Abstract
Background
Fast‐track cardiac care is a complex intervention involving several components of care during cardiac anaesthesia and in the postoperative period, with the ultimate aim of early extubation after surgery, to reduce length of stay in the intensive care unit and in the hospital. Safe and effective fast‐track cardiac care may reduce hospital costs. This is an update of a Cochrane review first published in 2003, updated in 2012 and updated now in 2016.
Objectives
To determine the safety and effectiveness of fast‐track cardiac care compared with conventional (not fast‐track) care in adult patients undergoing cardiac surgery. Fast‐track cardiac care intervention includes administration of low‐dose opioid‐based general anaesthesia or use of a time‐directed extubation protocol, or both.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 5), MEDLINE (January 2012 to May 2015), Embase (January 2012 to May 2015), the Cumulative Index to Nursing and Allied Health Literature (CINAHL; January 2012 to May 2015) and the Institute for Scientific Information (ISI) Web of Science (January 2012 to May 2015), along with reference lists of articles, to identify additional trials. We applied no language restrictions.
Selection criteria
We included all randomized controlled trials of adult cardiac surgical patients (coronary artery bypass grafts, aortic valve replacement, mitral valve replacement) that compared fast‐track cardiac care and conventional (not fast‐track) care groups. We focused on the following fast‐track interventions, which were designed for early extubation after surgery: administration of low‐dose opioid‐based general anaesthesia during cardiac surgery and use of a time‐directed extubation protocol after surgery. The primary outcome was risk of mortality. Secondary outcomes included postoperative complications, reintubation within 24 hours of surgery, time to extubation, length of stay in the intensive care unit and in the hospital, quality of life after surgery and hospital costs.
Data collection and analysis
Two review authors independently assessed trial quality and extracted study data. We contacted study authors for additional information. We calculated a Peto odds ratio (OR) for risk of mortality and used a random‐effects model to report risk ratio (RR), mean difference (MD) and 95% confidence intervals (95% CIs) for all secondary outcomes.
Main results
We included 28 trials (4438 participants) in the updated review. We considered most participants to be at low to moderate risk of death after surgery. We assessed two studies as having low risk of bias and 11 studies high risk of bias. Investigators reported no differences in risk of mortality within the first year after surgery between low‐dose versus high‐dose opioid‐based general anaesthesia groups (OR 0.53, 95% CI 0.25 to 1.12; eight trials, 1994 participants, low level of evidence) and between a time‐directed extubation protocol versus usual care (OR 0.80, 95% CI 0.45 to 1.45; 10 trials, 1802 participants, low level of evidence).
Researchers noted no significant differences between low‐dose and high‐dose opioid‐based anaesthesia groups in the following postoperative complications: myocardial infarction (RR 0.98, 95% CI 0.48 to 1.99; eight trials, 1683 participants, low level of evidence), stroke (RR 1.17, 95% CI 0.36 to 3.78; five trials, 562 participants, low level of evidence) and tracheal reintubation (RR 1.77, 95% CI 0.38 to 8.27; five trials, 594 participants, low level of evidence).
Comparisons with usual care revealed no significant differences in the risk of postoperative complications associated with a time‐directed extubation protocol: myocardial infarction (RR 0.59, 95% CI 0.27 to 1.31; eight trials, 1378 participants, low level of evidence), stroke (RR 0.85, 95% CI 0.33 to 2.16; 11 trials, 1646 participants, low level of evidence) and tracheal reintubation (RR 1.34, 95% CI 0.74 to 2.41; 12 trials, 1261 participants, low level of evidence).
Although levels of heterogeneity were high, low‐dose opioid anaesthesia was associated with reduced time to extubation (reduction of 4.3 to 10.5 hours, 14 trials, 2486 participants, low level of evidence) and length of stay in the intensive care unit (reduction of 0.4 to 7.0 hours, 12 trials, 1394 participants, low level of evidence). Use of a time‐directed extubation protocol was associated with reduced time to extubation (reduction of 3.7 to 8.8 hours, 16 trials, 2024 participants, low level of evidence) and length of stay in the intensive care unit (reduction of 3.9 to 10.5 hours, 13 trials, 1888 participants, low level of evidence). However, these two fast‐track care interventions were not associated with reduced total length of stay in the hospital (low level of evidence).
Authors' conclusions
Low‐dose opioid‐based general anaesthesia and time‐directed extubation protocols for fast‐track interventions have risks of mortality and major postoperative complications similar to those of conventional (not fast‐track) care, and therefore appear to be safe for use in patients considered to be at low to moderate risk. These fast‐track interventions reduced time to extubation and shortened length of stay in the intensive care unit but did not reduce length of stay in the hospital.
Keywords: Adult; Humans; Coronary Artery Bypass; Heart Valve Prosthesis Implantation; Intubation, Intratracheal; Aortic Aneurysm; Aortic Aneurysm/surgery; Aortic Valve; Aortic Valve/surgery; Controlled Clinical Trials as Topic; Early Ambulation; Length of Stay; Mitral Valve; Mitral Valve/surgery; Randomized Controlled Trials as Topic; Risk; Time Factors
Plain language summary
Fast‐track interventions of low‐dose opioid‐based general anaesthesia and early tracheal extubation in adults undergoing cardiac surgery
Review question
Fast‐track cardiac care involves early removal, within eight hours of heart surgery, of the tube that provides mechanical breathing support (called early tracheal extubation) to enable cardiac surgery. This review examined evidence on the effectiveness and safety of fast‐track care compared with conventional (not fast‐track) care. We have updated the published evidence that we identified in 2012. It is now current to March 2016.
Background
In the past, adults were given high‐dose opioid‐based anaesthesia for cardiac surgery and were provided with mechanical breathing support overnight in an intensive care unit after surgery. Now, many surgical units remove the tube that provides mechanical breathing support when the patient is on the operating table or within hours after cardiac surgery. They use time‐directed protocols for removing breathing support. Some patients recover in an intensive care unit (ICU) or in a dedicated unit outside the ICU. It is important to improve hospital efficiency by using safe fast‐track interventions.
Study characteristics
We found 28 relevant randomized controlled studies, conducted between 1994 and 2015. Most of the 4438 adults who participated in these studies were undergoing first‐time elective coronary artery graft bypass or valve replacement surgery, or both. They were at low to moderate risk of death after surgery. Eighteen studies examined the use of low‐dose opioid‐based general anaesthesia. Sixteen studies assessed how effective the protocols were in guiding staff to remove the tube that provided breathing support within eight hours after surgery.
Key findings and quality of evidence
We found no differences in risk of death in the first year after surgery (18 trials, 3796 participants) nor in complications after surgery such as the need to replace the tracheal tube after surgery (17 trials, 1855 participants) and occurrence of myocardial infarction (16 trials, 3061 participants) or stroke (16 trials, 2208 participants), when we examined both types of interventions. Occurrences of acute renal failure, major bleeding, sepsis and wound infection also were not different. We rated the quality of evidence as low for both mortality and postoperative complications.
Tracheal tubes were removed from adults in the fast‐track care group up to a half day earlier than for those in the conventional care group. The fast‐track group spent less time in the intensive care unit, but length of time spent in the hospital was similar between groups. The quality of evidence was low because of study limitations and unexplained variation in study findings. Large trials were few, and only one trial was designed to study postoperative effects of myocardial infarction, stroke or death.
Our results did not apply to ‘high‐risk' patients who had multiple concurrent health problems or to settings in which a short‐acting opioid (remifentanil) was used for general anaesthesia.
Conclusion
Fast‐track cardiac care is safe in patients considered to be at low to moderate risk of death after surgery.
Summary of findings
Summary of findings for the main comparison. Low‐dose opioid‐based GA vs high‐dose opioid‐based GA.
| Low‐dose opioid‐based general anaesthesia compared with high‐dose opioid‐based general anaesthesia for adults undergoing cardiac surgery | ||||||
|
Patient or population: adult cardiac surgical patients
Setting: people undergoing various cardiac surgical procedures in hospitals in Europe, North America, Asia, Australasia and Middle East Intervention: low‐dose opioid‐based general anaesthesia Comparison: high‐dose opioid‐based general anaesthesia | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with high‐dose opioid‐based general anaesthesia | Risk with low‐dose opioid‐based general anaesthesia | |||||
| Mortality ‐ Death at any time after surgery | Low | OR 0.53 (0.25 to 1.12) | 1994 (8 RCTs) | ⊕⊕⊝⊝ LOWa,b | No death recorded in 3 trials | |
| 10 per 1000 | 5 per 1000 (3 to 11) | |||||
| Moderate | ||||||
| 30 per 1000 | 16 per 1000 (8 to 33) | |||||
| High | ||||||
| 110 per 1000 | 61 per 1000 (30 to 122) | |||||
| Postoperative myocardial infarction | Study population | RR 0.98 (0.48 to 1.99) | 1683 (8 RCTs) | ⊕⊕⊝⊝ LOWa,b | No postoperative myocardial infarction recorded in 2 trials | |
| 30 per 1000 | 30 per 1000 (15 to 60) | |||||
| Postoperative stroke | Study population | RR 1.17 (0.36 to 3.78) | 562 (5 RCTs) | ⊕⊕⊝⊝ LOWb,c | No stroke recorded in 1 trial | |
| 18 per 1000 | 21 per 1000 (6 to 67) | |||||
| Postoperative tracheal reintubation | Study population | RR 1.77 (0.38 to 8.27) | 594 (5 RCTs) | ⊕⊕⊝⊝ LOWb,d | No reintubation recorded in 3 trials | |
| 7 per 1000 | 12 per 1000 (3 to 55) | |||||
| Time to extubation (hours) | Mean time to extubation (hours) was 5.2 to 35.1 | Mean time to extubation (hours) in the intervention group was 7.4 lower (10.51 lower to 4.29 lower). | ‐ | 2486 (14 RCTs) | ⊕⊕⊝⊝ LOWe,f | |
| Length of intensive care unit stay (hours) | Mean length of intensive care unit stay (hours) was 2.6 to 112.8. | Mean length of intensive care unit stay (hours) in the intervention group was 3.7 lower (6.98 lower to 0.41 lower). | ‐ | 1394 (12 RCTs) | ⊕⊕⊝⊝ LOWf,g | |
| Length of hospital stay (days) | Mean length of hospital stay (days) was 5.1 to 27.0. | Mean length of hospital stay (days) in the intervention group was 0.3 lower (1.04 lower to 0.43 higher). | ‐ | 913 (8 RCTs) | ⊕⊕⊝⊝ LOWf,h | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group using the EuroSCORE risk classification (Michel 2003) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aOf the 8 trials, 2 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations).
bOptimal information size not met (downgrade 1 point owing to imprecision).
cOf the 5 trials, 1 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations).
dOf the 5 trials, 2 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations).
eOf the 14 trials, 4 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations).
fUnexplained reasons for high heterogeneity.
gOf the 12 trials, 3 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations).
hOf the 8 trials, 3 had ≥ 1 high risk of bias domain (downgrade 1 point owing to study limitations).
Summary of findings 2. Time‐directed extubation protocol vs usual care.
| Time‐directed extubation protocol compared with usual care for adults undergoing cardiac surgery | ||||||
| Patient or population: adult cardiac surgical patients Setting: people undergoing various cardiac surgical procedures in hospitals in Europe, North America, Asia and Middle East Intervention: time‐directed extubation protocol Comparison: usual care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with usual care | Risk with time‐directed extubation protocol | |||||
| Mortality ‐ Death at any time after surgery | Low | OR 0.80 (0.45 to 1.45) | 1802 (10 RCTs) | ⊕⊕⊝⊝ LOWa,b | No deaths recorded in 4 trials. Low to moderate heterogeneity (I2 = 37%) may be explained by the inclusion of a trial (Reyes 1997) that had the highest rate of mortality of all trials considered. When excluded, the OR changed to 0.31 (95% CI 0.11 to 0.90, P = 0.03, I2 = 0%). | |
| 10 per 1000 | 8 per 1000 (5 to 14) | |||||
| Moderate | ||||||
| 30 per 1000 | 24 per 1000 (14 to 43) | |||||
| High | ||||||
| 110 per 1000 | 90 per 1000 (53 to 152) | |||||
| Postoperative myocardial infarction | Study population | RR 0.59 (0.27 to 1.31) | 1378 (8 RCTs) | ⊕⊕⊝⊝ LOWb,c | No postoperative myocardial infarction was recorded in 1 trial. | |
| 61 per 1000 | 36 per 1000 (16 to 80) | |||||
| Postoperative stroke | Study population | RR 0.85 (0.33 to 2.16) | 1646 (11 RCTs) | ⊕⊕⊝⊝ LOWb,d | No stroke was recorded in 2 trials. | |
| 12 per 1000 | 10 per 1000 (4 to 26) | |||||
| Postoperative reintubation | Study population | RR 1.34 (0.74 to 2.41) | 1261 (12 RCTs) | ⊕⊕⊝⊝ LOWb,e | No reintubation was recorded in 3 trials. | |
| 28 per 1000 | 38 per 1000 (21 to 68) | |||||
| Time to extubation (hours) | Mean time to extubation (hours) was 3.4 to 18.9. | Mean time to extubation (hours) in the intervention group was 6.25 lower (8.84 lower to 3.67 lower). | ‐ | 2024 (16 RCTs) | ⊕⊕⊝⊝ LOWf,g | No variation in time to extubation in the early extubation group in 1 trial |
| Length of intensive care unit stay (hours) | Mean length of intensive care unit stay (hours) was 17.9 to 95.0. | Mean length of intensive care unit stay (hours) in the intervention group was 7.16 lower (10.45 lower to 3.88 lower). | ‐ | 1888 (13 RCTs) | ⊕⊕⊝⊝ LOWf,h | No variation in length of ICU stay in early extubation group in 1 trial |
| Length of hospital stay (days) | Mean length of hospital stay (days) was 5.1 to 13.0. | Mean length of hospital stay (days) in the intervention group was 0.44 lower (1.04 lower to 0.16 higher). | ‐ | 1334 (8 RCTs) | ⊕⊕⊝⊝ LOWf,i | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group using the EuroSCORE risk classification (Michel 2003) and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aOf the 10 trials, 3 had 1 high risk of bias domain (downgrade 1 point owing to study limitations).
bOptimal information size not met (downgrade 1 point owing to imprecision).
cOf the 8 trials, 3 had 1 high risk of bias domain (downgrade 1 point owing to study limitations).
dOf the 11 trials, 5 had 1 high risk of bias domain (downgrade 1 point owing to study limitations).
dOf the 12 trials, 5 had 1 high risk of bias domain (downgrade 1 point owing to study limitations).
fUnexplained reasons for high heterogeneity.
gOf the 16 trials, 6 had 1 high risk of bias domain (downgrade 1 point owing to study limitations).
hOf the 13 trials, 5 had 1 high risk of bias domain (downgrade 1 point owing to study limitations).
iOf the 8 trials, 2 had 1 high risk of bias domain (downgrade 1 point owing to study limitations).
Background
Description of the condition
In the past, cardiac surgical patients were ventilated overnight following surgery and were given a regimen of high‐dose opioid‐based anaesthesia and postoperative analgesia (Hawkes 2003). However, in the early 1990s, fast‐track cardiac anaesthesia (FTCA) was introduced to address the increasing demand for cardiac surgery with limited medical facilities and available resources. Although the volume of coronary artery bypass grafting (CABG) surgery performed in the United States peaked in 1998, 219,000 patients underwent a total of 397,000 CABG procedures in 2010 (American Heart Association 2016). In many units, patients are now extubated (i.e. the tube that allows mechanical breathing support is removed) on the operating table or within hours after cardiac surgery via time‐directed formalized weaning protocols, and they recover in a dedicated unit outside the intensive care unit (ICU) setting (Ender 2008; Nougarede 2004; Probst 2014; Salah 2015) as part of a fast‐track programme.
Description of the intervention
The label 'fast‐track' originally referred to the use of low‐dose opioid‐based general anaesthesia in cardiac surgical patients to carry connotations of excitement and rapid advancement (Silbert 2009). Although no standard definition of FTCA is known, it is generally accepted that it involves the use of a combination of short‐acting hypnotic drugs with reduced doses of opioids, or the use of short‐acting opioids such as remifentanil (Myles 2003; van Mastrigt 2006b), with the ultimate aim of extubation within eight hours after cardiac surgery. Several authors arbitrarily defined the criteria for early extubation within eight hours (Berry 1998; Michalopoulos 1998), but no physiological or pathological reasons have been proposed to explain why this time point was adopted.
Normothermic temperature management and use of an extubation protocol with the intention to extubate a patient within a specified time period are considered fast‐track strategies (van Mastrigt 2006b).
In this systematic review, fast‐track cardiac care is defined as a complex intervention involving several components of care during cardiac anaesthesia and in the postoperative period, with the ultimate aim of early extubation after surgery to reduce length of stay in the ICU and in the hospital. These components of fast‐track cardiac care include administration of low‐dose opioid‐based general anaesthesia, use of a time‐directed extubation protocol, or both. Although safe and effective fast‐track cardiac care may reduce hospital costs, the incidence of fast‐track failure after cardiac surgery ranges from 11% (Lee 2013) to 16% (Constantinides 2006).
How the intervention might work
Early tracheal extubation after surgery is a key component of fast‐track cardiac management. Early extubation reduces the patient's length of stay in the ICU and in the hospital, resulting in reduced hospital costs and improved hospital efficiency (Hawkes 2003). Although high intraoperative opioid doses are used to suppress hormonal and metabolic stress responses to surgery, the opioids may have cumulative effects and can depress respiration and prolong ventilation times (Maddali 2006), whereas combining a short‐acting hypnotic with a low‐dose opioid‐based anaesthesia can avoid these problems without compromising patient recovery. A time‐directed extubation protocol improves efficiency of practice by following an expert consensus guideline to reduce variations in (1) decisions about when the patient is ready for weaning (the process leading to discontinuation of mechanical ventilation support), (2) the process of reducing ventilatory support and (3) criteria for deciding whether patients are ready to be extubated (Blackwood 2014).
Why it is important to do this review
In an earlier version of this Cochrane review (Hawkes 2003), which included six trials, review authors found no evidence of a difference between early and conventionally late extubated patients for the following outcomes: risk of mortality in ICU (risk ratio (RR) 0.80, 95% confidence interval (CI) 0.42 to 1.52); risk of mortality at 30 days after surgery (RR 1.20, 95% CI 0.63 to 2.27); risk of myocardial ischaemia (RR 0.96, 95% CI 0.71 to 1.30); and risk of reintubation within 24 hours of surgery (RR 5.93, 95% CI 0.72 to 49.14). Times spent in ICU and in hospital were significantly shorter for patients who were extubated early (‐7.02 hours, 95% CI ‐7.42 to ‐6.61; ‐1.08 days, 95% CI ‐1.35 to ‐0.82, respectively) (Hawkes 2003).
In another systematic review of the safety and effectiveness of FTCA in 10 trials (Myles 2003), the FTCA group spent less time in ICU (‐5.4 hours, 95% CI ‐10.5 to ‐0.3) than the conventional group given opioid‐based anaesthesia. However, investigators reported no significant reduction in hospital stay (‐0.61 days, 95% CI ‐1.51 to 0.28). Risk of mortality was similar between FTCA (1.2%) and conventional care (2.7%) groups (RR 0.51, 95% CI 0.23 to 1.13) (Myles 2003). A meta‐regression of randomized clinical trials of fast‐track treatment in cardiac patients showed that the introduction of an early extubation protocol was an independent predictor of decreased ICU stay and hospital stay (van Mastrigt 2006b).
In the last Cochrane review update of 25 trials (n = 4118) (Zhu 2012), we found no differences in risk of mortality within the first year after surgery between low‐dose and high‐dose opioid‐based general anaesthesia groups (RR 0.58, 95% CI 0.28 to 1.18), and between early extubation protocol versus usual care groups (RR 0.84, 95% CI 0.40 to 1.75). We noted no significant differences between low‐dose and high‐dose opioid‐based anaesthesia groups for the following postoperative complications: myocardial infarction (RR 0.98, 95% CI 0.48 to 1.99), reintubation (RR 1.77, 95% CI 0.38 to 8.27), acute renal failure (RR 1.19, 95% CI 0.33 to 4.33), major bleeding (RR 0.48, 95% CI 0.16 to 1.44) and stroke (RR 1.17, 95% CI 0.36 to 3.78). Comparison with usual care revealed no significant differences in risk of the following postoperative complications associated with a time‐directed extubation protocol: myocardial infarction (RR 0.94, 95% CI 0.55 to 1.60), reintubation (RR 1.91, 95% CI 0.90 to 4.07), acute renal failure (RR 0.77, 95% CI 0.19 to 3.10), major bleeding (RR 0.80, 95% CI 0.45 to 1.44), stroke (RR 0.87, 95% CI 0.31 to 2.46), major sepsis (RR 1.25, 95% CI 0.08 to 19.75) and wound infection (RR 0.67, 95% CI 0.25 to 1.83). Although levels of heterogeneity were high, both low‐dose opioid anaesthesia and use of time‐directed extubation protocols were associated with reductions in time to extubation (3.0 to 10.5 hours) and in length of stay in the intensive care unit (0.4 to 8.7 hours). However, these fast‐track care interventions were not associated with reductions in total length of stay in the hospital. One high‐quality cost‐effectiveness analysis conducted in a randomized controlled trial showed that early extubation was likely to be cost‐effective.
The rationale for conducting this Cochrane review update was to include findings from the most recent trials on risks and benefits of interventions commonly used as part of a fast‐track cardiac programme.
Objectives
To determine the safety and effectiveness of fast‐track cardiac care compared with conventional (not fast‐track) care in adult patients undergoing cardiac surgery. Fast‐track cardiac care intervention includes administration of low‐dose opioid‐based general anaesthesia or use of a time‐directed extubation protocol, or both.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCTs) comparing fast‐track care (interventions with the aim of early extubation within eight hours after surgery) with conventional (not fast‐track) care when extubation occurred after eight hours following cardiac surgery. We focused on RCTs that compared the use of low‐dose opioid‐based general anaesthesia versus high‐dose opioid‐based general anaesthesia, and early extubation based on time‐directed protocols versus usual care for extubation.
Types of participants
We included adults undergoing cardiac surgery (CABG, aortic valve procedures, mitral valve procedures or a combination of these) with or without cardiopulmonary bypass. We excluded studies that involved children (age limits defined by each study) or participants undergoing surgery for aortic aneurysm repair.
Types of interventions
As in the previous reviews (Hawkes 2003; Zhu 2012), we chose eight hours as the defined time limit for early tracheal extubation because this definition was frequently presented in the literature published in the 1990s. As rapid advances and changes in cardiac anaesthesia and surgical techniques have occurred since the 1990s, we did not exclude studies that compared interventions designed for early extubation (within four hours after surgery).
For the purpose of this review, FTCA involves the use of low‐dose opiate (fentanyl ≤ 20 μg/kg or equivalent) (Myles 2003) or short‐acting opioid supplemented with propofol or etomidate, or volatile anaesthesia with or without a protocol for early extubation within eight hours. Conventional cardiac anaesthesia was defined by the use of high‐dose opioids (fentanyl ≥ 20 μg/kg or equivalent) with propofol or etomidate, or volatile anaesthesia with or without a protocol for extubation within a specified time after surgery. We excluded trials with remifentanil as, unlike other opioids, it has a short half‐life and does not accumulate after prolonged administration (Howie 2003); its use in cardiac surgery has been reviewed elsewhere (Greco 2012). We excluded studies that examined major regional blockade (epidural or intrathecal), as the effectiveness of thoracic epidural in cardiac surgery has been reviewed in another Cochrane systematic review (Svircevic 2013). We also excluded studies that compared normothermia and hypothermia during cardiopulmonary bypass in adult cardiac surgery, as the risks and benefits have been reviewed elsewhere (Ho 2011).
Types of outcome measures
If a study did not report any of the following prespecified outcome data, we excluded that study from the systematic review.
Primary outcomes
Mortality
1. Risk of mortality in the ICU.
2. Risk of hospital mortality.
3. Risk of mortality at 30 days.
4. Risk of mortality at one year.
5. Risk of mortality at any time point.
Secondary outcomes
Postoperative complications
For the following postoperative complications, we used individual study definitions, which may vary between trials.
1. Risk of postoperative myocardial infarction.
2. Risk of stroke.
3. Risk of acute renal failure.
4. Risk of major bleeding.
5. Risk of major sepsis.
6. Risk of wound infection.
7. Risk of reintubation.
Patient‐centred outcomes
1. Quality of life at one month.
2. Quality of life at one year.
Service outcomes
1. Time to extubation.
2. ICU length of stay.
3. Hospital length of stay.
4. Inpatient costs (USD).
Costs were estimated at 2015 USD values. The reported currency was converted to 2015 USD with the ‘CCEMG – EPPI‐Centre Cost Converter’ (v.1.5) (a free web‐based tool available at http://eppi.ioe.ac.uk/costconversion/default.aspx).
We performed a separate meta‐analysis for each of the primary and secondary outcomes listed above for the following interventions used in fast‐track cardiac care.
1. Low‐dose opioid‐based general anaesthesia versus high‐dose opioid‐based general anaesthesia.
2. Early extubation via a time‐directed protocol versus usual extubation care.
Search methods for identification of studies
Electronic searches
We searched the following databases for relevant trials.
Cochrane Central Register of Controlled Trials (CENTRAL; 2015, Issue 5) (The Cochrane Library); see Appendix 1.
MEDLINE (Ovid SP) (January 2012 to 28 May 2015); see Appendix 2.
Embase (Ovid SP) (January 2012 to 28 May 2015); see Appendix 3.
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (via EBSCOhost) (January 2012 to 28 May 2015); see Appendix 4.
Institute for Scientific Information (ISI) Web of Science (January 2012 to 28 May 2015); see Appendix 5.
We used medical subject heading (MeSH) terms for MEDLINE and other headings appropriate to other databases, such as ‘cardiac surgery', ‘extubation' and ‘fast‐track'. We combined our subject search filter with the Cochrane highly sensitive search strategy for identifying RCTs, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), when searching in MEDLINE. We adopted this RCT filter to searches in other databases and applied no language restrictions.
Searching other resources
We searched the reference lists of retrieved articles, trials and reviews (Blackwood 2014; Meades 2001; Myles 2003; van Mastrigt 2006b). We also searched the World Health Organization Clinical Trials Registry and ClinicalTrial.gov on 29 December 2015.
Data collection and analysis
Selection of studies
We selected trials included in the systematic review on the basis of search strategy. Two review authors (WTW and AL) independently scanned the titles and abstracts of reports identified by electronic searches to produce a list of possibly relevant studies. We used the Rayyan application to manage the screening process (Elmagarmid 2014). We obtained full‐text versions, and two review authors (WTW and AL) used a standardized data collection form to independently assess them for inclusion. We resolved disagreements between review authors by meetings for discussion.
Data extraction and management
Two review authors (VKWL and AL) independently extracted data using the Cochrane Anaesthesia, Critical and Emergency Care Review Group data extraction form adapted for this review. We collected data on the types and doses of drugs used in anaesthesia, as well as on patient population and type of cardiac surgery.
Assessment of risk of bias in included studies
Two review authors (VKWL and AL) independently assessed the quality of studies by applying the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We graded risk of bias for each study in the domains of sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and comparison of baseline characteristics entered into a ‘Risk of bias' table (Higgins 2011). We graded each domain as ‘yes' (low risk of bias), ‘no' (high risk of bias) or ‘unclear' (uncertain risk of bias) according to the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We defined a high‐quality trial as one in which all domains were considered to be at low risk of bias, and a low‐quality trial as a trial in which one or more of these domains were rated as having high risk of bias.
We used the GRADE approach to rate the overall quality of evidence for seven outcomes (death at any time after surgery, postoperative myocardial infarction, postoperative stroke, postoperative tracheal reintubation, time to extubation, length of intensive care unit stay, length of hospital stay) (Summary of findings table 1; Summary of findings table 2) as high, moderate, low or very low (Guyatt 2011). We downgraded the quality of evidence from high if we noted study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates and potential publication bias (Guyatt 2011). We upgraded the quality of evidence when we observed a large effect (RR < 0.5 or RR > 2) in the absence of plausible confounders (Higgins 2011).
Measures of treatment effect
Summary estimates reported included risk ratio (RR), mean difference (MD) and associated 95% confidence intervals (95% CIs). For rare outcomes, such as death, we estimated Peto's odd ratio (OR) to combine data as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
If more than one group met intervention or control group criteria, we combined the data to create a single pair‐wise comparison. For dichotomous outcomes, we summed both sample sizes and numbers of participants with events across groups (Higgins 2011). For continuous outcomes, we combined means and standard deviations using methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In Slogoff and Keat's study (Slogoff 1989), we combined enflurane, halothane and isoflurane groups into a single early extubation group.
Unit of analysis issues
None.
Dealing with missing data
We contacted the first authors of included trials to obtain missing data that were necessary for meta‐analysis. We calculated missing standard deviations from standard errors, confidence intervals and interquartile ranges, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We calculated standard deviations for service outcomes in studies by van Mastrigt and colleagues using published confidence intervals (van Mastrigt 2006a, 2010).
Assessment of heterogeneity
As only randomized controlled trials were included in this systematic review, we would not expect methodological heterogeneity to be problematic. We observed clinical heterogeneity in the interventions compared, but if we found a comparable body of trials amenable to meta‐analysis, we calculated a summary estimate and displayed pooled results graphically. We assessed statistical heterogeneity between trials by using the I2 statistic. We defined low, moderate and high levels of heterogeneity as I2 values of 25%, 50% and 75%, respectively (Higgins 2003). When we found evidence of large heterogeneity, we attempted to explain the reason for it and rechecked the data for possible data entry errors.
Assessment of reporting biases
Using STATA statistical software (Stata Corporation, College Station, Texas, USA, version 14), we constructed a contour‐enhanced funnel plot to correctly identify publication bias separate from other causes of funnel plot asymmetry when we included more than 10 trials (Peters 2008). This was performed for mortality at any time with opioid‐based cardiac anaesthesia and with time‐directed extubation protocols. We used the Egger's test to test for funnel plot asymmetry with time to extubation outcomes (Egger 1997).
Data synthesis
We used a DerSimonian and Laird random‐effects model and Review Manager 5.3 software to combine data for continuous and dichotomous outcomes. We reported risk ratio (RR), mean difference (MD), 95% confidence interval (CI) and P value. However, we calculated Peto odds ratio (OR) and 95% CI were calculated in pooling mortality data, as many studies reported no deaths in either or both trial arms, and we expected the event rate to be low.
Subgroup analysis and investigation of heterogeneity
We undertook exploratory a priori subgroup analyses for trials examining the use of time‐directed protocols for early extubation. In an attempt to assess the safety associated with fast‐track recovery units, we compared subgroups for early extubation in settings inside and outside ICU and risk of reintubation. To test whether subgroups were different from one another, we tested the interaction using Review Manager 5.3 software (Deeks 2010).
Sensitivity analysis
We conducted a sensitivity analysis for trials with low risk of bias to estimate the robustness of results for mortality at any time within one year in studies examining FTCA and time‐directed extubation protocols.
Summary of findings
We used the principles of the GRADE system to assess the quality of the body of evidence associated with specific outcomes (Guyatt 2011). The GRADE approach appraises the quality of a body of evidence on the basis of the extent to which one can be confident that an estimate of effect or association reflects the outcome being assessed (Guyatt 2011). Assessment of the quality of a body of evidence considers within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of the effect estimates and risk of publication bias (Guyatt 2011). Using the GRADE software, we constructed 'Summary of findings' tables for comparison of the following specific outcomes: mortality, postoperative myocardial infarction, stroke, tracheal reintubation, time to extubation, length of ICU stay and length of hospital stay.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The search identified six studies for full‐text review. For this systematic review, we brought forward 62 trials (25 included and 37 excluded) from our previous Cochrane review (Zhu 2012). The process is shown in Figure 1. We found no ongoing studies.
1.
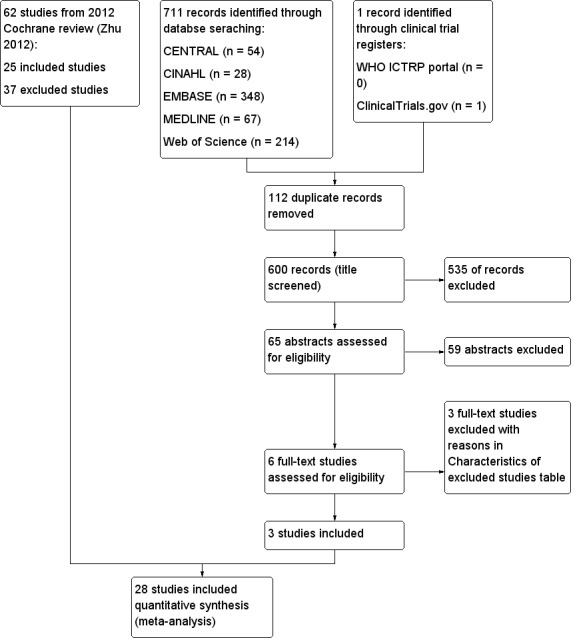
Study flow diagram.
Included studies
We included in the updated review a total of 28 RCTs involving 4438 participants. One trial was multi‐centred (Silbert 2006). The sample size of these studies ranged from 35 (Nicholson 2002) to 1012 (Slogoff 1989). The trials were conducted in the United States (Engoren 1998; Slogoff 1989), Canada (Cheng 1996a,1996b, 2003; Dumas 1999; Nicholson 2002; Quasha 1980), Australia (Myles 1997; Myles 2002; Silbert 1998; Silbert 2006), France (Nougarede 2004), Italy (Simeone 2002), United Kingdom (Bell 1994; Berry 1998; Sherry 1996), Netherlands (van Mastrigt 2006a, 2010), Spain (Reyes 1997), Sweden (Pettersson 2004), Greece (Michalopoulos 1998), Oman (Maddali 2006), Japan (Kadoi 2003; Sakaida 1998), Hong Kong (Gruber 2008; Zhu 2015), Germany (Probst 2014), Egypt (Salah 2015) and Taiwan (Chang 2007; Lu 2003). Most participants were undergoing first‐time elective coronary artery graft bypass or valvular replacement surgical procedures, or both. In two studies (Nicholson 2002; Reyes 1997), participants were undergoing emergency, semi urgent or urgent cardiac surgery. Two studies recruited participants with low cardiac output (Bell 1994; Sherry 1996).
Eighteen studies involved the use of low‐dose opioid‐based anaesthesia (Bell 1994; Berry 1998; Chang 2007; Cheng 1996a,1996b, 2003; Engoren 1998; Kadoi 2003; Lu 2003; Maddali 2006; Michalopoulos 1998; Myles 1997; Myles 2002; Probst 2014; Sakaida 1998; Sherry 1996; Silbert 1998; Silbert 2006; Slogoff 1989; Zhu 2015). Sixteen studies involved the use of time‐directed extubation protocols (Bell 1994; Berry 1998; Cheng 1996a,1996b, 2003; Dumas 1999; Gruber 2008; Michalopoulos 1998; Nicholson 2002; Nougarede 2004; Pettersson 2004; Probst 2014; Quasha 1980; Reyes 1997; Salah 2015; Simeone 2002; van Mastrigt 2006a, 2010; Zhu 2015).
Participants were extubated in the ICU, except in three studies in which early extubation occurred on the operating table (Nougarede 2004; Salah 2015), or in the postanaesthesia care unit (Nicholson 2002). Three studies provided follow‐up for participants for one year (Cheng 1996a,1996b, 2003; Silbert 2006; van Mastrigt 2006a, 2010); the remaining trials followed participants during their ICU stay or until the end of their hospital stay.
Unpublished data for four studies (Myles 2002; Reyes 1997; Sakaida 1998; Zhu 2015), were included in this updated review. We received no unpublished data after writing to the authors of new included trials.
Excluded studies
A total of 40 studies did not meet the inclusion criteria for the reasons shown in the Characteristics of excluded studies table.
Ongoing studies
We found no ongoing studies.
Studies awaiting classification
We found no studies awaiting classification.
Risk of bias in included studies
See Characteristics of included studies. Two studies had low risk of bias (Cheng 1996a,1996b, 2003; van Mastrigt 2006a, 2010), as all key domains were rated 'yes'. Eleven studies had high risk of bias (Bell 1994; Dumas 1999; Engoren 1998; Gruber 2008; Maddali 2006; Quasha 1980; Salah 2015; Sakaida 1998; Sherry 1996; Silbert 1998; Zhu 2015), as one or more domains were rated 'no'. A 'Risk of bias' graph and summary are provided in Figure 2 and Figure 3, respectively.
2.
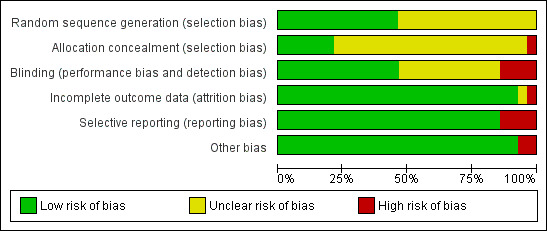
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
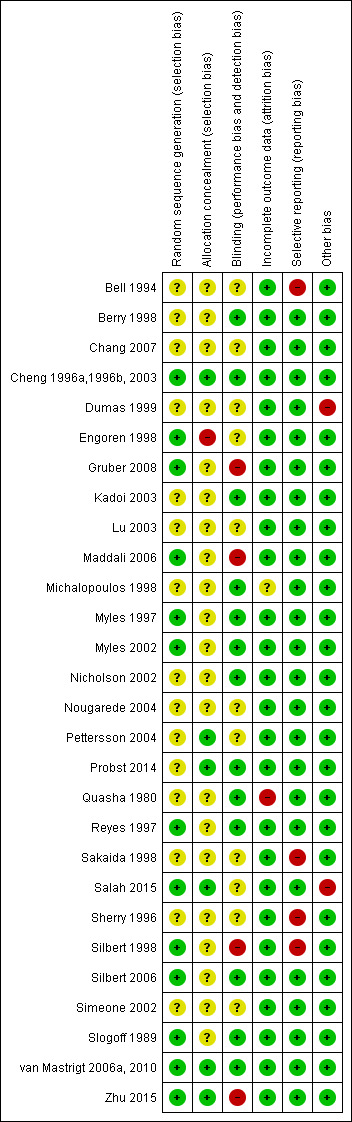
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Ten studies used computer‐generated random numbers to determine allocation sequences (Cheng 1996a,1996b, 2003; Engoren 1998; Gruber 2008; Maddali 2006; Reyes 1997; Salah 2015; Silbert 1998; Slogoff 1989; van Mastrigt 2006a, 2010; Zhu 2015), and three studies used random number tables (Myles 1997; Myles 2002; Silbert 2006). One study reported inadequate allocation concealment (Engoren 1998).
Blinding
Four studies did not use blinding (Gruber 2008; Maddali 2006; Silbert 1998; Zhu 2015).
Incomplete outcome data
One study omitted two participants from the outcome analysis (Quasha 1980).
Selective reporting
Three studies did not report mortality and complications outcomes (Bell 1994; Sakaida 1998; Sherry 1996), and one study showed inconsistency in outcome reporting (Silbert 1998).
Other potential sources of bias
Demographic and intraoperative characteristics were comparable in most studies.
Effects of interventions
Dose of opioid‐based cardiac anaesthesia
Primary outcomes
Mortality (Analysis 1.1)
Investigators reported short‐term (ICU and hospital stay) and long‐term (one‐year) mortality. Three studies recorded no deaths (Engoren 1998; Michalopoulos 1998; Myles 1997). Berry 1998 showed no significant differences between low‐dose and high‐dose opioid groups for risk of death in the ICU (OR 0.13, 95% CI 0.01 to 2.06). The overall risk of death at hospital discharge was less than 2%, and researchers found no significant differences between low‐dose and high‐dose opioid groups (OR 0.58, 95% CI 0.24 to 1.39; seven trials, 1896 participants) (Analysis 1.1). At one‐year follow‐up, risk of death was similar between groups (OR 0.55, 95% CI 0.17 to 1.82; two trials, 446 participants) in two studies (Cheng 1996a,1996b, 2003; Silbert 2006) (Analysis 1.1).
1.1. Analysis.
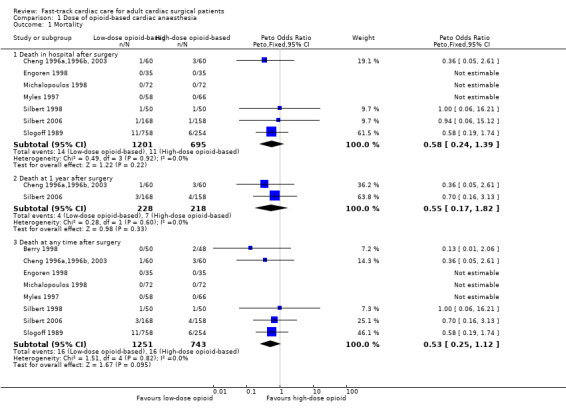
Comparison 1 Dose of opioid‐based cardiac anaesthesia, Outcome 1 Mortality.
When death was reported at several time periods within a study, we used the data from the longest follow‐up. Data from 1994 participants show that eight trials examined the risk of mortality after surgery at any time within a year (Berry 1998; Cheng 1996a,1996b, 2003; Engoren 1998; Michalopoulos 1998; Myles 1997; Silbert 1998; Silbert 2006; Slogoff 1989). We found no heterogeneity between the eight studies (I2 = 0%) (Analysis 1.1) and similar risk of death between groups (OR 0.53, 95% CI 0.25 to 1.12, P = 0.10). We downgraded the evidence from high to low because two trials had one or more high risk of bias domains along with imprecision.
As the meta‐analysis included fewer than 10 studies, we did not construct a contour‐enhanced funnel plot graph to examine the presence of publication bias. Sensitivity analysis showed that the risk of mortality at any time within a year in a study with low risk of bias was not different between groups (OR 0.36, 95% CI 0.05 to 2.61) (Cheng 1996a,1996b, 2003).
Secondary outcomes
Postoperative complications (Analysis 1.2)
Myocardial infarction (Analysis 1.2.1)
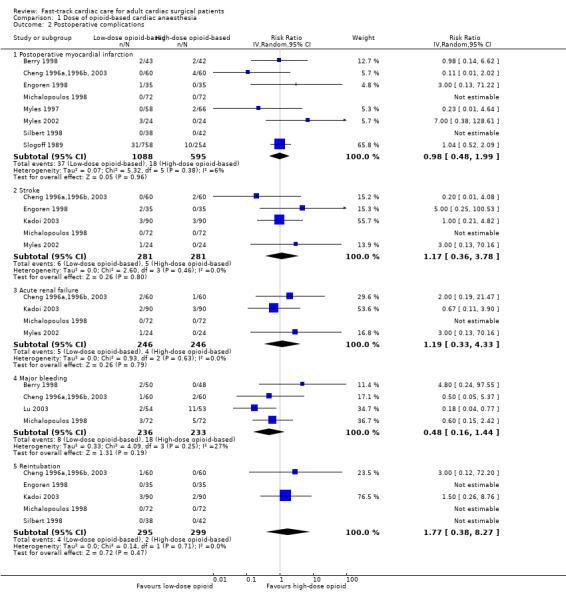
The overall incidence of postoperative myocardial infarction was 3.3% (95% CI 2.5% to 4.2%). Two studies stated that no participants had postoperative myocardial infarction (Michalopoulos 1998; Silbert 1998). Eight studies involving 1683 participants reported no significant difference in the risk of postoperative myocardial infarction between low‐dose and high‐dose opioid groups (RR 0.98, 95% CI 0.48 to 1.99; P = 0.96) (Berry 1998; Cheng 1996a,1996b, 2003; Engoren 1998; Michalopoulos 1998; Myles 1997; Myles 2002; Silbert 1998; Slogoff 1989). Heterogeneity between studies was low (I2 = 6%). We downgraded the evidence from high to low because of imprecision and because two trials had one or more domains at high risk of bias.
Stroke (Analysis 1.2.2)
The overall incidence of postoperative stroke was 2.0% (95% CI 1.0% to 3.4%). One study reported no participants with stroke after surgery (Michalopoulos 1998). The risk of stroke after surgery was similar between low‐dose and high‐dose opioid groups (RR 1.17, 95% CI 0.36 to 3.78; P = 0.80), and we found no heterogeneity (I2 = 0%) between the five studies involving 562 participants (Cheng 1996a,1996b, 2003; Engoren 1998; Kadoi 2003; Michalopoulos 1998; Myles 2002) (Analysis 1.2). We downgraded the evidence from high to low because of imprecision, and because one trial had one or more high risk of bias domains.
1.2. Analysis.
Comparison 1 Dose of opioid‐based cardiac anaesthesia, Outcome 2 Postoperative complications.
Acute renal failure (Analysis 1.2.3)
The overall incidence of acute renal failure after surgery was 1.8% (95% CI 0.9% to 3.3%). Michalopoulos 1998 reported no participants with acute renal failure after surgery. We found no significant difference in the risk of postoperative acute renal failure between low‐dose and high‐dose opioid groups (RR 1.19, 95% CI 0.33 to 4.33; P = 0.79) in four studies involving 492 participants (Cheng 1996a,1996b, 2003; Kadoi 2003; Michalopoulos 1998; Myles 2002) (Analysis 1.2). We downgraded the quality of evidence to moderate owing to imprecision.
Major bleeding (Analysis 1.2.4)
The overall incidence of major bleeding after cardiac surgery was 5.5% (95% CI 3.7% to 7.9%). Researchers reported no significant difference in the risk of major bleeding after surgery between low‐dose and high‐dose opioid groups (RR 0.48, 95% CI 0.16 to 1.44; P = 0.19), and heterogeneity between studies was low (I2 = 27%) in a random‐effects model of four studies involving 469 participants (Berry 1998; Cheng 1996a,1996b, 2003; Lu 2003; Michalopoulos 1998) (Analysis 1.2). We downgraded the quality of evidence to moderate owing to imprecision.
Major sepsis
Michalopoulos 1998 reported no participants with major sepsis.
Wound infection
Michalopoulos 1998 reported no wound infection. Myles 2002 observed no significant difference in the risk of postoperative wound infection between low‐dose and high‐dose opioid groups (RR 2.00, 95% CI 0.19 to 20.61).
Reintubation within 24 hours of surgery (Analysis 1.2.5)
No participants required reintubation in three studies (Engoren 1998; Michalopoulos 1998; Silbert 1998). The overall risk of reintubation in the low‐dose opioid group was 1.4% (95% CI 0.4% to 3.2%). This risk of reintubation in the low‐dose opioid group was not significantly higher than risk in the high‐dose opioid group (RR 1.77, 95% CI 0.38 to 8.27; P = 0.47) in five studies involving 594 participants (Cheng 1996a,1996b, 2003; Engoren 1998; Kadoi 2003; Michalopoulos 1998; Silbert 1998) (Analysis 1.2). These studies were homogenous (I2 = 0%). We downgraded the evidence from high to low because of imprecision, and because two trials had one or more high risk of bias domains.
Service outcomes (Analysis 1.3)
Time to extubation (Analysis 1.3.1)
Two studies did not report interquartile range around the median time to extubation (Bell 1994; Sherry 1996). In Bell 1994, investigators extubated the low‐dose opioid group 8.54 hours before the high‐dose opioid group (P < 0.0005). Researchers in Sherry 1996 extubated the low‐dose opioid group 4.84 hours before the high‐dose opioid group, but whether this difference was significant was not clear. We did not include these two studies in the time to extubation meta‐analysis.
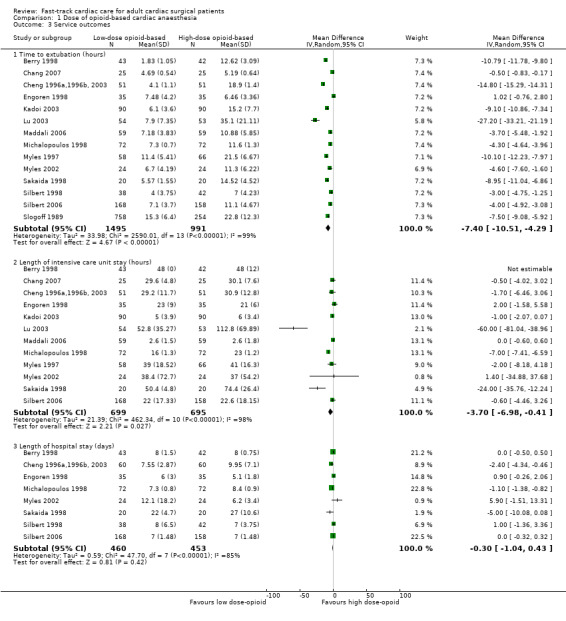
Comparison with the high‐dose opioid group revealed that the low‐dose opioid group was associated with a reduction in time to extubation (MD ‐7.40 hours, 95% CI ‐10.51 to ‐4.29; P < 0.0001) in 14 studies involving 2486 participants (Berry 1998; Chang 2007; Cheng 1996a,1996b, 2003; Engoren 1998; Kadoi 2003; Lu 2003; Maddali 2006; Michalopoulos 1998; Myles 1997; Myles 2002; Sakaida 1998; Silbert 1998; Silbert 2006; Slogoff 1989). The mean difference in time to extubation ranged from ‐27.20 hours (95% CI ‐33.21 to ‐21.19) (Lu 2003), favouring the low‐dose opioid group, to 1.02 hours (95% CI ‐0.76 to 2.80) (Engoren 1998), favouring the high‐dose opioid group. The Egger's test showed no evidence of funnel plot asymmetry (P = 0.40). Heterogeneity among the 14 studies was high (total n = 2486) when results were pooled (I2 = 99%) (Analysis 1.3). We downgraded the evidence from high to low because four trials had one or more high risk of bias domains and high heterogeneity among the studies was not explained.
1.3. Analysis.
Comparison 1 Dose of opioid‐based cardiac anaesthesia, Outcome 3 Service outcomes.
Intensive care unit length of stay (Analysis 1.3.2)
Berry 1998 described little variability in length of stay in the ICU in the early extubation group. Although Bell 1994 reported no variability, participants in the low‐dose opioid group were discharged from the ICU sooner than participants in the high‐dose opioid group (median 4.5 hours; P = 0.005). Analysis of 12 studies involving 1394 participants revealed that the low‐dose opioid group was associated with shorter ICU length of stay (MD ‐3.70, 95% CI ‐6.98 to ‐0.41 hours; P = 0.03) (Berry 1998; Chang 2007; Cheng 1996a,1996b, 2003; Engoren 1998; Kadoi 2003; Lu 2003; Maddali 2006; Michalopoulos 1998; Myles 1997; Myles 2002; Sakaida 1998; Silbert 2006). The mean difference in ICU length of stay ranged from ‐60.00 hours (95% CI ‐81.04 to ‐38.96), favouring the low‐dose opioid group (Lu 2003), to 2.00 hours (95% CI ‐1.58 to 5.58), favouring the conventional extubation group (Engoren 1998). Heterogeneity between the 12 studies (total n = 1394) was high when pooled (I2 = 98%). We downgraded the evidence from high to low because three trials had one or more high risk of bias domains and high heterogeneity among studies was not explained.
Hospital length of stay (Analysis 1.3.3)
All studies except two (Cheng 1996a,1996b, 2003; Michalopoulos 1998) reported no significant differences in hospital length of stay between groups. When we pooled the eight studies involving 913 participants, we found that the length of stay in the hospital was not significantly different between low‐dose and high‐dose opioid groups (MD ‐0.30 days, 95% CI ‐1.04 to 0.43; P = 0.42). However, heterogeneity between the studies was large (I2 = 85%). We downgraded the evidence from high to low because three trials had one or more high risk of bias domains and high heterogeneity among studies could not be explained.
Cost
We did not pool studies, as each study measured costs on different aspects of cardiac anaesthesia care. The low‐dose opioid‐based general anaesthesia intervention was associated with a reduction in departmental cost savings for uncomplicated CABG surgery (MD 2015 USD ‐2016, 95% CI ‐3247 to ‐785) (Cheng 1996a,1996b, 2003). The total hospital cost in Myles 2002 was similar between the low‐dose opioid group (mean 2015 USD 15,744 ± 4234) and the high‐dose opioid group (mean 2015 USD 14,641 ± 3376), with MD of 2015 USD 1103 (95% CI ‐1142 to 3348). The total cost of drugs was similar between the low‐dose opioid group (mean 2015 USD 117 ± 32) and the high‐dose opioid group (mean 2015 USD 128 ± 47), with MD of 2015 USD 11 (95% CI ‐35 to 13). The cost of ICU nursingand of drugs used in the operating theatre and in the ICU in the low‐dose opioid group was not significantly different from the cose in the high‐dose opioid group (MD 2015 USD ‐93, 95% CI ‐7 to 192) (Sherry 1996).
Time‐directed extubation protocol
Primary outcomes
Mortality (Analysis 2.1)
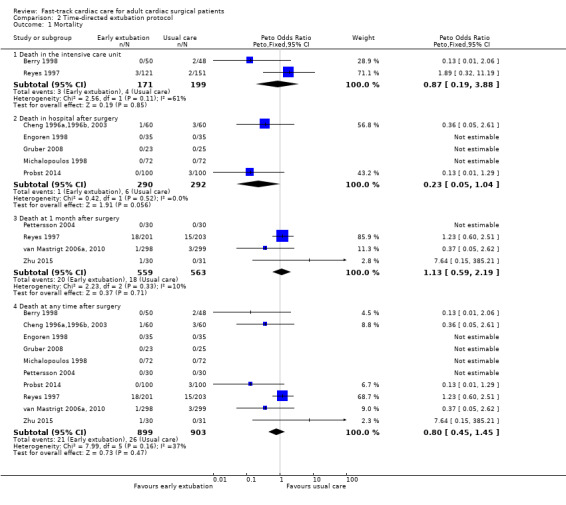
Investigators reported no significant difference between early and usual care (late extubation) groups for risk of death in the ICU in two studies involving 370 participants (OR 0.87, 95% CI 0.19 to 3.88) (Analysis 2.1). Three studies recorded no deaths in hospital after surgery (Engoren 1998; Gruber 2008; Michalopoulos 1998). Pooling of data from showed that risk of death in the hospital after surgery was similar between groups (OR 0.23, 95% CI 0.05 to 1.04; five trials, 582 participants) (Analysis 2.1). At one month after surgery, risk of death was similar (< 4%) in early extubation and usual care (late extubation) groups (OR 1.13, 95% CI 0.59 to 2.19; four trials, 1122 participants) (Analysis 2.1).
2.1. Analysis.
Comparison 2 Time‐directed extubation protocol, Outcome 1 Mortality.
When death was reported at several time periods within a study, we used the data from longest follow‐up. We pooled 10 studies involving 1802 participants for outcome analysis (Berry 1998; Cheng 1996a,1996b, 2003; Engoren 1998; Gruber 2008; Michalopoulos 1998; Pettersson 2004; Probst 2014; Reyes 1997; van Mastrigt 2006a, 2010; Zhu 2015). Heterogeneity between the studies was moderate (I2 = 37%) (Analysis 2.1). Researchers showed no difference in risk of mortality after surgery at any time within a year between early and late extubation groups (OR 0.80, 95% CI 0.45 to 1.45; P = 0.47) (Analysis 2.1). We downgraded the evidence from high to low because three trials had one or more high risk of bias domains and showed imprecision.
Sensitivity analysis showed that risk of mortality at any time within a year in a study with low risk of bias was not significantly different between early and late extubation groups (RR 0.37, 95% CI 0.05 to 2.62) (van Mastrigt 2006a, 2010). Heterogeneity in mortality at any time after surgery may be due in part to inclusion of the Reyes 1997 trial, which found exceptionally high mortality compared with other studies; when we excluded this study, the OR estimate favoured the time‐directed protocol group with much lower heterogeneity (OR 0.31, 95% CI 0.11 to 0.90; P = 0.03, I2 = 0%) and moderate quality of evidence (downgraded owing to study limitations). As fewer than 10 studies with estimates were included in the meta‐analysis, we did not construct a contour‐enhanced funnel plot graph to examine the presence of publication bias.
Secondary outcomes
Postoperative complications (Analysis 2.2)
Myocardial infarction (Analysis 2.2.1)
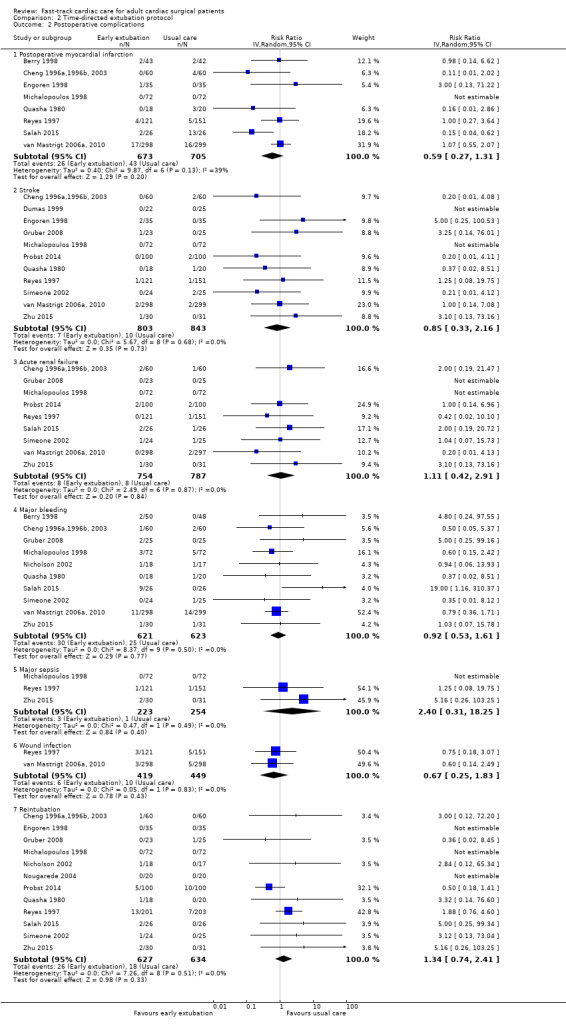
The overall incidence of postoperative myocardial infarction was 5.0% (95% CI 3.9% to 6.3%). One study stated that no participants had postoperative myocardial infarction (Michalopoulos 1998). We pooled eight studies involving 1378 participants for analysis and found no difference in risk of postoperative myocardial infarction between early extubation and usual care (late extubation) groups (RR 0.59, 95% CI 0.27 to 1.31; P = 0.20) (Analysis 2.2). Heterogeneity between studies was moderate (I2 = 39%). We downgraded the evidence from high to low because of imprecision, and because three trials had one or more high risk of bias domains.
2.2. Analysis.
Comparison 2 Time‐directed extubation protocol, Outcome 2 Postoperative complications.
Stroke (Analysis 2.2.2)
The overall incidence of postoperative stroke was 1.0% (95% CI 0.6% to 1.6%). Two studies reported that no participants had stroke after surgery (Dumas 1999; Michalopoulos 1998). Pooling of 11 studies involving 1646 participants for analysis revealed that risk of stroke after surgery was similar between time‐directed protocol and usual care groups (RR 0.85, 95% CI 0.33 to 2.16; P = 0.73) (Cheng 1996a,1996b, 2003; Dumas 1999; Engoren 1998; Gruber 2008; Michalopoulos 1998; Probst 2014; Quasha 1980; Reyes 1997; Simeone 2002; van Mastrigt 2006a, 2010; Zhu 2015). We found no heterogeneity (I2 = 0%) between the 11 studies involving 1646 participants (Analysis 2.2), and we downgraded the evidence from high to low because of imprecision, and because five trials had one or more high risk of bias domains.
Acute renal failure (Analysis 2.2.3)
The overall incidence of postoperative acute renal failure was 1.0% (95% CI 0.6% to 1.6%). Two studies reported that no participants had acute renal failure after surgery (Gruber 2008; Michalopoulos 1998). Pooling of nine studies involving 1541 participants for analysis showed no difference in the risk of postoperative acute renal failure between early extubation and late extubation groups (RR 1.11, 95% CI 0.42 to 2.91; P = 0.84) and these trials were homogeneous (I2 = 0%) (Analysis 2.2). We downgraded the evidence from high to low because of imprecision and because three trials had one or more high risk of bias domains.
Major bleeding (Analysis 2.2.4)
The overall incidence of major bleeding after cardiac surgery was 4.4% (95% CI 3.4% to 5.7%). Investigators showed no difference in the risk of major bleeding after surgery between early extubation and usual care (late extubation) groups (RR 0.92, 95% CI 0.53 to 1.61; P = 0.77). We noted no heterogeneity (I2 = 0%) between 10 studies involving 1244 participants (Berry 1998; Cheng 1996a,1996b, 2003; Gruber 2008; Michalopoulos 1998; Nicholson 2002; Quasha 1980; Salah 2015; Simeone 2002; van Mastrigt 2006a, 2010; Zhu 2015) (Analysis 2.2). We downgraded the evidence from high to low because of imprecision, and because four trials had one or more high risk of bias domains.
Major sepsis (Analysis 2.2.5)
The overall incidence of sepsis was 1.8% (95% CI 0.3% to 2.0%). Michalopoulos 1998 reported no participants with major sepsis. We found no difference in the risk of postoperative major sepsis between early extubation and usual care (late extubation) groups (RR 2.40, 95% CI 0.31 to 18.25; P = 0.40) in a random‐effects model of three studies involving 477 participants (Michalopoulos 1998; Reyes 1997; Zhu 2015) (Analysis 2.2). We found no heterogeneity between studies (I2 = 0%), and we downgraded the evidence from high to low because of imprecision, and because one trial had one or more high risk of bias domains.
Wound infection (Analysis 2.2.6)
The overall incidence of postoperative wound infection was 1.8% (95% CI 1.1% to 2.9%). We noted no difference in the risk of postoperative wound infection between early extubation and usual care (late extubation) groups (RR 0.67, 95% CI 0.25 to 1.83; P = 0.43) and no heterogeneity between the two trials (I2 = 0%) involving 868 participants (Reyes 1997; van Mastrigt 2006a, 2010) (Analysis 2.2). The level of evidence was moderate owing to imprecision.
Reintubation within 24 hours of surgery (Analysis 2.2.7)
No participants required reintubation in three studies (Engoren 1998; Michalopoulos 1998; Nougarede 2004). Reyes 1997 and Probst 2014 contributed to 42.8% and 32.1% of the total data pooled for this outcome, respectively. Risk of reintubation in the time‐directed extubation protocol group was 4.1% (95% CI 2.8% to 5.9%). This risk of reintubation in the time‐directed extubation protocol group was not significantly higher than risk in the usual care (late extubation) group (RR 1.34, 95% CI 0.74 to 2.41; P = 0.33) in 12 studies involving 1261 participants (Cheng 1996a,1996b, 2003; Engoren 1998; Gruber 2008; Michalopoulos 1998; Nicholson 2002; Nougarede 2004; Probst 2014; Quasha 1980; Reyes 1997; Salah 2015; Simeone 2002; Zhu 2015) (Analysis 2.2). We found no heterogeneity between studies (I2 = 0%) and no subgroup differences (P = 0.58) in risk of reintubation according to where extubation occurred (Figure 4). We downgraded the evidence from high to low because of imprecision, and because five trials had one or more high risk of bias domains.
4.
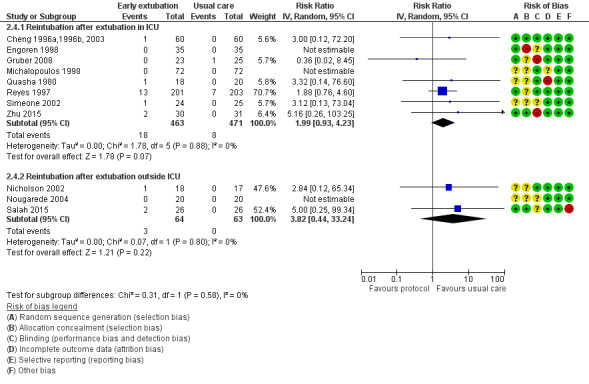
Forest plot of comparison: 2 Time‐directed extubation protocols, outcome: 2.4 Subgroup analysis.
Patient‐centred outcome
Quality of life within one year
Only one study reported quality of life within one year using the visual analogue scale (0 to 100) in the EuroQoL Group Quality of Life Questionnaire (EQ5D) instrument (van Mastrigt 2006a, 2010). The change in quality of life from baseline (one day before surgery) to one month after surgery between early extubation (9.99 ± 21.76) and usual care (late extubation) groups (7.00 ± 18.99) was similar (MD 2.99, 95% CI ‐0.98 to 6.96; P = 0.14). The change in quality of life from baseline to one year after surgery between early extubation (5.20 ± 17.23) and usual care (late extubation) groups (6.45 ± 16.19) was also similar (MD ‐1.25, 95% CI ‐4.50 to 2.00; P = 0.45).
Service outcomes (Analysis 2.3)
Time to extubation (Analysis 2.3.1)
In the early extubation group in two studies (Nougarede 2004; Salah 2015), investigators extubated participants immediately after surgery. Participants were extubated in the ICU in all but two trials (Nicholson 2002; Probst 2014), in which participants were extubated in the postanaesthesia care unit. The MD in time to extubation was ‐2.60 hours (95% CI ‐2.88 to ‐2.32) in Nicholson 2002 to ‐6.47 hours (95%CI ‐7.32 to ‐5.62) in Probst 2014.
We pooled 16 studies involving 2024 participants for analysis (Berry 1998; Cheng 1996a,1996b, 2003; Dumas 1999; Engoren 1998; Gruber 2008; Michalopoulos 1998; Nicholson 2002; Nougarede 2004; Pettersson 2004; Probst 2014; Quasha 1980; Reyes 1997; Salah 2015, Simeone 2002; van Mastrigt 2006a, 2010; Zhu 2015) (Analysis 2.3). Comparison with usual care revealed that use of a time‐directed extubation protocol was associated with a reduction in intubation time of ‐6.25 hours (95% CI ‐8.84 to ‐3.67; P < 0.001). The MD in the time to extubation group ranged from ‐16.00 hours (95% CI ‐17.64 to ‐14.36) (Quasha 1980), favouring the time‐directed extubation protocol group, to 1.02 hours (95% CI ‐0.76 to 2.80) (Engoren 1998), favouring the usual care group. The Egger's test showed no evidence of funnel plot asymmetry (P = 0.47). When pooled, heterogeneity between the 16 studies (total n = 2024) was high (I2 = 99%). We downgraded the evidence from high to low because six trials had one or more high risk of bias domains and high heterogeneity among studies was not explained.
2.3. Analysis.
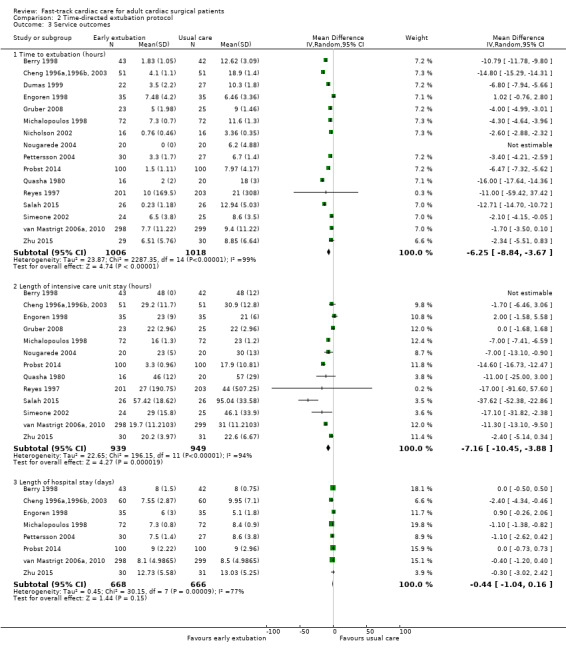
Comparison 2 Time‐directed extubation protocol, Outcome 3 Service outcomes.
Intensive care unit length of stay (Analysis 2.3.2)
We pooled 13 studies involving 1888 participants for analysis (Berry 1998; Cheng 1996a,1996b, 2003; Engoren 1998; Gruber 2008; Michalopoulos 1998; Nougarede 2004; Probst 2014; Quasha 1980; Reyes 1997; Salah 2015, Simeone 2002; van Mastrigt 2006a, 2010; Zhu 2015). The time‐directed extubation protocol intervention group was associated with a shorter ICU length of stay (MD ‐7.16, 95% CI ‐10.45 to ‐3.88 hours; P = 0.000019) (Analysis 2.3). The MD in the ICU length of stay ranged from ‐37.62 hours (95% CI ‐52.38 to ‐22.86), favouring the time‐directed extubation protocol group (Salah 2015) to 2.00 hours (95% CI ‐1.68 to 5.58), favouring the usual care group (Engoren 1998). Heterogeneity between the 13 studies involving 1888 participants was high when pooled (I2 = 94%). We downgraded the evidence from high to low because five trials had one or more high risk of bias domains and high heterogeneity among studies was not explained.
Hospital length of stay (Analysis 2.3.3)
Two studies found a significant difference in hospital length of stay between groups (Cheng 1996a,1996b, 2003; Michalopoulos 1998). Pooling of the eight studies involving 1334 participants showed that length of stay in the hospital was not different between time‐directed extubation protocol and usual care groups (MD ‐0.44, 95% CI ‐1.04 to 0.16; P = 0.15) (Berry 1998; Cheng 1996a,1996b, 2003; Engoren 1998; Michalopoulos 1998; Pettersson 2004; Probst 2014; van Mastrigt 2006a, 2010; Zhu 2015) (Analysis 2.3). However, heterogeneity between studies was large (I2 = 77%). We downgraded the evidence from high to low because two trials had one or more high risk of bias domains, and because reasons for high heterogeneity among studies were not explained.
Cost
We did not pool studies, as each study measured costs on different aspects of fast‐track cardiac anaesthesia care. The early extubation intervention was associated with a reduction in departmental cost savings for uncomplicated CABG surgery (MD 2015 USD ‐2016, 95% CI ‐3247 to ‐785) (Cheng 1996a,1996b, 2003).
Van Mastright and colleagues (van Mastrigt 2006a, 2010) reported that early extubation was associated with a reduction in total hospital cost (nutrition, laundry, accommodation, cleaning, overheads, equipment, staff, material and medication) (MD 2015 USD ‐1102, 95% CI ‐2117 to ‐233). Furthermore, the cost‐effectiveness (cost/change in quality‐adjusted life months) analysis found that 98% of bootstrapped incremental cost‐effectiveness ratios showed greater improvement in quality of life and lower costs for participants with early extubationcompared with participants with late extubation (van Mastrigt 2006a, 2010).
Discussion
Summary of main results
In this update, we added to the body of evidence three trials (Probst 2014; Salah 2015; Zhu 2015) ‐ all on the effects of a time‐directed extubation protocol. In this systematic review of 28 randomized controlled trials involving 4438 particpants, we found that fast‐track cardiac care was associated with a similar risk of mortality after surgery when compared with conventional (not fast‐track) care (Table 1; Table 2). The incidence of mortality at any time after surgery was generally low (< 5%). We found moderate‐quality evidence of reduced risk of death associated with a time‐directed protocol intervention when a trial (Reyes 1997) with a high mortality rate of 8% was excluded from the main meta‐analysis. This post hoc sensitivity analysis is a new finding of this review.
We noted no increase in risk of myocardial infarction, acute renal failure, stroke, major bleeding, sepsis or wound infection (Table 1; Table 2), and found that the overall quality of evidence for postoperative complications was low. We also found evidence of low quality for the reduction in time to extubation (up to 11 hours earlier) and length of stay in the intensive care unit (ICU) (up to 10 hours earlier). It should be noted that patient transfer out of ICU to the ward is dependent on the availability of beds on the ward in some settings, which may explain the high heterogeneity observed. Clinically important reductions in length of stay in the hospital were not associated with fast‐track cardiac care, and we rated this evidence as low quality.
We found limited evidence on the effects of early extubation following a time‐directed protocol on hospital cost and on quality of life within one year after cardiac surgery. Nevertheless, a formal cost‐effectiveness analysis in one trial showed that early tracheal extubation was cost‐effective (van Mastrigt 2006a, 2010).
Overall completeness and applicability of evidence
We considered most of the study participants to be at low to moderate risk of operative mortality (based on the European system for cardiac operative risk evaluation criteria), and most were undergoing elective cardiac surgery. This may explain why low rates of mortality and postoperative complications were found in most trials included in this systematic review. We found few large trials, and all, except one (Slogoff 1989), were not designed to detect an effect on myocardial infarction, stroke or death. Thus, it is likely that this systematic review was underpowered to detect significant risk reductions. For example, a post hoc power analysis using G*Power software (Faul 2014) showed that the power to detect a difference in the risk of mortality at any time after surgery with the use of time‐directed extubation protocols was 11%.
Results of this review are unlikely to be applicable to high‐risk patients, who required significantly longer time to extubation (about one hour) than low‐risk patients when the same fast‐track cardiac anaesthesia technique was used in a cohort study of 1162 participants (Alhan 2003). Of note, length of stay in the ICU and in hospital was similar for high‐risk patients and low‐risk patients (Alhan 2003). Risk factors associated with fast‐track cardiac surgery protocol failure (ICU readmission or failure to directly transfer patients to an intermediate care unit after surgery) include preoperative American Society of Anesthesiologists' Physical Status > 3, New York Heart Association class > III and operative time > 267 minutes (Kiessling 2013).
Our systematic review highlights the paucity of high‐quality cost‐analysis studies on fast‐track cardiac care. A propensity‐matched cohort study of 652 participants showed that extubation in the operating room was associated with a 23% decrease in overall postoperative costs (mean difference 2014 USD ‐1013, 95% CI ‐1597 to ‐429) compared with extubation in the ICU within 12 hours after surgery (Badhwar 2014). Reductions in cost were due to shorter length of stay in the ICU and hospital with similar risks of postoperative complications among patients extubated in the operating room (Badhwar 2014).
Remifentanil, an opioid with rapid onset and offset of action, is used frequently in fast‐track cardiac anaesthesia (Greco 2012). The trials in this systematic review did not include the use of remifentanil; this limits the applicability of evidence to settings in which remifentanil is used as part of the fast‐track cardiac anaesthesia technique. A meta‐analysis of remifentanil trials in cardiac surgery showed that remifentanil was associated with reduced time to extubation and length of hospital stay with no increase in risk of mortality when compared with use of fentanyl or sufentanil during general anaesthesia (Greco 2012).
Quality of the evidence
The quality of evidence as rated by the GRADE approach is shown in Table 1 and Table 2 (Guyatt 2011). A limitation of this systematic review is that 11 of the 28 trials had one or more high risk of bias domains. Blinding and selective reporting introduced the most common risks of bias. Overall, evidence on the association between fast‐track cardiac care and mortality was of low quality.
Evidence on the association between fast‐track cardiac care and major postoperative complications was of low quality, as review authors included high risk of bias trials in the various meta‐analyses and noted imprecision. Although we observed a clinically important difference between groups in time to extubation, the evidence was of low quality owing to inclusion of high risk of bias trials and large inconsistencies of effect between trials. Only one high‐quality trial examined changes in quality of life after cardiac surgery associated with a time‐directed extubation protocol (van Mastrigt 2006a, 2010).
Potential biases in the review process
We cannot rule out the presence of publication bias in this systematic review. We did not attempt to handsearch conference proceedings. As we identified fewer than 10 trials with risk estimates of mortality after surgery, it was impossible for review authors to assess publication bias using a contour‐enhanced funnel plot. Nevertheless, we found no funnel asymmetry for time to extubation outcomes for the comparison of opioid‐based general anaesthesia and time‐directed protocol interventions.
Caution is required in interpreting results of the post hoc sensitivity analysis on exclusion of a trial (Reyes 1997) from the meta‐analysis on risk of death at any time after surgery, and on use of time‐directed protocols (odds ratio (OR) 0.31, 95% confidence interval (CI) 0.11 to 0.90). We are unsure why the excluded trial (Reyes 1997) had a higher mortality rate than the other trials considered, irrespective of trial arm.
Agreements and disagreements with other studies or reviews
Although investigators measured mortality at different time points, the risk of mortality associated with fast‐track cardiac care in this systematic review did not change the conclusions reported in previous systematic reviews (Hawkes 2003; Meades 2001; Myles 2003; Zhu 2012). In a retrospective cohort of 7989 participants comparing low‐dose and high‐dose opioid‐based general anaesthesia (Svircevic 2009), the adjusted odds ratio for mortality was 0.92 (95% confidence interval (CI) 0.65 to 1.32). The significant post hoc sensitivity analysis result contrasts with findings of a consensus conference on fast‐track cardiac anaesthesia (FTCA) that found no significant reduction in risk of mortality in any of the trials screened (Landoni 2011).
Previous systematic reviews found no evidence of increased risk of major complications after surgery associated with FTCA (Myles 2003; Zhu 2012). Our relative risks of myocardial infarction, major sepsis, acute renal failure, stroke and major bleeding were similar, but showed greater precision. Therefore, we conclude that FTCA is safe and comparable with conventional (not fast‐track) care.
The time to extubation result in this systematic review was comparable with that in previous systematic reviews (Meades 2001; Myles 2003; Zhu 2012) and in a large retrospective cohort study of 7989 participants in which the fast‐track cardiac anaesthesia group was extubated six hours earlier than the high‐dose opioid group (Svircevic 2009). A previous review suggested that the introduction of an early extubation protocol, in conjunction with low‐dose opioid anaesthetic techniques and normothermic temperature management, would be essential for decreasing intensive care unit (ICU) and hospital stays (van Mastrigt 2006b).
This systematic review found four studies examining early extubation outside the ICU setting (Nicholson 2002; Nougarede 2004; Probst 2014; Salah 2015). Careful selection of patients for immediate extubation in the operating room rarely resulted in tracheal reintubation and was associated with shorter ICU and hospital length of stay in two propensity‐matched cohort studies (Chamchad 2010; Badhwar 2014).
As health‐related quality of life instruments and timing of these assessments varied in 10 studies, the authors of a previous review (van Mastrigt 2006b) did not pool the results. The main limitation of the previous review was that pain and cognitive function were the only main dimensions of quality of life considered in the postoperative period to six months after surgery (van Mastrigt 2006b). In contrast, we included a trial that used a valid and reliable generic health‐related quality of life tool (van Mastrigt 2006a, 2010) and found high quality of evidence for a small but non‐significant improvement in quality of life at one year after surgery associated with early extubation.
We did not pool our cost analyses but found that early extubation was likely to be cost‐effective in one high‐quality study (van Mastrigt 2006a, 2010). A propensity‐matched cohort study of 652 participants showed that extubation in the operating room was associated with a 23% decrease in overall postoperative costs (mean difference 2014 USD ‐1013, 95% CI ‐1597 to ‐429) compared with extubation in the ICU within 12 hours after surgery (Badhwar 2014).
Authors' conclusions
Implications for practice.
Low quality evidence suggests that adults who are considered to be at low to moderate risk of operative mortality can benefit from fast‐track care after undergoing uncomplicated cardiac surgery. The evidence in our review highlights the main benefit associated with fast‐track cardiac anaesthesia (FTCA): a clinically important reduction in time to extubation and intensive care unit (ICU) stay without increased risk of mortality or major complications after surgery. Although the risks of morbidity and mortality associated with FTCA are low, the randomized controlled trials included in this systematic review were not usually adequately powered to detect small to moderate group differences. We found no subgroup differences in the risk of reintubation based on the location where extubation occurred. We found limited evidence on the quality of life associated with FTCA and on the cost‐effectiveness of FTCA.
Implications for research.
Fast‐track cardiac anaesthesia is now the global standard of care (Silbert 2009). This review highlights the need for future, adequately powered trials to include long‐term cost analyses and health‐related quality of life outcomes after cardiac surgery. The results of fast‐track treatment provided in a postanaesthetic care unit (“Leipzig fast‐track concept”), instead of admission to an ICU, are encouraging (Ender 2008; Nicholson 2002; Probst 2014). The need for accurate fast‐track failure risk prediction models in cardiac surgery appears to be substantial. If a threshold probability of fast‐track failure of between 5% and 20% is used to determine who should be admitted to the ICU or to the fast‐track recovery unit, we would expect an increase in ICU bed utilization from 23% to 67%, even after adjustments for the negative consequences of unplanned ICU admissions (Lee 2013). An overview of Cochrane and non‐Cochrane systematic reviews of common interventions used before, during and after cardiac surgery would be worthwhile, to summarize the effectiveness of perioperative interventions that are included in a well‐established fast‐track cardiac programme.
Blackwood 2014 conducted a systematic review on the use of weaning protocols for reducing the duration of mechanical ventilation in critically ill adult patients and identified only one relevant, small, randomized controlled trial (Simeone 2002). Additional trials, similar to Gruber 2008 and Zhu 2015, are required to address the effects of weaning protocols based on different modes of mechanical ventilation on death, adverse events, duration of mechanical ventilation and length of stay in the ICU and hospital in cardiac surgical patients; these studies would need to be adequately powered and must provide clear descriptions of all aspects of study methods.
What's new
| Date | Event | Description |
|---|---|---|
| 28 May 2016 | New citation required but conclusions have not changed | In this updated review, we made the following changes to the previously published review (Zhu 2012). 1. We found 3 new completed trials (Probst 2014; Salah 2015; Zhu 2015). 2. Members of the review team changed from the previous review to the present update. Zhu left the review team, Wong and Lai joined the review team. 3. Instead of using a random‐effects model to estimate the risk ratio of mortality, we used the Peto method to estimate the odds ratio, as the mortality event was rare. |
| 28 May 2015 | New search has been performed | We performed a new search and reran the search until May 2015. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 5 September 2012 | New search has been performed | We updated the search. In general, our review of 25 RCTs reached the same conclusions that were reached by Hawkes et al (Hawkes 2003). We focused on the effectiveness of low‐dose opioid general anaesthesia and a time‐directed extubation protocol as interventions designed for early extubation after surgery. We included more trials and thus provided more precise estimates on mortality, postoperative complications and length of stay. |
| 5 September 2012 | New citation required but conclusions have not changed | This review is an update of a previous Cochrane systematic review that included 6 RCTs (Hawkes 2003). The previous review authors Claire A Hawkes, Srinivasan Dhileepan and David Foxcroft decided not to update the review (Hawkes 2003); the new review authors Fang Zhu, Anna Lee and Yee Eot Chee updated this version. |
| 5 September 2012 | New search has been performed | We changed the title to "Fast‐track cardiac care for adult cardiac surgical patients" from "Early extubation for adult cardiac surgical patients" (Hawkes 2003), to reflect various interventions designed to alter extubation timing. |
| 5 September 2012 | New search has been performed | In this updated systematic review, we applied several new statistical methods (contour‐enhanced funnel plot, Egger test), risk of bias tables and figures and summary of finding tables that were not included in Hawkes 2003. We also extended our search strategy to include additional electronic databases. |
| 1 August 2008 | Amended | We converted the review to new review format. |
Notes
None.
Acknowledgements
We would like to thank Rodrigo Cavallazzi (Content Editor), Nathan Pace (Statistical Editor) Janet Wale (Consumer Editor) and Jane Cracknell (Managing Editor) for help and editorial advice provided during preparation of this updated systematic review.
Thanks to Dr John Carlisle, Prof Nathan Pace, Dr Karen Hovhannisyan, Dr R Peter Alston, Prof Paul Myles, Dr Vesna Svircevic and Ms Jane Cracknell for help and editorial advice provided during the previous update (Zhu 2012). Thanks to Dr Zhirajr Mokini for translating a study (Nougarede 2004) from French to English.
We would like to acknowledge the work done in the original Cochrane review by Claire Hawkes, Srinivasan Dhileepan and David Foxcroft (Hawkes 2003), and in the previous update by Fang Zhu (Zhu 2012).
Appendices
Appendix 1. Search strategy for CENTRAL, The Cochrane Library
#1 MeSH descriptor Cardiac Surgical Procedures explode all trees #2 MeSH descriptor Thoracic Surgery, this term only #3 MeSH descriptor Coronary Artery Bypass explode all trees #4 (heart or cardiac):ti,ab #5 (#1 OR #2 OR #3 OR #4) #6 MeSH descriptor Anesthesia Recovery Period explode all trees #7 (removal near endotracheal) #8 fast near track #9 early near extubation #10 (#6 OR #7 OR #8 OR #9) #11 (#5 AND #10)
Appendix 2. Search strategy for MEDLINE (Ovid SP)
1. exp Cardiac‐Surgical‐Procedures/ or exp Thoracic‐Surgery/ or exp Coronary‐Artery‐Bypass/ 2. (heart or cardiac).ti,ab. 3. 1 or 2 4. exp Anesthesia‐Recovery‐Period/ 5. ((removal adj3 endotracheal) or (fast adj3 track) or (early adj3 extubation)).mp. 6. 4 or 5 7. 6 and 3 8. ((randomised controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 9. 8 and 7
Appendix 3. Search strategy for Embase (Ovid SP)
1. heart surgery/ or thorax surgery/ or exp coronary artery bypass graft/ 2. (heart or cardiac).ti,ab. 3. 2 or 1 4. exp anesthetic recovery/ 5. ((removal adj3 endotracheal) or (fast adj3 track) or (early adj3 extubation)).mp. 6. 5 or 4 7. 4 and 6 8. ((((singl* or doubl* or tripl*) adj3 blind) or crossover).ti,ab. or multicenter.ab. or placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) not (animals not (humans and animals)).sh. 9. 8 and 7
Appendix 4. Search strategy for CINAHL (EBSCOhost)
S1 (MH "Heart Surgery+") S2 (MH "Thoracic Surgery") S3 (MH "Coronary Artery Bypass+") S4 TI ( heart or cardiac ) or AB ( heart or cardiac ) S5 S1 or S2 or S3 or S4 S6 (MM "Anesthesia Recovery") S7 TX ( removal and endotracheal ) or TX fast track or TX ( early and extubation ) S8 S6 or S7 S9 S5 and S8 S10 (MM "Random Assignment") or (MH "Clinical Trials+") S11 (MM "Placebos") S12 (MM "Double‐Blind Studies") or (MM "Single‐Blind Studies") or (MM "Triple‐Blind Studies") S13 (MM "Multicenter Studies") S14 (MM "Crossover Design") S15 TI ( random* or placebo* or multi?center or crossover ) or AB ( random* or placebo* or multi?center or crossover ) or TI trial* or AB ( controlled and study ) S16 S10 or S11 or S12 or S13 or S14 or S15 S17 S9 and S16
Appendix 5. Search strategy for ISI Web of Science
#1 TS=((cardiac or heart or thora*) SAME (surg* or operat*)) or TS=((coronary artery) SAME bypass) #2 TS=(Anesthesia SAME Recovery) or TS=(removal SAME endotracheal) or TS=fast track or TS=(early SAME extubation) #3 #2 AND #1 #4 TS=(random* or placebo* or crossover or multi?center or VOLUNTEER*) or TI=trial* or TS= ((SINGL* or DOUBL* or TREBL* or TRIPL*) SAME (BLIND* or MASK*)) #5 #4 AND #3
Data and analyses
Comparison 1. Dose of opioid‐based cardiac anaesthesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 8 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Death in hospital after surgery | 7 | 1896 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.24, 1.39] |
| 1.2 Death at 1 year after surgery | 2 | 446 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.17, 1.82] |
| 1.3 Death at any time after surgery | 8 | 1994 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.25, 1.12] |
| 2 Postoperative complications | 10 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Postoperative myocardial infarction | 8 | 1683 | Risk Ratio (IV, Random, 95% CI) | 0.98 [0.48, 1.99] |
| 2.2 Stroke | 5 | 562 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.36, 3.78] |
| 2.3 Acute renal failure | 4 | 492 | Risk Ratio (IV, Random, 95% CI) | 1.19 [0.33, 4.33] |
| 2.4 Major bleeding | 4 | 469 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.16, 1.44] |
| 2.5 Reintubation | 5 | 594 | Risk Ratio (IV, Random, 95% CI) | 1.77 [0.38, 8.27] |
| 3 Service outcomes | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Time to extubation (hours) | 14 | 2486 | Mean Difference (IV, Random, 95% CI) | ‐7.40 [‐10.51, ‐4.29] |
| 3.2 Length of intensive care unit stay (hours) | 12 | 1394 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐6.98, ‐0.41] |
| 3.3 Length of hospital stay (days) | 8 | 913 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐1.04, 0.43] |
Comparison 2. Time‐directed extubation protocol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 10 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Death in the intensive care unit | 2 | 370 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.19, 3.88] |
| 1.2 Death in hospital after surgery | 5 | 582 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.04] |
| 1.3 Death at 1 month after surgery | 4 | 1122 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.13 [0.59, 2.19] |
| 1.4 Death at any time after surgery | 10 | 1802 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.45, 1.45] |
| 2 Postoperative complications | 15 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Postoperative myocardial infarction | 8 | 1378 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.27, 1.31] |
| 2.2 Stroke | 11 | 1646 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.33, 2.16] |
| 2.3 Acute renal failure | 9 | 1541 | Risk Ratio (IV, Random, 95% CI) | 1.11 [0.42, 2.91] |
| 2.4 Major bleeding | 10 | 1244 | Risk Ratio (IV, Random, 95% CI) | 0.92 [0.53, 1.61] |
| 2.5 Major sepsis | 3 | 477 | Risk Ratio (IV, Random, 95% CI) | 2.40 [0.31, 18.25] |
| 2.6 Wound infection | 2 | 868 | Risk Ratio (IV, Random, 95% CI) | 0.67 [0.25, 1.83] |
| 2.7 Reintubation | 12 | 1261 | Risk Ratio (IV, Random, 95% CI) | 1.34 [0.74, 2.41] |
| 3 Service outcomes | 16 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Time to extubation (hours) | 16 | 2024 | Mean Difference (IV, Random, 95% CI) | ‐6.25 [‐8.84, ‐3.67] |
| 3.2 Length of intensive care unit stay (hours) | 13 | 1888 | Mean Difference (IV, Random, 95% CI) | ‐7.16 [‐10.45, ‐3.88] |
| 3.3 Length of hospital stay (days) | 8 | 1334 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.04, 0.16] |
| 4 Subgroup analysis | 11 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Reintubation after extubation in ICU | 8 | 934 | Risk Ratio (IV, Random, 95% CI) | 1.99 [0.93, 4.23] |
| 4.2 Reintubation after extubation outside ICU | 3 | 127 | Risk Ratio (IV, Random, 95% CI) | 3.82 [0.44, 33.24] |
2.4. Analysis.
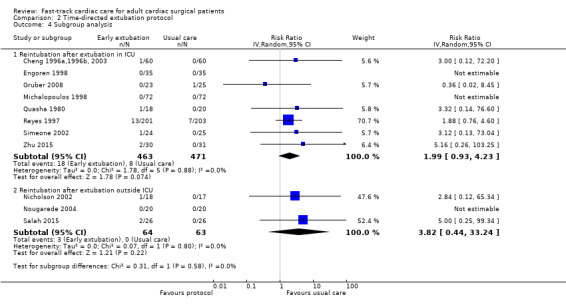
Comparison 2 Time‐directed extubation protocol, Outcome 4 Subgroup analysis.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bell 1994.
| Methods | Parallel‐group randomized controlled trial, conducted in England. Study dates not reported | |
| Participants | 39 elective cardiac (coronary artery and/or valve) surgical patients with low cardiac output (defined as cardiac index < 2.5 L/min/m2 and a minimal pulmonary capillary wedge pressure of 7 mmHg) without hepatic or renal impairment | |
| Interventions | Low‐dose opioid, early extubation (fentanyl 15 μg/kg and propofol, extubated within 8 hours) in 20 participants High‐dose opioid, usual care (fentanyl 60 μg/kg and midazolam, extubated after more than 8 hours) in 19 participants Details of weaning protocol and who decided when to extubate were not given. |
|
| Outcomes | Time to extubation Length of stay in the ICU |
|
| Notes | Median time to extubation and length of stay in the intensive care unit were reported with no interquartile range, thus data were not used in the meta‐analysis. Study authors did not report mortality outcomes or postoperative complications. No power calculation was done. Funding source was J.F. Blades and Zeneca Pharma, Wilmslow, Cheshire. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal from the study during follow‐up |
| Selective reporting (reporting bias) | High risk | Study authors did not report mortality outcomes or postoperative complications. |
| Other bias | Low risk | Baseline participant characteristics (age, sex, weight, surface area, duration of surgery, type of surgery) were comparable. |
Berry 1998.
| Methods | Parallel‐group blinded randomized controlled trial, conducted in the United Kingdom. Study dates not reported | |
| Participants | 98 elective coronary artery bypass graft (CABG) patients, with no ECG abnormalities, not older than 71 years, with no digitalis therapy, no left or right bundle branch block, no morbid obesity (BMI > 35), with FEV1 or FVC > 50%, no poor left ventricular function. Patients were excluded from the study after surgery if they failed to meet the following criteria: haemodynamically stable; blood loss < 120 mL/h and arterial PO2 ≤ 7 kPa with an inspired oxygen concentration of 50%; and PEEP ≤ 5 cm H2O. Patients with < 10 hours of acceptable number of beats in the postbypass period and with abnormal QRS complexes (i.e. not artefact, ventricular ectopics or bundle branch block) were excluded from the ECG analysis. | |
| Interventions | Low‐dose opioid, early extubation (within 8 hours) in 50 participants, but ECG analysis complete in 43 participants. Anaesthesia with fentanyl 15 μg/kg and isoflurane HIgh‐dose opioid, usual care (more than 8 hours) in 48 participants, but ECG analysis complete in 42 participants. Anaesthesia with fentanyl 50 μg/kg and isoflurane Details of weaning protocol and who decided when to extubate were not given. |
|
| Outcomes | Time to extubation
Risk of mortality in the ICU
Risk of postoperative myocardial ischaemia (defined non‐fatal myocardial infarction as concentrations of MB isoenzyme of creatine kinase (CK‐MB) levels > 130 IU/L and development of a new Q‐wave or new left bundle branch block on the postoperative 12‐lead ECG) Risk of major bleeding Hospital length of stay Length of stay in the ICU |
|
| Notes | Of 50 participants in the early extubation group, 3 failed early extubation in the designated period of 8 hours. Of 50 participants in the early extubation group, 2 had "excess blood loss (≥ 120 mL/h)". Power calculation was done. Details of any declarations of conflict of interest among study authors were not provided. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "On arrival in the operating room, patients were allocated to enter early extubation (group E) or late extubation (group L) according to a prepared randomization schedule" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Assessors were blinded to the diagnosis of myocardial ischaemic outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons and details for 13 dropouts were given. These data were excluded from further analysis. |
| Selective reporting (reporting bias) | Low risk | Reported all collected outcomes |
| Other bias | Low risk | Used multi‐variate analysis to examine ST depression to adjust for hypertension imbalance between 2 groups. All other participant characteristics and intraoperative surgical details were similar between groups. |
Chang 2007.
| Methods | Parallel‐group randomized controlled trial, conducted in Taiwan. Study dates not reported | |
| Participants | 50 patients undergoing elective off‐pump coronary artery bypass graft surgery. Patients were excluded if they had severe ventilatory impairment, history of renal failure, diabetes mellitus or American Society of Anesthesiologists' physical status of IV or above, or had undergone a previous cardiac surgical procedure. | |
| Interventions | Low‐dose opioid anaesthesia in 25 participants (isoflurane‐based anaesthesia with mean fentanyl 2.9 ± 0.2 μg/kg) High‐dose opioid anaesthesia in 25 participants (fentanyl‐propofol‐based anaesthesia with mean fentanyl 21.8 ± 2.7 μg/kg) Details of weaning protocol and who decided when to extubate were not given. |
|
| Outcomes | Time to extubation Length of stay in ICU |
|
| Notes | No power calculation was done. Funding was provided by a grant from Tri‐Service General Hospital (grant number: TSGH‐C92‐81). Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal from the study during follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported all collected outcomes |
| Other bias | Low risk | Baseline participant characteristics (age, sex, height, weight, duration of anaesthesia, ejection fraction) were comparable. |
Cheng 1996a,1996b, 2003.
| Methods | Parallel‐group double‐blinded randomized controlled trial, conducted in Canada. Study conducted from May 1992 to May 1994 | |
| Participants | 120 elective primary coronary artery bypass graft patients younger than 75 years of age, with LV function grades I to IV, no previous cardiac surgery, no allergy to propofol, no left bundle branch block or digitalis therapy, no documented myocardial infarction within previous 3 weeks, no active congestive cardiac failure, no inotropic therapy within 24 hours of surgery, no current intra‐aortic balloon pump, no severe hepatic disease, no renal insufficiency, no severe chronic obstructive pulmonary disease, no history of stroke or seizure | |
| Interventions | Low‐dose opioid, early extubation group: fentanyl 15 μg/kg, isoflurane and propofol anaesthesia with extubation 1 to 6 hours after surgery in 60 participants HIgh‐dose opioid, usual care group: fentanyl 50 μg/kg, isoflurane and midazolam anaesthesia with extubation on the day after surgery in 60 participants Details of weaning protocol and who decided when to extubate were not given. |
|
| Outcomes | Risk of mortality in hospital Risk of mortality at 1 year Risk of tracheal reintubation Risk of myocardial infarction (defined as either or both of the following findings: CK‐MB levels > 50 IU/L and representing > 8% of total CK or major 12‐lead ECG changes (including new Q‐wave > 0.04 seconds in duration and > 1 mm in depth, ST‐segment elevation or depression > 2 mm lasting 48 hours and a symmetrical T‐wave inversion persisting for 48 hours) from baseline in ≥ 2 leads) Risk of major bleeding Risk of stroke (defined as sudden onset of focal neurological deficit, symptoms of focal neurological deficit persisting > 24 hours, or both, as documented by a neurologist) Risk of renal failure (requiring dialysis or haemofiltration) Time to extubation Length of stay in the ICU Length of stay in hospital Hospital costs |
|
| Notes | Departmental actual cost savings in uncomplicated CABG surgery used for analysis (1996b, Table 9), converted to USD 2015 values. No power calculation done. Study was supported, in part, by a grant from Anesthesia Patient Safety Foundation, American Society of Anesthesiologists, 1995; a 1995 Investigator Award from the Society of Cardiovascular Anesthesiologists granted to first study author; and a grant from Zeneca Pharma, Canada, to second study author for the ICU sedation medication cost analysis. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly and consecutively allocated according to a computer‐generated randomization code to early (study) or conventional (control) groups" |
| Allocation concealment (selection bias) | Low risk | "Concealed in an envelope until anaesthesia was induced" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The surgeon and the research assistant collecting the data, but not the anaesthesiologist providing the clinical care, were blinded to the group assignments". Radiologist was blinded to study group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Nine participants in each group did not meet extubation criteria within the defined period. |
| Selective reporting (reporting bias) | Low risk | All collected outcomes reported |
| Other bias | Low risk | "There were no differences in demographic data, including the distribution of left ventricular function, between the early and conventional group" |
Dumas 1999.
| Methods | Parallel‐group randomized controlled trial, conducted in Canada. Study conducted from October 1995 to September 1996 | |
| Participants | 48 elective coronary artery bypass surgery patients who were younger than 75 years old, French speaking, living within a 60‐ to 70‐km radius of the hospital. Exclusion of those with “psychologic or psychiatric antecedents; prescription of lithium and/or antidepressant medication; alcohol abuse; chronic renal failure; ejection fraction less than 30%; valvuloplasty; significant peripheral vascular disease; uncontrolled hypertension; chronic obstructive pulmonary disease; and combined surgeries” | |
| Interventions | Early extubation (< 8 hours after surgery) in 22 participants Usual care (8 to 24 hours after surgery) in 25 participants Details of who decided when to extubate were not given. |
|
| Outcomes | Risk of stroke Time to extubation |
|
| Notes | This study focused on postoperative cognitive dysfunction. Time to extubation was defined as "delay between arrival at the intensive care unit and the start of withdrawal from anaesthetic/analgesic agents". Weaning protocol was the same in both groups, but timing was different, as described in the paper. No power calculation was done. Study was supported, in part, by the Department of Anesthesia, Faculty of Medicine, University of Montreal, Canada. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly assigned to one of two groups" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawals given |
| Selective reporting (reporting bias) | Low risk | All collected outcomes reported |
| Other bias | High risk | Most demographics, co‐morbidities and surgical data were similar between groups. However, early extubation groups were 6 years younger than conventional extubation groups and had lower prevalence of carotid bruit. |
Engoren 1998.
| Methods | Parallel‐group randomized controlled trial, conducted in the United States. Study dates not reported | |
| Participants | 70 patients undergoing primary coronary artery bypass surgery. Excluded were those with concomitant valve, carotid artery or other co‐incident surgery. | |
| Interventions | Low‐dose opioid (isoflurane anaesthesia with a continuous infusion of propofol and mean fentanyl dose 13.7 μg/kg) in 35 participants High‐dose opioid (isoflurane anaesthesia and mean fentanyl dose 21.0 μg/kg) in 35 participants Details of who decided when to extubate were not given in the paper. |
|
| Outcomes | Risk of mortality in hospital Risk of myocardial infarction Risk of stroke Risk of tracheal reintubation Time to extubation Length of stay in the ICU Length of stay in hospital Cost of anaesthesia drugs |
|
| Notes | Weaning protocol was the same in both groups, but timing was different, as described in the paper. Power calculation was performed. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized by a computer‐generated scheme" |
| Allocation concealment (selection bias) | High risk | "..and the anaesthesia provider was informed of the assignment while preparing the room for anaesthesia" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "..and the anaesthesia provider was informed of the assignment while preparing the room for anaesthesia" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants withdrew from the study. |
| Selective reporting (reporting bias) | Low risk | All collected outcomes reported |
| Other bias | Low risk | "Patients were very similar in baseline demographics (Table 1), although anaesthesia and cardiopulmonary bypass times were slightly longer in the propofol group" |
Gruber 2008.
| Methods | Parallel‐group randomized controlled trial, conducted in Hong Kong. Study dates not reported | |
| Participants | 50 adults undergoing elective coronary artery bypass graft (CABG). Excluded were patients with LV ejection fraction > 30%, concomitant vascular or aortic surgery, older than 80 years, chronic pulmonary artery disease requiring bronchodilator therapy, significant hepatic disease (alanine aminotransferase or aspartate aminotransferase > 150 U/L), renal failure or history of seizure and stroke | |
| Interventions | Early extubation (within 6 hours using adaptive‐support ventilation) in 23 participants Usual care (after 7 hours with pressure‐regulated volume‐controlled ventilation with auto‐mode) in 25 participants Details of who decided when to extubate were not given in the paper. |
|
| Outcomes | Risk of mortality in hospital Time to extubation Length of stay in the ICU Risk of stroke Risk of major bleeding Risk of tracheal reintubation Risk of acute renal failure (defined as new requirement for renal replacement) |
|
| Notes | Anaesthesia (low‐dose fentanyl and midazolam, with propofol or sevoflurane) in both groups was the same. Details of weaning protocol were given in the paper. Same weaning protocol was given to both groups, but timing was different. Power calculation was performed. Funding source was the department. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | High risk | "This was an unblinded randomized controlled trial" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawal given |
| Selective reporting (reporting bias) | Low risk | All outcomes collected were reported. |
| Other bias | Low risk | Participant demographics, anaesthesia and surgical procedures were similar between groups. However, participants in the intervention group had lower LVEF and higher EuroSCORE compared with participants in the control group. |
Kadoi 2003.
| Methods | Parallel‐group randomized controlled trial, conducted in Japan. Study dates not reported | |
| Participants | 180 elective coronary artery bypass graft (CABG) patients with no history of cerebrovascular disease, diabetes, psychiatric illness, renal disease or active liver disease. Patients were excluded if they had moderate or severe atherosclerotic lesions in the ascending aorta or carotid artery stenosis confirmed by preoperative ultrasonography and magnetic resonance imaging. | |
| Interventions | Low‐dose opioid (propofol infusion) in 90 participants High‐dose opioid (fentanyl infusion, mean fentanyl 58 ± 15 μg/kg) in 90 participants Details of weaning protocol and who decided when to extubate were not given. |
|
| Outcomes | Risk of tracheal reintubation (for pneumonia) Risk of acute renal failure (creatinine > 3 mg/dL) Risk of stroke (defined as clinical evidence of focal cerebral infarction including hemiparesis, visual or gait disturbance, mental changes or a combination of these) Time to extubation Length of ICU stay |
|
| Notes | Both groups had anaesthesia induced by midazolam 0.3 mg/kg, fentanyl 10 μg/kg and vecuronium 0.2 mg/kg. Power calculation was done. Study was supported, in part, by a grant of first study author from the Japanese Ministry of Science and Education. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Neurological examiner was blinded to group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participant follow‐up was complete except for neuropsychological assessments at 6 months. |
| Selective reporting (reporting bias) | Low risk | All outcomes collected were reported. |
| Other bias | Low risk | "No significant differences in demographic data between the two groups except for phenylephrine dosage" |
Lu 2003.
| Methods | Parallel‐group randomized controlled trial, conducted in Taiwan. Study dates not reported | |
| Participants | 107 patients undergoing elective coronary artery bypass graft surgery. Patients were excluded if they had severe ventilatory impairment or history of renal failure or diabetes mellitus, or if they had undergone a previous cardiac surgical procedure. | |
| Interventions | Low‐dose opioid anaesthesia in 54 participants (isoflurane‐based anaesthesia with mean fentanyl 4.4 ± 0.2 μg/kg) High‐dose opioid anaesthesia in 53 participants (fentanyl‐midazolam‐based anaesthesia; mean fentanyl was 66.4 ± 3.2 μg/kg) Details of weaning protocol and who decided when to extubate were not given. |
|
| Outcomes | Time to extubation Length of stay in ICU Risk of postoperative bleeding (> 100 mL/h drainage) |
|
| Notes | No power calculation was done. Study was supported, in part, by a grant from Tri‐Service General Hospital (grant number: TSGH‐C90‐1). Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal from the study during follow‐up |
| Selective reporting (reporting bias) | Low risk | Reported all collected outcomes |
| Other bias | Low risk | Baseline participant characteristics (age, sex, height, weight, duration of anaesthesia, duration of cardiopulmonary bypass, cardiac output, cardiac index, ejection fraction, aortic clamping time) were comparable. |
Maddali 2006.
| Methods | Parallel 3‐arm randomized controlled trial, conducted in Oman. Study conducted from January 2004 to June 2004 | |
| Participants | 180 primary CABG patients with ejection fraction > 30% and no known hypersensitivity to opioids, IV propofol, benzodiazepines or non‐steroidal antiinflammatory drugs. Excluded patients had morbid obesity, neurological condition making pain assessment difficult to evaluate after surgery, significant arrhythmias, congestive cardiac failure, preoperative intra‐aortic balloon pump or severely impaired organ function. | |
| Interventions | Low‐dose opioid group: fentanyl (mean total dose 16.5 μg/kg) and diclofenac 75 mg suppository in 60 participants High‐dose opioid group: continuous fentanyl infusion (mean total perioperative dose 39 μg/kg) in 60 participants Details of who decided when to extubate were not given. |
|
| Outcomes | Time to extubation Length of postcardiac surgical unit stay |
|
| Notes | Group 3: remifentanil (1 μg/kg/min and bolus IV fentanyl 1 μg/kg) in 60 participants. Remifentanil group was not included in analysis, as this is a high‐dose opioid with a short half‐life. Participants' stay in postcardiac surgical unit was considered equivalent to stay in ICU, as participants were weaned off mechanical ventilation. All groups had the same weaning protocol. Power calculation was done. No details were provided about funding source or any declarations of conflict of interest among study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned, using a computer‐generated randomization chart, to three groups..." |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | High risk | "Open labelled" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four participants excluded with reasons given |
| Selective reporting (reporting bias) | Low risk | All collected outcomes were reported. |
| Other bias | Low risk | Demographic and surgical data were similar between groups. |
Michalopoulos 1998.
| Methods | Parallel‐group single‐blinded randomized controlled trial, conducted in Greece. Study dates not reported | |
| Participants | 144 elective coronary artery bypass patients younger than 70 years of age, with ejection fraction ≥ 35%, New York Heart Classification Class I to III, with normal preoperative respiratory function. Excluded were those with chronic renal failure, hepatic failure or cerebral dysfunction, and those who underwent redo CABG surgery | |
| Interventions | Low‐dose opioid, early extubation (4 to 7 hours) in 72 participants. Anaesthesia included fentanyl 15 to 20 μg/kg at induction and 5 μg/kg for maintenance, followed by ICU sedation and analgesia (morphine and propofol) for 2 hours. High‐dose opioid, usual care (extubation 8 to 14 hours) in 72 participants. Anaesthesia included fentanyl 50 μg/kg at induction and 10 to 15 μg/kg for maintenance, followed by ICU sedation and analgesia (morphine and midazolam) for 6 hours. Details of who decided when to extubate were not given. |
|
| Outcomes | Risk of mortality in hospital Risk of myocardial infarction (new and persistent Q waves at ECG associated with an abrupt rise in CPK, CPK‐MB and troponin values) Risk of stroke Risk of sepsis Risk of major bleeding (blood loss > 500 mL during the first 6 postoperative hours, or blood loss necessitating transfusion of > 3 red cell units during the first 12 postoperative hours) Risk of acute renal failure Risk of tracheal reintubation Time to extubation Length of stay in the ICU Length of hospital stay |
|
| Notes | No power calculation was done. No details were provided about funding source or any declarations of conflict of interest among study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Operative and postoperative complications were assessed blinded to the randomization of the allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given |
| Selective reporting (reporting bias) | Low risk | All outcomes collected and reported |
| Other bias | Low risk | Participant characteristics between groups were similar for gender, smoking, age, preoperative LVEF (%) and NYHA classification |
Myles 1997.
| Methods | Parallel‐group randomized controlled trial, conducted in Australia. Study dates not reported | |
| Participants | 129 elective CABG surgical patients. Excluded were those undergoing concurrent valvular surgery or considered at very high risk (clinical severity score > 9) because they were more likely to require continued sedation and longer stay in the ICU. Also excluded were those with preexisting left bundle branch block or pacemaker in situ preventing ST‐segment diagnosis of perioperative myocardial ischaemia. | |
| Interventions | Low‐dose opioid (propofol infusion and mean fentanyl 15.1 μg/kg) in 58 participants High‐dose opioid (enflurane 0.2% to 1% and mean fentanyl 31.3 μg/kg) in 66 participants Details of weaning protocol and who decided when to extubate were not given. |
|
| Outcomes | Risk of hospital mortality Risk of postoperative myocardial infarction (new Q waves in ≥ 2 ECG leads, as detected by an independent and blinded cardiologist and creatine kinase‐MB fraction > 5%) Time to extubation in ICU Length of stay in the intensive care unit |
|
| Notes | Power calculation was done. Funding was provided by research grants from the Australian and New Zealand College of Anaesthetists, and the Research Committee of the Alfred Hospital. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We randomized patients after stratification according to the surgeon's angiographic assessment of contractility to maximize equality of both groups. Randomization was determined by a table of random numbers" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "ICU staff were blinded to group identity" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "...a total of 129 patients (103 male, 26 female) were enrolled in this study, with subsequent exclusion of 5 patients" |
| Selective reporting (reporting bias) | Low risk | All outcomes collected and reported |
| Other bias | Low risk | "The groups were well‐matched for age, clinical severity score, myocardial contractility, preinduction haemodynamics and duration of surgery. Despite stratification and randomization, there were more women in the enflurane based anaesthesia group and those in the propofol group had a longer aortic cross‐clamp time" |
Myles 2002.
| Methods | Parallel 3‐arm double‐blinded randomized controlled trial, conducted in Australia. Study dates not reported | |
| Participants | 87 elective coronary artery bypass patients younger than 75 years of age. Excluded were those with body weight > 100 kg, allergic to trial medications, at high risk of mortality, uncontrolled hypertension or hypotension, congestive cardiac failure or an ejection fraction < 25%, with atrioventricular or left bundle branch block detected on preoperative electrocardiogram or with a pacemaker in situ. | |
| Interventions | Low‐dose opioid (propofol and mean fentanyl 15 μg/kg) in 24 participants High‐dose opioid (propofol and mean fentanyl 28 μg/kg) in 24 participants Details of who decided when to extubate were not given. |
|
| Outcomes | Risk of postoperative myocardial infarction (new Q waves in ≥ 2 ECG leads as detected by an independent and blinded cardiologist) Risk of wound infection (unpublished) Risk of stroke Risk of acute renal failure (requiring dialysis or haemofiltration) (unpublished) Time to extubation in ICU Length of stay in ICU Length of hospital stay (unpublished) Total hospital cost (OT drug cost, OT other cost, ICU total cost) |
|
| Notes | Data from remifentanil (0.85 μg/kg/min) were not included as they did not fit the criteria for the early extubation group nor the conventional extubation group (as per correspondence with Prof Myles). Complete total hospital cost data were available for 46 participants (high cost outliers in 3 participants not included in analysis). Power calculation was done. First study author was supported by a National Health and Medical Research Council Practitioner Fellowship award; study was supported by the Alfred Hospital Wholetime Medical Specialists Scheme and Glaxo Wellcome. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization code was created by table of random numbers, and participants were stratified to high‐ or low‐risk groups. |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The clinical trial unit or hospital pharmacy department who prepared solutions according to the randomization code maintained blinding of the study drug preparations" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 87 participants were enrolled, and 10 participants were excluded (1 participant withdrew consent before surgery, 2 had their surgery deferred and 7 failed to receive their allotted study medication) |
| Selective reporting (reporting bias) | Low risk | All outcomes collected and reported |
| Other bias | Low risk | Perioperative factors were similar between groups. |
Nicholson 2002.
| Methods | Parallel‐group randomized controlled trial, conducted in Canada. Study conducted from September 1997 to March 1998 | |
| Participants | 35 patients undergoing elective or semi urgent coronary artery bypass grafting surgery, aged younger than 80 years with a normal preoperative chest radiograph. Excluded were patients who underwent emergency surgery, those with significant valvular heart disease requiring surgical repair, with previous CABG or heart valve surgery and with poor LVEF (EF < 35%), poor preoperative pulmonary function (FEV1/FVC < 60% or FEV1 < 1.5 L), active congestive heart failure, preoperative renal insufficiency (serum creatinine > 180 µmol/L) or body mass index > 35 | |
| Interventions | Early extubation (within 1 hour after surgery) in 17 participants Usual care (extubation done 3 hours after surgery) in 18 participants Details of who decided when to extubate were not given. |
|
| Outcomes | Risk of tracheal reintubation Risk of major bleeding Time to extubation |
|
| Notes | Participants were extubated in the Recovery Room rather than in the ICU. Maximum time to meet extubation criteria was 90 minutes in the early extubation group and 6 hours in the conventional extubation group. Both groups had the same sufentanil‐based general anaesthesia. No power calculation was done. No details about funding source were given. Details of any declarations of conflict of interest among study authors were not provided.or any declarations of conflict of interest among authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Chest radiologist and pulmonary technologist were blinded to the study group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants withdraw from the study for not meeting extubation criteria. |
| Selective reporting (reporting bias) | Low risk | All outcomes collected were reported. |
| Other bias | Low risk | "Preoperative and intraoperative variables were comparable between two groups" |
Nougarede 2004.
| Methods | Parallel‐group randomized controlled trial, conducted in France. Study conducted from June 2001 to October 2002 | |
| Participants | 51 patients scheduled for coronary artery bypass (CABG) and/or aortic valve replacement surgery were enrolled. Excluded were patients undergoing repeat cardiac surgery, likely to have a difficult intubation or with insulin‐dependent diabetes, severe pulmonary hypertension, acquired or congenital coagulation or chronic conditions likely to require more than 48 hours in the ICU after surgery | |
| Interventions | Early extubation (target controlled infusion of propofol, sufentanil (mean dose 62 μg), monitoring of anaesthetic depth by BIS index spectral analysis of electroencephalogram and extubation immediately after surgery) in 20 participants Usual care (anaesthesia technique chosen by attending anaesthetist with higher sufentanil dose (mean dose 120 μg), extubated between 4 and 6 hours after surgery) in 20 participants Details of who decided when to extubate were not given. |
|
| Outcomes | Risk of tracheal reintubation Time to extubation Length of stay in the ICU Total cost of drugs and disposable medical devices |
|
| Notes | French paper. No power calculation was done. No details were given about funding source. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for 11 withdrawals given |
| Selective reporting (reporting bias) | Low risk | Outcomes collected and reported |
| Other bias | Low risk | Preoperative characteristics were similar between the 2 groups. |
Pettersson 2004.
| Methods | Parallel‐group randomized controlled trial, conducted in Sweden. Study dates not reported | |
| Participants | 60 elective coronary artery bypass or aortic valve replacement surgery patients | |
| Interventions | Early extubation (about 2 hours after surgery) in 30 participants Usual care (about 6 hours after surgery) in 30 participants Details of who decided when to extubate were not given. |
|
| Outcomes | Risk of mortality at 30 days after surgery Time to extubation Length of stay in hospital |
|
| Notes | Both groups received the same propofol and remifentanil‐based anaesthesia. The study focused on postoperative pain scores. No power calculation was done. No details about funding source were given. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Three patients in late extubation group excluded because of incomplete data protocol" |
| Selective reporting (reporting bias) | Low risk | All outcomes collected and reported |
| Other bias | Low risk | Participant demographics, anaesthesia drugs used and type of surgery were similar between the 2 groups. |
Probst 2014.
| Methods | Parallel‐group single‐blinded randomized controlled trial, conducted in Germany. Study conducted from May 2008 to July 2009 | |
| Participants | 200 patients undergoing elective cardiac surgery (coronary artery bypass graft with or without valve surgery) who were haemodynamically stable The attending anaesthesiologist and the cardiac surgeon in consensus excluded those who were in cardiogenic shock, were dialysis dependent or had an additive EuroSCORE > 10 before surgery, as well as those with impaired left ventricular function (ejection fraction < 35%), cardiac assist devices preoperative or postoperative and cardiopulmonary instability (high inotropic support, lactate > 5 mmol/L, Horowitz index < 200) after surgery |
|
| Interventions | Early extubation group (100 participants): postoperative care in post‐anaesthetic care unit (PACU) with high physician‐to‐patient ratio of 1:3. Weaning protocol was driven by physician with good compliance. Participants were extubated as soon as criteria were met. Usual care group (100 patients): postoperative care in ICU with low physician‐to‐patient ratio of 1:12. Weaning protocol was mainly driven by nurse. Compliance with protocol was dependent on actual workload in the ICU. Participant was extubated when criteria were met, and when the overall situation in the ICU was favourable, as estimated by the physician. Extubation criteria (same for both groups): conscious and obeys commands, stable spontaneous ventilation with pressure support of 10 to 12 cm H2O, positive end‐expiratory pressure (PEEP) 5 cm H2O, fraction of inspired oxygen (FiO2) ≤ 0.4, haemodynamically stable, not bleeding (drain output ≤ 100 mL/h) and no significant electrocardiographic abnormalities |
|
| Outcomes | Time to extubation Length of stay in the ICU or in the PACU according to treatment allocation Length of stay in hospital Risk of tracheal reintubation Risk of renal failure (increase in postoperative serum creatinine ≥ 3 times the preoperative value, or serum creatinine > 150 μmol/L) Risk of stroke (new transient or permanent motor or sensory deficit of central origin or unexplained coma) Risk of hospital mortality |
|
| Notes | Power calculation done. Funding from Leipzig Heart Center, University of Leipzig. Study authors declare no conflict of interest in the study. Not included in reintubation subgroup analysis, as extubation occurred in different locations (PACU or ICU) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes picked out of a box |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Medical and nursing staff in ICU and PACU had been informed about the design and conduct of the study but were not informed as to which patients were enrolled in the study. Data collection and analysis were performed by an independent person who was not part of the anaesthetic, surgical or ICU team, and who was not blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 423 patients consented to participated in the study at the premedication visit, 223 were excluded at the end of surgery because they met exclusion criteria at the end of surgery, or because PACU and ICU beds were not simultaneously available. |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes collected |
| Other bias | Low risk | Groups were comparable at baseline except for duration of surgery and anaesthesia time. |
Quasha 1980.
| Methods | Parallel‐group randomized controlled trial, conducted in the United States. Study dates not reported | |
| Participants | 38 elective coronary artery bypass graft patients with normal and slightly impaired left ventricular function | |
| Interventions | Early extubation within 8 hours of surgery in 18 participants Usual care: extubation applied in the morning of the first day after surgery in 20 participants Details of weaning protocol and who decided when to extubate were not given. |
|
| Outcomes | Risk of tracheal reintubation Risk of postoperative myocardial infarction (increase in myocardial enzyme values, positive technetium pyrophosphate scan and meeting predefined electrocardiographic criteria) Risk of major bleeding (necessitating surgical control) Risk of stroke Time to extubation Length of stay in the ICU |
|
| Notes | Inhalation‐based anaesthesia was the same in both groups, but weaning criteria were applied earlier in the early extubation group. No power calculation was done. Study was supported, in part, by UPHS Grant (grant number: GMS‐15571‐10,11). Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Assessors for diagnosis of myocardial infarction were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Two participants failed to meet the early extubation criteria and were excluded from data analysis on time to extubation. |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes collected |
| Other bias | Low risk | Baseline characteristics were similar for age, weight, gender, preoperative ventricular function and preoperative pulmonary function. |
Reyes 1997.
| Methods | Parallel‐group blinded randomized controlled trial, conducted in Spain. Study conducted from February 1994 to March 1995 | |
| Participants | 404 elective, urgent and emergency coronary artery bypass graft (CABG) patients, CABG + valve, valve surgery | |
| Interventions | Early extubation (first trial for spontaneous ventilation 6 hours after ICU admission) in 201 participants Usual care (first trial for spontaneous ventilation at 08:00 h on the day after surgery) in 203 participants Doctor decided when to extubate. |
|
| Outcomes | Risk of mortality in the ICU Risk of mortality at 30 days after hospital discharge Risk of postoperative myocardial infarction (new Q wave in ECG together with increases in CPK‐MB enzyme in sequential determinations with a typical pattern) Risk of wound infection (purulent discharge or positive culture of exudate from sternal wound) Risk of acute renal failure (plasma creatinine increase ≥ 2 mg/dL above preoperative level) Risk of stroke (new and persistent neurological deficit or central nervous system damage documented on CT scan or nuclear MRI) Risk of sepsis (pathogen isolated in blood culture not related to local infection, with fever > 38.0°C or hypotension) Risk of tracheal reintubation Time to extubation Length of stay in the ICU |
|
| Notes | Weaning protocol was the same in both groups, but timing was different, as described in the paper. Same high‐dose opioid‐based general anaesthesia was given in both groups. No power calculation was done. Study was supported by Fondo de Investigacion Sanitaria de la Seguridad Social (grant number: 94/0178). Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated allocation schedule was applied to randomly assign in blocks of 20 to early extubation or conventional extubation group" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Chest radiograph and ECGs were examined by two independent observers unaware of the patient group and blinded of each other's interpretation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants were unavailable for follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes collected and reported |
| Other bias | Low risk | Participants' characteristics, preoperative and operative characteristics, were similar between groups. Of the 35 perioperative characteristics examined, only the prevalence of obesity and hypertension differed between groups. |
Sakaida 1998.
| Methods | Parallel‐group randomized controlled trial, conducted in Japan. Study dates not reported | |
| Participants | 40 elective coronary artery bypass graft, mitral valve or aortic valve replacement surgery patients | |
| Interventions | Low‐dose opioid (mean fentanyl 7.6 ± 1.6 μg/kg and isoflurane/nitrous oxide) in 20 participants High‐dose opioid (mean fentanyl 99 ± 5.8 μg/kg and nitrous oxide) in 20 participants Details of who decided when to extubate were not given. |
|
| Outcomes | Time to extubation Length of stay in the ICU Length of stay in hospital |
|
| Notes | Japanese article. No power calculation was done. No details about funding source were given. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal from the study |
| Selective reporting (reporting bias) | High risk | Mortality and postoperative complications not reported |
| Other bias | Low risk | Preoperative and intraoperative characteristics were similar in the 2 groups: age, sex, height, weight, body surface area, ejection fraction, duration of surgery, duration of anaesthesia, CPB duration and aortic clamping time. |
Salah 2015.
| Methods | Parallel‐group randomized controlled trial, conducted in Egypt. Study conducted from February 2011 to October 2013 | |
| Participants | 52 elective open heart surgery adult (> 18 years) patients Excluded were patients undergoing emergency/redo operations, patients already intubated preoperatively and patients with preoperative uncontrolled diabetes (HbA1C > 5.9 mg/dL), cardiogenic shock, poor left ventricular function (ejection fraction < 45%), severe pulmonary hypertension (pulmonary artery systolic pressure > 55 mmHg) or severe renal impairment (creatinine clearance < 50 mL/min), or on regular dialysis, and patients deliberately kept intubated for haemodynamic instability and/or concerns of postoperative bleeding |
|
| Interventions | Early extubation group (26 participants): Inhalational anaesthetic concentration was reduced gradually to 0.4 expired minimum alveolar concentration at completion of surgery, and residual muscle relaxation was antagonized with neostigmine (0.05 mg/kg) and atropine (0.02 mg/kg) if extubation criteria were met in the operating theatre. Participants were transferred to ICU after extubation Extubation criteria for early extubation: SpO2 > 95% with FiO2 < 0.6, ETCO2 < 50 mmHg, spontaneous respiratory rate < 24 min and train of four (TOF) > 90% Usual care group (26 participants): Participants were transferred to the ICU intubated and sedated with propofol infusion (50 to 70 μg/kg/min) and morphine (10 to 20 μg/kg/h) while mechanical ventilation was continued in 26 participants. Sedation was discontinued in the ICU according to local ICU protocol. Extubation criteria for usual care group: awake and able to respond comprehensively to simple verbal commands, haemodynamically stable, normal ventilatory mechanics, acid‐base status, PaO2 and PaCO2 at an inspired FiO2 of 0.4 |
|
| Outcomes | Time to extubation Length of stay in the ICU Risk of tracheal reintubation Risk of myocardial ischaemia (ST‐segment elevation or depression on ECG, creatinine kinase, creatinine kinase‐myocardial band and troponin I) Risk of bleeding (excessive mediastinal bleeding defined as 400 mL in the first hour, 200 mL/h for the first 6 hours or total drainage of 1000 mL at any time) Risk of acute renal failure (diminished urine output < 0.5 mL/kg/h and/or rising creatinine level) |
|
| Notes | Power calculation was done. No details about funding source were given. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed by computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes were used for concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals from the study |
| Selective reporting (reporting bias) | Low risk | All outcomes collected and reported |
| Other bias | High risk | More participants in the conventional group had DM, and they had longer operating time, bypass time and cross‐clamping time and consumed more morphine during the operation compared with the early extubation group |
Sherry 1996.
| Methods | Parallel‐group randomized controlled trial, conducted in England from June 1991 to September 1992 | |
| Participants | 77 elective cardiac (CABG and/or valve replacement) surgical patients, with low cardiac output | |
| Interventions | Low‐dose opioid (fentanyl at 15 μg/kg and propofol 4 to 8 mg/kg/h) in 37 participants High‐dose opioid (fentanyl at 60 μg/kg and midazolam 3 to 6 mg) in 33 participants Details of weaning protocol and who decided when to extubate were not given. |
|
| Outcomes | Time to extubation Length of stay in the ICU Total hospital cost (ICU nursing costs and OT/ICU drug costs) | |
| Notes | Results reported without variability measures (standard deviation, standard errors), thus could not be included in meta‐analyses, except for total hospital costs. No power calculation was done. Funding source was Zeneca Pharma. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawal of 7 participants from the study are given in the text (3 patients in early extubation group and 4 in the conventional group did not follow protocol). |
| Selective reporting (reporting bias) | High risk | Postoperative complications and mortality not reported. Variability measures not reported for most outcomes |
| Other bias | Low risk | All characteristics were comparable for age, weight, type of operation and ventricular function and blood gases, except gender distribution. |
Silbert 1998.
| Methods | Parallel‐group randomized controlled trial, conducted in Australia. Study dates not reported | |
| Participants | 100 elective coronary artery bypass graft patients, with good and moderate left ventricular function. Excluded were patients with concurrent valve disease, poor myocardial function, associated systemic illness, contraindications to early extubation (e.g. respiratory disease) or communication problems (language or psychiatric) | |
| Interventions | Low‐dose opioid (fentanyl 15 μg/kg with propofol infusion) in 38 participants High‐dose opioid (fentanyl 50 μg/kg with propofol or additional fentanyl infusion) in 46 participants Decision to extubate when criteria were met was made in consultation with the attending doctor. |
|
| Outcomes | Risk of mortality at hospital discharge Time to extubation Risk of tracheal reintubation Risk of myocardial infarction (increases in CPK‐MB levels (CPK‐MB > 50 IU/L, CPK‐MB/CPK > 8%) or new Q waves) Length of hospital stay |
|
| Notes | Weaning criteria were reported and applied to both groups. One participant from each group died, and all were withdrawn from the study. One participant in the early extubation group died 2 months after surgery. No power calculation was done. Study was supported, in part, by a research grant provided by the Australian Society of Anaesthetists and Abbott Australasia, and by a grant‐in‐aid provided by ICI Pharmaceuticals. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed by computer‐generated random numbers (in blocks of 4) to conventional extubation group or early extubation group. |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | High risk | "No attempt was made to blind ICU staff to treatment group, as a pilot study had previously shown that those patients in the early extubation group became readily apparent" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clear description of withdrawals from study before and after anaesthesia was given |
| Selective reporting (reporting bias) | High risk | ICU length of stay stated as collected outcome, but no results given in text |
| Other bias | Low risk | Demographic data similar for both groups |
Silbert 2006.
| Methods | Parallel‐group double‐blinded randomized controlled trial, conducted at 3 hospitals in Australia. Study conducted from June 2001 to December 2003 | |
| Participants | 350 elective first‐time coronary artery bypass graft patients, 55 years of ag or older, with no previous neurological deficit and able to undergo neuropsychological testing | |
| Interventions | Low‐dose opioid (mean fentanyl 9.9 ± 1.1 μg/kg) in 168 participants High‐dose opioid (fentanyl 50.0 ± 2.4 μg/kg) in 158 participants Details of who decided when to extubate were not given. |
|
| Outcomes | Risk of hospital mortality Risk of mortality at 3 months and 1 year Time to extubation Length of time in the ICU Length of stay in hospital |
|
| Notes | For purposes of analysis, hospital mortality and 1‐year mortality data were used. Power calculation was done. Funding sources were the National Health and Medical Research Council, Canberra, Australian Capital Territory, Australia (Project Grant No. 140510), and a National Health and Medical Research Council Practitioner Fellowship awarded to the seventh review author. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables and stratification by institution (3 sites) |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Neurological test administered by a trained interviewer, who was blinded to participants' allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants who withdrew were described and the reasons given. |
| Selective reporting (reporting bias) | Low risk | All outcomes collected and reported |
| Other bias | Low risk | Demographic data were similar for the 2 groups. |
Simeone 2002.
| Methods | Parallel‐group randomized controlled trial, conducted in Italy. Study conducted from February 1999 to November 1999 | |
| Participants | 49 elective coronary artery bypass graft, aortic or mitral valve surgery patients | |
| Interventions | Early extubation (weaning protocol with aim of extubation within 9 hours after surgery) in 24 participants Usual care (weaning according to physicians' subjective clinical judgement) in 25 participants Physicians decided when to extubate. |
|
| Outcomes | Risk of tracheal reintubation Risk of stroke (e.g. aphasia, right side paralysis) Risk of major bleeding Risk of acute renal failure Time to extubation Length of stay in the ICU |
|
| Notes | Weaning protocol was the same in both groups, but timing was different, as described in the paper. No power calculation was done. No details about the funding source were given. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants withdrew from the study. |
| Selective reporting (reporting bias) | Low risk | All outcomes collected and reported |
| Other bias | Low risk | Preoperative risk score, participants' characteristics, surgical time, illness severity score (APACHE II and III) and preoperative respiratory parameters were comparable between groups. |
Slogoff 1989.
| Methods | Parallel 4‐arm randomized controlled trial, conducted in the United States. Study conducted from September 1985 to July 1987 | |
| Participants | 1012 patients 21 to 75 years of age, scheduled for elective CABG by 4 participating surgeons. Excluded were patients with previous cardiac operation, emergency operation, operations performed in addition to CABG, severe systemic non‐cardiac disease other than diabetes and hypertension, history of allergy to any drugs that might be administered and preoperative EEG that precluded diagnosis of ischaemia, such as LBBB. | |
| Interventions | Low‐dose opioid (fentanyl 10 μg/kg and enflurane, halothane or Isoflurane) in 758 participants High‐dose opioid (mean sufentanil 28 ± 4 μg/kg) in 254 participants Details of weaning protocol and who decided when to extubate were not given. |
|
| Outcomes | Risk of hospital mortality Risk of postoperative myocardial infarction (new Q waves ≥ 0.04 seconds, or extended old Q waves, or LBBB presence, and CPK‐MB > 80U) Time to extubation |
|
| Notes | Enflurane, halothane and isoflurane groups were combined as a single low‐dose opioid group for meta‐analyses. Power calculation was done. Details of any declarations of conflict of interest among study authors were not provided. Study was supported in part by Janssen Pharmaceutica. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Primary drug assignment was randomized for each four surgeons from four different tables of random number" |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "All data were collected by trained observers who did not participate in patients care" "The ECG traces were reviewed by one investigator who was unaware of the patient, anaesthetic, operative event, or intervention" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Data of all patients were pooled and subjected to stepwise discriminant analysis of all factors listed in table 1" |
| Selective reporting (reporting bias) | Low risk | All outcomes collected and reported. "The five patients who died of PMI were included in the group considered to have PMI; the 12 others were included in the group without PMI" |
| Other bias | Low risk | "Of 34 characteristics compared, the only significant differences between primary anaesthesia groups were in the incidence of history of chronic hypertension and mean preoperative systolic blood pressure" |
van Mastrigt 2006a, 2010.
| Methods | Parallel‐group single‐blinded randomized clinical trial, conducted in Netherlands. Study conducted from February 2001 to March 2003 | |
| Participants | 600 patients admitted to the University Hospital for CABG surgery in the period from March 2001 until February 2003 with low risk stratified by Parsonnet score | |
| Interventions | Early extubation (short‐stay ICU treatment and extubation < 8 hours) in 300 participants Usual care (stay overnight in the ICU as usual practice and extubated) in 300 participants ICU physicians decided when to extubate. |
|
| Outcomes | Risk of death at 30 days Risk of myocardial infarction Risk of major bleeding Risk of wound infection Risk of stroke Risk of acute renal failure Time to extubation Length of stay in the ICU Length of stay in hospital Total hospital cost (nutrition, laundry, accommodation, cleaning, overheads, equipment, staff, material and medication) Change in quality of life (ED‐5D) at 1 month and 1 year from baseline |
|
| Notes | One participant in the early extubation group died during surgery and was excluded from study author's main analysis. During surgery, participants were anaesthetized with "total intravenous infusions of propofol and a short acting opioid." Quality of life data were taken from 2010 paper (n = 408). Power calculation was done. No details about funding source were given. Details of any declarations of conflict of interest among study authors were not provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A random list of permuted blocks (with a length of 10) was generated for either control (n=300) or SSIC (n=300) by a computer" |
| Allocation concealment (selection bias) | Low risk | The generated sequence was entered "sequentially into numbered, opaque, sealed envelopes sealed with tape" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and the surgical team were blinded for group assignments. The physician was not blinded but was not aware that ICU readmission was one of the 2 primary endpoints of the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for 3 withdrawals were given. |
| Selective reporting (reporting bias) | Low risk | All death and perioperative complications collected and reported |
| Other bias | Low risk | Demographic, co‐morbidity and operative data were comparable between the 2 groups. |
Zhu 2015.
| Methods | Parallel‐group unblinded randomized clinical trial, conducted in Hong Kong. Study conducted from May 2012 to March 2013 | |
| Participants | 68 patients 18 to 80 years of age, scheduled for elective cardiac valvular surgery (isolated valve surgery and valve surgery combined with another cardiac surgical procedure). Excluded were patients with acute or chronic obstructive pulmonary disease, serum creatinine concentration > 200 μmol/L, serum aspartate transaminase concentration > 80 U/L, left ventricular ejection fraction < 30% and history of seizures or stroke before surgery; as well as patients with chest tube drainage > 500 mL/h, reoperation, myocardial infarction, need for high‐dose inotropes or vasopressors or intra‐aortic balloon pump and with refractory hypoxaemia with arterial oxygen tension‐to‐fractional inspired oxygen concentration ratio < 150 mmHg after surgery. |
|
| Interventions | Early extubation group (25 participants): Paralysis was reversed and sedation was stopped in ICU. Weaning protocol using Adaptive Support Ventilation (ASV) with algorithm provided was used within 8 hours after ICU admission. Ventilation management was directed by duty physicians after 8 hours. Usual care group (25 participants): Paralysis was reversed and sedation was stopped in ICU. Weaning was directed by duty physicians. Extubated criteria (same in both groups): responsive and cooperative; Fio2 < 40%; PaO2/FiO2 > 150 mmHg; positive end‐expiratory pressure (PEEP) ≤ 5 cm H2O; haemodynamically stable; urine output exceeds 0.5 mL kg−1 h−1; last hour chest tube drainage < 100 mL; no uncontrolled arrhythmia; and rectal temperature above 36.0°C |
|
| Outcomes | Time to extubation Length of stay in the ICU Length of stay in hospital Risk of bleeding (unpublished data) Risk of stroke Risk of sepsis (unpublished data) Risk of acute renal failure (defined as new haemofiltration and dialysis) Risk of tracheal reintubation Risk of hospital mortality |
|
| Notes | The 2 ventilators were borrowed from Hamilton Medical GA, Rhäzuns, Switzerland. Power calculation was done. Funding source was the department. Details of any declarations of conflict of interest among study authors were not provided. Intention‐to‐treat analysis data reported in the paper were extracted for meta‐analyses. Study authors had access to raw RCT data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed by computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes containing treatment allocation were opened after the participant's arrival to the ICU. |
| Blinding (performance bias and detection bias) All outcomes | High risk | ICU staff and outcome assessor not blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Thirty‐four participants were randomized to each group, but 4 participants in the ASV group and 3 in the control group were excluded postoperatively with reasons given; protocol violations occurred in 5 participants in the ASV group and in 3 participants in the control group. |
| Selective reporting (reporting bias) | Low risk | All outcomes collected and reported |
| Other bias | Low risk | Demographic, preoperative and intraoperative parameters were comparable in the 2 groups, except for increased prevalence of preoperative atrial fibrillation in the usual care group and increased total dose of intraoperative propofol in the early extubation group. |
APACHE: Acute Physiology And Chronic Health Evaluation; ASV: Adaptive Support Ventilation; BIS: bispectral index; BMI: body mass index; CABG: coronary artery bypass grafting; CK: creatine kinase; CK‐MB: creatine kinase MB isoenzyme; cm: centimeter; CPB: cardiopulmonary bypass; CPK: creatine phosphokinase; CPK‐MB: creatine phosphokinase MB isoenzyme; CT scan: computed tomography scan; DM: diabetes mellitus; ECG: electrocardiography; ED‐5D: health questionnaire providing a simple descriptive profile and a single index value for health status; ETCO2: end‐tidal carbon dioxide; EuroSCORE: European System for Cardiac Operative Risk Evaluation; FEV1: forced expiratory volume in one second; FiO2: fractional inspiratory oxygen; FVC: forced vital capacity; h: hour; HbA1C: glycated haemoglobin; ICU: intensive care unit; IU/L: international unit per litre; IV: intravenous; km: kilometre; LBBB: left bundle branch block; LV: left ventricle; LVEF: left ventricular ejection fraction; mg: milligram; mg/dL: milligram per decilitre; mL/h: millilitre per hour; MRI: magnetic resonance imaging; n: number of participants; NYHA: New York Heart Association; OT: operating theatre; PACU: post‐anaethetic care unit; PaO2: arterial partial pressure of oxygen; PEEP: positive end‐expiratory pressure; PMI: postoperative myocardial infarction; PO2: partial pressure of oxygen; QRS: QRS complex in electrocardiography; RCT: randomized controlled trial; SpO2: arterial oxygen saturation; SSIC: short stay intensive care; ST: ST segment in electrocardiography; TOF: train of four; μg/kg: microgram per kilogram; μg/kg/min: microgram per kilogram per minute; μmol/L: micromole per litre; U: unit; U/L: unit per litre; UPHS: University of Pennsylvania Health System; USD: United States dollar.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anastasiadis 2013 | Intervention not relevant. Group A included participants who were operated on with the minimal extracorporeal circulation circuit, whereas those in Group B underwent surgery on conventional cardiopulmonary bypass. |
| Anderson 2010 | Not a randomized controlled trial |
| Cagli 2003 | Turkish study. Not a randomized controlled trial |
| Cheng 2001 | Comparison of remifentanil group (induction with remifentanil 1 μg/kg/min/isoflurane/propofol; remifentanil maintenance dose of 1 μg/kg) vs fentanyl group (induction with fentanyl 10 μg/kg bolus, fentanyl maintenance dose of 2 μg/kg). Both groups had isoflurane or enflurane and propofol maintenance. The type of anaesthesia given in both groups was considered fast‐track cardiac anaesthesia by study authors. |
| Delphin 2007 | RCT comparing sevoflurane vs isoflurane as the primary anaesthetic agent. Both groups were given a total of fentanyl 5 μg/kg during surgery. |
| El Tahan 2013 | All groups were given low‐dose sufentanil. |
| Ender 2008 | Investigators used a historical control instead of a randomized controlled trial study design. |
| Engoren 2001 | All participants recruited received low‐dose opioid‐based general anaesthesia (Group 1: median fentanyl dose 9.0 μg/kg; Group 2: median sufentanil dose 1.7 μg/kg; Group 3: median remifentanil 88 μg/kg and fentanyl 2.9 μg/kg). All participants aimed to be extubated by 6:30 am on the first postoperative day. No high‐dose opioid comparison group was included. All groups had the same weaning protocol. |
| Farag 2006 | Study did not recruit cardiac patients undergoing surgery. |
| Forestier 2003 | Five groups had differing predicted sufentanil effect site concentrations (0.5 ng/mL, 0.75 ng/mL, 1 ng/mL, 1.25 ng/mL and 1.5 ng/mL) with target propofol concentration of 1.5 μg/mL. No details about mean weight were given for each group. Corresponding cumulative mean (SD) sufentanil doses (μg/kg) were 165 (20), 229 (38), 300 (49), 355 (56) and 440 (107). All groups had the same weaning protocol with the aim of extubation within 8 hours of surgery. No high‐dose opioid or time‐directed extubation protocol comparative groups were included to meet systematic review inclusion criteria. |
| Gerlach 2002 | Comparison of sufentanil (mean 3.0 ± 0.6 μg/kg) vs remifentanil (mean 96.6 ± 39.1 μg/kg with clonidine); both groups had propofol infusion. Remifentanil used was high dose but has a short plasma half‐life. No details of weaning protocol provided. The type of anaesthesia given in both groups was considered fast‐track cardiac anaesthesia by study authors. |
| Gozdzik 2003 | Article in Polish. Not able to get full text |
| Hall 1991 | Comparison of Group A (propofol‐sufentanil) and Group B (enflurane‐sufentanil anaesthesia), but this anaesthesia regimen was not given for fast‐tracking purposes. Mean time to extubation in Group A was 26.9 ± 15.7 hours and for Group B was 29.5 ± 27.0 hours, respectively |
| Hansdottir 2006 | Comparison of Group A (combined thoracic epidural analgesia and general anaesthesia followed by patient‐controlled thoracic epidural analgesia) and Group B (general anaesthesia followed by patient‐controlled analgesia with intravenous morphine). Does not fit systematic review inclusion criteria |
| Heck 2000 | Comparison of different doses of sufentanil during induction. Study ended 2 minutes after endotracheal intubation. |
| Heijmans 2007 | Comparison of 4 groups (alfentanil, high‐dose remifentanil, low‐dose remifentanil, thoracic epidural group); all fast‐track anaesthetic techniques. All groups had the same weaning protocol. No relevant outcomes were reported in the paper. |
| Howie 2003 | Randomized to 1 of 3 remifentanil groups: Group 1 (1 μg/kg/min), Group 2 (2 μg/kg/min) and Group 3 (3 μg/kg/min). Total remifentanil doses administered (μg/kg) for Group 1, Group 2 and Group 3 were 716 ± 231, 879 ± 204 and 1174 ± 301, respectively. All groups aimed to be extubated within 6 hours after ICU admission. |
| Jacobsohn 2005 | Does not fit inclusion criteria for systematic review. Intrathecal low‐dose morphine compared with intrathecal normal saline |
| Kataoka 2007 | Not a randomized controlled trial |
| Knapik 2006 | Comparison of remifentanil‐based anaesthesia (0.5 μg/kg/min) and fentanyl 2.5 μg/kg/h supplemented with additional standard bolus doses 5 μg/kg during induction and before skin incision. Does not fit inclusion criteria for systematic review. No relevant outcomes |
| Koslov 1995 | Not a randomized controlled trial. The trial was excluded in published reviews (Hawkes 2003; Zhu 2012). |
| Lehmann 2003 | Retracted article |
| Lena 2005 | Comparison of Group 1: remifentanil and spinal analgesia (low‐dose morphine and clonidine) and Group 2: sufentanil without spinal analgesia. Does not fit systematic review inclusion criteria |
| Lena 2008 | Comparison of Group 1: remifentanil and spinal analgesia (low‐dose morphine and clonidine) and Group 2: sufentanil without spinal analgesia. Does not fit systematic review inclusion criteria |
| Lison 2007 | Comparison of isoflurane (0.4 to 0.8 vol%) together with either remifentanil group (1 μg/kg/min) or sulfentanil group (1 μg/kg for induction, 0.5 μg/kg for skin incision, then 0.02 μg/kg/min). Both groups were given what were considered fast‐track cardiac anaesthesia regimens with expected extubation within 8 hours after surgery. No late extubation group for comparison. Both groups had the same weaning protocol. |
| McDonald 2005 | Comparison of parasternal block and local anaesthetic infiltration of the sternotomy wound and mediastinal tube sites with levobupivacaine vs placebo. Both groups were given a desflurane‐based, low‐dose opioid anaesthetic. Does not fit systematic review inclusion criteria |
| Mollhoff 2001 | Multi‐centre RCT comparing high‐dose remifentanil by continuous infusion vs an intermittent bolus fentanyl regimen, both given in combination with propofol. No details about average or cumulative opioid doses used during surgery to classify 'high‐' versus 'low‐'dose opioid‐based cardiac anaesthesia |
| Murphy 2009 | Comparison of morphine 40 mg and fentanyl 600 μg as part of a standardized opioid‐isoflurane anaesthetic. Both groups considered as low‐dose opioid. In both groups, decisions on weaning, tracheal extubation, ICU and hospital discharge were standardized and were made by the surgical team, who were blinded to treatment allocation. Both groups were extubated after 8 hours following surgery (i.e. both groups were not fast‐tracked). |
| Najafi 2008 | Not a randomized controlled trial |
| Oliver 2011 | Postoperative sedation and analgesia regimen comparisons. All groups had the same standardized weaning protocol. |
| Puri 2003 | Bispectral index monitoring was the focus of this randomization. |
| Ramsay 1994 | Comparison of 3 high‐dose opioid‐based anaesthesia groups: (1) isoflurane and mean sufentanil 5 μg/kg, (2) enflurane and mean sufentanil 5 μg/kg, (3) no volatile agent and mean sufentanil 10.8 μg/kg. Study authors measured the risk of perioperative myocardial infarction. Data for postoperative myocardial infarction were not available. Fast‐track extubation was not the intention of the study. |
| Rose 2014 | Not a randomized controlled trial |
| Royse 2003 | Comparison of high thoracic epidural analgesia and intravenous morphine analgesia |
| Shroff 1997 | Comparison of Group 1 (10 μg/kg morphine and 25 μg fentanyl intrathecally preoperatively) vs Group 2 (no intrathecal opioid but 25 to 50 μg/kg fentanyl given intraoperatively) |
| Sulzer 2001 | Comparison of adaptive support ventilation vs synchronized intermittent mandatory ventilation. Both groups had formalized weaning protocols. |
| Tempe 1995 | Elective closed mitral valvotomy surgery. Does not fit participants' inclusion criteria |
| Tempe 2011 | Similar fentanyl total dose used between propofol and isoflurane groups (mean 1230 ± 170 μg and mean 1252 ± 218 μg, respectively). Early extubation was not the focus of the study. |
| Yorulmaz 2005 | Article in Turkish. No full text available |
| Zeydanoglu 2005 | Article in Turkish. No full text available |
ICU: intensive care unit; ng/mL: nanogram per millilitre; RCT: randomized controlled trial ; SD: standard deviation; μg/kg: microgram per kilogram; μg/kg/min: microgram per kilogram per minute; vol: volume.
Differences between protocol and review
Inclusion criteria and mortality time points in this updated systematic review are different from those in the original review by Hawkes et al (Hawkes 2003). This update and our previous updated review (Zhu 2012) includes more types of postoperative adverse outcomes, health‐related quality of life and healthcare costs. These patient‐centred outcomes are important for clinical decision making.
Contributions of authors
Designing the update for the review: Wai‐Tat Wong (WTW), Veronica Ka Wai Lai (VKWL), Yee Eot Chee (YEC), Anna Lee (AL).
Co‐ordinating the review: AL.
Screening search results: WTW, AL.
Organizing retrieval of papers: VKWL, AL.
Screening retrieved papers against inclusion criteria: WTW, AL.
Appraising quality of papers: VKWL, AL.
Abstracting data from papers: VKWL, AL.
Writing to authors of papers for additional information: AL.
Managing data for the review: VKWL, AL.
Entering data into Review Manager (RevMan 5.3): VKWL.
Checking data entry in Review Manager (RevMan 5.3): WTW, AL.
Analysing RevMan statistical data: VKWL, AL.
Performing there statistical analyses not using RevMan: VKWL, AL.
Interpreting data: all review authors.
Making statistical inferences: all review authors.
Writing the review: all review authors.
Providing guidance on the review: AL.
Securing funding for the review: not applicable.
Sources of support
Internal sources
Department of Anaesthesia and Intensive Care, The Chinese University of Hong Kong, Hong Kong.
External sources
No sources of support supplied
Declarations of interest
WTW, VKWL and YEC declare no potential conflict of interests in this review. AL co‐authored a previous related systematic review (Myles 2003), along with Professor Paul Myles; two of his trials are included in this updated review (Myles 1997; Myles 2002). As AL was also co‐author of a trial included in this updated review (Zhu 2015), WTW and VKWL independently performed data extraction.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bell 1994 {published data only}
- Bell J, Sartain J, Wilkinson GAL, Sherry KM. Propofol and fentanyl anaesthesia for patients with low cardiac output state undergoing cardiac surgery: comparison with high‐dose fentanyl anaesthesia. British Journal of Anaesthesia 1994;73(2):162‐6. [PUBMED: 7917729] [DOI] [PubMed] [Google Scholar]
Berry 1998 {published data only}
- Berry PD, Thomas SD, Mahon SP, Jackson M, Fox MA, Fabri B, et al. Myocardial ischaemia after coronary artery bypass grafting: early vs late extubation. British Journal of Anaesthesia 1998;80(1):20‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chang 2007 {published data only}
- Chang FL, Lin SL, Tsai CS, Yeh CC, Wu CT, Wong CS. Closed‐circuit isoflurane‐based anesthesia provides better fast‐tracking anesthesia than fentanyl/propofol‐based anesthesia for off‐pump coronary artery bypass graft surgery. Acta Anaesthesiologica Taiwanica 2007;45:135‐9. [PUBMED: 17972615] [PubMed] [Google Scholar]
Cheng 1996a,1996b, 2003 {published data only}
- Cheng DC, Wall C, Djaiani G, Peragallo RA, Carroll J, Li C, et al. Randomized assessment of resource use in fast‐track cardiac surgery 1‐year after hospital discharge. Anesthesiology 2003;98(3):651‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cheng DCH, Karski J, Peniston C, Asokumar B, Raveendran G, Carroll J, et al. Morbidity outcome in early versus conventional tracheal extubation after coronary artery bypass grafting: a prospective randomized controlled trial. Journal of Thoracic and Cardiovascular Surgery 1996;112(3):755‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cheng DCH, Karski J, Peniston C, Raveendran G, Asokumar B, Carroll J, et al. Early tracheal extubation after coronary artery bypass graft surgery reduces costs and improves resource use. Anesthesiology 1996;85(6):1300‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dumas 1999 {published data only}
- Dumas A, Dupuis GH, Searle N, Cartier R. Early versus late extubation after coronary artery bypass grafting: effects on cognitive function. Journal of Cardiothoracic and Vascular Anesthesia 1999;13(2):130‐5. [PUBMED: 10230943 ] [DOI] [PubMed] [Google Scholar]
Engoren 1998 {published data only}
- Engoren MC, Kraras C, Garzia F. Propofol‐based versus fentanyl‐isoflurane‐based anesthesia for cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 1998;12(2):177‐81. [PUBMED: 9583550] [DOI] [PubMed] [Google Scholar]
Gruber 2008 {published data only}
- Gruber PC, Gomersall CD, Leung P, Joynt GM, Ng SK, Ho KM, et al. Randomized controlled trial comparing adaptive‐support ventilation with pressure‐regulated volume‐controlled ventilation with automode in weaning patients after cardiac surgery. Anesthesiology 2008;109(1):81‐7. [PUBMED: 18580176] [DOI] [PubMed] [Google Scholar]
Kadoi 2003 {published data only}
- Kadoi Y, Saito S, Kunimoto F, Goto F, Fujita N. Comparative effects of propofol versus fentanyl on cerebral oxygenation state during normothermic cardiopulmonary bypass and postoperative cognitive dysfunction. The Annals of Thoracic Surgery 2003;75(3):840‐6. [PUBMED: 12645704] [DOI] [PubMed] [Google Scholar]
Lu 2003 {published data only}
- Lu CC, Ho ST, Wang JJ, Wong CS, Tsai CS, Chang SY, et al. Minimal low‐flow isoflurane‐based anesthesia benefits patients undergoing coronary revascularization via preventing hyperglycemia and maintaining metabolic homeostasis. Acta Anaesthesiologica Sinica 2003;41:165‐72. [PUBMED: 14768513] [PubMed] [Google Scholar]
Maddali 2006 {published data only}
- Maddali MM, Kurian E, Fahr J. Extubation time, hemodynamic stability, and postoperative pain control in patients undergoing coronary artery bypass surgery: an evaluation of fentanyl, remifentanil, and nonsteroidal antiinflammatory drugs with propofol for perioperative and postoperative management. Journal of Clinical Anesthesia 2006;18(8):605‐10. [PUBMED: 17175431] [DOI] [PubMed] [Google Scholar]
Michalopoulos 1998 {published data only}
- Michalopoulos A, Nikolaides C, Antzaka C, Deliyanni M, Smirli A, Geroulanos S, et al. Change in anaesthesia practice and postoperative sedation shortens ICU and hospital length of stay following coronary artery bypass surgery. Respiratory Medicine 1998;92(8):1066‐70. [PUBMED: 9893777] [DOI] [PubMed] [Google Scholar]
Myles 1997 {published data only}
- Myles PS, Buckland MR, Weeks AM, Bujor MA, McRae R, Langley M, et al. Hemodynamic effects, myocardial ischemia, and timing of tracheal extubation with propofol‐based anesthesia for cardiac surgery. Anesthesia and Analgesia 1997;84(1):12‐9. [PUBMED: 8988992] [DOI] [PubMed] [Google Scholar]
Myles 2002 {published and unpublished data}
- Myles PS, Hunt JO, Fletcher H, Watts J, Bain D, Silvers A, et al. Remifentanil, fentanyl, and cardiac surgery: a double‐blinded, randomized, controlled trial of costs and outcomes. Anesthesia and Analgesia 2002;95(4):805‐12. [PUBMED: 12351249] [DOI] [PubMed] [Google Scholar]
Nicholson 2002 {published data only}
- Nicholson DJ, Kowalski SE, Hamilton GA, Meyers MP, Serrette C, Duke PC. Postoperative pulmonary function in coronary artery bypass graft surgery patients undergoing early tracheal extubation: a comparison between short‐term mechanical ventilation and early extubation. Journal of Cardiothoracic and Vascular Anesthesia 2002;16(1):27‐31. [PUBMED: 11854874] [DOI] [PubMed] [Google Scholar]
Nougarede 2004 {published data only}
- Nougarede B, Caillet C, Bizouarn P, Maupeti JC, Blanloeil Y, Furic I. Impact of propofol and sufentanil target controlled infusion with monitoring of bispectral index on drugs and medical device costs in operating room and intensive care unit, for routine cardiac surgery with cardiopulmonary bypass. Journal de Pharmacie Clinique 2004;23:219‐26. [Google Scholar]
Pettersson 2004 {published data only}
- Pettersson PH, Settergren G, Owall A. Similar pain scores after early and late extubation in heart surgery with cardiopulmonary bypass. Journal of Cardiothoracic Vascular Anesthesia 2004;18(1):64‐7. [PUBMED: 14973802] [DOI] [PubMed] [Google Scholar]
Probst 2014 {published data only}
- Probst S, Cech C, Haentschel D, Scholz M, Ender J. A specialized post‐anaesthetic care unit improves fast‐track management in cardiac surgery: a prospective randomized trial. Critical Care 2014;18(4):468‐79. [PUBMED: 25123092] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quasha 1980 {published data only}
- Quasha AL, Loeber N, Feeley TW, Ullyot DJ, Roizen MF. Postoperative respiratory care: a controlled trial of early and late extubation following coronary artery bypass grafting. Anesthesiology 1980;52(2):135‐41. [PUBMED: 6986104] [PubMed] [Google Scholar]
Reyes 1997 {published and unpublished data}
- Reyes A, Vega G, Blancas R, Morato B, Moreno J, Torrecilla C, et al. Early vs conventional extubation after cardiac surgery with cardiopulmonary bypass. Chest 1997;112(1):193‐201. [PUBMED: 9228376] [DOI] [PubMed] [Google Scholar]
Sakaida 1998 {published and unpublished data}
- Sakaida K. [Isoflurane anesthesia with combined use of low dose fentanyl for open heart surgery]. Masui 1998;47(5):576‐84. [PUBMED: 9621668] [PubMed] [Google Scholar]
Salah 2015 {published data only}
- Salah M, Hosny H, Salah M, Saad H. Impact of immediate versus delayed tracheal extubation on length of ICU stay of cardiac surgical patients, a randomized trial. Heart, Lung and Vessels 2015;7(4):311‐9. [CTG: NCT02491749] [PMC free article] [PubMed] [Google Scholar]
Sherry 1996 {published data only}
- Sherry KM, McNamara J, Brown JS, Drummond M. An economic evaluation of propofol/fentanyl compared with midazolam/fentanyl on recovery in the ICU following cardiac surgery. Anaesthesia 1996;51(4):312‐7. [PUBMED: 8686815] [DOI] [PubMed] [Google Scholar]
Silbert 1998 {published data only}
- Silbert BS, Santamaria JD, O'Brien JL, Blyth CM, Kelly WJ, Molnar RR, et al. Early extubation following coronary artery bypass surgery: a prospective randomized controlled trial. Chest 1998;113:1481‐8. [PUBMED: 9631781] [DOI] [PubMed] [Google Scholar]
Silbert 2006 {published data only}
- Silbert BS, Scott DA, Evered LA, Lewis MS, Kalpokas M, Maruff P, et al. A comparison of the effect of high‐ and low‐dose fentanyl on the incidence of postoperative cognitive dysfunction after coronary artery bypass surgery in the elderly. Anesthesiology 2006;104(6):1137‐45. [PUBMED: 16732083] [DOI] [PubMed] [Google Scholar]
Simeone 2002 {published data only}
- Simeone F, Biagioli B, Scolletta S, Marullo AC, Marchet‐Ti L, Caciorgna M, et al. Optimization of mechanical ventilation support following cardiac surgery. Journal of Cardiovascular Surgery (Torino) 2002;43(5):633‐41. [PUBMED: 12386574] [PubMed] [Google Scholar]
Slogoff 1989 {published data only}
- Slogoff S, Keats AS. Randomized trial of primary anesthetic agents on outcome of coronary artery bypass operations. Anesthesiology 1989;70(2):179‐88. [PUBMED: 2521549] [DOI] [PubMed] [Google Scholar]
van Mastrigt 2006a, 2010 {published data only}
- Mastrigt GA, Heijmans J, Severens JL, Fransen EJ, Roekaerts P, Voss G, et al. Short‐stay intensive care after coronary artery bypass surgery: randomized clinical trial on safety and cost‐effectiveness. Critical Care Medicine 2006;34(1):65‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Mastrigt GA, Joore MA, Nieman FH, Severens JL, Maessen JG. Health‐related quality of life after fast‐track treatment results from a randomized controlled clinical equivalence trial. Quality of Life Research 2010;19(5):631‐42. [PUBMED: 20340049] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2015 {published and unpublished data}
- Zhu F, Gomersall CD, Ng SK, Underwood MJ, Lee A. A randomized controlled trial of adaptive support ventilation mode to wean patients after fast‐track cardiac valvular surgery. Anesthesiology 2015;122(4):832‐40. [PUBMED: 25569810] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anastasiadis 2013 {published data only}
- Anastasiadis K, Asteriou C, Antonitsis P, Argiriadou H, Grosomanidis V, Kyparissa M, et al. Enhanced recovery after elective coronary revascularization surgery with minimal versus conventional extracorporeal circulation: a prospective randomized study. Journal of Cardiothoracic and Vascular Anesthesia 2013;27(5):859‐64. [PUBMED: 23791499] [DOI] [PubMed] [Google Scholar]
Anderson 2010 {published data only}
- Anderson J, Henry L, Hunt S, Ad N. Bispectral index monitoring to facilitate early extubation following cardiovascular surgery. Clinical Nurse Specialist 2010;24(3):140‐8. [PUBMED: 20404622] [DOI] [PubMed] [Google Scholar]
Cagli 2003 {published data only}
- Cagli K, Uncu H, Iscan Z, Altintas G, Karadeniz U, Vural K, et al. The efficiency of fast track protocol in elderly patients who underwent coronary artery surgery. Anadolu Kardiyologi Dergisi. 2003;3(1):8‐12, AXVII. [MEDLINE: ] [PubMed] [Google Scholar]
Cheng 2001 {published data only}
- Cheng DC, Newman MF, Duke P, Wong DT, Finegan B, Howie M, et al. The efficacy and resource utilization of remifentanil and fentanyl in fast‐track coronary artery bypass graft surgery: a prospective randomized, double‐blinded controlled, multi‐center trial. Anesthesia and Analgesia 2001;92:1094‐102. [PUBMED: 11323328] [DOI] [PubMed] [Google Scholar]
Delphin 2007 {published data only}
- Delphin E, Jackson D, Gubenko Y, Botea A, Esrig B, Fritz W, et al. Sevoflurane provides earlier tracheal extubation and assessment of cognitive recovery than isoflurane in patients undergoing off‐pump coronary artery bypass surgery. Journal of Cardiothoracic and Vascular Anesthesia 2007;21(5):690‐5. [PUBMED: 17905275] [DOI] [PubMed] [Google Scholar]
El Tahan 2013 {published data only}
- Tahan MR, Khidr AM. Low target sufentanil effect‐site concentrations allow early extubation after valve surgery. Journal of Cardiothoracic and Vascular Anesthesia 2013;27(1):63‐70. [PUBMED: 22406043] [DOI] [PubMed] [Google Scholar]
Ender 2008 {published data only}
- Ender J, Borger MA, Scholz M, Funkat AK, Anwar N, Sommer M, et al. Cardiac surgery fast‐track treatment in a postanesthetic care unit: six‐month results of the Leipzig fast‐track concept. Anesthesiology 2008;109:61‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Engoren 2001 {published data only}
- Engoren M, Luther G, Fenn‐Buderer N. A comparison of fentanyl, sufentanil, and remifentanil for fast‐track cardiac anesthesia. Anesthesia and Analgesia 2001;93(4):859‐64. [PUBMED: 11574346] [DOI] [PubMed] [Google Scholar]
Farag 2006 {published data only}
- Farag E, Chelune GJ, Schubert A, Mascha EJ. Is depth of anesthesia, as assessed by the Bispectral Index, related to postoperative cognitive dysfunction and recovery?. Anesthesia and Analgesia 2006;103(3):633‐40. [PUBMED: 16931673] [DOI] [PubMed] [Google Scholar]
Forestier 2003 {published data only}
- Forestier F, Hirschi M, Rouget P, Rigal JC, Videcoq M, Girardet P, et al. Propofol and sufentanil titration with the bispectral index to provide anesthesia for coronary artery surgery. Anesthesiology 2003;99(2):334‐46. [PUBMED: 12883406] [DOI] [PubMed] [Google Scholar]
Gerlach 2002 {published data only}
- Gerlach K, Uhlig T, Hüppe M, Kraatz E, Saager L, Schmitz A, et al. Remifentanil‐clonidine‐propofol versus sufentanil‐propofol anesthesia for coronary artery bypass surgery. Journal of Cardiothoracic and Vascular Anesthesia 2002;16(6):703‐8. [PUBMED: 12486650] [DOI] [PubMed] [Google Scholar]
Gozdzik 2003 {published data only}
- Gozdzik W, Durek G, Maslanka P, Adamik B, Namieta K, Kubler A, et al. Perioperative endocrine stress response in patients undergoing CABG surgery under remifentanil propofol total intravenous anaesthesia. Anestezjologia Intensywna Terapia 2003;35:157‐64. [Google Scholar]
Hall 1991 {published data only}
- Hall RI, Murphy JT, Moffitt EA, Landymore R, Pollak PT, Poole L. A comparison of the myocardial metabolic and haemodynamic changes produced by propofol‐sufentanil and enflurane‐sufentanil anaesthesia for patients having coronary artery bypass graft surgery. Canadian Journal of Anesthesia 1991;38(8):996‐1004. [PUBMED: 1836422] [DOI] [PubMed] [Google Scholar]
Hansdottir 2006 {published data only}
- Hansdottir V, Philip J, Olsen MF, Eduard C, Houltz E, Ricksten SE. Thoracic epidural versus intravenous patient‐controlled analgesia after cardiac surgery: a randomized controlled trial on length of hospital stay and patient‐perceived quality of recovery. Anesthesiology 2006;104(1):142‐51. [PUBMED: 16394700] [DOI] [PubMed] [Google Scholar]
Heck 2000 {published data only}
- Heck M, Kumle B, Boldt J, Lang J, Lehmann A, Saggau W. Electroencephalogram bispectral index predicts hemodynamic and arousal reactions during induction of anesthesia in patients undergoing cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2001;14(6):693‐7. [PUBMED: 11139111] [DOI] [PubMed] [Google Scholar]
Heijmans 2007 {published data only}
- Heijmans J, Fransen E, Buurman W, Maessen J, Roekaerts P. Comparison of the modulatory effects of four different fast‐track anesthetic techniques on the inflammatory response to cardiac surgery with cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anesthesia 2007;21(4):512‐8. [PUBMED: 17678776] [DOI] [PubMed] [Google Scholar]
Howie 2003 {published data only}
- Howie MB, Michelsen LG, Hug CC Jr, Porembka DT, Jopling MW, Warren SM. Comparison of three remifentanil dose‐finding regimens for coronary artery surgery. Journal of Cardiothoracic and Vascular Anesthesia 2003;17(1):51‐9. [PUBMED: 12635061] [DOI] [PubMed] [Google Scholar]
Jacobsohn 2005 {published data only}
- Jacobsohn E, Lee TW, Amadeo RJ, Syslak PH, Debrouwere RG, Bell D, et al. Low‐dose intrathecal morphine does not delay early extubation after cardiac surgery. Canadian Journal of Anesthesia 2005;52(8):848‐57. [PUBMED: 16189338] [DOI] [PubMed] [Google Scholar]
Kataoka 2007 {published data only}
- Kataoka G, Murai N, Kodera K, Sasaki A, Asano R, Ikeda M, et al. Clinical experience with Smart Care after off‐pump coronary artery bypass for early extubation. Journal of Artificial Organs 2007;10(4):218‐22. [PUBMED: 18071851] [DOI] [PubMed] [Google Scholar]
Knapik 2006 {published data only}
- Knapik M, Knapik P, Nadziakiewicz P, Misiołek H, Saucha W, Walaszczyk M, et al. Comparison of remifentanil or fentanyl administration during isoflurane anesthesia for coronary artery bypass surgery. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research 2006;12(8):133‐8. [PUBMED: 16865075] [PubMed] [Google Scholar]
Koslov 1995 {published data only}
- Kozlov IA, Markin SM, Piliaeva IE, Alferov AV. [Early cessation of artificial ventilation of the lungs (extubation of the trachea in the operating room) in patients undergoing surgery with artificial circulation]. Anesteziologiia i Reanimatologiia 1995;March‐April(2):16‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Lehmann 2003 {published data only}
- Lehmann A, Karzau J, Boldt J, Thaler E, Lang J, Isgro F. Bispectral index‐guided anesthesia in patients undergoing aortocoronary bypass grafting. Anesthesia and Analgesia 2003;96(2):336‐43. [PUBMED: 12538174] [DOI] [PubMed] [Google Scholar]
Lena 2005 {published data only}
- Lena P, Balarac N, Arnulf JJ, Bigeon JY, Tapia M, Bonnet F. Fast‐track coronary artery bypass grafting surgery under general anesthesia with remifentanil and spinal analgesia with morphine and clonidine. Journal of Cardiothoracic and Vascular Anesthesia 2005;19(1):49‐53. [PUBMED: 15747269] [DOI] [PubMed] [Google Scholar]
Lena 2008 {published data only}
- Lena P, Balarac N, Lena D, Chapelle A, Arnulf JJ, Mihoubi A, et al. Fast‐track anesthesia with remifentanil and spinal analgesia for cardiac surgery: the effect on pain control and quality of recovery. Journal of Cardiothoracic Vascular Anesthesia 2008;22(4):536‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lison 2007 {published data only}
- Lison S, Schill M, Conzen P. Fast‐track cardiac anesthesia: efficacy and safety of remifentanil versus sufentanil. Journal of Cardiothoracic and Vascular Anesthesia 2007;21(1):35‐40. [PUBMED: 17289477] [DOI] [PubMed] [Google Scholar]
McDonald 2005 {published data only}
- McDonald SB, Jacobsohn E, Kopacz DJ, Desphande S, Helman JD, Salinas F, et al. Parasternal block and local anesthetic infiltration with levobupivacaine after cardiac surgery with desflurane: the effect on postoperative pain, pulmonary function, and tracheal extubation times. Anesthesia and Analgesia 2005;100(1):25‐32. [PUBMED: 15616047] [DOI] [PubMed] [Google Scholar]
Mollhoff 2001 {published data only}
- Möllhoff T, Herregods L, Moerman A, Blake D, MacAdams C, Demeyere R, et al. Comparative efficacy and safety of remifentanil and fentanyl in 'fast track' coronary artery bypass graft surgery: a randomized, double‐blind study. British Journal of Anaesthesia 2001;87(5):718‐26. [PUBMED: 11878522] [DOI] [PubMed] [Google Scholar]
Murphy 2009 {published data only}
- Murphy GS, Szokol JW, Marymont JH, Greenberg SB, Avram MJ, Vender JS, et al. Morphine‐based cardiac anesthesia provides superior early recovery compared with fentanyl in elective cardiac surgery patients. Anesthesia and Analgesia 2009;109(2):311‐9. [PUBMED: 19608797] [DOI] [PubMed] [Google Scholar]
Najafi 2008 {published data only}
- Najafi M. Fast‐track method in cardiac surgery: evaluation of risks and benefits of continuous administration technique. Singapore Medical Journal 2008;49(6):470‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Oliver 2011 {published data only}
- Oliver WC Jr, Nuttall GA, Murari T, Bauer LK, Johnsrud KH, Hall Long KJ, et al. A prospective, randomized, double‐blind trial of 3 regimens for sedation and analgesia after cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2011;25:110‐9. [PUBMED: 20850348] [DOI] [PubMed] [Google Scholar]
Puri 2003 {published data only}
- Puri GD, Murthy SS. Bispectral index monitoring in patients undergoing cardiac surgery under cardiopulmonary bypass. European Journal of Anaesthesiology 2003;20(6):451‐6. [PUBMED: 12803261] [DOI] [PubMed] [Google Scholar]
Ramsay 1994 {published data only}
- Ramsay JG, DeLima LG, Wynands JE, O'Connor JP, Ralley FE, Robbins GR. Pure opioid versus opioid‐volatile anesthesia for coronary artery bypass graft surgery: a prospective, randomized, double‐blind study. Anesthesia and Analgesia 1994;78(5):867‐75. [PUBMED: 8160983] [DOI] [PubMed] [Google Scholar]
Rose 2014 {published data only}
- Rose L, Schultz MJ, Cardwell CR, Jouvet P, McAuley DF, Blackwood B. Automated versus non‐automated weaning for reducing the duration of mechanical ventilation for critically ill adults and children. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD009235.pub3; PUBMED: 24915581] [DOI] [PMC free article] [PubMed] [Google Scholar]
Royse 2003 {published data only}
- Royse C, Royse A, Soeding P, Blake D, Pang J. Prospective randomised trial of high thoracic epidural analgesia for coronary artery bypass surgery. The Annals of Thoracic Surgery 2003;75(1):93‐100. [PUBMED: 12537199] [DOI] [PubMed] [Google Scholar]
Shroff 1997 {published data only}
- Shroff A, Rooke GA, Bishop MJ. Effects of intrathecal opioid on extubation time, analgesia, and intensive care unit stay following coronary artery bypass grafting. Journal of Clinical Anesthesia 1997;9(5):415‐9. [PUBMED: 9257210] [DOI] [PubMed] [Google Scholar]
Sulzer 2001 {published data only}
- Sulzer CF, Chioléro R, Chassot PG, Mueller XM, Revelly JP. Adaptive support ventilation for fast tracheal extubation after cardiac surgery: a randomized controlled study. Anesthesiology 2001;95(6):1339‐45. [PUBMED: 11748389] [DOI] [PubMed] [Google Scholar]
Tempe 1995 {published data only}
- Tempe D, Cooper A, Mohan JC, Nigam M, Tomar AS, Ramesh K, et al. Closed mitral valvotomy and elective ventilation in the postoperative period: effect of mild hypercarbia on right ventricular function. Journal of Cardiothoracic and Vascular Anesthesia 1995;9(5):552‐7. [PUBMED: 8547558] [DOI] [PubMed] [Google Scholar]
Tempe 2011 {published data only}
- Tempe DK, Dutta D, Garg M, Minhas H, Tomar A, Virmani S. Myocardial protection with isoflurane during off‐pump coronary artery bypass grafting: a randomized trial. Journal of Cardiothoracic and Vascular Anesthesia 2011;25(1):59‐65. [PUBMED: 20580572] [DOI] [PubMed] [Google Scholar]
Yorulmaz 2005 {published data only}
- Yorulmaz V, Arar C, Turan N, Pamukcu Z. Comparison of effects of sevoflurane and high‐dose fentanyl anesthesia on hemodynamic profile and postoperative recovery in coronary artery surgery. Turk Anesteziyoloji ve Reanimasyon Dernegi Dergisi 2005;33(1):63‐8. [Google Scholar]
Zeydanoglu 2005 {published data only}
- Zeydanoglu S, Bilir A, Ekemen S, Tanriverdi B. Comparison of remifentanil and fentanyl on early extubation and recovery at coronary artery bypass grafting. Anesteziyoloji ve Reanimasyon Uzmanları Derneği 2005;13(4):237‐42. [Google Scholar]
Additional references
Alhan 2003
- Alhan C, Toruman F, Karabulut EH, Tarcan S, Dagdelens S, Eren N, et al. Fast track recovery of high risk coronary bypass surgery patients. European Journal of Cardiothoracic Surgery 2003;23(5):678‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
American Heart Association 2016
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Executive Summary: Heart Disease and Stroke Statistics‐2016 Update: A Report From the American Heart Association. Circulation 2016;133(4):447‐54. [PUBMED: 26811276] [DOI] [PubMed] [Google Scholar]
Badhwar 2014
- Badhwar V, Esper S, Brooks M, Mulukutla S, Hardison R, Mallios D, et al. Extubating in the operating room after adult cardiac surgery safely improves outcomes and lowers costs. Journal of Thoracic and Cardiovascular Surgery 2014;148(6):3101‐9. [PUBMED: 25173117] [DOI] [PubMed] [Google Scholar]
Blackwood 2014
- Blackwood B, Burns KE, Cardwell CR, O'Halloran P. Protocolized versus non‐protocolized weaning for reducing the duration of mechanical ventilation in critically ill adult patients. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD006904.pub3; PUBMED: 25375085] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chamchad 2010
- Chamchad D, Horrow JC, Nachamchik L, Sutter FP, Samuels LE, Trace CL, et al. The impact of immediate extubation in the operating room after cardiac surgery on intensive care and hospital length of stay. Journal of Cardiothoracic and Vascular Anesthesia 2010;24:780‐4. [PUBMED: 20650657] [DOI] [PubMed] [Google Scholar]
Constantinides 2006
- Constantinides VA, Tekkis PP, Fazil A, Kaur K, Leonard R, Platt M, et al. Fast‐track failure after cardiac surgery: development of a prediction model. Critical Care Medicine 2006;34(12):2875‐82. [PUBMED: 17075376] [DOI] [PubMed] [Google Scholar]
Deeks 2010
- Deeks JJ, Higgins JPT. Statistical algorithms in Review Manager 5. http://www.cochrane.org/sites/default/files/uploads/handbook/Statistical_Methods_in_RevMan5‐1.pdf (accessed 16 March 2016).
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elmagarmid 2014
- Elmagarmid A, Fedorowicz Z, Hammady H, Ilyas I, Khabsa M, Ouzzani M. Rayyan: a systematic reviews web app for exploring and filtering searches for eligible studies for Cochrane Reviews. Evidence‐Informed Public Health: Opportunities and Challenges. Abstracts of the 22nd Cochrane Colloquium. Hyderabad, India: John Wiley & Sons, 2014.
Faul 2014 [Computer program]
- Faul F. G*Power 3.1.9.2. Universitat Kiel, Germany, 2014.
Greco 2012
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Ho 2011
- Ho KM, Tan JA. Benefits and risks of maintaining normothermia during cardiopulmonary bypass in adult cardiac surgery: a systematic review. Cardiovascular Therapeutics 2011;29:260‐79. [PUBMED: 20041882] [DOI] [PubMed] [Google Scholar]
Kiessling 2013
- Kiessling AH, Huneke P, Reyher C, Bingold T, Zierer A, Moritz A. Risk factor analysis for fast track protocol failure. Journal of Cardiothoracic Surgery 2013;Mar 15(8):47. [PUBMED: 23497403 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Landoni 2011
- Landoni G, Augoustides JG, Guarracino F, Santini F, Ponschab M, Pasero D, et al. Mortality reduction in cardiac anesthesia and intensive care: results of the first International Consensus Conference. Acta Anaesthesiologica Scandinavica 2011;55(3):259‐66. [PUBMED: 21288207] [DOI] [PubMed] [Google Scholar]
Lee 2013
- Lee A, Zhu F, Underwood MJ, Gomersall CD. Fast‐track failure after cardiac surgery: external model validation and implications to ICU bed utilization. Critical Care Medicine 2013;41(5):1205‐13. [PUBMED: 23388511 ] [DOI] [PubMed] [Google Scholar]
Meades 2001
- Meade MO, Guyatt G, Butler R, Elms B, Hand L, Ingram A, et al. Trials comparing early vs late extubation following cardiovascular surgery. Chest 2001;120(6 Suppl):445S‐53S. [PUBMED: 11742964] [DOI] [PubMed] [Google Scholar]
Michel 2003
- Michel P, Roques F, Nashef SA, EuroSCORE Project Group. Logistic or additive EuroSCORE for high‐risk patients?. European Journal of Cardiothoracic Surgery 2003;23(5):684‐7. [PUBMED: 12754018] [DOI] [PubMed] [Google Scholar]
Peters 2008
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour‐enhanced meta‐analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991‐6. [PUBMED: 18538991] [DOI] [PubMed] [Google Scholar]
Silbert 2009
- Silbert BS, Myles PS. Is fast‐track cardiac anesthesia now the global standard of care?. Anesthesia and Analgesia 2009;108(3):689‐91. [PUBMED: 19224767] [DOI] [PubMed] [Google Scholar]
Svircevic 2009
- Svircevic V, Nierich AP, Moons KGM, Bruinsma GJBB, Kalkman CJ, Dijk D. Fast‐track anesthesia and cardiac surgery: a retrospective cohort study of 7989 patients. Anesthesia and Anagesia 2009;108(3):727‐33. [PUBMED: 19224776] [DOI] [PubMed] [Google Scholar]
Svircevic 2013
- Svircevic V, Passier MM, Nierich AP, Dijk D, Kalkman CJ, Heijden GJ. Epidural analgesia for cardiac surgery. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD006715.pub2; PUBMED: 23740694] [DOI] [PubMed] [Google Scholar]
van Mastrigt 2006b
- Mastrigt GA, Maessen JG, Heijmans J, Severens JL, Prins MH. Does fast‐track treatment lead to a decrease of intensive care unit and hospital length of stay in coronary artery bypass patients? A meta‐regression of randomized clinical trials. Critical Care Medicine 2006;34(6):1624‐34. [PUBMED: 16614584] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hawkes 2003
- Hawkes CA, Dhileepan S, Foxcroft D. Early extubation for adult surgical patients. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003587] [DOI] [PubMed] [Google Scholar]
Myles 2003
- Myles PS, Daly DJ, Djaiani G, Lee A, Cheng DCH. A systematic review of the safety and effectiveness of fast‐track cardiac anesthesia. Anesthesiology 2003;99(4):982‐7. [PUBMED: 14508335] [DOI] [PubMed] [Google Scholar]
Zhu 2012
- Zhu F, Lee A, Chee YE. Fast‐track cardiac care for adult cardiac surgical patients. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD003587.pub2; PUBMED: 23076899] [DOI] [PubMed] [Google Scholar]


