Abstract
Background
'Keratinocyte cancer' is now the preferred term for the most commonly identified skin cancers basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC), which were previously commonly categorised as non‐melanoma skin cancers (NMSC). Keratinocyte cancer (KC) represents about 95% of malignant skin tumours. Lifestyle changes have led to increased exposure to the sun, which has, in turn, led to a significant increase of new cases of KC, with a worldwide annual incidence of between 3% and 8%. The successful use of preventive measures could mean a significant reduction in the resources used by health systems, compared with the high cost of the treatment of these conditions. At present, there is no information about the quality of the evidence for the use of these sun protection strategies with an assessment of their benefits and risks.
Objectives
To assess the effects of sun protection strategies (i.e. sunscreen and barrier methods) for preventing keratinocyte cancer (that is, basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC) of the skin) in the general population.
Search methods
We searched the following databases up to May 2016: the Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase, and LILACS. We also searched five trial registries and the bibliographies of included studies for further references to relevant trials.
Selection criteria
We included randomised controlled clinical trials (RCTs) of preventive strategies for keratinocyte cancer, such as physical barriers and sunscreens, in the general population (children and adults), which may provide information about benefits and adverse events related to the use of solar protection measures. We did not include trials focused on educational strategies to prevent KC or preventive strategies in high‐risk groups. Our prespecified primary outcomes were BCC or cSCC confirmed clinically or by histopathology at any follow‐up and adverse events.
Data collection and analysis
Two review authors independently selected studies for eligibility using Early Review Organizing Software (EROS). Similarly, two review authors independently used predesigned data collection forms to extract information from the original study reports about the participants, methods of randomisation, blinding, comparisons of interest, number of participants originally randomised by arm, follow‐up losses, and outcomes, and they assessed the risk of bias. We resolved any disagreement by consulting a third author and contacted trial investigators of identified trials to obtain additional information. We used standard methodological procedures expected by Cochrane.
Main results
We included one RCT (factorial design) that randomised 1621 participants.
This study compared the daily application of sunscreen compared with discretionary use of sunscreen, with or without beta‐carotene administration, in the general population. The study was undertaken in Australia; 55.2% of participants had fair skin, and they were monitored for 4.5 years for new cases of BCC or cSCC assessed by histopathology. We found this study to be at low risk of bias for domains such as allocation, blinding, and incomplete outcome data. However, we found multiple unclear risks related to other biases, including an unclear assessment of possible interactions between the effects of the different interventions evaluated (that is, sunscreen and beta‐carotene). We found no difference in terms of the number of participants developing BCC (n = 1621; risk ratio (RR) 1.03, 95% confidence interval (CI) 0.74 to 1.43) or cSCC (n = 1621; RR 0.88, 95% CI 0.50 to 1.54) when comparing daily application of sunscreen with discretionary use, even when analyses were restricted to groups without beta‐carotene supplementation. This evidence was of low quality, which means that there is some certainty that future studies may alter our confidence in this evidence.
We reported adverse events in a narrative way and included skin irritation or contact allergy.
We identified no studies that evaluated other sun protection measures, such as the use of sun‐protective clothing, sunglasses, or hats, or seeking the shade when outdoors.
Authors' conclusions
In this review, we assessed the effect of solar protection in preventing the occurrence of new cases of keratinocyte cancer. We only found one study that was suitable for inclusion. This was a study of sunscreens, so we were unable to assess any other forms of sun protection. The study addressed our prespecified primary outcomes, but not most of our secondary outcomes. We were unable to demonstrate from the available evidence whether sunscreen was effective for the prevention of basal cell carcinoma (BCC) or cutaneous squamous cell carcinoma (cSCC).
Our certainty in the evidence was low because there was a lack of histopathological confirmation of BCC or cSCC in a significant percentage of cases. Amongst other sources of bias, it was not clear whether the study authors had assessed any interaction effects between the sunscreen and beta‐carotene interventions. We think that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Plain language summary
Sun protection (including sunscreens) to prevent basal cell carcinoma and cutaneous squamous cell carcinoma of the skin
What is the aim of this review?
The aim of this Cochrane Review was to find out if using topical sunscreen and physical barrier methods (such as sun‐protective clothing, hats, sunglasses, and the active search for shade when outdoors) compared with no specific precautionary activity prevented the development of basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC) of the skin in adults and children.
What was studied in this review?
Keratinocyte cancer (BCC and cSCC of the skin) is the most commonly identified type of skin cancer. The main risk is exposure to ultraviolet radiation, which is a component of sunlight. Prevention has become an important way to manage this cancer, so it is important to assess the effectiveness of methods used to prevent keratinocyte cancer in the general population. In this review, we assessed the effects of using topical sunscreen and physical barrier methods (such as sun‐protective clothing, hats, sunglasses, and the active search for shade when outdoors) compared with no specific precautionary interventions aimed at preventing the development of BCC and cSCC in adults and children.
We searched the medical literature up to May 2016 for randomised controlled trials that evaluated preventive strategies. We found only one study suitable for inclusion. This study compared the daily application of sunscreen (with or without beta‐carotene, which is a precursor of vitamin A) compared with the occasional use of sunscreen (with or without beta‐carotene) in the general population, without restriction by gender or age. The study was undertaken in Australia, where 1621 participants, 55% of them with fair skin, were monitored for 4.5 years for new cases of BCC or cSCC assessed by histopathology (which is a method used to detect cancerous cells under the microscope).
What are the main results of this review?
We found no difference between the number of people who developed BCC or cSCC in the two groups over the time period of the trial. So, there did not seem to be a difference in applying sunscreen daily compared with using it occasionally.
Key messages
Our one included study was a study of sunscreens, so we were unable to assess any other forms of sun protection.
We identified no studies that evaluated other sun protection measures, such as the use of sun‐protective clothing, sunglasses, or hats, or seeking the shade when outdoors.
We did not find evidence for the effectiveness of daily sunscreen for preventing BCC or cSCC compared with the occasional use of sunscreen. The certainty of the evidence was low, which means that future studies may alter this result.
Side effects from the sunscreen used with or without the addition of beta‐carotene included a low percentage of cases of contact allergy and skin irritation.
How up to date is this review?
This review included studies identified up to May 2016.
Summary of findings
Summary of findings for the main comparison. Daily application of sunscreen compared with discretionary use for preventing basal cell and cutaneous squamous cell skin cancers.
| Daily application of sunscreen compared with discretionary use (including beta‐carotene use) for preventing basal cell and cutaneous squamous cell skin cancers | ||||||
| Patient or population: general participants Settings: outpatient Intervention: daily application of sunscreen (including beta‐carotene use) Comparison: discretionary use (including beta‐carotene use) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Discretionary use (including beta‐carotene use) | Daily application of sunscreen (including beta‐carotene use) | |||||
| Basal cell carcinoma Confirmed clinically or by histopathology Follow‐up: 4.5 years | 78 per 1000 |
80 per 1000 (58 to 111) |
RR 1.03 (95% CI 0.74 to 1.43) | 1621 (1 study) | ⊕⊕⊝⊝ Low¹,² | The study authors reported narratively that the incidence was similar, even when analysis was restricted to cases of BCC diagnosed histologically. Also, the study authors reported a similar risk when groups receiving beta‐carotene were excluded (IDR 0.96, 95% CI 0.59 to 1.57). |
| Cutaneous squamous cell carcinoma Confirmed clinically or by histopathology Follow‐up: 4.5 years | 31 per 1000 |
27 per 1000 (15 to 48) |
RR 0.88 (95% CI 0.50 to 1.54) | 1621 (1 study) | ⊕⊕⊝⊝ Low¹,² | The study authors reported an IDR of 0.74 (95% CI 0.39 to 1.38) when the analyses were restricted to histologically diagnosed cSCC. Also, the study authors reported a similar risk when groups receiving beta‐carotene were excluded (IDR 0.74, 95% CI 0.31 to 1.77). |
| Adverse events Number of participants with adverse events Follow‐up: 4.5 years | See comment | See comment | ‐ | 1621 (1 study) | ⊕⊕⊝⊝ Low¹,² | This was reported narratively for the daily sunscreen use group only. The most frequent adverse events were contact allergy or skin irritation (25 participants), skin greasiness (10 participants), and interference with perspiration or stinging eyes after facial perspiration (6 participants). |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BCC: basal cell carcinoma; CI: confidence interval; cSCC: cutaneous squamous cell carcinoma; IDR: incidence density ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
¹Downgraded by one level due to other potential sources of bias, including an unclear assessment of interaction between the effects of the different interventions evaluated (that is, sunscreen and beta‐carotene). ²Downgraded by one level due to indirectness because it is not clear whether these results are applicable to the wider population. Also, these results included cases not confirmed histologically (by clinical examination only).
Background
Description of the condition
Basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC) were previously commonly categorised as non‐melanoma skin cancers (NMSC), but now the preferred term 'keratinocyte cancer' is used (Albert 2003). Throughout the review, we have used this term when we refer to BCC and cSCC collectively.
Keratinocyte cancer (KC) is a term that includes BCC and cSCC of the skin. Together, KC represents about 95% of all skin cancers (Dubas 2013). Since 1960, changes in lifestyle have led to increased exposure to the sun, which has in turn led to a significant increase of new cases of KC, with a worldwide annual incidence of between 3% and 8% (Glass 1989; Ricotti 2009). Around 1.2 million keratinocyte cancers are diagnosed per year in the USA (Ricotti 2009). In Australia, the estimated incidence is 400 cases per 100,000 inhabitants (Johnson 1992; Stern 2010). In Colombia, an equatorial country with a predominant population of phototype III (skin type), a significant increase in KC figures has been documented, rising from 23 cases per 100,000 inhabitants in 2003 to 41 cases per 100,000 in 2007 (Sánchez 2011). In Europe, there is an increasing trend in the incidence of keratinocyte cancer, with exceptional cases such as Switzerland where the highest rate of cSCC has been documented in the whole continent, rising from 14.2 cases per 100,000 inhabitants in 1978 to 28.9 cases per 100,000 inhabitants in 1997 (Lomas 2012).
The action of ultraviolet radiation on the keratinocyte layer of cells in the epidermis is the common site of the development of cSCC and BCC lesions (Preston 1992). The mechanism involves damage to DNA and its repair system, specifically the formation of pyrimidine dimers (the bases that are part of the structure of DNA), as well as mutations of p53 tumour suppressor genes (Preston 1992). Basal cell carcinoma is characterised by a slow rate of growth and extremely low probability of metastasis; however, this condition can compromise wide areas of tissue, cartilage, and bone and produce local damage (Rubin 2005). By contrast, the potential for distant metastasis of cSCC is greater, with up to 5% of lesions metastasising within five years (Miller 2010).
The diagnosis of KC is based on clinical examination of the lesion and confirmation by histopathology (Motley 2003; Telfer 2008), and is classified into two large groups: basal cell carcinomas and cutaneous squamous cell carcinomas (Gloster 1996). The American Cancer Society reports that around 50% to 65% of KCs are classified as BCC, and 20% to 25%, as cSCC (Kornek 2013). Basal cell carcinoma is a tumour of epithelial (outer layer of the skin) origin, which rarely metastasises (ranging from an incidence of 0.0028% to 0.1%) (Glass 1989), but has significant disease burden and considerable costs (Green 2010; Sánchez 2011; Stern 2010). The main risk factor for developing BCC is exposure to ultraviolet radiation (UV) (Corona 2001). Other factors, such as sun exposure during work, skin cancer family history, personal history of sunburn, presence of actinic keratoses, and having skin phototypes I to III, have also been associated with its onset (Lear 1997; Sánchez 2012). By contrast, cSCC is a locally invasive malignant tumour with a great potential to spread to distant parts of the body (Lansbury 2010). Presence of cSCC has been associated with different factors, such as chronic exposure to UV radiation, history of burns and occupational exposure, inherited skin conditions (albinism or xeroderma pigmentosum), and human papilloma virus infection, among other factors (Green 2010; Sánchez 2013).
Description of the intervention
Prevention is an important component in the management of KC and includes measures to avoid and reduce the consequences of exposure to the sun (Kornek 2013). These measures are broadly divided into sunscreens and physical barriers.
Sunscreen agents include all the products designed to reduce contact between the skin and UV radiation (Mulliken 2012). The ability of sunscreens to prevent the damage related to UV radiation is measured by the sun protection factor (SPF) (Mulliken 2012). Briefly, the SPF is defined as the time needed to produce sunburn when the sunscreen is applied to the skin, divided by the time needed to cause sunburn when nothing is applied to the skin (Schalka 2011). The sun protection factor is accepted as the worldwide standard for the assessment of protection against the erythemogenic (reddening) effects of UVB and UVA radiation (Schalka 2011). The effectiveness of a sunscreen is dependent on such characteristics as specific ingredients, general formulation, water‐resistance, time over which the solar filter has been exposed to the sun, and the quantity of sunscreen applied (Saki 2012). Similarly, numerous studies have confirmed that instances of sunburn throughout life increase the risk of developing skin cancer (Sánchez 2012; Sánchez 2013; Zanetti 1996). UVB generates direct changes in DNA (Ravanat 2001). So, sunscreens that block UVB radiation would potentially increase the time of sun exposure without sunburn and, in theory, reduce the risk of developing skin cancer. The level of protection against UVB can be quantified by measuring the MDE (minimum dose of radiation that can produce erythema, which is reddening of the skin) and the SPF. The SPF is the result of the relationship between the minimal erythema dose (MED) with and without protection (MDE with sunscreen/MDE without sunscreen) (Jansen 2013a). Then, the question is to define the SPF that is able to decrease the risk of DNA damage resulting from sunburn and thus prevent the occurrence of new cases of KC.
Physical barriers can also help in preventing and minimising the harmful effects of UV radiation (Gasparro 1998; Latha 2013). Some authors have proposed that such measures could be more effective than sunscreen to prevent skin cancer (Linos 2011). Physical barriers include photoprotection with special clothes made of different materials, which provide protection against both UVB and UVA radiation (Diaz 2013). Polyester is the material with the highest ultraviolet light absorption capacity, while cotton has the lowest capacity (Diaz 2013). Hats can provide protection to the head and neck depending on their size and shape and the materials from which they are made (Klostermann 2013). Sunglasses, depending on their size, shape, and ability to block UV radiation, are effective in protecting the periorbital region, usually on the lower eyelid and inner edge, which are both areas highly exposed to solar radiation (Klostermann 2013). Finally, the active search for shade may be from a physical barrier, such as from a roof or the use of an umbrella when outdoors. These could provide significant protection against UV radiation without modifying exposure to visible light (Burnett 2012; Cooley 2013).
How the intervention might work
Sun protection strategies, such as those previously mentioned, act by blocking or diminishing the contact of UVA and UVB radiation with the skin, thus, avoiding DNA damage and the development of keratinocyte cancer (Sambandan 2011). Although there is a larger proportion of UVA (wavelength = 320 nm to 400 nm) radiation in the solar spectrum, UVB radiation (290 nm to 320 nm) causes most of the acute and chronic biological damage to the skin (Cole 2001). Any strategy suitable for wide implementation should protect against both types of radiation (Latha 2013). An intervention, such as beta‐carotene supplementation, has been evaluated in its role as a protective agent of the skin, for example, in the prevention of sunburn (Kopcke 2008).
Why it is important to do this review
Ultraviolet radiation is a risk factor in the development of KC (Lucas 2006), which has an effect on the quality of life of those with the condition, as well as having serious cost consequences for countries' health systems. The use of preventive measures could mean a significant reduction of the resources used by health systems compared with the high cost that the treatment of KCs and their aftermath entails. At present, there is no information about the evidence for the use of these sun protection strategies with an assessment of their benefits and harms.
Our review aims to systematically assess the effectiveness and related adverse events of these protective elements, providing information on the possible routes for controlling the increasing incidence of keratinocyte cancer in the general population.
The plans for this review were published as a protocol 'Sun protection for preventing basal cell and squamous cell skin cancers' (Sánchez 2014).
Objectives
To assess the effects of sun protection strategies (i.e. sunscreen and barrier methods) for preventing keratinocyte cancer (that is, basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (cSCC) of the skin) in the general population.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled clinical trials that may provide information about benefits and adverse events related to the use of solar protection elements, such as physical barriers and sunscreens, as preventive strategies for keratinocyte cancer.
We did not include trials focused on educational strategies to prevent KC (Langbecker 2014), nor preventive strategies in high‐risk groups (for example, people with actinic keratoses, organ transplant recipients, etc.), because there are other Cochrane Reviews published or in development that focus on these specific issues (Bath‐Hextall 2007; Morales‐Sánchez 2016).
Types of participants
We included trials focused on the general population (children and adults) without restriction by gender or age. We did not include trials focusing on special populations (for example, people with actinic keratoses, organ transplant recipients, etc.).
Types of interventions
Studies included one or more of the following interventions versus no intervention, placebo, or other interventions.
Sunscreens with any sun protection factor (SPF).
Wearing hats, sunglasses, and special clothing outdoors.
Staying out of the sun (e.g. use of shady places, roofs, and umbrellas outdoors, etc.).
Types of outcome measures
Primary outcomes
Basal cell carcinoma (BCC) confirmed clinically or histopathologically at any follow‐up.
Cutaneous squamous cell carcinoma (cSCC) confirmed clinically or histopathologically at any follow‐up.
Adverse events (e.g. dermatitis from sunscreens, acne secondary to the use of sunscreens, dermatitis from the use of hats and clothes, vitamin D deficiency from lack of exposure to the sun, etc.) reported by a number of participants or individually.
We have explained our reason for the addition of 'clinical' to our two primary outcomes in the Differences between protocol and review section.
Secondary outcomes
Number of self‐reported sunburns or skin lesions, defined by each study, at the end of follow‐up.
Actinic or solar keratoses at any follow‐up.
Total hours of ultraviolet radiation exposure at the end of follow‐up.
Total hours outdoors in peak exposure times at the end of follow‐up.
Minimal erythema dose (MED) at the end of follow‐up.
Participant's compliance with preventive strategies at the end of the trial.
Search methods for identification of studies
We aimed to identify all relevant randomised controlled trials (RCTs) regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
We searched the following databases up to 10 May 2016:
the Cochrane Skin Group Specialised Register using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) 2016, Issue 5, in the Cochrane Library using the strategy in Appendix 2;
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3;
Embase via Ovid (from 1974) using the strategy in Appendix 4; and
LILACS (Latin American and Caribbean Health Science Information database, from 1982) using the strategy in Appendix 5.
Trial registries
We searched the following trials registries and portals up to 10 May 2016:
the metaRegister of Controlled Trials (www.controlled‐trials.com);
the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov);
the Australian New Zealand Clinical Trials Registry (www.anzctr.org.au);
the World Health Organization International Clinical Trials Registry Platform (www.who.int/trialsearch); and
the EU Clinical Trials Register (www.clinicaltrialsregister.eu).
We used the terms "non melanoma skin cancer", "basal cell carcinoma", "squamous cell carcinoma", "bowen disease", and "actinic keratosis".
Searching other resources
References from included studies
We checked the bibliographies of included studies for further references to relevant trials.
Adverse effects
We did not perform a separate search for adverse effects of the target intervention, but we examined data on adverse effects from the included study that we identified.
Data collection and analysis
Due to the lack of studies, we were unable to carry out analyses that we had planned in our protocol. We have explained this in the Differences between protocol and review section.
Selection of studies
Two review authors (MO and GS) independently selected studies for eligibility with Early Review Organizing Software (EROS) (Ciapponi 2011; Glujovsky 2010; Glujovsky 2011). We checked the titles and abstracts of all of the retrieved studies to determine if they fulfilled the inclusion criteria previously proposed. We assessed the full texts of identified studies to confirm their final inclusion. We resolved all disagreements by involving a third author (IA‐R). We were not blinded to characteristics such as the authors' names, institutions, or the journal of publication at any stage of the review. We recorded the reasons for our exclusion of potential studies in the 'Characteristics of excluded studies' tables (Higgins 2011).
Data extraction and management
Two review authors (CS and JG) used predesigned data collection forms to retrieve information, such as randomisation methods, blinding of participants and personnel, comparisons, number of participants by arm, and follow‐up losses, from the original study in an independent way (Higgins 2011). We tested this format prior to extended use. We resolved any disagreement by discussion with a third review author (JN). We entered extracted data into Review Manager 5 for further analyses (Review Manager 2012).
Assessment of risk of bias in included studies
Two authors (ARH and IA‐R) used the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions to independently assess the risk of bias of the included trial (Higgins 2011). We took six domains into consideration: random sequence generation; allocation concealment; blinding of participants, personnel, and outcome assessment; incomplete outcome data; selective reporting; and other biases. We resolved any disagreement by discussion with a third author (GS). We assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings (Higgins 2011). We summarised the information in a 'Risk of bias' table, which is an extension of the 'Characteristics of included studies' table. We planned to explore the impact of the level of bias through undertaking sensitivity analyses (see the Sensitivity analysis section), but we did not perform this analysis because of the number of studies included.
Measures of treatment effect
We presented results of dichotomous outcomes (such as BCC and cSCC) as summary risk ratios (RR) with 95% confidence intervals (CIs), as well as the number needed to treat for an additional harmful outcome (NNTH) as an absolute measure of harm, and NNTH as the reciprocal of risk differences (RD) (McQuay 1998). We also planned that for continuous outcomes (such as the number of sunburns and total hours of ultraviolet radiation exposure), we would report the mean difference (MD) with its corresponding 95% CI, but these outcomes were not reported (see Differences between protocol and review section).
Unit of analysis issues
We did not expect to find any unit of analysis issues as we did not expect to find cross‐over studies. In the case of within‐participant trials (e.g. split face) related to the effectiveness of the sunscreen, we planned to report the results in a narrative way and not include this information in planned meta‐analyses. However, we found that information about these kinds of tumours can be expressed as number of participants affected, as well as total number of tumours by groups.
Dealing with missing data
We planned to retrieve levels of attrition of information if possible. We planned to explore, by using sensitivity analyses, the impact of trials with high levels of attrition in the assessment of treatment effects. If possible, we carried out analyses, as far as possible on an intention‐to‐treat (ITT) basis (i.e. we attempted to include in the analyses all randomised participants in the denominator of the assessed groups).
Assessment of heterogeneity
We planned to investigate heterogeneity in the first instance through visual examination of measures of treatment effect forest plots. Main sources of heterogeneity could include skin phototype, duration of follow‐up, and gender and age groups. We planned to evaluate statistical heterogeneity by means of the I² statistic. This statistic estimates the percentage of total variation across included trials due to heterogeneity rather than sampling error (Higgins 2003; Higgins 2011). We planned to explore heterogeneity if the I² statistic was greater than 30%, and in cases where the I² statistic was more than 80%, we did not plan to present pooled results.
Assessment of reporting biases
We planned to create funnel plots of the primary outcomes to provide a visual assessment of reporting bias if at least 10 trials were available (Higgins 2011; Sterne 2011). Also, we planned to use two tests to assess asymmetry in the corresponding funnel plot: the regression asymmetry test (Egger 1997) and the adjusted rank correlation test (Begg 1994). However, we did not perform this analysis because of the number of studies included.
Data synthesis
We planned to summarise the findings using random‐effects models with the DerSimonian and Laird method and carry out statistical analyses using Review Manager 5 (Review Manager 2012). We planned not to present a pooled result if we identified substantial heterogeneity (I² statistic was greater than 80%). Also, we planned to conduct a trial sequential analysis (TSA), which combines an information size calculation (cumulated sample sizes of included trials) for meta‐analysis with the threshold of statistical significance. We planned to conduct a TSA using the TSA software on binary outcomes (Brok 2009; Pogue 1997; Pogue 1998; Thorlund 2009; Wetterslev 2008) and apply trial sequential monitoring boundaries according to a heterogeneity‐adjusted required information size, based on an a priori 10% relative risk reduction (RRR) employing alpha = 0.05 and beta = 0.20 (CTU 2011; Thorlund 2011). However, we did not perform these analyses due to the number of studies included, and we present the results in a narrative way, with figures to illustrate the main information.
Subgroup analysis and investigation of heterogeneity
We planned to undertake subgroup analysis and perform interaction tests to check for subgroup differences where this assessment would be meaningful. For the primary outcomes, we planned to consider subgroup analyses for the following factors, as appropriate:
gender;
age groups (i.e. less than 18 years, 18 to 40 years, 41 to 60 years, greater than 60 years);
skin phototype (i.e. I, II, III, IV, V, VI);
hair colour phenotype (i.e. redhead, blonde, black, etc.);
eye colour phenotype (i.e. blue, green, black, brown, etc.);
duration at follow‐up (less than one year, between one to two years, between two to five years, more than five years); and
history of skin cancer or precancerous lesions (personal and family).
However, we did not perform these analyses because of the number of studies included (see Differences between protocol and review section).
Sensitivity analysis
We planned to perform a sensitivity analysis using those trials classified as having a low risk of bias (Higgins 2011) in three core domains: allocation concealment, incomplete outcome data, and blinding of outcome assessment. However, we did not perform this analysis because of the number of studies included (see Differences between protocol and review section).
'Summary of findings' tables
We used the guidelines of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group (Guyatt 2008) to assess the quality of the evidence related to primary outcomes, and developed a 'Summary of findings' table with the GRADE profiler software. The GRADE system assesses the quality of evidence based on the extent to which users can be confident that an association reflects the item being evaluated (Guyatt 2008). Assessment of the quality of evidence included consideration of the risk of bias, heterogeneity, directness of the evidence, risk of publication bias, and precision of effect estimates (Guyatt 2011; Guyatt 2011a; Guyatt 2011b; Guyatt 2011c; Guyatt 2011d; Guyatt 2011e; Guyatt 2011f; Guyatt 2011g).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We identified 482 records from the database searches after removal of duplicates. We excluded 452 records based on titles and abstracts and identified 30 records for further evaluation in full text. We excluded a further 21 records (See Characteristics of excluded studies). We included one trial, Green 1999, with data reported in nine different references (Figure 1). We did not find additional references from trial registers or other sources of information.
1.
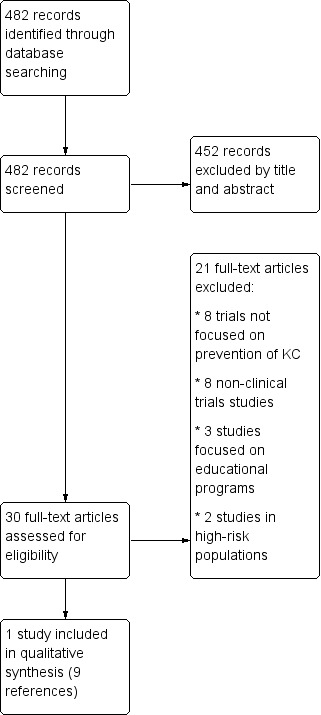
Study flow diagram
Included studies
We included one study, which compared daily application of sunscreen versus discretionary use (mostly for recreational use). We did not find studies of children or studies comparing sunscreen at any sun protection factor (SPF) with no intervention or placebo. Also, we did not identify studies about interventions such as wearing hats, sunglasses, or sun‐protective clothing outdoors, or staying out of the sun (use of shady places, roofs, umbrellas, etc.).
Design
We included one study that randomly assigned 1621 people and included 1383 with full skin examination in the analysis (see the Characteristics of included studies section). This study was a randomised 2x2 factorial trial developed in Australia, which evaluated the following four interventions: daily sunscreen and beta‐carotene supplementation (30 mg beta‐carotene tablet each day); daily sunscreen and placebo; discretionary sunscreen and beta‐carotene supplementation (30 mg beta‐carotene tablet each day); and discretionary sunscreen and placebo. The use of placebo cream alone was considered "unethical"; thus, the daily application of sunscreen was compared with the discretionary use of sunscreen. However, the study authors effectively reduced the comparisons to two by combining the groups (see below).
Participants
Adult participants were selected from a population‐based prevalence survey of skin cancer conducted in 1986, with 43.7% being men; the average age was 48.77 years. Fifty‐two per cent of the participants were fair skinned; 18.7% had an occupation that they carried out mainly outdoors; and 27% had a previous diagnosis of skin cancer.
Sample size and setting
One thousand two hundred and one eligible residents of Nambour were randomised to the different groups of this trial. The study authors presented analysis based on 1383 trial participants who had full skin examination during the follow‐up period. The study authors estimated that a minimum of 1600 participants needed to be enrolled to detect a 36% reduction of basal cell carcinoma (BCC) and a 59% reduction of cutaneous squamous cell carcinoma (cSCC).
Interventions
Four groups were evaluated.
Participants in group one received instructions for daily use of SPF 16 sunscreen and a 30 mg beta‐carotene tablet to be taken each day.
Participants in group two received instructions for daily use of SPF 16 sunscreen and a 30 mg placebo tablet each day.
The study authors added groups one and two together to obtain results for all participants with daily administration of SPF 16 sunscreen (daily sunscreen group: number of participants randomised = 812).
Participants in group three received a 30 mg beta‐carotene tablet each day and received instructions to use sunscreen on a discretionary basis.
Participants in group four received a 30 mg placebo tablet each day and received instructions to use sunscreen on a discretionary basis.
The study authors added groups three and four together to obtain results for all participants with discretionary administration of SPF 16 sunscreen (non‐daily sunscreen group: number of participants randomised = 809).
One thousand three hundred and eighty‐three participants completed the full follow‐up period of 4.5 years (85.3%). We included the 1621 participants originally allocated to our analysis (intention‐to‐treat analysis). Losses by group ranged from 12.3% to 17.1%. The administration of sunscreen on a daily basis involved the application of a layer of sunscreen to all exposed sites of the head, neck, arms, and hands every morning.
Outcomes
The primary outcome of our sole included study was the number of new cases of BCC and cSCC (Green 1999). Secondary outcomes were change in the prevalence of solar keratosis (as full body counts and per sunscreen site counts), adverse events (main reported complaints), and the degree of photoageing. The researchers assessed the participants' compliance with sunscreen use every three months by comparing the weight of the sunscreen provided with the average rate of consumption for a group of non‐participants. Likewise, the use of sunscreen in the control group was measured through a standard questionnaire delivered annually. Two follow‐up clinics in 1994 (two years after the beginning of the study) and 1996 (at the end of the study) assessed the participants, and all lesions identified at those times were evaluated by histopathology. Local doctors documented the rest of the lesions on prespecified cards.
Excluded studies
We excluded 21 studies for reasons reported in the 'Characteristics of excluded studies' tables after assessment of the full text of the report and most frequently because they did not evaluate the effectiveness of sun protection strategies in the prevention of keratinocyte cancer.
Risk of bias in included studies
We assessed the one included study for risk of bias and reported the judgments for the individual domains in the 'Risk of bias' table (see Figure 2).
2.
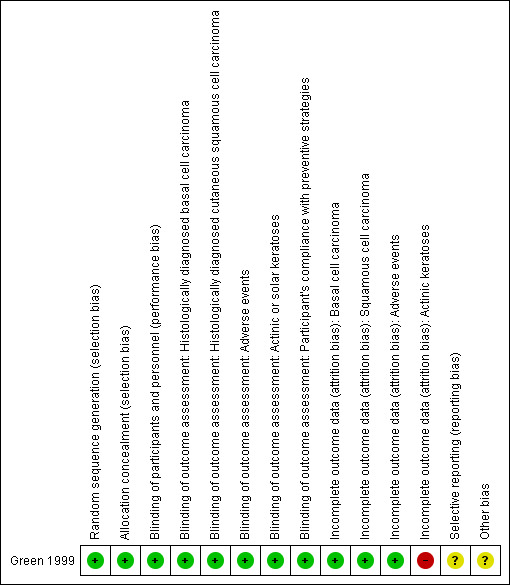
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
Regarding randomisation issues, Green 1999 declared, "A customized randomization computer program was used to assign all attending the baseline study clinics to one of four treatment groups." Likewise, regarding allocation concealment, they added, "The treatment code was known only to the investigator who generated it and the two people who packaged the tablets for distribution. None of these people had contact with participants." We therefore judged these two domains to be at low risk of bias.
Blinding
Regarding blinding of participants, personnel, and assessment of outcomes, the authors of Green 1999 reported that the treatment code was known only to the investigator who generated it and to the two people who packaged the tablets for distribution. None of these people had contact with participants. Likewise, a single dermatopathologist histologically examined all clinically diagnosed skin cancers during these follow‐up surveys. Dermatologists involved in the study survey were unaware of the treatment allocation. We judged these domains at low risk of bias.
Incomplete outcome data
Regarding attrition bias for our primary outcomes, Green 1999 declared: "At the end of the study in year 5, 238 (15%) participants had withdrawn without a complete skin examination by a dermatologist in the follow‐up period." The percentage of losses to follow‐up was similar for all groups, and it was the same for all primary outcomes (group one: 12.37%; group two: 17.15%; group three: 14.1%; group four: 15.01%). We judged this domain at low risk of bias.
By contrast, the number of participants lost to follow up was bigger for actinic keratosis (ranging from 28% to 36%), and the risk was considered high for this outcome. One thousand three hundred and eighty‐three participants completed the full follow‐up period of 4.5 years, but we included the 1621 participants originally randomised under intention‐to‐treat (ITT) analysis.
Selective reporting
In a secondary reference, Green 1999 reported the association between possible predictors of sunscreen use and frequency of sunscreen application, including general information on variables such as time outdoors on weekdays in summer and burns during the trial, but this information was not reported in a sufficient way to enable analysis. So, we judged this domain to be at unclear risk of bias.
Other potential sources of bias
We identified other potential sources of bias in the study, including an unclear assessment of the interaction between the effects of the different interventions evaluated (that is, sunscreen and beta‐carotene), an unclear impact of multiple posthoc analyses not planned a priori (repeated significance testing), and an unclear impact of clinical versus histological diagnosis of keratinocyte cancer. We did not consider funding as a source of bias as the Public Health Research and Development Committee of the National Health and Medical Research Council of Australia provided funding.
Effects of interventions
See: Table 1
We only found information from one study comparing the daily application of sunscreen (in which participants applied a layer of sunscreen themselves every morning to all uncovered areas on the head, neck, arms, and hands, reapplying after substantial sweating, bathing, or extended sun exposure) versus discretionary use (mostly for recreational use).
For the main analyses, Green 1999 included all participants in the trial, including those who received beta‐carotene tablets. They combined groups one and two to obtain results for all participants with daily administration of SPF 16 sunscreen (i.e. those participants who received instructions for daily use of SPF 16 sunscreen and a 30 mg beta‐carotene tablet to be taken each day, plus those participants who received instructions for daily use of SPF 16 sunscreen and a 30 mg placebo tablet each day). These were compared to all participants (groups three and four) with discretionary administration of SPF sunscreen (i.e. those participants who received a 30 mg beta‐carotene tablet each day and received instructions to use sunscreen on a discretionary basis, plus those participants who received a 30 mg placebo tablet each day and received instructions to use sunscreen on a discretionary basis).
For BCC and cSCC, the trial authors provided information for cases confirmed histopathologically in terms of incidence density ratios (IDR); these estimates are provided below as supplementary information. For actinic keratosis (AK), authors combined all lesions on the whole body at each examination (full body counts) and also added all lesions on the sites to which sunscreen was applied (sunscreen site counts). Also, Green 1999 provided supplementary analyses for BCC and cSCC excluding participants assigned to beta‐carotene tablets (i.e. groups one and three). However, there was not enough information about the number of cases per arm to estimate risk ratios (RR). Information about IDR (which was the effect measure reported by the study authors) is provided as supplementary information.
We present the quality of the evidence in Table 1.
Primary outcomes
Basal cell carcinoma (BCC) confirmed clinically or histopathologically at any follow‐up
The incidence of new BCC was similar in the daily‐application group (812 participants randomly assigned) compared with the discretionary‐use group (809 participants randomly assigned) (RR 1.03, 95% confidence interval (CI) 0.74 to 1.43; Analysis 1.1). This evidence was of low quality, which means that there is some certainty that future studies may alter our confidence in this evidence.
1.1. Analysis.
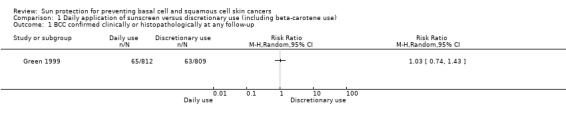
Comparison 1 Daily application of sunscreen versus discretionary use (including beta‐carotene use), Outcome 1 BCC confirmed clinically or histopathologically at any follow‐up.
It was reported in a narrative way that the incidence was similar, even when authors restricted analysis to cases of BCC "that had been diagnosed histologically". The study authors also provided information in terms of IDR when they excluded groups receiving beta‐carotene (that is, the authors excluded groups one and three), but they did not find differences between groups (IDR 0.96, 95% CI 0.59 to 1.56).
Cutaneous squamous cell carcinoma (cSCC) confirmed clinically or histopathologically at any follow‐up
The incidence of new cSCC was similar in the daily‐application group (812 participants randomly assigned) compared with the discretionary‐use group (809 participants randomly assigned) (RR 0.88, 95% CI 0.50 to 1.54; Analysis 1.2). This evidence was of low quality, which means that there is some certainty that future studies may alter our confidence in this evidence.
1.2. Analysis.
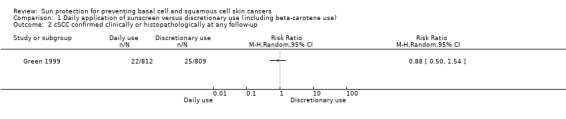
Comparison 1 Daily application of sunscreen versus discretionary use (including beta‐carotene use), Outcome 2 cSCC confirmed clinically or histopathologically at any follow‐up.
Likewise, the incidence rate of histologically diagnosed cSCC, based on 40 participants with pathological confirmation, was similar in both groups (IDR 0.74, 95% CI 0.39 to 1.38). The study authors also provided information in terms of IDR when they excluded groups receiving beta‐carotene (that is, the authors excluded groups one and three), but they found no differences between groups (IDR 0.74, 95% CI 0.31 to 1.77).
Adverse events
In a narrative report, Green 1999 stated that the main complaints made by the daily sunscreen use group were related to skin irritation or contact allergy (25 participants out of 812) and skin oiliness (10 participants out of 812). This evidence was of low quality, which means that there is some certainty that future studies may alter our confidence in this evidence. Adverse events related to the discretionary‐use group were not reported.
Secondary outcomes
We did not find information about the number of self‐reported sunburns or skin lesions, total hours of ultraviolet radiation exposure, total hours outdoors in peak exposure times, or minimal erythema dose (MED). Our sole included study only reported the following secondary outcomes.
Actinic or solar keratoses at any follow‐up
The rate of change in the total number (full body count) of prevalent actinic keratoses between 1994 and 1996 was similar in the daily‐application group (n = 812 participants randomly assigned) and in the discretionary‐use group (n = 809 participants randomly assigned; 559 participants analysed) (RR 0.95, 95% CI 0.75 to 1.20; Analysis 1.3).
1.3. Analysis.
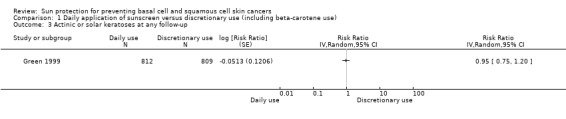
Comparison 1 Daily application of sunscreen versus discretionary use (including beta‐carotene use), Outcome 3 Actinic or solar keratoses at any follow‐up.
Participant's compliance with preventive strategies at the end of the trial
In a narrative report, Green 1999 stated, "75% of participants assigned to daily sunscreen use were applying sunscreen to their neck, arms and hands at least 3 to 4 days a week and those people not assigned to the sunscreen group were applying sunscreen to head, neck and arms not at all or no more than 1 or 2 days a week." For the daily use group, the median daily weight of sunscreen applied on average throughout the trial was 1.5 g/d (range 0 to 7.4 g/d), but the median decreased as the trial progressed (1992 = 1.67 g and 1996 = 1.22 g).
Discussion
We have summarised the evidence in Table 1.
Summary of main results
In this review, we only identified one study, which evaluated whether regular daily use of sunscreen in comparison with discretionary or occasional use prevented new cases of basal cell carcinoma (BCC), cutaneous squamous cell carcinoma (cSCC), and our secondary outcome actinic keratoses at follow‐up (Green 1999). The study was undertaken in Nambour in South East Queensland (Australia); the researchers also studied adverse events and adherence related to the use of sunscreen. They found that sun protection factor (SPF) 16 sunscreen applied on exposed sites of the body, such as the head, neck, and upper limbs, every morning and repeated after heavy sweating or bathing made no difference in terms of reducing the occurrence of new cases of BCC and cSCC confirmed or not by histopathology at 4.5 years, in comparison with discretionary use (low‐quality evidence; see Table 1). We did not identify studies comparing the use of sunscreen against no use of sunscreen.
Adverse events associated with the use of sunscreen in the daily sunscreen use group, such as occasional local reactions, like contact dermatitis; the feeling of oily skin; and interference with perspiration, were documented.
We identified no studies that evaluated other sun protection measures or regimens, such as wearing sun‐protective clothing, glasses, or hats, or seeking the shade when outdoors.
Overall completeness and applicability of evidence
Although we searched for randomised controlled trials (RCTs) of sun protection strategies aimed at preventing the development of cutaneous squamous cell carcinoma, basal cell carcinoma, and actinic keratoses, we found only one study, which was on the use of sunscreen. Limitations of this study were that it was restricted to only one SPF (SPF 16); it was in a population at higher risk of keratinocyte cancer due to the majority of participants having fair skin (55.2% were fair) and therefore a higher risk of sun damage and developing keratinocyte cancer (KC); and it did not address several of the prespecified secondary outcomes planned in our review.
Our review did not find differences between two sunscreen regimens (daily use versus discretionary use) in terms of the number of participants with new cases of BCC or cSCC, so to date, available evidence regarding the effectiveness and safety of preventive strategies for cSCC and BCC is scarce. This result could be attributed to a short period of exposure to sunscreen (4.5 years) and a limited tracking time. Given the above, it is possible that the clinical assessment did not last long enough to demonstrate that a protective effect can reduce the occurrence of BCC.
Cutaneous squamous cell carcinoma lesions are strongly related to sun exposure. It is estimated that 90% of these occur in body sites that are usually exposed to the sun (Miller 2010). Preventing early stages of carcinogenesis would indicate the benefit of performing preventive procedures from childhood with a longer follow‐up period (Zelen 1988). The pattern of BCC‐causing sun exposure seems to be different from that of cSCC, because although it is accepted that ultraviolet exposure (UV) is the main causal factor, the accurate relationship between the amount, timing, and pattern of exposure is unknown (i.e. exposure age, number of years, intensity of exposure, etc.) (Roewert‐Huber 2007). Although cSCC lesions show a higher metastatic profile in comparison with basal cell carcinoma lesions, there are BCC histologic patterns that might be locally aggressive without correlation with its metastatic risk (Roewert‐Huber 2007). However, reduced sunlight exposure can be deleterious for some populations, producing long‐term harms such as vitamin D deficiency and depression, which is a reason for not recommending reduced exposure to sunlight as a general measure (Maslin 2014).
The results of this study highlight that there are peculiarities in populations that could change the estimate of the effect, among which, phototype, type of radiation, geographic location (altitude above sea level, ozone layer, and latitude), and the number of hours spent outdoors are worth noting (Holman 1984). Thus, the benefits of using sunscreen may vary from one population to another. Finally, we must emphasise that Green 1999 conducted an evaluation of effectiveness with only one sun protection factor (SPF 16) and a once a day application scheme, which today would be considered inadequate (Pissavini 2013).
Quality of the evidence
The results of this single study suggest that using sunscreen on a daily basis, compared with discretionary use, does not reduce the onset of new cases of basal cell or cutaneous squamous cell carcinoma. This evidence was of low quality, which means that there is some certainty that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. We downgraded the risk of bias due to a number of potential risks that had an unclear impact on the results, including an unclear assessment of interaction between the effects of the different interventions evaluated (that is, sunscreen and beta‐carotene), an unclear impact of multiple posthoc analyses not planned a priori (repeated significance testing), and an unclear impact of clinical versus histological diagnosis of keratinocyte cancer. We deemed other considerations such as inconsistency, imprecision, and publication bias as not serious, but readers should consider these issues when considering the main results of this trial.
Potential biases in the review process
This review was comprehensive in aiming to identify clinical trials addressing the issue of the effectiveness and safety of sun protection measures for the prevention of cSCC and BCC. In general, we followed most of the strategies recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) regarding identification of relevant studies. However, the number of references identified was low, demonstrating the poor level of research at trial level in this area. This is understandable given the difficulty of long‐term population experiments, which are needed to adequately identify incident cases of KC. Also, the effectiveness of sun protection measures is often studied in conjunction with educational strategies to maximise adherence to preventive strategies, but this review did not address the educational aspect.
In this review, we made some changes from the protocol, which may be sources of bias. We expanded the criteria for confirmation of BCC and cSCC lesions, because histopathological confirmation of keratinocyte cancer could be a difficulty in population‐based studies with large sample sizes, and then, clinical confirmation is more likely to be used. Despite expanding our criteria, we considered that there was a lack of applicability of our results to a wider population, so we downgraded for indirectness. This change could be considered a source of bias in this review.
Agreements and disagreements with other studies or reviews
van der Pols 2006 contained the results of a follow‐up study of the cohort of people participating in the Nambour study eight years after the cessation of the trial. Including the time of the trial, a 12‐year follow‐up was completed. Comparing the group of participants originally assigned to daily sunscreen use with the group not assigned to daily sunscreen use, the author found that sunscreen significantly reduced the incidence of cSCC in both the number of people affected (RR 0.65, 95% CI 0.45 to 0.94) and the number of tumours diagnosed (RR 0.59, 95% CI 0.38 to 0.90). However, it was not possible to demonstrate, even during this monitoring period, that the regular application of sunscreen could reduce the occurrence of new cases of BCC, which may take many years to develop (van der Pols 2006). Although our plan was to assess the development of BCC or cSCC at any follow‐up period, it was clear to us (because of the participants' compliance with the preventive strategies) that the information reported in this study was a follow‐up of a cohort of participants who did not necessarily use sunscreen as planned in the trial.
Bimczok 2007 found that the minimum dose of sunscreen to be effective was SPF 15, and the minimum amount should be 2 mg/cm². However, subsequent studies have reported that the general population applies close to 1 mg/cm², which would affect the effectiveness of the intervention (Jansen 2013a; Jansen 2013b). In 2011, the U.S. Food and Drug Administration (FDA) recommended the use of sunscreen with a SPF higher than 15, and that same year, the National Institute for Health and Care Excellence (NICE) in the UK published a guide for the prevention of skin cancer in which it too recommended the use of sunscreen with a SPF higher than 15 (NICE 2011). Pissavini and collaborators, who after studying the population's patterns of sunscreen use and ultraviolet (UV) exposure, recommended the use of SPF 30, have recently supplemented these results, given that the effectiveness of this measure is related to frequency of use and the dose used (Pissavini 2013).
Authors' conclusions
Implications for practice.
In different countries, it has been documented that there has been a progressive increase in the rates of skin cancer (Lomas 2012; Sánchez 2011), driving the implementation of strategies that may help to control this growing phenomenon. The present systematic review explored the evidence related to the effect of sun protection measures against the occurrence of new cases of keratinocyte cancer, but was unable to find evidence for the effectiveness of sunscreen to prevent the development of basal cell carcinoma lesions (BCC) or cutaneous squamous cell carcinoma (cSCC) in the participants (Green 1999).
Implications for research.
Despite the fact that well‐planned studies need to be performed to address the main objective of this review, the widespread use of sunscreen may make it more difficult to run clinical trials where placebo creams are used as the control. However, other important questions remain, such as the optimal application frequency and minimum sun protection factor for the adequate prevention of cSCC and BCC, as well as the safety of the different sunscreen brands and their effects when applied to the skin. Future studies should also include histological confirmation of keratinocyte cancer (KC) cases, in order to obtain an accurate estimation of incident cases of cSCC or BCC, as well as the harms and possible adverse effects related to reduced sun exposure, such as vitamin D deficiency and depression.
Another difficulty is the proper monitoring of participants, with sufficient time to fully identify incident cases, which in the case of BCC could be longer than for cSCC. Population studies should be designed to assess follow‐up periods longer than 10 years to establish the effectiveness of sunscreen in preventing KC, because current studies have had short follow‐up periods. Although these short‐term outcome variables could be assumed as "proxy" in the incidence of KC, they do not allow for the generation of definitive conclusions given that they provide insufficient evidence. In addition, it would be worthwhile to evaluate the effect of multiple barrier measures combined with the use of sunscreen. It is important to remark that the design and conduct of these interventions need to be delivered within a suitable behavioural change framework. Such complex interventions require understanding the lessons learned from existing educational campaigns and behavioural motivation studies in these areas.
With regard to other protective measures (such as wearing sun‐protective clothing, glasses, or hats, or seeking the shade when outdoors), clinical studies need to be developed to evaluate their effectiveness and safety. These could be carried out in combination with the use of sunscreens and also in special groups, such as children or those with predominant skin phototypes I to III who have a higher risk of developing skin cancers.
The design of future studies in sun protection might be improved with the following suggestions.
Evaluating different sun protection factors and different application regimens in order to establish what is the best sunscreen regimen to avoid onset of KC.
Combining different sun protection strategies compared with sunscreen use in order to assess their effectiveness and safety.
Comparing other sun protection strategies (such as wearing sun‐protective clothing, glasses, or hats, and seeking the shade when outdoors) versus sunscreen in order to assess whether there are differences between these interventions in terms of effectiveness and safety.
Running campaigns to prevent skin cancer in different populations, especially those including the predominant skin phototypes I to III who have a higher risk of developing it.
Adding adverse events as an important endpoint in the assessment of all of these preventive strategies.
What's new
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | External source of support amended with more detail |
Acknowledgements
We are grateful to Hywel Williams, Finola Delamere, Elizabeth Doney, Helen Scott, and Laura Prescott from the Cochrane Skin Group for their assistance and advice in writing this review. We and the Cochrane Skin Group editorial base wish to thank Luigi Naldi, who was the Cochrane Dermatology Editor for this review; Thomas Chu, who was the statistical editor; Ching‐Chi Chi, who was the methods editor; the clinical referees, Eleni Linos and An‐Wen Chan; and the consumer referee, Jack Tweed.
Appendices
Appendix 1. Cochrane Skin Group Specialised Regiser (CRS)
(basal cell carcinoma or squamous cell carcinoma or keratinocyte carcinoma or bowen* or actinic keratos*) and (sunblock* or sun tan lotion* or suntan lotion* or sun screen* or sunburn cream* or sun cream* or block out or sunscreen* or hat or hats or sunglasses or protective cloth* or protective garment* or umbrella* or parasol* or sunshade* or canop* or tree or trees or shade or shadow)
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 "non melanoma skin cancer":ti,ab #2 nmsc:ti,ab #3 ((keratinocyte next cancer*) or (basal next keratinocyte*) or "keratinocyte carcinoma"):ti,ab #4 MeSH descriptor: [Skin Neoplasms] this term only #5 MeSH descriptor: [Acanthoma] this term only #6 MeSH descriptor: [Carcinoma, Basal Cell] this term only #7 (basal next cell next carcinoma*):ti,ab #8 bcc:ti,ab #9 (basal next cell next cancer*):ti,ab #10 ((jacob* or rodent) next ulcer*):ti,ab #11 basolioma*:ti,ab #12 {or #1‐#11} #13 ("squamous cell" next (carcinoma* or epithelioma*)):ti,ab #14 epidermoid next carcinoma*:ti,ab #15 scc:ti,ab #16 MeSH descriptor: [Carcinoma, Squamous Cell] this term only #17 {or #13‐#16} #18 (skin or epiderm* or cutaneous):ti,ab #19 MeSH descriptor: [Skin] this term only #20 MeSH descriptor: [Skin Neoplasms] this term only #21 {or #18‐#20} #22 #17 and #21 #23 ((senile or solar or actinic) next keratos*):ti,ab #24 MeSH descriptor: [Keratosis, Actinic] this term only #25 MeSH descriptor: [Bowen's Disease] this term only #26 Bowen* next disease:ti,ab #27 "bowenoid papulosis":ti,ab #28 "morbus bowen":ti,ab #29 {or #23‐#28} #30 #12 or #22 or #29 #31 MeSH descriptor: [Sunscreening Agents] this term only #32 MeSH descriptor: [Eye Protective Devices] explode all trees #33 MeSH descriptor: [Protective Clothing] this term only #34 (sunblock* or (sun next tan next lotion*) or (suntan next lotion*) or (sun next screen*) or (sunburn next cream*) or (sun next cream*) or "block out" or sunscreen*):ti,ab #35 (sunglasses or (sun next glasses)):ti,ab #36 shadow:ti,ab #37 shade:ti,ab #38 (hat or hats):ti,ab #39 (umbrella* or parasol* or sunshade* or canopy or canopies or tree or trees):ti,ab #40 (sun and protective and (cloth* or garment*)):ti,ab #41 (photo protective and (garment* or cloth*)):ti,ab #42 {or #31‐#41} #43 #30 and #42
Appendix 3. MEDLINE (Ovid) search strategy
1. nmsc.ti,ab. 2. non melanoma skin cancer$.ti,ab. 3. keratinocyte cancer$.ti,ab. 4. basal keratinocyte$.ti,ab. 5. skin neoplasms/ or acanthoma/ 6. carcinoma, basal cell/ or neoplasms, basal cell/ 7. basal cell carcinoma$.ti,ab. 8. bcc.ti,ab. 9. basal cell cancer$.ti,ab. 10. rodent ulcer$.ti,ab. 11. Jacob$ ulcer$.ti,ab. 12. basalioma$.ti,ab. 13. or/6‐12 14. carcinoma, squamous cell/ or neoplasms, squamous cell/ 15. squamous cell carcinoma$.ti,ab. 16. epidermoid carcinoma$.ti,ab. 17. squamous cell epithelioma$.ti,ab. 18. scc.ti,ab. 19. or/14‐18 20. Skin/ 21. (skin or epiderm$ or cutaneous).ti,ab. 22. Skin Neoplasms/ 23. 20 or 21 or 22 24. 19 and 23 25. Keratosis, Actinic/ 26. senile keratos?s.ti 27. solar keratos?s.ti,ab. 28. (actinic adj3 keratos?s).ti,ab. 29. or/25‐28 30. Bowen's Disease/ 31. Bowen$ disease.ti,ab. 32. bowenoid papulosis.ti,ab. 33. morbus bowen.ti,ab. 34. 30 or 31 or 32 or 33 35. 1 or 2 or 3 or 4 or 5 or 13 or 24 or 29 or 34 36. Sunscreening Agents/ 37. (sunblock$ or sun tan lotion$ or suntan lotion$ or sun screen$ or sunburn cream$ or sun cream$ or block out or sunscreen$).ti,ab. 38. Eye Protective Devices/ 39. (sunglasses or sun glasses).ti,ab. 40. Protective Clothing/ 41. (sun protective and (cloth$ or garment$)).ti,ab. 42. (photo protective and (garment$ or cloth$)).ti,ab. 43. hat$1.ti,ab. 44. (umbrella$ or parasol$ or sunshade$ or canop$3 or tree$1).ti,ab. 45. shade.ti,ab. 46. shadow.ti,ab. 47. or/36‐46 48. randomised controlled trial.pt. 49. controlled clinical trial.pt. 50. randomized.ab. 51. placebo.ab. 52. drug therapy.fs. 53. randomly.ab. 54. trial.ab. 55. groups.ab. 56. or/48‐55 57. exp animals/ not humans.sh. 58. 56 not 57 59. 35 and 47 and 58
[Lines 48‐58: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 4. Embase (Ovid) search strategy
1. nmsc.ti,ab. 2. non melanoma skin cancer$.ti,ab. 3. keratinocyte cancer$.ti,ab. 4. basal keratinocyte$.ti,ab. 5. keratinocyte carcinoma/ 6. acanthoma/ 7. skin tumor/ 8. basal cell carcinoma$.ti,ab. 9. basal cell carcinoma/ 10. bcc.ti,ab. 11. basal cell cancer$.ti,ab. 12. rodent ulcer$.ti,ab. 13. Jacob$ ulcer$.ti,ab. 14. basalioma$.ti,ab. 15. or/1‐14 16. squamous cell carcinoma$.ti,ab. 17. squamous cell carcinoma/ 18. epidermoid carcinoma$.ti,ab. 19. squamous cell epithelioma$.ti,ab. 20. scc.ti,ab. 21. or/16‐20 22. Skin/ 23. (skin or epiderm$ or cutaneous).ti,ab. 24. skin tumor/ 25. or/22‐24 26. 21 and 25 27. senile keratos?s.ti,ab. 28. actinic keratosis/ 29. solar keratos?s.ti,ab. 30. (actinic adj3 keratos?s).ti,ab. 31. Bowen$ disease.ti,ab. 32. Bowen disease/ 33. bowenoid papulosis.ti,ab. 34. morbus bowen.ti,ab. 35. or/27‐34 36. 15 or 26 or 35 37. (sunblock$ or sun tan lotion$ or suntan lotion$ or sun screen$ or sunburn cream$ or sun cream$ or block out or sunscreen$).ti,ab. 38. (sunglasses or sun glasses).ti,ab. 39. (sun protective and (cloth$ or garment$)).ti,ab. 40. (photo protective and (garment$ or cloth$)).ti,ab. 41. hat$1.ti,ab. 42. (umbrella$ or parasol$ or sunshade$ or canop$3 or tree$1).ti,ab. 43. shade.ti,ab. 44. shadow.ti,ab. 45. skin protection/ 46. sunscreen/ or skin protective agent/ 47. sunglasses/ 48. protective clothing/ 49. sunlight protection/ 50. or/37‐49 51. crossover procedure.sh. 52. double‐blind procedure.sh. 53. single‐blind procedure.sh. 54. (crossover$ or cross over$).tw. 55. placebo$.tw. 56. (doubl$ adj blind$).tw. 57. allocat$.tw. 58. trial.ti. 59. randomised controlled trial.sh. 60. random$.tw. 61. or/51‐60 62. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 63. human/ or normal human/ 64. 62 and 63 65. 62 not 64 66. 61 not 65 67. 36 and 50 and 66
Appendix 5. LILACS search strategy
("basal cell carcinoma" or "carcinoma basocelular" or "epitelioma basocelular" or "squamous cell carcinoma" or "epitelioma espinocelular") and (sunblock$ or sun tan lotion$ or suntan lotion$ or sun screen$ or sunburn cream$ or sun cream$ or block out or sunscreen$ or filtro solar or hat or hats or sunglasses or protective cloth$)
In LILACS we searched using the Controlled clinical trials topic‐specific query filter.
Data and analyses
Comparison 1. Daily application of sunscreen versus discretionary use (including beta‐carotene use).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 BCC confirmed clinically or histopathologically at any follow‐up | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 cSCC confirmed clinically or histopathologically at any follow‐up | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Actinic or solar keratoses at any follow‐up | 1 | Risk Ratio (Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Green 1999.
| Methods | Design: 2x2 factorial design Country: Australia Multisite: no International: no Treatment duration: 4.5 years Follow‐up: 4.5 years Random unit: participants Analysis unit: participants, tumours |
|
| Participants |
Inclusion criteria Residents of Nambour (South East Queensland), aged between 20 and 69 when they took part in a skin cancer survey in 1986. To be eligible for the current study, the original survey participants had to participate in a second survey in 1992, undergo a complete skin examination by a dermatologist with removal of all diagnosed skin cancers, and give written consent to take part in this randomised trial until 1996. Exclusion criteria Participants who were taking vitamin supplements containing beta‐carotene and those who reported that they were already applying sunscreen on a strict daily basis were excluded. Participant groups 1621 participants were randomised to 1 of 4 groups (Green 1994: the original protocol reported 1626 participants): Basal cell carcinoma, cutaneous squamous cell carcinoma, adverse events
Actinic keratosis
Frequency of sunscreen used
Demographic characteristics
|
|
| Interventions | 4 groups:
*Daily sunscreen: self‐application of a layer to all exposed sites on the head, neck, arms, and hands every morning (reapplication was advised after heavy sweating, bathing, or long sun exposure) |
|
| Outcomes |
Primary outcomes
Secondary outcomes
Assessment of participants' compliance with preventive strategies at the end of the trial (Neale 2002) through a questionnaire: weight and frequency of sunscreen used |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A customized randomization computer program was used to assign all attending the baseline study clinics to one of four treatment groups." (Page 516 ‐ Green 1994) |
| Allocation concealment (selection bias) | Low risk | Quote: "The treatment code was known only to the investigator who generated it and the two people who packaged the tablets for distribution. None of these people had contact with participants." (Page 724 ‐ Green 1999) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The treatment code was known only to the investigator who generated it and the two people who packaged the tablets for distribution. None of these people had contact with participants." (Page 724 ‐ Green 1999) |
| Blinding of outcome assessment: Histologically diagnosed basal cell carcinoma | Low risk | Quote: "At follow‐up clinics in 1994 and 1996, [a] collaborating dermatologist, unaware of treatment allocation, re‐examined active participants... All skin cancers clinically diagnosed during these follow‐up surveys were examined histologically by a single dermatopathologist." (Page 724 ‐ Green 1999) |
| Blinding of outcome assessment: Histologically diagnosed cutaneous squamous cell carcinoma | Low risk | Quote: "At follow‐up clinics in 1994 and 1996, [a] collaborating dermatologist, unaware of treatment allocation, re‐examined active participants... All skin cancers clinically diagnosed during these follow‐up surveys were examined histologically by a single dermatopathologist." (Page 724 ‐ Green 1999) |
| Blinding of outcome assessment: Adverse events | Low risk | Quote: "At follow‐up clinics in 1994 and 1996, [a] collaborating dermatologist, unaware of treatment allocation, re‐examined active participants." (Page 724 ‐ Green 1999) |
| Blinding of outcome assessment: Actinic or solar keratoses | Low risk | Quote: "Complete skin examinations were carried out in February 1992, August 1994 and August 1996 by dermatologists involved in the study survey but unaware of treatment allocation." (Page 452 ‐ Darlington 2003) |
| Blinding of outcome assessment: Participant's compliance with preventive strategies | Low risk | Quote: "Compliance is assessed on a 3‐monthly basis when supplies of sunscreen and tablets are replenished. Compliance with the daily sunscreen regimen is measured by comparing the weight of sunscreen used to an empirical standard usage rate derived from average consumption of the sunscreen when used by a group of non participants according to the study protocol. Sunscreen used by people in the control group is monitored by responses to a standard questionnaire delivered annually to participants to obtain information about sun exposure habits including frequency of use of sunscreen in the previous 12 months." (Pages 516 to 517 ‐ Green 1994) Quote: "To estimate compliance with the sunscreen protocol, participants completed a questionnaire in the third and fifth years of the trial that asked about average frequency of sunscreen use in a normal week, and about outdoor behavior. In addition, the measured weights of all returned sunscreen bottles used by those in the daily sunscreen group were recorded every 3 months." (Page 724 ‐ Green 1999) |
| Incomplete outcome data (attrition bias) Basal cell carcinoma | Low risk | Quote: "...at the end of the study in year 5, 238 (15%) participants had withdrawn without a complete skin examination by a dermatologist in the follow‐up period." (Page 725 ‐ Green 1999) |
| Incomplete outcome data (attrition bias) Squamous cell carcinoma | Low risk | Quote: "...at the end of the study in year 5, 238 (15%) participants had withdrawn without a complete skin examination by a dermatologist in the follow‐up period." (Page 725 ‐ Green 1999) |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Quote: "...at the end of the study in year 5, 238 (15%) participants had withdrawn without a complete skin examination by a dermatologist in the follow‐up period." (Page 725 ‐ Green 1999) |
| Incomplete outcome data (attrition bias) Actinic keratoses | High risk |
|
| Selective reporting (reporting bias) | Unclear risk | Neale 2002 reported the association between possible predictors of sunscreen use and frequency of sunscreen application, including information on variables such as time outdoors on weekdays in summer and burns during the trial, but this information was not reported in a full way in other references. |
| Other bias | Unclear risk | The impact of multiple posthoc analysis (repeated significance testing) was unclear. The report of the number of participants randomised (1626 versus 1621) was unclear. The impact of differences in sample size estimations and final sample obtained (different estimations in Green 1994 and Green 1999) was unclear. The impact of clinical versus histologic diagnosis of skin cancer was unclear. Quote: "67% of all interim skin cancers were diagnosed histologically, and 33% were clinically diagnosed." (Page 725 ‐ Green 1999) The impact of missing data was unclear. Quote: "Analyses have been based on the 1383 trial participants (85%) who had outcome data based on at least one complete skin examination by a dermatologist in 1994 or 1996." (Page 725 ‐ Green 1999) The assessment of interaction between the effects of the different interventions (sunscreen and beta‐carotene) was unclear. |
SD = standard deviation. SPF = sun protection factor.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Autier 1999 | This study assessed if the sun protection factor (SPF) had an influence on sun exposure duration. |
| Bauer 2005 | This study focused on the prevention of melanocytic nevi by means of an educational strategy plus free provision of sunscreen. |
| Bauer 2014 | This study assessed the acceptance and usability of sunscreens during outdoor work. |
| Buller 2011 | The study assessed the effectiveness of a programme at school for reducing sun exposure. |
| Carrera 2013 | The study evaluated the effectiveness of a topical sunscreen in preventing the different UV effects on nevi. |
| Dobbinson 2009 | The study assessed whether students use or avoid newly shaded areas installed at schools, without assessment of the prevention of basal or squamous cell carcinomas. |
| Duffy 2013 | The reference presented a trial protocol about educational sun protection strategies. |
| Dupuy 2005 | The trial assessed the influence of sun protection factor and the information about protection (label) on sun‐exposure behaviour. |
| Giles‐Corti 2004 | The study showed the implementation of sun protection policies at school. |
| Glanz 2010 | This study showed the effects of a mailed intervention on skin cancer prevention and skin self‐examination behaviours of adults. |
| Gordon 2009 | This was an evaluation of the cost‐effectiveness of a skin cancer prevention initiative based on regular sunscreen use, using primary data from a randomised controlled trial. |
| Hirst 2012 | This study focused on the lifetime health costs and benefits of sunscreen promotion in the primary prevention of skin cancers, including melanoma. |
| Janda 2014 | This study identified current practice of sun protection and factors associated with effective use in 4 outdoor worker industries in Queensland, Australia. |
| Lagerlund 2006 | This was a cross‐sectional survey that assessed sun protection behaviours, including the use of sunglasses. |
| Manion 1997 | This was a survey about knowledge, attitudes, and actual behaviour related to sun safety. |
| Marks 1995 | The trial included participants with at least 1 solar keratosis. |
| Mayer 2007 | This trial assessed an intervention including educational strategies. |
| Schalka 2009 | This was an evaluation of the amount of 2 sunscreens applied, including the same ingredients at different concentrations. |
| Thompson 1993 | The trial included participants with at least 1 to 30 solar keratoses at the beginning. |
| Vuong 2014 | This study evaluated the feasibility and acceptability of an opportunistic skin cancer prevention intervention in general practice. |
| Wolf 2003 | The study focused on calculating human in vivo immune protection factors of 2 sunscreen preparations in a model of ultraviolet‐induced local suppression of the induction of contact hypersensitivity to 2,4‐dinitrochlorobenzene. |
SPF = sun protection factor. UV = ultraviolet.
Differences between protocol and review
-
Due to scarcity and nature of data (one single study was identified and included in our review), we were not able to implement the following methods:
sensitivity analysis of 'Risk of bias' results;
analysis of heterogeneity;
assessment of reporting biases;
subgroup analyses; or
trial sequence analysis and summarising the findings using random‐effects models with the DerSimonian and Laird method to carry out statistical analyses using Review Manager 5 (Review Manager 2012).
-
We were not able to identify numerical information about the following continuous outcomes:
the number of self‐reported sunburns or skin lesions;
total hours of ultraviolet radiation exposure;
total hours outdoors in peak exposure times; or
minimal erythema dosis (MED).
We expanded on our primary outcomes by adding confirmation by 'clinical' criteria of basal cell carcinoma (BCC) lesions and cutaneous squamous cell carcinoma (cSCC) lesions, because histopathological confirmation of keratinocyte cancer could be a difficulty in population‐based studies with large sample sizes, and then, clinical confirmation is more likely to be used. However, we thought that this was a source of indirectness, and we downgraded the evidence related to these outcomes in the corresponding Table 1.
We added a 'Summary of findings' table, which was not planned in the protocol. However, these are now recommended in Cochrane Reviews to assess the quality of evidence.
Contributions of authors
IAR was the contact person with the editorial base. GS, IAR, and RDM co‐ordinated contributions from the co‐authors and wrote the final draft of the review. GS, JN, ARH, CSR, JG, MO, and IAR screened papers against eligibility criteria. GS, JN, ARH, CSR, JG, MO, IAR, and RDM appraised the quality of papers. GS, IAR, JN, and ARH extracted data for the review and sought additional information about papers. IAR, RDM, and GS entered data into RevMan. GS, JN, ARH, CSR, JG, MO, IAR, and RDM analysed and interpreted data. GS, IAR, JN, and ARH worked on the methods sections. JN, MO, JG, and CSR drafted the clinical sections of the background and responded to the clinical comments of the referees. GS, IAR, JN, and ARH responded to the methodology and statistics comments of the referees. KG was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers. GS is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
Fundación Universitaria de Ciencias de la Salud, Colombia.
Centro Dermatológico Federico Lleras Acosta, Colombia.
External sources
-
Colombian Ministry of Health and Social Protection, Colombia.
Programa Nacional de Ciencia, Tecnología e Innovación en Salud, Departamento Administrativo de Ciencia Tecnología e Innovación en Salud, Colciencias, Convocatoria 563/2012, Grant number 469‐2012, Code 5007‐563‐35261
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Guillermo Sánchez: nothing to declare. John Nova: nothing to declare. Andrea Esperanza Rodriguez‐Hernandez: nothing to declare. Roger David Medina: nothing to declare. Carolina Solorzano‐Restrepo: nothing to declare. Jenny Gonzalez: nothing to declare. Miguel Olmos: nothing to declare. Kathie Godfrey: nothing to declare. Ingrid Arevalo‐Rodriguez: nothing to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Green 1999 {published data only}
- Darlington S, Williams G, Neale R, Frost C, Green A. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Archives of Dermatology 2003;139(4):451‐5. [PUBMED: 12707092] [DOI] [PubMed] [Google Scholar]
- Green A, Battistutta D, Hart V, Leslie D, Marks G, Williams G, et al. The Nambour Skin Cancer and Actinic Eye Disease Prevention Trial: design and baseline characteristics of participants. Controlled Clinical Trials 1994;15(6):512‐22. [PUBMED: 7851112] [DOI] [PubMed] [Google Scholar]
- Green A, Williams G, Neale R. Daily use of beta carotene supplements did not prevent skin cancer, but daily sunscreen use reduced the incidence of squamous cell carcinomas: Commentary. Evidence‐Based Medicine 2000;5(3):91. [DOI: 10.1136/ebm.5.3.91] [DOI] [Google Scholar]
- Green A, Williams G, Neale R, Hart V, Leslie D, Parsons P, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal‐cell and squamous‐cell carcinomas of the skin: a randomised controlled trial. Lancet 1999;354(9180):723‐9. [PUBMED: 10475183] [DOI] [PubMed] [Google Scholar]
- Hughes MC, Williams GM, Baker P, Green AC. Sunscreen and prevention of skin aging: a randomized trial. Annals of Internal Medicine 2013;158(11):781‐90. [PUBMED: 23732711] [DOI] [PubMed] [Google Scholar]
- Neale R, Williams G, Green A. Application patterns among participants randomized to daily sunscreen use in a skin cancer prevention trial. Archives of Dermatology 2002;138(10):1319‐25. [PUBMED: 12374537] [DOI] [PubMed] [Google Scholar]
- Pandeya N, Purdie DM, Green A, Williams G. Repeated occurrence of basal cell carcinoma of the skin and multifailure survival analysis: follow‐up data from the Nambour Skin Cancer Prevention Trial. American Journal of Epidemiology 2005;161(8):748‐54. [PUBMED: 15800267] [DOI] [PubMed] [Google Scholar]
- Pols JC, Williams GM, Neale RE, Clavarino A, Green AC. Long‐term increase in sunscreen use in an Australian community after a skin cancer prevention trial. Preventive Medicine 2006;42(3):171‐6. [PUBMED: 16325898] [DOI] [PubMed] [Google Scholar]
- Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiology, Biomarkers & Prevention 2006;15(12):2546‐8. [PUBMED: 17132769] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Autier 1999 {published data only}
- Autier P, Dore J, Negrier S, Lienard D, Panizzon R, Lejeune FJ, et al. Sunscreen use and duration of sun exposure: a double‐blind, randomized trial. Journal of the National Cancer Institute 1999;91(15):1304‐9. [PUBMED: 10433619] [DOI] [PubMed] [Google Scholar]
Bauer 2005 {published data only}
- Bauer J, Buttner P, Wiecker TS, Luther H, Garbe C. Interventional study in 1,232 young German children to prevent the development of melanocytic nevi failed to change sun exposure and sun protective behavior. International Journal of Cancer 2005;116(5):755‐61. [PUBMED: 15849749] [DOI] [PubMed] [Google Scholar]
Bauer 2014 {published data only}
- Bauer A, Hault K, Puschel A, Ronsch H, Knuschke P, Beissert S. Acceptance and usability of different sunscreen formulations among outdoor workers: a randomized, single‐blind, cross‐over study. Acta Dermato‐Venereologica 2014;94(2):152‐6. [PUBMED: 23995520] [DOI] [PubMed] [Google Scholar]
Buller 2011 {published data only}
- Buller DB, Reynolds KD, Ashley JL, Buller MK, Kane IL, Stabell CL, et al. Motivating public school districts to adopt sun protection policies: a randomized controlled trial. American Journal of Preventive Medicine 2011;41(3):309‐16. [PUBMED: 21855746] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrera 2013 {published data only}
- Carrera C, Puig‐Butille JA, Aguilera P, Ogbah Z, Palou J, Lecha M, et al. Impact of sunscreens on preventing UVR‐induced effects in nevi: in vivo study comparing protection using a physical barrier vs sunscreen. JAMA Dermatology 2013;149(7):803‐13. [PUBMED: 23699984] [DOI] [PubMed] [Google Scholar]
Dobbinson 2009 {published data only}
- Dobbinson SJ, White V, Wakefield MA, Jamsen KM, Livingston PM, English DR, et al. Adolescents' use of purpose built shade in secondary schools: cluster randomised controlled trial. BMJ 2009;338:b95. [PUBMED: 19223344] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffy 2013 {published data only}
- Duffy SA, Ronis DL, Waltje AH, Choi SH. Protocol of a randomized controlled trial of sun protection interventions for operating engineers. BMC Public Health 2013;13:273. [PUBMED: 23530608] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dupuy 2005 {published data only}
- Dupuy A, Dunant A, Grob JJ, Reseau d'Epidemiologie en Dermatologie. Randomized controlled trial testing the impact of high‐protection sunscreens on sun‐exposure behavior. Archives of Dermatology 2005;141(8):950‐6. [PUBMED: 16103322] [DOI] [PubMed] [Google Scholar]
Giles‐Corti 2004 {published data only}
- Giles‐Corti B, English DR, Costa C, Milne E, Cross D, Johnston R. Creating SunSmart schools. Health Education Research 2004;19(1):98‐109. [PUBMED: 15020549] [DOI] [PubMed] [Google Scholar]
Glanz 2010 {published data only}
- Glanz K, Schoenfeld ER, Steffen A. A randomized trial of tailored skin cancer prevention messages for adults: Project SCAPE. American Journal of Public Health 2010;100(4):735‐41. [PUBMED: 20167900] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gordon 2009 {published data only}
- Gordon LG, Scuffham PA, Pols JC, McBride P, Williams GM, Green AC. Regular sunscreen use is a cost‐effective approach to skin cancer prevention in subtropical settings. Journal of Investigative Dermatology 2009;129(12):2766‐71. [PUBMED: 19536149] [DOI] [PubMed] [Google Scholar]
Hirst 2012 {published data only}
- Hirst NG, Gordon LG, Scuffham PA, Green AC. Lifetime cost‐effectiveness of skin cancer prevention through promotion of daily sunscreen use. Value in Health 2012;15(2):261‐8. [PUBMED: 22433757] [DOI] [PubMed] [Google Scholar]
Janda 2014 {published data only}
- Janda M, Stoneham M, Youl P, Crane P, Sendall MC, Tenkate T, et al. What encourages sun protection among outdoor workers from four industries?. Journal of Occupational Health 2014;56(1):62‐72. [PUBMED: 24270927] [DOI] [PubMed] [Google Scholar]
Lagerlund 2006 {published data only}
- Lagerlund M, Dixon HG, Simpson JA, Spittal M, Taylor HR, Dobbinson SJ. Observed use of sunglasses in public outdoor settings around Melbourne, Australia: 1993 to 2002. Preventive Medicine 2006;42(4):291‐6. [PUBMED: 16487999] [DOI] [PubMed] [Google Scholar]
Manion 1997 {published data only}
- Manion IG, Cloutier PF, Klassen TP. Ultraviolet radiation and safety behaviours at an outdoor community event. Canadian Journal of Public Health. Revue Canadienne de Sante Publique 1997;88(2):119‐22. [PUBMED: 9170691] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marks 1995 {published data only}
- Marks R, Foley PA, Jolley D, Knight KR, Harrison J, Thompson SC. The effect of regular sunscreen use on vitamin D levels in an Australian population. Results of a randomized controlled trial. Archives of Dermatology 1995;131(4):415‐21. [PUBMED: 7726582] [PubMed] [Google Scholar]
Mayer 2007 {published data only}
- Mayer JA, Slymen DJ, Clapp EJ, Pichon LC, Eckhardt L, Eichenfield LF, et al. Promoting sun safety among US Postal Service letter carriers: impact of a 2‐year intervention. American Journal of Public Health 2007;97(3):559‐65. [PUBMED: 17267715] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schalka 2009 {published data only}
- Schalka S, dos Reis VM, Cuce LC. The influence of the amount of sunscreen applied and its sun protection factor (SPF): evaluation of two sunscreens including the same ingredients at different concentrations. Photodermatology, Photoimmunology & Photomedicine 2009;25(4):175‐80. [PUBMED: 19614894] [DOI] [PubMed] [Google Scholar]
Thompson 1993 {published data only}
- Cockburn J, Thompson SC, Marks R, Jolley D, Schofield P, Hill D. Behavioural dynamics of a clinical trial of sunscreens for reducing solar keratoses in Victoria, Australia. Journal of Epidemiology & Community Health. 1997;51(6):716‐21. [PUBMED: 9519139] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SC, Jolley D, Marks R. Reduction of solar keratoses by regular sunscreen use. New England Journal of Medicine 1993;329(16):1147‐51. [PUBMED: 8377777] [DOI] [PubMed] [Google Scholar]
Vuong 2014 {published data only}
- Vuong K, Trevena L, Bonevski B, Armstrong BK. Feasibility of a GP delivered skin cancer prevention intervention in Australia. BMC Family Practice 2014;15:137. [PUBMED: 25070692] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolf 2003 {published data only}
- Wolf P, Hoffmann C, Quehenberger F, Grinschgl S, Kerl H. Immune protection factors of chemical sunscreens measured in the local contact hypersensitivity model in humans. Journal of Investigative Dermatology 2003;121(5):1080‐7. [PUBMED: 14708610] [DOI] [PubMed] [Google Scholar]
Additional references
Albert 2003
- Albert MR, Weinstock MA. Keratinocyte carcinoma. CA: a Cancer Journal for Clinicians 2003;53(5):292‐302. [PUBMED: 14570228] [DOI] [PubMed] [Google Scholar]
Bath‐Hextall 2007
- Bath‐Hextall Fiona J, Leonardi‐Bee J, Somchand N, Webster Angela C, Dellit J, Perkins W. Interventions for preventing non‐melanoma skin cancers in high‐risk groups. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD005414.pub2; CD005414] [DOI] [PMC free article] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [MEDLINE: ] [PubMed] [Google Scholar]
Bimczok 2007
- Bimczok R, Gers‐Barlag H, Mundt C, Klette E, Bielfeldt S, Rudolph T, et al. Influence of applied quantity of sunscreen products on the sun protection factor‐‐a multicenter study organized by the DGK Task Force Sun Protection. Skin Pharmacology & Physiology 2007;20(1):57‐64. [PUBMED: 17035723 ] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive‐‐Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burnett 2012
- Burnett ME, Hu JY, Wang SQ. Sunscreens: obtaining adequate photoprotection. Dermatologic Therapy 2012;25(3):244‐51. [PUBMED: 22913442] [DOI] [PubMed] [Google Scholar]
Ciapponi 2011
- Ciapponi A, Glujovsky D, Bardach A, García Martí S, Comande D. EROS: a new software for early stage of systematic reviews. HTAi 2011 Conference. Rio de Janeiro, Brazil, 2011. [DOI: 10.1016/j.jval.2011.08.1689] [DOI]
Cole 2001
- Cole C. Sunscreen protection in the ultraviolet A region: how to measure the effectiveness. Photodermatology, Photoimmunology & Photomedicine 2001;17(1):2‐10. [PUBMED: 11169170] [DOI] [PubMed] [Google Scholar]
Cooley 2013
- Cooley JH, Quale LM. Skin cancer preventive behavior and sun protection recommendations. Seminars in Oncology Nursing 2013;29(3):223‐6. [PUBMED: 23958220] [DOI] [PubMed] [Google Scholar]
Corona 2001
- Corona R, Dogliotti E, D'Errico M, Sera F, Iavarone I, Baliva G, et al. Risk factors for basal cell carcinoma in a Mediterranean population: role of recreational sun exposure early in life. Archives of Dermatology 2001;137(9):1162‐8. [PUBMED: 11559211] [DOI] [PubMed] [Google Scholar]
CTU 2011 [Computer program]
- Central Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Central Trial Unit, 2011. [http://ctu.dk/tsa/]
Darlington 2003
- Darlington S, Williams G, Neale R, Frost C, Green A. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Archives of Dermatology 2003;139(4):451‐5. [PUBMED: 12707092] [DOI] [PubMed] [Google Scholar]
Diaz 2013
- Diaz JH, Nesbitt LT. Sun exposure behavior and protection: recommendations for travelers. Journal of Travel Medicine 2013;20(2):108‐18. [PUBMED: 23464719] [DOI] [PubMed] [Google Scholar]
Dubas 2013
- Dubas LE, Ingraffea A. Nonmelanoma skin cancer. Facial Plastic Surgery Clinics of North America 2013;21(1):43‐53. [PUBMED: 23369588] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gasparro 1998
- Gasparro FP, Mitchnick M, Nash JF. A review of sunscreen safety and efficacy. Photochemistry & Photobiology 1998;68(3):243‐56. [PUBMED: 9747581] [PubMed] [Google Scholar]
Glass 1989
- Glass AG, Hoover RN. The emerging epidemic of melanoma and squamous cell skin cancer. JAMA 1989;262(15):2097‐100. [PUBMED: 2795783] [PubMed] [Google Scholar]
Gloster 1996
- Gloster HM Jr, Brodland DG. The epidemiology of skin cancer. Dermatologic Surgery 1996;22(3):217‐26. [PUBMED: 8599733] [DOI] [PubMed] [Google Scholar]
Glujovsky 2010
- Glujovsky D, Bardach A, García Martí S, Comande D, Ciapponi A. New Software for Early Stage of Systematic Reviews. XVIII Cochrane Colloquium. The Joint Colloquium of the Cochrane & Campbell Collaborations. Keystone Resort, Colorado, USA, 2010.
Glujovsky 2011
- Glujovsky D, Bardach A, García Martí S, Comande D, Ciapponi A. EROS: a new software for early stage of systematic reviews (Poster PRM2). Value in Health. ISPOR 3rd Latin America Conference, Mexico City, Mexico, 8‐10 September 2011 2011;14(7):A564. [Google Scholar]
Green 1994
- Green A, Battistutta D, Hart V, Leslie D, Marks G, Williams G, et al. The Nambour Skin Cancer and Actinic Eye Disease Prevention Trial: design and baseline characteristics of participants. Controlled Clinical Trials 1994;15(6):512‐22. [PUBMED: 7851112] [DOI] [PubMed] [Google Scholar]
Green 2010
- Green AC, McBride P. Squamous cell carcinoma of the skin (non‐metastatic). Clinical Evidence (Online) 2010;2010:1709. [PUBMED: 21733203] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. Journal of Clinical Epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, et al. GRADE guidelines: 5. Rating the quality of evidence‐‐publication bias. Journal of Clinical Epidemiology 2011;64(12):1277‐82. [PUBMED: 21802904] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence‐‐indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence‐‐study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] [DOI] [PubMed] [Google Scholar]
Guyatt 2011d
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Guyatt 2011e
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011f
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011g
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. Journal of Clinical Epidemiology 2011;64(4):395‐400. [PUBMED: 21194891] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011.
Holman 1984
- Holman CD, Evans PR, Lumsden GJ, Armstrong BK. The determinants of actinic skin damage: problems of confounding among environmental and constitutional variables. American Journal of Epidemiology 1984;120(3):414‐22. [PUBMED: 6475917] [DOI] [PubMed] [Google Scholar]
Jansen 2013a
- Jansen R, Osterwalder U, Wang SQ, Burnett M, Lim HW. Photoprotection: part II. Sunscreen: development, efficacy, and controversies. Journal of the American Academy of Dermatology 2013;69(6):867. [PUBMED: 24238180] [DOI] [PubMed] [Google Scholar]
Jansen 2013b
- Jansen R, Wang SQ, Burnett M, Osterwalder U, Lim HW. Photoprotection: part I. Photoprotection by naturally occurring, physical, and systemic agents. Journal of the American Academy of Dermatology 2013;69(6):853. [PUBMED: 24238179] [DOI] [PubMed] [Google Scholar]
Johnson 1992
- Johnson TM, Rowe DE, Nelson BR, Swanson NA. Squamous cell carcinoma of the skin (excluding lip and oral mucosa). Journal of the American Academy of Dermatology 1992;26(3 Pt 2):467‐84. [PUBMED: 1564155] [DOI] [PubMed] [Google Scholar]
Klostermann 2013
Kopcke 2008
- Kopcke W, Krutmann J. Protection from sunburn with beta‐Carotene‐‐a meta‐analysis. Photochemistry and photobiology 2008;84(2):284‐8. [PUBMED: 18086246] [DOI] [PubMed] [Google Scholar]
Kornek 2013
- Kornek T, Augustin M. Skin cancer prevention. Journal of the German Society of Dermatology 2013;11(4):283‐96; quiz 297‐8. [PUBMED: 23574893] [DOI] [PubMed] [Google Scholar]
Langbecker 2014
- Langbecker D, Diaz A, Chan Raymond J, Marquart L, Hevey D, Hamilton J. Educational programmes for primary prevention of skin cancer. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD011061] [DOI] [Google Scholar]
Lansbury 2010
- Lansbury L, Leonardi‐Bee J, Perkins W, Goodacre T, Tweed JA, Bath‐Hextall FJ. Interventions for non‐metastatic squamous cell carcinoma of the skin. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD007869.pub2; PUBMED: 20393962] [DOI] [PMC free article] [PubMed] [Google Scholar]
Latha 2013
- Latha MS, Martis J, Shobha V, Sham Shinde R, Bangera S, Krishnankutty B, et al. Sunscreening agents: a review. Journal of Clinical & Aesthetic Dermatology 2013;6(1):16‐26. [PUBMED: 23320122] [PMC free article] [PubMed] [Google Scholar]
Lear 1997
- Lear JT, Tan BB, Smith AG, Bowers W, Jones PW, Heagerty AH, et al. Risk factors for basal cell carcinoma in the UK: case‐control study in 806 patients. Journal of the Royal Society of Medicine 1997;90(7):371‐4. [PUBMED: 9290417] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linos 2011
- Linos E, Keiser E, Fu T, Colditz G, Chen S, Tang JY. Hat, shade, long sleeves, or sunscreen? Rethinking US sun protection messages based on their relative effectiveness. Cancer Causes & Control 2011;22(7):1067‐71. [PUBMED: 21637987] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lomas 2012
- Lomas A, Leonardi‐Bee J, Bath‐Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. British Journal of Dermatology 2012;166(5):1069‐80. [PUBMED: 22251204] [DOI] [PubMed] [Google Scholar]
Lucas 2006
- Lucas R, McMichael T, Smith W, Armstrong B. Solar ultraviolet radiation: Global burden of disease from solar ultraviolet radiation. World Health Organization, Environmental Burden of Disease Series, No. 13 2006.
Maslin 2014
- Maslin DL. Do suncreens protect us?. International Journal of Dermatology 2014;53(11):1319‐23. [PUBMED: 25208462] [DOI] [PubMed] [Google Scholar]
McQuay 1998
- McQuay HJ, Moore RA. An Evidence‐Based Resource for Pain Relief. Oxford: Oxford University Press, 1998. [Google Scholar]
Miller 2010
- Miller SJ, Alam M, Andersen J, Berg D, Bichakjian CK, Bowen G, et al. Basal cell and squamous cell skin cancers. Journal of the National Comprehensive Cancer Network 2010;8(8):836‐64. [PUBMED: 20870631] [DOI] [PubMed] [Google Scholar]
Morales‐Sánchez 2016
- Morales‐Sánchez MA, Peralta‐Pedrero ML, Jurado‐Santa Cruz F, Pomerantz H, Allen V, Barajas‐Nava LA. Interventions for preventing keratinocyte cancer in high‐risk groups not receiving immunosuppressive therapy. Cochrane Database of Systematic Reviews 2016, Issue 6. [DOI: 10.1002/14651858.CD012266] [DOI] [Google Scholar]
Motley 2003
- Motley R, Kersey P, Lawrence C, British Association of Dermatologists, British Association of Plastic Surgeons. Multiprofessional guidelines for the management of the patient with primary cutaneous squamous cell carcinoma. British Journal of Plastic Surgery 2003;56(2):85‐91. [PUBMED: 12791348] [DOI] [PubMed] [Google Scholar]
Mulliken 2012
- Mulliken JS, Russak JE, Rigel DS. The effect of sunscreen on melanoma risk. Dermatologic Clinics 2012;30(3):369‐76. [PUBMED: 22800545] [DOI] [PubMed] [Google Scholar]
Neale 2002
- Neale RE, Green AC. Measuring behavioral interventions by questionnaires and prospective diaries: an example of sunscreen use. Epidemiology 2002;13(2):224‐7. [PUBMED: 11880765] [DOI] [PubMed] [Google Scholar]
NICE 2011
- National Institute for Health and Clinical Excellence. Skin cancer prevention: information, resources and environmental changes. NICE guidelines [PH 32], January 2011. www.nice.org.uk/guidance/ph32. Manchester, UK, (accessed 23 September 2015).
Pissavini 2013
- Pissavini M, Diffey B. The likelihood of sunburn in sunscreen users is disproportionate to the SPF. Photodermatology, Photoimmunology & Photomedicine 2013;29(3):111‐5. [PUBMED: 23651270] [DOI] [PubMed] [Google Scholar]
Pogue 1997
- Pogue JM, Yusuf S. Cumulating evidence from randomized trials: utilizing sequential monitoring boundaries for cumulative meta‐analysis. Controlled Clinical Trials 1997;18(6):580‐93; discussion 661‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pogue 1998
- Pogue J, Yusuf S. Overcoming the limitations of current meta‐analysis of randomised controlled trials. Lancet 1998;351(9095):47‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Preston 1992
- Preston DS, Stern RS. Nonmelanoma cancers of the skin. New England Journal of Medicine 1992;327(23):1649‐62. [PUBMED: 1435901] [DOI] [PubMed] [Google Scholar]
Ravanat 2001
- Ravanat JL, Douki T, Cadet J. Direct and indirect effects of UV radiation on DNA and its components. Journal of Photochemistry & Photobiology. B ‐ Biology 2001;63(1‐3):88‐102. [PUBMED: 11684456] [DOI] [PubMed] [Google Scholar]
Review Manager 2012 [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, 2012.
Ricotti 2009
- Ricotti C, Bouzari N, Agadi A, Cockerell CJ. Malignant skin neoplasms. Medical Clinics of North America 2009;93(6):1241‐64. [PUBMED: 19932329] [DOI] [PubMed] [Google Scholar]
Roewert‐Huber 2007
- Roewert‐Huber J, Lange‐Asschenfeldt B, Stockfleth E, Kerl H. Epidemiology and aetiology of basal cell carcinoma. British Journal of Dermatology 2007;157(Suppl 2):47‐51. [EMBASE: 2007579513] [DOI] [PubMed] [Google Scholar]
Rubin 2005
- Rubin AI, Chen EH, Ratner D. Basal‐cell carcinoma. New England Journal of Medicine 2005;353(21):2262‐9. [DOI: 10.1056/NEJMra044151] [DOI] [PubMed] [Google Scholar]
Saki 2012
- Saki M, Toulany M, Sihver W, Zenker M, Heldt JM, Mosch B, et al. Cellular and molecular properties of (90)Y‐labeled cetuximab in combination with radiotherapy on human tumor cells in vitro. Strahlentherapie und Onkologie 2012;188(9):823‐32. [PUBMED: 22875052] [DOI] [PubMed] [Google Scholar]
Sambandan 2011
- Sambandan DR, Ratner D. Sunscreens: an overview and update. Journal of the American Academy of Dermatology 2011;64(4):748‐58. [PUBMED: 21292345] [DOI] [PubMed] [Google Scholar]
Schalka 2011
- Schalka S, Reis VM. Sun protection factor: meaning and controversies. Anais Brasileiros de Dermatologia 2011;86(3):507‐15. [PUBMED: 21738968] [DOI] [PubMed] [Google Scholar]
Stern 2010
- Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence‐based model. Archives of Dermatology 2010;146(3):279‐82. [PUBMED: 20231498] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sánchez 2011
- Sánchez G, Nova J, Hoz F, Castañeda C. Incidence of skin cancer in Colombia from 2003‐2007 [Incidencia de cáncer de piel en Colombia, años 2003‐2007]. Piel 2011;26(4):171‐7. [DOI: 10.1016/j.piel.2010.10.028] [DOI] [Google Scholar]
Sánchez 2012
- Sánchez G, Nova J, Hoz F. Risk factors for basal cell carcinoma: a study from the national dermatology center of Colombia [Factores de riesgo de carcinoma basocelular. Un estudio del Centro Nacional de Dermatologia de Colombia]. Actas Dermo‐Sifiliográficas 2012;103(4):294‐300. [PUBMED: 22078143] [DOI] [PubMed] [Google Scholar]
Sánchez 2013
- Sánchez G, Nova J. Risk factors for squamous cell carcinoma, a study by the national dermatology centre of Colombia. Actas Dermo‐Sifiliográficas 2013;104(8):672‐8. [PUBMED: 23968667] [DOI] [PubMed] [Google Scholar]
Sánchez 2014
- Sánchez G, Nova J, Rodriguez‐Hernandez AE, Solorzano‐Restrepo C, Gonzalez J, Olmos M, et al. Sun protection for preventing basal cell and squamous cell skin cancers. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD011161] [DOI] [PMC free article] [PubMed] [Google Scholar]
Telfer 2008
- Telfer NR, Colver GB, Morton CA. Guidelines for the management of basal cell carcinoma. British Journal of Dermatology 2008;159(1):35‐48. [PUBMED: 18593385] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis‐‐a simulation study. PLoS One [Electronic Resource] 2011;6(10):e25491. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Pols 2006
- Pols JC, Williams GM, Pandeya N, Logan V, Green AC. Prolonged prevention of squamous cell carcinoma of the skin by regular sunscreen use. Cancer Epidemiology, Biomarkers & Prevention 2006;15(12):2546‐8. [PUBMED: 17132769] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zanetti 1996
- Zanetti R, Rosso S, Martinez C, Navarro C, Schraub S, Sancho‐Garnier H, et al. The multicentre south European study 'Helios' I: Skin characteristics and sunburns in basal cell and squamous cell carcinomas of the skin. British Journal of Cancer 1996;73(11):1440‐6. [EMBASE: 1996180527] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zelen 1988
- Zelen M. Are primary cancer prevention trials feasible?. Journal of the National Cancer Institute 1988;80(18):1442‐4. [PUBMED: 3054126] [DOI] [PubMed] [Google Scholar]


