Abstract
Background
Epithelial ovarian cancer (EOC) is often diagnosed at an advanced stage, requiring primary cytoreductive surgery and combination chemotherapy for its first‐line management. Currently, the recommended standard first‐line chemotherapy is platinum‐based, usually consisting of carboplatin and paclitaxel (PAC/carbo). Pegylated liposomal doxorubicin (PLD) is an improved formulation of doxorubicin that is associated with fewer and less severe side effects than are seen with non‐modified doxorubicin. In combination with carboplatin, PLD has recently been shown to improve progression‐free survival compared with PAC/carbo in women with relapsed, platinum‐sensitive EOC. It is therefore important to know whether any survival benefit can be attributed to PLD when it is used in the first‐line setting.
Objectives
To evaluate the role of PLD, alone or in combination, in first‐line chemotherapy for women with EOC.
Search methods
We searched The Cochrane Gynaecological Cancer Group's Trial Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE from January 1990 to February 2013. In addition, we searched online trial registries for ongoing trials and abstracts of studies presented at relevant scientific meetings from 2000 onwards.
Selection criteria
We included all randomised controlled trials (RCTs) that compared PLD alone or in combination with other agent/s (e.g. carboplatin) versus other agent/s for first‐line chemotherapy in women with EOC who may or may not have undergone primary cytoreductive surgery.
Data collection and analysis
Two review authors independently selected trials, extracted data and assessed the risk of bias for each included trial. We obtained updated trial data when possible.
Main results
We included two large trials. One trial compared three‐weekly PLD and carboplatin (PLD/carbo) with PAC/carbo. The other trial included four experimental arms, one of which was PLD plus PAC/carbo, that were compared with the standard PAC/carbo regimen. We did not combine results of these two trials in the meta‐analysis. We considered the two studies to be at low risk of bias.
For the comparison PLD/carbo versus PAC/carbo (820 women; stages Ic to IV), no statistically significant differences in progression‐free survival (PFS) (hazard ratio [HR] 1.01, 95% confidence interval [CI] 0.85 to 1.19) or overall survival (OS) (HR 0.94, 95% CI 0.78 to 1.13) were noted between study arms. Severe anaemia (risk ratio [RR] 2.74, 95% CI 1.54 to 4.88) and thrombocytopenia (RR 8.09, 95% CI 3.93 to 16.67) were significantly more common with PLD/carbo, whereas alopecia (RR 0.09, 95% CI 0.06 to 0.14) and severe neurotoxicity (RR 0.09, 95% CI 0.01 to 0.66) were significantly more common with PAC/carbo. Quality of life scores were not significantly different.
For the comparison PLD/PAC/carbo versus PAC/carbo (1726 women; stage III/IV), it is important to note that PLD was given for alternate cycles only (i.e. every 6 weeks). No statistically significant difference in PFS (HR 0.98, 95% CI 0.88 to 1.09) or OS (HR 0.95, 95% CI 0.84 to 1.08) between these two treatment arms was reported. However, women in the triplet arm experienced significantly more severe haematological adverse events (anaemia, thrombocytopenia, neutropenia and febrile neutropenia) compared with those given standard treatment.
No RCTs evaluated single‐agent PLD for first‐line treatment of EOC.
Authors' conclusions
PLD/carbo is a reasonable alternative to PAC/carbo for the first‐line treatment of EOC. Although three‐weekly PLD/carbo may be associated with increased dose delays and discontinuations compared with the standard PAC/carbo regimen, it might be more acceptable to women who wish to avoid alopecia or those at high risk of neurotoxicity. No survival benefits appear to be associated with the alternating triplet regimen, and the additional toxicity associated with adding PLD to PAC/carbo limits further investigation. Further studies are needed to establish the safest, most effective PLD/carbo regimen for newly diagnosed disease.
Plain language summary
A modified formulation of doxorubicin for the treatment of newly diagnosed ovarian cancer
Background
PLD is an improved formulation of an anticancer drug that has been around since the 1960s. When used with carboplatin (carbo), it has been shown to improve survival in women with epithelial ovarian cancer (EOC) that has come back (relapsed) six months or longer after the last platinum (carbo)‐based treatment.
Methods
We wanted to find out whether PLD was also useful for the treatment of newly diagnosed EOC. We searched the literature from 1990 to January 2013 for relevant studies and included two studies in this review.
Study characteristics
One study compared PLD plus carbo given to women every three weeks versus the standard treatment (paclitaxel (PAC)/carbo every three weeks), and the other added PLD to the standard treatment and compared it with standard treatment only (the latter study also included other treatments not relevant to this review). These studies spanned three years and included 820 and 4100 women, respectively. Most women in these studies had advanced cancer and had undergone surgery to remove as much of the cancer as possible.
Key findings
Women receiving the PLD/carbo treatment and those given the standard treatment survived for a similar period, but PLD/carbo caused more women to experience low blood counts (anaemia and low platelets) that often led to a delay in treatment or the need to stop treatment. However, PLD/carbo caused far fewer women to experience hair loss and neuropathy (nerve damage causing symptoms such as tingling, numbness, pain, loss of sensation and/or coordination), and so it might help women who find these side effects unacceptable or intolerable. We concluded that three‐weekly PLD/carbo is a reasonable alternative to standard platinum‐based treatment for newly diagnosed EOC, but more research is needed to establish the safest and most effective dosage and dose frequency.
Adding PLD to standard treatment (PAC/carbo) every six weeks did not help women with newly diagnosed ovarian cancer survive longer and was associated with worse effects on blood counts that increased the chance of infection; therefore this triple drug treatment cannot be recommended.
Quality of the evidence
We considered the evidence related to survival of women after they are treated with PLD/carbo or PAC/carbo, and the evidence related to adverse drug effects to be of high quality.
Summary of findings
for the main comparison.
| PLD/carbo compared with PAC/carbo for first‐line treatment of epithelial ovarian cancer | |||||
|
Patient or population: women with newly diagnosed EOC (stage Ic to IV) Settings: hospital Intervention: PLD (30 mg/m²)/carbo every three weeks Comparison: PAC/carbo every three weeks | |||||
| Outcomes | Assumed risk* | Corresponding risk | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) |
| PFS¹ | HR 1.01 (0.85 to 1.19) |
820 (1) |
⊕⊕⊕⊕ high | ||
| OS² | HR 0.94 (0.78 to 1.13) |
820 (1) |
⊕⊕⊕⊕ high | ||
| Anaemia (grade 3/4) | 37 per 1000 | 100 per 1000 (57 to 181) |
RR 2.74 (1.54 to 4.88) |
803 (1) | ⊕⊕⊕⊕ high |
| Thrombocytopenia (grade 3/4) | 20 per 1000 | 162 per 1000 (79 to 333) |
RR 8.09 (3.93 to 16.67) |
803 (1) | ⊕⊕⊕⊕ high |
| Alopecia (grade 2) | 595 per 1000 | 54 per 1000 (36 to 83) |
RR 0.09 (0.06 to 0.14) |
803 (1) | ⊕⊕⊕⊕ high |
| Neuropathy (grade 3/4) | 29 per 1000 | 3 per 1000 (0 to 19) |
RR 0.09 (0.01 to 0.66) |
803 (1) | ⊕⊕⊕⊕ high |
| *The assumed risk is the median risk for the control group in the one included study. CI: Confidence interval; HR: Hazard Ratio; RR: Risk Ratio. | |||||
| GRADE Working Group grades of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
¹Relative median PFS in MITO‐2 2011 (all participants) was 16.8 months and 19.0 months for PAC/carbo and PLD/carbo, respectively (published 2011 data). MITO‐2 2011 also performed exploratory analyses of a high‐risk group (defined as stage IV, or stage III with residual disease > 1 cm). The relative median PFS for this high‐risk group was 11 versus 11.8 months for PAC/carbo and PLD/carbo, respectively (unpublished 2013 data).
²Relative median OS in MITO‐2 2011 (all participants) was 53.2 months and 61.6 months for PAC/carbo and PLD/carbo, respectively (published 2011 data). The relative median PFS for the high‐risk group defined above was 29 months versus 30.3 months for PAC/carbo and PLD/carbo, respectively (unpublished 2013 data).
Background
Description of the condition
Worldwide, nearly 225,000 women are diagnosed with ovarian cancer each year, making it the eighth most common cancer in women. The estimated risk of getting the disease is approximately 1% in developed countries (Europe, Northern America, Australia/New Zealand and Japan) and 0.5% in the rest of the world (GLOBOCAN 2008).
Epithelial ovarian cancer (EOC) usually has a relatively asymptomatic onset and, initially, an inconspicuous progression. Symptoms characteristically are non‐specific and include abdominal swelling and pain, early satiety, weight loss, changes in bowel habit and urinary urgency and frequency (NICE 2011). This absence of a clear clinical profile results in diagnosis with advanced‐stage disease for most women (FIGO (International Federation of Gynecology and Obstetrics) III and IV; FIGO 2009), giving EOC the poorest prognosis of all gynaecological cancers, with five‐year relative survival rates of only 37% and 54% in Europe and America, respectively (EUROCARE‐4 2009; SEER 2007). However, if women are diagnosed at FIGO stage I, they have a 90% chance of surviving the next five years (SEER 2007). The need to improve detection of early disease was recognised over 30 years ago, and this recognition led to the development of screening protocols based on vaginal examination, measurement of serum CA125 levels and ultrasonography (Campbell 1989; Jacobs 1988). However, although studies using refined diagnostic techniques, such as transvaginal ultrasound, are still under way (Menon 2009), it is not yet clear whether implementation of a screening programme would lower mortality rates and benefit affected women. Around 90% of all ovarian malignancies are EOC; other types include germ cell, stromal cell and Müllerian tumours (SEER 2007). Primary diagnostic tests for EOC consist of measurement of serum CA125 levels and ultrasonography. If disease is suspected, women undergo imaging with computed tomography (CT), magnetic resonance imaging (MRI) and biopsy or exploratory laparotomy, when the tumour is histologically classified and staged and all macroscopic disease is removed (NICE 2011).
Further standard treatment is disease‐dependent. Women who present with low‐grade stage I tumours might not undergo chemotherapy, those with high‐grade stage I disease may receive platinum‐based therapy alone and those with disease at all other stages receive intravenous platinum‐based chemotherapy, often in combination with paclitaxel (PAC) (NICE 2011). Carboplatin (Carbo) is favoured over cisplatin because it is less toxic and has equivalent efficacy (NICE 2003). Bevacizumab, an angiogenesis inhibitor, has been approved for use in advanced EOC in Europe (EMA 2011) and is now an option for many women. Adding bevacizumab as concurrent and maintenance therapy to the standard PAC/carbo regimen has been shown to improve progression‐free survival in the first‐line treatment of EOC (ICON7, Perren 2011; GOG 218, Burger 2011). Bevacizumab appears to benefit primarily women at high risk of disease progression (Burger 2011; Perren 2011; ), and its use in clinical practice should be weighed against potential reduction in quality of life (Stark 2013). Ways of improving PAC/carbo scheduling (e.g. by weekly dosing) with or without bevacizumab are currently under investigation (ICON8; GOG 262).
Surgery plus standard chemotherapy has a response rate of 70% to 80%. However, 55% to 75% of responders will relapse within two years of treatment completion (NICE 2003).
Description of the intervention
Doxorubicin hydrochloride is a cytotoxic drug that belongs to the anthracycline family and has been available since the 1960s (EMA 2010). It was originally used in the first‐line treatment of EOC in the 1970s, when in vitro experiments showed a dose‐response relationship in EOC cell lines. Activity against EOC was subsequently proven in clinical trials (A'Hern 1995; OCMP 1991; Ozols 1980). Despite its potent antineoplastic activity, clinical use of doxorubicin has been limited by its associated side effects, in particular haematological toxicity and irreversible cardiac damage. Pegylated liposomal doxorubicin (PLD) is a formulation of liposomal doxorubicin that is coated in polyethylene glycol (PEG), which reduces the rate at which the active drug is broken down (Gabizon 2001) and makes it less toxic to heart muscle.
In the UK, PLD is currently licensed for the treatment of advanced EOC in women for whom platinum‐based chemotherapy has failed (platinum‐resistant EOC; EMA 2010). In these women, PLD may be given intravenously at a dose of 50 mg/m² once every four weeks for six cycles if tolerated, and if the disease does not progress (EMA 2010). However, 50 mg/m² is generally considered to be too toxic; therefore, in clinical practice, lower doses are usually given to reduce drug‐related adverse effects (40 mg/m² as a single agent and 30 mg/m² in combination therapy). The main toxicities associated with PLD are nausea, palmar‐plantar erythema or hand‐foot syndrome (redness and soreness of palms of hands and soles of feet), stomatitis and myelosuppression (Janssen‐Cilag 2011).
PLD in combination with platinum has been shown to be a better alternative to PAC/carbo in women with platinum‐sensitive relapsed disease (CALYPSO 2010; HeCOG 2010). Compared with standard three‐weekly PAC/carbo treatment, a four‐weekly PLD/carbo regimen results in improved progression‐free survival in these women and is better tolerated. In CALYPSO 2010, significantly fewer women experienced complete hair loss, hypersensitivity reactions and neuropathies in the PLD arm compared with the PAC arm, and women in the PLD arm were less likely to discontinue treatment. Because PLD/carbo is a better alternative to PAC/carbo for relapsed EOC, this might also be the case when it is used as first‐line chemotherapy.
How the intervention might work
Anthracyclines interact with DNA, adversely affecting all cell functions that rely on DNA. Furthermore, they interact with cell membranes, altering their functions and generating hydrogen peroxide and hydroxy radicals, which are highly destructive to cells (Zunino 2002). The PEG coating of PLD represents a hydrophilic barrier that protects the liposomes from detection by the reticuloendothelial system and increases the time that the active drug remains in circulation (Gabizon 1997; Gabizon 2001). The size of the liposomes prevents PLD from entering tissues with tight capillary junctions, such as the heart and gastrointestinal tract; therefore it causes less toxicity compared with non‐modified doxorubicin, while leading to increased concentrations within the tumour (Waterhouse 2001).
Why it is important to do this review
PLD is a formulation of a proven chemotherapeutic agent with an improved efficacy and safety profile. Good evidence supports its use in the treatment of relapsed EOC, in combination with carboplatin in platinum‐sensitive disease and as a single agent in platinum‐resistant disease (Lawrie 2013). This represents a strong rationale for testing PLD in the first‐line setting. After a large randomised controlled trial (RCT) of PLD/carbo for first‐line treatment of EOC had been completed (MITO‐2 2011), we considered it important to review the evidence related to PLD as first‐line chemotherapy for newly diagnosed EOC.
Objectives
To evaluate the role of pegylated liposomal doxorubicin, alone or in combination, in first‐line chemotherapy for women with EOC.
Methods
Criteria for considering studies for this review
Types of studies
RCTs.
Types of participants
Women (aged 18 and older) with EOC who may or may not have undergone primary cytoreductive surgery.
Types of interventions
PLD (50 mg or less every three or more weeks) alone or in combination with other agent/s (e.g. carbo) versus other agent/s as first‐line chemotherapy.
Types of outcome measures
Primary outcomes
Progression‐free survival (PFS): survival until disease progression.
Secondary outcomes
Overall survival (OS): survival until death from any cause.
Severe adverse events, classified according to CTCAE 2006, including specific haematological, gastrointestinal, genitourinary, dermatological, neurological, pulmonary and other severe adverse events.
Symptom control (e.g. haematopoietic growth factors, transfusions, antiemetics, dose delays and reductions).
Quality of life (QoL).
Search methods for identification of studies
Electronic searches
We searched the following electronic databases (also see Cochrane Gynaecological Cancer Group methods used in reviews):
The Cochrane Gynaecological Cancer Group's Trial Register
The Cochrane Central Register of Controlled Trials (CENTRAL)
MEDLINE
EMBASE
The CENTRAL, MEDLINE and EMBASE search strategies, based on terms related to the review topic, are presented in Appendix 1; Appendix 2; and Appendix 3, respectively. As PLD was recently developed, we searched databases for articles published from January 1990 until February 2013.
Searching other resources
We searched the metaRegister of Controlled Trials (mRCT) (www.controlled‐trials.com), www.clinicaltrials.gov and the Physicians Data Query (PDQ) (www.cancer.gov/clinicaltrials) for ongoing trials. We also looked for abstracts of studies presented at relevant scientific meetings from 2000 onwards, including the American Society of Clinical Oncologists (ASCO), the European Society of Medical Oncologists (ESMO) and the European Society of Gynaecologic Oncologists (ESGO) Annual Meetings, using the zetoc.mimas.ac.uk website. When necessary, we contacted the main investigators of relevant trials to request for further information. In addition, we checked the citation lists of included studies to identify other relevant reports/studies.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to a reference management database (Reference Manager version 10) and removed duplicates. Two review authors (Theresa A Lawrie (TAL) and Clemens Thoma (CT)) reviewed the remaining records independently to identify potentially relevant trials. We excluded studies that clearly did not meet the inclusion criteria and obtained the full text of potentially relevant trials. Three review authors (CT, TAL and Roy Rabbie (RR)) independently assessed these identified trials for eligibility. Had there been any disagreements related to eligibility, we would have involved Jo Morrison (JM) in the process. For excluded studies, we documented the reasons for exclusion.
Data extraction and management
We designed and piloted a data extraction form for the review. Two review authors (RR and CT) independently extracted data from included studies. These data were checked by TAL. When disagreement arose between reviewers, JM was asked to resolve it.
For included studies, we extracted the following data when possible.
Author, year of publication and journal citation.
Country.
Setting.
Inclusion and exclusion criteria.
Study design, methodology.
Duration of follow‐up.
-
Study population:
Total number enrolled.
Participant characteristics.
Age.
Comorbidities.
-
EOC details at diagnosis:
FIGO stage.
Histological cell type.
Tumour grade.
Performance status.
Extent of disease.
Total number of intervention groups.
-
Intervention details:
Details of PLD, including dose, regimen, frequency and number of cycles.
Comparison details, including type of control and dose, regimen, frequency and number of cycles.
Proportions of participants who received all/part/none of the intended treatment.
Delays in treatment.
Risk of bias in study (see Assessment of risk of bias in included studies).
-
Outcomes—PFS, OS, QoL, symptom control and adverse events:
For each outcome: outcome definition (with diagnostic criteria if relevant).
Unit of measurement (if relevant).
For scales: upper and lower limits, and whether high or low score is good.
Results: number of participants allocated to each intervention group.
For each outcome of interest: sample size and missing participants.
Assessment of risk of bias in included studies
We assessed the risk of bias in included RCTs using The Cochrane Collaboration's tool and the criteria specified in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This included assessment of the following domains.
-
Selection bias:
Random sequence generation.
Allocation concealment.
-
Performance bias:
Blinding of participants and personnel (participants and treatment providers).
-
Detection bias:
Blinding of outcome assessment.
-
Attrition bias:
Incomplete outcome data: We recorded the proportion of participants whose outcomes were not reported at the end of the study and considered > 20% attrition to indicate high risk of bias.
-
Reporting bias:
Selective reporting of outcomes.
Other possible sources of bias.
Two review authors (RR and CT) independently applied the 'Risk of bias' tool (Appendix 4), and differences were resolved by discussion or by appeal to a third review author (TAL or JM). We have presented results in a 'Risk of bias' summary graph and have interpreted the results of the meta‐analyses in the light of findings with respect to risk of bias.
Measures of treatment effect
We used the following measures of the effect of treatment.
For time‐to‐event data, we used hazard ratios (HRs).
For dichotomous outcomes (e.g. adverse events), we extracted the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed at endpoint to estimate a risk ratio (RR).
For continuous outcomes (e.g. QoL measures), we extracted the mean difference (MD) and the standard deviation (SD) between the final value of the outcome measure in each treatment arm at the end of follow‐up. If standard deviations of final values were not available, we used change scores if SDs were available. If no SDs were available, we omitted these trials.
When possible, we extracted data relevant to an intention‐to‐treat analysis (ITT), in which participants were analysed in the groups to which they were assigned. When time‐to‐event outcomes were assessed by more than one method (i.e. independent radiology review, investigator assessment or independent oncology review), we used the independent radiology review data and noted any differences in effect size and direction, compared with the other methods, as reported in the text.
Unit of analysis issues
The unit of analysis was usually the individual participant; however, when data were presented per treatment cycle (e.g. for dose delays) we also extracted these data.
Dealing with missing data
We did not impute missing data.
Assessment of heterogeneity
We did not assess statistical heterogeneity between trials, as trials were insufficient to allow performance of meta‐analyses. In future versions of this review, if meta‐analyses are possible, we will assess heterogeneity by visual inspection of forest plots, by estimation of the percentage of heterogeneity between trials that cannot be ascribed to sampling variation (Higgins 2003) and by formal statistical testing of the significance of the heterogeneity (Deeks 2001). In each meta‐analysis, we will regard heterogeneity as substantial if I² is greater than 50% and either T² is greater than zero, or if a low P value (less than 0.10) is obtained in the Chi² test. If evidence of substantial heterogeneity is noted, we will investigate the possible reasons for this and report them.
Data synthesis
We used random‐effects models with inverse variance weighting for all analyses (DerSimonian 1986). As insufficient clinically similar trials were available, we could not perform a meta‐analysis; however, in future versions of this review, we plan to pool trial results as follows.
For time‐to‐event data, we will pool HRs using the generic inverse variance facility of RevMan 2012.
For dichotomous outcomes, we will pool the RRs.
For continuous outcomes, we will pool the mean differences (MDs) between treatment arms at the end of follow‐up if all trials measure the outcome on the same scale; otherwise we will pool standardised mean differences (SMDs).
Subgroup analysis and investigation of heterogeneity
Trials were insufficient for meta‐analyses of subgroup data; however, we recorded available subgroup data for survival outcomes as follows.
Stage of disease: early (FIGO Ia, Ib, Ic, IIa, IIb) and advanced (FIGO III, IV).
No residual disease, optimal staging (residual disease ≤ 1 cm), suboptimal staging (residual disease > 1 cm) and no surgery.
Age < 70 and ≥ 70 years.
Sensitivity analysis
Studies were insufficient to allow sensitivity analysis to be performed.
Results
Description of studies
Results of the search
The search strategy identified a total of 2267 reference hits, which were reduced to 1665 after de‐duplication. By screening titles and abstracts, we identified 16 citations as potentially eligible for this review. After evaluating full texts of these citations, we included two trials (14 citations) and excluded two trials (Figure 1). An update of the search (Oct 2016) revealed a further 192 unique references. By screening titles and bastracts, we identified xx citations as potentially eligible for the review. fter evaulating full text of these studies, both were excluded and no further studies were included in the review.
1.
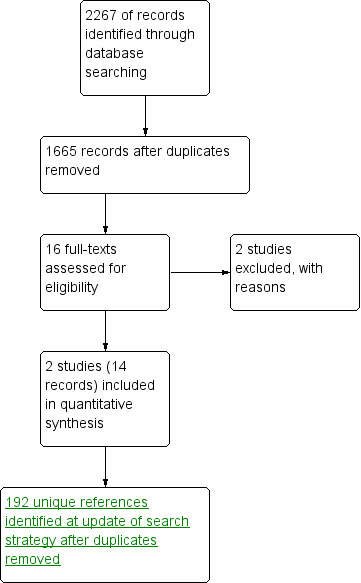
Study flow diagram of searches to February 2013.
Included studies
Two trials, MITO‐2 2011 and GOG0182/ICON 5 2009, met the inclusion criteria and contributed data to the analyses. Both were multi‐centre phase III RCTs that randomly assigned 820 and 1726 participants, respectively. MITO‐2 2011 compared the combination of three‐weekly PLD/carbo with standard three‐weekly PAC/carbo, whereas GOG0182/ICON 5 2009 compared a triplet combination of PAC/carbo/PLD with the same standard therapy. We attempted to contact the investigators of both trials to obtain additional information and data. The investigators of MITO‐2 2011 responded and supplied unpublished updated survival and subgroup data for this review. The trials are described below and in the Characteristics of included studies section of the review.
Participants
Both trials included women with histologically proven advanced EOC; however, GOG0182/ICON 5 2009 also included women with primary peritoneal carcinoma (similar in histology and treatment to the most common histological subtype of EOC) (13%). MITO‐2 2011 included stages Ic to IV and GOG0182/ICON 5 2009 stages III to IV, according to the FIGO scoring system. However, less than 20% of the women in MITO‐2 2011 had FIGO stage I/II disease. Median age and histological characteristics were comparable between the two trials. All women in GOG0182/ICON 5 2009 had undergone primary cytoreduction, whereas 18% of women in MITO‐2 2011 had had no surgery. Twenty‐eight per cent and 30% of women in MITO‐2 2011 and GOG0182/ICON 5 2009, respectively, had undergone suboptimal cytoreduction, as defined by residual tumour > 1 cm.
Interventions
Women in the standard treatment arms of both trials received carboplatin (dosed according to the Calvert formula) and paclitaxel (175 mg/m²) every three weeks. In the experimental treatment arms, PLD was given intravenously on day one at a dose of 30 mg/m² in both trials. In MITO‐2 2011, PLD was administered every three weeks, whereas in GOG0182/ICON 5 2009, women received PLD only on alternate cycles (i.e. 6‐weekly). Women in MITO‐2 2011 underwent three cycles of treatment, and those with stable or responding disease continued for a further three cycles. In GOG0182/ICON 5 2009, women were assigned to eight cycles of chemotherapy, and at least four cycles of carboplatin and paclitaxel were maintained in the standard and experimental arms.
Outcomes
In both studies, PFS and OS were the primary and secondary outcomes, respectively, and adverse events were reported as graded by National Cancer Institute Common Toxicity Criteria version 2.0 (CTCAE 2006). In addition, MITO‐2 2011 evaluated response in accordance with Response Evaluation Criteria in Solid Tumours (RECIST) version 1.0 (Therasse 2000) and quality of life using the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire C30 (QLQ‐C30) (Aaronson 1993). In GOG0182/ICON 5 2009, toxicities were reported in the form of a bar chart with the number of participants (%) as the y‐axis. As more detailed data were not available, we used the data from this bar chart to estimate the relative rates of toxicity in each treatment arm.
Excluded studies
We excluded two studies because they were not RCTs (see Characteristics of excluded studies).
Risk of bias in included studies
In general, we considered the two included studies to be at low risk of bias. Both were large, multi‐centre, international studies with central and regional coordinating offices. For MITO‐2 2011, randomisation was computer‐generated with allocation by central telephone assignment (low risk of selection bias), attrition was low except for quality of life data (low risk of attrition bias), prespecified and expected outcomes were reported (low risk of reporting bias) and baseline characteristics were similar between groups. For GOG0182/ICON 5 2009, allocation concealment was not clearly stated, and we were unable to obtain this information from the authors. However, randomisation was computer‐generated in stratified blocks, attrition was low, prespecified and expected outcomes were reported and baseline characteristics were similar in both of the extracted arms. Both included studies were open‐label studies, and independent outcome evaluation was not described in either study; this may have predisposed these trials to performance or detection bias, or both (high risk of performance bias).
Effects of interventions
See: Table 1
PLD/carbo versus PAC/carbo
Only one trial (MITO‐2 2011), which included 820 women with newly diagnosed EOC, contributed data for this comparison. We used updated, unpublished survival data (August 2013) that we obtained from the MITO‐2 2011 investigators for this review.
Survival
Overall, no statistically significant differences were noted between the PLD/carbo arm and the PAC/carbo arm with respect to PFS (HR 1.01; 95% CI 0.85 to 1.19; Analysis 1.1; high‐quality evidence) or OS (HR 0.94; 95% CI 0.78 to 1.13; Analysis 1.2; high‐quality evidence).
1.1. Analysis.
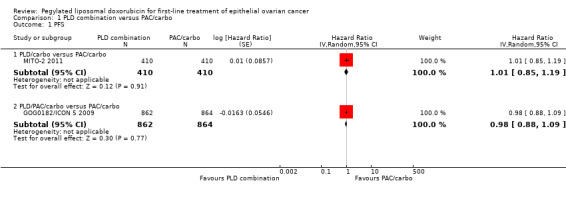
Comparison 1 PLD combination versus PAC/carbo, Outcome 1 PFS.
1.2. Analysis.
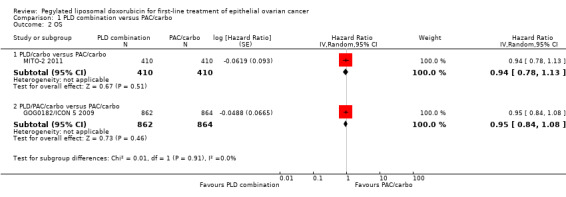
Comparison 1 PLD combination versus PAC/carbo, Outcome 2 OS.
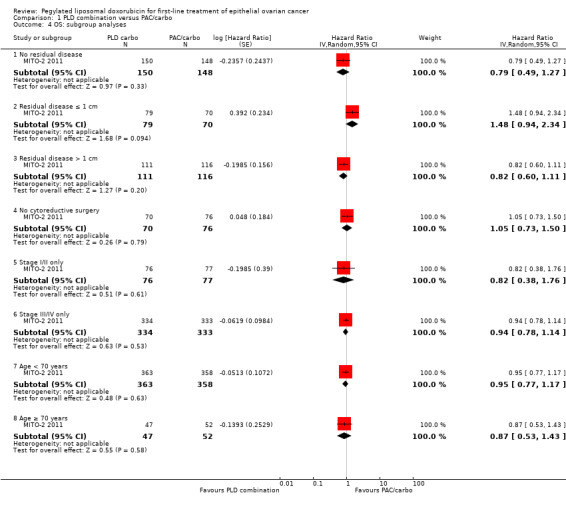
Exploratory 'ad hoc' subgroup analyses performed by the investigators revealed no significant differences between comparison arms in any of the subgroup analyses (Eastern Cooperative Oncology Group Performance Status (ECOG PS), age, stage, tumour histology and residual disease).
Further exploratory subgroup analyses were performed by the MITO‐2 2011 investigators upon our request, in which participants were separated into low‐ and high‐risk groups according to FIGO stage and residual disease (women with stage IV disease or stage III with residual disease > 1 cm were considered at high risk of disease progression). No significant differences in PFS and OS results were noted between treatment arms for participants included in different risk groups.
Toxicity
Women in the PLD/carbo arm compared with the PAC/carbo arm were significantly more likely to experience the following.
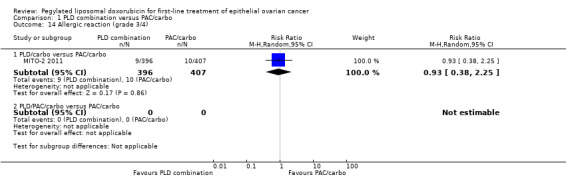
Anaemia (grade 3/4): RR 2.74; 95% CI 1.54 to 4.88 (Analysis 1.7).
Thrombocytopenia (grade 3/4): RR 8.09; 95% CI 3.93 to 16.67 (Analysis 1.8).
1.7. Analysis.
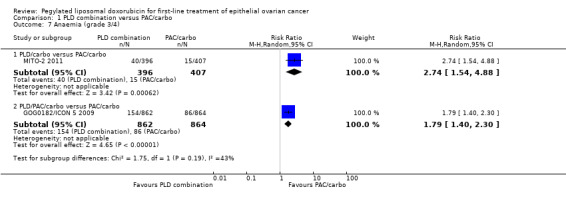
Comparison 1 PLD combination versus PAC/carbo, Outcome 7 Anaemia (grade 3/4).
1.8. Analysis.
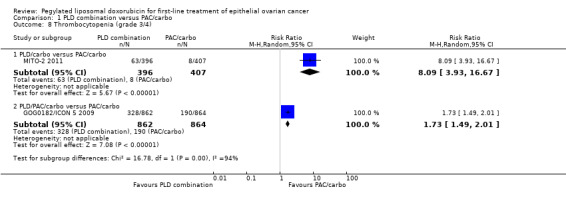
Comparison 1 PLD combination versus PAC/carbo, Outcome 8 Thrombocytopenia (grade 3/4).
And they were significantly less likely to experience these conditions.
Alopecia (grade 2): RR 0.09; 95% CI 0.06 to 0.14 (Analysis 1.9).
Neuropathy (grade 3/4): RR 0.09; 95% CI 0.01 to 0.66 (Analysis 1.13).
1.9. Analysis.
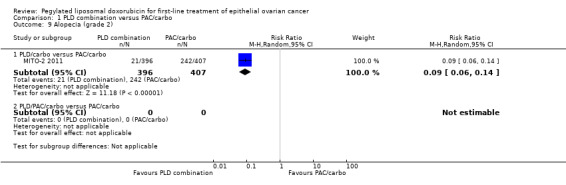
Comparison 1 PLD combination versus PAC/carbo, Outcome 9 Alopecia (grade 2).
1.13. Analysis.
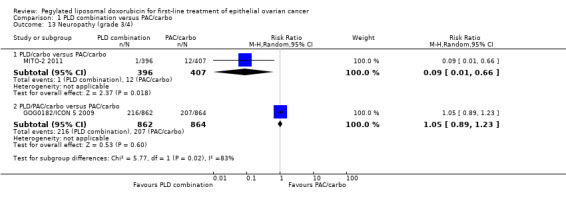
Comparison 1 PLD combination versus PAC/carbo, Outcome 13 Neuropathy (grade 3/4).
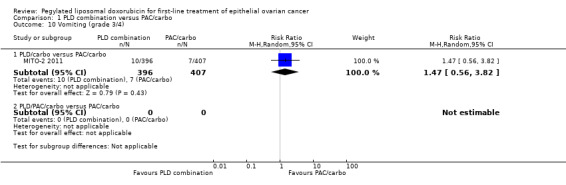
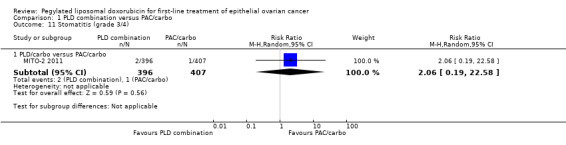
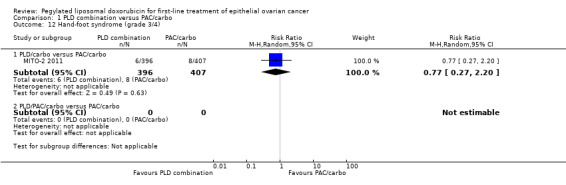
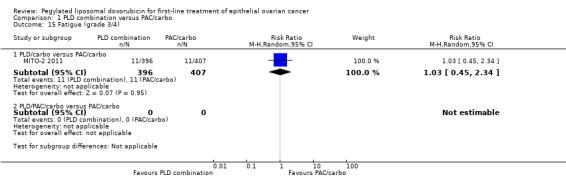
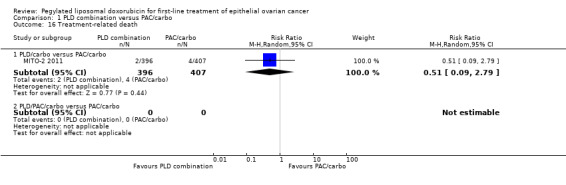
Although the risk ratio of neutropenia (grade 3/4) seemed to favour the PLD/carbo arm (RR 0.87; 95% CI 0.75 to 1.01), this did not translate into a statistically significant reduction in febrile neutropenia. No statistically significant differences were noted between study arms in the risk ratio of other severe adverse events, including allergic reactions, vomiting, stomatitis, hand‐foot syndrome and treatment‐related death (2 vs 4 deaths in the PLD vs PAC arm, respectively).
Dose delays and discontinuation
Women in the PLD/carbo arm were more likely to experience dose delays than women in the standard treatment arm (3636 cycles; RR 3.01; 95% CI 2.61 to 3.47; Analysis 1.17) and were more likely to discontinue treatment as the result of toxicity or refusal (RR 1.83; 95% CI 1.09 to 3.07; Analysis 1.18). The most frequent cause of toxicity related to delays was reported to be haematological toxicity.
1.17. Analysis.
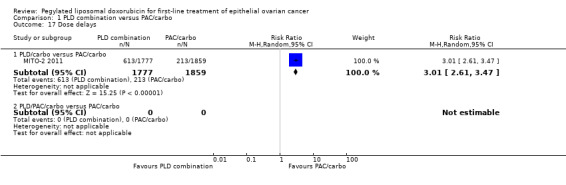
Comparison 1 PLD combination versus PAC/carbo, Outcome 17 Dose delays.
1.18. Analysis.
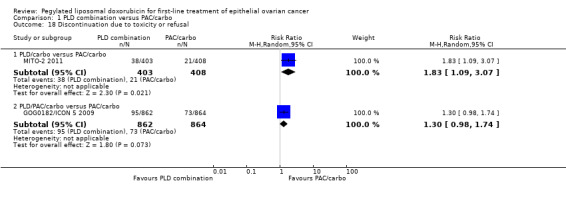
Comparison 1 PLD combination versus PAC/carbo, Outcome 18 Discontinuation due to toxicity or refusal.
Quality of life
Global quality of life scores were not statistically significantly different between the two groups, although these data suffered from high attrition rates.
PLD/PAC/carbo versus PAC/carbo
Only one trial (GOG0182/ICON 5 2009), which included 1726 women with newly diagnosed EOC, contributed data for this comparison.
Survival
No statistically significant differences between the PLD/PAC/carbo arm and the PAC/carbo arm were noted with respect to PFS (HR 0.98; 95% CI 0.88 to 1.09; Analysis 1.1) or OS (HR 0.95; 95% CI 0.84 to 1.08; Analysis 1.2). The results of any potential subgroup analyses were not available for this review.
Toxicity
Women in the PLD/PAC/carbo arm compared with the PAC/carbo arm were significantly more likely to experience the following.
Febrile neutropenia (grade 3/4): RR 2.25; 95% CI 1.72 to 2.94 (Analysis 1.5).
Neutropenia (grade 4): RR 1.13; 95% CI 1.06 to 1.22 (Analysis 1.6).
Anaemia (grade 3/4): RR 1.79; 95% CI 1.40 to 2.30 (Analysis 1.7).
Thrombocytopenia (grade 3/4): RR 1.73; 95% CI 1.49 to 2.01 (Analysis 1.8).
1.5. Analysis.
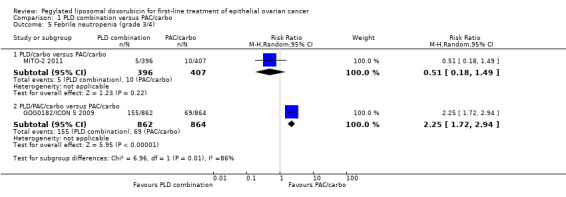
Comparison 1 PLD combination versus PAC/carbo, Outcome 5 Febrile neutropenia (grade 3/4).
1.6. Analysis.
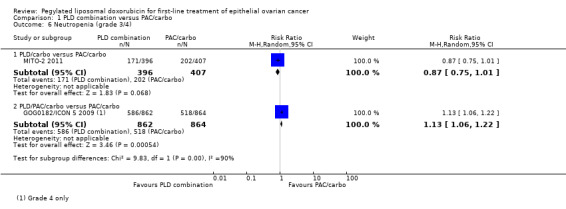
Comparison 1 PLD combination versus PAC/carbo, Outcome 6 Neutropenia (grade 3/4).
Dose delays and discontinuation
No statistically significant differences were noted between the PLD/PAC/carbo and PAC/carbo arms with regard to discontinuation due to toxicity or refusal (RR 1.30; 95% CI 0.98 to 1.74; Analysis 1.18), although the point estimate favours PAC/carbo.
Quality of life
These data were not available.
Discussion
Summary of main results
PLD/carbo versus PAC/carbo
We included one large study of women with advanced EOC, which used a PLD dose of 30 mg/m² in the experimental arm in combination with carboplatin every three weeks. No statistically significant differences in survival outcomes (PFS and OS) were reported between the treatment arms. PLD/carbo was associated with significantly more anaemia and thrombocytopenia than was PAC/carbo, whereas PAC/carbo was associated with significantly more alopecia and neuropathy. Women receiving PLD/carbo were significantly more likely to experience dose delays than women in the standard treatment arm and were significantly more likely to discontinue treatment as the result of toxicity or refusal.
PLD/PAC/carbo versus PAC/carbo
We included one study, which used a PLD dose of 30 mg/m² administered in alternate cycles (every six weeks) only as part of a triplet regimen in combination with paclitaxel and carboplatin. The addition of PLD to the combination of PAC/carbo produced no additional survival benefit over the standard regimen. However, the experimental triplet was associated with significantly more haematological toxicity, including anaemia, thrombocytopenia, neutropenia and febrile neutropenia.
Overall completeness and applicability of evidence
We consider the evidence sufficient to show that PLD/carbo is as effective with regard to survival outcomes as the standard PAC/carbo regimen in first‐line chemotherapy for women with advanced EOC. This effect appears to apply equally to women at lower and higher risk of disease progression, based on the stage and amount of residual disease present.
We did not prespecify subgrouping of the review results by performance status and histology. These subgroups were reported by MITO‐2 2011, however, and these investigators reported no significant differences between treatment groups in either subgroup analysis.
With regard to the triplet regimen of PLD/PAC/carbo, we consider current evidence provided by the GOG0182/ICON 5 2009 trial to be sufficient to show that no additional survival benefit is derived by adding PLD to the standard regimen on alternate cycles. Although the overall PLD dose intensity of this regimen was suboptimal (5 mg/m²/wk), further research using three‐weekly cycling (instead of six‐weekly cycling) is unlikely to be conducted, given the high rates of haematological toxicity experienced by women in this trial.
No RCTs have evaluated single‐agent PLD for first‐line treatment of EOC, probably because platinum‐based therapy is well established. However, PLD alone may be useful for women in whom platinum therapy is unsuitable (e.g. those with platinum hypersensitivity or renal dysfunction). This requires further investigation. Research efforts are currently focused in part on improving the scheduling of PAC/carbo, as weekly paclitaxel alongside three‐weekly carboplatin may be more effective (Katsumata 2009). Similarly, improvements in PLD/carbo scheduling might reduce adverse events associated with this drug combination.
Quality of the evidence
We considered the evidence regarding survival for PLD/carbo versus PAC/carbo to be of high quality (see Table 1). Although the published MITO‐2 2011 trial analysis was performed when fewer events had occurred than planned (556 instead of 632 PFS events), we obtained updated data from the investigators in August 2013, which support the earlier findings.
With regard to GOG0182/ICON 5 2009, although the quality of evidence related to alternate cycle dosing of PLD is high, we downgraded the quality of survival outcome effects to moderate because of the limited applicability of these results.
In general, we considered the quality of evidence related to severe adverse events to be high; although only two contributing studies were identified, the numbers of women enrolled in these well‐conducted trials were adequate to allow evaluation of the relative risks of common adverse events.
Potential biases in the review process
To our knowledge, no biases were present in the review process.
Agreements and disagreements with other studies or reviews
In a recent review and meta‐analysis of PLD for relapsed EOC, PLD/carbo administered every four weeks was found to be better than standard three‐weekly PAC/carbo for the treatment of relapsed platinum‐sensitive disease, with respect to survival and adverse events (Lawrie 2013). Two well‐conducted studies including 1164 women (CALYPSO 2010; HeCOG 2010) contributed data to these meta‐analyses, which found that women receiving PLD/carbo had an average increase in PFS of 15% (95% CI 3 to 26) compared with women receiving PAC/carbo for relapsed platinum‐sensitive disease. As was found in the present review, the risk ratio of severe haematological adverse events was significantly higher in the PLD/carbo arm than in the PAC/carbo arm. However, the associated risk ratio of thrombocytopenia was substantially lower with PLD/carbo in the recurrent EOC review (RR 2.69 vs RR 8.09). Furthermore, women in the recurrent EOC review were more likely to discontinue treatment as the result of toxicity/refusal if they received PAC/carbo, conversely to the present review.
These discrepancies between the adverse event findings of these reviews are most likely due to differences in dose densities of PLD: Three‐weekly cycles of PLD (30 mg/m²) were used in the first‐line treatment study (MITO‐2 2011), whereas four‐weekly cycles (30 mg/m²) were used in CALYPSO 2010. The use of additional therapies for supportive care (e.g. granulocyte colony stimulating factor [G‐CSF], erythropoietin) and, possibly, differences in the indications for discontinuation of treatment due to toxicity may also have contributed to these findings.
Similar to the findings of CALYPSO 2010 and HeCOG 2010, the occurrence of severe hand‐foot syndrome was not a significant problem in MITO‐2 2011. CALYPSO 2010 and Markman 2010 also reported lower rates of platinum‐associated hypersensitivity reactions (HSRs) with PLD/carbo; however, this was not a finding of MITO‐2 2011.
ICON7 (Perren 2011) reported a statistically significant improvement in PFS when bevacizumab was added to PAC/carbo compared with PAC/carbo alone. This improvement with bevacizumab was greatest in women at high risk for progression (15.9 vs 10.5 months; HR 0.68; P < 0.001; Perren 2011). For a similarly defined high‐risk group, MITO‐2 2011 investigators found no significant difference in PFS (11.8 vs 11 months; HR 0.97; P = 0.8; unpublished data). This suggests that bevacizumab plus PAC/carbo may be associated with improved survival compared with PLD/carbo. Final survival results of ICON7 are awaited with interest. A network meta‐analysis that directly and indirectly compares the various chemotherapy options available for the treatment of primary EOC is needed to clarify the relative effectiveness of these regimens and to guide future research. Network meta‐analysis was outside the scope of this review.
Authors' conclusions
Implications for practice.
Three‐weekly PLD/carbo is a reasonable alternative to PAC/carbo in the first‐line treatment of EOC, particularly for women in whom PAC‐induced alopecia and/or neuropathies are unacceptable. However, haematological adverse events, such as anaemia and thrombocytopenia, occur more frequently with PLD, and the availability and cost of appropriate supportive therapy to ameliorate these toxicities need to be considered. The alternating triplet regimen (PLD/PAC/carbo) is associated with significantly more toxicity than is standard treatment (PAC/carbo), with no significant improvement in survival, and therefore has no place in clinical practice.
Implications for research.
The following studies of PLD for first‐line treatment of ovarian cancer may be of value.
Adding bevacizumab to PLD/carbo compared with other bevacizumab combinations.
Alternating PLD and PAC (weekly or three‐weekly) with carbo.
Three‐weekly PLD/carbo regimens compared with four‐weekly regimens.
Single‐agent PLD in women for whom platinum‐based treatment is unsuitable.
A network meta‐analysis review with direct and indirect comparisons of the various chemotherapy options available for primary EOC.
What's new
| Date | Event | Description |
|---|---|---|
| 11 December 2019 | Review declared as stable | No new studies expected in this topic area. |
Acknowledgements
We would like to thank the following people for their contributions to the review process.
The MITO‐2 2011 trial investigators for supplying updated survival data and unpublished subgroup data for this trial.
Managing Editors, Gail Quinn and Clare Jess, of the Cochrane Gynaecological Cancer Group for their administrative support.
Trial Search Co‐ordinator, Jane Hayes, for performing the electronic searches.
The library staff at the Royal United Hospital for assistance with the sourcing of reference material.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Gynaecological Cancer Group. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, the NHS or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
MeSH descriptor Ovarian Neoplasms explode all trees
ovar* near/5 (cancer* or neoplas* or tumor* or tumour* or carcinoma* or malignan*)
(#1 OR #2)
MeSH descriptor Doxorubicin explode all trees
doxorubicin
caelyx
doxil
(#4 OR #5 OR #6 OR #7)
(#3 AND #8)
Appendix 2. MEDLINE search strategy
MEDLINE Ovid
exp Ovarian Neoplasms/
(ovar* adj5 (cancer* or neoplas* or tumor* or tumour* or carcinoma* or malignan*)).mp.
1 or 2
exp Doxorubicin/
doxorubicin.mp.
caelyx.mp.
doxil.mp.
myocet.mp.
4 or 5 or 6 or 7 or 8
3 and 9
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
clinical trials as topic.sh.
randomly.ab.
trial.ti.
11 or 12 or 13 or 14 or 15 or 16 or 17
10 and 18
exp animals/ not humans.sh.
19 not 20
key: mp = protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier, pt = publication type, ab = abstract, ti = title, sh = subject heading
Appendix 3. EMBASE search strategy
EMBASE Ovid
exp ovary tumor/
(ovar* adj5 (cancer* or neoplas* or tumor* or tumour* or carcinoma* or malignan*)).mp.
1 or 2
exp doxorubicin/
doxorubicin.mp.
caelyx.mp.
doxil.mp.
myocet.mp.
4 or 5 or 6 or 7 or 8
3 and 9
crossover procedure/
randomized controlled trial/
single blind procedure/
random*.mp.
factorial*.mp.
(crossover* or cross over* or cross‐over).mp.
placebo*.mp.
(doubl* adj blind*).mp.
(singl* adj blind*).mp.
assign*.mp.
allocat*.mp.
volunteer*.mp.
11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22
10 and 23
key: mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
Appendix 4. 'Risk of bias' tool
We applied this tool to included studies to assess the risk of bias:
1. Random sequence generation
Low risk of bias (e.g. participants assigned to treatments on basis of a computer‐generated random sequence or a table of random numbers).
High risk of bias (e.g. participants assigned to treatments on basis of date of birth, clinic ID number or surname, or no attempt to randomly assign participants).
Unclear risk of bias (e.g. not reported, information not available).
2. Allocation concealment
Low risk of bias (e.g. allocation sequence could not be foretold).
High risk of bias (e.g. allocation sequence could be foretold by participants, investigators or treatment providers).
Unclear risk of bias (e.g. not reported).
3. Blinding of participants and personnel
Low risk of bias if participants and personnel were adequately blinded.
High risk of bias if participants were not blinded to the interventions that they received.
Unclear risk of bias if this was not reported or was unclear.
4. Blinding of outcomes assessors
Low risk of bias if outcome assessors were adequately blinded.
High risk of bias if outcome assessors were not blinded to the intervention that the participant received.
Unclear risk of bias if this was not reported or was unclear.
5. Incomplete outcome data
Low risk of bias if fewer than 20% of participants were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms.
High risk of bias if more than 20% of participants were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms.
Unclear risk of bias if loss to follow‐up was not reported.
6. Selective reporting of outcomes
Low risk of bias (e.g. reports all outcomes specified in the protocol).
High risk of bias (e.g. it is suspected that outcomes have been selectively reported).
Unclear if it is unclear whether outcomes have been selectively reported.
7. Other bias
Low risk of bias if you do not suspect any other source of bias and the trial appears to be methodologically sound.
High risk of bias if you suspect that the trial was prone to an additional bias.
Unclear risk of bias if you are uncertain whether an additional bias may have been present.
Data and analyses
Comparison 1. PLD combination versus PAC/carbo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PFS | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 PLD/carbo versus PAC/carbo | 1 | 820 | Hazard Ratio (Random, 95% CI) | 1.01 [0.85, 1.19] |
| 1.2 PLD/PAC/carbo versus PAC/carbo | 1 | 1726 | Hazard Ratio (Random, 95% CI) | 0.98 [0.88, 1.09] |
| 2 OS | 2 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 PLD/carbo versus PAC/carbo | 1 | 820 | Hazard Ratio (Random, 95% CI) | 0.94 [0.78, 1.13] |
| 2.2 PLD/PAC/carbo versus PAC/carbo | 1 | 1726 | Hazard Ratio (Random, 95% CI) | 0.95 [0.84, 1.08] |
| 3 PFS: subgroup analyses | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 No residual disease | 1 | 298 | Hazard Ratio (Random, 95% CI) | 0.93 [0.67, 1.29] |
| 3.2 Residual disease ≤ 1 cm | 1 | 149 | Hazard Ratio (Random, 95% CI) | 1.39 [0.96, 2.01] |
| 3.3 Residual disease > 1 cm | 1 | 227 | Hazard Ratio (Random, 95% CI) | 0.92 [0.70, 1.22] |
| 3.4 No cytoreductive surgery | 1 | 146 | Hazard Ratio (Random, 95% CI) | 1.01 [0.72, 1.41] |
| 3.5 Stage I/II only | 1 | 153 | Hazard Ratio (Random, 95% CI) | 0.96 [0.56, 1.65] |
| 3.6 Stage III/IV only | 1 | 667 | Hazard Ratio (Random, 95% CI) | 0.99 [0.84, 1.16] |
| 3.7 Age < 70 years | 1 | 721 | Hazard Ratio (Random, 95% CI) | 0.95 [0.80, 1.13] |
| 3.8 Age ≥ 70 years | 1 | 99 | Hazard Ratio (Random, 95% CI) | 1.42 [0.92, 2.19] |
| 4 OS: subgroup analyses | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 No residual disease | 1 | 298 | Hazard Ratio (Random, 95% CI) | 0.79 [0.49, 1.27] |
| 4.2 Residual disease ≤ 1 cm | 1 | 149 | Hazard Ratio (Random, 95% CI) | 1.48 [0.94, 2.34] |
| 4.3 Residual disease > 1 cm | 1 | 227 | Hazard Ratio (Random, 95% CI) | 0.82 [0.60, 1.11] |
| 4.4 No cytoreductive surgery | 1 | 146 | Hazard Ratio (Random, 95% CI) | 1.05 [0.73, 1.50] |
| 4.5 Stage I/II only | 1 | 153 | Hazard Ratio (Random, 95% CI) | 0.82 [0.38, 1.76] |
| 4.6 Stage III/IV only | 1 | 667 | Hazard Ratio (Random, 95% CI) | 0.94 [0.78, 1.14] |
| 4.7 Age < 70 years | 1 | 721 | Hazard Ratio (Random, 95% CI) | 0.95 [0.77, 1.17] |
| 4.8 Age ≥ 70 years | 1 | 99 | Hazard Ratio (Random, 95% CI) | 0.87 [0.53, 1.43] |
| 5 Febrile neutropenia (grade 3/4) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 PLD/carbo versus PAC/carbo | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.18, 1.49] |
| 5.2 PLD/PAC/carbo versus PAC/carbo | 1 | 1726 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [1.72, 2.94] |
| 6 Neutropenia (grade 3/4) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 PLD/carbo versus PAC/carbo | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.75, 1.01] |
| 6.2 PLD/PAC/carbo versus PAC/carbo | 1 | 1726 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.06, 1.22] |
| 7 Anaemia (grade 3/4) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 PLD/carbo versus PAC/carbo | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 2.74 [1.54, 4.88] |
| 7.2 PLD/PAC/carbo versus PAC/carbo | 1 | 1726 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.40, 2.30] |
| 8 Thrombocytopenia (grade 3/4) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 PLD/carbo versus PAC/carbo | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 8.09 [3.93, 16.67] |
| 8.2 PLD/PAC/carbo versus PAC/carbo | 1 | 1726 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.49, 2.01] |
| 9 Alopecia (grade 2) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 PLD/carbo versus PAC/carbo | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.06, 0.14] |
| 9.2 PLD/PAC/carbo versus PAC/carbo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Vomiting (grade 3/4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10.1 PLD/carbo versus PAC/carbo | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.56, 3.82] |
| 10.2 PLD/PAC/carbo versus PAC/carbo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Stomatitis (grade 3/4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 PLD/carbo versus PAC/carbo | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.19, 22.58] |
| 12 Hand‐foot syndrome (grade 3/4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 12.1 PLD/carbo versus PAC/carbo | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.27, 2.20] |
| 12.2 PLD/PAC/carbo versus PAC/carbo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Neuropathy (grade 3/4) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 PLD/carbo versus PAC/carbo | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 0.66] |
| 13.2 PLD/PAC/carbo versus PAC/carbo | 1 | 1726 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.23] |
| 14 Allergic reaction (grade 3/4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14.1 PLD/carbo versus PAC/carbo | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.38, 2.25] |
| 14.2 PLD/PAC/carbo versus PAC/carbo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Fatigue (grade 3/4) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 15.1 PLD/carbo versus PAC/carbo | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.45, 2.34] |
| 15.2 PLD/PAC/carbo versus PAC/carbo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Treatment‐related death | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 16.1 PLD/carbo versus PAC/carbo | 1 | 803 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.09, 2.79] |
| 16.2 PLD/PAC/carbo versus PAC/carbo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Dose delays | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 PLD/carbo versus PAC/carbo | 1 | 3636 | Risk Ratio (M‐H, Random, 95% CI) | 3.01 [2.61, 3.47] |
| 17.2 PLD/PAC/carbo versus PAC/carbo | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Discontinuation due to toxicity or refusal | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 PLD/carbo versus PAC/carbo | 1 | 811 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.09, 3.07] |
| 18.2 PLD/PAC/carbo versus PAC/carbo | 1 | 1726 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.98, 1.74] |
1.3. Analysis.
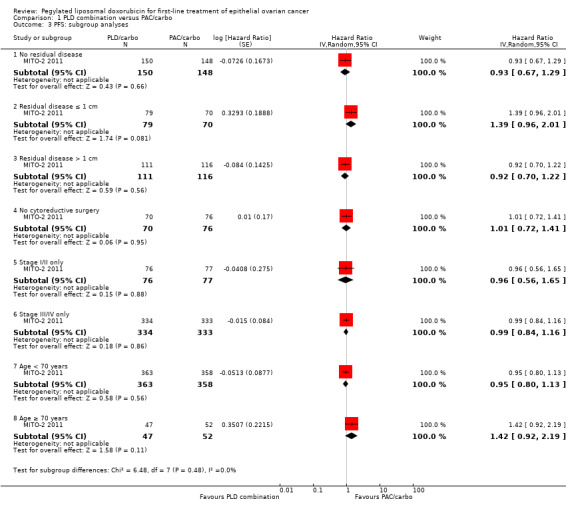
Comparison 1 PLD combination versus PAC/carbo, Outcome 3 PFS: subgroup analyses.
1.4. Analysis.
Comparison 1 PLD combination versus PAC/carbo, Outcome 4 OS: subgroup analyses.
1.10. Analysis.
Comparison 1 PLD combination versus PAC/carbo, Outcome 10 Vomiting (grade 3/4).
1.11. Analysis.
Comparison 1 PLD combination versus PAC/carbo, Outcome 11 Stomatitis (grade 3/4).
1.12. Analysis.
Comparison 1 PLD combination versus PAC/carbo, Outcome 12 Hand‐foot syndrome (grade 3/4).
1.14. Analysis.
Comparison 1 PLD combination versus PAC/carbo, Outcome 14 Allergic reaction (grade 3/4).
1.15. Analysis.
Comparison 1 PLD combination versus PAC/carbo, Outcome 15 Fatigue (grade 3/4).
1.16. Analysis.
Comparison 1 PLD combination versus PAC/carbo, Outcome 16 Treatment‐related death.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
GOG0182/ICON 5 2009.
| Methods | International phase III RCT (Australia, New Zealand, United Kingdom and Italy). Accrual from January 2001 to September 2004 | |
| Participants | 4100 women with EOC or primary peritoneal carcinoma. Women had to have FIGO stage III or IV and optimal (≤ 1 cm) or suboptimal residual disease. Other inclusion criteria included GOG performance status ≤ 2, absolute neutrophil count ≥ 1500/microL, platelets ≥ 100,000/microL and creatinine ≤ 1.5× institutional upper limit of normal (ULN), bilirubin ≤ 1.5× ULN, AST and alkaline phosphatase ≤ 2.5× ULN and baseline sensory or motor neuropathy grade 1 or lower, according to National Cancer Institute Common Toxicity Criteria version 2. Patients with history of breast cancer were eligible, provided they were disease ‐free for at least 3 years without contraindications for protocol‐based chemotherapy. Patients who had early‐stage synchronous endometrial cancer were also eligible, provided was no more than minimum invasion was noted without high‐grade features. | |
| Interventions | Five treatment arms with 8 cycles (C1‐8) each: 1. Carbo AUC 6/PAC 175 mg/m² (C1‐8); administered on day 1 (D1) (control arm). 2. Carbo AUC 5/PAC 175 mg/m² (C1‐8; D1) plus gemcitabine 800 mg/m² (C1‐8; D1/8). 3. Carbo AUC 5/PAC 175 mg/m² (C1‐8; D1) plus PLD 30 mg/m² alternate cycles (C1/3/5/7; D1) (experimental arm). 4. Carbo AUC 5/topotecan 125 mg/m² (C1‐4; Carbo D3, topotecan D1/2/3) followed by Carbo AUC 6/PAC 175 mg/m² (C5‐8; D1). 5. Carbo AUC 6/gemcitabine 1000 mg/m² (C1‐4; Carbo D8, gemcitabine D1/8) followed by Carbo AUC 6/paclitaxel 175 mg/m² (C5‐8; D1). | |
| Outcomes | Primary: PFS, OS. Secondary: toxicity (graded according to National Cancer Institute Common Toxicity Criteria version 2.0), complications, dose intensity, cumulative dose delivery. |
|
| Notes | Overall, approximately 30% of women had suboptimal cytoreduction and < 25% had measurable residual disease. For the purposes of this review, we extracted data for arms 1 (864 women) and 3 (862 women). We were unable to obtain additional data from the study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated stratified block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Independent evaluation not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition |
| Selective reporting (reporting bias) | Low risk | Prespecified and expected outcomes were reported |
| Other bias | Unclear risk | Alternate dosing of PLD limits interpretation of these data Baseline characteristics of groups were similar |
MITO‐2 2011.
| Methods | Open‐label, multi‐centre phase III RCT (Italy, Portugal, Turkey). Accrual dates January 2003 to November 2007, with median follow‐up time of 40 months | |
| Participants | 820 women with cytological or histological diagnosis of EOC (stage Ic to IV FIGO). Included if < 75 years, Eastern Cooperative Oncology Group performance status ≤ 2, life expectancy ≥ 3 months and adequate bone marrow, kidney and liver function | |
| Interventions | Standard arm: Carbo AUC 5 and PAC 175 mg/m² Experimental arm: Carbo AUC 5 and PLD 30 mg/m² (diluted in 250 mL 5% glucose and infused over 60 minutes, after completion of Carbo treatment) |
|
| Outcomes | Primary: PFS Secondary: OS, treatment activity, toxicity (according to National Cancer Institute Common Toxicity Criteria version 2.0), QoL (EORTC QLQ‐C30 questionnaire) |
|
| Notes | Included stage Ic and II. Overall, approximately 46% had suboptimal cytoreduction (residual disease > 1 cm) and 18% had no surgery We obtained updated, unpublished survival data (August 2013) from the investigators for this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated minimisation method |
| Allocation concealment (selection bias) | Low risk | Central telephone assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Independent evaluation not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low attrition except for QoL data |
| Selective reporting (reporting bias) | Low risk | Prespecified and expected outcomes were reported |
| Other bias | Low risk | Baseline characteristics of groups were similar |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Pontamianou 2005 | Not an RCT |
| SWOG S9912 2009 | Not an RCT |
Differences between protocol and review
None.
Contributions of authors
TAL and CT selected studies. TAL, RR and CT extracted data and contributed to the writing of the text. JM reviewed the drafts and suggested revisions. All authors approved the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Department of Health, UK.
NHS Cochrane Collaboration Programme Grant Scheme CPG‐10/4001/12
Declarations of interest
None.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
GOG0182/ICON 5 2009 {published data only}
- Bookman MA. Erratum. Journal of Clinical Oncology 2009;27(13):2305. [Google Scholar]
- Bookman MA and the Gynecologic Cancer InterGroup (GCIG). GOG0182‐ICON5: 5‐arm phase III randomized trial of paclitaxel (P) and carboplatin (C) vs combinations with gemcitabine (G), PEG‐lipososomal doxorubicin (D), or topotecan (T) in patients (pts) with advanced‐stage epithelial ovarian (EOC) or primary peritoneal (PPC) carcinoma. Journal of Clinical Oncology, 2006 ASCO Annual Meeting Proceedings Part I. 2006; Vol. 24, 18S (June 20 Suppl):5002.
- Bookman MA and the Gynecologic Cancer InterGroup (GCIG). GOG0182‐ICON5: 5‐arm phase III randomized trial of paclitaxel (P) and carboplatin (C) vs combinations with gemcitabine (G), PEG‐lipososomal doxorubicin (D), or topotecan (T) in patients (pts) with advanced‐stage epithelial ovarian (EOC) or primary peritoneal (PPC) carcinoma [abstract]. Journal of Clinical Oncology 2006;24(Suppl 18):A‐5002:256s. [Google Scholar]
- Bookman MA, Brady MF, McGuire WP, Harper PG, Alberts DS, Friedlander M, et al. Evaluation of new platinum‐based treatment regimens in advanced‐stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. Journal of Clinical Oncology 2009;27(9):1419‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookman MA, Greer BE, Ozols RF. Optimal therapy of advanced ovarian cancer: carboplatin and paclitaxel vs. cisplatin and paclitaxel (GOG 158) and an update on GOG0 182‐ICON5. International Journal of Gynecological Cancer 2003;13(6):735‐40. [DOI] [PubMed] [Google Scholar]
- Copeland LJB and the Gynecologic Cancer Intergroup (GCIG). Clinical trials of newer regimens for treating ovarian cancer: the rationale for Gynecologic Oncology Group Protocol GOG 182‐ICON5. Gynecologic Oncology 2003;90:(2 Part 2):S1‐7. [DOI] [PubMed] [Google Scholar]
MITO‐2 2011 {published data only}
- Pignata S, Scambia G, Ferrandina G, Savarese A, Sorio R, Breda E, et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as first‐line treatment for patients with ovarian cancer: the MITO‐2 randomized phase III trial. Journal of Clinical Oncology 2011;29(27):3628‐35. [DOI] [PubMed] [Google Scholar]
- Pignata S, Scambia G, Savarese A, Breda E, Scollo P, Vivo R, et al. Safety of a 3‐weekly schedule of carboplatin plus pegylated liposomal doxorubicin as first line chemotherapy in patients with ovarian cancer: preliminary results of the MITO‐2 randomized trial. BioMedCentral (BMC) Cancer 2006;6:202. http://www.biomedcentral.com/1471‐2407/6/202.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignata S, Scambia G, Savarese A, Breda E, Sorio R, Pisano C, et al. Carboplatin and pegylated liposomal doxorubicin for advanced ovarian cancer: preliminary activity results of the MITO‐2 phase III trial. Oncology 2009;76(1):49‐54. [DOI] [PubMed] [Google Scholar]
- Pignata S, Scambia G, Savarese A, Breda E, Sorio R, Vernaglia Lombardi A, et al. Carboplatin plus paclitaxel versus carboplatin plus Stealth liposomal doxorubicin in patients with advanced ovarian cancer: preliminary activity results of the MITO‐2 randomized multicenter trial [Abstract]. Journal of Clinical Oncology; 2007 ASCO Annual Meeting. 2007; Vol. 25:18S(abstract 5532).
- Pignata S, Scambia G, Savarese A, Sorio R, Breda E, Ferrandina G, et al. Carboplatin plus paclitaxel (CP) versus carboplatin plus stealth liposomal doxorubicin (CLD) in patients with advanced ovarian cancer (AOC): activity and safety results of the MITO‐2 randomized multicenter trial [abstract]. Journal of Clinical Oncology; 2009 ASCO Annual Meeting. 2009; Vol. 27:15S(Suppl; abstract LBA5508).
- Pignata S, Scambia G, Savarese A, Sorio R, Breda E, Legge F, et al. Carboplatin (C) plus paclitaxel (P) versus carboplatin plus pegylated liposomal doxorubicin (PLD) in patients with advanced ovarian cancer (AOC): final analysis of the MITO‐2 randomized multicenter trial. Journal of Clinical Oncology; 2010 ASCO Annual Meeting. 2010; Vol. 28:18s, 2010 (Suppl; abstract LBA5033).
- Pignata S, Scambia G, Savarese A, Sorio R, Breda E, Legge F, et al. Carboplatin plus paclitaxel versus carboplatin plus pegylated liposomal doxorubicin as firstline treatment for patients with ovarian cancer: the MITO‐2 (multicentre Italian trials in ovarian cancer) randomized phase III trial. Annals of Oncology; 35th ESMO Congress, Milan, Italy. 2010.
- Pignata S, Scambia G, Savarese A, Zagonel V, Gebbia V, Scollo P, et al. Carboplatin plus paclitaxel (CP) versus carboplatin plus Stealth liposomal doxorubicin (CLD) in patients with advanced ovarian cancer (AOC): preliminary safety results of the MITO‐2 randomized multicenter trial. Journal of Clinical Oncology; 2005 ASCO Annual Meeting. 2005; Vol. 23:16S (Suppl, abstract 5014).
References to studies excluded from this review
Pontamianou 2005 {published data only}
- Potamianou A, Androulakis N, Papakotoulas P, Toufexi H, Latoufis C, Kouroussis C, et al. Sequential combination of paclitaxel‐carboplatin and paclitaxel‐liposomal doxorubicin as a first‐line treatment in patients with ovarian cancer. A multicenter phase II trial. Oncology 2005;69(4):348‐53. [DOI] [PubMed] [Google Scholar]
SWOG S9912 2009 {published data only}
- Smith HO, Moon J, Wilczynski SP, Tiersten AD, Hannigan EV, Robinson WR, et al. Southwest Oncology Group Trial S9912: intraperitoneal cisplatin and paclitaxel plus intravenous paclitaxel and pegylated liposomal doxorubicin as primary chemotherapy of small‐volume residual stage III ovarian cancer. Gynecologic Oncology 2009;114(2):206‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
A'Hern 1995
- A'Hern R, Gore ME. The impact of doxorubicin on survival in advanced ovarian cancer. Journal of Clinical Oncology 1995;13:726‐32. [DOI] [PubMed] [Google Scholar]
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993;85(5):365‐76. [DOI] [PubMed] [Google Scholar]
Burger 2011
- Burger RA, Brady MF, Bookman MA, Fleming GF, Monk BJ, et al. for the Gynecologic Oncology Group. Incorporation of bevacizumab in the primary treatment of ovarian cancer. The New England Journal of Medicine 2011;365:2473‐83. [DOI] [PubMed] [Google Scholar]
CALYPSO 2010
- Pujade‐Lauraine E, Wagner U, Aavall‐Lundqvist E, Gebski V, Heywood M, Vasey PA, et al. Pegylated liposomal doxorubicin and carboplatin compared with paclitaxel and carboplatin for patients with platinum‐sensitive ovarian cancer in late relapse. Journal of Clinical Oncology 2010;28(20):3323‐9. [DOI] [PubMed] [Google Scholar]
Campbell 1989
- Campbell S, Bhan V, Royston P, Whitehead MI, Collins WP. Transabdominal ultrasound screening for early ovarian cancer. BMJ 1989;299(6712):1363‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
CTCAE 2006
- CTCAE. Common Terminology Criteria for Adverse Events v3.0 (CTCAE), 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf.
Deeks 2001
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining for heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Healthcare: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
EMA 2010
- European Medicines Agency. EPARs for authorised medicinal products for human use. http://www.emea.europa.eu/docs/en_GB/document_library/EPAR_‐_Summary_for_the_public/human/000089/WC500020173.pdf 2010 (accessed 24 January 2013).
EMA 2011
- European Medicines Agency. Avastin (bevacizumab) product page. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Information/human/000582/WC500029271.pdf.
EUROCARE‐4 2009
- Sant M, Allemani C, Santaquilani M, Knijn A, Marchesi F, Capocaccia R. EUROCARE‐4. Survival of cancer patients diagnosed in 1995‐1999. Results and commentary. European Journal of Cancer 2009;45(6):931‐91. [DOI] [PubMed] [Google Scholar]
FIGO 2009
- FIGO CinGO. Current FIGO staging for cancer of the vagina, fallopian tube, ovary, and gestational trophoblastic neoplasia. International Journal of Gynecology and Obstetrics 2009;105(1):3‐4. [DOI] [PubMed] [Google Scholar]
Gabizon 1997
- Gabizon A, Martin F. Polyethylene glycol‐coated (pegylated) liposomal doxorubicin: rationale for use in solid tumours. Drugs 1997;54(Suppl 4):15‐21. [DOI] [PubMed] [Google Scholar]
Gabizon 2001
- Gabizon AA. Stealth liposomes and tumour targeting: one step further in the quest for the magic bullet. Clinical Cancer Research 2001;7:223‐5. [PubMed] [Google Scholar]
GLOBOCAN 2008
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide. IARC CancerBase No. 10 [Internet]. Lyon, France: International Agency for Research on Cancer; 2010. http://globocan.iarc.fr. Lyon, France.
HeCOG 2010
- Bafaloukos D, Linardou H, Aravantinos G, Papadimitriou G, Bamias A, Fountzilas G, et al. A randomized phase II study of carboplatin plus pegylated liposomal doxorubicin versus carboplatin plus paclitaxel in platinum sensitive ovarian cancer patients: a Hellenic Cooperative Oncology Group study. BMC Medicine 2010;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Jacobs 1988
- Jacobs I, Bridges J, Reynolds C, Stabile I, Kemsley P, Grudzinskas J, et al. Multimodal approach to screening for ovarian cancer. The Lancet 1988;331(8580):268‐71. [DOI] [PubMed] [Google Scholar]
Janssen‐Cilag 2011
- Janssen‐Cilag P, Ltd. CAELYX Product Information. http://www.janssen.com.au/Products/Caelyx. Amended 4 January 2011 (accessed 24 January 2013).
Katsumata 2009
- Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose‐dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open‐label, randomised controlled trial. The Lancet 2009;374(9698):1331‐8. [DOI] [PubMed] [Google Scholar]
Lawrie 2013
- Lawrie TA, Bryant A, Cameron A, Gray E, Morrison J. Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD006910.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Markman 2010
- Markman M, Moon J, Wilczynski S, Lopez AM, Rowland KM, Michelin DP, et al. Single agent carboplatin versus carboplatin plus pegylated liposomal doxorubicin in recurrent ovarian cancer: final survival results of a SWOG (S0200) phase 3 randomized trial. Gynecologic Oncology 2010;116(3):323‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Menon 2009
- Menon U, Gentry‐Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). The Lancet Oncology 2009;10(4):327‐40. [DOI] [PubMed] [Google Scholar]
NICE 2003
- National Institute for Health and Clinical Excellence. Guidance on the use of paclitaxel in the treatment of ovarian cancer (TA55). London: National Institute for Health and Clinical Excellence, 2003.
NICE 2011
- National Institute for Health and Clinical Excellence. Ovarian cancer: the recognition and initial management of ovarian cancer (CG122). London: National Institute for Health and Clinical Excellence 2011.
OCMP 1991
- Ovarian Cancer Meta‐analysis Project. Cyclophosphamide plus cisplatin versus cyclophosphamide, doxorubicin and cisplatin chemotherapy of ovarian carcinoma. Journal of Clinical Oncology 1991;9:1668‐74. [DOI] [PubMed] [Google Scholar]
Ozols 1980
- Ozols RF, Wilson JK, Weltz MD, Grotzinger KR, Myers CE, Young RC. Inhibition of human ovarian cancer colony formation by adriamycin and its major metabolites. Cancer Research 1980;40:4109‐12. [PubMed] [Google Scholar]
Perren 2011
- Perren TJ, Swart AM, Pfisterer J, Ledemann JA, Pujade‐Lauraine E, Kristensen G, and the ICON7 Investigators. A phase 3 trial of bevacizumab in ovarian cancer. New England Journal of Medicine 2011;365:2484‐96. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
SEER 2007
- Kosary CL. Chapter 16: Cancer of the ovary. In: Ries LAG, Young JL, Keel GE, Eisner MP, Lin YD, Horner M‐J editor(s). Cancer Survival Among Adults: US SEER Program, 1988‐2001. Bethesda: US Department of Health and Human Services; National Cancer Institute, 2007:133‐44. [Google Scholar]
Stark 2013
- Stark D, Nankivell M, Pujade‐Lauraine E, Kristensen G, Elit L, Stockler M, et al. Standard chemotherapy with or without bevacizumab in advanced ovarian cancer: quality‐of‐life outcomes from the International Collaboration on Ovarian Neoplasms (ICON7) phase 3 randomised trial. The Lancet Oncology 2013;14(3):236‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Therasse 2000
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute 2000;92(3):205‐16. [DOI] [PubMed] [Google Scholar]
Waterhouse 2001
- Waterhouse DN, Tardi PG, Mayer LD, Bally MB. A comparison of liposomal formulations of doxorubicin with drug administered in free form: changing toxicity profiles. Drug Safety 2001;24:903‐20. [DOI] [PubMed] [Google Scholar]
Zunino 2002
- Zunino F, Pratesi G. Antitumour antibodies. Oxford Textbook of Oncology. 2nd Edition. Oxford, UK: Oxford University Press, 2002:715‐27. [Google Scholar]


