Abstract
Background
Usual‐type vulval intraepithelial neoplasia (uVIN) is a pre‐cancerous condition of the vulval skin. Also known as high‐grade VIN, VIN 2/3 or high‐grade vulval squamous intraepithelial lesion (HSIL), uVIN is associated with high‐risk subtype human papilloma virus (HPV) infection. The condition causes distressing vulval symptoms in the majority of affected women and may progress to vulval cancer, therefore is usually actively managed. There is no consensus on the optimal management of uVIN. High morbidity and recurrence rates associated with surgical treatments make less invasive treatments highly desirable.
Objectives
To determine which interventions are the most effective, safe and tolerable for treating women with uVIN.
Search methods
We searched the Cochrane Gynaecological Cancer Group Trials Register, Cochrane Central Register of Controlled Trials (CENTRAL), Issue 8 2015, MEDLINE and EMBASE (up to 1 September 2015). We also searched registers of clinical trials, abstracts of scientific meetings, reference lists of included studies and contacted experts in the field.
Selection criteria
Randomised controlled trials (RCTs) that assessed medical and surgical interventions in women with uVIN. If no RCTs were available, we included non‐randomised studies (NRSs) with concurrent comparison groups that controlled for baseline case mix in multivariate analysis.
Data collection and analysis
We used Cochrane methodology with two review authors independently extracting data and assessing risk of bias. Where possible, we synthesised data in meta‐analyses using random‐effects methods. Network meta‐analysis was not possible due to insufficient data.
Main results
We included six RCTs involving 327 women and five NRSs involving 648 women. The condition was variously named by investigators as uVIN, VIN2/3 or high‐grade VIN. Five RCTs evaluated medical treatments (imiquimod, cidofovir, indole‐3 carbinol), and six studies (one RCT and five NRSs) evaluated surgical treatments or photodynamic therapy. We judged two RCTs and four NRSs to be at a high or unclear risk of bias; we considered the others at relatively low risk of bias. Types of outcome measures reported in NRSs varied and we were unable to pool NRS data.
Medical interventions: Topical imiquimod was more effective than placebo in achieving a response (complete or partial) to treatment at five to six months post‐randomisation (three RCTs, 104 women; risk ratio (RR) 11.95, 95% confidence interval (CI) 3.21 to 44.51; high‐quality evidence). At five to six months, a complete response occurred in 36/62 (58%) and 0/42 (0%) women in the imiquimod and placebo groups, respectively (RR 14.40, 95% CI 2.97 to 69.80). Moderate‐quality evidence suggested that the complete response was sustained at one year (one RCT, nine complete responses out of 52 women (38%)) and beyond, particularly in women with smaller VIN lesions. Histologically confirmed complete response rates with imiquimod versus cidofovir at six months were 45% (41/91) and 46% (41/89), respectively (one RCT, 180 women; RR 1.00, 95% CI 0.73 to 1.37; moderate‐quality evidence). Twelve‐month data from this trial are awaited; however, interim findings suggested that complete responses were sustained at 12 months. Only one trial reported vulval cancer at one year (1/24 and 2/23 in imiquimod and placebo groups, respectively). Adverse events were more common with imiquimod than placebo and dose reductions occurred more frequently in the imiquimod group than in the placebo group (two RCTs, 83 women; RR 7.77, 95% CI 1.61 to 37.36; high‐quality evidence). Headache, fatigue and discontinuation were slightly more common with imiquimod than cidofovir (moderate‐quality evidence). Quality of life scores reported in one trial (52 women) were not significantly different for imiquimod and placebo. The evidence of effectiveness of topical treatments in immunosuppressed women was scant. There was insufficient evidence on other medical interventions.
Surgical and other interventions: Low‐quality evidence from the best included NRS indicated, when data were adjusted for confounders, that there was little difference in the risk of VIN recurrence between surgical excision and laser vaporisation. Recurrence occurred in 51% (37/70) of women overall, at a median of 14 months, and was more common in multifocal than unifocal lesions (66% versus 34%). Vulval cancer occurred in 11 women (15.1%) overall at a median of 71.5 months (9 to 259 months). The risk of vulval cancer did not differ significantly between excision and laser vaporisation in any of the NRSs; however, events were too few for robust findings. Alternative surgical procedures that might be as effective include Cavitron ultrasonic surgical aspiration (CUSA) and loop electrosurgical excision (LEEP) procedures, based on low‐ to very low‐quality evidence, respectively. Very low‐quality evidence also suggested that photodynamic therapy may be a useful treatment option.
We found one ongoing RCT of medical treatment (imiquimod) compared with surgical treatment.
Authors' conclusions
Topical treatment (imiquimod or cidofovir) may effectively treat about half of uVIN cases after a 16‐week course of treatment, but the evidence on whether this effect is sustained is limited. Factors predicting response to treatment are not clear, but small lesions may be more likely to respond. The relative risk of progression to vulval cancer is uncertain. However, imiquimod and cidofovir appear to be relatively well tolerated and may be favoured by some women over primary surgical treatment.
There is currently no evidence on how medical treatment compares with surgical treatment. Women who undergo surgical treatment for uVIN have about a 50% chance of the condition recurring one year later, irrespective of whether treatment is by surgical excision or laser vaporisation. Multifocal uVIN lesions are at a higher risk of recurrence and progression, and pose greater therapeutic dilemmas than unifocal lesions. If occult cancer is suspected despite a biopsy diagnosis of uVIN, surgical excision remains the treatment of choice. If occult cancer is not a concern, treatment needs to be individualised to take into account the site and extent of disease, and a woman's preferences. Combined modalities may hold the key to optimal treatment of this complex disease.
Keywords: Adult; Female; Humans; Aminoquinolines; Aminoquinolines/therapeutic use; Antineoplastic Agents; Antineoplastic Agents/therapeutic use; Carcinoma in Situ; Carcinoma in Situ/drug therapy; Carcinoma in Situ/pathology; Carcinoma in Situ/surgery; Cidofovir; Cytosine; Cytosine/analogs & derivatives; Cytosine/therapeutic use; Disease Progression; Imiquimod; Indoles; Indoles/therapeutic use; Laser Therapy; Neoplasm Recurrence, Local; Organophosphonates; Organophosphonates/therapeutic use; Photochemotherapy; Prospective Studies; Randomized Controlled Trials as Topic; Retrospective Studies; Treatment Outcome; Vulvar Neoplasms; Vulvar Neoplasms/drug therapy; Vulvar Neoplasms/pathology; Vulvar Neoplasms/surgery
Plain language summary
Medical and surgical treatments for usual‐type vulval intraepithelial neoplasia (uVIN)
What is the issue?
Usual‐type vulval intraepithelial neoplasia (uVIN) is a pre‐malignant condition affecting the vulval skin, which has the potential for progression to vulval cancer. Most patients have distressing symptoms that include itching, burning and soreness of the vulva, and painful intercourse. There may be white, brown, or red colour changes of the skin, breaks in the skin, or skin thickening. Usual‐type VIN is associated with infection with a virus called human papilloma virus (HPV or wart virus). Treatments are aimed at relieving distressing symptoms and ensuring that the condition does not become cancerous. The most common treatment option has been surgery to remove the affected skin areas. Surgery, however, does not guarantee a cure, can be disfiguring, and may result in physical and psychological problems. Alternatives include the use of laser technology to destroy the layer of affected skin, which may give better cosmetic results, but usually does not yield a specimen to exclude cancer. It may also be ineffective in treating uVIN that extends into hair follicles. Non‐surgical treatment alternatives include topical creams and gels, and HPV vaccines. This review aimed to assess the effectiveness and safety of these treatments.
What did we do? We searched the literature from 1946 to September 2015 for randomised controlled trials (RCTs) and non‐randomised studies (NRSs) of uVIN treatment.
What did we find?
We included six RCTs involving 327 women and five NRSs involving 648 women. Five RCTs evaluated medical treatments (imiquimod, cidofovir, indole‐3 carbinol), and six studies (one RCT and five NRSs) evaluated surgical treatments or photodynamic therapy.
We pooled data from three similar trials involving 104 women and found topical imiquimod cream to be more effective than placebo in clearing uVIN after a 16‐week course (58% cleared with imiquimod versus 0% with placebo). Most studies did not include long‐term follow‐up, but findings from one small study suggested that most women in whom uVIN was completely cleared at six months were likely to sustain this response by 12 months and beyond; however, more evidence is needed. Moderate‐quality evidence suggested that topical cidofovir gel has a similar effect to imiquimod on clearing uVIN lesions at six months (complete response rates were 46% and 45%, respectively). Again, we are uncertain about the longer‐term effects and more evidence is needed. Side effects of imiquimod included vulval pain, redness and swelling, usually managed by reducing the frequency of applications. Headaches and tiredness occurred more frequently with imiquimod than cidofovir. The evidence for imiquimod was of moderate to high quality, and that for cidofovir was of moderate quality. Very few women were immunosuppressed, therefore we cannot be certain whether these topical treatments will be as effective in these patients.
Low‐quality evidence showed that surgical excision and laser vaporisation were probably equally effective in removing uVIN lesions. However, uVIN recurrence after treatment was common, occurring in about half of women treated. The risk of vulval cancer did not differ significantly between these treatments, but there were too few cases for firm conclusions. Alternative surgical procedures that might be as effective include CUSA (ultrasonic surgical aspiration) and LEEP (loop electrosurgical excision procedure), based on low‐ to very low‐quality evidence, respectively. Very low‐quality evidence also suggested that photodynamic therapy may also be a useful treatment option.
We found no evidence on the effectiveness of medical treatment versus surgery, or of other treatments, such as HPV vaccines; however, we identified five ongoing trials that may provide important evidence in the future.
Our conclusions
Imiquimod or cidofovir as a 16‐week course appears to be effective against uVIN in about half of women treated, but more evidence is needed to prove that this effect is sustained in the longer term. It remains unknown whether topical treatments are as effective as surgery. Surgical excision and laser vaporisation may be equally effective treatments for uVIN, but about half of women will experience uVIN recurrence after either treatment. If cancer is suspected, despite a diagnosis of uVIN, surgical excision remains the treatment of choice. If cancer is not suspected, treatment should be individualised, taking into account a woman's preferences. Long‐term follow‐up after any treatment is essential.
Summary of findings
Summary of findings for the main comparison. Summary of findings for imiquimod versus placebo.
| Imiquimod compared with placebo for usual‐type vulval intraepithelial neoplasia | |||||
|
Patient or population: women with usual‐type vulval intraepithelial neoplasia Settings: outpatient Intervention: imiquimod 5% cream Comparison: placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk (risk in study population) | Corresponding risk | ||||
| Placebo | Imiquimod | ||||
| Response to treatment at 5 to 6 months ‐ overall response | 24 per 1000 | 285 per 1000 (76 to 1000) | RR 11.95 (3.21 to 44.51) | 104 (3 studies) | ⊕⊕⊕⊕ high |
| Response to treatment at 5 to 6 months ‐ complete response | 0 per 1000 | not estimable | RR 14.40 (2.97 to 69.80) | 104 (3 studies) | ⊕⊕⊕⊕ high |
| Response to treatment at 12 months ‐ overall response | 87 per 1000 | 791 per 1000 (207 to 1000) | RR 9.10 (2.38 to 34.77) | 47 (1 study) | ⊕⊕⊕⊝ moderate1 |
| Response to treatment at 12 months ‐ complete response | 0 per 1000 | Not estimable | RR 18.24 (1.12 to 296.41) | 47 (1 study) | ⊕⊕⊕⊝ moderate1 |
| Progression to vulval cancer at 12 months | 56 per 1000 | 28 per 1000 (3 to 288) | RR 0.48 (0.05 to 4.93) | 47 (1 study) | ⊕⊕⊝⊝ low1,2 |
| HPV DNA persistence | 923 per 1000 |
397 per 1000 (240 to 665) |
RR 0.43 (0.26 to 0.72) | 47 (1 study) | ⊕⊕⊕⊕ high |
| Pain ‐ any grade | 269 per 1000 | 922 per 1000 (484 to 1000) | RR 3.43 (1.80, 6.52) | 52 (1 study) | ⊕⊕⊕⊕ high |
| Dose reductions | 28 per 1000 | 218 per 1000 (45 to 1000) | RR 7.77 (1.61 to 37.36) | 83 (2 studies) | ⊕⊕⊕⊕ high |
| The risk in the cidofovir group is based on the assumed risk on the comparison group and the relative effect of the intervention and its 95% CI. CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded due to imprecision.
2Downgraded due to sparse data (few events).
Summary of findings 2. Summary of findings for imiquimod versus cidofovir.
| Imiquimod compared with cidofovir for usual‐type vulval intraepithelial neoplasia | |||||
|
Patient or population: women with usual‐type vulval intraepithelial neoplasia Settings: outpatient Intervention: imiquimod 5% cream Comparison: 1% cidofovir gel | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk (risk in study population) | Corresponding risk | ||||
| Cidofovir | Imiquimod | ||||
| Response to treatment at 6 months ‐ overall response | 618 per 1000 |
569 per 1000 (451 to 729) |
RR 0.92 (0.73 to 1.18) |
180 (1 study) | ⊕⊕⊕⊝ moderate1 |
| Response to treatment at 6 months ‐ complete response | 461 per 1000 | 461 per 1000 (336 to 631) |
RR 1.00 (0.73 to 1.37) |
180 (1 study) | ⊕⊕⊕⊝ moderate1 |
| Pain ‐ any grade | 516 per 1000 | 598 per 1000 (475 to 758) | RR 1.16 (0.92 to 1.47) | 168 (1 study) | ⊕⊕⊕⊝ moderate1 |
| Fatigue ‐ any grade | 607 per 1000 | 759 per 1000 (619 to 941) |
RR 1.25 (1.02 to 1.55) |
168 (1 study) |
⊕⊕⊕⊝ moderate1 |
| Headache ‐ any grade | 440 per 1000 | 656 per 1000 (493 to 871) |
RR 1.49 (1.12 to 1.98) |
168 (1 study) |
⊕⊕⊕⊝ moderate1 |
| Total serious adverse events | 369 per 1000 | 465 per 1000 (325 to 668) |
RR 1.26 (0.88 to 1.81) |
168 (1 study) |
⊕⊕⊕⊝ moderate1 |
| Treatment discontinuation | 126 per 1000 | 168 per 1000 (82 to 345) |
RR 1.33 (0.65 to 2.74) |
176 (1 study) |
⊕⊕⊕⊝ moderate1 |
| *The risk in the cidofovir group is based on the assumed risk on the comparison group and the relative effect of the intervention and its 95% CI. CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Downgraded due to imprecision.
Summary of findings 3. Summary of findings for surgical interventions and photodynamic therapy.
| Surgical interventions compared with photodynamic therapy or other interventions for usual‐type vulval intraepithelial neoplasia | |||||
|
Patient or population: women with usual‐type vulval intraepithelial neoplasia Settings: hospital or clinic Intervention: surgical excision, laser vaporisation or other options Comparison: surgical excision, laser vaporisation or other surgical procedure | |||||
| Intervention versus comparison | Outcomes | Relative effect (95% CI) or P value | No of participants (studies) | Quality of the evidence (GRADE) | Comments |
| Surgical excision versus laser vaporisationor other | Recurrence‐free survival | No difference P value = 0.142 |
73 women (1 study) | ⊕⊕⊝⊝ low1 | This NRS had a relatively low risk of bias and adjusted appropriately on multivariate analysis |
| Laser vaporisation versus CUSA | Recurrence rate |
RR 1.53 (0.56 to 4.15) |
30 women (1 study) | ⊕⊕⊝⊝ low2 | This RCT lacked power to detect a difference |
| Photodynamic therapy versus laser vaporisationand surgical excision | Disease‐free survival | No difference P value = 0.67 |
52 women (1 study) |
⊕⊝⊝⊝ very low3 | This NRS had serious design limitations |
| LEEP versus laser vaporisation and surgical excision | Recurrence‐free survival | No difference P value = 0.194 |
62 women (1 study) | ⊕⊝⊝⊝ very low3 | This NRS had serious design limitations |
| CI: confidence interval; CUSA: Cavitron ultrasonic surgical aspiration; LEEP: loop electrosurgical excision procedure; NRS: non‐randomised study; RCT: randomised controlled trial; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1Evidence from a non‐randomised study that adjusted for confounders.
2Downgraded twice due to imprecision.
3Evidence from a non‐randomised study with a high risk of bias.
Background
This topic was originally reviewed as two separate Cochrane topics, namely 'Medical interventions for high‐grade vulval intraepithelial neoplasia' (Pepas 2015), and 'Surgical interventions for high‐grade vulval intraepithelial neoplasia' (Kaushik 2014). The original protocols have been combined here to enable comparisons between the various types of interventions (medical, surgical and other interventions) to be made. As the terminology of vulval intraepithelial neoplasia (VIN) has evolved over the years with our understanding of the biology and natural history of the condition (Wilkinson 2015; Table 4), for the purpose of this new combined review, we used the term usual‐type VIN (uVIN) (2004 terminology; Sideri 2005), to include lesions also referred to in the literature as VIN 2 and VIN 3, high‐grade VIN and vulval high‐grade squamous intraepithelial lesions (HSIL) (2014 terminology ISSVD 2014).
1. Terminology changes for vulval intraepithelial neoplasia (VIN).
| ISSVD 1986 | ISSVD 2004 | LAST 2012 |
| VIN 1 | Flat condyloma or HPV effect | LSIL |
| VIN 2 | VIN, usual type a. VIN, warty type b. VIN, basaloid type c. VIN, mixed (warty/basaloid) type |
HSIL |
| VIN 3 | ||
| Differentiated VIN | VIN, differentiated type |
Table derived from ISSVD 2014.
Abbreviations: HSIL: high‐grade squamous intraepithelial lesion; ISSVD: International Society for the Study of Vulvovaginal Disease; LSIL: low‐grade squamous intraepithelial lesions
Description of the condition
Vulval intraepithelial neoplasia (VIN) is a term used for chronic pre‐cancerous skin conditions affecting the vulva. It can affect women at any age but the peak incidence occurs under the age of 50 (Jones 2005; Nygard 2014; de Sanjose 2013). There are two distinct types, a type related to human papilloma virus (HPV) infection (usual‐type VIN (uVIN)) and a type related to chronic skin conditions such as lichen sclerosis (differentiated‐type VIN (dVIN)); clinicopathological overlap occurs in a small proportion of cases (less than 1%) (de Sanjose 2013; McCluggage 2009). uVIN accounts for approximately 90% of VIN lesions. It has previously been graded as low‐grade (VIN 1) or high‐grade (VIN 2/3) depending on the thickness of vulval epithelium containing undifferentiated cells; however, VIN 1 was removed from the 2004 VIN classification as these lesions have little known risk of invasive carcinoma (Sideri 2005; Wilkinson 2015). uVIN is associated with high‐risk HPV types, particularly HPV 16 (Reyes 2014; de Sanjose 2013), and precedes almost all vulval squamous cell carcinomas (SCC) in younger women (Reyes 2014; Sideri 2005; van der Avoort 2006).
Recently the International Society for the Study of Vulvovaginal Disease (ISSVD) recommended new terminology, with VIN 1 renamed low‐grade squamous intraepithelial lesions (LSIL) and uVIN renamed high‐grade squamous intraepithelial lesions (HSIL) (ISSVD 2014; Wilkinson 2015); however, VIN 2/3 and uVIN are still widely used terms in the United Kingdom (UK) and elsewhere.
uVIN has a variable appearance on clinical examination, frequently presenting as multifocal raised plaques or papules, which may be white, brown or red (Reyes 2014; Preti 2015). Other visible changes including skin thickening (hyperkeratosis), splitting (fissuring) and ulceration. Women usually present with distressing vulval symptoms, including itching, pain, burning and dyspareunia, therefore impaired sexual functioning and psychological morbidity are frequent associated features (Dominiak‐Felden 2013; Shylasree 2008). Recurrence after treatment is common, with studies reporting recurrence rates of between 25% and 51% (Fehr 2013; Jones 2005; van Esch 2013; Wallbillich 2012).
Vulval SCC in younger women is increasing, driven primarily by the increasing incidence of uVIN (De Vuyst 2009; Dittmer 2011; Joura 2000; Lai 2014; Reyes 2014). In England, there has been a statistically significant increase in the age‐standardised risk of vulval cancer from approximately 2 to 2.5 per 100,000 women from 1990 to 2009 (Lai 2014), in agreement with similar trends noted in other countries (Baandrup 2011; Dittmer 2011; Jones 1997; Joura 2000; Judson 2006). Reported vulval cancer rates after treatment range from 2% to 15% (Fehr 2013; Jones 2005; van Esch 2013; Van Seters 2005; Wallbillich 2012).
Recurrence and progression of uVIN have been associated with smoking (Fehr 2013), multifocality (lesions at more than one site) (Fehr 2013; van Esch 2013), and large lesion size (Wallbillich 2012). The evidence for an association with positive surgical margins is conflicting (Jones 2005; Modesitt 1998; Wallbillich 2012; Yu 2014). Higher recurrence and progression rates have been reported in immunocompromised women (Fehr 2013; van Esch 2013; Wallbillich 2012), supporting the increasing interest in the immunological aspects of VIN, both as predictors of recurrence and progression, and to facilitate new immunotherapeutic approaches (van Esch 2012; van Esch 2015).
Description of the intervention
Surgical interventions
VIN lesions have historically been managed by surgical excision or ablative techniques to remove visible lesions (Kauffman 1995). Surgical excision remains the standard of care for small, well‐circumscribed uVIN lesions (BASHH guidelines 2014), but is not optimal for treating multifocal lesions, which are common and more problematic (Stern 2012); these tend to require more extensive surgery to remove all affected skin, e.g. skinning or simple vulvectomy. Surgical excision disrupts the normal structure of the vulva, often having a negative impact on sexual function and quality of life (Andreasson 1986; Aerts 2012)
Ablative techniques, such as carbon dioxide laser vaporisation, whereby a focused laser beam destroys the affected vulval skin, may offer greater precision and better cosmetic results, but some studies have reported a greater risk of treatment failure and recurrence with laser vaporisation (Steiner 2012; Wallbillich 2012). Laser vaporisation may not be appropriate for hair‐bearing areas, where hair follicle depth can extend to 3 mm or more (Committee 2011). A further disadvantage of ablative techniques is that, unlike surgical excision, no tissue is yielded for histological examination. Hence, when ablative procedures are planned, multiple biopsies of the lesion/s are necessary before the procedure to exclude microinvasion (Preti 2015).
Cavitron ultrasonic surgical aspiration (CUSA) is a technique that uses a handheld device to aspirate the affected epidermal tissue (von Gruenigen 2007). This technique yields a specimen for histology, therefore may have an advantage over laser vaporisation.
Medical interventions
Several medical interventions have been used to treat VIN in the past. Agents utilised prior to the 1990s have largely been disregarded due to either their inefficacy or their unacceptable side effect profile. These include chemotherapeutic agents such as topical 5‐fluorouracil (Sillman 1985), bleomycin (Roberts 1980), and trinitrochlorobenzene (Foster 1981). Alpha‐interferon (α‐IFN) was investigated in the 1980s and early 1990s; however, its high cost and side effects limited its use (Spirtos 1990).
In recent decades, various other medical interventions have been investigated. These include the immune response modulator, imiquimod, which was developed and licensed for the treatment of genital warts (Moore 2001). In an associated Cochrane review of medical interventions for VIN (Pepas 2015), we found good evidence that imiquimod was better than placebo in achieving a complete response at five to six months after treatment; however, longer‐term efficacy and safety data were lacking. In the UK, uVIN is an unlicensed indication for imiquimod (BASHH guidelines 2014).
Pepas 2015 also included a small randomised controlled trial (RCT) that compared two doses of phytochemical indole‐3‐carbinol (I3C), a natural compound that is present in large concentrations in cruciferous vegetables (cabbage, broccoli, Brussels sprouts and cauliflower); however, the review found insufficient evidence on the effectiveness of this compound (Naik 2006).
Another agent, cidofovir, a potent antiviral, has been used with some success in treating high‐grade anal intra‐epithelial neoplasia (AIN) (Stier 2013), but few studies have been conducted to demonstrate its efficacy in treating VIN (Tristram 2005). Recently, a randomised phase 2 trial of cidofovir compared with imiquimod reported promising activity, safety and feasibility results (Tristram 2014), and longer‐term follow‐up data are expected.
Prophylactic vaccination with the quadrivalent vaccine (HPV 6/11/16/18) has been shown to reduce the risk of HPV‐related disease including uVIN and vaccination programmes are expected to lead to future reductions in uVIN prevalence (Joura 2007). Vaccines may also have a role to play in the treatment of uVIN and various types, including peptide (Kenter 2009), and recombinant virus vaccines (Baldwin 2003), have been investigated. Limited evidence suggests that vaccination with a synthetic peptide vaccine targeting specific HPV 16/18 onco proteins can induce clinical responses, as well as clear HPV infection, in women with uVIN (Kenter 2009).
Photodynamic therapy, not strictly a medical intervention, has been evaluated in a number of small non‐randomised studies with variable results (Dougherty 1998; Fehr 2001; Hillemanns 2000). Proponents of this modality report that it is well tolerated and has the advantage over surgical modalities in that the appearance of the vulva is preserved (Fehr 2001). A non‐randomised phase II trial evaluating sequential imiquimod and photodynamic therapy has reported encouraging results (Winters 2008).
How the intervention might work
Surgical interventions aim to remove or destroy the abnormal tissue. However, as uVIN is linked to persistent HPV infection, surgery does not consistently affect a cure and may miss non‐visible lesions. Immune response modifiers, such as imiquimod, α‐IFN and vaccines, aim to destroy abnormal cells indirectly by enhancing the body's immune response to HPV. Topical imiquimod does this by activating dendritic cells and increasing local cytokine secretion including interferons, tumour necrosis factor α and interleukins (van Esch 2012), whereas vaccines need to stimulate a broad HPV‐specific cytotoxic T‐cell response to be effective against uVIN (Baldwin 2003; Stern 2012; van Esch 2012). Cidofovir is a broad‐spectrum antiviral agent that inhibits viral replication, and probably mediates its effect in uVIN by causing apoptosis of HPV‐infected cells (Stern 2012; Tristram 2005).
Natural compounds such as I3C and green tea extract (sinecatechins) have antioxidant properties. I3C has been shown to increase production of the anti‐proliferative metabolite 2‐hydroxyestrone, whilst decreasing production of 16‐alpha‐hydroxyestrone (a carcinogen) in rodents and humans (Newfield 1998). Photodynamic therapy causes direct destruction of uVIN lesions using the interaction between a tumour‐localising photo‐sensitiser, e.g. 5‐aminolaevulinic acid (ALA), and light of an appropriate wavelength to bring about molecular oxygen‐induced cell death (Dougherty 1998). In addition to lesion destruction, photodynamic therapy also induces local inflammation and activates T cells (van Esch 2012), therefore has both ablative and immunogenic modes of action.
Why it is important to do this review
There is currently no consensus on the optimal and most acceptable management of uVIN, largely due to a lack of high‐quality evidence to guide practice. The management of women with uVIN is complicated by the broad age range of women affected, the frequently multifocal nature of this condition and the high risk of recurrence. Surgical interventions are often disfiguring and associated with significant psychosexual morbidity, whereas medical interventions and photodynamic therapy have the advantage in that there is minimal alteration in the appearance of the vulva. uVIN is increasingly being diagnosed in younger women for whom surgical options may not be acceptable. Although new medical options have been developed to target uVIN, to date only limited evidence has emerged regarding their effectiveness and safety, and none are currently licensed in the UK for uVIN treatment. In addition, medical treatments generally require treatment to be administered over a prolonged course of time, and are frequently associated with significant distressing treatment‐related side effects.
A Cochrane review of surgical interventions for VIN, by this author team, found insufficient evidence from RCTs to determine the effectiveness and safety of CO₂ laser vaporisation versus CUSA, and no evidence regarding the comparative effectiveness and safety of other surgical approaches (Kaushik 2014). Another review of RCTs of medical interventions concluded that whilst imiquimod appeared to be effective in the short term, longer‐term data on effectiveness and safety were needed (Pepas 2015). Neither of these previous reviews had the scope to compare medical and surgical interventions. Since the previous Cochrane reviews were published, several new studies have been registered with online trial registries and one ongoing study has published results. Therefore, an updated review of both surgical and non‐surgical approaches was needed to determine which treatments are the most effective, safe and acceptable for uVIN, and to guide future research into treatment options for this increasingly common condition.
Objectives
To determine which interventions are the most effective, safe and tolerable for treating women with high‐grade VIN (uVIN).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) preferentially. Where we did not identify RCTs for treatment comparisons, we included non‐randomised studies (NRSs) with concurrent comparison groups, namely quasi‐randomised trials, non‐randomised trials, prospective and retrospective cohort studies, and case series of 20 or more women. We excluded case‐control studies, uncontrolled observational studies and case series of fewer than 20 women. In order to minimise selection bias we only included studies that used statistical adjustment for baseline case mix (age, VIN grade, lesion size/focality, immune system status) using multivariate analysis.
Types of participants
Women aged over 18 years with a confirmed histological diagnosis of uVIN, VIN 2 or 3, high‐grade VIN or vulval HSIL, which was either unifocal or multifocal. We excluded studies of women with a histological diagnosis of Paget's disease, vulval carcinoma and VIN 1.
Types of interventions
1. Surgical interventions
Excision (including wide local excision and simple vulvectomy)
Ablation (e.g. CO₂ laser vaporisation, CUSA, cryotherapy)
2. Medical interventions
Immune modulating drugs, e.g. imiquimod
Antiviral drugs, e.g. cidofovir
HPV vaccines
Other drug treatments, e.g. I3C
3. Other interventions, e.g. photodynamic therapy
We included studies comparing any, or combinations, of the above interventions with each other or no active intervention (observation only or placebo).
Types of outcome measures
Primary outcomes
Response to treatment (based on clinical or histological, or clinical and histological assessment of resolution, regression, persistence or progression of VIN)
Recurrence of VIN on long‐term follow‐up
Progression to vulval cancer
Secondary outcomes
HPV‐DNA persistence
Quality of life, as measured by a validated scale, e.g. European Quality of Life Index Version 5D (EQ‐5D) (EuroQoL 1990)
Sexual function, assessed using a validated tool, e.g. the Sabbatsberg sexual self rating scoring system (Garrat 1995; Naransingh 2000)
Control of local symptoms (pain, pruritis, erosion/ulceration, superficial dyspareunia, other); and systemic symptoms (fatigue, headache, other)
-
Severe adverse events classified according to CTCAE 2006:
direct surgical morbidity (death within 30 days; injury to bladder, ureter, vascular system, small bowel or rectum; wound healing; febrile morbidity; haematoma; local infection; indwelling catheter)
surgically related systemic morbidity (chest infection, thromboembolic event (deep vein thrombosis and pulmonary embolism), cardiac event (cardiac ischaemia and cardiac failure), cerebrovascular accident)
drug‐ or photodynamic therapy‐related toxicity, including pain, ulceration, skin reactions, fatigue and other effects
long‐term pain
recovery: unscheduled re‐admission to hospital, delayed discharge
other
Treatment discontinuation or dose reductions
Patient satisfaction (as measured by investigators)
Due to differences in types of interventions, not all outcomes apply to all interventions, e.g. response to treatment is usually assessed for medical not surgical treatments.
Search methods for identification of studies
We did not apply language restrictions to any of the searches.
Electronic searches
Please refer to the methods of the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers (CGNOC) Group (http://gnoc.cochrane.org/). The following electronic databases were searched:
Cochrane Gynaecological Cancer Review Group Trial Register;
Cochrane Central Register of Controlled Trials (CENTRAL);Issue 8, 2015
MEDLINE to September 2015
EMBASE to September 2015
The MEDLINE, EMBASE and CENTRAL search strategies aimed to identify all RCTs and NRSs involving interventions in women with high‐grade VIN (Appendix 1; Appendix 2; Appendix 3). In addition, we identified studies for classification on PubMed and, using the 'related articles' feature, we carried out further searches for published articles.
Searching other resources
Unpublished and grey literature
We searched www.controlled‐trials.com/rct, www.clinicaltrials.gov, www.cancer.gov/clinicaltrials and the World Health Organization (WHO) International Clinical Trials Registry Platform (www.who.int/ictpr) for ongoing trials. We approached the principal investigators of ongoing trials to confirm trial end dates and, where appropriate, to obtain unpublished data.
Reference lists and correspondence
We checked the reference lists of included studies and contacted experts in the field to identify further reports of trials.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to the reference management database Endnote® (EndNote 2013). Theresa Lawrie (TL) removed duplicates and those studies that clearly did not meet the inclusion criteria; two review authors (TL and Andy Bryant (AB)) independently examined the remaining references. We obtained copies of the full papers of 54 potentially relevant references. Two review authors assessed the eligibility of retrieved papers independently (TL and AB or Manas Chakrabarti (MC)). We resolved disagreements by discussion or by appeal to a fourth review author (Andy Nordin (AN)). We documented reasons for excluding studies.
Data extraction and management
We recorded the following data from included studies on a pre‐designed Microsoft Excel® spreadsheet:
author, year of publication and journal citation (including language)
country
setting
inclusion and exclusion criteria
study design and methodology
-
study population:
total number of participants enrolled;
total number of participants analysed;
mean (standard deviation (SD)) or median (range) age of participants;
proportion of participants with recurrent uVIN;
proportion of participants with previous anogenital neoplasia;
proportion of smokers (previous and current);
proportion immunocompromised
-
VIN details:
VIN terminology used
proportion of high‐grade lesions (uVIN)
proportion of lesions HPV‐DNA positive
lesion size (mean (SD) or median (range))
proportion of unifocal and multifocal lesions
intervention details: surgical (excision/ablation), medical (immune modulators/antivirals/vaccine/other), photodynamic therapy, or other versus observation/control
risk of bias in study (see below)
duration of follow‐up
-
outcomes – response to treatment, quality of life, sexual function, symptom assessment and adverse events:
for each outcome: outcome definition (with diagnostic criteria if relevant); sample size; missing participants; number of participants allocated to each intervention group
for scales: unit of measurement, upper and lower limits, and whether high or low score is good
We extracted outcome data as follows:
For dichotomous outcomes (e.g. adverse events or number of participants with disease recurrence), we extracted the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed at endpoint, in order to estimate a risk ratio (RR).
For continuous outcomes (e.g. subjective pain), we extracted the final value and standard deviation of the outcome of interest and the number of participants assessed at endpoint in each treatment arm at the end of follow‐up at one year, in order to estimate the mean difference (MD; if trials measured outcomes on the same scale) or standardised mean differences (SMD; if trials measured outcomes on different scales) between treatment arms and the standard error.
We extracted both unadjusted and adjusted statistics for RCTs, where reported, and adjusted statistics only for non‐RCTs in accordance with inclusion criteria. Where possible, all data extracted were those relevant to an intention‐to‐treat analysis, in which participants were analysed in the groups to which they were assigned. We noted the time points at which outcomes were collected and reported. We resolved differences between review authors by discussion or by appeal to a third review author when necessary.
We managed data in the same way as for the two previous Cochrane reviews of medical and surgical interventions for high‐grade VIN (Pepas 2015 and Kaushik 2014 respectively); therefore, where possible, we shared previously extracted data from studies included in these separate reviews with this new review.
Assessment of risk of bias in included studies
We assessed the risk of bias of included RCTs using Cochrane's tool and the criteria specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) (Appendix 4). This included assessment of:
sequence generation;
allocation concealment;
blinding;
incomplete outcome data;
selective reporting of outcomes;
other possible sources of bias.
For non‐randomised controlled studies, provided study findings were adjusted for important differences in baseline characteristics, we assessed the risk of bias for primary outcomes using the Cochrane 'Risk of bias' tool for non‐randomised studies of interventions (ACROBAT‐NRSI) (Sterne 2014) (Appendix 5):
confounding;
selection of participants;
measurement of interventions;
departure from interventions;
missing data;
measurement of outcomes;
selective reporting of outcomes.
Two review authors applied the 'Risk of bias' tools independently and resolved differences by discussion or by appeal to a third review author. We excluded NRSs assessed as having a critical risk of bias. We summarised results in risk of bias summaries for RCTs and included NRSs. We interpreted and graded the results of meta‐analyses in light of the findings with respect to risk of bias.
Measures of treatment effect
We used the following measures of the effect of treatment:
For dichotomous data, we used the RR with 95% confidence intervals (CIs).
For continuous data, we used the MD or SMD between treatment arms with 95% CIs.
Dealing with missing data
We did not impute missing outcome data. Where possible we attempted to contact trial authors to request missing data.
Assessment of heterogeneity
We assessed heterogeneity between studies in each meta‐analysis by visual inspection of forest plots: by estimation of the percentage heterogeneity between trials that cannot be ascribed to sampling variation (Higgins 2003), by a formal statistical test of the significance of the heterogeneity (Deeks 2001), and, where possible, by subgroup analyses. If there was evidence of substantial heterogeneity, we investigated and reported the possible reasons for this.
Assessment of reporting biases
If there were 10 or more studies in meta‐analyses we planned to investigate reporting biases (such as publication bias) using funnel plots; however, there were fewer than 10 studies in all meta‐analysis.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014), using random‐effects models with inverse variance weighting for all meta‐analyses (DerSimonian 1986). Results are presented as the average treatment effect with its 95% CI, and the estimates of T² and I². We planned multiple‐treatments meta‐analyses to synthesise studies making different comparisons of interventions. However, due to a lack of data for several key comparisons, this was not possible. In addition, the data from NRSs could not be pooled and are presented as a narrative.
We created a 'Summary of findings' table in RevMan 2014 using the GRADE approach (GRADE 2008). For assessments of the overall quality of evidence for each outcome that included pooled data from RCTs only, we downgraded the evidence from 'high quality' by one level for serious (or by two for very serious) study limitations (risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias. We graded evidence from sound NRSs as low quality, and downgraded this to very low quality for serious limitations. We included the following outcomes in the 'Summary of findings' table:
Response to treatment
Recurrence
Progression to vulval cancer
Severe adverse events
Subgroup analysis and investigation of heterogeneity
It was not possible to perform subgroup analyses according to lesion size and focality. We considered factors such as age, stage, HPV DNA status, immune status, length of follow‐up and risk of bias status in interpretation of any heterogeneity. When we identified substantial heterogeneity, we investigated it using sensitivity analyses and considered whether an overall summary was meaningful.
Sensitivity analysis
We performed sensitivity analysis by excluding studies at high risk of bias.
Results
Description of studies
Results of the search
We conducted electronic searches in June 2014 (3352 records), on 30 March 2015 (171 records) and on 1 September 2015 (118 records), which yielded a total of 3641 de‐duplicated records. We searched Medline from 1946 to August week 3 2015 and Embase from 1980 to week 35 2015. After excluding obviously irrelevant records on title and abstract, a shortlist of 246 records remained. On further screening by two review authors (TL and AB), we identified 54 records for full‐text retrieval and classification. Two authors (TL and AB or MC) classified 14 full texts (pertaining to 11 studies) as included studies, and excluded 39 full texts (pertaining to 38 studies). One conference abstract of a NRS remains unclassified whilst we await publication of the full study manuscript (Satmary 2013). See Figure 1. Searches of clinical trial registries identified five ongoing trials (EUCTR2008‐008251‐42‐NL; EUCTR2011‐003134‐13‐NL; ISRCTN98495886; NCT01861535; NTR1526).
1.
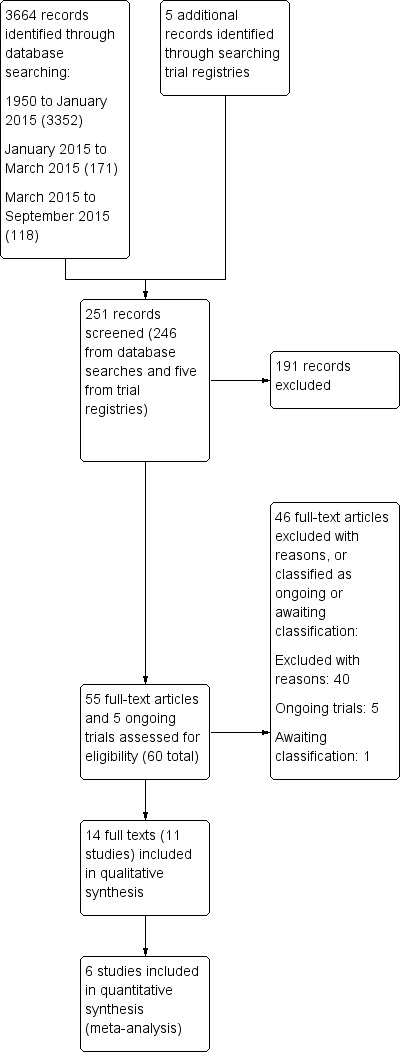
Study flow diagram (searches to September 2015).
Included studies
We included six RCTs (Mathiesen 2007; Naik 2006; Sterling 2005; Tristram 2014; Van Seters 2008; von Gruenigen 2007), and five NRSs (Fehr 2001; Fehr 2013; Leufflen 2013; van Esch 2013; Vlastos 2002).
RCTs
Five RCTs evaluated medical treatments and one evaluated surgical treatments as follows:
Mathiesen 2007 (31 participants), Sterling 2005 (21 participants) and Van Seters 2008 (52 participants) evaluated topical imiquimod versus placebo;
Tristram 2014 (180 participants) evaluated topical cidofovir versus imiquimod;
Naik 2006 (13 participants) evaluated different doses of the natural compound I3C; and
von Gruenigen 2007 (30 participants) evaluated carbon dioxide (CO2) laser vaporisation versus ultrasonic surgical aspiration (CUSA).
Mathiesen 2007, Sterling 2005 and Van Seters 2008 were conducted in Denmark, the United Kingdom (UK) and the Netherlands, respectively, and were double‐blinded, placebo‐controlled trials. The Sterling 2005 trial was published as an abstract only, with scant information. We contacted the authors in October 2010 for a previous review (Pepas 2015); however, we did not receive any further details. Tristram 2014 was an open‐label, multi‐centre, phase 2 trial conducted in 32 centres in the UK. Naik 2006 was a randomised, open‐label trial conducted in a single centre in Gateshead, UK, in which participants were randomised to receive one of two different dosage regimens of I3C without a placebo control. von Gruenigen 2007 was conducted in the United States of America (USA) and included 110 participants with vulval or vaginal dysplasia, of whom 30 were classified as having VIN 2/3 by the investigators and are included in this review.
Patient characteristics
All six trials randomised women with histologically proven VIN. The investigators of all RCTs used the older histological definitions, namely VIN 2 or 3 (Mathiesen 2007; Tristram 2014; Van Seters 2008; von Gruenigen 2007), or high‐grade VIN (Naik 2006; Sterling 2005). None of the studies used the term uVIN or HSIL. The proportion of women with HPV DNA detected were reported for Mathiesen 2007, Tristram 2014, and Van Seters 2008 as 58%, 84% and 96%, respectively, with test results missing for a few women in Mathiesen 2007 and Tristram 2014. One HPV DNA positive woman in the imiquimod arm of Van Seters 2008 had co‐existing lichen sclerosis and one had a histological diagnosis of VIN 1. Sterling 2005 reported that "HPV was detected in almost all women...with the majority shown to harbour HPV 16". HPV DNA status was not reported in Naik 2006. Limited data on 30 women with VIN2/3 were provided to us by trial authors of von Gruenigen 2007 (this trial also included women with VIN 1 and vaginal intraepithelial neoplasia (VAIN)) on request in 2010 and it is not clear what proportion of these women were HPV DNA‐positive. Data on the separate characteristics of these 30 participants were not available.
There were no significant differences in most reported baseline characteristics between the study groups of these RCTs. Women in most studies had a mean or median age of between 45 and 50 years old, except for Van Seters 2008, in which the median age of enrolled women was between 39 and 44 years. In Mathiesen 2007, Naik 2006, Tristram 2014 and Van Seters 2008, active smokers accounted for approximately 80%, 75%, 60% and 88% of the samples, respectively. The proportion of active smokers was not reported in Sterling 2005.
Nine women (three in the cidofovir arm and six in the imiquimod arm) were 'immunocompromised' in Tristram 2014, and two women in Van Seters 2008 had received corticosteroid cream prior to enrolment in the study. Immune deficiency or immunosuppressive treatment were exclusion criteria for Van Seters 2008 and Mathiesen 2007, respectively. Participants in Sterling 2005 were described as immunocompetent. Naik 2006 similarly noted that women were immunocompetent and there was no significant difference in menopausal status between women randomised to the two study arms.
The proportion of women with recurrent VIN lesions was 46% and 71% in Tristram 2014 and Van Seters 2008, respectively, and multifocal lesions were present in 51%, and 100% of participants in these trials, respectively. In addition, 40% and 62% of women in Tristram 2014 and Van Seters 2008 had previous anogenital neoplasia, respectively. In Mathiesen 2007, 29% of participants had multifocal lesions (five of 21 in the imiquimod group and four of 10 in the control group). Multifocal lesions occurred in three out of 12 women (25%) in Naik 2006. Fifty‐three per cent of all participants in von Gruenigen 2007 had received prior therapy for intraepithelial disease and most (93%) had multifocal disease. Focality and previous VIN were not reported in Sterling 2005.
However, significantly more women reported vulval pain at baseline in the imiquimod group compared with the placebo group of Van Seters 2008. Similarly, more women reported vulval pain at baseline in the cidofovir group of the Tristram 2014 trial, compared with the imiquimod group.
Interventions
Mathiesen 2007, Sterling 2005 and Van Seters 2008 randomised participants to receive either topical imiquimod 5% cream or placebo. In Mathiesen 2007, 21 women received imiquimod and 10 received placebo. All participants applied topical treatment for 16 weeks. The regimen involved application once a week for two weeks, twice a week during the following two weeks and, if tolerated, three times a week for the last 12 weeks. The endpoint of the study was two months after the end of treatment (24 weeks from randomisation).
In Van Seters 2008, 26 women received imiquimod and 26 received placebo. The women applied the treatment overnight twice a week for a period of 16 weeks. They were advised to use topical sulphur precipitate 5% in zinc oxide the day after treatment application to avoid superinfection. In both these trials, women were reviewed every fourth week and a post‐treatment biopsy was taken after six months (24 weeks from randomisation). Further assessments were performed at seven months and 12 months following treatment, after which the randomisation code was revealed.
In Sterling 2005, 15 women received imiquimod and six received placebo. It was not possible to ascertain the frequency of application, however active treatment continued for 16 weeks. Histological assessment was carried out eight weeks after the start of treatment and four weeks after the completion of treatment (20 weeks from randomisation).
Tristram 2014 randomised women to receive either 1% cidofovir gel or topical 5% imiquimod cream self applied overnight three times a week for a maximum of 24 weeks. Women were assessed at 6, 12, 18 and 24 weeks during treatment. Post‐treatment assessment was either six weeks after the end of treatment or six weeks after a complete response or disease progression. Two biopsy specimens were taken to assess histological response and test for HPV DNA. Women with a complete response were followed up every six months (6, 12, 18, 24 months) to the end of the study; however, at the time of writing, only the six‐week follow‐up results were available. Cross‐over to the other modality was allowed for women who failed to respond to the primary randomised treatment.
In Naik 2006, of the women completing the trial (three women dropped out, one could not access the medication and two did not attend the six‐month follow‐up), six were randomised to receive I3C 200 mg/day and seven received 400 mg/day. Vitamin C was also administered at the discretion of the treating clinician and five patients were prescribed this. Participants were reviewed at six weeks, three months and six months. Histological assessment was performed at six months (24 weeks).
von Gruenigen 2007 compared CO2 laser vaporisation with CUSA. CO2 laser surgery was performed to a depth of tissue destruction of 1 mm in non‐hairy vulval regions and 3 mm in hairy regions. CUSA was performed using the Cavitron Ultrasonic Surgical Aspirator Excel System (Valley‐lab, Boulder, Colorado, USA), which is a handheld device that vibrates and aspirates tissue to the reticular layer of the dermis. Procedures were performed in an outpatient setting, with participants given standard discharge instructions regarding postoperative care.
Outcomes
Response to treatment was the primary outcome in all RCTs, with the exception of von Gruenigen 2007. Histological response was determined by a repeat biopsy either from the lesion or lesions, if still present, or from the area where a lesion had been at initial assessment, when it had regressed entirely. Clinical response was varyingly defined as a reduction of the size of the lesion(s) at vulvoscopic assessment.
Van Seters 2008 classified clinical responses as either a complete response or partial response. Partial responses were further subdivided into a strong partial response (76% to 99% reduction in lesion size) or a weak partial response (26% to 75% reduction in lesion size), or no response (reduction in lesion size of 25% or less). Histological response was described as change from high‐grade VIN to a lower grade or complete clearance. Mathiesen 2007 and Sterling 2005 both defined responses as either complete response, which was defined as complete histological and clinical clearance, partial response (> 50% clearance) and no response (< 50% clearance). Tristram 2014 defined response according to adapted RECIST (Response Evaluation Criteria in Solid Tumours) criteria, whereby a partial response was at least a 30% decrease in the sum of the diameters of target lesions, taking as reference the baseline sum diameters, and progressive disease is at least a 20% increase in the sum of the diameters, or the appearance of one or more new lesions (Eisenhauer 2009). Naik 2006 commented on the size of the lesions and histological assessments without grouping responses further.
The only trial to report progression to invasive cancer 12 months after randomisation was Van Seters 2008, which also described the proportion of initially HPV‐positive women who cleared the virus at the end of the study period, and measured quality of life by means of validated questionnaires administered at baseline, 20 weeks and 12 months. The questionnaires used to assess quality of life were: the mental health scale of the Medical Outcomes Study 36‐Item Short‐Form General Health Survey (ranging from 0 to 100, with higher numbers indicating a better health‐related quality of life); the overall quality of life scale of the European Organisation for Research and Treatment of Cancer (EORTC) quality of life questionnaire (QLQ‐C30) used to assess generic and cancer‐specific health‐related quality of life; and the EORTC QLQBR23 to assess body image and sexuality.
Outcomes in von Gruenigen 2007 were recurrence after one year of follow‐up, pain, scarring, wound healing and adverse effects. Pain was assessed using a visual analogue scale one week after treatment.
Participants in Tristram 2014, Mathiesen 2007 and Van Seters 2008 were asked to keep a diary of compliance with treatment and side effects. Adherence was reported as an outcome in Tristram 2014 and was assessed at six weeks and 24 weeks in terms of the median number of applications up to those time points. All studies reported side effects, with the exception of Sterling 2005. Tristram 2014 graded adverse effects according to CTCAE 2006 criteria, whereas Van Seters 2008 did not grade most adverse effects, but distinguished erythema and erosion as mild‐to‐moderate or severe. Naik 2006 asked women to report symptoms of pruritus and pain using a visual analogue scale at recruitment and at each subsequent visit.
NRSs
Two or more treatment options were assessed in these five studies, most of which have critical design limitations (see Assessment of risk of bias in included studies) for the evaluation of relative effectiveness:
Fehr 2001 was a prospective feasibility study of photodynamic therapy (15 women with VIN 3) from a university hospital in Switzerland conducted between 1997 and 1999, compared with a retrospective cohort as controls (from 1992 to 1998) that included 37 women with VIN 3 who underwent laser vaporisation (30 procedures) or surgical excision (27 procedures). The photodynamic therapy intervention involved the use of 10 g of 10% ALA gel spread over the entire vulva. The vulva was then covered with a nonadherent dressing. Light application was performed using a dye laser; thereafter, women were advised to apply silver sulfadiazine cream after sitz baths twice a day. Women in the control group underwent one or more procedures and the mean duration of follow‐up was 35 months, compared with 12 months in the photodynamic therapy group. Women undergoing laser vaporisation were more likely than those undergoing photodynamic therapy to have multifocal disease at baseline (77% versus 60%; P value = 0.08). Authors performed multivariate analysis by treatment modality for the outcome 'disease‐free survival at 12 months after treatment'. Other study outcomes, including pain and response to treatment, were assessed for the photodynamic therapy group only.
Fehr 2013 was a retrospective study of a series of 411 women with high‐grade VIN or VAIN who were treated at four colposcopy clinics in Switzerland between 1977 and 2011. Women underwent laser vaporisation (270 women),surgical excision (114 women), vulvectomy (19 women) and other treatments, e.g. photodynamic therapy or imiquimod (eight women). Mean follow‐up time was 85 months and primary outcomes were biopsy‐proven recurrence (≥ 12 months) and progression to vulval cancer. Multifocality at baseline was 25%; however, baseline characteristics were not reported separately for the treatment groups. Multivariate analyses were performed and multinomial logistic regression models (stepwise backward) were used to control for potential confounders including age, immune status, focality, grade, type of treatment and smoking behaviour on discrete outcomes.
Leufflen 2013 was a retrospective study of a series of 50 women with high‐grade VIN (41 with uVIN and nine with dVIN) who underwent treatment at a French hospital between 1995 and 1999, involving surgery (cold knife or laser including partial or total vulvectomy; 24 women) or laser vaporisation (26 women). Follow‐up was every four to six months after treatment for two years; thereafter, it varied. Younger age, uVIN, smoking, multifocality and multicentricity were all significantly more common in the laser vaporisation group at baseline (P value < 0.5). Surgery was the first choice in treating unifocal VIN and dVIN, and laser vaporisation was used for all women with multicentric VIN. Mean follow‐up time was 5.6 years and primary outcomes were response to treatment, recurrence and progression to vulval cancer. Risk of recurrence according to patient characteristics and treatments was estimated using Cox proportional hazards regression models. Recurrence‐free survival was compared using the log‐rank test.
van Esch 2013 was a retrospective study of 73 women with uVIN who were treated at a Dutch university hospital between 1990 to 2012. Treatments included surgical excision (31 women),laser vaporisation (25 women), imiquimod (six women) and laser vaporisation plus excision (eight women). As with Leufflen 2013, more unifocal lesions were treated with excision compared with other modalities (P value = 0.105). Median follow‐up time was 49 months and primary outcomes were recurrence, recurrence‐free survival and progression to vulval cancer. uVIN was multifocal in 44% of women. Baseline characteristics were not reported separately for the treatment groups; however, recurrence‐free survival was adjusted for multifocal disease, smoking, HPV status, immune status and body mass index (BMI).
Vlastos 2002 was a retrospective study of a series of 62 women with multifocal VIN 2 and VIN 3 with no previous treatment who underwent treatment between 1995 and 1999 in the USA. Treatments included loop electrosurgical excision procedure (LEEP) (20 women), surgical excision (22 women) and laser vaporisation (20 women). Median follow‐up time was 37 months and outcomes included recurrence, time to recurrence, response rates and progression to vulval cancer. Multifocality was present in 50%, 68% and 75% of women in the LEEP, excision and laser vaporisation groups, respectively. Authors used Cox proportional hazards methods and logistic regression to compare the significance of variables predicting response to treatment.
For more details of these included studies see Characteristics of included studies.
Excluded studies
We excluded 39 studies for the following reasons:
an uncontrolled observational study (four studies; Abdullah 2015; Caglar 1986; Daayana 2009; Wright 1987);
a NRS of more than one treatment with no appropriate statistical adjustment for confounders (27 studies; Bar‐Am 1993; Ben David 1996; Brown 2005; Bruchim 2007; Cabrera Diaz 2011; Caglar 1982; Ferenczy 1994; Fiorica 1988; Frega 2013; Hillemanns 2006; Jones 1994; Jones 2005; Leuchter 1984; Li 2005; Penna 2002; Ribeiro 2012; Rodolakis 2003; Shafi 1989; Sideri 1999; Steiner 2012; Van Beurden 1998; Wallbillich 2012; Wee‐Stekly 2013; Wright 1987; Yu 2014; Zawislak 2006; Zhang 2009);
type of participants did not match review criteria (three studies; Garland 2013; Joura 2012; Krebs 1986).
a review (one study; Iavazzo 2008);
a case report (one study; Ballester 2012);
a cross‐over RCT with critical design flaws in which all participants received the active treatment and were analysed as a single cohort (one study; Spirtos 1990).
a letter to the editor (two studies; Bakri 1995; van Bogaert 2015).
Risk of bias in included studies
RCTs
We assessed RCTs using the Cochrane 'Risk of bias' tool in Appendix 4. We judged two imiquimod versus placebo trials to be at a low risk of bias overall (Mathiesen 2007; Van Seters 2008). We judged the trials Naik 2006 and Sterling 2005 to be at potentially high risk of bias as they provided scant methodological details on which to base risk of bias judgements. Sterling 2005 has only been published in abstract form so we were unable to properly assess its risk of bias. To our knowledge the complete details of this study remain unpublished. Both Tristram 2014 and von Gruenigen 2007 were open‐label trials; however, we did not consider the lack of blinding as a serious design limitation in these trials (see Figure 2) and we judged these trials to be at low risk of bias overall.
2.
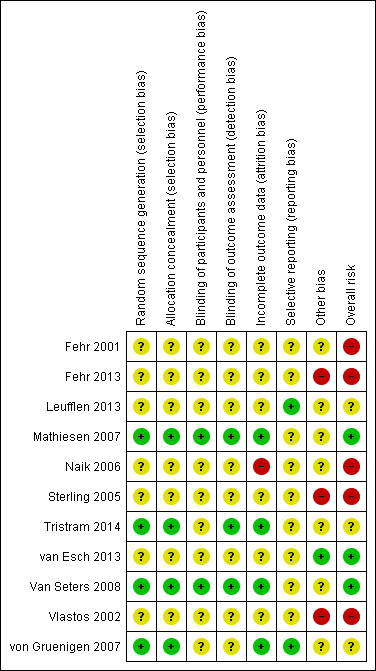
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Four trials reported the method of generation of the sequence of random numbers used to allocate women to treatment arms and this allocation was adequately concealed (Mathiesen 2007; Tristram 2014; Van Seters 2008; von Gruenigen 2007). These trials also had minimal attrition.
Mathiesen 2007 and Van Seters 2008 were both double‐blind trials in which the participants, healthcare professionals and outcome assessors were blinded; Tristram 2014 was an open‐label trial, and blinding was unclear in the remaining four trials. It was not clear whether all trials reported all the outcomes that they assessed and it was unclear whether any other bias may have been present. Intention‐to‐treat data were not reported in full in Tristram 2014. We were unsuccessful in obtaining these data from the investigators.
NRSs
We assessed NRSs using the Cochrane 'Risk of bias' tool for non‐randomised studies of interventions (ACROBAT‐NRSI; Appendix 5). We considered most to have serious design limitations and we judged them to be at high (Fehr 2001; Fehr 2013; Vlastos 2002), or unclear (Leufflen 2013), risk of bias for assessing the effectiveness of treatment on VIN recurrence. We considered van Esch 2013 to be at a low risk of bias relative to the other NRSs (see Characteristics of included studies).
Fehr 2001 compared prospective cases of women undergoing photodynamic therapy with retrospective cases of laser vaporisation or surgical excision. In this study, confounders were reported separately by treatment group and adjusted for in multivariate analysis. However, no effect estimates of the multivariate findings are reported and the authors stated that the power of the analysis was low. Intervention status was not well defined as 15 women in the control group had more than one type of procedure, i.e. the unit of analysis was procedures, not women. Therefore, re‐treatment of a patient on recurrence with the same or a different intervention was entered as a separate intervention/patient. The duration of follow‐up differed substantially between the photodynamic therapy and control arms. There was insufficient information to make a firm judgement but this study was potentially at a high risk of bias due to possible selective reporting bias, missing information and the other design limitations.
Fehr 2013 compared laser vaporisation with surgical excision, vulvectomy and other treatments. We had risk of bias concerns about the measurement of interventions and outcomes in this study. Women who received both laser vaporisation and excision in the first year were analysed in the laser vaporisation group and the actual number of women who received both treatments was not reported. Women in the laser vaporisation group may therefore have had more extensive treatment during the course of the first year and, therefore, this group may not be comparable to the excision only group. Early recurrences and progressions occurring in the first year were not counted but rather considered to have had inappropriate or insufficient initial treatment requiring immediate re‐treatment. We also had concerns about selective reporting bias with possible multiple intervention outcome testing to produce the odds ratios (ORs) for recurrence and progressions. The number of women included in these analyses was not reported in Table 2 of the article, and the findings for excision versus laser vaporisation was reported, not surgery (excision + vulvectomy) versus laser vaporisation, as in Table 1 of the article. These limitations might have biased results in the direction of laser vaporisation. We therefore judged this study to be at a high risk of bias.
Leufflen 2013 compared laser vaporisation with surgical excision. Interventions and outcomes were clearly defined, but participants included nine women with dVIN, which was more likely to be treated with surgical excision (seven out of nine women). Women in the laser vaporisation group were more likely to be younger and to have uVIN, multicentric and multifocal disease. We assessed risk of bias in relation to the outcome recurrence‐free survival as unclear risk as the report does not state which baseline variables were adjusted for, and it was not clear whether the reported recurrence‐free survival was adjusted for multifocality and the other important variables that differed significantly between the treatment groups.
van Esch 2013 compared surgical excision with laser vaporisation, excision and laser vaporisation combined, and imiquimod. Outcomes were clearly defined. Women with unifocal lesions were more likely to have excision than other modalities; however, this and other main confounders (smoking, HPV status, immune status and BMI) were adjusted for in a multivariate analysis of recurrence‐free survival. Precise effect estimates were not reported and analyses were probably underpowered. However, we judged this study to be at a relatively low risk of bias for the multivariate recurrence‐free survival analysis.
Vlastos 2002 compared LEEP, surgical excision and laser vaporisation. In this study report, the text stated that all women had multifocal disease, which was contradicted by tabulated data, indicating that 10/20, 15/22 and 15/20 women in the LEEP, excision and laser vaporisation groups had multifocal VIN, respectively. These data on multifocality were not statistically significantly different between groups (P value = 0.27). The authors stated that they controlled for age, age at first intercourse and number of sexual partners; however, it was unclear whether findings were adjusted for multifocality and other important factors. We considered the potential for bias in the measurement of outcomes to represent a serious risk as there was no information on how recurrence was monitored or measured. Median duration of follow‐up was significantly longer for the laser vaporisation group compared with the other treatment groups and there were more recurrences in this group. Precise data on the effect of treatments on recurrence were missing from the report. We judged this study to be at a potentially high risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3
Evidence from randomised controlled trials (RCTs)
1. Topical imiquimod versus placebo
Response to treatment at five to six months after randomisation
Three RCTs assessed 104 participants (Mathiesen 2007; Sterling 2005; Van Seters 2008). At five to six months after randomisation, women in the imiquimod group were more likely to have experienced a complete or partial response to treatment than women in the placebo group (risk ratio (RR) 11.95, 95% confidence interval (CI) 3.21 to 44.51; high‐quality evidence). There were 36/62 and 0/42 complete responders in the topical imiquimod and placebo groups, respectively (RR 14.40, 95% CI 2.97 to 69.80; Analysis 1.1).
1.1. Analysis.
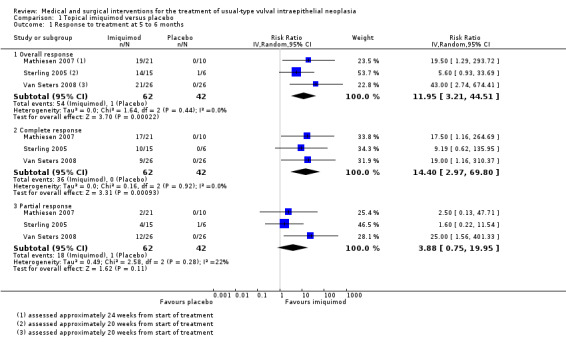
Comparison 1 Topical imiquimod versus placebo, Outcome 1 Response to treatment at 5 to 6 months.
Response to treatment at 12 months after randomisation
One study reported 12‐month data (Van Seters 2008). Two women in the imiquimod group and three women in the placebo group were lost to follow‐up. Overall response (complete or partial response) to treatment at 12 months was higher in the imiquimod group than the placebo group (RR 9.10, 95% CI 2.38 to 34.77; Analysis 1.2; moderate‐quality evidence). There were 9/24 and 0/23 complete responders in the topical imiquimod and placebo groups, respectively. Women in the imiquimod arm of this trial were followed up for at least five years (median 7.2 years) and, out of the nine complete responders, one developed vulval intraepithelial neoplasia (VIN) recurrence four years after randomisation (Terlou 2011); the others remained disease‐free. The authors noted that lesion size at study entry was smaller in these complete responders.
1.2. Analysis.
Comparison 1 Topical imiquimod versus placebo, Outcome 2 Response to treatment at 12 months.
Progression to vulval cancer at 12 months after randomisation
Only one trial reported this outcome (Van Seters 2008). There was no difference in rates of progression to vulval cancer at 12 months between the imiquimod and placebo groups (1/24 versus 2/23 events, respectively; RR 0.50, 95% CI 0.05 to 5.18; Analysis 1.3; low‐quality evidence).
1.3. Analysis.
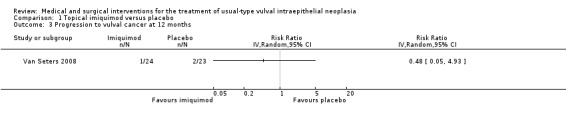
Comparison 1 Topical imiquimod versus placebo, Outcome 3 Progression to vulval cancer at 12 months.
HPV DNA persistence
One trial reported HPV clearance, which occurred significantly more often in the imiquimod group than the placebo group. Eleven out of 26 women in the imiquimod group and 24/26 women in the placebo group had HPV DNA persistence, respectively; RR 0.43, 95% CI 0.26 to 0.72; Analysis 1.4; high‐quality evidence).
1.4. Analysis.
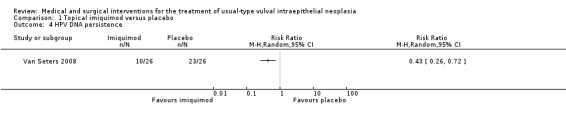
Comparison 1 Topical imiquimod versus placebo, Outcome 4 HPV DNA persistence.
Quality of life
One study reported quality of life and did not find any differences between the treatment and the placebo groups in any of the quality of life outcomes, including self reported health‐related quality of life, body image or sexuality scores at baseline, 20 weeks and at 12 months (Van Seters 2008). None of the other trials reported on quality of life.
Severe adverse events (SAE)
Severe adverse events were not reported; however, one study with 52 participants reported 'side effects' data for fatigue, headache, erythema, erosion, oedema, pain and pruritis (Van Seters 2008); however, the severity of these was not graded according to CTCAE 2006. Mathiesen 2007 (31 participants) reported 'local side effects' (Analysis 1.12), and Sterling 2005 did not report adverse event data.
1.12. Analysis.
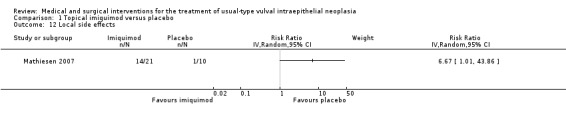
Comparison 1 Topical imiquimod versus placebo, Outcome 12 Local side effects.
There was no difference between imiquimod and placebo for the following outcomes (all low‐quality evidence):
Fatigue: RR 2.00, 95% CI 0.69 to 5.83 (Analysis 1.5).
Headache: RR 1.41, 95% CI 0.51 to 3.85 (Analysis 1.6).
1.5. Analysis.
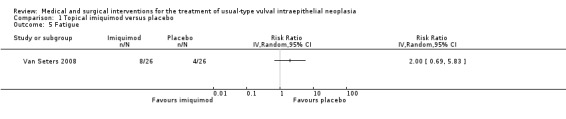
Comparison 1 Topical imiquimod versus placebo, Outcome 5 Fatigue.
1.6. Analysis.
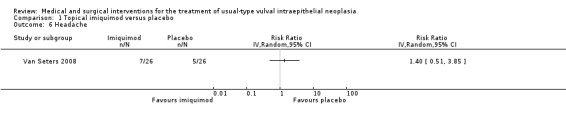
Comparison 1 Topical imiquimod versus placebo, Outcome 6 Headache.
The following outcomes were different and favoured the placebo group (moderate‐quality evidence):
Erythema (redness): RR 10.00, 95% CI 2.60 to 38.50 (Analysis 1.7).
Erosion/ulceration: RR 3.40, 95% CI 1.47 to 7.84 (Analysis 1.8).
Oedema: RR 23.00, 95% CI 1.43 to 371.00 (Analysis 1.9).
Pain or pruritis (itchiness): RR 3.43, 95% CI 1.80 to 6.52 (Analysis 1.10; Analysis 1.11).
Local side effects: RR 6.67, 95% CI 1.01 to 43.86 (Analysis 1.12).
No side effects: RR 0.08, 95% CI 0.01 to 0.55 (Analysis 1.13).
1.7. Analysis.
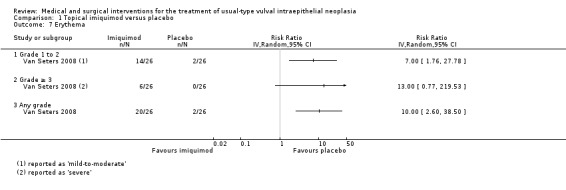
Comparison 1 Topical imiquimod versus placebo, Outcome 7 Erythema.
1.8. Analysis.
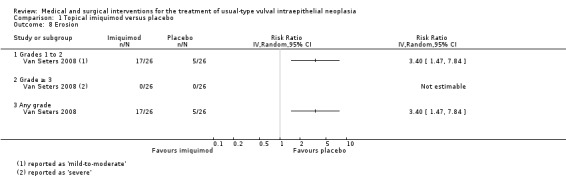
Comparison 1 Topical imiquimod versus placebo, Outcome 8 Erosion.
1.9. Analysis.
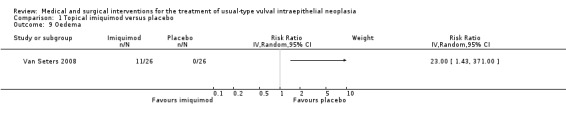
Comparison 1 Topical imiquimod versus placebo, Outcome 9 Oedema.
1.10. Analysis.
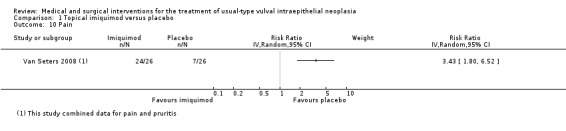
Comparison 1 Topical imiquimod versus placebo, Outcome 10 Pain.
1.11. Analysis.
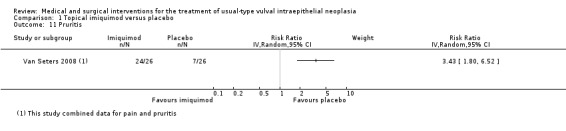
Comparison 1 Topical imiquimod versus placebo, Outcome 11 Pruritis.
1.13. Analysis.
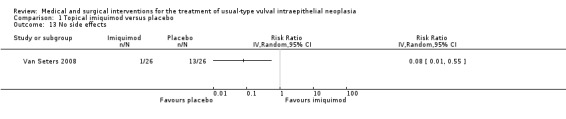
Comparison 1 Topical imiquimod versus placebo, Outcome 13 No side effects.
Dose reductions
Women who received imiquimod were more likely to require dose reductions compared with the placebo group (two studies, 83 participants; RR 7.77, 95% CI 1.61 to 37.36; I2 = 0%; Analysis 1.14).
1.14. Analysis.
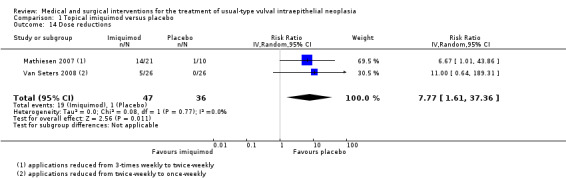
Comparison 1 Topical imiquimod versus placebo, Outcome 14 Dose reductions.
Other review outcomes were not reported.
2. Topical imiquimod versus topical cidofovir
One randomised trial with 180 participants evaluated this comparison, comparing topical 5% imiquimod cream with 1% cidofovir gel applied three times per week for 24 weeks (Tristram 2014). The results are reported below. We considered most of the resulting evidence to be of a moderate quality, mainly due to imprecision.
Response to treatment at six months after randomisation
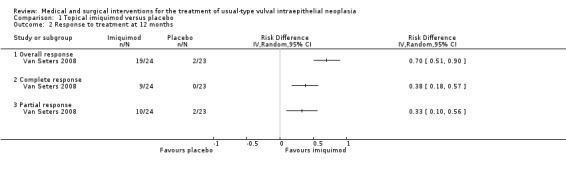
There was no difference in overall response between the imiquimod and cidofovir study groups (180 participants; RR 0.92, 95% CI 0.73 to 1.18; Analysis 2.1; moderate‐quality evidence), or for complete and partial response data separately. This phase two study was not powered to demonstrate a difference in response rates between the two treatment modalities. The histologically confirmed complete response rate at six months was 45% (41/91) and 46% (41/89), respectively (RR 1.00, 95% CI 0.73 to 1.37).
2.1. Analysis.
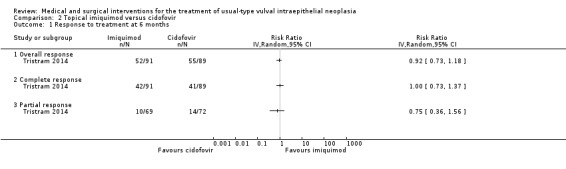
Comparison 2 Topical imiquimod versus cidofovir, Outcome 1 Response to treatment at 6 months.
Response to treatment at 12 months after randomisation
Twelve‐month follow‐up of all participants was incomplete at the time of writing. However, interim findings suggested that complete responders in both treatment groups at six months were likely to sustain the complete response at 12 months. A sustained complete response at 12 months was reported in 25/32 women (78%) who responded at six months in the imiquimod group and 20/23 women (87%) in the cidofovir group. Thus, 82% of 55 women with a complete response at six months after randomisation sustained the complete response at 12 months. More data are expected from this trial.
Progressive disease
There was no difference between imiquimod and cidofovir study groups in the proportion of women experiencing an increase in lesion size despite treatment (180 participants; RR 1.30, 95% CI 0.55 to 3.11, Analysis 2.2; low‐quality evidence).
2.2. Analysis.
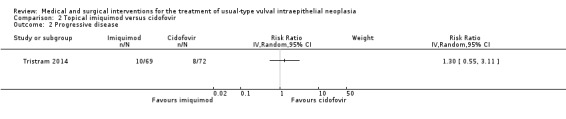
Comparison 2 Topical imiquimod versus cidofovir, Outcome 2 Progressive disease.
Investigators also reported that fewer women in the imiquimod group developed new lesions during treatment compared with the cidofovir group (RR 0.55, 95% CI 0.28 to 1.09; Analysis 2.3, not statistically significant). However, due to flaws in the data collection process they were uncertain whether these lesions occurred at the site of treatment, therefore it was not possible to establish whether these data represented new or progressive disease.
2.3. Analysis.
Comparison 2 Topical imiquimod versus cidofovir, Outcome 3 New lesions during treatment.
HPV DNA persistence
These data were not collected in this trial.
Severe adverse events (SAEs)
There was no difference between imiquimod and cidofovir for the following outcomes (all moderate‐quality evidence):
Total SAEs: RR 1.26, 95% CI 0.88 to 1.81 (Analysis 2.4).
Vulval pain: RR 1.08, 95% CI 0.93 to 1.25 (Analysis 2.5).
Pruritis: RR 1.03, 95% CI 0.90 to 1.17 (Analysis 2.6).
Erosion: RR 0.88, 95% CI 0.63 to 1.22 (Analysis 2.7).
Skin reactions: RR 0.88, 95% CI 0.46 to 1.68 (Analysis 2.10).
2.4. Analysis.
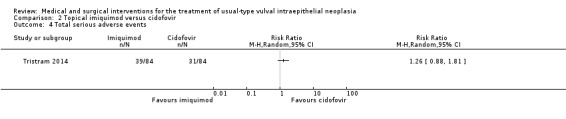
Comparison 2 Topical imiquimod versus cidofovir, Outcome 4 Total serious adverse events.
2.5. Analysis.
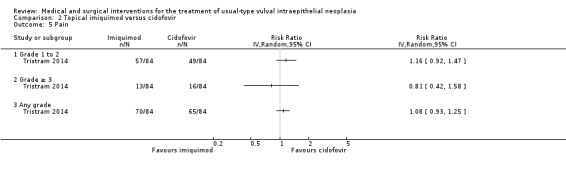
Comparison 2 Topical imiquimod versus cidofovir, Outcome 5 Pain.
2.6. Analysis.
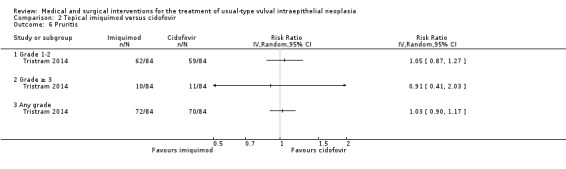
Comparison 2 Topical imiquimod versus cidofovir, Outcome 6 Pruritis.
2.7. Analysis.
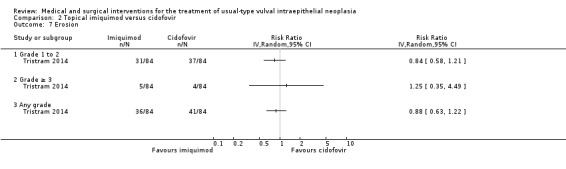
Comparison 2 Topical imiquimod versus cidofovir, Outcome 7 Erosion.
2.10. Analysis.
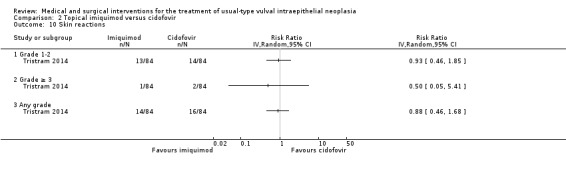
Comparison 2 Topical imiquimod versus cidofovir, Outcome 10 Skin reactions.
The following outcomes favoured cidofovir over imiquimod:
Fatigue: RR 1.25, 95% CI 1.02 to 1.55 (Analysis 2.8).
Headache: RR 1.49, 95% CI 1.12 to 1.98 (Analysis 2.9).
2.8. Analysis.
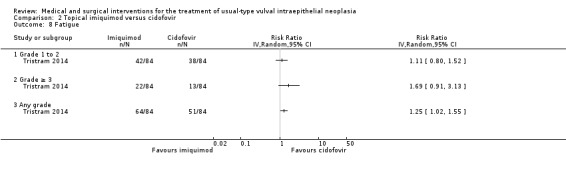
Comparison 2 Topical imiquimod versus cidofovir, Outcome 8 Fatigue.
2.9. Analysis.
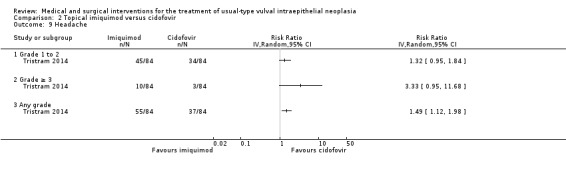
Comparison 2 Topical imiquimod versus cidofovir, Outcome 9 Headache.
Treatment discontinuation
There was no difference between the imiquimod and cidofovir study groups in the proportion of women requiring a dosage reduction or treatment cessation (15/89 versus 11/87, respectively; RR 1.33, 95% CI 0.65 to 2.74; Analysis 2.11; moderate‐quality evidence).
2.11. Analysis.
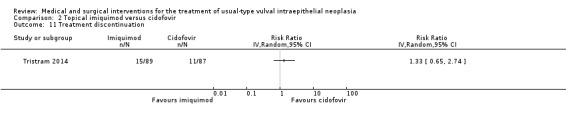
Comparison 2 Topical imiquimod versus cidofovir, Outcome 11 Treatment discontinuation.
Other review outcomes were not reported.
3. Laser vaporisation versus Cavitron ultrasonic surgical aspiration (CUSA)
Only one trial, including 30 women, contributed data for this comparison (von Gruenigen 2007).
Disease recurrence after one year of follow‐up
There was no difference in disease recurrence after one year of follow‐up between women who had laser vaporisation and those who had CUSA (RR 1.53, 95% CI 0.56 to 4.15; Analysis 3.1), even when the findings were adjusted for age, history of dysplasia and smoking status (adjusted odds ratio 0.68, 95% CI 0.27 to 1.83; low‐quality evidence). The trial lacked statistical power due to the small number of women in each group and the low number of observed events.
3.1. Analysis.
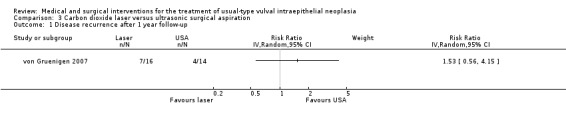
Comparison 3 Carbon dioxide laser versus ultrasonic surgical aspiration, Outcome 1 Disease recurrence after 1 year follow‐up.
Severe adverse events
The severity of these adverse events was not graded according to CTCAE 2006. There was no difference between laser surgery and CUSA in the following reported outcomes:
Pain: mean difference (MD) 1.70, 95% CI ‐26.80 to 23.40 (Analysis 3.2).
Scarring: 5/16 versus 0/14 in the laser and CUSA groups, respectively.
Dysuria or burning on micturition: RR 0.66, 95% CI 0.18 to 2.44 (Analysis 3.3).
Adhesions: 1/16 versus 0/14 in the laser and CUSA groups, respectively.
Infection (yeast, urinary tract infection, other): RR 0.88, 95% CI 0.14 to 5.42 (Analysis 3.4).
Abnormal discharge: RR 1.75, 95% CI 0.18 to 17.29 (Analysis 3.5).
Eschar: RR 0.88, 95% CI 0.14 to 5.42 (Analysis 3.6).
3.2. Analysis.
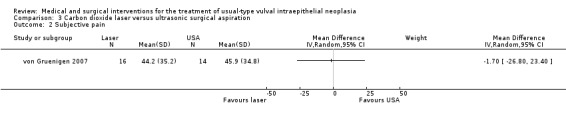
Comparison 3 Carbon dioxide laser versus ultrasonic surgical aspiration, Outcome 2 Subjective pain.
3.3. Analysis.
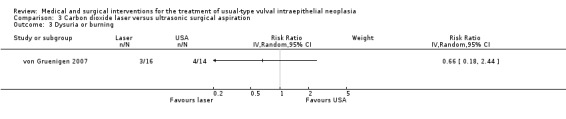
Comparison 3 Carbon dioxide laser versus ultrasonic surgical aspiration, Outcome 3 Dysuria or burning.
3.4. Analysis.
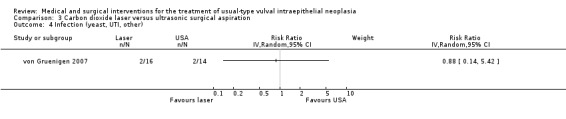
Comparison 3 Carbon dioxide laser versus ultrasonic surgical aspiration, Outcome 4 Infection (yeast, UTI, other).
3.5. Analysis.
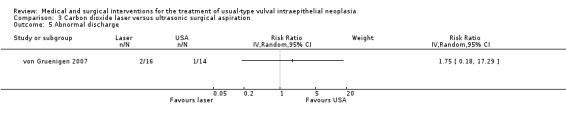
Comparison 3 Carbon dioxide laser versus ultrasonic surgical aspiration, Outcome 5 Abnormal discharge.
3.6. Analysis.
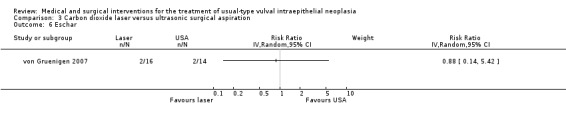
Comparison 3 Carbon dioxide laser versus ultrasonic surgical aspiration, Outcome 6 Eschar.
Other review outcomes were not reported.
4. Indole‐3‐carbinol (I3C) 200 mg/day versus 400 mg/day
The trial of Naik 2006 reported that there were no differences in any of the outcomes between the six women taking 200 mg/day of I3C and the six on 400 mg/day. Both groups reported significant improvement in symptoms of pruritus and pain. However, nine out of 10 women followed up for six months still had high‐grade VIN after biopsy. The authors did not report to which of the two doses these women had been randomised. The trial reported only one case of mild bowel upset, which was in a woman who received the high‐dose regimen.
Evidence from non‐randomised studies (NRSs)
5. Photodynamic therapy (PDT) versus laser vaporisation and surgical excision
One study evaluated this comparison (Fehr 2001). Comparative data for disease‐free survival (DFS) were described in the study report without effect estimates, as follows: "Analysis of DFS after treatment revealed no statistically significant difference between patients treated with PDT and patients treated with laser evaporation or local excision (P=0.67)." "In the laser group, 52% were recurrence free at 12 months versus 60% in the local excision group and 50% in the PDT group." "In … multivariate analysis, treatment modality was not associated with reduced DFS." We judged this to be very low‐quality evidence, due to design limitations.
6. Laser vaporisation versus excision
Three NRSs evaluated this comparison with respect to VIN recurrence and progression to vulval cancer (Fehr 2013; Leufflen 2013; van Esch 2013). Treatment effect findings for these outcomes were reported in different ways and it was not possible to pool these data.
Recurrence
Leufflen 2013 reported recurrence‐free survival at one year follow‐up as time to recurrence (laser vaporisation versus excision; hazard ratio (HR) 5.9, 95% CI 1.3 to 26.3; P value < 0.01), and the findings favoured excision over laser vaporisation as initial VIN treatment. It is not clear whether the recurrence‐free survival data reported in Leufflen 2013 were adjusted for all important confounders (including differentiated‐type VIN (dVIN) and multifocality); therefore this finding should be interpreted with caution. The overall recurrence rate one year after treatment was 22% (11 women) and the mean time to recurrence following either treatment was 21.7 months (95% CI 15.1 to 21.3 months).
In Fehr 2013, 123/411 women (30%) experienced recurrent disease at least one year after initial diagnosis. The authors reported that the relative odds of recurrence favoured laser vaporisation over excision (excision versus laser vaporisation; odds ratio (OR) 1.79, 95% CI 1.11 to 2.91; P value = 0.017).
In van Esch 2013, 37/73 women (51%) experienced recurrence at a median time of 14 months (range, 1 to 168 months) after initial treatment. Sixteen recurrences occurred out of 33 women in the excision group (48.5%) and 14 occurred out of 25 women in the laser vaporisation treated group (56%). Overall, time to recurrence was longer in the excision group and, on univariate analysis, recurrence‐free survival favoured excision as first treatment. However, on multivariate analysis (adjusted for multifocal disease, smoking, human papilloma virus (HPV) status, immune status and body mass index) there was no difference in recurrence‐free survival according to treatment type (P value = 0.142), which also included imiquimod and laser plus excision options. The authors presented the data as a Kaplan Meier curve with a P value and not as a comparative effect estimate (HR).
The evidence from Fehr 2013 and Leufflen 2013 of treatment effect on recurrence is contradictory and of a very low quality, mainly due to design limitations of the NRSs. The evidence in van Esch 2013 of no difference between the types of treatment in recurrence‐free survival is of a better quality than the other two studies and we graded this evidence as higher quality than that from the other two NRSs (low‐quality evidence). However, this finding lacks power and should be interpreted with caution.
Progression to vulval cancer
In Fehr 2013 and van Esch 2013, 24 women (5.8%) and 11 women (15.1%) developed invasive disease during follow‐up, respectively. The mean time to invasive disease was 82 months (standard deviation (SD) 74; range 13 to 290 months) in Fehr 2013, and the median time to invasive disease was 71.5 months (range 9 to 259 months) in van Esch 2013. The risk of progression to invasive disease (PFS) did not differ significantly according to treatment type in either study (Fehr 2013: excision versus laser vaporisation; OR 2.09, 95% CI 0.89 to 5.37; P value = 0.126; and van Esch 2013, P value = 0.20). In van Esch 2013, only univariate Cox analysis was performed for PFS due to the small numbers involved. One woman (2%) in Leufflen 2013 developed invasive cancer 30 months after treatment. We considered the evidence on PFS overall for this treatment comparison to be of a very low quality due to design limitations.
Severe adverse events
Severe adverse events of treatment were not reported in these retrospective studies.
Other review outcomes were not reported.
7. Loop electrosurgical excision (LEEP) versus surgical excision and laser vaporisation
Recurrence
Only one NRS involving 62 women evaluated the relative effects of LEEP, conventional surgical excision and laser vaporisation on time to recurrence for 20, 22 and 20 women, respectively (Vlastos 2002). Overall, 15 women in the cohort experienced recurrences during follow‐up. Mean time to recurrence was 16 months, 15 months and 25 months for LEEP, excision and laser vaporisation, respectively, at a mean time to follow‐up of 20, 32 and 51 months, respectively. Recurrence‐free survival was not different between treatment types and was reported as a P value only (P value = 0.194) with the following text: "There were no statistically significant differences among the therapies for time to recurrence, whether compared…. as LEEP versus wide local excision (WLE), LEEP versus LV, and WLE versus LV." We judged this to be very low‐quality evidence. We did not include the recurrence rate data from this study, which favoured LEEP and excision over laser vaporisation, as these data did not appear to be adjusted for multifocality (which was less frequent in the LEEP group) and the laser vaporisation group was followed up for a significantly longer period of time.
Progression to vulval cancer
There were no cases of vulval cancer during follow‐up in any of the treatment groups in Vlastos 2002.
Severe adverse events
Adverse effect data were inadequately reported and could not be extracted. However, the authors also reported that LEEP was performed on an outpatient basis and was associated with statistically significantly less hospital stay than the other modalities (0/20 versus 9/22 versus 18/20 for LEEP, WLE and laser vaporisation, respectively; P value = 0.0001).
Other review outcomes were not reported.
8. Excision versus imiquimod
Limited data from van Esch 2013, which could potentially contribute to this comparison, were too sparse to be meaningful.
Discussion
Summary of main results
Five randomised controlled trials (RCTs) (297 women) evaluated medical interventions (imiquimod, cidofovir and I3C) and one RCT (30 women) compared two surgical modalities (laser vaporisation versus Cavitron ultrasonic surgical aspiration (CUSA)). Evidence derived from these RCTs ranged from low to high quality. No RCTs compared the standard of care (surgical excision) to medical treatments, other surgical modalities or photodynamic therapy. We therefore looked to five non‐randomised studies (NRSs) to provide some evidence on these comparisons. However, data from these NRSs were presented in different ways and meta‐analysis was not possible for the relevant review outcomes. Thus, we considered the evidence derived from these NRSs on the relative effects of these interventions on vulval intraepithelial neoplasia (VIN) recurrence and progression to vulval cancer to be of low to very low quality.
Medical interventions
Imiquimod versus placebo
Three trials involving 104 women contributed data to this comparison (Table 1). At five to six months from the start of treatment, women with usual‐type VIN (uVIN) who received imiquimod were more likely to achieve complete or partial clearance of lesions (risk ratio (RR) 11.95, 95% confidence interval (CI) 3.21 to 44.51; high‐quality evidence). Complete response at five to six months occurred in 36/62 women (58%) in the imiquimod group compared with 0/42 (0%) in the control group. Evidence from one study suggested that a complete response at six months was likely to be sustained at 12 months (complete response 9/24 (37.5%) in the imiquimod group versus 0/23 controls; RR 9.10, 95% CI 2.38 to 34.77) and longer, particularly in women with small VIN lesions. Imiquimod was associated with more local side effects than placebo, including localised pain, oedema, erythema and erosion. None of the women experiencing side effects discontinued treatment; rather, side effects were managed by reducing the number of applications. Only one trial reported progression to vulval cancer at 12 months, which occurred in one out of 24 women in the imiquimod arm and two out of 23 women in the placebo arm. The same trial also reported increased clearance of human papilloma virus (HPV) infection with imiquimod treatment. There was no difference in quality of life measures between groups in this trial.
Imiquimod versus cidofovir
Moderate‐quality evidence from one well‐conducted RCT involving 180 women suggested that cidofovir was as effective as imiquimod with respect to response rates at six months after randomisation (approximately 46% complete response overall), but the study was not powered to demonstrate a difference between the two treatment modalities and longer follow‐up data are still to be reported. Interim findings from 55 complete responders suggested that complete response may be sustained at 12 months in both treatment groups. Adverse effects occurred with similar frequency between the treatment groups, with the exception of fatigue and headache, which were more common with imiquimod (Table 2).
Other medical interventions
Based on the limited evidence from one small trial of 3‐indole‐carbinol, it is unclear whether this natural compound has any role to play in the treatment of VIN. We found no evidence on HPV vaccines to treat uVIN; however, we identified one ongoing trial of an HPV vaccine (Gardasil®) plus imiquimod versus imiquimod only EUCTR2008‐008251‐42‐NL.
Surgical interventions
Low‐quality evidence suggested that there may be little difference between excision and laser vaporisation procedures in the risk of VIN recurrence or progression to vulval cancer. However, these NRSs were underpowered to detect a difference. Rates of recurrence ranged from 22% to 51%, with the wide range probably due to methodological limitations and differences between included NRSs. The best NRS was at a low risk of bias and findings were adjusted for important confounders (van Esch 2013). Recurrence occurred in 51% of women (37/70) overall, at a median of 14 months, and was more common in multifocal than unifocal lesions (66% versus 34%). Vulval cancer occurred in 11 women (15.1%) overall at a median of 71.5 months (9 to 259 months); however due to the small number of events, multivariate analysis was not performed for this outcome. The risk of vulval cancer did not differ significantly between excision and laser vaporisation in any of the other NRSs included; again, events were too few for robust findings.
Medical interventions or photodynamic therapy versus surgical interventions
We found no evidence on the relative effectiveness of medical treatment options compared with surgical treatment options. Very low‐quality evidence from one NRS suggested that photodynamic therapy may be as effective as surgical options (laser vaporisation or excision) and more evidence is needed to support this finding.
Overall completeness and applicability of evidence
Currently, the evidence on the use of medical treatments, such as imiquimod and cidofovir, for uVIN is encouraging, but many uncertainties remain. Trials of imiquimod versus placebo excluded immunosuppressed women and it remains uncertain whether uVIN lesions in these women will respond to topical treatments as well as immunocompetent women. The trial of imiquimod versus cidofovir reported complete responses at six months after randomisation in three out of eight immunosuppressed women (37.5%) suggesting that these topical treatments may also benefit these women, but more evidence from long‐term studies is needed.
Most RCTs of medical interventions reported response rates and there were very few available data on the effect of treatments on the risk of vulval cancer. Only one RCT to date has followed complete responders for more than five years (Van Seters 2008), and the findings suggested that a complete and sustained response may be more likely with smaller lesions. However, due to the limited data available, we were unable to draw conclusions about possible differences in the effectiveness of medical treatment according to focality or lesion size. It remains unclear what the optimal dose regimens should be for medical treatment, what type of lesions are most likely to respond to treatment and how treatment‐related symptoms are best relieved. Medical treatments generally require treatment to be administered over a prolonged course of time, and may be associated with distressing treatment‐related side effects. Therefore, whilst the evidence suggests that they are reasonably well‐tolerated, more evidence is needed on quality of life and women's satisfaction with treatment relative to other treatment modalities.
Various investigators have expressed concerns that non‐excisional therapeutic options (including laser vaporisation, photodynamic therapy and topical treatments) may be associated with higher recurrence and progression rates, as reported rates of occult (microinvasive) vulval cancer following histological examination of excised VIN specimens range from 12% to 22 % (Husseinzadeh 1999; Modesitt 1998; Sideri 1999). This review did not find any evidence to address these concerns. Longer‐term data from the Tristram 2014 trial are awaited with interest.
The evidence comparing the effectiveness of laser vaporisation and surgical excision is conflicting and many uncertainties remain regarding the relative effectiveness of these treatments. This is largely because the choice of treatment in the included retrospective NRSs was usually determined by lesion characteristics, with surgical excision being favoured by clinicians for unifocal (and dVIN lesions), and laser favoured for multifocal lesions. Even though the investigators performed multivariate analysis, NRS quality varied and findings were inconsistent. We considered one NRS that found no statistically significant difference in recurrence rates between excision and laser vaporisation to be the best evidence for the relative effectiveness of these two modalities (van Esch 2013).
Alternative surgical procedures tested that might be as effective as surgical excision and laser vaporisation on recurrence rates included Cavitron ultrasonic surgical aspiration (CUSA) and loop electrosurgical excision (LEEP) procedures, but the evidence is sparse and the applicability uncertain. Limited evidence suggested that these alternative procedures may also have other benefits, such as lower costs, shorter hospital stay and a specimen yielded for histology.
Medical interventions versus surgical interventions
We found no evidence on the effectiveness of medical interventions relative to surgical interventions. However, we identified one ongoing Austrian trial assessing imiquimod versus surgery (excision or ablation) for uVIN, which is due to complete in September 2016. The investigators plan to recruit 110 women (NCT01861535).
Other interventions
Very low‐quality evidence on photodynamic therapy relative to surgical treatments suggested that photodynamic therapy may be as effective in treating uVIN. An ongoing trial of photodynamic therapy versus imiquimod for uVIN (or lichen sclerosis) may make an important contribution to the body of evidence (EUCTR2011‐003134‐13‐NL).
The role for natural compounds, e.g. IC3, SR‐T100 (solanum incanum extract) and sinecatechins (from green tea leaves), if any, in uVIN is still unknown. An ongoing study, ISRCTN98495886, is currently evaluating SR‐T100, a traditional Chinese herbal medicine that has purportedly been used to treat cancer for centuries and is reported to induce apoptosis in squamous carcinoma cells (Wu 2011) for VIN. Other ongoing trials are evaluating HPV vaccination in combination with imiquimod (EUCTR2008‐008251‐42‐NL; NTR1526), and photodynamic therapy versus imiquimod (EUCTR2011‐003134‐13‐NL) for women with uVIN.
Quality of the evidence
We graded the evidence on the effectiveness of imiquimod for achieving and sustaining a complete response as high and moderate quality, respectively, and the evidence for the effectiveness of cidofovir as moderate quality. Low‐quality evidence showed that excision and laser vaporisation were equally effective, and very low quality evidence showed that there was no difference in progression to vulval cancer between these two surgical modalities; however, the analyses lacked power. Low‐quality evidence suggested that CUSA was similarly effective to laser vaporisation, and very low‐quality evidence suggested that LEEP or photodynamic therapy were comparable to laser vaporisation and excision in preventing recurrence.
Potential biases in the review process
In the protocol we stated that we would only include NRSs that used statistical adjustment for baseline case mix using multivariable analyses. Extent of disease and multifocality were significant confounders of NRSs involving VIN treatment, as these variables often influenced the type of treatment chosen in practice. Hence, we excluded most of the comparative NRSs for not using appropriate statistical adjustment of confounders where important differences in baseline case mix were evident or suspected. Although it was not stated in the protocol, we extracted the log hazard ratio (HR) and its variance for time‐to‐event outcomes (e.g. time to recurrence), if reported instead of or as well as dichotomous outcomes in the NRSs. These data were reported as a narrative and could not be pooled.
In one included NRS (Vlastos 2002), the text stating that "Lesions...were multifocal in all patients" was contradicted by a table showing multifocal VIN as a variable with data indicating proportions of 50%, 68% and 75% for the LEEP, excisional and laser ablation interventions, respectively. We attempted to obtain clarity from the authors regarding these contradictory data but were unsuccessful. However, we gave the study the benefit of the doubt and assumed that the text was correct, thereby enabling its inclusion in the review in the absence of the authors controlling for multifocality. As mentioned, none of the NRSs contributed data to meta‐analysis and we judged this study to be at a high risk of bias.
We excluded one prospective NRS comparing 32 women treated with imiquimod and 36 treated with cold knife excision (Frega 2013), based on our inclusion criteria requiring adjustment of NRSs for baseline case mix, and an unsuccessful attempt to contact the authors for clarity. Women with immunosuppression were excluded and other baseline characteristics between the groups did not appear to be significantly different, except perhaps for VIN grade (81% of women in the imiquimod group had VIN 3 versus 61% in the excision group), which was positively associated with recurrence in this study. Had statistical adjustments been performed, the study might have made a greater contribution to the 'medical versus surgical' debate. After a five‐year follow‐up, recurrence plus treatment failure in the imiquimod group was 69% (22/32) compared with 45% (17/38) in the surgery group. Complete response was lower with imiquimod at 31% (10/32) versus 55% (21/38) in the surgery group (and lower than that reported in the included RCTs). Surgical conversion occurred in 53% of women (17/32) in the imiquimod group, with two women in the imiquimod group and three in the surgery group developing invasive cancer in spite of treatment.
We excluded an older RCT of 5‐fluorouracil (5‐FU) as maintenance treatment compared with no additional treatment following ablation/removal of the lesion/s in 90 women (Krebs 1986). Participants in this trial had vulval or vaginal HPV‐related lesions including condylomata accuminata, and the proportion of participants with vulval lesions and VIN 2/3 was not clear. Various previous treatments were used alone and in combination to achieve a complete response (including podophyllin, 5‐FU, cryotherapy, local excision and laser) before women were randomised to receive 5‐FU cream or no 5‐FU cream. Krebs 1986 reported a statistically significant difference in recurrences in favour of the 5‐FU maintenance group of this study (six in the treated group versus 17 in the untreated group), with 87% of recurrences detected within six months of follow‐up (mean follow‐up was 14.4 months). 5‐FU in the context of maintenance, applied once every two weeks for six months, was apparently well tolerated and no "undesirable side effects" were reported or observed. After much discussion, we excluded the trial on the basis that the participants did not clearly fulfil the inclusion criteria for this review. However, the combination of surgical treatment with 'maintenance' medical treatment has not been evaluated in any other trials of uVIN treatment, and so this trial is of interest.
We also excluded a study that evaluated both laser vaporisation and LEEP in the same participants with VIN 3 (Ferenczy 1994). In this study, half of each lesion was treated with one modality and the other half treated with the other modality. The investigators concluded that LEEP and laser vaporisation produced similar success rates; however, participants underwent multiple treatments with either modality and we could not attribute data to one modality or the other; therefore, we excluded this study. It is unlikely that including this study would have changed the assessment of the quality of the evidence on recurrence rates for LEEP versus laser vaporisation, which we judge to be very low‐quality evidence.
Agreements and disagreements with other studies or reviews
Interpretation of head‐to‐head comparisons of treatment effectiveness using NRSs is difficult as the clinical presentation of uVIN is very heterogeneous and the choice of treatment has usually been influenced by several factors, particularly lesion focality, lesion size and clinician/patient preferences. Among NRSs, unifocality was frequently associated with surgical excision, whereas multifocality was associated with combined interventions and/or medical or ablative interventions. Adjusting for baseline case mix helps to reduce the effect of such confounding but does not guarantee robust results.
In this review, both RCTs and NRSs consistently found that multifocality was the greatest risk factor for uVIN recurrence and progression to vulval cancer. Immunosuppression was also strongly associated with progression to vulval cancer in two of the included NRSs (Fehr 2013; van Esch 2013). As few women in the included RCTs of medical treatment were immunosuppressed, it is uncertain whether the findings on effectiveness apply to immunosuppressed women with uVIN and this needs further study.
As mentioned above, we excluded one NRS that did not adjust for baseline case mix (Frega 2013). In this study, women undergoing treatment for uVIN (imiquimod or surgery) were followed up for at least five years, and the overall findings favoured surgery over imiquimod. In the imiquimod arm, the complete response rate was initially 13/32 (41%) after one course of imiquimod, and then two partial responders achieved a complete response after a second course, which increased the complete response to 47%, which is similar to the rates reported in the included RCTs. However, five women in this study subsequently experienced uVIN recurrence and underwent surgery. The sustained response rate of 31% (10 out of 32 women) over the five‐year period was lower than that suggested by the long‐term follow‐up data of Van Seters 2008 of 37.5%. In addition, these data do not confirm the Van Seters 2008 findings, that a complete response is likely to be sustained, as a third of complete responders in Frega 2013 had uVIN recurrence. Women who sustained a complete response in Van Seters 2008 had small lesions. Factors that predict complete response to medical treatment need further exploration. In addition, these inconsistent findings highlight the importance of long‐term follow‐up of all women who undergo treatment for uVIN.
Another excluded retrospective study identified women who underwent treatment for HPV‐related cervical disease or had a diagnosis of vulval or vaginal HPV‐related disease from two large HPV vaccination RCTs (Joura 2012). The study assessed the relative impact of vaccination and placebo on subsequent HPV‐related disease (including cervical intraepithelial neoplasia (CIN), genital warts, vaginal intraepithelial neoplasia (VAIN) and VIN). However, it was not clear what proportion of women had uVIN at baseline or subsequently, as women with genital warts and/or VAIN were included. Among women who had a baseline diagnosis of vulval or vaginal disease (including warts), a reduced rate of any subsequent HPV‐related disease (including CIN and warts) was noted in the vaccination group compared with the placebo group (70/211 (20%) versus 163/422 (31%)) and the impact on vulval disease was driven mainly by a reduction in the incidence in genital warts. Rates of subsequent "VIN 2/3 or VAIN 2/3" were 2.6% and 3.4% for the vaccination and placebo groups, respectively. Although this study did not meet our inclusion criteria, its findings are of interest. They suggest that vaccination programmes may have an impact, not only on preventing HPV‐related disease, but also on improving outcomes in women with pre‐existing HPV‐related disease, such as uVIN.
We did not include trials of the effectiveness of prophylactic vaccinations on preventing uVIN as this was beyond the scope of the review. However, findings from a RCT comparing HPV vaccination plus imiquimod versus imiquimod only are awaited with interest (EUCTR2008‐008251‐42‐NL).
We did not find any comparative studies evaluating smoking cessation interventions to treat or prevent recurrence of uVIN. However, smoking is a known risk factor for the development of uVIN and, like van Esch 2013, we believe that smoking cessation interventions should be integral to uVIN management.
Authors' conclusions
Implications for practice.
Topical treatment (imiquimod or cidofovir) may effectively treat about half of usual‐type vulval intraepithelial neoplasia (uVIN) cases after a 16‐week course of treatment, but the evidence on whether this effect is sustained is limited, and the risk of progression to vulval cancer is not known. However, topical imiquimod and cidofovir appear to be well tolerated and may be favoured by some women over surgical treatment. Surgical treatment remains available for women who fail to respond to initial medical management.
There may be little difference between surgical excision and laser vaporisation in the risk of recurrence and progression. Women who undergo surgical treatment for uVIN have about a 50% chance of the condition recurring, irrespective of whether treatment is by excision or laser vaporisation, and the risk of progression to vulval cancer may be as high as 15%. We found insufficient evidence to show whether one method of surgical excision (cold knife, loop electrosurgical excision (LEEP), laser, Cavitron ultrasonic surgical aspiration (CUSA)) was better than another in terms of effectiveness, adverse effects and quality of life.
There is currently no evidence on how medical treatment compares with surgical treatment. Multifocal uVIN lesions are at a higher risk of recurrence and progression, and pose greater therapeutic dilemmas than unifocal lesions. If occult cancer is suspected despite a biopsy diagnosis of uVIN, surgical excision remains the treatment of choice. If occult cancer is not a concern, treatment needs to be individualised to take into account the site and extent of disease and a woman's preferences. All women undergoing treatment for uVIN require biopsies prior to treatment to exclude occult cancer and continued follow‐up after treatment to monitor for disease progression.
Implications for research.
Several ongoing randomised controlled trials (RCTs) are due to report results in the next three years and are likely to make important contributions to the body of evidence on uVIN treatment:
EUCTR2008‐008251‐42‐NL: imiquimod plus human papilloma virus (HPV) vaccination (Gardasil®) versus imiquimod alone
EUCTR2011‐003134‐13‐NL: photodynamic therapy versus imiquimod
ISRCTN98495886: epigallocatechin‐3 (green tea compound) versus placebo
NCT01861535: imiquimod versus surgery
NTR1526: HPV vaccination (synthetic HPV16 E6/E7 peptides) with imiquimod versus HPV vaccination without imiquimod
In particular, evidence from the trial of imiquimod versus surgery is eagerly awaited. RCTs in this disease are complex and challenging to conduct, due to the variable presentation of the disease, the challenges with treatment compliance, the subjectivity of clinical assessment particularly with regards to disease response, and the pain and discomfort associated with the diagnostic biopsies necessary to confirm disease response at any particular disease site. Due to the difficulty in conducting RCTs for this rare condition, and the anticipated reduction in the incidence of uVIN as a result of routine prophylactic HPV vaccination, further RCTs may not be justified. However, combined interventions may hold the key to the optimal treatment of this complex disease and studies comparing combined interventions with single interventions (e.g. surgery plus imiquimod versus surgery alone) on recurrence and progression would be of value. Longer‐term follow‐up of participants in recent and ongoing RCTs is needed. Factors that predict complete response to medical treatment need further exploration. In addition, there is a need for good qualitative research on the various treatment options for uVIN, with respect to women's satisfaction, quality of life and cosmetic results.
What's new
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
Acknowledgements
We thank Co‐ordinating Editor, Jo Morrison, for clinical and editorial advice; Trials Search Co‐ordinator, Jane Hayes, for designing the search strategy; and Managing Editors Gail Quinn and Clare Jess for their contribution to the editorial process. We would also like to thank Susan Cooper, Raj Naik and Philipp Harter for their contribution to the peer review process.
This project was supported by the National Institute of Health Research (NIHR) directly commissioned Cochrane Incentive funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service or the Department of Health.
Appendices
Appendix 1. MEDLINE search strategy
Ovid MEDLINE 1946 to Aug week 3 2015
(VIN or VIN2 or VIN3).mp.
(vulva* adj5 intraepithelial neoplasia).mp.
1 or 2
exp Vulva/
vulva*.mp.
4 or 5
exp Precancerous Conditions/
(pre‐cancer* or precancer*).mp.
dysplasia.mp.
unifocal.mp.
multifocal.mp.
exp Carcinoma in Situ/
carcinoma in situ.mp.
7 or 8 or 9 or 10 or 11 or 12 or 13
6 and 14
3 or 15
key: mp=title, original title, abstract, name of substance word, subject heading word
Appendix 2. EMBASE search strategy
Original search: EMBASE Ovid 1980 to week 35, 2015.
(VIN or VIN2 or VIN3).mp.
(vulva* adj5 intraepithelial neoplasia).mp.
1 or 2
exp Vulva/
vulva*.mp.
4 or 5
exp Precancer/
(pre‐cancer* or precancer*).mp.
dysplasia.mp.
unifocal.mp.
multifocal.mp.
exp Carcinoma in Situ/
carcinoma in situ.mp.
8 or 11 or 7 or 13 or 10 or 9 or 12
6 and 14
3 or 15
key: mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name
Appendix 3. CENTRAL search strategy
Original search: CENTRAL Issue 3, 2010. Updated search: Issue 8, 2015.
(VIN or VIN2 or VIN3):ti,ab,kw
(vulva* near/5 intraepithelial neoplasia):ti,ab,kw
(#1 OR #2)
MeSH descriptor Vulva explode all trees
vulva*
(#4 OR #5)
MeSH descriptor Precancerous Conditions explode all trees
pre‐cancer* or precancer*
dysplasia
unifocal
multifocal
MeSH descriptor Carcinoma in Situ explode all trees
carcinoma in situ
(#7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13)
(#6 AND #14)
(#3 OR #15)
key: ti,ab,kw = title, abstract, keyword
Appendix 4. Methods for assessing bias in randomised trials
Two review authors independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
high risk of bias (e.g. open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel;
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses that we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; 'as treated' analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
[Note: You may like to specify the level of missing data used to assess that a study is at low risk of bias, for example, a cut‐off point of 20%, which is the most commonly used value. You may need to specify different levels of missing data that will be assessed as adequate for different outcomes or sets of outcomes. See Handbook section 8.13.]
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as:
low risk of bias (where it is clear that all of the study's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study's pre‐specified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by 1 to 5 above)
We described for each included study any important concerns we had about other possible sources of bias, e.g. extreme baseline imbalance. We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ see 'Sensitivity analysis'.
Appendix 5. Methods for assessing bias in non‐randomised studies
ACROBAT‐NRSI includes criteria for assessing bias in NRSs due to the following:
Confounding: potential confounders include age, VIN grade, smoking, and focality. We will assess the study to be at low risk of bias, if all of these characteristics are reported and no differences between the groups are present; at moderate risk of bias, if at least two of these characteristics are reported and adjusted for; at serious risk of bias, if at least one of the characteristics is not appropriately measured or adjusted for; and at critical risk of bias, if confounding characteristics are not controlled for. If no information is available as to whether confounding is present, we will assess as 'No information on which to base judgement'.
Selection of participants: if all eligible participants were included in the study and follow‐up and start of the intervention coincided for all participants, we will assess the study to be at low risk of bias for this item; if selection into the study may have been related to the intervention or the outcome, or, if start of intervention and follow‐up did not coincide, but appropriate adjustments were made, we will assess the study to be at moderate risk of bias; if selection into the study was related to intervention and outcome or start of intervention and follow‐up did not coincide, and a potentially important amount of follow‐up time is missing, we will assess the study to be at serious risk of bias; and if selection into the study was strongly related to intervention and outcome or start of intervention and follow‐up did not coincide and a substantial amount of follow‐up time is likely to be missing, we will assess the study to be at critical risk of bias. If no information is available as to whether bias in the selection of participants is present, we will assess as 'No information on which to base judgement'.
Measurement of interventions: if the intervention status is well defined and based solely on the information collected at the time of the intervention, we will assess the study as low risk of bias for this item; if intervention status was well defined, but some aspects of the assignments were determined retrospectively, we will assess the study as at moderate risk of bias; if intervention status was not well defined, or major aspects of the assignments were determined in a way that could have been affected by the knowledge of the outcome, we will assess the study as at serious risk of bias; if an extremely high amount of misclassification of intervention status is present or suspected, we will assess the study as at critical risk of bias. If no definition of intervention or explanation of the source of information about the intervention is given, we will assess as 'No information on which to base judgement'.
Departures from intended interventions: if no bias is expected due to departures from the intended intervention, we will assess the study to be at low risk of bias for this item; if bias due to departures from the intended intervention is expected, and switches, co‐interventions and some risk of bias items with intervention fidelity are appropriately measured and adjusted for, we will assess the study to be at moderate risk of bias for this item; if departures, switches, co‐interventions or risk of bias items with implementation fidelity are apparent and not adjusted for in the analyses, we will assess the study to be at serious risk of bias; and if substantial departures from the intended intervention are present, we will assess the study to be at critical risk of bias. If no information is reported on whether there is departure from the intended intervention, we will assess as 'No information on which to base judgement'.
Missing data: if data are reasonably complete or proportions and reasons for missing data were similar across intervention groups, we will assess the study to be at low risk of bias; if proportions or reasons for missing participants differ across interventions and missing data were not addressed in the analysis, we will assess the study as at moderate risk of bias; if proportions or reasons for missing participants differed substantially across interventions and were not appropriately addressed, we will assess the study to be at serious risk of bias; and if critical differences existed between interventions with regard to missing data that were not or could not be addressed through analysis, we will assess the study as at critical risk of bias. If no information is reported about missing data or the potential for data to be missing we will assess as 'No information on which to base judgement'.
Measurement of outcomes: if the methods of outcome assessment were comparable across studies and unlikely to be influenced by knowledge of the intervention or the outcomes assessors were unaware of the intervention received, we will assess the study to be at a low risk of bias for this item; if the measurement of outcome was only minimally influenced by knowledge of the intervention, we will assess the study to be at moderate risk of bias; if the method of outcome assessment was not comparable or was subjective, we will assess the study at serious risk of bias; and, if outcome measurement was so different between intervention groups that reasonable comparison was not possible, we will assess the study as having critical risk of bias. If no information is reported about the methods of outcome measurement, we will assess as 'No information on which to base judgement'.
Selective reporting of outcomes: if there is clear evidence that reported results correspond to all intended/pre‐specified outcomes, we will assess the study to be at low risk of bias for this item; if the outcomes reported are consistent with an a priori plan and there is no indication that selection of the cohort or subgroups for analysis and reporting was on the basis of results, we will assess the study to be at moderate risk of bias for this item; if outcome measures are inconsistent, or there is a high risk of selective reporting from multiple analyses, or the cohort or subgroup is selected from a larger study and appears to be reported on the basis of results, we will assess the study as at serious risk of bias; and, if there is evidence or strong suspicion of selective reporting, or if unreported results are likely to be substantially different from reported results, we will assess the study to be at critical risk of bias. If there is too little information on which to make a judgement, we will assess as 'No information on which to base judgement'.
Data and analyses
Comparison 1. Topical imiquimod versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response to treatment at 5 to 6 months | 3 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Overall response | 3 | 104 | Risk Ratio (IV, Random, 95% CI) | 11.95 [3.21, 44.51] |
| 1.2 Complete response | 3 | 104 | Risk Ratio (IV, Random, 95% CI) | 14.40 [2.97, 69.80] |
| 1.3 Partial response | 3 | 104 | Risk Ratio (IV, Random, 95% CI) | 3.88 [0.75, 19.95] |
| 2 Response to treatment at 12 months | 1 | Risk Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Overall response | 1 | Risk Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Complete response | 1 | Risk Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Partial response | 1 | Risk Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Progression to vulval cancer at 12 months | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 4 HPV DNA persistence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Fatigue | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6 Headache | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 7 Erythema | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Grade 1 to 2 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Grade ≥ 3 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Any grade | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Erosion | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Grades 1 to 2 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Grade ≥ 3 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Any grade | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Oedema | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 10 Pain | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 11 Pruritis | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 12 Local side effects | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 13 No side effects | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 14 Dose reductions | 2 | 83 | Risk Ratio (IV, Random, 95% CI) | 7.77 [1.61, 37.36] |
Comparison 2. Topical imiquimod versus cidofovir.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Response to treatment at 6 months | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Overall response | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Complete response | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Partial response | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Progressive disease | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 3 New lesions during treatment | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4 Total serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Pain | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 5.1 Grade 1 to 2 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Grade ≥ 3 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Any grade | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pruritis | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Grade 1‐2 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Grade ≥ 3 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 Any grade | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Erosion | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 7.1 Grade 1 to 2 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Grade ≥ 3 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 Any grade | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Fatigue | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Grade 1 to 2 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Grade ≥ 3 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Any grade | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Headache | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 9.1 Grade 1 to 2 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Grade ≥ 3 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Any grade | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Skin reactions | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Grade 1‐2 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Grade ≥ 3 | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.3 Any grade | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Treatment discontinuation | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Carbon dioxide laser versus ultrasonic surgical aspiration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease recurrence after 1 year follow‐up | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 2 Subjective pain | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3 Dysuria or burning | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 4 Infection (yeast, UTI, other) | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5 Abnormal discharge | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 6 Eschar | 1 | Risk Ratio (IV, Random, 95% CI) | Subtotals only |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fehr 2001.
| Methods | Prospective feasibility study with retrospective cohort as controls conducted at University Hospital in Zurich, Switzerland | |
| Participants | 15 women with biopsy proven VIN3 were recruited for the prospective study between August 1997 and December 1999. 37 women with biopsy proven VIN3 were identified from clinic records between 1992 and 1998. Altogether these women underwent 57 procedures (30 LV and 27 excision) Mean age: 40.5 (8.3) PDT; 36.5 (8.1) LV; 43.7 (11.6) excision (P value = 0.06) Recurrent disease: 12/15 PDT; 15/30 LV; 13/27 excision (P value = 0.10) Multifocality: 9/15 PDT; 23/30 LV; 13/27 excision (P value = 0.08) Immunosuppression: 3/15 PDT; 6/30 LV; 4/27 excision (P value = 0.86) Smoking: 11/15 PDT; 22/30 LV; 19/27 excision (P value = 0.96) No other baseline variables were reported |
|
| Interventions |
Group 1 (15 women): PDT with 10 g of 10% ALA gel spread over entire vulva. The vulva was covered with a non‐adherent dressing. Light application was performed using a dye laser. Women were advised to apply silver sulfadiazine cream after sitz baths twice a day thereafter. Group 2 (30 procedures): LV Group 3 (27 procedures): local excision Mean follow‐up was 12 months (SD 8 months) for group 1 and 35 months (SD 19 months) for groups 2 and 3 |
|
| Outcomes | Disease‐free survival at 12 months Pain and response was assessed for group 1 only |
|
| Notes | A feasibility and comparative efficacy NRS. Authors hypothesised that PDT has advantages over LV and excision because it may preserve vulval morphology, reduce pain and healing time because normal tissue is spared due to selective photosensitisation of the lesions, multiple lesions can be treated simultaneously by the application of the photosensitiser and light to the entire vulva, and microscopic or subclinical lesions can be treated simultaneously, thereby decreasing relapse rate. Persistence of VIN3 occurred in 4/15 women in the PDT group. Findings suggested an unpredictable occurrence of pain during and after treatment which may have been related to extent of disease. Authors concluded that the technique needed refinement but had potential due to short healing time and vulval preservation. We assessed risk of bias for the outcome DFS "Analysis of DFS after treatment revealed no statistically significant difference between patients treated with PDT and patients treated with laser evaporation or local excision (P=0.67) (Fig. 1)." "In the laser group, 52% were recurrence free at 12 months versus 60% in the local excision group and 50% in the PDT group." "In … multivariate analysis, treatment modality was not associated with reduced DFS." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not a RCT |
| Allocation concealment (selection bias) | Unclear risk | See other bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | See other bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See other bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | See other bias |
| Selective reporting (reporting bias) | Unclear risk | See other bias |
| Other bias | Unclear risk | Confounders were reported separately by intervention group and multivariate analysis was reportedly done to adjust for them, however no effect estimates of the multivariate findings are reported ‐ it is simply stated that DFS was not associated with treatment modality on multivariate analysis. It is stated also stated that the power of the analysis was low. Intervention status was not well defined as 15 women in the control group (groups and 3) had more than one type of procedure, i.e. the unit of analysis was procedures, not women. Therefore, re‐treatment of a patient on recurrence with the same or a different intervention was entered as a separate intervention/patient. Duration of follow‐up differed between the PDT and control arms with follow‐up of 2 years and 7 years, respectively. |
| Overall risk | High risk | Insufficient information to make a judgement but probably at a high risk of bias due to missing information and confounding |
Fehr 2013.
| Methods | Retrospective study of 411 women with high‐grade VIN or VAIN who were treated at 4 colposcopy clinics in Switzerland between 1977 and 2011 Multivariate analyses were performed and multinomial logistic regression models (stepwise backward) were used to control for potential confounders including age, immune status, focality, grade, type of treatment and smoking behaviour on discrete outcomes |
|
| Participants | Women with biopsy‐proven high‐grade VIN or VAIN. Only patients with a follow‐up of 12 months or longer after initial diagnosis were included in the analysis. Women were excluded if they had a history of either invasive vulval, vaginal, anal or cervical cancer, but not CIN. Patients with invasive cancer diagnosed within 1 year of VIN diagnosis were also excluded "in order to minimize falsely detecting preexisting invasive disease due to incorrect initial diagnosis." Age: mean 46 years (SD 14, range: 17 to 90) VIN grade: mostly high‐grade VIN (n = 381; 93%) but also vaginal intraepithelial neoplasia (n = 30; 7%) Smoking: n = 173 (42%) were smokers HPV status: not documented Immunocompetence: n = 29 immunosuppressed Recurrent disease: not reported Focality: n = 103 (25%) multifocal, n = 308 unifocal (75%) Variables were not reported separately for treatment groups |
|
| Interventions |
Group 1: CO2 laser vaporisation (n = 270) Group 2: surgical excision (n = 114) Group 3: vulvectomy (n = 19) Group 4: other treatments including PDT and imiquimod All positive margins were re‐excised "Follow‐up visits were usually scheduled every six months for the first five years and then on an annual basis." Mean follow‐up time was 85 months (SD 56 months; range 13 to 389 months) |
|
| Outcomes | Biopsy‐proven recurrence (≥ 12 months); progression to vulval cancer | |
| Notes | The purpose of this study was to examine risk factors for recurrence and progression. Only patients with a follow‐up of 12 months or longer after initial diagnosis were included in the analysis. "If a patient had both excision and biopsy combined with laser evaporation during the first year, laser evaporation was considered the initial treatment since it is the more comprehensive type of therapy." We assessed risk of bias for the adjusted findings for the outcomes 'recurrence' and 'progression' There was a relatively low rate of multifocality (25%) in this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not a RCT |
| Allocation concealment (selection bias) | Unclear risk | See other bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | See other bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See other bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | See other bias |
| Selective reporting (reporting bias) | Unclear risk | See other bias |
| Other bias | High risk | We had risk of bias concerns about the measurement of interventions and outcomes. Patients who received both LV and excision in the first year were analysed in the LV group and the number of women who received both treatments was not reported. Women in the LV group may therefore have had more extensive treatment during the course of the first year and this group may not be comparable to the excision only group. Early recurrences and progressions occurring in the first year were not counted but rather considered to have had inappropriate or insufficient initial treatment requiring immediate retreatment. We also had concerns about selective reporting bias with possible multiple intervention outcome testing to produce the ORs for recurrence and progressions. The number of women included in these analyses was not reported in Table 2, and the findings for excision versus LV were reported, not surgery (excision + vulvectomy) versus LV, as in Table 1.These limitations might have biased results in the direction of LV. |
| Overall risk | High risk | For the reasons described under 'other bias' |
Leufflen 2013.
| Methods | A retrospective NRS of 50 women with histologically proven uVIN or dVIN treated at a French hospital between 1995 and 1999. Multivariate analyses were performed. Risks of recurrence according to patient characteristics and treatments were estimated using Cox proportional hazards regression models. Recurrence‐free survival was compared using the log‐rank test. | |
| Participants | 50 women with VIN who underwent treatment by surgery (cold knife or laser including partial or total vulvectomy) or LV Mean age: 54.9 (16.4) for surgery versus 39.1 (14.7) for LV VIN type: uVIN (n = 41) or dVIN (n = 9) Smoking: 10/24 (surgery) versus 19/26 (LV) HPV status: NR Immunosuppressed: 2/24 (surgery) versus 3/26 (LV) Recurrent disease: 6/24 (surgery) versus 8/26 (LV) Focality: 13/24 (surgery) versus 21/26 (LV) Multicentric: 0/24 (surgery) versus 13/26 (LV) Younger age, smoking, multifocality and multicentricity were all significantly more common in the LV group (P value < 0.5). Median and mean duration of follow‐up was 4.4 years (range 0.8 to 18.4 years) and 5.6 years, respectively. It is not clear whether follow‐up duration was different between groups as these data were not reported. |
|
| Interventions | Group 1 (n = 24): surgery by cold knife or laser including partial or total vulvectomy Group 2 (n = 26): laser vaporisation (LV), 1 to 4 sessions during the first year Follow‐up was every 4 to 6 months after treatment for 2 years; thereafter it may have varied |
|
| Outcomes | Complete response, partial response, recurrence, progression to cancer CR was defined as the absence of any vulval disorder during the first 3 months after the end of treatment. PR defined as the persistence of VIN or SCC within the first 3 months after treatment. Recurrence was defined as re‐appearance of VIN or SCC after the first 3 months. |
|
| Notes | Surgery was the first choice in treating unifocal VIN and dVIN. 2 women had skinning vulvectomy, the rest had partial vulvectomy. Women with multicentric VIN were treated with LV only. We assessed risk of bias for the outcome RFS only. All other outcomes, including response, and recurrence rates, were at a critical risk of confounding (there were statistically significantly more uVIN, multifocal and multicentric lesions in the LV group) as the findings for these outcomes did not appear to be adjusted for and we have not included these data. We emailed the author for clarification but no response was received by the time of publication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not a RCT |
| Allocation concealment (selection bias) | Unclear risk | See other bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | See other bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See other bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | See other bias |
| Selective reporting (reporting bias) | Low risk | See other bias |
| Other bias | Unclear risk | Interventions and outcomes were clearly defined but participants included dVIN, which was more likely to be treated with surgery. Women in the LV group were more likely to be younger, and have multicentric and multifocal disease. We assessed the risk of bias in relation to the outcome recurrence‐free survival as unclear as the report does not state which baseline variables were adjusted for, and it is not clear whether the reported RFS was adjusted for multifocality and the other important variables listed above. |
| Overall risk | Unclear risk | We wrote to the author on 9 October 2015 for clarification |
Mathiesen 2007.
| Methods | RCT, single‐centre; randomisation ratio 2:1 31 women (21 imiquimod arm and 10 in placebo arm) |
|
| Participants |
Age: mean 47.8 years, range 21 to 68 VIN grade/type: VIN 2 (n = 2), VIN 3 (n = 29) Smoking status: active (n = 25), former (n = 3), never (n = 2), unknown (n = 1) HPV status: positive (n = 18), negative (n = 8), missing (n = 5) Immunocompetence: not reported but excluded women on immunosuppressive treatment and those with HIV Recurrent disease: not reported Focality: unifocal lesion (n = 22), multifocal lesions (n = 9; 5/21 and 4/10) |
|
| Interventions | Imiquimod versus placebo Treatment for 16 weeks (once a week for 2 weeks, then twice a week the following 2 weeks, if tolerated 3 times a week for the last 12 weeks) |
|
| Outcomes | Response to treatment Compliance with treatment Local side effects |
|
| Notes | Review every 4 weeks and a biopsy taken if suspicion of progression. Response to treatment was assessed histologically at 2 (24 weeks), 6 and 12 months (+/‐ repeat biopsy) after treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed by a computer programme at the study randomisation centre" |
| Allocation concealment (selection bias) | Low risk | "The medicines were then packed into sachets at the University Hospital of Aarhus pharmacy in accordance with the randomisation list. The randomisation list was not available to the investigators until the last patient included had been evaluated clinically and histologically 2 months after end of treatment". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The treatment modality was blinded to the pathologist as well as to the investigators and to the patients". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The treatment modality was blinded to the pathologist as well as to the investigators and to the patients". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | % analysed: 31/31 (100%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Side effects were poorly reported |
| Overall risk | Low risk | Low risk for most criteria |
Naik 2006.
| Methods | RCT, single centre 13 women randomised |
|
| Participants |
Age: mean 44.6 years, range: 26 to 63 VIN grade: all 13 high‐grade Smoking: smokers (n = 9), non‐smokers (n = 3) HPV status: not documented Recurrent disease: not reported Focality: unifocal (n = 9), multifocal (n = 3) |
|
| Interventions | Oral indole‐3‐carbinol: 200 mg/day versus 400 mg/day for 6 months | |
| Outcomes | Response to treatment: 6 weeks, 12 weeks and 6 months Urine: 2‐hydroxyestrone:16‐alpha‐hydroxyestrone ratio Symptom improvement Side effects |
|
| Notes | Vitamin C was commenced at the discretion of the investigator | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | % analysed: 10/13 (77%) "One patient was withdrawn from the study at the 6‐week visit as there was difficulty in obtaining the I3C and two women did not attend the 6‐month review." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
| Overall risk | High risk | Insufficient information |
Sterling 2005.
| Methods | RCT conducted in 2 UK hospitals 21 women randomised |
|
| Participants |
Age: mean 47 years, range 26 to 63 years VIN grade: all "high grade" VIN Smoking: not documented HPV status: "almost all women" were positive All "immunocompetent" Recurrent disease: not reported Focality: not reported |
|
| Interventions | Imiquimod (n = 15) versus placebo (n = 6) for 16 weeks | |
| Outcomes | Response to treatment assessed at 8 weeks and 20 weeks after randomisation | |
| Notes | Abstract only, not full paper, with little information and data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method and ratio are unclear and not reported in abstract |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Stated "double‐blind" but details lacking |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "All completed the treatment and biopsies" but details lacking |
| Selective reporting (reporting bias) | Unclear risk | Abstract only with little information and data |
| Other bias | High risk | To our knowledge, this study remains unpublished. Baseline characteristics were not reported. |
| Overall risk | High risk | Unpublished to date |
Tristram 2014.
| Methods | (RT3VIN) Phase 2 open‐label RCT conducted in 32 teaching hospitals in Wales and England between October 2009 to January 2013 (ISRCTN 34420460) 180 women randomised Duration of follow‐up: 2‐years (6 weeks for preliminary report) |
|
| Participants |
Age: cidofovir group: median 48 years, IQR range 42 to 52; imiquimod group: median 46 years, range 41 to 55 VIN grade: all had VIN 3 with at least 1 measurable lesion with longest diameter ≥ 20 mm Smoking status: 50/89 (56%) current smokers in cidofovir group, 56/91 (62%) in imiquimod group HPV status: 84% proven HPV DNA‐positive in both groups (75/89 versus 76/91) Immunocompetence: 3/89 (3%) immunocompromised in cidofovir group, 6/91 (7%) in imiquimod group Recurrent disease: 42/89 (47%) in cidofovir group versus 40/91 (44%) in imiquimod group Focality: multifocal = 45/89 (51%) in cidofovir group versus 46/91 (51%) in imiquimod group |
|
| Interventions |
Group 1 (89 women): 1% cidofovir gel supplied as a 10 g tube, to last 6 weeks Group 2 (91 women): 5% imiquimod (one 250 mg sachet per application) Topical treatment was to be self applied 3 times per week for a maximum of 24 weeks. Participants were advised to apply the treatment at night and wash it off with aqueous cream and water the next day. |
|
| Outcomes |
Primary: histologically confirmed CR at follow‐up visit (6 weeks post‐treatment, maximum of 30 weeks from start of treatment) Secondary: adherence at 6 weeks and 24 weeks during treatment SAEs up to 6 weeks post‐treatment Prediction of response according to HPV status Recurrence of VIN 3 at 12 months Assessed at 6, 12, 18, and 24 weeks during treatment. Post‐treatment assessment was either 6 weeks after the end of treatment or 6 weeks after a complete response or disease progression. 2 biopsy specimens were taken to assess histological response and test for HPV DNA. Women with a complete response were followed up every 6 months (6, 12, 18, 24 months) to the end of the study. |
|
| Notes | Trial was primarily a feasibility study and was not powered to detect differences in efficacy between cidofovir and imiquimod. Adherence was comparable between arms at 6 weeks (88% for cidofovir versus 86% for imiquimod). Number of treatment applications over the 24‐week treatment stage was not significantly different 67.5 (64 to 71) versus 63.0 (50 to 67) for cidofovir and imiquimod, respectively. New lesions arose in 19/87 (21%) and 11/91 (12%) in women in the cidofovir and imiquimod groups, respectively, however, it was not known whether these occurred within the treatment areas or not. The presence of HPV DNA 16, focality, recurrence of disease or smoking did not predict response to treatment. Investigators did not assess response according to lesion size. "Cidofovir had slightly fewer reported toxic effects." Most were grade 2. Investigators did not collect QoL data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation "in a 1:1 ratio…via a central computerised system by stratified minimisation (with an 80:20 random element)". Stratified by hospital, focality and first presentation or recurrent disease. |
| Allocation concealment (selection bias) | Low risk | Central computerised randomisation requires registration of the participant before revealing the allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label: "we did not mask the treatment allocation from patients or investigators" Cidofovir gel has a distinct appearance from imiquimod |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Open‐label but response was histologically confirmed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 women withdrew from the cidofovir group and 7 from the imiquimod group before the 6‐week visit and could not be included in the 6‐week analyses. A further 2 women in each group were lost to follow‐up before 24 weeks |
| Selective reporting (reporting bias) | Unclear risk | Pre‐specified outcomes were reported. Per protocol and partial ITT analyses were reported, therefore additional ITT response data were requested. |
| Other bias | Unclear risk | Baseline characteristics were balanced across study arms. Final trial results have not yet been reported. |
| Overall risk | Unclear risk | Low to moderate risk overall |
van Esch 2013.
| Methods | Retrospective study of 73 women with uVIN treated at Leiden University Medical Centre 1990 to 2012 | |
| Participants | 73 women with uVIN Age: mean 43 years, range 19 to 84 years VIN grade: VIN 2 17/73 (23%); VIN 3 56/73 (77%) Smoking: current 48/73 (66%) HPV status: 63/73 (86%) positive, 5 not tested and 5 negative Immune status: 11/73 (15%) immunosuppressed Multicentric disease: 55/73 (75%) including abnormal cervical cytology (24%), CIN (42.55%), CIN + VAIN (5.5%) and cervical cancer (2.7%) Focality: 30/73 (44%) multifocal, 38/73 (52%) unifocal |
|
| Interventions |
Group 1 (31 women): excision Group 2 (25 women): laser Group 3 (6 women): imiquimod Group 4 (8 women): laser + excision |
|
| Outcomes | Recurrence, recurrence‐free survival and progression to vulval cancer Median follow‐up was 49 months |
|
| Notes | The study aim was to identify clinical characteristics associated with recurrence and progression after initial treatment. More unifocal lesions were treated with excision compared with other modalities (P value = 0.105). Median time to recurrence was 14 months. On univariate analysis, RFS favoured the excision and imiquimod therapy group. However, when RFS was adjusted for multifocal disease, smoking, HPV status, immune status and BMI, excision as first therapy was no longer favoured (P value = 0.142). 11 women developed invasive SCC at a median of 71.5 months after initial diagnosis of uVIN. Progression to SCC from a persistent uVIN lesion occurred in 2 women and in 9 women occurred after successful treatment with no residual lesions. 7 of these lesions were microinvasive SCCs. Type of first treatment showed no difference on univariate analysis. 4 women who developed SCC were immunocompromised and the time to progression was shorter (median 54 months) but not significantly so on Cox univariate analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not a RCT |
| Allocation concealment (selection bias) | Unclear risk | See other bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | See other bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See other bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | See other bias |
| Selective reporting (reporting bias) | Unclear risk | See other bias |
| Other bias | Low risk | Outcomes were clearly defined. Women with unifocal lesions were more likely to have excision than other modalities; however, this and other main confounders (smoking, HPV status, immune status and BMI) were adjusted for in a multivariate analysis of RFS. We had some concerns that 38 women were excluded from the initial dataset due to missing baseline demographic information and no further information was provided on these women. Probably due to the small group sizes, excision and imiquimod treatment RFS data were combined on univariate analysis and compared with laser and laser plus excision combined. The effect demonstrated, in favour of excision as first therapy, was not confirmed on multivariate analysis; however, these effect estimates were not reported and analyses were probably underpowered. |
| Overall risk | Low risk | We assessed this study as having a relatively low risk of bias |
Van Seters 2008.
| Methods | Placebo‐controlled, double‐blind, parallel‐arm RCT 52 women randomised; 26 to each study group |
|
| Participants |
Age: intervention group: median 39 years, range 22 to 56; placebo group: median 44 years, range 31 to 71 (P value = 0.08) VIN grade: VIN 2 (n = 4; 2 versus 2), VIN 3 (n = 47; 23 versus 24), not reported (n = 1) Smoking status: smokers (n = 46; 23 versus 23) and non‐smokers (n = 6; 3 versus 3) HPV status: positive (n = 50; 25 versus 25), negative (n = 2) Immunocompetence: excluded women with immunodeficiency Recurrent disease (previous surgical treatment): (n = 37; 18 versus 19) Focality: 100% multifocal |
|
| Interventions | Imiquimod versus placebo for 16 weeks (twice weekly) | |
| Outcomes | Primary: clinical response at 20 weeks defined as a reduction in lesion size of more than 25% Secondary: histological regression to a lower grade at 20 weeks, clearance of HPV, relief of clinical symptoms QoL Side effects of treatment Response to treatment was assessed at 20 weeks (biopsy), 7 months and 12 months (+/‐ repeat biopsy) Investigators also reported progression to invasive SCC |
|
| Notes | Women were advised to use sulphur precipitate 5% in zinc oxide ointment the day after application to avoid superinfection 4‐weekly review with biopsy if suspicion of progression Complete responders were followed up for more than 5 years. At 5‐year follow‐up, 3 women in the imiquimod group had developed SCC, 2 with initially a partial response and 1 non‐responder. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was carried out by 3M Pharmaceuticals in blocks of four (with a two‐by‐two design) without stratification." |
| Allocation concealment (selection bias) | Low risk | "Except for cases of serious side effects, the randomization code was not broken until all women had been seen at 12 months." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind, randomized clinical trial". Clinical response was evaluated by 2 gynaecologists with the use of photographs to avoid bias relating to awareness of side effects of imiquimod. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All biopsy evaluations were reviewed independently by two experienced gynecologic pathologists who were unaware of the clinical data" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% analysed at 20 weeks All but 3 women were followed up for 12 months |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Among complete responders in the imiquimod arm, 1 woman's VIN lesions disappeared spontaneously after the initial biopsy and, after histological review, another woman had VIN 1 not VIN2/3. This may have biased the complete responder outcome in favour of imiquimod. There was also a slight imbalance in age between the 2 groups (P value = 0.08). |
| Overall risk | Low risk | A well‐conducted RCT |
Vlastos 2002.
| Methods | A retrospective NRS of 74 women with histologically proven VIN treated by one of the authors between 1995 and 1999. Data for the subgroup with no previous treatment were analysed in this paper (n = 62). Multivariate analyses were performed. | |
| Participants | 62 women with multifocal VIN 2 and VIN 3 with no previous treatment Median age: 44.4, 48.3 and 43.5 respectively for LEEP, WLE and LV groups VIN grade: "VIN 2 to 3 ...in all patients" Smoking status: 12/20; 18/22; 16/20 for LEEP, WLE and LV, respectively HPV status: NR Immunocompetence: NR Recurrent disease (previous treatment): none had been previously treated Focality*: "multifocal in all patients" |
|
| Interventions |
Group 1 (20): loop electrosurgical excision procedure (LEEP) Group 2 (22): wide local excision Group 3 (20): CO2 laser vaporisation (LV) |
|
| Outcomes | Recurrence, time to recurrence, response rates, vulval cancer Duration of follow‐up ranged from 1 to 175 months with a median of 37 months overall |
|
| Notes | *The text on multifocality was contradicted by data in table 3 (page 235) which indicated that 10/20, 15/22 and 15/20 women in the LEEP, WLE and LV groups had multifocal VIN, respectively. We attempted to obtain clarity from the authors in this regard but were unsuccessful. Data on adverse events were inadequately reported and could not be extracted as they applied to the whole group (n = 74) and not the subgroup of 62 that was the focus of the report Authors used Cox proportional hazards methods and logistic regression to compare the significance of variables predicting response No cases of vulval cancer occurred during follow‐up in the subgroup that was analysed. LEEP was performed as an outpatient and was associated with statistically significantly less hospital stay than the other modalities (0/20 versus 9/22 versus 18/20 for LEEP, WLE and LV, respectively; P value = 0.0001). No data for meta‐analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not a RCT |
| Allocation concealment (selection bias) | Unclear risk | None |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | None |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | None |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | Unclear risk | Mainly the data pertaining to a subgroup of women with no previous treatment were reported. Recurrences were reported as being "significantly fewer with LEEP and WLE than with the other procedures" but these groups were followed up for a shorter period than the LV group. The finding for time to recurrence was reported as not statistically significantly difference (log‐rank test P value = 0.194) but no HRs were provided. |
| Other bias | High risk | We considered the potential for confounding to represent a serious risk of bias. The text on multifocality was contradicted by data in table 3 (page 235), which indicated that 10/20, 15/22 and 15/20 women in the LEEP, WLE and LV groups, respectively, had multifocal VIN. It is unclear from the report which information is correct, however these data on multifocality were not significantly different between groups (P value = 0.27). Authors did control for age, age at first intercourse and number of sexual partners, however. We also considered the potential for bias in the measurement of outcomes to represent a serious risk. There was no information on how recurrence was monitored or measured. (Median) duration of follow‐up was significantly longer for the LV group compared with the other treatment groups and there were more recurrences in this group. Precise data on effect of treatments on recurrence were missing from the report. |
| Overall risk | High risk | Based on a serious risk of confounding and bias in the measurement of outcomes, and moderate risk of selective reporting bias |
von Gruenigen 2007.
| Methods | Multicentre randomised controlled trial conducted in the USA between 2000 and 2005 Age (50 years or younger and older than 50 years) and site location were used as stratification variables in the randomisation assignment |
|
| Participants | 30 women with high‐grade VIN of a total of 110, which included those with VIN 1 and all‐grade vaginal intraepithelial neoplasia; 16 of the 30 women with high‐grade VIN were randomised to carbon dioxide (CO2) laser surgery and 14 women to ultrasonic surgical aspiration | |
| Interventions |
Interventions: Group 1: CO2 laser surgery. Depth of tissue destruction was 1 mm in non‐hairy vulval regions and 3 mm in hairy vulval regions Group 1: ultrasonic surgical aspiration. Surgery was performed with the Cavitron Ultrasonic Surgical Aspirator Excel System (Valley‐lab, Boulder, Colorado, USA). The handheld tool vibrates and contains separate irrigation and suction channels. Lesions were removed to the reticular layer of the dermis. Surgeries were performed in an outpatient setting, with participants given standard discharge instructions regarding postoperative care. The use of topical postoperative symptom control therapies (e.g. silver sulfadiazine) were ordered at the discretion of the attending physician. All participants were seen preoperatively and treated by 1 of 3 gynaecological oncologists |
|
| Outcomes |
|
|
| Notes | Participants returned every 3 months for 1 year for a pelvic examination and cytology in order to assess recurrence Follow‐up colposcopy and biopsy were used at the discretion of the treating physician 53% of participants treated in this study had received prior therapy for intraepithelial disease |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Blocked randomisation was carried out by a computer‐generated table of random numbers corresponding to treatment assignment." |
| Allocation concealment (selection bias) | Low risk | "Randomisation assignment was given to the treating physician by personnel not involved in the patient’s medical care." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Percentage analysed: 30/30 (100%) |
| Selective reporting (reporting bias) | Low risk | Trial authors provided us with data for VIN 2 or higher‐grade women on request |
| Other bias | Unclear risk | Insufficient information to assess whether an additional risk of bias exists |
| Overall risk | Unclear risk | Moderate risk due to apparent/unclear lack of blinding |
ALA: 5‐aminolaevulinic acid BMI: body mass index CIN: cervical intraepithelial neoplasia
CO2: carbon dioxide CR: complete response dVIN: differentiated‐type vulval intraepithelial neoplasia DFS: disease‐free survival HPV: human papilloma virus HR: hazard ratio HRT: hormone replacement therapy IQR: interquartile range ITT: intention‐to‐treat LEEP: loop electrosurgical excision procedure LV: carbon dioxide laser vaporisation NR: not reported NRS: non‐randomised study OR: odds ratio PDT: photodynamic therapy PR: partial response QoL: quality of life RCT: randomised controlled trial RFS: recurrence‐free survival SAE: serious adverse event SCC: squamous cell carcinoma SD: standard deviation uVIN: usual‐type vulval intraepithelial neoplasia VAIN: vaginal intraepithelial neoplasia VIN: vulval intraepithelial neoplasia WLE: wide local excision
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdullah 2015 | Retrospective non‐comparative study (conference abstract only) |
| Bakri 1995 | A letter to the Editor regarding Jones 1994 |
| Ballester 2012 | A case report |
| Bar‐Am 1993 | Excluded due to design limitations. This study compared high‐ versus low‐power CO2 laser for 3 clinical indications, women with CIN2/3 (group 1, n = 56), "patients with benign vulvar and perineal HPV lesions" (group 2, n = 83), and men with penile shaft warts (group 3, n = 65). Although each group was randomised and analysed separately, the proportion of women with VIN 2/3 was not stated and the sample could have included women with VIN 1/condyloma accuminata mainly. |
| Ben David 1996 | Excluded due to design limitations. A retrospective chart review of 102 women treated for VIN 3. Treatments received included laser vaporisation (n = 52), excision (n = 31) and a combination of the two. The findings were not adjusted for baseline characteristics, which were not described separately for the treatment groups. |
| Brown 2005 | Excluded due to design limitations. A retrospective study of different surgical managements for VIN2 and VIN3. The findings was not adjusted for baseline characteristics, which differed between treatment groups. |
| Bruchim 2007 | Excluded due to design limitations. A retrospective study comparing recurrence and response rates for women treated with surgery (WLE or skinning vulvectomy), LEEP, CO2 laser ablation and imiquimod. Findings were not adjusted for baseline characteristics, which differed between treatment groups. |
| Cabrera Diaz 2011 | Excluded due to design limitations. Conference abstract of a retrospective review of 101 women with VIN 2/3 between 1994 and 2010. Treatments included wide local excision, laser ablation and imiquimod. Multivariate analysis was not performed. |
| Caglar 1982 | Excluded due to design limitations. A retrospective study of 50 women with carcinoma in situ treated variously with wide local excision, skinning vulvectomy, vulvectomy, CO2 laser treatment or 5‐fluorouracil treatment. Findings were not controlled for baseline characteristics. |
| Caglar 1986 | A retrospective study of 24 women with vulval carcinoma in‐situ who underwent skinning vulvectomy between 1974 and 1984. Extent (partial or total) depended on extent of vulval involvement. |
| Daayana 2009 | Conference abstract of two phase 2 non‐comparative studies of imiquimod and HPV therapeutic vaccination and imiquimod and PDT (Daayana 2010 and Winters 2008, respectively). |
| Ferenczy 1994 | Twenty‐eight women with VIN 3 lesions were treated with both CO2 laser and LEEP, applied to half of each VIN lesion. A randomisation procedure was used to assign one of the two sides to LEEP or CO2 laser. A complete response was observed in 12/25 women after a single treatment. Of thirteen recurrences, two occurred in areas treated with LEEP, one occurred in areas treated with CO2 laser, and ten occurred in areas treated with both LEEP and CO2 laser after relapse prior to the 9‐month assessment. Consequently, these data were very difficult to interpret as effects could not be attributed to one treatment or the other. |
| Fiorica 1988 | Excluded due to design limitations. A retrospective study of 125 women with carcinoma in‐situ between 1961 and 1984. Diverse cohort characteristics and multivariate analysis was not performed. |
| Frega 2013 | Excluded due to design limitations. A non‐randomised study of cold‐knife excision (n = 40; including 4 lost to follow‐up) versus imiquimod (n = 40; 2 lost to follow‐up and 6 withdrawn due to side effects) applied twice weekly for 16 weeks for VIN2/3. Women were followed up 6‐monthly for 5 years. Recurrence plus treatment failure in the imiquimod group was 69% (22/32) compared with 45% (17/38) in the surgery group. Complete response was lower at 31% (10/32) versus 55% (21/38). Surgical conversion occurred in 53% (17/32) of women in the imiquimod group, with 2 women in the imiquimod group and 3 in the surgery group developing invasive cancer in spite of treatment. Findings were not adjusted for patient baseline characteristics, which differed between the groups for VIN grade. |
| Garland 2013 | A conference abstract of a RCT evaluating the effect of a HPV vaccine compared with control on preventing VIN and VAIN lesions. |
| Hillemanns 2006 | Excluded due to design limitations. A retrospective study describing a series of 93 women treated for VIN 1‐3 between 1991 to 2001 by various modalities including laser vaporisation, PDT, excision and vulvectomy. Minimal follow‐up duration was 36 months. Recurrence rate was 40% overall and lowest for vulvectomy (0%). Treatment groups differed significantly according to risk factors for recurrence including multifocality and multicentricity (multifocal disease was more common in the vulvectomy group; multicentric disease was least common in the excision group) and these differences were not controlled for. |
| Iavazzo 2008 | A review of imiquimod for VIN and VAIN |
| Jones 1994 | Excluded due to design limitations. A retrospective descriptive study of a series of 113 women treated for VIN 3 between 1961 and 1993. Multivariate analysis was not performed. |
| Jones 2005 | Excluded due to design limitations. A retrospective descriptive study of a series of 405 women treated for VIN 2/3 between 1962 and 2003. Multivariate analysis was not performed. |
| Joura 2012 | Excluded due to design limitations. A retrospective study of women identified from the FUTURE I and II HPV vaccination trials who underwent treatment for HPV‐related cervical disease or had a diagnosis of vulval or vaginal HPV‐related disease. The study assessed the relative impact on subsequent HPV‐related disease (including CIN, genital warts, VAIN and VIN) of vaccination and placebo. Among women who had a baseline diagnosis of vulval or vaginal disease (including warts), a reduced rate of any subsequent HPV‐related disease (including CIN and warts) was noted in the vaccination group compared with the placebo group (70/211 (20%) versus 163/422 (31%)); however, the impact on vulval disease was driven mainly by a reduction in the incidence in genital warts. Rates of subsequent VIN 2/3 or VAIN 2/3 were 2.6% and 3.4%, for the vaccination and placebo groups, respectively. It was not clear what proportion of women had VIN 2/3 at baseline or subsequently, as women with genital warts and/or VAIN were included in the analyses. |
| Krebs 1986 | Excluded due to design limitations. A RCT of 5‐fluorouracil (5‐FU) as maintenance treatment following ablation/removal of the lesion/s in 90 women. Participants had vulval or vaginal HPV‐related lesions, with histology described as koilocytes, dysplasia and koilocytes, or dysplasia. As such it was not clear what proportion of the participants had VIN 2/3. In addition, various initial treatments were used alone and in combination to achieve the initial response (including podophyllin, 5‐FU, cryotherapy, local excision, laser and combinations of these treatments) before women were randomised to receive 5‐FU or no 5‐FU cream. Therefore the participants and interventions did not clearly fulfil the inclusion criteria for this review. |
| Leuchter 1984 | Excluded due to design limitations. A retrospective descriptive study of the treatment of carcinoma‐in‐situ between 1960 and 1982. Multivariate analysis was not performed. |
| Li 2005 | Excluded due to design limitations. A retrospective study of 24 women with VIN who underwent surgical excision including simple vulvectomy. Multivariate analysis was not performed. |
| Penna 2002 | Excluded due to design limitations. A retrospective study describing CO2 laser treatment (vaporisation and excisional) of 63 women with VIN 1‐3. Multivariate analysis was not performed. |
| Ribeiro 2012 | Excluded due to design limitations. A retrospective study of 29 women with VIN treated with laser ablation (n = 11), laser excision (n = 9), wide local excision (n = 7), vulvectomy (n = 1), imiquimod (n = 1). Multivariate analysis was not performed. |
| Rodolakis 2003 | Excluded due to design limitations. A retrospective study of 113 women diagnosed with VIN 1‐3 between 1986 and 1995. VIN 2/3 cases accounted for 56.7% and a variety of treatment modalities were used including laser CO2 treatment (vaporisation, excision, combination and skinning vulvectomy) and surgical treatment (excision, skinning vulvectomy, simple and radical vulvectomy). Multifocality was significantly associated with recurrence and multivariate analysis was not performed. |
| Shafi 1989 | Excluded due to design limitations. A retrospective study of 46 women with VIN. Treatments included laser skinning vulvectomy, local excision or simple vulvectomy. Multivariate analysis was not performed. |
| Sideri 1999 | Excluded due to design limitations. A retrospective study of CO2 laser vaporisation (n = 14) versus laser excision (n = 38) in women with VIN1‐3. Treatment choice was based on operator preference and multivariate analysis was not performed to control for confounders. Laser excision had a higher cure rate than laser vaporisation. 2 women in the laser vaporisation group progressed to cancer 5 and 7 years after treatment. 4 women in the laser excision group were found to have vulval cancer on histopathology examination of the excised specimen (12% unrecognised early invasion rate). 2 women had a repeat procedure for recurrence 2 and 3 years after primary treatment. |
| Spirtos 1990 | Excluded due to design limitations. This was a randomised trial that evaluated the use of nonoxynol‐9 gel compared with no gel in 21 women with VIN 3 receiving treatment with α‐IFN. All women received α‐IFN; nonoxynol‐9 was hypothesised to enhance the absorption of α‐IFN. Therefore, this trial was not evaluating an active treatment. Women who did not respond to treatment were crossed over to the alternative treatment arm. There was a lack of a clear time line of treatment for each patient and the duration of treatment in each arm for the crossed over patients was unclear. Overall, 14 out of 18 patients had a complete or partial response to α‐IFN treatment. Due to poor methodology, it was not possible to determine whether nonoxynol‐9 had any effect (good or bad) on the success of treatment. |
| Steiner 2012 | Excluded due to design limitations. A retrospective study of recurrence rates for laser vaporisation (n = 44) and surgical excision (n = 24) among 68 women with VIN 1‐3. Baseline characteristics were not described and it is not clear from this conference abstract whether multivariate analysis was performed. Recurrence rates were 72% for laser versus 41% for surgical excision but treatment selection was based on doctors' and patients' preferences. |
| Van Beurden 1998 | Excluded due to design limitations. A retrospective study of extensive versus restricted surgery for VIN 3. Baseline characteristics differed between treatment groups and were not controlled for. |
| van Bogaert 2015 | A letter to the editor about the prevalence of anogenital preinvasive and invasive lesions in South Africa |
| Wallbillich 2012 | Excluded due to design limitations. A retrospective chart review of 303 women treated for VIN2/3 between 1993 and 2011. Treatments received included laser vaporisation (n = 40), excision (n = 176), a combination of the two (n = 24), imiquimod (n = 22) and excision with imiquimod (n = 10). The findings on recurrence were not adjusted for baseline characteristics, which were not described separately for the treatment groups. |
| Wee‐Stekly 2013 | Excluded due to design limitations. Conference abstract only. An audit of 21 cases of VIN 1‐3 with heterogeneous treatment modalities. |
| Wright 1987 | A non‐comparative retrospective study of CO2 laser vaporisation for VIN 3. |
| Yu 2014 | Excluded due to design limitations. A retrospective study of 64 women with uVIN, 52 of whom underwent wide local excision and 12 who underwent simple vulvectomy. Treatment differed according to lesion characteristics (all lesions in the vulvectomy group were multifocal). |
| Zawislak 2006 | Excluded due to design limitations. An audit of 97 women with VIN 1‐3 between 1989 and 1999 treated variously initially, including local excision (n = 45), vulvectomy (n = 4), topical steroids (n = 20), 5‐fluorouracil (n = 4) and other modalities. |
| Zhang 2009 | Excluded due to design limitations. A retrospective descriptive study of 35 women with VIN 3 treated with different types of surgery including wide local excision or simple vulvectomy. Only 4 recurrences occurred with median follow‐up of 1 to 166 months. We were unable to get the full text of this paper but it appears that treatment groups were not compared. |
CO2: carbon dioxide CIN: cervical intraepithelial neoplasia
HPV: human papilloma virus LEEP: loop electrosurgical excision procedure PDT: photodynamic therapy RCT: randomised controlled trial uVIN: usual‐type vulval intraepithelial neoplasia VAIN: vaginal intraepithelial neoplasia VIN: vulval intraepithelial neoplasia WLE: wide local excision
Characteristics of studies awaiting assessment [ordered by study ID]
Satmary 2013.
| Methods | Retrospective study using multivariate analysis to assess the independent risk factors for recurrence/persistence while controlling for confounders |
| Participants | 788 women with VIN 2/3 and carcinoma in‐situ. Excluded VIN 1 and women with evidence of micro‐invasive disease. |
| Interventions | Laser ablation (153), excision (431), laser and excision (15), medical and surgical treatment (44), other (145) |
| Outcomes | Persistence/recurrence, vulval cancer |
| Notes | Conference abstract only. Author contacted for more info. 100/431 (23%) in excision‐only group and 42/153 (27.5%) in laser‐only group had recurrence/persistence (unadjusted). Awaiting manuscript in progress. |
VIN: vulval intraepithelial neoplasia
Characteristics of ongoing studies [ordered by study ID]
EUCTR2008‐008251‐42‐NL.
| Trial name or title | Clinical and immunological effects of imiquimod and HPV‐vaccination compared to imiquimod alone in women with uVIN ‐ effects of HPV‐vaccination and imiquimod in VIN patients |
| Methods | Double‐blind, parallel‐arm, placebo‐controlled RCT |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Arm 1: HPV vaccination (Gardasil®) followed by topical applications of imiquimod (5% cream) Arm 2: placebo vaccination (saline) followed by topical applications of imiquimod (5% cream) |
| Outcomes | Clinical efficacy (measured by reduction in lesion size, histological regression to normal tissue and relief of symptoms); systemic and local immunological response (immune cell counts, cytokine production, HPV‐specific antibody titers); HPV DNA presence (0 and 36 weeks); QoL at baseline and 36 weeks |
| Starting date | 2009 |
| Contact information | M.I.E.van_Poelgeest@lumc.nl |
| Notes | This trial has been completed. Results are awaited. |
EUCTR2011‐003134‐13‐NL.
| Trial name or title | 5‐Aminolevulinic acid photodynamic therapy for the treatment of pre‐malignant disorders of the vulva (uVIN and lichen sclerosis (LS)) |
| Methods | Parallel‐arm, open‐label RCT |
| Participants | Included if:
Excluded if:
|
| Interventions | Arm 1: low‐dose light fractionated 5‐aminolevulinic acid (gel) and photodynamic therapy (ALA‐PDT) Arm 2: imiquimod (5% cream) |
| Outcomes | Primary: clinical response to the treatment in VIN or LS lesions after the end of ALA‐PDT treatment measured by:
Secondary (assessed at 4 weeks post‐treatment):
|
| Starting date | — |
| Contact information | l.blok@erasmusmc.nl |
| Notes | Emailed 9 February 2015 for more details (none received) |
ISRCTN98495886.
| Trial name or title | A trial investigating the use of Veregen (EPIgallocatechin‐3‐gallate) in the treatment of Vulval Intraepithelial Neoplasia (EPIVIN) |
| Methods | Parallel‐arm, placebo‐controlled, double‐blind, phase 2 RCT |
| Participants | 56 women with uVIN 3 Inclusion criteria:
*All histological material generated by this study will be assessed by Specialist Consultant in Gynaecological Pathology, 10% of biopsies will be independently reviewed by a second pathologist Exclusion criteria:
|
| Interventions | Arm 1: Veregen cream (a botanical drug product derived from green tea leaves, camellia sinensis), 3 times daily for a maximum of 16 weeks Arm 2: placebo cream |
| Outcomes | Primary (assessed at 32 weeks from start of treatment): histological resolution Secondary (assessed at 2, 4, 8, 16, 32 and 52 weeks from start of treatment): objective response, safety, compliance, acceptability, need for further treatment and QoL |
| Starting date | 18 September 2014; estimated end date 18 September 2016 |
| Contact information | b.kaur@bham.ac.uk |
| Notes | Emailed 9 February 2015 |
NCT01861535.
| Trial name or title | Primary imiquimod treatment versus surgery for vulval intraepithelial neoplasia (PITVIN) |
| Methods | Parallel‐arm, open‐label, phase 3 RCT |
| Participants | 110 women > 18 years old with usual type VIN with visible, measurable lesions, who are using contraception (for premenopausal women) Excluded if:
|
| Interventions | Arm 1: imiquimod cream self administered for 4 to 6 months. Applied overnight once a week for 2 weeks, then twice a week for the following 2 weeks and, if tolerated, 3 times a week for the last weeks. In case of severe side effects the number of applications can be reduced; a treatment‐free period of no more than 1 week is permitted. Arm 2: primary surgery. The type of surgery (excision or ablation) will be based on clinical findings and surgeon's judgement. After excision the specimen will be histologically analysed to assess resection margins and rule out invasion. |
| Outcomes | Primary: complete clinical response Secondary: clinical response/lesion size (no, weak partial, strong partial, complete); histological response; extent of surgery (number, type and extent); HPV status; anxiety and sexual activity questionnaires; histochemical analysis of markers of immunity; aesthetic results (photos will be taken); symptomatology (assessed monthly to 6 months) Assessed at 6 and 12 months |
| Starting date | June 2013 |
| Contact information | gerda.trutnovsky@medunigraz.at |
| Notes | Other IDs: EUCTR2012‐002052‐17‐AT Still recruiting on 12‐11‐2015 |
NTR1526.
| Trial name or title | Randomised controlled study on the effects of imiquimod, a TLR 7 activating agent, on the HPV16‐specific immune response following HPV16 E6/E.7 synthetic long peptides vaccination in women with HPV16 positive high‐grade vulvar/vaginal intraepithelial neoplasia |
| Methods | Parallel‐arm, open‐label RCT |
| Participants | Included if:
Excluded if:
|
| Interventions | Arm 1: HPV vaccination (synthetic HPV16 E6/E7 peptides) followed by topical imiquimod to the vaccination site at 1 and 48 hours after each vaccination Arm 2: HPV vaccination without imiquimod |
| Outcomes | Primary: immunological response Secondary: safety and clinical response Assessed 3 weeks after second vaccination, and 3 weeks, 3 months and 12 months after last vaccination |
| Starting date | October 2008 |
| Contact information | Gemma Kenter: g.g.kenter@lumc.nl |
| Notes | Emailed on 9 February 2015 for more details (none received) |
HPV: human papilloma virus LS: lichen sclerosis QoL: quality of life RCT: randomised controlled trial uVIN: usual‐type vulval intraepithelial neoplasia VAIN: vaginal intraepithelial neoplasia VIN: vulval intraepithelial neoplasia
Contributions of authors
Theresa Lawrie contributed to study selection, data extraction and writing the manuscript. Andy Bryant contributed to study selection, review methodology and data checking. Manas Chakrabarti contributed to study selection, data extraction and checking. Litha Pepas and Sonali Kaushik contributed to data extraction and manuscript content. Andy Nordin contributed to study selection and manuscript content. All authors read and approved the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
National Institute for Health Research, UK.
Declarations of interest
Andy Nordin was a co‐investigator in the Tristram 2014 trial Theresa Lawrie: none declared Manas Chakrabarti: none declared Andy Bryant: none declared Sonali Kaushik: none declared Litha Pepas: none declared
Edited (no change to conclusions)
References
References to studies included in this review
Fehr 2001 {published data only}
- Fehr MK, Hornung R, Schwarz VA, Simeon R, Haller U, Wyss P. Photodynamic therapy of vulvar intraepithelial neoplasia III using topically applied 5‐aminolevulinic acid. Gynecologic Oncology 2001;80(1):62‐6. [DOI] [PubMed] [Google Scholar]
Fehr 2013 {published data only}
- Fehr MK, Baumann M, Mueller M, Fink D, Heinzl S, Imesch P, et al. Disease progression and recurrence in women treated for vulvovaginal intraepithelial neoplasia. Journal of Gynecologic Oncology 2013;24(3):236‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leufflen 2013 {published data only}
- Leufflen L, Baermann P, Rauch P, Routiot T, Bezdetnava L, Guillemin F, et al. Treatment of vulvar intraepithelial neoplasia with CO2 laser vaporization and excision surgery. Journal of Lower Genital Tract Disease 2013;17(4):446‐51. [DOI] [PubMed] [Google Scholar]
Mathiesen 2007 {published data only}
- Mathiesen O. "Topical imiquimod can reverse vulvar intraepithelial neoplasia: A randomised, double blinded study" ‐ an answer. Gynecologic Oncology 2008;109(3):431. [DOI] [PubMed] [Google Scholar]
- Mathiesen O, Buus SK, Cramers M. Topical imiquimod can reverse vulvar intraepithelial neoplasia: a randomised, double‐blinded study. Gynecologic Oncology 2007;107(2):219‐22. [DOI] [PubMed] [Google Scholar]
Naik 2006 {published data only}
- Naik R, Nixon S, Lopes A, Godfrey K, Hatem MH, Monaghan JM. A randomized phase II trial of indole‐3‐carbinol in the treatment of vulvar intraepithelial neoplasia. International Journal of Gynecological Cancer 2006;16(2):786‐90. [DOI] [PubMed] [Google Scholar]
Sterling 2005 {published data only}
- Sterling JC, Smith NA, Loo WJ, Cohen C, Neill S, Nicholson A, et al. Randomized, doubleblind, placebo‐controlled trial for treatment of high grade vulval intraepithelial neoplasia with imiquimod [Abstract]. Journal of the European Academy of Dermatology and Venereology: JEADV. 12‐15th October, The 14th Congress of the European Academy of Dermatology and Venereology, London, UK. 2005; Vol. 19(Suppl 2):22.
Tristram 2014 {published data only}
- Tristram A, Hurt CN, Madden T, Powell N, Man S, Hibbitts S, et al. Activity, safety, and feasibility of cidofovir and imiquimod for treatment of vulval intraepithelial neoplasia (RT(3)VIN): a multicentre, open‐label, randomised, phase 2 trial. Lancet Oncology 2014;15(12):1361‐8. [DOI] [PubMed] [Google Scholar]
van Esch 2013 {published data only}
- Esch EMG, Dam MCI, Osse MEM, Putter H, Trimbos B, Fleuren G, et al. Clinical characteristics associated with development of recurrence and progression in usual‐type vulvar intraepithelial neoplasia. International Journal of Gynecological Cancer 2013;23(8):1476‐83. [DOI] [PubMed] [Google Scholar]
Van Seters 2008 {published data only}
- Terlou A, Seters M, Santegoets LAM, Heijmans‐Antonissen C, Blok LJ, Beurden M, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod: results of long‐term follow‐up (> 5 years). Reproductive Sciences (Thousand Oaks, Calif), 57th Annual Scientific Meeting of the Society for Gynecologic Investigation, SGI 2010 Orlando, FL United States. SAGE Publications Inc., 2010.
- Terlou A, Seters M, Ewing PC, Aaronson NK, Gundy CM, Heijmans‐Antonissen C, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod: seven years median follow‐up of a randomized clinical trial. Gynecologic Oncology 2011;121(1):157‐62. [DOI] [PubMed] [Google Scholar]
- Seters M, Beurden M, Kate FJ, Beckmann I, Ewing PC, Eijkemans MJ, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod. New England Journal of Medicine 2008;358(14):1465‐73. [DOI] [PubMed] [Google Scholar]
Vlastos 2002 {published data only}
- Vlastos AT, Levy LB, Malpica A, Follen M. Loop electrosurgical excision procedure in vulvar intraepithelial neoplasia treatment. Journal of Lower Genital Tract Disease 2002;6(4):232‐8. [DOI] [PubMed] [Google Scholar]
von Gruenigen 2007 {published data only}
- Gruenigen VE, Gibbons HE, Gibbins K, Jenison EL, Hopkins MP. Surgical treatments for vulvar and vaginal dysplasia: a randomized controlled trial. Obstetrics and Gynecology 2007;109(4):942‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdullah 2015 {published data only}
- Abdullah S, Hanafiah A, McNally O. A clinicopathological review of VIN: a retrospective review of the management of vulvar intraepithelial neoplasia (VIN) over a 15 year period. BJOG, RCOG World Congress 2015 Brisbane, QLD Australia. 2015.
Bakri 1995 {published data only}
- Bakri Y, Dimitrievich E. Vulvar intraepithelial neoplasia III: a clinical study of the outcome in 113 cases with relation to the later development of invasive vulvar carcinoma. Obstetrics and Gynecology 1995;85(3):481‐2. [PubMed] [Google Scholar]
Ballester 2012 {published data only}
- Ballester I, Belinchon I, Guijarro J, Oltra F, Toledo F, Cuesta L. Photodynamic therapy of vulvar intraepithelial neoplasia using methyl aminolevulinate. Journal of Dermatological Treatment 2012;23(2):156‐8. [DOI] [PubMed] [Google Scholar]
Bar‐Am 1993 {published data only}
- Bar‐Am A, Lessing JB, Niv J, Brenner SH, Peyser MR. High‐ and low‐power CO2 lasers comparison of results for three clinical indications. Journal of Reproductive Medicine 1993;38(6):455‐8. [PubMed] [Google Scholar]
Ben David 1996 {published data only}
- Ben David Y, Lickrish GM, Rosen BP, Murphy JK, Walters M, Depetrillo AD. Vulvar intra‐epithelial neoplasia‐treatment outcome. International Journal of Gynecological Cancer 1996;6(2):145‐8. [Google Scholar]
Brown 2005 {published data only}
- Brown JV 3rd, Goldstein BH, Rettenmaier MA, Aylward MM, Graham CL, Micha JP. Laser ablation of surgical margins after excisional partial vulvectomy for VIN: effect on recurrence. Journal of Reproductive Medicine 2005;50(5):345‐50. [PubMed] [Google Scholar]
Bruchim 2007 {published data only}
- Bruchim I, Gotlieb WH, Mahmud S, Tunitsky E, Grzywacz K, Ferenczy A. HPV‐related vulvar intraepithelial neoplasia: outcome of different management modalities. International Journal of Gynaecology and Obstetrics 2007;99(1):23‐7. [DOI] [PubMed] [Google Scholar]
Cabrera Diaz 2011 {published data only}
- Cabrera Diaz S, Bradbury Lobato M, Centeno Mediavilla C, Fontalba Rojas, M, Xercavins J, Gil‐Moreno A. Vulvar intraepithelial neoplasia: risk factors, recurrence and progression in 101 cases. International Journal of Gynecological Cancer, 17th International Meeting of the European Society of Gynaecological Oncology, ESGO 2011, Milan, Italy. Lippincott Williams and Wilkins, 2011.
Caglar 1982 {published data only}
- Caglar H, Tamer S, Hreshchyshyn MM. Vulvar intraepithelial neoplasia. Obstetrics and Gynecology 1982;60(3):346‐9. [PubMed] [Google Scholar]
Caglar 1986 {published data only}
- Caglar H, Delgado G, Hreshchyshyn MM. Partial and total skinning vulvectomy in treatment of carcinoma in situ of the vulva. Obstetrics and Gynecology 1986;68(4):504‐7. [PubMed] [Google Scholar]
Daayana 2009 {published data only}
- Daayana S, Winters U, Stern P, Kitchener H. Vulval intraepithelial neoplasia‐immunotherapy phase II clinical trials. International Journal of Gynaecology and Obstetrics 2009;107 Suppl 2:S148. [Google Scholar]
Ferenczy 1994 {published data only}
- Ferenczy A, Wright Jr TC, Richart RM. Comparison of CO2 laser surgery and loop electrosurgical excision/fulguration procedure (LEEP) for the treatment of vulvar intraepithelial neoplasia (VIN). International Journal of Gynecological Cancer 1994;4(1):22‐8. [DOI] [PubMed] [Google Scholar]
Fiorica 1988 {published data only}
- Fiorica JV, Cavanagh D, Marsden DE, Shepherd JH, Ruffolo EH, Songster CL. Carcinoma in situ of the vulva: 24 years' experience in southwest Florida. Southern Medical Journal 1988;81(5):589‐93. [DOI] [PubMed] [Google Scholar]
Frega 2013 {published data only}
- Frega A, Sesti F, Sopracordevole F, Biamonti A, Scirpa P, Milazzo GN, et al. Imiquimod 5% cream versus cold knife excision for treatment of VIN 2/3: a five‐year follow‐up. European Review for Medical and Pharmacological Sciences 2013;17(7):936‐40. [PubMed] [Google Scholar]
Garland 2013 {published data only}
- Garland SM. Efficacy of the HPV‐16/18 AS04‐adjuvanted vaccine against vulvar/vaginal intraepithelial Neoplasia. Journal of Lower Genital Tract Disease, 22nd World Congress of the International Society for the Study of Vulvovaginal Disease, ISSVD 2013 Rome, Italy 2013;17(6 Suppl 2):s110. [Google Scholar]
Hillemanns 2006 {published data only}
- Hillemanns P, Wang X, Staehle S, Michels W, Dannecker C. Evaluation of different treatment modalities for vulvar intraepithelial neoplasia (VIN): CO(2) laser vaporization, photodynamic therapy, excision and vulvectomy. Gynecologic Oncology 2006;100(2):271‐5. [DOI] [PubMed] [Google Scholar]
Iavazzo 2008 {published data only}
- Iavazzo C, Pitsouni E, Athanasiou S, Falagas ME. Imiquimod for treatment of vulvar and vaginal intraepithelial neoplasia. International Journal of Gynaecology and Obstetrics 2008;101(1):3‐10. [DOI] [PubMed] [Google Scholar]
Jones 1994 {published data only}
- Jones RW, Rowan DM. Vulvar intraepithelial neoplasia III: a clinical study of the outcome in 113 cases with relation to the later development of invasive vulvar carcinoma. Obstetrics and Gynecology 1994;84(5):741‐5. [PubMed] [Google Scholar]
Jones 2005 {published data only}
- Jones RW, Rowan DM, Stewart AW. Vulvar intraepithelial neoplasia: aspects of the natural history and outcome in 405 women. Obstetrics and Gynecology 2005;106(6):1319‐26. [DOI] [PubMed] [Google Scholar]
Joura 2012 {published data only}
- Joura EA, Garland SM, Paavonen J, Ferris DG, Perez G, Ault KA, et al. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: retrospective pooled analysis of trial data. BMJ 2012;344:e1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krebs 1986 {published data only}
- Krebs HB. Prophylactic topical 5‐fluorouracil following treatment of human papillomavirus‐associated lesions of the vulva and vagina. Obstetrics and Gynecology 1986;68(6):837‐41. [PubMed] [Google Scholar]
Leuchter 1984 {published data only}
- Leuchter RS, Townsend DE, Hacker NF, Pretorius RG, Lagasse LD, Wade ME. Treatment of vulvar carcinoma in situ with the CO2 laser. Gynecologic Oncology 1984;19(3):314‐22. [DOI] [PubMed] [Google Scholar]
Li 2005 {published data only}
- Li H, Zhang WH, Wu LY, Znang R, Bai P. Clinicopathologic study of 24 patiens with vulvar intraepithelial neoplasia III [Chinese]. Chung‐Hua Chung Liu Tsa Chih [Chinese Journal of Oncology] 2005;27(5):306‐8. [PubMed] [Google Scholar]
Penna 2002 {published data only}
- Penna C, Fallani MG, Fambrini M, Zipoli E, Marchionni M. CO2 laser surgery for vulvar intraepithelial neoplasia. Excisional, destructive and combined techniques. Journal of Reproductive Medicine 2002;47(11):913‐8. [PubMed] [Google Scholar]
Ribeiro 2012 {published data only}
- Ribeiro F, Figueiredo A, Paula T, Borrego J. Vulvar intraepithelial neoplasia: evaluation of treatment modalities. Journal of Lower Genital Tract Disease 2012;16(3):313‐7. [DOI] [PubMed] [Google Scholar]
Rodolakis 2003 {published data only}
- Rodolakis A, Diakomanolis E, Vlachos G, Iconomou T, Protopappas A, Stefanidis C, et al. Vulvar intraepithelial neoplasia (VIN)‐‐diagnostic and therapeutic challenges. European Journal of Gynaecological Oncology 2003;24(3‐4):317‐22. [PubMed] [Google Scholar]
Shafi 1989 {published data only}
- Shafi MI, Luesley DM, Byrne P, Samra JS, Redman CW, Jordan JA, et al. Vulval intraepithelial neoplasia‐‐management and outcome. British Journal of Obstetrics and Gynaecology 1989;96(11):1339‐44. [DOI] [PubMed] [Google Scholar]
Sideri 1999 {published data only}
- Sideri M, Spinaci L, Spolti N, Schettino F. Evaluation of CO(2) laser excision or vaporization for the treatment of vulvar intraepithelial neoplasia. Gynecologic Oncology 1999;75(2):277‐81. [DOI] [PubMed] [Google Scholar]
Spirtos 1990 {published data only}
- Spirtos NM, Smith LH, Teng NN. Prospective randomized trial of topical alpha‐interferon (alpha‐interferon gels) for the treatment of vulvar intraepithelial neoplasia III. Gynecologic Oncology 1990;37(1):34‐8. [DOI] [PubMed] [Google Scholar]
- Spirtos NM, Smith LH, Teng NNH. A prospective, randomized trial of topical interferon‐alpha (INF) gels for the treatment of vulvar intraepithelial neoplasia III (VIN III) [abstract]. Gynecologic Oncology 1989;32(1):112, Abstract 76. [DOI] [PubMed] [Google Scholar]
Steiner 2012 {published data only}
- Steiner PH, Jochumsen KM. Treatment of vulvar intraepithelial neoplasia‐laser ablation or surgical resection?. International Journal of Gynecological Cancer, 14th Biennial Meeting of the International Gynecologic Cancer Society, IGCS 2012 Vancouver, BC Canada. 2012.
Van Beurden 1998 {published data only}
- Beurden M, Vange N, Kate FJW, Craen AJM, Schilthuis MS, Lammes FB. Restricted surgical management of vulvar intraepithelial neoplasia 3: Focus on exclusion of invasion and on relief of symptoms. International Journal of Gynecological Cancer 1998;8(1):73‐7. [DOI] [PubMed] [Google Scholar]
van Bogaert 2015 {published data only}
- Bogaert LJ. Anogenital preinvasive and invasive lesions in the Limpopo Province of South Africa. Sexually Transmitted Infections 2015;91(4):293. [DOI] [PubMed] [Google Scholar]
Wallbillich 2012 {published data only}
- Wallbillich JJ, Rhodes HE, Milbourne AM, Munsell MF, Frumovitz M, Brown J, et al. Vulvar intraepithelial neoplasia (VIN 2/3): comparing clinical outcomes and evaluating risk factors for recurrence. Gynecologic Oncology 2012;127(2):312‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wee‐Stekly 2013 {published data only}
- Wee‐Stekly WW, Chia YN, Yam KL. Vulvar intraepithelial neoplasia: our experience on diagnosis and treatment. BJOG, RCOG World Congress 2013 Liverpool, United Kingdom. Blackwell Publishing Ltd, 2013.
Wright 1987 {published data only}
- Wright VC, Davies E. Laser surgery for vulvar intraepithelial neoplasia: principles and results. American Journal of Obstetrics and Gynecology 1987;156(2):374‐8. [DOI] [PubMed] [Google Scholar]
Yu 2014 {published data only}
- Yu G, Lan Z, Li XC, Jin HM, Wang CY, Lang JH. Surgical treatment of usual type vulvar intraepithelial neoplasia: a study at three academic hospitals. Chinese Medical Journal 2014;127(4):784‐6. [PubMed] [Google Scholar]
Zawislak 2006 {published data only}
- Zawislak AA, Price JH, Dobbs SP, McClelland HR, McCluggage WG. The management of vulval intraepithelial neoplasia in Northern Ireland. International Journal of Gynecological Cancer 2006;16:780‐5. [DOI] [PubMed] [Google Scholar]
Zhang 2009 {published data only}
- Zhang GY. Clinical analysis of 35 cases of vulvar intraepithelial neoplasia grade III [Chinese]. Chung‐Hua Fu Chan Ko Tsa Chih [Chinese Journal of Obstetrics & Gynecology] 2009;44(3):163‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
Satmary 2013 {published data only}
- Satmary W, Natarajan S, Bebchuk JD, Holschneider CH. A retrospective analysis of 788 patients with vulvar intraepithelial Neoplasia: clinical, demographic and pathologic factors associated with disease persistence, recurrence and progression to cancer. Journal of Lower Genital Tract Disease, 22nd World Congress of the International Society for the Study of Vulvovaginal Disease, ISSVD 2013 Rome, Italy. 2013.
References to ongoing studies
EUCTR2008‐008251‐42‐NL {published data only}
- Poelgeest M, Helmerhorst T. Clinical and immunological effects of imiquimod and HPV‐vaccination compared to imiquimod alone in female patients with usual type VIN ‐ effects of HPV‐vaccination and imiquimod in VIN patients. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2008‐008251‐42 (accessed 6 February 2015) 2009.
EUCTR2011‐003134‐13‐NL {published data only}
- Blok L. 5‐Aminolevulinic acid photodynamic therapy for the treatment of pre‐malignant disorders of the vulva. https://www.clinicaltrialsregister.eu/ctr‐search/search?query=eudract_number:2011‐003134‐13 (accessed 9 February 2015) 2011.
ISRCTN98495886 {published data only}
- Kaur B. Phase II clinical trial investigating the use of EPIgallocatechin‐3‐gallate (Veregen) in the treatment of vulval intraepithelial neoplasia. http://www.isrctn.com/ISRCTN98495886 (accessed 9 February 2015) 2013.
NCT01861535 {published data only}
- Trutnovsky G, Tamussino K. Primary imiquimod treatment versus surgery for vulvar intraepithelial neoplasia. https://clinicaltrials.gov/show/NCT01861535 (accessed 9 February 2015) 2013.
NTR1526 {published data only}
- Kenter G, Nijman HW. Randomised controlled study on the effects of imiquimod, a TLR 7 activating agent, on the HPV16‐specific immune response following HPV16 E6/E.7 synthetic long peptides vaccination in women with HPV16 positive high grade vulvar/vaginal intraepithelial neoplasia. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=1526 (accessed 9 February 2015) 2008.
Additional references
Aerts 2012
- Aerts L, Enzlin P, Vergote I, Verhaeghe J, Poppe W, Amant F. Sexual, psychological, and relational functioning in women after surgical treatment for vulvar malignancy: a literature review. Journal of Sexual Medicine 2012;9(2):361‐71. [DOI] [PubMed] [Google Scholar]
Andreasson 1986
- Andreasson B, Moth I, Jensen SB, Bock JE. Sexual function and somatopsychic reactions in vulvectomy‐operated women and their partners. Acta Obstetricia et Gynecologica Scandinavica 1986;65(1):7‐10. [DOI] [PubMed] [Google Scholar]
Baandrup 2011
- Baandrup L, Varbo A, Munk C, Johansen C, Frisch M, Kjaer SK. In situ and invasive squamous cell carcinoma of the vulva in Denmark 1978‐2007 ‐ a nationwide population‐based study. Gynecologic Oncology 2011;122(1):45‐9. [DOI] [PubMed] [Google Scholar]
Baldwin 2003
- Baldwin PJ, Burg SH, Boswell CM, Offringa R, Hickling JK, Dobson J, et al. Vaccinia‐expressed human papillomavirus 16 and 18 e6 and e7 as a therapeutic vaccination for vulval and vaginal intraepithelial neoplasia. Clinical Cancer Research 2003;9(14):5205‐13. [PubMed] [Google Scholar]
BASHH guidelines 2014
- Clinical Effectiveness Unit of the British Association of Sexual Health and HIV. National guidelines on the management of vulval conditions. http://www.bashh.org/documents/UK%20national%20guideline%20for%20the%20management%20of%20vulval%20conditions%202014.pdf (accessed 3 February 2015).
Committee 2011
- The Committee in Gynecologic Practice of the American College of Obstetricians and Gynecologists and the American Society for Colposcopy and Cervical Pathology. Management of vulvar intraepithelial neoplasia. American Society for Colposcopy and Cervical Pathology 2011;Number 509:1‐4. [Google Scholar]
CTCAE 2006
- CTCAE. Common Terminology Criteria for Adverse Events v3.0. http://ctep.cancer.gov/forms/CTCAEv3.pdf (accessed 9 August 2006).
Daayana 2010
- Daayana A, Elkord E, Winters U, Pawlita M, Roden R, Stern PL, et al. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. British Journal of Cancer 102;7:1129‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Sanjose 2013
- Sanjose S, Alemany L, Ordi J, Tous S, Alejo M, Bigby SM, et al. Worldwide human papillomavirus genotype attribution in over 2000 cases of intraepithelial and invasive lesions of the vulva. European Journal of Cancer 2013;49(16):3450‐61. [DOI] [PubMed] [Google Scholar]
De Vuyst 2009
- Vuyst H, Clifford GM, Nascimento MC, Madeleine MM, Franceschi S. Prevalence and type distribution of human papillomavirus in carcinoma and intraepithelial neoplasia of the vulva, vagina and anus: a meta‐analysis. International Journal of Cancer 2009;124(7):1626‐36. [DOI] [PubMed] [Google Scholar]
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context :. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Dittmer 2011
- Dittmer C, Katalinic A, Mundhenke C, Thill M, Fischer D. Epidemiology of vulvar and vaginal cancer in Germany. Archives of Gynecology and Obstetrics 2011;284(1):169‐74. [DOI] [PubMed] [Google Scholar]
Dominiak‐Felden 2013
- Dominiak‐Felden G, Cohet C, Atrux‐Tallau S, Gillet H, Tristram A, Fiander A. Impact of human papillomavirus‐related genital diseases on quality of life and psychosocial wellbeing: results of an observational, health‐related quality of life study in the UK. BMC Public Health 2013;13:1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dougherty 1998
- Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, et al. Photodynamic therapy. Journal of National Cancer Institute 1998;90(12):889‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenhauer 2009
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer 2009;45:228‐47. [DOI] [PubMed] [Google Scholar]
EndNote 2013 [Computer program]
- Thomas Reuters. Website Endnote.com. EndNote X7. Version 7.02. Thomas Reuters. Website Endnote.com, 2013.
EuroQoL 1990
- The EuroQoL Group. EuroQol – a new facility for the measurement of health‐related quality of life. Health Policy 1990;16:199‐208. [DOI] [PubMed] [Google Scholar]
Foster 1981
- Foster DC, Woodruff JD. The use of dinitrochlorobenzene in the treatment of vulvar carcinoma in situ. Gynecological Oncology 1981;11(3):330‐9. [DOI] [PubMed] [Google Scholar]
Garrat 1995
- Garrat AM, Torgerson DJ, Wyness J, Hall MH, Reid DM. Measuring sexual function in premenopausal women. British Journal of Obstetrics and Gynaecology 1995;102:311‐5. [DOI] [PubMed] [Google Scholar]
GRADE 2008 [Computer program]
- Jan Brozek, Andrew Oxman, Holger Schünemann. GRADEpro. Version 3.6 for Windows. Jan Brozek, Andrew Oxman, Holger Schünemann, 2008.
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hillemanns 2000
- Hillemanns P, Untch M, Dannecker C, Baumgartner R, Stepp H, Diebold J, et al. Photodynamic therapy of vulvar intraepithelial neoplasia using 5‐aminolevulinic acid. International Journal of Cancer 2000;85(5):649‐53. [DOI] [PubMed] [Google Scholar]
Husseinzadeh 1999
- Husseinzadeh N, Recinto C. Frequency of invasive cancer in surgically excised vulvar lesions with intraepithelial neoplasia (VIN 3). Gynecologic Oncology 1999;73(1):119‐20. [DOI] [PubMed] [Google Scholar]
ISSVD 2014
- ISSVD. 2014 Bibliography. Current ISSVD terminology. http://issvd.org/wordpress/wp‐content/uploads/2014/02/2014‐BIBLIOGRAPHY‐CURRENT‐ISSVD‐TERMINOLOGYrev.pdf 2014.
Jones 1997
- Jones RW, Baranyai J, Stables S. Trends in squamous cell carcinoma of the vulva: the influence of vulvar intraepithelial neoplasia. Obstetrics and Gynecology 1997;90(3):448‐52. [DOI] [PubMed] [Google Scholar]
Joura 2000
- Joura EA, Lösch A, Haider‐Angeler MG, Breitenecker G, Leodolter S. Trends in vulvar neoplasia. Increasing incidence of vulvar intraepithelial neoplasia and squamous cell carcinoma of the vulva in young women. Journal of Reproductive Medicine 2000;45(8):613‐5. [PubMed] [Google Scholar]
Joura 2007
- Joura EA, Leodolter S, Hernandez‐Avila M, Wheeler CM, Perez G, Koutsky LA, et al. Efficacy of a quadrivalent prophylactic human papilloma virus (types 6, 11, 16, and 18) L1 virus‐like particle vaccine against high‐grade vulval and vaginal lesions: a combined analysis of three clinical trials. Lancet 2007;369:1693‐702. [DOI] [PubMed] [Google Scholar]
Judson 2006
- Judson PL, Habermann EB, Baxter NN, Durham SB, Virnig BA. Trends in the incidence of invasive and in situ vulvar carcinoma. Obstetrics and Gynecology 2006;107(5):1018‐22. [DOI] [PubMed] [Google Scholar]
Kauffman 1995
- Kauffman RH. Intraepithelial neoplasia of the vulva. Gynecological Oncology 1995;15(1):8‐21. [DOI] [PubMed] [Google Scholar]
Kaushik 2014
- Kaushik S, Pepas L, Nordin A, Bryant A, Dickinson HO. Surgical interventions for high‐grade vulval intraepithelial neoplasia. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD007928.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kenter 2009
- Kenter GG, Welters MJ, Valentijn AR, Lowik MJ, Berends‐van der Meer DM, Vloon AP, et al. Vaccination against HPV‐16 oncoproteins for vulvar intraepithelial neoplasia. New England Journal of Medicine 2009;361(19):1838‐47. [DOI] [PubMed] [Google Scholar]
Lai 2014
- Lai J, Elleray R, Nordin A, Hirschowitz L, Rous B, Gildea C, et al. Vulval cancer incidence, mortality and survival in England: age‐related trends. BJOG 2014;121(6):728‐38. [DOI] [PubMed] [Google Scholar]
McCluggage 2009
- McCluggage WG. Recent developments in vulvovaginal pathology. Histopathology 2009;54(2):156‐73. [DOI] [PubMed] [Google Scholar]
Modesitt 1998
- Modesitt SC, Waters AB, Walton L, Fowler W, Le L. Vulvar intraepithelial neoplasia III: occult cancer and the impact of margin status on recurrence. Obstetrics and Gynecology 1998;92(6):962‐6. [DOI] [PubMed] [Google Scholar]
Moore 2001
- Moore RA, Edwards JE, Hopwood J, Hicks D. Imiquimod for the treatment of genital warts: a quantitative systematic review. BMC Infectious Diseases 2001;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naransingh 2000
- Narayansingh GV, Cumming GP, Parkin DP, McConell DT, Honey E, Kolhe PS. Flap repair: an effective strategy for minimising sexual morbidity associated with the surgical management of vulval intraepithelial neoplasia. Journal of the Royal College of Surgeons of Edinburgh 2000;45(2):81‐4. [PubMed] [Google Scholar]
Newfield 1998
- Newfield L, Bradlow HL, Sepkovic DW, Auborn K. Estrogen metabolism and the malignant potential of human papilloma virus immortalised keratinocytes. Proceedings of the Society for Experimental Biology and Medicine 1998;217:322‐6. [DOI] [PubMed] [Google Scholar]
Nygard 2014
- Nygård M, Hansen BT, Dillner J, Munk C, Oddsson K, Tryggvadottir L, et al. Targeting human papillomavirus to reduce the burden of cervical, vulvar and vaginal cancer and pre‐invasive neoplasia: establishing the baseline for surveillance. PloS One 2014;9(2):e88323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pepas 2015
- Pepas L, Kaushik S, Bryant A, Nordin A, Dickinson HO. Medical interventions for high‐grade vulval intraepithelial neoplasia. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD007924.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Preti 2015
- Preti M, Igidbashian S, Costa S, Cristoforoni P, Mariani L, Origoni M, et al. VIN usual type ‐ from the past to the future. ecancermedicalscience 2015;29(9):531. [DOI: 10.3332/ecancer.2015.531] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Reyes 2014
- Reyes MC, Cooper K. An update on vulvar intraepithelial neoplasia: terminology and a practical approach to diagnosis. Journal of Clinical Pathology 2014;67:290‐4. [DOI] [PubMed] [Google Scholar]
Roberts 1980
- Roberts JA, Watring WG, Lagasse LD. Treatment of vulvar intraepithelial neoplasia (VIN) with local bleomycin. Cancer Clinical Trials 1980;3(4):351‐4. [PubMed] [Google Scholar]
Shylasree 2008
- Shylasree TS, Karanjgaokar V, Tristram A, Wilkes AR, MacLean AB, Fiander AN. Contributions of demographic, psychological and disease‐related factors to quality of life in women with high‐grade vulval intraepithelial neoplasia. Gynecologic Oncology 2008;110:185‐9. [DOI] [PubMed] [Google Scholar]
Sideri 2005
- Sideri M, Jones RW, Wilkinson EJ, Preti M, Heller DS, Scurry J, et al. Squamous vulvar intraepithelial neoplasia: 2004 modified terminology, ISSVD Vulvar Oncology Subcommittee. Journal of the Reproductive Medicine 2005;50(11):807‐10. [PubMed] [Google Scholar]
Sillman 1985
- Sillman FH, Sedlis A, Boyce JG. A review of lower genital intraepithelial neoplasia and the use of topical 5‐fluorouracil. Obstetrical and Gynecological Survey 1985;40(4):190‐220. [DOI] [PubMed] [Google Scholar]
Stern 2012
- Stern PL, Burg SH, Hampson IN, Broker T, Fiander A, Lacey CJ, et al. Therapy of human papillomavirus‐related disease. Vaccine 2012;30:F71‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2014
- Sterne JAC, Higgins JPT, Reeves BC, on behalf of the development group for ACROBAT‐NRSI. A Cochrane risk of bias tool: for non randomised studies of interventions (ACROBAT‐NRSI). Version 1.0.0. Available from http://www.riskofbias.info 2014 (accessed 1 December 2014).
Stier 2013
- Stier EA, Goldstone SE, Einstein MH, Jay N, Berry JM, Wilkin T, et al. Safety and efficacy of topical cidofovir to treat high‐grade perianal and vulvar intraepithelial neoplasia in HIV‐positive men and women. AIDS 2013;27(4):545‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Terlou 2011
- Terlou A, Seters M, Ewing PC, Aaronson NK, Gundy CM, Heijmans‐Antonissen C, et al. Treatment of vulvar intraepithelial neoplasia with topical imiquimod: seven years median follow‐up of a randomized clinical trial. Gynecologic Oncology 2011;121(1):157‐62. [DOI] [PubMed] [Google Scholar]
Tristram 2005
- Tristram A, Fiander A. Clinical responses to cidofovir applied topically to women with high grade vulval intraepithelial neoplasia. Gynecologic Oncology 2005;99(3):652‐5. [DOI] [PubMed] [Google Scholar]
van der Avoort 2006
- Avoort I, Shirango H, Hoevenaars BM, Grefte JM, Hullu JA, Wilde PC, et al. Vulvar squamous cell carcinoma is a multifactorial disease following two separate and independent pathways. International Journal of Gynecological Pathology 2006;25(1):22‐9. [DOI] [PubMed] [Google Scholar]
van Esch 2012
- Esch EM, Welters MJ, Jordanova ES, Trimbos JB, Burg SH, Poelgeest MI. Treatment failure in patients with HPV 16‐induced vulvar intraepithelial neoplasia: understanding different clinical responses to immunotherapy. Expert Review of Vaccines 2012;11(7):821‐40. [DOI] [PubMed] [Google Scholar]
van Esch 2015
- Esch EM, Poelgeest MI, Trimbos JB, Fleuren GJ, Jordanova ES, Burg SH. Intraepithelial macrophage infiltration is related to a high number of regulatory T cells and promotes a progressive course of HPV‐induced vulvar neoplasia. International Journal of Cancer 2015;136(4):E85‐94. [DOI] [PubMed] [Google Scholar]
Van Seters 2005
- Seters M, Beurden M, Craen AJ. Is the assumed natural history of vulvar intraepithelial neoplasia III based on enough evidence? A systematic review of 3322 published patients. Gynecologic Oncology 2005;97(2):645‐51. [DOI] [PubMed] [Google Scholar]
Wilkinson 2015
- Wilkinson EJ, Cox JT, Selim MA, O'Connor DM. Evolution of terminology for human‐papillomavirus‐infection‐related vulvar squamous intraepithelial lesions. Journal of Lower Genital Tract Disease 2015;19(1):81‐7. [DOI] [PubMed] [Google Scholar]
Winters 2008
- Winters U, Daayana S, Lear JT, Tomilson AE, Elkord E, Stern PL, et al. Clinical and immunologic results of a phase II trial of sequential imiquimod and photodynamic therapy for vulval intraepithelial neoplasia. Clinical Cancer Research 2008;14(16):5292‐9. [DOI] [PubMed] [Google Scholar]
Wu 2011
- Wu CH, Liang CH, Shiu LY, Chang LC, Lin TS, Lan CC, et al. Solanum incanum extract (SR‐T100) induces human cutaneous squamous cell carcinoma apoptosis through modulating tumor necrosis factor receptor signaling pathway. Journal of Dermatological Science 2011;63(2):83‐92. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Nordin 2015
- Nordin A, Lawrie, TA, Pepas L, Kaushik S, Bryant A. Medical and surgical interventions for the treatment of usual‐type vulval intraepithelial neoplasia. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD011837] [DOI] [PMC free article] [PubMed] [Google Scholar]


