Abstract
Background
This is an updated version of the original Cochrane review published in Issue 1, 2010. Pelvic lymphadenectomy is associated with significant complications including lymphocyst formation and related morbidities. Retroperitoneal drainage using suction drains has been recommended as a method to prevent such complications. However, this policy has been challenged by the findings from recent studies.
Objectives
To assess the effects of retroperitoneal drainage versus no drainage after pelvic lymphadenectomy on lymphocyst formation and related morbidities in gynaecological cancer patients.
Search methods
We searched the Cochrane Gynaecological Cancer Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL 2013, Issue 12) in The Cochrane Library, electronic databases MEDLINE (Nov Week 3, 2013), EMBASE (2014, week 1), and the citation lists of relevant publications. The latest searches were performed on 10 January 2014.
Selection criteria
Randomised controlled trials (RCTs) that compared the effect of retroperitoneal drainage versus no drainage after pelvic lymphadenectomy in gynaecological cancer patients. Retroperitoneal drainage was defined as placement of passive or active suction drains in pelvic retroperitoneal spaces. No drainage was defined as no placement of passive or active suction drains in pelvic retroperitoneal spaces.
Data collection and analysis
We assessed studies using methodological quality criteria. For dichotomous data, we calculated risk ratios (RRs) and 95% confidence intervals (CIs). We examined continuous data using mean difference (MD) and 95% CI.
Main results
Since the last version of this review, no new studies have been identified for inclusion. The review included four studies with 571 participants. Considering the short‐term outcomes (within four weeks after surgery), retroperitoneal drainage was associated with a comparable rate of overall lymphocyst formation when all methods of pelvic peritoneum management were considered together (two studies, 204 patients; RR 0.76, 95% CI 0.04 to 13.35). When the pelvic peritoneum was left open, the rates of overall lymphocyst formation (one study, 110 patients; RR 2.29, 95% CI 1.38 to 3.79) and symptomatic lymphocyst formation (one study, 137 patients; RR 3.25, 95% CI 1.26 to 8.37) were higher in the drained group. At 12 months after surgery, the rates of overall lymphocyst formation were comparable between the groups (one study, 232 patients; RR 1.48, 95% CI 0.89 to 2.45). However, there was a trend toward increased risk of symptomatic lymphocyst formation in the group with drains (one study, 232 patients; RR 7.12, 95% CI 0.89 to 56.97). The included trials were of low to moderate risk of bias.
Authors' conclusions
Placement of retroperitoneal tube drains has no benefit in prevention of lymphocyst formation after pelvic lymphadenectomy in patients with gynaecological malignancies. When the pelvic peritoneum is left open, the tube drain placement is associated with a higher risk of short and long‐term symptomatic lymphocyst formation.
Keywords: Female; Humans; Drainage; Drainage/methods; Genital Neoplasms, Female; Genital Neoplasms, Female/surgery; Lymph Node Excision; Lymph Node Excision/adverse effects; Lymphocele; Lymphocele/etiology; Lymphocele/prevention & control; Randomized Controlled Trials as Topic; Retroperitoneal Space; Suction; Suction/methods
Drains versus no drains after pelvic lymphadenectomy to prevent lymphocyst formation in patients with gynaecological cancer
Background
Pelvic lymphadenectomy, the procedure to remove lymph nodes surrounding major blood vessels in the pelvis, is an important component of the surgical management of gynaecological cancers. However, it can lead to complications, especially lymphocyst formation (collection of lymphatic fluid in the pelvis) and its related consequences such as leg swelling, blockage of the ureter, pelvic pain, clot formation in the leg and pelvic vein, bowel motility disorder, and infection. Without clear evidence, placement of suction drains to remove lymphatic fluid that accumulates in the operative area between the peritoneum and the posterior abdominal wall has been traditionally recommended to prevent such complications.
Review question
The aim of this review is to compare the effects of drains versus no drains in preventing lymphocyst formation following pelvic lymphadenectomy.
Main findings
The searches were updated in January 2014. We identified four studies (571 particpants) for inclusion. The participants were primarily those who had cancer of the cervix and endometrium, with only one study also including patients with cancer of the ovary. The findings have demonstrated that placement of suction drains is not effective in preventing lymphocysts, especially when the peritoneum (pelvic lining) is left open. In fact, such practice increases the risk of short and long‐term lymphocyst formation with related symptoms.
Quality of the evidence
The review includes four good‐quality (low to moderate risk of bias) clinical trials in its final analysis.
Summary of findings
Summary of findings for the main comparison.
Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy for gynaecological malignancies
| Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy for gynaecological malignancies | ||||||
| Patient or population: patients with gynaecological malignancies Settings: hospital Intervention: retroperitoneal drainage versus no drainage after pelvic lymphadenectomy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy | |||||
| Short‐term lymphocyst formation: both asymptomatic and symptomatic within 4 weeks after surgery | Study population | RR 0.76 (0.04 to 13.35) | 204 (2 studies) | ⊕⊕⊕⊕ high | ||
| 172 per 1000 | 131 per 1000 (7 to 1000) | |||||
| Medium risk population | ||||||
| 161 per 1000 | 122 per 1000 (6 to 1000) | |||||
| Short‐term lymphocyst formation: symptomatic within 4 weeks after surgery | Study population | RR 3.25 (1.26 to 8.37) | 237 (2 studies) | ⊕⊕⊕⊕ high1 | ||
| 43 per 1000 | 140 per 1000 (54 to 360) | |||||
| Medium risk population | ||||||
| 36 per 1000 | 117 per 1000 (45 to 301) | |||||
| Short‐term lymphocyst formation: both asymptomatic and symptomatic at 8 weeks after surgery | Study population | RR 0.72 (0.3 to 1.71) | 180 (2 studies) | ⊕⊕⊕⊕ high | ||
| 112 per 1000 | 81 per 1000 (34 to 192) | |||||
| Medium risk population | ||||||
| 110 per 1000 | 79 per 1000 (33 to 188) | |||||
| Long‐term lymphocyst formation: both asymptomatic and symptomatic at 12 months after surgery | Study population | RR 1.48 (0.89 to 2.45) | 232 (1 study) | ⊕⊕⊕⊕ high | ||
| 171 per 1000 | 253 per 1000 (152 to 419) | |||||
| Medium risk population | ||||||
| 171 per 1000 | 253 per 1000 (152 to 419) | |||||
| Long‐term lymphocyst formation: symptomatic at 12 months after surgery | Study population | RR 7.12 (0.89 to 56.97) | 232 (1 study) | ⊕⊕⊕⊕ high2 | ||
| 9 per 1000 | 64 per 1000 (8 to 513) | |||||
| Medium risk population | ||||||
| 9 per 1000 | 64 per 1000 (8 to 513) | |||||
| Febrile morbidity | Study population | RR 1.76 (0.87 to 3.55) | 571 (4 studies) | ⊕⊕⊕⊕ high | ||
| 39 per 1000 | 69 per 1000 (34 to 138) | |||||
| Medium risk population | ||||||
| 39 per 1000 | 69 per 1000 (34 to 138) | |||||
| Pelvic infection | Study population | RR 0.42 (0.11 to 1.62) | 571 (4 studies) | ⊕⊕⊕⊕ high3 | ||
| 18 per 1000 | 8 per 1000 (2 to 29) | |||||
| Medium risk population | ||||||
| 19 per 1000 | 8 per 1000 (2 to 31) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1The RR for this effect is 3.25 (> 2). 2The RR for this outcome is very high (7.12; > 5), but the 95% CI is wide. 3The RR for this outcome is low (0.42; < 0.5), but the 95% CI is wide.
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Charoenkwan 2010).
Pelvic lymphadenectomy, the removal of all or most of the lymph nodes surrounding major pelvic blood vessels, is an important component of the surgical management of gynaecological malignancies, particularly those originating from the cervix, endometrium, and ovary. Knowledge of the metastatic status of the removed pelvic lymph nodes provides valuable staging and prognostic information that guides postoperative adjuvant treatment. In addition, removal of both bulky and microscopic metastatic nodes can potentially improve treatment outcome. However, the procedure is associated with postoperative complications, among which lymphocyst formation and its related morbidities is important (Benedetti‐Panici 1997; Yamamoto 2000).
A lymphocyst, defined as a collection of lymphatic fluid in the retroperitoneal space (the space between the peritoneum and the posterior abdominal wall), is well known as a specific complication of pelvic lymphadenectomy (Benedetti‐Panici 1997; Ilancheran 1988; Livingston 1980; Petru 1989). The reported incidence of lymphocyst following gynaecological cancer surgery varies from 1% to 29% (Lopes 1995). These differences can be explained by the use of various surgical techniques (methods and instruments used to remove the lymph nodes and secure lymphatic vessels; sharp dissection with suture ligation or coagulation), the difference in extent of lymphadenectomy, and the use of different diagnostic methods (ultrasonography or computerised tomography (CT) scan) (Srisomboon 2002; Yamamoto 2000). The mechanism of lymphocyst formation is unknown. However, previous radiotherapy, node metastasis, and prophylactic heparin have been considered as possible risk factors (Yamamoto 2000). Most lymphocysts occur within the first two to four weeks after surgery (Conte 1990). Although frequently asymptomatic, lymphocysts can lead to leg oedema, ureteral obstruction, pelvic pain, deep venous thrombosis, ileus (intestinal stasis), secondary infection, and fistula (abnormal passage that connects an abscess, cavity, or hollow organ to the body surface or to another hollow organ) (Franchi 2007).
Retroperitoneal drainage has been traditionally recommended as a method to prevent lymphocyst formation and associated postoperative morbidities (Symmond 1961; Symmond 1966; Van Nagell 1976). The procedure is performed by the placement of passive or active suction drains to remove lymphatic fluid or blood that accumulate in the operative fields. This practice has become a surgical dogma. However, recent studies have challenged this policy by demonstrating that there was no advantage to the routine use of retroperitoneal drainage following radical hysterectomy and pelvic lymphadenectomy (Franchi 2007; Lopes 1995; Patsner 1995; Srisomboon 2002). In fact, the drain itself, as a foreign body, could disturb the reparative and absorptive functions of the peritoneum and contribute to the formation of lymphocyst (Maitland 1970).
Our aim is to determine whether there is clear evidence to support the omission of retroperitoneal drainage following pelvic lymphadenectomy in patients with gynaecological malignancies.
Objectives
To assess the effects of retroperitoneal drainage versus no drainage after pelvic lymphadenectomy on lymphocyst formation and related morbidities in gynaecological cancer patients.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCTs) that compared the effect of retroperitoneal drainage versus no drainage after pelvic lymphadenectomy in gynaecological cancer patients. However, we excluded quasi‐RCTs. The trials that were included in the final analysis had clear random allocation criteria with appropriate allocation concealment. Also, the included studies did not have significant violations of allocation procedure and exclusions after allocation.
Types of participants
Participants were patients with gynaecological malignancies who underwent pelvic lymphadenectomy with or without para‐aortic lymphadenectomy as part of their surgical treatment, regardless of lymphadenectomy approaches (transperitoneal or extraperitoneal) and surgical approach (open or laparoscopic).
Types of interventions
Main intervention
Retroperitoneal drainage ‐ defined as placement of passive or active suction drains in pelvic retroperitoneal spaces following pelvic lymphadenectomy, regardless of drainage route (transabdominal or transvaginal), surgical management of pelvic peritoneum (left open or sutured closed), and surgical management of vaginal stump (open or closed).
Comparison intervention
No drainage ‐ defined as no placement of passive or active suction drains in pelvic retroperitoneal spaces following pelvic lymphadenectomy, regardless of surgical management of pelvic peritoneum (left open or sutured closed), and surgical management of vaginal stump (open or closed).
Types of outcome measures
We recorded the following outcomes where the information was available.
Primary outcomes
Rate of lymphocyst formation; asymptomatic and symptomatic (categorical data). The diagnosis of lymphocyst must be made by imaging studies such as ultrasound, computerised tomography (CT), or magnetic resonance imaging (MRI). The techniques used for diagnosis were described.
Secondary outcomes
Rate of related postoperative morbidities: febrile morbidity, wound infection, wound dehiscence, leg oedema, deep venous thrombosis, bowel obstruction, fistula formation (categorical data).
Blood transfusion (categorical data).
Change of related serum chemistry: protein and albumin level (continuous data), albumin infusion (categorical data).
Time to the return of bowel sound (continuous data).
Duration of surgery (continuous data).
Postoperative hospital stay (continuous data).
Search methods for identification of studies
Electronic searches
We obtained all articles which described RCTs of retroperitoneal drainage versus no drainage after pelvic lymphadenectomy in gynaecological cancer patients from the following sources.
We searched the Cochrane Gynaecological Group Cancer Specialised Register for any relevant trials.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2013, Issue 12) in all fields (Appendix 1).
The MEDLINE electronic database (November week 3, 2013) (Appendix 2).
The EMBASE electronic database (January week 1, 2014) (Appendix 3).
We performed the latest searches on 10 January 2014.
Searching other resources
We searched the citation lists of relevant publications, systematic reviews, review articles, abstracts of scientific meetings, and included studies.
We conducted personal communication with experts, specialists in the field, and the study authors of relevant publications in an attempt to identify unpublished studies.
The search strategies used have been developed and executed by the author team.
Data collection and analysis
Selection of studies
Both review authors (KC and CK) undertook the study selection. Both authors screened the titles and abstracts of articles found in the search. We discarded studies that were clearly ineligible but aimed to be overly inclusive rather than risk losing relevant studies. We then obtained full‐text copies of the eligible articles. Both review authors independently assessed whether the studies met the inclusion criteria, with disagreements resolved by discussion. We sought further information from the study authors where papers contained insufficient information to make a decision about eligibility.
Data extraction and management
The review authors independently extracted information using the pre‐designed data extraction form. We resolved discrepancies by discussion. For each included trial, we collected information regarding the location of the study, methods of the study, the participants (age range, eligibility criteria), the nature of the interventions, and data relating to the outcomes specified above. Where possible, we sought missing data from study authors.
Assessment of risk of bias in included studies
Methodological quality
The two review authors then assessed the quality of all eligible studies independently, following the guidelines of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2008), with discrepancies resolved by discussion. We conducted the assessment by detailed description of the following standardised items.
Was the assigned treatment adequately concealed prior to allocation? (Appendix 4)
Were the treatment and control group comparable at entry?
Were the outcomes of patients who withdrew or were excluded after allocation described and included in an 'intention‐to‐treat' (ITT) analysis?
Were the subjects blinded to assignment status following allocation?
Were the treatment providers blinded to assignment status?
Were the care programmes, other than the trial options, identical?
Were the outcome assessors blinded to assignment status?
Were the withdrawals or loss to follow‐up less than 10% of the study population?
We rated each item as follows:
clearly yes: rate A;
not sure: rate B (seek details from study authors);
clearly no: rate C.
The information regarding methodological quality is presented graphically, providing a context for discussing the reliability of the results (Figure 1; Figure 2). We examined the funnel plot for each outcome for the possibility of publication bias.
Figure 1.
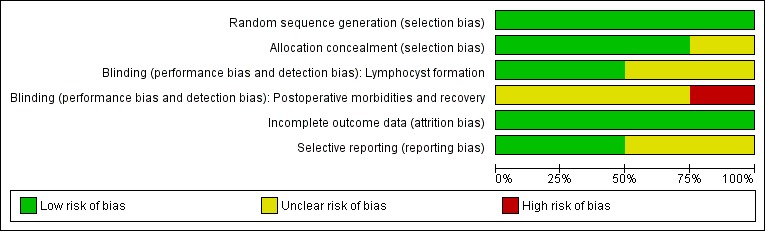
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Figure 2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Data synthesis
We performed statistical analysis in accordance with the guidelines for statistical analysis developed by The Cochrane Collaboration (Deeks 2008).
Subgroup analysis and investigation of heterogeneity
We examined heterogeneity (variations) between the results of different studies by inspecting the forest plot of a meta‐analysis for variation in effects. We also considered formal statistical tests, such as the statistical tests of homogeneity of 2 x 2 tables and the I2 value, in conjunction with the graphical approaches to determine between‐study differences.
For categorical data, we expressed results for each study as a risk ratio (RR) with 95% confidence interval (CI) and combined these for meta‐analysis with RevMan 5 software (RevMan 2012).
For continuous data, we expressed results from each study as a mean difference (MD) with 95% CI and combined these for meta‐analysis with RevMan 5 software. Meta‐analytic methods for continuous data assume that the underlying distribution of the measurements is normal. Where data were clearly skewed and results reported in the publication as median and range with non‐parametric tests of significance, we reported the results separately in the Results section of the review.
For meta‐analysis, we used the fixed‐effect or random‐effects model depending on the outcome of the tests of homogeneity.
Sensitivity analysis
The authors planned to perform sensitivity analyses where there was uncertainty or disagreement regarding inclusion of studies, data extraction, and missing data/drop‐outs.
Results
Description of studies
Results of the search
We considered 12 studies to be relevant and chose them for further assessment. We obtained copies of the full text of these potentially relevant references and both authors independently assessed these for eligibility.
We excluded six studies because they were not RCTs (see Characteristics of excluded studies).
We excluded two RCTs because the randomisation was not between retroperitoneal drainage and no drainage after pelvic lymphadenectomy. Franchi 1997 compared closure of pelvic and parietal peritoneum (with placement of a T‐shape suction drain through the vagina) to no peritoneal closure (but the vagina closed and two abdominal drains placed). Morice 2001 compared placement of a low‐pressure drain in the aortic area to no placement of para‐aortic drain after complete para‐aortic lymphadenectomy. However, most patients had suction drains placed in the pelvis.
We included four RCTs in the meta‐analysis (Benedetti‐Panici 1997; Franchi 2007; Lopes 1995; Srisomboon 2002) (see Figure 3 and Characteristics of included studies).
Figure 3.
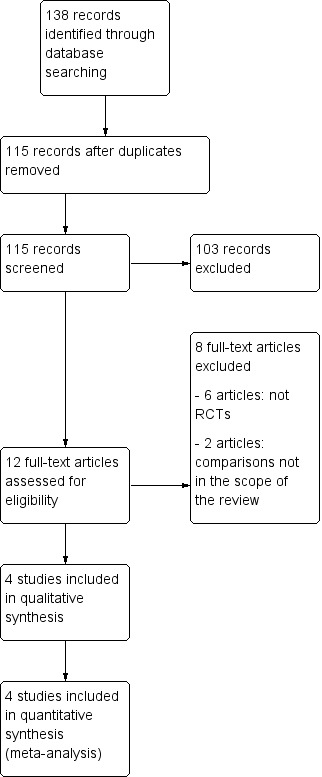
Study flow diagram.
Included studies
Participants
The review consists of a total of 571 women. The number of participants for the included studies was 100 (Lopes 1995), 137 (Benedetti‐Panici 1997), 100 (Srisomboon 2002), and 234 (Franchi 2007). The countries represented were the United Kingdom (Lopes 1995), Italy (Benedetti‐Panici 1997), and Thailand (Srisomboon 2002). Franchi 2007 is a European Organisation for Research and Treatment of Cancer‐Gynaecological Cancer Group (EORTC‐GCG) multi‐institutional trial that recruited participants from 12 European cancer centres. In three studies (Franchi 2007; Lopes 1995; Srisomboon 2002), the majority of participants were patients with early‐stage cervical carcinoma. In Benedetti‐Panici 1997, the participants' diagnosis was almost equally distributed between endometrial (early), cervical (early and locally advanced), and ovarian carcinoma (all stages). Baseline characteristics of the participants were comparable between the two groups in all studies.
Interventions
For participants randomised to the use of drains, two suction drains were placed retroperitoneally alongside the site of node dissection and brought out through the abdominal wall in Benedetti‐Panici 1997, Lopes 1995, and Srisomboon 2002. In Franchi 2007, two passive or active suction drains were placed in the retroperitoneal fossa and inserted via the vagina or the abdominal route, according to the institution's policy. The drains were removed when the loss was less than 50 mL in 24 hours in three studies (Benedetti‐Panici 1997; Franchi 2007; Srisomboon 2002), and less than 100 mL in 24 hours in Lopes 1995.
In three studies, the pelvic peritoneum was left open in all cases (Benedetti‐Panici 1997; Franchi 2007; Lopes 1995). In Srisomboon 2002, however, the pelvic peritoneum was closed in the drain group and left open in the no drain group.
In Benedetti‐Panici 1997, Franchi 2007 and Srisomboon 2002, the vaginal cuff was primarily closed in all participants. In Lopes 1995, the vaginal cuff edge was over sewn, leaving the vaginal vault open.
Outcomes
Three studies provided data on short‐term lymphocyst formation rate (Lopes 1995 (overall; asymptomatic and symptomatic together); Benedetti‐Panici 1997 (overall and symptomatic); Srisomboon 2002 (overall; asymptomatic and symptomatic together)). However, the surveillance schedule appeared different among studies. In Lopes 1995, an abdominal ultrasound scan was performed approximately eight weeks after the surgery to identify any asymptomatic Iymphocysts. In Srisomboon 2002, transabdominal and transvaginal ultrasounds were performed at four, eight, and 12 weeks after surgery to detect lymphocyst formation. Weekly abdominal and pelvic ultrasounds were performed on 110 of 137 patients to detect lymphocyst formation in Benedetti‐Panici 1997. Lymphocysts were followed up in order to assess their clinical behaviour. When no lymphocysts were detected at the third examination, monitoring was discontinued.
The only study that provided data on rate of long‐term lymphocyst formation (both asymptomatic and symptomatic) was Franchi 2007.
Risk of bias in included studies
Allocation
Allocation concealment appeared clearly adequate in Srisomboon 2002, in which randomisation was performed by using sequentially numbered, sealed, opaque assignment envelopes. We considered the concealment of allocation potentially adequate in Benedetti‐Panici 1997 and Lopes 1995, although further details on the method were not obtained. In Lopes 1995, the random allocation was done using numbered envelopes kept in the operating theatre. However, the authors did not specifically describe whether or not the envelopes were sealed and opaque. In Benedetti‐Panici 1997, it was stated that "Randomization was centralized and computer‐based". Franchi 2007 recruited participants from 12 European cancer centres; there was no detailed explanation on allocation concealment method.
Blinding
For the outcome of lymphocyst formation, the outcome assessors were unaware of the ongoing study in Benedetti‐Panici 1997, and unaware of the group allocation in Srisomboon 2002. The blinding of outcome assessors was not reported in Lopes 1995 and Franchi 2007.
For the postoperative morbidities and recovery outcome, the blinding of participants, treatment providers, and outcome assessors was not documented in Benedetti‐Panici 1997, Franchi 2007, and Lopes 1995. In Srisomboon 2002, the participants, treatment providers, and outcome assessors were not blinded.
Incomplete outcome data
Incomplete data on lymphocyst formation outcome were addressed in all studies (see the corresponding 'Risk of bias' tables under Characteristics of included studies).
Selective reporting
In Lopes 1995 and Srisomboon 2002, the outcomes prespecified in the Methods section were reported. In the other two studies, the rate of asymptomatic lymphocyst formation (Benedetti‐Panici 1997), and the rate of asymptomatic and symptomatic lymphocyst formation at one month after surgery (Franchi 2007), was not specifically reported.
The 'Risk of bias' graph and 'Risk of bias' summary are shown in Figure 1 and Figure 2.
Effects of interventions
See: Table 1
For the outcomes with data available, the number of studies contributing usable data ranged from one to four. When compared to no drainage, retroperitoneal drainage after pelvic lymphadenectomy was associated with the following.
Comparable rate of overall (asymptomatic and symptomatic) lymphocyst formation within four weeks after surgery, when all methods of pelvic peritoneum management (left open in all cases or closed in the drain group) were considered together (two studies Benedetti‐Panici 1997 and Srisomboon 2002, 204 patients; risk ratio (RR) 0.76, 95% confidence interval (CI) 0.04 to 13.35 (Analysis 1.1)). However, when the pelvic peritoneum was left open in all cases, the rate of overall lymphocyst formation within four weeks after surgery was significantly higher in the drain group (one study Benedetti‐Panici 1997, 110 patients; RR 2.29, 95% CI 1.38 to 3.79 (Analysis 1.1)).
Significantly higher rate of symptomatic lymphocyst formation within four weeks after surgery when all methods of pelvic peritoneum management (left open in all cases or closed in the drain group) were considered together as well as when the pelvic peritoneum was left open in all cases (two studies Benedetti‐Panici 1997 and Srisomboon 2002, 237 patients; RR 3.25, 95% CI 1.26 to 8.37 (Analysis 1.2)).
Comparable rate of overall (asymptomatic and symptomatic) lymphocyst formation at eight weeks after surgery, either when all methods of pelvic peritoneum management (left open in all cases or closed in the drain group) were considered together (two studies Lopes 1995 and Srisomboon 2002, 180 patients; RR 0.72, 95% CI 0.30 to 1.71 (Analysis 1.3)) or when each method of peritoneum management was considered separately (one study Lopes 1995 with pelvic peritoneum left open in all cases, 91 patients; RR 0.89, 95% CI 0.35 to 2.26 (Analysis 1.3) and one study Srisomboon 2002 with pelvic peritoneum closed in the drain group, 89 patients; RR 0.19, 95% CI 0.01 to 3.79 (Analysis 1.3)).
Comparable rate of overall (asymptomatic and symptomatic) lymphocyst formation at 12 weeks after surgery when the pelvic peritoneum was closed in the drain group (one study Srisomboon 2002, 83 patients; RR 0.13, 95% CI 0.01 to 2.38 (Analysis 1.4)).
Comparable rate of overall (asymptomatic and symptomatic) lymphocyst formation at 12 months after surgery (one study Franchi 2007, 232 patients; RR 1.48, 95% CI 0.89 to 2.45 (Analysis 1.5)) and a trend toward increased risk of symptomatic lymphocyst formation at 12 months after surgery (one study Franchi 2007, 232 patients; RR 7.12, 95% CI 0.89 to 56.97 (Analysis 1.6)) when the pelvic peritoneum was left open in all cases.
Comparable rate of postoperative febrile morbidity, regardless of the methods of pelvic peritoneum management (four studies Benedetti‐Panici 1997, Franchi 2007, Lopes 1995, and Srisomboon 2002, 571 patients; RR 1.76, 95% CI 0.87 to 3.55 (Analysis 1.7)).
Comparable rate of postoperative pelvic infection regardless of the methods of pelvic peritoneum management (four studies Benedetti‐Panici 1997, Franchi 2007, Lopes 1995, and Srisomboon 2002, 571 patients; RR 0.42, 95% CI 0.11 to 1.62 (Analysis 1.8)).
Comparable rate of postoperative wound infection when all methods of pelvic peritoneum management (left open in all cases or closed in the drain group) were considered together (two studies Franchi 2007 and Srisomboon 2002, 334 patients; RR 0.29, 95% CI 0.07 to 1.18 (Analysis 1.9)). However, when the pelvic peritoneum was left open in all cases, there was a significant decrease in the rate of postoperative wound infection in the drain group (one study Franchi 2007, 234 patients; RR 0.13, 95% CI 0.02 to 0.98 (Analysis 1.9)).
Comparable rate of wound dehiscence (two studies Benedetti‐Panici 1997 and Lopes 1995, 237 patients; RR 0.99, 95% CI 0.14 to 6.89 (Analysis 1.10)), fistula formation (three studies Benedetti‐Panici 1997, Franchi 2007, and Lopes 1995, 471 patients; RR 1.24, 95% CI 0.34 to 4.57 (Analysis 1.11), bowel obstruction (two studies Franchi 2007 and Srisomboon 2002, 334 patients; RR 0.20, 95% CI 0.01 to 4.12 (Analysis 1.12)), leg oedema (one study Benedetti‐Panici 1997, 137 patients; RR 2.71, 95% CI 0.75 to 9.77 (Analysis 1.13)), deep venous thrombosis (two studies Benedetti‐Panici 1997 and Franchi 2007, 371 patients; RR 2.02, 95% CI 0.38 to 10.84 (Analysis 1.14)), symptomatic ascites (one study Benedetti‐Panici 1997, 137 patients; RR 0.68, 95% CI 0.12 to 3.92 (Analysis 1.15)), and need for blood transfusion (two studies, Benedetti‐Panici 1997 and Srisomboon 2002, 237 patients; RR 0.91, 95% CI 0.68 to 1.21 (Analysis 1.16)).
Comparable duration of surgery (Srisomboon 2002, 100 patients; mean difference 4.20 minutes, 95% CI ‐15.35 to 23.75 (Analysis 1.17)). Similarly, there was no significant difference in duration of surgery in the two studies that reported this outcome as a median (10 minutes; 190 minutes in the drain group, 180 minutes in the no drain group in Benedetti‐Panici 1997 and five minutes; 240 minutes in the drain group, 245 minutes in the no drain group in Franchi 2007).
Comparable time interval to the return of bowel sounds (Srisomboon 2002, 100 patients; mean difference 0.14 day, 95% CI ‐0.11 to 0.39 (Analysis 1.18)). Similarly, there was no significant difference in the time interval to the return of bowel sounds in the two studies that reported this outcome as a mean only (Lopes 1995, 0.02 day; 1.04 day in the drain group, 1.02 day in the no drain group) and as a median (Franchi 2007, 0 day; two days in the drain group, two days in the no drain group).
Comparable postoperative decrease of total protein (‐0.1 mg%; 0.6 mg% in the drain group, 0.7 mg% in the no drain group) and albumin (‐0.1 mg%; 0.5 mg% in the drain group, 0.6 mg% in the no drain group) in the only study that reported this outcome as a median (Benedetti‐Panici 1997).
Comparable duration of postoperative hospital stay (two studies Lopes 1995 and Srisomboon 2002, 200 patients; mean difference 0.20 day, 95% CI ‐0.34 to 0.74 (Analysis 1.19)). However, the postoperative hospital stay was significantly longer in the drain group in a study that reported this outcome as a median (four days; 11 days in the drain group, seven days in the no drain group) (Benedetti‐Panici 1997).
Analysis 1.1.
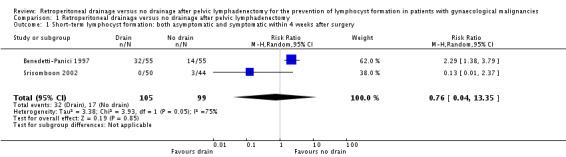
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 1 Short‐term lymphocyst formation: both asymptomatic and symptomatic within 4 weeks after surgery.
Analysis 1.2.
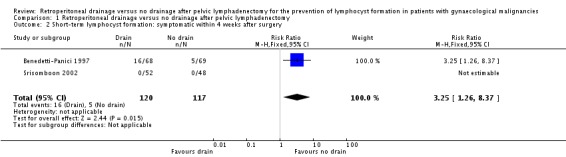
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 2 Short‐term lymphocyst formation: symptomatic within 4 weeks after surgery.
Analysis 1.3.
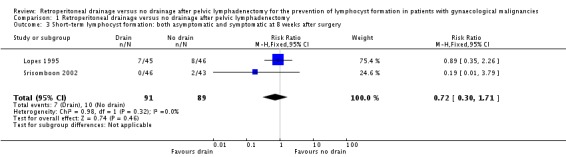
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 3 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 8 weeks after surgery.
Analysis 1.4.
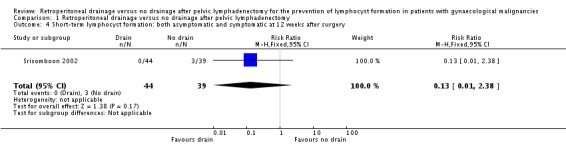
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 4 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 12 weeks after surgery.
Analysis 1.5.
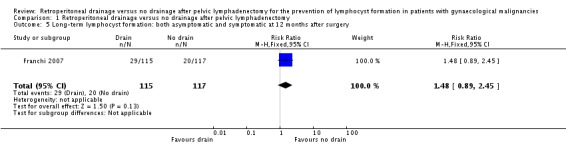
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 5 Long‐term lymphocyst formation: both asymptomatic and symptomatic at 12 months after surgery.
Analysis 1.6.
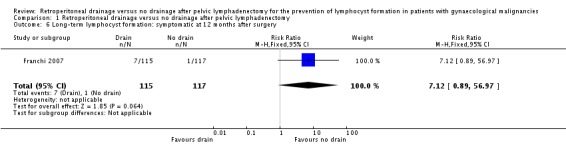
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 6 Long‐term lymphocyst formation: symptomatic at 12 months after surgery.
Analysis 1.7.
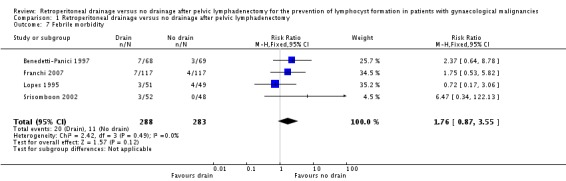
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 7 Febrile morbidity.
Analysis 1.8.
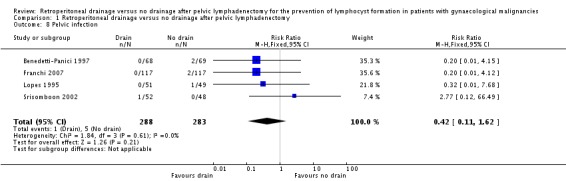
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 8 Pelvic infection.
Analysis 1.9.
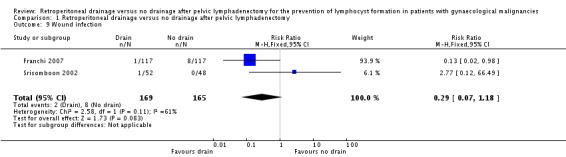
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 9 Wound infection.
Analysis 1.10.
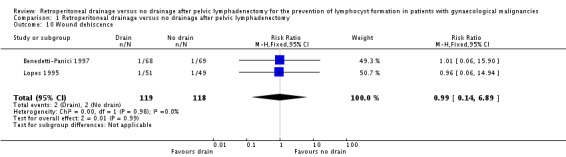
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 10 Wound dehiscence.
Analysis 1.11.
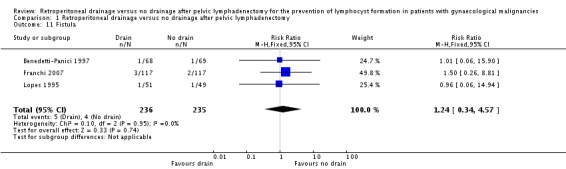
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 11 Fistula.
Analysis 1.12.
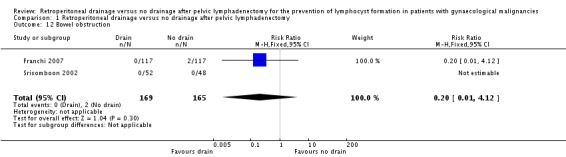
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 12 Bowel obstruction.
Analysis 1.13.
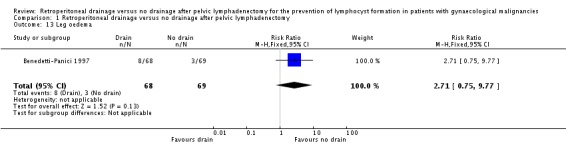
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 13 Leg oedema.
Analysis 1.14.
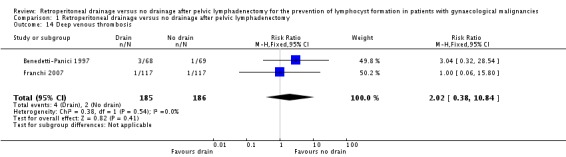
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 14 Deep venous thrombosis.
Analysis 1.15.
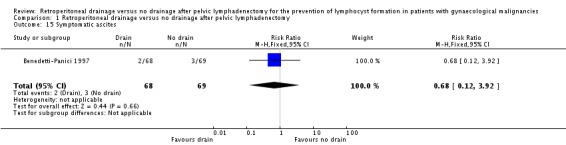
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 15 Symptomatic ascites.
Analysis 1.16.
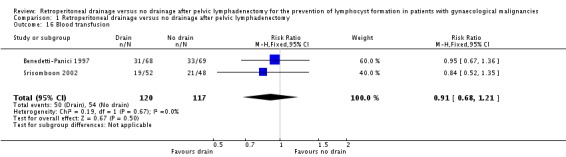
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 16 Blood transfusion.
Analysis 1.17.
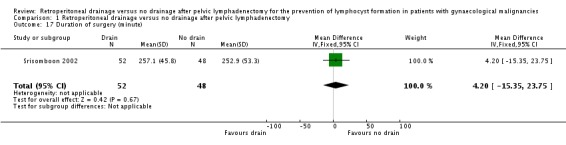
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 17 Duration of surgery (minute).
Analysis 1.18.
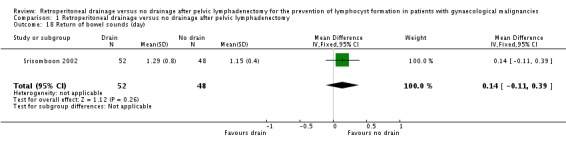
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 18 Return of bowel sounds (day).
Analysis 1.19.
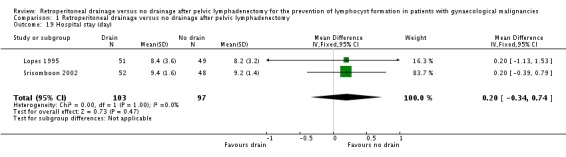
Comparison 1 Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy, Outcome 19 Hospital stay (day).
Discussion
Summary of main results
The incidence of lymphocyst formation varied among the included studies. This could be due to different surgical techniques, and also due to different methods and criteria for diagnosis. The available evidence has suggested that placement of retroperitoneal drains does not reduce the incidence of lymphocyst formation after pelvic lymphadenectomy in gynaecological cancer patients. In fact, if the pelvic peritoneum is not closed, the drain placement is associated with a higher incidence of symptomatic lymphocyst formation within a month and potentially so at 12 months following surgery. This could be explained by the drain itself acting as a foreign body and interfering with reparative and absorptive function of the pelvic peritoneum. The practice of leaving the pelvic peritoneum open appears to be an effective alternative method of pelvic retroperitoneal drainage, which allows lymphatic fluid to be reabsorbed by the peritoneal surface without the need to insert foreign material into the surgical site. Of note, when the pelvic peritoneum is left open, fluid produced from the pelvic lymphadenectomy area may freely circulate in the abdominal cavity. Ascites could develop as a result of imbalance between fluid production from the retroperitoneal area and fluid absorption by the peritoneal surface. Having the tube drain in place might lower the risk of developing ascites. However, the rate of symptomatic ascites was evidently the same between the patients with a drain (3%) and without a drain (4%) (Benedetti‐Panici 1997).
Cost‐effectiveness is an important issue that has not been adequately addressed in the studies on effects of drainage on lymphocyst formation. However, one would assume that drainage requires more materials, more personnel, and more time to spend supervising postoperative care. All of these together increase costs of health care. In addition, patients' comfort is another issue that need special attention. One could assume that patients without a drain would be more comfortable and more ambulant.
Leaving the vaginal vault open is another method of passive retroperitoneal drainage. In one included study (Lopes 1995), this practice was applied in combination with non‐closure of the pelvic peritoneum. In that case, it was found that patients without tube drains had a higher incidence of serosanguinous fluid loss through the vagina in the first postoperative day (12% versus 4%). After the first day, the loss continued in 4% of the patients without the tube drains and 2% of those with the tube drains. There are no data on the effect of leaving the vaginal vault open alone as a preventive measure for lymphocyst formation following pelvic lymphadenectomy.
Overall completeness and applicability of evidence
The number of eligible studies that examined the effects of retroperitoneal drainage following pelvic lymphadenectomy is limited. In addition, only a small number of studies contributed data for individual outcome measures. Furthermore, there were differences in the detail of the surgical procedures among the included trials, for example, method of pelvic peritoneum management (open or closed), vaginal vault management (open or closed), type of drains (active or passive), route of drains (abdominal or vaginal), exact location for placement of drains, and criteria for removal of drains. One important discrepancy of surgical technique among the included studies should be noted. In Srisomboon 2002, the pelvic peritoneum was sutured closed in the drain group and left open in the no drain group, whereas in the other three included studies, the pelvic peritoneum was left open in all patients regardless of the group allocation. Closing the pelvic peritoneum in the group with drains and leaving it open in the group without drains is the comparison of two sets of surgical management strategies that consider both placement of the tube drains and management of the pelvic peritoneum together, not the drain placement alone. Therefore, when this study is combined with the other studies in the final analysis, the result should be interpreted with this discrepancy in mind and bias in favour of the drain group is expected. However, the result of the final analysis appeared to be in the same direction whether this study was included or excluded. This finding would confirm that placement of retroperitoneal tube drains has no benefit in preventing lymphocyst formation. This information should be applicable to a wide range of gynaecological cancer operations that include pelvic lymphadenectomy as a part of the entire procedure.
Quality of the evidence
In general, the methodological quality of the included studies was acceptable, with some limitations. The allocation concealment appeared adequate or potentially adequate in all trials. Incomplete data on the main outcome, i.e. lymphocyst formation, were addressed in all studies. The important limitation of the included studies is the process of blinding. According to characteristics of the study intervention (retroperitoneal tube drainage), blinding of patients and care provider is difficult. On the other hand, blinding of outcome assessors is possible. However, for the diagnosis of lymphocyst formation by ultrasound, blinding of the outcome assessors was clearly documented in only two of the four included studies (Benedetti‐Panici 1997; Srisomboon 2002). Although, there are objective criteria for ultrasound diagnosis of pelvic lymphocyst, the final diagnostic decision sometimes involves a subjective component. In that case, lack of blinding could introduce bias to the results. Also, for the postoperative morbidities and recovery outcome, the blinding of participants, treatment providers, and outcome assessors was not documented in all included studies.
The possibility of publication bias should be kept in mind. In an attempt to assess its presence, we examined the funnel plots for all main outcomes. The findings were not suggestive of publication bias. However, we did not reach a meaningful conclusion on this issue due to the limited number of included studies.
Agreements and disagreements with other studies or reviews
There are a few retrospective and prospective non‐RCTs that examine the effect of retroperitoneal tube drainage on the prevention of lymphocyst formation after pelvic lymphadenectomy in gynaecological cancer patients. The results and conclusions from these studies are in keeping with the findings from the present review. In Jensen 1993, the records of 115 early‐stage cervical cancer patients were retrospectively reviewed. There was no difference between the patients with drains and without drains in the mean operative time, mean estimated blood loss, transfusion rate, febrile morbidity rates, incidence of pelvic cellulitis, length of postoperative ileus, and total hospital stay. However, the patients with drains had an increased rate of rehospitalisation and morbidity directly related to the presence of the drains. Patsner 1995 is a prospective non‐RCT comparing the effects of closed‐suction drainage versus no drainage after radical abdominal hysterectomy and bilateral pelvic lymphadenectomy for 120 stage IB cervical cancer patients. In this study, all operations were performed in a uniform manner by one surgeon. The vaginal cuff was closed and the pelvic peritoneum was left open in all cases. In addition, an omental J‐flap was brought into the pelvis in all patients and secured to the pelvic floor. There was no increase in postoperative lymphocyst formation, pelvic infection, and fistula in the group without drains. Of note, the rate of lymphocyst formation was 6.7% in the drain group and 0% in the no drain group. The exact role of the omental J‐flap in prevention of lymphocyst formation is unknown. In another prospective non‐RCT that compared the effects of closed suction pelvic drainage versus no suction drainage following pelvic/pelvic‐para‐aortic lymphadenectomy for 143 patients with various gynaecological malignancies (Bafna 2001), rates of lymphocyst formation were comparable between the study groups (7.3% in the drain group and 2.7% in the no drain group). The pelvic peritoneum was sutured closed in the drain group and left open in the no drain group.
Authors' conclusions
Since the last published version of this review, no new studies were found for inclusion. The findings from this review have demonstrated that retroperitoneal tube drain placement has no benefit in prevention of lymphocyst formation after pelvic lymphadenectomy in patients with gynaecological malignancies. Furthermore, the evidence suggests that when the pelvic peritoneum is left open, the tube drain placement is associated with a higher risk of short and long‐term symptomatic lymphocyst formation. The practice of leaving the pelvic peritoneum open appears to be an effective alternative method of pelvic retroperitoneal drainage.
Due to the consistent data on the effect of retroperitoneal tube drain placement for the prevention of lymphocyst formation, further research on this issue would be unnecessary. However, the role of leaving the vaginal vault open, and using the omental transposition/omental J‐flap alone or in combination with no‐closure of the pelvic peritoneum, in reducing lymphocyst formation and related morbidities will be interesting topics for future research.
Acknowledgements
We thank Clare Jess, Managing Editor, for her contribution to the editorial process, and Jane Hayes, Information Manager, for her help with literature search.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Gynaecological Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Genital Neoplasms, Female explode all trees\\ #2 ((gynae* or gyne* or genital* or pelvi* or ovar* or uter* or fallopian* or vulva* or endometr* or vagina* or cervi*) near/5 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma*)) #3 (#1 OR #2) #4 MeSH descriptor Lymph Node Excision explode all trees #5 lymphadenectom* #6 (lymph* and node* and (excis* or dissect*)) #7 MeSH descriptor Lymphocele, this term only #8 (lymphocele* or lymphocyst* or (lymph* and cyst*)) #9 (#4 OR #5 OR #6 OR #7) #10 MeSH descriptor Drainage explode all trees #11 MeSH descriptor Retroperitoneal Space, this term only #12 (drain* or retroperitone*) #13 (#10 OR #11 OR #12) #14 (#3 AND #9 AND #13)
Appendix 2. MEDLINE search strategy
MEDLINE Ovid
1 exp Genital Neoplasms, Female/ 2 ((gynae* or gyne* or genital* or pelvi* or ovar* or uter* or fallopian* or vulva* or endometr* or vagina* or cervi*) adj5 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma*)).mp. 3 1 or 2 4 exp Lymph Node Excision/ 5 lymphadenectom*.mp. 6 (lymph* and node* and (excis* or dissect*)).mp. 7 Lymphocele/ 8 (lymphocele* or lymphocyst* or (lymph* and cyst*)).mp. 9 4 or 5 or 6 or 7 or 8 10 *Drainage/ 11 Retroperitoneal Space/ 12 (drain* or retroperitone*).mp. 13 10 or 11 or 12 14 3 and 9 and 13 15 randomized controlled trial.pt. 16 controlled clinical trial.pt. 17 randomized.ab. 18 placebo.ab. 19 clinical trials as topic.sh. 20 randomly.ab. 21 trial.ti. 22 15 or 16 or 17 or 18 or 19 or 20 or 21 23 14 and 22
key: mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier pt=publication type ab=abstract ti=title sh=subject heading
Appendix 3. EMBASE search strategy
EMBASE Ovid
1 exp female genital tract tumor/ 2 ((gynae* or gyne* or genital* or pelvi* or ovar* or uter* or fallopian* or vulva* or endometr* or vagina* or cervi*) adj5 (cancer* or tumor* or tumour* or neoplas* or malignan* or carcinoma* or adenocarcinoma*)).mp. 3 1 or 2 4 exp lymphadenectomy/ 5 lymphadenectom*.mp. 6 (lymph* and node* and (excis* or dissect*)).mp. 7 lymphocele/ 8 (lymphocele* or lymphocyst* or (lymph* and cyst*)).mp. 9 4 or 5 or 6 or 7 or 8 10 exp surgical drainage/ 11 retroperitoneum/ 12 (drain* or retroperitone*).mp. 13 10 or 11 or 12 14 3 and 9 and 13 15 crossover procedure/ 16 double‐blind procedure/ 17 randomized controlled trial/ 18 single‐blind procedure/ 19 random*.mp. 20 factorial*.mp. 21 (crossover* or cross over* or cross‐over*).mp. 22 placebo*.mp. 23 (double* adj blind*).mp. 24 (singl* adj blind*).mp. 25 assign*.mp. 26 allocat*.mp. 27 volunteer*.mp. 28 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 29 14 and 28
key: [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
Appendix 4. Specific criteria for allocation concealment rating
Clearly yes: rate A
Some form of centralised randomisation scheme, such as having to provide participant details by phone to receive treatment group allocation
A scheme controlled by a pharmacy or nutritional unit
An on‐site computer system, given that allocations are in a locked unreadable file which can be accessed only after inputting participant details
Assignment envelopes, provided that they are sequentially numbered, sealed, and opaque
Other combinations which appear to provide assurance of adequate concealment
Unclear: rate B
Assignment envelopes, without description of adequate safeguards
Use of a 'list' or 'table'
Flip of a coin
A trial in which the description suggests adequate concealment, but other features are suspicious ‐ for example, markedly unequal controls and trial groups
Stated random, but unable to obtain further details
Clearly no: rate C
Alternation
Case record numbers, dates of birth, day of the week, or any other such approach
Any allocation procedure transparent before assignment, such as an open list of random numbers
Data and analyses
Comparison 1.
Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term lymphocyst formation: both asymptomatic and symptomatic within 4 weeks after surgery | 2 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.04, 13.35] |
| 2 Short‐term lymphocyst formation: symptomatic within 4 weeks after surgery | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [1.26, 8.37] |
| 3 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 8 weeks after surgery | 2 | 180 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.30, 1.71] |
| 4 Short‐term lymphocyst formation: both asymptomatic and symptomatic at 12 weeks after surgery | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.01, 2.38] |
| 5 Long‐term lymphocyst formation: both asymptomatic and symptomatic at 12 months after surgery | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.48 [0.89, 2.45] |
| 6 Long‐term lymphocyst formation: symptomatic at 12 months after surgery | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.12 [0.89, 56.97] |
| 7 Febrile morbidity | 4 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.76 [0.87, 3.55] |
| 8 Pelvic infection | 4 | 571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.11, 1.62] |
| 9 Wound infection | 2 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.07, 1.18] |
| 10 Wound dehiscence | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.14, 6.89] |
| 11 Fistula | 3 | 471 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.34, 4.57] |
| 12 Bowel obstruction | 2 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.12] |
| 13 Leg oedema | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.71 [0.75, 9.77] |
| 14 Deep venous thrombosis | 2 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.38, 10.84] |
| 15 Symptomatic ascites | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.12, 3.92] |
| 16 Blood transfusion | 2 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.68, 1.21] |
| 17 Duration of surgery (minute) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 4.20 [‐15.35, 23.75] |
| 18 Return of bowel sounds (day) | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.11, 0.39] |
| 19 Hospital stay (day) | 2 | 200 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.34, 0.74] |
What's new
Last assessed as up‐to‐date: 10 January 2014.
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 1, 2010
| Date | Event | Description |
|---|---|---|
| 11 February 2015 | Amended | Contact details updated. |
| 3 June 2014 | Amended | Author contact details amended. |
| 30 April 2014 | New search has been performed | The review was updated. The top‐up literature search was performed on 10 January 2014. |
| 30 April 2014 | New citation required but conclusions have not changed | No new relevant studies met the inclusion criteria. Therefore, further analysis was unnecessary and the conclusion remains the same. |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Benedetti‐Panici 1997
| Methods | RCT. Randomisation was determined using a computer‐based method. The groups were comparable at entry. There was no exclusion after allocation and no withdrawal. Weekly abdominal and pelvic ultrasound was performed on 110 of 137 patients to detect lymphocyst formation. | |
| Participants | Patients with FIGO stage I‐IV ovarian carcinoma (43 patients), stage IB‐III cervical carcinoma (45 patients), and stage I‐II endometrial carcinoma (49 patients). The patients underwent intensive surgical staging/tumour reductive surgery/second‐look laparotomy (ovarian carcinoma) or Piver type I/II radical hysterectomy (endometrial carcinoma) or Piver type III/IV radical hysterectomy (cervical carcinoma) plus bilateral pelvic or pelvic and para‐aortic lymphadenectomy Study setting: the Catholic University of Rome and the 'S. Carlo di Nancy' Hospital, Rome, Italy |
|
| Interventions | Patients were randomised intraoperatively to the use of drains (68 patients) or no drain (69 patients). For those randomised to drains, 2 retroperitoneal low‐pressure closed‐suction drains were placed. For pelvic/para‐aortic lymphadenectomy, the first drain was placed at the insertion of the mesenteric artery down to the left external iliac artery and the second at the level of the paracaval area from the point where the ovarian vein enters the cava down to the right external iliac artery. For pelvic lymphadenectomy, the cranial part of the drains was at the level of the ipsilateral common iliac vessels. The drains were removed when the loss was less than 50 mL in 24 hours | |
| Outcomes | There was a significant increase in complication (43% versus 22%) in the drain group, mainly related to lymphocyst. The hospital stay was shorter in the group not drained | |
| Notes | In all cases, the pelvic peritoneum and the peritoneum along the paracolic gutters were left open | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | It was stated that "Randomization was centralized and computer‐based." |
| Allocation concealment (selection bias) | Low risk | It was stated that "Randomization was centralized and computer‐based." The allocation concealment was probably adequate |
| Blinding (performance bias and detection bias) Lymphocyst formation | Low risk | Weekly ultrasound pelvic and abdominal examinations were performed; the ultrasound operator was unaware of the ongoing study |
| Blinding (performance bias and detection bias) Postoperative morbidities and recovery | Unclear risk | The blinding of participants, treatment providers, and outcome assessors was not documented |
| Incomplete outcome data (attrition bias) Short‐term lymphocyst formation | Low risk | It was stated that the weekly ultrasound examinations to detect lymphocyst formation were performed on 110 patients operated on at the Catholic University (of 137 patients in the study) |
| Selective reporting (reporting bias) | Unclear risk | The rate of asymptomatic lymphocyst formation was not specifically addressed |
Franchi 2007
| Methods | Multicentre RCT (12 European cancer centres). Randomisation was determined using a computer‐based method. Stratification was performed per centre. The groups were comparable at entry. There was no exclusion after allocation and no withdrawal. Ultrasonography or computerised tomography (CT) scan was performed at 1 and 12 months postoperatively on all patients to identify lymphocyst formation | |
| Participants | Patients with FIGO stage IA1‐IIA cervical carcinoma (198 patients), endometrial carcinoma (35 patients), and vaginal carcinoma (1 patient). All had radical hysterectomy and bilateral pelvic lymphadenectomy Study setting: European cancer centres (the EORTC trial) |
|
| Interventions | Patients were randomised following surgery to either pelvic drainage (117 patients) or no drainage (117 patients). For those randomised to drains, 2 passive or active suction drains were placed in the retroperitoneal fossa and inserted via the vagina or the abdominal route, according to the institution's policy. The drains were removed when the loss was less than 50 mL in 24 hours | |
| Outcomes | No difference in the incidence of postoperative lymphocyst formation or postoperative complications was found between the 2 groups. The late (12 months) incidence of symptomatic lymphocysts was 5.9% in the drain group versus 0.9% in the no drain group (P = 0.06) | |
| Notes | In all cases, the vaginal cuff was primarily closed and the pelvic peritoneum was left open | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence generation was probably adequate. The participants were recruited from 12 European cancer centres. Stratification was performed per centre |
| Allocation concealment (selection bias) | Unclear risk | There was no detailed explanation on allocation concealment method |
| Blinding (performance bias and detection bias) Lymphocyst formation | Unclear risk | At 1 and 12 months postoperatively, imaging was performed by ultrasonography or CT scan to detect lymphocyst formation. The blinding of outcome assessors was not documented |
| Blinding (performance bias and detection bias) Postoperative morbidities and recovery | Unclear risk | The blinding of participants, treatment providers, and outcome assessors was not documented |
| Incomplete outcome data (attrition bias) Short‐term lymphocyst formation | Low risk | 2 missing cases in the drains group at 12‐month follow‐up visit were documented |
| Selective reporting (reporting bias) | Unclear risk | The rate of asymptomatic and symptomatic lymphocyst formation at 1 month after surgery was not specifically reported |
Lopes 1995
| Methods | RCT. Randomisation was determined using a random number table. The groups were comparable at entry. There was no exclusion after allocation and no withdrawal. An abdominal ultrasound scan was performed approximately 8 weeks after the surgery to identify any asymptomatic Iymphocysts | |
| Participants | Patients with FIGO stage IA‐IIB cervical carcinoma (95 patients) and stage IIB endometrial carcinoma (5 patients). All had Piver type II radical hysterectomy and bilateral pelvic lymphadenectomy Study setting: Regional Department of Gynaecological Oncology, Gateshead, United Kingdom |
|
| Interventions | Patients were randomised immediately before abdominal closure to the use of drains (51 patients) or no drain (49 patients). For those randomised to drains, 2 suction drains were inserted, 1 through each iliac fossa, and placed alongside the site of node dissection. The drains were usually removed when the loss was less than 100 mL in 24 hours | |
| Outcomes | The detection of lymphocysts by ultrasound and clinical examination in the drain group (15.6% and 5.9%, respectively) was not significantly different from the group not drained (17.4% and 6.1%, respectively). Postoperative morbidity was not different between the groups | |
| Notes | In each case, the vaginal cuff edge was over sewn, leaving the vaginal vault open, and the pelvis was not reperitonised Of the 100 patients randomised, 8 defaulted their ultrasound scan; 5 were in the drained group and 3 were not. Of these 8, 2 (1 in each group) were assessed further; 1 had a magnetic resonance imaging scan and another had a laparotomy, both showing no evidence of lymphocysts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was determined using a random number table |
| Allocation concealment (selection bias) | Low risk | The random allocation was done using numbered envelopes kept in the operating theatre. The allocation concealment was probably adequate although the authors did not specifically describe whether the envelopes were sealed and opaque or not |
| Blinding (performance bias and detection bias) Lymphocyst formation | Unclear risk | An abdominal ultrasound scan was performed approximately 8 weeks after surgery to identify any asymptomatic lymphocysts. Also, the clinical detection of lymphocysts at subsequent review visits was documented. However, any attempt to blind outcome assessors was not demonstrated |
| Blinding (performance bias and detection bias) Postoperative morbidities and recovery | Unclear risk | The blinding of participants, treatment providers, and outcome assessors was not documented |
| Incomplete outcome data (attrition bias) Short‐term lymphocyst formation | Low risk | It was stated that "Of the 100 patients randomized, eight defaulted their ultrasound scan; five were in the drained group and three were not." Also, it was stated that "the scan was reported as unsatisfactory in one patient who had been drained." |
| Selective reporting (reporting bias) | Low risk | — |
Srisomboon 2002
| Methods | RCT. Randomisation was conducted by block randomisation. The groups were comparable at entry. There was no exclusion after allocation and no withdrawal. Transabdominal and transvaginal ultrasound were performed at 4, 8, and 12 weeks after surgery to detect lymphocyst formation | |
| Participants | Patients with FIGO stage IA2‐IIA cervical carcinoma (100 patients). All had Piver type II/III radical hysterectomy and bilateral pelvic lymphadenectomy Study setting: Chiang Mai University Hospital, Chiang Mai, Thailand |
|
| Interventions | Patients were randomised immediately before abdominal closure to the use of drains with closure of pelvic peritoneum (52 patients) or no drain with pelvic peritoneum left open (48 patients). For those randomised to drains, 2 low‐pressure closed‐suction drains were placed along the side of node dissection and brought out extraperitoneally through the anterior abdominal wall lateral to the rectus muscle margins. The drains were removed when the loss was less than 50 mL in 24 hours | |
| Outcomes | Asymptomatic lymphocysts were sonographically detected at 4, 8, and 12 weeks postoperatively in 6.8%, 4.6%, and 7.7% of patients, respectively, in the group not drained, whereas none were found in the drain group (P = 0.2). Postoperative morbidity was not significantly different between the 2 groups | |
| Notes | The vaginal vault was primarily closed in all cases Of 52 patients in the drained group, 50, 46, and 44 patients had ultrasound done at 4, 8, and 12 weeks after surgery, respectively, as scheduled. Of 48 patients in the no drain group, 44, 43, and 39 patients underwent ultrasound examination at each scheduled visit |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The method of block randomisation was used |
| Allocation concealment (selection bias) | Low risk | The random allocation was done using sealed, numbered envelopes kept in the operating theatre |
| Blinding (performance bias and detection bias) Lymphocyst formation | Low risk | The operators of transabdominal and transvaginal ultrasound to detect lymphocyst formation were not aware of the group allocation |
| Blinding (performance bias and detection bias) Postoperative morbidities and recovery | High risk | The participants, treatment providers, and outcome assessors were not blinded |
| Incomplete outcome data (attrition bias) Short‐term lymphocyst formation | Low risk | The number of participants who had postoperative ultrasound performed at each visit as scheduled was reported. Accordingly, the number of missing cases was realised |
| Selective reporting (reporting bias) | Low risk | — |
CT = computerised tomography EORTC = European Organisation for Research and Treatment of Cancer FIGO = International Federation of Gynecology and Obstetrics RCT = randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bafna 2001 | Not a RCT |
| Franchi 1997 | RCT comparing closure of pelvic and parietal peritoneum (with placement of a T‐shape suction drain through the vagina) to no peritoneal closure (but the vagina closed and 2 abdominal drains placed) |
| Jensen 1993 | Not a RCT |
| Morice 2001 | RCT comparing placement of a low‐pressure drain in the aortic area to no placement of para‐aortic drain after complete para‐aortic lymphadenectomy. However, most patients had suction drains placed in the pelvis |
| Orr 1986 | Not a RCT |
| Patsner 1995 | Not a RCT |
| Patsner 1999 | Not a RCT |
| Yamamoto 2000 | Studied the effect of the procedure to prevent vaginal shortening on lymphocyst formation. A closed‐suction drain was placed in each paravesical space for all patients. Not a RCT |
RCT = randomised controlled trial
Contributions of authors
Kittipat Charoenkwan: took the lead in writing the review, selected trials for inclusion, extracted data, and performed statistical analysis and interpretation of data. Chumnan Kietpeerakool: selected trials for inclusion, extracted data, performed statistical analysis and interpretation of data, and commented on drafts of the review.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Kittipat Charoenkwan is a co‐author of the article 'A prospective randomized study comparing retroperitoneal drainage with no drainage and no peritonization following radical hysterectomy and pelvic lymphadenectomy for invasive cervical cancer' published in the Journal of Obstetrics and Gynaecology Research 2002;28(3):149‐53.
Edited (no change to conclusions)
References
References to studies included in this review
- Benedetti‐Panici P, Maneschi F, Butillo G, D'Andrea G, Palumbo VS, Conte M, et al. A randomized study comparing retroperitoneal drainage with no drainage after lymphadenectomy in gynecologic malignancies. Gynecologic Oncology 1997;65:478‐82. [DOI] [PubMed] [Google Scholar]
- Franchi M, Trimbos J, Zanaboni F, Velden J, Reed N, Coens C, et al. Randomised trial of drains versus no drains following radical hysterectomy and pelvic lymph node dissection: a European Organisation for Research and Treatment of Cancer‐Gynaecological Cancer Group (EORTC‐GCG) study in 234 patients. European Journal of Cancer 2007;43:1265‐8. [DOI] [PubMed] [Google Scholar]
- Lopes AB, Hall JR, Monaghan JM. Drainage following radical hysterectomy and pelvic lymphadenectomy: dogma or need?. Obstetrics and Gynecology 1995;86:960‐3. [DOI] [PubMed] [Google Scholar]
- Srisomboon J, Phongnarisorn C, Suprasert P, Cheewakriangkrai C, Siriaree S, Charoenkwan K. A prospective randomized study comparing retroperitoneal drainage with no drainage and no peritonization following radical hysterectomy and pelvic lymphadenectomy for invasive cervical cancer. Journal of Obstetrics and Gynaecology Research 2002;28(3):149‐53. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Bafna UD, Umadevi K, Savitha M. Closed suction drainage versus no drainage following pelvic lymphadenectomy for gynecological malignancies. International Journal of Gynecological Cancer 2001;11:143‐6. [DOI] [PubMed] [Google Scholar]
- Franchi M, Ghezzi F, Zanaboni F, Scarabelli C, Beretta P, Donadello N. Nonclosure of peritoneum at radical abdominal hysterectomy and pelvic node dissection: a randomized study. Obstetrics and Gynecology 1997;90(4):622‐7. [DOI] [PubMed] [Google Scholar]
- Jensen JK, Lucci JA 3rd, DiSaia PJ, Manetta A, Berman ML. To drain or not to drain: a retrospective study of closed‐suction drainage following radical hysterectomy with pelvic lymphadenectomy. Gynecologic Oncology 1993;51(1):46‐9. [DOI] [PubMed] [Google Scholar]
- Morice P, Lassau N, Pautier P, Haie‐Meder C, Lhomme C, Castaigne D. Retroperitoneal drainage after complete paraaortic lymphadenectomy for gynecologic cancer: a randomized trial. Obstetrics and Gynecology 2001;97(2):243‐7. [DOI] [PubMed] [Google Scholar]
- Orr JW Jr, Barter JF, Kilgore LC, Soong SJ, Shingleton HM, Hatch KD. Closed suction pelvic drainage after radical pelvic surgical procedures. American Journal of Obstetrics and Gynecology 1986;155(4):867‐71. [DOI] [PubMed] [Google Scholar]
- Patsner B. Closed‐suction drainage versus no drainage following radical abdominal hysterectomy with pelvic lymphadenectomy for stage IB cervical cancer. Gynecologic Oncology 1995;57:232‐4. [DOI] [PubMed] [Google Scholar]
- Patsner B. Routine retroperitoneal drainage is not required for uncomplicated pelvic lymphadenectomy for uterine cancer. European Journal of Gynaecological Oncology 1999;20(2):87‐9. [PubMed] [Google Scholar]
- Yamamoto R, Saitoh T, Kusaka T, Todo Y, Takeda M, Okamoto K, et al. Prevention of lymphocyst formation following systematic lymphadenectomy. Japanese Journal of Clinical Oncology 2000;30(9):397‐400. [PubMed] [Google Scholar]
Additional references
- Conte M, Benedetti‐Panici P, Guariglia L, Scambia G, Greggi S, Mancuso S. Pelvic lymphocele following radical para‐aortic and pelvic lymphadenectomy for cervical carcinoma: incidence rate and percutaneous management. Obstetrics and Gynecology 1990;76:268‐71. [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated September 2008]. Higgins JPT, Green S (editors). The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [Updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
- Ilancheran A, Monaghan J. Pelvic lymphocyst ‐ a 10‐year experience. Gynecologic Oncology 1988;29:333‐6. [DOI] [PubMed] [Google Scholar]
- Livingston W, Confer D, Smith R. Large lymphoceles resulting from retroperitoneal lymphadenectomy. Journal of Urology 1980;124:543‐6. [DOI] [PubMed] [Google Scholar]
- Maitland A, Mathieson A. Suction drainage. A study in wound healing. British Journal of Surgery 1970;57:193‐7. [DOI] [PubMed] [Google Scholar]
- Petru E, Tamussino K, Lahousen M, Winter R, Pickel H, Haas J. Pelvic and para‐aortic lymphocysts after radical surgery because of cervical and ovarian cancer. American Journal of Obstetrics and Gynecology 1989;161:937‐41. [DOI] [PubMed] [Google Scholar]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
- Symmond R, Pratt J. Prevention of fistulas and lymphocysts in radical hysterectomy. Preliminary report of a new technique. Obstetrics and Gynecology 1961;17:57‐64. [PubMed] [Google Scholar]
- Symmond R. Morbidity and complications of radical hysterectomy with pelvic lymph node dissection. American Journal of Obstetrics and Gynecology 1966;94:663‐78. [DOI] [PubMed] [Google Scholar]
- Nagell J, Schweitz D. Surgical adjuncts in radical hysterectomy and pelvic lymphadenectomy. Surgery, Gynecology & Obstetrics 1976;143:735‐7. [PubMed] [Google Scholar]
References to other published versions of this review
- Charoenkwan K, Kietpeerakool C. Retroperitoneal drainage versus no drainage after pelvic lymphadenectomy for the prevention of lymphocyst formation in patients with gynaecological malignancies. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007387.pub2] [DOI] [PubMed] [Google Scholar]


