Abstract
Background
Peritonitis is the most frequent serious complication of continuous ambulatory peritoneal dialysis (CAPD). It has a major influence on the number of patients switching from CAPD to haemodialysis and has probably restricted the wider acceptance and uptake of CAPD as an alternative mode of dialysis.
This is an update of a review first published in 2000.
Objectives
This systematic review sought to determine if modifications of the transfer set (Y‐set or double bag systems) used in CAPD exchanges are associated with a reduction in peritonitis and an improvement in other relevant outcomes.
Search methods
We searched the Cochrane Renal Group's Specialised Register through contact with the Trials Search Co‐ordinator. Studies contained in the Specialised Register are identified through search strategies specifically designed for CENTRAL, MEDLINE and EMBASE. Date of last search: 22 October 2013.
Selection criteria
Randomised controlled trials (RCTs) or quasi‐RCTs comparing double bag, Y‐set and standard peritoneal dialysis (PD) exchange systems in patients with end‐stage kidney disease.
Data collection and analysis
Data were abstracted by a single investigator onto a standard form and analysed by Review Manager. Analysis was by a random effects model and results were expressed as risk ratio (RR) or mean difference (MD) with 95% confidence intervals (CI).
Main results
Twelve eligible trials with a total of 991 randomised patients were identified. Despite the large total number of patients, few trials covered the same interventions, small numbers of patients were enrolled in each trial and the methodological quality was suboptimal. Y‐set and twin‐bag systems were superior to conventional spike systems (7 trials, 485 patients, RR 0.64, 95% CI 0.53 to 0.77) in preventing peritonitis in PD.
Authors' conclusions
Disconnect systems should be the preferred exchange systems in CAPD.
Keywords: Humans; Equipment Design; Kidney Failure, Chronic; Kidney Failure, Chronic/therapy; Peritoneal Dialysis, Continuous Ambulatory; Peritoneal Dialysis, Continuous Ambulatory/adverse effects; Peritoneal Dialysis, Continuous Ambulatory/instrumentation; Peritoneal Dialysis, Continuous Ambulatory/methods; Peritonitis; Peritonitis/etiology; Peritonitis/prevention & control; Randomized Controlled Trials as Topic
Plain language summary
Y‐set and double bag systems offer the most protection against peritonitis during continuous ambulatory peritoneal dialysis (CAPD)
People with advanced kidney disease may be treated with CAPD where a catheter is permanently inserted into the peritoneum (lining around abdominal contents) through the abdominal wall and sterile fluid is drained in and out a few times each day. The most common serious complication is infection of the peritoneum ‐ peritonitis. This may be caused by bacteria accidentally being transferred from the catheter. This review of trials compared three types of connecting systems (used to connect the bags and the catheter) and found the Y‐set and double bag exchange systems are the most effective in preventing peritonitis.
Background
Continuous ambulatory peritoneal dialysis (CAPD) has been used as an alternative to haemodialysis for patients with end‐stage renal disease (ESRD) since 1976 (Popovich 1976). It may be used as the first choice dialysis therapy and in a number of countries including the United Kingdom a significant proportion of the ESRD population are treated by this modality in preference to chronic haemodialysis. A peritoneal dialysis (PD) exchange involves draining CAPD dialysate solution into and out of the peritoneal cavity using a permanently implanted PD catheter and a transfer or connection system. Peritonitis is the most common serious complication of PD and is the leading cause of technique failure requiring a switch to haemodialysis (CANUSA 1996). Undertaking a PD exchange is one of the key points during CAPD when micro‐organisms can be inadvertently transferred via the lumen of the peritoneal catheter into the peritoneal space (intraluminal route) causing peritonitis. The CAPD transfer system used may therefore have an important bearing on both the incidence of peritonitis and CAPD technique failure.
There are three main types of catheter connecting systems. In the "standard" or straight connecting system the catheter is connected to the dialysate solution bag using a straight piece of tubing and a "spike" or a luer lock device. At each exchange a new connection is made and the bag is drained. The empty bag is rolled up and remains attached until the next exchange when the process is repeated. The second type of transfer system is the Y‐set in which the patient disconnects (disconnect system) from the bags between exchanges. When a new exchange is due a Y‐connection with one limb connected to an empty bag and one to a bag containing fresh dialysate is used (Buoncristiani 1980; Buoncristiani 1993; Buoncristiani 1996). During an exchange the peritoneal dialysate is first drained from the peritoneal cavity into the empty bag. Before introducing the fresh dialysis solution into the peritoneal cavity the Y‐connecting system is first flushed with fresh dialysis solution and drained into the drainage bag. This allows any bacteria to be flushed into the spent fluid. The fresh fluid is then introduced into the peritoneal cavity and the Y‐connector is disconnected from the CAPD catheter. The early Y‐set technique, in addition, flushed the system with a disinfectant, a hypochlorite, during each exchange (Buoncristiani 1983). The third system, the double bag (twin bag) system, is a further development of the Y‐set disconnect systems. With this system the connection with the fresh dialysis solution bag is already made and the patient has to perform one less connection procedure (Balteau 1991; Bazzato 1980). It has been suggested that use of the Y‐set transfer or double bag systems will lead to a reduced frequency of CAPD peritonitis (Buoncristiani 1983) and some (Golper 1996; Port 1992) observational studies have indicated an association between use of the standard connect system and a significantly increased risk of peritonitis. At present a considerable proportion of CAPD patients, continue to use the standard system. Other techniques, such as the Ultraviolet Germicidal System (Churchill 1991; Nolph 1985), in‐line bacteriological filters (Churchill 1991; Slingeneyer 1983) and heat sterilisation (Churchill 1991; Durand 1995) have been developed and used in an attempt to reduce peritonitis rates; they were not considered as part of this review.
Objectives
To evaluate the evidence that supports the use of the Y‐set (and modifications) and double bag systems for the prevention of peritonitis in PD patients.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) and quasi‐RCTs investigating the effect of the Y‐set or double‐bag systems to prevent PD peritonitis.
Types of participants
Adult and paediatric patients undergoing PD treatment.
Types of interventions
Double bag (experimental group) and/or Y‐set (experimental group) versus standard CAPD exchange systems (control group).
Double bag (experimental group) versus Y‐set (control group).
We included studies where disinfectant had or had not been used to flush and/or to be retained in the elements of the Y‐set system. For either the Y‐set or double bag systems the sequence of the exchanges could vary:
drain/flush/fill or
flush/drain/fill.
Types of outcome measures
Number of patients experiencing peritonitis and peritonitis rate (number of episodes per patient months on treatment) (primary outcome)
Number of patients experiencing exit‐site/tunnel infections and exit‐site/tunnel infection rate (number of episodes per patient months on treatment)
Number of patients in whom CAPD catheters were removed
Number of patients switching to haemodialysis
Number of patients hospitalised and average number of days of hospitalisation
Measures of quality of life and patient preference
All‐cause mortality
Search methods for identification of studies
We searched the Cochrane Renal Group's Specialised Register on 23 October 2013 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
The Cochrane Renal Group’s Specialised Register contains studies identified from several sources:
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Details of these strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about the Cochrane Renal Group. See Appendix 1 for search terms used in strategies for this review.
For search strategies used in our previous review please see Daly 2000.
Data collection and analysis
The full‐text of each relevant identified study were assessed independently by two assessors for subject relevance and methodological quality using a standard form. Details concerning method of random allocation, blinding, description of withdrawals and dropouts, and whether data were analysed on an intention to treat basis were noted.
Data abstraction
Data on predetermined outcome measures were abstracted from included studies using a standard form, by a single assessor and data‐entry on REVIEW MANAGER 4.2.3 was performed. All data were independently checked from the original papers by a second investigator. A third investigator performed data abstraction and requested additional unpublished or unclear information from the authors of all included trials at the time of updating the present review.
Study quality
The quality of included studies was assessed by two independent investigators without blinding to authorship or journal using the checklist developed by the Cochrane Renal Group. Discrepancies were resolved by discussion with a third investigator. The quality items assessed were allocation concealment, blinding of investigators, participants and outcome assessors, intention‐to‐treat analysis, and the completeness to follow‐up.
Quality checklist
Allocation concealment
Adequate (A): Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study
Unclear (B): Randomisation stated but no information on method used is available
Inadequate (C): Method of randomisation used such as alternate medical record numbers or unsealed envelopes; any information in the study that indicated that investigators or participants could influence intervention group
Blinding
Blinding of investigators: Yes/no/not stated
Blinding of participants: Yes/no/not stated
Blinding of outcome assessor: Yes/no/not stated
Blinding of data analysis: Yes/no/not stated
The above are considered not blinded if the treatment group can be identified in > 20% of participants because of the side effects of treatment.
Intention‐to‐treat analysis
Yes: Specifically reported by authors that intention‐to‐treat analysis was undertaken and this was confirmed on study assessment.
Yes: not specifically stated but confirmed on study assessment
No: Not reported and lack of intention‐to‐treat analysis confirmed on study assessment (Patients who were randomised were not included in the analysis because they did not receive the study intervention, they withdrew from the study or were not included because of protocol violation).
No: Stated, but not confirmed upon study assessment
Not stated
Completeness to follow‐up
Percent of participants excluded or lost to follow‐up.
Statistical assessment
Data from individual trials were analysed using the risk ratio (RR) measure and its 95% confidence intervals (CI) for dichotomous outcomes and the mean difference (MD) and its 95% CI for continuous outcomes. Subgroup analysis was planned to explore potential sources of variability in observed treatment effect where possible (paediatric versus adult population, diabetic versus non diabetic, trial quality items, timing of peritonitis or other outcome). Heterogeneity of treatment effects between studies was formally tested using the Q (heterogeneity χ²) and the I² statistics. When appropriate, summary estimators of treatment effects were calculated using a random effects model with RR and its 95% CI. Where data on the number of subjects with events (e.g. number of subjects with one or more episodes of peritonitis) were available, the RR was calculated as the ratio of the incidence of the event (one or more episodes) in the experimental treatment group over the incidence in the control group. Where data on the number of episodes were available, then the RR was calculated as the ratio of the rate of the outcome (e.g. the peritonitis rate) in the experimental treatment group (given by number of episodes of the outcome over total patient months on PD) over the rate in the control group. It was also planned that if sufficient RCTs were identified, an attempt would be made to assess for publication bias using a funnel plot (Egger 1997).
Results
Description of studies
We identified twelve RCTs comparing double bag, Y‐set and standard transfer systems with a total of 991 randomised patients (Cheng 1994; Churchill 1989; Dryden 1992; Harris 1996; Kiernan 1995; Li 1996; Li 1999; Lindholm 1988; Maiorca 1983; Monteon 1998; Owen 1992; Rottembourg 1987). One study (Monteon 1998) compared all three system types, seven compared only Y‐set with standard systems (Cheng 1994; Churchill 1989; Li 1996; Lindholm 1988; Maiorca 1983; Owen 1992; Rottembourg 1987), one compared only double bag with standard systems (Dryden 1992) and three compared only double bag with Y‐set systems (Harris 1996; Kiernan 1995; Li 1999). Some of the trials' reports did not include data relevant to all this review's outcomes or reported these data in a manner that precluded inclusion in the meta‐analyses (e.g. standard deviations were not available). Of all authors which were contacted for clarification and requests of additional information, only one replied (Harris 1996). As a consequence, some of the meta‐analyses include fewer studies and fewer patients than might be expected. The publication of the studies were fairly evenly spread over a 16 year period from 1983 (Maiorca 1983) to 1999 (Li 1999).
A search performed in January 2005 identified three potential studies which were not relevant to this review (Huang 2001; Li 2002; Ong 2003). In this 2013 updated review, we excluded six new trials (Bailie 1990; Burkart 1990; de Fijter 1994; Lee 1997; Tan 2005; Wong 2006); one is awaiting classification (Correa‐Rotter 1997a).
Risk of bias in included studies
Details of the methodological quality of the included RCTs are outlined in the description of included studies. Only four studies described the method of randomisation. One described a probably secure method of random allocation (Monteon 1998, central list of random numbers with order of allocation sent to participating centres in sealed envelopes). Two did not completely describe their method of random allocation (Churchill 1989, "variable blocking factor, by the coordinating centre", no other details given; Maiorca 1983, "closed envelope system", no other details given). Cheng 1994 described an unclear method of random allocation (random number tables but "investigators were not blind to what treatment previously recruited patients received", i.e. the next treatment could be anticipated). All twelve had parallel designs. Only two (Cheng 1994; Lindholm 1988) failed to describe withdrawals and dropouts. None of the study reports clearly stated that data were analysed on an intention to treat basis, although for only four (Harris 1996; Li 1999; Maiorca 1983; Monteon 1998) was it clear that analysis was not on an intention‐to‐treat basis. Blinding or masking was infrequently described and none of the studies stated specifically that patients, healthcare providers or outcome assessors were masked/blinded to the intervention.
Effects of interventions
Y‐set versus standard spike systems
The use of the Y‐set compared to standard spike systems was associated with a significantly lower risk of peritonitis (Analysis 1.1 (7 trials, 485 patients): RR 0.64, 95% CI 0.53 to 0.77), peritonitis rate (Analysis 1.2 (8 trials, 7417 patient‐months): RR 0.49, 95% CI 0.40 to 0.61) but no difference in exit‐site/tunnel infection (Analysis 1.3 (3 trials, 226 patients): RR 1.02, 95% CI 0.72 to 1.46) and rate (Analysis 1.4 (2 trials, 2841 patient‐months): RR 1.24, 95% CI 0.91 to 1.69).
1.1. Analysis.
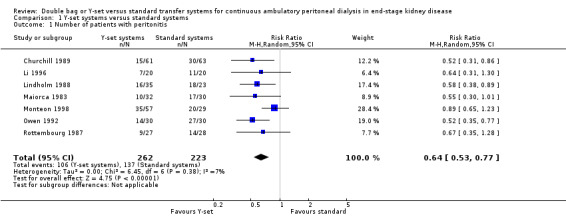
Comparison 1 Y‐set systems versus standard systems, Outcome 1 Number of patients with peritonitis.
1.2. Analysis.
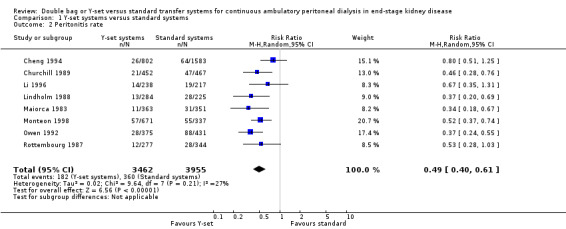
Comparison 1 Y‐set systems versus standard systems, Outcome 2 Peritonitis rate.
1.3. Analysis.
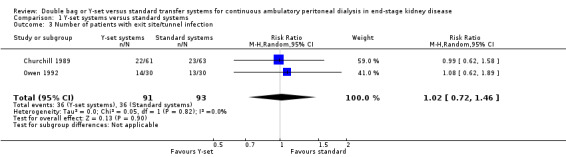
Comparison 1 Y‐set systems versus standard systems, Outcome 3 Number of patients with exit site/tunnel infection.
1.4. Analysis.
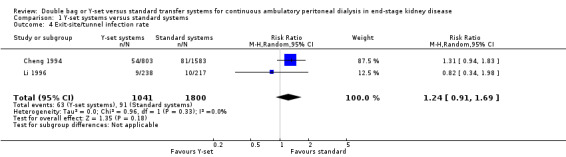
Comparison 1 Y‐set systems versus standard systems, Outcome 4 Exit‐site/tunnel infection rate.
A number of different definitions of "technique failure" (Analysis 1.5, Analysis 1.6) reported in the studies were considered in this review including: (i) switch to haemodialysis, (ii) switch to different transfer set and (iii) no longer on allocated treatment for whatever reason. Overall, there was no significant difference in the risk of technique failure with the Y‐set compared to standard spike systems with any of these definitions. There was also no difference in the risk of catheter removal (Analysis 1.7 (1 trial, 40 patients): RR 0.33, 95% CI 0.04 to 2.94). Only a single study which compared Y‐set with standard systems reported data on all‐cause (Analysis 1.8 (1 study, 69 patients): RR 1.12, 95% CI 0.81 to 1.56) and peritonitis‐related (Analysis 1.9 (1 study, 69 patients): RR 0.61, 95% CI 0.26 to 1.44) hospitalisation, and showed no significant difference in the risk. There was also no significant difference in the risk of all‐cause mortality with the Y‐set compared to standard spike systems (Analysis 1.10 (5 trials, 355 patients): RR 1.03, 95% CI 0.48 to 2.21). Heterogeneity was not significant in any of these analyses.
1.5. Analysis.
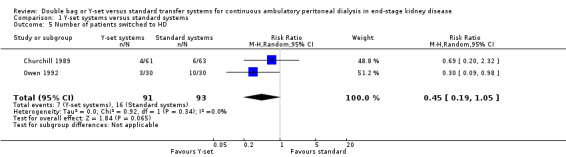
Comparison 1 Y‐set systems versus standard systems, Outcome 5 Number of patients switched to HD.
1.6. Analysis.
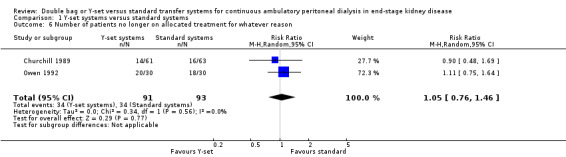
Comparison 1 Y‐set systems versus standard systems, Outcome 6 Number of patients no longer on allocated treatment for whatever reason.
1.7. Analysis.
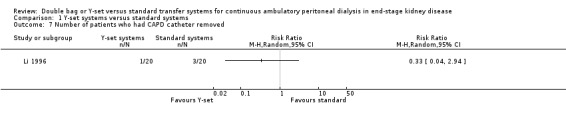
Comparison 1 Y‐set systems versus standard systems, Outcome 7 Number of patients who had CAPD catheter removed.
1.8. Analysis.
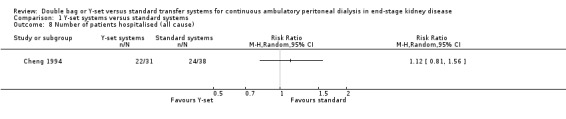
Comparison 1 Y‐set systems versus standard systems, Outcome 8 Number of patients hospitalised (all cause).
1.9. Analysis.
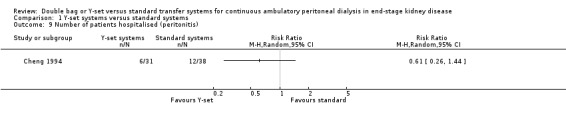
Comparison 1 Y‐set systems versus standard systems, Outcome 9 Number of patients hospitalised (peritonitis).
1.10. Analysis.
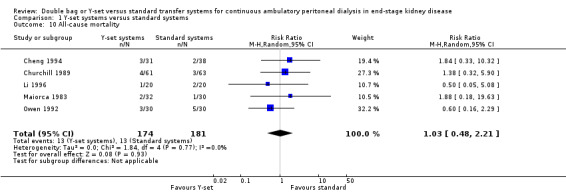
Comparison 1 Y‐set systems versus standard systems, Outcome 10 All‐cause mortality.
Double bag versus standard systems
There was no statistically significant difference with double bag systems compared to standard systems for the risk of peritonitis (Analysis 2.1 (2 trials, 170 patients): RR 0.43, 95% CI 0.29 to 0.62), the peritonitis rate (Analysis 2.2 (2 trials, 2110 patient‐months): RR 0.31, 95% CI 0.20 to 0.47), technique failure (Analysis 2.3 (1 trial, 80 patients): RR 1.00, 95% CI 0.06 to 15.44), exit‐site/tunnel infection (Analysis 2.4 (1 trial, 80 patients): RR 0.75, 95% CI 0.18 to 3.14) and all‐cause mortality (Analysis 2.5 (1 trial, 80 patients): RR 1.00, 95% CI 0.21 to 4.66) with no significant heterogeneity in any analysis.
2.1. Analysis.
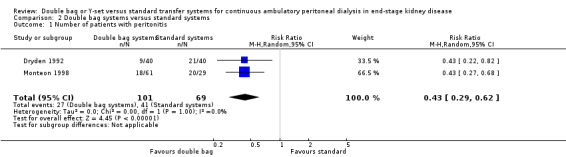
Comparison 2 Double bag systems versus standard systems, Outcome 1 Number of patients with peritonitis.
2.2. Analysis.
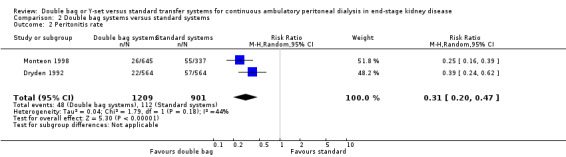
Comparison 2 Double bag systems versus standard systems, Outcome 2 Peritonitis rate.
2.3. Analysis.
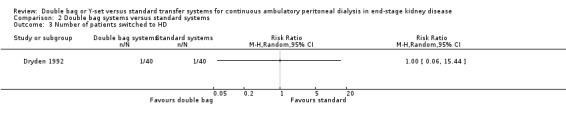
Comparison 2 Double bag systems versus standard systems, Outcome 3 Number of patients switched to HD.
2.4. Analysis.
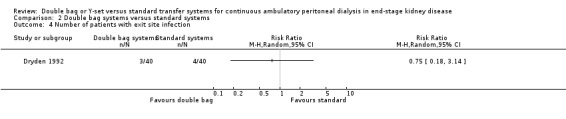
Comparison 2 Double bag systems versus standard systems, Outcome 4 Number of patients with exit site infection.
2.5. Analysis.
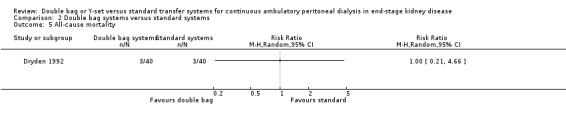
Comparison 2 Double bag systems versus standard systems, Outcome 5 All‐cause mortality.
Y‐set or double bag systems versus standard systems
The combined analysis of Y‐set or double bag systems compared to standard systems demonstrated a significant reduction in the risk of peritonitis (Analysis 3.1 (8 trials, 626 patients): RR 0.58, 95% CI 0.49 to 0.68) and peritonitis rate (Analysis 3.2 (11 trials, 10082 patient‐months): RR 0.55, 95% CI 0.42 to 0.32) but no significant difference in the risk of exit‐site/tunnel infection (Analysis 2.3 (3 trials, 264 patients): RR 1.00, 95% CI 0.71 to 1.42) and rate (Analysis 3.4 (2 trials, 2841 patient‐months): RR 1.24, 95% CI 0.91 to 1.69), catheter removal (Analysis 3.5 (1 trial, 40 patients): RR 0.33, 95% CI 0.04 to 2.94), technique failure by various definitions (Analysis 3.6 and Analysis 3.7), the number of patients hospitalised due to any cause (Analysis 3.8 (1 trial, 69 patients): RR 1.12, 95% CI 0.81 to 1.56) or number of patients hospitalised due to peritonitis (Analysis 3.9 (1 trial, 69 patients): RR 0.61, 95% CI 0.26 to 1.44) and all‐cause mortality (Analysis 3.10 (6 trials, 435 patients): RR 1.03, 95% CI 0.52 to 2.03). There was significant heterogeneity in the analysis of peritonitis rate (heterogeneity χ² = 26.78, P = 0.003, I² = 62.7%) caused mainly by the trial of Kiernan 1995 which had a shorter follow‐up duration compared to all others.
3.1. Analysis.
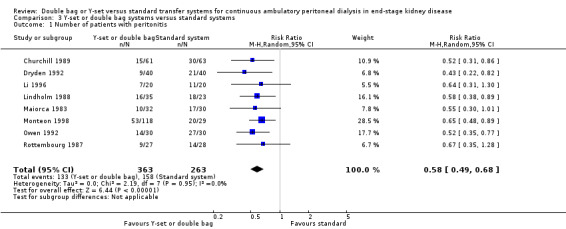
Comparison 3 Y‐set or double bag systems versus standard systems, Outcome 1 Number of patients with peritonitis.
3.2. Analysis.
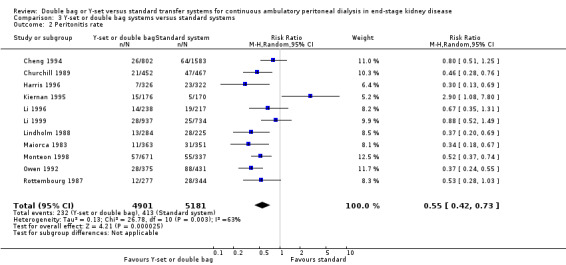
Comparison 3 Y‐set or double bag systems versus standard systems, Outcome 2 Peritonitis rate.
3.4. Analysis.
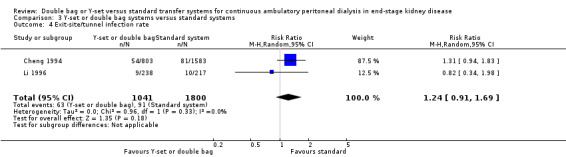
Comparison 3 Y‐set or double bag systems versus standard systems, Outcome 4 Exit‐site/tunnel infection rate.
3.5. Analysis.
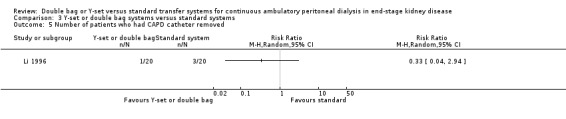
Comparison 3 Y‐set or double bag systems versus standard systems, Outcome 5 Number of patients who had CAPD catheter removed.
3.6. Analysis.
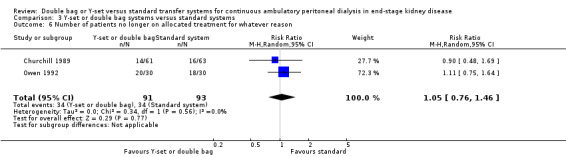
Comparison 3 Y‐set or double bag systems versus standard systems, Outcome 6 Number of patients no longer on allocated treatment for whatever reason.
3.7. Analysis.
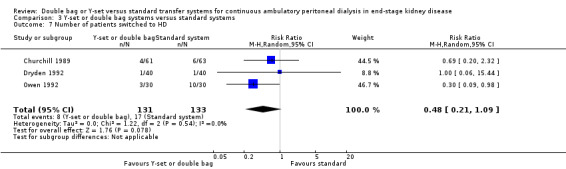
Comparison 3 Y‐set or double bag systems versus standard systems, Outcome 7 Number of patients switched to HD.
3.8. Analysis.
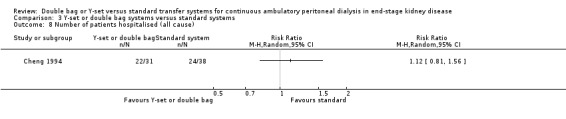
Comparison 3 Y‐set or double bag systems versus standard systems, Outcome 8 Number of patients hospitalised (all cause).
3.9. Analysis.
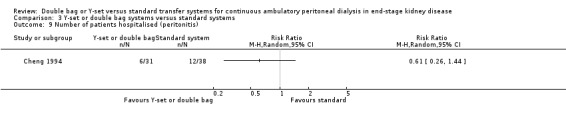
Comparison 3 Y‐set or double bag systems versus standard systems, Outcome 9 Number of patients hospitalised (peritonitis).
3.10. Analysis.
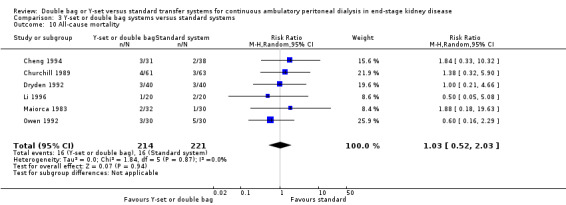
Comparison 3 Y‐set or double bag systems versus standard systems, Outcome 10 All‐cause mortality.
Double bag systems versus Y‐set systems
There was no significant difference with double bag compared to Y‐set for the risk of peritonitis (Analysis 4.1 (3 trials, 292 patients): RR 0.59, 95% CI 0.35 to 1.01), peritonitis rate (Analysis 4.2 (4 trials, 4319 patients‐months): RR 0.90, 95% CI 0.49 to 1.66), exit‐site/tunnel infection rate (Analysis 4.3 (2 trials, 2319 patient‐months): RR 1.04, 95% CI 0.52 to 2.06), technique failure by various definitions (Analysis 4.4, Analysis 4.5). There was also no difference in catheter removal/replacement (Analysis 4.6 (1 trial 63 patients): RR 0.10, 95% CI 0.01 to 1.81) and all‐cause mortality (Analysis 4.7 (2 trials, 193 patients): RR 0.98 95% CI 0.25 to 3.43). The analysis of peritonitis rate showed significant heterogeneity (heterogeneity χ² = 12.24, P = 0.007, I² = 75.5%) which is imputable to the trial of Kiernan 1995. This trial had a shorter follow‐up duration compared to all others.
4.1. Analysis.
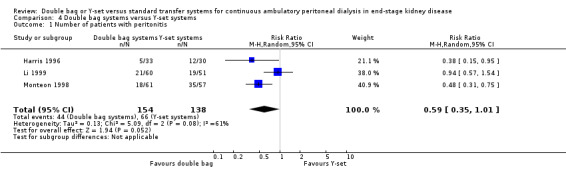
Comparison 4 Double bag systems versus Y‐set systems, Outcome 1 Number of patients with peritonitis.
4.2. Analysis.
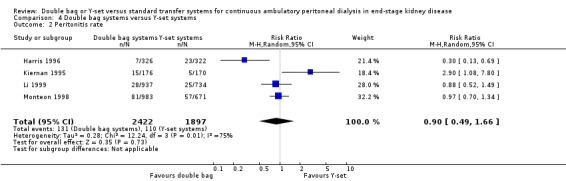
Comparison 4 Double bag systems versus Y‐set systems, Outcome 2 Peritonitis rate.
4.3. Analysis.
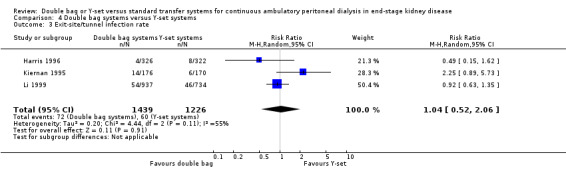
Comparison 4 Double bag systems versus Y‐set systems, Outcome 3 Exit‐site/tunnel infection rate.
4.4. Analysis.
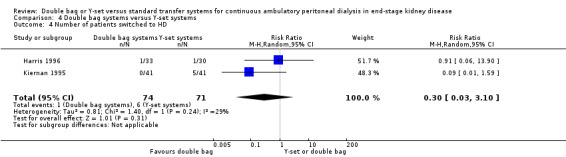
Comparison 4 Double bag systems versus Y‐set systems, Outcome 4 Number of patients switched to HD.
4.5. Analysis.
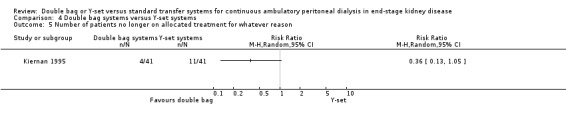
Comparison 4 Double bag systems versus Y‐set systems, Outcome 5 Number of patients no longer on allocated treatment for whatever reason.
4.6. Analysis.
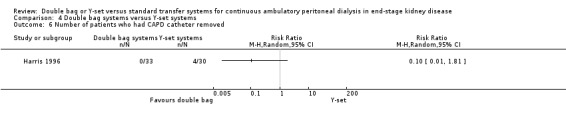
Comparison 4 Double bag systems versus Y‐set systems, Outcome 6 Number of patients who had CAPD catheter removed.
4.7. Analysis.
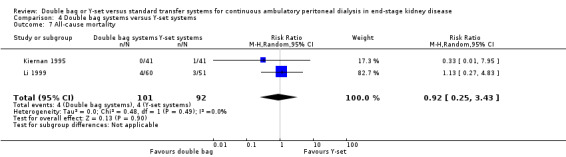
Comparison 4 Double bag systems versus Y‐set systems, Outcome 7 All‐cause mortality.
Quality of Life
Only two studies reported quality of life data (Harris 1996; Li 1999). Both compared a double bag with a Y‐set system. Harris 1996, using a Lickert scale, reported significantly greater "ease of use" and Li 1996, using a 6‐item questionnaire, reported significantly greater "patient acceptability" with the double bag system. It was unclear how well‐validated these instruments of assessment are in this particular setting.
Discussion
This systematic review of double bag or Y‐set versus standard transfer systems for CAPD has demonstrated that disconnect (double bag and Y‐connection) systems are superior to conventional spike (or luer lock) connect systems for the prevention of peritonitis. There was no statistically significant advantage of twin bag systems compared with Y‐systems, although the former were associated with a trend towards fewer affected patients with peritonitis (P = 0.05).
To our knowledge, the present study represents the most comprehensive systematic review of the relative benefits and harms of double bag or Y‐set systems in PD patients. Two additional reviews have now been published and relate to the use of anti‐microbial strategies (Strippoli 2004a) and other catheter‐related interventions including catheter type and surgical techniques for insertion of the PD catheter (Strippoli 2004b) used to prevent PD peritonitis.
The most likely reason for the observation that disconnect systems are superior to conventional spike systems is a reduction of inadvertent peritoneal microbial contamination during connections with Y‐set and twin bag systems as a result of the "flush before fill" manoeuvre (Bazzato 1980). Although the elimination of one connection procedure by twin bag systems should theoretically further reduce peritonitis episodes beyond that achieved by Y‐connection systems, this was unable to be demonstrated in our study. In contrast in a previous version of this review (Daly 2000) we reported a significantly lower risk of experiencing peritonitis episodes with double bag systems compared with Y‐systems (odds ratio 0.44, 95% CI 0.27 to 0.71). This apparent disparity may be partly explained by the more conservative statistical approach adopted in the present analysis (random effects model) compared with the previous (fixed effects model). In order to better assess the robustness of our statistical findings, we also additionally evaluated peritonitis rates as episodes/month (rather than just number of patients experiencing peritonitis) and again demonstrated no statistically significant differences between the two disconnect systems. Similar findings were observed for the other outcome measures evaluated, including exit‐site/tunnel infections, catheter removal, technique survival and all‐cause mortality.
These results support the recommendations of the British Renal Association (BRA) and the Caring for Australians with Renal Impairment (CARI) guidelines against the use of conventional spike connection systems. Although the International Society of Peritoneal Dialysis and K/DOQI clinical practice guidelines make no specific recommendations about connection methodology, spike and luer lock connect system usage has generally been declining in recent years. In the United Kingdom, the use of connect PD systems has decreased from 22% in 1998 to less than 1% in 2002 (Ansell 1998). A similar experience has been reported in Australia and New Zealand (Johnson 2003).
The strength of this investigation is that it represents a comprehensive systematic review based on a previous publication of a detailed protocol, rigid inclusion criteria for RCTs only, and a comprehensive multi‐database literature search. Data extraction, data analysis and method quality assessment were performed by two independent investigators, and consistency was checked with an additional reviewer. Furthermore, infectious outcomes were separately examined in terms of rates per patient‐month (Table 1; Table 2) and the number of patients affected in order to maximise statistical power and to verify the robustness of statistical analyses.
1. Number of patient‐months on CAPD per episode of peritonitis.
| Study | Double bag systems | Y‐set systems | Standard systems |
| Cheng 1994 | ‐‐ | 30.8 | 21.5 |
| Churchill 1989 | ‐‐ | 21.53 | 9.93 |
| Li 1996 | ‐‐ | 17.0 | 11.4 |
| Lindholm 1988 | ‐‐ | 22.0 | 8.0 |
| Maiorca 1983 | ‐‐ | 33.0 | 11.3 |
| Owen 1992 | ‐‐ | 13.4 | 4.9 |
| Rottembourg 1987 | ‐‐ | 23.0 | 12.3 |
| Dryden 1992 | 25.0 | ‐‐ | 9.7 |
| Monteon 1998 | 24.8 | 11.8 | 6.1 |
| Harris 1996 | 46.4 | 14.0 | ‐‐ |
| Kiernan 1995 | 33.9 | 11.7 | ‐‐ |
| Li 1999 | 33.5 | 29.4 | ‐‐ |
2. Number of patient‐months on CAPD per episode of exit‐site infection.
| Study | Double bag systems | Y‐set systems | Standard systems |
| Cheng 1994 | ‐‐ | 14.9 | 16.4 |
| Li 1996 | ‐‐ | 26.4 | 21.6 |
| Kiernan 1995 | 28.3 | 12.5 | ‐‐ |
| Li 1999 | 17.4 | 16.0 | ‐‐ |
The main weakness of this study was the relative paucity of quality RCTs identified. The vast majority of studies evaluated failed to specify whether randomisation allocation was concealed, outcome assessors were blinded or data were analysed on an intention to treat basis. Many studies were small and often short in duration, so that the possibility of a type 2 statistical error for some of the less frequently observed outcome measures (e.g. catheter loss) could not be excluded. Moreover, evidence of trial heterogeneity was found in some analyses of peritonitis rates (twin bag versus Y‐set), which most likely reflected significant inter‐trial variation in durations of follow‐up. These issues reduce the strength of the conclusions that have been drawn in this review.
Authors' conclusions
Implications for practice.
Disconnect (twin bag and Y‐set) systems are superior to conventional spike systems with respect to the prevention of peritonitis. No clear advantage of twin bag over Y‐set systems could be shown, although available trials were limited.
Implications for research.
Peritonitis is the most frequent serious complication of CAPD. It is essential that innovations in CAPD technique or technology designed to reduce peritonitis rates are subject to rigorous assessment by well‐designed RCTs. This review demonstrates that the above interventions have been very poorly studied to date. There is an obvious need in this area for well‐designed, RCTs (with clear descriptions of trial methodologies). The double bag and Y‐set systems should be the standard against which future design modifications in CAPD transfer technology are compared. If more than one intervention is being considered clinical trials should be designed to identify clearly to which intervention beneficial or adverse effects can be attributed. There is likely to be no additional research benefit in further trials comparing double bag, Y‐set and standard CAPD exchange systems.
What's new
| Date | Event | Description |
|---|---|---|
| 26 September 2016 | Amended | Minor edit to included and excluded studies |
| 22 September 2016 | Review declared as stable | There have been no new or ongoing studies since 1999, therefore this review is no longer being updated. Correa‐Rotter 1997, listed as awaiting assessment has now been excluded |
History
Protocol first published: Issue 2, 2001 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 25 November 2013 | New citation required but conclusions have not changed | Our conclusions remained unchanged |
| 25 November 2013 | New search has been performed | New search performed. In this 2013 updated review, we excluded six new trials (Bailie 1990; Burkart 1990; de Fijter 1994; Lee 1997; Tan 2005; Wong 2006); one is awaiting classification (Correa‐Rotter 1997a). |
| 29 September 2008 | Amended | Converted to new review format. |
| 20 November 2005 | Amended | Searched for new trials, none identified |
Notes
There have been no new or ongoing studies since 1999, therefore this review is no longer being updated. Correa‐Rotter 1997, listed as awaiting assessment has now been excluded.
Acknowledgements
This review was funded by the NHS Executive Research and Development Health Technology Assessment Programme. The Health Services Research Unit and the Health Economics Research Unit are funded by the Chief Scientist's Office, Scottish Executive Health Department. The authors would like to thank Carol Ritchie and Gloria Montague for secretarial support.
We also wish to gratefully acknowledge the contributions of Marion Campbell, Alison MacLeod, Adrian Grant and Paul Lawrence to earlier versions of this review.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL | #1 ("Y" near/2 set):ti,ab,kw #2 (y‐tub*):ti,ab,kw #3 (y‐connect* or (y near/1 connect*)):ti,ab,kw #4 (disconnect* near/2 system*):ti,ab,kw #5 (twin near/3 bag*):ti,ab,kw #6 (double near/3 bag*):ti,ab,kw #7 "O‐set":ti,ab,kw #8 conventional:ti,ab,kw #9 spike*:ti,ab,kw #10 b‐set:ti,ab,kw #11 {or #1‐#10} #12 "continuous ambulatory peritoneal dialysis":ti,ab,kw #13 CAPD:ti,ab,kw #14 peritonitis:ti,ab,kw #15 {or #12‐#14} #16 #11 and #15 |
| MEDLINE |
|
| EMBASE |
|
Data and analyses
Comparison 1. Y‐set systems versus standard systems.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with peritonitis | 7 | 485 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.53, 0.77] |
| 2 Peritonitis rate | 8 | 7417 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.40, 0.61] |
| 3 Number of patients with exit site/tunnel infection | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.72, 1.46] |
| 4 Exit‐site/tunnel infection rate | 2 | 2841 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.91, 1.69] |
| 5 Number of patients switched to HD | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.19, 1.05] |
| 6 Number of patients no longer on allocated treatment for whatever reason | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.76, 1.46] |
| 7 Number of patients who had CAPD catheter removed | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Number of patients hospitalised (all cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Number of patients hospitalised (peritonitis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 All‐cause mortality | 5 | 355 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.48, 2.21] |
Comparison 2. Double bag systems versus standard systems.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with peritonitis | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.29, 0.62] |
| 2 Peritonitis rate | 2 | 2110 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.20, 0.47] |
| 3 Number of patients switched to HD | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Number of patients with exit site infection | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 All‐cause mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Y‐set or double bag systems versus standard systems.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with peritonitis | 8 | 626 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.49, 0.68] |
| 2 Peritonitis rate | 11 | 10082 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.42, 0.73] |
| 3 Number of patients with exit‐site/tunnel infection | 3 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.71, 1.42] |
| 4 Exit‐site/tunnel infection rate | 2 | 2841 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.91, 1.69] |
| 5 Number of patients who had CAPD catheter removed | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Number of patients no longer on allocated treatment for whatever reason | 2 | 184 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.76, 1.46] |
| 7 Number of patients switched to HD | 3 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.21, 1.09] |
| 8 Number of patients hospitalised (all cause) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 9 Number of patients hospitalised (peritonitis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 10 All‐cause mortality | 6 | 435 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.52, 2.03] |
3.3. Analysis.
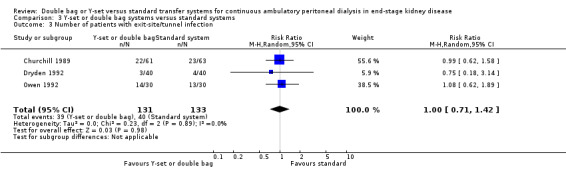
Comparison 3 Y‐set or double bag systems versus standard systems, Outcome 3 Number of patients with exit‐site/tunnel infection.
Comparison 4. Double bag systems versus Y‐set systems.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of patients with peritonitis | 3 | 292 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.35, 1.01] |
| 2 Peritonitis rate | 4 | 4319 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.49, 1.66] |
| 3 Exit‐site/tunnel infection rate | 3 | 2665 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.52, 2.06] |
| 4 Number of patients switched to HD | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.03, 3.10] |
| 5 Number of patients no longer on allocated treatment for whatever reason | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Number of patients who had CAPD catheter removed | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 All‐cause mortality | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.25, 3.43] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cheng 1994.
| Methods | RCT Method of random allocation: not described. However, states "since the investigators were not blind to the treatment previously recruited patients received, there might be a tendency to recruit older, less educated and diabetic patients with poorer eyesight and manual dexterity when treatment with UXVD was anticipated and to recruit younger and more educated patients when treatment with the O‐set was anticipated." Masking: not mentioned. Description of withdrawals/dropouts: not mentioned. Analysis on intention to treat basis: unclear. Duration of follow‐up: treatment group ‐ 802 patient‐months; control group ‐ 838 patient‐months. |
|
| Participants | All 100 patients starting CAPD over two years in a tertiary referral and satellite centre. 38 in treatment and 31 in control groups (also 31 in UVXD group). Exclusion criteria: age <10 or >70 years, living related transplant anticipated within 6 months of treatment. Unable to afford Y‐set. Male:female ratio: treatment group ‐ 20:18; control group ‐ 17:14. Age (mean, range): treatment group ‐ 34.9 (21‐59) years; control group ‐ 46.1 (23‐65) years. Primary renal disease: treatment group ‐ glomerulonephritis (11), pyelonephritis (3), diabetes mellitus (3), SLE/vasculitis/myeloma (1), unknown (13). Co‐morbidity: treatment group ‐ diabetes (1); control group (3). |
|
| Interventions | Y‐set (O‐set)(treatment) vs conventional spike (control) vs UVXD (excluded from this review). Sodium hypochlorite disinfectant (Amuchina) was retained in O‐set connections between exchanges. |
|
| Outcomes | Peritonitis Exit site infections Hospitalisation Costs | |
| Notes | A third group used UVXD ‐ data from this group not included in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
Churchill 1989.
| Methods | RCT Method of random allocation ‐ at co‐ordinating centre "using variable blocking factor". Masking: not mentioned. Withdrawals/dropouts: numbers and reasons clearly stated; treatment group ‐ 14 ( transplant ‐ 5; haemodialyses ‐ 4; intermittent peritoneal dialysis ‐ 1; recovery of renal function ‐ 1; discontinued dialysis ‐ 3); control group ‐ 16 (transplant ‐ 3; haemodialysis ‐ 6; intermittent peritoneal dialysis ‐ 3; recovery of renal function ‐ 1; discontinued dialysis ‐ 3). Analysis on intention to treat basis: unclear. Duration of follow‐up: treatment group ‐ 452 patient‐months; control group ‐ 467 patient‐months. |
|
| Participants | 124 new CAPD patients. 61 were in treatment group and 63 in control group. Exclusion criteria: age <18, likely to die in 6 months, previous complications on CAPD. Male:female ratio: not given. age ‐ not given. |
|
| Interventions | Y‐connector (plus Amuchina)(treatment) vs standard spike (Baxter II and III)(control). | |
| Outcomes | Peritonitis Exit site infections Technique survival (Kaplan‐ Meier). | |
| Notes | Hypochlorite disinfectant in Y‐set. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Dryden 1992.
| Methods | RCT Method of random allocation: not described. Masking: not mentioned Description of withdrawals and dropouts: numbers and reasons clearly stated; treatment group ‐ 12 (died ‐ 3; transplant ‐ 5; haemodialysis ‐ 1; recurrent infection ‐ 1; catheter failure ‐1; recovery of renal function ‐ 1); control group ‐ 12 (died ‐ 3; transplant ‐ 5; haemodialysis ‐ 1; recurrent infections ‐ 2; catheter failure ‐ 1). Analysis on intention to treat basis: unclear. Duration of follow‐up (mean, range):14.1 (3 ‐ 36) months. treatment group ‐ 564 ‐ patient‐months; control group ‐ 564 patient‐months. |
|
| Participants | 80 CAPD patients (new and established) at a single centre 40 in treatment and 40 in control groups. Male:female ratio: treatment group ‐ 23:17; control group ‐ 26:14. Age (mean, range): 49 (20 ‐ 67) years. Co‐morbidity: treatment group ‐ diabetes (5); control group (7). |
|
| Interventions | Y‐set (Freeline Solo) using drain/flush/fill (treatment) vs standard (Baxter II)(control). | |
| Outcomes | Peritonitis Exit site infections | |
| Notes | Hypochlorite not used in this system. Povidone iodine cap protectors used. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Harris 1996.
| Methods | RCT Method of random allocation: not described but states "patients stratified into three groups". Masking: not mentioned Description of withdrawals and dropouts: numbers and reasons clearly stated. Overall ‐ 10 (died ‐ 2; transplant ‐ 3; recovery of renal function ‐ 1; blindness ‐ 1; transfer to other unit ‐ 1; haemodialysis ‐ 2). It also stated " no differences in reasons for early termination between patients on the basic Y or Freeline Solo systems". Analysis on intention to treat basis: data from Group 3 were not analysed because of its high dropout rate but data from the other two groups "were analysed on an intention to treat basis". Duration of follow‐up (mean, SD): treatment group: group 1 ‐ 9.1(3.9) months; group 2 ‐ 9.9(3.5) months; control group: group 1 8.5(3.2) months; group 2 ‐ 11.5(3.6) months treatment group ‐ 328 patient‐months; control group ‐ 303 patient‐months. |
|
| Participants | New and established CAPD patients ‐ group 1 ‐ new patients (39); group 2 ‐ established patients with no peritonitis or exit site infections in previous 12 months or with new catheter (24); group 3 ‐ established patients with 1‐3 catheter‐related infections in previous 12 months (9). Because of high dropout rate data from group 3 were not reported nor analysed. Overall: 33 in treatment group and 30 in control group. Inclusion criteria: ≥ 18 years old; ability to perform exchanges without nursing assistance; informed consent. Exclusion criteria: within 2 months of catheter‐related infection; more than 3 separate catheter‐related infections in previous year unless catheter subsequently replaced. Male:female ratio: treatment group ‐ 13:20; control group ‐ 11:19. Age (mean, SD): treatment group ‐ group 1 ‐ 55(17) years; group 2 ‐ 59(11) years; control group ‐ group 1 ‐ 51(17) years; group 2 ‐ 60(15) years. Co‐morbidity: treatment group ‐ diabetes (9); control group ‐ diabetes (6). |
|
| Interventions | Double bag (Baxter Freeline Solo)(treatment) vs Basic Y (control). All had double‐cuffed silastic Tenckhoff catheters. Catheters immobilised. |
|
| Outcomes | Peritonitis Exit site infections Cost Training time Hospitalisation Catheter removal "Ease of use" scale | |
| Notes | Data from group 3 not reported nor analysed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Kiernan 1995.
| Methods | RCT Method of random allocation: not described but states "stratified for race and than randomly assigned". Masking: patients and healthcare workers not masked. Description of withdrawals and dropouts: numbers and reasons clearly stated; treatment group ‐ 4 (transplant ‐ 1; CAPD drainage problems ‐ 2; recovery of renal function ‐ 1); control group ‐ 15 (haemodialysis ‐ 5; transplant ‐ 3; transfer to other unit ‐ 1; CCPD ‐ 1; died ‐ 1). Analysis on intention to treat basis: unclear. Duration of follow‐up (mean, SD): treatment group ‐ 4.1(2.6) months; control group ‐ 4.3(2.5) months. |
|
| Participants | 35 new and 47 established CAPD patients in single unit. 41 in treatment and 41 in control groups. Male:female ratio: Age (mean,SD): treatment group ‐ 54.1(15.2) years; control group ‐ 55.2(14.5) years. Co‐morbidity: treatment group ‐ diabetes (15); HIV positive (4); cardiovascular disease (20); control group ‐ diabetes (10); HIV positive (4); cardiovascular disease (13). |
|
| Interventions | UltraTwin bag system (Baxter) (luer lock between Tenckhoff and double bag, no spiking required, flush/drain/fill)(treatment) vs Ultra Y‐set system (Baxter) (luer lock between catheter and Y‐set, new dialysate bag spiked, flush/drain/fill)(control). | |
| Outcomes | Peritonitis | |
| Notes | Treatment group ‐ whites (19); African‐americans (22); control group ‐ whites (18); African‐americans (23). Study terminated at interim analysis (300 patient‐months) because of statistically significant difference in infection rates between groups. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Li 1996.
| Methods | RCT Method of random allocation: not described. Masking: states single‐blinded but unclear who is blinded patients, healthcare providers or healthcare assessors. Description of withdrawals and dropouts: numbers and reasons clearly stated; total ‐ 4 (died ‐ 3; transplant ‐ 1) Analysis on intention to treat basis: unclear Duration of follow‐up: 12 months |
|
| Participants | 40 new ESRD patients in single unit. 20 in treatment group and 20 in control group. Male:female ratio: treatment group ‐ 9:11; control group ‐ 6:14. Age (mean,SD): treatment group ‐ 50(8) years; control group 47(15) years. Co‐morbidity: treatment group ‐ diabetes (5); HBsAg carriers (2); control group ‐ diabetes (4); HBsAg carriers (3). |
|
| Interventions | Y‐set (Ultraset, Baxter) with drain/flush/fill (treatment) vs conventional spike (control). 3 exchanges per day. |
|
| Outcomes | Peritonitis Exit site infections Hospitalisation Duration of training Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Li 1999.
| Methods | RCT Method of random allocation: not described; states randomisation was single‐blinded but remains unclear who was blinded. Masking: states single‐ blinded study but unclear who was blinded. Description of withdrawals and dropouts: numbers and reasons clearly stated ‐ treatment group ‐ 9 (opted not to use Y‐set ‐ 7; incomplete data ‐ 2). Analysis on intention to treat basis: no Duration of follow‐up: all patients followed for at least 12 months. Treatment group ‐ 937 patient‐months; control group ‐ 734 patient‐months. |
|
| Participants | 120 new ESRD patients admitted to CAPD programme. 60 in treatment group and 60 in control group (data on only 51 reported, see above). Male:female ratio: treatment group ‐ 28:32; control group ‐ 26:25. Age (mean,SD): treatment group ‐ 53.4(13.4) years; control group 49.3(14.2) years. Primary renal disease: treatment group ‐ glomerulonephritis (14); diabetes (11); adult polycystic kidney disease (2); hypertension (3); renal stone disease (5); others/unknown (25); control group ‐ glomerulonephritis (13); diabetes (11); adult polycystic kidney disease (4); hypertension (3); renal stone disease (0); others/unknown (20). Co‐morbidity: treatment group ‐ diabetes (15); HBsAg carriers (10); control group ‐ diabetes (14); HBsAg carriers (5). |
|
| Interventions | Double bag disconnect system (Ultrabag, Baxter) "pre‐assembled and sterilised as single unit". (treatment) vs Y‐set disconnect system (Ultraset, Baxter)(control). 3 exchanges per day. |
|
| Outcomes | Peritonitis Exit site infections Patient acceptance Hospitalisation Catheter removal Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lindholm 1988.
| Methods | RCT Method of random allocation: not described. Masking: not mentioned. Description of withdrawals and dropouts: not mentioned. Analysis on intention to treat basis: unclear. Duration of follow‐up: treatment group ‐ 284 patient‐months; control group 255 patient‐months. |
|
| Participants | 58 patients with ESRD. 35 in treatment group and 23 in control group. Co‐morbidity: overall ‐ diabetes (15). |
|
| Interventions | Y‐set (5F "safe‐lock" take‐off system, Fresenius, flush/drain/fill)(treatment) vs conventional CAPD systems (control). | |
| Outcomes | Peritonitis | |
| Notes | Authors stated that "we had problems with this study. The number of patients was envisaged to be 140, 58 were enrolled." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Maiorca 1983.
| Methods | RCT Method of random allocation: "closed envelope system" but no other details reported. Masking: not mentioned. Description of withdrawals and dropouts: number and reasons clearly stated ‐ treatment group ‐ 6 (died ‐ 2; withdrawn because additives to CAPD bags ‐ 1; CAPD stopped because of loss of ultrafiltration ‐ 1; patient request to withdraw from trial ‐ 1; progressive mental deterioration ‐ 1); control group (died ‐ 1; withdrawn because additives to CAPD bags ‐ 1; recurrent peritonitis and switched to Y‐set ‐ 4; CAPD stopped because of loss of ultrafiltration ‐ 1; patient request to withdraw from trial ‐ 1; withdrew because of recurrent exit site infections ‐ 2). Analysis on intention to treat basis: no Duration of follow‐up (mean,SD): treatment group ‐ 11.3(5.6)(range 3‐24) months; control group ‐ 11.7(6.1)(range 3‐24) months. treatment group ‐ 363 patient‐months; control group ‐ 351 patient‐months. |
|
| Participants | 62 new CAPD patients . 32 in treatment and 30 in control groups. Exclusion criteria: diabetes Male:female ratio: treatment group ‐ 17:15; control group ‐ 19:11. Age (mean,SD): treatment group ‐ 55.1(14.3)(range 12‐75) years; control group 55.5(17.5)(range 14‐80) years. |
|
| Interventions | Y‐connector with disinfectant (sodium hypochlorite, Amuchina) (Travenol, Lessines)(flush/drain/fill) (treatment) vs Standard (Travenol spike) (control). | |
| Outcomes | Peritonitis Mortality | |
| Notes | Hypochlorite used. Kaplan‐Meir analysis also reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Monteon 1998.
| Methods | RCT Method of random allocation: "done centrally by one of the researchers following the Moses‐Oakford method as described by Meinert, with the use of a list of random numbers. The order of allocation was sent to each participant centre in sealed envelopes. A copy of the assignment list was kept in the coordinating centre. At least three audit visits were done to each centre by trained nurses to certify that the envelopes were used in the order provided and that their content remained unknown to the personnel of the centre until the initiation of the treatment." Uneven randomisation in proportion double bag:Y‐set:standard system ‐ 2:2:1. Masking: none Description of withdrawals and dropouts: number and reasons clearly stated ‐ total ‐ 59 (randomised but did not begin study ‐ 7; transplant ‐ 10; died ‐ 23; technique failure ‐ 8; others ‐ 11) states "no significant difference in dropouts or deaths among the groups" Analysis on intention to treat basis: no Duration of follow‐up: "time at risk per patient" ‐ double bag ‐ 10.6 months; Y‐set 11.8 months; standard system ‐ 11.6 months. Total follow‐up: double bag ‐ 645.1 patient‐months; Y‐set ‐ 670.8 patient‐months; standard system ‐ 337.4 patient‐months. |
|
| Participants | 154 new ESRD
patients 147/154 randomised patients began study: double bag ‐ 61; Y‐set ‐ 57; standard system ‐ 29. Exclusion criteria: previous abdominal surgery, abdominal hernias, diverticulosis, cancer, AIDS. Male:female ratio: double bag ‐ 33:28; Y‐set ‐ 37:20; standard system ‐ 15:14. Age (mean,SD): double bag ‐ 43.6(21.9) years; Y‐set ‐ 43.2(21.3) years; standard system ‐ 39.7(19.0) years. Co‐morbidity: diabetes ‐ double bag (20), Y‐set (19), standard system (15). "High risk, low educational and socioeconomic levels with high prevalence of malnutrition." |
|
| Interventions | Double bag vs Y‐set vs standard spike system. | |
| Outcomes | Peritonitis Exit site infections Hospitalisation Catheter removal Cost | |
| Notes | Kaplan‐Meir analysis also reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Owen 1992.
| Methods | RCT Method of random allocation: not described. Masking: not mentioned. Description of withdrawals and dropouts: numbers and reasons clearly stated ‐ treatment group ‐ 20 (died ‐ 3; transplant ‐ 11; haemodialysis ‐ 3; intermittent peritoneal dialysis or other CAPD system ‐ 3); control ‐ 18 (died ‐ 5; transplanted ‐ 3; haemodialysis ‐ 10; transferred to intermittent peritoneal dialysis or CAPD ‐ 0). Analysis on intention to treat basis: unclear. Duration of follow‐up (median, range): treatment group ‐ 9 (1‐33) months; control group ‐ 14 (1‐34) months. treatment group ‐ 375 patient‐months; control group ‐ 430 patient‐months. |
|
| Participants | 60 patients commencing CAPD. 30 in treatment group and 30 in control group. Inclusion criteria: "patients who could tolerate 2L fluid exchanges". Exclusion criteria: "Significant physical disabilities or severe visual impairment". Male:female ratio: treatment group ‐ 16:14; control group ‐ 15:15. Age (median, range): treatment group ‐ 54 (11‐79) years; control group ‐ 56 (16‐75) years. |
|
| Interventions | Y‐set modification (O‐system "modification of the Y‐system with the ends of the connector joined to each other to form a closed circuit between exchanges"; flush before fill, ends soaked in hypochlorite prior to exchange) vs standard system (luer lock, System II, Baxter) | |
| Outcomes | Peritonitis. Exit site infection. Technique failure Training time | |
| Notes | Povidone iodine caps used. Kaplan‐Meir analysis also reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Rottembourg 1987.
| Methods | RCT Method of random allocation: not described. Masking: not mentioned. Description of withdrawals and dropouts: not mentioned. Analysis on intention to treat basis: unclear. Duration of follow‐up: treatment group ‐ 277 patient‐months (range 2‐24); control group ‐ 344 patient‐months (range, 2‐24). |
|
| Participants | 55 new CAPD patients 27 patients in treatment group and 28 patients in control group. Exclusion criteria: three new patients excluded because they were "returning to Africa". Male:female ratio: treatment group ‐ 12:15; control group ‐ 16:12. Age (mean,SD): treatment group ‐ 48.6(13.2) years; control group ‐ 54.6(14.6) years. Primary renal disease: treatment group ‐ diabetes (9); glomerulonephritis (6), interstitial nephritis (8); others/unknown (3); nephroangiosclerosis (1); control group ‐ diabetes (10); glomerulonephritis (11), interstitial nephritis (3); others/unknown (2); nephroangiosclerosis (2) |
|
| Interventions | Y‐set systems (3 different systems used ‐ Y‐set disposable disconnect system, one time use, two connections only, no disinfectant used, flush/drain/fill (Travenol); O‐set reusable system, no disinfectant, reused for one month (Travenol) and 5F safe‐lock system, flush/drain/fill (Fresenius)) (treatment) vs standard systems (luer lock or Reverse system (Travenol) or safe‐lock with spray of chlorhexidine (Fresenius) (control). | |
| Outcomes | Peritonitis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amato 1999 | Not RCT though reports data from two previously published RCTs |
| Bailie 1990 | Compares O‐set with UVXD germicidal system |
| Balteau 1991 | Not RCT |
| Buoncristiani 1989 | Not RCT |
| Burkart 1990 | Compared standard spike with Ultraset |
| Cancarini 1995 | RCT but patients randomised to "flushing" versus "flushing plus in line disinfectant" not to Y‐set, double‐bag or standard spike systems |
| Correa‐Rotter 1997 | Abstract only publication, no data available September 2016 |
| de Fijter 1994 | Compared Y‐connector CAPD with CCPD |
| Durand 1995 | Not RCT |
| Garcia‐Lopez 1994 | Not RCT |
| Hall 1989 | Possible quasi‐RCT but compares two non‐disconnect systems |
| Holley 1994 | Not RCT |
| Huang 2001 | Not RCT |
| Junor 1989 | Not RCT |
| Lee 1997 | Not RCT |
| Lempert 1986 | Not RCT |
| Ong 2003 | Comparisons not relevant to this review. Study compared two Y‐disconnect systems. |
| Orange 1987 | Not RCT |
| Piraino 1993 | Not RCT |
| Port 1992 | Not RCT |
| Ryckelynck 1988 | Probable RCT but patients randomised to "soaking" or not "soaking" connectors in povidone iodine |
| Smith 1997 | Not RCT and specifically concerns automated peritoneal dialysis and not CAPD |
| Smith 1997a | Not RCT and specifically concerns automated peritoneal dialysis and not CAPD |
| Tan 2005 | Compared ANDY.disc system to Ultrabag system |
| Tielens 1993 | Not RCT |
| Viglino 1989 | RCT but comparing Y‐set and modification of Y‐set (Y‐set with two short branches) no comparison with standard spike or double‐bag systems |
| Viglino 1993 | RCT but comparing Y‐set and modification of Y‐set (T‐set with disinfectant) no comparison with standard spike or double‐bag systems |
| Wong 2006 | Compared ANDY‐Disc system with UltraBag system |
| Wust 1985 | Not RCT |
Contributions of authors
Study conception (including funding): MacLeod A, Grant A , Daly C, Donaldson C, Campbell M, Khan I, Lawrence P
Protocol development: Campbell M, Cody J, Daly C, Donaldson C, Grant A, Vale L, Lawrence P, MacLeod A, Wallace S, Khan I
Literature search: Wallace S, Daly C, Lawrence P, Cody J, Khan I, Vale L, MacLeod A
Data extraction and analysis: Daly C, Campbell M, Khan I, Cody J
Writing of draft report: Daly C
Updating the review: Strippoli GFM (2004)
Editorial role and agreement of final manuscript: Campbell M, Cody J, Daly C, Donaldson C, Grant A, Vale L, Lawrence P, MacLeod A, Wallace S, Khan I
Sources of support
Internal sources
University of Aberdeen, UK.
Health Services Research Unit, UK.
Health Economics Research Unit, UK.
External sources
NHS Executive Research and Development Programme, UK.
Declarations of interest
Sheila Wallace is a Trials Search Co‐ordinator for the Cochrane Incontinence Review Group, whose single largest funder is the UK National Institute for Health Research (NIHR). The exploratory meeting to set up the Cochrane Incontinence Review Group was funded by Zeneca Pharmaceuticals in 1995.
Following completion of this review, an unrestricted educational grant from Janssen Cilag funded a further six systematic reviews related to ESKD and erythropoietin.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Cheng 1994 {published data only}
- Cheng IK, Chan CY, Cheng SW, Poon JF, Ji YL, Lo WK, et al. A randomized prospective study of the cost‐effectiveness of the conventional spike, O‐set, and UVXD techniques in continuous ambulatory peritoneal dialysis. Peritoneal Dialysis International 1994;14(3):255‐60. [EMBASE: 1994264793] [PubMed] [Google Scholar]
Churchill 1989 {published data only}
- Churchill DN, Canadian CAPD Clinical Trials Group. Randomised clinical trial comparing peritonitis rates among new CAPD patients using the Y set disinfectant system to standard systems [abstract]. Kidney International 1989;35(1):268. [Google Scholar]
- Churchill DN, Taylor DW, Vas SI, Singer J, Beecroft ML, Wu G, et al. Peritonitis in continuous ambulatory peritoneal dialysis (CAPD): a multi‐centre randomized clinical trial comparing the Y connector disinfectant system to standard systems. Canadian CAPD Clinical Trials Group. Peritoneal Dialysis International 1989;9(3):159‐63. [MEDLINE: ] [PubMed] [Google Scholar]
Dryden 1992 {published data only}
- Dryden MS, McCann M, Wing AJ, Phillips I. Controlled trial of a Y‐set dialysis delivery system to prevent peritonitis in patients receiving continuous ambulatory peritoneal dialysis. Journal of Hospital Infection 1992;20(3):185‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harris 1996 {published data only}
- Harris DC, Yuill EJ, Byth K, Chapman JR, Hunt C. Twin‐ versus single‐bag disconnect systems: infection rates and cost of continuous ambulatory peritoneal dialysis. Journal of the Amercian Society of Nephrology 1996;7(11):2392‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kiernan 1995 {published data only}
- Kiernan L, Kliger A, Gorban‐Brennan N, Juergensen P, Tesin D, Vonesh E, et al. Comparison of continuous ambulatory peritoneal dialysis‐related infections with different “Y‐tubing” exchange systems. Journal of the American Society of Nephrology 1995;5(10):1835‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Li 1996 {published data only}
- Li PK, Chan TH, So WY, Wang AYM, Leung CB, Lai KN. Comparisons of Y‐set disconnect system (Ultraset) versus conventional spike system in uremic patients on CAPD: outcome and cost analysis. Peritoneal Dialysis International 1996;16(Suppl 1):S368‐S70. [MEDLINE: ] [PubMed] [Google Scholar]
Li 1999 {published data only}
- Li PK, Law MC, Chan WK, Szeto CC, Chen YL, Lui SF, et al. Comparison of two double‐bag systems in continuous ambulatory peritoneal dialysis ‐ a prospective randomised controlled multi‐center study [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):312a. [Google Scholar]
- Li PK, Law MC, Chow KM, Chan WK, Szeto CC, Cheng YL, et al. Comparison of clinical outcome and ease of handling in two double‐bag systems in continuous ambulatory peritoneal dialysis ‐ a prospective randomized controlled multi‐center study [abstract]. Journal of the American Society of Nephrology 2002;13(Program & Abstracts):43a. [DOI] [PubMed] [Google Scholar]
- Li PK, Law MC, Chow KM, Chan WK, Szeto CC, Cheng YL, et al. Comparison of clinical outcome and ease of handling in two double‐bag systems in continuous ambulatory peritoneal dialysis: a prospective, randomized, controlled, multicenter study. American Journal of Kidney Diseases 2002;40(2):373‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Li PK, Szeto CC, Law MC, Chau KF, Fung KS, Leung CB, et al. Comparison of double‐bag and Y‐set disconnect systems in continuous ambulatory peritoneal dialysis: a randomized prospective multicenter study. American Journal of Kidney Diseases 1999;33(3):535‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lindholm 1988 {published data only}
- Lindholm T, Simonsen O, Krutzen L, Leusen R, Jordans JG, Moyy JM, et al. Evaluation of a new take‐off system: a prospective randomized multicenter study. Advances in Peritoneal Dialysis 1988;4:264‐5. [Google Scholar]
Maiorca 1983 {published data only}
- Maiorca R, Cantaluppi A, Cancarini GC, Scalamogna A, Broccoli R, Graziani G, et al. Prospective controlled trial of a Y‐connector and disinfectant to prevent peritonitis in continuous ambulatory peritoneal dialysis. Lancet 1983;2(8351):642‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Maiorca R, Cantaluppi A, Cancarini GC, Scalamogna A, Strada A, Graziani G, et al. 'Y' connector system for prevention of peritonitis in CAPD: a controlled study. Proceedings of the European Dialysis & Transplant Association 1983;20:223‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Monteon 1998 {published data only}
- Monteon F, Correa‐Rotter R, Paniagua R, Amato D, Hurtado ME, Medina JL, et al. Prevention of peritonitis with disconnect systems in CAPD: A randomized controlled trial. Kidney International 1998;54(6):2123‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Owen 1992 {published data only}
- Owen JE, Walker RG, Lemon J, Brett L, Mitrou D, Becker GJ. Randomized study of peritonitis with conventional versus O‐set techniques in continuous ambulatory peritoneal dialysis. Peritoneal Dialysis International 1992;12(2):216‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Rottembourg 1987 {published data only}
- Rottembourg J, Brouard R, Issad I, Allouache M, Jacobs C. Prospective randomised study about Y connectors in CAPD patients. Advances in Peritoneal Dialysis 1987:107‐13. [Google Scholar]
- Rottembourg J, Brouard R, Issad I, Allouache M, Nguyen J, Montassine MC, et al. Prevention of peritonitis during continuous ambulatory peritoneal dialysis. Value of disconnectable systems [Prevention des peritonites au cours de la dialyse peritoneale continue ambulatoire. Interet des systemes deconnectables]. Presse Medicale 1988;17(26):1349‐53. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Amato 1999 {published data only}
- Amato D, Paniagua R. Comparison of double‐bag and Y‐set disconnect systems in CAPD. American Journal of Kidney Diseases 1999;34(2):402‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bailie 1990 {published data only}
- Bailie GR, Rasmussen R, Hollister A, Eisele G. Incidence of CAPD peritonitis in patients using UVXD or O‐set systems. Clinical Nephrology 1990;33(5):252‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Balteau 1991 {published data only}
- Balteau P, Peluso FP, Coles GA, Michel C, Mignon FM, Tranaeus AP, et al. Design and testing of the Baxter Integrated Disconnect Systems. Peritoneal Dialysis International 1991;11(2):131‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Buoncristiani 1989 {published data only}
- Buoncristani U. The Y‐set with disinfectant is here to stay. Peritoneal Dialysis International 1989;9(3):149‐50. [MEDLINE: ] [PubMed] [Google Scholar]
Burkart 1990 {published data only}
- Burkart JM, Hylander B, Durnell‐Figel T, Roberts D. Comparison of peritonitis rates during long‐term use of standard spike versus Ultraset in continuous ambulatory peritoneal dialysis (CAPD). Peritoneal Dialysis International 1990;10(1):41‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Cancarini 1995 {published data only}
- Cancarini G, Catizone L, Fellini G, Feriani M, Quarello F, The Italian Study Group on CAPD connecting systems. Peritonitis prevention in CAPD. A flushing vs flushing + in line disinfectant comparison. Peritoneal Dialysis International 1995;15(Suppl 4):51. [Google Scholar]
Correa‐Rotter 1997 {published data only}
- Correa‐Rotter R, Monteon FJ, Paniagua R, Amato D, Moran JE. Peritonitis prevention with disconnect systems in CAPD: a prospective, randomized, controlled, multi‐center clinical trial [abstract]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):262A. [Google Scholar]
de Fijter 1994 {published data only}
- Fijter CW, Oe LP, Nauta JJ, Meulen J, Verbrugh HA, Verhoef J, et al. Clinical efficacy and morbidity associated with continuous cyclic compared with continuous ambulatory peritoneal dialysis. Annals of Internal Medicine 1994;120(4):264‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fijter CW, Oe LP, Meulen J, Nauta JJ, Donker AJ. Outcome in continuous ambulatory peritoneal dialysis with y‐set (CAPD‐Y) versus continuous cyclic peritoneal dialysis (CCPD): a prospective, randomized comparison [abstract]. Journal of the American Society of Nephrology 1992;3(3):408. [Google Scholar]
- Fijter CW, Oe PL, Nauta JJ, Meulen J, ter Wee PM, Snoek FJ, et al. A prospective, randomized study comparing the peritonitis incidence of CAPD and Y‐connector (CAPD‐Y) with continuous cyclic peritoneal dialysis (CCPD). Advances in Peritoneal Dialysis 1991;7:186‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Fijter CW, Oe PL, Verbrugh HA, Nauta JJ, Meulen J, Verhoef J, et al. Continuous cyclic peritoneal dialysis: clinical efficacy and comparison with continuous ambulatory peritoneal dialysis. Nederlands Tijdschrift Voor Geneeskunde 1995;139(13):658‐64. [EMBASE: 1995102998] [Google Scholar]
- Fijter CW, Oe PL, Nauta JJ, Meulen J, ter Wee PM, Snoek FJ, et al. Peritoneal dialysis‐related peritonitis: A prospective, randomized comparison between continuous ambulatory PD with Y‐connector (CAPD‐Y) and continuous cyclic PD (CCPD) [abstract]. Nephrology Dialysis Transplantation 1992;7(2):166‐7. [Google Scholar]
- Fijter CW, Snoek FJ, Oe PL, Meulen L, Nauta JJ, Verbrugh HA, et al. Has continuous cyclic peritoneal dialysis surplus value over CAPD‐Y regarding clinical outcome? [abstract]. Nephrology Dialysis Transplantation 1993;8(9):1020. [Google Scholar]
Durand 1995 {published data only}
- Durand PY, Chanliau J, Gamberoni J, Kessler M. Connectology for treatment by peritoneal dialysis for chronic renal insufficiency [La connectologie pour le traitement par dialyse peritoneale de l’insuffisance renale chronique]. Nephrologie 1995;16(1):37‐44. [MEDLINE: ] [PubMed] [Google Scholar]
Garcia‐Lopez 1994 {published data only}
- Garcia‐Lopez E, Mendoza‐Guevara A, Morales A, Aguilar‐Kitzu A, Vincenio LM, Hernandez‐Fernandez M, et al. Comparison of peritonitis rates in children on CAPD with spike connector versus two disconnect systems. Advances in Peritoneal Dialysis 1994;10:300‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Hall 1989 {published data only}
- Hall LJ, Kinney RA, Taber TE, Hegeman TF. Comparison of two non‐disconnect CAPD delivery systems. Advances in Peritoneal Dialysis 1989;5:227‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Holley 1994 {published data only}
- Holley JL, Bernardini J, Piraino B. Infecting organisms in Continuous Ambulatory Peritoneal Dialysis patients on the Y‐set. American Journal of Kidney Diseases 1994;23(4):569‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Huang 2001 {published data only}
- Huang JW, Hung KY, Yen CJ, Wu KD, Tsai TJ. Comparison of infectious complications in peritoneal dialysis patients using either a twin‐bag system or automated peritoneal dialysis. Nephrology Dialysis Transplantation 2001;16(3):604‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Junor 1989 {published data only}
- Junor BJ. CAPD disconnect systems. Blood Purification 1989;7(2‐3):156‐66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 1997 {published data only}
- Lee CC, Tsai CJ, Hong JJ. Twinbag disconnect system in continuous ambulatory peritoneal dialysis patients [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A183. [Google Scholar]
Lempert 1986 {published data only}
- Lempert KD, Kolb JA, Swartz RD, Campese V, Golper TA, Winchester JF, et al. Multicenter trial to evaluate the use of the CAPD "O" set. ASAIO Transactions 1986;32(1):557‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ong 2003 {published data only}
- Ong LM, Lim TO, Hooi LS, Morad Z, Tan PC, Wong HS, et al. A randomized, multicenter, open‐label trial to establish therapeutic equivalence between the Carex and Ultra disconnect systems in patients on continuous ambulatory peritoneal dialysis. Peritoneal Dialysis International 2003;23(Suppl 2):S139‐S43. [EMBASE: 2004037352] [PubMed] [Google Scholar]
Orange 1987 {published data only}
- Orange GV, Henderson IS, Marshall EA. Effectiveness of the flush technique in CAPD disconnect systems. International Journal of Artificial Organs 1987;10(3):185‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Piraino 1993 {published data only}
- Piraino B, Holley JL, Bernardini J. Are exit‐site infection rates lower with disconnect systems. Peritoneal Dialysis International 1993;13(1):67‐76. [MEDLINE: ] [PubMed] [Google Scholar]
Port 1992 {published data only}
- Port FK, Held PJ, Nolph KD, Turenne MN, Wolfe RA. Risk of peritonitis and technique failure by CAPD connection technique: a national study. Kidney International 1992;42(4):967‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ryckelynck 1988 {published data only}
- Ryckelynck JP, Verger C, Cam G, Faller B, Pierre D. Importance of the flush effect in disconnect systems. Advances in Peritoneal Dialysis 1988;4(6):282‐4. [Google Scholar]
- Verger C, Faller B, Ryckelynck JPH, Cam G, Pierre D. Efficacy of continuous ambulatory peritoneal dialysis y‐line systems without disinfectant and standard systems on peritonitis prevention: a multicentre prospective controlled trial [abstract]. Nephrology Dialysis Transplantation 1987;2(5):455‐6. [Google Scholar]
Smith 1997 {published data only}
- Smith CA. Does flush before fill decrease the incidence of peritonitis in our cycler population?. Journal CANNT 1997;7(2):20‐22. [MEDLINE: ] [PubMed] [Google Scholar]
Smith 1997a {published data only}
- Smith CA. Reduced incidence of peritonitis by utilizing "flush before fill" in APD. Advances in Peritoneal Dialysis 1997;13:225‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Tan 2005 {published data only}
- Tan SH, Huang XH, Chan YH, Lim S, Liew AS, Ng TG, et al. A prospective comparison of peritonitis between two disconnect CAPD systems in a single centre [abstract]. Nephrology 2005;10(Suppl):A69‐A70. [Google Scholar]
Tielens 1993 {published data only}
- Tielens E, Nube MJ, Vet JA, Limbeek J, Hofman X, Steffens A, et al. Major reduction of CAPD peritonitis after the introduction of the twin‐bag system. Nephrology Dialysis Transplantation 1993;8(11):1237‐43. [MEDLINE: ] [PubMed] [Google Scholar]
Viglino 1989 {published data only}
- Viglino G, Colombo A, Scalamogna A, Cavalli PL, Guerra L, Renzetti G, et al. Prospected randomized study of two Y devices in continuous ambulatory peritoneal dialysis (CAPD). Peritoneal Dialysis International 1989;9(3):165‐168. [MEDLINE: ] [PubMed] [Google Scholar]
Viglino 1993 {published data only}
- Viglino G, Colombo A, Cantu P, Camerini C, Catione L, Bonello F, et al. In vitro and in vivo efficacy of a new connector device for continuous ambulatory peritoneal dialysis. Peritoneal Dialysis International 1993;13(Suppl 2):S148‐S51. [PubMed] [Google Scholar]
Wong 2006 {published data only}
- Wong HS, Ong LM, Lim TO, Hooi LS, Morad Z, Ghazalli R, et al. A randomized, multicenter, open‐label trial to determine peritonitis rate, product defect, and technique survival between ANDY‐Disc and UltraBag in patients on CAPD. American Journal of Kidney Diseases 2006;48(3):464‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wust 1985 {published data only}
- Wust J, Binswanger U, Bichsel G. Prevention of peritonitis during continuous ambulatory peritoneal dialysis (CAPD) by Y‐connected double bag systems. Life Support Systems 1985;3 Suppl 1:107‐11. [MEDLINE: ] [PubMed] [Google Scholar]
Additional references
Ansell 1998
- Ansell D, Feest T. UK Renal Registry Report 2002. http://www.renalreg.com/Downloads.html.
Bazzato 1980
- Bazzato G, Landini S, Coli U, Lucatello S, Fracasso A, Maoracchiello M. A new technique of continuous ambulatory peritoneal dialysis (CAPD): double‐bag system for freedom to the patient and significant reduction of peritonitis. Clinical Nephrology 1980;13(6):251‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Buoncristiani 1980
- Buoncristiani U, Cozzari M, Carobi C, Quintaliani G, Barbarossa D, Paolo N. Semicontinuous semiambulatory peritoneal dialysis. Proceedings of the European Dialysis & Transplant Association 1980;17:328‐32. [MEDLINE: ] [PubMed] [Google Scholar]
Buoncristiani 1983
- Buoncristiani U, Paolo N. Autosterilizing CAPD connection systems. Nephron 1983;35(4):244‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Buoncristiani 1993
- Buoncristiani U. Continuous ambulatory peritoneal dialysis connection systems. Peritoneal Dialysis International 1993;13 Suppl(2):139‐45. [MEDLINE: ] [PubMed] [Google Scholar]
Buoncristiani 1996
- Buoncristiani U. Birth and evolution of the “Y” set. ASAIO Journal 1996;42(1):8‐11. [MEDLINE: ] [PubMed] [Google Scholar]
CANUSA 1996
- Anonymous. Adequacy of dialysis and nutrition in continuous peritoneal dialysis: association with clinical outcomes. Canada‐USA (CANUSA) Peritoneal Dialysis Study Group. Journal of the American Society of Nephrology 1996;7(2):198‐207. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Churchill 1991
- Churchill DN. CAPD peritonitis: a critical appraisal of prophylactic strategies. Seminars in Dialysis 1991;4(2):94‐100. [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315(109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Golper 1996
- Golper TA, Brier ME, Bunke M, Schreiber MJ, Bartlett DK, Hamilton RW, et al. Risk factors for peritonitis in long‐term peritoneal dialysis: the Network 9 peritonitis and catheter survival studies. American Journal of Kidney Diseases 1996;28(3):428‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnson 2003
- Johnson DW. Peritoneal Dialysis. In: McDonald SP, Russ GF editor(s). ANZDATA Registry Report 2003. Adelaide: Australia and New Zealand Dialysis and Transplantation Registry, 2003:39‐59. [Google Scholar]
Nolph 1985
- Nolph KD, Prowant B, Serkes KD, et al. A randomized multicenter clinical trial to evaluate the effects of ultraviolet germicidal system on peritonitis rate in continuous ambulatory peritoneal dialysis. Peritoneal Dialysis Bulletin 1985;5(1):19‐24. [1985093789] [Google Scholar]
Popovich 1976
- Popovich RP, Moncrief JW, Decherd JF, Bonar JB, Pyle WK. The definition of a novel/wearable equilibrium peritoneal dialysis technique (Abstract). American Society of Artificial Internal Organs 1976;5:64. [Google Scholar]
Slingeneyer 1983
- Slingeneyer A, Mion C. Peritonitis prevention in continuous ambulatory peritoneal dialysis: long term efficacy of a bacteriological filter. Proceedings of the European Dialysis & Transplant Association 1983;19:388‐96. [MEDLINE: ] [PubMed] [Google Scholar]
Strippoli 2004a
- Strippoli GFM, Tong A, Johnson D, Schena FP, Craig JC. Antimicrobial agents for preventing peritonitis in peritoneal dialysis patients. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004679.pub2] [DOI] [PubMed] [Google Scholar]
Strippoli 2004b
- Strippoli GFM, Tong A, Johnson D, Schena FP, Craig JC. Catheter type, placement and insertion techniques for preventing peritonitis in peritoneal dialysis patients. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004680.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Daly 2000
- Daly C, Campbell M, Cody J, Grant A, Donaldson C, Vale L, et al. Double bag or Y‐set versus standard transfer systems for continuous ambulatory peritoneal dialysis in end‐stage renal disease. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD003078] [DOI] [PubMed] [Google Scholar]


