Abstract
Background
Ovarian cancer is the eighth most common cancer in women and it is usually diagnosed at an advanced stage. The majority of ovarian tumours are epithelial in origin. Women with relapsed epithelial ovarian cancer (EOC) often have a reduced performance status with a limited life expectancy, therefore maintaining quality of life with effective symptom control is the main purpose of treatment. Drug treatment of relapsed disease is directed by the platinum‐free interval: relapsed platinum‐sensitive disease is usually re‐treated with platinum‐based therapy and platinum‐resistant disease challenged with non‐platinum drugs. However, the side‐effects of chemotherapy agents may be severe and optimal treatment regimens are unclear. Pegylated liposomal doxorubicin (PLD), which contains a cytotoxic drug called doxorubicin hydrochloride is one of several treatment modalities that may be considered for single‐agent treatment of relapsed EOC, or used in combination with other drugs.
Objectives
To assess the efficacy and safety of PLD in women with relapsed epithelial ovarian cancer (EOC).
Search methods
We searched the Cochrane Gynaecological Cancer Group (CGCG) trials register, CENTRAL, MEDLINE and EMBASE from 1990 to February 2013. We also searched online registers of clinical trials, abstracts of scientific meetings and reference lists of included studies.
Selection criteria
Randomised controlled trials (RCTs) that evaluated PLD in women diagnosed with relapsed epithelial ovarian cancer.
Data collection and analysis
Two review authors independently abstracted data to a pre‐designed data collection form and assessed the risk of bias according to the Cochrane Handbook for Systematic Reviews of Interventions guidelines. Where possible, we pooled collected data in meta‐analyses using RevMan 5.2 software.
Main results
We included 14 RCTs that evaluated PLD alone or in combination with other drugs. Four RCTs contributed no data to the meta‐analyses. Two studies compared PLD plus carboplatin (carbo) to paclitaxel (PAC)/carbo in women with platinum‐sensitive relapsed EOC. Overall survival (OS) was similar for these treatments, however progression‐free survival (PFS) was longer with PLD/carbo (1164 participants; hazard ratio (HR) 0.85, 95% confidence interval (CI) 0.74 to 0.97; I² = 7%; P value 0.01). PLD/carbo was associated with significantly more anaemia and thrombocytopenia than PAC/carbo, whereas PAC/carbo was associated with significantly more alopecia, neuropathies, hypersensitivity reactions and arthralgias/myalgias. PLD/carbo was well‐tolerated and women receiving this treatment were significantly less likely to discontinue treatment than those receiving PAC/carbo (two studies, 1150 participants; risk ratio (RR) 0.38, 95% CI 0.26 to 0.57; I² = 0%; P < 0.00001).
Five studies compared other agents to PLD alone. None of these agents were associated with significantly better survival or severe adverse‐event profiles than PLD. Topotecan and gemcitabine were associated with significantly more haematological severe adverse events than PLD, and patupilone was associated with significantly more severe neuropathies and diarrhoea. Severe hand‐foot syndrome (HFS) occurred consistently more frequently with PLD than the other drugs.
Three studies compared PLD combination treatment to PLD alone. Two combinations resulted in a significantly longer PFS compared with PLD alone: trabectedin (TBD)/PLD (one study, 672 women; HR 0.79, 95% CI 0.65 to 0.96; P value 0.02) and vintafolide (EC145)/PLD (one study, 149 women; HR 0.63, 95% CI 0.41 to 0.97; P value 0.04). TBD/PLD appeared to benefit the partially platinum‐sensitive subgroup only. Further studies are likely to have an important impact on our confidence in these estimates. TBD/PLD was associated with significantly more haematological and gastrointestinal severe adverse events than PLD alone, whereas EC145/PLD appeared to be well‐tolerated.
For platinum‐resistant relapsed EOC, the median PFS and OS for single‐agent PLD across seven included studies was 15 weeks and 54 weeks, respectively. Severe HFS occurred significantly more frequently in women receiving a 50 mg/m² dose of PLD than those receiving less than 50 mg/m² (17% versus 2%, respectively; P value 0.01).
Authors' conclusions
In platinum‐sensitive relapsed epithelial ovarian cancer, PLD/carbo is more effective than PAC/carbo and is better tolerated; PLD/carbo should therefore be considered as first‐line treatment in women with platinum‐sensitive relapsed EOC. PLD alone is a useful agent for platinum‐resistant relapsed EOC, however it remains unclear how it compares with other single agents for this subgroup and in what order these agents should be used. There is insufficient evidence to support the use of PLD in combination with other agents in platinum‐resistant relapsed EOC.
Plain language summary
A coated, longer‐lasting form of doxorubicin hydrochloride for the treatment of recurrent ovarian cancer
Background
The choice of chemotherapy in women with relapsed epithelial ovarian cancer (EOC) is influenced by the duration of the platinum‐free interval, the length of time from the last platinum‐based cycle to the time of disease progression. Women who relapse within one month of receiving platinum therapy or who progress on therapy are considered to be platinum‐refractory; women who relapse between one and six months after platinum therapy are considered to be platinum‐resistant; and women who relapse more than six months after platinum therapy are considered to be platinum‐sensitive. The latter group is further subgrouped by women who relapse between six and 12 months after platinum therapy (partially platinum‐sensitive) and those who relapse after 12 months.
Doxirubicin hydrochloride is an anti‐cancer drug that works by interfering with cancer cell DNA. A newer form of doxorubicin called pegylated liposomal doxorubicin (PLD) has been developed with a coating that allows it to reach higher concentrations in cancer cells and with less adverse effects on the heart.
Review question
We conducted this review to determine whether PLD was effective and safe compared with other drugs used for relapsed EOC.
Main findings
We searched electronic databases and other resources for studies of PLD for relapsed ovarian cancerEOC, and included 14 studies up to October 2012. Most of these studies (12/14) were funded by drug manufacturers with a commercial interest in PLD (two studies) or the comparator drugs (10 studies). For women with platinum‐sensitive relapsed EOC, we pooled data from two studies (1164 participants) that compared carboplatin plus PLD (PLD/carbo) with standard treatment (paclitaxel plus carbo (PAC/carbo)). Women survived for a similar length of time overall on these two treatments but the cancer took longer to progress in those receiving PLD/carbo. Women who received PLD experienced more severe low blood cell counts than the standard treatment. By comparison, women in the standard treatment group experienced more severe hair loss, nerve damage, allergic reactions, and joint and muscle pain. More women in the standard treatment group stopped treatment early suggesting that PLD/carbo was better tolerated than standard treatment. We concluded that PLD/carbo was a better treatment option than PAC/carbo for platinum‐sensitive relapsed EOC.
Five studies compared PLD to five other chemotherapy drugs. The numbers of participants in these studies ranged from 97 to 829 women and we did not pool these data. PLD worked as least as well as the other agents and was comparatively well‐tolerated. In all studies, hand‐foot syndrome (HFS: swollen, painful, red, cracked and peeled soles and palms) occurred more frequently in the PLD group.
Three studies compared PLD plus another drug (canfosfamide (CAN), vintafolide (EC145) or trabectedin (TBD)) to PLD alone. The final results of the CAN study were not reported. The numbers of participants in the other studies ranged from 149 to 672 women and we did not pool these data. Women receiving the PLD/TBD combination treatment progressed six weeks later than those getting PLD only, however they did not live longer overall, and the combination treatment was associated with additional harmful effects. EC145 may improve survival in women with platinum‐resistant relapsed ovarian cancer when combined with PLD; this combination is currently under investigation in a large trial. Although HFS can be severely disabling, we noted that it occurred much less frequently when lower doses of PLD were used.
Quality of the evidence
We consider the evidence related to the longer time to cancer progression with PLD/carbo for platinum‐sensitive relapsed ovarian cancer to be of a high quality. There is currently insufficient evidence to support the use of other PLD combination treatments in relapsed EOC.
Summary of findings
Background
Description of the condition
Ovarian cancer is the eighth most common cancer in women worldwide and is responsible for approximately 225,500 new cancer cases per annum (Jemel 2011). In Europe it is the fifth most common cancer in women and the sixth most common cause of cancer deaths (Ferlay 2013). The cumulative risk of getting the disease is approximately 1% in developed countries (Europe, Northern America, Australia/New Zealand and Japan) and 0.5% in the rest of the world (GLOBOCAN 2008), and the risk increases with age.
As the disease is characterised by the absence of early specific symptoms, approximately 60% to 70% of women with ovarian cancer are diagnosed with FIGO stages III to IV (ICBP 2012), having widespread tumour dissemination within and beyond the abdominal cavity (Jemal 2008) (see Table 4 for FIGO staging). For stage I ovarian cancer, the five‐year survival rate approaches 90% (SEER 2007), whereas the five‐year survival rate for stage IV is less than 20% (SEER 2007). Overall, in Europe and the United States, for women with any stage of ovarian cancer, the five‐year survival rate is around 40% (EUROCARE 2003; SEER 2007).
1. FIGO staging of ovarian cancer*.
| Stage | Extent of tumour | Substage | Details |
| I | Limited to ovaries | Ia | Limited to 1 ovary, no tumour on surface or capsule rupture, no positive ascites |
| Ib | Limited to both ovaries, no tumour on surface or capsule rupture, no positive ascites | ||
| Ic | Stage Ia or Ib but with capsule ruptured, tumour on ovarian surface or positive peritoneal washings/ascites | ||
| II | Limited to 1 or both ovaries with pelvic extension | IIa | Extension, metastases to uterus, tubes, or a combination |
| IIb | Extension to other pelvis tissues | ||
| II c | Stage IIa or IIb with tumour on the surface of 1 or both ovaries, or with capsule ruptured, or with positive peritoneal washings/ascites | ||
| III | Limited to abdomen with histologically confirmed peritoneal implants outside the pelvis or positive nodes, or both, or extension to small bowel or omentum | IIIa | Tumour grossly limited to the true pelvis with negative regional lymph nodes, microscopic seeding of abdominal peritoneal surfaces or extension to small bowel or mesentery |
| IIIb | Macroscopic metastases < 2 cm; negative regional lymph nodes | ||
| IIIc | Macroscopic metastases > 2 cm or positive regional lymph nodes, or both | ||
| IV | Distant metastases | Growth outside the abdominal cavity (e.g. lung, liver parenchyma (superficial liver metastases is stage III)) |
FIGO: International Federation of Gynaecology and Obstetrics. * From FIGO 2009.
Epithelial ovarian cancer (EOC) accounts for approximately 90% of all ovarian tumours (SEER 2007). The standard treatment involves surgical removal and cytoreduction of the tumour followed by platinum‐based chemotherapy in combination with paclitaxel (ESMO 2010; Hennessy 2009); carboplatin is favoured over cisplatin due to its less toxic adverse‐effect profile (ESMO 2010; NICE 2003). Although most tumours (70% to 80%) initially respond to first‐line chemotherapy, most responders eventually relapse and will require further chemotherapy (NICE 2003). The choice of subsequent chemotherapy in women with relapsed EOC is influenced by the duration of the platinum‐free interval, the length of time from the last platinum‐based cycle to the time of disease progression. Women who relapse within one month of receiving platinum therapy or who progress on therapy are considered to be platinum‐refractory; women who relapse between one and six months after platinum therapy are considered to be platinum‐resistant; and women who relapse more than six months after platinum therapy are considered to be platinum‐sensitive (Pfisterer 2006). The latter group is further subgrouped by women who relapse between six and 12 months after platinum therapy (partially platinum‐sensitive) and those who relapse after 12 months.
In women with relapsed platinum‐sensitive disease it is standard practice to re‐treat with platinum‐based therapy (PAC/carbo) unless allergic to platinum compounds, and provided that there is no residual neurological toxicity (NICE 2005). This follows ICON‐4, which reported a median progression‐free survival (PFS) of three months longer in the combination arm compared with the platinum‐only arm (13 versus 10 months), and median overall survival (OS) of five months longer in the combination arm (29 versus 24 months) than the platinum‐only arm.
For the group of women with platinum‐resistant relapsed EOC, non‐platinum agents may be used including paclitaxel, topotecan, gemcitabine and pegylated liposomal doxorubicin (PLD) (Naumann 2011; NICE 2005). However, response rates in this group are poor (10% to 15%) and OS is approximately 12 months (Naumann 2011).
Description of the intervention
Doxorubicin hydrochloride is a cytotoxic drug that has been available since the 1960s and belongs to the group 'anthracyclines' (EMA 2010). Its main mode of action is to bind with topoisomerase II and DNA, forming a complex which results in lethal double‐stranded DNA breaks (Zunino 2002). Although anthracyclines are effective anti‐tumour agents, they are known to cause cardiotoxicity (Zunino 2002). Liposomal doxorubicin was developed with the aim of reducing the risk of cardiotoxicity compared with conventional doxorubicin whilst preserving its anti‐tumour effect (Theodoulou 2004). PLD is a formulation of liposomal doxorubicin coated in polyethylene glycol (PEG). This hydrophilic coating protects the liposomes from detection by the body's reticular endothelial system, reducing the rate at which the active substance is broken down, and increasing its circulating half‐life compared with conventional and liposomal doxorubicin (Gabizon 2001). Pegylated liposomes are small enough to extravasate out of leaky tumour vasculature (CAELYX PI) and the lack of functional lymphatic drainage results in high uptake and retention of PLD by the tumour. In addition, the increased circulating time conferred by the pegylation increases the number of passes the drug makes though the tumour microvasculature, which ultimately results in a higher delivered dose to the tumour (Gabizon 2001). Compared with conventional doxorubicin, PLD is associated with a significantly lower risk of cardiotoxicity, which is thought to be due to the tight capillary junctions in the cardiac muscle that limit the concentrations of the drug in this tissue (Theodoulou 2004).
For women with relapsed ovarian cancer, PLD is recommended at a starting dose of 50 mg/m² intravenously every four weeks for six cycles if tolerated and if the disease does not progress (EMA 2010). However, several recent studies have used lower doses, particularly when PLD has been combined with other agents (30 to 45 mg/m²; CALYPSO 2010; HeCOG 2010; OVA‐301 2010), in an attempt to reduce side‐effects, and a dose of 40 mg/m² every four weeks is commonly used in clinical practice. The most common side‐effect of PLD is nausea (EMA 2010), however, other side‐effects frequently associated with PLD include palmar‐plantar erythrodysesthesia (also known as hand‐foot syndrome), stomatitis and neutropenia (abnormally low number of circulating white blood cells ‐ neutrophils) (CAELYX PI; EMA 2010). Hand‐foot syndrome usually occurs after two or three cycles and can be severely disabling, leading to dose reductions or discontinuation. Grade 3 to 4 severity is reported to occur in approximately 20% of women who start PLD therapy at the 50 mg/m² dose (Lorusso 2007). Numerous approaches to hand‐foot syndrome management have been described, however, there is an absence of high‐quality evidence to support these strategies (von Moos 2008).
Why it is important to do this review
PLD has been incorporated into relapsed ovarian cancer treatment guidelines and in the UK is currently recommended as a treatment option for women whose disease does not respond to, and those women whose disease relapses within 12 months from, initial platinum‐based therapy (NICE 2005); however several studies have been completed since the publication of these guidelines. Recently published studies include CALYPSO 2010 and HeCOG 2010, that favourably compared PLD plus carboplatin to paclitaxel plus carboplatin for platinum‐sensitive disease (i.e. relapsed ovarian cancer occurring greater than six months from prior treatment) in terms of survival. Thus, it is possible that existing guidelines require updating.
PLD manufacturers recently experienced production problems (DOXIL 2011) and manufacture was halted for almost two years, resulting in the disruption of individual care and the suspension of some ongoing trials (INOVATYON; TRINOVA‐2). Production has now resumed and PLD is no longer a hypothetical option. However, the optimal dosing regimen of PLD remains unclear, as does the relative efficacy and adverse effects of PLD compared with, and combined with, other new agents. Drug treatment of relapsed EOC is a very dynamic field and, to our knowledge, a systematic review of PLD has not been conducted, and is necessary. By conducting a comprehensive systematic review of randomised controlled trials (RCTs) of PLD in women with platinum‐sensitive or platinum‐resistant relapsed EOC, we aimed to evaluate its efficacy and safety compared with other chemotherapy options.
Objectives
To evaluate the efficacy and safety of PLD in women with relapsed EOC.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
Women with relapsed EOC of any stage, including patients with both platinum‐sensitive and platinum‐resistant disease.
Types of interventions
PLD in combination with platinum‐based therapy versus platinum‐based therapy with another agent, e.g. PLD plus carboplatin versus paclitaxel (PAC) plus carboplatin.
Other chemotherapy agent(s) versus PLD, e.g. topotecan (TOP) versus PLD.
PLD plus other agent(s) versus PLD alone or with placebo, e.g. trabectedin (TBD) plus PLD versus PLD.
Types of outcome measures
Primary outcomes
Progression‐free survival (PFS): survival until disease progression
Overall survival (OS): survival until death from all causes
Secondary outcomes
Severe adverse events, classified according to CTCAE 2006 including haematological, gastrointestinal, genitourinary, dermatological, neurological, pulmonary, and other severe adverse events
Quality of life (QoL)
Symptom control, including dose reductions and delays
Search methods for identification of studies
We sought papers in all languages and obtained translations when necessary:
Electronic searches
We searched the following electronic databases (also see Cochrane Gynaecological Cancer Group methods used in reviews):
The Cochrane Gynaecological Cancer Group's Trial Register
Cochrane Central Register of Controlled Trials (CENTRAL Issue 1, 2013
MEDLINE (1990 to February week 2, 2013)
EMBASE (1990 to 2013 week 07)
The MEDLINE, EMBASE and CENTRAL search strategies, based on terms related to the review topic, are presented in Appendix 1, Appendix 2 and Appendix 3 respectively. As PLD has been recently developed, searches before 1990 would not have been relevant; therefore databases were searched from 1990 until February 2013. We identified all relevant articles on PubMed and, using the 'related articles' feature, we carried out a further search for newly published articles.
Searching other resources
We searched the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com/rct), www.clinicaltrials.gov and the Physicians Data Query (PDQ) (www.cancer.gov/clinicaltrials) for ongoing trials, and we searched the abstracts of ASCO Annual Meetings from 2000 to 2012. Where necessary, we attempted to contact the main investigators of relevant ongoing trials for further information. In addition, we checked the citation lists of included studies to identify other reports/studies.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to the reference management database (Reference Manager version 10) and removed duplicates. The remaining records were examined independently by review author Tess Lawrie (TL) and Julia Dawson (see Acknowledgements) to identify potentially relevant trials. We excluded studies that clearly did not meet the inclusion criteria and obtained the full text of potentially relevant trials. Review authors TL and Jo Morrison (JM) independently assessed these identified trials for eligibility. Where there were any disagreements, we involved a third review author (Andy Bryant (AB)) in the process. Where we excluded studies, we documented the reasons for exclusion.
Data extraction and management
For included studies, we abstracted the following data where possible.
Author, year of publication and journal citation (including language)
Country
Setting
Inclusion and exclusion criteria
Study design, methodology
-
Study population
total number enrolled
patient characteristics
age
previous therapy (including platinum sensitivity or resistance)
co‐morbidities
-
Ovarian cancer details at diagnosis
FIGO stage
histological cell type
tumour grade
performance status
extent of disease
Total number of intervention groups
-
Intervention details
details of PLD including dose, regimen, frequency and the number of cycles
comparison details including type of control and dose, regimen, frequency and number of cycles, if appropriate
Proportion of participants who received all/ part/none of the intended treatment
Delays in treatment
Risk of bias in study (see Assessment of risk of bias in included studies)
Duration of follow‐up
-
Outcomes – overall survival, PFS, QoL, symptom control and adverse events
for each outcome: outcome definition (with diagnostic criteria if relevant)
unit of measurement (if relevant)
for scales: upper and lower limits, and whether high or low score is good
results: Number of participants allocated to each intervention group
for each outcome of interest: sample size; missing participants
Data abstraction of outcome data from each trial
Data on outcomes were extracted as follows.
For time‐to‐event data (OS and PFS), we abstracted the hazard ratio (HR), log of the hazard ratio (log(HR)) and its standard error (SE) from trial reports where possible. If these were not reported, we attempted to estimate them from other reported statistics using the methods of Parmar 1998 (e.g. number of events in each arm and log‐rank P‐value comparing the relevant outcomes in each arm). If it was not possible to estimate the HR, we abstracted the number of patients in each treatment arm who experienced the outcome of interest at a specific time point, in order to estimate a risk ratio (RR).
For dichotomous outcomes (e.g. adverse events), we abstracted the number of patients in each treatment arm who experienced the outcome of interest and the number of patients assessed at endpoint, in order to estimate a RR.
For continuous outcomes (e.g. QoL measures), we abstracted the mean difference (MD) and standard deviation (SD) between the final value of the outcome measure in each treatment arm at the end of follow‐up. If SDs of final values were not available, change scores were used if their SDs were available. If no SDs were available, these trials were omitted from the analyses.
Where possible, we extracted data relevant to an intention‐to‐treat analysis (ITT), in which participants were analysed in groups to which they were assigned. Where time‐to‐event outcomes were assessed by more than one method, e.g. independent radiology review, investigator assessment or independent oncology review, we used the independent radiology review data. We noted the time points at which outcomes were collected and reported. Where data from several time points were reported, we used the data from the last assessment in our meta‐analyses if appropriate. Where a trial evaluated the same drug in two or more different doses versus PLD, we extracted the combined data and the individual data of the most efficacious dose/regimen versus PLD.
Two review authors (TL and AB) independently extracted data from the selected trials using piloted data extraction forms specially designed for the review. Where there was disagreement between the two review authors, this was resolved by discussion with JM.
Assessment of risk of bias in included studies
The risk of bias in included RCTs was assessed using The Cochrane Collaboration's tool and the criteria specified in chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This included assessment of:
-
Selection bias:
random sequence generation
allocation concealment
-
Performance bias
blinding of participants and personnel (patients and treatment providers)
-
Detection bias
blinding of outcome assessment
-
Attrition bias
incomplete outcome data: We recorded the proportion of participants whose outcomes were not reported at the end of the study and considered greater than 20% attrition to be at a high risk of bias
-
Reporting bias
selective reporting of outcomes
Other possible sources of bias
The 'Risk of bias' tool was applied independently by two review authors (TL and AB) and differences were resolved by discussion or by appeal to a third review author (JM). Results are presented in a 'Risk of bias' summary graph and the results of the meta‐analyses were interpreted in light of the findings with respect to risk of bias.
Measures of treatment effect
We used the following measures of the effect of treatment.
For time‐to‐event data, we used the HR.
For dichotomous outcomes, we used the RR.
For continuous outcomes, we planned to use the MD between treatment arms.
Dealing with missing data
We did not impute missing outcome data.
Assessment of heterogeneity
Heterogeneity between trials was assessed by visual inspection of forest plots, by estimation of the percentage heterogeneity between trials that cannot be ascribed to sampling variation (Higgins 2003), and by a formal statistical test of the significance of the heterogeneity (Deeks 2001). We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics and regarded heterogeneity as substantial if the I² was greater than 50% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test. If there was evidence of substantial heterogeneity, we investigated the possible reasons for this and reported it.
Assessment of reporting biases
There was an insufficient number of included studies to adequately evaluate the potential for small study effects, such as publication bias, using funnel plots.
Data synthesis
When sufficient clinically similar trials were available, we pooled their results in meta‐analyses.
For time‐to‐event data, we pooled HRs using the generic inverse variance facility of RevMan 5.2.
For any dichotomous outcomes, we pooled the RRs.
For continuous outcomes, we planned to pool the MDs between the treatment arms at the end of follow‐up if all trials measured the outcome on the same scale, otherwise we planned to pool standardised mean differences (SMDs).
We used random‐effects models with inverse variance weighting for all meta‐analyses (Dersimonian 1986).
Subgroup analysis and investigation of heterogeneity
The RCTs were grouped by Types of interventions. Where the types of interventions differed within a comparison, e.g. other drugs versus PLD, we subgrouped data by the comparator drug and did not combine subgroup data. We had planned to subgroup survival outcomes by platinum sensitivity, however, this was not possible due to insufficient data.
Sensitivity analysis
We had planned to perform sensitivity analyses for survival outcomes by excluding trials which were at a high risk of bias. However, most of the studies at a high risk of bias had no useable data and so could not be included in meta‐analyses. Therefore, sensitivity analyses were not performed.
Results
Description of studies
Results of the search
We identified 1602 unique references by the database searches and 17 trials by the trial registry searches (Figure 1). We screened the abstracts of 185 records and obtained the full text of 109 potentially eligible publications, including the trial registry records. After evaluating these full texts we excluded seven studies (20 records) (see Characteristics of excluded studies) and added the details of the 16 ongoing trials to the Characteristics of ongoing studies section of the review (18 records). Fourteen completed RCTs (72 records) met our inclusion criteria. One of these was not yet published in full (PRECEDENT 2013); we contacted the investigators and obtained a copy of the unpublished manuscript. We also obtained additional unpublished data from the investigators of two other studies (Kaye 2012; MITO‐3 2008).
1.
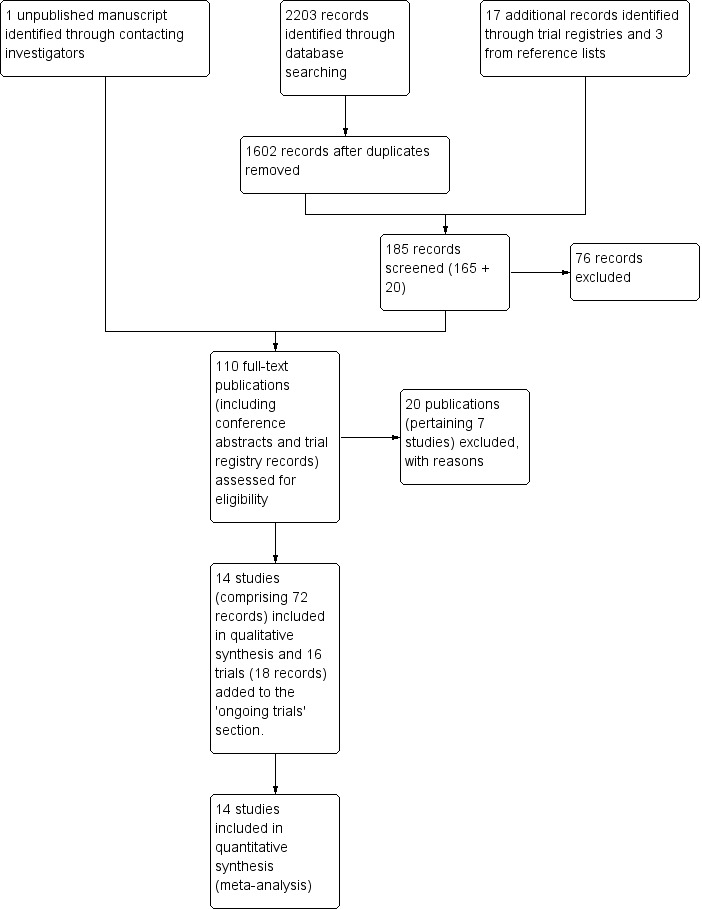
Study flow diagram of searches to 15 October 2012.
Included studies
A. Studies of PLD plus carboplatin versus platinum therapy plus another agent or alone
We included three studies in this comparison (SWOG S0200 2008; HeCOG 2010; CALYPSO 2010). All were multicentre RCTs randomising 61, 204 and 976 participants respectively. HeCOG 2010 was a phase II study and the other two studies were phase III. SWOG S0200 2008 was terminated early, when only 61 out of 900 women had been randomised, due to poor accrual.
Participants
These studies were conducted in women with platinum‐sensitive relapsed EOC, i.e. women in whom relapse occurred more than six months after completion of a course of platinum‐based chemotherapy. The median platinum‐free interval was greater then 12 months in all three studies. The majority (greater than 80%) of women in these studies had received only one prior platinum line. In HeCOG 2010 and CALYPSO 2010, 90% and 100% of participants respectively had also received prior taxane therapy, compared with only 9/61 women (15%) in SWOG S0200 2008. Other participant characteristics in these studies at baseline, including age and performance status, were similar.
Interventions
Two studies randomised women to PLD plus carboplatin (carbo) or paclitaxel (PAC) plus carbo (HeCOG 2010; CALYPSO 2010), and SWOG S0200 2008 compared PLD plus carbo with carbo alone. PLD was administered at a dose of 30 mg/m² in SWOG S0200 2008 and CALYPSO 2010, and at 45 mg/m² in HeCOG 2010. A standard premedication of corticosteroids and anti‐emetics was given to women in HeCOG 2010 and CALYPSO 2010. However, women in the PAC/carbo arm of the CALYPSO 2010 study also received additional premedication to prevent hypersensitivity reactions (HSRs). In the SWOG S0200 2008 protocol, premedication was optional and it is not clear what proportion of women received it.
Outcomes
PFS, OS and toxicity were primary or secondary outcomes in all studies, except for HeCOG 2010, which evaluated response rate as the primary outcome. Adverse events were assessed using CTCAE 2006 version 3.0, or an earlier version in all studies. Other outcomes included survival times, i.e. the median time to progression (TTP) and the median time to death (TTD). One study (CALYPSO 2010) evaluated participant quality of life (QoL) at baseline and at several time points after randomisation, using the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ‐C30).
B. Studies of other drug(s) versus PLD
We included seven trials in this comparison (ASSIST‐3 2007; Colombo 2012; Gordon 2001; Kaye 2012; MITO‐3 2008; Mutch 2007; O'Byrne 2002). All were phase III multicentre RCTs, except for one phase II trial (Kaye 2012), with the number of participants ranging from 97 (Kaye 2012) to 829 (Colombo 2012). Two studies (ASSIST‐3 2007; O'Byrne 2002) were published only as conference abstracts and contributed no data to our analyses despite the accrual of 247 and 214 participants, respectively (see Risk of bias in included studies).
Participants
Three studies included women with platinum‐resistant relapsed EOC only (relapse within six months; ASSIST‐3 2007; Colombo 2012; Mutch 2007); two studies included women with platinum‐resistant relapsed EOC and partially platinum‐sensitive relapsed EOC (relapse within 12 months; Kaye 2012; MITO‐3 2008); and two studies included all women with relapsed EOC (Gordon 2001; O'Byrne 2002). Women were eligible for these trials if they had progressed on platinum‐based regimens (ASSIST‐3 2007; Gordon 2001; Kaye 2012; Mutch 2007; O'Byrne 2002) or platinum‐taxane based regimens (Colombo 2012; MITO‐3 2008). Age and performance status of participants in these studies were similar.
Interventions
The following chemotherapy agents were evaluated in comparison to PLD, which served as the active control:
gemcitabine (GEM): Mutch 2007 (195 women); MITO‐3 2008 (153 women);
topotecan (TOP): Gordon 2001 (481 women);
canfosfamide (CAN) plus carbo: ASSIST‐3 2007 (247 women);
olaparib (OLA): Kaye 2012 (97 women);
patupilone (PAT): Colombo 2012 (829 women); and
paclitaxel (PAC): O'Byrne 2002 (214 women).
PLD was administered intravenously in all these studies at a dose of 50 mg/m², except for MITO‐3 2008, in which a dose of 40 mg/m² was used. In MITO‐3 2008, a corticosteroid premedication was administered to all participants; for the other studies premedication was either optional (Kaye 2012), not given (Colombo 2012) or not described.
Outcomes
PFS and OS were the primary or secondary outcomes in all studies except for MITO‐3 2008, which evaluated TTP as the primary outcome. Most studies also reported grade 3 to 4 adverse events using CTCAE 2006 or an earlier version. QoL was evaluated as a secondary outcome in five studies using either the Functional Assessment of Cancer Therapy ‐ Ovarian (FACT‐O) questionnaire or the EORTC QLQ‐C30. Other outcomes that were frequently reported included the overall response rate (ORR), complete response rate (CR) and partial response rate.
C. Studies of PLD plus other drug/s versus PLD alone
We included four studies in this comparison (ASSIST‐5 2010; PRECEDENT 2013; M200 2009; OVA‐301 2010). M200 2009 did not contribute any data to the analyses.
Participants
Two studies included women with platinum‐resistant relapsed EOC only (ASSIST‐5 2010; PRECEDENT 2013) and two studies included all women with relapsed EOC (M200 2009; OVA‐301 2010). Women in the OVA‐301 2010 study had received only one prior platinum‐based chemotherapy regimen, whereas the other studies included women who had received up to two previous platinum‐based regimens.
Interventions
In these studies, one of the following agents was combined with PLD in the experimental arm and evaluated in comparison to PLD, which served as the active control
canfosfamide (CAN): ASSIST‐5 2010 (125 women);
trabectedin (TBD): OVA‐301 2010 (672 women);
volociximab (M200): M200 2009 (127 women); and
vintafolide (EC145): PRECEDENT 2013 (162 women).
Women in the TBD/PLD arm of OVA‐301 2010 also received a corticosteroid premedication, whereas women in the PLD arm of this study did not.
Outcomes
PFS was the primary outcome of all these studies with secondary outcomes including OS, adverse events (according to CTCAE 2006) and ORR. OVA‐301 2010 also evaluated QoL.
Excluded studies
We excluded six studies either because they were not RCTs (Cherchi 2003; Kavanagh 2004; Palaia 2006; Scarfone 2006) or because they evaluated PLD for first‐line drug treatment of EOC (GOG0182/ICON 5; MITO‐2 2011). One additional RCT (ASSIST‐1 2009) was excluded for methodological reasons, as the allocation of participants to PLD treatment was not a truly random process. For further details of these excluded studies, see Characteristics of excluded studies.
Risk of bias in included studies
See Figure 2 for review authors' judgements about each risk of bias item for each included study.
2.
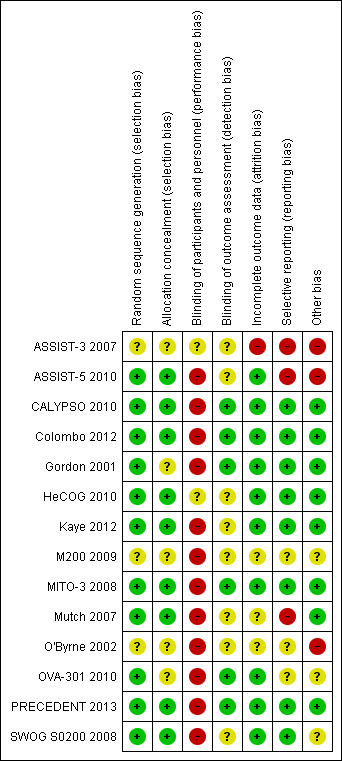
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Most studies were multicentre studies with central randomisation and treatment allocation after registration with the organising centre, and were therefore at a low risk of selection bias. The method of randomisation and allocation were not described in three studies that were published as conference abstracts only (ASSIST‐3 2007; M200 2009; O'Byrne 2002) and one other full‐text publication (Gordon 2001).
Blinding
All of the included studies were open‐label, i.e. the participants and attending healthcare professionals were aware of the associated group allocation; therefore, all studies were at a high risk of performance bias. All included studies assessed disease progression according to Response Evaluation Criteria in Solid Tumors (RECIST) and/or Gynecologic Cancer Intergroup (GCIG) criteria (CA‐125) (Therasse 2000; Rustin 1996); however, in most studies, it was not clear what methods, if any, were used to minimise detection bias ‐ only six studies reported assessor blinding or independent radiologist or oncologist review (CALYPSO 2010; Colombo 2012; Gordon 2001; MITO‐3 2008; OVA‐301 2010; PRECEDENT 2013).
Incomplete outcome data
Attrition rates were high in ASSIST‐3 2007 for primary outcomes and we were unable to use these data. Three other studies did not clearly state the total numbers of participants evaluated per outcome (i.e. denominators were missing) (M200 2009; Mutch 2007; O'Byrne 2002). Attrition rates for QoL outcomes were universally high (greater than 20%) in the seven studies that reported this outcome (CALYPSO 2010; Colombo 2012; Gordon 2001; Kaye 2012; MITO‐3 2008; Mutch 2007; OVA‐301 2010).
Selective reporting
Most included studies reported their pre‐specified outcomes. Three studies reported only limited data in the abstracts of conference proceedings that could not be adequately evaluated for reporting bias (ASSIST‐3 2007; M200 2009; O'Byrne 2002); to our knowledge, these studies have not been published in full.
ASSIST‐5 2010 was temporarily put on hold in June 2007 to review the results of the single‐agent trial (ASSIST‐1 2009). The clinical hold was released in October 2007 but the sponsor decided not to enrol any additional patients and closed the trial early (planned enrolment = 244, actual enrolment = 125). Overall survival data for ASSIST‐5 2010 have not been published and, to our knowledge, neither have the review findings. The drug manufacturer concerned, Telik, did not respond to our queries, therefore, we considered the canfosfamide studies to be at a high risk of reporting bias.
Other potential sources of bias
Since the results of O'Byrne 2002 and ASSIST‐3 2007 have not been published in full, there is a potentially high risk of bias associated with the non‐publication of these studies. O'Byrne 2002 enrolled women with relapsed EOC (platinum‐sensitive or platinum‐resistant) to PLD or PAC. As previous therapy with PLD or PAC was an exclusion criterion, once PAC/carbo became a first‐line chemotherapy combination option for EOC (NICE 2003), accrual was slow and the study became largely irrelevant. However, 220 women (out of a target of 438) were randomised and started on treatment and, ideally, the results of this terminated study should have been published. We were unsuccessful in our attempts to obtain these data or further information. Similarly, we were unable to obtain missing data for ASSIST‐3 2007, despite several attempts to contact the investigators and Telik.
SWOG S0200 2008 (PLD/carbo versus carbo alone for platinum‐sensitive relapsed EOC) was another study that closed early due to slow accrual following the release of the initial ICON‐4 results, that showed the combination of PAC/carbo to be superior to carbo alone for women with platinum‐sensitive relapsed EOC, and for other reasons. SWOG S0200 2008 is therefore limited by a small sample size (61 evaluable participants). However, unlike the O'Byrne 2002 study, the investigators of SWOG S0200 2008 published their final results in full.
Most included studies (12/14) were funded by drug manufacturers with a commercial interest in PLD (CALYPSO 2010; SWOG S0200 2008) or the comparator drugs (ASSIST‐3 2007; ASSIST‐5 2010; Colombo 2012; Gordon 2001; Kaye 2012; M200 2009; Mutch 2007; O'Byrne 2002; OVA‐301 2010; PRECEDENT 2013). The exceptions were HeCOG 2010 and MITO‐3 2008.
Effects of interventions
See: Table 1; Table 2; Table 3
for the main comparison.
| PLD/carbo compared with PAC/carbo for platinum‐sensitive relapsed ovarian cancer | ||||||
|
Patient or population: women with platinum‐sensitive relapsed ovarian cancer Settings: inpatient or outpatient setting Intervention: PLD/carbo Comparison: PAC/carbo | ||||||
| Outcomes | Illustrative comparative survival or risk rates* (95% CI included for RR) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| PAC/carbo | PLD/carbo | |||||
| Progression‐free survival | Median PFS¹ = 40 weeks (9 months) | Median PFS¹ = 48 weeks (11 months) | HR 0.85 (0.74 to 0.97) | 1164 (2) | ⊕⊕⊕⊕ high | P value 0.01. Low statistical heterogeneity between studies. |
| Overall survival | Median OS¹ = 141 weeks (33 months) | Median OS¹ = 132 weeks (31 months) | HR 1.01 (0.88 to 1.17) | 1164 (2) | ⊕⊕⊕⊝ moderate | P value 0.85. Low statistical heterogeneity between studies. We downgraded this evidence due to post‐study treatment differences between the groups in the CALYPSO 2010 study which may have impacted the results in the direction of the PAC/carbo arm. |
| SAE ‐ Hand‐foot syndrome (grade 3) | 3 per 1000 | 13 per 1000 (3 to 60) |
RR 4.30 (0.92 to 20.15) | 1140 (2) |
⊕⊕⊕⊝ moderate | P value 0.06. We downgraded the quality of this evidence due to the rarity of grade 3 events in these two studies. |
| SAE ‐ Hair loss (grade 2)² | 840 per 1000 | 76 per 1000 (50 to 126) |
RR 0.09 (0.06 to 0.15) | 1140 (2) |
⊕⊕⊕⊕ high | P < 0.00001. |
| Discontinuation due to toxicity | 144 per 1000 | 55 per 1000 (37 to 82) | RR 0.38 (0.26, 0.57) | 1150 (2) | ⊕⊕⊕⊕ high | P < 0.00001. Low statistical heterogeneity between studies. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; HR: hazard ratio; PFS: progression‐free survival; OS: overall survival; PLD: pegylated liposomal doxorubicin; PAC: paclitaxel; carbo: carboplatin | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
The basis of the assumed risk was the median control group risk across studies, and the corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI), unless otherwise noted.
¹ These illustrative values, rounded to the nearest week and month, are taken from CALYPSO 2010.
² Grade 2 is the highest grade of alopecia according to the CTCAE 2006.
2.
| PLD‐based combination treatment compared with PLD alone for relapsed ovarian cancer | ||||||
|
Patient or population: women with platinum‐resistant (PR) or platinum‐sensitive (PS) relapsed ovarian cancer Settings: inpatient or outpatient setting Intervention: PLD plus other drug Comparison: PLD alone | ||||||
| Outcomes | Illustrative comparative survival* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| PLD alone | PLD plus other drug | |||||
|
PFS: PR and PS disease TBD/PLD versusPLD |
Median PFS = 25 weeks | Median PFS = 31 weeks | HR 0.79 (0.65 to 0.96) | 672 (1) | ⊕⊕⊕⊝ moderate | We downgraded this evidence as meta‐analysis was not possible and subgroup analysis indicated that the survival benefit only related to the PPS subgroup. This finding therefore has limited clinical applicability as the standard treatment for the PS subgroup is PAC/carbo or PLD/carbo. |
|
PFS: PPS disease only PLD/TBD versusPLD |
Median PFS = 24 weeks | Median PFS = 32 weeks | HR 0.65 (0.45 to 0.93) | 208 (1) |
⊕⊕⊕⊝ moderate | We downgraded this evidence as meta‐analysis was not possible and the data was a subgroup analysis of the original study in which the sample sizes for the subgroup arms differed by 30%. This finding has limited clinical applicability as the standard treatment for the PPS subgroup is PAC/carbo or PLD/carbo. |
|
OS: PR and PS disease TBD/PLD versusPLD |
Median OS = 81 weeks (19 months) | Median OS = 95 weeks (22 months) | HR 0.86 (0.72 to 1.02) | 672 (1) | ⊕⊕⊕⊝ moderate | We downgraded this evidence as meta‐analysis was not possible and PFI baseline characteristics differed between the groups (women in the PLD only arm had significantly longer PFIs; P value 0.008). This may have biased the results of this study in favour of the PLD only arm. |
|
PFS: PR disease only EC145/PLD versusPLD |
Median PFS = 12 weeks | Median PFS = 21 weeks | HR 0.63 (0.41, 0.97) | 149 (1) |
⊕⊕⊝⊝ low | We downgraded this evidence as meta‐analysis was not possible and the source of the data was a single, phase II open‐label study. |
|
OS: PR disease only EC145/PLD versusPLD |
Median OS = 72 weeks | Median OS = 60 weeks | HR 1.01 (0.68, 1.50) | 149 (1) |
⊕⊝⊝⊝ very low | We downgraded this evidence as meta‐analysis was not possible and the source of the data was a single, phase II open‐label study. The study was not powered to evaluate OS. |
| *The illustrative comparative survival times are derived from the OVA‐301 2010 and PRECEDENT 2013 trial results and do not reflect a relative effect of the experimental intervention per se. CI: confidence interval; RR: risk ratio; HR: hazard ratio; PFS: progression‐free survival; OS: overall survival; PFI: platinum‐free interval; PPS: partially platinum‐sensitive; PR: platinum‐resistant; PS: platinum‐sensitive; PLD: pegylated liposomal doxorubicin; TBD: trabectedin; EC145: vintafolide | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
3.
| Adverse events related to PLD dose (<50 mg/m²and 50 mg/m²) in studies that compared PLD alone with non‐PLD agent/s for relapsed ovarian cancer | ||||||
|
Patient or population: women with relapsed ovarian cancer Settings: inpatient or outpatient setting Intervention: PLD Comparison: other non‐PLD drug/s | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| non‐PLD agent/s | PLD alone | |||||
| SAE: HFS (grade 3) subgrouped by PLD dose | < 50 mg/m²PLD dose every 4 weeks |
RR 4.63 (1.32 to 16.19) RR 50.75 (12.57 to 204.97) |
1344 (4) 1544 (4) |
⊕⊕⊕⊕
high ⊕⊕⊕⊕ high |
Tests for subgroup differences were significant (P value 0.01). | |
| < 1 per 1000 | 5 per 1000 (1 to16) | |||||
| 50 mg/m²PLD dose every 4 weeks | ||||||
| < 1 per 1000 | 51 per 1000 (13 to 205) | |||||
| SAE: Stomatitis (grade 3 to 4) subgrouped by PLD dose | <50 mg/m²PLD dose every 4 week |
RR 2.22 (0.87 to 5.67) RR 12.19 (4.62 to 32.20) |
1283
(4) 1544 (4) |
⊕⊕⊕⊕
high ⊕⊕⊕⊕ high |
Tests for subgroup differences were significant (P value 0.01). | |
| 1 per 1000 | 2 per 1000 (1 to 6) | |||||
| 50 mg/m²PLD dose every 4 weeks | ||||||
| 1 per 1000 | 12 per 1000 (5 to 32) | |||||
| *The basis for the assumed risk is the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; PLD: pegylated liposomal doxorubicin | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
A. PLD plus carboplatin versus carboplatin ± other drug/s
Three studies with a total of 1201 assessable participants contributed data to these meta‐analyses. Outcomes were subgrouped by the active control and comparison group, i.e. carbo alone (one study; 61 participants) or PAC/carbo (two studies; 1164 participants). We did not combine subgroup data.
Survival and efficacy
Progression‐free survival
PLD/carbo versuscarbo alone: The PLD/carbo regimen resulted in a significantly longer PFS that the carbo alone regimen (one study, 61 participants; hazard ratio (HR) 0.52, 95% confidence interval (CI) 0.31 to 0.88; Analysis 1.1).
1.1. Analysis.
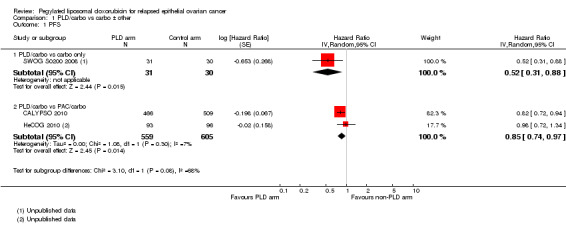
Comparison 1 PLD/carbo vs carbo ± other, Outcome 1 PFS.
PLD/carbo versusPAC/carbo: The PLD/carbo regimen resulted in a significantly longer PFS than the PAC/carbo regimen (two studies, 1164 participants; HR 0.85, 95% CI 0.74 to 0.97; I² = 7%; P value 0.01; Analysis 1.1).
Overall survival
There was no significant difference in OS between treatment arms for the PLD/carbo versus carbo alone comparison (one study, 61 participants; HR 0.69, 95% CI 0.40 to 1.21; Analysis 1.2) or for the PLD/carbo versus PAC/carbo meta‐analysis (two studies, 1164 participants; HR 1.01, 95% CI 0.88 to 1.17; I² = 0%; P value 0.85; Analysis 1.2).
1.2. Analysis.
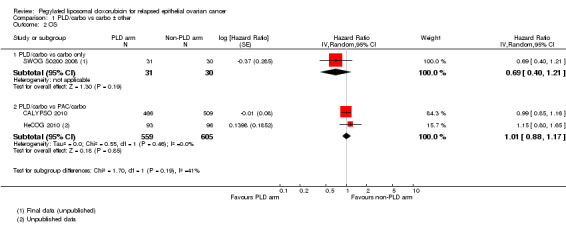
Comparison 1 PLD/carbo vs carbo ± other, Outcome 2 OS.
Safety and adverse events
PLD/carbo versuscarbo alone: Women in the combination arm were statistically significantly more likely than those in the carbo alone arm to experience neutropenia and thrombocytopenia (reduced numbers of platelets) in the one small study that evaluated this comparison (SWOG S0200 2008).
PLD/carbo versusPAC/carbo: Women receiving the PLD/carbo regimen were statistically significantly more likely than those receiving the PAC/carbo regimen to experience the following:
anaemia (grade 3 to 4): two studies,1140 participants; risk ratio (RR) 1.59, 95% CI 1.02 to 2.50; I² = 0%; P value 0.04 (Analysis 1.4); and
thrombocytopenia (grade 3 to 4): two studies, 1140 participants; RR 2.69, 95% CI 1.83 to 3.96; I² = 0%; P < 0.00001 (Analysis 1.7).
1.4. Analysis.
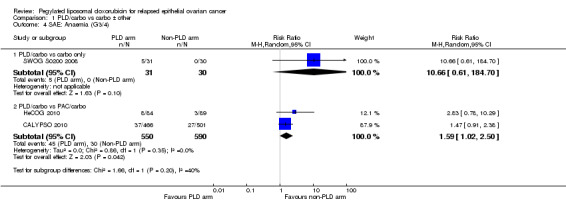
Comparison 1 PLD/carbo vs carbo ± other, Outcome 4 SAE: Anaemia (G3/4).
1.7. Analysis.
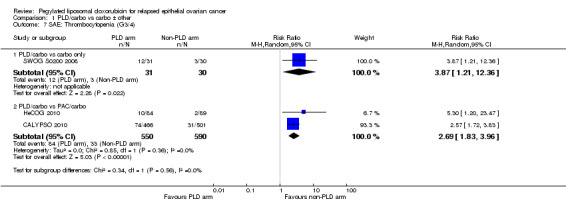
Comparison 1 PLD/carbo vs carbo ± other, Outcome 7 SAE: Thrombocytopenia (G3/4).
They were also statistically significantly less likely to experience the following:
alopecia (grade 2): two studies, 1140 participants; RR 0.09, 95% CI 0.06 to 0.15; I² = 44%; P < 0.00001 (Analysis 1.10);
neuropathy (grade 3 to 4): two studies, 1140 participants; RR 0.20, 95% CI 0.08 to 0.50; I² = 0%; P value 0.0005 (Analysis 1.11);
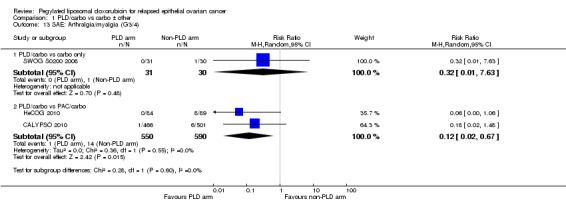
arthralgia/myalgia (grade 3 to 4): two studies, 1140 participants; RR 0.12, 95% CI 0.02 to 0.67; I² = 0%; P value 0.02 (Analysis 1.13); and
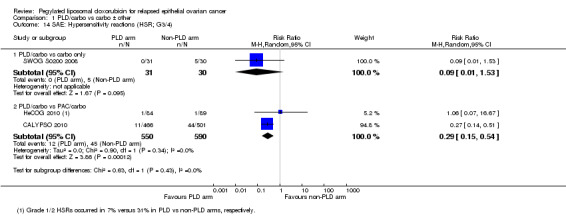
hypersensitivity reactions (HSRs; grade 3 to 4): two studies, 1140 participants; RR 0.29, 95% CI 0.15 to 0.54; I² = 0%; P value 0.0001 (Analysis 1.14).
1.10. Analysis.
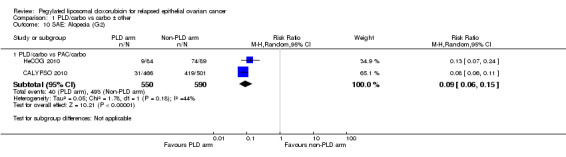
Comparison 1 PLD/carbo vs carbo ± other, Outcome 10 SAE: Alopecia (G2).
1.11. Analysis.
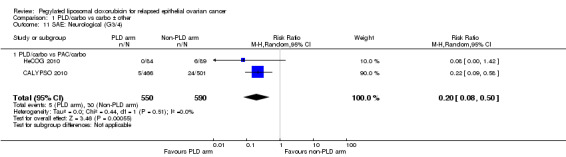
Comparison 1 PLD/carbo vs carbo ± other, Outcome 11 SAE: Neurological (G3/4).
1.13. Analysis.
Comparison 1 PLD/carbo vs carbo ± other, Outcome 13 SAE: Arthralgia/myalgia (G3/4).
1.14. Analysis.
Comparison 1 PLD/carbo vs carbo ± other, Outcome 14 SAE: Hypersensitivity reactions (HSR; G3/4).
There were trends towards more hand‐foot syndrome (grade 3) in the PLD/carbo group (RR 4.30, 95% CI 0.92 to 20.15; Analysis 1.3) compared with the PAC/carbo group, and more grade 3 to 4 stomatitis (RR 2.27, 95% CI 0.82 to 6.29; Analysis 1.8), however, these did not reach statistical significance.
1.3. Analysis.
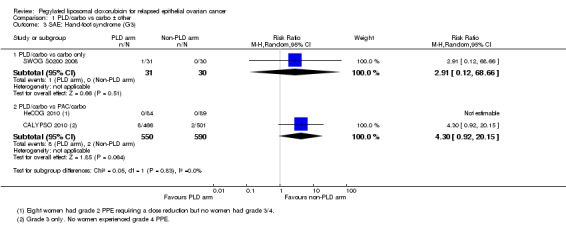
Comparison 1 PLD/carbo vs carbo ± other, Outcome 3 SAE: Hand‐foot syndrome (G3).
1.8. Analysis.
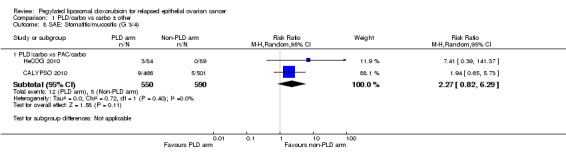
Comparison 1 PLD/carbo vs carbo ± other, Outcome 8 SAE: Stomatitis/mucositis (G 3/4).
There were no other statistically significant differences between treatment arms with regard to other serious adverse effects, including neutropenia, febrile neutropenia, vomiting and fatigue.
Discontinuation due to toxicity: Women in the PAC/carbo group were statistically significantly more likely to discontinue treatment due to toxicity than women in the PLD/carbo group (two studies, 1150 participants; RR 0.38, 95% CI 0.26 to 0.57; I² = 0%; P < 0.00001; Analysis 1.17)
1.17. Analysis.
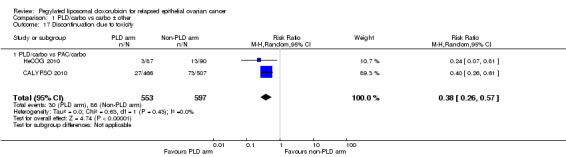
Comparison 1 PLD/carbo vs carbo ± other, Outcome 17 Discontinuation due to toxicity.
Quality of Life
Only one study (CALYPSO 2010) reported QoL outcomes, therefore we were unable to perform meta‐analyses. The mean change in global health scores from baseline scores was significantly better at three months post‐randomisation in the PLD/carbo group versus the PAC/carbo group (P value 0.01), but not at six months. Scores for peripheral neuropathy (P < 0.001), other chemotherapy side‐effects (P < 0.001) and body image (P value 0.02) were significantly worse in the PAC/carbo group at six months. These QoL data suffered from high attrition rates (greater than 30%).
Symptom control
Dosing delays/reductions
We could not perform a meta‐analysis for this outcome due to insufficient data. HeCOG 2010 (PLD dose = 45 mg/m²) reported significantly more dosing delays (26/85 versus 12/89; P value 0.006) and reductions (29/85 versus 4/89) in the PLD/carbo arm than in the PAC/carbo arm, mainly due to haematological toxicities. In comparison, CALYPSO 2010 (PLD dose = 30 mg/m²) investigators found that dosing delays were not significantly different between these regimens (reported as 7% versus 5% for PLD/carbo and PAC/carbo, respectively).
Supportive treatment
CALYPSO 2010 reported that supportive treatment use, including granulocyte colony stimulating factor (G‐CSF), erythropoietin and transfusions, was similar in the two treatment arms. There was no statistically significant difference in the use of antibiotics (Analysis 1.18). We were unable to perform meta‐analysis for 'blood transfusions required', however we noted a trend towards more transfusions in the PLD/carbo arms of two studies (Analysis 1.20). Only HeCOG 2010 reported G‐CSF data (Analysis 1.19), which was not significantly different between the treatment arms.
1.18. Analysis.
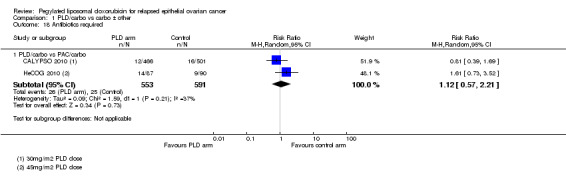
Comparison 1 PLD/carbo vs carbo ± other, Outcome 18 Antibiotics required.
1.20. Analysis.
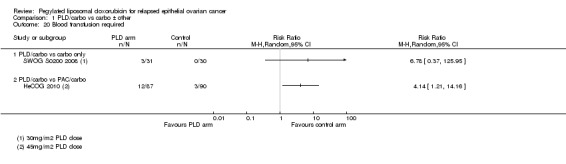
Comparison 1 PLD/carbo vs carbo ± other, Outcome 20 Blood transfusion required.
1.19. Analysis.
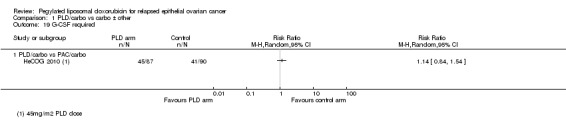
Comparison 1 PLD/carbo vs carbo ± other, Outcome 19 G‐CSF required.
B. Other drug(s) versus PLD
Five out of seven studies contributed data to the analyses. These studies were clinically heterogeneous in terms of the comparative intervention (e.g. GEM, TOP, OLA, PAT) and the platinum‐free interval, therefore in all analyses, we subgrouped studies by the comparative intervention and evaluated subtotals only.
Survival and efficacy
Progression‐free survival
Only two studies published HRs for PFS (Kaye 2012; Colombo 2012). We estimated HRs from the raw data of one study (MITO‐3 2008) and from the published Kaplan‐Meier curve of another (Gordon 2001). There was only one study per subgroup and we did not combine these data.
There were no significant differences in PFS between treatment arms in the GEM versus PLD, TOP versus PLD, OLA versus PLD, or PAT versus PLD subgroups (Analysis 2.1).
2.1. Analysis.
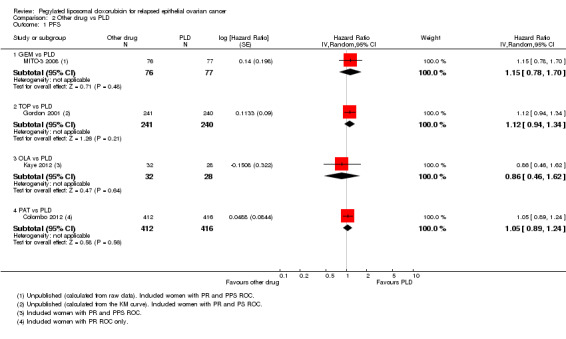
Comparison 2 Other drug vs PLD, Outcome 1 PFS.
Overall survival
Five out of seven of these studies reported OS as median OS time or median time to death (TTD) (see Table 5). Four studies comparing four different drugs (GEM, TOP, OLA and PAT) to PLD reported HRs for OS (Colombo 2012; Gordon 2001; Mutch 2007; Kaye 2012). We estimated HRs for one other study (MITO‐3 2008) using raw data provided by the investigators. We did not combine subgroup data.
2. Platinum sensitivity status and median survival times in participants of included studies.
| Platinum‐resistant data (PFI ≤6 months) | |||||||||
| STUDY NAME | Other drug arm | PLD arm | N (other drug) | N (PLD) | Median TTP for other arm in weeks | Median TTP for PLD arm in weeks | Median TTD for other arm in weeks | Median TTD for PLD arm in weeks | Comment |
| Colombo 2012 | PAT | PLD | 412 | 416 | 16 | 16 | 57 | 54 | 17% of these women had non‐measurable disease. |
| Mutch 2007 | GEM | PLD | 99 | 96 | 15 | 13 | 54 | 58 | 36% of these women with non‐measurable disease. |
| Gordon 2001 | TOP | PLD | 125 | 130 | 14 | 9 | 41 | 36 | It is unclear why survival in the PLD arm of this PR subgroup is so much shorter than that of the other trials. |
| ASSIST‐3 2007 | CAN/carbo | PLD | NA | NA | 15 | 15 | NA | NA | Limited available data. Additional data were requested from Telik but not obtained. |
| Kaye 2012 | OLA | PLD | 16 | 14 | NA | NA | NA | NA | Small study, subgroup data not available. |
| MITO‐3 2008 | GEM | PLD | 43 | 43 | NA | NA | NA | NA | Subgroup data not available. |
| PRECEDENT 2013 | EC145/PLD | PLD | 100 | 49 | 21 | 12 | 60 | 72 | Unpublished OS data. Study was not adequately powered to assess OS. |
| OVA‐301 2010 | TBD/PLD | PLD | 118 | 124 | 17 | 16 | 61 | 53 | Subgroup analysis was pre‐planned for PFS but was exploratory for OS. |
| ASSIST‐5 2010 | CAN/PLD | PLD | 65 | 60 | 24 | 16 | NA | NA | Pre‐planned subgroup analysis favoured the CAN/PLD group for PFS. Final OS results were not published. Additional data were requested from Telik but not obtained. |
| Partially platinum‐sensitive data (PFI 6‐12 months) | |||||||||
| CALYPSO 2010 | PAC/carbo | PLD/carbo | 183 | 161 | 38 | 40 | NA | NA | PFS HR = 0.73 (95% CI 0.58 to 0.90, P value 0.004) from Gladieff 2012; OS HR = 1.01 (0.80 to 1.28) from Wagner 2012. |
| OVA‐301 2010 | TBD/PLD | PLD | 123 | 90 | 32 | 24 | 96 | 71 | TTP data from Poveda 2011 and exploratory TTD data from Monk 2012. PFS HR = 0.65 (95% CI 0.45 to 0.92; P value 0.015; OS HR =0.64 (95% CI 0.47 to 0.86; P value 0.0027). |
| Platinum‐sensitive data (PFI > 6months) | |||||||||
| Gordon 2001 | TOP | PLD | 111 | 109 | 23 | 29 | 70 | 108 | Exploratory analysis. The greatest effect was seen in the PPS subgroup (N=112; HR = 1.58, 95% CI 1.07‐2.34; P value 0.021). |
| OVA‐301 2010 | TBD/PLD | PLD | 215 | 202 | 39 | 32 | 116 | 103 | Subgroup analysis was pre‐planned for PFS but was exploratory for OS. |
| SWOG S0200 2008 | carbo | PLD/carbo | 30 | 31 | 34 | 51 | 77 | 133 | Small study which closed early. |
| HeCOG 2010 | PAC/carbo | PLD/carbo | 96 | 93 | 46 | 51 | 126 | 106 | |
| CALYPSO 2010 | PAC/carbo | PLD/carbo | 509 | 466 | 40 | 48 | 141 | 132 | |
| Platinum‐resistant and platinum‐sensitive data combined | |||||||||
| MITO‐3 2008 | GEM | PLD | 76 | 77 | 20 | 16 | 51 | 56 | PR + PPS. |
| Kaye 2012 | OLA | PLD | 32 | 33 | 38 | 30 | NA | 76 | PR + PPS. Unpublished TTD data obtained from investigators. Phase II study not powered to assess survival. |
| Gordon 2001 | TOP | PLD | 235 | 239 | 17 | 16.1 | 60 | 63 | PR + PS. |
| O'Byrne 2002 | PAC | PLD | 107 | 107 | 22 | 22 | 56 | 46 | PR + PS; preliminary data. |
| OVA‐301 2010 | TBD/PLD | PLD | 337 | 335 | 31 | 25 | 95 | 81 | PR + PS. |
Conversions from published data (months to weeks) were performed assuming one month to be 4.3 weeks, and then rounding the answer to the nearest week.
*This is from the comparison CAN versus active control (PLD and TOP data combined). The PLD group had an improved PFS compared with the TOP group but we were unable to obtain separate data.
Abbreviations: NA = not available; ; HR = hazard ratio; OS = overall survival; TTP = time to progression; TTD = time to death; PFI = platinum‐free interval; PR = platinum‐resistant (recurrence within 6 months of platinum‐based therapy); PPS = partially platinum‐sensitive (recurrence of 7 to 12 months of platinum‐based therapy); PS = platinum‐sensitive (recurrence >12 months after platinum‐based therapy); PRef = platinum‐refractory (recurrence within 1 month of, or during, platinum‐based therapy); PLD = pegylated liposomal doxorubicin; GEM = gemcitabine; TOP = topotecan; TBD = trabectedin; CAN = canfosfamide; PAT = patupilone; OLA = olaparib; PAC = paclitaxel; carbo = carboplatin
All the subgroups consisted of only one study, except for the GEM versus PLD subgroup. There was no statistically significant difference in OS between the GEM and PLD arms (two studies, 348 participants; HR 1.23, 95% CI 0.81 to 1.88; I² = 73%; P value 0.33; Analysis 2.2), although the point estimate favoured the PLD arm. None of the individual studies in any of the other subgroups showed a statistically significant difference in OS between the experimental and PLD arms, except for the study of TOP versus PLD (Gordon 2001), where OS was significantly longer in the PLD arm (481 women; HR 1.23, 95% CI 1.01 to 1.50; Analysis 2.2).
2.2. Analysis.
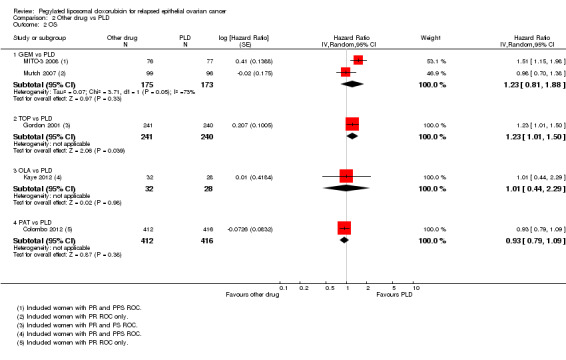
Comparison 2 Other drug vs PLD, Outcome 2 OS.
Safety and adverse events
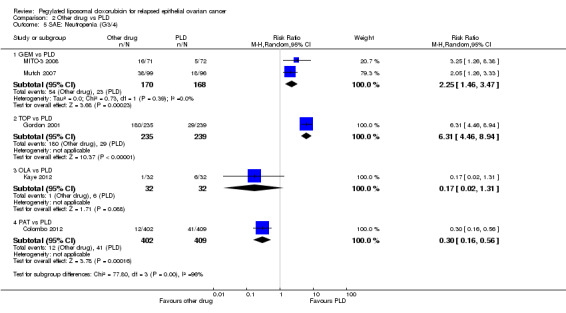
Analyses were subgrouped by intervention type and most subgroups comprised only one study. We did not pool data. The statistically significant differences between interventions with regard to G3 to 4 severe adverse events were as follows (by subgroup):
GEM versus PLD (two studies; 338 women):
hand‐foot syndrome, RR 0.07 (95% CI 0.01 to 0.54) in favour of GEM (Analysis 2.3);
neutropenia, RR 2.25 (95% CI 1.46 to 3.47) in favour of PLD (Analysis 2.5).
2.3. Analysis.
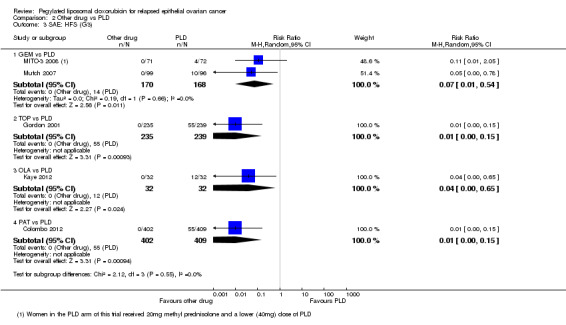
Comparison 2 Other drug vs PLD, Outcome 3 SAE: HFS (G3).
2.5. Analysis.
Comparison 2 Other drug vs PLD, Outcome 5 SAE: Neutropenia (G3/4).
TOP versus PLD (one study; 474 women):
hand‐foot syndrome, RR 0.01 (95% CI 0.00 to 0.15) in favour of TOP (Analysis 2.3);
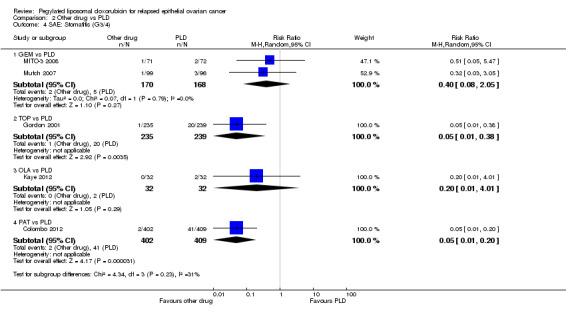
stomatitis, RR 0.05 (95% CI 0.01 to 0.38) in favour of TOP (Analysis 2.4);
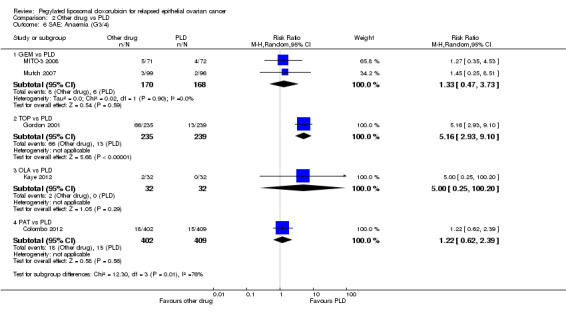
anaemia, RR 5.16 (95% CI 2.93 to 9.10) in favour of PLD (Analysis 2.6);
neutropenia, RR 6.31 (95% CI 4.46 to 8.94) in favour of PLD (Analysis 2.5);
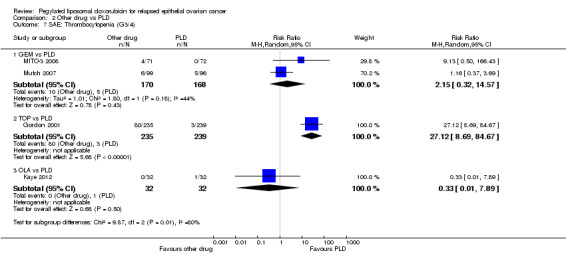
thrombocytopenia, RR 27.12 (95% CI 8.69 to 84.67) in favour of PLD (Analysis 2.7);
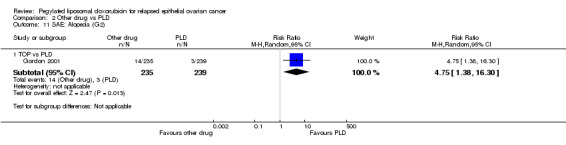
alopecia, RR 4.75 (95% CI 1.38 to 16.30) in favour of PLD (Analysis 2.11).
2.4. Analysis.
Comparison 2 Other drug vs PLD, Outcome 4 SAE: Stomatitis (G3/4).
2.6. Analysis.
Comparison 2 Other drug vs PLD, Outcome 6 SAE: Anaemia (G3/4).
2.7. Analysis.
Comparison 2 Other drug vs PLD, Outcome 7 SAE: Thrombocytopenia (G3/4).
2.11. Analysis.
Comparison 2 Other drug vs PLD, Outcome 11 SAE: Alopecia (G2).
OLA versus PLD (one study; 64 women):
hand‐foot syndrome, RR 0.04 (95% CI 0.00 to 0.65) in favour of OLA (Analysis 2.3).
PAT versus PLD (one study; 811 women):
hand‐foot syndrome, RR 0.01 (95% 0.00 to 0.15) in favour of PAT (Analysis 2.3);
stomatitis, RR 0.05 (95% 0.01 to 0.20) in favour of PAT (Analysis 2.4);
neutropenia, RR 0.30 (95% CI 0.16 to 0.56) in favour of PAT (Analysis 2.5);
peripheral neuropathy, RR 12.72 (95% CI 3.03 to 53.34) in favour of PLD (Analysis 2.10);
diarrhoea, RR 11.64 (95% CI 5.97 to 22.69) in favour of PLD (Analysis 2.12).
2.10. Analysis.
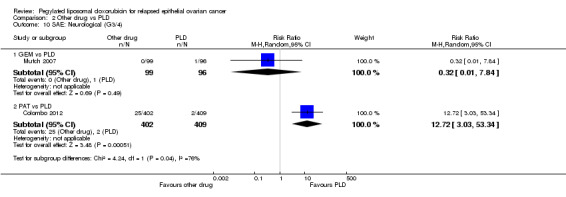
Comparison 2 Other drug vs PLD, Outcome 10 SAE: Neurological (G3/4).
2.12. Analysis.
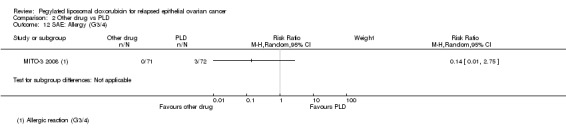
Comparison 2 Other drug vs PLD, Outcome 12 SAE: Allergy (G3/4).
Quality of Life
Five studies reported this outcome after pre‐specifying the use of FACT‐O (Colombo 2012; Kaye 2012; Mutch 2007) and QLQ‐C30 (Gordon 2001; MITO‐3 2008) questionnaires; however meta‐analysis was not possible due to, either, insufficient data reported, or a high attrition rate in women completing the questionnaires, resulting in a potentially high risk of bias.
No significant differences were reported in the change of QoL scores from baseline in Gordon 2001; Kaye 2012 and Mutch 2007. In MITO‐3 2008, where 79% of women completed QoL questionnaires at baseline and one other time, global QoL scores were statistically significantly higher in the PLD arm at the follow‐up assessments (better physical and emotional functioning, and fatigue scores) compared with women in the GEM arm. Similarly, mean well‐being scores were higher in the PLD arm compared with the PAT arm in Colombo 2012. However, it is not clear whether this difference was statistically significant; furthermore, this outcome suffered from high and unequal attrition (fewer women in the PLD arm completed the follow‐up questionnaire) in this study.
Dose delays/reductions
Four studies reported this outcome, however we did not combine the data due to the substantial heterogeneity of the experimental interventions. In Gordon 2001, women in the PLD arm were significantly less likely to experience dose delays or reductions than women in the TOP arm (Analysis 2.14; Analysis 2.15). There were no significant differences in dose delays or reductions in any of the other studies that reported this outcome, namely Colombo 2012, Kaye 2012 and MITO‐3 2008.
2.14. Analysis.
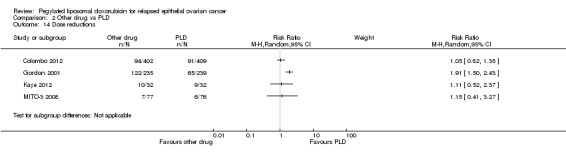
Comparison 2 Other drug vs PLD, Outcome 14 Dose reductions.
2.15. Analysis.
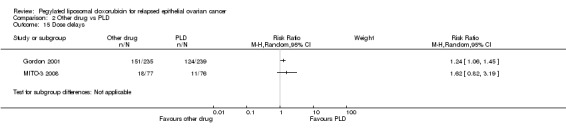
Comparison 2 Other drug vs PLD, Outcome 15 Dose delays.
C. PLD plus other drug/s versus PLD alone
Three studies compared combination treatment in women with platinum‐resistant relapsed EOC only (ASSIST‐5 2010; PRECEDENT 2013) and all women (platinum‐resistant and platinum‐sensitive) with relapsed EOC (OVA‐301 2010). Due to the heterogeneity of chemotherapy agents and participants, we did not combine data.
Survival and efficacy
Progression‐free survival
TBD/PLD versus PLD (one study, 672 participants): PFS was significantly longer in the combination arm compared with PLD alone (HR 0.79, 95% CI 0.65 to 0.96; P value 0.02; Analysis 3.1).
3.1. Analysis.
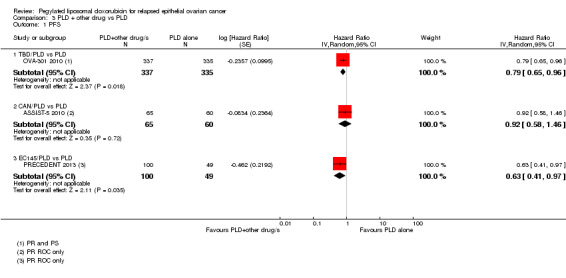
Comparison 3 PLD + other drug vs PLD, Outcome 1 PFS.
EC145/PLD versus PLD (one study, 149 participants): PFS was significantly longer in the combination arm compared with PLD alone (HR 0.63, 95% CI 0.41 to 0.97; P value 0.04; Analysis 3.1).
Overall survival
TBD/PLD versus PLD (one study, 672 participants): OS was not significantly different between the treatment arms. However, the point estimate favoured the combination treatment (HR 0.86, 95% CI 0.72 to 1.02; P value 0.09; Analysis 3.3).
3.3. Analysis.
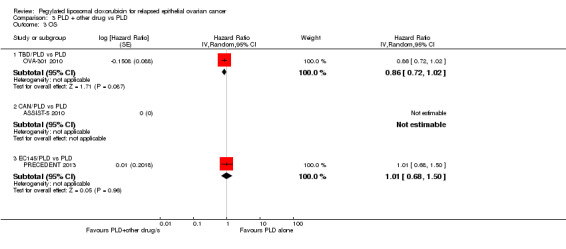
Comparison 3 PLD + other drug vs PLD, Outcome 3 OS.
EC145/PLD versus PLD (one study, 149 participants): OS was not significantly different between the treatment arms (HR 1.01, 95% CI 0.68 to 1.50; P value 0.96; Analysis 3.3), however, this study was not powered to detect a difference.
Safety/adverse events
TBD/PLD versus PLD:
Women in the combination arm were significantly more likely than those in the PLD only arm (333 versus 330 women respectively) to experience the following G3 to 4 adverse events:
anaemia: RR 2.54, 95% CI 1.45 to 4.43; P value 0.001; Analysis 3.4;
neutropenia: RR 2.80, 95% CI 2.25 to 3.48; P < 0.00001; Analysis 3.5;
thrombocytopenia: RR 7.56, 95% CI 3.67 to 15.54; P < 0.00001; Analysis 3.6; and
vomiting: RR 4.81, 95% CI 2.16 to 10.70; Analysis 3.7.
3.4. Analysis.
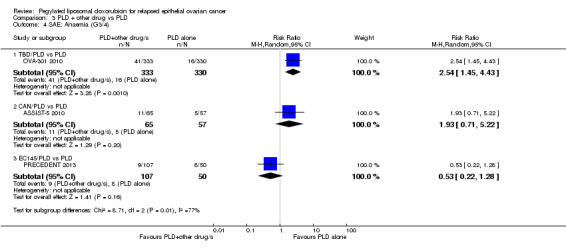
Comparison 3 PLD + other drug vs PLD, Outcome 4 SAE: Anaemia (G3/4).
3.5. Analysis.
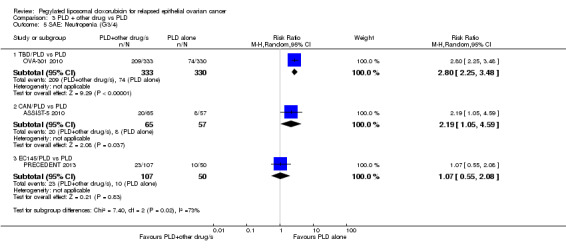
Comparison 3 PLD + other drug vs PLD, Outcome 5 SAE: Neutropenia (G3/4).
3.6. Analysis.
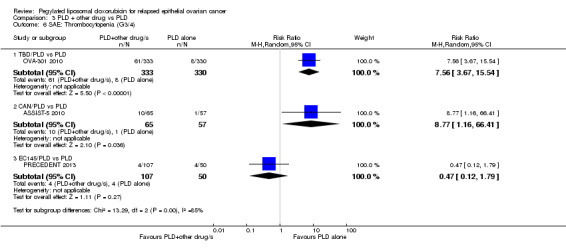
Comparison 3 PLD + other drug vs PLD, Outcome 6 SAE: Thrombocytopenia (G3/4).
3.7. Analysis.
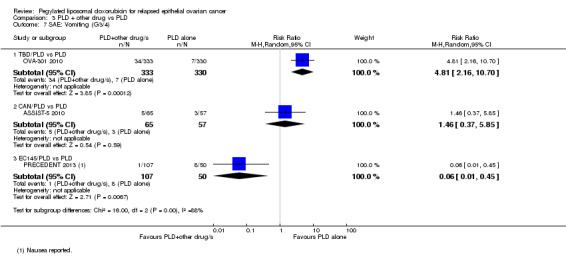
Comparison 3 PLD + other drug vs PLD, Outcome 7 SAE: Vomiting (G3/4).
Women in the combination arm were significantly less likely than those in the PLD only arm to experience the following grade 3 to 4 adverse events:
hand‐foot syndrome: RR 0.20, 95% CI 0.11 to 0.35; P < 0.00001; Analysis 3.8; and
Stomatitis:RR 0.17 95% CI 0.05 to 0.59; P value 0.005; Analysis 3.9.
3.8. Analysis.
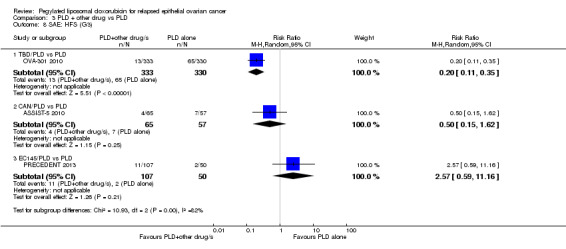
Comparison 3 PLD + other drug vs PLD, Outcome 8 SAE: HFS (G3).
3.9. Analysis.
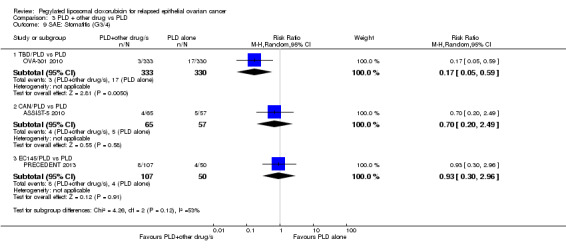
Comparison 3 PLD + other drug vs PLD, Outcome 9 SAE: Stomatitis (G3/4).
There was no statistically significant difference between the treatment groups with regard to alopecia or neuropathy, however, women in the TBD/PLD arm were significantly more likely to have raised serum bilirubin and alkaline phosphatase levels indicative of hepatotoxicity.
EC145/PLD versus PLD:
In our analyses, there were no statistically significant differences in the rates of severe adverse events (grade 3 to 4) between the EC145/PLD and PLD alone arms, except for nausea which was significantly worse in the PLD alone arm (RR 0.06, 95% CI 0.01 to 0.45; Analysis 3.7). The investigators, however, reported that abdominal pain, leukopenia and peripheral neuropathy of all grades occurred more frequently in the EC145/PLD combination group.
Quality of Life
The OVA‐301 2010 trial assessed QoL using the QLQ‐C30 to assess the change from baseline to end‐of‐treatment. There were no significant differences in any of the individual items on the scale or the global QoL score, however there was more than 20% missing data for these outcomes.
Symptom control
Dose delays/reductions
ASSIST‐5 2010 reported significantly more dose delays in the combination arm (CAN/PLD) than the PLD only arm of this study (Analysis 2.15). In OVA‐301 2010, the incidence of dose reductions was similar between arms, however, the incidence of dose delays were more frequent with the combination arm than the PLD only arm and occurred most commonly due to drug‐related adverse events (Monk 2010); precise data were not published. The most common adverse event leading to cycle delay was neutropenia for both arms.
Supportive treatment
OVA‐301 2010 did not report precise data on supportive treatment, however 42% of women in the TBD/PLD combination arm required G‐CSF compared with 17% in the PLD alone arm. Women in the combination arm were also given anti‐emetic premedication which was not routinely administered to the PLD arm.
D. Exploratory Analyses
PLD dose and hand‐foot syndrome
We analysed the rates of severe hand‐foot syndrome (grade 3) in all included studies that compared PLD (alone or in combination) with a non‐PLD treatment arm, and subgrouped the studies by PLD dose (less than 50 mg/m² and 50 mg/m² or more). Four studies in each of the two subgroups included 653 and 776 women, respectively. The incidence of hand‐foot syndrome (grade 3) was significantly lower in the less than 50 mg/m² subgroup (2% versus 17%; tests for subgroup differences were significant: P value 0.01; Analysis 4.1).
4.1. Analysis.
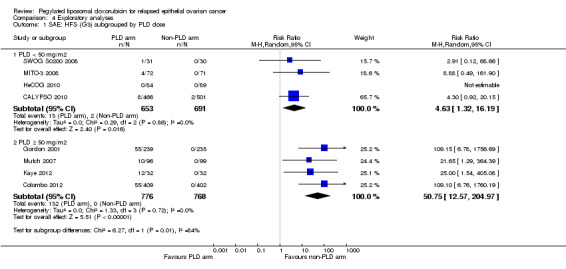
Comparison 4 Exploratory analyses, Outcome 1 SAE: HFS (G3) subgrouped by PLD dose.
PLD dose and stomatitis
Similarly, we pooled rates of stomatitis (grade 3 to 4) and performed a meta‐analysis subgrouped by PLD dose. The subgroups were statistically significantly different (P value 0.01; Analysis 4.2), with an incidence of severe stomatitis of 2% and 6% for the low dose and 50 mg/m² dose, respectively.
4.2. Analysis.
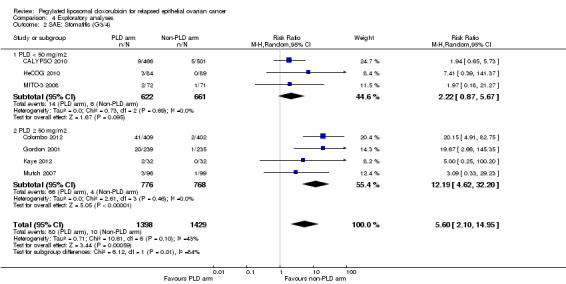
Comparison 4 Exploratory analyses, Outcome 2 SAE: Stomatitis (G3/4).
Discussion
Summary of main results
PLD/carbo versus PAC/carbo for platinum‐sensitive relapsed ovarian cancer (EOC)
Two studies were included, which used PLD doses of 30 and 45 mg/m² four‐weekly, respectively in women with platinum‐sensitive relapsed EOC. Overall survival (OS) was similar for the PLD/carbo and PAC/carbo treatments, however, PFS was longer with the PLD/carbo combination. PLD/carbo was associated with significantly more anaemia and thrombocytopenia than PAC/carbo, whereas PAC/carbo was associated with significantly more alopecia, neuropathies, hypersensitivity reactions and arthralgias/myalgias. Women receiving PLD/carbo were significantly less likely to discontinue treatment than those receiving PAC/carbo (see Table 1).
Other drugs versus PLD
Five studies contributed data: only the 'GEM versus PLD' subgroup included more than one study and all studies used a four‐weekly PLD dose of 50 mg/m². For all 'other drug' interventions (GEM,TOP, OLA and PAT), PFS was comparable with that of the PLD arms, however we did not pool these data. Similarly, OS was not significantly different for any of the comparative interventions, with the exception of the 'TOP versus PLD' comparison where the OS results of one large study statistically significantly favoured the PLD arm. TOP and GEM were associated with significantly more haematological severe adverse events than PLD, and PAT was associated with significantly more severe neuropathies and diarrhoea. The incidence of hand‐foot syndrome was statistically significantly higher in the PLD arms of all subgroup comparisons.
Other drugs plus PLD versus PLD
Three studies compared PLD combination treatment to PLD alone. Combination treatments resulted in a significantly longer PFS compared with the PLD alone for the TBD/PLD and the EC145/PLD treatments, but not for CAN/PLD. The CAN/PLD study (ASSIST‐5 2010) closed early and, since final OS has not been reported, we considered it to be at a high risk of bias. PFS subgroup analysis performed by the OVA‐301 2010 investigators found that a statistically significant benefit in PFS occurred in the partial platinum‐sensitive subgroup only. The increase in PFS did not translate into a statistically significant increase in OS in either study, although the point estimate of OVA‐301 2010 favoured the TBD/PLD arm. The phase II EC145/PLD study (PRECEDENT 2013) was not powered to evaluate OS (see Table 2).
TBD/PLD was associated with significantly more haematological and gastrointestinal severe adverse events than the PLD alone group, however less hand‐foot syndrome and stomatitis were experienced in the combination arm. The reasons for the latter are unclear, however they may be due to the lower PLD dose intensity in the TBD/PLD arm (10 mg/m² versus 12.5 mg/m² per week), or be due to an increase in the haematologically‐induced treatment delays that resulted in a lower dose intensity. severe adverse events with EC145/PLD were not significantly different to those occurring with PLD alone.
Overall completeness and applicability of evidence
PLD/carbo versus PAC/carbo for platinum‐sensitive relapsed EOC
There is sufficient high quality evidence to show that the PLD/carbo combination results in similar OS to the standard PAC/carbo regimen for platinum‐sensitive disease, and improved PFS. In CALYPSO 2010, 90% of women received post‐progression treatment and the proportion of women in the PAC/carbo arm who received PLD as post‐study therapy (68%) was significantly higher than the proportion of women in the PLD/carbo arm who received PAC (43%; P < 0.001); this may have influenced OS HRs in the direction of the PAC/carbo arm. PLD/carbo was well‐tolerated compared with PAC/carbo. The majority of women receiving PAC/carbo regimen experienced complete hair loss, compared with less than 10% in the PLD/carbo arm. At the 30 mg/m² dose, the incidence of grade 3 hand‐foot syndrome was not significantly difference between the two regimens. There was no statistically significant difference in neutropenia in the meta‐analysis of two studies, although evidence from CALYPSO 2010 suggests that this difference is significant if a 30 mg/m² dose is used, and favours the PLD/carbo combination.
Other drugs versus PLD
Most of these studies included women with platinum‐resistant relapsed EOC in whom other agents (GEM, OLA and PAT) were compared with PLD as the active control agent, and which produced similar survival results to PLD. Exceptions were the TOP versus PLD study (Gordon 2001) that included women with platinum‐resistant and platinum‐sensitive relapsed EOC and found an improved OS with PLD compared with TOP; and MITO‐3 2008 (GEM versus PLD) that included women with platinum‐resistant and partial platinum‐sensitive relapsed EOC and in which PLD was similarly associated with a significantly longer OS compared with GEM. The OLA versus PLD study (Kaye 2012) was a phase II study that was not powered to assess OS and research is ongoing for this agent. These studies confirm PLD as a good choice for women in whom single‐agent therapy is a treatment option. PLD has been compared with PAC in only one study that was never published in full (O'Byrne 2002). Since PAC is still the most frequently used single agent for platinum‐resistant relapsed EOC, an RCT comparing these drugs would be extremely helpful. Indeed the timing of PLD relative to other treatments cannot be assessed from the current evidence.
PLD plus other drugs versus PLD alone
More evidence is needed to determine whether the survival benefits of combination treatment in relapsed EOC are worth the often considerable adverse drug effects. In OVA‐301 2010 (TBD/PLD versus PLD alone), OS was not significantly improved in the combination arm despite a statistically significant difference in PFS. The improvement in PFS equated to six weeks longer to progression in the combination group which, given the additional haematological and other toxicities, may not be worth the potential severe adverse‐event‐associated decrease in quality of life. The RR of vomiting in favour of the PLD only arm is likely to be underestimated in our analysis as women in the TBD/PLD arm also received anti‐emetic premedication. OVA‐301 2010 investigators performed subgroup analyses which suggested that the combination treatment may confer a significant PFS and OS benefit (exploratory analysis) to the partial platinum‐sensitive subgroup only (see Table 5). This evidence has limited applicability as the standard treatment of women with platinum‐sensitive relapsed EOC is PAC/carbo (or PLD/carbo). A further study of TBD/PLD has been commenced (INOVATYON) comparing TBD/PLD versus standard treatment (PLD/carbo) for this subgroup of women. Although this study had to be suspended due to the PLD shortage, we understand that enrolment is expected to resume (personal communication with investigators). More data should be published on the additional supportive treatment required during and following treatment with TBD/PLD.
To date, the only combination treatment to have shown a significant benefit in PFS over PLD alone in platinum‐resistant relapsed EOC is EC145/PLD (21 weeks versus 12 weeks). In this phase II study (PRECEDENT 2013), this combination appeared to be well‐tolerated and did not result in a significant increase in severe adverse events, however the investigators reported a statistically significant increase in some adverse events (leucopenia (reduced numbers of white blood cells), neutropenia, abdominal pain and peripheral neuropathy) when all grades were considered. Due to the small size of the study, OS results are unreliable. Thus the evidence is very incomplete and should be clarified following the completion of a large, ongoing, double‐blind, placebo‐controlled trial (PROCEED).
For completeness, we included the ASSIST‐5 2010 study, however, we considered it to be at a high risk of selective reporting and other bias. Based on a review of ASSIST‐1 2009 data, this trial was closed early and, to our knowledge, neither the ASSIST‐1 2009 review findings nor the overall survival results for either trial have been published. We were unsuccessful in obtaining these missing data from the pharmaceutical company (Telik). We consider it unlikely that further studies of this agent will be conducted in relapsed EOC, despite claims that it is well‐tolerated and active in platinum‐refractory and platinum‐resistant relapsed EOC (Kavanagh 2010).
Quality of the evidence
PLD has been tested in more than 14 RCTs against standard chemotherapy regimens and novel agents for relapsed EOC. PLD is an effective alternative to PAC, when combined with carboplatin, for combination therapy of platinum‐sensitive disease, producing better PFS and similar OS times to PAC/carbo. We consider this evidence to be of a high and moderate quality, respectively (Table 1). In combination treatment, PLD is well‐tolerated in lower dose regimens, which do not adversely affect survival compared with other standard regimens. In addition, there is high quality evidence to show that PLD/carbo causes much less neuropathy and alopecia than PAC/carbo.
As a single‐agent in platinum‐resistant relapsed EOC, median time to progression across all the studies included in this review was 15 weeks (range nine to 16) and median time to death was 54 weeks (range 36 to 72) (Table 5). PLD is a good option for single‐agent salvage treatment of platinum‐resistant relapsed EOC when compared to other non‐PLD regimens, however, most of these treatment comparisons comprised only one study and meta‐analyses could not be performed. Currently the quality of the evidence in favour of other PLD‐combination therapy for platinum‐resistant (EC145/PLD) and partial platinum‐sensitive relapsed EOC (TBD/PLD) is moderate to very low (Table 2), therefore, we suggest that these PLD‐combinations only be administered within clinical trials, pending further evidence.
Potential biases in the review process
We attempted to prevent bias in the review by including grey literature and making every effort to obtain missing data from the investigators; however, we were unable to obtain OS data for Mutch 2007, or any statistically useful data for ASSIST‐3 2007, M200 2009 and O'Byrne 2002. We considered the latter three studies to be at a high risk of bias. They contributed no data but we included them to avoid repeating the publication bias to which they may have been subject.
For most included studies, it was not possible to subgroup data according to the platinum‐free interval as stratification had not been performed at the randomisation stage, subgroup results were exploratory, or because there were too few studies to perform meta‐analyses. Exploratory results from MITO‐3 2008 (GEM versus PLD) suggest that OS was significantly longer in the PLD arm of the partial platinum‐sensitive subgroup only. Similarly, in OVA‐301 2010, the TBD/PLD arm was associated with a longer PFS and OS compared with the PLD arm in the partial platinum‐sensitive subgroup only. Although we did not analyse these data separately, we do not consider this to be a major source of bias; further research is needed here. There are several ongoing studies evaluating PLD treatments for specific subgroups of relapsed EOC (e.g.INOVATYON and PROCEED), therefore subgroup analyses should be possible in future versions of this review, when there are more data available.
Some studies only reported severe adverse events if they occurred at a rate of more than 5% or 10%. Where this occurred, we attempted to obtain these unpublished data, however it was not always possible. Therefore, where we estimated rates of severe adverse events or an 'assumed risk' based on the cumulative reported risk rates (e.g. in 'Summary of findings' tables), these rates may be slightly over‐estimated. Furthermore, uncommon severe adverse events may therefore not be represented in this review.
Agreements and disagreements with other studies or reviews
Hand‐foot syndrome can be a severely debilitating adverse effect of PLD use and there is little consensus on its management, although dose reduction is considered to have the greatest effect (von Moos 2008). Rose 2005 suggested the optimum dose of PLD to be 40 mg/m² and this has since been recommended by a European panel of experts (von Moos 2008) and others, some of whom have raised serious ethical concerns about the use of the 50 mg/m² dose (Markman 2010), which remains the approved dose of the US Food and Drug Administration (Markman 2010) and European Medicines Agency (EMA 2010). It is apparently for this reason that most ongoing studies utilise the 50 mg/m2 dose when comparing new agents to PLD (see Characteristics of ongoing studies).
In this review, grade 3 hand‐foot syndrome events occurred with an overall frequency of 2% in women receiving a PLD dose of less than 50 mg/m² and 17% in those receiving a 50 mg/m² dose which, although only an exploratory analysis, seems to strongly support the argument for a lower dose (see Table 3). These subgroup data were homogenous (I² = 0%). It was not possible to perform similar exploratory analyses on survival outcomes due to the heterogeneity of the participants (platinum‐resistant and platinum‐sensitive relapsed EOC) and chemotherapy agents evaluated, however PLD administered at the 40 mg/m² dose has not been associated with reduced efficacy (Markman 2010). The review evidence relating to PLD dose is confounded by the routine use of corticosteroid premedication in all the studies employing lower dose regimens. Corticosteroids might prevent or ameliorate hand‐foot syndrome, however their role, if any, is uncertain and evidence from RCTs is lacking (Farr 2012; von Moos 2008).
In the OVA‐301 2010 study, which we did not include in the exploratory hand‐foot syndrome analysis because PLD was given to both arms, women in the combination arm (TBD/PLD) experienced significantly less hand‐foot syndrome than women in the PLD only arm. As with our exploratory analysis, this reduction may have been due to the lower dose of PLD used in the combination arm (30 mg/m²) or to the corticosteroid premedication that was given to theTBD arm only, or indeed to the experimental agent (TBD) itself. Similarly, ASSIST‐5 2010 reported significantly lower rates of hand‐foot syndrome in the combination arm (CAN/PLD) compared with PLD alone (50 mg/m² dose in both arms), however all women in this study (including the PLD only group) received corticosteroid premedication. Therefore, the reason for the decrease in hand‐foot syndrome observed in these combination studies remains unclear. It may even be due to the increased rates of bone marrow suppression that occur more frequently with combination treatment, rather than the experimental agents per se. Furthermore, these were open‐label studies and detection bias may have played a role. Since improving quality of life is the main aim of treatment in women with platinum‐resistant relapsed EOC, in the absence of a randomised trial comparing 40 mg/m² and 50 mg/m² dosage regimens, we agree with von Moos 2008 and Markman 2010 in the support of a 40 mg/m² dose for single‐agent studies, and recommend that ongoing studies reconsider their PLD dose regimens.
Authors' conclusions
Implications for practice.
For platinum‐sensitive disease, PLD/carbo has greater efficacy than PAC/carbo and treatment guidelines should be updated to include this combination as first‐line treatment. Although there was no statistically significant difference in OS, PLD/carbo was associated with a longer PFS and was better tolerated than the standard PAC/carbo regimen. The findings of this review support the continued use of PLD as a single‐agent for platinum‐resistant relapsed EOC, although it is still unclear how PLD compares with PAC and other agents for platinum‐resistant relapsed EOC and in what order these agents should be used. In combination with TBD, PFS was increased in women with partial platinum‐sensitive relapsed EOC compared with PLD alone, however it is not clear how TBD/PLD compares to standard treatment for this subgroup. TBD/PLD was associated with significantly more toxicity compared to PLD alone and further evidence is needed before this treatment can be recommended. Ongoing trials should clarify whether PLD‐based combination treatments, e.g. EC145/PLD, are of significant benefit to women with platinum‐resistant relapsed EOC. We support the use of a PLD dose of not more than 40 mg/m² every four weeks to reduce the incidence of severe hand‐foot syndrome in clinical practice and ongoing studies.
Implications for research.
The canfosfamide studies (ASSIST‐1 2009; ASSIST‐3 2007; ASSIST‐5 2010) illustrate the importance of conducting large, double‐blind RCTs; the results of phase II studies may suggest survival benefits but can be misleading because of the small numbers of participants and the risk of bias associated with open‐label studies. A greater effort should be made by investigators to use double‐blind trial methodology in multinational phase III trials. Although the shortage of PLD has had a significant impact on ovarian cancer research in the past two years, the following phase III trials are expected to resume participant enrolment (see Characteristics of ongoing studies).
For women with partially platinum‐sensitive relapsed EOC:
INOVATYON: PLD (30 mg/m²)/TBD versus PLD (30 mg/m²)/carbo
MITO‐8: PLD (40 mg/m²) followed by PAC/carbo versus PAC/carbo followed by PLD(40 mg/m²); (this trial also evaluates GEM and TOP).
For women with platinum‐resistant relapsed EOC:
PROCEED: PLD (50 mg/m²)/EC145 versus PLD (50 mg/m²)/placebo
AURELIA: liposomal doxorubicin (LD) plus PAC plus TOP versus bevacizumab plus LD plus PAC plus TOP.
For women with partially platinum‐sensitive or platinum‐resistant relapsed EOC:
TRINOVA‐2: AMG 386/PLD (50 mg/m²) versus PLD (50 mg/m²)/placebo.
For women with advanced OC in remission:
AGOG06‐001: maintenance PLD (30 mg/m²)/carbo versus no maintenance.
For women with platinum‐resistant relapsed EOC, an RCT of PLD versus PAC is desirable. Such a trial could also include a randomised and blinded comparison of PLD doses (40 mg/m² versus 50 mg/m²).
There is currently little evidence to support the use of liposomal doxorubicin (Myocet) as a substitute for PLD in ovarian cancer treatment. A phase II study comparing Myocet plus gemcitabine with Myocet alone in platinum‐resistant relapsed EOC is apparently ongoing (NCT01100372), and two other ongoing studies (AURELIA; IMC‐383/NCT00913835) are evaluating Myocet against other experimental interventions (see Characteristics of ongoing studies).
Regional cooling (Mangili 2008) and corticosteroid premedication may prevent hand‐foot syndrome (Lorusso 2007); given the numerous ongoing studies utilising PLD, there appears to be ample opportunity to conduct a concurrent randomised study to determine whether these preventative measures are helpful.
What's new
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 7, 2013
| Date | Event | Description |
|---|---|---|
| 1 April 2015 | Amended | Contact details updated. |
| 11 February 2015 | Amended | Contact details updated. |
| 27 March 2014 | Amended | Contact details updated. |
| 15 October 2012 | Amended | New search performed. |
| 24 June 2008 | Amended | Converted to new review format. |
Acknowledgements
We are grateful to the following people for their generous assistance.
The Managing Editors, Clare Jess and Gail Quinn, and the rest of the CGCG team for administrative and editorial support.
Jane Hayes for co‐ordinating the trials search.
The librarians at the Royal United Hospital Library, Bath, UK, for obtaining most of these references for us.
Nicoletta Colombo for supplying Qol data for Colombo 2012.
Gabriella Ferrandina for supplying raw PFS data from MITO‐3 2008.
Wendel Naumann for allowing us access to the unpublished manuscript of PRECEDENT 2013 and for providing additional unpublished data.
Eric Pujade‐Lauraine and Nicholas Gane for additional data on side‐effects from CALYPSO 2010.
Julia Dawson, who assisted with the sifting of the database searches.
Appendices
Appendix 1. MEDLINE search strategy
Medline Ovid
exp Ovarian Neoplasms/
(ovar* adj5 (cancer* or neoplas* or tumor* or tumour* or carcinoma* or malignan*)).mp.
1 or 2
exp Doxorubicin/
doxorubicin.mp.
caelyx.mp.
doxil.mp.
myocet.mp.
4 or 5 or 6 or 7 or 8
3 and 9
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
clinical trials as topic.sh.
randomly.ab.
trial.ti.
11 or 12 or 13 or 14 or 15 or 16 or 17
10 and 18
exp animals/ not humans.sh.
19 not 20
key: mP value protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier, pt=publication type, ab=abstract, ti=title, sh=subject heading
Appendix 2. EMBASE search strategy
EMBASE Ovid
exp ovary tumor/
(ovar* adj5 (cancer* or neoplas* or tumor* or tumour* or carcinoma* or malignan*)).mp.
1 or 2
exp doxorubicin/
doxorubicin.mp.
caelyx.mp.
doxil.mp.
myocet.mp.
4 or 5 or 6 or 7 or 8
3 and 9
crossover procedure/
randomized controlled trial/
single blind procedure/
random*.mp.
factorial*.mp.
(crossover* or cross over* or cross‐over).mp.
placebo*.mp.
(doubl* adj blind*).mp.
(singl* adj blind*).mp.
assign*.mp.
allocat*.mp.
volunteer*.mp.
11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
10 and 23
key: mP value title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
Appendix 3. CENTRAL search strategy
CENTRAL
MeSH descriptor Ovarian Neoplasms explode all trees
ovar* near/5 (cancer* or neoplas* or tumor* or tumour* or carcinoma* or malignan*)
(#1 OR #2)
MeSH descriptor Doxorubicin explode all trees
doxorubicin
caelyx
doxil
(#4 OR #5 OR #6 OR #7)
(#3 AND #8)
Data and analyses
Comparison 1. PLD/carbo vs carbo ± other.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PFS | 3 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 PLD/carbo vs carbo only | 1 | 61 | Hazard Ratio (Random, 95% CI) | 0.52 [0.31, 0.88] |
| 1.2 PLD/carbo vs PAC/carbo | 2 | 1164 | Hazard Ratio (Random, 95% CI) | 0.85 [0.74, 0.97] |
| 2 OS | 3 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 PLD/carbo vs carbo only | 1 | 61 | Hazard Ratio (Random, 95% CI) | 0.69 [0.40, 1.21] |
| 2.2 PLD/carbo vs PAC/carbo | 2 | 1164 | Hazard Ratio (Random, 95% CI) | 1.01 [0.88, 1.17] |
| 3 SAE: Hand‐foot syndrome (G3) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 PLD/carbo vs carbo only | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 2.91 [0.12, 68.66] |
| 3.2 PLD/carbo vs PAC/carbo | 2 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 4.30 [0.92, 20.15] |
| 4 SAE: Anaemia (G3/4) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 PLD/carbo vs carbo only | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 10.66 [0.61, 184.70] |
| 4.2 PLD/carbo vs PAC/carbo | 2 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.02, 2.50] |
| 5 SAE: Febrile neutropenia (G3/4) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 PLD/carbo vs carbo only | 1 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.03, 12.37] |
| 5.2 PLD/carbo vs PAC/carbo | 1 | 967 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.31, 1.23] |
| 6 SAE: Neutropenia (G3/4) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 PLD/carbo vs carbo only | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 14.52 [2.04, 103.16] |
| 6.2 PLD/carbo vs PAC/carbo | 2 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.60, 1.36] |
| 7 SAE: Thrombocytopenia (G3/4) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 PLD/carbo vs carbo only | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 3.87 [1.21, 12.36] |
| 7.2 PLD/carbo vs PAC/carbo | 2 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 2.69 [1.83, 3.96] |
| 8 SAE: Stomatitis/mucositis (G 3/4) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 PLD/carbo vs PAC/carbo | 2 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 2.27 [0.82, 6.29] |
| 9 SAE: Vomiting (G 3/4) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 PLD/carbo vs carbo only | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 2.91 [0.12, 68.66] |
| 9.2 PLD/carbo vs PAC/carbo | 2 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.44, 4.42] |
| 10 SAE: Alopecia (G2) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 PLD/carbo vs PAC/carbo | 2 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.06, 0.15] |
| 11 SAE: Neurological (G3/4) | 2 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.08, 0.50] |
| 11.1 PLD/carbo vs PAC/carbo | 2 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.08, 0.50] |
| 12 SAE: Fatigue (G3/4) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 PLD/carbo vs carbo only | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.26, 8.09] |
| 12.2 PLD/carbo vs PAC/carbo | 2 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.66, 1.57] |
| 13 SAE: Arthralgia/myalgia (G3/4) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 PLD/carbo vs carbo only | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.63] |
| 13.2 PLD/carbo vs PAC/carbo | 2 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 0.12 [0.02, 0.67] |
| 14 SAE: Hypersensitivity reactions (HSR; G3/4) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 PLD/carbo vs carbo only | 1 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.53] |
| 14.2 PLD/carbo vs PAC/carbo | 2 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.15, 0.54] |
| 15 SAE: Treatment‐related death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 15.1 PLD/carbo vs PAC/carbo | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 16 QoL: Global health score (mean change) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17 Discontinuation due to toxicity | 2 | 1150 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.26, 0.57] |
| 17.1 PLD/carbo vs PAC/carbo | 2 | 1150 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.26, 0.57] |
| 18 Antibiotics required | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 PLD/carbo vs PAC/carbo | 2 | 1144 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.57, 2.21] |
| 19 G‐CSF required | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 19.1 PLD/carbo vs PAC/carbo | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20 Blood transfusion required | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 20.1 PLD/carbo vs carbo only | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 20.2 PLD/carbo vs PAC/carbo | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.5. Analysis.
Comparison 1 PLD/carbo vs carbo ± other, Outcome 5 SAE: Febrile neutropenia (G3/4).
1.6. Analysis.
Comparison 1 PLD/carbo vs carbo ± other, Outcome 6 SAE: Neutropenia (G3/4).
1.9. Analysis.
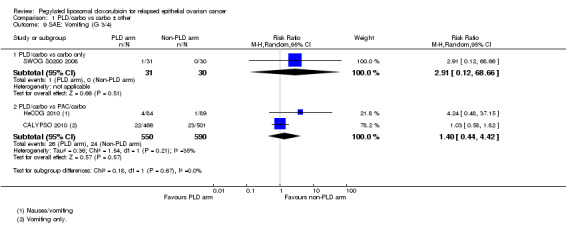
Comparison 1 PLD/carbo vs carbo ± other, Outcome 9 SAE: Vomiting (G 3/4).
1.12. Analysis.
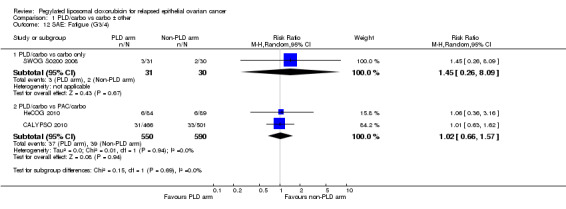
Comparison 1 PLD/carbo vs carbo ± other, Outcome 12 SAE: Fatigue (G3/4).
1.15. Analysis.
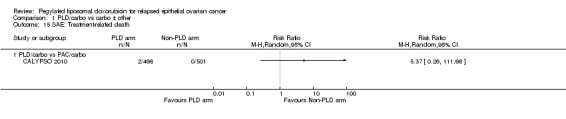
Comparison 1 PLD/carbo vs carbo ± other, Outcome 15 SAE: Treatment‐related death.
1.16. Analysis.
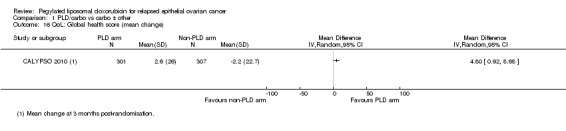
Comparison 1 PLD/carbo vs carbo ± other, Outcome 16 QoL: Global health score (mean change).
Comparison 2. Other drug vs PLD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PFS | 4 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 GEM vs PLD | 1 | 153 | Hazard Ratio (Random, 95% CI) | 1.15 [0.78, 1.70] |
| 1.2 TOP vs PLD | 1 | 481 | Hazard Ratio (Random, 95% CI) | 1.12 [0.94, 1.34] |
| 1.3 OLA vs PLD | 1 | 60 | Hazard Ratio (Random, 95% CI) | 0.86 [0.46, 1.62] |
| 1.4 PAT vs PLD | 1 | 828 | Hazard Ratio (Random, 95% CI) | 1.05 [0.89, 1.24] |
| 2 OS | 5 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 GEM vs PLD | 2 | 348 | Hazard Ratio (Random, 95% CI) | 1.23 [0.81, 1.88] |
| 2.2 TOP vs PLD | 1 | 481 | Hazard Ratio (Random, 95% CI) | 1.23 [1.01, 1.50] |
| 2.3 OLA vs PLD | 1 | 60 | Hazard Ratio (Random, 95% CI) | 1.01 [0.44, 2.29] |
| 2.4 PAT vs PLD | 1 | 828 | Hazard Ratio (Random, 95% CI) | 0.93 [0.79, 1.09] |
| 3 SAE: HFS (G3) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 GEM vs PLD | 2 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.07 [0.01, 0.54] |
| 3.2 TOP vs PLD | 1 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.01 [0.00, 0.15] |
| 3.3 OLA vs PLD | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.00, 0.65] |
| 3.4 PAT vs PLD | 1 | 811 | Risk Ratio (M‐H, Random, 95% CI) | 0.01 [0.00, 0.15] |
| 4 SAE: Stomatitis (G3/4) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 GEM vs PLD | 2 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.08, 2.05] |
| 4.2 TOP vs PLD | 1 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.38] |
| 4.3 OLA vs PLD | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 4.01] |
| 4.4 PAT vs PLD | 1 | 811 | Risk Ratio (M‐H, Random, 95% CI) | 0.05 [0.01, 0.20] |
| 5 SAE: Neutropenia (G3/4) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 GEM vs PLD | 2 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [1.46, 3.47] |
| 5.2 TOP vs PLD | 1 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 6.31 [4.46, 8.94] |
| 5.3 OLA vs PLD | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.31] |
| 5.4 PAT vs PLD | 1 | 811 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.16, 0.56] |
| 6 SAE: Anaemia (G3/4) | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 GEM vs PLD | 2 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.47, 3.73] |
| 6.2 TOP vs PLD | 1 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 5.16 [2.93, 9.10] |
| 6.3 OLA vs PLD | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 100.20] |
| 6.4 PAT vs PLD | 1 | 811 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.62, 2.39] |
| 7 SAE: Thrombocytopenia (G3/4) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 GEM vs PLD | 2 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [0.32, 14.57] |
| 7.2 TOP vs PLD | 1 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 27.12 [8.69, 84.67] |
| 7.3 OLA vs PLD | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.89] |
| 8 SAE: Vomiting (G3/4) | 4 | 1213 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.72, 2.65] |
| 8.1 GEM vs PLD | 2 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.10, 11.12] |
| 8.2 OLA vs PLD | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.22, 4.59] |
| 8.3 PAT vs PLD | 1 | 811 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.84, 2.32] |
| 9 SAE: Fatigue/asthenia (G3/4) | 4 | 1213 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.73, 3.03] |
| 9.1 GEM vs PLD | 2 | 338 | Risk Ratio (M‐H, Random, 95% CI) | 3.44 [0.47, 24.92] |
| 9.2 OLA vs PLD | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.18, 3.09] |
| 9.3 PAT vs PLD | 1 | 811 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.82, 1.93] |
| 10 SAE: Neurological (G3/4) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 GEM vs PLD | 1 | 195 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.01, 7.84] |
| 10.2 PAT vs PLD | 1 | 811 | Risk Ratio (M‐H, Random, 95% CI) | 12.72 [3.03, 53.34] |
| 11 SAE: Alopecia (G2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 TOP vs PLD | 1 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 4.75 [1.38, 16.30] |
| 12 SAE: Allergy (G3/4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13 SAE: Diarrhoea (G3/4) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 OLA vs PLD | 1 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 4.01] |
| 13.2 PAT vs PLD | 1 | 811 | Risk Ratio (M‐H, Random, 95% CI) | 11.64 [5.97, 22.69] |
| 14 Dose reductions | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15 Dose delays | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
2.8. Analysis.
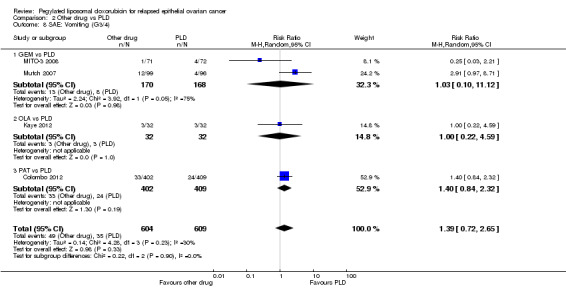
Comparison 2 Other drug vs PLD, Outcome 8 SAE: Vomiting (G3/4).
2.9. Analysis.
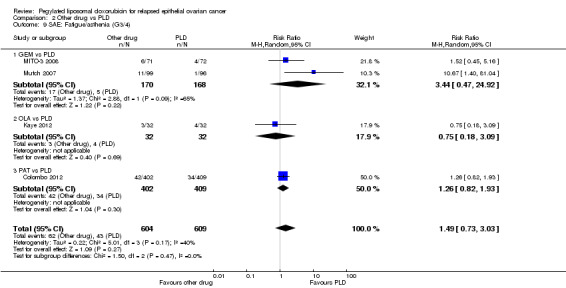
Comparison 2 Other drug vs PLD, Outcome 9 SAE: Fatigue/asthenia (G3/4).
2.13. Analysis.
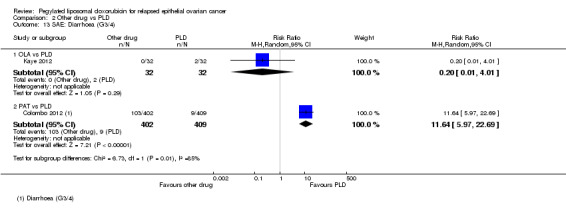
Comparison 2 Other drug vs PLD, Outcome 13 SAE: Diarrhoea (G3/4).
Comparison 3. PLD + other drug vs PLD.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PFS | 3 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 TBD/PLD vs PLD | 1 | 672 | Hazard Ratio (Random, 95% CI) | 0.79 [0.65, 0.96] |
| 1.2 CAN/PLD vs PLD | 1 | 125 | Hazard Ratio (Random, 95% CI) | 0.92 [0.58, 1.46] |
| 1.3 EC145/PLD vs PLD | 1 | 149 | Hazard Ratio (Random, 95% CI) | 0.63 [0.41, 0.97] |
| 2 PFS: PPS subgroup only | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 PLD/TBD vs PLD | 1 | 208 | Hazard Ratio (Random, 95% CI) | 0.65 [0.45, 0.93] |
| 3 OS | 3 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 TBD/PLD vs PLD | 1 | Hazard Ratio (Random, 95% CI) | 0.86 [0.72, 1.02] | |
| 3.2 CAN/PLD vs PLD | 1 | Hazard Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 EC145/PLD vs PLD | 1 | Hazard Ratio (Random, 95% CI) | 1.01 [0.68, 1.50] | |
| 4 SAE: Anaemia (G3/4) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 TBD/PLD vs PLD | 1 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 2.54 [1.45, 4.43] |
| 4.2 CAN/PLD vs PLD | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.71, 5.22] |
| 4.3 EC145/PLD vs PLD | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.22, 1.28] |
| 5 SAE: Neutropenia (G3/4) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 TBD/PLD vs PLD | 1 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 2.80 [2.25, 3.48] |
| 5.2 CAN/PLD vs PLD | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [1.05, 4.59] |
| 5.3 EC145/PLD vs PLD | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.55, 2.08] |
| 6 SAE: Thrombocytopenia (G3/4) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 TBD/PLD vs PLD | 1 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 7.56 [3.67, 15.54] |
| 6.2 CAN/PLD vs PLD | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 8.77 [1.16, 66.41] |
| 6.3 EC145/PLD vs PLD | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.12, 1.79] |
| 7 SAE: Vomiting (G3/4) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 TBD/PLD vs PLD | 1 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 4.81 [2.16, 10.70] |
| 7.2 CAN/PLD vs PLD | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.37, 5.85] |
| 7.3 EC145/PLD vs PLD | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.06 [0.01, 0.45] |
| 8 SAE: HFS (G3) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 TBD/PLD vs PLD | 1 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.11, 0.35] |
| 8.2 CAN/PLD vs PLD | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.50 [0.15, 1.62] |
| 8.3 EC145/PLD vs PLD | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 2.57 [0.59, 11.16] |
| 9 SAE: Stomatitis (G3/4) | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 TBD/PLD vs PLD | 1 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.05, 0.59] |
| 9.2 CAN/PLD vs PLD | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.20, 2.49] |
| 9.3 EC145/PLD vs PLD | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.30, 2.96] |
| 10 SAE: Alopecia (G2) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 TBD/PLD vs PLD | 1 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.60, 1.34] |
| 10.2 CAN/PLD vs PLD | 1 | 122 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 SAE: Abdominal pain (G3/4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 EC145/PLD vs PLD | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [0.41, 8.48] |
| 12 SAE: Neuropathy (G3/4) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 TBD/PLD vs PLD | 1 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.85, 2.31] |
| 12.2 EC145/PLD vs PLD | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 4.25 [0.23, 77.45] |
| 13 SAE‐related death | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 TBD/PLD vs PLD | 1 | 663 | Risk Ratio (M‐H, Random, 95% CI) | 2.48 [0.48, 12.68] |
| 13.2 EC145/PLD vs PLD | 1 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Dose reductions | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 CAN/PLD vs PLD | 1 | 535 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.53, 2.14] |
| 15 Dose delays | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 CAN/PLD vs PLD | 1 | 535 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.00, 2.26] |
3.2. Analysis.
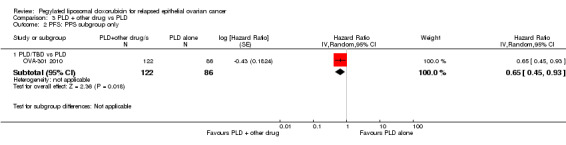
Comparison 3 PLD + other drug vs PLD, Outcome 2 PFS: PPS subgroup only.
3.10. Analysis.
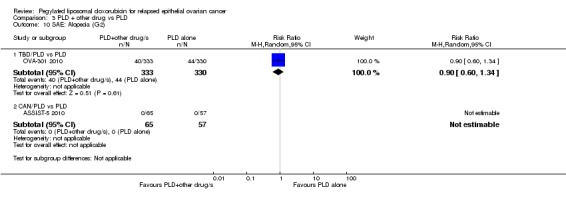
Comparison 3 PLD + other drug vs PLD, Outcome 10 SAE: Alopecia (G2).
3.11. Analysis.
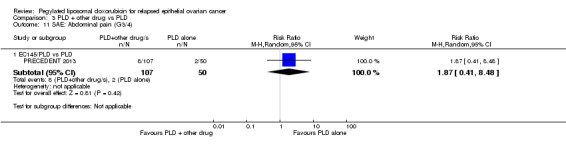
Comparison 3 PLD + other drug vs PLD, Outcome 11 SAE: Abdominal pain (G3/4).
3.12. Analysis.
Comparison 3 PLD + other drug vs PLD, Outcome 12 SAE: Neuropathy (G3/4).
3.13. Analysis.
Comparison 3 PLD + other drug vs PLD, Outcome 13 SAE‐related death.
3.14. Analysis.
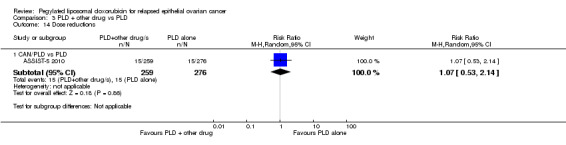
Comparison 3 PLD + other drug vs PLD, Outcome 14 Dose reductions.
3.15. Analysis.
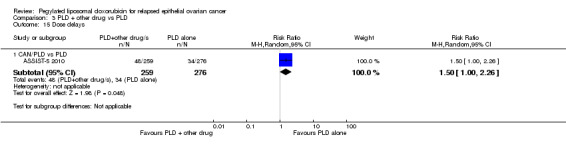
Comparison 3 PLD + other drug vs PLD, Outcome 15 Dose delays.
Comparison 4. Exploratory analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 SAE: HFS (G3) subgrouped by PLD dose | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 PLD < 50 mg/m2 | 4 | 1344 | Risk Ratio (M‐H, Random, 95% CI) | 4.63 [1.32, 16.19] |
| 1.2 PLD ≥ 50 mg/m2 | 4 | 1544 | Risk Ratio (M‐H, Random, 95% CI) | 50.75 [12.57, 204.97] |
| 2 SAE: Stomatitis (G3/4) | 7 | 2827 | Risk Ratio (M‐H, Random, 95% CI) | 5.60 [2.10, 14.95] |
| 2.1 PLD < 50 mg/m2 | 3 | 1283 | Risk Ratio (M‐H, Random, 95% CI) | 2.22 [0.87, 5.67] |
| 2.2 PLD ≥ 50 mg/m2 | 4 | 1544 | Risk Ratio (M‐H, Random, 95% CI) | 12.19 [4.62, 32.20] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ASSIST‐3 2007.
| Methods | Phase III multicentre RCT (ID not found on trial registries); abstract only; no further methodological details. | |
| Participants | 247 women with PR ROC (resistant and refractory) with measurable disease (RECIST), who had progressed on 2 platinum regimens. | |
| Interventions | Arm 1: CAN (750 mg/m²) and carboplatin (AUC 5) (carbo) Arm 2: PLD (50 mg/m²) IV q4wks until progression |
|
| Outcomes | ORR, PFS, safety and QoL | |
| Notes | Published results included the following statements with little supporting data:
Overall median survival had not been reached at the time of the 2007 ASCO proceedings where these results were reported. Subgroup analyses of women with time from last carbo dose (TFP) = 6 months 'reported large differences in ORR and QoL and statistical significance in PFS and survival' in favour of the experimental group (CAN/carbo), but this subgroup consisted of 19 women in each group and 58% (11/19) of the CAN/carbo arm were censored (compared with 3/19 in the PLD arm). We emailed Dr Rose and Telik, Inc in November 2012 for further information and final survival and safety data but received no response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | ORR results differed between clinician and independent radiological assessments, however it is not stated which assessment was used in the analyses. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Insufficient data. The abstract states ' 25% of patients discontinued treatment without documented progression'. Final results not reported. Censoring imbalance. |
| Selective reporting (reporting bias) | High risk | Preliminary results were reported at ASCO 2007 with scant useful data. Overall survival was not reported. |
| Other bias | High risk | Publication bias. We were unable to obtain any useful data despite several attempts to contact the first author and Telik. We assessed the overall risk of bias of this study as high. |
ASSIST‐5 2010.
| Methods | Phase III multicentre RCT (US, Brazil, Belgium, UK). Accrual from Sept 2006 to June 2007. Followed up every 8 weeks. (ID: NCT00350948) | |
| Participants | 125 women with PR ROC. Included if: ≥ 18 years old; 1 or 2 previous platinum‐based chemo regimens given; measurable disease defined by RECIST; ECOG PS 0,1 or 2; and adequate bone marrow reserves and cardiac, renal and hepatic function were required. Bulky disease was defined as tumour mass ≥ 5cm. | |
| Interventions | Arm 1: CAN (1000 mg/m²) IVI for 30 min followed by PLD (50 mg/m²) on day 1 every 28 days Arm 2: PLD (50 mg/m²) IVI for 60 min on day 1 every 28 days |
|
| Outcomes | Primary: PFS Secondary: ORR, SAE (NCI‐CTCAE v3.0) |
|
| Notes | This study was temporarily put on hold in June 2007 to review the results of the single‐agent trial (ASSIST‐1) in PR ROC was reviewed. The clinical hold was released in October 2007 but the sponsor decided not enrol any additional patients. Patients requiring dose reductions for HFS and stomatitis were 15% and 4% respectively, in the intervention arm compared with 42% and 25% respectively in the PLD arm; i.e. CAN appeared to decrease the rate of HFS and stomatitis when combined with PLD. Premedication (ondansetron and IV corticosteroids) was the same in both arms. For the exploratory subgroup of PR ROC women with platinum‐refractory or primary platinum resistance (i.e. excluding secondary platinum resistance), the difference in PFS was significantly in favour of arm 1 (HR = 0.55; P value 0.0425). Also in this subgroup, median survival for arm 1 was 11.8 months versus 7.8 months in arm 2. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation with stratification for ECOG PS, prior best response to platinum‐based chemotherapy and bulky disease. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It is not stated whether assessors were blind to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/60 women in the PLD arm did not receive any study drug and so were not included in the SAE analyses. |
| Selective reporting (reporting bias) | High risk | OS was not reported as there was an insufficient number of death events at the time of reporting. We requested final OS data from Telik Inc but received no reply to our queries. |
| Other bias | High risk | This trial closed early. Planned enrolment = 244, actual enrolment = 125. See notes above. As a result of the clinical hold, 35 patients (21 in combo arm and 14 in PLD arm) were not able to complete their assigned therapy as per protocol. |
CALYPSO 2010.
| Methods | Phase III open‐label multicentre non‐inferiority RCT. Accrual from Apr 2005 to Sept 2007. (ID: NCT00538603) | |
| Participants | 976 women with PS ROC (recurrence > 6 months after first or second line platinum‐based chemotherapy and had received a taxane). included if ECOG ≤ 2; previous taxane therapy; measurable or assessable disease; life‐expectancy of at least 12 weeks; and adequate bone marrow, renal and hepatic function. Patients with pre‐existing peripheral neuropathy grade > 1 were excluded. | |
| Interventions | Arm 1 (509 women): carbo (AUC 5) + PAC (175 mg/m²) q3wks Arm 2 (466 women): carbo (AUC 5) + PLD (30 mg/m²) q4wks Premedication of antiemetics (5HT agonist) and dexamethasone was to given to all women; those in the carbo/PAC arm also received clemastine and ranitidine. |
|
| Outcomes | Primary: PFS Secondary: OS, SAE, QoL (QLQ C30 and OV 28) assessed at baseline, 3,6, 9 and 12 months |
|
| Notes | Overall, severe non‐haematological toxicity occurred in 36.8% of the PAC/carbo arm compared with 28.4% of the PLD/carbo arm (P < 0.01). Significantly fewer severe allergic reactions (grade 3 to 4) were observed in the PLD/carbo arm than in the PAC/carbo arm: 2.4% versus 8.8%, respectively (P < 0.001) (see Joly 2011). Significantly more women in the PAC/carbo arm discontinued treatment before six cycles had been completed (110/507 versus 70/466), mainly due to toxicity (73/507 women versus 27/466 women; P < 0.001). In total, 90% of women received post‐progression treatment, 69% received two or more lines. The proportion of women in the PAC/carbo arm who received PLD as post‐study therapy (68%) was significantly higher than the proportion of women in the PLD/carbo arm who received PAC (43%; P < 0.001); this may have influenced OS HRs in the direction of the PAC/carbo arm. We obtained unpublished data on non‐haematological adverse effects (grade 3 to 4) from the investigators. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally randomised. Randomisation was in permuted blocks of 6, with stratification by measurable disease, treatment free interval (6‐12 versus >12 months) and centre. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Evaluation assessments were independently reviewed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition for survival and toxicity outcomes. Regarding QoL data, 79% of women in the carbo/PAC arm and 84% of women in the carbo/PLD arm had QoL data at baseline and one other point in the study. The most complete data set (< 20% missing data) was available at 3 months post‐randomisation, therefore we used these data. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes were reported. |
| Other bias | Low risk | Baseline characteristics were similar and arms were well balanced for stratification factors. Imbalance in treatment allocation (509 versus 467) was consistent with chance. |
Colombo 2012.
| Methods | Phase III open‐label RCT conducted in 22 countries; accrual between Nov 2005 and Mar 2009 ID: NCT00262990 | |
| Participants | 829 women with PR ROC following ≤ 3 platinum‐taxane based regimens. Measurable and non‐measurable disease (but CA125 elevated at baseline); ovarian, fallopian and primary peritoneal cancer included. Excluded if peripheral neuropathy, unresolved bowel obstruction or diarrhoea had within 7 days of start of treatment. | |
| Interventions | Arm 1: PAT (10 mg/m²) IVI q3wk Arm 2: PLD (50 mg/m²) IVI q4wk No routine premedication was given to either arm. |
|
| Outcomes | Primary: OS Secondary: PFS, ORR, SAE |
|
| Notes | Women were assessed 8‐weekly; median follow‐up was 27 months. Arms received a median of 4.5 and 3 cycles for PAT and PLD respectively. Median TTP was 15.9 weeks for both arms. Median time to death was 56.6 weeks versus 54.4 weeks in favour of PAT. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation. |
| Allocation concealment (selection bias) | Low risk | Allocation via an interactive voice response system. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded central review of results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Very few women lost to follow‐up and low attrition (< 20%) in most analyses. As with other studies, QoL data suffered from high attrition rates and therefore we could not use it in the meta‐analyses. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported. |
| Other bias | Low risk | Baseline characteristics were similar. |
Gordon 2001.
| Methods | Phase III multicentre open‐label RCT with 104 sites in USA and Europe that recruited participants between May 1997 to March 1999. | |
| Participants | 481 women with ROC (PS or PR) who had recurred or failed first‐line platinum‐based chemotherapy; with measurable disease, or measurable and assessable disease; adequate bone marrow, renal, hepatic and cardiac function; Karnofsky performance status ≥ 60%; expected to live > 3 months | |
| Interventions | Arm 1: PLD 50 mg/m² IVI over 1 hour, q4wk Arm 2: TOP 1.5 mg/m²/d IVI over 30 min x 5d, q3wk |
|
| Outcomes | Primary: PFS Secondary: ORR, OS, SAE, QoL (QLQ‐C30) |
|
| Notes | Seven women received no treatment after randomisation and were excluded from most analyses. G‐CSF was given to women who experienced febrile neutropenia, prophylactically in the following cycles; 29.1 % TOP versus 4.6% PLD received G‐CSF. The Investigators concluded that PLD was the treatment of choice among non‐platinum agents for women with ROC, especially platinum‐sensitive disease. 72% and 74% of women in the TOP and PLD groups, respectively, received prior taxane therapy. Median TTP was 17 weeks versus 16.1 weeks in favour of the TOP arm. Median time to death was 59.7 weeks versus 62.7 weeks in favour of the PLD arm. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation; stratified by platinum sensitivity and bulky disease. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent radiological review used for primary outcome (PFS). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition low for primary outcomes (high for QoL data). |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. Censoring = 13%. |
| Other bias | Low risk | Baseline characteristics were similar. |
HeCOG 2010.
| Methods | Phase II RCT of the Hellenic Cooperative Oncology Group. Accrual from Oct 199 to Dec 2005. (ID: ACTRN12609000436279) | |
| Participants | 189 women with PS ROC (≥ 6 months after platinum‐based chemotherapy). Included if ECOG 0‐2; life expectancy ≥ 3 months; and adequate bone marrow, renal, hepatic function. Patients with residual neurotoxicity from previous platinum and/or taxane chemotherapy and those with other cancers were excluded | |
| Interventions | Arm 1: carbo (AUC 5) + PAC 175 mg/m² over 3 hours, q3wks Arm 2: carbo (AUC 5) + PLD 45 mg/m², q4wks Standard premedication included dexamethasone, dyphenhydramine and ranitidine for both groups, although the PAC group received both an oral (12 hours prior) and an IV dose (30 min prior to PAC administration). Six cycles intended. |
|
| Outcomes | Primary: ORR (WHO criteria or CA‐125 Rustin's criteria) and toxicity Secondary: TTP, OS |
|
| Notes | 204 women were randomised but 15 were subsequently considered to be ineligible and excluded. Median follow‐up 43.6 months (95% CI 0.1 to 74.8). 88% and 93% respectively received previous taxane‐containing therapy. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation. |
| Allocation concealment (selection bias) | Low risk | Central randomisation/allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition (< 20%). Fifteen post‐randomisation exclusions due to non‐eligibility including other cancers, non‐measurable disease without CA‐125 elevations. Eleven lost medical records, (5 in CP arm and 6 in CLD arm); 8 and 5 women lost to follow‐up respectively. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. |
| Other bias | Low risk | None noted. Baseline characteristics were similar. |
Kaye 2012.
| Methods | Phase II open‐label multicentre RCT; 1:1:1 ratio (ID: NCT00628251) | |
| Participants | 97 women with ROC within 12 months of receiving platinum‐based chemotherapy with confirmed BRCA1/2 germline mutations; one or more measurable lesion; ECOG PS 0‐2; estimated life expectancy ≥ 16 weeks; adequate bone marrow, hepatic and renal function. Excluded if previous PARP inhibitors or anthracyclines; brain metastases; other malignant disease; persistent toxic effects of treatment; LVEF < 50% | |
| Interventions | Arm 1: OLA 200 mg bd continuously (32 women) Arm 2: OLA 400 mg bd continuously (32 women) Arm 3: PLD 50 mg/m² IVI q4wk (33 women) |
|
| Outcomes | Primary: PFS (RECIST‐assessed) Secondary: ORR, duration of treatment response, tumour size, OS, SAE, QoL (FACT‐O) |
|
| Notes | PARP nuclear enzymes facilitate DNA repair. Olaparib is a PARP inhibitor selective for homologous‐recombination‐deficient cells, such as those with BRCA1/2 deficiency. The primary outcome was reported for the olaparib arms combined and individually, versus the PLD arm. We used the results from the OLA 400 mg arm versus PLD. Median time to progression was 38 weeks versus 30 weeks in favour of OLA. Median time to death was not calculable for the OLA group and was 76 weeks for the PLD group (unpublished data). Corticosteroids and serotonin antagonists were given to 22/33 (67%) and 14/33 (42%) of the women in the PLD group respectively versus 12.5 % and 12.5% of the OLA group respectively, but it was not possible to determine whether they were given as premedication or at another time (unpublished information). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, block randomisation, stratified according to BRCA status and platinum sensitivity (≤ 6 months and > 6 months). |
| Allocation concealment (selection bias) | Low risk | Allocation via an Interactive Voice Response System |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | 'Centrally reviewed tumour assessments' were used for analyses; investigator‐assessed primary outcome; assessor blinding/independence not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the PLD arm, 5/33 discontinued treatment for unknown reasons versus 1/64 in the olaparib arm. Otherwise, attrition rates seem low. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. Results are not reported for platinum‐sensitive subgroups; these data were requested from the lead investigator on the 6/12/12. |
| Other bias | Low risk | Baseline characteristics were similar except that more women in Arm 2 had received > 2 prior chemotherapy regimens. |
M200 2009.
| Methods | Multicentre open‐label RCT; enrolment in USA from July 2007 to Oct 2008. (ID: NCT00635193) | |
| Participants | 127 women with stage III/IV PS or PR ROC. Maximum of 2 prior chemotherapy treatments (at least one of which was platinum/taxane based); at least one measurable lesion to assess response by RECIST. | |
| Interventions | Volociximab (M200) is an anti‐angiogenic integrin inhibitor/monoclonal antibody. Two dosage regimes were tested combined with PLD versus PLD alone: Arm 1: PLD 40 mg/m² q4wk (66 women) Arm 2: M200 15 mg/kg qwk + PLD 40 mg/m² q4wk (34 women) Arm 3: M200 15 mg/kg q2wk + PLD 40 mg/m² q4wk (27 women) |
|
| Outcomes | Efficacy, safety and tolerability | |
| Notes | No useable data. Results were reported as follows: 'The most common Grade 3 to 4 AEs (≥5% in any group) were abdominal pain, intestinal obstruction, ascites, fatigue, hypoalbuminemia, and cytopenias. The incidence of AEs was balanced across treatment groups. "There were no CRs; PRs were 16%, 18%, and 19%....Preliminary analysis of PFS suggested that there was a low probability of detecting a statistically significant difference in favor of V+PLD, so the study was closed to enrollment." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Efficacy and safety were not clearly detailed in the ASCO 2009 abstract which is the only publication for this study. |
| Selective reporting (reporting bias) | Unclear risk | Baseline data were not reported. |
| Other bias | Unclear risk | Limited information was available and results have not been published in full. Dr Obrocea of Abbott Laboratories was emailed on 28/11/2012 for final study data. |
MITO‐3 2008.
| Methods | Phase III multicentre RCT; accrual from Jan 2003 to Jan 2007. | |
| Participants | 153 women with ROC that had relapsed within 12 months (PPS and PR ROC) of receiving one platinum/paclitaxel regimen. Women had measurable or assessable disease (RECIST), adequate hepatic, renal, cardiac and bone marrow function, no prior malignancies, and were expected to live > 3months. | |
| Interventions | Arm 1: GEM (1000 mg/m²) days 1, 5, 8, 15, q4wk Arm 2: PLD (40 mg/m²) IVI, q4wk Methylprednisolone 20 mg was given as premedication to the PLD arm. |
|
| Outcomes | Primary: TTP (time to progression) Secondary: OS, ORR, SAE, QoL (QLQ‐C30) |
|
| Notes | Trial used a lower (40 mg/m²) dose of PLD to minimise SAEs. Post‐progression treatment was only documented in 36 participants so OS data difficult to interpret. Median TTP was 20 weeks versus 16 weeks in favour of GEM. Median time to death was 51 weeks versus 56 weeks in favour of PLD. HR for OS and PFS not given but requested from Dr Ferrandina on 3/12/12. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation. |
| Allocation concealment (selection bias) | Low risk | Random assignment by central telephone service. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and physicians not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary outcome was TTP. PFS/OS were not reported clearly with HRs but we were able to obtain these from the investigators in January 2013. |
| Other bias | Low risk | Treatment groups were well balanced for baseline characteristics. |
Mutch 2007.
| Methods | Phase III open‐label multicentre RCT; accrual from July 2002 to May 2004 at 44 sites in the USA. | |
| Participants | 195 women with PR ROC who had received 1‐2 prior platinum‐based chemotherapy regimens with measurable (RECIST) or assessable disease (Zubrod performance status of 0 to 2 and adequate bone marrow, hepatic and neurological function. | |
| Interventions | Arm 1: GEM (1000 mg/m²) IV day 1, 8 q3wk Arm 2: PLD (50 mg/m²) IV q4wk |
|
| Outcomes | Primary: PFS Secondary: OS, SAE (NCI‐CTCAE v 2.0) QoL (FACT‐O) |
|
| Notes | If participants experienced disease progression, unacceptable toxicity or if cumulative PLD dose exceeded 500 mg/m², they crossed over to the alternative drug. Median follow‐up was 29.2 months. 99% of women had received prior taxane. Median TTP was 15.4 weeks versus 13.3 weeks in favour of the GEM arm. Median time to death was 54.4 versus 57.9 weeks in favour of the PLD arm. PFS and OS were not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Independent assessment/blinding not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number of events/(total number evaluated) and censoring was not described for the primary outcome (PFS) or OS. Attrition for QoL outcomes not reported. Additional data requested from authors 4/12/12. |
| Selective reporting (reporting bias) | High risk | HRs, number of events, and censoring was not described for the primary outcome (PFS) or OS. Limited (non‐comparative) QoL data reported. Additional data requested from authors 4/12/12. |
| Other bias | Low risk | Baseline characteristics were similar. |
O'Byrne 2002.
| Methods | Phase III multicentre RCT. Accrual May 1997 to April 2000. (ID: NCT00653952) | |
| Participants | 438 women with ROC (PS or PR) that had 1 prior course of platinum‐based non‐taxane containing chemotherapy and evaluable disease. Prior therapy with PLD or PAC was an exclusion criterion. | |
| Interventions | Arm 1: PLD 50 mg/m² IVI over 60 min q4wk (D) Arm 2: Paclitaxel 175 mg/m² over 3 hours q3wk (P) |
|
| Outcomes | OS, PFS and SAE | |
| Notes | This study is listed as 'Terminated' on the NCT registry after enrolling 220 women. The only published report is an ASCO 2002 abstract which had no data that could be included in our meta‐analyses. Results were reported as follows: 'A preliminary analysis indicates that the overall progression‐free survival rates are similar between the two arms (D: 21.7 versus P: 22.4 weeks; P value 0.15). The overall response rates for D and P are 17.8% and 22.4%, respectively (P value 0.34). Median overall survival times are 45.7 weeks for D and 56.1 weeks for P (P value 0.44). No significant difference was seen in median progression‐free or overall survival for platinum sensitive or refractory patients in either treatment arm. The overall number of adverse events was equivalent in either arm. Nausea and vomiting, stomatitis and plantar‐palmar erythrodysesthesia were seen more frequently with D whereas alopecia, myalgia, arthralgia and paraesthesiae occurred more commonly with P. These findings clearly indicate that D has comparable efficacy to P in taxane naive patients with ROC. D may be particularly suitable for those patients with musculoskeletal disorders or for whom the prospect of alopecia has a significant adverse psychological effect.' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. Stratified prospectively for platinum sensitivity and bulky disease. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Assessor blinding not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information given to assess this risk. |
| Selective reporting (reporting bias) | Unclear risk | Very limited results were reported; see notes section above. Baseline characteristics stated as 'well‐matched'. |
| Other bias | High risk | 214 women were enrolled and yet the study was terminated. The reason for termination was 'poor accrual' and the final results for the 214 women were, to our knowledge, never published. Janssen Oncology emailed on 29/11/12 for more data (no response). |
OVA‐301 2010.
| Methods | Phase III multicentre RCT (21 countries); recruited from April 2005 to May 2007. Participants were followed up every 8 weeks. | |
| Participants | 672 women with PR ROC (PFI < 6 months) and women with PS ROC (PFI ≥ 6 months), excluding platinum refractory patients. Planned enrolment was 650 women. Included if measurable disease was present (defined by RECIST); only 1 prior platinum‐based regimen received; ECOG PS 0,1 or 2; PFI based on radiological evaluation; no other major medical conditions. |
|
| Interventions | Arm 1: Dexamethasone (IVI 20 mg) + PLD (30 mg/m²) IVI for 90 min +TBD (1.1 mg/m²) IVI for 3‐hours, every 21 days Arm 2: PLD (50 mg/m²) IVI for 90 min every 28 days |
|
| Outcomes | Primary: PFS Secondary: OS, ORR, duration of response, SAE (NCI‐CTCAE v3.0) Tertiary: QoL |
|
| Notes | Growth factor was necessary in 42% arm 1 versus 17% arm 2 to treat neutropenia (precise figures were not given). There were more withdrawals in the TBD arm than the PLD alone arm due to patient choice or adverse events (126 versus 89 participants). Dexamethasone was given to the TBD group only to reduce hepatic toxicity (personal communication). When results were subgrouped by platinum sensitivity, only women in the PS ROC group experienced significantly longer PFS with arm 1; i.e. TBD + PLD offered no significant additional benefit over PLD alone for women with PR ROC. Similarly for OS, only the PPS ROC subgroup of arm 1 had a statistically significantly longer OS than the arm 2 subgroup (HR 0.59; 95% CI 0.42 to 0.82; P value 0.0015). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central block randomisation (1:1) with stratification by platinum sensitivity and ECOG PS. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent radiological assessment and oncologist review. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All expected outcomes were reported, although missing data was >20% for QoL outcomes. |
| Selective reporting (reporting bias) | Unclear risk | The reduced rate of PLD toxicity reported in the TBD + PLD arm could have been due to the premedication drug dexamethasone (and not TBD) that was given to the experimental group, or due to the lower dose of PLD used. This was not mentioned in any of the trial publications. |
| Other bias | Unclear risk | Women in arm 2 had a significantly longer PFI than arm 1 (P value 0.009) which may have biased the survival data in the direction of PLD alone. When the investigators adjusted OS results for the PFI and other prognostic factors in ad hoc exploratory analyses, the adjusted OS produced a statistically significant result in favour of arm 1 (HR = 0.82; 95% CI 0.69 to 0.98; P value 0.0285). |
PRECEDENT 2013.
| Methods | Phase II open‐label multicentre RCT; randomisation ratio EC145 (Vintafolide) + PLD to PLD was 2:1; recruitment between Sept 2008 and June 2010 in USA, Canada and Poland. | |
| Participants | 162 women with PR ROC (149 had measurable disease); ≥18 years; ECOG performance status of 0‐2; measurable disease; ≤ 2 prior systemic cytotoxic regimens and adequate organ function. Excluded if prior exposure to PLD, folate‐receptor (FR) targeted therapy or vinca‐containing compounds; recent surgery; serious comorbidities; concurrent malignancy. | |
| Interventions | Arm 1 (100 women): EC145 (2.5 mg IV days 1,3 and 5, weeks 1 and 3, q4wk) + PLD (50 mg/m²) q4wk Arm 2 (49 women): PLD (50 mg/m²) IV q4wk EC145 is a folate‐linked vinca alkaloid. Premedication was optional, but considered not necessary for EC145 administration. |
|
| Outcomes | Primary: PFS assessed within 12 months following completion of accrual using RECIST and clinical findings Secondary: OS assessed within 18 months after PFS analysis; ORR; safety and tolerability; correlation between therapeutic response and 99mTc‐EC20 levels. |
|
| Notes | We contacted the investigators, who gave us access to their unpublished manuscript and provided us with additional unpublished data. The Independent radiologic committee (IRC) assessment in women with more than one CT scan correlation was 74%. PFS was not significantly different between the treatment groups for the IRC assessment except for the subgroup of folate‐receptor positive women. One woman in each group required growth factor support (unpublished data). Median OS was unusually long in the PLD only arm (16.8 months) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation 2:1 EC145/PLD:PLD. Stratified according to primary or secondary platinum resistance, treatment centre, and baseline CA‐125 (<200 versus ≥200 U/ml). |
| Allocation concealment (selection bias) | Low risk | Central randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was based upon investigator assessment using RECIST criteria, however blinded assessment was performed by an IRC to check for investigator bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Censoring due to clinical progression was 12% and 10% for treatment arms respectively. Eight women in the EC145 arm were withdrawn from EC145 due to treatment related AEs (7.5%) but were included in ITT analyses. Women with non‐measurable disease (13) were included in the safety analyses but excluded from the survival analyses. |
| Selective reporting (reporting bias) | Low risk | All pre‐specified outcomes reported. Sensitivity analysis performed for primary outcome. |
| Other bias | Low risk | Baseline characteristics were similar between the arms except for the number of tumour lesions, which was greater in the EC145 arm, however this was not a prognostic factor for shorter PFS. |
SWOG S0200 2008.
| Methods | Phase III multicentre RCT. Accrual from Aug 2002 and Dec 2004. (ID:NCT00043082) | |
| Participants | 61 women with PS ROC or peritoneal cancer; a progression‐free and platinum‐free interval of 6 to 24 months according to RECIST or GCIG CA‐125 criteria; progression following first‐line platinum based CT and up to 12 courses of non‐platinum containing consolidation treatment; Zubrod performance status 0‐1. | |
| Interventions | Arm 1: PLD (30 mg/m²) IV plus carbo IV (AUC = 5 mg/mL/min) q4wk Arm 2: carbo IV (AUC = 5 mg/mL/min) q4wk Patients could receive a premed of intravenous dexamethasone (20 mg) plus IV granisetron before carbo dose, and further dexa on days 2,3, and 4. G‐CSF was allowed to treat G3 to 4 neutropenia when it occurred, and then subsequently to prevent it. |
|
| Outcomes | Primary: OS Secondary: PFS, ORR, toxicity |
|
| Notes | The accrual goal was 900 but study was discontinued due to slow accrual. Unpublished final survival data related to the 2010 publication was received from investigators on 13/12/12. PFS was significantly improved by the addition of PLD to carbo. The final OS was not statistically significantly different between treatment arms, in contrast to the earlier report of 2008 where OS was significantly longer in the PLD/carbo arm. Despite using a lower dose of PLD, this trial had a relatively high rate of haematological SAEs (G3 to 4) in the PLD/carbo arm compared with the carbo alone arm (neutropenia 48% versus 3%; anaemia 16% versus 0%; thrombocytopenia 39% versus 10%). Eight women in the carbo arm had allergic reactions (any grade) compared with 0 in the PLD/carbo arm. The HFS rate was 3/31 (10%) in the PLD/carbo arm. The proportion of women in each group who received a dexamethasone premed was not described. Investigators concluded that PLD/carbo dosing interval was more convenient than the PAC/carbo and GEM/carbo alternatives; that PLD was well tolerated with no significant HFS problems; and that 'administering PLD with carboplatin appears to substantially reduce the incidence of platinum‐associated hypersensitivity reactions'. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Central randomisation. |
| Allocation concealment (selection bias) | Low risk | Central allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition. |
| Selective reporting (reporting bias) | Low risk | Final HRs for survival were not published, however the investigators provided us with these unpublished data. |
| Other bias | Unclear risk | This study closed early due to insufficient accrual and the final sample size was not powered to detect a survival difference. |
AE: adverse events; ASCO: American Society of Clinical Oncology; CAN: canfosfamide; bd: twice daily; carbo: carboplatin; CLD: carbo liposomal doxorubicin; CI: confidence interval; CT: computed tomography; ECOG PS: Eastern Cooperative Oncology Group Performance Status; FACT‐O: Functional Assessment of Cancer Therapy; GEM: gemcitabine; G‐CSF: granulocyte Colony‐stimulating factor; HFS: hand‐foot syndrome; HR: hazard ratio; IRC: Independent radiologic committee; ITT: intention‐to‐treat; IV: intravenous; IVI: intravenous infusion; LVEF: left ventricular ejection fraction; NCI‐CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events; OLA: olaparib; ORR: objective response rate; OS: overall survival; PARP: Poly (ADP‐ribose); polymerase; PAT: patupilone; PFI: platinum‐free interval; PFS: progression free survival; PLD: pegylated liposomal doxorubicin; PR: partial response; PR ROC: platinum refractory relapsed ovarian cancer; QoL: quality of life; RCT: randomised controlled trial; RECIST: Response Evaluation Criteria in Solid Tumours; ROC: relapsed ovarian cancer; SAE: serious adverse events; TBD: trabectedin; TFP: trifluoperazine; TOP: topotecan; TTP: time to progression; q3wks: every three weeks; q4wks: every four weeks; bd: twice daily.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ASSIST‐1 2009 | RCT of canfosfamide versus topotecan or PLD, however allocation of the control intervention (TOP or PLD) depended on previous treatment and therefore was not a random process. |
| Cherchi 2003 | Not an RCT. |
| GOG0182/ICON 5 | A multicentre RCT to evaluate new platinum‐based combination treatments, including PLD, GEM and TOP for first‐line treatment of advanced stage OC, not relapsed OC. |
| Kavanagh 2004 | Not an RCT. |
| MITO‐2 2011 | A multicentre RCT of carboplatin plus paclitaxel versus carboplatin plus PLD as first‐line treatment for OC, not relapsed OC. |
| Palaia 2006 | Not an RCT. |
| Scarfone 2006 | Not an RCT. |
Abbreviations: RCT = randomised controlled trial; OC = ovarian cancer; ROC = relapsed ovarian cancer; PR ROC = platinum refractory relapsed ovarian cancer; PLD = pegylated liposomal doxorubicin; GEM = gemcitabine; TOP = topotecan
Characteristics of ongoing studies [ordered by study ID]
ABT‐888/NCT01113957.
| Trial name or title | A trial of ABT‐888 in combination with temozolomide versus pegylated liposomal doxorubicin alone in ovarian cancer |
| Methods | Phase II open‐label multicentre RCT |
| Participants | 150 women with recurrent high grade serous OC; must be PR or unable to tolerate platinum‐based therapy |
| Interventions | ABT‐888 + temozolomide versus PLD |
| Outcomes | Primary: ORR based on tumour measurements and CA125 levels (assessed every 3 months for 3 years) Secondary: PFS, OS, 12‐month survival rate, 6‐month PFS rate, duration of response, safety and tolerability, QoL |
| Starting date | Mar 2010 |
| Contact information | Yan Luo (Abbott): yan.luo@abbott.com |
| Notes | End date: Mar 2013 |
AGOG06‐001.
| Trial name or title | Phase III RCT of maintenance pegylated liposomal doxorubicin (PLD)/carboplatin versus without in patients with advanced ovarian cancer. (ANZCTR reg. ID: ACTRN12607000329460) |
| Methods | Open‐label RCT; central, computerised, block randomisation with allocation concealment; stratified by residual tumour after primary surgery and baseline CA‐125 |
| Participants | 290 women with advanced OC in complete remission after first‐line chemotherapy |
| Interventions | Maintenance PLD (30 mg/m²)/carboplatin (AUC 4) in 28‐day cycles for 6 courses versus observation (no treatment) after complete remission of first‐line chemotherapy |
| Outcomes | Primary: PFS Secondary: OS, QoL, safety profile |
| Starting date | June 2007 |
| Contact information | TTY BIOPHARM and the Asian Gynecologic Oncology group (AGOG) Chang Gung Memorial Hospital, Taiwan. DR C‐H Lai: laich46@cgmh.org.tw |
| Notes | As at 16/11/12, 49 women enrolled. |
ATI0918/NCT01715168.
| Trial name or title | A cross‐over bioequivalence study of intravenously administered ATI0918 and DOXIL/CAELYX in patients with ovarian cancer |
| Methods | Phase I single‐blind RCT |
| Participants | 40 women with ROC |
| Interventions | PLD (50 mg/m²) versus ATI‐0918 |
| Outcomes | Pharmaco‐equivalence outcomes |
| Starting date | Oct 2012 |
| Contact information | Karen Kuhn: kkuhn@ockham.com |
| Notes | May 2013 |
AURELIA.
| Trial name or title | AURELIA: A study of Avastin (Bevacizumab) added to chemotherapy in patients with platinum‐resistant ovarian cancer (NCT ID: NCT00976911) |
| Methods | Phase III open‐label multicentre RCT |
| Participants | 300 women with PR ROC |
| Interventions | LD+paclitaxel+TOP versus BEV+LD+paclitaxel+TOP |
| Outcomes | Primary: PFS Secondary: ORR, QoL, SAE |
| Starting date | Oct 2009 |
| Contact information | Hoffmann‐La Roche: genentechclinicaltrials@druginfo.com |
| Notes |
NCT00976911 End Date: Dec 2013 |
HECTOR.
| Trial name or title | Topotecan plus carboplatin (TC) versus standard therapy with paclitaxel plus carboplatin (PC) or gemcitabine plus carboplatin (GC) or carboplatin plus pegylated doxorubicin (PLDC): a randomised phase III trial of the NOGGO‐AGO‐Germany‐AGO Austria and GEICO‐GCIG intergroup study (HECTOR) |
| Methods | Phase III multicentre RCT |
| Participants | 550 women with PS ROC. |
| Interventions | Arm 1:TC Arm 2: GC or PC or PLDC |
| Outcomes | Primary: PFS Secondary: toxicity |
| Starting date | Accrual from Feb 2007 to Dec 2009 |
| Contact information | Sehouli@aol.com |
| Notes | Interim data of the first 200 women were presented at ASCO 2012. However, approx. 78% of control arm received GC and it is not clear how many participants, if any, received the PLDC intervention. We emailed the lead investigator for more information on 13/11/12 and 30/11/12. |
IMC‐383/NCT00913835.
| Trial name or title | A study of liposomal doxorubicin with or without IMC‐3G3 in platinum‐refractory or resistant advanced ovarian cancer |
| Methods | Phase II open‐label RCT |
| Participants | 125 women with ROC |
| Interventions | IMC‐383 + LD versus LD |
| Outcomes | Primary: PFS Secondary: OS, ORR, duration of response, SAE |
| Starting date | Jun 2009 |
| Contact information | ClinicalTrials@ ImClone.com |
| Notes | End Date: Oct 2011 |
INOVATYON.
| Trial name or title | Phase III international, randomised study of trabectedin plus pegylated liposomal doxorubicin (PLD) versus carboplatin plus PLD in patients with ovarian cancer progressing within 6‐12 months of last platinum (ID: NCT01379989) |
| Methods | Phase III multicentre RCT |
| Participants | 558 women with ROC 6‐12 months after completion of first line treatment with platinum‐based chemotherapy |
| Interventions | trabectedin + PLD (30 mg/m²) versus carboplatin + PLD (30 mg/m²). |
| Outcomes | Primary: OS Secondary: PFS, ORR, CA125, duration of response, time to subsequent CT, safety |
| Starting date | Jun 2010 |
| Contact information | Nicoletta Colombo |
| Notes | End Date: Dec 2017 Suspended due to the PLD shortage in 2011/12 |
MITO‐8.
| Trial name or title | Liposomal doxorubicin versus carboplatin/paclitaxel in patients with ovarian cancer recurrence between 6 and 12 months after previous platinum based therapy: phase III randomised multicentre study (ID: NCT00657878) |
| Methods | Phase III open‐label multicentre RCT |
| Participants | 250 women with PS ROC |
| Interventions | PLD 40 mg/m² (or GEM or TOP) followed by CP versus CP followed by PLD 40 mg/m² (or GEM or TOP) |
| Outcomes | Primary: OS Secondary: PFS, QoL, ORR, toxicity |
| Starting date | Apr 2008 |
| Contact information | Marilina Piccirillo: marilina.piccirillo@usc‐intnapoli.net |
| Notes | End Date: Nov 2014. As at 8/3/13, 149 women were enrolled. |
NCT01100372.
| Trial name or title | Randomised phase II AGO‐study comparing combined liposomal doxorubicin (Myocet) and gemcitabine (Gemzar) With liposomal doxorubicin (Myocet) monotherapy in platinum‐refractory and platinum‐resistant epithelial cancer of the ovary, fallopian tube and the peritoneum (Other IDs: CDR0000669716/ MUI‐AGO‐10/ EUDRACT‐2008‐008746‐20/ EU‐21028) |
| Methods | Phase II open‐label multicentre RCT |
| Participants | 154 women with PR ROC |
| Interventions | LD + GEM versus LD |
| Outcomes | Primary: CR, PR Secondary: QoL, PFS, OS, toxicity |
| Starting date | Jun 2009 |
| Contact information | Alain Zeimet (Innsbruck Universitaetsklinik) |
| Notes | May 2014 |
NCT01666444.
| Trial name or title | VTX‐2337 and pegylated liposomal doxorubicin (PLD) in patients with recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancer |
| Methods | Phase II double blind multicentre RCT |
| Participants | 210 women with PR ROC |
| Interventions | VTX‐2337 + PLD versus PLD + placebo |
| Outcomes | Primary: OS Secondary: PFS, toxicity, ORR, DCR |
| Starting date | Aug 2012 |
| Contact information | Bradley Monk: bradley.monk@chw.edu |
| Notes | End Date: Mar 2016 |
NGR018.
| Trial name or title | Phase II study of NGR‐hTNF in combination with doxorubicin in platinum‐resistant ovarian cancer (ID: NCT01358071) |
| Methods | Phase II open‐label multicentre RCT |
| Participants | 100 women with PR ROC |
| Interventions | NGR‐hTNF + PLD (50 mg/m²) or doxorubicin versus PLD (50 mg/m²) or doxorubicin |
| Outcomes | Primary: PFS Secondary: OS, RR, DCR, DDC, safety and toxicity |
| Starting date | Jun 2011 |
| Contact information | Antonio Lambiase (MolMed) |
| Notes | End Date: Dec 2012 |
PROCEED.
| Trial name or title | Study for women with platinum resistant ovarian cancer evaluating EC145 in combination with Doxil |
| Methods | Phase III multicentre RCT |
| Participants | 640 women with PR ROC (including 500 folate‐receptor positive women) |
| Interventions | EC145 + PLD (50 mg/m²) versus PLD (50 mg/m²) + placebo |
| Outcomes | Primary: PFS assessed at 6 week intervals to weeks 24 then at 8 week intervals using RECIST v1.1 Secondary: OS, SAE up to 26 months |
| Starting date | Apr 2011 |
| Contact information | Binh Nguyen (Endocyte) |
| Notes | End Date: May 2015 |
PROVE.
| Trial name or title | PROVE A randomised phase II trial of standard carboplatin‐based chemotherapy with or without panitumumab in platinum‐sensitive recurrent ovarian cancer (NCT ID: NCT01388621) |
| Methods | Phase II open‐label RCT |
| Participants | 140 women with ROC |
| Interventions | Panitumumab + carbo + PLD or GEM versus carbo + PLD or GEM |
| Outcomes | Primary: PFS Secondary: OS, duration of response, SAE, translational research |
| Starting date | Oct 2011 |
| Contact information | Sascha M Neugebauer; info@wisp.de |
| Notes | End Date: Jul 2015 |
SGI‐110/NCT01696032.
| Trial name or title | A randomised, controlled, open‐label, phase 2 trial of SGI‐110 and carboplatin in subjects with platinum‐resistant recurrent ovarian cancer |
| Methods | Phase II open‐label RCT |
| Participants | 116 women with PR ROC |
| Interventions | SGI‐110 + carbo versus PLD (40 mg/m²) or TOP or paclitaxel |
| Outcomes | Primary; SAE, PFS, CA125 Secondary: ORR, duration of response, OS |
| Starting date | Oct 2012 |
| Contact information | Astex Pharmaceuticals; Medpace Recruitment Center |
| Notes | End Date: Jul 2014 |
TRINOVA‐2.
| Trial name or title | Trial IN OVArian cancer‐2: A phase 3, randomised, double‐blind trial of pegylated liposomal doxorubicin (PLD) plus AMG 386 or placebo in women with recurrent partially platinum sensitive or resistant epithelial ovarian, primary peritoneal, or fallopian tube cancer (ID: NCT01281254) |
| Methods | Phase III multicentre double‐blind RCT |
| Participants | 380 women with recurrent PPS or PR epithelial ovarian, primary peritoneal or fallopian tube cancer |
| Interventions | AMG 386 + PLD (50 mg/m²) versus PLD (50 mg/m²) + placebo |
| Outcomes | Primary: OS Secondary: PFS, ORR, CA125, duration of response, time to subsequent CT, safety |
| Starting date | Jan 2011 |
| Contact information | AMGEN |
| Notes | End Date: Jun 2018 Suspended due to the PLD shortage |
Volasertib/NCT01121406.
| Trial name or title | Phase II randomised trial of the polo‐like kinase 1 inhibitor BI 6727 (Volasertib) monotherapy versus investigator´s choice chemotherapy in ovarian cancer patients resistant or refractory to platinum‐based cytotoxic therapy |
| Methods | Phase II open‐label multicentre RCT |
| Participants | 100 women with PR OC |
| Interventions | BI 6726 versus paclitaxel or GEM or TOP or PLD |
| Outcomes | Primary: DCR at week 24 Secondary: PFS, adverse events, QoL |
| Starting date | Apr‐10 |
| Contact information | Boehringer Ingelheim Pharmaceuticals |
| Notes | Active, not recruiting. End date: May‐13 |
Abbreviations: OC = ovarian cancer; ROC = relapsed ovarian cancer; PR ROC = platinum refractory relapsed ovarian cancer; PLD = pegylated liposomal doxorubicin; LD = liposomal doxorubicin; GEM = gemcitabine; TOP = topotecan; PFS = progression‐free survival; OS = overall survival; ORR = objective response rate; DCR = disease control rate; DDC = duration of disease control; CR = complete response; PR = partial response; QoL = quality of life; RCT = randomised controlled trial; SAE = serious adverse event;
Differences between protocol and review
In 'Types of Interventions' we have included 'PLD in combination with other agent/s versus PLD alone or with placebo' in the review, whereas this comparison was not included in the protocol. In addition, we have removed the comparison 'PLD versus best supportive care', which was included in the protocol.
Contributions of authors
Tess Lawrie selected and classified studies, abstracted and entered data and wrote the first draft of the review. Andy Bryant abstracted and checked data, provided statistical and methodological support and reviewed the first draft. Jo Morrison helped to classify studies, reviewed the first draft and contributed to the text. Alison Cameron and Emma Gray co‐wrote the protocol and reviewed the first draft. All authors approved the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Department of Health, UK.
NHS Cochrane Collaboration Programme Grant Scheme CPG‐10/4001/12
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
ASSIST‐3 2007 {published data only}
- Rose P, Edwards R, Finkler N, Seiden M, Duska L, Krasner C, et al. Phase 3 Study: Canfosfamide (C, TLK286) plus carboplatin (P) vs liposomal doxorubicin (D) as 2nd line therapy of platinum (P) resistant ovarian cancer (OC). Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part I. 2007; Vol. 25, 18S (June 20 Supplement), 2007: LBA5529.
ASSIST‐5 2010 {published data only}
- Vergote I, Finkler NJ, Hall JB, Melnyk O, Edwards RP, Jones M, et al. Randomized phase III study of canfosfamide in combination with pegylated liposomal doxorubicin compared with pegylated liposomal doxorubicin alone in platinum‐resistant ovarian cancer. International Journal of Gynecological Cancer 2010;20(5):772‐80. [DOI] [PubMed] [Google Scholar]
- Vergote I, et al. Phase 3 study of canfosfamide (TLK 286) plus pegylated liposomal doxorubicin (PLD) vs PLD as second‐line therapy in platinum (P) refractory or resistant ovarian cancer. Journal of Clinical Oncology; 2009 ASCO annual meeting. 2009; Vol. 27(15S): abstr 5552.
CALYPSO 2010 {published and unpublished data}
- Alexandre J, Brown C, Coeffic D, Raban N, Pfisterer J, Mäenpää J, et al. CA‐125 can be part of the tumour evaluation criteria in ovarian cancer trials: experience of the GCIG CALYPSO trial. British Journal of Cancer 2012;106(4):633‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre J, Brown C, Priou F, Rauglaudre G, Pfisterer J, Maenpaa J, et al. Should CA 125 still be part of tumour evaluation criteria in ovarian cancer trials? Experience of the GCIG calypso trial. Annals of Oncology, 35th ESMO Congress, Milan, Italy. 2010; Vol. 21(suppl 8):viii 307: abstr. 981P.
- Baumann KH, Pujade‐Lauraine E, Jackisch C, Lubbe D, du Bois A, Brown C, et al. Therapy expectations of patients with recurrent platinum‐sensitive ovarian cancer‐a German substudy of the calypso/ago‐ovar 2.9 trial. International Journal of Gynecological Cancer, 17th International Meeting of ESGO. 2011.
- Brundage M, Gropp M, Mefti F, Mann K, Lund B, Gebski V, et al. Health‐related quality of life in recurrent platinum‐sensitive ovarian cancer‐‐results from the CALYPSO trial. Annals of Oncology 2012;23(8):2020‐7. [DOI] [PubMed] [Google Scholar]
- Gladieff L, Ferrero A, Rauglaudre G, Brown C, Vasey P, Reinthaller A, et al. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in partially platinum‐sensitive ovarian cancer patients: results from a subset analysis of the CALYPSO phase III trial. Annals of Oncology 2012;23(5):1185‐9. [DOI] [PubMed] [Google Scholar]
- Joly F, Ray‐Coquard I, Fabbro M, Donoghoe M, Boman K, Sugimoto A, et al. Decreased hypersensitivity reactions with carboplatin‐pegylated liposomal doxorubicin compared to carboplatin‐paclitaxel combination: analysis from the GCIG CALYPSO relapsing ovarian cancer trial. Gynecologic Oncology 2011;122(2):226‐32. [DOI] [PubMed] [Google Scholar]
- Kurtz J, Hilpert F, Dorum A, Veillard A, Elit L, Buck M, et al. Can elderly patients with recurrent ovarian cancer (ROC) be treated with a platinum‐based doublet? Results from the CALYPSO trial. Journal of Clinical Oncology. 2010 ASCO Annual Meeting, 2010; Vol. 28:15s, (suppl; abstr 5031).
- Kurtz JE, Kaminsky MC, Floquet A, Veillard AS, Kimmig R, Dorum A, et al. Ovarian cancer in elderly patients: carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in late relapse: a Gynecologic Cancer Intergroup (GCIG) CALYPSO sub‐study. Annals of Oncology 2011;22(11):2417‐23. [DOI] [PubMed] [Google Scholar]
- Lee CK. Ovar 2.9: A Phase III study compare PLD‐doxorubicine‐carboplatin (CD) with carboplatin‐paclitaxel (CP) in recurrent platin‐sensible ovarian cancer. A GCIG study. Archives of Gynecology and Obstetrics, Proceedings of the 58th Congress of the German Society for Gynecology and Obstetrics (DGGG). 2011.
- Lee CK, Friedlander M, Brown C, Gebski VJ, Georgoulopoulos A, Vergote I, et al. Early decline in cancer antigen 125 as a surrogate for progression‐free survival in recurrent ovarian cancer. Journal of the National Cancer Institute 2011;103(17):1338‐42. [DOI] [PubMed] [Google Scholar]
- Lee CK, Gurney H, Brown C, Sorio R, Donadello N, Tulunay G, et al. Carboplatin‐paclitaxel‐induced leukopenia and neuropathy predict progression‐free survival in recurrent ovarian cancer. British Journal of Cancer 2011;105(3):360‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Simes RJ, Brown C, Lord S, Wagner U, Plante M, et al. Prognostic nomogram to predict progression‐free survival in patients with platinum‐sensitive recurrent ovarian cancer. British Journal of Cancer 2011;105(8):1144‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahner S, Meier W, Du Bois A, Brown C, Lorusso D, Ferrero A, et al. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in very platinum‐sensitive ovarian cancer patients: Results from a subset analysis of the CALYPSO phase III GCIG trial. Journal of Clinical Oncology. 2011 ASCO Annual Meeting, 2011; Vol. 29 (suppl; abstr 5059).
- Marth C, Alexandre J, Hanker LC, Brown C, Kaern J, Heywood M, et al. Pegylated liposomal doxorubicin and carboplatin (C‐PLD) versus paclitaxel and carboplatin (C‐P) in platinum‐sensitive ovarian cancer (OC) patients (pts): Treatment at recurrence and overall survival (OS) final analysis from CALYPSO phase III GCIG trial. Journal of Clinical Oncology. 2011 ASCO Annual Meeting, 2011; Vol. 29: (suppl; abstr 5052).
- Pujade‐Lauraine E, Mahner S, Kaern J, Gebski V, Heywood M, Vasey P, et al. A randomized, phase III study of carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in relapsed platinum‐sensitive ovarian cancer (OC): CALYPSO study of the Gynecologic Cancer Intergroup (GCIG). Journal of Clinical Oncology. 2009 ASCO Annual Meeting, 2009; Vol. 27:18s (suppl; abstr LBA5509).
- Pujade‐Lauraine E, Wagner U, Aavall‐Lundqvist E, Gebski V, Heywood M, Vasey PA, et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum‐sensitive ovarian cancer in late relapse. Journal of Clinical Oncology 2010;28(20):3323‐9. [DOI] [PubMed] [Google Scholar]
- Simes R, Lee CK, Mirza M, Sauthier P, Georgopoulos A, Vergote I, et al. The value of early decrease in CA125 levels as a prognostic or surrogate marker for disease progression in patients with recurrent ovarian cancer. Results from the calypso study. Asia‐Pacific Journal of Clinical Oncology, 37th Annual Meeting of the Clinical Oncological Society of Australia (COSA). 2010; Vol. 6 (Suppl 3):150‐253: Abstr. 262.
- Vasey P. A GCIG randomized phase III study of carboplatin (C) & pegylated liposomal doxorubicin (PLD) (C‐D) vs carboplatin (C) & paclitaxel (P) (C‐P): CALYPSO results in partially platinum‐sensitive ovarian cancer (OC) patients. European Journal of Cancer, Joint 15th ECCO‐34th ESMO Multidiciplinary Congress, Berlin, Germany. 2009.
- Wagner U, Marth C, Largillier R, Kaern J, Brown C, Heywood M, et al. Final overall survival results of phase III GCIG CALYPSO trial of pegylated liposomal doxorubicin and carboplatin vs paclitaxel and carboplatin in platinum‐sensitive ovarian cancer patients. British Journal of Cancer 2012;107(4):588‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Åvall‐Lundqvist E, Wimberger P, Gladieff L, Gebski V, Huober JB, Floquet A, et al. Pegylated liposomal doxorubicin (PLD)‐carboplatin (C) (C‐D) vs paclitaxel‐carboplatin (C‐P) in relapsing sensitive ovarian cancer (OC): A 500‐patient interim safety analysis of the CALYPSO GCIG Intergroup phase III study. Journal of Clinical Oncology. 2008 ASCO Annual Meeting, 2008; Vol. 26 (May 20 suppl; abstr 5565).
Colombo 2012 {published data only}
- Colombo N, Kutarska E, Dimopoulos M, Bae DS, Rzepka‐Gorska I, Bidzinski M, et al. Randomized, open‐Label, phase III study comparing patupilone (EPO906) with pegylated liposomal doxorubicin in platinum‐refractory or ‐resistant patients with recurrent epithelial ovarian, primary fallopian tube, or primary peritoneal cancer. Journal of Clinical Oncology 2012;30(31):3841‐7. [DOI] [PubMed] [Google Scholar]
Gordon 2001 {published data only}
- Capri S, Cattaneo G. Cost‐minimization analysis of pegylated liposomal doxorubicin versus topotecan for the treatment of ovarian cancer in Italy. Clinical therapeutics 2003;25(6):1826‐45. [DOI] [PubMed] [Google Scholar]
- Coleman RL, Gordon A, Barter J, Sun S, Rackoff W, Herzog TJ. Early changes in CA125 after treatment with pegylated liposomal doxorubicin or topotecan do not always reflect best response in recurrent ovarian cancer patients. The Oncologist 2007;12(1):72‐8. [DOI] [PubMed] [Google Scholar]
- Gordon A. Overall survival advantage for pegylated liposomal doxorubicin compared to topotecan in recurrent epithelial ovarian cancer [abstract]. European Journal of Cancer; ECCO Conference. 2003; Vol. 1(5):S51.
- Gordon A, Sun S, Rackoff W. Incidence of adverse events in women (</=65 or >65 years) with recurrent ovarian cancer receiving pegylated liposomal doxorubicin or topotecan [abstract]. Gynecologic Oncology. 2006; Vol. 101 (1 suppl 1):S59:60.
- Gordon AN, Fleagle JT, Guthrie D, Parkin DE, Gore ME, Lacave AJ, et al. Recurrent epithelial ovarian carcinoma: a randomized phase III study of pegylated liposomal doxorubicin versus topotecan. Journal of Clinical Oncology 2001;19(14):3312‐22. [DOI] [PubMed] [Google Scholar]
- Gordon AN, Tonda M, Sun S, Rackoff W, on behalf of the Doxil Study 30–49 investigators. Long‐term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecologic Oncology 2004;95(1):1‐8. [DOI] [PubMed] [Google Scholar]
HeCOG 2010 {published and unpublished data}
- Bafaloukos D, Linardou H, Aravantinos G, Papadimitriou G, Bamias A, Fountzilas G, et al. A randomized phase II study of carboplatin plus pegylated liposomal doxorubicin versus carboplatin plus paclitaxel in platinum sensitive ovarian cancer patients: a Hellenic Cooperative Oncology Group study. BMC Medicine 2010;8:3:Accessed on 9/11/2012 at http://www.biomedcentral.com/1741‐7015/8/3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kaye 2012 {published data only}
- Kaye S, Kaufman B, Lubinski J, Matulonis U, Gourley C, Karlan B, et al. Phase II study of the oral parp inhibitor olaparib (AZD2281) versus liposomal doxorubicin in ovarian cancer patients with BRCA1 and/or BRCA2 mutations. Annals of Oncology; Proceedings of the 35th ESMO Congress. NCT00628251, 2010; Vol. 21(suppl 8, abstr 9710):viii 304.
- Kaye SB, Lubinski J, Matulonis U, Ang JE, Gourley C, Karlan BY, et al. Phase II, open‐label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP‐ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. Journal of Clinical Oncology 2012;30(4):372‐9. [DOI] [PubMed] [Google Scholar]
M200 2009 {published data only}
- Vergote IB, Colombo N, Kutarska E, Campo J, Pippitt C, Casado A, et al. Phase II study comparing volociximab (an antiangiogenic antibody) and pegylated liposomal doxorubicin (PLD) with PLD alone in recurrent ovarian or primary peritoneal cancer. Journal of Clinical Oncology. 2009; Vol. 27:15s, (suppl; abstr 5560).
MITO‐3 2008 {published and unpublished data}
- Ferrandina G, Ludovisi M, Lorusso D, Pignata S, Breda E, Savarese A, et al. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. Journal of Clinical Oncology 2008;26(6):890‐6. [DOI] [PubMed] [Google Scholar]
Mutch 2007 {published data only}
- Mutch DG, Orlando M, Goss T, Teneriello MG, Gordon AN, McMeekin SD, et al. Randomized phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in patients with platinum‐resistant ovarian cancer. Journal of Clinical Oncology 2007;25(19):2811‐8. [DOI] [PubMed] [Google Scholar]
- Mutch DG, Orlando M, Goss T, Wang Y, Lilly E, Teneriello MG, et al. Randomized phase III trial of gemcitabine versus pegylated liposomal doxorubicin for patients with platinum‐resistent taxane‐pretreated ovarian cancer as second or third line therapy [abstract]. Gynecologic Oncology. 2006; Vol. 101:S14 (1 Suppl 1):5.
O'Byrne 2002 {published data only}
- O'Byrne KJ, Bliss P, Graham JD, Gerber J, Vasey PA, Khanna S, et al. A phase III study of Doxil/Caelyx versus paclitaxel in platinum‐treated, taxane‐naive relapsed ovarian cancer. Proceedings of the American Society of Clinical Oncologists. 2002; Vol. 21:808.
OVA‐301 2010 {published data only}
- Bidzinski M, Poveda A, et al. Influence of an independent review on PFS and response assessments in a phase III clinical trial in relapsed ovarian cancer. European Journal of cancer, Supplement, Proceedings of the Joint 15th ECCO ‐ 34th ESMO Multidisciplinary Congress, Berlin. 2009:var. pagings.
- Boman K, Colombo N. Tolerability of trabectedin (TR) plus pegylated liposomal doxorubicin (PLD) in platinum sensitive (p‐s) vs. platinum resistant (P‐R) patients (PTS) with relapsed ovarian cancer. Annals of Oncology; 35th ESMO Congress, Milan, Italy. 2010:var. pagings.
- Brundage M, Gropp M, Mefti F, Mann K, Lund B, Gebski V, et al. Health‐related quality of life in recurrent platinum‐sensitive ovarian cancer‐‐results from the CALYPSO trial. Annals of Oncology 2012;23(8):2020‐7. [DOI] [PubMed] [Google Scholar]
- Colombo N. Efficacy of trabectedin in platinum‐sensitive‐relapsed ovarian cancer: new data from the randomized OVA‐301 study. International Journal of Gynecological Cancer 2011;21(Suppl 1):S12‐6. [DOI] [PubMed] [Google Scholar]
- Diebolder H, Runnebaum I. Extending platinum‐free interval (PFI) in partially platinum‐sensitive (PPS) patients (pts) with recurrent ovarian cancer (ROC) treated with trabectedin (Yondelis) plus pegylated liposomal doxorubicin (Caelyx [PLD]) combination versus PLD alone: Results from a PPS cohort of the OVA‐301 phase III study. Archives of Gynecology and Obstetrics; 58th Congress of the German Society for Gynaecology and Obstetrics (DGGG). 2010.
- Gore M, Vergote I, Vasanthan S, Chan S, Arranz JA, Colombo N, et al. Cost‐effectiveness of trabectedin in combination with pegylated liposomal doxorubicin for the treatment of women with relapsed platinum‐sensitive ovarian cancer in the UK: Analysis based on the final survival data. European Journal of Cancer; 2011 ECCO Multidisciplinary Cancer Congress, Stockholm, Sweden. 2011; Vol. var. pagings.
- Herzog TJ, Vermorken JB, Pujade‐Lauraine E, Li J, Bayever E, et al. Correlation of CA‐125 and RECIST evaluation in recurrent ovarian cancer (ROC): results from a randomized phase III study of trabectedin (T) with pegylated liposomal doxorubicin (PLD) versus PLD alone [abstract]. Journal fo Clinical Oncology; 2009 ASCO Annual Meeting. 2009; Vol. 27:15s (suppl; abstr 5550).
- Herzog TJ, Vermorken JB, Pujade‐Lauraine E, Provencher DM, Jagiello‐Gruszfeld A, Kong B, et al. Correlation between CA‐125 serum level and response by RECIST in a phase III recurrent ovarian cancer study. Gynecologic Oncology 2011;122(2):350‐5. [DOI] [PubMed] [Google Scholar]
- Kaye SB, Colombo N, Monk BJ, Tjulandin S, Kong B, Roy M, et al. Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer delays third‐line chemotherapy and prolongs the platinum‐free interval. Annals of Oncology 2011;22(1):49‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong B, Biakhov M, Kelley JL, Nunez J, Lebedinsky C, Parekh TV, et al. Influence of tumor control on tumor‐related events (TRE) in relapsed ovarian cancer (ROC): results from OVA‐301, a randomized phase III study of trabectedin (Tr) with pegylated liposomal doxorubicin (PLD) versus PLD alone [abstract]. Journal of Clinical Oncology; 2010 ASCO Annual Meeting. 2010; Vol. 28:7s (supple; abstr e15529).
- Krasner CN, Poveda A, Herzog T, Vermorken J, Monk B, Zintl P, et al. Health‐related quality of life/patient‐reported outcomes in relapsed ovarian cancer: results from a randomized phase III study of trabectedin witj pegylated doxorubicin (PLD) versus PLD alone [abstract]. Journal fo Clinical Oncology; 2009 ASCO Annual Meeting. 2009; Vol. 27:15s (suppl; abstr 5526).
- Krasner CN, Poveda A, Herzog TJ, Vermorken JB, Kaye SB, Nieto A, et al. Patient‐reported outcomes in relapsed ovarian cancer: results from a randomized Phase III study of trabectedin with pegylated liposomal doxorubicin (PLD) versus PLD alone. Gynecologic Oncology 2012;127(1):161‐7. [DOI] [PubMed] [Google Scholar]
- Kurtz JE, Kaminsky MC, Floquet A, Veillard AS, Kimmig R, Dorum, A, et al. Ovarian cancer in elderly patients: carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in late relapse: a Gynecologic Cancer Intergroup (GCIG) CALYPSO sub‐study. Annals of Oncology 2011;22(11):2417‐23. [DOI] [PubMed] [Google Scholar]
- Meerpohl H. Tolerability of long‐term use of trabectedin in combination with pegylated liposomal doxorubicin (PLD) in patients (pts) with relapsed ovarian cancer (ROC). Archives of Gynecology and Obstetrics; 58th Congress of the German Society for Gynaecology and Obstetrics (DGGG). 2010.
- Monk BJ. A randomized phase III study of trabectedin with pegylated liposomal doxorubicin (PLD) versus PLD in relapsed, recurrent ovarian cancer (OC). Annals of Oncology; Joint ECCO 15‐ 34th ESMO Congress. 2009.
- Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia F, et al. Final survival results of the randomized phase III study of trabectedin with pegylated liposomal doxorubicin (PLD) versus PLD in recurrent ovarian cancer [abstract]. Journal of Clinical Oncology; 2011 ASCO Annual Meeting 2011;29:(suppl; abstr 5046). [Google Scholar]
- Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, et al. Trabectedin plus pegylated liposomal Doxorubicin in recurrent ovarian cancer. Journal of Clinical Oncology 2010;28(19):3107‐14. [DOI] [PubMed] [Google Scholar]
- Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, et al. Trabectedin plus pegylated liposomal doxorubicin (PLD) versus PLD in recurrent ovarian cancer: Overall survival analysis. European Journal of Cancer 2012;48(15):2361‐8. [DOI] [PubMed] [Google Scholar]
- Poveda A, Kaye SB, McCormack RT, Wang S, Ricci D, Broderick E, et al. Circulating tumor cells (CTC) in a study of relapsed/recurrent advanced ovarian cancer: An exploratory analysis in the ova‐301 phase III study of pegylated liposomal doxorubicin (PLD) compared with trabectedin and PLD. Journal of Clinical Oncology, 2009 ASCO Annual Meeting. 2009; Vol. 27:15s (suppl; abstr 5551).
- Poveda A, Monk BJ, Kaye SB, Vermorken JB, Nieto A, Gomez J, et al. Prediction of overall survival (OS) adjusted by continuous platinum‐free interval (PFI) at fixed timepoints in patients with recurrent ovarian cancer (ROC): results from OVA‐301. European Journal of cancer, Supplement, Proceedings of the 2011 ECCO Multidisciplinary Congress, Berlin. 2011:Abstr:E16‐0428.
- Poveda A, Monk BJ, Kaye SB, Vermorken JB, Vergote IB, Runnebaum IB, et al. Prediction of progression‐free survival (PFS) adjusted by continuous platinum‐free interval (PFI) at fixed timepoints in patients with recurrent ovarian cancer (ROC): Results from OVA‐301. Journal of Clinical Oncology, 2011 ASCO Annual Meeting. 2011; Vol. 29:(suppl; abstr 5067).
- Poveda A, Tjulandin S, Kong B, Roy M, Chan S, Filipczyk‐Cisarz E, et al. Extending platinum‐free interval (PFI) in partially platinum‐sensitive (PPS) patients (pts) with recurrent ovarian cancer (ROC) treated with trabectedin (Tr) plus pegylated liposomal doxorubicin (Tr+PLD) versus PLD alone: results from a PPS cohort of a phase III study [abstract]. Journal of Clinical Oncology, 2010 ASCO Annual Meeting. 2010; Vol. 28:15s, (suppl; abstr 5012).
- Poveda A, Vergote I, Tjulandin S, Kong B, Roy M, Chan S, et al. Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer: outcomes in the partially platinum‐sensitive (platinum‐free interval 6‐12 months) subpopulation of OVA‐301 phase III randomized trial. Annals of Oncology 2011;22(1):39‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero I, Colombo N, Kaye SB, Arranz J, Roszak A, Provencher DM, et al. Tolerability of long‐term use of trabectedin (Tr) in combination with pegylated liposomal doxorubicin (PLD) in patients (pts) with relapsed ovarian cancer (ROC). Journal of Clinical Oncology; 2010 ASCO Annual Meeting. 2010; Vol. 28:15s, 2010 (suppl; abstr 5121).
- Runnebaum IB. Extending platinum‐free interval (PFI) in partially platinum‐sensitive (PPS) patients (pts) with recurrent ovarian cancer (ROC) treated with trabectedin (Tr) plus pegylated liposomal doxorubicin (Tr + PLD) versus PLD alone: Results from a PPS cohort of a phase III study. Archives of Gynecology and Obstetrics; 58th Congress of the German Society for Gynaecology and Obstetrics (DGGG). 2010.
- Vergote. Safety analysis of trabectedin in combination with pegylated liposomal doxorubicin (PLD) vs PLD alone in ovarian cancer patients 65 years of age and older. European Journal of cancer, Supplement, Proceedings of the Joint 15th ECCO ‐ 34th ESMO Multidisciplinary Congress, Berlin. 2009:var pagings.
- Vergote I, Bidzinski M, Kelley J, Vasanthan S, Runnebaum I, Vermorken J, et al. Health‐Related Quality of Life (HRQoL) / Patient Reported Outcomes (PRO) of patients with partially platinum sensitive (PPS) recurrent ovarian cancer (ROC) treated in a randomized phase III trial of trabectedin and PLD vs PLD alone (OVA‐301): An Exploratory Analysis. European Journal of Cancer, Supplement, Proceedings of the 2011 ECCO Multidisciplinary Congress, Berlin. 2011:abstr: 8.029.
PRECEDENT 2013 {published data only}
- Naumann RW, Coleman RL, Burger RA, Herzog TJ, Morris R, Sausville E, et al. PRECEDENT: A randomized phase II trial comparing EC145 and pegylated liposomal doxorubicin (PLD) in combination, versus PLD alone, in subjects with platinum‐resistant ovarian cancer. Journal of Clinical Oncology. 2011; Vol. 29: (suppl; abstr 5045). [DOI] [PubMed]
- Naumann RW, Coleman RL, Burger RA, Sausville EA, Kutarska E, Ghaman de SA, et al. PRECENDET: A randomised phase II trial comparing EC145 (Vintafolide) and pegylated liposomal doxorubicin (PLD) in combination, versus PLD alone, in patients with platinum‐resistant ovarian cancer. Unpublished manuscript (submitted to the Journal of Clinical Oncology) 2013 (In press). [DOI] [PubMed]
- Naumann RW, Symanowski J, Kutarska E, Sharad G, Nashat G, Pasquale S, et al. EC20 imaging predicts response in a randomized trial comparing pegylated liposomal doxorubicin (PLD) +/‐ EC145 in platinum‐resistant ovarian cancer. International Journal of Gynaecological Cancer, Proceedings of the 17th International Meeting of ESGO. 2011; Vol. 21(suppl 3).
- Naumann RW, Symanowski JT, Ghamande SA, Gabrail NY, Gilbert MG, Teneriello G, et al. PRECEDENT: A randomized phase II trial comparing EC145 and pegylated liposomal doxorubicin (PLD) in combination, versus PLD alone, in subjects with platinum‐resistant ovarian cancer [abstract]. Journal of Clinical Oncology, 2010 ASCO Annual Meeting Proceedings. 2010; Vol. 28:18S, (suppl; abstr LBA5012b). [DOI] [PubMed]
SWOG S0200 2008 {published and unpublished data}
- Alberts DS, Liu PY, Wilczynski S, Clouser M, Lopez A, Lange M, et al. Phase III randomized trial of pegylated liposomal doxorubicin plus carboplatin versus carboplatin in platinum‐sensitive patients with recurrent epithelial ovarian or peritoneal carcinoma after failure of initial platinum‐based chemotherapy: Southwest Oncology Group Protocol S0200 [Abstract]. Journal of Clinical Oncology, 2007 ASCO Annual Meeting Proceedings Part 1. 2007; Vol. 25 (18S). [DOI] [PMC free article] [PubMed]
- Alberts DS, Liu PY, Wilczynski SP, Clouser MC, Lopez AM, Michelin DP, et al. Randomized trial of pegylated liposomal doxorubicin (PLD) plus carboplatin versus carboplatin in platinum‐sensitive (PS) patients with recurrent epithelial ovarian or peritoneal carcinoma after failure of initial platinum‐based chemotherapy (Southwest Oncology Group Protocol S0200). Gynecologic Oncology 2008;108(1):90‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markman M, Moon J, Wilczynski S, Lopez AM, Rowland KM, Michelin DP, et al. Single agent carboplatin versus carboplatin plus pegylated liposomal doxorubicin in recurrent ovarian cancer: final survival results of a SWOG (S0200) phase 3 randomized trial. Gynecologic Oncology 2010;116(3):323‐325. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
ASSIST‐1 2009 {published and unpublished data}
- Vergote I, Finkler N, Campo J, Lohr A, Hunter J, Matei D, et al. Single agent, canfosfamide (C, TLK286) vs pegylated liposomal doxorubicin or topotecan in 3rd‐line treatment of platinum refractory or resistant ovarian cancer: phase III study results [Abstract]. Journal of Clinical Oncology; 2007 ASCO Annual Meeting. 2007; Vol. 25:18S (Suppl, abstr. LBA5528).
- Vergote, I, Finkler N, Campo J, Lohr A, Hunter J, Matei D, et al. Phase 3 randomised study of canfosfamide (Telcyta, TLK286) versus pegylated liposomal doxorubicin or topotecan as third‐line therapy in patients with platinum‐refractory or ‐resistant ovarian cancer. European Journal of Cancer 2009;45(13):2324‐32. [DOI] [PubMed] [Google Scholar]
Cherchi 2003 {published data only}
- Cherchi PL, Capobianco G, Fattorini F, Canetto AM, Milia L, Cosso M, et al. Second‐line therapy with topotecan and pegylated liposomal doxorubicin vs. topotecan slone in patients with recurrent ovarian cancer. International Journal of Gynecological Cancer. 2003 IGCS Annual Meeting, 2003; Vol. 13(Suppl):53.
GOG0182/ICON 5 {published data only}
- Bookman MA. Erratum. Journal of Clinical Oncology 2009;27(13):2305. [Google Scholar]
- Bookman MA. GOG0182‐ICON5: 5‐arm phase III randomized trial of paclitaxel (P) and carboplatin (C) vs combinations with gemcitabine (G), PEG‐lipososomal doxorubicin (D), or topotecan (T) in patients (pts) with advanced‐stage epithelial ovarian (EOC) or primary peritoneal (PPC) carcinoma. [Abstract]. Journal of Clinical Oncology. 2006; Vol. 24 (Suppl 18): A‐5002, 256s.
- Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, et al. Evaluation of new platinum‐based treatment regimens in advanced‐stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. Journal of Clinical Oncology 2009;27(9):1419‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookman MA, Greer BE, Ozols RF. Optimal therapy of advanced ovarian cancer: carboplatin and paclitaxel vs. cisplatin and paclitaxel (GOG 158) and an update on GOG0 182‐ICON5. International Journal of Gynecological Cancer 2003;13(6):735‐40. [DOI] [PubMed] [Google Scholar]
- Bookman MA, Gynecologic Cancer InterGroup (GCIG). GOG0182‐ICON5: 5‐arm phase III randomized trial of paclitaxel (P) and carboplatin (C) vs combinations with gemcitabine (G), PEG‐lipososomal doxorubicin (D), or topotecan (T) in patients (pts) with advanced‐stage epithelial ovarian (EOC) or primary peritoneal (PPC) carcinoma. Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings Part I. 2006; Vol. 24, 18S (June 20 Supplement):5002.
- Copeland LJB, Gynecologic Cancer Intergroup (GCIG). Clinical trials of newer regimens for treating ovarian cancer: the rationale for Gynecologic Oncology Group Protocol GOG 182‐ICON5. Gynecologic Oncology 2003;90(2 Part 2):S1‐7. [DOI] [PubMed] [Google Scholar]
Kavanagh 2004 {published data only}
- Kavanagh JJ, Garcia A, Choi H, Gershenson DM, Lewis L, Mascavage J, et al. Efficacy of TLK286 (Telcyta) alone and Inparaplatin® or Doxil® (Caelyx®) in platinum resistant ovarian cancer ‐ results from 3 phase 2 studies [abstract]. International Journal of Gynaecological Cancer. 2004; Vol. 14 (suppl 1):abstr 95:28.
MITO‐2 2011 {published data only}
- Pignata S, Scambia G, Ferrandina G, Savarese A, Sorio R, Breda E, et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first‐line treatment for patients with ovarian cancer: the MITO‐2 randomized phase III trial. Journal of Clinical Oncology 2011;29(27):3628‐35. [DOI] [PubMed] [Google Scholar]
- Pignata S, Scambia G, Savarese A, Breda E, Scollo P, Vivo R, et al. Safety of a 3‐weekly schedule of carboplatin plus pegylatedliposomal doxorubicin as first line chemotherapy in patients withovarian cancer: preliminary results of the MITO‐2 randomized trial. BMC Cancer 2006;6:202:This article is available from: http://www.biomedcentral.com/1471‐2407/6/202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignata S, Scambia G, Savarese A, Breda E, Sorio R, Pisano C, et al. Carboplatin and pegylated liposomal doxorubicin for advanced ovarian cancer: preliminary activity results of the MITO‐2 phase III trial. Oncology 2009;76(1):49‐54. [DOI] [PubMed] [Google Scholar]
- Pignata S, Scambia G, Savarese A, Breda E, Sorio R, Vernaglia Lombardi A, et al. Carboplatin plus paclitaxel versus carboplatin plus Stealth liposomal doxorubicin in patients with advanced ovarian cancer: preliminary activity results of the MITO‐2 randomized multicenter trial [Abstract]. Journal of Clinical Oncology; 2007 ASCO Annual meeting. 2007; Vol. 25:18S (abstr. 5532).
- Pignata S, Scambia G, Savarese A, Sorio R, Breda E, Ferrandina G, et al. Carboplatin plus paclitaxel (CP) versus carboplatin plus stealth liposomal doxorubicin (CLD) in patients with advanced ovarian cancer (AOC): activity and safety results of the MITO‐2 randomized multicenter trial [abstract]. Journal of Clinical Oncology; 2009 ASCO Annual Meeting. 2009; Vol. 27:15S (suppl; abstr LBA5508).
- Pignata S, Scambia G, Savarese A, Sorio R, Breda E, Legge F, et al. Carboplatin (C) plus paclitaxel (P) versus carboplatin plus pegylated liposomal doxorubicin (PLD) in patients with advanced ovarian cancer (AOC): Final analysis of the MITO‐2 randomized multicenter trial. Journal of Clinical Oncology; 2010 ASCO Annual Meeting. 2010; Vol. 28:18s, 2010 (suppl; abstr LBA5033).
- Pignata S, Scambia G, Savarese A, Sorio R, Breda E, Legge F, et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as firstline treatment for patients with ovarian cancer: The mito‐2 (multicentre Italian trials in ovarian cancer) randomized phase III trial. Annals of Oncology; 35th ESMO Congress, Milan, Italy. 2010; Vol. var. pagings.
- Pignata S, Scambia G, Savarese A, Zagonel V, Gebbia V, Scollo P, et al. Carboplatin plus paclitaxel (CP) versus carboplatin plus Stealth liposomal doxorubicin (CLD) in patients with advanced ovarian cancer (AOC): preliminary safety results of the MITO‐2 randomized multicenter trial. Journal of Clinical Oncology; 2005 ASCO Annual Meeting. 2005; Vol. 23:16S (Suppl, Abstr. 5014).
Palaia 2006 {published data only}
- Palaia I, Angioli R, Calcagno M, Zullo MA, Plotti F, Basile S, et al. Liposome‐encapsulated doxorubicin citrate in previously treated recurrent/metastatic gynecologic malignancies. International Journal of Gynecological Cancer. 2006; Vol. 16(S3): 799.
Scarfone 2006 {published data only}
- Scarfone, G, Presti M, Scarabelli C, Polverino GP, Polonio N, Bertoglio S, et al. Pegylated liposomal doxorubicin alone or in combination with platinum compounds in recurrent ovarian cancer after first line chemotherapy containing paclitaxel and carboplatin. International Journal of Gynecological Cancer. 2006; Vol. 16(S3):668.
References to ongoing studies
ABT‐888/NCT01113957 {published data only}
- Abbott. A trial of ABT‐888 in combination with temozolomide versus pegylated liposomal doxorubicin alone in ovarian cancer [Ongoing]. Accessed on 12/11/12 at http://www.http://clinicaltrials.gov/show/NCT01113957.
AGOG06‐001 {published data only}
- Lai C‐H. Phase III randomised trial of maintenance pegylated liposomal doxorubicin/carboplatin versus without in patients with advanced ovarian cancer [Ongoing]. Australia and New Zealand Clinical Trials Registry; 2010; Vol. ACTRN12607000329460:Accessed on 20/11/12 at http://www.anzctr.org.au/ACTRN12607000329460.aspx.
ATI0918/NCT01715168 {published data only}
- Kuhn K. A Crossover bioequivalence study of intravenously administered ATI0918 and DOXIL/CAELYX in patients with ovarian cancer. Accessed on 14/11/12 at ttp://www.clinicaltrials.gov/ct2/show/record/NCT01715168.
AURELIA {published data only}
- Hoffmann‐La Roche. AURELIA: A study of Avastin (Bevacizumab) added to chemotherapy in patients with platinum‐resistant ovarian cancer. Accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT00976911.
HECTOR {published data only}
- Meier W, Lichtenegger W, Marth C, Gonzalez‐Martin AJ, Harter P, Tome O, et al. Topotecan plus carboplatin versus standard therapy with paclitaxel plus carboplatin (PC) or gemcitabin plus carboplatin (GC) or carboplatin plus pegylated doxorubicin (PLDC): A planed 200‐pt interim safety analysis of the NOGGO‐AGO‐Germany‐AGO Austria and GEICO‐GCIG Intergroup Study (HECTOR). Journal of Clinical Oncology, 2010 ASCO Annual Meeting. 2010; Vol. 28:15s, (suppl; abstr 5071).
- Sehouli J, Meier W, Wimberger P, Chekerov R, Belau A, Mahner S, et al. Topotecan plus carboplatin vesus standard therapy with paclitaxel plus carboplatin (PC) or gemcitabin plus carboplatin (GC) or carboplatin plus pegylated doxorubicin (PLDC): a randomized phase III trial of the NOGGO‐AGO‐Germany‐AGO Austria and GEICO‐GCIG intergroup study (HECTOR) [abstract]. Journal of Clinical Oncology, 2012 ASCO Annual Meeting. 2012; Vol. 30 (suppl, abstr 5031).
IMC‐383/NCT00913835 {published data only}
- ImClone. A study of liposomal doxorubicin with or without IMC‐3G3 in platinum‐refractory or resistant advanced ovarian cancer. Accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT00913835.
- McGuire WP, Shah GD, Loizos N, Youssoufian H, Rowinsky EK, Gore ME, et al. Randomized phase II trial of pegylated liposomal doxorubicin (PLD) with or without anti‐platelet‐derived growth factor receptor‐alpha (PDGFR‐alpha) monoclonal antibody IMC‐3G3 in platinum‐refractory/resistant advanced ovarian cancer. Journal of Clinical Oncology. 2010; Vol. 28:15s, (suppl; abstr TPS256).
INOVATYON {published data only}
- Colombo N. INOVATYON STUDY ‐International, randomized study in patients with ovarian cancer. Accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT01379989.
MITO‐8 {published data only}
- NCT00657878. Liposomal doxorubicin versus carboplatin/paclitaxel in patients with ovarian cancer recurrence between 6 and 12 months after previous platinum based therapy: Phase III randomized multicenter study [Ongoing]. Accessed 13/11/12 at http://clinicaltrials.gov/ct2/show/study/NCT00657878.
NCT01100372 {published data only}
- Zeimet A. Randomized phase II AGO‐study comparing combined liposomal doxorubicin (Myocet) and gemcitabine (Gemzar) with liposomal doxorubicin (Myocet) monotherapy in platinum‐refractory and platinum‐resistant epithelial cancer of the ovary, fallopian tube and the peritoneum. Accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT01100372.
NCT01666444 {published data only}
- Monk B. VTX‐2337 and pegylated liposomal doxorubicin (PLD) in patients with recurrent or persistent epithelial ovarian, fallopian tube or primary peritoneal cancer. Accessed on 14/11/12 at http://clinicaltrials.gov/ct2/show/record/NCT01666444.
NGR018 {published data only}
- MolMed. Phase II study of NGR‐hTNF in combination with doxorubicin in platinum‐resistant ovarian cancer (NGR018). Accessed on 14/11/12 at http://clinicaltrials.gov/show/NCT01358071.
PROCEED {published data only}
- Endocyte. Study for women with platinum resistant ovarian cancer evaluating EC145 in combination with doxil. Accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT01170650.
PROVE {published data only}
- Sehouli J. PROVE A randomized phase II trial of standard carboplatin‐based chemotherapy with or without panitumumab in platinum‐sensitive recurrent ovarian cancer. Accessed on 14/11/12 at http://clinicaltrials.gov/ct2/show/NCT01388621.
SGI‐110/NCT01696032 {published data only}
- Medpace Recruitment Center. A randomized, controlled, open‐label, phase 2 trial of SGI‐110 and carboplatin in subjects with platinum‐resistant recurrent ovarian cancer. accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT01696032.
TRINOVA‐2 {published data only}
- AMGEN. Trial IN OVArian cancer‐2. Accessed on 14/11/12 at http://www.clinicaltrials.gov/ct2/show/NCT01281254.
Volasertib/NCT01121406 {published data only}
- Boehringer Ingelheim Pharmaceuticals. Phase II randomized trial of the polo‐like kinase 1 inhibitor BI 6727 (Volasertib) monotherapy versus investigator´s choice chemotherapy in ovarian cancer patients resistant or refractory to platinum‐based cytotoxic therapy [Ongoing]. Accessed on 12/11/12 at http://clinicaltrials.gov/ct2/show/NCT01121406.
Additional references
CAELYX PI
- Janssen‐Cilag Pty, Ltd. CAELYX Product Information. http://www.janssen.com.au/Products/Caelyx Amended 4 January 2011:(Accessed 24 January 2013).
CTCAE 2006
- CTCAE. Common Terminology Criteria for Adverse Events. (http://ctep.cancer.gov/forms/CTCAEv3.pdf) 9th August 2006; Vol. v3.0 (CTCAE).
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining for heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith g, Altman DG editor(s). Systematic Reviews in Healthcare: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
Dersimonian 1986
- Dersimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
DOXIL 2011
- Janssen Products. Important update regarding the availability of DOXIL. http://www.doxil.com/sites/default/files/DOXIL_DHCP_letter_July2011.pdf 2011:(Accessed 24/1/13).
EMA 2010
- European Medicines Agency. EPARs for authorised medicinal products for human use EPARs for authorised medicinal products for human use. http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_‐_Summary_for_the_public/human/000089/WC500020173.pdf 2010; Vol. accessed 24 January 2013.
ESMO 2010
- Colombo N, Peiretti M, Parma G, Lapresa M, Mancari R, Carinelli S, et al. on behalf of the ESMO Guidelines Working Group. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology 2010;21(suppl 5):v23‐30. [DOI] [PubMed] [Google Scholar]
EUROCARE 2003
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, et al the EUROCARE Working Group. EUROCARE‐3: survival of cancer patients diagnosed 1990‐94 ‐ results and commentary. Annals of Oncology 2003;14 (Supplement 5):v61‐v118. [DOI] [PubMed] [Google Scholar]
Farr 2012
- Podlekareva Farr K, Safwat A. Palmar‐plantar erythodysesthesia associated with chemotherapy and its treatment. Case Reports in Oncology 2011;4:229‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferlay 2013
- Ferlay J. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. European Journal of Cancer 2013;49(6):1374‐403. [DOI] [PubMed] [Google Scholar]
FIGO 2009
- FIGO Committee in Gynecologic Oncology. Current FIGO staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia. International Journal of Gynecology and Obstetrics 2009;105:3‐4. [DOI] [PubMed] [Google Scholar]
Gabizon 2001
- Gabizon AA. Stealth liposomes and tumor targeting: one step further in the quest for the magic bullet. Clinical Cancer Research 2001;7(2):223‐5. [PubMed] [Google Scholar]
Gladieff 2012
- Gladieff L, Ferrero A, Rauglaudre G, Brown C, Vasey P, Reinthaller A, et al. Carboplatin and pegylated liposomal doxorubicin versus carboplatin and paclitaxel in partially platinum‐sensitive ovarian cancer patients: results from a subset analysis of the CALYPSO phase III trial. Annals of Oncology 2012;23(5):1185‐9. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2008
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide. IARC CancerBase No. 10 [Internet]. Lyon, France, 2010; Vol. International Agency for Research on Cancer:http://globocan.iarc.fr.
Hennessy 2009
- Hennessy BT, Coleman RL, Markman M. Ovarian Cancer. Lancet 2009;374:1371‐82. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICBP 2012
- Maringe C, Walters S, Butler J, Coleman MP, Hacker N, Hanna L, et al. Stage at diagnosis and ovarian cancer survival: evidence from the International Cancer Benchmarking Partnership. Gynecologic Oncology 2012;127(1):75‐82. [DOI] [PubMed] [Google Scholar]
ICON‐4
- Parmar MK, Ledermann JA, Colombo N, du Bois A, Delaloye JF, Kristensen GB, et al. Paclitaxel plus platinum‐based chemotherapy versus conventional platinum‐based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO‐OVAR‐2.2 trial. Lancet 2003;361:2099–106. [DOI] [PubMed] [Google Scholar]
Jemal 2008
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA: A Cancer Journal for Clinicians 2008;58:71‐96. [DOI] [PubMed] [Google Scholar]
Jemel 2011
- Jemel A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer Journal 2011;61(2):69‐90. [DOI] [PubMed] [Google Scholar]
Kavanagh 2010
- Kavanagh JJ, Levenbach CF, Ramires PT, Wolf JL, Moore CL, Jones MR, et al. Phase 2 study of canfosfamide in combination with pegylated liposomal doxorubicin in platinum and paclitaxel refractory or resistant epithelial ovarian cancer. Journal of Hematology & Oncology; www.jhoonline.org/content/3/1/9 2010;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lorusso 2007
- Lorusso D, Stefano A, Carone V, Fagotti A, Pisconti S, Scambia G. Pegylated liposomal doxorubicin‐related palmar‐plantar erthrodysesthesia ('hand‐foot syndrome'). Annals of Oncology 2007;18(7):1159‐64. [DOI] [PubMed] [Google Scholar]
Mangili 2008
- Mangili G, Petrone M, Gentile C, Marzi P, Vigano R, Rabaiotti E. Prevention strategies in palmar‐plantar erythodysesthesia onset: the role of regional cooling. Gynecologic Oncology 2008;108:332‐5. [DOI] [PubMed] [Google Scholar]
Markman 2010
- Markman M. Serious ethical dilemma of single‐agent peylyated liposomal doxorubin employed as a control arm in ovarian cancer chemotherapy trials. Journal of Clinical Oncology 2010;28(19):e319‐20. [DOI] [PubMed] [Google Scholar]
Monk 2010
- Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, et al. Trabectedin plus pegylated liposomal Doxorubicin in recurrent ovarian cancer. Journal of Clinical Oncology 2010;28(19):3107‐14. [DOI] [PubMed] [Google Scholar]
Monk 2012
- Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, et al. Trabectedin plus pegylated liposomal doxorubicin (PLD) versus PLD in recurrent ovarian cancer: Overall survival analysis. European Journal of Cancer 2012;48(15):2361‐8. [DOI] [PubMed] [Google Scholar]
Naumann 2011
- Naumann RW, Coleman RL. Management strategies for recurrent platinum‐resistant ovarian cancer. Drugs 2011;71:1397‐412. [DOI] [PubMed] [Google Scholar]
NICE 2003
- National Institute for Clinical Excellence. Guidance on the use of paclitaxel in the treatment of ovarian cancer. NICE: Technology Appraisal Guidance‐ No. 55 2003.
NICE 2005
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Pfisterer 2006
- Pfisterer J, Lederman JA. Management of platinum‐sensitive recurrent ovarian cancer. Seminars in Oncology 2006;33(2 Suppl 6):S12‐6. [DOI] [PubMed] [Google Scholar]
Poveda 2011
- Poveda A, Vergote I, Tjulandin S, Kong B, Roy M, Chan S, et al. Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer: outcomes in the partially platinum‐sensitive (platinum‐free interval 6‐12 months) subpopulation of OVA‐301 phase III randomized trial. Annals of Oncology 2011;22(1):39‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rose 2005
- Rose PG. Pegylated liposomal doxorubicin: optimizing the dosing schedule in ovarian cancer. Oncologist 2005;10:205‐14. [DOI] [PubMed] [Google Scholar]
Rustin 1996
- Rustin GJ, Nelstrop AE, Tuxen MK, Lambert HE. Defining progression of ovarian carcinoma during follow‐up according to CA 125: a North Thames Ovary Group Study. Annals of Oncology 1996;7:361‐4. [DOI] [PubMed] [Google Scholar]
SEER 2007
- Kosary CL. Chapter 16: Cancer of the ovary. In: Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M‐J editor(s). Cancer Survival Among Adults: US SEER Program, 1988‐2001. Bethesda: US Department of Health and Human Services; National Cancer Institute, 2007:133‐144. [Google Scholar]
Theodoulou 2004
- Theodoulou M, Hudis C. Cardiac profiles of liposomal anthracyclines. Cancer 2004;100(10):2052‐63. [DOI] [PubMed] [Google Scholar]
Therasse 2000
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumours: European Organisation for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;92:205‐16. [DOI] [PubMed] [Google Scholar]
von Moos 2008
- Moos R, Thuerlimann BJK, Aapro M, Rayson D, Harrold K, Sehouli J, et al. Pegylated liposomal doxorubicin‐associated hand‐foot syndrome: recommendations of an international panel of experts. European Journal of Cancer 2008;44:781‐90. [DOI] [PubMed] [Google Scholar]
Wagner 2012
- Wagner U, Marth C, Largillier R, Kaern J, Brown C, Heywood M, et al. Final overall survival results of phase III GCIG CALYPSO trial of pegylated liposomal doxorubicin and carboplatin vs paclitaxel and carboplatin in platinum‐sensitive ovarian cancer patients. British Journal of Cancer 2012;107(4):588‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zunino 2002
- Zunino F, Pratesi G. Antitumour Antibodies. In: Souhami RL, Tannock I, Hohenberger P, Horiot J‐C editor(s). Oxford Textbook of Oncology. 2nd Edition. Oxford: Oxford University Press, 2002:715‐27. [Google Scholar]


