Abstract
Background
Hyperkalaemia occurs in outpatients and in between 1% and 10% of hospitalised patients. When severe, consequences include arrhythmia and death.
Objectives
To review randomised evidence informing the emergency (i.e. acute, rather than chronic) management of hyperkalaemia
Search methods
We searched MEDLINE (1966‐2003), EMBASE (1980‐2003), The Cochrane Library (issue 4, 2003), and SciSearch using the text words hyperkal* or hyperpotass* (* indicates truncation). We also searched selected journals and abstracts of meetings. The reference lists of recent review articles, textbooks, and relevant papers were reviewed for additional potentially relevant titles.
Selection criteria
All selection was performed in duplicate. Articles were considered relevant if they were randomised, quasi‐randomised or cross‐over randomised studies of pharmacological or other interventions to treat non‐neonatal humans with hyperkalaemia, reporting on clinically‐important outcomes, or serum potassium levels within the first six hours of administration.
Data collection and analysis
All data extraction was performed in duplicate. We extracted quality information, and details of the patient population, intervention, baseline and follow‐up potassium values. We extracted information about arrhythmias, mortality and adverse effects. Where possible, meta‐analysis was performed using random effects models.
Main results
None of the studies of clinically‐relevant hyperkalaemia reported mortality or cardiac arrhythmias. Reports focused on serum potassium levels. Many studies were small, and not all intervention groups had sufficient data for meta‐analysis to be performed. On the basis of small studies, inhaled beta‐agonists, nebulised beta‐agonists, and intravenous (IV) insulin‐and‐glucose were all effective, and the combination of nebulised beta agonists with IV insulin‐and‐glucose was more effective than either alone. Dialysis is effective. Results were equivocal for IV bicarbonate. K‐absorbing resin was not effective by four hours, and longer follow up data on this intervention were not available from RCTs.
Authors' conclusions
Nebulised or inhaled salbutamol, or IV insulin‐and‐glucose are the first‐line therapies for the management of emergency hyperkalaemia that are best supported by the evidence. Their combination may be more effective than either alone, and should be considered when hyperkalaemia is severe. When arrhythmias are present, a wealth of anecdotal and animal data suggests that IV calcium is effective in treating arrhythmia. Further studies of the optimal use of combination treatments and of the adverse effects of treatments are needed.
Keywords: Humans; Emergency Treatment; Adrenergic beta‐Agonists; Adrenergic beta‐Agonists/therapeutic use; Albuterol; Albuterol/therapeutic use; Bicarbonates; Bicarbonates/therapeutic use; Glucose; Glucose/therapeutic use; Hyperkalemia; Hyperkalemia/therapy; Hypoglycemic Agents; Hypoglycemic Agents/therapeutic use; Infusions, Intravenous; Insulin; Insulin/therapeutic use; Randomized Controlled Trials as Topic; Renal Dialysis
Plain language summary
Emergency interventions for hyperkalaemia
No trials with clinically‐important outcomes were identified. Many of the studies were conducted in convenience samples of patients. Many of the trials were methodologically flawed. Adverse events were rarely reported. Decrease in serum potassium was the most frequently reported outcome: for this outcome beta agonists and intravenous insulin‐and‐glucose were effective. The combination may be more effective than either alone.
Background
Hyperkalaemia is defined as an excess concentration of potassium ions in the extracellular fluid compartment. Hyperkalaemia occurs in outpatients, and between 1% and 10% of hospitalised patients (Moore 1989; Paice 1983; Shemer 1983), often due to renal failure, hyperglycaemia, or inappropriate use of potassium supplements and other drugs that affect potassium homeostasis (Acker 1998; Moore 1989; Paice 1983; Shemer 1983). Commonly used therapies include intravenous (IV) calcium salts to reverse membrane polarization abnormalities (Greenberg 1998; Schwartz 1978; Weiner 1998); bicarbonate (Halperin 1998), insulin (Zierler 1987) or beta‐2 agonists (Williams 1985) to shift potassium from the extracellular to the intracellular compartment; and resins (Berlyne 1966;Frohnert 1968; Johnson 1976Scherr 1961) or dialysis (Blumberg 1988) to remove potassium from the extracellular compartment.
Patients with hyperkalaemia may experience weakness, fatigue, distal paraesthesiae, and respiratory depression (Freeman 1993). Changes in the electrocardiogram (ECG) include peaked T waves, QRS widening, and diminished P waves (Browning 1981). Severe hyperkalaemia can cause the QRS complex to merge with the T wave, leading to a sine wave pattern. This may progress to atrioventricular dissociation, ventricular tachycardia or fibrillation, and death. Treatment for hyperkalaemia has been shown to vary from patient to patient within an institution, without obvious rationale (Acker 1998). Variations in recommendations for treatment of hyperkalaemia were observed in a survey of nephrology program directors (Iqbal 1989). No previous systematic review of this question has appeared.
Objectives
To summarise randomised controlled trials (RCTs) and quasi‐RCTs of acute interventions (calcium, insulin, beta‐2 agonists, bicarbonate, ion‐exchange resins and dialysis) in the treatment of patients with hyperkalaemia, with respect to the outcomes: serum potassium, ECG changes, arrhythmia, adverse effects of therapy and death.
To identify whether the addition of beta‐2 agonists to IV insulin is safe (few adverse effects) and effective (results in greater lowering of serum potassium than IV insulin alone).
To identify whether the addition of IV insulin to beta‐2 agonists is safe (few adverse effects) and effective (results in greater lowering of serum potassium than beta‐2 agonists alone).
To identify whether the addition of sodium bicarbonate to other agents of combinations is safe (few adverse effects) and effective (results in greater lowering of serum potassium than other agents or combinations alone).
To identify areas in which further investigation is required.
Methods
Criteria for considering studies for this review
Types of studies
RCTs, quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) and randomised cross‐over studies were included.
Types of participants
Inclusion criteria
Non‐neonatal (> one month old) humans (hyperkalaemia in neonates appears to be a distinct clinical problem and we did not wish to include studies of neonates).
Exclusion criteria
Artificially‐induced hyperkalaemia.
Types of interventions
All interventions used in the emergency (acute, rather than chronic) treatment of hyperkalaemia were studied. Pharmacological or other agents used to lower plasma (K+) or to treat or prevent adverse effects of hyperkalaemia. Intervention was consistent between patients and described in sufficient detail to be reproducible. At least one intervention given to five or more subjects.
Types of outcome measures
The following outcomes were of interest:
plasma or serum potassium concentrations (pre‐intervention and post‐intervention),
arrhythmias,
ECG changes,
adverse effects of interventions,
death.
Outcomes within the first six hours of the intervention were studied.
Search methods for identification of studies
We searched the following databases:
MEDLINE(1966‐2003),
EMBASE (1980‐2003),
The Cochrane Library (Issue 4, 2003)
Cochrane Renal Group's specialised register of trials,
SciSearch.
Articles were identified through the keyword search: hyperkal* OR hyperpotass*. In MEDLINE, the strategy was refined by combination (Boolean 'and') with an expert search strategy that has been shown to identify studies of therapeutic interventions with 98% sensitivity (Haynes 1994). We searched the journals American College of Physicians Journal Club and Evidence Based Medicine using the text words potassium, hyperkalemia/c and hyperkalaemia/c. Abstracts from the American Society of Nephrology meeting between 1996 and 2003 were searched using the same text words. We reviewed the reference lists of chapters describing the management of hyperkalemia in the following textbooks: Brenner and Rector's The Kidney (Brenner 2000), the Oxford Textbook of Nephrology (Davison 1998), Schrier's Diseases of the Kidney (Schrier 1993), and the online reference UpToDate. We also reviewed the reference lists of recent review articles on hyperkalaemia and all relevant studies. Studies were not excluded on the basis of language of publication.
Data collection and analysis
Thedatabase searching, identification of relevant articles, quality assessment and data extraction was performed independently in duplicate by two reviewers (BAM and WADS; DL and KT). Titles and abstracts were reviewed independently in duplicate and full text obtained for possibly relevant articles. Final decisions were made independently by the two reviewers on the basis of full text. Disagreements were resolved by group discussion including CMC, and consensus was reached in each case. Where data is available in text or tabulated form, this was extracted in preference to values displayed graphically. Where relevant outcomes (e.g. serum potassium) were reported only graphically, data were extracted from photographically enlarged copies of graphs. With the exception of data extracted from graphs, differences were resolved by consensus. For data extracted from graphs, the mean values of those obtained by each of the two reviewers were used, unless one set was clearly in error on comparison. Correspondence with study authors was attempted to complete missing data. Studies were not excluded on the basis of author, affiliation, language, journal or publication year. Final decisions to include or exclude based upon an examination of the full text of each identified article, along with additional information from authors who responded to questions.
Statistical assessment
Random effects models were used to combine outcomes for interventions versus placebo and for comparisons between interventions. If available, change in serum potassium from baseline to serum potassium 30 (± 5) minutes and 60 (± 5) minutes after the intervention were to be combined. Dichotomous outcomes (arrhythmia, death) were to be presented for the total follow‐up period.
Subgroup analysis
We had prespecified a subgroup analysis summarising RCTs only (and excluding quasi‐RCTs), however, no quasi‐RCTs were identified. We had also planned to examine, if data permitted, whether effects were different in convenience samples (e.g. haemodialysis patients studied before a routine treatment), compared with patients requiring clinical treatment for hyperkalaemia; in end‐stage renal disease (ESRD) patients compared with patients with renal function; in diabetics compared with nondiabetics; for route of administration of beta‐2 agonists (IV versus nebulised versus aerosolised); and for hypertonic sodium bicarbonate compared with isotonic sodium bicarbonate. However, because of the small number of studies that provided data that could be combined, none of these subgroup analyses was possible.
Quality assessment
The methodological quality of each relevant study was characterised independently by two pairs of reviewers (BAM and WADS for studies before 1999; DL and KT for studies from 1999).
Studies were categorised as:
RCTs,
randomised crossover trials, or
quasi‐RCTs.
The following information was extracted:
allocation concealment,
blinding of participants, blinding of investigators, blinding of outcome assessment,
intention‐to‐treat analysis,
completeness of follow‐up (> 80%).
We did not combine these quality items into a composite score. Studies were not excluded from the review on the basis of quality.
Heterogeneity
We planned to examine heterogeneity using Chi² and considering P < 0.10 as statistically significant, and the I² statistic (Higgins 2003).
Cross‐over studies
We planned to use data from the time period prior to crossing over. However, we found none of the studies reported the two time periods separately, and we extracted the data as reported for all the patients.
Possible duplicate publications
When it appeared that there might be overlap in patient populations between two reports, we contacted authors to clarify the issue. If they did not respond, we used only data from the more recent publication.
Results
Description of studies
From MEDLINE (1966‐1998) the search strategy identified 282 citations, of which two relevant studies were identified by both reviewers (McClure 1994; Ngugi 1997). There were no disagreements (Kappa = 1.0). From the period of 1999 to 2003, 239 citations were identified in MEDLINE using the search strategy. These were reviewed in duplicate and two relevant articles were identified by each reviewer (Kappa = 1.0) (Mahajan 2001; Mandelberg 1999).
An EMBASE search from 1980 to 2003 identified 1234 articles. After deduplication against MEDLINE, two additional RCTs were identified by both reviewers (Kappa = 1.0) (Gruy‐Kapral 1998; Pancu 2003). Cochrane search retrieved 219 articles. Nine relevant articles, six of which had not previously been identified in MEDLINE or EMBASE, were identified by both reviewers (Kappa = 1.0) (Allon 1989; Allon 1990; Allon 1995; Allon 1996; Gutzwiller 2003; Liou 1994). One of these articles excluded 'non‐responders' from the analysis (Liou 1994). Data from this study are not considered further for this reason (a post‐hoc exclusion). Kappa for this search was 0.94.
ACP Journal Club, Evidence‐Based Medicine, the abstracts of the American Society of Nephrology, UpToDate, textbooks and review articles did not produce any additional studies that met the relevance criteria.
One other article was identified in the authors' files that was not identified by any of the search strategies (Zehnder 2001).
No studies reported the clinically‐important outcomes of arrhythmia or death.
Most studies reported serum potassium levels at different time points after the initiation of the intervention. Most of the interventions studied had been examined in only one trial, and therefore combining data across studies was not possible. However, four studies comparing inhaled salbutamol (albuterol) with placebo were identified (Allon 1989; Allon 1996; Mandelberg 1999; Pancu 2003). Of these, one study compared two different doses (10 mg and 20 mg) of inhaled salbutamol with placebo (Allon 1989). We chose the data from the higher of the two doses for inclusion in the meta‐analysis. A random effects model was used to combine outcomes for inhaled salbutamol versus placebo. The primary outcome was serum potassium at 30 minutes. Other time points as reported in the original papers have also been also shown. In one of the studies of inhaled salbutamol included in the meta‐analysis, standard deviations were given for the treatment group but not for the control group (Mandelberg 1999). In this case, the standard deviations for the control group were assumed to equal those of the control group at each time point. For a number of other comparisons, standard error of the means were given. In one case, neither standard error or standard deviations were given (Ngugi 1997). In this case, the missing standard deviations were entered as zeros and the data were not included in the pooled analyses.
Risk of bias in included studies
Twelve studies were included in this review. Eight were randomised crossover trials (Allon 1989; Allon 1990; Allon 1995; Allon 1996; Gruy‐Kapral 1998; Gutzwiller 2003; McClure 1994; Zehnder 2001); four were RCTs with placebo (Pancu 2003; Mandelberg 1999) or without placebo (Mahajan 2001; McClure 1994; Ngugi 1997).
Allocation concealment was adequate in four studies (Allon 1989; Allon 1995; Allon 1996; Mandelberg 1999). The intervention was reproducibly described in all twelve studies. Three studies ( Allon 1989; Mandelberg 1999; Pancu 2003) used blinding of investigators and patients to treatment. There was one single blinded study (Ngugi 1997). Cointerventions were not described in any study.
One study (Pancu 2003) reported either standard deviations or standard errors without stating which were used. Another study did not consistently report variances (Ngugi 1997). In some cases it was not explicitly stated whether standard error or standard deviation was reported for some or all of the values extracted. In these cases, we assumed that authors had used the same metric throughout, so that if standard errors were mentioned anywhere in the paper, the data were treated as standard errors in our analysis. In some papers, the standard deviation of absolute post‐treatment values in the different groups were not reported. In these cases, we calculated the standard deviation of the absolute potassium value at each time point from the standard error of the difference, sample size per group, and standard deviation of the absolute value at baseline (Norman 1993).
Effects of interventions
None of the studies of clinically‐relevant hyperkalaemia reported mortality or cardiac arrhythmias. Reports focussed on serum potassium levels. Characteristics are shown in the table of included studies. All appeared to be studies of convenience samples of patients with modest hyperkalaemia and either acute or chronic renal insufficiency. No study reported the emergency management of life‐threatening hyperkalaemia. A number of studies did not report absolute values of serum potassium for time points later than baseline, or did not report variance in a usable way. Some papers reported change in potassium and standard error of the change, rather than the absolute values of potassium, and standard errors or standard deviations of the absolute values at different time points. These problems limited the number of studies from which combinable data could be extracted. For this reason, effect sizes and statistical significance for each individual study are described below, and are shown in the additional Table 1‐ Narrative summary of results for efficacy and harm.
1. Narrative summary of results for efficacy and harm.
| Study | Intervention | Efficacy | Adverse Effects |
| Gruy‐Kapral 1998 | Resin‐cathartics | The serum potassium concentrations before the various treatments ranged from 3.4 to 5.7 mEq/L. None of the resin‐cathartic regiments caused a fall in average serum potassium concentrations, compared with prepreatment values. | None reported |
| Gutzwiller 2003 | Haemodialysis with blood flow of 200, 250, 300 mL/min | Potassium removal was 53.0 ± 2.4, 63.4 ± 2.6, and 74.2 ± 3.8 mMol with blood flows of 200, 250, and 300 mL/min, respectively. Kt/V increased from 1.10 ± 0.14 to 1.22 ± 0.14 (10.9%) and finally to 1.39 ± 0.16 (26.4%), P = 0.0001, with increasing blood flow. | None reported |
| Mahajan 2001 | Aminophylline infusion versus insulin‐dextrose infusion aminophyllin infusion versus insulin‐dextrose infusion | IV aminophylline lowered plasma potassium from 6.48 ± 0.39 mEq/L to 5.92 ± 0.40 mEq/L at 180 min (P < 0.001 versus basal) and 6.05 ± 0.53 mEq/L at 360 min (P < 0.01 versus basal). IV insulin‐dextrose lowered plasma potassium from 6.59 ± 0.31 mEq/L to 5.76 ± 0.32 mEq/L (P < 0.001 versus basal) and 5.84 ± 0.21 mEq/L (P < 0.001 versus basal). Aminophylline is effective for acute treatment of hyperkalaemia but it is less effective than insulin‐dextrose infusion. | There was one episode of hypoglycaemia in the insulin‐glucose group. No other side effects were observed. |
| Mandelberg 1999 | 1200 μg salbutamol through MDS‐1 | Potassium levels rose from 5.5 mEq/L to 5.55 mEq/L after 1 min following completion of treatment and then decreased steadily. Potassium level decreased to 5.1 mEq/L by 60 min. The consistent reduction serium potassium levels started 3‐5 min following delivery of salbutamol. | The heart rate increased from 83 ± 2 at baseline to 89 ± 2 at 1 min was greatest at 95 ± 2 at 15 min after salbutamol inhalation. The heart rate declined slightly after 1 h. |
| McClure 1994 | Salbutamol given IV versus salbutamol nebuliser | Within 30 min of the first dose, the mean plasma potassium concentration fell significantly by 0.87 and 0.61 mmol/L after IV and nebulised administration respectively. Sixty minutes after the second dose the plasma potassium was significantly reduced by a further 0.28 and 0.53 mmol/L respectively. At the end point of the study (300 min) the level of reduction in plasma potassium in the nebulised group was significantly greater than in the group receiving salbutamol IV (1.19 compared with 0.7 mmol/L). | No major side effects were observed. Of the 10 patients who received IV salbutamol 6 had mild short lasting tremor, and 1 had mild vasomotor flushing. Of the 10 patients who received nebulized salbutamol, 4 had tremor, 2 had tremor with nausea, and one had mild vasomotor flushing. |
| Ngugi 1997 | Dextrose and insulin, versus bicarbonate, versus salbutamol versus 2 regimen combination treatments | Of the single therapeutic approaches, at 2 h, the decrease in serum potassium caused by insulin with glucose was 0.90 ± 0.45 mmol/L (P < 0.001) which was comparable to that caused by salbutamol 0.90 ± 0.56 mmol/L (P < 0.001). Amongst the 2 regimen combinations, at 2 hours the decrease caused by salbutamol and insulin with glucose of 1.18 ± 0.69 mmol/L (P < 0.001) was comparable to that of insulin with glucose and bicarbonate of 1.19 ± 0.50 mmol/L (P = 0.002). | None reported |
| Pancu 2003 | Nebulised albuterol versus nebulised levalbuterol | Immediately after nebulisation, only levalbuterol showed a significant decrease in potassium level (P = 0.024). Serum potassium was lowered from a baseline value of 3.9 ± 0.3 mEq/L to 3.6 ± 0.5 at 30 min post‐treatment and to 3.6 ± 0.4mEq/L at 60 min post‐treatment with albuterol, and from 4.1 ± 0.3 mEq/L to 3.8 ± 0.2 at 30 min and to 3.6 ± 0.2 mEq/L at 60 min with levalbuterol. No significant difference was found when the albuterol and levalbuterol groups were compared. Levalbuterol caused fewer adverse effects. | Seven patients in the albuterol group had side effects (7 had tremor, 5 nervousness, 5 palpitations, 4 had tachycardia). The 4 patients with tachycarida had heart rates between 105 and 125 beats/min. Two patients in the levalbuterol group reported tremor. One patient in the normal saline group reported nervousness and palpitations. |
| Zehnder 2001 | Dialysate containing 0, 1, and 2 mmol/L of potassium | Serum potassium at completion of dialysis was lower with 0K compared with 1K and 2K, achieving 2.7 ± 0.1, 3.0 ± 0.2 and 3.5 ± 0.1mmol/L (P < 0.001) respectively. The amount of potassium removed was significantly different between the 3 regimens used. Potassium removal was 117.1 ± 10.3 mmol for 0K, 80.2 ± 6.2 mmol for 1K, and 63.3 ± 5.2 mmol for 2K (P < 0.001). Potassium free dialysate removed 85% more potassium than 2K and 46% more than 1K. | None reported. |
| Allon 1990 | IV insulin with glucose, nebulised albuterol or combination of IV insulin with glucose and nebulised albuterol | IV insulin with glucose produced a fall in plasma potassium apparent within 15 min of drug administration and persisted for at least one hour. Nebulised albuterol resulted in a decrease in plasma K within 30 min of initiating treatment. The mean decrease in plasma potassium was not different at 30, 45, or 60 min following the 2 respective treatments. The maximal decrease was 0.65 ± 0.09 and 0.66 ± 0.12 mmol/L after insulin with glucose and albuterol, respectively. The magnitude of fall in plasma potassium was greater with combined IV insulin and glucose and nebulized albuterol. the maximal decrease in mean plasma potassium concentration after insulin with glucose plus albuterol was 1.21 ± 0.19 mmol/L. | There were no statistically significant changes in blood pressure or heart rate following any of the treatments. Combined drug therapy (IV insulin with glucose and nebulised salbutamol) was associated with a increase in heart rate of 15.1 ± 6.0 beats/min at 60 min (P < 0.02). |
| Allon 1996 | Isotonic bicarbonate, insulin and albuterol, and the above in combination with bicarbonate | IV bicarbonate did not decrease plasma potassium during 60 min of observation. IV insulin with dextrose decreased plasma potassium 0.5 mmol/L (P < 0.001) within 15 min and progressed for the duration of infusion. The magnitude of change at 60 min did not differ whether insulin was given with isotonic bicarbonate or saline (‐0.81 ± 0.15 mmol/L versus ‐0.85 ± 0.06 mmol/L; P = 0.65). Nebulised albuterol decreased plasma potassium by 0.2 mmol/L at 15 min and progressed for the duration of the infusion. The magnitude of change at 60 min did not differ whether albuterol was given with isotonic bicarbonate or saline (0.71 ± 0.16 mmol/L versus ‐0.53 ± 0.15 mmol/L; P = 0.18). | The 3 protocols that included bicarbonate administration resulted in significant increases in blood bicarbonate (+3.5 ± 0.5 mmol/L, +3.1 ± 0.5 mmol/L, and +2.2 ± 0.5 mmol/L, P < 0.005) and pH (+0.05 ± 0.01, +0.03 ± 0.01, and +0.03 ± 0.01, P < 0.01). Administration of insulin with glucose increased plasma glucose to high physiologic concentrations (P < 0.05) at all time points between 15 and 60 min. Nebulised albuterol produced a mild increase in plamsa glucose (P < 0.05) at time points between 30 and 60 min. |
| Allon 1989 | Nebulised albuterol (10 mg or 20 mg) or placebo | Nebulised albuterol therapy resulted in a significant fall in the plasma potassium concentration within 30 min of drug administration that persisted for at least 2 hours. The maximal mean decrease in the potassium concentration was 0.62 ± 0.09 mmol/L after the 10 mg dose and 0.98 ± 0.14 mmol/L after the 20 mg dose (P < 0.001 for both comparisons). At all periods between 30 and 120 min, the decrease in the plasma potassium concentraion was significantly greater than that measured in the corresponding period of placebo administration. The potassium‐lowering effect of the 20 mg dose was greater than that of the 10 mg dose, but this difference achieved statistical significance only at 120 min. | No significant changes in blood pressure or heart rate with albuterol treatment. All patients denied palpitations, tremor, or chest pain after inhalation of albuterol. One patient had mild anxiety after a 20 mg dose of albuterol. |
| Allon 1995 | Nebulised albuterol (20 mg) or placebo in addition to hemodialysis treatment | Plasma potassium decreased significantly 30 min after nebulised albuterol therapy compared to placebo in the pre‐dialysis period. Dialysis decreased plasma potassium in both groups, but plasma potassium in the albuterol treatment group has persistently lowered level than placebo. However, the total potassium removal is significantly less in the albuterol group. | Albuterol produced a significant increase in heart rate 30 minutes after its administration ( 79 ± 4 pretreatment, 103 ± 5 predialysis/30 min after administration, P < 0.001). Rare unifocal premature ventricular contractions were noted in 5/7 patients in the albuterol protocol and in 2/7 patients in the control protocol during the last 2 hours of dialysis. One patient with a history of paroxsymal atrial fibrillation developed asymptomatic atrial fibrillation after albuterol administration that resolved spontaneously. |
We contacted two authors in the hope of clarifying uncertainties or finding additional data, of whom one responded.
Beta‐agonists
Eight studies (Allon 1989; Allon 1990; Allon 1995; Allon 1996; Mandelberg 1999; McClure 1994; Ngugi 1997; Pancu 2003) investigated the efficacy of beta‐agonists in the treatment of hyperkalaemia (Analysis 1.1; Analysis 2.1; Analysis 3.1).
1.1. Analysis.
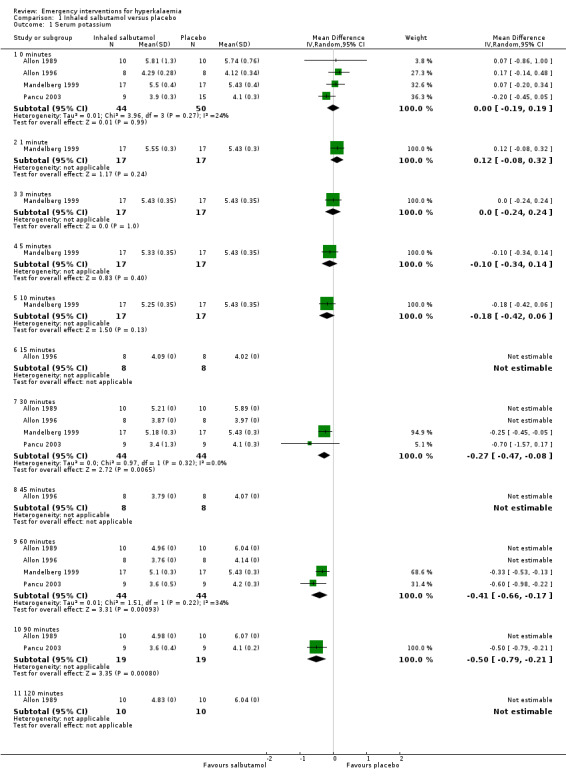
Comparison 1 Inhaled salbutamol versus placebo, Outcome 1 Serum potassium.
2.1. Analysis.
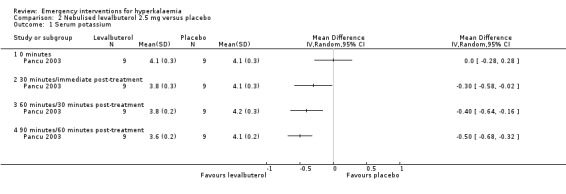
Comparison 2 Nebulised levalbuterol 2.5 mg versus placebo, Outcome 1 Serum potassium.
3.1. Analysis.
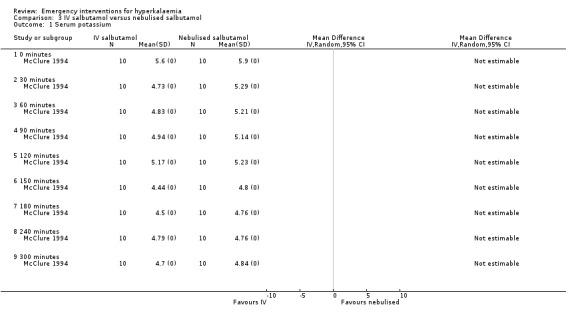
Comparison 3 IV salbutamol versus nebulised salbutamol, Outcome 1 Serum potassium.
Inhaled or nebulised beta‐agonists were effective, compared with placebo, by 30 minutes, and at all time points beyond that. Individual study results, not amenable to meta analysis, provided further information about dose‐response, method of administration and time course of action (Allon 1989; Allon 1990; Allon 1995; Allon 1996; Mandelberg 1999; McClure 1994; Ngugi 1997; Pancu 2003). A dose‐response relationship in maximum serum potassium reduction was observed: potassium values in the group receiving 20 mg nebulised salbutamol (albuterol) were lower than in the group receiving 10 mg throughout the experiment, reaching statistical significance (P < 0.05) at 120 minutes (Allon 1989). Compared with the placebo control, salbutamol (albuterol) and levalbuterol were equally effective in reducing serum potassium at 30 minutes and 60 minutes after drug introduction (Pancu 2003).
When comparing IV administration of salbutamol with nebulised salbutamol, no statistically significant differences were observed between groups. Based on the reporting of the variance estimates in this study, an effect estimate could not be calculated for this review (McClure 1994). For both routes of delivery, a second dose at 120 minutes caused further reductions in serum potassium (McClure 1994). Paradoxical elevation of serum potassium of 0.1 mmol/L or more was observed in 59% of patients one minute after administration of inhaled salbutamol (albuterol) in one study (Mandelberg 1999). In these patients, potassium returned to baseline by three minutes, and then fell progressively, reaching a statistically significant difference compared with placebo between five and 10 minutes.
No direct comparisons of the safety and efficacy of MDS‐I with either nebulised or IV salbutamol were available.
Insulin in combination with glucose infusion
We were unable to perform meta‐analysis for this intervention. However, four individual studies (Allon 1990; Allon 1996; Mahajan 2001; Ngugi 1997) investigated the efficacy of insulin with glucose in the treatment of emergency hyperkalaemia. Insulin with glucose was effective by 15 minutes in studies which assessed this time point (Allon 1990; Allon 1996), and at 30 minutes in all studies (Analysis 4.1; Analysis 14.1; Analysis 15.1; Analysis 16.1).
4.1. Analysis.
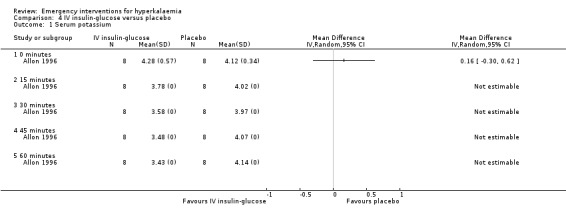
Comparison 4 IV insulin‐glucose versus placebo, Outcome 1 Serum potassium.
14.1. Analysis.
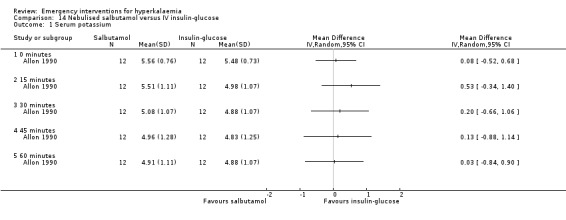
Comparison 14 Nebulised salbutamol versus IV insulin‐glucose, Outcome 1 Serum potassium.
15.1. Analysis.
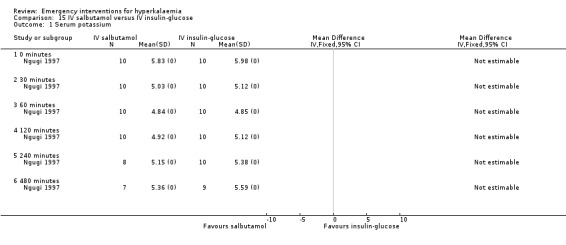
Comparison 15 IV salbutamol versus IV insulin‐glucose, Outcome 1 Serum potassium.
16.1. Analysis.
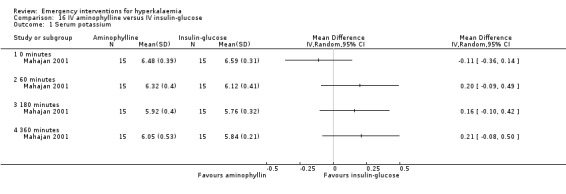
Comparison 16 IV aminophylline versus IV insulin‐glucose, Outcome 1 Serum potassium.
Resin potassium binders
One study (Gruy‐Kapral 1998) addressed the effects of sodium polystyrene sulfonate on serum potassium in chronic renal failure patients. No differences in serum potassium were observed at four hours when resin was compared with placebo. No useful information on later time points could be extracted from this study, because oral potassium was administered at four hours (Analysis 5.1; Analysis 6.1; Analysis 7.1; Analysis 8.1).
5.1. Analysis.
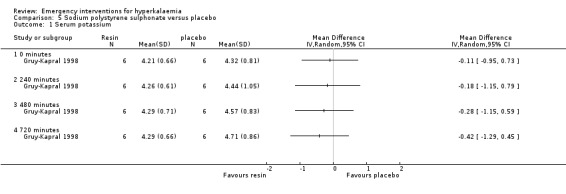
Comparison 5 Sodium polystyrene sulphonate versus placebo, Outcome 1 Serum potassium.
6.1. Analysis.
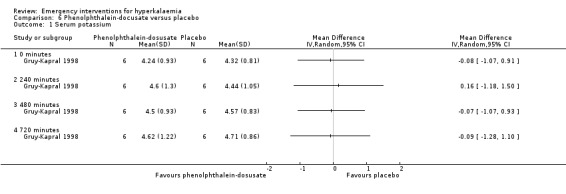
Comparison 6 Phenolphthalein‐docusate versus placebo, Outcome 1 Serum potassium.
7.1. Analysis.
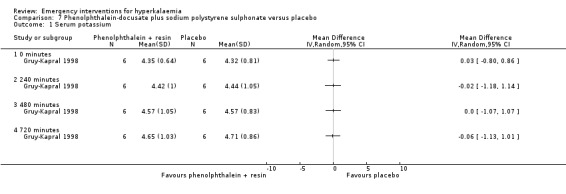
Comparison 7 Phenolphthalein‐docusate plus sodium polystyrene sulphonate versus placebo, Outcome 1 Serum potassium.
8.1. Analysis.
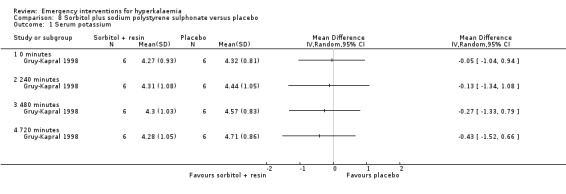
Comparison 8 Sorbitol plus sodium polystyrene sulphonate versus placebo, Outcome 1 Serum potassium.
Bicarbonate infusion
One study (Ngugi 1997) compared bicarbonate infusion with insulin and glucose and with salbutamol (albuterol) infusion, and found that it lowered serum potassium levels by 0.47 ± 0.31 mmol/L at 30 minutes (P = 0.001) and at all other time points. However in this study, the bicarbonate was less effective in reducing serum potassium than either of the alternatives (insulin, salbutamol). However, in the only placebo‐controlled study (Allon 1996) bicarbonate therapy did not lower serum potassium at any time points out to 60 minutes (P = 0.60). Variance estimates for the absolute values were not available in either of these publications.
Dialysis
Two studies (Gutzwiller 2003; Zehnder 2001) investigated the utility of haemodialysis in serum potassium removal. The use of low‐potassium (1 mmol/L and 2 mmol/L potassium chloride) and potassium‐free dialysate was shown to be safe and effective in reducing serum potassium (Zehnder 2001). High potassium removal did not impair dialysis efficiency. There was a greater amount of potassium removed during dialysis with increasing blood flow (Gutzwiller 2003) (Analysis 10.1; Analysis 11.1; Analysis 12.1; Analysis 13.1).
10.1. Analysis.
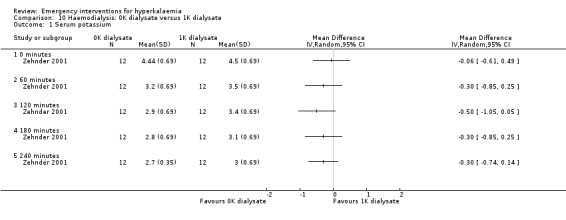
Comparison 10 Haemodialysis: 0K dialysate versus 1K dialysate, Outcome 1 Serum potassium.
11.1. Analysis.
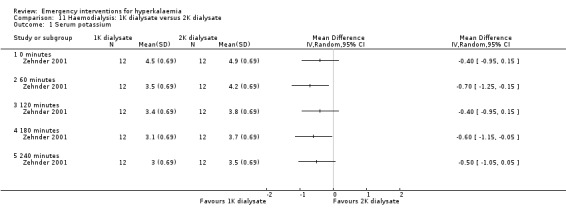
Comparison 11 Haemodialysis: 1K dialysate versus 2K dialysate, Outcome 1 Serum potassium.
12.1. Analysis.
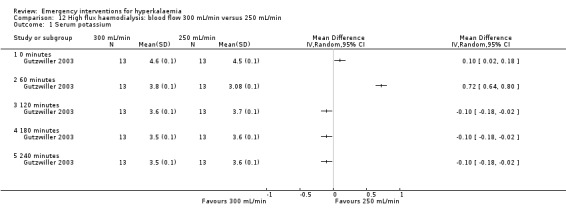
Comparison 12 High flux haemodialysis: blood flow 300 mL/min versus 250 mL/min, Outcome 1 Serum potassium.
13.1. Analysis.
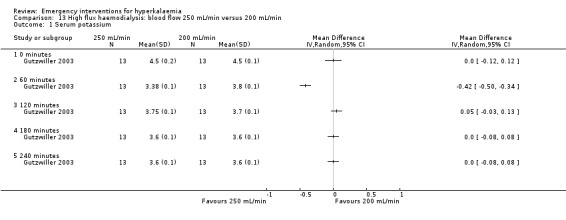
Comparison 13 High flux haemodialysis: blood flow 250 mL/min versus 200 mL/min, Outcome 1 Serum potassium.
Other
There were no placebo‐controlled trials of aminophylline. In a comparison of aminophylline with IV insulin‐glucose no significant difference was observed between groups (Mahajan 2001) (Analysis 16.1).
Combination of agents
One study (Allon 1990) showed that the addition of nebulised salbutamol to insulin and glucose is superior to either treatment alone at 30, 45 and 60 minutes, in the original between‐group analysis of change in potassium concentration reported by the authors. This effect is not evident in our figure which, for consistency across studies, compares absolute potassium levels at different time points (Analysis 14.1).
One study (Ngugi 1997) compared a number of different combinations of agents with single agents. It was not possible to calculate effect sizes or compare groups since no estimates of variance were provided in this study. The authors did not specify the statistical procedures used, but concluded that there was no statistically significant difference between any of the treatment arms that they investigated (P = 0.794). Attempts to contact the authors for further information were unsuccessful. Allon 1995 found statistically significant differences in potassium levels when salbutamol used in combination with dialysis was compared with dialysis alone (Analysis 25.1).
25.1. Analysis.
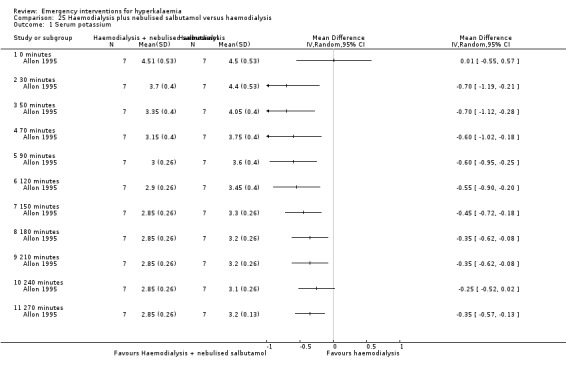
Comparison 25 Haemodialysis plus nebulised salbutamol versus haemodialysis, Outcome 1 Serum potassium.
The addition of IV bicarbonate to IV insulin or nebulised albuterol did not provide any greater lowering effect of serum potassium compared with either treatment alone (Allon 1996). Based on the reporting of the variance estimates in this study, an effect estimate could not be calculated for this review.
In addition to summary graphs in MetaView, the effect on serum potassium for individual study arms, categorised by intervention, are shown in addtional Figure 1; Figure 2; Figure 3; Figure 4; Figure 5; Figure 6; Figure 7; Figure 8; Figure 9; Figure 10; Figure 11).
1.
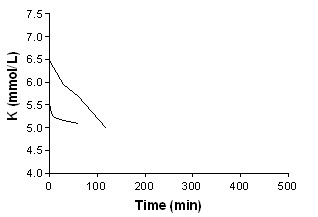
Beta‐agonist aerosolized
2.
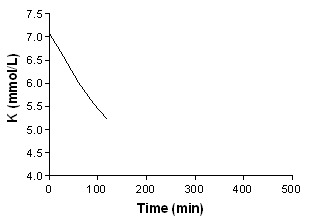
Beta‐agonist aerosolized + insulin
3.
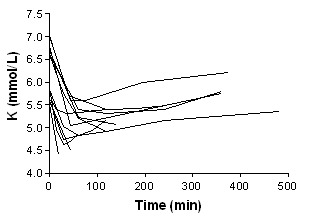
Beta‐agonist IV
4.
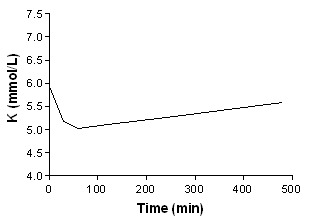
Beta‐agonist + bicarbonate
5.
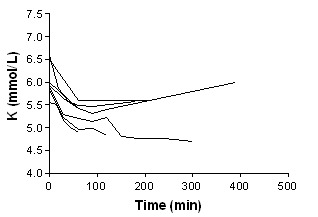
Beta‐agonist nebulised
6.
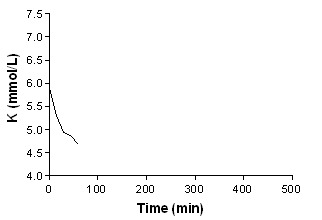
Beta‐agonist nebulised + insulin
7.
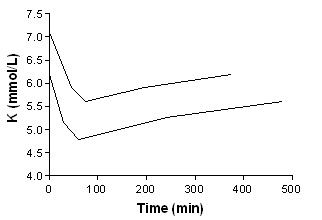
Beta agonist IV + insulin
8.
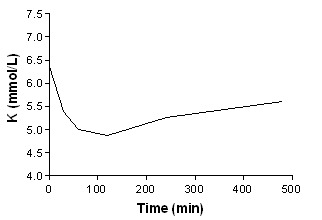
Beta‐agonist Iv + insulin + bicarbonate
9.
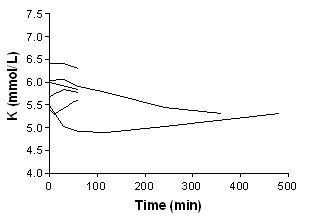
Bicarbonate
10.
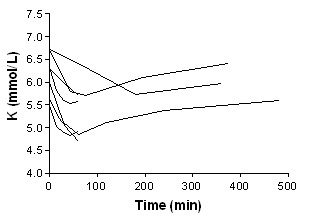
Insulin
11.
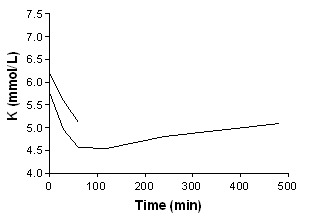
Insulin + bicarbonate
Adverse effects
Adverse effects are summarised in additional Table 1 ‐ Narrative summary of results for efficacy and harm. No major adverse effects were reported with any intervention. Side effects with salbutamol (albuterol) included tremor, vasomotor flushing (McClure 1994) nervousness, palpitations, (Pancu 2003) mild anxiety (Allon 1989).and increases in heart rate (Allon 1990; Allon 1995; Mandelberg 1999; Pancu 2003). In one study (Pancu 2003), 4/9 patients had tachycardia with heart rates between 105 and 125 beats/minute. One patient, who had a previous history of paroxysmal atrial fibrillation, had an episode during treatment with salbutamol (albuterol). There was a single episode of hypoglycaemia with insulin use in one of the studies (Mahajan 2001). In Allon 1996 all three protocols that included bicarbonate therapy were associated with increases in serum bicarbonate (3.5 ± 0.5 mmol/L, 3.1 ± 0.5 mmol/L, 2.2 ± 0.5 mmol/L, P < 0.005) and pH (0.05 ± 0.01, 0.03 ± 0.01, 0.03 ± 0.01; P < 0.01).
Discussion
The evidence
Because of the deleterious cardiac effects of hyperkalaemia, its management is an emergency intervention. We reviewed the evidence for various agents widely used for this problem. Heterogeneity in recommendations of experts in this scenario has been documented (Iqbal 1989) and is in keeping with the lack of clear evidence for the optimal regime.
There is evidence that the commonly practiced management options (salbutamol, insulin with glucose, dialysis) are effective. Salbutamol is effective when administered intravenously, nebulised, or as metered dose inhaler with spacer (MDS‐I). While bicarbonate was found to be effective in one study (Ngugi 1997), it did not lower serum potassium below baseline in another (Allon 1996). Though resins are widely used clinically, there was no randomised evidence for their efficacy in an emergency setting, and one study showed no benefit (Gruy‐Kapral 1998). Aminophylline, which is not commonly used in emergency management of hyperkalaemia, was found to be as effective as insulin and glucose, and safe. Combination of treatment modalities produced greater magnitudes of potassium lowering effect (Allon 1990; Allon 1995). One study (Allon 1996) found that when IV bicarbonate was added to salbutamol or to IV insulin‐glucose therapy there was no additional benefit. Based on the reporting of variance estimates in this study, an effect estimate could not be calculated for this review.
No studies were identified which examined the role of increasing urine output (e.g. by fluid resuscitation, if appropriate; or by loop diuretics) to promote potassium excretion. Resins did not appear to be effective at four hours, though the dose of resin (30 g) used in the one study of this subject is lower than doses commonly used in clinical practice for the emergency management of hyperkalaemia. Since the site of action of resin is thought to be the colon, it is also possible that resins are not effective in the first few hours when given orally, but require longer to act. We are aware of two non‐randomised studies that provided evidence of a potassium‐lowering effect at 24 h exists for calcium resonium and sodium polystyrene sulphonate (Kayexalate) (Berlyne 1966; Scherr 1961). Dialysis with a low potassium bath (Zehnder 2001) and high blood flow rate (Gutzwiller 2003) was effective and safe.
No studies discussed the use of calcium salts given intravenously. The prevention of arrhythmia in the setting of hyperkalaemia was first observed by Ringer in 1883 (Ringer 1883) and corroborated by Winkler (Winkler 1939) and Thuillier (Thuillier 1952). Meroney (Meroney 1955) found IV calcium to be effective both in the treatment and prophylaxis of hyperkalaemia in humans. By the time of Chamberlain's classic review article on the emergency treatment of hyperkalaemia (Chamberlain 1964), the role of IV calcium in this setting appears to have been established.
Quality of the evidence
There were three double‐blinded studies (Allon 1989; Mandelberg 1999; Pancu 2003). Allocation concealment was unclear in most of the studies. The sample sizes were small. Most studies did not comment on the number of participants completing the studies. In one study (Ngugi 1997), 90% of the patients completed the study period, the other 10% were started on dialysis before the end of the study period. Cointerventions were not explicitly described in any of the papers.
Publication bias
Given the small numbers of studies in each analysis we were not able to perform any analyses for publication bias. Publication bias may well exist in this literature, and would be expected to result in overestimating treatment effects.
Heterogeneity
Tests for heterogeneity are underpowered in analysis which include as few studies as are included in our analysis, and we do not think that much weight can be ascribed to the absence of statistically significant heterogeneity in our analyses.
Generalizability
Most of studies were in patients with ESRD which serves as a convenient model because patients are often mildly hyperkalaemic when due for dialysis. In addition, the absence of significant renal function simplifies the experiment, and increases generalizability to patients with reduced renal function, whether acute or chronic, in whom problems with hyperkalaemia are more likely. There is no good biological reason why treatment modalities that are effective in ESRD patients would not be applicable to the general population; however, it is possible that treatments that are ineffective or of equivocal effectiveness (e.g. IV bicarbonate) in patients with ESRD might still be effective in patients with normal renal function. With the exception of dialysis, the treatment options are generally convenient, widely available, and feasible.
Limitations of current evidence
All the studies reported surrogate outcomes for efficacy, and not all commented on clinically‐important adverse events. With the possible exception of Ngugi 1997, all studies appeared to have been conducted in convenience samples of mildly‐hyperkalaemic individuals. Studies were in general small, and few comparisons of interventions were studied repeatedly. In many cases, standard deviations or standard errors were not reported numerically and were extracted from graphs. In one case, no variance estimates were reported. In other cases, whether error bars represented standard deviations or standard errors was not clearly reported and was inferred from data reported elsewhere in the same article. Statistical comparisons provided in the original article frequently reported changes from baseline and the statistical significance of this comparison, rather than the comparison between randomised groups. Very few data are available on the optimal combination of agents. No data were found on the role of IV calcium salts.
Limitations in this review
This review relied on authors' reporting on the quality of the study and the accuracy of the results. We did not contact all the authors for clarification or for original data, when the publication was unclear. We were unable to extract data from the first time period, before crossing over, from those studies which were cross over studies. This results in the inclusion of data that were not truly independent in the meta‐analysis. We chose to leave these studies in the analysis because in each case we felt the wash‐out period was adequate (usually 2‐3 days) and because they represent a significant proportion of the available data.
Importance of review
We are not aware of any previous meta‐analysis on this common medical problem. This review serves as a useful summary for clinicians and researchers.
Authors' conclusions
Implications for practice.
The use of a beta‐agonist appeared to be effective in rapid reduction of hyperkalaemia in both the setting of chronic renal failure adults and in children. Salbutamol is effective when given intravenously), in a nebulised form and as a multi‐dose inhaler.
IV insulin and glucose is effective and has a rapid onset of action.
The evidence for the use of bicarbonate in hyperkalaemia is equivocal and we do not recommend its use as monotherapy. If used in conjunction with other treatments, the possible effects on pH and extracellular volume must be carefully considered in the assessment of the risk‐benefit ratio for an individual patient.
Combining insulin‐glucose with salbutamol (albuterol) probably leads to greater reductions in serum potassium than either alone.
Each of the treatments above relies on shifting potassium from extracellular to intracellular compartments in order to minimize its effects on the electrical stability of membranes. In animal and human studies, IV calcium stabilizes membranes and reduces the arrhythmic threshold. Though no randomised evidence exists to support its use, we recommend that calcium chloride (10 mL of 10%) be given in the presence of ECG changes or arrhythmia and repeated as needed. There is no randomised evidence that potassium‐exchange resins are effective. In the absence of gastrointestinal pathology, these agents are safe, and may be instituted for their possible effects at 24 hours, but should not be relied upon for rapid effects. If medical therapy fails, or is deemed to be only a temporising measure because of concurrent renal failure, dialysis should be considered. Low‐potassium and potassium‐free dialysate (Zehnder 2001) and high blood flow (Gutzwiller 2003) are safe and effective in these circumstances.
Implications for research.
The current body of evidence lacks large RCTs that are double‐blinded, with clear allocation concealment. The dose‐response relationship observed for salbutamol (albuterol) deserves further study, particularly with respect to optimising the reduction in serum potassium while minimizing adverse effects. The observation of additive benefits from the combination of insulin‐glucose with salbutamol (albuterol) should be confirmed in a larger study. The evidence for bicarbonate is unclear, and well‐designed randomized trials that address the effectiveness of isotonic and hypertonic bicarbonate when added nebulised salbutamol or to insulin and glucose are needed. A RCT of sodium polystyrene sulfonate (Kayexalate) and of calcium resonium, examining the time course of action of these agents would be of value.
Trials in patients with clinically‐important hyperkalaemia, though difficult to conduct, could compare therapies known to be effective on the basis of studies of convenience samples, and collect information on clinically‐important outcomes such as harm, arrhythmia and death.
What's new
| Date | Event | Description |
|---|---|---|
| 15 September 2016 | Review declared as stable | This review has been replaced by "Pharmacological interventions for the acute management of hyperkalaemia in adults". 10.1002/14651858.CD010344.pub2 |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 13 May 2009 | Amended | Contact details updated. |
| 29 September 2008 | Amended | Converted to new review format. |
Notes
September 2016: this review has been replaced by "Pharmacological interventions for the acute management of hyperkalaemia in adults". 10.1002/14651858.CD010344.pub2
Acknowledgements
We thank Ms Ruth Mitchell for searching the Cochrane Renal Groups Specialised Register of Trials.
Data and analyses
Comparison 1. Inhaled salbutamol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 0 minutes | 4 | 94 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.19, 0.19] |
| 1.2 1 minute | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.08, 0.32] |
| 1.3 3 minutes | 1 | 34 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.24, 0.24] |
| 1.4 5 minutes | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.34, 0.14] |
| 1.5 10 minutes | 1 | 34 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.42, 0.06] |
| 1.6 15 minutes | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 30 minutes | 4 | 88 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.47, ‐0.08] |
| 1.8 45 minutes | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.9 60 minutes | 4 | 88 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.66, ‐0.17] |
| 1.10 90 minutes | 2 | 38 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.79, ‐0.21] |
| 1.11 120 minutes | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Nebulised levalbuterol 2.5 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 30 minutes/immediate post‐treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 60 minutes/30 minutes post‐treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 90 minutes/60 minutes post‐treatment | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. IV salbutamol versus nebulised salbutamol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 30 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 90 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 120 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 150 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 180 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 240 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 300 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. IV insulin‐glucose versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 15 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 30 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 45 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Sodium polystyrene sulphonate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 240 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 480 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 720 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Phenolphthalein‐docusate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 240 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 480 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 720 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Phenolphthalein‐docusate plus sodium polystyrene sulphonate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 240 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 480 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 720 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 8. Sorbitol plus sodium polystyrene sulphonate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 240 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 480 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 720 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. IV bicarbonate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 15 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 30 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 45 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.
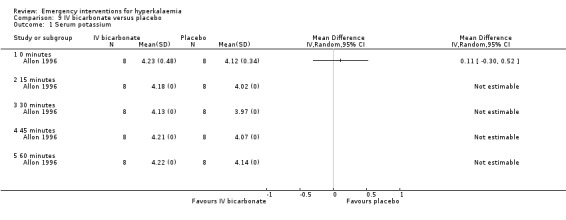
Comparison 9 IV bicarbonate versus placebo, Outcome 1 Serum potassium.
Comparison 10. Haemodialysis: 0K dialysate versus 1K dialysate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 120 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 180 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 240 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 11. Haemodialysis: 1K dialysate versus 2K dialysate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 120 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 180 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 240 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 12. High flux haemodialysis: blood flow 300 mL/min versus 250 mL/min.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 120 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 180 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 240 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 13. High flux haemodialysis: blood flow 250 mL/min versus 200 mL/min.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 120 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 180 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 240 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 14. Nebulised salbutamol versus IV insulin‐glucose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 15 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 30 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 45 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 15. IV salbutamol versus IV insulin‐glucose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 30 minutes | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 60 minutes | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 120 minutes | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 240 minutes | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 480 minutes | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 16. IV aminophylline versus IV insulin‐glucose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 180 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 360 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 17. IV bicarbonate versus IV insulin‐glucose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 30 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 120 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 240 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 480 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
17.1. Analysis.
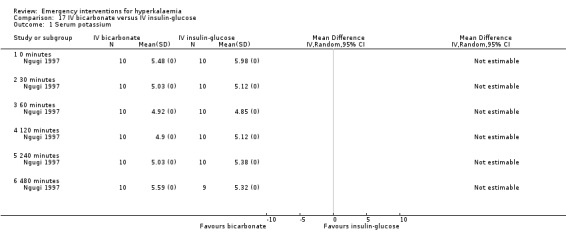
Comparison 17 IV bicarbonate versus IV insulin‐glucose, Outcome 1 Serum potassium.
Comparison 18. IV bicarbonate plus nebulised salbutamol versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 15 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 30 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 45 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
18.1. Analysis.
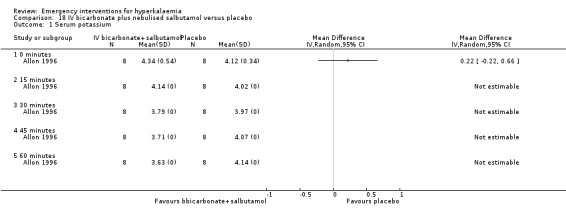
Comparison 18 IV bicarbonate plus nebulised salbutamol versus placebo, Outcome 1 Serum potassium.
Comparison 19. IV bicarbonate plus IV insulin‐glucose versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 15 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 30 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 45 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
19.1. Analysis.
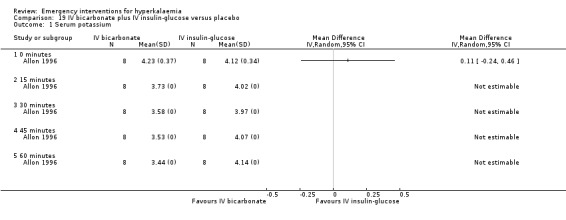
Comparison 19 IV bicarbonate plus IV insulin‐glucose versus placebo, Outcome 1 Serum potassium.
Comparison 20. IV insulin‐glucose plus nebulised salbutamol versus IV insulin‐glucose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 15 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 30 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 45 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
20.1. Analysis.
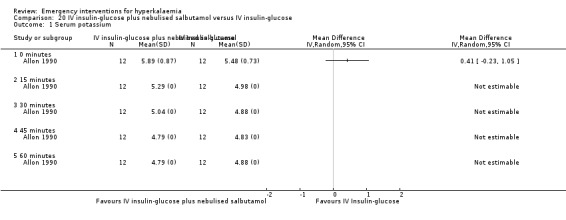
Comparison 20 IV insulin‐glucose plus nebulised salbutamol versus IV insulin‐glucose, Outcome 1 Serum potassium.
Comparison 21. IV insulin‐glucose plus IV salbutamol versus IV insulin‐glucose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 30 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 120 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 240 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 480 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
21.1. Analysis.
Comparison 21 IV insulin‐glucose plus IV salbutamol versus IV insulin‐glucose, Outcome 1 Serum potassium.
Comparison 22. IV insulin‐glucose plus IV bicarbonate versus IV insulin‐glucose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 0 minutes | 2 | 36 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.52, 0.42] |
| 1.2 15 minutes | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 30 minutes | 2 | 36 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 45 minutes | 1 | 16 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 60 minutes | 2 | 36 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 120 minutes | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 240 minutes | 1 | 20 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 480 minutes | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
22.1. Analysis.
Comparison 22 IV insulin‐glucose plus IV bicarbonate versus IV insulin‐glucose, Outcome 1 Serum potassium.
Comparison 23. IV bicarbonate plus IV salbutamol versus IV insulin‐glucose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 30 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 120 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 240 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 480 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
23.1. Analysis.
Comparison 23 IV bicarbonate plus IV salbutamol versus IV insulin‐glucose, Outcome 1 Serum potassium.
Comparison 24. IV insulin‐glucose plus IV bicarbonate plus IV salbutamol versus IV insulin‐glucose.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 30 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 120 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 240 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 480 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
24.1. Analysis.
Comparison 24 IV insulin‐glucose plus IV bicarbonate plus IV salbutamol versus IV insulin‐glucose, Outcome 1 Serum potassium.
Comparison 25. Haemodialysis plus nebulised salbutamol versus haemodialysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 30 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 50 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 70 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 90 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 120 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 150 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 180 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 210 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 240 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 270 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 26. IV insulin‐glucose plus nebulised salbutamol versus nebulised salbutamol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum potassium | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 0 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 15 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 30 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 45 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 60 minutes | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
26.1. Analysis.
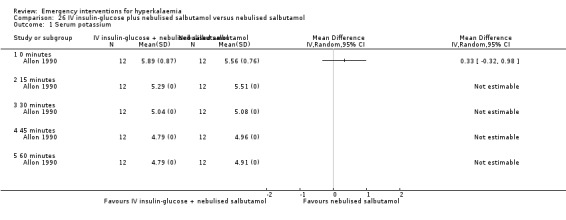
Comparison 26 IV insulin‐glucose plus nebulised salbutamol versus nebulised salbutamol, Outcome 1 Serum potassium.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allon 1989.
| Methods | Prospective double‐blind, placebo controlled study, outpatient haemodialysis clinic at a univeristy medical centre, paried student t‐tests wtih bonferroni correction for repeated comparisons | |
| Participants | 10 Patients on maintenance haemodialysis who had chronic hyperkalaemia | |
| Interventions | Nebulised albuterol therapy (10 mg or 20 mg) or placebo (saline) | |
| Outcomes | Plasma potassium concentration, also serially measured blood pressure and pulse | |
| Notes | Allocation concealment felt to be adequate only after clarification from author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Allon 1990.
| Methods | Randomised prospective cross‐over design, study conducted at the University of Oklahoma Health Sciences Center and Veterans Administration Medical Center | |
| Participants | 12 Nondiabetic hyperkalaemic patients on stable haemodialysis regiment | |
| Interventions | a) regular insulin 10 units with glucose 50 mL of 50% solution IV over 5 minutes, b) nebulised treatment of albuterol 20 mg in 4 mL normal saline inhaled over 10 minute period, c) combined regimen consisting of intravenous insulin and dextrose as well as a nebulised albuterol treatment | |
| Outcomes | Plasma potassium, Plasma glucose Plasma insulin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Allon 1995.
| Methods | Randomised, prospective cross‐over design | |
| Participants | 7 Patients on chronic haemodialysis | |
| Interventions | Haemodialysis with nebulised albuterol 20 mg, 30 minutes Pre‐dialysis versus haemodialysis only | |
| Outcomes | Plasma potassium Glucose BUN Insulin | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Allon 1996.
| Methods | Randomised, prospective cross‐over design, study conducted at the University of Alabama at Birmingham and the Veterans Administration Medical Center in Birmingham, ANOVA | |
| Participants | 8 Nondiabetic chronic haemodialysis patients | |
| Interventions | There were 6 experimental protocols: 1. isotonic sodium bicarbonate at 90 mmol/h, 2. isotonic saline at 90 mmol/h, 3. isotonic bicarbonate (in 10% dextrose) at 90 mmol/h + IV insulin (5mU/kg/min), 4. isotonic saline (in 10% dextrose) at 90 mmol/h +IV insulin (5mU/kg/min), 5. isotonic bicarbonate at 90 mmol/h + nebulised albuterol (10 mg in 2.0 mL saline, nebulised over 10 minutes), and 6. isotonic saline at 90 mmol/h + nebulised albuterol (10mg in 2.0 mL saline, nebulised over 10 minutes) | |
| Outcomes | Plasma potassium | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Gruy‐Kapral 1998.
| Methods | Randomised controlled cross‐over trial, 5 studies on 5 separate experimental days Study conducted in Dallas, Texas, USA, One way ANOVA and t‐test | |
| Participants | 6 CRF patients on haemodialysis | |
| Interventions | 1). 8 gelatin capsules with 500 mL of water, versus 2). sodium polystyrene sulfonate (30 g of resin with 500 mL of water) versus 3). phenolphthalein‐docusate (8 tablets with 500 mL of water) versus 4). phenolphthalein‐docusate plus resin (8 tablets, 30 g of resin with 500 mL of water) versus 5). sorbitol plus resin (60 g of sorbitol, 30 g of resin with 500 mL of water) | |
| Outcomes | Faecal potassium output Serum potassium concentration | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Gutzwiller 2003.
| Methods | Randomised, cross‐over prospective study | |
| Participants | 13 ESRD patients on haemodialysis | |
| Interventions | blood flows (Qb) of 200, 250, 300 mL/min, for 39 standardised high‐flux haemodialysis treatments | |
| Outcomes | Phosphate and potassium mass removal, Urea Phosphate Potassium | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Mahajan 2001.
| Methods | Randomised controlled trial Study conducted in India, Paired and unpaired student t‐test | |
| Participants | 30 ESRD patients admitted to Pt BD Sharma PGIMS Hospital | |
| Interventions | Group A: aminophylline infusion Group B: insulin‐dextrose infusion | |
| Outcomes | Serum potassium Serum glucose | |
| Notes | Aminophylline is a methylxanthine derivative Vitals monitored and ECG taken No serious side‐effects observed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Mandelberg 1999.
| Methods | Randomised double‐blind placebo controlled cross‐over trial conducted in Israel Fisher's exact, post‐hoc t‐test | |
| Participants | 17 CRF patients referred to Nehprology Unit between October 1, 1997 and March 31, 1998 for haemodialysis | |
| Interventions | Group 1: 1200 ug salbutamol through MDS‐I followed by placebo versus Group 2: placebo, then 1200 μg salbutamol through MDS‐I | |
| Outcomes | Serum potassium Insulin levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
McClure 1994.
| Methods | Randomised cross‐over study, Study conducted in the United Kingdom | |
| Participants | 11 Children (aged 5‐18 years) with end stage chronic renal failure | |
| Interventions | Two doses of salbutamol (separated by two hours) given intravenously (4 μg/kg) and on a separate date, of salbutamol administered by nebuliser (2.5 mg if the child weighed below 25 kg, 5 mg if above) was observed | |
| Outcomes | Plasma potassium | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | D ‐ Not used |
Ngugi 1997.
| Methods | Randomised Controlled Trial, Study conducted in Nairobi | |
| Participants | 10 Patients with acute renal failure and 60 patients with chronic renal failure, both groups with hyperkalaemia | |
| Interventions | Intervention A: Insulin 10U IV + 25 g glucose IV Intervention B: NaCO3 1 mmol/mL (8.4%) @ 3.3 mL/min x 15 min Intervention C: Albuterol 0.5 mL IV + 2.5 g glucose IV Intervention D: Interventions A + B Intervention E: Interventions B + C Intervention F: Interventions A + C Intervention G: Interventions A + B + C | |
| Outcomes | Serum potassium | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Pancu 2003.
| Methods | Randomised double‐blind placebo controlled trial Study conducted in USA, ANOVA across and within group | |
| Participants | 27 healthy volunteers, 9 subjects in each of the 3 study groups | |
| Interventions | Nebulised albuterol 10 mg over 30 minutes versus Nebulised levalbuterol 2.5 mg over 30 minutes, versus Nornal saline in equivalent volumes | |
| Outcomes | Serum potassium | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Zehnder 2001.
| Methods | Prospective randomised cross‐over study | |
| Participants | 12 Non‐diabetic haemodialysis patients | |
| Interventions | Dialysates containing 0, 1, and 2 mmol/L of potassium | |
| Outcomes | Mass removal of potassium and serum potassium | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Liou 1994 | Non‐responders of salbutamol excluded from the study |
Contributions of authors
Mahoney & Smith conducted the first phase of the review, up to 2000.
Lo and Tsoi conducted the second phase of the review, 2000‐2004.
Tonelli researched the non‐randomized literature for calcium studies to include in the discussion.
Clase supervised the project.
All reviewers were involved in writing and reviewed the full manuscript.
Declarations of interest
None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Allon 1989 {published data only}
- Allon M, Dunlay R, Copkney C. Nebulized albuterol for acute hyperkalemia in patients on hemodialysis. Annals of Internal Medicine 1989;110(6):426‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Allon 1990 {published data only}
- Allon M, Copkney C. Albuterol and insulin for treatment of hyperkalemia in hemodialysis patients. Kidney International 1990;38(5):869‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Allon 1995 {published data only}
- Allon M, Shanklin N. Effect of albuterol treatment on subsequent dialytic potassium removal. American Journal of Kidney Diseases 1995;26(4):607‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Allon 1996 {published data only}
- Allon M, Shanklin N. Effect of bicarbonate administration on plasma potassium in dialysis patients: interactions with insulin and albuterol. American Journal of Kidney Diseases 1996;28(4):508‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gruy‐Kapral 1998 {published data only}
- Gruy‐Kapral C, Emmett M, Santa Ana CA, Porter JL, Fordtran JS, Fine KD. Effect of single dose resin‐cathartic therapy on serum potassium concentration in patients with end‐stage renal disease. Journal of the American Society of Nephrology 1998;9(10):1924‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gutzwiller 2003 {published data only}
- Gutzwiller JP, Schneditz D, Huber AR, Schindler C, Garbani E, Zehnder CE. Increasing blood flow increases kt/V(urea) and potassium removal but fails to improve phosphate removal. Clinical Nephrology 2003;59(2):130‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mahajan 2001 {published data only}
- Mahajan SK, Mangla M, Kishore K. Comparison of aminophylline and insulin‐dextrose infusions in acute therapy of hyperkalemia in end‐stage renal disease patients. Journal of the Association of Physicians of India 2001;49:1082‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Mandelberg 1999 {published data only}
- Mandelberg A, Krupnik Z, Houri S, Smetana S, Gilad E, Matas Z, et al. Salbutamol metered‐dose inhaler with spacer for hyperkalemia: how fast? How safe?. Chest 1999;115(3):617‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McClure 1994 {published data only}
- McClure RJ, Prasad VK, Brocklebank JT. Treatment of hyperkalaemia using intravenous and nebulised salbutamol. Archives of Disease in Childhood 1994;70(2):126‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ngugi 1997 {published data only}
- Ngugi NN, McLigeiyo SO, Kayima JK. Treatment of hyperkalaemia by altering the transcellular gradient in patients with renal failure: effect of various therapeutic approaches. East African Medical Journal 1997;74(8):503‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Pancu 2003 {published data only}
- Pancu D, LaFlamme M, Evans E, Reed J. Levalbuterol is as effective as racemic albuterol in lowering serum potassium. The Journal of Emergency Medicine 2003;25(1):13‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zehnder 2001 {published data only}
- Zehnder C, Gutzwiller JP, Huber A, Schindler C, Schneditz D. Low‐potassium and glucose‐free dialysis maintains urea but enhances potassium removal. Nephrology Dialysis Transplantation 2001;16(1):78‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Liou 1994 {published data only}
- Liou HH, Chiang SS, Wu SC, Yang WC, Huang TP. Intravenous infusion or nebulization of salbutamol for treatment of hyperkalemia in patients with chronic renal failure. Chung Hua i Hsueh Tsa Chih ‐ Chinese Medical Journal 1994;53(5):276‐81. [MEDLINE: ] [PubMed] [Google Scholar]
Additional references
Acker 1998
- Acker CG, Johnson JP, Palevsky PM, Greenberg A. Hyperkalemia in hospitalized patients: causes, adequacy of treatment, and results of an attempt to improve physician compliance with published therapy guidelines. Archives of Internal Medicine 1998;158(8):917‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Berlyne 1966
- Berlyne GM, Janabi K, Shaw AB, Hocken AG. Treatment of hyperkalemia with a calcium‐resin. Lancet 1966;1(7420):169‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blumberg 1988
- Blumberg A, Weidmann P, Shaw S, Gnadinger M. Effect of various therapeutic approaches on plasma potassium and major regulating factors in terminal renal failure. American Journal of Medicine 1988;85(4):507‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brenner 2000
- Brenner BM. The Kidney. 6th Edition. Saunders, 2000. [Google Scholar]
Browning 1981
- Browning JJ, Channer KS. Hyperkalaemic cardiac arrhythmia caused by potassium citrate mixture. British Medical Journal Clinical Research Ed 1981;283(6303):1366. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chamberlain 1964
- Chamberlain MJ. Emergency treatment of hyperkalaemia. Lancet 1964;18:464‐7. [DOI] [PubMed] [Google Scholar]
Davison 1998
- Davison AM. Oxford textbook of clinical nephrology. Oxford University Press, 1998. [Google Scholar]
Freeman 1993
- Freeman SJ, Fale AD. Muscular paralysis and ventilatory failure caused by hyperkalaemia. British Journal of Anaesthesia 1993;70(2):226‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Frohnert 1968
- Frohnert PP, Johnson WJ, Mueller GJ, Tauxe WN, McCall JT. Resin treatment of hyperkalemia. I. Exchange properties of a cation exchange resin (calcium cycle). Journal of Laboratory & Clinical Medicine 1968;71(5):834‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Greenberg 1998
- Greenberg A. Hyperkalemia: treatment options. Seminars in Nephrology 1998;18(1):46‐57. [MEDLINE: ] [PubMed] [Google Scholar]
Halperin 1998
- Halperin ML, Kamel KS. Potassium. Lancet 1998;352(9122):135‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haynes 1994
- Haynes RB, Wilczynski N, McKibbon KA, Walker CJ, Sinclair JC. Developing optimal search strategies for detecting clinically sound studies in MEDLINE. Journal of the American Medical Informatics Association 1994;1(6):447‐58. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7417):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Iqbal 1989
- Iqbal Z, Friedman EA. Preferred therapy of hyperkalemia in renal insufficiency: survey of nephrology training‐program directors. New England Journal of Medicine 1989;320(1):60‐1. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Johnson 1976
- Johnson K, Cazee C, Gutch C, Ogden D. Sodium polystyrene sulfonate resin candy for control of potassium in chronic dialysis patients. Clinical Nephrology 1976;5(6):266‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Meroney 1955
- Meroney WH, Herndon RF. The management of acute renal insufficiency. JAMA 1955:877‐83. [DOI] [PubMed] [Google Scholar]
Moore 1989
- Moore ML, Bailey RR. Hyperkalaemia in patients in hospital. New Zealand Medical Journal 1989;102(878):557‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Norman 1993
- Norman GR, Streiner DL. Comparing two groups: the t test. In: Norman GR, Streiner DL editor(s). Biostatistics: the Bare Essentials. 1st Edition. St Louis: Mosby, 1993:58‐63. [Google Scholar]
Paice 1983
- Paice B, Gray JMB, McBribe D, Donnelly T, Dawson DH. Hyperkalaemia in patients in hospital. British Medical Journal Clinical Research Ed 1983;286(6372):1189‐92. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ringer 1883
- Ringer S. A further contribution regarding the influence of the different constituents of the blood on the contraction of the heart. Journal of Physiology 1883;4:29‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scherr 1961
- Scherr L. Management if hyperkalemia with a cation exchange resin. New England Journal of Medicine 1961;264:115‐9. [DOI] [PubMed] [Google Scholar]
Schrier 1993
- Schrier RW, Goschalk CW. Diseases of the Kidney. 5th Edition. Little, Brown, 1993. [Google Scholar]
Schwartz 1978
- Schwartz AB. Potassium‐related cardiac arrhythmias and their treatment. Angiology 1978;29(3):194‐205. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shemer 1983
- Shemer J, Modan M, Ezra D, Cabili S. Incidence of hyperkalemia in hospitalized patients. Israel Journal of Medical Sciences 1983;19(7):659‐61. [MEDLINE: ] [PubMed] [Google Scholar]
Thuillier 1952
- Thuillier J, Hazard J. Correction of electrographic signs of hyperpotassiumemia by calcium chloride. Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales 1952;146:840‐3. [PubMed] [Google Scholar]
Weiner 1998
- Weiner DI. Hyperkalemia: A potential silent killer. Journal of the American Society of Nephrology 1998;9(8):1535‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Williams 1985
- Williams ME, Gervino EV, Rosa RM, Landsberg L, Young JB, Silva P, et al. Catecholamine modulation of rapid potassium shifts during exercise. New England Journal of Medicine 1985;312(13):823‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Winkler 1939
- Winkler AW, Hoff HE, Smith PK. Factors affecting the toxicity of potassium. American Journal of Physiology 1939;127:430‐6. [Google Scholar]
Zierler 1987
- Zierler K. Insulin hyperpolarizes rat myotube primary culture without stimulating glucose uptake. Diabetes 1987;36(9):1035‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Clase 2001
- Clase CM, Smith WAD, Mahoney BA, Tonelli M. Emergency interventions for hyperkalaemia. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD003235] [DOI] [PMC free article] [PubMed] [Google Scholar]


