Abstract
Background
Pre‐cancerous lesions of cervix (cervical intraepithelial neoplasia (CIN)) are usually treated with excisional or ablative procedures. In the UK, the National Health Service (NHS) cervical screening guidelines suggest that over 80% of treatments should be performed in an outpatient setting (colposcopy clinics). Furthermore, these guidelines suggest that analgesia should always be given prior to laser or excisional treatments. Currently various pain relief strategies are employed that may reduce pain during these procedures.
Objectives
To assess whether the administration of pain relief (analgesia) reduces pain during colposcopy treatment and in the postoperative period.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 2), MEDLINE (1950 to March week 3, 2016) and Embase (1980 to week 12, 2016) for studies of any design relating to analgesia for colposcopic management. We also searched registers of clinical trials, abstracts of scientific meetings, reference lists of included studies and contacted experts in the field.
Selection criteria
Randomised controlled trials (RCTs) that compared all types of pain relief before, during or after outpatient treatment to the cervix, in women with CIN undergoing loop excision, laser ablation, laser excision or cryosurgery in an outpatient colposcopy clinic setting.
Data collection and analysis
We independently assessed study eligibility, extracted data and assessed risk of bias. We entered data into Review Manager 5 and double checked it for accuracy. Where possible, we expressed results as mean pain score and standard error of the mean with 95% confidence intervals (CI) and synthesised data in a meta‐analysis.
Main results
We included 19 RCTs (1720 women) of varying methodological quality in the review. These trials compared a variety of interventions aimed at reducing pain in women who underwent treatment for CIN, including cervical injection with lignocaine alone, lignocaine with adrenaline, buffered lignocaine with adrenaline, prilocaine with felypressin, oral analgesics (non‐steroidal anti‐inflammatory drugs (NSAIDs)), inhalation analgesia (gas mixture of isoflurane and desflurane), lignocaine spray, cocaine spray, local application of benzocaine gel, lignocaine‐prilocaine cream (EMLA cream) and transcutaneous electrical nerve stimulation (TENS).
Most comparisons were restricted to single trial analyses and were under‐powered to detect differences in pain scores between treatments that may or may not have been present. There was no difference in pain relief between women who received local anaesthetic infiltration (lignocaine 2%; administered as a paracervical or direct cervical injection) and a saline placebo (mean difference (MD) ‐13.74; 95% CI ‐34.32 to 6.83; 2 trials; 130 women; low quality evidence). However, when local anaesthetic was combined with a vasoconstrictor agent (one trial used lignocaine plus adrenaline while the second trial used prilocaine plus felypressin), there was less pain (on visual analogue scale (VAS)) compared with no treatment (MD ‐23.73; 95% CI ‐37.53 to ‐9.93; 2 trials; 95 women; low quality evidence). Comparing two preparations of local anaesthetic combined with vasoconstrictor, prilocaine plus felypressin did not differ from lignocaine plus adrenaline for its effect on pain control (MD ‐0.05; 95% CI ‐0.26 to 0.16; 1 trial; 200 women). Although the mean (± standard deviation (SD)) observed blood loss score was less with lignocaine plus adrenaline (1.33 ± 1.05) compared with prilocaine plus felypressin (1.74 ± 0.98), the difference was not clinically as the overall scores in both groups were low (MD 0.41; 95% CI 0.13 to 0.69; 1 trial; 200 women). Inhalation of gas mixture (isoflurane and desflurane) in addition to standard cervical injection with prilocaine plus felypressin resulted in less pain during the LLETZ (loop excision of the transformation zone) procedure (MD ‐7.20; 95% CI ‐12.45 to ‐1.95; 1 trial; 389 women). Lignocaine plus ornipressin resulted in less measured blood loss (MD ‐8.75 ml; 95% CI ‐10.43 to ‐7.07; 1 trial; 100 women) and a shorter duration of treatment (MD ‐7.72 minutes; 95% CI ‐8.49 to ‐6.95; 1 trial; 100 women) than cervical infiltration with lignocaine alone. Buffered solution (sodium bicarbonate buffer mixed with lignocaine plus adrenaline) was not superior to non‐buffered solution of lignocaine plus adrenaline in relieving pain during the procedure (MD ‐8.00; 95% CI ‐17.57 to 1.57; 1 trial; 52 women).
One meta‐analysis found no difference in pain using VAS between women who received oral analgesic and women who received placebo (MD ‐3.51; 95% CI ‐10.03 to 3.01; 2 trials; 129 women; low quality evidence).
Cocaine spray was associated with less pain (MD ‐28.00; 95% CI ‐37.86 to ‐18.14; 1 trial; 50 women) and blood loss (MD 0.04; 95% CI 0 to 0.70; 1 trial; 50 women) than placebo.
None of the trials reported serious adverse events and majority of trials were at moderate or high risk of bias (13 trials).
Authors' conclusions
Based on two small trials, there was no difference in pain relief in women receiving oral analgesics compared with placebo or no treatment (MD ‐3.51; 95% CI ‐10.03 to 3.01; 129 women). We consider this evidence to be of a low to moderate quality. In routine clinical practice, intracervical injection of local anaesthetic with a vasoconstrictor (lignocaine plus adrenaline or prilocaine plus felypressin) appears to be the optimum analgesia for treatment. However, further high quality, adequately powered trials should be undertaken in order to provide the data necessary to estimate the efficacy of oral analgesics, the optimal route of administration and dose of local anaesthetics.
Plain language summary
Pain relief for women with pre‐cancerous changes of the cervix (cervical intraepithelial neoplasia (CIN)) undergoing outpatient treatment
What is the issue? Treatment for CIN is usually undertaken in an outpatient colposcopy clinic to remove the pre‐cancerous cells from the cervix (lower part of the womb). It commonly involves lifting the cells off the cervix with electrically heated wire (diathermy) or laser, or destroying the abnormal cells with freezing methods (cryotherapy). This is potentially a painful procedure.
The aim of the review The purpose of this review is to determine which, if any, pain relief should be used during cervical colposcopy treatment.
What evidence did we find?
We identified 19 clinical trials up to March 2016 and these reported different forms of pain relief before, during and after colposcopy. Evidence from two small trials showed that women having a colposcopy treatment had less pain and blood loss if the cervix was injected with a combination of a local anaesthetic medicine and a medicine that causes blood vessels to constrict (narrow), compared with placebo (a pretend treatment). Although taking oral pain‐relieving medicines (e.g. ibuprofen) before treatment on the cervix in the colposcopy clinic is recommended by most guidelines, evidence from two small trials did not show that this practice reduced pain during the procedure.
Quality of the evidence Most of the evidence was of a low to moderate quality and further research may change these findings.
Additionally, we were unable to obtain evidence with regards to how much of the local anaesthetic or method of administering local anaesthetic into the cervix.
What are the conclusions? There is a need for high quality trials with sufficient numbers of women in order to provide the data necessary to determine which pain relief should be used during outpatient colposcopy treatment.
Summary of findings
Background
Description of the condition
Cervical cancer is the second most common cancer among women up to 65 years of age and is the most frequent cause of death from gynaecological cancers worldwide. A woman's risk of developing cervical cancer by the age of 65 years ranges from 0.69% in high income countries to 1.38% in low to moderate income countries (GLOBOCAN 2008). In Europe, about 60% of women with cervical cancer are alive five years after diagnosis (EUROCARE 2003). Cervical screening has all the characteristics of a good screening programme. There are effective screening tests, such as the traditional cytological approach (Pap smear) for diagnosing pre‐invasive and early invasive disease, or new methodologies, such as human papillomavirus (HPV) testing, which try to improve sensitivity and specificity. In addition, there are effective surgical treatments for pre‐invasive and early invasive disease that dramatically alter prognosis. As cervical screening is relatively inexpensive, non‐invasive and treatment of pre‐invasive disease requires only simple surgical techniques, screening is cost‐effective and has been clearly demonstrated to reduce mortality in countries with well‐organised screening programmes (Peto 2004).
One previous Cochrane review examined the effectiveness of different modalities of treatment for pre‐invasive disease (Martin‐Hirsch 2010). In the review, each modality of treatment was assessed for its ability to eradicate disease and associated morbidity. Current treatment for cervical intraepithelial neoplasia (CIN) is by local ablative therapy or by excisional methods, depending on the nature and extent of the disease. There is an international consensus that the majority of these procedures can be performed within the colposcopy clinic in an outpatient setting. In the UK, the National Health Service (NHS) cervical screening guidelines suggest that over 80% of treatments should be performed in a clinic setting (NHSCSP 2004; NHSCSP 2010). Furthermore, these guidelines also suggest that analgesia should always be given prior to laser or excisional treatments.
Description of the intervention
Therapies that are available to treat pre‐malignant lesions of the cervix in outpatient settings include loop diathermy excision, laser ablation or excision, and cryotherapy (Martin‐Hirsch 2010). Studies have reported variable outcomes with different types of pain relief for these procedures. The choice of pain relief in these studies varies from no analgesia to intracervical infiltration with anaesthetic agent (e.g. lignocaine ‐ buffered and non‐buffered ‐ or prilocaine) with or without vasopressor agents (e.g. adrenaline or felypressin) (Lee 1986; Johnson 1989; Kizer 2014). Other methods studied are oral therapy with non‐steroidal anti‐inflammatory drugs (NSAIDs) (Frega 1994), local spray with cocaine (Mikhail 1988) or lignocaine (xylocaine) spray (Vanichtantikul 2013), topical benzocaine gel (Lipscomb 1995), inhalation of gas mixture of isoflurane and desflurane (Cruickshank 2005), local anaesthetic cream (EMLA cream) (Sarkar 1993), and transcutaneous electrical nerve stimulation (TENS) (Crompton 1992).
How the intervention might work
The possible mechanisms proposed in the literature to explain pain during cervical laser vaporisation includes pain mediated through peripheral pain fibres in the cervix, stimulated by heat energy, with or without pain caused by increased uterine contractions, probably because of the release of prostaglandins. The interventions may work by blocking the pain pathways. The nerve supply to the cervix is unclear, but the richest supply appears to be at the level of internal os. The ectocervix appears to be relatively insensitive to extremes of temperature with few specialised nerve fibres (Jordan 1976). Pain stimuli from the cervix and vagina are conducted by visceral afferent fibres to the S2 to S4 spinal ganglia via the pudendal and pelvic splanchnic nerves, along with parasympathetic fibres (Moore 2006).
Why it is important to do this review
There now appears to be a consensus that analgesia should be administered before treatment to the cervix. Currently, there is no systematic review or meta‐analysis evaluating whether administering analgesia reduces the pain experienced by women undergoing outpatient treatment. Most guidelines are also not explicit on the nature of optimum analgesia for intra‐ and postoperative pain relief. Analgesia is commonly administered intra‐ or para‐cervically using fine dental needles. Other routes of administering analgesics evaluated are TENS, peri‐operative NSAIDs and inhalation analgesia.
Objectives
To assess whether the administration of pain relief (analgesia) reduces pain during colposcopy treatment and in the postoperative period.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Women with CIN undergoing loop excision, laser ablation, laser excision or cryosurgery treatment of the cervix in an outpatient colposcopy clinic setting.
Types of interventions
All types of pain relief before, during or after outpatient treatment to the cervix, compared with no pain relief or another type of pain relief. We excluded studies that included treatment performed under general anaesthetic.
Types of outcome measures
Primary outcomes
Presence or absence of pain, as a dichotomous outcome, or the degree of pain, measured by visual analogue scale (VAS) or categorical scales.
Secondary outcomes
Speed of procedure (in minutes).
Blood loss (either in millilitres (ml) or categorical scale as none, mild or minimal, heavy, troublesome or as dichotomous data).
Any moderate or severe adverse effects (dizziness, fainting, shaking, delayed discharge, etc.).
Search methods for identification of studies
We searched for papers in all languages and undertook translations if necessary.
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2016, Issue 2), MEDLINE (1950 to March week 3, 2016), Embase (1980 to week 12, 2016) for studies of any design relating to analgesia for colposcopic management. The electronic literature search strategies for CENTRAL, MEDLINE and EMBASE are summarised in Appendix 1, Appendix 2, and Appendix 3, respectively.
We identified all relevant articles on PubMed and used the 'related articles' feature to search for newly published articles.
Searching other resources
Registries of randomised trials
We searched the following registries for ongoing trials: Metaregister (www.controlled-trials.com), Physicians Data Query (www.ncbi.nlm.nih.gov/), www.clinicaltrials.gov, and www.cancer.gov/clinicaltrials.
We used ZETOC to search conference proceedings and abstracts (zetoc.mimas.ac.uk).
Handsearching
We handsearched the citation lists of included studies, key textbooks, previous systematic reviews and reports of conferences in the following sources:
Gynecologic Oncology (Annual Meeting of the American Society of Gynecologic Oncologist);
International Journal of Gynecological Cancer (Annual Meeting of the International Gynecologic Cancer Society);
British Society for Colposcopy and Cervical Cytology (BSCCP) Annual Meeting.
Data collection and analysis
Selection of studies
Two review authors (KG, AB) scanned the titles and abstracts (when available) of all reports identified through the electronic searches. For studies appearing to meet the inclusion criteria, or for which there were insufficient information in the title and abstract to make a clear decision, we obtained the full report. Two review authors (KG, AB) independently assessed the full reports obtained from all the electronic and other methods of searching to establish whether the studies met the inclusion criteria. We resolved disagreements by discussion. Where resolution was not possible, we consulted a third review author (PM‐H). All studies meeting the inclusion criteria underwent validity assessment and data extraction using a standardised proforma. We recorded studies rejected at this or subsequent stages in the Characteristics of excluded studies table, and recorded reasons for exclusion.
Data extraction and management
Two review authors (KG, AB) independently extracted the data using specially designed data extraction forms. The data extraction forms were piloted on several papers and modified as required before use. We discussed any disagreements and consulted a third review author (PM‐H) when necessary. We contacted study authors for clarification or missing information if necessary and excluded data until further clarification was available or if we could not reach agreement.
For included studies, we extracted data as recommended in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This included data on the following:
author, year of publication, country of origin, source of study funding and journal citation (including language);
setting;
details of the participants including demographic characteristics (e.g. age, co‐morbidities, etc.), total number enrolled, and criteria for inclusion and exclusion;
CIN details at diagnosis;
details of the type of intervention;
risk of bias in study (see below);
duration of follow‐up;
-
details of the outcomes reported (pain, blood loss, adverse events), including method of assessment, and time intervals (see below):
for each outcome: outcome definition (with diagnostic criteria if relevant);
unit of measurement (if relevant);
for scales: upper and lower limits, and whether high or low score was good;
results: number of participants allocated to each intervention group;
for each outcome of interest: sample size; missing participants;
the time points at which outcomes were collected and reported.
We extracted data on outcomes as below:
for dichotomous outcomes (e.g. pain, adverse events), we extracted the number of women in each treatment arm who experienced the outcome of interest and the number of women assessed at endpoint, in order to estimate a risk ratio (RR) and 95% confidence interval (CI);
for continuous outcomes (e.g. blood loss), we extracted the final value and standard deviation (SD) of the outcome of interest and the number of women assessed at endpoint in each treatment arm at the end of follow‐up, in order to estimate the mean difference (MD) (if trials measured outcomes on the same scale) and 95% CI or standardised mean differences (if trials measured outcomes on different scales) between treatment arms and its standard error.
Where possible, all data extracted were those relevant to an intention‐to‐treat (ITT) analysis, in which participants were analysed in groups to which they were assigned.
Assessment of risk of bias in included studies
We assessed the risk of bias in included RCTs in accordance with guidelines in the Cochrane Handbook for Systematic Reviews of Interventions using the Cochrane 'Risk of bias' tool and the criteria specified in Chapter 8 (Higgins 2011). This included assessment of:
sequence generation;
allocation concealment;
blinding (of participants, healthcare providers and outcome assessors);
-
incomplete outcome data:
-
we recorded the proportion of women whose outcomes were not reported at the end of the study. We coded the satisfactory level of loss to follow‐up for each outcome as:
low risk of bias, if less than 20% of women were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms;
high risk of bias, if more than 20% of women were lost to follow‐up or reasons for loss to follow‐up were different between treatment arms;
unclear risk of bias, if loss to follow‐up was not reported;
-
selective reporting of outcomes;
other possible sources of bias.
Two review authors (KG, AB) independently applied the 'Risk of bias' tool and resolved differences by discussion or by appeal to a third review author (PM‐H). We summarised results in both a 'Risk of bias' graph and a 'Risk of bias' summary. Results of meta‐analyses were interpreted with consideration of the findings with respect to risk of bias.
Measures of treatment effect
We used the following measures of the effect of treatment:
for dichotomous outcomes, we used the RR;
for continuous outcomes, we used the MD between treatment arms.
Unit of analysis issues
Two review authors (KG, AB) reviewed any unit of analysis issues according to Higgins 2011 and resolved differences by discussion.
Dealing with missing data
We did not impute missing outcome data for the primary outcome. If data were missing or only imputed data were reported, we contacted trial authors to request data on the outcomes only among women who were assessed.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials that could not be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001), and, when possible, by subgroup analyses. If there was evidence of substantial heterogeneity, we investigated and reported the possible reasons.
Data synthesis
We characterised each trial by its type of analgesia and route of administration. Furthermore, we classified the assessment of pain or any other outcomes on whether dichotomous or continuous outcomes were used. We performed meta‐analysis when the interventions, route of administration and outcome measures were clinically similar.
For dichotomous outcomes, we calculated the RR for each trial and then pooled them.
For continuous outcomes, we pooled the MDs between the treatment arms at the end of follow‐up if all trials measured the outcome on the same scale, otherwise we pooled standardised mean differences.
If any trials had multiple treatment groups, we divided the 'shared' comparison group into the number of treatment groups and comparisons between each treatment group and treated the split comparison group as independent comparisons.
We used random‐effects models with inverse variance weighting for all meta‐analyses (DerSimonian 1986).
Summary of findings and assessment of the certainty of the evidence
Results
Description of studies
Results of the search
This review was first published in 2012 (Gajjar 2012). The original search strategy identified 232 unique references (Figure 1). Two review authors (KG, AB) screened the abstracts and articles that obviously did not meet the inclusion criteria and identified 20 articles as potentially eligible for inclusion in this Cochrane review. We performed full‐text screening of these 20 references and excluded two references for reasons described in the Characteristics of excluded studies table. The remaining 18 references pertaining to 17 completed RCTs met our inclusion criteria and are described in the Characteristics of included studies table.
1.
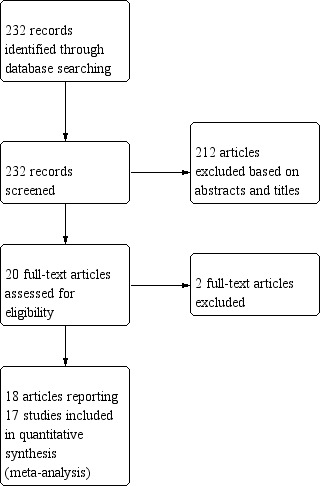
Study flow diagram of search results (September 2010).
For the 2016 review update, the search strategy identified an additional 109 unique references (Figure 2). After title and abstract screening these references, we retrieved four full‐text articles. We excluded one (Bogani 2014; see Characteristics of excluded studies table), and one study is awaiting classification until additional information is obtained from the study author (Diab 2015). Details of this study are described in Characteristics of studies awaiting classification table. We have contacted the author to request quantitative data and details of the measures used to quantify pain and anxiety, however, to date we have not received a reply. We included the remaining two studies in the review (Characteristics of included studies table).
2.

Study flow diagram of search results for review update April 2016.
Included studies
This updated review included the 17 trials from the original review (Al‐Kurdi 1985; Connell 2000; Crompton 1992; Cruickshank 2005; Diakomanolis 1997; Duncan 2005; Frega 1994; Howells 2000; Johnson 1989; Johnson 1996; Lee 1986; Lipscomb 1995; Mikhail 1988; Rogstad 1992; Sammarco 1993; Sarkar 1993; Winters 2009), and two trials identified from the 2016 search (Kizer 2014; Vanichtantikul 2013). The 19 trials randomised 1753 women, and assessed 1720 at the end of the trials (Characteristics of included studies table; Table 4).
1. Overview of included studies.
| Study | Participants' characteristics | Interventions | Outcomes | Notes |
| Al‐Kurdi 1985 | 97 women, undergoing CO2 laser treatment for CIN Age: 18 to 50 years |
Intervention (n = 50): 2 tablets naproxen sodium 550 mg Comparison (n = 47): 2 placebo tablets Given no less than 30 minutes before procedure |
Pain relief: VAS on 10 cm scale; VRS (none, very slight, mild, moderate, severe) Speed of procedure Use of analgesia in first 24 hours |
Self reported adverse effects very minor (aches and pains at 24 hours) and not included in analysis |
| Connell 2000 | 30 women undergoing LLETZ Age: 20 to 64 years |
Intervention (n = 15): lignocaine hydrochloride 10% spray Comparison (n = 15): saline spray Both groups received 4.4 ml local anaesthetic (prilocaine hydrochloride 30 mg/ml + felypressin 0.54 µg/ml) in the cervix |
Pain relief: VAS on 100 mm scale; 4 point categorical scale 1 to 4 (1 = not painful; 2 = slightly painful; 3 = moderately painful; 4 = severely painful) |
Outcome reported on categorical scale was not included in the analysis |
| Crompton 1992 | 98 women undergoing CO2 laser treatment for CIN Linear analogue anxiety and HAD anxiety/depression personality trait scores (Zigmond 1983), age and parity recorded to assess group comparability 3 arm trial |
TENS (n = 34) TENS + direct infiltration of 2 ml lignocaine 2% + Octapressin (prilocaine 30 mg/ml + felypressin 0.03 IU/ml) 1:10,000 (0.03 IU/ml) (n = 29) Direct infiltration of 2 ml lignocaine 2% + Octapressin (n = 35) |
Pain relief: VAS on 120 mm scale | Median pain score on 120 mm VAS; however, authors converted it to percentage for reporting |
| Cruickshank 2005 | 389 women undergoing LLETZ treatment for CIN Age mean: intervention: 32.7 years; comparison: 31.5 years |
Intervention group (n = 195): isoflurane + desflurane gases Comparison (n = 194): placebo (air) Both groups received infiltration of the cervix with prilocaine hydrochloride (30 mg/ml) + Octapressin (prilocaine 30 mg/ml plus felypressin 0.03 IU/ml) (0.54 mg/ml) |
Pain relief: VAS on 100 mm scale Heavy vaginal bleeding (yes/no) Anxiety using HAD scale |
Acceptability, satisfaction, helpfulness and willingness to undergo procedures in future ‐ not included in analysis |
| Diakomanolis 1997 | 100 women undergoing CO2 laser for CIN Age median: intervention: 28 years; comparison: 28.5 years |
Intervention (n = 50): 30 ml of 1:30 POR8 (vasoconstrictor) + lignocaine 1% solution Comparison (n = 50): 30 ml of lignocaine 1% solution |
Pain relief: VRS (none, moderate, severe) Intra‐operative blood loss Duration of procedure |
Adverse effects (transient hypertension and sweating) |
| Duncan 2005 | 97 women undergoing treatment with Semm coagulator Intervention: n = 46, mean age 31.3 years (SD 8.4); comparison: n = 47, mean age 32.6 years (SD 8.0) Nullipara: intervention: 14 (30.4%); comparison: 10 (21.3%) Married/cohabiting: intervention: 20 (43.5%); comparison: 22 (46.8%) CIN (1/2/3/unspecified) details (number (%)): intervention: HPV/CIN1 = 17/46 (37%), CIN2,3 = 29/46 (63%), microinvasion = 0/46; comparison: HPV/CIN1 = 17/47 (36.2%), CIN2,3 = 29/47 (61.7%), microinvasion = 1/47 (2.1%) |
Intervention (n = 46): 5 ml vials prilocaine 3% (30 mg/ml) + felypressin 0.03 IU/ml Comparison (n = 47): normal saline |
Pain relief: 11 point analogue scale ( 1 to 3, mild; 4 to 7, moderate; 8 to 10, severe pain) |
Details of anticipated pain excluded from the analysis |
| Frega 1994 | 63 women undergoing CO2 laser vaporisation for CIN 3 arm trial |
Intervention (n = 21): naproxen sodium 550 mg 30 minutes before treatment Comparison 1 (n = 21): placebo 30 minutes before treatment Comparison 2 (n = 21): no drug |
Pain relief: VAS 100 mm scale |
‐ |
| Howells 2000 | 200 women, aged 20 to 60 years, undergoing LLETZ for CIN (final histology negative intervention: 4/94 women; comparison: 8/106 women) Age mean (SD): intervention: 36.6 years (10.3); comparison: 34.6 years (9.7) |
Intervention (n = 94): prilocaine 3% (30 mg/ml) + felypressin 0.03 IU/ml (Citanest) Comparison (n = 106): lignocaine (xylocaine) 2% + adrenaline 1:80,000 |
Duration of procedure Degree of bleeding (0 = none; 5 = heavy) Pain relief: participant reported (0 = none; 5 = unbearable) |
Other adverse effects, e.g. feeling faint, nausea and shaking, scored in a similar way (0 = none; 5 = a great deal) |
| Johnson 1989 | 70 women undergoing CO2 laser ablation for cervical dysplastic lesion Size of transformation zone recorded as a score out of 2: intervention: 1.4; comparison: 1.2 |
Bilateral paracervical block by injecting 10 ml into the paracervical tissues Intervention (n = 35): lignocaine 2% Comparison (n = 35): normal saline |
Pain relief: VAS on 120 mm scale and objective scoring by nurse and attending operator Blood loss (recorded as a score) |
Anxiety and depression HAD scores (Zigmond 1983) and premenstrual syndrome scores also recorded |
| Johnson 1996 | 44 women undergoing CO2 laser treatment for CIN | Intervention (n = 23): 10 ml paracervical lignocaine 2% Comparison (n = 21): 2 ml lignocaine 2% directly into the TZ |
Pain relief: VAS (expressed as percentage) and objective scoring by nurse and laser operator |
‐ |
| Kizer 2014 | 52 women undergoing loop excision of the cervix for treatment in colposcopy clinic setting Age mean: intervention: 32.3 years; comparison 32.4 years |
Intervention (n = 28): buffered solution of lignocaine 1% + 1:100,000 adrenaline Comparison (n = 24): non‐buffered solution of lignocaine 1% + 1:100,000 adrenaline |
Pain relief: pain scores reported as mean, SD, median and range using 100 mm VAS during the injection, during procedure and cramping pain after procedure |
Adverse effects not reported in paper but mentioned in results section of clinical trials website No complications observed in either group |
| Lee 1986 | 50 women undergoing laser vaporisation of cervix for CIN | Intervention (n = 25): ectocervix infiltrated with 2 ml prilocaine 3% + 0.03 IU/ml felypressin (Citanest) Control (n = 25): no analgesia or anaesthesia |
Pain relief: VAS on 100 mm scale (VRS none, mild, moderate or severe) Blood loss: none, slight, moderate, troublesome |
Adverse effects, e.g. sweating, nausea, dizziness and cramps, also reported but not included in analysis as they were minor adverse effects |
| Lipscomb 1995 | 50 women scheduled for the loop electrosurgical excision for treatment of CIN Age mean (SD): intervention: 29.5 (10.5); comparison: 28.4 (8.9) Parity mean (SD): intervention: 2.1 (2.1); comparison: 2.3 (1.6) Loop passes: mean (SD): intervention: 1.2 (0.4); comparison: 1.3 (0.6) Positive margins: mean (SD): intervention: 2/25; comparison: 3/25 |
Intervention (n = 25): cervical application of benzocaine 20% gel Comparison (n = 25): placebo gel After 1 minute of gel application, 4 ml of lignocaine 1% + adrenaline 1:100,000 was injected in cervix |
Pain relief: VAS on 10 cm scale |
‐ |
| Mikhail 1988 | 50 women undergoing laser vaporisation of the cervix for CIN Age mean (SD): intervention: 27.4 (3.9); comparison: 26.7 (4.6) Parity mean (SD): intervention 0.9 (1.24); comparison: 1 (1) |
Intervention (n = 25): cervix was sprayed with 3 to 4 ml of a cocaine 10% solution Comparison (n = 25): cervix sprayed with a similar quantity of preservative alone |
Duration of procedure Blood loss (minimal, moderate, severe) Pain relief: VAS on 100 mm scale and VRS (none, mild, moderate or severe) |
‐ |
| Rogstad 1992 | 60 women undergoing cold coagulation for cervical abnormalities | Intervention (n = 29): 2 ml lignocaine 2% Comparison (n = 31): normal saline |
Pain relief: VAS on 0 to 10 scale and VRS |
Other outcomes, e.g. pain of injection and 3 to 6 weeks' follow‐up questionnaire of pain and bleeding were excluded from the analysis |
| Sammarco 1993 | 45 women undergoing cryocoagulation with liquid nitrogen using cryo‐2000 by double‐freeze technique for CIN | Intervention (n = 19): 2 to 3 ml of lignocaine 1% + adrenaline 1:100,000 dilution Comparison (n = 26): no treatment Both groups received single dose of ketoprofen 75 mg, within 1 hour of the procedure; 2 women received naproxen sodium 550 mg |
Pain relief: VAS on 100 mm scale Mean VAS score recorded by nurses was not included in analysis owing to high risk of bias |
‐ |
| Sarkar 1993 | 70 women undergoing laser treatment for CIN Age mean (SD): intervention: 27.8 years (6.3); comparison: 28 years (5.4) |
Intervention (n = 35): EMLA cream (lignocaine 2.5% + prilocaine 2.5%) Comparison (n = 35): placebo cream |
Pain relief: assessed by McGill's pain questionnaire (Melzack 1975) and VAS Blood loss (none, mild, moderate, troublesome) |
Minor adverse experiences during treatment, e.g. feeling hot, sweating, dizziness, fainting and sickness, not included in analysis |
| Vanichtantikul 2013 | 101 women
undergoing loop excision treatment for CIN Age median: intervention: 47 years (range 28 to 69 years); comparison: 48 years (range 28 to 85 years) Parity median: 2 (range 0 to 5) in both groups |
Intervention (n = 50): 40 mg of lignocaine 10% spray Comparison (n = 51): 1.8 ml of lignocaine 2% in 1:100,000 adrenaline injected submucosally |
Pain relief: 10 cm VAS after speculum insertion, during local injection or spray application, during loop excision and 30 minutes after procedure. Pain scores reported as median and range for all categories Pain scores further stratified using 2 cm cut‐off for the size of loop and these data reported as mean and SD only for loop size ≥ 2 cm |
No complications observed in any participants |
| Winters 2009 | 60 women undergoing LLETZ for CIN | Intervention (n = 30): prilocaine 3% + felypressin injected deep into the cervical stroma around TZ Comparison (n = 30): prilocaine 3% + felypressin injected superficial followed by deep into the cervical stroma around TZ Same amount used for both groups |
Pain relief: VAS on 100 mm scale |
Pain experienced during local anaesthetic injection also evaluated |
CIN: cervical intraepithelial neoplasia; HAD: Hospital Anxiety and Depression; HPV: human papillomavirus; LLETZ: loop excision of the transformation zone; n: number of participants; SD: standard deviation; TENS: transcutaneous electrical nerve stimulation; VAS: visual analogue scale; VRS: verbal rating score; TZ: transformation zone.
Design
All trials were conducted as single centre trials in a colposcopy clinic setting. Various pain relief interventions were reported in the 19 included trials. Two trials investigated cervical injection (intracervical block (Johnson 1989) and paracervical block (Rogstad 1992)) with anaesthetic agent (lignocaine 2%) compared with saline (Johnson 1989; Rogstad 1992). Three trials used preparations made up of local anaesthetic plus vasoconstrictor (Duncan 2005; Lee 1986; Sammarco 1993). One of these three trials used cervical injection with lignocaine 1% mixed with 1:100,000 dilution of adrenaline given submucosally and compared it with no treatment (Sammarco 1993), while the two other trials reported cervical injection with a different anaesthetic agent (prilocaine 30 mg/ml) mixed with vasoconstrictor (felypressin 0.03 IU/ml) compared with no treatment or placebo (Duncan 2005; Lee 1986). One trial investigated lignocaine 1% plus vasoconstrictor (1:30 of ornipressin in lignocaine 1% solution) compared to lignocaine 1% alone to evaluate the effects on the blood loss during the procedure (Diakomanolis 1997). One trial evaluated pain relief during the loop excision of the transformation zone (LLETZ) procedure with buffered local anaesthetic with vasoconstrictor compared to non‐buffered local anaesthetic with vasoconstrictor injected locally into the cervix (Kizer 2014). A buffered solution of local anaesthetic was prepared immediately before use by mixing 9 ml of lignocaine 1% plus adrenaline 1:100,000 with 1 ml of a 1 mEq/ml (8.4%) solution of sodium bicarbonate buffer while the non‐buffered solution contained 10 ml of lignocaine with adrenaline.
Three trials investigated the method of cervical injection. One trial compared local anaesthetic combined with vasoconstrictor (prilocaine 3% plus felypressin) administered by deep and superficial injection with deep injection alone (Winters 2009), while another trial compared paracervical injection of lignocaine 2% with direct injection (Johnson 1996). One trial investigated two different preparations of anaesthetic agent with vasoconstrictor (prilocaine 30 mg/ml plus felypressin 0.03 IU/ml compared with lignocaine 2% plus adrenaline 1:80,000) (Howells 2000).
Two trials reported the use of oral analgesia with NSAID (naproxen sodium 550 mg), given 30 minutes to one hour before treatment, compared to placebo or no treatment (Al‐Kurdi 1985; Frega 1994), with one trial using a single dose of naproxen sodium 550 mg (Frega 1994) while the other trial used double the dose (1100 mg) (Al‐Kurdi 1985).
One trial used a gas mixture (isoflurane 0.3% and desflurane 1%) as inhalation agent, in addition to standard cervical injection of local anaesthetic plus vasoconstrictor (prilocaine 30 mg/ml plus felypressin 0.03 IU/ml, also known as Octapressin) (Cruickshank 2005).
A further five trials used topical application of gel, cream and sprays for their anaesthetic effects during the treatment on cervix (Connell 2000; Lipscomb 1995; Mikhail 1988; Sarkar 1993; Vanichtantikul 2013). One trial looked at the effects of benzocaine 20% gel compared to placebo gel (Lipscomb 1995) and the other trial compared EMLA cream, which is a local anaesthetic cream consisting of a mixture of lignocaine 2.5% and prilocaine 2.5% (Sarkar 1993) to a placebo cream. Mikhail 1988 compared 3 to 4 ml of a cocaine 10% spray as a surface anaesthesia to a placebo solution (preservative) for its effects on pain relief. Out of the two trials using 10% lignocaine hydrochloride spray on the cervix (Connell 2000; Vanichtantikul 2013), one trial compared the effectiveness of 10% lignocaine spray with injection of 2% lignocaine with 1:100,000 adrenaline (Vanichtantikul 2013). In the trial of Connell 2000, women were randomised to receive either lignocaine 10% spray or saline spray in addition to standard cervical infiltration using prilocaine 30 mg/ml plus felypressin 0.03 IU/ml. One trial investigated TENS, which is a non‐invasive method (Crompton 1992).
Participant characteristics
The age of the women in the included trials ranged from 17 to 85 years; the mean age across the trials ranged from 27 to 35 years. However, Vanichtantikul 2013 reported a median age of 47 years (range 28 to 69 years) in the control group and 48 years (28 to 85 years) in the intervention (lignocaine spray) group. Most of the studies included both pre‐ and postmenopausal women, although two trials excluded perimenopausal or postmenopausal women, or both (Crompton 1992; Johnson 1989). Other common exclusion criteria were: various allergies, pregnancy and previous treatment to the cervix. Concomitant use of highly protein‐bound drug was an exclusion criterion in one trial with oral analgesia using NSAID (Al‐Kurdi 1985), while another trial using a gas mixture of isoflurane and desflurane excluded women taking monoamine‐oxidase inhibitors or women driving themselves home from the clinic (Cruickshank 2005). The trial of Kizer 2014 excluded women using prescription analgesics within seven days of scheduled procedure. Other reasons for exclusion included pelvic inflammatory disease, cardiac pacemaker (Crompton 1992), bronchial asthma (Al‐Kurdi 1985), cervical and vaginal infection, drug abuse, history of neurological deficit (Vanichtantikul 2013), cardiac conditions, hypertension and epilepsy (Diakomanolis 1997).
Eight trials described parity for intervention and control groups (Crompton 1992; Cruickshank 2005; Duncan 2005; Howells 2000; Johnson 1989; Kizer 2014; Lipscomb 1995; Vanichtantikul 2013). Number of nulliparous women recruited in these trials ranged from 18% to 48%. Two trials reported the number of children, which ranged from no children up to five (Lipscomb 1995; Mikhail 1988). Three trials reported marital status (Duncan 2005; Mikhail 1988; Sarkar 1993). Two trials provided the usage of contraception (Howells 2000; Johnson 1989). Sixty‐five per cent of women in the intervention group and 72% in the control group used contraception in the trial of Howells 2000. The use of oral contraceptive pills in Johnson 1989 was 47% in the intervention group compared to 53% in the control group.
Cruickshank 2005 used deprivation scores, while median anxiety Hospital Anxiety and Depression (HAD) score (Zigmond 1983) and median depression HAD score (Zigmond 1983) was used to compare the characteristics of intervention and control groups in the Crompton 1992 and Johnson 1989 trials. The Johnson 1989 trial also used anxiety VAS (Zigmond 1983) and premenstrual syndrome scores (no reference provided). Lee 1986 also used the Anxiety score (Spielberger 1970). Only one trial compared the groups for smoking status (Howells 2000).
Three trials reported smear grades as well as final histology with CIN grades (Howells 2000; Vanichtantikul 2013; Winters 2009). Lipscomb 1995 and Winters 2009 reported positive margins of excised cervical specimen after treatment. Crompton 1992 reported the size of the cervical pre‐invasive lesion in participants' characteristics, while Howells 2000, Kizer 2014, and Vanichtantikul 2013 reported the size of the loop excised. Vanichtantikul 2013 also reported the volume of the cone excised. Howells 2000 and Kizer 2014 provided number of passes of loop diathermy. Lipscomb 1995 reported mean number of loop passes per person in trial and control group.
The trial of Kizer 2014 reported the volume of anaesthetic agent used in both groups.
In nine of the 19 trials, women underwent laser ablation of the cervix (Al‐Kurdi 1985; Crompton 1992; Diakomanolis 1997; Frega 1994; Johnson 1989; Johnson 1996; Lee 1986; Mikhail 1988; Sarkar 1993), seven trials used LLETZ (Connell 2000; Cruickshank 2005; Howells 2000; Kizer 2014; Lipscomb 1995; Vanichtantikul 2013; Winters 2009), one trial used cryotherapy (Sammarco 1993), and two trials used cold coagulation with Semm Coagulator to treat the cervix (Duncan 2005; Rogstad 1992).
Outcomes
The diverse nature of the interventions in the trials precluded direct comparison apart from two trials comparing oral analgesia versus control, it was possible to combine the pain relief outcome reported on VAS (Al‐Kurdi 1985; Frega 1994).
Pain relief reported on visual analogue scale
Fifteen trials reported the degree of pain relief during the procedure as VAS (Al‐Kurdi 1985; Connell 2000; Cruickshank 2005; Frega 1994; Johnson 1989; Johnson 1996; Kizer 2014; Lee 1986; Lipscomb 1995; Mikhail 1988; Rogstad 1992; Sammarco 1993; Sarkar 1993; Vanichtantikul 2013; Winters 2009). All 19 trials used VAS to assess pain immediately after the procedure. In addition, one trial reported VAS for scoring pain after insertion of speculum, after spray or injection of local anaesthetic solution and 30 minutes after the procedure (Vanichtantikul 2013). The pain scores were further stratified according to the size of the excised loop. Kizer 2014 reported VAS for pain due to injection of local anaesthetic solution and cramping pain after procedure in addition to VAS pain scores immediately after the procedure. Seven trials used a 100‐mm or 10‐cm linear analogue scale, where 0 was no pain at all and 100 (or 10 in the 10‐cm scale) was worst pain imaginable (Al‐Kurdi 1985; Cruickshank 2005; Kizer 2014; Lipscomb 1995; Sarkar 1993; Vanichtantikul 2013; Winters 2009). One trial reported pain relief on 120‐mm linear VAS, which was converted to percentages (Johnson 1989). Connell 2000, Johnson 1996, and Vanichtantikul 2013 reported pain relief as VAS; however, the values were median and interquartile range (IQR), rather than mean and SD. Sammarco 1993 and Vanichtantikul 2013 reported VAS on an 11‐point scale (0 to 10 or 10‐cm scale) where 0 was no pain and 10 was severe pain.
Pain relief reported on verbal rating scores
Five trials reported pain relief on verbal rating score (VRS) categorised as none, mild, moderate or severe (Al‐Kurdi 1985; Diakomanolis 1997; Duncan 2005; Lee 1986; Mikhail 1988).
Pain relief reported on other categorical scales
In addition to VAS, Johnson 1989 and Johnson 1996 reported pain relief as an objective score, given by the attending nurse and laser operator on a categorical scale of 0 to 2. The attending colposcopist of another trial scored pain on a categorical scale (0 = none to 4 = severe) as well as by women undergoing treatment (0 = none to 5 = unbearable) (Howells 2000). The Sarkar 1993 trial measured pain scores for pain relief after treatment and not just during treatment. However, the time scale for carrying out the pain score was not specified. The trial of Al‐Kurdi 1985 asked women whether additional analgesics were required within the first 24 hours. Cruickshank 2005 asked women whether additional pain relief was required after treatment. It would appear that this was asked at six months' follow‐up, which carries a risk of recall bias. Owing to this risk, we did not include these data in the analysis.
Blood loss during treatment
Seven trials reported blood loss as none, mild, moderate and troublesome (Crompton 1992; Cruickshank 2005; Diakomanolis 1997; Howells 2000; Lee 1986; Mikhail 1988; Sarkar 1993). The Diakomanolis 1997 trial explicitly specified the method of measuring blood loss, while other trials reported blood loss subjectively as scored by the operator on a categorical scale (0 = none to 5 = heavy/troublesome) (Crompton 1992; Cruickshank 2005; Howells 2000; Lee 1986; Mikhail 1988; Sarkar 1993). One trial reported estimated blood loss as volume range with categories of 0 ml, 1 to 5 ml, 6 to 10 ml and more than 10 ml (Kizer 2014).
Speed of procedure (or duration of treatment)
Four trials reported speed of procedure (Diakomanolis 1997; Howells 2000; Lee 1986; Sarkar 1993).
Anxiety
Preoperative anxiety is one of the most significant risk factors for experiencing pain during cervical colposcopy treatment (Johnson 1994). Four trials measured anxiety levels preoperatively in both arms. Three trials measured anxiety using HAD scores (Crompton 1992; Cruickshank 2005; Johnson 1989), while a fourth trial (Lee 1986) used a different scale (Spielberger State Anxiety Inventory; Spielberger 1970).
Excluded studies
We excluded three references after obtaining the full text, for the following reasons.
The trial of Sarkar 1990 was not an RCT and it was not controlled for placebo effects. This trial reported use of EMLA cream (lignocaine‐prilocaine cream) for pain relief during cervical laser treatment.
Sharp 2009 did not compare pain relief interventions. This was an observational study nested within an RCT in which women completed a questionnaire about their experiences at colposcopy, colposcopy and biopsy, and colposcopy and LLETZ treatment.
Bogani 2014 was not an RCT and the intervention was for pain relief for different procedure (colposcopically guided biopsies rather than excisional or ablative procedures).
For further details of all the excluded studies, see the Characteristics of excluded studies table.
Risk of bias in included studies
Six trials were at low risk of bias, as they satisfied at least five of the criteria that we used to assess risk of bias (Connell 2000; Cruickshank 2005; Johnson 1989; Johnson 1996; Kizer 2014; Mikhail 1988). Nine trials were at moderate risk of bias as they satisfied three or four of the criteria (Al‐Kurdi 1985; Diakomanolis 1997; Duncan 2005; Howells 2000; Lee 1986; Lipscomb 1995; Sarkar 1993; Winters 2009). The trials of Rogstad 1992 and Vanichtantikul 2013 were at high risk of bias as they only satisfied two of the criteria and a further three trials were also at high risk of bias as they only satisfied one criterion (Crompton 1992; Frega 1994; Sammarco 1993) (see Figure 3; Figure 4).
3.

'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
4.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study.
Allocation
Ten trials reported the method of generation of the sequence of random numbers used to allocate women to treatment arms (Connell 2000; Cruickshank 2005; Johnson 1989; Johnson 1996; Kizer 2014; Lipscomb 1995; Mikhail 1988; Rogstad 1992; Vanichtantikul 2013; Winters 2009), but three of these trials did not report concealment of this allocation sequence from participants and healthcare professionals involved in the trial (Lipscomb 1995; Mikhail 1988; Rogstad 1992). Five trials did not report on either the method of sequence generation or concealment of allocation (Al‐Kurdi 1985; Connell 2000; Frega 1994; Lee 1986; Sammarco 1993; Sarkar 1993). In the trials of Duncan 2005and Howells 2000, it was unclear whether the method of assigning women to treatment groups was carried out using an adequate method of sequence generation, but the allocation was adequately concealed. The trial of Crompton 1992 did not report sequence generation details but did state that the allocation was not concealed.
Blinding
Four trials reported blinding of participants, healthcare professionals and outcome assessors (Cruickshank 2005; Johnson 1989; Johnson 1996; Kizer 2014), whereas this information was not reported in five trials (Al‐Kurdi 1985; Duncan 2005; Frega 1994; Lee 1986; Rogstad 1992). Five trials confirmed blinding of participants and healthcare professionals, but it was unclear whether the outcome assessor was blinded (Connell 2000; Diakomanolis 1997; Lipscomb 1995; Mikhail 1988; Sarkar 1993). Four trials confirmed that participants and/or healthcare professionals were not blinded but did not report whether the outcome assessor was blinded or not (Crompton 1992; Howells 2000; Sammarco 1993; Winters 2009). In the trial of Vanichtantikul 2013 comparing a spray with local injection, operator blinding was not possible.
Incomplete outcome data
At least 80% of the women who were enrolled were assessed at endpoint in all 19 trials.
Selective reporting
It was not certain whether three trials reported all the outcomes that they assessed (Crompton 1992; Lipscomb 1995; Sammarco 1993), but in 10 trials it appeared that additional pertinent outcomes should have been reported and their omission left a gap in the evidence (Connell 2000; Cruickshank 2005; Diakomanolis 1997; Duncan 2005; Frega 1994; Howells 2000; Johnson 1989; Johnson 1996; Rogstad 1992; Vanichtantikul 2013; Winters 2009). The remaining five trials seemed to report all relevant outcomes related to the subject matter.
Other potential sources of bias
No other form of bias appeared likely in 10 trials (Al‐Kurdi 1985; Connell 2000; Cruickshank 2005; Duncan 2005; Howells 2000; Johnson 1989; Lee 1986; Lipscomb 1995; Mikhail 1988; Winters 2009). Additional forms of bias may have been possible in the trials of Sammarco 1993 and Sarkar 1993 in the way some analyses were undertaken, but it was unclear whether this was the case in the remaining five trials.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings 1. Local anaesthetic infiltration (lignocaine 2%) compared with saline injection for pain relief during outpatient colposcopy treatment.
| Local anaesthetic infiltration (lignocaine 2%) compared with saline injection for pain relief during outpatient colposcopy treatment | ||||||
|
Patient or population: women undergoing outpatient colposcopy treatment Settings: outpatient clinic Intervention: local anaesthetic infiltration (lignocaine 2%) Comparison: saline injection | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect MD (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Saline injection | Local anaesthetic infiltration with lignocaine 2% | |||||
| Pain scores during procedure (VAS: 0 to 100) | The mean VAS score ranged across control groups was 30 to 53 | The mean VAS score in the intervention groups was 27 to 29 | 13.74 lower (34.32 lower to 6.83 higher) | 130 (2 studies) | ⊕⊕⊝⊝ low1,2 | Lower VAS score represents less pain |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Allocation concealment and blinding of outcome assessment were not described in one of the included studies.
2 Adverse events not reported.
Summary of findings 2. Local anaesthetic plus vasoconstrictor compared with no analgesia for pain relief during outpatient colposcopy treatment.
| Local anaesthetic plus vasoconstrictor compared with no analgesia for pain relief during outpatient colposcopy treatment | ||||||
|
Patient or population: women undergoing outpatient colposcopy treatment Settings: outpatient clinic Intervention: local anaesthetic + vasoconstrictor Comparison: no analgesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect MD (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No analgesia | Local anaesthetic + vasoconstrictor | |||||
| Pain scores during procedure (VAS: 0 to 100) | The mean VAS score ranged across control groups was 42.7 to 43 | The mean VAS score in the intervention groups was 11.6 to 26 |
23.73 lower (9.93 lower to 37.53 lower) |
95 (2 studies) | ⊕⊕⊝⊝ low1,2 | Lower VAS score represents less pain |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Allocation concealment and blinding of outcome assessment were not described in one of the included studies. Participants and personnel were not blinded in the second study.
2 Adverse events not reported.
Summary of findings 3. Oral analgesia compared with placebo for pain relief during outpatient colposcopy treatment.
| Oral analgesia compared with placebo for pain relief during outpatient colposcopy treatment | ||||||
|
Patient or population: women undergoing outpatient colposcopy treatment Settings: outpatient clinic Intervention: oral analgesia Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect MD (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Oral analgesia | |||||
| Pain scores during procedure (VAS: 0 to 100) | The mean VAS score ranged across control groups was 21 to 41 | The mean VAS score in the intervention groups was 19 to 36 |
3.51 lower (10.03 lower to 3.01 higher) |
129 (2 studies) | ⊕⊝⊝⊝ very low1,2,3 | Lower VAS score represents less pain |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Allocation concealment and blinding of outcome assessment were not described in one of the included studies.
2 Adverse events not reported.
3 Some inconsistencies regarding presentation of the data in one study.
Local anaesthetic versus placebo
Two trials compared local anaesthetic (lignocaine 2%) alone versus placebo (saline injection) (Johnson 1989; Rogstad 1992). Johnson 1989 used lignocaine 2% injection for paracervical block while the trial of Rogstad 1992 used lignocaine 2% for direct injection in the cervix.
Pain scores during procedure (VAS)
Rogstad 1992 found that women who received local anaesthetic had less pain during treatment than women who received placebo (MD ‐24.00; 95% CI ‐35.44 to ‐12.56; 60 women), whereas Johnson 1989 found no difference between the groups (MD ‐3.00; 95% CI ‐16.03 to 10.03; 70 women) (Analysis 1.1).
1.1. Analysis.

Comparison 1: Local anaesthetic (lignocaine 2% injection) versus control (saline injection), Outcome 1: Pain scores during procedure (VAS)
Moderate to severe pain during procedure
Rogstad 1992 found that women who received local anaesthetic reported less moderate or severe pain during treatment than women who received placebo (RR 0.36; 95% CI 0.18 to 0.71; 60 women) (Analysis 1.2).
1.2. Analysis.

Comparison 1: Local anaesthetic (lignocaine 2% injection) versus control (saline injection), Outcome 2: Moderate to severe pain
Local anaesthetic plus vasoconstrictor versus control
Three trials compared local anaesthetic plus vasoconstrictor versus control, but variations in both the interventions or control groups, or both, meant that the trials could not be pooled in a meta‐analysis (Duncan 2005; Lee 1986; Sammarco 1993).
Pain scores during procedure
Meta‐analysis of two trials, assessing 95 women, found that women who received local anaesthetic plus vasoconstrictor (prilocaine 3% with felypressin 0.03 IU/ml (Lee 1986) and lignocaine 1% with adrenaline 1:100,000 dilution (Sammarco 1993)) had less pain during treatment than women who received no treatment (MD ‐23.73; 95% CI ‐37.53 to ‐9.93) (Analysis 2.1). The percentage of the variability in effect estimates that is because of heterogeneity rather than chance may represent substantial heterogeneity (I2 = 63%). In Sammarco 1993, women in both intervention arm and control arm received the oral analgesic ketoprofen 75 mg single dose within one hour of receiving treatment.
2.1. Analysis.

Comparison 2: Local anaesthetic plus vasoconstrictor versus control, Outcome 1: Pain scores during procedure (VAS: 0‐100)
Moderate or severe pain during procedure
Two trials reporting pain relief with local anaesthetic plus vasoconstrictor versus control using VRS showed contrasting results (Duncan 2005; Lee 1986). Duncan 2005 found that women who received local anaesthetic with vasoconstrictor (5 ml vials of prilocaine 3% (30 mg/ml) with felypressin 0.03 IU/ml) reported less moderate or severe pain during treatment than women who received placebo. Lee 1986 found no difference in the same outcome between women who received vasoconstrictor with local anaesthetic (2 ml of prilocaine 3% with felypressin 0.03 IU/ml) and those who received no treatment (RR 0.12; 95% CI 0.04 to 0.37 and RR 0.73; 95% CI 0.42 to 1.27 (Analysis 2.2) for local anaesthetic with vasoconstrictor versus placebo or no treatment, respectively). Whether the difference could be attributable to varying dosage of anaesthetic agents (5 ml in Duncan 2005 trial versus 2 ml in Lee 1986 trial) is worth considering. We identified no other trials on optimal dosage to address this issue. Also of note, the method of cervical treatment differed in these two trials. Women in Lee 1986 received cervical treatment with laser vaporisation while in Duncan 2005 the women received treatment with Semm coagulator (high‐temperature electro‐cautery).
2.2. Analysis.

Comparison 2: Local anaesthetic plus vasoconstrictor versus control, Outcome 2: Moderate or severe pain
Blood loss (subjective) during procedure
Lee 1986 found no difference in the risk of troublesome bleeding between women who received local anaesthetic plus vasoconstrictor (2 ml of prilocaine 3% plus 0.03 IU/ml of felypressin 0.03 IU/ml) and women who received no treatment (RR 0.40; 95% CI 0.09 to 1.87) (Analysis 2.3). However, the blood loss was not measured and was a subjective impression by the operator.
2.3. Analysis.

Comparison 2: Local anaesthetic plus vasoconstrictor versus control, Outcome 3: Troublesome bleeding
Local anaesthetic plus vasoconstrictor versus local anaesthetic injection alone
Diakomanolis 1997 compared local anaesthetic plus vasoconstrictor (30 ml of ornipressin 1:30 plus lignocaine 1% solution) with local anaesthetic alone (30 ml of lignocaine 1% solution).
Moderate or severe pain during procedure
Diakomanolis 1997 found no difference in the risk of moderate or severe pain between women who received local anaesthetic with vasoconstrictor and women who received local anaesthetic alone (RR 1.20; 95% CI 0.57 to 2.52) (Analysis 3.1).
3.1. Analysis.

Comparison 3: Local anaesthetic plus vasoconstrictor versus local anaesthetic injection alone, Outcome 1: Moderate or severe pain
Blood loss (measured) during procedure
Diakomanolis 1997 found that women who received vasoconstrictor with local anaesthetic had less measured blood loss during treatment than women who received local anaesthetic alone (MD ‐8.75 ml; 95% CI ‐10.43 to ‐7.07) (Analysis 3.2). In this trial, the amount of solution used for cervical injection of 30 ml was higher than what is generally used. Unlike the subjective evaluation of blood loss in other trials by the operator, trial of Diakomanolis 1997 reported the actual measured volume of blood loss.
3.2. Analysis.

Comparison 3: Local anaesthetic plus vasoconstrictor versus local anaesthetic injection alone, Outcome 2: Blood loss (volume)
Speed of procedure (duration of treatment)
Diakomanolis 1997 found that duration of treatment was less in women who received vasoconstrictor with local anaesthetic than women who received local anaesthetic alone (MD ‐7.72; 95% CI ‐8.49 to ‐6.95) (Analysis 3.3).
3.3. Analysis.

Comparison 3: Local anaesthetic plus vasoconstrictor versus local anaesthetic injection alone, Outcome 3: Duration of treatment
Local anaesthetic plus vasoconstrictor (prilocaine (local anaesthetic) with felypressin (vasoconstrictor)) versus lignocaine (local anaesthetic) with adrenaline (vasoconstrictor))
Howells 2000 compared two types of local anaesthetic with vasoconstrictor. More specifically, it reported a comparison of prilocaine 3% with felypressin 0.03 IU/ml versus lignocaine 2% with adrenaline 1:80,000.
Pain scores during procedure (using 6‐point categorical scale)
Howells 2000 found no difference in pain scores when measured using a 6‐point categorical scale between women who received prilocaine plus felypressin and women who received lignocaine plus adrenaline (MD ‐0.05; 95% CI ‐0.26 to 0.16) (Analysis 4.1).
4.1. Analysis.

Comparison 4: Prilocaine plus felypressin versus lignocaine plus adrenaline, Outcome 1: Pain (using 6 category scale)
Blood loss during procedure
Howells 2000 found that women who received prilocaine plus felypressin had more mean blood loss during treatment than women who received lignocaine plus adrenaline (MD 0.41; 95% CI 0.13 to 0.69) (Analysis 4.2). However, the observed difference is unlikely to be clinically significant and the assessment of blood loss was by subjective 0‐ to 5‐point scoring and not the actual measured loss.
4.2. Analysis.

Comparison 4: Prilocaine plus felypressin versus lignocaine plus adrenaline, Outcome 2: Blood loss (0‐5 scale)
Deep plus superficial versus deep cervical injection
One trial compared deep plus superficial injection with deep injection alone (Winters 2009).
Pain scores during procedure (VAS: 0 to 100)
Winters 2009 found no difference in pain scores when measured using a VAS between women who received deep and superficial injection and women who received deep cervical injection (MD ‐4.90; 95% CI ‐11.51 to 1.71) (Analysis 5.1).
5.1. Analysis.

Comparison 5: Deep plus superficial versus deep cervical injection, Outcome 1: Pain scores during procedure (VAS: 0‐100)
Oral analgesic versus placebo or no treatment
Two trials reported a comparison of naproxen sodium 550 mg tablets given at least 30 minutes before treatment (oral analgesic) versus placebo (Al‐Kurdi 1985; Frega 1994). The trial of Frega 1994 also included a third arm, which had randomised women to no drug.
Pain scores during procedure
Meta‐analysis of the two trials assessing 129 women, found no difference in pain scores when measured using a VAS between women who received oral analgesic and women who received placebo (MD ‐3.51; 95% CI ‐10.03 to 3.01 (Analysis 6.1) (Al‐Kurdi 1985; Frega 1994). The percentage of the variability in effect estimates that was because of heterogeneity rather than sampling error (chance) was not important (I2 = 0%). The trial of Frega 1994 also found no difference in pain scores between oral analgesic versus no treatment (MD ‐4.00; 95% CI ‐13.69 to 5.69 (Analysis 6.1).
6.1. Analysis.

Comparison 6: Oral analgesic versus control, Outcome 1: Pain scores (VAS: 0‐100)
Moderate to severe pain during procedure
Al‐Kurdi 1985 found no difference in the moderate or severe pain experienced during treatment between women who received oral analgesic and women who received placebo (RR 0.82; 95% CI 0.60 to 1.13) (Analysis 6.2).
6.2. Analysis.

Comparison 6: Oral analgesic versus control, Outcome 2: Moderate to severe pain
Pain relief required in first 24 hours
The trial of Al‐Kurdi 1985 found that women who received oral analgesic for pain relief during colposcopy were less likely to use additional pain relief within the first 24 hours following treatment than women who received placebo (RR 0.12; 95% CI 0.03 to 0.47) (Analysis 6.3).
6.3. Analysis.

Comparison 6: Oral analgesic versus control, Outcome 3: Pain relief required in first 24 hours
Inhalation analgesia versus placebo or no treatment
Cruickshank 2005 reported a comparison of a gas mixture (isoflurane and desflurane) as inhalation analgesia versus placebo (air) (in addition to standard cervical injection with prilocaine 30 mg/ml plus felypressin 0.03 IU/ml).
Pain scores during procedure
Cruickshank 2005 found that women who received gas mixture for pain relief (in addition to standard cervical injection with prilocaine 30 mg/ml plus felypressin 0.03 IU/ml) had less pain during treatment than women who received placebo (MD ‐7.20; 95% CI ‐12.45 to ‐1.95) (Analysis 7.1).
7.1. Analysis.

Comparison 7: Inhalation analgesia versus placebo, Outcome 1: Pain scores (VAS: 0‐100)
Haemorrhage during procedure
Cruickshank 2005 found no difference in the risk of heavy vaginal bleeding between women who received gas mixture and those who received placebo (RR 1.17; 95% CI 0.83 to 1.64) (Analysis 7.2).
7.2. Analysis.

Comparison 7: Inhalation analgesia versus placebo, Outcome 2: Heavy vaginal bleeding
Anxiety (HAD score) during procedure
Cruickshank 2005 found no difference in anxiety scores between women who received gas mixture and women who received placebo (MD 0.01; 95% CI ‐0.80 to 0.82) (Analysis 7.3).
7.3. Analysis.

Comparison 7: Inhalation analgesia versus placebo, Outcome 3: Anxiety ‐ HAD score
Topical application (benzocaine gel, EMLA cream, cocaine spray and lignocaine spray) versus control
Five trials reported comparisons of anaesthetic topical application versus control agent, but variations in the interventions meant that the trials were could not be pooled in a meta‐analysis (Connell 2000; Lipscomb 1995; Mikhail 1988; Sarkar 1993; Vanichtantikul 2013).
Topical (gel/cream) versus placebo
Two trials reported topical gel or cream versus placebo (Lipscomb 1995; Sarkar 1993).
Pain scores during procedure
Lipscomb 1995 found no difference in pain scores when measured using a VAS between women who received anaesthetic topical gel (20% benzocaine) and women who received placebo (MD ‐9.00; 95% CI ‐68.59 to 50.59) (Analysis 8.1). Women in both intervention and placebo arm received pre‐procedure oral analgesia in addition to injecting 4 ml of lignocaine 1% (mixed with adrenaline 1:100,000) in four quadrants of the cervix.
8.1. Analysis.

Comparison 8: Topical application versus placebo, Outcome 1: Pain scores during procedure (VAS: 0‐100)
Speed of procedure (duration of treatment)
Sarkar 1993 found no difference in the duration of treatment between women who received anaesthetic topical cream (EMLA cream ‐ mixture of lignocaine 2.5% and prilocaine 2.5%) and women who received placebo (MD 0.10; 95% CI ‐1.38 to 1.58) (Analysis 8.2).
8.2. Analysis.

Comparison 8: Topical application versus placebo, Outcome 2: Duration of treatment
Cocaine spray versus placebo
Mikhail 1988 reported a comparison of cocaine spray versus placebo.
Pain scores during procedure (VAS: 0 to 100)
Mikhail 1988 found that women who received cocaine spray for pain relief had less pain during treatment than women who received placebo (MD ‐28.00; 95% CI ‐37.86 to ‐18.14) (Analysis 9.1).
9.1. Analysis.

Comparison 9: Cocaine spray versus placebo, Outcome 1: Pain scores during procedure (VAS: 0‐100)
Moderate to severe pain during procedure
Mikhail 1988 found that women who received cocaine spray experienced less moderate or severe pain during treatment than women who received placebo (RR 0.57; 95% CI 0.37 to 0.89) (Analysis 9.2).
9.2. Analysis.

Comparison 9: Cocaine spray versus placebo, Outcome 2: Moderate to severe pain
Blood loss during procedure (troublesome bleeding)
Mikhail 1988 found that women who received cocaine spray had less risk of troublesome bleeding following treatment than women who received placebo. No women in the cocaine spray arm and 11 out of 25 women in the placebo arm had troublesome bleeding. We did not calculate the RR; the default zero‐cell correction within Review Manager 5 would bias the result of the meta‐analysis towards no difference between cocaine spray and placebo (Analysis 9.3).
9.3. Analysis.

Comparison 9: Cocaine spray versus placebo, Outcome 3: Troublesome bleeding
Lignocaine spray versus cervical injection with lignocaine with adrenaline
Vanichtantikul 2013 compared 40 mg 10% lignocaine spray alone with 1.8 ml of 2% lignocaine plus 1:100,000 adrenaline.
Pain scores during procedure
Vanichtantikul 2013 reported pain scores using 10 cm VAS scoring system between women who received 40 mg 10% lignocaine spray and women who received 1.8 ml of 2% lignocaine with 1:100,000 adrenaline. Pain during the procedure was reported as median rather than mean pain scores. As such, the median pain scores were not included in the analysis but were summarised separately. However, pain during the procedure was reported as means following stratification of data by loop size (less than 2 cm and 2 cm or greater), and these data were included in the analysis.
TENS, local anaesthetic alone and TENS plus local anaesthetic injection
Crompton 1992 compared three treatments; TENS, TENS plus cervical infiltration with local anaesthetic with a vasoconstrictor (2 ml of lignocaine 2% plus Octapressin (prilocaine 30 mg/ml plus felypressin 0.03 IU/ml)) injection and local anaesthetic injection alone. As results of pain relief were reported as median with IQR, they were not included in analysis but were summarised separately.
Troublesome blood loss during procedure
Crompton 1992 found no difference in the risk of troublesome vaginal bleeding between women who received TENS, TENS plus local anaesthetic and local anaesthetic alone (TENS versus TENS plus local anaesthetic: RR 2.56; 95% CI 0.28 to 23.29; TENS versus local anaesthetic alone: RR 0.77; 95% CI 0.19 to 3.20; TENS plus local anaesthetic versus local anaesthetic alone: RR 0.30; 95% CI 0.04 to 2.55) (Analysis 10.1; Analysis 11.1; Analysis 12.1).
10.1. Analysis.

Comparison 10: TENS versus TENS plus local anaesthetic injection, Outcome 1: Troublesome blood loss
11.1. Analysis.

Comparison 11: TENS versus local anaesthetic injection, Outcome 1: Troublesome blood loss
12.1. Analysis.

Comparison 12: TENS plus local versus local anaesthetic injection, Outcome 1: Troublesome blood loss
Buffered solution of local anaesthetic plus vasoconstrictor versus non‐buffered solution of local anaesthetic plus vasoconstrictor
Kizer 2014 compared submucosal cervical injection of a buffered solution of local anaesthetic plus vasoconstrictor agent (mixture of 9 ml of lignocaine with adrenaline 1:100,000 mixed with 1 ml of a 1 mEq/ml (8.4%) solution of sodium bicarbonate buffer) with the non‐buffered solution of lignocaine plus adrenaline.
Pain scores during procedure
Kizer 2014 found no difference in the pain scores on VAS scale between women who received buffered local anaesthetic plus vasoconstrictor and women who received non‐buffered solution of local anaesthetic plus vasoconstrictor agent (MD ‐8.00; 95% CI ‐17.57 to 1.57) (Analysis 13.1).
13.1. Analysis.

Comparison 13: Buffered versus non‐buffered local anaesthetic with vasoconstrictor, Outcome 1: Pain scores (VAS: 0‐100)
Studies or analyses, or both, included within the review but not in the forest plots
Pain scores (VAS and objective pain scores)
Connell 2000; Crompton 1992; and Johnson 1996 reported pain on VAS scales using median and IQR. Johnson 1996 also reported objective pain scores by attending nurse and colposcopist.
Lignocaine spray versus placebo
Connell 2000 compared 0.5 ml of lignocaine 10% spray in addition to standard cervical infiltration with prilocaine 30 mg/ml plus felypressin 0.03 IU/ml versus placebo. The trial reported the results of pain relief using a VAS scale as median and IQR. The results showed that application of lignocaine spray had no effect on pain scores (P value = 0.38). The medians with IQR of the VAS scale for lignocaine spray versus placebo were 40.0 (IQR 21.25 to 63.25) for lignocaine spray and 36.0 (IQR 17.5 to 49.5) for placebo.
Lignocaine spray versus local anaesthetic plus vasoconstrictor
Vanichtantikul 2013 compared four puffs (10 mg each) of 10% lignocaine spray versus standard 1.8 ml of 2% lignocaine with adrenaline 1:100,000 cervical injection. The trial reported the results of pain relief during the intervention (spray versus injection) during the procedure and 30 minutes after the procedure using a 10 cm VAS score as median and range.
Pain scores during the procedure
The median pain scores with IQR using 10 cm VAS during procedure were 3.0 (IQR 0.0 to 9.5) for lignocaine spray and 4.0 (IQR 0.0 to 8.2) for lignocaine plus adrenaline injection (P value = 0.11).
Pain scores 30 minutes after the procedure
The median pain scores with IQR after 30 minutes of completion of the procedure were 2.6 (IQR 0.0 to 7.8) for lignocaine spray and 1.9 (IQR 0.0 to 8.5) for lignocaine plus adrenaline injection (P value = 0.84).
Pain scores during application of spray versus injection
The median with IQR for the VAS pain score was 0.0 (IQR 25.7 to 3.0) for lignocaine spray and 1.9 (IQR 23.5 to 7.6) for lignocaine plus adrenaline injection (P value < 0.01).
TENS, local anaesthetic injection and TENS plus local anaesthetic injection
Crompton 1992 compared three treatments; TENS, TENS plus cervical infiltration with local anaesthetic with a vasoconstrictor (2 ml of lignocaine 2% plus Octapressin (prilocaine 30 mg/ml plus felypressin 0.03 IU/ml) injection and local anaesthetic injection alone (2 ml of lignocaine 2%).
The results of pain relief using VAS were reported as median pain scores and IQR (24 (IQR 10 to 42) for TENS; 17 (IQR 7 to 30) for local anaesthetic alone; 18 (IQR 8 to 31) for TENS plus local anaesthetic). The median pain score for the group assigned TENS only was higher than the median score for the group given direct infiltration of local anaesthetic alone (U = ‐1.57; P value = 0.12).
Paracervical versus intracervical injection in the transformation zone of cervix with lignocaine
Johnson 1996 compared direct infiltration in the transformation zone (TZ) with 2 ml of lignocaine 2% versus paracervical block with lignocaine 2% using 5 ml on each side of the cervix. This trial reported pain relief on a VAS expressed as median and IQRs. The median linear analogue pain scores (IQR) for direct infiltration was 14% (IQR 6% to 29%) for direct infiltration and 30% (IQR 21% to 47%) for paracervical block (Mann‐Whitney Z = 2.79; P value = 0.005) suggesting direct infiltration was associated with lower pain scores. The trial also reported objective pain scores as scored by the attending nurse and colposcopist. The objective pain score for direct injection with local anaesthetic was slightly lower (23 women; 0 (IQR 0 to 0.25)) than the score associated with paracervical lignocaine injection (21 women; 0 (IQR 0 to ‐0.75); Mann‐Whitney test Z = 0.23; P value = 0.8).
Discussion
Summary of main results
Nineteen RCTs (1720 women) met the inclusion criteria and were assessed in the review. These trials compared a variety of interventions aimed at reducing pain in women who underwent treatment for CIN in colposcopy clinic settings, including cervical injection with lignocaine alone, lignocaine plus adrenaline, lignocaine plus adrenaline in sodium bicarbonate buffer, prilocaine plus felypressin, oral analgesics (NSAID), inhalation analgesia (gas mixture of isoflurane and desflurane), lignocaine spray, cocaine spray, local application of benzocaine 20% gel, EMLA cream and TENS.
Use of lignocaine 2% for cervical injection (as direct injection or paracervical block) showed no overall benefit in pain relief as compared to placebo, with one trial (Rogstad 1992) showing a beneficial effect while another trial (Johnson 1989) found no benefit. Use of the local anaesthetic prilocaine with a vasoconstrictor (felypressin) showed a reduction in pain on VAS (Duncan 2005; Lee 1986). However, one trial found no benefit when the pain was assessed with VRS (Lee 1986). This trial also reported no reduction in blood loss with prilocaine plus felypressin. However, the blood loss was not measured and it was the subjective impression of the operator. It is also worth noting that this trial, though randomised, was not a double‐blind controlled trial and only had a small sample size (25 in the intervention arm and 25 in the placebo arm) (Lee 1986). The addition of a vasoconstrictor agent (ornipressin) to anaesthetic agent (lignocaine 1%) resulted in less measured blood loss and reduction of the duration of procedure (Diakomanolis 1997). Direct cervical injection with local anaesthetic (lignocaine 2%) resulted in better pain relief than placebo (Rogstad 1992) and paracervical block (Johnson 1996). Superficial injection of local anaesthetic in the cervix before deep injection did not result in any better pain relief (Winters 2009). Buffering of lignocaine with adrenaline did not give better pain relief as compared to non‐buffered lignocaine 1% with adrenaline 1:100,000.
Oral analgesia with an NSAID before the procedure did not result in better pain relief, although one trial reported that the women were less likely to use oral analgesics at home within the first 24 hours of treatment (Al‐Kurdi 1985).
Inhalation of gas mixture (isoflurane and desflurane) in addition to standard cervical injection with prilocaine 30 mg/ml plus felypressin 0.03 IU/ml resulted in less pain during the LLETZ procedure with no effect on blood loss or HAD anxiety scores (Cruickshank 2005).
EMLA local anaesthetic cream did not result in better pain relief compared to placebo (Sarkar 1993). Spraying of the cervix with cocaine spray before treatment resulted in better pain relief and less troublesome bleeding (Mikhail 1988). Use of topical gel (benzocaine 20%) (Lipscomb 1995) or lignocaine spray in addition to standard cervical injection prilocaine 30 mg/ml plus felypressin 0.03 IU/ml did not result in any benefit (Connell 2000).
On comparison of different preparations of local anaesthetic mixed with vasoconstrictor, prilocaine with felypressin did not differ from lignocaine with adrenaline for its effect on pain control (Howells 2000). Mean observed blood loss was less in the lignocaine with adrenaline group compared with the prilocaine with felypressin group.
The use of TENS on its own or combined with local anaesthetic injection during cervical laser therapy did not appear to be of any benefit (Crompton 1992).
The trials reporting these outcomes noticed no serious adverse effects. The reported adverse effects were feeling faint, shaking, dizziness, abdominal cramps, sweating, feeling hot, weakness, and moderate, transient hypertension. Prilocaine with felypressin caused fewer adverse effects (mainly shaking and fainting) than lignocaine with adrenaline in one trial (Howells 2000).
Overall completeness and applicability of evidence
This review consisted of many single trial analyses of small numbers of women, which limits the conclusions that can be drawn. Some of the trials included use of more than one type of pain relief intervention such as preoperative oral analgesics in addition to cervical infiltration. In modern day colposcopy practice, commonly used interventions for pain relief are local anaesthetic infiltration with vasopressin followed by large loop excision of the cervix, cryotherapy, laser ablation or conisation with a knife. In order to improve quantification of the benefits of these interventions in relief of pain and other symptoms (blood loss, etc.) without adverse effects, larger RCTs are required.
Measurement of pain
The trials used several validated scales for the measurement of pain, which may influence the accuracy of the outcome as complexity of the rating task for the measure influences the sensitivity and specificity. It is thought that a VAS reflects pain experienced during operative procedures more accurately (Huskisson 1983). Fifteen trials used VAS to report pain relief (Al‐Kurdi 1985; Connell 2000; Cruickshank 2005; Frega 1994; Johnson 1989; Johnson 1996; Kizer 2014; Lee 1986; Lipscomb 1995; Mikhail 1988; Rogstad 1992; Sammarco 1993; Sarkar 1993; Vanichtantikul 2013; Winters 2009). Sammarco 1993 reported VAS using an 11‐point scale rather than 0 to 100 VAS scoring system. Similarly, Vanichtantikul 2013 reported pain scores on VAS using a 0 to 10 cm scale. In addition to VAS, two trials reported pain relief as an objective score given by the attending nurse and laser operator on a categorical scale of 0 to 2 (Johnson 1989; Johnson 1996). The other trial scored pain by the attending colposcopist on a categorical scale (0 to 4) as well as by women undergoing treatment (0 to 5) (Howells 2000). Sarkar 1993 reported pain utilising McGill's pain questionnaire, on a categorical scale to grade pain, cramp and backache caused by the laser treatment. Five trials reported pain relief on VRS categorised as none, mild, moderate or severe (Al‐Kurdi 1985; Diakomanolis 1997; Duncan 2005; Lee 1986; Mikhail 1988).
There was an element of under reporting, especially where specific mean and SDs were not stated. Several trials reported pain as a graphical representation without numerical values, which is a form of under reporting. Such selective outcome reporting must be taken into consideration when interpreting the results. The trials of Johnson 1996, Connell 2000, and Sarkar 1993 reported pain relief as VAS, but the values were median and IQR rather than mean and SD and, therefore, these data could not be converted to mean pain scores. Similarly, Vanichtantikul 2013 reported mean and SD only for pain scores for the subgroup with loop size of 2 cm or more. Four trials reported pain relief outcomes as a graphical representation that required calculation of mean and SD (Frega 1994; Lee 1986; Mikhail 1988; Rogstad 1992). Three trials used graphical representation of data without numerical values (Frega 1994; Mikhail 1988; Lee 1986). The major limitation of the review and interpretation of the results was the presence of selective outcome reporting.
Quality of the evidence
We conducted GRADE assessments for the primary outcome. Overall, the quality of evidence was low for pain relief reported on a VAS. We downgraded the quality of evidence because of an unclear or high risk of bias with regards to allocation concealment, blinding of participants and personnel, incomplete outcome data and other biases.
This review incorporated evidence from 19 RCTs that assessed 1720 women. Effective pain relief from local anaesthesia is dependent on various factors, including route of administration, concentration and classification of drug, and the time interval between the administration of the analgesic and start of the procedure. These factors differed between the trials. This review was unable to establish the time interval between administration of injection and start of the procedure from the trial data.
Owing to the heterogeneity of the outcomes and treatments considered, there were many single trial analyses and limited consistent data available to carry out comparisons between trials. The majority of the included trials were underpowered to demonstrate a significant effect and some trials did not include a power calculation in their methodologies. As the majority of comparisons relied on single trials that were underpowered, the treatment effects should ideally be examined by conducting further studies.
Potential biases in the review process
We performed a comprehensive search, including electronic databases and a thorough search of the grey literature. Two review authors independently sifted all references and extracted data. We restricted the included studies to RCTs as they provide the strongest level of evidence available. Hence, we have attempted to reduce bias in the review process.
The greatest threat to the validity of the review is likely to be the possibility of publication bias (i.e. studies that did not find the treatments to have been effective may not have been published). We were unable to assess this possibility as the meta‐analyses included a limited number of the included trials (two out of 17 included trials).
Agreements and disagreements with other studies or reviews
The review authors found no other systematic reviews in this field and we did not identify any other retrospective controlled studies using these outcomes.
Authors' conclusions
Implications for practice.
Oral analgesia, EMLA cream (benzocaine gel, lignocaine‐prilocaine cream), transcutaneous electrical nerve stimulation (TENS), lignocaine spray or benzocaine gel did not provide any benefit in pain relief during cervical colposcopy treatment. Spraying of the cervix with cocaine spray before treatment resulted in better pain relief during the procedure and also less troublesome bleeding. Local anaesthetic agent combined with a vasoconstrictor agent resulted in better pain control compared with placebo and was associated with less blood loss. Buffered injection of lignocaine with adrenaline was not superior to non‐buffered lignocaine with adrenaline. Mean observed blood loss score was less with lignocaine plus adrenaline as compared with prilocaine plus felypressin. Direct cervical injection of local anaesthetic with a vasoconstrictor agent resulted in reduction in pain scores during treatment and should be considered for all cervical colposcopy treatment for cervical intraepithelial neoplasia (CIN). However, we could draw no conclusions with regards to optimum number of sites to inject in the cervix, depth of injection in the cervix (superficial, deep, or both) and dose of the agent used. In terms of adverse effects, combination of prilocaine with felypressin caused fewer adverse effects than lignocaine with adrenaline. Inhalation of gas mixture in addition to standard pain relief injection appears to have additional pain relief benefit. In routine clinical practice, intracervical injection of analgesic with a vasoconstrictor, particularly those related to vasopressin, appeared to be the optimum analgesia for treatment.
Implications for research.
Oral analgesia and the individual topical agents such as EMLA cream, lignocaine spray or benzocaine gel appeared to provide little benefit over placebo or no treatment for pain relief during colposcopy. However, this evidence came from small trials with methodological shortcomings, therefore, we consider this evidence to be of a low quality.
Further available evidence suggested that a local anaesthetic combined with a vasoconstrictor agent reduced pain and measured blood loss, therefore, this treatment should be offered to women undergoing colposcopy. This evidence was of moderate quality and further research will have an important impact on our confidence in these findings.
Further high‐quality, adequately powered trials should be undertaken in order to provide the data necessary to estimate the optimal route of administration and dose of local anaesthetics.
What's new
| Date | Event | Description |
|---|---|---|
| 8 June 2020 | Amended | Most recent search date 3 April 2020. One new study and one ongoing study has been identified but the new information is unlikely to change the review findings (see Studies awaiting classification). The conclusions of this Cochrane Review are therefore still considered up to date. |
History
Protocol first published: Issue 3, 2006 Review first published: Issue 10, 2012
| Date | Event | Description |
|---|---|---|
| 29 March 2016 | New search has been performed | Searches updated in March 2016. |
| 29 March 2016 | New citation required but conclusions have not changed | Two new studies identified for inclusion; overall conclusions remain unchanged. |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
| 24 June 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Jane Hayes and Jo Platt, Information Specialists, for designing and running the search strategies, Clare Jess and Gail Quinn, Managing Editors, for their contributions to the editorial process and Jo Morrison, Co‐ordinating Editor, for her clinical and editorial advice.
The National Institute for Health Research supported this project via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Analgesia explode all trees #2 MeSH descriptor Anesthetics explode all trees #3 MeSH descriptor Analgesics explode all trees #4 MeSH descriptor Pain explode all trees with qualifiers: DT,TH #5 MeSH descriptor Anti‐Inflammatory Agents, Non‐Steroidal explode all trees #6 analgesia or analgesic* #7 anesthetic* or anaesthetic* #8 anti‐inflammator* #9 transcutaneous electrical nerve stimulation or TENS #10 pain next/3 (relief or drug* or therap* or treat*) #11 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10) #12 MeSH descriptor Uterine Cervical Neoplasms explode all trees #13 MeSH descriptor Cervical Intraepithelial Neoplasia explode all trees #14 MeSH descriptor Uterine Cervical Dysplasia explode all trees #15 cervi* near/5 (intraepithel* or dysplasia or cancer* or tumor* or tumour* or neoplas* or carcinoma* or malignan*) #16 CIN or CIN1 or CIN2 or CIN3 #17 (#12 OR #13 OR #14 OR #15 OR #16) #18 MeSH descriptor Colposcopy explode all trees #19 colposcop* #20 LLETZ #21 LEEP #22 excis* or ablat* or laser* or cryosurg* #23 (#18 OR #19 OR #20 OR #21 OR #22) #24 (#11 AND #17 AND #23)
Appendix 2. MEDLINE search strategy
MEDLINE Ovid
1 exp Analgesia/ 2 exp Anesthetics/ 3 exp Analgesics/ 4 exp Pain/dt, th [Drug Therapy, Therapy] 5 exp Anti‐Inflammatory Agents, Non‐Steroidal/ 6 (pain adj3 (relief or drug* or therap* or treat*)).mp. 7 (analgesia or analgesic*).mp. 8 (anesthetic* or anaesthetic*).mp. 9 anti‐inflammator*.mp. 10 (Transcutaneous Electrical Nerve Stimulation or TENS).mp. 11 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 12 exp Uterine Cervical Neoplasms/ 13 Cervical Intraepithelial Neoplasia/ 14 Uterine Cervical Dysplasia/ 15 (cervi* adj5 (intraepithel* or dysplasia or cancer* or tumor* or tumour* or neoplas* or carcinoma* or malignan*)).mp. 16 (CIN or CIN1 or CIN2 or CIN3).mp. 17 12 or 13 or 14 or 15 or 16 18 Colposcopy/ 19 colposcop*.mp. 20 LLETZ.mp. 21 LEEP.mp. 22 (excis* or ablat* or laser* or cryosurg*).mp. 23 18 or 19 or 20 or 21 or 22 24 11 and 17 and 23
key: mp=title, original title, abstract, name of substance word, subject heading word, unique identifier
Appendix 3. Embase search strategy
Embase Ovid
1 exp analgesia/ 2 exp anesthetic agent/ 3 exp analgesic agent/ 4 exp pain/dt, th [Drug Therapy, Therapy] 5 exp nonsteroid antiinflammatory agent/ 6 (pain adj3 (relief or drug* or therap* or treat*)).mp. 7 (analgesia or analgesic*).mp. 8 (anesthetic* or anaesthetic*).mp. 9 anti‐inflammator*.mp. 10 transcutaneous nerve stimulation/ 11 (transcutaneous electrical nerve stimulation or TENS).mp. 12 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 13 exp uterine cervix tumor/ 14 uterine cervix dysplasia/ 15 uterine cervix carcinoma in situ/ 16 (cervi* adj5 (intraepithel* or dysplasia or cancer* or tumor* or tumour* or neoplas* or carcinoma* or malignan*)).mp. 17 (CIN or CIN1 or CIN2 or CIN3).mp. 18 13 or 14 or 15 or 16 or 17 19 colposcopy/ 20 colposcop*.mp. 21 LLETZ.mp. 22 LEEP.mp. 23 (excis* or ablat* or laser* or cryosurg*).mp. 24 19 or 20 or 21 or 22 or 23 25 12 and 18 and 24
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer
Data and analyses
Comparison 1. Local anaesthetic (lignocaine 2% injection) versus control (saline injection).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain scores during procedure (VAS) | 2 | 130 | Mean Difference (IV, Random, 95% CI) | ‐13.74 [‐34.32, 6.83] |
| 1.1.1 Paracervical block vs. placebo | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐3.00 [‐16.03, 10.03] |
| 1.1.2 Direct cervical infiltration vs. placebo | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐24.00 [‐35.44, ‐12.56] |
| 1.2 Moderate to severe pain | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Comparison 2. Local anaesthetic plus vasoconstrictor versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Pain scores during procedure (VAS: 0‐100) | 2 | 95 | Mean Difference (IV, Random, 95% CI) | ‐23.73 [‐37.53, ‐9.93] |
| 2.1.1 Lignocaine + adrenaline | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐31.10 [‐43.74, ‐18.46] |
| 2.1.2 Prilocaine + felypressin | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐17.00 [‐28.19, ‐5.81] |
| 2.2 Moderate or severe pain | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.2.1 vs. placebo | 1 | 92 | Risk Ratio (IV, Random, 95% CI) | 0.12 [0.04, 0.37] |
| 2.2.2 vs. no treatment | 1 | 50 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.42, 1.27] |
| 2.3 Troublesome bleeding | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Comparison 3. Local anaesthetic plus vasoconstrictor versus local anaesthetic injection alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Moderate or severe pain | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3.2 Blood loss (volume) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.3 Duration of treatment | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 4. Prilocaine plus felypressin versus lignocaine plus adrenaline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Pain (using 6 category scale) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.2 Blood loss (0‐5 scale) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 5. Deep plus superficial versus deep cervical injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Pain scores during procedure (VAS: 0‐100) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 6. Oral analgesic versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Pain scores (VAS: 0‐100) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1.1 vs. placebo | 2 | 129 | Mean Difference (IV, Random, 95% CI) | ‐3.51 [‐10.03, 3.01] |
| 6.1.2 vs. no treatment | 1 | 32 | Mean Difference (IV, Random, 95% CI) | ‐4.00 [‐13.69, 5.69] |
| 6.2 Moderate to severe pain | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6.3 Pain relief required in first 24 hours | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Comparison 7. Inhalation analgesia versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Pain scores (VAS: 0‐100) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.2 Heavy vaginal bleeding | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 7.3 Anxiety ‐ HAD score | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 8. Topical application versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Pain scores during procedure (VAS: 0‐100) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.2 Duration of treatment | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 9. Cocaine spray versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Pain scores during procedure (VAS: 0‐100) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 9.2 Moderate to severe pain | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 9.3 Troublesome bleeding | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Comparison 10. TENS versus TENS plus local anaesthetic injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Troublesome blood loss | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Comparison 11. TENS versus local anaesthetic injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Troublesome blood loss | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Comparison 12. TENS plus local versus local anaesthetic injection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Troublesome blood loss | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Comparison 13. Buffered versus non‐buffered local anaesthetic with vasoconstrictor.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Pain scores (VAS: 0‐100) | 1 | 52 | Mean Difference (IV, Random, 95% CI) | ‐8.00 [‐17.57, 1.57] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Kurdi 1985.
| Study characteristics | ||
| Methods | Prospective randomised double‐blind trial Setting: single centre |
|
| Participants | 97 women satisfied the inclusion criteria and were entered into the study. 50 were allotted naproxen sodium treatment and 47 were given a placebo. Women were generally healthy and undergoing CO2 laser treatment for CIN for the first time Exclusion criteria: pregnancy, lactation, a history of bronchial asthma or allergic diathesis and concomitant use of highly protein bound drugs All women were assessed following laser treatment but 2 women from the naproxen sodium group failed to return their 24‐hour questionnaire Age: 18‐50 years 3 women from each group failed to complete their laser treatment because of pain and were subsequently given local or general anaesthetics. Their response to the laser treatment was recorded and included in the analysis |
|
| Interventions | Intervention: 2 naproxen sodium 550 mg or Comparison: 2 placebo tablets In both groups, treatment given at least 30 minutes before the CO2 laser treatment of the cervix was performed. The procedure mainly started within 60 minutes of taking the tablets. Laser treatment was performed as previously described (Lowles 1983), and the duration of laser treatment and laser working time were recorded |
|
| Outcomes | VAS: a 10‐cm VAS, which ranged from no pain to the worst pain ever experienced Pain intensity measured using both a VAS and VRS (none, very slight, mild, moderate, severe) Speed of procedure reported as total treatment time Various other outcomes not specified in our protocol |
|
| Notes | Analgesic use following treatment (in the naproxen sodium group only 2/48 women used analgesics compared to 17/47 women in the placebo group) Self reported adverse effects were very minor (aches and pains at 24 hours) and not included in analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Unclear risk | Not reported |
| Allocation: sequence concealment | Unclear risk | Not reported |
| Performance and detection: blinding All outcomes | Unclear risk | Not reported |
| Detection: blind outcome assessment All outcomes | Unclear risk | Not reported |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 97/97 (100%) women analysed for pain. 2 women in treatment arm did not reply to 24‐hour questionnaire, but we assessed women immediately after treatment to eliminate recall bias |
| Reporting: unreported outcomes | Low risk | Pertinent outcomes were reported in the trial |
| Other: anything else | Low risk | No additional form of bias was likely |
Connell 2000.
| Study characteristics | ||
| Methods | Prospective, randomised, double‐blind, placebo‐controlled study Setting: colposcopy clinics at teaching hospital |
|
| Participants | Women undergoing biopsy or loop excision under local anaesthetic for cytological abnormalities. Of the 51 women entered into the study, 19 had a biopsy performed and were excluded from analysis. 32 had a LLETZ and were included for analysis. 16 were randomised to receive solution A (lignocaine spray) and 16 received solution B (saline). 2 women in group failed to complete the second VAS so 30 women were included in the final analysis ‐ 15 in each of 2 groups Age: 20‐64 years |
|
| Interventions | Intervention: lignocaine hydrochloride 10% spray (labelled 'A') Comparison: saline (labelled 'B') Multiple atomiser bottles made up with solution and labelled 'A' or 'B'. The spray was primed and operator depressed the spray 4 times applying approximately 0.5 ml of solution to the cervix. At least 1 minute later, 1.1 ml of local anaesthetic (prilocaine hydrochloride 30 mg/ml + felypressin 0.54 μg/ml) was injected with a dental syringe and needle. In the LLETZ group injection was into 4 quadrants, the total volume being 4.4 ml |
|
| Outcomes | Pain measured using 100‐mm VAS score. The women marked the line with a cross as soon as the injections had been performed Pain assessed with a 4‐point categorical scale: 1 = not painful; 2 = slightly painful; 3 = moderately painful; 4 = severely painful |
|
| Notes | Pain was only reported on VAS. The outcome reported on categorical scale was not included in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Low risk | "Randomisation was by stratified computer‐generated numbers" |
| Allocation: sequence concealment | Low risk | To ensure blinding, the bottles made up by a pharmacist who also sealed the code in an envelope |
| Performance and detection: blinding All outcomes | Low risk | The attending doctor, the nursing staff, the woman and the investigator all blinded to the identification of the solution used |
| Detection: blind outcome assessment All outcomes | Unclear risk | Not reported |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 94% (30/32) women analysed for all outcomes |
| Reporting: unreported outcomes | High risk | Blood loss and duration of procedure not reported. Outcome for categorical scale of pain relief not available to carry out comparison |
| Other: anything else | Low risk | No additional risk of bias likely |
Crompton 1992.
| Study characteristics | ||
| Methods | Prospective randomised 3‐arm controlled clinical trial Setting: colposcopy unit adapted to run randomised trials |
|
| Participants | 100 women with a colposcopic diagnosis of CIN recruited. They had a gynaecological interview, colposcopy and a colposcopically directed biopsy. Linear analogue anxiety and HAD anxiety/depression personality trait scores (Zigmond 1983), age and number of vaginal deliveries recorded to assess group comparability Exclusion criteria: history of treatment for CIN, other cervical surgery or pelvic inflammatory disease, postmenopausal women and women with cardiac pacemakers. 2 other women refused to enter the trial Mean (SD) age at trial entry: TENS alone: n = 34, 31.8 years (SD = 9); local anaesthetic alone: n = 35, 32.6 years (SD = 9); TENS + local anaesthetic: n = 29, 30.1 years (SD = 8) % women who were nullipara: TENS alone: 48%; local anaesthetic alone: 44%; TENS + local anaesthetic: 35% Median anxiety HAD score (interquartile range): TENS alone: 6 (5 to 11), local anaesthetic alone: 7 (4 to 9), TENS + local anaesthetic: 6.5 (4 to 8) Median depression HAD score (interquartile range): TENS alone: 3 (1 to 4), local anaesthetic alone: 2 (1 to 4), TENS + local anaesthetic: 3 (1 to 3) |
|
| Interventions | Women were allocated to 1 of 3 groups:
2 ml of lignocaine 2% + Octapressin was injected from a dental syringe via a 30‐gauge needle into 4 points on the TZ to a depth of 3 to 5 mm. Microtens TENS pads (Neen Pain Management Systems, Norfolk, UK) were applied 20 minutes before treatment. 4 conductive silicone polymer electrodes were applied using conducting gel and tape fixative; 2 anterior to the abdominal wall just above the symphysis pubis and 1 on each side of the sacrum. The electrodes were connected to an 80Hz nerve stimulator (pulse width 210 µs) by a cable. The single channel amplitude control was activated by the women under instruction. Initially they were encouraged to experience a tingling sensation and then they increased the amplitude until it became uncomfortable. They were given approximately 20 minutes to experiment with the device until they were called into the second room for laser treatment. All the treatments were carried out in this second room by a second operator. The entire ectocervical TZ was either ablated to a depth of approximately 7 mm or excised with the aid of skin hooks using a 35W CO2 laser (spot size 1.5 mm) |
|
| Outcomes | At end of procedure, surgeon gave a further explanation of the treatment and scored the pain experienced by the women using 120mm VAS. The scores were converted into percentages At end of procedure, the women offered TENS were given a simple questionnaire. They were asked to answer 'Yes' or 'No' to indicate whether or not they found the TENS each of the following: (1) comfortable, (2) unpleasant, (3) helpful, (4) frightening, (5) soothing or (6) pain relieving |
|
| Notes | Median pain score was on 120mm VAS; however, authors converted it to percentage for reporting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Unclear risk | Not reported |
| Allocation: sequence concealment | High risk | "The block randomisation code was held by one investigator who then allocated treatment" |
| Performance and detection: blinding All outcomes | High risk | "It was impossible to conceal the use of TENS from the surgeon and patients but we had intended to 'blind' the attendants to the use of local anaesthesia. Injections of lignocaine were given in a separate room before the laser surgery was carried out by a different attendant but the surgeon was able to identify points where local anaesthetic had been given" |
| Detection: blind outcome assessment All outcomes | Unclear risk | Not reported |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 98% (98/100) women analysed for all outcomes |
| Reporting: unreported outcomes | Unclear risk | Median pain scores reported rather than mean and adverse events were not reported |
| Other: anything else | Unclear risk | Insufficient information to permit judgement |
Cruickshank 2005.
| Study characteristics | ||
| Methods | Prospective double‐blind RCT Setting: colposcopy clinic serving a regional population in single‐centre setting |
|
| Participants | 396 women scheduled for treatment of CIN by LLETZ. All women attending for investigation of an abnormal smear were screened and women suitable for treatment at their first visit ('see and treat'). Most women were seen for initial colposcopic assessment with directed punch biopsies only and treatment at a later appointment if necessary Exclusion criteria: pregnancy, currently taking a monoamine oxidase inhibitor or if they had to drive home from the clinic themselves Mean age at trial entry: 32.7 years (SD 9.8) in the isoflurane + desflurane group and 31.5 (SD 9.1) in the placebo group Deprivation score details (395 women): Class 1 (least deprived): 82 (20.7%); Class 2: 72 (18.2%); Class 3: 53 (13.4%); Class 4: 37 (9.4%); Class 5: 36 (9.1%); Class 6: 16 (4.0%); Class 7: 46 (11.6%); not classified: 53 (13.4%) Parity details: no children: 158 (40.3%); 1 to 5 children: 234 (59.7%) |
|
| Interventions | Intervention: isoflurane + desflurane gases (n = 195) Comparison: placebo (air) (n = 194) Both gases were self administered using a demand valve regulator (Ohmeda) as is used for Entonox. Slight odour of the trial gas was masked by a small amount of peppermint oil smeared inside the facemask for intervention and placebo gas administration. Women instructed to use the gas before the procedure began and to continue to use the gas according to their own requirements. Exhaled gas scavenged using standard equipment (Ohmeda). Infiltration of the cervix with prilocaine hydrochloride (30 mg/ml) + Octapressin (prilocaine 30 mg/ml + felypressin 0.03 IU/ml) (0.54 mg/ml) was started approximately 2 minutes after the start of inhalation. 2 to 3 ampoules were used at the clinical discretion of the colposcopist depending on the size of the cervical lesion. A number of different colposcopists performed treatment and were evenly distributed between the 2 arms |
|
| Outcomes | Pain measured using VAS (0 to 100, where 100 was worst pain imaginable) Heavy vaginal bleeding Anxiety using HAD Various other outcomes not specified in our protocol |
|
| Notes | Women were followed up immediately after colposcopy and at 6 months after. We did not report 6‐month data as recall bias was likely to be a problem "Took pain killer for stomach pain" ‐ this outcome did not mention the time limit from procedure and so it was excluded from analysis (isoflurane + desflurane: 66/175; placebo group: 66/173) 9/175 women in isoflurane + desflurane group and 7/173 women in placebo group had difficulty returning to normal activity after colposcopy 14/175 women in isoflurane + desflurane group and 15/173 women in placebo group contacted on‐call service with problem related to treatment These 2 outcomes were not included in forest plots since descriptions were vague and full details were not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Low risk | "The random allocation of women to the cylinder code used computer‐generated random numbers" |
| Allocation: sequence concealment | Low risk | "The random allocation of women ... used ... a series of opaque sequentially numbered envelopes" |
| Performance and detection: blinding All outcomes | Low risk | "The trial and clinic staff and trial participants were blinded to the contents of the cylinders, and peppermint oil was applied to the facemask prior to use" |
| Detection: blind outcome assessment All outcomes | Low risk | "The subject matter was tabulated by an assessor blinded to the randomisation of each individual" |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 348/395 (88%) women analysed for heavy vaginal bleeding outcome. Other outcomes assessed > 88% of women in the trial |
| Reporting: unreported outcomes | High risk | Adverse events of gas not reported |
| Other: anything else | Low risk | No additional form of bias likely |
Diakomanolis 1997.
| Study characteristics | ||
| Methods | Randomised double‐blind study Setting: single centre |
|
| Participants | 100 women randomly allocated to 1 of 2 groups. All underwent laser excision of TZ for CIN. All women included in the study had abnormal Pap smears, abnormal colposcopic findings, histologically confirmed CIN and were premenopausal Exclusion criteria: history of coronary disease, epilepsy and chronic hypertension Median age vasoconstrictor + lignocaine group: 28 years (range 17 to 50) Median age lignocaine only group: 28.5 years (range 19 to 51) |
|
| Interventions | Intervention: vasoconstrictor + lignocaine:50 women who underwent laser excision using 30 ml of a 1:30 POR8 (vasoconstrictor) + lignocaine 1% solution. The ectocervix was infiltrated with solution just before the start of procedure using a 30 gauge dental needle on a dental syringe to a depth of 3 to 4 cm Comparison: lignocaine only: 50 women who underwent laser excision received 30 ml of lignocaine 1% solution |
|
| Outcomes | Intraoperative blood loss measured with a glass blood measure (maximum volume 60 ml) (used in paediatric surgery) set in the suction apparatus Postoperative haemorrhage measured with weighing the blood that soaked the pads Early haemorrhage defined as bleeding occurring within 4 days of operation that required intervention to stop bleeding Late haemorrhage defined as after 4 days Pain relief recorded as VRS (none, moderate and severe) postoperatively Operative time of each procedure. After the procedure, all women were contacted by telephone 1 week later |
|
| Notes | Other outcomes were not included in analysis e.g. hypertension. Hypertension occurred in 7 women in vasoconstrictor + lignocaine group and 2 women in lignocaine only group Note: the 30 ml of local anaesthetic + vasoconstrictor or local anaesthetic alone is considered a higher than average amount used to infiltrate the cervix in pain relief for colposcopic management |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Unclear risk | Not reported |
| Allocation: sequence concealment | Low risk | "The randomization was performed as the central pharmacy of the hospital during the preparation and distribution of both medications used" |
| Performance and detection: blinding All outcomes | Low risk | "The surgeon was not aware of the medication that was used" |
| Detection: blind outcome assessment All outcomes | Unclear risk | Not reported |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 100/100 (100%) women analysed for all outcomes |
| Reporting: unreported outcomes | High risk | Pain was not analysed using VAS |
| Other: anything else | Unclear risk | An additional form of bias was unlikely |
Duncan 2005.
| Study characteristics | ||
| Methods | Double‐blind randomised prospective placebo‐controlled trial Setting: single centre |
|
| Participants | Out of 100 women who met the criteria and approached 93 were enrolled in the study. 46 in intervention arm and 47 in comparison arm. 100 consecutive women attending the colposcopy clinic and expected to undergo colposcopically directed biopsy and treatment with Semm coagulator were approached. 7 did not meet the eligibility criteria Exclusion criteria: history of allergy to local anaesthetic, unsuitable for treatment at first colposcopy examination, previous treatment to cervix or pregnant Mean (SD) age at trial entry: intervention: n = 46, mean age = 31.3 years (SD 8.4); comparison: n = 47, mean age = 32.6 years (SD 8.0) Nullipara: intervention: 14 women (30.4%); comparison: 10 women (21.3%) Married/cohabiting: intervention: 20 women (43.5%); comparison: 22 women (46.8%) CIN (1/2/3/unspecified) details (number (%)):
|
|
| Interventions | Intervention: prilocaine 3% (30 mg/ml) + felypressin 0.03 IU/ml Comparison: normal saline Externally identical numbered 5 ml vials of prilocaine + felypressin or normal saline prepared in‐house in pharmacy department along with randomised opaque sealed envelopes each containing number of vial. Colposcopic examinations performed by 1 of the authors. Once treatment decision was taken, vial opened from sealed envelope and injected circumferentially in TZ of cervix. Volume noted. Treatment performed with SEMM coagulator |
|
| Outcomes | Pain was recorded on 11 point analogue scale where 0 was no pain at all and 10 indicated the worst pain imaginable. Each woman was asked to complete 4 such scales: expected and actual sensation for biopsy and treatment. Pain scores of 1 to 3 were classified as mild, 4 to 7 as moderate and 8 to 10 as severe | |
| Notes | Details of anticipated pain was excluded from the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Unclear risk | Not reported |
| Allocation: sequence concealment | Low risk | "Externally identical, numbered vials of active medications or normal saline were prepared by the in house pharmacy department along with randomised opaque sealed envelopes, each containing the number of a vial. Pharmacy retained the key to the vial contents until the end of the trial" |
| Performance and detection: blinding All outcomes | Unclear risk | Labelled as double‐blind placebo‐controlled trial, but details were not documented in materials and methods section |
| Detection: blind outcome assessment All outcomes | Unclear risk | See above |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 92/93 (99%) women analysed for pain related to treatment. Data from 1 woman missing in intervention group |
| Reporting: unreported outcomes | High risk | Adverse event not reported |
| Other: anything else | Low risk | An additional form of bias unlikely |
Frega 1994.
| Study characteristics | ||
| Methods | Randomised study | |
| Participants | 63 women affected by CIN of various degrees were randomly divided into 1 of 3 groups in order to evaluate the pain experienced during laser vaporisation of the lesion. All women premenopausal. Aged: 19 to 39 years |
|
| Interventions | Intervention: naproxen sodium 550 mg 30 minutes before treatment (n = 21) Comparison 1: placebo 30 minutes before treatment (n = 21) Comparison 2: no drug (n = 21) |
|
| Outcomes | Severity of pain assessed using 0 to 100 mm VAS at end of procedure | |
| Notes | Mean and SD for each group was calculated from figure 1 on page 189 of the publication, using GraphPad Prism software package, where the graphs were enlarged allowing an accurate estimate of each women's pain score Since the trial included 3 arms, the shared intervention group was divided out approximately evenly among the comparisons. Hence, for pain outcome on VAS, the total number of women in the drug group was divided up into 2 (the total number of 21 in the group was halved and rounded up to 11) and the means and SDs were left unchanged (see Higgins 2011, Chapter 16.5.4) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Unclear risk | Not reported |
| Allocation: sequence concealment | Unclear risk | Not reported |
| Performance and detection: blinding All outcomes | Unclear risk | Not reported |
| Detection: blind outcome assessment All outcomes | Unclear risk | Not reported |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 63/63 (100%) women analysed for all outcomes |
| Reporting: unreported outcomes | High risk | Median rather than mean used for pain. Adverse events not reported |
| Other: anything else | Unclear risk | Insufficient information to permit judgement |
Howells 2000.
| Study characteristics | ||
| Methods | Prospective RCT Setting: colposcopy clinic |
|
| Participants | 200 consecutive women referred by general practitioners with abnormal cervical cytology (n = 180) or clinically suspicious abnormality (n = 20) enrolled. Inclusion criteria: women aged 20 to 60 years; no previous treatment to the cervix who required treatment Mean age (SD) (prilocaine + felypressin vs. lignocaine + adrenaline): 94 women; 36.6 years (10.3) vs. 106 women; 34.6 years (9.7) Menopausal status (prilocaine + felypressin vs. lignocaine + adrenaline): premenopausal: 81 (86%) vs. 96 (91%); postmenopausal: 12 (13%) vs. 9 (8%); missing data: 1 (1%) vs. 1 (1%) Contraception (prilocaine + felypressin vs. lignocaine + adrenaline): no: 37 (39%) vs. 44 (42%); yes: 61 (65%) vs. 76 (72%); missing data: 0 vs. 1 (1%) Smear grade (prilocaine + felypressin vs. lignocaine + adrenaline):
Nullipara (prilocaine + felypressin vs. lignocaine + adrenaline): 17 (18%) vs. 24 (23%) Colposcopic findings (prilocaine +us felypressin vs. lignocaine + adrenaline): normal: 13 (14%) vs. 15 (14%); low grade: 25 (27%) vs. 29 (27%); high grade: 49 (52%) vs. 53 (50%); uncertain: 5 (5%) vs. 7 (7%); ? invasion: 1 (1%) vs. 1 (1%); missing data: 1 (1%) vs. 1 (1%) Final histology (prilocaine + felypressin vs. lignocaine + adrenaline): normal: 4 (4%) vs. 8 (8%); low grade: 36 (38%) vs. 31 (29%); high grade: 51 (55%) vs. 63 (59%); others: 3 (3%) vs. 2 (2%); missing data: 0 vs. 2 (2%) Final histology negative (prilocaine + felypressin vs. lignocaine + adrenaline): 4/94 (4%) vs. 8/106 (8%); exclusion from analysis not possible so included in analysis Local anaesthetic volume (ml) (prilocaine + felypressin vs. lignocaine + adrenaline): 5.02 ml vs. 4.83 ml Loop passes (prilocaine + felypressin vs. lignocaine + adrenaline): 1 pass: 71 (76%) vs. 80 (75%); 2 passes: 19 (20%) vs. 18 (17%); 3 passes: 2 (2%) vs. 4 (4%) Loop size (prilocaine + felypressin vs. lignocaine + adrenaline): small: 10 (11%) vs. 14 (13%); medium: 80 (85%) vs. 87 (82%); large: 3 (3%) vs. 1 (1%); missing data: 1 (1%) vs. 4 (4%) |
|
| Interventions | Intervention (n = 94): prilocaine 3% (30 mg/ml) + felypressin 0.03 IU/ml Comparison (n = 106): lignocaine (xylocaine) 2% + adrenaline 1:80,000 |
|
| Outcomes | Duration of treatment calculated from start of the loop excision to end of ball diathermy used to achieve haemostasis Discomfort: colposcopist scored his or her perception of the discomfort experienced by women in a scale of ordered categories (0 = none; 4 = severe) Degree of bleeding: colposcopist scored degree of bleeding caused by the procedure (0 = none; 5 = heavy) Perception of pain: following treatment, women answered a questionnaire on their perception of pain during the administration of the local anaesthetic and during their treatment in a scale of ordered categories (0 = none; 5 = unbearable) Other adverse effects, such as feeling faint, nausea and shaking, scored in a similar way (0 = none; 5 = a great deal). The scores were then added to derive an overall score |
|
| Notes | Missing data (prilocaine + felypressin vs. lignocaine + adrenaline): 1 (1%) vs. 2 (2%) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Unclear risk | "The women were randomised by an independent observer using simple randomisation" |
| Allocation: sequence concealment | Low risk | "The women were randomised ... using simple randomisation with opaque sealed envelopes" |
| Performance and detection: blinding All outcomes | High risk | "The colposcopists were aware of the identity of the local anaesthetic solutions" |
| Detection: blind outcome assessment All outcomes | Unclear risk | Not reported |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 200/200 (100%) women |
| Reporting: unreported outcomes | High risk | Trial authors reported important outcomes but these could have been reported using more appropriate methods (e.g. continuous data for pain and blood loss, rather than using logistic regression for non‐parametric data) |
| Other: anything else | Low risk | No additional form of bias likely |
Johnson 1989.
| Study characteristics | ||
| Methods | Prospective double‐blind randomised placebo‐controlled clinical trial | |
| Participants | 70 women with a new colposcopic and histological diagnosis of a cervical dysplastic lesion suitable for laser ablation Exclusion criteria: previous cervical surgery, > 1 colposcopic examination, menopausal or peri‐menopausal status, sensitivity to lignocaine, woman refusing paracervical injection or refusing to be recruited into the trial, or vaginal involvement of the lesion |
|
| Interventions | Intervention: lignocaine 2% Comparison: normal saline Numbered vials. A bilateral paracervical block was delivered by injecting 10 ml into the paracervical tissues |
|
| Outcomes | Pain: at end of procedure and after a further explanation, the women scored their pain on a 120 mm VAS Pain: objectively scored by attending nurse who assessed the woman's level of vocalisation (2 = moan/cry; 1 = gasp; 0 = no vocalisation), muscle tension of the upper limbs (2 = the clenching the bed etc.; 1 = making a fist; 0 = relaxed), thigh movements (2 = adduction; 1 = twitchy; 0 = relaxed) Movements of the thigh and perineal movement (2 = bottom movement up the bed; 1 = speculum twitches; 0 = no movement/relaxed) scored by laser operator. Size of the TZ Blood loss Anxiety and depression HAD scores Premenstrual syndrome scores |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Low risk | "Consenting patients were then randomised to receive either 2% lignocaine or normal saline from a numbered vial. Each vial could only be identified at the end of the study by its number which was allocated prospectively according to a block randomised code" |
| Allocation: sequence concealment | Low risk | "Each vial could only be identified at the end of the study by its number which was allocated prospectively" |
| Performance and detection: blinding All outcomes | Low risk | Trial labelled as a placebo‐controlled double‐blind trial. "Laser ablation of the entire transformation zone to a depth of approximately 7 mm was performed with a continuous fine beam (spot size 1.5 mm) 35 W CO, laser by a separate surgeon in a second suite" |
| Detection: blind outcome assessment All outcomes | Low risk | "Pain was objectively scored by the attending nurse who assessed the woman's level of vocalization ... muscle tension of the upper limbs, thigh movements ... The laser operator independently scored movements of the thigh as well as perineal movement" |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 70/70 (100%) women |
| Reporting: unreported outcomes | High risk | Adverse events of paracervical injections not reported, Pain inadequately reported |
| Other: anything else | Low risk | No additional form of bias likely |
Johnson 1996.
| Study characteristics | ||
| Methods | Double‐blind randomised clinical trial Setting: colposcopic clinic specifically adapted to run clinical trials |
|
| Participants | 44 (23 in intervention group and 21 in comparison group) women referred following abnormal smear and underwent colposcopic examination and biopsy before being recruited. No participant refused entry in the trial but trial terminated prematurely when the laser surgeon realised that he could identify women given direct infiltration by looking for the injection mark Exclusion criteria: past cervical surgery, past cervical atypia, vaginal involvement with lesion, menopausal, reluctance to take part in trial |
|
| Interventions | Intervention: 10 ml of paracervical lignocaine 2% injection Comparison: 2 ml of lignocaine 2% injection directly into the TZ |
|
| Outcomes | Pain scored on VAS at the end of the procedure by women Pain objectively scored by attending nurse and laser operator independently |
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Low risk | "Consenting women were block randomised to receive either 10ml of paracervical 2% lignocaine or 2ml of 2% lignocaine injected directly into the transformation zone" |
| Allocation: sequence concealment | Low risk | "Neither nurses, clerical officers responsible for appointments, nor the laser surgeon had access to this code. The worker responsible for randomisation obtained consent, drew the allocation code from a box" |
| Performance and detection: blinding All outcomes | Low risk | "The worker responsible for randomisation ... gave the local anaesthetic in a room separate from the laser suite" |
| Detection: blind outcome assessment All outcomes | Low risk | "Pain was objectively scored by the attending nurse who assessed the woman's level of vocalization ... muscle tension of the upper limbs and thigh movements. The laser operator independently scored movements of the thigh as well as perineal movement" |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 44/44 (100%) women |
| Reporting: unreported outcomes | High risk | Adverse events of paracervical injections not reported and pain inadequately reported |
| Other: anything else | Unclear risk | No woman refused entry to the trial, but study terminated prematurely when the laser surgeon realised that he could identify women given direct infiltration by looking for the injection marks. Up to this point, the study was a true double‐blind, randomised trial. This is not necessarily a source of bias but we were unsure whether any additional source of bias may have been present |
Kizer 2014.
| Study characteristics | ||
| Methods | Prospective double‐blind RCT Setting: single centre |
|
| Participants | Out of 58 women who were assessed for eligibility, 2 were excluded as they did not meet inclusion criteria and 4 women were excluded as they did not complete the questionnaire correctly. 52 women aged ≥ 18 years undergoing loop excision of the cervix were included in the analysis Exclusion criteria: anatomy unsuitable for safe loop excision procedure in clinic settings, inability to tolerate loop excision under local anaesthesia, pregnancy, inability to understand spoken or written English, refusal to consent, incarceration, mental incapacity, anticoagulant or antiplatelet therapy or known bleeding diathesis, and use of prescription analgesics within 7 days of scheduled loop excision Women taking low‐dose aspirin (81 mg) daily eligible Mean age (SD) in intervention group (n = 28): 32.3 years (7.6) Mean age (SD) in control group (n = 24): 32.4 years (10.3) |
|
| Interventions | Intervention: 1 ml of 1 mEq/ml (8.4%) solution of sodium bicarbonate buffer + 9 ml of lignocaine + adrenaline Comparison: 10 ml of lignocaine + adrenaline without the buffer Both injected into the cervix subepithelially using a 27 gauge needle before the loop excision of cervix. Prepared syringes used within 30 minutes of preparation |
|
| Outcomes | Pain intensity within 30 minutes of completion of the procedure and after instruction by the investigators by women 100 mm Likert VAS. Separate scales marked to represent the intensity of pain from injection itself, pain from loop excision and cramping. Pain scores reported as mean, SD, median and range | |
| Notes | 4 women from the comparison arm who did not complete the questionnaire correctly excluded from analysis. This information was not available in the paper but was obtained via the registered trials website page (NCT01405768; www.Clinicaltrials.gov) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Low risk | Computerised random number generation |
| Allocation: sequence concealment | Low risk | Treatment assignments sequenced accordingly, and prepared sealed opaque envelopes opened in order by a registered nurse who did not participate in the loop excision procedure |
| Performance and detection: blinding All outcomes | Low risk | Syringes for intervention and comparison groups prepared by a nurse in a separate room adjacent to the loop excision room. Neither the treating clinician nor the women aware of allocation |
| Detection: blind outcome assessment All outcomes | Low risk | Study members instructed participants to report intensity of pain on a 100 mm Likert scale within 30 minutes of completion of procedure. Participant's treatment allocation revealed to study personnel only once the data analysis completed |
| Attrition: incomplete outcome data All outcomes | High risk | 4 participants excluded as they did not complete the questionnaire correctly. All 4 women belonged to the comparison arm |
| Reporting: unreported outcomes | Low risk | Adverse events not reported in the paper but the online information from registered trials website page (NCT01405768; www.Clinicaltrials.gov) results suggested no adverse events |
| Other: anything else | Low risk | No other additional form of bias likely |
Lee 1986.
| Study characteristics | ||
| Methods | RCT | |
| Participants | 50 women undergoing laser vaporisation of cervix for CIN. All women were premenopausal Age: 19 to 39 years |
|
| Interventions | Intervention (n = 25): ectocervix was infiltrated with 2 ml prilocaine 3% + felypressin 0.03 IU/ml immediately before the procedure Comparison (n = 25): women received no analgesia or anaesthesia Using a 30 gauge dental needle on a dental syringe, infiltration around the periphery of the TZ performed immediately before the procedure. Local anaesthetic employed in the comparison group only when significant pain experienced |
|
| Outcomes | Severity of pain assessed at the end of the procedure using VAS and VRS. VAS consisted of a 100 mm line drawn on plain paper representing pain ranging from 'no pain at all' to 'pain as much as you can imagine'. Women marked a point on the line at the end of the procedure that they felt corresponded to the pain they experienced. The VRS consisted of a choice of 4 descriptions, none, mild, moderate or severe Blood loss during the procedure: recorded as none, slight, moderate and troublesome |
|
| Notes | Other outcome measures included pain while receiving the injection and the level of anxiety before the procedure was measured before the woman undressed using the Spielberger state anxiety inventory. Adverse effects such as sweating, nausea, dizziness and cramps also reported. This was not included in the analysis Mean and SD for each group calculated from figure 1 on page 968 of the publication, using GraphPad Prism software package, where the graph was enlarged allowing an accurate estimate of each woman's pain score |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Unclear risk | Not reported |
| Allocation: sequence concealment | Unclear risk | Not reported |
| Performance and detection: blinding All outcomes | Unclear risk | Not reported |
| Detection: blind outcome assessment All outcomes | Unclear risk | Not reported |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 50/50 (100%) women |
| Reporting: unreported outcomes | Low risk | Pertinent outcomes reported in the trial |
| Other: anything else | Unclear risk | No additional form of bias likely |
Lipscomb 1995.
| Study characteristics | ||
| Methods | Prospective double‐blind RCT | |
| Participants | 50 women scheduled for the loop excision for treatment of cervical dysplasia. All agreed to take part. Age and parity was comparable in both groups Age: mean (SD) (intervention vs. comparison): 29.5 years (10.5) vs. 28.4 years (8.9) Parity: mean (SD) (intervention vs. comparison): 2.1 (2.1) vs. 2.3 (1.6) Loop passes: mean (SD) (intervention vs. comparison): 1.2 (0.4) vs. 1.3 (0.6) Positive margins: mean (SD) (intervention vs. comparison): 2/25 vs. 3/25 |
|
| Interventions | Intervention (n = 25): cervical application of benzocaine 20% gel Comparison (n = 25): placebo gel before the procedure In addition, all women also received pre‐procedural oral analgesia ketorolac tromethamine 10 mg orally 30 minutes before procedure. After 1 minute of gel application, 1 ml of lignocaine 1% + adrenaline 1:100,000 injected in 1 ml volumes into the cervical stroma at the 12, 3, 6 and 9 o'clock positions (total 4 ml) with a 25 gauge needle on a needle extender |
|
| Outcomes | Pain: immediately after procedure, women rated pain from injection and pain from loop excision procedure on a standard VAS. 10 cm horizontal line with vertical cross bars at each endpoint. Endpoints labelled 'no pain' and 'worst pain possible' | |
| Notes | Other outcomes, e.g. number of passes of the loop or details of margins of the loop, not included for analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Low risk | "By use of computer‐generated numbers, patients were randomized to one of two groups" |
| Allocation: sequence concealment | Unclear risk | Not reported |
| Performance and detection: blinding All outcomes | Low risk | "Both patient and physician were unaware which gel the syringe contained" |
| Detection: blind outcome assessment All outcomes | Unclear risk | Not reported |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 50/50 (100%) women |
| Reporting: unreported outcomes | Unclear risk | Adverse events of gel not reported |
| Other: anything else | Low risk | No additional form of bias likely |
Mikhail 1988.
| Study characteristics | ||
| Methods | Randomised prospective double‐blind placebo controlled trial | |
| Participants | 50 women undergoing laser vaporisation of the cervix for CIN. Age: mean (SD) (intervention vs. comparison): 27.4 years (3.9) vs. 26.7 years (4.57) Parity: mean (SD): (intervention vs. comparison): 0.9 (1.24) vs. 1 (1) |
|
| Interventions | Intervention (n = 25): cervix sprayed with 3 to 4 ml of a cocaine 10% solution preserved in nipasept (a mixture of the methyl, ethyl and propyl esters of p‐hydroxybenzoic acid) Comparison (n = 25): sprayed with a similar quantity of the preservative alone No indication on the spray to identify the solution. When necessary, additional pain relief was given by the local infiltration of prilocaine by hypodermic injection. 1 to 2 ml of the solution sprayed on the cervix and repeated as necessary through the procedure |
|
| Outcomes | Time taken to complete the treatment and assessment of the blood loss Severity of the pain experienced assessed at end of procedure using standard 100mm VAS (Huskisson 1983) and VRS. VRS consisted of 4 categories ‐ none, mild, moderate or severe Blood loss assessed subjectively by the operator as minimal, moderate and severe |
|
| Notes | Mean and SD for each group calculated from figure 1 on page 471 of the publication, using GraphPad Prism software package, where the graph was enlarged allowing an accurate estimate of each woman's pain score | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Low risk | "The patients were allocated to their groups by a computer‐generated random list" |
| Allocation: sequence concealment | Unclear risk | Not reported |
| Performance and detection: blinding All outcomes | Low risk | "There was no indication on the spray to identify the solution ... The randomized and double‐blind nature of the trial eliminated observer bias" |
| Detection: blind outcome assessment All outcomes | Unclear risk | Not reported |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 50/50 (100%) women |
| Reporting: unreported outcomes | Low risk | No reason to suspect outcomes were selectively reported |
| Other: anything else | Low risk | No additional form of bias likely |
Rogstad 1992.
| Study characteristics | ||
| Methods | Randomised placebo‐controlled double‐blind trial | |
| Participants | 60 women who were scheduled to undergo cold coagulation for cervical abnormalities | |
| Interventions | Intervention (n = 21): cervix infiltrated with 2 ml of lignocaine 2% before cold coagulation Comparison (n = 31): cervix infiltrated with 2 ml of normal saline before cold coagulation |
|
| Outcomes | Degree of pain felt measured by VRS and VAS | |
| Notes | Other outcomes like pain of injection and 3 to 6 weeks' follow‐up questionnaire of pain and bleeding were excluded from the analysis Mean and SD for each group calculated from figure on page 942 of the publication, using GraphPad Prism software package, where the graph was enlarged allowing an accurate estimate of each woman's pain score |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Low risk | "The trial was randomised, placebo controlled and double‐blind. Randomisation was by computerised generation of random numbers" |
| Allocation: sequence concealment | Unclear risk | Not reported |
| Performance and detection: blinding All outcomes | Unclear risk | Labelled as double‐blind placebo‐controlled trial, but details not documented in paper |
| Detection: blind outcome assessment All outcomes | Unclear risk | Not reported |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 60/60 (100%) women analysed for pain outcome |
| Reporting: unreported outcomes | High risk | Adverse events not reported |
| Other: anything else | Unclear risk | Insufficient information to permit judgement |
Sammarco 1993.
| Study characteristics | ||
| Methods | Prospective RCT | |
| Participants | Each women was evaluated by colposcopy with biopsy and had a histological diagnosis of cervical dysplasia. They were scheduled to undergo cryosurgery. Cryosurgery was carried out with liquid nitrogen using Cryo 2000 (Valleylab, Boulder, Colorado) by double freeze technique with a 3 minute freeze and 5 minute thaw cycle Exclusion criteria: nulliparous women, girls < 16 years and women with allergies Women with no endocervical disease and lesions < 3 cm were eligible |
|
| Interventions | Both control and intervention group received a single dose of ketoprofen 75 mg (a non‐steroidal anti‐inflammatory drug) within 1 hour of procedure, 2 women received naproxen sodium 550 mg Intervention: injection of 2 to 3 ml of lignocaine + 1:100,000 dilution of adrenaline, which was administered submucosally at the 2 and 10 o'clock positions with 25 gauge needle 1 minute prior to the cryosurgery Comparison: no further analgesia |
|
| Outcomes | Pain by VAS with 0 representing no pain and 10 representing severe pain | |
| Notes | Mean VAS score recorded by nurses was not included in analysis owing to high risk of bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Unclear risk | Not reported |
| Allocation: sequence concealment | Unclear risk | Not reported |
| Performance and detection: blinding All outcomes | High risk | "The study was limited since neither the nurse nor the patient was blinded" |
| Detection: blind outcome assessment All outcomes | Unclear risk | Not reported |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 45/49 (92%) women analysed for pain outcomes |
| Reporting: unreported outcomes | Unclear risk | Adverse events were not reported |
| Other: anything else | High risk | Nulliparous women excluded from the study. Women with no endocervical disease and lesions of < 3 cm were eligible "Four of the original 49 study patients were excluded from the final data analysis since they recorded a higher pain score prior to the procedure than after the procedure and therefore recorded a negative pain score for unexplained reasons. This included 2 patients in the control group and 2 patients in the study group" |
Sarkar 1993.
| Study characteristics | ||
| Methods | Prospective, random allocation, double‐blind, placebo‐controlled trial | |
| Participants | Women undergoing laser treatment for CIN in the colposcopy and laser clinic Exclusion criteria: known or suspected hypersensitivity to local anaesthetics of amide type, concomitant treatment with analgesic medication, inability to complete assessment forms and woman's refusal to be recruited into the trial Age: mean (SD) (intervention vs. comparison): 27.8 years (6.3) vs. 28 years (5.4) |
|
| Interventions | Intervention (35 women): EMLA cream Comparison (35 women): placebo cream Creams supplied in visually identical metal tubes that were identified by participant number. 10 minutes before the start of the laser treatment, 10 ml of cream was applied to the cervix and surrounding area |
|
| Outcomes | Severity of pain experienced during the treatment assessed at end of treatment using McGill's pain questionnaire (Melzack 1975), and VAS (Huskisson 1983) Blood loss during the procedure reported as none, mild, moderate and troublesome |
|
| Notes | Minor adverse experiences during treatment, e.g. feeling hot, sweating, dizziness, fainting and sickness, not included in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Unclear risk | Not reported |
| Allocation: sequence concealment | Unclear risk | Not reported |
| Performance and detection: blinding All outcomes | Low risk | "The EMLA and the placebo cream were supplied in visually identical metal tubes which were identified by patient number" |
| Detection: blind outcome assessment All outcomes | Unclear risk | Not reported |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 68/70 (97%) women analysed for pattern of pain outcome |
| Reporting: unreported outcomes | Low risk | Pertinent outcomes were reported in the trial |
| Other: anything else | High risk | "When expressing the 'present pain intensity', some patients indicated a score between categories, so extra categories were created, such as 1 to 5, 2.5 etc". Such analyses are therefore dubious. Furthermore, "When patients were asked to describe their present pain by choosing specific words from McGill’s pain questionnaire (Melzack, 1975), the EMLA treated group tended to select words from fewer categories. The average number of words selected by the EMLA group was 3.83, compared with 5.06 for the placebo group (P < 0.05)". This probably applied to a mean ordinal score rating rather than mean number of words chosen, but this was unclear. |
Vanichtantikul 2013.
| Study characteristics | ||
| Methods | RCT comparing lignocaine spray vs. lignocaine submucosal injection Setting: single centre |
|
| Participants | Women undergoing loop excision for any degree of CIN detected from cytology, histology or both. 101 women participated with no drop‐outs. Exclusion criteria: known hypersensitivity to local anaesthesia, pregnancy, heart disease using a pacemaker or cardiac arrhythmia, history of neurological deficit, drug abuse and local infection (cervical or vaginal infection). Loop excision was performed in the outpatient setting by gynaecological oncology fellows using the uniform conventional technique. Participants were similar with respect to age and parity Median age: 47 years (range 28 to 69 years) in intervention group and 48 years (range 28 to 85 years) in comparison group. Median parity 2 (range 0 to 5) in both groups. Proportion of women who had punch biopsy done at the time of colposcopy higher in intervention group (37.3% in intervention group vs. 16.0% in comparison group; P value = 0.02). 1 woman in each group had undergone prior loop excision. Loop diameter, cone volume, additional tophat excision and final histology were comparable between groups. |
|
| Interventions | Intervention (n = 50): 4 puffs (40 mg, 10 mg per puff) lignocaine (xylocaine) 10% spray applied thoroughly to the ectocervix. Comparison (n = 51): 1.8 ml (36 mg) lignocaine injection 2% + 1:100,000 adrenaline injected submucosally using a standard pressure syringe injector with a 27 gauge needle tip at 3, 6, 9 and 12 O'clock locations of the ectocervix. |
|
| Outcomes | Pain: research assistant asked the woman to rate her pain according to a standard 10 cm VAS at different points during the procedure. This included pain scores at the beginning of the procedure immediately after speculum insertion (baseline pain score), immediately after administering anaesthetic agents (post‐anaesthesia pain score), immediately after accomplishing cone excision before proceeding with the remaining steps of the entire procedure (excision pain score), and at 30 minutes after the excision (post‐excision pain score). VAS consisted of a 10 cm line scaled from 0 (no pain) to 10 (worst possible pain) Primary outcomes: excision pain score and its difference from the baseline pain score The pain scores on VAS for pain relief during the procedure reported as median and range rather than mean and SD. Pain scores further stratified for size of loop excision (< 2 cm vs. ≥ 2 cm). Results reported as mean and SD only for loop size ≥ 2 cm, however, the study did not specify the number of participants in each group After the procedure, the women were observed for 30 minutes for any evidence of complications before being discharged home |
|
| Notes | There was a discrepancy in the number of participants in table of characteristics and the pain scores table | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Low risk | "Participants were randomly assigned into two groups according to a computer‐generated random allocation sequence." |
| Allocation: sequence concealment | Low risk | "Sequentially numbered, sealed opaque envelopes were used to provide allocation concealment." |
| Performance and detection: blinding All outcomes | High risk | "The blinding process was incomplete. An attempt was made to blind the participants from the assigned allocation during the procedure. However, complete blinding of the participants would not be achievable as a result of the nature of the procedure. Also, blinding of the operators would not be practical in this situation." |
| Detection: blind outcome assessment All outcomes | Unclear risk | Not reported. |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 101/101 (100%) women. |
| Reporting: unreported outcomes | High risk | Discrepancy in the number of participants in tables of characteristics and pain score tables. |
| Other: anything else | Unclear risk | Insufficient information to permit judgement |
Winters 2009.
| Study characteristics | ||
| Methods | RCT Setting: colposcopy clinic |
|
| Participants | 60 women scheduled to have LLETZ carried out for CIN recruited to have the anaesthetic injection in the cervix before procedure by 2 different techniques Referral smear (intervention vs. control): mild: 8/32 vs. 5/32; moderate: 12/32 vs. 10/32; severe: 8/32 vs. 7/32; borderline: 2/32 vs. 3/32; inadequate: 2/32 vs. 0/32; glandular abnormality: 0/32 vs. 1/32 LLETZ histology: CIN1: 5/32 vs. 1/32; CIN2: 7/32 vs. 7/32; CIN3: 15/32 vs. 17/32; inflammation: 3/32 vs. 0/32; CGIN: 2/32 vs. 0/32; adenocarcinoma: 0/32 vs. 1/32 Margins: both negative: 24/32 (75%) vs. 17/32 (65%); positive endocervical margin: 0/32 vs. 2/32; positive ectocervical margin: 5/32 vs. 6/32; both positive: 1/32 vs. 0/32; uncertain: 2/32 vs. 1/32 |
|
| Interventions | Both groups received 8.8 ml (4 ampoules) of prilocaine 3% + felypressin (Citanest, AstraZeneca, UK). Intervention: 1 x 2.2 ml ampoule of prilocaine + felypressin injected just under the epithelium, in 4 areas circumferentially, in order to raise a blanch. Then 3 x 2.2 ml ampoules injected in 8 places circumferentially deep into the cervical stroma Comparison: 4 x 2.2 ml ampoules of prilocaine + felypressin injected deep into the cervical stroma at 8 equally spaced points around the circumference of the cervical TZ Both using a 35 mm 27 gauge dental needle |
|
| Outcomes | Pain following completion of treatment indicated on separate 100 mm VAS for pain during administration of local anaesthetic and then during LLETZ procedure | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation: sequence generation | Low risk | "The block randomisation code was computer generated" |
| Allocation: sequence concealment | Low risk | "Randomisation was performed by opening sequentially numbered, sealed envelopes in order of recruitment" |
| Performance and detection: blinding All outcomes | High risk | "Participants were blinded to the technique of administration of local anaesthetic, by necessity the colposcopist could not be blinded to this" |
| Detection: blind outcome assessment All outcomes | Unclear risk | Not reported |
| Attrition: incomplete outcome data All outcomes | Low risk | % analysed: 58/60 (97%) women |
| Reporting: unreported outcomes | High risk | Adverse events of injections not reported |
| Other: anything else | Low risk | No additional form of bias likely |
CGIN: cervical glandular intraepithelial neoplasia; CIN: cervical intraepithelial neoplasia; HAD: Hospital Anxiety and Depression scale; HPV: human papillomavirus; LLETZ: large loop excision of the transformation zone; n: number of women; RCT: randomised controlled trial; SD: standard deviation; TENS: transcutaneous electric nerve stimulation; TZ: transformation zone; VAS: visual analogue scale; VRS: verbal rating score.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bogani 2014 | Not an RCT |
| Sarkar 1990 | Not an RCT |
| Sharp 2009 | Pain relief interventions were not part of trial scope |
RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
Diab 2015.
| Methods | Prospective double‐blinded randomised control trial |
| Participants | 234 women scheduled to have outpatient treatment of cervical intraepithelial neoplasia using LLETZ |
| Interventions | 117 women randomised to 2 site cervical infiltration (superficial followed by deep infiltration) during LLETZ and 117 women randomised to multiple site infiltration |
| Outcomes | Pain score, participant anxiety, amount of blood loss. No quantitative data given |
| Notes | Contacted author to ask for quantitative data and details of measures used to quantify pain and anxiety |
NCT 03100565.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Öz 2015.
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
LLETZ: large loop excision of the transformation zone.
Differences between protocol and review
We did not identify any ongoing trials so we removed the following sentence from the 'Searching other resources section':
"If ongoing trials which have not been published are identified through these searches, the principal investigators will be approached for relevant data."
The review included 19 trials but comparisons were restricted to single trial analyses or meta‐analysis of few trials so we removed the following section on reporting biases:
"Assessment of reporting biases Funnel plots corresponding to meta‐analysis of the primary outcome will be examined to assess the potential for small study effects. When there is evidence of small‐study effects, publication bias will be considered as only one of a number of possible explanations. If these plots suggest that treatment effects may not be sampled from a symmetric distribution, as assumed by the random effects model, sensitivity analyses will be performed using fixed effects models."
We performed no subgroup analyses so we removed the following section:
"Subgroup analysis and investigation of heterogeneity If possible, subgroup analysis will be performed, grouping the trials by different routes of administering analgesia i.e. oral, injectable or inhalation and pain relief for different treatment types. Factors such as age, CIN grade, length of follow‐up, adjusted/unadjusted analysis will be considered in interpretation of any heterogeneity."
We did not carry out sensitivity analysis. We had specified the following in the protocol:
"Sensitivity analysis We will perform sensitivity analyses excluding studies at moderate or high risk of bias."
Contributions of authors
KG ‐ primary author. PMH ‐ editing and clinical expertise. AB ‐ statistical and methodological support. GO ‐ data interpretation and editing of the updated review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Department of Health, UK
NHS Cochrane Collaboration programme Grant Scheme CPG‐10/4001/12
Declarations of interest
KG ‐ none known. PMH ‐ none known AB ‐ None known GO ‐ recipient of a MCRC Clinical Research Training Fellowship, and previously received a Wellbeing of Women Entry Level Scholarship.
Edited (no change to conclusions)
References
References to studies included in this review
Al‐Kurdi 1985 {published data only}
- AI-Kurdi M, Hare MJ, Lowles I, Douglas CP. The effect of a prostaglandin-synthetase inhibitor, naproxen sodium, and a placebo on the pain response to carbon dioxide laser treatment of the uterine cervix. Journal of Obstetrics and Gynaecology 1985;5:260-2. [Google Scholar]
Connell 2000 {published data only}
- Connell RJ, Creighton SM, Cutner A. The effect of local anaesthetic spray on the pain associated with local anaesthetic injection, prior to biopsy or loop diathermy to the cervix in the outpatient colposcopy clinic. BJOG: An International Journal of Obstetrics & Gynaecology 2000;107(5):686-8. [DOI] [PubMed] [Google Scholar]
Crompton 1992 {published data only}
- Crompton AC, Johnson N, Dudek U, Batra N, Tucker A. Is transcutaneous electrical nerve stimulation of any value during cervical laser treatment? British Journal of Obstetrics & Gynaecology 1992;99(6):492-4. [DOI] [PubMed] [Google Scholar]
Cruickshank 2005 {published data only}
- Anthony GB, Ross J, Tunstall M, Alexander D, Graham W, Fitzmaurice A, et al. A double blinded randomised controlled trial comparing standard analgesia versus standard analgesia and isodesox for the out-patient treatment of cervical intra-epithelial neoplasia. Journal of Obstetrics and Gynaecology 2002;22(1):108. [Google Scholar]
- Cruickshank ME, Anthony GB, Fitzmaurice A, McConnell D, Graham W, Alexander DA, et al. A randomised controlled trial to evaluate the effect of self-administered analgesia on women's experience of outpatient treatment at colposcopy. BJOG: An International Journal of Obstetrics & Gynaecology 2005;112(12):1652-8. [DOI] [PubMed] [Google Scholar]
Diakomanolis 1997 {published data only}
- Diakomanolis E, Stefanidis KS, Rodolakis A, Sakellaropoulos G. Local vasoconstrictive effect of ornipressin (POR8) in laser conization of the cervix: a randomized study. Journal of Gynecologic Surgery 1997;13(4):187-90. [Google Scholar]
Duncan 2005 {published data only}
- Duncan ID, McKinley CA, Pinion SB, Wilson SM. A double-blind, randomized, placebo-controlled trial of prilocaine and felypressin (Citanest and Octapressin) for the relief of pain associated with cervical biopsy and treatment with the Semm coagulator. Journal of Lower Genital Tract Disease 2005;9(3):171-5. [DOI] [PubMed] [Google Scholar]
Frega 1994 {published data only}
- Frega A, Stentella P, Di Renzi F, Gallo G, Palazzetti PL, Del Vescovo M, et al. Pain evaluation during carbon dioxide laser vaporization for cervical intraepithelial neoplasia: a randomized trial. Clinical & Experimental Obstetrics & Gynecology 1994;21(3):188-91. [PubMed] [Google Scholar]
Howells 2000 {published data only}
- Howells RE, Tucker H, Millinship J, Shroff JF, Dhar KK, Jones PW, et al. A comparison of the side effects of prilocaine with felypressin and lignocaine with adrenaline in large loop excision of the transformation zone of the cervix: results of a randomised trial. BJOG: An International Journal of Obstetrics & Gynaecology 2000;107(1):28-32. [DOI] [PubMed] [Google Scholar]
Johnson 1989 {published data only}
- Johnson N, Crompton AC, Ramsden SV. The efficacy of paracervical injections of lignocaine before laser ablation of the cervical transformation zone. A randomized placebo-controlled double-blind clinical trial. British Journal of Obstetrics & Gynaecology 1989;96(12):1410-2. [DOI] [PubMed] [Google Scholar]
Johnson 1996 {published data only}
- Johnson N, Crompton AC, Doodek U. Comparing paracervical with direct infiltration of lignocaine for cervical laser surgery. Journal of Gynecologic Surgery 1996;12(3):197-9.
Kizer 2014 {published data only}
- Kizer NT, Zhao Q, Peipert JF, Ioffe Y, Massad LS. A randomized trial of buffered versus nonbuffered lidocaine with epinephrine for cervical loop excision. Journal of Lower Genital Tract Disease 2014;18(1):8-12. [DOI] [PubMed] [Google Scholar]
Lee 1986 {published data only}
- Lee ET, Ozumba EN, Bevan JR. A randomized trial of Citanest with Octapressin for relief of pain associated with laser vaporization of the cervix. British Journal of Obstetrics & Gynaecology 1986;93(9):967-9. [DOI] [PubMed] [Google Scholar]
Lipscomb 1995 {published data only}
- Lipscomb GH, McCord ML, Bain KW, Ling FW. The effect of topical 20% benzocaine on pain during loop electrosurgical excision of the cervix. American Journal of Obstetrics & Gynecology 1995;173(3 Pt 1):772-4. [DOI] [PubMed] [Google Scholar]
Mikhail 1988 {published data only}
- Mikhail MG, Bevan JR. A randomized trial of the use of cocaine spray to provide pain relief during laser vaporization of the cervix. British Journal of Obstetrics & Gynaecology 1988;95(5):469-72. [DOI] [PubMed] [Google Scholar]
Rogstad 1992 {published data only}
- Rogstad KE, White DJ, Ahmed-Jushuf IH. Efficacy of lignocaine analgesia during treatment to the cervix. Lancet 1992;340(8825):942. [DOI] [PubMed] [Google Scholar]
Sammarco 1993 {published data only}
- Sammarco MJ, Hartenbach EM, Hunter VJ. Local anaesthesia for cryosurgery on the cervix. Journal of Reproductive Medicine 1993;38(3):170-2. [PubMed] [Google Scholar]
Sarkar 1993 {published data only}
- Sarkar PK, Williams JH, Davies Humphreys J. A double-blind placebo-controlled trial of EMLA cream for the relief of pain associated with laser vapourisation for cervical pre-malignant conditions. Journal of Obstetrics and Gynaecology 1993;13(5):370-3.
Vanichtantikul 2013 {published data only}
- Vanichtantikul A, Charoenkwan K. Lidocaine spray compared with submucosal injection for reducing pain during loop electrosurgical excision procedure: a randomized controlled trial. Obstetrics and Gynaecology 2013;122(3):553-7. [DOI] [PubMed] [Google Scholar]
Winters 2009 {published data only}
- Winters U, Keating PJ. A randomised controlled trial comparing two different local anaesthetic injection techniques prior to LLETZ. Journal of Obstetrics & Gynaecology 2009;29(6):539-41. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bogani 2014 {published data only}
- Bogani G, Serati M, Cromi A, Di Naro E, Casarin J, Pinelli C, et al. Local anaesthetic versus forced coughing at colposcopic-guided biopsy: a prospective study. European Journal of Obstetrics Gynecology & Reproductive Biology 2014;181:15-9. [DOI] [PubMed] [Google Scholar]
Sarkar 1990 {published data only}
- Sarkar PK. Topical anaesthesia with lignocaine-prilocaine cream (EMLA) for carbon dioxide treatment to the cervix - a pilot study. British Journal of Clinical Practice 1990;44(9):352-3. [PubMed] [Google Scholar]
Sharp 2009 {published data only}
- Sharp L, Day N, Marteau T, Parmar M, Patnick J, Woodman C. Biopsy and selective recall compared with immediate large loop excision in management of women with low grade abnormal cervical cytology referred for colposcopy: multicentre randomised controlled trial. BMJ 2009;339(7716):330. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Diab 2015 {published data only (unpublished sought but not used)}
NCT 03100565 {published data only}
- Lidocaine spray versus coughing for pain relief during colposcopy. https://clinicaltrials.gov/show/NCT03100565 2017.
Öz 2015 {published data only}
- Öz M, Korkmaz E, Cetinkaya N, Baş S, Özdal B, Mutlu M, et al. Comparison of topical lidocaine spray with placebo for pain relief in colposcopic procedures: A randomized, placebo-controlled, double-blind study. Journal of Lower Genital Tract Disease 2015;19(3):212-4. [DOI] [PubMed] [Google Scholar]
Additional references
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In: Egger M, Davey Smith G, Altman DG, editors(s). Systematic Reviews in Health Care: Meta-Analysis in Context. 2nd edition. London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
EUROCARE 2003
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, et al and the EUROCARE Working Group. EUROCARE-3: survival of cancer patients diagnosed 1990-94 - results and commentary. Annals of Oncology 2003;14 (Supplement 5):v61-v118. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2008
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM, International Agency for Research on Cancer. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide, 2010. IARC CancerBase No. 10, globocan.iarc.fr (accessed 29 August 2012).
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Huskisson 1983
- Huskisson EC. Pain Measurement and Assessment. New York: Raven Press, 1983. [Google Scholar]
Johnson 1994
- Johnston N, Crompton AC. Who finds cervical laser therapy painful? Gynecologic Oncology 1994;52:44-9. [DOI] [PubMed] [Google Scholar]
Jordan 1976
- Jordan JA, Singer A. The Cervix. London: Saunders, 1976. [Google Scholar]
Lowles 1983
- Lowles IE, Al-Kurdi M, Hare MJ. Women's recollection of pain during and after carbon-dioxide laser treatment to the uterine cervix. British Journal of Obstetrics and Gynaecology 1983;90:1157-9. [DOI] [PubMed] [Google Scholar]
Martin‐Hirsch 2010
- Martin-Hirsch PL, Paraskevaidis E, Kitchener HC. Surgery for cervical intraepithelial neoplasia. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD001318.pub2] [DOI] [PubMed] [Google Scholar]
Melzack 1975
- Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975;1:277-99. [DOI] [PubMed] [Google Scholar]
Moore 2006
- Moore KL, Dalley AF. Clinical Orientated Anatomy. 5th edition. London: Lippincott Williams & Wilkins, 2006. [Google Scholar]
NHSCSP 2004
- Luesley D. Standards and Quality in Colposcopy. Programme (1996). NHSCSP Publication no. 2. Sheffield, NHS Cervical Screening Programme, 2004.
NHSCSP 2010
- Luesley D. Colposcopy and Programme Management: Guidelines for the NHS Cervical Screening Programme; 2nd edition. NHSCSP Publication no. 20, 2010.
Peto 2004
- Peto J, Gilham C, Fletcher O, Matthews FE. The cervical cancer epidemic that screening has prevented in the UK. Lancet 2004;364(9430):249-56. [DOI] [PubMed] [Google Scholar]
Spielberger 1970
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologists Press, 1970. [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression scale. Acta Psychiatrica Scandinavica 1983;67:361-70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gajjar 2012
- Gajjar K, Martin-Hirsch PPL, Bryant A. Pain relief for women with cervical intraepithelial neoplasia undergoing colposcopy treatment. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD006120.pub3] [DOI] [PubMed] [Google Scholar]


