Abstract
Background
Tension‐type headache (TTH) affects about 1 person in 5 worldwide. It is divided into infrequent episodic TTH (fewer than one headache per month), frequent episodic TTH (two to 14 headaches per month), and chronic TTH (15 headache days a month or more). Paracetamol (acetaminophen) is one of a number of analgesics suggested for acute treatment of headaches in frequent episodic TTH.
Objectives
To assess the efficacy and safety of paracetamol for the acute treatment of frequent episodic TTH in adults.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (CRSO), MEDLINE, EMBASE, and the Oxford Pain Relief Database to October 2015, and also reference lists of relevant published studies and reviews. We sought unpublished studies by asking personal contacts and searching online clinical trial registers and manufacturers' websites.
Selection criteria
We included randomised, double‐blind, placebo‐controlled studies (parallel‐group or cross‐over) using oral paracetamol for symptomatic relief of an acute episode of TTH. Studies had to be prospective, with participants aged 18 years or over, and include at least 10 participants per treatment arm.
Data collection and analysis
Two review authors independently assessed studies for inclusion and extracted data. We used the numbers of participants achieving each outcome to calculate the risk ratio (RR) and number needed to treat for one additional beneficial outcome (NNT) or one additional harmful outcome (NNH) for oral paracetamol compared to placebo or an active intervention for a range of outcomes, predominantly those recommended by the International Headache Society (IHS).
We assessed the evidence using GRADE (Grading of Recommendations Assessment, Development and Evaluation) and created 'Summary of findings' tables.
Main results
We included 23 studies, all of which enrolled adults with frequent episodic TTH. Twelve studies used the IHS diagnostic criteria or similar, six used the older classification of the Ad Hoc Committee, and five did not describe specific diagnostic criteria but generally excluded participants with migraines. Participants had moderate or severe pain at the start of treatment. While 8079 people with TTH participated in these studies, the numbers available for any analysis were lower than this because outcomes were inconsistently reported and because many participants received active comparators.
None of the included studies were at low risk of bias across all domains considered, although for most studies and domains this was likely to be due to inadequate reporting rather than poor methods. We judged five studies to be at high risk of bias for incomplete outcome reporting, and seven due to small size.
For the IHS preferred outcome of being pain free at two hours the NNT for paracetamol 1000 mg compared with placebo was 22 (95% confidence interval (CI) 15 to 40) in eight studies (5890 participants; high quality evidence), with no significant difference from placebo at one hour. The NNT was 10 (7.9 to 14) for pain‐free or mild pain at two hours in five studies (5238 participants; high quality evidence). The use of rescue medication was lower with paracetamol 1000 mg than with placebo, with an NNTp to prevent an event of 7.8 (6.0 to 11) in six studies (1856 participants; moderate quality evidence). On limited data, the efficacy of paracetamol 500 mg to 650 mg was not superior to placebo, and paracetamol 1000 mg was not different from either ketoprofen 25 mg or ibuprofen 400 mg (low quality evidence).
Adverse events were not different between paracetamol 1000 mg and placebo (RR 1.1 (0.94 to 1.3); 5605 participants; 11 studies; high quality evidence). Studies reported no serious adverse events.
The quality of the evidence using GRADE comparing paracetamol 1000 mg with placebo was moderate to high. Where evidence was downgraded it was because a minority of studies reported the outcome. For comparisons of paracetamol 500 mg to 650 mg with placebo, and of paracetamol 1000 mg with active comparators, we downgraded the evidence to low quality or very low quality because of the small number of studies and events.
Authors' conclusions
Paracetamol 1000 mg provided a small benefit in terms of being pain free at two hours for people with frequent episodic TTH who have an acute headache of moderate or severe intensity.
Plain language summary
Oral paracetamol for treatment of acute episodic tension‐type headache in adults
Bottom line
This review found that few people with two to 14 tension‐type headaches a month get good pain relief from taking paracetamol 1000 mg. There are questions about how studies of this type of headache are conducted. These questions involve the type of people chosen for the studies, and the types of outcomes reported. This limits the usefulness of the results, especially for people who just have an occasional headache.
Background
People with frequent episodic tension‐type headache have between two and 14 headaches every month. Tension‐type headache stops people concentrating and working properly, and results in much disability. When headaches do occur, they get better over time, even without treatment.
Paracetamol is a commonly used painkiller, available without prescription (over the counter) in most parts of the world. The usual dose is 1000 mg (usually two tablets) taken by mouth.
Study characteristics
In October 2015, we searched the medical literature and found 23 studies involving 8079 participants looking at paracetamol for frequent episodic tension‐type headache. About 6000 participants were involved in comparisons between paracetamol 1000 mg and placebo (a dummy tablet). Results were usually reported two hours after taking the medicine or placebo. The International Headache Society recommends the outcome of being pain free two hours after taking a medicine, but other outcomes are also suggested. Few studies reported pain free at two hours or other outcomes, so there was limited information to analyse for some outcomes.
Key results
The outcome of being pain free at two hours was reported by 24 in 100 people taking paracetamol 1000 mg, and in 19 out of 100 people taking placebo, meaning that only 5 in 100 people benefited because of paracetamol 1000 mg (high quality evidence). The outcome of being pain free or having only mild pain at two hours was reported by 59 in 100 people taking paracetamol 1000 mg, and in 49 out of 100 people taking placebo (high quality evidence), meaning that only 10 in 100 people benefited because of paracetamol 1000 mg.
About 10 in 100 people taking paracetamol 1000 mg reported having a side effect, which was the same as with placebo (9 in 100 people) (high quality evidence). Most side effects were mild or moderate in intensity. No side effects were serious.
We found a very small amount of information comparing paracetamol 500 mg or 650 mg with placebo, and comparing paracetamol 1000 mg with other painkillers. There was no difference between any of these treatments.
Quality of the evidence
The quality of the evidence was moderate or high for paracetamol 1000 mg compared with placebo, and low or very low for paracetamol 500 mg to 650 mg compared with placebo, and for paracetamol 1000 mg compared with other painkillers. High quality evidence means that we are very certain about the results. Low quality evidence means that we are very uncertain about the results.
Summary of findings
Summary of findings for the main comparison. Paracetamol 1000 mg compared with placebo for episodic tension‐type headache.
| Paracetamol 1000 mg compared with placebo for episodic tension‐type headache | ||||||
|
Patient or population: adults with episodic tension‐type headache Settings: community Intervention: paracetamol 1000 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with comparator | Probable outcome with intervention |
RR
(95% CI) NNT or NNH (95% CI) |
No. of studies, attacks, events | Quality of the evidence (GRADE) | Comments |
| Pain‐free at 2 hours | 190 in 1000 | 240 in 1000 | RR 1.3 (1.1 to 1.4) NNT 22 (15 to 40) |
8 studies 5890 attacks 1285 events |
High | Adequate numbers of studies and events Consistent direction of results |
| Pain‐free at 1 hour | 51 in 1000 | 60 in 1000 | RR 1.2 (0.90 to 1.5) NNT not calculated |
4 studies 4717 attacks 269 events |
Moderate | Downgraded because few studies reported, and modest number of events Some inconsistency in direction of response Dominated by 1 study |
| Pain‐free at 4 hours | 440 in 1000 | 560 in 1000 | RR 1.2 (1.16 to 1.3) NNT 8.2 (6.6 to 11) |
4 studies 4909 attacks 2577 events |
Moderate | Downgraded because few studies reported, but large number of events, tight CIs Consistent direction of results Dominated by 1 study |
| Use of rescue medication | 300 in 1000 | 170 in 1000 | RR 0.58 (0.50 to 0.69) NNTp 7.7 (6.0 to 11) |
6 studies 1856 attacks 422 events |
Moderate | Downgraded because few studies reported, and modest number of events Consistent direction of results |
| Pain‐free or mild pain at 2 hours | 490 in 1000 | 590 in 1000 | RR 1.2 (1.15 to 1.3) NNT 10 (7.9 to 14) |
5 studies 5238 attacks 2910 events |
High | Few studies reported, but large number of events, tight CIs 1 small study showed different direction of response |
| Any adverse event | 86 in 1000 | 100 in 1000 | RR 1.1 (0.94 to 1.3) NNH not calculated |
11 studies 5605 attacks 528 events |
High | Adequate numbers of studies and events Consistent direction of results (no effect) |
| Serious adverse events | No events reported | No events reported | ‐ | 15 studies, estimated 5147 participants in comparisons | Moderate | Downgraded because no events reported in 5147 comparisons Rate of serious adverse events unlikely to be > 1 in 1700 (Eypasch 1995) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTH: number needed to treat for one additional harmful outcome; NNT: number needed to treat for one additional beneficial outcome; NNTp: number needed to treat to prevent one harmful outcome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Summary of findings 2. Paracetamol 500 mg to 650 mg compared with placebo for episodic tension‐type headache.
| Paracetamol 500 mg to 650 mg compared with placebo for episodic tension‐type headache | ||||||
|
Patient or population: adults with episodic tension‐type headache Settings: community Intervention: paracetamol 500 mg to 650 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with comparator | Probable outcome with intervention |
RR
(95% CI) NNT or NNH (95% CI) |
No. of studies, attacks, events | Quality of the evidence (GRADE) | Comments |
| Pain‐free at 2 hours | No data | No data | ‐ | ‐ | ‐ | ‐ |
| Pain‐free at 1 hour | No data | No data | ‐ | ‐ | ‐ | ‐ |
| Pain‐free at 4 hours | No data | No data | ‐ | ‐ | ‐ | ‐ |
| Use of rescue medication | 370 in 1000 | 280 in 1000 | RR 0.76 (0.55 to 1.1) NNTp not calculated |
2 studies 301 attacks 99 events |
Low | Few studies and events Consistent direction of results (no effect) 1 study had high attrition |
| Pain‐free or mild pain at 2 hours | 530 in 1000 | 590 in 1000 | RR 1.1 (0.90 to 1.4) NNT not calculated |
2 studies 275 attacks 154 events |
Low | Few studies and events Consistent direction of results (no effect) |
| Any adverse event | 110 in 1000 | 140 in 1000 | RR 1.3 (0.71 to 2.5) NNH not calculated |
2 studies 301 attacks 38 events |
Low | Few studies and events Consistent direction of results (no effect) 1 study had high attrition |
| Serious adverse events | No events reported | No events reported | ‐ | 5 studies estimated 463 participants in comparisons |
Very low | 0 events reported in only 463 comparisons Rate of serious adverse events unlikely to be > 1 in 155 (Eypasch 1995) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNH: number needed to treat for one additional harmful outcome; NNT: number needed to treat for one additional beneficial outcome; NNTp: number needed to treat to prevent one harmful outcome; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
Background
This review is based on a template for reviews of drugs used for acute treatment of frequent episodic tension‐type headache (TTH) in adults. The aim is for all reviews to use the same methods.
Headaches are a commonly reported problem in community‐based surveys worldwide. The lifetime prevalence of headache is estimated to be greater than 90% (Steiner 2004), and the annual prevalence rate is estimated to be 46% in the general adult population (Stovner 2007). Variations in reported prevalence may result from differences in study design, population, inclusion or exclusion of cases of infrequent episodic TTH, overlap with probable migraine, cultural and environmental differences, or even genetic factors (Sahler 2012). TTH is more common than migraine, a finding replicated across the world (Oshinaike 2014; Vos 2012).
The management of people with headaches is largely neglected (Rasmussen 2001; Steiner 2011), and may be fragmented by the involvement of clinicians from different medical specialities (neurology; ear, nose and throat; ophthalmology; psychiatry). Because headache is rarely life‐threatening and headache pain is generally mild to moderate in intensity, people often self medicate and do not seek formal care from health services (Rasmussen 2001).
Headache can be either primary (no underlying cause) or secondary (due to other systemic or local causes) (Green 2009). TTH belongs to the group of primary headaches and is seen in nearly one‐third of people experiencing headaches; the large number of people affected imposes a significant burden on the healthcare system (Stovner 2007). Generally, episodes of TTH are mild to moderate in intensity, and self limiting, but in a small group of people they may be more severe and disabling (Green 2009). People with longer lasting or more severe headaches may seek help in a clinical setting, but the majority of people do not do so, resulting often in inadequate and inappropriate management (Kernick 2008). In one community‐based telephone survey to determine medication patterns of 274 people with frequent headache, only 1% used prescription medication. The majority reported using over‐the‐counter (OTC) analgesics (paracetamol (acetaminophen): 56% and aspirin: 15%), and the perceived effectiveness of OTC medication was approximately 7 on a scale of 0 to 10 (Forward 1998). There is a greater propensity to develop analgesic abuse among people who self medicate with OTC preparations, particularly those with frequent TTH. This calls for developing treatment and management guidelines that bring about substantial and sustained pain relief with minimal adverse effects.
Professional strategies for the management of TTH have typically been extrapolated from those used for migraine; the World Health Organization (WHO) essential drug list, for example, does not include indications for the management of TTH (WHO 2015). In 2010, the British Association for the Study of Headache (BASH) and the European Federation of Neurological Societies (EFNS) updated or published guidelines for the management of TTH (BASH 2010; Bendtsen 2010); there is also German and Austrian guidance (Haag 2011). The guidelines reflect ongoing systematic efforts to bridge the gap between clinical trial evidence and clinical practice with the aim of improving practice. While these guidelines represent a step forward, there are, nonetheless, issues relating to the quality and methodological limitations of individual studies.
People with TTH and migraine have more work absence than people without headaches (Lyngberg 2005); there is also considerable loss of productivity (Cristofolini 2008; Pop 2002). Headache‐related characteristics include significant problems with headache management, disability, pain, worry, and dissatisfaction with care, as well as greater use of medical services and worse general health (Harpole 2005).
Description of the condition
TTH has been known by several names, including tension headache, muscle contraction headache, psychomyogenic headache, stress headache, ordinary headache, essential headache, idiopathic headache, and psychogenic headache (IHS 2004). TTH is diagnosed mainly by the absence of features found in other headache types, especially migraine. The third edition of the International Classification of Headache Disorders (ICHD‐3 beta) distinguishes between episodic and chronic varieties of TTH (IHS 2013). Chronic TTH is diagnosed when headache occurs on 15 days or more per month on average for three months or more (180 or more days per year); otherwise TTH is considered to be episodic.
Acute treatment with analgesics is more appropriate for episodic TTH, while both pharmacological and non‐pharmacological treatments are used for managing chronic TTH. Structural changes in the brain have been reported in people with chronic TTH (Fumal 2008). Furthermore, management of TTH in children and adolescents raises diverse clinical issues (establishing diagnoses, dosages, nature of preparation, pharmacodynamics, etc; Monteith 2010). For all of these reasons, the proposed review will focus on the acute treatment of episodic TTH in adults.
Diagnosis
Episodic TTH is subdivided into infrequent and frequent types (IHS 2013).
Infrequent episodic TTH is diagnosed by the following criteria.
At least 10 episodes occurring on fewer than one day per month (fewer than 12 days per year) and satisfying criteria 2 through 4.
Headache lasting from 30 minutes to seven days.
-
Headache has at least two of the following characteristics:
bilateral location;
pressing/tightening (non‐pulsating) quality;
mild or moderate intensity;
not aggravated by routine physical activity such as walking or climbing stairs.
-
Both of the following:
no nausea or vomiting (anorexia may occur);
no more than one of photophobia or phonophobia.
Not attributed to another disorder.
Frequent episodic TTH is defined when at least 10 episodes of headache occur on at least one day but fewer than 15 days per month for at least three months (at least 12 and fewer than 180 days per year), and when criteria 2 to 5, above, are also met.
Prevalence
The Global Burden of Diseases Study 2010 found global prevalence of TTH as 21%, making it the second most prevalent condition after dental caries, and slightly more prevalent than migraine (Vos 2012).
In previous studies, the one‐year prevalence of infrequent episodic TTH in one Danish study of 4000 people aged 40 years was 48.2%, while that of frequent episodic TTH was 34% (Russell 2005). Overall annual prevalence of TTH in the US was estimated to be 38%, with a higher incidence among women (prevalence ratio of 1:1.2; Schwartz 1998). In Canada, the estimated prevalence was 29% (Edmeads 1993). One study conducted in Chile reported that TTH constituted 72% of all recurrent headaches, with a total prevalence of 27% (95% confidence interval (CI) 25% to 29%). Nearly 1 in 4 (24%) participants had episodic TTH, and prevalence was greater among women when compared to men (35% for women versus 18% for men; Lavados 1998).
Causation
The exact pathogenesis of TTH is still unknown and is said to be multifactorial, including central dysfunction of pain processing pathways and peripheral myofascial factors. There is a general agreement that peripheral myofascial nociception disturbances play a greater role in the pathogenesis of both frequent and infrequent episodic TTH (Fernández‐de‐las‐Peñas 2010; Fumal 2008).
Description of the intervention
Paracetamol (acetaminophen) was first identified as the active metabolite of two older antipyretic drugs, acetanilide and phenacetin, in the late nineteenth century. It became available in the UK on prescription in 1956, and without prescription (OTC) in 1963 (PIC 2015). Since then, it has become one of the most popular antipyretic and analgesic drugs worldwide, and is often also used in combination with other drugs. OTC medications are less expensive, more accessible, and have favourable safety profiles relative to many prescription treatments.
Despite a low incidence of adverse effects, paracetamol has a recognised potential for hepatotoxicity and is thought to be responsible for approximately half of all cases of liver failure in the UK (Hawton 2001), and about 40% in the US (Norris 2008). One study evaluating all cases of acute liver failure leading to registration for transplantation (ALFT) across seven European countries for a three‐year period showed that paracetamol overdose was responsible for one sixth of cases of ALFT; however, this varied considerably between each country (Gulmez 2015). Acute paracetamol hepatotoxicity at therapeutic doses has been judged to be extremely unlikely, despite reports of so‐called 'therapeutic misadventure' (Prescott 2000). It has been observed that non‐overdose ALFT is more likely to follow therapeutic‐dose paracetamol exposure than similar nonsteroidal anti‐inflammatory drug (NSAID) exposure (Gulmez 2013). Legislative changes were introduced in the UK to restrict pack sizes and the maximum number of tablets permitted in OTC sales (CSM 1997) on the basis of evidence that poisoning was lower in countries that restrict availability (Gunnell 1997; Hawton 2001). The contribution of these changes, which are inconvenient and costly (particularly to people with chronic pain), to any observed reductions in incidence of liver failure or death, remains uncertain (Bateman 2014a; Bateman 2014b; Hawkins 2007; Hawton 2013). There have been concerns over the safety of paracetamol in people with compromised hepatic function (people with severe alcoholism, cirrhosis, or hepatitis), but these have not been substantiated (Dart 2000; PIC 2015).
The use of paracetamol during pregnancy has been questioned following reports that it is linked to behavioural problems and hyperkinetic disorders in children whose mothers took it during pregnancy (Liew 2014), and suggestions that it can interfere with sex hormones (Mazaud‐Guittot 2013).
In one analysis of single dose studies in migraine, there was no evidence that adverse events were more common with paracetamol 1000 mg than with placebo, and no serious adverse events occurred with paracetamol alone (Derry 2013).
Oral paracetamol has long been used as a first‐line analgesic for a variety of acute and chronic conditions, although some systematic reviews and meta‐analyses have suggested that there is no good evidence for a clinically relevant benefit of paracetamol (as monotherapy) in many of these pain conditions (Machado 2015; Moore 2014a). The benefit risk of paracetamol has been called into question, especially in view of limited or absent evidence of efficacy, and growing evidence about risk (Moore 2016).
How the intervention might work
The lack of significant anti‐inflammatory activity of paracetamol implies a mode of action distinct from that of NSAIDs; yet, despite years of use and research, the mechanisms of action of paracetamol are not fully understood. NSAIDs act by inhibiting the activity of cyclooxygenase (COX), now recognised to consist of two isoforms (COX‐1 and COX‐2), which catalyses the production of prostaglandins responsible for pain and inflammation. Paracetamol has previously been shown to have no significant effects on COX‐1 or COX‐2 (Schwab 2003), but is now being considered as a selective COX‐2 inhibitor (Hinz 2008). Significant paracetamol‐induced inhibition of prostaglandin production has been demonstrated in tissues in the brain, spleen, and lung (Botting 2000; Flower 1972). A 'COX‐3 hypothesis', wherein the efficacy of paracetamol is attributed to its specific inhibition of a third COX isoform enzyme, COX‐3 (Botting 2000; Chandrasekharan 2002), has little credibility, and a central mode action of paracetamol is thought to be likely (Graham 2005).
Why it is important to do this review
Episodic TTH is ubiquitous, affecting a large proportion of adults. Despite being generally mild to moderate in intensity, headache results in considerable suffering to the affected person and contributes overall to a significant loss of productivity to society (Mannix 2001; Rasmussen 2001; Steiner 2004; Stovner 2007). Seeking relief, people generally self medicate with one or more medicines, and OTC medicines are often used (Forward 1998). Paracetamol is a readily accessible OTC analgesic. As a generic drug, paracetamol could be a drug of choice for management of TTH, particularly in low‐resource settings. It has some efficacy in individual studies and one systematic review (Moore 2014b; Schachtel 1996).
Two guidelines on the management of TTH have reviewed the effectiveness of different treatments, both using a consensus methodology because the amount of randomised trial evidence is limited (Moore 2014b). The BASH guidelines are based on a limited review of studies (BASH 2010), and the EFNS guidelines are based on a more detailed and thorough literature search (Bendtsen 2010). The EFNS guidelines represent an improvement over the BASH guidelines in that they used a standard published protocol for developing management guidelines (Brainin 2004). That protocol strongly recommends active and frequent consultation of The Cochrane Library. However, there were no published Cochrane reviews on the acute management of frequent episodic TTH until the publication of a review of ibuprofen for this indication (Derry 2015). One non‐Cochrane systematic review by Verhagen and others followed methods similar to those used in Cochrane reviews and evaluated the efficacy and tolerability of analgesics for the acute treatment of episodes of TTH in adults (Verhagen 2006), but the authors analysed a non‐standard outcome of "pain relief or recovery over 2 to 6 hours" as the main efficacy outcome.
Reviews explicitly adopting Cochrane methods and evaluating the more focused outcomes recommended in the updated guidelines of the IHS for controlled trials of drugs in TTH are clearly important (IHS 2010). One survey of TTH study methods and reporting demonstrated that these are seldom adhered to in clinical trials, and studies of aspirin, ibuprofen, ketoprofen, and paracetamol used a variety of outcomes including, occasionally, IHS‐preferred outcomes (Moore 2014b).
Objectives
To assess the efficacy and safety of paracetamol for the acute treatment of frequent episodic TTH in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, double‐blind, placebo‐controlled studies (parallel‐group or cross‐over) in any setting using paracetamol for symptomatic relief of an acute episode of TTH. Studies had to be prospective. We accepted studies reporting treatment of consecutive headache episodes if they reported outcomes for the first, or each, episode separately. We included trials regardless of publication status or language of publication. We included studies conducted in any setting (home, clinic, doctor's surgery, community centre, etc) as long as it was clear that treatment was for an acute episode of TTH.
Cross‐over studies are well‐suited to study acute episodic TTH and eliminate within‐person variation; however, they pose challenges during analysis related to drop‐outs, inadequate reporting (reporting only the first period), and inappropriate reporting (reporting as parallel‐group trials instead of paired observations). We included cross‐over trials only if there was adequate washout (at least 48 hours) between treatments and after ascertaining that the participants were adequately randomised to the first treatment period.
We excluded trials using alternation, date of birth, hospital record number, or other 'quasi‐random' methods of allocation of treatment.
Types of participants
Study participants were adults (at least 18 years of age) with frequent episodic TTH. We excluded studies involving participants with chronic TTH.
The diagnosis of episodic TTH ideally conformed to IHS criteria (IHS 2013). We considered other definitions if they conformed in general to IHS diagnostic criteria and reasonably distinguished TTH from other headache types by specifying distinctive features of TTH; for example, absence of nausea or vomiting, mild to moderate head pain, character and location of pain, absence of obvious phonophobia or photophobia and aura, and differentiated from chronic daily headache.
We analysed data only for people with acute TTH episodes. Studies including people with 'mixed' migraine and TTH or 'combination' headaches would have posed problems, as these terms may refer to people with discrete episodes of migraine and discrete episodes of TTH, or to people with headaches that (in the view of the investigators) combined features of migraine and TTH. The IHS criteria assign a dual diagnosis of migraine and TTH or 'probable migraine', respectively, to such people. Where participants experienced both migraine and TTH, they were required to be able to distinguish between them and to treat only TTH. We excluded secondary headache disorders using criteria based on ICHD (IHS 2013).
Types of interventions
Included studies had to have at least one arm in which paracetamol was given orally for acute treatment of an episode of TTH. There was no restriction on dose. Included studies could use either a single dose to treat a discrete headache episode or investigate different dosing strategies. We looked primarily for studies using paracetamol alone, but also for studies that used paracetamol in combination with another active oral treatment.
A placebo comparator is essential to demonstrate that paracetamol is effective in this condition. The placebo used had to be identical to paracetamol in appearance (size, colour, etc) and the number of tablets, or a double‐dummy technique should be used. All the active‐controlled trials also included a placebo treatment arm.
Types of outcome measures
Primary and secondary outcomes selected for analysis reflected the most recent guidelines for controlled trials of drugs in TTH issued by the IHS (IHS 2010).
Primary outcomes
Pain‐free rate at two hours using any standard method of pain assessment and without the use of rescue medication.
Secondary outcomes
Pain‐free rate at different time points, without the use of rescue medication. We used one hour, four hours, and 24 hours as clinically important endpoints and analysed them separately.
Pain Intensity Difference (PID) and Sum of Pain Intensity Difference (SPID), at two hours, without the use of rescue medication.
Pain‐free or mild pain at two hours (equivalent to headache response in migraine trials); this is an outcome regarded as useful by most people with acute or chronic pain (Moore 2013).
Use of rescue medication. When participants use rescue medication they are considered to have withdrawn from the study because of a lack of efficacy.
Adverse events: number of participants with any adverse event, identity and rates (if data permitted) of specific adverse events, serious adverse events, and number of withdrawals due to adverse events.
Search methods for identification of studies
Electronic searches
We searched the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL) (via CRSO) on 14 October 2015.
MEDLINE (via Ovid, 1946 to 14 October 2015).
EMBASE (via Ovid, 1974 to 14 October 2015).
Oxford Pain Relief Database (Jadad 1996a) on 14 October 2015.
Appendix 1 shows the search strategy for CENTRAL, Appendix 2 for MEDLINE, and Appendix 3 for EMBASE.
Searching other resources
We searched the International Clinical Trials Registry Platform (apps.who.int/trialsearch/) and ClinicalTrials.gov (ClinicalTrials.gov) for completed or ongoing trials using the key words 'headache' or 'cephalalgia' or their variations (using wildcards). We also examined web‐based clinical trials registries of relevant manufacturers and drug companies including GlaxoSmithKline, Novartis, Bayer, and Reckitt Benckiser.
We searched the reference lists of all eligible trials and previous systematic reviews for additional studies, and asked personal contacts for information about any unpublished and ongoing studies known to them.
Data collection and analysis
Selection of studies
Two review authors independently reviewed the titles and abstracts of all studies identified through searching to exclude any that clearly did not satisfy inclusion criteria, and read full copies of the remaining studies to identify those suitable for inclusion. We resolved disagreements by discussion or by referral to a third review author for independent review and a final decision.
Data extraction and management
We adapted the Cochrane Pain, Palliative and Supportive Care Review Group's (PaPaS) data extraction form to suit the requirements of this review. Two review authors independently extracted data from each study using the form. We resolved disagreements and uncertainties by discussion. It was not necessary to involve a third review author. One review author entered data into Review Manager 5 (RevMan 2014).
Assessment of risk of bias in included studies
We used the Oxford Quality Score (Jadad 1996b) as the basis for inclusion, limiting inclusion to studies that were randomised and double‐blind as a minimum. We reported the scores for each study in the Characteristics of included studies table.
Two review authors independently assessed the risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions, Chapter 8 (Higgins 2011), and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions before assignment determines whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We assessed the methods as: low risk of bias (telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (open list).
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received as: low risk of bias (study stated that it was blinded and described the method used to achieve blinding, identical tablets, matched in appearance and smell); unclear risk of bias (study stated that it was blinded but did not provide an adequate description of how it was achieved). We excluded studies that were not double‐blind.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (fewer than 10% of participants did not complete the study or the study used 'baseline observation carried forward' analysis, or both); unclear risk of bias (used 'last observation carried forward' analysis); high risk of bias (used 'completer' analysis).
Size of study (checking for possible biases confounded by small size). Small studies have been shown to overestimate treatment effects, probably because the conduct of small studies is more likely to be less rigorous, allowing critical criteria to be compromised (Dechartres 2013; Nüesch 2010). We assessed studies as being at low risk of bias (200 participants or greater per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (fewer than 50 participants per treatment arm).
Measures of treatment effect
We used risk ratio (RR) to establish statistical difference, and the numbers needed to treat for an additional beneficial outcome (NNT) and pooled percentages as absolute measures of benefit or harm.
We used the following terms to describe adverse outcomes in terms of harm or prevention of harm.
When significantly fewer adverse outcomes occurred with paracetamol than with control (placebo or active), we used the term the number needed to treat to prevent one event (NNTp).
When significantly more adverse outcomes occurred with paracetamol compared with control (placebo or active), we used the term the number needed to treat for an additional harmful outcome or to cause one event (NNH).
We have reported continuous data as the mean difference, with 95% confidence intervals (CIs), where appropriate. As anticipated, we did not carry out any analysis of continuous data.
Unit of analysis issues
The unit of analysis was the individual participant.
Dealing with missing data
The most likely source of missing data was expected to be cross‐over studies; we planned to use only first‐period data where possible, but where those data were not provided, we treated the results as if they were parallel group results, and commented on this.
For all outcomes, we carried out analyses, as far as possible, on a modified intention‐to‐treat basis in which we included all participants who were randomised and received an intervention. Where studies reported sufficient information, we re‐included missing data in the analyses undertaken. We noted where there were substantial amounts of missing data in any study, and planned to perform sensitivity analyses to investigate their effect in any analyses.
Assessment of heterogeneity
We assessed heterogeneity of response rates using L'Abbé plots, a visual method for assessing differences in results of individual studies (L'Abbé 1987). Where we pooled data, we reported the I2 statistic.
Assessment of reporting biases
We planned to assess publication bias by examining the number of participants in trials with zero effect (RR = 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level (Moore 2008). In this case, we specified a clinically useful level as an NNT of 10 or greater for the outcome 'pain‐free at two hours', and NNT of 8 or greater for 'pain‐free or mild pain at two hours'. In the event, the NNTs were higher than these pre‐specified levels, so this was not possible.
Data synthesis
We planned to analyse studies using a single dose of paracetamol in established pain of at least moderate intensity separately from studies in which medication was taken before pain was well established, or in which a second dose of medication was permitted. In the event, all the studies treated established headaches and almost all reported a mean baseline pain of moderate intensity. None specifically treated early, or when pain was mild. Only one study allowed a second dose of study medication, and that study did not contribute data for analysis.
We carried out all analyses according to dose (1000 mg or 500 mg to 650 mg) and compared paracetamol with placebo or an active comparator. We combined data for analysis only for comparisons and outcomes where there were at least two studies and 200 participants (Moore 1998). We calculated the RRs for benefit or harm with 95% CIs using a fixed‐effect model (Morris 1995). We calculated NNT, NNTp, and NNH with 95% CIs using the pooled number of events by the method of Cook and Sackett (Cook 1995). We assumed a statistically significant difference from control when the 95% CI of the RR for benefit or harm included the number one.
We used the Z test to determine significant differences between the two doses of paracetamol (Tramèr 1997).
We have described data from comparisons and outcomes with only one study or fewer than 200 participants in the text and summary tables where appropriate for information and comparison, but did not analyse them quantitatively.
Quality of the evidence
Two review authors independently rated the quality of each outcome. We used the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system to assess the quality of the evidence related to the key outcomes listed in Types of outcome measures (Appendix 4; Chapter 12, Higgins 2011).
'Summary of findings' tables
We included 'Summary of findings' tables to present the main findings in a transparent and simple tabular format. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the outcomes of pain‐free at two hours, pain‐free at one and four hours, pain‐free or mild pain at two hours, participants with any adverse event, and participants with serious adverse events.
Subgroup analysis and investigation of heterogeneity
Possible issues for subgroup analysis were dose, formulation, and route of administration. A minimum of two studies and 200 participants had to be available for any subgroup analysis.
Sensitivity analysis
We planned sensitivity analysis for study quality (Oxford Quality Score of 2 versus 3 or more). A minimum of two studies and 200 participants had to be available for any sensitivity analysis.
Results
Description of studies
Results of the search
Our searches identified 532 potentially relevant reports in CENTRAL, 540 in MEDLINE, 1748 in EMBASE, and three in clinical trial registries. After removing duplicates and screening titles and abstracts, we obtained and read 27 full reports. Of these, we included 23 of these reports (Dahlöf 1996; Diener 2014; Friedman 1987; Gatoulis 2012; Gilbert 1976; Göbel 1996; Göbel 1998; Göbel 2001; Mehlisch 1998; Migliardi 1994; Miller 1987; NCT01755702; NL9701; Packman 2000; Peters 1983; Pini 2008; Prior 2002; Schachtel 1991; Schachtel 1996; Steiner 1998; Steiner 2003; Thorpe 1970; Ward 1991) and excluded four (de Souza Carvalho 2012; Diener 2005; NCT01552798; Wójcicki 1977). In addition, RB provided the clinical trial report for one unpublished study that satisfied our inclusion criteria (NL9701) (Figure 1).
1.
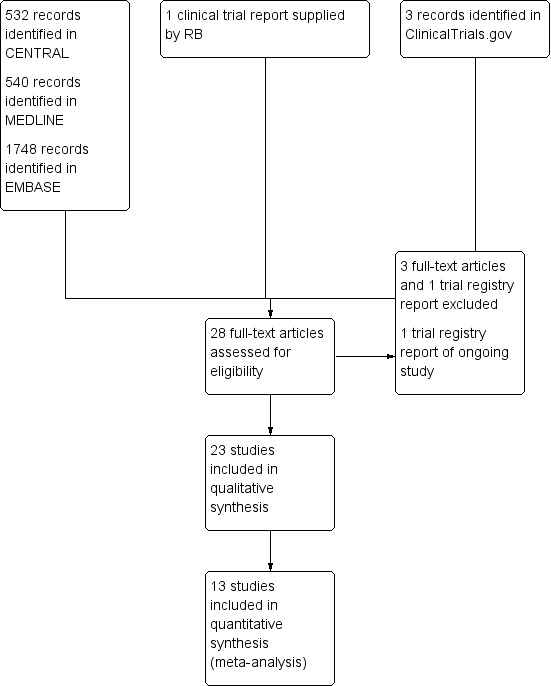
Study flow diagram.
One ongoing study is testing paracetamol 1000 mg plus caffeine 130 mg compared with placebo and ibuprofen 400 mg (NCT01842633) (see Characteristics of ongoing studies).
Included studies
One article reported on six individual studies with similar methods, but combined the results (Migliardi 1994). Another article subsequently reported combined results for four of these studies, providing additional efficacy data (Diener 2014). For the purposes of this review, we refer to studies 1 to 4 as Diener 2014 and studies 5 and 6 as Migliardi 1994, and count them as two included studies. We included 23 studies (8079 participants; 7701 in efficacy analyses), all of which enrolled adult participants with frequent episodic TTH (see Characteristics of included studies table).
Eleven studies specified using the IHS diagnostic criteria (Dahlöf 1996; Gatoulis 2012; Göbel 1996; Göbel 1998; Mehlisch 1998; NL9701; Packman 2000; Pini 2008; Prior 2002; Steiner 1998; Steiner 2003), and one other reported criteria in line with those of the IHS (NCT01755702). Six studies used the older classification of the Ad Hoc Committee (Ad Hoc Committee 1962), (Diener 2014; Friedman 1987; Migliardi 1994; Miller 1987; Schachtel 1991; Schachtel 1996). Five studies did not describe specific diagnostic criteria (Gilbert 1976; Göbel 2001; Peters 1983; Thorpe 1970; Ward 1991). Of these, the investigators of one study (Göbel 2001) had previously described using IHS criteria in other studies. Two studies described excluding headaches of other origin including migraine (Peters 1983; Ward 1991), and one study described a typical TTH history without other causes (Thorpe 1970). We included these six studies in the review, with the intention to carry out sensitivity analyses if any of them contributed to analyses.
All studies reported mean baseline pain of at least moderate intensity, except one in which it was not reported (Thorpe 1970). None of the studies reported the average headache frequency of participants.
Ten studies used a cross‐over design (Dahlöf 1996; Diener 2014; Gilbert 1976; Göbel 1996; Göbel 1998; Göbel 2001; Migliardi 1994; NCT01755702; Pini 2008; Ward 1991), and 13 used a parallel‐group design (Friedman 1987; Gatoulis 2012; Mehlisch 1998; Miller 1987; NL9701; Packman 2000; Peters 1983; Prior 2002; Schachtel 1991; Schachtel 1996; Steiner 1998; Steiner 2003; Thorpe 1970). All but one study used a single dose of medication to treat a discrete headache episode. The one exception to this was Thorpe 1970, which permitted the use of a second dose. None of the cross‐over studies reported first period data separately. In most of the studies, participants treated a single episode with any one intervention, but in Diener 2014 and Migliardi 1994 participants treated two episodes with each intervention. Four cross‐over studies specified a washout of at least 48 hours between doses (Dahlöf 1996; Diener 2014; Gilbert 1976; Migliardi 1994), whereas the others did not specify periods between treatments. We included these six other studies in the review, with the intention to carry out sensitivity analyses if any of them contributed to analyses (Göbel 1996; Göbel 1998; Göbel 2001; NCT01755702; Pini 2008; Ward 1991).
The studies did not consistently report the outcomes of interest. Eight studies reported pain‐free at two hours, four studies reported pain‐free at one hour, and three studies reported pain‐free at four hours. Five studies reported pain‐free or mild pain at two hours (including "total or worthwhile effect at two hours"), and 15 studies reported some measure of PID. Seventeen studies reported on adverse events, and eight studies reported on the use of rescue medication.
Sixteen studies used paracetamol 1000 mg (Dahlöf 1996; Diener 2014; Göbel 1996; Göbel 1998; Göbel 2001; Mehlisch 1998; Migliardi 1994; NCT01755702; NL9701; Packman 2000; Peters 1983; Prior 2002; Schachtel 1991; Schachtel 1996; Steiner 1998; Steiner 2003). Two studies used paracetamol 500 mg (Dahlöf 1996; Steiner 2003). Two studies used paracetamol 650 mg (Gilbert 1976; Miller 1987). One used paracetamol 648 mg (Ward 1991). Nine studies used paracetamol given in combination with other medications. One study used paracetamol 600 mg plus codeine 60 mg (Friedman 1987). One study used paracetamol 300 mg plus codeine 30 mg (Gatoulis 2012). One study used paracetamol 650 mg plus phenyltoloxamine citrate 60 mg (Percogesic) (Gilbert 1976). Two studies used paracetamol 1000 mg plus peppermint oil 10 mg (Göbel 1996; Göbel 2001). One study used paracetamol 500 mg plus aspirin 500 mg plus caffeine 130 mg (Diener 2014). Three studies used paracetamol 1000 mg plus caffeine 130 mg (Migliardi 1994; NCT01755702; Pini 2008). One study used both paracetamol 648 mg plus caffeine 65 mg, and paracetamol 648 mg plus caffeine 130 mg (Ward 1991). One study used paracetamol 650 mg plus butalbital 100 mg plus caffeine 80 mg (Fioricet) (Friedman 1987). One used paracetamol plus aspirin plus caffeine plus isobutylallylbarbituric acid; Fiorinal‐Pa) (Thorpe 1970).
Active comparators were:
ketoprofen 12.5 mg, 25 mg, and 50 mg (Dahlöf 1996; Mehlisch 1998; Steiner 1998);
phenyltoloxamine 60 mg (Gilbert 1976);
peppermint oil 10 g (Göbel 1996; Göbel 1998; Göbel 2001);
aspirin 500 mg (Steiner 2003);
aspirin 650 mg (Peters 1983);
aspirin 1000 mg (Gatoulis 2012; Steiner 2003);
aspirin 1000 mg plus caffeine 64 mg (Schachtel 1991);
naproxen 375 mg (Prior 2002);
naproxen sodium 550 mg (Miller 1987; Pini 2008);
ibuprofen 400 mg (NCT01755702; Packman 2000; Schachtel 1996), presumably as ibuprofen acid.
The total number of participants in the 23 studies was 7164, with 5141 in 13 parallel‐group studies and 2023 in 10 cross‐over studies (though some of these participants contributed more than one headache episode). Because outcomes were inconsistently reported and because many participants received active comparators, the number of participants with data for analyses for paracetamol was therefore much smaller than the total number.
Excluded studies
We excluded four studies (see Characteristics of excluded studies table). Two studies treated participants with either TTHs or migraine headaches, and did not report results separately (de Souza Carvalho 2012; Diener 2005). One study treated "common idiopathic headache", which we considered insufficiently classified, and it was not clearly randomised (Wójcicki 1977). The fourth study was terminated early, with only nine participants enrolled, and had no results (NCT01552798).
Risk of bias in included studies
Figure 2 presents a summary of the risk of bias assessment.
2.
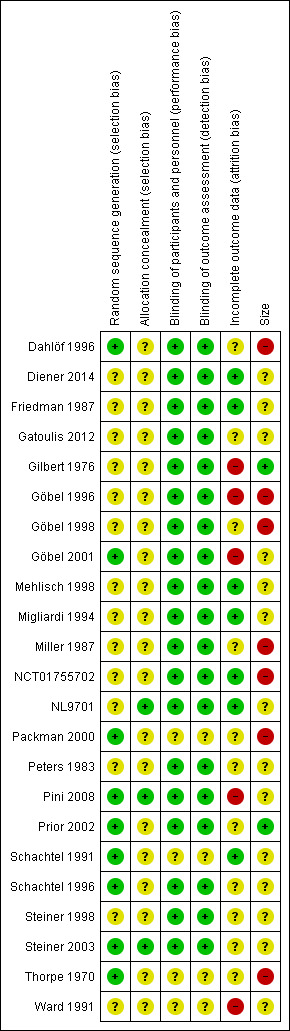
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
All studies were randomised, but only eight adequately described the methods used to generate the random sequence (Dahlöf 1996; Packman 2000; Pini 2008; Prior 2002; Schachtel 1991; Schachtel 1996; Steiner 2003; Thorpe 1970). Three studies adequately described the method used to conceal the random allocation (NL9701; Pini 2008; Steiner 2003).
Blinding
All studies were double blind, and 19 adequately described the methods used to conceal the intervention from participants and personnel (Dahlöf 1996; Diener 2014; Friedman 1987; Gatoulis 2012; Gilbert 1976; Göbel 1996; Göbel 1998; Göbel 2001; Mehlisch 1998; Migliardi 1994; Miller 1987; NCT01755702; NL9701; Peters 1983; Pini 2008; Prior 2002; Schachtel 1996; Steiner 1998; Steiner 2003).
Incomplete outcome data
Seven studies convincingly accounted for all participants in the primary outcome (Diener 2014; Friedman 1987; Mehlisch 1998; Migliardi 1994; NCT01755702; NL9701; Schachtel 1991). Three studies were at high risk of bias due to their use of completer analysis (Göbel 1998; Göbel 2001; Ward 1991). We judged the remaining studies to be at unclear risk of bias due to a lack of information.
Other potential sources of bias
Two studies enrolled 200 or more participants per treatment arm (low risk of bias; Gilbert 1976; Prior 2002). Seven studies all included at least one treatment arm with fewer than 50 participants (high risk of bias: Dahlöf 1996; Göbel 1996; Göbel 1998; Miller 1987; NCT01755702; Packman 2000; Thorpe 1970). The remaining 14 studies had a minimum of between 50 and 199 per treatment arm (unclear risk of bias; Diener 2014 (in individual studies); Friedman 1987; Gatoulis 2012; Göbel 2001; Mehlisch 1998; Migliardi 1994 (in individual studies); NL9701; Peters 1983; Pini 2008; Schachtel 1991; Schachtel 1996; Steiner 1998; Steiner 2003; Ward 1991).
Effects of interventions
Appendix 5 (efficacy) and Appendix 6 (adverse events and withdrawals) show the results for individual studies. A summary of results for comparisons of paracetamol with placebo is presented in a summary table at the end of this section.
Paracetamol 1000 mg versus placebo
Sixteen studies compared paracetamol 1000 mg with placebo.
Pain‐free at two hours
Eight studies (5890 attacks or participants) contributed data for pain‐free at two hours (Dahlöf 1996; Diener 2014; NCT01755702; NL9701; Prior 2002; Schachtel 1991; Schachtel 1996; Steiner 1998).
The proportion of attacks/participants who were pain‐free at two hours with paracetamol was 24% (867/3681, range 8.6% to 56%).
The proportion of attacks/participants who were pain‐free at two hours with placebo was 19% (418/2209, range 1.3% to 53%).
The RR for paracetamol 1000 mg compared with placebo was 1.3 (95% CI 1.1 to 1.4); the NNT was 22 (15 to 40) (Figure 3).
3.
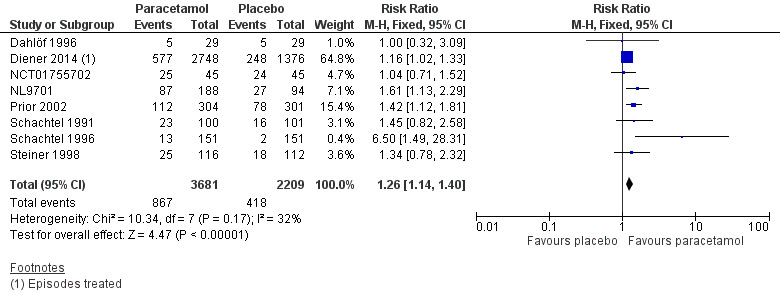
Forest plot of comparison: 1 Paracetamol 1000 mg versus placebo, outcome: 1.1 Pain‐free at 2 hours.
We assessed the evidence for this outcome to be of high quality according to GRADE because there were adequate numbers of studies and events and the direction of results was consistent.
Pain‐free at one hour
Four studies (4717 attacks or participants) contributed data for pain‐free at one hour (Diener 2014; NCT01755702; Schachtel 1991; Schachtel 1996).
The proportion of attacks/participants who were pain‐free or had only mild pain at one hour with paracetamol was 6.0% (183/3044; range 0% to 11%).
The proportion of attacks/participants who were pain‐free or had only mild pain at one hour with placebo was 5.1% (86/1673; range 0% to 16%).
The RR for paracetamol 1000 mg compared with placebo was 1.2 (95% CI 0.90 to 1.5); the NNT was not calculated (Analysis 1.2).
1.2. Analysis.
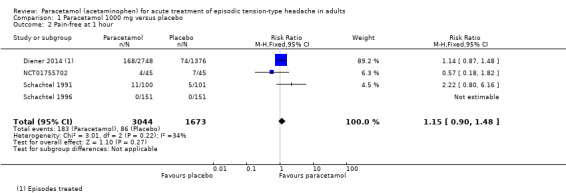
Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 2 Pain‐free at 1 hour.
We downgraded the evidence for this outcome from high to moderate quality because few studies reported it and there was a modest number of events. There was some inconsistency in the direction of response, and the results were dominated by one study.
Pain‐free at four hours
Four studies (4909 attacks or participants) contributed data for pain‐free at four hours (Diener 2014; NL9701; Schachtel 1991; Schachtel 1996).
The proportion of attacks/participants who were pain‐free at four hours with paracetamol was 57% (1810/3187, range 34% to 77%).
The proportion of attacks/participants who were pain‐free at four hours with placebo was 45% (767/1722, range 7.3% to 59%).
The RR for paracetamol 1000 mg compared with placebo was 1.2 (95% CI 1.16 to 1.3); the NNT was 8.2 (6.6 to 11) (Analysis 1.3).
1.3. Analysis.
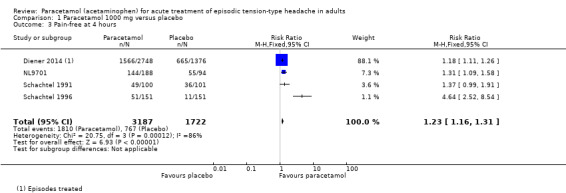
Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 3 Pain‐free at 4 hours.
We downgraded the evidence for this outcome from high to moderate quality because few studies reported it and, although there was a large number of events, the direction of results was consistent, and the confidence intervals were tight, the analysis was dominated by one study (Diener 2014, 84% of participants) and the I2 statistic was 86%. One of the smaller studies had a particularly low placebo event rate, which probably accounts for the high I2 statistic. It had almost identical inclusion criteria and methods to the other small study, and the low placebo event rate could well be due to random chance, given its small size.
Pain‐free at 24 hours
No studies reported pain‐free at 24 hours.
Pain intensity difference at two hours
Eleven studies reported some measure of PID, but no analysis was possible because they used different scales and recorded at different time points. Eight studies reported a statistically significant difference between paracetamol and placebo (Göbel 1996; Migliardi 1994; NL9701; Prior 2002; Schachtel 1991; Schachtel 1996; Steiner 1998; Steiner 2003), and three no significant difference (Dahlöf 1996; Mehlisch 1998; NCT01755702). None commented on the clinical significance of the findings.
Pain‐free or mild pain at two hours
Five studies (5238 attacks or participants) contributed data for pain‐free or mild pain at two hours (Dahlöf 1996; Diener 2014; Prior 2002; Steiner 1998; Steiner 2003).
The proportion of attacks/participants who were pain‐free or had only mild pain at two hours with paracetamol was 59% (1958/3308, range 38% to 71%).
The proportion of attacks/participants who were pain‐free or had only mild pain at two hours with placebo was 49% (952/1930, range 36% to 55%).
The RR for paracetamol 1000 mg compared with placebo was 1.2 (95% CI 1.15 to 1.3); the NNT was 10 (7.9 to 14) (Figure 4).
4.
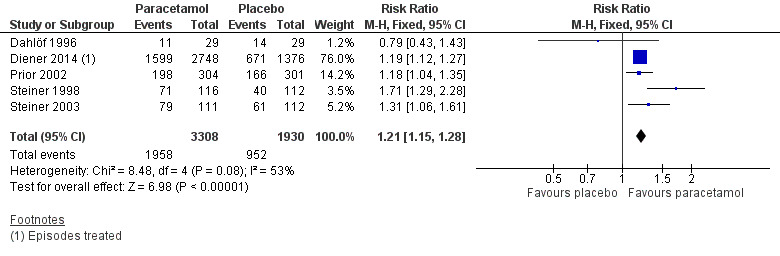
Forest plot of comparison: 1 Paracetamol 1000 mg versus placebo, outcome: 1.4 Pain‐free or mild pain at 2 hours.
We assessed the evidence for this outcome to be of high quality because, although few studies reported it, there was a large number of events and the CIs were tight. One very small study showed a different direction of response, which is likely to be due to random chance.
Use of rescue medication
Six studies (1856 attacks or participants) provided data for the use of rescue medication with paracetamol 1000 mg versus placebo (Mehlisch 1998; NL9701; Prior 2002; Schachtel 1991; Steiner 1998; Steiner 2003). The time frames over which these were reported varied from two hours to 24 hours, but it seems likely that participants with an inadequate response would have taken rescue medication soon after it was allowed, and due to the limited amount of data available we have combined these for the analysis. There was no obvious heterogeneity (I2 = 8%).
The proportion of participants who used rescue medication with paracetamol was 17% (164/985; range 2.0% to 46%).
The proportion of participants who used rescue medication with placebo was 30% (258/871; range 13% to 72%).
The RR for paracetamol 1000 mg compared with placebo was 0.58 (95% CI 0.50 to 0.69); the NNTp was 7.7 (6.0 to 11) (Analysis 1.5).
1.5. Analysis.
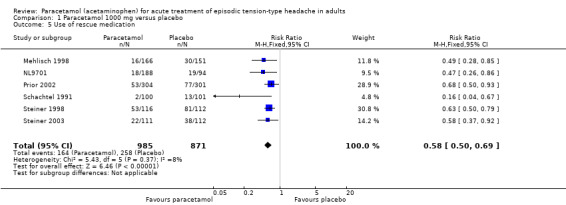
Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 5 Use of rescue medication.
Nine studies did not report use of rescue medication (Dahlöf 1996; Diener 2014; Göbel 1996; Göbel 1998; Göbel 2001; Migliardi 1994; Packman 2000; Peters 1983; Schachtel 1996), while NCT01755702 reported that the median time to use of rescue medication was 130 minutes with paracetamol 1000 mg compared with 62 minutes with placebo.
We downgraded the evidence for this outcome from high to moderate quality because few studies reported it and there was a modest number of events. There was consistency in the direction of response.
Adverse events
Any adverse events
Eleven studies (5605 attacks or participants) contributed data for any adverse events (Diener 2014; Mehlisch 1998; Migliardi 1994; NCT01755702; NL9701; Peters 1983; Prior 2002; Schachtel 1991; Schachtel 1996; Steiner 1998; Steiner 2003).
The proportion of attacks/participants who experienced any adverse event with paracetamol was 10% (337/3373; range 0% to 17%).
The proportion of attacks/participants who experienced any adverse event with placebo was 8.6% (191/2232; range 0.66% to 13%).
The RR for paracetamol 1000 mg compared with placebo was 1.1 (95% CI 0.94 to 1.3); the NNH was not calculated (Analysis 1.6).
1.6. Analysis.
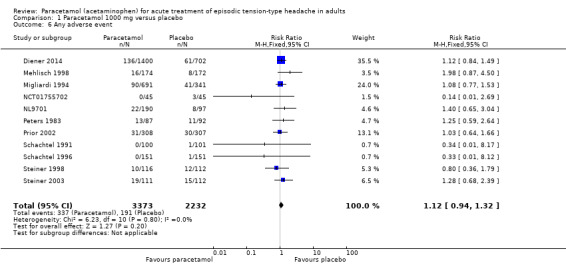
Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 6 Any adverse event.
We assessed the evidence for this outcome to be of high quality because there were adequate numbers of studies and events and the direction of results was consistent (no effect).
Gastrointestinal adverse events
Ten studies (5526 attacks or participants) contributed data for gastrointestinal adverse events (Diener 2014; Mehlisch 1998; Migliardi 1994; NL9701; Peters 1983; Prior 2002; Schachtel 1991; Schachtel 1996; Steiner 1998; Steiner 2003). One of these studies reported only nausea as a subgroup of adverse events, and gave no indication as to other gastrointestinal events; therefore, we included nausea figures in this analysis (Steiner 1998).
The proportion of attacks/participants who experienced any gastrointestinal adverse event with paracetamol was 4.6% (155/3335; range 0% to 8.0%).
The proportion of attacks/participants who experienced any gastrointestinal adverse event with placebo was 3.8% (84/2191; range 0.66% to 5.6%).
The RR for paracetamol 1000 mg compared with placebo was 1.1 (95% CI 0.86 to 1.5); the NNH was not calculated (Analysis 1.7).
1.7. Analysis.
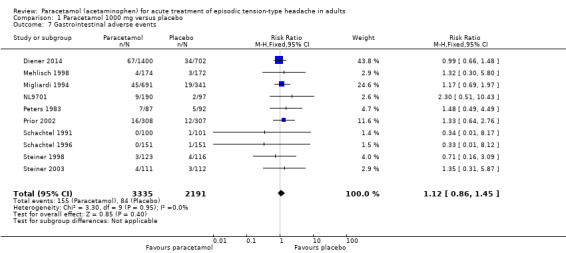
Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 7 Gastrointestinal adverse events.
Removing Steiner 1998 (which reported only nausea as a subgroup of adverse events) from the analysis did not change the result.
We downgraded the evidence for this outcome from high to moderate quality because, although there was an adequate number of studies and the direction of results was consistent (no effect), there was a moderate number of events.
Dizziness adverse events
Four studies (4036 attacks or participants) contributed data for dizziness adverse events (Diener 2014; Migliardi 1994; NL9701; Prior 2002).
The proportion of attacks/participants who experienced any dizziness adverse events with paracetamol was 1.6% (42/2589; range 1.6% to 1.9%).
The proportion of attacks/participants who experienced any dizziness adverse events with placebo was 1.1% (16/1447; range 0.98% to 1.2%).
The RR for paracetamol compared with placebo was 1.5 (95% CI 0.83 to 2.6); the NNH was not calculated (Analysis 1.8).
1.8. Analysis.
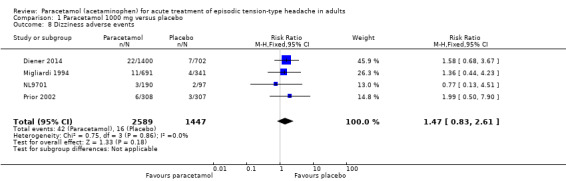
Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 8 Dizziness adverse events.
We downgraded the evidence for this outcome from high to low quality because there were few studies and events. The direction of results was consistent (no effect).
Serious adverse events
There were no serious adverse events in comparisons using paracetamol 1000 mg (15 studies, estimated 5147 participants). We estimated that the rate of serious adverse events is unlikely to be greater than 1 in 1700 people (Eypasch 1995).
We judged the evidence for this outcome to be moderate quality because, although there were no events reported, there were large numbers of studies and participants.
Adverse event withdrawals
One study reported an adverse event withdrawal (for tinnitus and indigestion) in a participant taking paracetamol 1000 mg (Dahlöf 1996). Eleven studies reported no adverse event withdrawals (Diener 2014; Göbel 1996; Göbel 1998; Göbel 2001; Migliardi 1994; Packman 2000; Schachtel 1991; Schachtel 1996; Steiner 1998; Steiner 2003), and one did not specifically report this outcome (Peters 1983). NCT01755702 reported no withdrawals during treatment periods, but there were three during the first washout period, and five during the second washout period. The reasons for withdrawal and the active treatment given before the washout were not reported.
We downgraded the evidence for this outcome from high to low quality because of poor reporting and small numbers of events.
Paracetamol 500 mg to 650 mg versus placebo
Five studies compared paracetamol 500 mg to 650 mg with placebo (Dahlöf 1996; Gilbert 1976; Miller 1987; Steiner 2003; Ward 1991). Due to the small amount of data available, we pooled studies in this dose range.
Pain‐free at two hours
Only one study reported pain‐free at two hours; 17% (5/29) of participants were pain‐free at two hours with both paracetamol 500 mg and placebo (Dahlöf 1996).
Pain‐free at other time points
No studies reported pain‐free outcomes at one, four, or 24 hours.
Pain intensity difference at two hours
Four studies reported some information about PIDs at two hours. Dahlöf 1996 reported a group mean difference between paracetamol and placebo of about 15/100 participants, Steiner 2003 a difference of about 0.3/10 participants, and Ward 1991 a difference of 10/100 participants, while Miller 1987 reported that the mean SPID was not significantly different at any time point. Gilbert 1976 did not report PID at two hours. None of the studies commented on the clinical significance of the findings.
Pain‐free or mild pain at two hours
Two studies (275 attacks or participants) contributed data for pain‐free or mild pain at two hours (Dahlöf 1996; Steiner 2003).
The proportion of attacks/participants who were pain‐free or had only mild pain at two hours with paracetamol was 59% (79/134; range 41% to 64%).
The proportion of attacks/participants who were pain‐free or had only mild pain at two hours with placebo was 53% (67/105; range 48% to 54%).
The RR for paracetamol 500 mg to 650 mg compared with placebo was 1.1 (95% CI 0.90 to 1.4); the NNT was not calculated (Analysis 2.1).
2.1. Analysis.
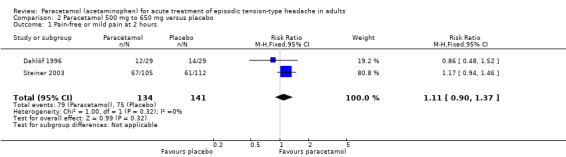
Comparison 2 Paracetamol 500 mg to 650 mg versus placebo, Outcome 1 Pain‐free or mild pain at 2 hours.
We downgraded the evidence for this outcome from high to low quality because there were few studies and events. The direction of effect was consistent (no effect).
Use of rescue medication
Two studies (301 participants) reported on use of rescue medication, one within six hours (Miller 1987), and one at two hours (Steiner 2003).
The proportion of participants who used rescue medication with paracetamol was 28% (42/148; range 26% to 35%).
The proportion of participants who used rescue medication with placebo was 37% (57/153; range 34% to 46%).
The RR for paracetamol 500 mg to 650 mg compared with placebo was 0.76 (95% CI 0.55 to 1.1). The NNTp was not calculated (Analysis 2.2).
2.2. Analysis.
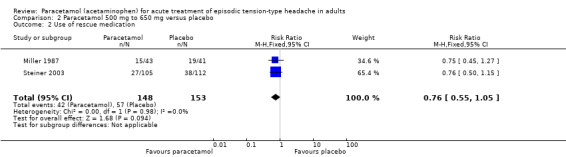
Comparison 2 Paracetamol 500 mg to 650 mg versus placebo, Outcome 2 Use of rescue medication.
We downgraded the evidence for this outcome from high to low quality because there were few studies and events, and one study had a high attrition rate. The direction of effect was consistent (no effect).
Adverse events
Any adverse events
Two studies (301 participants) contributed data for any adverse events (Miller 1987; Steiner 2003).
The proportion of participants who experienced any adverse event with paracetamol was 14% (21/148; range 9.3% to 16%).
The proportion of participants who experienced any adverse event with placebo was 11% (17/153; range 4.9% to 13%).
The RR for paracetamol 500 mg to 650 mg compared with placebo was 1.3 (95% CI 0.71 to 2.4). The NNT was not calculated (Analysis 2.3).
2.3. Analysis.
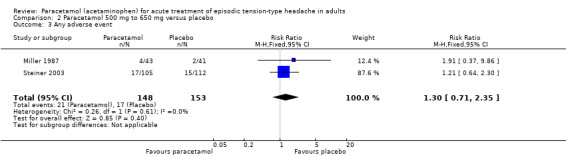
Comparison 2 Paracetamol 500 mg to 650 mg versus placebo, Outcome 3 Any adverse event.
The number of participants in this comparison only just reached our threshold for carrying out the analysis. We downgraded the evidence for this outcome from high to low quality because there were few studies and events, and one study had a high attrition rate. The direction of effect was consistent (no effect).
There were insufficient data for analysis of any specific adverse events.
Serious adverse events
There were no serious adverse events in comparisons using paracetamol 500 mg or 650 mg (five studies, estimated 463 participants). We estimated that the rate of serious adverse events is unlikely to be greater than 1 in 155 participants (Eypasch 1995).
We judged the evidence for this outcome to be of very low quality because there were few studies and participants on which to base our estimate.
Adverse event withdrawals
One study did not provide any information about adverse event withdrawals (Ward 1991), and three studies reported no adverse event withdrawals in comparisons of paracetamol 500 mg to 650 mg with placebo (Dahlöf 1996; Miller 1987; Steiner 2003). The remaining study reported that two participants dropped out after the first treatment period because of adverse events, but did not specify which of the four treatments they had received (Gilbert 1976).
We downgraded the evidence for this outcome from high to very low quality because of poor reporting and small numbers of studies and events.
Subgroup analyses
We planned subgroup analysis for dose, route of administration, and formulation. We carried out all analyses by dose (1000 mg or 500 mg to 650 mg), but all studies used the oral route of administration, and only one study reported on formulation (Packman 2000, which used ibuprofen liquigel), so no further subgroup analysis was possible.
Sensitivity analyses
We planned to carry out sensitivity analysis for study quality (Oxford Quality Score of 2/5 versus 3/5 or more), but only one study scored 2/5 (Ward 1991), and it did not contribute to any analyses.
We carried out post‐hoc sensitivity analyses for studies that did not report clear diagnostic criteria (Gilbert 1976; Göbel 2001; Peters 1983; Thorpe 1970; Ward 1991), or did not specify a washout period between treatments in cross‐over studies (Göbel 1996; Göbel 1998; Göbel 2001; NCT01755702; Pini 2008; Ward 1991). Of these studies, only Gilbert 1976 and NCT01755702 contributed data for analyses, and removing them did not change the results.
| Summary of results for paracetamol versus placebo | ||||
| Outcome/intervention | Studies | Participants/attacks | RR (95% CI) | NNT (95% CI) |
| Pain‐free at 2 hours | ||||
| Paracetamol 1000 mg | 8 | 5890 | 1.3 (1.1 to 1.4) | 22 (15 to 40) |
| Pain‐free at 1 hours | ||||
| Paracetamol 1000 mg | 4 | 4717 | 1.2 (0.90 to 1.5) | Not calculated |
| Pain‐free at 4 hours | ||||
| Paracetamol 1000 mg | 4 | 4909 | 1.2 (1.15 to 1.3) | 8.2 (6.6 to 11) |
| Pain‐free or mild pain at 2 hours | ||||
| Paracetamol 1000 mg | 5 | 5238 | 1.2 (1.15 to 1.3) | 10 (7.9 to 14) |
| Paracetamol 500‐650 mg | 2 | 275 | 1.1 (0.90 to 1.4) | Not calculated |
| NNTp (95% CI) | ||||
| Use of rescue medication | ||||
| Paracetamol 1000 mg | 6 | 1856 | 0.58 (0.50 to 0.69) | 7.7 (6.0 to 11) |
| Paracetamol 500‐650 mg | 2 | 301 | 0.76 (0.55 to 1.1) | Not calculated |
| NNH (95% CI) | ||||
| Any adverse event | ||||
| Paracetamol 1000 mg | 11 | 5605 | 1.1 (0.94 to 1.3) | Not calculated |
| Paracetamol 500‐650 mg | 2 | 301 | 1.3 (0.71 to 2.4) | Not calculated |
| Gastrointestinal adverse events | ||||
| Paracetamol 1000 mg | 10 | 5526 | 1.1 (0.86 to 1.5) | Not calculated |
| Dizziness | ||||
| Paracetamol 1000 mg | 4 | 4036 | 1.5 (0.83 to 2.6) | Not calculated |
| NNH: number needed to treat for an additional harmful event; NNT: number needed to treat for an additional beneficial event; NNTp: number needed to treat to prevent an event. | ||||
Paracetamol 1000 mg versus paracetamol 500 mg
Two studies (274 attacks or participants) included both 500 mg and 1000 mg treatment arms (Dahlöf 1996; Steiner 1998), but the only outcome that could be compared was pain‐free or no pain at two hours.
The proportion of attacks/participants who were pain‐free or had only mild pain at two hours with paracetamol 1000 mg was 64% (90/140; range 38% to 71%).
The proportion of attacks/participants who were pain‐free or had only mild pain at two hours with paracetamol 500 mg was 59% (79/134; range 41% to 64%).
The RR for paracetamol 1000 mg compared with 500 mg was 1.1 (95% CI 0.91 to 1.3) (Analysis 3.1). The NNT was not calculated.
3.1. Analysis.
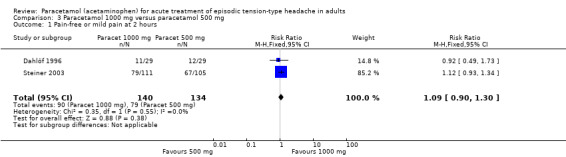
Comparison 3 Paracetamol 1000 mg versus paracetamol 500 mg, Outcome 1 Pain‐free or mild pain at 2 hours.
Indirect comparison with placebo also showed no significant difference between the doses (Z = 0.6673, P value = 0.43).
We downgraded the evidence for this comparison from high to very low quality because of small numbers of studies and events.
Paracetamol 1000 mg versus other active comparators
Three studies compared paracetamol 1000 mg with ketoprofen 25 mg (Dahlöf 1996; Mehlisch 1998; Steiner 1998). Two studies compared paracetamol 1000 mg with ibuprofen 400 mg (NCT01755702; Schachtel 1996). Only single studies provided usable data for comparing paracetamol 1000 mg with other active comparators, so no other analyses were done.
Appendix 5 and Appendix 6 show results for individual studies.
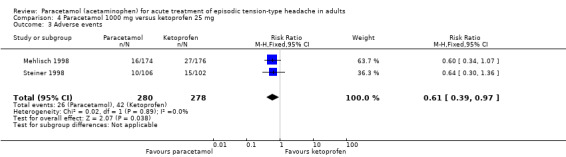
Paracetamol 1000 mg versus ketoprofen 25 mg
Three studies compared paracetamol 1000 mg with ketoprofen 25 mg (Dahlöf 1996; Mehlisch 1998; Steiner 1998).
Pain‐free at two hours
Two studies (276 attacks or participants) contributed data for pain‐free at two hours (Dahlöf 1996; Steiner 1998).
The proportion of attacks/participants who were pain‐free at two hours with paracetamol was 21% (30/145; range 17% to 22%).
The proportion of attacks/participants who were pain‐free at two hours with ketoprofen was 27% (36/131; range 27% to 28%).
The RR for paracetamol 1000 mg compared with ketoprofen 25 mg was 0.75 (95% CI 0.49 to 1.2). The NNT was not calculated (Analysis 4.1).
4.1. Analysis.
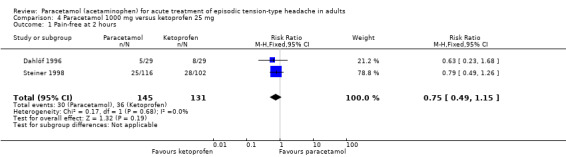
Comparison 4 Paracetamol 1000 mg versus ketoprofen 25 mg, Outcome 1 Pain‐free at 2 hours.
We downgraded the evidence for this outcome from high to very low quality because of small numbers of studies and events.
Pain‐free at one, four, and 24 hours
No studies reported pain‐free at one, four, and 24 hours.
Pain intensity difference at two hours
Three studies reported no significant difference in pain intensity at two hours between paracetamol 1000 mg and ketoprofen 25 mg.
Pain‐free or mild pain at two hours
Two studies (276 attacks or participants) reported pain‐free or mild pain at two hours (Dahlöf 1996; Steiner 1998).
The proportion of attacks/participants who were pain‐free or had only mild pain at two hours with paracetamol was 57% (83/145; range 41% to 61%).
The proportion of attacks/participants who were pain‐free or had only mild pain at two hours with ketoprofen was 66% (86/131; range 52% to 70%).
The RR for paracetamol 1000 mg compared with ketoprofen 25 mg was 0.87 (95% CI 0.72 to 1.04) (Analysis 4.2). The NNT was not calculated.
4.2. Analysis.
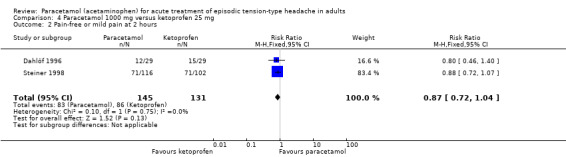
Comparison 4 Paracetamol 1000 mg versus ketoprofen 25 mg, Outcome 2 Pain‐free or mild pain at 2 hours.
We downgraded the evidence for this outcome from high to very low quality because of small numbers of studies and events.
Use of rescue medication
Mehlisch 1998 reported that 16/166 participants used rescue medication over four hours with paracetamol, and 7/156 with ketoprofen 25 mg. Steiner 1998 reported that 53/116 participants used rescue medication over two to 24 hours with paracetamol, and 44/102 with ketoprofen 25 mg.
Adverse events
Any adverse event
Two studies (558 participants) contributed data for any adverse event (Mehlisch 1998; Steiner 1998).
The proportion of participants who experienced any adverse event with paracetamol was 9.3% (26/280; range 9.2% to 9.4%).
The proportion of participants who experienced any adverse event with ketoprofen was 15% (42/278; range 14.7% to 15.3%).
The RR for paracetamol 1000 mg compared to ketoprofen 25 mg was 0.61 (95% CI 0.39 to 0.97); the NNTp was 17 (8.9 to 240) (Analysis 4.3). For every 17 participants treated, one would not experience an adverse event with paracetamol who would have done with ketoprofen.
4.3. Analysis.
Comparison 4 Paracetamol 1000 mg versus ketoprofen 25 mg, Outcome 3 Adverse events.
We downgraded the evidence for this outcome from high to very low quality because of small numbers of studies and events.
There were insufficient data for any analysis of individual adverse events.
Serious adverse events
None of the studies reported any serious adverse events.
Adverse event withdrawals
Dahlöf 1996 reported one adverse event withdrawal with paracetamol 1000 mg (tinnitus and indigestion). There were no other adverse event withdrawals in these studies.
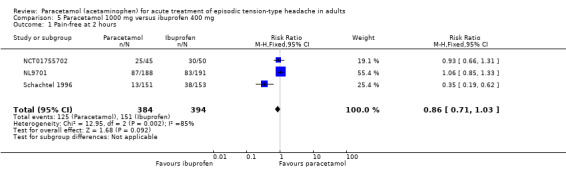
Paracetamol 1000 mg versus ibuprofen 400 mg
Pain‐free at two hours
Three studies (778 attacks or participants) contributed data for pain‐free at two hours (NCT01755702; NL9701; Schachtel 1996).
The proportion of attacks/participants who were pain‐free at two hours with paracetamol was 33% (125/384; range 8.6% to 56%).
The proportion of attacks/participants who were pain‐free at two hours with ibuprofen was 38% (151/394; range 25% to 60%).
The RR for paracetamol 1000 mg compared with ibuprofen 400 mg was 0.86 (95% CI 0.71 to 1.03); the NNH was not calculated (Analysis 5.1).
5.1. Analysis.
Comparison 5 Paracetamol 1000 mg versus ibuprofen 400 mg, Outcome 1 Pain‐free at 2 hours.
These three studies showed a high degree of variability in response rates, between 9% and 53% for paracetamol 1000 mg, and between 25% and 60% for ibuprofen 400 mg. Placebo response rates in the same studies ranged between 1% and 53%. There was clear statistical heterogeneity between these three studies; the I2 for this analysis was 85%. There was no obvious clinical heterogeneity, other than the mean age of the participants being 20 years, 30 years, and 40 years, though this factor is unlikely in itself to be the source of any heterogeneity.
We downgraded the evidence for this outcome from high to low quality because, although there was a modest number of attacks or participants, there were few studies and events and there was inconsistency in response.
Pain‐free at one hour
Two studies contributed data for pain‐free at one hour. In NCT01755702, 4/45 participants were pain‐free at two hours with paracetamol 1000 mg, and 7/50 with ibuprofen 400 mg, while in Schachtel 1996, the numbers were 0/151 with paracetamol and 4/153 with ibuprofen (estimated from a graph). There were too few events for sensible analysis.
Pain‐free at four hours
Schachtel 1996 reported that 51/151 participants were pain‐free at four hours with paracetamol 1000 mg, and 96/153 participants with ibuprofen 400 mg. NCT01755702 did not report this outcome and there were insufficient data for analysis.
Two studies (683 participants) contributed data for pain‐free at four hours (NL9701; Schachtel 1996).
The proportion of attacks/participants who were pain‐free at four hours with paracetamol was 58% (195/339; range 34% to 77%).
The proportion of participants who were pain‐free at two hours with ibuprofen was 69% (238/344; range 63% to 74%).
The RR for paracetamol 1000 mg compared with ibuprofen 400 mg was 0.83 (95% CI 0.74 to 0.93); the NNH was 8.6 (5.3 to 22) (Analysis 5.2).
5.2. Analysis.
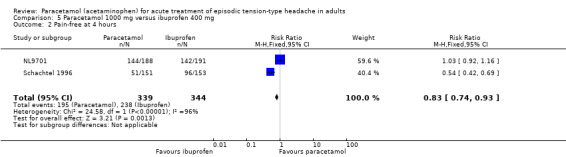
Comparison 5 Paracetamol 1000 mg versus ibuprofen 400 mg, Outcome 2 Pain‐free at 4 hours.
As with the outcome of pain‐free at two hours, there was clear statistical heterogeneity between these studies, with no obvious clinical cause: the I2 statistic was 96%. We downgraded the evidence for this outcome from high to very low quality because, although there was a modest number of attacks or participants, there were few studies and events and there was inconsistency in response.
Pain‐free at 24 hours
No studies reported pain‐free at 24 hours.
Pain intensity difference at two hours
No studies reported PID at two hours.
Pain‐free or mild pain at two hours
No studies reported pain‐free or mild pain at two hours.
Use of rescue medication
NCT01755702 reported a slightly shorter median time to use of rescue medication with paracetamol 1000 mg (130 minutes) than with ibuprofen 400 mg (150 minutes). Schachtel 1996 did not report any information on use of rescue medication.
Adverse events
Any adverse event
Two studies contributed data for any adverse event. NCT01755702 reported 0/45 participants experiencing adverse events with paracetamol 1000 mg, and 4/50 with ibuprofen 400 mg, while Schachtel 1996 reported no adverse events in 151 participants with paracetamol 1000 mg and 153 participants with ibuprofen 400 mg. There were too few events for sensible analysis.
Serious adverse events
Neither study reported serious adverse events.
Adverse event withdrawals
There were no adverse event withdrawals during treatment periods in either of the studies.
Paracetamol used in combination with other medications
Studies used paracetamol in combination with codeine (Friedman 1987; Gatoulis 2012), with phenyltoloxamine citrate (Percogesic; Gilbert 1976), with peppermint oil (Göbel 1996; Göbel 2001), with caffeine (Migliardi 1994; NCT01755702; Pini 2008; Ward 1991), with aspirin and caffeine (Migliardi 1994), and as Fiorinal‐Pa (aspirin, caffeine, isobutylallylbarbituric acid, paracetamol; Thorpe 1970). However, none of these studies provided sufficient data to do any analyses.
Appendix 5 and Appendix 6 present results for individual studies.
Discussion
Summary of main results
The primary outcome of this review was pain‐free at two hours using any standard method of pain assessment and without the use of rescue medication, reflecting the updated guidelines for controlled trials of drugs in TTH issued by the IHS (IHS 2010). We included 23 studies, with over 8000 participants, of which about 6000 were in comparisons of paracetamol 1000 mg versus placebo. Participants had moderate or severe pain at the start of treatment, and took just one treatment dose per headache episode.
The NNT for the outcome pain‐free at two hours for paracetamol 1000 mg compared with placebo was 22 (95% CI 15 to 40) (high quality evidence). There was no significant difference from placebo at the earlier time point of one hour (RR 1.2 (0.90 to 1.5)) (moderate quality evidence). There was a better (lower) NNT for the outcome pain‐free or mild pain at two hours (10 (7.9 to 14); high quality evidence), and also for pain‐free at four hours (8.2 (6.6 to 11); moderate quality evidence). Pain‐free or mild pain at two hours is easier to achieve than pain‐free at two hours, and is an outcome regarded as useful by most people with acute or chronic pain (Moore 2013), but even this result might be regarded as being on the borderline of what is generally considered clinically useful as few more people attain this outcome with paracetamol than with placebo. There were insufficient data for any analysis of outcomes assessing mean pain intensity differences (PID and SPID), but where information was reported, paracetamol was usually numerically or statistically significantly better than placebo. Overall differences between paracetamol 1000 mg and placebo were not great (between 4/100 and 15/100). There was clearly some small benefit from paracetamol in this condition, but the clinical significance of the benefit was difficult to assess. There is growing evidence of small or absent benefit and growing evidence about risks for paracetamol generally (Moore 2016).
Fewer participants used rescue medication with paracetamol 1000 mg than with placebo, giving an NNTp of 7.7 (6.0 to 11) (moderate quality evidence). There was no significant difference in the number of participants who experienced adverse events with paracetamol 1000 mg compared with placebo (high quality evidence). Analysis of gastrointestinal adverse events (moderate quality evidence) and dizziness (low quality evidence) showed no significant differences between paracetamol 1000 mg and placebo (see Table 1).
Data were combined for analysis for the small number of studies using paracetamol doses between 500 and 650 mg. No significant difference was found between paracetamol 500 mg to 650 mg and placebo for the outcomes of pain‐free or mild pain at two hours, use of rescue medication, or participants with adverse events (low quality evidence). There were insufficient data for analysis for any other outcomes (see Table 2).
Direct comparison of paracetamol 1000 mg and 500 mg within studies did not show a significant difference between the doses for pain‐free or mild pain at two hours (low quality evidence), and neither did indirect comparisons with placebo (P value = 0.43). This is not unexpected as a dose‐response curve for paracetamol is known to be difficult to demonstrate in a clinical setting, although it has been done in large data sets (McQuay 2007; Moore 2015).
A small number of studies included sufficient data to compare paracetamol 1000 mg with the active comparators ketoprofen 25 mg and ibuprofen 400 mg. Based on this very limited information, there was no difference between paracetamol and ketoprofen for the outcome of pain‐free at two hours, or for pain‐free or mild pain at two hours, but fewer participants experienced adverse events with paracetamol than with ketoprofen (NNTp 17(8.9 to 240) (all low quality evidence). Again, based on very limited information, there was no difference between paracetamol and ibuprofen for pain‐free at two hours (low quality evidence), but a statistical difference was found for pain‐free at four hours (very low quality evidence). There were very variable response rates in the three trials comparing these two drugs. There was a broader observation that, at standard doses, ibuprofen is a more effective analgesic than paracetamol in a wide range of conditions (Moore 2014a). There were no data, or insufficient data for sensible analysis, for any other outcomes for these two comparators, or for any other active comparators.
Overall completeness and applicability of evidence
IHS recommendations regarding outcomes of headache trials are well regarded (IHS 2010), and often, if not always, followed (Bendtsen 2010; Moore 2014b). Studies included in this review largely predated those recommendations and were inconsistent in reporting them, which limited the ability to draw useful conclusions about the efficacy of paracetamol, either alone or in combination with other agents, compared with placebo or active comparators. Of the 23 included studies, only 15 reported the IHS preferred outcome of pain‐free rate at two hours (IHS 2010). In our results, there was a lower (better) NNT for the secondary outcome of pain‐free at four hours, suggesting the beneficial effects of paracetamol extend beyond this preferred time point, but only four studies contributed data to this analysis, which was dominated by one large cross‐over study, limiting confidence in this result.
Although two doses of paracetamol were considered in this review, most of the information was for the higher dose of 1000 mg, which is the standard dose for adult pain relief in most circumstances. More information on different doses, use of paracetamol in combination with another agent, and different formulations, would improve our understanding of the role of paracetamol in TTH.
None of the included studies provided information on the mean number of headaches experienced by participants before study entry, although all studies required participants to have frequent episodic TTH. This is defined as anywhere between two and 14 headache days month. We do not know whether the participants in these studies were typically experiencing two to five, or 10 to 14 headaches a month. This might influence the efficacy of treatments tested in these TTH studies, but we do not know because the information was missing. Nor do we know if these results are applicable to people with infrequent episodic TTH (one headache per month or less), which may represent a large proportion of people experiencing this type of headache, who do not consult their doctors or need medical management, but who use simple analgesics for pain relief.
The overwhelming majority of participants in the included studies had moderate or severe baseline pain. There is good reason for this in clinical trials, because it gives sensitivity to demonstrate a reduction in pain. However, the pain of TTH is usually described as being of mild to moderate intensity (IHS 2010), so the participants in these trials may represent a population with headaches that are more severe and possibly more difficult to treat than is the norm.
To understand these important methodological points, analysis of clinical trials at the level of the individual participant is required, using substantial amounts of data. Such an analysis seems unlikely at present, but would probably be highly informative for the development of existing IHS guidance (Bendtsen 2010).
Quality of the evidence
The quality of findings ranks from moderate to high for paracetamol 1000 mg compared with placebo, and from very low to low for paracetamol 500 mg to 650 mg compared with placebo, and for paracetamol 1000 mg compared with other active interventions.
All included studies were both randomised and double‐blind; none were considered at high risk of bias for study conduct. Inconsistent reporting of outcomes, the small number of studies for the lower dose and for active comparators, and the small size of some studies were the major problems that limited analyses and downgraded our assessments of the quality of the results. For the most part, the direction of results was consistent within analyses.
Potential biases in the review process
Ten of the included studies (four contributed to analyses) were cross‐over studies, and in the absence of first period data we included data from all treatment periods. TTH is a suitable condition to study with cross‐over trials, and washout periods were appropriate for the treatments investigated. While this may introduce unknown biases (Elbourne 2002), the data from these studies were consistent with data from others for both beneficial and harmful outcomes.
We carried out extensive searches to identify relevant studies, and consulted widely and internationally for an earlier review (Moore 2014b). We think it unlikely that there is a substantial number of studies that we have missed or are unpublished, and estimate that even if a similar number of participants existed in studies with no effect, there would still be a small beneficial effect overall for paracetamol 1000 mg relative to placebo. In the circumstance of the limited efficacy from the results of this review, the potential effects of unpublished data with no treatment effect are largely irrelevant.
Agreements and disagreements with other studies or reviews
These results are broadly in agreement with previous reviews that concluded that ibuprofen, paracetamol, and ketoprofen were better than placebo (Moore 2014b; Verhagen 2006), as well as the guideline from the EFNS, which recommends ibuprofen as drug of choice among NSAIDs or paracetamol or aspirin for acute treatment of TTH (Bendtsen 2010). That guideline was not based on a systematic review. The German evidence‐based recommendations for self medication of migraine and TTH were based on systematic reviews (Haag 2011), and included only seven studies that recruited at least some people with TTH. For self medication of TTH, it recommended paracetamol in combination with other analgesics or caffeine, but not paracetamol alone.
Authors' conclusions
Implications for practice.
For people with frequent episodic tension‐type headache
Paracetamol 1000 mg may relieve headache pain, but the chance of the pain being relieved entirely by two hours is low, about 2 in 10 (24%), but this is only very slightly greater than the proportion who took placebo (about 1 in 5, or 19%) We do not know how or if these results can be extrapolated to people with an occasional headache.
For clinicians
It may be that a different formulation of paracetamol, or a combination with a nonsteroidal anti‐inflammatory drug, or caffeine might be better, but evidence on this is lacking.
For policy makers
There is insufficient information on drugs, doses, formulations, or outcomes for policy makers to be able to make strong recommendations. Policy should reflect the finding of no or little clinically useful benefit.
For funders
Because of the very limited information and small degree of efficacy found for paracetamol, it is highly unlikely that the treatment could be regarded as cost effective even considering the low price of this drug.
Implications for research.
General
Frequent episodic tension‐type headache is common and debilitating. The amount and reporting of evidence was limited by reporting issues, particularly of outcomes; this is a general finding for all TTH studies, not just those involving paracetamol. It is not sufficient just to call for more studies. What is needed is a better understanding of TTH studies, in terms of the outcomes that can be reported from clinical trials, and often are not, and the differential effects of treatments in people with different degrees of headache frequency. This can be done from individual participant‐level analyses. Given that a number of modern studies have been completed or are underway, this would appear to be the research priority before new studies are commissioned.
Design
The design of studies was generally good, although some were small. Future studies should be adequately powered to detect the magnitude of any effect, not simply a statistical difference from placebo.
Measurement (endpoints)
The measurement of pain is not a major issue as most studies, especially modern studies, have used standard pain intensity and pain relief scales. What is at issue are the outcomes reported using those pain measurements. It is not clear that the International Headache Society‐preferred outcome of being free of pain at two hours is entirely appropriate, and while it is reasonable by analogy with migraine, it requires substantiating.
Comparison between active treatments
No authoritative comparisons between active treatments is possible in the present state of knowledge.
Feedback
Feedback submitted, 2 August 2016
Summary
Date of Submission: 02‐Aug‐2016
Name: Stephen Senn
Email Address: stephen.senn@lih.lu
Affiliation: Luxembourg Institute of Health
Role: Head of competence unit for methodology and statistics
Comment: The summary states "The outcome of being pain free or having only mild pain at two hours was reported by 59 in 100 people taking paracetamol 1000 mg, and in 49 out of 100 people taking placebo (high quality evidence), meaning that only 10 in 100 people benefited because of paracetamol 1000 mg." The conclusion, which may or may not be true (but is almost certainly false) does not, however, necessarily follow from the stated facts. For example a simple exponential distribution model with a mean headache duration of 2.97 hours duration under placebo and 2.24 under paracetamol would produce the probabilities quoted for two hours. This corresponds to a reduction in mean headache time of about 3/4 of an hour. See https://errorstatistics.com/2016/08/02/s‐senn‐painful‐dichotomies‐guest‐post/ for a discussion. The model would be consistent with a reduction of approximately 25% 100x(2.97‐2.24)/2.97 on headache duration and such a model could in turn, in theory, be consistent with the following situation: every time any patient has a headache he or she will reduce the duration of that headache by 1/4 by taking paracetamol rather than nothing. Of course, this is also unlikely to be true, since combination of the exponential model and proportional reduction is the simplest consistent with the stated facts. However, the point is that nothing supports the conclusion in the quoted paragraph.
This sort of error is very common in Cochrane reviews and is fed, in my opinion, by an obsession.with numbers needed to treat and the illusion that these are easy to interpret. Further explanation of the issues will be found in the blog here: https://errorstatistics.com/2014/07/26/s‐senn‐responder‐despondency‐myths‐of‐personalized‐medicine‐guest‐post/ Further discussion of the approaches necessary to establish the individual element of response will be found in my paper http://onlinelibrary.wiley.com/doi/10.1002/sim.6739/abstract and in the context of pain control in Gewandter et al http://www.ncbi.nlm.nih.gov/pubmed/24865794. A simple explanation is also given in my paper, Three Things Every Medical Writer Should Know about Statistics: http://eprints.gla.ac.uk/8107/1/id8107.pdf.
As far as I am aware there is no conflict of interest. However, I maintain a full declaration of interest here http://www.senns.demon.co.uk/Declaration_Interest.htm so that any reader can check for him or herself.
Reply
Response prepared by the author Professor Andrew Moore.
Thank you for this feedback. We have followed your writing on responders, and have a degree of sympathy for them and have looked carefully to see how results in pain might or might not transgress. We will respond in two ways. First a response narrowly related to paracetamol in tension‐type headache (TTH), and second, a broader response for pain in general.
Paracetamol in TTH
One might anticipate that headache trials would report results as the average time taken for headache to resolve with an active treatment compared with placebo. That would be reasonable, as the common experience is that even the most severe headache resolves eventually – though there can be a very long tail and the average may not accurately represent the experience of all. And, of course, headaches experienced at different times may have different causes, so be different even in the same person, and the response to an active therapy may be different in the same person depending on the headache and other factors.
We would have investigated all that had the necessary outcomes been reported in the papers, but they generally were not. The ideal would be patient data at the individual level, but these are not available to us. Even though TTH is one of the most common conditions (not just painful conditions), the research into treatments has been poor, and outcomes reported generally not of much use to patients or clinicians. We know because we surveyed the area before embarking on a series of Cochrane reviews, in part to understand how we might most usefully assemble evidence for comparison [Pain. 2014 Nov;155(11):2220‐8].
The International Headache Society has long held that the most appropriate outcome is a combination of pain intensity and time – namely low pain intensity by a certain time after medicine has been taken. This was no whim, but was based on some solid research demonstrating it to be an outcome that people with headache wanted, and that they thought worthwhile. Moreover, we have conducted a systematic review of the outcomes that people with pain (of any origin) want from treatment, and they almost universally say that they want low pain, and quickly please [Anaesthesia. 2013 Apr;68(4):400‐12].
So for TTH generally, we are using an outcome that people with headache want to know about.
Pain responders more generally
We have been examining the question of how people with pain respond to treatment by analysing data at the level of the individual patient, often with data sets extending to thousands of patients. What we find is quite clear – namely that in the context of any trial or condition we have examined, we see a dichotomy between people who have a very good early response in terms of sharply reduced pain intensity, and those who do not and whose pain intensity levels remain at or above the initial value. Those who respond early generally continue to have low pain unless they discontinue because of adverse events, while those not responding early never respond during the period of the trial, which for chronic pain is typically three months. We have seen this in acute postoperative pain, fibromyalgia, back pain, and osteoarthritis. Acute pain responses have been published [Eur J Pain. 2015 Feb;19(2):187‐92]. And lest it be forgotten, the degree of pain relief needed to be a responder is substantial, is that considered important by people with pain, and also by members of the public who do not have pain. Admittedly, not all this research is yet published because of ill‐health, but we regard it as solid.
Furthermore, for people with chronic pain, response in terms of pain is associated with improved sleep, less depression, better quality of life, and increased ability to work. Non‐response for pain is associated with none of those changes – all of which, to reiterate, are based on analysis at the level of the individual patient. This is true whatever the treatment, and links between pain response and other benefits are also evident with placebo.
This accords with clinical and patient experience. Surveys show that most people with chronic pain continue to have significant pain despite being on treatment for a long time, but that a change in therapy can result in significant pain reduction for some, and that also is accompanied with quality of life and other benefits. People lucky enough to have low pain on treatment can often continue to benefit for many years, and that can be supported by test retest interventions.
We believe our approach is firmly based on what people want, and is supported by very considerable detailed analysis of large, high quality clinical studies of long duration. In pain, across the board, we see that responses to therapy are typically ‘all or none’. The average result is one that describes the experience of few people, and we long ago showed how taking average results in such circumstances can lead to erroneous conclusions [Pain. 1996 Feb;64(2):331‐5]. So we stand by our approach and can find nothing erroneous in the evidence we have assembled. Pain is not blood pressure, and thought experiments around blood pressure are probably not relevant.
One final comment about pharmacogenomics. It is a difficult subject and one where we have no detailed knowledge. However, there are examples in pain of some tragic experiences that can be firmly pinned onto genetic causes. The science underpinning our understanding of pain and its treatment can and should be questioned (we do). What is undoubtedly the case, though, is that in humans there are very considerable inter‐individual variability that we should expect to result in different experiences in our response to drugs.
Contributors
Feedback Editor Kate Seers, Managing Editor Anna Erskine, and Co‐ordinating Editor Christopher Eccleston.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 24 January 2018 | Review declared as stable | See Published notes |
History
Protocol first published: Issue 9, 2015 Review first published: Issue 6, 2016
| Date | Event | Description |
|---|---|---|
| 1 September 2016 | Amended | Track changes removed in Figure 1 |
| 5 August 2016 | Feedback has been incorporated | See Feedback |
| 22 June 2016 | Amended | Typo 'medial' changed to 'medical' in PLS. |
Notes
A restricted search in January 2018 did not identify any potentially relevant studies likely to change the conclusions. The authors and editors are confident that further research will not change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We wish to thank for supplying the clinical trial report for one unpublished study, which we were able to include.
The Oxford Pain Research Trust provided institutional support for this review.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Group. Disclaimer: The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy (via CRSO)
MESH DESCRIPTOR Acetaminophen EXPLODE ALL TREES (1751)
((acetaminophen or paracetamol or Panadol or Tylenol):TI,AB,KY (5197)
1 or 2 (5197)
MESH DESCRIPTOR headache (1520)
MESH DESCRIPTOR headache disorders EXPLODE ALL TREES (1816)
(headache* or cephalgi* or cephalalgi*):TI,AB,KY (16408)
4 or 5 or 6 (16973)
3 and 7 (532)
Appendix 2. MEDLINE search strategy (via Ovid)
Acetaminophen/ (15078)
(acetaminophen or paracetamol or Panadol or Tylenol).mp. (20624)
1 or 2 (20624)
Headache/ (23647)
exp Headache Disorders/ (28590)
(headache* or cephalgi* or cephalalgi*).mp. (67972)
4 or 5 or 6 (80512)
randomized controlled trial.pt. (413275)
controlled clinical trial.pt. (91856)
randomized.ab. (305168)
placebo.ab. (158098)
drug therapy.fs. (1845475)
randomly.ab. (216110)
trial.ab. (317473)
groups.ab. (1362061)
or/8‐15 (3481184)
3 and 7 and 16 (540)
Appendix 3. EMBASE search strategy (via Ovid)
Paracetamol/ (69353)
(acetaminophen or paracetamol or Panadol or Tylenol).mp. (73787)
1 or 2 (73787)
exp headache/ (155260)
exp "headache and facial pain"/ (226009)
(headach* or cephalgi* or cephalalgi*).mp. (205956)
4 or 5 or 6 (244515)
random*.tw. (1030843)
factorial*.tw. (26537)
cross?over*.tw. (55045)
placebo*.tw. (227508)
(doubl* adj blind*).tw. (162111)
assign*.tw. (274924)
allocat*.tw. (98684)
Crossover Procedure/ (44714)
Double‐blind procedure/ (126638)
Randomized Controlled Trial/ (388338)
or 8‐17 (1463601)
3 and 7 and 18 (1784)
Appendix 4. GRADE: criteria for assigning grade of evidence
The GRADE system uses the following criteria for assigning grade of evidence (GRADEpro GDT 2015).
High = further research is very unlikely to change our confidence in the estimate of effect.
Moderate = further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low = further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low = any estimate of effect is very uncertain.
We decrease grade if we find:
a serious (‐1) or very serious (‐2) limitation to study quality;
important inconsistency (‐1);
some (‐1) or major (‐2) uncertainty about directness;
imprecise or sparse data (‐1);
a high probability of reporting bias (‐1).
We increase grade if we find:
strong evidence of association ‐ significant relative risk of > 2 (< 0.5) based on consistent evidence from two or more; observational studies, with no plausible confounders (+1);
very strong evidence of association ‐ significant relative risk of > 5 (< 0.2) based on direct evidence with no major threats to validity (+2);
evidence of a dose response gradient (+1);
that all plausible confounders would have reduced the effect (+1).
Appendix 5. Summary of outcomes in individual studies: efficacy
| Study ID | Treatment | Pain‐free at 2 h | Pain‐free at 1 h | Pain‐free at 4 h | Pain‐free at 24 h | ≤ Mild pain at 2 h | PID at 2 h |
| Dahlöf 1996 | (1) Paracetamol 500 mg (2) Paracetamol 1000 mg (3) Ketoprofen 25 mg (4) Ketoprofen 50 mg (5) Placebo |
PP analysis (29 participants who completed all 5 attacks without major violation) (1) 17% = 5/29 (2) 17% = 5/29 (3) 28% = 8/29 (4) 32% = 9/29 (5) 17% = 5/29 |
No data | No data | No data | From graph (1) 41% = 12/29 (2) 38% = 11/29 (3) 50% = 15/29 (4) 68% = 20/29 (5) 48% = 14/29 |
No significant difference between paracetamol and placebo Mean PID at 2 h about 15/100 Ketoprofen 50 mg significantly different from placebo, but not 25 mg |
| Diener 2014 (studies 1‐4 in Migliardi 1994) | (1) Paracetamol 1000 mg (2) Paracetamol 1000 mg + aspirin 500 mg + caffeine 130 mg (3) Placebo |
(1) 21.0% (2) 28.5% (3) 18.0% |
(1) 6.1% (2) 8.6% (3) 3.1% |
(1) 57.0% (2) 65.9% (3) 48.3% |
No data | (1) 58.2% (2) 66.6% (3) 48.8% |
No data |
| Friedman 1987 | (1) Butalbital 100 mg + caffeine 80 mg + paracetamol 650 mg (Fioricet) (2) Paracetamol 600 mg + codeine 60 mg (3) Placebo |
(1) 27% = 18/66 (2) 25% = 16/65 (3) 12% = 8/67 |
(1) 9.1% = 6/66 (2) 11% = 7/65 (3) 6% = 4/67 |
No data | No data | No data | No data |
| Gatoulis 2012 | (1) Paracetamol 300 mg + codeine 30 mg (2) Aspirin 1000 mg (3) Placebo |
No data | No data | No data | No data | No data | SPID 4 and 6 significantly better for both active treatments than placebo (P value < 0.001) Active treatments equivalent for PID measures |
| Gilbert 1976 | (1) Paracetamol 650 mg (2) Phenyltoloxamine 60 mg (3) Paracetamol 650 mg + phenyltoloxamine citrate 60 mg (Percogesic) (4) Placebo |
No data | No data | No data | No data | No data | No data |
| Göbel 1996 | (1) Paracetamol 1000 mg (2) Peppermint oil solution 10 g (3) Peppermint oil + paracetamol (4) Placebo |
No data | No data | No data | No data | No data | Significant reduction with both active treatments compared to placebo. No significant difference between active treatments. Effects of combination additive "but below significance threshold" |
| Göbel 1998 | (1) Paracetamol 1000 mg (2) Peppermint oil combination (distillate of oelum menthae piperitae, oleum cajeputi, oleum eucalypti, oleum juniperi, and oleum gaultheriae) (3) Placebo |
No data | No data | No data | No data | No data | No data |
| Göbel 2001 | (1) Paracetamol 1000 mg (2) Peppermint oil (oleum menthae piperitae) solution Ll 170, 10 g (3) Peppermint oil + paracetamol (4) Placebo |
No data | No data | No data | No data | No data | No data |
| Mehlisch 1998 | (1) Paracetamol 1000 mg (2) Ketoprofen 12.5 mg (3) Ketoprofen 25 mg (4) Placebo |
No data | No data | No data | No data | No data | Mean PID for (3) > (2) > (1) > (4). No significant difference at 2 h |
| Migliardi 1994 | Studies 5, 6 (1) Paracetamol 1000 mg (2) Paracetamol 1000 mg + caffeine 130 mg (3) Placebo |
No data | No data | No data | No data | No data | Mean PID and TOTPAR significantly better for (2) than (1) or (3). (1) significantly better than (3) for most summary measures |
| Miller 1987 | (1) Paracetamol 650 mg (2) Naproxen sodium 550 mg (3) Placebo |
No data | No data | No data | No data | No data | Mean SPID for (1) not significantly different from (3) at any time point. (2) significantly better than (1) and (3) from 1 h |
| NCT01755702 | (1) Paracetamol 1000 mg (2) Paracetamol 1000 mg + caffeine 130 mg (3) Ibuprofen 400 mg (4) Placebo |
(1) 25/45 (2) 29/47 (3) 30/50 (4) 24/45 |
(1) 4/45 (2) 15/47 (3) 7/50 (4) 7/45 |
No data | No data | No data | PID 2 h: (1) 2.94 (SD 1.20) (2) 2.94 (SD 1.11) (3) 3.08 (SD 1.10) (4) 2.67 (SD 1.24) SPID 2 h: (1) 4.32 (SD 1.98) (2) 4.42 (SD 1.88) (3) 4.56 (SD 1.73) (4) 3.69 (SD 1.70) |
| NL9701 | (1) Paracetamol 1000 mg (2) Ibuprofen 400 mg (3) Placebo |
(1) 87/188 (2) 83/91 (3) 27/94 |
No data | (1) 144/188 (2) 142/191 (3) 55/94 |
No data | No data | ‐ |
| Packman 2000 | (1) Paracetamol 1000 mg (2) Ibuprofen liquigel 400 mg (3) Placebo |
No data | No data | Pain‐free at 3 h (1) 32% (2) 75% (3) 13% |
No data | No data | No data |
| Peters 1983 | (1) Paracetamol 1000 mg (2) Aspirin 650 mg (3) Placebo |
No data | No data | No data | No data | No data | No data |
| Pini 2008 | (1) Paracetamol 1000 mg + caffeine 130 mg (2) Naproxen sodium 550 mg (3) Placebo |
No data | No data | No data | No data | No data | (1) and (2) better than (3) for measures of PID and pain relief (P value < 0.05), but not different from each other |
| Prior 2002 | (1) Paracetamol 1000 mg (2) Naproxen 375 mg (3) Placebo |
(1) 112/304 (2) 93/295 (3) 78/301 |
No data | No data | No data | (1) 198/304 (2) 182/295 (3) 166/301 |
Mean PID at 2 h (4‐point scale): (1) 1.45 (2) 1.3 (3) 1.1 SPID 6 h: (1) 9.14 (SE 0.34) (2) 8.81 (SE 0.35) (3) 7.42 (SE 0.34) Active treatments significantly better than placebo (P value < 0.02) |
| Schachtel 1991 | (1) Paracetamol 1000 mg (2) Aspirin 1000 mg + caffeine 64 mg (3) Placebo |
From Fig 3 (1) 23/100 (2) 31/101 (3) 16/101 |
From Fig 3 (1) 11/100 (2) 15/101 (3) 5/101 |
(1) 49/100 (2) 69/101 (3) 36/101 |
No data | No data | From Fig 1, at 2 h (100 mm VAS) (1) 38 (SE about 4) (2) 45 (SE about 4) (3) 29 (SE about 5) Active treatments significantly better than placebo (P value < 0.01) |
| Schachtel 1996 | (1) Paracetamol 1000 mg (2) Ibuprofen 400 mg (3) Placebo |
From Fig 3 (1) 13/151 (2) 38/153 (3) 2/151 |
From Fig 3 (best estimate) (1) 0/151 (2) 4/153 (3) 0/151 |
(1) 51/151 (2) 96/153 (3) 11/151 |
No data | No data | At 2 h (100 mm VAS) (1) 31 (2) 48 (3) 12 Active treatments significantly better than placebo (P value < 0.001). Ibuprofen better than placebo (P value < 0.01) |
| Steiner 1998 | (1) Paracetamol 1000 mg (2) Ketoprofen 25 mg (3) Placebo |
(1) 25/116 (2) 28/102 (3) 18/112 |
No data | No data | No data | Total relief + worthwhile effect (1) 71/116 (2) 71/102 (3) 40/112 |
From Fig 1, at 2 h (100 mm VAS) (1) 26 (2) 30 (3) 17 Active treatments significantly better than placebo |
| Steiner 2003 | (1) Paracetamol 500 mg (2) Paracetamol 1000 mg (3) Aspirin 500 mg (4) Aspirin 1000 mg (5) Placebo |
No data | No data | No data | No data | Total relief + worthwhile effect (1) 67/105 (2) 79/111 (3) 78/111 (4) 78/103 (5) 61/112 |
From Fig 5, at 2 h (scale 0‐10) (1) 3.0 (2) 3.5 (3) 3.6 (4) 3.9 (5) 2.7 Active treatments significantly better than placebo |
| Thorpe 1970 | (1) Fiorinal‐Pa (aspirin + caffeine + isobutylallylbarbituric acid + paracetamol) (2) Placebo |
No data | No data | No data | No data | No data | Drop of ≥ 2 grades in pain intensity (1) 21/25 (2) 7/27 |
| Ward 1991 | (1) Paracetamol 648 mg (2) Paracetamol 648 mg + caffeine 65 mg (3) Paracetamol 648 mg + caffeine 130 mg (4) Caffeine 65 mg (5) Caffeine 130 mg (6) Placebo |
No data | No data | No data | No data | No data | At 2 h (0‐100) (1) 22 (2) 27 (3) 28 (4) 22 (5) 23 (6) 12 All active treatments significantly better than placebo (P value < 0.01) |
| Fig: Figure; h: hour; PID: pain intensity difference (from baseline); PP: per‐protocol; SD: standard deviation; SE: standard error; SPID: summed pain intensity difference; TOTPAR: total pain relief; VAS: visual analogue scale. | |||||||
Appendix 6. Summary of outcomes in individual studies: adverse events, withdrawals, and rescue medication
| Study ID | Treatment | Any AE | Serious AE | AE withdrawals | Rescue medication |
| Dahlöf 1996 | (1) Paracetamol 500 mg (2) Paracetamol 1000 mg (3) Ketoprofen 25 mg (4) Ketoprofen 50 mg (5) Placebo |
No data Total of 30 adverse events reported within 24 h of treating 178 attacks Most of mild or moderate severity No difference between groups |
None | (2) 1/29 (tinnitus and indigestion) 10 participants did not complete all 5 attacks 1 participant had a major protocol violation (took medication < 72 h after previous dose) |
Not reported, but in Methods, so measured |
|
Diener 2014 (studies 1‐4 in Migliardi 1994) |
(1) Paracetamol 1000 mg (2) Paracetamol 1000 mg + aspirin 500 mg + caffeine 130 mg (3) Placebo |
(1) 136/1400 (2) 241/1400 (3) 61/702 |
None | None | No data |
| Friedman 1987 | (1) Butalbital 100 mg + caffeine 80 mg + paracetamol 650 mg (Fioricet) (2) Paracetamol 600 mg + codeine 60 mg (3) Placebo |
No difference between groups | None | None | No data |
| Gatoulis 2012 | (1) Paracetamol 300 mg + codeine 30 mg (2) Aspirin 1000 mg (3) Placebo |
(1) 57/233 (2) 38/223 (3) 19/103 Most mild or moderate Dizziness, somnolence, nausea most common ‐ not different from placebo |
None | None | Time to use of rescue medication not different from (3) for (1), but significantly longer for (2) than (1) Proportion of participants taking rescue medication with (1) significantly different from (3) only at 3 h; (2) significantly different from (3) from 2 h |
| Gilbert 1976 | (1) Paracetamol 650 mg (2) Phenyltoloxamine 60 mg (3) Paracetamol 650 mg + phenyltoloxamine citrate 60 mg (Percogesic) (4) Placebo |
Reports participants with individual AEs, not number with any AE Events: (1) 26 (2) 34 (3) 43 (4) 22 |
None | 2 participants dropped out after first period due to AEs ‐ group not given | No data |
| Göbel 1996 | (1) Paracetamol 1000 mg (2) Peppermint oil solution 10 g (3) Peppermint oil + paracetamol (4) Placebo |
None | None | None | No data |
| Göbel 1998 | (1) Paracetamol 1000 mg (2) Peppermint oil combination (distillate of oelum menthae piperitae, oleum cajeputi, oleum eucalypti, oleum juniperi, and oleum gaultheriae) (3) Placebo |
None | None | None | No data |
| Göbel 2001 1 | (1) Paracetamol 1000 g (2) Peppermint oil (oleum menthae piperitae) solution Ll 170, 10 g (3) Peppermint oil + paracetamol (4) Placebo |
Reports number of events, not number of participants with any AE (1) 15 (4) 13 |
None | None | No data |
| Mehlisch 1998 | (1) Paracetamol 1000 mg (2) Ketoprofen 12.5 mg (3) Ketoprofen 25 mg (4) Placebo |
(1) 16/174 (2) 18/181 (3) 27/176 (4) 8/172 |
None | None 72 participants not included in efficacy analysis: 5 protocol violations, 67 did not record data properly |
At 4 h: (1) 16/166 (2) 10/158 (3) 7/156 (4) 30/151 |
| Migliardi 1994 | Studies 5, 6 (1) Paracetamol 1000 mg (2) Paracetamol 1000 mg + caffeine 130 mg (3) Placebo |
(1) 90/691 (2) 144/692 (3) 41/341 Most were stomach upset, nervousness, dizziness. All transient |
None | None | No data |
| Miller 1987 | (1) Paracetamol 650 mg (2) Naproxen sodium 550 mg (3) Placebo |
(1) 4/43 (2) 7/40 (3) 2/41 None considered "clinically significant" [Note: total number of participants given as 128 ‐ do not know to which groups additional 4 belonged] |
None | None | At 6 h: (1) 15/43 (2) 7/40 (3) 19/41 |
| NCT01755702 | (1) Paracetamol 1000 mg (2) Paracetamol + caffeine 1000 + 130 mg (3) Ibuprofen 400 mg (4) Placebo |
(1) 0/45 (2) 2/47 (3) 4/50 (4) 3/45 |
None | Withdrawal during washout periods: period 1 = 3 period 2 = 5 Reasons and groups not given. No withdrawals during treatment periods (except for remedication ‐ lack of efficacy) |
Median (range) time (minutes) to use (1) 129.5 (129‐130) (2) 119 (119‐119) (3)150 (126‐211) (4) 62 (62‐149) |
| NL9701 | (1) Paracetamol 1000 mg (2) Ibuprofen 400 mg (3) Placebo |
(1) 22/190 (2) 23/194 (3) 8/97 |
None | 32 participants not analysed as did not take medication 8 participants excluded due to major protocol violations No withdrawals following treatment (except for remedication ‐ lack of efficacy) |
At 6 h: (1) 18/188 (2) 25/191 (3) 19/94 |
| Packman 2000 | (1) Paracetamol 1000 mg (2) Ibuprofen liquigel 400 mg (3) Placebo |
None | None | None | No data |
| Peters 1983 | (1) Paracetamol 1000 mg (2) Aspirin 650 mg (3) Placebo |
Number of participants with AEs not reported. No difference between groups Mostly mild |
None | None (38 protocol violations) |
No data |
| Pini 2008 | (1) Paracetamol 1000 mg + caffeine 130 mg (2) Naproxen sodium 550 mg (3) Placebo |
(1) 36.6% (2) 31.2% (3) 36.6% Denominator unclear. Most mild or moderate: nervousness, nausea, drowsiness, and fatigue most common Global assessment of tolerability (very good or excellent): (1) 45.7% (2) 51.6% (3) 41.7% |
None | (1) 1/98 (2) 0/94 (3) 0/98 |
(1) 4.8% (2) 3.3% (3) 10% Denominator unclear |
| Prior 2002 | (1) Paracetamol 1000 mg (2) Naproxen 375 mg (3) Placebo |
(1) 31/308 (2) 35/300 (3) 30/307 |
None | None | At 6 h: (1) 53/304 (2) 49/295 (3) 77/301 Mean time to use (minutes) (1) 324 (2) 326 (3) 308 |
| Schachtel 1991 | (1) Paracetamol 1000 mg (2) Aspirin 1000 mg + caffeine 64 mg (3) Placebo |
(1) 0/100 (2) 0/101 (3) 1/101 |
None | None | At 4 h: (1) 2/100 (2) 2/101 (3) 13/101 |
| Schachtel 1996 | (1) Paracetamol 1000 mg (2) Ibuprofen 400 mg (3) Placebo |
(1) 0/151 (2) 0/153 (3) 1/151 |
None | None | No data |
| Steiner 1998 | (1) Paracetamol 1000 mg (2) Ketoprofen 25 mg (3) Placebo |
(1) 10/116 (2) 15/102 (3) 12/112 |
None | None | 2‐24 h: (1) 53/116 (2) 44/102 (3) 81/112 |
| Steiner 2003 | (1) Paracetamol 500 mg (2) Paracetamol 1000 mg (3) Aspirin 500 mg (4) Aspirin 1000 mg (5) Placebo |
(1) 17/105 (2) 19/111 (3) 21/111 (4) 19/103 (5) 15/112 All mild, transient |
None | None | After 2 h: (1) 27/105 (2) 22/111 (3) 18/111 (4) 16/103 (5) 38/112 |
| Thorpe 1970 | (1) Fiorinal‐Pa (aspirin + caffeine + isobutylallylbarbituric acid + paracetamol) (2) Placebo |
No data | No data | No data | No data |
| Ward 1991 | (1) Paracetamol 648 mg (2) Paracetamol 648 mg + caffeine 65 mg (3) Paracetamol 648 mg + caffeine 130 mg (4) Caffeine 65 mg (5) Caffeine 130 mg (6) Placebo |
No data | No data | No data | No data |
| AE: adverse event; h: hour. | |||||
Data and analyses
Comparison 1. Paracetamol 1000 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 hours | 8 | 5890 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [1.14, 1.40] |
| 2 Pain‐free at 1 hour | 4 | 4717 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.90, 1.48] |
| 3 Pain‐free at 4 hours | 4 | 4909 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.23 [1.16, 1.31] |
| 4 Pain‐free or mild pain at 2 hours | 5 | 5238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.15, 1.28] |
| 5 Use of rescue medication | 6 | 1856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.50, 0.69] |
| 6 Any adverse event | 11 | 5605 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.94, 1.32] |
| 7 Gastrointestinal adverse events | 10 | 5526 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.86, 1.45] |
| 8 Dizziness adverse events | 4 | 4036 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.83, 2.61] |
1.1. Analysis.
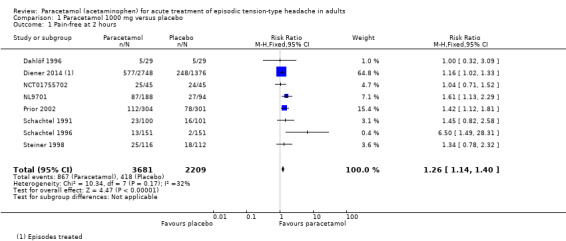
Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 1 Pain‐free at 2 hours.
1.4. Analysis.
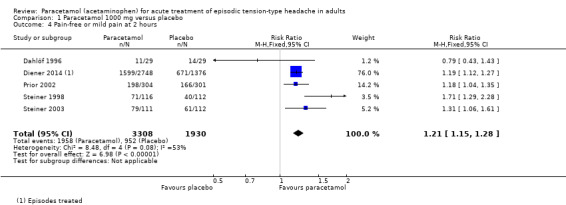
Comparison 1 Paracetamol 1000 mg versus placebo, Outcome 4 Pain‐free or mild pain at 2 hours.
Comparison 2. Paracetamol 500 mg to 650 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free or mild pain at 2 hours | 2 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.90, 1.37] |
| 2 Use of rescue medication | 2 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.55, 1.05] |
| 3 Any adverse event | 2 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.71, 2.35] |
Comparison 3. Paracetamol 1000 mg versus paracetamol 500 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free or mild pain at 2 hours | 2 | 274 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.90, 1.30] |
Comparison 4. Paracetamol 1000 mg versus ketoprofen 25 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 hours | 2 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.49, 1.15] |
| 2 Pain‐free or mild pain at 2 hours | 2 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.72, 1.04] |
| 3 Adverse events | 2 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.39, 0.97] |
Comparison 5. Paracetamol 1000 mg versus ibuprofen 400 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 hours | 3 | 778 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.71, 1.03] |
| 2 Pain‐free at 4 hours | 2 | 683 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.74, 0.93] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dahlöf 1996.
| Methods | R, DB, PC, cross‐over study 5 episodes treated, 1 with each study drug Minimum of 72 h between attacks Diary cards to record details of each attack and response |
|
| Participants | Episodic TTH (IHS), ≥ 1 year, 2‐8 headaches/month
Age range 18‐70 years
Excl: relevant gastrointestinal history or asthma, concomitant NSAID, antiepileptics, chloramphenicol, probenecid, analgesic hypersensitivity
n = 40
M 13, F 27
Mean age 45 years (range 19‐56 years) Baseline PI 56/100 to 60/100 |
|
| Interventions | Paracetamol 500 mg Paracetamol 1000 mg Ketoprofen 25 mg Ketoprofen 50 mg Placebo No medication within 2 h of test medication Rescue medication (usual) allowed after 2 h |
|
| Outcomes | PI: 100 mm VAS and 5‐point VRS PF2, HR2, PID2 Patient global at 2 h: 4‐point VRS Tension/neck muscle stiffness: 5‐point VRS AEs Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Determined according to a Latin square cross‐over design" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐dummy method" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double‐dummy method" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 10/40 participants not included in PP analysis (reported). Reports "similar" result for ITT analysis |
| Size | High risk | < 50 participants per treatment arm (29) |
Diener 2014.
| Methods | R, DB (DD), PC, 2‐period cross‐over study 2 treatment periods in each of which 2 episodes treated with 1 medication; 48 h between same medications, 1 week between different medications Headache ≥ mod intensity |
|
| Participants | Episodic tension headache (ad hoc criteria), ≥ 4 and ≤ 10 episodes/month Age range 18‐65 years Excl: chronic, recurrent, continuous, post‐traumatic, or migraine headache Study 1, n = 446 (437 efficacy) Study 2, n = 475 (447) Study 3, n = 449 (432) Study 4, n = 415 (401) M 301, F 1416 Mean age 34 years (SD 9.8) Baseline pain ≥ mod |
|
| Interventions | Studies 1, 2, 3, 4 Paracetamol 1000 mg, n = 1376 (2748 episodes) Paracetamol 500 mg + aspirin 500 mg + caffeine 130 mg, n = 1369 (2737 episodes) Placebo, n = 689 (1376 episodes) | |
| Outcomes | PI: 4‐point scale over 4 h PR: 5‐point scale over 4 h AEs |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Use of the double‐dummy technique" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Use of the double‐dummy technique" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | BOCF or WOCF for withdrawals |
| Size | Unclear risk | 50‐200 participants per treatment arm (161‐358 in individual studies) |
Friedman 1987.
| Methods | R, DB, PC, parallel groups Single dose to treat 1 episode |
|
| Participants | Tension headache (ad hoc criteria), 6 per month for ≥ 3 months
n = 212 M 25, F 187 Mean age 37 years (range 18‐65 years) Mean pretreatment severity mod/severe |
|
| Interventions | Paracetamol 600 mg + codeine 60 mg, n = 65 Fioricet (butalbital 100 mg + caffeine 80 mg + paracetamol 650 mg), n = 66 Placebo, n = 67 |
|
| Outcomes | PI, SPID over 4 h Participant reporting complete relief at 0.5, 1, 2, 3, 4 h AE |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Given two identical capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Given two identical capsules" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | WOCF for withdrawals |
| Size | Unclear risk | 50‐200 participants per treatment arm (65‐67) |
Gatoulis 2012.
| Methods | R, DB, AC, and PC, parallel groups 1 episode treated with a single dose of study medication when pain was ≥ mod intensity Participant diary |
|
| Participants | Episodic TTH (IHS), 2‐10 episodes/month. Able to distinguish from migraine Excl: known allergy to study interventions, previous non‐response to active interventions, significant medical history, recent head injury n = 559 (safety population), 487 (efficacy) M 183, F 304 Mean age 37 years (range 18‐66 years) Baseline PI: 76% mod, 24% severe |
|
| Interventions | Paracetamol 300 mg + codeine 30 mg, n = 233 Aspirin 1000 mg, n = 223 Placebo, n = 103 Rescue medication (usual treatment) allowed. No timing given |
|
| Outcomes | PI: 4‐point scale (0.5, 1, 2, 3, 4 5, 6 h) PR: 5‐point scale |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Individuals were randomly assigned". Method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double dummy method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double dummy method |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 7‐14% attrition. Imputation method not reported |
| Size | Unclear risk | 50‐200 participants in each treatment arm (103‐233) |
Gilbert 1976.
| Methods | R, DB, AC, and PC, cross‐over study 4 episodes treated, 1 with each study medication, 48 h washout |
|
| Participants | Occasional tension headache associated with anxiety and irritability, 2‐20 attacks/month (median 6) n = 208 (206 completed) M 6, F 200 Mean age 19 years (range 18‐65 years) All but 1 participant usually experienced ≥ mod headache severity |
|
| Interventions | Paracetamol 650 mg Paracetamol 650 mg + phenyltoloxamine citrate 60 mg (Percogesic) Phenyltoloxamine 60 mg Placebo |
|
| Outcomes | PI, tension, anxiety, and irritability: 4‐point scale at baseline Maximum relief of each symptom: 5‐point scale Time to maximum relief: 5‐point scale (0 in 4 h, over 2 h, 1‐2 h, 0.5‐1 h, within 30 minutes) Patient global AEs |
|
| Notes | Oxford Quality Score: R1, DB2, W0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Identical looking tablets" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Identical looking tablets" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No mention of withdrawals or imputation method |
| Size | Low risk | > 200 participants per treatment arm (≥ 206 maximum) |
Göbel 1996.
| Methods | R, DB, AC and PC, cross‐over study 4 episodes treated, 1 with each study medication |
|
| Participants | Tension headache according to ICD‐10 or according to IHS Age range 18‐65 years n = 41 completers (of 54) M 13, F 28 Mean age 34 (SD 13) years Days with tension headache per month: 5 (SD 6) |
|
| Interventions | Paracetamol 1000 mg Peppermint oil solution 10 g Paracetamol plus peppermint oil Placebo Application of peppermint or placebo oils on forehead and temples, repeated 15 and 30 minutes after start of treatment |
|
| Outcomes | PI: 5‐point scale (0‐4) Impairment due to headache: 5‐point scale (0‐4) AEs |
|
| Notes | Oxford Quality Score: R2, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Balanced randomisation, Latin squares |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Capsules of identical appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Capsules of identical appearance |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis (41/54) |
| Size | High risk | < 50 participants per treatment arm (41) |
Göbel 1998.
| Methods | R, DB, AC and PC, cross‐over study 3 episodes treated, 1 with each study medication |
|
| Participants | Tension headache according to ICD‐10 (G 44.2) or according to IHS (Code 2) Age range 18‐65 years n = 38 M 15, F 23 Mean age 52 (SD 18) Days with headache per month: 13.7 (SD 10.3) |
|
| Interventions | Paracetamol 1000 mg Peppermint oil combination (distillate of oelum menthae piperitae, oleum cajeputi, oleum eucalypti, oleum juniperi, and oleum gaultheriae) Placebo Application of peppermint or placebo oils on forehead and temples, repeated 15 and 30 minutes after start of treatment |
|
| Outcomes | PI: 4‐point scale (0‐3) Impairment due to headache: 4‐point scale (0‐3) AEs |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Balanced randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Capsules of identical appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Capsules of identical appearance |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Apparently no withdrawals, no mention of imputation |
| Size | High risk | < 50 participants per treatment arm (≤ 38) |
Göbel 2001.
| Methods | R, DB, AC and PC, cross‐over study 4 episodes treated, 1 with each study medication |
|
| Participants | Tension headache Age range 18‐65 years n = 129 (105 completers included in analysis) |
|
| Interventions | Paracetamol 1000 mg Peppermint oil (oleum menthae piperitae) solution Ll 170, 10 g Peppermint oil plus paracetamol Placebo Application of solution on forehead and temples, repeated 15 and 30 minutes after start of treatment |
|
| Outcomes | PI: 5‐point scale (0‐4) AEs |
|
| Notes | Oxford Quality Score: R2, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced randomisation. Latin squares |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Capsules of identical appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Capsules of identical appearance |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis (105/129) |
| Size | Unclear risk | 50‐200 participants per treatment arm (105) |
Mehlisch 1998.
| Methods | R, DB, PC, parallel‐group study 1 episode treated with a single dose of study medication when pain was ≥ mod intensity |
|
| Participants | Episodic tension headache (IHS), 1‐10 episodes/month Excl: pregnancy/lactating; history of other headache types; significant medical history; chronic analgesia use; drug dependence; hypersensitivity to study medications n = 737 randomised, 703 took medication (631 in analysis) M 201, F 430 Mean age 32 years Baseline pain 88% mod, 12% severe |
|
| Interventions | Paracetamol 1000 mg, n = 174 (166) Ketoprofen 12.5 mg, n = 181 (158) Ketoprofen 25 mg, n = 176 (156) Placebo, n = 172 (151) Rescue medication allowed after 2 h |
|
| Outcomes | PI: 4‐point scale over 4 h PR: 5‐point scale over 4 h Meaningful relief. Functional impairment. Patient global AEs |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double dummy method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double dummy method |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | WOCF for withdrawals, LOCF for other, linear interpolation for missing points |
| Size | Unclear risk | 50‐200 participants per treatment arm (151‐166) |
Migliardi 1994.
| Methods | R, DB (DD), PC, 2‐period cross‐over study 2 treatment periods in each of which 2 episodes treated with 1 medication; 48 h between same medications, 1 week between different medications Headache ≥ mod intensity |
|
| Participants | Episodic tension headache (ad hoc criteria), mean 6 or 7 episodes/month Excl: chronic, recurrent, continuous, post‐traumatic or migraine headache Age range 18‐65 years Study 5, n = 441 (415) Study 6, n = 442 (423) M:F ratio about 1:5 (all studies) Baseline pain ≥ mod |
|
| Interventions | Studies 5, 6 Paracetamol 1000 mg Paracetamol 1000 mg + caffeine 130 mg Placebo Rescue medication allowed after 2 h |
|
| Outcomes | PI: 4‐point scale over 4 h PR: 5‐point scale over 4 h AEs |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Use of the double‐dummy technique" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Use of the double‐dummy technique" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | WOCF for withdrawals |
| Size | Unclear risk | 50‐200 participants per treatment arm (162‐337 in individual studies) |
Miller 1987.
| Methods | R, DB, PC, parallel‐group study 1 episode treated with a single dose of study medication when pain was ≥ mod intensity |
|
| Participants | Tension headache (ad hoc criteria) n = 149 Groups balanced for age and sex Age range 18‐65 years Baseline pain mod or greater; significantly greater for naproxen than paracetamol |
|
| Interventions | Paracetamol 650 mg, n = 50 (43) Naproxen sodium 550 mg, n = 51 (40) Placebo, n = 48 (41) Rescue medication allowed after 2 h |
|
| Outcomes | PI: 10 cm VAS over 12 h (6 h analysed) PR: 10 cm VAS Patient global |
|
| Notes | Oxford Quality Score: R1, DB2, W0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double dummy method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double dummy method |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | LOCF for withdrawals. Approximately 17% of participants not eligible for efficacy analysis (lost to follow‐up, non‐compliance, protocol violations). Did not contribute data to any efficacy analysis |
| Size | High risk | < 50 participants per treatment arm (≤ 48) |
NCT01755702.
| Methods | R, DB (DD), AC, PC, cross‐over study. Washout time not specified Up to 3 episodes treated, 1 dose per episode |
|
| Participants | Episodic TTH, 4‐10 episodes/month in previous 3 months, usually ≥ mod intensity (untreated), previously responsive to OTC medication Age range 18‐65 years n = 66 M 22, F 44 Mean age 42 years (SD 12) |
|
| Interventions | Paracetamol 1000 mg Paracetamol 1000 mg + caffeine 130 mg Ibuprofen 400 mg Placebo |
|
| Outcomes | PR: 5‐point scale PF at 1 and 2 h Rescue medication AEs |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double dummy method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double dummy method |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All treated attacks analysed |
| Size | High risk | < 50 participants in each treatment group (45‐50) |
NL9701.
| Methods | R, DB (DD), AC, PC, parallel group 1 episode treated with a single dose of study medication when pain was ≥ mod intensity, with no significant nausea, and no headache within previous 48 h |
|
| Participants | Episodic TTH (IHS), ≥ 1 year, 2‐10 episodes/month, aged 18‐65 years, in good health Excl: known hypersensitivity to any test medication, other contraindications, chronic pain, ≥ 1 migraine episode/month n = 513 (473 for efficacy, 481 for safety analyses) M 125, F 348 Mean age 29 years (range 18‐63 years) Baseline PI: 85% mod, 15% severe |
|
| Interventions | Paracetamol 1000 mg, n = 188 Ibuprofen 400 mg, n = 191 Placebo, n = 94 |
|
| Outcomes | PF at 2, 4, 24 h SPID at 2 h Use of rescue medication AEs |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not described, "randomisation list" |
| Allocation concealment (selection bias) | Low risk | Tablets packaged independently and supplied in blister packs with tear‐off labels. Allocated sequentially |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double dummy method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double dummy method |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Appropriate imputation for missing data points |
| Size | Unclear risk | 50‐200 participants per treatment arm (94‐191) |
Packman 2000.
| Methods | R, DB, PC, parallel‐group 1 episode treated with a single dose of study medication when pain was ≥ mod intensity |
|
| Participants | Episodic tension headache (IHS), 4‐15 headaches per month Excl: habituation to analgesics, recurrent migraines, menstrual headaches, contraindications to aspirin, other NSAIDs or paracetamol n = 154 M 37, F 117 Mean age about 40 years Baseline pain ≥ moderately severe or ≥ 66/100 |
|
| Interventions | Paracetamol 1000 mg, n = 62 Ibuprofen liquigel 400 mg, n = 60 Placebo, n = 32 |
|
| Outcomes | PI: 4‐point scale at 2 and 3 h PR: 5‐point scale at 2 and 3 h Perceptible and meaningful relief AEs |
|
| Notes | Oxford Quality Score: R2, DB1, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated code" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details of blinding not reported and medicines were liquigels, caplets, or placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of blinding not reported and medicines were liquigels, caplets, or placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals but imputation not mentioned |
| Size | High risk | < 50 participants per treatment arm (32‐62) |
Peters 1983.
| Methods | R, DB, PC, parallel‐group trial 1 episode treated with a single dose of study medication when pain was ≥ mod intensity |
|
| Participants | Headache (tension and tension‐vascular) clinically moderately severe, impairing efficiency, previously responsive to OTC medication: divided into tension headache and tension‐vascular headache Excl: significant medical history; history other headache type; headache severe enough to render bed bound; intolerance to study medications n = 307 completed study (269 evaluated) M 53, F 216 (evaluated participants) Mean age 32 years Baseline pain moderately severe |
|
| Interventions | Paracetamol 1000 mg, n = 87 Aspirin 650 mg, n = 90 Placebo, n = 92 No rescue medication allowed for 6 h |
|
| Outcomes | PI: 3‐point scale over 6 h PR: 4‐point scale over 6 h AEs |
|
| Notes | Oxford Quality Score: R1, DB2, W0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method not reported Participants not randomised according to type of headache |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double dummy method. "The tablets of each size were identical in appearance" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double dummy method "The tablets of each size were identical in appearance" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | May be participants randomised who did not complete. No mention of imputation |
| Size | Unclear risk | 50‐200 participants per treatment arm (87‐92) |
Pini 2008.
| Methods | R, DB, PC, cross‐over trial 3 consecutive episodes treated with 3 different medications |
|
| Participants | Episodic TTH (IHS), 4‐14 days/month, no nausea, vomiting, photo‐ or phonophobia. Previous response to OTC medication. Daily consumption ≥ 2 cups of coffee Excl: contraindications to study medication; migraine or post‐traumatic headache; overuse of analgesics; significant medical history n = 99 (93 for safety, 81 for efficacy) M 40, F 59 Mean age 35 years (SD 10, range 19‐64 years) Baseline PI: 59% mod, 16% mild, 25% severe |
|
| Interventions | Paracetamol 1000 mg + caffeine 130 mg Naproxen sodium 550 mg Placebo Rescue medication (ibuprofen 600 mg) to be taken at 2 h if necessary |
|
| Outcomes | PI: 4‐point scale over 4 h PR: 5‐point scale over 4 h Rescue medication Patient global evaluation: 5‐point scale at 4 h Use of rescue medication AEs |
|
| Notes | Oxford Quality Score: R2, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation code" |
| Allocation concealment (selection bias) | Low risk | "Assigned in sequential order of entry"; "access to the randomization code was strictly controlled and treatment assignment remained unknown to all parties until formal database lock" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "double‐dummy method"; "identical boxes"; "matched supplies, identical in colour, size, shape and taste" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "double‐dummy method"; "identical boxes"; "matched supplies, identical in colour, size, shape and taste" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis included only those taking all 3 interventions. LOCF imputation. 10 participants did not provide pain relief data, with no reason given |
| Size | Unclear risk | 50‐200 participants per treatment arm (94‐98) |
Prior 2002.
| Methods | R, DB, PC, parallel‐group study 1 episode treated with a single dose of study medication when pain was ≥ mod intensity |
|
| Participants | Episodic tension headache (IHS), usually relieved by OTC medication, < 15 episodes/month (range 4‐10) Excl: headache satisfying alternative diagnosis; daily analgesic use; serious medical conditions n = 915 (900 evaluated) M 254, F 646 Age range 18‐87 years Baseline pain ≥ mod |
|
| Interventions | Paracetamol 1000 mg, n = 304 Naproxen 375 mg, n = 295 Placebo, n = 301 Rescue medication allowed after 1 h |
|
| Outcomes | PI: 4‐point scale over 6 h PR: 5‐point scale over 6 h Meaningful relief Patient global evaluation: 5‐point scale at 6 h PF at 2 h (post hoc) Use of rescue medication AEs |
|
| Notes | Oxford Quality Score: R2, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated randomisation schedule" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Two capsules that were identical in colour, size and shape" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Two capsules that were identical in colour, size and shape" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Excl and withdrawals evenly distributed, BOCF for PR, but 'ITT' does not appear to include withdrawals |
| Size | Low risk | > 200 participants per treatment arm (≥ 300) |
Schachtel 1991.
| Methods | R, DB, PC, parallel‐group study 1 episode treated with a single dose of study medication when pain was ≥ mod intensity |
|
| Participants | Tension headache (ad hoc criteria) ≥ 2/month. Previous satisfactory relief with OTC medication Excl: migraine headaches; hypersensitivity to paracetamol, aspirin, or caffeine; pregnant or breastfeeding n = 327 (302 eligible) M 114, F 188 Mean age 21 years (range 18‐65 years) Baseline pain ≥ mod, mean 69/100 |
|
| Interventions | Paracetamol 1000 mg, n = 100 Aspirin 1000 mg + caffeine 64 mg, n = 101 Placebo, n = 101 Rescue medication allowed after 2 h |
|
| Outcomes | PI: 100 mm VAS over 4 h PR: 6‐point scale over 4 h Complete relief ‐ number with and time to Preference over usual medication |
|
| Notes | Oxford Quality Score: R2, DB1, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated randomization code" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Excl < 8%, mostly mild headaches. BOCF for PR |
| Size | Unclear risk | 50‐200 participants per treatment arm (100‐101) |
Schachtel 1996.
| Methods | R, DB, PC, parallel‐group study 1 episode treated with a single dose of study medication when pain was ≥ mod intensity |
|
| Participants | Tension headache (ad hoc criteria) ≥ 2/month. Previous satisfactory relief with OTC medication Excl: history of migraine; gastrointestinal/hepatic/renal disease; menstrual dysfunction; pregnant; breastfeeding; intolerance to paracetamol or ibuprofen n = 613 screened, 455 analysed M 170, F 285 Mean age 21 years Baseline pain ≥ mod, mean 71/100 |
|
| Interventions | Paracetamol 1000 mg, n = 151 Ibuprofen 400 mg, n = 153 Placebo, n = 151 |
|
| Outcomes | PI: 100 mm VAS over 4 h PR: 6‐point scale over 4 h Complete relief ‐ number with and time to |
|
| Notes | Oxford Quality Score: R2, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated randomization code" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Identically appearing opaque capsules" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Identically appearing opaque capsules" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No mention of imputation |
| Size | Unclear risk | 50‐200 participants per treatment arm (151‐153) |
Steiner 1998.
| Methods | R, DB, PC, parallel‐group study 1 episode treated with a single dose of study medication when pain was ≥ mod intensity, 1‐12 h after onset of headache |
|
| Participants | Episodic tension headache (IHS) (< 15/month) Excl: migraine; pregnant/breastfeeding; history gastrointestinal ulcer/haemorrhage; history alcohol/med misuse n = 348 (339 for efficacy) M 72, F 267 Median age 42 years (range 18‐74 years) Mean baseline pain 60/100 (17‐100) |
|
| Interventions | Paracetamol 1000 mg, n = 123 Ketoprofen 25 mg, n = 109 Placebo, n = 116 Rescue medication allowed after 2 h |
|
| Outcomes | PI: 100 mm VAS over 2 h PR: 7‐point scale at 2 h Patient global evaluation: 5‐point scale at 2 h Functional ability: 4‐point scale at 2 and 4 h, and time to return to normal function Use of rescue medication at/after 2 h AEs |
|
| Notes | Oxford Quality Score: R1, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy method. "matching placebos" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐dummy method. "matching placebos" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis reported, but no mention of imputation |
| Size | Unclear risk | 50‐200 participants per treatment arm (109‐123) |
Steiner 2003.
| Methods | R, DB, PC, parallel‐group study 1 episode treated with a single dose of study medication when pain was ≥ mod intensity, 1‐12 h after onset of headache |
|
| Participants | Episodic tension headache (IHS), < 15/month Excl: migraine; pregnant/breastfeeding; history gastrointestinal ulcer/haemorrhage; history alcohol/med misuse n = 542 M:F ratio about 30:70 Mean age 40 years (SD 12; range 16‐65 years) Mean baseline pain ≥ 57/100 (median ≥ 60/100) |
|
| Interventions | Paracetamol 500 mg, n = 105 Paracetamol 1000 mg, n = 111 Aspirin 500 mg, n = 111 Aspirin 1000 mg, n = 103 Placebo, n = 112 Rescue medication allowed after 2 h |
|
| Outcomes | PI: 100 mm VAS PR: 7‐point scale Functional ability: 4‐point scale, and time to return to normal function Patient global evaluation: 5‐point scale AEs |
|
| Notes | Oxford Quality Score: R2, DB2, W1 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated list" |
| Allocation concealment (selection bias) | Low risk | Assigned in numerical sequence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double dummy method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double dummy method |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis reported, with LOCF imputation |
| Size | Unclear risk | 50‐200 participants per treatment arm (103‐112) |
Thorpe 1970.
| Methods | R, DB, PC, parallel‐group study 1 episode treated with up to 2 doses of study medication. Capsules taken at onset on headache |
|
| Participants | Typical history of tension headache without other causes* n = 52 (completed to extent that reports could be analysed) No demographic data Baseline pain not reported |
|
| Interventions | Fiorinal‐Pa (aspirin + caffeine + isobutylallylbarbituric acid + paracetamol), n = 25 Placebo, n = 27 |
|
| Outcomes | PI: 5‐point scale 4 h after 1st dose Response = reduction ≥ 2 grades on PI AEs |
|
| Notes | * Lance JW. Treatment of chronic tension headache. Lancet 1964;1:1236‐9 Oxford Quality Score: R2, DB1, W0 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "According to tables set out by Smart". Judged adequate |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported on participants who "completed to extent that reports could be analysed". Unclear what this means. No mention of withdrawals or imputation |
| Size | High risk | < 50 participants per treatment arm (25‐27) |
Ward 1991.
| Methods | R, DB, AC, PC, cross‐over study 4 episodes treated, each with 1 of 4 study interventions |
|
| Participants | Non‐migrainous headaches, ≥ 6/month of > mild intensity n = 60 completed (53 for efficacy) M 17, F 36 Mean age 37 years (range 20‐60 years) Baseline pain ≥ 48/100 |
|
| Interventions | Paracetamol 648 mg Paracetamol 648 mg + caffeine 65 mg Paracetamol 648 mg + caffeine 130 mg Caffeine 65 mg Caffeine 130 mg Placebo |
|
| Outcomes | PI: 10 cm VAS over 2 h Profile of mood states over 2 h |
|
| Notes | Oxford Quality Score: R1, DB1, W0 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation method not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Completer analysis |
| Size | Unclear risk | 50‐200 participants per treatment arm (≤ 53) |
AC: active controlled; AE: adverse event; BOCF: baseline observation carried forward; DB: double‐blind; DD: double‐dummy; Excl: exclusions; F: female; h: hour; HR: headache relief; ICD: International Classification of Diseases; IHS: International Headache Society; ITT: intention to treat; M: male; mod: moderate; N: number of participants in study; n: number of participants in treatment arm; NSAID: nonsteroidal anti‐inflammatory drug; OTC: over‐the‐counter; PC: placebo controlled; PF: pain‐free: PI: pain intensity; PID: pain intensity difference; PP: per protocol; PR: pain relief; R: randomised; SD: standard deviation; SPID: summed pain intensity difference; TTH: tension‐type headache; VAS: visual analogue scale; VRS: verbal rating scale; W: withdrawals; WOCF: worst observation carried forward.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| de Souza Carvalho 2012 | Mixed headaches. Data not available separately for 17 participants with tension headache |
| Diener 2005 | Mixed tension (16%) and migraine (84%) headaches. Data not reported separately |
| NCT01552798 | Study terminated with only 9 participants enrolled. No results |
| Wójcicki 1977 | "Common idiopathic headache". Not clearly randomised |
Characteristics of ongoing studies [ordered by study ID]
NCT01842633.
| Trial name or title | A Study to Assess Efficacy Over Placebo and Speed of Onset of Pain Relief of New Paracetamol and Caffeine Tablets as Compared to Ibuprofen in Episodic Tension Type Headache |
| Methods | R, DB (DD), AC, PC, parallel group |
| Participants | Episodic tension‐type headache, ≥ 2 episodes/month, ≥ moderate intensity, normal duration ≥ 4 h Age 18‐65 years Estimated enrolment 300 |
| Interventions | Paracetamol 1000 mg + caffeine 130 mg Ibuprofen 400 mg Placebo |
| Outcomes | TOTPAR PID, SPID Rescue medication |
| Starting date | April 2013 |
| Contact information | GSKClinicalSupportHD@gsk.com |
| Notes | Estimated completion date March 2015 |
AC: active controlled; DB: double‐blind; DD: double‐dummy; h: hour; PC: placebo controlled; PID: pain intensity difference; R: randomised; SPID: summed pain intensity difference; TOTPAR: total pain relief.
Differences between protocol and review
For the full review, we specified a time point of two hours for pain intensity difference outcomes. In practice we were unable to carry out any analysis for this outcome, and results are presented in Appendix 5 for the measures closest to two hours.
We had planned to exclude data from outcomes where results from 10% or more of participants were missing with no acceptable reason provided or apparent. We chose not to do this in the full review as we felt this is taken into account in the risk of bias and quality of evidence assessments. In the event, it only affected one study (Miller 1987).
We added 'use of rescue medication' to our list of secondary outcomes, in keeping with other reviews in this series. Use of rescue medication equates to withdrawal due to lack of efficacy.
We did not state in the protocol that we would include a 'Summary of findings table'. This has been added to the methods for the full review.
Contributions of authors
All authors participated in writing the protocol.
GS and SD carried out searches, identified studies for inclusion, and carried out data extraction and analyses.
All authors were involved in writing the review.
Sources of support
Internal sources
-
The Oxford Pain Research Trust, UK.
Institutional support
External sources
No sources of support supplied
Declarations of interest
GS: none known.
SD: none known.
RAM: has received grant support from RB relating to individual patient level analyses of trial data on ibuprofen in acute pain and the effects of food on drug absorption of analgesics (2013), and from Grünenthal relating to individual patient level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015).
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Dahlöf 1996 {published data only}
- Dahlöf CG, Jacobs LD. Ketoprofen, paracetamol and placebo in the treatment of episodic tension‐type headache. Cephalalgia 1996;16(2):117‐23. [PUBMED: 8665578] [DOI] [PubMed] [Google Scholar]
Diener 2014 {published data only}
- Diener HC, Gold M, Hagen M. Use of a fixed combination of acetylsalicylic acid, acetaminophen and caffeine compared with acetaminophen alone in episodic tension‐type headache: meta‐analysis of four randomized, double‐blind, placebo‐controlled, crossover studies. Journal of Headache and Pain 2014;15:76. [DOI: 10.1186/1129-2377-15-76] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliardi JR, Armellino JJ, Friedman M, Gillings DB, Beaver WT. Caffeine as an analgesic adjuvant in tension headache (studies 1‐4). Clinical Pharmacology and Therapeutics 1994;56(5):576‐86. [DOI: 10.1038/clpt.1994.179] [DOI] [PubMed] [Google Scholar]
Friedman 1987 {published data only}
- Friedman AP, DiSerio F. Symptomatic treatment of chronically recurring tension headache: a placebo‐controlled, multicenter investigation of Fioricet® and acetaminophen with codeine. Clinical Therapeutics 1987;10(1):69‐81. [PUBMED: 3329967] [PubMed] [Google Scholar]
Gatoulis 2012 {published data only}
- Gatoulis SC, Voelker M, Fisher M. Assessment of the efficacy and safety profiles of aspirin and acetaminophen with codeine: results from 2 randomized, controlled trials in individuals with tension‐type headache and postoperative dental pain. Clinical Therapeutics 2012;34(1):138‐48. [PUBMED: 22169050] [DOI] [PubMed] [Google Scholar]
Gilbert 1976 {published data only}
- Gilbert MM, Sola Pool N, Schecter C. Analgesic/calmative effects of acetaminophen and phenyltoloxamine in treatment of simple nervous tension accompanied by headache. Current Therapeutic Research, Clinical and Experimental 1976;20(1):53‐8. [PUBMED: 821705] [PubMed] [Google Scholar]
Göbel 1996 {published data only}
- Göbel H, Fresenius J, Heinze A, Dworschak M, Soyka D. Effectiveness of oleum menthae piperitae and paracetamol in therapy of headache of the tension type [in German] [Effektivität von oleum menthae piperitae und von Paracetamol in der Therapie des Kopfschmerzes vom Spannungstyp]. Nervenarzt 1996;67(8):672‐81. [PUBMED: 8805113] [DOI] [PubMed] [Google Scholar]
Göbel 1998 {published data only}
- Göbel H, Heinze A, Lurch A, Dworschak M. Essential oils in the therapy of tension headache [in German]. Zeitschrift fur Allgemeinmedizin 1998;74(4):223‐8. [Google Scholar]
Göbel 2001 {published data only}
- Göbel H, Heinze A, Dworschak M, Heinze‐Kuhn K, Stolze H. Analgesic efficacy and tolerability of locally applied Oleum menthae piperitae preparation LI 170 in patients with migraine or tension‐type headache [German] [Wirksamkeit und verträglichkeit von oleum‐menthaepiperitae‐lösung LI 170 bei kopfschmerz vom spannungstyp und migräne]. Zeitschrift fur Allgemeinmedizin 2001;77(6):287‐295. [Google Scholar]
Mehlisch 1998 {published data only}
- Mehlisch DR, Weaver M, Fladung B. Ketoprofen, acetaminophen, and placebo in the treatment of tension headache. Headache 1998;38(8):576‐89. [DOI: 10.1046/j.1526-4610.1998.3808579.x] [DOI] [PubMed] [Google Scholar]
Migliardi 1994 {published data only}
- Migliardi JR, Armellino JJ, Friedman M, Gillings DB, Beaver WT. Caffeine as an analgesic adjuvant in tension headache (studies 5 and 6). Clinical Pharmacology & Therapeutics 1994;56(5):576‐86. [DOI: 10.1038/clpt.1994.179] [DOI] [PubMed] [Google Scholar]
Miller 1987 {published data only}
- Miller DS, Talbot CA, Simpson W, Korey A. A comparison of naproxen sodium, acetaminophen and placebo in the treatment of muscle contraction headache. Headache 1987;27(7):392‐6. [DOI: ] [DOI] [PubMed] [Google Scholar]
NCT01755702 {published data only}
- GlaxoSmithKline. A study to assess the efficacy of paracetamol taken in combination with caffeine for the treatment of episodic tension type headache, 2015. www.clinicaltrials.gov/ct2/show/NCT01755702 (accessed 22 December 2015).
NL9701 {published data only}
- Daniels SE (Study investigator). A randomised, double‐blind, placebo‐controlled, double‐dummy, parallel group comparison of commercial ibuprofen (single dose of 400 mg) versus commercial paracetamol (single dose of 1000 mg) in patients with tension type headache. Supplied by Reckitt Benckiser 2001.
Packman 2000 {published data only}
- Packman B, Packman E, Doyle G, Cooper S, Ashraf E, Koronkiewicz K, et al. Solubilized ibuprofen: evaluation of onset, relief, and safety of a novel formulation in the treatment of episodic tension‐type headache. Headache 2000;40(7):561‐7. [DOI: 10.1046/j.1526-4610.2000.00087.x] [DOI] [PubMed] [Google Scholar]
Peters 1983 {published data only}
- Peters BH, Fraim CJ, Masel BE. Comparison of 650 mg aspirin and 1,000 mg acetaminophen with each other, and with placebo in moderately severe headache. American Journal of Medicine 1983;74(6):36‐42. [DOI: 10.1016/0002-9343(83)90526-0] [DOI] [PubMed] [Google Scholar]
Pini 2008 {published data only}
- Pini LA, Bene E, Zanchin G, Sarchielli P, Trapani G, Prudenzano MP, et al. Tolerability and efficacy of a combination of paracetamol and caffeine in the treatment of tension‐type headache: a randomised, double‐blind, double‐dummy, cross‐over study versus placebo and naproxen sodium. Journal of Headache and Pain 2008;9(6):367‐73. [PUBMED: 18815727] [DOI] [PMC free article] [PubMed] [Google Scholar]
Prior 2002 {published data only}
- Prior MJ, Cooper KM, May LG, Bowen DL. Efficacy and safety of acetaminophen and naproxen in the treatment of tension‐type headache. A randomised, double‐blind, placebo‐controlled trial. Cephalgia 2002;20(9):740‐8. [PUBMED: 12421160 ] [DOI] [PubMed] [Google Scholar]
Schachtel 1991 {published data only}
- Schachtel BP, Thoden WR, Konerman JP, Brown A, Chaing DS. Headache pain model for assessing and comparing the efficacy of over‐the‐counter analgesic agents. Clinical Pharmacology & Therapeutics 1991;50(3):322‐9. [DOI: 10.1038/clpt.1991.143] [DOI] [PubMed] [Google Scholar]
Schachtel 1996 {published data only}
- Schachtel BP, Furey SA, Thoden WR. Nonprescription ibuprofen and acetaminophen in the treatment of tension‐type headache. Journal of Clinical Pharmacology 1996;36(12):1120‐5. [DOI: 10.1002/j.1552-4604.1996.tb04165.x] [DOI] [PubMed] [Google Scholar]
Steiner 1998 {published data only}
- Steiner TJ, Lange R. Ketoprofen (25 mg) in the symptomatic treatment of episodic tension‐type headache: double‐blind placebo‐controlled comparison with acetaminophen (1000 mg). Cephalgia 1998;18(1):38‐43. [PUBMED: 9601623] [DOI] [PubMed] [Google Scholar]
Steiner 2003 {published data only}
- Steiner TJ, Lange R, Voelker M. Aspirin in episodic tension‐type headache: placebo‐controlled dose‐ranging comparison with paracetamol. Cephalgia 2003;23(1):59‐66. [PUBMED: 12534583] [DOI] [PubMed] [Google Scholar]
Thorpe 1970 {published data only}
- Thorpe P. Controlled and uncontrolled studies on "Fiorinal‐PA" for symptomatic relief in tension headache. Medical Journal of Australia 1970;2(4):180‐1. [PUBMED: 4917129] [DOI] [PubMed] [Google Scholar]
Ward 1991 {published data only}
- Ward N, Whitney C, Avery D, Dunner D. The analgesic effects of caffeine in headache. Pain 1991;44(2):151‐5. [DOI: 10.1016/0304-3959(91)90129-L] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
de Souza Carvalho 2012 {published data only}
- Souza Carvalho D, Barea LM, Kowacs PA, Fragoso YD. Efficacy and tolerability of combined dipyrone, isometheptene and caffeine in the treatment of mild‐to‐moderate primary headache episodes. Expert Review of Neurotherapeutics 2012;12(2):159‐67. [DOI: 10.1586/ern.11.193] [DOI] [PubMed] [Google Scholar]
Diener 2005 {published data only}
- Diener HC, Peil H, Aicher B. The efficacy and tolerability of a fixed combination of acetylsalicylic acid, paracetamol, and caffeine in patients with severe headache: a post‐hoc subgroup analysis from a multicentre, randomized, double‐blind, single‐dose, placebo‐controlled parallel group study. Cephalalgia 2011;31(14):1466‐76. [DOI: 10.1177/0333102411419682] [DOI] [PubMed] [Google Scholar]
- Diener HC, Pfaffenrath V, Pageler L, Peil H, Aicher B. The fixed combination of acetylsalicylic acid, paracetamol and caffeine is more effective than single substances and dual combination for the treatment of headache: a multicentre, randomized, double‐blind, single‐dose, placebo‐controlled parallel group study. Cephalalgia 2005;25(10):776‐87. [DOI: 10.1111/j.1468-2982.2005.00948.x] [DOI] [PubMed] [Google Scholar]
NCT01552798 {published data only}
- Bayer. A double‐blind, randomized, parallel, placebo‐controlled trial assessing the analgesic efficacy of a single dose of fast release aspirin 1000 mg and acetaminophen 1000 mg in tension type headache pain, 2014. www.clinicaltrials.gov/ct2/show/NCT01552798 (accessed 22 December 2015).
Wójcicki 1977 {published data only}
- Wójcicki J, Samochowiec L, Lawczyński L, Szwed G, Olszewska M. A double‐blind comparative evaluation of aspirin, paracetamol and paracetamol + caffeine (Finimal) for their analgesic effectiveness. Archivum Immunologiae et Therapiae Experimentalis (Warsz) 1977;25(2):175‐9. [PUBMED: 326219] [PubMed] [Google Scholar]
References to ongoing studies
NCT01842633 {published data only}
- GlaxoSmithKline. A study to assess efficacy over placebo and speed of onset of pain relief of new paracetamol and caffeine tablets as compared to ibuprofen in episodic tension type headache, 2014. www.clinicaltrials.gov/ct2/show/record/NCT01842633 (accessed 22 December 2015).
Additional references
Ad Hoc Committee 1962
- Ad Hoc Committee on Classification of Headache. Classification of headache. JAMA 1962;179:717‐8. [Google Scholar]
BASH 2010
- MacGregor EA, Steiner TJ, Davies PTG, for the British Association for the Study of Headache. Guidelines for all healthcare professionals in the diagnosis and management of migraine, tension‐type, cluster, and medication overuse headache. 3rd edition (1st revision), 2010. www.bash.org.uk/wp‐content/uploads/2012/07/10102‐BASH‐Guidelines‐update‐2_v5‐1‐indd.pdf (accessed 22 December 2015).
Bateman 2014a
- Bateman DN, Carroll R, Pettie J, Yamamoto T, Elamin ME, Peart L, et al. Effect of the UK's revised paracetamol poisoning management guidelines on admissions, adverse reactions, and costs of treatment. British Journal of Clinical Pharmacology 2014;78(3):610‐8. [DOI: 10.1111/bcp.12362] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bateman 2014b
- Bateman DN. Pack size and paracetamol overdose: 16 years later. Clinical Toxicology 2014;52:821‐3. [DOI: 10.3109/15563650.2014.952432] [DOI] [PubMed] [Google Scholar]
Bendtsen 2010
- Bendtsen L, Evers S, Linde M, Mitsikostas DD, Sandrini G, Schoenen J. EFNS guideline on the treatment of tension‐type headache ‐ report of an EFNS taskforce. European Journal of Neurology 2010;17(11):1318‐25. [DOI: 10.1111/j.1468-1331.2010.03070.x] [DOI] [PubMed] [Google Scholar]
Botting 2000
- Botting RM. Mechanism of action of acetaminophen: is there a cyclooxygenase 3?. Clinical Infectious Diseases 2000;31(5):S203‐10. [DOI: 10.1086/317520] [DOI] [PubMed] [Google Scholar]
Brainin 2004
- Brainin M, Barnes M, Baron JC, Gilhus NE, Hughes R, Selmaj K, et al. for the Guideline Standards Subcommittee of the EFNS Scientific Committee. Guidance for the preparation of neurological management guidelines by EFNS scientific task forces‐revised recommendations 2004. European Journal of Neurology 2004;11(9):577‐81. [DOI: 10.1111/j.1468-1331.2004.00867.x] [DOI] [PubMed] [Google Scholar]
Chandrasekharan 2002
- Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, et al. COX‐3, a cyclooxygenase‐1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure and expression. Proceedings of the National Academy of Sciences of the United States of America 2002;99(21):13926‐31. [DOI: 10.1073/pnas.162468699] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452‐4. [DOI: 10.1136/bmj.310.6977.452] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cristofolini 2008
- Cristofolini A, Dalla Serra P, Scherillo G, Orrico D, Micciolo R. The prevalence of headache in a population of health care workers and the effects on productivity costs. La Medicina del Lavoro 2008;99(1):8‐15. [PubMed] [Google Scholar]
CSM 1997
- Committee on Safety of Medicines. Medicines Control Agency. Paracetamol and aspirin. Current Problems in Pharmacovigilance 1997;23:9. [Google Scholar]
Dart 2000
- Dart RC, Kuffner EK, Rumack BH. Treatment of pain or fever with paracetamol (acetaminophen) in the alcoholic patient: a systematic review. American Journal of Medicine 2000;7(2):123‐34. [PUBMED: 11319580] [DOI] [PubMed] [Google Scholar]
Dechartres 2013
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta‐epidemiological study. BMJ 2013;346:f2304. [DOI: 10.1136/bmj.f2304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2013
- Derry S, Moore RA. Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008040.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2015
- Derry S, Wiffen PJ, Moore RA, Bendtsen L. Ibuprofen for acute treatment of episodic tension‐type headache in adults. Cochrane Database of Systematic Reviews 2015, Issue 7. [DOI: 10.1002/14651858.CD011474.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Edmeads 1993
- Edmeads J, Findlay H, Tugwell P, Pryse‐Phillips W, Nelson RF, Murray TJ. Impact of migraine and tension‐type headache on life‐style, consulting behaviour, and medication use: a Canadian population survey. Canadian Journal of Neurological Sciences 1993;20(2):131‐7. [PUBMED: 8334575] [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI: 10.1093/ije/31.1.140] [DOI] [PubMed] [Google Scholar]
Eypasch 1995
- Eypasch E, Lefering R, Kum CK, Troidl H. Probability of adverse events that have not yet occurred: a statistical reminder. BMJ 1995;311(7005):619‐20. [DOI: 10.1136/bmj.311.7005.619] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fernández‐de‐las‐Peñas 2010
- Fernández‐de‐las‐Peñas C, Madeleine P, Caminero AB, Cuadrado ML, Arendt‐Nielsen L, Pareja JA. Generalized neck‐shoulder hyperalgesia in chronic tension‐type headache and unilateral migraine assessed by pressure pain sensitivity topographical maps of the trapezius muscle. Cephalalgia 2010;30(1):77‐86. [DOI: 10.1111/j.1468-2982.2009.01901.x] [DOI] [PubMed] [Google Scholar]
Flower 1972
- Flower RJ, Vane JR. Inhibition of prostaglandin synthetase in brain explains the anti‐pyretic activity of paracetamol (4‐acetamidophenol). Nature 1972;240(5381):410‐1. [PUBMED: 4564318] [DOI] [PubMed] [Google Scholar]
Forward 1998
- Forward SP, McGrath PJ, MacKinnon D, Brown TL, Swann J, Currie EL. Medication patterns of recurrent headache sufferers: a community study. Cephalalgia 1998;18(3):146‐51. [DOI: 10.1046/j.1468-2982.1998.1803146.x] [DOI] [PubMed] [Google Scholar]
Fumal 2008
- Fumal A, Schoenen J. Tension‐type headache: current research and clinical management. Lancet Neurology 2008;7(1):70‐83. [DOI: 10.1016/S1474-4422(07)70325-3] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University. GRADEpro Guideline Development Tool. McMaster University, 2015.
Graham 2005
- Graham GG, Scott KF. Mechanism of action of paracetamol. American Journal of Therapeutics 2005;12(1):46‐55. [DOI: 10.1097/00045391-200501000-00008] [DOI] [PubMed] [Google Scholar]
Green 2009
- Green MW. Primary and secondary headaches. In: Rowland LP, Pedley TA editor(s). Merritt's Neurology. 12th Edition. Philadelphia: Lippincott Williams and Wilkins, 2009:951‐60. [ISBN: 9780781791861] [Google Scholar]
Gulmez 2013
- Gulmez SE, Larrey D, Pageaux GP, Lignot S, Lassalle R, Jové J, et al. Transplantation for acute liver failure in patients exposed to NSAIDs or paracetamol (acetaminophen): the multinational case‐population SALT study. Drug Safety 2013;36(2):135‐44. [DOI: 10.1007/s40264-012-0013-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gulmez 2015
- Gulmez SE, Larrey D, Pageaux GP, Bernuau J, Bissoli F, Horsmans Y, et al. Liver transplant associated with paracetamol overdose: results from the seven‐country SALT study. British Journal of Clinical Pharmacology 2015;80(3):599‐606. [DOI: 10.1111/bcp.12635] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunnell 1997
- Gunnell D, Hawton K, Garnier V, Bismuth C, Fagg J. Use of paracetamol for suicide and non‐fatal poisoning in the UK and France: are restrictions on availability justified?. Journal of Epidemiology and Community Health 1997;51(2):175‐9. [PUBMED: 9196648] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haag 2011
- Haag G, Diener HC, May A, Meyer C, Morck H, Straube A, et al. Self‐medication of migraine and tension‐type headache: summary of the evidence‐based recommendations of the Deutsche Migräne und Kopfschmerzgesellschaft (DMKG), the Deutsche Gesellschaft für Neurologie (DGN), the Österreichische Kopfschmerzgesellschaft (ÖKSG) and the Schweizerische Kopfwehgesellschaft (SKG). Journal of Headache and Pain 2011;12(2):201‐17. [DOI: 10.1007/s10194-010-0266-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harpole 2005
- Harpole LH, Samsa GP, Matchar DB, Silberstein SD, Blumenfeld A, Jurgelski AE. Burden of illness and satisfaction with care among patients with headache seen in a primary care setting. Headache 2005;45(8):1048‐55. [DOI: 10.1111/j.1526-4610.2005.05186.x] [DOI] [PubMed] [Google Scholar]
Hawkins 2007
- Hawkins LC, Edwards JN, Dargan PI. Impact of restricting paracetamol pack sizes on paracetamol poisoning in the United Kingdom: a review of the literature. Drug Safety 2007;30(6):465‐79. [PUBMED: 17536874] [DOI] [PubMed] [Google Scholar]
Hawton 2001
- Hawton K, Townsend E, Deeks J, Appleby L, Gunnell D, Bennewith O, et al. Effects of legislation restricting pack sizes of paracetamol and salicylate on self poisoning in the United Kingdom: before and after study. BMJ 2001;322(7296):1203‐7. [DOI: 10.1136/bmj.322.7296.1203] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hawton 2013
- Hawton K, Bergen H, Simkin S, Dodd S, Pocock P, Bernal W, et al. Long term effect of reduced pack sizes of paracetamol on poisoning deaths and liver transplant activity in England and Wales: interrupted time series analyses. BMJ 2013;346:f403. [DOI: 10.1136/bmj.f403] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hinz 2008
- Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase‐2 inhibitor in man. Federation of American Societies for Experimental Biology Journal 2008;22(2):383‐90. [DOI: 10.1096/fj.07-8506com] [DOI] [PubMed] [Google Scholar]
IHS 2004
- Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders: 2nd edition. Cephalalgia 2004;24 Suppl 1:1‐160. [DOI: 10.1111/j.1468-2982.2003.00824.x] [DOI] [PubMed] [Google Scholar]
IHS 2010
- Bendtsen L, Bigal ME, Cerbo R, Diener HC, Holroyd K, Lampl C, et al. on behalf of the International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in tension‐type headache: second edition. Cephalalgia 2010;30(1):1‐16. [DOI: 10.1111/j.1468-2982.2009.01948.x] [DOI] [PubMed] [Google Scholar]
IHS 2013
- Headache Classification Committee of the International Headache Society (IHS). The international classification of headache disorders, 3rd edition (beta version). Cephalalgia 2013;33(9):629‐808. [DOI: 10.1177/0333102413485658] [DOI] [PubMed] [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66(2‐3):239‐46. [DOI: 10.1016/0304-3959(96)03033-3] [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI: 10.1016/0197-2456(95)00134-4] [DOI] [PubMed] [Google Scholar]
Kernick 2008
- Kernick D, Stapley S, Hamilton W. GPs' classification of headache: is primary headache underdiagnosed?. British Journal of General Practice 2008;58(547):102‐4. [DOI: 10.3399/bjgp08X264072] [DOI] [PMC free article] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107(2):224‐33. [DOI: 10.7326/0003-4819-107-2-224] [DOI] [PubMed] [Google Scholar]
Lavados 1998
- Lavados PM, Tenhamm E. Epidemiology of tension‐type headache in Santiago, Chile: a prevalence study. Cephalalgia 1998;18(8):552‐8. [DOI: 10.1046/j.1468-2982.1998.1808552.x] [DOI] [PubMed] [Google Scholar]
Liew 2014
- Liew Z, Ritz B, Rebordosa C, Lee PC, Olsen J. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatrics 2014;168(4):313‐20. [DOI: 10.1001/jamapediatrics.2013.4914] [DOI] [PubMed] [Google Scholar]
Lyngberg 2005
- Lyngberg AC, Rasmussen BK, Jørgensen T, Jensen R. Secular changes in health care utilization and work absence for migraine and tension‐type headache: a population based study. European Journal of Epidemiology 2005;20(12):1007‐14. [DOI: 10.1007/s10654-005-3778-5] [DOI] [PubMed] [Google Scholar]
Machado 2015
- Machado GC, Maher CG, Ferreira PH, Pinheiro MB, Lin CW, Day RO, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta‐analysis of randomised placebo controlled trials. BMJ 2015;350:h1225. [DOI: 10.1136/bmj.h1225] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mannix 2001
- Mannix LK. Epidemiology and impact of primary headache disorders. Medical Clinics of North America 2001;85(4):887‐95. [DOI: 10.1016/S0025-7125(05)70349-7] [DOI] [PubMed] [Google Scholar]
Mazaud‐Guittot 2013
- Mazaud‐Guittot S, Nicolas Nicolaz C, Desdoits‐Lethimonier C, Coiffec I, Ben Maamar M, Balaguer P, et al. Paracetamol, aspirin, and indomethacin induce endocrine disturbances in the human fetal testis capable of interfering with testicular descent. Journal of Clinical Endocrinology and Metabolism 2013;98(11):1757‐67. [DOI: 10.1210/jc.2013-2531] [DOI] [PubMed] [Google Scholar]
McQuay 2007
- McQuay HJ, Moore RA. Dose‐response in direct comparisons of different doses of aspirin, ibuprofen and paracetamol (acetaminophen) in analgesic studies. British Journal of Clinical Pharmacology 2007;63(3):271‐8. [DOI: 10.1111/j.1365-2125.2006.02723.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Monteith 2010
- Monteith TS, Sprenger T. Tension type headache in adolescence and childhood: where are we now?. Current Pain and Headache Reports 2010;14(6):424‐30. [DOI: 10.1007/s11916-010-0149-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5] [Google Scholar]
Moore 2013
- Moore RA, Straube S, Aldington D. Pain measures and cut‐offs ‐ 'no worse than mild pain' as a simple, universal outcome. Anaesthesia 2013;68(4):400‐12. [DOI: 10.1111/anae.12148] [DOI] [PubMed] [Google Scholar]
Moore 2014a
- Moore RA, Derry S, Wiffen PJ, Straube S, Aldington DJ. Overview review: comparative efficacy of oral ibuprofen and paracetamol (acetaminophen) across acute and chronic pain conditions. European Journal of Pain 2014;19(9):1213‐23. [DOI: 10.1002/ejp.649] [DOI] [PubMed] [Google Scholar]
Moore 2014b
- Moore RA, Derry S, Wiffen PJ, Straube S, Bendtsen L. Evidence for efficacy of acute treatment of episodic tension‐type headache: methodological critique of randomised trials for oral treatments. Pain 2014;155(11):2220‐8. [DOI: 10.1016/j.pain.2014.08.009] [DOI] [PubMed] [Google Scholar]
Moore 2015
- Moore RA, Derry S, Aldington D, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults ‐ an overview of Cochrane reviews. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD008659.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2016
- Moore RA, Moore N. Paracetamol and pain: the kiloton problem. European Journal of Hospital Pharmacy 2016;April. [DOI: 10.1136/ejhpharm-2016-000952] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with Confidence ‐ Confidence Intervals and Statistical Guidelines. London: British Medical Journal, 1995:50‐63. [ISBN: 0‐7279‐0222‐0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Norris 2008
- Norris W, Paredes AH, Lewis JH. Drug‐induced liver injury in 2007. Current Opinion in Gastroenterology 2008;24(3):287‐97. [DOI: 10.1097/MOG.0b013e3282f9764b] [DOI] [PubMed] [Google Scholar]
Nüesch 2010
- Nüesch E, Trelle S, Reichenbach S, Rutjes AW, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341:c3515. [DOI: 10.1136/bmj.c3515] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oshinaike 2014
- Oshinaike O, Ojo O, Okubadejo N, Ojelabi O, Dada A. Primary headache disorders at a tertiary health facility in Lagos, Nigeria: prevalence and consultation patterns. Biomedical Research International 2014;2014:782915. [DOI: 10.1155/2014/782915] [DOI] [PMC free article] [PubMed] [Google Scholar]
PIC 2015
- PharmWeb. Paracetamol Information Centre. www2.pharmweb.net/pwmirror/pwy/paracetamol/pharmwebpic.html (accessed 22 December 2015).
Pop 2002
- Pop PH, Gierveld CM, Karis HA, Tiedink HG. Epidemiological aspects of headache in a workplace setting and the impact on the economic loss. European Journal of Neurology 2002;9(2):171‐4. [DOI] [PubMed] [Google Scholar]
Prescott 2000
- Prescott LF. Therapeutic misadventure with paracetamol: fact or fiction?. American Journal of Therapeutics 2000;7(2):99‐114. [PUBMED: 11319578] [DOI] [PubMed] [Google Scholar]
Rasmussen 2001
- Rasmussen BK. Epidemiology of headache. Cephalalgia 2001;21(7):774‐7. [PUBMED: 11595011] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Russell 2005
- Russell MB. Tension‐type headache in 40‐year‐olds: a Danish population‐based sample of 4000. Journal of Headache and Pain 2005;6(6):441‐7. [DOI: 10.1007/s10194-005-0253-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sahler 2012
- Sahler K. Epidemiology and cultural differences in tension‐type headache. Current Pain and Headache Research 2012;16(6):525‐32. [DOI: 10.1007/s11916-012-0296-5] [DOI] [PubMed] [Google Scholar]
Schwab 2003
- Schwab JM, Schluesener HJ, Laufer S. COX‐3: just another COX or the solitary elusive target of paracetamol?. Lancet 2003;361(9362):981‐2. [DOI: 10.1016/S0140-6736(03)12841-3] [DOI] [PubMed] [Google Scholar]
Schwartz 1998
- Schwartz BS, Stewart WF, Simon D, Lipton RB. Epidemiology of tension‐type headache. JAMA 1998;279(5):381‐3. [DOI: 10.1001/jama.279.5.381] [DOI] [PubMed] [Google Scholar]
Steiner 2004
- Steiner TJ. Lifting the burden: the global campaign against headache. Lancet Neurology 2004;3(4):204‐5. [DOI: 10.1016/S1474-4422(04)00703-3] [DOI] [PubMed] [Google Scholar]
Steiner 2011
- Steiner TJ, Stovner LJ, Dua T, Birbeck GL, Jensen R, Katsarava Z, et al. Time to act on headache disorders. Journal of Headache and Pain 2011;12(5):501‐3. [DOI: 10.1007/s10194-011-0368-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stovner 2007
- Stovner LJ, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher AI, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 2007;27(3):193‐210. [DOI: 10.1111/j.1468-2982.2007.01288.x] [DOI] [PubMed] [Google Scholar]
Tramèr 1997
- Tramèr MR, Reynolds DJM, Moore RA, McQuay HJ. Impact of covert duplicate results on meta‐analysis: a case study. BMJ 1997;315(7109):635‐9. [DOI: 10.1136/bmj.315.7109.635] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verhagen 2006
- Verhagen AP, Damen L, Berger MY, Passchier J, Merlin V, Koes BW. Is any one analgesic superior for episodic tension‐type headache?. Journal of Family Practice 2006;55(12):1064‐72. [PUBMED: 17137543] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI: 10.1016/S0140-6736(12)61729-2] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2015
- World Health Organization. WHO model list of essential medicines, 19th List, 2015. www.who.int/medicines/publications/essentialmedicines/en/index.html (accessed 22 December 2015).


