Abstract
Background
Several clinical trials of vitamin D to prevent asthma exacerbation and improve asthma control have been conducted in children and adults, but a meta‐analysis restricted to double‐blind, randomised, placebo‐controlled trials of this intervention is lacking.
Objectives
To evaluate the efficacy of administration of vitamin D and its hydroxylated metabolites in reducing the risk of severe asthma exacerbations (defined as those requiring treatment with systemic corticosteroids) and improving asthma symptom control.
Search methods
We searched the Cochrane Airways Group Trial Register and reference lists of articles. We contacted the authors of studies in order to identify additional trials. Date of last search: January 2016.
Selection criteria
Double‐blind, randomised, placebo‐controlled trials of vitamin D in children and adults with asthma evaluating exacerbation risk or asthma symptom control or both.
Data collection and analysis
Two review authors independently applied study inclusion criteria, extracted the data, and assessed risk of bias. We obtained missing data from the authors where possible. We reported results with 95% confidence intervals (CIs).
Main results
We included seven trials involving a total of 435 children and two trials involving a total of 658 adults in the primary analysis. Of these, one trial involving 22 children and two trials involving 658 adults contributed to the analysis of the rate of exacerbations requiring systemic corticosteroids. Duration of trials ranged from four to 12 months, and the majority of participants had mild to moderate asthma. Administration of vitamin D reduced the rate of exacerbations requiring systemic corticosteroids (rate ratio 0.64, 95% CI 0.46 to 0.90; 680 participants; 3 studies; high‐quality evidence), and decreased the risk of having at least one exacerbation requiring an emergency department visit or hospitalisation or both (odds ratio (OR) 0.39, 95% CI 0.19 to 0.78; number needed to treat for an additional beneficial outcome, 27; 963 participants; 7 studies; high‐quality evidence). There was no effect of vitamin D on % predicted forced expiratory volume in one second (mean difference (MD) 0.48, 95% CI ‐0.93 to 1.89; 387 participants; 4 studies; high‐quality evidence) or Asthma Control Test scores (MD ‐0.08, 95% CI ‐0.70 to 0.54; 713 participants; 3 studies; high‐quality evidence). Administration of vitamin D did not influence the risk of serious adverse events (OR 1.01, 95% CI 0.54 to 1.89; 879 participants; 5 studies; moderate‐quality evidence). One trial comparing low‐dose versus high‐dose vitamin D reported two episodes of hypercalciuria, one in each study arm. No other study reported any adverse event potentially attributable to administration of vitamin D. No participant in any included trial suffered a fatal asthma exacerbation. We did not perform a subgroup analysis to determine whether the effect of vitamin D on risk of severe exacerbation was modified by baseline vitamin D status, due to unavailability of suitably disaggregated data. We assessed two trials as being at high risk of bias in at least one domain; neither trial contributed data to the analysis of the outcomes reported above.
Authors' conclusions
Whilst we are confident that Vitamin D reduced the risk of asthma exacerbation in these trials (high quality GRADE assessment), we recognise that there is uncertainty about how these findings might be applied in practice. More research is needed to clarify whether there is a difference in effect between adults and children and with respect to asthma severity, baseline vitamin D status and doses.
Plain language summary
Vitamin D to prevent asthma attacks
Review question
Does vitamin D prevent asthma attacks or improve control of asthma symptoms or both?
Background
Low blood levels of vitamin D (the 'sunshine vitamin') have been linked to an increased risk of asthma attacks in children and adults with asthma. Several clinical trials have been conducted to test whether vitamin D might prevent asthma attacks and improve control of asthma symptoms in children and adults, but results from studies with the most scientifically sound designs have not previously been evaluated as a group.
Included studies
We included seven trials involving 435 children and two trials involving 658 adults in the review from searches run up to January 2016. Of these, one trial involving 22 children and two trials involving 658 adults contributed to the analysis of the rate of severe asthma attacks. Study duration ranged from four to 12 months, and the majority of those taking part had mild or moderate asthma. All of the studies compared vitamin D with placebo.
Key results
People given vitamin D experienced fewer asthma attacks needing treatment with oral steroids. The average number of attacks per person per year went down from 0.44 to 0.28 with vitamin D (high‐quality evidence). Vitamin D reduced the risk of attending hospital with an acute asthma attack from 6 per 100 to around 3 per 100 (high‐quality evidence).
Vitamin D had little or no effect on lung function or day‐to‐day asthma symptoms (high‐quality evidence). We found that vitamin D did not increase the risk of serious adverse events at the doses that were tested (moderate‐quality evidence).
We based all of these findings on studies judged to be of high quality.
Conclusion
Vitamin D has been found to offer some protection against severe asthma attacks in adults with mild to moderate asthma. Further trials focusing on children and people who experience frequent severe asthma attacks are needed before definitive clinical recommendations can be made.
Summary of findings
Summary of findings for the main comparison. Vitamin D versus placebo for the management of asthma (all studies).
| Vitamin D versus placebo for the management of asthma (all studies) | ||||||
|
Patient or population: children and adults with predominantly mild to moderate asthma Setting: primary and secondary care Intervention: vitamin D3 administered orally over study duration of 4 to 12 months Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with vitamin D | |||||
| Rate ratio, exacerbations requiring systemic corticosteroids assessed with: number of events per participant per year. Follow‐up: 6 to 12 months | Study population | RR 0.64 (0.46 to 0.90) | 680 (3 RCTs) | ⊕⊕⊕⊕ HIGH | Evidence based primarily on adults with mild to moderate asthma | |
| 0.44 events per person per year1 | 0.28 events per person per year (0.20 to 0.40) | |||||
| People with 1 or more exacerbations requiring ED visit or hospitalisation or both. Follow‐up: 6 to 12 months | Study population | OR 0.39 (0.19 to 0.78) | 963 (7 RCTs) | ⊕⊕⊕⊕ HIGH | Evidence based primarily on children and adults with mild to moderate asthma | |
| 63 per 1000 | 25 per 1000 (13 to 50) | |||||
| FEV1, % predicted. Follow‐up: 6 to 12 months | The mean FEV1, % predicted was 85.62% | The mean FEV1, % predicted in the intervention group was 0.48% more (0.93 fewer to 1.89 more) | ‐ | 387 (4 RCTs) | ⊕⊕⊕⊕2 HIGH | Evidence based primarily on children and adults with mild to moderate asthma |
| ACT/C‐ACT score. Follow‐up: 6 to 12 months | The mean ACT/C‐ACT score was 20 points | The mean ACT/C‐ACT score in the intervention group was 0.08 points fewer (0.7 fewer to 0.54 more) | ‐ | 713 (3 RCTs) | ⊕⊕⊕⊕2 HIGH | Evidence based primarily on adults with mild to moderate asthma |
| People with fatal asthma exacerbation. Follow‐up: 6 to 12 months | Study population | Not estimable | 963 (7 RCTs) | ⊕⊕◯◯3 LOW |
No fatal asthma exacerbations occurred in included studies | |
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| People with 1 or more serious adverse event due to any cause. Follow‐up: 6 to 12 months | Study population | OR 1.01 (0.54 to 1.89) | 879 (5 RCTs) | ⊕⊕⊕◯4 MODERATE |
Evidence based primarily on children and adults with mild to moderate asthma | |
| 48 per 1000 | 49 per 1000 (27 to 87) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACT, Asthma Control Test; C‐ACT, Childhood Asthma Control Test; CI, confidence interval; ED, emergency department; FEV1, forced expiratory volume in one second; OR, odds ratio; RCT, randomised controlled trial; RR, rate ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect | ||||||
1The event rate in participants randomised to placebo was estimated by calculating the weighted mean of event rates reported in placebo arms of included studies. 2Despite null effects of the intervention on these outcomes, we are confident that the true effect lies close to the estimates, as 95% confidence intervals for these estimates are very narrow. 3Downgraded two levels due to imprecision (no events occurred in included studies). 4Downgraded one level due to imprecision (wide confidence intervals).
Background
Description of the condition
Asthma is a chronic inflammatory condition of the airways, characterised by recurrent attacks of breathlessness, wheezing, cough, and chest tightness, commonly termed 'exacerbations'. The prevalence of asthma varies widely between countries. In children, the prevalence of severe asthma symptoms ranges from 0% (India) to 20.3% (Costa Rica) (Lai 2009); in adults, the prevalence of doctor‐diagnosed asthma ranges from 0.2% (China) to 21.0% (Australia) (To 2012). Exacerbations represent the major cause of morbidity and mortality in people with asthma (Johnston 2006). Asthma exacerbations are commonly classified as severe when they require treatment with systemic corticosteroids and/or when they result in emergency department attendance, hospitalisation, or death (Reddel 2009). Common precipitants of asthma exacerbation include acute respiratory infections and exposure to allergens and particulates (Singh 2006).
Description of the intervention
Vitamin D is a fat‐soluble micronutrient that has two 'parent' forms: cholecalciferol (vitamin D3) and ergocalciferol (vitamin D2). Cholecalciferol is synthesised in human skin from its precursor molecule 7‐dehydrocholesterol on exposure to ultraviolet B (UVB) radiation in sunlight; it may also be ingested, either in the diet (primarily from eating oily fish or vitamin D‐fortified foods) or as vitamin D supplements. Ergocalciferol is the plant and fungal form of the vitamin, which may be ingested in the diet (primarily by eating fungi) or as vitamin D supplements. In situations where cutaneous exposure to UVB radiation of appropriate intensity is limited (for example during winter at latitudes above 34ºN or below 34ºS, or in settings where people do not regularly expose their skin to sunlight), dietary sources of vitamin D or vitamin D supplements or both may be required to meet the body’s vitamin D requirement (Holick 2007).
Following cutaneous synthesis or ingestion, both forms of parent vitamin D undergo metabolism to form 25‐hydroxyvitamin D (25(OH)D), the major circulating vitamin D metabolite whose serum concentration indicates vitamin D status. 25‐hydroxylation may occur in the liver and in extra‐hepatic tissues, including leucocytes (Holick 2007). Serum 25(OH)D concentrations less than 50 nmol/L are widely accepted to indicate vitamin D deficiency; concentrations less than 25 nmol/L represent profound deficiency. Concentrations of 50 to 74 nmol/L may represent a milder state of inadequate vitamin D status, commonly termed ‘vitamin D insufficiency’. 25(OH)D undergoes a second hydroxylation step at the 1‐alpha position to form the active vitamin D metabolite 1,25‐dihydroxyvitamin D (1,25(OH)2D), the steroid hormone and active vitamin D metabolite that mediates the biological actions of vitamin D by binding the vitamin D receptor to regulate gene expression (Holick 2007). This 1‐alpha hydroxylation step is catalysed by the enzyme CYP27B1, which is expressed in many tissues including the kidney, leucocytes, and pulmonary epithelium; expression of CYP27B1 in leucocytes and pulmonary epithelium is up‐regulated in response to infection and inflammation.
This review included studies evaluating the effects of administration, by any route and at any dose, of vitamin D3, vitamin D2, 25(OH)D, or 1,25(OH)2D. Vitamin D3, vitamin D2, and 25(OH)D are usually administered orally; the ‘parent compounds’ vitamin D3 and vitamin D2 may also be given intramuscularly. Intramuscular administration of a bolus dose of vitamin D induces a slower increase and a lower peak in serum 25(OH)D than oral administration of the same dose (Romagnoli 2008), consequently this route of administration is not widely employed in clinical trials of vitamin D. The functional in vivo half‐life of 25(OH)D in the circulation is one to two months; accordingly, it takes at least three months to attain steady‐state concentrations of 25(OH)D in response to daily administration of vitamin D (Heaney 2003). Due to the relatively long half‐life of 25(OH)D, parent vitamin D and 25(OH)D may be administered intermittently as well as daily; weekly and monthly dosing regimens are often employed, and more widely spaced dosing regimens are also sometimes used. However, dosing less frequently than monthly results in large non‐physiological fluctuations in serum 25(OH)D concentration, which may cause undesirable effects (Hollis 2013; Martineau 2012; Vieth 2009). The influence of dosing interval on biological responses to administration of vitamin D is an area of active research in the field.
How the intervention might work
About 1 billion people worldwide are estimated to have 25(OH)D levels of less than 75 nmol/L (Holick 2007). Inadequate vitamin D status has been reported to be common among people with asthma in a variety of settings. Cross‐sectional, Brehm 2012, and cohort, Brehm 2010 and Confino‐Cohen 2014, studies have demonstrated independent associations between inadequate vitamin D status and increased risk of exacerbations. Administration of vitamin D3, vitamin D2, or 25(OH)D results in increased circulating concentrations of 25(OH)D. This 25(OH)D acts as a substrate for CYP27B1 expressed in the kidney and multiple extra‐renal tissues. Of particular relevance for asthma, CYP27B1 expression in the airway and leucocytes is induced during infection and inflammation, so that the active vitamin D metabolite 1,25(OH)2D is synthesised locally in the lung. 1,25(OH)2D ligates the vitamin D receptor (VDR) to induce antimicrobial activity (for example by induction of antimicrobial peptide expression), Greiller 2015 and Martineau 2007, and exert anti‐inflammatory activity (for example by induction of the anti‐inflammatory cytokine IL‐10, suppression of proinflammatory tumour necrosis factor and interferon‐γ‐ inducible chemokines, and inhibition of lipopolysaccharide‐induced synthesis of reactive oxygen species) (Coussens 2012; Lan 2014; Mann 2014). This combination of antimicrobial, antiviral, and anti‐inflammatory activity might decrease the risk of exacerbations, which are often precipitated by respiratory infection and which are characterised by dysregulated pulmonary inflammation. Of particular relevance to asthma, 1,25(OH)2D has been shown to inhibit TH17 cytokine production and enhance responsiveness to inhaled corticosteroids for production of interleukin‐10 ex vivo in people with asthma (Nanzer 2014; Xystrakis 2006). These findings raise the possibility that administration of vitamin D or 25(OH)D may therefore have a role in reducing exacerbation risk and improving symptom control in combination with inhaled corticosteroids, as well as independently. However, controversy exists regarding what serum 25(OH)D concentration, if any, is optimum for reducing the risk of asthma exacerbations.
Why it is important to do this review
There is considerable interest in the potential of administration of vitamin D to reduce exacerbation risk and improve asthma symptom control. Several published trials of vitamin D in children with asthma have reported statistically significant reductions in exacerbation rates among children randomised to the intervention arm (Majak 2011; Urashima 2010; Yadav 2014), two trials in adults have also reported non‐statistically significant trends towards reduced exacerbation rates in their intervention arms (Castro 2014; Martineau 2015). Meta‐analysis of these trials has the potential to increase statistical power to detect effects of administering vitamin D on exacerbation risk. However, definitions of severe exacerbation differ between trials, and published meta‐analyses in the field have utilised the variable definitions reported in primary publications rather than adopting a unified definition for this outcome across studies (Luo 2015; Riverin 2015; Xiao 2015). These meta‐analyses also included some non‐placebo‐controlled trials (Baris 2014; Darabi 2013), as well as trials of relatively short duration (less than 12 weeks) (De Groot 2015; Schou 2003). We therefore conducted a meta‐analysis that was restricted to double‐blind, placebo‐controlled trials of at least 12 weeks' duration to determine the effect of vitamin D on the primary outcome of exacerbation treated with systemic corticosteroids.
Objectives
To evaluate the efficacy of administration of vitamin D and its hydroxylated metabolites in reducing the risk of severe asthma exacerbations (defined as those requiring treatment with systemic corticosteroids) and improving asthma symptom control.
Methods
Criteria for considering studies for this review
Types of studies
We reviewed double‐blind, randomised, placebo‐controlled trials of at least 12 weeks’ duration. We did not include studies focusing only on bone outcomes, which we considered to provide very limited insights into asthma morbidity. We included studies reported as full text and unpublished data. Where eligible studies were published as abstracts only, we contacted the authors to request the full text of the trial report; where full text was unavailable, we listed such studies as 'ongoing'.
Types of participants
We included children and adults with a clinical diagnosis of asthma, based on the presence of characteristic symptoms and signs (wheeze, shortness of breath, chest tightness, or cough) and variable airflow obstruction. We imposed no restrictions regarding disease severity, baseline vitamin D status, or duration of treatment with asthma medication.
Types of interventions
The review was open to studies in which vitamin D3, vitamin D2, 25(OH)D, or 1,25(OH)2D was administered at any dose.
Types of outcome measures
Primary outcomes
Incidence of severe asthma exacerbations, defined as those requiring treatment with systemic corticosteroids
Secondary outcomes
Incidence of asthma exacerbations precipitating an emergency department visit or requiring hospital admission or both
End‐study Asthma Control Test (ACT) score
End‐study % predicted forced expiratory volume in one second (FEV1)
Incidence of any severe adverse event, irrespective of causation
Incidence of fatal asthma exacerbation
Incidence of asthma exacerbation as defined in the study protocol
End‐study % eosinophils in induced sputum or bronchoalveolar lavage
End‐study peak expiratory flow rate
Incidence of adverse reactions attributed to administration of vitamin D or its metabolites
Proportion of participants withdrawing from the trial
We would have meta‐analysed the following secondary outcomes had sufficient data been available.
Time off school or work due to asthma symptoms
Beta2‐agonist inhaler use
End‐study asthma quality of life as judged by use of a validated instrument
End‐study fractional exhaled nitric oxide concentration
End‐study airway reactivity
Costs from the perspective of healthcare providers
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group's Specialised Register (CAGR), which is maintained by the information specialist for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED, and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details). We searched all records in the CAGR using the search strategy in Appendix 2.
We also conducted searches of ClinicalTrials.gov (www.ClinicalTrials.gov), the World Health Organization trials portal (www.who.int/ictrp/en/), the ISRCTN registry (www.isrctn.com/), the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au/), and the UMIN Clinical Trials Registry (www.umin.ac.jp/ctr/). We searched all databases from their inception to 6 January 2016, and imposed no restriction on language of publication.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We searched relevant manufacturers' websites for trial information.
We searched for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed), but did not find any. We also contacted a panel of international experts for additional references and information on trials in progress.
Data collection and analysis
Selection of studies
Two people (Adrian R Martineau (ARM) and either Christopher J Cates (CJC) or Andrea Takeda (AT)) independently screened for inclusion the titles and abstracts of all the potentially relevant studies identified as a result of the search, coding them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We then retrieved the full‐text study reports/publication, and two people (ARM and either CJC or AT) independently screened the full text, identifying studies for inclusion and identifying and recording reasons for exclusion of the ineligible studies. Any disagreements were resolved through discussion or by consultation with other members of the review team (Christopher J Griffiths (CJG) and Aziz Sheikh (AS)) or both. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Moher 2009).
Data extraction and management
We used a data collection form for study characteristics and outcome data which was piloted on at least one study in the review. Two review authors (ARM and one of CJC, CJG, and Alex P Griffiths (APG)) extracted study characteristics from each included study. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: number, mean age, age range, gender, body mass index, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (ARM and one of CJC, CJG, and APG) independently extracted outcome data from each included study. If outcome data were not reported in a usable way, we noted this in the 'Characteristics of included studies' table. We resolved disagreements by consensus or by involving a third person (CJG or AS). One review author (ARM) transferred data into the RevMan 2015 file. We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A second review author (CJC) checked study characteristics for accuracy against the trial reports.
Assessment of risk of bias in included studies
The 'Risk of bias' assessment for the study authored by ARM and CJG, Martineau 2015, was performed by Ulugbek Nurmatov (UN) and CJC. For all other studies, two review authors (ARM and one of CJC and APG) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (AS). We assessed the risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other biases, including study size
We graded each potential source of bias as high, low, or unclear and provided a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each of the domains listed. Where information on risk of bias related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table. When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to a published protocol (Martineau 2015b), and have reported any deviations from it in the Differences between protocol and review section.
Measures of treatment effect
We analysed event rates as rate ratios (RR), dichotomous data as odds ratios (OR), and times to first event as hazard ratios (HR). We analysed other continuous outcome measures as mean difference (MD) or standardised mean difference (SMD). We used generic inverse variance meta‐analysis where adjusted measures of treatment effect from individual trials were included. We entered data presented as a scale with a consistent direction of effect. For analyses of outcomes in which no events occurred in some studies, we also calculated risk differences (RD). We undertook meta‐analyses only where this was meaningful, that is if the treatments, participants, and the underlying clinical question were similar enough for pooling to make sense.
Where multiple trial arms were reported in a single trial, we included only the relevant arms. If two comparisons (for example drug A versus placebo and drug B versus placebo) had been combined in the same meta‐analysis, we would have halved the control group to avoid double‐counting.
For outcomes measured at different time points, we included the longest time point after randomisation.
Unit of analysis issues
If data had been expressed in unconventional units of analysis, we would have converted them to conventional units, liaising with the authors where required.
Dealing with missing data
We contacted investigators or study sponsors in order to verify key study characteristics and to obtain missing numerical outcome data where possible. We asked all investigators to provide data relating to the incidence of fatal asthma exacerbations and exacerbations requiring treatment with systemic corticosteroids or emergency department attendance/hospitalisation or both where these were not reported in the manuscript or abstract.
Assessment of heterogeneity
We used the I2 statistic to measure heterogeneity among the trials in each analysis. Where we identified substantial heterogeneity (I2 greater than 40%), we assessed the value of exploring possible causes by using a prespecified subgroup analysis. However, limitations of the available data (for example where data for participants within different subgroups could not be disaggregated, or where numbers of participants or events or both within a subgroup were small) precluded the conduct of such subgroup analyses.
Assessment of reporting biases
Had we been able to pool more than 10 trials, we would have created and examined a funnel plot to explore possible small‐study biases.
Data synthesis
Given significant heterogeneity between studies, we used a random‐effects model for the primary analysis. We performed sensitivity analyses using fixed‐effect models for outcomes where the two models yielded different results. We analysed all data by intention‐to‐treat. We synthesised event rates as RRs, dichotomous data as ORs, and times to first event as HRs. We synthesised other continuous outcome measures as MD or SMD. We calculated the number needed to treat for an additional beneficial outcome (NNTB) using the Visual Rx NNT calculator (www.nntonline.net/visualrx/) where meta‐analysis of dichotomous outcomes revealed a statistically significant beneficial effect of allocation to vitamin D. We would have similarly calculated the number needed to treat for an additional harmful outcome (NNTH) if meta‐analysis of dichotomous outcomes had revealed statistically significant harmful effects of vitamin D. We used means and standard deviations (SDs) when available. Where data were not reported we approached the study authors. We would have extracted values from graphs had study authors not responded.
'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: incidence of asthma exacerbation treated with systemic corticosteroids; incidence of asthma exacerbation requiring emergency department attendance or hospitalisation for asthma or both; end‐study % predicted FEV1; end‐study ACT score; incidence of fatal asthma exacerbation; and incidence of serious adverse events due to any cause. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence as it related to the studies which contributed data to the meta‐analyses for the prespecified outcomes. Where data from primary studies conducted by review authors contributed to a given outcome, the quality of the evidence was assessed by review authors who were not involved with those primary studies (CJC and AS). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions,Higgins 2011, using GRADEpro GDT 2014 software. We justified all decisions to down‐ or upgrade the quality of studies using footnotes where necessary.
Subgroup analysis and investigation of heterogeneity
We prespecified that we would carry out the following subgroup analyses for the outcome of exacerbation treated with systemic corticosteroids (Martineau 2015b).
Baseline vitamin D status (e.g. serum 25(OH)D < 50 nmol/L versus ≥ 50 nmol/L).
Age (e.g. children aged < 5 years versus 5 to 16 years versus adults).
Severity of asthma and concomitant asthma treatment being taken (e.g. taking versus not taking inhaled corticosteroids, taking versus not taking leukotriene receptor antagonists).
The dose (e.g. daily equivalent of < 400 IU versus 400 to 2000 IU versus > 2000 IU) and form of vitamin D administered (e.g. cholecalciferol versus calcitriol).
The frequency of administration (e.g. daily versus intermittent bolus doses).
Genetic variation in pathways of vitamin D metabolism, transport, and signalling (e.g. GC 2/2 versus 2/1 versus 1/1 genotype for the GC polymorphism of the vitamin D binding protein).
Body mass index (e.g. < 25 kg/m2 versus ≥ 25 kg/m2).
However, limitations of the available data (for example where data for participants within different subgroups could not be disaggregated, or where numbers of participants or events or both within a subgroup were small) precluded the conduct of such subgroup analyses.
Had we conducted these subgroup analyses, we would have used the formal test for subgroup interactions in RevMan 2015.
Sensitivity analysis
We carried out the following sensitivity analyses.
Exclusion of publications assessed as being at high risk of bias in one or more of the following domains: sequence generation, allocation concealment, blinding, completeness of outcome data, or selective outcome reporting.
Analyses using fixed‐effect models were performed for outcomes where such models yielded results different from those generated by random‐effects models (Table 2).
1. Sensitivity analysis: random‐effects versus fixed‐effect models.
| Analysis | Random‐effects model | Fixed‐effect model |
| People with 1 or more exacerbations requiring systemic corticosteroids (risk difference) | (RD ‐0.01, 95% CI ‐0.04 to 0.02) | (RD ‐0.03, 95% CI ‐0.07 to 0.01) |
| ACT/C‐ACT score | (MD ‐0.08, 95% CI ‐0.70 to 0.54) | (MD ‐0.09, 95% CI ‐0.64 to 0.46) |
| People with 1 or more serious adverse event due to any cause | (OR 1.01, 95% CI 0.54 to 1.89) | (OR 1.00, 95% CI 0.54 to 1.85) |
| People with 1 or more study‐defined exacerbation | (OR 0.53, 95% CI 0.28 to 0.99) | (OR 0.66, 95% CI 0.48 to 0.91) |
| % eosinophils, lower airway | (MD ‐0.38, 95% CI ‐1.92 to 1.15) | (MD ‐0.26, 95% CI ‐1.35 to 0.83) |
| Peak expiratory flow rate | (MD 3.16, 95% CI ‐13.40 to 19.72) | (MD 4.09, 95% CI ‐1.34 to 9.52) |
| People withdrawing from the trial | (OR 1.07, 95% CI 0.73 to 1.58) | (OR 1.09, 95% CI 0.74 to 1.59) |
Sensitivity analyses are presented only for those outcomes where results of analyses using random‐effects versus fixed‐effect models are non‐identical.
Abbreviations: CI, confidence interval; MD, mean difference; OR, odds ratio; RD, risk difference.
Results
Description of studies
Results of the search
See Figure 1 for full details.
1.
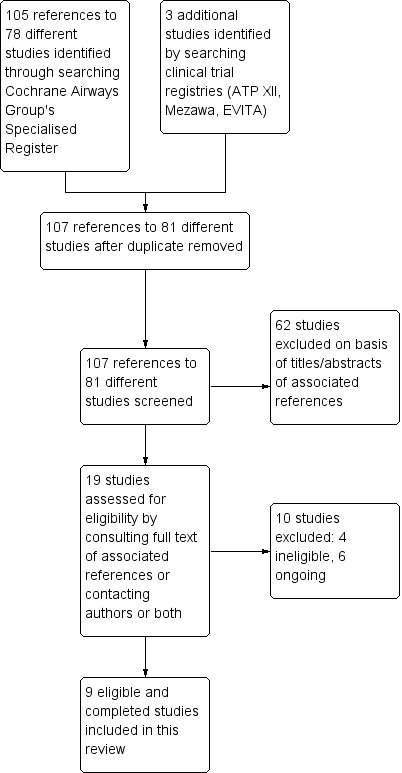
Study flow diagram.
We identified a total of 105 references to 78 different studies by searching the CAGR and an additional three studies by searching clinical trial registries. After removing one duplicate reference, we screened 107 references to 81 different studies for eligibility. We excluded 62 studies on the basis of the titles or the abstracts or both of the associated references. We assessed the remaining 19 studies for eligibility by consulting the full text of associated references or contacting study authors or both; we then excluded 10 more studies, four of which did not meet eligibility criteria for inclusion and six of which we classified as ongoing. We have presented the reasons for excluding potentially relevant studies in the Characteristics of excluded studies table.
Included studies
See Characteristics of included studies for full details. Nine completed studies including a total of 1093 participants with asthma met the inclusion criteria for this review (Castro 2014; Jensen 2016; Lewis 2012; Majak 2009; Majak 2011; Martineau 2015; Tachimoto 2016; Urashima 2010; Yadav 2014).
Study design
All included studies were double‐blind randomised controlled trials with a parallel‐group design, open to male and female participants of any ethnic background; five were conducted at a single centre (Jensen 2016; Lewis 2012; Majak 2009; Majak 2011; Yadav 2014), and four were multicentre studies (Castro 2014; Martineau 2015; Tachimoto 2016; Urashima 2010). All studies recruited in secondary care, and one study also recruited in primary care (Martineau 2015). Study duration ranged from four months, in Urashima 2010, to 12 months, in Lewis 2012, Majak 2009, and Martineau 2015. All trials were restricted to individuals with a physician diagnosis of asthma; two trials additionally based eligibility on evidence of reversible or variable airway obstruction (Castro 2014; Martineau 2015). Current treatment with inhaled corticosteroids was a requirement for three trials (Castro 2014; Majak 2009; Martineau 2015), and an exclusion criterion for one trial (Majak 2011); one trial excluded participants who had received oral corticosteroid therapy in the year prior to enrolment (Urashima 2010). All of the remaining trials included at least some participants who were taking inhaled corticosteroids.
Only one trial included baseline vitamin D status as an eligibility criterion (Castro 2014, which excluded people with baseline 25(OH)D concentration greater than or equal to 75 nmol/L), but six trials had exclusion criteria relating to maximum permitted pre‐trial or concomitant supplemental vitamin D intake or both (Castro 2014; Jensen 2016; Majak 2009; Majak 2011; Martineau 2015; Tachimoto 2016).
Participants
Seven studies involved 435 children (Jensen 2016; Lewis 2012; Majak 2009; Majak 2011; Tachimoto 2016; Urashima 2010; Yadav 2014), and two studies involved 658 adults (Castro 2014; Martineau 2015). Participants were ethnically diverse, reflecting the broad range of geographic settings: Canada (Jensen 2016), India (Yadav 2014), Japan (Tachimoto 2016; Urashima 2010), Poland (Majak 2009; Majak 2011), the UK (Martineau 2015), and the USA (Castro 2014; Lewis 2012). The majority of participants had mild/moderate asthma, and a minority had severe asthma. Where measured, mean/median baseline serum 25(OH)D concentration ranged from 48 nmol/L, in Castro 2014, to 89 nmol/L, in Majak 2011; a small minority of participants had serum 25(OH)D concentrations in the profoundly deficient range (less than 25 nmol/L).
Intervention
All studies administered oral vitamin D3 (cholecalciferol) to participants in the intervention arm. There was considerable heterogeneity in vitamin D dosage regimens employed. Four studies, Lewis 2012, Majak 2011, Tachimoto 2016, and Urashima 2010, used exclusively daily dosing regimens ranging from 500 IU/day, in Majak 2011, to 1200 IU/day, in Urashima 2010. Of the other studies, one used weekly dosing (Majak 2009), one used monthly dosing (Yadav 2014), one used two‐monthly dosing (Martineau 2015), and two gave a bolus dose at the start of the study, followed by daily dosing (Castro 2014; Jensen 2016). One study administered low‐dose vitamin D (400 IU/day) to participants in both the control arm and intervention arm; participants in the intervention arm of this study received an additional bolus of 100,000 IU vitamin D at the start of the study (Jensen 2016). For the six trials in which vitamin D was given daily (with or without additional bolus doses) (Castro 2014; Jensen 2016; Lewis 2012; Majak 2011; Tachimoto 2016; Urashima 2010), the median daily dose was 900 IU/day, ranging from 400 IU/day, in Jensen 2016, to 4000 IU/day, in Castro 2014. Where vitamin D status was assessed, the intervention resulted in an interarm difference in follow‐up serum 25(OH)D concentration on at least one follow‐up time point in four studies (Castro 2014; Jensen 2016; Martineau 2015; Tachimoto 2016), but not in three others (Lewis 2012; Majak 2009; Majak 2011).
Outcomes
Seven trials reported asthma exacerbation as an outcome measure (Castro 2014; Jensen 2016; Majak 2011; Martineau 2015; Tachimoto 2016; Urashima 2010; Yadav 2014). Definitions of exacerbation varied significantly between trials. Authors of seven trials provided data on exacerbations requiring treatment with systemic corticosteroids for the purposes of this review (Castro 2014; Jensen 2016; Majak 2009; Majak 2011; Martineau 2015; Tachimoto 2016; Urashima 2010).
Excluded studies
See Characteristics of excluded studies for full details.
Risk of bias in included studies
An overview of 'Risk of bias' judgements is shown in Figure 2.
2.
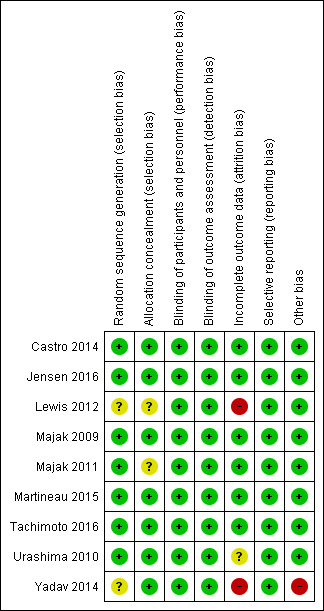
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Two studies did not report the method of sequence generation (Lewis 2012; Yadav 2014), and two studies did not report the method of allocation concealment (Lewis 2012; Majak 2011). We have therefore classified the risk of selection bias for these studies as 'unclear'. We assessed the risk of selection bias for the remaining studies as low.
Blinding
It appears that participants and study personnel, including those who administered the intervention, have been effectively blinded to allocation for all studies; accordingly, we assessed the risk of performance and detection bias as low for all studies.
Incomplete outcome data
One‐third of participants in the study by Lewis et al were lost to follow‐up (Lewis 2012); we have therefore assessed the risk of attrition bias as high for this study. The study by Yadav et al reports that 18 out of 100 participants were lost to follow‐up, but follow‐up data for 100 participants was presented for the final follow‐up visit (Yadav 2014). This discrepancy led us to assess the risk of attrition bias as being high for this study. We assessed the study by Urashima et al as being at unclear risk of attrition bias (Urashima 2010); although rates of loss were comparable between arms for this trial as a whole (50 out of 217 intervention arm, 46 out of 213 control arm), they were not reported for the subgroup of participants with doctor‐diagnosed asthma. We assessed the risk of attrition bias for the remaining studies as low.
Selective reporting
We found no evidence of selective reporting for any of the included studies, and have therefore assessed the risk of reporting bias as low for all studies.
Other potential sources of bias
In the study by Yadav et al (Yadav 2014), we noted a marked change in classification of asthma severity between the six‐month time point and earlier time points. This suggested a high risk of misclassification bias operating at the final follow‐up time point. We identified no other potential sources of bias for the remaining included trials.
Effects of interventions
See: Table 1
See Table 1.
Vitamin D versus placebo: all eligible trials
Nine trials with a total of 1093 participants (435 children and 658 adults) contributed to this comparison for at least one outcome. Three trials with a total of 680 participants (22 children and 658 adults) contributed to this comparison for analysis of the rate of exacerbations requiring systemic corticosteroids.
Primary outcome
Asthma exacerbation treated with systemic corticosteroids
Analyses including all participants
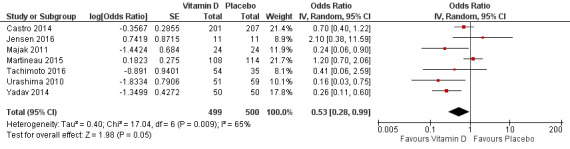
Administration of vitamin D was associated with a statistically significant reduction in the rate of asthma exacerbations treated with systemic corticosteroids (RR 0.64, 95% confidence interval (CI) 0.46 to 0.90; 680 participants; 3 studies; high‐quality evidence; Analysis 1.1; Figure 3). We found weaker evidence to suggest a benefit of vitamin D for the outcomes of time to first such exacerbation (HR 0.69, 95% CI 0.48 to 1.00; 658 participants; 2 studies; moderate‐quality evidence; Analysis 1.2) and proportion of participants experiencing one or more such exacerbation (OR 0.74, 95% CI 0.49 to 1.10; 933 participants; 7 studies; moderate‐quality evidence; Analysis 1.3); 95% confidence intervals included or spanned 1.00 for these outcomes. Of note, trials conducted in adults contributed a disproportionate amount of data to these analyses (Castro 2014; Martineau 2015); severe exacerbations were only seen in two out of five trials that enrolled children (Jensen 2016; Tachimoto 2016), and the total numbers of such events were small. Also of note, only one child in the trial by Tachimoto et al experienced such an event (Tachimoto 2016), therefore RRs and HRs for this study could not be calculated. Time‐to‐event data for calculation of HRs were not available for the other paediatric trial that saw any such events (Jensen 2016).
1.1. Analysis.
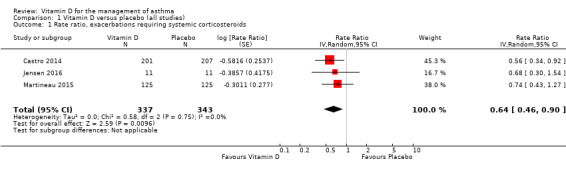
Comparison 1 Vitamin D versus placebo (all studies), Outcome 1 Rate ratio, exacerbations requiring systemic corticosteroids.
3.
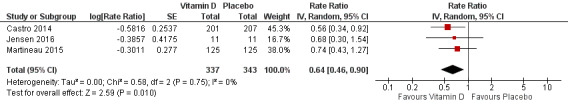
Forest plot of comparison: 1 Vitamin D versus placebo (all studies), outcome: 1.1 Rate ratio, exacerbations requiring systemic corticosteroids.
1.2. Analysis.
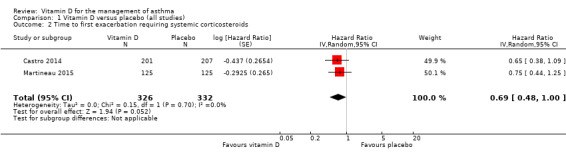
Comparison 1 Vitamin D versus placebo (all studies), Outcome 2 Time to first exacerbation requiring systemic corticosteroids.
1.3. Analysis.
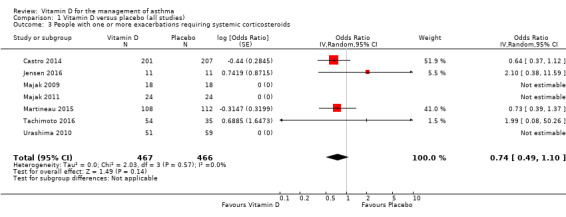
Comparison 1 Vitamin D versus placebo (all studies), Outcome 3 People with one or more exacerbations requiring systemic corticosteroids.
Subgroup analyses
Lack of access to individual participant data precluded conduct of prespecified subgroup analyses for the outcome of severe asthma exacerbation according to baseline vitamin D status, asthma severity, concomitant asthma treatment, body mass index, and genetic variation in the vitamin D pathway.
We did not conduct prespecified subgroup analyses for different age groups (children aged less than 5 years versus 5 to 16 years versus adults) due to a lack of severe exacerbations arising in trials that enrolled children. We did not conduct subgroup analyses for different dosing frequencies as some studies combined bolus and daily dosing strategies and could not be classified (Castro 2014; Jensen 2016), and the number of remaining studies within each subcategory was small. We did not perform subgroup analyses for different dose sizes due to the small number of studies and events arising within each subcategory.
All trials investigated effects of vitamin D3, which precluded the conduct of subgroup analysis by type of vitamin D administered.
Secondary outcomes
Asthma exacerbation precipitating emergency department visit or requiring hospitalisation or both
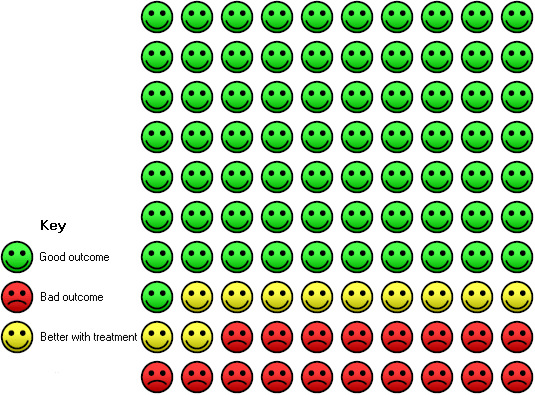
Administration of vitamin D was associated with a statistically significant reduction in the proportion of participants experiencing an asthma exacerbation precipitating an emergency department visit or hospital admission or both (OR 0.39, 95% CI 0.19 to 0.78; NNTB 27, 95% CI 20 to 76; 963 participants; 7 studies; high‐quality evidence; Analysis 1.5; Figure 4). The expected result in 100 people given vitamin D for an average of 7 months is shown in the Cates plot in Figure 5: in comparison with 6 out of 100 with this outcome on placebo, this fell to 3 out of 100 (95% CI 1 to 5) on vitamin D.
1.5. Analysis.
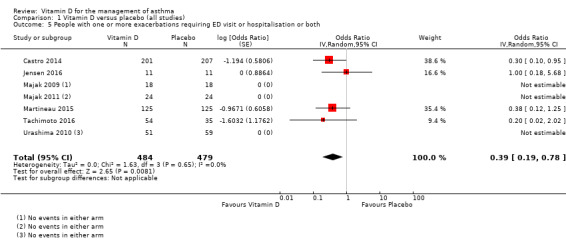
Comparison 1 Vitamin D versus placebo (all studies), Outcome 5 People with one or more exacerbations requiring ED visit or hospitalisation or both.
4.
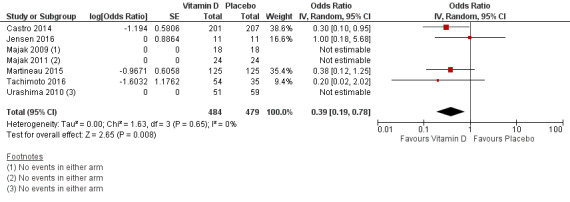
Forest plot of comparison: 1 Vitamin D versus placebo (all studies), outcome: 1.5 People with one or more exacerbations requiring ED visit or hospitalisation or both.
5.
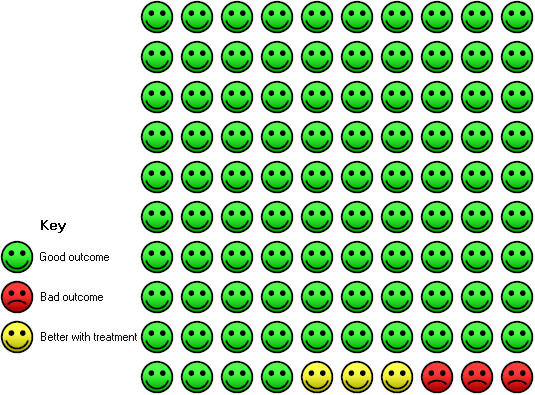
In the control group 6 out of 100 people had a visit to ED or hospitalisation over 8 months, compared to 3 (95% CI 1 to 5) out of 100 on vitamin D.
As only two of the trials conducted in children reported any such events (Jensen 2016; Tachimoto 2016), results of this analysis were primarily driven by the findings of the two trials conducted in adults (Castro 2014; Martineau 2015).
ACT scores
We saw no effect of vitamin D on ACT scores (MD ‐0.08, 95% CI ‐0.70 to 0.54; 713 participants; 3 studies; high‐quality evidence; Analysis 1.6).
1.6. Analysis.
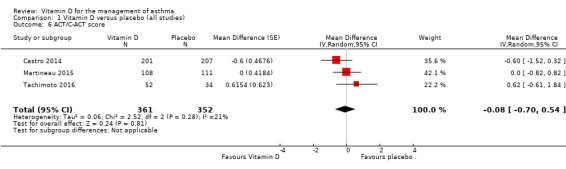
Comparison 1 Vitamin D versus placebo (all studies), Outcome 6 ACT/C‐ACT score.
FEV1, % predicted
There was no overall effect of vitamin D on % predicted FEV1 (MD 0.48, 95% CI ‐0.93 to 1.89; 387 participants; 4 studies; high‐quality evidence; Analysis 1.8). We did not include data from one trial that investigated FEV1 as an outcome measure in this meta‐analysis because absolute values were reported instead of % predicted values for this study (Castro 2014). Of note, vitamin D did not influence absolute values of FEV1 in this study (change in pre‐albuterol FEV1 [L] in intervention vs. control arm over the course of the study: ‐0.07 [95% CI ‐0.14 to 0.01] vs. ‐0.04 [‐0.11 to 0.03], P = 0.64).
1.8. Analysis.
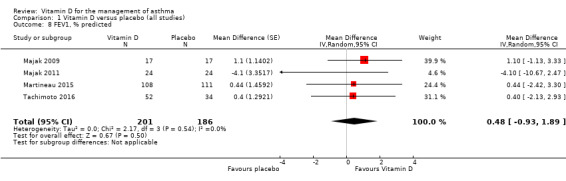
Comparison 1 Vitamin D versus placebo (all studies), Outcome 8 FEV1, % predicted.
Serious adverse event, any cause
Administration of vitamin D did not influence the incidence of serious adverse events of any cause (OR 1.01, 95% CI 0.54 to 1.89; 879 participants; 5 studies; I2 = 0%; moderate‐quality evidence; Analysis 1.9)
1.9. Analysis.
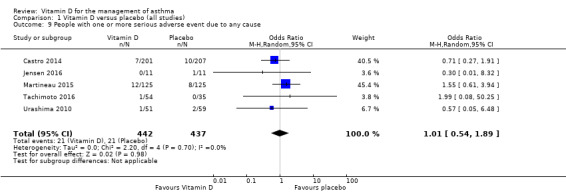
Comparison 1 Vitamin D versus placebo (all studies), Outcome 9 People with one or more serious adverse event due to any cause.
Fatal asthma exacerbations
No participant in any of the included trials suffered a fatal asthma exacerbation, therefore we saw no effect of the intervention on this outcome (RD 0.00, 95% CI ‐0.01 to 0.01; 963 participants; 7 studies; I2 = 0%; low quality evidence; Analysis 1.7).
1.7. Analysis.
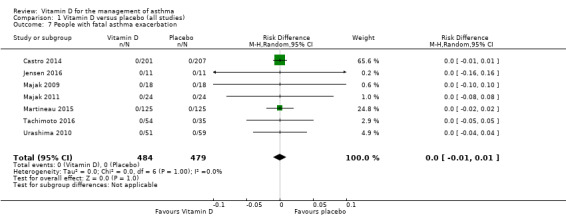
Comparison 1 Vitamin D versus placebo (all studies), Outcome 7 People with fatal asthma exacerbation.
Asthma exacerbation as defined in primary trial protocols
The definitions of asthma exacerbations used in individual trials are summarised in Table 3. Administration of vitamin D reduced the risk of experiencing at least one such exacerbation (OR 0.53, 95% CI 0.28 to 0.99; NNTB 9, 95% CI 6 to 483; 999 participants; 7 studies; moderate‐quality evidence; Figure 6; Analysis 1.10), but there was considerable heterogeneity in study definitions of exacerbation, and I2 was high (65%). The expected result in 100 people given vitamin D for an average of 8 months is shown in the Cates plot in Figure 7: in comparison with 29 out of 100 with this outcome on placebo, this fell to 18 out of 100 (95% CI 10 to 29) on vitamin D.
2. Definitions of asthma exacerbation used in primary trials.
| Study | Definition |
| Castro 2014 | Meeting criteria for treatment failure and 1 or more of the following:
|
| Jensen 2016 | Exacerbation requiring rescue oral corticosteroids, documented in medical or pharmacy records or both |
| Lewis 2012 | Exacerbation not defined or reported in study manuscript |
| Majak 2009 | Exacerbation not defined or reported in study manuscript; authors confirmed that no exacerbations requiring systemic corticosteroid treatment occurred in the study |
| Majak 2011 | Reported but not defined in study manuscript; authors confirmed that no exacerbations requiring systemic corticosteroid treatment occurred in the study |
| Martineau 2015 | Deterioration in asthma resulting in (A) treatment with oral corticosteroids, or (B) hospital admission or emergency department treatment, or (C) decrease in the morning PEFR to more than 25% below the mean run‐in value on 2 or more consecutive days |
| Tachimoto 2016 | Worsening of asthma symptoms prompting a need for a change in asthma treatment (from authors) |
| Urashima 2010 | Asthma attack that included wheezing, improved by inhalation of a beta‐stimulant in participants who already had a diagnosis of asthma; authors confirmed that no exacerbations requiring systemic corticosteroid treatment occurred in the study |
| Yadav 2014 | Reported but not defined in study manuscript |
FEV1, forced expiratory volume in one second; PEFR, peak expiratory flow rate.
6.
Forest plot of comparison: 1 Vitamin D versus placebo (low risk of bias), outcome: 1.10 People with one or more study‐defined exacerbations.
1.10. Analysis.
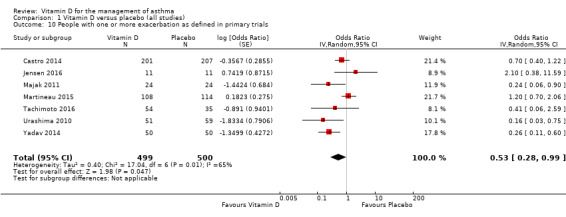
Comparison 1 Vitamin D versus placebo (all studies), Outcome 10 People with one or more exacerbation as defined in primary trials.
7.
In the control group 29 out of 100 people had a study‐defined exacerbation over 7 months, compared to 18 (95% CI 10 to 29) out of 100 on Vitamin D.
Lower airway eosinophilia
Vitamin D did not influence mean eosinophil count in the lower airway (MD ‐0.38, 95% CI ‐1.92 to 1.15; 525 participants; 3 studies; high‐quality evidence; Analysis 1.11).
1.11. Analysis.
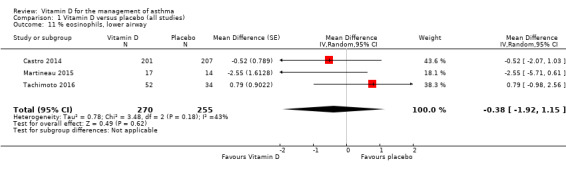
Comparison 1 Vitamin D versus placebo (all studies), Outcome 11 % eosinophils, lower airway.
Peak expiratory flow rate
Vitamin D did not influence mean end‐study peak expiratory flow rate (MD 3.16, 95% CI ‐13.40 to 19.72; 302 participants; 2 studies; high‐quality evidence; Analysis 1.12).
1.12. Analysis.
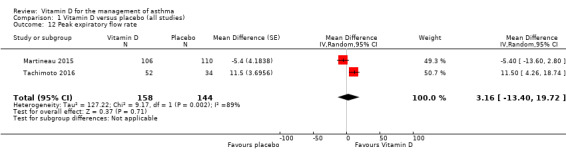
Comparison 1 Vitamin D versus placebo (all studies), Outcome 12 Peak expiratory flow rate.
Adverse reaction to vitamin D
Two participants in one trial experienced hypercalciuria (Jensen 2016), an adverse event that is recognised as an adverse reaction to vitamin D; this event arose in one participant in the intervention arm and one participant in the control arm of a study in which low‐dose vitamin D was administered in both arms. No other study reported episodes of hypercalciuria or any other adverse events potentially attributable to administration of vitamin D.
Withdrawals
We saw no difference in the proportion of participants withdrawing from trials between intervention and control arms, but the confidence intervals were wide (OR 1.07, 95% CI 0.73 to 1.58; 1093 participants; 9 studies; moderate‐quality evidence; Analysis 1.14).
1.14. Analysis.
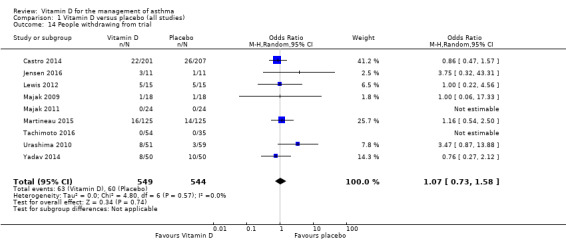
Comparison 1 Vitamin D versus placebo (all studies), Outcome 14 People withdrawing from trial.
Time off school or work
One trial conducted in adults investigated the outcome of work absence due to asthma exacerbation or upper respiratory infection (Martineau 2015). Allocation to vitamin D did not influence such work absence when measured as time to first event (adjusted hazard ratio 0.77, 95% CI 0.53 to 1.10), event rate (adjusted rate ratio 0.86, 95% CI 0.50 to 1.46), or proportion of participants with at least one such absence (adjusted odds ratio 0.77, 95% CI 0.45 to 1.30). No trial conducted in children investigated the outcome of time off school due to asthma symptoms.
Use of inhaled beta2‐agonists
One trial conducted in adults investigated the effects of vitamin D on the number of uses of inhaled relief medication per 24 hours (Martineau 2015). Allocation to vitamin D did not influence this outcome at 12 months (adjusted ratio of geometric means 1.00, 95% CI 0.77 to 1.28).
Asthma quality of life
Two trials conducted in adults investigated the effects of vitamin D on respiratory quality of life. Martineau et al reported that administration of vitamin D modestly improved respiratory quality of life as evidenced by adjusted interarm differences in total St George's Respiratory Questionnaire (SGRQ) score of ‐3.9 points at 2 months (P = 0.005), ‐3.7 points at 6 months (P = 0.038), and ‐3.3 points at 12 months (P = 0.060; P for allocation‐time interaction = 0.026). These reductions were associated with statistically significant decreases in component scores for the impacts dimension of the SGRQ at two months (P = 0.05) and six months (P = 0.005; P for allocation‐time interaction = 0.030) (Martineau 2015). Of note, the minimum clinically important difference for this score is around 4 points (Jones 2005). Castro et al reported no effect of the intervention on the Asthma Bother Profile score: the adjusted mean change in score was ‐1.0 (95% CI ‐2.7 to 0.7) in the intervention arm versus ‐2.4 (95% CI ‐4.0 to ‐0.7) in the placebo arm; P = 0.16) (Castro 2014). Data from these two different instruments were unsuitable for pooling and were therefore not meta‐analysed.
Fractional exhaled nitric oxide concentration (FeNO)
One trial conducted in adults investigated the effects of vitamin D on FeNO. Martineau et al reported that administration of vitamin D had no effect on mean FeNO concentrations at 12 months (ratio of geometric means −1.4, 95% CI −6.8 to 3.9) (Martineau 2015).
Other immunological biomarkers of asthma control
One trial conducted in adults investigated the effects of vitamin D on concentrations of inflammatory markers in induced sputum supernatants. Martineau et al reported that administration of vitamin D had no effect on supernatant concentrations of a panel of 17 inflammatory markers whose concentrations were detectable, measured at 2 and 12 months (Martineau 2015). Another trial conducted in adults investigated the effects of vitamin D on function of myeloid cells and CD4+ Tcells in peripheral blood, but found no effect (Castro 2014).
Airway reactivity
One trial conducted in adults investigated the effects of vitamin D on airway reactivity. Castro et al reported that administration of vitamin D had no effect on the provocative concentration of methacholine at which FEV1 decreased by 20% (PC20): the adjusted mean change in log base 2 transformed PC20 (doubling dilutions) was 0.70 (95% CI 0.38 to 1.03) in the intervention arm versus 0.74 (95% CI 0.41 to 1.07) in the placebo arm; P = 0.87 (Castro 2014).
Costs from the perspective of healthcare providers
One trial conducted in adults investigated the effects of vitamin D on health economic outcomes. Martineau et al reported that administration of vitamin D had no effect on total costs associated with asthma/upper respiratory infection over 12 months (adjusted mean difference GBP 66.78, 95% CI GBP ‐263.47 to GBP 397.03).
Vitamin D versus placebo: sensitivity analysis excluding trials at high risk of bias
Neither of the two trials assessed as being at high risk of bias contributed data relating to incidence of exacerbation treated with systemic corticosteroids or exacerbation precipitating emergency department attendance or hospitalisation or both. One trial assessed as being at high risk of bias reported effects of vitamin D on the proportion of participants experiencing at least one study‐defined exacerbation (Yadav 2014). When this trial was excluded in a sensitivity analysis, the effect of vitamin D on this outcome was no longer statistically significant (OR 0.64, 95% CI 0.34 to 1.21; 899 participants; 6 studies; moderate‐quality evidence; Analysis 2.1).
2.1. Analysis.
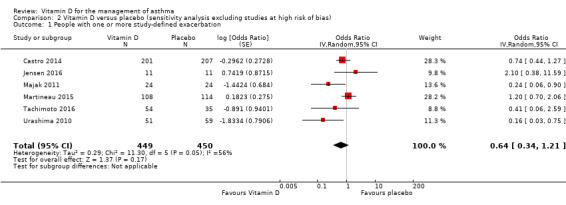
Comparison 2 Vitamin D versus placebo (sensitivity analysis excluding studies at high risk of bias), Outcome 1 People with one or more study‐defined exacerbation.
Both trials assessed as being at high risk of bias reported effects of vitamin D on the proportion of participants withdrawing from the trial (Lewis 2012; Yadav 2014). When these trials were excluded in a sensitivity analysis, the effect of vitamin D on this outcome remained null (OR 1.17, 95% CI 0.73 to 1.88; 963 participants; 7 studies; I2 = 7%; moderate‐quality evidence; Analysis 2.2).
2.2. Analysis.
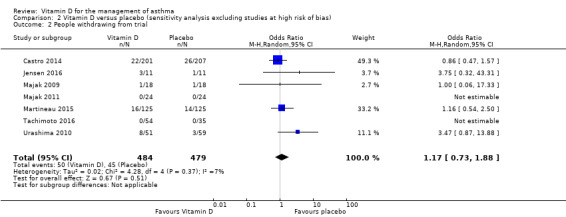
Comparison 2 Vitamin D versus placebo (sensitivity analysis excluding studies at high risk of bias), Outcome 2 People withdrawing from trial.
Vitamin D versus placebo: sensitivity analysis using fixed‐effect model
Random‐effects and fixed‐effect models yielded non‐identical but similar results for seven secondary outcomes. Results of analyses performed using each model are presented in Table 2
Discussion
Summary of main results
This systematic review and meta‐analysis incorporated evidence from 435 children and 658 adults participating in nine double‐blind, randomised, placebo‐controlled trials of vitamin D supplementation; of these, one trial involving 22 children and two trials involving 658 adults contributed to the analysis of the rate of exacerbations requiring systemic corticosteroids. Administration of vitamin D resulted in a clinically and statistically significant reduction in the rate of asthma exacerbations requiring treatment with systemic corticosteroids (RR 0.64, 95% CI 0.46 to 0.90; 680 participants; 3 studies; high‐quality evidence; we define a clinically significant reduction in an adverse outcome as being one that patients and clinicians consider large enough to justify a change in treatment). Administration of vitamin D also resulted in a clinically and statistically significant reduction in the risk of asthma exacerbations resulting in emergency department attendance or hospitalisation or both (OR 0.39, 95% CI 0.19 to 0.78; 963 participants; 7 studies; high‐quality evidence). Of note, only two of the trials conducted in children reported any severe exacerbations (Jensen 2016; Tachimoto 2016), and both of these trials were relatively small (22 and 89 participants, respectively). Accordingly, results of this analysis were primarily driven by the findings of the two trials conducted in adults (Castro 2014; Martineau 2015). It should also be noted that three out of seven studies for which data on emergency department attendance or hospitalisation or both were available did not report any such events (Majak 2009; Majak 2011; Urashima 2010).
In contrast to the protective effects demonstrated against severe exacerbation, we saw no effect of vitamin D on ACT score (MD ‐0.08, 95% CI ‐0.70 to 0.54; 713 participants; 3 studies; high‐quality evidence) or % predicted FEV1 (MD 0.48, 95% CI ‐0.93 to 1.89; 387 participants; 4 studies; high‐quality evidence). Vitamin D did not influence the risk of any serious adverse event, although the 95% confidence interval for this outcome was wide (OR 1.01, 95% CI 0.54 to 1.89; 879 participants; 5 studies; I2 = 0%; moderate‐quality evidence). No fatal asthma exacerbations were reported in any trial included in this meta‐analysis.
Overall completeness and applicability of evidence
This review incorporated evidence from a relatively modest number of studies, and results should not be generalised to patient populations who are not represented. Specifically, there is a relative lack of evidence on the effects of vitamin D in people with severe asthma, as evidenced by the modest number of exacerbations resulting in emergency department attendance or hospitalisation or both, and the absence of fatal exacerbations. This caveat applies particularly to the studies enrolling children: only 13 of the 305 children included in analysis of the primary outcome experienced an exacerbation that was treated with systemic corticosteroids, as compared with 118 of 628 adults. Consequently, the finding that vitamin D protected against severe asthma exacerbation is based primarily on results of trials conducted in adults, and therefore should not be generalised to paediatric populations. Moreover, the review does not provide evidence about optimum vitamin D doses and circulating 25(OH)D concentrations.
This review was limited to the inclusion of aggregate data from published manuscripts, which prevented us from conducting any of the subgroup analyses prespecified in the study protocol. Consequently we are unable to comment on whether effects of the intervention are modified by factors such as asthma severity or baseline vitamin D status. Populations with proven profound vitamin D deficiency (serum 25(OH)D less than 25 nmol/L) were also poorly represented in the studies eligible for inclusion in this review; this is particularly significant given that baseline vitamin D status may modify the effects of administering vitamin D on exacerbation risk, a phenomenon that has been reported in people with chronic obstructive pulmonary disease (Lehouck 2012; Martineau 2015a). Conversely, a trend towards increased risk of exacerbation when vitamin D is given to those with higher baseline vitamin D status has been reported (Janssens 2013; Martineau 2015a). Further research to clarify whether baseline vitamin D status modifies effects of vitamin D on exacerbation risk, including individual patient data meta‐analysis of existing datasets, is needed before definitive clinical recommendations can be made.
Despite these reservations regarding external validity, there is less reason to doubt the internal validity of our findings: these are based on double‐blind, placebo‐controlled trials assessed as being at low risk of bias. Moreover, we found effects of vitamin D on risk of exacerbation to be consistent when this outcome was expressed in different ways (RR (Analysis 1.1) versus HR (Analysis 1.2) versus OR (Analysis 1.3)), and when different definitions of exacerbation were used (exacerbations treated with systemic corticosteroids (Analysis 1.1) versus those defined according to study protocols (Analysis 1.10)). For outcomes where vitamin D was found not to have an effect (% predicted FEV1, ACT score), 95% confidence intervals were narrow (Analysis 1.8; Analysis 1.6), effectively ruling out a clinically important effect in the populations studied. The contrast between favourable effects of vitamin D on exacerbation versus null effects of this intervention on other measures of asthma control is striking, and it has implications for choice of outcome measures in future trials. Given that the majority of asthma exacerbations are precipitated by viral upper respiratory infections (Johnston 2006), it seems likely that vitamin D's mechanism of action relates either to prevention of such infections, or to interruption of pathways by which such events trigger exacerbations (Greiller 2015).
Quality of the evidence
This review was restricted to double‐blind, placebo‐controlled trials; consequently, we assessed all included studies as being at low risk of performance bias and detection bias. We assessed two studies as being at high risk of bias in at least one domain. As neither of these studies contributed data to the primary outcome of this meta‐analysis, we regard the evidence contributing to analysis of the effects of vitamin D on the risk of severe asthma exacerbation as high quality. We considered at length whether the evidence for exacerbations should be downgraded for imprecision or for indirectness, and while there are reasonable arguments for doing so, the eventual consensus of the author team was that neither imprecision nor indirectness posed a serious enough threat to our confidence in the result of this meta‐analysis to warrant a downgrade. The quality of the evidence relating to adverse event outcomes was lower. Specifically, evidence regarding fatal exacerbations was downgraded two levels to 'low' due to imprecision, as no such events occurred in any included study. Evidence relating to incidence of serious adverse events was downgraded one level due to 'moderate' for imprecision, as confidence intervals for the pertinent odds ratio were relatively wide (0.54 to 1.89).
Potential biases in the review process
We searched multiple databases for eligible studies using prespecified criteria, and this strategy led to identification of unpublished data which are included in this review. As for any review of randomised controlled trials, publication bias may have favoured publication of trials reporting favourable results of vitamin D on asthma outcomes. The total number of studies included in this review is relatively modest, and we identified a further six eligible trials that are ongoing; a repetition of the review in the short to medium term will determine whether or not promising results from meta‐analysis of early trials are reinforced by subsequent studies.
Agreements and disagreements with other studies or reviews
We are aware of three other systematic reviews that have synthesised evidence from randomised controlled trials of vitamin D in people with asthma.
The study by Riverin et al (Riverin 2015) included data from eight trials in children, five of which we included in our review (Lewis 2012; Majak 2009; Majak 2011; Urashima 2010; Yadav 2014) and three of which we excluded either on the grounds that they were not placebo controlled (Baris 2014; Darabi 2013), or because duration of follow‐up was less than 12 weeks (Schou 2003). Data from Tachimoto et al (Tachimoto 2016), included in this meta‐analysis, were not included in Riverin 2015. Riverin 2015 reported a reduction in risk of study‐defined asthma exacerbation with vitamin D (RR 0.41, 95% CI 0.27 to 0.63; 378 participants; 3 studies), which was deemed of low quality. No effect of the intervention was seen on asthma symptom scores or lung function.
The study by Luo et al included data from seven trials in both children and adults (Luo 2015), four of which we included in our review, and three of which we excluded on the grounds that they did not report asthma control outcomes (Worth 1994), they were not placebo controlled (Baris 2014), or because duration of follow‐up was less than 12 weeks (De Groot 2015). Luo et al excluded four studies included in our review (Lewis 2012; Majak 2011; Tachimoto 2016; Urashima 2010). This meta‐analysis reported no effect of vitamin D on risk of study‐defined asthma exacerbation (RR 0.66, 95% CI 0.32 to 1.37; 820 participants; 3 studies).
The study by Xiao et al focused primarily on effects of vitamin D on risk of acute respiratory infection (Xiao 2015), but it also investigated risk of asthma exacerbation in children as a secondary outcome. This analysis included only two trials (Majak 2011; Urashima 2010), which reported a protective effect of vitamin D against "asthma exacerbation triggered by respiratory infection" (RR 0.28, 95% CI 0.12 to 0.64; 2 studies; n not reported).
The findings of our study seem to be in keeping with those of Riverin 2015 and Xiao 2015, but contrast with those of Luo 2015. Disparities in results may be attributable to the inclusion of different primary trials in the different meta‐analyses. In addition, the other meta‐analyses used heterogeneous definitions of asthma exacerbation, as defined by the primary trial, rather than imposing a universal definition (exacerbation treated with systemic corticosteroids), as we did.
Authors' conclusions
Implications for practice.
We found a clinically and statistically significant protective effect of vitamin D against severe exacerbation of asthma and no convincing evidence of an increase in serious adverse events. Trials predominantly enrolled people with mild or moderate asthma, therefore those with severe asthma are under‐represented. Additionally, trials in children made a relatively minor contribution to findings of the review relating to severe exacerbations. Consequently, particular caution should be taken in generalising our findings to people who have recurrent severe asthma exacerbations and to those aged less than 16 years.
Furthermore, it is not yet clear whether beneficial effects of administering vitamin D are experienced by all people with asthma, or whether this result is driven by favourable effects that are confined to particular subgroups (for example those with lower baseline vitamin D status, or frequent exacerbations). Studies in chronic obstructive pulmonary disease have shown a trend towards increased risk of exacerbation when vitamin D is given to those with higher baseline vitamin D status (Janssens 2013; Martineau 2015a). Further research to clarify this issue, including individual patient data meta‐analysis of existing datasets, is needed before definitive clinical recommendations can be made.
Implications for research.
As discussed above, meta‐analysis of individual patient data from the trials included in this review may potentially elucidate clinically significant subgroup effects. Such a project is ongoing (AVID‐Asthma IPDMA), with results expected later in 2016.
We highlight that the optimum vitamin D dose or circulating 25(OH)D level that protects against asthma exacerbations is as yet unknown and requires additional primary studies to determine. There is also a need for new primary randomised controlled trials in populations that are under‐represented in the current review, specifically in vitamin D‐deficient children and adults who experience recurrent severe exacerbations. Eligibility criteria should be guided by findings of subgroup analyses from individual patient data meta‐analysis, which may reveal groups who are more likely to experience benefit or harm from the intervention than others. Our review suggests that such studies are more likely to find effects of vitamin D on exacerbations requiring treatment with systemic corticosteroids than on other outcome measures. These studies should measure participants' vitamin D status both at baseline and at follow‐up to allow determination of whether effects of administering vitamin D are dependent on baseline or attained serum 25(OH)D concentrations or both.
Feedback
Concerns over judgement of the quality and interpretation of evidence, 25 September 2016
Summary
1a) This is an interesting review on an important topic. However, the authors have not yet completed the analysis and the SOF is misleading. For example, for the main outcome, exacerbations requiring steroids, the authors give an RR of 0.63 and say it is high quality evidence.
1b) On the table, high quality evidence is defined as “High quality: We are very confident that the true effect lies close to that of the estimate of the effect”. But then the authors say in their conclusion that “further research…is needed to clarify this issue”. Therefore it is not high quality evidence.
2) Digging deeper, the analysis (comparison 1) only includes 22 children so any statement about vitamin D throughout this abstract should specify the effects are only in adults. Indeed the SOF is misleading as it says “children and adults”. Much better in my view to do a SOF for children (where you would NOT KNOW‐it would all be low or very low quality evidence for this outcome) and a SOF for adults.
3a) Then with adults: 45% (probably more when you have excluded the children) of the weight comes from a study where ALL the participants were vitamin D deficient, with 408 adults. 38% comes from Martineau, who is lead author of the review. As it is inverse variance, it's not clear to the reader how common these events are in the two groups, this would also help interpretation.
3b) So you need to either exclude the adults that are vitamin D deficient, OR downgrade on indirectness, and if you are extending the results to general populations, and over half the data comes from vitamin D deficient adults, then you would need to downgrade by two. That means in adults, the evidence for the main outcome is low quality evidence, and in children, probably very low. The terminology in the abstract and review should be suitably adjusted to address this uncertainty.
4) As Martineau is such a large part of the results, I think the SOF and interpretation should be done by the other authors independent of the person who carried out the original trial, to assure the readers of transparency and avoiding the conflict of interest with trialists as authors.
I hope this is useful to you in amending the review.
Reply
We thank Professor Garner for his comments on our review. In response:
1a) We respectfully disagree with the contention that ‘the authors have not yet completed the analysis and the SOF is misleading’. The aggregate data meta‐analysis specified in the review protocol has indeed been completed. Our assessment remains that the evidence is of high quality. The outcome in question is based on studies assessed by authors who were independent of each contributing study as being at low risk of bias in seven separate domains; the evidence was not inconsistent or imprecise; there was no evidence of publication bias; and there were no grounds to downgrade for indirectness (please see response to comment 3b for more detail on the issue of indirectness).
1b) Our comments with respect to the need for further research relate specifically to the issue of whether or not beneficial effects of vitamin D are restricted to individuals with lower baseline 25‐hydroxyvitamin D levels. In this meta‐analysis of aggregate data we did not have access to the data necessary to run this sub‐group analysis. This does not imply that the evidence from the aggregate data meta‐analysis is not of high quality: it simply means that analysis of the data available could not address the question relating to sub‐group effects.
2) Comparison 1 is based primarily, but not exclusively, on data from trials conducted in adults. However, since it contains data from one trial conducted in children, it would be misleading to say that effects are only seen in adults. We therefore stand by our comment in the Summary of Findings table that the evidence for this comparison is ‘based primarily on adults with mild to moderate asthma’. We agree that a sub‐group analysis to evaluate effects of vitamin D in adults vs. children will be of interest when sufficient data are available to power it. However, as stated in Methods (Assessment of Heterogeneity), we did not conduct sub‐group analyses where the number of events within a subgroup was small; this was the case for the outcome of severe asthma exacerbations in trials conducted in children. Larger trials of vitamin D to prevent severe asthma exacerbation in children are on‐going, and we hope that sufficient data from these trials will be available to power this sub‐group analysis in a future Cochrane review.
3a) We employed inverse variance where possible as this method allows for inclusion of adjusted effects, which are potentially more precise than unadjusted effects.
3b) Attempting to exclude study participants who are vitamin D deficient as suggested would raise a number of problems. First, individual patient data were not available to us for all studies during conduct of this review: thus, such participants could not be consistently excluded. Second, thresholds defining inadequate vitamin D status are controversial and not universally agreed. For example, Professor Garner contends that all the participants in the study by Castro et al1 were vitamin D deficient. However, this study enrolled participants with serum 25‐hydroxyvitamin D levels < 75 nmol/L (30 ng/ml) – whereas the threshold concentration of serum 25‐hydroxyvitamin D defining vitamin D deficiency is regarded as being 50 nmol/L (20 ng/ml) by the US Institute of Medicine 2 and 25 nmol/L by the UK Department of Health. 3 Conversely, some experts regard 25‐hydroxyvitamin D concentrations <100 nmol/L as being sub‐optimal. 4 Third, in order to conduct a sub‐group analysis rigorously, one would want to test the effect of vitamin D in individuals with 25‐hydroxyvitamin D levels below vs. above a given threshold, and then to perform a test for interaction to establish whether effects of the intervention differed between groups. Simply excluding those with higher baseline 25‐hydroxyvitamin D levels would prevent such an analysis.
With respect to the question of indirectness, Guyatt et al have suggested four ways in which evidence can be indirect. 5 Considering each in turn:
a) Applicability – i.e. participants in included trials may differ from patients of interest. The participants of trials included in our review were diverse in terms of ethnicity, age, gender and asthma severity. We acknowledge that children were under‐represented in the rate analysis for severe asthma exacerbation; however, in order to downgrade for indirectness on these grounds, one would have to argue that biological or social factors in study populations are sufficiently different from non‐trial populations that one might expect substantial differences in the magnitude of effect between study participants vs. patients seen in clinical practice.5 The likely mechanism by which vitamin D prevents asthma exacerbations is by enhancing innate immune responses to viral respiratory pathogens that precipitate such exacerbations; we do not have good reason to suspect that this effect would be substantially different between children vs. adults. This interpretation is borne out by primary clinical trials showing that vitamin D supplementation can prevent acute respiratory infection in children (e.g. 6) and by other analyses published in the Cochrane review, e.g. the analysis showing protective effects of vitamin D against study‐defined exacerbations (Figure 6) – five trials in children contributed to this analysis, contributing 56.9% of the weight. Thus, we are not persuaded that downgrading on applicability is indicated.
b) The intervention tested may differ from the intervention of interest. Again, we have no reason to think that the various vitamin D supplementation regimens investigated in trials incorporated in the review would differ substantially from the range of different regimens used in clinical practice.
c) Outcomes may differ from those of primary interest. This does not apply – the primary outcome of the review (severe asthma exacerbation) is of interest in clinical practice, and is not a surrogate.
d) Interventions available to clinicians have not been tested in head‐to‐head comparisons. Again, this does not apply, since trials investigated effects of adding vitamin D supplementation to standard asthma therapy.
4) We note that Professor Garner questions the objectivity of one of the four authors (Martineau) who acted as investigators in trials that contributed primary data to this review. We reiterate that risk of bias assessments for each study were conducted by review authors who were independent of these studies, where applicable. With regard to overall interpretation of our findings, this represents a consensus that was reached between all authors, and that was approved by 5 independent reviewers and 3 independent Cochrane editors; issues relating to GRADE criteria were discussed within the group and with Cochrane editors, and the issue of indirectness was explicitly addressed. We do not therefore feel that it is appropriate to exclude one member of the review team when responding to comments on our collective work.
To reflect the perceived disparity between our GRADE assessment of high quality and the uncertainty about how these findings might be applied in practice we have made minor adjustments to the text of the conclusions of the abstract and the plain language summary and the quality of the evidence section of the discussion.
References
Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA. 2014; 311(20): 2083‐91.
Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academy Press; 2011.
Department of Health. Department of Health Report on Health and Social Subjects, No. 49. Nutrition and bone health with particular reference to calcium and vitamin D. London; 1998.
Bischoff‐Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson‐Hughes B. Estimation of optimal serum concentrations of 25‐hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006; 84(1): 18‐28.
Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence‐‐indirectness. J Clin Epidemiol. 2011; 64(12): 1303‐10.
Camargo CA, Jr., Ganmaa D, Frazier AL, Kirchberg FF, Stuart JJ, Kleinman K, et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics. 2012; 130(3): e561‐7.
Contributors
Prof Paul Garner, Coordinating Editor for Cochrane Infectious Diseases, Department of Clinical Sciences, Liverpool School of Tropical Medicine
Chicken or the Egg; vitamin D might effect lung development, or vice versa, 25 September 2016
Summary
Vitamin D status may play an important role in the development of foetal lungs (1). Maternal vitamin D status during pregnancy may be associated with the risk of asthma in childhood (2). And, also maternal vitamin D intake as well as vitamin D levels in blood were inversely associated with respiratory tract infections and other wheezing illnesses (3). Smoking is a well known risk factor for vitamin D deficiency. Brot et al. (4) have found a significant negative association between smoking and serum levels of 25(OH)D, and 1,25(OH)2D. Smokers had on average an approximately 10% decrease of circulating levels of 25(OH)D and 1,25(OH)2D (4). Airway epithelium converts 25(OH)D (storage form) to 1,25(OH)2D (active form). Smoking decreases the production of the active form of 1,25(OH)2D in lung epithelial cells (5). In light of these knowledge, it would be beneficial to evaluate patient’s smoking habits with regard to response of vitamin D administration.
References
Hart PH, Lucas RM, Walsh JP, et al. Vitamin D in fetal development: findings from a birth cohort study. Pediatrics 2015;135:e167‐73
Duijts L. Fetal and infant origins of asthma. Eur J Epidemiol 2012;27:5‐14.
Miyake Y, Tanaka K, Okubo H, Sasaki S, Arakawa M. Maternal consumption of dairy products, calcium, and vitamin D during pregnancy and infantile allergic disorders. Ann Allergy Asthma Immunol 2014; 113: 82‐7.
Brot C, Jorgensen NR, Sorensen OH. The influence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr. 1999; 53: 920‐6.
Sundar IK, Rahman I. Vitamin d and susceptibility of chronic lung diseases: role of epigenetics. Front Pharmacol. 2011 Aug 30;2:50. doi: 10.3389/fphar.2011.00050.
Reply
We thank Dr Cerit for the comments on our review. We note the findings reported in the literature that have been highlighted, and acknowledge that smoking may modify response to vitamin D supplementation for the reasons stated. In this meta‐analysis of aggregate data we did not have access to the data necessary to run this sub‐group analysis, but we will consider adding it to on‐going meta‐analysis of individual patient data from these trials if significant further data become available (http://www.crd.york.ac.uk/prospero/display_record.asp?ID=CRD42014013953).
Contributors
Zeynep Cerit, Near East University, Turkey.
What's new
| Date | Event | Description |
|---|---|---|
| 23 April 2019 | Amended | An author added in error at the previous amendment was removed. |
History
Protocol first published: Issue 3, 2015 Review first published: Issue 9, 2016
| Date | Event | Description |
|---|---|---|
| 13 September 2017 | Amended | We discovered that two exacerbations requiring steroids had been misclassified in Martineau 2015, so the IRR for this study has been corrected from 0.70 (95% CI 0.41 to 1.21) to 0.74 (0.43 to 1.26). The pooled IRR has also been corrected from from 0.63 (0.45 to 0.88) to 0.64 (0.46 to 0.90). |
| 15 November 2016 | Feedback has been incorporated | Two pieces of feedback received and authors have added two responses. No changes made to the review. |
Acknowledgements
The review authors thank Andrea Takeda for assistance with assessment of eligible trials for inclusion; Iwona Stelmach and Pawel Majak (Medical University of Lodz, Poland), Mario Castro (Washington University School of Medicine, St Louis, MO, USA), Tonya S King (Penn State University, Hershey, PA, USA), and Thomas B Casale (Creighton University Medical Center, Omaha, NE, USA) for providing unpublished data relating to their studies; and Emma Welsh and Elizabeth Stovold of the Cochrane Airways Group, who provided advice on protocol content and search structure, respectively.
Julia AE Walters and Rebecca Normansell were the Editors for this review and commented critically on it.
The Background and Methods sections of this review were based on a standard template used by the Cochrane Airways group.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to retrieve trials from the CAGR
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Vitamin D Explode All
#6 MeSH DESCRIPTOR Vitamin D Deficiency Explode All
#7 "vitamin d"
#8 #5 or #6 or #7
#9 #4 and #8
(in search line #1, MISC1 refers to the field in the record where the reference has been coded for condition, in this case, asthma)
Data and analyses
Comparison 1. Vitamin D versus placebo (all studies).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Rate ratio, exacerbations requiring systemic corticosteroids | 3 | 680 | Rate Ratio (Random, 95% CI) | 0.64 [0.46, 0.90] |
| 2 Time to first exacerbation requiring systemic corticosteroids | 2 | 658 | Hazard Ratio (Random, 95% CI) | 0.69 [0.48, 1.00] |
| 3 People with one or more exacerbations requiring systemic corticosteroids | 7 | 933 | Odds Ratio (Random, 95% CI) | 0.74 [0.49, 1.10] |
| 4 People with one or more exacerbations requiring systemic corticosteroids (risk difference) | 7 | 933 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.04, 0.02] |
| 5 People with one or more exacerbations requiring ED visit or hospitalisation or both | 7 | 963 | Odds Ratio (Random, 95% CI) | 0.39 [0.19, 0.78] |
| 6 ACT/C‐ACT score | 3 | 713 | Mean Difference (Random, 95% CI) | ‐0.08 [‐0.70, 0.54] |
| 7 People with fatal asthma exacerbation | 7 | 963 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 8 FEV1, % predicted | 4 | 387 | Mean Difference (Random, 95% CI) | 0.48 [‐0.93, 1.89] |
| 9 People with one or more serious adverse event due to any cause | 5 | 879 | Odds Ratio (M‐H, Random, 95% CI) | 1.01 [0.54, 1.89] |
| 10 People with one or more exacerbation as defined in primary trials | 7 | 999 | Odds Ratio (Random, 95% CI) | 0.53 [0.28, 0.99] |
| 11 % eosinophils, lower airway | 3 | 525 | Mean Difference (Random, 95% CI) | ‐0.38 [‐1.92, 1.15] |
| 12 Peak expiratory flow rate | 2 | 302 | Mean Difference (Random, 95% CI) | 3.16 [‐13.40, 19.72] |
| 13 People with one or more adverse reactions attributed to vitamin D | 5 | 879 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.01, 0.01] |
| 14 People withdrawing from trial | 9 | 1093 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.73, 1.58] |
1.4. Analysis.
Comparison 1 Vitamin D versus placebo (all studies), Outcome 4 People with one or more exacerbations requiring systemic corticosteroids (risk difference).
1.13. Analysis.
Comparison 1 Vitamin D versus placebo (all studies), Outcome 13 People with one or more adverse reactions attributed to vitamin D.
Comparison 2. Vitamin D versus placebo (sensitivity analysis excluding studies at high risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 People with one or more study‐defined exacerbation | 6 | 899 | Odds Ratio (Random, 95% CI) | 0.64 [0.34, 1.21] |
| 2 People withdrawing from trial | 7 | 963 | Odds Ratio (M‐H, Random, 95% CI) | 1.17 [0.73, 1.88] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Castro 2014.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial Multicentre, 28 weeks long 4‐week run‐in period, prior asthma treatments discontinued 48 dropped out from study due to consent withdrawal, treatment failure, and asthma‐related adverse event Analysed by intention‐to‐treat |
|
| Participants | 9 academic medical centres in the USA, AsthmaNet network Predominantly white/black with some Hispanic and Asian N = 408. 130 m, 278 f. Mean age 39.7 yrs Inclusion criteria:
Asthma entry criteria:
Exclusion criteria:
|
|
| Interventions | Treatment (n = 201): Oral vitamin D3, 100,000 IU bolus once, then 4,000 IU/day for 28 weeks, added to inhaled ciclesonide (320 μg/d). Control (n = 207): Placebo soft gelatin capsules matching in appearance, added to inhaled ciclesonide (320 μg/d). Median 25(OH)D concentration at baseline: 47 nmol/L. Mean serum 25(OH)D concentration, intervention arm: 105 nmol/L (12 weeks), 107 nmol/L (20 weeks), 105 nmol/L (28 weeks). |
|
| Outcomes |
Primary outcome: Time to first asthma treatment failure. Treatment failure defined as 1 or more of the following:
Secondary outcomes:
|
|
| Notes | Grants awarded by the National Heart and Lung Institute. Ciclesonide and levalbuterol were provided without cost by Sunovion Pharmaceuticals Inc. The National Institutes of Health (NIH) program officers participated in the design and conduct of the study, and did not participate in the collection, management, analysis, and interpretation of the data. The authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Study was "double‐masked" and active and placebo capsules were matched in appearance. Randomisation code was held by the Data Co‐ordinating Centre; the Data Safety Monitoring Board oversaw the trial and reviewed data as the trial progressed in aggregate (group A and B) then unblinded at the end. Allocation was kept concealed until the last participant completed the trial (information from trial report and principal investigator) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, placebo controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind, placebo controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low rates of withdrawal overall, which were seen equally between study arms (17/201 in active arm vs 23/207 in control arm discontinued the study) |
| Selective reporting (reporting bias) | Low risk | No suggestion of selective outcome reporting; outcomes detailed in Methods were reported in Results. However, we did not have access to the original protocol |
| Other bias | Low risk | Nil |
Jensen 2016.
| Methods | Single‐centre, double‐blind, randomised, placebo‐controlled trial of 6 months’ duration. Concomitant asthma medications were not discontinued during the trial, and analysis was by intention‐to‐treat. There was no run‐in period. The trial was a pilot study, powered to compare the proportion of participants achieving serum 25(OH)D concentration ≥ 75 nmol/L. Target enrolment was 17 per arm, actual enrolment was 11 per arm; enrolment was discontinued on receipt of funding for the substantive trial for which this was the pilot | |
| Participants | Participants (n = 22) were recruited from the asthma clinic, hospital wards, and emergency department of the Sainte‐Justine University Health Centre, Montreal, Canada, and randomised to intervention vs control arms of the study in equal numbers. Baseline characteristics were well matched, other than an excess of eczema among participants randomised to vitamin D3 vs placebo. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Active intervention (n = 11): 100,000 IU vitamin D3 oral bolus at baseline, followed by 400 IU vitamin D3 IU orally daily. Control intervention (n = 11): oral placebo at baseline, followed by 400 vitamin D3 IU orally daily. Mean serum 25(OH)D concentration, intervention arm: 62 nmol/L (baseline), 157 nmol/L (10 days) |
|
| Outcomes |
Primary outcome: The mean group change in total serum 25(OH)D from baseline to 3 months. Secondary outcomes:
|
|
| Notes | Note that low‐dose vitamin D was administered to participants in both intervention and control arms of this trial. Unpublished full text obtained from corresponding author. No conflict of interest identified. Funding: Thrasher Research Fund | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Group assignment, recorded on a sequentially numbered list, was allocated by the Sainte‐Justine Hospital Research Pharmacy, which held the randomisation code. To maintain blinding, the intervention and placebo dose were identical in colour, appearance, volume, taste, and packaging. All research personnel, physicians, nurses, participants and their parents were blinded to group allocation. The code was not broken until the study trial was complete (information from principal investigator) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, placebo controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind, placebo controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 21/22 participants were included in analysis of primary outcome |
| Selective reporting (reporting bias) | Low risk | All prespecified primary and secondary outcomes were reported in the main paper. All exploratory/additional outcomes were also reported, with the exception of the duration of exacerbations and viral infections and the severity of exacerbations (due to poor questionnaire completion rate, as well as space restrictions for the manuscript). The additional outcome of cytokine profile is to be reported separately (information from principal investigator; original study protocol was not obtained) |
| Other bias | Low risk | Nil. Information on risk of bias for this trial relates to unpublished data |
Lewis 2012.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial (pilot study) Single‐centre, 12 months long Run‐in period not described Concomitant medication: current daily controller asthma medication 10 dropped out of study (reasons not provided) Analysis by intention‐to‐treat not specified |
|
| Participants | Omaha, Nebraska, USA Majority black/Hispanic N = 30, sex distribution not described m/f, age range 6 to 17 yrs Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Treatment (n = 15): Oral vitamin D3, 1000 IU/d for 12 months.
Control (n = 15): Placebo (specifications not given) daily for 12 months. Study dates not described. Mean serum 25(OH)D concentration, intervention arm: 30 nmol/L (baseline), 68 nmol/L (6 months, summer), 70 nmol/L (12 months, winter). All 25(OH)D concentrations above estimated from figure |
|
| Outcomes | Primary outcomes:
Seconday outcomes: Not given. |
|
| Notes | Disclosures: Authors have nothing to disclose. Funding sources: Funding provided by LB595 State of Nebraska Tobacco Settlement funds to Creighton University | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High rates of loss to follow‐up (10/30 participants) |
| Selective reporting (reporting bias) | Low risk | No suggestion of selective outcome reporting: results were reported for outcomes listed as having been investigated in the study report. However, we did not have access to the original protocol |
| Other bias | Low risk | Nil |
Majak 2009.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial Single‐centre, 12 months long Concomitant medication was continued except: inhaled long‐acting beta2‐agonists, leukotriene modifiers, beta‐blockers, multivitamin supplements, and systemic corticosteroids Run‐in period: September 2005 to March 2006 Analysed on ITT basis |
|
| Participants | Lodz, Poland Polish nationals Total N = 54 N = 36 used for data extraction, 22 m, 14 f. Age range 6 to 12 yrs Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Treatment (n = 18): SIT with prednisone 20 mg + oral vitamin D3, 1000 IU/week for 3 months. Control (n = 18): SIT with prednisone 20 mg + placebo for 3 months. SIT with placebo only group (n = 18) was not included as did not allow direct comparison of effect of vitamin D. Study dates: April 2006 to April 2007. Mean serum 25(OH)D concentration, intervention arm: 80 nmol/L (baseline), 82 nmol/L (3 months) |
|
| Outcomes |
Primary outcomes: Inhaled steroid‐sparing effect of SIT (dose reduction). Secondary outcomes:
|
|
| Notes | This study was funded by grant 502‐12‐760 and 503‐2056‐1 from the Medical University of Lodz, Poland. No conflict of interest to declare |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Active intervention drugs and placebo were blinded by the hospital pharmacy. The double‐blind code was not revealed until the end of the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, placebo controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Doube blind, placebo controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low rates of loss to follow‐up, equal between study arms (1/18 for D3 + steroid arm vs 1/18 for steroid arm) |
| Selective reporting (reporting bias) | Low risk | No suggestion of selective outcome reporting: outcomes listed in Methods are reported in Results. However, we did not have access to the trial protocol |
| Other bias | Low risk | Nil |
Majak 2011.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial Single‐centre, 6 months long Run‐in period: 6 months, concomitant medication discontinued No drop‐out, all participants completed follow‐up |
|
| Participants | Lodz, Poland Mainly Polish nationals N = 48. 32 m, 16 f. Mean age 11.5 yrs, range 5 to 18 yrs Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Treatment (n = 24): budesonide 800 mg daily administered as a dry inhaled powder and oral vitamin D3 500 IU daily. Control (n = 24): budesonide 800 mg daily administered as a dry inhaled powder and oral placebo daily. Mean serum 25(OH)D concentration, intervention arm: 90 nmol/L (baseline), 94 nmol/L (6 months) |
|
| Outcomes |
Primary outcomes:
Secondary outcome: Serum vitamin D status at various time points. |
|
| Notes | Supported by grant nos. 502‐12‐760 and 503‐2056‐1 from the Medical University of Lodz, Poland. Disclosure of potential conflict of interest: The authors have declared that they have no conflict of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double blind, placebo controlled |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double blind, placebo controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up |
| Selective reporting (reporting bias) | Low risk | Nil to suggest selective reporting: results were reported for outcomes listed as having been investigated in the study report. However, we did not have access to the original protocol |
| Other bias | Low risk | Nil |
Martineau 2015.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial Multicentre, 12 months long Run‐in period: At least 2 weeks, concomitant medication continued 31 did not complete: 17 withdrew consent, 13 lost to follow‐up, and 1 died Study analysed on ITT basis |
|
| Participants | London, UK Majority (202/250) white British N = 250. 109 m, 141 f. Mean age 47.9 yrs Inclusion criteria: Medical‐record diagnosis of asthma treated with ICS. Exclusion criteria:
|
|
| Interventions | Treatment (n = 125): six 2‐monthly oral doses of 6 mL Vigantol oil (Merck Serono, Darmstadt, Germany) containing 3 mg (120,000 IU) vitamin D3. Control (n = 125): six 2‐monthly oral doses of 6 mL organoleptically identical placebo (Miglyol oil, Caesar & Loretz, Hilden, Germany). Mean serum 25(OH)D concentration, intervention arm: 50 nmol/L (baseline), 61.2 nmol/L (2 months), 69.4 nmol/L (12 months) |
|
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
|
| Notes | Funded by the National Institute for Health Research’s Programme Grants for Applied Research Programme (ref RP‐PG‐0407‐10398). No competing interests to declare |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed by manufacturer (Nova Laboratories). Manufacturer and independent data monitoring committee held copies of the randomisation code, which was not revealed to investigators until database lock at the end of the trial. All personnel involved in recruitment and medication delivery were blinded to randomisation (information from trial report and principal investigator) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants contributed data to analysis of co‐primary outcomes. Rates of loss to follow‐up were comparable between arms (8/125 in intervention arm vs 5/125 control arm) |
| Selective reporting (reporting bias) | Low risk | Results of all analyses specified in protocol and relating to asthma control are reported; results of analyses relating to symptoms of allergic rhinitis will be reported elsewhere. We had access to the study protocol |
| Other bias | Low risk | Nil |
Tachimoto 2016.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial Multicentre, 6 months long Run‐in period: Not described, concomitant medication continued No drop‐out, all participants completed follow‐up Study analysed on ITT basis |
|
| Participants | Tokyo, Japan Predominantly Japanese N = 89. 50 m, 39 f. Mean age 9.9 yrs Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Treatment (n = 54): vitamin D3 800 IU/day orally for 2 months. Control (n = 39): daily oral placebo for 2 months. Mean serum 25(OH)D concentration, intervention arm: 71 nmol/L (baseline), 86 nmol/L (2 months), 77 nmol/L (6 months) |
|
| Outcomes |
Primary outcome: Changes in asthma control levels defined by GINA. Secondary outcomes:
|
|
| Notes | This study was supported by the Ministry of Education, Culture, Sports, Science and Technology in the Japan‐Supported Program for the Strategic Research Foundation at Private Universities and the Jikei University School of Medicine as well by JSPH KAKENHI Grant Number: 23591553 KAKENHI. All the authors declare no conflicts of interest | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Person performing blinding had no clinical involvement in the trial. Randomisation code was kept by independent data management committee and was not revealed to staff or participants until the trial was complete (information from trial report and principal investigator) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | Nil to suggest selective reporting: outcomes listed in Methods are reported in Results. However, the trial protocol was not accessed |
| Other bias | Low risk | Nil. Information on risk of bias for this trial relates to unpublished data |
Urashima 2010.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial Multicentre, 24 weeks long Run‐in period: Not described, concomitant medication continued 96 were lost to follow‐up, no reasons provided Study analysed on ITT basis |
|
| Participants | 12 hospitals in Japan N = 430. 242 m, 188 f. Mean age 10.2 yrs, range 6 to 15 yrs Number with diagnosed asthma: 110 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Treatment (n = 217): 3 tablets twice daily (total: 1200 IU vitamin D3/day). Control (n = 213): 3 tablets twice daily (placebo tablets identical in appearance). Those with asthma on treatment n = 51. Those with asthma on placebo n = 59. Vitamin D status not assessed |
|
| Outcomes |
Primary outcome: Influenza A, diagnosed by influenza antigen testing. Secondary outcomes:
|
|
| Notes | Funded by the Jikei University School of Medicine. None of the authors had any conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from staff and participants. Randomisation code was kept by independent data management committee and was not revealed to staff or participants until the trial was complete (information from trial report and principal investigator) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Rates of loss comparable between arms for the trial as a whole (50/217 intervention arm, 46/213 control arm), but not reported for subgroup of participants with doctor‐diagnosed asthma |
| Selective reporting (reporting bias) | Low risk | Nil to suggest selective reporting: outcomes listed in Methods are reported in Results. However, the trial protocol was not accessed |
| Other bias | Low risk | Nil |
Yadav 2014.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group trial Single‐centre, 6 months long Run‐in period: Not described, concomitant medication continued 18 were lost to follow‐up, reasons not provided Study analysed by intention‐to‐treat |
|
| Participants | Rohtak, India Indian N = 100. 49 m, 51 f. Mean age 9.6 yrs, range 5 to 13 yrs Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Treatment (n = 50): oral vitamin D3 (cholecalciferol) 60,000 IU per month for 6 months. Control (n = 50): placebo powder in the form of glucose sachet. Vitamin D status not assessed |
|
| Outcomes |
Primary outcome: Change in the level of asthma severity according to GINA guidelines. Secondary outcomes:
|
|
| Notes | No details on funding provided. Authors declare no conflict of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not reported |
| Allocation concealment (selection bias) | Low risk | Allocation concealed in opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind, placebo‐controlled trial |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10/50 children in control arm and 8/50 in active arm were lost to follow‐up, but data for these 'lost' children are presented at the 6‐month time point (end of study) |
| Selective reporting (reporting bias) | Low risk | Nil to suggest selective reporting: outcomes listed in Methods are reported in Results. However, we did not have access to the trial protocol |
| Other bias | High risk | Marked change in classification of asthma severity between 6‐month time point and earlier time points suggests likelihood of misclassification bias operating at end‐study time point |
25(OH)D, 25‐hydroxyvitamin D; ACT, Asthma Control Test; ASUI, Asthma Symptom Utility Index; ATAQ, Asthma Therapy Assessment Questionnaire; COPD, chronic obstructive pulmonary disease; FeNO, fractional exhaled nitric oxide concentration; FEV1, forced expiratory volume in one second; GFR, glomerular filtration rate; ICS, inhaled corticosteroids; IgE, immunoglobulin E; ITT, intention to treat; IU, international unit (40 IU vitamin D = 1 microgram vitamin D); PC20, provocative concentration of methacholine at which FEV1 decreased by 20%; PEFR, peak expiratory flow rate; SCRG, St George's Respiratory Questionnaire; SIT, specific immunotherapy; URTI, upper respiratory tract infection.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alansari 2015 | Not placebo controlled |
| Arshi 2014 | Not placebo controlled |
| Bantz 2015 | Single‐blind study |
| Bar Yoseph 2015 | Duration < 12 weeks |
| Baris 2014 | Not placebo controlled |
| Breitenbuecher 2012 | Duration < 12 weeks |
| Darabi 2013 | Not placebo controlled |
| De Groot 2015 | Duration < 12 weeks |
| Goldring 2013 | Primary prevention study |
| Lakatos 2000 | Bone outcomes only |
| Litonjua 2014 | Primary prevention study, protocol only |
| McDonald 2006 | Bone outcomes only |
| Menon 2014 | Not placebo controlled |
| Nanzer 2014 | Duration < 12 weeks |
| Price 2015 | Duration < 12 weeks |
| Rajanandh 2015 | Not placebo controlled |
| Schou 2003 | Duration < 12 weeks |
| Thijs 2011 | Duration < 12 weeks |
| Torres 2013 | Duration < 12 weeks |
| Utz 1976 | Duration < 12 weeks |
| Worth 1994 | Bone outcomes only |
| Yemelyanov 2001 | Bone outcomes only |
Characteristics of ongoing studies [ordered by study ID]
NCT01419262.
| Trial name or title | Vitamin D Outcomes and Interventions In Toddlers |
| Methods | Double‐blind, placebo‐controlled RCT |
| Participants | Children aged 1 to 5 years |
| Interventions | 2000 vs 400 IU vitamin D3 orally daily |
| Outcomes | Upper respiratory infections (primary), asthma exacerbations in subgroup (secondary) |
| Starting date | September 2011 |
| Contact information | Dr Jonathon Maguire, St Michael's Hospital, Toronto, Canada |
| Notes |
NCT01728571.
| Trial name or title | LungVITamin D and OmegA‐3 Trial |
| Methods | Double‐blind, placebo‐controlled RCT |
| Participants | Adults aged 50 years or older |
| Interventions | 2000 IU vitamin D3 orally daily (factorial design with marine omega‐3 fatty acids) |
| Outcomes | Asthma exacerbations and symptoms in subgroup |
| Starting date | July 2010 |
| Contact information | Prof Diane Gold, Brigham and Women's Hospital, Boston, USA |
| Notes |
NCT02197702.
| Trial name or title | Vitamin D in Preschoolers With Viral‐induced Asthma (NCT02197702) |
| Methods | Double‐blind, placebo‐controlled RCT |
| Participants | Children aged 1 to 5 years with physician‐diagnosed asthma |
| Interventions | Vitamin D (100,000 IU) given in a 2 ml oral dose at baseline and 3.5 months |
| Outcomes | Proportion of children with ≥ 1 asthma exacerbation requiring rescue oral corticosteroids |
| Starting date | September 2014 |
| Contact information | Francine M Ducharme, St Justine's Hospital, Montreal, Canada |
| Notes |
NCT02424552.
| Trial name or title | Effect of Vitamin D as add‐on Therapy for Vitamin D Insufficient Patients With Severe Asthma |
| Methods | Double‐blind, placebo‐controlled RCT |
| Participants | Adults with physician‐diagnosed severe asthma |
| Interventions | 100,000 IU vitamin D3 bolus, followed by 4000 IU daily, both orally |
| Outcomes | Corticosteroid dose (primary), asthma exacerbations (secondary) |
| Starting date | June 2015 |
| Contact information | Dr Stephanie Korn, Johannes Gutenberg University, Mainz, Germany |
| Notes |
NCT02428322.
| Trial name or title | Trial of Vitamin D3 Supplementation in Paediatric Asthma |
| Methods | Double‐blind, placebo‐controlled RCT |
| Participants | Children aged 6 to 16 years with physician‐diagnosed asthma |
| Interventions | 2000 IU vitamin D3 orally daily |
| Outcomes | Paediatric ACT (primary) |
| Starting date | October 2013 |
| Contact information | Dr Basil Elnazir, National Children's Hospital, Dublin, Ireland |
| Notes |
Patella 2013.
| Trial name or title | Vitamin D3 associated to lactobacillus reuteri improves effects of allergen immunotherapy in asthmatic children |
| Methods | Double‐blind, placebo‐controlled RCT |
| Participants | Children with asthma and house dust mite allergy, age not stated |
| Interventions | Vitamin D, dose not stated |
| Outcomes | Asthma symptoms, FeNO, "medication scores" |
| Starting date | Not reported |
| Contact information | Prof Vincenzo Patella, Agropoli Hospital, Agropoli, Italy |
| Notes | Information from published abstract only |
UMIN000004160.
| Trial name or title | A randomized, double blind, comparative study of vitamin D3 versus placebo in small children with asthma to prevent asthma attack |
| Methods | Double‐blind, placebo‐controlled RCT |
| Participants | Children aged 2 to 5 years with physician‐diagnosed asthma |
| Interventions | 600 IU vitamin D3 orally daily |
| Outcomes | Asthma exacerbations, C‐ACT score |
| Starting date | October 2010 |
| Contact information | Prof Mitsuyoshi Urashima, Jikei University School of Medicine, Tokyo, Japan |
| Notes |
ACT, Asthma Control Test; C‐ACT, Childhood Asthma Control Test; FeNO, fractional exhaled nitric oxide; IU, international unit (40 IU vitamin D = 1 microgram vitamin D); RCT, randomised controlled trial.
Differences between protocol and review
The protocol specified that studies published as abstract only would be included, with a note to the effect that they were pending definitive evaluation as and when fuller reports became available (Martineau 2015b). In conducting the review, where studies were published as abstracts only, we contacted the study authors requesting full text of the trial report. Where this was unavailable, we listed such studies as 'ongoing'.
The protocol specified that exacerbations precipitating emergency department attendance versus hospitalisation would be analysed separately (Martineau 2015b). However, due to difficulties in differentiating such events, this outcome was pooled in the current analysis.
The protocol did not specify that we would meta‐analyse hazard ratios or that we would use generic inverse variance meta‐analysis (Martineau 2015b); however, we employed both techniques in the review.
The protocol did not specify that risk difference would be calculated for some analyses. This was added so that studies where no events occurred could be included.
Contributions of authors
Adrian R Martineau (ARM) and Chris J Griffiths (CJG) wrote the protocol; Christopher J Cates (CJC), Aziz Sheikh (AS), and Ulugbek Nurmatov (UN) commented on it. Mitsuyoshi Urashima (MU) and Megan Jensen (MJ) contributed unpublished data. ARM, Alex P Griffiths (APG), CJC and UN assessed eligibility of trials for inclusion, extracted data, and performed 'Risk of bias' assessments. ARM entered data into Review Manager 5.3 for statistical analysis, which CJC cross‐checked. ARM drafted the manuscript, and all review authors critically evaluated it for important intellectual content and gave final approval of the version to be published.
Sources of support
Internal sources
-
Internal funds, UK.
Chris Griffiths
-
Christopher Cates, UK.
supported by St George's, University of London
External sources
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure, Cochrane Programme Grant, or Cochrane Incentive funding to Cochrane Airways. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health, UK.
Declarations of interest
ARM, MU, MJ, and CJG all acted as investigators in one or more clinical trials contributing data to this review. The 'Risk of bias' assessment for the study authored by ARM and CJG was performed independently by UN and CJC (Martineau 2015). For all other studies, ARM and one of CJC and APG independently assessed the risk of bias for each study. Where data from primary studies conducted by review authors contributed to a given outcome, the quality of the evidence was assessed by review authors who were not involved with those primary studies (CJC and AS).
Edited (no change to conclusions)
References
References to studies included in this review
Castro 2014 {published data only}
- Castro M, King TS, Kunselman SJ, Cabana MD, Denlinger L, Holguin F, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA 2014;311(20):2083‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger LC, King TS, Cardet JC, Craig T, Holguin F, Jackson DJ, et al. Vitamin D supplementation and the risk of colds in patients with asthma. American Journal of Respiratory and Critical Care Medicine 2015;[Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denlinger LC, King TS, Cardet JC, Craig TJ, Holguin F, Kraft M, et al. Vitamin D supplementation and the risk of colds in patients with asthma. Journal of Allergy and Clinical Immunology. 2015; Vol. 135:AB109. [DOI] [PMC free article] [PubMed]
- Jiao J, King TS, McKenzie M, Bacharier LB, Dixon AE, Codispoti CD, et al. Effects of vitamin D3 supplementation in adults with asthma complicated by sinonasal disease. American Journal of Respiratory and Critical Care Medicine. 2015; Vol. 191:A4148.
- Jiao J, King TS, McKenzie M, Bacharier LB, Dixon AE, Codispoti CD, et al. Vitamin D3 therapy in patients with asthma complicated by sinonasal disease: Secondary analysis of the Vitamin D Add‐on Therapy Enhances Corticosteroid Responsiveness in Asthma trial. Journal of Allergy and Clinical Immunology 2016;138(2):589‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore WC, King TS, Bleecker ER, Meyers DA, Peters SP, Wenzel SE. Sarp clinical clusters predict steroid responsiveness and risk of asthma exacerbations in the asthma net vida (vitamin D in asthma) trial. American Journal of Respiratory and Critical Care Medicine. 2015; Vol. 191:A6055.
- Reid B, Girodet P, Boomer JS, Abdel‐Gadir A, Zheng K, Wechsler ME, et al. Vitamin D3 treatment of vitamin D insufficient asthmatics does not alter immune cell function. Journal of Allergy and Clinical Immunology 2016;138(1):286‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jensen 2016 {unpublished data only}
- Jensen M, Mailhot G, Alos N, Rousseau E, White J, Khamessan A, et al. Vitamin D intervention in preschoolers with viral‐induced asthma (DIVA): a pilot randomised controlled trial. American Journal of Respiratory and Critical Care Medicine 2015;191:A3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen ME, Mailhot G, Alos N, Rousseau E, White J, Khamessan A, et al. Vitamin D intervention in preschoolers with viral‐induced asthma (DIVA): a pilot randomised controlled trial. Trials 2016;17:353. [DOI: 10.1186/s13063-016-1483-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewis 2012 {published data only}
- Lewis E, Fernandez C, Nella A, Hopp R, Gallagher JC, Casale TB. Relationship of 25‐hydroxyvitamin D and asthma control in children. Annals of Allergy, Asthma & Immunology 2012;108(4):281‐2. [DOI] [PubMed] [Google Scholar]
- Lewis E, Fernandez C, Nella AA, Hopp R, Casale T, Gallagher C. The relationship of vitamin D and asthma in children. Journal of Allergy and Clinical Immunology. 2011; Vol. 127:Suppl 1.
Majak 2009 {published data only}
- Majak P, Jerzyiska J, Smejda K, Stelmach I, Timler D, Stelmach W. Correlation of vitamin D with Foxp3 induction and steroid‐sparing effect of immunotherapy in asthmatic children. Annals of Allergy, Asthma & Immunology 2012;109(5):329‐35. [DOI] [PubMed] [Google Scholar]
- Majak P, Rychlik B, Stelmach I. The effect of oral steroids with and without vitamin D3 on early efficacy of immunotherapy in asthmatic children. Clinical & Experimental Allergy 2009;39(12):1830‐41. [DOI] [PubMed] [Google Scholar]
Majak 2011 {published data only}
- Majak P, Olszowiec‐Chlebna M, Smejda K, Stelmach I. Vitamin D supplementation in children may prevent asthma exacerbation triggered by acute respiratory infection. Journal of Allergy and Clinical Immunology 2011;127(5):1294‐6. [DOI] [PubMed] [Google Scholar]
Martineau 2015 {published data only}
- Martineau AR, MacLaughlin BD, Hooper RL, Barnes NC, Jolliffe DA, Choudhury AB, et al. Double‐blind multi‐centre randomised controlled trial of vitamin D3 supplementation in adults with inhaled corticosteroid‐treated asthma (ViDiAs). Thorax. 2014:A51‐2.
- Martineau AR, MacLaughlin BD, Hooper RL, Barnes NC, Jolliffe DA, Greiller CL, et al. Double‐blind randomised placebo‐controlled trial of bolus‐dose vitamin D3 supplementation in adults with asthma (ViDiAs). Thorax 2015;70(5):451‐7. [DOI] [PubMed] [Google Scholar]
Tachimoto 2016 {published and unpublished data}
- Tachimoto H, Mezawa H, Segawa T, Akiyama N, Ida H, Urashima M. Improved control of childhood asthma with low‐dose, short‐term vitamin D supplementation: a randomized, double‐blind, placebo‐controlled trial. Allergy 2016;71(7):1001‐9. [DOI] [PubMed] [Google Scholar]
Urashima 2010 {published data only}
- Urashima M, Segawa T, Okazaki M, Kurihara M, Wada Y, Ida H. Randomized trial of vitamin D supplementation to prevent seasonal influenza A in school children. The American Journal of Clinical Nutrition 2010;91(5):1255‐60. [DOI] [PubMed] [Google Scholar]
Yadav 2014 {published data only}
- Yadav M, Mittal K. Effect of vitamin D supplementation on moderate to severe bronchial asthma. Indian Journal of Pediatrics 2014;81(7):650‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alansari 2015 {published data only}
- Alansari K, Alattar M, Ibrahim KY, Davidson BL, Elnair MB, Mohamed AN. A randomized trial of vitamin D to reduce pediatric asthma exacerbations. American Journal of Respiratory and Critical Care Medicine. 2015; Vol. 191:A2621.
Arshi 2014 {published data only}
- Arshi S, Fallahpour M, Nabavi M, Bemanian MH, Javad‐Mousavi SA, Nojomi M, et al. The effects of vitamin D supplementation on airway functions in mild to moderate persistent asthma. Annals of Allergy, Asthma & Immunology 2014;113(4):404‐9. [DOI] [PubMed] [Google Scholar]
Bantz 2015 {published data only}
- Bantz SK, Dy T, Herzog R. Vitamin D deficiency in a young, atopic pediatric population. Journal of Allergy and Clinical Immunology. 2015; Vol. 135 (2 Suppl 1):AB148.
Baris 2014 {published data only}
- Baris S, Kiykim A, Ozen A, Tulunay A, Karakoc‐Aydiner E, Barlan IB. Vitamin D as an adjunct to subcutaneous allergen immunotherapy in asthmatic children sensitized to house dust mite. Allergy 2014;69(2):246‐53. [DOI] [PubMed] [Google Scholar]
- Safa B, Karakoc‐Aydiner E, Cagan H, Kiykim A, Tulunay A, Akkoc T, et al. 25 (OH) vitamin D3 as an adjunct to subcutaneous allergen immunotherapy: Is it effective?. 31st Congress of the European Academy of Allergy and Clinical Immunology; 2012 June 16‐20; Geneva. 2012.
Bar Yoseph 2015 {published data only}
- Bar Yoseph R, Livnat G, Schnapp Z, Hakim F, Dabbah H, Goldbart A. The effect of vitamin D on airway reactivity and inflammation in asthmatic children: a double‐blind placebo‐controlled trial. Pediatric Pulmonology 2015;50(8):747‐53. [DOI: 10.1002/ppul.23076] [DOI] [PubMed] [Google Scholar]
- Bar‐Yoseph R, Livnat G, Schnapp Z, Dabbah H, Goldbart A, Bentur LPY. The effect of vitamin D therapy on airway reactivity and airway inflammation in asthmatic children. European Respiratory Society Annual Congress; 2013 Sept 7‐11; Barcelona, Spain. 2013; Vol. 42, Suppl 57:1159.
Breitenbuecher 2012 {published data only}
- Breitenbuecher A, Voit U, Miedinger D, Chhajed P, Krapf R, Leuppi J. Calcitriol‐treatment in patients with severe persistent asthma: A randomized, placebo‐controlled study. Respiration; International Review of Thoracic Diseases 2014;87(6):523‐4. [Google Scholar]
- Breitenbuecher A, Voit U, Miedinger D, Leuppi J, Krapf R. Substitution of vitamin D in patients with moderate to severe persistent asthma: A randomized, placebo‐controlled pilot study. European Respiratory Journal 2012;40:Suppl 56 4698. [Google Scholar]
Darabi 2013 {published data only}
- Darabi B, Moin M, Pourpak Z. The effect of vitamin D supplementation over asthma outcome. 2nd International Congress of Immunology; 2013 Feb 19‐21; Tehran. 2013.
De Groot 2015 {published data only}
- Groot JC, Roon ENH, Storm H, Veeger NJ, Bel EHD, Brinke A. The effect of vitamin D on airway inflammation in non‐atopic asthma. American Journal of Respiratory and Critical Care Medicine. 2014; Vol. 189:A1390.
- Groot JC, Roon EN, Storm H, Veeger NJ, Zwinderman AH, Hiemstra PS, et al. Vitamin D reduces eosinophilic airway inflammation in non‐atopic asthma. Journal of Allergy and Clinical Immunology 2015;135(3):670‐5. [DOI] [PubMed] [Google Scholar]
- Groot JC, Roon EN, Storm H, Bel EH, Brinke A. The effect of a single high dose vitamin D3 on neutrophilic airway inflammation in nonatopic asthma. European Respiratory Journal. 2012; Vol. 40 Suppl 56:P1790.
Goldring 2013 {published data only}
- Goldring ST, Griffiths CJ, Martineau AR, Robinson S, Yu C, Poulton S, et al. Prenatal vitamin D supplementation and child respiratory health: a randomised controlled trial. PLoS One 2013;8(6):e66627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lakatos 2000 {published data only}
Litonjua 2014 {published data only}
- Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, Harshfield BJ, et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemporary Clinical Trials 2014;38(1):37‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2006 {published data only}
- McDonald CF, Matthews S, Seeman E. A two year double blind placebo controlled prospective study of the effects of calcitriol on bone mineral density (BMD) in patients with asthma. Proceedings of the Thoracic Society of Australia & New Zealand, Annual Scientific Meeting; 2003 April 4‐9; Adelaide. 2003:P022.
- McDonald CF, Zebaze RMD, Seeman E. Calcitriol does not prevent bone loss in patients with asthma receiving corticosteroid therapy: a double‐blind placebo‐controlled trial. Osteoporosis International 2006;17(10):1546‐51. [DOI] [PubMed] [Google Scholar]
Menon 2014 {published data only}
- Menon B, Nima G, Dogra V, Mittal A, Kaur C, Mittal U. Evaluation of vitamin D in bronchial asthma and the effect of vitamin D supplementation on asthma severity and control: A randomised control trial. European Respiratory Journal. 2014; Vol. 44 Suppl 58:P4049.
Nanzer 2014 {published data only}
- Chambers ES, Nanzer AM, Pfeffer PE, Richards DF, Martineau AR, Griffiths CJ, et al. Calcitriol restores glucocorticoid responsiveness in steroid resistant asthmatics through reduction of IL‐17A. Immunology. 2014; Vol. 143:50.
- Chambers ES, Nanzer AM, Pfeffer PE, Richards DF, Timms PM, Martineau AR, et al. Distinct endotypes of steroid‐resistant asthma characterized by IL‐17A (high) and IFN‐gamma (high) immunophenotypes: Potential benefits of calcitriol. Journal of Allergy and Clinical Immunology 2015;136(3):628‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers ES, Nanzer AM, Richards DF, Freeman A, Ryanna K, Griffiths C, et al. Serum 25(OH)D levels can predict Foxp3+ treg frequency and steroid responsiveness in severe asthmatics. Immunology 2011;135:S1. [Google Scholar]
- Nanzer AM, Chambers ES, Ryanna K, Freeman AT, Colligan G, Richards DF, et al. The effects of calcitriol treatment in glucocorticoid‐resistant asthma. Journal of Allergy and Clinical Immunology 2014;133(6):1755‐7. [DOI] [PubMed] [Google Scholar]
Price 2015 {published data only}
- Price OJ, Hull JH, Howatson G, Robson‐Ansley P, Ansley L. Vitamin D and omega‐3 polyunsaturated fatty acid supplementation in athletes with exercise‐induced bronchoconstriction: A pilot study. Expert Review of Respiratory Medicine 2015;9(3):369‐78. [DOI] [PubMed] [Google Scholar]
Rajanandh 2015 {published data only}
- Nageswari AD, Rajanandh MG, Priyanka RK, Rajasekhar P. Effect of vitamin D3 on mild to moderate persistent asthmatic patients: A randomized controlled pilot study. Perspectives in Clinical Research 2014;5(4):167‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajanandh MG, Nageswari AD, Prathiksha G. Effectiveness of vitamin D3 in severe persistent asthmatic patients: A double blind, randomized, clinical study. Journal of Pharmacology and Pharmacotherapeutics 2015;6(3):142‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schou 2003 {published data only}
- Schou AJ, Heuck C, Wolthers OD. Does vitamin D administered to children with asthma treated with inhaled glucocorticoids affect short‐term growth or bone turnover?. Pediatric Pulmonology 2003;36(5):399‐404. [DOI] [PubMed] [Google Scholar]
- Wolthers OD, Schou AJ, Heuck C. A double blind trial of vitamin‐D in children with asthma treated with inhaled budesonide. European Respiratory Society 9th Annual Congress; 1999 Oct 9‐13; Madrid. 1999.
Thijs 2011 {published data only}
- Thijs W, Janssen K, Verhoosel RM, Papapoulos SE, Cessie S, Middeldorp S, et al. Effect of vitamin D treatment on antimicrobial peptides in asthma patients and healthy controls. ERJ 2011;38(55):4888. [Google Scholar]
Torres 2013 {published data only}
- Torres J, Martinez M, Chavez E, Garcia D, Fabiano F, Hernandez J. Vitamin D levels in peripheral blood in patients with mild to moderate bronchial asthma. European Academy of Allergy and Clinical Immunology and World Allergy Organization World Allergy and Asthma Congress; 2013 June 22‐26; Milan Italy. 2013:68.
Utz 1976 {published data only}
- Utz G, Hauck AM. Oral application of calcium and vitamin D2 in allergic bronchial asthma. MMW Munch Med Wochenschr 1976;118(43):1395‐8. [PubMed] [Google Scholar]
Worth 1994 {published data only}
- Worth H, Stammen D, Keck E. Therapy of steroid‐induced bone loss in adult asthmatics with calcium, vitamin D, and a diphosphonate. American Journal of Respiratory and Critical Care Medicine 1994;150(2):394‐7. [DOI] [PubMed] [Google Scholar]
Yemelyanov 2001 {published data only}
- Yemelyanov A, Shevelev S, Murzin B, Shubin S. Efficacy and safety of calcium and vitamin D in treatment of steroid osteoporosis in asthmatic patients. European Respiratory Journal 2001;18:S33. [Google Scholar]
References to ongoing studies
NCT01419262 {published data only}
- Maguire JL, Birken CS, Loeb MB, Mamdani M, Thorpe K, Hoch JS, et al. DO IT Trial: vitamin D outcomes and interventions in toddlers ‐ a TARGet Kids! randomized controlled trial. BMC Pediatrics 2014;8(14):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01419262. DO IT Trial: Vitamin D outcomes and interventions in toddlers (DO IT). https://clinicaltrials.gov/ct2/show/NCT01419262 (accessed 11 August 2016).
NCT01728571 {published data only}
- NCT01728571. LungVITamin D and OmegA‐3 Trial (lungVITAL) (lungVITAL). https://clinicaltrials.gov/ct2/show/NCT01728571 (accessed 11 August 2016).
NCT02197702 {published data only}
- NCT02197702. Vitamin D in preschoolers with viral‐induced asthma (DIVA). https://clinicaltrials.gov/ct2/show/NCT02197702 (accessed 11 August 2016).
NCT02424552 {published data only}
- NCT02424552. Effect of vitamin D as add‐on therapy for vitamin D insufficient patients with severe asthma (EVITA). https://clinicaltrials.gov/ct2/show/NCT02424552 (accessed 11 August 2016).
NCT02428322 {published data only}
- Hutchinson K, Kerley C, Elnazir B, Couglan D, Greally P, Rochev Y, et al. A randomized, double‐blind, placebo‐controlled trial of vitamin D for Irish children with asthma: Baseline data. Irish Journal of Medical Science. 2014; Vol. 183 (9 Suppl 1):S443.
- Kerley C, Greally P, Coughlan D, Elnazir BPY. A randomized, double‐blind, placebo‐controlled trial of vitamin D3 for Irish children with asthma: Baseline data. European Respiratory Journal. 2014; Vol. 44, Suppl 58:1172.
- Kerley CP, Hutchinson K, Greally P, Coghlan D, Elnazir B. The effects of vitamin D supplementation on pulmonary function, disease severity and markers of inflammation in childhood asthmatics: A randomized, double‐blind, placebo‐controlled trial. Irish Journal of Medical Science. 2014; Vol. 183 11 Suppl 1:S522.
- NCT02428322. Trial of Vitamin D3 Supplementation in Paediatric Asthma (NCHVitDAst). https://clinicaltrials.gov/ct2/show/NCT02428322 (accessed 11 August 2016).
Patella 2013 {published data only}
- Florio G, Rubano A, Patella V. Effect of sublingual specific immunotherapy associated to vitamin D3 and lactobacillus reuteri in asthmatic teens. American Journal of Respiratory and Critical Care Medicine. 2015; Vol. 191:A4269.
- Patella V, Florio G, Palmieri M. Vitamin D3 associated to lactobacillus reuteri improves effects of allergen immunotherapy in asthmatic children. European Respiratory Society 23rd Annual Congress; 2013 Sep 7‐11; Barcelona. 2013.
UMIN000004160 {published data only}
- UMIN000004160. A randomized, double blind, comparative study of vitamin D3 versus placebo in small children with asthma to prevent asthma attack. https://upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_his_list.cgi?recptno=R000004995 (accessed 11 August 2016).
Additional references
AVID‐Asthma IPDMA
- Martineau AR, Jolliffe DA, Hooper RL, Khan KS, Griffiths CJ, Camargo CA Jr. Individual patient data meta‐analysis of randomised controlled trials of vitamin D supplementation to prevent acute respiratory infection and acute exacerbations of asthma and COPD. http://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42014013953 (accessed 11 August 2016).
Brehm 2010
- Brehm JM, Schuemann B, Fuhlbrigge AL, Hollis BW, Strunk RC, Zeiger RS, et al. Serum vitamin D levels and severe asthma exacerbations in the Childhood Asthma Management Program study. Journal of Allergy and Clinical Immunology 2010;126(1):52‐8 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brehm 2012
- Brehm JM, Acosta‐Perez E, Klei L, Roeder K, Barmada M, Boutaoui N, et al. Vitamin D insufficiency and severe asthma exacerbations in Puerto Rican children. American Journal of Respiratory and Critical Care Medicine 2012;186(2):140‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Confino‐Cohen 2014
- Confino‐Cohen R, Brufman I, Goldberg A, Feldman BS. Vitamin D, asthma prevalence and asthma exacerbations: a large adult population‐based study. Allergy 2014;69(12):1673‐80. [DOI] [PubMed] [Google Scholar]
Coussens 2012
- Coussens AK, Wilkinson RJ, Hanifa Y, Nikolayevskyy V, Elkington PT, Islam K, et al. Vitamin D accelerates resolution of inflammatory responses during tuberculosis treatment. Proceedings of the National Academy of Sciences 2012;109(38):15449‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 11 August 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Greiller 2015
- Greiller CL, Martineau AR. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015;7(6):4240‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heaney 2003
- Heaney RP, Davies KM, Chen TC, Holick MF, Barger‐Lux MJ. Human serum 25‐hydroxycholecalciferol response to extended oral dosing with cholecalciferol. American Journal of Clinical Nutrition 2003;77:204‐10. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at www.cochrane‐handbook.org.
Holick 2007
- Holick MF. Vitamin D deficiency. The New England Journal of Medicine 2007;357(3):266‐81. [DOI] [PubMed] [Google Scholar]
Hollis 2013
- Hollis BW, Wagner CL. The role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. Journal of Clinical Endocrinology and Metabolism 2013;98(12):4619‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Janssens 2013
- Janssens W, Decramer M, Mathieu C, Korf H. Vitamin D and chronic obstructive pulmonary disease: hype or reality?. The Lancet Respiratory Medicine 2013;1(10):804‐12. [DOI] [PubMed] [Google Scholar]
Johnston 2006
- Johnston NW, Sears MR. Asthma exacerbations 1: epidemiology. Thorax 2006;61(8):722‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2005
- Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005;2(1):75‐9. [DOI] [PubMed] [Google Scholar]
Lai 2009
- Lai CK, Beasley R, Crane J, Foliaki S, Shah J, Weiland S. Global variation in the prevalence and severity of asthma symptoms: phase three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009;64(6):476‐83. [DOI] [PubMed] [Google Scholar]
Lan 2014
- Lan N, Luo G, Yang X, Cheng Y, Zhang Y, Wang X, et al. 25‐hydroxyvitamin d3‐deficiency enhances oxidative stress and corticosteroid resistance in severe asthma exacerbation. PLoS ONE 2014;9(11):e111599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lehouck 2012
- Lehouck A, Mathieu C, Carremans C, Baeke F, Verhaegen J, Eldere J, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease. Annals of Internal Medicine 2012;156:105‐14. [DOI] [PubMed] [Google Scholar]
Luo 2015
- Luo J, Liu D, Liu C‐T. Can vitamin D supplementation in addition to asthma controllers improve clinical outcomes in patients with asthma? A meta‐analysis. Medicine (Baltimore) 2015;94(50):e2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mann 2014
- Mann EH, Chambers ES, Pfeffer PE, Hawrylowicz CM. Immunoregulatory mechanisms of vitamin D relevant to respiratory health and asthma. Annals of the New York Academy of Sciences 2014;1317:57‐69. [DOI] [PubMed] [Google Scholar]
Martineau 2007
- Martineau AR, Wilkinson KA, Newton SM, Floto RA, Norman AW, Skolimowska K, et al. IFN‐gamma‐ and TNF‐independent vitamin D‐inducible human suppression of mycobacteria: the role of cathelicidin LL‐37. Journal of Immunology 2007;178(11):7190‐8. [DOI] [PubMed] [Google Scholar]
Martineau 2012
- Martineau AR. Bolus‐dose vitamin D and prevention of childhood pneumonia. The Lancet 2012;379(9824):1373‐5. [DOI] [PubMed] [Google Scholar]
Martineau 2015a
- Martineau AR, James WY, Hooper RL, Barnes NC, Jolliffe DA, Greiller CL, et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double‐blind, randomised controlled trial. The Lancet Respiratory Medicine 2015;3(2):120‐30. [DOI] [PubMed] [Google Scholar]
Martineau 2015b
- Martineau A, Takeda A, Nurmatov U, Sheikh A, Griffiths CJ. Vitamin D for the management of asthma. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD011511] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):Epub 2009 Jul 21. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reddel 2009
- Reddel HK, Taylor DR, Bateman ED, Boulet LP, Boushey HA, Busse WW, et al. An official American Thoracic Society/European Respiratory Society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. American Journal of Respiratory and Critical Care Medicine 2009;180(1):59‐99. [DOI] [PubMed] [Google Scholar]
RevMan 2015 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2015.
Riverin 2015
- Riverin BD, Maguire JL, Li P. Vitamin D supplementation for childhood asthma: a systematic review and meta‐analysis. PLoS ONE 2015;10(8):e0136841. [DOI] [PMC free article] [PubMed] [Google Scholar]
Romagnoli 2008
- Romagnoli E, Mascia ML, Cipriani C, Fassino V, Mazzei F, D'Erasmo E, et al. Short and long‐term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. Journal of Clinical Endocrinology and Metabolism 2008;93(8):3015‐20. [DOI] [PubMed] [Google Scholar]
Singh 2006
- Singh AM, Busse WW. Asthma exacerbations 2: aetiology. Thorax 2006;61(9):809‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
To 2012
- To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, et al. Global asthma prevalence in adults: findings from the cross‐sectional world health survey. BMC Public Health 2012;12:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vieth 2009
- Vieth R. How to optimize vitamin D supplementation to prevent cancer, based on cellular adaptation and hydroxylase enzymology. Anticancer Research 2009;29(9):3675‐84. [PubMed] [Google Scholar]
Xiao 2015
- Xiao L, Xing C, Yang Z, Xu S, Wang M, Du H, et al. Vitamin D supplementation for the prevention of childhood acute respiratory infections: a systematic review of randomised controlled trials. British Journal of Nutrition 2015;114:1026‐34. [DOI] [PubMed] [Google Scholar]
Xystrakis 2006
- Xystrakis E, Kusumakar S, Boswell S, Peek E, Urry Z, Richards DF, et al. Reversing the defective induction of IL‐10‐secreting regulatory T cells in glucocorticoid‐resistant asthma patients. Journal of Clinical Investigation 2006;116(1):146‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]


