Abstract
Background
Atypical squamous cells of undetermined significance (ASCUS) and low‐grade squamous intra‐epithelial lesions (LSIL) are minor lesions of the cervical epithelium, detectable by cytological examination of cells collected from the surface of the cervix of a woman.
Usually, women with ASCUS and LSIL do not have cervical (pre‐) cancer, however a substantial proportion of them do have underlying high‐grade cervical intra‐epithelial neoplasia (CIN, grade 2 or 3) and so are at increased risk for developing cervical cancer. Therefore, accurate triage of women with ASCUS or LSIL is required to identify those who need further management.
This review evaluates two ways to triage women with ASCUS or LSIL: repeating the cytological test, and DNA testing for high‐risk types of the human papillomavirus (hrHPV) ‐ the main causal factor of cervical cancer.
Objectives
Main objective
To compare the accuracy of hrHPV testing with the Hybrid Capture 2 (HC2) assay against that of repeat cytology for detection of underlying cervical intraepithelial neoplasia of grade 2 or worse (CIN2+) or grade 3 or worse (CIN3+) in women with ASCUS or LSIL. For the HC2 assay, a positive result was defined as proposed by the manufacturer. For repeat cytology, different cut‐offs were used to define positivity: Atypical squamous cells of undetermined significance or worse (ASCUS+), low‐grade squamous intra‐epithelial lesions or worse (LSIL+) or high‐grade squamous intra‐epithelial lesions or worse (HSIL+).
Secondary objective
To assess the accuracy of the HC2 assay to detect CIN2+ or CIN3+ in women with ASCUS or LSIL in a larger group of reports of studies that applied hrHPV testing and the reference standard (coloscopy and biopsy), irrespective whether or not repeat cytology was done.
Search methods
We made a comprehensive literature search that included the Cochrane Register of Diagnostic Test Accuracy Studies; the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), MEDLINE (through PubMed), and EMBASE (last search 6 January 2011). Selected journals likely to contain relevant papers were handsearched from 1992 to 2010 (December). We also searched CERVIX, the bibliographic database of the Unit of Cancer Epidemiology at the Scientific Institute of Public Health (Brussels, Belgium) which contains more than 20,000 references on cervical cancer.
More recent searches, up to December 2012, targeted reports on the accuracy of triage of ASCUS or LSIL with other HPV DNA assays, or HPV RNA assays and other molecular markers. These searches will be used for new Cochrane reviews as well as for updates of the current review.
Selection criteria
Studies eligible for inclusion in the review had to include: women presenting with a cervical cytology result of ASCUS or LSIL, who had undergone both HC2 testing and repeat cytology, or HC2 testing alone, and were subsequently subjected to reference standard verification with colposcopy and colposcopy‐directed biopsies for histologic verification.
Data collection and analysis
The review authors independently extracted data from the selected studies, and obtained additional data from report authors.
Two groups of meta‐analyses were performed: group I concerned triage of women with ASCUS, group II concerned women with LSIL.
The bivariate model (METADAS‐macro in SAS) was used to assess the absolute accuracy of the triage tests in both groups as well as the differences in accuracy between the triage tests.
Main results
The pooled sensitivity of HC2 was significantly higher than that of repeat cytology at cut‐off ASCUS+ to detect CIN2+ in both triage of ASCUS and LSIL (relative sensitivity of 1.27 (95% CI 1.16 to 1.39; P value < 0.0001) and 1.23 (95% CI 1.06 to 1.4; P value 0.007), respectively. In ASCUS triage, the pooled specificity of the triage methods did not differ significantly from each other (relative specificity: 0.99 (95% CI 0.97 to 1.03; P value 0.98)). However, the specificity of HC2 was substantially, and significantly, lower than that of repeat cytology in the triage of LSIL (relative specificity: 0.66 (95% CI 0.58 to 0.75) P value < 0.0001).
Authors' conclusions
HPV‐triage with HC2 can be recommended to triage women with ASCUS because it has higher accuracy (significantly higher sensitivity, and similar specificity) than repeat cytology. When triaging women with LSIL, an HC2 test yields a significantly higher sensitivity, but a significantly lower specificity, compared to a repeat cytology. Therefore, practice recommendations for management of women with LSIL should be balanced, taking local circumstances into account.
Summary of findings
Background
Target condition being diagnosed
Cervical cancer is the third most common cancer in women worldwide. It is estimated that, in 2008, approximately 530,000 women developed cervical cancer and that 275,000 died from the disease (Arbyn 2011). Cervical cancer primarily affects younger women, with the majority of cases becoming apparent between the ages of 30 and 50 years (Yang 2004). Nevertheless, of all malignant tumours cervical cancer is the one that is most easily preventable by screening. Cervical cancer screening is primarily performed using a Papanicolaou test, that is by taking a 'Pap smear'. Microscopical examination of these Pap smears may reveals cytological abnormalities, which may be classified as atypical, low‐ or high‐grade. By application of a confirmation test, colposcopy and histological examination of colposcopy‐targeted biopsies, cervical precancer can be identified. Cervical precancer is graded histologically as cervical intra‐epithelial neoplasia (CIN), grade CIN1, CIN2 or CIN3. CIN2 and CIN3 are often joined together as high‐grade CIN. The subsequent treatment of women with high‐grade CIN prevents development of cancer (Miller 1993). Through well‐organized screening and management of detected lesions, the incidence of cervical cancer can be reduced to a low level (IARC 2005; Arbyn 2009a).
Women with cytological lesions require further follow‐up or treatment, or both, depending on the severity of the lesion. Women with high‐grade cytological lesions should be referred immediately for further examination using the reference standard test that involves colposcopy (observation technique that can identify potential precancerous and cancerous lesions) and histological (tissue) examination of colposcopy‐targeted biopsies (Wright 2002; Jordan 2009). However, management of women with minor cytologic lesions remains controversial (Cox 1998; Cox 2005; Sawaya 2005; Soutter 1994; Stoler 2001). Until recently, follow‐up recommendations for women with atypical squamous cells of undetermined significance (ASCUS, or ASC‐US) and low‐grade squamous intra‐epithelial lesions (LSIL) varied from conservative repeat cytology (Robertson 1988; Coleman 1993; Flannelly 1994), to immediate referral for colposcopy and biopsy (Richart 1987; Noumoff 1987; Richart 1993).
The natural history of minor cytological lesions is difficult to predict on the basis of cytomorphological (appearance of cells) grounds. These lesions often regress spontaneously without treatment (Narod 1991; Ostor 1993; Melnikow 1998). Therefore, referring all women with minor cytological lesions for further gynaecological examination would mean an increase in over‐diagnosis and over‐treatment (Murdoch 1992; Ferenczy 1995). Over‐referral would also cause unnecessary anxiety in women (Wilkinson 1990), with substantial increases in costs to the healthcare system (Ferenczy 1995). Moreover, lack of availability of colposcopic services at affordable prices often makes such a policy unrealistic.
Although most women with an ASCUS or LSIL smear result do not have clinically significant disease, a substantial proportion of them do have histopathologically‐confirmed high‐grade cervical intra‐epithelial neoplasia (CIN) (Cox 1995; Wright 1995; Kinney 1998). From a population of women screened in the USA, it was estimated that one third of CIN were discovered on follow‐up of a previous smear with ASCUS (Kinney 1998). An appropriate triage (prioritising) method should identify those women that have, or will develop, a cervical cancer precursor. At the same time, an accurate triage would reduce the risk of over‐diagnosis and over‐treatment, and should limit adverse obstetric outcomes associated with excision of CIN lesions (Arbyn 2008b; Kyrgiou 2006).
Given the evidence concerning the etiological (causative) role of high‐risk human papillomavirus (HPV) infections in the development of cervical cancer and its precursors (zur Hausen 1994; Walboomers 1999; Bosch 2002; IARC 2007), HPV testing has been proposed as an alternative triage method to distinguish between women with minor cytological lesions who need referral for colposcopy, and those who can be referred back to the normal screening schedule (Wright 1995; Cox 1998).
Index test(s)
The index test of interest is the B‐probe of the Hybrid Capture 2 assay (HC2, Qiagen, Gaithersburg, MD, USA), which detects DNA of 13 high‐risk HPV (hrHPV) types. HC2 contains a cocktail of 13 RNA probes that hybridise viral DNA of the following hrHPV types: HPV16, HPV18, HPV31, HPV33, HPV35, HPV39, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59, and HPV68. The A‐probe of HC2, which targets five low‐risk HPV types (HPV6, HPV11, HPV42, HPV43, and HPV44) is not considered in the current systematic review.
HC2 is a sandwich hybridisation technique based on type‐specific full genomic HPV RNA probes hybridising with DNA from human papillomaviruses in the test material (Lorincz 1997). DNA‐RNA hybrids are captured by immobilised antibodies against RNA‐DNA hybrids, coated on the surface of microplates. The antibody is conjugated to alkaline phosphatase to leave a chemiluminescent substrate, that yields a light signal measured with a luminometer. The intensity of light emission, expressed in relative light units (RLU), provides a semi‐quantitative measure proportional to the amount of target HPV DNA present. The system is calibrated by positive and negative control samples provided by the manufacturer. The RLU corresponding to the average light intensity for the positive control sample is fixed at '1'. In standard conditions of the HC2 test, RLU = 1 corresponds to a detection threshold of 1 pg of HPV, 16 DNA/mL, or 5000 copies of HPV genome per sample.
HC2 is a standardised kit that can be easily used in a large range of laboratories. It is the only commercially available HPV test that is approved by the US Food and Drugs Administration for triage of women with ASCUS, or, in primary cervical cancer screening, for women older than 30.
Alternative test(s)
Repetition of cervical cytology is the conventional method of triage women with ASCUS or LSIL. When repeat cytology shows cervical abnormality again, three to six months after the first observation of ASCUS or LSIL, women are referred for further diagnostic investigations.
Rationale
Clinicians need an accurate triage method to decide whether a woman with minor cervical cytological abnormalities needs further investigation with colposcopy and biopsies. Testing for hrHPV DNA or repeating the cytological test are two possible triage methods.
In this Cochrane review the review authors will update previous meta‐analyses, where accuracy estimates of triage tests were pooled separately. Newer hierarchical or multilevel models, adapted by the Diagnostic Test Accuracy Working Group of The Cochrane Collaboration, will be applied (Macaskill 2010). These models account for the intrinsic negative correlation between sensitivity and specificity and for the usually considerable within‐ and inter‐study heterogeneity in test accuracy meta‐analyses.
Objectives
Main objective
For studies where both triage methods, i.e. repeat cytology and the HC2 assay, were assessed:
To assess the sensitivity and specificity of triage with HC2 and with repeat cytology to detect underlying cervical intraepithelial neoplasia of grade 2 or worse (CIN2+), or grade 3 or worse (CIN3+), in women with an index smear showing ASCUS (triage group I) or LSIL (triage group II), and to compare the accuracy of both triage methods.
Secondary objectives
To assess the accuracy of the HC2 assay to detect CIN2+ or CIN3+ in women with ASCUS or LSIL in a larger group of studies that investigated hrHPV testing, irrespective whether repeat cytology was done.
Investigation of sources of heterogeneity
The following sources of heterogeneity were investigated:
Different cytological classification systems used to categorise equivocal and mild cytological abnormalities.
Characteristics of the study population (study location, age distribution).
Properties of the HPV testing (collection device, transport medium).
Properties of repeat cytology (collection device, preparation method (conventional or liquid‐based), cytological thresholds).
Blinding of interpreters for other test results.
Procedures of reference standard verification.
Methods
Criteria for considering studies for this review
Types of studies
Two types of studies were considered:
Studies with concomitant testing where all study participants were tested with the HC2 assay and repeat cytology, followed by verification with the reference standard; and studies where all study participants were tested only with HC2 followed by verification with the reference standard (coloscopy and biopsy).
Randomised clinical trials (RCTs) where study participants were randomised to HPV‐based triage or repeat cytology, and where, subsequently, all women were submitted to verification with the reference standard (coloscopy and biopsy).
Participants
Participants were women with a cervical cytology result of ASCUS (triage group I) or LSIL (triage group II) detected in the framework of cervical cancer screening. For a discussion on the cytological definitions of ASCUS and LSIL the reader is referred to Appendix 1.
Index tests
The index test was the B probe of the HC2 assay, which detects DNA of 13 hrHPV types (see Index test(s)).
For the main meta‐analysis on the accuracy of triage with HC2, the review authors only considered the standard cut‐off of test positivity as defined by the manufacturer. This standard cut‐off of test positivity corresponds with a light signal of the test sample equivalent to that of a positive control containing 1 pg/mL of HPV DNA (RLU = 1).
Comparator tests
The conventional strategy to triage women with ASCUS or LSIL is to repeat the cytology test. Usually triage is done within six months after the first observation of ASCUS or LSIL. However, in practice this delay can vary between three to 12 months. When the result of the repeat cytology test is positive, women are referred for further diagnostic investigation with the reference standard. Three possible cut‐offs to define a positive repeat cytology result will be distinguished: ASCUS+, LSIL+, and high‐grade squamous intraepithelial lesion or worse (HSIL+). Cervical cytology testing can be performed with: (1) the Pap smear, where cellular material scraped from the transformation zone of the cervix is transferred to a glass slide and fixed, or (2) using liquid cytology, where scraped cervical cells are transferred into a vial with fixating fluid (Arbyn 2007; Arbyn 2008a).
Comparison of the accuracy of repeat cytology (comparator test) with HC2 (index test) was an optional selection criterion for inclusion in the review.
Target conditions
Presence of histologically‐confirmed high‐grade CIN was the target outcome of disease. Two separate outcomes were distinguished:
CIN2 or worse disease (CIN2, CIN3, invasive squamous cervical cancer, and adenocarcinoma of the cervix (CIN2+));
CIN3 or worse (CIN3, invasive squamous cervical cancer, and adenocarcinoma of the cervix (CIN3+)).
CIN3 is the most relevant clinical outcome, since it is considered to be an obvious precursor of cervical cancer, whereas CIN2 is a mixed condition that is less reproducible, with a lower probability of progression to cervical cancer. However, the outcome CIN2+ is reported more often than CIN3+. See Appendix 1 for a more detailed discussion on the natural history of the different degrees of CIN.
Reference standards
The following reference standards were considered as acceptable for judging on presence or absence of the target condition: colposcopy and colposcopy‐directed punch biopsies or excision biopsies by large loop excision of the transformation zone or conization, with or without endocervical curettage, followed by histological verification of the biopsy specimen. Cases where no biopsy was taken, because colposcopy was negative and satisfactory, were considered as being free of CIN2+.
An overview of the key elements of the Cochrane review is summarised in Table 5.
1. Key elements of the systematic review.
| Study questions | What is the accuracy of HPV DNA testing and repeat cytology to detect CIN2, or worse (CIN2+), disease in women with ASCUS or LSIL? |
|
Patient population |
Women with a cervical cytology result showing ASCUS or LSIL. |
| Prior testing | Prior screening, screening for first time, or not documented. |
| Setting | Cervical cancer screening using cytology and follow‐up of women with minor cytological lesions. |
| Index test | Triage with HPV DNA testing using HC2 (test positivity defined as RLU > 1). |
|
Comparator test |
Triage with repeat cytology considered at 3 cut‐offs (ASCUS+, LSIL+, HSIL+). |
| Target disease | Cervical cancer precursors: CIN2+ or CIN3+. |
| Reference standard | Colposcopy with colposcopy‐targeted biopsies or biopsies from all cases, considering the histological result as the outcome, where available, but accepting negative colposcopy as sufficient ascertainment for absence of CIN2+ or CIN3+ when no biopsies were taken. |
| Importance | ASCUS and LSIL indicate an increased risk for having or developing cervical precancer. Nevertheless, frequently no underlying or incipient precursors CIN2+ can be identified. Therefore, accurate triage methods are needed that identify women with cervical precancer who are at increased risk for developing cancer, but that also avoid over‐diagnosis and over‐treatment. |
| Studies |
1) Triage of ASCUS Outcome CIN2+: 39 studies (including 13,196 women) allowing evaluation of the accuracy of HC2; 10 of these studies (including 5261 women) permitted investigation of the accuracy of repeat cytology. Outcome CIN3+: 17 studies (6144 women) with accuracy of HC2; 4 of these studies (2726 women) allowed assessment of accuracy of both HC2 and repeat cytology. 2) Triage of LSIL Outcome CIN2+: 24 studies (including 9983 women) allowed evaluation of the accuracy of HC2; 6 of these studies (including 1591 women) also allowed assessment of the accuracy of repeat cytology. Outcome CIN3+: 14 studies (8253 women) with accuracy of HC2; 4 of these studies (1295 women) permitted assessment of accuracy of both HC2 and repeat cytology. |
Abbreviations
ASCUS = atypical squamous cells of undetermined significance
ASCUS+ = ASCUS or worse
CIN2 = cervical intraepithelial neoplasia of grade 2
CIN2+ = cervical intraepithelial neoplasia of grade 2 or worse
CIN3+ = cervical intraepithelial neoplasia of grade 3 or worse
HC2 = Hybrid Capture 2 assay
HPV = human papillomavirus
HSIL+ = high‐grade squamous intraepithelial lesion or worse
LSIL = low‐grade squamous intra‐epithelial lesions
LSIL+ = LSIL or worse
RLU = relative light units
Search methods for identification of studies
A systematic literature search identified articles published between 1992 and 2010 that contain data allowing assessment of the research questions. A previous meta‐analysis revealed that in all retrieved triage studies published before 1992, only obsolete HPV testing systems were used; these systems are not used in current practice (Arbyn 2002; Arbyn 2004a).
No effort was made to identify studies where the only triage performed was by repeating Pap smears because:
Before the 1990s disparate cytological classification systems were used to categorise cervical abnormalities, impeding pooling of data (Lundberg 1989).
For reports published since the adaption of The Bethesda‐System (TBS) (Lundberg 1989), recommendations for follow up of ASCUS involved repeat cytology with colposcopy if repeat cytology was abnormal, or direct referral for colposcopy and biopsy, which was considered as over‐management (Kurman 1994).
In the context of the evaluation of HPV‐based triage, studies had to be designed to include submission to verification by the reference standard for all participants.
Electronic searches
Studies were retrieved from the following electronic bibliographical databases: Cochrane Register of Diagnostic Test Accuracy Studies (up to published issue 12, 2011); the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, up to published issue 12, 2011), MEDLINE (through PubMed, from January 1992 to January 2011), and EMBASE (from January 1992 to January 2011). The search strategies for all the databases can be found in Appendix 2.
More recent searches, up to December 2012, targeted reports on the accuracy of triage of ASCUS or LSIL with other HPV DNA assays (Arbyn 2012), or HPV RNA assays (Arbyn 2013) and other molecular markers (Roelens 2012). These searches will be used for new Cochrane reviews as well as for updates of the current review. The recent references retrieved by these recent searches can be found in Studies awaiting classification.
The search string for PubMed‐MEDLINE was saved into My NCBI, an electronic search tool developed by the National Center for Biotechnology Information (NCBI) at the National Library of Medicine, which saves searches and automatically retrieves newer references not yet picked up at previous searches. An auto‐alert was also set up in EMBASE.
The review authors acknowledge the possible limitations of the electronic database searches. The structure of the search strategy (index test AND target condition AND triage concept), in which the triage concept is also combined with the Boolean operator 'AND', may restrict the search. This option to increase specificity includes a risk of missing relevant studies. Therefore, other methods and resources were used to reduce this risk (see below).
Searching other resources
The 'related articles' feature in PubMed was used, from the original included studies; likewise for Scopus, to retrieve articles that cite the originally included studies.
Retrieval of reports was extended by manual searching of the reference lists of included papers, and by screening the tables of contents (for 1992 to December 2010) of the following journals: American Journal of Obstetrics and Gynecology; Cancer Cytopathology; Diagnostic Cytopathology; Gynecologic Oncology; and Obstetrics and Gynecology; which are journals that, according to previous meta‐analysis, contributed multiple references.
We used the bibliographic database CERVIX of the Unit of Cancer Epidemiology at the Scientific Institute of Public Health (Brussels, Belgium) containing more than 20,000 references, mostly on cervical cancer, as an additional source.
The Trials Search Co‐ordinator of the Cochrane Gynaecological Cancer Review Group requested a search from Mrs Ruth Mitchell, the Trials Search Co‐ordinator of the Cochrane Renal Group, who is managing and developing the Cochrane Register of Diagnostic Test Accuracy Studies on behalf of The Cochrane Collaboration.
Data collection and analysis
Selection of studies
References were selected if they fulfilled the conditions for study selection, namely if they:
Included women with ASCUS or LSIL.
Used triage testing with HC2.
Verified with the reference test.
Used triage with repeat cytology (optional).
One selection criterion, triage by repeat cytology as the comparator test, was optional. See Criteria for considering studies for this review. For studies where no repeat cytology was done, only the accuracy of HC2 testing was assessed.
Three review authors (MA, CS and JR) verified inclusion and exclusion of eligible studies independently and discussed any discordance. If no consensus could be reached, other review authors (PMH or EP) were consulted.
Data extraction and management
After conversion of the cytological classification into the 1988 version of TBS, and separation of data by triage group (as explained in Participants), the numbers of true‐positives, false‐positives, false‐negatives, and true‐negatives defined at the considered thresholds were extracted from each study. The main review authors (MA, CS (until 2009) and JR (after 2009)) separately extracted data from the selected studies and subsequently discussed the extracted data where there were differences. Additional data on the absolute numbers of false and true positives and negatives were obtained from report authors in cases where accuracy parameters were reported but where the absolute number of false and true positives and negatives could not be derived or computed from the reported data.
Besides the quality issues (see Assessment of methodological quality), other trial properties were extracted from the included studies and summarised in comprehensive tables (Characteristics of included studies and Appendix 3).
Assessment of methodological quality
Methodological quality
The methodological quality of selected studies were assessed using the QUADAS guidelines (Whiting 2003). For each study, a methodological quality table was completed. Table 6 contains an explanation of how the QUADAS items should be understood in terms of triage of women with minor cervical cytology lesions.
2. QUADAS quality assessment table.
| Quality item | Definition | Comment |
| Representative spectrum | Was the spectrum of participants representative of the women who will receive the test in practice? | By restricting the study participants to the two triage groups, the spectrum necessarily coincides with the clinical indication of HPV testing: women with a cervical cytology result showing squamous atypia (ASCUS = triage group I) or mild dysplasia (LSIL = triage group II). However within the spectrum of clinical groups we can distinguish:
|
| Acceptable reference test | Is the reference standard likely to classify the target condition correctly? | The following possible reference standards were distinguished:
The type of biopsy (punch biopsy or excision biopsy) was recorded. |
| Acceptable delay between tests | Is the time period between reference standard and index test short enough to be reasonably sure that the target condition did not change between the two tests? | The delay between the index triage with HC2 (and repeat cytology if done) and colposcopy and biopsy will be assessed. The delay should be no more than 6 months. Also the delay between the finding of a case with ASCUS or LSIL and the triage test was noted. |
| Partial verification avoided | Did the whole sample, or a random selection of the sample, receive verification using a reference standard of diagnosis? | By imposing complete verification for all participants, verification bias should be avoided. In randomised trials, verification is restricted to all women with positive results in the triage test, but this will not involve bias of relative sensitivity or relative PPV. |
| Differential verification avoided | Did participants receive the same reference standard regardless of the index test result? | For studies where the index triage test (HC2 assay) and the comparator triage test (repeat cytology) were used, all subjects should be submitted to the same type of verification with reference standard used to verify presence or absence of CIN2+ or CIN3+. |
| Incorporation avoided | Was the reference standard independent of the index test? (i.e. the index test did not form part of the reference standard.) | By imposing complete verification by colposcopy and histological interpretation for all participants, we avoided incorporation of the triage test in the outcome assessment. |
| Reference standard results blinded | Were the reference standard results interpreted without knowledge of the results of the index or comparator tests? | It was noted whether or not colposcopists and histologists were aware of the HC2 or repeat cytology result. |
| Index test results blinded | Were the index test or comparator test results interpreted without knowledge of the results of the reference standard or the other test? | Since the HC2‐result is generated by the assay, based on light emission measured by a luminometer (expressed as a relative light unit, a quantified computer‐generated measure), objective assessment of the index triage test is assured. Independence of repeat cytology interpretation towards the HC2 result was noted. |
| Relevant clinical information | Were the same clinical data available when test results were interpreted as would be available when the test is used in practice? | The most essential clinical information is the triage group (ASCUS or LSIL), which, in principle, always is available in clinical practice. |
| Uninterpretable results reported | Were non‐interpretable or intermediate test results reported? | Given the definition of the quantitatively defined cut‐off for a positive HC2 test, no problems are expected regarding interpretation. Occurrence of unsatisfactory HC2 (insufficient material, for instance) was notified. For repeat cytology, presence of inadequate preparations will be recorded. |
| Withdrawals explained | Were withdrawals from the study explained? | Loss of participants for whom no outcome could be obtained was recorded. |
Abbreviations
ASCUS = atypical squamous cells of undetermined significance
BSCC = British Society of Clinical Cytology
CIN = cervical intraepithelial neoplasia
CIN2+ = cervical intraepithelial neoplasia of grade 2 or worse
CIN3+ = cervical intraepithelial neoplasia of grade 3 or worse
HC2 = Hybrid Capture 2 assay
HPV = human papillomavirus
HPV+ = human papillomavirus positive
LSIL = low‐grade squamous intra‐epithelial lesions
PPV = positive predictive value
Other covariate information
All quality issues and other study characteristics were coded to allow subgroup meta‐analysis and by including covariates in multi‐variate regressions (see Investigations of heterogeneity).
Where possible, age‐stratified data were extracted to study the variation of test accuracy with age.
Statistical analysis and data synthesis
Separate analyses were performed for the two triage groups (ASCUS, LSIL) and the two disease thresholds (CIN2+, CIN3+).
Absolute accuracy
METADAS, an SAS macro for meta‐analysis of diagnostic accuracy studies, was used to compute the pooled sensitivity and specificity and to plot the summary receiver operating curves (SROC) curve, with summary point and corresponding 95% confidence ellipse (Takwoingi 2009). METADAS can fit two statistical models: the hierarchical summary receiver operating curves (HSROC) and the bivariate model (Rutter 2002; Reitsma 2005; Chu 2006). For the computation of the pooled absolute sensitivity and specificity of the evaluated triage tests separately, we fitted the bivariate model. In this case, all tests were considered as dichotomous variables (positive or negative result considering the standard cut‐off of positivity).
Relative accuracy
Again, for computation of the relative sensitivity and specificity, we used the bivariate model in METADAS by adding a covariate for test, which estimates differences in logit sensitivity and logit specificity (Reitsma 2005; Takwoingi 2009).
Where no convergence was reached (in general when a small number of studies were included, in studies assessing both repeat cytology and HC2), accuracy parameters were estimated by omitting the correlation between the logit of true positivity rate (TPR) and the logit of the false positivity rate (FPR).
Investigations of heterogeneity
Multiple regressions were performed using the METADAS macro with each time point providing another covariate to verify the influence of study population and test characteristics on the accuracy estimates. If convergence failed for the bivariate model, the correlation between the logit TPR and logit FPR was removed to run separate univariate analyses, which should give consistent estimates of the means and variances of model parameters with some loss in efficiency (Riley 2007).
In the studies that provided data stratified by age group, particular attention was given to variation of test accuracy according to age.
To verify whether conclusions were robust over all subcategories of equivocal cervical cytology, as defined through different cytology classification systems, the review authors performed subgroup meta‐analyses restricting selected studies by the classification system used for reporting cervical cytology results (see Assessment of methodological quality: other covariates).
Sensitivity analyses
The influence of outlying results was addressed by repeating the meta‐analysis omitting the studies with extreme results. A sensitivity analysis was also performed by excluding randomised trials where not all cases were submitted to verification with the reference test. Finally, the absolute sensitivity and specificity estimates were pooled separately for the studies where only the HC2 assay was evaluated and where the comparator test (repeat cytology) was not applied.
Assessment of reporting bias
Publication bias
We used the effective sample size funnel plot and associated regression test of asymmetry to detect publication bias (Deeks 2005). These approaches are more appropriate in systematic reviews of diagnostic tests than the standard tests developed to detect publication bias in meta‐analyses of randomised trials of healthcare interventions. It has been shown that the rank correlation test (Begg 1994), and the asymmetry regression test (Egger 1997), suffer from serious degrees of bias when applied to test accuracy studies (Deeks 2005).
Results
Results of the search
We identified 2938 references from the electronic searches in the Cochrane Register of Diagnostic Test Accuracy Studies, the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library), MEDLINE (through Pubmed) EMBASE and CERVIX databases. After initial evaluation of their titles, 2641 studies were excluded. We read the abstracts of the remaining 297 references, and the full papers of those that appeared to be potentially eligible. We excluded an additional 254 studies after reading the abstracts and/or full papers. A summary of the search results, with the main reasons for excluding publications, is shown in Figure 1.
1.
Flowchart of retrieval, selection and exclusion of studies.
Included studies
We identified 39 different studies, allowing computation of the accuracy of HC2 triage in women with ASCUS: (Manos 1999; Bergeron 2000; Lytwyn 2000; Shlay 2000; Rebello 2001; Morin 2001; Zielinski 2001; Pretorius 2002; Kulasingam 2002; Solomon 2001/Sherman 2002; Wensveen 2003; Lonky 2003; Ordi 2003; Guyot 2003; Cuzick 2003; Andersson 2005; Davis‐Devine 2005; Nieh 2005; Giovannelli 2005; Chen 2005b; Dalla Palma 2005; Kiatpongsan 2006; Bergeron 2006; Holladay 2006; Kelly 2006; Monsonego 2006; You 2007; Ronco 2007; Cuschieri 2007; De Francesco 2008; Szarewski 2008; Siddiqui 2008; Monsonego 2008; Lee 2009; Cattani 2009; Silverloo 2009; Huang 2009; Denton 2010; Del Mistro 2010).The accuracy of HC2 in triage of LSIL women could be evaluated in 24 studies (Bergeron 2000; Lytwyn 2000; Lee 2001; Rebello 2001; Zielinski 2001; Pretorius 2002; Kulasingam 2002; Sherman 2002; Guyot 2003; Andersson 2005; Chen 2005b; Holladay 2006; Monsonego 2006; You 2007; Ronco 2007; De Francesco 2008; Szarewski 2008; Monsonego 2008; Lee 2009; Cattani 2009; Huang 2009; Denton 2010; Voss 2010; Castle 2010a).
Only 10 of the 39 selected ASCUS studies presented data on triage by repetition of the Pap smear (Manos 1999; Bergeron 2000; Lytwyn 2000; Solomon 2001; Morin 2001; Kulasingam 2002; Andersson 2005; Monsonego 2008; Silverloo 2009; Del Mistro 2010), and only six of the 24 selected LSIL triage studies (Bergeron 2000; Lytwyn 2000; Kulasingam 2002; Sherman 2002; Andersson 2005; Monsonego 2008).
From the ALTS (ASCUS‐LSIL Triage Study) study, we selected a first report for the extraction of accuracy data on ASCUS triage for the outcome of CIN2+ (Solomon 2001). For information on accuracy of ASCUS triage for outcome CIN3+ and LSIL triage (all outcomes), a second report of the ALTS study was used (Sherman 2002), which was completed by data sets received directly from the trial authors. More data sets were received from S Anderson, P Castle, A Lytwyn, J Monsonego, J Pretorius, G Ronco, M Schiffman and A Szarewski.
Description of the studies
Details of the study designs, the characteristics of the enrolled women and the applied tests (sampling devices, transport media, preparation methods of the repeat smear) are summarised in Characteristics of included studies and in Appendix 3.
Study design
Three studies were randomised controlled trials (Lytwyn 2000; Solomon 2001; Sherman 2002; Cuzick 2003). In all other studies, a concomitant testing design was used, where enrolled women received the HPV test, a repeat smear (if done) and the reference standard.
Study size
In total, 13,196 women with ASCUS, included in 39 studies, were triaged with HC2 at the standard test cut‐off (1 pg/mL) and 5261 of them also received a repeat Pap smear. Twenty‐one studies were small, each contributing fewer than 200 women; 11 studies were of intermediate size, each contributing between 200 and 500 women; and seven studies were large, each contributing more than 500 women. One of the large studies, the ASCUS‐LSIL Triage Study (ALTS), enrolled more than 2300 women (Solomon 2001; Sherman 2002).
Nine‐thousand nine‐hundred and eighty‐three women with LSIL, included in 24 studies were triaged with HC2 (at 1 pg/mLl) and 1591 of them also received a repeat smear. Fourteen studies were small with fewer than 200 women, seven studies were of intermediate size ranging 200 to 499 women, and three trials were large with more than 500 women.
Clinical setting and population characteristics
In all studies, women were recruited in colposcopy clinics or from gynaecologic services to which they had been referred because of cytologic abnormalities. In three studies, the referred women with ASCUS cytology had repeated atypical cytology (Rebello 2001; Zielinski 2001; Guyot 2003). One study included only women with ASCUS occurring after two sequential normal smears (Morin 2001). Fifteen studies excluded women with a history of CIN, cervical surgery, or biopsy (Lytwyn 2000; Solomon 2001; Morin 2001; Zielinski 2001; Kulasingam 2002; Sherman 2002; Cuzick 2003; Davis‐Devine 2005; Chen 2005b; Kiatpongsan 2006; Ronco 2007; De Francesco 2008; Szarewski 2008; Cattani 2009; Huang 2009).
Women with atypical squamous or atypical glandular endocervical cells were included in two studies (Shlay 2000; Wensveen 2003). Enrolment of women with equivocal cytology was restricted to ASC‐US cases, in fifteen studies where the 2001 Bethesda System (TBS 2001) was used (Pretorius 2002; Davis‐Devine 2005; Giovannelli 2005; Dalla Palma 2005; Kiatpongsan 2006; Bergeron 2006; Holladay 2006; Kelly 2006; You 2007; Siddiqui 2008; Monsonego 2008; Huang 2009; Denton 2010; Del Mistro 2010; Castle 2010a).
Preparation of the repeat Pap test
A conventional Pap smear was used as cytologic triage method in six studies (Bergeron 2000; Lytwyn 2000; Morin 2001; Andersson 2005; Silverloo 2009; Del Mistro 2010). A liquid‐based cytology specimen was prepared in four other studies (Manos 1999; Kulasingam 2002; Monsonego 2008; Solomon 2001/Sherman 2002).
Methodological quality of included studies
The overall methodological quality of all included studies is summarized in Figure 2. Overall, the quality of included studies was good with average negative scores for the 11 QUADAS items varying between 0% and 1à%; equivocal scores varying between 2% and 71% and positive scores varying between 29% and 95% of included studies (Figure 3). The clinical spectrum of participants was clearly representative in 74% of included studies and unclear in 21%. The reference standard was of acceptable quality in 95%, unclear in 5%, and never of unacceptable quality. The delay between triage testing and verification with the reference standard was short in 69%, unreported in 26% and long in 5%. Partial verification was avoided in 88%, unclear or not avoided in 12%, whereas differential verification was clearly absent in 86% and unclear in 5%. Incorporation bias was avoided in 93% and unclear in 5%. Blinding of the reference triage tests was reported in 52% of the studies and unreported in 45%. For the 8th QUADAS criterion (blinding of triage test), it was accepted that all HC2 interpretations were blinded, since results are generated automatically and, therefore, only studies including repeat cytology could be judged as potentially blinded, not‐blinded or blinding unclear. Using this principle, 86% of the studies were considered as blinded, and for the other 14% it was unclear whether the comparator test (repeat cytology) was blinded towards the index test (HC2). Only 36% of studies reported on uninterpretable results, and 29% either explained any withdrawals or were clear that there were no withdrawals.
2.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.

Methodological quality summary: review authors' judgements about each QUADAS item for each included study.
Findings
The main findings concerning the primary questions about the absolute and relative accuracy measures derived from studies where both triage methods (HC2 and repeat cytology) were used are presented first, and are summarised in the Summary of Results tables.
Subsequently, we assess the absolute accuracy of triage with HC2, derived from a larger group of studies that also included evaluations without repeat cytology (secondary objective). The results are summarised in tables included in the appendices.
1 Primary objective: accuracy of HC2 and repeat cytology in studies where both triage methods were applied
1.1 Triage of ASCUS
1.1.1 Absolute accuracy of HC2 triage of ASCUS cases
For the studies where both HC2 testing and repeat cytology were done, the absolute sensitivity of HC2, pooled from 10 studies, was 90.9% (95% CI 85.7 to 94.4%) for CIN2+. The pooled sensitivity from four studies where the outcome was CIN3+ was 94.8% (95% CI 89.6 to 97.5%) (Table 3). The pooled specificity was 60.7% (95% CI 52.9 to 68.0%) and 56.6% (95% CI 39.4 to 72.3%) for predicting absence of CIN2+ or CIN3+, respectively.
Summary of findings 3. Summary of the main meta‐analyses of the absolute and relative accuracy of HC2 and repeat cytology used to triage women with ASCUS.
| Studies where HC2 triage and repeat cytology were applied | |||||||
| Triage test | Test cut‐off | Outcome | Studies | Accuracy parameter | Pooled estimate | (95% CI) | Analysis |
| HC2 | RLU > (1 pg/mL) | CIN2+ | 10 | Absolute sensitivity | 90.9% | (85.7 to 94.4%) | 29 |
| 10 | Absolute specificity | 60.7% | (52.9 to 68.0%) | ||||
| CIN3+ | 4 | Absolute sensitivity* | 94.8% | (89.6 to 97.5%) | 30 | ||
| 4 | Absolute specificity* | 56.6 % | (39.4 to 72.3%) | ||||
| Repeat cytology | ASCUS+ | CIN2+ | 10 | Absolute sensitivity | 71.5% | (62.9 to 78.8%) | 17 |
| 10 | Absolute specificity | 68.4% | (59.9 to 75.8%) | ||||
| CIN3+ | 4 | Absolute sensitivity* | 77.9% | (64.0 to 87.6%) | 20 | ||
| 4 | Absolute specificity* | 57.4% | (40.3 to 73.0%) | ||||
| HC2 (RLU > 1) vs repeat cytology (ASCUS+) | CIN2+ | 10 | Relative sensitivity | 1.27 | (1.16 to 1.39) | 1 | |
| 10 | Relative specificity | 0.99 | (0.97 to 1.03) | ||||
| CIN3+ | 4 | Relative sensitivity* | 1.14 | (1.06 to 1.22) | 4 | ||
| 4 | Relative specificity* | 0.99 | (0.89 to 1.09) | ||||
*Univariate analyses using the bivariate model run separately for sensitivity and specificity.
Abbreviations
ASCUS+ = ASCUS or worse
CIN2+ = cervical intraepithelial neoplasia of grade 2 or worse
CIN3+ = cervical intraepithelial neoplasia of grade 3 or worse
HC2 = Hybrid Capture 2 assay
RLU = relative light units
1.1.2 Absolute accuracy of cytology triage of ASCUS cases
The pooled sensitivity dropped substantially when the test threshold was increased: 71.5% (95% CI 62.9 to 78.8%) at ASCUS+, 44.1% (95% CI 33.3 to 55.5%) at LSIL+, and 15.8% (95% CI 6.5 to 33.6%) at HSIL+ for endpoint CIN2+; and 77.9% (95% CI 64.0 to 87.6%) at ASCUS+, 53.5% (95% CI 17.8 to 85.9%) at LSIL+, and 33.2% (95% CI 6.1 to 79.2%) at HSIL+ for endpoint CIN3+. The pooled specificity of repeat cytology rose with increasing test threshold: between 68.4% (95% CI 59.9 to 75.8%) at ASCUS+, and 98.3% (95% CI 96.7 to 99.1%) at HSIL+, for excluding CIN2+; and between 57.4% (95% CI 40.36 to 73.0%) at ASCUS+, and 95.6% (95% CI 93.6 to 97.1%) at HSIL+, for excluding CIN3+ (Table 3; Appendix 4).
1.1.3 Relative accuracy of HC2 compared to repeat cytology in triage of ASCUS cases
In order to compute the relative sensitivity and specificity of HC2 versus repeat cytology at different cut‐offs, the triage test (HC2 or repeat cytology) was added as a covariate in the bivariate model.
Triage of ASCUS cases with HC2 was 27% more sensitive than repetition of the Pap smear at the lowest cytological cut‐off (ASCUS+) for detecting CIN2+ (relative sensitivity: 1.27; 95% CI 1.16 to 1.39; P value < 0.0001) (Table 3; Figure 4). This contrast rose further when the cut‐off of the repeated smear increased. The specificity of a repeat smear at the cut‐off ASCUS+ was nearly identical (relative specificity: 0.99; 95% CI 0.97 to 1.03) to the specificity of HC2 for exclusion of CIN2+. At higher cytological cut‐offs, HC2 became progressively less specific than repeat cytology (Figure 5).
4.

Analysis 1: Summary ROC‐plot (Bivariate model with test as covariate): Sensitivity and specificity of triage of ASCUS with HC2 (black) (at RLU>1) versus repeat cytology (red) at cut‐off ASCUS+ for an outcome of underlying CIN2+ based on within study comparisons.
5.

Analysis 2: Summary ROC‐plot (Bivariate model with test as covariate): Sensitivity and specificity of triage of LSIL with HC2 (black) (at RLU>1) versus repeat cytology (red) at cut‐off ASCUS+ for an outcome of underlying CIN2+ based on within study comparisons.
Due to failure of convergence, the relative accuracy measures for detection of CIN3+ had to be computed by removing the correlation parameter from the bivariate model. HC2 was more sensitive than repeat cytology for detection of CIN3+, and the difference rose with increasing cytological cut‐off: ratios ranging from 1.13 (95% CI: 1.06 to 1.22) at cut‐off ASCUS, to 2.82 (95% CI: 0.79 to 10) at cut‐off HSIL. However, the specificity of HC2 for the outcome of CIN3+ was similar to repeat cytology at ASCUS, but became significantly lower at higher cytological cut‐offs: ratios ranging from 1.13 (95% CI: 1.06 to 1.22) at cut‐off ASCUS, to 2.82 (95% CI: 0.79 to 10) at cut‐off HSIL (Table 7).
3. Triage of ASCUS: relative sensitivity and specificity of HC2 versus repeat cytology.
| Cut‐off repeat cytology | Studies | Relative sensitivity (95% CI) | Relative specificity (95% CI) | P value | |
| Effect on sensitivity** | Effect on specificity** | ||||
| Outcome CIN2+ | |||||
| ASCUS+ | 10 | 1.27 (1.16 to 1.39) | 0.99 (0.97 to 1.03) | < 0.001 | 1.000 |
| LSIL+ | 6 | 2.19 (1.71 to 2.81) | 0.69 (0.64 to 0.75) | < 0.001 | < 0.001 |
| HSIL+ | 5 | 8.06 (4.46 to 14.58) | 0.62 (0.55 to 0.69) | < 0.001 | < 0.001 |
| Oucome CIN3+ | |||||
| ASCUS+ | 4* | 1.14 (1.06 to 1.22) | 0.99 (0.89 to 1.09) | 0.01 | 0.6 |
| LSIL+ | 4* | 1.74 (0.86 to 3.54) | 0.62 (0.55 to 0.72) | 0.08 | 0.004 |
| HSIL+ | 4* | 2.82 (0.79 to 10) | 0.50 (0.46, 0.55) | 0.07 | < 0.001 |
* Univariate analyses using the bivariate model run separately for sensitivity and specificity with test as covariate.
** Likelihood ratio test for the bivariate model with versus without the covariate 'test'.
Abbreviations
ASCUS = atypical squamous cells of undetermined significance
ASCUS+ = ASCUS or worse
CIN2+ = cervical intraepithelial neoplasia of grade 2 or worse
CIN3+ = cervical intraepithelial neoplasia of grade 3 or worse
HC2 = Hybrid Capture 2 assay
HSIL+ = positive for high‐grade squamous intraepithelial lesion or worse
LSIL+ = low‐grade squamous intra‐epithelial lesions or worse
1.2 Triage of LSIL
1.2.1 Absolute accuracy of HC2 triage of LSIL cases
In the studies where both triage methods were applied, the sensitivity of HC2 was high: 96.2% (95% CI 91.4 to 98.3%) and 97.5% (95% CI 69.6 to 99.8%) for CIN2+ and CIN3+, respectively (Table 4). HC2 triage in LSIL cases showed a low pooled specificity: 27.7% (95% CI 20.9 to 35.7%) and 24.8% (95% CI 7.32 to 58.1%) for predicting absence of CIN2+ and CIN3+, respectively.
Summary of findings 4. Summary of the main meta‐analyses of the absolute and relative accuracy of HC2 and repeat cytology used to triage women with LSIL.
| Studies where HC2 triage and repeat cytology were applied | |||||||
| Triage test | Test cut‐off | Outcome | Studies | Accuracy parameter | Pooled estimate | (95% CI) | Analysis |
| HC2 | RLU > 1 (1 pg/mL) | CIN2+ | 6 | Absolute sensitivity | 96.2% | (91.4 to 98.3%) | 31 |
| 6 | Absolute specificity | 27.7% | (20.9 to 35.7%) | ||||
| CIN3+ | 4 | Absolute sensitivity* | 97.5% | (69.6 to 99.8%) | 32 | ||
| 4 | Absolute specificity* | 24.8% | (7.3 to 58.1%) | ||||
|
Repeat cytology |
ASCUS+ | CIN2+ | 6 | Absolute sensitivity | 77.1% | (59.5 to 88.5%) | 23 |
| 6 | Absolute specificity | 51.2% | (34.5 to 67.6%) | ||||
| CIN3+ | 4 | Absolute sensitivity* | 84.6% | (48.6 to 97.0%) | 26 | ||
| 4 | Absolute specificity* | 44.4% | (16.0 to 76.9%) | ||||
| HC2 (RLU > 1) vs repeat cytology (ASCUS+) | CIN2+ | 10 | Relative sensitivity | 1.23 | (1.06 to 1.43) | 7 | |
| 10 | Relative specificity | 0.66 | (0.58 to 0.75) | ||||
| CIN3+ | 4 | Relative sensitivity* | 1.15 | (0.89 to 1.48) | 10 | ||
| 4 | Relative specificity* | 0.56 | (0.37 to 0.84) | ||||
*Univariate analyses using the bivariate model run separately for sensitivity and specificity.
Abbreviations
ASCUS+ = ASCUS positive
CIN2+ = cervical intraepithelial neoplasia of grade 2 or worse
CIN3+ = cervical intraepithelial neoplasia of grade 3 or worse
HC2 = Hybrid Capture 2 assay
RLU = relative light units
> = less than
1.2.2 Absolute accuracy of cytology triage of LSIL cases
The pooled sensitivity for prediction of the presence of CIN2+ dropped with increasing cut‐off: from 77.1% (95% CI 59.5 to 88.5%) at ASCUS+, to 31.6% (95% CI 18.2 to 49.0%) at HSIL+; and from 84.6% (95% CI 48.6 to 97.0%) at ASCUS+, to 41.9% (95% CI 24.8 to 61.2%) at HSIL+, for prediction of CIN3+ lesions. The specificity rose with increasing cytological cut‐off (Table 4; Appendix 5).
1.2.3 Relative accuracy of HC2 compared to repeat cytology in triage of LSIL cases
The sensitivity of HC2 for CIN2+ was significantly higher than that of repeat cytology at cut‐off ASCUS+ (P value 0.007) (relative sensitivity: 1.23 (95% CI 1.06 to 1.43)). The specificity of HC2, on the other hand, was substantially and significantly lower than that of repeat cytology at cut‐off ASCUS+ (P value < 0.0001) (relative specificity: 0.66 (95% CI 0.58 to 0.75) (Figure 6; Table 4). At higher cytological thresholds, contrasts increased (progressively higher relative sensitivity and lower relative specificity). For the outcome CIN3+, the relative accuracy estimates were similar, but the differences between both triage tests were not always significant (Table 8).
6.

Analysis 7: Summary ROC‐plot (Bivariate model with test as covariate): Sensitivity and specificity of triage of ASCUS with HC2 (black) (at RLU>1) versus repeat cytology (red) at cut‐off LSIL+ for an outcome of underlying CIN2+ based on within study comparisons.
4. Triage of LSIL: relative sensitivity and specificity of HC2 versus repeat cytology.
| Cut‐off repeat cytology | Studies | Relative sensitivity (95% CI) | Relative specificity (95% CI) | P value | |
| Effect on sensitivity** | Effect on specificity** | ||||
| Outcome CIN2+ | |||||
| ASCUS+ | 6 | 1.23 (1.06 to 1.43) | 0.66 (0.58 to 0.75) | < 0.001 | < 0.001 |
| LSIL+ | 4* | 1.55 (1.02 to 2.36) | 0.42 (0.32 to 0.55) | 0.04 | 0.001 |
| HSIL+ | 4* | 3.06 (1.88 to 4.99) | 0.42 (0.32 to 0.55) | 0.003 | < 0.001 |
| Outcome CIN3+ | |||||
| ASCUS+ | 4* | 1.15 (0.89 to 1.38) | 0.56 (0.37 to 0.84) | 0.14 | 0.02 |
| LSIL+ | 4* | 1.36 (0.88 to 2.11) | 0.38 (0.22 to 0.63) | 0.09 | 0.01 |
| HSIL+ | 4* | 2.33 (1.47 to 3.68) | 0.24 (0.13 to 0.42) | 0.02 | 0.009 |
* Univariate analyses using the bivariate model run separately for sensitivity and specificity with test as covariate.
** Likelihood ratio test for the bivariate model with versus without the covariate 'test'.
Abbreviations
ASCUS+ = atypical squamous cells of undetermined significance or worse
CIN2+ = cervical intraepithelial neoplasia of grade 2 or worse
CIN3+ = cervical intraepithelial neoplasia of grade 3 or worse
HC2 = Hybrid Capture 2 assay
HSIL+ = positive for high‐grade squamous intraepithelial lesion or worse
LSIL+ = low‐grade squamous intra‐epithelial lesions or worse
2. Secondary objective: accuracy of HC2 irrespective of whether or not repeat cytology was done
2.1 Absolute accuracy in triage of ASCUS
In the 39 retrieved studies, the absolute sensitivity of triage with HC2 varied from 60% (Andersson 2005), to 100% for the detection of CIN2+ (Guyot 2003; Cuzick 2003; Davis‐Devine 2005; Holladay 2006; Szarewski 2008),and from 75% (Andersson 2005; Cattani 2009), to 100% for detection of CIN3+ (Zielinski 2001; Wensveen 2003; Szarewski 2008; Siddiqui 2008). The pooled sensitivities were 90.4% (95% CI 88.1 to 92.3%) and 93.7% (95% CI 90.4 to 95.9%) for detecting CIN2+ and CIN3+, respectively (Appendix 4).
The specificity of HC2 varied from 31% (Nieh 2005), to 80% for the detection of CIN2+ (Giovannelli 2005), and from 25% (Lee 2009), to 70% for detection of CIN3+ (Pretorius 2002; Ronco 2007). The pooled specificities were 58.3% (95% CI 53.6 to 62.9%) and 52.3% (95% CI 45.7 to 58.7%) for predicting absence of CIN2+ or CIN3+, respectively.
The pooled accuracy estimates of HC2 from the 29 studies where only HPV‐based triage was done, were not significantly different from the 10 studies where both triage methods were assessed (P value 0.534, and P value 0.250 for CIN2+ and CIN3+, respectively).
2.2 Absolute accuracy in triage of LSIL
In the 24 retrieved studies, the absolute sensitivity of triage with HC2 varied from 80% (Cattani 2009), to 100% for the detection of CIN2+ (Lytwyn 2000; Zielinski 2001; Kulasingam 2002; Chen 2005b; Holladay 2006; Szarewski 2008; Monsonego 2008; Lee 2009; Voss 2010), and from 76% (De Francesco 2008) to 100% for detection of CIN3+ (Zielinski 2001; Kulasingam 2002; Andersson 2005; Chen 2005b; Holladay 2006; Ronco 2007; Szarewski 2008; Monsonego 2008; Lee 2009).
The pooled absolute sensitivities were 95.4% (95% CI 94.0.1 to 96.5%) and 96.4% (95% CI 90.5 to 98.7%) for detecting CIN2+ and CIN3+, respectively (Appendix 5).
The specificity varied between 16% (Chen 2005b) and 58% for confirming absence of CIN2+ (Lee 2009), and between 15% (Huang 2009) and 47% for confirming absence of CIN3+ (Lee 2009). The pooled specificities were 27.8% (95% CI 23.8 to 32.1%) and 23.7% (95% CI 19.4 to 28.7%) for CIN2+ and CIN3+, respectively.
There was no significant difference in pooled accuracy measures of HC2 between studies where both triage methods were applied and studies where only HC2 triage was applied (P value 0.715, and P value 0.450 for the outcomes CIN2+ and CIN3+, respectively).
3. Influence of study characteristics
When no convergence was reached using the bivariate model, including covariates, univariate analyses were run without the correlation parameter to investigate the influence of study and test characteristics on the sensitivity and specificity. Results of the heterogeneity analysis can be found in Appendix 6.
The heterogeneity analysis by covariate was performed only when the groups compared contained at least five studies in one group and at least three studies in the other group. Most often, the absolute accuracy of triage with HC2 or repeat cytology did not change significantly by covariate, except in the following cases:
Triage of ASCUS with HC2 for outcome CIN2+, by sampling device and transport medium:
HC2 was less sensitive when the sample was collected with a brush than with a broom: relative sensitivity = 0.92 (95% CI: 0.88 to 0.97);
HC2 was less specific when the transport medium was Preservcyt compared to specimen transport medium (STM): relative sensitivity = 0.72 (95% CI: 0.55 to 0.95).
Triage of ASCUS with repeat cytology for outcome CIN2+, by continent:
Repeat cytology was less sensitive (0.79; 95% CI: 0.63 to 0.99), but more specific (1.27; 95% CI: 1.02 to 1.58), in studies conducted in Europe compared to America.
Triage of LSIL with HC2 for outcome CIN2+, by sampling device and continent:
HC2 was less sensitive (0.94; 95% CI: 0.91 to 0.98) when a brush was used compared to broom;
the specificity varied by continent (P value 0.04): 21.4% in America (95% CI: 16.7% to 27.0%), 24.7% in Asia (95% CI: 16.8% to 34.9%), and 33.1% in Europe (95% CI: 27.8% to 38.8%).
Triage of LSIL with HC2 for the outcome CIN3+, by continent:
HC2 was more specific in Europe than in America (1.50; 95% CI: 1.01 to 2.21).
4. Influence of age
Age‐stratified data on the accuracy of detection of high grade CIN were published in only three studies (Sherman 2002; Ronco 2007; Castle 2010a). However, no pooled analysis was possible from the published data because different cut‐offs were used to define age strata. From the ASCUS‐LSIL Triage Study (Solomon 2001), and the Italian NTCC study, we obtained non‐published five‐year age‐stratified data for the outcomes of CIN2+ directly from Dr M Schiffman (National Cancer Institute, Bethesda, MD) and Dr G Ronco (Istituto Oncologica, Torino, Italy). Similarly stratified data could be extracted from the published paper of Castle 2010a for LSIL triage.
The HSROC analysis failed to calculate the age‐specific absolute and relative accuracy measures for the triage of women with ASCUS, since only two studies provided data (Sherman 2002; Ronco 2007).
The age‐specific absolute and relative sensitivity and specificity and confidence intervals for LSIL triage are shown in Appendix 7. The sensitivity did not vary significantly by age group. However the specificity of HC2 always increased by age. For instance, the pooled specificity of triaging LSIL women with HC2 for excluding CIN2+ was 18.0% (95% CI 15.6% to 20.6%) in women younger than 30, and 43.7% (95% CI 24.4% to 65.2%) in women aged 50 years or older.
5. Publication bias or sample size effects
Table 9 shows the intercept and its 95% CI of the asymmetry regression assessing the relationship between the effective sample size and the logarithm of the diagnostic odds ratio (DOR) for all triage groups (ASCUS, LSIL), triage tests (HC2, repeat cytology) and outcomes (CIN2+ and CIN3+). An intercept significantly different from zero suggests publication bias or sample size effects. A negative intercept significantly different from zero (‐4.69 (95% CI ‐10.23 to ‐1.15), P value 0.015) was observed only in triage of ASCUS with HC2 for an outcome of CIN2+. This means that larger studies tended to show a larger overall accuracy (DOR). The asymmetry regression plot (Figure 7) indicates that the regression was influenced by one large study with high DOR (ALTS study of Solomon 2001). Omission of the ALTS study made the intercept statistically non significant from zero, but did not alter the intercept substantially: ‐4.00 (95% CI ‐9.59 to 1.60). These findings are suggestive of a positive relationship between diagnostic accuracy and sample size. The ALTS trial was one of the best designed triage studies with high‐quality disease certification. The data show the opposite of the usual publication bias where excessive accuracy in small published studies is unbalanced by non‐published small studies with low accuracy.
5. Publication bias and sample size effects.
| Triage group | Triage test | Outcome | No studies | Regression test asymmetry | ||
| Intercept | CI | P value | ||||
| ASCUS | HC2 | CIN2+ | 39 | ‐5.69 | (‐10.23, ‐1.15) | 0.015 |
| CIN3+ | 17 | ‐4.98 | (‐12.29, 2.33) | 0.167 | ||
| Repeat cytology | CIN2+ | 10 | 0.50 | (‐3.29, 4.29) | 0.769 | |
| CIN3+ | 4 | 6.20 | (‐8.07, 20.47) | 0.202 | ||
| LSIL | HC2 | CIN2+ | 24 | 3.06 | (‐4.73, 10.84) | 0.424 |
| CIN3+ | 14 | 5.21 | (‐3.26, 13.68) | 0.205 | ||
| Repeat cytology | CIN2+ | 6 | 10.84 | (‐69.05, 90.72) | 0.726 | |
| CIN3+ | 4 | 10.59 | (‐138.10, 159.27) | 0.310 | ||
Abbreviations
ASCUS = atypical squamous cells of undetermined significance
CIN2+ = cervical intraepithelial neoplasia of grade 2 or worse
CIN3+ = cervical intraepithelial neoplasia of grade 3 or worse
HC2 = Hybrid Capture 2 assay
LSIL = low‐grade squamous intra‐epithelial lesions
7.

Assymmetry regression plot showing the relation between the effective sample size and the logarithm of the diagnostic odds ratio of HC2 triage of ASCUS for the detection of CIN2+
A negative intercept was also observed In triage of ASCUS for CIN3+ with HC2, but this was not statistically significant (P value > 0.05). In LSIL triage with HC2, the intercepts were not significantly different from zero.
Triage with newer repeat cytology showed significant intercepts, but assessment was based on a small number of studies (10 or less), therefore, sample size effects cannot be excluded with certainty.
6. Sensitivity analyses
6.1 Triage of women with ASCUS
Rather low values of sensitivity for CIN2+ were reported in two studies (Andersson 2005; Giovannelli 2005): 60% and 75%, respectively. These two studies were small, and their omission resulted in a change in the pooled estimate of sensitivity of only 0.3%. The specificity of HC2 for CIN2+ was extremely low in one study, at 27% (Lee 2009). Exclusion of this study, yielded a small and non‐significant increase of the pooled specificity: from 58.3% (95% CI 53.8% to 62.9%) to 58.8% (95% CI 54.1% to 63.4%).
Deletion of the ALTS trial from the meta‐analysis yielded a minor insignificant decrease in the pooled absolute sensitivity of HC2 for CIN2+ from 90.4% (95% CI 88.2% to 92.3%) to 89.5% (95% CI 87.4% to 91.3%), whereas the specificity increased from 58.3% (95% CI 53.6% to 62.9%) to 58.6% (95%CI 53.7% to 63.3%).
The overall sensitivity and specificity pooled from the 29 studies where only HPV triage data were available were 89.9% (95%CI 86.9% to 92.2%) and 57.4% (95% CI 51.5% to 63.2%), respectively, and were not significantly different from the 10 studies where both triage tests were used (P values of 0.53 and 0.55, respectively).
6.2. Triage of women with LSIL
There were no studies that showed outlying results for sensitivity or specificity. Deletion of the ALTS trial yielded no changes in the pooled absolute sensitivity of HC2 for CIN2+ 95.4% to 95.2% (95%CI 93.7% to 96.4%) and an insignificant increase in pooled absolute specificity from 27.8% to 28.3% (95%CI 24.2% to 32.8%). The overall sensitivity and specificity pooled from the 18 studies where only HPV triage data were available were 95.2% (95%CI 93.6% to 96.4%) and 27.9% (95%CI 23.0% to 33.3%), respectively, and were not significantly different from the six studies for which both triage tests were available (P values of 0.97 and 0.45 respectively).
Discussion
The current Cochrane review corroborates the conclusions from our previous meta‐analyses which all indicated that HC2 triage of women with ASCUS predicts presence of underlying high‐grade CIN with greater accuracy than a repeat Pap smear considering ASCUS+ as cut‐off (significantly higher sensitivity, similar specificity) (Arbyn 2004a; Arbyn 2004b; Arbyn 2005).
However, the conclusions concerning the triage of LSIL differ from previous reviews, where HC2 triage showed no significant gain in sensitivity but a substantial and statistically significant loss in specificity compared to repeat cytology (Arbyn 2002; Arbyn 2006). In the current Cochrane review, with the inclusion of more studies, the lower specificity of HC2 triage of LSIL was confirmed, but a significant gain in sensitivity was revealed.
Below, we will discuss how robust and generalisable these findings are.
1. Consistency of the findings
1.1 Triage of women with ASCUS
The accuracy of cytological triage was described in a minority of studies: for instance, 39 studies assessed the sensitivity and specificity of HC2 for CIN2+, but only 10 of them evaluated repeat cytology (at cut‐off ASCUS or worse) as well. However, no significant inter‐study heterogeneity was found in the absolute sensitivities. Moreover, the fact that the sensitivity of HC2 did not differ between the 10 studies where both triage methods were evaluated and the 29 studies that only offered virological triage, provides considerable weight regarding the consistency and the generalisability of the study results. We included one randomised trial, the ALTS study (Sherman 2002; Solomon 2001), where not all women in the HPV‐triage and cytology‐arms were verified. However, in the HPV arm, the CIN2+ and CIN3+ detection rates were similar to those in the colposcopy arm where all women were submitted to the reference standard. Moreover, the pooled accuracy estimates of the meta‐analyses did not change significantly after exclusion of the ALTS study.
1.2 Triage of women with LSIL
As for ASCUS above, the pooled accuracy estimates for LSIL triage did not change after omission of the ALTS study.
Only six studies reported data of virological and cytological triage of women with LSIL. However, there was no significant difference between the pooled accuracy of these six studies and the pooled accuracy of the 18 studies reporting only HC2 data.
LSIL is usually the manifestation of a productive HPV infection with low potential for neoplastic transformation (Zuna 2005). Therefore, HPV DNA testing nearly always yields positive results, limiting its capacity to distinguish between cases with, or without, severe underlying or developing lesions. The proportion of LSIL observed in women with a positive HC2 test reported in the included studies ranged from 55% to 89%. The test positivity rates were consistently higher than in ASCUS. Because of the high hrHPV positivity rate (83%), enrolment of LSIL women in the ALTS trial was interrupted (ALTS Group 2000). Moss found 89% positive HC2 results in women under 35 years of age with mild dyskaryosis on Pap smears, 69% in women between the ages of 35 and 49, and 51% in women aged 50 or over (Moss 2006). The specificity for the outcome CIN2+ in the ALTS study was 16% in women under 29 years of age, and 30% in women of 29 years or older (Sherman 2002).
2. Low specificity of all triage methods
The specificity of triage with HC2 or by repeat Pap smears at a low cytologic threshold ranged from moderate, in the case of ASCUS, to poor, in the case of LSIL. Colposcopy of all triage positive women generates considerable costs. Therefore, there is a need for specific triage tests for women with LSIL, with high predictive values, that allow for the identification of women at increased risk for cervical cancer.
Virological triage could be made more specific by increasing the viral load cut‐off, by adding a second triage test, by choosing an alternative triage test, or by excluding young women.
Viral load cut‐off
Very few published data are available concerning HC2 triage at higher test thresholds. Increasing the cut‐off from 1 pg/mL to 10 pg/mL, in the ALTS, yielded a gain in specificity for exclusion of CIN2+ of 12% in triage of ASCUS, and 11% in triage of LSIL. This gain in specificity was accompanied by a loss in sensitivity of 9% for women with ASCUS or 10% for women with LSIL (Sherman 2002). Guyot observed an increase in specificity (13.6%, 95% CI to ‐15.4 to 42.6%), without loss in sensitivity, by raising the HC2 cut‐off to 3 pg/mL in a small‐sized triage study where the included women had persistent borderline smears (Guyot 2003). Rebello triaged women with two smears showing borderline or mild dyskaryosis with HC2 at 1 pg/mL, 2 pg/mLand 4 pg/mL and found sensitivities for CIN2+ of 108/116 (93%), 106/116 (91%), and 99/116 (85%) respectively, and specificities of 119/217 (55%), 124/217 (57%), and 134/217 (62%) (Rebello 2001). Ronco made the same conclusions after observing an increase of specificities of HC2 for CIN2+ among women with ASCUS at 1 pg/mL, 2 pg/mL, 4 pg/mL, 10 pg/mL and 20 pg/mL, of 70.9%, 75.5%, 78.0%, 81.1% and 83.6%, respectively. In contrast, sensitivities decreased with higher positivity threshold of HC2 (96.2%, 96.2%, 88.5%, 84.6%, and 73.1%, respectively) (Ronco 2007). In women with LSIL, the specificities of HC2 for outcome CIN2+ increased from 48.3% to 55.6%, whereas the sensitivity dropped from 96.9% to 90.6% when raising the HC2 cut‐off from 1 pg/mL to 20 pg/mL (Ronco 2007).
Targeted HPV types
The composition of the cocktail of HPV type‐specific probes, the analytical capacity to pick up DNA from target types, and cross‐reactivity with non‐target types influence the specificity of HPV triage. A posteriori HPV typing of the ALTS samples, taken at enrolment, indicated that genotyping for more than 10 HPV genotypes resulted in serious loss in the specificity, with almost no additional gain in sensitivity (Schiffman 2005a). Schiffman compared HC2 accuracy for CIN3+ with that of polymerase chain reaction (PCR) amplification with L1 consensus primer PGMY09/11 followed by reverse‐line blot hybridisation for 13 high‐risk types in triage of women with ASCUS (Schiffman 2005b). Sensitivity and specificity of HC2 for CIN3+, corrected for the insensitivity of colposcopy, was 92% and 53%, whereas the PCR showed ‐ surprisingly ‐ a lower sensitivity (87%) and a higher specificity (56%) (P value for differences in sensitivity and specificity < 0.001). The specificity for a cumulative diagnosis (defined over 0 to two years after enrolment) could be increased substantially by identifying HPV 16 (see below) (Castle 2005).
Triage with other molecular markers
Testing of ASCUS women for E6/E7 transcripts of HPV types 16, 18, 31, 33, and 45 using real‐time multiplex Nucleic acid sequence based amplification (NASBA) (Pretect HPV Proofer, Norchip, AS, Klokkarstua, Norway) yielded an equal sensitivity (100% (2/2)) but a higher specificity (83%; 95% CI 70.7% to 91.8%) for subsequent CIN2+ than triaging with GP5+/GP6+ consensus PCR (specificity of 56%; 95% CI 41.3% to 69.5%) (P value for difference in specificity: 0.003) (Molden 2005).
Carozzi could reduce the false positivity rate of HPV triage by a factor of approximately 2.5 by p16‐immunostaining slides from women with minor cytological lesions testing HPV‐positive. This policy was accompanied with loss in sensitivity for CIN2+ of 12% (Carozzi 2006).
Nieh tested ASCUS cases for p16‐overexpression, and with HC2 and found an insignificantly higher sensitivity and a significantly higher specificity for p16 (sensitivity of p16 was 20/21, sensitivity of HC2 was 18/21 (P value for McNemar's Chi2 = 0.157); specificity of p16 was 14/45, specificity of HC2 was 25/45 (P value for McNemar's Chi2 = 0.012) (Nieh 2005). However, Longatto‐Filho found equal sensitivity for CIN2+ (3/3), but lower specificity for p16 staining (19/40) compared to HPV testing (27/40) in a small series of ASCUS cases (P value for Pearson's Chi2 = 0.070) (Longatto‐Filho 2005). Triage of ASCUS or LSIL with new molecular markers is the target of ongoing research and systematic reviews should be performed as soon as more studies become available.
Influence of age
The influence of age on the accuracy could be assessed in only two studies for triage of ASCUS, and in three studies for triage of LSIL. Multivariate analyses identified a significant increase in specificity by age in triage of LSIL when the outcome was CIN3+. The increase in specificity reflects the drop in HPV test positivity rate with increasing age. However, in LSIL triage, the drop in test positivity, and consequent increase in specificity in older women, varied in size between the studies. In one study, the test positivity rates were 57% and 38%, and the specificity rates were 45% and 66%, in the 30 to 39 years and over 50 years age groups, respectively (Ronco 2007). In another study, the age variation in the test positivity and specificity was limited, with a test positivity rate of 76% and a specificity of 25% in women aged 50 or older (Castle 2010a).
3. Verification bias
According to the study selection criteria, colposcopy was performed on all subjects, and, therefore, in principle, no verification bias could occur. In the ALTS, results from women in the HPV arm were not verified when the HC2 test was negative, if results from repeat cytology ranged from normal to LSIL, and if no suspect macroscopic lesions were observed. However, colposcopy was performed on all women in a second arm of the ALTS. We can conclude that it is probable that no ‐ or very few cases ‐ of CIN2+ were missed in the HPV arm, and that there is no evidence of verification bias in the ALTS because the cross‐sectional detection rates of histologically confirmed CIN2+ were 11.3% (95% CI 9.5% to 13.2%) for women in the colposcopy arm, and 11.7% (95% CI 9.9% to 13.7%) for women in the HPV arm (Sherman 2002; Solomon 2001). Three other studies could be considered as potentially suffering from verification bias because of incomplete verification with the reference test (Dalla Palma 2005; Holladay 2006; Silverloo 2009). Indeed, the low specificity (37%) and the rather high sensitivity (94%) reported in triage of ASCUS participants by Dalla Palma 2005 could be explained by some degree of verification bias. The other two studies did not show an outlying low specificity (Holladay 2006, Silverloo 2009). Moreover, the multivariate regression analysis did not reveal "partial verification" as a significant factor that explained the inter‐study heterogeneity of the accuracy.
It should be recognised that certain studies described accuracy in series of women with ASCUS and LSIL who all were submitted to verification without providing details about non‐verified women, giving the impression that partial verification was completely avoided.
4. Validity of the reference standard
We used histology as the reference standard and accepted negative satisfactory colposcopy as evidence for absence of high‐grade CIN when no biopsies were taken. This definition of the reference standard might be imperfect. Recent data have shown that the sensitivity of colposcopy for high‐grade CIN might be considerably lower than usually believed. Mitchell estimated, from a meta‐analysis including nine studies, that the average sensitivity and specificity of colposcopy in detecting CIN2+ was 96% and 48% respectively (Mitchell 1998). However, in most studies included in this meta‐analysis, biopsy taking was triggered by a positive colposcopic interpretation. Since biopsy taking was correlated to a positive colposcopic impression, the sensitivity estimation of colposcopy for detection of CIN2+ is inflated artificially (Arbyn 2009b). In one particular study, conducted in China, a more unbiased assessment of colposcopic accuracy was revealed. Biopsies were taken not only from colposcopically‐suspect areas but also from the four quadrants of the transformation zone in colposcopically negative cases (Pretorius 2004). Moreover, endo‐cervical curettage was performed in every woman. In this study the sensitivity of colposcopy‐directed biopsy for CIN2+ in women with satisfactory colposcopy was 57% (95% CI 52% to 62%). In the ALTS, immediate colposcopy at enrolment detected 64% (95% CI 57% to 71%) of the two‐year clinical cumulative diagnoses of CIN2+ (ALTS Group 2003a).
The fact that histological interpretation of biopsy material is also prone to error has been widely documented in the literature (Ismail 1989; Robertson 1989; O'Sullivan 1998; Stoler 2001).
Misclassifications in colposcopy and histology may yield biased estimates of the accuracy of triage tests, if the correlation of test and reference standard ratings are dependent on disease status (Pepe 2004). However, if reference standard classification errors are independent, test accuracy estimates will not be affected. In the Chinese study, Pretorius showed that the sensitivity of HPV testing was similar when either the usual or the improved reference standard was used (Pretorius 2006).
5. Choice of the endpoints: CIN2+ or CIN3+
All included studies described accuracy for CIN2+ but only half of them also provided data for the outcome of CIN3+. Nevertheless, CIN3+ might be a more relevant endpoint, since its potential to regress spontaneously is more limited than CIN2 (Morrison 1992; Ostor 1993; Schiffman 2003). Moreover, CIN2, is less reproducible and comprises over‐classified CIN1 (Ismail 1989; Robertson 1989). It is possible that HPV triage leads to detection of additional CIN lesions which may, predominantly, be at lower risk of progression than those usually detected after an HSIL smear (Sherman 2003). The fact that the pooled relative sensitivities (HC2/repeat cytology) were 1.33 (for CIN2+) and 1.21 (for CIN3+), in the four studies where both outcomes were documented (Kulasingam 2002; Sherman 2002; Andersson 2005; Monsonego 2008) provides some evidence for the hypothesis that HPV triage results in a small amount of over‐diagnosis. Sherman noted that CIN3 lesions, found after enrolment in the ALTS study as a consequence of HC2 testing, were overwhelmingly small and showed less gland involvement than those found after a HSIL repeat smear (Sherman 2003).
6. Previous reviews and meta‐analyses
Conclusions from the current Cochrane review on triage of women with ASCUS are in agreement with findings from previous meta‐analyses conducted by the review authors and by others (Cuzick 1999; ANAES 2002; Arbyn 2004b; Arbyn 2006), which were already accepted as evidence for recent European and American guidelines for management of women with ASCUS or borderline cervical cytology (ASCCP 2006; Wright 2007; European Commission 2008; Jordan 2008b; Solomon 2009; ACOG 2009; Partridge 2010; Stampler 2010).
The findings on triage of women of LSIL are different from previous meta‐analyses, where it was concluded that triage with HC2 was not more sensitive, but substantially less specific than repeat cytology (Arbyn 2005; Arbyn 2006). Therefore HC2 was not recommended in guiding management of women with low‐grade cytological lesions (Wright 2007; Jordan 2008b). The current Cochrane Review demonstrated significantly higher sensitivity of HC2 for CIN2+ and CIN3+. This new finding could justify recommending the use of HC2 to decide whether women need referral to colposcopy. However, the practice recommendations for management of LSIL should be based on local cost‐effectiveness analyses, the local prevalence of HPV in LSIL, and compliance of women with follow‐up recommendations, and should be restricted in situations where access to colposcopy referral is limited and/or expensive. Where sufficient colposcopy capacity exists, and where colposcopy is not excessively expensive, direct referral of LSIL women to colposcopy is a defendable option. The American Society for Colposcopy and Cervical Pathology (ASCCP) does not recommend reflex HPV triage, but proposes to refer to colposcopy (Wright 2002). If colposcopy and/or biopsy are normal, or only reveal CIN1, an HPV test at 12 months or two repeat smears after the initial LSIL smear is recommended. Postponing triage, is another option, allowing viral clearance ‐ over a period of six to 12 months this can vary from 18% to 45% (Bais 2005) ‐ and reducing the need for colposcopy. This latter recommendation is conditioned by good compliance with follow‐up recommendations. HPV DNA testing certainly is not useful in young women with LSIL, given the very high prevalence of HPV infection and the very high probability of regression unrelated to initial HPV status (Woodman 2001; Moscicki 2004). HPV triage in older women, using higher viral load cut‐off, might be an acceptable management option, but more age‐specific accuracy data are needed to define the age cut‐off. In the USA, LSIL triage with hrHPV testing is only recommended for postmenopausal women (Solomon 2009).
Summary of main results
Triage of women with a cytological test result of ASCUS (or ASC‐US) by means of the HC‐2 assay is more accurate (i.e. more sensitive, and equally specific) than repetition of the cytological test to detect underlying CIN2, or worse, and CIN3, or worse.
Triage of women with a cytological test result of LSIL by means of the HC‐2 assay is more sensitive, but substantially less specific, than repetition of the cytological test to detect CIN2+ or CIN3+. The specificity of HC2 improves for older women, but a clear universal age cut‐off (older than 35, 40 or 45 years) cannot be defined because of the low number of studies with age‐specific data, and the heterogeneity amongst them.
Strengths and weaknesses of the review
The following strong and weak elements of the current review are recognised:
Strengths
We retrieved a large number of studies that evaluated the accuracy of HC2‐based triage of women with ASCUS, allowing us to run models with covariates.
We took different cytological classifications into consideration (BSCC, TBS89 and TBS01).
The quality of reporting in retrieved studies was good, with negative scores for the diverse QUADAS items varying between zero and 10%.
All trial authors who were contacted and asked to provide additional data responded positively.
We used robust modern statistical procedures recommended by the DTA‐group of the Cochrane Collaboration.
Weaknesses
A low number of studies contained accuracy data on HC2 and repeat cytology (only 10 in ASCUS triage for outcome CIN2+, and even fewer for the other situations).
Different classification systems were used to categorise cervical lesions throughout the literature. However, this potential weakness did not influence accuracy estimates, as shown in multivariate analyses.
The reference standard was not always uniformly defined ‐ and varied between studies ‐ from systematic biopsies taken from all eligible women; to colposcopy in all women, and biopsy only in case of colposcopic suspicion, completed with endocervical brushing or curettage in case of unsatisfactory colposcopy; accepting negative satisfactory colposcopy as sufficient ascertainment of absence of CIN2+. Moreover, techniques for taking biopsies varied between conization, large loop excision and multiple or single targeted biopsies. Verification of the outcome was not always performed within a short time (six months) after triage testing, and sometimes follow‐up cytology was also used to verify outcomes. We addressed all these potentially influencing issues in subgroup meta‐analyses, or multivariate regression, but they did not influence outcomes significantly.
The inter‐study variation in accuracy was large, particularly for specificity. In most situations, no significant explanatory factors could be identified.
The evaluation of the variation of the test accuracy by age could not be assessed from the published data, however, accuracy data, requested from trial authors, and equally stratified by age group, allowed an increase of the specificity of HC2 by age to be identified. This could offer the possibility of using HC2 in older women with LSIL, however, due to the large inter‐study variability no universal age cut‐off could be identified to justify precise recommendations.
We did not evaluate the accuracy of repeat cytology or HC2 in women with other equivocal cytology categories such as ASC‐H (atypical squamous cells of undetermined significance, where HSIL cannot be excluded) and AGC (atypical glandular cells), as hoped in the original protocol, since this would have contributed additional complexity.
Applicability of findings to the review question
The clinical impact of our findings is simulated in Table 1 for ASCUS triage, and in Table 2 for LSIL triage using a population of 1000 women. We assumed a low, moderate or high prevalence of disease, and accepted the pooled sensitivity and specificity values estimated from the current meta‐analyses (Table 3 and Table 4). To assess the clinical utility of triaging, we computed the risk of disease in women testing positive (PPV) or negative (cNPV = complement of NPV = 1‐NPV). The PPV should be sufficiently high to justify referral to colposcopy with biopsy‐taking, possibly followed by treatment, whereas the cNPV should be sufficiently low to justify safe referral of triage‐negative women to the normal screening schedule.
Summary of findings 1. Clinical impact of applying triage tests in a population of 1000 ASCUS patients with a given prevalence of disease.
| Test | Outcome |
Sensitivity/ Specificity |
Prevalence |
Useful re‐ ferrals |
Missed cases |
Unneces‐ sary referrals |
True reas‐ surance |
Post‐test risk |
|
| Pre‐test risk |
TP | FN | FP | TN | if test+ (PPV) |
if test‐ (1‐NPV) |
|||
|
Repeat cytology (at ASCUS+) |
CIN2+ | 71.5%/ 68.4% |
5% | 36 | 14 | 300 | 650 | 10.7% | 2.1% |
| 10% | 72 | 28 | 284 | 616 | 20.2% | 4.4% | |||
| 15% | 107 | 43 | 269 | 581 | 28.5% | 6.9% | |||
| CIN3+ | 77.9%/ 57.4% |
2% | 16 | 4 | * | * | * | 0.7% | |
| 6% | 47 | 15 | * | * | * | 2.7% | |||
| 10% | 78 | 27 | * | * | * | 5.0% | |||
|
HC2 (RLU > 1) |
CIN2+ | 90.9%/ 60.7% |
5% | 45 | 5 | 307 | 643 | 12.8% | 0.8% |
| 10% | 91 | 9 | 291 | 609 | 23.8% | 1.5% | |||
| 15% | 136 | 14 | 274 | 576 | 33.1% | 2.4% | |||
| CIN3+ | 94.8%/ 56.6% |
2% | 19 | 1 | * | * | * | 0.2% | |
| 6% | 66 | 4 | * | * | * | 0.7% | |||
| 10% | 114 | 6 | * | * | * | 1.2% | |||
The clinical impact of applying HC2 in stead of repeat cytology at cut‐off ASCUS+ is assessed for a population of 1000 ASCUS patients with low, intermediate and high prevalence of CIN2+ (5%, 10% or 15%) or CIN3+ (2%, 6% or 10%) accepting the sensitivity and specificity values derived from the meta‐analyses.
*The number of unnecessarily referred and truly reassured cases was not computed for the outcome CIN3+, since CIN2 cannot be considered as an obvious false‐positive case.
Abbreviations
ASCUS+ = ASCUS or worse
CIN2+ = cervical intraepithelial neoplasia of grade 2 or worse
CIN3+ = cervical intraepithelial neoplasia of grade 3 or worse
FN = false negatives
FP = false positives
HC2 = Hybrid Capture 2 assay
NPV = negative predictive value
PPV = positive predictive value
RLU = relative light units
TN = true negatives
TP = true positives
Summary of findings 2. Clinical impact of applying triage tests in a population of 1000 LSIL patients with a given prevalence of disease.
| Test | Outcome |
Sensitivity/ Specificity |
Preval‐ ence |
Useful referrals |
Missed cases |
Unneces‐ sary referrals |
True reas‐ surance |
Post‐test risk |
|
| Pretest risk |
TP | FN | FP | TN | + (PPV) |
‐ (1‐NPV) |
|||
|
Repeat cytology (at ASCUS+) |
CIN2+ |
77.1%/ 51.2% |
10% | 77 | 23 | 438 | 461 | 14.9% | 4.8% |
| 20% | 154 | 46 | 390 | 410 | 28.3% | 10.1% | |||
| 25% | 193 | 57 | 366 | 3844 | 34.5% | 12.9% | |||
| CIN3+ |
84.6%/ 44.4% |
5% | 42 | 8 | * | * | * | 1.9% | |
| 10% | 85 | 15 | * | * | * | 3.6% | |||
| 15% | 127 | 23 | * | * | * | 5.7% | |||
|
HC2 (RLU > 1) |
CIN2+ |
96.2%/ 27.7% |
10% | 96 | 4 | 572 | 328 | 14.2% | 1.2% |
| 20% | 192 | 8 | 509 | 291 | 27.2% | 2.7% | |||
| 25% | 127 | 23 | 477 | 273 | 33.2% | 3.2% | |||
| CIN3+ |
97.5%/ 24.8% |
5% | 49 | 1 | * | * | * | 0.4% | |
| 10% | 98 | 2 | * | * | * | 0.9% | |||
| 15% | 146 | 4 | * | * | * | 1.9% | |||
The clinical impact of applying HC2 in stead of repeat cytology at cut‐off ASCUS+ is computed for a population of 1000 LSIL patients with low, intermediate and high prevalence of CIN2+ (10%, 20% or 25%) or CIN3+ (5%, 10% or 15%) accepting the sensitivity and specificity values derived from the meta‐analyses.
*The number of unnecessarily referred and truly reassured cases was not computed for the outcome CIN3+, since CIN2 cannot be considered as an obvious false‐positive case.
Abbreviations
ASCUS+ = ASCUS or worse
CIN2+ = cervical intraepithelial neoplasia of grade 2 or worse
CIN3+ = cervical intraepithelial neoplasia of grade 3 or worse
FN = false negatives
FP = false positives
HC2 = Hybrid Capture 2 assay
NPV = negative predictive value
PPV = positive predictive value
RLU = relative light units
TN = true negatives
TP = true positives
Triage of ASCUS
In triage of women with ASCUS (Table 1), a negative HC2 test always corresponds with a risk (1‐NPV) for CIN2+ which is lower than 2.5% (and lower than 1.5% for CIN3+). A negative repeat Pap smear still is associated with a risk of CIN2+ of more than 4%, and possibly as high as 7% in moderate and high prevalence situations. Depending on the local prevalence, repeat cytology misses between 14 and 43 CIN2+ cases/1000 ASCUS women, whereas the HC2 misses only five to 14 CIN2+/1000 ASCUS women. Corresponding figures for the outcome of CIN3+ show repeat cytology misses four to 27 cases, and HC2 misses only one to six cases in a population of 1000 ASCUS women.
A positive HC2 test corresponds with a risk (PPV) of CIN2+ that is similar to, or even slightly higher than, a positive repeat cytology result. However, both tests result in a considerable number of unnecessary referrals (around 300 per 1000 women with ASCUS). More than 576 women (range of 576 to 650) per 1000 ASCUS women, with a negative test result can be reassured that they are free of disease (CIN2+).
Triage of LSIL
In triage of women with LSIL (Table 2), a negative HC2 test corresponds with a risk (1‐NPV) for CIN2+ that ranges between 1.2% and 3.2% (and for CIN3+ between 0.4% and 1.9%). A negative repeat Pap smear still is associated with a risk of CIN2+ in the range of 5% to 13% (and for CIN3+ the range is 2% to 6%). Depending on the local prevalence, repeat cytology misses between 23 and 57 per 1000 LSIL women, whereas the HC2 misses only four CIN2 cases, in a low‐prevalence situation, and 23 CIN2+ cases in a high‐prevalence situation per 1000 LSIL women. Corresponding figures for the outcome of CIN3+ show that repeat cytology misses eight to 23 cases and HC2 misses one to four per 1000 LSIL women.
A positive HC2 test corresponds with a risk (PPV) of CIN2+ that is lower than a positive repeat cytology result: the difference is about 1%. In spite of the low specificity of both tests in triage of LSIL, the PPV is slightly higher than in triage of ASCUS, which is due to the higher prevalence of underlying CIN2+. However, given the high HPV positivity rate in LSIL women, a positive HC2 test results in approximately 500 or more unnecessary referrals per 1000 LSIL women. A positive repeat Pap smear results in about 100 fewer unnecessary referrals than a positive HC2 test (still about 300 to 400). HC2 triage in LSIL yields substantially more false positive and fewer true negative cases compared to repeat cytology.
The finding of higher sensitivity, but lower specificity, makes it difficult to recommend whether or not to use HC2 in LSIL triage The decision should be based on the local costs of HPV tests and colposcopy, the local prevalence of HPV in LSIL, and the compliance of women with follow‐up recommendations, and should be restricted to situations where access to colposcopy referral is limited and/or expensive. Where sufficient colposcopy capacity exists, and where colposcopy is not excessively expensive, direct referral of LSIL women to colposcopy is a defendable option.
Tests as sensitive ‐ but more specific ‐ than HC2, or more sensitive and more specific than repeat cytology, would be very welcome to triage women with LSIL.
Authors' conclusions
Implications for practice.
We conclude that virological testing using HC2 is a more accurate method than repeat cytology to triage women with ASCUS (atypical squamous cells of undetermined significance). Moreover, when the collected cervical specimen is a liquid sample, HC2 can be applied on the same sample using remnant material, avoiding an additional visit to take a new cytological specimen.
LSIL (low‐grade squamous intra‐epithelial lesions) triage with HC2 is more sensitive than triage with repeat cytology, but its specificity is substantially lower than that of repeat cytology. HC2 could be considered for triage of older women with LSIL, but no precise universal evidence‐based guidelines can be formulated at present.
Implications for research.
Since no good reflex method is currently available to triage women with LSIL, research aiming to find specific markers that allow identification of women with LSIL who are at risk of having, or developing, high‐grade intraepithelial neoplasia, should be considered as a priority.
A substantial number of research reports are available that illustrate the strong correlation between severity of cervical lesions, infection with high‐risk human papillomavirus types (in particular HPV types 16 and 18), presence of viral mRNA and over‐expression of certain cell‐cycle regulating proteins, such as p16 or Ki‐67. Nevertheless, there is a need for studies in which the central research question concerns the diagnostic and prognostic roles of these markers. Systematic reviews on triage of ASCUS and low‐grade cytological lesions using these markers need to be performed as soon as new studies become available.
Authors of papers and editors of journals should be encouraged to produce age‐stratified results for the histological outcomes of CIN2+ and CIN3+.
The current review was restricted to triage of ASCUS and LSIL. An additional new review to address the accuracy of HPV‐based triage in more rare cytological abnormalities, such as AGC (atypical glandular cells) and ASC‐H (atypical squamous cells of undetermined significance, where HSIL cannot be excluded), is warranted.
Individual patient‐data meta‐analyses of the larger triage reports could already provide better insight into optimising viral‐load cut‐offs and age‐delimitations of triage strategies. Current ongoing primary screening trials, comparing cytology‐based screening with HPV‐based screening or combined HPV and cytology screening, will provide high‐quality information on the performance of different triage strategies for women with minor cytological lesions (Davies 2006).
What's new
| Date | Event | Description |
|---|---|---|
| 25 March 2021 | Review declared as stable | This review has been superseded by Cytology versus HPV testing for cervical cancer screening in the general population (https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD008587.pub2/full). |
History
Protocol first published: Issue 4, 2009 Review first published: Issue 3, 2013
Acknowledgements
The review authors acknowledge the agencies and institutes that have contributed financially to the systematic review, as mentioned in Sources of support. The financial support has made it possible to conduct the review, by mobilising sufficient human resources. The grant obtained from the Cochrane Gynaecological Cancer Review Group (CGCRG) was of crucial importance, as well as the diplomatic and administrative support of Clare Jess, Gail Quin, Chris Williams and Jo Morrison of the CGCRG Editorial Team. In particular we thank Yemisi Takwoingi (Birmingham) for running the bivariate model with the NLMIXED procedure (SAS) when METADAS did not converge. We are grateful to Bernadette Claus, Loes Houthuys and Els Delporte (of the Scientific Institute of Public Health, Brussels, Belgium) for their assistance in obtaining and classifying literature references.
We would like to acknowledge the following trial authors who provided additional accuracy data that were not available in the published reports: S Anderson, P Castle, A Lytwyn, J Monsonego, J Pretorius, G Ronco, M Schiffman and A Szarewski.
Appendices
Appendix 1. Classifications used for reporting cervical epithelial lesions
Cytological classification
In the current review two groups of participants are considered: triage group I (women with equivocal cervical lesions or ASCUS) and triage group II (women with low‐grade cytological grade cytological lesions or LSIL).
In triage group I, participants are women with atypical squamous cells of undetermined significance (ASCUS), as defined in the 1988 version of The Bethesda System (TBS) (Lundberg 1989). In the 1991 version of TBS (Luff 1992), subclassification of the equivocal category of ASCUS was proposed: (a) atypical squamous cells favouring a benign reactive process squamous cells of undetermined significance (ASC‐R); (b) undetermined significance and (c) neoplasia cannot be excluded. In TBS‐2001, the first subcategory (ASC‐R) was included with “negative for neoplasia or malignancy”, whereas the second and third subcategories were identified as ASC‐US (with hyphen; atypical squamous cells of undetermined significance) and ASC‐H (atypical squamous cells where a high grade lesion cannot be excluded), respectively. For the current meta‐analysis, if possible the number of ASCUS cases will be computed from the respective subcategories. In publications using TBS‐2001, where this was not possible, only data on ASC‐US cases will be extracted. Studies reporting data exclusively on ASC‐R or ASC‐H will be excluded from the main meta‐analysis, but will be included in subgroup meta‐analyses.
Glandular cytological lesions (AGUS (atypical glandular cells of undermined significance), and AGC (atypical glandular cells)) are rare findings that are not the focus of the current review. However, when triage data concern women with squamous or glandular atypia and the outcome of glandular lesions is not documented separately, the whole triage group will be considered as ASCUS. Studies reporting data exclusively on glandular atypia will be excluded from the main meta‐analysis but will be included in subgroup meta‐analyses.
In triage group II, participants will be women with low‐grade squamous intraepithelial lesions (LSIL). The definition of low‐grade squamous intraepithelial lesions remained unchanged in the successive versions of TBS.
The terminology of the British Society of Clinical Cytology (BSCC), used by NHSCSP (Evans 1986), will be translated into TBS‐1988. The BSCC terms borderline cytology and mild dyskaryosis will be considered as similar to ASCUS and LSIL, respectively (Dudding 2002).
Table 7 contains all the categories of cervical neoplastic changes (from normal to cancer), used in different classification systems. It formed the basis for translation of different terminologies into TBS 1988, used in the current systematic review.
Histological classification
Throughout the current review, the CIN nomenclature is used to describe histologically‐confirmed dysplasia of the cervical squamous epithelium (Richart 1973), whereas TBS is used to describe cytological cervical lesions (see Table 7 and Appendix 1). CIN1 (mild dysplasia) has a low potential of progression to cancer (Holowaty 1999; Ostor 1993). Moreover, cytological, virological and molecular profiles of CIN1 appear to be similar to that of epithelium without CIN (Wentzensen 2008). On the other hand, CIN2 (moderate dysplasia), and in particular CIN3 (severe dysplasia and carcinoma in situ), indicate a considerable risk of developing cancer (Holowaty 1999; Ostor 1993). Consensus exists to recommend treatment of CIN2 and CIN3 (Jordan 2008a), and, therefore, they are often included within the term high‐grade CIN. However, it is clinically relevant to distinguish CIN2 from CIN3 (Herbert 2008). CIN2 is an intermediate condition, which contains over‐called CIN1 and under‐called CIN3 (Sherman 2002). CIN3 (encompassing severe dysplasia and carcinoma in situ) is a more progressive and reproducible histological diagnosis than CIN2 (Ismail 1989; Ostor 1993). Therefore, in the current review, the authors will assess the accuracy separately for both outcomes:
CIN2 or worse disease (CIN2, CIN3, invasive squamous cervical cancer and adenocarcinoma of the cervix) (CIN2+));
CIN3 or worse (CIN3, invasive squamous cervical cancer and adenocarcinoma of the cervix (CIN3+)).
| WHO (WHO 2003) | CIN (Richart 1973) | BSCC 1986 (NHSCSP 1995) | TBS 1988 (Lundberg 1989) | TBS 2001 (Solomon 2002) |
| Normal | Absence of CIN | No dyskaryotic cells | Within normal limits | Negative for epithelial abnormality, encompassing ASC‐R |
| Atypia | Atypia | Borderline nuclear change | ASCUS | |
| ASC‐US | ||||
| ASC‐H | ||||
| Atypical glandular cells | Cervical glandular intraepithelial neoplasia (CGIN) | Borderline nuclear change, glandular | AGUS | Atypical glandular cells |
| Koylocytosis | Condyloma | Borderline nuclear change with koylocytes | LSIL | LSIL |
| Mild dysplasia | CIN1 | Mild dyskaryosis | ||
| Moderate dysplasia | CIN2 | Moderate dyskaryosis | HSIL | HSIL |
| Severe dysplasia | CIN3 | Severe dyskaryosis | ||
| Carcinoma in situ (CIS) | ||||
| Adenocarcinoma in situ (AIS) | CGIN | Glandular neoplasia | AGUS | AIS |
| Invasive carcinoma | Invasive carcinoma | Invasive carcinoma | Invasive carcinoma | Invasive carcinoma |
Footnote
is adapted from Herbert 2007 and NHSCSP 1995. In the current review, we used the CIN classification to identify histological outcomes and the Bethesda system to describe cytological cervical abnormalities.
Appendix 2. Search strategies used in PubMed MEDLINE, CENTRAL, EMBASE AND CERVIX
Search string in CENTRAL:(http://onlinelibrary.wiley.com/o/cochrane/) and PubMed MEDLINE (http://www.ncbi.nlm.nih.gov/sites/entrez):
((cervix OR cervical OR cervico*) AND (cancer OR carcinoma OR neoplas* OR dysplas* OR squamous OR CIN[tw] OR CINII*[tw] OR CIN2*[tw] OR CINIII*[tw] OR CIN3[tw] OR SIL[tw] OR HSIL[tw] OR H‐SIL[tw] OR LSIL[tw] OR L‐SIL OR ASCUS[tw] OR “ASC R”[tw] OR “ASC US”[tw] OR “ASC H”[tw])) AND (HPV OR “human papillomavirus” OR papillomaviridae OR papillomavirus infections[MeSH Terms] OR “hybrid capture” OR HC2 OR “HC 2” OR HCII OR “HC II” OR DNA OR viral OR virolog*) AND (triage OR management OR followup OR “follow up”)
Search string in EMBASE (http://www.embase.com):
'cervix'/exp OR cervic* AND ('cancer'/exp OR cancer OR 'carcinoma'/exp OR carcinoma OR 'neoplasia'/exp OR neoplasia OR 'dysplasia'/exp OR dysplasia OR cin OR sil) OR ('cervix'/exp OR cervix AND ('neoplasm'/exp OR neoplasm)) AND ('hpv'/exp OR hpv OR 'wart virus'/exp OR 'wart virus' OR ('hpv'/exp OR hpv AND ('dna'/exp OR dna)) OR ('hpv'/exp OR hpv AND viral) OR 'human papillomavirus'/exp OR 'human papillomavirus' OR 'hybrid'/exp OR hybrid AND capture OR 'hc2 assay' OR hc2 OR 'hc 2' OR hcii OR 'hc ii') AND ('triage'/exp OR triage OR 'management'/exp OR management OR 'follow up'/exp OR 'follow up')
The EMBASE string was saved using the name "ascuslsiltriage" by setting a weekly email alert.
In the CERVIX database, the following keywords were used: ((cervix AND cancer) OR (cervical cancer)) AND (ASC‐US OR ASCUS OR LSIL) AND HPV AND triage
Appendix 3. Characteristics of included studies (additional tables)
Additional characteristics of included studies
| Author, Year | Study location | Study population | Exclusion criteria | Details of diagnosis of ASCUS/LSIL cases |
Positivity criterion HPV test |
Reference standard |
| Manos, 1999 | 12 gynaecology clinics belonging to the Kaiser Permanente Medical Care Programme (N‐California, USA) | Screened women recalled for a smear showing ASCUS | Pregnancy, treatment cervical neoplasia < 6 months before, recalling impossible | Papette brush + cytobrush if stenosis | 1 pg/mL HPV DNA | Colposcopy with biopsy and/or ECC on all cases. ECC on cases in which no lesion requiring biopsy was seen |
| Bergeron, 2000 | 41 private gynaecologists working with 1 laboratory in Paris (France) | Screened women with index smear showing ASCUS or LSIL | Biopsy containing only endocervical cylindrical cells | No information | 1 pg/mL HPV DNA | Colposocpy and biopsies taken from the TZ |
| Lytwyn, 2000 | Family practices & 1 university health clinic in Ontario (Canada) | Women having a screen test result of ASCUS or LSIL | Expected low compliance, absence of cervix, previous diagnosis HSIL/AGUS/GIN/ cancer, previous surgery on cervix, current vaginal/vulvar neoplasia, immunosuppression, current indication for gynaecologic surgery | No information | 1 pg/mL HPV DNA | Directed biopsies of abnormal looking areas, ECC if abnormality extending into the endocervical canal or if repeat cytology = HSIL with normal colposcopy. No biopsy if colposcopy normal & ECC not indicated |
| Shlay, 2000 | Women's clinic in Denver (USA) | Indigent women referred for ASCUS or AGUS | Menstruation, pregnancy, refusal of consent | No information | 1 pg/mL HPV DNA | Biopsy from colposcopically abnormal areas. ECC and endocervical brush from all subjects |
| Lee, 2001 | University hospital Seoul (South‐Korea) | Women screened for cervical carcinoma and precancerous cervical lesions |
Women without subsequent HC2 testing | Cervicovaginal cytology, Cervex brush | 1 pg/mL HPV DNA | Colposcopy and colposcopy‐directed biopsy |
| Morin, 2001 | Service of gynaecology University of Quebec (Canada) |
Women with diagnosis of ASCUS after 2 consecutive normal smears | Pregnancy, previous biopsy or treatment on the cervix | No information | 1pg/mL HPV DNA |
Colposcopy and directed biopsies or ECC on all lesions of the cervix |
| Rebello, 2001 | Colposcopy clinic in Edinburgh (Scotland) | Women with persistent borderline of mildly dyskaryotic smears | No information | No information | 1, 2 and 4 pg/mL HPV DNA | Large loop excision on all patients |
| Solomon, 2001 | Gynaecology clinics at Birmingham, Oklahoma, Pittsburgh, Seattle (USA) |
Women enrolled on average 2 months after an index smear showing ASCUS enrolled in ALTS study | Pregnancy, ablative surgery or excisional therapy on the cervix | No information | 1 pg/mL HPV DNA | Biopsy from colposcopically suspected CIN, ECC if indicated (TZ or extent of lesion not completely visible). CIN2+ lesions treated with LEEP. Quality review of histology |
| Zielinski, 2001 | General practitioners (GPs) and outpatient clinic in Walcheren (the Netherlands) | Women consulting at GPs or outpatient clinic | Known history of cervical pathology, glandular lesion, lost to follow‐up | No information | 1 pg/mL HPV DNA | Colposcopically‐directed biopsies when a lesion was visible |
| Kulasingam, 2002 | Planned Parenthood clinics in Washington (USA) | Consecutive enrolment of women presenting for annual examinations at 1 of 3 Planned Parenthood clinics | No history of hysterectomy, chronic immune suppression, treatment of CIN, women younger than 18 or older than 50 years |
LBC | 1 pg/mL HPV DNA | Colposcopy, biopsy from visible lesions, 12 o'clock biopsies if no visible lesions |
| Pretorius, 2002 | Southern California Permanente clinics (USA) |
Women selected out of a cohort of women who underwent colposcopy. All women had abnormal cytology (ASCUS or LSIL) |
Colposcopy performed for reasons other than evaluation of abnormal Pap smear (e.g. follow‐up treatment of CIN) No specimens for HPV testing |
No information | 1 pg/mL HPV DNA | Colposcopy, biopsy of all abnormalities, endocervical
curettage if not pregnant |
| Sherman, 2002 | Gynaecology clinics at Birmingham, Oklahoma, Pittsburgh, Seattle (USA) | 2198 women with previous ASCUS 848 women with previous LSIL enrolled in ALTS study | Pregnancy, ablative surgery or excisional therapy on the cervix | No information | 1 pg/mL and 10 pg/mL HPV DNA | See Solomon, 2001 |
| Cuzick, 2003 | 161 family practices associated with 5 referral centres (Birmingham, Edinburgh, London, Manchester, Mansfield (UK) | Women with a screening result of borderline cytology/positive for hrHPV, randomised to immediate colposcopy or surveillance by repeat HPV testing, cytology, colposcopy at 12 months | Previous CIN treatment, previous (< 3 years before enrolment) abnormal cytology result | Conventional cytology (extended tip spatula) | 1 pg/mL HPV DNA | Colposcopy and target biopsies. Negative colposcopy
without biopsy accepted as negative outcome |
| Guyot, 2003 | Colposcopy clinic in West‐ Middlesex (UK) | Women referred to colposcopy clinic for minor cytological abnormalities in their cervical smear | Previous LLETZ, inadequate biopsy specimen, low cell count in LBC | No information | 1 and 3 pg/mL HPV DNA | Colposcopy on all and punch biopsies if colposcopically‐abnormal result |
| Lonky, 2003 | No information (USA) |
Women who underwent pelvic examination and conventional Pap smear | No information | Conventional Pap smear obtained with wooden spatula and endocervical brush | 1 pg/mL HPV DNA | Colposcopy on all, biopsy if suspicious lesion, ECC if colposcopy was unsatisfactory |
| Ordi, 2003 | No information (Spain) |
Women referred to colposcopy because of ASCUS or SIL or AIS diagnosis in the previous 6 months | No information | Ayre spatula | 1 pg/mL HPV DNA | Colposcopy on all, biopsy if suspicious lesion |
| Wensveen, 2003 | Gynaecologic outpatients clinic in The Hague (the Netherlands) | Women with ASCUS/AGUS on cervical smears | Pregnant women, women with HIV | Cervical smear | 1 pg/mL HPV DNA | Colposcopy and biopsy or ECC |
| Andersson, 2005 | Gynaecologic departments of 3 university hospitals in Stockholm (Sweden) | Women with atypia or low‐grade lesions detected at a population based screening | None reported | No information | 1 pg/mL HPV DNA | Colposcopy, biopsy from visible lesions, 12 o'clock biopsies if no visible lesions |
| Chen, 2005 | Colposcopy hospital in Mackay (Taiwan) | Women with mildly abnormal Pap results, either ASCUS or LSIL, who were referred to a Taiwanese colposcopy clinic | Subjects were excluded if they had a prior cytologic diagnosis of HSIL or had been treated for (CIN) | No information | 1 pg/mL HPV DNA | Colposcopy‐directed biopsies |
| Dalla Palma, 2005 | Pathology institute in Trentino (Italy) | Women attending a population‐based free organised screening, with equivocal cytological findings | No information | No information | 1.0 pg/mL HPV DNA | Colposcopy and biopsy |
| Davis‐Devine, 2005 | Gynaecology department of the Carle Clinic, Illinois (USA) | Women with an abnormal SurePath result within the preceding year | CIN treatment. Women with no intact cervix |
SurePath (Rovers cervex‐brush) | 1 pg/mL HPV DNA | Colposcopy and biopsy |
| Giovanelli, 2005 | Pathology Service of Palermo (Italy) | Women presenting to the Pathology Service consecutively enrolled when ASC‐US or AFR cytological abnormalities were found | No colposcopy | No information | 1 pg/mL HPV DNA | Colposcopy and colposcopy‐directed biopsy when necessary |
| Nieh, 2005 | No information (Taiwan) |
Women who had a routine Pap smear diagnosis of ASCUS | No information | Pap smear, cervical cytobrush | 1 pg/mL HPV DNA | Histological diagnosis through follow‐up biopsies |
| Bergeron, 2006 | Laboratory in Paris (France) | Screened women with index smear showing ASCUS | Women with no histology follow‐up data available | No information | 1 pg/mL HPV DNA | Colposcopy and biopsy if colposcopically‐suspicious |
| Holladay, 2006 | Center for cytopathology research and molecular diagnostics (USA) | 400 randomly selected ThinPrep specimens | Specimens not stored at room temperature or volume of the specimen < 12 mL | No information | 1 pg/mL HPV DNA | Histological diagnosis if available |
| Kelly, 2006 | Johns Hopkins Cytopathology laboratory, Baltimore (USA) | Residual cervical cytology samples | Specimens with no follow‐up biopsy data available after 6 months of cytology specimen procurement. Samples stored for more than 1 year |
No information | 1 pg/mL HPV DNA | Colposcopy and biopsy |
| Kiatpongsan, 2006 | King Chulalongkorn Memorial Hospital in Bangkok (Thailand) | Women with cytological smears showing ASCUS cytologic within the past 2 months |
Pregnant women and known cases of high‐grade precancerous cervical lesions (CIN2 or worse (CIN2+)) |
No information | 1 pg/mL HPV DNA | Colposcopy‐directed cervical biopsy |
| Monsonego, 2006 | Colposcopy center Institute Alfred Fournier in Paris (France) | Women with abnormal Pap smear or previous/current HPV‐related disease referred to colposcopy | All patients in whom Pap smear, colposcopy or biopsy could not be evaluated for technical reasons Immunosuppressed/pregnant women |
No information | 1 pg/mL HPV DNA | Colposcopy and biopsy (LEEP, punch biopsy) when indicated |
| Cushieri, 2007 | 15 GP practices in Edinburgh area (Scotland) | Women attending for routine cytology screening | Women with no follow‐up pathology records available for a minimum of 3 years subsequent to the borderline cytology diagnosis | LBC | 1 pg/mL HPV DNA | Colposcopy and biopsy |
| Ronco, 2007 | 9 cervical cancer screening centers in Italy | Women with ASCUS, AGUS or LSIL diagnosis selected out of a large randomised controlled trial of 9 screening programmes | Women who were pregnant, underwent hysterectomy or had been treated for CIN within 5 years Women younger than 25 or older than 60 years | Conventional cytology and LBC | 1, 2, 4, 10 and 20 pg/mL HPV DNA | Colposcopy, and biopsy when indicated |
| You, 2007 | Department of Gynecology, the Third Hospital of Peking University and the Maternal and Child Health Hospital of Haidian District of Beijing (China) | Selected women with abnormal cytology who had received HPV‐test and biopsy | No information | LBC | 1 pg/mL HPV DNA | Colposcopy‐directed biopsy |
| De Francesco, 2008 | Main hospital of Brescia (Italy) | Women attending routine cervical screening, with abnormal Pap smear results | Pregnant women, women with no intact uterus, a treatment for SILs or a history of chronic illness |
Cytobrush, STM | 1 pg/mL HPV DNA | Colposcopy and colposcopy‐directed punch biopsy |
|
Monsonego, 2008 |
Colposcopy clinic in Paris (France) | Women with abnormal Pap smear referred to colposcopy | No information | No information | 1 pg/mL HPV DNA | Colposcopy on all, biopsies if abnormal colposcopy (LEEP or punch biopsy) |
| Siddiqui, 2008 | Cytopathology laboratory Emory University Hospital, Atlanta (USA) | Cervical cytology samples of consecutive women with ASCUS cytology, selected out of all the samples collected between 2006‐2007 | No information | Residual cervical/vaginal cytology specimens in SurePath preservative | Threshold at RLU 1 (RLU between 1‐2.5 = equivocal, RLU > 2.5 = positive) | Histological diagnosis of cervical/vaginal biopsy |
| Szarewski, 2008 | Colposcopy clinics at the Hammersmith and St Mary’s Hospitals in London (UK) | Women referred to colposcopy because of an abnormal Pap smear | Pregnant women, women treated for CIN, hysterectomy | No information | 1 pg/mL HPV DNA | Colposcopy and histology |
| Cattani, 2009 | Colposcopy hospital and gynaecological oncology unit, Rome & Campobasso (Italy) | Women who attended secondary screening | Pregnant women or
women under treatment for invasive cervical cancer Women who didn't underwent colposcopy and HPV testing |
Cytology, conventional Pap smear | 1 pg/mL HPV DNA | Colposcopy, colposcopy‐directed biopsy (punch biopsies), cone specimens by LLETZ or cytological surveillance |
| Huang, 2009 | 5 colposcopy clinics across Canada | Women with abnormal cytology of any grade referred for colposcopy, or had a history of abnormal cytology, who were being followed‐up in colposcopy clinics per the routine standard of care | Women younger than 16 years or women who had a CIN‐treatment | No information | 1 pg/mL HPV DNA | Colposcopy and biopsy |
| Lee, 2009 | Colposcopy clinic (South‐Korea) | Consecutive women who visited a colposcopy clinic | No information | No information | 1 pg/mL HPV DNA | Colposcopy, colposcopy‐directed biopsy |
| Silverloo, 2009 | No information (Sweden) |
Consecutive women with ASCUS diagnosis on a primary organised cytological screening | No information | No information | 1 pg/mL HPV DNA | Colposcopy, Pap‐smear, cervical biopsy, ECC |
| Castle, 2010 | Health maintenance organization (Kaiser Permanente, Northern California (USA) | Women with abnormal cytology, aged 30 years or older, who attended cervical cancer screening between 2003‐2008 | Women older than 65 years, women with HPV+ results and negative cytology |
Conventional Pap smear | 1 pg/mL HPV DNA | Colposcopy and LEEP if colposcopically suspicious |
| Del Mistro, 2010 | 5 screening centres in Veneto region (Italy) | Women with ASCUS diagnosis on organised cytological screening | No information | Pap smear | 1 pg/mL HPV DNA | Colposcopy with punch biopsy when indicated |
| Denton, 2010 | 5 anatomic pathology laboratories (Switzerland & Italy) | Retrospectively collected, consecutive specimens from women with ASCUS or LSIL cytology | Damaged samples, contamination of slides with blood/mucus/inflammatory cells, age sample > 36 months, women with no follow‐up biopsy data available |
LBC (ThinPrep) | 1 pg/mL HPV DNA | Histological diagnosis on re‐cut tissue block specimens |
| Voss, 2010 | Mayo Clinic, Rochester (USA) | Previously collected, residual cervical cytology ThinPrep vials randomly selected for research testing from women who underwent same‐day colpo‐biopsy or had follow‐up biopsy data available | No information | LBC | 1 pg/mL HPV DNA | Colposcopy‐directed biopsy |
Footnotes
See Appendix 3 for explanation of acronyms and abbreviations
Technical details of the triage tests
| Author, year | Blinding of testing | Potential verification bias | Interval index‐enrolment tests | Collection device for repeat cytology | Device for collection of material for HPV testing | Transport medium for HPV test |
| Manos, 1999 | Colposcopy/histology was blinded to triage test results | No | 67 days | Papette brush | Papette brush | Thinprep |
| Bergeron, 2000 | Pathologist was masked to the other test results | No | 2 months | Ayre spatula + cytobrush | Cone brush | STM |
| Lytwyn, 2000 | Histology blinded from HPV and repeat cytology | No | 6 months for cytology | Extended tip spatula (sometimes + cytobrush) | ‐ | ‐ |
| Shlay, 2000 | Histology blinded to clinic and HPV results | No | ‐ | ‐ | ‐ | ‐ |
| Lee, 2001 | All histologic diagnoses blinded to HPV status | Unclear | ‐ | Cervex brush | Dacron swab | STM |
| Morin, 2001 | Not documented | No | ‐ | ‐ | Dacron brush | STM |
| Rebello, 2001 | Not documented | No | ‐ | ‐ | Cone brush | STM |
| Solomon, 2001 | Histology not blinded to cytology. Colposcopy repeated when histology < cytology. No statement on independence of histo/viro. In arm 2 almost all cases were HPV+ | Yes | 2 months | Papette brush | Papette | Thinprep |
| Zielinski, 2001 | Not documented | No | ‐ | ‐ | ‐ | ‐ |
| Kulasingam, 2002 | Gold standard not blinded to cytological or HPV testing | No | ‐ | Ayre spatula + cytobrush | Dacron swab | STM |
| Pretorius, 2002 | Not documented | No | ‐ | ‐ | Cone brush or Dacron swab (if pregnant) | STM |
| Sherman, 2002 | See Solomon, 2001 | Yes | 2 months | Papette brush | Papette | Thinprep |
| Cuzick, 2003 | Histology read locally, but reviewed centrally in a blinded fashion by one pathologist |
Yes. Only women with negative cytology and HPV test were randomly selected for colposcopy |
‐ | ‐ | Cone brush | STM |
| Guyot, 2003 | HPV testing, colposcopy and histology interpretation were blinded | No | < 3 months | Papette brush | Papette brush | PreservCyt |
| Lonky, 2003 | Pathology blinded to HPV test and cytology results | No | 3‐4 weeks | ‐ | Cone brush | STM |
| Ordi, 2003 | Not documented | No | 6 months | ‐ | Cervical brush (Digene cervical sampler) | STM |
| Wensveen, 2003 | Not documented | No | < 12 weeks | ‐ | ‐ | ‐ |
| Andersson, 2005 | Not documented | No | 4‐6 months | Cervical brush | Cervical brush | STM |
| Chen, 2005 | Not documented | No | ‐ | ‐ | ‐ | ‐ |
| Dalla Palma, 2005 | Probably not, as only cases with positive cytology and/or HC2 had colposcopy and biopsy | Yes. Only cases with positive cytology and/or HC2 had colposcopy and biopsy |
‐ | ‐ | ‐ | PreservCyt |
| Davis‐Devine, 2005 | All cytologic and histologic materials were reviewed without knowledge of the study design | No | 7 days | Broom device (Rovers cervex brush) | Broom device (Rovers cervex brush) | SurePath |
| Giovanelli, 2005 | Not documented | No | Co‐collection | ‐ | Ecto‐cervical wooden spatula and endo‐cervical cytobrush |
STM |
| Nieh, 2005 | Not documented | No | ‐ | ‐ | Cytobrush | STM |
| Bergeron, 2006 | Not documented | No | 6‐18 months | Cervical brush | Cervical brush | Cyto‐screen liquid |
| Holladay, 2006 | Histo‐confirmation was blinded to p16 and HC2 results | Yes. Only a part of the cases had histologic outcome available. The accuracy data were computed on these histologically‐verified cases |
< 2 months? 8 months | ‐ | ‐ | ThinPrep |
| Kelly, 2006 | Colposcopy and histology of biopsies blinded to HPV status and immunostaining results | No | ‐ | ‐ | ‐ | PreservCyt |
| Kiatpongsan, 2006 | HC2 test blinded to histopathology and vice versa. | No | 2 months | ‐ | Cytobrush | STM |
| Monsonego, 2006 | Yes | Possible differential verification | 3 months | ‐ | Cone brush | ‐ |
| Cushieri, 2007 | Not documented | No | Unclear, 3‐3.5 year follow‐up | ‐ | ‐ | PreservCyt |
| Ronco, 2007 | Yes | No | Co‐testing | Ayre spatula | Ayre spatula | PreservCyt |
| You, 2007 | Not documented | No | ‐ | ‐ | ‐ | ‐ |
| De Francesco, 2008 | Not documented | No | ‐ | Cytobrush | Cytobrush | STM |
| Monsonego, 2008 | Yes | No | 2‐3 months | Broom‐like collection device | HC2 collection device | Universal Collecting Medium |
| Siddiqui, 2008 | Not mentioned | No | < 2 weeks | ‐ | ‐ | SurePath |
| Szarewski, 2008 | Unclear | No | Median: 2.4 months | ‐ | Cervex broom | PreservCyt |
| Cattani, 2009 | Pathologists involved in cytological and histological assessments were not involved in testing for HPV. HPV tests were blinded to results of cytology and histology | No | ‐ | ‐ | Cervical brush | PreservCyt/ThinPrep |
| Huang, 2009 | Colposcopy and histology were blinded to HPV status. HPV tests were carried out independent of each other | No | 1‐3 months | Cervex‐brush | Cervex‐brush | PreservCyt |
| Lee, 2009 | Not documented | No | Cervex brush | ‐ | PreservCyt | |
| Silverloo, 2009 | Yes | Yes. Cytology/HPV negative women did not undergo colposcopy/biopsy, but had a repeat Pap smear |
< 3 months | ‐ | ‐ | ‐ |
| Castle, 2010 | Yes, results of tests were blinded | No | ‐ | ‐ | ‐ | ‐ |
| Del Mistro, 2010 | Pap smears at enrolment were blinded to the results of HPV tests | No | 3 months | ‐ | ‐ | ‐ |
| Denton, 2010 | Histology diagnoses were blinded to HPV and p16 status | No | 6 months | ‐ | ‐ | ‐ |
| Voss, 2010 | Not documented | No | ‐ | ‐ | ‐ | PreservCyt |
Appendix 4. Summary of all the meta‐analyses of the absolute accuracy in ASCUS triage
| Studies where HC2 triage and repeat cytology were applied | |||||||
| Triage test | Test cut‐off | Outcome | Studies | Accuracy parameter | Pooled estimate | (95% CI) | Range |
| HC2 | RLU > 1 (1 pg/mL) | CIN2+ | 10 | Sensitivity | 90.9% | (85.7% to 94.4%) | 60%‐96% |
| 10 | Specificity | 60.7% | (52.9% to 68.0%) | 39%‐79% | |||
| CIN3+ | 4* | Sensitivity | 94.8% | (89.6% to 97.5%) | 75%‐96% | ||
| 4* | Specificity | 56.6% | (39.4% to 72.3%) | 38%‐58% | |||
| Repeat cytology | ASCUS+ | CIN2+ | 10 | Sensitivity | 71.5% | (62.9% to 78.8%) | 40%‐94% |
| 10 | Specificity | 68.4% | (59.9% to 75.8%) | 45%‐90% | |||
| CIN3+ | 4* | Sensitivity | 77.9% | (64.0% to 87.6%) | 67%‐86% | ||
| 4* | Specificity | 57.4% | (40.3% to 73.0%) | 42%‐90% | |||
| LSIL+ | CIN2+ | 6 | Sensitivity | 44.1% | (33.3% to 55.5%) | 16%‐61% | |
| 6 | Specificity | 90.1% | (83.3% to 94.4%) | 76%‐96% | |||
| CIN3+ | 4* | Sensitivity | 53.5% | (17.8% to 85.9%) | 25%‐67% | ||
| 4 | Specificity | 79.9% | (73.4% to 85.2)%) | 75%‐96% | |||
| HSIL+ | CIN2+ | 5 | Sensitivity | 15.8% | (6.5% to 33.6%) | 8%‐50% | |
| 5 | Specificity | 98.3% | (96.7% to 99.1%) | 97%‐99% | |||
| CIN3+ | 4* | Sensitivity | 33.2% | (6.1% to 79.2%) | 0‐58% | ||
| 4* | Specificity | 95.6% | (93.6% to 97.1%) | 95%‐99% | |||
| Studies where HC2 triage was applied with or without repeat cytology | |||||||
| HC2 | RLU > 1 (1 pg/mL) | CIN2+ | 39 | Sensitivity | 90.4% | (88.1% to 92.3%) | 60%‐100% |
| 39 | Specificity | 58.3% | (53.6% to 62.9%) | 27%‐79% | |||
| CIN3+ | 17 | Sensitivity | 93.7% | (90.4% to 95.9%) | 75%‐100% | ||
| 17 | Specificity | 52.3% | (45.7% to 58.7%) | 33%‐70% | |||
Footnotes
*Univariate analyses using the bivariate model run separately for sensitivity and specificity.
Appendix 5. Summary of all the meta‐analyses of the absolute accuracy in LSIL triage
| Studies where HC2 triage and repeat cytology were applied | |||||||
| Triage test | Triage cut‐off | Outcome | Studies | Accuracy parameter | Pooled estimate | (95% CI) | Range |
| HC2 | RLU > 1 (1 pg/mL) | CIN2+ | 6 | Sensitivity | 96.2% | (91.4 to 98.3%) | 89%‐100% |
| 6 | Specificity | 27.7% | (20.9 to 35.7%) | 19%‐44% | |||
| CIN3+ | 4* | Sensitivity | 97.5% | (69.6 to 99.8%) | 97%‐100% | ||
| 4* | Specificity | 24.8% | (7.3 to 58.1%) | 17%‐29% | |||
|
Repeat cytology |
ASCUS+ | CIN2+ | 6 | Sensitivity | 77.1% | (59.5 to 88.5%) | 33%‐100% |
| 6 | Specificity | 51.2% | (34.5% to 67.6%) | 23%‐83% | |||
| CIN3+ | 4* | Sensitivity | 84.6% | (48.6% to 97.0%) | 64%‐90% | ||
| 4* | Specificity | 44.4% | (16.0% to 76.9%) | 21%‐82% | |||
| LSIL+ | CIN2+ | 4* | Sensitivity | 62.1% | (33.4% to 84.3%) | 21%‐80% | |
| 4* | Specificity | 66.9% | (51.5% to 79.4%) | 48%‐91% | |||
| CIN3+ | 4* | Sensitivity | 71.6% | (33.4% to 92.7%) | 36%‐83% | ||
| 4* | Specificity | 61.8% | (33.1% to 84.1%) | 44%‐91% | |||
| HSIL+ | CIN2+ | 4* | Sensitivity | 31.6% | (18.2% to 49.0%) | 12%‐42% | |
| 4* | Specificity | 96.6% | (93.6% to 98.3%) | 93%‐99% | |||
| CIN3+ | 4* | Sensitivity | 41.9% | (24.8% to 61.2%) | 21%‐50% | ||
| 4* | Specificity | 93.8% | (86.1% to 97.4%) | 91%‐99% | |||
| Studies where HC2 triage was applied with or without repeat cytology | |||||||
| HC2 | RLU > 1 (1 pg/mL) | CIN2+ | 24 | Sensitivity | 95.4% | (94.0% to 96.5%) | 80%‐100% |
| 24 | Specificity | 27.8% | (23.8% to 32.1%) | 16%‐58% | |||
| CIN3+ | 14 | Sensitivity | 96.4% | (90.5% to 98.7%) | 76%‐100% | ||
| 14 | Specificity | 23.7% | (19.4% to 28.7%) | 15%‐47% | |||
Appendix 6. Heterogeneity analysis
The heterogeneity analysis of the accuracy of the triage tests by covariate was performed when the compared groups contained at least three studies in one group and at least five studies in another group. The influence of the following covariates on the accuracy could be addressed: use of the cytological classification system (Bethesda versions 1989 or 2001 and BSCC); collection device used to collect cellular material for the HPV test (brush or broom); blinding of tests (yes or unclear); transport medium to store the sample for virological testing (Preservcyt, STM),
7.1 Influence of covariates on the accuracy of ASCUS triage by HC2, outcome CIN2+ or CIN3+
| Analysis |
Number of studies |
Summary sensitivity (95% CI) | Summary specificity (95% CI) |
P value LR test** |
| ASCUS triage with HC2 for CIN2+: Classification system for reporting of cervical cytology | ||||
| BSCC | 5 | 86.5 (74.3‐93.4) | 64.4 (50.7‐76.1) | 0.8 |
| Bethesda 1989 | 23 | 91.0 (88.0‐93.3) | 56.8 (50.4‐63.0) | |
| Bethesda 2001 | 11 | 90.3 (85.6‐93.6) | 58.8 (49.7‐67.4) | |
| ASCUS triage with HC2 for CIN2+: device to collect sample for HPV testing* | ||||
| Broom | 5 | 94.9 (91.3‐97.0) | 57.2 (43.4‐69.9) | 0.03 |
| Brush | 16 | 87.5 (83.4‐90.7) | 59.4 (51.8‐66.6) | |
| Ratio brush vs broom | 0.92 (0.88‐0.97), P = 0.002 | 1.04 (0.80‐1.36), P = 0.8 | ||
| ASCUS triage with HC2 for CIN2+: blinding | ||||
| Yes | 21 | 91.4 (88.4 93.8) | 60.4 (53.7‐66.7) | 0.3 |
| Unclear | 17 | 89.0 (84.9‐92.1) | 56.1 (48.6‐63.4) | |
| Ratio unclear vs blinded | 0.97 (0.93‐1.02), P = 0.3 | 0.93 (0.78‐1.10), P = 0.4 | ||
| ASCUS triage with HC2 for CIN2+: transport medium | ||||
| STM | 14 | 85.9 (81.0‐89.7) | 61.5 (53.4‐69.0) | 0.006 |
| Preservcyt | 9 | 91.7 (86.4‐95.1) | 44.4 (34.0‐55.3) | |
| Ratio Preservcyt vs STM | 1.07 (1.00‐1.14), P = 0.06 | 0.72 (0.55‐0.95), P = 0.02 | ||
| ASCUS triage with HC2 for CIN3+: Classification system for reporting of cervical cytology | ||||
| Bethesda 1989 | 13 | 92.9 (88.0‐95.9) | 51.3 (43.1‐59.4) | 0.6 |
| Bethesda 2001 | 3 | 96.3 (84.2‐99.2) | 51.1 (35.4‐66.6) | |
| Ratio Bethesda 2001 vs 1989 | 1.04 (0.96‐1.11), P = 0.3 | 1.00 (0.70‐1.42), P = 1.0 | ||
| ASCUS triage with HC2 for CIN3+: continent * | ||||
| America | 6 | 95.9 (91.8‐98.0) | 54.0 (43.3‐64.5) | 0.1 |
| Europe | 9 | 90.2 (82.8‐94.7) | 51.3 (41.8‐60.6) | |
| Ratio Europe vs America | 0.94 (0.88‐1.01), P = 0.09 | 0.95 (0.72‐1.24), P = 0.7 | ||
| ASCUS triage with HC2 for CIN3+: blinding* | ||||
| Yes | 8 | 91.4 (85.3‐95.1) | 52.6 (42.1‐62.9) | 0.3 |
| Unclear | 8 | 95.2 (90.5‐97.6) | 52.4 (41.5‐63.0) | |
| Ratio unclear vs blinded | 1.04 (0.98‐1.11), P = 0.2 | 1.0 (0.75‐1.33), P = 1.0 | ||
| ASCUS triage with HC2 for CIN3+: transport medium* | ||||
| STM | 7 | 91.0 (82.3‐95.7) | 55.6 (44.1‐66.5) | 0.4 |
| Preservcyt | 5 | 93.2 (81.3‐97.8) | 45.4 (31.4‐60.2) | |
| Ratio Preservcyt vs STM | 1.02 (0.92‐1.14), P = 0.6 | 0.82 (0.56‐1.20), P = 0.3 | ||
*Univariate analyses using the bivariate model run separately for sensitivity and specificity.
**Likelihood ratio test for model with and without the covariate.
7.2 Influence of covariates on the accuracy of ASCUS triage by repeat cytology, outcome CIN2+
| Analysis |
Number of studies |
Summary sensitivity (95% CI) | Summary specificity (95% CI) |
P value LR test** |
| ASCUS with repeat cytology at cut‐off ASCUS+ for CIN2+: continent* | ||||
| America | 5 | 80.1 (70.5‐87.1) | 59.8 (48.5‐70.1) | 0.007 |
| Europe | 5 | 62.9 (49.6‐74.5) | 75.8 (65.9‐83.6) | |
| Ratio Europe vs America | 0.79 (0.63‐0.99), P = 0.04 | 1.27 (1.02‐1.58), P = 0.04 | ||
*Univariate analyses using the bivariate model run separately for sensitivity and specificity.
**Likelihood ratio test for model with and without the covariate.
7.3 Influence of covariates on the accuracy of LSIL triage by HC2, outcome CIN2+ or CIN3+
| Analysis |
Number of studies |
Summary sensitivity (95% CI) | Summary specificity (95% CI) |
P value LR test** |
| LSIL triage with HC2 for CIN2+: Classification system for reporting of cervical cytology* | ||||
| BSCC | 3 | 96.1 (88.1‐98.8) | 33.8 (22.6‐47.1) | 0.2 |
| Bethesda 1989 | 16 | 95.8 (93.6‐97.3) | 29.8 (25.2‐34.8) | |
| Bethesda 2001 | 5 | 96.2 (93.0‐98.0) | 19.5 (14.0‐26.4) | |
| LSIL triage with HC2 for CIN2+: device to collect sample for HPV testing* | ||||
| Broom | 3 | 98.4 (95.8‐99.4) | 24.8 (17.5‐33.8) | 0.003 |
| Brush | 9 | 92.8 (89.1‐95.3) | 32.6 (26.8‐38.9) | |
| Ratio brush vs broom | 0.94 (0.91‐0.98), P = 0.005 | 1.32 (0.90‐1.92), P = 0.1 | ||
| LSIL triage with HC2 for CIN2+: continent* | ||||
| America | 8 | 96.5 (95.2‐97.5) | 21.4 (16.7‐27.0) | 0.04 |
| Asia | 4 | 96.1 (90.5‐98.4) | 24.7 (16.8‐34.9) | |
| Europe | 12 | 95.0 (92.7‐96.6) | 33.1 (27.8‐38.8) | |
| LSIL triage with HC2 for CIN2+: Classification system for reporting of cervical cytology* | ||||
| BSCC | 3 | 96.1 (88.1‐98.8) | 33.8 (22.6‐47.1) | 0.2 |
| Bethesda 1989 | 16 | 95.8 (93.6‐97.3) | 29.8 (25.2‐34.8) | |
| Bethesda 2001 | 5 | 96.2 (93.0‐98.0) | 19.5 (14.0‐26.4) | |
| LSIL HC2 CIN2+: device to collect sample for HPV testing* | ||||
| Broom | 3 | 98.4 (95.8‐99.4) | 24.8 (17.5‐33.8) | 0.003 |
| Brush | 9 | 92.8 (89.1‐95.3) | 32.6 (26.8‐38.9) | |
| Ratio brush vs broom | 0.94 (0.91‐0.98), P = 0.005 | 1.32 (0.90‐1.92), P = 0.1 | ||
| LSIL HC2 CIN2+: continent* | ||||
| America | 8 | 96.5 (95.2‐97.5) | 21.4 (16.7‐27.0) | 0.04 |
| Asia | 4 | 96.1 (90.5‐98.4) | 24.7 (16.8‐34.9) | |
| Europe | 12 | 95.0 (92.7‐96.6) | 33.1 (27.8‐38.8) | |
| LSIL triage with HC2 for CIN3+: blinding* | ||||
| Yes | 13 | 95.4 (93.1‐97.0) | 29.3 (23.7‐35.6) | 0.5 |
| Unclear | 10 | 96.5 (94.2‐98.0) | 26.4 (20.3‐33.5) | |
| Ratio unclear vs blinded | 1.01 (0.98‐1.04), P = 0.4 | 0.90 (0.65‐1.25), P = 0.5 | ||
| LSIL triage with HC2 for CIN2+: transport medium | ||||
| STM | 8 | 93.6 (87.6‐96.9) | 33.1 (26.3‐40.6) | 0.4 |
| Preservcyt | 7 | 97.2 (93.0‐98.9) | 31.9 (24.5‐40.3) | |
| Ratio Preservcyt vs STM | 1.04 (0.98‐1.09), P = 0.2 | 0.96 (0.69‐1.34) P = 0.8 | ||
| LSIL triage with HC2 for CIN3+: continent | ||||
| America | 5 | 96.0 (88.5‐98.7) | 18.1 (13.1‐24.5) | 0.1 |
| Europe | 8 | 96.3 (86.4‐99.1) | 27.1 (21.2‐33.8) | |
| Ratio Europe vs America | 1.00 (0.95‐1.06), P = 0.9 | 1.50 (1.01‐2.21), P = 0.04 | ||
| LSIL triage with HC2 for CIN3+: blinding | ||||
| Yes | 7 | 96.1 (84.2‐99.1) | 23.7 (17.2‐31.8) | 1.0 |
| Unclear | 6 | 95.9 (87.8‐98.7) | 24.8 (17.1‐34.5) | |
| Ratio Unclear vs Blinded | 1.00 (0.94‐1.06), P = 0.9 | 1.04 (0.65‐1.67), P = 0.8 | ||
| LSIL triage with HC2 for CIN3+: transport medium | ||||
| STM | 5 | 96.5 (74.2‐99.6) | 26.2 (18.2‐36.2) | 0.5 |
| Preservcyt | 5 | 99.2 (71.9‐100) | 28.6 (19.7‐39.7) | |
| Ratio Presercyt vs STM | 1.03 (0.95‐1.11), P = 0.4 | 1.09 (0.67‐1.79), P = 0.7 | ||
*Univariate analyses using the bivariate model run separately for sensitivity and specificity.
**Likelihood ratio test for model with and without the covariate.
7.4. Influence of covariates on the accuracy of LSIL triage by repeat cytology
No analyses performed because of the small number of studies.
Appendix 7. Influence of age on the accuracy of HC2
| Triage group | Outcome | Age group | Estimate of pooled sensitivity (95% CI) | Estimate of pooled specificity (95% CI) | Relative sensitivity (95% CI) | P value (relative sensitivity) | Relative specificity (95% CI) | P value (relative specificity) |
| ASCUS | CIN2+ | < 30 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| 30‐39 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| 40‐49 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| 50+ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| CIN3+ | < 30 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | |
| 30‐39 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| 40‐49 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| 50+ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ||
| LSIL | CIN2+ | < 30 | 96.2% (93.4% to 97.8%) | 18.0% (15.6% to 20.6%) | 0.99 (0.97 to 1.02) | 0.81 | 0.64 (0.32 to 1.31) | 0.222 |
| 30‐39 | 96.5% (94.6% to 97.7%) | 27.9% (12.9% to 50.5%) | reference | reference | reference | reference | ||
| 40‐49 | 95.4% (91.6% to 97.5%) | 40.3% (21.4% to 62.5%) | 0.99 (0.96 to 1.02) | 0.416 | 1.44 (0.60 to 3.48) | 0.416 | ||
| 50+ | 95.0% (85.9% to 98.3%) | 43.7% (24.4% to 65.2%) | 0.98 (0.93 to 1.04) | 0.592 | 1.56 (0.66 to 3.68) | 0.304 | ||
| CIN3+ | < 30 | 95.8% (91.7% to 97.9%) | 16.3% (14.0% to 18.9%) | 1.02 (0.97 to 1.07) | 0.410 | 0.71 (0.41 to 1.22) | 0.214 | |
| 30‐39 | 93.9% (89.6% to 96.5%) | 22.9% (13.2% to 36.7%) | reference | reference | reference | reference | ||
| 40‐49 | 95.2% (87.7% to 98.2%) | 28.5% (13.2% to 51.0%) | 1.01 (0.96 to 1.07) | 0.653 | 1.24 (1.11 to 1.39) | 0.0002 | ||
| 50+ | 93.0% (78.2% to 98.0%) | 43.4% (22.5% to 67.0%) | 0.99 (0.90 to 1.09) | 0.832 | 1.90 (0.89 to 4.02) | 0.095 |
Appendix 8. List of abbreviations
AGC: atypical glandular cells AGUS: atypical glandular cells of undetermined significance AIS: adenocarcinoma in situ ALTS: ASCUS‐LSIL Triage Study ASC: atypical squamous cells (comprises ASC‐US and ASC‐H) ASC‐H: atypical squamous cells, HSIL cannot be ruled out ASC‐R: atypical squamous cells favouring a benign reactive process squamous cells of undetermined significance ASC‐US: atypical squamous cells of undetermined significance ASCUS: atypical squamous cells of undetermined significance (comprises ASC‐R, ASC‐US and ASC‐H) BSCC: Brittish Society of Clinical Cytology CGIN: cervical glandular intraepithelial neoplasia CI (95%) confidence interval CIN: cervical intra‐epithelial neoplasia CIS: carcinoma in situ DNA: deoxyribo‐nucleic acid DOR: diagnostic odds ratio ECC: endocervical curettage FN: false negative FP: false positive HC: Hybrid Capture HC2: Hybrid Capture‐II (2nd generation assay) HCT: Hybrid Capture tube‐based assay HPV: human papillomavirus hrHPV: high‐risk HPV HSIL: high‐grade squamous intraepithelial lesion HSROC: hierarchical summary receiver operating characteristic LBC: liquid‐based cytology LEEP: loop electrosurgical excision procedure LLETZ: large loop excision of the transformation zone LSIL: low‐grade squamous intraepithelial lesion NASBA: nucleic acid sequence‐based amplification NHSCSP: National Health Service Cervical Screening Programme
NILM: negative for intraepithelial lesion or malignancy (=normal cytology) NPV: negative predictive value PCR: polymerase chain reaction PPV: positive predictive value RLU: relative light units RNA: ribo‐nucleic acid ROC: receiver operating characteristic SROC‐curve: summary receiver operating characteristic curve STM: specimen transport medium TBS: The Bethesda System TN: true negative TP: true positive TZ: transformation zone VIA: visual inspection after application of acetic acid
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
1. Test.

Triage ASCUS with HC2, CIN2+ (all studies)
2. Test.

Triage of ASCUS with HC2, CIN2+ (studies with both tests)
3. Test.
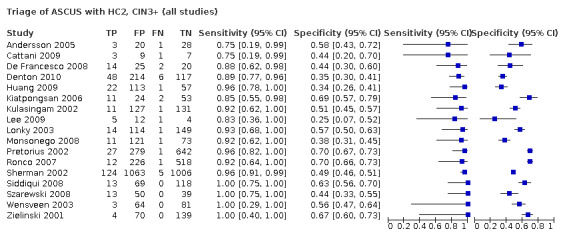
Triage of ASCUS with HC2, CIN3+ (all studies)
4. Test.

Triage of ASCUS with HC2, CIN3+ (studies with both tests)
5. Test.

Triage of ASCUS with repeat cytology (cut‐off ASCUS+), CIN2+
6. Test.

Triage of ASCUS with repeat cytology (cut‐off LSIL+), CIN2+
7. Test.

Triage of ASCUS with repeat cytology (cut‐off HSIL+), CIN2+
8. Test.

Triage of ASCUS with repeat cytology (cut‐off ASCUS+), CIN3+
9. Test.

Triage of ASCUS with repeat cytology (cut‐off LSIL+), CIN3+
10. Test.

Triage of ASCUS with repeat cytology (cut‐off HSIL+), CIN3+
11. Test.

Triage LSIL with HC2, CIN2+ (all studies)
12. Test.

Triage of LSIL with HC2, CIN2+ (studies with both tests)
13. Test.

Triage of LSIL with HC2, CIN3+ (all studies)
14. Test.

Triage of LSIL with HC2, CIN3+ (studies with both tests)
15. Test.
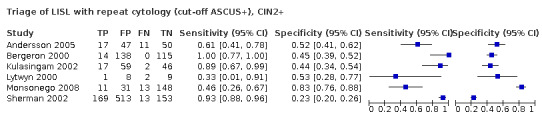
Triage of LISL with repeat cytology (cut‐off ASCUS+), CIN2+
16. Test.

Triage of LSIL with repeat cytology (cut‐off LSIL+), CIN2+
17. Test.

Triage of LSIL with repeat cytology (cut‐off HSIL+), CIN2+
18. Test.

Triage of LSIL with repeat cytology (cut‐off ASCUS+), CIN3+
19. Test.

Triage of LSIL with repeat cytology (cut‐off LSIL+), CIN3+
20. Test.

Triage of LSIL with repeat cytology (cut‐off HSIL+), CIN3+
25. Test.
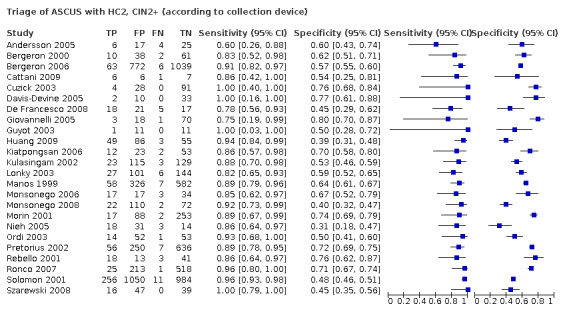
Triage of ASCUS with HC2, CIN2+ (according to collection device)
26. Test.

Triage of ASCUS with HC2, CIN2+ (according to transport medium)
27. Test.

Triage of ASCUS with HC2, CIN2+ (<30y)
28. Test.

Triage of ASCUS with HC2, CIN2+ (30‐39y)
29. Test.

Triage of ASCUS with HC2, CIN2+ (40‐49y)
30. Test.

Triage of ASCUS with HC2, CIN2+ (50y or older)
31. Test.

Triage of ASCUS with HC2, CIN3+ (<30y)
32. Test.

Triage of ASCUS with HC2, CIN3+ (30‐39y)
33. Test.

Triage of ASCUS with HC2, CIN3+ (40‐49y)
34. Test.

Triage of ASCUS with HC2, CIN3+ (50y or older)
35. Test.

Triage of LSIL with HC2, CIN2+ (<30y)
36. Test.

Triage of LSIL with HC2, CIN2+ (30‐39y)
37. Test.

Triage of LSIL with HC2, CIN2+ (40‐49y)
38. Test.

Triage of LSIL with HC2, CIN2+ (50y or older)
39. Test.

Triage of LSIL with HC2, CIN3+ (<30y)
40. Test.
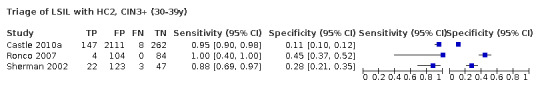
Triage of LSIL with HC2, CIN3+ (30‐39y)
41. Test.

Triage of LSIL with HC2, CIN3+ (30‐49y)
42. Test.

Triage of LSIL with HC2, CIN3+ (50y or older)
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Andersson 2005.
| Study characteristics | ||
| Clinical features and settings | Women with low‐grade atypia in the Stockholm area. Population‐based cytology screening. |
|
| Participants | Women with ASCUS or LSIL. | |
| Study design | 177 women (52 ASCUS, 125 LSIL) tested with HC2 and repeat cytology. All women underwent colposcopy and punch biopsies were taken in all cases. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+ and CIN3+. Reference standard: colposcopy and biopsy from all women. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: repeat cytology (conventional Pap test). |
|
| Follow‐up | Colposcopy verification and triage tests were performed 4–6 months after observation of ASCUS or LSIL | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Consecutive enrolment of women with low‐grade atypia, detected at a population‐based screening. |
| Acceptable reference standard? All tests | Yes | Colposcopy and histological examination of colposcopically directed biopsies. |
| Acceptable delay between tests? All tests | Yes | Reference standard verification performed by enrolment, together with the index and comparator test. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsies, independent of the HC2 result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Unclear | Not reported. |
| Relevant clinical information? All tests | Yes | Women with known low‐grade atypia. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Bergeron 2000.
| Study characteristics | ||
| Clinical features and settings | Opportunistic screening. 41 gynaecological practices collaborating with a private cytological laboratory in Paris (France). |
|
| Participants | Women with ASCUS or LSIL. | |
| Study design | 378 women (111 ASCUS, 267 LSIL) tested with HC2 and repeat cytology. All women underwent colposcopy and biopsies were taken in all cases (type of biopsies not reported). | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy and biopsy from all women. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: repeat cytology (conventional Pap test). PCR and Southern blot hybridisation (not evaluated in the current review). |
|
| Follow‐up | Two months between referral cytological test and enrolment. Colposcopy verification and triage tests were performed simultaneously. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Women referred to colposcopy because of ASCUS or LSIL cytology result. |
| Acceptable reference standard? All tests | Yes | Colposcopy and histological examination of biopsies. |
| Acceptable delay between tests? All tests | Yes | Less than 2 months between first test and enrolment tests. Reference standard verification performed concurrently with index and comparator tests. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsies (reference standard), independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Yes | Colposcopy and histology were blinded to HC2 and repeat cytology results. |
| Index test results blinded? All tests | Yes | HC2 and repeat cytology were assessed independently. |
| Relevant clinical information? All tests | Yes | Women with known low‐grade atypia (ASCUS and LSIL) referred to colposcopy. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Bergeron 2006.
| Study characteristics | ||
| Clinical features and settings | Opportunistic screening. | |
| Participants | Women with ASCUS cytology. | |
| Study design | 1880 women (ASCUS) were tested with HC2 and repeat cytology. All women underwent colposcopy and if a colposcopic suspicious lesion was found, biopsies were taken. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy and/or biopsy from all women. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | 6‐18 months. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Women diagnosed with ASCUS cytology. |
| Acceptable reference standard? All tests | Yes | Colposcopy and histological examination of biopsies. |
| Acceptable delay between tests? All tests | Yes | 6‐18 months between first test and enrolment tests. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and/or the same type of biopsies, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Yes | The HC2 test interpretation is machine‐based. |
| Relevant clinical information? All tests | Yes | Women with known ASCUS. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Castle 2010a.
| Study characteristics | ||
| Clinical features and settings | CC screening from 2003‐2008 at Kaiser Permanente, USA. | |
| Participants | Women aged ≥ 30 years who underwent CC screening between 2003‐2008 at Kaiser Permanente hospital. | |
| Study design | Women were screened using conventional Pap tests and an HC2 test. Women with HPV+ and abnormal cervical cytology (ASC‐H, ASCUS, LSIL, HSIL, AGC) routinely underwent colposcopy. If colposcopy showed a CIN2+ lesion, Loop Electrical Excision Procedure (LEEP) was performed. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy and histology. HPV+ women with abnormal cytology and HPV‐ women with abnormal cytology (ASC‐H, LSIL, HSIL, AGC) underwent colposcopy. HPV‐ women with ASCUS cytology were re‐screened after 6‐12 months. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | HPV‐negative women with ASCUS were followed‐up by retesting after 6‐12 months and did not receive verification if retesting was negative. Therefor data on ASCUS triage was not included in the review. | |
| Notes | We only included the data for LSIL triage, because women with ASCUS cytology and HPV‐ results did not undergo colposcopy. Age‐stratified data available. |
|
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Women attending cervical cancer screening. (Note: only women > 30 years included.) |
| Acceptable reference standard? All tests | Yes | Colposcopy and LEEP, if a colposcopic suspicious lesion was found. |
| Acceptable delay between tests? All tests | Unclear | Delay not reported. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | No | Women with LSIL, AGC, ASC‐H or HSIL cytology were referred to colposcopy irrespective of the HPV testing results. In contrast, women with ASCUS cytology and HPV+ results underwent colposcopy while women with ASCUS and HPV‐ test results, did not undergo colposcopy. For this meta‐analysis we did not include the ASCUS data. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Yes | Colposcopy was blinded to HPV test results. |
| Index test results blinded? All tests | Yes | The HC2 test interpretation is machine‐based. |
| Relevant clinical information? All tests | Yes | Women with abnormal cytology were referred to colposcopy. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Cattani 2009.
| Study characteristics | ||
| Clinical features and settings | Secondary screening in Italy. | |
| Participants | Women with ASCUS or LSIL. | |
| Study design | Cross‐sectional study enrolling women referred to colposcopy with HC2 and mRNA testing for viral genes E6 and E7 using the NucliSens EasyQ HPV assay. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: all women underwent colposcopy. If a suspicious lesion was found, a biopsy was taken. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: NucliSens EasyQ HPV mRNA test (not evaluated in the current review). No repeat cytology data available. |
|
| Follow‐up | No information provided. | |
| Notes | Separated ASCUS and LSIL data were obtained from the author. | |
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Women diagnosed with ASCUS or LSIL cytology. |
| Acceptable reference standard? All tests | Yes | Colposcopy, colposcopy‐directed biopsy (punch biopsies), cone specimens by LLETZ or cytological surveillance. |
| Acceptable delay between tests? All tests | Unclear | Delay not reported. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of reference standard, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Yes | Pathologists involved in cytological and histological assessments were not involved in testing for HPV. |
| Index test results blinded? All tests | Yes | The HC2 test interpretation is machine‐based. |
| Relevant clinical information? All tests | Yes | Women who underwent a secondary screening. |
| Uninterpretable results reported? All tests | Yes | All except 2 Pap smears were of satisfactory quality. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Chen 2005b.
| Study characteristics | ||
| Clinical features and settings | Women with mildly abnormal Pap results, either ASCUS or LSIL, who were referred to a colposcopy clinic, in Taiwan. | |
| Participants | Women with ASCUS or LSIL cytology. | |
| Study design | 266 women with abnormal cytology (160 ASCUS and 106 LSIL) underwent colposcopy‐directed biopsy. The women were tested for the presence of HPV DNA with the HC2 test. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy‐directed biopsy. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | There was no follow‐up. All triage tests and verification were performed simultaneously. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Women with mildly abnormal Pap results, either ASCUS or LSIL, who were referred to the colposcopy clinic. |
| Acceptable reference standard? All tests | Yes | Colposcopy‐directed biopsy. |
| Acceptable delay between tests? All tests | Yes | HPV test, Pap smear and colposcopy‐directed biopsy were performed at the same time. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsies, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Yes | The HC2 test interpretation is machine‐based. |
| Relevant clinical information? All tests | Yes | Women with ASCUS or LSIL cytology, referred to a colposcopy clinic. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Cuschieri 2007.
| Study characteristics | ||
| Clinical features and settings | Prospective study in multiple General Practitioners' practices in the UK. | |
| Participants | Women with ASCUS cytology diagnosis. | |
| Study design | 190 women diagnosed with ASCUS were tested with HC2 and AMPLICOR. Biopsies were taken from all women. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy and biopsy from all women. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: AMPLICOR (not evaluated in the current review). No repeat cytology data available. |
|
| Follow‐up | Average time of follow‐up: 3.5 years. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Women with borderline changes attending routine screening. |
| Acceptable reference standard? All tests | Yes | Colposcopy and biopsy performed on all borderline (ASCUS) cases. |
| Acceptable delay between tests? All tests | Unclear | Followed up for 3‐3.5 years. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsies, independent of the HC2 result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Yes | The HC2 test interpretation is machine‐based. |
| Relevant clinical information? All tests | Yes | Women referred to colposcopy because of abnormal cytology. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Cuzick 2003.
| Study characteristics | ||
| Clinical features and settings | Multicenter screening study: 161 family practices in the UK associated with 5 referral centres. | |
| Participants | Women with ASCUS cytology diagnosis (restricted to immediate colposcopy arm). | |
| Study design | 11,085 women (aged 30‐60 years) were screened with cytology and HC2 testing. Women with borderline cytology or positive for high‐risk HPV with negative cytology were randomised to immediate colposcopy or to surveillance by repeat HPV testing, cytology, and colposcopy at 12 months. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | For this review, data were selected for women with borderline cytology and included in the immediate colposcopy arm. Women in the surveillance arm (including follow‐up over 12 months) were ignored. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Women attending for routine cervical screening, without abnormal smear in the past 3 years or treatment for CIN. |
| Acceptable reference standard? All tests | Yes | Colposcopy. |
| Acceptable delay between tests? All tests | Unclear | Delay was not reported. |
| Partial verification avoided? All tests | Yes | A random sample of women with negative cytology and HPV test in the surveillance arm was selected for colposcopy. However, for this review, verification bias was avoided by restriction to the direct colposcopy arm. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy (reference standard), independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the colposcopy. |
| Reference standard results blinded? All tests | Yes | Histology was read locally, but was reviewed centrally in a blinded fashion by one pathologist. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women with abnormal cytology, referred to colposcopy. |
| Uninterpretable results reported? All tests | Yes | Inadequate smears or colposcopy, lost or damaged HPV sample/cytology slides. |
| Withdrawals explained? All tests | Yes | Women who did not return for repeat test, of whom the sample was lost or damaged. Participants who withdrew consent, did not attend colposcopy/cytology/HPV test or who had inadequate testing. |
Dalla Palma 2005.
| Study characteristics | ||
| Clinical features and settings | Screening population, of whom > 2% had ASCUS. | |
| Participants | Women with ASCUS cytology. | |
| Study design | 909 women (aged 25‐65 years) with equivocal cytological findings were tested for HPV DNA testing (HC2). All women with positive cytology and/or positive HC2 result, underwent colposcopy and biopsy. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy and biopsy. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | No information provided. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Women attending an organised cervical screening. |
| Acceptable reference standard? All tests | Yes | Colposcopy and biopsy (only cases with positive cytology or positive HC2 result, or both). |
| Acceptable delay between tests? All tests | Unclear | Delay was not reported. |
| Partial verification avoided? All tests | No | Only cases with positive cytology or positive HC2 result, or both, underwent colposcopy and biopsy. |
| Differential verification avoided? All tests | Yes | The same type of colposcopy and biopsy was performed on all women who were referred to colposcopy. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Unclear | Probably not, as only cases with positive cytology or HC2, or both, had colposcopy and biopsy. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women with known ASCUS/ASCUS and ASC‐H. |
| Uninterpretable results reported? All tests | Yes | HPV test invalid because the quantity of residual PreservCyt < 4 mL. |
| Withdrawals explained? All tests | Yes | Not all the women who were asked to undergo colposcopy participated. |
Davis‐Devine 2005.
| Study characteristics | ||
| Clinical features and settings | Women with an abnormal SurePath result within the preceding year attending a gynaecology department in the USA. | |
| Participants | Women with abnormal SurePath results (ASCUS, ASC‐H, AGUS). | |
| Study design | 45 women with ASCUS cytology diagnosis underwent colposcopy and cervical biopsy. The presence of HPV DNA was tested with HC2 testing and the Inform HPV assay (=in situ hybridisation assay). | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy and cervical biopsy. |
|
| Index and comparator tests |
Index test: HC2. Comparator test: inform HPV assay (not evaluated in the current review). No repeat cytology data available. |
|
| Follow‐up | HPV testing and colposcopy verification performed at enrolment. No further follow‐up. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Women 18‐60 years old with an intact cervix, and abnormal SurePath result within the preceding year. |
| Acceptable reference standard? All tests | Yes | Colposcopy and cervical biopsy. |
| Acceptable delay between tests? All tests | Yes | Colposcopy and biopsy within 10 minutes of the Pap sample, used for both cytologic examination as HPV testing. |
| Partial verification avoided? All tests | Yes | All women were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | Each woman received the same type of colposcopy and biopsies, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Yes | All cytologic and histologic materials were reviewed without knowledge of the study design. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women with ASC (atypical squamous cells (comprises ASC‐US and ASC‐H)) or worse. |
| Uninterpretable results reported? All tests | Yes | 1 x unsatisfactory (Pap), 8 x < 5000 squamous cells (Inform HPV), 1 x insufficient tissue (PCR). |
| Withdrawals explained? All tests | Yes | Women excluded from the study because of insufficient residual material for HC2 testing. |
De Francesco 2008.
| Study characteristics | ||
| Clinical features and settings | Routine cervical screening in a hospital in Italy. | |
| Participants | Women with abnormal Pap smear results (ASCUS or LSIL). | |
| Study design | Women with abnormal Pap smear results were tested for HPV DNA with HC2 and AMPLICOR test. All women underwent colposcopy and directed punch biopsies were taken. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+ and CIN3+. Reference standard: colposcopy and directed punch biopsies. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: AMPLICOR (not evaluated in the current review). No repeat cytology data available. |
|
| Follow‐up | No information provided. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | 213 women with abnormal cytology out of 3900 who came for routine cervical screening. |
| Acceptable reference standard? All tests | Yes | Colposcopy and directed punch biopsies. |
| Acceptable delay between tests? All tests | Unclear | Specimens for HC2 were collected during Pap visit, timing of colposcopy and biopsy not reported. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsies, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women with abnormal cytology referred to colposcopy. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Del Mistro 2010.
| Study characteristics | ||
| Clinical features and settings | Organised cytological screening in 5 Italian screening centres. | |
| Participants | Women with ASCUS cytology diagnosis. | |
| Study design | 749 women with ASCUS cytology tested for HPV DNA with HC2 test and repeat cytology samples (conventional cytology). All women underwent colposcopy. If a suspicious colposcopic lesion was found, punch biopsies were taken. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+ and CIN3+. Reference standard: colposcopy (n = 749) with punch biopsy when indicated (n = 338). |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: repeat cytology (conventional cytology). |
|
| Follow‐up | Re‐examination after 12 months (colposcopy, Pap and HC2). Re‐examination after 6 months (Pap and HC2) if any positive finding at enrolment tests. |
|
| Notes | Data available for 2 age groups (< 35 years and ≥35 years). | |
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Women with ASCUS selected from organized cytological screening. |
| Acceptable reference standard? All tests | Yes | Colposcopy (n = 742) (with punch biopsy when indicated, n = 338). |
| Acceptable delay between tests? All tests | Yes | About 3 months (median 72.2 days) between initial Pap test and enrolment tests. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsy, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based on the histological interpretation of the biopsy. A colposcopic negative result was diagnosed as negative for high‐grade CIN lesions. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Yes | Pap smears at enrolment were blinded to the results of HPV test. |
| Relevant clinical information? All tests | Yes | Women with ASCUS cytology diagnosis referred to colposcopy. |
| Uninterpretable results reported? All tests | Yes | 2.2% inadequate Pap smears (at enrolment), 2.4% inadequate biopsies. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Denton 2010.
| Study characteristics | ||
| Clinical features and settings | Cervical ASCUS or LSIL samples retrospectively collected from 5 anatomy pathology laboratories in Switzerland and Italy. | |
| Participants | Women with ASCUS or LSIL cytology. | |
| Study design | Retrospective diagnostic case‐control study. p16 immunostaining and HC2 test performed on 810 ASCUS/LSIL samples. Histological data for all 810 cases was available. |
|
| Target condition and reference standard(s) |
Outcome condition: CIN2+ and CIN3+. Reference standard: histology of colposcopic‐directed biopsies (punch/cone biopsies or ECC). |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: p16 immunostaining (not evaluated in the current review). No repeat cytology data available. |
|
| Follow‐up | The follow‐up period between index cytology of ASCUS or LSIL and triage/verification tests was less than six months. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Study contains separate results for ASCUS and LSIL cases. |
| Acceptable reference standard? All tests | Yes | Histologic diagnosis on re‐cut tissue block specimens (coming from colposcopic directed biopsies (punch/cone biopsies or ECC)). |
| Acceptable delay between tests? All tests | Yes | All tests and reference verification were simultaneous; < 6 months between initial cytology and enrolment tests. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women underwent the same reference standard, independent of the index test results. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on histologic interpretation of biopsies. |
| Reference standard results blinded? All tests | Yes | Colposcopy and histological diagnoses were blinded to HPV and p16 status. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women referred to colposcopy because of abnormal cytology. |
| Uninterpretable results reported? All tests | Yes | 135 samples (out of 945 initial samples) excluded because of sample age. Total number of samples included was 810. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Giovannelli 2005.
| Study characteristics | ||
| Clinical features and settings | Consecutive enrolment of women with ASCUS cytological abnormalities presenting to the Pathology Service (Palermo). . | |
| Participants | Women with ASCUS cytology diagnosis. | |
| Study design | 100 women with ASCUS were consecutively included in the study. All participants underwent colposcopy, followed by biopsy when suspicious colposcopic lesion(s) found, and screened for HPV infection by the combined use of HC2 and PCR with MY09/11 primers. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy and colposcopically‐directed biopsy. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: PCR with MY09/11 primers (not evaluated in the current review). No repeat cytology data available. |
|
| Follow‐up | Colposcopy and HPV testing took place 4‐5 weeks after finding the ASCUS cases. No further follow‐up. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Women with ASCUS cytology. |
| Acceptable reference standard? All tests | Yes | Colposcopy and colposcopically directed biopsy (when necessary). |
| Acceptable delay between tests? All tests | Yes | Participants underwent HPV typing, gynaecological examination, colposcopy (within 4‐5 weeks of the initial diagnosis) and, when necessary, colposcopically‐directed biopsy. Samples for HPV test and Pap smear were co‐collected. |
| Partial verification avoided? All tests | Yes | All subjects received the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy or biopsy, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on histologic interpretation of the biopsy. If colposcopy was negative, women were diagnosed as negative for high‐grade CIN lesions. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women with ASCUS cytology referred to colposcopy. |
| Uninterpretable results reported? All tests | Yes | 4 samples from the ASCUS group were unsuitable for PCR assay, and were excluded. |
| Withdrawals explained? All tests | Yes | 4 women with ASCUS did not undergo colposcopy and were excluded from the analysis. |
Guyot 2003.
| Study characteristics | ||
| Clinical features and settings | Women referred to the colposcopy clinic of West Middlesex University Hospital for minor cytological abnormalities in their cervical smears. | |
| Participants | Women with ASCUS or LSIL cytology diagnosis. | |
| Study design | 146 women (110 with mild dyskaryosis and 23 with persistent borderline changes) evaluated with HC2, all were referred for colposcopy. Punch biopsies taken from suspicious areas of the cervix. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy and punch biopsy from suspicious areas of the cervix. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | Collection for HC2 testing and colposcopy verification were performed simultaneously. No further follow‐up. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Women with ASCUS or LSIL cytology referred to colposcopy. |
| Acceptable reference standard? All tests | Yes | Colposcopy and punch biopsy from suspicious areas of the cervix. |
| Acceptable delay between tests? All tests | Yes | Less than three months between abnormal Pap smear and colposcopy (median = 4 weeks). Pap smear and HPV test were performed simultaneously. |
| Partial verification avoided? All tests | Yes | All subjects received the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and (if necessary) biopsy, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. Women with a negative colposcopy were diagnosed as negative for high‐grade CIN lesions. |
| Reference standard results blinded? All tests | Yes | The histopathologists were blinded to HPV results. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women were referred to the colposcopy clinic for minor cytological abnormalities in their cervical smear. |
| Uninterpretable results reported? All tests | Yes | 2 women had an inadequate biopsy, 5 had low cell count in the liquid‐based cytology, which did not allow an additional HPV test, and were excluded. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Holladay 2006.
| Study characteristics | ||
| Clinical features and settings | Randomly selected ThinPrep cytological specimens, setting not reported. | |
| Participants | Women with normal cytology (NILM), ASCUS, LSIL or HSIL cytology diagnosis. | |
| Study design | 400 ThinPrep cytological specimens randomly selected (100 normal (NILM), 100 ASCUS, 100 LSIL, 100 HSIL). p16 immunostaining and HC2 test performed on these specimens. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: we can assume that verification was done as usually recommended with colposcopy and biopsies, but no information was provided. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: p16 immunostaining (not evaluated in the current review). No repeat cytology data available. |
|
| Follow‐up | Follow‐up interval between finding ASCUS and HC2 triage and between ASCUS finding and colposcopy verification was <8 months. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Randomly selected ThinPrep specimens. |
| Acceptable reference standard? All tests | Unclear | We can assume that verification was done as usually recommended with colposcopy and biopsies, but no information was provided. |
| Acceptable delay between tests? All tests | Yes | Delay between specimen collection and index test‐comparator test was < 2‐8 months. Delay between initial cytology diagnosis and colposcopy‐biopsy outcome: ≤ 6 months. |
| Partial verification avoided? All tests | No | Only a part of the ASCUS (48/100) and LSIL (76/100) cases had a histologic interpretation available. The accuracy was computed on these histologically‐verified cases. |
| Differential verification avoided? All tests | Unclear | Not reported. |
| Incorporation avoided? All tests | Unclear | Not reported. |
| Reference standard results blinded? All tests | Yes | Histologic confirmation was determined by board‐certified pathologists without knowledge of the p16 or HC2 results. |
| Index test results blinded? All tests | Yes | Interpretation of p16 was blinded. Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | No | Apparently 400 study slides were selected from recently stored ThinPrep cytological specimen. |
| Uninterpretable results reported? All tests | Yes | Specimens with inadequate cellularity or cellular preservation inadequacies on the immuno‐stained slide were deemed inconclusive and were excluded from accuracy calculations. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Huang 2009.
| Study characteristics | ||
| Clinical features and settings | Women attending 5 colposcopy clinics in Canada. | |
| Participants | Women with abnormal cervical cytology referred to colposcopy. | |
| Study design | 193 women with ASCUS and 170 with LSIL cytology referred to colposcopy. Biopsies were taken. HC2 HPV test and Abbott RT PCR performed on all women | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+ and CIN3+. Reference standard: colposcopy and biopsy (type of biopsies not reported). |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: Abbott RT PCR and Linear Array (not evaluated in the current review). No repeat cytology data available. |
|
| Follow‐up | Longitudinal component of study not reported yet. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Women attending a colposcopy clinic, with abnormal cytology results (ASCUS or LSIL). |
| Acceptable reference standard? All tests | Yes | Colposcopy on all participants, followed by biopsies on all (type of biopsy not reported). |
| Acceptable delay between tests? All tests | Yes | All tests and reference verification were simultaneous.1‐3 months between initial cytology and enrolment tests. All cases expected to be followed for a further 2 years. Longitudinal follow‐up not included in current paper. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsy, independent of the index test results. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Yes | Colposcopy and histology of biopsies were blinded to HPV status. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women referred to colposcopy because of abnormal cytology. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Kelly 2006.
| Study characteristics | ||
| Clinical features and settings | Residual cytology samples from women with abnormal cytology, collected in a cytopathology laboratory. | |
| Participants | Women with abnormal cytology and follow‐up biopsy within 6 months of cytologic enrolment. | |
| Study design | 317 residual cytology samples (156 NLIM, 55 ASCUS, 1 AGUS, 24 ASC‐H, 53 LSIL, 28 HSIL) were selected in a cytopathology laboratory. Priority given to those with available follow‐up biopsy results. ProExC immunostaining and HC2 test performed on these samples. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+ Reference standard: colposcopy, histological assessment of biopsies on women with abnormal cytology. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: ProExC immunostaining (not evaluated in the current review). No repeat cytology data available. |
|
| Follow‐up | Follow‐up period between index cytology and verification: < 6 months. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Unclear | Residual cytology specimens (priority was given to those with abnormal cytology). |
| Acceptable reference standard? All tests | Yes | Colposcopy and biopsy on all ASCUS cases. Type of biopsy not reported. |
| Acceptable delay between tests? All tests | Yes | Delay between LBC and biopsy was < 6 months. All tests (index and comparator) were performed within 1 year after cytology specimen collection. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women were verified with the same reference standard, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Yes | Colposcopy and histology of biopsies blinded to HPV status and immunostaining results. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women referred to colposcopy because of abnormal cytology. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Kiatpongsan 2006.
| Study characteristics | ||
| Clinical features and settings | Enrolment of ASCUS cases (diagnosis within the 2 months previous to enrolment) at outpatient gynaecological and family planning clinics of King Chulalongkorn Memorial Hospital. | |
| Participants | Women with ASCUS cytology diagnosis. | |
| Study design | Enrolled all new cases with cytologic smears showing ASCUS that presented in King Chulalongkorn Memorial Hospital, excluding known cases of HSILs and pregnancies. All women underwent colposcopy and had colposcopic‐directed cervical biopsies taken. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopic‐directed cervical biopsy. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | No data provided. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Unclear | Very little information provided about background, or reasons for inclusion of participants. Women with ASCUS cytology. |
| Acceptable reference standard? All tests | Yes | Colposcopic‐directed cervical biopsy or ECC. |
| Acceptable delay between tests? All tests | Unclear | Delay was not reported. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopic‐directed cervical biopsy, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy or the ECC. |
| Reference standard results blinded? All tests | Yes | The gynaecologic pathologists examined and reviewed the pathologic slides without knowing the HC2 test results. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | All women with known ASCUS referred to colposcopy. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Kulasingam 2002.
| Study characteristics | ||
| Clinical features and settings | Women attending the Planned Parenthood clinics in Washington State. | |
| Participants | Women with ASCUS or LSIL cytology diagnosis. | |
| Study design | 4075 women were screened simultaneously using thin‐layer Pap and HPV DNA testing by a PCR‐based method and HC2. Women with a positive screening test, and a random sample of women with negative screening results, were referred for colposcopy and biopsy. | |
| Target condition and reference standard(s) |
Outcome condition: CIN3+. Reference standard: colposcopy or biopsy. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: repeat cytology (liquid‐based cytology) and HPV PCR (not evaluated in the current review). |
|
| Follow‐up | The average time between enrolment tests and colposcopy verification was 3 months. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Consecutive enrolment of women presenting for annual examinations at 1 of 3 Planned Parenthood clinics in Washington State. |
| Acceptable reference standard? All tests | Yes | Colposcopy and biopsy. |
| Acceptable delay between tests? All tests | Yes | The average time between the screening visit (gynaecologic examination, cervical cytology, HPV DNA testing) and the colposcopy visit was 3 months. |
| Partial verification avoided? All tests | Yes | Women were referred for colposcopy and biopsy if they had ASCUS, AGUS, LSIL or HSIL; or a positive PCR test result. A 45% random sample of the first 1000 women with negative Pap and HPV DNA test results was invited to have colposcopy performed. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and cervical biopsy, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy or the ECC. |
| Reference standard results blinded? All tests | No | Reference standard not blinded to cytology or HPV testing. |
| Index test results blinded? All tests | Unclear | Not reported. |
| Relevant clinical information? All tests | No | The pathologists had no knowledge of other laboratory or clinical data. |
| Uninterpretable results reported? All tests | Yes | Inadequate Pap samples, insufficient HPV DNA samples, missing HPV DNA test results, women who did not undergo biopsy. |
| Withdrawals explained? All tests | Yes | 283 eligible women refused to participate. |
Lee 2001.
| Study characteristics | ||
| Clinical features and settings | Women screened for cervical carcinoma and precancerous cervical lesions at Korea University Ansan Hospital. | |
| Participants | Women with ASCUS and LSIL. | |
| Study design | 66 women with a cervical cytology result of LSIL who received a Pap test and HC2 test. Colposcopy and colposcopy‐directed biopsy of hysterectomy was performed on all women. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy and colposcopy‐directed biopsy or hysterectomy (for women with normal cytology, performed to treat other benign diseases). |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | No information provided. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Unclear | A subset of 457 women out of the 2967 screened for cervical carcinoma and precancerous cervical lesions during 1 year. |
| Acceptable reference standard? All tests | Yes | Colposcopy and colposcopy‐directed biopsy (in participants with LSIL or HSIL, or with ASCUS and high‐risk HPV) or hysterectomy (for WNL and BCC women, performed to treat other benign diseases). |
| Acceptable delay between tests? All tests | Unclear | Delay was not reported. |
| Partial verification avoided? All tests | Unclear | Histological results only available for 206 participants out of the cohort of 457 women. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and cervical biopsy, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy or the hysterectomy. |
| Reference standard results blinded? All tests | Yes | Two pathologists confirmed all histologic diagnoses without any knowledge of the HPV status. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Clinical data were recorded from the participants' medical records. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Lee 2009.
| Study characteristics | ||
| Clinical features and settings | Consecutive women who visited a colposcopy clinic in Korea. | |
| Participants | Women with abnormal cytology. | |
| Study design | 74 women consecutively visited the clinic and underwent LBC. They also were tested for HPV DNA with HC2, Linear Array, HPV DNA Chip test, cycle sequencing. All women also underwent colposcopy and colposcopy‐directed biopsy. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+ and CIN3+. Reference standard: colposcopy and colposcopy‐directed biopsies. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: HPV DNA Chip test, Linear Array, cycle sequencing (not evaluated in the current review). No repeat cytology data available. |
|
| Follow‐up | Enrollment tests and verification were performed simultaneously. No further follow‐up. | |
| Notes | Additional data obtained from author. Women had LSIL lesions. | |
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Consecutive women who visited the colposcopy clinic. |
| Acceptable reference standard? All tests | Yes | Colposcopy, colposcopy‐directed biopsy. |
| Acceptable delay between tests? All tests | Unclear | Delay was not reported. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsies, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index/comparator test does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women visiting a colposcopy clinic. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Yes | 3 cases of nonsquamous lesion, adenocarcinoma in situ, endometrial cancer, and malignant mixed mullerian tumour were excluded from the analysis of HPV DNA test of squamous cervical lesions. |
Lonky 2003.
| Study characteristics | ||
| Clinical features and settings | Women with abnormal cytology diagnosis, setting not defined. | |
| Participants | Women with ASCUS or LSIL. | |
| Study design | 8170 women screened with Pap smears; all ASCUS cases (278) underwent colposcopy and/or colposcopically directed punch biopsies. HC2 testing was performed on all of them. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy or colposcopically directed punch biopsy. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | Sample collection for HC2 testing and colposcopy verification were performed simultaneously. No further follow‐up. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | 278 women with ASCUS out of 8170 women screened. |
| Acceptable reference standard? All tests | Yes | Colposcopy and biopsy. |
| Acceptable delay between tests? All tests | Yes | All participants were referred for colposcopy within 3‐4 weeks of their initial ASCUS result. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsy (if necessary), independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. If no suspicious colposcopic lesion was found, women were diagnosed as negative for high‐grade CIN lesions. |
| Reference standard results blinded? All tests | Yes | The investigators of the Department of Pathology, Women's and Children's Hospital of the University of Southern California were blinded to the antecedent referral Pap smear result, the result of HC2 testing, and the histologic diagnosis rendered at Keiser Permanente Medical Center. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women with ASCUS cytology referred to colposcopy. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Lytwyn 2000.
| Study characteristics | ||
| Clinical features and settings | Community‐based randomised trial (52 community‐based family practices and 1 university student health clinic in Ontario). Randomly assignment of women with ASCUS or LSIL. |
|
| Participants | Women with ASCUS or LSIL. | |
| Study design | Random assignment of 212 women (16‐50 years) with diagnosis of ASCUS or LSIL on cervical cytology screening to undergo either immediate HPV DNA testing or a repeat Pap test in 6 months. Colposcopy was performed on all women, and directed biopsy or ECC was also performed. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy, regardless of the cytology results, or directed biopsy or ECC. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: repeat cytology (conventional cytology). |
|
| Follow‐up | Repeat cytology after 6 months; HPV testing was done soon after the index smear. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | By recruiting women from primary care practices, the results of this trial are more generalized to a primary care setting than those obtained in a colposcopy referral population. |
| Acceptable reference standard? All tests | Yes | Colposcopy and/or directed biopsy or ECC. |
| Acceptable delay between tests? All tests | Yes | Immediately after randomisation, the family physician obtained material for the HPV DNA testing; repeat Pap smears were taken after 6 months. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the results of the colposcopic examination and/or the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Yes | Two expert gynaecologic pathologists, blinded to the HPV or repeat Pap test result, independently reviewed all specimens. |
| Index test results blinded? All tests | Yes | The colposcopists, physicians and the participants were blinded to the HPV test results. |
| Relevant clinical information? All tests | Unclear | Women with known low‐grade abnormality on screening Pap test. |
| Uninterpretable results reported? All tests | Yes | A swab from 1 woman did not have sufficient material for testing; 14 women did not return for repeat cytology, the specimen from 1 woman was not received by the laboratory and could not be traced, 2 women presented after the 6 months and were referred immediately for colposcopy. |
| Withdrawals explained? All tests | Yes | Some women withdrew from the trial, for instance those women who did not present for colposcopy or the repeat Pap test. |
Manos 1999.
| Study characteristics | ||
| Clinical features and settings | A cohort of 995 ASCUS women out of 46,009 women from the Kaiser Permanente Medical Care Program (12 gynaecology clinics at 4 medical centres), who had a routine cervical Pap examination. | |
| Participants | Women with ASCUS. | |
| Study design | Women with ASCUS Pap smear results (995) at routine screening, all had LBC, HPV testing, and subsequent repeat Pap tests and colposcopy with histologic evaluation. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy with biopsy and/or ECC. |
|
| Index and comparator tests |
Index test: HC2. Comparator test: repeat cytology (liquid‐based cytology). |
|
| Follow‐up | Mean interval of 67 days between index smear and repeat cytology/colposcopy. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Kaiser Permanente membership is demographically similar to the US census‐enumerated population in the Bay Area Metropolitan Statistical Area, so population is representative for Northern California, with the exception that extremes are not represented. |
| Acceptable reference standard? All tests | Yes | Colposcopy and biopsy and/or ECC performed on all participants. The reported results of the HPV tests are those taken at the same time as the initial index smear. |
| Acceptable delay between tests? All tests | Yes | Colposcopy examinations with repeat Pap specimen collection was conducted after a median of 67 days (range, 12‐240 days) after the initial Pap examination. HPV DNA testing was performed independently on PreservCyt and the specimen transport medium (STM) specimens that were collected at the initial Pap examination. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsy/ECC, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological examination of the biopsy. |
| Reference standard results blinded? All tests | Yes | Examinations were conducted without knowledge of the women's HPV or ThinPrep Pap results. Histopathologic diagnoses were made without knowledge of HPV results. |
| Index test results blinded? All tests | Unclear | Not reported. |
| Relevant clinical information? All tests | Yes | Women who underwent routine cervical cytology screening. |
| Uninterpretable results reported? All tests | Yes | Histology specimens from 17 participants were considered insufficient. 5 of the PreservCyt specimens had insufficient material for HPV testing after slide preparation. For 16 women a repeat Pap result was not available. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Monsonego 2006.
| Study characteristics | ||
| Clinical features and settings | Women with abnormal Pap smear or previous/current HPV‐related disease who attended a colposcopy centre in France. | |
| Participants | Women with abnormal Pap smear results, women with ASCUS or LSIL. | |
| Study design | Women with abnormal Pap smear results were selected and underwent colposcopy and biopsy. HPV DNA was tested with HC2 testing. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy (n = 389), LEEP cone biopsy (n = 344) or directed punch biopsy (n = 43), repeat Pap/colposcopy after 4 months (n = 2). |
|
| Index and comparator tests |
Index test: HC2 assay. comparator test: no repeat cytology data available. |
|
| Follow‐up | Sample collection for HC2 testing and colposcopy verification were performed simultaneously. No further follow‐up. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | 389 women with abnormal Pap smear, or previous/current HPV‐related disease. |
| Acceptable reference standard? All tests | Yes | Colposcopy and LEEP cone biopsy (if indicated). |
| Acceptable delay between tests? All tests | Yes | HC2 within 3 months after enrolment (referral) Pap test. HC2 sample taken just before colposcopy/cone biopsy. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | No | ‐ Leep if: a) Pap showed HSIL, b) atypical transformation zone, c) endocervical lesion + unsatisfactory colposcopy, d) squamocolumnar junction > 3 mm within endocervix. ‐ Directed punch biopsy if: a) normal Pap + external genital warts, b) follow‐up after CIN + abnormal colposcopy. ‐ Repeat Pap + colposcopy if: a) abnormal Pap + satisfactory normal colposcopy. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological examination of the biopsy. |
| Reference standard results blinded? All tests | Yes | Pathologists were unaware of the HPV DNA status. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women with abnormal cytology referred to colposcopy. |
| Uninterpretable results reported? All tests | Yes | If Pap smear, colposcopy or biopsy could not be evaluated for technical reasons, the woman was excluded from the study. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Monsonego 2008.
| Study characteristics | ||
| Clinical features and settings | Women with abnormal cytology who attended a colposcopy clinic in France. | |
| Participants | Women with ASCUS, LSIL, HSIL, AGC or ASC‐H cytology diagnosis. | |
| Study design | 575 women had abnormal cytology and underwent colposcopy. Biopsies taken from the women with abnormal colposcopies (n = 520). The presence of HPV DNA was tested through HC2 testing and Linear Array. Also repeat ThinPrep smears were taken. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+ and CIN3+. Reference standard: colposcopy, and biopsy if colposcopic abnormal results. |
|
| Index and comparator tests |
Index test: HC2 assay Comparator test: repeat cytology (liquid‐based cytology), Linear Array (not evaluated in the current review). |
|
| Follow‐up | Sample collection for HC2 testing and colposcopy verification were performed simultaneously. No further follow‐up. | |
| Notes | Data for different triage‐groups received from trial authors. | |
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Study contains separate results for ASCUS and LSIL cases (also for ASC‐H cases). Women attended a colposcopy clinic. |
| Acceptable reference standard? All tests | Yes | Colposcopy on all followed by biopsies on 520 subjects. Type of biopsy: LEEP or punch biopsy. |
| Acceptable delay between tests? All tests | Yes | All tests and reference verification were simultaneous (2‐3 months after initial Pap‐smear). |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women underwent the same type of colposcopy (and punch biopsy if indicated), independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. Women with no suspicious colposcopic lesions were diagnosed as negative for high‐grade CIN lesions. |
| Reference standard results blinded? All tests | Yes | Colposcopy and histology of biopsies were blinded to HPV status. |
| Index test results blinded? All tests | Unclear | Not reported. |
| Relevant clinical information? All tests | Yes | Women referred to colposcopy because of abnormal cytology. |
| Uninterpretable results reported? All tests | Yes | See Table I of the paper: 30 equivocal results for colposcopy were registered, as well as 1 unsatisfactory result. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Morin 2001.
| Study characteristics | ||
| Clinical features and settings | Part of a case‐control study (university clinic of Quebec) evaluating determinants of concomitant CIN in women with a cytologic diagnosis of ASCUS in conventional Pap smears. | |
| Participants | Women with ASCUS. | |
| Study design | 360 women cytologically diagnosed with ASCUS referred for colposcopy who had a repeat Pap smear, a biopsy, when necessary, and HPV testing. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy with directed biopsy or ECC. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: repeat cytology (conventional cytology). |
|
| Follow‐up | No information provided. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Unclear | Women with ASCUS selected out of a case‐control study. |
| Acceptable reference standard? All tests | Yes | Colposcopy and biopsy or ECC. |
| Acceptable delay between tests? All tests | Unclear | Delay was not reported. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsy/ECC, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Unclear | Not reported. |
| Relevant clinical information? All tests | Yes | Women with known ASCUS on initial Pap smear. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Nieh 2005.
| Study characteristics | ||
| Clinical features and settings | Women who had a routine Pap smear diagnosis of ASCUS in Taiwan. | |
| Participants | Women with ASCUS cytology diagnosis. | |
| Study design | 66 women with ASCUS Pap smear were tested for the presence of HPV DNA through HC2 testing and p16 immunostaining. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: histological diagnosis (unclear: done through follow‐up biopsy?). |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: p16 immunostaining (not evaluated in the current review). No repeat cytology data available. |
|
| Follow‐up | No information provided. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Unclear | 66 routine selected ASCUS smears, but source/setting not documented. |
| Acceptable reference standard? All tests | Unclear | Histological diagnosis through follow‐up biopsy. |
| Acceptable delay between tests? All tests | Unclear | Delay was not reported. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Unclear | Not reported. |
| Incorporation avoided? All tests | Yes | Outcome was based only on histology results for all cases. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Unclear | Women with abnormal cytology. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained, but this is not applicable since this was a selected series of Pap smears. |
Ordi 2003.
| Study characteristics | ||
| Clinical features and settings | 1005 women referred to colposcopy, in a cervical pathology unit, as a result of ASCUS or LSIL cytology. | |
| Participants | Women with cytological diagnosis of ASCUS or LSIL. | |
| Study design | Women with ASCUS or LSIL cytology were referred to colposcopy. HC2 HPV testing was performed on all participants and a biopsy was taken if colposcopy was abnormal. If colposcopy was negative, diagnosis was based on cytology. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy and biopsy if suspicious colposcopic lesions. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | Follow‐up period of 6 months on average between index cytology result of ASCUS or LSIL and HC2 testing and colposcopy verification. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Women referred to cervical pathology institute between 1999‐2002 because of an abnormal (ASCUS, LSIL) cytology result. |
| Acceptable reference standard? All tests | Yes | Colposcopy on all. Biopsy if colposcopy was abnormal. |
| Acceptable delay between tests? All tests | Yes | 6 months. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy (and biopsy if necessary), independent of the index test result. |
| Incorporation avoided? All tests | Unclear | For women with a negative colposcopy, final diagnosis was based on cytology results. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women referred to colposcopy because of an abnormal cytology result. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Pretorius 2002.
| Study characteristics | ||
| Clinical features and settings | Women who had colposcopy performed by 1 of 21 physicians at the Southern California Permanente clinics in Fontana (California). | |
| Participants | Women with ASCUS or LSIL. | |
| Study design | Colposcopy and HPV testing (HC2) performed on 1309 women for evaluation of abnormal Pap smears. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy and/or biopsy or ECC. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: first generation Hybrid Capture test (not evaluated in the current review). No repeat cytology data available. |
|
| Follow‐up | Colposcopy verification and HPV testing were performed simultaneously. No further follow‐up. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Unclear | Women selected out of a cohort of women who underwent colposcopy. All women had abnormal cytology (ASCUS or LSIL). |
| Acceptable reference standard? All tests | Yes | Colposcopy and/or biopsy or ECC. |
| Acceptable delay between tests? All tests | Yes | Delay was not reported. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsy/ECC, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women with abnormal Pap smears (ASCUS, LSIL, HSIL or AGUS). |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Yes | 153 women with colposcopy for reasons other than for evaluation of an abnormal Pap smear were excluded, 80 refused to participate, 83 women without a specimen for the HPV test were excluded from the study. |
Rebello 2001.
| Study characteristics | ||
| Clinical features and settings | 333 consecutive women referred for colposcopy with persistent borderline, or mildly dyskaryotic, smears were tested for HPV with HC2. | |
| Participants | Women with ASCUS or LSIL. | |
| Study design | 75 ASCUS and 117 LSIL cases with persistent borderline smears and mild dyskaryosis referred for colposcopy were tested for hrHPV (HC2). | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: large loop excisions of the transformation zone. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | No information provided. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | No | Study population with repeated abnormal smears. If the study had been a triage of women with only 1 abnormal smear, the prevalence of high grade disease would probably be lower. |
| Acceptable reference standard? All tests | Yes | Large loop excisions were performed on all participants. |
| Acceptable delay between tests? All tests | Unclear | Delay was not reported. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women underwent the same type of large loop excisions, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the large loop excision. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women with persistent borderline or mild dyskaryosis. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Ronco 2007.
| Study characteristics | ||
| Clinical features and settings | Women with ASCUS, AGUS or LSIL cytology diagnosis, selected out of a large randomised controlled trial of 9 screening programmes in Italy. | |
| Participants | Women with ASCUS, AGUS or LSIL cytology. | |
| Study design | 757 women with ASCUS/AGUS and 485 with LSIL cytology, underwent colposcopy and biopsy. All these women were tested for the presence of HPV DNA through HC2 testing. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+ and CIN3+. Reference standard: colposcopy and eventually biopsy (type of biopsies not reported). |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | Follow‐up up to one year after triage testing. | |
| Notes | Age‐specific data reported. | |
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Selected samples (with valid HPV test and colposcopy) out of a large randomised controlled trial. |
| Acceptable reference standard? All tests | Yes | Colposcopy, followed by biopsy when indicated. Type of biopsy not reported. |
| Acceptable delay between tests? All tests | No | Same cervical cell sample for LBC and HPV testing. Included histologically‐confirmed lesions detected within 1 year from referral to colposcopy. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | No | Did not report who just had colposcopy and who also had a biopsy (suspicious areas were biopsied). |
| Incorporation avoided? All tests | Yes | Index test does not form part of the reference standard. |
| Reference standard results blinded? All tests | Yes | Histology was independently reviewed, blinded to HPV test and cytology results. (But colposcopists had access to participants' notes, both for cytology and HPV). |
| Index test results blinded? All tests | Yes | Slides were read without knowledge of HPV results, HPV testing was blind to cytology. |
| Relevant clinical information? All tests | Yes | Women with ASCUS/AGUS and LSIL referred to colposcopy. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Sherman 2002.
| Study characteristics | ||
| Clinical features and settings | Enrolled 3488 women with ASCUS and 1572 women with LSIL reported by community laboratories. | |
| Participants | Women with ASCUS or LSIL. | |
| Study design | 2198 ASCUS and 848 LSIL cases, referred to colposcopy, were subjected to HPV testing and repeat thin layer cytopathology. | |
| Target condition and reference standard(s) |
Outcome condition: CIN3+. Reference standard: colposcopy and biopsy or ECC. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: repeat cytology (liquid‐based cytology). |
|
| Follow‐up | Women are enrolled an average of 2 months after the index smear was obtained. Colposcopy in arm 2 (for HPV‐positive women) took place on average 8 weeks after enrolment while less than 1 day in arm 1 (where all women received colposcopy). | |
| Notes | Reported age‐specific data. | |
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | The findings in this study should apply to most US screening populations in which cytopathology is performed with standard diagnostic criteria, because the study was large, with a broad representation of the US population and quality‐control components, . |
| Acceptable reference standard? All tests | Yes | Colposcopy and biopsy or ECC. |
| Acceptable delay between tests? All tests | Yes | The cervical sample collected at the pelvic examination was used to prepare a thin‐layer slide and for HPV DNA testing. The time interval between referral smear to collection of cells for HPV testing could exceed 65 days. No information was available about the colposcopy. |
| Partial verification avoided? All tests | No | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsy/ECC, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy or ECC. |
| Reference standard results blinded? All tests | Yes | Not reported. |
| Index test results blinded? All tests | Unclear | Not reported. |
| Relevant clinical information? All tests | Yes | Women with known ASCUS or LSIL. |
| Uninterpretable results reported? All tests | Yes | 153 women with missing HPV results, 18 with missing cytopathology results, and 4 with both results missing, were excluded from the analysis. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Shlay 2000.
| Study characteristics | ||
| Clinical features and settings | Women from a local clinic within the Denver Health system (the major provider of health care for the indigent population in Denver, Colorado), with a single atypical Pap smear diagnosis. | |
| Participants | Women with ASCUS/AGUS. | |
| Study design | 195 consenting women referred for colposcopy because of atypia on Pap smears. Before colposcopy, a cervical swab was collected for HPV testing (HC2). | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy and/or biopsy and ECC. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | Enrolment smears were collected 64 days (median; range: 12‐430 days) after the index smear. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | No | Study at the Women's Care Clinic at Denver Health, the major provider of health care for the indigent population in Denver, Colorado. |
| Acceptable reference standard? All tests | Yes | Colposcopy and/or biopsy and ECC. |
| Acceptable delay between tests? All tests | Yes | Study visits (colposcopy and collection of specimens for HPV testing) performed a median of 64 days (range 12‐430 days) after atypical Pap smear results. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsy/ECC, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy or ECC. |
| Reference standard results blinded? All tests | Yes | Laboratory personnel were unaware whether women participated in the study and were blinded to the HPV test results. |
| Index test results blinded? All tests | Yes | Laboratory personnel were blinded to the biopsy results. |
| Relevant clinical information? All tests | Yes | Women with known atypia on the initial Pap smear. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Siddiqui 2008.
| Study characteristics | ||
| Clinical features and settings | Cervical cytology samples of women with ASCUS were selected out of all the samples collected in a cytopathology laboratory in the USA between 2006‐2007. | |
| Participants | Women with ASCUS cytology. | |
| Study design | 200 samples of women with ASCUS were selected. All these women underwent colposcopy and biopsy. All samples tested for the presence of HPV DNA with ProExC immunostaining and HC2 test. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+ and CIN3+. Reference standard: colposcopy and biopsy (type of biopsies not reported). |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: ProExC immunostaining (not evaluated in the current review). No repeat cytology data available. |
|
| Follow‐up | Triage tests and colposcopy verification were performed simultaneously. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | 200 women with ASCUS cervical cytology selected out of samples collected between 2006‐2007. |
| Acceptable reference standard? All tests | Yes | Colposcopy on all, followed by biopsies on all subjects. Type of biopsy not reported. |
| Acceptable delay between tests? All tests | Yes | All tests and reference verification were simultaneous. < 1 month between initial cytology and enrolment tests. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women underwent the same colposcopy, independent of the index test results. |
| Incorporation avoided? All tests | Yes | Index/comparator tests did not form part of the reference standard. The reference standard outcome was based only on the histological verification of the biopsies. |
| Reference standard results blinded? All tests | Unclear | Not reported; separate clinical protocol for colposcopy‐biopsy. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women referred to colposcopy because of abnormal cytology. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Silverloo 2009.
| Study characteristics | ||
| Clinical features and settings | Women with ASCUS diagnosis on primary organised cytological screening in Sweden. | |
| Participants | Women with ASCUS cytology. | |
| Study design | 197 women with ASCUS tested with the HC2 assay and a new Pap smear taken. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: 1) If cyto+ and/or HPV+, then colposcopy, new Pap‐smear, cervical biopsy and ECC. 2) If cyto‐/HPV‐, then 3‐yearly organised screenings via Pap‐smear. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: repeat cytology (conventional cytology). |
|
| Follow‐up | Re‐examination after 3 months, 2 cervical smears: primary assessment via cytology, secondary assessment via hrHPV test. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Consecutive ASCUS women from an organized screening program. |
| Acceptable reference standard? All tests | Yes | Colposcopy, biopsy and ECC for women who were cytology+ and/or HPV+, repeated cytology after 3 years for women who were cytology‐ and HPV‐. |
| Acceptable delay between tests? All tests | Unclear | Not documented. |
| Partial verification avoided? All tests | No | Cyto/HPV‐ women did not undergo colposcopy/biopsy, but repeat cytology was performed. |
| Differential verification avoided? All tests | No | According to the result of the index and comparator test, women received colposcopy/biopsy or repeat cytology after 3 years. |
| Incorporation avoided? All tests | No | Repeat cytology as reference test for 1 group, equal to the primary screenings test. |
| Reference standard results blinded? All tests | Yes | Colposcopy blinded to triage results (whether cytology+ or HPV+). |
| Index test results blinded? All tests | Yes | Index/comparator test performed and interpreted before reference test. |
| Relevant clinical information? All tests | Yes | ASCUS information was available. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Yes | 15 women could not be re‐examined 3 years later in the organized screening. |
Solomon 2001.
| Study characteristics | ||
| Clinical features and settings | Multicenter, randomised trial. Woman with ASCUS or LSIL referred to one of 4 clinics throughout the US. |
|
| Participants | Women with ASCUS cytology. | |
| Study design | 3488 women with a referral diagnosis of ASCUS either underwent 1) immediate colposcopy, 2) triage to colposcopy based on HPV (HC2) and thin‐layer cytology results, or 3) triage based on cytology results alone. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+ and CIN3+. Reference standard: colposcopy and biopsy for any colposcopically‐suspected CIN. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: repeat cytology (liquid‐based cytology). |
|
| Follow‐up | Women are enrolled an average of 2 months after the index smear was obtained. Colposcopy in arm 2 (for HPV‐positive women) took place on average 8 weeks after enrolment while less than 1 day in arm 1 (where all women received colposcopy). | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | A large study of 3488 women referred to one of 4 clinics throughout the US. |
| Acceptable reference standard? All tests | Yes | Colposcopy and biopsy for any colposcopically‐suspected CIN. |
| Acceptable delay between tests? All tests | Yes | Women were enrolled an average of 2 months after the index smear was obtained. Colposcopy in arm 1 after < 1 day, and in arm 2 after 8 weeks (median). |
| Partial verification avoided? All tests | No | All women assigned to arm 1 referred for colposcopy and/or biopsy or ECC, regardless of the cytology results. In arm 2 (HPV triage), colposcopy was performed only if the enrolment HPV test was positive, or missing, or any cytology was HSIL+. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsy/ECC, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test does not form part of the reference standard. The reference standard outcome was based only on the histological result. |
| Reference standard results blinded? All tests | Yes | For histology slides, the Pathology Quality Control Group review protocol included review by a quality control pathologist who was masked to the original diagnosis. (In contrast to the immediate colposcopy arm (arm 1), in arm 2 and 3, colposcopists were aware of the triage test results at the time of colposcopy, which may have influenced clinical assessment of the cervix.) |
| Index test results blinded? All tests | Unclear | Not reported. |
| Relevant clinical information? All tests | Yes | Previous histological diagnoses were given. |
| Uninterpretable results reported? All tests | Yes | 164 HPV tests not done (missing), most often because of an insufficient amount of residual specimen in the PreservCyt vial. |
| Withdrawals explained? All tests | Yes | Some women triaged to colposcopy refused the procedure or were lost to follow‐up (1.2% in arm 1, 6.1% in arm 2, and 6.9% in arm 3). |
Szarewski 2008.
| Study characteristics | ||
| Clinical features and settings | Women referred for colposcopy to 2 colposcopic clinics in the UK, as the result of an abnormal Pap smear. | |
| Participants | Women with abnormal Pap smear results. | |
| Study design | 953 women with abnormal Pap smear results (104 ASCUS, 617 LSIL) had colposcopy and were tested for the presence of HPV via the following tests: HC2, AMPLICOR, Linear Array, clinical array, PreTect Proofer, P16 immunostaining and APTIMA. Histological data were available for the women. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+ and CIN3+. Reference standard: colposcopy and histology. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: AMPLICOR, APTIMA, Linear Array, clinical array, PreTect Proofer, p16 immunostaining (not evaluated in the current review). No repeat cytology data available. |
|
| Follow‐up | Collection for triage tests and colposcopy verification were performed simultaneously. Varabiable follow‐up period between index cytology and referral. | |
| Notes | Separate data for ASCUS and LSIL triage groups were received from the authors. | |
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Study contained results for 953 women referred for colposcopy and histology as a result of abnormal cervical smears. Separate data for different triage groups were received from trial authors. |
| Acceptable reference standard? All tests | Yes | Colposcopy and histology. |
| Acceptable delay between tests? All tests | Unclear | Median delay between cytological screening and reference test was 2.4 months, the range of delays was 0.6‐50 months. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women underwent the same type of colposcopy, independent of the index test results. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological data available for all cases. |
| Reference standard results blinded? All tests | Unclear | Histopathology was blinded to test results, but the pathologist had access to cytology results. |
| Index test results blinded? All tests | Yes | All molecular tests were blinded to the cytology and histopathology results. Not mentioned if the tests were blinded to each other. |
| Relevant clinical information? All tests | Yes | Women referred to colposcopy because of abnormal cytology. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Voss 2010.
| Study characteristics | ||
| Clinical features and settings | Women with LSIL diagnosis who had biopsy within 1 year after cytological enrolment in a clinic (USA). | |
| Participants | Women with LSIL diagnosis. | |
| Study design | Out of the total number of women who had biopsy within 1 year after cytological enrolment (n = 204), 115 women with LSIL diagnosis were chosen. The presence of HPV DNA in these women was tested through Multiprobe FISH and HC2 testing. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy and biopsy (type of biopsies not reported). |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: Multiprobe FISH (not evaluated in the current review). No repeat cytology data available. |
|
| Follow‐up | Follow‐up period between HC2 tests and colposcopy verification was less than one year.. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Unclear | 115 women with LSIL cytology selected from group of 204 women who had biopsy within 1 year after cytological enrolment. |
| Acceptable reference standard? All tests | Yes | Colposcopy on all, followed by biopsies on all 115 subjects. Type of biopsy not reported. |
| Acceptable delay between tests? All tests | Unclear | Delay between all tests and reference verification was not specifically reported. Follow‐up biopsies done within 1 year after enrolment. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women underwent the same type of colposcopy, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on histologic interpretation of the biopsies. |
| Reference standard results blinded? All tests | Yes | Colposcopy and histology of biopsies were blinded to HPV status. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Unclear | Not reported. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Yes | 3 withdrawals reported during follow‐up. |
Wensveen 2003.
| Study characteristics | ||
| Clinical features and settings | Prospective cohort study at the gynaecologic outpatients clinic of the Medical Center Haaglanden (the Netherlands). | |
| Participants | Women with ASCUS/AGUS. | |
| Study design | 148 women with ASCUS or AGUS on cervical smears evaluated by colposcopy, histological sampling, and HPV testing (HC2). | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy and biopsy or ECC. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | Colposcopy verification and triage tests were performed simultaneously. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Unclear | 85% of the population was immigrant, so the cohort may not really representative for the Dutch population. |
| Acceptable reference standard? All tests | Yes | Colposcopy and biopsy or ECC. |
| Acceptable delay between tests? All tests | Yes | No more then 12 weeks between the Pap smear and intake tests. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsy/ECC, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women with known ASCUS/AGUS. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Yes | 3 women were pregnant, 1 was HIV positive, in 11 cases there was a delay of more than 12 weeks between the Pap smear and the intake, 8 because of a normal or dysplastic smear after review, 36 did not arrive for intake or colposcopy, 2 because of a missed HPV test, 1 because of a carcinoma of the corpus uteri. |
You 2007.
| Study characteristics | ||
| Clinical features and settings | Women with abnormal cytology and who received HPV test and biopsy were selected out of the women who visited the gynaecology or maternal health department of a Chinese hospital. | |
| Participants | Women with abnormal cytology. | |
| Study design | 2152 women (selected from 20,000 women) with abnormal cytology (1171 ASCUS, 109 ASC‐H, 656 LSIL), were tested for the presence of HPV DNA via HC2 testing and taking of biopsies. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy‐directed biopsy. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | No information provided. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Yes | Study contained separate results for ASCUS, ASC‐H and LSIL cases. Selected samples of 2152 women with abnormal cytology + biopsy + HPV test. |
| Acceptable reference standard? All tests | Yes | Colposcopy‐directed biopsy. |
| Acceptable delay between tests? All tests | Unclear | Not reported. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women underwent the same type of colposcopy and biopsy, independent of the index test results. |
| Incorporation avoided? All tests | Yes | Index test (HC2) does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsies. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women with abnormal cytology (ASCUS, ASC‐H, LSIL). |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals reported, not applicable (selected sample). |
Zielinski 2001.
| Study characteristics | ||
| Clinical features and settings | Participants recruited from general practitioners or from a gynaecological outpatient clinic (The Netherlands), with a borderline or mild dyskaryosis result of their Pap smear. | |
| Participants | Women with ASCUS or LSIL. | |
| Study design | 278 women with smears read as borderline, or mild dyskaryosis, referred for colposcopy. HC2 performed on a cervical scrape taken at the first visit before colposcopy and follow‐up. Biopsies were taken if a lesion was observed. | |
| Target condition and reference standard(s) |
Outcome condition: CIN2+. Reference standard: colposcopy and colposcopically‐directed biopsy. |
|
| Index and comparator tests |
Index test: HC2 assay. Comparator test: no repeat cytology data available. |
|
| Follow‐up | Median follow‐up time of 1.4 years (range 0‐4.5 years). All cytologically positive and HPV+ women followed‐up at 6‐monthly intervals. | |
| Notes | ||
| Methodological quality | ||
| Item | Authors' judgement | Support for judgement |
| Representative spectrum? All tests | Unclear | Participants recruited from general practitioners or from a gynaecological outpatient clinic in Walcheren (The Netherlands). |
| Acceptable reference standard? All tests | Yes | Colposcopy. |
| Acceptable delay between tests? All tests | Yes | All participants were referred to the gynaecologist for a colposcopy within 3 months. |
| Partial verification avoided? All tests | Yes | All subjects were verified with the reference standard. |
| Differential verification avoided? All tests | Yes | All women received the same type of colposcopy and biopsy, independent of the index test result. |
| Incorporation avoided? All tests | Yes | Index test does not form part of the reference standard. The reference standard outcome was based only on the histological interpretation of the biopsy. |
| Reference standard results blinded? All tests | Unclear | Not reported. |
| Index test results blinded? All tests | Yes | Interpretation of the HC2 test is machine‐based. |
| Relevant clinical information? All tests | Yes | Women with known borderline or mild dyskaryosis smears. |
| Uninterpretable results reported? All tests | Unclear | There were no cases of uninterpretable results reported. |
| Withdrawals explained? All tests | Unclear | No withdrawals were reported, or explained. |
Abbreviations
< = less than ≥ = greater than or equal to AGC = atypical glandular cells AGUS = atypical glandular cells of undermined significance
AIS: adenocarcinoma in situ ASC‐H = atypical squamous cells of undetermined significance, where HSIL cannot be excluded ASCUS = atypical squamous cells of undetermined significance CC = cervical cancer CIN2+ = cervical intraepithelial neoplasia of grade 2 or worse CIN3+ = cervical intraepithelial neoplasia of grade 3 or worse
CIS: carcinoma in situ ECC = endocervical curettage HC2 = Hybrid Capture 2 assay HPV = human papillomavirus HPV+ = human papillomavirus positive HPV‐ = human papillomavirus negative HSIL = high‐grade squamous intraepithelial lesion LBC = liquid‐based cytology LEEP = loop electrical excision procedure LSIL = low‐grade squamous intra‐epithelial lesions Pap = Papanicolaou test PCR = polymerase chain reaction
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adam 1998 | Triage concerns women with ASCUS or LSIL. No distinct data provided to separate the 2 triage groups. HPV triage test was not HC2, but HPV Profile. |
| Aerssens 2009 | Follow‐up study, no triage of women with minor cytological lesions. |
| Ahmed 2008 | No HPV testing done, only follow‐up over time with follow‐up cytology and colposcopy/biopsy provided according to follow‐up cytology. |
| Alameda 2010 | Discrepancy between reported and computed numbers of false positives and true negatives . |
| Allen 2009 | No verification was done. Compared hrHPV (HC2) rates in ASCUS women from a private clinic (low‐risk) with women from a public clinic (high‐risk). |
| Antonishyn 2009 | No HC2 testing was done. HPV triage done with several other HPV markers (genotyping, mRNA). |
| Arbyn 2010 | Comments on TOMBOLA trial. |
| Armah 2009 | Only HPV‐positive cases included. |
| Atkins 2006 | Contained only the outcome of cancer from the ALTS study, already included in Solomon 2001. |
| Bais 2005 | Triage concerned women with ASCUS or LSIL. No distinct data available to separate the 2 triage groups. HPV triage test was not HC2 but GP5+6+ PCR followed by identification of 14 high‐risk types using EIA. Longitudinal outcome at 6 months (only for women with CIN2+ at baseline) and at 12 months for all women was provided. |
| Baseman 2008 | Primary screening, not a triage study. |
| Bavin 1993 | HPV triage test was not HC2 but PCR. |
| Beccati 2008 | Study sample too small, contained marker data for Ki67 and ProExcy for women with ASCUS, ASC‐H or LSIL. |
| Bellinson 2009 | No triage of ASCUS, LSIL. Primary screening with HPV testing on self samples using visual inspection after application of acetic acid (VIA) as a triage method. |
| Bello 2009 | No triage of ASCUS, LSIL. Colposcopy setting without cytological subgroups distinguished. HPV genotyping, but no HC2. |
| Bentley 2006 | No primary data, ongoing study from the TOMBOLA group. |
| Berkhof 2006a | Triage test was PCR (GP5+/6+) and not HC2. |
| Berkhof 2006b | Model to evaluate cervical screening strategies, with no primary data. |
| Bewtra 2005 | The HPV test evaluated was an in situ hybridization (ISH) method (Ventana Inform HPV Test, Tucson, Arizona, US A). Included women with ASCUS and histologically‐confirmed CIN in follow‐up biopsies. No histological gold standard information available for all cases. |
| Biu 2009 | Evaluated HPV in women with ASCUS or ASC‐H with colpo/biopsy data. Test was a genotyping assay, not HC2. |
| Bjerre 2008 | HPV and cytology results grouped together. Triage group not defined. No histopathology results available for all women. Only relative sensitivity could be calculated. |
| Boardman 2005 | Only age‐specific (high‐risk) HC2 positivity rates were presented. No gold standard‐verified accuracy data were presented. |
| Boardman 2006 | Only age‐specific (high‐risk) HC2 positivity rates were presented. No gold standard‐verified accuracy data were presented. Only risk factors for CIN2+ were provided. |
| Bohmer 2002 | Triage concerned women with ASCUS, LSIL or other lesions. No separate data for the ASCUS or LSIL triage groups could be extracted since the used Munich cytological classification system did not allow translation into equivalent categories of The Bethesda System. |
| Bollen 1997 | Triage concerned women with ASCUS, LSIL or other lesions. No separate data for the ASCUS or LSIL triage groups were provided. |
| Botezatu 2009 | Measurement of HPV16/18 viral load. ASCUS/LSIL grouped together, no separate data available. HPV test was not HC2. No colposcopy or biopsy was performed. |
| Boulanger 2006 | Review article, no primary data available. Article provides guidelines to manage ASCUS. |
| Brismar 2009 | No HC2 performed. HPV triage with linear array testing. |
| Brown 2009 | No HC2 testing, but PCR for HPV triage. |
| Bruner 2004 | Only age‐specific (high‐risk) HC2 positivity rates presented. No gold standard‐verified accuracy data presented. |
| Burgis 2009 | Paper discussed guidelines for adolescents. No triage data. |
| Carozzi 2005 | HPV triage test was not HC2, but a type‐specific PCR, based on E6/E7 amplification of 8 high‐risk HPV types. |
| Carozzi 2006 | Triage concerned women with ASCUS, LSIL or other lesions. No distinct data provided to separate the two triage groups. HPV triage test was not HC2, but a PCR based on E6/E7 amplification of 8 high‐risk HPV types and p16 immunostaining of residual material remnant after preparation of a liquid‐based smear. |
| Carozzi 2008 | HPV testing with triage by p16. Data available only for HPV+ women. |
| Carvalho 2010 | Type‐specific PCR and sequencing. No HC2 testing. |
| Castle 2008 | More relevant data published in other papers (Solomon 2001), already included in the meta‐analysis. |
| Castle 2009a | Concerned negative predictive values of primary screening tests (Hybrid Capture tube test, VIA, cervicography). No triage data. |
| Castle 2009b | No triage of ASCUS, LSIL. Focus on primary screening in the US with HPV and cytology for women over 30 years of age. |
| Castle 2010b | ALTS study with focus on the odds ratio between hrHPV infection and subsequent CIN3+ according to the quality of histological ascertainment. |
| Castle 2010c | Included only patients with CIN3+ diagnosis from the clinical laboratories in the ALTS study. The quality‐centre (QC) diagnosis was analysed versus HPV typing and other variables. |
| Chaiwongkot 2007 | Different PCRs compared for hrHPV positivity. No histological outcomes reported. |
| Chen 2005a | Triage concerned women with AGC. This study was included in the sub‐group meta‐analysis for specific categories of equivocal cytology. |
| Chesebro 1997 | HPV triage test was not HC2, but HC1. |
| Cheung 2010 | p63 and p73 expression in different categories of cytological lesions. Progression and regression in women with ASCUS or LSIL evaluated according to the p63:73 status. No accuracy data presented. |
| Chivukula 2006 | Triage concerns women with atypical squamous cells where HSIL cannot be ruled out (ASC‐H). |
| Conesa‐Zamora 2010 | No HC2 testing. Polymorphism of FcGR3A gene compared in LSIL versus HSIL cytology cases. No histological outcome reported. Association between polymorphism and HPV genotypes also evaluated. |
| Constandinou‐Williams2010 | No data for ASCUS or LSIL women. Evaluated risk of developing cytological cervical abnormalities as a function of type‐specific viral load of HPV (quantitative PCR GP5+/6+). |
| Coste 2003 | No separate accuracy data for the criterion HC2 or repeat cytology were presented in the colposcopy group. |
| Cotton 2006 | Described only the design of an RCT regarding different management options for women with borderline or low‐grade abnormal cytology. |
| Cotton 2010 | HPV testingwas not performed with HC2 but with GP5+/6+ PCR. HPV testing performed on archived residual material and the result was blinded (no diagnostic process was initiated on HPV+ women). |
| Cox 1992 | Triage concerned women with ASCUS, LSIL or other lesions. No separate data for the ASCUS or LSIL triage groups were provided. |
| Cox 1995 | HPV triage test was not HC2, but HC1. |
| Crabtree 2002 | Only HC2+ positive cases with ASCUS and ASC‐H were verified. Only the PPVs for different degrees of CIN were evaluated. |
| Cricca 2009 | No HC2 but mRNA testing and no HPV negative cases included. |
| Del Mistro 2007 | Conference report, no primary data. |
| Del Pino 2009 | No ASCUS/LSIL triage, but triage of histologically confirmed CIN cases with p16 immunostaining. |
| Derchain 2004 | Triage concerned women with atypical glandular cells (AGC). |
| Diaz‐Montes 2007 | Compares histological outcomes of AGC in conventional Pap smears and LBC. For a subset of women, HPV testing was done, but the histological outcome for HPV triage was insufficiently detailed (no CIN category given). |
| Difurio 2010 | No HPV testing, only Pap test. |
| Dockter 2009 | Women with abnormal cytology were grouped together, no separate data for ASCUS, LSIL. |
| Duerr 2006 | Study compared women with and without human immunodeficiency virus (HIV) infection: prevalence of ASCUS, HC2+ positivity rate and cumulative incidence of cervical lesions among ASCUS cases. No gold standard‐verified accuracy data were provided. |
| Duncan 2005 | HPV testing done in only a small fraction of the women. Outcomes were not differentiated by HPV test result. |
| Einstein 2010 | No HC2 data available. |
| Ekalaksananan 2006 | No separate accuracy data available for ASCUS and LSIL group. |
| Eleuterio 2007 | No index cytology, apart from biopsies, no HC2 results were shown. |
| Eltoum 2005 | Study contained information of HC2 positivity rate in ASCUS cases, but, due to incomplete verification, no accuracy parameters could be presented. The presentation of other rates and ratios was unclear. |
| Evans 2006 | HPV triage test was not HC2, but PCR. Outcome was restricted to biopsy‐confirmed cases yielding accuracy estimates suffering from verification bias. |
| Fadare 2009 | No triage of ASCUS or LSIL, but follow‐up of women with CIN1 biopsies. |
| Fait 1998 | HPV triage test was not HC2, but HC1. |
| Fait 2000 | HPV triage test was not HC2, but HC1. |
| Farag 2008 | Follow‐up with HPV testing, no triage. |
| Feng 2005 | This study evaluated the influence of pretreatment of bloody liquid‐based specimens with acetic acid on HC2 positivity in ASCUS cases. No gold standard‐verified accuracy data were presented. |
| Feng 2007a | Outcome only available for HPV+ women. |
| Feng 2007b | No data for ASCUS or LSIL. Evaluated association between HPV infection, epidermal growth factor receptor (EGFR) status in normal, CIN and cervical cancer biopsies. |
| Feng 2008 | Only data about HPV+ women, no data about HPV negative women. Risk for verification bias. |
| Ferenczy 1996 | Triage concerned women with ASCUS, LSIL or other lesions. No separate data for the ASCUS or LSIL triage groups were provided. |
| Ferris 1998a | HPV triage test was not HC2, but HC1. |
| Ferris 1998b | HC2 was not defined at the standard cut‐off but at 0.3 pg/mL. |
| Fletcher 2009 | Adolescent population. Almost no colposcopy verification in HPV subjects. |
| Fröberg 2008 | Triage test was a linear array, no HC2 results. |
| Ge 2009 | Only follow‐up of HPV+ women. |
| Getman 2009 | In vitro experience to derive analytical sensitivity of APTIMA vs HC2. Test positivity in clinical ASCUS samples without verification. |
| Goff 1993 | HPV triage test was not HC2, but ViraType. |
| Griesser 2009 | Only HPV+ mild and modarate dyskaryosis cases followed with L1 immunostaining. |
| Guarisi 2009 | Cohort study on incidence of CIN in ASCUS/LSIL cases smokers vs non smokers. No triage data. |
| Guimaraes 2005 | Study patients had CIN1 biopsy confirmed at baseline instead of an LSIL smear. HPV triage test was not HC2, but PCR. The study addressed correlation between protein markers p16 and bcl‐2 with HPV16 and HPV18 in CIN1 progressors and CIN1‐non progressors. |
| Guo 2004 | Compareed positivity‐rates and PPV for CIN2+ of p16 and HC2 in a LSIL group and a HSIL group. |
| Guo 2007 | Triage test was Inform HPV (Ventana), no HC2 results. |
| Guo 2008 | Triage test was PCR (GP5+/GP6+), no HC2 results. |
| Gupta 2007 | No HPV DNA results included. |
| Gupta 2010 | No HPV testing. |
| Gustavsson 2009 | No verification with gold standard; HC2 and hpVIR real time PCR compared for virological concordance and by cytological categories. |
| Halfon 2007 | No gold standard verification. Addressed HPV test concordance (HC2 and AMPLICOR). |
| Halfon 2010a | Data not separated into ASCUS or LSIL triage groups. |
| Halfon 2010b | Data not separated into ASCUS or LSIL triage groups. |
| Hall 1996 | Triage concerned women with ASCUS or LSIL. No separate data for the two triage groups were provided. HPV triage test was not HC2, but HCT. |
| Harvey 2009 | No separate data available for ASCUS or LSIL triage groups. |
| Hatch 1995 | Triage concerned women with ASCUS, LSIL or other lesions. No separate data provided for the ASCUS or LSIL triage groups. |
| Herrington 1995 | Triage concerned women with ASCUS or LSIL. No separate data provided for the 2 triage groups. HPV triage test was not HC2, but PCR and in situ hybridisation. |
| Ho 2003 | Triage concerned women with ASCUS or LSIL. No separate data provided for the 2 triage groups. HPV triage test was not HC2, but PCR and HPV typing |
| Howard 2008 | Economic evaluation. |
| Howell 2010 | Review concerning the evolution of atypical squamous cells (ASC) terminology |
| Hughes 2002 | HPV triage test was not HC2, but PCR. |
| Irvin 2005 | Triage concerned women with AGC. This study was included in the sub‐group meta‐analysis for specific categories of equivocal cytology. |
| Jamison 2009 | No HC2 testing was done. Linear array results correlated with cytology. No verification with gold standard. |
| Jarboe 2010 | No HPV negative cases included. Only comparison of women with ASCUS with low or high viral load. |
| Jeantet 2009 | Focus on analytical accuracy of NucliSENS EasyQ HPV v1 test and concordance with PreTect HPV‐Proofer. No triage data. No HC2 testing. |
| Jeronimo 2007 | Triage test was PGMY 09/11 instead of HC2, contained cervicography and HPV16 data, other results from the ALTS study already included in the Solomon 2001 and Sherman 2002 papers. |
| Julian 2006 | No primary data available. |
| Kapeu 2009 | Case‐control study addressing smoking as risk factor for CC. No triage data available. |
| Kaufman 1997a | Triage concerned women with ASCUS or LSIL. No separate data provided for the 2triage groups. HPV triage test was not HC2, but HPV Profile. |
| Kaufman 1997b | Triage concerned women with ASCUS or LSIL. No separate data provided for the 2 triage groups. HPV triage test was not HC2, but HPV Profile. |
| Kavoussi 2009 | No triage was done; CC screening with liquid cytology in women with developmental disabilities. |
| Keegan 2009 | No gold standard verification, compared positivity rates in different cytological categories for HC2 and PreTect HPV‐Proofer. |
| Kendall 2005 | Presented only age‐specific (high‐risk) HC2 positivity rates. No gold standard‐verified accuracy data presented. |
| Kitchener 2008 | Follow‐up of women, no triage. |
| Knoepp 2007 | Examined histological outcomes in a group with mixed cytology index diagnoses and with an equivocal HC2 result. No HPV‐ cases were included. All were a priori borderline cytology and HPV+. |
| Knoepp 2010 | Only histological follow‐up data available for HPV+ cases. Cases with equivocal hrHPV results (low load) compared with higher load. |
| Kong 2007 | Material of atypical squamous metaplasia was histological and not cytological. |
| Kovacs 2009 | No ASCUSLSIL triage data. The paper describes the natural history of HPV infection by type of HPV infection |
| Krambeck 2008 | Triage test was PCR, no HC2 results. |
| Krane 2004 | Triage concerned women with AGC. |
| Kulasingam 2006 | No primary data (ALTS data and medical care costs). |
| Kumar 2007 | Study sample too small. |
| Kuperman 2000 | HPV triage test was not HC2, but HC1. |
| Layfield 2005 | Comparison of costs of HC2 versus INFORM HPV test (in situ hybridisation). |
| Lee 2006 | Compared outcomes of triage: repeat smear vs HPV vs immediate colposcopy or combinations in 50 ‐/+ year old women with ASCUS. Incomplete verification. Colposcopy or biopsy in ASC‐H (incomplete verification and no HPV data). |
| Lee 2007 | No HPV test results. Discussed impact of LBC and TBS2001 on PPV of atypical smears. |
| Legood 2006 | No primary data, modelling study, no outcome results for all subjects. |
| Leo 2009 | No HC2, Locked Nucleic Acid real time PCR genotyping compared with InnoLiPA. No triage data available. |
| Liang 2010 | Papillary SIL: distribution of markers in hrHPV+ cases and lrHPV+ cases. |
| Lim 2010 | No HC2 testing. Selected population where all LSIL patients had histologically‐confirmed CIN1. |
| Liman 2005 | Triage concerned women with ASC‐H. |
| Lin 2000 | HC2 not defined at the standard cut‐off but at 0.3 pg/mL. |
| Lin 2010 | HC2, PCR‐reverse line blot hybridization, methylation‐specific PCR. Markers correlated to cytology and to histology, but histology not stratified separately for ASCUS or LSIL triage groups. |
| Longatto‐Filho 2005 | Limited to ASCUS/HPV+ cases. Outcome poorly documented, 2 tests used; HC2 or PCR, no separate data for HC2. Described KI67/p16 correlations. |
| Martin 2009 | No clinical samples. Gene expression profiles (RNA, proteins) in cell cultures. |
| Massad 2004 | HPV triage test was not HC2, but PCR, and concerned triage of HIV positive women with ASCUS. |
| Massad 2009 | No HC2 testing, focus on colposcopy grading. No triage data (ALTS). |
| Maucort‐Boulch 2010 | ALTS study: focussed on the persistence of HPV as a function of age and incidence/prevalence status. No ASCUS triage data presented. |
| McHale 2007 | Descibed outcome of ASC‐H without relating to HPV status. |
| Melnikow 2006 | Editorial, no primary data. |
| Mesher 2010 | Primary screening (HART study), no triage. |
| Mirasoli 2009 | Histological distinction between CIN categories using p16. No ASCUS triage data. No HC2 testing. |
| Mo 2008 | No histological outcomes. |
| Mokhtar 2008 | No HPV testing, ASC‐H cases followed‐up by cytology and by biopsy for some of the cases. |
| Molden 2005 | HPV triage test was not HC2, but PreTect HPV‐Proofer (test for mRNA coding for E6/7 from 5 high‐risk types). |
| Monsonego 2005 | No HC2 testing, but AMPLICOR and LBC. |
| Monsonego 2008a | Contained complete verification data for a series of women with WNL, ASCUS, LSIL, and HSIL tested with p16,HC2 and linear array. No separate accuracy data available for ASCUS or LSIL triage groups. |
| Moore 2010 | No accuracy data, provided risk CIN3+ in 21‐24 year old women with ASCUS, HPV+ and LSIL from ALTS study. |
| Moscicki 2008 | Study about the management and treatment of women with minor cytological abnormalities, no HC2 testing results. |
| Mould 2000 | Triage concerns women with ASCUS or LSIL. No separate data proivided for the 2 triage groups. HPV triage test was not HC2 but HC1. |
| Nassar 2008 | Outcome threshold was not defined. Where it was defined, data not stratified by cytological category. |
| Noel 2006 | Review article, no primary data available. |
| Nomelini 2007 | Results for ASCUS and LSIL triage groups could not be separated. |
| Nuovo 2008 | No outcome available with CIN2+ details, only CIN+ outcomes were reported. |
| Nyirjesy 1998 | Triage concerned women with ASCUS or LSIL. No separate data provided for the 2 triage groups. HPV triage test was not HC2 but HC1. |
| Ogilvie 2010 | Preliminary results, might give data in the future. |
| Oliveira 2004 | Triage concerned only women with AGC. The outcome was the cytological result of a repeat smear. No gold standard‐verified data provided. |
| Oliveira 2010 | HPV prevalence (and typing) and cyto‐virological correlation in Brazilian students. No histological outcome reported. |
| Onuma 2006 | Only pregnant women with ASC‐H cytology included. Incomplete verification. |
| Ou 2007 | Only abstract available, article in Chinese. Insufficient data were given. |
| Ozsaran 2003 | HPV triage test was not HC2, but HC1. |
| Pajtler 2010 | No separate accuracy data for ASCUS group. No point in requesting separate data, since only 11 ASCUS women included. |
| Pambuccian 2002 | HPV triage test was not HC2, but PCR. |
| Paternoster 2008 | No HC2 triage test; no gold standard‐verification. |
| Patton 2008 | No general screening population. Addressed outcome in ASC‐H according to hormonal status. |
| Peng 2006 | Compared outcomes in triage versus no triage situation vs situation where HPV testing always used; incomplete verification in triage situation. |
| Perrons 2005 | Follow‐up and post treatment data; no accuracy parameters could be derived. |
| Philips 2006 | No primary data, questionnaire about consequences of introduction of HPV triage into screening. |
| Pisal 2003 | Triage concerned women with ASCUS or LSIL. No separate data provided for the 2 triage groups. |
| Poljak 2009 | No ASCUS or LSIL triage; concordance between Abbott RealTime PCR and HC2 testing. |
| Pretet 2008 | Triage test was INNO‐LiPA, no HC2 results. |
| Quddus 2002 | Only CIN1+ outcome documented (cervical intraepithelial neoplasia of grade 1 or worse), triage of atypical squamous metaplasia (ASM), was not ASCUS. |
| Quddus 2009 | Addressed ASCUS and AGUS rates, %hrHPV among these and the detection rate of histological outcomes before and after introduction of ThinPrep Imager. |
| Qureshi 2003 | Triage concerned women with ASCUS or LSIL. No separate data provided for the 2 triage groups. |
| Rabelo‐Santos 2009 | Not HC2 testing, but Roche reverse line blot assay. |
| Rana 2004 | Longitutidinal cytological or histological outcomes of borderline or mild dyskaryosis. No HPV testing done. |
| Raskin 2009 | No HC2 testing. |
| Rauber 2008 | No HPV testing. L1 immunostaining correlated with regression/progression. |
| Recio 1998 | HPV triage test was not HC2, but HC1. |
| Reesink‐Peters 2003 | Triage concerned women with ASCUS, LSIL or other lesions. No separate data provided for the ASCUS or LSIL triage groups. HPV triage test not HC2, but PCR and detection of telomerase. |
| Riethmuller 2008 | Follow‐up, no triage. |
| Rijkaart 2009 | No complete accuracy data. Only cumulative detection of CIN2/3+ in cytology versus HPV‐based follow‐up; PPV and colposcopy referral. |
| Robova 2007 | hrHPV test was not specified. |
| Rodriguez 2008 | Data restricted to HPV triage negative patients and no colposcopy data (gold standard‐verification) available, only follow‐up Pap tests. |
| Ronnett 1999 | Triage concerned women with AGC. |
| Rosini 2007 | 3 groups were not separated. Outcome was based on colposcopy. Only DNA/RNA test and cytology were documented with crude data. |
| Rowe 2004 | Study evaluatesd only the HC2‐positivity rate in women with different categories of equivocal squamous or glandular abnormalities or LSIL. No gold standard‐verified accuracy data were presented. |
| Saad 2006 | Only women of peri and post menopausal age included. |
| Safaeian 2007 | Documented accuracy of HPV/cytology testing at 12 months among HPV‐ASCUS women; also gave accuracy of HPV testing for cumulative CIN2/3+ according to age group. |
| Samarawardana 2010 | No HC2, but type‐specific PCR and p16. |
| Santos 2003 | Triage concerned women with ASCUS or LSIL. No separate data provided for the 2 triage groups. |
| Santos 2006 | Patients had histologically confirmed CIN1, not LSIL. |
| Saqi 2006 | Triage concerned women with AGC. |
| Sargent 2010 | Accuracy data for triage of borderline/mild dyskaryosis (nested in ARTISTIC trial) were not presented separately. |
| Sarian 2009 | Natural history study: risk of HPV infection and lesions related to smoking. No triage data. |
| Sarode 2003 | In the ASCUS group (including women with ASC‐H, ASC‐R (atypical squamous cells which are probably reactive) or ASCUS), verification was done only if HC2+. In the LSIL group, all women were verified but the data were not separated from the ASCUS groups.. |
| Schiffman 2006 | No primary data, comment on Legood and Moss paper. |
| Schledermann 2008 | No HC2 testing. |
| Schmitz 2009 | No triage data. No HC2. Compared analytical accuracy genotyping realtime PCR (and concordance with GP5/6+ PCR). |
| Schnatz 2006 | Systematic review of outcomes of AGC. No HPV testing. |
| Seme 2006 | Repeated borderline virological HC2 results. No gold standard outcome; objective was to determine concordance between HC2/PCR and typing. |
| Sharpless 2005a | Adherence of guidelines for management of AGC. |
| Sharpless 2005b | Outcomes of AGC. No HPV testing. |
| Sharpless 2009 | Systematic review on HPV triage in AGC. |
| Sheriff 2007 | Economic analysis, no primary data. |
| Shi 2009 | No ASCUS or LSIL triage. Longitudinal absolute risk of CIN2+ after 1st screening round. |
| Shidham 2007 | Did not contain ASCUS or LSIL data, only LSIL‐H (LSIL lesions which might be high‐grade). Contained HPV and histological data, only PPV could be calculated, verification bias. |
| Shin 2008 | Index result for ASCUS and LSIL were grouped together and could not be separated. |
| Siddiqi 2009 | Extremely low sensitivity of HC2 and nearly perfect sensitivity of ProExC. |
| Siddiqui 2008a | No complete gold standard‐verification. |
| Sideri 1998 | HPV triage test was not HC2, but HC1. |
| Singh 2009 | No HC2; PCR test and 2 protein markers (p53,Bcl‐2) evaluated for prediction of progression. |
| Six 2008 | PCR, serology ‐ vaccination. |
| Slawson 1994 | HPV triage test was not HC2, but ViraPap. |
| Sorbye 2010 | Only histological follow‐up data available for mrRNA+ cases: only PPV could be evaluated. |
| Srodon 2005 | Triage concerned women with ASC‐H. |
| Steinman 2008 | No gold standard verification. |
| Stemberger‐Papic 2010 | All samples were HC2 positive. The natural history was evaluated according to L1 immunochemistry. No separate data presented for LSIL. |
| Stevens 2007 | Complete accuracy data for HC2, linear array HPV genotyping, AMPLICOR PCR, but not presented separately for ASCUS or LSIL triage groups. |
| Stoler 2001 | Documented only high HC2 rate in LSIL. |
| Stoler 2007 | No primary data. |
| Suba 2009 | Comment;. no primary data. |
| Sun 2006 | Triage test was DNA image cytometry, no HC2 testing; article in Chinese. |
| Sung 2010 | Addressed p16 in ASCUS and ASC‐H for subsequent CIN. Incomplete HPV test data, HPV data not linked to follow‐up outcome. |
| Tambouret 2008 | Histological outcome for some subjects and follow‐up cytology for others. No separate data for ASCUS/LSIL. Triage test was HC2 and ProEx C. |
| Tang 2009 | The test used was: Abbot rtPCR in CIN3, cancer and cyto‐negative subjects. No triage data. |
| Tarkkanen 2007 | ASCUS‐LSIL could not be separated in the index smear. |
| Thrall 2008 | HPV triage was HC2, but histological verification was overwhelmingly based on a positive HC2 result resulting in extremely low specificity estimates due to verification bias. |
| Thrall 2010 | No gold standard verification of HPV testing on all participants; triage in women > 30 years of age. |
| TOMBOLA 2009a | No HPV testing accuracy data presented in this paper. |
| TOMBOLA 2009b | No HPV testing accuracy data presented in this paper. |
| TOMBOLA 2009c | No HPV testing accuracy data presented in this paper. |
| Torres 2009 | Study of prevalence of HPV (HC2) in a CC screening population, with correlation with cytology. No verification data. |
| Tu 2009 | No HC2 testing. Telomerase presence documented separately in different categories of cytology and histology. |
| Vijayaraghavan 2010 | 0nly cost‐effectiveness results of HPV16/18 triage of HPV+ women. |
| Vince 2001 | HC2 test positivity rate was documented for women with LSIL, but no gold standard‐verified outcome data were provided. |
| Walker 2006 | Contained data only on the risk of CIN3 by follow up (ALTS), other data from this study already included in Solomon 2001 and Sherman 2002. |
| Wang 2007 | Article in Chinese, only abstract available, HPV test not specified. |
| Wensveen 2006 | ASCUS and AGUS were grouped together. The accuracy data for triage separated for ASCUS and LSIL were reported in Wensveen 2003. |
| Wheeler 2006 | Triage test was PCR instead of HC2, results of ALTS study already included in Solomon 2001 and Sherman 2002. |
| Wong 2008 | No gold standard‐verification. Addressesses HPV test concordance (HC2 and Inv2). |
| Wong 2008a | No colposcopy/biopsy results. Study focusses on morphological correlates for lr & hrHPV infection. No triage data. |
| Wright 1995 | HPV triage test was not HC2, but HC1. |
| Wright 1998 | Triage test was HC1, secondary publication of Wright 1995. |
| Wright 2006 | HPV triage was HC2, but histological verification was overwhelmingly based on a positive HC2 result resulting in extremely low specificity estimates due to verification bias. |
| Wu 2006 | Gold standard‐verification was incomplete. |
| Xi 2009 | This report of the ALTS study does not describe the perforamce of HC2 but focuses on the viral load of HPV18. |
| Yarandi 2009 | No HC2 testing, but PCR. |
| Yu 2010 | No HPV testing. P16, Ki67 and L1 immunostaining in LBC and cell block were correlated with histological outcomes. |
| Zhao 2009a | No HPV triage investigated. |
| Zhao 2009b | No ASCUS/LSIL smears, but unsatisfactory Pap smears. |
| Zivadinovic 2009 | Incomplete HPV testing and histological outcomes. |
Abbreviations
AGC = atypical glandular cells
AGUS = atypical glandular cells of undermined significance
AMPLICOR = PCR‐based test for detection of 13 high‐risk HPVgenotypes
ASC‐H = atypical squamous cells of undetermined significance, where HSIL cannot be excluded
ASCUS = atypical squamous cells of undetermined significance
ASM = atypical squamous metaplasia
CC = cervical cancer
CIN2+ = cervical intraepithelial neoplasia of grade 2 or worse
CIN3+ = cervical intraepithelial neoplasia of grade 3 or worse
HC2 = Hybrid Capture 2 assay
HPV = human papillomavirus
HPV+ = human papillomavirus positive
HSIL = high‐grade squamous intraepithelial lesion
LBC = liquid‐based cytology
LSIL = low‐grade squamous intra‐epithelial lesions
Pap = Papanicolaou test
PCR = polymerase chain reaction
PPV = positive predictive value
VIA =
Characteristics of studies awaiting classification [ordered by study ID]
Alameda 2011.
| Clinical features and settings | |
| Participants | Women with ASCUS. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test: p16 immunocytochemistry. |
| Follow‐up | |
| Notes |
Barcelos 2011.
| Clinical features and settings | |
| Participants | Women with ASC‐US and ASC‐H. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2 Comparator test: review of cytological specimen. |
| Follow‐up | |
| Notes |
Belinson 2011.
| Clinical features and settings | |
| Participants | Women participating in a primary screening trial, involving self‐sampling and sampling by a clinician. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | |
| Follow‐up | Index test: HC2. Comparator tests: Cervista and MALDI‐TOF [PCR‐based mass array matrix‐assisted laser desorption/ionization time‐offlight mass spectrometry system]). |
| Notes | Data requested from authors separately for women with ASC‐US and for women with LSIL. |
Chao 2013.
| Clinical features and settings | |
| Participants | Women with ASC‐US or ASC‐H. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test: methylation markers. |
| Follow‐up | |
| Notes |
Clad 2011.
| Clinical features and settings | |
| Participants | Women with ASCUS or LSIL. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test: APTIMA hrHPV RNA assay. |
| Follow‐up | |
| Notes | Data requested from authors separately for women with ASC‐US and for women with LSIL. |
Cuzick 2013.
| Clinical features and settings | |
| Participants | Women with LSIL. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test: COBAS‐4800 assay. |
| Follow‐up | |
| Notes |
Dona 2012.
| Clinical features and settings | |
| Participants | Women referred to colposcopy. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: Linear Array. Comparator test: p16/Ki67 cytoimmunochemistry. |
| Follow‐up | |
| Notes | Data requested from authors separately for women with ASC‐US and for women with LSIL. |
Dufresne 2011.
| Clinical features and settings | |
| Participants | Women with ASC‐US |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2 Comparator tests: AMPLICOR, Linear Array and COBAS‐4800 |
| Follow‐up | |
| Notes |
Edgerton 2011.
| Clinical features and settings | |
| Participants | Women with ASCUS. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test: p16 immunocytochemistry. |
| Follow‐up | |
| Notes |
Gustinucci 2012.
| Clinical features and settings | |
| Participants | |
| Study design | Women with ASC‐US, ASC‐H or LSIL. |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test: p16 cyto‐immunochemistry. |
| Follow‐up | |
| Notes |
Heider 2011.
| Clinical features and settings | |
| Participants | Women with LSIL. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. No comparator tests. |
| Follow‐up | |
| Notes |
Huang 2012.
| Clinical features and settings | |
| Participants | Women with mildly cytologic abnormalities |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | |
| Follow‐up | Index test: EasyChip HPV Blot |
| Notes | Data requested from authors separately for women with ASC‐US and for women with LSIL. |
Ibanez 2012.
| Clinical features and settings | |
| Participants | Women with ASCUS. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. |
| Follow‐up | |
| Notes |
Jakobsson 2012.
| Clinical features and settings | |
| Participants | Women with ASC‐US. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: AMPLICOR; |
| Follow‐up | |
| Notes |
Jiang 2011.
| Clinical features and settings | |
| Participants | Women with ASC‐US and LSIL. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: PCR (ABI 7500 PCR system, Applied Biosystems, USA) detecting nine hrHPV types. |
| Follow‐up | |
| Notes |
Koliopoulos 2012.
| Clinical features and settings | |
| Participants | Women with ASC‐US or LSIL. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test:PreTect HPV‐Proofer. Comparator test: flow cytometry for E6&E7 mRNA (OncoTect) |
| Follow‐up | |
| Notes |
Lapierre 2012.
| Clinical features and settings | |
| Participants | Women with ASC‐US |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test: Linear Array, COBAS‐4800 assay. |
| Follow‐up | |
| Notes |
Levi 2011.
| Clinical features and settings | |
| Participants | Women with LSIL. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. No comparator test. |
| Follow‐up | |
| Notes |
Loghavi 2012.
| Clinical features and settings | |
| Participants | Women with ASC‐US or LSIL. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: Cervista/Invader test. Comparator test: p16/Ki69 cytoimmunochemistry. |
| Follow‐up | |
| Notes |
Ma 2011.
| Clinical features and settings | |
| Participants | Women with ASC‐US or LSIL. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test; p16 immunocytochemistry. |
| Follow‐up | |
| Notes |
Monsonego 2011.
| Clinical features and settings | |
| Participants | Women with diverse cytology results participating in a screening trial. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test:APTIMA hrHPV RNA assay, Linear Array. |
| Follow‐up | |
| Notes | Data requested from authors separately for women with ASC‐US and for women with LSIL. |
Nasioutziki 2011.
| Clinical features and settings | |
| Participants | Women with ASC‐US or LSIL. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test: p16 immunocytochemistry. |
| Follow‐up | |
| Notes |
Origoni 2012.
| Clinical features and settings | |
| Participants | Women with ASC‐US. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. |
| Follow‐up | |
| Notes |
Ovestad 2011.
| Clinical features and settings | |
| Participants | Women with ASC‐US or LSIL. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test; AMPLICOR; Comparator test;APTIMA hrHPV RNA assay, COBAS‐4800 assay and Pretect HPV Proofer. |
| Follow‐up | |
| Notes |
Pista 2011.
| Clinical features and settings | |
| Participants | Women with ASC‐US and LSIL. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. No comparator test. |
| Follow‐up | |
| Notes | Data requested from authors. |
Ratnam 2011.
| Clinical features and settings | |
| Participants | Women with diverse cytology results participating in a screening trial. and women referred to colposcopy. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test:APTIMA hrHPV RNA assay, Linear Array and PreTect HPV Proofer. |
| Follow‐up | |
| Notes | Data requested from authors separately for women with ASC‐US and for women with LSIL. |
Rodriguez 2012.
| Clinical features and settings | |
| Participants | Women with ASC‐US being HC2+. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. |
| Follow‐up | |
| Notes |
Schmidt 2011.
| Clinical features and settings | |
| Participants | Women with ASC‐US or LSIL. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test: p16 immunocytochemistry. |
| Follow‐up | |
| Notes |
Soderlund‐Strand 2011.
| Clinical features and settings | |
| Participants | Women low‐grade cervical lesions |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: PCR amplification using 5/bioGP 6 primers followed by luminex‐based identification of multiple high‐ and low‐risk HPV types. |
| Follow‐up | |
| Notes |
Stoler 2011.
| Clinical features and settings | |
| Participants | Women with ASC‐US |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test: COBAS‐4800. |
| Follow‐up | |
| Notes |
Stoler 2013.
| Clinical features and settings | |
| Participants | Women with ASC‐US. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test:APTIMA HPV mRNA assay.. |
| Follow‐up | |
| Notes |
Szarewski 2012.
| Clinical features and settings | |
| Participants | Women with diverse abnormal cytology referred to colposcopy. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator tests: p16 cytoimmunochemistry,Abbott RealTime hrHPV PCR, Cobas‐4800, APTIMA hrHPV RNA assay,BD HPV tests, APTIMA hrHPV RNA test and,PreTect HPV‐Proofer. |
| Follow‐up | |
| Notes | Data requested from authors separated for women with ASC‐US and for women with LSIL. |
Tsoumpou 2010.
| Clinical features and settings | |
| Participants | Women with LSIL. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: PCR test (CLART [(Clinical Array Technology] including identification for 35 HPV types). Comparator test: p16 immunocytochemistry. |
| Follow‐up | |
| Notes |
Valasoulis 2011.
| Clinical features and settings | |
| Participants | Women with LSIL. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: p16 cytoimmunochemistry. |
| Follow‐up | |
| Notes |
Waldstrom 2011.
| Clinical features and settings | |
| Participants | Women with ASC‐US or LSIL |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: Linear Array. Comparator test: APTIMA hrHPV RNA assay and p16/Ki67 immunocytochemistry. |
| Follow‐up | |
| Notes |
Wentzensen 2012.
| Clinical features and settings | |
| Participants | Women referred to colposcopy. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index: Linear Array. Comparator test: p16/Ki67 immunocytochemistry. |
| Follow‐up | |
| Notes | Data requested from authors separately for women with ASC‐US and for women with LSIL. |
Wong 2011.
| Clinical features and settings | |
| Participants | Women with ASC‐US. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test: Abott real time PCR for hrHPV. |
| Follow‐up | |
| Notes |
Wong 2012.
| Clinical features and settings | |
| Participants | Women with ASC‐US. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test: Linear Array and GenoFlow human papillomavirus assay. |
| Follow‐up | |
| Notes |
Wu 2010.
| Clinical features and settings | |
| Participants | Women participating in a primary screening trial. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test: APTIMA hrHPV RNA assay. |
| Follow‐up | |
| Notes | Data requested from authors separately for women with ASC‐US and for women with LSIL. |
Ziemke 2012.
| Clinical features and settings | |
| Participants | Women with LSIL. |
| Study design | |
| Target condition and reference standard(s) | |
| Index and comparator tests | Index test: HC2. Comparator test: p16/Ki67 cytoimmunocytochemistry. |
| Follow‐up | |
| Notes |
Differences between protocol and review
The original review protocol also proposed to evaluate the accuracy of HC2 triage of women with ASC‐H cytology (atypical squamous cells where HSIL cannot be excluded) and AGC cytology (atypical glandular cells). Given the large quantity and complexity of studies addressing triage of ASCUS and LSIL, we preferred to focus on the main triage groups in this review. Triage of ASC‐H and AGC, and also triage of ASCUS and LSIL, with other HPV assays and molecular markers will be the target of a future, separate meta‐analysis.
Contributions of authors
Conception of the systematic review: M Arbyn, F Buntinx, P Martin‐Hirsch, E Paraskevaidis and W Prendiville. Study design: M Arbyn and F Buntinx. Retrieval of references: M Arbyn, C Simoens, J Roelens and P Martin‐Hirsch. Checking eligibility of references: M Arbyn, C Simoens and J Roelens. Extraction of data: M Arbyn, C Simoens and J Roelens. Methodological support: F Buntinx. Statistical analysis: M Arbyn and J Roelens. Writing of the protocol: M Arbyn, C Simoens. Critical review of the protocol: P Martin‐Hirsch, E Paraskevaidis and W Prendiville.
Sources of support
Internal sources
-
Scientific Institute of Public Health, Brussels, Belgium
Bibliographic support to obtain literature references, secretarial and logistic support in organising contacts and meetings with coauthors, and to store and sort bibliographic references
External sources
-
Gynaecological Cancer Cochrane Collaboration Review Group, UK
Financial support to conduct four Cochrane reviews
-
European Comission: DG Sanco (Luxembourg, Grand‐Duchy of Luxembourg) and 7th Framework Programme of DG Research (Brussels, Belgium
Financial support received via the ECCG Network (European Cooperation on development and implementation of Cancer screening and prevention Guidelines) from DG SANCO: grant number 2006322 and via the PREHDICT Network, from FP7: grant number 242061.
-
DWTC/SSTC (Federal Service for Science, Technology and Culture), Belgium
Financial support for recruitment and training of a statistician
-
IWT (Institute for the Promotion of Innovation by Science and Technology in Flanders) ‐ project number 060081, Belgium
Financial support to collect data for mathematical modelling of HPV infection (natural history, cost‐effectiveness of HPV screening and vaccination)
-
Belgian Foundation Against Cancer, Belgium
Financial support to conduct methodological research on evaluation of emerging screening methods and to continue systematic reviews on cervical cancer prevention methods
-
IARC (International Agency for Research on Cancer), Lyon, France
Financial support to update recommendations on cancer screening in the European Code Against Cancer and to develop criteria for evaluating the diagnostic accuracy of new cancer screening tests
Declarations of interest
All review authors declare that they do not have any conflicts of interest regarding the test assays discussed in the systematic review.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Andersson 2005 {published and unpublished data}
- Andersson S, Dillner L, Elfgren K, Mints M, Persson M, Rylander E. A comparison of the human papillomavirus test and Papanicolaou smear as a second screening method for women with minor cytological abnormalities. Acta Obstetricia et Gynecologica Scandinavica 2005;84(10):996-1000. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bergeron 2000 {published data only}
- Bergeron C, Jeannel D, Poveda J, Cassonnet P, Orth G. Human papillomavirus testing in women with mild cytologic atypia. Obstetrics and Gynecology 2000;95:821-7. [DOI] [PubMed] [Google Scholar]
Bergeron 2006 {published data only}
- Bergeron C, Cas F, Fagnani F, Contrepas A, Wadler R, Poveda JD. Assessment of human papillomavirus testing on liquid-based CYTO-screen system for women with atypical squamous cells of undetermined significance. Effect of age. Gynécologie, Obstétrique and Fertilité 2006;34:312-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Castle 2010a {published and unpublished data}
- Castle PE, Fetterman B, Thomas CJ, Shaber R, Poitras N, Lorey T, et al. The age-specific relationships of abnormal cytology and human papillomavirus DNA results to the risk of cervical precancer and cancer. Obstetrics and Gynecology 2010;116:76-84. [DOI] [PubMed] [Google Scholar]
Cattani 2009 {published data only}
- Cattani P, Zannoni GF, Ricci C, D'Onghia S, Trivellizzi IN, Di Franco A, et al. Clinical performance of human papillomavirus E6 and E7 mRNA testing for high-grade lesions of the cervix. Journal of Clinical Microbiology 2009;47:3895-3901. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2005b {published data only}
- Chen HS, Yang YC, Su TH, Wang TY, Huang YW. Human papillomavirus testing (Hybrid Capture II) to detect high-grade cervical intraepithelial neoplasia in women with mildly abnormal Papanicolaou results. Taiwanese Journal of Obstetrics and Gynecology 2005;44:252-7. [MEDLINE: ] [Google Scholar]
Cuschieri 2007 {published data only}
- Cuschieri KS, Grahamb C, Moorea C, Cubie HA. Human Papillomavirus testing for the management of low-grade cervical abnormalities in the UK—Influence of age and testing strategy. Journal of Clinical Virology 2007;38(1):14-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cuzick 2003 {published data only}
- Cuzick J, Szarewski A, Cubie H, Hulman G, Kitchener H, Luesley D, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet 2003;362:1871-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dalla Palma 2005 {published data only}
- Dalla Palma P, Pojer A, Girlando S. HPV triage of women with atypical squamous cells of undetermined significance: a 3 year experience in an Italian organized programme. Cytopathology 2005;16:22-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Davis‐Devine 2005 {published data only}
- Davis-Devine S, Day SJ, Freund GG. Test performance comparison of inform HPV and hybrid capture 2 high-risk HPV DNA tests using the SurePath liquid-based Pap test as the collection method. American Journal of Clinical Pathology 2005;124:24-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Francesco 2008 {published data only}
- De Francesco MA, Gargiulo F, Schreiber C, Ciravolo G, Salinaro F, Manca N. Comparison of the AMPLICOR human papillomavirus test and the hybrid capture 2 assay for detection of high-risk human papillomavirus in women with abnormal PAP smear. Journal of Virological Methods 2008;147:10-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Del Mistro 2010 {published data only}
- Del Mistro A, Frayle-Salamanca H, Trevisan R, Matteucci M, Pinarello A, Zambenedetti P, et al. Triage of women with atypical squamous cells of undetermined significance (ASC-US): Results of an Italian multicentric study. Gynecologic Oncology 2010;117:77-88. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Denton 2010 {published data only}
- Denton KJ, Bergeron C, Klement P Trunk MJ, Keller T, Ridder R. The sensitivity and specificity of p16(INK4a) cytology vs HPV testing for detecting high-grade cervical disease in the triage of ASC-US and LSIL pap cytology results. American Journal of Clinical Pathology 2010;134:12-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Giovannelli 2005 {published data only}
- Giovannelli L, Capra G, Lama A, Bustinto T, Genco A, Valenti FM. Atypical squamous cells of undetermined significance-favour reactive compared to atypical squamous cells of undetermined significance-favour dysplasia: association with cervical intraepithelial lesions and human papillomavirus infection. Journal of Clinical Virology 2005;33(4):281-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyot 2003 {published and unpublished data}
- Guyot A, Karim S, Kyi MS, Fox J. Evaluation of adjunctive HPV testing by hybrid capture II(R) in women with minor cytological abnormalities for the diagnosis of CIN2/3 and cost comparison with colposcopy. BMC Infectious Diseases [electronic resource] 2003;3(23):1-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holladay 2006 {published data only}
- Holladay EB, Logan S, Arnold J, Knesel B, Smith GD. A comparison of the clinical utility of p16(INK4a) immunolocalization with the presence of human papillomavirus by hybrid capture 2 for the detection of cervical dysplasia/neoplasia. Cancer 2006;108:451-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Huang 2009 {published data only}
- Huang S, Erickson B, Tang N, Mak WB, Salituro J, Robinson J, et al. Clinical performance of Abbott RealTime High Risk HPV test for detection of high-grade cervical intraepithelial neoplasia in women with abnormal cytology. Journal of Clinical Virology 2009;45(Suppl 1):S19-23. [DOI] [PubMed] [Google Scholar]
Kelly 2006 {published data only}
- Kelly D, Kincaid E, Fansler Z, Rosenthal DL, Clark DP. Detection of cervical high-grade squamous intraepithelial lesions from cytologic samples using a novel immunocytochemical assay (ProExtrade mark C). Cancer 2006;108(6):494-500. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kiatpongsan 2006 {published data only}
- Kiatpongsan S, Niruthisard S, Mutirangura A, Trivijitsilp P, Vasuratna A, Chaithongwongwatthana S, et al. Role of human papillomavirus DNA testing in management of women with atypical squamous cells of undetermined significance. International Journal of Gynecological Cancer 2006;16(1):262-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kulasingam 2002 {published and unpublished data}
- Kulasingam SL, Hughes JP, Kiviat NB, Mao C, Weiss NS, Kuypers JM, et al. Evaluation of human papillomavirus testing in primary screening cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002;288(14):1749-57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lee 2001 {published data only}
- Lee NW, Kim D, Park JT, Kim A. Is the human papillomavirus test in combination with the Papanicolaou test useful for management of patients with diagnosis of atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesions? Archives of Pathology and Laboratory Medicine 2001;125(11):1453-7. [DOI] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee JK, Kim MK, Song SH, Hong JH, Min KJ, Kim JH, et al. Comparison of human papillomavirus detection and typing by Hybrid Capture 2, Llnear Array, DNA Chip, and cycle sequencing in cervical swab samples. International journal of Gynecological Cancer 2009;19:266-72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lonky 2003 {published data only}
- Lonky NM, Felix JC, Naidu YM, Wolde-Tsadik G. Triage of atypical squamous cells of undetermined significance with Hybrid Capture II: colposcopy and histologic human papillomavirus correlation. Obstetrics and Gynecology 2003;101(3):481-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lytwyn 2000 {published and unpublished data}
- Lytwyn A, Sellors JW, Mahony JB. Comparison of human papillomavirus DNA testing and repeat Papanicolaou test in women with low-grade cervical cytologic abnormalities: a randomized trial. Canadian Medical Association Journal 2000;163:701-7. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Manos 1999 {published data only}
- Manos MM, Kinney WK, Hurley LB, Sherman ME, Shieh-Ngai J, Kurman RJ, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 1999;281:1605-10. [DOI] [PubMed] [Google Scholar]
Monsonego 2006 {published data only}
- Monsonego J, Pintos J, Semaille C, Beumont M, Dachez R, Zerat L, et al. Human papillomavirus testing improves the accuracy of colposcopy in detection of cervical intraepithelial neoplasia. International Journal of Gynecological Cancer 2006;16(2):591-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Monsonego 2008 {published and unpublished data}
- Monsonego J, Pollini G, Evrard MJ, Sednaoui P, Monfort L, Zerat L, et al. Detection of human papillomavirus genotypes among high-risk women: a comparison of Hybrid Capture and Llnear Array tests. Sexually Transmitted Diseases 2008;35:521-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morin 2001 {published data only}
- Morin C, Bariati C, Bouchard C, Fortier M, Roy M, Moore L, et al. Managing atypical squamous cells of undetermined significance in Papanicolaou smears. Journal of Reproductive Medicine 2001;46:799-805. [PubMed] [Google Scholar]
Nieh 2005 {published data only}
- Nieh S, Chen SF, Chu TY, Lai HC, Lin YS, Fu E, et al. Is p16(INK4A) expression more useful than human papillomavirus test to determine the outcome of atypical squamous cells of undetermined significance-categorized Pap smear? A comparative analysis using abnormal cervical smears with follow-up biopsies. Gynecologic Oncology 2005;97:35-40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ordi 2003 {published data only}
- Ordi J, Puig-Tintore LM, Torne A, Sanz S, Esteve R, Romagosa C, et al. Contribution of high risk human papillomavirus testing to the management of premalignant and malignant lesions of the uterine cervix. Medicina Clínica 2003;121(12):441-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pretorius 2002 {published and unpublished data}
- Pretorius RG, Peterson P, Novak S, Azizi F, Sadeghi M, Lorincz AT. Comparison of two signal-amplification DNA tests for high-risk HPV as an aid to colposcopy. Journal of Reproductive Medicine 2002;47(4):290-6. [MEDLINE: ] [PubMed] [Google Scholar]
Rebello 2001 {published data only}
- Rebello G, Hallam N, Smart G, Farquharson D, McCafferty J. Human papillomavirus testing and the management of women with mildly abnormal cervical smears: an observational study. BMJ 2000;322:894-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ronco 2007 {published data only}
- Ronco G, Cuzick J, Segnan N, Brezzi S, Carozzi F, Folicaldi S, et al. HPV triage for low grade (L-SIL) cytology is appropriate for women over 35 in mass cervical cancer screening using liquid based cytology. European Journal of Cancer 2007;43:476-80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sherman 2002 {published and unpublished data}
- Sherman ME, Schiffman M, Cox JT. Effects of age and human papilloma viral load on colposcopy triage: data from the randomised atypical squamous cells of undetermined significance/low-grade intraepithelial lesion triage study (ALTS). Journal of the National Cancer Institute 2002;94:102-7. [DOI] [PubMed] [Google Scholar]
Shlay 2000 {published data only}
- Shlay JC, Dunn T, Byers T, Baron AE, Douglas JM Jr. Prediction of cervical intraepithelial neoplasia grade 2-3 using risk assessment and human papillomavirus testing in women with atypia on Papanicolaou smears. Obstetrics and Gynecology 2000;96:410-6. [DOI] [PubMed] [Google Scholar]
Siddiqui 2008 {published data only}
- Siddiqui MT, Hornaman K, Cohen C, Nassar A. ProEx C immunocytochemistry and high-risk human papillomavirus DNA testing in Papanicolaou tests with atypical squamous cell (ASC-US) cytology: correlation study with histologic biopsy. Archives of Pathology & Laboratory Medicine 2008;132:1648-52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Silverloo 2009 {published data only}
- Silverloo I, Andrae B, Wilander E. Value of high-risk HPV-DNA testing in the triage of ASCUS. Acta Obstetricia et Gynecologica Scandinavica 2009;88:1006-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Solomon 2001 {published data only}
- Solomon D, Schiffman M, Tarone B. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance (ASCUS): baseline results from a randomized trial. Journal of the National Cancer Institute 2001;93:293-9. [DOI] [PubMed] [Google Scholar]
Szarewski 2008 {published and unpublished data}
- Szarewski A, Ambroisine L, Cadman L, Austin J, Ho L, Terry G, et al. Comparison of predictors for high-grade cervical intraepithelial neoplasia in women with abnormal smears. Cancer Epidemiology, Biomarkers & Prevention 2008;17:3033-43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Voss 2010 {published data only}
- Voss JS, Kipp BR, Campion MB, Sokolova IA, Henry MR, Halling KC, et al. Assessment of fluorescence in situ hybridization and hybrid capture 2 analyses of cervical cytology specimens diagnosed as low grade squamous intraepithelial lesion for the detection of high grade cervical intraepithelial neoplasia. Analytical and Quantitative Cytology and Histology 2010;32:121-30. [MEDLINE: ] [PubMed] [Google Scholar]
Wensveen 2003 {published data only}
- Wensveen C, Kagie M, Veldhuizen R, De Groot C, Denny L, Zwinderman K, et al. Detection of cervical intraepithelial neoplasia in women with atypical squamous or glandular cells of undetermined significance cytology: a prospective study. Acta Obstetricia et Gynecologica Scandinavica 2003;82(9):883-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
You 2007 {published data only}
- You K, Liang X, Qin F, Guo Y, Geng L. High-risk human papillomavirus DNA testing and high-grade cervical intraepithelial lesions. The Australian & New Zealand Journal of Obstetrics & Gynaecology 2007;47:141-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zielinski 2001 {published data only}
- Zielinski GD, Snijders PJF, Rozendaal L, Voorhorst FJ, Runsink AP, De Schipper FA, et al. High-risk HPV testing in women with borderline and mild dyskaryosis: long-term follow-up data and clinical relevance. The Journal of Pathology 2001;195:300-6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adam 1998 {published data only}
- Adam E, Kaufman RH, Berkova Z. Is human papillomavirus testing an effective triage method for detection of high-grade (grade 2 or 3) cervical intraepithelial neoplasia? Obstetrics and Gynecology 1998;92:601-7. [DOI] [PubMed] [Google Scholar]
Aerssens 2009 {published data only}
- Aerssens A, Claeys P, Beerens E, Garcia A, Weyers S, Van Renterghem L, et al. Prediction of recurrent disease by cytology and HPV testing after treatment of cervical intraepithelial neoplasia. Cytopathology 2009;20:27-35. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ahmed 2008 {published data only}
- Ahmed AS, Goumalatsos G, Akbar N, Lawton FG, Savvas M. Outcome analysis of 4 years' follow-up of patients referred for colposcopy with one smear showing mild dyskaryosis. Cytopathology 2008;19:94-105. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Alameda 2010 {published data only}
- Alameda F, Alameda F, Piujan L, Lioveras B, Bellosillo B, Larrazabal F, et al. The value of p16 in ASCUS cases: A retrospective study using frozen cytologic material. Diagnostic Cytopathology 2011;39:110-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Allen 2009 {published data only}
- Allen GL, Klobocista MM, Sugarman S, Gravel K, Feldman D, Schnatz PF. Prevalence of high-risk human papillomavirus in an inner-city population with atypical squamous cells of undetermined significance. Journal of Lower Genital Tract Disease 2009;13:63-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Antonishyn 2009 {published data only}
- Antonishyn NA, Horsman GB, Kelln RA, Severini A. Human papillomavirus typing and viral gene expression analysis for the triage of women with abnormal results from Papanicolaou test smears to colposcopy. Archives of Pathology & Laboratory Medicine 2009;133:1577-86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arbyn 2010 {published data only}
- Arbyn M, Martin-Hirsch P, Wentzensen N. Human papillomavirus-based triage of women showing a cervical cytology result of borderline or mild dyskaryosis. British Journal of Obstetrics and Gynaecology 2010;117:641-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Armah 2009 {published data only}
- Armah H, Austin RM, Dabbs D, Zhao C. Follow-up findings for women with human papillomavirus-positive and atypical squamous cells of undetermined significance screening test results in a large women's hospital practice. Archives of Pathology & Laboratory Medicine 2009;133:1426-30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Atkins 2006 {published data only}
- Atkins KA, Jeronimo J, Stoler MH. Description of patients with squamous cell carcinoma in the atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesion triage study. Cancer 2006;5:212-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bais 2005 {published data only}
- Bais AG, Rebolj M, Snijders PJ, De Schipper FA, Meulen DA, Verheijen RH, et al. Triage using HPV-testing in persistent borderline and mildly dyskaryotic smears: proposal for new guidelines. International Journal of Cancer 2005;116(1):122-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baseman 2008 {published data only}
- Baseman JG, Kulasingam SL, Harris TG, Hughes JP, Kiviat NB, Mao C, et al. Evaluation of primary cervical cancer screening with an oncogenic human papillomavirus DNA test and cervical cytologic findings among women who attended family planning clinics in the United States. American Journal of Obstetrics and Gynecology 2008;199:26-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bavin 1993 {published data only}
- Bavin PJ, Giles JA, Deery A. Use of semi-quantitative PCR for human papillomavirus DNA type 16 to identify women with high grade cervical disease in a population presenting with a mildly dyskaryotic smear report. British Journal of Cancer 1993;67(3):602-5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beccati 2008 {published data only}
- Beccati MD, Buriani C, Pedriali M, Rossi S, Nenci I. Quantitative detection of molecular markers ProEx C (minichromosome maintenance protein 2 and topoisomerase IIa) and MIB-1 in liquid-based cervical squamous cell cytology. Cancer 2008;114:196-203. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bellinson 2009 {published data only}
- Belinson JL, Pretorius RG, Enerson C, Garcia F, Cruz EP, Belinson SE, et al. The Mexican Cervical Cancer Screening Trial: self-sampling for human papillomavirus with unaided visual inspection as a secondary screen. International Journal of Gynecological Cancer 2009;19:27-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bello 2009 {published data only}
- Bello BD, Spinillo A, Alberizzi P, Cesari S, Gardella B, D'Ambrosio G, et al. Cervical infections by multiple human papillomavirus (HPV) genotypes: Prevalence and impact on the risk of precancerous epithelial lesions. Journal of Medical Virology 2009;81:703-12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bentley 2006 {published data only}
- Bentley E, Cotton SC, Cruickshank ME, Duncan I, Gray NM, Jenkins D, et al. Refining the management of low-grade cervical abnormalities in the UK National Health Service and defining the potential for human papillomavirus testing: a commentary on emerging evidence. Journal of Lower Genital Tract Diseases 2006;10(1):26-38. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berkhof 2006a {published data only}
- Berkhof J, Bulkmans NW, Bleeker MC, Bulk S, Snijders PJ, Voorhorst FJ, et al. Human papillomavirus type-specific 18-month risk of high-grade cervical intraepithelial neoplasia in women with a normal or borderline/mildly dyskaryotic smear. Cancer Epidemiology, Biomarkers & Prevention 2006;15:1268-73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berkhof 2006b {published data only}
- Berkhof J, Bruijne MC, Zielinski GD, Bulkmans NW, Rozendaal L, Snijders PJ, et al. Evaluation of cervical screening strategies with adjunct high-risk human papillomavirus testing for women with borderline or mild dyskaryosis. International Journal of Cancer 2006;118:1759-68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bewtra 2005 {published data only}
- Bewtra C, Xie Q, Soundararajan S, Gatalica Z, Hatcher L. Genital human papillomavirus testing by in situ hybridization in liquid atypical cytologic materials and follow-up biopsies. Acta Cytologica 2005;49:127-31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Biu 2009 {published data only}
- Biu H, Zhao J, Li KM, Liao QP. [The significance of HPV-DNA genotyping assays in the ASC]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2009;23:296-8. [MEDLINE: ] [PubMed] [Google Scholar]
Bjerre 2008 {published data only}
- Bjerre P, Silfverdal L, Dillner L, Hagmar B, Edvardsson H, Dillner J, et al. A randomized trial of basing treatment on human papillomavirus and/or cytology results in low-grade cervical lesion triage. American Journal of Obstetrics and Gynecology 2008;199:24-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boardman 2005 {published data only}
- Boardman LA, Stanko C, Weitzen S, Sung J. Atypical squamous cells of undetermined significance: human papillomavirus testing in adolescents. Obstetrics and Gynecology 2005;105:741-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boardman 2006 {published data only}
- Boardman LA, Weitzen S, Stanko C. Atypical squamous cells of undetermined significance, human papillomavirus, and cervical intraepithelial neoplasia 2 or 3 in adolescents: ASC-US, age, and high-grade cervical neoplasia. Journal of Lower Genital Tract Disease 2006;10:140-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bohmer 2002 {published data only}
- Bohmer G, Petry KU, Iftner T, Brummer O, Gunter HH, Flemming P, et al. Detection of human papillomavirus DNA using hybrid capture does not allow sufficient triaging of recurrent atypical pap smear classified as Pap III D. Zentralblatt für Gynäkologie 2002;124(2):111-5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bollen 1997 {published data only}
- Bollen LJ, Tjong-A-Hung SP, Van der Velden J, Brouwer K, Mol BW, ten Kate FJ, et al. Human papillomavirus deoxyribonucleic acid detection in mildly or moderately dysplastic smears: a possible method for selecting patients for colposcopy. American Journal of Obstetrics and Gynecology 1997;177:548-53. [DOI] [PubMed] [Google Scholar]
Botezatu 2009 {published data only}
- Botezatu A, Socolov D, Goia CD, Iancu IV, Ungureanu C, Huica I, et al. The relationship between HPV16 and HPV18 viral load and cervical lesions progression. Roumanian Archives of Microbiology and Immunology 2009;68:175-82. [MEDLINE: ] [PubMed] [Google Scholar]
Boulanger 2006 {published data only}
- Boulanger JC, Sevestre H. [ASCUS: an update]. Gynécologie, Obstétrique & Fertilité 2006;34:44-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brismar 2009 {published data only}
- Brismar-Wendel S, Froberg M, Hjerpe A, Andersson S, Johansson B. Age-specific prevalence of HPV genotypes in cervical cytology samples with equivocal or low-grade lesions. British Journal of Cancer 2009;101:511-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2009 {published data only}
- Brown CR, Leon ML, Munoz K, Fagioni A, Amador LG, Frain B, et al. Human papillomavirus infection and its association with cervical dysplasia in Ecuadorian women attending a private cancer screening clinic. Brazilian Journal of Medical and Biological Research 2009;42:629-36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bruner 2004 {published data only}
- Bruner KS, Davey DD. ASC-US and HPV testing in women aged 40 years and over. Diagnostic Cytopathology 2004;31(5):358-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burgis 2009 {published data only}
- Burgis JT, Brown J, Menon S, Bacon JL. Cervical cytology screening and management of abnormal cytology in adolescents. Journal of the South Carolina Medical Association 2009;105:16-9. [MEDLINE: ] [PubMed] [Google Scholar]
Carozzi 2005 {published data only}
- Carozzi FM, Confortini M, Cecchini S, Bisanzi S, Cariaggi MP, Pontenani G, et al. Triage with human papillomavirus testing of woman with cytologic abnormalities prompting referral for colposcopy assessment.. Cancer Cytopathology 2005;105:2-7. [DOI] [PubMed] [Google Scholar]
Carozzi 2006 {published data only}
- Carozzi F, Cecchini S, Confortini M, Becattini V, Cariaggi MP, Pontenani G, et al. Role of P16 (INK4a) expression in identifying CIN2 or more severe lesions among HPV-positive patients referred for colposcopy after abnormal cytology. Cancer 2006;108:119-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carozzi 2008 {published data only}
- Carozzi F, Confortini M, Palma PD, Del Mistro A, Gillio-Tos A, De Marco L, et al. Use of p16-INK4A over expression to increase the specificity of human papillomavirus testing: a nested sub study of the NTCC randomised controlled trial. Lancet Oncology 2008;9:937-45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carvalho 2010 {published data only}
- Carvalho NO, Castillo DM, Perone C, Januario JN, Melo VH, Brasileiro FG. Comparison of HPV genotyping by type-specific PCR and sequencing. Memórias do Instituto Oswaldo Cruz 2010;105:73-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Castle 2008 {published data only}
- Castle PE, Gravitt PE, Solomon D, Wheeler CM, Schiffman M. Comparison of Linear Array and Line Blot assay for detection of human papillomavirus and diagnosis of cervical precancer and cancer in the atypical squamous cell of undetermined significance and low-grade squamous intraepithelial lesion triage study. Journal of Clinical Microbiology 2008;46:109-17. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castle 2009a {published data only}
- Castle PE, Rodriguez AC, Burk RD, Herrero R, Hildesheim A, Solomon D, et al. Neither one-time negative screening tests nor negative colposcopy provides absolute reassurance against cervical cancer. International Journal of Cancer 2009;125:1649-56. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castle 2009b {published data only}
- Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Five-year experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstetrics and Gynecology 2009;113:595-600. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castle 2010b {published data only}
- Castle PE, Schiffman M, Wheeler CM, Wentzensen N, Gravitt PE. Impact of improved classification on the association of human papillomavirus with cervical precancer. American Journal of Epidemiology 2010;171:155-63. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Castle 2010c {published data only}
- Castle PE, Schiffman M, Wheeler CM, Wentzensen N, Gravitt PE. Human papillomavirus genotypes in cervical intraepithelial neoplasia grade 3. Cancer Epidemiology, Biomarkers & Prevention 2010;19:1675-81. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaiwongkot 2007 {published data only}
- Chaiwongkot A, Pientong C, Ekalaksananan T, Kongyingyoes B, Thinkhamrop J, Yuenyao P, et al. Evaluation of primers and PCR performance on HPV DNA screening in normal and low grade abnormal cervical cells. Asian Pacific Journal of Cancer Prevention 2007;8:279-82. [MEDLINE: ] [PubMed] [Google Scholar]
Chen 2005a {published data only}
- Chen SF, Yang SF, Chu TY, Lai HC, Lin YW, Bai CY et al. Which test is a better strategy to determine the outcome of atypical glandular cell-categorized Pap smears? Immunocytochemical p16INK4A expression or human papillomavirus test--a retrospective cohort study. Gynecologic Oncology 2005;99(3):578-84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chesebro 1997 {published data only}
- Chesebro MJ, Everett WD, Lorincz AT. High-risk human papilloma virus testing of women with cytological low-grade squamous intraepithelial lesions. Journal of Lower Genital Tract Disease 1997;1:234-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cheung 2010 {published data only}
- Cheung AN, Tsun KL, Ng KM, Szeto E, Siu MK, Wong ES, et al. P634A4 and TAp73 immunocytochemistry in liquid-based cervical cytology--potential biomarkers for diagnosis and progress prediction of cervical neoplasia. Modern Pathology 2010;23:559-66. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chivukula 2006 {published data only}
- Chivukula M, Shidham V. ASC-H in Pap test- definitive categorization of cytomorphological spectrum. CytoJournal [electronic resource] 2006;3(1):1-14. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Conesa‐Zamora 2010 {published data only}
- Conesa-Zamora P, Santaclara V, Gadea-Ninoles E, Ortiz-Reina S, Perez-Guillermo M. Association of polymorphism in FcGR3A gene and progression of low-grade precursor lesions of cervical carcinoma. Human Immunology 2010;71:314-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Constandinou‐Williams2010 {published data only}
- Constandinou-Williams C, Collins SI, Roberts S, Young LS, Woodman CB, Murray PG. Is human papillomavirus viral load a clinically useful predictive marker? A longitudinal study. Cancer Epidemiology, Biomarkers & Prevention 2010;19:832-7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coste 2003 {published data only}
- Coste J, Cochand-Priollet B, Cremoux P, Le Gales C, Cartier I, Molinie V, et al. Cross sectional study of conventional cervical smear, monolayer cytology, and human papillomavirus DNA testing for cervical cancer screening. BMJ 2003;326:733-6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cotton 2006 {published data only}
- Cotton SC, Sharp L, Little J, Duncan I, Alexander L, Cruickshank ME, et al. Trial of management of borderline and other low-grade abnormal smears (TOMBOLA): Trial design. Contemporary Clinical Trials 2006;27:449-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cotton 2010 {published data only}
- Cotton S, Sharp L, Little J, Cruickshank M, Seth R, Smart L, et al. The role of human papillomavirus testing in the management of women with low-grade abnormalities: multicenter randomised controlled trial. British Journal of Obstetrics and Gynaecology 2010;117:645-59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cox 1992 {published data only}
- Cox JT, Schiffman MH, Winzelberg AJ, Patterson JM. An evaluation of human papillomavirus testing as part of referral to colposcopy clinics. Obstetrics and Gynecology 1992;80:389-95. [PubMed] [Google Scholar]
Cox 1995 {published data only}
- Cox JT, Lorincz AT, Schiffman MH, Sherman ME, Cullen A, Kurman RJ. Human papillomavirus testing by hybrid capture appears to be useful in triaging women with a cytologic diagnosis of atypical squamous cells of undetermined significance. American Journal of Obstetrics and Gynecology 1995;172:946-54. [DOI] [PubMed] [Google Scholar]
Crabtree 2002 {published data only}
- Crabtree D, Unkraut A, Cozens D, Smith T, Lucas C, Pennington D, et al. Role for HPV testing in ASCUS: a cytologic-histologic correlation. Diagnostic Cytopathology 2002;27:382-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cricca 2009 {published data only}
- Cricca M, Venturoli S, Leo E, Costa S, Musiani M, Zerbini M. Molecular analysis of HPV 16 E6I/E6II spliced mRNAs and correlation with the viral physical state and the grade of the cervical lesion. Journal of Medical Virology 2009;81:1276-82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Del Mistro 2007 {published data only}
- Del Mistro A. ASC-US diagnosis and triage. Gynecologic Oncology 2007;107:S2-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Del Pino 2009 {published data only}
- Pino M, Garcia S, Fuste V, Alonso I, Fuste P, Torne A, et al. Value of p16(INK4a) as a marker of progression/regression in cervical intraepithelial neoplasia grade 1. American Journal of Obstetrics and Gynecology 2009;201:488. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Derchain 2004 {published data only}
- Derchain SF, Rabelo-Santos SH, Sarian LO, Zeferino LC, Oliveira Zambeli ER, do Amaral Westin MC, et al. Human papillomavirus DNA detection and histological findings in women referred for atypical glandular cells or adenocarcinoma in situ in their Pap smears. Gynecologic Oncology 2004;95(3):618-23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Diaz‐Montes 2007 {published data only}
- Diaz-Montes TP, Farinola MA, Zahurak ML, Bristow RE, Rosenthal DL. Clinical utility of atypical glandular cells (AGC) classification: cytohistologic comparison and relationship to HPV results. Gynecologic Oncology 2007;104:366-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Difurio 2010 {published data only}
- Difurio MJ, Mailhiot T, Sundborg MJ, Nauschuetz KK. Comparison of the clinical significance of the Papanicolaou test interpretations LSIL cannot rule out HSIL and ASC-H. Diagnostic Cytopathology 2010;38:313-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dockter 2009 {published data only}
- Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. Journal of Clinical Virology 2009;45:S55-S61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Duerr 2006 {published data only}
- Duerr A, Paramsothy P, Jamieson DJ, Heilig CM, Klein RS, Cu-Uvin S, et al. Effect of HIV infection on atypical squamous cells of undetermined significance. Clinical Infectious Diseases 2006;42:855-61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Duncan 2005 {published data only}
- Duncan LD, Jacob SV. Atypical squamous cells, cannot exclude a high-grade squamous intraepithelial lesion: the practice experience of a hospital-based reference laboratory with this new Bethesda system diagnostic category. Diagnostic Cytopathology 2005;32:243-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Einstein 2010 {published data only}
- Einstein MH, Martens MG, Garcia FAR, Ferris DG, Mitchell AL, Day SP, et al. Clinical validation of the Cervista HPV HR and 16/18 genotyping tests for use in women with ASC-US cytology. Gynecologic Oncology 2010;118:116-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ekalaksananan 2006 {published data only}
- Ekalaksananan T, Pientong C, Sriamporn S, Kongyingyoes B, Pengsa P, Kleebkaow P, et al. Usefulness of combining testing for p16 protein and human papillomavirus (HPV) in cervical carcinoma screening. Gynecologic Oncology 2006;103:62-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eleuterio 2007 {published data only}
- Eleuterio J Jr, Giraldo PC, Goncalves AK, Cavalcante DI, Almeida Ferreira FV, Mesquita SM, et al. Prognostic markers of high-grade squamous intraepithelial lesions: the role of p16INK4a and high-risk human papillomavirus. Acta Obstetrica Gynecologica Scandinava 2007;86:94-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eltoum 2005 {published data only}
- Eltoum IA, Chhieng DC, Roberson J, McMillon D, Partridge EE. Reflex human papilloma virus infection testing detects the same proportion of cervical intraepithelial neoplasia grade 2-3 in young versus elderly women. Cancer 2005;105:194-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Evans 2006 {published data only}
- Evans MF, Adamson CS, Papillo JL, St John TL, Leiman G, Cooper K. Distribution of human papillomavirus types in ThinPrep Papanicolaou tests classified according to the Bethesda 2001 terminology and correlations with patient age and biopsy outcomes. Cancer 2006;106:1054-64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fadare 2009 {published data only}
- Fadare O, Rodriguez R. The significance of marked nuclear atypia in grade 1 cervical intraepithelial neoplasia. Human Pathology 2009;40:1487-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fait 1998 {published data only}
- Fait G, Daniel Y, Kupferminc MJ, Geva E, Ron IG, Lessing JB, et al. Does typing of human papillomavirus assist in the triage of women with repeated low-grade, cervical cytologic abnormalities? Gynecologic Oncology 1998;70:319-22. [DOI] [PubMed] [Google Scholar]
Fait 2000 {published data only}
- Fait G, Kupferminc MJ, Daniel Y, Lessing JB, Niv J, Bar-Am A. Contribution of human papillomavirus testing by hybrid capture in the triage of women with repeated abnormal pap smears before colposcopy referral.. Gynecologic Oncology 2000;79:177-80. [DOI] [PubMed] [Google Scholar]
Farag 2008 {published data only}
- Farag R, Redline R, Abdul-Karim FW. Value of combining HPV-DNA testing with follow-up Papanicolaou smear in patients with prior atypical squamous cells of undetermined significance. Acta Cytologica 2008;52:294-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Feng 2005 {published data only}
- Feng J, Husain M. Reflex high-risk human papillomavirus DNA testing (Hybrid Capture 2) of bloody ThinPrep specimens with atypical squamous cells of undetermined significance interpretation. Cancer 2005;105:452-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Feng 2007a {published data only}
- Feng J, Husain M. Outcomes of women with atypical squamous cells of undetermined significance and high-risk human papillomavirus DNA. Acta Cytologica 2007;51(5):730-4. [DOI] [PubMed] [Google Scholar]
Feng 2007b {published data only}
- Feng SY, Zhang YN, Liu JH, Liu JG, Yan M. [Expression of epidermal growth factor receptor and the correlation with HPV16/18 infection in cervical intraepithelial neoplasia and cervical carcinoma]. Zhonghua Zhong Liu Za Zhi 2007;29(10):759-63. [PubMed] [Google Scholar]
Feng 2008 {published data only}
- Feng J, Al Abbadi MA, Bandyopadhyay S, Salimnia H, Husain M. Significance of high-risk human papillomavirus DNA-positive atypical squamous cells of undetermined significance pap smears in perimenopausal and postmenopausal women. Acta Cytologica 2008;52(4):434-8. [DOI] [PubMed] [Google Scholar]
Ferenczy 1996 {published data only}
- Ferenczy A, Franco E, Arseneau J, Wright TC, Richart RM. Diagnostic performance of Hybrid Capture human papillomavirus deoxyribonucleic acid assay combined with liquid-based cytologic study. American Journal of Obstetrics and Gynecology 1996;175:651-6. [DOI] [PubMed] [Google Scholar]
Ferris 1998a {published data only}
- Ferris DG, Wright TC, Litaker MS, Richart RM, Lorincz AT, Sun XW, et al. Triage of women with ASCUS and LSIL on Pap smear reports: management by repeat Pap smear, HPV DNA testing, or colposcopy? The Journal of Family Practice 1998;46:125-34. [PubMed] [Google Scholar]
Ferris 1998b {published data only}
- Ferris DG, Wright TC, Litaker MS, Richart RM, Lorincz AT, Sun XW, et al. Comparison of two tests for detecting carcinogenic HPV in women with Papanicolaou smear reports of ASCUS and LSIL. The Journal of Family Practice 1998;46:136-41. [PubMed] [Google Scholar]
Fletcher 2009 {published data only}
- Fletcher AH, Wilkinson EJ, Knapik JA. Oncogenic human papillomavirus testing in an adolescent population with atypical squamous cells of undetermined significance. Journal of Lower Genital Tract Disease 2009;13(1):28-32. [DOI] [PubMed] [Google Scholar]
Fröberg 2008 {published data only}
- Fröberg M, Johansson B, Hjerpe A, Andersson S. Human papillomavirus "reflex" testing as a screening method in cases of minor cytological abnormalities. British Journal of Cancer 2008;99(4):563-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ge 2009 {published data only}
- Ge Y, Smith D, Schwartz MR, Mody DR. Image-guided ThinPrep Papanicolaou tests and co-testing with high-risk human papillomavirus in women aged 30 years and older in a low-risk private practice population. Cancer Cytopathology 2009;117(5):326-32. [DOI] [PubMed] [Google Scholar]
Getman 2009 {published data only}
- Getman D, Aiyer A, Dockter J, Giachetti C, Zhang F, Ginocchio CC. Efficiency of the APTIMA HPV Assay for detection of HPV RNA and DNA targets. Journal of Clinical Virology 2009;45 Suppl 1:S49-54. [DOI] [PubMed] [Google Scholar]
Goff 1993 {published data only}
- Goff BA, Muntz HG, Bell DA, Wertheim I, Rice LW. Human papillomavirus typing in patients with Papanicolaou smears showing squamous atypia. Gynecologic Oncology 1993;48:384-8. [DOI] [PubMed] [Google Scholar]
Griesser 2009 {published data only}
- Griesser H, Sander H, Walczak C, Hilfrich RA. HPV vaccine protein L1 predicts disease outcome of high-risk HPV+ early squamous dysplastic lesions. American Journal of Clinical Pathology 2009;132(6):840-5. [DOI] [PubMed] [Google Scholar]
Guarisi 2009 {published data only}
- Guarisi R, Sarian LO, Hammes LS, Longatto-Filho A, Derchain SF, Roteli-Martins C, et al. Smoking worsens the prognosis of mild abnormalities in cervical cytology. Acta Obstetricia et Gynecologica Scandinavica 2009;88(5):514-20. [DOI] [PubMed] [Google Scholar]
Guimaraes 2005 {published data only}
- Guimaraes MC, Goncalves MA, Soares CP, Bettini JS, Duarte RA, Soares EG. Immunohistochemical expression of p16INK4a and bcl-2 according to HPV type and to the progression of cervical squamous intraepithelial lesions. The Journal of Histochemistry and Cytochemistry: Official Journal of the Histochemistry Society 2005;53:509-16. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guo 2004 {published data only}
- Guo M, Hu L, Baliga M, He Z, Hughson MD. The predictive value of p16(INK4a) and hybrid capture 2 human papillomavirus testing for high-grade cervical intraepithelial neoplasia. American Journal of Clinical Pathology 2004;122(6):894-901. [DOI] [PubMed] [Google Scholar]
Guo 2007 {published data only}
- Guo M, Patel SJ, Chovanec M, Jan YJ, Tarco E, Bevers TB, et al. A human papillomavirus testing system in women with abnormal Pap results: a comparison study with follow-up biopsies. Acta Cytologica 2007;51(5):749-54. [DOI] [PubMed] [Google Scholar]
Guo 2008 {published data only}
- Guo M, Lin CY, Gong Y, Cogdell DE, Zhang W, Lin E, et al. Human papillomavirus genotyping for the eight oncogenic types can improve specificity of HPV testing in women with mildly abnormal Pap results. Modern Pathology 2008;21:1037-43. [DOI] [PubMed] [Google Scholar]
Gupta 2007 {published data only}
- Gupta S, Sodhani P, Chachra KL, Singh V, Sehgal A. Outcome of "Atypical squamous cells" in a cervical cytology screening program: implications for follow up in resource limited settings. Diagnostic Cytopathology 2007;35(11):677-80. [DOI] [PubMed] [Google Scholar]
Gupta 2010 {published data only}
- Gupta N, Srinivasan R, Nijhawan R, Rajwanshi A, Dey P, Suri V, et al. Atypical squamous cells and low-grade squamous intraepithelial lesion in cervical cytology: cytohistological correlation and implication for management in a low-resource setting. Cytopathology 2011;22(3):189-94. [DOI] [PubMed] [Google Scholar]
Gustavsson 2009 {published data only}
- Gustavsson I, Juko-Pecirep I, Backlund I, Wilander E, Gyllensten U. Comparison between the Hybrid Capture 2 and the hpVIR real-time PCR for detection of human papillomavirus in women with ASCUS or low grade dysplasia. Journal of Clinical Virology 2009;45(2):85-9. [DOI] [PubMed] [Google Scholar]
Halfon 2007 {published data only}
- Halfon P, Trepo E, Antoniotti G, Bernot C, Cart-Lamy P, Khiri H, et al. Prospective evaluation of the Hybrid Capture 2 and AMPLICOR human papillomavirus (HPV) tests for detection of 13 high-risk HPV genotypes in atypical squamous cells of uncertain significance. Journal of Clinical Microbiology 2007;45(2):313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halfon 2010a {published data only}
- Halfon P, Benmoura D, Agostini A, Khiri H, Martineau A, Penaranda G, et al. Relevance of HPV mRNA detection in a population of ASCUS plus women using the NucliSENS EasyQ HPV assay. Journal of Clinical Virology 2010;47(2):177-81. [DOI] [PubMed] [Google Scholar]
Halfon 2010b {published data only}
- Halfon P, Benmoura D, Khiri H, Penaranda G, Blanc B, Riggio D, et al. Comparison of the clinical performance of carcinogenic HPV typing of the Linear Array and Papillocheck HPV-screening assay. Journal of Clinicla Virology 2010;47(1):38-42. [DOI] [PubMed] [Google Scholar]
Hall 1996 {published data only}
- Hall S, Lorincz A, Shah F, Sherman ME, Abbas F, Paull G et al. Human papillomavirus DNA detection in cervical specimens by hybrid capture: correlation with cytologic and histologic diagnoses of squamous intraepithelial lesions of the cervix. Gynecologic Oncology 1996;62:353-9. [DOI] [PubMed] [Google Scholar]
Harvey 2009 {published data only}
- Harvey M, Stout S, Starkey CR, Hendren R, Holt S, Miller GC. The clinical performance of Invader technology and SurePath when detecting the presence of high-risk HPV cervical infection. Journal of Clinical Virology 2009;45 Suppl 1:S79-83. [DOI] [PubMed] [Google Scholar]
Hatch 1995 {published data only}
Herrington 1995 {published data only}
- Herrington CS, Evans MF, Hallam NF, Charnock FM, Gray W, McGee ODJ. Human papillomavirus status in the prediction of high-grade cervical intraepithelial neoplasia with persistent low-grade cervical cytological abnormalities. British Journal of Cancer 1995;71:206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 2003 {published data only}
- Ho L, Terry G, Londesborough P, Cuzick J, Lorenzato F, Singer A. Human papillomavirus DNA detection in the management of women with twice mildly abnormal cytological smears. Journal of Medical Virology 2003;69:118-21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Howard 2008 {published data only}
- Howard K, Salkeld G, McCaffery K, Irwig L. HPV triage testing or repeat Pap smear for the management of atypical squamous cells (ASCUS) on Pap smear: is there evidence of process utility? Health Economics 2008;17(5):593-605. [DOI] [PubMed] [Google Scholar]
Howell 2010 {published data only}
- Howell LP, Wilton M, Bishop J, Afify A. Living with uncertainty: equivocal Pap test results and the evolution of ASC terminology. Diagnostic Cytopathology 2010;38(3):221-32. [DOI] [PubMed] [Google Scholar]
Hughes 2002 {published data only}
- Hughes SA, Sun D, Gibson C, Bellerose B, Rushing L, Chen H, et al. Managing atypical squamous cells of undetermined significance (ASCUS): human papillomavirus testing, ASCUS subtyping, or follow-up cytology? American Journal of Obstetrics and Gynecology 2002;83:396-403. [DOI] [PubMed] [Google Scholar]
Irvin 2005 {published data only}
- Irvin W, Evans SR, Andersen W, Jazaeri A, Taylor P, Stoler M. The utility of HPV DNA triage in the management of cytological AGC. American Journal of Obstetrics and Gynecology 2005;193(2):559-65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Jamison 2009 {published data only}
- Jamison J, Wilson RT, Carson J. The evaluation of human papillomavirus genotyping in cervical liquid-based cytology specimens; using the Roche Linear Array HPV genotyping assay. Cytopathology 2009;20(4):242-8. [DOI] [PubMed] [Google Scholar]
Jarboe 2010 {published data only}
- Jarboe EA, Venkat P, Hirsch MS, Cibas ES, Crum CP, Garner EI. A weakly positive human papillomavirus Hybrid Capture II result correlates with a significantly lower risk of cervical intraepithelial neoplasia 2,3 after atypical squamous cells of undetermined significance cytology. Journal of Lower Genital Tract Disease 2010;14(3):174-8. [DOI] [PubMed] [Google Scholar]
Jeantet 2009 {published data only}
- Jeantet D, Schwarzmann F, Tromp J, Melchers WJ, Wurff AA, Oosterlaken T, et al. NucliSENS EasyQ HPV v1 test - testing for oncogenic activity of human papillomaviruses. Journal of Clinical Virology 2009;45(Suppl 1):S29-37. [DOI] [PubMed] [Google Scholar]
Jeronimo 2007 {published data only}
- Jeronimo J, Massad LS, Schiffman M. Visual appearance of the uterine cervix: correlation with human papillomavirus detection and type. American Journal of Obstetrics and Gynecology 2007;197(1):47-8. [DOI] [PubMed] [Google Scholar]
Julian 2006 {published data only}
- Julian TM, Dexeus S, Kitchener HC, Shier RM. Clinical question: ask the experts. A 45-year-old woman seeks treatment at your clinic with atypical squamous cells from Pap smear results. Journal of Lower Genital Tract Disease 2006;10(1):64-5. [DOI] [PubMed] [Google Scholar]
Kapeu 2009 {published data only}
- Kapeu AS, Luostarinen T, Jellum E, Dillner J, Hakama M, Koskela P, et al. Is smoking an independent risk factor for invasive cervical cancer? A nested case-control study within Nordic biobanks. American Journal of Epidemiology 2009;169(4):480-8. [DOI] [PubMed] [Google Scholar]
Kaufman 1997a {published data only}
- Kaufman RH, Adam E. Does typing of human papillomavirus assist in the triage of women with repeated low-grate, cervical cytologic abnormalities? Gynecologic Oncology 1997;70:317-8. [DOI] [PubMed] [Google Scholar]
Kaufman 1997b {published data only}
- Kaufman RH, Adam E, Icenogle J, Reeves WC. Human papillomavirus testing as triage for atypical squamous cells of undetermined significance and low-grade squamous intraepithelial lesions: Sensitivity, specificity, and cost-effectiveness. American Journal of Obstetrics and Gynecology 1997;177:930-6. [DOI] [PubMed] [Google Scholar]
Kavoussi 2009 {published data only}
- Kavoussi SK, Smith YR, Ernst SD, Quint EH. Cervical cancer screening with liquid cytology in women with developmental disabilities. Journal of Women's Health 2009;18(1):115-8. [DOI] [PubMed] [Google Scholar]
Keegan 2009 {published data only}
- Keegan H, Mc Inerney J, Pilkington L, Gronn P, Silva I, Karlsen F, et al. Comparison of HPV detection technologies: Hybrid capture 2, PreTect HPV-Proofer and analysis of HPV DNA viral load in HPV16, HPV18 and HPV33 E6/E7 mRNA positive specimens. Journal of Virological Methods 2009;155(1):61-6. [DOI] [PubMed] [Google Scholar]
Kendall 2005 {published data only}
- Kendall BS, Bush AC, Olsen CH, Zahn CM. Reflex high-risk human papillomavirus testing for women with atypical squamous cells of undetermined significance in cytologic smears: effects since implementation in a large clinical practice. American Journal of Clinical Pathology 2005;123:524-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kitchener 2008 {published data only}
- Kitchener H, Walker P, Nelson L, Hadwin R, Patnick J, Anthony G, et al. HPV testing as an adjunct to cytology in the follow up of women treated for cervical intraepithelial neoplasia. British Journal of Obstetrics and Gynaecology 2008;115:1001-7. [DOI] [PubMed] [Google Scholar]
Knoepp 2007 {published data only}
- Knoepp SM, Kuebler DL, Wilbur DC. Resolution of equivocal results with the Hybrid Capture II high-risk HPV DNA test: a cytologic/histologic review of 191 cases. Diagnostic Molecular Pathology 2007;16(3):125-9. [DOI] [PubMed] [Google Scholar]
Knoepp 2010 {published data only}
- Knoepp SM, Kuebler DL, Wilbur DC. Correlation between hybrid capture II high-risk human papillomavirus DNA test chemiluminescence intensity from cervical samples with follow-up histologic results: a cytologic/histologic review of 367 cases. Cancer Cytopathology 2010;118(4):209-17. [DOI] [PubMed] [Google Scholar]
Kong 2007 {published data only}
- Kong CS, Balzer BL, Troxell ML, Patterson BK, Longacre TA. p16INK4A immunohistochemistry is superior to HPV in situ hybridization for the detection of high-risk HPV in atypical squamous metaplasia. The American Journal of Surgical Pathology 2007;31(1):33-43. [DOI] [PubMed] [Google Scholar]
Kovacs 2009 {published data only}
- Kovacs K, Varnai AD, Bollmann M, Bankfalvi A, Szendy M, Speich N, et al. A 7.5-year prospective study of longer than 18 months type-specific human papillomavirus persistence in a routine cytology-based cervical screening population of about 31,000 women in West Germany. European Journal of Cancer Prevention 2009;18(4):307-15. [DOI] [PubMed] [Google Scholar]
Krambeck 2008 {published data only}
- Krambeck WM, Cadide RM, Dalmarco EM, Cordova CM. HPV detection and genotyping as an earlier approach in cervical cancer screening of the female genital tract. Clinical and Experimental Obstetrics & Gynecology 2008;35(3):175-8. [PubMed] [Google Scholar]
Krane 2004 {published data only}
- Krane JF, Lee KR, Sun D, Yuan L, Crum CP. Atypical glandular cells of undetermined significance. Outcome predictions based on human papillomavirus testing. American Journal of Clinical Pathology 2004;121(1):87-92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kulasingam 2006 {published data only}
- Kulasingam SL, Kim JJ, Lawrence WF, Mandelblatt JS, Myers ER, Schiffman M, et al. Cost-effectiveness analysis based on the atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesion Triage Study (ALTS). Journal of the National Cancer Institute 2006;98(2):92-100. [DOI] [PubMed] [Google Scholar]
Kumar 2007 {published data only}
- Kumar K, Iyer VK, Bhatla N, Kriplani A, Verma K. Comparative evaluation of smear cytology & hybrid capture II for the diagnosis of cervical cancer. The Indian Journal of Medical Research 2007;126(1):39-44. [PubMed] [Google Scholar]
Kuperman 2000 {published data only}
- Kuperman L, Krumholz BA. The triage of women with ASCUS cytology using human papillomavirus DNA testing. Journal of Lower Genital Tract Disease 2000;4(1):1-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Layfield 2005 {published data only}
- Layfield LJ, Qureshi MN. HPV DNA testing in the triage of atypical squamous cells of undetermined significance (ASCUS): cost comparison of two methods. Diagnostic Cytopathology 2005;33(2):138-43. [DOI] [PubMed] [Google Scholar]
Lee 2006 {published data only}
- Lee SJ, Jung KL, Lee JW, Song SY, Kim BG, Lee JH, et al. Analyses of atypical squamous cells refined by the 2001 Bethesda System: the distribution and clinical significance of follow-up management. International Journal of Gynecological Cancer 2006;16(2):664-9. [DOI] [PubMed] [Google Scholar]
Lee 2007 {published data only}
- Lee CY, Ng WK. A follow-up study of atypical squamous cells in gynecologic cytology using conventional Papanicolaou smears and liquid-based preparations: the impact of the Bethesda System 2001. American Journal of Clinical Ppathology 2007;127(4):548-55. [DOI] [PubMed] [Google Scholar]
Legood 2006 {published data only}
- Legood R, Gray A, Wolstenholme J, Moss S. Lifetime effects, costs, and cost effectiveness of testing for human papillomavirus to manage low grade cytological abnormalities: modelling study. BMJ 2006;332(7533):79-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leo 2009 {published data only}
- Leo E, Venturoli S, Cricca M, Musiani M, Zerbini M. High-throughput two-step LNA real time PCR assay for the quantitative detection and genotyping of HPV prognostic-risk groups. Journal of Clinical Virology 2009;45(4):304-10. [DOI] [PubMed] [Google Scholar]
Liang 2010 {published data only}
- Liang CW, Lin MC, Hsiao CH, Lin YT, Kuo KT. Papillary squamous intraepithelial lesions of the uterine cervix: human papillomavirus-dependent changes in cell cycle expression and cytologic features. Human Pathology 2010;41(3):326-35. [DOI] [PubMed] [Google Scholar]
Lim 2010 {published data only}
- Lim EH, Ng SL, Li JL, Chang AR, Ng J, Ilancheran A, et al. Cervical dysplasia: assessing methylation status (Methylight) of CCNA1, DAPK1, HS3ST2, PAX1 and TFPI2 to improve diagnostic accuracy. Gynecologic Oncology 2010;119(2):225-31. [DOI] [PubMed] [Google Scholar]
Liman 2005 {published data only}
- Liman AK, Giampoli EJ, Bonfiglio TA. Should women with atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion, receive reflex human papillomavirus-DNA testing? Cancer 2005;105:457-60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lin 2000 {published data only}
- Lin CT, Tseng CJ, Lai CH, Hsueh S, Huang HJ, Law KS. High-risk HPV DNA detection by Hybrid Capture II. An adjunctive test for mildly abnormal cytologic smears in women > or = 50 years of age. Journal of Reproductive Medicine 2000;45:345-50. [PubMed] [Google Scholar]
Lin 2010 {published data only}
- Lin CJ, Lai HC, Wang KH, Hsiung CA, Liu HW, Ding DC, et al. Testing for methylated PCDH10 or WT1 is superior to the HPV test in detecting severe neoplasms (CIN3 or greater) in the triage of ASC-US smear results. American Journal of Obstetrics and Gynecology 2011;204(1):e1-7. [DOI] [PubMed] [Google Scholar]
Longatto‐Filho 2005 {published data only}
- Longatto-Filho A, Utagawa ML, Shirata NK, Pereira SM, Namiyama GM, Kanamura CT, et al. Immunocytochemical expression of p16INK4A and Ki-67 in cytologically negative and equivocal pap smears positive for oncogenic human papillomavirus. International Journal of Gynecological Pathology 2005;24(2):118-24. [DOI] [PubMed] [Google Scholar]
Martin 2009 {published data only}
- Martin CM, Astbury K, McEvoy L, O'Toole S, Sheils O, O'Leary JJ. Gene expression profiling in cervical cancer: identification of novel markers for disease diagnosis and therapy. Methods in Molecular Biology 2009;511:333-59. [DOI] [PubMed] [Google Scholar]
Massad 2004 {published data only}
- Massad LS, Schneider MF, Watts DH, Strickler HD, Melnick S, Palefsky J, et al. HPV testing for triage of HIV-infected women with Papanicolaou smears read as atypical squamous cells of uncertain significance. Journal of Women's Health 2004;13(2):147-53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Massad 2009 {published data only}
- Massad LS, Jeronimo J, Katki HA, Schiffman M. The accuracy of colposcopic grading for detection of high-grade cervical intraepithelial neoplasia. Journal of Lower Genital Tract Disease 2009;13(3):137-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maucort‐Boulch 2010 {published data only}
- Maucort-Boulch D, Plummer M, Castle PE, Demuth F, Safaeian M, Wheeler CM, et al. Predictors of human papillomavirus persistence among women with equivocal or mildly abnormal cytology. International Journal of Cancer 2010;126(3):684-91. [DOI] [PubMed] [Google Scholar]
McHale 2007 {published data only}
- McHale MT, Souther J, Elkas JC, Monk BJ, Harrison TA. Is atypical squamous cells that cannot exclude high-grade squamous intraepithelial lesion clinically significant? Journal of Lower Genital Tract Disease 2007;11(2):86-9. [DOI] [PubMed] [Google Scholar]
Melnikow 2006 {published data only}
- Melnikow J, Birch S. Human papillomavirus triage of atypical squamous cells of undetermined significance: cost-effective, but at what cost? Journal of the National Cancer Institute 2006;98(2):82-3. [DOI] [PubMed] [Google Scholar]
Mesher 2010 {published data only}
- Mesher D, Szarewski A, Cadman L, Cubie H, Kitchener H, Luesley D, et al. Long-term follow-up of cervical disease in women screened by cytology and HPV testing: results from the HART study. British Journal of Cancer 2010;102:1405-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mirasoli 2009 {published data only}
- Mirasoli M, Guardigli M, Simoni P, Venturoli S, Ambretti S, Musiani M, et al. Multiplex chemiluminescence microscope imaging of P16(INK4A) and HPV DNA as biomarker of cervical neoplasia. Analytical and Bioanalytical Chemistry 2009;394(4):981-7. [DOI] [PubMed] [Google Scholar]
Mo 2008 {published data only}
- Mo LZ, Monnier-Benoit S, Kantelip B, Petitjean A, Riethmuller D, Pretet JL, et al. Comparison of AMPLICOR and Hybrid Capture II assays for high risk HPV detection in normal and abnormal liquid-based cytology: use of INNO-LiPA Genotyping assay to screen the discordant results. Journal of Clinical Virology 2008;41(2):104-10. [DOI] [PubMed] [Google Scholar]
Mokhtar 2008 {published data only}
- Mokhtar GA, Delatour NL, Assiri AH, Gilliatt MA, Senterman M, Islam S. Atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion: cytohistologic correlation study with diagnostic pitfalls. Acta Cytologica 2008;52(2):169-77. [DOI] [PubMed] [Google Scholar]
Molden 2005 {published data only}
- Molden T, Nygard JF, Kraus I, Karlsen F, Nygard M, Skare GB, et al. Predicting CIN2+ when detecting HPV mRNA and DNA by PreTect HPV-proofer and consensus PCR: a 2-year follow-up of women with ASCUS or LSIL pap smear. International Journal of Cancer 2005;114(6):973-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Monsonego 2005 {published data only}
- Monsonego J, Bohbot JM, Pollini G, Krawec C, Vincent C, Merignargues I, et al. Performance of the Roche AMPLICOR(R) Human papillomavirus (HPV) test in prediction of cervical intraepithelial neoplasia (CIN) in women with abnormal PAP smear. Gynecologic Oncology 2005;99:160-8. [DOI] [PubMed] [Google Scholar]
Monsonego 2008a {published data only}
- Monsonego J, Pollini G, Evrard MJ, Sednaoui P, Monfort L, Quinzat D, et al. Linear array genotyping and hybrid capture II assay in detecting human papillomavirus genotypes in women referred for colposcopy due to abnormal Papanicolaou smear. International Journal of STD & AIDS 2008;19(6):385-92. [DOI] [PubMed] [Google Scholar]
Moore 2010 {published data only}
- Moore G, Fetterman B, Cox JT, Poitras N, Lorey T, Kinney W, et al. Lessons from practice: risk of CIN 3 or cancer associated with an LSIL or HPV-positive ASC-US screening result in women aged 21 to 24. Journal of Lower Genital Tract Diseases 2010;14(2):97-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moscicki 2008 {published data only}
- Moscicki AB. Conservative management of adolescents with abnormal cytology and histology. Journal of the National Comprehensive Cancer Network 2008;6(1):101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mould 2000 {published data only}
- Mould TAJ, Singer A, Gallivan S. Quantitative detection of oncogenic HPV DNA using hybrid capture to triage borderline and mildly dyskaryostic Papanicolaou smears. European Journal of Gynaelogical Oncology 2000;21:245-8. [PubMed] [Google Scholar]
Nassar 2008 {published data only}
- Nassar A, O'Reilly K, Cohen C, Siddiqui MT. Comparison of p16(INK4A) and Hybrid Capture(R) 2 human papillomavirus testing as adjunctive tests in liquid-based gynecologic SurePathtrade mark preparations. Diagnostic Cytopathology 2008;36(3):142-8. [DOI] [PubMed] [Google Scholar]
Noel 2006 {published data only}
- Noel JC, Bucella D, Fayt I, Romero-Munoz MR, Simon P. Contribution of HPV sequences detection in cervical carcinoma screening. Annales de Pathologie 2006;26(5):389-96. [DOI] [PubMed] [Google Scholar]
Nomelini 2007 {published data only}
- Nomelini RS, Barcelos AC, Michelin MA, Adad SJ, Murta EF. Utilization of human papillomavirus testing for cervical cancer prevention in a university hospital. Cadernos de Saúde Pública 2007;23(6):1309-18. [DOI] [PubMed] [Google Scholar]
Nuovo 2008 {published data only}
- Nuovo GJ, Bartholomew D, Jung WW, Han IK, Um T, Grabarz DF, et al. Correlation of Pap smear, cervical biopsy, and clinical follow-up with an HPV typing microarray system. Diagnostic Molecular Pathology 2008;17(2):107-11. [DOI] [PubMed] [Google Scholar]
Nyirjesy 1998 {published data only}
- Nyirjesy I, Billingley FS, Forman MR. Evaluation of atypical and low-grade cervical cytology in private practice. Obstetrics and Gynecology 1998;92:601-7. [DOI] [PubMed] [Google Scholar]
Ogilvie 2010 {published data only}
- Ogilvie GS, Van Niekerk DJ, Krajden M, Martin RE, Ehlen TG, Ceballos K, et al. A randomized controlled trial of Human Papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer 2010;10(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliveira 2004 {published data only}
- Oliveira ER, Derchain SF, Rabelo-Santos SH, Westin MC, Zeferino LC, Campos EA, et al. Detection of high-risk human papillomavirus (HPV) DNA by Hybrid Capture II in women referred due to atypical glandular cells in the primary screening. Diagnostic Cytopathology 2004;31:19-22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Oliveira 2010 {published data only}
- Oliveira LH, Ferreira MD, Augusto EF, Melgaco FG, Santos LS, Cavalcanti SM, et al. Human papillomavirus genotypes in asymptomatic young women from public schools in Rio de Janeiro, Brazil. Revista da Sociedade Brasileira de Medicina Tropical 2010;43(1):4-8. [DOI] [PubMed] [Google Scholar]
Onuma 2006 {published data only}
- Onuma K, Saad RS, Kanbour-Shakir A, Kanbour AI, Dabbs DJ. Clinical implications of the diagnosis "atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion" in pregnant women. Cancer 2006;108(5):282-7. [DOI] [PubMed] [Google Scholar]
Ou 2007 {published data only}
- Ou H, Bian ML, Zhang XY, Chen QY, Li M, Chen Y, et al. [Application of high-risk human papillomavirus testing in women with abnormal cytology]. Zhongguo yi xue ke xue yuan xue bao. Acta Academiae Medicinae Sinicae 2007;29(5):608-11. [PubMed] [Google Scholar]
Ozsaran 2003 {published data only}
- Ozsaran AA, Dikmen Y, Akercan F, Zekioglu O, Terek MC, Mgoyi L, et al. The triage of squamous cell abnormalities of cervical cytology by human papilloma virus screening. European Journal of Gynaelogical Oncology 2003;24(6):535-8. [MEDLINE: ] [PubMed] [Google Scholar]
Pajtler 2010 {published data only}
- Pajtler M, Milicic-Juhas V, Milojkovic M, Topolovec Z, Curzik D, Mihaljevic I. Assessment of HPV DNA test value in management women with cytological findings of ASC-US, CIN1 and CIN2. Collegium Antropologicum 2010;34(1):81-6. [PubMed] [Google Scholar]
Pambuccian 2002 {published data only}
- Pambuccian SE, Lundeen SJ, Woronzoff K, Rohlader J, Kjeldahl K, Curran C, et al. Reflex HPV testing of ASCUS patients: initial experience with PCR-based HPV identification and typing. In: American Society of Cytopathology , editors(s). 50th Annual Scientific Meeting of American Society of Clinical Cytology. Salt Lake City, 2002. [MEDLINE: ]
Paternoster 2008 {published data only}
- Paternoster DM, Cester M, Resente C, Pascoli I, Nanhorngue K, Marchini F, et al. Human papilloma virus infection and cervical intraepithelial neoplasia in transplanted patients. Transplantation Proceedings 2008;40(6):1877-80. [DOI] [PubMed] [Google Scholar]
Patton 2008 {published data only}
- Patton AL, Duncan L, Bloom L, Phaneuf G, Zafar N. Atypical squamous cells, cannot exclude a high-grade intraepithelial lesion and its clinical significance in postmenopausal, pregnant, postpartum, and contraceptive-use patients. Cancer 2008;114(6):481-8. [DOI] [PubMed] [Google Scholar]
Peng 2006 {published data only}
- Peng Y, Wang HH. Impact of reflex HPV testing on interpretation and management of ThinPrep Pap tests. Diagnostic Cytopathology 2006;34(8):585-8. [DOI] [PubMed] [Google Scholar]
Perrons 2005 {published data only}
- Perrons C, Brink N, Jalal H, Watts P, Jelley R. The impact of high risk human papillomavirus testing in an inner London colposcopy clinic. Journal of Medical Virology 2005;76(4):576-82. [DOI] [PubMed] [Google Scholar]
Philips 2006 {published data only}
- Philips Z, Avis M, Whynes DK. Introducing HPV triage into the English cervical cancer screening program: consequences for participation. Women & Health 2006;43(2):17-34. [DOI] [PubMed] [Google Scholar]
Pisal 2003 {published data only}
- Pisal N, Sindos M, Chow C, Singer A. Triage by HPV-DNA testing: is it useful in women with persistent minor smear abnormalities? Acta Obstetricia et Gynecologica Scandinavica 2003;82(6):575-7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Poljak 2009 {published data only}
- Poljak M, Kovanda A, Kocjan BJ, Seme K, Jancar N, Vrtacnik-Bokal E. The Abbott RealTime High Risk HPV test: comparative evaluation of analytical specificity and clinical sensitivity for cervical carcinoma and CIN 3 lesions with the Hybrid Capture 2 HPV DNA test. Acta Dermatovenerologica Alpina, Panonica, et Adriatica 2009;18(3):94-103. [PubMed] [Google Scholar]
Pretet 2008 {published data only}
- Pretet JL, Jacquard AC, Saunier M, Clavel C, Dachez R, Gondry J, et al. Human papillomavirus genotype distribution in low-grade squamous intraepithelial lesions in France and comparison with CIN2/3 and invasive cervical cancer: the EDiTH III study. Gynecologic Oncology 2008;110(2):179-84. [DOI] [PubMed] [Google Scholar]
Quddus 2002 {published data only}
- Quddus MR, Zhang S, Sung CJ, Liu F, Neves T, Struminsky J, et al. Utility of HPV DNA detection in thin-layer, liquid-based tests with atypical squamous metaplasia. Acta Cytologica 2002;46(5):808-12. [DOI] [PubMed] [Google Scholar]
Quddus 2009 {published data only}
- Quddus MR, Neves T, Reilly ME, Steinhoff MM, Sung CJ. Does the ThinPrep Imaging System increase the detection of high-risk HPV-positive ASC-US and AGUS? The Women and Infants Hospital experience with over 200,000 cervical cytology cases. CytoJournal 2009;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qureshi 2003 {published data only}
- Qureshi MN, Rudelli RD, Tubbs RR, Biscotti CV, Layfield LJ. Role of HPV DNA testing in predicting cervical intraepithelial lesions: comparison of HC HPV and ISH HPV. Diagnostic Cytopathology 2003;29(3):149-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rabelo‐Santos 2009 {published data only}
- Rabelo-Santos SH, Derchain SF, Villa LL, Costa MC, Sarian LO, do Amaral Westin MC, et al. Human papillomavirus-specific genotypes in cervical lesions of women referred for smears with atypical glandular cells or adenocarcinoma in situ. International Journal of Gynecological Pathology 2009;28(3):272-8. [DOI] [PubMed] [Google Scholar]
Rana 2004 {published data only}
- Rana DN, Marshall J, Desai M, Kitchener C, Perera DM, El Teraifi H, et al. Five-year follow-up of woman with borderline and mildly dyskaryotic cervical smears. Cytopathology 2004;15:263-70. [DOI] [PubMed] [Google Scholar]
Raskin 2009 {published data only}
- Raskin GA, Petrov SV, Orlova RV, Mal'tseva LI, Akhmetzianova AV, Farrakhova LN, et al. [Comparison of efficacy of combined use of liquid cytology and immunohistochemical assay of p16ink4 and standard procedure and high risk evaluation by polymerase chain reaction of human papillomavirus infection in diagnosing cervical dysplasia and carcinoma]. Voprosy Onkologii 2009;55(2):192-5. [PubMed] [Google Scholar]
Rauber 2008 {published data only}
- Rauber D, Mehlhorn G, Fasching PA, Beckmann MW, Ackermann S. Prognostic significance of the detection of human papilloma virus L1 protein in smears of mild to moderate cervical intraepithelial lesions. European Journal of Obstetrics, Gynecology, and Reproductive Biology 2008;140(2):258-62. [DOI] [PubMed] [Google Scholar]
Recio 1998 {published data only}
- Recio FO, Sahai Srivastava BJ, Wong C, Hempling RE, Eltabbakh GH, Piver MS. The clinical value of digene hybrid capture HPV DNA testing in a referral-based population with abnormal pap smears. European Journal of Gynaelogical Oncology 1998;19:203-8. [PubMed] [Google Scholar]
Reesink‐Peters 2003 {published data only}
- Reesink-Peters N, Helder MN, Wisman GB, Knol AJ, Koopmans S, Boezen HM. Detection of telomerase, its components, and human papillomavirus in cervical scrapings as a tool for triage in women with cervical dysplasia. Journal of Clinical Pathology 2003;56:31-5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riethmuller 2008 {published data only}
- Riethmuller D, Gabelle C, Ramanah R, Sautiere JL, Pretet JL, Schaal JP, et al. [Importance of human papillomavirus (HPV) screening in the follow-up after CIN2-3 treatment]. Journal de Gynécologie, Obstétrique et Biologie de la Reproduction 2008;37(4):329-37. [DOI] [PubMed] [Google Scholar]
Rijkaart 2009 {published data only}
- Rijkaart DC, Berkhof J, Kemenade FJ, Rozendaal L, Verheijen RH, Bulk S, et al. Comparison of HPV and cytology triage algorithms for women with borderline or mild dyskaryosis in population-based cervical screening (VUSA-screen study). International Journal of Cancer 2009;126(9):2175-81. [DOI] [PubMed] [Google Scholar]
Robova 2007 {published data only}
- Robova H, Rob L, Pluta M, Kacirek J, Slavik V, Skapa P, et al. [Progression and regression low grade intraepithelial squamous lesions in context of positivity of high risk human papillomavirus]. Ceská Gynekologie 2007;72(5):347-50. [PubMed] [Google Scholar]
Rodriguez 2008 {published data only}
- Rodriguez R, Fadare O. Longitudinal cytological follow-up of patients with a Papanicolaou test interpretation of "atypical squamous cells of undetermined significance" that was followed by a negative reflex test for high-risk human papillomavirus types. International Journal of Gynecological Pathology 2008;27(1):108-12. [DOI] [PubMed] [Google Scholar]
Ronnett 1999 {published data only}
- Ronnett BM, Manos M, Ransley JE, Fetterman B, Kinney W, Hurley A. Atypical glandular cells of undetermined significance (AGUS): cytopathologic features, histopathologic results, and human papillomavirus DNA detection. Human Pathology 1999;30:816-25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rosini 2007 {published data only}
- Rosini S, Zappacosta R, Di Bonaventura G, Caraceni D, Pilla D, Di Girolamo G, et al. Management and triage of women with human papillomavirus infection in follow-up for low-grade cervical disease: association of HPV-DNA and RNA-based methods. International Journal of Immunopathology and Pharmacology 2007;20(2):341-7. [DOI] [PubMed] [Google Scholar]
Rowe 2004 {published data only}
- Rowe LR, Aldeen W, Bentz JS. Prevalence and typing of HPV DNA by Hybrid Capture II in women with ASCUS, ASC-H, LSIL, and AGC on ThinPrep Pap tests. Diagnostic Cytopathology 2004;30:426-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Saad 2006 {published data only}
- Saad RS, Dabbs DJ, Kordunsky L, Kanbour-Shakir A, Silverman JF, Liu Y, et al. Clinical significance of cytologic diagnosis of atypical squamous cells, cannot exclude high grade, in perimenopausal and postmenopausal women. American Journal of Clinical Pathology 2006;126(3):381-8. [DOI] [PubMed] [Google Scholar]
Safaeian 2007 {published data only}
- Safaeian M, Solomon D, Wacholder S, Schiffman M, Castle P. Risk of precancer and follow-up management strategies for women with human papillomavirus-negative atypical squamous cells of undetermined significance. Obstetrics and Gynecology 2007;109(6):1325-31. [DOI] [PubMed] [Google Scholar]
Samarawardana 2010 {published data only}
- Samarawardana P, Dehn DL, Singh M, Franquemont D, Thompson C, Gaido L, et al. p16(INK4a) is superior to high-risk human papillomavirus testing in cervical cytology for the prediction of underlying high-grade dysplasia. Cancer Cytopathology 2010;118(3):146-56. [DOI] [PubMed] [Google Scholar]
Santos 2003 {published data only}
- Santos AL, Derchain SF, Martins MR, Sarian LO, Martinez EZ, Syrjanen KJ. Human papillomavirus viral load in predicting high-grade CIN in women with cervical smears showing only atypical squamous cells or low-grade squamous intraepithelial lesion. Sao Paulo Medical Journal 2003;121(6):238-43. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Santos 2006 {published data only}
- Santos AL, Derchain SF, Sarian LO, Martins MR, Morais SS, Syrjanen KJ. Performance of Pap smear and human papilloma virus testing in the follow-up of women with cervical intraepithelial neoplasia grade 1 managed conservatively. Acta Obstetricia et Gynecologica Scandinavica 2006;85(4):444-50. [DOI] [PubMed] [Google Scholar]
Saqi 2006 {published data only}
- Saqi A, Gupta PK, Erroll M, Babiac A, Blackmun D, Mansukhani M, et al. High-risk human papillomavirus DNA testing: a marker for atypical glandular cells. Diagnostic Cytopathology 2006;34(3):235-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sargent 2010 {published data only}
- Sargent A, Bailey A, Turner A, Almonte M, Gilham C, Baysson H, et al. Optimal threshold for a positive hybrid capture 2 test for detection of human papillomavirus: data from the ARTISTIC trial. Journal of Clinical Microbiology 2010;48(2):554-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarian 2009 {published data only}
- Sarian LO, Hammes LS, Longatto-Filho A, Guarisi R, Derchain SF, Roteli-Martins C, et al. Increased risk of oncogenic human papillomavirus infections and incident high-grade cervical intraepithelial neoplasia among smokers: experience from the Latin American screening study. Sexually Transmitted Diseases 2009;36(4):241-8. [DOI] [PubMed] [Google Scholar]
Sarode 2003 {published data only}
- Sarode VR, Werner C, Gander R, Foster B, Fulmer A, Saboorian MH, et al. Reflex human papillomavirus DNA testing on residual liquid-based (TPPT trade mark ) cervical samples. Cancer 2003;99:149-55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schiffman 2006 {published data only}
- Schiffman M, Castle PE. When to test women for human papillomavirus. BMJ 2006;332(7533):61-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schledermann 2008 {published data only}
- Schledermann D, Andersen BT, Bisgaard K, Dohse M, Ejersbo D, Hoelund B, et al. Are adjunctive markers useful in routine cervical cancer screening? Application of p16(INK4a) and HPV-PCR on ThinPrep samples with histological follow-up. Diagnostic Cytopathology 2008;36(7):453-9. [DOI] [PubMed] [Google Scholar]
Schmitz 2009 {published data only}
- Schmitz M, Scheungraber C, Herrmann J, Teller K, Gajda M, Runnebaum IB, et al. Quantitative multiplex PCR assay for the detection of the seven clinically most relevant high-risk HPV types. Journal of Clinical Virology 2009;44(4):302-7. [DOI] [PubMed] [Google Scholar]
Schnatz 2006 {published data only}
- Schnatz PF, Guile M, O'sullivan DM, Sorosky JI. Clinical significance of atypical glandular cells on cervical cytology. Obstetrics and Gynecology 2006;107(3):701-8. [DOI] [PubMed] [Google Scholar]
Seme 2006 {published data only}
- Seme K, Fujs K, Kocjan BJ, Poljak M. Resolving repeatedly borderline results of Hybrid Capture 2 HPV DNA Test using polymerase chain reaction and genotyping. Journal of Virological Methods 2006;134(1-2):252-6. [DOI] [PubMed] [Google Scholar]
Sharpless 2005a {published data only}
- Sharpless KE, Schnatz PF, Mandavilli S, Greene JF, Sorosky JI. Dysplasia associated with atypical glandular cells on cervical cytology. Obstetrics and Gynecology 2005;105(3):494-500. [DOI] [PubMed] [Google Scholar]
Sharpless 2005b {published data only}
- Sharpless KE, Schnatz PF, Mandavilli S, Greene JF, Sorosky JI. Lack of adherence to practice guidelines for women with atypical glandular cells on cervical cytology. Obstetrics and Gynecology 2005;105(3):501-6. [DOI] [PubMed] [Google Scholar]
Sharpless 2009 {published data only}
- Sharpless KE, O'sullivan DM, Schnatz PF. The utility of human papillomavirus testing in the management of atypical glandular cells on cytology. Journal of Lower Genital Tract Disease 2009;13(2):72-8. [DOI] [PubMed] [Google Scholar]
Sheriff 2007 {published data only}
- Sheriff SK, Petry KU, Ikenberg H, Crouse G, Mazonson PD, Santas CC. An economic analysis of human papillomavirus triage for the management of women with atypical and abnormal Pap smear results in Germany. European Journal of Health Economics 2007;8(2):153-60. [DOI] [PubMed] [Google Scholar]
Shi 2009 {published data only}
- Shi JF, Belinson JL, Zhao FH, Pretorius RG, Li J, Ma JF, et al. Human papillomavirus testing for cervical cancer screening: results from a 6-year prospective study in rural China. American Journal of Epidemiology 2009;170(6):708-16. [DOI] [PubMed] [Google Scholar]
Shidham 2007 {published data only}
- Shidham VB, Kumar N, Narayan R, Brotzman GL. Should LSIL with ASC-H (LSIL-H) in cervical smears be an independent category? A study on SurePathtrade mark specimens with review of literature. CytoJournal 2007;4(7):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shin 2008 {published data only}
- Shin EK, Lee SR, Kim MK, Kang EJ, Ju W, Lee SN, et al. Immunocytochemical staining of p16(ink4a) protein as an adjunct test in equivocal liquid-based cytology. Diagnostic Cytopathology 2008;36(5):311-6. [DOI] [PubMed] [Google Scholar]
Siddiqi 2009 {published data only}
- Siddiqi A, Spataro M, McIntire H, Akhtar I, Baliga M, Flowers R, et al. Hybrid capture 2 human papillomavirus DNA testing for women with atypical squamous cells of undetermined significance Papanicolaou results in SurePath and ThinPrep specimens. Cancer Cytopathology 2009;117(5):318-25. [DOI] [PubMed] [Google Scholar]
Siddiqui 2008a {published data only}
- Siddiqui MT, Cohen C, Nassar A. Detecting high-grade cervical disease on ASC-H cytology: role of BD ProEx C and Digene Hybrid Capture II HPV DNA testing. American Journal of Clinical Pathology 2008;130(5):765-70. [DOI] [PubMed] [Google Scholar]
Sideri 1998 {published data only}
- Sideri M, Spinaci L, Schettino F, Mezzetti M, Robertson C, Spolti N, et al. Risk factors for high-grade cervical intraepithelial neoplasia in patients with mild cytological dyskarosis: human papillomavirus testing versus multivariate tree analysis of demographic data. Cancer Epidemiology, Biomarkers and Prevention 1998;7:237-41. [MEDLINE: ] [PubMed] [Google Scholar]
Singh 2009 {published data only}
- Singh M, Srivastava S, Singh U, Mathur N, Shukla Y. Co-expression of p53 and Bcl-2 proteins in human papillomavirus-induced premalignant lesions of the uterine cervix: correlation with progression to malignancy. Tumour Biology 2009;30(5-6):276-85. [DOI] [PubMed] [Google Scholar]
Six 2008 {published data only}
- Six L, Leodolter S, Sings HL, Barr E, Haupt R, Joura EA. Prevalence of human papillomavirus types 6, 11, 16 and 18 in young Austrian women - baseline data of a phase III vaccine trial. Wiener Klinische Wochenschrift 2008;120(21-22):666-71. [DOI] [PubMed] [Google Scholar]
Slawson 1994 {published data only}
- Slawson DC, Bennett JH, Simon LJ, Herman JM. Should all women with cervical atypia be referred for colposcopy: a HARNET study. Harrisburgh Area Research Network. Journal of Family Practice 1994;38:387-92. [PubMed] [Google Scholar]
Sorbye 2010 {published data only}
- Sorbye SW, Fismen S, Gutteberg T, Mortensen ES. Triage of women with minor cervical lesions: data suggesting a "test and treat" approach for HPV E6/E7 mRNA testing. PLoS ONE 2010;5(9):e12724. [DOI: 10.1371] [DOI] [PMC free article] [PubMed] [Google Scholar]
Srodon 2005 {published data only}
- Srodon M, Parry DH, Ronnett BM. Atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion. Cancer 2005;108:32-8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Steinman 2008 {published data only}
- Steinman S, Smith D, Chandler N, Dhurandhar B, Di Filippo L, Scheiber-Pact M, et al. Morphologic, patient and interpreter profiles of high-risk human papillomavirus-positive vs.-negative cases of atypical squamous cells of undetermined significance. Acta Cytologica 2008;52(3):279-85. [DOI] [PubMed] [Google Scholar]
Stemberger‐Papic 2010 {published data only}
- Stemberger-Papic S, Vrdoljak-Mozetic D, Ostojic DV, Rubesa-Mihaljevic R, Manestar M. Evaluation of the HPV L1 capsid protein in prognosis of mild and moderate dysplasia of the cervix uteri. Collegium Antropologicum 2010;34(2):419-23. [PubMed] [Google Scholar]
Stevens 2007 {published data only}
- Stevens MP, Garland SM, Rudland E, Tan J, Quinn MA, Tabrizi SN. Comparison of the Digene Hybrid Capture 2 assay and Roche AMPLICOR and LINEAR ARRAY human papillomavirus (HPV) tests in detecting high-risk HPV genotypes in specimens from women with previous abnormal Pap smear results. Journal of Clinical Microbiology 2007;45(7):2130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoler 2001 {published data only}
- Stoler MH, Schiffman MA. Interobserver reproducibility of cervical cytologic and histologic interpretations. JAMA 2001;285(11):1500-5. [DOI] [PubMed] [Google Scholar]
Stoler 2007 {published data only}
- Stoler M, Castle P, Solomon D, Schiffman M. The authors' reply: The expanded use of HPV testing in gynecologic practice per ASCCP-guided management requires the use of well-validated assays. American Journal of Clinical Pathology 2007;128:884-6. [DOI] [PubMed] [Google Scholar]
Suba 2009 {published data only}
- Suba EJ, Cibas ES, Raab SS. HPV screening for cervical cancer in rural India. The New England Journal of Medicine 2009;361(3):304-6. [DOI] [PubMed] [Google Scholar]
Sun 2006 {published data only}
- Sun XR, Che DY, Tu HZ, Li D, Wang J. [Assessment of cervical intraepithelial neoplasia (CIN) lesions by DNA image cytometry]. Zhonghua zhong liu za zhi [Chinese Journal of Oncology] 2006;28(11):831-5. [PubMed] [Google Scholar]
Sung 2010 {published data only}
- Sung CO, Kim SR, Oh YL, Song SY. The use of p16(INK4A) immunocytochemistry in "Atypical squamous cells which cannot exclude HSIL" compared with "Atypical squamous cells of undetermined significance" in liquid-based cervical smears. Diagnostic Cytopathology 2010;38(3):168-71. [DOI] [PubMed] [Google Scholar]
Tambouret 2008 {published data only}
- Tambouret RH, Misdraji J, Wilbur DC. Longitudinal clinical evaluation of a novel antibody cocktail for detection of high-grade squamous intraepithelial lesions on cervical cytology specimens. Archives of Pathology & Laboratory Medicine 2008;132(6):918-25. [DOI] [PubMed] [Google Scholar]
Tang 2009 {published data only}
- Tang N, Huang S, Erickson B, Mak WB, Salituro J, Robinson J, et al. High-risk HPV detection and concurrent HPV 16 and 18 typing with Abbott RealTime High Risk HPV test. Journal of Clinical Virology 2009;45(Suppl 1):S25-8. [DOI] [PubMed] [Google Scholar]
Tarkkanen 2007 {published data only}
- Tarkkanen J, Auvinen E, Nieminen P, Malmi R, Vartiainen J, Timonen T, et al. HPV DNA testing as an adjunct in the management of patients with low grade cytological lesions in Finland. Acta Obstetricia et Gynecologica Scandinavica 2007;86(3):367-72. [DOI] [PubMed] [Google Scholar]
Thrall 2008 {published data only}
- Thrall MJ, Russell DK, Bonfiglio TA, Hoda RS. Use of the ThinPrep(R) Imaging System does not alter the frequency of interpreting Papanicolaou tests as atypical squamous cells of undetermined significance. CytoJournal 2008;5:10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thrall 2010 {published data only}
- Thrall MJ, Smith DA, Mody DR. Women >or=30 years of age with low grade squamous intraepithelial lesion (LSIL) have low positivity rates when co-tested for high-risk human papillomavirus: should we reconsider HPV triage for LSIL in older women? Diagnostic Cytopathology 2010;38:407-10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
TOMBOLA 2009a {published data only}
- TOMBOLA Group. Cytological surveillance compared with immediate referral for colposcopy in management of women with low grade cervical abnormalities: multicentre randomised controlled trial. BMJ 2009;339:b2546-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
TOMBOLA 2009b {published data only}
- TOMBOLA Group. Biopsy and selective recall compared with immediate large loop excision in management of women with low grade abnormal cervical cytology referred for colposcopy: multicentre randomised controlled trial. BMJ 2009;339:b2548-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
TOMBOLA 2009c {published data only}
- TOMBOLA Group. Options for managing low grade cervical abnormalities detected at screening: cost effectiveness study. BMJ 2009;339:b2549-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Torres 2009 {published data only}
- Torres LM, Paez M, Insaurralde A, Rodriguez MI, Castro A, Kasamatsu E. Detection of high risk human papillomavirus cervical infections by the Hybrid Capture in Asunci¢n, Paraguay. The Brazilian Journal of Infectious Disease 2009;13:203-6. [DOI] [PubMed] [Google Scholar]
Tu 2009 {published data only}
- Tu Z, Zhang A, Wu R, Jiang J, Li Y, Wulan N, et al. Genomic amplification of the human telomerase RNA gene for differential diagnosis of cervical disorders. Cancer Genetics and Cytogenetics 2009;191(1):10-6. [DOI] [PubMed] [Google Scholar]
Vijayaraghavan 2010 {published data only}
- Vijayaraghavan A, Efrusy MB, Goodman KA, Santas CC, Huh WK. Cost-effectiveness of using human papillomavirus 16/18 genotype triage in cervical cancer screening. Gynecologic Oncology 2010;119:237-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vince 2001 {published data only}
- Vince A, Ivanisevic M, Harni V, Skalko D, Jeren T. Molecular detection of human papillomavirus in women with minor-grade cervical cytology abnormalities. Journal of Clinical Virology 2001;20(1-2):91-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walker 2006 {published data only}
- Walker JL, Wang SS, Schiffman M, Solomon D. Predicting absolute risk of CIN3 during post-colposcopic follow-up: results from the ASCUS-LSIL Triage Study (ALTS). American Journal of Obstetrics and Gynecology 2006;195:341-8. [DOI] [PubMed] [Google Scholar]
Wang 2007 {published data only}
- Wang XL, Zhang R, Wu LY, Li SM, Huang MN, Li N. [The clinical significance and management of cervico-cytologically diagnosed ASCUS/LSIL.]. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 2007;21(3):267-9. [PubMed] [Google Scholar]
Wensveen 2006 {published data only}
- Wensveen CW, Kagie MJ, Veldhuizen RW, Trimbos JB, Boon ME. Combining HPVand MIB-1 tests reduces the number of colposcopies in women with equivocal cytology. Acta Obstetricia et Gynecologica Scandinavica 2006;85(12):1491-5. [DOI] [PubMed] [Google Scholar]
Wheeler 2006 {published data only}
- Wheeler CM, Hunt WC, Schiffman M, Castle PE. Human papillomavirus genotypes and the cumulative 2-year risk of cervical precancer. The Journal of Infectious Diseases 2006;194(9):1291-9. [DOI] [PubMed] [Google Scholar]
Wong 2008 {published data only}
- Wong AK, Chan RC, Nichols WS, Bose S. Human papillomavirus (HPV) in atypical squamous cervical cytology: the Invader HPV test as a new screening assay. Journal of Clinical Microbiology 2008;46(3):869-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2008a {published data only}
- Wong NK, Ng FY, Leung G. Cytological distinction between high-risk and low-risk human papillomavirus infections in SurePath liquid-based cell preparations. Journal of Clinical Pathology 2008;61(12):1317-22. [DOI] [PubMed] [Google Scholar]
Wright 1995 {published data only}
- Wright TC, Sun XW, Koulos J. Comparison of management algorithms for the evaluation of women with low-grade cytologic abnormalities. Obstetrics and Gynecology 1995;85:202-10. [DOI] [PubMed] [Google Scholar]
Wright 1998 {published data only}
- Wright TC, Lorincz AT, Ferris DG, Richart RM, Ferenczy A, Mielzynska I, et al. Reflex papillomavirus deoxyribonucleic acid testing in women with abnormal Papanicolaou smears. American Journal of Obstetrics and Gynecology 1998;178:962-6. [DOI] [PubMed] [Google Scholar]
Wright 2006 {published data only}
- Wright JD, Rader JS, Davila R, Powell MA, Mutch DG, Gao F, et al. Human papillomavirus triage for young women with atypical squamous cells of undetermined significance. Obstetrics and Gynecology 2006;107(4):822-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wu 2006 {published data only}
- Wu HH, Allen SL, Kirkpatrick JL, Elsheikh TM. Reflex high-risk human papilloma virus DNA test is useful in the triage of women with atypical squamous cells cannot exclude high-grade squamous intraepithelial lesion. Diagnostic Cytopathology 2006;34(10):707-10. [DOI] [PubMed] [Google Scholar]
Xi 2009 {published data only}
- Xi LF, Koutsky LA, Castle PE, Wheeler CM, Galloway DA, Mao C, et al. Human papillomavirus type 18 DNA load and 2-year cumulative diagnoses of cervical intraepithelial neoplasia grades 2-3. Journal of the National Cancer Institute 2009;101(3):153-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yarandi 2009 {published data only}
- Yarandi F, Shojaei H, Eftekhar Z, Izadi-Mood N. Comparison of three management strategies for patients with atypical squamous cells of undetermined significance, after six months delay: a three-year experience in an Iranian university hospital. The Australian & New Zealand Journal of Obstetrics & Gynaecology 2009;49(2):207-10. [DOI] [PubMed] [Google Scholar]
Yu 2010 {published data only}
- Yu L, Wang L, Zhong J, Chen S. Diagnostic value of p16INK4A, Ki-67, and human papillomavirus L1 capsid protein immunochemical staining on cell blocks from residual liquid-based gynecologic cytology specimens. Cancer Cytopathology 2010;118(1):47-55. [DOI] [PubMed] [Google Scholar]
Zhao 2009a {published data only}
- Zhao C, Austin RM. High-risk human papillomavirus DNA test results are useful for disease risk stratification in women with unsatisfactory liquid-based cytology pap test results. Journal of Lower Genital Tract Disease 2009;13(2):79-84. [DOI] [PubMed] [Google Scholar]
Zhao 2009b {published data only}
- Zhao C, Florea A, Onisko A, Austin RM. Histologic follow-up results in 662 patients with Pap test findings of atypical glandular cells: results from a large academic women's hospital laboratory employing sensitive screening methods. Gynecologic Oncology 2009;114(3):383-9. [DOI] [PubMed] [Google Scholar]
Zivadinovic 2009 {published data only}
- Zivadinovic R, Lilic V, Dordevic B, Stanojevic Z, Petric A, Lilic G. [The role of colposcopy and typization of human papillomavirus in further diagnostic proceedings in patients with ASC-US cytological finding of the uterine cervix]. Vojnosanitetski Pregled 2009;66(8):651-5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Alameda 2011 {published data only}
- Alameda F, Alameda F, Piujan L, Lioveras B, Bellosillo B, Larrazabal F, et al. The value of p16 in ASCUS cases: A retrospective study using frozen cytologic material. Diagnostic Cytopathology 2011;39(2):110-4. [DOI] [PubMed] [Google Scholar]
Barcelos 2011 {published data only}
- Barcelos AC, Michelin MA, Adad SJ, Murta EF. Atypical squamous cells of undetermined significance: Bethesda classification and association with human papillomavirus. Infectious Diseases in Obstetrics and Gynecology 2011;2011(904674):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Belinson 2011 {published data only}
- Belinson JL, Wu R, Belinson SE, Qu X, Yang B, Du H, et al. A population-based clinical trial comparing endocervical high-risk HPV testing using hybrid capture 2 and Cervista from the SHENCCAST II Study. American Journal of Clinical Pathology 2011;135(5):790-5. [DOI] [PubMed] [Google Scholar]
Chao 2013 {published data only}
- Chao TK, Ke FY, Liao YP, Wang HC, Yu CP, Lai HC. Triage of cervical cytological diagnoses of atypical squamous cells by DNA methylation of paired boxed gene 1 (PAX1). Diagnostic Cytopathology 2013;41(1):41-6. [DOI] [PubMed] [Google Scholar]
Clad 2011 {published data only}
- Clad A, Reuschenbach M, Weinschenk J, Grote R, Rahmsdorf J, Freudenberg N. Performance of the APTIMA high-risk HPV mRNA assay in a referral population in comparison with Hybrid Capture 2 and cytology. Journal of Clinical Microbiology 2011;49(3):1071-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuzick 2013 {published data only}
- Cuzick J, Thomas CJ, Zhang G, Einstein MH, Stoler M, Trupin S, et al. Human papillomavirus testing for triage of women with low-grade squamous intraepithelial lesions. International Journal of Cancer 2013;132(4):959-66. [DOI] [PubMed] [Google Scholar]
Dona 2012 {published data only}
- Dona MG, Vocaturo A, Giuliani M, Ronchetti L, Rollo F, Pescarmona E, et al. p16/Ki-67 dual staining in cervico-vaginal cytology: Correlation with histology, Human Papillomavirus detection and genotyping in women undergoing colposcopy. Gynecologic Oncology 2012;126(2):198-202. [DOI] [PubMed] [Google Scholar]
Dufresne 2011 {published data only}
- Dufresne S, Sauthier P, Mayrand MH, Petignat P, Provencher D, Drouin P, et al. Human papillomavirus (HPV) DNA triage of women with atypical squamous cells of undetermined significance with Amplicor HPV and Hybrid Capture 2 assays for detection of high-grade lesions of the uterine cervix. Journal of Clinical Microbiology 2011;49(1):48-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edgerton 2011 {published data only}
- Edgerton N, Cohen C, Siddiqui MT. Evaluation of CINtec PLUS(R) testing as an adjunctive test in ASC-US diagnosed SurePath(R) preparations. Diagnostic Cytopathology 2013;41(1):35-40. [DOI] [PubMed] [Google Scholar]
Gustinucci 2012 {published data only}
- Gustinucci D, Passamonti B, Cesarini E, Butera D, Palmieri EA, Bulletti S, et al. Role of p16 cytology testing as an adjunct to enhance the diagnostic specificity and accuracy in human papillomavirus-positive women within an organized cervical cancer screening program. Acta Cytologica 2012;56(5):506-14. [DOI] [PubMed] [Google Scholar]
Heider 2011 {published data only}
- Heider A, Austin RM, Zhao C. HPV Test Results Stratify Risk for Histopathologic Follow-Up Findings of High-Grade Cervical Intra-Epithelial Neoplasia in Women with Low-Grade Squamous Intra-Epithelial Lesion Pap Results. Acta Cytologica 2011;55(1):48-53. [DOI] [PubMed] [Google Scholar]
Huang 2012 {published data only}
- Huang LW, Lin YH, Pan HS, Seow KM, Lin CY. Human papillomavirus genotyping as a predictor of high-grade cervical dysplasia in women with mildly cytologic abnormalities: A two-year follow-up report. Diagnostic Cytopathology 2012;40(8):673-7. [DOI] [PubMed] [Google Scholar]
Ibanez 2012 {published data only}
- Ibanez R, Moreno-Crespi J, Sarda M, Autonell J, Fibla M, Gutierrez C, et al. Prediction of cervical intraepithelial neoplasia grade 2+ (CIN2+) using HPV DNA testing after a diagnosis of atypical squamous cells of undetermined significance (ASC-US) in Catalonia, Spain. BMC Infectious Diseases 2012;12(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jakobsson 2012 {published data only}
- Jakobsson M, Tarkkanen J, Auvinen E, Hakkinen R, Laurila P, Tapper AM. Colposcopy referral rate can be reduced by high-risk human papillomavirus triage in the management of recurrent atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion cytology in Finland. International Journal of STD & AIDS 2012;23(7):485-9. [DOI] [PubMed] [Google Scholar]
Jiang 2011 {published data only}
- Jiang L, Zeng Y, Li J, Wang H, Xia Y, Fang X, et al. Performance of high-risk human papillomavirus testing in the triage of abnormal cervical cytology among Chinese younger women in Shanghai, China. Asian Pacific Journal of Cancer Prevention 2011;12(11):2963-7. [PubMed] [Google Scholar]
Koliopoulos 2012 {published data only}
- Koliopoulos G, Chrelias C, Pappas A, Makridimas S, Kountouris E, Alepaki M, et al. The diagnostic accuracy of two methods for E6&7 mRNA detection in women with minor cytological abnormalities. Acta Obstetricia et Gynecologica Scandinavica 2012;91(7):794-801. [DOI] [PubMed] [Google Scholar]
Lapierre 2012 {published data only}
- Lapierre SG, Sauthier P, Mayrand MH, Dufresne S, Petignat P, Provencher D, et al. Human papillomavirus (HPV) DNA triage of women with atypical squamous cells of undetermined significance with cobas 4800 HPV and Hybrid Capture 2 tests for detection of high-grade lesions of the uterine cervix. Journal of Clinical Microbiology 2012;50(4):1240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levi 2011 {published data only}
- Levi AW, Harigopal M, Hui P, Schofield K, Chhieng DC. Use of high-risk human papillomavirus testing in patients with low-grade squamous intraepithelial lesions. Cancer Cytopathology 2011;119(4):228-34. [DOI] [PubMed] [Google Scholar]
Loghavi 2012 {published data only}
- Loghavi S, Walts AE, Bose S. CINtec(R) plus dual immunostain: a triage tool for cervical pap smears with atypical squamous cells of undetermined significance and low grade squamous intraepithelial lesion. Diagnostic Cytopathology 2012;Epub ahead of print:10.1002/dc.22900. [DOI: 10.1002/dc.22900] [DOI] [PubMed] [Google Scholar]
Ma 2011 {published data only}
- Ma YY, Cheng XD, Zhou CY, Qiu LQ, Chen XD, Lu WG, et al. Value of P16 expression in the triage of liquid-based cervical cytology with atypical squamous cells of undetermined significance and low-grade squamous intraepithelial lesions. Chinese Medical Journal 2011;124(16):2443-7. [PubMed] [Google Scholar]
Monsonego 2011 {published data only}
- Monsonego J, Hudgens MG, Zerat L, Zerat JC, Syrjanen K, Halfon P, et al. Evaluation of oncogenic human papillomavirus RNA and DNA tests with liquid based cytology in primary cervical cancer screening (The FASE study). International Journal of Cancer 2011;129(3):691-701. [DOI] [PubMed] [Google Scholar]
Nasioutziki 2011 {published data only}
- Nasioutziki M, Daniilidis A, Dinas K, Kyrgiou M, Valasoulis G, Loufopoulos PD, et al. The evaluation of p16INK4a immunoexpression/immunostaining and human papillomavirus DNA test in cervical liquid-based cytological samples. International Journal of Gynecological Cancer 2011;21(1):79-85. [DOI] [PubMed] [Google Scholar]
Origoni 2012 {published data only}
- Origoni M, Carminati G, Rolla S, Clementi M, Sideri M, Sandri MT, et al. Human papillomavirus viral load expressed as relative light units (RLU) correlates with the presence and grade of preneoplastic lesions of the uterine cervix in atypical squamous cells of undetermined significance (ASCUS) cytology. European Journal of Clinical Microbiology & Infectious Diseases 2012;31(9):2401-6. [DOI] [PubMed] [Google Scholar]
Ovestad 2011 {published data only}
- Ovestad IT, Vennestrom U, Andersen L, Gudlaugsson E, Munk AC, Malpica A, et al. Comparison of different commercial methods for HPV detection in follow-up cytology after ASCUS/LSIL, prediction of CIN2-3 in follow up biopsies and spontaneous regression of CIN2-3. Gynecological Oncology 2011;123(2):278-83. [DOI] [PubMed] [Google Scholar]
Pista 2011 {published data only}
- Pista A, Verdasca N, Oliveira A. Clinical performance of the CLART human papillomavirus 2 assay compared with the hybrid capture 2 test. Journal of Medical Virology 2011 Feb;83(2):272-6. [DOI] [PubMed] [Google Scholar]
Ratnam 2011 {published data only}
- Ratnam S, Coutlee F, Fontaine D, Bentley J, Escott N, Ghatage P, et al. Aptima HPV E6/E7 mRNA test is as sensitive as Hybrid Capture 2 Assay but more specific at detecting cervical precancer and cancer. Journal of Clinical Microbiology 2011;49(2):557-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriguez 2012 {published data only}
- Rodriguez EF, Reynolds JP, Jenkins SM, Winter SM, Henry MR, Nassar A. Atypical squamous cells of undetermined significance in patients with HPV positive DNA testing and correlation with disease progression by age group: an institutional experience. International Journal of Clinical and Experimental Pathology 2012;5(5):428-35. [PMC free article] [PubMed] [Google Scholar]
Schmidt 2011 {published data only}
- Schmidt D, Bergeron C, Denton KJ, Ridder R. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL Papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathology 2011;119(3):158-66. [DOI] [PubMed] [Google Scholar]
Soderlund‐Strand 2011 {published data only}
- Soderlund-Strand A, Eklund C, Kemetli L, Grillner L, Tornberg S, Dillner J, et al. Genotyping of human papillomavirus in triaging of low-grade cervical cytology. American Journal of Obstetrics and Gynecology 2011;205(2):145-6. [DOI] [PubMed] [Google Scholar]
Stoler 2011 {published data only}
- Stoler MH, Wright TC, Sharma A, Apple R, Gutekunst K, Wright TL. High-risk human papillomavirus testing in women with ASC-US cytology: results from the ATHENA HPV study. American Journal of Clinical Pathology 2011;135(3):468-75. [DOI] [PubMed] [Google Scholar]
Stoler 2013 {published data only}
- Stoler MH, Wright TC Jr, Cuzick J, Dockter J, Reid JL, Getman D, et al. APTIMA HPV assay performance in women with atypical squamous cells of undetermined significance cytology results. American Journal of Obstetrics and Gynecology 2013;208(2):144.e1-8. [DOI: 10.1016/j.ajog.2012.12.003] [DOI] [PubMed] [Google Scholar]
Szarewski 2012 {published data only}
- Szarewski A, Mesher D, Cadman L, Austin J, Ashdown-Barr L, Ho L, et al. A comparison of seven tests for high grade cervical intraepithelial neoplasia in women with abnormal smears: the Predictors 2 study. Journal of Clinical Microbiology 2012;50(6):1867-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsoumpou 2010 {published data only}
- Tsoumpou I, Valasoulis G, Founta C, Kyrgiou M, Nasioutziki M, Daponte A, et al. High-risk human papillomavirus DNA test and p16(INK4a) in the triage of LSIL: a prospective diagnostic study. Gynecologic Oncology 2010 Dec 29;121:49-53. [DOI] [PubMed] [Google Scholar]
Valasoulis 2011 {published data only}
- Valasoulis G, Tsoumpou I, Founta C, Kyrgiou M, Dalkalitsis N, Nasioutziki M, et al. The role of p16INK4a immunostaining in the risk assessment of women with LSIL cytology: a prospective pragmatic study. European Journal of Gynaecological Oncology 2011;32(2):150-2. [PubMed] [Google Scholar]
Waldstrom 2011 {published data only}
- Waldstrom M, Ornskov D. Clinical performance of a human papillomavirus messenger RNA test (Aptima HPV assay) on residual material from archived 3-year-old PreservCyt samples with low-grade squamous intraepithelial lesion. Archives of Pathology & Laboratory Medicine 2011;135(8):1052-6. [DOI] [PubMed] [Google Scholar]
Wentzensen 2012 {published data only}
- Wentzensen N, Schwartz L, Zuna RE, Smith KM, Mathews C, Gold M, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clinical Cancer Research 2012;18(15):4154-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 2011 {published data only}
- Wong OG, Lo CK, Szeto E, Cheung AN. Efficacy of Abbott RealTime High Risk HPV test in evaluation of atypical squamous cells of undetermined significance from an Asian screening population. Journal of Clinical Virology 2011;51(2):136-8. [DOI] [PubMed] [Google Scholar]
Wong 2012 {published data only}
- Wong OG, Lo CK, Chow JN, Tsun OK, Szeto E, Liu SS, et al. Comparison of the GenoFlow human papillomavirus (HPV) test and the Linear Array assay for HPV screening in an Asian population. Journal of Clinical Microbiology 2012;50(5):1691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2010 {published data only}
- Wu R, Belinson SE, Du H, Na W, Qu X, Wu R, et al. Human papillomavirus messenger RNA assay for cervical cancer screening: the Shenzhen Cervical Cancer Screening Trial I. International Journal of Gynecological Cancer 2010;20(8):1411-4. [DOI] [PubMed] [Google Scholar]
Ziemke 2012 {published data only}
- Ziemke P, Marquardt K. Immunocytochemistry of p16(INK4a) and Ki-67 as adjunctive method for routine gynecological cytology of mild and moderate dysplasia. Pathologe 2012;Epub ahead of print:Jul 4. [DOI] [PubMed] [Google Scholar]
Additional references
ACOG 2009
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 109: Cervical cytology screening. Obstetrics and Gynecology 2009;114(6):1409-20. [DOI] [PubMed] [Google Scholar]
ALTS Group 2000
- ALTS Group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. Journal of the National Cancer Institute 2000;92:397-402. [DOI] [PubMed] [Google Scholar]
ALTS Group 2003a
- ALTS Group. Results of a randomised trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. American Journal of Obstetrics and Gynecology 2003;188:1383-92. [DOI] [PubMed] [Google Scholar]
ANAES 2002
- Agence nationale d'accréditation et d'évaluation en santé (ANAES). Conduite à tenir devant une patiente ayant un frottis cervico-utérin anormal. Service Recommandations et Références Professionnelles et Service Evaluation Economique. ANAES 2002.
Arbyn 2002
- Arbyn M, Buntinx F, Van Ranst M, Cortiñas Abrahantes J. Triage of women with atypical or low-grade cytological abnormalities of the cervix by HPV testing: systematic review and meta-analysis. IPH/EPI-REPORTS Nr. 2001-019, 1-101. 2002.
Arbyn 2004a
- Arbyn M, Buntinx F, Van Ranst M, Paraskevaidis E, Martin-Hirsch P, Dillner J. Virologic versus cytologic triage of women with equivocal Pap smears: a meta-analysis of the accuracy to detect high-grade intraepithelial neoplasia. Journal of the National Cancer Institute 2004;96:280-93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arbyn 2004b
- Arbyn M, Dillner J, Van Ranst M, Buntinx F, Martin-Hirsch P, Paraskevaidis E. Have we resolved how to triage equivocal cervical cytology? Journal of the National Cancer Institute 2004;96(18):1401-2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arbyn 2005
- Arbyn M, Paraskevaidis E, Martin-Hirsch P, Prendiville W, Dillner J. Clinical utility of HPV DNA detection: triage of minor cervical lesions, follow-up of women treated for high-grade CIN. An update of pooled evidence. Gynecologic Oncology 2005;99(Suppl 3):7-11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arbyn 2006
- Arbyn M, Sasieni P, Meijer CJLM, Clavel C, Koliopoulos G, Dillner J. Clinical applications of HPV testing: a summary of meta-analyses. Vaccine 2006;24(S3):78-89. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Arbyn 2007
- Arbyn M, Herbert A, Schenck U, Nieminen P, Jordan J, McGoogan E, et al. European guidelines for quality assurance in cervical cancer screening: recommendations for collecting samples for conventional and liquid-based cytology. Cytopathology 2007;18(3):133-9. [DOI] [PubMed] [Google Scholar]
Arbyn 2008a
- Arbyn M, Bergeron C, Klinkhamer P, Martin-Hirsch P, Siebers AG, Bulten J. Liquid compared with conventional cervical cytology: a systematic review and meta-analysis. Obstetrics and Gynecology 2008;111(1):167-77. [DOI] [PubMed] [Google Scholar]
Arbyn 2008b
- Arbyn M, Kyrgiou M, Simoens C, Raifu AO, Koliopoulos G, Martin-Hirsch P, et al. Peri-natal mortality and other severe adverse pregnancy outcomes associated with treatment of cervical intraepithelial neoplasia: a meta-analysis. BMJ 2008;337:a1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arbyn 2009a
- Arbyn M, Rebolj M, Kok IM, Becker N, O'Reilly M, Andrae B. Challenges for organising cervical screening programmes in the 15 old member states of the European Union. European Journal of Cancer 2009;45(15):2640-8. [DOI] [PubMed] [Google Scholar]
Arbyn 2009b
- Arbyn M, Ronco G, Cuzick J, Wentzensen, N, Castle, PE. How to evaluate emerging technologies in cervical cancer screening? International Journal of Cancer 2009;125(11):2489-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arbyn 2011
Arbyn 2012
- Arbyn M, Ronco G, Anttila A, Meijer CJLM, Poljak M, Ogilvie G, et al. Evidence regarding HPV testing in secondary prevention of cervical cancer. Vaccine 2012;30(Suppl 5):F88-99. [DOI] [PubMed] [Google Scholar]
Arbyn 2013
- Arbyn M, Roelens J, Cuschieri K, Cuzick J, Szarewski A, Ratnam S, et al. The APTIMA HPV assay versus the Hybrid Capture II test in triage of women with ASC-US or LSIL cervical cytology: a meta-analysis of the diagnostic accuracy. International Journal of Cancer 2013;132(1):101-8. [DOI] [PubMed] [Google Scholar]
ASCCP 2006
- American Society of Colposcopy and Cervical Pathology. Consensus Conference Bulletin Board: ASC-US and ASC-H. In: American Society of Colposcopy and Cervical Pathology. 2006:http://www.asccp.org/consensus/bulletin/.
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088-101. [MEDLINE: ] [PubMed] [Google Scholar]
Bosch 2002
- Bosch FX, Lorincz AT, Munoz N, Meijer CJ, Shah KV. Bosch FX, Lorincz AT, Munoz N, Meijer CJ, Shah KV. Journal of Clinical Pathology 2002;55(4):244-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castle 2005
- Castle PE, Solomon D, Schiffman M, Wheeler CM. Human papillomavirus type 16 infections and 2-year absolute risk of cervical precancer in women with equivocal or mild cytologic abnormalities. Journal of the National Cancer Institute 2005;97:1066-71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chu 2006
- Chu H, Cole SR. Bivariate meta-analysis of sensitivity and specificity with sparse data: a generalized linear mixed model approach. Journal of Clinical Epidemiology 2006;59(12):1331-2. [DOI] [PubMed] [Google Scholar]
Coleman 1993
- Coleman D, Day N, Douglas G. European guidelines for quality assurance in cervical cancer screening. European Journal of Cancer 1993;29A(Suppl 4):S1-38. [PubMed] [Google Scholar]
Cox 1998
- Cox JT. HPV testing: is it useful in triage of minor Pap abnormalities? Journal of Family Practice 1998;46:121-4. [PubMed] [Google Scholar]
Cox 2005
- Cox JT. Management of women with cervical cytology interpreted as ASC-US or as ASC-H. Clinical Obstetrics and Gynecology 2005;48:160-77. [DOI] [PubMed] [Google Scholar]
Cuzick 1999
- Cuzick J, Sasieni P, Davies P, Adams J, Normand C, Frater A, et al. A systematic review of the role of human papillomavirus testing within a cervical screening programme. Health Technology Assessment 1999;3:1-204. [PubMed] [Google Scholar]
Davies 2006
- Davies P, Arbyn M, Dillner J, Kitchener H, Ronco G, Hakama M. A report on the current status of European research on the use of human papillomavirus testing for primary cervical cancer screening. International Journal of Cancer 2006;118:791-6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Deeks 2005
- Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology 2005;58(9):882-93. [DOI] [PubMed] [Google Scholar]
Dudding 2002
- Dudding N, Sutton J. BSCC Terminology Conference, Koilocytosis and Mild Dyskaryosis. Cytopathology 2002;13(6):379-81. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test.. BMJ 1997;315:629-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
European Commission 2008
- European Cancer Network (ECN): Arbyn M , Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, et al. European Guidelines for Quality Assurance in Cervical Cancer Screening. 2nd edition. Luxembourg: Office for Official Publications of the European Communities, 2008. [Google Scholar]
Evans 1986
- Evans DMD, Hudson EA, Brown CL, Boddington MM, Hughes HE, Mackenzie EFD, et al. Terminology in gynaecological cytopathology: report of the working party of the BSCC. Journal of Clinical Pathology 1986;39:933-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferenczy 1995
- Ferenczy A. Viral testing for genital human papillomavirus infections: recent progress and clinical potentials (review). International Journal Gynecological Cancer 1995;5:321-6. [DOI] [PubMed] [Google Scholar]
Flannelly 1994
- Flannelly G, Anderson D, Kitchener HC. Management of women with mild and moderate cervical dyskaryosis. BMJ 1994;308:1399-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herbert 2007
- Herbert A, Bergeron C, Wiener H, Schenck U, Klinkhamer PJ, Arbyn M. European guidelines for quality assurance in cervical cancer screening: recommendations for cervical cytology terminology. Cytopathology 2007;18(4):213-9. [DOI] [PubMed] [Google Scholar]
Herbert 2008
Holowaty 1999
- Holowaty P, Miller AB, Rohan T, To T. Natural history of dysplasia of the uterine cervix. Journal of the National Cancer Institute 1999;91(3):252-8. [DOI] [PubMed] [Google Scholar]
IARC 2005
- IARC. Cervix cancer screening. IARC Handbooks of Cancer Prevention. Vol. 10. Lyon (France): IARC, 2005. [Google Scholar]
IARC 2007
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Human Papillomaviruses. Vol. 90. Lyon (France): IARC, 2007. [Google Scholar]
Ismail 1989
- Ismail SM, Colclough AB, Dinnen JS, Eakins D, Evans DM, Gradwell E, et al. Observer variation in histopathological diagnosis and grading of cervical intraepithelial neoplasia [see comments]. BMJ 1989;298:707-10. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jordan 2008a
- Jordan J, Martin-Hirsch P, Arbyn M, Schenck U, Baldauf JJ, Anttila A, et al. Chapter 6: Management of abnormal cervical cytology. In: Arbyn M, Anttila A, Jordan J, Ronco G, Schenck U, Segnan N, et al, European Commission, editors(s). European Guidelines for Quality Assurance in Cervical Cancer Screening. 2nd edition. Luxembourg: Office for Official Publications of the European Communities, 2008:191-232. [Google Scholar]
Jordan 2008b
- Jordan J, Arbyn M, Martin-Hirsch P, Schenck U, Baldauf J, Da Silva D, et al. European guidelines for quality assurance in cervical cancer screening: recommendations for clinical management of abnormal cervical cytology, part 1. Cytopathology 2008;19(6):342-54. [DOI] [PubMed] [Google Scholar]
Jordan 2009
- Jordan J, Martin-Hirsch P, Arbyn M, Schenck U, Baldauf J-J, Da Silva D, et al. European guidelines for management of abnormal cervical cytology, Part 2. Cytopathology 2009;20:5-16. [DOI] [PubMed] [Google Scholar]
Kinney 1998
- Kinney WK, Manos MM, Hurley LB, Ransley JE. Where's the high grade cervical neoplasia? The importance of minimally abnormal Papanicolaou diagnoses. Obstetrics and Gynecology 1998;91:973-6. [DOI] [PubMed] [Google Scholar]
Kurman 1994
- Kurman RJ, Henson DE, Herbst AL, Noller KL, Schiffman MH. Interim guidelines for management of abnormal cervical cytology. JAMA 1994;271:1866-9. [PubMed] [Google Scholar]
Kyrgiou 2006
- Kyrgiou M, Koliopoulos G, Martin-Hirsch P, Arbyn M, Prendiville W, Paraskevaidis E. Obstetric outcomes after conservative treatment for intra-epithelial or early invasive cervical lesions: a systematic review and meta-analysis of the literature. Lancet 2006;367(9509):489-98. [DOI] [PubMed] [Google Scholar]
Lorincz 1997
- Lorincz AT. Methods of DNA hybridisation and their clinical applicability to human papillomavirus detection. In: Franco E, Monsonego J, editors. New developments in cervical cancer screening and prevention. Blackwell Science, 1997. [Google Scholar]
Luff 1992
- Luff RD. National Cancer Institute. The Bethesda system for reporting cervical/vaginal cytologic diagnoses: a report of the 1991 Bethesda workshop. Human Pathology 1992;23:719-21. [DOI] [PubMed] [Google Scholar]
Lundberg 1989
- Lundberg GD. The 1988 Bethesda system for reporting cervical/vaginal cytologic diagnoses.. JAMA 1989;262:931-4. [PubMed] [Google Scholar]
Macaskill 2010
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and presenting results. In: Deeks JJ, Bossuyt PM, Gatsonis C, editors(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy; Version 1.0. The CochraneCollaboration, Available from: http://srdta.cochrane.org/.. Chichester: John Wiley and Sons, 2010:1-61. [Google Scholar]
Melnikow 1998
- Melnikow J, Nuovo J, Willan AR, Chan BK, Howell LP. Natural history of cervical squamous intraepithelial lesions: meta-analysis. Obstetrics and Gynecology 1998;92:727-35. [DOI] [PubMed] [Google Scholar]
Miller 1993
- Miller AB. Cervical cancer screening programmes. Managerial guidelines. Geneva: World Health Organisation, 1993. [Google Scholar]
Mitchell 1998
- Mitchell MF, Schottenfeld D, Tortolero-Luna G, Cantor SB, Richards-Kortum R. Colposcopy for the diagnosis of squamous intraepithelial lesions: a meta-analysis. Obstetrics and Gynecology 1998;91:626-31. [DOI] [PubMed] [Google Scholar]
Morrison 1992
- Morrison AS. Screening in chronic disease. In: Monographs in Epidemiology and Biostatistics. USA: Oxford University Press, Inc., 1992:1-254. [Google Scholar]
Moscicki 2004
- Moscicki AB, Shiboski S, Hills NK, Powell KJ, Jay N, Hanson EN, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet 2004;364:1678-83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moss 2006
- Moss S, Gray A, Legood R, Vessey M, Patnick J, Kitchener H. Effect of testing for human papillomavirus as a triage during screening for cervical cancer: observational before and after study. BMJ 2006;332:83-5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murdoch 1992
- Murdoch JB, Grimshaw RN, Morgan PR, Monaghan JM. The impact of loop diathermy on management of early invasive cervical cancer. International Journal of Gynecological Cancer 1992;2:129-33. [DOI] [PubMed] [Google Scholar]
Narod 1991
- Narod SA, Thompson DW, Jain M, Wall C, Green LM, Miller AB. Dysplasia and the natural history of cervical cancer: early results of the Toronto Cohort Study. European Journal of Cancer 1991;27:1411-6. [DOI] [PubMed] [Google Scholar]
NHSCSP 1995
- National Health Service Cancer Screening Programme (NHSCSP). Achievable standards, benchmarks for reporting, criteria for evaluating cervical cytopathology. Workshop report. Cytopathology 1995;6 Suppl 2:1-32. [PubMed] [Google Scholar]
Noumoff 1987
- Noumoff JS. Atypia in cervical cytology as a risk factor for intraepithelial neoplasia. American Journal of Obstetrics and Gynecology 1987;156:628-31. [DOI] [PubMed] [Google Scholar]
O'Sullivan 1998
- O'Sullivan JP. Observer variation in gynaecological cytopathology. Cytopathology 1998;9:6-14. [DOI] [PubMed] [Google Scholar]
Ostor 1993
- Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. International Journal of Gynaecologic Pathology 1993;12:186-92. [PubMed] [Google Scholar]
Partridge 2010
Pepe 2004
- Pepe MS. Incomplete data and imperfect reference tests. In: Pepe MS, editors(s). The statistical evaluation of medical tests for classification and prediction. Oxford: Oxford University Press, 2004:168-213. [MEDLINE: ] [Google Scholar]
Pretorius 2004
- Pretorius RG, Zhang WH, Belinson JL, Huang MN, Wu LY, Zhang X, et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. American Journal of Obstetrics and Gynecology 2004;191:430-4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pretorius 2006
- Pretorius RG, Kim RJ, Belinson JL, Elson P, Qiao YL. Inflation of sensitivity of cervical cancer screening tests secondary to correlated error in colposcopy. Journal of Lower Genital Tract Diseases 2006;10:5-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AWS, Scholten RJPM, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58:982-90. [DOI] [PubMed] [Google Scholar]
Richart 1973
- Richart M. Cervical intraepithelial neoplasia. Pathology Annual 1973;8:301-23. [PubMed] [Google Scholar]
Richart 1987
- Richart RM. Causes and management of cervical intraepithelial neoplasia. Cancer 1987;60:1951-9. [DOI] [PubMed] [Google Scholar]
Richart 1993
- Richart RM, Wright TC. Controversies in the management of low-grade cervical intraepithelial neoplasia. Cancer 1993;71:1413-21. [DOI] [PubMed] [Google Scholar]
Riley 2007
- Riley RD, Abrams KR, Lambert PC, Sutton AJ, Thompson JR. An evaluation of bivariate random-effects meta-analysis for the joint synthesis of two correlated outcomes. Statistics in Medicine 2007;26(1):78-97. [DOI] [PubMed] [Google Scholar]
Robertson 1988
- Robertson JH, Woodend BE, Crozier EH, Hutchinson J. Risk of cervical cancer associated with mild dyskaryosis. BMJ 1988;297:18-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robertson 1989
- Robertson AJ, Anderson JM, Beck JS, Burnett RA, Howatson SR, Lee FD, et al. Observer variability in histopathological reporting of cervical biopsy specimens. Journal of Clinical Pathology 1989;42:231-8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roelens 2012
- Roelens J, Reuschenbach M, Knebel-Doeberitz M, Wentzensen N, Bergeron C, Arbyn M. p16INK4a immunocytochemistry versus HPV testing for triage of women with minor cytological abnormalities: a systematic review and meta-analysis. Cancer Cytopathology 2012;120(5):294-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rutter 2002
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Statistics in Medicine 2002;20(19):2865-84. [DOI] [PubMed] [Google Scholar]
Sawaya 2005
- Sawaya GF. A 21-year-old woman with atypical squamous cells of undetermined significance. JAMA 2005;294(17):2210-18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schiffman 2003
- Schiffman M, Kjaer SK. Natural history of anogenital human papillomavirus infection and neoplasia. Journal of the National Cancer Institute. Monographs 2003;31:14-9. [DOI] [PubMed] [Google Scholar]
Schiffman 2005a
- Schiffman MA, Khan MJ, Solomon D, Herrero R, Wacholder S, Hildesheim A, et al. A study of the impact of adding HPV types to cervical cancer screening and triage tests. Journal of the National Cancer Institute 2005;97:147-50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schiffman 2005b
- Schiffman M, Wheeler CM, Dasgupta A, Solomon D, Castle PE. A comparison of a prototype PCR assay and hybrid capture 2 for detection of carcinogenic human papillomavirus DNA in women with equivocal or mildly abnormal Papanicolaou smears. American Journal of Clinical Pathology 2005;124:722-32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sherman 2003
- Sherman ME, Wang SS, Tarone R, Rich L, Schiffman M. Histopathologic extent of cervical intraepithelial neoplasia 3 lesions in the atypical squamous cells of undetermined significance low-grade squamous intraepithelial lesion triage study: implications for subject safety and lead-time bias. Cancer Epidemiology, Biomarkers and Prevention 2003;12:372-9. [PubMed] [Google Scholar]
Solomon 2002
- Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 Bethesda system: terminology for reporting results of cervical cytology.. JAMA 2002;287:2114-9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Solomon 2009
Soutter 1994
- Soutter WP. Controversies in management: management of women with mild dyskaryosis. BMJ 1994;309:591-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stampler 2010
- Stampler KM, Dunton CJ. Cervical neoplasia guidelines: United States and Europe compared. Journal of Lower Genital Tract Disease 2010;14(2):142-7. [DOI] [PubMed] [Google Scholar]
Takwoingi 2009 [Computer program]
- Diagnostic Test Accuracy Working Group METADAS: a SAS macro for meta-analysis of diagnostic accuracy studies [Accessible via: http://srdta.cochrane.org/Files/Website/METADAS_Readme_v1.0_beta.pdf]. Takwoingi Y. Diagnostic Test Accuracy Working Group, 2009.
Walboomers 1999
- Walboomers JMM, Jacobs MV, Manos M, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. Journal of Pathology 1999;189:12-9. [DOI] [PubMed] [Google Scholar]
Wentzensen 2008
- Wentzensen N, Schiffman M, Dunn ST, Zuna RE, Walker J, Allen RA, et al. Grading the severity of cervical neoplasia based on combined histopathology, cytopathology, and HPV genotype distribution among 1,700 women referred to colposcopy in Oklahoma. International Journal of Cancer 2009;124(4):964-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2003
- Whiting P, Rutjes AWS, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Medical Research Methodology 2003;3(25):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2003
- WHO. Pathology and Genetics of Tumours of the Breast and Female Genital Organs. Lyon: IARCPress, 2003. [Google Scholar]
Wilkinson 1990
- Wilkinson C, Jones JM, Mcbride J. Anxiety caused by abnormal result of cervical smear test: a controlled trial. BMJ 1990;300(6722):440. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woodman 2001
- Woodman CBJ, Collins S, Winter H, Bailey A, Elis J, Prior P, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet 2001;357:1831-6. [DOI] [PubMed] [Google Scholar]
Wright 2002
- Wright TC, Cox JT, Massad LS, Wilkinson EJ. Consensus guidelines for the management of women with cervical cytological abnormalities. JAMA 2002;287:2120-9. [DOI] [PubMed] [Google Scholar]
Wright 2007
- Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D. 2006 consensus guidelines for the management of women with abnormal cervical cancer screening tests. American Journal of Obstetrics and Gynecology 2007;197(4):346-55. [DOI] [PubMed] [Google Scholar]
Yang 2004
- Yang BH, Bray FI, Parkin DM, Sellors JW, Zhang Z-F. Cervical cancer as a priority for prevention in different world regions: an evaluation using years of life lost. International Journal of Cancer 2004;109:418-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zuna 2005
- Zuna RE, Wang SS, Rosenthal DL, Jeronimo J, Schiffman M, Solomon D. Determinants of human papillomavirus-negative, low-grade squamous intraepithelial lesions in the atypical squamous cells of undetermined significance/low-grade squamous intraepithelial lesions triage study (ALTS). Cancer 2005;105:253-62. [DOI] [PubMed] [Google Scholar]
zur Hausen 1994
- zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Current Topics in Microbiology and Immunology 1994;186:131-56. [DOI] [PubMed] [Google Scholar]



