Abstract
Background
Cognitive deficits are common in people who have received cranial irradiation and have a serious impact on daily functioning and quality of life. The benefit of pharmacological and non‐pharmacological treatment of cognitive deficits in this population is unclear.
Objectives
To assess the effectiveness of interventions for preventing or ameliorating cognitive deficits in adult patients treated with cranial irradiation.
Search methods
In August 2014. we searched the Cochrane Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and PsycINFO and checked the reference lists of included studies. We also searched for ongoing trials via ClinicalTrials.gov, the Physicians Data Query and the Meta Register of Controlled Trials.
Selection criteria
We included randomised controlled trials (RCTs) that evaluated pharmacological or non‐pharmacological interventions in cranial irradiated adults, with objective cognitive functioning as a primary or secondary outcome measure.
Data collection and analysis
Two review authors (JD, KZ) independently extracted data from selected studies and carried out a 'Risk of bias' assessment. Cognitive function, fatigue and mood outcomes were reported. No data were pooled.
Main results
Sixteen studies were identified for possible inclusion in the review, six of which were included. Three studies investigated prevention and three studies investigated amelioration. Due to differences between studies in the interventions being evaluated, a meta‐analysis was not possible. Two studies investigated a pharmacological intervention for the prevention of cognitive deficits; memantine compared with placebo, and d‐threo‐methylphenidate HCL compared with placebo. In the first study the primary cognitive outcome of memory at six months did not reach significance, but there was significant improvement in overall cognitive function compared to placebo, with similar adverse events across groups. The second study found no statistically significant difference between arms, with few adverse events. The third study investigated a rehabilitation program for the prevention of cognitive deficits but did not carry out a statistical comparison of cognitive performance between groups.
Three studies investigated the use of a pharmacological intervention for the treatment of cognitive deficits; methylphenidate compared with modafinil, two different doses of modafinil, and donepezil compared with placebo. The first study found improvements in cognitive function in both the methylphenidate and modafinil arms; few adverse events were reported. The second study combined treatment arms and found improvements across all cognitive tests, however, a number of adverse events were reported. Both studies were limited by a small sample size. The third study did not find an improvement in the primary cognitive outcome of overall performance, but did find improvement in an individual test of memory, compared to placebo; adverse events were not reported. No non‐pharmacological studies for the amelioration of cognitive deficits were eligible. There were a number of limitations across studies but few without high risks of bias.
Authors' conclusions
There is supportive evidence that memantine may help prevent cognitive deficits for adults with brain metastases receiving cranial irradiation. There is supportive evidence that donepezil may have a role in treating cognitive deficits in adults with primary or metastatic brain tumours who have been treated with cranial irradiation. Patient withdrawal affected the statistical power of both studies. Further research that tries to minimise the withdrawal of consent, and subsequently reduce the requirement for imputation procedures, may offer a higher quality of evidence.
There is no strong evidence to support any non‐pharmacological interventions (medical or cognitive/behavioural) in the prevention or amelioration of cognitive deficits. Non‐randomised studies appear promising but are as yet to be conclusive via translation into high quality evidence. Further research is required.
Keywords: Adult, Humans, Benzhydryl Compounds, Benzhydryl Compounds/therapeutic use, Cognition Disorders, Cognition Disorders/etiology, Cognition Disorders/prevention & control, Cognition Disorders/therapy, Cranial Irradiation, Cranial Irradiation/adverse effects, Indans, Indans/therapeutic use, Memantine, Memantine/therapeutic use, Methylphenidate, Methylphenidate/therapeutic use, Nootropic Agents, Nootropic Agents/therapeutic use, Piperidines, Piperidines/therapeutic use, Randomized Controlled Trials as Topic
Interventions for preventing and ameliorating cognitive deficits in adults treated with cranial irradiation
Background
Problems with mental activities (cognitive deficits) are common in patients who have received radiation to the brain for a primary or secondary (metastatic) brain tumour, or to help prevent a tumour spreading to the brain from elsewhere in the body. This toxic side effect of brain radiation may be acute (during treatment) or early after treatment (one to six months) and may be reversible. However, late toxicities may occur many months or years later and are generally irreversible and are slowly progressive. Late cognitive deficits, such as memory loss, problems planning tasks or behavioural changes, can have a serious impact on quality of life and the ability to carrying out activities normally. Interventions to help prevent or treat these late radiation toxicities may improve a patient's well‐being.
Study Characteristics
In August 2014 we searched four literature databases. Six randomised controlled trials (RCTs), in which patients were randomly assigned to the intervention or a comparison group (control group), were eligible for inclusion. Each trial assessed different interventions, so results were not combined. The largest trial investigated the medical drug memantine in 508 patients with a metastatic brain tumour. Another trial investigated donepezil in 198 patients with a primary or secondary brain tumour. The other trials were smaller and investigated modafinil and methylphenidate. We found one psychological intervention for preventing cognitive deficits during brain radiation. There is one ongoing medical drug trial recruiting participants. There were many non‐randomised and non‐controlled trials that offer promising results for further exploration using an RCT method.
Key findings
Findings into the efficacy of memantine offer supportive evidence for preventing cognitive deficits in patients with a secondary brain tumour receiving brain irradiation. Findings into the efficacy of donepezil offer some support for its use in the amelioration of cognitive deficits in patients with a primary or secondary tumour previously treated with radiation. The remaining studies did not have a sufficient number of participants to provide reliable results. The drugs used had few side effects (adverse events), although these were not reported well. Recruitment and retention of trial participants for these medical drug studies is difficult.
Quality of the evidence
We found limitations in the evidence across studies, most medical drug randomised controlled trials had a low risk of bias, whereas the psychological interventions were at a high risk of bias.
Background
Description of the condition
Cognition refers to the mental abilities that require the high‐level processing of sensory information. Such abilities include memory, executive function, thought, sensory perception, visuo‐spatial processing, concentration, attention, intellectual function, behaviour, personality and mood (Gilroy 2000). Cognitive dysfunction (or deficit) in any of these areas can have a significant impact on a person's ability to function in day‐to‐day life, including work performance, language and communication, social interactions and independent living (Meyers 1998).
Cognitive deficits are common among patients who have received cranial irradiation (Taphoorn 2004) to treat primary or metastatic brain tumours, or as prevention (prophylaxis) of other cancers. Both the brain tumour itself and tumour treatment can cause cognitive deficits (Taphoorn 2004). Over 80% of primary and metastatic brain tumour patients have self‐reported cognitive concerns regarding memory or concentration (Lidstone 2003; Mukand 2001). For example, in a prospective study, cognitive functioning was assessed objectively using neuropsychological testing in patients receiving cranial irradiation for the therapeutic treatment of brain metastases. Results demonstrated cognitive deficits in the domains of learning, delayed recall and recognition six to eight weeks following radiotherapy when compared to baseline scores (Welzel 2008). In another study, patients with lung cancer receiving prophylactic cranial irradiation demonstrated reduced cognitive functioning on subjective and objective measures at six‐ and 12‐month follow‐up assessments when compared to baseline scores (Gondi 2013). A randomised controlled trial (RCT) also documented significant cognitive deficits four months after whole brain radiotherapy (WBRT) compared to patients treated with radiosurgery alone (Chang 2009).
Neurotoxic effects of cranial irradiation
Radiation can be delivered to the brain injury using large focused doses (stereotactic radiation), as part of standard fractionated treatments, or to the whole brain (WBRT). Potential risk factors for cognitive decline following brain radiation include receiving fractionated radiation doses greater than 2 Gy, higher total radiation dose, larger brain volume of irradiation, using a divided‐dose schedule and longer overall treatment time (Lee 2002). Other risk factors may include either combined or subsequent chemotherapy use, age, with those fewer than seven years or greater than 60 years old at higher risk, and comorbid vascular risk factors such as diabetes and hypertension (Crossen 1994; Szerlip 2011). In the identification of treatment‐related neurotoxicity it is important to distinguish symptoms from tumour progression, recurrence or metastases, since continuation of treatment may lead to irreversible central nervous system (CNS) injury (Dietrich 2008).
The neurotoxic effects of brain radiation can be divided into acute, early‐delayed and late‐delayed radiation encephalopathy (Sheline 1980). Acute radiation encephalopathy occurs as a result of disruption to the blood‐brain barrier leading to accumulation of fluid in the tissue (vasogenic oedema). Corticosteroids are used at this stage, and may improve symptoms of somnolence and headache, and prevent further neurologic decline. Early‐delayed radiation encephalopathy may occur at one to six months following completion of treatment, and symptoms of short‐term memory and attentional deficits are seen alongside drowsiness and worsening of pre‐existing neurological deficits. A return to baseline is often found within 12 months (Vigliani 1996). This phase is associated with blood‐brain barrier disruption and with reversible damage to the myelin sheath (Sheline 1980). In contrast to early complications, late‐delayed radiation encephalopathy is viewed as irreversible. This complication occurs months to years following radiation therapy and manifests as white matter lesions (i.e. leukoencephalopathy). In more severe forms it can manifest or lead to a formation of dead brain tissue which, as a result, can lead to a pressure effect and associated neurological dysfunction (Fink 2012).
The precise relationship between initial acute changes and late/chronic radiation damage to the brain is unknown. Clinically, late radiation damage is characterised by progressive mental slowing and impairment in attention and memory, with less commonly gait ataxia, urinary incontinence, apathy, and pyramidal and extrapyramidal signs (Taphoorn 2003). These cognitive deficits increase in incidence and severity over time (Klein 2002). However the exact incidence is hard to distinguish due to the range of neuropsychological tests, the population and the time at which patients are followed up (Taphoorn 2004). For example, up to 90% of adult brain tumour patients who survive for more than six months following WBRT therapy develop (some form of) cognitive impairment (Crossen 1994), and in up to 5% of long‐term survivors the cognitive impairment progresses to dementia necessitating admission to a nursing home (DeAngelis 1989; Vigliani 1996). The incidence of severe cognitive deficits/late delayed radiation encephalopathy is even higher in patients with primary CNS lymphoma, reaching nearly 100% in patients older than 60 years old (Abrey 1998). Due to these adverse effects of cranial irradiation, the benefit of radiotherapy treatment for patients with a more favourable prognosis, such as with a low‐grade glioma (LGG), or as prophylactic cranial irradiation for small cell lung carcinoma, has been the subject of much debate in the past decade (Gondi 2013).
The mechanism of cranial irradiation‐induced cognitive impairment
The mechanisms by which radiation causes cognitive decline, particularly in learning and memory, have been proposed to relate to metabolic changes, white matter changes and radionecrosis, as well as changes in neuronal function, particularly synaptic plasticity, and long‐lasting damage to hippocampal neurogenesis (Greene‐Schloesser 2013). Of those, impaired white matter radiation changes and neurogenesis are the most thoroughly studied.
The primary mechanism of delayed radiation‐induced white matter changes is associated with secondary endothelial damage and microvascular ischaemic insult (Lyubimova 2004), accompanied by a reduction in the proliferative capacity of glial cells (van der Maazen 1993). This leads to a decrease in the volume of cerebral white matter, which is directly associated with cognitive decline (Correa 2004; Mulhern 2004; Reddick 2006). This has been confirmed in a longitudinal study that found medulloblastoma patients receiving a cranial irradiation dose of 36 Gy to show more rapid cerebral white matter volume decrease than those receiving a cranial irradiation dose of 23.4 Gy (Palmer 2002). Rarely, these white matter lesions can increase in size and may progress to frank white matter necrosis characterised by focal cavitations in the white matter within the radiated fields (Anscher 1991). Treatment of radionecrosis involves surgical excision and steroid therapy, and recent studies using bevacizumab, an angiogenesis inhibitor, have also reported high rates of clinical and radiological responses, albeit with small sample sizes (Gonzales 2007; Levin 2011; Torcuator 2009; Wang 2012).
Neurogenesis refers to self‐renewing cells that may produce neurons, glial cells and cells that give rise to restricted cell types (lineage‐restricted precursor cells) throughout life, associated with normal hippocampal functioning (Zhao 2008). This was explored in a post‐mortem study in patients with medulloblastoma that found significantly lower neurogenesis in patients treated with radiotherapy two to 23 years prior to analysis, compared to controls matched for age and sex (Monje 2007). Therefore, radiotherapy strategies that attempt to spare the crucial areas of neurogenesis may produce better cognitive outcomes, compared to WBRT (Dietrich 2008; Peiffer 2011), and are currently being conducted.
Measuring cognitive deficits
Wefel 2011 recommends a core battery of validated neuropsychological tests to assess cognitive function. These include the Hopkins Verbal Learning Test‐Revised (HVLT‐R) (Benedict 1998) to assess learning and memory, Trail Making Test (TMT), (Reitan 1992) to assess processing speed and executive function, and the Controlled Oral Word Association test of the Multilingual Aphasia Examination (COWA), (Benton 1989) to assess verbal fluency. Other tests have also been used, such as digit span and digit symbol (Wechsler 1981) to assess working memory. Cognitive function has also been assessed through the use of brief mental status evaluations, such as the Mini‐Mental State Examination (MMSE), (Folstein 1975). Whilst the MMSE is often shorter than neuropsychological testing, it has been associated with poor sensitivity in detecting cognitive deficits (Meyers 2003). Other studies have used subjective patient reports of cognitive concerns, such as in memory and concentration (Lidstone 2003; Mukand 2001). An additional consistent finding from the research literature is that correlations between subjectively assessed cognitive symptoms and objectively determined cognitive functioning are quite modest, with correlation coefficients generally ranging from 0.20 to 0.30 (Klein 2002).These are suggested to be confounded by some patients' lack of awareness regarding their cognitive impairments, and correlations with fatigue and depression, rather than cognitive test performance (Cull 1996).
Differences in the time points at which cognitive functioning is measured are also present, both in pharmacological and non‐pharmacological intervention studies. One study carried out assessments at baseline, and at four weeks of modafinil or methylphenidate use (Gehring 2012a), whereas another continued to follow up patients at eight, 16, 24 and 52 weeks following initiation of the drug memantine (Brown 2013). In cognitive rehabilitation studies, patients were assessed at baseline and at the end of a two‐week intervention and at three months (Locke 2008). These studies also demonstrate the variations in duration of the intervention.
The variations in tools available, use of both objective and subjective measures, differences in time points at which cognitive functioning is measured and the differences in intervention duration highlight the caution that must be taken when combining and generalising results and conclusions.
Description of the intervention
This review included all interventions that aim to:
prevent, or
ameliorate
any cognitive deficits in patients who have received therapeutic or prophylactic cranial irradiation prior to, or during, participation in the study. These may include pharmacological and non‐pharmacological (medical, psychological or behavioural) interventions for the management of cognitive deficits.
Pharmacological
We defined pharmacological interventions as a drug given by any route at any therapeutic dose with the intention of preventing or ameliorating cognitive deficits in persons who have received cranial irradiation.
Studies investigating the pharmacological prevention of cognitive impairment frequently occur in patients undergoing cranial irradiation during participation. For example, memantine, used in the treatment of Alzheimer's Disease (Robinson 2006), and lithium, used in the treatment of psychiatric disorders (Cipriani 2013) and in patients with cancer (Khasraw 2012), have both been investigated for their neuroprotective role during irradiation.
Studies of pharmacological treatment for cognitive impairment after cranial irradiation have largely focused on psychostimulants, including methylphenidate and modafinil. Objective cognitive functioning and patient‐reported outcomes of fatigue, mood and quality of life have been used to assess the efficacy of methylphenidate and modafinil in brain tumour patients, 83% of whom had received cranial irradiation (Gehring 2012a). Donepezil, used in the treatment of Alzheimer's Disease, has also been investigated for its use in the treatment of cognitive symptoms in brain‐irradiated adults (Shaw 2006).
Non‐pharmacological
We defined non‐pharmacological interventions as any non‐drug intervention given with the intention of ameliorating or preventing cognitive deficits during or following cranial irradiation. These can include, but are not limited to, medical, psychological and behavioural interventions, as well as alternative interventions such as the use of dietary supplements.
Medical interventions include any biomedical intervention given to a person in which the intervention is not primarily investigating cancer treatment or control. For example, one study explored the use of hyperbaric oxygen therapy in cranial irradiated brain tumour patients using 31 neuropsychological tests (Hulshof 2002).
Psychological interventions may include (but are not limited to) retraining, education and compensation strategies. A randomised clinical trial investigating the use of cognitive rehabilitation in glioma patients, 61% of whom had received cranial irradiation, investigated computer‐based retraining and compensatory strategies. Objective and subjective cognitive functioning, as well as perceived burden and mental fatigue, were assessed (Gehring 2009).
Behavioural interventions can include exercise, as well as behavioural modification interventions.
Dietary supplements such as Ginkgo biloba have also been investigated in irradiated brain tumour patients (Attia 2012).
How the intervention might work
Clinical trials have explored the prevention and treatment of cognitive deficits by targeting pharmacological, psychological or behavioural pathways, as well as other biological pathways.
Pharmacological
Pharmacological interventions may prevent cognitive deficits via their neuroprotective role during WBRT such as memantine, an N‐Methyl‐D‐aspartate receptor antagonist (Brown 2013), and lithium, found to reduce oxidative distress via the glutathione system (Machado‐Vieira 2007).
Pharmacological interventions may ameliorate cognitive deficits via their involvement in critical neurotransmitter pathways. Methylphenidate is a CNS stimulant found to have a positive effect on attention due to its action on the brain centre for attention control, the fronto‐striatal network, by increasing dopamine and noradrenaline concentrations (Volkow 2002). Another centrally acting drug is donepezil, a reversible cholinesterase inhibitor involved in inhibiting the breakdown of the neurotransmitter acetylcholine. This may have a cognitive enhancing effect by prolonging and improving cholinergic function, associated with learning and memory (Steinberg 2011).
Non‐pharmacological
Medical interventions have also been considered to help prevent or treat cognitive deficits. Hyperbaric oxygen therapy has been used to improve damage to the nervous system by stimulating angiogenesis, the process through which new blood vessels are formed from pre‐existing blood vessels (Gill 2004).
Psychological interventions may help prevent and improve cognitive deficits by retraining cognitive capacities such as attention and memory, or via compensation strategies such as memory aids. These interventions target the plasticity of the brain, via restoration or reorganisation of function (Miotto 2013; Mora 2013). For example, Cicerone 2011 reviewed 370 cognitive rehabilitation interventions and found supportive evidence for its role in patients with traumatic brain injury and stroke.
Behavioural interventions, such as exercise, may also help ameliorate or prevent cognitive deficits. Exercise has been associated with increases in cerebral blood flow, increased hippocampal neurogenesis, changes in neurotransmitter release and arousal levels and brain structure, and particularly through the involvement of Brain Derived Neurotrophic Factor (Gligoroska 2012).
Other non‐pharmacological interventions, such as those involving diet modifications, may also play a role in improving cognitive functioning. The dietary supplement Ginkgo biloba has been associated with regulating signalling pathways, cellular metabolism and gene transcription (Smith 2003).
Why it is important to do this review
As anti‐cancer treatments become more effective and readily available across treatment centres, patients live longer disease‐free but with long‐term sequelae of the disease and the neurotoxic side effects of treatment (Cochran 2012).Greater emphasis is now being placed on quality of life and with the establishment of neurocognitive function as a predictor of survival (Meyers 2000) and quality of life (Mitchell 2010), cognitive functioning is an essential outcome measure. There is currently no standard policy to direct treatment, and there are no systematic reviews of preventive measures or interventions for cognitive problems specifically associated with cranial irradiation in adult cancer survivors. With even mild cognitive impairment leading to negative functional and psychiatric consequences, especially if persistent and untreated, it is important to identify ways to reduce the long‐term impact of cranial irradiation on neuropsychological function.
Objectives
To assess the effectiveness of interventions for preventing or ameliorating cognitive deficits in adult patients treated with cranial irradiation.
Methods
Criteria for considering studies for this review
Types of studies
Prevention
For studies investigating the prevention of cognitive deficits, we searched for any studies fulfilling the following criteria:
randomised controlled trial (RCT) or non‐randomised controlled trial (non‐RCT), including cluster and cross‐over controlled trials;
they have included a control group or comparison group receiving no intervention for cognitive function, standard care, or are compared with a normative data control group;
they involve an intervention aimed at the prevention of cognitive deficit in adults who are all receiving cranial irradiation during participation;
they include cognitive performance, as assessed by neuropsychological tests (and not self‐report), as the primary outcome, or include cognitive performance as the secondary outcome to an alternative primary quality of life measure (e.g. fatigue, mood).
We included studies in which cognitive functioning was measured at baseline and following intervention at any time point.
Whilst we included studies that investigated the preventative role of an intervention during cranial irradiation, we did not include those where the intervention being investigated was cranial irradiation itself, associated with treating the tumour or improving tumour control. Such excluded studies included those on:
hippocampal sparing techniques;
techniques limiting radiation dosage to healthy tissue (e.g. intensity‐modulating radiation therapy);
the addition of chemotherapy agents (e.g. motexafin gadolinium).
Although these techniques can be associated with reduced or limited cognitive side effects, these techniques would best fit a separate Cochrane systematic review investigating the effect of dose of radiotherapy in causing cognitive problems.
Amelioration
For studies investigating the amelioration of cognitive deficits, we included any studies fulfilling the following criteria:
randomised controlled trial (RCT) or non‐randomised controlled trial (non‐RCT), including cluster and cross‐over controlled trials;
they have included a control group or comparison group receiving no intervention for cognitive function, standard care, or are compared with a normative data control group;
they involve an intervention for ameliorating cognitive function in adults to which the majority (> 80%) have received cranial irradiation prior to participation;
they include cognitive performance, as assessed by neuropsychological tests, as the primary outcome, or include cognitive performance as the secondary outcome to an alternative primary quality of life measure (e.g. fatigue, mood);
cognitive functioning has been measured at baseline and following intervention initiation at any time point.
To improve the relevance of the review, we included non‐RCTs in our search. These studies are described in the excluded studies section. They were not included in the main body of evidence but offer preliminary findings for the justification of further research.
Types of participants
Prevention
For studies investigating the prevention of cognitive deficits, we included studies that involved adult patients (aged 18 years and over), who had undergone cranial irradiation (whole brain or partial brain radiation) during participation in the study, for the treatment of primary or secondary brain cancer, or prophylactic treatment for other cancers.
Since these studies refer to interventions for preventing cognitive deficits, the presence of cognitive deficits at baseline was not an inclusion criterion. However, we only included studies where cognitive functioning was assessed via neuropsychological testing both prior to and following the start of the intervention.
Amelioration
For studies investigating the amelioration of cognitive deficits, we included studies that involved adult patients (aged 18 years and over) with impairment in at least one cognitive domain, who had previously undergone cranial irradiation (whole brain or partial brain radiation) prior to participation in the study for the treatment of primary or secondary brain cancer, or prophylactic treatment for other cancers. Participants could have received cranial irradiation during childhood, but had to be an adult (aged 18 years and over) during participation in the study. Cognitive impairment was determined prior to participation via neuropsychological testing.
We also included studies that involved only a subset of patients who had undergone cranial irradiation in the review, if this group formed a large majority (> 80%) of the study population or had been explored via subgroup analyses.
Types of interventions
Studies that were included could have utilised pharmacological (e.g. stimulants, or neuro‐protective agents) or medical (e.g. hyperbaric oxygen therapy) approaches, or psychological (e.g. cognitive rehabilitation) or behavioural (e.g. exercise) interventions, targeted to prevent or ameliorate radiation‐related cognitive deficits.
Pharmacological interventions
We investigated the effectiveness of any drug given by any route for any duration, and at any therapeutic dose, with the objective of preventing or treating cognitive deficits in patients who had received, or were receiving, cranial irradiation. Such drugs are likely to include psychostimulants (e.g. methylphenidate, modafinil), and might include drugs to treat cognitive deficits in other neurological conditions (e.g. donepezil, memantine). For ethical reasons, studies involving drugs may not automatically include a placebo arm. To increase the relevance of the review we included studies without a placebo arm if the study involved a group of participants who have been randomised to a control group of some kind (e.g. treatment as usual, another active drug or allocation to a waiting list), or that have been compared to normative control data with correction of practice effects caused by repeated neuropsychological testing.
Non‐pharmacological interventions
For medical interventions, we investigated any medical intervention, such as hyperbaric oxygen therapy, which aimed to prevent or improve cognitive deficits in patients who had received, or were receiving, cranial irradiation.
For psychological and behavioural interventions, we reviewed any cognitive and/or behavioural treatment given with the intention or preventing or treating cognitive deficits in patients who had received, or were receiving, cranial irradiation; these could include, but were not limited to, retraining, education or teaching of compensation strategies, physical exercise interventions or dietary supplements.
Types of outcome measures
Primary outcomes
The primary outcome was cognitive performance; this could be a general or composite cognitive score or individual cognitive test scores using validated neuropsychological tests (e.g. HVLT‐R, COWA). In studies involving preventative interventions, we determined efficacy as a statistically significant improvement in cognitive functioning, or no change/decline from baseline. In studies involving treatment interventions, we determined efficacy as a statistically significant improvement, or no change, in cognitive functioning from baseline. To increase the relevance of the review, we did not restrict eligible reviews with respect to the time point at which cognitive functioning was measured at baseline or at follow‐up. We noted and discussed the time points at which cognitive functioning was measured.
Secondary outcomes
Self‐reported cognitive functioning via interviews or questionnaires.
General functioning including mood/psychiatric symptoms (e.g. Hospital Anxiety and Depression Scale), self reported fatigue (e.g. Brief Fatigue Inventory) and quality of life measurements (e.g. FACT‐Br).
Adverse events (e.g. nausea, skin reactions, headache).
We noted and reviewed the secondary outcomes if recorded, but these were not eligibility criteria for this review.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases for published studies and conference abstracts:
the Cochrane Register of Controlled Trials (CENTRAL, 2014, Issue 8);
MEDLINE (1950 to August 2014);
EMBASE (1980 to August 2014);
PsycINFO (1974 to August 2014).
The search strategies are listed in Appendix 1 (MEDLINE), Appendix 2 (EMBASE), Appendix 3 (PsycINFO) and Appendix 4 (CENTRAL). The search strategies were not restricted by year of publication, language or publication type.
Searching other resources
We searched the reference lists of included studies.
We searched for ongoing trials using ClinicalTrials.gov (www.clinicaltrials.gov), the Physicians Data Query (www.cancer.gov/clinicaltrials) and the metaRegister of Controlled Trials (www.controlled‐trials.com/mrct).
Data collection and analysis
Selection of studies
We used the reference management database EndNote to download all titles and abstracts retrieved by electronic searching. We removed duplicates and two review authors (JD, KZ) independently examined the remaining references. The review authors were not blinded to the authors or affiliations of the studies. We excluded studies clearly not meeting the inclusion criteria and obtained full‐text copies of potentially relevant references. Two review authors (JD, KZ) independently assessed the eligibility of retrieved papers, with disagreements resolved by discussion with a third author (KG). We documented reasons for exclusion of studies.
Data extraction and management
Data extraction
We used the recommendations from the Cochrane Handbook for Systematic Reviews of Interventions to abstract data from included trials using a data extraction form specifically designed for this review (Higgins 2011). Two review authors (JD, KZ) completed data abstraction independently. Differences between review authors were resolved by discussion.
Data abstracted included the following:
article details (author, year of publication, journal, country and language);
methodology (study design, participant recruitment method, inclusion and exclusion criteria, informed consent, ethical approval, statistical analyses);
population demographics (geographical location, setting, age, gender, ethnicity, total number included in trial and analyses);
details of participants health status (including disease status, tumour pathology, tumour treatment details, antiepileptic medication, corticosteroid use);
intervention (characteristics such as drug dose, preparation and route of administration, frequency and duration, detail of providers);
outcomes (primary and secondary outcomes assessed, method and timing of assessments);
results of cognitive functioning measure (neuropsychological test performance);
results of other outcome measures (including self reported cognitive questionnaires, quality of life, depression, fatigue and adverse events);
risk of bias.
Where possible, all data extracted were those relevant to an intention‐to‐treat (ITT) analysis, in which participants are analysed in the groups to which they are assigned.
Data management
We used Review Manager 5.3 to collate data (RevMan 2014). For continuous outcomes (e.g. cognitive performance and quality of life measures), we extracted the final value and standard deviation, and the number of patients assessed at endpoint for each treatment arm to estimate the mean difference between treatment arms and its standard error. We noted and reviewed the time points for outcome assessment. Where participant and study details were missing; we noted these as a potential limitation of the study.
Assessment of risk of bias in included studies
We used the Cochrane Handbook for Systematic Reviews of Interventions 'Risk of bias' tool to assess the risk of bias in included studies (Higgins 2011), including the assessment of:
selection bias: random sequence generation and allocation concealment;
performance bias: blinding of participants, personnel (patients and treatment providers) and outcome assessors;
attrition bias: incomplete outcome data;
reporting bias: selective reporting of outcomes;
other possible sources of bias.
A full 'Risk of bias' item list with specific criteria for each item can be found in Appendix 5.
We interpreted and reported all bias criteria as having a low, high or unclear risk of bias. We reported an unclear risk of bias when insufficient information was provided, or when uncertainty over the potential for bias was present. Two review authors (JD, KZ) applied the 'Risk of bias' tool independently and resolved differences by discussion. We summarised results in a 'Risk of bias' graph and 'Risk of bias' summary and interpreted the results with respect to risk of bias.
Measures of treatment effect
For continuous outcomes, we used the mean difference (MD) with 95% confidence interval (CI). We planned to use the standardised mean difference with 95% CIs to combine trials that measured the same outcome, but used different methods.
For dichotomous outcomes we used the risk ratio (RR) with 95% CI.
Dealing with missing data
We did not impute missing outcome data for any outcomes.
Assessment of heterogeneity
We aimed to asses heterogeneity between studies by a formal statistical test to indicate the significance of the heterogeneity (Deeks 2001). We planned to Investigate and report heterogeneity according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and via visual inspection of forest plots.
Assessment of reporting biases
Two review authors (JD, KZ) reviewed and recorded reporting bias. We aimed to exam funnel plots, if a meta‐analysis that included more than 10 trials was possible, to assess potential small study effects, such as publication bias.
Data synthesis
If sufficient clinically similar trials had been available, we intended to combine data for meta‐analysis using the Cochrane Review Manager software 5.3 (RevMan 2014), as follows:
for continuous outcomes, we planned to pool MDs between treatment arms at the end of follow‐up if trials measured the outcome on the same scale and at the same primary study endpoint, otherwise we planned to pool SMDs;
we intended to use random‐effects models for all meta‐analyses, with 95% CIs (DerSimonian 1986);
for dichotomous data, we planned to pool RRs (RevMan 2014).
Subgroup analysis and investigation of heterogeneity
If sufficient data had been available, we would have reviewed studies separately using the following categories:
drug dose;
World Health Organization (WHO) tumour grade (low‐grade/high‐grade).
Sensitivity analysis
If sufficient data had been available, we would have considered the following factors:
differing study quality (high or low risk of bias);
different classes of agents, doses or scheduling differences.
We anticipated that additional types of sensitivity analyses would have been identified during the conduct of the review.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification; Characteristics of ongoing studies
Results of the search
Details can be found in Figure 1.
Figure 1.
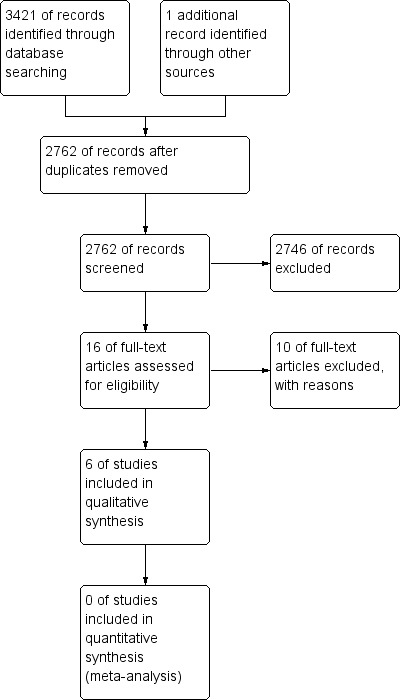
Study flow diagram.
We found 2762 citations using the initial search strategy following de duplication of the results. Upon screening of titles, this narrowed the results to 16 articles. Six studies were included in this review. Four published trials met our inclusion criteria for analysis; two trials investigating the prevention of cognitive deficits and two trials investigating the amelioration of cognitive deficits. In addition, we identified one study when searching clinical trial databases for ongoing trials. Three conference abstracts were also identified, data were available for one study (Kaleita 2006) and, following correspondence, data were obtained for another study (Rapp 2013); the third study is awaiting classification (Shaw 2013).
Included studies
For detailed information on included studies see the 'Characteristics of included studies' table.
Prevention
Two included studies investigated a pharmacological intervention for the prevention of cognitive deficits during cranial irradiation (Brown 2013; Butler 2007). One included study investigated a cognitive rehabilitation and problem‐solving program for the prevention of cognitive deficits in primary brain tumour patients receiving radiotherapy (Locke 2008).
Pharmacological Studies
One study recruited 554 eligible patients with brain metastases primarily from lung cancer, with breast, colon and other cancers also included; 46 patients did not meet the inclusion criteria, therefore 508 participants were allocated to intervention or placebo (Brown 2013). This was greater than the calculated 221 participants required in each arm to reach 80% statistical power. One study recruited 68 of the 81 projected patients, calculated for a 90% statistical power. Patients had a primary (N = 33) or metastatic brain tumour (Butler 2007); further details concerning the brain tumour ere not reported. Both studies recruited participants in the United States, and both studies were multi‐centre studies involving four centres (Butler 2007), and 143 centres, including Canada (Brown 2013). Both studies reported obtaining ethical approval and informed consent from participants and recorded adverse events. Cranial irradiation schedule requirements varied between studies with patients receiving 37.5 Gy of WBRT via 15 fractions of 2.5 Gy (Brown 2013), or receiving partial or WBRT of at least 25 Gy in at least 10 fractions of 1.8 to 3.0 Gy/fraction (Butler 2007). Both studies reported using a randomisation method, which was confirmed via correspondence as random number generation using a computer program. Both studies reported the use of a double‐blinding and allocation concealment technique, which was also confirmed via correspondence through the use of a pharmaceutical company providing matched drug containers.
Interventions included d‐threo‐methylphenidate (d,l‐MPH; Butler 2007) and memantine (Brown 2013). Both studies compared the interventions with a matched placebo. Both studies included dose escalation techniques, continued for eight (Butler 2007) or 24 (Brown 2013) weeks. Dose reduction and withdrawal techniques were included if patients experienced severe adverse events (Butler 2007) or when the patient's creatine clearance declined (Brown 2013).
Both studies assessed cognitive functioning using the MMSE. One study included a neuropsychological test battery that assessed memory, processing speed, executive function and verbal fluency (Brown 2013). One study also included self‐report measures of fatigue, depression and quality of life (Butler 2007). Timing of outcome assessment varied between studies, with patients assessed at baseline and then at eight, 16, 24 weeks of drug use (Brown 2013), or at the end of radiation therapy and at eight weeks of drug use (Butler 2007). Both studies carried out a final follow‐up assessment after the drug was stopped, at 12 (Brown 2013) and 52 (Butler 2007) weeks.
Non‐Pharmacological Studies
Locke 2008 recruited 19 participants receiving cranial irradiation for the treatment of a primary brain tumour (17 glioma, two meningioma). Recruitment was carried out at a single radiation oncology clinic. Ethical approval was obtained and informed consent sought. Patients were required to have a caregiver available to accompany them to each follow‐up to complete a quality of life questionnaire. Radiation schedule requirements were not reported. The use of a randomisation method was reported, however this was abandoned due to low accrual and the final three participants were enrolled into the intervention arm. Due to the nature of the study, participants were not blinded. Blinding of personnel was not reported.
The intervention included six 50‐minute sessions of cognitive rehabilitation and six 50‐minute sessions of problem‐solving therapy over two weeks, compared with standard medical care. The cognitive rehabilitation intervention was particularly aimed at memory. This involved the education and use of a calendar to compensate for cognitive problems. The problem‐solving intervention involved the education and training of a positive problem‐solving model via constructive thinking, using feelings as cues and reversed advocacy role play.
The primary aim of the study was to assess the tolerability and feasibility of the program. This was assessed through the use of the Mayo‐Portland Adaptability Inventory (Malec 2003), primarily used in the evaluation of rehabilitation programs designed for patients with acquired brain injury, and via patient feedback questionnaires. Cognitive functioning was assessed using the cognitive test battery Repeatable Battery for the Assessment of Neuropsychological Status (R‐BANS; Randolph 1998). Self‐reported quality of life, mood and fatigue were also assessed. Assessments were taken at baseline, following the two‐week intervention, and at three months.
Amelioration
Three pharmacological studies were included that investigated the treatment of cognitive deficits (Gehring 2012a; Kaleita 2006; Rapp 2013). No non‐pharmacological studies were eligible.
Pharmacological Studies
The first study recruited 30 of 30 expected patients with a primary brain tumour, 87% of whom had received radiotherapy (Kaleita 2006); the distribution of tumour grade was almost equal between grade II, III and IV tumours, with two patients with a grade I tumour. The second study recruited 34 of the 75 planned patients with a primary brain tumour, calculated to have 90% statistical power; 24 patients were included in the analysis (21 glioma, one medulloblastoma, one primary CNS lymphoma, one hemangiopericytoma); 83% of whom had received cranial irradiation (Gehring 2012a). The third study recruited 198 of the required 200 patients, required to reach 90% statistical power, from 26 sites; 66% had a primary brain tumour, 27% a metastatic brain tumour and 8% had received prophylactic cranial irradiation (Rapp 2013). Two studies reported their results as a conference abstract (Kaleita 2006; Rapp 2013). All three studies were conducted in the United States. Ethical approval and informed consent was reported for two studies (Gehring 2012a; Rapp 2013) and all reported adverse events. Two studies did not restrict patients to those receiving cranial irradiation (Gehring 2012a; Kaleita 2006). In one study, patients were eligible to participate following partial or whole brain irradiation of 30 Gy or greater (Rapp 2013). The use of a randomisation method was reported by all studies, and correspondence confirmed this and was through the use of a computer program in two studies (Gehring 2012a; Rapp 2013). Two studies used double‐blinding (Kaleita 2006; Rapp 2013) and one study also reported an allocation concealment method via a pharmaceutical company (Rapp 2013). One study used an open‐label design, although all treatment arms were experimental (Gehring 2012a).
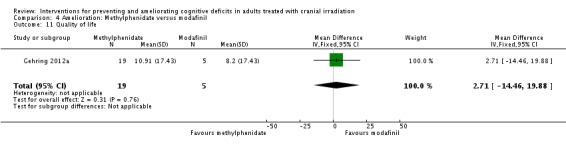
Intervention arms varied between studies. Gehring 2012a included three intervention arms using two forms of methylphenidate (immediate release; sustained release), compared to a modafinil arm. Rapp 2013 included one intervention arm of donepezil, with an increasing dosage from 5 mg/day for six weeks and 10 mg/day for 18weeks if tolerated, compared with placebo. Kaleita 2006 compared two dosages of modafinil followed by an extended treatment phase using a titrated dose between 50 and 600 mg/day for eight weeks.
All studies assessed cognitive functioning using neuropsychological testing, and one also calculated a cognitive composite score (Rapp 2013). All studies included self‐reported measures of mood and fatigue, and one also included a measure of quality of life (Gehring 2012a). Assessments were taken at baseline and at four weeks of drug use (Gehring 2012a), at baseline, 12 and 24 weeks of drug use (Rapp 2013) or at baseline and at one, three, four, eight and 12 weeks of drug use (Kaleita 2006). Assessments were not carried out following withdrawal of the drug, but were recorded in one study during a washout period prior to the extension phase (Kaleita 2006).
Non‐Pharmacological Studies
No studies were eligible.
Excluded studies
For detailed information on excluded studies see the 'Characteristics of excluded studies' table.
An initial screening of the search results was carried out, and reasons for excluding publications were the following:
studies were not intervention studies, such as reviews, comments or correspondence;
studies were conducted in a paediatric population;
studies were evaluating different cranial irradiation schedules, such as hippocampal sparing techniques (see Types of interventions);
studies did not assess cognitive functioning as the primary outcome, or as the secondary outcome to another quality of life measure (e.g. fatigue, mood);
cognitive functioning was assessed via a self‐reported measure only, and not via neuropsychological testing.
After this initial screening, the full‐text articles were retrieved of the remaining 16 potential studies. From these full‐text articles a further seven studies were excluded:
three prevention studies investigating methylphenidate (Meyers 1998), donepezil (Shaw 2006) and Ginkgo biloba (Attia 2012) did not include a comparison group e.g. control group or comparison with normative data;
two prevention studies investigating hyperbaric oxygen therapy (Schellart 2011) and Vitamin E (Chan 2003) did not randomise participants to treatment arms;
two amelioration studies investigating modafinil (Boele 2013) and a cognitive rehabilitation program (Gehring 2009) did not include a majority (> 80%) of participants who had received cranial irradiation or did not analyse these patients separately.
Following discussion, a further three RCTs were removed. A brief description of each study is provided below.
Levin 2011 was excluded as the primary aim was improvement of radionecrosis via magnetic resonance imaging (MRI) imaging. Whilst cognitive impairment is one symptom of radionecrosis, other neurological symptoms may be present, as well as, or instead of, cognitive impairment. This study did not require patients to have a cognitive deficit prior to participation. Nineteen patients with a primary brain tumour (grade II‐III) with neurological signs or symptoms of radiation necrosis were randomly assigned to receive bevacizumab or a matched placebo. All 11 patients who received bevacizumab showed an improvement in neurological symptoms after six weeks, including memory, compared to no symptom improvements in the seven control patients.
Jatoi 2005 was excluded due to only one of the nine recruited participants completing the study, with only two participants receiving the intervention and four participants placebo at the first assessment following intervention initiation. Patients were also removed from the intervention group, and the study, following worsening of cognition or depression, as this indicated failure of the intervention. This study investigated the prevention of cognitive deficits using combined donepezil and Vitamin E in nine of the 104 projected small cell lung cancer patients receiving prophylactic cranial irradiation. Cognitive functioning was assessed using the MMSE, and self‐report measures of functional capacity, depression and quality of life were also included. Descriptive results were reported for one month, three months and six months of drug use. Stable cognitive function was reported in all but one patient, and stable depression and quality of life in all but one patient. Three participants withdrew after the baseline measure, three after one month and two after three months. The study was closed early, resulting in a smaller sample size than expected. Additional reasons for poor accrual were attributed to the strict inclusion criteria resulting in few eligible patients, lack of tolerability immediately following high intensity cancer treatment and the focus on prevention of cognitive deficits in all patients rather than treatment for those already cognitively impaired.
Hulshof 2002 was excluded following correspondence with an author as, contrary to what the study reported, randomisation had not been carried out. This study investigated the amelioration of cognitive deficits in seven patients following cranial irradiation; the reason for cranial irradiation not reported. Cognitive functioning was assessed using neuropsychological testing, and no statistically significant improvements were found.
Studies Awaiting Classifications
One study, for which data could not be obtained, was published in conference proceedings and was identified in the initial search (Shaw 2013). The study investigated the use of armodafinil in improving cognitive functioning in patients who had received cranial irradiation, compared with a placebo drug. The study is described in more detail in the 'Characteristics of studies awaiting classification' table.
Ongoing Studies
The search for ongoing studies found one RCT. Umphrey 2013 is investigating the use of armodafinil in patients with high grade glioma following surgery and radiotherapy. This ongoing study is described in more detail in the 'Characteristics of ongoing studies' table. There are several ongoing studies for the treatment of cognitive deficits in patients with a primary brain tumour, however cranial irradiation is not an inclusion criteria. Thus we cannot pre‐determine if these studies will be eligible for inclusion following completion.
Risk of bias in included studies
We assessed studies using the Cochrane 'Risk of bias' tool (Higgins 2011). Between the two review authors (JD, KZ) there was agreement in the 'Risk of bias' scores following discussion. Attempts to contact authors were carried out where there was an risk of unclear bias. A summary of the risk of bias is presented in Figure 2 and Figure 3.
Figure 2.
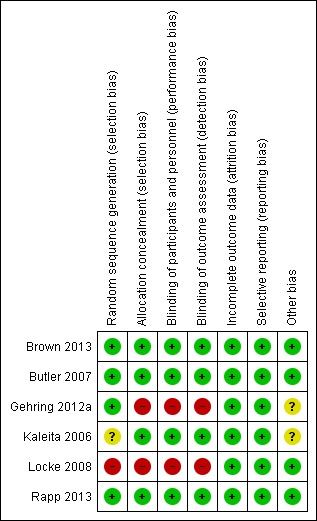
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Figure 3.
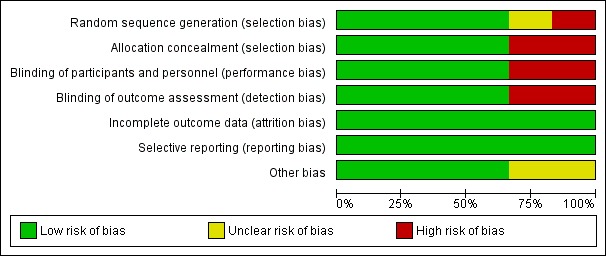
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Three of the four pharmacological studies were at a low risk of bias and used a stratification randomisation method and a pharmaceutical company to create identical drug containers (Brown 2013; Butler 2007; Rapp 2013). One study was at an unclear risk of bias reported the use of a randomisation method, however the method used could not be identified (Kaleita 2006). Two studies were at a high risk of bias: one study used an open‐label design (Gehring 2012a); one non‐pharmacological study reported the use of a randomisation method, but this was abandoned due to low accrual (Locke 2008).
Blinding
Four of the five pharmacological studies were at a low risk of bias and blinded participants and personnel to the intervention group (Brown 2013; Butler 2007; Kaleita 2006; Rapp 2013); one study was at a high risk of bias and used an open‐label design (Gehring 2012a). The non‐pharmacological study was at a high risk of bias; blinding of participants to the intervention was not possible due to the nature of the intervention, however blinding of assessors was also not carried out (Locke 2008).
Incomplete outcome data
The majority of studies included reasons for participant drop‐out, which were unlikely to be related to the outcomes and all studies were judged at a low risk of bias. Three studies performed intention‐to‐treat analysis (Brown 2013; Butler 2007; Rapp 2013). One study was terminated early (Butler 2007). Two studies recruited the projected number of participants (Brown 2013; Kaleita 2006). However, no study was able to include the number of participants required to reach the desired statistical power due to withdrawal from participation as a result of patient death, tumour progression or withdrawal of consent. One study reported the use of a multiple imputation procedure via the Markov chain Monte Carlo method for patients still alive at the time of assessments; this included the imputation of scores for 47% of recruited participants at 24 weeks (Brown 2013). One study reported the use of an imputation method in all participants who provided at least baseline data (Rapp 2013). Two studies carried out statistical comparisons between patients who withdrew consent, and those who remained in the study (Brown 2013; Butler 2007). Butler 2007 reported that patients who dropped out were significantly older and with worse performance status, but not in any of the outcome measures. Brown 2013 reported that patients who could not complete cognitive assessments were more likely to have worse neurological function and a shorter survival time and had not undergone surgery or radiosurgery.
Selective reporting
All outcomes were reported by all included studies and therefore were all judged at a low risk of bias. It was noted that some studies did not report assessments carried out following withdrawal of the drug (Brown 2013; Butler 2007) or report interim assessments (Kaleita 2006; Rapp 2013).
Other potential sources of bias
An unclear risk of bias was reported for two studies. Due to a small sample size, two studies combined intervention groups of participants receiving different forms of the drug methylphenidate (Gehring 2012a) and different doses of modafinil (Kaleita 2006) when analysing results.
Effects of interventions
Study interventions and comparisons were heterogenous, and were not sufficiently clinically similar to pool data. Six studies were identified for inclusion in the review; due to differences in interventions and aim of interventions (prevention versus amelioration) investigated, including the use of drugs with different modes of actions and dosage schedules, and one study's use of a non‐pharmacological intervention, the results are reviewed separately in this review.
Prevention
Pharmacological Studies
The large differences in the mode of action of the drugs investigated, and the unavailability of mean changes in scores, standard deviations or P values, meant pooling of the data was inappropriate. Therefore, results of the studies are reviewed separately and the data are reviewed as reported in the study.
Brown 2013 compared memantine, which acts on the glutamatergic system as an N‐Methyl‐D‐aspartate receptor antagonist, with placebo intervention. Of the 508 eligible patients, 55 patients withdrew consent and 271 patients died prior to completion of the study. Percentages of patients who missed assessments escalated from 41% to 57% from eight‐ to 52‐week assessments. Overall, 31% and 33% of participants completed 24 weeks of drug use as per the study protocol in the memantine and control groups respectively. Imputation was carried out for participants still alive at the time of missed assessment. Therefore, mean cognitive decline was reported for 280 participants with brain metastases at eight, 16 and 24 weeks assessments. Wilcoxon rank‐sum test, Gray’s Test, Cox proportional hazards regression model, stratified log‐rank test statistical analyses were performed. The primary endpoint was HVLT‐R, which did not reach significance (P value = 0.59); this was attributed to attrition. Median change and interquartile ranges were also reported for individual neuropsychological tests at 24 weeks, which are summarised in Table 6. There was significantly less decline in the memantine arm for processing speed (Median difference = .29, P value = .01) and delayed recognition (Median difference = .72, P value = .01). A cognitive functioning composite score was also calculated and the median change was ‐0.41 (interquartile range (IQR) ‐1.30 to 0.12) in the control group and ‐0.03 (IQR ‐0.90 to 0.72) in the intervention group at 24 weeks, which was reported to be significantly different (P value = 0.02). This indicated a stability of cognitive function in the intervention group and a decline in the control group. The most common adverse events were fatigue, alopecia, nausea and headache; there was no difference in the adverse events reported between groups (RR 1.00; 95% CI 0.76 to 1.32). It was noted that more participants in the memantine group were receiving steroids at entry into the study than the control group (P value = .05), which may have played a role in the expression of cognitive deficits, although this difference in steroid use did not continue over time.
Table 1.
Summary of findings: Memantine versus placebo
|
Cognitive functioning measure (standardised scores) |
Memantine | Placebo | P | ||
| N | Median change after 24 weeks (IQR) | N | Median change after 24 weeks (IQR) | ||
| Short‐term verbal memory | 77 | ‐0.23 (‐1.16 to 0.70) | 90 | ‐0.415 (‐1.86 to 0.46) | 0.21 |
| Long‐term verbal memory (recall) | 76 | 0 (‐1.67 to 0.59) | 90 | ‐0.90 (‐2.22 to 0.55) | 0.06 |
| Long‐term verbal memory (recognition) | 76 | 0 (‐1.12 to 1.43) | 90 | ‐0.72 (‐2.73 to 0.71) | 0.01* |
| Verbal Fluency | 78 | ‐0.10 (‐0.62 to 0.53) | 90 | ‐0.16 (‐0.83 to 0.61) | 0.31 |
| Trail making test A | 76 | 0.08 (‐1.01 to 1.82) | 92 | ‐0.37 (‐2.08 to 0.50) | 0.02* |
| Trail making test B | 74 | ‐0.45 (‐2.37 to 1.04) | 90 | ‐0.49 (‐2.60 to 0.62) | 0.30 |
| Cognitive composite score | 73 | ‐0.03 (‐0.90 to 0.72) | 90 | ‐0.41 (‐1.30 to 0.12) | 0.02* |
* P < .05
IQR: interquartile range
Butler 2007 evaluated d,l‐MPH, a central nervous system stimulant that acts as a norepinephrine‐dopamine reuptake inhibitor, compared with placebo. Two patients withdrew consent prior to receiving the intervention, 11 patients withdrew consent following the baseline assessment; 12 following radiation and 11 after four weeks. Participant withdrawal of consent after eight weeks was not reported. This study was terminated early due to low accrual. Results were reported for 32 participants at baseline, at post‐radiation and at four, eight and 12 weeks follow‐up; four‐ and 12‐week follow‐up assessments were only reported for the fatigue outcome. Two sample t‐tests, analysis of covariance, mixed‐model analysis of covariance and autoregressive covariance structure statistical analyses were performed. Mean cognitive functioning, as assessed with the MMSE, in the control group ranged from 26.5 (3.39 SD) to 27.8 (6.12 SD) at the end of radiation to 25.6 (11.54 SD) at eight weeks follow‐up. Mean overall cognitive functioning in the intervention group ranged from 27.2 (2.92 SD) at baseline to 26.4 (5.92 SD) at the end of radiation to 23.3 (10.46 SD) at eight weeks follow‐up. This difference was published as not significant and the standard deviation of mean change could not be calculated as P values were not reported. No other measures of cognitive performance were used, and it is noted that the MMSE is not considered a reliable measure of cognitive function (Meyers 2003). Fatigue was the primary outcome in this study, but was not found to be significantly different between groups at eight weeks follow‐up of drug use (MD 3.30, 95% CI ‐10.37 to 16.97). Depression and quality of life were also assessed, reporting no significant difference between groups, although again P values were not provided. Four adverse events were reported in total, although they were not all reported specifically to the arm and therefore the risk ratio could not be calculated; two patients experienced nausea and vomiting, one patient experienced tachycardia (control arm) and one patient was withdrawn from the study due to an increase in liver enzymes.
Non‐Pharmacological Studies
Locke 2008 evaluated a two‐week cognitive rehabilitation and problem‐solving program, compared with standard care. Six patients withdrew consent prior to completion of the study due to time commitments, tumour progression and the unwillingness for a caregiver to attend appointments. One patient reported fatigue during the study and withdrew participation. Results were reported for 13 participants and their caregivers at post‐intervention and three months follow‐up. Cognitive functioning, assessed using the R‐BANS, was only reported at baseline and post‐intervention. This was due to the majority of participants choosing a telephone follow‐up for their final clinic appointment, and subsequently cognitive assessment could not be carried out. Wilcoxon signed rank statistical tests were used to assess functional status only. Means and standard deviations for the R‐BANS subtest were reported for baseline and post‐intervention; mean change and standard deviation of mean change were not reported. The mean total cognitive functioning score remained stable at 73 at baseline (SD 13.4) and post‐intervention (SD 9.3) in the control groups and improved at baseline from 79 (SD 20.0) to 80 (SD 18.6) at post‐intervention in the intervention group; a statistical comparison was not carried out. Control participants were reported as more significantly impaired in cognitive functioning than intervention participants at baseline (P value = 0.03). Mood, fatigue, quality of life and caregiver burden measures were also recorded, but again no statistical comparisons were made. The primary outcome of the study was strategy implementation and patient satisfaction; 7/8 of the intervention group participants were using strategies at least once per week at the eight‐week follow‐up and 7/8 of both the patients and the caregivers found the intervention 'very helpful'.
Amelioration
Pharmacological Studies
Due to the differences in control groups or differences in the drug investigated, pooling of the data was inappropriate. Therefore results of the studies are reviewed separately.
Gehring 2012a compared two CNS stimulants, in three treatment arms; sustained‐release methylphenidate, immediate‐release methylphenidate and modafinil. Six patients were excluded from the study; three due to tumour progression (two methylphenidate arm; one modafinil arm), one due to infection‐related delirium requiring hospitalisation (modafinil arm), one due to nausea (modafinil arm), one due to steroid‐induced hyperactivity (modafinil arm). Four additional patients did not complete the study; three withdrew prior to receiving the intervention (two methylphenidate arm; one modafinil arm), one missed the follow‐up appointment (methylphenidate arm). There were significantly more males in the modafinil group, compared to the methylphenidate group (P value = .03). Results were reported for 24 participants at four weeks of treatment. Eighty‐three per cent of participants had received cranial irradiation prior to participation and 62.5% were receiving chemotherapy during participation. An exploratory statistical analysis approach was used via t‐tests and repeated measures analyses of covariance. Due to low accrual, the two methylphenidate arms were combined during analysis; further analysis also combined all interventions and compared findings with normative data. Practice effect adjusted reliable change index was used to assess individual change in cognitive test scores relative to baseline. The mean cognitive functioning scores comparison for each test is summarised in Analysis 4.2; digit span (MD 0.38; 95% CI 0.03 to 0.73), favouring methylphenidate, and trail making test A (MD ‐2.48, 95% CI ‐4.82 to ‐0.14), favouring modafinil were the only tests to show a significant difference between groups. The two stimulant groups together demonstrated significant improvement of individual trail making test B scores P value = < .01), corrected for practice effects. Mood, fatigue, quality of life and sleep also significantly improved in both groups, but with no significant difference between groups (see Analysis 4.5; Analysis 4.6; Analysis 4.8; Analysis 4.9; Analysis 4.11). None of the patients who completed the study experienced adverse events in either treatment arm.
Analysis 4.2.
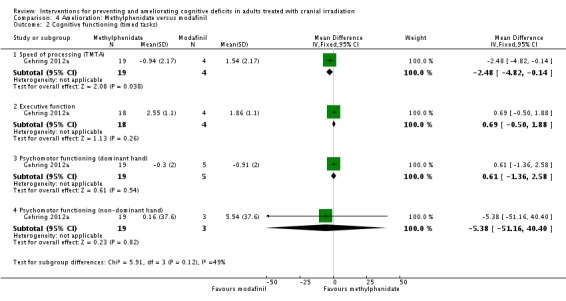
Comparison 4 Amelioration: Methylphenidate versus modafinil, Outcome 2 Cognitive functioning (timed tasks).
Analysis 4.5.
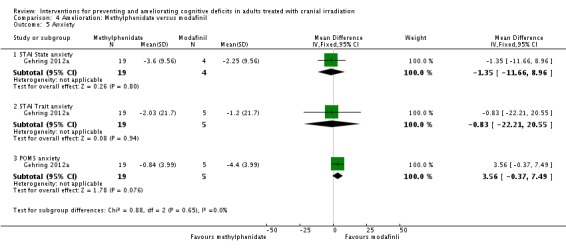
Comparison 4 Amelioration: Methylphenidate versus modafinil, Outcome 5 Anxiety.
Analysis 4.6.
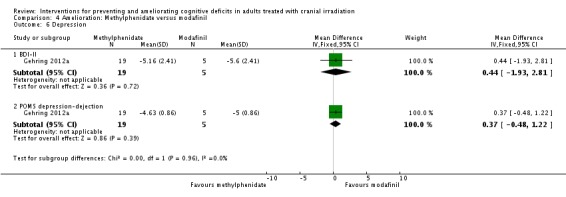
Comparison 4 Amelioration: Methylphenidate versus modafinil, Outcome 6 Depression.
Analysis 4.8.
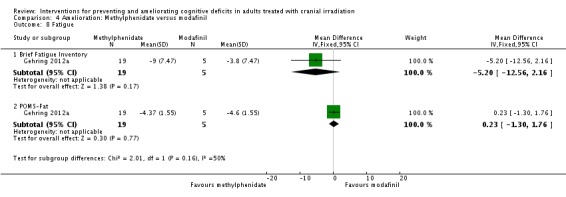
Comparison 4 Amelioration: Methylphenidate versus modafinil, Outcome 8 Fatigue.
Analysis 4.9.
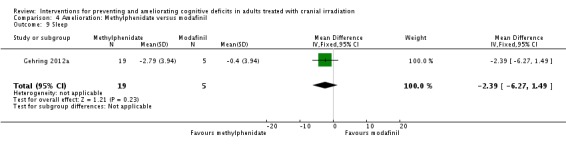
Comparison 4 Amelioration: Methylphenidate versus modafinil, Outcome 9 Sleep.
Analysis 4.11.
Comparison 4 Amelioration: Methylphenidate versus modafinil, Outcome 11 Quality of life.
Kaleita 2006 compared two doses of the CNS stimulant modafinil; 200 mg/day or 400 mg/day in divided doses. Results were reported for 30 participants at baseline, and at eight and 12 weeks of drug use. Groups were combined for statistical analysis, and paired t‐tests and Wilcoxon Signed Rank tests were used to assess change from baseline. A significant improvement from baseline was seen at 12 weeks across all cognitive test; trail making test A (P value =.002) and B ( P value < .0001), verbal fluency (P value = .002) and symbol digit modalities ‐oral (P value = .006) and ‐manual (P value = .004). Significant differences were also found at eight weeks. Improvements in fatigue and mood were found at eight and 12 weeks. Adverse events were reported, however the distribution of adverse events between treatment arms was not reported. Thirteen participants experienced symptoms of headache, eight of insomnia, seven of dizziness, seven of dry mouth, five of depressed consciousness and four or nausea.
Rapp 2013 compared donepezil, a reversible acetylcholinesterase inhibitor, with placebo. Fifty‐three participants withdrew from the study; reasons were not reported. Results were presented as a conference abstract for 145 participants at 24 weeks follow‐up. Cognitive functioning was assessed using neuropsychological testing. Chi‐square, Fisher exact and Wilcoxon rank‐sum statistical tests were used. Imputation was carried out in all participants who provided at least baseline data. The mean cognitive functioning score comparison for each test is reported in Analysis 5.2. The primary outcome was the calculated cognitive composite score, in which both groups were found to significantly improve after 24 weeks; there was no significant difference between groups (MD 0.03, 95% CI ‐0.05 to 0.11). A significant difference was found in tests of long‐term memory recognition (MD 0.57, 95% CI 0.07 to 1.07), long‐term memory discrimination (MD 0.94, 95% CI 0.27 to 1.61), and dominant hand psychomotor functioning (MD ‐11.93, 95% CI ‐21.51 to ‐2.35) favouring donepezil. Adverse events were not reported.
Analysis 5.2.
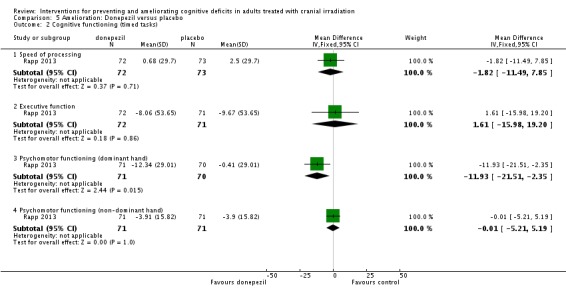
Comparison 5 Amelioration: Donepezil versus placebo, Outcome 2 Cognitive functioning (timed tasks).
Non‐Pharmacological Studies
No eligible studies were identified.
Discussion
Summary of main results
The aim of this review was to evaluate the effect of any pharmacological or non‐pharmacological intervention on cognitive functioning during or following cranial irradiation. We included three heterogeneous randomised controlled trials (RCTs) for the prevention of cognitive deficits, recruiting a total of 641 patients and three heterogeneous RCTs for the amelioration of cognitive deficits, recruiting a total of 199 patients.
Prevention
The two trials that compared drug versus placebo used very different drug agents, dosage schedules and time points for follow‐up; a meta‐analysis was therefore inappropriate.
Brown 2013 compared memantine in a large sample size from 143 centres across the United States and Canada, and carried out imputation in participants who had not withdrawn from the study as a result of death. Significant differences between groups were found, with overall cognitive function remaining stable in the intervention group and declining in the control group. Butler 2007 compared d,l‐MPH in a small sample size with a high drop‐out rate; no difference was found between the intervention group and control group in cognitive functioning. Further, no differences were found between the groups in depression, fatigue and quality of life.
The one trial that investigated a cognitive rehabilitation and problem‐solving program, compared with standard care, had the primary aim of assessing the tolerability and feasibility of the intervention (Locke 2008). Therefore, no statistical comparisons were made of the difference between groups in cognitive functioning. Consequently, this study provides very little evidence for the prevention of cognitive deficits, but does offer a tolerable and feasible intervention for further investigation.
Amelioration
The three trials that investigated the amelioration of cognitive deficits used different drug agents and/or control groups and different time points for follow‐up; a meta‐analysis was therefore inappropriate.
Gehring 2012a compared two forms of methylphenidate with modafinil, in a small sample size. Inconsistent, differential effects were found in cognitive performance between groups in attention, favouring methylphenidate and processing speed, favouring modafinil. However, when treatment groups were combined, there was evidence of a beneficial effect on test performance in speed of processing and executive function requiring divided attention. Further, no differences were found between groups in fatigue, mood, sleep or quality of life.
Kaleita 2006 also combined intervention arms, and found an overall significant improvement in all cognitive assessments at eight and 12 weeks, compared to baseline. Improvements were also found in measures of mood and fatigue. A number of adverse events were reported.
Rapp 2013 with a larger sample size than either Gehring 2012a or Kaleita 2006 compared donepezil with a placebo from 26 sites. Significant differences were noted between groups in long‐term memory recognition and discrimination, and dominant hand psychomotor functioning, favouring donepezil.
No studies were eligible for the amelioration of cognitive deficits via a non‐pharmacological intervention.
Overall completeness and applicability of evidence
We included six RCTs examining the efficacy of interventions for the prevention or amelioration of cognitive deficits in adults treated with cranial irradiation. Due to differences in drug agents, it was inappropriate to combine data into a meta‐analysis. Many studies were restricted by the following limitations, which therefore reduces the applicability of the evidence.
Many studies were limited by low accrual. Studies recruited from few centres and were unable to reach the necessary number of participants required for sufficient statistical power (Butler 2007; Gehring 2012a; Kaleita 2006; Locke 2008). It may be that patients may be reluctant to take any additional medication, especially if they are not subjectively experiencing cognitive impairment at the time of enrolment.
All studies were limited by high attrition rates. One study was able to recruit the projected participants required for 80% statistical power with the involvement of 143 centres (Brown 2013). However, 34% of patients died at 24 weeks of drug use and 47% of the remaining 280 participants missed their assessment appointment. It is important to understand the reasons for missed appointments. For example, Brown 2013 reported the length of time to carry out the cognitive assessments (20 minutes) may have played a role in participant drop‐out. Further, in studies that focus on prevention, rather than treatment, a lack of interest may be found in patients not experiencing cognitive impairment at that stage. Therefore, it is important to make sufficient attempts to keep patients engaged in study participation, such as by emphasising the importance of ongoing cognitive function assessment.
Failure to report all study and patient characteristics meant limitations remained a concern. The lack of reporting of mean differences and standard deviation, or P values, meant reported results as per the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) could not be carried out in four studies (Brown 2013; Butler 2007; Kaleita 2006; Locke 2008). Further correspondence to obtain this data was unsuccessful. The use and changes in use of medications associated with cognitive functioning were rarely reported. Only one study reported the use, and change in use of steroids during participation (Brown 2013), which reported that more intervention participants were receiving steroids at study entry than control participants. No studies reported the use, or change in use of anti‐epileptic drugs during participation. As these drugs may also play a role in cognitive functioning, findings may be attributable to changes in these medications during the study period. Assessment of cognitive functioning following withdrawal of the drug may have provided additional information relating to the efficacy of the drug. Two studies carried out post‐drug withdrawal assessments (Brown 2013; Butler 2007) but these data were not reported.
Quality of the evidence
Overall, this review summarises limited evidence for the effect of pharmacological and non‐pharmacological interventions for the prevention or amelioration of cognitive deficits in adults treated with cranial irradiation. Two of the studies were at high risk of bias in three domains, with additional domains at an unclear risk of bias (Gehring 2012a; Locke 2008). Blinding was not carried out in Gehring 2012a or in Locke 2008 and randomisation could not be concluded or was abandoned, respectively. Four RCTs were at a low risk of bias across all (Brown 2013; Butler 2007; Rapp 2013) or almost all (Kaleita 2006) domains.
Potential biases in the review process
We carried out extensive searches in four databases, which included published studies and conference proceedings as well as searching the reference lists of included studies. However, we may have failed to identify all eligible studies, particularly those that have not been published. We were somewhat successful in our attempts to obtain further information about the methodology of the studies by emailing the authors. Overall, our attempts to obtain further data on the included studies did not allow us to report all studies as per the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We were unsuccessful in our attempts to obtain data from one study that has yet to publish their results, described in the Characteristics of studies awaiting classification.
Agreements and disagreements with other studies or reviews
In the search results we found one other review reporting interventions for preventing or ameliorating cognitive deficits in adults treated with cranial irradiation (Tallet 2013), but did not find any additional studies from the review that were eligible to include in this review.
Other reviews identified were associated more generally with all brain tumour patients, all cancer patients, or more widely in all clinical conditions, who had not necessarily received cranial irradiation. For example, Gehring 2008 conducted a systematic review of interventions for cognitive deficits in patients with all types of brain tumour. Davis 2013 and Gehring 2012b conducted reviews of ongoing studies investigating pharmacological interventions to treat cancer patients with cognitive dysfunction. Challman 2000 reviews the use of methylphenidate in many clinical conditions, including patients with a brain tumour. The studies included in these reviews did not provide additional evidence specifically for cranial irradiated patients. Similar conclusions were made, emphasising that the evidence is limited by significant methodological limitations; such as the absence of a control group, lack of statistical comparisons between groups when control groups were present (due to small sample sizes), the use of unreliable cognitive tests and the overall need for larger trials with more statistical power. Gehring 2012b suggested the use of home assessments or Internet‐based programs as potential solutions to help with accrual and attrition.
Authors' conclusions
One study provided supportive evidence that memantine is beneficial in the prevention of cognitive deficits in patients receiving cranial irradiation and could therefore be considered safe for use as a preventative agent; adverse events were similar across groups. These findings relate specifically to patients with brain metastases receiving whole brain radiotherapy (WBRT). One study provided supportive evidence that donepezil is beneficial in the improvement of cognitive deficits in primary or metastatic brain tumour following cranial irradiation, although adverse events were not reported. However, it is noted that these supportive findings were for secondary outcomes; negative findings were found for each study's primary outcome, with the primary endpoint nearly reaching statistical significance for memantine. Two studies offered some evidence for the role of central nervous system (CNS) stimulants in improving cognitive deficits with few adverse events; these studies were limited by a small sample size. There is currently no evidence to suggest non‐pharmacological interventions are beneficial in the prevention or amelioration of cognitive deficits in adults treated with cranial irradiation.
High drop‐out rates are to be expected in studies examining prophylactic cranial irradiation, and therapeutic cranial irradiation. This is due to progression of systemic cancer or primary brain tumours leading to death, disability or affecting quality of life resulting in study withdrawal. Further research is necessary to determine whether memantine is effective in other patient populations receiving cranial irradiation, including patients with primary brain tumours and patients with tumours elsewhere in the body receiving prophylactic cranial irradiation. Further research is required in other pharmacological agents, for example, the use of methylphenidate and modafinil are still to be clarified. Sufficient attempts to recruit and maintain sufficient numbers of participants is essential. Appropriate control groups are necessary to rule out practice effects and determine the efficacy compared to standard care. It is also important to identify and report patient characteristics and other medications, such as steroid and anti‐epileptic drug use, which are associated with impaired cognitive function.
Only one non‐pharmacological intervention study could be included in this review, which offers preliminary findings of safety and tolerability for a cognitive rehabilitation program. Further research is needed to determine the efficacy of cognitive rehabilitation in this patient population. Other studies that have investigated cognitive rehabilitation programs in patients groups with other types of brain tumour offer detailed descriptions of cognitive interventions that could be investigated (Gehring 2010; Gehring 2011; Hassler 2010; Zucchella 2013).
Lastly, non‐RCTs and observational studies reporting other non‐pharmacological interventions including hyperbaric oxygen therapy and dietary supplements such as Vitamin E and Ginkgo biloba also provide descriptive methodology that could be explored further using appropriate control groups, blinding, and when funding is more accessible.
Acknowledgements
With thanks to the Cochrane Neuro‐oncology Group for their contribution and continued support and feedback throughout the editorial process.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Gynaecological Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
The review is funded in part by a grant from the NIHR Cochrane Incentive Scheme.
The review is funded in part by a grant from the Edinburgh Lothian Health Foundation.
Appendices
Appendix 1. MEDLINE search strategy
1 exp Cranial Irradiation/ 2 ((crani* or head or skull) adj5 (irradiat* or radiat* or radiotherap*)).mp. 3 exp Brain Neoplasms/ 4 exp Glioma/ 5 (brain adj5 (tumor* or tumour* or malignan* or neoplas* or carcinoma* or cancer* or metastas*)).mp. 6 (glioma* or astrocytoma* or ependymoma* or ganglioma* or gliosarcoma* or medulloblastoma* or oligodendroglioma* or meningioma*).mp. 7 1 or 2 or 3 or 4 or 5 or 6 8 exp Cognition Disorders/ 9 exp Neurobehavioral Manifestations/ 10 exp Mental Processes/ 11 exp Neuropsychological Tests/ 12 (cognit* or neurocognit* or neuropsycholog* or memory or neurobehavior* or neurobehaviour* or problem solving or attention or concentrat*).mp. 13 8 or 9 or 10 or 11 or 12 14 7 and 13 15 randomized controlled trial.pt. 16 controlled clinical trial.pt. 17 randomized.ab. 18 placebo.ab. 19 clinical trials as topic.sh. 20 randomly.ab. 21 trial.ti. 22 15 or 16 or 17 or 18 or 19 or 20 or 21 23 14 and 22
Key:
mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier pt=publication type ab=abstract fs=floating subheading
Appendix 2. EMBASE search strategy
1 skull irradiation/ 2 ((crani* or head or skull) adj5 (irradiat* or radiat* or radiotherap*)).mp. 3 exp brain tumor/ 4 exp glioma/ 5 (brain adj5 (tumor* or tumour* or malignan* or neoplas* or carcinoma* or cancer* or metasta*)).mp. 6 (glioma* or astrocytoma* or ependymoma* or ganglioma* or gliosarcoma* or medulloblastoma* or oligodendroglioma* or meningioma*).mp. 7 1 or 2 or 3 or 4 or 5 or 6 8 exp cognitive defect/ 9 exp mental function/ 10 neuropsychological test/ 11 (cognit* or neurocognit* or neuropsycholog* or memory or neurobehavior* or neurobehaviour* or problem solving or attention or concentrat*).mp. 12 8 or 9 or 10 or 11 13 7 and 12 14 exp controlled clinical trial/ 15 crossover procedure/ 16 double‐blind procedure/ 17 randomized controlled trial/ 18 single‐blind procedure/ 19 random*.mp. 20 factorial*.mp. 21 (crossover* or cross over* or cross‐over*).mp. 22 placebo*.mp. 23 (double* adj blind*).mp. 24 (singl* adj blind*).mp. 25 assign*.mp. 26 allocat*.mp. 27 volunteer*.mp. 28 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 29 13 and 28
key:
mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
Appendix 3. PsycINFO search strategy
1 ((crani* or head or skull) adj5 (irradiat* or radiat* or radiotherap*)).mp. 2 exp Brain Neoplasms/ 3 glioma/ 4 (brain adj5 (tumor* or tumour* or malignan* or neoplas* or carcinoma* or cancer* or metastas*)).mp. 5 (glioma* or astrocytoma* or ependymoma* or ganglioma* or gliosarcoma* or medulloblastoma* or oligodendroglioma* or meningioma*).mp. 6 1 or 2 or 3 or 4 or 5 7 cognitive impairment/ 8 exp cognitive processes/ 9 exp neuropsychological assessment/ 10 (cognit* or neurocognit* or neuropsycholog* or memory or neurobehavior* or neurobehaviour* or problem solving or attention or concentrat*).mp. 11 7 or 8 or 9 or 10 12 6 and 11 13 clinical trials/ 14 (random* or trial* or crossover* or cross over or double blind or single blind or placebo* or assign* or allocat*).mp. 15 13 or 14 16 12 and 15
key:
mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures
Appendix 4. CENTRAL search strategy
#1 MeSH descriptor: [Cranial Irradiation] explode all trees #2 (crani* or head or skull) near/5 (irradiat* or radiat* or radiotherap*) #3 MeSH descriptor: [Brain Neoplasms] explode all trees #4 MeSH descriptor: [Glioma] explode all trees #5 (brain near/5 (tumor* or tumour* or malignan* or neoplas* or carcinoma* or cancer* or metastas*)) #6 (glioma* or astrocytoma* or ependymoma* or ganglioma* or gliosarcoma* or medulloblastoma* or oligodendroglioma* or meningioma*) #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MeSH descriptor: [Cognition Disorders] explode all trees #9 MeSH descriptor: [Neurobehavioral Manifestations] explode all trees #10 MeSH descriptor: [Mental Processes] explode all trees #11 MeSH descriptor: [Neuropsychological Tests] explode all trees #12 (cognit* or neurocognit* or neuropsycholog* or memory or neurobehavior* or neurobehaviour* or problem solving or attention or concentrat*) #13 #8 or #9 or #10 or #11 or #12 #14 #7 and #13
Appendix 5. 'Risk of bias' item list
1.Selection bias
1.1 Random sequence generation
Low risk of bias, e.g. participants assigned to treatments on the basis of a computer‐generated random sequence or a table of random numbers.
High risk of bias, e.g. participants assigned to treatments on the basis of date of birth, clinic ID number or surname, or no attempt to randomise participants.
Unclear risk of bias, e.g. not reported or information not available.
1.2. Allocation concealment
Low risk of bias, e.g. where the allocation sequence could not be foretold.
High risk of bias, e.g. allocation sequence could be foretold by patients, investigators or treatment providers.
Unclear risk of bias, e.g. not reported or unclear.
2. Performance bias
2.1. Blinding of participants and personnel
Assessment of blinding was restricted to pharmacological interventions, since it would not be possible to blind participants and treatment providers to the non‐pharmacological interventions.
Low risk of bias if participants and personnel were adequately blinded.
High risk of bias if participants were not blinded to the intervention that the participant received.
Unclear risk of bias if this was not reported or unclear.
2.2. Blinding of outcomes assessors
Low risk of bias if outcome assessors were adequately blinded.
High risk of bias if outcome assessors were not blinded to the intervention that the participant received.
Unclear risk of bias if this was not reported or unclear.
2.3 Differences between the care provided to groups
Low risk of bias, e.g. both groups were followed on similar schedules other than intervention of interest.
High risk of bias, e.g. each group was followed according to different schedules.
Unclear risk of bias, e.g. not reported or unclear.
3. Incomplete outcome data
We recorded the proportion of participants whose outcomes were not reported at the end of the study. We coded a satisfactory level of loss to follow‐up for each outcome as follows.
Low risk of bias, if the reasons patients were lost to follow‐up were similar in both treatment arms such that it is unlikely to be related to the outcome of interest.
High risk of bias, if the reasons patients were lost to follow‐up differed between treatment arms such that it is likely to be related to the outcome of interest.
Unclear risk of bias if loss to follow‐up was not reported.
4. Selective reporting of outcomes
Low risk of bias, e.g. study reports all outcomes specified in the protocol.
High risk of bias, e.g. it is suspected that outcomes have been selectively reported.
Unclear risk of bias, e.g. it is unclear whether outcomes have been selectively reported.
5. Other bias
Low risk of bias if we did not suspect any other potential sources of bias.
High risk of bias if we suspected that the trial was prone to an additional bias.
Unclear risk of bias if we were uncertain whether an additional bias may have been present.
Data and analyses
Comparison 1.
Prevention: Memantine versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive functioning composite score | Other data | No numeric data | ||
| 2 Cognitive functioning sub‐tests | Other data | No numeric data | ||
| 2.1 Short‐term verbal memory | Other data | No numeric data | ||
| 2.2 Long‐term verbal memory (recall) | Other data | No numeric data | ||
| 2.3 Long‐term verbal memory (recognition) | Other data | No numeric data | ||
| 2.4 Trail making test A | Other data | No numeric data | ||
| 2.5 Trail making test B | Other data | No numeric data | ||
| 2.6 Verbal fluency | Other data | No numeric data | ||
| 3 Adverse events | 1 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.76, 1.32] |
Analysis 1.1.
Comparison 1 Prevention: Memantine versus placebo, Outcome 1 Cognitive functioning composite score.
Cognitive functioning composite score
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| Brown 2013 |
Analysis 1.2.
Comparison 1 Prevention: Memantine versus placebo, Outcome 2 Cognitive functioning sub‐tests.
Cognitive functioning sub‐tests
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 | Heading 6 | Heading 7 |
|---|---|---|---|---|---|---|---|
| Short‐term verbal memory | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Long‐term verbal memory (recall) | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Long‐term verbal memory (recognition) | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Trail making test A | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Trail making test B | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Verbal fluency | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
| Brown 2013 | |||||||
Analysis 1.3.
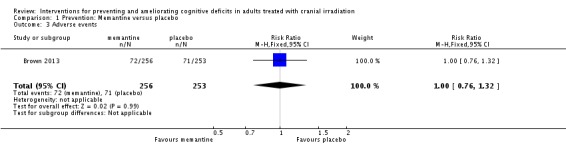
Comparison 1 Prevention: Memantine versus placebo, Outcome 3 Adverse events.
Comparison 2.
Prevention: d‐threo‐methylphenidate versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 General cognitive functioning | Other data | No numeric data | ||
| 2 Depression | Other data | No numeric data | ||
| 3 Fatigue | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 3.30 [‐10.37, 16.97] |
| 4 Quality of life | Other data | No numeric data | ||
| 4.1 FACT | Other data | No numeric data | ||
| 4.2 Brain subscale | Other data | No numeric data |
Analysis 2.1.
Comparison 2 Prevention: d‐threo‐methylphenidate versus placebo, Outcome 1 General cognitive functioning.
General cognitive functioning
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| Butler 2007 |
Analysis 2.2.
Comparison 2 Prevention: d‐threo‐methylphenidate versus placebo, Outcome 2 Depression.
Depression
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| Butler 2007 |
Analysis 2.3.
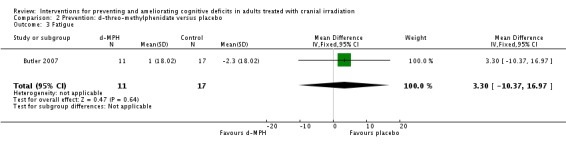
Comparison 2 Prevention: d‐threo‐methylphenidate versus placebo, Outcome 3 Fatigue.
Analysis 2.4.
Comparison 2 Prevention: d‐threo‐methylphenidate versus placebo, Outcome 4 Quality of life.
Quality of life
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| FACT | |||||
| Butler 2007 | |||||
| Brain subscale | |||||
| Butler 2007 | |||||
Comparison 3.
Prevention: Cognitive rehabilitation versus standard care
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive functioning | Other data | No numeric data | ||
| 2 Cognitive functioning sub‐test | Other data | No numeric data | ||
| 2.1 Attention | Other data | No numeric data | ||
| 2.2 Short‐term verbal memory | Other data | No numeric data | ||
| 2.3 Short‐term visual memory | Other data | No numeric data | ||
| 2.4 Long‐term verbal/visual memory | Other data | No numeric data | ||
| 2.5 Language | Other data | No numeric data | ||
| 3 Mood | Other data | No numeric data | ||
| 4 Fatigue | Other data | No numeric data | ||
| 5 Functional capacity | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | ‐8.55 [‐22.57, 5.47] |
| 6 Quality of life | 1 | 13 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐21.55, 33.55] |
Analysis 3.1.
Comparison 3 Prevention: Cognitive rehabilitation versus standard care, Outcome 1 Cognitive functioning.
Cognitive functioning
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| Locke 2008 |
Analysis 3.2.
Comparison 3 Prevention: Cognitive rehabilitation versus standard care, Outcome 2 Cognitive functioning sub‐test.
Cognitive functioning sub‐test
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| Attention | |||||
| Locke 2008 | |||||
| Short‐term verbal memory | |||||
| Locke 2008 | |||||
| Short‐term visual memory | |||||
| Locke 2008 | |||||
| Long‐term verbal/visual memory | |||||
| Locke 2008 | |||||
| Language | |||||
| Locke 2008 | |||||
Analysis 3.3.
Comparison 3 Prevention: Cognitive rehabilitation versus standard care, Outcome 3 Mood.
Mood
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| Locke 2008 |
Analysis 3.4.
Comparison 3 Prevention: Cognitive rehabilitation versus standard care, Outcome 4 Fatigue.
Fatigue
| Study | Heading 1 | Heading 2 | Heading 3 | Heading 4 | Heading 5 |
|---|---|---|---|---|---|
| Locke 2008 |
Analysis 3.5.
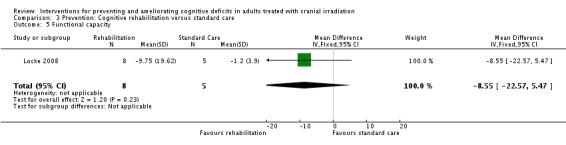
Comparison 3 Prevention: Cognitive rehabilitation versus standard care, Outcome 5 Functional capacity.
Analysis 3.6.
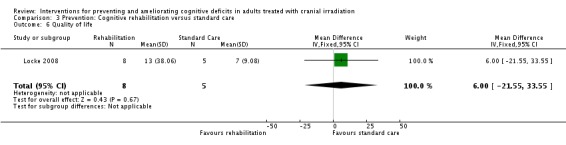
Comparison 3 Prevention: Cognitive rehabilitation versus standard care, Outcome 6 Quality of life.
Comparison 4.
Amelioration: Methylphenidate versus modafinil
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive functioning (calculated score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Attention (Digit Span) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [0.03, 0.73] |
| 1.2 Speed of processing (Digit symbol) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [‐0.30, 1.04] |
| 2 Cognitive functioning (timed tasks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Speed of processing (TMTA) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐2.48 [‐4.82, ‐0.14] |
| 2.2 Executive function | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.69 [‐0.50, 1.88] |
| 2.3 Psychomotor functioning (dominant hand) | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.61 [‐1.36, 2.58] |
| 2.4 Psychomotor functioning (non‐dominant hand) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐5.38 [‐51.16, 40.40] |
| 3 Cognitive functioning (total score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Verbal fluency | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 0.33 [‐0.16, 0.82] |
| 3.2 Short‐term verbal memory | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.35, 1.19] |
| 3.3 Long‐term verbal memory (recall) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [0.00, 1.24] |
| 3.4 Long‐term verbal memory (recognition) | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | 1.62 [‐0.56, 3.80] |
| 4 Self‐reported confusion | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐1.04 [‐4.76, 2.68] |
| 5 Anxiety | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 STAI State anxiety | 1 | 23 | Mean Difference (IV, Fixed, 95% CI) | ‐1.35 [‐11.66, 8.96] |
| 5.2 STAI Trait anxiety | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.83 [‐22.21, 20.55] |
| 5.3 POMS anxiety | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 3.56 [‐0.37, 7.49] |
| 6 Depression | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 BDI‐II | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.44 [‐1.93, 2.81] |
| 6.2 POMS depression‐dejection | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [‐0.48, 1.22] |
| 7 Anger | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐5.99, 4.99] |
| 8 Fatigue | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Brief Fatigue Inventory | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐5.2 [‐12.56, 2.16] |
| 8.2 POMS‐Fat | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐1.30, 1.76] |
| 9 Sleep | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | ‐2.39 [‐6.27, 1.49] |
| 10 Activity | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 1.94 [‐1.79, 5.67] |
| 11 Quality of life | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 2.71 [‐14.46, 19.88] |
Analysis 4.1.
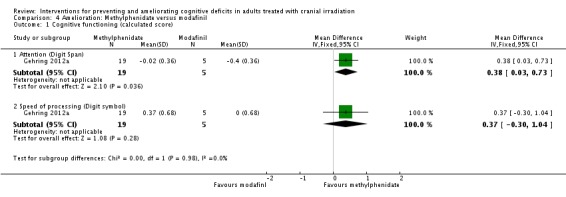
Comparison 4 Amelioration: Methylphenidate versus modafinil, Outcome 1 Cognitive functioning (calculated score).
Analysis 4.3.
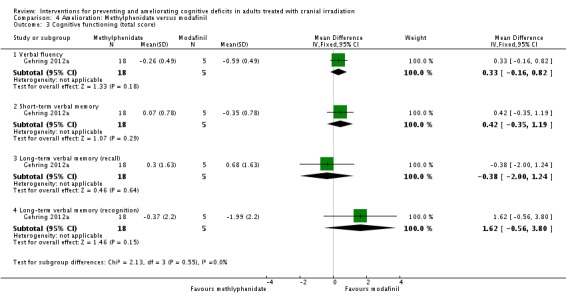
Comparison 4 Amelioration: Methylphenidate versus modafinil, Outcome 3 Cognitive functioning (total score).
Analysis 4.4.
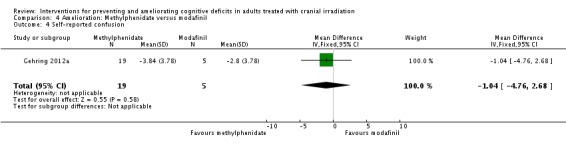
Comparison 4 Amelioration: Methylphenidate versus modafinil, Outcome 4 Self‐reported confusion.
Analysis 4.7.
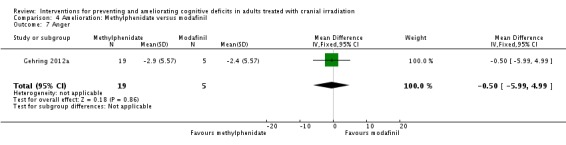
Comparison 4 Amelioration: Methylphenidate versus modafinil, Outcome 7 Anger.
Analysis 4.10.
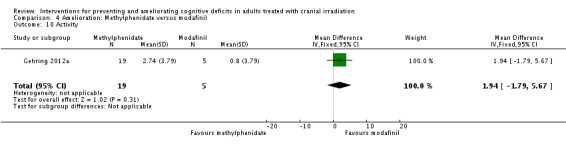
Comparison 4 Amelioration: Methylphenidate versus modafinil, Outcome 10 Activity.
Comparison 5.
Amelioration: Donepezil versus placebo
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cognitive composite score | 1 | 144 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.05, 0.11] |
| 2 Cognitive functioning (timed tasks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Speed of processing | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐1.82 [‐11.49, 7.85] |
| 2.2 Executive function | 1 | 143 | Mean Difference (IV, Fixed, 95% CI) | 1.61 [‐15.98, 19.20] |
| 2.3 Psychomotor functioning (dominant hand) | 1 | 141 | Mean Difference (IV, Fixed, 95% CI) | ‐11.93 [‐21.51, ‐2.35] |
| 2.4 Psychomotor functioning (non‐dominant hand) | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐5.21, 5.19] |
| 3 Cognitive functioning (total score) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Attention (Digit span (total)) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐1.12, 0.80] |
| 3.2 Attention (Digit span (backward)) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.4 [‐1.01, 0.21] |
| 3.3 Attention (Digit Span (forward)) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.57, 0.69] |
| 3.4 Verbal fluency | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.92 [‐3.19, 1.35] |
| 3.5 Short‐term verbal memory | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.32 [‐0.93, 1.57] |
| 3.6 Long‐term verbal memory (recall) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.35 [‐0.36, 1.06] |
| 3.7 Long‐term verbal memory (retention) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 3.96 [‐4.40, 12.32] |
| 3.8 Long‐term verbal memory (recognition) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.57 [0.07, 1.07] |
| 3.9 Long‐term verbal memory (discrimination) | 1 | 145 | Mean Difference (IV, Fixed, 95% CI) | 0.94 [0.27, 1.61] |
| 3.10 Short‐term visual memory (copy) | 1 | 144 | Mean Difference (IV, Fixed, 95% CI) | ‐0.69 [‐1.63, 0.25] |
| 3.11 Short‐term visual memory (recall) | 1 | 144 | Mean Difference (IV, Fixed, 95% CI) | ‐0.50 [‐1.51, 0.51] |
| 3.12 Long‐term visual memory (retention) | 1 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐0.39 [‐1.48, 0.70] |
Analysis 5.1.
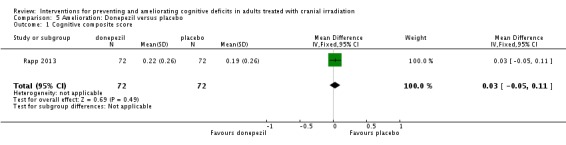
Comparison 5 Amelioration: Donepezil versus placebo, Outcome 1 Cognitive composite score.
Analysis 5.3.
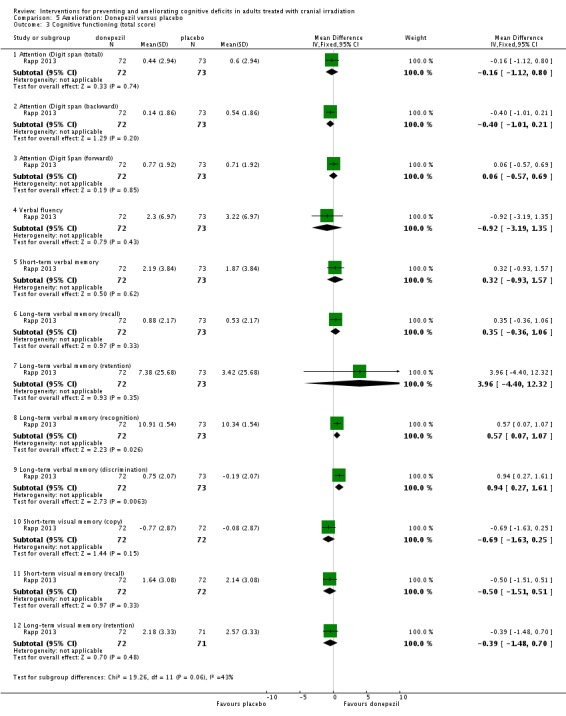
Comparison 5 Amelioration: Donepezil versus placebo, Outcome 3 Cognitive functioning (total score).
What's new
Last assessed as up‐to‐date: 28 August 2014.
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
History
Protocol first published: Issue 10, 2014 Review first published: Issue 12, 2014
| Date | Event | Description |
|---|---|---|
| 20 April 2015 | Amended | Minor editorial amendments to text. |
Differences between protocol and review
Parts of the methods section of this review are based on a standard template established by the Cochrane Neuro‐oncology Group.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brown 2013
| Methods | Randomised controlled trial, parallel arm, double‐blind, stratification by recursive partitioning analysis class and prior surgical therapy. | |
| Participants |
Inclusion criteria: Adult patients; pathologically proven diagnosis of solid malignancy within 5 years of registration; brain metastases visible on contrast‐enhanced MRI (or a contrast‐enhanced CT for patients unable to have an MRI) with stable systemic disease 3 months prior to study entry; receiving 37.5 Gy of WBRT via 15 fractions of 2.5 Gy; KPS ≥ 70; serum creatinine ≤ 3 mg/dL, creatinine clearance ≥ 30 mL/min, total bilirubin ≤ 2.5 mg/dL, blood urea nitrogen (BUN) 20 mg/dL; MMSE score > 18; negative serum pregnancy test. Exclusion criteria: Memantine allergy, current alcohol or drug abuse, chronic use of benzodiazepines, severe active comorbidity. No. randomised: Memantine: 278; placebo: 276. Follow‐up: 8, 16, 24 and 52 weeks. Setting: 143 centres in the United States and Canada |
|
| Interventions | Treatment arm schedule: Week 1: 5 mg oral memantine taken in the morning Week 2: 10 mg oral memantine taken in divided dosage (5 mg morning, 5 mg night) Week 3: 15 mg oral memantine taken in divided dosage (10 mg morning, 5 mg night) Week 4‐24: 20 mg oral memantine taken in divided dosage (10 mg morning, 10 mg night) Control Arm: Matched placebo The dosage was lowered to 10 mg twice daily, as week 2, if creatinine clearance decreased to 30 mL/min, and was continued at this dosage if creatine clearance fell below 5 mL/min following a weekly recheck. |
|
| Outcomes | Cognitive function (HVLT‐R, COWA, TMT) | |
| Notes | Efficacy reported via median change and interquartile ranges. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The Zelen treatment allocation scheme was used to stratify patients according to according to recursive partitioning analysis (RPA) class and prior surgical therapy.Within each stratum, patients were randomised in a 1:1 ratio to placebo or memantine.” "A computer at RTOG headquarters randomly generated the sequences for the randomization" (obtained via correspondence). |
| Allocation concealment (selection bias) | Low risk | "The placebo was actually provided by the company (Forest Pharma) and it was impossible to tell which was placebo and which was active drug." (obtained via correspondence). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding carried out. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding carried out. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "All eligible patients randomised to the study were included (intent‐to‐treat analysis)...The multiple imputation procedure employing the Markov chain Monte Carlo method was also used to determine values for all remaining living patients missing assessments." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None. |
Butler 2007
| Methods | Randomised controlled trial, parallel arm, double‐blind, stratification by tumour type, treatment and KPS. | |
| Participants |
Inclusion criteria: Aged 18 years or over; histologically confirmed metastatic or primary brain tumour; KPS > 70; life expectancy > 3months; haemoglobin > 10.0, white blood cell count > 1,500, platelets > 75,000, planned partial or WBRT at a total dose of > 25 Gy via > 10 fractions of 180‐300 c Gy. Exclusion criteria: Serious medical or psychiatric illness that would prevent informed consent; completion of protocol therapy or QoL questionnaires; history of hypersensitivity to d,l‐MPH; history of steroid psychosis; history of/currently taking medication for ADD, anxiety disorder, schizophrenia or substance abuse; currently taking antidepressants; family history or active Tourette's Syndrome; history or active glaucoma; history of RT; undergoing craniospinal axis irradiation; hypertension or other CV disease requiring antihypertensives or other CV medications; pregnant of breast‐feeding. No. randomised: d,l‐MPH: 34; placebo: 34. Follow‐up: end of RT and at 4, 8 and 12 weeks. Setting: four centres in the United states. |
|
| Interventions | Treatment arm schedule: Day 1: 10 mg oral d,l‐MPH taken in divided doses (5 mg before breakfast, 5 mg before 6 pm) Day 5‐7 to Day 10: 20 mg oral d,l‐MPH taken in divided doses (10 mg before breakfast, 10 mg before 6pm) Day 10‐14 to Week 8: 30 mg to oral d‐threo‐methylphenidate taken in divided doses (15 mg before breakfast, 15 mg before 6pm) Control Arm: Matched placebo |
|
| Outcomes | Cognitive function (MMSE) Fatigue (FACIT‐Fatigue sub‐scale) QoL (FACT, FACT‐Br, FACIT‐Fatigue) Depression (CESDS) |
|
| Notes | Study funded by pharmaceutical company and closed prematurely due to withdrawal of funding and low accrual. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were stratified by tumour type, treatment and KPS and randomized within strata to one of the two treatment arms with equal probability." "Randomised by computer program" (obtained via correspondence). |
| Allocation concealment (selection bias) | Low risk | "Used a 3rd party research pharmacy that knew which group patient was assigned to and mailed drug or placebo in containers that were labelled to include instructions for use." (obtained via correspondence). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding carried out. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding carried out. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "We did do intent to treat analysis. The dropouts were due to disease progression either in the brain or systemically ... not due to toxicity of intervention" (obtained via correspondence). |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None. |
Gehring 2012a
| Methods | Randomised controlled trial, three arms, open‐label, stratification by tumour location. | |
| Participants |
Inclusion criteria: age > 18; KPS > 70; primary brain tumour; subjective complaint of cognitive decline or fatigue; being considered for stimulant therapy by their neuro‐oncologist; the ability to speak and understand English or Spanish. Exclusion criteria: current use of psychostimulants, monoamine oxidase inhibitors, anticoagulants, drugs similar to erythropoietin, or illicit drugs; history of hypersensitivity reaction to methylphenidate or modafinil; history of uncontrolled seizures, cardiac or pulmonary disease, or hypertension (systolic > 140 mm Hg, diastolic > 90 mm Hg, or not on a stable dose of anti‐hypertensive medication for the past month; severe headaches; current glaucoma, narcolepsy, Tourette’s syndrome, major psychiatric diagnosis, alcohol or drug abuse; current use of herbals/supplements for fatigue relief, e.g. Ginkgo biloba, ginseng, St John's Wort, dehydroepiandrosterone; unstable dose of antidepressants; comorbidities or medications that in the treating physician’s opinion could potentially interfere with safe administration of MPH or MOD. No. randomised: methylphenidate: 24; modafinil: 10. Follow‐up: 4 weeks. Setting: one cancer centre in the United States. |
|
| Interventions | Arm I: 10 mg of oral methylphenidate (immediate release) taken in divided doses for 4 weeks Arm II: 18 mg or oral methylphenidate (sustained release) taken in the morning for 4 weeks Arm III: 200 mg of oral modafinil taken in the morning for 4 weeks. |
|
| Outcomes | Cognitive function (WAIS‐III Digit span and Digit symbol, TMT, HVLT‐R, grooved pegboard, MAE COWA). Fatigue (BFI, POMS‐Fat, POMS‐Vig sub‐scales) Sleep disturbance (BSDS) Mood (POMS, BDI‐II, STAI) QoL (FACT general and brain modules, FIM) |
|
| Notes | Arm I and II combined for statistical analysis. Second drug used as control arm, rather than placebo. Groups also compared with normative data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were stratified by tumour location (i.e., right verses left hemisphere) and randomly assigned to one of three conditions." "a computer performed the randomization" (obtained via correspondence). |
| Allocation concealment (selection bias) | High risk | An open‐label design was used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | An open‐label design was used. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | An open‐label design was used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across treatment groups, and reasons for missing outcome data similar between treatment groups and unlikely to be related to outcomes measured. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Possible recruitment bias: "PBT patients were considered eligible for participation if… being considered for stimulant therapy by their neuro‐oncologist." |
Kaleita 2006
| Methods | Randomised controlled trial, parallel arm, double‐blind. | |
| Participants |
Inclusion criteria: aged 21‐65, primary brain tumour, receiving treatment in the UCLA Neuro‐Oncology Program, prior neurosurgical resection, radiotherapy, and/or cytotoxic or cytostatic chemotherapy, mild to severe fatigue and/or attention/memory impairment, as measured by the Clinical Global Impression of Severity Scale, able to speak English, capable of completing self‐rating scales and one‐on‐one psychometric tests, at least 30 days since prior stimulants (e.g., amphetamines or methylphenidate), negative pregnancy test and use of contraception if fertile, concurrent conventional chemotherapy (e.g., carboplatin, lomustine, temozolomide), glucocorticoids (e.g. dexamethasone) and tamoxifen allowed. Exclusion criteria: significant hepatic disease, significant renal disease, severe cognitive impairment, other terminal illness, emergency patient, institutional resident, prisoner or parolee, UCLA students or staff, pregnant or nursing, concurrent irinotecan, concurrent participation in UCLA experimental chemotherapy trials, prior modafinil, concurrent experimental anticancer medication, concurrent tricyclic antidepressants and/or monoamine oxidase inhibitors. No. randomised: 30 (each arm not reported). Follow‐up: 1, 3, 4, 8 and 12 weeks. Setting: one centre in the United states. |
|
| Interventions | Arm I: 200 mg/day modafinil in divided doses for 3 weeks Arm II: 400 mg/day modafinil in divided doses for 3 weeks Both arms then completed a 1‐week wash out period, followed up 200 mg/day modafinil for 3 days, followed by a titrated dose for 8 weeks. |
|
| Outcomes | Cognitive function (TMT, SDM, verbal fluency test) Fatigue (Fatigue severity scale, Visual analogue fatigue scale, modified fatigue impact scale) Mood (HDS) |
|
| Notes | All participants required to have mild or severe fatigue. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Use of a randomisation method reported but not described. |
| Allocation concealment (selection bias) | Low risk | Blinding carried out. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding carried out. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding carried out. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Projected accrual successful. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | All participants required to have mild or severe fatigue. |
Locke 2008
| Methods | Randomised controlled trial, parallel arm, unblinded. | |
| Participants |
Inclusion criteria: 18 years of age or older; mild‐to‐moderate cognitive impairment based on a combination of quantitative neuropsychological test data from the clinical assessment and the clinical judgement of the evaluation neuropsychologist; prognosis of at least 6 months of life; ability to attend sessions at our medical centre for 2 weeks; designated caregiver available to attend all sessions; receiving radiation therapy. No. randomised: Cognitive rehabilitation program: 7; standard care: 12. Follow‐up: post‐intervention and 3 months. Setting: one radiation oncology clinic in the United states. |
|
| Interventions | Treatment arm schedule: 1. Six 50‐minute sessions of cognitive rehabilitation carried out over 2 weeks, involving the learning and use of a calender as a compensatory aid. 2. Six 50‐minute sessions of problem‐solving carried out over 2 weeks, involving education of a model of stress and learning positive problem‐solving management techniques. Control arm: standard care. |
|
| Outcomes | Cognitive function (RBANS) QoL (CQOLC, LASA) Mood (POMS) Fatigue (BFI) Functional capacity (FACT‐BR, MPAI‐4) |
|
| Notes | No cognitive function statistical comparisons were made between groups. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Patients were randomized by our randomization center at the time of their enrollment. The randomization center is an entirely separate group of personnel from those recruiting and enrolling patients for the study" (obtained via correspondence). However, "Due to low accrual and anticipation of the ending of the enrolment period, the last three patient/caregiver dyads were not randomized and were enrolled directly into the intervention group." |
| Allocation concealment (selection bias) | High risk | "randomization was not pre‐scheduled. That is, they were randomized patient by patient as they enrolled so I could not foresee their group because it had not been determined yet." (obtained via correspondence). However, "Due to low accrual and anticipation of the ending of the enrolment period, the last three patient/caregiver dyads were not randomized and were enrolled directly into the intervention group." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the intervention, blinding of patients to the treatment or control group was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | It is unclear who carried out the assessments, and whether they were blinded, but it is likely that is was the neuropsychologist, master’s level behavioural therapist or master’s level psychology study personnel that had been involved in delivering the intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar reasons between groups for missing data. "Most patients did not return at that time for in‐person follow‐up…so most patients did not complete the R‐BANS at follow‐up." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None. |
Rapp 2013
| Methods | Randomised controlled trial, parallel arm, double‐blind, stratification according to whole‐brain vs partial‐brain irradiation type and by study site. | |
| Participants |
Inclusion criteria: adults > 18 years; primary or metastatic brain tumour; completed a course of fractionated partial or whole brain irradiation of at least 30 Gy > 6 months prior to enrolment; no imaging evidence of disease progression within 6 months prior to enrolment; life expectancy > 6 months; ECOG score > 2; Exclusion criteria: currently taking cognition enhancing medications; planned treatment for the next 6 months; pregnant. No. randomised: donepezil: 99; placebo: 99. Follow‐up: 24 weeks Setting: two academic medical centres, 21 Community Clinical Oncology Programs (CCOPs), 3 Cancer Trial Support Unit sites (United States). |
|
| Interventions | Treatment arm schedule: Week 1‐6: 5 mg oral donepezil Week 7‐24: 10 mg oral donepezil if tolerated. Control arm: Matched oral placebo |
|
| Outcomes | Cognitive functioning (HVLT‐R, COWA, Digit Span, mROCF, TMT, grooved pegboard) | |
| Notes | Conference abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was generated using nQuery Advisor"... "Patients were stratified by accruing site (academic centers vs CCOP sites) and type of radiation (whole vs partial) and assigned within strata to receive donepezil or a placebo with equal probability using variably sized permuted block randomization." (obtained via correspondence). |
| Allocation concealment (selection bias) | Low risk | "Drug and placebo were over encapsulated and distributed to the study sites by Biologics Inc., Raleigh, NC" (obtained via correspondence). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding carried out. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding carried out. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis carried out. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None. |
Scales MMSE Mini‐Mental State Examination; FACIT‐Fatigue sub‐scale The Functional Assessment of Chronic Illness Therapy ‐ Fatigue sub‐scale; FACT Functional Assessment of Cancer Therapy; FACT‐Br Functional Assessment of Cancer Therapy ‐ Brain; FACIT‐Fatigue Functional Assessment of Chronic Illness Therapy ‐ Fatigue; CESDS Center for Epidemiological Studies Depression Scale; WAIS‐III Digit Span and Digit Symbol Wechsler Adult Intelligence Scale III Digit Span and Digit Symbol sub‐tests; TMT Trail making test parts A and B; HVLT‐R Hopkin's Verbal Learning Test‐Revised;COWA Controlled Oral Word Association Test; BFI Brief Fatigue Inventory; POMS Profile of Mood States; POMS‐Fat POMS‐Fatigue sub‐sale; POMS‐Vig POMS‐Vigilance sub‐scale; BSDS Brief Sleep Disturbance Scale; BDI‐II Beck's Depression Inventory‐IIl; STAI State‐Trait Anxiety Inventory; FIM Functional Independence Measure; HDS Hamilton Depression Scale; RBANS Repeatable Battery of Assessment of Neuropsychological Status; CQOLC Caregiver Quality of Life Index‐Cancer; LASA Linear Analogue Self‐Assessment; MPAI‐4 Mayo‐Portland Adaptability Inventory‐4. Other ADD attention deficit disorder; CT computed tomography; CV cardiovascular; MRI magnetic resonance imaging; RT radiotherapy; WBRT whole brain radiotherapy.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Attia 2012 | Not a controlled trial. |
| Boele 2013 | Study included > 20% non‐irradiation patients and did not analyse cranial irradiation patients separately. |
| Chan 2003 | Patients not randomised. |
| Gehring 2009 | Study included > 20% non‐irradiation patients and did not analyse cranial irradiation patients separately. |
| Hulshof 2002 | Patients not randomised. |
| Jatoi 2005 | 1 participant completed the study. |
| Levin 2011 | The primary outcome not cognitive functioning or other quality of life measure. |
| Meyers 1998 | Not a controlled trial. |
| Schellart 2011 | Patients not randomised. |
| Shaw 2006 | Not a controlled trial. |
Characteristics of studies awaiting assessment [ordered by study ID]
Shaw 2013
| Methods | Phase II double‐blind placebo‐controlled RCT. Patients were randomly assigned to receive armodafinil or a placebo during cranial irradiation and for four weeks following RT. Patients completed assessments at baseline, at the end of RT, and 4 weeks after the end of RT. Intervention toxicity was recorded. |
| Participants | Patients with a primary brain tumour receiving a total RT dose of 45 Gy during participation. |
| Interventions | Arm I: 150 mg/day Armodafinil Arm II: Placebo |
| Outcomes | Fatigue (BFI, FACIT‐Fatigue) Sleepiness (ESS) Quality of life (FACT, FACT‐brain) Cognitive function (Wake Forest Cognitive Function Battery) |
| Notes | In the armodafinil treatment arm, fatigue, sleepiness and quality of life scores significantly improved at the end of RT, compared with the placebo arm. |
Scales BFI Brief Fatigue Inventory;FACIT‐Fatigue Functional Assessment of Chronic Illness Therapy ‐ Fatigue; ESS Epworth Sleepiness Scale; FACT Functional Assessment of Cancer Therapy
Characteristics of ongoing studies [ordered by study ID]
Umphrey 2013
| Trial name or title | Armodafinil in reducing cancer‐related fatigue in patients with high grade glioma |
| Methods | Phase III double‐blind placebo‐controlled RCT. Patients are randomly assigned to receive one of two doses of armodafinil or a placebo for 8 weeks. |
| Participants |
Inclusion criteria: aged ≥ 18yrs; glioblastoma multiforme, anaplastic astrocytoma, gliosarcoma, or anaplastic oligodendroglioma; clinically stable (stable/improved KPS compared to the prior month); completed radiation therapy > 21 days and ≤ 24 months prior to enrolment; ≥ 6 score on the worst fatigue question of the BFI (Brief Fatigue Inventory); previous surgery (gross total or subtotal resection) or biopsy; negative serum pregnancy test done ≤ 7 days prior to registration; ability to complete questionnaire(s) by themselves or with assistance, ECOG PS 0, 1, 2 or 3; provide informed written consent; willing to return to enrolling institution for follow‐up (during the Active Monitoring Phase of the study)
; stable dose of corticosteroid ≤ 28 days prior to registration. Exclusion criteria: History of hypersensitivity to other psychostimulants; history of steroid psychosis; history of/currently taking medications for attention deficit hyperactivity disorder, severe anxiety disorder, schizophrenia, or substance abuse by patient record and/or self‐report; currently taking medications to treat fatigue including psychostimulants, antidepressants, acupuncture (antidepressants used to treat items other than fatigue (such as hot flushes or depression) are allowed if the patient has been on a stable dose for ≥ 30 days and plans to continue for the duration of the trial); anticipating surgery; laboratory evidence of hypothyroidism with an elevated thyroid stimulating hormone (TSH) concentration in the blood > 5.0 mlU/L; profound anaemia (haemoglobin level of < 10 g/dL) ≤ 28 days prior to registration; clinical depression per physician discretion; active/history of Tourette's syndrome or tic disorder, glaucoma, intractable epilepsy or uncontrolled seizure disorder; history of myocardial infarction, unstable angina, left ventricular hypertrophy or mitral valve prolapse syndrome; use of strong or moderate inhibitors of cytochrome P450 3A4 (CYP3A4) ≤ 7 days prior to registration; use of medications or substances that are inducers of CYP3A4 ≤ 7 days prior to registration. Follow‐up: 8 weeks Setting: 92 centres in the United States. |
| Interventions | Arm I: 150 mg Armodafinil Arm II: 250 mg Armodafinil Arm III: Placebo |
| Outcomes | Patient‐reported fatigue (BFI) Cognitive functioning (SDM, COWA, TMT, FACT‐Cog) Quality of life (LASA). |
| Starting date | 2013 |
| Contact information | Recruitment is being carried out in 92 centres. Study Chair: Alyx Umphrey Mayo Clinic Rochester Minnesota United States 55905 507‐538‐7623 |
| Notes | ClinicalTrials.gov Identifier: NCT01781468 Current status: Recruiting participants. |
Scales BFI Brief Fatigue Inventory; SDM Symbol Digit Modalities Test; COWA Controlled Oral Work Association Test; TMT Trail making test; FACT‐Cog Functional Assessment of Cancer Therapy‐Cognitive Function; LASA Linear Analogue Self Assessment
Contributions of authors
| Develop and run the search strategy | JD, KZ, KG |
| Obtain copies of trials | JD, KZ |
| Select which trials to include | JD, KZ, KG |
| Extract data from trials (2 people) | JD, KZ |
| Enter data into RevMan | JD, KZ |
| Carry out the analyses | JD, KZ |
| Interpret the analyses | All authors |
| Draft the final review | All authors |
| Update the review | All authors |
Sources of support
Internal sources
No sources of support supplied
External sources
NIHR Cochrane Incentive Scheme Award 2014, UK.
Declarations of interest
Julia Day: nothing to declare Karolis Zienius: nothing to declare Martin Taphoorn: nothing to declare Jing Li: nothing to declare Karin Gehring: nothing to declare David Grosshans: nothing to declare Robin Grant: nothing to declare Paul Brown: nothing to declare
Edited (no change to conclusions)
References
References to studies included in this review
- Brown PD, Pugh S, Laack NN, Wefel JS, Khuntia D, Meyers C, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole‐brain radiotherapy: a randomized, double‐blind, placebo‐controlled trial. Neuro‐Oncology 2013;15(10):1429‐37. [DOI: 10.1093/neuonc/not114] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JM Jr, Case LD, Atkins J, Frizzell B, Sanders G, Griffin P, et al. A phase III, double‐blind, placebo‐controlled prospective randomised clinical trial of d‐threo‐methylphenidate HCl in brain tumour patients receiving radiation therapy. Internation Journal of Radiation Oncology *Biology *Physics 2007;69(5):1496‐501. [DOI: 10.1016/j.ijrobp.2007.05.076] [DOI] [PubMed] [Google Scholar]
- Gehring K, Patwardhan SY, Collins R, Groves MD, Etzel CJ, Meyers CA, et al. A randomized trial on the efficacy of methylphenidate and modafinil for improving cognitive functioning and symptoms in patients with a primary brain tumor. Journal of Neuro‐Oncology 2012;107(1):165‐74. [DOI: 10.1007/s11060-011-0723-1] [DOI] [PubMed] [Google Scholar]
- Kaleita TA, Wellisch DK, Graham CA, Steh B, Nghiemphu, Ford JM, et al. Pilot study of modafinil for treatment of neurobehavioral dysfunction and fatigue in adult patients with brain tumors. Journal of Clinical Oncology. 2006; Vol. 24, issue 18S:1503. [Google Scholar]
- Locke DEC, Cerhan JH, Wu W, Malec JF, Clark MM, Rummans TA, et al. Cognitive rehabilitation and problem‐solving to improve quality of life of patients with primary brain tumours: a pilot study. Journal of Supportive Oncology 2008;6(8):383‐91. [PubMed] [Google Scholar]
- Rapp SR, Case D, Peiffer A, Naughton MJ, Stieber VW, Bayer GK, et al. Phase III randomized, double‐blind, placebo‐controlled trial of donepezil in irradiated brain tumor survivors. Journal of Clinical Oncology. 2013; Vol. 31:2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Attia A, Rapp SR, Case LD, D'Agostino R, Lesser G, Naughton M, et al. Phase II study of Ginkgo biloba in irradiated brain tumor patients: effect on cognitive function, quality of life, and mood. Journal of Neuro‐Oncology 2012;109:357‐63. [DOI: 10.1007/s11060-012-0901-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boele FW, Douw L, Groot M, Thuijl HF, Cleijne W, Heimans JJ, et al. The effect of modafinil on fatigue, cognitive functioning, and mood in primary brain tumor patients: a multicenter randomized controlled trial. Neuro‐Oncology 2013;15(10):1420‐8. [DOI: 10.1093/neuonc/not102] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AS, Cheung MC, Law SC, Chan JH. Phase II study of alpha‐tocopherol in improving the cognitive function of patients with temporal lobe radionecrosis. Cancer 2004;100(2):398‐404. [DOI: 10.1002/cncr.11885] [DOI] [PubMed] [Google Scholar]
- Gehring K, Sitskoorn MM, Gundy CM, Sikkes SAM, Klein M, Postma TJ, et al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. Journal of Clinical Oncology 2009;27(22):3712‐22. [DOI: 10.1200/JCO.2008.20.5765] [DOI] [PubMed] [Google Scholar]
- Hulshof MCCM, Stark NM, Kleij A, Sminia P, Smeding HMM, Gonzalez DG. Hyperbaric oxygen therapy for cognitive disorders after irradiation of the brain [Hyperbare oxygenierung bei patienten mit kognitiven störungen nach hirnbestrahlung]. Strahlentherapie und Onkologie 2002;178(4):192‐8. [DOI: 10.1007/s00066-002-0916-9] [DOI] [PubMed] [Google Scholar]
- Jatoi A, Kahanic SP, Frytak S, Schaefer P, Foote RL, Sloan J, et al. Donepezil and vitamin E for preventing cognitive dysfunction in small cell lung cancer patients: preliminary results and suggestions for future study designs. Supportive Care in Cancer 2005;13:66‐9. [DOI: 10.1007/s00520-004-0696-0] [DOI] [PubMed] [Google Scholar]
- Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized double‐blind placebo‐controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. International Journal of Radiation Oncology *Biology *Physics 2011;79(5):1487‐95. [DOI: 10.1016/j.ijrobp.2009.12.061] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers CA, Weitzner MA, Valentine AD, Levin VA. Methylphenidate therapy improves cognition, mood and function of brain tumor patients. Journal of Clinical Oncology 1998;16(7):2522‐7. [DOI] [PubMed] [Google Scholar]
- Schellart NAM, Reits D, Kleij, Stalpers LJA. Hyperbaric oxygen treatment improved neurophysiologic performance in brain tumor patients after neurosurgery and radiotherapy: a preliminary report. Cancer 2011;117(15):3434‐44. [DOI: 10.1002/cncr.25874] [DOI] [PubMed] [Google Scholar]
- Shaw EG, Rosdhal R, D'Agostino RB Jr, Lovato J, Naughton MJ, Robbins ME, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. Journal of Clinical Oncology 2006;24(9):1415‐20. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Shaw EG, Case D, Bryant D, Grisell D, Lesser G, Monitto DC, et al. Phase II double‐blind placebo‐controlled study of armodafinil for brain radiation induced fatigue. Journal of Clinical Oncology. 2013; Vol. 13:9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
- Umphrey ABP. Armodafinil in reducing cancer‐related fatigue in patients with high grade glioma. ClinicalTrials.gov2013; Vol. NCT01781468.
Additional references
- Abrey LE, DeAngelis LM, Yahalom J. Long‐term survival in primary CNS lymphoma. Journal of Clinical Oncology 1998;16(3):859‐63. [DOI] [PubMed] [Google Scholar]
- Anscher MS, Swift PS, Gaspar LE, Marks LB. Radiation injury of the brain and spinal cord. In: Wilkins RH, Rengachary SS editor(s). Neurosurgery. New York: McGraw‐Hill, 1996:1921‐36. [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test‐revised: normative data and analysis of inter‐form and test‐retest reliability. Clinical Neuropsychologist 1998;12(1):43‐55. [Google Scholar]
- Benton AL, Hamsher KS. Multilingual Aphasia Examination. Iowa City, IA, AJA Associates1989.
- Challman TD, Lipsky JJ. Methylphenidate: its pharmacology and uses. Mayo Clinic Proceedings 2000;75(7):711‐21. [DOI] [PubMed] [Google Scholar]
- Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole‐brain irradiation: a randomised controlled trial. The Lancet Oncology 2009;10(11):1037‐44. [DOI: 10.1016/S1470-2045(09)70263-3] [DOI] [PubMed] [Google Scholar]
- Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, et al. Evidence‐based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Archives of Physical Medicine and Rehabilitation 2011;92(4):519‐30. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Hawton K, Stockton S, Geddes JR. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta‐analysis. BMJ: British Medical Journal 2013;346:1‐13. [DOI] [PubMed] [Google Scholar]
- Cochran DC, Chan MD, Aklilu M, Lovato JF, Alphonse NK, Bourland JD, et al. The effect of targeted agents on outcomes in patients with brain metastases from renal cell carcinoma treated with Gamma Knife surgery. Journal of Neurosurgery 2012;116(5):978‐83. [DOI: 10.3171/2012.2.JNS111353] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa DD, DeAngelis LM, Shi W, Thaler H, Glass A, Abrey LE. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology 2004;62(4):548‐55. [PUBMED: 14981169] [DOI] [PubMed] [Google Scholar]
- Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation‐induced encephalopathy. Journal of Clinical Oncology 1994;12(3):627‐42. [DOI] [PubMed] [Google Scholar]
- Cull A, Hay C, Love SB, Mackie M, Smets E, Stewart M. What do cancer patients mean when they complain of concentration and memory problems?. British Journal of Cancer 1996;74(10):1674‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J, Ahlberg FM, Berk M, Ashley DM, Khasraw M. Emerging pharmacotherapy for cancer patients with cognitive dysfunction. BMC Neurology 2013;13(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeAngelis LM, Delattre JY, Posner JB. Radiation‐induced dementia in patients cured of brain metastases. Neurology 1989;39(6):789. [DOI: 10.1212/WNL.39.6.789] [DOI] [PubMed] [Google Scholar]
- Deeks JJ. Systematic reviews in health care: systematic reviews of evaluations of diagnostic and screening tests. BMJ 2001;323(7305):157‐62. [DOI: 10.1136/bmj.323.7305.157] [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
- Dietrich J, Monje M, Wefel J, Meyers C. Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. The Oncologist 2008;13(12):1285‐95. [DOI: 10.1634/thoncologist.2008-0130] [DOI] [PubMed] [Google Scholar]
- Fink J, Born D, Chamberlain MC. Radiation necrosis: relevance with respect to treatment of primary and secondary brain tumors. Current Neurology and Neuroscience Reports 2012;12(3):276‐85. [DOI: 10.1007/s11910-012-0258-7] [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state": a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189‐98. [DOI] [PubMed] [Google Scholar]
- Gehring K, Sitskoorn MM, Aaronson NK, Taphoorn MJB. Interventions for cognitive deficits in adults with brain tumours. The Lancet Neurology 2008;7(6):548‐60. [DOI] [PubMed] [Google Scholar]
- Gehring K, Aaronson NK, Taphoorn MJ, Sitskoorn MM. Interventions for cognitive deficits in patients with a brain tumor: an update. Expert Review of Anticancer Therapy 2010;10(11):1779‐95. [DOI] [PubMed] [Google Scholar]
- Gehring K, Aaronson NK, Gundy CM, Taphoorn MJB, Sitskoorn MM. Predictors of neuropsychological improvement following cognitive rehabilitation in patients with gliomas. Journal of International Neuropsychological Society 2011;17(2):256‐66. [DOI] [PubMed] [Google Scholar]
- Gehring K, Roukema JA, Sitskoorn MM. Review of recent studies on interventions for cognitive deficits in patients with cancer. Expert Review of Anticancer Therapy 2012;12(2):255‐69. [DOI: 10.1586/era.11.202] [DOI] [PubMed] [Google Scholar]
- Gill AL, Bell CNA. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM: An International Journal of Medicine 2004;97(7):385‐95. [DOI: 10.1093/qjmed/hch074] [DOI] [PubMed] [Google Scholar]
- Gilroy J. Basic Neurology. 3rd Edition. New York: McGraw‐Hill Health Professions Division, 2000. [Google Scholar]
- Gligoroska JP, Manchevska S. The effect of physical activity on cognition ‐ physiological mechanisms. Materia Socio Medica 2012;24(3):198‐202. [PUBMED: PMC363396] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondi V, Paulus R, Bruner DW, Meyers CA, Gore EM, Wolfson A, et al. Decline in tested and self‐reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of radiation therapy oncology group randomized trials 0212 and 0214. International Journal of Radiation Oncology* Biology* Physics 2013;86(4):656‐64. [DOI: 10.1016/j.ijrobp.2013.02.033] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales J, Kumar AJ, Conrad CA, Levin VA. Effect of bevacizumab on radiation necrosis of the brain. International Journal of Radiation Oncology* Biology* Physics 2007;67(2):323‐6. [DOI: 10.1016/j.ijrobp.2006.10.010] [DOI] [PubMed] [Google Scholar]
- Greene‐Schloesser D, Moore E, Robbins ME. Molecular pathways: radiation‐induced cognitive impairment. Clinical Cancer Research 2013;19(9):2294‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassler MR, Elandt K, Preusser M, Lehrner J, Binder P, Dieckmann K, et al. Neurocognitive training in patients with high‐grade glioma: a pilot study. Journal of Neuro‐Oncology 2010;97(1):109‐15. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Khasraw M, Ashley D, Wheeler G, Berk M. Using lithium as a neuroprotective agent in patients with cancer. BMC Medicine 2012;10(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Heimans JJ, Ploeg HM, Grit J, Muller M, Postma TJ, et al. Effect of radiotherapy and other treatment‐related factors on mid‐term to long‐term cognitive sequelae in low‐grade gliomas: a comparative study. Lancet 2002;360(9343):1361‐8. [DOI] [PubMed] [Google Scholar]
- Lee AWM, Kwong DLW, Leung SF, Tung SY, Sze WM, Sham JST, et al. Factors affecting risk of symptomatic temporal lobe necrosis: significance of fractional dose and treatment time. International Journal of Radiation Oncology* Biology* Physics 2002;53(1):75‐85. [DOI: 10.1016/S0360-3016(02)02711-6] [DOI] [PubMed] [Google Scholar]
- Lidstone V, Butters E, Seed PT, Sinnott C, Beynon T, Richards M. Symptoms and concerns amongst cancer outpatients: identifying the need for specialist palliative care. Palliative Medicine 2003;17(7):588‐95. [DOI] [PubMed] [Google Scholar]
- Lyubimova N, Hopewell JW. Experimental evidence to support the hypothesis that damage to vascular endothelium plays the primary role in the development of late radiation‐induced CNS injury. British Journal of Radiology 2004;77(918):488‐92. [PUBMED: 15151969] [DOI] [PubMed] [Google Scholar]
- Machado‐Vieira R, Andreazza AC, Viale CI, Zanatto V, Cereser V, Vargas RDS, et al. Oxidative stress parameters in unmedicated and treated bipolar subjects during initial manic episode: a possible role for lithium antioxidant effects. Neuroscience Letters 2007;421(1):33‐6. [DOI] [PubMed] [Google Scholar]
- Malec JF, Kragness M, Evans RW, Finlay KL, Kent A, Lezak M. Further psychometric evaluation and revision of the Mayo‐Portland Adaptability Inventory in a national sample. Journal of Head Trauma Rehabilitation 2003;18(6):479‐92. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Hess KR, Yung WA, Levin VA. Cognitive function as a predictor of survival in patients with recurrent malignant glioma. Journal of Clinical Oncology 2000;18(3):646. [DOI] [PubMed] [Google Scholar]
- Meyers CA, Wefel JS. The use of the Mini‐Mental State Examination to assess cognitive functioning in cancer trials: no ifs, ands, buts, or sensitivity. Journal of Clinical Oncology 2003;21(19):3557‐8. [DOI] [PubMed] [Google Scholar]
- Miotto EC, Savage CR, Evans JJ, Wilson BA, Martin MGM, Balardin JB, et al. Semantic strategy training increases memory performance and brain activity in patients with prefrontal cortex lesions. Clincal Neurology and Neurosurgery 2013;115(3):309‐16. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, Kemp S, Benito‐León J, Reuber M. The influence of cognitive impairment on health‐related quality of life in neurological disease. Acta Neuropsychiatrica 2010;22(1):2‐13. [DOI: 10.1111/j.1601-5215.2009.00439.x] [DOI] [Google Scholar]
- Monje ML, Vogel H, Masek M, Ligon KL, Fisher PG, Palmer TD. Impaired human hippocampal neurogenesis after treatment for central nervous system malignancies. Annals of Neurology 2007;62(5):515‐20. [DOI: 10.1002/ana.21214] [DOI] [PubMed] [Google Scholar]
- Mora F. Successful brain aging: plasticity, environmental enrichment, and lifestyle. Dialogues in Clinical Neuroscience 2013;15(1):45‐52. [PUBMED: PMC3622468] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukand JA, Blackinton DD, Crincoli MG, Lee JJ, Santos BB. Incidence of neurologic deficits and rehabilitation of patients with brain tumors. American Journal of Physical Medicine & Rehabilitation 2001;80(5):346‐50. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. The Lancet Oncology 2004;5(7):399‐408. [DOI] [PubMed] [Google Scholar]
- Palmer SL, Reddick WE, Glass JO, Gajjar A, Goloubeva O, Mulhern RK. Decline in corpus callosum volume among pediatric patients with medulloblastoma: longitudinal MR imaging study. American Journal of Neuroradiology 2002;23(7):1088‐94. [PUBMED: 12169462] [PMC free article] [PubMed] [Google Scholar]
- Peiffer AM, Lyrer CM, Greene‐Schloesser D, Kearns WT, Hinson WH, Tattor SB, et al. Normal tissue complication modeling of the brain: dose‐volume histogram analyses of neurocognitive outcomes of two CCOP trials. Neuro‐Oncology. 2011; Vol. 13:iii73‐5. [DOI: 10.1093/neuonc/nor166] [DOI] [Google Scholar]
- Randolph C. Repeatable battery for the assessment of neuropsychological status (RBANS). Psychological Corporation, San Antonio1998. [DOI] [PubMed]
- Reddick WE, Shan ZY, Glass JO, Helton S, Xiong X, Wu S, et al. Smaller white‐matter volumes are associated with larger deficits in attention and learning among long‐term survivors of acute lymphoblastic leukemia. Cancer 2006;106(4):941‐9. [PUBMED: 16411228] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan RM. Trail making test manual for administration and scoring. Reitan Neuropsychology Laboratory, Tucson1989.
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Robinson DM, Keating GM. Memantine: a review of its use in Alzheimer's disease. Drugs 2006;66(11):1515‐34. [PUBMED: 16906789] [DOI] [PubMed] [Google Scholar]
- Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. International Journal of Radiation Oncology* Biology* Physics 1980;6(9):1215‐28. [DOI] [PubMed] [Google Scholar]
- Smith JV, Luo Y. Studies on molecular mechanisms of Ginkgo biloba extract. Applied Microbiology and Biotechnology 2004;64(4):465‐72. [DOI: 10.1007/s00253-003-1527-9] [DOI] [PubMed] [Google Scholar]
- Steinberg M, Lyketsos CG. Pharmacological therapies in Alzheimer's Disease. In: Abou‐Saleh MT, Katona C, Kumar A editor(s). Principles and Practice of Geriatric Psychiatry. 3rd Edition. Oxford: John Wiley & Sons, 2010. [DOI: 10.1002/9780470669600.ch52] [DOI] [Google Scholar]
- Szerlip N, Rutter C, Ram N, Yovino S, Kwok Y, Maggio W, et al. Factors impacting volumetric white matter changes following whole brain radiation therapy. Journal of Neuro‐Oncology 2011;103(1):111‐9. [PUBMED: 20725847] [DOI] [PubMed] [Google Scholar]
- Tallet A, Dhermain F, Taillia H, Ricard D, Mornex F, Métellus P. Cognition and radiation therapy for brain metastases: a new paradigm to define [Cognition et radiothérapie dans les métastases cérébrales: un nouveau paradigme à définir]. Bulletin du Cancer 2013;100(1):63‐9. [DOI: 10.1684/bdc.2012.1682] [DOI] [PubMed] [Google Scholar]
- Taphoorn MJB. Neurocognitive sequelae in the treatment of low‐grade gliomas. Seminars in Oncology 2003;30(6):45‐8. [PUBMED: 14765385] [DOI] [PubMed] [Google Scholar]
- Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumours. The Lancet Neurology 2004;3(3):159‐68. [DOI] [PubMed] [Google Scholar]
- Torcuator R, Zuniga R, Mohan YS, Rock J, Doyle T, Anderson J, et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. Journal of Neuro‐Oncology 2009;94(1):63‐8. [DOI] [PubMed] [Google Scholar]
- Maazen RWM, Kleiboer BJ, Verhagen I, Kogel AJ. Repair capacity of adult rat glial progenitor cells determined by an in vitro clonogenic assay after in vitro or in vivo fractionated irradiation. International Journal of Radiation Biology 1993;63(5):661‐6. [PUBMED: 8099113] [DOI] [PubMed] [Google Scholar]
- Vigliani MC, Sichez N, Poisson M, Delatrre JY. A prospective study of cognitive functions following conventional radiotherapy for supratentorial gliomas in young adults: 4‐year results. International Journal of Radiation Oncology* Biology* Physics 1996;35(3):527‐33. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang G, Ding Y, Gatley SJ. Mechanism of action of methylphenidate: insights from PET imaging studies. Journal of Attention Disorders 2002;6:S31‐43. [DOI] [PubMed] [Google Scholar]
- Wang Y, Pan L, Sheng X, Mao Y, Yao Y, Wany E, et al. Reversal of cerebral radiation necrosis with bevacizumab treatment in 17 Chinese patients. European Journal of Medical Research 2012;17(1):25. [DOI: 10.1186/2047-783X-17-25] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler. Wechsler Adult Intelligence Scale‐Revised. Psychological Corporation, San Antonio1981.
- Wefel JS, Vardy J, Ahles T, Schagen SB. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. The Lancet Oncology 2011;12(7):703‐8. [DOI: 10.1016/S1470-2045(10)70294-1] [DOI] [PubMed] [Google Scholar]
- Welzel G, Fleckenstein K, Schaefer J, Hermann B, Kraus‐Tiefenbacher U, Mai SK, et al. Memory function before and after whole brain radiotherapy in patients with and without brain metastases. International Journal of Radiation Oncology* Biology* Physics 2008;72(5):1311‐8. [DOI: 10.1016/j.ijrobp.2008.03.009] [DOI] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell 2008;132(4):645‐60. [DOI] [PubMed] [Google Scholar]
- Zucchella C, Capone A, Codella V, Nunzio AM, Vecchione C, Sandrini G, et al. Cognitive rehabilitation for early post‐surgery inpatients affected by primary brain tumor: a randomized, controlled trial. Journal of Neuro‐Oncology 2013;114(1):93‐100. [DOI] [PubMed] [Google Scholar]


