Abstract
Background
Cancer is a common disease and radiotherapy is one well‐established treatment for some solid tumours. Hyperbaric oxygenation therapy (HBOT) may improve the ability of radiotherapy to kill hypoxic cancer cells, so the administration of radiotherapy while breathing hyperbaric oxygen may result in a reduction in mortality and recurrence.
Objectives
To assess the benefits and harms of radiotherapy while breathing HBO.
Search methods
In March 2011 we searched The Cochrane Central Register of Controlled Trials (CENTRAL), (The Cochrane Library, Issue 3), MEDLINE, EMBASE, DORCTHIM and reference lists of articles.
Selection criteria
Randomised and quasi‐randomised studies comparing the outcome of malignant tumours following radiation therapy while breathing HBO versus air.
Data collection and analysis
Three review authors independently evaluated the quality of the relevant trials and extracted the data from the included trials.
Main results
Nineteen trials contributed to this review (2286 patients: 1103 allocated to HBOT and 1153 to control). With HBOT, there was a reduction in mortality for head and neck cancers at both one year and five years after therapy (risk ratio (RR) 0.83, P = 0.03, number needed to treat (NNT) = 11; and RR 0.82, P = 0.03, NNT = 5 respectively), as well as improved local tumour control at three months (RR with HBOT 0.58, P = 0.006, NNT = 7). The effect of HBOT varied with different fractionation schemes. Local tumour recurrence was less likely with HBOT at one year (head and neck: RR 0.66, P < 0.0001, NNT = 5), two years (uterine cervix: RR 0.60, P = 0.04, NNT = 5) and five years (head and neck: (RR 0.77, P = 0.01, NNT = 6). Any advantage is achieved at the cost of some adverse effects. There was a significant increase in the rate of both severe radiation tissue injury (RR 2.35, P < 0.0001, (number needed to harm (NNH) = 8) and the chance of seizures during therapy (RR 6.76, P = 0.03, NNH = 22) with HBOT.
Authors' conclusions
There is some evidence that HBOT improves local tumour control and mortality for cancers of the head and neck, and local tumour recurrence in cancers of the head and neck, and uterine cervix. These benefits may only occur with unusual fractionation schemes. HBOT is associated with significant adverse effects including oxygen toxic seizures and severe tissue radiation injury. The methodological and reporting inadequacies of the studies included demand a cautious interpretation. More research is needed for head and neck cancer, but is probably not justified for bladder cancer. There is little evidence available concerning malignancies at other anatomical sites on which to base a recommendation.
Keywords: Female; Humans; Male; Radiation Tolerance; Bronchial Neoplasms; Bronchial Neoplasms/mortality; Bronchial Neoplasms/radiotherapy; Combined Modality Therapy; Combined Modality Therapy/methods; Esophageal Neoplasms; Esophageal Neoplasms/mortality; Esophageal Neoplasms/radiotherapy; Head and Neck Neoplasms; Head and Neck Neoplasms/mortality; Head and Neck Neoplasms/radiotherapy; Hyperbaric Oxygenation; Hyperbaric Oxygenation/adverse effects; Hyperbaric Oxygenation/methods; Neoplasm Recurrence, Local; Neoplasm Recurrence, Local/epidemiology; Neoplasms; Neoplasms/mortality; Neoplasms/radiotherapy; Randomized Controlled Trials as Topic; Rectal Neoplasms; Rectal Neoplasms/mortality; Rectal Neoplasms/radiotherapy; Time Factors; Urinary Bladder Neoplasms; Urinary Bladder Neoplasms/mortality; Urinary Bladder Neoplasms/radiotherapy; Uterine Cervical Neoplasms; Uterine Cervical Neoplasms/mortality; Uterine Cervical Neoplasms/radiotherapy
High pressure oxygen breathing during radiotherapy for cancer treatment
Breathing oxygen while at raised pressure in a closed chamber (hyperbaric oxygen or HBO) may increase the effectiveness of radiotherapy and thus improve mortality and reduce tumour regrowth. We found some evidence that people with head and neck cancer are less likely to die within five years if they are treated this way, and evidence that regrowth of tumour at the original site is less likely for head and neck, and cervical cancer. However, Hyperbaric oxygen therapy (HBOT) may only be effective when radiotherapy is given in an unusually small number of sessions, each with a relatively high dose. HBOT does not appear to work for other cancers studied. Our conclusions are based on 19 randomised trials with over 2000 patients.
Background
Description of the condition
Invasive cancer continues to be a major world health problem. According to World Health Organization (WHO) statistics, more than 10 million people are diagnosed with cancer every year, and it is estimated there will be 15 million new cases every year by 2020. Cancer causes 6 million deaths every year or 12% of deaths worldwide (WHO 2004), and being associated with approximately 0.5 million deaths each year it is the second leading cause of death in the USA (ACS 2004; Hotes 2003). Radiotherapy is a well established treatment of suitable malignancies in a wide variety of anatomical areas. In the USA, approximately 1.2 million new cases are diagnosed annually, and about 50% of these will be treated with radiation (Jemal 2002).
Description of the intervention
Hyperbaric oxygen therapy (HBOT) is relatively widely available in North America (where there are more than 300 facilities registered with the Undersea and Hyperbaric Medical Society (UHMS)), Russia, China and Cuba, but is less well established in Europe and Australasia (UHMS 2001). Treatment involves placing the patient in a compression chamber, increasing the environmental pressure within the chamber, and administering 100% oxygen for respiration. In this way, it is possible to deliver a greatly increased pressure of oxygen to the tissues. Typically, treatments for tumour oxygen sensitisation involve pressurisation to between 2.0 and 4.0 atmospheres absolute (ATA) for periods between 20 and 30 minutes for pre‐oxygenation, following which the radiation therapy is delivered while the patient continues to breathe oxygen at pressure. A range of radiation fractionation and dosing schemes has been suggested.
How the intervention might work
Many, if not all, solid tumours include regions where there is significant hypoxia and it has been established for some years that these areas of hypoxia are resistant to therapy (Gray 1953; Overgaard 1996). A body of evidence exists to suggest that this radioresistance can be overcome by a variety of measures including increasing oxygen pressure within the tumour (e.g. high oxygen content breathing, administration of red blood cells), and administration of radiation sensitising agents (e.g. nitroimidazoles such as nimorazole) (Bush 1986; Grau 1992; Overgaard 1994; Rubin 1979). The effectiveness of such measures remains controversial, and despite more than 10,000 patients in total being randomised to a variety of treatment and control groups, no clinically important benefits of these treatments have been conclusively demonstrated. One review with meta‐analysis suggested a reduction in tumour recurrence at the site irradiated, and in the lymph nodes draining that site when all methods to modify tumour hypoxia were combined and compared to control, with an odds ratio (OR) of 0.83 (95% confidence interval (CI) 0.77 to 0.89) (Overgaard 1996). The search strategy, inclusion and exclusion criteria for trials, definition of outcomes and statistical methods of this review were not clear from that report.
One attractive method for increasing oxygen pressure in hypoxic areas is the administration of 100% oxygen at greater than one atmosphere total pressure, a procedure known as hyperbaric oxygenation (HBO). HBO was first used for this purpose in the 1960s and reported by Churchill‐Davidson (Churchill 1968). The technique of administering radiation whilst confined in a hyperbaric chamber was adopted in a number of centres around the world, but inherent difficulties with the physical requirements and the advent of orally administered agents to improve tumour sensitivity to radiation led to the abandonment of this combined approach during the 1980s.
Why it is important to do this review
These decisions were made despite the publication of a number of promising clinical trials with HBO, and it has been suggested HBOT was abandoned before a measured evaluation was made of the true clinical impact (Overgaard 1996). While many of the trials using HBO were included in the Overgaard 1996 review, we believe a structured systematic search may reveal further evidence, and we are aware of at least two randomised trials published after 1996 (Dische 1999; Haffty 1999).
HBOT is associated with some risk of adverse effects including damage to the ears, sinuses and lungs from the effects of pressure, temporary worsening of myopia, claustrophobia and oxygen poisoning. Although serious adverse events are rare, HBO cannot be regarded as an entirely benign intervention. It has further been suggested that HBOT may increase the incidence and/or rate of growth of local recurrence or remote metastatic disease in patients with a history of malignancy, although a recent comprehensive review fails to support these concerns (Feldmeier 2003). For all these reasons, we believe a review may clarify the true value, if any, of HBOT in this area.
Objectives
The aim of this review was to assess the evidence for the benefit of simultaneously combining radiation therapy and HBOT for the treatment of solid tumours.
-
Does the addition of HBO to radiation therapy:
reduce mortality at any time following therapy?
increase local tumour response?
reduce the incidence of local recurrence?
reduce the incidence of metastatic spread?
improve the quality of life (QoL) for these patients?
Does sensitisation to radiation therapy with HBO, compared to other agents, produce any of the benefits above?
Is HBO administration safe in this setting?
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) or quasi‐RCTs that:
compared the effect of simultaneous HBOT and radiation therapy to regimens employing radiation therapy while breathing air, or
compared the effect of simultaneous HBOT and radiation therapy to regimens employing another sensitising therapy and radiation therapy.
Types of participants
Patients with solid tumours where radiation therapy is indicated. We did not impose any restrictions on the basis of age or gender.
Types of interventions
We included studies that compared treatment regimens which included HBO with similar regimens that excluded HBO, with or without the use of other sensitisers. Where cointerventions or fractionation regimens differed significantly between studies we clearly stated this and discussed the implications, or performed an appropriate subgroup analysis.
We accepted studies of HBO administered in a compression chamber at any pressure above 1.0 ATA, either simultaneously with, or immediately following radiation therapy.
Types of outcome measures
Primary outcomes
Mortality rate at any time.
Complete or partial failure to control local tumour at any time.
Local recurrence rate at any time.
Metastatic disease at any time.
Secondary outcomes
Quality of life (QoL) assessment.
Adverse effects of HBOT
Specific to combined HBOT/radiation therapy
Acute tissue reaction in irradiated area.
Late tissue injury in irradiated area.
Pain scores.
General relating to HBO
Visual disturbance (short and long term).
Barotrauma (aural, sinus, pulmonary in the short and long term).
Oxygen toxicity (short term).
Any other recorded adverse effects would be reported and discussed.
Search methods for identification of studies
Electronic searches
It was our intention to capture both published and unpublished studies.
We searched the following (from inception) in November 2004 and then repeated the searches in September 2008 and March 2011 (not CINAHL): Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2011, Issue 1), MEDLINE (week 1, 2011), EMBASE (week 11, 2011), and an additional database developed in our hyperbaric facility (the Database of Randomised Trials in Hyperbaric Medicine, Bennett 2011). We searched CINAHL in 2004 and 2008, but not 2011. The search strategy was broad and the strategies we used are given in Appendix 1, Appendix 2, Appendix 3 and Appendix 4.
Searching other resources
In addition we made a systematic search for relevant controlled trials in specific hyperbaric literature sources as follows.
We contacted experts in the field and leading hyperbaric therapy centres (as identified by personal communication and searching the Internet) and asked for additional relevant data in terms of published or unpublished randomised trials.
We handsearched relevant hyperbaric textbooks (Jain 2009; Kindwall 2008; Mathieu 2006; Neuman 2008), journals (Undersea and Hyperbaric Medicine, Hyperbaric Medicine Review, South Pacific Underwater Medicine Society (SPUMS) Journal, European Journal of Hyperbaric Medicine and Aviation and Space and Environmental Medicine Journal) and conference proceedings (Undersea and Hyperbaric Medical Society, SPUMS, European Undersea and Baromedical Society and International Congress of Hyperbaric Medicine) published since 1980.
We contacted authors of relevant studies to request details of unpublished or ongoing investigations.
We examined the reference list of all trials for inclusion in this review.
We considered all languages. We contacted authors if there was any ambiguity about the published data.
Data collection and analysis
Selection of studies
Two review authors (MB and RS) scanned the records retrieved by the initial search to identify trials that met the inclusion criteria. The same two review authors retrieved and reviewed the full‐text articles for the purpose of applying inclusion criteria independently. In all instances, differences of opinion were to be resolved by discussion among the reviewers and referral to a third reviewer (CM) for a decision. This was not necessary, however.
Data extraction and management
Two review authors (MB and RS) used standardisation forms to independently extract data from the studies. Extracted data included the following characteristics: methods (number eligible and randomised, adequacy of randomisation, allocation concealment, blinding, and completeness of follow‐up); participant characteristics and exclusions; interventions; outcomes (dichotomous variables (number with outcome of interest); and continuous variables (mean and standard deviation). We attempted to contact primary authors when missing data were encountered or if necessary data were not clearly stated. The review authors resolved all differences by discussion.
Assessment of risk of bias in included studies
We assessed study quality by using an adaptation of the method outlined in Schulz 1995. Results from the study quality are presented in a descriptive manner. We assessed the following characteristics.
Adequacy of the randomisation process: A ‐ Adequate sequence generation is reported using random number tables, computer random number generator, coin tossing, or shuffling. B ‐ Did not specify one of the adequate reported methods in (A) but mentioned the randomisation method. C ‐ Other methods of allocation that appear to be unbiased.
Adequacy of the allocation concealment process: A ‐ Adequate measures to conceal allocations such as central randomisation; serially numbered, opaque, sealed envelopes; or other description that contained convincing elements of concealment. B‐ Unclearly concealed trials in which the author either did not report an allocation concealment approach at all, or reported an approach that did not fall into one of the categories in (A). C‐ Inadequately concealed trials in which method of allocation is not concealed such as alternation methods or use of case record numbers.
Potential for selection bias after allocation: A‐ Trials where an intention‐to‐treat (ITT) analysis is possible and few losses to follow‐up are noted. B‐ Trials which reported exclusions (as listed in (A) but exclusions were less than 10%). C‐ No reporting on exclusions or exclusions greater than 10% or wide differences in exclusions between groups.
Level of masking (treatment provider, patient, outcome assessor): A‐ Double‐ or triple‐blind. B‐ Single‐blind. C‐ Non‐blind.
Sensitivity analysis
We used a fixed‐effect model where there was no evidence of significant heterogeneity between studies and a random‐effects model when such heterogeneity was likely (DerSimonian 1986). We considered the appropriateness of meta‐analysis in the presence of significant clinical or statistical heterogeneity. We tested heterogeneity using the I2 statistic and assumed significant heterogeneity if I2 was greater than 40% (more than 40% of the variability in outcome between trials could not be explained by sampling variation) (Higgins 2003). Where appropriate data were available or could be extracted, we intended to compare survival over time using the lgHR and variance (Parmar 1998). For proportions (dichotomous outcomes), we used RR. We would have converted continuous data to the weighted mean difference (WMD) using the inverse variance method and calculated an overall WMD. We tested selection bias using a funnel plot, depending on the number of clinical trials included in the individual outcomes.
We considered sensitivity analysis on the basis of the presence or absence of clear allocation concealment, however this was not appropriate.
Where appropriate data existed, we performed subgroup analyses based on:
age ‐ adults versus children (less than 16 years);
dose of oxygen received (pressure less than 2.5 ATA versus greater than or equal to 2.5 ATA);
dose and fractionation of radiation therapy: large fractions (total dose over 12 or fewer fractions) versus conventional fractions (total dose over 12 fractions); and
simultaneous versus sequential administration of HBOT.
Results
Description of studies
Results of the search
In our original searches in 2004 and 2008, we identified a combined 106 publications apparently dealing with the use of HBOT in conjunction with therapeutic radiotherapy. A further search (combined strategy with that for a related Cochrane review, 'Hyperbaric oxygen therapy for late radiation tissue injury' (Bennett 2005) update in progress) located another 180 publications. Following identification and deletion of duplicate publications, we culled this list to 226 publications. Initial examination of the titles suggested 123 were not relevant to this review, leaving 103 publications for which we retrieved the abstracts, where available. Examination of the abstracts determined that 60 were not relevant to this review. We retrieved the full reports of the remaining 43 possible comparative trials. After appraisal, we further excluded five as reviews without new data and 19 as abstracts or interim reports of randomised controlled trials (RCTs) where the data was reported more fully in another publication (see table 'Characteristics of excluded studies'). We accepted the remaining 19 trials for this review (see Figure 1 for the study flow details).
Figure 1.
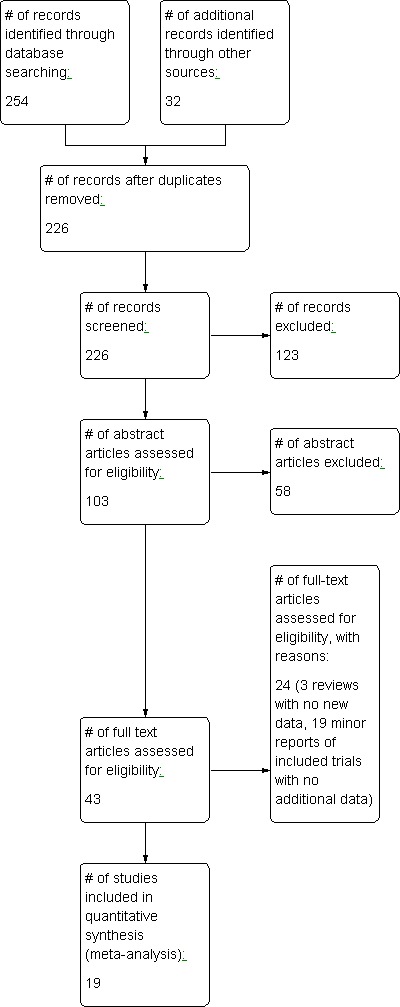
Study flow diagram.
Included studies
The included trials were published between 1967 and 1999, and the review authors are unaware of any ongoing RCTs in the area. The trials report data concerning the treatment of malignant tumours from several different sites: head and neck (Berry 1979; Chang 1973; Haffty 1999; Henk 1977a; Henk 1986; Sause 1979; Sealy 1986; Shigematsu 1973; Tobin 1971; Van Den Brenk 1968); uterine cervix (Brady 1981; Dische 1999; Fletcher 1977; Glassburn 1974; Tobin 1971; Ward 1979; Watson 1978); urinary bladder (Cade 1967; Cade 1978; Plenk 1972; Tobin 1971; Van Den Brenk 1968); bronchus (Cade 1967); rectum (Tobin 1971); brain (Tobin 1971); and oesophagus (Tobin 1971). In total, these trials enrolled 2286 subjects, of which 1103 were allocated to receive HBO and 1153 to control (no allocation information was available on 30 subjects). The largest trial (Dische 1999) accounts for 14.7% of cases in this review and the smallest (Berry 1979) for 1%. (See table: Characteristics of included studies).
The dose of oxygen per treatment session in the HBO arm was remarkably uniform, with all trials except one administering external beam radiation therapy at 3 ATA for between 30 and 40 minutes. The exception was Haffty 1999 who used oxygen at 4 ATA and required all patients to be anaesthetised and intubated because of the risk of oxygen toxic seizures. However, the total number of treatment sessions varied widely. The shortest fractionation scheme was two sessions only, separated by three weeks (Haffty 1999) and the longest was 40 sessions over eight weeks (Cade 1967; Cade 1978). External beam radiation dose also varied widely in both arms of the studies with a range from 2600 rads (Haffty 1999) to 7000 rads (Shigematsu 1973) for the control groups and from 2300 rads (Haffty 1999) to 6000 rads (Cade 1967; Cade 1978) for the HBO groups. Most studies of the treatment of uterine cervical cancer also included intracavity placement of radioactive material, the exception being Tobin 1971. One trial examined the efficacy of HBO plus a second sensitising agent, misonidazole (Sealy 1986).
None of the included studies employed a sham therapy, so no comparisons between the efficacy of HBO and air breathing during radiotherapy were blinded to either patients or treatment providers.The follow‐up period varied between trials, ranging from six months (Van Den Brenk 1968) to 10 years (Haffty 1999), although most studies followed subjects for between two and five years. All included studies reported at least one outcome of interest. Of the outcomes identified above, these trials reported data on all four primary outcomes, and on adverse effects of therapy, but not the secondary outcome of QoL.
Other outcomes (including non‐clinical) reported include: selected cause mortality (Henk 1977a), development of radiation tissue effects (Henk 1977a; Shigematsu 1973), disease‐free survival (Fletcher 1977), survival according to histology (Cade 1978), development of new primary malignancy (Sealy 1986), relationship between dose and morbidity (Brady 1981; Dische 1999) and the incidence of salvage surgery (Henk 1986; Sause 1979).
Risk of bias in included studies
Details of the quality assessment are given in the table Characteristics of included studies. In general, study quality was assessed as fair with regard to methodology. The significance of variations in quality detailed below is unclear and given that we were able to pool relatively few analyses, we did not use study quality as a basis for sensitivity analysis.
Randomisation
Randomisation procedures were described as by centrally supplied sealed envelopes in Berry 1979; Cade 1967; Cade 1978; Dische 1999; Henk 1986; Ward 1979 and Watson 1978. Although not stated in the report, it is likely this is true also of Henk 1977a, as this trial was undertaken under the auspices of the same group (British Medical Council). Three trials (Chang 1973; Haffty 1999; Sealy 1986) also employed a sealed envelope system, while Plenk 1972 used a random number table and Tobin 1971 a card drawn by a disinterested person. The method of randomisation was not stated in four studies (Brady 1981; Fletcher 1977; Glassburn 1974; Sause 1979) and was quasi‐random in two studies: Shigematsu 1973 employed a method based on the registration number, while Van Den Brenk 1968 used birth date.
Concealment of allocation
Allocation concealment appeared adequate for the British Medical Council trials but in none of the remaining studies is there a clear indication that the investigators were unable to predict the prospective group to which a participant would be allocated.
Subject baseline characteristics
Subjects entered into all trials had proven malignancies where radiotherapy was the treatment of choice in the anatomical area of interest to the particular trial. Many trials included only subjects who were less than 75 years old. Details of staging are given in the table Characteristics of included studies, but were generally reasonably consistent across trials.
Blinding
None of the studies included were blinded in any way.
Intention‐to‐treat (ITT) analysis
Nine studies reported no losses to follow‐up (Berry 1979; Cade 1967; Chang 1973; Fletcher 1977; Glassburn 1974; Haffty 1999; Shigematsu 1973; Van Den Brenk 1968; Watson 1978). Two studies reported analysing patients randomised to receive HBO in the control group (Berry 1979; Ward 1979), while 10 studies reported losses to follow‐up, none of which appear in analysis in those reports. The highest proportion of lost subjects was in Plenk 1972, who lost 22 subjects at final follow‐up, 55% of the total enrolled. We have performed sensitivity analysis using best and worse case scenarios, where possible, for dichotomous outcomes involving those studies with losses to follow‐up.
None of the included studies specifically indicated an ITT approach, however 8 of 19 studies (see above) reported full follow‐up and did not report any protocol violation.
Effects of interventions
Primary outcomes
1. Death rate
All trials reported mortality rate at some time, and therefore contribute to this outcome. There was insufficient data in any trial to permit calculation of survival over time using the log hazard ratio (lgHR).
One year mortality
Mortality at one year with head and neck cancer (Analysis 1.1)
Nine trials reported this outcome (Berry 1979; Chang 1973; Haffty 1999; Henk 1977a; Henk 1986; Sealy 1986; Shigematsu 1973; Tobin 1971; Van Den Brenk 1968), for 710 subjects after exclusion of withdrawals (31% of the total subjects in this review), with 339 (48%) allocated to HBOT and 371 (52%) to control. Over all fractionation schemes, there was a statistically significant reduction in the proportion of subjects dying within one year after receiving radiation therapy with HBOT (RR of death with HBOT was 0.83; 95% CI 0.70 to 0.98, P = 0.03). There was no evidence of substantial heterogeneity between trials overall (I2 = 0%), but some heterogeneity for the trials using fewer than 12 sessions of HBO compared to more than 12 using air (I2 = 39%), so these results are achieved using a random effects model. There is an absolute risk reduction of 9.2% when using HBOT (number needed to treat (NNT) to avoid one death = 11; 95% CI 7 to 52).
The reduction in risk of death overall is sensitive to the allocation of withdrawals (best case scenario: RR 0.73; 95% CI 0.62 to 0.85, P = 0.0001 (Analysis 1.2); worst case scenario: RR 0.93; 95% CI 0.76 to 1.15, P = 0.51 (Analysis 1.3). However, the risk for those receiving 12 fractions with HBO versus more than 12 fractions in air is not sensitive to allocation of withdrawals (worst case scenario: RR 0.72; 95% CI 0.56 to 0.92, P = 0.01).
Analysis 1.2.
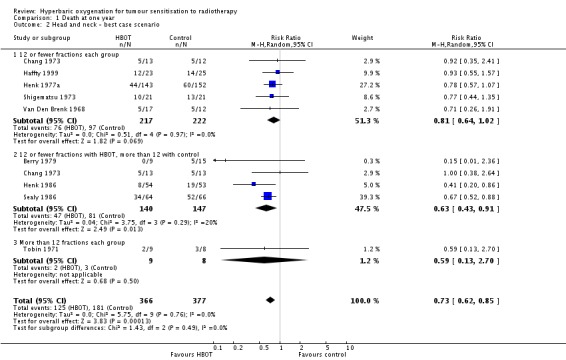
Comparison 1 Death at one year, Outcome 2 Head and neck ‐ best case scenario.
Analysis 1.3.
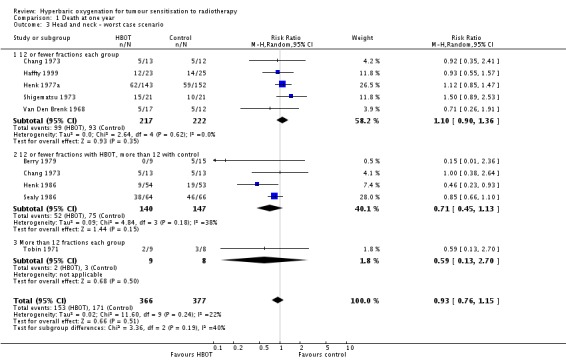
Comparison 1 Death at one year, Outcome 3 Head and neck ‐ worst case scenario.
Mortality at one year with cancer of the uterine cervix (Analysis 1.4)
Four trials reported this outcome (Dische 1999; Tobin 1971; Ward 1979; Watson 1978), for 751 subjects after exclusion of withdrawals (33% of the total subjects in this review), with 348 (46%) allocated to HBOT and 384 (54%) to control. There was no statistically significant reduction in the proportion of subjects dying within one year after receiving radiation therapy with HBOT (RR 0.88; 95% CI 0.69 to 1.11, P = 0.27), neither did subgroup analysis suggest any benefit with different fractionation schemes. There was no evidence of substantial heterogeneity between trials overall (I2 = 0%) and this result is achieved using a fixed effects model. The risk of death was not sensitive to the allocation of withdrawals (best case scenario: RR 0.87; 95% CI 0.69 to 1.10, P = 0.25 (Analysis 1.5); worst case scenario: RR 0.91; 95% CI 0.72 to 1.15, P = 0.43 (Analysis 1.6)).
Analysis 1.5.
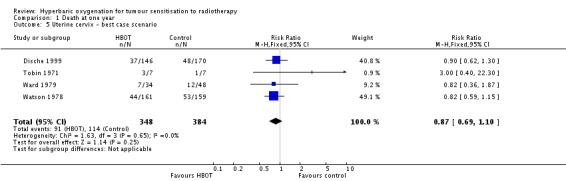
Comparison 1 Death at one year, Outcome 5 Uterine cervix ‐ best case scenario.
Analysis 1.6.
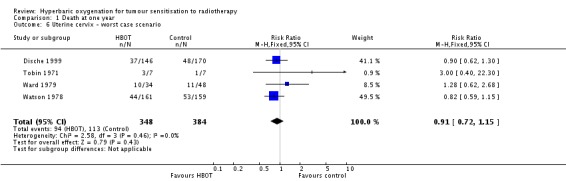
Comparison 1 Death at one year, Outcome 6 Uterine cervix ‐ worst case scenario.
Mortality at one year with cancer of the urinary bladder (Analysis 1.7)
Four trials reported this outcome (Cade 1967; Cade 1978; Plenk 1972; Van Den Brenk 1968), for 330 subjects after exclusion of withdrawals (14% of the total subjects in this review), with 165 allocated to both HBOT and control. There was no statistically significant reduction in the proportion of subjects dying within one year after receiving radiation therapy with HBOT (RR 0.97; 95% CI 0.74 to 1.27, P = 0 .82), neither did subgroup analysis suggest any benefit with different fractionation schemes. There was moderate heterogeneity between trials overall (I2 = 39%) (fixed‐effect model). The risk of death was not sensitive to the allocation of withdrawals (best case scenario: RR 0.92; 95% CI 0.71 to 1.21, P = 0.56 (Analysis 1.8); worst case scenario: RR 1.03; 95% CI 0.78 to 1.34, P = 0.86 (Analysis 1.9)).
Analysis 1.8.
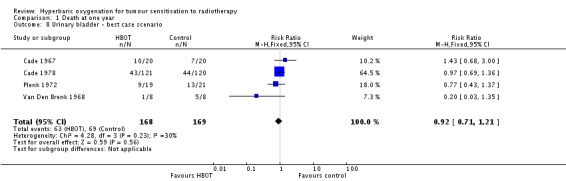
Comparison 1 Death at one year, Outcome 8 Urinary bladder ‐ best case scenario.
Analysis 1.9.
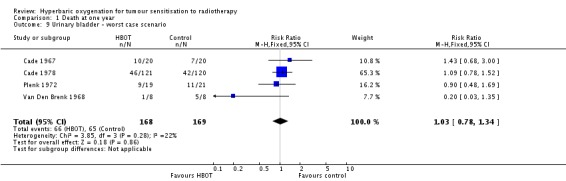
Comparison 1 Death at one year, Outcome 9 Urinary bladder ‐ worst case scenario.
Mortality at one year with carcinoma of the bronchus (Analysis 1.10)
One trial reported this outcome (Cade 1967), involving 49 subjects after exclusion of withdrawals (2% of the total subjects in this review), with 25 (51%) allocated to HBOT and 24 (49%) to control. There was no statistically significant difference in the proportion of subjects dying within one year after receiving radiation therapy with HBOT (RR 1.09; 95% CI 0.72 to 1.64, P = 0.69).
Mortality at one year with carcinoma of the rectum (Analysis 1.11)
One trial reported this outcome (Tobin 1971), involving four subjects (0.2% of the total subjects in this review), with two allocated to both HBOT and control. Both subjects died following HBOT and one of those receiving the control. There was no statistically significant difference in the proportion of subjects dying within one year after receiving radiation therapy with HBOT (RR 1.67; 95% CI 0.48 to 5.76, P = 0.42).
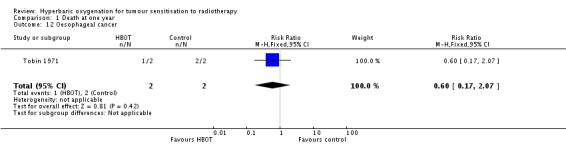
Mortality at one year with carcinoma of the oesophagus (Analysis 1.12)
One trial reported this outcome (Tobin 1971), involving four subjects (0.2% of the total subjects in this review), with two allocated to both HBOT and control. One subject died following HBOT and both of those receiving the control. There was no statistically significant difference in the proportion of subjects dying within one year after receiving radiation therapy with HBOT (RR 0.2; 95% CI 0.00 to 8.82, P = 0.4).
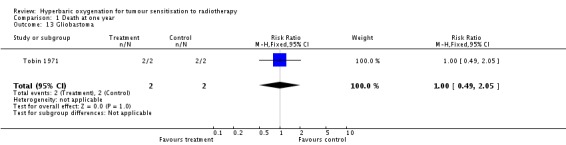
Mortality at one year with glioblastoma (Analysis 1.13)
One trial reported this outcome (Tobin 1971), involving four subjects (0.2% of the total subjects in this review), with two allocated to both HBOT and control. All subjects died within one year, making analysis unhelpful.
Mortality at two years
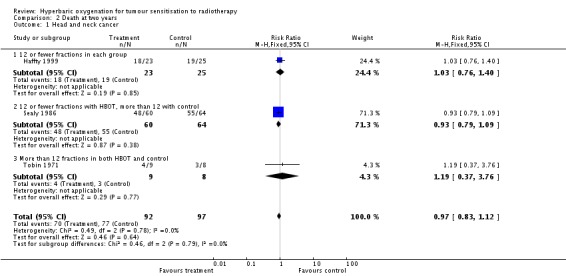
Mortality at two years with head and neck cancer (Analysis 2.1)
Three trials reported this outcome (Haffty 1999; Sealy 1986; Tobin 1971) for 189 subjects after exclusion of withdrawals (8% of the total subjects in this review), with 92 (49%) allocated to HBOT and 97 (51%) to control. Sealy 1986 contributes 65% of the weight to this analysis. There was no statistically significant reduction in the proportion of subjects dying within two years after receiving radiation therapy with HBOT (RR 0.84; 95% CI 08.3 to 1.12, P = 0.64), neither did subgroup analysis suggest any benefit with different fractionation schemes. There was no evidence of substantial heterogeneity between trials overall (I2 = 0%) and this result is achieved using a fixed effects model. The reduction in risk of death is not sensitive to the allocation of withdrawals (best case scenario: RR 0.92; 95% CI 0.79 to 1.07, P = 0.28 (Analysis 2.2); worst case scenario: RR 1.00; 95% CI 0.86 to 1.15, P = 0.97 (Analysis 2.3)).
Analysis 2.2.
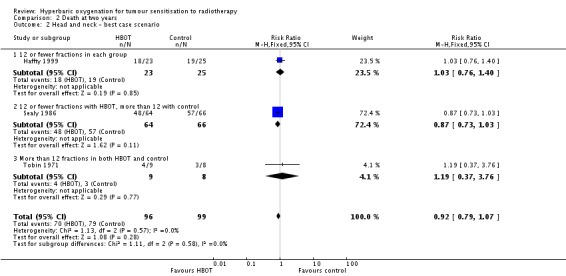
Comparison 2 Death at two years, Outcome 2 Head and neck ‐ best case scenario.
Analysis 2.3.
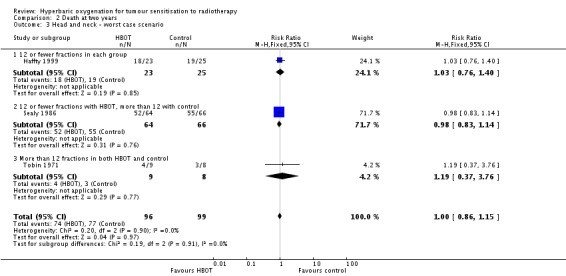
Comparison 2 Death at two years, Outcome 3 Head and neck ‐ worst case scenario.
Mortality at two years with cancer of the uterine cervix (Analysis 2.4)
Four trials reported this outcome (Fletcher 1977; Glassburn 1974; Tobin 1971; Watson 1978) for 607 subjects after exclusion of withdrawals (27% of the total subjects in this review), with 294 (48%) allocated to HBOT and 313 (52%) to control. There was no statistically significant reduction in the proportion of subjects dying within two years after receiving radiation therapy with HBOT (RR 0.94; 95% CI 0.76 to 1.15, P = 0.53), neither did subgroup analysis suggest any benefit with different fractionation schemes. There was evidence of moderate heterogeneity between trials overall (I2 = 36%) (random‐effects model). No trials had suffered any losses to follow‐up after randomisation.
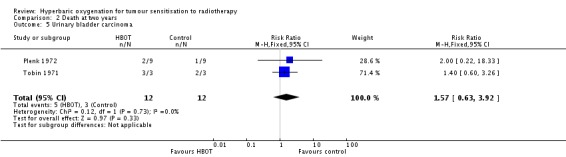
Mortality at two years with urinary bladder carcinoma (Analysis 2.5 comparison 2, outcomes 05, 06, 07)
Two trials reported this outcome (Plenk 1972; Tobin 1971) for 24 subjects after exclusion of withdrawals (1% of the total subjects in this review), with 12 allocated to both HBOT and control. Plenk 1972 contributes 71% of the weight to this analysis. There was no statistically significant difference in the proportion of subjects dying within two years after receiving radiation therapy with HBOT (RR 1.57; 95% CI 0.63 to 3.92, P = 0.33). There was no evidence of substantial heterogeneity between trials overall (I2 = 0%) (fixed‐effect model). The risk of death with HBOT is sensitive to the allocation of the large number of losses to follow‐up in the Plenk 1972 trial (best case scenario: RR 0.47; 95% CI 0.04 to 5.24, P = 0.54 (Analysis 2.6); worst case scenario: RR 5.18; 95% CI 2.18 to 12.31, P = 0.0002 (Analysis 2.7)).
Analysis 2.6.
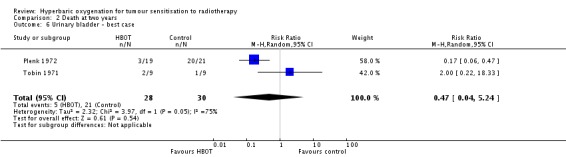
Comparison 2 Death at two years, Outcome 6 Urinary bladder ‐ best case.
Analysis 2.7.

Comparison 2 Death at two years, Outcome 7 Urinary bladder ‐ worst case.
Mortality at five years
Mortality at five years with head and neck cancer (Analysis 3.1)
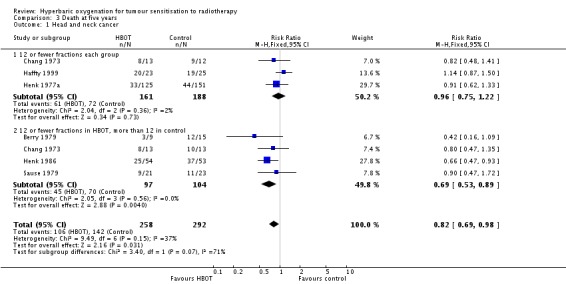
Six trials reported this outcome (Berry 1979; Chang 1973; Haffty 1999; Henk 1977a; Henk 1986; Sause 1979) for 550 subjects after exclusion of withdrawals (24% of the total subjects in this review), with 258 (47%) allocated to HBOT and 292 (53%) to control. Over all fractionation schemes there was a statistically significant reduction in the proportion of subjects dying within five years after receiving radiation therapy with HBOT (RR 0.82; 95% CI 0.69 to 0.98, P = 0.03), however subgroup analysis by fractionation scheme suggests the benefit may be restricted to those who receive 12 or fewer fractions when compared to those who receive a standard fractionation scheme of more than 12 sessions (RR in this group 0.69; 95% CI 0.53 to 0.89, P = 0.004; RR for 12 or fewer fractions in each group 0.96; 95% CI 0.75 to 1.22, P = 0.73). There was moderate heterogeneity between trials overall (I2 = 37%), however little evidence for heterogeneity within each subgroup of fraction schemes(fixed‐effect model). There is an absolute risk reduction of 7.5% (NNT = 14; 95% CI 7 to infinity) overall, but a 20.9% reduction for those who receive 12 or fewer fractions when compared to those who receive a standard fractionation scheme of more than 12 sessions (NNT = 5; 95% CI 3 to 14).
The overall reduction in risk of death is sensitive to the allocation of withdrawals (best case scenario: RR 0.77; 95% CI 0.64 to 0.92, P = 0.004 (Analysis 3.2); worst case scenario: RR 0.96; 95% CI 0.81 to 1.13, P = 0.6 (Analysis 3.3)), however, the risk for those receiving 12 fractions with HBO versus more than 12 fractions in air is not sensitive to allocation of withdrawals (worst case scenario: RR 0.75; 95% CI 0.59 to 0.96, P = 0.02).
Analysis 3.2.
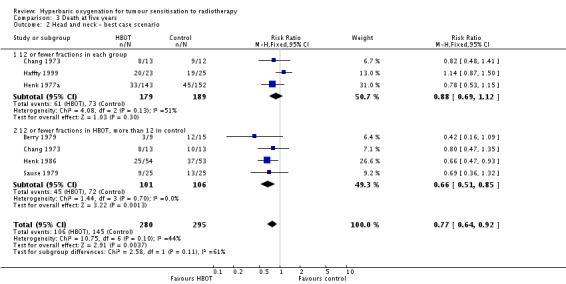
Comparison 3 Death at five years, Outcome 2 Head and neck ‐ best case scenario.
Analysis 3.3.
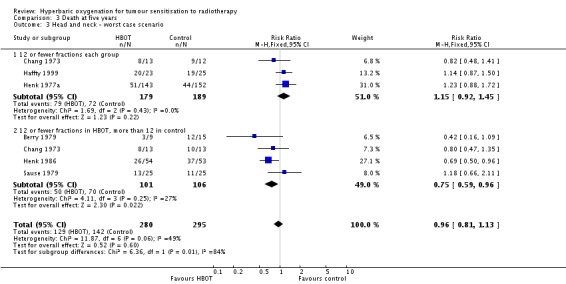
Comparison 3 Death at five years, Outcome 3 Head and neck ‐ worst case scenario.
Mortality at five years with cancer of the uterine cervix (Analysis 3.4))
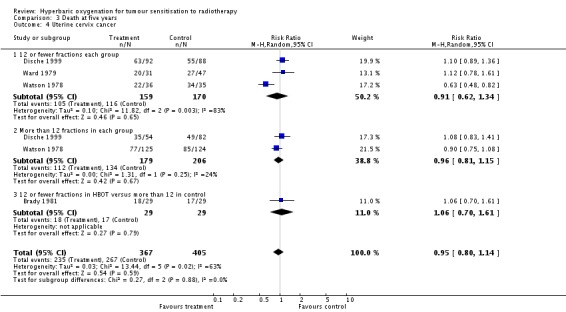
Four trials reported this outcome (Brady 1981; Dische 1999; Ward 1979; Watson 1978) for 772 subjects after exclusion of withdrawals (34% of the total subjects in this review), with 367 (48%) allocated to HBOT and 405 (52%) to control. There was no significant reduction in the proportion of subjects dying within five years after receiving radiation therapy with HBOT (RR 0.95; 95% CI 0.80 to 1.14, P = 0.59). There was considerable heterogeneity between trials (I2 = 63%) for which Watson 1978 is largely responsible (suggesting a strong beneficial effect of HBOT) (random‐effects model). The result was not sensitive to the allocation of withdrawals (best case scenario: RR 0.92; 95% CI 0.77 to 1.09, P = 0.32 (Analysis 3.5); worst case scenario: RR 0.98, 95% CI 0.81 to 1.18, P = 0.8 (Analysis 3.6)).
Analysis 3.5.
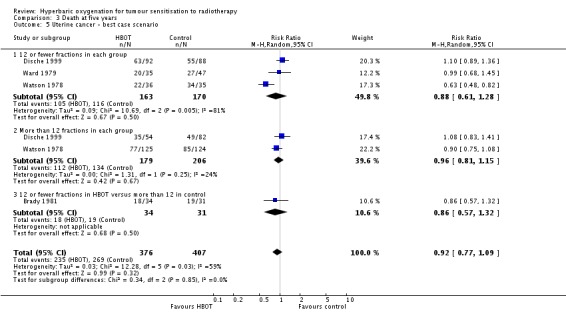
Comparison 3 Death at five years, Outcome 5 Uterine cancer ‐ best case scenario.
Analysis 3.6.
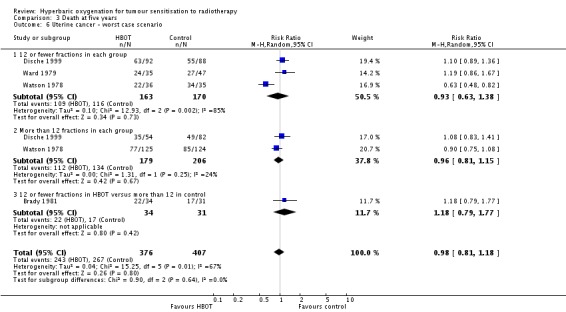
Comparison 3 Death at five years, Outcome 6 Uterine cancer ‐ worst case scenario.
Mortality at five years with urinary bladder cancer (Analysis 3.7))
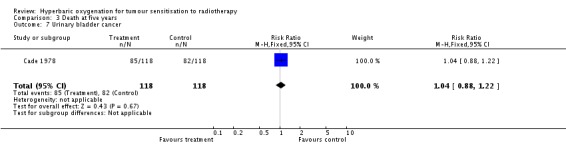
One trial reported this outcome (Cade 1978) for 236 subjects after exclusion of withdrawals (10% of the total subjects in this review), with 118 allocated to each of HBOT and control. There was no reduction in the proportion of subjects dying within five years after receiving radiation therapy with HBOT (RR 1.04; 95% CI 0.88 to 1.22, P = 0.67). We could not perform a sensitivity analysis for the five subjects lost to analysis due to lack of information about original allocation.
2. Failure to control local tumour
Failure to control local tumour at three months in head and neck cancer (Analysis 4.1))
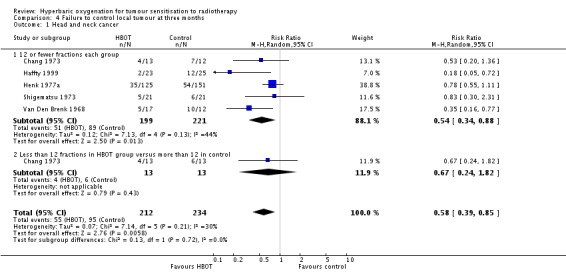
Four trials reported this outcome (Haffty 1999; Henk 1977a; Shigematsu 1973; Van Den Brenk 1968) for 446 subjects after exclusion of withdrawals (20% of the total subjects in this review), with 212 (48%) allocated to HBOT and 234 (52%) to control. Over all fractionation schemes there was a statistically significant improvement in the chance of local tumour control at three months following radiation therapy with HBOT (RR of failure with HBOT 0.58; 95% CI 0.39 to 0.85, P = 0.006). Subgroup analysis by fractionation scheme suggests the magnitude of benefit remains similar, but statistical significance is restricted to a comparison between those who receive 12 or fewer fractions in both groups (RR in this group 0.54; 95% CI 0.34 to 0.88, P = 0.01; RR for 12 or fewer fractions in HBOT versus more than 12 with control 0.67; 95% CI 0.24 to 1.82, P = 0.43). There was moderate heterogeneity between trials overall (I2 = 26%) (fixed‐effect model). There is an absolute risk reduction of 15% when using HBOT (NNT to avoid one failure to control = 7; 95% CI 5 to 17). The overall reduction in failure to control tumour is marginally sensitive to the allocation of withdrawals (best case scenario: RR 0.57; 95% CI 0.41 to 0.78, P = 0.0005 (Analysis 4.2); worst case scenario: RR 0.59; 95% CI 0.35 to 1.00, P = 0.05 (Analysis 4.3)).
Analysis 4.2.
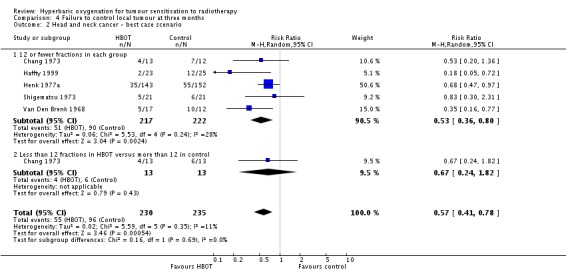
Comparison 4 Failure to control local tumour at three months, Outcome 2 Head and neck cancer ‐ best case scenario.
Analysis 4.3.

Comparison 4 Failure to control local tumour at three months, Outcome 3 Head and neck ‐ worst case scenario.
3. Local recurrence
Local recurrence at one year
Local recurrence at one year with head and neck cancer (Analysis 5.1)
Five trials reported this outcome (Haffty 1999; Henk 1977a; Henk 1986; Sealy 1986; Shigematsu 1973) for 714 subjects after exclusion of withdrawals (31% of the total subjects in this review), with 338 (47%) allocated to HBOT and 376 (53%) to control. Over all fractionation schemes there was a statistically significant reduction in the incidence of local tumour recurrence following radiation therapy with HBOT (RR 0.66; 95% CI 0.56 to 0.78, P < 0.00001). Subgroup analysis by fractionation scheme suggests the benefit is independent of fractionation scheme (RR with fewer than 12 fractions in each group 0.62; 95% CI 0.50 to 0.77, P < 0.0001; RR for 12 or fewer fractions in HBOT versus more than 12 with control 0.73; 95% CI 0.56 to 0.94, P = 0.01). There was no evidence of heterogeneity between trials overall (I2 = 0%) (fixed‐effect model). There is an absolute risk reduction of 21.1% when using HBOT (NNT to avoid one recurrence = 5; 95% CI 4 to 8). The overall reduction in failure to control tumour is not sensitive to the allocation of withdrawals (best case scenario: RR 0.61; 95% CI 0.51 to 0.71, P < 0.00001 (Analysis 5.2); worst case scenario: RR 0.75; 95% CI 0.65 to 0.87, P = 0.0002 (Analysis 5.3)). Figure 2
Analysis 5.2.
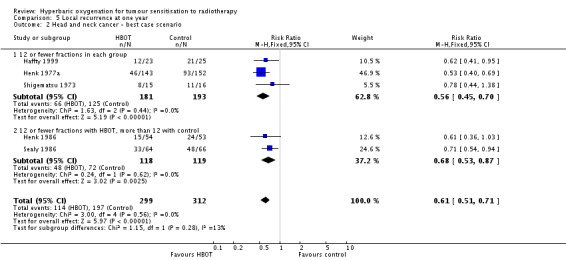
Comparison 5 Local recurrence at one year, Outcome 2 Head and neck cancer ‐ best case scenario.
Analysis 5.3.
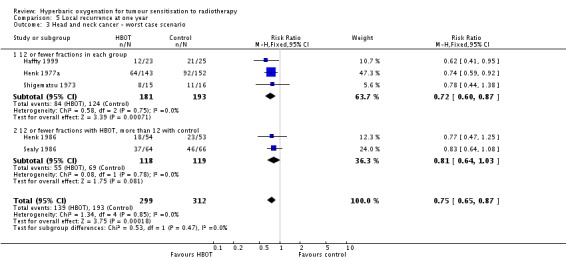
Comparison 5 Local recurrence at one year, Outcome 3 Head and neck cancer ‐ worst case scenario.
Figure 2.
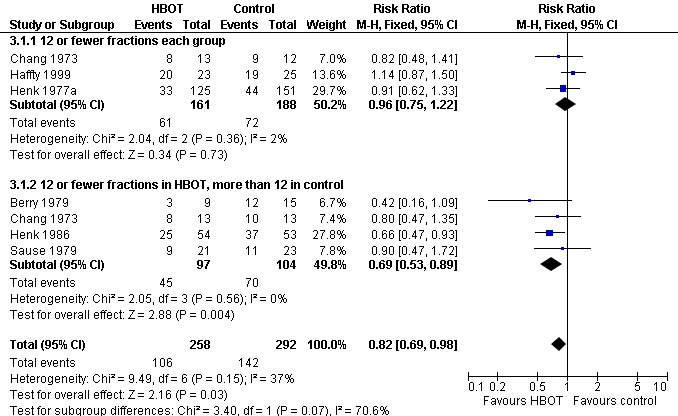
Forest plot of comparison: Death at five years for head and neck cancer: outcome 3.1
Local recurrence at one year with cancer of the uterine cervix (Analysis 5.4)
Three trials reported this outcome (Dische 1999; Ward 1979; Watson 1978) for 714 subjects after exclusion of withdrawals (31% of the total subjects in this review), with 338 (47%) allocated to HBOT and 376 (53%) to control. Over all fractionation schemes there was no statistically significant reduction in the incidence of local tumour recurrence following radiation therapy with HBOT (RR 0.82; 95% CI 0.63 to 1.06, P = 0.13), with little difference between subgroups with different fractionation schemes. There was evidence of moderate heterogeneity between trials overall (I2 = 23%), but significant heterogeneity when comparing groups who had received fewer than 12 fractions (I2 = 37%) (random‐effects model). The risk of recurrence was not sensitive to the allocation of those lost to follow‐up (best case scenario: RR 0.81; 95% CI 0.63 to 1.02, P = 0.08 (Analysis 5.5); worst case scenario: RR 0.87; 95% CI 0.63 to 1.19, P = 0.38 (Analysis 5.6)).
Analysis 5.5.
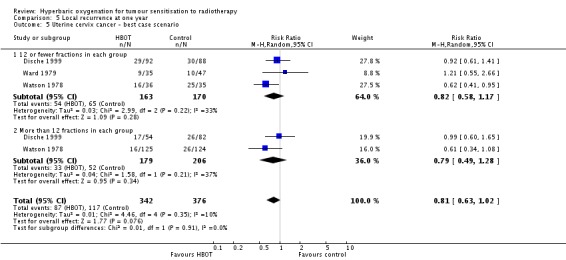
Comparison 5 Local recurrence at one year, Outcome 5 Uterine cervix cancer ‐ best case scenario.
Analysis 5.6.
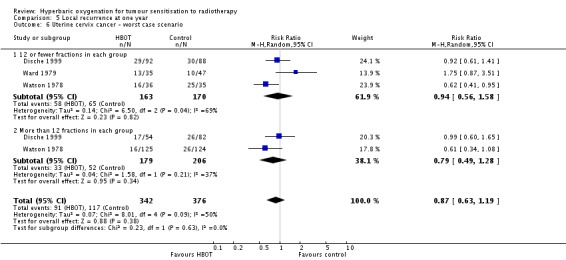
Comparison 5 Local recurrence at one year, Outcome 6 Uterine cervix cancer ‐ worst case scenario.
Local recurrence at two years
Local recurrence at two years with head and neck cancer (Analysis 6.1)
One trial reported this outcome (Haffty 1999) for 48 subjects (2% of the total subjects in this review), with 23 (48%) allocated to HBOT and 25 (52%) to control. There was no significant reduction in the incidence of local tumour recurrence following radiation therapy with HBOT (RR 0.83; 95% CI 0.60 to 1.14, P = 0.25).
Local recurrence at two years with cancer of the uterine cervix (Analysis 6.2)
Two trials reported this outcome (Glassburn 1974; Watson 1978) for 360 subjects after exclusion of withdrawals (16% of the total subjects in this review), with 178 (49%) allocated to HBOT and 182 (51%) to control. Watson 1978 contributes 73% of the weight to this analysis. Over all fractionation schemes there was a statistically significant reduction in the incidence of local tumour recurrence following radiation therapy with HBOT (RR 0.60; 95% CI 0.38 to 0.97, P = 0.04), however subgroup analysis by fractionation scheme suggests the benefit may be restricted to those who receive 12 or fewer fractions in each group (RR in this group 0.53; 95% CI 0.37 to 0.77, P = 0.0007; RR for more than 12 fractions in each group 0.68; 95% CI 0.26 to 1.73, P = 0.41). There was evidence of significant heterogeneity between trials overall (I2 = 67%) (random‐effects model). Overall, there is a risk reduction of 23% when using HBOT (NNT to avoid one recurrence = 5; 95% CI 4 to 8), while the reduction for the comparison between groups receiving fewer than 12 fractions was 41.3%, (NNT = 3; 95% CI 2 to 5). There were no losses to follow‐up for any of these studies.
Local recurrence at five years
Local recurrence at five years with head and neck cancer (Analysis 7.1)
Five trials reported this outcome (Berry 1979; Haffty 1999; Henk 1977a; Henk 1986; Sause 1979) for 495 subjects after exclusion of withdrawals (22% of the total subjects in this review), with 229 (46%) allocated to HBOT and 266 (54%) to control. Over all fractionation schemes there was a statistically significant reduction in the incidence of local tumour recurrence following radiation therapy with HBOT (RR 0.77; 95% CI 0.62 to 0.95, P = 0.01). Subgroup analysis by fractionation scheme suggests the benefit may be restricted to those trials comparing fewer than 12 fractions in each group (RR with fewer than 12 fractions in each group 0.74; 95% CI 0.62 to 0.88, P = 0.0009; RR for 12 or fewer fractions in HBOT versus more than 12 with control 0.75; 95% CI 0.39 to 1.43, P = 0.38). There was evidence of moderate heterogeneity between trials overall (I2 = 32%), and substantial heterogeneity for those trials comparing fewer than 12 fractions in HBOT with 12 or more fractions in control (I2 = 63%) (random‐effects model). Overall, there is an absolute risk reduction of 19% when using HBOT (NNT to avoid one recurrence = 6; 95% CI 4 to 11), and the absolute risk reduction is also 19% for trials comparing fewer than 12 fractions in each group (NNT = 6; 95% CI 4 to 12). The overall reduction in failure to control tumour is sensitive to the allocation of withdrawals (best case scenario: RR 0.70; 95% CI 0.57 to 0.86, P = 0.0008 (Analysis 7.2); worst case scenario: RR 0.84; 95% CI 0.66 to 1.06, P = 0.14 (Analysis 7.3)).
Analysis 7.2.

Comparison 7 Local recurrence at five years, Outcome 2 Head and neck cancer ‐ best case scenario.
Analysis 7.3.
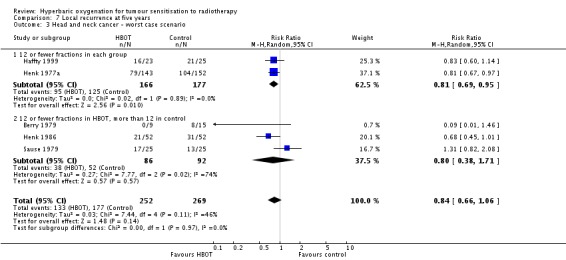
Comparison 7 Local recurrence at five years, Outcome 3 Head and neck cancer ‐ worst case scenario.
Local recurrence at five years with cancer of the uterine cervix (Analysis 7.4)
Four trials reported this outcome (Brady 1981; Dische 1999; Ward 1979; Watson 1978) for 772 subjects (34% of the total subjects in this review), with 367 (48%) allocated to HBOT and 405 (52%) to control. There was no significant reduction in the incidence of local tumour recurrence following radiation therapy with HBOT (RR 0.85; 95% CI 0.65 to 1.13, P = 0.27). Subgroup analysis did not suggest benefit with any particular fractionation scheme. There was evidence of significant heterogeneity between trials overall (I2 = 68%) (random‐effects model). The analysis is sensitive to the allocation of withdrawals (best case scenario: RR 0.83; 95% CI 0.72 to 0.97, P = 0.02 (Analysis 7.5); worst case scenario: RR 0.89; 95% CI 0.76 to 1.03, P = 0.11 (Analysis 7.6)).
Analysis 7.5.
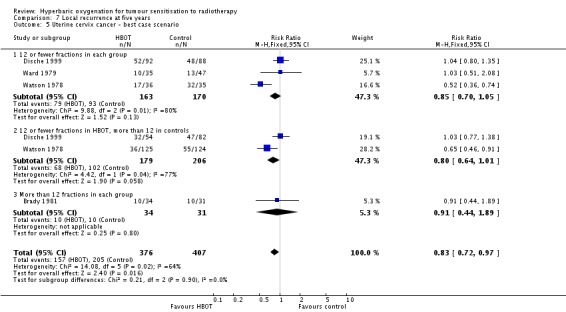
Comparison 7 Local recurrence at five years, Outcome 5 Uterine cervix cancer ‐ best case scenario.
Analysis 7.6.
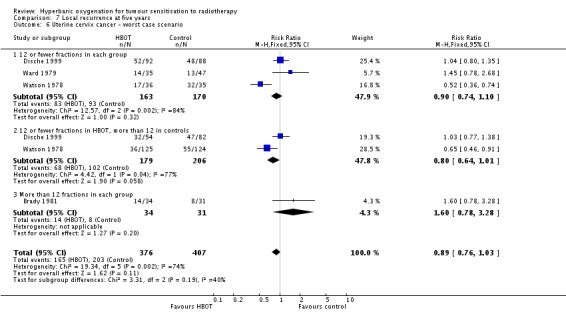
Comparison 7 Local recurrence at five years, Outcome 6 Uterine cervix cancer ‐ worst case scenario.
4. Development of metastasis
Metastases at one year
Metastases at one year with cancer of the uterine cervix (Analysis 8.1)
One trial reported this outcome (Watson 1978) for 320 subjects (23% of the total subjects in this review), with 161 (50.3%) allocated to HBOT and 159 (49.7%) to control. There were no withdrawals or losses to follow‐up. There was no significant reduction in the incidence of metastases following radiation therapy with HBOT (RR 0.79; 95% CI 0.52 to 1.19, P = 0.26). Subgroup analysis did not suggest benefit with any particular fractionation scheme.
Metastases at two years
Metastases at two years with cancer of the uterine cervix (Analysis 9.1)
Three trials reported this outcome (Fletcher 1977; Glassburn 1974; Watson 1978) for 522 subjects (23% of the total subjects in this review), with 251 (48%) allocated to HBOT and 271 (52%) to control. There were no withdrawals or losses to follow‐up. There was no significant reduction in the incidence of metastases following radiation therapy with HBOT (RR 1.05; 0.84 to 1.31, P = 0.70).
Metastases at two years with cancer of the urinary bladder (Analysis 9.2)
Two trials reported this outcome (Cade 1967; Plenk 1972) for 80 subjects (2% of the total subjects in this review), with 25 (51%) allocated to HBOT and 24 (49%) to control. However, Plenk 1972 reported no patients with metastases and so did not contribute to the analysis. There were no withdrawals or losses to follow‐up. There was no significant difference in the incidence of metastases following radiation therapy with HBOT (RR 2.00; 95% CI 0.58 to 6.91, P = 0.27).
Metastases at two years with cancer of the bronchus (Analysis 9.3)
One trial reported this outcome (Cade 1967) for 49 subjects (3.5% of the total subjects in this review), with 39 (51%) allocated to HBOT and 41 (49%) to control. There were no withdrawals or losses to follow‐up. There was no significant difference in the incidence of metastases following radiation therapy with HBOT (RR 1.04; 95% CI 0.60 to 1.80, P = 0.89).
Metastases at five years
Metastases at five years with cancer of the head and neck (Analysis 10.1)
One trial reported this outcome (Chang 1973) for 50 subjects (2% of the total subjects in this review), with 26 (52%) allocated to HBOT and 24 (48%) to control. There were no withdrawals or losses to follow‐up. There was no significant reduction in the incidence of metastases following radiation therapy with HBOT (RR 0.46; 95% CI 0.09 to 2.30, P = 0.34).
Metastases at five years with cancer of the uterine cervix (Analysis 10.2)
Three trials reported this outcome (Brady 1981; Ward 1979; Watson 1978) for 456 subjects after exclusion of withdrawals, (20% of the total subjects in this review), with 221 (49%) allocated to HBOT and 235 (51%) to control. Watson 1978 contributes 83% of the weight of this analysis. Over all fractionation schemes there was no significant difference in the incidence of metastases following radiation therapy with HBOT (RR 0.79; 0.50 to 1.26, P = 0.32). Subgroup analysis by fractionation scheme suggests there may be a benefit when comparing 12 or fewer fractions in each group (RR 0.67; 95% CI 0.45 to 0.99, P = 0.05), but not for other comparisons (RR with 12 or fewer fractions with HBOT versus more than 12 with control RR 0.07; 95% CI 0.00 to 1.12, P = 0.06 and RR for more than 12 fractions in each group 0.99; 95% CI 0.78 to 1.26, P = 0.95). There was evidence of considerable heterogeneity between trials overall (I2 = 58%) (random‐effects model).
The risk of recurrence was not sensitive to the allocation of those lost to follow‐up (best case scenario: RR 0.76; 95% CI 0.46 to 1.26, P = 0.28 (Analysis 10.3); worst case scenario: RR 0.85; 95% CI 0.56 to 1.31, P = 0.46 (Analysis 10.4)).
Analysis 10.3.
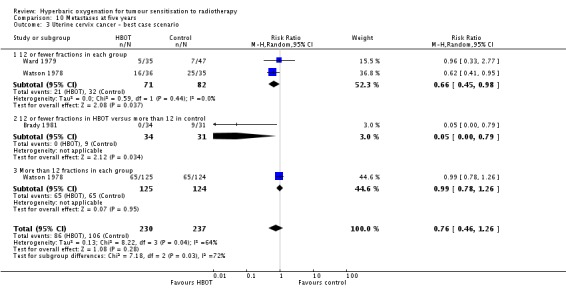
Comparison 10 Metastases at five years, Outcome 3 Uterine cervix cancer ‐ best case scenario.
Analysis 10.4.
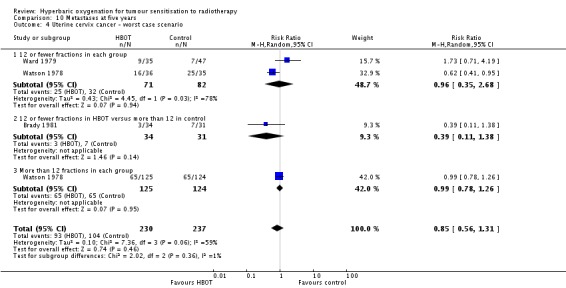
Comparison 10 Metastases at five years, Outcome 4 Uterine cervix cancer ‐ worst case scenario.
5. Adverse effects
Death from radiation tissue effects (Analysis 11.1)
Two trials reported this outcome (Dische 1999; Watson 1978) for 633 subjects after exclusion of withdrawals (28% of the total subjects in this review), with 307 (49%) allocated to HBOT and 326 (51%) to control. There was no significant increase in the chance of death due to radiation tissue injury following HBOT (RR 1.64; 95% CI 0.89 to 3.03, P = 0.11).
Severe radiation tissue injury (Analysis 11.2)
Seven trials reported this outcome (Brady 1981; Haffty 1999; Henk 1986; Sause 1979; Sealy 1986; Watson 1978; Ward 1979) for 779 subjects after exclusion of withdrawals (34% of the total subjects in this review), with 379 (48%) allocated to HBOT and 400 (52%) to control. There was a statistically significant increase in the chance of severe radiation tissue injury following HBOT (RR 2.35; 95% CI 1.66 to 3.33, P < 0.00001). There was little heterogeneity between trials overall (I2 = 15%) (fixed‐effect model). There is an absolute risk increase of 12% when using HBOT (number needed to harm (NNH) to cause one severe injury = 8; 95% CI 4 to 15). The increased risk of injury is not sensitive to the allocation of withdrawals (best case scenario: RR 1.94; 95% CI 1.39 to 2.69, P < 0.0001 (Analysis 11.3); worst case scenario: RR 2.69; 95% CI 1.92 to 3.77, P < 0.00001 (Analysis 11.4)).
Analysis 11.3.
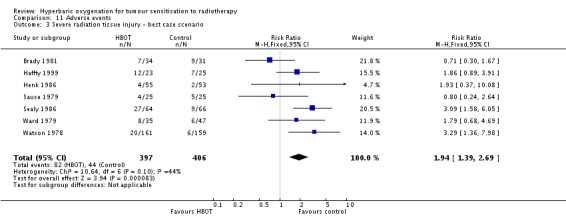
Comparison 11 Adverse events, Outcome 3 Severe radiation tissue injury ‐ best case scenario.
Analysis 11.4.
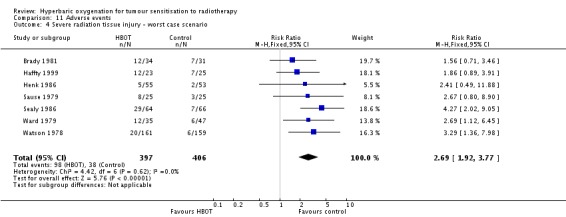
Comparison 11 Adverse events, Outcome 4 Severe radiation tissue injury ‐ worst case scenario.
Acute central nervous system toxicity (Analysis 11.5)
Four trials reported this outcome (Cade 1967; Chang 1973; Plenk 1972; Sealy 1986) for 331 subjects after exclusion of withdrawals (15% of the total subjects in this review), with 150 (45%) allocated to HBOT and 181 (55%) to control. There was a statistically significant increase in the chance of severe radiation tissue injury following HBOT (RR 6.76; 95% CI 1.16 to 39.31, P = 0.03). There was no evidence of important heterogeneity between trials overall (I2 = 0%) (fixed‐effect model). There is an absolute risk increase of 5% when using HBOT (NNH to cause one episode = 22; 95% CI 11 to 44). The increased risk of injury is sensitive to the allocation of withdrawals (best case scenario: RR 3.00; 95% CI 0.81 to 11.10, P = 0.1 (Analysis 11.6); worst case scenario: RR 9.74; 95% CI 1.73 to 54.98, P = 0.01 (Analysis 11.7)).
Analysis 11.6.
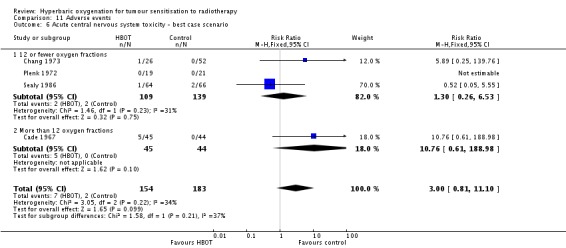
Comparison 11 Adverse events, Outcome 6 Acute central nervous system toxicity ‐ best case scenario.
Analysis 11.7.
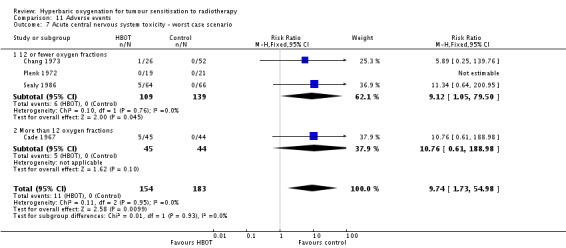
Comparison 11 Adverse events, Outcome 7 Acute central nervous system toxicity ‐ worst case scenario.
Middle ear barotrauma (Analysis 11.8))
Only one trial reported this outcome (Cade 1967) for 89 subjects (4% of the total subjects in this review), with 45 allocated to HBOT and 44 to control. There were no losses to follow‐up or withdrawals. The chance of suffering middle ear barotrauma was not statistically significantly increased with HBOT (RR 6.85; 95% CI 0.36 to 128.83, P = 0.20).
Discussion
Summary of main results
We were able to pool data for a number of clinical outcomes of interest, however interpretation of some results was complicated by consideration of the fractionation scheme through subgroup analysis. For head and neck cancer, there was an overall reduction in the risk of dying at both one year and five years after therapy (RR 0.83, P = 0.03, NNT = 11; and RR 0.82, P = 0.03, NNT = 5 respectively), and evidence of improved local tumour control immediately following irradiation (RR with HBOT 0.58, P = 0.006, NNT = 7). For mortality however, at both times this difference largely reflected an advantage when comparing a small number of fractions while breathing HBO (less than 12) versus the more standard scheme of 20 to 25 fractions breathing air. When considering only the comparison between all subjects who received fewer than 12 fractions in each group, there is no advantage of HBOT (RR at one year 0.93, P = 0.53; RR at five years 0.96; P = 0.73). Any possible benefit of HBO must therefore be interpreted in the knowledge of the most effective fractionation scheme in air. If there is a mortality benefit from reduction in fractionation scheme alone, then HBO may not contribute to this benefit. Our results must, therefore be interpreted with caution. There was no evidence of benefit with respect to mortality or early tumour control for other anatomical sites.
These trials also suggest an advantage following HBOT in the chance of experiencing local tumour recurrence at one year (head and neck: RR 0.66, P < 0.0001, NNT = 5), two years (uterine cervix: RR 0.60, P = 0.04, NNT = 5) and five years (head and neck: RR 0.77, P = 0.01, NNT = 6), but no such advantage in the incidence of metastatic disease for any anatomical site at any time. Any advantage of the combined therapy seems to be achieved at the cost of some adverse effects. Although the chance of dying from severe radiation injury is not significantly increased (RR 1.64, P = 0.11), there was a significant increase in the rate of both severe radiation tissue injury (RR 2.35, P < 0.0001, NNH = 8) and the chance of seizures during therapy (RR 6.76, P = 0.03, NNH = 22).
Overall completeness and applicability of evidence
In total, there were 2286 subjects available for evaluation using our planned comparisons. There were 785 subjects with head and neck tumours, 1089 with carcinoma of the cervix and 343 with carcinoma of the bladder. While there were sufficient numbers to form some impression of treatment impact for these tumours, there were only 49 subjects with carcinoma of the bronchus and four each of glioblastoma, carcinoma of the rectum and carcinoma of the oesophagus. Therefore the trials in this review have low power to assess the impact of HBO on these tumours.
We included data from 19 trials investigating the treatment of various malignancies with radiation therapy while breathing HBO, and we believe these represent all randomised human trials in this area, both published and unpublished, at the time of searching the databases. We did not locate further trials when we repeated the searches in September 2008 and March 2011. Ten trials included subjects with head and neck cancers, seven trials of carcinoma of the uterine cervix, five trials of carcinoma of the urinary bladder and one trial each with carcinoma of the bronchus, glioblastoma, cancer of the oesophagus and cancer of the rectum. We found some evidence that radiotherapy with HBO reduces the one and five year mortality rate and local tumour recurrence, along with improved early local tumour control for head and neck cancer, and two year local recurrence for carcinoma of the cervix. We also found evidence of significant adverse effects with HBOT, particularly the incidence of oxygen toxic seizures and the chance of suffering severe radiation injury. There were no reliable data from these trials to confirm any beneficial effect of HBOT for other malignancies studied, nor on the incidence of metastatic disease for cancers of any primary site.
These trials were published over a 32‐year period up to 1999, mainly drawing subjects from the UK and the USA. We had planned to perform subgroup analyses with respect to age, dose of oxygen, dose of radiation therapy and the temporal relationship of the two therapies. After appraisal of these trials however, we were only able to conduct a subgroup analysis for the different fractionation schemes employed. Specifically, there were no children included and no trials used a sequential approach to HBOT and radiation therapy, while the dose of oxygen administered was remarkably uniform per session.
Quality of the evidence
Although study quality varied across these trials, in general the methodology was reasonable as 13 of the 19 included trials employed a reliable method of randomisation and allocation concealment. Although none of the trials employed a sham therapy, most of our clinical outcomes, such as mortality and cancer recurrence, were unlikely to be subject to participant or observer bias. Some major problems for this review were the poor reporting of methodological quality in some of these trials, variability in entry criteria, the nature and timing of outcomes, and poor reporting of outcomes. In particular, there is a possibility of bias due to different fractionation schemes and radiation doses across the trials, as well as a general failure to report data suitable for comparison of survival over time using the lgHR. None of the trials blinded participants, investigators or outcome assessors to treatment.
Potential biases in the review process
All of these findings are subject to a potential publication bias. While we have made every effort to locate further unpublished data, it remains possible that this review is subject to a positive publication bias, with generally favourable trials more likely to achieve reporting. With regard to any effect on the QoL for these patients, we have located little relevant data.
Agreements and disagreements with other studies or reviews
Our findings are in general agreement with previously published reviews of the area. In his review of modifying agents designed to sensitise tumours to the effect of radiotherapy, Overgaard has suggested that HBOT was abandoned before a measured evaluation was made of the true clinical impact (Overgaard 1996). This decision seems to have been based more on convenience and logistics rather than a demonstrated superiority of alternative sensitising agents.
Authors' conclusions
There is some evidence that HBOT improves local tumour control and mortality for cancers of the head and neck, as well as reducing the chance of local tumour recurrence in cancers of the head, neck and uterine cervix. There is however, also some evidence that these outcomes may be related to the use of unusual fractionation schemes, and these benefits should be interpreted with caution. HBOT also appears to be associated with significant adverse effects including oxygen toxic seizures and severe tissue radiation injury. Thus, the routine use of HBO in these patients cannot be justified by this review. The methodological and reporting inadequacies of the primary studies included in this review demand a cautious interpretation.
Given the findings of improved tumour control and mortality with the use of HBO for patients with cancers of the head, neck and uterine cervix, there is a case for large randomised trials of high methodological rigour in order to define the true extent of benefit (if any) from the administration of HBO for these cancers at appropriate fractionations schemes. Specifically, such trials must employ appropriate fractionation schemes in both arms in order to clearly define any benefits of HBOT as opposed to novel fractionation. The effect of differing oxygen dosage and effect of other therapies administered simultaneously is not known. Any future trials would need to consider in particular:
appropriate sample sizes with power to detect expected differences;
careful definition and selection of target patients;
appropriate range of oxygen doses per treatment session (pressure and time);
use of an effective sham therapy where appropriate and ethical;
effective and explicit blinding of outcome assessors;
appropriate outcome measures including all those listed in this review;
careful elucidation of any adverse effects; and
the cost‐utility of the therapy.
Acknowledgements
We thank Gail Quinn and Clare Jess (Managing Editors) of the Cochrane Gynaecological Cancer Review Group (CGCRG) for their assistance in the preparation of this review. Jane Hayes (Information Manager) for the CGCRG for designing and executing the search strategy. We are particularly grateful for the extensive editorial assistance of Heather Dickinson for the original review.
Appendices
Appendix 1. MEDLINE search strategy
1 Hyperbaric Oxygenation/ 2 (hyperbaric and oxygen*).mp. 3 (hbo or hbot).mp. 4 (high adj3 (pressure or tension)).mp. 5 ((multiplace or monoplace) and chamber*).mp. 6 1 or 2 or 3 or 4 or 5 7 exp Radiotherapy/ 8 radiotherap*.mp. 9 radiation.mp. 10 irradiat*.mp. 11 radiotherapy.fs. 12 7 or 8 or 9 or 10 or 11 13 randomized controlled trial.pt. 14 controlled clinical trial.pt. 15 randomized.ab. 16 placebo.ab. 17 clinical trials as topic.sh.˜ 18 randomly.ab. 19 trial.ti. 20 13 or 14 or 15 or 16 or 17 or 18 or 19 21 6 and 12 and 20
key:
mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier pt=publication type ab=abstract sh=subject heading ti=title
Appendix 2. EMBASE search strategy
1 hyperbaric oxygen/ 2 (hyperbaric and oxygen*).mp. 3 (hbo or hbot).mp. 4 (high adj3 (pressure or tension)).mp. 5 ((multiplace or monoplace) and chamber*).mp. 6 1 or 2 or 3 or 4 or 5 7 cancer radiotherapy/ 8 exp radiotherapy/ 9 radiotherap*.mp. 10 radiation.mp. 11 irradiat*.mp. 12 rt.fs. 13 7 or 8 or 9 or 10 or 11 or 12 14 crossover procedure/ 15 randomized controlled trial/ 16 single blind procedure/ 17 random*.mp. 18 factorial*.mp. 19 (crossover* or cross over* or cross‐over*).mp. 20 placebo*.mp. 21 (doubl* adj blind*).mp. 22 (singl* adj blind*).mp. 23 assign*.mp. 24 allocat*.mp. 25 volunteer*.mp. 26 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 27 6 and 13 and 26
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer
Appendix 3. CINAHL search strategy
1. exp Radiotherapy/ 2. exp brachytherapy/. 3. exp radiation‐sensitising agents/ 4. (radiation or radiother*).mp. 5. 1 or 2 or 3 or 4 6. exp HYPERBARIC OXYGENATION/ 7. (high adj5 (pressur$ or oxygen$)).mp. 8. hyperbaric$.mp. 9. 6 or 7 or 8 10. oxygen$.mp. 11. 9 and 10 12. (HBO or HBOT).mp. 13. multiplace chamber$.mp. 14. monoplace chamber*.mp. 15. 11 or 12 or 13 or 14 16. 5 and 15 17. (random$ or controlled clinical trial or groups).mp. 18. 16 and 17
Appendix 4. CENTRAL search strategy
#1 MeSH descriptor Hyperbaric Oxygenation, this term only #2 hyperbaric and oxygen* #3 hbo and hbot #4 high near/3 (pressure or tension) #5 (multiplace or monoplace) and chamber* #6 (#1 OR #2 OR #3 OR #4 OR #5) #7 MeSH descriptor Radiotherapy explode all trees #8 radiotherap* #9 radiation #10 irradiat* #11 Any MeSH descriptor with qualifier: RT #12 (#7 OR #8 OR #9 OR #10 OR #11) #13 (#6 AND #12)
Data and analyses
Comparison 1.
Death at one year
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Head and neck cancer | 9 | 710 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.70, 0.98] |
| 1.1 12 or fewer fractions each group | 5 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.74, 1.17] |
| 1.2 12 or fewer fractions with HBOT, more than 12 with control | 4 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.41, 1.08] |
| 1.3 More than 12 fractions each group | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.13, 2.70] |
| 2 Head and neck ‐ best case scenario | 9 | 743 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.62, 0.85] |
| 2.1 12 or fewer fractions each group | 5 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.64, 1.02] |
| 2.2 12 or fewer fractions with HBOT, more than 12 with control | 4 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.43, 0.91] |
| 2.3 More than 12 fractions each group | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.13, 2.70] |
| 3 Head and neck ‐ worst case scenario | 9 | 743 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.76, 1.15] |
| 3.1 12 or fewer fractions each group | 5 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.90, 1.36] |
| 3.2 12 or fewer fractions with HBOT, more than 12 with control | 4 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.45, 1.13] |
| 3.3 More than 12 fractions each group | 1 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.13, 2.70] |
| 4 Uterine cervix cancer | 4 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.11] |
| 4.1 12 or fewer fractions each groups | 3 | 329 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.70, 1.38] |
| 4.2 More than 12 fractions each group | 3 | 399 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.57, 1.10] |
| 5 Uterine cervix ‐ best case scenario | 4 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.69, 1.10] |
| 6 Uterine cervix ‐ worst case scenario | 4 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.72, 1.15] |
| 7 Urinary bladder cancer | 4 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.27] |
| 7.1 12 or fewer fractions each group | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.35] |
| 7.2 12 or fewer fractions with HBOT, more than 12 with control | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.44, 1.51] |
| 7.3 More than 12 fractions each group | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.68, 3.00] |
| 7.4 Mixed fractionation scheme | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.73, 1.44] |
| 8 Urinary bladder ‐ best case scenario | 4 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.71, 1.21] |
| 9 Urinary bladder ‐ worst case scenario | 4 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.78, 1.34] |
| 10 Bronchial cancer | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.72, 1.64] |
| 11 Rectal cancer | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.48, 5.76] |
| 12 Oesophageal cancer | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.17, 2.07] |
| 13 Gliobastoma | 1 | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.49, 2.05] |
Analysis 1.1.

Comparison 1 Death at one year, Outcome 1 Head and neck cancer.
Analysis 1.4.
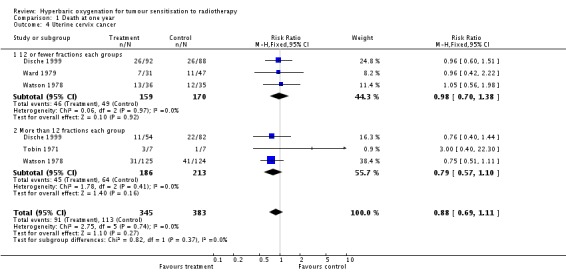
Comparison 1 Death at one year, Outcome 4 Uterine cervix cancer.
Analysis 1.7.
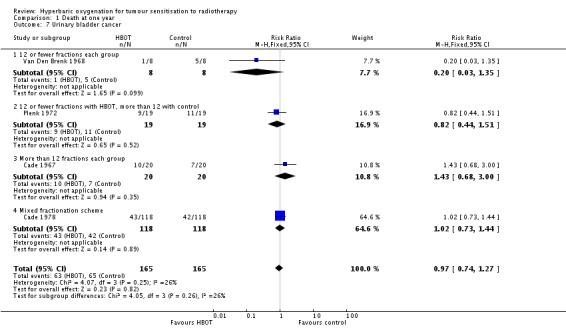
Comparison 1 Death at one year, Outcome 7 Urinary bladder cancer.
Analysis 1.10.

Comparison 1 Death at one year, Outcome 10 Bronchial cancer.
Analysis 1.11.
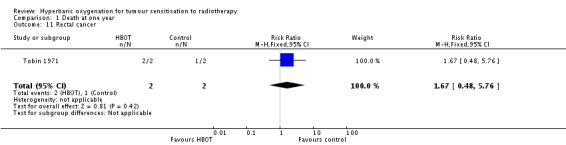
Comparison 1 Death at one year, Outcome 11 Rectal cancer.
Analysis 1.12.
Comparison 1 Death at one year, Outcome 12 Oesophageal cancer.
Analysis 1.13.
Comparison 1 Death at one year, Outcome 13 Gliobastoma.
Comparison 2.
Death at two years
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Head and neck cancer | 3 | 189 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.83, 1.12] |
| 1.1 12 or fewer fractions in each group | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.76, 1.40] |
| 1.2 12 or fewer fractions with HBOT, more than 12 with control | 1 | 124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.09] |
| 1.3 More than 12 fractions in both HBOT and control | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.37, 3.76] |
| 2 Head and neck ‐ best case scenario | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.79, 1.07] |
| 2.1 12 or fewer fractions in each group | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.76, 1.40] |
| 2.2 12 or fewer fractions with HBOT, more than 12 with control | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.73, 1.03] |
| 2.3 More than 12 fractions in both HBOT and control | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.37, 3.76] |
| 3 Head and neck ‐ worst case scenario | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.86, 1.15] |
| 3.1 12 or fewer fractions in each group | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.76, 1.40] |
| 3.2 12 or fewer fractions with HBOT, more than 12 with control | 1 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.83, 1.14] |
| 3.3 More than 12 fractions in both HBOT and control | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.37, 3.76] |
| 4 Uterine cervix cancer | 4 | 607 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.76, 1.15] |
| 4.1 12 or fewer fractions in HBOT and control | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.47, 1.09] |
| 4.2 More than 12 fractions in HBOT and control | 4 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.81, 1.21] |
| 5 Urinary bladder carcinoma | 2 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [0.63, 3.92] |
| 6 Urinary bladder ‐ best case | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.04, 5.24] |
| 7 Urinary bladder ‐ worst case | 2 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.18 [2.18, 12.31] |
Analysis 2.1.
Comparison 2 Death at two years, Outcome 1 Head and neck cancer.
Analysis 2.4.

Comparison 2 Death at two years, Outcome 4 Uterine cervix cancer.
Analysis 2.5.
Comparison 2 Death at two years, Outcome 5 Urinary bladder carcinoma.
Comparison 3.
Death at five years
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Head and neck cancer | 6 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 1.1 12 or fewer fractions each group | 3 | 349 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.75, 1.22] |
| 1.2 12 or fewer fractions in HBOT, more than 12 in control | 4 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.53, 0.89] |
| 2 Head and neck ‐ best case scenario | 6 | 575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.64, 0.92] |
| 2.1 12 or fewer fractions in each group | 3 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.12] |
| 2.2 12 or fewer fractions in HBOT, more than 12 in control | 4 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.51, 0.85] |
| 3 Head and neck ‐ worst case scenario | 6 | 575 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.81, 1.13] |
| 3.1 12 or fewer fractions each group | 3 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.92, 1.45] |
| 3.2 12 or fewer fractions in HBOT, more than 12 in control | 4 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.96] |
| 4 Uterine cervix cancer | 4 | 772 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.80, 1.14] |
| 4.1 12 or fewer fractions each group | 3 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.62, 1.34] |
| 4.2 More than 12 fractions in each group | 2 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.81, 1.15] |
| 4.3 12 or fewer fractions in HBOT versus more than 12 in control | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.70, 1.61] |
| 5 Uterine cancer ‐ best case scenario | 4 | 783 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.77, 1.09] |
| 5.1 12 or fewer fractions in each group | 3 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.61, 1.28] |
| 5.2 More than 12 fractions in each group | 2 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.81, 1.15] |
| 5.3 12 or fewer fractions in HBOT versus more than 12 in control | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.57, 1.32] |
| 6 Uterine cancer ‐ worst case scenario | 4 | 783 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.81, 1.18] |
| 6.1 12 or fewer fractions in each group | 3 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.63, 1.38] |
| 6.2 More than 12 fractions in each group | 2 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.81, 1.15] |
| 6.3 12 or fewer fractions in HBOT versus more than 12 in control | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.79, 1.77] |
| 7 Urinary bladder cancer | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.88, 1.22] |
Analysis 3.1.
Comparison 3 Death at five years, Outcome 1 Head and neck cancer.
Analysis 3.4.
Comparison 3 Death at five years, Outcome 4 Uterine cervix cancer.
Analysis 3.7.
Comparison 3 Death at five years, Outcome 7 Urinary bladder cancer.
Comparison 4.
Failure to control local tumour at three months
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Head and neck cancer | 5 | 446 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.39, 0.85] |
| 1.1 12 or fewer fractions each group | 5 | 420 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.34, 0.88] |
| 1.2 Less than 12 fractions in HBOT group versus more than 12 in control | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.24, 1.82] |
| 2 Head and neck cancer ‐ best case scenario | 5 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.41, 0.78] |
| 2.1 12 or fewer fractions in each group | 5 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.36, 0.80] |
| 2.2 Less than 12 fractions in HBOT versus more than 12 in control | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.24, 1.82] |
| 3 Head and neck ‐ worst case scenario | 5 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.35, 1.00] |
| 3.1 12 or fewer fractions in each group | 5 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.30, 1.05] |
| 3.2 Less than 12 fractions in HBOT versus more than 12 in control | 1 | 26 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.24, 1.82] |
Analysis 4.1.
Comparison 4 Failure to control local tumour at three months, Outcome 1 Head and neck cancer.
Comparison 5.
Local recurrence at one year
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Head and neck cancer | 5 | 582 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.56, 0.78] |
| 1.1 12 or fewer fractions each group | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.50, 0.77] |
| 1.2 12 or fewer fractions with HBOT, more than 12 with control | 2 | 227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.57, 0.94] |
| 2 Head and neck cancer ‐ best case scenario | 5 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.51, 0.71] |
| 2.1 12 or fewer fractions in each group | 3 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.45, 0.70] |
| 2.2 12 or fewer fractions with HBOT, more than 12 with control | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.53, 0.87] |
| 3 Head and neck cancer ‐ worst case scenario | 5 | 611 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.65, 0.87] |
| 3.1 12 or fewer fractions in each group | 3 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.87] |
| 3.2 12 or fewer fractions with HBOT, more than 12 with control | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.03] |
| 4 Uterine cervix cancer | 3 | 714 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.06] |
| 4.1 12 or fewer fractions each group | 3 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.57, 1.27] |
| 4.2 more than 12 fractions in each group | 2 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.49, 1.28] |
| 5 Uterine cervix cancer ‐ best case scenario | 3 | 718 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.63, 1.02] |
| 5.1 12 or fewer fractions in each group | 3 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.58, 1.17] |
| 5.2 More than 12 fractions in each group | 2 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.49, 1.28] |
| 6 Uterine cervix cancer ‐ worst case scenario | 3 | 718 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.63, 1.19] |
| 6.1 12 or fewer fractions in each group | 3 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.56, 1.58] |
| 6.2 More than 12 fractions in each group | 2 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.49, 1.28] |
Analysis 5.1.
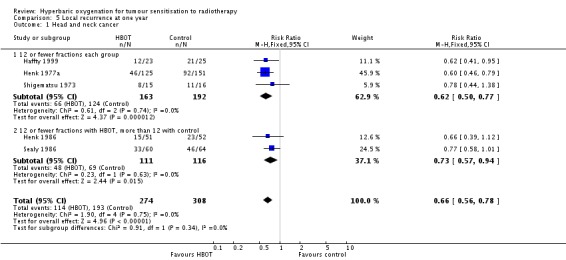
Comparison 5 Local recurrence at one year, Outcome 1 Head and neck cancer.
Analysis 5.4.
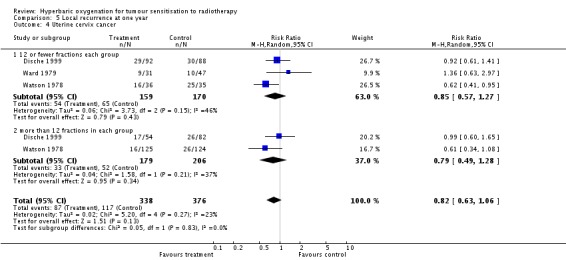
Comparison 5 Local recurrence at one year, Outcome 4 Uterine cervix cancer.
Comparison 6.
Local recurrence at two years
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Head and neck cancer | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.60, 1.14] |
| 2 Uterine cervix cancer | 2 | 360 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.38, 0.97] |
| 2.1 12 or fewer fractions in each group | 1 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.37, 0.77] |
| 2.2 More than 12 fractions in each group | 2 | 289 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.26, 1.73] |
Analysis 6.1.
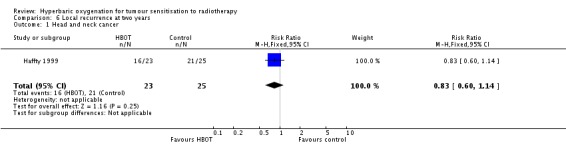
Comparison 6 Local recurrence at two years, Outcome 1 Head and neck cancer.
Analysis 6.2.
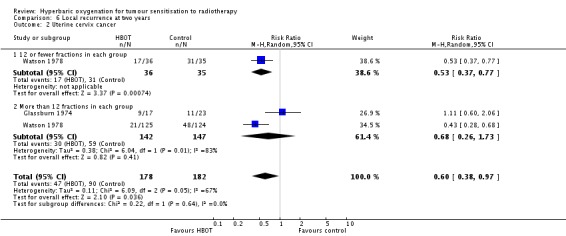
Comparison 6 Local recurrence at two years, Outcome 2 Uterine cervix cancer.
Comparison 7.
Local recurrence at five years
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Head and neck cancer | 5 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.62, 0.95] |
| 1.1 12 or fewer fractions in each group | 2 | 324 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.88] |
| 1.2 12 or fewer fractions in HBOT, more than 12 in control | 3 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.43] |
| 2 Head and neck cancer ‐ best case scenario | 5 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.57, 0.86] |
| 2.1 12 or fewer fractions in each group | 2 | 343 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.52, 0.94] |
| 2.2 12 or fewer fractions in HBOT, more than 12 in control | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.43, 1.12] |
| 3 Head and neck cancer ‐ worst case scenario | 5 | 521 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.66, 1.06] |
| 3.1 12 or fewer fractions in each group | 2 | 343 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.69, 0.95] |
| 3.2 12 or fewer fractions in HBOT, more than 12 in control | 3 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.38, 1.71] |
| 4 Uterine cervix cancer | 4 | 772 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.65, 1.13] |
| 4.1 12 or fewer fractions each group | 3 | 329 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.49, 1.41] |
| 4.2 12 or fewer fractions in HBOT group versus more than 12 fractions in control | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.58, 2.71] |
| 4.3 12 or more fractions in each group | 2 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.32] |
| 5 Uterine cervix cancer ‐ best case scenario | 4 | 783 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.72, 0.97] |
| 5.1 12 or fewer fractions in each group | 3 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.70, 1.05] |
| 5.2 12 or fewer fractions in HBOT, more than 12 in controls | 2 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.64, 1.01] |
| 5.3 More than 12 fractions in each group | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.44, 1.89] |
| 6 Uterine cervix cancer ‐ worst case scenario | 4 | 783 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.03] |
| 6.1 12 or fewer fractions in each group | 3 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.74, 1.10] |
| 6.2 12 or fewer fractions in HBOT, more than 12 in controls | 2 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.64, 1.01] |
| 6.3 More than 12 fractions in each group | 1 | 65 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.78, 3.28] |
Analysis 7.1.

Comparison 7 Local recurrence at five years, Outcome 1 Head and neck cancer.
Analysis 7.4.
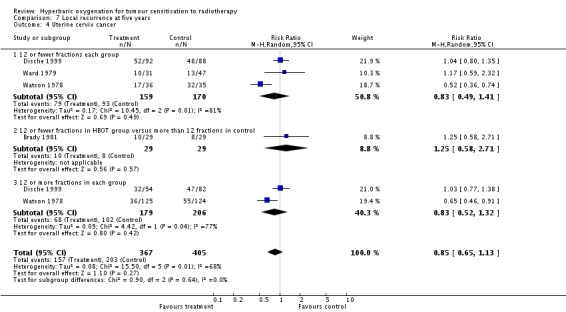
Comparison 7 Local recurrence at five years, Outcome 4 Uterine cervix cancer.
Comparison 8.
Metastases at one year
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine cervix cancer | 1 | 320 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.52, 1.19] |
| 1.1 10 or fewer fractions | 1 | 71 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.35, 2.73] |
| 1.2 11 or more fractions | 1 | 249 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.48, 1.19] |
Analysis 8.1.

Comparison 8 Metastases at one year, Outcome 1 Uterine cervix cancer.
Comparison 9.
Metastases at two years
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine cervix cancer | 3 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.84, 1.31] |
| 1.1 More than 12 fractions in each group | 3 | 522 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.84, 1.31] |
| 2 Urinary bladder carcinoma | 2 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.58, 6.91] |
| 3 Carcinoma of the bronchus | 1 | 49 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.60, 1.80] |
Analysis 9.1.
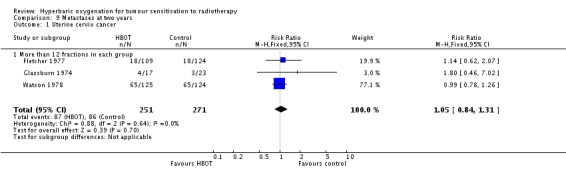
Comparison 9 Metastases at two years, Outcome 1 Uterine cervix cancer.
Analysis 9.2.
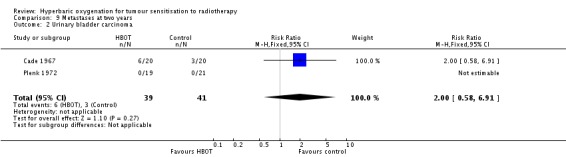
Comparison 9 Metastases at two years, Outcome 2 Urinary bladder carcinoma.
Analysis 9.3.
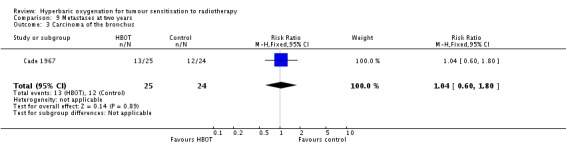
Comparison 9 Metastases at two years, Outcome 3 Carcinoma of the bronchus.
Comparison 10.
Metastases at five years
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Head and neck carcinoma | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.09, 2.30] |
| 2 Uterine cervix cancer | 3 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.50, 1.26] |
| 2.1 12 or fewer fractions each group | 2 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.45, 0.99] |
| 2.2 12 or fewer fractions in HBOT group versus more than 12 fractions in control | 1 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.00, 1.12] |
| 2.3 More than 12 fractions in each group | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.78, 1.26] |
| 3 Uterine cervix cancer ‐ best case scenario | 3 | 467 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.46, 1.26] |
| 3.1 12 or fewer fractions in each group | 2 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.45, 0.98] |
| 3.2 12 or fewer fractions in HBOT versus more than 12 in control | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.00, 0.79] |
| 3.3 More than 12 fractions in each group | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.78, 1.26] |
| 4 Uterine cervix cancer ‐ worst case scenario | 3 | 467 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.56, 1.31] |
| 4.1 12 or fewer fractions in each group | 2 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.35, 2.68] |
| 4.2 12 or fewer fractions in HBOT versus more than 12 in control | 1 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.11, 1.38] |
| 4.3 More than 12 fractions in each group | 1 | 249 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.78, 1.26] |
Analysis 10.1.
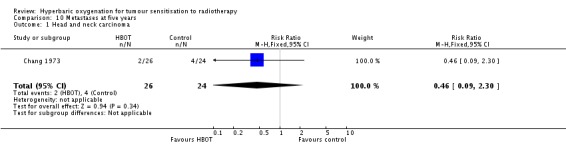
Comparison 10 Metastases at five years, Outcome 1 Head and neck carcinoma.
Analysis 10.2.
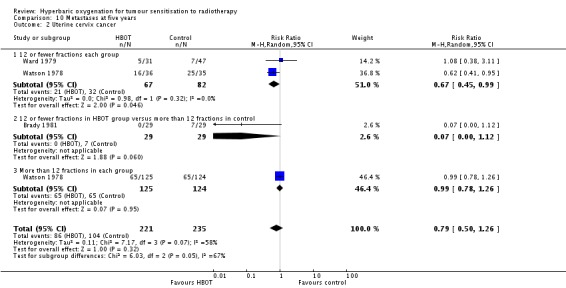
Comparison 10 Metastases at five years, Outcome 2 Uterine cervix cancer.
Comparison 11.
Adverse events
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death through radiation tissue injury | 2 | 633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.89, 3.03] |
| 2 Severe radiation tissue injury | 7 | 779 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.35 [1.66, 3.33] |
| 3 Severe radiation tissue injury ‐ best case scenario | 7 | 803 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.39, 2.69] |
| 4 Severe radiation tissue injury ‐ worst case scenario | 7 | 803 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.69 [1.92, 3.77] |
| 5 Acute central nervous system oxygen toxicity | 4 | 331 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.76 [1.16, 39.31] |
| 5.1 12 or fewer oxygen fractions | 3 | 242 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.30 [0.47, 39.60] |
| 5.2 More than 12 oxygen fractions | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.76 [0.61, 188.98] |
| 6 Acute central nervous system toxicity ‐ best case scenario | 4 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.81, 11.10] |
| 6.1 12 or fewer oxygen fractions | 3 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.26, 6.53] |
| 6.2 More than 12 oxygen fractions | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.76 [0.61, 188.98] |
| 7 Acute central nervous system toxicity ‐ worst case scenario | 4 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.74 [1.73, 54.98] |
| 7.1 12 or fewer oxygen fractions | 3 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.12 [1.05, 79.50] |
| 7.2 More than 12 oxygen fractions | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.76 [0.61, 188.98] |
| 8 Middle ear barotrauma | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.85 [0.36, 128.83] |
Analysis 11.1.
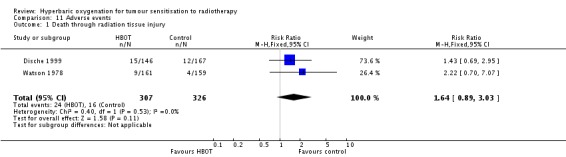
Comparison 11 Adverse events, Outcome 1 Death through radiation tissue injury.
Analysis 11.2.
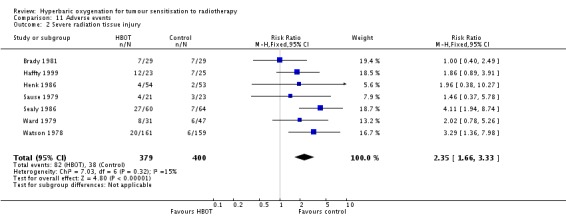
Comparison 11 Adverse events, Outcome 2 Severe radiation tissue injury.
Analysis 11.5.
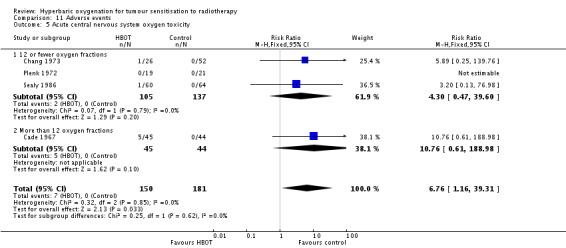
Comparison 11 Adverse events, Outcome 5 Acute central nervous system oxygen toxicity.
Analysis 11.8.
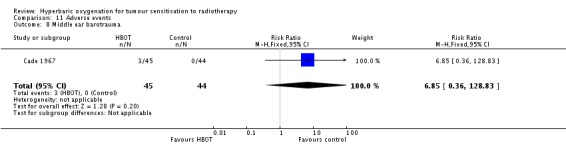
Comparison 11 Adverse events, Outcome 8 Middle ear barotrauma.
What's new
Last assessed as up‐to‐date: 6 January 2012.
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
History
Protocol first published: Issue 1, 2004 Review first published: Issue 4, 2005
| Date | Event | Description |
|---|---|---|
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
| 21 February 2012 | New citation required but conclusions have not changed | New searches executed March 2011 but no new studies identified. |
| 6 January 2012 | New search has been performed | Text updated and study flow diagram added. |
| 4 October 2008 | New search has been performed | Review updated, no new trials identified when searches were re‐run on 27 September 2008. |
| 30 April 2008 | Amended | Converted to new review format. |
| 16 July 2005 | New citation required and conclusions have changed | Substantive amendment |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Berry 1979
| Methods | RCT with allocation concealment and randomisation through central sealed envelope allocation. Patient, outcome assessors and treating team all aware of allocation at the start of treatment. No indication of power calculation | |
| Participants | 24 adults with SCC of the head and neck where radiotherapy was the treatment of choice. 11 allocated to HBOT and 13 to control. No dropouts, but two participants crossed from HBOT to control after refusing HBOT | |
| Interventions |
|
|
| Outcomes | Death at 1 and 5 years, local recurrence at 5 years | |
| Notes | Also see Berry 1968 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
Brady 1981
| Methods | RCT with allocation concealment not clear, method of randomisation not stated. Patient, outcome assessors and treating team all aware of allocation. No indication of power calculation | |
| Participants | 65 adults with Stage IIb to IVa carcinoma of the uterine cervix where radiotherapy was the treatment of choice. 34 allocated to HBOT and 31 to control. Several participants refused HBOT and there were only 19 of 34 available for analysis in HBOT group and 29 of 31 in the control group | |
| Interventions |
|
|
| Outcomes | Death at 4 years, local recurrence at 4 years, metastases at 4 years, late radiation tissue injuries | |
| Notes | Trial stopped due to poor accrual Schulz rating: randomisation: C; allocation concealment: B; selection bias: C; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Cade 1967
| Methods | RCT with allocation concealment and randomisation by centrally generated card method. Two separate studies reported ‐ one for carcinoma of the bronchus and one for carcinoma of the urinary bladder. Patient, outcome assessors and treating team all aware of allocation at start of therapy course. No indication of power calculation | |
| Participants |
No drop‐outs or losses to follow‐up in either trial |
|
| Interventions |
|
|
| Outcomes | Death 1 year, metastatic disease 1 to 2 years, oxygen toxicity data (combined for both trials) | |
| Notes | Also reported in McEwen 1968 Schulz rating: randomisation: A; allocation concealment: A; selection bias: A; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Cade 1978
| Methods | Mulitcentred RCT with allocation concealment and randomisation by centrally generated envelope method. Patient, outcome assessors and treating team all aware of allocation at start of therapy course. No indication of power calculation | |
| Participants | 241 adults with carcinoma of the urinary bladder spread to vagina or rectum. Losses not accounted for, final analysis 118 in each group (5 lost) | |
| Interventions | Different regimens of treatment were used in each of the four centres and also varied within some centres during the course of the trial. No individual centre or fractionation data is given
|
|
| Outcomes | Death at 1 and 5 years | |
| Notes | Also see Kirk 1976, Wiernik 1974 and Dische 1973 Schulz rating: randomisation: A; allocation concealment: A; selection bias: B; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Chang 1973
| Methods | RCT with allocation concealment and randomisation through sealed envelope method. Patient, outcome assessors and treating team all aware of allocation at the start of treatment. No indication of power calculation | |
| Participants | 51 previously untreated adults with advanced (T3 and T4) carcinomas of the soft palate and adjacent structures. 26 allocated to HBOT and 25 to control. No dropouts or losses to analysis | |
| Interventions |
|
|
| Outcomes | Death at 1 and 5 years, early local tumour control, metastatic disease at 5 years, oxygen toxicity. HBOT group results split between the two controls for analysis | |
| Notes | Schulz rating: randomisation: A; allocation concealment: B; selection bias: A; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Dische 1999
| Methods | RCT with allocation concealment and randomisation by centrally generated envelope method. Patient, outcome assessors and treating team all aware of allocation. No indication of power calculation | |
| Participants | 335 adults with Stage IIb or III carcinoma of the uterine cervix where radiotherapy was the treatment of choice. 146 allocated to HBOT and 170 to control. 19 participants lost to follow‐up and group not indicated | |
| Interventions | Four different treatment regimens used. Where individual centre data is given it is used in analysis
|
|
| Outcomes | Death 1 and 5 years, locoregional control 1 and 5 years, death by late radiation effects at 5 years | |
| Notes | See also Bennett 1977. 27 fraction HBOT schema discontinued after interim analysis did not suggest any benefit Schulz rating: randomisation: A; allocation concealment: A; selection bias: B; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Fletcher 1977
| Methods | RCT stratified for node involvement and clinical stage, with allocation concealment not clear, method of randomisation not stated. Patient, outcome assessors and treating team all aware of allocation. No indication of power calculation | |
| Participants | 233 adults with Stage IIb to IVa carcinoma of the uterine cervix where radiotherapy was the treatment of choice. 109 allocated to HBOT and 124 to control. No dropouts or losses to follow‐up | |
| Interventions |
|
|
| Outcomes | Death at 2 years, metastatic disease at 2 years | |
| Notes | An interim report that does not seem to have been reported in a complete paper to date. Also see Lindberg 1973 and Fletcher 1975 Schulz rating: randomisation: C; allocation concealment: B; selection bias: A; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Glassburn 1974
| Methods | RCT with allocation concealment not clear, method of randomisation not stated. Patient, outcome assessors and treating team all aware of allocation. No indication of power calculation. Participants excluded if second primary, prior radiotherapy or contraindication to HBOT | |
| Participants | 40 adults with Stage III or IV carcinoma of the uterine cervix where radiotherapy was the treatment of choice. 17 allocated to HBOT and 23 to control. No dropouts or losses to follow‐up | |
| Interventions |
|
|
| Outcomes | Death at 27 months, local tumour recurrence at 27 months, metastases at 27 months | |
| Notes | An interim report that does not seem to have been reported in a complete paper to date. Also see Faust 1969 Schulz rating: randomisation: C; allocation concealment: B; selection bias: A; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Haffty 1999
| Methods | RCT using sealed envelopes but allocation concealment not clear. Patient, outcome assessors and treating team all aware of allocation after start of therapy | |
| Participants | 48 adults with SCC of the head and neck where radiotherapy was the treatment of choice. 23 allocated to HBOT and 25 to control | |
| Interventions |
|
|
| Outcomes | Death at 1, 2 and 5 years, local tumour control, recurrence rate, complications | |
| Notes | Very unusual radiation regimen Schulz rating: randomisation: A; allocation concealment: B; selection bias: A; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Henk 1977a
| Methods | RCT stratified by site of tumour (nasal and oral, laryngeal, laryngopharyngeal and other) with allocation concealment not clear, method of randomisation not stated. Patient, outcome assessors and treating team all aware of allocation. No indication of power calculation | |
| Participants | 295 adults with SCC of the head and neck where radiotherapy was the treatment of choice. 143 allocated to HBOT and 152 to control Dropouts identified (18 from HBOT group, 1 from control) but not included in analysis | |
| Interventions |
|
|
| Outcomes | Death at 1 to 5 years, local control of tumour at 3 months, local recurrence rates at 1 to 5 years, significant radiation tissue effects at 6 months | |
| Notes | Other reports of this trial in Henk 1974, Henk 1975, Kunkler 1968 Schulz rating: randomisation: C; allocation concealment: B; selection bias: B; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Henk 1986
| Methods | RCT stratified by site of tumour (mouth, oropharynx, nasal sinus, nasopharynx, larynx, hypopharynx and middle ear). Allocation concealment and randomisation achieved by centrally supplied sealed envelopes. Patient, outcome assessors and treating team all aware of allocation after trial started. No indication of power calculation | |
| Participants | 107 adults with SCC of the head and neck where radiotherapy was the treatment of choice. 54 allocated to HBOT and 53 to control. Dropouts identified (1 from HBOT group) but not included in analysis | |
| Interventions |
|
|
| Outcomes | Death at 1 and 5 years, recurrence at 1 and 4 years, late radiation tissue effects at 5 years | |
| Notes | Other reports of this trial in Henk 1974, Henk 1975, Henk 1977 Schulz rating: randomisation: A; allocation concealment: A; selection bias: A; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Plenk 1972
| Methods | RCT using random number table, allocation concealment not clear. Patient, outcome assessors and treating team all aware of allocation. No indication of power calculation | |
| Participants | 40 adults with carcinoma of the urinary bladder. 19 allocated to HBOT and 21 to control. More than 50% loss to follow‐up at two years | |
| Interventions |
|
|
| Outcomes | Death at one and two years, oxygen toxicity | |
| Notes | Schulz rating: randomisation: A; allocation concealment: B; selection bias: C; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sause 1979
| Methods | RCT of previously untreated head and neck SCC with allocation concealment not clear, method of randomisation not stated. Patient, outcome assessors and treating team all aware of allocation. No indication of power calculation | |
| Participants | 50 adults with SCC of the head and neck where radiotherapy was the treatment of choice. Group allocation unclear but six dropouts and 21 analysed in HBOT group, 23 in control | |
| Interventions |
|
|
| Outcomes | Death at 2 to 8 years, local tumour control and late radiation tissue injury | |
| Notes | 5 participants excluded from analysis because they died from 'intercurrent disease' prior to 2 year follow‐up Schulz rating: randomisation: C; allocation concealment: B; selection bias: B; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Sealy 1986
| Methods | RCT stratified by sex, site of tumour, extent of node involvement and histology. Allocation concealment achieved by sealed envelopes prepared by an individual not otherwise involved in the study. Patient, outcome assessors and treating team all aware of allocation. No indication of power calculation | |
| Participants | 130 adults with SCC of the mouth or fixed lymph nodes in the neck where radiotherapy was the treatment of choice. 64 allocated to HBOT and 66 to control. Dropouts identified (4 from HBOT group, 2 from control) but not included in analysis | |
| Interventions |
|
|
| Outcomes | Death at one and two years, local recurrence at one year, toxic reactions to therapy and oxygen toxicity | |
| Notes | Other report of this trial in Sealy 1978. Schulz rating: randomisation: B; allocation concealment: A; selection bias: B; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Shigematsu 1973
| Methods | RCT stratified by tumour stage and possibly allocation was actually achieved by quasi‐random method. No indication of allocation concealment. Patient, outcome assessors and treating team all aware of allocation after treatment started. No indication of power calculation | |
| Participants | 42 adults with SCC of the maxillary sinus. 21 allocated to both HBOT and control. No dropouts from therapy or losses to follow‐up All patients had myringotomies prior to compression | |
| Interventions |
|
|
| Outcomes | Death at one year, local early tumour control, recurrence at one year | |
| Notes | Schulz rating: randomisation: C; allocation concealment: B; selection bias: A; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Tobin 1971
| Methods | RCT with randomisation by card drawn by an individual not involved with the study. Allocation probably made in a concealed manner after randomisation. Patient, outcome assessors and treating team all aware of allocation after trial started. No indication of power calculation. Several different tumours studied: head and neck, uterine cervix, urinary bladder, rectal, brain and oesophagus | |
| Participants |
A further three patients allocated to HBOT were incomplete when trial ceased and have not been analysed |
|
| Interventions |
|
|
| Outcomes | Death at 1 and 2 years | |
| Notes | Trial terminated after explosive decompression of the chamber due to degradation of chamber wall from radiation Schulz rating: randomisation: B; allocation concealment: A; selection bias: A; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Van Den Brenk 1968
| Methods | Pseudo‐RCT with allocation to group by birth date. No allocation concealment. Two separate studies reported ‐ one for carcinoma of the head and neck and one for carcinoma of the urinary bladder. Patient, outcome assessors and treating team all aware of allocation. No indication of power calculation | |
| Participants |
No drop‐outs or losses to follow‐up in either trial |
|
| Interventions |
Both conducted at 3ATA breathing 100% oxygen, total compression time about 40 minutes |
|
| Outcomes | Death at 6 months, local tumour control early | |
| Notes | Schulz rating: randomisation: C; allocation concealment: C; selection bias: A; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
Ward 1979
| Methods | RCT with allocation concealment and randomisation by centrally generated envelope method. Patient, outcome assessors and treating team all aware of allocation. No indication of power calculation | |
| Participants | 82 adults with Stage IIb or III carcinoma of the uterine cervix where radiotherapy was the treatment of choice. 39 allocated to HBOT and 43 to control. Four dropouts not analysed because treatment incomplete plus five participants crossed over from HBOT to control group when they refused HBOT | |
| Interventions |
|
|
| Outcomes | Death at 1 and 5 years, local recurrence at 1 and 5 years, metastatic disease at 5 years, radiation tissue injury | |
| Notes | Also see Ward 1978, 1973 and 1974 Schulz rating: randomisation: A; allocation concealment: A; selection bias: C; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Watson 1978
| Methods | Mulitcentred RCT with allocation concealment and randomisation by centrally generated envelope method. Patient, outcome assessors and treating team all aware of allocation at start of therapy course. No indication of power calculation | |
| Participants | 320 adults with Stage III to IVa carcinoma of the uterine cervix where radiotherapy was the treatment of choice. 161 allocated to HBOT and 159 to control. No dropouts or losses to follow‐up | |
| Interventions | Different regimens of treatment were used in each of the four centres. Where individual centre data is given it is used in analysis
|
|
| Outcomes | Death at 1, 2 and 5 years, local recurrence at 5 years, metastatic disease at 1 and 5 years, late radiation tissue effects and severe tissue reactions | |
| Notes | Also see Wiernik 1974 and Dische 1974 Schulz rating: randomisation: A; allocation concealment: A; selection bias: A; blinding: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
ATA ‐ atmospheres absolute HBOT ‐ hyperbaric oxygen therapy RCT ‐ randomised controlled trial Rads ‐ SCC ‐ squamous cell carcinoma
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bennett 1977 | More fully reported in Dische 1999 |
| Berry 1968 | More fully reported in Berry 1979 and Ward 1979 |
| Dische 1973 | More fully reported in Cade 1978 |
| Dische 1974 | More fully reported in Watson 1978 |
| Dische 1979 | A summary of several trials with no new data |
| Dische 1991 | A summary of several trials with no new data |
| Faust 1970 | More fully reported in Glassburn 1974 |
| Fletcher 1975 | More fully reported in Fletcher 1977 |
| Henk 1974 | More fully reported in Henk 1977a and Henk 1986 |
| Henk 1975 | More fully reported in Henk 1977a and Henk 1986 |
| Henk 1977b | More fully reported in Henk 1986 |
| Kirk 1976 | More fully reported in Cade 1978 |
| Kunkler 1968 | More fully reported in Henk 1977a |
| Lindberg 1973 | More fully reported in Fletcher 1977 |
| Mayer 2005 | Review, no new data |
| McEwen 1968 | More fully reported in Cade 1967 |
| McEwen 1972 | More fully reported in Cade 1967 |
| MRCWP 1978 | Summary of trials with no new data |
| Overgaard 2007 | Review, no new data |
| Sealy 1978 | More fully reported in Sealy 1986 |
| Ward 1973 | More fully reported in Ward 1979 |
| Ward 1974 | More fully reported in Ward 1979 |
| Ward 1978 | More fully reported in Ward 1979 |
| Wiernik 1973 | More fully reported in Watson 1978 and Cade 1978 |
Contributions of authors
Michael Bennett: Principal author, conception, search strategy and execution, data extraction and critical appraisal, Hyperbaric Medicine content expert, statistical analysis. Robert Smee: Co‐author, data extraction and critical appraisal, Radiation Oncology content expert. John Feldmeier: Co‐author, Radiation Oncology and Hyperbaric Medicine content expert. Chris Milross: Co‐author Background and Discussion, Radiation Oncology content expert.
Sources of support
Internal sources
No specific support provided, Australia.
External sources
No sources of support, Not specified.
Declarations of interest
None known. Michael Bennett is a hyperbaric physician who regularly treats patients with late radiation tissue injury, while John Feldmeier has previous hyperbaric experience. Chris Milross, John Feldmeier and Robert Smee are radiation oncologists who refer patients with late radiation tissue injury for hyperbaric oxygen therapy (HBOT).
Edited (no change to conclusions)
References
References to studies included in this review
- Berry G, Dixon B, Ward A. The Leeds results for radiotherapy in HBO for carcinoma of the head and neck. Clinical Radiology 1979;30:591‐2. [DOI] [PubMed] [Google Scholar]
- Brady LW, Plenk HP, Hanley JA, Glassburn JR, Kramer S, Parker RG. Hyperbaric oxygen therapy for carcinoma of the cervix ‐ stages IIb, IIA, IIIB and IVA: results of a randomised study by the radiation therapy oncology group. International Journal of Radiation Oncology, Biology Physics 1981;7:991‐8. [DOI] [PubMed] [Google Scholar]
- Cade IS, McEwen JB. Megavoltage radiotherapy in hyperbaric oxygen. A controlled trial. Cancer 1967;20:817‐21. [DOI] [PubMed] [Google Scholar]
- Cade IS, Dische S, Watson ER, Wiernik G, Sutherland I. Hyperbaric oxygen and radiotherapy: a Medical Research Council trial in carcinoma of the bladder. British Journal of Radiology 1978;51:876‐8. [DOI] [PubMed] [Google Scholar]
- Chang CH, Conley JJ, Herbert C. Radiotherapy of advanced carcinoma of the oropharyngeal region under hyperbaric oxygenation. An interim report. American Journal of Roetgenology 1973;117:509‐15. [DOI] [PubMed] [Google Scholar]
- Dische S, Saunders M, Sealy R, Werner I, Verma N, Foy C, et al. Carcinoma of the cervix and the use of hyperbaric oxygen with radiotherapy: a report of a randomised controlled trial. Radiotherapy and Oncology 1999;53:93‐8. [10665784] [DOI] [PubMed] [Google Scholar]
- Fletcher GH, Lindberg RD, Caderao JB, Wharton JT. Hyperbaric oxygen as a radiotherapeutic adjuvant in advanced cancer of the uterine cervix. Preliminary results of a randomised trial. Cancer 1977;39:617‐23. [DOI] [PubMed] [Google Scholar]
- Glassburn JR, Damsker JI, Brady LW, Faust DS, Antionades J, Prasavinichai, et al. Hyperbaric oxygen and radiation in the treatment of advanced cervical cancer. Fifth International Hyperbaric Congress Proceedings. Burnaby, BC: Simon Fraser University, 1974; Vol. II:813‐9. [74‐75484] [Google Scholar]
- Haffty BG, Hurley R, Peters LJ. Radiation therapy with hyperbaric oxygen at 4 atmospheres pressure in the management of squamous cell carcinoma of the head and neck: results of a randomized clinical trial. The Cancer Journal from Scientific American 1999;5:341‐7. [10606475] [PubMed] [Google Scholar]
- Henk JM, Kunkler PB, Smith CW. Radiotherapy and hyperbaric oxygen in head and neck cancer. Final report of first controlled clinical trial. Lancet 1977;2(8029):101‐3. [DOI] [PubMed] [Google Scholar]
- Henk JM. Late results of a trial of hyperbaric oxygen and radiotherapy in head and neck cancer: a rationale for hypoxic cell sensitisers?. International Journal of Radiation Oncology, Biology Physics 1986;12:1339‐41. [DOI] [PubMed] [Google Scholar]
- Plenk HP. Hyperbaric radiation therapy. Preliminary results of a randomised study of cancer of the urinary bladder and review of the "oxygen experience". American Journal of Roetgenology, Radium Therapy and Therapeutic Nuclear Medicine 1972;114:152‐7. [PubMed] [Google Scholar]
- Sause WT, Plenk HP. Radiation therapy of head and neck tumours: a randomised study of treatment in air versus hyperbaric oxygen. International Journal of Radiation Oncology, Biology Physics 1979;5:1833‐6. [DOI] [PubMed] [Google Scholar]
- Sealy R, Cridland S, Barry L, Norris R. Irradiation with misonidazole and hyperbaric oxygen: final report on a randomised controlled trial in advanced head and neck cancer. International Journal of Radiation Oncology, Biology Physics 1986;12:1343‐6. [DOI] [PubMed] [Google Scholar]
- Shigematsu Y, Fuchihata H, Makino T, Inoue T. Radiotherapy with reduced fraction in head and neck cancer, with special reference to hyperbaric oxygen radiotherapy in maxillary sinus carcinoma (a controlled study). In: Sugahara T, Rivesz I, Scott O editor(s). Radiobiology and Radiotherapy. Tokyo: Ishiyaku, 1973:180‐7. [Google Scholar]
- Tobin DA, Vermund H. A randomised study of hyperbaric oxygen as an adjunct to regularly fractionated radiation therapy for clinical treatment of advanced neoplastic disease. American Journal of Roetgenology, Radium and Therapeutic Nuclear Medicine 1971;3(3):613‐21. [DOI] [PubMed] [Google Scholar]
- Brenk HA. Hyperbaric oxygen in radiation therapy. An investigation of dose‐effect relationships in tumour response and tissue damage. American Journal of Roetgenology 1968;102:8‐26. [PubMed] [Google Scholar]
- Ward AJ, Dixon B. Carcinoma of the cervix: results of a hyperbaric oxygen trial associated with the use of the cathetron. Clinical Radiology 1979;30:383‐7. [DOI] [PubMed] [Google Scholar]
- Watson ER, Dische S, Cade IS, Wiernik G, Sutherland I. Hyperbaric oxygen and radiotherapy: a Medical Research Council trial in carcinoma of the cervix. British Journal of Radiology 1978;51:879‐87. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Bennett MH. The treatment of Stage III squamous carcinoma of the cervix in air and hyperbaric oxygen. Proceedings of the British Institute of Radiology. London: British Journal of Radiology, 1977; Vol. 51:38. [Google Scholar]
- Berry G. A clinical appraisal of hyperbaric oxygen in head and neck cancer. Abstracts of papers read at a meeting on 'An Assessment of Hyperbaric Oxygen Therapy. January 18, 1968'. Proceedings of the British Insitutue of Radiology 1978;51:150. [Google Scholar]
- Dische S. The hyperbaric oxygen chamber in the radiotherapy of carcinoma of the bladder. British Journal of Radiology 1973;46:13‐7. [DOI] [PubMed] [Google Scholar]
- Dische S. The hyperbaric oxygen chamber in the radiotherapy of carcinoma of the uterine cervix. British Journal of Radiology 1974;47:99‐107. [DOI] [PubMed] [Google Scholar]
- Dische S. Hyperbaric oxygen: the Medical Research Council trials and their clinical significance. British Journal of Radiology 1979;51:888‐94. [DOI] [PubMed] [Google Scholar]
- Dische S. What have we learnt from hyperbaric oxygen?. Radiotherapy and Oncology 1991;20(Suppl 1 ):71‐4. [DOI] [PubMed] [Google Scholar]
- Faust DS, Brady LW, Kazem I, Germon PA. Hybaroxia and radiation therapy in carcinoma of the cervix (stage III and IV): a clinical trial. Proceedings of the Fourth International Hyperbaric Congress. Tokyo: Igaku Shoin, 1970:410‐4. [Google Scholar]
- Fletcher GH, Lindberg RD, Caderao JB, Wharton JT. The preliminary results of a randomised trial of patients with late stage cancer of the uterine cervix using hyperbaric tank or atmospheric oxygen. Proceedings of the American Radium Society. 1975:341‐6. [Google Scholar]
- Henk JM. Overcoming the oxygen effect: hyperbaric oxygen or protracted fractionation. Fifth International Hyperbaric Congress Proceedings. Burnaby, BC: Simon Fraser University, 1974; Vol. II:794‐801. [74‐75484] [Google Scholar]
- Henk JM. The influence of oxygen and hypoxia on laryngeal cancer management. Laryngoscope 1975;85:1134‐44. [DOI] [PubMed] [Google Scholar]
- Henk JM, Smith CW. Radiotherapy and hyperbaric oxygen in head and neck cancer. Interim report of second clinical trial. Lancet 1977;8014:104‐5. [DOI] [PubMed] [Google Scholar]
- Kirk J, Wingate G, Watson E. High‐dose effects in the treatment of carcinoma of the bladder under air and hyperbaric oxygen conditions. Clinical Radiology 1976;27:137‐44. [DOI] [PubMed] [Google Scholar]
- Kunkler PB, Boulis‐Wassif S, Shah NK, Sutherland WH, Smith C. A controlled trial of hyperbaric oxygen in the radiotherapy of head and neck tumours. British Journal of Radiology 1968;41(487):557. [PubMed] [Google Scholar]
- Lindberg RD, Fletcher GH, Caderao JB. Present status of high‐pressure oxygen in radiotherapy. Proceedings of the XIII International Congress of Radiology. Amsterdam: Excerpta Medica, 1973; Vol. 1:665‐70. [Google Scholar]
- Mayer R, Hamilton‐Farrell MR, Kleij AJ, Schmutz J, Granstrom G, Sicko Z, et al. Hyperbaric oxygen and radiotherapy. Strahlentherapie und Onkologie 2005;181(2):113‐23. [DOI] [PubMed] [Google Scholar]
- McEwen JB. Clinical trials of hyperbaric oxygen and radiotherapy. British Journal of Radiology 1968;41(487):556. [PubMed] [Google Scholar]
- McEwen J. Hyperbaric oxygen as an adjunct to megavoltage radiotherapy. British Journal of Radiology. 1972; Vol. 45. [PubMed] [Google Scholar]
- The Medical Research Council Working Party. Radiotherapy and Hyperbaric Oxygen. Lancet 1978; 2 (8095):881‐4. [PubMed] [Google Scholar]
- Overgaard J. Hypoxic radiosensitization: adored and ignored. Journal of Clinical Oncology 2007;25(26):4066‐74. [DOI] [PubMed] [Google Scholar]
- Sealy R. A preliminary clinical study in the use of misonidazole in cancer of the head and neck. British Journal of Cancer 1978;37(Suppl III):314‐7. [PMC free article] [PubMed] [Google Scholar]
- Ward AJ, Dixon B, Stubbs B, Ridout EJ, Madan S. Hyperbaric oxygen and radiotherapy for squamous cell carcinoma of the cervix. Fifth International Hyperbaric Oxygen Congress Proceedings. Burnaby BC: Simon Fraser University, 1973; Vol. II:802‐12. [Google Scholar]
- Ward AJ, Stubbs B, Dixon B. Carcinoma of the cervix: establishment of a hyperbaric oxygen trial associated with the use of the cathetron. British Journal of Radiology 1974;47:319‐25. [DOI] [PubMed] [Google Scholar]
- Ward AJ, Dixon B, Stubbs B. A clinical appraisal of hyperbaric oxygen in cervix cancer. Proceedings of the British Institute of Radiotherapy 1978;51:150‐1. [Google Scholar]
- Wiernik G, Perrins D. Hyperbaric oxygen and radiotherapy in advanced carcinoma of the cervix and bladder. Fifth International Hyperbaric Congress Proceedings. Burnaby BC: Simon Fraser University, 1974; Vol. II:820‐9. [Google Scholar]
Additional references
- American Cancer Society. Cancer facts and figures 2004. American Cancer Society. Atlanta, GA: American Cancer Society, 2004. [Google Scholar]
- Bennett MH, Feldmeier J, Hampson N, Smee R, Milross C. Hyperbaric oxygen therapy for late radiation tissue injury. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD005005] [DOI] [PubMed] [Google Scholar]
- Bennett M, Connor D. The database of randomised controlled trials in diving and hyperbaric medicine. www.hboevidence.com; accessed April 20112011. [PubMed]
- Bush RS. The significance of anemia in clinical radiation therapy. International Journal of Radiation Oncology, Biology, Physics 1986;12(11):2047‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Churchill‐Davidson I. The oxygen effect in radiotherapy ‐ historical review. Frontiers of Radiation Therapeutic Oncology 1968;1:1‐15. [Google Scholar]
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
- Feldmeier J, Carl U, Hartmann K, Sminia P. Hyperbaric oxygen: does it promote growth or recurrence of malignancy?. Undersea and Hyperbaric Medicine 2003;30(1):1‐18. [MEDLINE: ] [PubMed] [Google Scholar]
- Grau C, Horsman MR, Overgaard J. Improving the radiation response in a C3H mouse mammary carcinoma by normobaric oxygen and carbogen breathing. International Journal of Radiation Oncology, Biology, Physics 1992;22(3):415‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Gray LH, Conger AD, Ebert M. The concentration of oxygen dissolved in the tissues at the time of irradiation as a factor in radiotherapy. British Journal of Radiotherapy 1953;26:638‐48. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotes JL, Wu X‐C, McLaughlin C. Cancer in North America, 1996 ‐ 2000. Vol. 2: Mortality, Springfield, IL: North American Association of Central Cancer Registries, 2003. [Google Scholar]
- Jain KK. Textbook of Hyperbaric Medicine. 4. New York: Hogrefe and Huber, 2009. [Google Scholar]
- Jemal A, Thomas A, Murray T, Thun M. Cancer statistics. CA: A Cancer Journal for Clinicians 2002;52:23‐47. [MEDLINE: ; pISSN: 0007‐9235] [DOI] [PubMed] [Google Scholar]
- Kindwall EP, Whelan HT. Hyperbaric Medical Practice. 3. Flagstaff, Az: Best Publishing, 2008. [Google Scholar]
- Mathieu D. Handbook on Hyperbaric Medicine. 1st Edition. Dordrecht, Netherlands: Springer, 2009. [Google Scholar]
- Neuman T, Thom S. Physiology and Medicine of Hyperbric Oxygen Therapy. 1st Edition. Philadelphia, PA: Saunders, 2008. [Google Scholar]
- Overgaard J. Clinical evaluation of nitroimidazoles as modifiers of hypoxia in solid tumours. Oncology Research 1994;6:509‐18. [MEDLINE: ] [PubMed] [Google Scholar]
- Overgaard J, Horsman MR. Modification of hypoxia‐induced radioresistance in tumors by the use of oxygen and sensitizers. Seminars in Radiation Oncology 1996;6(1):10‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rubin P, Hanley J, Keys HM, Marcial V, Brady L. Carbogen breathing during radiation therapy. The RTOG study. International Journal of Radiation Oncology, Biology, Physics 1979;5(11‐12):1963‐70. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
- Undersea and Hyperbaric Medical Society. Hyperbaric chambers in North and Central America: A directory of hyperbaric treatment chambers. Undersea and Hyperbaric Medical Society, 2000. [Google Scholar]
- World Health Organisation sites: Cancer. www.who.int/cancer/en/2004.


