MC, Pieper CF, Sloane R, Snyder DC, et al. Lifestyle intervention development study to improve physical function in older adults with cancer: Outcomes from Project LEAD. Journal of Clinical Oncology 2006;24:3465‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Demark‐Wahnefried 2008 {published data only}
- Demark‐Wahnefried W, Case LD, Blackwell K, Marcom PK, Kraus W, Aziz N, et al. Results of a diet/exercise feasibility trial to prevent adverse body composition change in breast cancer patients on adjuvant chemotherapy. Clinical Breast Cancer 2008;8:70‐9. [DOI] [PubMed] [Google Scholar]
Dimeo 1999 {published data only}
- Dimeo FC, Stieglitz RD, Novelli‐Fischer U, Fetscher S, Keul J. Effects of physical activity on the fatigue and psychologic status of cancer patients during chemotherapy. Cancer 1999;85(10):2273‐7. [PubMed] [Google Scholar]
Duijts 2009 {published data only}
- Duijts SF, Oldenburg HS, van BM, Aaronson NK. Cognitive behavioral therapy and physical exercise for climacteric symptoms in breast cancer patients experiencing treatment‐induced menopause: design of a multicenter trial. BMC Women's Health 2009;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duijts 2012 {published data only}
- Duijts SFA, Beurden M, Oldenburg HSA, Hunter MS, Kieffer JM, Stuiver MM, et al. Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment‐induced menopausal symptoms in patients with breast cancer: Results of a randomized, controlled, multicenter trial. Journal of Clinical Oncology 2012;30(33):4124‐33. [DOI] [PubMed] [Google Scholar]
Emami 2012 {published data only}
- Emami H, Rahnama N, Nuri R, Damirchi A, Rahmani‐Nia F, fshar‐Nejad T. Effect of combination exercise training on sex hormone binding globulin in postmenopausal women with breast cancer. Gazzetta Medica Italiana Archivio per le Scienze Mediche 2012;171:633‐8. [Google Scholar]
Ergun 2013 {published data only}
- Ergun M, Eyigor S, Karaca B, Kisim A, Uslu R. Effects of exercise on angiogenesis and apoptosis‐related molecules, quality of life, fatigue and depression in breast cancer patients. European Journal of Cancer Care 2013;22(5):626‐37. [DOI] [PubMed] [Google Scholar]
Fairey 2003 {published data only}
- Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Effects of exercise training on fasting insulin, insulin resistance, insulin‐like growth factors, and insulin‐like growth factor binding proteins in postmenopausal breast cancer survivors: a randomized controlled trial. Cancer Epidemiology, Biomarkers & Prevention 2003;12(8):721‐7. [PubMed] [Google Scholar]
Fairey 2005 {published data only}
- Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. Journal of Applied Physiology 2005;98(4):1534‐40. [DOI] [PubMed] [Google Scholar]
Fairey 2005a {published data only}
- Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Martin BS, et al. Effect of exercise training on C‐reactive protein in postmenopausal breast cancer survivors: a randomized controlled trial. Brain, Behavior, and Immunity 2005;19(5):381‐8. [DOI] [PubMed] [Google Scholar]
Fernandez 2013 {published data only}
- Fernandez‐Lao C, Cantarero‐Villanueva I, Ariza‐Garcia A, Courtney C, Fernandez‐de‐las‐Penas C, Arroyo‐Morales M. Water versus land‐based multimodal exercise program effects on body composition in breast cancer survivors: A controlled clinical trial. Supportive Care in Cancer 2013;21:521‐30. [DOI] [PubMed] [Google Scholar]
Galantino 2010 {published data only}
- Galantino ML, Schmid P, Botis S, Dagan C, Leonard SM, Milos A. Exploring wellness coaching and traditional group support for breast cancer survivors: A pilot study. Rehabilitation Oncology 2010;28(1):19‐25. [Google Scholar]
Given 2002 {published data only}
- Given B, Given C, McCorkle R, Kozachik S, Cimprich B, Rahbar M, et al. Pain and fatigue management: results of a nursing randomized clinical trial. Oncology Nursing Forum 2002;29(6):949‐56. [DOI] [PubMed] [Google Scholar]
Gomes 2011 {published data only}
- Gomes A, Fernandes EL, Carvalho MP, Fabricio VC. Effectiveness of brief exercise orientation program on breast cancer induced fatigue. European Journal of Cancer 2011;47:S238‐9. [Google Scholar]
Gomez 2011 {published data only}
- Gómez AM, Martínez C, Fiuza‐Luces C, Herrero F, Pérez M, Madero L, et al. Exercise training and cytokines in breast cancer survivors. International Journal of Sports Medicine 2011;32(6):461‐7. [DOI] [PubMed] [Google Scholar]
Griffith 2009 {published data only}
- Griffith K, Wenzel J, Shang J, Thompson C, Stewart K, Mock V. Impact of a walking intervention on cardiorespiratory fitness, self‐reported physical function, and pain in patients undergoing treatment for solid tumors. Cancer 2009;115(20):4874‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mock V, Krumm S, Belcher A, Stewart K, DeWeese T, Shang J, et al. Exercise during prostate cancer treatment: effects on functional status and symptoms. Oncology Nursing Forum 2007;34(1):189–90. [Google Scholar]
- Mock V, Ours C, Hall S, Bositis A, Tillery M, Belcher A, et al. Using a conceptual model in nursing research ‐ mitigating fatigue in cancer patients. Journal of Advanced Nursing 2007;58(5):503‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel JA, Griffith KA, Shang J, Thompson CB, Hedlin H, Stewart KJ, et al. Impact of a home‐based walking intervention on outcomes of sleep quality, emotional distress, and fatigue in patients undergoing treatment for solid tumors. The Oncologist 2013;18(4):476‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann 2013 {published data only}
- Hartmann I, Lethan C, Dalvad E, Koehler U, Sigaard L, Dieperink K. Fatigue in breast cancer patients undergoing curative radiotherapy treatment. Supportive Care in Cancer 2013;21:S60. [Google Scholar]
Hatchett 2013 {published data only}
- Hatchett A, Hallam JS, Ford MA. Evaluation of a social cognitive theory‐based email intervention designed to influence the physical activity of survivors of breast cancer. Psycho‐Oncology 2013;22(4):829‐36. [DOI] [PubMed] [Google Scholar]
Herrero 2006 {published data only}
- Herrero F, San Juan AF, Fleck SJ, Balmer J, Perez M, Canete S, et al. Combined aerobic and resistance training in breast cancer survivors: A randomized, controlled pilot trial. International Journal of Sports Medicine 2006;27(7):573‐80. [DOI] [PubMed] [Google Scholar]
Ho 1986 {published data only}
- Ho C. Psychological adaptation and coping resources of breast cancer patients: Comparisons across three treatment modalities. Dissertation‐Abstracts‐International. Dissertation‐Abstracts‐International, Vol 47 (6‐B): 2617, 1986; Vol. 47:2617.
Huang 2014 {published data only}
- Huang HP, Wen FH, Tsai JC, Lin YC, Shun SC, Chang HK, et al. Adherence to prescribed exercise time and intensity declines as the exercise program proceeds: findings from women under treatment for breast cancer. Supportive Care in Cancer 2015;23(7):2061‐71. [DOI] [PubMed] [Google Scholar]
Irwin 2008 {published data only}
- Irwin ML, Cadmus L, Alvarez‐Reeves M, O'Neil M, Mierzejewski E, Latka R, et al. Recruiting and retaining breast cancer survivors into a randomized controlled exercise trial: the Yale Exercise and Survivorship Study. Cancer 2008;112(11 Suppl):606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Irwin 2009 {published data only}
- Irwin ML, Alvarez‐Reeves M, Cadmus L, Mierzejewski E, Mayne ST, Yu H, et al. Exercise improves body fat, lean mass, and bone mass in breast cancer survivors. Obesity 2009;17(8):1534‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Irwin 2009a {published data only}
- Irwin ML, Varma K, Alvarez‐Reeves M, Cadmus L, Wiley A, Chung GG, et al. Randomized controlled trial of aerobic exercise on insulin and insulin‐like growth factors in breast cancer survivors: the Yale Exercise and Survivorship study. Cancer Epidemiology, Biomarkers & Prevention 2009;18(1):306‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Janelsins 2011 {published data only}
- Janelsins MC, Davis PG, Wideman L, Katula JA, Sprod LK, Peppone LJ, et al. Effects of Tai Chi Chuan on insulin and cytokine levels in a randomized controlled pilot study on breast cancer survivors. Clinical Breast Cancer 2011;11(3):161‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2006 {published data only}
- Kim CJ, Kang DH, Smith BA, Landers KA. Cardiopulmonary responses and adherence to exercise in women newly diagnosed with breast cancer undergoing adjuvant therapy. Cancer Nursing 2006;29(2):156‐65. [DOI] [PubMed] [Google Scholar]
Kleine‐Tebbe 2006 {unpublished data only}
- Kleine‐Tebbe A. [Untersuchung zum Einfluss eines Bewegungstrainings auf die Leistungsfähigkeit, die Lebensqualität und den Immunstatus, sowie die Rezidivrate im Vergleich zu einem Entspannungsverfahren bei Frauen mit Mammakarzinom während der adjuvanten Chemotherapie] (Influence of exercise training vs. relaxation training on performance, quality of life, immune status and rate of recurrence in women with breast cancer during adjuvant chemotherapy)]. Personal communication February 2006 and September 2013.
Kohler 2008 {published data only}
- Kohler N, Damm F, Bauer K, Klee C, Rach S, Lintz D, et al. Supportive effects of moderate physical exercise during cytotoxic/endocrine treatment of breast cancer patients ‐ Results of a pilot study. Geburtshilfe und Frauenheilkunde 2008;68(8):805‐13. [Google Scholar]
Kovacic 2011 {published data only}
- Kovacic T, Kovacic M. Impact of relaxation training according to yoga in daily life (registered trademark) system on self‐esteem after breast cancer surgery. Journal of Alternative and Complementary Medicine 2011;17(12):1157‐64. [DOI] [PubMed] [Google Scholar]
Latikka 1997 {published data only}
- Latikka P, Pukkala E, Vihko V. Exercise and breast cancer. Duodecim 1997;113(4):317‐22. [PubMed] [Google Scholar]
Latka 2009 {published data only}
- Latka RN, varez‐Reeves M, Cadmus L, Irwin ML. Adherence to a randomized controlled trial of aerobic exercise in breast cancer survivors: the Yale exercise and survivorship study. Journal of Cancer Survivorship 2009;3(3):148‐57. [DOI] [PubMed] [Google Scholar]
Lauridsen 2005 {published data only}
- Lauridsen MC, Christiansen P, Hessov I. The effect of physiotherapy on shoulder function in patients surgically treated for breast cancer: a randomized study. Acta Oncologica 2005;44(5):449‐57. [DOI] [PubMed] [Google Scholar]
Lee 2006 {published data only}
- Lee TI, Chen HH, Yeh ML. Effects of chan‐chuang qigong on improving symptom and psychological distress in chemotherapy patients. The American Journal of Chinese Medicine 2006;34(1):37‐46. [DOI] [PubMed] [Google Scholar]
Ligibel 2006 {published data only}
- Ligibel JA, Chen W, Keshaviah A, Adloff K, Partridge A, Salinardi T, et al. The impact of an exercise intervention on body composition, fat distribution, and weight in breast cancer survivors. Journal of Clinical Oncology 2006;24(18 Suppl):25s‐s. [DOI] [PubMed] [Google Scholar]
Ligibel 2008 {published data only}
- Ligibel JA, Campbell N, Partridge A, Chen WY, Salinardi T, Chen H, et al. Impact of a mixed strength and endurance exercise intervention on insulin levels in breast cancer survivors. Journal of Clinical Oncology 2008;26(6):907‐12. [DOI] [PubMed] [Google Scholar]
Ligibel 2009 {published data only}
- Ligibel JA, Giobbie‐Hurder A, Olenczuk D, Campbell N, Salinardi T, Winer EP, et al. Impact of a mixed strength and endurance exercise intervention on levels of adiponectin, high molecular weight adiponectin and leptin in breast cancer survivors. Cancer Causes & Control 2009;20(8):1523‐8. [DOI] [PubMed] [Google Scholar]
MacVicar 1986 {published data only}
- MacVicar MG, Winningham ML. Promoting the functional capacity of cancer patients. Cancer Bulletin 1986;38:235‐9. [Google Scholar]
Mamom 2012 {published data only}
- Mamom J. Effectiveness of an education‐combining exercise programme for chemotherapy‐related fatigue in women with breast cancer. European Journal of Cancer 2012;48:S6. [Google Scholar]
Martin 2013 {published data only}
- Martin E, Battaglini C, Groff D, Naumann F. Improving muscular endurance with the MVe Fitness Chair in breast cancer survivors: a feasibility and efficacy study. Journal of Science & Medicine in Sport 2013;16(4):372‐6. [DOI] [PubMed] [Google Scholar]
Maryam 2010 {published data only}
- Maryam A, Fazlollah A, Eesa M, Ebrahim H, Abbas VF. The effect of designed exercise programme on quality of life in women with breast cancer receiving chemotherapy. Scandinavian Journal of Caring Sciences 2010;24:251‐8. [DOI] [PubMed] [Google Scholar]
McGuire 2011 {published data only}
- McGuire R, Waltman N, Zimmerman L. Intervention components promoting adherence to strength training exercise in breast cancer survivors with bone loss. Western Journal of Nursing Research 2011;33(5):671‐89. [DOI] [PubMed] [Google Scholar]
McKenzie 2003 {published data only}
- McKenzie DC, Kalda AL. Effect of upper extremity exercise on secondary lymphedema in breast cancer patients: a pilot study. Journal of Clinical Oncology 2003;21(3):463‐6. [DOI] [PubMed] [Google Scholar]
Milecki 2013 {published data only}
- Milecki P, Hojan K, Ozga‐Majchrzak O, Molinska‐Glura M. Exercise tolerance in breast cancer patients during radiotherapy after aerobic training. Wspolczesna Onkologia 2013;17(2):205‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moadel 2007 {published data only}
Mock 1994 {published data only}
- Mock V, Burke MB, Sheehan P, Creaton EM, Winningham ML, McKenney Tedder S, et al. A nursing rehabilitation program for women with breast cancer receiving adjuvant chemotherapy. Oncology Nursing Forum 1994;21(5):899‐907. [PubMed] [Google Scholar]
Mock 1997 {published data only}
- Mock V, Dow KH, Meares CJ, Grimm PM, Dienemann JA, Haisfield Wolfe ME, et al. Effects of exercise on fatigue, physical functioning, and emotional distress during radiation therapy for breast cancer. Oncology Nursing Forum 1997;24(6):991‐1000. [PubMed] [Google Scholar]
Mock 2001 {published data only}
- Mock V, Pickett M, Ropka ME, Muscari Lin E, Stewart KJ, Rhodes VA, et al. Fatigue and quality of life outcomes of exercise during cancer treatment. Cancer Practice 2001;9(3):119‐27. [DOI] [PubMed] [Google Scholar]
Mock 2002 {published data only}
- Mock V. Fatigue and physical functioning during breast cancer treatment. Oncology Nursing Forum 2002;29:338. [PubMed] [Google Scholar]
Moller 2013 {published data only}
- Moller T, Lillelund C, Andersen C, Ejlertsen B, Norgaard L, Christensen KB, et al. A randomised feasibility study in patients with colon and breast cancer. BMJ Open 2013;3(11):e003556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller T, Lillelund C, Andersen C, Bloomquist K, Christensen KB, Ejlertsen B, et al. The challenge of preserving cardiorespiratory fitness in physically inactive patients with colon or breast cancer during adjuvant chemotherapy: a randomised feasibility study. BMJ Open Sport & Exercise Medicine 2015;1(1):e000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulero 2008 {published data only}
- Mulero Portela AL, Colon Santaella CL, Cruz Gomez C, Burch A. Feasibility of an exercise program for Puerto Rican women who are breast cancer survivors. Rehabilitation Oncology 2008;26(2):20‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Murtezani 2014 {published data only}
- Murtezani A, Ibraimi Z, Bakalli A, Krasniqi S, Disha ED, Kurtishi I. The effect of aerobic exercise on quality of life among breast cancer survivors: A randomized controlled trial. Journal of Cancer Research and Therapeutics 2014;10(3):658. [DOI] [PubMed] [Google Scholar]
Musanti 2012 {published data only}
- Musanti R. A study of exercise modality and physical self‐esteem in breast cancer survivors. Medicine & Science in Sports & Exercise 2012;44(2):352‐61. [DOI] [PubMed] [Google Scholar]
Mustian 2002 {published data only}
- Mustian KM, Katula JA, Gill DL. Exercise: complementary therapy for breast cancer rehabilitation. In: Hall RL editor(s). Exercise and Sport in Feminist Therapy: Constructing Modalities and Assessing Outcomes. New York: Haworth Press, 2002:105‐18. [Google Scholar]
Mustian 2006 {published data only}
- Mustian KM, Morrow GR, Yates J, Gillies L, Boles C. A randomized controlled pilot of home‐based exercise (HBEX) versus standard care (SC) among breast cancer (BC) and prostate cancer (PC) patients receiving radiation therapy (RTH). Journal of Clinical Oncology 2006;24(18 Suppl):8504. [Google Scholar]
Pickett 2002 {published data only}
- Pickett M, Mock V, Ropka ME, Cameron L, Coleman M, Podewils L. Adherence to moderate‐intensity exercise during breast cancer therapy. Cancer Practice 2002;10(6):284‐92. [DOI] [PubMed] [Google Scholar]
Pinto 2003 {published data only}
- Pinto BM, Clark MM, Maruyama NC, Feder SI. Psychological and fitness changes associated with exercise participation among women with breast cancer. Psycho‐Oncology 2003;12(2):118‐26. [DOI] [PubMed] [Google Scholar]
Pinto 2008 {published data only}
- Pinto BM, Rabin C, Papandonatos GD, Frierson GM, Trunzo JJ, Marcus BH. Maintenance of effects of a home‐based physical activity program among breast cancer survivors. Supportive Care in Cancer 2008;16(11):1279‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinto 2009 {published data only}
- Pinto BM, Rabin C, Dunsiger S. Home‐based exercise among cancer survivors: adherence and its predictors. Psycho‐Oncology 2009;18(4):369‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rabin 2006 {published data only}
- Rabin C, Pinto B, Frierson G. Mediators of a randomized controlled physical activity intervention for breast cancer survivors. Journal Sport Exercise Psychology 2006;28:269‐84. [Google Scholar]
Raghavendra 2007 {published data only}
- Raghavendra RM, Nagarathna R, Nagendra HR, Gopinath KS, Srinath BS, Ravi BD, et al. Effects of an integrated yoga programme on chemotherapy‐induced nausea and emesis in breast cancer patients. European Journal of Cancer Care 2007;16(6):462‐74. [DOI] [PubMed] [Google Scholar]
Rahnama 2010 {published data only}
- Rahnama N, Nouri R, Rahmaninia F, Damirchi A, Emami H. The effects of exercise training on maximum aerobic capacity, resting heart rate, blood pressure and anthropometric variables of postmenopausal women with breast cancer. Journal of Research in Medical Sciences 2010;15(2):78‐83. [PMC free article] [PubMed] [Google Scholar]
Rao 2008 {published data only}
- Rao RM, Telles S, Nagendra HR, Nagarathna R, Gopinath K, Srinath S, et al. Effects of yoga on natural killer cell counts in early breast cancer patients undergoing conventional treatment. Comment to: recreational music‐making modulates natural killer cell activity, cytokines, and mood states in corporate employees Masatada Wachi, Masahiro Koyama, Masanori Utsuyama, Barry B. Bittman, Masanobu Kitagawa, Katsuiku Hirokawa Med Sci Monit, 2007; 13(2): CR57‐70. Medical Science Monitor 2008;14(2):LE3‐4. [PubMed] [Google Scholar]
Rao 2009 {published data only}
- Rao MR, Raghuram N, Nagendra HR, Gopinath KS, Srinath BS, Diwakar RB, et al. Anxiolytic effects of a yoga program in early breast cancer patients undergoing conventional treatment: a randomized controlled trial. Complementary Therapies in Medicine 2009;17(1):1‐8. [DOI] [PubMed] [Google Scholar]
Rogers 2011 {published data only}
- Rogers LQ, Markwell S, Hopkins‐Price P, Vicari S, Courneya KS, Hoelzer K, et al. Reduced barriers mediated physical activity maintenance among breast cancer survivors. Journal of Sport & Exercise Psychology 2011;33(2):235‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sandel 2005 {published data only}
- Sandel SL, Judge JO, Landry N, Faria L, Ouellette R, Majczak M. Dance and movement program improves quality‐of‐life measures in breast cancer survivors. Cancer Nursing 2005;28(4):301‐9. [DOI] [PubMed] [Google Scholar]
Schmitz 2005 {published data only}
- Schmitz KH, Ahmed RL, Hannan PJ, Yee D. Safety and efficacy of weight training in recent breast cancer survivors to alter body composition, insulin, and insulin‐like growth factor axis proteins. Cancer Epidemiology, Biomarkers & Prevention 2005;14(7):1672‐80. [DOI] [PubMed] [Google Scholar]
Schwartz 1999 {published data only}
- Schwartz AL. Fatigue mediates the effects of exercise on quality of life. Quality of Life Research 1999;8(6):529‐38. [DOI] [PubMed] [Google Scholar]
Schwartz 2001 {published data only}
- Schwartz AL, Mori M, Gao R, Nail LM, King ME. Exercise reduces daily fatigue in women with breast cancer receiving chemotherapy. Medicine & Science in Sports & Exercise 2001;33(5):718‐23. [DOI] [PubMed] [Google Scholar]
Scott 2013 {published data only}
- Scott E, Daley AJ, Doll H, Woodroofe N, Coleman RE, Mutrie N. Effects of an exercise and hypocaloric healthy eating program on biomarkers associated with long‐term prognosis after early‐stage breast cancer: a randomized controlled trial. Cancer Causes & Control 2013;24:181‐91. [DOI] [PubMed] [Google Scholar]
Segar 1998 {published data only}
- Segar ML, Katch VL, Roth RS, Garcia AW, Portner TI, Glickman SG, et al. The effect of aerobic exercise on self‐esteem and depressive and anxiety symptoms among breast cancer survivors. Oncology Nursing Forum 1998;25(1):107‐13. [PubMed] [Google Scholar]
Shaw 2003 {published data only}
- Shaw E, Demark‐Wahnefried W, Andersen R. STRENGTH (Survival TRaining for ENhancing Total Health): Phase II randomized pilot study of distance medicine‐based exercise and dietary approach to prevent body composition change during adjuvant chemotherapy in patients with stage I, II or IIIA breast cancer. http://www.clinicaltrials.gov/ct/show/NCT00068458?order=47 (accessed 1 October 2003).
So 2006 {published data only}
Sprod 2012 {published data only}
- Sprod LK, Janelsins MC, Palesh OG, Carroll JK, Heckler CE, Peppone LJ, et al. Health‐related quality of life and biomarkers in breast cancer survivors participating in Tai chi chuan. Journal of Cancer Survivorship 2012;6(2):146‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stevinson 2009 {published data only}
- Stevinson C. Inconclusive trial on yoga for anxiety among breast cancer patients: Commentary. Focus on Alternative and Complementary Therapies 2009;14(2):123‐4. [Google Scholar]
Swenson 2009 {published data only}
- Swenson KK, Nissen MJ, Anderson E, Shapiro A, Schousboe J, Leach J. Effects of exercise vs bisphosphonates on bone mineral density in breast cancer patients receiving chemotherapy. The Journal of Supportive Oncology 2009;7(3):101‐7. [PubMed] [Google Scholar]
Swenson 2010 {published data only}
- Swenson KK, Nissen MJ, Henly SJ. Physical activity in women receiving chemotherapy for breast cancer: adherence to a walking intervention. Oncology Nursing Forum 2010;37(3):321‐30. [DOI] [PubMed] [Google Scholar]
Twiss 2009 {published data only}
- Twiss JJ, Waltman NL, Berg K, Ott CD, Gross GJ, Lindsey AM. An exercise intervention for breast cancer survivors with bone loss. Journal of Nursing Scholarship 2009;41(1):20‐7. [DOI] [PubMed] [Google Scholar]
Vadiraja 2009 {published data only}
- Vadiraja HS, Rao MR, Nagarathna R, Nagendra HR, Rekha M, Vanitha N, et al. Effects of yoga program on quality of life and affect in early breast cancer patients undergoing adjuvant radiotherapy: a randomized controlled trial. Complementary Therapies in Medicine 2009;17(5‐6):274‐80. [DOI] [PubMed] [Google Scholar]
Vadiraja 2010 {published data only}
- Vadiraja HS, Rao MR, Nagarathna R, Nagendra HR, Rekha M, Vanitha N, et al. Yoga leads to improved QoL in breast cancer patients receiving radiotherapy. Focus on Alternative and Complementary Therapies 2010;15(2):149‐50. [Google Scholar]
Vallance 2007 {published data only}
- Vallance JK, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. Journal of Clinical Oncology 2007;25(17):2352‐9. [DOI] [PubMed] [Google Scholar]
Vallance 2008 {published data only}
- Vallance JK, Courneya KS, Plotnikoff RC, Dinu I, Mackey JR. Maintenance of physical activity in breast cancer survivors after a randomized trial. Medicine & Science in Sports & Exercise 2008;40(1):173‐80. [DOI] [PubMed] [Google Scholar]
Vallance 2008a {published data only}
- Vallance JK, Courneya KS, Plotnikoff RC, Mackey JR. Analyzing theoretical mechanisms of physical activity behavior change in breast cancer survivors: results from the activity promotion (ACTION) trial. Annals of Behavioral Medicine 2008;35(2):150‐8. [DOI] [PubMed] [Google Scholar]
Vincent 2013 {published data only}
- Vincent F, Labourey JL, Leobon S, Antonini MT, Lavau‐Denes S, Tubiana‐Mathieu N. Effects of a home‐based walking training program on cardiorespiratory fitness in breast cancer patients receiving adjuvant chemotherapy: a pilot study. European Journal of Physical and Rehabilitation Medicine 2013;49(3):319‐29. [PubMed] [Google Scholar]
Waltman 2010 {published data only}
- Waltman NL, Twiss JJ, Ott CD, Gross GJ, Lindsey AM, Moore TE, et al. The effect of weight training on bone mineral density and bone turnover in postmenopausal breast cancer survivors with bone loss: A 24‐month randomized controlled trial. Osteoporosis International 2010;21(8):1361‐9. [DOI] [PubMed] [Google Scholar]
Wang 2010 {published data only}
- Wang YJ. Effects of a six‐week home‐based walking program on Taiwanese women newly diagnosed with early stage breast cancer [PhD thesis]. Buffalo, USA: State University of New York at Buffalo, 2010:143. [Google Scholar]
- Wang YJ, Boehmke M, Wu YWB, Dickerson SS, Fisher N. Effects of a 6‐week walking program on Taiwanese women newly diagnosed with early‐stage breast cancer. Cancer Nursing 2011;34(2):E1‐13. [DOI] [PubMed] [Google Scholar]
Wilkie 2003 {published data only}
- Wilkie DJ, Schwartz AL, Huang H‐Y, Ko N‐Y, Liao W‐C, Hairabedian D, et al. Innovations in Cancer Communication. Proceedings of the American Public Health Association, 131st Annual Meeting; 2003 Nov 15‐19; San Francisco. San Francisco: American Public Health Association, 2003.
Yeh 2006 {published data only}
- Yeh ML, Lee TI, Chen HH, Chao TY. The influences of Chan‐Chuang Qi‐gong therapy on complete blood cell counts in breast cancer patients treated with chemotherapy. Cancer Nursing 2006;29(2):149‐55. [DOI] [PubMed] [Google Scholar]
Yuen 2007 {published data only}
- Yuen HK, Sword D. Home‐based exercise to alleviate fatigue and improve functional capacity among breast cancer survivors. Journal of Allied Health 2007;36(4):e257‐75. [PubMed] [Google Scholar]
References to studies awaiting assessment
Lotzke 2016 {published data only}
- Lotzke D, Wiedemann F, Rodrigues Recchia D, Ostermann T, Sattler D, Ettl J, et al. Iyengar‐yoga compared to exercise as a therapeutic intervention during (neo)adjuvant therapy in women with stage I‐III breast cancer: health‐related quality of life, mindfulness, spirituality, life satisfaction, and cancer‐related fatigue. Evidence‐Based Complementary and Alternative Medicine 2016;2016:5931816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann F, Buessing A, Halle M, Kiechle M, Kohls N, Ostermann T, et al. Iyengar yoga compared to exercise in women with stage I‐III breast cancer: Feasibility of therapeutic interventions during adjuvant cytotoxic or endocrine therapy. Journal of Clinical Oncology 2013;31(15):e20623. [CRSREF: 3244155] [Google Scholar]
Petrella 2012 {published data only}
- Petrella TM, Laredo S, Oh P, Marzolini S, Warner E, Dent R, et al. A pilot study evaluating the benefits and feasibility of an exercise program for breast cancer patients receiving adjuvant chemotherapy. Cancer Research 2012;72(24):P2‐12. [Google Scholar]
References to ongoing studies
ACTRN12614000051640 {published data only}
- ACTRN12614000051640. Preventing ‘chemo‐brain’: Can exercise mitigate chemotherapy‐induced cognitive impairment in breast cancer patients?. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365594 (accessed 28 October 2015).
NCT01943695 {published data only}
- NCT01943695. Supervised aerobic training during or after adjuvant therapy for operable breast cancer. clinicaltrials.gov/ct2/show/NCT01943695 (accessed 28 October 2015).
NCT02117011 {published data only}
- NCT02117011. Effects of a structured exercise program on cancer‐related fatigue in women receiving radiation therapy for breast cancer. clinicaltrials.gov/ct2/show/NCT02117011 (accessed 28 October 2015).
NCT02159157 {published data only}
- NCT02159157. A randomized, controlled trial to determine the effects of an exercise intervention on physical activity during chemotherapy for patients with early stage breast cancer. clinicaltrials.gov/ct2/show/NCT02159157 (accessed 28 October 2015).
NCT02240836 {published data only}
- NCT02240836. Energy Balance and Breast Cancer Aspects‐II (EBBA‐II). clinicaltrials.gov/ct2/show/NCT02240836 (accessed 28 October 2015).
NCT02252991 {published data only}
- NCT02252991. Adaptated Physical Activity in Cancerology (APACAN). clinicaltrials.gov/ct2/show/study/NCT02252991 (accessed 28 October 2015).
NCT02350582 {published data only}
- NCT02350582. e‐CUIDACHEMO: Telerehabilitation during chemotherapy in breast cancer. clinicaltrials.gov/ct2/show/study/NCT02350582 (accessed 28 October 2015).
Additional references
ACSM 2000
- Franklin BA, Whaley MH (editors). ACSM's Guidelines for Exercise Testing and Prescription. 6th Edition. Philadelphia: Lippincott Williams & Wilkins, 2000. [Google Scholar]
Bourke 2014
- Bourke L, Homer KE, Thaha MA, Steed L, Rosario DJ, Robb KA, et al. Interventions to improve exercise behaviour in sedentary people living with and beyond cancer: a systematic review. British Journal of Cancer 2014;110(4):831‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2015
- Brown LC, Mutter RW, Halyard MY. Benefits, risks, and safety of external beam radiation therapy for breast cancer. International Journal of Women's Health 2015;7:449‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carayol 2013
- Carayol M, Bernard P, Boiche J, Riou F, Mercier B, Cousson‐Gelie F, et al. Psychological effect of exercise in women with breast cancer receiving adjuvant therapy: what is the optimal dose needed?. Annals of Oncology 2013;24(2):291‐300. [DOI] [PubMed] [Google Scholar]
Colcombe 2003
- Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta‐analytic study. Psychological Science 2003;14(2):125‐30. [DOI] [PubMed] [Google Scholar]
Courneya 2007
- Courneya KS, Segal RJ, Mackey JR, Gelmon K, Reid RD, Friedenreich CM, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. Journal of Clinical Oncology 2007;25(28):4396‐404. [DOI] [PubMed] [Google Scholar]
Craig 2008
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M, et al. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:a1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cramp 2012
- Cramp F, Byron‐Daniel J. Exercise for the management of cancer‐related fatigue in adults. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD006145.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Jong 2002
- Jong N, Courtens AM, Abu‐Saad HH, Schouten HC. Fatigue in patients with breast cancer receiving adjuvant chemotherapy: a review of the literature. Cancer Nursing 2002;25(4):283‐97. [DOI] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Higgins, JPT, Altman DG (editors). Chapter 8: Analysing and presenting results. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 4.2.5 [updated May 2005]. The Cochrane Collaboration, 2005. Available from www.cochrane‐handbook.org.
Gargon 2014
- Gargon E, Gurung B, Medley N, Altman DG, Blazeby JM, Clarke M, et al. Choosing important health outcomes for comparative effectiveness research: a systematic review. PLoS ONE 2014;9(6):e99111. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEproGDT
- GRADEproGDT: GRADEpro Guideline Development Tool [Software]. McMaster University, 2015 (developed by Evidence Prime, Inc). Available from www.gradepro.org.
Hayes 2013
- Hayes SC, Rye S, DiSipio T, Yates P, Bashford J, Pyke C. Exercise for health: A randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment‐related side effects following breast cancer. Breast Cancer Research and Treatment 2013;137(1):175‐86. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holley 2000
- Holley S. Cancer‐related fatigue. Suffering a different fatigue. Cancer Practice 2000;8(2):87‐95. [DOI] [PubMed] [Google Scholar]
Ioannidis 2004
- Ioannidis JPA, Evans SJW, Gøtzsche PC, O’Neill RT, Altman DG, Schulz K, et al. Better Reporting of Harms in Randomized Trials: An Extension of the CONSORT Statement. Annals of Internal Medicine 2004;141(10):781‐7. [DOI] [PubMed] [Google Scholar]
Irwin 2003
- Irwin ML, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer 2003;97(7):1746‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jereczek‐Fossa 2001
- Jereczek‐Fossa BA, Marsiglia HR, Orecchia R. Radiotherapy‐related fatigue: how to assess and how to treat the symptom. A commentary. Tumori 2001;87(3):147‐51. [DOI] [PubMed] [Google Scholar]
Lee 2011
- Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ‐SF): a systematic review. International Journal of Behavioral Nutrition and Physical Activity 2011;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meneses‐Echavez 2015
- Meneses‐Echavez JF, Gonzalez‐Jimenez E, Ramirez‐Velez R. Effects of supervised exercise on cancer‐related fatigue in breast cancer survivors: a systematic review and meta‐analysis. BMC Cancer 2015;15:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mishra 2012
- Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health‐related quality of life for people with cancer during active treatment. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD008465.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCCN 2004
Newman 2003
- Newman LA, Sabel M. Advances in breast cancer detection and management. The Medical Clinics of North America 2003;87(5):997‐1028. [DOI] [PubMed] [Google Scholar]
O'Shaughnessy 2003
- O'Shaughnessy J. Chemotherapy‐related cognitive dysfunction in breast cancer. Seminars in Oncology Nursing 2003;19(4 Suppl 2):17‐24. [DOI] [PubMed] [Google Scholar]
Partridge 2001
- Partridge AH, Burstein HJ, Winer EP. Side effects of chemotherapy and combined chemohormonal therapy in women with early‐stage breast cancer. Journal of the National Cancer Institute. Monographs 2001;30:135‐42. [DOI] [PubMed] [Google Scholar]
Patrick 2003
- Patrick DL, Ferketich SL, Frame PS, Harris JJ, Hendricks CB, Levin B, et al. National Institutes of Health State‐of‐the‐Science Conference Statement: Symptom Management in Cancer: Pain, Depression, and Fatigue, July 15‐17, 2002. Journal of the National Cancer Institute 2003;95(15):1110‐7. [DOI] [PubMed] [Google Scholar]
Pinto 2002
- Pinto BM, Trunzo JJ, Reiss P, Shiu SY. Exercise participation after diagnosis of breast cancer: trends and effects on mood and quality of life. Psycho‐Oncology 2002;11(5):389‐400. [DOI] [PubMed] [Google Scholar]
Rayson 2008
- Rayson D, Richel D, Chia S, Jackisch C, Vegt S, Suter T. Anthracycline‐trastuzumab regimens for HER2/neu‐overexpressing breast cancer: current experience and future strategies. Annals of Oncology 2008;19(9):1530‐9. [DOI] [PubMed] [Google Scholar]
Rogers 2004
- Rogers LQ, Matevey C, Hopkins‐Price P, Shah P, Dunnington G, Courneya KS. Exploring social cognitive theory constructs for promoting exercise among breast cancer patients. Cancer Nursing 2004;27(6):462‐73. [DOI] [PubMed] [Google Scholar]
Rogers 2005
- Rogers LQ, Shah P, Dunnington G, Greive A, Shanmugham A, Dawson B, et al. Social cognitive theory and physical activity during breast cancer treatment. Oncology Nursing Forum 2005;32(4):807‐15. [DOI] [PubMed] [Google Scholar]
Rogers 2006
- Rogers LQ, Courneya KS, Verhulst S, Markwell S, Lanzotti V, Shah P. Exercise barrier and task self‐efficacy in breast cancer patients during treatment. Supportive Care in Cancer 2006;14(1):84‐90. [DOI] [PubMed] [Google Scholar]
Rugo 2003
- Rugo HS, Ahles T. The impact of adjuvant therapy for breast cancer on cognitive function: current evidence and directions for research. Seminars in Oncology 2003;30(6):749‐62. [DOI] [PubMed] [Google Scholar]
Rutqvist 2004
- Rutqvist LE. Adjuvant endocrine therapy. Best practice & research. Clinical Endocrinology & Metabolism 2004;18(1):81‐95. [DOI] [PubMed] [Google Scholar]
Salmon 2001
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clinical Psychology Review 2001;21(1):33‐61. [DOI] [PubMed] [Google Scholar]
Schwartz 2007
- Schwartz AL, Winters‐Stone K, Gallucci B. Exercise effects on bone mineral density in women with breast cancer receiving adjuvant chemotherapy. Oncology Nursing Forum 2007;34(3):627‐33. [DOI] [PubMed] [Google Scholar]
Segal 2001
- Segal R, Evans W, Johnson D, Smith J, Colletta S, Gayton J, et al. Structured exercise improves physical functioning in women with stages I and II breast cancer: results of a randomized controlled trial. Journal of Clinical Oncology 2001;19:657‐65. [DOI] [PubMed] [Google Scholar]
Spiegel 1997
- Spiegel D. Psychosocial aspects of breast cancer treatment. Seminars in Oncology 1997;24(1 Suppl 1):1‐47. [PubMed] [Google Scholar]
Tchen 2003
- Tchen N, Juffs HG, Downie FP, Yi QL, Hu H, Chemerynsky I, et al. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. Journal of Clinical Oncology 2003;21(22):4175‐83. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Markes 2006
- Markes M, Brockow T, Resch KL. Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD005001.pub2] [DOI] [PubMed] [Google Scholar]


