Abstract
Background
Air travel might increase the risk of deep vein thrombosis (DVT). It has been suggested that wearing compression stockings might reduce this risk. This is an update of the review first published in 2006.
Objectives
To assess the effects of wearing compression stockings versus not wearing them for preventing DVT in people travelling on flights lasting at least four hours.
Search methods
For this update the Cochrane Vascular Information Specialist (CIS) searched the Specialised Register (10 February 2016). In addition, the CIS searched the Cochrane Register of Studies (CENTRAL (2016, Issue 1)).
Selection criteria
Randomised trials of compression stockings versus no stockings in passengers on flights lasting at least four hours. Trials in which passengers wore a stocking on one leg but not the other, or those comparing stockings and another intervention were also eligible.
Data collection and analysis
Two review authors independently selected trials for inclusion and extracted data. We sought additional information from trialists where necessary.
Main results
One new study that fulfilled the inclusion criteria was identified for this update. Eleven randomised trials (n = 2906) were included in this review: nine (n = 2821) compared wearing graduated compression stockings on both legs versus not wearing them; one trial (n = 50) compared wearing graduated compression tights versus not wearing them; and one trial (n = 35) compared wearing a graduated compression stocking on one leg for the outbound flight and on the other leg on the return flight. Eight trials included people judged to be at low or medium risk of developing DVT (n = 1598) and two included high‐risk participants (n = 1273). All flights had a duration of more than five hours.
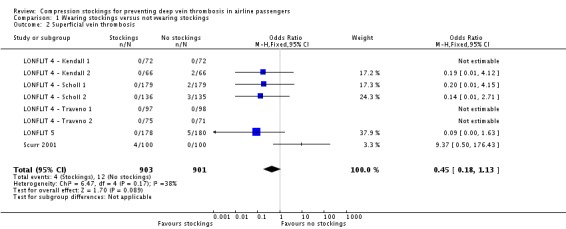
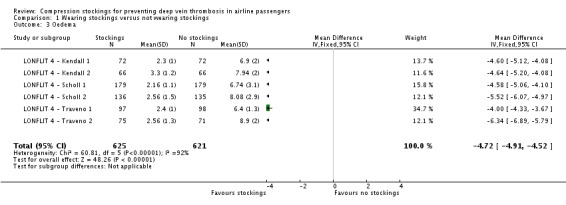
Fifty of 2637 participants with follow‐up data available in the trials of wearing compression stockings on both legs had a symptomless DVT; three wore stockings, 47 did not (odds ratio (OR) 0.10, 95% confidence interval (CI) 0.04 to 0.25, P < 0.001; high‐quality evidence). There were no symptomless DVTs in three trials. Sixteen of 1804 people developed superficial vein thrombosis, four wore stockings, 12 did not (OR 0.45, 95% CI 0.18 to 1.13, P = 0.09; moderate‐quality evidence). No deaths, pulmonary emboli or symptomatic DVTs were reported. Wearing stockings had a significant impact in reducing oedema (mean difference (MD) −4.72, 95% CI −4.91 to −4.52; based on six trials; low‐quality evidence). A further two trials showed reduced oedema in the stockings group but could not be included in the meta‐analysis as they used different methods to measure oedema. No significant adverse effects were reported.
Authors' conclusions
There is high‐quality evidence that airline passengers similar to those in this review can expect a substantial reduction in the incidence of symptomless DVT and low‐quality evidence that leg oedema is reduced if they wear compression stockings. Quality was limited by the way that oedema was measured. There is moderate‐quality evidence that superficial vein thrombosis may be reduced if passengers wear compression stockings. We cannot assess the effect of wearing stockings on death, pulmonary embolism or symptomatic DVT because no such events occurred in these trials. Randomised trials to assess these outcomes would need to include a very large number of people.
Keywords: Humans, Aircraft, Bandages, Travel, Aerospace Medicine, Edema, Edema/etiology, Randomized Controlled Trials as Topic, Venous Thrombosis, Venous Thrombosis/prevention & control
Compression stockings for preventing deep vein thrombosis (DVT) in airline passengers
Background
In the last few years, there has been increasing interest in whether compression stockings (or 'flight socks') reduce the risk of deep vein thrombosis (DVT; blood clots in the legs) and other circulatory problems in airline passengers. The stockings are worn throughout the flight and are similar to those known to be effective in patients lying in bed after an operation. By applying a gentle pressure, to the ankle in particular, compression stockings help blood to flow. Pressure combined with leg movement helps blood in superficial (surface) veins to move to the deep veins and back to the heart. The blood is then less likely to clot in the deep veins, which could be fatal if the clot moves to the lungs.
Study characteristics and key results
This review included eleven trials (2906 participants) and we were able to combine the data from nine trials with a total of 2637 participants (current to February 2016). Almost half of the participants were randomly assigned to wearing stockings for a flight lasting at least five hours while the other half did not wear stockings.
None of the passengers developed a DVT with symptoms (slowly developing leg pain, swelling and increased temperature) and no serious events (a blood clot in their lungs (pulmonary embolism) or dying) were reported. Passengers were carefully assessed after the flight to detect any problems with the circulation of blood in their legs, even if they had not noticed any problems themselves. Wearing compression stockings resulted in a large reduction in symptomless DVT among airline passengers who were allocated to wear compression stockings compared to those allocated not to wear compression stockings. This difference in symptomless DVT between the two groups is equivalent to a reduction in the risk from a few tens per thousand passengers to two or three per thousand. People who wore stockings had less swelling in their legs (oedema) than those who did not wear them. Fewer passengers developed superficial vein thrombosis when wearing compression stockings than those not wearing stockings. Not all the trials reported on possible problems with wearing stockings but in those that did, the researchers said that the stockings were well‐tolerated, without any problems.
Quality of the evidence
High‐quality evidence shows that airline passengers wearing compression stockings develop less symptomless DVT and low‐quality evidence shows that leg swelling is reduced when compared to not wearing compression stockings. Quality of the evidence was limited by the way that swelling was measured. There is moderate‐quality evidence that superficial vein thrombosis may be reduced in passengers who wear compression stockings. We cannot assess the effect of wearing stockings on death, pulmonary embolism or symptomatic DVT because no such events occurred in these trials. Randomised trials to assess these outcomes would need to include a very large number of people.
Summary of findings
Summary of findings for the main comparison.
Compression stockings compared with no compression stockings for people taking long haul flights
| Does wearing compression stockings prevent deep vein thrombosis in people taking long haul flights? | ||||||
| Patient or population: passengers on a long haul flight (more than 4 hours) Setting: long haul flights Intervention: wearing compression stockings1 Comparison: not wearing stockings | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with not wearing compression stockings | Risk with wearing compression stockings | |||||
| Symptomatic deep vein thrombosis (DVT) Follow‐up period immediately post flight to 48 hours |
0 participants developed symptomatic DVT in these studies | Not estimable | 2821 (9 studies) |
Not estimable² | ||
| Symptomless DVT Follow‐up period immediately post flight to 48 hours |
Low‐risk population3 | OR 0.10 (0.04 to 0.25) | 2637 (9 RCTs) | ⊕⊕⊕⊕ HIGH | ||
| 10 per 1000 | 1 per 1000 (0 to 3) | |||||
| High‐risk population2 | ||||||
| 30 per 1000 | 3 per 1000 (1 to 8) | |||||
| Pulmonary embolism (PE) Follow‐up period immediately post flight to 48 hours |
0 participants developed symptomatic PE in these studies | Not estimable | 2821 (9 studies) |
Not estimable2 | ||
| Death Follow‐up period immediately post flight to 48 hours |
0 participants died in these studies | Not estimable | 2821 (9 studies) |
Not estimable2 | ||
| Superficial vein thrombosis Follow‐up period immediately post flight to 48 hours |
Study population | OR 0.45 (0.18 to 1.13) | 1804 (8 RCTs) | ⊕⊕⊕⊝ MODERATE4 | ||
| 13 per 1000 | 6 per 1000 (2 to 15) | |||||
| Oedema Follow‐up period immediately post flight Post flight values measured on a scale from 0 (no oedema) to 10 (maximum oedema) |
The mean oedema score ranged across control groups from 6 to 9 |
The mean oedema score in the intervention groups was on average 4.7 lower (4.9 lower to 4.5 lower) | ‐ | 1246 (6 RCTs) | ⊕⊕⊝⊝ LOW5 | It was not possible to pool data from an additional 2 studies (Hagan 2008; Loew 1998). These both reported reduced oedema post flight in the stocking group6 |
| Adverse effects arising from the use of compression stockings Follow‐up period immediately post flight |
The tolerability of the stockings was described as very good with no complaints of side effects in 4 studies | Not estimable | 1182 (4 studies) |
Not estimable | None of the trials reported adverse effects, apart from 4 cases of superficial vein thrombosis in varicose veins in the knee region that were compressed by the upper edge of the stocking in 1 trial. However, the meta‐analysis of the data on this outcome from this trial and 7 others found a non‐statistically significant difference (see above) | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; DVT: deep vein thrombosis; PE: pulmonary embolism; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Stockings in the nine trials included in the meta‐analysis were below‐knee compression stockings. In four trials the compression strength was 20 to 30 mmHg at the ankle. It was 10 to 20 mmHg in the other four trials. One trial not included in the meta‐analysis used graduated compression tights. See Characteristics of included studies for details.
2 If there are very few or no events and the number of participants is large, judgement about the quality of evidence (particularly judgements about precision) may be based on the absolute effect. Here the quality rating may be considered 'high' if the outcome was appropriately assessed and the event, in fact, did not occur in 2821 studied participants.
3 Two trials recruited high‐risk participants defined as those with previous episodes of DVT, coagulation disorders, severe obesity, limited mobility due to bone or joint problems, neoplastic disease within the previous two years, large varicose veins or, in one of the studies, participants taller than 190 cm and heavier than 90 kg. The incidence for seven trials that excluded high‐risk participants was 1.45% and the incidence for the two trials that recruited high‐risk participants (with at least one risk factor) was 2.43%. We have rounded these off to 10 and 30 per 1000 respectively.
4 Downgraded by one level ‐ the confidence interval crosses no difference and does not rule out a small increase.
5 Downgraded by two levels ‐ the measurement of oedema was not validated or blinded to the intervention. All of these studies included in the meta‐analysis were conducted by the same investigators.
6 In Hagan 2008, oedema was measured by calculating the change in ankle circumference before and after landing; stockings versus no stockings. Loew 1998 used a clinical scale and between‐individual comparison as the passengers wore a stocking on one leg only.
Background
Description of the condition
Deep vein thrombosis (DVT) occurs where there is a partial or total blockage of the deep venous system of the body by blood clot, usually in the legs. The symptoms of DVT do not usually develop immediately and diagnosis can be problematic (Anand 1998). However, typical signs and symptoms of DVT, and associated superficial thrombophlebitis (tenderness caused by inflamed veins), may include redness of the lower legs, a swollen or painful calf or thigh, fever and discolouration of the skin over the affected area. If left untreated, people with DVT are at risk of developing a pulmonary embolism (when part of the clot/thrombus breaks away and lodges in the lungs), which can be fatal. In a review of medical records, for a population‐based inception cohort of 2218 patients in Minnesota who had a DVT or pulmonary embolism between 1966 and 1990, Silverstein et al concluded that the annual incidence of DVT was 48 per 100,000 people and the figure for pulmonary embolism was 69 per 100,000 (Silverstein 1998). More recent articles report incidence rates for leg DVT alone ranging from 45 to 117 per 100,000 person‐years (Cannegieter 2006; Heit 2016; Tagalakis 2013).
Venous thrombosis related to air travel was first reported in 1954 in a 54‐year old doctor, who developed DVT following a 14‐hour flight (Homans 1954). In 1977, Symington called this condition 'economy class syndrome', believing that prolonged sitting in confined conditions was a major factor in developing venous thrombosis (Symington 1977). Recent publications report the risk of venous thromboembolism (VTE) is increased 1.5 to 3 fold (Timp 2015), by approximately two fold (Cannegieter 2006; WRIGHT project 2007), or four fold (Kuipers 2007), following long‐haul travel. Kuipers and colleagues also reported that the "absolute risk of a symptomatic event within 4 weeks of flights longer than 4 h is 1/4600 flights" and "the risk of severe pulmonary embolism (PE) occurring immediately after air travel increases with duration of travel, up to 4.8 per million in flights longer than 12 h" (Kuipers 2007).
A number of factors have been suggested as possible causes of any increase in the risk of developing DVT when flying (Adi 2003). These can be classified as:
travel‐related: for example, prolonged immobilisation in narrow economy class seats with limited leg room, insufficient fluid intake, dehydration and low humidity (Giangrande 2001; Milne 1992); coagulation activation caused by immobilisation (Kuipers 2007),
person‐related: for example, hereditary or acquired prothrombotic clotting disorders, previous DVT, older age, recent surgery or trauma (particularly orthopaedic), cancer, smoking, pregnancy, chronic heart disease, obesity, extremes of height and use of oral contraceptives (Bihari 2001; Cannegieter 2006; Geroulakos 2001; Landgraf 1999).
Description of the intervention
It has been suggested that the use of compression stockings during long‐haul flights may help to reduce the risk of developing DVT. It has also been suggested that standing up or walking around occasionally in flight, drinking plenty of water and performing leg‐stretching exercises may also help to reduce a person's risk (Geroulakos 2001). Aspirin and low‐dose heparin have also been suggested as preventative strategies (Giangrande 2001). Another Cochrane review examines the effects of graduated compression stockings in patients at risk of developing DVT in hospitalised patients (Sachdeva 2014). The review analysed 19 randomised trials and showed that graduated compression stockings are effective in reducing the risk of developing DVT in hospitalised patients. Furthermore, a review of observational studies on the relationship between air travel and DVT also included a systematic review of randomised trials to prevent DVT. The reviewers did their search in September 2002 (Adi 2003) and found two randomised trials of wearing versus not wearing compression stockings (LONFLIT 2; Scurr 2001).
How the intervention might work
Compression stockings are thought to reduce the risk of DVT by exerting graduated pressure on the leg, with the pressure being greatest at the ankle. This, when combined with muscular activity in the limb, is thought to displace blood from the superficial venous system to the deep venous system. This, in turn, reduces blood stasis that can lead to clotting and increases the velocity and volume of blood flow in the deep venous system, thereby potentially preventing thrombosis (Sachdeva 2014).
Why it is important to do this review
There has been increased research interest in the issue of DVT in airline passengers in recent years. For example, as well as the review by Adi 2003 and Adi 2004 and another by Ansari 2005, the World Health Organization announced the launch of a research programme to investigate the relationship between air travel and venous thrombosis in May 2002 (WHO 2002). The findings of this report show that the increased risk of VTE observed in passengers on long–haul flights is due to extended periods of immobility. As the number of people taking long‐haul flights is increasing, and as these passengers will have known or unknown thrombosis risk factors, they concluded that "air travel–related VTE is an important public health issue" (WRIGHT project 2007).
Objectives
To assess the effects of wearing compression stockings versus not wearing them for preventing DVT in people travelling on flights lasting at least four hours.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials that compare people wearing compression stockings versus people not wearing them during flights lasting at least four hours. Randomised trials in which people wore a stocking on one leg and not the other are included for any leg‐specific outcomes but are not eligible for any meta‐analyses of outcomes (such as pulmonary embolism (PE) and death). This is because it would not be possible to know which leg had contributed to the particular outcome.
Types of participants
Any passenger on a flight of more than four hours' continuous duration. This includes people of both sexes, all ages, all risk factors, irrespective of any other interventions they may have used for the prevention of deep vein thrombosis (DVT). Flights in any direction are eligible, as are both daytime and night‐time flights.
Types of interventions
The primary analyses are of unconfounded randomised trials in which the only difference between the groups is the allocation to wear, or not wear, compression stockings. This includes trials in which no other form of prevention was used, and trials in which other forms of prevention were available equally to both groups. If confounded randomised trials, in which some people were allocated to wear stockings and other people were allocated to an alternative form of prevention, had been found (none were), subsidiary analyses would have been performed for these.
Types of outcome measures
Primary outcomes
Diagnosis of symptomatic or symptomless DVT (by ultrasound, venogram or isotope)
Secondary outcomes
Diagnosis of pulmonary embolism (by ventilation perfusion lung scan, pulmonary angiogram, spiral computed tomography (CT) scanning, or postmortem examination)
Death
Superficial vein thrombosis
Oedema
Adverse effects arising from the use of compression stockings
Search methods for identification of studies
Electronic searches
For this update the Cochrane Vascular Information Specialist (CIS) searched the Specialised Register (10 February 2016). In addition the CIS searched the Cochrane Register of Studies (CRS) www.metaxis.com/CRSWeb/Index.asp (CENTRAL (2016, Issue 1)). See Appendix 1 for details of the search strategy used to search the CRS. The Specialised Register is maintained by the CIS and is constructed from weekly electronic searches of MEDLINE, Embase, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Vascular module in the Cochrane Library (www.cochranelibrary.com).
The following trial databases were searched by the CIS (10 February 2016) for details of ongoing and unpublished studies:
World Health Organization International Clinical Trials Registry (apps.who.int/trialsearch/)
ClinicalTrials.gov (clinicaltrials.gov/)
ISRCTN Register (www.isrctn.com/)
See Appendix 2 for details of the search strategy used.
Searching other resources
We checked the bibliographies of relevant studies and reviews identified by the search to check for any additional trials. We also checked the website of the World Health Organization (WHO) (www.who.int/), for information on the ongoing WHO Research into Global Hazards of Travel project (WRIGHT project 2007).
Data collection and analysis
Selection of studies
For this update, two review authors (CB and MC) screened the titles and abstracts of all retrieved records to identify obvious exclusions (i.e. records that were found by our electronic searches but were clearly irrelevant to this review). We obtained full copies for reports that might relate to eligible studies and two review authors (CB and MC) assessed these to determine if they met the inclusion criteria for the review. CB and MC resolved any disagreements through discussion.
Data extraction and management
Using a pre‐specified data extraction form, CB performed data extraction and MC confirmed this independently. Information extracted included:
descriptive data for the people in the trial (including age, sex and all reported risk factors);
details of the interventions (including type of compression stocking; the duration, direction and time of day of the flight; and any additional interventions for the prevention of deep vein thrombosis or confounding interventions);
outcomes, as described in Types of outcome measures above.
Assessment of risk of bias in included studies
One review author (CB) and a member of the Cochrane Vascular editorial base (MS) independently evaluated all included studies for quality, using Cochrane's tool for assessing risk of bias (Higgins 2011). This tool provides judgements made on six domains, which include randomisation sequence generation, allocation concealment methods, blinding (participants, personnel and outcome assessors), incomplete outcome data, selective outcome reporting, and any other relevant biases. The two investigators performed evaluations of low risk, unclear risk, or high risk for each domain for every included study and resolved any disagreements through discussion.
Measures of treatment effect
For dichotomous outcomes we calculated odds ratios (ORs) with 95% confidence intervals (CIs). For continuous data, we planned a meta‐analysis using mean differences (MD) with standard deviations (SDs) and 95% CIs.
Unit of analysis issues
The unit of analysis for this review was the individual participant. One trial randomly allocated passengers to wear a stocking on one leg during an outward flight and on the other leg on the return journey. Only leg‐specific outcome data were used in this case.
Dealing with missing data
We based analysis on an intention‐to‐treat basis and therefore all randomised participants of interest from the included studies were to be included in the analysis. Where data were missing, we investigated if these were described within the reports and if evenly distributed between the groups.
Assessment of heterogeneity
Statistical heterogeneity was assessed both visually within the forest plots and by using the I² statistic as described in Chapter 9 of Higgins 2011. Heterogeneity was taken to be substantial with an I² value of 50% to 75% and considerable with an I² value of 75% or more.
Assessment of reporting biases
If sufficient trials had been identified we intended to assess reporting bias using a funnel plot. As fewer than 10 studies reported on any one outcome this was not possible for this 2016 update.
Data synthesis
The decision about whether or not to combine the results of individual studies depended on an assessment of heterogeneity. The studies were assessed for homogeneity of study design and when they were judged to be sufficiently homogeneous in their design, a meta‐analysis was carried out and the statistical heterogeneity was assessed. The preferred statistical analysis was the OR and the fixed‐effect model, using the statistical software in Cochrane's Review Manager software (RevMan), but other analyses were considered in the light of the very low event rates in the included studies. If considerable heterogeneity was detected we planned to use the random‐effects model.
As noted above, unconfounded trials (i.e. allocation to wearing stockings versus not wearing them with no other differences between the groups) would have been analysed separately from confounded trials (i.e. allocation to wearing stockings versus another intervention), if any of the latter had been found (none were found).
Subgroup analysis and investigation of heterogeneity
If sufficient data had been available, subgroup analyses would have been performed separating participants into different groups on the basis of risk of DVT and into different time periods following the flight, with the main focus of the analyses expected to be the period within one week of the flight. However, no data were available from the trials for outcomes measured beyond this period of follow‐up.
Sensitivity analysis
We carried out sensitivity analysis using both RevMan and Stata, to determine if the results of the meta‐analysis were robust due to the small or low overall event rate in some of the included trials.
Summary of findings
A table summarising the best evidence of relevant outcomes was constructed for comparison of compression stockings versus not wearing compression stockings. Study populations consisting of passengers at low or medium risk and high risk of developing DVTs were considered. The most important and clinically relevant outcomes (both desirable and undesirable) that were thought to be essential for decision‐making were selected for the Table 1. These are described in the Types of outcome measures and include symptomatic and symptomless DVT; PE; death; superficial vein thrombosis; oedema and adverse effects from the use of compression stockings. Assumed control intervention risks were calculated by the mean number of events in the control groups of the selected studies for each outcome. The system developed by the GRADE working group was used for grading the quality of evidence as high, moderate, low and very low, based on within‐study risk of bias, directness of evidence, heterogeneity, precision of effects estimates, and risk of population bias (GRADE 2004). We used GRADEpro (gradepro.org) software to create the 'Summary of findings' table.
Results
Description of studies
Results of the search
See Figure 1.
Figure 1.
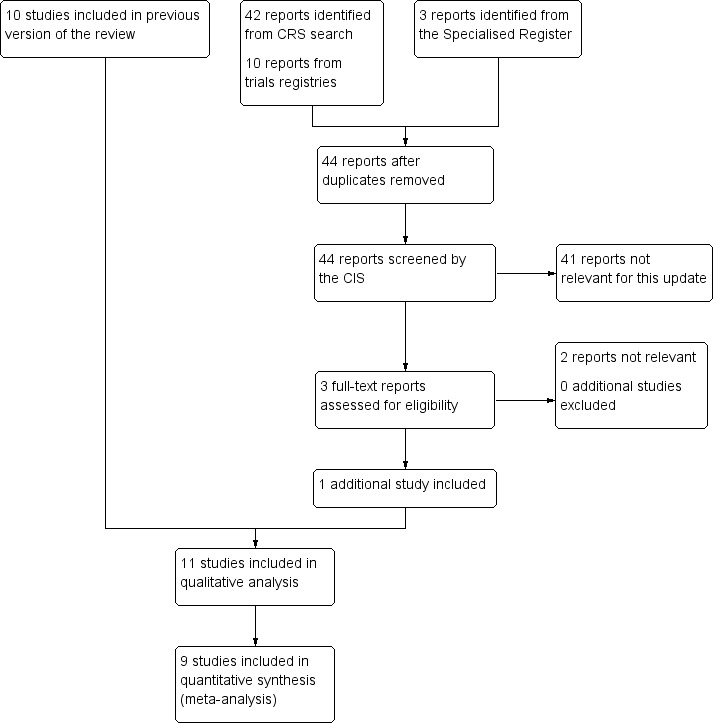
Study flow diagram.
Included studies
For this update we included one additional study: Hagan 2008. Therefore in total we identified 11 randomised controlled trials with a combined total of 2906 participants that were eligible for this review (Hagan 2008; Loew 1998; LONFLIT 2; LONFLIT 4 ‐ Kendall 1; LONFLIT 4 ‐ Kendall 2; LONFLIT 4 ‐ Scholl 1; LONFLIT 4 ‐ Scholl 2; LONFLIT 4 ‐ Traveno 1; LONFLIT 4 ‐ Traveno 2; LONFLIT 5; Scurr 2001).
There were 10 unconfounded trials in which participants were allocated to either wear stockings on both legs or neither (Hagan 2008; LONFLIT 2; LONFLIT 4 ‐ Kendall 1; LONFLIT 4 ‐ Kendall 2; LONFLIT 4 ‐ Scholl 1; LONFLIT 4 ‐ Scholl 2; LONFLIT 4 ‐ Traveno 1; LONFLIT 4 ‐ Traveno 2; LONFLIT 5; Scurr 2001). Eight of these trials were part of the LONFLIT series of studies into the incidence and prevention of DVT in air travel. These studies were done by an international group of researchers, with investigators in Pescara (Italy), London and Melbourne; further details about the group are available in the articles referenced in this review.
Six of the studies were different parts of LONFLIT 4 (LONFLIT 4 ‐ Kendall 1; LONFLIT 4 ‐ Kendall 2; LONFLIT 4 ‐ Scholl 1; LONFLIT 4 ‐ Scholl 2; LONFLIT 4 ‐ Traveno 1; LONFLIT 4 ‐ Traveno 2). The other four trials were LONFLIT 2, LONFLIT 5 and the trials by Scurr 2001 and Hagan 2008. See the Characteristics of included studies for details.
A total of 2871 participants were randomised in the 10 unconfounded trials identified. Eight of the trials recruited a total of 1598 participants who were judged to be at low or medium risk of a DVT (Hagan 2008; LONFLIT 4 ‐ Kendall 1; LONFLIT 4 ‐ Kendall 2; LONFLIT 4 ‐ Scholl 1; LONFLIT 4 ‐ Scholl 2; LONFLIT 4 ‐ Traveno 1; LONFLIT 4 ‐ Traveno 2; Scurr 2001). These studies excluded passengers with previous episodes of DVT, coagulation disorders, limited mobility due to bone or joint problems, neoplastic disease, varicose veins or participants taller than 190 cm and heavier than 90 kg, those advised to wear graduated compression tights in flight, or on medication for cardiovascular disease, diabetes or hypertension. The other two trials recruited a total of 1273 high‐risk participants (LONFLIT 2; LONFLIT 5). LONFLIT 5 excluded participants taller than 190 cm, or weighing more than 90 kg, or having recent/presence of thrombosis, severe bone, joint, or mobility problems, severe hypertension, or clinical disease requiring treatment. The exclusion criteria of LONFLIT 2 were not clear. The LONFLIT trials were reported to have been conducted during 2001 to 2003. The Scurr trial was described as ongoing in a report published in early 2001 (Scurr 2001a); but the actual start and finish dates for recruitment were not reported (Scurr 2001b). Hagan 2008 was conducted between April and October 2006. In all the trials the flight duration was at least five hours and passengers allocated to wear stockings were told to wear these for the duration of the flight. In the LONFLIT 2 trial, participants were advised to put the stockings on 6 to 10 hours before the flight. In the other trials they were advised to put them on within a few hours before the flight.
All unconfounded trials, except Hagan 2008, assessed incidence of symptomless DVT within a few days of the flight but information on the assessment of symptomatic DVT, pulmonary embolism and death is not reported for all of them. Symptomless DVT was assessed by ultrasound (LONFLIT 2; LONFLIT 4 ‐ Kendall 1; LONFLIT 4 ‐ Kendall 2; LONFLIT 4 ‐ Scholl 1; LONFLIT 4 ‐ Scholl 2; LONFLIT 4 ‐ Traveno 1; LONFLIT 4 ‐ Traveno 2; Scurr 2001) or D‐dimer testing and fibrinogen tests (LONFLIT 5).
Nine trials used below‐knee compression stockings (LONFLIT 2; LONFLIT 4 ‐ Kendall 1; LONFLIT 4 ‐ Kendall 2; LONFLIT 4 ‐ Scholl 1; LONFLIT 4 ‐ Scholl 2; LONFLIT 4 ‐ Traveno 1; LONFLIT 4 ‐ Traveno 2; LONFLIT 5; Scurr 2001). In four trials the compression strength was 20 to 30 mm Hg at the ankle (LONFLIT 2; LONFLIT 4 ‐ Kendall 1; LONFLIT 4 ‐ Kendall 2; Scurr 2001). The compression strength was 10 to 20 mmHg in a further five trials (LONFLIT 4 ‐ Scholl 1; LONFLIT 4 ‐ Scholl 2; LONFLIT 4 ‐ Traveno 1; LONFLIT 4 ‐ Traveno 2; LONFLIT 5). Hagan 2008 used full leg length stockings (tights) with compression strength of about 5 mmHg at the ankle, 17 to 20 mmHg at the calf, 10 mmHg above the knee and 4 mmHg at the buttocks.
In addition to the 10 unconfounded trials, we found one trial (n = 35) in which participants were randomly allocated to wear a stocking on one leg during an outward flight and on the other leg on the return journey. This trial used class II compression stockings, with the flights lasting approximately 14.5 hours. Participants were also randomised to receive dried vine leaves (Antistax) versus diuretics versus no drugs. However, owing to the strong effect of the diuretics on the outward flight, these were not used on the return journey and results of the effects of the stocking on the return flight were reported for the nine patients this affected (Loew 1998).
We also identified two conference abstracts for studies from the LONFLIT group which reported on research involving 420 high‐risk participants comparing stockings versus low molecular weight heparin versus control (Belcaro 2002a), and 400 high‐risk participants comparing aspirin versus low molecular weight heparin versus low molecular weight heparin and stockings versus control (Belcaro 2002b). However, correspondence with the first author of these abstracts, Gianni Belcaro, in 2005 suggests that the relevant data from these comparisons have been used within our analyses for the LONFLIT trials described above.
Excluded studies
No additional studies were excluded for the 2016 update. One trial was excluded previously as it was not randomised (Iwama 2002).
Risk of bias in included studies
Figure 2.
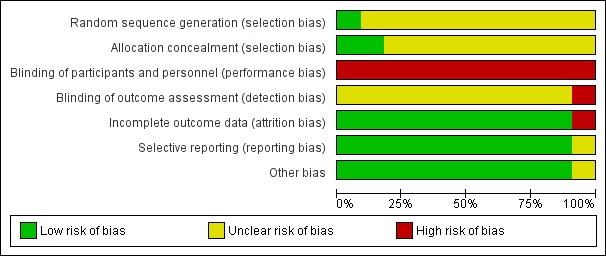
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Figure 3.
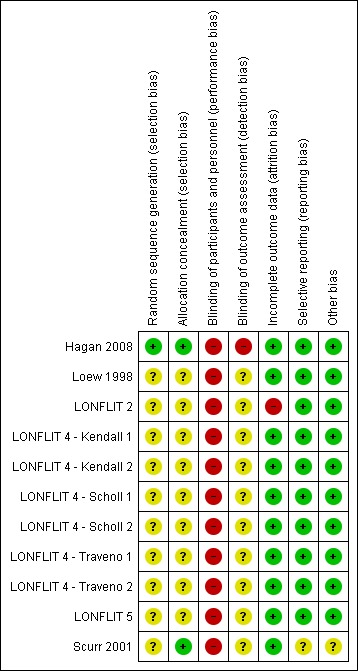
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
All 11 trials were described as randomised but only Hagan 2008 provided sufficient information to be judged as being at low risk of selection bias (Hagan 2008; Loew 1998; LONFLIT 2; LONFLIT 4 ‐ Kendall 1; LONFLIT 4 ‐ Kendall 2; LONFLIT 4 ‐ Scholl 1; LONFLIT 4 ‐ Scholl 2; LONFLIT 4 ‐ Traveno 1; LONFLIT 4 ‐ Traveno 2; LONFLIT 5; Scurr 2001). All 11 trials were at high risk of bias as they did not blind the participants to which group they had been randomised. All trials reported some losses to follow‐up mostly due to poor compliance or flight connection problems.
Allocation
Only Hagan 2008 was judged as being at low risk of bias for random sequence generation and allocation concealment. Scurr 2001 was judged as being at low risk of allocation concealment but did not provide adequate information on the randomisation method used. All the remaining trials provided insufficient information on these domains and were therefore assessed as being at unclear risk of selection bias (Loew 1998; LONFLIT 2; LONFLIT 4 ‐ Kendall 1; LONFLIT 4 ‐ Kendall 2; LONFLIT 4 ‐ Scholl 1; LONFLIT 4 ‐ Scholl 2; LONFLIT 4 ‐ Traveno 1; LONFLIT 4 ‐ Traveno 2; LONFLIT 5).
Blinding
Given the intervention was to wear or not wear a compression stocking or stockings it was not possible to blind the passengers. All studies were assessed as being at high risk of performance bias given the subjective nature of some of the outcomes. Blinding of outcome assessment was not described in eight studies. Scurr 2001 described adequate blinding techniques for DVT detection only and so was assessed as being at low risk of detection bias for this outcome, but unclear overall. Hagan 2008 was at high risk of detection bias as outcomes were self‐reported. The remaining nine trials did not describe outcome assessment in sufficient detail and so were at an unclear risk of detection bias (Loew 1998; LONFLIT 2; LONFLIT 4 ‐ Kendall 1; LONFLIT 4 ‐ Kendall 2; LONFLIT 4 ‐ Scholl 1; LONFLIT 4 ‐ Scholl 2; LONFLIT 4 ‐ Traveno 1; LONFLIT 4 ‐ Traveno 2; LONFLIT 5).
Incomplete outcome data
See the table 'Characteristics of included studies' for details on losses to follow‐up in each trial. In summary, all trials reported losses to follow‐up, largely due to poor compliance or flight connection problems. Outcome data were typically unavailable for less than 10% of participants, with only LONFLIT 4 ‐ Traveno 2 (19 of 165, 12%) and Scurr 2001 (31 of 231, 13%) reporting higher losses than this in trials of wearing stockings on both legs versus neither but numbers were similar between the treatment and control groups. In LONFLIT 2, 52 of 885 (6%) of participants were lost to follow‐up but it is not clear if these were evenly distributed between the groups, so LONFLIT 2 was given a high risk of bias judgement.
Selective reporting
Ten included studies reported on all the expected outcomes and were judged as being at low risk of reporting bias (Hagan 2008; Loew 1998; LONFLIT 2; LONFLIT 4 ‐ Kendall 1; LONFLIT 4 ‐ Kendall 2; LONFLIT 4 ‐ Scholl 1; LONFLIT 4 ‐ Scholl 2; LONFLIT 4 ‐ Traveno 1; LONFLIT 4 ‐ Traveno 2; LONFLIT 5). Scurr 2001 did not pre‐specify what outcomes they would measure and only reported on DVT. This domain was therefore assessed as being at an unclear risk of bias.
Other potential sources of bias
A gender imbalance between the compression stockings and no compression stockings group (70% female versus 53% respectively) was reported in Scurr 2001. It is not clear if this could affect the outcomes. No other potential sources of bias were identified.
Effects of interventions
See: Table 1
Symptomatic deep vein thrombosis
None of the 2821 participants in the nine trials of wearing compression stockings on both legs versus neither were reported to have developed a symptomatic DVT. Hagan 2008 did not report on this outcome.
Symptomless deep vein thrombosis
Of the 2821 participants randomised into the nine trials of wearing compression stockings on both legs versus not wearing them, follow‐up data were available for 2637 (LONFLIT 2; LONFLIT 4 ‐ Kendall 1; LONFLIT 4 ‐ Kendall 2; LONFLIT 4 ‐ Scholl 1; LONFLIT 4 ‐ Scholl 2; LONFLIT 4 ‐ Traveno 1; LONFLIT 4 ‐ Traveno 2; LONFLIT 5; Scurr 2001). Among these, 50 people were reported to have developed a symptomless DVT, which was detected by the investigations done within the trials, either using ultrasound or D‐dimer testing and fibrinogen tests. Three of these people had been allocated to wear stockings and the remaining 47 people were not wearing stockings.
In three of the nine trials, no symptomless DVTs were found in any of the participants, regardless of whether they wore compression stockings or not (LONFLIT 4 ‐ Kendall 1; LONFLIT 4 ‐ Traveno 1; LONFLIT 4 ‐ Traveno 2). The overall incidence of symptomless DVT was 2.43% in the two trials that recruited high‐risk participants (29 among the 1191 participants with follow‐up, distributed as follows: three in the compression stockings group and 26 in the no compression stockings group) and 1.45% in the seven trials that recruited people judged to be at low or medium risk (21 among the 1446 participants with follow‐up: two in the compression stockings group and 19 in the no compression stockings group).
There was no evidence of statistical heterogeneity among the results of the trials and the combined estimate of the effect of wearing compression stockings versus not wearing them is an OR of 0.10 (95% CI 0.04 to 0.25, P < 0.001, high‐quality evidence). However, because of the very low overall event rate, the fact that some trials had zero events and the fact that some trials had a small number of events in one group and none in the other, we explored the stability of this result depending upon the assumptions made when calculating estimate of effect.
The default method used to calculate an OR in RevMan is the Mantel‐Haenszel method, which adds a continuity correction of 0.5 to groups in which there were no events. This may be too high for the present circumstances. Statistical work on continuity corrections in meta‐analyses of sparse data has concluded that the "Mantel‐Haenszel summary estimates using the alternative continuity correction factors gave the least biased results for all group size imbalances. Logistic regression was virtually unbiased for all scenarios and gave good coverage properties. The Peto method provided unbiased results for balanced treatment groups but bias increased with the ratio of the study arm sizes. The Bayesian fixed‐effect model provided good coverage for all group size imbalances. The two alternative continuity corrections outperformed the constant correction factor in nearly all situations. The inverse variance method performed consistently badly, irrespective of continuity correction used." (Sweeting 2004).
It is not possible to explore this further using the statistical tools available in RevMan so we performed sensitivity analyses using Stata, using the Mantel‐Haenszel method with various small continuity corrections, logistic regression and the Peto method for comparison. These special analyses were done using data from an earlier version of our meta‐analysis that had slightly fewer events and a smaller number of participants from the LONFLIT 5 trial, and an overall estimate of effect of 0.07 (0.02 to 0.22). However, the general finding that the choice of analysis technique makes little important difference to the overall conclusions would still hold. The recalculated ORs using the Mantel‐Haenszel method converged to a steady level of 0.04 (95% confidence interval 0.01 to 0.18) as the continuity correction was diminished to zero. This was identical to the one obtained using logistic regression. The result using the Peto method was 0.15 (95% confidence interval 0.09 to 0.28, P < 0.001).
Pulmonary embolism
None of the 2821 participants in the nine trials of wearing stockings on both legs versus neither were reported to have developed a pulmonary embolism. Hagan 2008 did not report on this outcome.
Death
None of the 2821 participants in the nine trials of wearing stockings on both legs versus neither were reported to have died. Hagan 2008 did not report on this outcome.
Superficial vein thrombosis
Eight trials (1804 participants with follow‐up) assessed superficial vein thrombosis (LONFLIT 4 ‐ Kendall 1; LONFLIT 4 ‐ Kendall 2; LONFLIT 4 ‐ Scholl 1; LONFLIT 4 ‐ Scholl 2; LONFLIT 4 ‐ Traveno 1; LONFLIT 4 ‐ Traveno 2; LONFLIT 5; Scurr 2001). Sixteen people developed superficial vein thrombosis: four in the compression stockings group and 12 in the no compression stockings group. The OR was non‐significant: 0.45 (95% CI 0.18 to 1.13, P = 0.09; participants = 1804; studies = 8; I² = 38%; moderate‐quality evidence). All four in the compression stockings group were in the Scurr 2001 trial, which noted that these occurred in varicose veins in the knee region which were compressed by the upper edge of the stocking (Scurr 2001b). No superficial vein thromboses were found in the control group of the Scurr 2001 trial.
Oedema
Of the included studies, only Hagan 2008, Loew 1998 and the LONFLIT 4 trials reported assessments of leg oedema (LONFLIT 4 ‐ Kendall 1; LONFLIT 4 ‐ Kendall 2; LONFLIT 4 ‐ Scholl 1; LONFLIT 4 ‐ Scholl 2; LONFLIT 4 ‐ Traveno 1; LONFLIT 4 ‐ Traveno 2). The six separate randomised LONFLIT comparisons measured oedema using a score based on oedema tests, ankle circumference and volume, and swelling and discomfort as assessed by the participant. The score had a maximum (worst) value of 10 and was assessed before and after the flight. Within each comparison, the randomised groups had similar oedema scores before the flight (mean values of approximately 1), and the final values (rather than a change score) are used in our analyses. These final values were approximately 2 or 3 for people in the compression stockings group, compared to 6 to 9 in the group allocated not to wear compression stockings. As reported by the trialists, each of the randomised comparisons showed a significant reduction in oedema in their own right, both on objective measures and on subjective measures reported by the participants. In each comparison, there was a small increase in oedema for participants in the compression stockings group but a much larger increase for the no compression stockings group. The longer flights showed the greatest differences between the randomised groups. When combined, although there is significant heterogeneity among the results of the individual comparisons, the overall result clearly indicates a large and significant benefit for passengers who were allocated to wear compression stockings, compared to those allocated not to wear them (mean difference (MD) −4.72, 95% CI −4.91 to −4.52; participants = 1246; studies = 6; I² = 92%; P < 0.001; low‐quality evidence).
In the Hagan 2008 trial, oedema was measured by calculating the change of ankle circumference (before and after landing) between those wearing compression stockings versus those not wearing compression stockings. Hagan 2008 reported that there was a decrease in ankle swelling compared with not wearing compression stockings (MD −0.19 cm, 95% CI −0.33 to −0.065 cm; P = 0.012).
The Loew trial also studied oedema, within its randomised between‐individual comparison of wearing a compression stocking on one leg only (Loew 1998). These trialists assessed clinical oedema using a three point scale: 1 = none; 2 = slight; 3 = definite. They found that the leg on which a compression stocking was worn had less oedema than the other leg, and reported "oedema was most significant in the non‐stockinged leg". Before flying, the oedema ratings were none: 56, slight: 5 and definite: 0 for the leg on which a stocking would be worn compared to 57, 4 and 0 respectively for the other leg. After the flight, the scores for the leg on which a stocking had been worn had worsened slightly to none: 48, slight: 10, definite: 3; but the scores for the other leg were much worse at none: 30, slight: 22 and definite: 9.
It was not possible to pool the data from Hagan 2008 and Loew 1998 with data from the LONGFLIT 4 trials due to different methods used to measure oedema.
Adverse effects arising from the use of compression stockings
Some of the reports of the LONFLIT trials commented on possible adverse effects resulting from wearing compression stockings. In these reports, the tolerability of the stockings was described as very good with no complaints of side effects (LONFLIT 4 ‐ Scholl 1; LONFLIT 4 ‐ Scholl 2; LONFLIT 4 ‐ Traveno 1; LONFLIT 5). None of the other trials reported adverse effects of wearing the stockings, apart from the effect on superficial vein thrombosis in the Scurr 2001 trial, as noted above.
Discussion
Summary of main results
This review provides relatively precise estimates for a very large reduction in symptomless DVT among airline passengers who were allocated to wear compression stockings compared to those allocated not to wear such stockings.
The choice of statistical analysis — in the circumstances we encountered of having rare events that are very unevenly distributed between the treatment groups — is potentially controversial. However, as shown in the results section, whichever method is used there is a highly statistically significant, large effect of wearing stockings compared to not wearing them, equivalent to the odds of a symptomless DVT being decreased by approximately 90%. This might relate to, for example, a reduction in the risk of a symptomless deep vein thrombosis from about 10 to 30 per 1000 to 1 to 3 per 1000 long‐haul passengers. There is also a large and significant reduction in leg oedema associated with the wearing of stockings although the quality of the evidence for this was deemed to be low. There is moderate‐quality evidence that superficial vein thrombosis may be reduced if passengers wear compression stockings. The studies reported no cases of symptomatic DVT, PE or deaths.
There is no robust evidence to indicate that the different types of stockings assessed in the trials included in this review vary in their effects, nor that particular subgroups of people similar to those in these trials would not experience this benefit from wearing these stockings. A reliable investigation of these issues would require larger randomised trials, direct randomisation of different types of stockings and trials in which a wider range of participants are recruited. There also does not appear to be any significant increase in adverse effects associated with wearing the stockings in the types of people assessed.
Overall completeness and applicability of evidence
The relevance of the substantial reduction in symptomless DVT for outcomes such as death, pulmonary embolism and symptomatic deep vein thrombosis cannot be assessed from this review because there were no such events in any of the included trials. This may be because the trials involved special additional tests on all participants which, by identifying symptomless DVT, may have led to effective management and thereby prevented more serious consequences. It is also possible that death, pulmonary embolism and symptomatic DVT would have been so rare among the people in these trials that, even without the special diagnostic tests and subsequent treatments, no such events would have been recorded. Randomised trials to assess these outcomes would likely need to include a very large number of people. Therefore, this review provides a clear guide to the large effects of compression stockings on reducing symptomless DVT and oedema, but is unable to assess the impact this has on outcomes that might be judged of more relevance to airline passengers and the people who care for them.
Quality of the evidence
This review provides high‐quality evidence that wearing graduated compression stockings reduces the risk of developing a symptomless DVT when travelling on a long‐haul flight (over four hours). There is moderate‐quality evidence that wearing compression stockings may reduce risk of developing superficial vein thrombosis. The quality of evidence was downgraded because the confidence interval crosses no difference and does not rule out a small increase. Post‐flight oedema was reduced in passengers who wore compression stockings but we have graded the quality of this evidence as low. This is because the measurement technique used in the pooled data was not blinded or validated, and was carried out by the same investigators. As the methods from a further two studies used were different, we were unable to pool the data. However these additional studies also reported reduced oedema in passengers (or a leg) wearing compression stockings. As discussed above, we were unable to assess the quality of the evidence for the outcomes of PE, death and symptomatic DVT as no events occurred.
Potential biases in the review process
The search used was comprehensive and we have included all relevant studies. However, the possibility remains that some relevant trials may have been missed. Two review authors independently performed study selection and data extraction in order to minimise bias in the review process. The inclusion and exclusion criteria set out in the protocol were strictly adhered to in order to limit subjectivity (Clarke 2003). We followed Cochrane processes as described by Higgins 2011 for assessing the risk of bias. Our analyses are based mainly on assessments of symptomless DVT which were identified through special tests and, if found, led to additional interventions for the participants. The reliability of the diagnosis of symptomless DVT is dependent on the quality of the test used. This can lead to different rates of false positives and false negatives for different tests. However, because we used randomised trials in which the assessment of participants in both groups of each trial involved the same diagnostic technique, the possibility of mis‐diagnosis will have been the same for both groups and will not have introduced bias within the trials.
Agreements and disagreements with other studies or reviews
We are aware of other systematic reviews on this topic. Hsieh 2005 appears to have used similar methods to us and did not find any eligible studies that we had not already identified. Philbrick 2007 included case‐control studies, cohort studies and randomised controlled trials which reported on travel as a risk factor for VTE; or tested preventive measures (including pharmacological agents) for travel‐related VTE. They concluded that compression stockings "prevented travel‐related VTE (P < 0.05 in 4 of 6 studies)". One cross‐over trial compared intermittent pneumatic compression devices, compression stockings and periodic exercise in participants who were immobilized for eight hours (to mimic long‐haul travel, Coppens 2007). Similar to our findings, this study reported that compression stockings decreased the amount of oedema experienced by wearers and suggested that this may play a role in preventing VTE.
Authors' conclusions
High‐quality evidence shows that airline passengers similar to those in the trials in this review can expect a substantial reduction in their risk of a symptomless DVT if they wear compression stockings. Wearing stockings might reduce the incidence of this outcome from a few tens per thousand passengers, to two or three per thousand. There is moderate‐quality evidence that superficial vein thrombosis may be reduced if passengers wear compression stockings. Low‐quality evidence shows that passengers who wear stockings will also experience less oedema in their legs. However, this review is unable to identify whether these effects of wearing stockings translate into effects on outcomes such as death, pulmonary embolism and symptomatic DVT.
This review shows that the question of the effects on symptomless DVT of wearing versus not wearing compression stockings in the types of people studied in these trials should now be regarded as answered. Further research may be justified to investigate the relative effects of different strengths of stockings or of stockings compared to other preventative strategies. Further randomised trials to address the remaining uncertainty about the effects of wearing versus not wearing compression stockings on outcomes such as death, pulmonary embolism and symptomatic DVT would need to be large. As suggested by Adi 2004, a study to assess whether airline travel itself is associated with an increased risk of symptomatic DVT might require several tens of thousands of participants and so a randomised trial to investigate a preventative strategy would probably require a sample size at least this large.
Feedback
Comment on LONFLIT Studies, 10 August 2009
Summary
These reviews include a number of papers by Gianni Belcaro who was erased from the UK medical register in June 2007. This was for "misconduct", which seems to have been that he included as co‐authors on his papers people who were not involved in the research. The GMC report does not suggest that data was falsified (//webcache.gmc‐uk.org/minutesfiles/3313.HTML).
Should you mention in the systematic reviews that the data may be suspect in Belcaro's papers?
Reply
The authors of this review have been informed of concerns that have been raised about the authorship of some research papers by the lead investigator for the LONFLIT trials (which provide most of the randomised evidence included in this review): Gianni Belcaro. They are considering the potential impact on the findings of the review. The contact author for this Cochrane review, Mike Clarke, has written to the Editor in Chief of Angiology, which published the reports for many of the LONFLIT studies, asking if they should have any concerns about the reliability of these articles.
2016 update
The Editor in Chief of Angiology raised no concerns about the reliability of these articles when contacted by Mike Clarke in response to the concerns raised about the authorship of some research papers by the lead investigator for the LONFLIT trials.
Contributors
Feedback: Michael Power (Guideline author)
Reply: Mike J Clarke on behalf of the review team
Acknowledgements
We dedicate this update to our good friend and colleague, Monica Kjeldstrom, who was an author on the protocol and first version of this review but died too young in 2014. We wish to thank Marlene Stewart (MS) from the Cochrane Vascular editorial base for helping to assess the risk of bias of the included studies. We wish to thank Karen Welch from the Cochrane Vascular editorial base for performing the searches for this review.
Appendices
Appendix 1. CRS search strategy
| #1 | MESH DESCRIPTOR Aerospace Medicine | 190 |
| #2 | MESH DESCRIPTOR Air Travel | 2 |
| #3 | MESH DESCRIPTOR Travel Medicine | 2 |
| #4 | MESH DESCRIPTOR Travel Nursing | 0 |
| #5 | MESH DESCRIPTOR Aircraft | 79 |
| #6 | MESH DESCRIPTOR Aviation EXPLODE ALL TREES | 230 |
| #7 | MESH DESCRIPTOR Transportation EXPLODE ALL TREES | 540 |
| #8 | (aviation or aviator* or airline* or aeroplane* or aircraft* or plane* or flying or flight* or travel* or passenger*):TI,AB,KY | 4068 |
| #9 | (long near2 haul):TI,AB,KY | 30 |
| #10 | (long near2 distance):TI,AB,KY | 196 |
| #11 | nonstop:TI,AB,KY | 4 |
| #12 | (non near2 stop):TI,AB,KY | 8 |
| #13 | (economy class):TI,AB,KY | 7 |
| #14 | (coach class):TI,AB,KY | 2 |
| #15 | (economy near2 seat):TI,AB,KY | 0 |
| #16 | sedentar*:TI,AB,KY | 3009 |
| #17 | (sitting or seated ):TI,AB,KY | 3880 |
| #18 | inactiv*:TI,AB,KY | 4778 |
| #19 | immobil*:TI,AB,KY | 1697 |
| #20 | MESH DESCRIPTOR Immobilization | 402 |
| #21 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 | 17254 |
| #22 | MESH DESCRIPTOR Bandages EXPLODE ALL TREES | 2108 |
| #23 | (stocking* or hosiery or tights or sock*):TI,AB,KY | 1287 |
| #24 | #22 OR #23 | 3123 |
| #25 | MESH DESCRIPTOR Venous Thrombosis | 751 |
| #26 | MESH DESCRIPTOR Thrombophlebitis EXPLODE ALL TREES | 1046 |
| #27 | MESH DESCRIPTOR Thromboembolism | 880 |
| #28 | MESH DESCRIPTOR Venous Thromboembolism | 220 |
| #29 | MESH DESCRIPTOR Thrombosis | 1209 |
| #30 | (thromboprophyla* or thrombus or thrombotic or thrombolic or *emboli* or thrombos*):TI,AB,KY | 16375 |
| #31 | (DVT or VTE):TI,AB,KY | 1510 |
| #32 | (blood near2 clot*):TI,AB,KY | 2224 |
| #33 | (blood near2 stasis):TI,AB,KY | 394 |
| #34 | (vein* near2 stasis):TI,AB,KY | 7 |
| #35 | (venous near2 stasis):TI,AB,KY | 115 |
| #36 | #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 | 18662 |
| #37 | #21 AND #24 AND #36 | 42 |
Appendix 2. Trial registries searches
| WHO | clinicaltrials.gov | ISRCTN | |
| flight AND stockings | 0 | 0 | 0 |
| travel AND stockings | 0 | 0 | 1 |
| flight AND tights | 0 | 0 | 0 |
| travel AND tights | 0 | 2 | 0 |
| flight AND oedema | 2 | 0 | 0 |
| flights AND edema | 2 | 0 | 0 |
| air AND travel AND thrombosis | 1 | 0 | 0 |
| air AND travel AND thromboembolism | 2 | 0 | 0 |
Data and analyses
Comparison 1.
Wearing stockings versus not wearing stockings
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomless deep vein thrombosis | 9 | 2637 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.04, 0.25] |
| 2 Superficial vein thrombosis | 8 | 1804 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.18, 1.13] |
| 3 Oedema | 6 | 1246 | Mean Difference (IV, Fixed, 95% CI) | ‐4.72 [‐4.91, ‐4.52] |
Analysis 1.1.
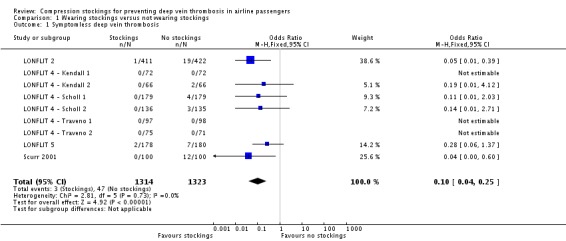
Comparison 1 Wearing stockings versus not wearing stockings, Outcome 1 Symptomless deep vein thrombosis.
Analysis 1.2.
Comparison 1 Wearing stockings versus not wearing stockings, Outcome 2 Superficial vein thrombosis.
Analysis 1.3.
Comparison 1 Wearing stockings versus not wearing stockings, Outcome 3 Oedema.
What's new
Last assessed as up‐to‐date: 10 February 2016.
| Date | Event | Description |
|---|---|---|
| 18 March 2016 | New citation required but conclusions have not changed | Search updated. One new study included. Review amended to reflect current Cochrane standards. 'Risk of bias' assessments and 'Summary of findings' table added. New author joined the review team. No change to conclusions. |
| 18 March 2016 | New search has been performed | Search updated. One new study included. |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 10 November 2009 | Amended | Contact details updated. |
| 11 August 2009 | Feedback has been incorporated | Comment on LONFLIT Studies |
| 4 June 2008 | Amended | Converted to new review format. |
| 4 April 2007 | Amended | One new included trial; added as a secondary reference to Scurr 2001. Updated dates of search in text. |
Differences between protocol and review
In previous versions of this review the method of concealment of allocation used in each identified study was assessed and categorized as A (adequate), B (unclear) or C (not concealed). For this update all included studies were assessed for risk of bias using Cochrane's 'Risk of bias' tool as described in Higgins 2011. In keeping with current Cochrane guidelines a 'Summary of findings' table was included in this update.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hagan 2008
| Methods | Trial design: open, randomised, crossover trial. Country principal investigators: Australia. Where trial conducted: Australia. Date of trial: between April and October 2006. Multi‐centre: no. Funder: SKINS Compression Garments, Sydney, NSW. Blinding: no. Lost to follow‐up: 3. | |
| Participants | Inclusion criteria: low to medium risk of DVT. Participants were aged 18 or older with confirmed booking on a flight (5 hours' minimum) with 48 hours between forward and return flight. Exclusion criteria: received medical advice to wear GCT in flight, previous history of DVT, on medication for cardiovascular disease, coagulation disorders, varicose veins, bone or joint problems, diabetes or hypertension, BMI ≥ 35, or neoplastic disease within previous 2 years. No. randomised: 50 No. analysed: 47. Age (years): 24 to 71 years. Sex: 35 M, 12 F. | |
| Interventions | Stocking was worn on both legs (tights) on either the outward flight or on the return. Type of stocking: SKINS travel and recovery garment, listed as class I medical device (SKINS Compression Garments Pty Ltd, Sydney, NSW; ID: 880116). 5 mmHg at ankle, 17 to 20 mmHg at calf, 10 mmHg above knee and 4 mmHg at buttocks. Length of flight: 9.6 hours (GCT), 9.7 hours (no GCT). Type of seat: not reported. Route and time of flight: not reported. Additional interventions: suggestions for in flight exercises given to both groups. | |
| Outcomes | Primary outcomes assessed: difference in change of ankle circumference (before flight and after landing) between control (no GCT) and treatment (GCT). Secondary outcomes assessed: leg pain, leg discomfort, perceived leg swelling, energy levels, alertness and ability to concentrate. |
|
| Notes | Funding by manufacturers, study designed by manufacturers, study authors independently collected, analysed and reported data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used "...a computer generated randomisation sequence" |
| Allocation concealment (selection bias) | Low risk | "Random allocation achieved by giving participants a sealed envelope with instructions..." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes self‐reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All reported and intention‐to‐treat analysis used |
| Selective reporting (reporting bias) | Low risk | All reported |
| Other bias | Low risk | No evidence of other bias |
Loew 1998
| Methods | Trial design: randomised controlled trial (single leg). Country principal investigators: Germany. Where trial conducted: Germany. Date of trial: not reported. Multi‐centre: no. Funder: not reported. Blinding: outcome assessor not blinded. Lost to follow‐up: none. | |
| Participants | Inclusion criteria: risk is not reported. Participants were going to and returning from the 10th Workshop of the German Association of Phlebologists in Namibia. Exclusion criteria: not clear. No. randomised: 35. No. analysed: 35 on the outward flight and 26 on the return flight. Age (years): mean 48. Sex: 20 M, 15 F. | |
| Interventions | Stocking was worn on 1 leg on outward flight and on the other leg on the return. Type of stocking: class II compression stocking (Sigvaris 902 A‐D KK1.2, Ganzoni). Length of flight: 14.5 hours. Type of seat: not reported. Route and time of flight: Frankfurt, Germany to Windhoek, Namibia and return flight (both flights were mostly at night). Additional interventions: participants were also randomised to receive either dried vine leaves (Antistax) for 7 days before the flight and on the day (13 people), diuretics on the day of the flight (9), or no drugs (13). Participants in the diuretics group were told not to take these drugs on the return flight. | |
| Outcomes | Outcomes assessed: limb volume, oedema status, subjective symptoms, phlebological status and clinical and Doppler findings. Measurements were made soon after landing on both flights. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but no further details. Medication randomised |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | reported as "Open". Not possible for GCT |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Subjective scores, states same doctors carried out all examinations. Unclear if these doctors were involved in other aspects of the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No evidence of other bias |
LONFLIT 2
| Methods | Trial design: randomised controlled trial (parallel). Country principal investigators: Italy, UK, Australia. Where trial conducted: UK. Date of trial: May to June 2001. Multi‐centre: yes. Funder: multiple sources. Blinding: outcome assessor not blinded. Lost to follow‐up: 52; dropouts due to low compliance or flight connection problems. | |
| Participants | Inclusion criteria: high‐risk participants defined as those with previous episodes of DVT, coagulation disorders, severe obesity, limited mobility due to bone or joint problems, neoplastic disease within the previous 2 years, or large varicose veins. Participants were recruited through flight shops. Exclusion criteria: not clear. No. randomised: 885. No. analysed: 833. Age (years): mean 44.8 (SD 9) range 20 to 80. Sex: 57% M, 43% F. | |
| Interventions | Stocking Group Type of stocking: below‐knee compression stocking with 25 mmHg of pressure at the ankle. Stockings were put on 6 to 10 hours before the flight. No. randomised: unclear. No. analysed: 411. Length of flight: average 12.4 hours, range 10 to 15. Type of seat: Economy, seat pitch 31 inches. Route and time of flight: not available. Additional interventions: participants were advised to move often, drink water (at least 1 glass every 2 hours), stretch limbs every hour for 2 minutes, not keep baggage in the space under the seats, avoid salty snacks, wear comfortable clothing. Control Group Type of control: no intervention. No. randomised: unclear. No. analysed: 422. Length of flight: average 12.4 hours, range 10 to 15. Type of seat: Economy, seat pitch 31 ins. Route and time of flight: not available. Additional interventions: the same advice as the stocking group was given. |
|
| Outcomes | Outcomes assessed: incidence of DVT. DVT diagnosis method: participants were scanned within 48 hours pre flight and 24 hours post flight using Sonosite scanners with 10 MHz probes (Sonosite, Bothell, WA, USA). B‐mode and power ultrasound were used to evaluate DVT. This was done by scanning the femoral and popliteal veins. Site of DVT: proximal vein, distal venous system, superficial system. Site of SVT: none developed. Other investigations performed: none. Additional complications reported: none. | |
| Notes | See Feedback. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but no further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported but not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not discussed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 52 lost to follow‐up ‐ no details given on which group these passengers belonged to. Although less than 10% these may not be evenly distributed |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No evidence of other bias |
LONFLIT 4 ‐ Kendall 1
| Methods | Trial design: randomised controlled trial (parallel). Country principal investigators: Italy, UK, Australia. Where trial conducted: UK. Date of trial: May to July 2002. Multi‐centre: yes. Funder: multiple sources. Blinding: outcome assessor not blinded. Lost to follow‐up: 2/74 (stocking), 4/76 (control); dropouts due to low compliance or flight connection problems. | |
| Participants | Inclusion criteria: low‐ to medium‐risk participants. Participants were recruited through flight shops. Exclusion criteria: high‐risk participants defined as those with previous episodes of DVT, coagulation disorders, severe obesity, limited mobility due to bone or joint problems, neoplastic disease within the previous 2 years, large varicose veins or participants taller than 190 cm and heavier than 90 kg. No. randomised: 150. No. analysed: 144. Age (years): mean 46 (stocking), 47 (control). Sex: 37 M, 35 F (stocking), 38 M, 34 F (control) ‐ based on number analysed. | |
| Interventions | Stocking Group Type of stocking: below‐knee Kendall travel sock with 20 to 30 mmHg of pressure at the ankle. Stockings were put on 2 to 3 hours before the flight. No. randomised: 74. No. analysed: 72. Length of flight: 7 to 8 hours. Type of seat: Economy, seat pitch 31 inches. Route and time of flight: London to New York. Additional interventions: suggestions were given to participants i.e. mild exercise, walking, drinking water and avoiding salty food and excessive baggage restricting leg motion. Control Group Type of control: no intervention No. randomised: 76 No. analysed: 72 Length of flight: 7 to 8 hours Type of seat: Economy, seat pitch 31 ins Route and time of flight: London to New York Additional interventions: the same advice as the stocking group was given. |
|
| Outcomes | Outcomes assessed: incidence of DVT, SVT and oedema score. DVT diagnosed by: pre‐ and post‐flight ultrasound scanning using Sonosite scanners with a 7.5 to 13 MHz, high‐resolution probe (Sonosite, Bothell, WA, USA). This was done by compressing the major veins (femoral, popliteal and tibial). Site of DVT: none developed. Site of SVT: not mentioned. Other investigations performed: oedema test (ankle circumference, volume, swelling, discomfort). Additional complications reported: increased oedema in the control group. | |
| Notes | See Feedback. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but no further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported but not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Oedema scale described but variation possible. Parametric and non‐parametric |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All accounted for, dropouts reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No evidence of other bias |
LONFLIT 4 ‐ Kendall 2
| Methods | Trial design: randomised controlled trial (parallel). Country principal investigators: Italy, UK, Australia. Where trial conducted: UK. Date of trial: May to July 2002. Multi‐centre: yes. Funder: multiple sources. Blinding: outcome assessor not blinded. Lost to follow‐up: 0/66 (stocking) 2/68 (control); dropouts due to low compliance or flight connection problems. | |
| Participants | Inclusion criteria: low‐ to medium‐risk participants. Participants were recruited through flight shops. Exclusion criteria: high‐risk participants defined as those with previous episodes of DVT, coagulation disorders, severe obesity, limited mobility due to bone or joint problems, neoplastic disease within the previous 2 years, large varicose veins or participants taller than 190 cm and heavier than 90 kg. No. randomised: 134 No. analysed: 132 Age (years): mean 47 (stocking), 46.9 (control). Sex: 34 M, 30 F (stocking), 34 M, 32 F (control) ‐ based on number analysed. | |
| Interventions | Stocking Group Type of stocking: below‐knee Kendall travel sock with 20 to 30 mmHg of pressure at the ankle. Stockings were put on 2 to 3 hours before the flight. No. randomised: 66 No. analysed: 66 Length of flight: 11 to 12 hours. Type of seat: Economy, seat pitch 31 inches. Route and time of flight: London to Phoenix. Additional interventions: suggestions were given to participants i.e. mild exercise, walking, drinking water and avoiding salty food and excessive baggage restricting leg motion. Control Group Type of control: no intervention No. randomised: 68 No. analysed: 66 Length of flight: 11 to 12 hours Type of seat: Economy, seat pitch 31 ins Route and time of flight: London to Phoenix Additional interventions: the same advice as the stocking group was given. |
|
| Outcomes | Outcomes assessed: incidence of DVT, SVT and oedema score. DVT diagnosed by: pre‐ and post‐flight ultrasound scanning using Sonosite scanners with a 7.5 to 13 MHz, high‐resolution probe (Sonosite, Bothell, WA, USA). This was done by compressing the major veins (femoral, popliteal and tibial). Site of DVT: none developed. Site of SVT: not mentioned. Other investigations performed: oedema test (ankle circumference, volume, swelling, discomfort). Additional complications reported: increased oedema in the control group. | |
| Notes | See Feedback. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but no further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported but not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported. Oedema scale described but variation possible, parametric and non‐parametric |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All accounted for, dropouts reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No evidence of other bias |
LONFLIT 4 ‐ Scholl 1
| Methods | Trial design: randomised controlled trial (parallel). Country principal investigators: Italy, UK, Australia. Where trial conducted: UK. Date of trial: May to July 2002. Multi‐centre: yes. Funder: multiple sources. Blinding: outcome assessor not blinded. Lost to follow‐up: 5/184 (stocking) 9/188 (control); dropouts due to low compliance or flight connection problems. | |
| Participants | Inclusion criteria: low‐ to medium‐risk participants. Participants were recruited through flight shops. Exclusion criteria: high‐risk participants defined as those with previous episodes of DVT, coagulation disorders, severe obesity, limited mobility due to bone or joint problems, neoplastic disease within the previous 2 years, large varicose veins or participants taller than 190 cm and heavier than 90 kg. No. randomised: 372 No. analysed: 358 Age (years): mean 49 (stocking), 48.4 (control). Sex: 101 M, 78 F (stocking), 98 M, 81 F (control) ‐ based on the number analysed. | |
| Interventions | Stocking Group Type of stocking: below‐knee Scholl flight sock UK with 14 to 17 mmHg of pressure at the ankle. Stockings were put on 2 to 3 hours before the flight. No. randomised: 184. No. analysed: 179. Length of flight: 7 to 8 hours. Type of seat: Economy, seat pitch 31 inches. Route and time of flight: London to New York. Additional interventions: suggestions were given to participants, i.e. mild exercise, walking, drinking water and avoiding salty food and excessive baggage restricting leg motion. Control Group Type of control: no intervention No. randomised: 188 No. analysed: 179 Length of flight: 7 to 8 hours Type of seat: Economy, seat pitch 31 ins Route and time of flight: London to New York Additional interventions: the same advice as the stocking group was given. |
|
| Outcomes | Outcomes assessed: incidence of DVT, SVT and oedema score. DVT diagnosed by: pre‐ and post‐flight ultrasound scanning using Sonosite scanners with a 7.5 to 13 MHz, high‐resolution probe (Sonosite, Bothell, WA, USA). This was done by compressing the major veins (femoral, popliteal and tibial). Site of DVT: not mentioned. Site of SVT: none developed. Other investigations performed: oedema test (ankle circumference, volume, swelling, discomfort). Additional complications reported: increased oedema in the control group. | |
| Notes | See Feedback. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but no further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Oedema measured both parametrically and non‐parametrically |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All accounted for and dropouts similar between groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Low risk | No evidence of other bias |
LONFLIT 4 ‐ Scholl 2
| Methods | Trial design: randomised controlled trial (parallel). Country principal investigators: Italy, UK, Australia. Where trial conducted: UK. Date of trial: May to July 2002. Multi‐centre: yes. Funder: multiple sources. Blinding: outcome assessor not blinded. Lost to follow‐up: 6/142 (stocking) 8/143 (control); dropouts due to low compliance or flight connection problems. | |
| Participants | Inclusion criteria: low‐ to medium‐risk participants. Participants were recruited through flight shops. Exclusion criteria: high‐risk participants defined as those with previous episodes of DVT, coagulation disorders, severe obesity, limited mobility due to bone or joint problems, neoplastic disease within the previous 2 years, large varicose veins or participants taller than 190 cm and heavier than 90 kg. No. randomised: 285. No. analysed: 271. Age (years): mean 48 (stocking), 47 (control). Sex: 89 M, 53 F (stocking), 87 M, 56 F (control) ‐ based on number randomised. | |
| Interventions | Stocking Group Type of stocking: below‐knee Scholl flight sock UK with 14 to 17 mmHg of pressure at the ankle. Stockings were put on 2 to 3 hours before the flight. No. randomised: 142. No. analysed: 136. Length of flight: 11 to 12 hours. Type of seat: Economy, seat pitch 31 inches. Route and time of flight: London to Phoenix. Additional interventions: suggestions were given to participants, i.e. mild exercise, walking, drinking water and avoiding salty food and excessive baggage restricting leg motion. Control Group Type of control: no intervention No. randomised: 143 No. analysed: 135 Length of flight: 11 to 12 hours Type of seat: Economy, seat pitch 31 ins Route and time of flight: London to Phoenix Additional interventions: the same advice as the stocking group was given. |
|
| Outcomes | Outcomes assessed: incidence of DVT, SVT and oedema score. DVT diagnosed by: pre‐ and post‐flight ultrasound scanning using Sonosite scanners with a 7.5 to 13 MHz, high‐resolution probe (Sonosite, Bothell, WA, USA). This was done by compressing the major veins (femoral, popliteal and tibial). Site of DVT: not mentioned. Site of SVT: none developed. Other investigations performed: oedema test (ankle circumference, volume, swelling, discomfort). Additional complications reported: increased oedema in the control group. | |
| Notes | See Feedback. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but no further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported but not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported, oedema scale described but variations possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All accounted for, dropouts reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No evidence of other bias |
LONFLIT 4 ‐ Traveno 1
| Methods | Trial design: randomised controlled trial (parallel). Country principal investigators: Italy, UK, Australia. Where trial conducted: UK. Date of trial: May to July 2002. Multi‐centre: yes. Funder: multiple sources. Blinding: outcome assessor not blinded. Lost to follow‐up: 6/103 (stocking) 10/108 (control); dropouts due to low compliance or flight connection problems. | |
| Participants | Inclusion criteria: low‐ to medium‐risk participants. Participants were recruited through flight shops. Exclusion criteria: high‐risk participants defined as those with previous episodes of DVT, coagulation disorders, severe obesity, limited mobility due to bone or joint problems, neoplastic disease within the previous 2 years, large varicose veins or participants taller than 190 cm and heavier than 90 kg. No. randomised: 211. No. analysed: 195. Age (years): mean 44.5 (stocking), 45 (control). Sex: 53 M, 44 F (stocking), 54 M, 44 F (control) ‐ based on number analysed. | |
| Interventions | Stocking Group Type of stocking: below‐knee Traveno stocking with 12 to 18 mmHg of pressure at the ankle. Stockings were put on 2 to 3 hours before the flight. No. randomised: 103. No. analysed: 97. Length of flight: 7 to 8 hours. Type of seat: Economy, seat pitch 31 inches. Route and time of flight: London to New York. Additional interventions: suggestions were given to participants, i.e. mild exercise, walking, drinking water and avoiding salty food and excessive baggage restricting leg motion. Control Group Type of control: no intervention No. randomised: 108 No. analysed: 98 Length of flight: 7 to 8 hours Type of seat: Economy, seat pitch 31 ins Route and time of flight: London to New York Additional interventions: the same advice as the stocking group was given. |
|
| Outcomes | Outcomes assessed: incidence of DVT, SVT and oedema score. DVT diagnosed by: pre‐ and post‐flight ultrasound scanning using Sonosite scanners with a 7.5 to 13 MHz, high‐resolution probe (Sonosite, Bothell, WA, USA). This was done by compressing the major veins (femoral, popliteal and tibial). Site of DVT: none developed. Site of SVT: none developed. Other investigations performed: oedema test (ankle circumference, volume, swelling, discomfort). Additional complications reported: increased oedema in the control group. | |
| Notes | See Feedback. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated randomised but no further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported, scale described but variation possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Selective reporting (reporting bias) | Low risk | All preplanned outcomes reported |
| Other bias | Low risk | No evidence of other bias |
LONFLIT 4 ‐ Traveno 2
| Methods | Trial design: randomised controlled trial (parallel). Country principal investigators: Italy, UK, Australia. Where trial conducted: UK. Date of trial: May to July 2002. Multi‐centre: yes. Funder: multiple sources. Blinding: outcome assessor not blinded. Lost to follow‐up: 8/83 (stocking), 11/82 (control); dropouts due to low compliance or flight connection problems. | |
| Participants | Inclusion criteria: low‐ to medium‐risk participants. Participants were recruited through flight shops. Exclusion criteria: high‐risk participants defined as those with previous episodes of DVT, coagulation disorders, severe obesity, limited mobility due to bone or joint problems, neoplastic disease within the previous 2 years, large varicose veins or participants taller than 190 cm and heavier than 90 kg. No. randomised: 165. No. analysed: 146. Age (years): mean 45 (stocking), 46 (control). Sex: 44 M, 31 F (stocking), 39 M, 32 F (control) ‐ based on number analysed. | |
| Interventions | Stocking Group Type of stocking: below‐knee Traveno stocking with 12 to 18 mmHg of pressure at the ankle. Stockings were put on 2 to 3 hours before the flight. No. randomised: 83. No. analysed: 75. Length of flight: 11 to 12 hours. Type of seat: Economy, seat pitch 31 inches. Route and time of flight: London to Phoenix. Additional interventions: suggestions were given to participants, i.e. mild exercise, walking, drinking water and avoiding salty food and excessive baggage restricting leg motion. Control Group Type of control: no intervention No. randomised: 82 No. analysed: 71 Length of flight: 11 to 12 hours Type of seat: Economy, seat pitch 31 ins Route and time of flight: London to Phoenix Additional interventions: the same advice as the stocking group was given. |
|
| Outcomes | Outcomes assessed: incidence of DVT, SVT and oedema score. DVT diagnosed by: pre‐ and post‐flight ultrasound scanning using Sonosite scanners with a 7.5 to 13 MHz, high‐resolution probe (Sonosite, Bothell, WA, USA). This was done by compressing the major veins (femoral, popliteal and tibial). Site of DVT: none developed. Site of SVT: not mentioned. Other investigations performed: oedema test (ankle circumference, volume, swelling, discomfort). Additional complications reported: increased oedema in the control group. | |
| Notes | See Feedback. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stated randomised but no further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported, scale described but variations possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 12% lost to follow‐up but accounted for and distributed equally between groups |
| Selective reporting (reporting bias) | Low risk | All preplanned outcomes reported |
| Other bias | Low risk | No evidence of other bias |
LONFLIT 5
| Methods | Trial design: randomised controlled trial (parallel). Country principal investigators: Italy, UK, Australia. Where trial conducted: UK. Date of trial: October 2002 to January 2003. Multi‐centre: yes. Funder: multiple sources. Blinding: outcome assessor not blinded. Lost to follow‐up: 13/191 (stocking), 17/197 (control); dropouts due to low compliance or flight connection problems. | |
| Participants | Inclusion criteria: 446 high‐risk participants were contacted. High risk included previous episodes of DVT or superficial vein thrombosis, coagulation disorders, severe obesity or limited mobility due to bone or joint problems, neoplastic disease within the previous 2 years, clinical cardiovascular disease and large varicose veins. Participants were recruited through flight shops. Exclusion criteria: participants taller than 190 cm, weighing more than 90 kg, recent or presence of thrombosis, severe bone, joint, or mobility problems, severe hypertension, or clinical disease requiring treatment. No. randomised: 388. No. analysed: 358. Age (years): mean 45, SD 12 (stocking); mean 45, SD 11(control). Sex: 55% M (stocking), 66% M (control) ‐ based on number analysed. | |
| Interventions | Stocking Group Type of stocking: below‐knee Scholl flight socks UK with 14 to 17 mmHg of pressure at the ankle. Stockings were put on 3 to 4 hours before the flight. No. randomised: 191. No. analysed: 178. Length of flight: 11 to 13 hours. Type of seat: Economy, seat pitch 31 inches. Route and time of flight: London to Narita (Japan). Additional interventions: suggestions were given to participants. An exercise plan and educational video were given to all participants which included advice on mild exercise, drinking regularly and avoiding placing baggage under seats. Control Group Type of control: no intervention. No. randomised: 197. No. analysed: 180. Length of flight: 11 to 13 hours. Type of seat: Economy, seat pitch 31 inches. Route and time of flight: London to Narita (Japan). Additional interventions: the same advice as the stocking group was given. |
|
| Outcomes | Outcomes assessed: incidence of DVT and SVT DVT diagnosed by: D‐dimer and fibrinogen tests which were performed before (within 12 hours) and after the flight (within 4 hours) (Dade Dimmertest, Latex Test, Behring, Germany). This was done by compressing the major veins (femoral, popliteal and tibial). Site of DVT: Stockings group: distal, below knee vein (2); Control group: location not reported for 7 in most recent report but the locations for the 6 reported in the first article were distal superficial femoral (3), popliteal (2), soleal (1). All DVTs were asymptomatic. Site of SVT: site not reported for the 5 (control). Other investigations performed: none reported. Additional complications reported: none. | |
| Notes | See Feedback. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but no further details |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All accounted for |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias | Low risk | No evidence of other bias |
Scurr 2001
| Methods | Trial design: randomised controlled trial (parallel). Country principal investigators: UK. Where trial conducted: UK. Date of trial: not reported. Multi‐centre: no. Funder: Stamford Hospital, London, UK; and Medi UK Ltd. Blinding: outcome assessor was blinded. Lost to follow‐up: 15/116 (stocking), 16/115 (control); dropouts due to low compliance or flight connection problems. | |
| Participants | Inclusion criteria: participants older than 50 years and travelling 2 sectors of at least 8 hours within 6 weeks. Participants were recruited by placing advertisements in local newspapers, travel shops and press releases. Preliminary screening included examination and medical questionnaire, those eligible were investigated for previous DVT by duplex ultrasound. Exclusion criteria: participants with previous episodes of venous thrombosis, those taking anticoagulants, those regularly wearing compression stockings, those with cardiorespiratory problems, or other serious illness. No. randomised: 231. No. analysed: 200. Age (years): median 61 (IQR 56 to 66) (stocking), 62 (IQR 56 to 68) (control). Sex: 34 M, 81 F (stocking), 55 M, 61 F (control) ‐ based on number randomised. | |
| Interventions | Stocking Group Type of stocking: below‐knee Class 1 German Hohenstein compression stocking with 20 to 30 mmHg of pressure at the ankle. Stockings were put on before the flight and removed on arrival. No. randomised: 116. No. analysed: 100. Length of flight: more than 8 hours. Type of seat: Economy. Route and time of flight: not reported. Additional interventions: none given but some participants were taking additional medication: aspirin (11); HRT (16); thyroxine (6); antihypertensives (12); antipeptic ulcer drugs (3). Control Group Type of control: no intervention. No. randomised: 115. No. analysed: 100. Length or flight: more than 8 hours. Type of seat: Economy. Route and time of flight: not reported. Additional interventions: none given but some participants were taking additional medication: aspirin (9); HRT (8); thyroxine (6); antihypertensives (10); antipeptic ulcer drugs (8). |
|
| Outcomes | Outcomes assessed: incidence of DVT and SVT. DVT diagnosed by: duplex ultrasound scanning which was performed during the pre screening process and within 48 hours after the flight. Site of DVT: not reported. Site of SVT: knee region which was compressed by the upper edge of the stocking, in 4 participants who all had varicose veins. No SVT in the control group. Other investigations performed: blood samples were taken before and after the flight. Additional complications reported: none. | |
| Notes | More females in stockings group (70% GCT versus 53% no GCT). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | States randomised but not reported |
| Allocation concealment (selection bias) | Low risk | Used sealed envelope |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Low risk for the only reported outcome of DVT as technician unaware of group. Unclear if assessor of other outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13% of participants lost to follow‐up, but these are evenly distributed between the groups |
| Selective reporting (reporting bias) | Unclear risk | Outcomes (apart from DVT) not reported fully |
| Other bias | Unclear risk | Gender imbalance at baseline despite randomisation. Unclear if this could impact the incidence of DVT. No other baseline imbalances identified |
BMI: body mass index DVT: deep vein thrombosis F: female GCS: graduated compression stockings GCT: graduated compression tights HRT: hormone replacement therapy IQR: inter‐quartile range M: male SD: standard deviation SVT: superficial vein thrombosis
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Iwama 2002 | Not randomised |
Contributions of authors
MC: initial draft of protocol, revision and approval of the protocol, initial screening of articles, selection of studies; data extraction for the original review, review and approval of update CB: selection of studies and data extraction, assessment of all included studies for risk of bias, revision of text for update SH: initial draft of the protocol, revision and approval of the protocol, initial screening of articles, selection of studies and data extraction for the original review, review and approval of update EJ: revision and approval of the protocol, selection of studies, data extraction and additional statistical analyses for the original review, review and approval of update AE: revision and approval of the protocol, design of the search strategy for original review, initial screening and selection of articles; data extraction for the original review, review and approval of update
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist Office
Declarations of interest
MC: none known CB: none known. CB is a member of Cochrane Vascular's editorial staff. To prevent any conflict‐of‐interest issues editorial decisions and activities related to this review were carried out by other editorial staff where appropriate SH: none known EJ: none known AE: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
- Hagan MJ, Lambert SM. A randomised crossover study of low ankle pressure graduated‐compression tights in reducing flight‐induced ankle oedema. Medical Journal of Australia 2008;188(2):81‐4. [DOI] [PubMed] [Google Scholar]
- Loew D, Gerlach HE, Altenkamper KH, Schneider B. Effect of long‐distance flights on oedema of the lower extremities. Phlebology 1998;13(2):64‐7. [Google Scholar]
- Belcaro G, Geroulakos G, Bucci M, Kennet AM, Winford M, Cesarone MR, et al. Venous thromboembolism from air travel. The LONFLIT Studies. Circulation 2001;104(17 Suppl 2):824. [Google Scholar]; Belcaro G, Geroulakos G, Sanctis M, Nicolaides AN, Incandela L, Cesarone MR, et al. Prevention of deep venous thrombosis on long‐haul flights. Journal of the American College of Cardiology 2002;39:212A. [Google Scholar]; Belcaro G, Geroulakos G, Nicolaides AN, Myers KA, Winford M. Venous thromboembolism from air travel: the LONFLIT study. Angiology 2001;52(6):369‐74. [DOI] [PubMed] [Google Scholar]
- Cesarone MR, Belcaro G, Errichi BM, Nicolaides AN, Geroulakos G, Ippolito E, et al. The LONFLIT4 Concorde Deep Venous Thrombosis and Edema Study: prevention with travel stockings. Angiology 2003;54(2):143‐54. [DOI] [PubMed] [Google Scholar]
- Cesarone MR, Belcaro G, Errichi BM, Nicolaides AN, Geroulakos G, Ippolito E, et al. The LONFLIT4 Concorde Deep Venous Thrombosis and Edema Study: prevention with travel stockings. Angiology 2003;54(2):143‐54. [DOI] [PubMed] [Google Scholar]
- Belcaro G, Cesarone MR, Shah SS, Nicolaides AN, Geroulakos G, Ippolito E, et al. Prevention of edema, flight microangiopathy and venous thrombosis in long flights with elastic stockings. A randomized trial: The LONFLIT 4 Concorde Edema‐SSL Study. Angiology 2002;53(6):635‐45. [DOI] [PubMed] [Google Scholar]
- Belcaro G, Cesarone MR, Geroulakos G, Nicolaides AN, Griffin M, Asahi M, et al. Prevention of long‐haul flight‐thrombosis with anti‐thrombotic flight‐stockings. Circulation 2003;108(17 Suppl):786. [Google Scholar]; Belcaro G, Cesarone MR, Shah SS, Nicolaides AN, Geroulakos G, Ippolito E, et al. Prevention of edema, flight microangiopathy and venous thrombosis in long flights with elastic stockings. A randomized trial: The LONFLIT 4 Concorde Edema‐SSL Study. Angiology 2002;53(6):635‐45. [DOI] [PubMed] [Google Scholar]
- Cesarone MR, Belcaro G, Nicolaides AN, Geroulakos G, Lennox A, Myers KA, et al. The LONFLIT4 Concorde Sigvaris Traveno Stockings in Long Flights (EcoTraS) Study: a randomized trial. Angiology 2003;54(1):1‐9. [DOI] [PubMed] [Google Scholar]
- Cesarone MR, Belcaro G, Nicolaides AN, Geroulakos G, Lennox A, Myers KA, et al. The LONFLIT4 Concorde Sigvaris Traveno Stockings in Long Flights (EcoTraS) Study: a randomized trial. Angiology 2003;54(1):1‐9. [DOI] [PubMed] [Google Scholar]
- Belcaro G, Cesarone MR, Nicolaides AN, Geroulakos G, Dugall M, Renzo A, et al. Prevention of flight‐related thrombosis with elastic stockings: The JPA‐Study, final analysis. Journal of the American College of Cardiology 2004;43(5 Suppl A):524a. [Google Scholar]; Belcaro G, Cesarone MR, Nicolaides AN, Ricci A, Geroulakos G, Shah SS, et al. Prevention of venous thrombosis with elastic stockings during long‐haul flights: the LONFLIT 5 JAP study. Clinical and Applied Thrombosis/Hemostasis 2003;9(3):197‐201. [DOI] [PubMed] [Google Scholar]
- DeLoughery TG. Wearing elastic compression stockings during long‐haul flights prevented the development of deep vein thrombosis. ACP Journal Club 2001;135(3):96. [Google Scholar]; Machin SJ, Mackie IJ, McDonald S, Bailey KS, Colleridge SP, Scurr JH. Airline travel: incidence and prevention of venous thrombosis. British Journal of Haematology 2001;113(Suppl 1):59. [Google Scholar]; Scurr JH, Machin SJ, Bailey‐King S. Wearing elastic compression stockings during long‐haul flights prevented the development of deep venous thrombosis. Evidence Based Medicine 2001;6(6):169. [Google Scholar]; Scurr JH, Machin SJ, Bailey‐King S, Mackie IJ, McDonald S, Smith PD. Frequency and prevention of symptomless deep‐vein thrombosis in long‐haul flights: a randomised trial. Lancet 2001;357(9267):1485‐9. [DOI] [PubMed] [Google Scholar]; Scurr JH, Smith PD, Machin S. Deep vein thrombosis in airline passengers ‐ the incidence of deep vein thrombosis and the efficacy of elastic compression stockings. Cardiovascular Surgery 2001;9(2):159‐61. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Iwama H, Furuta S, Ohmizo H. Graduated compression stocking manages to prevent economy class syndrome. American Journal of Emergency Medicine 2002;20(4):378‐80. [DOI] [PubMed] [Google Scholar]
Additional references
- Adi Y, Bayliss S, Rouse A, Lip G, Taylor R. Air travel as a risk factor for venous thromboembolism (VTE) and the effectiveness of preventative measures. www.publichealth.bham.ac.uk/wmhtac/pdf/DVT.pdf (accessed 8 June 2005).
- Adi Y, Bayliss S, Rouse A, Taylor RS. The association between air travel and deep vein thrombosis: systematic review and meta‐analysis. BMC Cardiovascular Disorders 2004;4(7):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand SS, Wells PS, Hunt D, Brill‐Edwards P, Cook D, Ginsberg JS. Does this patient have deep vein thrombosis?. JAMA 1998;279(14):1094‐9. [DOI] [PubMed] [Google Scholar]
- Ansari MT, Cheung BMY, Huang JQ, Eklof B, Karlberg JPE. Traveler's thrombosis: a systematic review. Journal of Travel Medicine 2005;12(3):142‐54. [DOI] [PubMed] [Google Scholar]
- Belcaro G, Cesarone MR, Nicolaides AN, Geroulakos G, Griffin M. Prevention of flight venous thrombosis in high risk subjects with stockings or one‐dose enoxaparine. Circulation 2002;106(Suppl II):721‐2. [Google Scholar]
- Belcaro G, Geroulakos G, Sanctis M, Nicolaides AN, Incandela L, Cesarone MR, et al. Prevention of deep venous thrombosis on long‐haul flights. Journal of the American College of Cardiology 2002;39(4 Suppl A):212A. [Google Scholar]
- Bihari I, Sandor T. Thromboembolism in travellers. Orvosi Hetilap 2001;142(45):2469‐73. [PubMed] [Google Scholar]
- Cannegieter SC, Doggen CJ, Houwelingen HC, Rosendaal FR. Travel‐related venous thrombosis: results from a large population‐based case control study (MEGA study). PLoS medicine 2006;3(8):e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppens M, Schreijer AJ, Berger FH, Cannegieter SC, Rosendaal FR, Büller HR. Mechanical prophylaxis for travellers' thrombosis: a comparison of three interventions that promote venous outflow. Journal of Thrombosis and Haemostasis 2007;5(7):1556‐7. [DOI] [PubMed] [Google Scholar]
- Geroulakos G. The risk of venous thromboembolism from air travel. BMJ 2001;322:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangrande PL. Air travel and thrombosis. International Journal of Clinical Practice 2001;55(10):690‐3. [PubMed] [Google Scholar]
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism. Journal of Thrombosis and Thrombolysis 2016;41(1):3‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from www.cochrane‐handbook.org.
- Homans J. Thrombosis of the deep leg veins due to prolonged sitting. New England Journal of Medicine 1954;250(4):148‐9. [DOI] [PubMed] [Google Scholar]
- Hsieh HF, Lee FP. Graduated compression stockings as prophylaxis for flight‐related venous thrombosis: systematic literature review. Journal of Advanced Nursing 2005;51(1):83‐98. [DOI] [PubMed] [Google Scholar]
- Kuipers S, Schreijer AJ, Cannegieter SC, Büller HR, Rosendaal FR, Middeldorp S. Travel and venous thrombosis: a systematic review. Journal of Internal Medicine 2007;262(6):615‐34. [DOI] [PubMed] [Google Scholar]
- Landgraf H. Economy class syndrome: fiction or fact?. Zeitschrift für Ärztliche Fortbildung und Qualitätssicherung 1999;93(7):503‐7. [PubMed] [Google Scholar]
- Milne R. Venous thromboembolism and travel: is there an association?. Journal of the Royal College of Physicians of London 1992;26(1):47‐9. [PMC free article] [PubMed] [Google Scholar]
- Philbrick JT, Shumate R, Siadaty MS, Becker DM. Air travel and venous thromboembolism: a systematic review. Journal of General Internal Medicine 2007;22(1):107‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachdeva A, Dalton M, Amaragiri SV, Lees T. Graduated compression stockings for prevention of deep vein thrombosis. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD001484.pub3] [DOI] [PubMed] [Google Scholar]
- Scurr JH, Smith PD, Machin S. Deep vein thrombosis in airline passengers ‐ the incidence of deep vein thrombosis and the efficacy of elastic compression stockings. Cardiovascular Surgery 2001;9(2):159‐61. [DOI] [PubMed] [Google Scholar]
- Scurr JH, Machin SJ, Bailey‐King S, Mackie IJ, McDonald S, Smith PD. Frequency and prevention of symptomless deep‐vein thrombosis in long‐haul flights: a randomized trial. Lancet 2001;357(9267):1485‐9. [DOI] [PubMed] [Google Scholar]
- Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'Fallon WM, Melton LJ III. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population based study. Archives of Internal Medicine 1998;158(6):585‐93. [DOI] [PubMed] [Google Scholar]
- Sweeting MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta‐analysis of sparse data. Statistics in Medicine 2004;23(9):1351‐75. [DOI] [PubMed] [Google Scholar]
- Symington IS, Stack BH. Pulmonary thromboembolism after travel. British Journal of Diseases of the Chest 1977;71(2):138‐40. [DOI] [PubMed] [Google Scholar]
- Tagalakis V, Patenaude V, Kahn SR, Suissa S. Incidence of and mortality from venous thromboembolism in a real‐world population: The Q‐VTE Study Cohort. American Journal of Medicine 2013;126(9):832, e13‐21. [DOI] [PubMed] [Google Scholar]
- Timp J, Le CS, Van HV, Rosendaal FR, Cannegieter SC. Long‐haul travel and the risk of recurrent venous thrombosis. Journal of Thrombosis and Haemostasis 2015;13(Suppl 2):725. [Google Scholar]
- WHO launches study of venous thrombosis and air travel. www.who.int/mediacentre/news/releases/release40/en/ (accessed 18 November 2002; updated 20 February 2006).
- World Health Organisation. WHO research into global hazards of travel (WRIGHT) project. www.who.int/cardiovascular_diseases/guidelines/WRIGHT_INFORMATION/en/ (accessed March 2016).
References to other published versions of this review
- Clarke M, Fischer M, Hopewell S, Juszczak E, Eisinga A. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004002] [DOI] [PubMed] [Google Scholar]
- Clarke MJ, Hopewell S, Juszczak E, Eisinga A, Kjeldstrøm M. Compression stockings for preventing deep vein thrombosis in airline passengers. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004002.pub2] [DOI] [PubMed] [Google Scholar]


