Abstract
Background
The presence of residual tumour after primary debulking surgery is the most important prognostic factor in patients with advanced ovarian cancer. In up to 60% of cases, residual tumour of more than 1 cm is left behind, stressing the necessity of accurately selecting those patients who should be treated with primary debulking surgery and those who should receive neoadjuvant chemotherapy instead.
Objectives
To determine if performing an open laparoscopy after the diagnostic work‐up of patients suspected of advanced ovarian cancer is accurate in predicting the resectability of disease.
Search methods
We searched MEDLINE, EMBASE, The Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Register of Diagnostic Test Accuracy Studies, MEDION and ISI Web of Science to February 2013. Furthermore, we checked references of identified primary studies and review articles.
Selection criteria
We included studies that evaluated the diagnostic accuracy of laparoscopy to determine the resectability of disease in patients who are suspected of advanced ovarian cancer and planned to receive primary debulking surgery.
Data collection and analysis
Two review authors assessed the quality of included studies using QUADAS‐2 and extracted data on study and patients' characteristics, index test, target condition and reference standard. Data for two‐by‐two tables were extracted and summarised graphically. Sensitivity and specificity and negative predictive values were calculated.
Main results
We included seven studies reporting on six cohorts. Between 27% to 64% of included patients per study were positive on laparoscopy (too extensive disease to warrant laparotomy) and between 36% to 73% were negative (disease suitable for debulking laparotomy). Only two studies avoided partial verification bias and provided data to calculate sensitivity and specificity, which did not justify meta‐analysis. These two studies had a sensitivity of 0.70 (95% confidence interval (CI) 0.57 to 0.82) and 0.71 (95% CI 0.44 to 0.90); however, the specificity of both studies was 1.00 (95% CI 0.90 to 1.00). In these two studies there were no false positives, i.e. no patients for whom laparoscopy indicated that major surgery would not be successful and should be avoided, whereas, in reality the patient could be successfully operated upon. Negative predictive values (NPV), for those patients who were diagnosed with having not too extensive disease correctly identified were 0.75 (95% CI 0.55 to 0.86) and 0.96 (95% CI 0.56 to 0.99) due to a different prevalence. Although the studies did report sufficient data to calculate NPVs, we judged these estimates too heterogeneous to meta‐analyse.
Three studies described the development or validation of a prediction model with a clear cut‐off for test positivity. Sensitivity and specificity of these prediction models were 0.30 to 0.70 and 0.89 to 1.00, respectively. However, one of these studies suffered from partial verification bias.
Authors' conclusions
Laparoscopy is a promising test, but the low number of studies and the differences between the included studies do not allow firm conclusions to be drawn from these data. Due to a difference in prevalence, there is a wide range in negative predictive values between studies. Two studies verified all patients. These imply a high specificity of laparoscopy in diagnosing resectability and have a good sensitivity. Both studies show that the use of criteria for unresectable disease will result in no patients inappropriately unexplored. However, there will still be patients undergoing unsuccessful primary laparotomy. Using a prediction model does not increase the sensitivity and will result in more unnecessarily explored patients, due to a lower specificity.
Keywords: Female; Humans; Laparoscopy; Chemotherapy, Adjuvant; Laparoscopes; Neoplasm, Residual; Ovarian Neoplasms; Ovarian Neoplasms/drug therapy; Ovarian Neoplasms/pathology; Ovarian Neoplasms/surgery; Randomized Controlled Trials as Topic; Tumor Burden; Validation Studies as Topic
Laparoscopy in diagnosing extensiveness of ovarian cancer
Background Ovarian cancer is a disease with a high mortality. Worldwide, approximately 200.000 women receive a diagnosis of ovarian cancer annually; of these 75% are at an advanced stage and 140.000 women die of this disease each year. Although response to primary treatment is high, most patients have recurrent disease and become resistant to treatment resulting in this high mortality.
Review question The diagnostic evaluation of a woman with suspected ovarian cancer includes a physical examination, an ultrasonography, an abdominal computed tomography (CT‐scan), and measurement of serum tumour marker CA‐125 and CEA. Standard treatment of ovarian cancer consists of primary cytoreductive surgery followed by six courses of taxane‐ and platinum‐based systemic chemotherapy. The goal of cytoreductive surgery is to resect all macroscopic tumour or at least to reduce the largest tumour residuals to less than a centimetre. When the diagnostic evaluation suggests that it is impossible to accomplish complete cytoreduction or when a patient is unable to sustain extensive surgery, than interval debulking surgery (IDS) could be an alternative. IDS implies three cycles of neoadjuvant chemotherapy (NACT) followed by cytoreductive surgery and another three courses of chemotherapy. However if at primary debulking it is not possible to remove all disease to at least residuals of less than one centimetre another laparotomy will be performed after three courses of chemotherapy.
The aim of this review was to investigate if laparoscopy is more accurate to diagnose extensiveness of disease than standard staging. If so, a useless and unnecessary primary laparotomy can be avoided and these patients can start immediately with neoadjuvant chemotherapy followed by interval surgery.
Main findings In total we identified 7 studies which reported on 6 cohorts of patients. In these studies 364 patients underwent a laparoscopy to evaluate the extensiveness of disease in the abdomen. All studies concluded that if at laparoscopy it was thought that removing macroscopic to at least residual disease of less than one centimetre was not feasible this was correct. However, even when performing a laparoscopy there are still patients primarily operated who have an unsuccessful debulking.
Quality of the evidence A limitation of this review is that only two studies performed the laparoscopy and the laparotomy in all patients. The other studies only performed a laparotomy when at laparoscopy it was thought that no optimal result was feasible. Most studies suffer therefore from verification bias, which makes it impossible to draw conclusion on sensitivity of this test. Three studies develop or validated a prediction model including laparoscopy. Using a prediction model does not increase the sensitivity and will result in more unnecessarily explored patients.
Background
Epithelial ovarian cancer is one of the leading causes of death in gynaecological malignancies and the seventh most common cancer in the world among women (Globocan 2008). Women are commonly diagnosed with the disease at an advanced stage, when the tumour has spread through the abdominal cavity or into the liver or the pleural fluid (Siegel 2012). Although the five‐year overall survival in the early stages is high, the five‐year overall survival in an advanced stage is around 30%. Despite an initial response rate of 80%, recurrence occurs in 70% of women, and the median survival for women with advanced disease is two to four years (Munkarah 2004).
Standard treatment of patients with advanced ovarian cancer is a combination of debulking surgery and chemotherapy consisting of carboplatin and paclitaxel. The aim of debulking surgery is to leave no visible macroscopic tumour. If this cannot be achieved, the diameter of separate residual tumour metastases should be as small as possible, ideally less than 1 cm in diameter, as survival is related to the size of the residual tumour (Bristow 2002; Du Bois 2009; Eisenkop 1998; Elattar 2011). Debulking surgery leaving the largest residual tumour metastasis greater than 1 cm in diameter is regarded as sub‐optimal.
An alternative strategy to primary surgery followed by chemotherapy is treatment with chemotherapy first, followed by surgery after three cycles of chemotherapy, followed by a further three cycles after interval surgery, so called neoadjuvant chemotherapy. Neoadjuvant chemotherapy is given in patients with co‐morbidities or, if primary surgery is likely to leave a large residual tumour (Morrison 2012; Vergote 2013). Also, if the first surgery was not a maximal attempt by an experienced gynaecological oncologist, an interval debulking may improve survival. These patients could be operated on again after three courses of chemotherapy, followed by another three courses of chemotherapy (Rose 2004). In these cases, primary debulking surgery may lead to surgical morbidity without any gain in survival (Vergote 2010). Ideally, primary surgery leaving residual tumour deposits of more than 1 cm should be avoided and women with disease which is not debulkable initially, should be treated with neoadjuvant chemotherapy.
Clinical pathway
Standard diagnostic work‐up of women with suspected advanced ovarian cancer consists of clinical judgement based on performance status and physical examination, ultrasonography, serum cancer antigen 125 (CA 125) measurement and computed tomography (CT) or magnetic resonance imaging (MRI) scan evaluation (Ramirez 2011). When women are thought to have operable disease, primary surgery can be attempted. However, the ability of the standard diagnostic work‐up to predict accurately who might benefit from primary surgery is low, resulting in 25% to 62% of primary surgery with residual tumour depositions of more than 1 cm in diameter (Gerestein 2009; Vergote 2010). This suggests that current diagnostic work‐up is not sufficiently accurate and should be improved. Staging laparotomy is the most accurate way to determine if the amount of tumour in the abdomen is too extensive to achieve complete surgery results. Yet, a laparotomy is a very invasive intervention for diagnostic purposes. Diagnostic laparoscopy is a less invasive surgical option for determining operability. In some institutes, laparoscopy is a standard procedure in the diagnostic work‐up, whilst in other institutes a laparoscopy is only performed when there is doubt about the resectability of tumour metastases. This surgical diagnostic procedure, under general anaesthesia, is an extra intervention with both risk of complications and increased costs. The overall risk of complications of a diagnostic laparoscopy is between 1% and 5% depending on the type of surgery and patient population (Chi 2004).
Role of index test(s)
So far, solid evidence is lacking that laparoscopy can reliably predict the outcome of debulking surgery. However, it is already the standard of care in many centres. If we are able to identify, prior to surgery, those women with ovarian cancer who have metastatic disease that is likely to be too extensive to be resected at debulking surgery, unnecessary surgery could be prevented. During laparoscopy the entire abdominal cavity is examined systematically, inspecting the ovaries, fallopian tubes, uterus, pelvic peritoneum, omentum, serosa and mesentery of the large and small bowel, spleen, liver surface, paracolic gutters and diaphragm. Those women who are diagnosed with too extensive disease to be removed during laparotomy will be treated with neoadjuvant chemotherapy (Figure 1). This would improve the rate of successfully operated women, limiting unnecessary morbidity and costs.
Figure 1.
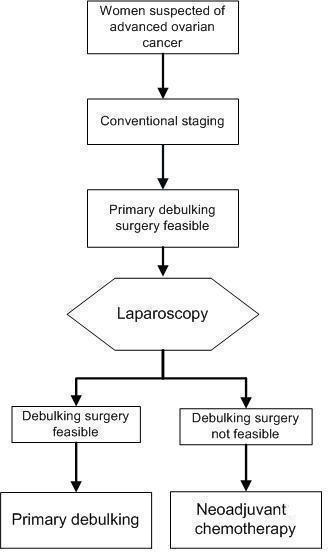
Laparoscopy is used as a triage test. If laparoscopy is positive indicating that the target condition is present (i.e. a direct debulking operation would be unsuccessful: residual cancer > 1 cm is left behind). For these patients a primary debulking could be avoided and they will be treated with neoadjuvant chemotherapy. The existing pathway would be that every patient will receive an explorative laparotomy where in this flow diagram the laparoscopy is placed.
Objectives
In this review we aim to determine if an open laparoscopy, after the conventional diagnostic work‐up of women suspected of advanced ovarian cancer, accurately predicts extensiveness and therefore, resectability of disease.
Methods
Criteria for considering studies for this review
Types of studies
We included studies that evaluated laparoscopy as a diagnostic added test to determine resectability of disease in women with suspected advanced ovarian cancer who were planned for primary debulking surgery after conventional staging. In these studies the extensiveness of disease diagnosed with a laparoscopy was compared with the diagnosis at laparotomy. Because of the bias which can be introduced by case‐control studies, these were excluded from the review.
We expected that there would be studies in which not all participants had undergone reference standard (i.e. laparotomy) when the index test was positive (i.e. unsuitable for debulking surgery as likely to lead to sub‐optimal result). Therefore, we also included studies in which only the participants who had a negative result of the index test (meaning that the tumour was not too extensively spread to be operated upon) underwent the reference standard.
Participants
Participants included women suspected of having advanced stage ovarian carcinoma (FIGO stage IIB, IIC, IIIA to C, IV), who were scheduled for primary debulking surgery and did not have contra‐indications for laparoscopy or laparotomy.
Index tests
The test under evaluation was an additional open diagnostic laparoscopy that was performed when a patient was planned for primary debulking surgery after conventional diagnostic work‐up. Conventional diagnostic work‐up consisted of physical and ultrasound examination, serum CA 125 measurement and/or CT or MRI scan.
A positive index test result means that the tumour was too extensively spread to be operated upon; a negative index test result means that the tumour, according to laparoscopy, was not too extensively spread to be resected (Figure 2).
Figure 2.
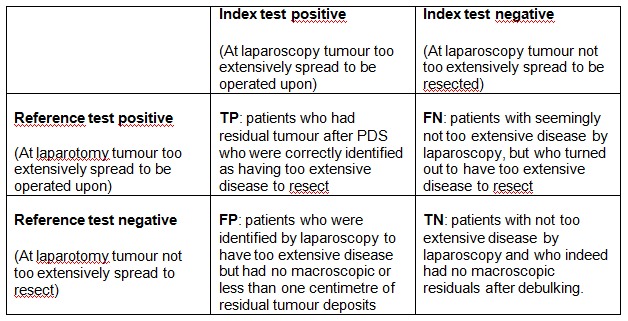
Two by two table of index test results by reference standard outcomes. TP= True Positive, TN= True Negative, FP= False Positive, FN= False Negative
Target conditions
The target condition was ovarian cancer deposits that could not be resected at laparotomy to at least less than 1 cm in diameter and therefore makes patients not suitable for primary debulking surgery. Examples of ovarian cancer deposits that cannot be resected include extensive peritoneal and mesenteric carcinomatoses (> 100 spots) or extensive metastases in the upper abdomen, such as bulky disease on the diaphragm or liver surfaces. The definition of ovarian cancer deposits that could not be resected at laparotomy was extracted from each study.
Reference standards
Laparotomy was the reference standard. When performing a laparotomy one is able to explore the entire abdominal cavity and to locate all tumour deposits. The goal at laparotomy is to remove all macroscopic tumour, therefore giving a true impression of the resectability of ovarian cancer deposits in the abdominal cavity.
Search methods for identification of studies
Electronic searches
For identifying any eligible studies, searches were run in the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL); the Cochrane Register of Diagnostic Test Accuracy Studies; MEDLINE (OvidSP), EMBASE (OvidSP), MEDION and Science Citation Index and Conference Proceedings Citation Index (ISI Web of Science), to February 2013. The search strategies can be found in Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5. No language restrictions were made.
Searching other resources
Manually searching the references of articles retrieved from the computerised databases and searching relevant review articles did not identify additional references.
Data collection and analysis
We followed the guidelines provided in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (DTA Handbook 2010).
Selection of studies
Two review authors (MR and MB) independently reviewed all citations identified by the search strategy described above, first by title and abstract and when necessary by review of the full text of the study report, to determine eligibility. To be eligible, each publication was assessed to determine if participants met the inclusion and exclusion criteria detailed above. Abstracts were included if sufficient information was provided to apply the selection criteria and if they included data for analysis.
Data extraction and management
A data extraction form was developed and piloted using a subset of the identified studies. Two authors (MR and MB) independently completed a data extraction form on all included studies. Any discrepancies were resolved by discussion. The following data were retrieved.
General information: title, journal, year, publication status, and study design (prospective versus retrospective).
Sample size: number of participants meeting the criteria and total number of participants included in analyses.
Baseline characteristics: age, disease stage as defined by the guidelines of FIGO.
Inclusion and exclusion criteria.
The index test: technique of laparoscopy and cut‐off for test positivity.
Reference standard test: reference standard used; if not all patients received reference standard, how many and what proportion of the total did not; definition of complete, optimal and suboptimal result at laparotomy.
Whether or not the laparoscopy or laparotomy was performed by a gynaecological‐oncologist or general gynaecologist.
Number of true positives (TP): patients who had residual tumour after primary debulking surgery (PDS) who were correctly identified as having too extensive disease to resect.
Number of true negatives (TN): patients with not too extensive disease by laparoscopy and who indeed had no macroscopic residuals after debulking.
Number of false positives (FP): patients who were identified by laparoscopy to have too extensive disease but had no macroscopic or less than 1 cm of residual tumour deposits.
Number of false negatives (FN): patients with seemingly not too extensive disease by laparoscopy, but who turned out to have too extensive disease to resect.
Number of missing, uninterpretable or doubtful results. Number of complete, optimal and suboptimal resections.
Side effects or complications due to the laparoscopy.
Assessment of methodological quality
Methodological quality of the eligible studies was assessed using QUADAS‐2 (Quality Assessment of Diagnostic Accuracy Studies), an evidence‐based tool for the assessment of quality in systematic reviews of diagnostic accuracy studies (Whiting 2006; Whiting 2011). The tool is based on items that cover a wide range of methodological issues in diagnostic test studies.
QUADAS‐2 comprises four domains: patient selection, index test, reference standard, and flow and timing. Each domain is assessed in terms of risk of bias, and the first three domains are also assessed in terms of concerns regarding applicability. Signalling questions are included to help judge risk of bias. The QUADAS‐2 tool is applied in four phases: summarise the review question (Appendix 6), tailor the tool and produce review‐specific guidance (Appendix 7), construct a flow diagram for the primary study, and judge bias and applicability.
The tool was independently piloted on two primary studies and no refinements were needed. After testing, the tool was used to rate all included studies. Quality assessment of studies was independently performed by review authors MR and MB. Any disagreement was resolved by consensus.
Statistical analysis and data synthesis
Data from each study is summarised in two by two tables of TP, FP, TN, FN and used to calculate sensitivity and specificity for each study. Studies providing insufficient data to construct two by two tables were used to present data on negative predicting values (NPV). NPV is defined as the number of patients with no residual disease who were correctly defined (TN) divided by the total number of patients who were thought to have no residual disease after debulking surgery (TN + FN) (Figure 2).
Individual study results are presented graphically by plotting the estimates of sensitivity and specificity if possible and negative predictive values (and their 95% confidence intervals). All plots were done using RevMan 5.2 and Excel.
Analyses were done in Excel and SAS statistical software (SAS Institute Inc, Cary, NC, USA).
Investigations of heterogeneity
The main sources of heterogeneity in diagnostic accuracy we expected to encounter were likely to be related to differences in the disease stage of patients included in the study or to the person performing the laparoscopy or laparotomy, or differences in conventional staging. However, sources of heterogeneity for sensitivity or specificity were not investigated because we retrieved only seven studies reporting on six cohorts. For example, in only three studies was the performer of the laparoscopy mentioned.
We did perform investigation for heterogeneity among estimates of NPV and among estimates of test positivity. Hereto, we used Cochran's Q‐test and used the I2 to estimate the amount of heterogeneity (Higgins 2002). We decided not to pool data if there turned out to be significant heterogeneity, based on the results of Cochrane's Q‐test (P value < 0.05 was considered to be statistically significant). The Q‐statistic was based on the weight (1/variance) and the logit of the negative predictive value of each study, estimate in a univariate model (only test negatives counted), because for most of these studies there were not enough data to do this in a bivariate model.
Sensitivity analyses
The most important form of bias that we expected to encounter was (both partial and differential) verification bias. However, sensitivity analyses could not be performed because too few studies were retrieved.
Assessment of reporting bias
Tests to detect publication bias are currently used for systematic reviews of clinical trials. However, similar tests have not been designed for reviews of diagnostic studies and in the absence of appropriate methodology, publication bias was not explored in our review.
Results
Results of the search
We identified 1972 citations from the electronic searches in MEDLINE, 2693 citations from EMBASE, and 60 from the Cochrane Central Register of Controlled Trials (CENTRAL). Searching MEDION, the Science Citation Index and Conference Proceedings Citation Index did not lead to any additional citations. The references of relevant reviews and primary diagnostic studies were checked. After initial evaluation, 16 full papers were retrieved, seven of which were finally considered eligible for the review. There were no disagreements between the review authors on studies eligible for the review. A summary of the search results, including the main reason for excluding papers is presented in Figure 3. Main reasons for exclusion were if studies did not report on diagnosis of unresectable disease or if they were not diagnostic studies (Characteristics of excluded studies). Furthermore, we retrieved one congress abstract for which the full article was available and was used in this review. No case‐control studies were identified by our search. Two studies reported on validation of an earlier developed prediction model. Because this model was based on diagnostic laparoscopic criteria only, it was evaluated as a diagnostic test. Details of all included studies on the design, setting, population, target condition and reference standard of each included study can be found in the Characteristics of included studies.
Figure 3.
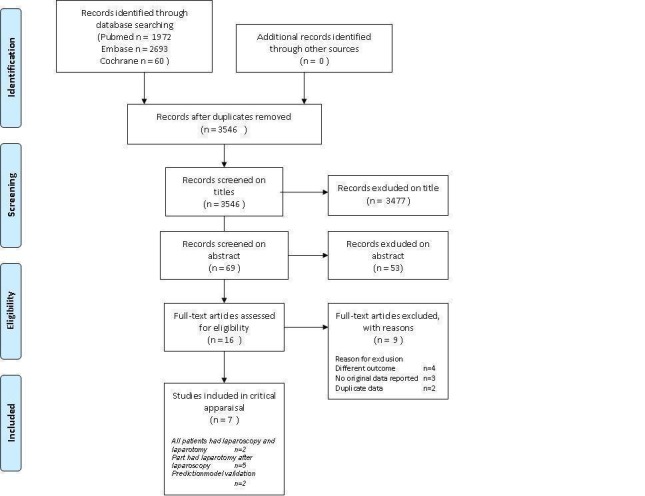
Study flow diagram: Results of the search for studies evaluating the diagnostic accuracy of laparoscopy to determine resectability of disease in patients who are suspected of advanced ovarian cancer and planned for primary debulking surgery after conventional staging.
Methodological quality of included studies
The results of the quality assessment are presented in Figure 4 and Figure 5 for the seven included studies. No study was judged as low risk of bias and low concern regarding applicability in all domains. Every study was judged on one domain to be at high or unclear risk of bias or having concerns regarding applicability. Except for two studies (Fagotti 2004; Fagotti 2008), in which a laparotomy was performed in all patients, all studies had a high risk of bias concerning flow and timing. However, these two studies have high concerns regarding applicability in the patient domain, because they included not solely patients before primary debulking, but also before interval debulking surgery and surgery for recurrence. Unfortunately it was not possible to analyse the primary debulking group of these two studies separately.
Figure 4.
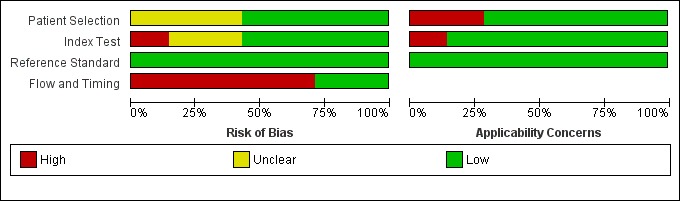
'Risk of bias' and applicability concerns graph: review authors' judgements about each domain presented as percentages across included studies
Figure 5.
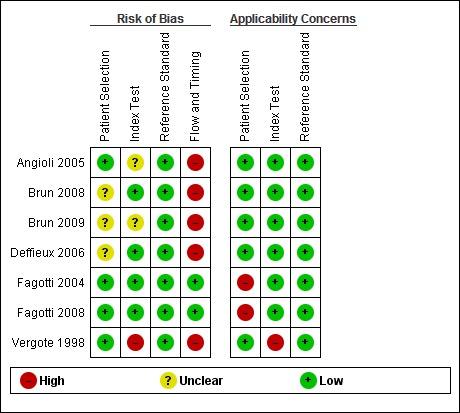
'Risk of bias' and applicability concerns summary: review authors' judgements about each domain for each included study
The study by Vergote et al (Vergote 1998) was judged at high risk of bias and high concern of applicability on more then one domain. There were concerns about bias because not all the patients received the reference test, but also high concern about bias and applicability because there was not a clear description of test positivity or threshold used at laparoscopy. In the studies of Angioli 2005 and Brun 2009, the threshold for test positivity of the index test was based on a prediction rather than presence of predefined criteria, therefore, these two studies were judged as unclear in risk of bias for index test.
Although in all studies the result of the reference standard was interpreted with the knowledge of the result of the index test, the risk of bias concerning the reference standard was scored as low. Not only was an explorative laparotomy performed in all index test negative patients to judge the extensiveness of disease, but also all the women judged as operable underwent debulking surgery. Therefore a true conclusion about the resectability of the tumour deposits could be drawn at the end of the surgical intervention. Because a primary debulking surgery leaving no or less than 1 cm of residual tumour is associated with a better prognosis, it is likely that all women, who were thought at laparotomy to be operable, underwent an attempt at debulking surgery.
Findings
We identified seven studies, describing six cohorts of patients, evaluating laparoscopy as a diagnostic test for extensiveness of disease in advanced ovarian cancer patients. Two studies described the validation of a prediction model based on laparoscopic disease criteria (Brun 2008; Fagotti 2008). These two studies were included in the final analyses because they had a clear cut‐off for test‐positivity based only on criteria which were diagnosed using a diagnostic laparoscopy. The prediction model validation study of Brun 2008 used the same population as described in the study of 2009 (Brun 2009).
In total, 364 women suspected of primary advanced ovarian cancer after conventional work‐up (range 15 to 113), who had a diagnostic laparoscopy to evaluate the possibility of primary debulking surgery were included in the studies. Two studies also included women undergoing laparoscopy prior to interval debulking surgery or surgery for recurrent disease (41 and three patients respectively) (Fagotti 2004; Fagotti 2008), therefore, the total number of included patients in all studies was 408. In all studies 179 patients were at laparoscopy diagnosed with unresectable disease. In four studies the index test positive patients (those who were thought to have too extensive disease to achieve residual disease of less than 1 cm at surgery did not undergo a laparotomy), thus 113 patients were not verified.
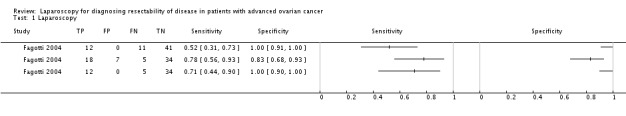
Only two studies performed a laparoscopy and laparotomy in all included patients. In these two studies all women who were diagnosed at laparoscopy with disease that was too extensive to be resected at debulking surgery were diagnosed as indeed having unresectable disease at laparotomy. These patients could have been treated with neoadjuvant chemotherapy straight away as shown in Figure 1. The specificity of laparoscopy in both studies was therefore 1, meaning that all women in whom the tumour could be successfully resected, showed not too extensive spread of tumour at laparoscopy. Sensitivity of laparoscopy in these two studies was 0.71 (95% confidence interval (CI) 0.44 to 0.90) and 0.70 (95% CI 0.57 to 0.82), which means that of all women with unresectable disease, 70% or 71% indeed showed too much spread of the tumour on laparoscopy. However, leaving 29% or 30% of women with unresectable disease at laparotomy because it was not diagnosed at laparoscopy. Fagotti 2004 did not even include all women in the analysis because in 13 cases laparoscopy could not diagnose the extensiveness of disease. The values given in the study of Fagotti 2004 were therefore based on only 80% of included cases, resulting in overestimation of sensitivity and specificity. Furthermore, 25% of included women in these two studies had laparoscopy before interval debulking surgery or because of recurrent disease.
In all studies, patients received a laparotomy when the laparoscopy had a negative test result (indicating resectable disease). However, the laparoscopy had a false negative test result in 4% to 30% of patients and these patients should not have received a laparotomy. Data for individual studies are presented in Figure 6. Negative predictive values (NPV) ranged from 69% to 96% (Figure 7), meaning that of all women in whom laparoscopy seemed to show that disease that was limited enough to be operated upon, 69% to 96% indeed had a successful operation afterwards, with no residual tumours larger than 1 cm. There was a large difference in the prevalence of negative test result ranging form 36% to 73%, which will have influenced the NPVs. The studies showing a high percentage of test negative results of the laparoscopy had a higher NPV (Figure 8). Because of considerable heterogeneity (Q = 30.32 (P value < 0.001); I2 = 84%), NPV’s were not pooled and the low number of studies hindered performing sensitivity analyses to correct for differences in reference standard threshold or partial verification.
Figure 6.
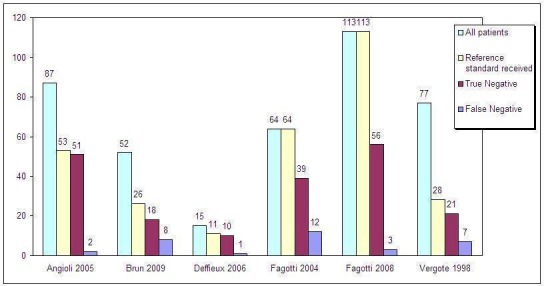
Absolute numbers of all patients, patients who received the reference standard, and false negative and true negative test results. Only Fagotti et al (2004 and 2008) validated all index test results, the other studies only verified test negatives.
Figure 7.
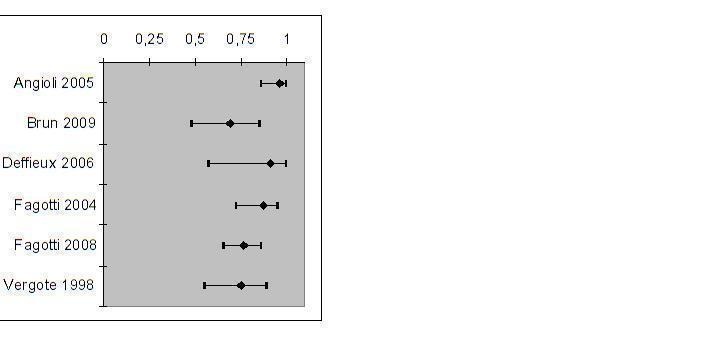
Negative Predictive Values and their 95% CIs for each primary cohort
Figure 8.
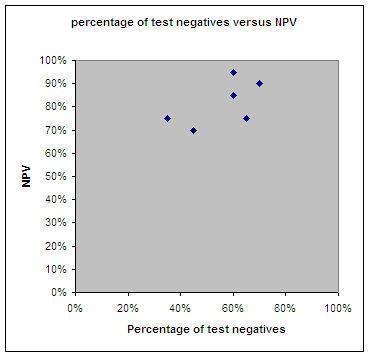
Percentage of test negative results and NPV of each included study
The percentage of test‐positives (those patients who were thought to have too extensive disease to have successful surgery and could start with neoadjuvant chemotherapy) ranged between 27% and 64% and test‐negatives ranged between 36% to 73% per study (Figure 9).
Figure 9.
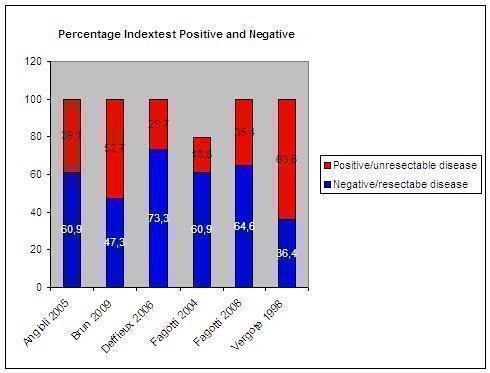
Percentage of patients per study with a negative and positive index test result. The percentage of positive index test results varied between 19% and 64%. Only Fagotti et al (2004 and 2008) validated these results. Both studies found no false positives. In the study of Fagotti 2004 13 patients were not evaluable by laparoscopy.
Although all studies included women suspected of advanced ovarian cancer, based on standard diagnostics, i.e. clinical and/or radiological examination, only one study evaluated the additional value of laparoscopy after clinical/radiological diagnostics with only clinical/radiological evaluation (Fagotti 2004). Adding laparoscopy to clinical/radiological evaluation resulted in more women considered to have resectable disease but who were found not to be operable at laparotomy than when only using clinical/radiological evaluation (NPV 89% versus 87%). Heterogeneity between the studies could be caused by a difference in the reason to perform a laparoscopy. Deffieux 2006 included only women in whom the preoperative evaluation of the extent of tumour was unsatisfactory. Fagotti et al (Fagotti 2004; Fagotti 2008) instead excluded patients with advanced ASA score and/or a large mass reaching the xiphoid because they were thought not to be operable by means of standard diagnostics. Another reason for heterogeneity could be due to the definition of the target condition, which was either any macroscopic residual tumour or deposits of more than 1 cm in diameter. Fagotti 2004, Fagotti 2008 and Vergote 1998 used resectable disease to less than 1 or 0.5 cm respectively, whereas the rest aimed at leaving no macroscopic tumour.
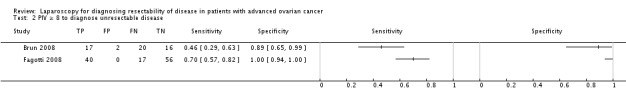
There were two studies identified by the search using a cut‐off value of test‐positivity derived from a prediction model using diagnostic criteria for extensiveness of disease diagnosed with a laparoscopy. Fagotti 2006 developed a prediction model using a laparoscopy‐based score using almost all women from the cohort of Fagotti 2004. In this prediction model peritoneal carcinosis, diaphragmatic disease, mesenteric disease, omental disease, stomach infiltration, bowel infiltration and liver metastases were used as diagnostic criteria. Presence of the disease was scored with two points and the total score was used to calculate the Predictive Index Value (PIV). A PIV of eight diagnosed too extensive disease (Data and analyses table). Criteria used to calculate the PIV are also presented in the Characteristics of included studies at the Index test section. This prediction model was externally validated by Fagotti 2008 in a new and larger cohort; sensitivity in this study was 0.70 (95% CI 0.57 to 0.82) and specificity 1.00 (95% CI. 0.94 to 1.00). Brun 2008 also performed an external validation of the prediction model developed by Fagotti et al in 2006 (Fagotti 2006). Sensitivity was 0.46 (95% CI 0.29 to 0.63) specificity was 0.89 (95% CI 0.65 to 0.99). Results from the study of Brun 2008 could lead to an over‐ or underestimation because only 26 of 55 women received a laparotomy verifying the diagnosis of the laparoscopy.
Summary of findings
Summary of findings.
Summary of findings table
| To determine if adding an open laparoscopy to the diagnostic work‐up of patients suspected of advanced ovarian cancer is accurate in predicting resectability of disease? Population: Patients suspected of advanced ovarian cancer Prior testing: Standard diagnostic work‐up consisting of clinical and radiological evaluation Index tests: Open diagnostic laparoscopy after conventional work‐up. Target condition: Tumour which could not be resected at laparotomy (extensive disease) Reference standard: Explorative laparotomy. Studies: Cohort studies (5) and development/validation prediction model (n = 3) | |||||||
| Cut off test‐positivity laparoscopy |
Sensitivity range of estimates |
Specificity range of estimates |
Prevalence of positive test result (range) |
Prevalence of negative test result (range) |
Negative Predictive Value (range) |
Number of participants |
Quality (QUADAS) |
| Prediction of surgery result based on different criteria of unresectability or estimation 5 studies, no pooled analysis | 0.70 to 0.71* | 1.00* | 41% (27%‐64%) |
56% (36%‐73%) |
69%‐96% | 295 | High risk of bias1 Applicability concerns2 |
|
PIV ≥ 8 2 studies, no pooled analysis |
0.30‐0.70 | 0.89‐1.00 | 28% (10%‐40%) | 72% (65%‐90%) | 44%‐76% | 165 | Low risk of bias High concerns Applicability2 |
* Only two studies performed the reference standard in all patients, sensitivity and specificity are based on these two studies 1Four studies did not perform the reference standard in test‐positive patients 2 Applicability concerns based on inclusion of not only patients planned for primary cytoreductive surgery or conventional diagnostic work‐up not conclusive
Discussion
Summary of main results
We found only seven studies describing the diagnostic accuracy of laparoscopy to diagnose unresectable disease in advanced ovarian cancer patients at primary debulking surgery. Only one study evaluated clinical/radiological diagnostics with clinical/radiological and laparoscopic diagnostics. The threshold for test accuracy was based on the presence of one or more criteria of unresectability in two studies, on a prediction made by the person who performed the laparoscopy in another two studies and one study did not describe the threshold used. Two more studies had a threshold for test‐positivity based on a cut‐off value calculated with a prediction model.
In the included studies, between 27% and 64% of the women were considered to have too extensive disease to be referred for laparotomy (the index test positives). The other 36% to 73% were considered to be suitable for laparotomy (the test negatives) and they underwent a laparotomy. At laparotomy, between 4% and 31% were found to have residual tumour left after surgery, suggesting that they could have been spared laparotomy. The numbers of patients who were sent for neoadjuvant therapy, but who should have had debulking therapy first (the number of false positives), is not known for most of the studies.
The two studies that did avoid partial verification (Fagotti 2004; Fagotti 2008) imply that laparoscopy has a high specificity for predicting optimal debulking surgery (leaving residual tumour of not more than 1 cm in diameter). With a sensitivity of 0.70 and 0.71 within these two studies it is a promising test. However, in Fagotti 2004, 13 women had an uninterpretable diagnostic laparoscopy. If these 13 women were added to the index test positive group sensitivity and specificity would be 0.78 (95% CI 0.56 to 0.93) and 0.83 (95% CI 0.68 to 0.93) respectively, resulting in more women not undergoing surgery who could have had optimal debulking surgery. When added to the group judged to have no residual disease after surgery, sensitivity decreased to 0.52 (95% CI 0.31 to 0.73) (Data and analyses, data table Fagotti 2004).
Because only two studies verified all their included patients using a laparotomy, of all 168 (46%) patients diagnosed positive at the index test, only 52 were verified. Of these 52, none were identified as false positive at laparotomy and in all, unnecessary laparotomies could have been prevented. The other 116 patients were treated with neoadjuvant chemotherapy straight away. However, because survival is not worse after treatment with neoadjuvant chemotherapy (Vergote 2010), we are more interested in the false negative predicted patients. These are the patients for whom laparoscopy could prevent unnecessary surgery and who should receive neoadjuvant chemotherapy straight away, but were incorrectly subjected to laparotomy. NPV in all seven studies ranged from 69% to 96%, which means that of every 100 women referred for surgery after laparoscopy, between four and 31 will turn out to have too extensive metastases to remove. These patients will have a laparotomy that was intended to be avoided by adding the laparoscopy. However, negative predictive values are influenced by the prevalence of disease in the population that is being tested and those studies with a high percentage of test negative patients had a high NPV and vice versa.
It is not possible to provide a pooled estimate of NPV and two (likely biased) studies providing sensitivity and specificity are inadequate to draw firm conclusions. Not only was there a difference in the cut‐off of index test positivity, but also the endpoint of surgery was different between studies, ranging between 1 cm, 0.5 cm, or leaving no macroscopic tumour. This is likely to have contributed to the heterogeneity.
Strengths and weaknesses of the review
This review is the first systematic review on the accuracy of a diagnostic laparoscopy in the work‐up of women with suspected advanced ovarian cancer. We performed an extensive search addressing all available databases. However, only a small number of studies was available on this subject, which precludes performing a meta‐analysis and correction for factors leading to bias. Unfortunately, only in two studies (Fagotti 2004; Fagotti 2008) was the reference standard performed in all women. Therefore, the data could not be pooled. In addition, we were not able to perform a sensitivity analysis to correct for this form of bias due to this small number of studies. Furthermore, the only two studies avoiding verification bias conducted their studies in a heterogeneous population, without presenting their results for the primary debulking surgery patients alone. Therefore it is not clear if their results would have been the same in a more homogeneous population. Another factor that could have introduced heterogeneity was the way in which clinical and/or radiological staging in the studies was used for inclusion. All studies performed clinical and/or radiological staging before performing a laparoscopy. However only Fagotti et al (Fagotti 2004; Fagotti 2008) in both studies clearly stated that if on radiological findings there was a high suspicion of too extensive disease, or if the patient was in a poor condition, women were not included in the study. Another study by Deffieux et al (Deffieux 2006) only performed the laparoscopy when earlier diagnostics were not conclusive (Deffieux 2006). Therefore, we were not able to draw firm conclusions about the true added value of laparoscopy after clinical and radiological evaluation. Another factor for heterogeneity could be due to the difference in endpoint of debulking surgery (complete absence of macroscopic disease or residual deposits of less than 1 cm).
Finally, methodological quality was judged with QUADAS‐2. This is the most recent available tool for assessing methodological quality of diagnostic accuracy studies. Signaling questions concerning quality of included studies were added to adjust the quality tool to make it suitable for this specific review. The overview of study quality of Figure 5 shows clearly that none of the studies did not suffer from any kind of bias or applicability concern.
Applicability of findings to the review question
The diagnostic performance of an open laparoscopy may seem better than standard diagnostic staging alone. However, based on the results of the studies described in this review, laparoscopy should not be a standard procedure in clinical practice. When at laparoscopy very extensive disease is diagnosed, this was confirmed by laparotomy. However, after performing a diagnostic laparoscopy some women will have unresectable disease at laparotomy. The question whether diagnostic laparoscopy should be added to the standard work‐up cannot be answered based on the result of currently available studies.
In some clinics laparoscopy is already a standard intervention in the diagnostic process of women with advanced ovarian cancer. Women, who are diagnosed by laparoscopy with unresectable disease, will be treated with neoadjuvant chemotherapy. However, even though some women, who could be debulked to less than 1 cm residual disease might not have primary surgery if they were assessed laparoscopically, this will not influence survival as neoadjuvant chemotherapy and interval debulking surgery is not deemed inferior to primary debulking surgery followed by chemotherapy (Vergote 2010). Based on the results of our review, we are not able to show that the proportion of primary debulking surgery leaving more than 1 cm of residual tumour is less if a diagnostic laparoscopy is routinely performed.
Authors' conclusions
At the moment, there is no conclusive data that laparoscopy can diagnose the extensiveness of disease. When using criteria for unresectable disease no women will have debulking surgery inappropriately withheld. However, there will be some women who will undergo an unsuccessful primary laparotomy. Using a prediction model does not increase the sensitivity and will result in more patients unsuccessful debulking surgery. No statement can be made on whether laparoscopy is more accurate than clinical and radiological diagnostic work‐up.
Future research should focus on the additional value of a diagnostic laparoscopy and whether it can avoid unnecessary primary laparotomy. Clear inclusion and exclusion criteria and cut‐off values for test positivity should be used to minimise heterogeneity and to be able to draw conclusions. The accuracy of clinical/radiological diagnostics compared to clinical/radiological diagnostics and additional laparoscopy should be evaluated with clear predictive values.
A randomised controlled trial is ongoing, aimed at evaluating the additional value of laparoscopy to avoid primary debulking surgery leaving residual tumour of more than 1 cm in women with advanced ovarian cancer, (NTR 2644) (Rutten 2012) and we await the results. As patients with bulky and unresectable disease who can not be operated to small residual disease at primary debulking surgery do not have a worse prognosis when treated with neoadjuvant chemotherapy, evidence is needed for how we should appropriately select patients and avoid undue morbidity.
Data
Presented below are all the data for all of the tests entered into the review.
Tests.
Data tables by test
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 Laparoscopy | 1 | 179 |
| 2 PIV ≥ 8 to diagnose unresectable disease | 2 | 168 |
Test 1.
Laparoscopy.
Test 2.
PIV ≥ 8 to diagnose unresectable disease.
Acknowledgements
We thank Jo Morrison for clinical expertise, Gail Quinn for editorial assistance and Jane Hayes from the Cochrane Gynaecological Cancer Review Group for her help in the search for trials for inclusion in this review.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Gynaecological Cancer Group.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health
Appendices
Appendix 1. MEDLINE search strategy
1 exp Ovarian Neoplasms/ 2 Fallopian Tube Neoplasms/ 3 ((ovar* or fallopian tube*) adj5 (cancer* or tumor* or tumour* or adenocarcinoma* or carcino* or cystadenocarcinoma* or choriocarcinoma* or malignan* or neoplas* or metasta* or mass or masses)).tw,ot. 4 (thecoma* or luteoma*).tw,ot. 5 1 or 2 or 3 or 4 6 exp Laparoscopy/ 7 laparoscop*.tw,ot. 8 celioscop*.tw,ot. 9 peritoneoscop*.tw,ot. 10 abdominoscop*.tw,ot. 11 6 or 7 or 8 or 9 or 10 12 5 and 11 13 exp animals/ not humans.sh. 14 12 not 13
key:
tw,ot.=textword, original title
Appendix 2. EMBASE search strategy
1 exp ovary tumor/ 2 uterine tube tumor/ 3 ((ovar* or fallopian tube*) adj5 (cancer* or tumor* or tumour* or adenocarcinoma* or carcino* or cystadenocarcinoma* or malignan* or neoplas* or metasta* or mass or masses)).tw,ot. 4 (thecoma* or luteoma*).tw,ot. 5 1 or 2 or 3 or 4 6 exp laparoscopy/ 7 laparoscop*.tw,ot. 8 celioscop*.tw,ot. 9 peritoneoscop*.tw,ot. 10 abdominoscop*.tw,ot. 11 6 or 7 or 8 or 9 or 10 12 5 and 11 13 (exp Animal/ or Nonhuman/ or exp Animal Experiment/) not Human/ 14 12 not 13
key: tw,ot =textword, original title
Appendix 3. CENTRAL search strategy
#1 MeSH descriptor Ovarian Neoplasms explode all trees #2 MeSH descriptor Fallopian Tube Neoplasms, this term only #3 ((ovar* or fallopian tube*) near/5 (cancer* or tumor* or tumour* or adenocarcinoma* or carcino* or cystadenocarcinoma* or malignan* or neoplas* or metasta* or mass or masses)) #4 thecoma* or luteoma* #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Laparoscopy explode all trees #7 laparoscop* #8 celioscop* #9 peritoneoscop* #10 abdominoscop* #11 (#6 OR #7 OR #8 OR #9 OR #10) #12 (#5 AND #11)
Appendix 4. MEDION (http://www.mediondatabase.nl/)
ICPC code for female genital system ‐ "X"
Appendix 5. Science Citation Index
All citations found though the searches in MEDLINE, EMBASE and CENTRAL were checked in Science Citation Index for articles which cited these articles.
Appendix 6. Quadas review question and inclusion criteria
| Category | Review Question | Inclusion Criteria |
| Patients | Women with advanced stage ovarian cancer who are thought to have resectable disease after conventional diagnostic work‐up |
Women suspected of advanced stage ovarian cancer |
| Index test |
Additional open laparoscopy | Diagnostic laparoscopy |
| Target Condition |
Non‐resectable disease | Non‐resectable disease for which a definition is given |
| Reference standard |
Laparotomy | Laparotomy |
| Outcome |
NA | Sufficient data to construct a 2 x 2 table |
| Study Design | NA | Diagnostic cohort study |
Appendix 7. Quality indicator
| Risk of Bias | Applicabillity | ||||
| Quality indicator | Notes | Quality indicator | Notes | ||
|
Domain 1 Patient Selection |
Could the selection of patients have introduced bias? (High/low/unclear) |
Are there concerns that the included patients and settings do not match the review question? (High/low/unclear) | |||
| 1. Was a consecutive or random sample of patients enrolled? |
“Yes” if a consecutive or random sample of patients was enrolled “No” if a selected group of patients was enrolled “Unclear” if there is insufficient information on enrollement, |
1. Were the patients diagnosed by conventional diagnostic work‐up for advanced stage ovarian cancer? |
“Yes” if patients were diagnosed by conventional diagnostic work‐up with advanced stage ovarian cancer. “No” if patients included in the trial are diagnosed with low‐stage disease (FIGO I or IIA) only. No high stage disease patients in the trial. “Unclear” if there is insufficient information on recruitment method, criteria for diagnosis of ovarian cancer. |
||
| 2. Did the study avoid inappropriate exclusions? |
“Yes” if there were no inappropriate exclusions “No” if there were inappropriate exclusions “Unclear” if there is insufficient information on exclusions |
2. Were the patients planned for primary cytoreductive surgery after conventional diagnostic work‐up? |
“Yes” if the patients were planned for primary cytoreductive surgery after conventional diagnostic work‐up? “No” if none of the patients were planned for primary cytoreductive surgery “Unclear” if there is insufficient information |
||
| Domain 2 Index Test |
Could the interpretation of the Index test have introduced bias? (High/low/unclear) | Are there concerns that the index test, its conduct or the interpretation differ from the review question? (High/low/unclear) | |||
| 1. Were the index test results interpreted without the knowledge of the results of the reference standard? |
This will always be rated as yes, because the index test is performed before the reference standard |
1. Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? |
“Yes” if all usual clinical data (except laparotomy results) are available when the index test is interpreted, including details of physical examination, serum tumor markers, and ultrasound and CT/MRI imaging. Also answer “yes” if one of the items is missing. “No” if clinical information (as mentioned by “yes”) was not available to the gynaecologist “Unclear” if insufficient information is reported. |
||
| 2. Was the threshold used prespecified? |
“Yes” if a clear description of the threshold is given which was specified before start of the study. “No” if no clear description is given before hand “Unclear” if there is insufficient information within the paper to determine whether or not a prespecified threshold was used |
2. Did the study provide a clear definition of what was considered to be a 'positive' result for the index test? |
“Yes” if a clear description is given about when the index test is positive or negative. (e.g. what the cut‐off for too extensive abdominal disease was) “No” if there is no clear description of what is classified as too extensive disease or not. “Unclear” if there is insufficient information within the paper to determine whether or not a defined threshold was used to a positive test result |
||
| 3. Did the whole sample, or a random selection of the sample, receive verification using a reference standard of diagnosis? | “Yes” if all patients underwent the reference standard (laparotomy) “No” if not all patients underwent reference standard, also those who were tested negative by indextest didn't undergo reference test. “Unclear” if insufficient information is provided. |
||||
| 4. Did patients receive the same reference standard regardless of the index test result? | “Yes” if patients who underwent reference standard had laparotomy. “No” if patients did not undergo laparotomy. “Unclear” if insufficient information is provided. |
||||
| Domain 3 Reference Standard |
Could the interpretation of the reference standard have introduced bias? (High/low/unclear) |
Are there concerns that the target condition as defined by the reference standard does not match the question? (High/low/unclear) |
|||
| 1. Is the reference standard likely to correctly classify the target condition? |
“Yes” if the reference standard is laparotomy. “No” if the reference standard used is not the one defined in the protocol. “Unclear” if the information is insufficient |
1. Did the study provide a clear definition of what was considered to be a 'positive' result for the reference standard? |
“Yes” if a clear description is given about when the reference standard is positive or negative. (e.g. what the cut‐off at laparotomy is for too extensive abdominal disease was) “No” if there is no clear description of what is classified as too extensive disease or not. “Unclear” if there is insufficient information within the paper to determine whether or not a defined threshold was used to a positive test result |
||
| 2. Were the reference standard results interpreted without the knowledge of the results of the index test? | “Yes” if the report stated that the reference test is performed by individuals who did not perform the index test. “No” if the reference test were done by the same person performing the index test. “Unclear” if not reported. |
||||
| Domain 4 Flow and Timing |
Could the patient flow have introduced bias? (High/low/unclear) | ||||
| 1. Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | “Yes” if the time period between the index test and reference standard is not longer than 3 weeks. “No” if the time period is more than 3 weeks for an unacceptable high proportion of patients. “Unclear” if the information on the timing of tests is not provided |
||||
| 2. Did all patients receive the same reference standard? |
“Yes” if all patients underwent the reference standard (laparotomy) “No” if not all patients underwent reference standard, also those who were tested negative by indextest didn’t undergo reference test. “Unclear” if insufficient information is provided. |
||||
| 3. Were all patients included in the analysis? |
“Yes” if for all patients entered in the study are included in the analysis. “No” if not all the patients in the study are included in the analysis “Unclear” if it is not clear whether all patients were accounted for. |
||||
| 4. Were withdrawals from the study reported? |
“Yes” if for all patients entered in the study is reported what happened during the study, also those who withdraw or answer “yes” if no withdrawals where reported and all patients who entered in the study results were reported. “No” if not all the patients in the study complete the study and these patients were not accounted for. “Unclear” if it is not clear whether all patients were accounted for. |
||||
What's new
Last assessed as up‐to‐date: 1 February 2013.
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
History
Protocol first published: Issue 4, 2012 Review first published: Issue 2, 2014
| Date | Event | Description |
|---|---|---|
| 1 April 2015 | Amended | Contact details updated. |
| 24 February 2015 | Amended | Contact details updated. |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
| 26 February 2014 | Amended | Contact details updated. |
Differences between protocol and review
We removed the subheading “Secondary objectives” because there were limited data on variation in test accuracy according to FIGO stage (FIGO stage IIB until IV or only stage IIIC and IV), and who performed the laparoscopy, a general gynaecologist or gynaecologist‐oncologist. Because definition of test positive, that is unresectable disease, is based on a judgement rather than measurement, we thought different thresholds would be used to define test positivity. Therefore, we planned to analyse and summarise the data using a hierarchical summary receiver operating characteristic (HSROC) model (Rutter 2001). However, because of the few studies we retrieved and the high heterogeneity, data could not be pooled and no meta‐analyses were performed.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Angioli 2005
| Study characteristics | |||
| Patient sampling | Retro‐ or prospective enrolment not known | ||
| Patient characteristics and setting | Sample size: 87 patients Mean age: 58 years (range 19‐79) Presentation: Patients with primary ovarian cancer FIGO Stage IIIC/IV, good nutrition status, WHO < 2, no contraindications for surgery, evaluation for optimal primary debulking surgery (RT = 0) Diagnostics before index test: physical/gynaecological examination, ultrasonography, CA 125, CT abdomen/pelvis, Thorax x‐ray/CT Kind of surgery: PDS 53; IDS 25: No debulking surgery: 9 Setting: Department of gynaecology, University hospital Rome, Italy |
||
| Index tests | Open diagnostic laparoscopy; examination of the whole abdominal cavity, biopsies for frozen section, performed by gynaecological oncologist. If judged resectable direct cytoreduction Cut‐off test‐positivity: prediction of complete absence of disease after cytoreduction Complications of index test: trocar metastasis 2 cases (6%), intraoperative complication 1 (3%) |
||
| Target condition and reference standard(s) | Target condition: possibility of leaving no macroscopic disease at debulking surgery Criteria for target condition: extensive peritoneal carcinomatosis/involvement of bowel mesentery/bulky disease diaphragm/ multiple liver metastases/heavily bleeding tumoral tissue Reference standard: Laparotomy.Test operators: gynaecological oncologist Percentage of patients reference standard performed: 61% Unresectable disease at laparotomy: 2 |
||
| Flow and timing | Time between reference standard and Index test: 0 day. | ||
| Comparative | |||
| Notes | 87 patients had a laparoscopy, 53 where indicated to be operable. Of these 51 had operable disease at laparotomy and 2 not. The other 34 patients were treated with NACT and 25 received an interval debulking surgery after 3 courses of chemotherapy. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Were the patients suspected of advanced ovarian cancer by conventional diagnostic work‐up? | Yes | ||
| Were patients planned for primary cytoreductive surgery after conventional diagnostic work‐up? | Yes | ||
| Low | |||
| DOMAIN 2: Index Test Diagnostic open laparoscopy | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | No | ||
| Did the study provide a clear definition of what was considered to be a "positive "result for the index test? | No | ||
| Low | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | |||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did patients receive the same reference standard regardless of the index test result? | No | ||
Brun 2008
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 55 patients Diagnostics before index test: physical/gynaecological examination, abdominal ultrasound, CA125, CT abdomen/pelvis, thorax x‐ray/CT, routine blood test. Mean age: 61 years; (range 21‐88) Presentation: Patients suspected of ovarian cancer FIGO III‐IV without contraindication for surgery Kind of surgery: 26 patients PDS Setting: Hospital Tenon, France. |
||
| Index tests | Diagnostic laparoscopy; examination of uterus and ovaries, peritoneal surfaces, paracolic gutters, small bowel and mesentery, liver surface, omentum, diaphragm, large bowel. Cut‐off test‐positivity: PIV of 8 or more Complications of index test: none reported |
||
| Target condition and reference standard(s) | Target condition: residual disease of more than 1 cm after surgery Criteria for target condition: no extensive peritoneal carcinomatosis/ involvement of bowel mesentery/bulky disease diaphragm/unresectable upper abdomen metastases Reference standard: Laparotomy. Test operators: gynaecological oncologist Percentage of patients reference standard performed: 26/55 Unresectable disease at laparotomy: 8 (of 26 operated) (29 NACT) |
||
| Flow and timing | Reference standard performed after index test, time between treatment not mentioned | ||
| Comparative | |||
| Notes | Retrospective external validation of the prediction model of Fagotti 2006. This was done in the same population as described in Brun 2009. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Were the patients suspected of advanced ovarian cancer by conventional diagnostic work‐up? | Yes | ||
| Were patients planned for primary cytoreductive surgery after conventional diagnostic work‐up? | Yes | ||
| Low | |||
| DOMAIN 2: Index Test Diagnostic open laparoscopy | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | ||
| Did the study provide a clear definition of what was considered to be a "positive "result for the index test? | Yes | ||
| Low | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | |||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Were all patients included in the analysis? | No | ||
| Did patients receive the same reference standard regardless of the index test result? | No | ||
Brun 2009
| Study characteristics | |||
| Patient sampling | Retrospective study | ||
| Patient characteristics and setting | Sample size: 55 patients Mean age: 62 years (range 21‐88) Presentation: Patients with primary ovarian cancer FIGO stage III/IV, no contraindication for surgery or malnutrition, evaluation for PDS Diagnostics before index test: physical/gynaecological examination, CA 125, CT abdomen/pelvis, Thorax x‐ray/CT, routine blood test Kind of surgery: PDS 26; IDS 26: No debulking surgery 3 Setting: Department of gynaecology hospital Tenon, Paris, France. |
||
| Index tests | Open diagnostic laparoscopy performed by 7 surgeons, 3 gynaecological oncologists, 4 non‐gynaecological surgeons. Frozen section of tumour/metastasis. In case of operability direct cytoreduction by laparotomy Cut‐off test‐positivity: absence of visible residual tumour was considered feasible Complications of index test: 1 trocar metastasis occurred in PDS group (2%) |
||
| Target condition and reference standard(s) | Target condition: macroscopic residual tumour. Criteria for target condition: extensive peritoneal carcinomatosis/involvement of bowel mesentery/bulky disease diaphragm/unresectable upper abdomen metastases. Reference standard: Laparotomy. Test operators: gynaecological oncologists and general gynaecologists. Percentage of patients in whom reference standard performed: 47% Unresectable disease at laparotomy: 12 |
||
| Flow and timing | Time between reference standard and Index test: 0 day | ||
| Comparative | |||
| Notes | Same population as Brun 2008. 52 patients had a diagnostic laparoscopy. 26 of these patients were considered suitable for laparotomy. However 8 had more than 1 cm of residual disease left after laparotomy. The other 26 patients received NACT and interval debulking surgery. Cytoreduction only when absence of visible residual tumour was considered feasible. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Were the patients suspected of advanced ovarian cancer by conventional diagnostic work‐up? | Yes | ||
| Were patients planned for primary cytoreductive surgery after conventional diagnostic work‐up? | Yes | ||
| Low | |||
| DOMAIN 2: Index Test Diagnostic open laparoscopy | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | ||
| Did the study provide a clear definition of what was considered to be a "positive "result for the index test? | Yes | ||
| Low | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | |||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Did patients receive the same reference standard regardless of the index test result? | No | ||
Deffieux 2006
| Study characteristics | |||
| Patient sampling | cross‐sectional study, enrolment not reported | ||
| Patient characteristics and setting | Sample size: 15 patients Mean age: 54 years (range 37‐75) Presentation: Patients with primary ovarian cancer FIGO stageIIIC/IV suspected of peritoneal carcinomatosis and in whom preoperative evaluation was unsatisfactory to define the possibility of achieving a complete cytoreduction, no massive disease at diaphragm/xiphoid or liver pedicle, planned for PDS Diagnostics before index test: physical/gynaecological examination, CT abdomen/pelvis, Thorax x‐ray/CT, Kind of surgery: PDS 11; IDS 0 No debulking surgery 4 Setting: Department of gynaecology hospital Tenon, Paris, France |
||
| Index tests | Open diagnostic laparoscopy; examination of the whole abdomen, focus on involvement of small bowel, liver pedicle and upper right diaphragmatic dome. Cut‐off test‐positivity: having one or more of the criteria for unresectability Complications of index test: none |
||
| Target condition and reference standard(s) | Target condition: unresectable carcinomatosis Criteria for target condition: involvement of bowel mesentery/bulky disease diaphragm/massive involvement of liver pedicle Reference standard: Laparotomy. Test operators: not reported Percentage of patients in whom reference standard performed:73% Unresectable disease at laparotomy: 1 |
||
| Flow and timing | Time between reference standard and index test: 0 day. | ||
| Comparative | |||
| Notes | 15 patients underwent diagnostic laparoscopy, of these 11 were considered operable. Ten of the patients were indeed successfully operated and one had too extensive disease. The other four patients were treated with NACT. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Were the patients suspected of advanced ovarian cancer by conventional diagnostic work‐up? | Yes | ||
| Were patients planned for primary cytoreductive surgery after conventional diagnostic work‐up? | Yes | ||
| Low | |||
| DOMAIN 2: Index Test Diagnostic open laparoscopy | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | ||
| Did the study provide a clear definition of what was considered to be a "positive "result for the index test? | Yes | ||
| Low | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | |||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did patients receive the same reference standard regardless of the index test result? | No | ||
Fagotti 2004
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 64 patients Mean age: 57.4 years (+/‐ 12,7) Presentation: Patients undergoing surgery for a suspected advanced ovarian or peritoneal cancer, exclusion advanced ASA or very large mass reaching xiphoid/occupying the abdominal wall or cavity. Included also if on radiologic and clinical criteria suspected of unresectable disease. Diagnostics before index test: physical/gynaecological examination, ultrasonography, CA 125, CT abdomen/pelvis, Thorax x‐ray/CT Kind of surgery: All patients received explorative laparotomy. Performed before: PDS 42; IDS 19: recurrence: 3 |
||
| Index tests | Open diagnostic laparoscopy, investigating frozen pelvis, omental cake, diaphragmatic or peritoneal extensive carcinomatosis, tumour diffusion to the large and small curvature of the stomach, large and/or small bowel mesentery disease, spleen and/or liver metastases, bulky lymph nodes. Cut‐off test‐positivity: absence of criteria for un resectability Complications of index test: none |
||
| Target condition and reference standard(s) | Target condition: resectability of tumour residual of less than 1 cm not possible Criteria for target condition: extensive peritoneal carcinomatosis/involvement of bowel mesentery/bulky disease diaphragm/ portal triad disease/unresectable upper abdominal metastasis Reference standard: Explorative laparotomy. Test operators: not reported percentage of patients Reference stand performed: 100% Unresectable disease at laparotomy: 23 |
||
| Flow and timing | Time between reference standard and index test: 0 day. | ||
| Comparative | |||
| Notes | Not only patients planned for primary surgery, but also planned for IDS or secondary surgery because of recurrence. After inclusion and laparotomy FIGO I‐II, III‐IV 9‐42 respectivily, 6 benign, 7 other tumour | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Were the patients suspected of advanced ovarian cancer by conventional diagnostic work‐up? | Yes | ||
| Were patients planned for primary cytoreductive surgery after conventional diagnostic work‐up? | No | ||
| High | |||
| DOMAIN 2: Index Test Diagnostic open laparoscopy | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | ||
| Did the study provide a clear definition of what was considered to be a "positive "result for the index test? | Yes | ||
| Low | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | |||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| Did patients receive the same reference standard regardless of the index test result? | Yes | ||
Fagotti 2008
| Study characteristics | |||
| Patient sampling | Prospective study | ||
| Patient characteristics and setting | Sample size: 113 patients Diagnostics before index test: physical/gynaecological examination, CA 125, CT abdomen/pelvis, Thorax x‐ray/CT, Performance status Mean age: 59 years (range 34‐81) Presentation: Patients suspected of advanced primary ovarian cancer Kind of surgery: primary (91) or interval debulking (22) surgery Setting: Division of Gynaecologic Oncology, University hospital, Rome, Italy |
||
| Index tests | Eight laparoscopic features investigated as potential indicators of surgical outcome; presence of ovarian masses, omental cake, peritoneal carcinomatosis, diaphragmatic carcinosis, mesenteric retraction, bowel infiltration, stomach infiltration, liver metastases. Cut‐off test‐positivity: PIV 8 or more ( based on presence of criteria) Complications of index test: none |
||
| Target condition and reference standard(s) | Target condition: possibility of leaving less than 1 cm macroscopic disease at debulking surgery Criteria for target condition: no extensive peritoneal carcinomatosis/no involvement of bowel mesentery/no bulky disease diaphragm/ no unresectable upper abdomen metastases, good performance status Reference standard: Laparotomy. Test operators: gynaecological oncologist Percentage of patients in whom reference standard performed: 100% Unresectable disease at laparotomy: 50% |
||
| Flow and timing | Time between ref standard and indextest not mentioned | ||
| Comparative | |||
| Notes | Validation of prediction model developed by Fagotti 2006 in prospective cohort. Also patients planned for IDS included in study. Primary and interval debulking patients were not separately analysed. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Were the patients suspected of advanced ovarian cancer by conventional diagnostic work‐up? | Yes | ||
| Were patients planned for primary cytoreductive surgery after conventional diagnostic work‐up? | No | ||
| High | |||
| DOMAIN 2: Index Test Diagnostic open laparoscopy | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | ||
| Did the study provide a clear definition of what was considered to be a "positive "result for the index test? | Yes | ||
| Low | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | |||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did patients receive the same reference standard regardless of the index test result? | Yes | ||
Vergote 1998
| Study characteristics | |||
| Patient sampling | Retrospective study design | ||
| Patient characteristics and setting | Sample size: 77 Diagnostics before index test: radiological examination Mean age: not mentioned Presentation: Patients with obvious metastatic disease of ovarian cancer on radiological examination, planned for PDS Kind of surgery: Primary debulking in 28 patients, IDS 31, 18 no surgery Setting: Division of Gynaecologic Oncology, University hospital, Brussels, Belgium |
||
| Index tests | Decision to give NACT of primary debulking with the help of an open laparoscopy Cut‐off test‐positivity: not reported Complications of index test: two port site metastases (3%) |
||
| Target condition and reference standard(s) | Target condition: unresectability leaving more than 0.5 cm of residual tumour Criteria for target condition: not mentioned Reference standard: Explorative laparotomy. Test operators: not reported Percentage of patients in whom reference standard performed: 36% (28 patients) Unresectable disease at laparotomy: 21% (6 patients) |
||
| Flow and timing | Time between ref standard and indextest not mentioned | ||
| Comparative | |||
| Notes | Decision to give NACT or PDS in all patients with advanced ovarian cancer, subgroup in paper about NACT or PDS retrospectively. 77 patients had a diagnostic laparoscopy. Of these 28 underwent a laparotomy and 21 had resectable disease. 49 patients received NACT and IDS. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| Were the patients suspected of advanced ovarian cancer by conventional diagnostic work‐up? | Yes | ||
| Were patients planned for primary cytoreductive surgery after conventional diagnostic work‐up? | Yes | ||
| Low | |||
| DOMAIN 2: Index Test Diagnostic open laparoscopy | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | No | ||
| Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | Yes | ||
| Did the study provide a clear definition of what was considered to be a "positive "result for the index test? | No | ||
| High | |||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| Low | |||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Did patients receive the same reference standard regardless of the index test result? | No | ||
ASA: American Society of Anesthesiology CA125: cancer antigen 125 CT: computed tomography IDS: interval debulking surgery NACT: neoadjuvant chemotherapy PDS: primary debulking surgery PIV: Predictive Index Value RT: Residual Tumour WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Batka 1993 | Different outcome |
| Bristow 2006 | No original data shown |
| Bruhat 1981 | No original data shown |
| Brun 2009a | Duplicate data; abstract of congress |
| Dagnini 1987 | Different outcome |
| Fagotti 2006 | Duplicate data; development of prediction model in study population of Fagotti 2004, which is included in the review |
| Gurrea 2010 | No data about evaluation of resectability |
| Nezhat 2010 | No data about evaluation of resectability |
| Vergote 2003 | No original data shown |
Characteristics of ongoing studies [ordered by study ID]
Rutten 2012
| Trial name or title | Laparoscopy to predict the result of primary cytoreductive surgery in advanced ovarian cancer patients (LapOvCa‐trial): a multicentre randomized controlled study |
| Target condition and reference standard(s) | target condition is residual disease > 1 cm; reference standard is laparotomy |
| Index and comparator tests | Laparoscopy |
| Starting date | May 2011 |
| Contact information | lapovca@studies‐obsgyn.nl |
| Notes |
Contributions of authors
Drafting the protocol: MJ Rutten, MM Leeflang, GG Kenter, BWJ Mol, MR Buist. Developing the search strategy: MJ Rutten, MM Leeflang, GG Kenter, BWlJ Mol, MR Buist. Searching for trials: MJ Rutten. Obtaining copies of trials: MJ Rutten. Selecting trials for inclusion: MJ Rutten, MR Buist. Extracting data: MJ Rutten, MR Buist. Carrying out analysis: MJ Rutten, MM Leeflang. Interpreting the analysis: MJ Rutten, MM Leeflang, GG Kenter, BWJ Mol, MR Buist. Drafting the final review: MJ Rutten, MM Leeflang, GG Kenter, BWJ Mol, MR Buist. Updating the review: MJ Rutten, MR Buist.
Sources of support
Internal sources
Academic Medical Center Amsterdam, Netherlands.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
- Angioli R, Palaia I, Zullo MA, Muzii L, Manci N, Calcagno M, et al. Diagnostic open laparoscopy in the management of advanced ovarian cancer. Gynecologic Oncology 2006;100(3):455‐61. [DOI] [PubMed] [Google Scholar]
- Brun JL, Rouzier R, Uzan S, Darai E. External validation of a laparoscopic‐based score to evaluate resectability of advanced ovarian cancers: clues for a simplified score. Gynecologic Oncology 2008;110(3):354‐9. [DOI] [PubMed] [Google Scholar]
- Brun JL, Rouzier R, Selle F, Houry S, Uzan S, Darai E. Neoadjuvant chemotherapy or primary surgery for stage III/IV ovarian cancer: contribution of diagnostic laparoscopy. BMC Cancer 2009;9:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffieux X, Castaigne D, Pomel C. Role of laparoscopy to evaluate candidates for complete cytoreduction in advanced stages of epithelial ovarian cancer. International Journal of Gynecological Cancer 2006;16 Suppl 1:35‐40. [DOI] [PubMed] [Google Scholar]
- Fagotti A, Fanfani F, Ludovisi M, Lo Voi R, Bifulco G, Testa AC, et al. Role of laparoscopy to assess the chance of optimal cytoreductive surgery in advanced ovarian cancer: a pilot study. Gynecologic Oncology 2005;96(3):729‐35. [DOI] [PubMed] [Google Scholar]
- Fagotti A, Ferrandina G, Fanfani F, Garganese G, Vizzielli G, Carone V, et al. Prospective validation of a laparoscopic predictive model for optimal cytoreduction in advanced ovarian carcinoma. American Journal of Obstetrics & Gynecology 2008;199(6):642‐6. [DOI] [PubMed] [Google Scholar]
- Vergote I, Wever I, Tjalma W, Gramberen M, Decloedt J, Dam P. Neoadjuvant chemotherapy or primary debulking surgery in advanced ovarian carcinoma: a retrospective analysis of 285 patients. Gynecologic Oncology 1998;71(3):431‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Batka M, Graf AH, Steiner H, Staudach A. [Laparotomy vs. pelviscopy in adnexa processes‐‐preoperative assessment, therapy planning and histology]. [German]. Gynakologisch‐Geburtshilfliche Rundschau 1993;33 Suppl 1:41‐3. [DOI] [PubMed] [Google Scholar]
- Bristow RE. Predicting "unresectable" ovarian cancer: Taking aim at a moving target. Gynaecologic Oncology 2006;100:449‐50. [DOI] [PubMed] [Google Scholar]
- Bruhat MA, Dal Soglio I, Mage G, Manhes H. Coelioscopy and ovarian cancer. Gynecologie 1981;32:191‐6. [Google Scholar]
- Brun J, Rouzier R, Uzan S, Darai E. External validation of a laparoscopic‐based score to evaluate resectability of advanced ovarian cancers: Clues for a simplified score. International Journal of Gynecology and Obstetrics 2009;Conference: 19th FIGO World Congress of Gynecology and Obstetrics Cape Town South Africa. Conference Start: 20091004 Conference End: 20091009. Conference Publication::S135. [Google Scholar]
- Dagnini G, Marin G, Caldironi MW, Piccigallo E, Miola E. Laparoscopy in staging, follow‐up, and restaging of ovarian carcinoma. Gastrointestinal Endoscopy 1987;33(2):80‐3. [DOI] [PubMed] [Google Scholar]
- Fagotti A, Ferrandina G, Fanfani F, Ercoli A, Lorusso D, Rossi M, et al. A laparoscopy‐based score to predict surgical outcome in patients with advanced ovarian carcinoma: a pilot study. Annals of Surgical Oncology 2006;13(8):1156‐61. [DOI] [PubMed] [Google Scholar]
- Gurrea M, Fuster S, Rodriguez E, Ruiz F, Domingo S, Pellicer A. Inclusion of diagnostic laparoscopy in the advanced ovarian cancer work algorithm. Gynecological Surgery 2010;Conference: 19th Annual Congress of the European Society for Gynaecological Endoscopy, ESGE Barcelona Spain. Conference Start: 20100929 Conference End: 20101002. Conference Publication::S41. [Google Scholar]
- Nezhat FR, DeNoble SM, Liu CS, Cho JE, Brown DN, Chuang L, et al. The safety and efficacy of laparoscopic surgical staging and debulking of apparent advanced stage ovarian, fallopian tube, and primary peritoneal cancers. Journal of the Society of Laparoendoscopic Surgeons 2010;14(2):155‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergote I. Controversies in surgery in ovarian cancer ‐ What is its real role?. European Journal of Cancer, Supplement 2003;1:115‐25. [Google Scholar]
References to ongoing studies
- Rutten MJ, Gaarenstroom KN, Gorp T, Meurs HS, Arts HJ, Bossuyt PM, et al. Laparoscopy to predict the result of primary cytoreductive surgery in advanced ovarian cancer patients (LapOvCa‐trial): a multicentre randomized controlled study. BMC Cancer 2012;12:31. [PUBMED: 22264278] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
- Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta‐analysis. Journal of Clinical Oncology 2002;20(5):1248‐59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Chi DS, Abu‐Rustum NR, Sonoda Y, Awtrey C, Hummer A, Venkatraman ES, et al. Ten‐year experience with laparoscopy on a gynecologic oncology service: analysis of risk factors for complications and conversion to laparotomy.. American Journal of Obstetrics and Gynecology 2004;191(4):1138‐45. [PUBMED: 15507933 ] [DOI] [PubMed] [Google Scholar]
- Du Bois A, Reuss A, Pujade‐Lauraine E, Harter P, Ray‐Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO‐OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO). Cancer 2009;115(6):1234‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Eisenkop SM, Friedman RL, Wang HJ. Complete cytoreductive surgery is feasible and maximizes survival in patients with advanced epithelial ovarian cancer: a prospective study. Journal of Gynaecologic Oncology 1998;62(2):103‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Elattar A, Bryant A, Winter‐Roach Brett A, Hatem M, Naik R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD007565.pub2; CD007565] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerestein CG, Spek DW, Eijkemans MJ, Bakker J, Kooi GS, Burger CW. Prediction of residual disease after primary cytoreductive surgery for advanced‐stage ovarian cancer: accuracy of clinical judgment. International Journal of Gynecological Cancer 2009;19(9):1511‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C and Parkin DM. International Agency for Research on Cancer. Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. GLOBOCAN 2008 v2.0. Available from: http://globocan.iarc.fr accessed on 08/07/2013.
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
- Morrison J, Haldar K, Kehoe S, Lawrie TA. Chemotherapy versus surgery for initial treatment in advanced ovarian epithelial cancer. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD005343.pub3; CD005343] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munkarah AR, Coleman RL. Critical evaluation of secondary cytoreduction in recurrent ovarian cancer. Gynecologic Oncology 2004;95(2):273‐80. [PUBMED: 15491746] [DOI] [PubMed] [Google Scholar]
- Ramirez I, Chon HS, Apte SM. The role of surgery in the management of epithelial ovarian cancer. Cancer Control 2011;18(1526‐2359 (Electronic), 1073‐2748 (Linking), 1):22‐30. [DOI] [PubMed] [Google Scholar]
- Rose PG, Nerenstone S, Brady MF, Clarke‐Pearson D, Olt G, Rubin SC, et al. Secondary surgical cytoreduction for advanced ovarian carcinoma. New England Journal of Medicine 2004;351(24):2489‐97. [DOI] [PubMed] [Google Scholar]
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Statistics in Medicine 2001;20(19):2865‐84. [PUBMED: 11568945] [DOI] [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: A Cancer Journal for Clinicians 2012;62(1):10‐29. [DOI] [PubMed] [Google Scholar]
- Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. New England Journal of Medicine 2010;363:943‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Vergote I, du Bois A, Amant F, Heitz F, Leunen K, Harter P. Neoadjuvant chemotherapy in advanced ovarian cancer: On what do we agree and disagree?. Gynecologic Oncology 2013;128(1):6‐11. [PUBMED: 23006973] [DOI] [PubMed] [Google Scholar]
- Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Medical Research Methodology 2006;6:9. [PUBMED: 16519814] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of internal medicine 2011;155(8):529‐36. [PUBMED: 22007046] [DOI] [PubMed] [Google Scholar]


