Abstract
Background
Asthma is one of the most common reasons for hospital admission among children and constitutes a significant economic burden. Use of non‐invasive positive pressure ventilation (NPPV) in the care of children with acute asthma has increased even though evidence supporting the intervention has been considered weak and clinical guidelines do not recommend the intervention. NPPV might be an effective intervention for acute asthma, but no systematic review has been conducted to assess the effects of NPPV as an add‐on therapy to usual care in children with acute asthma.
Objectives
To assess the benefits and harms of NPPV as an add‐on therapy to usual care (e.g. bronchodilators and corticosteroids) in children with acute asthma.
Search methods
We identified trials from the Cochrane Airways Group Specialised Register (CAGR). The Register contains trial reports identified through systematic searches of bibliographic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, CINAHL, AMED and PsycINFO, and by handsearching of respiratory journals and meeting abstracts. We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the WHO trials portal (www.who.int/ictrp/en/). We searched all databases from their inception to February 2016, with no restriction on language of publication.
Selection criteria
We included randomised clinical trials (RCTs) assessing NPPV as add‐on therapy to usual care versus usual care for children (age < 18 years) hospitalised for an acute asthma attack.
Data collection and analysis
Two review authors independently screened titles and abstracts. We retrieved all relevant full‐text study reports, independently screened the full text, identified trials for inclusion and identified and recorded reasons for exclusion of ineligible trials. We resolved disagreements through discussion or, if required, consulted a third review author. We recorded the selection process in sufficient detail to complete a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐analyses) flow diagram and 'Characteristics of excluded studies' table. We identified the risk of bias of included studies to reduce the risk of systematic error. We contacted relevant study authors when data were missing.
Main results
We included two RCTs that randomised 20 participants to NPPV and 20 participants to control. We assessed both studies as having high risk of bias; both trials assessed effects of bilateral positive airway pressure (BiPAP). Neither trial used continuous positive airway pressure (CPAP). Controls received standard care. Investigators reported no deaths and no serious adverse events (Grades of Recommendation, Assessment, Development and Evaluation (GRADE): very low quality of evidence due to serious risk of bias and serious imprecision of results). Both trials showed a statistically significant reduction in symptom score. One trial did not report a standard deviation (SD), but by using an estimated SD, we found a statistically significantly reduced asthma symptom score (mean difference (MD) ‐2.50, 95% confidence interval (CI) ‐4.70 to ‐0.30, P = 0.03, 19 participants, GRADE: very low quality of evidence). In the other trial, NPPV was associated with a lower total symptom score (5.6 vs 1.9, 16 participants, very low quality of evidence) before cross‐over, but investigators did not report an SD, nor could it be estimated from the first phase of the trial, before the cross‐over. These gains could be clinically relevant, as a reduction of three or more points in symptom score is considered a clinically meaningful change. Researchers documented five dropouts (12.5%), four of which were due to intolerance to NPPV, and one to respiratory failure requiring intubation. Owing to insufficient reporting in the latter trial and use of different scoring systems, it was not possible to conduct a meta‐analysis nor a Trial Sequential Analysis.
Authors' conclusions
Current evidence does not permit confirmation or rejection of the effects of NPPV for acute asthma in children. Large RCTs with low risk of bias are warranted.
Plain language summary
Non‐invasive positive pressure ventilation for acute asthma in children
Review question
We reviewed available evidence on non‐invasive positive pressure ventilation (NPPV) for children with acute asthma.
Background
Asthma is known to cause acute exacerbations, in which characteristic changes in the lungs predispose to respiratory difficulties and in some cases respiratory failure. This condition constitutes a significant economic burden and a major health issue worldwide. Evidence supporting this intervention has been considered weak, and the intervention is not recommended in clinical guidelines. Nevertheless, use of NPPV in the care of children with acute asthma has increased, and NPPV might be an effective intervention for acute asthma. Until now, no systematic review has summed up all the evidence provided by randomised clinical trials.
Study characteristics
The evidence is current to August 2016. We included two trials, with 40 participants. Included trials assessed the effects of one type of NPPV called bilevel positive airway pressure, which lasted for two and 24 hours, respectively, in the two trials.
Key results
Overall, we found that NPPV compared with no additional treatment, treatment as usual or placebo did not result in any benefit or harm regarding death from all causes, serious adverse events (i.e. major complications) or improvement in asthma symptoms. Five study participants did not tolerate the treatment, four because of discomfort and one because intubation was required. Current evidence cannot confirm or reject the effects of NPPV for treatment of children with acute asthma. Larger randomised clinical trials are warranted.
Quality of the evidence
The evidence behind our conclusions is of very low quality. The two studies had high risk of bias (i.e. the studies were conducted in a way that may skew results to the positive side). In addition, the two studies included few participants, making results of this review imprecise.
Summary of findings
for the main comparison.
| Non‐invasive positive pressure ventilation for children with acute asthma | ||||||
| Patient or population: children with acute asthma Setting: hospital Intervention: non‐invasive ventilation as add‐on therapy to standard care Comparison: standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with standard care | Risk with non‐invasive ventilation as add‐on therapy to standard care | |||||
| Mortality | Study population | not estimable | 16 (1 RCT) | ⊕⊝⊝⊝ very lowa | No deaths were seen. | |
| 0 per 1000 | 0 per 1000 | |||||
| Serious adverse events | Study population | not estimable | 35 (2 RCTs) | ⊕⊝⊝⊝ very lowa | No serious adverse events were reported | |
| not pooled | not pooled | |||||
| Asthma symptom score at the acute phase | not estimable | not pooled | ‐ | 35 (2 RCTs) | ⊕⊝⊝⊝ very lowa |
Basnet 2012: Children in NPPV group
had an improvement in their mean CAS
from 7 (median, 7; interquartile range,
6 to 8) at baseline to 1.6 (median, 2; interquartile
range, 2 to 2.8) at 24 hours vs mean
CAS from 6.9 (median, 7; interquartile
range, 6 to 8) to 4.1 (median, 3.5; interquartile
range, 3 to 5.5) in the standard
group (P < 0.01) Thill 2004: NPPV was associated with lower total CAS (5.6 vs 1.9) before cross‐over and lower scores for each individual component (accessory muscle use, wheeze and dyspnoea; all P < 0.01) |
| Pneumonia | Study population | not estimable | 19 (1 RCT) | ⊕⊝⊝⊝ very lowa | ||
| 0 per 1000 | 0 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded by 4 levels because of very serious risk of bias and very serious imprecision of results.
Background
The prevalence of asthma in children around the world has increased in recent decades (Masoli 2004; AIHW 2010; Asher 2014). Asthma affects approximately 334 million people globally (Asher 2014). The highest prevalence of asthma among children is seen in high‐income countries (Asher 2014). Recent reviews have concluded that asthma is the most common chronic disease in children, and is among the top 10 chronic conditions in global ranking of disability‐adjusted life‐years during mid‐childhood (Papadopoulos 2012; Asher 2014). In 2012, 14% of US children had at some point been diagnosed with asthma, and 8.3% had asthma during the study period (Bloom 2013; CDC 2013).
Millions of patients worldwide continue to have suboptimal asthma control, possibly as the result of suboptimal treatment (Papadopoulos 2012). A serial, cross‐sectional analysis revealed that asthma seems to account for 2.3% of all hospitalisations among children (Hasegawa 2013). Data from the Centers for Disease Control and Prevention (CDC) indicate that annual rates of emergency department treatment and hospitalisation have ranged from 7 to 9.5 and from 1.9 to 2.5 per 100 US children with asthma, respectively (Akinbami 2012). Current international mortality rates for children with asthma range from 0.0 to 0.7 per 100,000 children (Asher 2014). Great variance across countries is apparent, as demonstrated, for example, by the higher Australian mortality rate for asthma at 1.67 (95% confidence interval (CI) 1.51 to 1.85) per 100,000 population (AIHW 2010; Asher 2014). Several high‐risk populations have been noted, as prevalence seems to be affected by sex, ethnicity and socioeconomic status. In contrast to adults, predominance among males has been reported among children (Bloom 2013; Wade 2015). Children with a multiethnic background seem to have the highest prevalence, at 14.1%, compared with children of Asian ethnicity, who had the lowest prevalence at 5.2% (Akinbami 2012). Prevalence is also higher among children of lower socioeconomic status (Akinbami 2012; Bloom 2013).
The effect of asthma on quality of life was investigated among adults in a survey from 2010, on which the proportion of patients that rated their health as 'fair to poor' was 25% among adults with asthma compared with 14% among people without asthma (AIHW 2010). Another survey suggests that the effect of asthma on quality of life is even more profound among children aged 12 to 17 compared with adults, perhaps resulting from the need for greater limits on their daily activities and frequent absence from school (Alith 2015).
Description of the condition
Asthma is a chronic inflammatory respiratory condition that leads to persistent remodelling of the small airways and bronchial hyper‐responsiveness. This hyper‐responsiveness is an immune response that asthmatic individuals display to certain stimuli that seem to have little or no effect on individuals with normal airways (Bloemen 2007; Kumar 2009). Hyper‐responsiveness is seen as characteristic acute asthmatic exacerbations of reversible bronchoconstriction, dyspnoea, wheezing and coughing (Brannan 2012; Hedlin 2012; Liu 2014). The diagnosis is determined primarily by the history of the patient and the clinical presentation (Liu 2014). In younger paediatric patients, the diagnosis might, however, present a challenge, as several conditions among preschool children can cause an obstructive pattern, which in turn presents as asthma‐like respiratory symptoms (e.g. wheeze, cough, shortness of breath) (Dijk 2013; Cave 2014; Mantzouranis 2014).
During acute exacerbations of asthma, the lumen of the airways narrows as a result of smooth muscle contraction in synergy with increased mucus production and bronchial oedema (Kumar 2009). This narrowing of the lumen reduces airflow into and out of the lungs, potentially resulting in respiratory failure (Kumar 2009). Asthma is also known to cause air trapping, lung hyperinflation and atelectasis (partial collapse or incomplete inflation of the lung) as the result of mucus plugging and bronchoconstriction (Blanch 2005; Finder 2014). The airway obstruction causes an increase in alveolar pressure, the so‐called 'intrinsic positive end‐expiratory airway pressure (PEEPi)' (Pepe 1982; Blanch 2005; Caramez 2005; Tzoufi 2005; Graham 2007). This PEEPi is proportionately higher when airway obstruction becomes more severe (Aldrich 1993).
Although several genes have been associated with increased risk of asthma, the causes of asthma are not completely understood (Bisgaard 2009; Mantzouranis 2014). It is known that environmental exposure to allergens is essential for development of the sensitisation that is seen in asthma (Galli 2008; Hedlin 2012; Jackson 2012; Jobe 2014; Yoo 2014). This is exemplified by the fact that prevalence is higher among children living in rural areas than in children from urban areas (Douwes 2002; Mantzouranis 2014).
No curative treatment for asthma is known, although some children grow out of asthma (Papadopoulos 2012; GINA 2015). Conventional treatment depends on the severity of the condition. In an acute asthma attack/exacerbation, first‐line pharmacological treatment usually consists of bronchodilators (beta2‐agonists) and corticosteroids (Papadopoulos 2012; Sawicki 2014a; GINA 2015). Second‐line agents such as ipratropium bromide (anticholinergic agent) and magnesium sulphate are recommended and used in some cases (GINA 2015; Scarfone 2015). Care in acute situations most often seeks to promote bronchodilation and antagonise the mediators of inflammation and bronchoconstriction, thereby upholding sufficient ventilation (NHLBI 2007). Long‐term treatment focuses on prevention of asthma attacks. This is usually achieved with inhalation of corticosteroids, strategies to avoid triggers and smoking cessation, as well as use of long‐acting beta2‐agonists when warranted by the frequency of attacks (GINA 2015). Several asthma severity scores have been created to assess the severity of acute asthma exacerbations among children (Chalut 2000; Ducharme 2008; Alnaji 2014; Sawicki 2014b).
Description of the intervention
Respiratory support can be divided into invasive ventilation and non‐invasive ventilation (Pavone 2013). In invasive ventilation, air is given through an internal interface directly into the trachea (e.g. intubation, tracheostomy). In non‐invasive ventilation, air is given through an external interface (e.g. facemask, nasal cannula) (Pavone 2013).
Non‐invasive positive pressure ventilation (NPPV)
Non‐invasive positive pressure ventilation (NPPV) employs a full facial mask or nasal cannula that administers ventilatory support from a flow generator (Teague 2005; Pavone 2013). Non‐invasive positive pressure ventilation includes continuous positive airway pressure (CPAP) and bilevel positive airway pressure (BiPAP). The positive end‐expiratory airway pressure created by NPPV is distinct from the PEEPi described under Description of the condition and is sometimes referred to as an extrinsic intrinsic positive end‐expiratory airway pressure (PEEPe) (Graham 2007).
Continuous positive airway pressure (CPAP)
In CPAP, a single constant positive airway pressure is maintained throughout the respiratory cycle. Therefore, CPAP does not assist spontaneous inspiration of the patient (Boldrini 2012; Pavone 2013). Instead, higher intraluminal pressures prevent collapse in the upper airways, thereby promoting alveolar recruitment (Pavone 2013). The consequent alveolar recruitment increases functional residual capacity and counteracts the development of atelectasis (Pavone 2013). Through this mechanism, CPAP aims to improve oxygenation and reduce the workload for inspiratory muscles, thereby reducing the work of breathing (Shivaram 1987; Pavone 2013). A recent observational study suggested that CPAP might provide a beneficial effect for children with acute asthma by initiating autonomic modulation, thereby producing a bronchodilator effect that may go beyond the mechanical effect described previously (de Freitas 2013).
Bilevel positive airway pressure (BiPAP)
In BiPAP, pressure in inspiratory and expiratory phases of the respiratory cycle can be adjusted separately (Gupta 2010). When BiPAP is used, a positive expiratory airway pressure and an even higher inspiratory positive airway pressure are induced compared with physiological breathing (Pavone 2013). BiPAP therefore creates a difference between expiratory and inspiratory phases, which is thought to support the spontaneous inspiratory act of the patient (Gupta 2010; Pavone 2013).
Continuous positive airway pressure (CPAP) and BiPAP are not among the conventional interventions for asthma (GINA 2015). When patients do not respond to intensified conventional treatment (e.g. bronchodilators), they may require invasive ventilation (Lim 2012). Observational studies have reported that 10% to 12% of children admitted to the paediatric intensive care unit (PICU) with asthma required invasive ventilation (Malmstrom 2001; Bratton 2012). Invasive ventilation involves transfer to the intensive care unit and use of general anaesthesia and intubation. Invasive ventilation may result in complications such as damage to local tissue, difficulties weaning off the ventilator, ventilator‐associated pneumonia, pneumothorax, sinusitis, catheter‐related infection, urinary tract infection and bacteraemia (Fagon 1993; Brochard 1994; Esteban 1995; Guerin 1997; Nourdine 1999; Teague 2005). Invasive ventilation has been shown to be associated with higher morbidity compared with non‐invasive ventilation in intensive care unit patients in randomised clinical trials assessing outcomes such as the complications mentioned previously (Brochard 2002).
Both CPAP and BiPAP offer the advantage that they can be applied earlier and intermittently compared with invasive ventilation. NPPV may be efficient enough to reverse an acute exacerbation so invasive ventilation is avoided (Lim 2012). Several observational studies show a trend favouring NPPV for other causes of respiratory failure, including acute respiratory distress syndrome and acute lung injury (defined as acute hypoxaemic respiratory failure), as well as acute hypercapnic respiratory failure such as chronic obstructive pulmonary disease (COPD) and asthma (Brochard 2002; Keenan 2009). For these reasons, NPPV may prove beneficial in children with acute asthma.
How the intervention might work
Non‐invasive positive pressure ventilation (NPPV) may have a direct bronchodilating effect, may improve alveolar recruitment and may increase response to bronchodilators (Lin 1995; Wang 1996). At least some of the effect is thought to be independent of better drug dispersion (Buda 1979; Soroksky 2003; Tzoufi 2005). As described in Description of the condition, an intrinsic positive end‐expiratory airway pressure is created during an asthma attack, and NPPV is thought to counteract this intrinsic PEEP by creating an extrinsic PEEP (Broux 1991). The result consists of presumed improvement in airflow, re‐expansion of atelectatic lung segments, reversal of hyper‐inflation and supportive inspiratory muscles (Martin 1982; Shivaram 1987; Moloney 1999; Caramez 2005; Pavone 2013). This might in turn correct ventilation‐perfusion mismatches and reduce the work of breathing (Andersen 1979; Martin 1982; Broux 1991; Aldrich 1993; Soroksky 2003; Graham 2007).
A comparative study has shown that CPAP might reduce bronchial reactivity and sensitivity in histamine‐induced asthma (Lin 1995). Another small observational study suggested that CPAP might assist inspiratory muscles and reduce the work of breathing in methacholine‐induced asthma (Martin 1982).
Bilateral positive airway pressure has been shown to improve breathing, oxygenation, clinical asthma scores and alveolar ventilation in several observational studies (Akingbola 2002; Beers 2007; Mayordomo‐Colunga 2011; Williams 2011). Beers 2007 showed that BiPAP seemed to improve oxygenation and decrease the respiratory rate, while none of these children experienced a rise in respiratory rate nor a decrease in saturation. Observational studies also suggest that BiPAP might decrease the proportion of children who need intubation among children with severe asthma (Mayordomo‐Colunga 2011; Williams 2011).
Why it is important to do this review
Asthma is one of the most common causes of hospital admission for children and constitutes a significant economic burden (Braman 2006; Bahadori 2009; Jacob 2015). Use of NPPV has increased in the care of children with acute asthma (Carroll 2013) even though evidence is lacking (GINA 2015). Non‐invasive positive pressure ventilation might be an effective intervention for acute asthma, but no systematic review has been conducted to assess effects of NPPV as an add‐on therapy to usual care in children.
Objectives
To assess the benefits and harms of non‐invasive positive pressure ventilation (NPPV) as an add‐on therapy to usual care (e.g. bronchodilators and corticosteroids) in children with acute asthma.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials regardless of publication type, publication status, publication date and language.
Types of participants
We included children (aged < 18 years) hospitalised for an asthma attack (as defined by the trialists). We excluded children with a primary diagnosis of pneumonia, acute aspiration, bronchiolitis, cystic fibrosis or any ciliary dyskinetic syndrome.
Types of interventions
Any type of NPPV (including continuous positive airway pressure (CPAP) and bilevel positive airway pressure (BiPAP)). We included trials comparing NPPV as an add‐on therapy to usual care versus usual care.
We excluded trials that examined weaning off invasive ventilation and trials in which NPPV or invasive ventilation preceded enrolment of children into the trial.
Types of outcome measures
Primary outcomes
All‐cause mortality
Proportion of participants with a serious adverse event defined as any untoward medical occurrence that resulted in death, was life threatening, jeopardised the participant, was persistent or led to significant disability or prolonged hospitalisation (ICH‐GCP 1997)
Symptom score in the acute phase (e.g. Pediatric Asthma Severity Score (PASS), Pediatric Respiratory Assessment Measure (PRAM))
Secondary outcomes
Non‐serious adverse events (ICH‐GCP 1997)
Health‐related quality‐of‐life measures for long‐term follow‐up in children after major trauma, such as DISABKIDS, KIDSCREEN‐52 and PedsQL
Arterial blood gases and pH
Pneumonia diagnosed after randomisation (as defined by trialists)
Cost
We assessed symptom score in the acute phase at the time point of primary interest defined by study authors. If such a time point of primary interest was not defined, we planned to use the outcome assessed at the first time point after the start of the intervention, but before discharge.
All additional dichotomous and continuous outcomes were assessed at two time points.
Outcomes assessed at the time point closest to hospital discharge.
Outcomes assessed at maximal follow‐up (this was the time point of primary interest).
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Information Specialist for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases, including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and by handsearching of respiratory journals and meeting abstracts (see Appendix 1 for details). We searched all records in the CAGR using the search strategy provided in Appendix 2. See Appendix 1 and Appendix 2 for our exact search terms. We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/). We searched all databases from their inception up to February 2016, and we imposed no restriction on language of publication.
Searching other resources
We checked the reference lists of all relevant primary trials and reviews for additional references.
We tried to identify unpublished trials by searching the clinical trial registers of Europe and the USA, websites of pharmaceutical companies and websites of the US Food and Drug Administration (FDA) and the European Medicines Agency.
We searched for errata and retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Two review authors (SKK and JF) independently screened titles and abstracts. We retrieved all relevant full‐text study reports/publications, and two review authors (SKK and JF) independently screened the full text, identified trials for inclusion and identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion or, if required, we consulted a third review author (JCJ). We recorded the selection process in sufficient detail to complete a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐analyses) flow diagram (Moher 2009) and a Characteristics of excluded studies table.
Data extraction and management
We recorded trial characteristics and outcome data using data collection forms that had been piloted on at least one trial in the review. Two review authors (SKK and JF) extracted the following trial characteristics from included trials, when available.
Methods: trial design, total duration of the trial, number of trial centres and locations, trial setting, withdrawals and trial dates.
Participants: number of participants in each intervention group, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, pressure device, mask interface and concomitant medications.
Outcomes: primary and secondary outcomes specified and collected and time points reported.
Notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors (SKK and JF) independently extracted outcome data from included trials. We noted in the Characteristics of included studies table if outcome data were not reported in a useable way. We resolved disagreements by consensus or by consultation with a third person (JCJ). One review author (SKK) transferred data into the Review Manager (Review Manager 2014) file. We double‐checked that data were entered correctly by comparing data presented in the systematic review with data provided in the study reports. A second review author (JF) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
We used the instructions given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) in our evaluation of the methods and hence the risk of bias of included trials. Again, two review authors (SKK and JF) each independently assessed the included trials. We evaluated the methods in terms of generation of allocation sequence, allocation concealment, blinding of participants and treatment providers, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other sources of bias, because these components enabled classification of randomised trials with low risk of bias and high risk of bias. The latter trials tend to overestimate positive intervention effects and underestimate negative effects (Moher 1998; Kjaergard 2001; Gluud 2006; Wood 2008; Lundh 2012; Savovic 2012).
We classified trials according to the components below.
Allocation sequence generation
Low risk: if sequence generation was achieved using a computer random number generator or a random numbers table. Drawing lots, tossing a coin, shuffling cards and throwing dice were also considered adequate if performed by an independent adjudicator.
Unclear risk: if the method of randomisation was not specified, but the trial was still presented as randomised.
High risk: if the allocation sequence was not randomised or was only quasi‐randomised.
Allocation concealment
Low risk: if allocation of participants was performed by a central independent unit, an on‐site locked computer, identical‐looking numbered sealed envelopes or drug bottles or containers prepared by an independent pharmacist or investigator.
Uncertain risk: if the trial was classified as randomised but the allocation concealment process was not described.
High risk: if the allocation sequence was familiar to the investigators who assigned participants.
Blinding of participants and treatment providers
Low risk: if participants and treatment providers were blinded to intervention allocation and this was described.
Uncertain risk: if the procedure of blinding was insufficiently described.
High risk: if blinding of participants and treatment providers was not performed.
Blinding of outcome assessment
Low risk of bias: if it was mentioned that outcome assessors were blinded and this was described.
Uncertain risk of bias: if it was not mentioned whether outcome assessors in the trial were blinded, or if the extent of blinding was insufficiently described.
High risk of bias: if no blinding or incomplete blinding of outcome assessors was performed.
Incomplete outcome data
Low risk of bias: if missing data were unlikely to make treatment effects depart from plausible values. This could occur as (1) no drop‐outs or withdrawals reported for all outcomes, or (2) numbers and reasons for withdrawals and drop‐outs for all outcomes clearly stated and described as similar in both groups. Generally, we judged the trial as having low risk of bias due to incomplete outcome data if drop‐outs accounted for less than 5% of participants. However, the 5% cut‐off is not definitive.
Uncertain risk of bias: if information was insufficient for assessment of whether missing data were likely to induce bias in the results.
High risk of bias: if results were likely to be biased owing to missing data because the pattern of drop‐outs could be described as different in the two intervention groups, or because the trial used improper methods in dealing with missing data (e.g. last observation carried forward).
Selective outcome reporting
Low risk of bias: if a protocol was published before or at the time the trial was begun and investigators reported on the outcomes specified in the protocol. If no protocol was available, or if the protocol was published after the trial had begun, reporting of all‐cause mortality and serious adverse events granted the trial a grade of low risk of bias.
Uncertain risk of bias: if no protocol was published and investigators did not report on the outcomes all‐cause mortality and serious adverse events.
High risk of bias: if study authors did not report on the outcomes included in the protocol.
Other bias
Low risk of bias: if the trial appeared to be free of other components (e.g. academic bias, for‐profit bias) that could put it at risk of bias.
Unclear risk of bias: if it was unclear whether other risk of bias could affect trial results.
High risk of bias: if other factors in the trial could put it at risk of bias (e.g. authors have conducted trials on the same topic, for‐profit bias is present) .
Overall risk of bias
We assessed overall risk of bias in three groups, as it may be difficult to blind participants and treatment providers performing NPPV.
Low risk of bias: We classified the outcome result as having overall 'low risk of bias' only if all of the bias domains described in the previous paragraphs were classified as presenting low risk of bias.
Potentially medium risk of bias: We classified the outcome result as having overall 'potentially medium risk of bias' if all bias domains described in the previous paragraphs, excluding 'blinding of participants and personnel', were classified as presenting 'low risk of bias'.
High risk of bias: We classified the outcome result as having 'high risk of bias' if any of the bias risk domains described previously, excluding 'blinding of participants and personnel', were classified as presenting 'unclear' or 'high risk of bias'.
We assessed the domains 'Blinding of outcome assessment', 'Incomplete outcome data' and 'Selective outcome reporting' for each outcome. Thus, we planned to assess the bias risk for each result, as well as for each trial. We based our primary conclusions as well as our presentation in Table 1 on the results of our primary outcomes with low risk of bias.
We graded each potential source of bias as high, low or unclear and provided a quote from the trial report together with a justification for our judgement in the Risk of bias in included studies table. We summarised 'Risk of bias' judgements across different trials for each of the domains listed.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Korang 2016) and reported any deviations under Differences between protocol and review.
Measures of treatment effect
Dichotomous outcomes
We calculated risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcomes.
Continous outcomes
We calculated mean differences (MDs) and standardised mean differences (SMDs) with 95% CIs for continuous outcomes.
Dealing with missing data
Dichotomous outcomes
We did not impute missing values for any outcomes in our primary analysis. In two of our sensitivity analyses, we planned to impute data (see Sensitivity analysis).
Continuous outcomes
We primarily analysed scores assessed at single time points. If only changes from baseline scores were reported, we planned to analyse the results together with follow‐up scores (Higgins 2011). If standard deviations (SDs) were not reported, we planned to calculate SDs using data from the trial if possible. We did not use intention‐to‐treat data if the original report did not contain such data. We did not impute missing values for any outcomes in our primary analysis. In our sensitivity analysis for continuous outcomes, we planned to impute data (see Sensitivity analysis).
We contacted investigators and trial sponsors to verify key trial characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only).
Assessment of heterogeneity
We planned to visually inspect forest plots to assess signs of heterogeneity and to explore possible heterogeneity in our prespecified subgroup analyses. We inspected trial characteristics across trials to identify clinical heterogeneity. We planned to assess the presence of statistical heterogeneity by using the Chi2 test (threshold P < 0.10) and to measure the quantities of heterogeneity via the I2 statistic (Higgins 2002; Higgins 2003).
Assessment of reporting biases
We planned to use a funnel plot to assess reporting bias if we included 10 or more trials. We planned to visually inspect funnel plots to assess the risk of bias. For dichotomous outcomes, we planned to test asymmetry by using the Harbord test (Harbord 2006). For continuous outcomes, we planned to use the regression asymmetry test (Egger 1997) and the adjusted rank correlation (Begg 1994). However, we did not perform any of these tasks because data were insufficient.
Data synthesis
Meta‐analysis
We undertook this meta‐analysis according to the recommendations stated in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used the statistical software Review Manager 5 (Review Manager 2014) provided by Cochrane to analyse data.
We assessed our intervention effects by performing both random‐effects meta‐analyses (DerSimonian 1986) and fixed‐effect meta‐analyses (DeMets 1987). We used the more conservative point estimate of the two (Jakobsen 2014). We used three primary outcomes and, therefore, considered a P value of 0.025 or less as the threshold for statistical significance (Jakobsen 2014). We used an eight‐step procedure to assess whether thresholds for significance were crossed (Jakobsen 2014). We planned to base our primary conclusion on results with low risk of bias (Jakobsen 2014).
When multiple trial arms were reported in a single trial, we planned to include only the relevant arms. If two comparisons (e.g. CPAP vs usual care and BiPAP vs usual care) were combined in the same meta‐analysis, we planned to halve the control group to avoid double‐counting.
Trial Sequential Analysis
Traditional meta‐analysis runs the risk of random errors due to sparse data and repetitive testing of accumulating data during review updates. We therefore planned to perform Trial Sequential Analyses on the outcomes, to calculate the required information size and the cumulative Z‐curve breach of relevant trial sequential monitoring boundaries (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010; Thorlund 2011; TSA 2011). We wished to control risks of type I and type II errors. A more detailed description of Trial Sequential Analysis can be found at http://www.ctu.dk/tsa/.
For dichotomous outcomes, we planned to estimate the required information size based on the observed, unweighted proportion of participants with an outcome in the control group (the cumulative proportion of participants with an event in the control groups relative to all participants in the control groups), a relative risk reduction of 20%, an alpha of 2.5%, a beta of 20% and diversity as suggested by trials in the meta‐analysis. For continuous outcomes in the Trial Sequential Analysis, we planned to use the observed SD, a mean difference in the observed SD/2, risk of type I error of 2.5% and risk of type II error of 20%.
'Summary of findings' table
We created a 'Summary of findings' table using each of the prespecified outcomes. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence as it relates to the trials that contributed data to the meta‐analyses for prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and GRADEpro software. We justified all decisions to downgrade or upgrade the quality of studies by using footnotes, and we made comments to aid the reader's understanding of the review when necessary. We planned to present in the 'Summary of findings' table our results from trials with low risk of bias, as well as our results from all trials.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses for our primary outcomes.
High risk of bias trials versus low risk of bias trials.
Age of participants: neonates (zero to one month), infants (one month to one year), children of preschool age (one to five years), children of school age (five to 12 years), adolescents (over 12 years).
CPAP versus BIPAP.
Trials including children with ‘clearly defined severe asthma’ versus remaining trials.
Children who had good compliance with regular use of inhaled corticosteroids versus those who had poor or unknown compliance.
Children who had good compliance with regular use of long‐acting beta agonists versus those who had poor or unknown compliance.
Types of masks used to administer NPPV.
We planned to use the formal test for subgroup interactions in Review Manager (Review Manager 2014).
Sensitivity analysis
To assess the potential impact of missing data, we planned to perform the following sensitivity analyses on primary outcomes.
'Best‐worst‐case' scenario: We planned to assume that all participants lost to follow‐up in the experimental group survived, had no serious adverse events and had a beneficial outcome with regards to the symptom score; and that all participants with missing outcomes in the control group did not survive, had a serious adverse event and had a harmful outcome with regards to the symptom score.
'Worst‐best‐case' scenario: We planned to assume that all participants lost to follow‐up in the experimental group did not survive, had a serious adverse event and had a harmful outcome with regards to the symptom score; and that all participants lost to follow‐up in the control group survived, had no serious adverse event and had a beneficial outcome with regards to the symptom score.
When analysing continuous outcomes, we planned to define a ‘beneficial outcome’ as the group mean plus two standard deviations (SDs) of the group mean (we planned to then use one SD in another analysis), and a ‘harmful outcome’ as the group mean minus two SDs of the group mean (we planned to then use one SD in another analysis) (Jakobsen 2014).
We planned to present results of both scenarios in our review.
To assess the potential impact of missing SDs for continuous outcomes, we planned to perform the following sensitivity analysis.
When SDs are missing and it is not possible to calculate them, we planned to impute SDs from trials with similar populations and low risk of bias. If we found no such trials, we planned to impute SDs from trials with a similar population. As the final option, we would impute SDs from all trials.
We planned to present results of this scenario in our review.
Other post hoc sensitivity analyses might be warranted if we identified unexpected clinical or statistical heterogeneity during analysis of review results (Jakobsen 2014).
Results
Description of studies
We assessed all studies according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and the protocol for this review (Korang 2016). Characteristics of each study can be found in the Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies tables.
Results of the search
Our initial search identified 311 references. We found no duplicates. We deemed 33 studies relevant and obtained full texts for further evaluation (see Figure 1). Of these, we included two completed studies (Thill 2004; Basnet 2012). We added two ongoing trials to the review, but these trials have not yet reported any data (NCT02347462; NCT01497691).
1.
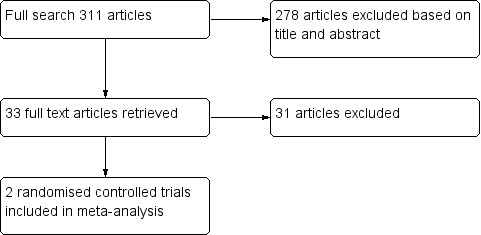
Flow diagram.
Included studies
Two trials met our inclusion criteria (Thill 2004; Basnet 2012). For detailed descriptions, see the Characteristics of included studies table.
Both were single‐centre trials conducted in a PICU setting in the United States.
Participants
The two studies included a total of 40 participants. The mean age of participants was six years (range, zero to 11 years), and the mean proportion of girls was 55%. We noted no statistically significant differences in any of the baseline characteristics described (Thill 2004; Basnet 2012). This lack of statistical significance very well could have been caused by random error due to small sample sizes or due to systematic error caused by failed randomisation.
Interventions
Both included trials used BiPAP. Basnet 2012 intervened with BiPAP for 24 hours, and Thill 2004 with BiPAP for two hours. Investigators gradually increased inspiratory positive airway pressure to 8 cm H2O in Basnet 2012, and to 10 cm H2O in Thill 2004. Both trials set end‐expiratory positive airway pressure to 5 cm H2O (Thill 2004; Basnet 2012) and first tried nasal masks; if this was unsuccessful, they applied full facemasks. Thill 2004 used an 'S/T machine' (Respironics, Murrysville, PA), whereas Basnet 2012 used 'Vision Bipap' (Respironics).
In both trials, both groups received similar planned standard care as a co‐intervention (Thill 2004; Basnet 2012). Neither of the included trials assessed the use of CPAP.
Co‐interventions
Participants in both trials received standard of care. This included continuous nebulised albuterol (as 10 mg/h (Thill 2004) or as 0.5 mg/kg, maximum 15 mg/kg (Basnet 2012)), intravenous methylprednisolone 1 to 2 mg/kg/d (maximum 80 mg/d) (Thill 2004; Basnet 2012) and supplemental oxygen. One trial also used adjunct therapy, which included magnesium sulfate and helium‐oxygen heliox mixtures (Basnet 2012).
Outcomes
Only Thill 2004 reported all‐cause mortality. Both trials reported serious adverse events and provided symptom scores (Thill 2004; Basnet 2012). Thill 2004 used the Clinical Asthma Score, and Basnet 2012 the Pediatric Asthma Severity Score.
Neither of these trials reported quality of life, costs or arterial blood gases/pH. Both trials reported on non‐serious adverse events (Thill 2004; Basnet 2012).
Excluded studies
We assessed 31 trials as relevant upon review of the abstract, but later excluded them upon review of the full publication. Eighteen of the excluded studies involved adult participants only (Anderson 1982; Branscomb 1982; Webber 1982; Frischknecht 1991; Christensen 1993; Aron 1999; Cross 2003; Soroksky 2003; de Miranda 2004; Bahera 2007; Soma 2008; Brandao 2009; Brandao 2009a; Chaudhry 2010; Sutherasan 2011; Hanekom 2012; Sutherasan 2013; Rondinel 2015). Three trials did not report the age of participants and did not mention inclusion of any children (Andersen 1982; Ferrari 2007; Filho 2009). We excluded four trials because the children were not hospitalised (Parkes 1997; Edwards 2003; Edwards 2004; Holbrook 2016). Three trials were not randomised (Thill 1998; Soma 2002; Basnet 2010), one trial did not include participants with asthma (Archis 2001) and in one trial, participants received something other than NPPV (Compagnoni 2000). One trial included children and adults but did not provide separate data for children (Fergusson 1983). See the Characteristics of excluded studies table for full information.
When participant age was unclear or separate data were not available for children, we contacted the study authors. However, we obtained no additional information on these trials.
Risk of bias in included studies
We assessed both included studies as having overall high risk of bias (see Figure 2). We contacted the authors of both trials for clarification, as some data were missing and several bias domains were unclear. We received no response from Basnet 2012, but Thill 2004 provided us with details concerning serious adverse events and the randomisation process.
2.
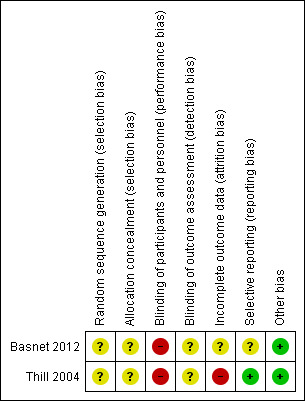
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Thill 2004 allocated participants with slips of paper drawn at random from an envelope, resulting in assessment of 'unclear' risk. Thill 2004 did not describe allocation concealment. Basnet 2012 did not describe how allocation sequence generation or allocation concealment was performed, and we judged risk in both domains as 'unclear'.
Blinding
Neither of the two included trials blinded participants or treatment providers. As we assessed several other bias domains as having high or uncertain risk, this did not affect the overall risk of bias in accordance with our protocol (Korang 2016). Neither trial described whether outcome assessors were blinded. However, both trials (Thill 2004; Basnet 2012) used independent investigators to assess symptom scores.
Incomplete outcome data
One trial reported four drop‐outs and did not include them in the analysis (Thill 2004). The other trial (Basnet 2012) performed a secondary intention‐to‐treat analysis but provided only a P value without full data for this analysis.
Selective reporting
One trial did not report mortality (Basnet 2012), resulting in an uncertain risk of bias. The other trial reported both mortality and serious adverse events (Thill 2004). Neither trial provided a pre‐published protocol.
Other potential sources of bias
Review authors observed no other biases.
Effects of interventions
See: Table 1
A total of two trials (Thill 2004; Basnet 2012) met all of the inclusion criteria. We were able to assess in part mortality, serious adverse events and symptom scores as primary outcomes in addition to the secondary outcomes of non‐serious adverse events and pneumonia.
Subgroup‐analyses based on risk, age, type of NPPV, type of mask and asthma severity were not performed, as the two trials were in the same category with regard to these subgroups. None of the trials described medical compliance.
Primary outcomes
All‐cause mortality
One trial with 16 participants (eight in the NPPV arm and eight in the control arm) assessed mortality (Thill 2004) and reported no events in either group (Analysis 1.1). Hence, we performed no meta‐analysis and no Trial Sequential Analysis (TSA).
1.1. Analysis.
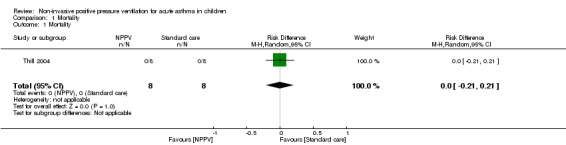
Comparison 1 Mortality, Outcome 1 Mortality.
Serious adverse events
Both trials assessed a total of 35 participants (17 in the NPPV arm and 18 in the control arm) for serious adverse events (Thill 2004; Basnet 2012) and reported no events in either group (Analysis 2.1). Hence, we performed no meta‐analysis and no TSA. Four participants in Thill 2004 did not complete the trial (one was intubated and three others reported discomfort with the BiPAP). However, researchers did not include these participants in their analysis.
2.1. Analysis.
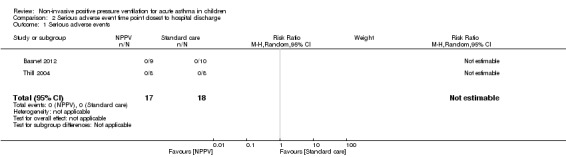
Comparison 2 Serious adverse event time point closest to hospital discharge, Outcome 1 Serious adverse events.
Symptom scores
Both trials assessed a total of 35 participants (17 in the NPPV arm and 18 in the control arm) for symptom scores (Thill 2004; Basnet 2012) (Analysis 3.1). However, neither the Basnet 2012 trial nor the first phase of the cross‐over trial (Thill 2004) reported standard deviations (SDs). We therefore had to estimate the standard error (SE) from the graph (Figure 2) in Basnet 2012 and then calculate the SD. We estimated the SE to be 0.985 (SD = 2.96) for the intervention group, and 0.539 (SD = 1.7) for the control group when assessing the primary time point of interest (zero to two hours). The calculated SD indicated that Basnet 2012 reported a reduced symptom score (mean difference (MD) ‐2.50, 95% confidence interval (CI) ‐4.70 to ‐0.30; P = 0.03, participants = 19, very low quality of evidence). Thill 2004 provided a figure illustrating symptom scores at the end of the intervention, for which a mean symptom score could be approximated as 1.9 in the NPPV group and 5.6 in the control group. According to our protocol, we would therefore try to impute SDs for the Thill 2004 trial. However, this could not be done because the two trials used different symptom score systems.
3.1. Analysis.
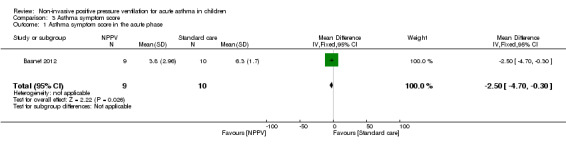
Comparison 3 Asthma symptom score, Outcome 1 Asthma symptom score in the acute phase.
Heterogeneity
As we performed no meta‐analysis, we did not estimate heterogeneity.
Trial Sequential Analysis
As we performed no meta‐analysis, we did not conduct a TSA.
Secondary outcomes
Non‐serious adverse events
The two trials assessed a total of 35 participants (17 in the NPPV arm and 18 in the control arm) (Thill 2004; Basnet 2012) for non‐serious adverse events (see Table 2).
1. Non‐serious adverse events.
| Trial | Events in experimental group | Events in control group |
| Thill 2004 | 4 (including 2 participants with mild nasal bridge pain, 1 with redness around the nose without skin breakdown and 1 with dry eyes) | 0 |
| Basnet 2012 | 0 | 0 |
Health‐related quality‐of‐life
No trials reported quality of life.
Arterial blood gases and pH
No trials reported blood gases or pH.
Pneumonia
One trial with 19 participants (nine in the NPPV arm and 10 in the control arm) assessed pneumonia (Basnet 2012) and reported no events in either group (Analysis 4.1). Hence, we performed no meta‐analysis.
4.1. Analysis.
Comparison 4 Pneumonia, Outcome 1 Pneumonia.
Cost
Neither trial reported cost.
Discussion
Summary of main results
We included two trials that randomised a total of 40 participants. We assessed these trials as having high risk of bias and a total of five drop‐outs. We were able to perform neither meta‐analysis nor Trial Sequential Analysis (TSA) on our primary outcome of mortality, as no events were reported. Both trials reported no serious adverse events. Meta‐analysis of symptom scores was not possible, as Thill 2004 was a cross‐over trial and did not report a standard deviation (SD), and we could not estimated the SD from before the cross‐over. We were not able to impute SDs from the Basnet 2012 trial, as investigators used two different types of symptom score systems. Both trials showed a statistically significant reduction in symptom scores. Basnet 2012 showed that non‐invasive positive pressure ventilation (NPPV) improved symptom scores (mean difference (MD) ‐2.50, 95% confidence interval (CI) ‐4.70 to ‐0.30, P = 0.03, 19 participants, GRADE: very low quality of evidence). In Thill 2004, NPPV was also associated with a lower total symptom score (5.6 vs 1.9, 19 participants, GRADE: very low quality of evidence) before cross‐over, although no SD was reported in the assessment before the cross‐over. Both trials favoured the NPPV group. Researchers reported five drop‐outs (12.5%), four of which were due to intolerance to NPPV, and one to respiratory failure/intubation. The benefits and harms of NPPV remain unclear owing to the lack of well‐powered trials and the high risk of systematic errors.
Observational studies and clinical experience
As already described in the Background, some observational studies suggest that NPPV might be a safe alternative to invasive ventilation in asthma and other types of respiratory failure (Keenan 2009). Some suggest beneficial effects on respiratory function with the use of NPPV for acute asthma in children (Beers 2007; Mayordomo‐Colunga 2011; Williams 2011). Use of NPPV in children with acute asthma has increased (Carroll 2013), perhaps because clinicians have had positive experiences with NPPV. However, observational studies and clinical expertise are insufficient to permit full assessment of the benefits and harms of an intervention (Jakobsen 2013; Hemkens 2016).
Choice of primary outcomes
Mortality is one of the most important outcomes for the patient. However, the mortality rate among children with acute asthma is low (Asher 2014). Therefore, a very large number of included participants would be required for investigators to detect any possible difference. Hence, one might question the relevance of all‐cause mortality as a primary outcome. The same argument might be made for serious adverse events. However, one must be careful when making assumptions, and it might be true that use of NPPV would increase the mortality rate because in theory this could delay necessary intubation. Therefore, we have chosen all‐cause mortality and serious adverse events as primary outcomes.
Our third primary outcome, symptom score, has been shown to provide a quick assessment of response to treatment among children with acute asthma when specific types of symptom scores are used (Chalut 2000; Ducharme 2008). Symptom score might also be a more realistic outcome for detecting differences between intervention groups. Nevertheless, one should always carefully consider the clinical implications and the clinical meaning of scores and scales when interpreting such results. The danger always exists that a given statistically significant result may not have clinical relevance. To assess clinical relevance, an observational study suggests that a reduction of three or more points should be considered a clinically meaningful change in the 12‐point Preschool Respiratory Assessment Measure (PRAM) score system (Ducharme 2008). The two symptom score systems observed in the included trials are eight‐point and nine‐point systems; therefore, a reduction of three or more points would surely represent a clinically significant change (Thill 2004; Basnet 2012).
Overall completeness and applicability of evidence
We were not able to perform any meta‐analyses. Therefore, we cannot conclude whether NPPV shows benefit or harm compared with standard care in children with acute asthma. More and larger randomised clinical trials with low risks of bias are needed to assess this.
Quality of the evidence
Heterogeneity
As no meta‐analysis was performed, we did not assess heterogeneity.
Risk of systematic error ('bias')
We found no studies and no outcome results with low risk of bias. Our results may show an overestimation of benefit and an underestimation of harm associated with nutritional support (Savovic 2012).
It was not possible to assess publication bias, as we included only two studies.
Risk of random error ('play of chance')
It was not possible to perform TSA, as we performed no meta‐analysis.
GRADE
We have assessed the quality of the evidence for each outcome by using the GRADE approach (Table 1). The GRADE assessment generally showed that evidence was of very low quality. Reasons for the GRADE assessment are given in the footnotes of the table (Table 1).
Potential biases in the review process
The main limitation of this Cochrane review is the paucity of evidence for the use of NPPV in children with asthma. We made numerous attempts to contact the authors of different trials to obtain raw data. However, our inability to obtain all relevant information might introduce a bias, which has the potential to alter the outcome of the meta‐analysis. In addition, biases that occur because of the methodological design of included studies might not be adequately accounted for, despite assessment by two independent review authors. Another potential limitation of this review design is that NPPV is often administered in an intensive care unit (ICU) setting, whereas usual care might be provided in a non‐ICU setting, accounting for risk of potential bias involving randomisation of participants not only to different intervention groups but also to different settings. However, this was not the case in the included trials, in which all randomised participants were treated in an ICU setting.
Agreements and disagreements with other studies or reviews
One review (Silva 2015) was published while this review was being conducted. This published review also included observational studies, and review authors conducts no meta‐analysis. The conclusion of review authors was that NPPV is applicable in the treatment of status asthmaticus in most paediatric patients unresponsive to standard treatment (Silva 2015). However, they also concluded that the current body of evidence is not conclusive, and that further high‐quality research is needed to estimate the effect of NPPV in children with acute asthma (Silva 2015).
Authors' conclusions
Implications for practice.
Current evidence does not allow confirmation or rejection of the effects of NPPV in children with acute asthma. If a child is admitted with acute asthma, and it is assessed that intubation is not indicated at the moment, one might consider NPPV as an add‐on therapy to standard care. Use of NPPV remains controversial, and additional research is required before changes to standard practice can be recommended.
Implications for research.
High‐quality randomised clinical trials are needed to assess the effects of NPPV in children with acute asthma. Such trials should:
randomise a sufficient number of participants to demonstrate a reliable result;
assess all‐cause mortality, serious adverse events, asthma symptom scores and quality of life;
be conducted with low risk of bias; and
blind treatment providers and participants through subtherapeutic (sham) NPPV; low pressures (e.g. < 5 cm H2O) can be used in an attempt to mask NPPV treatment (as demonstrated by Soroksky 2003) to eliminate the bias that lack of blinding. However, we cannot exclude the possibility that sham NPPV may be harmful; therefore, this recommendation should be considered only on a case‐by‐case basis.
Acknowledgements
Jimmy Chong was the Editor and commented critically on both the protocol and the full review.
The review author team would like to acknowledge the Airways editorial team for their professional and highly qualified comments and contributions throughout the process of writing this review.
The Background and Methods sections of this protocol are based on a standard template used by the Cochrane Airways Review Group.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Review Group. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, the National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| Embase (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials from the CAGR
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Positive‐Pressure Respiration Explode All
#6 NPPV
#7 NIPPV
#8 CPAP
#9 BiPAP
#10 non‐invasive or "non invasive"
#11 positive‐pressure or "positive pressure"
#12 "pressure support" or pressure‐support
#13 "positive airway" or positive‐airway
#14 airway NEXT pressure or airway‐pressure
#15 "pressure control" or pressure‐control
#16 bi‐level* or bilevel*
#17 ventilat* NEAR3 support*
#18 volume NEXT control or volume‐control
#19 ((nasal* OR mechanical*) NEAR3 ventilat*)
#20 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19
#21 #4 AND #20
[Note: in search line #1, MISC1 denotes the field in the record in which the reference has been coded for condiiton, in this case, asthma]
Data and analyses
Comparison 1. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 16 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [‐0.21, 0.21] |
Comparison 2. Serious adverse event time point closest to hospital discharge.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events | 2 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Asthma symptom score.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Asthma symptom score in the acute phase | 1 | 19 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐4.70, ‐0.30] |
Comparison 4. Pneumonia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pneumonia | 1 | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Basnet 2012.
| Methods | Randomised clinical trial, PICU, St. John’s Children’s Hospital (Springfield, IL), January 2009 to January 2010 | |
| Participants | 20 hospitalised children with known history of asthma admitted to the paediatric intensive care unit with status asthmaticus Male:Female = 11:9, mean age = 6 years (range, 3‐11 years) Exclusion criteria: participants with no previous history of asthma, absence of airway‐protective reflexes, absence of respiratory drive, need for emergent intubation as determined by the attending physician, facial or airway anomaly or injury precluding use of a tight‐fitting mask |
|
| Interventions |
Experimental group: BiPAP for 24 hours. Inspiratory positive airway pressure was gradually increased to 8 cm H2O to achieve a tidal volume of 6 to 9 mL/kg and end‐expiratory positive airway pressure to 5 cm H2O. Nasal masks were tried initially; if pressures of 8/5 could not be maintained, a full facemask was applied.
Control group: no intervention Co‐interventions: Participants in both groups continued to receive standard of care. All participants were started on continuous nebulised albuterol (0.5 mg/kg; maximum, 15 mg/kg), intravenous methylprednisolone 2 mg/kg/d (maximum, 80 mg/d) and supplemental oxygen to keep saturation at > 92%. Adjunct therapy, which included magnesium sulfate and helium‐oxygen heliox) mixtures, was provided at the attending physician’s discretion at any point in time 2 hours after study initiation, if CAS was greater than the score at initiation. |
|
| Outcomes | Pediatric Asthma Severity Score, heart rate, respiratory rate, transcutaneous oxygen saturation, need for supplemental oxygen set by the respiratory therapist to keep oxygen saturation at > 92% Side effects: (minor) nasal bridge pain and skin irritation, gastric insufflations, sinus and ear pain, dry eyes, (major) hypotension, pneumothorax, aspiration pneumonia. Need for adjunct therapy |
|
| Notes | Study authors were contacted on 19/2‐2016 by email (sbasnet@siumed.edu), and no response was received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Investigators used independent observers ‐ bedside respiratory therapists ‐ to apply the scoring system, but it was unclear whether they were blinded to the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although study authors used intention‐to‐treat data in a secondary analysis, and it would seem they have no missing data, they did not report the mean when including the missing participant, giving the trial a 10% drop‐out rate in the experimental group. |
| Selective reporting (reporting bias) | Unclear risk | No protocol could be obtained, and the trial did not report mortality. |
| Other bias | Low risk | The trial was "Supported, in part, by Central Research Committee, Southern Illinois University." No other bias was observed. |
Thill 2004.
| Methods | Randomised clinical cross‐over trial, Children’s Memorial Hospital Pediatric Intensive Care Unit (Chicago, IL) | |
| Participants | 20 hospitalised children with lower airway obstruction characterised by increased work of breathing, wheezing and dyspnoea, and a CAS > 3 Male:Female = unknown, mean age = 6 years (range, 0‐11 years) Exclusion criteria: presence of a tracheostomy tube, absence of airway protective reflexes, need for emergent intubation as determined by the attending physician, facial or airway anomaly or injury precluding use of a tight‐fitting mask, CAS > 8, discretion of the attending physician |
|
| Interventions |
Experimental group: received 2 hours of NPPV. NPPV was administered with a bilevel positive airway pressure ventilation S/T machine via a tight‐fitting nasal mask, fitted and held in place with head straps. If an appropriately sized nasal mask was not available, a full‐facemask was used. NPPV was initiated in spontaneous mode without a backup rate, with inspiratory positive airway pressure of 10 cm H2O and expiratory positive airway pressure of 5 cm H2O; settings remained unchanged throughout the course of the study period. Control group: conventional therapy Co‐interventions: All participants received conventional therapy for lower airway obstruction at the discretion of the attending physician, including supplemental oxygen via a high‐flow Venturi mask system, with FiO2 titrated by oxygen blender, inhaled beta2‐agonists (continuous nebulized albuterol, 10 mg/h) and intravenous corticosteroids (methylprednisolone, 1 to 2 mg/kg). No therapy was withdrawn or added during the study period. |
|
| Outcomes | Mortality, respiratory rate, Clinical Asthma Score (score: 0 to 9), oxygen saturation, transcutaneous CO2, adverse events, participant experience with NPPV | |
| Notes | Study authors were contacted on pthill65@gmail.com, and information regarding randomisation and serious adverse events was obtained. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The randomisation process to determine whether to start on or off BiPAP was done with slips of paper drawn at random from an envelope. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Four participants dropped out and were not accounted for in the analysis. |
| Selective reporting (reporting bias) | Low risk | No protocol was found, but the trial reported on serious adverse events and mortality. |
| Other bias | Low risk | No outside funding; labor was provided by the investigators, and computer and technical assistance through the resources of my Pediatric Critical Care fellowship programme and Northwestern University's Childrens Memorial Hospital (now Lurie Childrens). No additional biases were observed. |
BiPAP: bilateral positive airway pressure.
CAS: Clinical Asthma Score.
FiO2: fraction of inspired oxygen.
NPPV: non‐positive pressure ventilation.
PICU: paediatric intensive care unit.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andersen 1982 | The age of participants was unclear. No mention of children. Not able to contact study authors |
| Anderson 1982 | Participants were adults. |
| Archis 2001 | Participants did not have asthma. No mention of children |
| Aron 1999 | Participants were adults. |
| Bahera 2007 | Participants were adults. |
| Basnet 2010 | Not randomised |
| Brandao 2009 | Participants were adults. |
| Brandao 2009a | Participants were adults. |
| Branscomb 1982 | Participants were adults. |
| Chaudhry 2010 | Participants were adults. |
| Christensen 1993 | Participants were adults. |
| Compagnoni 2000 | No NPPV |
| Cross 2003 | Participants were adults. |
| de Miranda 2004 | Participants were adults. |
| Edwards 2003 | Participants were not hospitalised. |
| Edwards 2004 | Participants were not hospitalised. |
| Fergusson 1983 | Participants included adults and children with life‐threatening asthma. However, we were unable to extract separate data for the children, and it was not possible to establish contact with study authors. |
| Ferrari 2007 | The age of participants was unclear. No mention of children |
| Filho 2009 | The age of participants was unclear. No mention of children |
| Frischknecht 1991 | Participants were adults. |
| Hanekom 2012 | Participants were adults. |
| Holbrook 2016 | Participants were not hospitalised and were asthmatic participants in a chronic stable state. |
| Parkes 1997 | Participants were asthmatic patients in a chronic stable state. |
| Rondinel 2015 | Participants were adults. |
| Soma 2002 | Not randomised |
| Soma 2008 | Participants were adults. |
| Soroksky 2003 | Participants were adults. |
| Sutherasan 2011 | Participants were adults. |
| Sutherasan 2013 | Participants were adults. |
| Thill 1998 | Not a randomised trial |
| Webber 1982 | Participants were adults. |
Characteristics of ongoing studies [ordered by study ID]
NCT01497691.
| Trial name or title | Noninvasive Positive Airway Pressure in the Pediatric Emergency Department for the Treatment of Acute Asthma Exacerbations |
| Methods | Randomised clinical trial |
| Participants |
|
| Interventions | Intervention group will receive all standard of care therapies as per the paediatric ED Asthma Severity Protocol. All nebulised treatments will be given via the NIPPV/BiPAP machine. Settings will be adjusted on the basis of age and clinical presentation of the child. |
| Outcomes |
Primary outcome measure
Secondary outcome measures
|
| Starting date | 2013 January |
| Contact information | abby.m.williams@vanderbilt.edu |
| Notes |
NCT02347462.
| Trial name or title | BiPAP for the Treatment of Moderate to Severe Acute Asthma Exacerbations |
| Methods | Randomised clinical trial |
| Participants |
|
| Interventions | Children in the intervention group will receive BiPAP (Trilogy, Philips Respironics; spontaneous trigger mode) via nasal mask or full facemask. EPAP will be set at 5 cm H2O. IPAP will be titrated to achieve a tidal volume of 6 to 9 mL/kg. These settings will remain unchanged throughout the study period. |
| Outcomes |
Primary outcome measure
Secondary outcome measures
|
| Starting date | April 2015 |
| Contact information | mseear@cw.bc.ca |
| Notes |
BiPAP: bilateral positive airway pressure.
ED: emergency department.
EPAP: end‐expiratory positive airway pressure.
IPAP: inspiratory positive airway pressure.
NPPV: non‐positive pressure ventilation.
PCP: primary care physician
PRAM: Pediatric Respiratory Assessment Measure.
Differences between protocol and review
Mention of for‐profit bias was included under "other biases" in the section on Assessment of risk of bias in included studies, in accordance with the rest of the protocol.
Contributions of authors
Steven Kwasi Korang (SK) conceived, designed and drafted the protocol.
Joshua Feinberg (JF) provided advice and revised the protocol.
Jørn Wetterslev (JW) provided advice and revised the protocol.
Janus Christian Jakobsen (JCJ) conceived, designed and revised the protocol.
Sources of support
Internal sources
-
Copenhagen Trial Unit, Denmark.
The review was conducted during work hours
-
Paediatric Dept, Holbæk Sygehus, Denmark.
The review was conducted during work hours
External sources
The review authors declare that no such funding was received for this systematic review, Other.
Declarations of interest
Steven Kwasi Korang has no interest to declare.
Joshua Feinberg has no interest to declare.
Jørn Wetterslev is a member of the task force at the Copenhagen Trial Unit convened to develop the theory and software of Trial Sequential Analysis (TSA).
Janus Christian Jakobsen has no interest to declare.
New
References
References to studies included in this review
Basnet 2012 {published data only}
- Basnet S, Mander G, Andoh J, Klaska H, Verhulst S, Koirala J. Safety, efficacy, and tolerability of early initiation of noninvasive positive pressure ventilation in pediatric patients admitted with status asthmaticus: a pilot study. Pediatric Critical Care Medicine 2012;13(4):393‐8. [CENTRAL: 834472; CRS: 4900100000062937; EMBASE: 2012437313] [DOI] [PubMed] [Google Scholar]
Thill 2004 {published data only}
- Thill PJ, McGuire JK, Baden HP, Green TP, Checchia PA. Noninvasive positive‐pressure ventilation in children with lower airway obstruction. Pediatric Critical Care Medicine 2004;5(4):337‐42. [CENTRAL: 489916; CRS: 4900100000017175; PUBMED: 15215002] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andersen 1982 {published data only}
- Andersen JB, Klausen NO. A new mode of administration of nebulized bronchodilator in severe bronchospasm. European Journal of Respiratory Diseases. Supplement 1982;119:97‐100. [CENTRAL: 28284; CRS: 4900100000001266; EMBASE: 1982208248; PUBMED: 7047190] [PubMed] [Google Scholar]
Anderson 1982 {published data only}
- Anderson PB, Goude A, Peake MD. Comparison of salbutamol given by intermittent positive‐pressure breathing and pressure‐packed aerosol in chronic asthma. Thorax 1982;37(8):612‐6. [CENTRAL: 188486; CRS: 4900100000007135; EMBASE: 1982245924] [DOI] [PMC free article] [PubMed] [Google Scholar]
Archis 2001 {published data only}
- Archis C, Dunford M, Lazaris M, Jacques T, Skowronski G, Gotsopoulos H, et al. A randomised control trial of non‐invasive ventilation (NIV) in acute exacerbations of chronic airflow limitation (CAL) in a ward setting. Thoracic Society of Australia and New Zealand Annual Scientific Meeting March 16‐21; Brisbane. Brisbane, 2001. [CENTRAL: 340807; CRS: 4900100000010407]
Aron 1999 {published data only}
- Aron C, Moutaux G. [Non‐invasive ventilation in acute or chronic respiratory failure: a comparison of volumetric ventilation] [Ventilation non invasive dans les décompensations respiratoires aiguës des broncho‐pneumopathies chroniques obstructives. Comparaison de ventilations barométrique et volumétrique]. Revue des Maladies Respiratoires 1999;16(2):181‐7. [CENTRAL: 332371; CRS: 4900100000010182; 4900100000010182; PUBMED: 10339761] [PubMed] [Google Scholar]
Bahera 2007 {published data only}
- Bahera D, Agarwal R. Non invasive ventilation in acute asthma, 2007. http://clinicaltrials.gov/show/NCT00510991. [CRS: 4900100000040918]
Basnet 2010 {published data only}
- Basnet S, Mander G, Andoh J, Koirala J. Early initiation of noninvasive positive pressure ventilation in the management of children with status asthmaticus [Abstract]. Pediatric Critical Care Medicine. 2010; Vol. 11, issue 5:658. [CENTRAL: 774436; CRS: 4900100000025949; EMBASE: 70287754]
Brandao 2009 {published data only}
- Brandao DC, Lima VM, Filho VG, Silva TS, Campos TF, Dean E, et al. Reversal of bronchial obstruction with bi‐level positive airway pressure and nebulization in patients with acute asthma. Journal of Asthma 2009;46(4):356‐61. [CENTRAL: 697266; CRS: 4900100000023867; PUBMED: 19484669] [DOI] [PubMed] [Google Scholar]
Brandao 2009a {published data only}
- Brandao DC, Lima VM, Filho VG, Silva TS, Campos TF, Dean E, et al. Reversal of bronchial obstruction with bi‐level positive airway pressure and nebulization in patients with acute asthma. The Journal of Asthma 2009;46(4):356‐61. [PUBMED: 19484669] [DOI] [PubMed] [Google Scholar]
Branscomb 1982 {published data only}
- Branscomb BV. Metaproterenol solution in the treatment of asthmatic patients by intermittent positive pressure breathing. Journal of Clinical Pharmacology 1982;22(5‐6):231‐5. [CENTRAL: 190619; CRS: 4900100000007213; EMBASE: 1982167187] [DOI] [PubMed] [Google Scholar]
Chaudhry 2010 {published data only}
- Chaudhry D, Indora M, Sangwan V, Sehgal IPS, Chaudhry A, Chaudhry D, et al. Evaluation of non‐invasive ventilation in management of acute severe asthma [Abstract]. Thorax. 2010; Vol. 65, issue Suppl 4:S68. [CENTRAL: 783244; CRS: 4900100000026301; EMBASE: 70325758]
Christensen 1993 {published data only}
- Christensen EF, Norregaard O, Jensen LW, Dahl R. Inhaled beta 2‐agonist and positive expiratory pressure in bronchial asthma. Influence on airway resistance and functional residual capacity [ENGLISH]. Chest 1993;104(4):1108‐13. [CENTRAL: 96122; CRS: 4900100000004202; PUBMED: 8404176] [DOI] [PubMed] [Google Scholar]
Compagnoni 2000 {published data only}
- Compagnoni ML, Nava S. Bronchodilator efficacy during non‐invasive mechanical ventilation (NIMV). American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A556. [CENTRAL: 429057; CRS: 4900100000014613] [Google Scholar]
Cross 2003 {published data only}
- Cross AM, Cameron P, Kierce M, Ragg M, Kelly AM. Non‐invasive ventilation in acute respiratory failure: a randomised comparison of continuous positive airway pressure and bi‐level positive airway pressure. Emergency Medicine Journal 2003;20(6):531‐4. [CENTRAL: 475013; CRS: 4900100000016379; EMBASE: 2003491615; 4900100000016379; PUBMED: 14623840] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Miranda 2004 {published data only}
- Miranda AS, Silva COS, Beppu OS, Damasceno MCP, Vieira MDD. Noninvasive ventilation for continuous positive airway pressure (CPAP) associate to the bronchodilator in the treatment of the bronchial asthma crisis [Portuguese]. Fisioterapia em Movimento 2004;17(2):17‐27. [CENTRAL: 526157; CRS: 4900100000018938; 4900100000018938] [Google Scholar]
Edwards 2003 {published data only}
- Edwards N, Blyton DM, O'Meara T, Poulos L, Brannan JD, Sullivan E. Nocturnal nasal CPAP use improves respiratory function in moderate to severe asthma [Abstract]. Journal of Allergy and Clinical Immunology. 2003; Vol. 111, issue 2 Suppl:S215. [CENTRAL: 524763; CRS: 4900100000018694]
Edwards 2004 {published data only}
- Edwards N, Blyton D, Sullivan C. Asthma is improved with nocturnal nasal CPAP [abstract]. Internal Medicine Journal. 2004; Vol. 34, issue 3:A30. [CENTRAL: 591896; CRS: 4900100000020525; 4900100000020525]
Fergusson 1983 {published data only}
- Fergusson RJ, Carmichael J, Rafferty P, Willey RF, Crompton GK, Grant IW. Nebulized salbutamol in life‐threatening asthma: is IPPB necessary?. British Journal of Diseases of the Chest 1983;77(3):255‐61. [CENTRAL: 187253; CRS: 4900100000007077; EMBASE: 1983207875] [PubMed] [Google Scholar]
Ferrari 2007 {published data only}
- Ferrari R, Groff P, Agostinelli D, Bertele L, Lazzari R, Battista N. Acute asthma treatment by noninvasive CPAP in the emergency department [Abstract]. European Respiratory Journal. 2007; Vol. 30, issue Suppl 51:265s [E1665]. [CENTRAL: 642694; CRS: 4900100000022252]
Filho 2009 {published data only}
- Filho VG, Rodrigues Machado M, Brandao D, Alcoforado L, Galvao AM, Fregonezi Getal, et al. Coupling of noninvasive mechanical ventilation and nebulization during asthma exacerbations: higher amount of aerosol deposition into the airways or effective mechanical bronchodilation due to positive pressure? [Abstract]. European Respiratory Society Annual Congress, Vienna, Austria, September 12‐16. 2009:[P2292]. [CENTRAL: 756100; CRS: 4900100000025074]
Frischknecht 1991 {published data only}
- Frischknecht Christensen E, Norregaard O, Dahl R. Treatment of bronchial asthma with terbutaline inhaled by conespacer combined with positive expiratory pressure mask [ENGLISH]. Chest 1991;100(2):317‐21. [CENTRAL: 77251; CRS: 4900100000003468; PUBMED: 1864100] [DOI] [PubMed] [Google Scholar]
Hanekom 2012 {published data only}
- Hanekom SG, Aswegen H, Engelbrecht L, Becker PJ. Effect of continuous and bilevel noninvasive ventilation for acute asthma exacerbation: a randomized controlled trial [Abstract]. European Respiratory Journal. 2012; Vol. 40, issue Suppl 56:832s [4562]. [CENTRAL: 839364; CRS: 4900100000067971; EMBASE: 71923962]
Holbrook 2016 {published data only}
- Holbrook JT, Sugar EA, Brown RH, Drye LT, Irvin CG, Schwarz AR, et al. Effect of CPAP on airways reactivity in asthma: a randomized sham‐controlled clinical trial. Annals of the American Thoracic Society 2016 July 11 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
Parkes 1997 {published data only}
- Parkes SN, Bersten AD. Aerosol kinetics and bronchodilator efficacy during continuous positive airway pressure delivered by face mask. Thorax 1997;52(2):171‐5. [CENTRAL: 137392; CRS: 4900100000005612; 4900100000005612; PUBMED: 9059480] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rondinel 2015 {published data only}
- Rondinel TZ, Correa IF, Hoscheidt LM, Bueno MH, Silva LMC, Reppold CT, et al. Incentive spirometry combined with expiratory positive airway pressure improves asthma control and quality of life in asthma: a randomised controlled trial. Journal of Asthma 2015;52(2):220‐6. [CENTRAL: 1076705; CRS: 4900132000004120; EMBASE: 2015136465] [DOI] [PubMed] [Google Scholar]
Soma 2002 {published data only}
- Soma T, Hino M, Tanaka Y, Kudou S. Treatment of acute bronchospasm with noninvasive positive pressure ventilation (NIPPV). American Journal of Respiratory and Critical Care Medicine 2002;165(8 Suppl):A191. [CENTRAL: 384284; CRS: 4900100000011890; 4900100000011890] [Google Scholar]
Soma 2008 {published data only}
- Soma T, Hino M, Kida K, Kudoh S. A prospective and randomized study for improvement of acute asthma by non‐invasive positive pressure ventilation (NPPV). Internal Medicine (Tokyo, Japan) 2008;47(6):493‐501. [CENTRAL: 630834; CRS: 4900100000021853; PUBMED: 18344635] [DOI] [PubMed] [Google Scholar]
Soroksky 2003 {published data only}
- Soroksky A, Stav D, Shpirer I. A pilot prospective, randomized, placebo‐controlled trial of bilevel positive airway pressure in acute asthmatic attack. Chest 2003;123(4):1018‐25. [CENTRAL: 430949; CRS: 4900100000015109; PUBMED: 12684289] [DOI] [PubMed] [Google Scholar]
Sutherasan 2011 {published data only}
- Sutherasan Y, Kiatboonsri S, Kiatboonsri C, Theerawit P, Trakulsrichai S. The impact of continuous positive airway pressure on the treatment of acute exacerbation of asthma: randomised controlled trial of efficacy [Abstract]. Respirology (Carlton, Vic.). 2011; Vol. 16, issue Suppl 2:197 [134]. [CENTRAL: 833806; CRS: 4900100000054329; EMBASE: 70576352]
Sutherasan 2013 {published data only}
- Sutherasan Y, Kiatboonsri S, Theerawit P, Kiatboonsri C, Trakulsrichai S. Impact of continuous positive airway pressure on the treatment of acute exacerbation of asthma: a randomised controlled trial [Abstract]. Critical Care (London, England). BioMed Central Ltd, 2013; Vol. 17:S55‐6. [CENTRAL: 856471; CRS: 4900100000076318; EMBASE: 71030447]
Thill 1998 {published data only}
- Thill PJ, Baden HP, Green TP. The use of bilevel positive airway pressure in children with obstructive airways disease. Pediatrics 1998;102(3):696. [CENTRAL: 416765; CRS: 4900100000014021; 4900100000014021] [Google Scholar]
Webber 1982 {published data only}
- Webber BA, Collins JV, Branthwaite MA. Severe acute asthma: a comparison of three methods of inhaling salbutamol. British Journal of Diseases of the Chest 1982;76(1):69‐74. [CENTRAL: 258684; CRS: 4900100000007825; PUBMED: 7037036] [PubMed] [Google Scholar]
References to ongoing studies
NCT01497691 {unpublished data only}
- NCT01497691. Noninvasive positive airway pressure in the pediatric emergency department for the treatment of acute asthma exacerbations. https://clinicaltrials.gov/ct2/show/NCT01497691 (accessed 22 September 2016).
NCT02347462 {unpublished data only}
- NCT02347462. Bilevel positive airway pressure (BiPAP) for the treatment of moderate to severe acute asthma exacerbations. https://clinicaltrials.gov/ct2/show/NCT02347462 (accessed 22 September 2016).
Additional references
AIHW 2010
- Australian Institute of Health and Welfare. Australia's Health 2010. http://www.aihw.gov.au/WorkArea/DownloadAsset.aspx?id=6442452962 (accessed 30 September 2015).
Akinbami 2012
- Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001‐2010. NCHS Data Brief 2012;94:1‐8. [PUBMED: 22617340] [PubMed] [Google Scholar]
Akingbola 2002
- Akingbola OA, Simakajornboon N, Hadley Jr EF, Hopkins RL. Noninvasive positive‐pressure ventilation in pediatric status asthmaticus. Pediatric Critical Care Medicine 2002;3(2):181‐4. [PUBMED: 12780991] [DOI] [PubMed] [Google Scholar]
Aldrich 1993
- Aldrich TK, Hendler JM, Vizioli LD, Park M, Multz AS, Shapiro SM. Intrinsic positive end‐expiratory pressure in ambulatory patients with airways obstruction. The American Review of Respiratory Disease 1993;147(4):845‐9. [PUBMED: 8466118] [DOI] [PubMed] [Google Scholar]
Alith 2015
- Alith MB, Gazzotti MR, Montealegre F, Fish J, Nascimento OA, Jardim JR. Negative impact of asthma on patients in different age groups. Jornal Brasileiro de Pneumologia 2015;41(1):16‐22. [PUBMED: 25750670] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alnaji 2014
- Alnaji F, Zemek R, Barrowman N, Plint A. PRAM score as predictor of pediatric asthma hospitalization. Academic Emergency Medicine 2014;21(8):872‐8. [PUBMED: 25176153] [DOI] [PubMed] [Google Scholar]
Andersen 1979
- Andersen JB, Qvist J, Kann T. Recruiting collapsed lung through collateral channels with positive end‐expiratory pressure. Scandinavian Journal of Respiratory Diseases 1979;60(5):260‐6. [PUBMED: 392747] [PubMed] [Google Scholar]
Asher 2014
- Asher I, Pearce N. Global burden of asthma among children. International Journal of Tuberculosis and Lung Disease 2014;18(11):1269‐78. [PUBMED: 25299857] [DOI] [PubMed] [Google Scholar]
Bahadori 2009
- Bahadori K, Doyle‐Waters MM, Marra C, Lynd L, Alasaly K, Swiston J, et al. Economic burden of asthma: a systematic review. BMC Pulmonary Medicine 2009;9:24. [PUBMED: 19454036] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beers 2007
- Beers SL, Abramo TJ, Bracken A, Wiebe RA. Bilevel positive airway pressure in the treatment of status asthmaticus in pediatrics. American Journal of Emergency Medicine 2007;25(1):6‐9. [PUBMED: 17157675] [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PUBMED: 7786990] [PubMed] [Google Scholar]
Bisgaard 2009
- Bisgaard H, Bonnelykke K, Sleiman PM, Brasholt M, Chawes B, Kreiner‐Moller E, et al. Chromosome 17q21 gene variants are associated with asthma and exacerbations but not atopy in early childhood. American Journal of Respiratory and Critical Care Medicine 2009;179(3):179‐85. [PUBMED: 19029000] [DOI] [PubMed] [Google Scholar]
Blanch 2005
- Blanch L, Bernabe F, Lucangelo U. Measurement of air trapping, intrinsic positive end‐expiratory pressure, and dynamic hyperinflation in mechanically ventilated patients. Respiratory Care 2005;50(1):110‐23; discussion 123‐4. [PUBMED: 15636649] [PubMed] [Google Scholar]
Bloemen 2007
- Bloemen K, Verstraelen S, Heuvel R, Witters H, Nelissen I, Schoeters G. The allergic cascade: review of the most important molecules in the asthmatic lung. Immunology Letters 2007;113(1):6‐18. [PUBMED: 17765979] [DOI] [PubMed] [Google Scholar]
Bloom 2013
- Bloom B, Jones LI, Freeman G. No. 258. Summary health statistics for U.S. children: National Health Interview Survey, 2012 (Series 10. Data From the National Health Interview Survey). http://www.cdc.gov/nchs/data/series/sr_10/sr10_258.pdf (accessed 30 September 2015). [PUBMED: 24784481] [PubMed]
Boldrini 2012
- Boldrini R, Fasano L, Nava S. Noninvasive mechanical ventilation. Current Opinion in Critical Care 2012;18(1):48‐53. [PUBMED: 22186215] [DOI] [PubMed] [Google Scholar]
Braman 2006
- Braman SS. The global burden of asthma. Chest 2006;130(1 Suppl):4S‐12S. [PUBMED: 16840363] [DOI] [PubMed] [Google Scholar]
Brannan 2012
- Brannan JD, Lougheed MD. Airway hyperresponsiveness in asthma: mechanisms, clinical significance, and treatment. Frontiers in Physiology 2012;3:460. [PUBMED: 23233839] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bratton 2012
- Bratton SL, Newth CJ, Zuppa AF, Moler FW, Meert KL, Berg RA, et al. Critical care for pediatric asthma: wide care variability and challenges for study. Pediatric Critical Care Medicine 2012;13(4):407‐14. [PUBMED: 22067984] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brochard 1994
- Brochard L, Rauss A, Benito S, Conti G, Mancebo J, Rekik N, et al. Comparison of three methods of gradual withdrawal from ventilatory support during weaning from mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 1994;150(4):896‐903. [PUBMED: 7921460] [DOI] [PubMed] [Google Scholar]
Brochard 2002
- Brochard L. Noninvasive ventilation for acute respiratory failure. JAMA 2002;288(8):932‐5. [PUBMED: 12190351] [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61(8):763‐9. [PUBMED: 18411040] [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive. Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [PUBMED: 18824466] [DOI] [PubMed] [Google Scholar]
Broux 1991
- Broux R, Foidart G, Mendes P, Saad G, Fatemi M, D'Orio V, et al. Use of PEEP in management of life‐threatening status asthmaticus: a method for the recovery of appropriate ventilation‐perfusion ratio. Applied Cardiopulmonary Pathophysiology 1991;4(1):79‐83. [PUBMED: 10147542] [PubMed] [Google Scholar]
Buda 1979
- Buda AJ, Pinsky MR, Ingels NB Jr, Daughters GT 2nd, Stinson EB, Alderman EL. Effect of intrathoracic pressure on left ventricular performance. New England Journal of Medicine 1979;301(9):453‐9. [PUBMED: 460363] [DOI] [PubMed] [Google Scholar]
Caramez 2005
- Caramez MP, Borges JB, Tucci MR, Okamoto VN, Carvalho CR, Kacmarek RM, et al. Paradoxical responses to positive end‐expiratory pressure in patients with airway obstruction during controlled ventilation. Critical Care Medicine 2005;33(7):1519‐28. [PUBMED: 16003057] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carroll 2013
- Carroll CL, Sala KA. Pediatric status asthmaticus. Critical Care Clinics 2013;29(2):153‐66. [PUBMED: 23537669] [DOI] [PubMed] [Google Scholar]
Cave 2014
- Cave AJ, Atkinson LL. Asthma in preschool children: a review of the diagnostic challenges. Journal of the American Board of Family Medicine 2014;27(4):538‐48. [PUBMED: 25002008] [DOI] [PubMed] [Google Scholar]
CDC 2013
- Centers of Disease Control and Prevention. Most Recent Asthma Data. http://www.cdc.gov/asthma/most_recent_data.htm 2013 (accessed 30 September 2015).
Chalut 2000
- Chalut DS, Ducharme FM, Davis GM. The Preschool Respiratory Assessment Measure (PRAM): a responsive index of acute asthma severity. Journal of Pediatrics 2000;137(6):762‐8. [PUBMED: 11113831] [DOI] [PubMed] [Google Scholar]
de Freitas 2013
- Freitas D, Gomes EL, Costa D, Germano SM, Borges PV, Sampaio LMM. Effects of CPAP on clinical variables and autonomic modulation in children during an asthma attack. Respiratory Physiology & Neurobiology 2013;188(1):66‐70. [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods of combining randomized trials: strength and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Dijk 2013
- Dijk FN, Jongste JC, Postma DS, Koppelman GH. Genetics of onset of asthma. Current Opinion in Allergy and Clinical Immunology 2013;13(2):193‐202. [PUBMED: 23407123] [DOI] [PubMed] [Google Scholar]
Douwes 2002
- Douwes J, Pearce N. Asthma and the westernization 'package'. International Journal of Epidemiology 2002;31(6):1098‐102. [PUBMED: 12540698] [DOI] [PubMed] [Google Scholar]
Ducharme 2008
- Ducharme FM, Chalut D, Plotnick L, Savdie C, Kudirka D, Zhang X, et al. The Pediatric Respiratory Assessment Measure: a valid clinical score for assessing acute asthma severity from toddlers to teenagers. The Journal of Pediatrics 2008;152(4):476‐80, 480.e1. [PUBMED: 18346499] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Esteban 1995
- Esteban A, Frutos F, Tobin MJ, Alia I, Solsona JF, Valverdu I, et al. A comparison of four methods of weaning patients from mechanical ventilation. Spanish Lung Failure Collaborative Group. New England Journal of Medicine 1995;332(6):345‐50. [PUBMED: 7823995] [DOI] [PubMed] [Google Scholar]
Fagon 1993
- Fagon JY, Chastre J, Hance AJ, Montravers P, Novara A, Gibert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. American Journal of Medicine 1993;94(3):281‐8. [PUBMED: 8452152] [DOI] [PubMed] [Google Scholar]
Finder 2014
- Finder J. Atelectasis in children. http://www.uptodate.com/contents/atelectasis‐in‐children 2014 (accessed 30 September 2015).
Galli 2008
- Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature 2008;454(7203):445‐54. [PUBMED: 18650915] [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2015
- Global Strategy for Asthma Management and Prevention, Global Initiative for Asthma (GINA) 2015. http://www.ginasthma.org/local/uploads/files/GINA_Report_2015_Aug11.pdf (accessed 30 September 2015).
Gluud 2006
- Gluud LL. Bias in clinical intervention research. American Journal of Epidemiology 2006;163(6):493‐501. [PUBMED: 16443796] [DOI] [PubMed] [Google Scholar]
Graham 2007
- Graham AS, Chandrashekharaiah G, Citak A, Wetzel RC, Newth CJL. Positive end‐expiratory pressure and pressure support in peripheral airways obstruction. Intensive Care Medicine 2007;33(1):120‐7. [DOI] [PubMed] [Google Scholar]
Guerin 1997
- Guerin C, Girard R, Chemorin C, Varax R, Fournier G. Facial mask noninvasive mechanical ventilation reduces the incidence of nosocomial pneumonia. A prospective epidemiological survey from a single ICU. Intensive Care Medicine 1997;23(10):1024‐32. [PUBMED: 9407237] [DOI] [PubMed] [Google Scholar]
Gupta 2010
- Gupta D, Nath A, Agarwal R, Behera D. A prospective randomized controlled trial on the efficacy of noninvasive ventilation in severe acute asthma. Respiratory Care 2010;55(5):536‐43. [PUBMED: 20420722] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443‐57. [PUBMED: 16345038] [DOI] [PubMed] [Google Scholar]
Hasegawa 2013
- Hasegawa K, Tsugawa Y, Brown DF, Camargo CA Jr. Childhood asthma hospitalizations in the United States, 2000‐2009. The Journal of Pediatrics 2013;163(4):1127‐33.e3. [PUBMED: 23769497] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedlin 2012
- Hedlin G, Konradsen J, Bush A. An update on paediatric asthma. European Respiratory Review 2012;21(125):175‐85. [PUBMED: 22941882] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hemkens 2016
- Hemkens LG, Contopoulos‐Ioannidis DG, Ioannidis JP. Agreement of treatment effects for mortality from routinely collected data and subsequent randomized trials: meta‐epidemiological survey. BMJ (Clinical research ed.) 2016;352:i493. [PUBMED: 26858277] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
ICH‐GCP 1997
- ICH‐GCP 1997. International Conference on Harmonisation; Good Clinical Practice: Consolidated Guideline; Availability. https://www.gpo.gov/fdsys/pkg/FR‐1997‐05‐09/pdf/97‐12138.pdf (accessed 22 September 2016). [PUBMED: 11656783] 11656783
Jackson 2012
- Jackson DJ, Evans MD, Gangnon RE, Tisler CJ, Pappas TE, Lee WM, et al. Evidence for a causal relationship between allergic sensitization and rhinovirus wheezing in early life. American Journal of Respiratory and Critical Care Medicine 2012;185(3):281‐5. [PUBMED: 21960534] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacob 2015
- Jacob C, Bechtel B, Engel S, Kardos P, Linder R, Braun S, et al. Healthcare costs and resource utilization of asthma in Germany: a claims data analysis. European Journal of Health Economics 2015;epub ahead of print:1‐7. [DOI: 10.1007/s10198-015-0671-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jakobsen 2013
- Jakobsen JC, Gluud C. The necessity of randomized clinical trials. British Journal of Medicine & Medical Research 2013;3(4):1453‐68. [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14:120. [PUBMED: 25416419] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jobe 2014
- Jobe AH, Tibboel D. Update in pediatric lung disease 2013. American Journal of Respiratory and Critical Care Medicine 2014;189(9):1031‐6. [PUBMED: 24787065] [DOI] [PubMed] [Google Scholar]
Keenan 2009
- Keenan SP, Mehta S. Noninvasive ventilation for patients presenting with acute respiratory failure: the randomized controlled trials. Respiratory Care 2009;54(1):116‐26. [PUBMED: 19111111] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [PUBMED: 11730399] [DOI] [PubMed] [Google Scholar]
Kumar 2009
- Kumar V, Abbas AK, Aster J. Robbins & Cotran Pathologic Basis of Disease. 8th Edition. Philadelphia: Saunders, 2009. [Google Scholar]
Lim 2012
- Lim WJ, Mohammed Akram R, Carson KV, Mysore S, Labiszewski NA, Wedzicha JA, et al. Non‐invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD004360.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 1995
- Lin HC, Wang CH, Yang CT, Huang TJ, Yu CT, Shieh WB, et al. Effect of nasal continuous positive airway pressure on methacholine‐induced bronchoconstriction. Respiratory Medicine 1995;89(2):121‐8. [PUBMED: 7708996] [DOI] [PubMed] [Google Scholar]
Liu 2014
- Liu M. Pathogenesis of Asthma. http://www.uptodate.com/contents/pathogenesis‐of‐asthma 2014 (accessed 30 September 2015).
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Malmstrom 2001
- Malmstrom K, Kaila M, Korhonen K, Dunder T, Nermes M, Klaukka T, et al. Mechanical ventilation in children with severe asthma. Pediatric Pulmonology 2001;31(6):405‐11. [PUBMED: 11389571] [DOI] [PubMed] [Google Scholar]
Mantzouranis 2014
- Mantzouranis E, Papadopouli E, Michailidi E. Childhood asthma: recent developments and update. Current Opinion in Pulmonary Medicine 2014;20(1):8‐16. [PUBMED: 24240439] [DOI] [PubMed] [Google Scholar]
Martin 1982
- Martin JG, Shore S, Engel LA. Effect of continuous positive airway pressure on respiratory mechanics and pattern of breathing in induced asthma. American Review of Respiratory Disease 1982;126(5):812‐7. [PUBMED: 6216839] [DOI] [PubMed] [Google Scholar]
Masoli 2004
- Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59(5):469‐78. [PUBMED: 15080825] [DOI] [PubMed] [Google Scholar]
Mayordomo‐Colunga 2011
- Mayordomo‐Colunga J, Medina A, Rey C, Concha A, Menendez S, Arcos ML, et al. Non‐invasive ventilation in pediatric status asthmaticus: a prospective observational study. Pediatric Pulmonology 2011;46(10):949‐55. [PUBMED: 21520437] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [PUBMED: 9746022] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
Moloney 1999
- Moloney E, Kiely JL, McDonnell T, McNicholas WT. Nocturnal nasal intermittent positive pressure ventilation (NIPPV) therapy for chronic respiratory failure: long‐term effects. Irish Medical Journal 1999;92(6):401‐3. [PUBMED: 10598422] [PubMed] [Google Scholar]
NHLBI 2007
- National Heart, Lung, and Blood Institute (US). Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. http://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf 2007 (accessed 30 September 2015).
Nourdine 1999
- Nourdine K, Combes P, Carton MJ, Beuret P, Cannamela A, Ducreux JC. Does noninvasive ventilation reduce the ICU nosocomial infection risk? A prospective clinical survey. Intensive Care Medicine 1999;25(6):567‐73. [PUBMED: 10416907] [DOI] [PubMed] [Google Scholar]
Papadopoulos 2012
- Papadopoulos NG, Arakawa H, Carlsen KH, Custovic A, Gern J, Lemanske R, et al. International consensus on (ICON) pediatric asthma. Allergy 2012;67(8):976‐97. [PUBMED: 22702533] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pavone 2013
- Pavone M, Verrillo E, Caldarelli V, Ullmann N, Cutrera R. Non‐invasive positive pressure ventilation in children. Early Human Development 2013;89 Suppl 3:S25‐31. [PUBMED: 23958409] [DOI] [PubMed] [Google Scholar]
Pepe 1982
- Pepe PE, Marini JJ. Occult positive end‐expiratory pressure in mechanically ventilated patients with airflow obstruction: the auto‐PEEP effect. American Review of Respiratory Disease 1982;126(1):166‐70. [PUBMED: 7046541] [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Savovic 2012
- Savovic J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [PUBMED: 22945832] [DOI] [PubMed] [Google Scholar]
Sawicki 2014a
- Sawicki G. Acute asthma exacerbations in children: inpatient management. http://www.uptodate.com/contents/acute‐asthma‐exacerbations‐in‐children‐inpatient‐management (accessed 4 September 2014).
Sawicki 2014b
- Sawicki G, Haven K. Acute asthma exacerbations in children: home/office management and severity assessment. http://www.uptodate.com/contents/acute‐asthma‐exacerbations‐in‐children‐home‐office‐management‐and‐severity‐assessment (accessed 30 September 2015).
Scarfone 2015
- Scarfone RJ. Acute asthma exacerbations in children: emergency department management. http://www.uptodate.com/contents/acute‐asthma‐exacerbations‐in‐children‐emergency‐department‐management (accessed 25 January 2016).
Shivaram 1987
- Shivaram U, Donath J, Khan FA, Juliano J. Effects of continuous positive airway pressure in acute asthma. Respiration; International Review of Thoracic Diseases 1987;52(3):157‐62. [PUBMED: 3326082] [DOI] [PubMed] [Google Scholar]
Silva 2015
- Silva PS, Barreto SS. Noninvasive ventilation in status asthmaticus in children: levels of evidence [Ventilacao mecanica nao invasiva na crise de asma aguda grave em criancas: niveis de evidencias.]. Revista Brasileira de terapia intensiva 2015;27(4):390‐6. [PUBMED: 26761478] [DOI] [PMC free article] [PubMed] [Google Scholar]
Teague 2005
- Teague WG. Non‐invasive positive pressure ventilation: current status in paediatric patients. Paediatric Respiratory Reviews 2005;6(1):52‐60. [PUBMED: 15698817] [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses?. International Journal of Epidemiology 2009;38(1):276‐86. [PUBMED: 18824467] [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [PUBMED: 20865104] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA), 2011. ctu.dk/tsa/files/tsa_manual.pdf (accessed 7 January 2015).
TSA 2011
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis, 2011. ctu.dk/tsa/ (accessed 7 January 2015).
Tzoufi 2005
- Tzoufi M, Mentzelopoulos SD, Roussos C, Armaganidis A. The effects of nebulized salbutamol, external positive end‐expiratory pressure, and their combination on respiratory mechanics, hemodynamics, and gas exchange in mechanically ventilated chronic obstructive pulmonary disease patients. Anesthesia and Analgesia 2005;101(3):843‐50, table of contents. [PUBMED: 16116002] [DOI] [PubMed] [Google Scholar]
Wade 2015
- Wade A, Chang C. Evaluation and treatment of critical asthma syndrome in children. Clinical Reviews in Allergy & Immunology 2015;48(1):66‐83. [PUBMED: 24488329] [DOI] [PubMed] [Google Scholar]
Wang 1996
- Wang CH, Lin HC, Huang TJ, Yang CT, Yu CT, Kuo HP. Differential effects of nasal continuous positive airway pressure on reversible or fixed upper and lower airway obstruction. European Respiratory Journal 1996;9(5):952‐9. [PUBMED: 8793457] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [PUBMED: 18083463] [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in random‐effects model meta‐analyses. BMC Medical Research Methodology 2009;9:86. [PUBMED: 20042080] [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2011
- Williams AM, Abramo TJ, Shah MV, Miller RA, Burney‐Jones C, Rooks S, et al. Safety and clinical findings of BiPAP utilization in children 20 kg or less for asthma exacerbations. Intensive Care Medicine 2011;37(8):1338‐43. [PUBMED: 21567114] [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical research ed.) 2008;336(7644):601‐5. [PUBMED: 18316340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yoo 2014
- Yoo Y, Perzanowski MS. Allergic sensitization and the environment: latest update. Current Allergy and Asthma Reports 2014;14(10):465. [PUBMED: 25149167] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Korang 2016
- Korang SK, Feinberg J, Wetterslev J, Jakobsen Janus C. Non‐invasive positive pressure ventilation for acute asthma in children. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, 2016, issue 1. [DOI: 10.1002/14651858.CD012067; CD012067] [DOI] [PMC free article] [PubMed]


