Abstract
Background
Electronic cigarettes (ECs) are electronic devices that heat a liquid into an aerosol for inhalation. The liquid usually comprises propylene glycol and glycerol, with or without nicotine and flavours, and stored in disposable or refillable cartridges or a reservoir. Since ECs appeared on the market in 2006 there has been a steady growth in sales. Smokers report using ECs to reduce risks of smoking, but some healthcare organizations, tobacco control advocacy groups and policy makers have been reluctant to encourage smokers to switch to ECs, citing lack of evidence of efficacy and safety. Smokers, healthcare providers and regulators are interested to know if these devices can help smokers quit and if they are safe to use for this purpose. This review is an update of a review first published in 2014.
Objectives
To evaluate the safety and effect of using ECs to help people who smoke achieve long‐term smoking abstinence.
Search methods
We searched the Cochrane Tobacco Addiction Group's Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and PsycINFO for relevant records from 2004 to January 2016, together with reference checking and contact with study authors.
Selection criteria
We included randomized controlled trials (RCTs) in which current smokers (motivated or unmotivated to quit) were randomized to EC or a control condition, and which measured abstinence rates at six months or longer. As the field of EC research is new, we also included cohort follow‐up studies with at least six months follow‐up. We included randomized cross‐over trials, RCTs and cohort follow‐up studies that included at least one week of EC use for assessment of adverse events (AEs).
Data collection and analysis
We followed standard Cochrane methods for screening and data extraction. Our main outcome measure was abstinence from smoking after at least six months follow‐up, and we used the most rigorous definition available (continuous, biochemically validated, longest follow‐up). We used a fixed‐effect Mantel‐Haenszel model to calculate the risk ratio (RR) with a 95% confidence interval (CI) for each study, and where appropriate we pooled data from these studies in meta‐analyses.
Main results
Our searches identified over 1700 records, from which we include 24 completed studies (three RCTs, two of which were eligible for our cessation meta‐analysis, and 21 cohort studies). Eleven of these studies are new for this version of the review. We identified 27 ongoing studies. Two RCTs compared EC with placebo (non‐nicotine) EC, with a combined sample size of 662 participants. One trial included minimal telephone support and one recruited smokers not intending to quit, and both used early EC models with low nicotine content and poor battery life. We judged the RCTs to be at low risk of bias, but under the GRADE system we rated the overall quality of the evidence for our outcomes as ‘low’ or ‘very low’, because of imprecision due to the small number of trials. A ‘low’ grade means that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. A ‘very low’ grade means we are very uncertain about the estimate. Participants using an EC were more likely to have abstained from smoking for at least six months compared with participants using placebo EC (RR 2.29, 95% CI 1.05 to 4.96; placebo 4% versus EC 9%; 2 studies; 662 participants. GRADE: low). The one study that compared EC to nicotine patch found no significant difference in six‐month abstinence rates, but the confidence intervals do not rule out a clinically important difference (RR 1.26, 95% CI 0.68 to 2.34; 584 participants. GRADE: very low).
Of the included studies, none reported serious adverse events considered related to EC use. The most frequently reported AEs were mouth and throat irritation, most commonly dissipating over time. One RCT provided data on the proportion of participants experiencing any adverse events. The proportion of participants in the study arms experiencing adverse events was similar (ECs vs placebo EC: RR 0.97, 95% CI 0.71 to 1.34 (298 participants); ECs vs patch: RR 0.99, 95% CI 0.81 to 1.22 (456 participants)). The second RCT reported no statistically significant difference in the frequency of AEs at three‐ or 12‐month follow‐up between the EC and placebo EC groups, and showed that in all groups the frequency of AEs (with the exception of throat irritation) decreased significantly over time.
Authors' conclusions
There is evidence from two trials that ECs help smokers to stop smoking in the long term compared with placebo ECs. However, the small number of trials, low event rates and wide confidence intervals around the estimates mean that our confidence in the result is rated 'low' by GRADE standards. The lack of difference between the effect of ECs compared with nicotine patches found in one trial is uncertain for similar reasons. None of the included studies (short‐ to mid‐term, up to two years) detected serious adverse events considered possibly related to EC use. The most commonly reported adverse effects were irritation of the mouth and throat. The long‐term safety of ECs is unknown. In this update, we found a further 15 ongoing RCTs which appear eligible for this review.
Keywords: Humans, Middle Aged, Electronic Nicotine Delivery Systems, Electronic Nicotine Delivery Systems/adverse effects, Electronic Nicotine Delivery Systems/instrumentation, Smoking Prevention, Cohort Studies, Nicotine, Nicotine/administration & dosage, Nicotinic Agonists, Nicotinic Agonists/administration & dosage, Publication Bias, Randomized Controlled Trials as Topic, Smoking, Smoking/epidemiology, Smoking Cessation, Smoking Cessation/methods, Tobacco Use Cessation Products
Can electronic cigarettes help people stop smoking, and are they safe to use for this purpose?
Background
Electronic cigarettes (ECs) are electronic devices that produce an aerosol (commonly referred to as vapour) that the user inhales. This vapour typically contains nicotine without most of the toxins smokers inhale with cigarette smoke. ECs have become popular with smokers who want to reduce the risks of smoking. This review aimed to find out whether ECs help smokers stop smoking, and whether it is safe to use ECs to do this.
Study characteristics
This is an update of a previous review. The first review was published in 2014 and included 13 studies. For this update, we searched for studies published up to January 2016 and found 11 new studies. Only two of the included studies are randomized controlled trials and followed participants for at least six months. These provide the best evidence. The remaining 22 studies either did not follow participants for very long or did not put people into treatment groups so could not directly compare ECs with something else. These studies can tell us less about how ECs might help with quitting smoking but can tell us about short‐term safety. The two randomized trials, conducted in New Zealand and Italy, compared ECs with and without nicotine. We judged these studies to be at low risk of bias. In one study, people wanted to quit smoking, while in the other study they did not. The trial in people who wanted to quit smoking also compared ECs to nicotine patches.
Key results
Combined results from two studies, involving 662 people, showed that using an EC containing nicotine increased the chances of stopping smoking in the long term compared to using an EC without nicotine. We could not determine if EC was better than a nicotine patch in helping people stop smoking, because the number of participants in the study was low. More studies are needed to evaluate this effect. The other studies were of lower quality, but they supported these findings. None of the studies found that smokers who used EC short‐ to mid‐term (for two years or less) had an increased health risk compared to smokers who did not use ECs.
Quality of the evidence
The quality of the evidence overall is low because it is based on only a small number of studies, although these studies were well conducted. More studies of ECs are needed. Some are already underway.
Summary of findings
Summary of findings for the main comparison.
Electronic cigarettes for smoking cessation
| Electronic cigarettes (EC) for smoking cessation | ||||||
|
Patient or population: people defined as current smokers at enrolment into trials, motivated or unmotivated to quit Intervention: nicotine‐containing electronic cigarettes Comparison: placebo electronic cigarettes or nicotine replacement therapy (or for adverse events, uncontrolled) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| Control | Electronic cigarettes | |||||
| Cessation: Nicotine EC versus placebo EC2 assessed with exhaled CO Follow‐up: 6 ‐ 12 months | 40 per 1000 | 93 per 1000 (42 to 201) | RR 2.29 (1.05 to 4.96) | 662 (2 studies) | ⊕⊕⊝⊝ low3,4 | Only RCTs reported here. Some cohort data also available (see full review) but only RCTs provide efficacy data |
| Cessation: Nicotine EC versus nicotine replacement therapy assessed with exhaled CO Follow‐up: 6 months | 58 per 1000 | 73 per 1000 (39 to 135) | RR 1.26 (0.68 to 2.34) | 584 (1 study) | ⊕⊝⊝⊝ very low3,5 | As above |
|
Adverse events (AEs) Follow‐up: 6 ‐ 24 months |
Summary data not available. No studies reported serious AEs considered related to EC use. One RCT provided data on the proportion of participants experiencing any adverse events. The proportion of participants in the study arms experiencing adverse events was similar (ECs vs placebo EC: RR 0.97, 95% CI 0.71 to 1.34 (298 participants); ECs vs patch: RR 0.99, 95% CI 0.81 to 1.22 (456 participants)). The second RCT reported no statistically significant difference in the frequency of AEs at three‐ or 12‐month follow‐up between the EC and placebo EC groups. Cohort studies found mouth and throat irritation, dissipating over time, to be the most frequently reported AEs in EC users. | 1201 (11 studies (2 RCTs, 9 cohort)) |
⊕⊕⊝⊝ low6,7 | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1'Assumed risk' calculated as risk in control groups. 2'Placebo EC' refers to ECs which do not contain nicotine. 3Downgraded one level due to indirectness. The electronic cigarette used in Bullen 2013 was not very effective at delivering nicotine. 4Downgraded one level due to imprecision. Only two included studies, small number of events (< 300) in each arm.
5Downgraded two levels due to imprecision. Only one included study, with small number of events in each arm. 6Downgraded due to risk of bias. 11/13 included studies (cohort studies) judged to be at high risk of bias. 7Downgraded due to imprecision. Only one trial provided data for nicotine EC versus nicotine replacement therapy
Background
Throughout this review, we discuss two types of cigarettes: electronic and conventional tobacco cigarettes. To avoid confusion, all mention of smoking, smoking cessation, cigarette use, smoke intake, etc., concern conventional cigarettes. When the text concerns electronic cigarettes we use the abbreviation 'ECs'. EC users are sometimes described as vapers, and EC use as vaping. We refer to ECs that do not contain nicotine as placebo ECs.
Description of the condition
Stopping smoking is associated with large health benefits. Despite most smokers wanting to quit, many find it difficult to succeed in the long term. Almost half who try to quit without support will not manage to stop for even a week, and fewer than five per cent remain abstinent at one year after quitting (Hughes 2004).
Behavioural support and medications such as nicotine patches or gum increase the chances of quitting, but even with this additional support long‐term quit rates remain low (Cahill 2016; Hughes 2014; Lancaster 2005; Stead 2005; Stead 2006; Stead 2012). One of the limitations of current treatments is that none adequately addresses the sensory and behavioural aspects of smoking that smokers miss when they stop smoking (e.g. holding a cigarette in their hands, taking a puff, enjoyment of smoking, etc.). ECs may offer a way to overcome this limitation.
There is no doubt that people become dependent on tobacco, and find it difficult to stop smoking, primarily because of nicotine and its actions on the brain's reward system (Balfour 2004). However, other factors also contribute to tobacco dependence (Rose 2006). Sensory and behavioural cues provide additional reinforcement of smoking behaviour (Rose 1993; Rose 2000) and over time become almost as rewarding as nicotine. There are several lines of evidence to support this. Firstly, smokers appear to have a preference for cigarette smoke compared to other forms of nicotine delivery. This is partly related to its speed of nicotine delivery. However, even when nicotine is administered intravenously it does not provide the same level of satisfaction or reward as smoking (Rose 2000; Westman 1996). Secondly, the local sensory effects of smoking (e.g. the ‘scratch’ in the back of the throat) may be important for enjoyment and reward. Numbing the sensations of cigarette smoke by anaesthetizing the upper and lower respiratory tract leads to less enjoyment of smoking (Rose 1985). Conversely, products that mimic the sensory effects of smoking on the mouth and throat (such as citric acid, black pepper, and ascorbic acid) reduce craving and some withdrawal symptoms, at least in the short term (Levin 1993; Rose 1994; Westman 1995). Thirdly, de‐nicotinized cigarettes (DNCs), which have a very low content of nicotine (e.g. 0.08 mg instead of the normal 1 mg) and so have negligible or no central effects, have also been investigated for their role in aiding smoking cessation (Przulj 2013). Despite not delivering nicotine, DNCs are satisfying over the initial few days of abstinence from nicotine (Donny 2007; Pickworth 1999; Rose 2000). They also reduce tobacco withdrawal symptoms, including urges to smoke and low mood (Barrett 2010; Donny 2009; McRobbie 2016; Perkins 2010; Rose 2000), and have been shown to improve long‐term continuous abstinence rates in one study (Walker 2012).
Considering the other factors that contribute to tobacco dependence, there is interest in developing smoking cessation products that would not only help relieve the unpleasant effects of nicotine withdrawal but would also act as an effective substitute for smoking behaviour and the rituals and sensations that accompany smoking, without the health risks associated with the inhalation of tobacco smoke. The only pharmaceutical treatment available that has some of these characteristics is the nicotine inhalator. However, the inhalator does not have greater cessation efficacy than the other nicotine replacement therapy (NRT) products (Hajek 1999; Stead 2012). This may in part be due to the considerable effort (e.g. 20 minutes of continuous puffing) needed to provide nicotine blood concentrations consistent with other NRTs (Schneider 2001). Adherence to correct use of the inhalator is low compared to other NRTs (Hajek 1999). It is therefore possible that any advantage of sensorimotor replacement is diminished by low nicotine delivery and limited similarities between inhalator use and sensations of smoking (Bullen 2010).
Description of the intervention
ECs are electronic vaporizing devices that have in common the ability to heat a liquid, usually comprising propylene glycol and glycerol, with or without nicotine and flavours, and stored in disposable or refillable cartridges or a reservoir, into an aerosol for inhalation. The commonly‐used term for this aerosol is vapour, which we use throughout the review. ECs are currently being promoted by retailers to use instead of cigarettes when in smoke‐free environments, and to replace conventional cigarettes with a safer alternative.
ECs provide sensations similar to smoking a cigarette. They provide taste and throat sensations that are closer to smoking than those provided by the nicotine inhalator (Barbeau 2013). The vapour that looks like tobacco smoke is only visible when the user exhales after drawing on the mouthpiece, not when the device is being held.
There are hundreds of different brands and models of EC available. There is also wide variation in the composition of the fluid in the cartridge or in the EC reservoir (nicotine content, flavours and other components) (Goniewicz 2012; Goniewicz 2014). This makes a blanket assessment of cessation efficacy difficult. Conclusions should relate to the particular type of EC tested and the composition of the liquid being aerosolized.
Initial studies showed that the brands of EC tested delivered very low amounts of nicotine to naïve users (Bullen 2010; Eissenberg 2010; Vansickel 2010). However, the studies suggested that even in the absence of good nicotine delivery, these brands of EC could alleviate urges to smoke. One study allowed a comparison of EC and inhalator, although its main objective was a comparison of ECs with and without nicotine. Puffing for 20 minutes on the inhalator and puffing for five minutes on the EC had similar effects on desire to smoke after overnight abstinence (Bullen 2010). Later studies that have measured nicotine pharmacokinetics in both experienced (Vansickel 2013) and naïve (Vansickel 2012) EC users have found that some EC users can achieve blood nicotine levels similar to those achieved with smoking, albeit more slowly, and that their ability to do so often improves over time (Hajek 2015b).
At the time of writing, the most popular types of EC include 'cigalike' products that look like cigarettes and are easier to operate (they are disposable or use cartridges that are just screwed on) and 'tank' products that include a larger battery and a transparent container that users fill with an e‐liquid of their choice. The tank ECs provide better nicotine delivery, allow a wider choice of flavours and nicotine concentrations, and are typically used by experienced vapers who managed to switch to vaping altogether (ASH 2016; Dawkins 2013b; Farsalinos 2014; McNeill 2015). Observational evidence suggests smokers are more likely to successfully quit using tank models than with cigalikes, perhaps because of improved nicotine delivery in these models (Chen 2016; Hitchman 2015). EC types are also often grouped by 'generation': first‐generation devices are typically cigalikes; second‐generation devices are usually tank models; and third‐generation devices are tank models which, unlike second generation devices, allow users to adjust the voltage level of the product (see NCSCT EC briefing for further information and images of different product types).
Throughout this review we refer to a nicotine‐containing EC as ‘nicotine EC’ and to a nicotine‐free EC as ‘placebo EC’. The 'placebo' comparison is a test just of the nicotine effect and not of the potential sensorimotor replacement that the EC may provide.
Why it is important to do this review
Since ECs appeared on the market in 2006 there has been a steady growth in sales, with some commentators reporting that ECs are a threat to the sales of cigarettes (Herzog 2013). This growth in sales is reflected in population survey data from high‐income countries that show an increased awareness and use of ECs over time (ASH 2016; Agaku 2014; Ayers 2011; Gallus 2014; West 2016). Data from lower‐income countries also suggest high levels of EC use and awareness (Jiang 2016; Palipudi 2016). ECs are used almost exclusively by smokers or ex‐smokers (ASH 2016; Douptcheva 2013; West 2016). A small proportion of never‐smokers have reported trying or experimenting with ECs but they do not seem to progress to daily or even regular use (ASH 2016; CDC 2013; West 2016). Of smokers who try ECs, fewer than 15% become daily users (Douptcheva 2013; Kralikova 2012), which suggests that ECs are still not an entirely satisfying replacement for smoking.
Regulatory approaches being used for ECs currently vary widely, from no regulation to complete bans in countries including Singapore and Brazil. The US Food and Drug Administration has classified them as tobacco products and is preparing to implement a regulation that will restrict their sale and use (FDA 2016). The European Union has included ECs in their Tobacco Products Directive, except where therapeutic claims are made or in instances where they contain over 20 mg/nl of nicotine, when they will require medicines authorization (European Parliament 2014).There is now general agreement that EC use exposes the user to fewer toxicants than smoking tobacco cigarettes (McNeill 2015; RCP 2016). However, those calling for ECs to be stringently regulated (e.g. Grana 2014a; McKee 2016; WHO 2014) cite the lack of quality control measures, possible harms of second‐hand EC vapour inhalation, concerns that the products may be a gateway to smoking initiation, concerns that ECs may undermine smoke‐free legislation if used in smoke‐free spaces, and concerns regarding the involvement of the tobacco industry. However, other reviews of available data do not support these concerns or suggest that potential benefits outweigh potential disadvantages (Farsalinos 2014; Hajek 2014; McNeill 2015; RCP 2016).
Regarding safety, categorical statements about the toxicity of ECs are not possible because of the large number of devices and fluids available and the frequent addition of new products to the market. However, among those brands of EC that have been tested, levels of toxins have been found to be substantially lower than in cigarettes, and are present at levels that are unlikely to represent a significant risk to health to either the user or to bystanders (Hajek 2014; McNeill 2015). Short‐ to medium‐term use of ECs is associated with few adverse events (Bullen 2013; Caponnetto 2013a). Long‐term effects beyond 12 months are unknown, although based on what is known about liquid and vapour constituents and patterns of use, a recent report from the UK's Royal College of Physicians has concluded that using an EC is likely to be considerably safer than smoking (RCP 2016).
Smokers, healthcare providers and regulators are interested to know if these devices can help smokers quit and if it is safe to use them to do so. In particular, healthcare providers have an urgent need to know what advice they should give to people who smoke. The largest health gains are achieved from stopping smoking completely, as opposed to reducing cigarette consumption, and as such this review focuses on the effectiveness of ECs in aiding smoking cessation. There is also an opportunity to investigate if the EC has potential to aid reduction in cigarette consumption in those smokers who cannot or do not want to stop smoking altogether; this was covered in the previous version of this review (McRobbie 2014), but is now covered in a separate review (Stead 2007, update forthcoming).
Objectives
To evaluate the safety and effect of using electronic cigarettes (ECs) to help people who smoke achieve long‐term smoking abstinence.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) in which smokers are randomized to ECs or to a control condition, and which measure abstinence rates at six months or longer, to determine the efficacy of ECs in aiding smoking cessation and reduction. We anticipated that the search would return few RCTs and so we also considered the results from cohort follow‐up studies with six months' or longer follow‐up. In this and the previous version of the review, we include those observational cohort studies which survey existing smokers at baseline, some of whom are already dual users of EC and cigarettes. As discussed in further detail below, these studies are heavily confounded due to the nature of their design. In anticipation of further high‐quality studies becoming available, we will exclude this study design for efficacy outcomes in the next update of this review, and will only include those observational studies where an intervention has been provided.
For adverse events and biomarkers, we included randomized cross‐over trials and cohort follow‐up studies with follow‐up of greater than a week.
We included studies regardless of their publication status or language of publication.
Types of participants
People defined as current smokers at enrolment into the studies. Participants can be motivated or unmotivated to quit.
Types of interventions
We compare ECs with placebo ECs, ECs versus alternative smoking cessation aids, including NRT or no intervention, and ECs added to standard smoking cessation treatment (behavioural or pharmacological or both) with standard treatment alone. As relatively few controlled trials are currently available (some are underway), we also include uncontrolled studies which evaluate ECs (see Types of studies).
Types of outcome measures
Cessation at the longest follow‐up point, which was at least six months from the start of the intervention, measured on an intention‐to‐treat basis using the strictest definition of abstinence, preferring biochemically‐validated results where reported. We collected any data on adverse events at one week or longer, serious and non‐serious, from the included studies, including changes in relevant biomarkers.
Search methods for identification of studies
Electronic searches
We searched the following databases in January 2016:
Cochrane Tobacco Addiction Group Specialized Register
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2016, Issue 1)
MEDLINE (OVID SP) (2004 to 2016 January week 2, & MEDLINE in process/In data review Feb 1 2016)
Embase (OVID SP) (2004 to 2016 week 5)
PsycINFO (OVID SP) (2004 to 2016 January week 4)
For the first version of the review we also searched CINAHL (EBSCO Host) (2004 to July 2014). We did not search this database for this review update as it did not contribute additional search results to the first version of the review.
The search terms were broad and included e‐cig$ OR elect$ cigar$ OR electronic nicotine. The search for the 2016 update added the terms vape or vaper or vapers or vaping. The search strategy for MEDLINE (Ovid SP) is shown in Appendix 1.
The search date parameters are limited to 2004 to the present, due to the fact that ECs were not available before 2004.
Searching other resources
We searched the reference lists of studies found in the literature search and the metaRegister of controlled trials database (www.isrctn.com/page/mrct). We also contacted authors of known trials and other published EC studies.
Data collection and analysis
Selection of studies
Two review authors (from JHB, HM, LS or RB) independently prescreened all titles and abstracts obtained from the search, using a screening checklist. Where there was disagreement, we obtained the full‐text version and resolved the disagreement by discussion or by referral to a third review author (PH).
Two review authors (from JHB, HM and RB) obtained and independently screened full‐text versions of the potentially relevant papers for inclusion. We resolved any disagreements by discussion or with a third review author (PH).
Data extraction and management
Two review authors (from JHB, HM or LS) extracted data from the included studies, and checked them against each other. A third review author (PH) was available to review and resolve any discrepancies. We extracted data on:
Author
Date and place of publication
Study design
Inclusion and exclusion criteria
Setting
Summary of study participant characteristics
Summary of intervention and control conditions
Number of participants in each arm
Smoking cessation outcomes
Type of biochemical validation (if any)
Adverse events (AEs), serious adverse events (SAEs), and relevant biomarkers
Assessment time points
Risk of bias in the domains specified below
Additional comments
We adopted a broad focus to detect a variety of adverse events.
One review author then entered the data into Review Manager 5 software for analyses, and another checked them.
Assessment of risk of bias in included studies
Two review authors (JHB and HM or LS) independently assessed the risk of bias for each included study, following the approach recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This approach uses a domain‐based evaluation that addresses seven different areas: random sequence generation; allocation concealment; blinding of participants and providers; blinding of outcome assessment; incomplete outcome data; selective outcome reporting; and other potential sources of bias. We assigned a grade (low, high, or unclear) for risk of bias for each domain. We resolved disagreements by discussion or by consulting a third author (PH).
Measures of treatment effect
We analyzed dichotomous data by calculating the risk ratio (RR), using the longest follow‐up data reported. For cessation, we calculated the RR as ((number of events in intervention condition/intervention denominator) / (number of events in control condition/control denominator)) with a 95% confidence interval (CI).
We analyzed continuous data (other measures of tobacco exposure) by comparing the difference between the mean change from baseline to the longest follow‐up point in the intervention and control groups.
Unit of analysis issues
We extracted data on smoking outcomes only from RCTs in which individuals were the unit of randomization. In the case of trials with multiple arms, we combined all relevant experimental intervention groups of the study into a single group, and combined all relevant control intervention groups into a single control group.
We offer a narrative synthesis of data from cohort studies.
Dealing with missing data
For smoking cessation, we used a conservative approach as is standard for the Cochrane Tobacco Addiction Group, treating participants with missing data as still smoking. We based the proportion of people affected by adverse events on the number of people available for follow‐up, and not the number randomized.
Assessment of heterogeneity
We assessed the clinical and methodological diversity between studies to guide our decision as to whether data should be pooled. We were also guided by the degree of statistical heterogeneity, assessed by calculating the I² statistic (Higgins 2003); we considered a value greater than 50% as evidence of substantial heterogeneity.
Assessment of reporting biases
Reporting bias is best assessed using funnel plots, where 10 or more RCTs contribute to an outcome. However, there are currently insufficient studies to support this approach.
Data synthesis
We provide a narrative summary of the included studies. Where appropriate, we have pooled data from these studies in meta‐analyses. For dichotomous data, we used a fixed‐effect Mantel‐Haenszel model to calculate the risk ratio with a 95% confidence interval, in accord with the standard methods of the Cochrane Tobacco Addiction Group for cessation studies.
We had planned to calculate the summary estimates for continuous outcomes (e.g. biomarkers of tobacco exposure) using the inverse variance approach (also with a 95% CI). However, there were insufficient data with which to do so.
For adverse events, we originally planned to enter the most commonly‐reported adverse events into meta‐analyses to determine if there were any significant differences between the EC and control groups. We also originally planned to include data from cross‐over trials in a meta‐analysis using paired data obtained from reports. However, there were again insufficient data with which to do so, and hence we have summarized adverse event data narratively.
Subgroup analysis and investigation of heterogeneity
We had planned to undertake subgroup analyses to investigate differences between studies, such as:
Intensity of behavioural support used;
Type of control group (e.g. placebo EC, NRT);
Type of participants (e.g. experience of EC use).
However, there were too few studies to conduct such analyses. Should further studies become available in future, we will follow this approach.
Sensitivity analysis
We had planned to undertake sensitivity analyses to assess the effect of removing studies judged to be at high risk of bias. However, there were too few studies to conduct such analyses. Should further studies become available in subsequent updates, we will adopt this approach.
Summary of findings table
Following standard Cochrane methodology, we created a 'Summary of findings' table for both outcomes. For cessation, the 'Summary of findings' table only includes data from randomized controlled trials. Also following standard Cochrane methodology, we used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome, and to draw conclusions about the quality of evidence within the text of the review.
Results
Description of studies
Results of the search
Our bibliographic database searches identified 1704 non‐duplicate records. We found a further six records through screening references in the papers identified through electronic searches, and one further record through author contact. We screened all records and retrieved the full‐text papers of 117 potentially relevant studies. After screening and checking the full text of 117 papers, we identified 24 eligible completed studies (11 of which were new for this update) and eight ongoing studies. Searches of trials registers for this update identified a further 19 potentially relevant ongoing studies, making a total of 27 ongoing studies (Characteristics of ongoing studies). We excluded 46 studies after checking full‐text papers (Excluded studies). Secondary study reports, commentaries, and correspondence relating to included studies are linked to studies in the reference section. Figure 1 and Figure 2 present PRISMA flow charts for the update and the original review, respectively.
Figure 1.
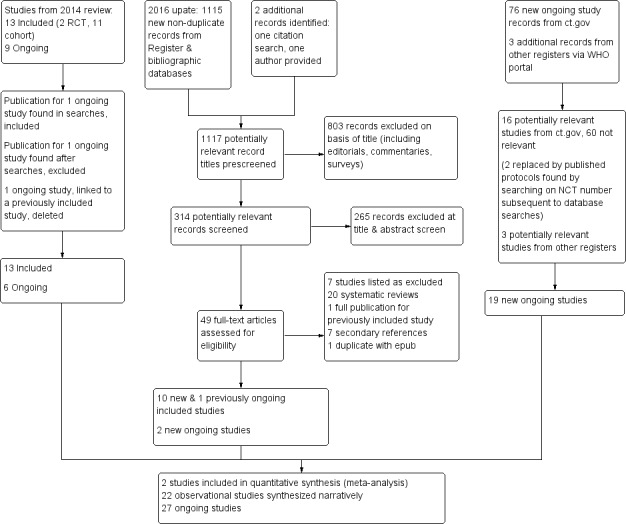
Study flow diagram for review update 2016
Figure 2.
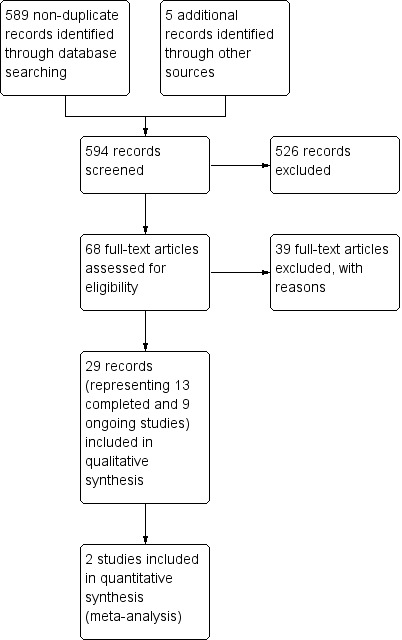
Study flow diagram for original review, 2014
The completed studies include three RCTs and 21 prospective cohort studies that describe abstinence at six months or longer or adverse events (AEs), or both. In one of the included studies (Choi 2014), the data come from the authors’ response to a criticism of their paper; the data had not been included in the original study report. One retrospective cohort study (Polosa 2014a) provided data on changes in respiratory parameters and symptoms in people with asthma that were using ECs. Although this used a retrospective design it used data from different time points and used routine clinical records that we deemed adequate for capturing data concerning adverse events.
In this update, we also collected information on systematic reviews (defined as having run a systematic search of at least one database) published within the update search period. Appendix 2 lists key features of the 14 reviews which met these criteria; we discuss these further in Agreements and disagreements with other studies or reviews.
Included studies
The key features of the included studies are summarized by study type below. Further details on each included study can be found in the Characteristics of included studies tables.
Randomized controlled trials
We identified only two completed randomized controlled trials (Bullen 2013; Caponnetto 2013a) which contribute data on cessation at six months or longer.
The ASCEND trial (Bullen 2013) randomized 657 smokers (middle‐aged, highly dependent, with one‐third being of New Zealand Maori origin) who wanted to quit to use either an Elusion brand EC (first‐generation technology) with cartridges containing 16 mg nicotine, or 21 mg/24‐hour nicotine patches, or an EC with cartridges without nicotine (placebo EC), for 12 weeks following a target quit date (TQD). The ECs were couriered to participants, and those allocated to the patch arm were mailed a voucher to exchange for NRT at a pharmacy, which is standard practice in New Zealand, but also received a voucher to cover the dispensing costs. All participants received an invitation to access phone‐ or text‐based support, although this was accessed by fewer than 10%. The EC used in this study delivered only low levels of nicotine. This was determined in a subsample of four participants, who had used the EC for at least one week, volunteered to give a baseline blood sample, and then use their EC, taking one puff every minute over 10 minutes. They then provided five further blood samples at approximately 10, 20, 30, and 60 minutes after the start of EC use. Pharmacokinetic analyses showed that plasma nicotine concentrations peaked (a median increase of 2.1 ng/ml from baseline) at 10 minutes after the start of EC use. Participants were followed up at six months post‐TQD and self‐reported abstinence was validated by carbon monoxide (CO) in expired breath, in line with the Russell Standard (West 2005). Participants who were still smoking at follow‐up were asked to report their daily cigarette consumption, and a change from baseline consumption was measured.
In the three‐arm ECLAT trial (Caponnetto 2013a), 300 smokers (again middle‐aged and highly dependent), who were not intending to quit smoking in the next 30 days, were randomized to use a 'Categoria' brand EC (model 401, which is no longer produced) with disposable cartridges containing 7.2 mg nicotine or 0 mg nicotine (placebo EC) for 12 weeks. The third arm used cartridges containing 7.2 mg nicotine for six weeks followed by 5.2 mg nicotine for another six weeks. The EC was presented simply as a healthier alternative to tobacco smoke, and could be freely used ad libitum (up to four cartridges per day) as a tobacco substitute. Participants were seen on eight occasions over 12 months, once at baseline and at seven follow‐up visits where they received more cartridges, handed in smoking diaries, and had CO and vital signs measured. Abstinence at 12 months was defined as complete self‐reported abstinence from tobacco smoking since the previous visit at six months, confirmed with CO less than 7 parts per million (ppm) at six and 12 months. Participants who were still smoking at follow‐up were asked to report their daily cigarette consumption, and a change from baseline consumption was measured.
New for this update is a further randomized controlled trial, Adriaens 2014. This three‐armed trial randomized 51 smokers not intending to quit in the near future to either the Joyetech e‐GO‐C second‐generation EC, the Kanger T2‐CC second‐generation EC, or to no treatment at baseline. EC groups were provided guidance on EC use and instructed to use the assigned EC ad libitum. Both groups were also provided with bottles of tobacco‐flavoured e‐liquid containing 18 mg/mL nicotine. At eight weeks, the control group was given the same EC provisions, but without instructions. Participants were followed up at three lab sessions over two months in which biomarkers, mood, adverse events and cessation were measured, as well as craving, withdrawal, and EC usage. Further data collection occurred at five and eight months from baseline. As all groups were provided with nicotine‐containing EC by six months, this study is not included in our meta‐analysis of smoking cessation outcomes and we report results narratively only.
Cohort studies
Six prospective intervention studies (three new for this update) described abstinence at six months or longer in smokers provided with ECs and/or instructions on EC use to reduce or stop smoking. Eight further studies (five new for this update) described abstinence in smokers who had tried or used ECs in the past at six months or longer from baseline (note, we will exclude this group of studies from the next version of this review, as higher‐quality data become available). Finally, seven studies (two new for this update) provide information on adverse events only.
Intervention studies
The first of the intervention studies recruited 14 smokers with schizophrenia from among inpatients at a psychiatric institution in Italy (Caponnetto 2013b). All had been smoking at least 20 cigarettes a day for at least the past 10 years and were not intending to quit. Participants were seen at baseline and provided with an EC ('Categoria' brand) with an initial four‐week supply of 7.4 mg nicotine cartridges. They were instructed to use their EC ad libitum (up to four cartridges a day), but no instruction on cessation or reduction was provided. Follow‐up was completed at 1, 2, 3, 6 and 12 months when cigarette consumption, CO, AEs and positive and negative symptoms of schizophrenia were measured. Further EC cartridges were supplied at one, two, and three months.
Another similarly designed study examined the effects of EC use over an extended period of time in 40 highly dependent middle‐aged smokers not wanting to quit smoking at any time in the next 30 days, recruited from among staff working in an Italian hospital (Polosa 2011). At baseline they were given an EC ('Categoria' brand) with a four‐week supply of 7.4 mg nicotine cartridges and instructed to use ad libitum (up to four cartridges a day). No instruction on cessation or reduction was provided. Participants were followed up at 1, 2, 3, 6, 18 and 24 months, when cigarette consumption, CO, and AEs were recorded. Additional EC cartridges could be requested at months one, two, and three.
The third study (Ely 2013) recruited 48 smokers, who wanted to quit or switch from cigarettes to ECs, from among 640 patients of a single family medical practice in Colorado (USA) who were recorded as current smokers. The intervention was based on the '5 As' and the transtheoretical model, and participants were informed of the range of treatment options at the start of the programme. They were provided with written information on 'blu cig' and 'smoke tip' ECs, regarding cost, availability, and nicotine dosage options. All participants used an EC, with 16 using bupropion and two using varenicline as well. Follow‐up was undertaken by telephone at two weeks, one, three and six months after the start of the intervention. No definition of abstinence was provided, nor were self‐reports biochemically verified.
The fourth study (Pacifici 2015), new for this update, recruited 34 adult smokers who had never received stop‐smoking support and were unmotivated to quit from a hospital‐based smoking cessation clinic in Italy. Participants were naïve to EC use at baseline and were provided with a commercially available EC over a period of four weeks, starting with a nicotine‐free e‐liquid before moving to a personally‐tailored nicotine dosage. Participants were offered a multicomponent medically‐assisted training programme for EC use, and were followed up at one, four and eight months where cessation, cigarettes per day, adverse events, exhaled CO, and nicotine concentration were measured.
The fifth study (Polosa 2014b), also new for this update and also based in a smoking cessation clinic in Italy, recruited 50 smokers unwilling to quit who had been smoking at least 15 cigarettes a day at baseline for at least 10 years. Participants were provided with second‐generation ECs with 9 mg/ml nicotine e‐liquid, and instructed to use the products ad libitum. No encouragement to quit smoking was provided, but participants were supported in charging, filling, activating and using the EC, with phone numbers provided for assistance. Thirty‐day, biochemically‐verified point prevalence abstinence, adverse events, cigarettes per day, exhaled CO and data on product usage and opinions of the product were collected at 4, 8, 12 and 24 weeks.
The final study (Polosa 2015), also new for this update, recruited 71 adult smokers making their first EC purchase from vape shops across Catania province in Italy. Participants were not provided with ECs but, upon purchasing an EC product of their choice, were instructed on how to set up and use the device and were given troubleshooting advice and a phone number for technical support. Participants were encouraged to use the EC in anticipation of reducing their daily cigarette consumption. Thirty‐day self‐reported point prevalence abstinence, details of product purchase, and cigarettes smoked per day were collected at six and 12 months.
Non‐intervention studies
We include three longitudinal web‐based surveys in this review. The first (Etter 2014) followed up smokers and EC users accessing websites selling or informing users about ECs and online EC forums. The survey was open to all nationalities, with 34% of respondents from the USA, 24% from France, 8% from the UK, 6% from Switzerland, and 28% from other countries. Three hundred and sixty‐seven participants who had completed a baseline questionnaire also completed a follow‐up survey one year later when they were asked to provide follow‐up data on EC use and smoking behaviour. Of these participants, 35 (10%) were occasional or daily smokers and daily EC users at baseline.
In the second web‐based survey, Grana 2014b recruited 949 current cigarette smokers (59% smoked within 30 minutes of waking and 69% never expected to quit or did not intend to quit in the next six months), who completed surveys at both baseline and one‐year follow‐up. At baseline 9% (n = 88) were using ECs (defined as use in the past 30 days). Self‐reported abstinence (not defined) was measured at one‐year follow‐up.
In the final web‐based survey, Brose 2015 recruited 4064 UK residents who had smoked in the past year, with 1769 followed up at 12 months. Twenty‐three per cent of participants were EC users at baseline, the majority of whom indicated they were using first‐generation ECs. At follow‐up, data were collected on quit attempts, reduction in cigarettes per day, and whether the participant considered him‐ or herself to be an 'ex‐smoker.'
Two longitudinal telephone‐based surveys are included in this review. In the first (Al‐Delaimy 2015), which is new for this update, California residents (USA) were recruited, who had smoked at least 100 cigarettes in their lifetime and smoked cigarettes 'at least some days' at baseline. At baseline, 83.6% were daily smokers, 236 had used ECs, and 306 indicated they would never use ECs. Self‐reported prolonged abstinence for one month or longer, quit attempts, and reduction were assessed at 12 months.
In the second study (Choi 2014), authors presented new data from a prospective cohort study of young adults recruited from Midwestern states of the USA in a response to a letter criticizing their main paper, which did not provide data on EC users and smoking outcomes. The letter reports on smoking cessation outcomes (not defined) in a sample of smokers who used ECs for one or more days in the last 30 days at baseline (no N given), comparing these to a sample of baseline smokers who had never used ECs at baseline. The main paper included 1379 participants (mean age 24) who had never used ECs, 17.8% of whom were reported to be current smokers.
A final three prospective studies used a range of follow‐up methods; all are new for this update. Borderud 2014 recruited 1074 patients presenting with cancer at a large US cancer centre who were referred to and completed intake assessment for the centre's tobacco cessation programme. All participants were offered multicomponent, evidence‐based behavioural and pharmacological treatment for tobacco dependence. At baseline, 26.5% of participants had used an EC within the last 30 days. Seven hundred and eighty‐one participants were followed up at six to 12 months from baseline, where self‐reported seven‐day point prevalence abstinence, cigarettes per day, and information on whether a participant had gone a day without smoking since baseline were collected.
In Manzoli 2015, which took place in community settings in Abruzzo, Italy, 491 tobacco smokers and 232 dual EC and tobacco smokers were followed up at 12 months, with further follow‐ups planned at 24, 36 and 60 months. At baseline, the mean EC nicotine dosage was 9.8 mg/ml, and the mean months of EC use amongst dual users was 8.6. Follow‐up measures included 30‐day sustained abstinence with CO verified in a subsample, and 30‐day abstinence from tobacco and EC.
Finally, Prochaska 2014 reports a secondary analysis of data from a randomized controlled trial in an inpatient psychiatric hospital in California, USA. Nine hundred and fifty‐six smokers of at least five cigarettes a day were recruited and randomized to different levels of behavioural support. At baseline, 11% of participants used an EC. This paper reports cessation measures (not defined) in EC and non‐EC users at the longest available follow‐up (not defined, but study length was 18 months).
Adverse event data only
We include seven short‐term cohort studies that report on adverse events. These studies are not included in smoking analyses due to short follow‐up. Again, further details can be found in the Characteristics of included studies tables.
Hajek 2015a offered an EC to 100 smokers joining a stop‐smoking service in London, UK. Participants were offered a choice of a ‘cigalike’ product (Gamucci, 1.6% or 2.2% nicotine per ml) or a tank model (EVOD, 1.8%; later replaced with Aspire product due to leakage issues), and 69% took up the offer. The ECs were provided alongside standard stop‐smoking service provisions, including an offer of stop‐smoking medications and weekly behavioural support. Adverse events were collected throughout. The study also measured abstinence at four weeks, cost, and client feedback.
Humair 2014 describes a prospective cohort study involving 17 participants (all highly dependent smokers, 82% with a mental illness), recruited from a university hospital outpatient clinic in Switzerland, who chose to use an EC to help them stop or reduce smoking. NRT or varenicline were used at some stage by 59% of participants in addition to EC. This study was available as an abstract only and thus we have limited detail on the methods and measures used to record adverse events.
McRobbie 2015 recruited 40 daily smokers who wanted to quit, from advertisements placed in free London newspapers. Participants attended a baseline session one week prior to their target quit date (TQD). On the TQD, participants were provided with ECs ('Green Smoke', first‐generation device, 2.4% nicotine cartridges). Two cartridges a day were supplied initially, with the supply later adjusted to actual usage. Participants attended weekly follow‐up sessions for four weeks, and received standard behavioural support. Cigarette consumption and CO readings collected at each session and urine samples for cotinine and 3‐hydroxypropylmercapturic acid (3‐HPMA) analysis were collected at baseline and at four weeks post‐TQD.
Nides 2014 recruited 29 smokers in good health and not intending to reduce or quit smoking in the next 30 days. The aim of this study was to investigate nicotine delivery and potential for smoking reduction or cessation. Participants were provided with a 10‐day supply of disposable ECs ('NJOY King Bold' brand containing 26 mg of nicotine) and instructed to use them ad libitum for a week. At the end of the week, 25 participants returned to the clinic, after abstaining from smoking and EC use for 12 hours. They undertook two series, an hour apart, of 10 puffs on their EC, and changes in plasma nicotine, heart rate and CO, and withdrawal symptoms were measured. Adverse events that occurred during the period of ad libitum use were also collected.
Oncken 2015 describes a randomized cross‐over study involving 27 non‐treatment‐seeking smokers of at least 10 cigarettes a day who were willing to try ECs for two weeks. Participants were prescribed Joye e‐GO C with 18 mg/ml nicotine, and crossed over at one week between menthol flavour and non‐menthol tobacco‐flavoured ECs. Participants were requested not to smoke during the study, but 60% reported intermittently using their normal cigarettes. At one and two weeks, blood pressure, heart rate, body plethysmography, static lung volumes, airways resistance (Raw) and specific conductance (sGaw) were measured after abstaining from EC for two hours and, subsequently, five minutes after inhaling an EC. Data on adverse events, nicotine concentrations and rates of cigarette and EC use were also collected.
Although not a prospective cohort study, Polosa 2014a allowed for extraction of data regarding adverse events. This study identified 18 participants with mild‐to‐moderate asthma who had previously smoked an average of 22 cigarettes a day, who reported regular EC use on at least two consecutive follow‐up visits, approximately six months apart, using a retrospective audit of clinical records from a respiratory outpatient clinic in Italy. Ten were using ECs only, and eight used ECs and smoked up to five cigarettes a day. The duration of EC use ranged from 10 to 14 months, and all started on first‐generation ECs, though the 'majority' switched to a "personal vaporiser" (second‐ or third‐generation). The authors collected data from four clinic visits: pre‐baseline (6 to 12 months prior to baseline); baseline visit (pre‐EC use), which occurred approximately six months prior to the first follow‐up visit; six‐month follow‐up; and 12‐month follow‐up. At each visit, participants were assessed by clinical history and examination, and by re‐evaluation of treatment adherence and efficacy. Information was gathered on asthma control, the number of exacerbations from the previous follow‐up visit, spirometry measurements, forced expiratory flow, and bronchial provocation tests assessing Airway Hyper Responsiveness (AHR) with methacholine (some participants only).
Van Staden 2013 recruited 15 healthy smokers of at least 10 cigarettes a day from a military hospital in South Africa. They were each provided with an EC ('Twisp eGo' 18 mg/ml nicotine) and asked to use this and to stop smoking for two weeks. Blood pressure, pulse, arterial and venous carboxyhaemoglobin saturation (COHb) and blood oxygen saturation were measured at baseline and two‐week follow‐up in 13 participants that attended both sessions.
Excluded studies
The reasons for exclusion of the 46 studies that we reviewed are briefly summarized below, but further detail can be found in the Characteristics of excluded studies table.
We ruled out the majority of excluded studies because the participants used ECs for less than a week, or the study report contained no information on cessation or adverse events. In these cases we were unable to determine if the excluded studies intended to measure these outcomes. In line with our protocol, we excluded cross‐sectional studies with data collected at one time point only, for reasons including inability to control for confounding variables and recall bias (see Agreements and disagreements with other studies or reviews for further discussion of potential biases).
Risk of bias in included studies
The risk of bias in the two RCTs which contribute to the cessation meta‐analysis (Bullen 2013; Caponnetto 2013a) was low across all domains. The only exception was in the reporting bias in Caponnetto 2013a, as it was unclear if the original intention was to combine the two nicotine‐containing EC groups or not. In the sample size calculation the authors compared the nicotine EC group with the placebo EC, but results are not reported in this way. In both studies the randomization procedures were adequate, biochemical validation of abstinence was used, and an intention‐to‐treat analysis was undertaken where all participants lost to follow‐up (LTFU) were considered to be smoking. The lost‐to‐follow‐up rate in Bullen 2013 was 22%. Although the patch group had higher LTFU and withdrawal than the EC group (patch: 27%; nicotine‐EC: 16%; placebo EC: 22%), there was minimal difference between the per‐protocol and ITT analyses and so we deemed attrition bias to be at low risk. LTFU rates were similar among the three arms at 12 months in Caponnetto 2013a (35% in 7.2 mg nicotine group; 37% in 5.4 mg nicotine group; 45% in no‐nicotine group). In the randomized cross‐over trial (Oncken 2015), we judged the risk of selection, performance and detection bias to be unclear, due to the limited amount of detail provided. We rated attrition and selection bias as low, with 20 out of 27 participants followed up and all expected outcomes reported. In Adriaens 2014, a further RCT not included in the cessation meta‐analysis, we judged allocation concealment and attrition bias to be unclear, due to limited detail available; we rated all other domains as low risk of bias.
We categorized all other included studies, by nature of their design, as being at high risk of selection bias. Ten of these did not blind participants or personnel and, given the nature of the study, follow‐up measures and contact with researchers, we judged them to be at risk of selection or performance bias or both. In the other studies, the lack of intervention or contact with researchers means that there is unlikely to be significant performance or detection bias. Rates of follow‐up were mixed in the non‐randomized studies, with four judged to be at risk of attrition bias because of high or differential levels of follow‐up. For many of the cohort studies we were unable to determine prespecified outcomes and hence rated these as being at unclear risk of reporting bias. One cohort study stated they collected data on adverse events, but did not provide any results for this outcome measure, and we judged it to be at high risk of reporting bias (Pacifici 2015). Finally, Ely 2013 did not provide a definition of abstinence and it was unclear if the completion of the programme was at six months after enrolment, or at an earlier time point. We therefore judged this study to be at high risk of other bias.
Details of 'Risk of bias' judgements for each domain of each included study can be found in the Characteristics of included studies table. Figure 3 illustrates judgements for each included study.
Figure 3.
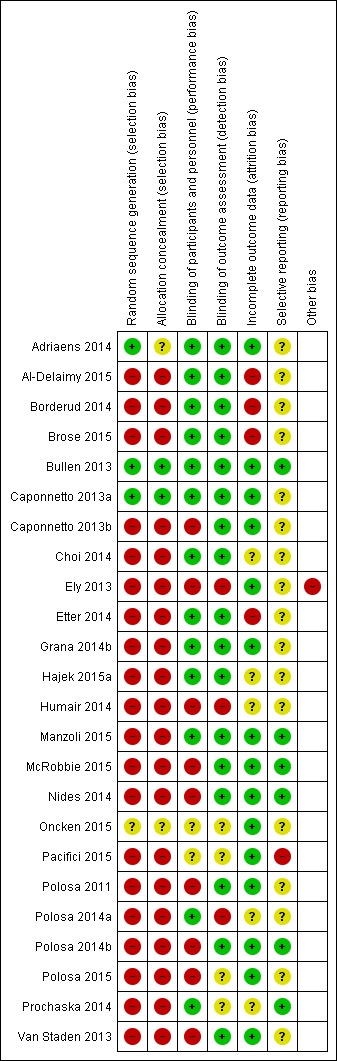
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
See: Table 1
In this section we have summarized the effects of ECs on smoking cessation and adverse events.
Cessation
Randomized controlled trials
In the trial comparing EC to patch (Bullen 2013) there was no significant difference in six‐month CO‐validated continuous abstinence between the treatment arms (7.3%, 5.8% and 4.1%, in the nicotine EC, patch and placebo EC arms respectively). We made two comparisons. The first compares abstinence rates between nicotine and placebo EC (7.3% versus 4.1%, risk ratio (RR) 1.77, 95% confidence interval (CI) 0.54 to 5.77; 362 participants; Analysis 1.1). The second compares abstinence rates between the nicotine EC and patch arms (7.3% versus 5.8%, RR 1.26, 95% CI 0.68 to 2.34; 584 participants; Analysis 1.2). Fewer than half of all participants across all groups accessed support (39.8%, 35.9%, and 35.6% in the nicotine EC, patch and placebo EC arms respectively).
Analysis 1.1.
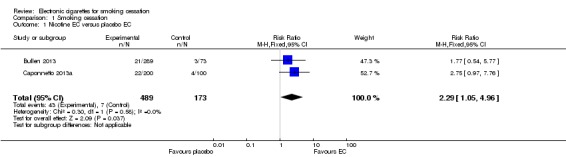
Comparison 1 Smoking cessation, Outcome 1 Nicotine EC versus placebo EC.
Analysis 1.2.
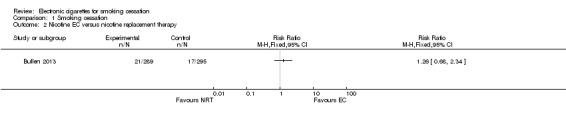
Comparison 1 Smoking cessation, Outcome 2 Nicotine EC versus nicotine replacement therapy.
In the other RCT (Caponnetto 2013a) one‐year abstinence rates (at least six months of not smoking and CO‐validated) were higher in the two nicotine EC arms (13% and 9%) compared with the placebo EC group (4%). In our analysis we combined the two nicotine EC arms and compared these with the placebo group. The difference was not statistically significant (11% versus 4%, RR 2.75, 95% CI 0.97 to 7.76; 300 participants; Analysis 1.1).
We combined data from the two studies comparing abstinence rates in nicotine versus placebo EC groups. There was no significant statistical heterogeneity between the studies (Chi² = 0.30, P = 0.58; I² = 0%) and pooled results showed use of a nicotine‐containing EC was associated with higher abstinence rates than placebo EC use (RR 2.29, 95% CI 1.05 to 4.96, 662 participants; Analysis 1.1).
Cohort studies
The abstinence rates from each cohort study are summarized in Table 4.
Table 1.
Summary of proportion of participants abstinent from smoking at follow‐up: cohort studies
| Study | Smokers motivated or unmotivated to quit? | Intervention vs relevant Control | % abstinent | ||||
| Cohort studies | 6‐month | 12‐month | 18‐month | 24‐month | Notes | ||
| Adriaens 20141 | Unmotivated to quit | Nicotine EC | 19.6% (10/51) | Data from 8 month follow‐up | |||
| Al‐Delaimy 2015 | Not defined. 43% intended to quit in next 6m | Had ever used nicotine EC at baseline | 5% (12/236) | Compared to 10.5% in never users | |||
| Borderud 2014 | Motivated to quit | Used EC in past 30 days at baseline | 14.5% | Average follow‐up 10 months. Compared to 30% in non EC users. Denominators for both groups not known, but ITT analysis | |||
| Caponnetto 2013b | Unmotivated to quit | Nicotine EC | 14% (2/14) | ||||
| Ely 2013 | Motivated to quit | Nicotine EC2 | 44% (21/48) | ||||
| Manzoli 2015 | Not defined | Nicotine EC | 16% (51/319) | Compared to 15% non‐users at baseline | |||
| Pacifici 2015 | Unmotivated to quit | Nicotine EC | 53% (18/34) | ||||
| Polosa 2011 | Unmotivated to quit | Nicotine EC | 23% (9/40) | 15% (6/40) | 13% (5/40) | ||
| Polosa 2014b | Unmotivated to quit | Nicotine EC | 36% (18/50) | ||||
| Polosa 2015 | Not defined | Nicotine EC | 42% (30/71) | 41% (29/71) | |||
| Cohort studies not allowing inclusion of non‐responders | |||||||
| Brose 2015 | Not defined. 46% attempted to quit in past 1 yr | Daily EC users at baseline | 8% (7/86) | Compared to 9.5% non‐daily EC users and 12.9% non‐users | |||
| Etter 2014 | Not defined | Daily EC users at baseline | 46% (16/35) | Response rate: 47% (367/773) completed follow‐up survey | |||
| Grana 2014b | Not defined | Used EC in the past 30 days (even once) at baseline | 10% (9/88) | Response rate: 81% completed follow‐up Abtsinence rate was 14% (119/861) in non‐EC users |
|||
| Choi 2014 | Not defined | Used EC for ≥ 1 day in the past 30 days at baseline | 11% | Response rate: unknown Abstinence rate was 17% in non‐EC users |
|||
| Prochaska 20141 | Not defined. 24% intended to quit smoking in next month | EC use at baseline via open‐ended question | 21% | Follow‐up period unclear, 12m is estimate. Denominator unclear. Compared to 19% not reporting EC use. | |||
1Technically an RCT but observational for purposes of EC analysis. 2All participants (N = 48) used an EC, but 16 also used bupropion and 2 used varenicline.
Intervention studies
Among the intervention cohort studies that enrolled smokers unmotivated to quit, Polosa 2011 reported abstinence rates (30‐day point prevalence, CO‐validated abstinence) of 22.5% at six months and 12.5% at two years. Pacifici 2015 reported cessation rates of 52.9% at 12 months, but did not define how cessation was measured. Polosa 2014b reported 36% (18/50) seven‐day point prevalence abstinence rates at 6 months, which were CO‐validated. In the study of highly‐dependent smokers with schizophrenia, 14% (2/14) achieved abstinence (CO‐validated) at one year (Caponnetto 2013b). In Ely 2013, 43.8% (21/48) of participants were abstinent from smoking at the completion of the six‐month programme. Of those that exclusively used ECs (n = 26), 50% (13) were abstinent, compared with 37.5% (6/16) of those who used both ECs and bupropion, and 100% (2/2) who used ECs with varenicline. In the one intervention cohort study in which motivation to quit was not defined (Polosa 2015), 42.2% of participants (30/71) were abstinent at six months, with similar numbers at 12 months (40.8%, 29/71; 30‐day, self‐reported point prevalence abstinence). In Adriaens 2014, a randomized controlled trial in which all participants were provided with nicotine‐containing ECs at eight weeks, and which we hence treat as a cohort study for cessation purposes, 19.6% of participants were abstinent at eight months (10/51) using CO validation.
Longitudinal surveys
The longitudinal surveys from the first version of this review contained relatively few smokers who were using ECs at baseline. Etter 2014 showed one‐year self‐reported abstinence rates of 45.7% (16/35) among the responders who used ECs at baseline. In Grana 2014b the one‐year abstinence rate was 10% (9/88) in smokers who had used ECs (at least once in the last 30 days) at baseline, compared with 13.8% (119/861) in non‐EC users. The difference between EC and non‐EC users was not statistically significant. No information was provided on whether people were using ECs for the purpose of cessation or reduction prior to baseline, or whether they used any EC at all during the follow‐up period. Choi 2014 only reported that 11% of smokers who had used ECs for one day or more in the last 30 days at baseline had quit smoking at one‐year follow‐up, compared with 17% of smokers who had never used ECs. After adjusting for demographics and baseline cigarette consumption, the odds of quitting were not significantly different between EC users and people who had never used ECs (odds ratio (OR) 0.93, 95% CI 0.19 to 4.63). Again, no information was provided on whether the participants used ECs during the follow‐up period.
Reflecting the increase in EC usage, some of the longitudinal surveys added during this review update had a higher baseline prevalence of EC use than those included previously. Al‐Delaimy 2015 found one‐year self‐reported prolonged abstinence (one month or longer) rates of 5% (12/236) in people who reported ever using EC at baseline, compared to 10.5% (32/306) in participants who indicated they would never use EC at both baseline and follow‐up; the authors report that ever use of EC predicted a lower likelihood of cessation in a multivariable analysis (OR 0.41, 95% CI 0.18 to 0.93). In Borderud 2014, 14.5% (denominator unknown) of participants who reported EC use in the past 30 days at baseline were abstinent at 12 months (self‐reported seven‐day point prevalence abstinence), compared with 30% of non‐EC users. In an ITT analysis correcting for a range of predictors, non‐EC users were found to be more likely to quit than EC users (OR 2.00, 95% CI 1.23 to 3.26), although there was no significant difference in a complete‐case analysis. It was not possible to calculate ITT data for Brose 2015; at one year, 8.1% of people who reported daily EC use at baseline (7/86) reported being ex‐smokers, compared to 9.5% (25/263) of people who reported non‐daily EC use at baseline and 12.9% (168/1307) of non‐EC users. Compared with non‐use, daily EC use at baseline was not significantly associated with cessation at follow‐up (OR 0.62, 95% CI 0.28 to 1.37), nor was non‐daily EC use. In Manzoli 2015, sustained (30‐day) smoking abstinence was reported at 12 months, with CO validation in a subsample of participants. The authors report there was no significant difference in abstinence between EC users and non‐users (summary statistic not provided), with 16% (51/319) of those who reported baseline EC use abstinent at 12 months compared with 15% (101/693) of people who did not use EC at baseline. Finally, Prochaska 2014 also did not find a significant difference in cessation (definition not provided) between those using EC and non‐users; at the longest available follow‐up point, 21% of people reporting EC use at baseline were abstinent, compared to 19% of those not reporting EC use at baseline (P = 0.726).
Crucially, this group of studies (the longitudinal surveys) share a serious limitation. As these studies only recruited current smokers, they excluded those people from the same population who tried ECs and stopped smoking (e.g. if 100 smokers tried ECs and 50 stopped smoking, these studies would only recruit the 50 who continued to smoke). Following up ‘treatment failures’ is likely to show a low treatment effect, even for treatments that are highly effective. To asses the effects of ECs on smoking, participants need to be recruited prior to initiating EC use. In future versions of this review, as higher‐quality data become available, we will no longer include this group of studies.
Adverse events
None of the RCTs or cohort studies reported any serious adverse events (SAEs) that were considered to be plausibly related to EC use.
Of the people available for six‐month follow‐up in the ASCEND trial (Bullen 2013), 44.4% of participants in the nicotine EC arm reported any AEs, compared with 44.7% and 45.6% in the patch and placebo EC arms respectively. Differences were not statistically significant (nicotine versus placebo EC: RR 0.97, 95% CI 0.71 to 1.34; 298 participants; Analysis 2.1; nicotine EC versus patch; RR 0.99, 95% CI 0.81 to 1.122; 456 participants; Analysis 2.2).
Analysis 2.1.
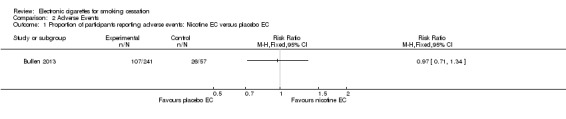
Comparison 2 Adverse Events, Outcome 1 Proportion of participants reporting adverse events: Nicotine EC versus placebo EC.
Analysis 2.2.
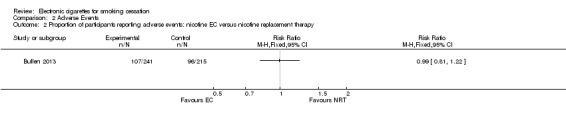
Comparison 2 Adverse Events, Outcome 2 Proportion of participants reporting adverse events: nicotine EC versus nicotine replacement therapy.
The ECLAT trial (Caponnetto 2013a) found no difference in frequency of AEs at three‐ or 12‐month follow‐up between the three groups. AEs were also measured at baseline, with the five most frequently reported being cough (26%), dry mouth (22%), shortness of breath (20%), throat irritation (17%), and headache (17%). In all groups the frequency of AEs decreased significantly over time, with the exception of throat irritation.
The cohort studies show a similar picture, with mouth and throat irritation being the most frequently reported AEs in EC users, most commonly dissipating over time. In Nides 2014, where participants used ECs for one week, 12 participants experienced 15 AEs and all but one (throat irritation) were classified as mild. After two weeks of use, Van Staden 2013 documented that 54% of participants (7/13) reported reduction in phlegm compared with baseline, whilst 31% (4/13) reported an increase. Changes in phlegm production could also be secondary to stopping smoking (the majority also reported an improved sense of taste, smell and an increase in appetite). There was one dropout due to illness (headache and fever), but it is unclear if this was deemed to be related to EC use or not. In Oncken 2015, where participants used ECs for two weeks with cross‐over at one week between menthol and non‐menthol tobacco‐flavoured e‐liquid, AEs included cough in 19% of participants (5/27) , mouth/throat irritation in 15% (4/27), nausea in 4% (1/27), headache in 4% (1/27), and “other” in 4% (1/27) (irritability, stomach cramps). This study reported one severe adverse event (itchy throat and cough) in a participant with a history of childhood asthma; the participant was discontinued from EC use and symptoms resolved. In Polosa 2011, which reported longer‐term follow‐up, the most commonly reported AEs were throat irritation (8.7%), mouth irritation (8.7%), dry cough (13.1%), dry mouth (4.3%), and headache (4.3%), which were stable throughout the study (percentages represent 24‐month data). Dizziness and nausea had been reported at the start of the study but disappeared by 24 months. In Polosa 2014b, where AEs were measured over six months of EC use, throat/mouth irritation (35.6%), dry throat/mouth (28.9%), headache (26.7%) and dry cough (22.2%) were frequently reported at study start but all decreased in frequency over time. In Hajek 2015a, where AEs were collected over four weeks, throat irritation and minor coughing were reported (incidence not quantified). The authors report one incident of a leak from the EVOD system which resulted in mouth irritation; medical treatment was not sought and the incident was resolved by washing the lip with water. Adriaens 2014 did not systematically collect data on AEs but did collect 'complaints' through online diaries; across all groups; these included bad taste, dry mouth/throat, irritated mouth/throat, dizziness, headache, nausea, and increased heart rate/palpitations, although rates were not provided. The authors note no significant change in Beck Depression Inventory scores (from 5.79 (standard deviation (SD) 8.35) at baseline to 4.94 (SD 8.76) at eight months). Humair 2014 reports only that participants did not experience any AEs. Pacifici 2015 reports measuring AEs but does not report the resulting data.
Effects on specific parameters
Eight studies report the effects of at least one week of EC use on more specific parameters.
In Adriaens 2014, which randomized participants to EC or control at baseline and then provided all participants with EC at eight weeks, authors report changes over time within groups but do not report direct between‐group comparisons. The EC groups showed an overall significant decrease in expired CO from the first lab session at week one to the second lab session at 4 weeks (P < 0.001), but not the control group (P = 0.10). At eight months (by which time all groups had received EC) there was a significant decrease in CO for all groups combined compared with baseline (P < 0.01, mean baseline CO 17.58 ppm (SD 7.17), mean CO at eight months 11.56 ppm (10.41)).
McRobbie 2015, a prospective cohort study in which all participants were provided with an EC, assessed the change in 3‐HPMA, the main metabolite of acrolein, excreted in urine after four weeks of EC use. Acrolein is a carcinogen and is present in cigarette smoke and some EC vapour (Bein 2011). There is a concern that people that use EC and smoke may be exposed to higher levels of acrolein than smoking alone. Of the 33 people that completed four‐week follow‐up, 16 were EC users only, and 17 were dual users. Both groups showed a significant decrease in 3‐HMPA in ng/mg creatinine (EC users: 1623 (SD 850) to 343 (SD 178), P < 0.001; Dual users: 2443 (SD 1105) to 969 (SD 807), P < 0.001). CO levels (ppm) also showed a significant decrease over time in both groups (EC users: 15 (SD 8) to 3 (SD 2), P < 0.001; Dual users: 23 (SD 11) to 11 (SD 8), P = 0.001).
Pacifici 2015 tested exhaled CO at one, four and eight months in an uncontrolled pre‐post pilot study. At one month, EC users showed a significant decline in exhaled CO; there was no significant change in non‐EC users (people who had opted not to use the EC provided). At four and eight months, exhaled CO had declined in EC and non‐EC users. Polosa 2011, a prospective cohort study in which all participants were provided with EC, measured exhaled CO and found a significant reduction in the average across the whole cohort of 23.5 to 8 ppm at 24 months (P = 0.011). Polosa 2014b, a further prospective cohort study in which all participants were provided with EC, also measured exhaled CO but report results graphically by group; at 24 weeks, CO appears to have significantly reduced amongst quitters and people reducing cigarette consumption by at least 50%, and appears to have remained stable in people who continued smoking at least half as many cigarettes as they had at baseline.
In the randomized cross‐over trial of menthol versus non‐menthol tobacco‐flavoured e‐liquid (Oncken 2015), the authors found no significant differences in airway function (Raw or sGaw) over the course of the two weeks compared to baseline (P > 0.09), or five minutes after inhalation of either type of EC (P > 0.1). There were also no significant changes in heart rate or blood pressure in either group at any time point.
In the retrospective study of smokers with asthma who had become regular EC users (Polosa 2014a), there was no evidence of harm. On the contrary, there were significant improvements in asthma control, measures of lung function, and airways hyper‐responsiveness both in EC users only (n = 10) and in dual users (n = 8) over the 12‐month follow‐up period. There was a slight decrease in the number of asthma exacerbations, but this was not statistically significant (1.17 to 0.78, P = 0.153).
Van Staden 2013, a short‐term pre‐post study which measured outcomes after two weeks of EC use, showed that smokers who switched to ECs had significant improvement in blood oxygen saturation (96.15% (SD 1.76) to 97.49% (SD 1.34); 1.34% increase, 95% CI 0.60 to 2.08; P = 0.002) and reduction in arterial (1.95%, 95% CI 0.47 to 3.44; P = 0.01) and venous (1.87%, 95% CI 0.38 to 3.36; P = 0.02) carboxyhaemoglobin levels.
Discussion
Summary of main results
This update includes a further 11 studies. However, no new randomized controlled trials (RCTs) evaluating smoking cessation at six months or longer were available, and the conclusions of this review have not substantively changed. As with the previous version of this review, a meta‐analysis that pooled the results of two randomized controlled trials (RCTs), covering 662 participants, showed that smokers who used nicotine electronic cigarettes (ECs) were significantly more likely to stop smoking than smokers using placebo ECs. The effect size (5%) is small, but not unusual given the low level of behavioural support provided. There was no evidence of statistical heterogeneity, despite the differences in study designs. In the one trial that evaluated it, a first‐generation EC with low nicotine delivery was as effective as nicotine patches in helping smokers to quit long‐term, but confidence intervals were wide.
Although the two RCTs were well conducted and judged to be at low risk of bias, we categorize the quality of the evidence overall as low, because of the small number of trials on which it is based (see Table 1). We would be more confident in the findings were there more studies available, and are encouraged by the increase in ongoing studies collected as part of this review update.
None of the included studies reported serious adverse events considered possibly related to EC use. One of the included studies detected a severe adverse event considered possibly related to EC use, which was the advent of itchy throat and cough in a participant with a history of childhood asthma. This resolved once EC use was discontinued (Oncken 2015). No studies detected a significant increase in adverse events in people using ECs. The most commonly reported AEs were local irritation of the throat and mouth. One of the RCTs (Caponnetto 2013a) measured AEs at baseline and then across the study duration, and showed that the frequency of respiratory symptoms (e.g. cough and shortness of breath) decreased over time, which is likely to be secondary to changes in cigarette smoking. This finding was supported by data from observational cohort studies.
Overall completeness and applicability of evidence
This is a new and rapidly evolving field of research. The search for the first version of this review captured almost 600 publications; for this update, our searches returned a further 1117 references. While we are confident that this represents the full range of data for the time period searched (to January 2016), there may be unpublished studies that we did not find. Despite the large number of publications returned, there were relatively few that contain empirical data and meet our inclusion criteria. The increase in ongoing studies suggests the evidence base will be strengthened in coming years.
We relied predominantly on RCTs for smoking cessation. Only two met our inclusion criteria. This limits the strength of our conclusions. We were unable to do many of the planned analyses because of insufficient data.
The designs of the two included RCTs limit the interpretation of the findings. The ECLAT study (Caponnetto 2013a) used only a placebo EC control, which does not allow comparison with standard smoking cessation treatments. The ASCEND trial (Bullen 2013) was more pragmatic, but also has some limitations. For example, few people accepted the offer of telephone‐based behavioural support. This is a likely reason for low absolute abstinence rates across all arms. The pragmatic nature of the study also resulted in some differences in the way that participants received their allocated product (EC was couriered directly to participants, whereas nicotine patches were supplied via a voucher that participants had to take to a community pharmacist). This approach has been criticized, as this difference may have influenced the outcomes (Grana 2014a). However, the trial was trying to replicate standard practice, and sensitivity analyses did not suggest that this was a mediator.
Both studies used first‐generation cartridge ‘cigalike’ ECs that were widely available at the time but that have now been surpassed by newer models. The EC used in the ASCEND trial (Bullen 2013) delivered little nicotine and not particularly quickly (Cmax of 1.3 ng/ml was achieved after 10 minutes of use). The EC used in the ECLAT trial (Caponnetto 2013a) also performed poorly and was discontinued before the trial was published. This may have yielded a more conservative estimate than would be seen with newer models. If these poorly‐performing EC products can assist smokers, products with better nicotine delivery may have better effects.
This update includes additional data on cessation from nine further studies. The newly‐added intervention cohort studies show a similar response to EC (with quit rates ranging from 14% in smokers with mental illness to 53% in a population of smokers unwilling to quit at the outset). This update also includes newly‐added longitudinal surveys. These studies share a serious limitation, as they include only continuing smokers at baseline, meaning people who have successfully used EC to quit prior to baseline are not included in the study populations; as higher‐quality data become available, we will not include this study type in future updates of this review. Of the seven longitudinal surveys which analyzed cessation at follow‐up based on EC use at baseline, five detected no significant difference based on baseline EC use, and two found that EC use at baseline was significantly associated with decreased rates of abstinence at follow‐up.
The adverse effects described in both the RCT and cohort studies are similar, regardless of the brand of EC used or nicotine content, with placebo and nicotine‐containing ECs showing similar numbers and types of adverse events in direct comparisons. They also reflect what is reported in survey data (Dawkins 2013b; Etter 2011), so we believe that they are broadly applicable to most EC brands. The common adverse effects, i.e. mouth and throat irritation, are likely to be caused by the propylene glycol (a humectant) and nicotine, which has a distinctive hot/peppery taste.
There has been concern raised that dual use may expose people to greater health risks, including higher nicotine levels. However, given that people who smoke like to maintain relatively stable blood nicotine levels (Russell 1990), receiving nicotine from an alternative source (i.e. EC) is likely to reduce nicotine intake from cigarettes, which should be accompanied by a reduction in smoke and toxin intake (Fagerström 2004). In a study assessing biochemical changes exclusively in dual users, there was a significant decrease in cotinine, exhaled carbon monoxide levels, and urinary 3‐HMPA (McRobbie 2015). These results are supported by longer‐term studies in smokers provided with ECs, which found decreases in exhaled carbon monoxide among dual users, and no significant increases in cotinine levels across the study populations (Adriaens 2014; Pacifici 2015; Polosa 2011; Polosa 2014b).
Quality of the evidence
The RCTs from which we extracted data for this review were conducted to a high standard, with adequate randomization, treatment allocation and blinding, and the abstinence data are reported in line with accepted standards, including biochemical validation of self‐reported smoking status. We consider these studies to be at an overall low risk of bias. However, as there were only two of them, the body of evidence is limited and we consider it to be low or very low quality by GRADE standards, because of the small number of trials. These GRADE ratings reflect low levels of confidence in the effect estimates presented in this review. This low level of certainty in the findings does not reflect issues with the quality of the individual studies, but rather reflects imprecision arising from low event rates and wide confidence intervals around the estimated effects, and some indirectness due to poor nicotine delivery in one of the devices tested.
It was unclear if the ECLAT trial (Caponnetto 2013a) intended to combine the two EC arms in the analysis or not. In sample size calculation they compared ECs with placebo ECs, but results are not reported in this way. The rationale for examining two very similar EC arms is not obvious to the review authors.
Both RCTs were underpowered. The sample for the ASCEND trial (Bullen 2013) was based on absolute six month quit rates of 20% and 30% for the patch and nicotine EC groups respectively. The effect size was estimated from the meta‐analysis of NRT trials, but the estimated patch group 20% quit rate, which was estimated from previous research undertaken in New Zealand where participants were recruited from among callers to the national Quitline, was clearly too optimistic. The ASCEND study recruited directly from the community and this population may not have been as committed to quitting, or the national Quitline data were based on a less rigorous standard (e.g. unvalidated self‐reported abstinence rate). The ECLAT trial (Caponnetto 2013a) also overestimated expected abstinence rates and the subsequent sample size (n = 300) was insufficient to detect significant differences.
The cohort studies that we included were all deemed to have high risks of bias, which is inherent in the study design. Some studies did not define abstinence outcomes or validated self‐reported smoking status, which further lowers our confidence in the findings. Data presented from these studies therefore needs to be interpreted with caution.
A major limitation common to several cohort studies (e.g. Choi 2014; Dutra 2014; Lee 2014; Popova 2013) is the definition of EC use, which is generally categorized as ‘ever use’ (e.g. ever tried, even just once) and ‘current’ use (used on at least one day in the last 30 days). 'Ever use' identifies experimentation, but oddly experimentation within the last 30 days would be captured as current use. Most of these studies were also unclear on the reasons for EC use (e.g. as part of a quit attempt, trying the new product out of curiosity, or to use when they cannot smoke) and failed to take into account other relevant factors (e.g. level of dependence) in their analyses. Perhaps most importantly, these studies excluded EC users who stopped smoking and so only followed up ‘treatment failures’. As such, causation cannot be inferred. As higher‐quality data become available, we will drop these studies from future versions of this review.
Potential biases in the review process
We consider the review process used to be robust, and do not believe we have introduced any biases. For outcome assessment, we followed the standard methods used for Cochrane Tobacco Addiction Review Group cessation reviews. Our search strategy included the Cochrane Tobacco Addiction Group Specialised Register and we were able to capture a number of ongoing studies. However, there may be unpublished data that our searches did not uncover. We also considered participants lost to follow‐up as smokers, which is best practice in this field of work.
Agreements and disagreements with other studies or reviews
When this review was initially published (McRobbie 2014), it was the first review of ECs to pool data and conduct a meta‐analysis. Since then, 14 systematic reviews of EC safety and/or efficacy for smoking cessation have been conducted (see Appendix 2). Three of these present meta‐analyses for smoking cessation, and of these, two included the same studies that we include: Rahman 2015a, which had virtually identical results to ours (RR 2.29. 95% CI 1.05 to 4.97), and Khoudigian 2016, which had similar results but marginally missed statistical significance as they included six‐ as opposed to 12‐month data from Caponnetto 2013a, in which the quit rate was slightly higher in the control group at six months than at 12 months (RR 2.02, 95% CI 0.97 to 4.22). The third meta‐analysis, conducted by Kalkhoren and Glantz (Kalkhoran 2016), has significantly different results from ours, concluding that, as currently being used, ECs are associated with significantly less quitting among smokers (OR 0.72, 95% CI 0.57 to 0.91). This review has generated considerable media attention and controversy within the academic community (Hajek 2016). The crucial difference between Kalkhoran's meta‐analysis and the other three meta‐analyses is that, rather than restricting the analysis to include RCTs only, the authors have included a range of study types, including cohort studies and cross‐sectional studies, as well as the two RCTs included in the other meta‐analyses. Kalkhoran and Glantz argue that the range of study types included in their meta‐analysis does not affect the validity of the result, as a sensitivity analysis by study type did not reveal a significant difference. However, given the paucity of RCTs (the sensitivity analysis compared 19 non‐randomized studies to two RCTs), there is very low power to detect any reasonable difference. This very low power explains why, despite the fact that the ORs for the RCTs and other trials were in opposite directions (0.67 versus 1.38), the comparison was not statistically significant.
There are various reasons why RCTs provide different answers from many observational studies in this area. These include variations in the effectiveness of ECs depending on the level of support provided, issues around definitions of baseline EC usage, and unexplored confounders. This is not an issue specific to ECs: cohort studies of NRT show clear evidence that failure to adjust for confounders leads to estimates that suggest NRT is ineffective, while including adjustment for variables related to tobacco dependence supports its effectiveness (West 2007). In addition, those studies which analyze results in smokers based on EC use at baseline have by the nature of their design already excluded people who have successfully quit using EC, and therefore only retain participants who, at entrance to the study, would be classed as 'treatment failures' or are in the midst of a cessation attempt involving cutting down to quit. Following the standard methods of the Cochrane Tobacco Addiction Group and the protocol for this review, we focused on evidence from randomized controlled trials for cessation outcomes, although we also analyzed cohort studies which provided interpretable data.
Despite their differences, the one area in which all systematic reviews of ECs for smoking cessation agree is that more evidence is needed. The majority of recent systematic reviews in this area sound a note of cautious optimism when it comes to the use of EC as a smoking cessation aid, but the evidence base is limited, particularly in comparison with smoking cessation treatments with established efficacy, such as traditional forms of nicotine replacement therapy, varenicline and bupropion (Cahill 2016). Uncertainty remains as to the long‐term safety profile of ECs, given their relatively new position in the market. Expert consensus broadly holds that, based on all available evidence, ECs are considerably safer than traditional cigarettes (McNeill 2015; RCP 2016), but further studies are needed to establish their safety profile compared with established smoking cessation aids.
Authors' conclusions
A limited number of randomized trials have been reported, so certainty about the effects is low. More data are needed to strengthen confidence in the estimates. There is evidence from the pooled results of two trials that electronic cigarettes (ECs) with nicotine, compared with placebo ECs, helped smokers to stop smoking long‐term. This corresponds to findings from placebo‐controlled trials of NRT (Stead 2012).
There is evidence from one trial that ECs may lead to six‐month quit rates similar to those achieved with NRT, but the confidence interval is wide. ECs are an evolving technology and the effects of newer devices with better nicotine delivery are unknown.
None of the included studies (short‐ to mid‐term, up to two years) detected serious adverse events considered possibly related to EC use. The most commonly reported adverse effects were irritation of the mouth and throat. The long‐term safety of ECs is unknown. In some studies, reductions in biomarkers were observed in smokers who switched to vaping consistent with reductions seen in smoking cessation.
Although the gold standard in examining the efficacy of medicines, including those used to help people stop smoking, is to compare active treatment with placebo, testing ECs containing nicotine against ECs without nicotine presents a rather conservative paradigm. This is because ECs provide nicotine replacement as well as behavioural and sensory replacement for cigarettes. As both of these elements are likely to be active ingredients of EC effects, ‘placebo‐controlled’ trials are in effect subtracting the sensorimotor element from EC efficacy. Although these sensorimotor effects may be important to many smokers, we do not know how much they might enhance quit rates. Existing evidence suggests that this may be only small (Bullen 2013; Przulj 2013). Although placebo ECs were important in testing ECs with metrics used in evaluating NRT products, future studies should focus on comparing ECs with ‘usual care’ or minimal treatment, and with alternative pharmacological and behavioural treatments. In this update, we found 15 ongoing RCTs with follow‐up of six months or longer, which include comparisons with pharmacological and behavioural treatments and 'usual care.'
Data are also needed on the proportions of smokers who successfully quit smoking with the help of ECs and who continue to use ECs long‐term, and the proportion who eventually become nicotine‐free. To assess the effects of ECs on smokers at the population level, data are needed on relationships between trajectories of vaping and smoking rates in countries where both products are available.
Given the variety of EC products on the market and the product evolution, future studies need to select ECs with good nicotine delivery that are representative of the best current standard in terms of reliability and user satisfaction.
Further RCTs also need to be adequately powered, and to consider providing ECs in a way that would be used in real‐world settings (e.g. taking into account individual preferences for strengths and flavours of e‐liquids and even EC devices).
Acknowledgements
JHB is funded by the National Institute of Health Research School for Primary Care Research.
Appendices
Appendix 1. MEDLINE search strategy
e‐cig$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
electr$ cigar$.mp.
electronic nicotine.mp.
(vape or vaper or vapers or vaping).ti,ab.
1 OR 2 OR 3 OR 4
Identical terms used for other databases.
Line 4 added to search strategy for 2016 update.
Appendix 2. Systematic reviews (2014 ‐ 2016) of EC safety or efficacy or both for smoking cessation
| Reference | Cessation reported? | Safety reported? | Study types included | Number of included studies | Summary of main conclusions |
| Gualano 2015 | Yes (narrative only) | Yes (narrative only) | "experimental and observational studies" | 12 | EC can reduce the number of cigarettes smoked and withdrawal symptoms AEs reported are mainly related to a short period of use Long‐term studies are needed to evaluate the effects of ECs after chronic exposure |
| Hagstrom 2014 | Yes (narrative only) | Yes (narrative only) | Not specified, but includes RCT and observational studies | 5 | Limited scientific data to support or refute their use as an option for smoking cessation Side effects were similar across studies, including cough, local irritation, and headache Length of follow‐up was too short to assess other, more concerning side effects such as respiratory disorders or malignancy |
| Harrell 2014 | Yes. Meta‐analysis: references systematic reviews only, OR 2.29, 95% CI 1.09 to 4.96 for nicotine EC v no‐nicotine EC | Yes (narrative only) | All | 20 | Further research is needed to examine the longer‐term safety, potential for long‐term use and efficacy as a cessation aid |
| Hua 2016 | No | Yes (narrative only) | Published case reports | 26 | EC use can be accompanied by negative and, less frequently, positive health effects ECs have their own set of health effects that need to be better characterized and understood |
| Kalkhoran 2016 | Yes. Meta‐analysis: includes 15 cohort, 3 cross‐sectional, 2 RCTs, OR for quitting 0.72, 95% CI 0.57 to 0.91 in EC users versus non‐EC users. | No | Clinical trials, cohort studies, cross‐sectional studies. Only studies with control groups included in meta‐analysis | 38 | As currently being used, ECs are associated with significantly less quitting among smokers ECs should not be recommended as effective smoking cessation aids until there is evidence that, as promoted and used, they assist smoking cessation |
| Khoudigian 2016 | Yes. Meta‐analysis (2 RCTs), nicotine versus non‐nicotine EC/other therapies RR 2.02, 95% CI 0.97 to 4.22 | Yes. Meta‐analysis (2 studies), nicotine versus non‐nicotine EC RR −0.09, 95% CI −0.28 to 0.46 | RCTs or comparative observational studies comparing EC to NRT or placebo | 5 | Limited low‐quality evidence of a non‐statistically significant trend toward smoking cessation in adults using nicotine EC compared with other therapies or placebo Larger, high‐quality studies are needed No trials reported increased withdrawal symptoms or incidence of AEs Safety of EC is inconclusive due to limited evidence |
| Lam 2015 | Yes (narrative only) | Yes (narrative only) | RCTs | 4 | EC may constitute an effective smoking cessation tool ECs have the potential to eliminate the harmful effects of tobacco smoking Long‐term effects of EC are uncertain |
| Malas 2016 | Yes (narrative only) | No | “empirical quantitative and qualitative papers” | 62 | Majority of studies demonstrate a positive relationship between EC use and smoking cessation Evidence remains inconclusive due to low quality of available research Well‐designed randomized controlled trials and longitudinal, population studies are needed |
| Meo 2014 | No | Yes (narrative only) | All | 28 | EC use unsafe and hazardous to human health Should not be used as a cessation tool |
| Orr 2014 | Yes (narrative only) | Yes (narrative only) | “clinical studies” | 6 | Limited evidence for the effectiveness of EC in smoking cessation EC may help modify smoking habits or reduce the number of cigarettes smoked Long‐term safety is unknown Concerns regarding increased poisoning exposures are alarming |
| Pisinger 2014 | No | Yes (narrative only) | “original publications describing a health‐related topic” | 76 | “Due to many methodological problems, severe conflicts of interest, the relatively few and often small studies, the inconsistencies and contradictions in results, and the lack of long‐term follow‐up no firm conclusions can be drawn on the safety of ECs. However, they can hardly be considered harmless.” |
| Rahman 2015a | Yes. Meta‐analysis (2 RCTs): Nicotine‐filled e‐cigarettes were more effective for cessation than those without nicotine (RR 2.29, 95%CI 1.05 to 4.97) | No | Published studies reporting cessation or reduction after EC use | 6 | Use of EC associated with smoking cessation and reduction, but included studies were heterogenous More randomized controlled trials are needed to assess effectiveness against other cessation methods |
| Rahman 2015b | Yes (narrative only) | Yes (narrative only) | All | 48 | Conclusions cannot be drawn due to insufficient evidence More research is needed in different population groups to assess long‐term safety and efficacy |
| Waghel 2015 | Yes (narrative only) | No | All English‐language clinical trials assessing cessation, reduction, desire to smoke, and/or withdrawal symptoms | 7 | Limited evidence available suggests EC may be effective as monotherapy for smoking cessation and reduction Superiority to nicotine replacement therapy was not proven |
Data and analyses
Comparison 1.
Smoking cessation
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Nicotine EC versus placebo EC | 2 | 662 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.29 [1.05, 4.96] |
| 2 Nicotine EC versus nicotine replacement therapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 2.
Adverse Events
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Proportion of participants reporting adverse events: Nicotine EC versus placebo EC | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Proportion of participants reporting adverse events: nicotine EC versus nicotine replacement therapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2016 | Amended | Clarification on outcome data from Adriaens ‐ no changes to conclusions |
History
| Date | Event | Description |
|---|---|---|
| 23 June 2016 | New search has been performed | Update search run January 2016, 11 new included studies added. Reduction removed as outcome, now covered in Harm Reduction review. |
| 23 June 2016 | New citation required but conclusions have not changed | 11 new included studies added; no changes to conclusions. |
Differences between protocol and review
Originally, the protocol did not specify a minimum follow‐up period for data on adverse events. The Methods section has been changed to clarify that we will exclude follow‐up data at less than a week.
The original version of this review included reduction as a secondary outcome. The 2016 update removed reduction as an outcome, to bring the review into line with other reviews of cessation treatments produced by the Cochrane Tobacco Addiction Group and to prevent substantial overlap with the update of the group's review of interventions for harm reduction (Stead 2007, update forthcoming).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: 3‐armed RCT (but for abstinence outcomes, treated as cohort in this review) Recruitment: Advertisement on university website, flyers on university campuses, emails to personnel and advertisement in local newspaper Setting: Community and laboratory, Belgium Inclusion criteria: Smoker for at least 3 years, smoking at least 10 factory‐made cpd, not intending to quit in the near future but willing to try a less unhealthy alternative Exclusion criteria: Diabetes, severe allergies, asthma or other respiratory diseases, psychiatric problems, dependence on chemicals other than nicotine, pregnancy, breast feeding, hypertension, CV disease, currently using any kind of smoking cessation therapy, prior use of EC |
|
| Participants | Total N: 48 provided data (51 consented, 50 attended any lab sessions, 2 further withdrawals) Randomized to: EC1 16, EC2 17, control 17 56% women, mean age 44, mean cpd 19, mean FTCD 5.79, all unwilling to quit with no baseline EC use |
|
| Interventions | Intervention: 2 intervention groups (EC1 and EC2) provided with 2nd‐generation EC and instructed to use EC or smoke ad libitum (EC1 group provided with Joyetech eGO‐C, EC2 group provided with Kanger T2‐CC) and provided guidance on EC use. For both types, provided 30 mL bottles of tobacco‐flavoured e‐liquid (Dekang “Turkish Blend”), containing 18 mg/mL of nicotine. 4 bottles at baseline replenished at 4 weeks, keep any remaining after 8 weeks Control: 6 bottles for 2 months at week 8 (half offered EC1, half offered EC2); no guidance on use |
|
| Outcomes | 3 lab sessions over 2 months (weeks 1, 4 and 8), plus online questionnaires, further follow‐up at 3 and 6m after last lab session Cessation: measured but definition not provided, validated with eCO 5 ppm or less Adverse events and biomarkers: eCO, salivary cotinine measured during lab sessions. Also collected “complaints” via online diaries, not EC‐specific Also collected craving and withdrawal symptoms via lab sessions, “benefits and complaints”, mood, EC usage |
|
| Notes | Not included in cessation meta‐analysis or interpreted as RCTs as does not meet our inclusion criteria for RCTs (6m comparison with non‐users/placebo). Reported narratively alongside cohort studies. At 2 months, before the control group received EC, CO‐validated quit rates were 34% vs 0% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization was performed by using a randomization tool available on the website www.randomizer.org (But high for abstinence outcome as non‐randomized for our purposes) |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unblinded but as this review only includes data on objective measurements and not cessation judged unlikely to affect outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unblinded but as this review only includes data on objective measurements and not cessation judged unlikely to affect outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 36 out of 48 completed follow‐up (11/16 in EC1 group, 12/17 in EC2 group, 13/17 in control group) |
| Selective reporting (reporting bias) | Unclear risk | Outcome reporting somewhat non‐traditional; for example, collecting complaints but not explicitly adverse events, and incidence of AEs not reported. Unable to find prospectively registered protocol |
| Methods | Design: Prospective cohort study Recruitment: Members of California Smokers Cohort (longitudinal survey), recruited proactively 2011 ‐ 2013 via telephone Setting: California, USA Inclusion criteria: state residents aged 18 ‐ 59 who had smoked at least 100 cigarettes during their lifetime and smoked cigarettes "at least some days" at baseline Exclusion criteria: Not stated |
|
| Participants | Total N: 1000 adult smokers (for this review, only include 236 ever EC users and 306 'will never use EC' respondents) 52.2% women; 30% 18 ‐ 44 years old, 70% 45 ‐ 59; 10% Hispanic, 73% non‐Hispanic white, 18% other; 83.6% daily smoker, 43% intended to quit smoking in next 6m |
|
| Interventions | Observational, no specific intervention. At baseline asked to indicate if they had used, might use, or would never use EC. Defined EC as “devices that look like cigarettes and contain nicotine but do not produce tobacco smoke; some brands are The Safe Cig, Green Smoke, and Blu.” | |
| Outcomes | Self‐reported prolonged abstinence for 1m or longer, assessed via phone at 12m Also measured quit attempts, reduction |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Observational study |
| Allocation concealment (selection bias) | High risk | Observational study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although there is no blinding, the study design and lack of intervention or contact with researchers mean that there is unlikely to be significantly impact on performance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Telephone report, unblinded, but given nature of the study differential misreport seems unlikely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Greater loss to follow‐up for 'will never use’ than users |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
| Methods | Design: Prospective observational cohort study Recruitment: Patients presenting with cancer at large US cancer centre; smokers referred to tobacco cessation programme (TCP). This study included all patients who completed TCP intake assessment, 2012 ‐ 2013 Setting: Cancer centre, USA Inclusion criteria: Smokers (smoked cigarettes or used other tobacco products within past 30 days) accepting cessation programme Exclusion criteria: none stated |
|
| Participants | Total N: 1074. 781 eligible for 6 ‐ 12m follow‐up 56.5% women, mean age 56, mean cpd 13, mean FTND 3.7. At baseline, 26.5% (285/1074) had used EC within last 30 days, 92% dual users |
|
| Interventions | All participants offered "multicomponent, evidence‐based behavioral and pharmacologic treatment for tobacco dependence”; plans differed by individual but offered up to 5 sessions of phone or in‐person counselling | |
| Outcomes | Follow‐up ranged from 6 to 12m after enrolling in TCP (mean 10m). Collected: Self‐reported 7‐day PP abstinence Gone at least 1 day without smoking CPD |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Observational |
| Allocation concealment (selection bias) | High risk | Observational |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although there is no blinding, the study design means that there is unlikely to be significantly impact on performance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report only but differential misreport across EC conditions judged to be unlikely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large number of participants (285) lost to follow‐up (of eligible, 59.5% followed up). A further 82 deceased “significantly higher percentage of E‐cigarette users dropped out of tobacco treatment and were lost to follow‐up than non–E‐cigarette users”. Complete‐case analysis not significant, ITT analysis significant. |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
| Methods | Design: Prospective cohort study Recruitment: National general population sample recruited from online market research organization, 2012 ‐ 2013 Setting: web‐based, UK Inclusion criteria: Smoked in the past year Exclusion criteria: not stated |
|
| Participants | Total N: 4064, 1769 followed up 50% women, mean age 43.4, mean cpd 12.9, 23% used EC at baseline, 46.3% attempted to quit in past year Of those using EC at baseline, majority used ‘first generation’ EC that were cigarette‐like in appearance (‘cigalikes’) |
|
| Interventions | None | |
| Outcomes | Reported being 'ex‐smoker' at 12m follow‐up Quit attempts 50% reduction in cpd |
|
| Notes | Baseline characteristics from Brown 2014a, but reports broader sample than that included here so some characteristics may be different from those reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Observational study |
| Allocation concealment (selection bias) | High risk | Observational study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although there is no blinding, the study design and lack of intervention or contact with researchers mean that there is unlikely to be significantly impact on performance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Online survey, differential misreport seems unlikely |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 43.3% (1759) followed up. 1687 used in analyses due to missing data or baseline pipe or cigar smoking. 1473 used in quit attempt analysis (further missing data) |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
| Methods | Design: 3 parallel groups RCT Recruitment: Smokers recruited from the community, via newspaper advertisements Setting: Research Unit, New Zealand Inclusion criteria: 18 years of age or older; Smoked 10 or more cpd over past year; Wanted to stop smoking Exclusion criteria: pregnant and breastfeeding women, people using cessation medicines or using other support to quit, heart attack, stroke, severe angina in the last 2 weeks, poorly‐controlled medical disorder, allergies, other chemical dependence |
|
| Participants | Total N: 657 62% women, mean age 42, ⅓ NZ Maori, smoking 18 cpd, mean FTND score 5.5 Lost to follow‐up at 6 months:
Discontinued treatment:
|
|
| Interventions | Randomized 4:4:1 to NEC, PATCH or PEC use for 13 weeks (from 1 week prior to TQD)
All participants referred to Quitline and received an invitation to access phone‐ or text‐based support. This was accessed by < 10% |
|
| Outcomes | Sustained (≤ 5 cigarettes allowed) validated (exhaled breath CO < 10 ppm) abstinence at 6 months ≥ 50% self‐reported reduction in baseline cigarettes at 6 months Participants reporting any adverse events Proportion of AEs that were serious Proportion of unrelated AEs |
|
| Notes | Accessed support: NEC: 115/289; PATCH: 106/295; PEC: 26/73 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised block randomization |
| Allocation concealment (selection bias) | Low risk | Computerised via study statistician |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | NEC and PEC were blind to treatment condition in relation to one another. No blinding for NEC/PEC vs PATCH conditions, but as NEC and PATCH were both active treatments performance bias judged unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | LTFU 22% (all considered smokers). Patch group had a higher LTFU and withdrawal than EC (loss to follow‐up 17% NEC, 27% patches, 22% PEC). However, minimal difference in per‐protocol and ITT analyses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
| Methods | Design: 3‐arm double‐blind randomized controlled trial: EC with 7.2 mg nicotine for 12 weeks; same for 6 weeks followed by 5.2 mg for 6 weeks: EC with no nicotine for 12 weeks Recruitment: Newspaper advertisements Setting: Outpatient clinic, Italy Inclusion criteria: Smoked at least 10 cpd for past 5 years; age 18 ‐ 70; in good health; not currently or intending to quit smoking in the next 30 days Exclusion criteria: symptomatic cardiovascular or respiratory disease; regular psychotropic medicine use; current or past history of alcohol abuse; use of smokeless tobacco or NRT; pregnant or breast feeding. |
|
| Participants | Total N: 300 36% women, mean age 44 (SD 12.5), mean cpd 20 (IQR: 15 ‐ 25) Lost to follow‐up at 12 months
No participants discontinued intervention |
|
| Interventions | EC presented as a healthier alternative to tobacco smoke and could be freely used, ad libitum (up to 4 cartridges per day) for 12 weeks, as a tobacco substitute EC used: 'Categoria' (model 401) with disposable cartridges
Baseline visit and up to 7 follow‐up visits to receive more cartridges, hand in diaries, measure CO and vital signs |
|
| Outcomes | Abstinence at 12 months (complete self‐reported abstinence from tobacco smoking since previous visit at 6 months, confirmed with CO < 7 ppm at 12 months) ≥ 50% reduction in baseline cigarettes at 12 months Recorded AEs thought to be related to tobacco smoking and EC at baseline and at each study visit (7 follow‐up visits over 12 weeks, plus at 24 and 52 weeks) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, block size 15 (5:5:5 ratio) |
| Allocation concealment (selection bias) | Low risk | Randomization carried out by pharmacy, who did not have direct contact with the participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. “Blinding was ensured by the identical external appearance of the cartridges. The hospital pharmacy was in charge of randomization and packaging of the cigarettes” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 211 (70.3%) and 183 (61%) attended 6‐ and 12‐month follow‐up (at 12m, 35% lost in 7.2 group; 37% lost in 5.4 group; 45% lost in no‐nicotine group) |
| Selective reporting (reporting bias) | Unclear risk | Unclear if original intention was to combine groups A+B or not. In sample size calculation they compared A+B with C, but results are not reported in this way |
| Methods | Design: Prospective cohort Recruitment: Inpatients at a psychiatric institution in Italy Inclusion criteria: Smoked ≥ 20 cpd for at least the past 10 years; diagnosis of schizophrenia Exclusion criteria: Alcohol and illicit drug use, recent myocardial infarction, angina pectoris, high blood pressure (BP > 140 mmHg systolic or 90 mmHg diastolic, or both), diabetes mellitus, severe allergies, poorly‐controlled asthma or other airway diseases |
|
| Participants | Total N: 14 57% women, mean age 44.6 (SD 12.5), mean pack years smoked 28.8 (SD 12.9) |
|
| Interventions | Seen at baseline, given EC ('Categoria' brand) with an initial 4‐week supply of 7.4 mg nicotine cartridges. Instructed to use ad libitum up to 4 cartridges per day. EC cartridges supplied at months 1, 2, and 3 No instruction on cessation or reduction was provided. |
|
| Outcomes | Follow‐up at 1, 2, 3, 6 and 12 months where cigarette consumption, CO, AEs and positive and negative symptoms of schizophrenia were measured Sustained reduction of ≥ 50% for at least 30 days at 12 months 30‐day point prevalence CO‐validated abstinence at 12 months Adverse events |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort; no randomization |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0/14 lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
| Methods | Design: Longitudinal survey (data from the Minnesota Adolescent Community Cohort) Recruitment: Participants selected via cluster random sampling of household phone numbers Setting: Telephone survey Inclusion criteria: Participants who completed the survey between October 2010 and March 2011 and provided follow‐up data 1 year later Exclusion criteria: none stated |
|
| Participants | Total N: 346 | |
| Interventions | Observational; no specific intervention. No data on nicotine content of ECs are provided | |
| Outcomes | Self‐reported smoking cessation at 1‐year follow‐up (not otherwise defined) | |
| Notes | This publication is a letter in response to a comment on the authors' original paper Choi 2014, and the details on methods are taken from this. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although there is no blinding, the study design and lack of intervention or contact with researchers mean that there is unlikely to be significantly impact on performance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although there is no blinding, the study design and lack of intervention or contact with researchers mean that there is unlikely to be significantly impact on detection |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unable to determine attrition bias |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
| Methods | Design: Prospective cohort Recruitment: Letter sent to family practice patients who were current smokers Setting: Single family practice, Colorado USA Inclusion criteria: Want to quit or switch from tobacco cigarettes to ECs Exclusion criteria: None reported |
|
| Participants | Letters sent to 640 patients, 48 chose to participate and 44 completed the programme, 4 were lost to follow‐up Of the 44 participants, 66% women, all non‐Hispanic/white, aged 20 ‐ 75 (30% were age 51 ‐ 60), 57% had a high school education or less |
|
| Interventions | The 6‐month smoking cessation programme was based on The '5 A's' model and transtheoretical model. Options for treatment were discussed with each participant at the start of the programme. All used an EC, with 16 using bupropion and 2 using varenicline as well Participants were provided with written information on “blu cig” and “smoke tip” ECs, regarding cost, availability, nicotine dosage options |
|
| Outcomes | Phone follow‐ups at 2 weeks, 1 month, 3 months, and 6 months At completion of programme (using ITT) Abstinence from smoking and EC use Abstinence from smoking but not EC use ≥ 50% reduction of baseline cigarette consumption (still using ECs) |
|
| Notes | No definition of abstinence provided Not clear if 'completed programme' was at 6 months |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/48 lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
| Other bias | High risk | No definition of abstinence provided Not clear if 'completed programme' was at 6 months. |
| Methods | Design: Longitudinal Internet survey Recruitment: Via websites selling or informing about ECs and online EC forums Setting: Online survey (open to all nationalities; of respondents, 34% US, 24% France, 8% UK, 6% Switzerland, 28% other countries) Inclusion criteria: Aged 18 years and older Exclusion criteria: none stated |
|
| Participants |
One‐month survey Total N: 477, mean age 42, 41% women, 59% had a diploma giving access to university, 28% daily or occasional smokers, 76% daily EC users. 50/477 occasional or daily smokers at baseline One‐year survey Total N: 367, mean age 43, 42% women, 59% had a diploma giving access to university, 24% daily or occasional smokers, 79% daily EC users. 35/367 occasional or daily smokers at baseline |
|
| Interventions | Observational; no specific intervention. Participants that had completed a baseline questionnaire were emailed one month and one year later and asked to provide follow‐up data on EC use and smoking behaviour | |
| Outcomes | From among those that were smoking cigarettes at baseline 7‐day PP abstinence from smoking at 12 months Smoking consumption (change from baseline) at 12 months |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although there is no blinding, the study design and lack of intervention or contact with researchers mean that there is unlikely to be significantly impact on performance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although there is no blinding, the study design and lack of intervention or contact with researchers mean that there is unlikely to be significantly impact on detection |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 28% (N = 367) for those who answered the baseline survey (N = 1329) provided data at 1‐year follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
| Methods | Design: Longitudinal web‐based survey Recruitment: Via Knowledge Networks (now GfK) probability‐based web‐enabled panel Setting: Web‐based survey, USA Inclusion criteria: Aged 18 years and older Exclusion criteria: none stated |
|
| Participants | Total N: 949 52.4% women, 90.8% having at least a high school education, 75.3% white, mean (SD) daily cigarette consumption 14.5 (9.7), 59% smoke within 30 minutes of waking, 69.4% never expecting to quit or intending to quit in the next 6 months 90.7% did not use (EC use within the last 30 days) an EC at baseline. No data on nicotine content of EC are provided |
|
| Interventions | Observational; no specific intervention | |
| Outcomes | Self‐reported smoking cessation at 1‐year follow‐up (not otherwise defined) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although there is no blinding, the study design and lack of intervention or contact with researchers mean that there is unlikely to be significantly impact on performance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although there is no blinding, the study design and lack of intervention or contact with researchers mean that there is unlikely to be significantly impact on detection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 81.3% of the participants of baseline survey completed follow‐up survey |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
| Methods | Design: Prospective cohort, intervention provided Recruitment: Smokers attending stop‐smoking service Setting: Stop‐smoking service, London, UK Inclusion criteria: All smokers joining stop‐smoking service |
|
| Participants | Total N: 100 (69 of whom accepted offer of EC) 38% women (those who accepted) 55% women (those who declined), mean age 41, mean cpd 14, all motivated to quit. EC use at baseline not specified but some who declined EC offer had used EC in the past |
|
| Interventions | EC: offered to all smokers joining service; offered choice of ‘cigalike’ (Gamucci, 1.6% or 2.2% nicotine per ml) product or tank model (EVOD, 1.8%; later replaced with Aspire product due to leakage issues). 69% of those offered received an EC on TQD Medication: Offered stop‐smoking medications including NRT and varenicline as in standard protocol. Of EC users 33% opted to also use NRT, 29% varenicline, 38% nothing. Support: weekly, as in standard protocol |
|
| Outcomes | Adverse events collected throughout, method for collection unclear Also collected: 4‐week biochemically‐validated abstinence, client feedback, cost |
|
| Notes | Study allows a comparison between users and non‐users of EC but follow‐up only 4 weeks so does not contribute to abstinence results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomized |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unblinded but given nature of the study judged unlikely to affect results |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unblinded but given nature of the study judged unlikely to affect results |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 26% lost in EC group, dropout rate in EC decliners not reported. Reasons for dropout not stated |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes authors set out to collect, no protocol available |
| Methods | Design: Prospective cohort Recruitment: People attending an outpatient clinic Setting: University hospital outpatient clinic, Switzerland Inclusion criteria: Wish to reduce tobacco use or had failed to stop smoking using varenicline, bupropion or NRT in past |
|
| Participants | TOTAL N: 17 mean 23 cpd, 82% had a psychiatric illness |
|
| Interventions | Offered an EC with nicotine 59% also reported using NRT or varenicline in addition to EC |
|
| Outcomes | Smoking cessation and reduction by at least 30% at 12 months (self‐report) Adverse events No significant side effects |
|
| Notes | Abstract only, hence little detail available Not clear if EC was provided by clinic or if participants had to buy their own |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding, no biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers lost to follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
| Methods | Design: Prospective cohort study Recruitment: Community, Abruzzo, Italy Setting: 2013, via GPs, EC shops, internet advertisements and social networks Inclusion criteria: Adults (30 – 75 years), smokers of at least 1 tobacco cigarette/day (tobacco smokers) for past 6m, users of any type of EC, inhaling at least 50 puffs weekly for past 6m (e‐smokers), or smokers of both tobacco and EC (smoked both tobacco and EC within the same week for the past 6 months) (dual smokers) Exclusion criteria: Age < 30 yrs and > 75 yrs; pregnancy or breastfeeding; illicit drug use, major depression, severe allergies, angina, and past episodes of smoking‐related major diseases |
|
| Participants | Total N: 1012 (includes only those smoking at baseline) 44.1% women, mean age 44.5, mean cpd 14.4 60% of EC users using to quit, 36.5% to reduce |
|
| Interventions | Observational only, no intervention provided. Mean EC nicotine dose 9.8 mg, mean EC daily puffs 130, mean months of EC use 8.6 | |
| Outcomes | 12m (Planned also 24, 36, 60m – this is noted as early data) 30‐day sustained abstinence, CO tested in 25% random sample of those declaring abstinence 30‐day abstinence from EC and tobacco |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Observational |
| Allocation concealment (selection bias) | High risk | Observational |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although there is no blinding, the study design and lack of intervention or contact with researchers mean that there is unlikely to be significantly impact on performance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | CO tested in 25% random sample of those declaring abstinence. Of those, 4% misreport (2 tobacco smokers, 1 e cig user) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 70.8% response rate overall |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. Authors initially planned follow‐up at 6m but funding was withdrawn |
| Methods | Design: Prospective cohort Recruitment: advertisements in free London newspapers Setting: Smokers' clinic, East London, UK Inclusion criteria: Daily smokers who want to quit, aged 18 and older Exclusion criteria: pregnant and breastfeeding women, current serious medical illness, EC use for more than 1 week in the past |
|
| Participants | Total N: 40 45% women, mean age 47 (SD 12), mean cpd 19 (SD 10), mean FTND 5.2 (SD 2.8), 65% in full‐time employment |
|
| Interventions | Participants attended baseline session 1 week prior to their TQD. On the TQD, participants were provided with an EC (Green Smoke, 1st generation device, 2.4% nicotine cartridges). 2 cartridges per day were supplied initially, with the supply adjusted to actual use later. Attended 4 weekly follow‐up sessions and received standard behavioural support | |
| Outcomes | Cigarette consumption and CO readings collected at each session. Urine sample for cotinine and 3‐HPMA analysis collected at baseline and 4 weeks post‐TQD Change in urinary 3‐HPMA (ng/mg creatinine) at 4 weeks Change in urinary cotinine (ng/mg creatinine) at 4 weeks Change in CO at 4 weeks |
|
| Notes | Previously McRobbie 2014, ID updated in this version to reflect 2015 publication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/40 participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes reported |
| Methods | Design: Open‐label non‐comparative study Setting: Clinical Trials Unit, USA Recruitment: Study site database and community advertisements Inclusion criteria: age 18 ‐ 65 years; good health; BMI 18 ‐ 35; smoking 10+ cpd; and CO > 10 ppm Exclusion criteria: pregnancy or breastfeeding; other drug dependency; use of any psychiatric or opioid medications; EC within the previous 14 days; use of NRT in last 30 days; want to reduce or quit smoking within the next 30 days |
|
| Participants | Total N: 29 44% women; mean age 43; mean cpd 20.1; mean FTND 4.5 |
|
| Interventions | Participants attended 3 clinic visits at 1‐week intervals Visit 1: Baseline Visit 2: Provided with 1st generation type ‐ 'NJOY® King Bold' (NJOY, Inc., Scottsdale, AZ), with 26 mg nicotine. Used ad libitum for 20 minutes in the clinic, then ad libitum use over the next week. Recorded use of regular cigarettes and puffs on EC Visit 3: Participants abstained from all sources of nicotine for 12 hours prior to visit |
|
| Outcomes | Adverse events | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants dropped out between visits 1 and 2. |
| Selective reporting (reporting bias) | Low risk | Planned comparisons reported |
| Methods | Design: Randomized cross‐over study Recruitment: Newspaper advertisements, radio announcements, and from local general medicine practices Setting: Lab‐based study, Connecticut, USA Inclusion criteria: non‐treatment‐seeking smokers who were willing to try EC for 2 weeks and abstain from conventional cigarette smoking. 18 – 55 years of age who smoked at least 10 cpd Exclusion criteria: pregnant, previous myocardial infarction or stroke, uncontrolled hypertension (blood pressure (BP) > 160/100), insulin‐dependent diabetes, COPD or current asthma, known allergy to propylene glycol |
|
| Participants | Total N: 27 45% women; mean age 42; 70% white; 15% Hispanic, 15% black; mean cpd 16; 45% had tried EC at baseline, 50% smoked menthol cigarettes |
|
| Interventions | Prescribed Joye eGo‐C (www.joyetech.com) and e‐Juice (18 mg/mL nicotine) procured from American eLiquid (www.americanliquid.com). Cross‐over study between menthol‐flavoured and non‐menthol tobacco‐flavoured EC. Requested not to smoke their regular cigarettes during study period; however majority (60%) reported intermittently smoking cigarettes during study | |
| Outcomes | Follow‐up at 1w and 2w BP, heart rate, body plethysmography, static lung volumes and airways resistance (Raw) and specific conductance (sGaw) – taken at lab visits after abstaining from EC for at least 2 hrs, then taken again after inhaling EC and repeated 5 mins later Adverse events also reported but method for measuring not stated Also measured nicotine concentrations, rates of cigarette and EC use |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated; "Subjects were then randomly assigned to use the menthol or plain e‐cigarette cartridge for one week, switching to the other cartridge for the second week" |
| Allocation concealment (selection bias) | Unclear risk | No detail given |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20/27 followed up |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
| Methods | Design: Uncontrolled pre‐post pilot study Recruitment: Word of mouth Setting: Hospital‐based smoking cessation clinic, Italy Inclusion criteria: Adult smokers unwilling to quit smoking tobacco cigarettes and who have never tried a quit smoking protocol and/or have refused any smoking cessation treatment Exclusion criteria: none stated |
|
| Participants | Total N: 34 47.1% women, mean age 40.6, mean cpd 21.5, no EC use at baseline, not motivated to quit |
|
| Interventions | EC: Participants were given commercially available EC (AVATAR device, Battery 550 mAh/3.9 V, W: 7.8, cartomizer with 2, 2 ohm resistance, tank capacity 1.5 mL, temperature of the aerosol: 55/65 degrees), 2 different chargers for each EC and PUFFIT e‐liquids with nicotine content matching the individual nicotine daily intake and tobacco and/or other flavours freely chosen by each participant W1: nicotine‐free e‐liquid W2&3: Own EC with personal nicotine dosage, encouraged to use as substitute for traditional cigarettes W4: Encouraged to forego all traditional cigarettes Throughout: assistance at any time of day from centre staff with any EC‐related problem, plus follow‐up group sessions and smartphone messaging application Behavioural support: Multi‐component medically‐assisted training programme with monitoring of nicotine intake as a biomarker of correct EC use, including Information about general working principles, safety and risks of EC, together with medically‐assisted face‐to‐face training on how to correctly use the device to absorb nicotine vapour |
|
| Outcomes | Follow‐up at 1, 4 and 8m Cessation (measure not defined) Adverse events Exhaled CO, COT, 3‐HCOT concentration cpd |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not controlled |
| Allocation concealment (selection bias) | High risk | Not controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if staff were blinded to participant EC usage, not clear how cessation was defined |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants followed up |
| Selective reporting (reporting bias) | High risk | AEs measured but not reported |
| Methods | Design: Prospective cohort Recruitment: Advertisments in local hospital in Catania, Italy Inclusion criteria: Healthy smokers 18 ‐ 60 years old, smoking ≥ 15 cpd for at least the past 10 years, and not wanting to quit smoking at any time in the next 30 days Exclusion criteria: History of alcohol and illicit drug use, psychiatric illness, recent myocardial infarction, angina pectoris, high blood pressure (BP > 140 mmHg systolic or 90 mmHg diastolic, or both), diabetes mellitus, severe allergies, poorly‐controlled asthma or other airways diseases |
|
| Participants | Total N: 40, hospital staff 35% women, mean age 42.9 (SD 8.8), median cpd 25 (IQR 20 ‐ 30), median FTND 6.0 (IQR 6 ‐ 8) |
|
| Interventions | Seen at baseline, given EC ('Categoria' brand) with an initial 4‐week supply of 7.4 mg nicotine cartridges. Instructed to use ad libitum up to 4 cartridges per day. EC cartridges supplied at months 1, 2, and 3 No instruction on cessation or reduction was provided |
|
| Outcomes | Follow‐up at 1, 2, 3, 6, 18 and 24 months where cigarette consumption, CO, and AEs were measured, incl. 30‐day PP CO‐validated abstinence at 6 months and CO‐validated abstinence at 18 & 24 months (not otherwise defined) Adverse events |
|
| Notes | Smoking cessation services provided to those who spontaneously asked for assistance with quitting. These participants were excluded from the study protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13/40 were lost to follow‐up, but used ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
| Methods | Design: Retrospective cohort (retrospective audit of clinical records) Recruitment: Review of medical records from a respiratory outpatient clinic in Italy from September 2012 until December 2013 Setting: Respiratory outpatient clinic, Italy Inclusion criteria: People with mild to moderate asthma reporting regular EC use on at least 2 consecutive follow‐up visits Exclusion criteria: None reported |
|
| Participants | Total N: 18, 39% (N = 7) women 10 were using EC only (3 women, mean age 36) 8 used ECs and smoked ≤ 5 cpd (4 women, mean age 42) Both groups smoked 22 cpd at baseline Duration of EC use 10 ‐ 14 months. N = 12 using them for > 1 year All started on 1st generation EC, but the 'majority' switched to a 'personal vaporiser' (2nd or 3rd generation) |
|
| Interventions | Observational; no specific intervention. First 2 observations prior to EC use, second 2 observations during EC use | |
| Outcomes | Data from 4 clinic visits were collected: (1) pre‐baseline (6 – 12 months prior to baseline); (2) baseline; (3) 6 (± 1) month follow‐up; and (4) 12 (± 2) month follow‐up. Visits 1 and 2 were pre‐EC use and visits 3 and 4 were during EC use At each visit, participants were assessed by clinical history and examination and re‐evaluation of treatment adherence and efficacy
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Retrospective cohort |
| Allocation concealment (selection bias) | High risk | Self‐selected sample |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although there is no blinding, the study design and lack of intervention means that there is unlikely to be significantly impact on performance |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemical validation undertaken |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not applicable; unclear if some participants attended first 3 visits but not 4th, and hence were excluded |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
| Methods | Design: Prospective cohort study Recruitment: Volunteers, leaflets, cessation service kiosk in hospital Setting: Smoking cessation clinic, Italy Inclusion criteria: Healthy smokers 18 – 60 years old, smoking ≥ 15 conventional cpd for at least 10 years, unwilling to quit Exclusion criteria: none stated |
|
| Participants | Total N: 50 40% women, mean age 41, mean cpd 25, mean FTND 6.0, no EC use at baseline, not motivated to quit |
|
| Interventions | EC: 2nd generation devices (personal vaporisers ‐ PVs): EGO/CE4 model, filled with tobacco aroma e‐Liquid containing 9 mg/ml nicotine; instructed to use the study products ad libitum (up to a maximum of 5 ml/day; i.e. half vial) Behavioural support: Participants were instructed how to charge, fill, activate and use the EC. Key troubleshooting was addressed and phone numbers were supplied for assistance. “No emphasis on encouragement, motivation and reward for the smoking cessation‐related efforts were provided during the study.” |
|
| Outcomes | 4, 8, 12 and 24w 30‐day PP verified by CO ≤ 10 ppm Adverse events Cpd, exhaled CO, reduction rates, product usage, and opinions of the EC products |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not controlled |
| Allocation concealment (selection bias) | High risk | Not controlled |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically‐verified abstinence, adverse events collected through study diaries |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 76% followed up, ITT analysis used, no significant differences in baseline characteristics between completers and those lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Unable to determine prespecified outcomes |
| Methods | Design: Prospective cohort Recruitment: Professional retail staff in participating vape shops Setting: 7 vape shops in Catania province, Italy Inclusion criteria: Adult smokers (≥ 18) making first purchase at participating vape shop (definition of smoker not stated) Exclusion criteria: none stated |
|
| Participants | Total N: 71 38% women, mean age 41.7, mean cpd 24.9, mean FTND 5, no EC use at baseline |
|
| Interventions | Instructed how to charge, fill, activate and use EC; key troubleshooting advice provided; phone number available for technical support. “Encouraged to use these products in anticipation of reducing the number of cig/day smoked” | |
| Outcomes | 6 and 12m follow‐up 30‐day PP abstinence via self‐report Details of product purchase Sustained 50% and 80% reduction in cpd from baseline |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not controlled |
| Allocation concealment (selection bias) | High risk | Not controlled |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear how final follow‐up measures collected |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 69% follow‐up at 12m. Participants lost to follow‐up considered as continuing smokers |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
| Methods | Design: Prospective observational study using data from a 3‐group randomized RCT Recruitment: 2009 ‐ 2013, recruited as part of clinical trial of smokers with serious mental illness Setting: Inpatient psychiatric hospital, California, USA Inclusion criteria: Daily smokers of 5+ cpd, patient at 100% smoke‐free acute care unit at psychiatric hospitals Exclusion criteria: non‐English speaking; medical contraindications to NRT use (pregnancy, recent myocardial infarction); and lack of capacity to consent as determined by a 3‐item screener of study purpose, risks, and benefits |
|
| Participants | Total N: 956
50% women; mean age 39; 15% Hispanic, 57% white, 24% African‐American, 5% Asian/Pacific Islander; 14% multiracial/other; mean cpd 17; 11% used EC at baseline, 24% intended to quit smoking in next month Psychiatric diagnoses were 27% unipolar depression, 32% bipolar depression, and 27% nonaffective psychotic disorder; other (14%). 68% met criteria for alcohol or illicit drug abuse or dependence |
|
| Interventions | RCT tested levels of behavioural support: Usual care; brief treatment; extended treatment. Treatment groups received tailored computer‐assisted intervention or on‐unit counselling. Extended group offered 10 sessions of CBT No significant differences in EC use by treatment group. All participants were provided NRT following hospitalization (3 months brief arm, 6 months extended arm) |
|
| Outcomes | Follow‐up at 3, 6, 12, 18m. This paper reports “latest follow‐up” Cessation measured but definition not described Cpd |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Observational for purpose of this analysis |
| Allocation concealment (selection bias) | High risk | Observational for purpose of this analysis |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although there is no blinding, the study design means that there is unlikely to be significantly impact on performance by EC use at baseline |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear how outcome measures were assessed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Follow‐up for larger RCT still ongoing, unclear what percentage of participants eligible for this analysis were followed up |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Methods | Design: Single‐group within‐subject design Recruitment: Participants from a military hospital in South Africa Inclusion criteria: Adult daily smokers of at least 10 cpd Exclusion criteria: History of lung disease |
|
| Participants | Total N: 15, mean age 38 years, smoked 20 cpd (range 10 ‐ 30), for an average of 17 years (range 5 ‐ 27) Total N: 13 completed the study (5 women) |
|
| Interventions | Participants were asked to use an EC only for 2 weeks (i.e. no cigarettes) EC: 'Twisp eGo' cartridge 0.8 ml containing 0.0144 mg of nicotine |
|
| Outcomes | The following measurements were taken at baseline and 2‐week follow‐up:
|
|
| Notes | Dropouts (N = 2) were due to illness (headache and fever) and undertaking a military course associated with high stress and exposure to others smoking, making it difficult to abstain from cigarettes The paper states that the EC cartridge contained 0.8 ml of solution with 0.0144 mg of nicotine. This would be an unusually low concentration of nicotine and we have assumed an error in units where milligrams should have been grams (0.0144 grams of nicotine would make the concentration 18 mg/ml) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/15 lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
AE: adverse event BMI: body mass index CO: carbon monoxide COT: cotinine cpd: cigarettes per day EC: electronic cigarette FTND: Fagerström Test for Nicotine Dependence IQR: interquartile range ITT: intention‐to‐treat LTFU: lost to follow‐up NEC: nicotine electronic cigarette NRT: nicotine replacement therapy PEC: placebo electronic cigarette PP: point prevalence SAE: serious adverse event SD: standard deviation TQD: target quit date UC: usual care
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adkison 2013 | Although this study uses a prospective cohort design, no data on EC use were collected at baseline, with EC use data only being available at follow‐up |
| Battista 2013 | Short‐term EC use only |
| Biener 2015 | Cohort study, but EC use evaluated retrospectively only |
| Brown 2014a | Cross‐sectional survey |
| Bullen 2010 | Short‐term EC use only |
| Chausse 2015 | Ineligible study design |
| Chorti 2012 | Short‐term EC use only |
| Czogala 2012 | Short‐term EC use only |
| Dawkins 2012 | Short‐term EC use only |
| Dawkins 2013a | Short‐term EC use only |
| Dawkins 2014 | Short‐term EC use only |
| Douptcheva 2013 | Longitudinal study, but no data are reported for smoking cessation or reduction or for adverse events |
| Dutra 2014 | Cross‐sectional survey |
| Eissenberg 2010 | Short‐term EC use only |
| Farsalinos 2012 | Short‐term EC use only |
| Farsalinos 2013a | Included people that had already stopped smoking conventional cigarettes |
| Farsalinos 2013b | Short‐term EC use only |
| Farsalinos 2013c | Short‐term EC use only |
| Farsalinos 2013d | Short‐term EC use only |
| Flouris 2012 | Short‐term EC use only |
| Flouris 2013 | Short‐term EC use only |
| Gmel 2016 | Cohort study, but EC use only evaluated retrospectively |
| James 2016 | Follow‐up at 12 weeks, AE data not collected |
| Kasza 2013 | Longitudinal study, but no data are reported for smoking cessation or reduction or for adverse events |
| Kouretas 2012 | Short‐term EC use only |
| Lee 2014 | Cross‐sectional survey |
| Marini 2014 | Short‐term EC use only |
| Miura 2015 | Tests a device which is not an EC |
| Palamidas 2014 | Short‐term EC use only |
| Pearson 2012 | Longitudinal study, but no data are reported for smoking cessation or reduction or for adverse events |
| Pokhrel 2013 | Cross‐sectional survey |
| Popova 2013 | Cross‐sectional survey |
| Schober 2014 | Short‐term EC use only |
| Siegel 2011 | Retrospective survey of 222 EC users that responded to a survey sent to 5000 new users of the 'Blu' EC. Likely to be a self‐selected sample |
| Tsikrika 2014 | Short‐term EC use only |
| Tzatzarakis 2013 | Short‐term EC use only |
| Vakali 2014 | Short‐term EC use only |
| Vansickel 2010 | Short‐term EC use only |
| Vansickel 2012 | Short‐term EC use only |
| Vansickel 2013 | Short‐term EC use only |
| Vardavas 2012 | Short‐term EC use only |
| Vickerman 2013 | Cross‐sectional survey |
| Wagener 2014 | EC use for up to 1 week, but does not report on any adverse events |
| Walele 2016a | RCT but follow‐up too short |
| Walele 2016b | RCT but follow‐up too short |
| Yan 2015 | Ineligible study design |
EC: electronic cigarette
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Smoking cessation and reduction In schizophrenia (the SCARIS study) |
| Methods | 3‐arm prospective 12m randomized controlled trial investigating efficacy and safety of EC Setting: psychiatric and smoking cessation centres, Italy Recruitment: local newspapers and radio/television advertisements |
| Participants | 153 participants, schizophrenic in stable phase of illness, smoked at least 10 cpd over previous 5 years, aged 18 ‐ 65, in good general health, not currently attempting to quit smoke or wishing to do so in next 6m Excluded if: use smokeless tobacco or NRT; pregnant or breastfeeding; current or recent (1 yr) history of drug or alcohol abuse; other significant co‐morbidities |
| Interventions | 12‐wk supply of: 1) EC, high nicotine (24 mg) 2) EC, no nicotine (0 mg, with tobacco aroma) 3) PAIPO nicotine‐free inhalator |
| Outcomes | Follow‐up visits at 4, 8, 12, 24 and 52 wks Outcome measures:
|
| Starting date | September 2014 |
| Contact information | Pasquale Caponnetto, p.caponnetto@unict.it |
| Notes |
| Trial name or title | An open‐label randomized pragmatic policy trial examining effectiveness of short‐term use of Nicotine Replacement Therapy (NRT) vs short‐ or long‐term use of NRT vs short‐ or long‐term use of NRT or electronic nicotine delivery systems for smoking cessation in cigarette smokers |
| Methods | Phase 3 blinded RCT Setting: Australia Recruitment: commercial market research panel |
| Participants | Target sample size: 1600 Current daily smokers (at least 6 cpd), can read and understand English, agree to try samples of nicotine products, willing to complete surveys, 18 years or older Excl. if currently treated for serious medical condition, pregnant or planning to become pregnant or breastfeed in next 12m |
| Interventions | a) Factsheet explaining relative harm of NRT compared to smoking, free sample of NRT, participant chooses preferences, has free for 3 wks then offered at subsidised rate for further 6m b) As (a) but with additional information provided c) As (a) but additional information on electronic cigarettes and emphasis on cessation, and may select electronic cigarettes as well as NRT |
| Outcomes | 6 and 12m, self‐report Continuous abstinence, NRT and EC use, interest in quitting smoking and in quitting NRT, cigarette consumption, product orders and use, quit attempts |
| Starting date | Feb 2014 |
| Contact information | Coral Gartner, c.gartner@uq.edu.au |
| Notes |
| Trial name or title | The efficacy of e‐cigarettes compared with nicotine replacement therapy, when used within the UK stop smoking service |
| Methods | Multicentre pragmatic randomized controlled trial to examine the efficacy of e‐cigarettes compared with nicotine replacement therapy Setting: UK stop‐smoking service Recruitment: participants attending UK stop‐smoking service |
| Participants | Target: 886 participants Aged 18 or older, current smoker accessing stop‐smoking service, able to read/write/understand English |
| Interventions | Smokers who want help to quit smoking will be individually randomized to receive usual care (UC; a choice of NRT combined with usual care behavioural support provided by a Stop Smoking Service) or EC with the same behavioural support |
| Outcomes | Primary: CO‐validated sustained abstinence rates at 52 wks post–TQD Secondary: sustained abstinence at 4 and 24 wks, 7‐day PP abstinence at 4, 24 and 52 wks, smoking reduction, treatment ratings, adverse reactions, cost efficacy |
| Starting date | April 2015 |
| Contact information | Anna Phillips, a.phillips@qmul.ac.uk |
| Notes |
| Trial name or title | Effect of an electronic cigarette for smoking reduction and cessation in Korean male smokers: a randomized, controlled study |
| Methods | Parallel single‐blinded randomized controlled trial Setting: Hospital, Korea Recruitment: not specified |
| Participants | Sample size not stated Men, 18 or older, at least 10 cpd for past year, smoked for at least 3 years, motivated to quit or reduce cigarette consumption. Excl. if history of serious disease or quit attempt in past 12m using NRT |
| Interventions | 1) 50‐min education sessions on smoking cessation and the use of smoking‐cessation aids, instructed to visit the medical office each month for evaluation and counselling by a health practitioner who was unaffiliated with the study. Participants supplied with eGo‐CTM EC from Ovale in 12‐wk supply 2) As (1) but instead of EC given nicotine gum in 12‐wk supply |
| Outcomes | Primary: continuous abstinence at 12 and 24 wks Secondary: 7‐day PP abstinence at 12 and 24 wks, cpd, adverse events |
| Starting date | May 2012 |
| Contact information | Yoo‐Seok Cheong, Dankook University Hospital |
| Notes |
| Trial name or title | Randomized controlled trial methods for novel tobacco products evaluation |
| Methods | Randomized parallel‐assignment double‐blind trial Setting: USA (2 sites) Recruitment: message boards, radio, print, web‐based advertising |
| Participants | Estimated enrolment: 520 Inclusion criteria: Age 21 ‐ 65, smoke > 9 cpd for at least 1 yr, smoke regular filtered cigarettes or machine‐rolled cigarettes with filter, CO > 9 ppm, no 'serious quit attempt' in past month, not planning to quit in next 6m, interested in reducing cig consumption Exclude if: pregnant or nursing, unstable or significant medical condition, use of non‐cigarette nicotine in past 7 days, uncontrolled mental illness or substance abuse |
| Interventions | For 24 wks: 1) Cigarette substitute (plastic tube, does not provide drug delivery) 2) EC with no nicotine (EGO EC) 3) As (2) but 8 mg/ml nicotine 4) As (2) but 36 mg/ml nicotine |
| Outcomes | Urinary NNAL and cotinine at 24 wks, biomarkers of oxidative stress, glutathione and 8 Isoprostanes |
| Starting date | June 2015 |
| Contact information | Thomas Eissenberg, Virginia Commonwealth University |
| Notes |
| Trial name or title | Benefits of tobacco free cigarette among heavy smokers undergoing a lung cancer screening program: a randomized controlled study |
| Methods | Randomized parallel‐assignment double‐blind trial Setting: Early lung cancer detection programme (Cosmos II) at European Institute of Oncology Recruitment: volunteers participating in screening programme |
| Participants | Estimated enrolment: 210 Inclusion criteria: Smokers > 10 cpd for > 10 years, motivated to reduce smoking, not already in cessation treatment Exclusion criteria:
|
| Interventions | All participants receive smoking cessation programme including a motivational interview and 3 months low‐intensity distance counselling 1) EC and activity tracker 2) Nicotine‐free EC and activity tracker 3) Activity tracker |
| Outcomes | At 6 and 12m Primary: pulmonary health Secondary: psychological well‐being, cpd, CO, daily activity, cough‐related QoL, lifestyle |
| Starting date | September 2014 |
| Contact information | Marianna Masiero, University of Milan |
| Notes |
| Trial name or title | Spain‐UK‐Czech E‐cigarette Study (SUKCES) |
| Methods | Randomized controlled trial, open‐label pilot study Setting: smoking cessation clinics in London, Madrid and Prague Recuitment: via smoking cessation clinics |
| Participants | 220 smokers seeking help to quit Inclusion criteria: 18 or older,want help to quit Exclusion criteria: pregnant or breastfeeding; enrolled in other research; currently using EC |
| Interventions | 1) standard care plus 4 wks EC supply 2) standard care only |
| Outcomes |
|
| Starting date | December 2013 |
| Contact information | Peter Hajek, p.hajek@qmul.ac.uk |
| Notes |
| Trial name or title | Smoking cessation in women with gynaecological conditions |
| Methods | Randomized controlled trial, open‐label feasibility study Setting: hospital clinic, USA Recruitment: in clinic |
| Participants | 30 women smokers with cervical dysplasia Inclusion criteria: women smokers of at least 10 cpd over past year, diagnosis of cervical dysplasia, cervical cancer, and lower genital tract dysplasia and cancer, aged 18 ‐ 65 Exclusion criteria: previous diagnoses or treatment for cancer (except for non‐melanoma skin cancer); stroke, heart disease, heart attack, or irregular heart beat; pregnancy and lactation; plan to continue to use other nicotine as well as study products; uncontrolled hypertension; using other stop‐smoking medication; taking prescription medicine for depression or asthma |
| Interventions | 1) NRT patch (21 mg for first 3 wks, 14 mg for 2nd 3 wks) plus nicotine gum (2 mg) or lozenges (2 mg) for 6 wks 2) EC device ('Blu' Cig) with refills to last 6 wks, number provided based on packs smoked a day x 1.5 Strength of EC reduced at 3 wks Both groups receive identical cessation counselling |
| Outcomes | At 6 and 12 wks via survey:
|
| Starting date | June 2013 |
| Contact information | Laura A Beebe, laura‐beebe@ouhsc.edu |
| Notes |
| Trial name or title | Electronic cigarettes or nicotine inhaler for smoking cessation |
| Methods | Randomized controlled trial, open‐label safety/efficacy study Setting and recruitment not specified, USA |
| Participants | 40 participants Inclusion criteria: 18 ‐ 60 years old, meet DSM‐IV criteria for nicotine dependence, seeking treatment for smoking cessation, smoking at least 15 cpd Exclusion criteria: DSM‐IV diagnosis of schizophrenia, schizoaffective disorder, or bipolar disorder; current diagnosis of major depressive disorder; current diagnosis for other psychiatric disorders that may require intervention over course of study; receiving treatment for nicotine dependence; pregnancy, lactation, or chance of pregnancy; unstable medical condition; substance abuse diagnosis; use of cannabis or alcohol on more than 20 days in past 30 days; suicide risk |
| Interventions | 4 wks: 1) ECs (2nd generation) with 24 mg nicotine cartridges, 1 ‐ 2 cartridges daily 2) Nicotine inhaler with 10 mg cartridges, max 16 cartridges per day |
| Outcomes | Over 4 wks:
|
| Starting date | December 2013 |
| Contact information | Barney Vaughan, vaughan@nyspi.columbia.edu |
| Notes |
| Trial name or title | A study to evaluate the safety profile of an e‐vapour product |
| Methods | Randomized, open‐label, multicentre trial |
| Participants | 420 participants Inclusion criteria: age 21 ‐ 65 years, BMI 18 ‐ 35 kg/m², established smokers (smoking 5 ‐ 30 cpd for at least 1 year), not wanting to quit Exclusion criteria: Use of NRT within 14 days, blood donation in previous 12 months, history of drug or alcohol abuse, HIV or hepatitis positive, medically unwell, pregnant women |
| Interventions | 12 wks: Experimental: Participants who switch from using conventional cigarettes to using an e‐vapour product (EVP). No further information available about this product Control: Participants who continue smoking their usual conventional cigarette |
| Outcomes | Over 12 wks: Primary
Secondary
|
| Starting date | December 2013 |
| Contact information | Robert Turner, robert.turner_cain@covance.com |
| Notes | Sponsor: Imperial Tobacco Group PLC |
| Trial name or title | Smoking cessation and reduction in depression (SCARID) |
| Methods | 3‐arm prospective 12m randomized controlled trial investigating efficacy and safety of ECs |
| Participants | 129 participants Inclusion criteria: diagnosis of major depressive disorder (MDD) (according to DSM‐5 criteria), smoke ≥ 10 cpd (for at least the past 5 years), age 18 ‐ 65 years, in good general health, unwilling to quit smoking in the next 30 days Exclusion criteria: use of smokeless tobacco or NRT or other smoking cessation therapies, pregnancy or breastfeeding, current or recent (< 1 yr) past history of alcohol or drug abuse or both, active suicidal intention, other significant co‐morbidities according to the Investigator's clinical assessment (e.g. cancer, acute myocardial infarction, unstable angina, severe cardiac arrhythmia, recent cerebrovascular incident, or severe atherosclerosis) |
| Interventions | 12‐wk supply of:
|
| Outcomes | Follow‐up visits at 4, 8, 12, 24 and 52 wks Outcome measures:
|
| Starting date | February 2015 |
| Contact information | Pasquale Caponnetto p.caponnetto@unict.it |
| Notes |
| Trial name or title | A study to evaluate the safety of electronic vapour products for 2 years |
| Methods | Open‐label, singe‐group assignment, multicentre trial |
| Participants | 420 participants Inclusion criteria: participated in NCT02029196, age 21 ‐ 65 years, BMI 18 ‐ 35 kg/m², established smokers (smoking 5 ‐ 30 cpd for at least 1 year) not wanting to quit, willingness to use the electronic vapourizer product for 2 years, no clinically significant abnormalities during the prior trial Exclusion criteria: use of NRT within 14 days, blood donation in previous 12 months, history of drug or alcohol abuse, HIV or hepatitis positive, medically unwell, pregnant women |
| Interventions | Use of e‐vapour product (EVP) for 2 years |
| Outcomes | Follow‐up visits at 1, 2, 3, 6, 9, 12, 15, 18, 21, and 24 months Primary
Secondary
|
| Starting date | May 2014 |
| Contact information | Robert Turner, robert.turner_cain@covance.com |
| Notes |
| Trial name or title | Acceptability, patterns of use and safety of electronic cigarette in people with mental illness: a pilot study |
| Methods | Single‐group safety/efficacy study Setting: London, UK, NHS mental health service trust Recruitment: by invitation |
| Participants | Estimated enrolment: 50 Inclusion criteria:
Exclude if:
|
| Interventions | Free disposable ECs will be provided during 6 weeks to smokers with serious mental illness |
| Outcomes | To 24 wks: Primary: EC use, acceptability, respiratory symptoms, cotinine, nitrosamines, side effects of antipsychotics, withdrawal symptoms, respiratory symptoms Secondary: Predictors of EC use, psychiatric symptoms, physical symptoms |
| Starting date | August 2014 |
| Contact information | Rocio Perez‐Iglesias |
| Notes |
| Trial name or title | A mixed method EMA assessment of cognition and behavior among new ENDS users: an observational cohort study |
| Methods | Observational cohort study Setting: community Recruitment: volunteers |
| Participants | Estimated enrolment: 120, 100 not intending to quit in next 30 days, 20 intending to quit Selected inclusion criteria:
|
| Interventions | Unclear whether participants will be encouraged to use EC or not |
| Outcomes | Wks 1 ‐ 3: Primary: cigarette use, EC use Secondary: motivation to quit |
| Starting date | August 2014 |
| Contact information | Jennifer Pearson, American Legacy Foundation |
| Notes | May not be eligible |
| Trial name or title | Randomized clinical trial to reduce harm from tobacco |
| Methods | Randomized parallel‐assignment efficacy study, single‐blind Setting: Recruitment: |
| Participants | Target 6000 participants Vitality beneficiaries, 18 or older, reported/tested positive for smoking, excluding participants who opt out |
| Interventions | a) Standardized Vitality programme aimed at promoting tobacco cessation. This programme includes existing employee benefits for quitting and the use of text/email messages to encourage tobacco cessation b) as (a), plus free EC c) as (b) plus access to free NRT, bupropion or varenicline d) as (c) plus incentives across 6m for testing negative for tobacco use e) as (c) plus provide money at start and lose money from this fund if they do not test negative across 6m |
| Outcomes | Primary: verified abstinence at 6m Secondary: abstinence at 1, 3 and 12m |
| Starting date | January 2015 |
| Contact information | Scott Halpern, University of Pennsylvania |
| Notes |
| Trial name or title | A trial of e‐cigarettes: natural uptake, patterns and impact of use |
| Methods | Randomized parallel‐assignment open‐label trial |
| Participants | Estimated enrolment 68 Inclusion criteria: age 18+, current smoker of at least 5 cpd for at least 1 year, at least some concern for health effects of smoking Exclude if:
|
| Interventions | 2/3 sample will be given EC (Blu) for a 3‐wk period, to use as much or as little as they would like 1/3 sample will not receive EC to sample and will continue smoking their regular cigarettes as much or as little as they would like |
| Outcomes | At 3 months: Primary: EC uptake and use, nicotine and cotinine, antecedents of EC use, use within smoking‐restricted areas Secondary: smoking abstinence, smoking reduction, quit attempts |
| Starting date | November 2014 |
| Contact information | Matthew Carpenter, Medical University of South Carolina |
| Notes |
| Trial name or title | Head‐to‐head comparison of personal vaporizers versus cigalike: prospective 6‐month randomized control design study (VAPECIG 2) |
| Methods | Randomized parallel‐assignment open‐label trial Setting: Italy, community |
| Participants | Estimated enrolment: 200 Inclusion criteria: (smokers) in good general health committed to follow trial procedures Exclude if:
|
| Interventions | Comparison between 2 types of EC; 'personal vaporizers' and 'cigalike' |
| Outcomes | 24 weeks: Smoking cessation, smoking reduction |
| Starting date | October 2014 |
| Contact information | Riccardo Polosa |
| Notes |
| Trial name or title | Evaluating the efficacy of e‐cigarette use for smoking cessation (E3) Trial |
| Methods | Randomized parallel‐assignment double‐blind trial Setting: community, Canada Recruitment: motivated volunteers |
| Participants | Estimated enrolment: 486 Inclusion criteria:
Exclude if:
|
| Interventions | Smoking cessation/relapse prevention counselling will be provided for all participants for a minimum of 30 minutes at baseline, 10 minutes during telephone follow‐ups, and 15 minutes at clinic visits (20 minutes at week 4). Counselling will consist of a number of approaches, including reviewing smoking history, development/revision of a quit plan, encouragement of self‐monitoring, review of triggers and challenges, and skill development. 1) Nicotine‐containing EC 2) Non‐nicotine EC 3) Counselling only |
| Outcomes | At 4, 12, 24 and 52 weeks: Primary: PP abstinence Secondary: multiple PP and continuous abstinence, change in cig consumption. Adverse events and dropouts (at 12 weeks) |
| Starting date | September 2016 |
| Contact information | Mark Eisenberg |
| Notes |
| Trial name or title | A pilot randomized controlled clinical trial ‐ "Electronic nicotine delivery device (e‐cigarette) for perioperative smoking cessation in veterans" |
| Methods | Randomized parallel‐assignment double‐blind pilot trial Setting: San Francisco Veterans Affairs Medical Center (SFVAMC), USA Recruitment: veterans awaiting surgery |
| Participants | Estimated enrolment: 30 Inclusion criteria:
Exclude if:
|
| Interventions | All participants receive < 2 minutes brief advice, referral to California Smokers' Helpline for proactive counselling and self‐help materials 1) 6‐week supply of EC (NJOY) 2) Prescription for 6‐week supply of NRT (Nicoderm CQ) |
| Outcomes | Primary: smoking status on day of surgery (1 ‐ 2 weeks post enrolment) Secondary: smoking status at 8 weeks (confirmed by CO), 6 months, smoking reduction, EC use at 6 months, dual use, cotinine, spirometry, postoperative complications, length of stay, adverse events, qualitative data |
| Starting date | August 2015 |
| Contact information | Susan Lee |
| Notes |
| Trial name or title | Electronic nicotine delivery systems (ENDS) as a smoking cessation treatment |
| Methods | Randomized parallel‐assignment double‐blind trial Setting: Smoking cessation research centre, USA Recruitment: volunteers |
| Participants | Estimated enrolment: 300 Inclusion criteria:
Exclusion criteria: multiple related to baseline health status |
| Interventions | 1) Nicotine EC + nicotine patch 2) Nicotine EC + placebo patch 3) Placebo (non‐nicotine) EC + nicotine patch Nicotine patches will be provided for 2 weeks before TQD and 8 weeks after at full dose then dose weaning for 4 weeks EC will be provided for 1 week before TQD and 8 weeks after, then instructed to reduce |
| Outcomes | Primary: abstinence at 4 ‐ 8 weeks from TQD Secondary: abstinence at 9 ‐ 12 weeks, 13 ‐ 16 weeks, 6 months All abstinence validated by CO |
| Starting date | January 2016 |
| Contact information | Al Salley: al.salley@duke.edu. PI Jed Rose |
| Notes |
| Trial name or title | Short term effects of electronic cigarettes in tobacco dependent adults |
| Methods | Randomized parallel‐assignment double‐blind trial Setting: community, USA Recruitment: from cessation clinics and chest clinics |
| Participants | Estimated enrolment: 40 Inclusion criteria: smoking 1 or more cpd Exclude if:
|
| Interventions | All participants receive nicotine patch and intensive counselling 1) Nicotine EC 2) Non‐nicotine EC |
| Outcomes | At 8 wks and 6m: Primary: change in daily smoking, change in CO Secondary: change in lung function |
| Starting date | October 2014 |
| Contact information | Stephen Baldassari |
| Notes |
| Trial name or title | A randomized‐controlled clinical trial to evaluate the effectiveness and safety of combining nicotine patches with e‐cigarettes (with and without nicotine) plus behavioural support, on smoking abstinence |
| Methods | Randomized parallel‐assignment double‐blind trial Setting: community, New Zealand Recruitment: volunteers |
| Participants | Estimated enrolment: 1809 Inclusion criteria:
Exclude if:
|
| Interventions | All participants will receive withdrawal‐oriented behavioural support for 6 weeks post‐quit 1) Nicotine patch for 14 weeks including 2 week prequit 2) Nicotine patch and nicotine‐free EC for 14 weeks 3) Nicotine patch and nicotine EC for 14 weeks |
| Outcomes | Primary: Continuous abstinence at 6 months with CO validation Secondary: Self‐reported continuous abstinence, PP abstinence, number of cigs, smoking reduction, time to relapse, withdrawal, self‐efficacy, use of other cessation methods/products, compliance, dual use, serious adverse events, opinions |
| Starting date | March 2015 |
| Contact information | Natalie Walker |
| Notes |
| Trial name or title | E‐cigarettes: dynamic patterns of use and health effects |
| Methods | Prospective observational study Setting: community, USA Recruitment: Smokers and dual EC and cigarette users |
| Participants | Estimated enrolment: 450 Inclusion criteria:
|
| Interventions | "We will conduct a 2‐year longitudinal cohort study comprising participants who smoke exclusively CCs (n = 175) and dual users of e‐cigs and CCs (n = 275)" |
| Outcomes | 'We will use state‐of‐the‐art ecological momentary assessments to determine: 1) dynamic patterns of e‐cig and CC use and related outcomes (e.g. dependence, withdrawal symptoms, CC quit attempts and quitting success); 2) episodic (affective, contextual, social) and stable person‐factor (lifestyle factors, demographics) variables that covary meaningfully with e‐cig and CC use and related outcomes; 3) biomarkers of tobacco and carcinogen exposure as well as other health‐related outcomes (e.g. reduced pulmonary function)." |
| Starting date | September 2015 |
| Contact information | PI Megan Piper |
| Notes |
| Trial name or title | The role of nicotine and non‐nicotine alkaloids in e‐cigarette use and dependence |
| Methods | Randomized parallel‐assignment double‐blind trial Setting: Smoking research clinic, USA Recruitment: volunteers |
| Participants | Estimated enrolment: 375 Inclusion criteria:
Exclude if: multiple, related to baseline health status |
| Interventions | 1) Switch to standard nicotine EC use for 8 wks 2) Switch to ECs with same nicotine but very low non‐nicotine alkaloid levels 3) Switch to ECs with very low nicotine and non‐nicotine alkaloids |
| Outcomes | Primary: CO levels at 8 wks Secondary: EC use, EC solution use, cig use, at 8 wks |
| Starting date | May 2016 |
| Contact information | Jed Rose |
| Notes | "This is not a smoking cessation study; smokers will not be asked to quit smoking, and e‐cigarettes will not be used as a medical device or therapy." |
| Trial name or title | Assessing the use of electronic cigarettes (e‐cigarettes) as a harm reduction strategy |
| Methods | Randomized parallel‐assignment double‐blind trial Setting: Community, USA Recruitment: Volunteers |
| Participants | Estimated enrolment: 100 Inclusion criteria: Exclude if: |
| Interventions | All participants will receive a 20 ‐ 30‐minute behavioural counselling consultation with a trained tobacco counsellor. Counsellors will review each participant's smoking pattern and offer tailored behavioural and environmental change strategies including specific smoking reduction strategy options. Participants will be given a supply of EC and followed for 3 weeks 1) Nicotine EC 2) Non‐nicotine EC |
| Outcomes | Primary: cpd and reduction at 3 wks Secondary: Adverse effects, use of other tobacco products, satisfaction, craving, withdrawal, up to 12 wks |
| Starting date | May 2015 |
| Contact information | Donna Shelley |
| Notes |
| Trial name or title | Changes in lung function parameters, bronchial reactivity, state of health and smoking behaviour associated with changing from conventional smoking to electronic cigarettes |
| Methods | Prospective observational study Setting: Community, Germany Recruitment: Vape shops and smoking cessation clinics |
| Participants | Estimated enrolment: 80 Inclusion criteria:
Exclude if: |
| Interventions | Comparison between: 1) Smokers who intend to start EC use for the first time 2) Smokers who intend to quit smoking within a clinical conducted smoking cessation programme |
| Outcomes | Primary: Lung function, QoL, respiratory tract inflammation |
| Starting date | October 2015 |
| Contact information | Tobias Rüther |
| Notes |
| Trial name or title | Evaluation of appeal and impact of e‐cigarettes among chronic smokers with smoking‐related cancers |
| Methods | Randomized open‐label study Setting: Medical centre, USA Recruitment: Patients with cancer |
| Participants | Estimated enrolment Inclusion criteria:
Exclude if:
|
| Interventions | Participants with be supplied with 1 of 2 models of EC (HALO brand), 1 cigalike, the other a tank model |
| Outcomes | Use of EC, CO, urine NNAL, at 3, 6, 9, 12 wks |
| Starting date | January 2016 |
| Contact information | Katie H Rice. PI James Sargent |
| Notes |
BMI: body mass index CO: carbon monoxide COPD: chronic obstructive pulmonary disease cpd: cigarettes per day CVD: cardiovascular disease EC: electronic cigarette ECG: electrocardiogram NNAL: carcinogen found in tobacco smoke NRT: nicotine replacement therapy PP: point prevalence QoL: quality of life TQD: target quit date wk: week yr: year
Contributions of authors
All authors contributed to the writing of this review. JHB, HM and LS extracted data, with discrepancies and disagreements referred to PH. As principal investigator of one of the included trials, CB was not involved with data extraction or assessment of study quality.
Sources of support
Internal sources
-
Queen Mary University of London, UK.
provides salary, office space and library resources for HM and PH
-
The University of Auckland, New Zealand.
provides salary, office space and library resources for CB
External sources
No sources of support supplied
Declarations of interest
Within the last three years HM has received honoraria for speaking at research symposia and received benefits in kind and travel support from, and has provided consultancy to, the manufacturers of smoking cessation medications.
Within the last three years PH has provided consultancy for and received research funding from GSK, Pfizer, Novartis and other manufacturers of smoking cessation medications.
Two authors (HM, CB) have additional declarations: CB and HM were investigators on a study of ECs from an EC manufacturer (Ruyan Group, Beijing and Hong Kong). Ruyan supplied the ECs used in the trial and contracted with Health NZ Ltd. to undertake the study. Health New Zealand Ltd funded The University of Auckland to conduct the trial, independently of Ruyan Group (Holdings) Ltd. The trial design conduct, analysis and interpretation of results were conducted independently of the sponsors. CB and HM were investigators on the ASCEND EC trial funded by the Health Research Council of New Zealand that used product supplied at no charge from PGM international, a retailer of ECs.
JHB, RB and LS have no conflicts of interest to declare.
Edited (no change to conclusions)
References
References to studies included in this review
- Adriaens K, Gucht D, Declerck P, Baeyens F. Effectiveness of the electronic cigarette: An eight‐week Flemish study with six‐month follow‐up on smoking reduction, craving and experienced benefits and complaints. International Journal of Environmental Research and Public Health 2014;11(11):11220‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Delaimy WK, Myers MG, Leas EC, Strong DR, Hofstetter CR. E‐cigarette use in the past and quitting behavior in the future: a population‐based study. American Journal of Public Health 2015;105(6):1213‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]; Donzelli A. E‐cigarettes may impair ability to quit, but other explanations are possible. American Journal of Public Health 2015;105(11):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton MK. Electronic cigarettes did not help patients with cancer stop smoking. CA: a cancer journal for clinicians 2015;65(2):85‐6. [DOI] [PubMed] [Google Scholar]; Borderud SP, Li Y, Burkhalter JE, Sheffer CE, Ostroff JS. Electronic cigarette use among patients with cancer: characteristics of electronic cigarette users and their smoking cessation outcomes. Cancer 2014;120(22):3527‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]; Fillon M. Electronic cigarettes might not help cancer patients quit smoking. Journal of the National Cancer Institute 2015;107(1):496. [DOI] [PubMed] [Google Scholar]
- Brose LS, Hitchman SC, Brown J, West R, McNeill A. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1‐year follow‐up. Addiction 2015;110(7):1160‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Brown J, West R, Beard E, Michie S, Shahab L, McNeill A. Prevalence and characteristics of e‐cigarette users in Great Britain: Findings from a general population survey of smokers. Addictive Behaviors 2014;39(6):1120‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]; Hitchman SC, Brose LS, Brown J, Robson D, McNeill A. Associations between e‐cigarette type, frequency of use, and quitting smoking: findings from a longitudinal online panel survey in Great Britain. Nicotine & Tobacco Research 2015;17(10):1187‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J. Do electronic cigarettes help smokers quit? Results from a randomised controlled trial [Abstract]. European Respiratory Society Annual Congress, 2013 September 7 ‐ 11, Barcelona, Spain 2013;42:215s‐[P1047]. [Google Scholar]; Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, et al. Electronic cigarettes and smoking cessation: a quandary? ‐ Authors' reply. Lancet 2014;383(9915):408‐9. [DOI] [PubMed] [Google Scholar]; Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013;382(9905):1629‐37. [DOI] [PubMed] [Google Scholar]; Bullen C, Williman J, Howe C, Laugesen M, McRobbie H, Parag V, et al. Study protocol for a randomised controlled trial of electronic cigarettes versus nicotine patch for smoking cessation. BMC Public Health 2013;13:210. [DOI] [PMC free article] [PubMed] [Google Scholar]; O'Brien B, Knight‐West O, Walker N, Parag V, Bullen C. E‐cigarettes versus NRT for smoking reduction or cessation in people with mental illness: Secondary analysis of data from the ASCEND trial. Tobacco Induced Diseases2014; Vol. 13, issue 1:5. [DOI] [PMC free article] [PubMed]
- Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12‐month randomized control design study. PloS One 2013;8(6):e66317. [DOI] [PMC free article] [PubMed] [Google Scholar]; Russo C, Cibella F, Caponnetto P, Campagna D, Maglia M, Frazzetto E, et al. Evaluation of post cessation weight gain in a 1‐year randomized smoking cessation trial of electronic cigarettes. Scientific Reports 2016;6:18763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caponnetto P, Auditore R, Russo C, Cappello GC, Polosa R. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12‐month pilot study. International Journal of Environmental Research and Public Health 2013;10(2):446‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]; Minutolo G, Caponnetto P, Auditore R, Russo C, Polosa R. Management of smoking reduction and cessation in inpatients with schizophrenia: Impact of electronic cigarettes. European Neuropsychopharmacology 2013;23:S581‐2. [Google Scholar]
- Choi K, Forster JL. Beliefs and experimentation with electronic cigarettes : a prospective analysis among young adults. American Journal of Preventive Medicine 2014;6(2):175‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]; Choi K. Forster JL. Authors' Response to: Context on use is needed before public health recommendations are made about e‐cigarettes. Americal Journal of Preventive Medicine 2014;46(6):e58‐59. [DOI] [PubMed] [Google Scholar]
- Ely J. Evaluation of the use of electric cigarettes in a rural smoking cessation program. Available online at: digitalunc.coalliance.org/fedora/repository/cogru:4161 2013 (accessed 1st November 2014).
- Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addictive Behaviors 2014;39(2):491‐4. [DOI] [PubMed] [Google Scholar]
- Grana RA, Popova L, Ling PM. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Internal Medicine 2014;174(5):812‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, Corbin L, Ladmore D, Spearing E. Adding e‐cigarettes to specialist stop‐smoking treatment: City of London pilot project. Journal of Addiction Research & Therapy 2015;6(244):(online ahead of print). [DOI: 10.4172/2155-6105.1000244] [DOI] [Google Scholar]
- Humair J‐P, Tango R. Can e‐cigarette help patients to reduce or stop smoking in primary care practice?. Journal of General Internal Medicine 2014;29:S480. [Google Scholar]
- Manzoli L, Flacco ME, Fiore M, Vecchia C, Marzuillo C, Gualano MR, et al. Electronic cigarettes efficacy and safety at 12 months: cohort study. PLoS One 2015;10(6):e0129443‐. [DOI] [PMC free article] [PubMed] [Google Scholar]; Manzoli L, Vecchia C, Flacco ME, Capasso L, Simonetti V, Boccia S, et al. Multicentric cohort study on the long‐term efficacy and safety of electronic cigarettes: study design and methodology. BMC public health 2013;13(1):883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRobbie H, Goniewicz M, Phillips A, Myers‐Smith K, West O, Hajek P. Effects of the use of electronic cigarettes with and without concurrent smoking on acrolein delivery POS4‐33. Society for Research on Nicotine and Tobacco, 20th Annual Meeting, Seattle, Washington. 2014:13. [www.srnt.org/conferences/2014/pdf/SRNT_2014_Rapids_C.pdf] [Google Scholar]; McRobbie H, Phillips A, Goniewicz ML, Smith KM, Knight‐West O, Przulj D, et al. Effects of switching to electronic ccigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prevention Research (Philadelphia, Pa.) 2015;8(9):873‐8. [DOI] [PubMed] [Google Scholar]
- Nides MA, Leischow SJ, Bhatter M, Simmons M. Nicotine blood levels and short‐term smoking reduction with an electronic nicotine delivery system. American Journal of Health Behavior 2014;38(2):265‐74. [DOI] [PubMed] [Google Scholar]
- Oncken CA, Litt MD, McLaughlin LD, Burki NA. Nicotine concentrations with electronic cigarette use: effects of sex and flavor. Nicotine & Tobacco Research 2015;17(4):473‐8. [DOI] [PubMed] [Google Scholar]; Swedeh MA, Oncken C, Burki NK. Acute effects of electronic cigarettes on airway function in human subjects. American Thoracic Society International Conference Abstracts 2014;189:A4089. [Google Scholar]
- Pacifici R, Pichini S, Graziano S, Pellegrini M, Massaro G, Beatrice F. Successful nicotine intake in medical assisted use of e‐cigarettes: a pilot study. International Journal of Environmental Research and Public Health 2015;12(7):7638‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Caponnetto P, Morjaria JB, Papale G, Campagna D, Russo C. Effect of an electronic nicotine delivery device (e‐cigarette) on smoking reduction and cessation: a prospective 6‐month pilot study. BMC Public Health 2011;11:786. [DOI] [PMC free article] [PubMed] [Google Scholar]; Polosa R, Morjaria JB, Caponnetto P, Campagna D, Russo C, Alamo A, et al. Effectiveness and tolerability of electronic cigarette in real‐life: a 24‐month prospective observational study. Internal and Emergency Medicine 2014;9(5):537‐46. [DOI] [PubMed] [Google Scholar]
- Polosa R, Morjaria J, Caponnetto P, Caruso M, Strano S, Battaglia E, et al. Effect of smoking abstinence and reduction in asthmatic smokers switching to electronic cigarettes: evidence for harm reversal. International Journal of Environmental Research & Public Health 2014;11(5):4965‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Caponnetto P, Maglia M, Morjaria JB, Russo C. Success rates with nicotine personal vaporizers: a prospective 6‐month pilot study of smokers not intending to quit. BMC Public Health 2014;14:1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Caponnetto P, Cibella F, Le‐Houezec J. Quit and smoking reduction rates in vape shop consumers: A prospective 12‐month survey. International Journal of Environmental Research and Public Health 2015;12(4):3428‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochaska JJ, Grana RA. E‐cigarette use among smokers with serious mental illness. PLoS One 2014;9(11):e113013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden SR, Groenewald M, Engelbrecht R, Becker PJ, Hazelhurst LT. Carboxyhaemoglobin levels, health and lifestyle perceptions in smokers converting from tobacco cigarettes to electronic cigarettes. South African Medical Journal 2013;103(11):865‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Adkison SE, O'Connor RJ, Bansal‐Travers M, Hyland A, Borland R, Yong HH, et al. Electronic nicotine delivery systems: international tobacco control four‐country survey. American Journal of Preventive Medicine 2013;44(3):207‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista L, Iorio M, Tancredi M, Acconcia MC, Torromeo C, Barilla F, et al. Cardiovascular effects of electronic cigarettes. Circulation 2013;128:A16755. [Google Scholar]
- Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population‐based sample of adult smokers: Association with smoking cessation and motivation to quit. Nicotine & Tobacco Research 2015;17(2):127‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Beard E, Kotz D, Michie S, West R. Real‐world effectiveness of e‐cigarettes when used to aid smoking cessation: a cross‐sectional population study. Addiction 2014;109(9):1531‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross‐over trial. Tobacco Control 2010;19(2):98‐103. [DOI] [PubMed] [Google Scholar]
- Chausse P, Naughton G, Dutheil F. Electronic cigarettes: the resistance value of the heating filament could be the key to lung toxicity. Chest 2015;148(1):e29‐30. [DOI] [PubMed] [Google Scholar]
- Chorti M, Poulianiti K, Jamurtas A, Kostikas K, Tzatzarakis M, Vynias D, et al. Effects of active and passive electronic and tobacco cigarette smoking on lung function (P08‐02). Toxicology Letters 2012;211:S64. [Google Scholar]
- Czogala J, Cholewinski M, Kutek A, Zielinska‐Danch W. [Evaluation of changes in hemodynamic parameters after the use of electronic nicotine delivery systems among regular cigarette smokers]. [Polish]. Przeglad lekarski 2012;69(10):841‐5. [PubMed] [Google Scholar]
- Dawkins L, Turner J, Hasna S, Soar K. The electronic‐cigarette: effects on desire to smoke, withdrawal symptoms and cognition. Addictive Behaviors 2012;37(8):970‐3. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Crowe E. Nicotine derived from the electronic cigarette improves time‐based prospective memory in abstinent smokers. Psychopharmacology 2013;227(3):377‐84. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology 2014;231(2):401‐7. [DOI] [PubMed] [Google Scholar]
- Douptcheva N, Gmel G, Studer J, Deline S, Etter JF. Use of electronic cigarettes among young Swiss men. Journal of Epidemiology & Community Health 2013;67(12):1075‐6. [DOI] [PubMed] [Google Scholar]
- Dutra LM, Glantz SA. Electronic cigarettes and conventional cigarette use among U.S. adolescents: a cross‐sectional study. JAMA Pediatrics 2014;168(7):610‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eissenberg T. Electronic nicotine delivery devices: ineffective nicotine delivery and craving suppression after acute administration. Tobacco Control 2010;19(1):87‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K, Tsiapras D, Kyrzopoulos S, Savvopoulou M, Avramidou E, Vasilopoulou D, et al. Acute effects of using an electronic nicotine‐delivery device (e‐cigarette) on myocardial function: comparison with the effects of regular cigarettes. European Heart Journal 2012;33:203. [DOI] [PMC free article] [PubMed] [Google Scholar]; Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Acute effects of using an electronic nicotine‐delivery device (electronic cigarette) on myocardial function: comparison with the effects of regular cigarettes. BMC Cardiovascular Disorders 2014;14:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluating nicotine levels selection and patterns of electronic cigarette use in a group of "vapers" who had achieved complete substitution of smoking. Substance Abuse: Research and Treatment 2013;7:139‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V. Impact of flavour variability on electronic cigarette use experience: an internet survey. International Journal of Environmental Research & Public Health 2013;10(12):7272‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos K, Tsiapras D, Kyrzopoulos S, Stefopoulos C, Spyrou A, Tsakalou M, et al. Immediate effects of electronic cigarette use on coronary circulation and blood carboxyhemoglobin levels: comparison with cigarette smoking. European Heart Journal 2013;34(Suppl 1):13. 22933568 [Google Scholar]
- Farsalinos K, Tsiapras D, Kyrzopoulos S, Spyrou A, Stefopoulos C, Romagna G, et al. Effects of electronic cigarette use on the elastic properties of the ascending aorta in healthy subjects: comparison with the effects of tobacco cigarettes. European Heart Journal Cardiovascular Imaging 2013;14(Suppl 2):ii203. [Google Scholar]
- Flouris AD, Poulianiti KP, Chorti MS, Jamurtas AZ, Kouretas D, Owolabi EO, et al. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food and Chemical Toxicology 2012;50(10):3600‐3. [DOI] [PubMed] [Google Scholar]
- Flouris AD, Chorti MS, Poulianiti KP, Jamurtas AZ, Kostikas K, Tzatzarakis MN, et al. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhalation Toxicology 2013;25(2):91‐101. [DOI] [PubMed] [Google Scholar]
- Gmel G, Baggio S, Mohler‐Kuo M, Daeppen JB, Studer J. E‐cigarette use in young Swiss men: is vaping an effective way of reducing or quitting smoking?. Swiss Medical Weekly 2016;146:w14271. [DOI] [PubMed] [Google Scholar]
- James SA, Meier EM, Wagener TL, Smith KM, Neas BR, Beebe LA. E‐Cigarettes for immediate smoking substitution in women diagnosed with cervical dysplasia and associated disorders. International Journal of Environmental Health Research 2016;13(3):E288. [DOI: 10.3390/ijerph13030288] [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT01842828. E‐Cigarettes as an addition to multi‐component treatment for tobacco dependence: a pilot study. clinicaltrials.gov/show/NCT01842828 (accessed 16 July 2014).
- Kasza KA, Bansal‐Travers M, O'Connor RJ, Compton WM, Kettermann A, Borek N, et al. Cigarette smokers' use of unconventional tobacco products and associations with quitting activity: findings from the ITC‐4 U.S. cohort. Nicotine & Tobacco Research 2013;16(6):672‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouretas D, Poulianiti K, Chorti M, Jamurtas A, Kostikas K, Tzatzarakis M, et al. Effects of electronic cigarette and tobacco cigarette smoking on complete blood count (P08‐03). Toxicology Letters 2012;211:S64. [DOI] [PubMed] [Google Scholar]
- Lee S, Grana RA, Glantz SA. Electronic cigarette use among Korean adolescents: a cross‐sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. Journal of Adolescent Health 2014;54(6):684‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini S, Buonanno G, Stabile L, Ficco G. Short‐term effects of electronic and tobacco cigarettes on exhaled nitric oxide. Toxicology and Applied Pharmacology 2014;278(1):9‐15. [DOI] [PubMed] [Google Scholar]
- Miura N, Yuki D, Minami N, Kakehi A, Futama Y. A study to investigate changes in the levels of biomarkers of exposure to selected cigarette smoke constituents in Japanese adult male smokers who switched to a non‐combustion inhaler type of tobacco product. Regulatory Toxicology and Pharmacology 2015;71(3):498‐506. [DOI] [PubMed] [Google Scholar]
- Palamidas A, Gennimata SA, Kaltsakas G, Tsikrika S, Vakali S, Gratziou C, et al. Acute effect of an e‐cigarette with and without nicotine on lung function. Tobacco Induced Diseases 2014;12(Suppl 1):A34. [Google Scholar]
- Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. e‐Cigarette awareness, use, and harm perceptions in US adults. American Journal of Public Health 2012;102(9):1758‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokhrel P, Fagan P, Little MA, Kawamoto CT, Herzog TA. Smokers who try e‐cigarettes to quit smoking: findings from a multiethnic study in Hawaii. American Journal of Public Health 2013;103(9):e57‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling PM, Popova L. Novel "Tobacco" product use and association with smoking cessation: A national study. Journal of General Internal Medicine 2012;27(2 Suppl):S254. [Google Scholar]; Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. American Journal of Public Health 2013;103(5):923‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schober W, Szendrei K, Matzen W, Osiander‐Fuchs H, Heitmann D, Schettgen T, et al. Use of electronic cigarettes (e‐cigarettes) impairs indoor air quality and increases FeNO levels of e‐cigarette consumers. International Journal of Hygiene and Environmental Health 2014;217(6):628‐37. [DOI] [PubMed] [Google Scholar]
- Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking‐cessation tool: results from an online survey. American Journal of Preventive Medicine 2011;40(4):472‐5. [DOI] [PubMed] [Google Scholar]
- Tsikrika S, Vakali S, Gennimata SA, Palamidas A, Kaltsakas G, Koulouris N, et al. Short term use of an e‐cig: influence on clinical symptoms, vital signs and eCO levels. Tobacco Induced Diseases 2014;12(Suppl 1):A30. [Google Scholar]
- Tzatzarakis MN, Tsitoglou KI, Chorti MS, Poulianiti KP, Jamurtas AZ, Koutedakis Y, et al. Acute and short term impact of active and passive tobacco and electronic cigarette smoking on inflammatory markers. Toxicology Letters 2013;221(Suppl):S86. [Google Scholar]
- Vakali S, Tsikrika S, Gennimata SA, Kaltsakas G, Palamidas A, Koulouris N, et al. E‐Cigarette acute effect on symptoms and airway inflammation: comparison of nicotine with a non‐nicotine cigarette. Tobacco Induced Diseases 2014;12(Suppl 1):A35. [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic "cigarettes": nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiology, Biomarkers & Prevention 2010;19(8):1945‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Weaver MF, Eissenberg T. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction 2012;107(8):1493‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine & Tobacco Research 2013;15(1):267‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short‐term pulmonary effects of using an electronic cigarette: Impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 2012;141(6):1400‐6. [DOI] [PubMed] [Google Scholar]
- Vickerman KA, Carpenter KM, Altman T, Nash CM, Zbikowski SM. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine & Tobacco Research 2013;15(10):1787‐91. [DOI] [PubMed] [Google Scholar]
- Wagener TL, Meier E, Hale JJ, Oliver ER, Warner ML, Driskill LM, et al. Pilot investigation of changes in readiness and confidence to quit smoking after E‐cigarette experimentation and 1 week of use. Nicotine & Tobacco Research 2014;16(1):108‐14. [DOI] [PubMed] [Google Scholar]
- Walele T, Sharma G, Savioz R, Martin C, Williams J. A randomised, crossover study on an electronic vapour product, a nicotine inhalator and a conventional cigarette. Part A: Pharmacokinetics. Regulatory Toxicity 2016;74:187‐92. [DOI] [PubMed] [Google Scholar]
- Walele T, Sharma G, Savioz R, Martin C, Williams J. A randomised, crossover study on an electronic vapour product, a nicotine inhalator and a conventional cigarette. Part B: Safety and subjective effects. Regulatory Toxicity 2016;74:193‐9. [DOI] [PubMed] [Google Scholar]
- Yan XS, D'Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regulatory Toxicology and Pharmacology 2015;71(1):24‐34. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Caponnetto P, Polosa R, Auditore R, Minutolo G, Signorelli M, Maglia M, et al. Smoking cessation and reduction in schizophrenia (SCARIS) with e‐cigarette: study protocol for a randomized control trial. Trials [electronic resource] 2014;15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT01979796. Antismoking effects of rlectronic cigarettes in subjects with schizophrenia and their potential influence on cognitive functioning: design of a randomized trial. Smoking Cessation And Reduction In Schizophrenia (The SCARIS Study). clinicaltrials.gov/show/NCT01979796 (accessed 16 July 2014).
- ACTRN12612001210864. An open‐label randomised pragmatic policy trial examining effectiveness of short‐term use of Nicotine Replacement Therapy (NRT) vs short‐ or long‐term use of NRT vs short‐ or long‐term use of NRT or electronic nicotine delivery systems for smoking cessation in cigarette smokers. ACTRN12612001210864 (accessed 15 August 2016). ; Fraser D, Borland R, Gartner C. Protocol for a randomised pragmatic policy trial of nicotine products for quitting or long‐term substitution in smokers. BMC Public Health 2015;15:1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN60477608. The efficacy of e‐cigarettes compared with nicotine replacement therapy, when used within the UK stop smoking service. ISRCTN60477608 2014 (accessed 15 August 2016).
- KCT0001277. Effect of an electronic cigarette for smoking reduction and cessation in Korean male smokers: a randomized, controlled study. KCT0001277 2014 (accessed 15 August 2016). [DOI] [PubMed]
- Lopez AA, Cobb CO, Yingst JM, Veldheer S, Hrabovsky S, Yen MS, et al. A transdisciplinary model to inform randomized clinical trial methods for electronic cigarette evaluation. BMC Public Health 2016;16(1):217. [CRS: 9400131000005107; PMCID:: PMC4778292; PUBMED: 26941050] [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT02342795. Randomized controlled trial methods for novel tobacco products evaluation. clinicaltrials.gov/show/NCT02342795 (accessed 17 February 2016). [CRS: 9400131000005070]
- Lucchiari C, Masiero M, Veronesi G, Maisonneuve P, Spina S, Jemos C, et al. Benefits of e‐cigarettes among heavy smokers undergoing a lung cancer screening program: randomized controlled trial protocol. JMIR Research Protocols 2016;5(1):e21. [CRS: 9400131000006459; PUBMED: 26842790] [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT02422914. Benefits of tobacco free cigarette among heavy smokers undergoing a lung cancer screening program: a randomized controlled study. clinicaltrials.gov/show/NCT02422914 (accessed 17 February 2016). [CRS: 9400131000005080]
- NCT01842828. E‐Cigarettes as an addition to multi‐component treatment for tobacco dependence: a pilot study.. clinicaltrials.gov/show/NCT01842828 (accessed 16 July 2014)..
- NCT01989923. Immediate smoking cessation for patients at risk for cervical dysplasia, cervical cancer and lower genital tract dysplasia and cancer ‐ a feasibility study comparing nicotine replacement therapy with the electronic nicotine delivery system. clinicaltrials.gov/show/NCT01989923 (accessed 16 July 2014).
- NCT02004171. Electronic nicotine delivery devices (ENDDs) or nicotine inhaler for smoking cessation. clinicaltrials.gov/show/NCT02004171 (accessed 16 July 2014).
- NCT02029196. A randomised, parallel group, multi‐centre study to evaluate the safety profile of the ITG EVP G1 product. clinicaltrials.gov/show/NCT02029196 (accessed 16 July 2014).
- NCT02124187. Smoking cessation and reduction in depression (SCARID). clinicaltrials.gov/show/NCT02124187 (accessed 16 July 2014).
- NCT02143310. A multi‐centre study to evaluate the safety of use of electronic vapour products for two years. clinicaltrials.gov/show/NCT02143310 (accessed 16 July 2014).
- NCT02212041. Acceptability, patterns of use and safety of electronic cigarette in people with mental illness: a pilot study. clinicaltrials.gov/show/NCT02212041 (accessed 17 February 2016). [CRS: 9400131000005045]
- NCT02261363. A mixed method EMA assessment of cognition and behavior among new ENDS users: an observational cohort study. clinicaltrials.gov/show/NCT02261363 2014 (accessed 15 August 2016). [CRS: 9400131000005078]
- NCT02328794. Randomized clinical trial to reduce harm from tobacco. clinicaltrials.gov/show/NCT02357173 (accessed 17 February 2016).
- NCT02357173. A trial of e‐cigarettes: natural uptake, patterns and impact of use. clinicaltrials.gov/show/NCT02357173 (accessed 17 February 2016). [CRS: 9400131000005049]
- NCT02398487. Head‐to‐head comparison of personal vaporizers versus cigalike: prospective 6‐month randomized control design study. clinicaltrials.gov/show/NCT02398487 (accessed 17 February 2016). [CRS: 9400131000005074]
- NCT02417467. Evaluating the efficacy of e‐cigarette use for smoking cessation (E3) trial. clinicaltrials.gov/show/NCT02417467 (accessed 17 February 2016). [CRS: 9400131000005055]
- NCT02482233. A pilot randomized controlled clinical trial ‐ "Electronic nicotine delivery device (e‐cigarette) for perioperative smoking cessation in veterans". clinicaltrials.gov/show/NCT02482233 (accessed 17 February 2016). [CRS: 9400131000005062]
- NCT02487953. Electronic nicotine delivery systems (ENDS) as a smoking cessation treatment. clinicaltrials.gov/show/NCT02487953 (accessed 17 February 2016). [CRS: 9400131000005072]
- NCT02498145. Short term effects of electronic cigarettes in tobacco dependent adults. clinicaltrials.gov/show/NCT02498145 (accessed 17 February 2016). [CRS: 9400131000005051]
- NCT02521662. A randomised‐controlled clinical trial to evaluate the effectiveness and safety of combining nicotine patches with e‐cigarettes (with and without nicotine) plus behavioural support, on smoking abstinence. clinicaltrials.gov/show/NCT02521662 (accessed 17 February 2016). [CRS: 9400131000005047]
- NCT02527980. E‐cigarettes: dynamic patterns of use and health effects. clinicaltrials.gov/show/NCT02527980 (accessed 17 February 2016). [CRS: 9400131000005064]
- NCT02590393. The role of nicotine and non‐nicotine alkaloids in e‐cigarette use and dependence. clinicaltrials.gov/show/NCT02590393 (accessed 17 February 2016). [CRS: 9400131000005059]
- NCT02628964. Assessing the use of electronic cigarettes (e‐cigarettes) as a harm reduction strategy. clinicaltrials.gov/show/NCT02628964 (accessed 17 February 2016). [CRS: 9400131000005053]
- NCT02635620. Changes in lung function parameters, bronchial reactivity, state of health and smoking behaviour associated with changing from conventional smoking to electronic cigarettes. clinicaltrials.gov/show/NCT02635620 (accessed 17 February 2016). [CRS: 9400131000005057]
- NCT02648178. Evaluation of appeal and impact of e‐cigarettes among chronic smokers with smoking‐related cancers. clinicaltrials.gov/show/NCT02648178 (accessed 17 February 2016). [CRS: 9400131000005068]
Additional references
- Agaku IT, King BA, Husten CG, Bunnell R, Ambrose BK, Hu SS, et al. Tobacco product use among adults ‐ United States, 2012‐2013. MMWR ‐ Morbidity & Mortality Weekly Report 2014;63(25):542‐7. [PMC free article] [PubMed] [Google Scholar]
- Action on Smoking and Health. Use of electronic cigarettes (vapourisers) among adults in Great Britain. www.ash.org.uk/files/documents/ASH_891.pdf (accessed 21 July 2016).
- Ayers JW, Ribisl KM, Brownstein JS. Tracking the rise in popularity of electronic nicotine delivery systems (electronic cigarettes) using search query surveillance. American Journal of Preventive Medicine 2011;40(4):448‐53. [DOI] [PubMed] [Google Scholar]
- Balfour D. The neurobiology of tobacco dependence: A preclinical perspective on the role of dopamine projections to the nucleus. Nicotine & Tobacco Research 2004;6(6):899‐912. [DOI] [PubMed] [Google Scholar]
- Barbeau AM, Burda J, Siegel M. Perceived efficacy of e‐cigarettes versus nicotine replacement therapy among successful e‐cigarette users: a qualitative approach. Addiction Science & Clinical Practice 2013;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SP. The effects of nicotine, denicotinized tobacco, and nicotine‐containing tobacco on cigarette craving, withdrawal, and self‐administration in male and female smokers. Behavorial Pharmacology 2010;21(2):144‐52. [DOI] [PubMed] [Google Scholar]
- Bein K, Leikauf GD. Acrolein ‐ a pulmonary hazard. Molecular Nutrition & Food Research 2011;55(9):1342‐60. [DOI] [PubMed] [Google Scholar]
- Cahill K, Lindson‐Hawley N, Thomas K, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 5. [DOI: 10.1002/14651858.CD006103.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Notes from the field: electronic cigarette use among middle and high school students ‐ United States, 2011‐2012. MMWR ‐ Morbidity and Mortality Weekly Report 2013;62(35):729‐30. [PMC free article] [PubMed] [Google Scholar]
- Chen C, Zhuang YL, Zhu SH. E‐cigarette design preference and smoking cessation: a U.S. population study. American Journal of Preventive Medicine2016 [Epub ahead of print]. [DOI: 10.1016/j.amepre.2016.02.002] [DOI] [PMC free article] [PubMed]
- Dawkins L, Turner J, Roberts A, Soar K. 'Vaping' profiles and preferences: an online survey of electronic cigarette users. Addiction 2013;108(6):1115‐25. [DOI] [PubMed] [Google Scholar]
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction 2007;102(2):324‐34. [DOI] [PubMed] [Google Scholar]
- Donny EC, Jones M. Prolonged exposure to denicotinized cigarettes with or without transdermal nicotine. Drug and Alcohol Dependence 2009;104(1‐2):23‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction 2011;106(11):2017‐28. [DOI] [PubMed] [Google Scholar]
- European Parliament and Council of the European Union. Directive 2014/40/EU of the European Parliament and of the Council of 3 April 2014 on the approximation of the laws, regulations and administrative provisions of the Member States concerning the manufacture, presentation and sale of tobacco and related products and repealing Directive 2001/37/EC. www.ncdlinks.org/pie‐elibrary/reports‐publications/?entryid43=35159&p=8 2014 (accessed 25th November 2014). [PubMed]
- Fagerström KO, Hughes JR. Nicotine concentrations with concurrent use of cigarettes and nicotine replacement: a review. Nicotine & Tobacco Research 2004;4(Suppl 2):S73‐9. [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Therapeutic Advances in Drug Safety 2014;5(2):67‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food, Drug Administration. Deeming tobacco products to be subject to the Federal Food, Drug, and Cosmetic Act, as amended by the Family Smoking Prevention and Tobacco Control Act. www.fda.gov/TobaccoProducts/Labeling/RulesRegulationsGuidance/ucm394909.htm 2016 (accessed 21st July 2016). [PubMed]
- Gallus S, Lugo A, Pacifici R, Pichini S, Colombo P, Garattini S, et al. E‐Cigarette awareness, use, and harm perception in Italy: a national representative survey. Nicotine & Tobacco Research2014; Vol. 16, issue 12:1541‐8. [DOI] [PubMed]
- Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine & Tobacco Research 2012;15(1):158‐66. [DOI] [PubMed] [Google Scholar]
- Goniewicz M L, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction 2014;109(3):500‐7. [DOI] [PubMed] [Google Scholar]
- Grana R, Benowitz N, Glantz SA. E‐cigarettes: a scientific review. Circulation 2014;129(19):1972‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualano MR, Passi S, Bert F, Torre G, Scaioli G, Siliquini R. Electronic cigarettes: assessing the efficacy and the adverse effects through a systematic review of published studies. Journal of Public Health 2015;37(3):488‐97. [DOI] [PubMed] [Google Scholar]
- Hagstrom K, Gannon D, Sobieraj D. Electronic cigarettes for smoking cessation. Connecticut Medicine 2014;78(7):435‐9. [PubMed] [Google Scholar]
- Hajek P, West R, Foulds J, Nilsson F, Burrows S, Meadow A. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Archives of Internal Medicine 1999;159(17):2033‐8. [DOI] [PubMed] [Google Scholar]
- Hajek P, Etter J‐F, Benowitz N, Eissenberg T, McRobbie H. Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction 2014;109(11):1801‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, Goniewicz ML, Phillips A, Myers Smith K, West O, McRobbie H. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine & Tobacco Research 2015;17(2):175‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, McRobbie H, Bullen C. E‐cigarettes and smoking cessation. Lancet Respiratory Medicine 2016;4(6):e23. [DOI] [PubMed] [Google Scholar]
- Harrell PT, Simmons VN, Correa JB, Padhya TA, Brandon TH. Electronic Nicotine Delivery Systems (“E‐cigarettes”) Review of Safety and Smoking Cessation Efficacy. Otolaryngology‐‐Head and Neck Surgery 2014;151(3):381‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog B. E cigs revolutionizing the tobacco industry. Convenience Store News 2013;49(9):94‐6. [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
- Hitchman SC, Brose LS, Brown J, Robson D, McNeill A. Associations between e‐cigarette type, frequency of use, and quitting smoking: findings from a longitudinal online panel survey in Great Britain. Nicotine and Tobacco Research 2015;17(10):1187‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua M, Talbot P. Potential health effects of electronic cigarettes: A systematic review of case reports. Preventive Medicine Reports 2016;4:169‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long‐term abstinence among untreated smokers. Addiction 2004;99(1):29‐38. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Stead LF, Hartmann‐Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD000031.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang N, Chen J, Wang MP, McGhee SM, Kwong AC, Lai VW, et al. Electronic cigarette awareness and use among adults in Hong Kong. Addictive Behaviors 2016;52:34‐8. [DOI] [PubMed] [Google Scholar]
- Kalkhoran S, Glantz SA. E‐cigarettes and smoking cessation in real‐world and clinical settings: a systematic review and meta‐analysis. Lancet Respiratory Medicine 2016;4(2):116‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoudigian S, Devji T, Lytvyn L, Campbell K, Hopkins R, O'Reilly D. The efficacy and short‐term effects of electronic cigarettes as a method for smoking cessation: a systematic review and a meta‐analysis. International Journal of Public Health 2016;61(2):257‐67. [DOI] [PubMed] [Google Scholar]
- Kralikova E, Kubatova S, Truneckova K, Kmetova A, Hajek P. The electronic cigarette: what proportion of smokers have tried it and how many use it regularly?. Addiction 2012;107(8):1528‐9. [DOI] [PubMed] [Google Scholar]
- Lam C, West A. Are electronic nicotine delivery systems an effective smoking cessation tool?. Canadian Journal of Respiratory Therapy 2015;51(4):93‐8. [PMC free article] [PubMed] [Google Scholar]
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001292.pub2] [DOI] [PubMed] [Google Scholar]
- Levin ED, Behm F, Carnahan E, LeClair R, Shipley R, Rose JE. Clinical trials using ascorbic acid aerosol to aid smoking cessation. Drug and Alcohol Dependence 1993;33(3):211‐23. [DOI] [PubMed] [Google Scholar]
- Malas M, Tempel J, Schwartz R, Minichiello A, Lightfoot C, Noormohamed A, et al. Electronic cigarettes for smoking cessation: a systematic review. Nicotine & Tobacco Research2016 [Epub ahead of print]. [DOI] [PubMed]
- McKee M, Daube M, Chapman S. E‐cigarettes should be regulated. Medical Journal of Australia 2016;204(9):331. [DOI] [PubMed] [Google Scholar]
- McNeill A, Brose LS, Calder R, Hitchman SC, Hajek P, McRobbie H. E‐cigarettes: an evidence update. A report commissioned by Public Health England. www.gov.uk/government/uploads/system/uploads/attachment_data/file/457102/Ecigarettes_an_evidence_update_A_report_commissioned_by_Public_Health_England_FINAL.pdf 2015 (accessed 20th August 2016).
- McRobbie H, Przulj D, Smith KM, Cornwall D. Complementing the standard multicomponent treatment for smokers with denicotinized cigarettes: a randomized trial. Nicotine & Tobacco Research 2016;18(5):1134‐41. [DOI] [PubMed] [Google Scholar]
- Meo SA, Al Asiri SA. Effects of electronic cigarette smoking on human health. European Review for Medical and Pharmacological Sciences 2014;18(21):3315‐9. [PubMed] [Google Scholar]
- Orr KK, Asal NJ. Efficacy of electronic cigarettes for smoking cessation. Annals of Pharmacotherapy 2014;48(11):1502‐6. [DOI] [PubMed] [Google Scholar]
- Palipudi KM, Mbulo L, Morton J, Bunnell R, Blutcher‐Nelson G, Kosen S, et al. Awareness and current use of electronic cigarettes in Indonesia, Malaysia, Qatar, and Greece: findings From 2011–2013 global adult tobacco surveys. Nicotine & Tobacco Research 2016;18(4):501‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Conklin CA, Sayette MA, Giedgowd GE. Acute negative affect relief from smoking depends on the affect situation and measure but not on nicotine. Biological Psychology 2010;67(8):707‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickworth WB, Fant RV, Nelson RA, Rohrer MS, Henningfield JE. Pharmacodynamic effects of new de‐nicotinized cigarettes. Nicotine & Tobacco Research 1999;1(4):357‐64. [DOI] [PubMed] [Google Scholar]
- Pisinger C, Dossing M. A systematic review of health effects of electronic cigarettes. Preventive Medicine 2014;69:248‐60. [DOI] [PubMed] [Google Scholar]
- Przulj D, McRobbie H, Hajek P. The effect of sensorimotor replacement on smoking cessation and craving. Open Addiction Journal 2013;5:41‐50. [Google Scholar]
- Rahman MA, Hann N, Wilson A, Mnatzaganian G, Worrall‐Carter L. E‐cigarettes and smoking cessation: evidence from a systematic review and meta‐analysis. PLoS ONE 2015;10(3):e0122544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Nik Mohamed MH, Jamshed SQ. Safety and effectiveness of electronic cigarettes: A narrative review. International Medical Journal of Malaysia 2015;22(3):122‐31. [Google Scholar]
- Tobacco Advisory Group of the Royal College of Physicians. Nicotine Without Smoke—Tobacco Harm Reduction. London: Royal College of Physicians, 2016. [Google Scholar]
- Rose JE, Tashkin DP, Ertle A, Zinser MC, Lafer R. Sensory blockade of smoking satisfaction. Pharmacology Biochemistry & Behavior 1985;23(2):289‐93. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Levin ED. Role of nicotine dose and sensory cues in the regulation of smoke intake. Pharmacology Biochemistry & Behavior 1993;44(4):891‐900. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Inhalation of vapor from black pepper extract reduces smoking withdrawal symptoms. Drug and Alcohol Dependence 1994;34(3):225‐9. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacology Biochemistry & Behavior 2000;67(1):71‐81. [DOI] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology 2006;184(3‐4):274‐85. [DOI] [PubMed] [Google Scholar]
- Russell MAH. Nicotine intake and its control over smoking. In: Wonnacott S, Russell MAH, Stolerman I editor(s). Nicotine Psychopharmacology: Molecular, Cellular and Behavioural Aspects. London: Oxford University Press, 1990:374‐418. [Google Scholar]
- Schneider NG, Olmstead RE, Franzon MA, Lunell E. The nicotine inhaler: clinical pharmacokinetics and comparison with other nicotine treatments. Clinical Pharmcokinetics 2001;40(9):661‐84. [DOI] [PubMed] [Google Scholar]
- Stead LF, Lancaster T. Group behaviour therapy programmes for smoking cessation. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001007.pub2] [DOI] [PubMed] [Google Scholar]
- Stead LF, Lancaster T. Nicobrevin for smoking cessation. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005990] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead LF, Lancaster T. Interventions to reduce harm from continued tobacco use. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD005231.pub2] [DOI] [PubMed] [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000146.pub4] [DOI] [PubMed] [Google Scholar]
- Waghel RC, Battise DM, Ducker ML. Effectiveness of electronic cigarettes as a tool for smoking cessation or reduction. Journal of Pharmacy Technology 2015;31(1):8‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker N, Howe C, Bullen C, Grigg M, Glover M, McRobbie H, et al. The combined effect of very low nicotine content cigarettes, used as an adjunct to usual Quitline care (nicotine replacement therapy and behavioural support), on smoking cessation: a randomized controlled trial. Addiction 2012;107(10):1857‐67. [DOI] [PubMed] [Google Scholar]
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100(3):299‐303. [DOI] [PubMed] [Google Scholar]
- West R, Zhou X. Is nicotine replacement therapy for smoking cessation effective in the “real world”? Findings from a prospective multinational cohort study. Thorax 2007;62(11):998‐1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R, Beard B, Brown J. Trends in electronic cigarette use in England: Smoking Toolkit Study. www.smokinginengland.info/latest‐statistics 2016 (accessed 20 August 2016).
- Westman EC, Behm FM, Rose JE. Airway sensory replacement combined with nicotine replacement for smoking cessation. A randomized, placebo‐controlled trial using a citric acid inhaler. Chest 1995;107(5):1358‐64. [DOI] [PubMed] [Google Scholar]
- Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacology Biochemistry & Behavior 1996;53(2):309‐15. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Electronic nicotine delivery systems: Report prepared by WHO for the sixth session of the Conference of the Parties to the WHO Framework Convention on Tobacco Control. apps.who.int/gb/fctc/PDF/cop6/FCTC_COP6_10‐en.pdf?ua=1 2014 (accessed 25 November 2014).
References to other published versions of this review
- McRobbie H, Bullen C, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010216] [DOI] [PubMed] [Google Scholar]
- McRobbie H, Bullen C, Hartmann‐Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database of Systematic Reviews 2014, Issue 12. [DOI: 10.1002/14651858.CD010216.pub2] [DOI] [PubMed] [Google Scholar]


